ABSTRACT
Recent metagenome-assembled genome (MAG) analyses have profoundly impacted Rickettsiology systematics. The discovery of basal lineages (novel families Mitibacteraceae and Athabascaceae) with predicted extracellular lifestyles exposed an evolutionary timepoint for the transition to host dependency, which seemingly occurred independent of mitochondrial evolution. Notably, these basal rickettsiae carry the Rickettsiales vir homolog (rvh) type IV secretion system and purportedly use rvh to kill congener microbes rather than parasitize host cells as described for later-evolving rickettsial pathogens. MAG analysis also substantially increased diversity for the genus Rickettsia and delineated a sister lineage (the novel genus Tisiphia) that stands to inform on the emergence of human pathogens from protist and invertebrate endosymbionts. Herein, we probed Rickettsiales MAG and genomic diversity for the distribution of Rickettsia rvh effectors to ascertain their origins. A sparse distribution of most Rickettsia rvh effectors outside of Rickettsiaceae lineages illuminates unique rvh evolution from basal extracellular species and other rickettsial families. Remarkably, nearly every effector was found in multiple divergent forms with variable architectures, indicating profound roles for gene duplication and recombination in shaping effector repertoires in Rickettsia pathogens. Lateral gene transfer plays a prominent role in shaping the rvh effector landscape, as evinced by the discovery of many effectors on plasmids and conjugative transposons, as well as pervasive effector gene exchange between Rickettsia and Legionella species. Our study exemplifies how MAGs can yield insight into pathogen effector origins, particularly how effector architectures might become tailored to the discrete host cell functions of different eukaryotic hosts.
IMPORTANCE
While rickettsioses are deadly vector-borne human diseases, factors distinguishing Rickettsia pathogens from the innumerable bevy of environmental rickettsial endosymbionts remain lacking. Recent metagenome-assembled genome (MAG) studies revealed evolutionary timepoints for rickettsial transitions to host dependency. The rvh type IV secretion system was likely repurposed from congener killing in basal extracellular species to parasitizing host cells in later-evolving pathogens. Our analysis of MAG diversity for over two dozen rvh effectors unearthed their presence in some non-pathogens. However, most effectors were found in multiple divergent forms with variable architectures, indicating gene duplication and recombination-fashioned effector repertoires of Rickettsia pathogens. Lateral gene transfer substantially shaped pathogen effector arsenals, evinced by the discovery of effectors on plasmids and conjugative transposons, as well as pervasive effector gene exchanges between Rickettsia and Legionella species. Our study exemplifies how MAGs yield insight into pathogen effector origins and evolutionary processes tailoring effectors to eukaryotic host cell biology.
KEYWORDS: Rickettsia, metagenome, type IV secretion system, effector, evolution
INTRODUCTION
Until recently, Order Rickettsiales (Alphaproteobacteria) contained three families harboring diverse obligate intracellular parasites (1). Rickettsiaceae and Anaplasmataceae are best studied and harbor invertebrate endosymbionts, human pathogens, and reproductive parasites (2–7). Midichloriaceae contains some arthropod-associated bacteria of unknown vertebrate pathogenicity (8), but most species are described from protists (9–14). Remarkably, Castelli and colleagues (15) described the first extracellular rickettsial species, “Candidatus Deianiraea vastatrix,” as a bacterium dependent on Paramecia and sharing many characteristics of the intracellular lifestyle. A new family, Deianiraeaceae, was proposed, calling into question the specific timepoint in rickettsial evolution wherein obligate intracellularity emerged from an obligate extracellular or facultative intracellular lifestyle.
Historically, Rickettsiales were widely considered a sister lineage to the mitochondrial progenitor, with this assemblage representing a basal branch of the Alphaproteobacteria (16–20). Pioneering work on rickettsial genomes identified decreased genome size and pseudogenization of genes within many metabolic pathways, processes termed “reductive genome evolution” that coincide with addiction to the eukaryotic cytosol (18, 21–25). This dogma for shared evolutionary history and convergence in genome reduction between mitochondria and Rickettsiales held for two decades while hundreds of diverse Rickettsiales genomes were sequenced (6, 26). However, more recent phylogenetic analysis of deep marine metagenome-assembled genomes (MAGs) illustrated that mitochondria likely originated outside of all described Alphaproteobacteria (27). Furthermore, a recent phylogenomic description of certain novel MAGs established two basal rickettsial lineages, families Mitibacteraceae and Athabascaceae, with features indicating an extracellular lifestyle not dependent on eukaryotic hosts (28). These landmark findings bolstered the growing trend for identifying mostly aquatic, protist-associated rickettsial species with traits (e.g., flagella, larger genome size, greater metabolic capacity, etc.) more characteristic of free-living and facultative intracellular bacteria but absent from the numerous genomes of well-characterized invertebrate- and vertebrate-associated rickettsial species (13, 15, 29–33). Importantly, a revised Rickettsiales phylogenetic framework now allows for assessing the evolutionary trajectories within five later-evolving rickettsial families for innovations that emerged from transitions to host dependency (34).
Estimated to have arisen ~1.9 billion years ago (35), Alphaproteobacteria are highly diversified in form and function yet rife with convergence in morphology and lifestyle through common adaptation to numerous environments, including eukaryotic cells (36). Alphaproteobacteria have few Order-level signatures, yet the Rickettsiales vir homolog (rvh) type IV secretion system (T4SS) is a bona fide rickettsial signature that functions in colonizing eukaryotic cells (37–41). The rvh T4SS is odd in its design (42, 43), with specialized duplications of some components hypothesized to autoregulate effector secretion (44, 45). Effectors have been experimentally characterized for species of Ehrlichia (46–49), Anaplasma (50–55), and Rickettsia (56–58). As the rvh T4SS is present in purportedly free-living Mitibacteraceae and Athabascaceae, Schön et al. (28) proposed that these rickettsiae utilize the rvh T4SS for killing congener microbes, provided their genomes harbor candidate rvh effectors with characteristics similar to effectors in other T4SS and type VI secretion system (T6SS) killing machines (59, 60). Thus, the five later-evolving families likely repurposed the rvh T4SS to secrete effectors that commandeer host cellular processes to support intracellular replication (or epicellular parasitism in the case of “Candidatus Deianiraea vastatrix” and likely other Deianiraeaceae species).
The existence of an ancient secretion machine (rvh), yet independent gain of its effectors later in evolution, prompted us to poll the ever-growing MAG diversity for clues on rvh effector origins. We focus on known or candidate effectors from the genus Rickettsia, as recent studies have considerably expanded Rickettsiaceae diversity. Genome sequences from “environmental” Rickettsiaceae species (i.e., those from protists, apicomplexans, diplomonads, crustaceans, and insects) have illuminated basal lineages of Rickettsiaceae that are critical for inferring the emergence of genomic traits in Orientia and Rickettsia pathogens (29, 61–65). Furthermore, phylogenetic analysis of genome sequences from novel genera “Candidatus Sarmatiella” (paramecium symbiont) (66) and “Candidatus Megaira” (symbionts of algae and ciliates) (31, 67) indicates that Orientia and Rickettsia species are more divergent than previously appreciated. Finally, a long-standing recognized basal lineage of Rickettsia termed “Torix Group,” which is highly diverse and widely present in non-blood-feeding arthropods (68–72), was recently classified as a new genus, “Candidatus Tisiphia,” in a study that identified many new provisional Rickettsia (and Tisiphia) species from MAG analyses of diverse arthropods (73).
We present phylogenomic and other in silico analyses that effectively demonstrate the utility of MAG data for not only inferring the origins of pathogen effectors but also for better understanding effector architectures (i.e., protein structure, domain composition, and organization) through enhanced predictive power from greater sequence diversity. Provided that many MAGs come from environmental sampling or eukaryotic microbes with no known human association, our approach stands to inform on the evolution of vertebrate pathogenesis not only for Rickettsiales but also any bacterial taxon wherein human pathogens evolved from non-pathogenic relatives.
RESULTS AND DISCUSSION
Mapping the acquisition of rickettsial effectors
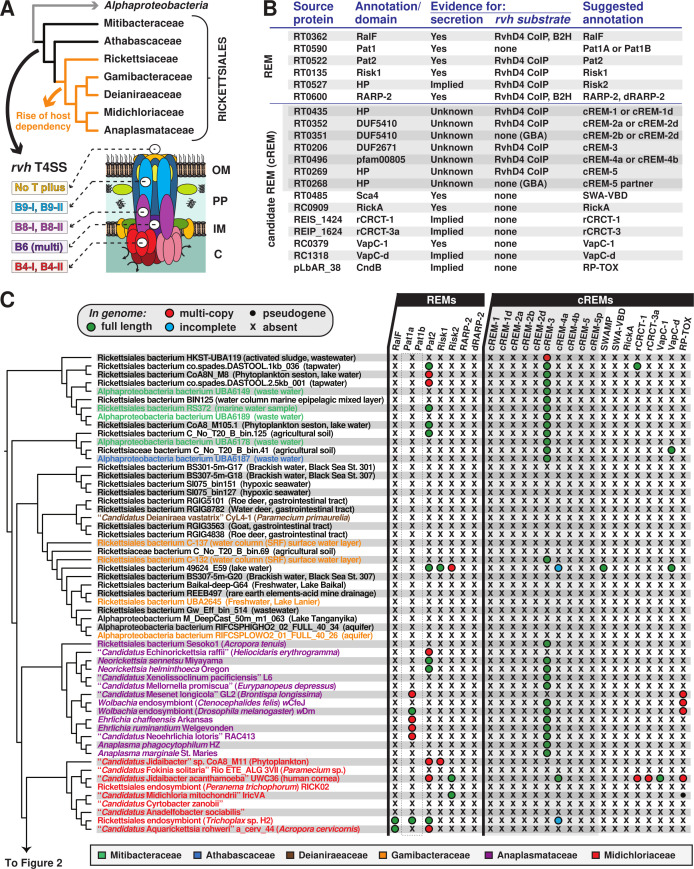
We hypothesize that the transition to an intracellular lifestyle necessitated the acquisition of a more diverse effector repertoire. Thus, to gain an appreciation of the origins and conservation of Rickettsia rvh T4SS effectors, we performed a phylogenomics analysis encompassing the newly appreciated rickettsial diversity (Fig. 1A). This initially involved creating a matrix of taxa (depicted by genomes and metagenomes) determined to encode the rvh T4SS (Fig. S1) and the distribution of effectors. Six rvh effector molecules (REMs: RalF, Pat1, Pat2, Risk1, RT0527, and RARP-2) and 14 candidate REMs (cREMs) were evaluated based on prior studies implicating their secretion and/or interaction with the rvh coupling protein (RvhD4) or presence of motifs known to target either congener bacteria or eukaryotic molecules (56–58, 74–81) (Fig. 1B). Our analyses added complexity for two REMs (Pat1 and RARP-2) and four cREMs based on the identification of duplications (cREM-1, cREM-2, and cREM-4), a partner protein (cREM-5), and a domain within the surface cell antigen (sca) Sca4 that we demonstrate to be widespread in non-Sca4 proteins (discussed further below). Collectively, a total of 26 proteins were analyzed within the phylogenomic framework (Fig. 1C and 2). A phylogeny was estimated from concatenated alignments of RvhB4-I and RvhB4-II proteins from 153 genome assemblies (Fig. 1C and 2 see Table S1 for sequence information; see Fig. S2 for entire phylogeny and related information). This collective matrix is an effective framework for mapping the earliest occurrence of these rvh effectors in the rickettsial tree, additionally identifying several likely origins for lateral gene transfer (LGT).
Fig 1.
Probing Rickettsiales diversity for the evolution of Rickettsia type IV secretion system effectors. (A) The atypical Rickettsiales vir homolog (rvh) T4SS is a hallmark of Rickettsiales that was present before the origin of host dependency (orange) (28, 34). Schema depicts recent genome-based phylogeny estimation (28). Rvh characteristics (38, 45) are described at the bottom and further in Fig. S1. (B) List of Rickettsia rvh effector molecules and candidate REMs. GBA, guilty by association (meaning a tandem gene with sequence similarities has experimental support for encoding a REM). “Implied” means analogous proteins are known to be secreted by other bacteria and/or the effector has strongly predicted host cell targets. Secretion, coimmunoprecipitation (CoIP), and bacterial two-hybrid (B2H) data are compiled from prior reports (56–58, 74–81). SWA, Schuenke-Walker antigen domain. (C) Phylogenomic analysis of Rickettsia REMs and cREMs in non-Rickettsiaceae lineages. The cladogram summarizes a phylogeny estimated from concatenated alignments for RvhB4-I and RvhB4-II proteins from 153 rickettsial assemblies (full tree, Fig. S2; sequence information, Table S1). Non-Rickettsiaceae lineages are shown (see Fig. 2 for Rickettsiaceae). The dashed box for Pat1 proteins indicates the inability to confidently discern Pat1a and Pat1b homology outside of Tisiphia and Rickettsia species (see Fig. 2 and 4). SWAMP, SWA modular proteins. Information for all REMs and cREMs is provided in Table S2.
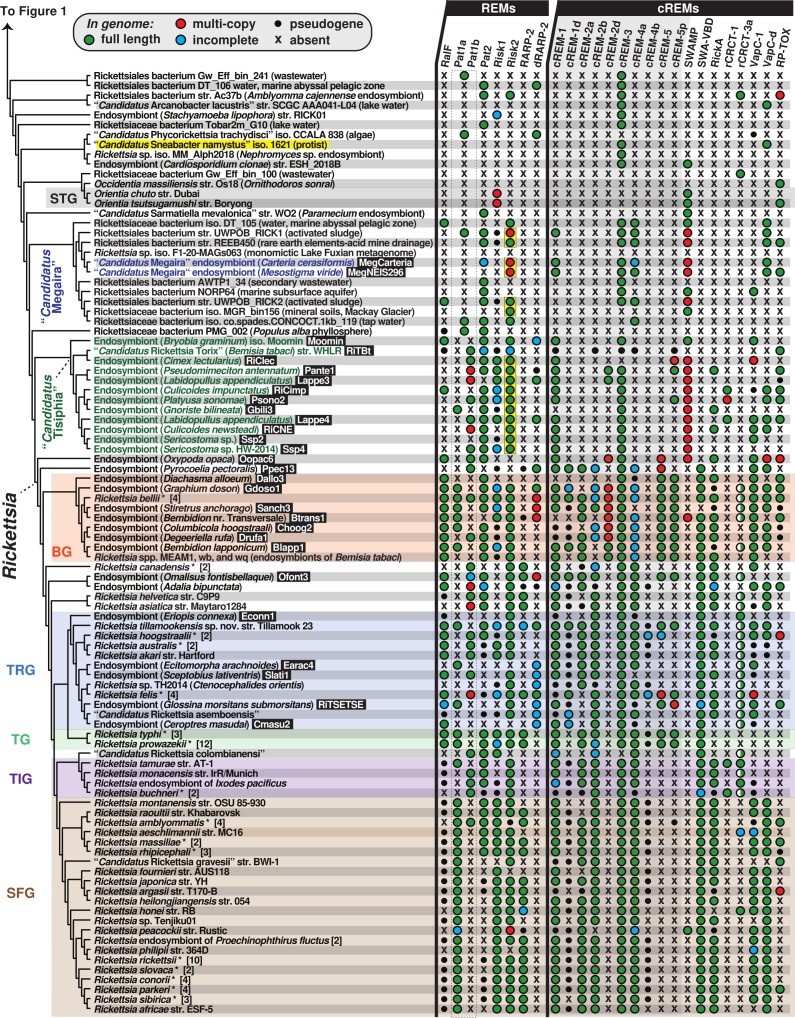
Fig 2.
Phylogenomic analysis of Rickettsia REMs and cREMs in Rickettsiaceae. Cladogram (continued from Fig. 1C) summarizes a phylogeny estimated from concatenated alignments for RvhB4-I and RvhB4-II proteins from 153 rickettsial assemblies (full tree, Fig. S2; sequence information, Table S1). “Candidatus Sneabacter namystus” (highlighted yellow) was manually added to the cladogram based on prior phylogeny estimation (82, 83) as this species lacks rvh genes but carries a T6SS (see Fig. S3). Black boxes provide short names for 29 MAGs from Davison et al. (73) (Note: the clade colored green comprises genus Tisiphia though genus name Rickettsia reflects NCBI taxonomy as of 26 February 2023). Asterisks depict multiple genome assemblies for a species. BG, Bellii Group; TRG, Transitional Group; TG, Typhus Group; TIG, Tamurae-Ixodes Group; and SFG, Spotted Fever Group. The dashed box for Pat1 proteins indicates the inability to discern Pat1a and Pat1b homology outside of Tisiphia and Rickettsia species (see Fig. 4). Yellow boxes denote Risk2 proteins that are appended to C-terminal Schuenke-Walker antigen (SWA) domains (see Fig. 5). SWAMP, SWA modular proteins; all other REMs and cREMs are described in Fig. 1B and Table S2). Half circles for rCRCT-3a depict the presence of one or more antidotes but no toxin.
Origins of REMs
An emerging diversity of bacterial Arf-GEFs
Bacterial mimicry of eukaryotic-like Sec7 domains (S7D) to function as guanine nucleotide exchange factors (GEFs) for host ADP-ribosylation factors (Arfs) was first described for Legionella pneumophila, which utilizes the dot/icm T4SS effector RalF to recruit and activate host Arf1 to the Legionella-containing vacuole (LCV) (84, 85). Certain Rickettsia genomes encode RalF proteins that are remarkably similar to Legionella counterparts across the S7D, as well as a Sec7 capping domain (SCD) that restricts access to the catalytic site (86–88). The SCD has high specificity for host membranes and differentially regulates effector subcellular localization for Legionella (the LCV) and Rickettsia (cytosolic leaflet of plasma membrane) RalF (89). Rickettsia RalF was the first characterized REM; its secretion during host cell invasion activates host Arf6 at the plasma membrane, a process driven by a unique C-terminal extension, termed variable with Pro-rich region (VPR), which interacts with host actin and phosphatidylinositol 4,5-biphosphate at entry foci (56, 90). The presence of ralF in the genomes of some Rickettsia pathogens but its absence in non-pathogenic species led to speculation that this REM may be a lineage-specific virulence factor (56, 90, 91). Furthermore, while species of Rickettsia and Legionella exchange genes in common intracellular environments (92, 93), the absence of ralF in any other known bacteria precluded insight into the origin of RalF and specifically the nature of Legionella- and Rickettsia-specific C-terminal architectures.
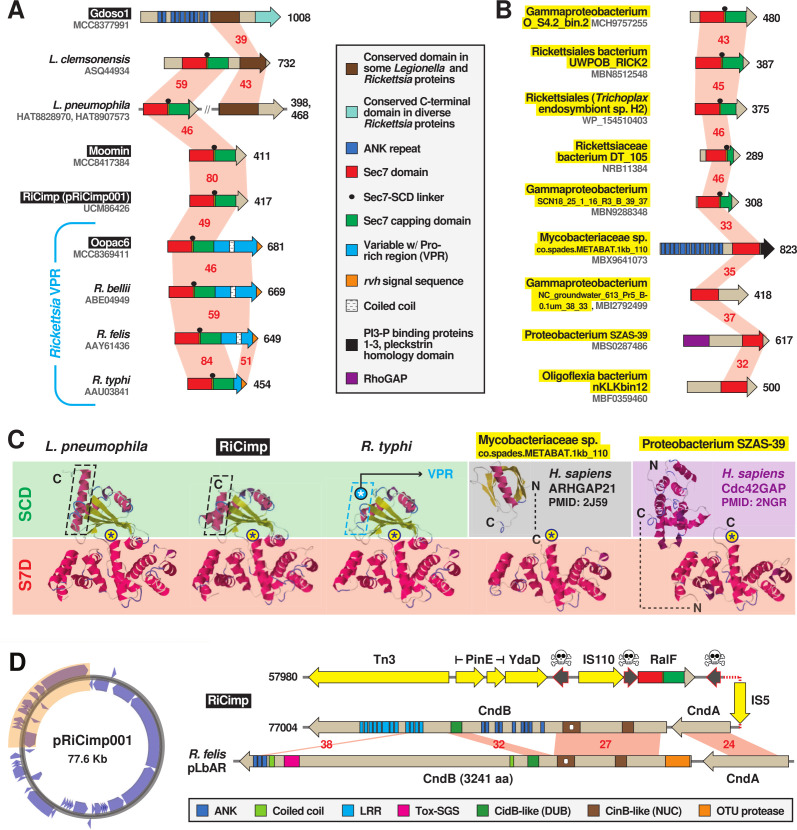
Our analyses provide clarity on RalF evolution by unearthing numerous bacterial analogs with novel S7D-containing architectures (Fig. 3; Fig. S4). First, an unusual Legionella RalF from Legionella clemsonensis was found to carry a conserved domain at its C-terminus that was also detected in a large ankyrin (ANK) repeat-containing protein of the Rickettsia endosymbiont of Graphium doson (Gdoso1) genome (Fig. 3A; Fig. S4A and B). This Gdoso1 protein also contains another conserved domain at its C-terminus that is widespread in Rickettsia genomes but lacks any associated annotation in public databases. These observations indicate frequent recombination in conjunction with the LGT of these diverse genes. Second, while the most basal Rickettsia species (endosymbiont of Oxypoda opaca, “Oopac6”) harbors a RalF with the Rickettsia-like C-terminal VPR, two Tisiphia species (endosymbionts of Bryobia graminum or “Moomin,” and Culicoides impunctatus or “RiCimp”) instead exhibit Legionella-like C-terminal domains (Fig. 3A and C; Fig. S4C through E). RiCimp RalF is encoded on a plasmid (pRiCimp001), which is unique among all other known RalF genes (Fig. 3D), supporting original speculation for RalF exchange between Legionella and Rickettsia species (84). Remarkably, pRiCimp001 also carries a toxin-antidote (TA) module highly similar to the plasmid-encoded TA module of Rickettsia felis str. LSU-Lb (94) (Fig. 3D), which we previously described as part of the mobilome shuttling reproductive parasitism (RP) genes across Rickettsia and Wolbachia (94) (discussed in section cREMs with characterized function).
Fig 3.
MAG analysis divulges novel diversity of bacterial Sec7-domain-containing proteins. Black boxes provide short names for MAGs from Davison et al. (73). These and additional newly discovered RalF-like proteins (highlighted yellow) substantially expand the prior recognized RalF diversity (56, 84, 88, 90). Structural models for proteins are found in Fig. S4D through F. (A and B) Insight from (A) novel Legionella and rickettsial architectures and (B) diverse RalF-like proteins discovered in MAGs. Red shading and numbers indicate percent aa identity across pairwise alignments (sequence information in Table S2). All protein domains are described in the gray inset. (C) Comparison of the Legionella pneumophila RalF structure (PDB 4C7P) (88) with predicted structures of S7D-SCD regions of RiCimp RalF (LF885_07310) and Rickettsia typhi RalF (RT0362), and S7Ds of Mycobacteriaceae sp. co.spades.METABAT.1kb_110 (K2 × 97_15435) and Proteobacterium SZAS-39 (JSR17_09325). The delineation of the Sec7 domain (S7D, red) and Sec7-capping domain (SCD, green if present) is shown with an approximation of the active site Glu (asterisk). Additional eukaryotic-like domains for the non-rickettsial proteins are noted. Modeling was done with Phyre2 (95). More detailed structural explanation can be found in Fig. S4C. (D) RiCimp plasmid pRiCimp001 carries RalF and a CindB/A toxin-antidote module similar to those characterized or implicated in reproductive parasitism (94). Gene region drawn to scale using the PATRIC compare region viewer tool (96). Yellow, transposases and other mobile elements; skull-and-crossbones, pseudogenes; other domains are described in the gray inset at the bottom. Plasmid map created with Proksee (https://proksee.ca/).
Finally, RalF proteins from three additional rickettsial species and two putative gammaproteobacterial species carry both the S7D and SCD but no C-terminal extensions (Fig. 3B; Fig. S4E and F). Four other novel S7D-containing proteins from non-rickettsial bacteria lack SCDs; however, two contain eukaryotic domains found in RHO GTPase-activating proteins (RHOGAP) that also target Arfs (Fig. 3B and C; Fig. S4F). All these discovered proteins have a highly conserved S7D and SCD (if present) and include most of the structural features that define RalF proteins (Fig. S4G). These collective characteristics attest to LGT disseminating the S7D-SCD architecture across divergent bacteria, with recurrent gains of additional domains tailored to eukaryotic cell functions (e.g., VPR, ANK, and RHOGAP). Our phylogenomics results indicate the acquisition of the Rickettsia-unique C-terminal VPR occurred early in Rickettsia evolution after divergence from Tisiphia spp., with multiple losses of RalF in more than half of the sequenced species (Fig. 2).
Patatins: divergent phospholipases are recurrent in Rickettsiales
Rickettsiae (and other Rickettsiales species exiting the phagosome and/or lysing host cells) require membranolytic effectors throughout the intracellular lifestyle. Phospholipase D (PLD) is a highly conserved enzyme with demonstrated membranolytic activity in a surrogate expression system (97), though its function during Rickettsia infection of host cells remains unresolved (98). PLD contains a N-terminal Sec signal (91), yet other phospholipase A2 (PLA2) enzymes (patatins Pat1 and Pat2) have sequence characteristics of rvh substrates (74), and Pat2 binds RvhD4 in coimmunoprecipitation assays (57) (Fig. 1B). Studies on R. typhi have shown that both Pat1 and Pat2 are secreted during host cell infection, require host cofactors for activation, and function early in infection by facilitating phagosome escape (74, 75). Recent work on Rickettsia parkeri Pat1 also demonstrated a role in phagosome escape in addition to facilitating avoidance of host polyubiquitination and autophagosome maturation, as well as promotion of actin-based motility and intercellular spread (99). R. parkeri lacks Pat2, which is slightly more restricted in Rickettsia genomes and possibly provides a function in host cell lysis for rickettsiae that do not spread intercellularly without host cell lysis (e.g., TG rickettsiae).
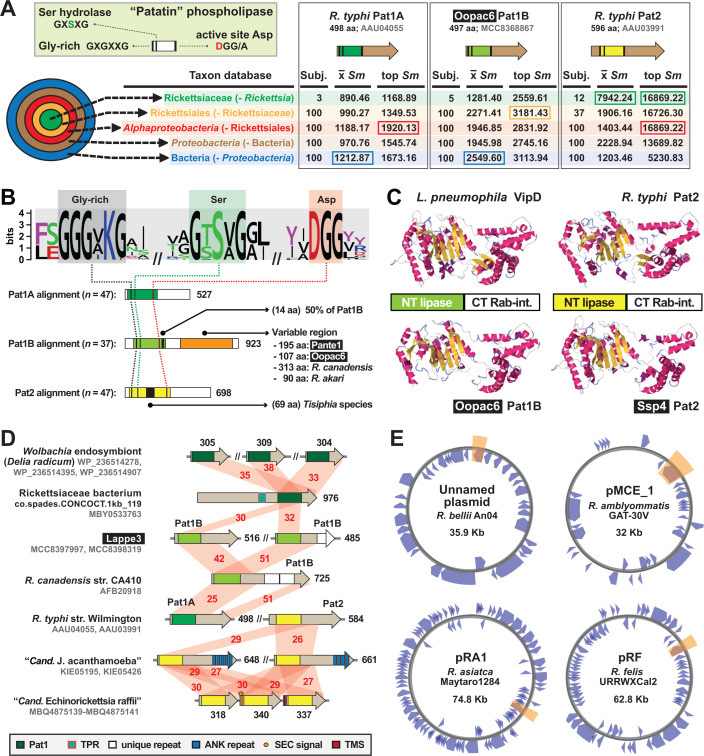
All patatins share a common active site architecture that is critical for PLA2 activity (Fig. 4A). Despite this, Pat1 and Pat2 are highly divergent outside of the patatin domain and have different origins based on phylogeny estimation (74). Furthermore, Pat1 proteins form two distinct groups, Pat1A and Pat1B, with pat1B found on plasmids and often recombining with chromosomal pat1 loci (74). Utilizing newly discovered rickettsial patatins from MAGs, we show that all three enzymes (Pat1A, Pat1B, and Pat2) have distinct sequence profiles, with Pat1B proteins having a high length variable C-terminal region relative to Pat1A and Pat2 enzymes (Fig. 4A and B). Despite this, Pat1B and Pat2 proteins have cryptic similarity across their C-terminal regions to support robust modeling to the crystal structure of L. pneumophila dot/icm T4SS effector VipD (100) (Fig. 4C). During L. pneumophila host cell infection, secreted VipD localizes to host endosomes, catalyzing the removal of phosphatidylinositol 3-phosphate from endosomal membranes (N-terminal patatin domain) and binding Rab5 or Rab25 (C-terminal domain), ultimately blocking endosome-LCV fusion (100–102). As with RalF, it is likely that Rickettsia Pat1 and Pat2 proteins have rudimentary analogous functions to VipD (targeting host membranes and binding host Rabs), but spatiotemporal and biochemical differences provided that rickettsiae lyse the phagosome and seemingly do not engage early endosome trafficking on par with Legionella species.
Fig 4.
Divergent patatin phospholipases are recurrent in rickettsial evolution. Black boxes provide short names for MAGs from Davison et al. (73). (A) PLA2 active site characteristics and divergent patatin forms. Green inset describes general patatin domain and active site architecture. HaloBlast results for Pat1A, Pat1B, and Pat2 (query sequences described at the top) are shown, with top-scoring halos boxed (full results in Table S4). (B) Sequence logo (103) showing conservation of the PLA2 active site motifs across Tisiphia and Rickettsia patatins (sequence information provided in Table S2). Pat1A, Pat1B, and Pat2 sequences were aligned separately with MUSCLE (104) (default parameters) with active site motifs compiled for conservation assessment. Features unique to each patatin are noted. (C) Legionella pneumophila VipD structure (PDBID: 4AKF) and modeling of three rickettsial patatins to VipD using Phyre2 (95). (D) Diverse architectures for select patatins. Red shading and numbers indicate percent aa identity across pairwise alignments (sequence information in Table S2). All protein domains are described in the gray inset. Dark green indicates Pat1 domains not grouped into A or B. (E) Four Rickettsia plasmids carry pat1B (shaded orange). Plasmid maps were created with Proksee (https://proksee.ca/).
We detected Pat1 and Pat2 proteins in several non-Rickettsiaceae genomes (Fig. 1C), with some genomes [e.g., novel sea urchin and cabbage root fly endosymbionts in the Anaplasmataceae (105, 106)] harboring duplications (Fig. 4D). Pat1 proteins from Rickettsiales species outside of the genera Tisiphia and Rickettsia could not be confidently assigned to either Pat1A or Pat1B (Fig. 1C and 2; dark green domains in Fig. 4D). Most species of Tisiphia and Rickettsia carry either Pat1A or Pat1B and/or Pat2 (Fig. 2). The only two species carrying all three distinct enzymes (Rickettsia bellii and Rickettsia amblyommatis) have Pat1B encoded on a plasmid (Fig. 4E). Overall, the patchwork distribution of these divergent enzymes, evidence for modular domain diversification, and presence on plasmids indicate that PLA2 activities for rickettsiae are lineage-specific and subject to continual patatin gene gain and loss throughout evolution. Furthermore, certain pat gene profiles may confer advantages in particular hosts.
Domain repurposing is risky business
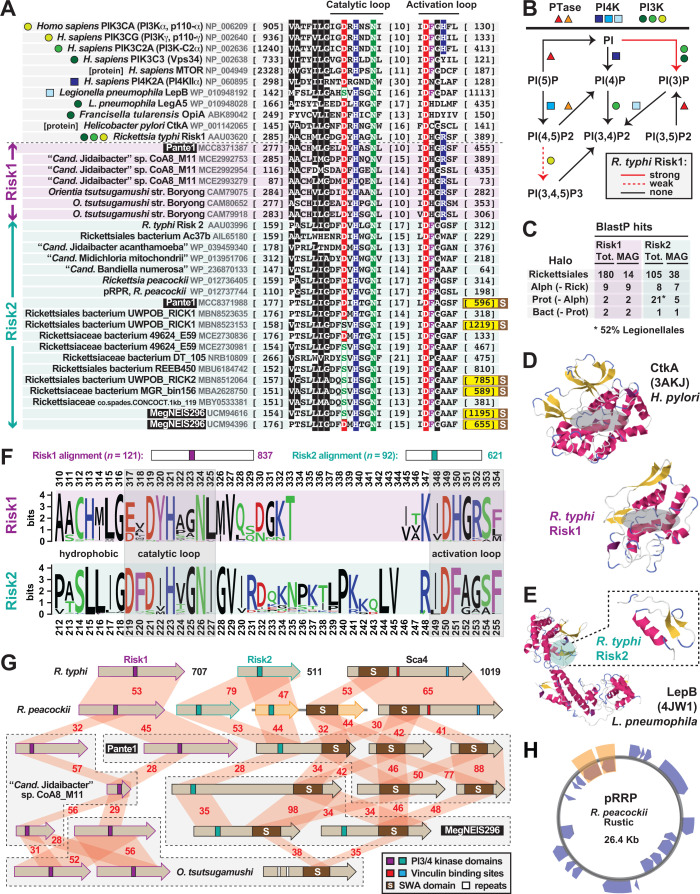
Bacterial pathogens can directly modify host membrane phosphatidylinositol (PI) composition by mimicking eukaryotic kinases, phosphatases, and phosphotransferases (107–110). Secreted PI kinases from intracellular pathogens R. typhi (Risk1), L. pneumophila (LegA and LepB), and Francisella tularensis (OpiA) alter the PI composition on phagosomes to prohibit maturation and fusion with lysosomes (57, 111, 112). Characterized as either PI3 (Risk1, LegA, and OpiA) or PI4 (LepB) kinases, these enzymes possess a similar PI3/4 active site architecture (pfam00454) analogous to eukaryotic PI kinases, as well as certain protein kinases, that function in a myriad of membrane-associated functions, including intracellular signaling and trafficking (113) (Fig. 5A and B). Subverting these host cell functions is highly advantageous to intracellular parasitism; thus, the dearth of identified PI3/4 kinase effectors likely reflects the cryptic nature of the PI3/4 active site within these proteins, which lack similarity outside of the PI3/4 active site domain (57, 112).
Fig 5.
Discovery of a novel cryptic rickettsial PI kinase exposes a widespread rickettsial surface antigen. Black boxes provide short names for MAGs from Davison et al. (73). Amino acid coloring is described in Fig. 3 legend. (A) Previous work (above dashed line) identified a Rickettsia PI kinase (Risk1) with a cryptic active site like human and other bacterial PI3/PI4 kinases, as well as related protein kinases. Colored shapes depict characterized substrate specificity (see panel B). Our study (below dashed line; select proteins shown) identified new rickettsial Risk1 proteins, as well as a second distinct PI kinase (Risk2) also prevalent in rickettsial genomes and MAGs (Fig. 1C and 2). All PI3/PI4 kinase domains were aligned using MUSCLE (104) (default parameters). Sequence information is provided in Table S2. Yellow highlighting on end coordinates denotes Risk2 proteins fused to a C-terminal SWA domain (see panel G and Fig. S5 for a full description of the SWA domain). (B) Mechanisms of phosphorylation on the PI inositol ring at 3′, 4′, and 5′ positions. Data for R. typhi Risk1 are shown by red arrows (57). (C) HaloBlast results (R. typhi Risk1 and Risk2 as queries) broken down to illustrate the presence of Rickettsia-like PI kinases in MAGs and the similarity between Risk2 and Legionella PI kinases (full data in Table S2). (D) Risk1 threads with high confidence [90.7%, 72% coverage; Phyre2 (95)] to the Helicobacter pylori proinflammatory kinase CtkA (114). (E) Risk2 threads with high confidence [85.1%, 9% coverage; Phyre2 (95)] to a limited region of LepB, a Rab GTPase-activating protein effector from L. pneumophila (115). (F) Risk1 and Risk2 proteins have cryptic and distinct PI3/4 active sites yet lack similarity outside of these regions. Logos depict individual alignments, which are summarized at the top and were performed with MUSCLE (104), default settings. (G) Many rickettsiae carry a diverse arsenal of PIK effectors, some of which are tethered to SWA domains. Six select species are shown with their full complement of PI3/4 kinase and SWA architectures. Red shading and numbers indicate percent aa identity across pairwise alignments (sequence information in Table S2). Rickettsia peacockii pRRP proteins are shaded orange (see panel H). (H) Plasmid pRRP of R. peacockii str. Rustic (116, 117) carries a divergent Risk2 gene that is adjacent to an ORF encoding a SWAMP (orange highlighting). Plasmid map created with Proksee (https://proksee.ca/).
BlastP and HMMER (118) analyses using only the Risk1 PI3/4 active site unearthed nearly 300 Rickettsiales proteins, with many genomes having multiple divergent kinases. Further inspection revealed the presence of a second conserved protein harboring the PI3/4 active site, which we named Rickettsia intracellular secreted kinase-2 (Risk2) (Fig. 5A). Notably, the R. typhi Risk2 protein (RT0527) was captured in the same RvhD4 coimmunoprecipitation assay that identified Risk1 as a REM (57) (Fig. 1B). HaloBlast analyses of full-length Risk1 and Risk2 proteins indicate distinct profiles, with Risk2 sharing low similarity to Legionellales kinases (Fig. 5C). Structural analyses corroborated this result, with limited regions of Risk1 and Risk2 modeling best to structures of Helicobacter pylori CtkA (114) and L. pneumophila LepB (111), respectively (Fig. 5E and F). Comparison of Risk1 and Risk2 PI3/4 active sites revealed (i) juxtapositioned aromatic residues in their catalytic loops, (ii) the presence of a positively charged residue in the activation loops of most Wortmannin-sensitive kinases (Risk1 and human class 1 and 2 PI3 kinases), and (iii) greater sequence length between catalytic and activation loops in Risk2 proteins (Fig. 5F). Furthermore, only LepB and some rickettsial Risk2 proteins have the catalytic loop Asp replaced by Ser (Fig. 5A). These collective observations indicate two divergent PI3/4 kinases encoded in most rickettsial genomes (Fig. 2), leading us to posit that Risk2 is a PI4 kinase that complements the PI3 kinase activity of Risk1 (57).
We determined a remarkable connection between Risk2 and another Rickettsia effector, Sca4, which is highly conserved in Rickettsia species and implicated in intercellular spread by reducing mechanotransduction at cell-cell junctions (76, 119). The Sca4 C-terminal region has eukaryotic-like vinculin-binding sites (VBSs) that reduce vinculin-α-catenin interactions, which facilitates neighboring cell engulfment of Rickettsia-induced protrusions. The N-terminal region, shown by Schuenke and Walker (120) to elicit anti-rickettsia antibodies (Pfam: 120_Rick_ant).
From many forms, one descendent
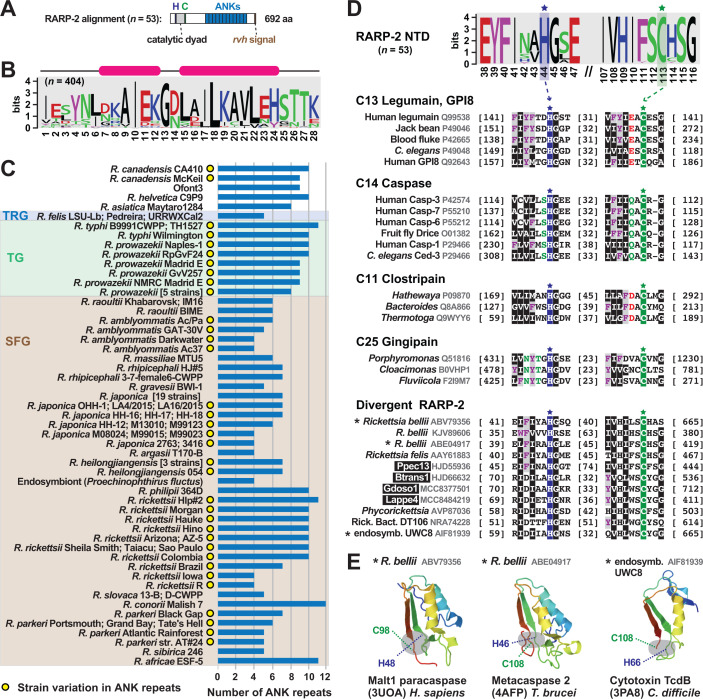
Early in host infection, pathogens R. typhi and R. rickettsii secrete the REM RARP-2, which traffics to the endoplasmic reticulum and Golgi apparatus, leading to trans-Golgi network (TGN) fragmentation and ultimately perturbed protein transport to the host cell surface (58, 121). Like RalF, RARP-2 has a C-terminal tail that binds RvhD4 (Fig. 1B); furthermore, the protein has well-delineated N-terminal protease and C-terminal ANK repeat domains (Fig. 6A). The protease domain has minimal analogy to clan CD protease families (C13 legumain, C14 caspase 1, C11 clostripain, and C25 gingipain R), which share a common fold that arranges a His and Cys catalytic dyad (122). This active site is essential for RARP-2 fragmentation of TGN (121) and also contributes to the lytic plaque phenotype of virulent R. rickettsii strains (58). The ANK repeat domain is atypical among most ANK repeat-containing proteins (123), as the composition of each repeat is highly similar in length and identity (Fig. 6B; Fig. S6A through C) despite a highly variable repeat number across orthologs, even at the strain level in most cases (Fig. 6C). RARP-2 active site mutants still traffic to perinuclear membranes, indicating that the ANK domain drives subcellular localization. However, shorter repeats (four in attenuated strains Iowa R. rickettsii) do not disrupt TGN fragmentation relative to those of pathogenic R. rickettsii (121), suggesting a larger ANK domain is required for proper localization.
Fig 6.
RARP-2 architecture is derived from multiple divergent forms. Black boxes provide short names for MAGs from Davison et al. (73). Amino acid coloring is described in Fig. 3 legend. Sequence logos constructed with WebLogo 3 (103). Sequence information in Table S2. (A) General architecture of RARP-2 proteins deduced from an alignment of 53 non-redundant RARP-2 proteins using MUSCLE (104) (default parameters). (B) Consensus sequence for the RARP-2 ANK repeat deduced from 404 repeats. (C) Depiction of the 53 non-redundant RARP-2 proteins with ANK domain repeat number provided. For brevity, some strain names are not shown for Rickettsia prowazekii: Chernikova, Katsinyian, Dachau, BuV67-CWPP, and Rp22; Rickettsia japonica: YH, DT-1, HH-1, HH06154, HH07124, HH07167, MZ08014, Nakase, PO-1, Tsuneishi, HH-13, HH06116, HH06125, LON-151, M11012, M14012, M14024, SR1567, and YH_M; Rickettsia heilongjiangensis: HCN-13, Sendai-29, and Sendai-58. (D) RARP-2 and dRARP-2 proteins possess N-terminal domain clan CD cysteine protease-like active sites (122). Sequences were manually aligned to illustrate the conservation across all diverse protein groups. “Rick. endo. UWC8,” endosymbiont of Acanthamoeba str. UWC8 (not shown in Fig. 1C but closely related to endosymbiont of Acanthamoeba str. UWC36 in the Midichloriaceae). (E) Insight on RARP-2/dRARP-2 structure. Asterisks indicate proteins from panel D that were used in Phyre2 (95) searches to identify template structures for modeling (124–126). A complete structure of R. typhi RARP-2 predicted with Alphafold (127, 128) corroborates these dRARP-2 models and indicates deviations on a common effector architecture (Fig. S6C).
Probing recently sequenced genomes and MAGs did not reveal RARP-2 sequences in the Bellii Group (BG) rickettsiae or other Rickettsiaceae genomes (Fig. 2), consistent with prior observations that RARP-2 is unique to later-evolving Rickettsia lineages (91). Yet, by focusing on the N-terminal protease domain, we discovered 56 divergent RARP-2 (dRARP-2) proteins that possess the clan CD active site architecture (Fig. 6D). These proteins were binned into six groups (Fig. S6D) that have very different ANK repeat domain identities (data not shown); furthermore, several could be modeled to structures of eukaryotic (124, 125) and prokaryotic (126) clan CD members (Fig. 6E). dRARP-2 proteins are predominantly found in BG rickettsiae and Tisiphia genomes but likely shuttle in the intracellular mobilome given that one is carried by a Midichloriaceae species [endosymbiont of Acanthamoeba str. UWC8 (129)]. Based on the discordant genomic distribution of RARP-2 and dRARP-2 (Fig. 1C and 2) and the strong bias of RARP-2 in vertebrate-associated species, we speculate that RARP-2 and dRARP-2 may be tailored for similar functions related to TGN fragmentation yet well diverged to allow recognition of targets specific to disparate eukaryotic hosts. This is reminiscent of the recent discovery that R. parkeri utilizes different factors for apoptosis induction in ticks versus mammals (130).
cREMs with unknown function
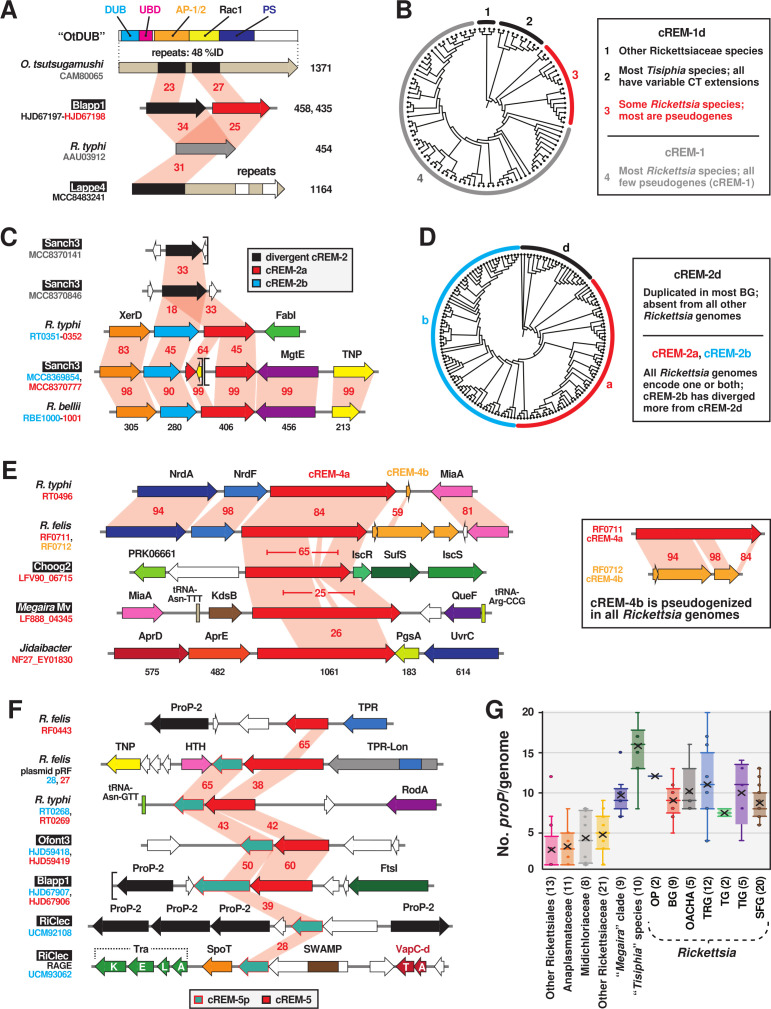
For five R. typhi hypothetical proteins previously shown to interact with RvhD4 (cREM-1–5; Fig. 1B), MAG analyses provided substantial clarity on the mechanisms of evolution shaping their architectures. Four of these proteins are described below in light of newfound gene fission/fusion and duplication events (cREM-1, cREM-2, and cREM-4), as well as a greater role of conjugative transposons shaping Rickettsia evolution (cREM-5). Unexpectedly, the small cREM-3 (~93 aa) was determined to have widespread conservation in Rickettsiales yet also exist in certain other Proteobacteria (Fig. 1C and 2). While likely not a REM, our analyses revealed a potential structure associated with this curious protein (Fig. S7C).
Cryptic gene fission and duplication obscured by rapid divergence
For cREM-1 and cREM-2, we utilized phylogeny estimation in conjunction with sequence analysis to predict gene fission (cREM-1) and duplication (cREM-2) events behind the evolution of these proteins. Neither protein was found outside of Rickettsiaceae (Fig. 1C and 2). cREM-1 proteins are streamlined from larger modular Tisiphia proteins that harbor the entire cREM-1 sequence as a domain; accordingly, we named these divergent cREM-1 (cREM-1d) (Fig. 7A). Some rickettsiae carry cREM-1 tandem duplications, though most genomes have one conserved gene and the second pseudogenized (Fig. S7B; red clade). Curiously, cREM-1 proteins have similarities to a repeat region within the Orientia tsutsugamushi effector OtDUB (Fig. 7A; Fig. S7A). This region in OtDUB binds clathrin adaptor-protein complexes AP-1 and AP-2 and harbors a cryptic Rac1 GEF domain (131–133). This indicates that cREM-1 proteins may have evolved from larger modular proteins with functions tailored to the eukaryotic cytosol, with repeat regions of these large effectors streamlining to smaller proteins encoded by tandem genes (Fig. 7B; Fig. S7A).
Fig 7.
Four candidate REMs are characterized by gene fission and duplication. Black boxes provide short names for 29 MAGs from Davison et al. (73). Gene regions were drawn to scale using the PATRIC compare region viewer tool (96). Sequence information is provided in Table S2. (A) Similarity between O. tsutsugamushi effector OtDUB (CAM80065), divergent cREM-1 (cREM-1d), and cREM-1 proteins. OtDUB characterized domains: deubiquitinase (light blue), ubiquitin-binding (pink), cryptic Rac 1-like guanine nucleotide exchange factor (yellow), clathrin adaptor-protein complexes AP-1 and AP-2 (orange), and phosphatidylserine-binding (gray) (5, 131, 134). Red shading and numbers indicate percent aa identity across pairwise alignments. (B) Cladogram depicting phylogeny estimation of 102 cREM-1d and cREM-1 proteins (see Fig. S7A for phylogram and methods). Inset describes cREM-1 proteins, with clade colors matching the protein colors in the schema in panel A. (C) Diversification of cREM-2 proteins via duplication. (D) Cladogram depicting phylogeny estimation of 158 cREM-2d, cREM-2a, and cREM-2b proteins (see Fig. S7B for phylogram and methods). Inset describes cREM-2 proteins, with clade colors matching the protein colors in the schema in panel C. (E) Ancient gene duplication of cREM-4 and location of cREM-4 genes in select Rickettsiales species. The cREM-4 pentapeptide repeat domain is illustrated in Fig. S7D. (F) cREM-5/5p loci occur in variable genomic regions, including plasmids and Rickettsiales amplified genetic elements (RAGEs). The complete RAGE for RiCle is illustrated in Fig. S7F. (G) Distribution of ProP genes in Rickettsiales genomes. OP, Rickettsia endosymbionts of Oxypoda opaca (Oopac6) and Pyrocoelia pectoralis (Ppec13); OACHA, Rickettsia endosymbionts of Omalisus fontisbellaquei (Ofont3) and Adalia bipunctata, Rickettsia canadensis, Rickettsia helvetica, and Rickettsia asiatica.
cREM-2 proteins belong to pfam17422 (DUF5410: specific to Rickettsia species). Our analyses identified a second DUF5410-like protein encoded adjacent to cREM-2 proteins in many Rickettsia genomes (Fig. 2). Neither of these tandem duplicates (designated cREM-2a and cREM2-b) contain Sec signal sequences or other predictable features (Fig. 7C). Furthermore, some BG rickettsiae and Tisiphia species harbor a third divergent cREM-2 (cREM-2d) that is absent from later-evolving Rickettsia lineages. With the assumption that cREM-2d is an ancestral form, phylogeny estimation indicates cREM-2b proteins are more divergent than cREM-2a proteins (Fig. 7D), though all three protein architectures share high conservation in dozens of residues within the central region of these proteins (Fig. S7B).
cREM-4 proteins also show evidence of an ancestral duplication (Fig. 7E), though no genomes contain a complete duplicate gene, indicating a consistent pseudogenization event that rapidly followed cREM-4 duplication (Fig. 2). Despite their large size (~950 aa), these proteins contain only one observable feature, a small internal pentapeptide repeat (PR). While widespread in diverse bacterial proteins, PR function is generally unknown, though some bacterial PR-containing proteins can interact with DNA-binding proteins (135) and contribute to virulence (136) (Fig. S7D). cREM-4 proteins are encoded in certain other Rickettsiaceae genomes, and like dRARP-2, a single Midichloriaceae species [“Candidatus Jidaibacter acanthamoeba” (30)] encodes a cREM-4 protein. While cREM-4 of BG rickettsiae lack the PR (Fig. S7D), nearly all later-evolving Rickettsia genomes encode a complete cREM-4 protein, indicating retention of a conserved function after an ancestral duplication.
LGT of cREM-5 as a two-gene module across select species
cREM-5 proteins were previously noted for their restricted distribution in TG rickettsiae and R. felis, which carry copies on the chromosome and plasmid pRF (137). Our analyses yielded several novel findings. First, while absent from any Spotted Fever Group (SFG) or Tamurae-Ixodes Group (TIG) rickettsiae, cREM-5 proteins are highly conserved in all BG rickettsiae genomes, as well as in a few Tisiphia genomes (Fig. 2). Second, most cREM-5 genes have an associated protein, cREM-5 partner (cREM-5p), encoded immediately downstream (Fig. 7F). Despite conserved regions (Fig. S7E), neither protein has detectable domains or similarity to proteins in other Rickettsiales (Fig. 2). Third, cREM-5/5p genes have a strong co-occurrence with PropP-2 genes (black, Fig. 7F). ProP (proline betaine transporters of the major facilitator superfamily) function in osmoregulation (138, 139) are proliferated in Rickettsia genomes, with seven conserved groups (PropP1-7) containing species-specific duplications (93, 140). Why specifically PropP-2 genes cluster near certain cREM-5/5p loci is unclear, though insight from MAGs illuminated a previously unrealized point in Rickettsiales evolution where ProP proliferation occurred (Fig. 7G).
Finally, cREM-5 modules are found in recombination hotspots and other less conserved genomic regions, indicating LGT behind their evolution in Rickettsia genomes. Aside from cREM-5/5p on plasmid pRF, one copy of cREM-5p from the RiClec (endosymbiont of Cimex lectularius) genome is found on a conjugative transposon termed Rickettsiales amplified genetic elements (RAGEs) (Fig. 7F). RAGEs are integrative and conjugative elements present on certain Rickettsia plasmids and chromosomes (93, 94, 141), as well as proliferated and scattershot in O. tsutsugamushi genomes (142, 143). Cargo genes, or those piggybacking on RAGEs at indiscriminate insertion sites, have functions mostly related to the stringent response and metabolism, defense and resistance, and adaptation to host cells (e.g., ProP genes are shuttled by RAGE). The addition of cREM-5p and SWAMPs, as well as a myriad of TA modules, to the list of RAGE cargo (see Fig. S7F) indicates that these mobile elements play a role in disseminating pathogenicity factors, which was previously unappreciated.
cREMs with characterized function
Several Rickettsia proteins that lack N-terminal Sec signals have either been well characterized for their roles in subverting host cell processes (e.g., Sca4 and RickA) or possess features that implicate them in targeting host molecules (e.g., VapC and other toxins within TA modules). Until secretory pathways for these molecules are characterized, we consider them cREMs (Fig. 1B). MAG analyses of these proteins have generated novel insight into the structure and evolution of domains targeting the host actin cytoskeleton. Furthermore, a greater appreciation for toxin architecture and distribution indicates the rvh T4SS may still function in congener killing despite the host-dependent lifestyle of the later-evolved Rickettsiales species.
Insight on Rickettsia interactions with host actin
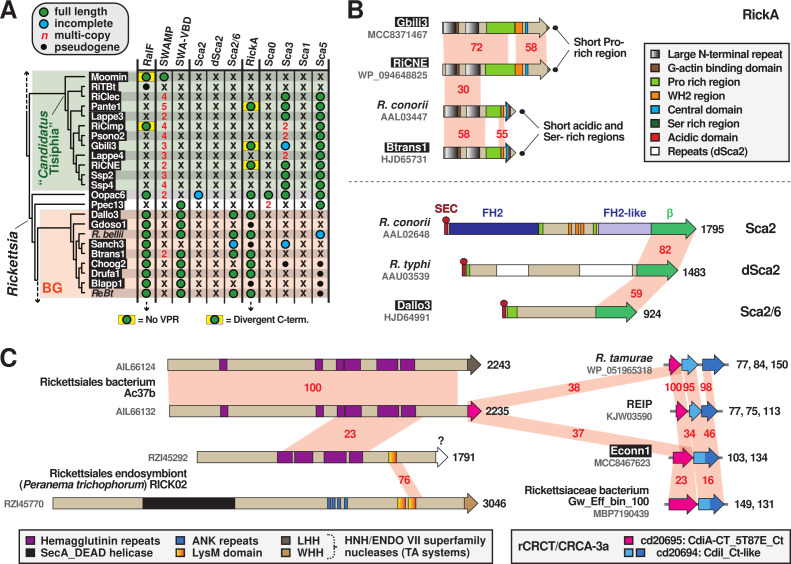
With their RalF proteins lacking VPRs and their SWAMPs lacking VBDs (Sca4), Tisiphia species may interact with host cell actin cytoskeleton differently than Rickettsia species (Fig. 8A). We analyzed another host actin-associated protein, RickA, which some Rickettsia species use for intracellular motility and possibly intercellular spread (144–147). While no association with the rvh T4SS has been characterized, RickA localizes to the bacterial surface in the absence of a Sec signal peptide (147). RickA directly activates host Arp2/3 complexes through an architecture that mimics host nucleation-promoting factors (NPF) (78, 148). We discovered several RickA proteins from Tisiphia MAGs that differ in their C-terminal architectures relative to SFG rickettsiae RickA proteins characterized in actin polymerization (Fig. 8B; Fig. S8A). The functional relevance of these differences is unclear. Surprisingly, we gained novel insight into the N-terminal structure of all RickA proteins. The substantial increase in diversity from MAGs illuminated a large (~95 aa) repeat region enclosing the G actin-binding domain, with each repeat highly conserved in hydrophobicity and predicted structure (Fig. 8B; Fig. S8B). We envisage that this conserved region may facilitate the docking of G actin to the G actin-binding domain and the overall positioning of the N-terminus to Wiskott-Aldrich syndrome protein homology 2 (WH2) motifs at the C-terminal region.
Fig 8.
MAG analyses lend insight on Rickettsia interactions with host actin cytoskeleton and rvh T4SS function. Black boxes provide short names for 29 MAGs from Davison et al. (73). (A) MAGs shed light on the evolution of Rickettsia factors behind host actin polymerization and invasion. Tisiphia and BG rickettsiae taxa, as well as SWAMP, SWA-VBD, RickA, and RalF info, are from Fig. 2. The passenger domains of Rickettsia conorii Sca2 (AAL02648), R. typhi dSca2 (AAU03539), R. bellii Sca2-6 (ABE05361), R. conorii Sca0 (AAL03811), R. typhi Sca3 (AAU03915), R. typhi Sca1 (AAU03504), and R. typhi Sca5 (AAU04158) were used in BlastP searches directly against Tisiphia and BG rickettsiae genomes. Passenger domains and linker sequences were delineated as previously shown (91). ReBt, Rickettsia spp. MEAM1, wb, and wq (endosymbionts of Bemisia tabaci). (B) Some Rickettsia genomes encode one or more host actin nucleation proteins. Top: Tisiphia and Rickettsia RickA proteins share a large N-terminal repeat domain but diverge at their C-termini.. Further details on RickA architecture are provided in Fig. S8. Bottom: Sca2, d-Sca2, and Sca2-6 proteins have a common autotransporter domain (β) but divergent passenger domains. FH2, formin homology 2. Sca2 mimics host formin actin nucleators (149) to recruit and polymerize actin for intracellular motility and intercellular spread (150, 151). The functions of dSca2 and Sca2/6 are unknown. (C) The mobile nature of CDI-like/Rhs-like C-terminal toxin/antidote (CRCT/CRCA) modules across diverse rickettsial genomes. Schema shows the integration of CRCT/CRCA modules into larger polymorphic toxins (hemagglutinin-like toxins, LysM-like peptidoglycan/chitin-targeting toxins, etc.), as well as CRCT/CRCA modules independent of larger toxins. The toxin warhead for RZI45292 is unknown. Further details are provided in Fig. S9.
Despite activation by RickA, the specific role of host Arp2/3 complexes during rickettsial infection is unclear, perhaps due to different species utilized across studies garnering contrasting results (146, 147, 152). Specifically, actin-based motility in certain Rickettsia species is carried out by a second NPF, Sca2, that polymerizes actin independent of Arp2/3 complexes (150, 151). The passenger domains of Sca2 mimic eukaryotic formins by elaborating ring-like structures to elongate actin, with intervening Pro-rich regions and WH2 domains incorporating profilin-actin for elongation and recruiting actin monomers for nucleation, respectively (149). Thus, at least for species carrying both RickA and Sca2 (most SFG rickettsiae), RickA-mediated Arp2/3 activation may play a greater role early in infection, possibly for inducing host cell filopodia formation during invasion (79, 151, 153, 154). Still, few Rickettsia species outside of SFG rickettsiae encode Sca2 proteins with intact formin-like passenger domains (Fig. 8B), and some of these species lack RickA genes as well (93, 116, 155) (Fig. 2 and 8A). This implies that host actin polymerization for motility is an expendable trait for most Rickettsia species, and that Arp2/3 recruitment and activation during invasion can be instigated by other bacteria-driven processes, i.e., Arf recruitment to the plasma membrane for inducing PI shifts required for filopodia formation (90).
MAG analyses indicate that, barring acquisitions via LGT, RickA and RalF were likely present before the diversification of major Rickettsia lineages, whereas Sca2 appeared later in Rickettsia evolution (Fig. 8A). Furthermore, we polled MAGs for the presence of genes encoding the four major autotransporters (Sca0, Sca1, Sca3, and Sca5) with known (or anticipated) functions in host cell binding and/or invasion (156–160). Remarkably, Sca3 is predominant in Tisiphia genomes despite a very limited distribution in Rickettsia species [restricted mostly to TG and TRG rickettsiae (91)]. Furthermore, BG rickettsiae are counter to most other rickettsiae in lacking both Sca0 and Sca5, the dominant proteins of the characterized Rickettsia S-layer (161). Collectively, these analyses show that Rickettsia factors described in host cell invasion and actin cytoskeleton subversion are sporadically encoded across genomes, indicating host specificity and/or expendability in their contribution to the intracellular lifestyle.
A repurposed or multi-purposed rvh T4SS?
Aside from secreting effectors that target host cellular processes, evidence is mounting for intracellular bacteria utilizing large contact-dependent growth inhibition (CDI) and recombination hotspot (Rhs) toxins for interbacterial antagonism (162, 163). We recently identified a few rickettsial genomes encoding specialized TA modules that some bacteria integrate into CDI and Rhs toxins to expand toxic activities (164–167). Widespread in bacteria, these CDI-like/Rhs-like C-terminal toxin and antidote (CRCT/A) modules are extremely polymorphic, variable at the species- and strain-levels, and found either associated with larger toxins or alone as small TA modules (168). The two types of Rickettsia CRCT/A (rCRCT/A) modules we identified, rCRCT/A-1 and rCRCT/A-3a, were once associated with large Rhs-like toxins that have mostly degraded (81). rCRCT/A-1 modules are highly divergent from other characterized CRCT/A modules and predominantly occur in Actinomycetia and Cyanobacteria genomes. Only two Rickettsia species, Rickettsia tamurae and Rickettsia buchneri, harbor rCRCT/A-1 modules; however, the “Cand. J. acanthamoeba” genome encodes one as an independent module and one integrated into a large modular hemagglutinin toxin with nuclease and peptidase domains. MAG analyses herein discovered several more rCRCT/A-1 modules mostly in Tisiphia genomes associated with pseudogenized hemagglutinin-like toxins (Fig. 1C and 2).
rCRCT/A-3a modules resemble the prototype CDI TA module (CdiA-CT/CdiI), wherein the nuclease CdiA-CT targets tRNAs in recipient cytosol (164). CdiA-CT/CdiI is associated with a large modular protein (CdiA) that joins with a second protein (CdiB) as a type Vb secretion system to deliver the toxin into neighboring bacteria (166, 168, 169). However, rCRCT/A-3a modules (and all Rickettsiales genomes) lack CdiB genes. This type of CRCT/A module is widespread in proteobacterial genomes (166). MAG analyses also revealed more rCRCT/A-3a modules in Rickettsia genomes and a much higher presence of single antidotes (Fig. 1C and 2), possibly indicating greater selection for defense against toxins versus toxin secretion. Additionally, a rCRCT/A-3a module was found integrated into a large hemagglutinin-like toxin in the Rickettsiales endosymbiont Ac37b, an early-branching Rickettsiaceae species that can co-infect ticks with SFG rickettsiae (Fig. 8B; Fig. S9). Remarkably, this species also carries an identical hemagglutinin-like toxin but with a divergent warhead of the HNH/ENDO VII nuclease superfamily, illustrating the integrative nature of diverse CRCT/A modules. Furthermore, two toxins in the genome of the Rickettsiales endosymbiont of Peranema trichophorum (Midichloriaceae) carry different C-terminal toxins, as well as lysin motifs (LysM) that often occur in cell wall-degrading enzymes (170). Collectively, our analyses illuminate diverse CRCT/A modules in the Rickettsiales mobilome that equip bacteria with weapons for interbacterial antagonism.
Rickettsiales species may also utilize filamentation induced by cAMP (FIC) proteins and type II TA modules for interbacterial antagonism. Some intracellular bacteria harbor FIC domain-containing proteins (163), and several human pathogens secrete effectors with FIC domains into host cells to subvert cellular processes (171). Furthermore, a recent report illustrated that Yersinia pseudotuberculosis utilizes an FIC domain effector, CccR, that alters conspecific gene expression and inhibits congener growth (172). Many Rickettsia genomes encode multiple divergent FIC proteins (Table S2); however, to our knowledge, none of these proteins are known to be secreted by rickettsiae. Similarly, Rickettsia species also harbor a myriad of diverse type II TA modules, with many found on RAGE (e.g., Fig. 7F; Fig. S7F) or plasmids (94, 173). Only one module, VapBC of R. felis, has been characterized. Structural analysis revealed the nature of antidote (VapB) binding to toxin (VapC) (174), and VapC possesses toxic RNase activity when expressed in bacterial or eukaryotic host cells. We previously showed that Rickettsia genomes encode VapBC and/or a divergent module (VapBC-d) (91), and MAG analysis confirmed this observation (Fig. 2). Furthermore, in light of the new genomic diversity, more discrete VapBC loci are encoded in many genomes (data not shown), as well as other type II TA modules (e.g., those encoding ParE, BrnT, and RatA toxins) that have yet to be characterized (Table S2).
Finally, MAG analyses doubled the number of Rickettsiaceae proteins harboring domains found in RP toxins, particularly those of Wolbachia cytoplasmic incompatibility-inducing nucleases (CinB) and deubiquitinases (CidB), as well as the Spiroplasma male killer toxin deubiquitinase (Spaid) (175–179) (Fig. S10). Many of these toxins are substantially large and modular, encoding numerous domains with uncharacterized effects on host cells (5, 180, 181) (e.g., see Fig. 3D). The increasing number of RP toxins (and antidotes when present) in rickettsiae, particularly in species associated with male-killing and parthenogenesis phenotypes, indicates undiscovered molecular mechanisms underpinning these modes of RP. Like the rCRCT/A modules, FIC toxins, and type II TA modules, these RP toxins all lack characterized secretion pathways.
While the rvh T4SS cannot be ruled out as a secretion pathway for any of these potential effectors, a T1SS conserved in all Rickettsiales (91) (and possibly other unappreciated routes) could also be involved. It is important to consider that all putative effectors, particularly those acquired by LGT, maybe in various stages of coevolving with novel secretory pathways and secretion systems. Thus, while there is strong selection for retaining rvh T4SS genes in rickettsial genomes, the presence of REMs and cREMs alone does not imply their secretion given the often-complex interactions between the T4SS machine components and translocated effectors that typically co-evolve to streamline recognition and secretion (182, 183).
Power and efficacy of MAG diversity
The inclusion of diverse MAGs in the assessment of rvh effector evolution has provided several key insights. First, like the rvh T4SS (42), many REMs and cREMs are often present as duplicate genes in rickettsial genomes. However, unlike the rvh machine, effector duplication seems to define basal lineages (Tisiphia, BG rickettsiae, and other Rickettsiaceae) and tends to lead to the retention of only one protein in the later-evolving Rickettsia groups. Still, divergent forms arising from duplication stand to inform on effector function, particularly if derived proteins are utilized for vertebrate cell infection.
Second, the sparse distribution of polled effectors outside of Rickettsia genomes indicates they originated after the divergence of rickettsial families. In some cases, analyses strongly implicate LGT for the acquisition of effectors, with a particular bias from Legionellales (e.g., RalF, patatins, and PIKs) and other aquatic microbes. This supports the “intracellular arena” hypothesis for the gain of similar effectors in divergent pathogens that occupy common hosts (i.e., protists and arthropods) (184). It also corroborates our earlier observations that LGT, particularly by RAGEs and plasmids, offsets reductive genome evolution in rickettsiae (26, 93). A more recent study reached a similar conclusion via the discovery of gene gain shaping Chlamydiae genome architecture, despite the reduced size of most chlamydial genomes (185). MAGs have also provided a greater appreciation for Legionellales diversity and revealed that the major host-adaptive features (i.e., the dot/icm T4SS and a few conserved effectors) were established in the last common Legionellales ancestor (186). This is consistent with the discovery by Schön et al. (28) of the rvh T4SS in ancestral Rickettsiales; however, it is premature to conclude that strict rvh repurposing from congener killing to facilitation of host parasitism occurred until the secretory pathways of the numerous effectors described above are experimentally determined.
Third, MAGs help bridge the gap between research on microbial ecology and human pathogeEnesis, revealing genome evolutionary and architectural traits that are underappreciated due to biases of clinical isolates or more common environmental strains on public databases (72, 73). Our discovery here of REMs and cREMS on novel plasmids and RAGEs accentuates this point, indicating that such genetic elements may be underestimated for roles in rickettsial biology due to the strong bias of high passage clinical isolates on databases. This is particularly relevant considering the recent demonstration that the Rickettsia regulator of actin motility (roaM) is often pseudogenized in highly passaged laboratory strains, suggesting serial passage in cell culture can eliminate essential genes lacking environmental selective pressure (in this case, the arthropod cytosol) (187).
Fourth, the most profound insight gained from our work shows how MAG analyses often illuminate novel architectures for well-studied virulence factors. Unearthing new effector designs provides clues on how general foundations are tailored to different hosts and host cell processes. This is epitomized by our discovery of novel RalF-like proteins with SCDs substituted for ARF-interacting domains, which not only fortifies the literature on Legionella and Rickettsia RalF-mediated host ARF recruitment (56, 84, 87, 89, 90) but also pinpoints the rise of the actin-targeting VPR regions in Rickettsia RalF proteins after the divergence from Tisiphia species. Combined with numerous other novel effector architectures identified herein, this highlights a remarkable recapitulation of mechanisms for mimicking eukaryotic functions that exist beyond Rickettsia and other human pathogens and are widespread in the environment. We assert that widening the comparative genomics lens will allow evolution, which has already matched effector form and function to host environments, to guide experimental designs and reinvigorate pathogen effector research.
Finally, as the landscape of Rickettsia pathogenesis undergoes gradual change due to virulence factor characterization and immunological studies (1, 2), the traditional designation of SFG and TG rickettsiae as the major lineages defining the genus has become grossly outdated. A substantial spike in TRG rickettsiae diversity (73, 188, 189), coupled with robust genome-based phylogeny estimations and phylogenomic analyses (28, 73), make the common ancestry of TG and TRG rickettsiae incontrovertible. Prior bias in genome sequences for SFG rickettsiae portrayed TG rickettsiae as unique by smaller genome size and greater pseudogenization relative to all other rickettsiae. However, our focus on rvh effectors across a highly diverse and unbiased genomic sampling shows that all the major Rickettsia groups (BG, TRG, TG, TIG, and SFG rickettsiae) have distinct evolutionary trajectories of gene gain, loss, and modification (Fig. 2). Thus, MAGs have exposed far greater Rickettsia diversity than previously realized, though long ago conjectured by environmental sampling of rickettsiae infecting non-blood-feeding eukaryotes (190). These data, as well as careful dissection of the attributes distinguishing Tisiphia and Rickettsia species, will be paramount for deciphering how human pathogens have emerged, possibly multiple times, from this veritable bevy of endosymbiont diversity. Furthermore, an understanding of environmental genomic richness, particularly in mobile element diversity, may help forecast the next serious rickettsial diseases to emerge.
Conclusion
Discovery and analyses of MAGs have greatly impacted the landscape of Rickettsiology, adding substantial diversity and dispelling the long-held dogma for an ancestral link to the mitochondrial ancestor. Despite predicted extracellular lifestyles, basal rickettsial species carry the rvh T4SS and likely use it as a congener killing machine. Our study coupled a robust evolutionary framework with the inspection of over two dozen known or predicted Rickettsia rvh T4SS effectors to provide insight on the origin of mechanisms for host cell subversion and obligate intracellular parasitism. Though focused on taxonomic scope, this experimental design is amenable to probing the origins of virulence factors in any human pathogen with representation in the diverse treasure trove of MAG data. At the bare minimum, our work demonstrates that utilizing MAGs in comparative approaches greatly enlightens dialogue on mechanisms of pathogenesis.
MATERIALS AND METHODS
Rickettsiales phylogeny estimation
Robust genome-based phylogeny estimations for Rickettsiales (28) and Rickettsia-Tisiphia (73) were used as benchmarks to evaluate our estimated phylogenies based on single or concatenated rvh proteins. We polled the rich MAG diversity on the NCBI database for the presence of vir-like T4SS genes possessing rvh hallmarks (38, 42, 45) (i.e., RvhB8, RvhB9, and RvhB4 duplication, multicopy RvhB6, no VirB5 analog; Fig. S1). Provided that many MAGs and certain genome assemblies are likely incomplete, we limited our data set to assemblies containing both RvhB4-I and RvhB-II, except for a few cases where strong evidence from other rvh genes indicated a Rickettsiales assembly. A total of 153 genome assemblies were retained for further analyses: (i) 93 Rickettsiaceae genome assemblies [including the 28 MAGs from Davison et al. (73) and another 15 previously unanalyzed MAGs], (ii) 14 and 9 genome assemblies from Anaplasmataceae and Midichloriaceae, respectively, (iii) the “Candidatus Deianiraea vastatrix” (Deianiraeaceae) genome assembly, and (iv) 33 environmental MAGs likely comprising Deianiraeaceae, Athabascaceae, or Mitibacteraceae [nine previously analyzed by Schön et al. (28)] (Table S1; Fig. S2).
Only RvhB4-I and RvhB4-II proteins were included in phylogeny estimation, as alignments of other Rvh proteins were extraordinarily variable across the selected taxa (data not shown). RvhB4-I and RvhB4-II proteins were separately aligned using MUSCLE (default parameters). Each alignment included Agrobacterium tumefaciens str. F4 VirB4, which was used as an outgroup to root estimated trees. RvhB4-I and RvhB4-II protein alignments were subsequently concatenated (1,974 total positions, “unmasked alignment”). TRIMAL (191) was used to create a second alignment with less conserved positions masked (1,613 total positions, “masked alignment”).
For both unmasked and masked alignments, a maximum likelihood-based phylogeny was estimated with PhyML (192), using the Smart Model Selection (193) tool to determine the best substitution matrix and model for rates across aa sites [LG (G + I + F) for both alignments]. Branch support was assessed with 1,000 pseudo-replications. Trees were drawn using FigTree (https://github.com/rambaut/figtree/) and manually modified using Adobe Illustrator. Final trees were manually adjusted to place “Candidatus Sneabacter namystus” (which lacks the rvh T4SS) in a position on the phylogram suggested by prior phylogeny estimation (82, 83). All terminal taxa were assigned names based on NCBI database taxonomy (as of 26 February 2023), with some “short names” taken from Davison et al. (73) (these are provided in black boxes throughout the figures). Rickettsial classification scheme (Scrub Typhus Group, Bellii Group, Transitional Group, Typhus Group, Tamurae-Ixodes Group, and Spotted Fever Group) follows our prior reports (81, 173).
Phylogenomics analysis
The RvhB4-based estimated phylogeny was used as a scaffold to complete a distribution matrix for REMs and cREMs. It was not our goal to assess the relative completeness of each MAG included in the matrix, but to only assess if MAGs and other genome assemblies possessing a rvh T4SSs also include counterparts (homologs or analogs) to Rickettsia REMs and cREMs. REM/cREM assignment is based on prior studies implicating their secretion and/or interaction with RvhD4 (by bacterial two-hybrid and/or coimmunoprecipitation assays) or the presence of motifs known to target either congener bacteria or eukaryotic molecules (56–58, 74–81). Analyses of some REMs and cREMs illuminated more complex gene structures (duplications, gene streamlining, and gene fusions) that prompted expansion of the total effector data set. A total of 26 proteins were analyzed within the final phylogenomic framework (Table S2).
In silico protein characterization
Analyses of each REM and cREM data set contained discrete workflows tailored to the level of effector conservation in Rickettsiales (and in some cases, other bacteria), prior studies that included bioinformatics analyses, and identification of gene duplication, streamlining, or gene fusion. All individual workflows are described in the pertinent figure legends and/or supplemental figure legends. Only general bioinformatics analyses are described below.
Data set compilation
Rickettsia REMs and cREMs were used as queries in Blastp searches to compile and analyze diverse proteins harboring significant similarity across the entire lengths of the queries. Analyses utilized our HaloBlast method, which is a combinatorial Blastp-based approach originally designed to interrogate proteins for LGT (26). HaloBlasting compiles Blastp subjects from restricted taxonomic searches that theoretically decrease in similarity by sampling lower levels of bacterial classification. A general search strategy for rickettsiae entails individual Blastp searches against six distinct taxonomic databases: (i) “Rickettsia” (NCBI taxid: 780)”; (ii) “Rickettsiales” (taxid: 766) excluding “Rickettsia”; (iii) “Alphaproteobacteria” (taxid: 28211) excluding “Rickettsiales”; (iv) “Proteobacteria” (taxid: 1224) excluding “Alphaproteobacteria”; (v) “Bacteria” (taxid: 2) excluding Proteobacteria”; and (vi) “minus bacteria.” Data subsets were constructed strictly using the NCBI taxid and following the NCBI taxid hierarchy to identify “daughter” taxonomic groups. Typically, 500 subjects (if available) are retained per search. All subjects from each search were separately ranked by Sm score (= b × I × Q, where b is the bit score of the match, I is the percent identity, and Q is the percent length of the query that aligned), a comparative sequence similarity score designed to de-emphasize highly significant matches to short stretches of the query in favor of longer stretches of similarity (26). The “halos” or separate database searches are then compared to one another to determine the taxon with the strongest similarity to the query sequences. These analyses usually make LGT apparent when divergent data sets contain top-ranking proteins more similar to the Rickettsia queries than more closely related data sets.
Protein characterization
Select proteins or domains (again, context-dependent) are typically compiled and aligned with MUSCLE using default parameters (104). To identify conserved regions, alignments are then visualized as sequence logos using WebLogo (103). Domain analyses are performed by cross-checking predictions from the NCBI Conserved Domains Database and EMBL’s Simple Modular Architecture Research Tool (194). In some cases, proteins were evaluated for N-terminal signal peptides (195) and transmembrane-spanning regions (196). Alignments shown in the figures and supplemental figures are manually assessed for conservation, typically considering 80% of a position conserved (alignment size-dependent), with amino acid coloring scheme and assignment as follows: black, hydrophobic (Ala, Val, Iso, Leu, Pro, Met, and Gly); gray, less hydrophobic (can include a minority of Try, Phe, and Tyr); red, negatively charged (Glu and Asp); green, hydrophilic (Cys, Asn, Gln, Ser, and Thr); purple, aromatic (Try, Phe, and Tyr); and blue, positively charged (His, Lys, and Arg). Individual protein schemas were generated using Illustrator of Biological Sequences (197) with manual adjustment in Adobe Illustrator.
Protein structures were predicted using the Protein Homology/analogY Recognition Engine V 2.0 (Phyre2) (95); in some cases, published structures were retrieved from the Protein Data Bank (198) and used in one-to-one threading mode with Phyre2. For some effectors, we also evaluated structures generated with Alphafold (127, 128). Finally, some regions of proteins were analyzed for predicted secondary structure using JPred (199).
Phylogenies were estimated for some REM and cREM data sets, which were compiled uniquely for each case and utilized HaloBlast to obtain non-rickettsial taxa (if available). Alignments were not masked since masking eliminated too many informative positions. Maximum likelihood-based phylogenies were estimated with PhyML (192), using the Smart Model Selection (193) tool to determine the best substitution matrix and model for rates across aa sites. Branch support was assessed with 1,000 pseudo-replications. Trees were visualized and drawn as described above.
ACKNOWLEDGMENTS
This work was supported with funds from the National Institute of Health/National Institute of Allergy and Infectious Diseases grant R21AI156762 and R21AI166832. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Joseph J. Gillespie, Email: Jgillespie@som.umaryland.edu.
Vaughn S. Cooper, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.00759-23.
Supplemental figures.
Additional supplemental figures.
RvhB4 sequences used for phylogeny estimation.
Supporting information for REMs and cREMs.
Supporting information for type VI secretion system analyses.
Supporting information for patatin analyses.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Salje J. 2021. Cells within cells: rickettsiales and the obligate intracellular bacterial lifestyle. Nat Rev Microbiol 19:375–390. doi: 10.1038/s41579-020-00507-2 [DOI] [PubMed] [Google Scholar]
- 2. Sahni A, Fang R, Sahni SK, Walker DH. 2019. Pathogenesis of rickettsial diseases: pathogenic and immune mechanisms of an endotheliotropic infection. Annu Rev Pathol Mech Dis 14:127–152. doi: 10.1146/annurev-pathmechdis-012418-012800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Narra HP, Sahni A, Walker DH, Sahni SK. 2020. Recent research milestones in the pathogenesis of human rickettsioses and opportunities ahead. Future Microbiol 15:753–765. doi: 10.2217/fmb-2019-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751. doi: 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- 5. Gillespie JJ, Driscoll TP, Verhoeve VI, Rahman MS, Macaluso KR, Azad AF. 2018. A tangled web: origins of reproductive parasitism. Genome Biol Evol 10:2292–2309. doi: 10.1093/gbe/evy159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gillespie JJ, Nordberg EK, Azad AA, Sobral BW. 2012. Phylogeny and comparative genomics: the shifting landscape in the genomics era, p 84–141. In Azad AF, Palmer GH (ed), Intracellular pathogens II: Rickettsiales. American Society of Microbiology, Boston. [Google Scholar]
- 7. Kaur R, Shropshire JD, Cross KL, Leigh B, Mansueto AJ, Stewart V, Bordenstein SR, Bordenstein SR. 2021. Living in the endosymbiotic world of Wolbachia: a centennial review. Cell Host Microbe 29:879–893. doi: 10.1016/j.chom.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mariconti M, Epis S, Gaibani P, Dalla Valle C, Sassera D, Tomao P, Fabbi M, Castelli F, Marone P, Sambri V, Bazzocchi C, Bandi C. 2012. Humans parasitized by the hard tick Ixodes ricinus are seropositive to Midichloria mitochondrii: is Midichloria a novel pathogen, or just a marker of tick bite? Pathog Glob Health 106:391–396. doi: 10.1179/2047773212Y.0000000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szokoli F, Castelli M, Sabaneyeva E, Schrallhammer M, Krenek S, Doak TG, Berendonk TU, Petroni G. 2016. Disentangling the taxonomy of Rickettsiales and description of two novel symbionts (“Candidatus Bealeia paramacronuclearis” and “Candidatus Fokinia cryptica”) sharing the cytoplasm of the ciliate protist Paramecium biaurelia. Appl Environ Microbiol 82:7236–7247. doi: 10.1128/AEM.02284-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Floriano AM, Castelli M, Krenek S, Berendonk TU, Bazzocchi C, Petroni G, Sassera D. 2018. The genome sequence of “Candidatus Fokinia solitaria”: insights on reductive evolution in Rickettsiales. Genome Biol Evol 10:1120–1126. doi: 10.1093/gbe/evy072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sassera D, Beninati T, Bandi C, Bouman EAP, Sacchi L, Fabbi M, Lo N. 2006. “Candidatus Midichloria mitochondrii”, an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. Int J Syst Evol Microbiol 56:2535–2540. doi: 10.1099/ijs.0.64386-0 [DOI] [PubMed] [Google Scholar]
- 12. Montagna M, Sassera D, Epis S, Bazzocchi C, Vannini C, Lo N, Sacchi L, Fukatsu T, Petroni G, Bandi C. 2013. “Candidatus midichloriaceae” fam. nov. (Rickettsiales), an ecologically: widespread clade of intracellular alphaproteobacteria. Appl Environ Microbiol 79:3241–3248. doi: 10.1128/AEM.03971-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giannotti D, Boscaro V, Husnik F, Vannini C, Keeling PJ. 2022. The “other” Rickettsiales: an overview of the family “Candidatus Midichloriaceae” Appl Environ Microbiol 88. doi: 10.1128/aem.02432-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klinges JG, Rosales SM, McMinds R, Shaver EC, Shantz AA, Peters EC, Eitel M, Wörheide G, Sharp KH, Burkepile DE, Silliman BR, Vega Thurber RL. 2019. Phylogenetic, genomic, and biogeographic characterization of a novel and ubiquitous marine invertebrate-associated Rickettsiales parasite, Candidatus Aquarickettsia rohweri, gen. nov., sp. nov. ISME J 13:2938–2953. doi: 10.1038/s41396-019-0482-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castelli M, Sabaneyeva E, Lanzoni O, Lebedeva N, Floriano AM, Gaiarsa S, Benken K, Modeo L, Bandi C, Potekhin A, Sassera D, Petroni G. 2019. Deianiraea, an extracellular bacterium associated with the ciliate Paramecium, suggests an alternative scenario for the evolution of Rickettsiales. ISME J 13:2280–2294. doi: 10.1038/s41396-019-0433-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viklund J, Ettema TJG, Andersson SGE. 2012. Independent genome reduction and phylogenetic reclassification of the oceanic SAR11 clade. Mol Biol Evol 29:599–615. doi: 10.1093/molbev/msr203 [DOI] [PubMed] [Google Scholar]
- 17. Emelyanov VV, Vyssokikh MY. 2006. On the nature of obligate intracellular symbiosis of rickettsiae--Rickettsia prowazekii cells import mitochondrial porin. Biochemistry (Mosc) 71:730–735. doi: 10.1134/s0006297906070054 [DOI] [PubMed] [Google Scholar]
- 18. Andersson SGE, Zomorodipour A, Andersson JO, Sicheritz-Pontén T, Alsmark UCM, Podowski RM, Näslund AK, Eriksson A-S, Winkler HH, Kurland CG. 1998. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396:133–140. doi: 10.1038/24094 [DOI] [PubMed] [Google Scholar]
- 19. Andersson SG. 1998. Bioenergetics of the obligate intracellular parasite Rickettsia prowazekii. Biochim Biophys Acta 1365:105–111. doi: 10.1016/s0005-2728(98)00050-4 [DOI] [PubMed] [Google Scholar]
- 20. Andersson SG, Kurland CG. 1998. Ancient and recent horizontal transfer events: the origins of mitochondria. APMIS Suppl 84:5–14. doi: 10.1111/j.1600-0463.1998.tb05641.x [DOI] [PubMed] [Google Scholar]
- 21. Andersson JO, Andersson SG. 1999. Insights into the evolutionary process of genome degradation. Curr Opin Genet Dev 9:664–671. doi: 10.1016/s0959-437x(99)00024-6 [DOI] [PubMed] [Google Scholar]
- 22. Fuxelius H-H, Darby A, Min C-K, Cho N-H, Andersson SGE. 2007. The genomic and metabolic diversity of Rickettsia. Res Microbiol 158:745–753. doi: 10.1016/j.resmic.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 23. Andersson JO, Andersson SG. 1999. Genome degradation is an ongoing process in Rickettsia. Mol Biol Evol 16:1178–1191. doi: 10.1093/oxfordjournals.molbev.a026208 [DOI] [PubMed] [Google Scholar]
- 24. Darby AC, Cho N-H, Fuxelius H-H, Westberg J, Andersson SGE. 2007. Intracellular pathogens go extreme: genome evolution in the Rickettsiales. Trends Genet 23:511–520. doi: 10.1016/j.tig.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 25. Fuxelius H-H, Darby AC, Cho N-H, Andersson SGE. 2008. Visualization of pseudogenes in intracellular bacteria reveals the different tracks to gene destruction. Genome Biol 9:1–15. doi: 10.1186/gb-2008-9-2-r42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Driscoll TP, Gillespie JJ, Nordberg EK, Azad AF, Sobral BW. 2013. Bacterial DNA sifted from the trichoplax adhaerens (Animalia: placozoa) genome project reveals a putative rickettsial endosymbiont. Genome Biol Evol 5:621–645. doi: 10.1093/gbe/evt036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martijn J, Vosseberg J, Guy L, Offre P, Ettema TJG. 2018. Deep mitochondrial origin outside the sampled alphaproteobacteria. Nature 557:101–105. doi: 10.1038/s41586-018-0059-5 [DOI] [PubMed] [Google Scholar]
- 28. Schön ME, Martijn J, Vosseberg J, Köstlbacher S, Ettema TJG. 2022. The evolutionary origin of host association in the Rickettsiales. Nat Microbiol 7:1189–1199. doi: 10.1038/s41564-022-01169-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martijn J, Schulz F, Zaremba-Niedzwiedzka K, Viklund J, Stepanauskas R, Andersson SGE, Horn M, Guy L, Ettema TJG. 2015. Single-cell genomics of a rare environmental alphaproteobacterium provides unique insights into rickettsiaceae evolution. ISME J 9:2373–2385. doi: 10.1038/ismej.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schulz F, Martijn J, Wascher F, Lagkouvardos I, Kostanjšek R, Ettema TJG, Horn M. 2016. A Rickettsiales symbiont of amoebae with ancient features. Environ Microbiol 18:2326–2342. doi: 10.1111/1462-2920.12881 [DOI] [PubMed] [Google Scholar]
- 31. Lanzoni O, Sabaneyeva E, Modeo L, Castelli M, Lebedeva N, Verni F, Schrallhammer M, Potekhin A, Petroni G. 2019. Diversity and environmental distribution of the cosmopolitan endosymbiont “Candidatus Megaira" Sci Rep 9:1179. doi: 10.1038/s41598-018-37629-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sassera D, Lo N, Epis S, D’Auria G, Montagna M, Comandatore F, Horner D, Peretó J, Luciano AM, Franciosi F, Ferri E, Crotti E, Bazzocchi C, Daffonchio D, Sacchi L, Moya A, Latorre A, Bandi C. 2011. Phylogenomic evidence for the presence of a flagellum and cbb(3) oxidase in the free-living mitochondrial ancestor. Mol Biol Evol 28:3285–3296. doi: 10.1093/molbev/msr159 [DOI] [PubMed] [Google Scholar]
- 33. Kang YJ, Diao XN, Zhao GY, Chen MH, Xiong Y, Shi M, Fu WM, Guo YJ, Pan B, Chen XP, Holmes EC, Gillespie JJ, Dumler SJ, Zhang YZ. 2014. Extensive diversity of Rickettsiales bacteria in two species of ticks from China and the evolution of the Rickettsiales. BMC Evol Biol 14:167. doi: 10.1186/s12862-014-0167-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verhoeve VI, Gillespie JJ. 2022. Origin of rickettsial host dependency unravelled. Nat Microbiol 7:1110–1111. doi: 10.1038/s41564-022-01187-9 [DOI] [PubMed] [Google Scholar]
- 35. Wang S, Luo H. 2021. Dating Alphaproteobacteria evolution with eukaryotic fossils. Nat Commun 12:1–9. doi: 10.1038/s41467-021-23645-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ettema TJG, Andersson SGE. 2009. The α-proteobacteria: the Darwin finches of the bacterial world. Biol Lett 5:429–432. doi: 10.1098/rsbl.2008.0793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rikihisa Y, Lin M, Niu H, Cheng Z. 2009. Type IV secretion system of Anaplasma phagocytophilum and Ehrlichia chaffeensis. Ann N Y Acad Sci 1166:106–111. doi: 10.1111/j.1749-6632.2009.04527.x [DOI] [PubMed] [Google Scholar]
- 38. Gillespie JJ, Brayton KA, Williams KP, Diaz MAQ, Brown WC, Azad AF, Sobral BW. 2010. Phylogenomics reveals a diverse Rickettsiales type IV secretion system. Infect Immun 78:1809–1823. doi: 10.1128/IAI.01384-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rancès E, Voronin D, Tran-Van V, Mavingui P. 2008. Genetic and functional characterization of the type IV secretion system in Wolbachia. J Bacteriol 190:5020–5030. doi: 10.1128/JB.00377-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rikihisa Y. 2017. Role and function of the type IV secretion system in Anaplasma and Ehrlichia species, p. 297–321. Current topics in microbiology and immunology. [DOI] [PubMed] [Google Scholar]
- 41. Rikihisa Y, Lin M. 2010. Anaplasma phagocytophilum and Ehrlichia chaffeensis type IV secretion and Ank proteins. Curr Opin Microbiol 13:59–66. doi: 10.1016/j.mib.2009.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gillespie J.J, Ammerman NC, Dreher-Lesnick SM, Rahman MS, Worley MJ, Setubal JC, Sobral BS, Azad AF. 2009. An anomalous type IV secretion system in Rickettsia is evolutionarily conserved. PLoS ONE 4:e4833. doi: 10.1371/journal.pone.0004833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sutten EL, Norimine J, Beare PA, Heinzen RA, Lopez JE, Morse K, Brayton KA, Gillespie JJ, Brown WC. 2010. Anaplasma marginale type IV secretion system proteins VirB2, VirB7, VirB11, and Vird4 are immunogenic components of a protective bacterial membrane vaccine. Infect Immun 78:1314–1325. doi: 10.1128/IAI.01207-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gillespie JJ, Phan IQH, Scheib H, Subramanian S, Edwards TE, Lehman SS, Piitulainen H, Rahman MS, Rennoll-Bankert KE, Staker BL, Taira S, Stacy R, Myler PJ, Azad AF, Pulliainen AT. 2015. Structural insight into how bacteria prevent interference between multiple divergent type IV secretion systems. mBio 6:e01867-15. doi: 10.1128/mBio.01867-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gillespie J.J, Phan IQH, Driscoll TP, Guillotte ML, Lehman SS, Rennoll-Bankert KE, Subramanian S, Beier-Sexton M, Myler PJ, Rahman MS, Azad AF. 2016. The Rickettsia type IV secretion system: unrealized complexity mired by gene family expansion. Pathog Dis 74:ftw058. doi: 10.1093/femspd/ftw058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rikihisa Y. 2021. The “biological weapons” of Ehrlichia chaffeensis: novel molecules and mechanisms to subjugate host cells. Front Cell Infect Microbiol 11:830180. doi: 10.3389/fcimb.2021.830180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yan Q, Zhang W, Lin M, Teymournejad O, Budachetri K, Lakritz J, Rikihisa Y. 2021. Iron robbery by intracellular pathogen via bacterial effector–induced ferritinophagy. Proc Natl Acad Sci USA 118:e2026598118. doi: 10.1073/pnas.2026598118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yan Q, Lin M, Huang W, Teymournejad O, Johnson JM, Hays FA, Liang Z, Li G, Rikihisa Y. 2018. Ehrlichia type IV secretion system effector Etf-2 binds to active RAB5 and delays endosome maturation. Proc Natl Acad Sci USA 115:E8977–E8986. doi: 10.1073/pnas.1806904115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu H, Bao W, Lin M, Niu H, Rikihisa Y. 2012. Ehrlichia type IV secretion effector ECH0825 is translocated to mitochondria and curbs ROS and apoptosis by upregulating host MnSOD. Cell Microbiol 14:1037–1050. doi: 10.1111/j.1462-5822.2012.01775.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim Y, Wang J, Clemens EG, Grab DJ, Dumler JS. 2022. Anaplasma phagocytophilum ankyrin A protein (AnkA) enters the nucleus using an importin-β-, RanGTP-dependent mechanism. Front Cell Infect Microbiol 12:828605. doi: 10.3389/fcimb.2022.828605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lockwood S, Voth DE, Brayton KA, Beare PA, Brown WC, Heinzen RA, Broschat SL. 2011. Identification of Anaplasma marginale type IV secretion system effector proteins. PLoS One 6:e27724. doi: 10.1371/journal.pone.0027724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu J, He M, Xu W, Li Y, Huang R, Wu S, Niu H. 2019. Development of TEM-1 β-Lactamase based protein translocation assay for identification of Anaplasma phagocytophilum type IV secretion system effector proteins. Sci Rep 9. doi: 10.1038/s41598-019-40682-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lin M, den Dulk-Ras A, Hooykaas PJJ, Rikihisa Y. 2007. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell Microbiol 9:2644–2657. doi: 10.1111/j.1462-5822.2007.00985.x [DOI] [PubMed] [Google Scholar]
- 54. Niu H, Kozjak-Pavlovic V, Rudel T, Rikihisa Y. 2010. Anaplasma phagocytophilum Ats-1 is imported into host cell mitochondria and interferes with apoptosis induction. PLoS Pathog 6:e1000774. doi: 10.1371/journal.ppat.1000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Park JM, Genera BM, Fahy D, Swallow KT, Nelson CM, Oliver JD, Shaw DK, Munderloh UG, Brayton KA. 2023. An Anaplasma phagocytophilum T4SS effector, AteA, is essential for tick infection. bioRxiv:2023.02.06.527355. doi: 10.1101/2023.02.06.527355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rennoll-Bankert KE, Rahman MS, Gillespie JJ, Guillotte ML, Kaur SJ, Lehman SS, Beier-Sexton M, Azad AF. 2015. Which way in? The ralf ARF-GEF orchestrates Rickettsia host cell invasion. PLoS Pathog 11:e1005115. doi: 10.1371/journal.ppat.1005115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Voss OH, Gillespie JJ, Lehman SS, Rennoll SA, Beier-Sexton M, Rahman MS, Azad AF. 2020. Risk1, a phosphatidylinositol 3-kinase effector, promotes Rickettsia typhi intracellular survival. mBio 11:1–22. doi: 10.1128/mBio.00820-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lehman SS, Noriea NF, Aistleitner K, Clark TR, Dooley CA, Nair V, Kaur SJ, Rahman MS, Gillespie JJ, Azad AF, Hackstadt T. 2018. The rickettsial ankyrin repeat protein 2 is a type IV secreted effector that associates with the endoplasmic reticulum. mBio 9:e00975-18. doi: 10.1128/mBio.00975-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Souza DP, Oka GU, Alvarez-Martinez CE, Bisson-Filho AW, Dunger G, Hobeika L, Cavalcante NS, Alegria MC, Barbosa LRS, Salinas RK, Guzzo CR, Farah CS. 2015. Bacterial killing via a type IV secretion system. Nat Commun 6:6453. doi: 10.1038/ncomms7453 [DOI] [PubMed] [Google Scholar]
- 60. Ho BT, Dong TG, Mekalanos JJ. 2014. A view to a kill: the bacterial type 6 secretion system. Cell Host Microbe 15:9–21. doi: 10.1016/j.chom.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Muñoz-Gómez SA, Hess S, Burger G, Lang BF, Susko E, Slamovits CH, Roger AJ. 2019. An updated phylogeny of the alphaproteobacteria reveals that the parasitic rickettsiales and holosporales have independent origins. eLife 8:eLife. doi: 10.7554/eLife.42535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yurchenko T, Ševčíková T, Přibyl P, El Karkouri K, Klimeš V, Amaral R, Zbránková V, Kim E, Raoult D, Santos LMA, Eliáš M. 2018. A gene transfer event suggests a long-term partnership between eustigmatophyte algae and a novel lineage of endosymbiotic bacteria. ISME J 12:2163–2175. doi: 10.1038/s41396-018-0177-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hunter ES, Paight C, Lane CE. 2020. Metabolic contributions of an alphaproteobacterial endosymbiont in the apicomplexan Cardiosporidium cionae. Front Microbiol 11:580719. doi: 10.3389/fmicb.2020.580719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tashyreva D, Prokopchuk G, Votýpka J, Yabuki A, Horák A, Lukeš J. 2018. Life cycle, ultrastructure, and phylogeny of new diplonemids and their endosymbiotic bacteria. mBio 9:e02447-17. doi: 10.1128/mBio.02447-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Prokopchuk G, Tashyreva D, Yabuki A, Horák A, Masařová P, Lukeš J. 2019. Morphological, ultrastructural, motility and evolutionary characterization of two new Hemistasiidae species. Protist 170:259–282. doi: 10.1016/j.protis.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 66. Castelli M, Lanzoni O, Nardi T, Lometto S, Modeo L, Potekhin A, Sassera D, Petroni G. 2021. ‘Candidatus Sarmatiella mevalonica’ endosymbiont of the ciliate paramecium provides insights on evolutionary plasticity among rickettsiales. Environ Microbiol 23:1684–1701. doi: 10.1111/1462-2920.15396 [DOI] [PubMed] [Google Scholar]
- 67. Schrallhammer M, Ferrantini F, Vannini C, Galati S, Schweikert M, Görtz H-D, Verni F, Petroni G. 2013. “Candidatus Megaira polyxenophila” gen. nov., sp. nov.: considerations on evolutionary history, host range and shift of early divergent rickettsiae. PLoS One 8:e72581. doi: 10.1371/journal.pone.0072581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Park E, Poulin R. 2020. Widespread torix Rickettsia in New Zealand amphipods and the use of blocking primers to rescue host COI sequences. Sci Rep 10:1–13. doi: 10.1038/s41598-020-73986-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pilgrim J, Ander M, Garros C, Baylis M, Hurst GDD, Siozios S. 2017. Torix group Rickettsia are widespread in Culicoides biting midges (Diptera: Ceratopogonidae), reach high frequency and carry unique genomic features. Environ Microbiol 19:4238–4255. doi: 10.1111/1462-2920.13887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thongprem P, Davison HR, Thompson DJ, Lorenzo-Carballa MO, Hurst GDD. 2021. Incidence and diversity of torix Rickettsia–Odonata symbioses. Microb Ecol 81:203–212. doi: 10.1007/s00248-020-01568-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang H-L, Lei T, Wang X-W, Maruthi MN, Zhu D-T, Cameron SL, Rao Q, Shan H-W, Colvin J, Liu Y-Q, Liu S-S. 2020. A newly recorded Rickettsia of the Torix group is a recent intruder and an endosymbiont in the whitefly Bemisia tabaci. Environ Microbiol 22:1207–1221. doi: 10.1111/1462-2920.14927 [DOI] [PubMed] [Google Scholar]
- 72. Pilgrim J, Thongprem P, Davison HR, Siozios S, Baylis M, Zakharov EV, Ratnasingham S, deWaard JR, Macadam CR, Smith MA, Hurst GDD. 2021. Torix Rickettsia are widespread in arthropods and reflect a neglected symbiosis. Gigascience 10:giab021. doi: 10.1093/gigascience/giab021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Davison HR, Pilgrim J, Wybouw N, Parker J, Pirro S, Hunter-Barnett S, Campbell PM, Blow F, Darby AC, Hurst GDD, Siozios S. 2022. Genomic diversity across the Rickettsia and ‘Candidatus Megaira’ genera and proposal of genus status for the torix group. Nat Commun 13:2630. doi: 10.1038/s41467-022-30385-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rahman MS, Gillespie JJ, Kaur SJ, Sears KT, Ceraul SM, Beier-Sexton M, Azad AF. 2013. Rickettsia typhi possesses phospholipase A2 enzymes that are involved in infection of host cells. PLoS Pathog 9:e1003399. doi: 10.1371/journal.ppat.1003399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rahman MS, Ammerman NC, Sears KT, Ceraul SM, Azad AF. 2010. Functional characterization of a phospholipase A2 homolog from Rickettsia typhi. J Bacteriol 192:3294–3303. doi: 10.1128/JB.00155-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Park H, Lee JH, Gouin E, Cossart P, Izard T. 2011. The Rickettsia surface cell antigen 4 applies mimicry to bind to and activate vinculin. J Biol Chem 286:35096–35103. doi: 10.1074/jbc.M111.263855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sears KT, Ceraul SM, Gillespie JJ, Allen ED, Popov VL, Ammerman NC, Rahman MS, Azad AF. 2012. Surface proteome analysis and characterization of surface cell antigen (Sca) or autotransporter family of Rickettsia typhi. PLoS Pathog 8:e1002856. doi: 10.1371/journal.ppat.1002856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gouin E, Egile C, Dehoux P, Villiers V, Adams J, Gertler F, Li R, Cossart P. 2004. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature 427:457–461. doi: 10.1038/nature02318 [DOI] [PubMed] [Google Scholar]
- 79. Reed SCO, Lamason RL, Risca VI, Abernathy E, Welch MD. 2014. Rickettsia actin-based motility occurs in distinct phases mediated by different actin nucleators. Curr Biol 24:98–103. doi: 10.1016/j.cub.2013.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Audoly G, Vincentelli R, Edouard S, Georgiades K, Mediannikov O, Gimenez G, Socolovschi C, Mège JL, Cambillau C, Raoult D. 2011. Effect of rickettsial toxin VapC on its eukaryotic host. PLoS One 6:e26528. doi: 10.1371/journal.pone.0026528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Verhoeve VI, Fauntleroy TD, Risteen RG, Driscoll TP, Gillespie JJ. 2022. Cryptic genes for interbacterial antagonism distinguish Rickettsia species infecting blacklegged ticks from other Rickettsia pathogens. Front cell infect Microbiol 12:880813. doi: 10.3389/fcimb.2022.880813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. George EE, Husnik F, Tashyreva D, Prokopchuk G, Horák A, Kwong WK, Lukeš J, Keeling PJ. 2020. Highly reduced genomes of protist endosymbionts show evolutionary convergence. Curr Biol 30:925–933. doi: 10.1016/j.cub.2019.12.070 [DOI] [PubMed] [Google Scholar]
- 83. George EE, Tashyreva D, Kwong WK, Okamoto N, Horák A, Husnik F, Lukeš J, Keeling PJ. 2022. Gene transfer agents in bacterial endosymbionts of microbial eukaryotes. Genome Biol Evol 14:evac099. doi: 10.1093/gbe/evac099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295:679–682. doi: 10.1126/science.1067025 [DOI] [PubMed] [Google Scholar]
- 85. Nagai H, Cambronne ED, Kagan JC, Amor JC, Kahn RA, Roy CR. 2005. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc Natl Acad Sci U S A 102:826–831. doi: 10.1073/pnas.0406239101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Folly-Klan M, Sancerne B, Alix E, Roy CR, Cherfils J, Campanacci V. 2015. On the use of Legionella/Rickettsia chimeras to investigate the structure and regulation of Rickettsia effector RalF. J Struct Biol 189:98–104. doi: 10.1016/j.jsb.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 87. Alix E, Chesnel L, Bowzard BJ, Tucker AM, Delprato A, Cherfils J, Wood DO, Kahn RA, Roy CR. 2012. The capping domain in RalF regulates effector functions. PLoS Pathog 8:e1003012. doi: 10.1371/journal.ppat.1003012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Amor JC, Swails J, Zhu X, Roy CR, Nagai H, Ingmundson A, Cheng X, Kahn RA. 2005. The structure of RalF, an ADP-ribosylation factor guanine nucleotide exchange factor from Legionella pneumophila, reveals the presence of a cap over the active site. J Biol Chem 280:1392–1400. doi: 10.1074/jbc.M410820200 [DOI] [PubMed] [Google Scholar]
- 89. Folly-Klan M, Alix E, Stalder D, Ray P, Duarte LV, Delprato A, Zeghouf M, Antonny B, Campanacci V, Roy CR, Cherfils J. 2013. A novel membrane sensor controls the localization and arfgef activity of bacterial RalF. PLoS Pathog 9:e1003747. doi: 10.1371/journal.ppat.1003747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rennoll-Bankert KE, Rahman MS, Guillotte ML, Lehman SS, Beier-Sexton M, Gillespie JJ, Azad AF. 2016. RalF-mediated activation of Arf6 controls Rickettsia typhi invasion by co-opting phosphoinositol metabolism. Infect Immun 84:3496–3506. doi: 10.1128/IAI.00638-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gillespie JJ, Kaur SJ, Rahman MS, Rennoll-Bankert K, Sears KT, Beier-Sexton M, Azad AF. 2015. Secretome of obligate intracellular Rickettsia. FEMS Microbiol Rev 39:47–80. doi: 10.1111/1574-6976.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ogata H, La Scola B, Audic S, Renesto P, Blanc G, Robert C, Fournier P-E, Claverie J-M, Raoult D. 2006. Genome sequence of Rickettsia bellii illuminates the role of amoebae in gene exchanges between intracellular pathogens. PLoS Genet. 2:e76. doi: 10.1371/journal.pgen.0020076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gillespie J.J, Joardar V, Williams KP, Driscoll TP, Hostetler JB, Nordberg E, Shukla M, Walenz B, Hill CA, Nene VM, Azad AF, Sobral BW, Caler E. 2012. A Rickettsia genome overrun by mobile genetic elements provides insight into the acquisition of genes characteristic of an obligate intracellular lifestyle. J Bacteriol 194:376–394. doi: 10.1128/JB.06244-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gillespie JJ, Driscoll TP, Verhoeve VI, Utsuki T, Husseneder C, Chouljenko VN, Azad AF, Macaluso KR. 2015. Genomic diversification in strains of Rickettsia felis isolated from different arthropods. Genome Biol Evol 7:35–56. doi: 10.1093/gbe/evu262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kelley LA, Sternberg MJE. 2009. Protein structure prediction on the web: a case study using the phyre server. Nat Protoc 4:363–371. doi: 10.1038/nprot.2009.2 [DOI] [PubMed] [Google Scholar]
- 96. Gillespie JJ, Wattam AR, Cammer SA, Gabbard JL, Shukla MP, Dalay O, Driscoll T, Hix D, Mane SP, Mao C, Nordberg EK, Scott M, Schulman JR, Snyder EE, Sullivan DE, Wang C, Warren A, Williams KP, Xue T, Seung Yoo H, Zhang C, Zhang Y, Will R, Kenyon RW, Sobral BW. 2011. Patric: the comprehensive bacterial bioinformatics resource with a focus on human pathogenic species. Infect Immun 79:4286–4298. doi: 10.1128/IAI.00207-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Whitworth T, Popov VL, Yu XJ, Walker DH, Bouyer DH. 2005. Expression of the Rickettsia prowazekii pld or tlyC gene in Salmonella enterica serovar typhimurium mediates phagosomal escape. Infect Immun 73:6668–6673. doi: 10.1128/IAI.73.10.6668-6673.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Driskell LO, Yu X, Zhang L, Liu Y, Popov VL, Walker DH, Tucker AM, Wood DO. 2009. Directed mutagenesis of the Rickettsia prowazekii pld gene encoding phospholipase D. Infect Immun 77:3244–3248. doi: 10.1128/IAI.00395-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Borgo GM, Burke TP, Tran CJ, Lo NTN, Engström P, Welch MD. 2022. A patatin-like phospholipase mediates Rickettsia parkeri escape from host membranes. Nat Commun 13:3656. doi: 10.1038/s41467-022-31351-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ku B, Lee K-H, Park WS, Yang C-S, Ge J, Lee S-G, Cha S-S, Shao F, Heo WD, Jung JU, Oh B-H. 2012. VipD of Legionella pneumophila targets activated Rab5 and Rab22 to interfere with endosomal trafficking in macrophages. PLoS Pathog 8:e1003082. doi: 10.1371/journal.ppat.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gaspar AH, Machner MP. 2014. VipD is a Rab5-activated phospholipase A1 that protects Legionella pneumophila from endosomal fusion. Proc Natl Acad Sci USA 111:4560–4565. doi: 10.1073/pnas.1316376111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lucas M, Gaspar AH, Pallara C, Rojas AL, Fernández-Recio J, Machner MP, Hierro A. 2014. Structural basis for the recruitment and activation of the Legionella phospholipase VipD by the host GTPase Rab5. Proc Natl Acad Sci USA 111:E3514–E3523. doi: 10.1073/pnas.1405391111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Crooks GE, Hon G, Chandonia J-M, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res 14:1188–1190. doi: 10.1101/gr.849004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Carrier TJ, Leigh BA, Deaker DJ, Devens HR, Wray GA, Bordenstein SR, Byrne M, Reitzel AM. 2021. Microbiome reduction and endosymbiont gain from a switch in sea urchin life history. Proc Natl Acad Sci USA 118:e2022023118. doi: 10.1073/pnas.2022023118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sontowski R, Poeschl Y, Okamura Y, Vogel H, Guyomar C, Cortesero A-M, van Dam NM. 2022. A high-quality functional genome assembly of Delia radicum L. (Diptera: Anthomyiidae) annotated from egg to adult. Mol Ecol Resour 22:1954–1971. doi: 10.1111/1755-0998.13594 [DOI] [PubMed] [Google Scholar]
- 107. Walpole GFW, Pacheco J, Chauhan N, Clark J, Anderson KE, Abbas YM, Brabant-Kirwan D, Montaño-Rendón F, Liu Z, Zhu H, Brumell JH, Deiters A, Stephens LR, Hawkins PT, Hammond GRV, Grinstein S, Fairn GD. 2022. Kinase-independent synthesis of 3-phosphorylated phosphoinositides by a phosphotransferase. Nat Cell Biol 24:708–722. doi: 10.1038/s41556-022-00895-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pizarro-Cerdá J, Kühbacher A, Cossart P. 2015. Phosphoinositides and host–pathogen interactions. Biochim Biophys Acta 1851:911–918. doi: 10.1016/j.bbalip.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 109. Pizarro-Cerdá J, Cossart P. 2004. Subversion of phosphoinositide metabolism by intracellular bacterial pathogens. Nat Cell Biol 6:1026–1033. doi: 10.1038/ncb1104-1026 [DOI] [PubMed] [Google Scholar]
- 110. Steiner B, Weber S, Hilbi H. 2018. Formation of the Legionella-containing vacuole: phosphoinositide conversion, GTPase modulation and ER dynamics. Int J Med Microbiol 308:49–57. doi: 10.1016/j.ijmm.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 111. Dong N, Niu M, Hu L, Yao Q, Zhou R, Shao F. 2016. Modulation of membrane phosphoinositide dynamics by the phosphatidylinositide 4-kinase activity of the Legionella LepB effector. Nat Microbiol 2:16236. doi: 10.1038/nmicrobiol.2016.236 [DOI] [PubMed] [Google Scholar]
- 112. Ledvina HE, Kelly KA, Eshraghi A, Plemel RL, Peterson SB, Lee B, Steele S, Adler M, Kawula TH, Merz AJ, Skerrett SJ, Celli J, Mougous JD. 2018. A phosphatidylinositol 3-kinase effector alters phagosomal maturation to promote intracellular growth of Francisella. Cell Host Microbe 24:285–295. doi: 10.1016/j.chom.2018.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li X, Anderson DE, Chang Y-Y, Jarnik M, Machner MP. 2022. VpdC is a ubiquitin-activated phospholipase effector that regulates Legionella vacuole expansion during infection. Proc Natl Acad Sci USA 119:e2209149119. doi: 10.1073/pnas.2209149119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kim DJ, Park K-S, Kim J-H, Yang S-H, Yoon JY, Han B-G, Kim HS, Lee SJ, Jang JY, Kim KH, Kim MJ, Song J-S, Kim H-J, Park C-M, Lee S-K, Lee BI, Suh SW. 2010. Helicobacter pylori proinflammatory protein up-regulates NF-kappaB as a cell-translocating Ser/Thr kinase. Proc Natl Acad Sci USA 107:21418–21423. doi: 10.1073/pnas.1010153107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yu Q, Hu L, Yao Q, Zhu Y, Dong N, Wang DC, Shao F. 2013. Structural analyses of Legionella LepB reveal a new GAP fold that catalytically mimics eukaryotic RasGAP. Cell Res 23:775–787. doi: 10.1038/cr.2013.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Felsheim RF, Kurtti TJ, Munderloh UG. 2009. Genome sequence of the endosymbiont Rickettsia peacockii and comparison with virulent Rickettsia rickettsii: identification of virulence factors. PLoS One 4:e8361. doi: 10.1371/journal.pone.0008361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Baldridge GD, Burkhardt NY, Felsheim RF, Kurtti TJ, Munderloh UG. 2008. Plasmids of the pRM/pRF family occur in diverse Rickettsia species. Appl Environ Microbiol 74:645–652. doi: 10.1128/AEM.02262-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Finn RD, Clements J, Eddy SR. 2011. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res 39:W29–W37. doi: 10.1093/nar/gkr367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lamason RL, Bastounis E, Kafai NM, Serrano R, Del Álamo JC, Theriot JA, Welch MD. 2016. Rickettsia Sca4 reduces vinculin-mediated intercellular tension to promote spread. Cell 167:670–683. doi: 10.1016/j.cell.2016.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Schuenke KW, Walker DH. 1994. Cloning, sequencing, and expression of the gene coding for an antigenic 120-kilodalton protein of Rickettsia conorii. Infect Immun 62:904–909. doi: 10.1128/iai.62.3.904-909.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Aistleitner K, Clark T, Dooley C, Hackstadt T. 2020. Selective fragmentation of the trans-golgi apparatus by Rickettsia rickettsii. PLoS Pathog 16:e1008582. doi: 10.1371/journal.ppat.1008582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Barrett AJ, Rawlings ND. 2001. Evolutionary lines of cysteine peptidases. Biol Chem 382:727–733. doi: 10.1515/BC.2001.088 [DOI] [PubMed] [Google Scholar]
- 123. Mosavi LK, Minor DL, Peng Z-Y. 2002. Consensus-derived structural determinants of the ankyrin repeat motif. Proc Natl Acad Sci USA 99:16029–16034. doi: 10.1073/pnas.252537899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yu JW, Jeffrey PD, Ha JY, Yang X, Shi Y. 2011. Crystal structure of the mucosa-associated lymphoid tissue lymphoma translocation 1 (MALT1) paracaspase region. Proc Natl Acad Sci USA 108:21004–21009. doi: 10.1073/pnas.1111708108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. McLuskey K, Rudolf J, Proto WR, Isaacs NW, Coombs GH, Moss CX, Mottram JC. 2012. Crystal structure of a Trypanosoma brucei metacaspase. Proc Natl Acad Sci USA 109:7469–7474. doi: 10.1073/pnas.1200885109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Puri AW, Lupardus PJ, Deu E, Albrow VE, Garcia KC, Bogyo M, Shen A. 2010. Rational design of inhibitors and activity-based probes targeting Clostridium difficile virulence factor TcdB. Chem Biol 17:1201–1211. doi: 10.1016/j.chembiol.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al. 2021. Highly accurate protein structure prediction with AlphaFold. Nature 596:583–589. doi: 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, et al. 2022. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res 50:D439–D444. doi: 10.1093/nar/gkab1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wang Z, Wu M. 2014. Complete genome sequence of the endosymbiont of Acanthamoeba strain UWC8, an amoeba endosymbiont belonging to the “Candidatus Midichloriaceae” family in Rickettsiales. Genome Announc 2:e00791-14. doi: 10.1128/genomeA.00791-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wang X-R, Burkhardt NY, Kurtti TJ, Oliver JD, Price LD, Cull B, Thorpe CJ, Thiel MS, Munderloh UG. 2021. Mitochondrion-dependent apoptosis is essential for Rickettsia parkeri infection and replication in vector cells. mSystems 6:e01209-20. doi: 10.1128/mSystems.01209-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Berk JM, Lee MJ, Zhang M, Lim C, Hochstrasser M. 2022. OtDUB from the human pathogen Orientia tsutsugamushi modulates host membrane trafficking by multiple mechanisms. Mol Cell Biol 42:e0007122. doi: 10.1128/mcb.00071-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Lim C, Berk JM, Blaise A, Bircher J, Koleske AJ, Hochstrasser M, Xiong Y. 2020. Crystal structure of a guanine nucleotide exchange factor encoded by the scrub typhus pathogen Orientia tsutsugamushi. Proc Natl Acad Sci USA 117:30380–30390. doi: 10.1073/pnas.2018163117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Adcox HE, Berk JM, Hochstrasser M, Carlyon JA. 2022. Orientia tsutsugamushi OtDUB is expressed and interacts with adaptor protein complexes during infection. Infect Immun 90:e0046922. doi: 10.1128/iai.00469-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Berk JM, Lim C, Ronau JA, Chaudhuri A, Chen H, Beckmann JF, Loria JP, Xiong Y, Hochstrasser M. 2020. A deubiquitylase with an unusually high-affinity ubiquitin-binding domain from the scrub typhus pathogen Orientia tsutsugamushi. Nat Commun 11:2343. doi: 10.1038/s41467-020-15985-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mérens A, Matrat S, Aubry A, Lascols C, Jarlier V, Soussy CJ, Cavallo JD, Cambau E. 2009. The Pentapeptide repeat proteins MfpAmt and QnrB4 exhibit opposite effects on DNA gyrase catalytic reactions and on the ternary gyrase-DNA-quinolone complex. J Bacteriol 191:1587–1594. doi: 10.1128/JB.01205-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kim N, Han G, Jung H, Lee HH, Park J, Seo YS. 2021. T6SS accessory proteins, including DUF2169 domain-containing protein and pentapeptide repeats protein, contribute to bacterial virulence in T6Ss droup_5 of Burkholderia glumae BGR1. Plants (Basel) 11:34. doi: 10.3390/plants11010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ammerman NC, Gillespie JJ, Neuwald AF, Sobral BW, Azad AF. 2009. A typhus group-specific protease defies reductive evolution in rickettsiae. J Bacteriol 191:7609–7613. doi: 10.1128/JB.01077-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. van der Heide T, Stuart MC, Poolman B. 2001. On the osmotic signal and osmosensing mechanism of an ABC transport system for glycine betaine. EMBO J 20:7022–7032. doi: 10.1093/emboj/20.24.7022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wood JM. 1999. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol Mol Biol Rev 63:230–262. doi: 10.1128/MMBR.63.1.230-262.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gillespie JJ, Williams K, Shukla M, Snyder EE, Nordberg EK, Ceraul SM, Dharmanolla C, Rainey D, Soneja J, Shallom JM, Vishnubhat ND, Wattam R, Purkayastha A, Czar M, Crasta O, Setubal JC, Azad AF, Sobral BS. 2008. Rickettsia phylogenomics: unwinding the intricacies of obligate intracellular life. PLoS ONE 3:e2018. doi: 10.1371/journal.pone.0002018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hagen R, Verhoeve VI, Gillespie JJ, Driscoll TP. 2018. Conjugative transposons and their cargo genes vary across natural populations of Rickettsia buchneri infecting the tick Ixodes scapularis. Genome Biol Evol 10:3218–3229. doi: 10.1093/gbe/evy247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Nakayama K, Yamashita A, Kurokawa K, Morimoto T, Ogawa M, Fukuhara M, Urakami H, Ohnishi M, Uchiyama I, Ogura Y, Ooka T, Oshima K, Tamura A, Hattori M, Hayashi T. 2008. The whole-genome sequencing of the obligate intracellular bacterium Orientia tsutsugamushi revealed massive gene amplification during reductive genome evolution. DNA Res 15:185–199. doi: 10.1093/dnares/dsn011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cho N-H, Kim H-R, Lee J-H, Kim S-Y, Kim J, Cha S, Kim S-Y, Darby AC, Fuxelius H-H, Yin J, Kim JH, Kim J, Lee SJ, Koh Y-S, Jang W-J, Park K-H, Andersson SGE, Choi M-S, Kim I-S. 2007. The Orientia tsutsugamushi genome reveals massive proliferation of conjugative type IV secretion system and host-cell interaction genes. Proc Natl Acad Sci USA 104:7981–7986. doi: 10.1073/pnas.0611553104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Heinzen RA, Hayes SF, Peacock MG, Hackstadt T. 1993. Directional actin polymerization associated with spotted fever group Rickettsia infection of Vero cells. Infect Immun 61:1926–1935. doi: 10.1128/iai.61.5.1926-1935.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Heinzen RA, Grieshaber SS, Van Kirk LS, Devin CJ. 1999. Dynamics of actin-based movement by Rickettsia rickettsii in Vero cells. Infect Immun 67:4201–4207. doi: 10.1128/IAI.67.8.4201-4207.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Van Kirk LS, Hayes SF, Heinzen RA. 2000. Ultrastructure of Rickettsia rickettsii actin tails and localization of cytoskeletal proteins. Infect Immun 68:4706–4713. doi: 10.1128/IAI.68.8.4706-4713.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gouin E, Gantelet H, Egile C, Lasa I, Ohayon H, Villiers V, Gounon P, Sansonetti PJ, Cossart P. 1999. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J Cell Sci 112 (Pt 11):1697–1708. doi: 10.1242/jcs.112.11.1697 [DOI] [PubMed] [Google Scholar]
- 148. Jeng RL, Goley ED, D’Alessio JA, Chaga OY, Svitkina TM, Borisy GG, Heinzen RA, Welch MD. 2004. A Rickettsia WASP-like protein activates the Arp2/3 complex and mediates actin-based motility. Cell Microbiol 6:761–769. doi: 10.1111/j.1462-5822.2004.00402.x [DOI] [PubMed] [Google Scholar]
- 149. Madasu Y, Suarez C, Kast DJ, Kovar DR, Dominguez R. 2013. Rickettsia Sca2 has evolved formin-like activity through a different molecular mechanism. Proc Natl Acad Sci USA 110:E2677–E2686. doi: 10.1073/pnas.1307235110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kleba B, Clark TR, Lutter EI, Ellison DW, Hackstadt T. 2010. Disruption of the Rickettsia rickettsii Sca2 autotransporter inhibits actin-based motility. Infect Immun 78:2240–2247. doi: 10.1128/IAI.00100-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Haglund CM, Choe JE, Skau CT, Kovar DR, Welch MD. 2010. Rickettsia Sca2 is a bacterial formin-like mediator of actin-based motility. Nat Cell Biol 12:1057–1063. doi: 10.1038/ncb2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Serio AW, Jeng RL, Haglund CM, Reed SC, Welch MD. 2010. Defining a core set of actin cytoskeletal proteins critical or actin-based motility of Rickettsia. Cell Host Microbe 7:388–398. doi: 10.1016/j.chom.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Balraj P, El Karkouri K, Vestris G, Espinosa L, Raoult D, Renesto P. 2008. RickA expression is not sufficient to promote actin-based motility of Rickettsia raoultii. PLOS ONE 3:e2582. doi: 10.1371/journal.pone.0002582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Martinez JJ, Cossart P. 2004. Early signaling events involved in the entry of Rickettsia conorii into mammalian cells. J Cell Sci 117:5097–5106. doi: 10.1242/jcs.01382 [DOI] [PubMed] [Google Scholar]
- 155. Oliver JD, Burkhardt NY, Felsheim RF, Kurtti TJ, Munderloh UG. 2014. Motility characteristics are altered for Rickettsia bellii transformed to overexpress a heterologous rickA gene. Appl Environ Microbiol 80:1170–1176. doi: 10.1128/AEM.03352-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Chan Y-Y, Riley SP, Martinez JJ. 2010. Adherence to and invasion of host cells by spotted fever group Rickettsia species. Front Microbiol 1:139. doi: 10.3389/fmicb.2010.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chan YGY, Cardwell MM, Hermanas TM, Uchiyama T, Martinez JJ. 2009. Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, c-Cbl, clathrin and caveolin 2-dependent manner. Cell Microbiol 11:629–644. doi: 10.1111/j.1462-5822.2008.01279.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hillman RD, Baktash YM, Martinez JJ. 2013. OmpA-mediated rickettsial adherence to and invasion of human endothelial cells is dependent upon interaction with α2β1 integrin. Cell Microbiol 15:727–741. doi: 10.1111/cmi.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Riley SP, Goh KC, Hermanas TM, Cardwell MM, Chan YGY, Martinez JJ. 2010. The Rickettsia conorii autotransporter protein sca1 promotes adherence to nonphagocytic mammalian cells. Infect Immun 78:1895–1904. doi: 10.1128/IAI.01165-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Martinez JJ, Seveau S, Veiga E, Matsuyama S, Cossart P. 2005. Ku70, a component of DNA-dependent protein kinase, is a mammalian receptor for Rickettsia conorii. Cell 123:1013–1023. doi: 10.1016/j.cell.2005.08.046 [DOI] [PubMed] [Google Scholar]
- 161. Carl M, Dasch GA. 1989. The importance of the crystalline surface layer protein antigens of rickettsiae in T-cell immunity. J Autoimmun 2 Suppl:81–91. doi: 10.1016/0896-8411(89)90119-4 [DOI] [PubMed] [Google Scholar]
- 162. Steele MI, Moran NA. 2021. Evolution of interbacterial antagonism in bee gut microbiota reflects host and symbiont diversification. mSystems 6:e00063-21. doi: 10.1128/mSystems.00063-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Massey JH, Newton ILG. 2022. Diversity and function of arthropod endosymbiont toxins. Trends Microbiol 30:185–198. doi: 10.1016/j.tim.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Poole SJ, Diner EJ, Aoki SK, Braaten BA, t’Kint de Roodenbeke C, Low DA, Hayes CS. 2011. Identification of functional toxin/immunity genes linked to contact-dependent growth inhibition (CDI) and rearrangement hotspot (Rhs) systems. PLoS Genet 7:e1002217. doi: 10.1371/journal.pgen.1002217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Zhang D, Iyer LM, Aravind L. 2011. A novel immunity system for bacterial nucleic acid degrading toxins and its recruitment in various eukaryotic and DNA viral systems. Nucleic Acids Res 39:4532–4552. doi: 10.1093/nar/gkr036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Aoki SK, Diner EJ, de Roodenbeke CT, Burgess BR, Poole SJ, Braaten BA, Jones AM, Webb JS, Hayes CS, Cotter PA, Low DA. 2010. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature 468:439–442. doi: 10.1038/nature09490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ruhe ZC, Subramanian P, Song K, Nguyen JY, Stevens TA, Low DA, Jensen GJ, Hayes CS. 2018. Programmed secretion arrest and receptor-triggered toxin export during antibacterial contact-dependent growth inhibition. Cell 175:921–933. doi: 10.1016/j.cell.2018.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Willett JLE, Ruhe ZC, Goulding CW, Low DA, Hayes CS. 2015. Contact-dependent growth inhibition (CDI) and CdiB/CdiA two-partner secretion proteins. J Mol Biol 427:3754–3765. doi: 10.1016/j.jmb.2015.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Aoki SK, Webb JS, Braaten BA, Low DA. 2009. Contact-dependent growth inhibition causes reversible metabolic downregulation in Escherichia coli. J Bacteriol 191:1777–1786. doi: 10.1128/JB.01437-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Joris B, Englebert S, Chu CP, Kariyama R, Daneo-Moore L, Shockman GD, Ghuysen JM. 1992. Modular design of the Enterococcus hirae muramidase-2 and Streptococcus faecalis autolysin. FEMS Microbiol Lett 70:257–264. doi: 10.1016/0378-1097(92)90707-u [DOI] [PubMed] [Google Scholar]
- 171. Veyron S, Peyroche G, Cherfils J. 2018. FIC proteins: from bacteria to humans and back again. Pathog Dis 76:12. doi: 10.1093/femspd/fty012 [DOI] [PubMed] [Google Scholar]
- 172. Wang D, Zhu L, Zhen X, Yang D, Li C, Chen Y, Wang H, Qu Y, Liu X, Yin Y, Gu H, Xu L, Wan C, Wang Y, Ouyang S, Shen X. 2022. A secreted effector with a dual role as a toxin and as a transcriptional factor. Nat Commun 13:1–15. doi: 10.1038/s41467-022-35522-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Gillespie JJ, Beier MS, Rahman MS, Ammerman NC, Shallom JM, Purkayastha A, Sobral BS, Azad AF. 2007. Plasmids and rickettsial evolution: insight from Rickettsia felis. PLoS One 2:e266. doi: 10.1371/journal.pone.0000266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Maté MJ, Vincentelli R, Foos N, Raoult D, Cambillau C, Ortiz-Lombardía M. 2012. Crystal structure of the DNA-bound VapBC2 antitoxin/toxin pair from Rickettsia felis. Nucleic Acids Res 40:3245–3258. doi: 10.1093/nar/gkr1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Beckmann JF, Sharma GD, Mendez L, Chen H, Hochstrasser M. 2019. The Wolbachia cytoplasmic incompatibility enzyme CIDB targets nuclear import and protamine-histone exchange factors. Elife 8:e50026. doi: 10.7554/eLife.50026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Chen H, Ronau JA, Beckmann JF, Hochstrasser M. 2019. A Wolbachia nuclease and its binding partner comprise a novel mechanism for induction of cytoplasmic incompatibility. Proc Natl Acad Sci USA, no. 44:22314–22321. doi: 10.1073/pnas.1914571116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. LePage DP, Metcalf JA, Bordenstein SR, On J, Perlmutter JI, Shropshire JD, Layton EM, Funkhouser-Jones LJ, Beckmann JF, Bordenstein SR. 2017. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 543:243–247. doi: 10.1038/nature21391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Beckmann JF, Ronau JA, Hochstrasser M. 2017. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat Microbiol 2:17007. doi: 10.1038/nmicrobiol.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Harumoto T, Lemaitre B. 2018. Male-killing toxin in a bacterial symbiont of Drosophila. Nature 557:252–255. doi: 10.1038/s41586-018-0086-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Driscoll TP, Verhoeve VI, Brockway C, Shrewsberry DL, Plumer M, Sevdalis SE, Beckmann JF, Krueger LM, Macaluso KR, Azad AF, Gillespie JJ. 2020. Evolution of Wolbachia mutualism and reproductive parasitism: insight from two novel strains that co-infect cat fleas. PeerJ 8:e10646. doi: 10.7717/peerj.10646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Beckmann JF, Bonneau M, Chen H, Hochstrasser M, Poinsot D, Merçot H, Weill M, Sicard M, Charlat S. 2019. The toxin–antidote model of cytoplasmic incompatibility: genetics and evolutionary implications. Trends Genet 35:175–185. doi: 10.1016/j.tig.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Cascales E, Christie PJ. 2003. The versatile bacterial type IV secretion systems. Nat Rev Microbiol 1:137–149. doi: 10.1038/nrmicro753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Cascales E, Christie PJ. 2004. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 304:1170–1173. doi: 10.1126/science.1095211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Bordenstein SR, Wernegreen JJ. 2004. Bacteriophage flux in endosymbionts (Wolbachia): infection frequency, lateral transfer, and recombination rates. Mol Biol Evol 21:1981–1991. doi: 10.1093/molbev/msh211 [DOI] [PubMed] [Google Scholar]
- 185. Dharamshi JE, Köstlbacher S, Schön ME, Collingro A, Ettema TJG, Horn M. 2023. Gene gain facilitated endosymbiotic evolution of Chlamydiae. Nat Microbiol 8:40–54. doi: 10.1038/s41564-022-01284-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Hugoson E, Guliaev A, Ammunét T, Guy L. 2022. Host adaptation in Legionellales is 1.9 Ga, coincident with eukaryogenesis. Mol Biol Evol 39:msac037. doi: 10.1093/molbev/msac037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Nock AM, Clark TR, Hackstadt T. 2022. Regulator of actin-based motility (RoaM) downregulates actin tail formation by Rickettsia rickettsii and is negatively selected in mammalian cell culture. mBio 13:e0035322. doi: 10.1128/mbio.00353-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Gauthier DT, Karpathy SE, Grizzard SL, Batra D, Rowe LA, Paddock CD. 2021. Characterization of a novel transitional group Rickettsia species (Rickettsia tillamookensis sp. nov.) from the western black-legged tick, Ixodes pacificus. Int J Syst Evol Microbiol 71:004880. doi: 10.1099/ijsem.0.004880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Blanton LS, Quade BR, Bouyer DH. 2019. Differentiation of Rickettsia felis and Rickettsia felis -like organisms via restriction fragment length polymorphism analysis. Vector Borne Zoonotic Dis 19:637–639. doi: 10.1089/vbz.2018.2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Perlman SJ, Hunter MS, Zchori-Fein E. 2006. The emerging diversity of Rickettsia. Proc Biol Sci 273:2097–2106. doi: 10.1098/rspb.2006.3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. doi: 10.1093/bioinformatics/btp348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 193. Lefort V, Longueville JE, Gascuel O. 2017. SMS: smart model selection in PhyML. Mol Biol Evol 34:2422–2424. doi: 10.1093/molbev/msx149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Letunic I, Bork P. 2018. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res 46:D493–D496. doi: 10.1093/nar/gkx922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi: 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 196. Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. doi: 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 197. Liu W, Xie Y, Ma J, Luo X, Nie P, Zuo Z, Lahrmann U, Zhao Q, Zheng Y, Zhao Y, Xue Y, Ren J. 2015. IBS: an illustrator for the presentation and visualization of biological sequences. Bioinformatics 31:3359–3361. doi: 10.1093/bioinformatics/btv362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. 2000. The protein data bank. Nucleic Acids Res 28:235–242. doi: 10.1093/nar/28.1.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Drozdetskiy A, Cole C, Procter J, Barton GJ. 2015. JPred4: a protein secondary structure prediction server. Nucleic Acids Res 43:W389–W394. doi: 10.1093/nar/gkv332 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figures.
Additional supplemental figures.
RvhB4 sequences used for phylogeny estimation.
Supporting information for REMs and cREMs.
Supporting information for type VI secretion system analyses.
Supporting information for patatin analyses.