Abstract
Background
Selumetinib is approved for the treatment of pediatric patients with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas (PN) in multiple countries, including the USA (≥ 2 years). Until recently, individuals had to take selumetinib twice daily (BID) in a fasted state. This study evaluated the effect of a low-fat meal on selumetinib PK parameters and gastrointestinal (GI) tolerability in adolescent participants with NF1-PN.
Methods
Eligible participants aged ≥ 12 to < 18 years took 25 mg/m2 selumetinib BID with a low-fat meal (T1) for 28 days, followed by a 7-day washout, and then administration in a fasted state (T2) for another 28 days. Primary objectives were to evaluate the effect of a low-fat meal on AUC0−12,ss and GI tolerability after multiple selumetinib doses in T1 versus T2. Key secondary objectives were additional PK parameters and adverse events (AEs).
Results
At primary data cut-off, all 24 participants completed T1, and 23 participants completed T2. There were no significant differences in AUC0−12,ss between T1 and T2. In T1 and T2, 29.2% and 33.3% participants, respectively, reported ≥ 1 GI AE. No GI AEs Grade ≥ 3, or serious AEs, or GI AEs resulting in treatment interruptions, discontinuation, or dose reductions were reported in T1 and T2.
Conclusions
Dosing selumetinib with a low-fat meal had no clinically relevant impact on selumetinib AUC0−12,ss nor GI tolerability in adolescents with NF1-PN.
Trial registration ClinicalTrials.gov ID
Keywords: gastrointestinal tolerability, neurofibromatosis type 1, pharmacokinetics, plexiform neurofibroma, selumetinib
Key Points.
Low-fat meals had no clinically relevant impact on selumetinib steady-state exposure.
Selumetinib maintains an acceptable PK/safety profile when taken with a low-fat meal.
This will allow flexibility in administration of selumetinib with respect to food.
Importance of the Study.
Currently, selumetinib is approved for pediatric patients with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas (PN) in multiple countries. The initial approved label required selumetinib to be taken on an empty stomach with no food 2 h before, and 1 h after each dose. Gastrointestinal (GI) events are common adverse reactions in patients who receive selumetinib. Results from the primary data cut-off for this study show that when selumetinib is taken with a low-fat meal, no clinically relevant impact on the PK of selumetinib and GI tolerability was observed. These results demonstrate that the current recommended dose of selumetinib can maintain an acceptable pharmacokinetic and safety profile if taken with a low-fat meal. The FDA and EMA have recently approved that selumetinib can now be taken with or without food.
Neurofibromatosis type 1 (NF1) is a genetic disorder that results from a pathological variant in the NF1 gene, which leads to the loss of function of neurofibromin, a tumor suppressor protein.1,2–4 NF1 affects approximately 1 in 3000 worldwide and is associated with a diverse range of clinical manifestations.5 Manifestations characteristic of NF1 include pigmented lesions, cutaneous and subcutaneous neurofibromas, brain tumors, and internal and peripheral nerve sheath tumors known as plexiform neurofibromas (PN).5,6 PN can be debilitating with associated symptoms including pain, disfigurement, and motor dysfunction.2 Complete surgical resection is often not possible, as it comes with risks, such as blood loss, nerve damage, and functional impairment.7,8 In 1 study, 13% of PN surgeries in patients with NF1 resulted in the manifestation of new symptoms, and 23% of tumors regrew significantly after surgery.7
Selumetinib (ARRY-142886, AZD6244) is a potent and highly selective MEK1/2 inhibitor, which blocks the RAS/RAF/MEK/ERK pathway signaling, thereby preventing PN growth and reducing PN volume.3,9–11 Selumetinib was first approved by the FDA for pediatric patients (age ≥ 2 years) with NF1 who have symptomatic, inoperable PN, and has since been approved by the regulatory bodies in multiple other countries.3,12–15 These approvals were based on results from the pivotal Phase 1/2 SPRINT trial, in which selumetinib demonstrated durable PN shrinkage and improvements in several quality of life measures.11–15
The initial approved label for selumetinib indicated that it must be administered twice daily (BID) on an empty stomach, requiring individuals to fast for 2 h before and 1 h after taking each dose. This dosing schedule resulted in a total of 6 h of fasting per day,3,4 which could have been inconvenient. The recommendation for administration of selumetinib while fasting, as indicated on the initial approved label, was based on multiple studies in adults without NF1.16,17
Data from 3 previous food effect studies have demonstrated that consumption of a meal reduces the extent of selumetinib absorption compared with administration in fasted conditions.16–18 The food effect studies conducted in healthy participants,16 and in participants with advanced solid tumors,17 showed that consumption of a high-fat meal reduced the rate of absorption of selumetinib, but did not reduce the extent of the absorption by an amount that was considered to be clinically relevant.16,17 In healthy participants, maximum observed plasma peak drug concentration (Cmax) was reduced by 50%, time to reach peak or maximum observed concentration (Tmax) was delayed by 1.5 h, and AUC was reduced by 16%.16 In participants with advanced solid tumors, Cmax was reduced by 62%,Tmax was delayed by 2.5 h, and AUC was reduced by 19%.17 A further study conducted in healthy adults showed that consumption of a low-fat, low-calorie meal reduced both the rate and extent of absorption after a single dose of selumetinib; Cmax was reduced by 60%, Tmax was delayed by 2.5 h, and AUC was reduced by 38%.18 Therefore, administration in the fed rather than the fasted state could affect exposure to, and absorption of, selumetinib.
As reported in previous studies, gastrointestinal (GI) adverse events (AEs) are commonly associated with selumetinib treatment.3,11,19 GI disturbances were the most commonly reported AEs in SPRINT, although these were mostly mild to moderate in severity (Grade 1/2)11; the most frequently occurring GI AEs included vomiting, abdominal pain, diarrhea, and nausea.3,11 Here, we present results from the primary data cut-off (DCO; April 6, 2022) for this Phase 1 study, which aimed to evaluate the effect of a low-fat meal on steady-state exposure and GI tolerability of selumetinib in adolescents with NF1 who have inoperable PN. This study evaluated the effect of a low-fat meal on selumetinib PK parameters and GI tolerability in adolescent participants with NF1-PN. The data from this study were assessed by the FDA and EMA, which contributed to the removal of the fasting requirement on the selumetinib label.3,4,20
Methods
Study Design and Participants
This was a Phase 1, single-arm, sequential period, food effect study, to evaluate the steady-state systemic exposure of selumetinib and GI tolerability when multiple doses were taken with a low-fat meal compared with a fasted state.
Eligible participants were adolescents aged ≥ 12 to < 18 years diagnosed with NF1 (per National Institutes of Health Consensus Development Conference Statement 1988)21 and inoperable PN. Participants who had prior treatment with a MEK inhibitor were eligible; those who had received an investigational medicinal product or other systemic PN treatment were required to undergo a washout period of 4 weeks or equivalent to 5 half-lives. Participants who had received radiotherapy within 6 weeks before selumetinib initiation were excluded. Full inclusion and exclusion criteria are provided in the Supplementary Material.
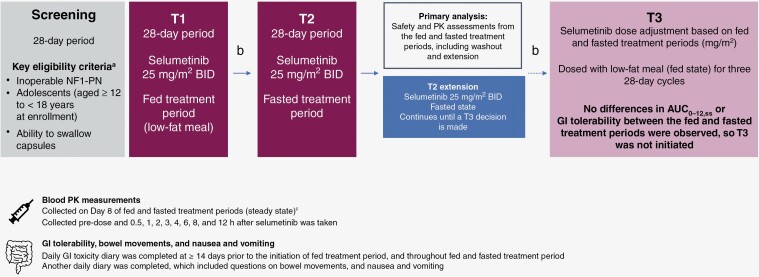
Participants were administered 25 mg/m2 selumetinib BID (Figure 1). A 28-day screening period was followed by a 28-day treatment period, during which participants were dosed in a fed state (T1; fed treatment period). During T1, participants were given selumetinib with a light meal or snack of approximately 500 calories containing < 15 g fat. Other than this low-fat meal, participants did not consume any other food in the 2 h before, and the 1 h after their dose of selumetinib. T1 was followed by a 7-day washout period. If any GI-related AEs had not recovered or returned to baseline after 7 days of washout, this period could be extended to 21 days at the discretion of the investigator. This washout period was followed by another 28-day treatment period, during which participants were dosed BID in a fasted state (T2; fasted treatment period), as per the initial approved label.3,4 Following this, an extension in the fasted state was initiated (T2 extension; fasted treatment period extension), which continued until results from the primary analysis (safety and PK assessments from the fed and fasted treatment periods) were available. In the T2 and T2 extension treatment periods, participants did not eat or drink (other than water) in the 2 h before, and 1 h after receiving selumetinib. Doses of selumetinib were taken approximately 12 h apart.
Figure 1.
Study design. aFull lists of the inclusion and exclusion criteria are included in the Supplementary Material; b7-day washout period (could be extended up to 21 days at the investigator’s discretion for GI-related AEs to return to baseline); cPK blood measurements were targeted for Day 8 but could be taken on any day between Days 4 and 14, assuming the participant had received at least 1 consecutive day of dosing immediately prior to the day the PK sample was taken on. AE, adverse event; AUC0–12,ss, area under the concentration-time curve from time zero to 12 h at steady state; BID, twice daily; GI, gastrointestinal; NF1, neurofibromatosis type 1; PK, pharmacokinetic; PN, plexiform neurofibroma.
Following T1 and T2, a third treatment period (T3) was embedded in the initial protocol development, whereby an adjustment of selumetinib dosing could be administered based on PK analyses of T1 and T2; as such, T3 would only occur if required (based on results from T1 and T2). By considering the flat exposure–response relationship at the investigated dose range of 20–30 mg/m2, the observed range of exposure, and the variability in SPRINT, it was expected that a < 30% change in AUC0–12 would not alter the benefit–risk profile of selumetinib. A significant reduction in exposure was defined as a > 30% decrease in the AUC of selumetinib when dosed with a low-fat meal compared with fasted treatment. Therefore, if the fed/fasted AUC geometric mean ratio (GMR) showed a ≥ 20% decrease and the lower 90% confidence interval (CI) of the ratio showed a ≥ 30% decrease in the fed state compared to fasted, then the effect of the low-fat meal on selumetinib exposure would have been considered clinically relevant, which would have triggered initiation of T3.
Participants who had stopped taking selumetinib for any reason other than death, loss to follow-up, or withdrawal of consent, would have had an end of treatment assessment, on the day of, or up to 7 days after, the day they stopped taking selumetinib and a safety follow-up assessment 30 days after the end-of-treatment visit. Details of the withdrawal criteria for this study are in the Supplementary Material.
Study Objectives and Assessments
The primary objectives were: (1) to evaluate the effect of a low-fat meal on the AUC0−12,ss of selumetinib capsules, and (2) to investigate GI tolerability after multiple selumetinib doses in adolescent patients with NF1 and inoperable PN. The secondary objectives were additional PK parameters in the fed and fasted states, and additional safety measures (including non-GI AEs, laboratory variables, and vital signs). Additional PK (fed vs fasted treatment period) endpoints were observed plasma concentrations and PK parameters for both selumetinib and its metabolite (N-desmethyl selumetinib) after multiple-dose administration. These included Cmax, the area under the concentration–time curve from zero to the last quantifiable concentration (AUClast), Tmax, and time to last observed quantifiable concentration (Tlast). Additional PK parameters for selumetinib only included apparent total clearance of drug from plasma after extravascular administration (CLss/F), and apparent volume of distribution at a steady-state following extravascular administration (Vss/F).
Pharmacokinetics
The primary PK parameter was assessed using GMR (1-sided 90% CI) of selumetinib AUC0–12,ss for the fed versus the fasted treatment period. In both treatment periods, blood PK measurements were collected pre-dose on Day 8 and then at 0.5, 1, 2, 3, 4, 6, 8, and 12 h after selumetinib was taken. Although measurements were targeted for Day 8, these could be taken on any day between Days 4 and 14, assuming the participant had received at least 1 consecutive day of dosing immediately prior to the day the PK sample was taken.
Selumetinib has a relatively short-half-life of 6.2 h and reaches steady state in approximately 1–2 days, with minimal accumulation.3 To be evaluable for PK analyses, participants must have received selumetinib, as per the dosing requirements (please see the Study Design and Participants section) the day prior to, and on the multiple-dose PK sampling day with no protocol deviations that could have affected the PK analysis; participants must also have had ≥ 1 post-dose quantifiable plasma concentration.
PK parameters were derived from plasma concentrations using non-compartmental methods in Phoenix® WinNonlin® Version 8.1.1.
Safety and Tolerability
GI symptoms were collected through a daily diary, and usage of GI concomitant medication(s) per the WHODrug Global B3-format.22 The daily GI diary incorporated the modified Bristol Stool Form Scale for Children23 (mBSFS-C) and the Nausea and Vomiting Symptom Rating Scale (adapted from the Children’s Cancer and Leukaemia Group).24 Bowel movements were assessed using the mBSFS-C scale.23 Nausea and vomiting were assessed by severity, frequency, and interference with daily life, based on the Nausea and Vomiting Symptom Rating Scale.24 Clinical safety assessments (clinical chemistry, hematology, urinalysis) and physical examinations were conducted. Weight, vital signs, electrocardiogram, echocardiogram or cardiac MRI, ophthalmologic assessment, and performance status were also assessed. AE-related assessments included occurrence/frequency, relationship to study intervention, and AEs leading to discontinuation of study intervention. All AEs were coded using MedDRA Version 24.1 and graded using Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. For each participant, only the occurrence with the highest observed grade for each AE was reported. AEs were assigned to a treatment period based on onset date; if an AE emerged in the fed treatment period and continued into the fasted treatment period, it would not be re-counted. However, if the AE emerged in the fed treatment period, resolved, and re-emerged in the fasted treatment period, it would be counted in both treatment periods.
Data and Statistical Analysis
All statistical analyses were performed using Statistical Analysis Software (SAS)® Version 9.4. PK analyses were based on the PK analysis set, which consisted of all participants who received at least 1 dose of selumetinib and had at least 1 post-dose quantifiable plasma concentration without any protocol deviations that affected PK analysis. PK analyses were performed on both selumetinib and N-desmethyl selumetinib analytes. For comparison between fed and fasted conditions of AUC0–12,SS, AUClast, and Cmax, a mixed-effects analysis of variance (ANOVA) was performed with log-transformed PK parameter as the response variable, treatment as a fixed effect, and participant as a random effect. The estimate of the difference in means on the log scale from the mixed-effects ANOVA was back-transformed to obtain the GMR (fed treatment period/fasted treatment period) and its 1-sided 90% CI. The geometric least squares (LS) mean for each period and the corresponding 2-sided 95% CIs were derived similarly. Comparison between fed and fasted conditions of Tmax was analyzed with the Hodges–Lehmann point estimate of median difference and associated 2-sided 90% CI. CLss/F and Vss/F were descriptively summarized and no statistical comparison between the treatment periods was made. Geometric mean selumetinib plasma concentration–nominal time profiles under fed and fasted conditions were plotted on a semi-logarithmic scale.
Safety analyses were based on the safety analysis set, which included all participants who received at least 1 dose of selumetinib. There were no inferential analyses for safety results; summary statistics were provided for safety endpoints.
Ethics
This study was performed in accordance with the Declaration of Helsinki, and was consistent with the International Council for Harmonisation Good Clinical Practice guidelines, applicable regulatory requirements, and the AstraZeneca policy on bioethics.
Informed Consent
Mandatory provision of signed and dated parent/legal guardian consent for the study along with the pediatric assent form (or consent form if the participant was deemed a “young adult” [aged 16 to 18 years]) was required, when applicable. For participants who reached the age of legal consent during the clinical study, notification was required, and a new consent form needed to be signed by the participant.
Results
The study started on July 21, 2021, and the primary DCO for this analysis was April 6, 2022.25 Results from the primary DCO (fed vs fasted) are presented herein.25 Additional analyses from this dataset may be available in future publications.
Participant Demographics and Baseline Characteristics
Overall, 25 participants were enrolled at 9 sites across 4 countries (Poland, Russia, Spain, and the USA). One participant failed screening; therefore, 24 participants were included in the safety and PK analysis sets. Participant demographics and baseline characteristics are shown in Table 1.
Table 1.
Participant demographics and clinical characteristics at baseline (safety analysis set)
| Characteristic | Participants (N = 24) |
|---|---|
| Sex, n (%) | |
| Female | 12 (50.0) |
| Male | 12 (50.0) |
| Age, median (range), years | 15 (12, 17) |
| Height, median (range), cm | 158.5 (146.0, 178.4) |
| Weight, median (range), kg | 50.2 (41.0, 86.0) |
| Body surface area, median (range), m2 | 1.5 (1.3, 2.0) |
| Ethnic group, n (%) | |
| Hispanic or Latino | 2 (8.3) |
| Not Hispanic or Latino | 22 (91.7) |
| Race, n (%) | |
| Asian | 1 (4.2) |
| White | 23 (95.8) |
| Country, n (%)a | |
| Poland | 9 (37.5) |
| Russia | 7 (29.2) |
| Spain | 4 (16.7) |
| USA | 4 (16.7) |
| NF1 diagnostic criteria, n (%)b | |
| Six or more café-au-lait macules | 20 (83.3) |
| Freckling in axilla or groin | 18 (75.0) |
| Two or more Lisch nodules | 18 (75.0) |
| First-degree relative with NF1 | 10 (41.7) |
| Optic glioma | 6 (25.0) |
| A distinctive osseous lesion | 4 (16.7) |
| Reasons for inoperable PN, n (%)c | |
| Close proximity to vital structures | 15 (62.5) |
| Invasiveness | 13 (54.2) |
| Encasement of vital structures | 9 (37.5) |
| High vascularity | 3 (12.5) |
| Lansky performance status score, median (range)d |
n=22 90 (80, 100) |
| Time from diagnosis of NF1 to start of selumetinib, median (range), ye |
11.7 (0, 16) |
| Time from diagnosis of inoperable PN to start of selumetinib, median (range), y | 5.4 (0, 12) |
| Previous PN-directed treatments, n (%)f | 14 (58.3) |
| Medical therapyg | 7 (29.2) |
| Surgery | 9 (37.5) |
aTotal percentage may not equal 100% due to rounding; bParticipants could have had more than 1 NF1 diagnostic criterion; cParticipants could have had more than 1 reason for inoperable PN; dThe Lansky scale was completed for participants aged ≤ 16 years at study entry. The Karnofsky scale was completed for participants aged ≥ 16 years at study entry. Both the Karnofsky and Lansky performance status scores range from 10 to 100, with higher scores indicating better functioning. Only participants with Karnofsky/Lansky scores ≥ 70 at enrollment were included in the study. Karnofsky scores were recorded for 2 participants included in the study for which the median (range) was 90.0 (80 to 100); eThe "start of selumetinib" refers to participants who received selumetinib treatment as part of the study, not participants who had received selumetinib as prior therapy; fParticipants could have had more than 1 modality; gSix of 7 participants who had previously received PN-directed medical therapy had received selumetinib.
NF1, neurofibromatosis type 1; PN, plexiform neurofibroma.
Participant Disposition
All 24 participants completed the fed treatment period (T1), and 23 participants completed the fasted treatment period (T2). The maximum dose of 50 mg BID (for participants with a body surface area [BSA] of ≥ 1.9 m2 at baseline3,4) was administered to 3 participants in the study. One participant did not complete T2 due to a dose reduction and was entered into the extension part of the study. However, as this patient started T2, data for all 24 participants are presented for both treatment periods in all text, tables, and figures, unless otherwise stated. Over the course of the study (including the fed treatment period, washout, fasted treatment period, and fasted treatment period extension), 3 participants (12.5%) met the withdrawal criteria (Supplementary Material); of these, 2 participants had a prolonged interruption for > 7 days while in the fasted treatment period extension, and the remaining participant had a dose reduction while in the fasted treatment period. Therefore, although these 3 participants continued to receive selumetinib in the fasted treatment period extension, and treatment was ongoing at data cut-off, these participants did not complete the fasted treatment period; consequently, PK statistical comparisons to the fed treatment period were not possible. No other participants had a dose reduction or prolonged dose interruption during either of the 28-day treatment periods. Participants with dose modifications were not included in the PK analysis as the doses that they received in the fed and fasted treatment periods were not comparable; however, these participants were included in the safety analysis as they received selumetinib in both treatment periods.
Pharmacokinetics
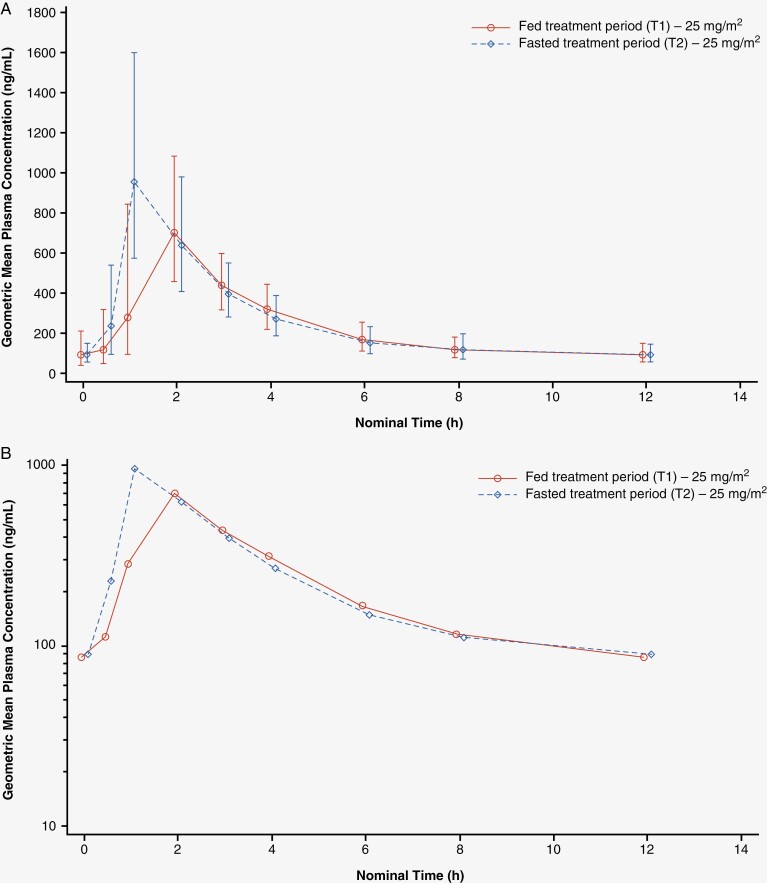
Full PK results for selumetinib are shown in Table 2 and full PK results for N-desmethyl selumetinib are shown in Supplementary Table 1. The geometric mean plasma concentration–time profiles following multiple doses of selumetinib under fed and fasted conditions are shown in Figure 2.
Table 2.
Summary of selumetinib PK parameters in the fed (T1) versus fasted (T2)a treatment periods at primary DCO (PK analysis set)
| PK parameter (n = 19) | Geometric LS mean (95% CI) | Geometric mean ratio (fed vs fasted, 1-sided 90% CI lower bound) |
|
|---|---|---|---|
| AUC0–12,ss (h*ng/mL) | Fed (T1) | 2910 (2508, 3376) | 0.919 (0.841) |
| Fasted (T2) | 3166 (2729, 3674) | ||
| AUClast (h*ng/mL) | Fed (T1) | 2909 (2500, 3385) | 0.931 (0.850) |
| Fasted (T2) | 3124 (2685, 3636) | ||
| C max (ng/mL) | Fed (T1) | 851 (709, 1021) | 0.763 (0.667) |
| Fasted (T2) | 1116 (929, 1339) | ||
| Geometric mean (geometric CV%) |
|||
|---|---|---|---|
| CLss/F (L/h) | Fed (T1) | 13.2 (32.6)b | – |
| Fasted (T2) | 12.3 (34.9)b | ||
| V ss/F (L) | Fed (T1) | 87.6 (37.9)c | – |
| Fasted (T2) | 74.6 (38.7)b | ||
| Median (range) | Estimated median difference (2-sided 90% CI) |
||
|---|---|---|---|
| T max (h) | Fed (T1) | 2.00 (0.92, 3.00)b | 0.57 (0.46, 1.00) |
| Fasted (T2) | 1.00 (0.55, 2.07)b |
aClinically implausible high concentrations of selumetinib were reported for 3 participants at 12 h post-dose in the fasted treatment period. The plasma concentration–time profiles for each participant from the last timepoint with a measurable concentration (8 h) to 12 h post-dose to estimate the AUC0–12,SS values were extrapolated. As the percentage extrapolation was small, and to maximize the data used in the statistical assessment of food effect, the AUC0–12,ss values for these 3 participant's T2 selumetinib profiles were considered to be representative of the primary endpoint parameter and, therefore, were included in the statistical analysis; bn = 21; cn = 20.
AUC0–12,ss, area under the concentration–time curve from time zero to 12 h at steady state; AUClast, area under the concentration–time curve from time zero to time of last quantifiable concentration; CI, confidence interval; CLss/F, apparent total clearance of drug from plasma after extravascular administration; Cmax, maximum observed plasma drug concentration; CV, coefficient of variation; DCO, data cut-off; LS, least squares; PK, pharmacokinetic; Tmax, time to reach peak or maximum observed concentration; Vss/F, apparent volume of distribution at steady state following extravascular administration.
Figure 2.
Geometric mean plasma concentrations of selumetinib versus time—multiple dose (A. Linear; B. Semi-logarithmic).a
aClinically implausible high concentrations of selumetinib were reported for 3 participants at 12 h post-dose in the fasted treatment period. The plasma concentration–time profiles for each participant from the last timepoint with a measurable concentration (8 h) to 12 h post-dose to estimate the AUC0–12,SS values were extrapolated. As the percentage extrapolation was small, and to maximize the data used in the statistical assessment of food effect, the AUC0–12,ss values for these 3 participants' T2 selumetinib profiles were considered to be representative of the primary endpoint parameter and, therefore, were included in the statistical analysis. AUC0–12,ss, area under the concentration–time curve from time zero to 12 h at steady state.
Of the 24 participants included in the PK analysis set, 19 (79.2%) were eligible for the PK statistical comparison analysis, having been compliant with the study protocol and provided evaluable PK data including the last required PK sample in both the fed and fasted treatment periods. The reasons for exclusion of 5 participants from the PK statistical comparison analysis have been included in the Supplementary Material.
There were no notable differences in AUC0–12,SS and AUClast for selumetinib in the fed and fasted treatment periods, with GMRs of 0.919 and 0.931, respectively. The lower bounds of the 1-sided 90% CI for both parameters were above 0.7 (AUC0–12,SS 0.841, AUClast 0.850), and therefore within the 30% threshold for a clinically relevant change (Table 2). The GMRs for AUC0−12,ss and AUClast for N-desmethyl selumetinib were 1.008 and 1.022; the lower bounds of the 1-sided 90% CI for both parameters were above 0.9 (AUC0−12,ss 0.910, AUClast 0.918) (Supplementary Table 1).
For selumetinib, the GMR for Cmax was 0.763, with a lower bound of the 1-sided 90% CI of 0.667 (Table 2). For N-desmethyl selumetinib, the GMR for Cmax was 0.839, with a lower bound of the 1-sided 90% CI of 0.723 (Supplementary Table 1). The median differences in Tmax between the fed and fasted treatment periods indicated that there was a delay in the Tmax for selumetinib of 0.57 h (90% CI: 0.46, 1.00) (Table 2). For N-desmethyl selumetinib, the median differences in Tmax between the fed and fasted treatment periods indicated that there was a delay in the Tmax of 0.49 h (90% CI: 0.44, 1.00) (Supplementary Table 1).
Clinically implausible high concentrations of selumetinib were reported for 3 participants at 12 h post-dose in the fasted treatment period, with values for selumetinib between 11- and 16-fold higher than at 8 h post-dose concentrations. The corresponding values for N-desmethyl selumetinib were between 7- and 10-fold higher than concentrations at 8 h post-dose. These anomalous concentrations could not be attributed to bioanalytical issues, nor was there any correspondence available from the study site to explain these values. These concentrations, for both selumetinib and N-desmethyl selumetinib, were excluded from PK parameter calculations, as well as concentration summary tables and figures. WinNonlin® software was used to extrapolate the plasma concentration–time profile from the last timepoint with a measurable concentration (8 h) to 12 h post-dose to estimate the AUC0–12,SS value. The resultant extrapolated areas from AUClast ranged from approximately 6% to 11%. As the percentage extrapolation was small, and to maximize the data used in the statistical assessment of food effect, the AUC0–12,ss values for these 3 participants’ T2 selumetinib profiles were considered to be representative of the primary endpoint parameter and, therefore, were included in the statistical analysis. A sensitivity analysis was performed where the statistical analysis that compared fed versus fasted PK parameters was conducted with these 3 participants excluded. This analysis showed that exclusion of these participants had no impact on the overall conclusion regarding a lack of food effect.
Exposure
In the fed and fasted treatment periods, the mean (SD) total (intended treatment length) days of exposure were 28.3 (1.12) and 28.3 (3.03), respectively. The mean (SD) actual (total intended minus dose interruptions) days of exposure were 28.3 (1.12) and 28.3 (3.03) in the fed and fasted treatment periods, respectively.
GI AEs
GI AEs for the fed treatment period plus washout and the fasted treatment period plus extension are included in Supplementary Table 2.
The incidence of GI AEs was similar in both treatment periods (Table 3). Seven participants (29.2%) in the fed treatment period reported any GI AE; 3 of these participants also reported any GI AE in the fasted treatment period. In the fasted treatment period, 8 participants (33.3%) reported at least 1 GI AE. No Grade ≥ 3 GI AEs were reported over the course of the study. There were no serious GI AEs or GI AEs resulting in treatment interruptions, discontinuation, or dose reduction.
Table 3.
Summary of AEs reported in fed (T1) and fasted (T2) treatment periods at primary DCO (safety analysis set)
| Participants with AE, n (%)a | Fed treatment period (T1; N = 24) |
Fasted treatment period (T2; N = 24) |
|---|---|---|
| Any AE | 19 (79.2) | 14 (58.3) |
| Any AE of CTCAE Grade 3 or higher | 0 | 0 |
| Any AE resulting in death | 0 | 0 |
| Any SAE (including events with an outcome of death) | 0 | 0 |
| Any AE leading to discontinuation | 0 | 0 |
| Any AE leading to interruption | 1 (4.2)b | 0 |
| Any AE leading to dose reduction | 1 (4.2)c | 0 |
| Any GI AE | 7 (29.2) | 8 (33.3) |
| Vomiting | 3 (12.5) | 3 (12.5) |
| Nausea | 2 (8.3) | 3 (12.5) |
| Diarrhea | 2 (8.3) | 1 (4.2) |
| Abdominal pain | 1 (4.2) | 1 (4.2) |
| Abdominal pain upper | 0 | 1 (4.2) |
| Constipation | 0 | 1 (4.2) |
| Dry mouth | 0 | 1 (4.2) |
| Stomatitis | 0 | 1 (4.2) |
| Non-GI AE d | ||
| Skin and subcutaneous lesions | 16 (66.7) | 6 (25.0) |
| Dermatitis acneiform | 12 (50.0) | 4 (16.7) |
| Alopecia | 2 (8.3) | 0 |
| Infections and infestations | 3 (12.5) | 2 (8.3) |
| COVID-19 | 1 (4.2) | 2 (8.3) |
| Any AE possibly related to treatmente | 16 (66.7) | 8 (33.3) |
| Skin and subcutaneous tissue lesions | 14 (58.3) | 4 (16.7) |
| Dermatitis acneiform | 10 (41.7) | 3 (12.5) |
| Acne | 1 (4.2) | 1 (4.2) |
| Alopecia | 2 (8.3) | 0 |
| Pruritis | 1 (4.2) | 0 |
| Rash | 1 (4.2) | 0 |
| Rash maculo-papular | 1 (4.2) | 0 |
| GI disorders | 4 (16.7) | 3 (12.5) |
| Nausea | 2 (8.3) | 2 (8.3) |
| Abdominal pain | 1 (4.2) | 1 (4.2) |
| Abdominal pain upper | 0 | 1 (4.2) |
| Diarrhea | 1 (4.2) | 0 |
| Dry mouth | 0 | 1 (4.2) |
| Vomiting | 1 (4.2) | 0 |
| Infections and infestations | 2 (8.3) | 1 (4.2) |
| Paronychia | 1 (4.2) | 1 (4.2) |
| Folliculitis | 1 (4.2) | 0 |
| Tinea pedis | 1 (4.2) | 0 |
| Investigations | 0 | 2 (8.3) |
| Blood creatine phosphokinase increased | 0 | 1 (4.2) |
| Ejection fraction decreased | 0 | 1 (4.2) |
| Musculoskeletal and connective tissue disorders | 1 (4.2) | 0 |
| Soft tissue swelling | 1 (4.2) | 0 |
AEs for the fed treatment period plus washout and the fasted treatment period plus fasted treatment period extension have been included in Supplementary Table S2.
aParticipants who reported multiple events in the same category were only counted once in that category, and participants with events in more than 1 category were counted once in each of those categories; bThis dose interruption was attributed to the onset of Grade 2 dermatitis acneiform during the fed state treatment period, and the onset of Grade 2 alopecia during the fasted treatment period extension; the dose interruption itself occurred during the fasted treatment period extension; cThe dose reduction occurred on Day 16 of the fed state treatment period, due to the onset of dermatitis acneiform; dOccurring in more than 5% of participants in either treatment period; eAs assessed by investigator.
AE, adverse event; COVID-19, coronavirus disease 2019; CTCAE, Common Terminology Criteria for Adverse Events; DCO, data cut-off; GI, gastrointestinal; SAE, serious adverse event.
The most frequently occurring GI AE experienced in both the fed and fasted treatment periods was vomiting (12.5%, 3 participants each). Nausea was reported by 2 participants (8.3%) and 3 participants (12.5%) in the fed and fasted treatment periods, respectively. In the fed treatment period, 2 participants (8.3%) experienced diarrhea; 1 participant (4.2%) reported diarrhea in the fasted treatment period. Upper abdominal pain, constipation, dry mouth, and stomatitis were all reported by 1 participant (4.2%) in the fasted treatment period; there were no reports of these AEs in the fed treatment period. Abdominal pain was reported by 1 participant (4.2%) in each treatment period.
Participant-Reported GI Outcomes
In both the fed and fasted treatment periods, the most commonly reported mBSFS-C type was 3 (Table 4).
Table 4.
GI PROs in the fed (T1) and fasted (T2) treatment periods at primary DCO (safety analysis set)
| PRO n (%) |
Fed treatment period (T1; N = 24) |
Fasted treatment period (T2; N = 24) |
|---|---|---|
| Type of mBSFS-Ca | ||
| Type 1 | 12 (50.0) | 8 (33.3) |
| Type 2 | 15 (62.5) | 14 (58.3) |
| Type 3 | 19 (79.2) | 19 (79.2) |
| Type 4 | 13 (54.2) | 9 (37.5) |
| Type 5 | 6 (25.0) | 7 (29.2) |
| Nausea | ||
| No | 24 (100.0) | 23 (95.8) |
| Yes, able to eat ok | 12 (50.0) | 9 (37.5) |
| Yes, able to eat but not as normal | 6 (25.0) | 8 (33.3) |
| Yes, I couldn’t eat but I could drink | 0 | 2 (8.3) |
| Yes, I couldn’t eat or drink | 0 | 0 |
| Vomiting | ||
| None | 24 (100.0) | 23 (95.8) |
| 1 vomit in 24 h | 7 (29.2) | 5 (20.8) |
| 2 to 5 vomits in 24 h | 2 (8.3) | 2 (8.3) |
| 6 to 10 vomits in 24 h | 0 | 0 |
| > 10 vomits in 24 h | 0 | 0 |
Percentages in each arm may not total 100% as it was possible for participants to report more than 1 symptom at different timepoints throughout the treatment period.
aFor each participant, average bowel movements per day in an mBSFS type was calculated by the total number of bowel movements in that mBSFS-C type recorded in his/her diary in the period divided by actual total number of diary days of the participant in the treatment period. Results > 9 are imputed as 9 for analyses.
AE, adverse event; DCO, data cut-off; GI, gastrointestinal; mBSFS-C, modified Bristol Stool Form Scale; PRO, participant-reported outcome.
Based on diary entries, no participants reported nausea that prevented them from eating and drinking concurrently in either the fed or fasted treatment period (Table 4). There were 2 participants (8.3%) in each treatment period who reported 2 to 5 episodes of vomiting over a 24-h period (Table 4).
Use of GI Medications
No GI medications were initiated by any participant to treat GI-related AEs in the fed treatment period. Two participants (8.3%) initiated medication to treat GI-related AEs in the fasted treatment period. One participant received macrogol (for 3 days from Day 51 of the study), to treat Grade 1 constipation. The other participant received almagate (for 7 days from Day 44 of the study) and esomeprazole (for 5 days from Day 50 of the study) to treat Grade 2 epigastric pain.
Non-GI AEs
Table 3 presents non-GI AEs reported by at least 5% of participants in either treatment period. AEs for the fed treatment period plus washout and the fasted treatment period plus extension are presented in Supplementary Table 2.
Skin and subcutaneous lesions were reported in 16 participants (66.7%) and 6 participants (25.0%) in the fed and fasted treatment periods, respectively, and were also the most commonly reported AEs categorized as possibly related to selumetinib treatment (fed: 14 participants [58.3%]; fasted: 4 participants [16.7%]; Table 3). For most patients who experienced dermatitis acneiform, this AE emerged in the fed treatment period and continued through the fasted treatment period and into the fasted treatment period extension.
Although there were no reports of alopecia in the fasted treatment period, 2 participants (8.3%) reported alopecia in the fed treatment period. Two participants (8.3%) in the fed treatment period and 1 participant (4.2%) in the fasted treatment period reported infections and infestations that were possibly related to treatment.
All AEs
Table 3 describes AEs, serious AEs, treatment-related AEs, and AEs that led to dose interruptions and reductions for the fed and fasted treatment periods. A summary of AEs occurring during the fed treatment period plus washout and fasted treatment period plus extension are presented in Supplementary Table 2. No new safety signals were observed in this study, and no clinically important changes were noted in laboratory values or vital signs.
Of the 24 participants evaluable for safety, 19 (79.2%) and 14 (58.3%) reported at least 1 AE in the fed and fasted treatment periods, respectively; none of these were Grade ≥ 3. In the fed and fasted treatment periods, 16 participants (66.7%) and 8 participants (33.3%), respectively, had at least 1 AE possibly related to study treatment.
No AE resulted in discontinuation of study treatment or death.
One participant experienced interruption of selumetinib treatment in the fasted treatment period extension due to Grade 2 dermatitis acneiform (onset during the fed treatment period; Day 13 of the study) and Grade 2 alopecia (onset during fasted treatment period extension; Day 96 of the study). Both AEs led to prolonged interruption of > 7 days, and the AE of alopecia led to a dose reduction.
Another participant experienced prolonged dose interruption because of a Grade 3 rash (onset during fasted treatment extension period; Day 78 of study). This was the only Grade ≥ 3 AE reported at primary DCO.
One participant (4.2%) experienced Grade 2 dermatitis acneiform (Day 8), which resulted in a dose reduction during the fed treatment period (Day 16 of the study). The participant’s dose was reduced from 35 mg BID to 25 mg BID, which remained the same until the end of the fed treatment period. The participant continued receiving 25 mg selumetinib BID throughout the fasted treatment period but did not complete this treatment period, and they were moved to the fasted treatment period extension on Day 50 of the study.
Discussion
Until recently, selumetinib was approved for administration in the fasted state based on previous studies showing potential differences in PK parameters between fed and fasted states, as well as differences in the incidence of GI AEs.3,4,16–18 This study evaluated the effects of a low-fat meal on the steady-state exposure and GI tolerability of selumetinib in adolescents with NF1 and inoperable PN. By the primary DCO for this study, administration of selumetinib in the fed state compared with the fasted state had no clinically relevant effect on PK parameters. Although taking selumetinib with a low-fat meal slightly reduced the rate of absorption (estimated median difference [2-sided 90% CI] for Tmax, 0.57 [0.46, 1.00]; GMR [1-sided 90% CI] for Cmax, 0.763 [0.667]), it did not affect the overall extent of absorption, as the AUC0–12,ss was only slightly lower in the fed treatment period compared with the fasted treatment period (GMR [1-sided 90% CI] for AUC0–12,ss was 0.919 [0.841]). As the lower bound of the 90% CI of the GMR was > 0.7, no clinically relevant effect of a low-fat meal on selumetinib AUC0–12,ss and AUClast was observed. Overall, as no clinically relevant difference in AUC0–12,ss between the fed and fasted treatment periods was observed, T3 was not initiated (Figure 1). The extrapolated AUC0–12,SS values from the T2 selumetinib profiles of the 3 participants with clinically high concentrations at 12 h post-dose were considered to be representative of the primary endpoint parameter. Similar conclusions on the effect of a low-fat meal on the PK parameters for N-desmethyl selumetinib were drawn from the selumetinib data obtained here. The concentration of metabolite was low, and it was excreted quickly. Therefore, although N-desmethyl selumetinib has a 3- to 5-fold greater potency for MEK1 inhibition than selumetinib, due to the lower systemic exposure in relation to selumetinib, the metabolite was not expected to contribute to the overall activity of selumetinib in patients with NF1-PN.26
Although previous studies evaluated the potential effect of food on selumetinib PK parameters, there were differences that included the evaluation of PK after a single dose, and study populations that were either healthy volunteers or adult patients who had advanced solid tumors.16–18 A recent pooled population PK analysis that included data from 15 clinical studies also demonstrated that selumetinib administration with a low-fat meal did not have a clinically relevant effect on selumetinib exposure. The same analysis demonstrated that selumetinib administration with a high-fat meal did not significantly affect selumetinib exposure, suggesting that these PK results were unlikely to be dependent on the fat content of the food when taken together with selumetinib; however, the population PK analysis did not assess the effect of food on GI tolerability.27
Data presented here demonstrate that administration of selumetinib capsules with a low-fat meal compared with administration in the fasted state showed no clinically relevant difference in terms of the occurrence of GI and non-GI AEs. Previous studies have indicated that AEs affecting the GI system are among the most common AEs associated with selumetinib treatment.11,19,28 In our study, a similar number of participants reported at least 1 GI AE in the fed and fasted treatment periods by the primary DCO. The GI AEs reported in this study were generally consistent with those commonly associated with selumetinib treatment3; for example, vomiting, nausea, diarrhea, and abdominal pain were commonly reported in both the fed and fasted treatment periods.
Although 14 participants (58.3%) reported at least 1 GI AE from the initiation of selumetinib treatment, including the washout period and the fasted treatment period extension, skin and subcutaneous AEs were the most commonly reported type of AE occurring in 20 participants (83.3%) (Supplementary Table 2). Dermatitis acneiform was the most commonly reported non-GI AE, and this AE has also been frequently reported in previous selumetinib studies.11,16,18,29 No new safety signals for selumetinib were identified; the AEs reported were similar in both the fed and fasted treatment periods and were comparable to previous studies.11,17
Given that previous PK analyses had indicated a flat PK–efficacy relationship, efficacy was not assessed as an endpoint in this study. By restricting this study to adolescents, the range of BSA bands in the cohort was reduced, thereby limiting any variability in dose levels. The fixed sequential treatment periods meant that it is not possible to rule out any potential sequencing effect. However, it would have made the study more complex for the young participants to comply with first taking selumetinib in the fasted state for 28 days, then switching to taking it with a light meal for another 28 days, before switching back to the fasting posology again. Furthermore, it would have required a greater number of participants to be accrued from adolescent patients in a rare disease population. Another limitation is that the AE collection method may have confounded safety results for the overall study (including washout and fasted treatment period extension). It should also be noted that blood PK measurements were only taken from each participant once in each treatment period; this was a patient-centric decision to ensure that participants and their caregivers, who often had to travel long distances to attend a specialist center for blood measurements, were not required to take extended periods out of school and work, thereby reducing any burden on them.
Based on results from previous studies,11,16,30 the initial approved label stated that selumetinib must be taken BID on an empty stomach with no food or drink 2 h before dosing and 1 h after dosing. However, results from the primary DCO of this study show that multiple dosing of selumetinib with a low-fat meal has no clinically relevant impact on selumetinib exposure or GI tolerability in adolescents with NF1-PN. Based on the contribution of these data, patients can now take selumetinib with or without food,3,4 without compromising PK or GI tolerability. Flexibility in dosing with respect to food could improve adherence to treatment, as fasting can be burdensome for young patients.
Supplementary Material
Supplementary material is available online at Neuro-Oncology (https://academic.oup.com/neuro-oncology).
Acknowledgments
The authors would like to thank the participants, their families, and the study investigators. We thank Anna Rigazio (Alexion, AstraZeneca Rare Disease) for her quality check and review of the manuscript. We also thank Million Arefayene (Alexion, AstraZeneca Rare Disease) for his review of the manuscript.
Contributor Information
David Viskochil, Department of Pediatrics, University of Utah, Salt Lake City, Utah, USA.
Mariusz Wysocki, Department of Pediatric Hematology and Oncology, Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University Torun, Jurasz University Hospital 1, Bydgoszcz, Poland.
Maria Learoyd, Clinical Pharmacology & Safety Sciences, AstraZeneca, Cambridge, UK.
Peng Sun, Alexion, AstraZeneca Rare Disease Clinical Development, NF and Bone Metabolism Therapeutic Area, Cambridge, UK.
Karen So, Alexion, AstraZeneca Rare Disease Clinical Development, NF and Bone Metabolism Therapeutic Area, Cambridge, UK.
Azura Evans, Alexion, AstraZeneca Rare Disease Clinical Development, NF and Bone Metabolism Therapeutic Area, Cambridge, UK.
Francis Lai, Quantitative Sciences, Alexion, AstraZeneca Rare Disease, Boston, Massachusetts, USA.
Héctor Salvador Hernàndez, Department of Pediatric Oncology and Hematology, Sant Joan de Déu Barcelona Hospital, Barcelona, Spain.
Funding
This study was funded by AstraZeneca; part of an alliance between AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (M.S.D.). Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Connie Feyerherm, MSci of OPEN Health Communications (London, UK), funded by Alexion, AstraZeneca Rare Disease.
Conflict of interest statement
D.V. received advisory support from AstraZeneca, grants or contracts from SpringWorks Therapeutics, Soleno Therapeutics, Levo Therapeutics, NFlection Therapeutics, and Takeda Pharmaceuticals; consulting fees from Sanofi Genzyme as well as payment or honoraria from SpringWorks Therapeutics and AstraZeneca. M.W. reports no conflicts of interest. H.S.H. has received advisory fees from AstraZeneca. M.L., P.S., K.S., and A.E. report employment at AstraZeneca. A.E., M.L., and K.S. own AstraZeneca stocks. F.L. owns AstraZeneca and AbbVie stocks.
Authorship statement
Conceptualization: M.W., M.L., P.S., K.S., A.E., and F.L.; resources: M.W., M.L., K.S., P.S., A.E., and F.L.; data curation: D.V., M.W., M.L., P.S., K.S., A.E., and F.L.; software: F.L.; formal analysis: D.V., M.L., P.S., K.S., A.E., and F.L.; supervision: M.W., M.L., K.S., P.S., A.E., H.S.H.; validation: M.W., M.L., P.S., K.S., A.E., and F.L.; investigation: D.V., M.W., M.L., P.S., A.E., and H.S.H.; visualization: M.W., M.L., P.S., K.S., A.E., F.L., and H.S.H.; methodology: M.L, P.S., K.S., A.E., and F.L.; writing—original draft: M.W., M.L., P.S., K.S., A.E., and F.L.; writing—review and editing: D.V., M.W., M.L., P.S., K.S., A.E., F.L., and H.S.H.
References
- 1. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13(8):834–843. [DOI] [PubMed] [Google Scholar]
- 2. Blakeley JO, Plotkin SR. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro Oncol. 2016;18(5):624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexion. Selumetinib (Koselugo) Full Prescribing Information. Available at https://alexion.com/Documents/koselugo_uspi.pdf. Accessed February 20, 2024.
- 4. European Medicines Agency. Koselugo. Available at https://www.ema.europa.eu/en/documents/product-information/koselugo-epar-product-information_en.pdf. Accessed February 20, 2024.
- 5. Bergqvist C, Servy A, Valeyrie-Allanore L, et al. ; NF France Network. Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet J Rare Dis. 2020;15(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tonsgard JH. Clinical manifestations and management of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13(1):2–7. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen R, Ibrahim C, Friedrich RE, et al. Growth behavior of plexiform neurofibromas after surgery. Genet Med. 2013;15(9):691–697. [DOI] [PubMed] [Google Scholar]
- 8. Azizi AA. Management and multi-specialty approach in the evolving treatment landscape of neurofibromatosis type 1 plexiform neurofibromas. EMJ. 2021;6(4):32–35. [Google Scholar]
- 9. Yeh TC, Marsh V, Bernat BA, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13(5):1576–1583. [DOI] [PubMed] [Google Scholar]
- 10. Jessen WJ, Miller SJ, Jousma E, et al. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest. 2013;123(1):340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020;382(15):1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. AstraZeneca. Koselugo (selumetinib) approved in US for paediatric patients with neurofibromatosis type 1 plexiform neurofibromas. Available at https://www.astrazeneca.com/media-centre/press-releases/2020/koselugo-selumetinib-approved-in-us-for-paediatric-patients-with-neurofibromatosis-type-1-plexiform-neurofibromas.html#. Accessed February 20, 2024.
- 13. AstraZeneca. Koselugo approved in China for paediatric patients with neurofibromatosis type 1 and plexiform neurofibromas. Available at https://www.astrazeneca.com/media-centre/press-releases/2023/koselugo-approved-in-china-for-paediatric-patients-with-neurofibromatosis-type-1-and-plexiform-neurofibromas.html. Accessed February 20, 2024.
- 14. AstraZeneca. Koselugo approved in Japan for paediatric patients with plexiform neurofibromas in neurofibromatosis type 1. Available at https://www.astrazeneca.com/media-centre/press-releases/2022/koselugo-approved-in-japan-for-paediatric-patients-with-plexiform-neurofibromas.html. Accessed February 20, 2024.
- 15. AstraZeneca. Koselugo approved in the EU for children with neurofibromatosis type 1 and plexiform neurofibromas. Available at https://www.astrazeneca.com/media-centre/press-releases/2021/koselugo-approved-in-the-eu-for-children-with-neurofibromatosis-type-1-and-plexiform-neurofibromas.html#. Accessed February 20, 2024.
- 16. Tomkinson H, McBride E, Martin P, et al. Comparison of the pharmacokinetics of the phase II and phase III capsule formulations of selumetinib and the effects of food on exposure: results from two randomized crossover trials in healthy male subjects. Clin Ther. 2017;39(11):2260–2275.e1. [DOI] [PubMed] [Google Scholar]
- 17. Leijen S, Soetekouw PM, Jeffry Evans TR, et al. A phase I, open-label, randomized crossover study to assess the effect of dosing of the MEK 1/2 inhibitor selumetinib (AZD6244; ARRY-142866) in the presence and absence of food in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;68(6):1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen-Rabbie S, Mattinson A, So K, Wang N, Goldwater R. Effect of food on capsule and granule formulations of selumetinib. Clin Transl Sci. 2022;15(4):878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gross AM, Glassberg B, Wolters PL, et al. Selumetinib in children with neurofibromatosis type 1 and asymptomatic inoperable plexiform neurofibroma at risk for developing tumor-related morbidity. Neuro Oncol. 2022;24(11):1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drugs.com. Koselugo dosage. Available at https://www.drugs.com/dosage/koselugo.html. Accessed February 21, 2024.
- 21. Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45(5):575–578. [PubMed] [Google Scholar]
- 22. WHODrug Global B3-format Sep 2021. [Google Scholar]
- 23. Lane MM, Czyzewski DI, Chumpitazi BP, Shulman RJ. Reliability and validity of a modified Bristol Stool Form Scale for children. J Pediatr. 2011;159(3):437–441.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Children’s Cancer and Leukaemia Group. Chemotherapy induced nausea and vomiting. Available at https://www.cclg.org.uk/csoir/chemotherapy-induced-nausea-and-vomiting. Accessed February 20, 2024.
- 25. ClinicalTrials.gov. Phase I study to assess the effect of food on the PK and gastrointestinal tolerability of selumetinib in adolescent children with neurofibromatosis type 1 related plexiform neurofibromas. Available at https://classic.clinicaltrials.gov/ct2/show/NCT05101148. Accessed February 20, 2024.
- 26. Schalkwijk S, Zhou L, Cohen-Rabbie S, et al. Population pharmacokinetics and exposure-response of selumetinib and its N-desmethyl metabolite in pediatric patients with neurofibromatosis type 1 and inoperable plexiform neurofibromas. Cancer Chemother Pharmacol. 2021;88(2):189–202. [DOI] [PubMed] [Google Scholar]
- 27. Arefayene M, Zuo P. A population pharmacokinetic assessment of the effect of food on selumetinib in patients with neurofibromatosis type 1-related plexiform neurofibromas and healthy volunteers. Clin Pharmacol Drug Dev. Published online April 9, 2024; doi: 10.1002/cpdd.1400. [DOI] [PubMed] [Google Scholar]
- 28. Baldo F, Magnolato A, Barbi E, Bruno I. Selumetinib side effects in children treated for plexiform neurofibromas: first case reports of peripheral edema and hair color change. BMC Pediatr. 2021;21(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balagula Y, Barth Huston K, Busam KJ, et al. Dermatologic side effects associated with the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886). Invest New Drugs. 2011;29(5):1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dymond AW, Howes C, Pattison C, et al. Metabolism, excretion, and pharmacokinetics of selumetinib, an MEK1/2 inhibitor, in healthy adult male subjects. Clin Ther. 2016;38(11):2447–2458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.