Abstract
Background
Both the triglyceride-glucose (TyG) index, as a surrogate marker of insulin resistance, and systemic inflammation are predictors of cardiovascular diseases; however, little is known about the coexposures and relative contributions of TyG index and inflammation to cardiovascular diseases. Using the nationally representative data from the China Health and Retirement Longitudinal Study (CHARLS), we conducted longitudinal analyses to evaluate the joint and mutual associations of the TyG index and high-sensitivity C-reactive protein (hsCRP) with cardiovascular events in middle-aged and older Chinese population.
Methods
This study comprised 8 658 participants aged at least 45 years from the CHARLS 2011 who are free of cardiovascular diseases at baseline. The TyG index was calculated as Ln [fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2]. Cardiovascular events were defined as the presence of physician-diagnosed heart disease and/or stroke followed until 2018.We performed adjusted Cox proportional hazards regression and mediation analyses.
Results
The mean age of the participants was 58.6 ± 9.0 years, and 3988 (46.1%) were females. During a maximum follow-up of 7.0 years, 2606 (30.1%) people developed cardiovascular diseases, including 2012 (23.2%) cases of heart diseases and 848 (9.8%) cases of stroke. Compared with people with a lower TyG index (< 8.6 [median level]) and hsCRP < 1 mg/L, those concurrently with a higher TyG and hsCRP had the highest risk of overall cardiovascular disease (adjusted hazard ratio [aHR], 1.300; 95% CI 1.155–1.462), coronary heart disease (aHR, 1.294; 95% CI 1.130–1.481) and stroke (aHR, 1.333; 95% CI 1.093–1.628), which were predominant among those aged 70 years or below. High hsCRP significantly mediated 13.4% of the association between the TyG index and cardiovascular disease, while TyG simultaneously mediated 7.9% of the association between hsCRP and cardiovascular risk.
Conclusions
The findings highlight the coexposure effects and mutual mediation between the TyG index and hsCRP on cardiovascular diseases. Joint assessments of the TyG index and hsCRP should be underlined for the residual risk stratification and primary prevention of cardiovascular diseases, especially for middle-aged adults.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02244-9.
Keywords: Triglyceride-glucose (TyG) index, Insulin resistance, Inflammation, High-sensitivity C-reactive protein (hsCRP), Cardiovascular disease
Background
Cardiovascular disease (CVD) is the largest contributor to global mortality [1]. In China, the prevalence of CVD has doubled since 1990, reaching nearly 94 million in 2016 [2]. There is an urgent need to understand the current epidemiological features and risk factors for the major types of CVD and the implications of these features for the primary prevention of CVD. Conventional risk factors in the Framingham risk score (FRS), such as age, cholesterol, hypertension, and smoking, account for most of the risk of CVD and have been used for risk assessment for decades [3]. However, approximately one-third of individuals with fewer risk factors develop CVD, and approximately 40% of individuals with low cholesterol levels die from coronary arterial events in the general population [4–6], highlighting the residual CVD risk [7]. Therefore, a wide array of other biomarkers for the refinement of risk assessment of CVD are promising.
Insulin resistance, characterized by a decrease in sensitivity or responsiveness to the metabolic actions of insulin, including insulin-mediated glucose disposal, has been widely identified as an independent risk factor for the development of CVD. The attributable risk to diabetes is relatively low than 10% [8], and that there is conflicting data on whether accounting for insulin resistance could further improve CVD risk prediction among different populations [9]. There is an acceleration of the incidence and prevalence of insulin resistance along with associated CVD [10]. The triglyceride-glucose (TyG) index has been proposed as a reliable surrogate indicator of insulin resistance [11, 12], which also plays an important role in CVD [13]. Recently, a considerable number of studies have provided strong evidence suggesting the predictive effect of the TyG index on CVD [14–16]. In addition, the TyG index is also closely associated with risk factors for cardiovascular diseases, such as arterial stiffness and hypertension [17, 18]. Notably, multifactorial risk factor evaluation and management are essential for the early prevention of CVD [19]. Systemic inflammation is often observed in CVD incidence [20]. A large body of evidence has accumulated supporting the use of high-sensitivity C-reactive protein (hsCRP) as a clinical measure of inflammation, which holds promise to enable risk stratification of CVD in clinical practice [21]. Previous studies have highlighted the need for combined assessment and management of chronic inflammation and atherogenic dyslipidemia in the primary prevention of CVD [20, 22]. A recent study found that adding hsCRP and TyG index to conventional risk model improved the risk reclassification for CVD, while there is no significant interaction between hsCRP and TyG index [23]. Our previous study suggested the combined evaluation of the TyG index and renal function for the risk reclassification of CVD, in which impaired renal function partially mediated the association between the TyG index and cardiovascular risk [24]. However, the mutual association between insulin resistance, inflammation and CVD remains unclear, given the possible mutual mediating effect between insulin resistance and inflammation in terms of CVD [25, 26].
To fill this knowledge gap, we therefore conducted a longitudinal study based on data from a prospective national cohort to examine the joint effect and risk reclassification capacity of the TyG index and hsCRP level with the onset of CVD. Together, we conducted a mediation analysis to underline the mutual mediating effect linking the TyG index and hsCRP level to the development of CVD.
Methods
Data source and study population
This current study was a secondary analysis of the China Health and Retirement Longitudinal Study (CHARLS), which is a national population-based cohort study (http://charls.pku.edu.cn/) among Chinese adults aged 45 years or older initiated in 2011. The following health surveys were issued every 2 years, with a total of four surveys through 2018. The participants were recruited from both rural and urban areas using a multistage stratified probability proportional-to-size sampling strategy and covered 150 counties or districts of 28 provinces in China. Details of the study design and cohort profile have been previously described [27].
At each survey, sociodemographic characteristics, medical history, and lifestyle behaviors were collected by the trained staff by face-to-face interviews using a standardized questionnaire [28]. In this current study, participants who underwent the first visit (2011–2012) were included as baseline and then followed at the three subsequent surveys (2013–2014, 2015–2016, 2017–2018). Those lacking necessary sociodemographic characteristics (age and sex), blood sample tests or data of cardiovascular diseases or cancer history at baseline were excluded. Finally, a total of 8 658 participants were included in the final analysis. The detailed inclusion and exclusion process is shown in Additional file 1: Fig. S1.
The CHARLS study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Peking University (IRB00001052-11015). All participants provided written informed consent before participating in the CHARLS study. This study was conducted following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Exposure
Fasting venous blood samples were collected by medical staff from the Chinese Centre for Disease Control and Prevention based on the standard protocol and subsequently tested at the central laboratory. Triglycerides and glucose were measured based on an enzymatic colorimetric test. The coefficient of variation of triglycerides was 1.5% within the assay. The coefficient of variation of glucose was 0.9% within the assay. The concentration of hsCRP was measured based on an immunoturbidimetric assay on a Hitachi 7180 chemistry analyzer (Hitachi, Tokyo, Japan). The coefficient of variation (CV) of blood marker measurement was < 5%. According to previous studies [16, 29], the TyG index was calculated as Ln [triglycerides (mg/dL) × glucose (mg/dL)/2].
Ascertainment of CVD events
The study outcome was the incidence of CVD events. In accordance with previous studies [30, 31], incident CVD events were assessed by the following standardized questions: “Have you been told by a doctor that you have been diagnosed with a heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems?” or “Have you been told by a doctor that you have been diagnosed with a stroke?” Participants who reported heart disease or stroke during the follow-up period were defined as having incident CVD. The date of CVD diagnosis was recorded as being between the date of the last interview and that of the interview reporting an incident CVD. The outcomes were assessed by rigorously trained interviewers through standardized questionnaires that are harmonized to international leading aging surveys in the Health and Retirement Study (HRS) and related international aging surveys, including the English Longitudinal Study of Aging (ELSA) and the Survey of Health, Aging and Retirement in Europe (SHARE). Quality control of data recording and checking was conducted to ensure data reliability.
Covariates
Baseline measurements of age, sex, education level, marital status, residence location, BMI, smoking, drinking and self-reported health conditions (hypertension and diabetes) were included as covariates in the current study. Educational level was categorized as “primary education,” “secondary education,” and “third education.” Marital status included “married” and “others.” Residence location included “urban” and “rural.” Smoking status was defined as “never smoking”, “current smoker” and “former smoker”. BMI was calculated as weight (in kilograms)/height^2 (in meters squared) [32]. Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or self-reported diagnosis history of hypertension or use of any antihypertensive medication [33]. Diabetes was defined as fasting glucose ≥ 7.0 mmol/L or self-reported diagnosis history of diabetes or use of any hypoglycemic medication [34].
Statistical analysis
Data are described as the means and standard deviation (SD) for continuous variables. Frequency with percentage was used to describe categorical variables. Baseline characteristics are summarized according to joint assessments of the TyG index (median value [8.6] as cutoff point) and hsCRP (1 mg/L as cutoff point) and compared among participants of four groups (TyG < median & hsCRP < 1 mg/L, TyG < median & hsCRP ≥ 1 mg/L, TyG ≥ median & hsCRP < 1 mg/L and TyG ≥ median & hsCRP ≥ 1 mg/L) using the χ2 test or analysis of variance, as appropriate. Due to lacking clinical cutoff point of TyG, we used the median value of TyG following previous studies [23, 24], and TyG levels above median should be interpreted as elevated values. Eighteen percent (1532 of 8658) of total data items were missing and were assumed to be missing at random.
We computed the person-time of follow-up for each participant from the date of the 2011 to 2012 survey (baseline) to the dates of the CVD diagnosis or the end of follow-up (2017 to 2018 survey), whichever came first. Incidence rates of CVD events per 1000 person-years were calculated. To determine the association of the TyG index and hsCRP with the development of CVD, multivariable-adjusted Cox proportional hazards models were used to calculate the hazard ratio (HR) with 95% confidence interval (CI), considering the time-to-event framework. The proportional hazards assumption was tested using Schoenfeld residuals, and no potential violation was observed. Age (continuous) and sex were adjusted in model 1, and in model 2, residence (rural, urban), education level (primary, secondary, third), marital status (married, others), smoking status (current, former, never), current drinking (yes, no), BMI (continuous), hypertension (yes, no) and diabetes (yes, no) were further adjusted. Then, we evaluated the effect of the TyG index on CVD events stratified by hsCRP level, and vice versa.
Subgroup analyses were conducted by age groups: 45–59 years, 60–69 years, and ~ 70 years. Three sensitivity analyses were conducted as follows: (1) we used 1.0 mg/L and 3.0 mg/L as the cutoff points of hsCRP [35]; thus, participants were divided into six groups together with the TyG index; (2) all analyses were repeated using the complete data set (7126 participants) without multiple imputations; and (3) we created 5 imputed data sets and pooled the results using multiple imputation of the chained equation Markov chain Monte Carlo method.
We conducted a mediation analysis to assess the direct and indirect associations between the TyG index and CVD events via higher hsCRP. In brief, a higher TyG index (≥ median value of 8.6) was used as a predictor variable (X), a higher hsCRP level (≥ 1.0 mg/L) was used as a mediator (M) and a CVD event was used as the outcome variable (Y), as widely used in previous studies to quantify the mediating effect [36]. Meanwhile, the mediating effect of hsCPR on CVD events through the TyG index was similarly evaluated.
All statistical analyses were performed using R software (version 4.2.1). Multiple imputation was performed using the ‘mice’ package. Mediation analysis was performed using the ‘mediation’ package, and Cox regression analysis was performed using the ‘survival’ package. A two-sided P value < 0.05 was considered statistically significant.
Results
A total of 8 658 participants in CHARLS from 2011 to 2018 were included in the final analyses. The mean (SD) age was 58.6 (9.0) years, including 3988 (46.1%) females. Table 1 and Additional file 1: Table S1 show the characteristics of the participants. At baseline, 1826 (21.1%) participants had a solely elevated TyG index, 1917 (22.1%) participants had a solely elevated hsCRP, and 2503 (28.9%) participants had both an elevated TyG index and hsCRP level. Compared to participants with low TyG and low hsCRP levels, those with high TyG index and hsCRP levels were more likely to be older, male, living in urban areas, and having diabetes. Additional file 1: Fig. S2 presents the codistribution of the TyG index and hsCRP stratified by incident CVD. Participants with CVD events were more likely to have both high TyG and hsCRP levels.
Table 1.
Characteristics of 8 658 participants according to TyG and hsCRP levels
| Characteristics | Overall | TyG < median & hsCRP < 1 mg/L | TyG ≥ median & hsCRP ≥ 1 mg/L | P value |
|---|---|---|---|---|
| Participants, No | 8658 | 2412 | 2503 | |
| Age, years, mean (SD) | 58.58 (9.01) | 57.78 (8.96) | 59.18 (8.92) | < 0.001 |
| Sex, Female, n (%) | 3988 (46.1) | 1165 (48.3) | 1037 (41.4) | < 0.001 |
| Residence, n (%) | < 0.001 | |||
| Rural | 6976 (80.7) | 2023 (84.0) | 1891 (75.6) | |
| Urban | 1672 (19.3) | 386 (16.0) | 611 (24.4) | |
| Marriage, married, n (%) | 7754 (89.6) | 2170 (90.0) | 2231 (89.1) | 0.108 |
| Educational level, n (%) | 0.603 | |||
| Primary | 5833 (67.4) | 1632 (67.7) | 1668 (66.7) | |
| Secondary | 1844 (21.3) | 511 (21.2) | 525 (21.0) | |
| Third | 975 (11.3) | 269 (11.2) | 308 (12.3) | |
| Smoking status, n (%) | 0.263 | |||
| Never | 5157 (59.6) | 1451 (60.3) | 1513 (60.5) | |
| Former | 629 (7.3) | 162 (6.7) | 199 (8.0) | |
| Current | 2862 (33.1) | 794 (33.0) | 789 (31.5) | |
| Current drinking, n (%) | 2991 (34.6) | 817 (34.0) | 857 (34.3) | 0.17 |
| BMIa, kg/m2 | ||||
| Continuous | 23.76 (6.03) | 23.89 (6.20) | 23.85 (6.05) | 0.333 |
| < 23.9 | 4018 (56.2) | 1129 (56.5) | 1142 (55.4) | 0.861 |
| 24–27.9 | 1559 (21.8) | 419 (21.0) | 467 (22.6) | |
| ≥ 28 | 1567 (21.9) | 452 (22.6) | 453 (22.0) | |
| Hypertension, n (%) | 3704 (42.8) | 1045 (43.3) | 1069 (42.7) | 0.905 |
| Antihypertensive, n (%) | 1289 (14.9) | 363 (15.0) | 353 (14.1) | 0.520 |
| Diabetes, n (%) | 1577 (18.2) | 216 (9.0) | 772 (30.8) | < 0.001 |
| Antidiabetic, n (%) | 254 (2.9) | 71 (2.9) | 77 (3.1) | 0.953 |
Data are presented as the mean (SD) or number (%), as appropriate. Number of missing: BMI (n = 1514); residence (n = 10); smoking (n = 10); drinking (n = 13); education (n = 6). Median TyG index: 8.6
SD standard deviation, BMI body mass index, TyG triglyceride-glucose index, hsCRP high-sensitivity C-reactive protein
aCalculated as weight in kilograms divided by height in meters squared
During a maximum follow-up of 7.0 years, 2606 (30.1%) people developed cardiovascular diseases, including 2012 (23.2%) cases of heart diseases and 848 (9.8%) cases of stroke. Additional file 1: Table S2 shows the effects of individual exposures of the TyG index and hsCRP level on CVD onset. The incidence rates of CVD were 41.5 per 1000 years among participants with both low TyG and hsCRP levels, 52.2 per 1000 years among participants with low TyG but high hsCRP levels, 48.3 per 1000 years among participants with low hsCRP levels but high TyG, and 59.2 per 1000 years among participants with high TyG and hsCRP levels. Figure 1 displays the Kaplan–Meier curves of the cumulative incidence of CVD in the overall study participants. Additional file 1: Fig. S3 presents the Kaplan–Meier curves of the cumulative incidence of coronary heart diseases and stroke. Table 2 shows the associations of the TyG index and hsCRP with incident CVD events. After adjusting for potential confounders (in model 2), people with solely elevated hsCPR, solely elevated TyG, and elevated hsCPR plus TyG were independently associated with a 19.3% (adjusted HR [aHR], 1.193; 95% CI 1.053–1.351), 16.8% (aHR, 1.168; 95% CI 1.027–1.327), and 30.0% (aHR, 1.300; 95% CI 1.155–1.462) increased risk of incident CVD compared to those with low hsCRP and TyG index. Similar results were found for coronary heart disease, while only people with both higher TyG and hsCRP levels had a significantly increased risk of stroke. Additional file 1: Table S3 shows the effects of coexposures of the TyG index and hsCRP level on CVD onset when hsCRP was divided into three groups.
Fig. 1.
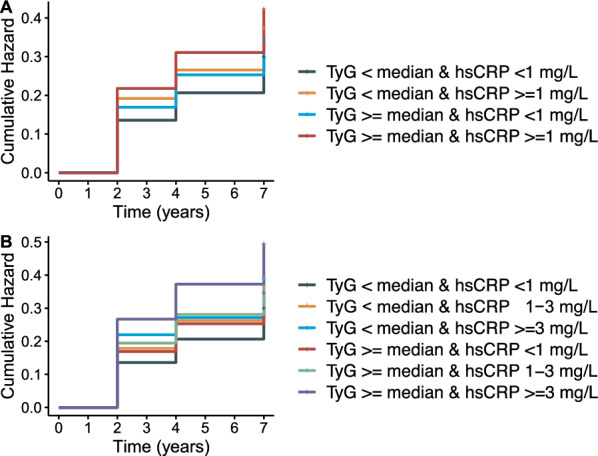
K‒M plot of cardiovascular diseases by TyG index and hsCRP level. TyG triglyceride-glucose index, hsCRP high-sensitivity C-reactive protein; median of TyG index: 8.6. a hsCRP was divided into two groups at 1 mg/L; b hsCRP was divided into three groups at 1 and 3 mg/L
Table 2.
Risk of cardiovascular disease upon coexposure stratified by the TyG index and hsCRP
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| CVD (cases/person-years) | ||||
| Group 1 (620/14922) | Ref | |||
| Group 2 (595/11408) | 1.234 (1.102–1.381) | < 0.001 | 1.193 (1.053–1.351) | 0.006 |
| Group 3 (531/10990) | 1.157 (1.03–1.299) | 0.014 | 1.168 (1.027–1.327) | 0.018 |
| Group 4 (860/14537) | 1.387 (1.25–1.538) | < 0.001 | 1.300 (1.155–1.462) | < 0.001 |
| CHD (cases/person-years) | ||||
| Group 1 (463/14922) | Ref | |||
| Group 2 (465/11408) | 1.292 (1.136–1.47) | < 0.001 | 1.245 (1.079–1.436) | 0.003 |
| Group 3 (413/10990) | 1.194 (1.045–1.363) | 0.009 | 1.189 (1.026–1.377) | 0.021 |
| Group 4 (671/14537) | 1.425 (1.266–1.605) | < 0.001 | 1.294 (1.130–1.481) | < 0.001 |
| Stroke (cases/person-years) | ||||
| Group 1 (216/14922) | Ref | |||
| Group 2 (189/11408) | 1.090 (0.896–1.326) | 0.388 | 1.115 (0.900–1.380) | 0.318 |
| Group 3 (165/10990) | 1.047 (0.855–1.283) | 0.656 | 1.067 (0.854–1.335) | 0.568 |
| Group 4 (278/14537) | 1.298 (1.086–1.551) | 0.004 | 1.333 (1.093–1.628) | 0.005 |
HR hazard ratio, CI confidence interval, BMI body mass index, TyG triglyceride-glucose index, hsCRP: high-sensitivity C-reactive protein, CVD cardiovascular disease, CHD coronary heart disease
Group 1: TyG < median & hs-CRP < 1 mg/L; Group 2: TyG < median & hs-CRP ≥ 1 mg/L; Group 3: TyG ≥ median & hs-CRP < 1 mg/L; Group 4: TyG ≥ median & hs-CRP ≥ 1 mg/L. Median TyG index: 8.6
Model 1: age and sex were adjusted; model 2: age, sex, residence, marriage, education level, BMI level, smoking status, current drinking, hypertension and diabetes were adjusted
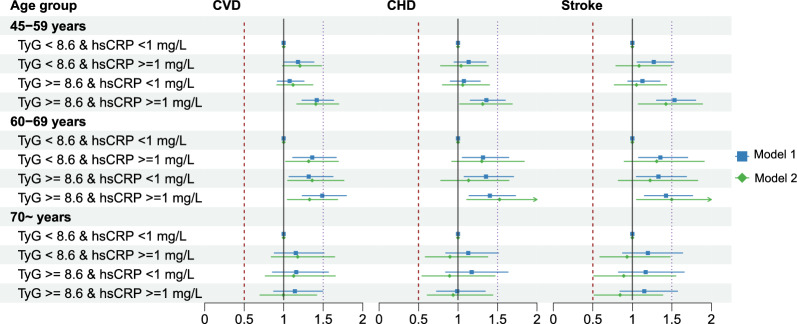
Regardless of hsCRP level, participants with a higher TyG index had a significantly increased risk of CVD events. Similarly, participants with higher hsCRP levels had a significantly increased risk of CVD events independent of the TyG index, as shown in Table 3. In terms of age groups, the effect of coexposure of the TyG index and hsCRP tended to be predominant among those aged 70 years old or younger, as shown in Fig. 2 and Additional file 1: Fig. S4. The results remained consistent among the complete data set without missing data (7126 participants) and multiple imputed data sets (Additional file 1: Table S4). In addition, we repeated the analysis among those free of diabetes, and the associations between joint TyG and hsCRP groups with CVD events were consistent (Additional file 1: Table S5). After further adjusting for the uses of antihypertensive and antidiabetic medications, we found that the association magnitudes were slightly exaggerated compared to the main analysis (Additional file 1: Table S6).
Table 3.
Risk reclassification of cardiovascular disease based on the TyG index and hsCRP
| CVD | CHD | Stroke | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Scenario 1 (N of participants) | ||||||
| hsCRP < 1 mg/L (N = 4 238) | ||||||
| TyG < median | – | – | – | |||
| TyG ≥ median | 1.159 (1.032–1.301) | 0.013 | 1.191 (1.043–1.361) | 0.010 | 1.058 (0.863–1.296) | 0.587 |
| hsCRP ≥ 1 mg/L (N = 4 420) | ||||||
| TyG < median | – | – | ||||
| TyG ≥ median | 1.121(1.009–1.245) | 0.034 | 1.105 (0.981–1.245) | 0.101 | 1.171 (0.972–1.411) | 0.097 |
| Scenario 2 (N of participants) | ||||||
| TyG < median (N = 4 329) | ||||||
| hsCRP < 1 mg/L | – | – | – | |||
| hsCRP ≥ 1 mg/L | 1.229 (1.098–1.377) | < 0.001 | 1.285 (1.129–1.463) | < 0.001 | 1.082 (0.889–1.316) | 0.434 |
| TyG ≥ median (N = 4 329) | ||||||
| hsCRP < 1 mg/L | – | – | – | |||
| hsCRP ≥ 1 mg/L | 1.198 (1.075–1.336) | 0.001 | 1.196 (1.058–1.352) | 0.004 | 1.243 (1.024–1.507) | 0.027 |
HR hazard ratio, CI confidence interval, BMI body mass index, TyG triglyceride-glucose index, hsCRP: high-sensitivity C-reactive protein, CVD cardiovascular disease, CHD coronary heart disease. Median TyG index: 8.6
Model 1: age and sex were adjusted; model 2: age, sex, residence, marriage, education level, BMI level, smoking status, current drinking, hypertension and diabetes were adjusted
Scenario 1: effect of TyG on cardiovascular diseases between hsCRP groups; scenario 2: effect of hsCRP on cardiovascular diseases between TyG groups
Fig. 2.
Age-associated risk of TyG index and hsCRP for cardiovascular disease onset. HR hazard ratio, CI confidence interval, BMI body mass index, TyG triglyceride-glucose index, hsCRP high-sensitivity C-reactive protein, CVD cardiovascular disease, CHD coronary heart disease. Number of participants: 45–59 years (n = 5 015); 60–69 years (n = 2 495); 70 ~ years (n = 1 148). Dots and lines represent the HR and 95% CI. Model 1: age and sex were adjusted; model 2: age, sex, residence, marriage, education level, BMI level, smoking status, current drinking, hypertension and diabetes were adjusted
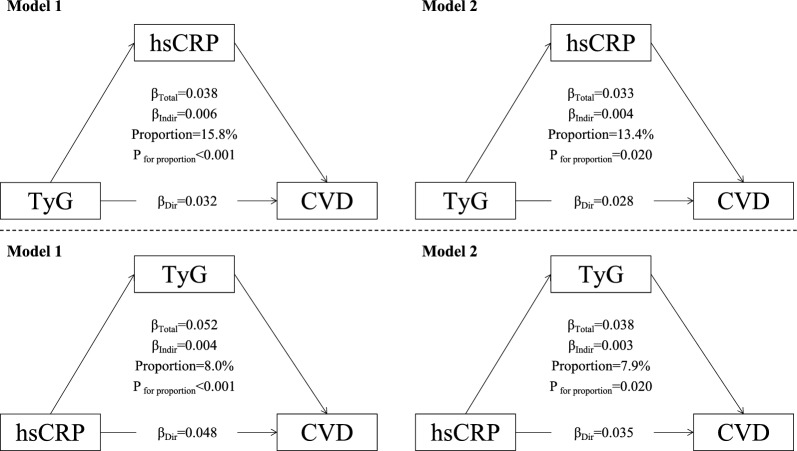
Figure 3 summarizes the mutual mediating effects linking TyG and hsCRP to CVD events. High hsCRP significantly mediated 13.4% (P = 0.020) of the association between a high TyG index and CVD events, while TyG simultaneously mediated 7.9% (P = 0.020) of the association between high hsCRP and cardiovascular risk in the fully adjusted model. In terms of coronary heart disease, the mediation proportions were 16.9% (P = 0.020) and 7.4% (P < 0.001), respectively (Additional file 1: Fig. S5). In terms of stroke, the mediation proportions were similar at 15.7% (P = 0.240) and 7.8% (P = 0.180), although the mutual mediation effects between TyG and hsCRP were not statistically significant (Additional file 1: Fig. S6). In terms of the predictive capacity of TyG index and hsCRP, we found that combining TyG and hsCRP could slightly improve the C-statistics than TyG or hsCRP alone, which suggests that TyG and hsCRP are mutually correlated (Additional file 1: Fig. S7).
Fig. 3.
Mutual mediation effects of the TyG index and hsCRP on cardiovascular diseases. TyG triglyceride-glucose index, hsCRP high-sensitivity C-reactive protein. Model 1: age and sex were adjusted; model 2: age, sex, residence, marriage, education level, BMI level, smoking status, current drinking, hypertension and diabetes were adjusted
Discussion
Among 8658 Chinese adults aged 45 years or above followed up to 7.0 years, coexposure to a higher TyG index and hsCRP was significantly associated with the highest risk of CVD events, especially among those aged 70 years or below. The TyG index can further restratify cardiovascular risk independently of hsCRP level, and vice versa. The associations persisted even after adjustment for other established cardiovascular risk factors. Importantly, our findings indicated a mutual mediation relationship between the TyG index and hsCRP in terms of atherosclerotic risk.
The positive associations of individual TyG index or hsCPR level with cardiovascular risk have been widely evaluated in previous studies. The Tehran Lipid and Glucose Study, the Kailuan Study, the National Health Insurance Service Study in Korea, and the Atherosclerosis Risk in Communities (ARIC) Study have reported that elevated levels of the baseline or long-term TyG index are associated with an increased risk of CVD events [37–42]. The cumulative exposure, variability, and progressive trajectory of the TyG index have also been linked with a higher risk of CVD events [40, 43–45]. In addition, there is an association of the TyG index with the established risk factors for CVD in several cohort studies. The Hanzhong Adolescent Hypertension Cohort study and our previous study found that the TyG index was independently associated with elevated arterial stiffness, which is an important predictor of CVD events [17, 46]. In addition, the effects of TyG on diabetes, carotid atherosclerosis, hypertension, liver diseases, renal function and frailty have also been reported [47–52].
On the other hand, chronic inflammation is another important risk factor for CVD. The role of inflammation in the propagation of atherosclerosis and susceptibility to CVD events is well established [53]. Of the wide array of inflammatory markers, hsCRP has received the most attention for its use in screening and risk reclassification of CVD. The Emerging Risk Factor Collaboration (ERFC) reviewed the association among hsCRP levels, CV risk factors, and vascular risk in 160,309 individuals from 54 prospective studies and found that hsCRP concentration is significantly associated with the risk of coronary heart disease, ischemic stroke, and vascular mortality [54]. A hsCRP level > 3 mg/L was independently associated with a 60% excess risk of the onset of coronary heart disease compared with levels < 1 mg/L after adjustment for all Framingham risk variables [55]. In the middle-aged Chinese population, hsCRP was associated with an increased risk of developing CVD [56].
There is an elusive link between insulin resistance and inflammation. Inflammation plays a key role in diabetogenesis and enhancing insulin resistance [25]. Inflammation also greatly mediates lipid metabolism, modifying the constitution and fraction of lipid profiles and deteriorating glucose levels [57], which constitute the current form of the TyG index. A recent study pointed out that inflammation assessed by hsCRP was a stronger predictor for risk of future CVD events and death than cholesterol, suggesting that inflammation and hyperlipidemia jointly contribute to atherothrombotic disease and the combined use of aggressive lipid-lowering and inflammation-inhibiting therapies to further reduce atherosclerotic risk [20]. Moreover, hsCRP and the TyG index have been used to develop the CRP-TyG index (CTI), synchronously reflecting the inflammation and insulin resistance status given the close correlation between inflammation and insulin resistance [58]. There is also accumulating evidence indicating that chronic inflammation as measured by hsCRP is associated with insulin resistance and other features of insulin resistance syndrome and then leads to increased cardiovascular risk [59]. The joint assessment of inflammation and the TyG index has been evaluated in cancer prognosis [60], while the joint effect on cardiovascular risk needs more data. Zhang et al. reported that the cumulative value of TyG and hsCRP may better identify moderate-to-severe asymptomatic intracranial arterial stenosis, as well as its severity and numerical burden [61]. This current study highlighted the coexposure effect of the TyG index and hsCRP on CVD events. People concurrently with a higher TyG and hsCRP had the highest risk of overall cardiovascular disease, coronary heart disease and stroke, which were predominant among those aged 70 years or below. Similarly, Lan et al. highlighted the need for age-specific combined assessment and management of chronic inflammation and dyslipidemia in primary prevention against diabetes, particularly for young adults [62]. In addition, the assessment of hsCRP level further stratifies the risk of CVD events irrelevant of baseline TyG level, and vice versa. These findings highlighted the need for combined evaluation of the TyG index and hsCRP for the primary prevention of CVD events in clinical practice.
In terms of the mutual association, our results indicated a significant mediating effect of the TyG index on CVD events partially through inflammation biomarkers. Mirzababaei et al. reported that inflammation can play an important role in the relationship between body shape indices and cardiometabolic risk factors among overweight and obese women [63]. Li et al. reported that in diabetic chronic coronary syndrome patients, insulin resistance and systemic inflammation synergistically increased the risk of CVD events, and systemic inflammation partially mediated the association between insulin resistance and clinical outcomes [64]. In addition, our findings indicated that hsCRP is a risk factor for CVD events partially via higher TyG levels, which is consistent with a review summarizing that the proinflammatory phenotype contributes to cardiovascular insulin resistance [65]. Similar to the coexposure pattern widely recognized between inflammation and hyperlipidemia to atherothrombotic disease [20], the current study underlined the implications of joint contributions between inflammation and insulin resistance as determinants for CVD events.
There are possible mechanisms underlining the complex correlation among the TyG index or insulin resistance, inflammation, and cardiovascular damage. Generally, insulin resistance plays an important role in dyslipidemia, metabolic cardiac alterations, and myocardial and vascular stiffness damage [66]. The presence of hsCRP within most atherosclerotic plaques and all acute myocardial infarction lesions, coupled with binding of hsCRP to lipoproteins and its capacity for pro-inflammatory complement activation, suggests that hsCRP may contribute to the pathogenesis of CVD events [67]. Polarization of macrophages and lymphocytes toward a pro-inflammatory phenotype can contribute to the progression of insulin resistance, possibly through the renin–angiotensin–aldosterone system, sympathetic activation and incretin modulators (e.g., DPP-4) and immune responses [65, 68].
Limitations
Several limitations of the current study should be acknowledged. First, owing to the observational study design, we could not confirm the causal association among the TyG index, inflammation biomarkers, and cardiovascular risk. However, both TyG and hsCRP have been validated widely as predictors of CVD events. The main aim of this study was to evaluate the coexposure effect and mutual mediation relationship between TyG and hsCRP in terms of cardiovascular risk. Second, we still cannot exclude the possibility of residual or unmeasured confounding bias, which could affect the estimation of effect size. Third, the diagnosis of cardiovascular events was self-reported. Medical records were not available in the CHARLS, and we were not able to differentiate the fatal and nonfatal CVD events. However, some other large-scale studies, such as the English Longitudinal Study of Aging, found that self-reported incident cardiovascular disease had good agreement with medical records. Fourth, we used the TyG index as a surrogate marker of insulin resistance. Further studies are needed for validation using data on measured insulin levels in other populations.
Conclusions
In summary, using a prospective and national cohort of Chinese adults, we found that inflammation significantly mediated the association of insulin resistance with cardiovascular risk, and vice versa. The findings highlight the coexposure effect of the TyG index and hsCRP level in terms of cardiovascular events and recommend the combined assessment of the TyG index and inflammation markers to further stratify cardiovascular risk.
Supplementary Information
Additional file 1: Table S1. Baseline characteristics of the study participants. Table S2. Risk of cardiovascular disease upon individual exposure stratified by TyG index and hsCRP. Table S3. Risk of cardiovascular disease upon co-exposure stratified by TyG index and hsCRP. Table S4. Sensitivity analyses of co-exposure for TyG index and hsCRP in terms of data missing. Table S5. Risk of cardiovascular disease upon coexposure stratified by the TyG index and hsCRP among those free of diabetes (7081 participants). Table S6. Risk of cardiovascular disease upon coexposure stratified by the TyG index and hsCRP further adjusting for medication uses. Figure S1. Flowchart and follow-up setting of this current study. Figure S2. Co-distribution of TyG index and hsCRP stratified by incident cardiovascular diseases or not. Figure S3. K-M plot of coronary heart disease and stroke by TyG index and hsCRP level. Figure S4. Age-associated risk of TyG index and hsCRP for cardiovascular diseases onset. Figure S5. Mutual mediation effects of TyG index and hsCRP on coronary heart disease. Figure S6. Mutual mediation effects of TyG index and hsCRP on stroke. Figure S7. Predictive capacity of TyG index and hsCRP on the cardiovascular risk.
Acknowledgements
This study used data from China Health and Retirement Longitudinal Study (CHARLS). We would like to thank the CHARLS research team for the time and effort into the CHARLS project.
Originality Of Content
All authors verify that all information and materials in the manuscript are original.
Abbreviations
- CHARLS
China Health and Retirement Longitudinal Study
- TyG
Triglyceride-glucose index
- hsCRP
High-sensitivity C-reactive protein
- BMI
Body mass index
Author contributions
Literature search: CC. Cui, ZJ. Wang, XY. Shang; Study conception and design: T. Zhang, ZY. Wu; Data collection: YT. Qi, TJ. Han; Data analysis and interpretation: N. Han, HK. Xu, YN. Zha; Manuscript writing and reviewing: CC. Cui, X. Wei; Study supervision: L. Liu, ZY. Wu.
Funding
None.
Availability of data and materials
The data sets used and/or analyzed during the current study are publicly available or from the corresponding author upon reasonable request. All authors verify that all information and materials in the manuscript are original.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Cancan Cui and Liu Lin are the first co-authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xin Wei, Email: weinxin@jlu.edu.cn.
Zhiyuan Wu, Email: zwu0@our.ecu.edu.au.
References
- 1.Sacco RL, Roth GA, Reddy KS, Arnett DK, Bonita R, Gaziano TA, Heidenreich PA, Huffman MD, Mayosi BM, Mendis S, et al. The heart of 25 by 25: achieving the goal of reducing global and regional premature deaths from cardiovascular diseases and stroke: a modeling study from the American heart association and world heart federation. Circulation. 2016;133(23):e674–690. doi: 10.1161/CIR.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Li Y, Zeng X, Wang H, Yin P, Wang L, Liu Y, Liu J, Qi J, Ran S, et al. Burden of cardiovascular diseases in China, 1990–2016: findings from the 2016 global burden of disease study. JAMA Cardiol. 2019;4(4):342–352. doi: 10.1001/jamacardio.2019.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the framingham heart study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB, Wilson PW. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290(7):891–897. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 5.Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, Ellis SG, Lincoff AM, Topol EJ. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290(7):898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 6.Smith SC., Jr Current and future directions of cardiovascular risk prediction. Am J Cardiol. 2006;97(2):28–32. doi: 10.1016/j.amjcard.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Magnussen C, Ojeda FM, Leong DP, Alegre-Diaz J, Amouyel P, Aviles-Santa L, De Bacquer D, Ballantyne CM, Bernabé-Ortiz A, Bobak M, et al. Global effect of modifiable risk factors on cardiovascular disease and mortality. N Engl J Med. 2023;389(14):1273–1285. doi: 10.1056/NEJMoa2206916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox CS, Coady S, Sorlie PD, D'Agostino RB, Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: the framingham heart study. Circulation. 2007;115(12):1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 9.Schmiegelow MD, Hedlin H, Stefanick ML, Mackey RH, Allison M, Martin LW, Robinson JG, Hlatky MA. Insulin resistance and risk of cardiovascular disease in postmenopausal women: a cohort study from the women’s health initiative. Circ Cardiovasc Qual Outcomes. 2015;8(3):309–316. doi: 10.1161/CIRCOUTCOMES.114.001563. [DOI] [PubMed] [Google Scholar]
- 10.Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, Sowers JR. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. doi: 10.1016/j.metabol.2021.154766. [DOI] [PubMed] [Google Scholar]
- 11.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 12.Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, Rizka A, Tarigan TJE, Harbuwono DS, Purnamasari D, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16(8):102581. doi: 10.1016/j.dsx.2022.102581. [DOI] [PubMed] [Google Scholar]
- 13.Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin JL, Cao YX, Wu LG, You XD, Guo YL, Wu NQ, Zhu CG, Gao Y, Dong QT, Zhang HW, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137–6146. doi: 10.21037/jtd.2018.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Won KB, Park EJ, Han D, Lee JH, Choi SY, Chun EJ, Park SH, Han HW, Sung J, Jung HO, et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol. 2020;19(1):34. doi: 10.1186/s12933-020-01008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Z, Wang J, Li Z, Han Z, Miao X, Liu X, Li X, Wang W, Guo X, Tao L. Triglyceride glucose index and carotid atherosclerosis incidence in the Chinese population: a prospective cohort study. Nutr Metab Cardiovasc Dis. 2021;31(7):2042–2050. doi: 10.1016/j.numecd.2021.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, Zhou D, Liu Y, Li Z, Wang J, Han Z, Miao X, Liu X, Li X, Wang W, et al. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol. 2021;20(1):134. doi: 10.1186/s12933-021-01330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritz J, Brozek W, Concin H, Nagel G, Kerschbaum J, Lhotta K, Ulmer H, Zitt E. The association of excess body weight with risk of ESKD is mediated through insulin resistance, hypertension, and hyperuricemia. J Am Soc Nephrol. 2022;33(7):1377–1389. doi: 10.1681/ASN.2021091263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, Yu S, Zhang H, Guo Z, Zheng Y, Xu Z, Li Z, Liu X, Li X, Chen S, et al. Combined evaluation of arterial stiffness, glycemic control and hypertension for macrovascular complications in type 2 diabetes. Cardiovasc Diabetol. 2022;21(1):262. doi: 10.1186/s12933-022-01696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, Bhatt DL, Pradhan AD, Glynn RJ, MacFadyen JG, Nissen SE. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401(10384):1293–1301. doi: 10.1016/S0140-6736(23)00215-5. [DOI] [PubMed] [Google Scholar]
- 21.Lawler PR, Bhatt DL, Godoy LC, Lüscher TF, Bonow RO, Verma S, Ridker PM. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J. 2021;42(1):113–131. doi: 10.1093/eurheartj/ehaa099. [DOI] [PubMed] [Google Scholar]
- 22.Lan Y, Chen G, Wu D, Ding X, Huang Z, Wang X, Balmer L, Li X, Song M, Wang W, et al. Temporal relationship between atherogenic dyslipidemia and inflammation and their joint cumulative effect on type 2 diabetes onset: a longitudinal cohort study. BMC Med. 2023;21(1):31. doi: 10.1186/s12916-023-02729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng G, Yang M, Xu L, Liu Y, Yu J, Zang Y, Shen S, Zheng X. Combined effects of high sensitivity C-reactive protein and triglyceride-glucose index on risk of cardiovascular disease among middle-aged and older Chinese: evidence from the China health and retirement longitudinal study. Nutr Metab Cardiovasc Dis. 2023;33(6):1245–1253. doi: 10.1016/j.numecd.2023.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Cui C, Liu L, Zhang T, Fang L, Mo Z, Qi Y, Zheng J, Wang Z, Xu H, Yan H, et al. Triglyceride-glucose index, renal function and cardiovascular disease: a national cohort study. Cardiovasc Diabetol. 2023;22(1):325. doi: 10.1186/s12933-023-02055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 26.Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, Jenny NS, Ouyang P, Rotter JI. Inflammation and the incidence of type 2 diabetes: the multi-ethnic study of atherosclerosis (MESA) Diabetes Care. 2010;33(4):804–810. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS) Int J Epidemiol. 2014;43(1):61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z, Zhang H, Miao X, Li H, Pan H, Zhou D, Liu Y, Li Z, Wang J, Liu X, et al. High-intensity physical activity is not associated with better cognition in the elder: evidence from the China health and retirement longitudinal study. Alzheimers Res Ther. 2021;13(1):182. doi: 10.1186/s13195-021-00923-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alizargar J, Bai CH, Hsieh NC, Wu SV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 2020;19(1):8. doi: 10.1186/s12933-019-0982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Zheng D, Li Z, Wu Z, Feng W, Cao X, Wang J, Gao Q, Li X, Wang W, et al. Association of depressive symptoms with incident cardiovascular diseases in middle-aged and older Chinese adults. JAMA Netw Open. 2019;2(12):e1916591. doi: 10.1001/jamanetworkopen.2019.16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie W, Zheng F, Yan L, Zhong B. Cognitive decline before and after incident coronary events. J Am Coll Cardiol. 2019;73(24):3041–3050. doi: 10.1016/j.jacc.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 32.He W, Li Q, Yang M, Jiao J, Ma X, Zhou Y, Song A, Heymsfield SB, Zhang S, Zhu S. Lower BMI cutoffs to define overweight and obesity in China. Obesity. 2015;23(3):684–691. doi: 10.1002/oby.20995. [DOI] [PubMed] [Google Scholar]
- 33.Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the joint national committee on the prevention, detection, evaluation, and treatment of high blood pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41(6):1178–1179. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 34.Standards of Medical Care in Diabetes-2020 Abridged for Primary Care Providers. Clin Diabetes 2020, 38(1):10–38. [DOI] [PMC free article] [PubMed]
- 35.Möhlenkamp S, Lehmann N, Moebus S, Schmermund A, Dragano N, Stang A, Siegrist J, Mann K, Jöckel K-H, Erbel R. Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality. J Am Coll Cardiol. 2011;57(13):1455–1464. doi: 10.1016/j.jacc.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z, Jiang Y, Li P, Wang Y, Zhang H, Li Z, Li X, Tao L, Gao B, Guo X. Association of impaired sensitivity to thyroid hormones with hyperuricemia through obesity in the euthyroid population. J Transl Med. 2023;21(1):436. doi: 10.1186/s12967-023-04276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran lipid and glucose study. Cardiovasc Diabetol. 2020;19(1):155. doi: 10.1186/s12933-020-01121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian X, Zuo Y, Chen S, Liu Q, Tao B, Wu S, Wang A. Triglyceride-glucose index is associated with the risk of myocardial infarction: an 11-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. 2021;20(1):19. doi: 10.1186/s12933-020-01210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park B, Lee YJ, Lee HS, Jung DH. The triglyceride-glucose index predicts ischemic heart disease risk in Koreans: a prospective study using national health insurance service data. Cardiovasc Diabetol. 2020;19(1):210. doi: 10.1186/s12933-020-01186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao JW, Hao QY, Gao M, Zhang K, Li XZ, Wang JF, Vuitton DA, Zhang SL, Liu PM. Triglyceride-glucose index in the development of peripheral artery disease: findings from the atherosclerosis risk in communities (ARIC) study. Cardiovasc Diabetol. 2021;20(1):126. doi: 10.1186/s12933-021-01319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–197. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- 42.Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361. doi: 10.1186/s12916-020-01824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Zuo Y, Qian F, Chen S, Tian X, Wang P, Li X, Guo X, Wu S, Wang A. Triglyceride-glucose index variability and incident cardiovascular disease: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):105. doi: 10.1186/s12933-022-01541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu X, Huang R, Lin Y, Guo Y, Xiong Z, Zhong X, Ye X, Li M, Zhuang X, Liao X. High triglyceride-glucose index in young adulthood is associated with incident cardiovascular disease and mortality in later life: insight from the CARDIA study. Cardiovasc Diabetol. 2022;21(1):155. doi: 10.1186/s12933-022-01593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Z, Ding X, Yue Q, Wang X, Chen Z, Cai Z, Li W, Cai Z, Chen G, Lan Y, et al. Triglyceride-glucose index trajectory and stroke incidence in patients with hypertension: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):141. doi: 10.1186/s12933-022-01577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan Y, Wang D, Sun Y, Ma Q, Wang K, Liao Y, Chen C, Jia H, Chu C, Zheng W, et al. Triglyceride-glucose index trajectory and arterial stiffness: results from Hanzhong adolescent hypertension cohort study. Cardiovasc Diabetol. 2022;21(1):33. doi: 10.1186/s12933-022-01453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park B, Lee HS, Lee YJ. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: a 12-year longitudinal study of the Korean Genome and Epidemiology Study cohort. Transl Res. 2021;228:42–51. doi: 10.1016/j.trsl.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. 2017;16(1):175. doi: 10.1186/s12944-017-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Che B, Zhong C, Zhang R, Pu L, Zhao T, Zhang Y, Han L. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: an analysis of UK biobank data. Cardiovasc Diabetol. 2023;22(1):34. doi: 10.1186/s12933-023-01762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivière B, Jaussent A, Macioce V, Faure S, Builles N, Lefebvre P, Géraud P, Picot MC, Rebuffat S, Renard E, et al. The triglycerides and glucose (TyG) index: a new marker associated with nonalcoholic steatohepatitis (NASH) in obese patients. Diabetes Metab. 2022;48(4):101345. doi: 10.1016/j.diabet.2022.101345. [DOI] [PubMed] [Google Scholar]
- 51.Yuan Y, Chen S, Lin C, Huang X, Lin S, Huang F, Zhu P. Association of triglyceride-glucose index trajectory and frailty in urban older residents: evidence from the 10-year follow-up in a cohort study. Cardiovasc Diabetol. 2023;22(1):264. doi: 10.1186/s12933-023-02002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amouzegar A, Honarvar M, Masoumi S, Tohidi M, Mehran L, Azizi F. Sex-specific trajectories of insulin resistance markers and reduced renal function during 18 years of follow-up: TLGS. J Clin Endocrinol Metab. 2023;108(6):e230–e239. doi: 10.1210/clinem/dgac735. [DOI] [PubMed] [Google Scholar]
- 53.Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, Blumenthal RS, Budoff MJ. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013;62(5):397–408. doi: 10.1016/j.jacc.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 54.Kaptoge S, Di Angelantonio E, Lowe G, Pepys M, Thompson S, Collins R, Danesh J. Emerging risk factors collaboration C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the US preventive services task force. Ann Intern Med. 2009;151(7):483–495. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 56.Dong Y, Wang X, Zhang L, Chen Z, Zheng C, Wang J, Kang Y, Shao L, Tian Y, Wang Z. High-sensitivity C reactive protein and risk of cardiovascular disease in China-CVD study. J Epidemiol Community Health. 2019;73(2):188–192. doi: 10.1136/jech-2018-211433. [DOI] [PubMed] [Google Scholar]
- 57.McGillicuddy FC, de la Llera MM, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119(8):1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruan GT, Xie HL, Zhang HY, Liu CA, Ge YZ, Zhang Q, Wang ZW, Zhang X, Tang M, Song MM, et al. A novel inflammation and insulin resistance related indicator to predict the survival of patients with cancer. Front Endocrinol. 2022;13:905266. doi: 10.3389/fendo.2022.905266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haffner SM. Pre-diabetes, insulin resistance, inflammation and CVD risk. Diabetes Res Clin Pract. 2003;61(Suppl 1):S9–s18. doi: 10.1016/S0168-8227(03)00122-0. [DOI] [PubMed] [Google Scholar]
- 60.Ruan GT, Xie HL, Gong YZ, Ge YZ, Zhang Q, Wang ZW, Zhang X, Zhang HY, Tang M, Song MM, et al. Prognostic importance of systemic inflammation and insulin resistance in patients with cancer: a prospective multicenter study. BMC Cancer. 2022;22(1):700. doi: 10.1186/s12885-022-09752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang N, Xiang Y, Zhao Y, Ji X, Sang S, Shao S, Ma X, Wang G, Lv M, Xue F, et al. Association of triglyceride-glucose index and high-sensitivity C-reactive protein with asymptomatic intracranial arterial stenosis: a cross-sectional study. Nutr Metab Cardiovasc Dis. 2021;31(11):3103–3110. doi: 10.1016/j.numecd.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Lan Y, Wu D, Cai Z, Xu Y, Ding X, Wu W, Lan S, Chen L, Guo Z, Balmer L, et al. Supra-additive effect of chronic inflammation and atherogenic dyslipidemia on developing type 2 diabetes among young adults: a prospective cohort study. Cardiovasc Diabetol. 2023;22(1):181. doi: 10.1186/s12933-023-01878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mirzababaei A, Abaj F, Khosravinia D, Ghorbani M, Valisoltani N, Clark CCT, Radmehr M, Mirzaei K. The mediatory effect of inflammatory markers on the association between a body shape index and body roundness index with cardiometabolic risk factor in overweight and obese women: a cross-sectional study. Front Nutr. 2023;10:1178829. doi: 10.3389/fnut.2023.1178829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li T, Wang P, Wang X, Liu Z, Zhang Z, Zhang Y, Wang Z, Feng Y, Wang Q, Guo X, et al. Inflammation and insulin resistance in diabetic chronic coronary syndrome patients. Nutrients. 2023 doi: 10.3390/nu15122808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62(11):1543–1552. doi: 10.1016/j.metabol.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirschfield GM, Pepys MB. C-reactive protein and cardiovascular disease: new insights from an old molecule. QJM. 2003;96(11):793–807. doi: 10.1093/qjmed/hcg134. [DOI] [PubMed] [Google Scholar]
- 68.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Baseline characteristics of the study participants. Table S2. Risk of cardiovascular disease upon individual exposure stratified by TyG index and hsCRP. Table S3. Risk of cardiovascular disease upon co-exposure stratified by TyG index and hsCRP. Table S4. Sensitivity analyses of co-exposure for TyG index and hsCRP in terms of data missing. Table S5. Risk of cardiovascular disease upon coexposure stratified by the TyG index and hsCRP among those free of diabetes (7081 participants). Table S6. Risk of cardiovascular disease upon coexposure stratified by the TyG index and hsCRP further adjusting for medication uses. Figure S1. Flowchart and follow-up setting of this current study. Figure S2. Co-distribution of TyG index and hsCRP stratified by incident cardiovascular diseases or not. Figure S3. K-M plot of coronary heart disease and stroke by TyG index and hsCRP level. Figure S4. Age-associated risk of TyG index and hsCRP for cardiovascular diseases onset. Figure S5. Mutual mediation effects of TyG index and hsCRP on coronary heart disease. Figure S6. Mutual mediation effects of TyG index and hsCRP on stroke. Figure S7. Predictive capacity of TyG index and hsCRP on the cardiovascular risk.
Data Availability Statement
The data sets used and/or analyzed during the current study are publicly available or from the corresponding author upon reasonable request. All authors verify that all information and materials in the manuscript are original.