Abstract
As the incidence of hepatocellular carcinoma (HCC) and subsequent treatments with liver-directed therapies rise, the complexity of assessing lesion response has also increased. The Liver Imaging Reporting and Data Systems (LI-RADS) treatment response algorithm (LI-RADS TRA) was created to standardize assessment of response after locoregional therapy (LRT) on contrast-enhanced CT or MRI. Originally created based on expert opinion, these guidelines are currently undergoing revision based on emerging evidence. While many studies support the use of LR-TRA for evaluation of HCC response after thermal ablation and intra-arterial embolic therapy, data suggests a need for refinements to improve assessment after radiation therapy. In this manuscript, we review expected MR imaging findings after different forms of LRT, clarify how to apply the current LI-RADS TRA by type of LRT, explore emerging literature on LI-RADS TRA, and highlight future updates to the algorithm.
Keywords: Hepatocellular Carcinoma, Locoregional therapy, Liver Imaging Data and Reporting System treatment response algorithm, treatment response assessment, LI-RADS TRA, LR-TR
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading causes of cancer-related deaths worldwide [1]. Management of HCC is variable, and while hepatic transplant offers the best outcomes, limitations include organ availability and other patient-related factors (eg. tumor stage and non-candidates for transplant). Other treatment options for HCC include surgical resection and minimally invasive locoregional therapies (LRT) including ablation, intra-arterial embolic therapy, radiation therapy, and systemic chemotherapy or immunotherapy. These can also be used for curative intent, palliative intent, or bridging and downstaging to transplant[2]. Given the correlation between treatment response and patient prognosis, it is essential to provide an accurate response assessment after treatment to help guide clinical management[3].
Imaging surveillance after HCC treatment is usually performed every 3 months with multiphasic contrast-enhanced CT or MRI [4–6]. The LI-RADS treatment response algorithm (LI-RADS TRA) was created in 2017 based largely on expert opinion to provide a standardized approach for treatment response assessment and to help guide clinical management. Emerging evidence on the validity of the LI-RADS TRA continues to accumulate, with several new publications reporting the diagnostic performance of the algorithm in predicting tumor viability on pathology, its correlation with patient survival, and inter-reader agreement when using LI-RADS TRA. In this review, we highlight expected MR imaging findings after different forms of LRT, clarify how to apply the current LI-RADS TRA based on the type of LRT, provide an update on the current LI-RADS TRA, highlight strengths and weaknesses of MRI for TRA after LRT for HCC, and suggest updates to improve future versions of the algorithm.
Review of LI-RADS TRA
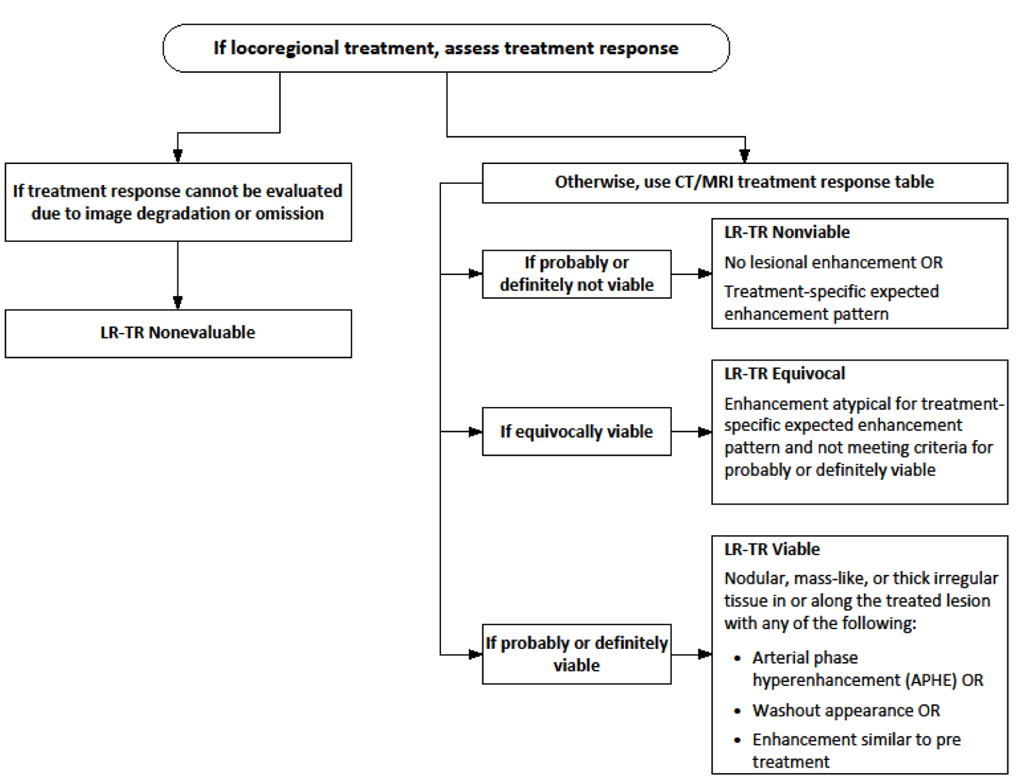
The LI-RADS TRA provides a standardized approach for image acquisition, interpretation, reporting, and data collection for HCC treated with LRT. It is unique in providing a lesion-level assessment of treated observations while other treatment response systems provide a patient-level assessment (mRECIST, EASL). This is important for this high-risk patient population in which metachronous disease is common, particularly when patients may undergo several different types of LRT over time, based on patient factors, stage of disease, and tumor factors like size and location. Each treated lesion must be evaluated for treatment response to best assess tumor burden for the purposes of bridging and downstaging for liver transplant.
Currently, treatment response assessment is performed by evaluating treated lesions with multiphasic contrast-enhanced CT or MRI with either extracellular or hepatobiliary contrast agents. The key imaging feature used to categorize treatment response is the presence or absence of masslike arterial phase hyperenhancement (APHE). Based on the LI-RADS TRA, additional imaging features used for response assessment include washout and enhancement similar to pre-treatment[7]. [Figure 1] Regardless of the type of LRT used, LI-RADS TRA v2018 categorizes lesions as LI-RADS Treatment Response (LR-TR) Viable, Nonviable, or Equivocal. By strict application of LI-RADS criteria, lack of enhancement of the treated lesion is compatible with Nonviable categorization. However, the imaging appearances of treated HCC vary depending on the type of LRT performed, and it is therefore essential for the radiologist to understand not only the expected post-therapy imaging appearances after each type of LRT, but also the type of treatment each lesion has received in order to accurately characterize treated versus nontreated disease and ensure appropriate patient management.
Figure 1:
LI-RADS Treatment Response Algorithm
Expected Imaging appearance of HCC treated with LRT
Broadly speaking, LRT can be divided into two main groups when assessing treatment response: non-radiation and radiation-based therapies. [Table 2] Non-radiation therapies include thermal ablation (ie., radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation), and intra-arterial embolic therapy (ie., transarterial embolization (TAE), conventional trans-arterial chemoembolization (cTACE), or drug eluting bead TACE (DEB-TACE))[8]. Radiation-based therapies include stereotactic body radiotherapy (SBRT) and transarterial radioembolization (TARE). To accurately assess the treatment response of HCC, radiologists must be aware that HCC appears different after treatment with non-radiation-based therapies compared to radiation-based therapies, due to differences in pathophysiology of cell death between the different treatment modalities. In the first group, cell death is achieved by coagulation necrosis from thermal destruction or ischemic and/or cytotoxic mechanisms from arterial embolic therapies. On the other hand, radiation-induced cell death can occur via one of two pathways: direct DNA damage resulting in immediate apoptosis and cell death, or indirect damage to the DNA resulting in cellular senescence [9, 10]. In the second pathway, cellular senescence results in metabolically active cells without the capacity to replicate, and thus the cells eventually die without tumor progression [9, 10]. Thus, the imaging appearance of irradiated HCC will often differ from other more conventional forms of LRT, as the senescent cells remain metabolically active and maintain vascular perfusion, albeit are ‘dead’ in terms of clonogenicity, as they cannot replicate and will eventually undergo apoptosis[9, 10].
Table 2:
Summary of imaging findings after LRT of HCC
| Expected post-treatment imaging appearance of successfully treated HCC | Imaging features suggestive of Viable Category | |
|---|---|---|
| Thermal Ablation (Figure 2 and 3) | • No intralesional enhancement • Smooth perilesonal rim of enhancement and/or parenchymal perfusional changes without masslike area(s) of enhancement |
Irregular masslike enhancement along the margin or intralesional (any degree, any phase) |
| Intra-arterial embolic therapy (TAE, cTACE, DEB-TACE) (Figure 4) | • No intralesional enhancement • Smooth perilesional rim of enhancement and/or parenchymal perfusional changes without masslike area(s) of enhancement |
Irregular masslike enhancement along the margin or intralesional (any degree, any phase) |
| SBRT (Figure 5) | • No intralesional enhancement • Smooth or geographic parenchymal perfusional changes without masslike area(s) of enhancement • Irregular intralesional masslike enhancement (any degree, any phase) any time after treatment AND definite stability or decrease in size and/or degree of intralesional enhancement |
Irregular intralesional masslike enhancement (any degree, any phase) at any time after treatment with new or increased size or degree of intralesional enhancing component(s) |
| TARE/y90 (Figure 6) | • No intralesional enhancement • Smooth or geographic parenchymal perfusional changes without masslike area(s) of enhancement • Irregular intralesional masslike enhancement (any degree, any phase) any time after treatment AND definite stability or decrease in size and/or degree of intralesional enhancement |
Irregular intralesional masslike enhancement (any degree, any phase) at any time after treatment with new or increased size or degree of intralesional enhancing component(s) |
Expected imaging appearance of non-radiation treated HCC
After RFA, MWA, or cryoablation, tumor necrosis is instantly achieved, resulting in loss of intralesional perfusion and enhancement, which would be categorized as LR-TR Nonviable. [Figure 2] Other key imaging features expected after these treatment strategies include: a thin continuous rim of smooth peripheral enhancement secondary to inflammation[11], geographic APHE within the parenchyma surrounding the treatment zone which can be isoenhancing or hyperenhancing on portal venous and delayed phases of imaging [12], and coagulative necrosis centrally within the treated lesions resulting in hyperintense signal on pre-contrast T1-weighted images [5, 11]. Subtraction images may be used to confirm a lack of central enhancement, particularly when coagulative necrosis is present. [Figure 2] The presence of these key imaging findings allow categorization of the treated tumor as LR-TR Nonviable according to LI-RADS TRA v2018.
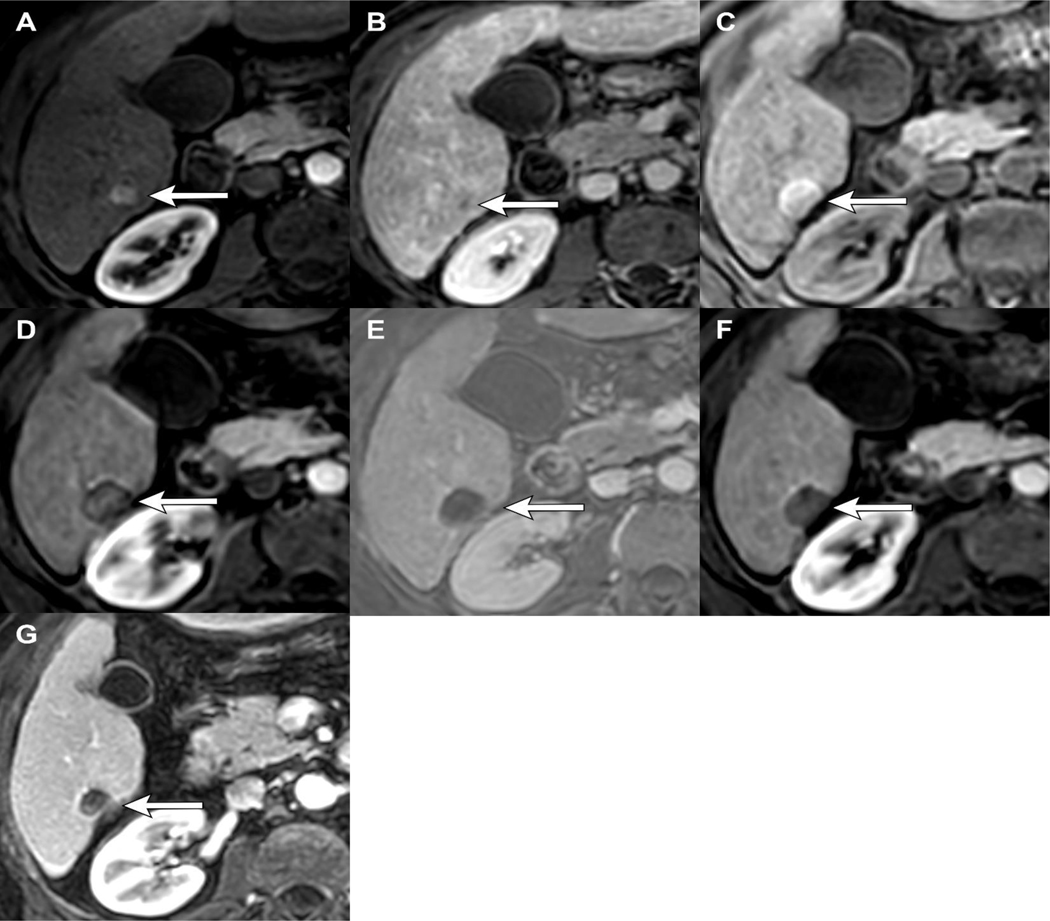
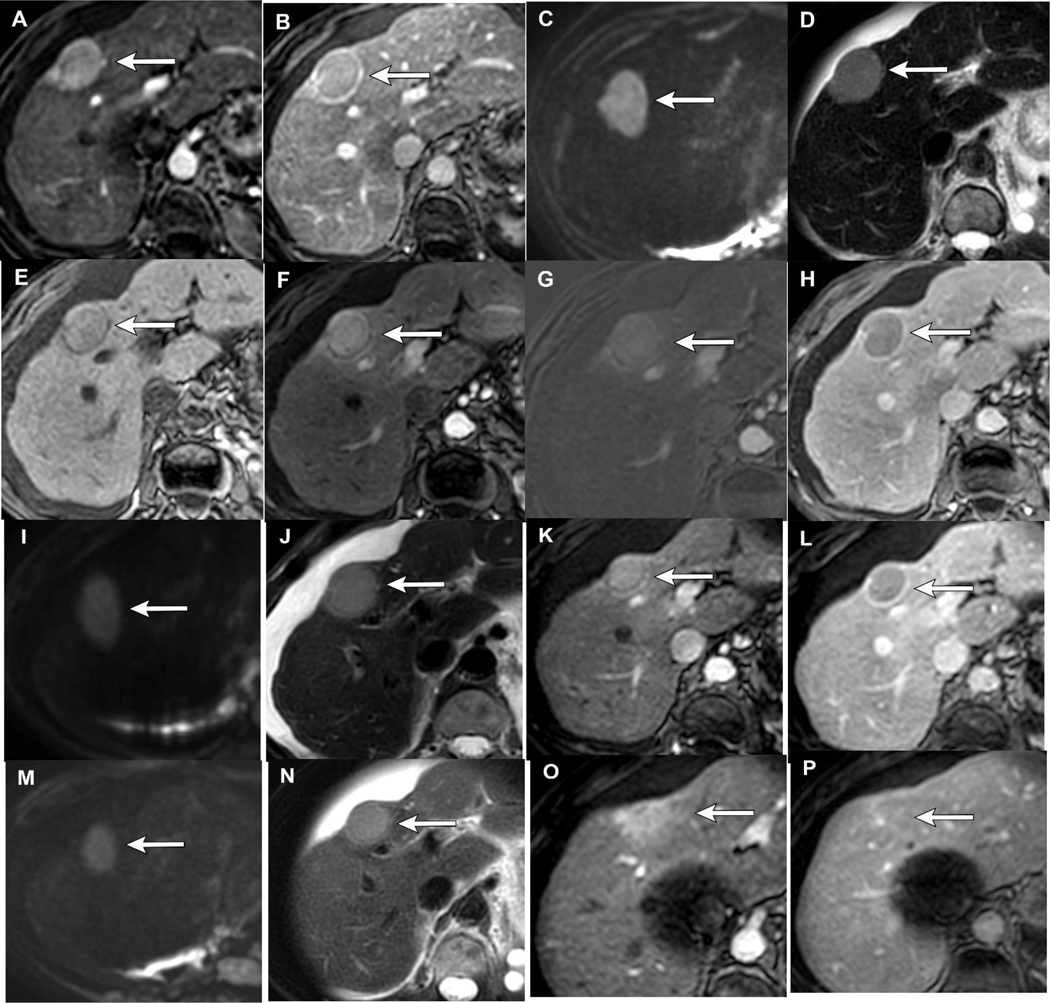
Figure 2.
LR-TR Nonviable after thermal ablation: 65-year-old male with HCV cirrhosis presenting with a 1.3cm LR 5 observation demonstrating APHE (A) and WO (B). 1-month post-MWA there is hyperintense signal within the treatment cavity seen on T1 pre-contrast MRI (C), compatible with coagulation necrosis. Arterial phase imaging demonstrates no enhancement (D), confirmed with subtraction images (E). Arterial (D) and portal venous phase of imaging (F) shows smooth peripheral rim enhancement which is an expected post-ablation finding, LR-TR Nonviable. Note, the treatment cavity is larger than the original tumor size, also an expected imaging finding which ensures adequate treatment of possible microscopic tumor along the treatment margin. 12 months post-MWA the lesion is smaller in size with no APHE (G), LR-TR Nonviable.
Early, transient apparent increase in the size of the treatment zone may occur post-treatment from intentional ablation of a zone larger than the tumor (after thermal ablation) or from underlying edema or hemorrhage (after arterial embolic therapy), typically followed by decreases in size over time on serial follow up exams[11]. Creation of an ablation margin at least 5–10 mm larger than the original tumor is essential to ensure adequate treatment margins encompassing any microscopic tumor to prevent early post-treatment recurrence[11]. Over time, the ablation cavity will slowly involute, although it usually does not completely resolves.
Following thermal ablation or intra-arterial embolic therapy, key imaging features of locally recurrent or residual viable HCC include irregular or masslike areas of APHE, APHE plus washout, washout alone, or enhancement similar to the pretreatment tumor. [11] Such cases are categorized as LR-TR Viable according to LI-RADS TRA v2018. [Figure 3] LR-TR Equivocal categorization is intended for lesions that do not have imaging criteria to suggest “probably viable” or “definitely viable” disease. LR-TR Equivocal is often used when there is subtle atypical enhancement in the hepatic parenchyma surrounding a treated lesion, which could reasonably represent either abnormal perfusion post-treatment versus true tumoral enhancement[11]. Equivocal categorization allows for a ‘wait and watch’ approach, during which subsequent reimaging can help to reassess the potential for viability. Unlike in the LI-RADS diagnostic algorithm, use of ancillary features (diffusion restriction, mildly hyperintense T2-weighted signal and hepatobiliary phase hypointensity) is not part of LI-RADS TRA v2018.
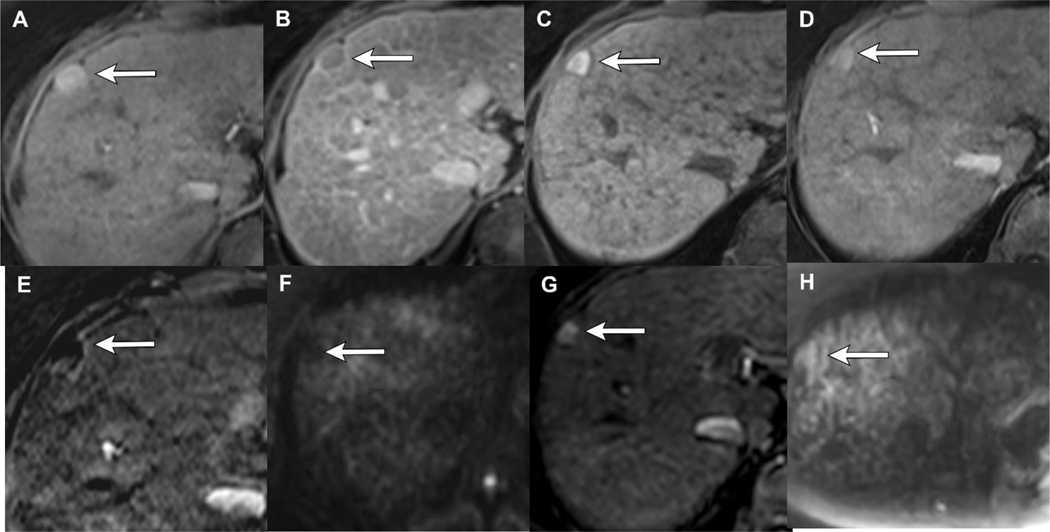
Figure 3.
LR-TR Viable after thermal ablation: Cirrhotic patient with 1.8 cm LR 5 HCC demonstrating APHE (A) and WO (B). 1-month post-MWA there is hyperintense signal on T1 pre-contrast MRI (C), compatible with coagulation necrosis. Arterial phase imaging demonstrates no enhancement (D), confirmed with subtraction images (E) and no restricted diffusion (F). 18 months post-MWA there is new mass-like nodular APHE along the margin of the treated lesion (G), LR-TR Viable. There is also new restricted diffusion corresponding to the area of nodular APHE (H).
CT and MRI are generally both regarded as acceptable for the follow-up of treated disease. However, one imaging pitfall after transarterial embolic therapy with iodized oil is that the embolic material appears hyperdense on non-contrast CT, which may obscure subtle areas of enhancement within and along the treatment zone, thus confounding evaluation for viable tumor[13] [Figure 4]. In these cases, MRI is a better imaging modality for treatment response assessment, as the iodized oil is not seen on MRI, and thus more accurate assessment of response can be provided[14]. On the other hand, an advantage of using iodized oil is that staining patterns within the targeted tumor can be a biomarker for treatment response by predicting lesion necrosis and outcomes in patients. Staining patterns within and around a targeted tumor, such as increased density of oil deposition within a targeted tumor, dense homogenous staining patterns, and a rim of ethiodized oil deposition surrounding the radiographically visible margin of the tumor are signs of excellent treatment response. [15–18] Such staining patterns have shown to be associated with lower rates of early post-treatment recurrence [15–18]. [Figure 4]
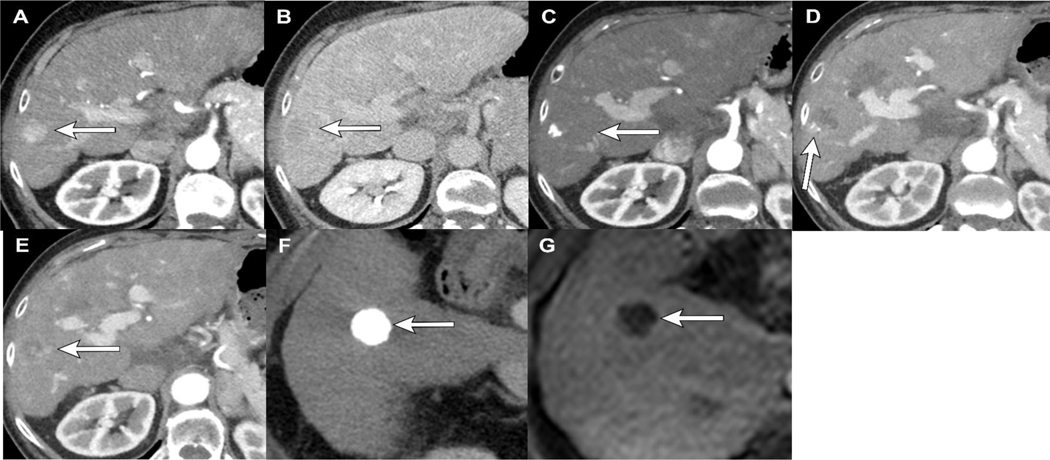
Figure 4.
LR-TR Viable and Nonviable categorization after cTACE: 47 year old female with cirrhosis and LR 5 HCC demonstrating APHE (A) and washout (B) pre-treatment. Intraprocedural CT after cTACE with ethiodized oil shows incomplete and heterogeneous staining of the tumor (C, D). 1 month post cTACE, there is significant masslike intralesional APHE (E), compatible with LR-TR Viable category. A second lesion in the same patient treated at a different time shows dense homogeneous staining of ethiodized oil at the time of treatment (F). 1 month follow-up MRI after cTACE shows complete tumor necrosis with no APHE (G), LR-TR Nonviable. Note that the dense ethiodized oil is not seen on MRI, thus if there were subtle areas of masslike APHE along the margin of the tumor, it would not be obscured and could easily be identified.
Expected imaging appearance of Radiation-treated HCC
In contrast, to ablation and embolic arterial therapy, lesions treated with radiation-based therapies (SBRT and TARE) have a distinct post-treatment imaging appearance based on the previously described complex mechanism of cell death. Some irradiated lesions exhibit immediate non-enhancement on MRI, compatible with an LR TR Nonviable categorization. However, a large proportion, greater than 50%, demonstrate irregular nodular or masslike APHE with or without washout, which can persist for a year or longer[19, 20]. [Figure 5] Emerging data suggests that enhancement and size of the treated lesion should gradually decrease over time if nonprogressing or nonviable, unlike viable tumors in which an increase in enhancement and/or size are imaging biomarkers for progression and should warrant discussion at multidisciplinary conference[21–23]. [Figure 6]
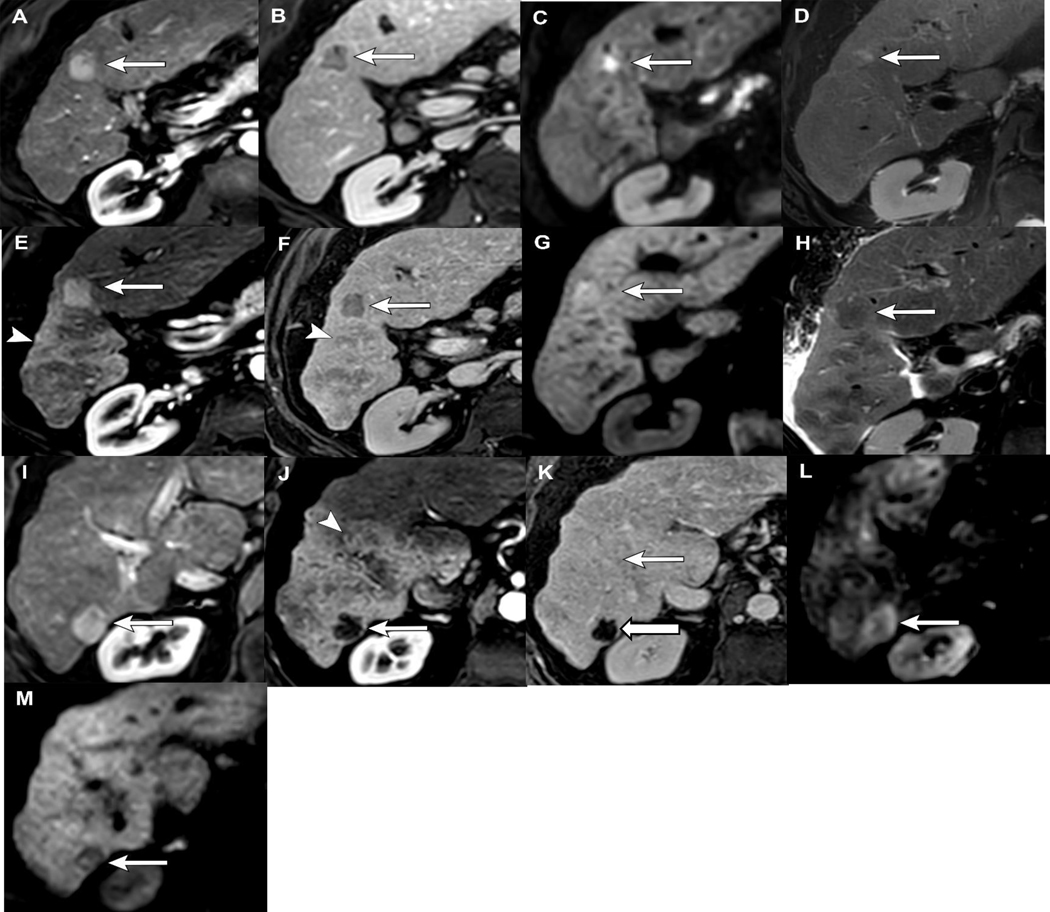
Figure 5.
Application of LI-RADS TRA v2018 after SBRT: 72-year-old female with alcohol induced cirrhosis presenting with a 3.3 cm LR 5 HCC with APHE (A), washout and capsule (B), restricted diffusion (C) and mild T2 hyperintense signal (D). 3 months post SBRT the treated lesion now measures 2.9 cm with T1 pre-contrast hyperintense signal (E), persistent APHE (F), confirmed with subtractions (G), washout and capsule (H), restricted diffusion (I) and mild T2 hyperintense signal (J), LR-TR Equivocal based on v2018, although nonprogressing. 10 months post-SBRT the treated observation measures 2.6 cm, with similar imaging features as the 3 month post-SBRT imaging, persistent APHE (K), washout and capsule (L), restricted diffusion (M) and mild T2 hyperintense signal (N), LR-TR Equivocal. Note the geographic arterial phase hyperenhancement in the parenchyma surrounding the treatment cavity (O) which becomes isoenhancing on PV phase of imaging (P), findings related to radiation induced changes in the parenchyma, not tumor progression, which would likely demonstrate areas of washout.
Figure 6.
Application of LI-RADS TRA v2018 after TARE: 78-year old male with cirrhosis, presenting with multi-focal HCC. Lesion 1: There is a 1.9 cm segment 5 LR 5 observation with APHE (A), washout (B), restricted diffusion (C) and mild T2 hyperintense signal (D). 12 months post-TARE the tumor is now 2.6cm with persistent APHE (E), washout (F), increasing restricted diffusion (G) and resolution of T2 signal (H). The increasing size of the arterially enhancing component makes this LR-TR Viable. This patient was retreated with MWA. Note, the presence of new diffusion could be used as an ancillary feature for upgrading the category in the new algorithm (not yet released). Lesion 2: 2.4 cm LR 5 observation with APHE (I), washout (not shown), and restricted diffusion (J) pre-treatment. 12 months post-TARE there is no enhancement of the tumor on arterial (K) or PV phase (L), and the restricted diffusion has almost completely resolved (M). Note the extensive geographic perfusional changes within the surrounding parenchyma (E, J) which becomes isoenhancing on PV/delayed phase of imaging (F, K), compatible with perfusional post-radiation changes, in contrast to viable tumor which would washout.
With strict application of LI-RADS TRA v2018 criteria, HCC treated with radiation-based therapy would be categorized as LR-TR Nonviable if there is no enhancement, LR-TR Equivocal if there is persistent post-treatment enhancement with stable to decreasing size, and LR-TR Viable if there is new or increasing enhancement and/or increasing size. Although LR-TR Equivocal lesions may harbor viable tumor after SBRT, they most often necrose over time. This natural history is different than LR-TR Equivocal categorization of lesions treated with non-radiation based treatment which frequently progress over time and often require retreatment [23–26]. This difference is likely a result of the different mechanisms of cell deathinduced by radiation versus non-radiation therapies.
Additional imaging findings commonly seen after radiation-based treatment include extensive perfusional changes within the liver parenchyma surrounding the treated tumor. Comparison of the pre-treatment imaging to the post-treatment imaging is essential to identify the margins and size of the treated tumor and distinguish peri-tumoral perfusional changes from viable tumor. Other findings that can help distinguish perfusional changes from tumor include that perfusional changes tend to be geographic in shape, hyperenhancing on arterial phase and hyperenhancing or isoenhancing to background parenchyma on portal venous and delayed phases of imaging, instead of a washout[5, 20, 27] [Figure 5, 6]. In contradistinction, the radiation treated tumor can demonstrate persistent enhancement with washout, although, as mentioned above, persistent APHE and washout are expected post-radiation changes[19]. One way to distinguish clinically significant viable tumor from expected post radiation changes is to evaluate change and size of APHE over time, specifically, if the area of questionable APHE and washout are new when compared to pre-treatment or preceding post-treatment imaging. On MRI, new APHE or increasing intensity of enhancement over time on post-treatment imaging follow-up studies is an important feature suggesting local recurrence and should prompt classification of a lesion as LR-TR Viable [11, 20, 23]. [Figure 6]
Emerging Evidence Assessing the Diagnostic Performance of the LI-RADS TRA
To be clinically useful, any treatment response classification system must be assessed for validity and performance. The LI-RADS TRA is a relatively new algorithm based on expert opinion, and thus validation studies assessing performance and survival are important to support its use clinically. There is significant emerging data assessing diagnostic performance of the LI-RADS TRA via inter-reader reliability and radiologic-pathologic correlation, particularly for local ablative and intra-arterial embolic therapies. Usefulness of a treatment response algorithm requires the need for high inter-reader agreement since the final interpretation dictates patient management. Additionlly, the performance of LR-TR categories to predict outcomes data such as overall survival (OS), time to progression (TTP), and disease-free survival (DFS) is important from a clinical perspective for patient management.
Diagnostic Performance of Locoablative and Non-Radiation Arterial-based Therapies
Several recent metanalyses have evaluated the diagnostic performance of LI-RADS TRA in predicting incomplete necrosis based on pathology as the gold standard and have shown excellent performance after ablation and intra-arterial embolic therapy. Perhaps the most comprehensive analysis performed by Kim et al included 8 studies evaluating the diagnostic performance of LI-RADS TRA[28]. This metanalysis of 851 treated HCCs demonstrated an inter-rater reliability k of 0.55–0.94, with a pooled k of 0.70 (95% CI, 0.58–0.82) on CT and MRI. When evaluating inter-rater reliability of MRI alone as an imaging modality for TRA, pooled k was 0.71 (95% CI, 0.53–0.89), suggesting substantial inter-rater reliability for MRI to assess TRA after LRT for HCC [28]. [Table 1]
Table 1:
Studies evaluating diagnostic performance and reliability of LIRADS TRA
| Study | Study Design | Publication year | # Of HCCs | # of total LRTs performed and analyzed | Imaging Modality | Interreader agreement (κ; 95CI) | Sensitivity (for LR TR Viable to detect incomplete necrosis) % | Specificity (for LR TR Viable to detect incomplete necrosis) % |
|---|---|---|---|---|---|---|---|---|
| Abdel Razek et al [53] | Prospective | 2020 | 112 | Ablation: 97 Arterial (cTACE): 25 |
MRI | 0.94 (0.89–1) | N/A | N/A |
| Chaudhry et al[31] | Retrospective | 2020 | 53 | Ablation: 53 | MRI | 0.71 (0.59–0.84) | 0.4–0.77 | 0.85–0.97 |
| Cools KS et al[32] | Retrospective | 2020 | 59 | Ablation: 59 | MRI | 0.75 (SE±0.09) | 0.30 | 1.0 |
| Kim SW et al[49] | Retrospective | 2020 | 183 | Ablation: 42 Arterial (cTACE or DEB TACE): 137 Ablation + Arterial: 4 |
MRI | 0.58 (0.49–0.67) | 0.41–0.67 | 0.98 |
| Park S et al[37] | Retrospective | 2020 | 138 | Ablation: 18 Arterial: 98 Ablation + Arterial: 22 |
CT or MRI | 0.72 (0.61–0.83) | 0.79 | 0.83 |
| Seo N et al[25] | Retrospective | 2020 | 206 | Ablation: 34 Arterial: 168 Ablation + Arterial: 4 |
CT or MRI | 0.56 (0.41– 0.72) | 0.56 | 0.92 |
| Shropshire EL [24]et al | Retrospective | 2019 | 63 | Arterial: 63 | CT or MRI | 0.55 (0.47–0.67) | 0.55 | 0.88 |
| Kim Dh et al[29] | Meta-analysis (5 studies: retrospective) | 2022 | 631 | TARE: 1 TACE: 1 Combination: 3 |
MRI (3 studies) CT and MRI (2) |
0.56–0.69 | 0.58* | 0.93** |
| Kim TH et al[30] | Meta-analysis (6 studies: retrospective) | 2021 | 534 | Ablation and TACE | MRI and CT | N/A | 0.56* | 0.91** |
LRT: Locoregional therapy, cTACE: conventional Transarterial chemoembolization, DEB TACE: Drug eluting beads TACE,
pooled sensitivity,
pooled specificity
A more recent study systematically evaluated the LR-TR Viable category for detection of pathologically viable HCC after non-radiation LRT. For this category, the pooled sensitivity and specificity was 58% (95% CI, 45%–70%) and 93% (95% CI, 88%– 96) for pathologic detection of incomplete necrosis[29]. In another meta-analysis using similar studies as the aforementioned study, they performed a subgroup analysis of LR-TR Viable categorization based on MRI as the imaging modality for detection of incomplete necrosis in 379 non-radiation treated HCCs and demonstrated sensitivity and specificity of 0.60 (0.46–0.74) and 0.90 (0.84–0.97), respectively[30]. Both of these studies evaluating radiology and pathology concordance of the current LR-TRA, suggest that the current LR-TRA is effective for detection of Viable HCC after non-radiation LRT. Furthermore, it has also been shown that interreader reliability for LR-TR categorization as a whole was moderate to substantial for MRI (k = 0.56–0.69), comparable to CT (k = 0.69)[29]. [Table 1] These findings suggest that readers with varying levels of experience can assign LI-RADS TRA categories on multiphase imaging with moderate to substantial agreement.
While most evidence suggests a high sensitivity of the LI-RADS TRA for detection of viable and nonviable disease, the data for the LR-TR Equivocal categorization remains less convincing. Most studies show higher sensitivities for predicting incomplete necrosis when the LR-TR Equivocal category is treated as viable rather than non-viable [24, 31]. For example, Chaudhry et al found a sensitivity of 81–87% for predicting incomplete necrosis when the equivocal category was considered viable versus 40–77% when considered nonviable, with little difference in specificity (81–85% versus 85–97%, respectively)[31]. Similarly, another study reported improved sensitivity, specificity, and positive predictive value (PPV) of 44%, 99% and 93% for predicting incomplete necrosis when the equivocal category was considered viable versus 30%, 86% and 67% when considered non-viable, respectively[32] A third study demonstrated that 71% of non-radiation treated HCC with LR TR Equivocal categorization had pathologically viable tumor on explant, thus increasing the evidence that LR TR Equivocal category warrants close follow-up because of the likelihood of persistent viable disease. [24].
A recent metanalysis by Kim et al. showed pooled sensitivities for the LR-TR Viable category alone for diagnostic detection of incomplete necrosis was 0.56 (95% CI 0.43–0.69) with a specificity of 0.91 (95% CI 0.84–0.96) and area under the curve of 0.86 (95% CI 0.82–0.88). However, when combining the LR-TR Viable and Equivocal categories for detection of incomplete necrosis on pathology, the pooled sensitivity and specificity was 0.73 (95% CI 0.60–0.84) and 0.82 (95% CI 0.74–0.88), respectively[30]. This is corroborated by a recent meta-analysis which reviewed TRA of HCC treated with ablation or embolic therapy and showed that the majority (70.5%) of LR TR Equivocal lesions were viable[33]
Based on the aforementioned data, most lesions categorized as equivocal after ablation or embolic therapy are likely incompletely necrotic following ablation and intra-arterial embolization. Low sensitivity is likely in part due to the challenges of conventional imaging to detect pathologic microscopic viable tumor. The LR-TR Equivocal category is most often applied in questionable areas of enhancement, and thus presumably represents small volumes of viable tumor with limited clinical impact, as HCC tumor doubling times are reported to be 70–180 days.[34] Therefore, we advocate for imaging surveillance intervals of 3 months after equivocal categorization, as viable tumor will eventually declare itself over time.
The above studies support the use of LI-RADS TRA v2018, but the agreement seen per feature of the LR-TR categories must also be evaluated to ensure the validity and clinical utility of the current algorithm. The key features of the LI-RADS TRA include APHE, washout, and enhancement similar to pretreatment, the latter two of which are novel features when evaluating treatment response as compared to other response classification systems (e.g. modified Response Evaluation Criteria in Solid Tumors (mRECIST) and European Association for the Study of Liver Disase (EASL)). Multiple studies show that APHE has the highest diagnostic accuracy for predicting incomplete necrosis (AUC 0.69, 95% CI: 0.58–0.80), whereas lack of APHE and decreased size have the highest accuracy for predicting complete necrosis (AUC 0.75, 95% CI: 0.62–0.88)[24, 31, 35, 36]. Studies evaluating LI-RADS TRA features show relatively high interreader agreement in APHE (κ = 0.71–0.80), washout (κ = 0.67–0.72), and enhancement similar to pretreatment (κ = 0.62 to 0.73)[25, 37, 38].
However, while interreader agreement on the presence of imaging features is high, the actual diagnostic performance of each individual imaging feature did not perform as well in terms of interreader agreement. In a metanalysis by Kim et al, they show that the pooled sensitivity for detection of incomplete necrosis was substantially higher using APHE (0.67 [95% CI 0.51–0.81]) than washout (0.43 [95% CI 0.26–0.62]) or enhancement similar to pretreatment (0.24 [95% CI 0.15–0.36]). Furthermore, in lesions with incomplete pathologic necrosis, the pooled proportion of lesions showing washout without APHE was 2%, enhancement similar to pretreatment without APHE was 1% and enhancement similar to pretreatment with neither APHE nor washout was 0%[30]. According to a metanalysis by Huh et al, pooled calculations of sensitivity for each LI-RADS TRA feature from 10 studies with a composite cohort of 971 patients and 1153 observations post-LRT showed that the presence of APHE provided the highest sensitivity (81%) for diagnosing viable HCC following LRT, while washout (55%) and enhancement similar to pretreatment (21%) demonstrated suboptimal performance; specificity amongst all 3 features was high (95–98%)[38]. This pooled analysis across a variety of post-LRT studies reinforces the singular importance of APHE in detection of viable tumor, which is the best indicator of incomplete necrosis, and the most agreed upon LI-RADS TRA feature. These data will be used for future modifications in the LI-RADS TRA.
Current evidence assessing diagnostic performance of LI-TRA with Radiation-based therapies
Studies have shown SBRT and TARE to be effective treatment options for downstaging or bridging to transplant[39–42]. While SBRT shows promising outcomes for HCC treatment, lack of prospective randomized comparative clinical trials for predicting survival outcomes limits widespread clinical adoption and inclusion of SBRT as a treatment modality in the updated Barcelona Clinic Liver Cancer (BCLC) staging system. However, strong evidence from the recent LEGACY (Local radioEmbolization using Glass Microspheres for the Assessment of Tumor Control with Y-90 ) trial has allowed for modification of the new BCLC 2022 staging system to incorporate TARE as a treatment option for HCC[43]. With increasing use of radiation as a form of LRT for HCC, accurate assessment of post-treatment imaging is critical to ensure appropriate patient management.
As mentioned above, HCC treated with radiation therapy usually demonstrates post-treatment persistent APHE, a feature LR-TRA uses to categorize lesions as viable. Thus, this imaging feature confounds treatment response assessment after radiation using current response assessment systems (LI-RADS TRA, mRECIST) and can result in a high rate of potential misclassification of treated disease as LR-TR Viable, which could result in unnecessary retreatment of already successfully treated HCC. One challenge in validating response assessment systems after radiation therapy is the paucity of studies correlating post-radiation imaging findings with pathology. Additionally, the mechanism of radiation-induced cell death complicates the application of imaging features to determine a final LR category. Since radiation can induce cellular senescence, cell death is not always immediate and evolves over time[44]. Thus, strict application of the current LI-RADS TRA would suggest that ‘viable’ categorization may be appropriate when radiation-treated HCC demonstrates persistent APHE; however, the clinical implication is different than disease radiologically categorized as ‘viable’ after conventional LRT.
To date, there is only one study evaluating the diagnostic performance of the LI-RADS TRA in assessing HCC viability after SBRT using radiology-pathology concordance[23]. In this study, decreasing APHE after treatment highly correlated with complete pathologic necrosis, although persistent post-treatment APHE was still seen in 45% of tumors that were completely necrotic on pathology[23]. Inter-reader agreement was fair (κ = 0.22) for LR-TRA categorization of HCC treated with SBRT. Additionally, LI-RADS TRA was able to predict complete (sensitivity 71–86%, specificity 85–96%, NPV = 85–96%) and incomplete tumor necrosis (sensitivity 88–96%, specificity 71–93%, PPV = 88–92%, with LR-TR Equivocal lesions considered viable)[23]. 67% of lesions categorized as LR-TR Equivocal were found to be incompletely necrotic at histopathology, a finding which is not unexpected since SBRT-treated HCC demonstrating persistent APHE on imaging were categorized as LR-TR Equivocal by the readers. Concordant with other prior results, they also found that a longer time to transplant from treatment resulted in greater loss of APHE (OR 0.68) and increasing degree of necrosis (OR 0.2). These findings suggest that radiated lesions categorized as ‘equivocal’ should be followed rather than immediately retreated, as pathologically viable tumor will usually necrose over time, given the normal course and pathophysiology of radiation induced cell death.
The data supporting TARE for HCC is more robust because of several large clinical trials. Like SBRT-treated HCC, the appearance of HCC treated with TARE is variable, ranging from complete necrosis immediately after treatment to varying degrees of intralesional APHE which can persist for some time after treatment [45]. Riaz et al show that 61% of HCCs treated with TARE exhibited complete pathologic necrosis despite residual nodular APHE on post-treatment imaging [22]. King et al evaluated the performance of LI-RADS TRA versus mRECIST in predicting treatment response after TARE in 57 patients with 77 HCCs and found that LI-RADS TRA was a better predictor of pathologic necrosis and treatment response[46], a finding which was confirmed by Violi et al[47]. Few studies have assessed the ability of LI-RADS TRA to predict overall survival following radiation-based therapies, and further longitudinal studies are needed.
Performance of Ancillary Features and Evidence Supporting the Use in LIRADS TRA
An emerging area of interest is the utility of ancillary features, including T2-weighted (T2W) signal abnormality, hepatobiliary phase (HBP) hypointensity, and restricted diffusion [48], in improving detection of viable tumor following LRT. Currently the LI-RADS TRA uses only major features (APHE and washout) for determining tumor viability after LRT. However, recent radiology-pathology studies demonstrate improved diagnostic performance for detection of viable tumor post-LRT using MRI-based ancillary features (AF), specifically mild T2 weighted hyperintensity, restricted diffusion and hepatobiliary phase hypointensity. Park et al evaluated the utility of adding MRI-based AF in category adjustment for detecting viable tumor as compared to LR-TR categorization using only major imaging features of APHE and washout on MRI[37]. They showed higher sensitivities of detecting tumor viability when using AFs (84% (91/108)) to upgrade to viable category as compared to enhancement alone on MRI (76% (82/108)) without differences in specificity[37]. Similarly, Kim et al showed an increase in the sensitivity for detecting viable tumor when using LI-RADS TRA with AF (sensitivity 64.5% without AF vs. 86.9% with AF) and no difference in specificity (98% vs. 97%)[49]. In both studies, the presence of AF corresponding to areas of questionable APHE allowed upgrading of the category from LR-TR Equivocal to LR-TR Viable, and had high concordance with pathologic viability. Both studies showed an overall decrease in use of LR-TR Equivocal categorization when adding AF to assess for tumor viability.
Additional smaller studies confirm improved detection of tumor viability with the addition of AFs. For example, in a radiology-pathology study of 181 HCCs, the presence of hepatobiliary phase hypointensity in a lesion categorized as LR-TR Viable or LR-TR Equivocal compared to LR-TR Viable alone demonstrated improved sensitivity for detection of viable tumor (65.6% versus 57%) with no significant difference in specificity (90.8% versus 94.3%)[50]. Another study showed that using restricted diffusion corresponding to equivocal areas of APHE improved sensitivity for detection of viable disease than when not using AF (92% versus 85%)[51] Overall, findings suggest that a modified TRA utilizing ancillary MR features may be superior to the LI-RADS TRA alone in detecting viable tumor and may result in lower use of ‘equivocal’ classification [52].
LIRADS TRA: Current Limitations and Future Directions
We have demonstrated a host of emerging data describing post-LRT imaging appearances, as well as supporting the validity of the LI-RADS TRA to predict necrosis. Most studies demonstrate excellent diagnostic performance of the LI-RADS TRA; however, emerging data also reveals key limitations. First, the current LI-RADS TRA must be modified to incorporate more accurate treatment response assessment after radiation therapy based on the unique pathophysiology of radiation induced cell death which results in persistent post-treatment APHE, a feature currently used to assign tumor viability based on all treatment response systems. Second, studies continue to demonstrate the utility of AFs in improving the sensitivity of viable tumor detection. Improved post-treatment categorization will ultimately impact patient management. Third, further guidance on treatment response assessment for HCC treated with combination LRT and systemic and immunotherapies must be clarified since they are not part of the current algorithm. This will likely require longitudinal studies to obtain enough evidence to validate or update existing LI-RADS TRA. Fourth, improved guidance on structured template reporting and an updated lexicon for LI-RADS TRA will be critical to allow for improved standardization and concordance in image interpretation in this cohort of patients. Finally, treatment response assessment using contrast enhanced ultrasound is currently under investigation and future studies are necessary in order to develop its utility in TRA.
Conclusion
The expanding number of available LRTs has increased the complexity of treatment response assessment. Post-treatment imaging for treatment response assessment is essential to guide patient management. Understanding the various expected post-treatment imaging findings specific to each form of LRT is key to accurately categorizing treated lesions. LI-RADS TRA provides a framework to describe treatment response using a lesion-level approach with emerging evidence suggesting high validity and reliability in using this algorithm after non-radiation-based therapies. Emerging data also reveals that the LI-RADS TRA should be used cautiously when evaluating treatment response after radiation-based therapies since persistent APHE is common and expected after irradiation of HCC. Furthermore, emerging data suggests that the use of AFs improves sensitivity for detection of viable disease, although the impact on overall survival and time to progression is currently unknown. This constellation of evidence has resulted in modifications of the existing LI-RADS TRA v2018, with a separate categorization system for radiation-treated HCC and incorporation of AFs to allow category upgrades, similar to the LI-RADS diagnostic algorithm. This new algorithm is currently in the final stages of approval by the LI-RADS treatment response working group, steering committee, and the American College of Radiology, and will be released in 2023.
Grant support:
No funding specific in this submission.
Richard Kinh Gian Do is employed at Memorial Sloan Kettering Cancer Center which receives funding through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Terminology:
- HCC
hepatocellular carcinoma
- LI-RADS
Liver Imaging Reporting and Data Systems
- LI-RADS TRA
Treatment response algorithm
- LRT
locoregional therapy
- APHE
arterial phase hyperenhancement
- LR-TR
LI-RADS Treatment Response
- RFA
radiofrequency ablation
- MWA
microwave ablation
- TAE
transarterial embolization
- cTACE
conventional trans-arterial chemoembolization
- DEB-TACE
drug eluting bead TACE
- SBRT
stereotactic body radiotherapy
- TARE
transarterial radioembolization
- OS
overall survival
- TTP
time to progression
- DFS
disease-free survival
- PPV
positive predictive value
- NPV
negative predictive value
- mRECIST
modified Response Evaluation Criteria in Solid Tumors
- EASL
European Association for the Study of Liver Disase
- HBP
hepatobiliary phase
- AF
ancillary features
- BCLC
Barcelona Clinic Liver Cancer
Footnotes
Mustafa Bashir. Research support to institution (industry): Carmot Therapeutics, Corcept Therapeutics, Madrigal Pharmaceuticals, Metacrine Inc, NGM Biopharmaceuticals, Siemens Healthineers
Richard Kinh Gian Do. Disclosure: consultant GE Healthcare, Bayer Healthcare
Charles Y. Kim. Disclosures: Medical advisory board: Boston Scientific, Genentech; Consultant: Medtronic
Contributor Information
Richa Patel, Department of Radiology, Stanford.
Anum Aslam, Department of Radiology, University of Michigan Medicine.
Neehar D. Parikh, Department of Internal Medicine.
Benjamin Mervak, Department of Radiology, University of Michigan Medicine.
Eman Mubarak, University of Michigan Medicine.
Lily Higgins, Department of Radiology, University of Michigan Medicine.
Kayli Lala, Department of Radiology, University of Michigan Medicine.
Jack F Conner, Department of Radiology, University of Toledo Medical Center.
Valerie Khaykin, Department of Radiology and Hepatology, University of Michigan Medicine.
Mustafa Bashir, Department of Radiology, Duke University Medical Center.
Richard Kinh Gian Do, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY.
Lauren M.B. Burke, Department of Radiology, University of North Carolina at Chapel Hill School of Medicine.
Elainea N. Smith, University of Alabama at Birmingham.
Charles Y. Kim, Department of Radiology, Duke University Medical Center.
Kimberly L. Shampain, Department of Radiology, University of Michigan Medicine.
Dawn Owen, Department of Radiation Oncology, Mayo Clinic Rochester.
Mishal Mendiratta-Lala, Department of Radiology, University of Michigan Medicine, 1500 E Medical Center Dr. UH B2 A209R, Ann Arbor, MI 48109.
References:
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, and Jemal A, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2018. 68(6): p. 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Heimbach JK, Kulik LM, Finn RS, et al. , AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology, 2018. 67(1): p. 358–380. [DOI] [PubMed] [Google Scholar]
- 3.Allard MA, Sebagh M, Ruiz A, et al. , Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol, 2015. 63(1): p. 83–92. [DOI] [PubMed] [Google Scholar]
- 4.Belghiti J. and Kianmanesh R, Surgical treatment of hepatocellular carcinoma. HPB (Oxford), 2005. 7(1): p. 42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voizard N, Cerny M, Assad A, et al. , Assessment of hepatocellular carcinoma treatment response with LI-RADS: a pictorial review. Insights Imaging, 2019. 10(1): p. 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The LI-RADS v2018 Manual. 2018; Available from: https://www.acr.org/-/media/ACR/Files/Clinical-Resources/LIRADS/LI-RADS-2018-Manual-5Dec18.pdf?la=en.
- 7.American College of Radiology, in Liver Imaging Reporting and Data System Version 2018 Manual. 2018.
- 8.Elhalawani H, Lin TA, Volpe S, et al. , Machine Learning Applications in Head and Neck Radiation Oncology: Lessons From Open-Source Radiomics Challenges . Front Oncol, 2018. 8: p. 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sia J, Szmyd R, Hau E, and Gee HE, Molecular Mechanisms of Radiation-Induced Cancer Cell Death: A Primer. Front Cell Dev Biol, 2020. 8: p. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, and Formenti SC, The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol, 2012. 2: p. 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aslam A, Do RKG, Kambadakone A, et al. , Hepatocellular carcinoma Liver Imaging Reporting and Data Systems treatment response assessment: Lessons learned and future directions. World J Hepatol, 2020. 12(10): p. 738–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan YS, Sun L, Zhou XP, Li X, and Zheng XH, Hepatocellular carcinoma treated with interventional procedures: CT and MRI follow-up. World J Gastroenterol, 2004. 10(24): p. 3543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian G, Yang S, Yuan J, et al. , Comparative efficacy of treatment strategies for hepatocellular carcinoma: systematic review and network meta-analysis. BMJ Open, 2018. 8(10): p. e021269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloeckner R, Otto G, Biesterfeld S, Oberholzer K, Dueber C, and Pitton MB, MDCT versus MRI assessment of tumor response after transarterial chemoembolization for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol, 2010. 33(3): p. 532–40. [DOI] [PubMed] [Google Scholar]
- 15.Dioguardi Burgio M, Sartoris R, Libotean C, et al. , Lipiodol retention pattern after TACE for HCC is a predictor for local progression in lesions with complete response. Cancer Imaging, 2019. 19(1): p. 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DY, Ryu HJ, Choi JY, et al. , Radiological response predicts survival following transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma. Aliment Pharmacol Ther, 2012. 35(11): p. 1343–50. [DOI] [PubMed] [Google Scholar]
- 17.Letzen BS, Malpani R, Miszczuk M, et al. , Lipiodol as an intra-procedural imaging biomarker for liver tumor response to transarterial chemoembolization: Post-hoc analysis of a prospective clinical trial . Clin Imaging, 2021. 78: p. 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miszczuk MA, Chapiro J, Geschwind JH, et al. , Lipiodol as an Imaging Biomarker of Tumor Response After Conventional Transarterial Chemoembolization: Prospective Clinical Validation in Patients with Primary and Secondary Liver Cancer . Transl Oncol, 2020. 13(3): p. 100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendiratta-Lala M, Gu E, Owen D, et al. , Imaging findings within the first 12 months of hepatocellular carcinoma treated With stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys, 2018. 102(4): p. 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendiratta-Lala M, Masch W, Owen D, et al. , Natural history of hepatocellular carcinoma after stereotactic body radiation therapy. Abdom Radiol (NY), 2020. [DOI] [PubMed]
- 21.Brook OR, Thornton E, Mendiratta-Lala M, et al. , CT Imaging Findings after Stereotactic Radiotherapy for Liver Tumors . Gastroenterol Res Pract, 2015. 2015: p. 126245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riaz A, Kulik L, Lewandowski RJ, et al. , Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology, 2009. 49(4): p. 1185–93. [DOI] [PubMed] [Google Scholar]
- 23.Mendiratta-Lala M, Aslam A, Maturen KE, et al. , LI-RADS Treatment Response Algorithm: Performance and Diagnostic Accuracy With Radiologic-Pathologic Explant Correlation in Patients With SBRT-Treated Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys, 2022. 112(3): p. 704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shropshire EL, Chaudhry M, Miller CM, et al. , LI-RADS treatment response algorithm: performance and diagnostic accuracy. Radiology, 2019. 292(1): p. 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo N, Kim MS, Park MS, et al. , Evaluation of treatment response in hepatocellular carcinoma in the explanted liver with Liver Imaging Reporting and Data System version 2017. Eur Radiol, 2020. 30(1): p. 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ormiston WEL, Yarmohammadi H, Lobaugh S, et al. , Post-treatment CT LI-RADS categories: predictors of overall survival in hepatocellular carcinoma post bland transarterial embolization. Abdom Radiol (NY), 2021. 46(8): p. 3738–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendiratta-Lala M, Masch W, Shankar PR, et al. , Magnetic Resonance Imaging Evaluation of Hepatocellular Carcinoma Treated With Stereotactic Body Radiation Therapy: Long Term Imaging Follow-Up. Int J Radiat Oncol Biol Phys, 2019. 103(1): p. 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DW, Choi SH, Lee JS, Kim SY, Lee SJ, and Byun JH, Interreader Reliability of Liver Imaging Reporting and Data System Treatment Response: A Systematic Review and Meta-Analysis . Diagnostics (Basel), 2021. 11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DH, Kim B, Choi JI, Oh SN, and Rha SE, LI-RADS Treatment Response versus Modified RECIST for Diagnosing Viable Hepatocellular Carcinoma after Locoregional Therapy: A Systematic Review and Meta-Analysis of Comparative Studies. Taehan Yongsang Uihakhoe Chi, 2022. 83(2): p. 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim TH, Woo S, Joo I, et al. , LI-RADS treatment response algorithm for detecting incomplete necrosis in hepatocellular carcinoma after locoregional treatment: a systematic review and meta-analysis using individual patient data. Abdom Radiol (NY), 2021. 46(8): p. 3717–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhry M, McGinty KA, Mervak B, et al. , The LI-RADS version 2018 mri treatment response algorithm: evaluation of ablated hepatocellular carcinoma. Radiology, 2020. 294(2): p. 320–326. [DOI] [PubMed] [Google Scholar]
- 32.Cools KS, Moon AM, Burke LMB, McGinty KA, Strassle PD, and Gerber DA, Validation of the Liver Imaging Reporting and Data System Treatment Response Criteria After Thermal Ablation for Hepatocellular Carcinoma. Liver Transpl, 2020. 26(2): p. 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta P, Bansal A, Das GC, et al. , Diagnostic accuracy of Liver Imaging Reporting and Data System locoregional treatment response criteria: a systematic review and meta-analysis. Eur Radiol, 2021. 31(10): p. 7725–7733. [DOI] [PubMed] [Google Scholar]
- 34.Nathani P, Gopal P, Rich N, et al. , Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut, 2021. 70(2): p. 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartnik K, Podgórska J, Rosiak G, Korzeniowski K, and Rowiński O, Inter-observer agreement using the LI-RADS version 2018 CT treatment response algorithm in patients with hepatocellular carcinoma treated with conventional transarterial chemoembolization. Abdom Radiol (NY), 2022. 47(1): p. 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kierans AS, Najjar M, Dutruel SP, et al. , Evaluation of the LI-RADS treatment response algorithm in hepatocellular carcinoma after trans-arterial chemoembolization. Clin Imaging, 2021. 80: p. 117–122. [DOI] [PubMed] [Google Scholar]
- 37.Park S, Joo I, Lee DH, et al. , Diagnostic Performance of LI-RADS Treatment Response Algorithm for Hepatocellular Carcinoma: Adding Ancillary Features to MRI Compared with Enhancement Patterns at CT and MRI. Radiology, 2020: p. 192797. [DOI] [PubMed]
- 38.Huh YJ, Kim DH, Kim B, Choi JI, and Rha SE, Per-Feature Accuracy of Liver Imaging Reporting and Data System Locoregional Treatment Response Algorithm: A Systematic Review and Meta-Analysis . Cancers (Basel), 2021. 13(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salem R, Johnson GE, Kim E, et al. , Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology, 2021. 74(5): p. 2342–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathew AS, Atenafu EG, Owen D, et al. , Long term outcomes of stereotactic body radiation therapy for hepatocellular carcinoma without macrovascular invasion. Eur J Cancer, 2020. 134: p. 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mastrocostas K, Fischer S, Munoz-Schuffenegger P, et al. , Radiological tumor response and histopathological correlation of hepatocellular carcinoma treated with stereotactic body radiation therapy as a bridge to liver transplantation. Abdom Radiol (NY), 2021. 46(4): p. 1572–1585. [DOI] [PubMed] [Google Scholar]
- 42.Schaub SK, Hartvigson PE, Lock MI, et al. , Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Current Trends and Controversies. Technol Cancer Res Treat, 2018. 17: p. 1533033818790217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reig M, Forner A, Rimola J, et al. , BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol, 2022. 76(3): p. 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiao Y, Cao F, and Liu H, Radiation-induced Cell Death and Its Mechanisms. Health Phys, 2022. 123(5): p. 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keppke AL, Salem R, Reddy D, et al. , Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. AJR Am J Roentgenol, 2007. 188(3): p. 768–75. [DOI] [PubMed] [Google Scholar]
- 46.King MJ, Tong A, Dane B, Huang C, Zhan C, and Shanbhogue K, Response assessment of hepatocellular carcinoma treated with yttrium-90 radioembolization: inter-reader variability, comparison with 3D quantitative approach, and role in the prediction of clinical outcomes. Eur J Radiol, 2020. 133: p. 109351. [DOI] [PubMed] [Google Scholar]
- 47.Vietti Violi N, Gnerre J, Law A, et al. , Assessment of HCC response to Yttrium-90 radioembolization with gadoxetate disodium MRI: correlation with histopathology. Eur Radiol, 2022. 32(9): p. 6493–6503. [DOI] [PubMed] [Google Scholar]
- 48.Cerny M, Chernyak V, Olivié D, et al. , LI-RADS Version 2018 Ancillary Features at MRI. Radiographics, 2018. 38(7): p. 1973–2001. [DOI] [PubMed] [Google Scholar]
- 49.Kim SW, Joo I, Kim HC, et al. , LI-RADS treatment response categorization on gadoxetic acid-enhanced MRI: diagnostic performance compared to mRECIST and added value of ancillary features. Eur Radiol, 2020. 30(5): p. 2861–2870. [DOI] [PubMed] [Google Scholar]
- 50.Kim YY, Kim MJ, Yoon JK, Shin J, and Roh YH, Incorporation of Ancillary MRI Features Into the LI-RADS Treatment Response Algorithm: Impact on Diagnostic Performance After Locoregional Treatment of Hepatocellular Carcinoma. AJR Am J Roentgenol, 2022. 218(3): p. 484–493. [DOI] [PubMed] [Google Scholar]
- 51.Yu JS, Kim JH, Chung JJ, and Kim KW, Added value of diffusion-weighted imaging in the MRI assessment of perilesional tumor recurrence after chemoembolization of hepatocellular carcinomas. J Magn Reson Imaging, 2009. 30(1): p. 153–60. [DOI] [PubMed] [Google Scholar]
- 52.Park S, Joo I, Lee DH, et al. , Diagnostic Performance of LI-RADS Treatment Response Algorithm for Hepatocellular Carcinoma: Adding Ancillary Features to MRI Compared with Enhancement Patterns at CT and MRI. Radiology, 2020. 296(3): p. 554–561. [DOI] [PubMed] [Google Scholar]
- 53.Abdel Razek AAK, El-Serougy LG, Saleh GA, Shabana W, and Abd El-Wahab R, Reproducibility of LI-RADS treatment response algorithm for hepatocellular carcinoma after locoregional therapy. Diagn Interv Imaging, 2020. [DOI] [PubMed]