Abstract
Introduction:
The health effects of alcohol are well established but the influence on pulmonary function remains debated. Studies indicate that small amounts of alcohol are beneficial and heavy consumption is harmful, suggesting a U-shaped association. Our objective is to determine whether there is an association between alcohol intake and changes in pulmonary function parameters, exploring the potential protective effect of moderate alcohol consumption and the harm caused by heavy drinking.
Methods:
A comprehensive search from PubMed, Embase, Cochrane and CINAHL was carried out, and studies were evaluated using the JBI methodological framework for scoping reviews. Two independent reviewers conducted parallel screening and data extraction. A data extraction form was utilised to organise key themes, with qualitative analysis and visual representation of the results.
Results:
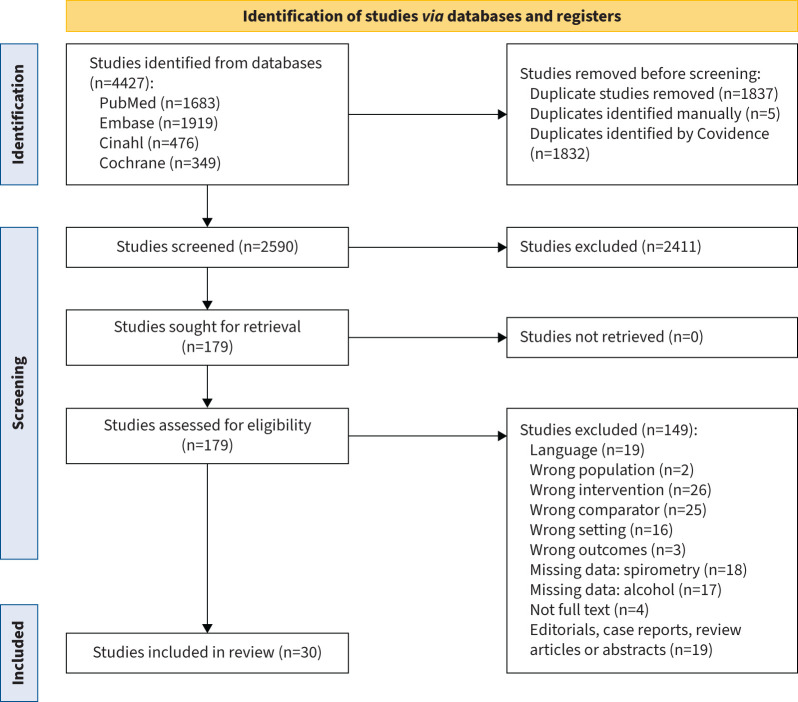
Among 4427 screened abstracts, 179 underwent full-text review, resulting in 30 eligible studies. Of these, 10 showed a negative effect, nine reported no impact, nine exhibited a positive effect and two indicated a nonlinear U-shaped association between alcohol consumption and pulmonary function parameters.
Conclusion:
While the U-shaped curve hypothesis remains unconfirmed by the current literature, there are notable associations. Heavy alcohol consumption appears to negatively affect pulmonary function, while low to moderate intake shows a positive influence in included studies. However, the diversity in study quality, the nonstandardised alcohol intake quantification and the confounding role of smoking challenge definitive conclusions. The need for consistent, long-term international studies is evident to further explore this relationship while addressing the complex interplay between alcohol and smoking.
Shareable abstract
This review found that while heavy drinking may harm lung function, moderate intake may be protective. Previous studies lack long-term follow-up as well as consistency in quantification of alcohol intake and handling of smoking as a confounding factor. https://bit.ly/3Isq4zy
Introduction
Alcohol, primarily ethanol, is known for its diverse acute and chronic physiological effects. When alcohol is consumed, it is rapidly absorbed into the bloodstream and subsequently distributed throughout the body, including the respiratory system.
Alcohol consumption is one of the most important treatable behaviours affecting public health in the world. An estimated 2.3 billion people are current drinkers and every year approximately 3 million deaths worldwide are caused by harmful use of alcohol [1]. While there is no safe lower limit for smoking and a linear dose–response relationship between the amount of tobacco smoked and the harm, a U-shaped dose response relationship between alcohol consumption and both cardiovascular and respiratory disease and overall mortality has been suggested [2–4]. However, there is strong evidence that heavy consumption is harmful [5] and a systematic review and meta-analysis found no significant reductions in risk of all-cause mortality in those who drank less than 25 g·day−1 of ethanol [6]. In 2023, The World Health Organization published a statement in The Lancet Public Health, stating “when it comes to alcohol there is no safe amount that does not affect health” [7, 8].
Alcohol abuse refers to a pattern of excessive and harmful alcohol consumption that can lead to physical, psychological and social problems. The definition of alcohol abuse varies across different diagnostic classifications, such as the International Classification of Diseases [9] and the Diagnostic and Statistical Manual of Mental Disorders [10]. The Alcohol Use Disorders Identification Test (AUDIT) [11] is widely used in healthcare settings worldwide to assess alcohol consumption patterns, identify potential alcohol-related problems and to determine the likelihood of alcohol use disorder (AUD). In clinical practice, the diagnosis of alcohol problems is based on medical history, clinical signs, detection of biological, psychological and social markers of alcohol damage, and possible specific blood tests, such as those examining gamma-glutamyl-transferase (GGT) and carbohydrate-deficient transferrin (CDT).
One fundamental aspect of alcohol abuse is the consumption of alcohol in quantities that exceed recommended guidelines or thresholds established by health authorities. There is no consensus on the standard drink size, as the modal standard drink is 10 g pure ethanol, but variations from 8 to 20 g are reported [12]. The same variability is seen for low-risk drinking guidelines ranging from 10–42 g·day−1 for women and 10–56 g·day−1 for men to 98–140 g·week−1 for women and 150–280 g·week−1 for men [12].
Research into the relationship between alcohol consumption and pulmonary function has yielded conflicting results, contributing to the complexity of this association. Chronic alcohol abuse has been associated with detrimental effects on lung health. These adverse effects include decreased cough reflex, impaired mucociliary clearance, increased susceptibility to pulmonary infections and increased risk of developing acute respiratory disease [13–15]. However, recent studies have suggested that light to moderate alcohol consumption may lead to improved pulmonary function and a reduced risk of symptom burden in COPD [4, 16]. These findings have sparked considerable interest and raised questions about the nature and extent of alcohol's impact on the respiratory system. However, investigating the association between alcohol consumption and pulmonary function is complex as the high prevalence of smokers in most studies is an important confounder. Also, factors such as the amount, frequency and duration of alcohol intake, beverage preference, together with overall health status, may contribute to variations in the observed outcomes. Therefore, understanding the nuanced relationship between alcohol and pulmonary function requires a comprehensive examination of available research and a synthesis of existing knowledge.
This scoping review aims to provide a comprehensive overview of the current state of research regarding the effects of alcohol on pulmonary function in individuals aged 18 and older to identify whether there is an association between alcohol intake and changes in pulmonary function parameters. We aim to explore the potential protective effect of small to moderate amounts of alcohol and the harmful effect of larger doses to investigate whether existing literature can confirm the theory of the U-shaped association.
Methods
Search strategy
According to the guidelines outlined in the JBI Manual for Evidence Synthesis [17], a research protocol was developed [18].
Reporting followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for Scoping Reviews guidelines [19]. The systematic search for methods to assess the effect of alcohol on pulmonary function was conducted in PubMed, Embase, CINAHL and Cochrane (1 March 2023) (details of the searches can be found in the supplementary material). The systematic search included MESH-terms “Respiratory Function Tests”, “Alcohol Drinking”, “Alcoholism” and “Alcoholic beverages”. The same search terms were used in Embase, CINAHL and Cochrane, with relevant search word terms and free word search, and finally a chain search. Synonyms, near-synonyms, acronyms and index terms for each keyword were also identified. The literature search was conducted under the supervision of a research librarian from Aalborg University with great experience in securing high-quality systematic research. Only studies in English, Danish, Norwegian or Swedish were included. There were no limitations concerning publication year. Reference lists of included studies were hand-searched and citation searching was conducted to identify additional relevant studies within the field.
Eligibility criteria
Full-text studies were included if 1) studies were observational, 2) participants were adults (aged ≥18 years), 3) papers held information about pulmonary function (as a minimum, data on forced expiratory volume in 1 s (FEV1)), alcohol consumption and smoking status (as never-smoker, former smoker or current smoker). Animal, in vitro, clinical and autopsy studies were excluded together with editorials, reviews, case reports and conference abstracts.
Review process: data collection and analyses
The articles were transferred to the software program Covidence and checked for duplicates [20]. Titles and abstracts identified from the search strategy were screened for eligibility by two reviewers. For all eligible abstracts, full-text articles were examined by two reviewers to determine whether they met the inclusion criteria or not. Any disagreements were discussed and, if no consensus could be reached, a third reviewer was consulted. Relevant data (study design, study setting, population, pulmonary function and alcohol consumption) from eligible articles were extracted by a reviewer and verified by a second reviewer. No formal assessment of the quality of the included articles was performed. To investigate the effect of alcohol on pulmonary function, we described the definitions of alcohol consumption, the definitions of pulmonary function changes and the different pulmonary function parameters.
Results
Study overview
Figure 1 describes the screening process. Of 4427 abstracts, 179 were selected for full-text review. A total of 30 articles reporting alcohol and pulmonary function in adults (aged ≥18 years) was included in the final dataset (figure 1) [4, 16, 21–48]. Table 1 shows the characteristics of the included studies. Geographically, 47% of the studies were conducted in North America [21, 24, 28, 30, 31, 34, 38, 41, 42, 44–47], 37% in Europe [4, 23, 25–27, 33, 35–37, 43, 48], 17% in Asia [16, 29, 32, 39, 40] and one study in Nigeria [22]. Timewise, 47% of the studies were published before 2000 [21–26, 31, 42–48]. The most recent studies were from 2022 [40, 41].
FIGURE 1.
Flow diagram of study selection process, as depicted by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [71].
TABLE 1.
Extracted results from the included studies in the review
| First author [ref.] | Country | Year | Study design | Subjects, n (male/female) | Alcohol consumption | Study population | Results |
| Negative effects | |||||||
| Emirgil [31] | USA | 1974 | Cross-sectional | 23 (19/4) | Pint years: pints per day times the number of years of intake | Selected group from detoxification unit | TLC, RV, VC and FEV1 declined with increasing alcohol consumption |
| Emirgil [42] | USA | 1977 | Cross-sectional | 44 (25/19) | Pint years: pints per day times the number of years of intake | Selected group from members of Alcoholics Anonymous | 64% of subjects had abnormal expiratory flow rates, 39% had an elevated value for the ratio of RV to TLC SBDC was abnormal in 16% |
| Sarić [43] | Yugoslavia USA |
1977 | Cross-sectional | 763 (763/0) | None or occasional: ≤0.5 L wine daily Daily: >1 L wine and spirits per day |
Selected group of workers from a ferromanganese factory, an electrode production factory and a light metal plant | Reduced FVC was primarily connected with age, and when all three factors (alcohol, smoking and age) were combined Reduced FEV1 was associated with alcohol consumption and age separately but not smoking habit Alcohol consumption combined with age and when all three factors (alcohol, smoking and age) were combined was statistically significant |
| Lebowitz [46] | USA | 1981 | Cross-sectional | 2637 (1164/1473) | Non-/light drinkers: <0.25 ounces·week−1 Moderate drinkers: 0.25–6.25 ounces·week−1 Heavy drinkers: >6.25 ounces·week−1 |
Population sample of white non-Mexican Americans residing in Tucson Arizona | Negative correlation in young male and female heavy smokers between total amount of alcohol consumed and FEV1 and FVC |
| Oleru [22] | USA Nigeria |
1987 | Cross-sectional | 60 (60/0) | Bottle-years: number of bottles of beer consumed per day times the number of years of intake | Selected group of workers in a cotton textile factory in Lagos | Lifetime alcohol intake was negatively correlated with pulmonary function and obstructive and restrictive pulmonary disease parameters Together with weight, alcohol bottle-years accounted for 18–22% of the variation in pulmonary function in a forward and reverse stepwise regression analysis |
| Lange [23] | Denmark | 1988 | Longitudinal | 8765 (3751/5014) | ALC1: Never or rarely a monthly drink ALC2: <30 g·week−1 ALC3: ≥30–<140 g·week−1 ALC4: ≥140–<350 g·week−1 ALC5: >350 g·week−1 |
Population-based study | Loss of FEV1 and FVC tended to be greater in the group with highest alcohol consumption compared to the other groups Alcohol consumption was positively correlated to the annual decrease in FEV1 and FVC |
| Zureik [26] | France | 1996 | Cross-sectional Longitudinal |
328 (328/0) | 1) 0–25 g·day−1 2) 26–60 g·day−1 3) >60 g·day−1 |
Selected group of policemen | In both 1980 and 1990 surveys age and height adjusted FEV1 was negatively associated with alcohol consumption and GGT Adjustment for covariates did not alter the results When daily alcohol consumption and log GGT were fitted as continuous variables in the multiple regression model, the test of trend was all significant VC displayed associations with alcohol consumption and GGT categories like those observed for FEV1 |
| Ström [25] | Sweden | 1996 | Cross-sectional | 478 (478/0) | 1) 0–40 g·week−1 2) 41–115 g·week−1 3) >115 g·week−1 |
Selected group of men born in even months in 1914 in Malmö | All men: after correction for smoking status and BMI, TLC and RV were significantly positively related to alcohol intake Current smokers: after correction for current tobacco consumption and BMI, TLC and RV were significantly positively correlated with alcohol intake Smokers with obstruction: after correction for current tobacco consumption and BMI, TLC and RV were significantly positively correlated with alcohol intake |
| Frantz [36] | Sweden | 2014 | Cross-sectional | 450 (185/265) | Based on AUDIT: 1) nondrinkers 2) moderate drinkers 3) hazardous drinkers CDT ≥2.0%: heavy alcohol consumption |
Population-based study | Heavy drinking (CDT level) compared to nonheavy drinkers were associated with lower FEV1/VC and DLCO For heavy and nonheavy drinkers, a significant difference was seen for DLCO when adjustments were made for several covariates After adjusting for crude lung function variables for covariates, a higher CDT was associated with lower FEV1, VC, FEV1/VC and DLCO Multiple regression showed an association between CDT and both FEV1 and DLCO in all alcohol drinkers but not in never-smokers |
| Sorli-Aguilar [37] | Spain | 2016 | Cross-sectional | 207 (91/116) | Units·week−1 Divided into: 1) lower tertile 2) middle tertile 3) upper tertile |
Randomised smokers without respiratory disease selected from 20 primary healthcare centres | More than two- or three-times prevalence of impaired lung function in the medium and highest tertiles of the alcohol-consumption pattern compared with the lowest The differences between tertiles were more intense in women |
| No effects | |||||||
| Cohen [44] | USA | 1980 | Cross-sectional | 2519 (1282/1237) | Based on quantity, frequency and maximal consumption: 1) light 2) moderate 3) heavy |
Population-based study | Unadjusted mean values of FEV1/FVC were significantly lower for heavy than for light drinkers The differences disappeared when adjustment was made for confounding factors There was also no evidence of an association between alcohol consumption and airway restriction |
| Sarkar [45] | USA | 1980 | Cross-sectional | 10 (9/1) | Pint-years: average daily consumption times number of years of alcoholism |
Selected group from the medical service of the Hospital for Joint Diseases and Medical Center, New York, and outpatients All were chronic heavy drinkers and nonsmokers |
Mean values in all pulmonary function studies were within normal limits |
| Sparrow [47] | USA | 1983 | Cross-sectional Longitudinal |
1067 (1067/0) | 1) 0–0.25 ounces·week−1 2) 0.26–6.25 ounces·week−1 3) >6.25 ounces·week−1 |
Selected group of white men from the Veterans Administration Outpatient Clinic in Boston | A multiple regression analysis indicated that alcohol consumption did not significantly influence baseline levels of FVC or FEV1 after controlling for covariates Alcohol consumption did not significantly influence follow-up levels of FVC or FEV1 after controlling several covariates |
| Lyons [48] | UK | 1986 | Case–control | 27 (21/6) | 1) 0–0.25 ounces·week−1 2) 0.26–6.25 ounces·week−1 3) >6.25 ounces·week−1 |
Selected subjects who were referred for assessment and treatment of various alcohol-related problems | No difference in pulmonary function between alcoholics and controls |
| Hoffstein [21] | Canada | 1987 | Longitudinal | 33 (32/1) | Alcoholics: 1) on average 80 g·day−1 or more of ethanol ≥3 months prior or 2) on average 160 g·day−1 ethanol ≥1 month prior |
Selected group of alcoholics admitted to the Addiction Research Foundation Clinical Institute within 5 days of their last drink | In smoking alcoholics, short-term abstinence from alcohol did not influence pulmonary function Mean values of FVC, FEV1 and ratio were within normal range |
| Garshick [24] | USA | 1989 | Cross-sectional | 165 (165/0) | Based on quantity and frequency kg·year−1 and g·month−1 Divided into: 1) lower tertile 2) middle tertile 3) upper tertile |
Selected population from the population of veterans in Southeastern Massachusetts Subjects recruited from the alcohol detoxification and rehabilitation wards The study cohort also included male hospital employees |
Alcohol consumption tended to have a negative effect on FEV1/height2 |
| Shin [29] | Korea | 2003 | Cross-sectional | 1160 (483/677) | 1) 0 drinks·week−1 2) 1–7 drinks·week−1 3) 8–21 drinks·week−1 4) ≥22 drinks·week−1 |
Population-based study | The odds of airway obstruction increased with increasing alcohol intake |
| Tang [32] | Hong Kong | 2005 | Cross-sectional | 300 (300/0) | AUDIT, cut-off score: 8 | Selected subjects from Hong Kong who sought compensation for pneumoconiosis | The drinking group had a higher unadjusted FEV1 predicted than the nondrinking group The differences between the FEV1 of the two groups was not significant after adjustments for covariates |
| Zifodya [41] | USA | 2022 | Cross-sectional | 350 (241/109) | 1) AUDIT, cut off score: 8 2) Lifetime alcohol exposure in grams 3) Early life alcohol use (frequency of alcohol use from 10 to 20 years old and from 21 to 30 years old) 4) Recent alcohol use (measured by whole-blood spot phosphatidyl ethanol level) 5) Alcohol use latent class: Heavy drinkers, former heavy drinkers, heavy drinkers with problems and low-risk drinkers |
Selected subjects of people living with HIV in Louisiana | In adjusted models, total lifetime alcohol use was not associated with FEV1, FVC or FEV1/FVC In multivariable models no association of AUDIT score with FEV1, FVC and ratio was found; a similar result was found related to early alcohol use |
| Positive effects | |||||||
| Tabak [27] | The Netherlands | 2001 | Cross-sectional | 13 651 (6279/7372) | 1) None or ≤1 drink·week−1 2) >1 drink·week−1 and ≤3 drinks·day−1 3) >3 drinks·day−1 |
Population-based study | In subjects with low alcohol consumption the FEV1 was higher than in nondrinkers |
| Schünemann [28] | USA | 2002 | Cross-sectional | 1555 (741/814) | 1) Never drinkers: <12 drinks in a lifetime 2) Not current drinkers: ≥12 or more drinks in a lifetime, no intake in the past 30 days 3) Current drinkers: alcohol intake in the past 30 days In current drinkers, grams of alcohol calculated |
Population-based study | Positive associations were found between recent and lifetime wine intake and FEV1 and FVC When analysing white and red wine intake separately, the association of lung function with red wine was weaker than for white wine |
| Sisson [30] | USA | 2005 | Cross-sectional | 15 294 (7135/8159) | 1) Lifetime never-drinker 2) Former heavy drinker 3) <5 drinks·month−1 4) 5–14 drinks·month−1 5) 15–30 drinks·month−1 6) 31–90 drinks·month−1 7) >90 drinks·month−1 |
Population-based study | Low to moderate alcohol intake was associated with better FVC and FEV1 in the absence of obstruction, consistent with reduced odds for lung restriction |
| Hansel [33] | France | 2010 | Cross-sectional | 149 773 (97 406/52 367) | 1) No consumption, 2) Low: fewer than 1 glass·day−1 3) Moderate: 1–3 glasses·day−1 4) High: >3 glasses·day−1 5) Former drinkers |
Population-based study | In both genders, respiratory function assessed by FEV1 was highest in moderate drinkers and lowest in never-drinkers Similar results after adjustment for tobacco consumption |
| Siedlinski [35] | The Netherlands | 2012 | Longitudinal | 3224 (1560/1664) | Grams of wine per day | Population-based study | The intake of white wine was associated with higher FEV1 level Significant interaction of pack-years smoked and white wine intake with the FEV1 This interaction reflected an association between white wine consumption and higher FEV1 in heavy smokers only White wine intake was significantly associated with a decreased risk of airway obstruction |
| Vasquez [38] | USA | 2018 | Longitudinal | 1333 (60–62% female participation in each survey) | Longitudinal drinking categories: 1) never-drinker 2) inconsistent drinker 3) persistent drinker Quantative drinking exposure (drinks·month−1): 1) none 2) <5 3) 5–<15 4) 15–<30 5) 30–<90 6) 90–<140 |
Population-based study of non-Hispanic white households in Arizona | After adjustment for several covariates, as compared to never drinkers, persistent drinkers had higher FVC but a lower ratio Differences were due to a slower decline of FVC among persistent than never-drinkers and these trends were present independent of smoking status Inconsistent drinking showed similar but weaker associations After adjustment for potential confounders, light to moderate alcohol consumption was associated with a significantly decreased rate of FVC decline over adult life and associated with protection from restriction |
| Choi [39] | Korea | 2020 | Cross-sectional | 3262 (1801/1461) | AUDIT, cut-off score: 8 | Population-based study | In nonsmokers, men with AUDIT score ≥8 demonstrated a significantly higher FEV1/FVC than those with AUDIT score <8 |
| Makino [16] | Japan | 2021 | Cross-sectional Longitudinal |
Cross-sectional: 6036 (3696/2340) Longitudinal: 1765 (1148/617) |
Based on quantity and frequency, g·week−1 were calculated and divided into: 1) never-drinker 2) light 3) moderate 4) heavy |
Population-based study | Moderate alcohol consumption was positively correlated with FEV1 and FVC in the cross-sectional study In the longitudinal study over 5 years higher baseline alcohol consumption, as well as increased alcohol intake over 5 years attenuated time-related deterioration of FVC without affecting total lung volume This effect was independent of smoking |
| Wang [40] | China | 2022 | Cross-sectional Longitudinal |
Cross-sectional: 16 268 (6451/9817) Longitudinal: 8914 (not available) |
Noncurrent drinkers: weekly intake previous 6 months; otherwise, they were regarded as nondrinkers, including never-drinkers and former drinkers Drinkers were further divided into: 1) moderate drinkers 2) heavy drinkers |
Population-based study | Compared with nondrinkers, moderate alcohol intake was significantly associated with increases in FEV1 and FVC, after adjusting for covariates Regarding beverage type, red wine was associated with increases in FEV1 and FVC in the total population Moderate alcohol intake was also associated with increases in FEV1 and FVC for liquor and red wine In the longitudinal analyses, moderate alcohol intake and red wine were associated with increases in FVC, respectively |
| U-shape association | |||||||
| Tabak [4] | The Netherlands | 2001 | Cross-sectional | 2953 (2953/0) | 1) No alcoholic drinks 2) ≤1 drink·week−1 3) >1 drink·week−1 and ≤3 drinks·day−1 4) >3 and ≤9 drinks·day−1 5) >9 drinks·day−1 |
Population-based study in Italy, the Netherlands and Finland | In Finland and the Netherlands, pulmonary function was higher in occasional and light drinkers (>0 and <30 g·day−1) compared with nondrinkers In Italy, very heavy drinkers had a lower FEV0.75 than moderate-to-heavy drinkers (>3 and <12 drinks·day−1) |
| Siu [34] | USA | 2010 | Cross-sectional | 177 721 (81 498/96 223) | 1) No alcohol 2) ≤2 drinks·day−1 3) 3–5 drinks·day−1 4) ≥6 drinks·day−1 |
Population-based study with members of a comprehensive health plan | Light to moderate drinkers of alcohol had better FEV1, FVC and FEV1/FVC than abstainers Heavier drinkers had worse lung function |
AUDIT: Alcohol Use Disorders Identification Test; BMI: body mass index; CDT: carbohydrate-deficient transferrin; DLCO: diffusing capacity of the lungs for carbon monoxide; FEV0.75: forced expiratory volume in 0.75 s; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; GGT: gamma-glutamyl-transferase; RV: residual volume; SBDC: single breath diffusing capacity; TLC: total lung capacity; VC: vital capacity.
Study methodology
All 30 studies were observational. A strictly cross-sectional study design was used in 21 (70%) of the studies [4, 22, 24, 25, 27–34, 36, 37, 39, 41–46], four (13%) used a combined cross-sectional and longitudinal study design [16, 26, 40, 47], four (13%) were strictly longitudinal (prospective follow-up) [21, 23, 35, 38] and one study was a case–control study [48].
Population characteristics
Study population sizes varied considerably. The largest study by Siu et al. [34] included 177 721 subjects. The smallest sample size was an observational study by Sarkar et al. [45] with 10 subjects. Gender distribution also varied greatly, eight studies only included men [4, 22, 24–26, 32, 43, 47] and, in six studies, more than two thirds of the included patients were men (65–97%) [21, 31, 33, 41, 45, 48]. Some of the studies had specified study populations. Six studies were conducted on subjects defined as chronic alcoholics [21, 31, 45], members of Alcoholics Anonymous [42] or patients receiving treatment for various alcohol-related diseases [24, 48]. The study by Sarkar et al. [45] was the only one conducted on nonsmoking subjects [45]. Sarić et al. [43] and Oleru et al. [22] conducted their studies on workers employed in different factories. In two studies, the study population had specific comorbidities, e.g. Tang et al. [32] included subjects with pneumoconiosis and Zifodya et al. [41] focused on subjects with HIV. Adjustments for socioeconomic status and/or education was only described in 10/30 studies [16, 26, 28, 30, 34, 37, 38, 44, 46, 47].
Pulmonary function characteristics
In six studies, both spirometry and body plethysmography, including results for FEV1, forced vital capacity (FVC), FEV1/FVC ratio, total lung capacity (TLC), diffusing capacity of the lungs for carbon monoxide (DLCO) and residual volume (RV) was carried out [21, 31, 36, 42, 45, 48].
In 18 studies, spirometry with measurements of FEV1, FVC and FEV1/FVC was carried out [4, 16, 22, 23, 26, 29, 30, 32, 34, 35, 37–41, 43, 44, 46]. Three studies only included FEV1 and FVC [24, 28, 47] and two studies only included FEV1 [27, 33].
Different definitions of obstructive and restrictive disease were used in the studies. Sisson et al. [30] defined airway obstruction as FEV1/FVC <0.7 and FEV1 less than 80%, and restrictive lung function as FEV1/FVC >0.7 and FVC <80%. Shin et al. [29] defined airway obstruction as FEV1/FVC <75% and the severity of the airway obstruction was further categorised depending on the value of FEV1. Lyons et al. [48] defined an FEV1/FVC <0.8 as indicative of an obstructive defect and DLCO <80% together with a low RV and a high FEV1/FVC as indicative of a restrictive defect.
Definition of alcohol consumption
In the following, we will describe differences in the reporting of consumption and the differences in the definition of one drink.
Four studies expressed alcohol consumption in terms of grams per day or week [23, 25, 35, 48]. Three of these studies further subdivided the subjects into different categories in terms of their alcohol consumption with varying definitions for each study [23, 25, 35].
In the study by Oleru et al. [22], alcohol consumption was expressed as lifetime intake in bottle-years, i.e. the average daily consumption in bottles of beer times the number of years of intake. Two studies used the measure pint-years defined as average daily consumption in pints times the number of years of alcoholism [31, 45]. Three studies used alcoholic drinks consumed per day/week and divided subjects into different categories depending on the number of alcoholic drinks [4, 34, 38]. Four studies used the AUDIT score to divide people into different categories [32, 36, 39, 41]. Frantz et al. [36] used an increase in CDT as an indicator of heavy alcohol consumption and Zureik et al. [26] used GGT as a biological marker of drinking. Schünemann et al. [28] and Sisson et al. [30] used extensive questionnaires regarding alcohol consumption during the previous 30 days and the participants’ lifetime.
19 studies included a specific definition of one drink [4, 21–24, 26–31, 33, 34, 38–40, 42, 47, 48]. Tabak et al. [4, 27] defined a drink as containing 10 g of alcohol and Shin et al. [29] defined one drink as equivalent to an intake of 12.5 g ethyl alcohol.
Definition of tobacco consumption
In 10 studies, the population was described as never, former or current smokers [4, 16, 28, 29, 32, 33, 35, 38, 40, 41] and 14 studies gave more detailed definitions [23, 24, 44, 46–48, 25, 26, 30, 34, 36, 39, 42, 43]. Three studies defined pack-years as the number of cigarettes per day/20 and the number of years smoking [27, 37, 46]. Another study defined a packet of cigarettes as 25 cigarettes [4].
Negative effect of alcohol on pulmonary function
As described in table 1, 10/30 studies found alcohol to have a negative effect on pulmonary function [22, 23, 25, 26, 31, 36, 37, 42, 43, 46]. In all of these studies, heavy alcohol consumption was associated with a negative effect on pulmonary function. Lange et al. [23] found, in a study of 8765 subjects, that loss of FEV1 and FVC was greater in a group consuming more than 350 g·week−1 of alcohol compared to the other groups. In a study by Frantz et al. [36] that included 450 subjects, it was found that heavy drinkers (defined from CDT score) had significantly lower DLCO and FEV1/FVC than nonheavy drinkers. This was also seen after adjustments for several covariates. Multiple regression showed an association between CDT and both FEV1 and DLCO in all alcohol drinkers except never-smokers. In six studies, smoking was identified as a confounder on the effect of alcohol on pulmonary function [25, 31, 36, 37, 42, 46]. The studies by Emirgil et al. [31, 42] included 91–96% smokers, Lebowitz et al. [46] only found a negative effect in heavy smokers and, in the study by Sorli-Aguilar et al. [37], all the subjects were smokers. Ström et al. [25] found, in a study of 478 subjects, that TLC and RV increased in relation to alcohol intake, but only in current smokers and smokers with obstruction.
No effect of alcohol on pulmonary function
As shown in table 1, nine studies found no significant association between alcohol consumption and pulmonary function parameters [21, 24, 29, 32, 41, 44, 45, 47, 48]. Although five studies found both negative and positive effects of alcohol intake on pulmonary function, these associations were no longer found when adjusting for covariates, such as smoking, socioeconomics and age [24, 29, 32, 44, 47]. This effect was seen in both light drinkers [29, 32, 47] and heavy drinkers [24, 44, 47]. The only study within this review involving nonsmokers, conducted by Sarkar et al. [45], found no association between alcohol consumption and pulmonary function. A recent study from 2022 by Zifodya et al. [41] found no association between the AUDIT score and FEV1, FVC and FEV1/FVC in multivariable analysis. Similar results were found when analysing subjects with frequent early alcohol use from the ages of 10–20 and 21–30 years old. In the study by Hoffstein et al. [21], there was no improvement in pulmonary function within 4 weeks of abstinence from alcohol. This is in line with the findings of Emirgil et al. [31] and Lyons et al. [48] where abstinence for periods averaging 9 days and 11 weeks did not alter pulmonary function.
Positive effect of alcohol on pulmonary function
As shown in table 1, nine studies found alcohol to have a positive effect on pulmonary function [16, 27, 28, 30, 33, 35, 38–40]. Six studies showed a positive association between low to moderate alcohol intake on pulmonary function compared to nondrinkers [16, 27, 30, 33, 38, 40]. Vasquez et al. [38] found that persistent drinkers had higher FVC compared to never-drinkers after adjustment for confounders. After adjustment for potential confounders, light to moderate alcohol consumption was associated with a significantly decreased rate of FVC decline throughout adult life. The cross-sectional studies by Wang et al. [40] and Makino et al. [16] both found that moderate alcohol intake was positively correlated with FEV1 and FVC. These two longitudinal studies found that moderate alcohol intake and red wine were associated with an increase in FVC and 5-year attenuated deterioration of FVC, respectively [16, 40]. The positive association between wine consumption and pulmonary function was also studied by Schünemann et al. [28] and Siedlinski et al. [35]. Schünemann et al. [28] found positive associations between recent and lifetime wine intake and FEV1% and FVC% [28]. When analysing white and red wine intake separately, the association between pulmonary function and red wine was weaker than for white wine. Siedlinski et al. [35] found that intake of white wine was associated with a higher FEV1 level and a decreased risk of airway obstruction. They found no association between red wine intake and FEV1, FVC or airway obstruction.
U-shaped association between alcohol consumption and pulmonary function
As shown in table 1, two studies studied the U-shaped association with pulmonary function [4, 34]. Tabak et al. [4] compared pulmonary function with alcohol consumption in three European countries. Analysis of data from 2953 middle-aged men from Finland, Italy and the Netherlands showed a U-shaped risk curve where reduced pulmonary function was observed among nondrinkers compared with occasional and light drinkers and among very heavy drinkers compared with moderate to heavy drinkers. Importantly, the U-shaped risk curve was independent of age, height, body mass index, smoking status or country. These findings were supported by Siu et al. [34] who found, in a study of 177 721 subjects, that mean FEV1, FVC and FEV1/FVC ratios were lower in both abstainers and the heaviest drinkers (six or more drinks per day), compared to subjects with an intermediate alcohol intake.
Discussion
In this systematic scoping review, 10/30 studies showed a negative effect on pulmonary function parameters, 9/30 studies showed no effect on pulmonary function parameters, 9/30 studies showed a positive effect on pulmonary function parameters and 2/30 studies showed a nonlinear U-shaped association of alcohol with pulmonary function parameters.
Negative or no effects of heavy alcohol consumption on pulmonary function
We found conflicting results regarding heavy alcohol consumption and pulmonary function. 10 studies found that heavy alcohol consumption was associated with a negative effect on pulmonary function [22, 23, 25, 26, 31, 36, 37, 42, 43, 46]. Six studies with heavy drinkers did not show an effect on pulmonary function [21, 24, 29, 45, 47, 48]. In three of those studies, the associations disappeared when adjusting for covariates [24, 29, 47]. In both groups of negative and no effect of alcohol on pulmonary function, the majority of the studies are more than 20 or 30 years old [21–26, 31, 42, 43, 45–48]. As such, there may be socioeconomic confounders that are unaccounted for in these studies. In addition, the majority of the studies in both groups had small study populations [21, 22, 24–26, 31, 36, 37, 42, 45, 48]. Selection bias may therefore also influence results. Not least, the studies had different definitions of alcohol consumption and of former and current alcoholism, making it difficult to compare the different studies. It is therefore very difficult to draw any conclusions based on these studies.
The studies by Lange et al. [23] and Sparrow et al. [47] had comparable study designs; however, they arrived at different results. Yet, they differed in terms of their definition of high alcohol consumption. Sparrow et al. [47] defined high intake as exceeding 6.25 ounces·week−1, whereas the threshold given by Lange et al. [23] was almost twice as high, at 350 g·week−1, which may have influenced the outcome. The population studied by Sparrow et al. [47] had no chronic medical conditions prior to inclusion, which hypothetically could exclude heavier drinkers. As such, this may still indicate a negative effect of a very high alcohol intake on pulmonary function and thereby still support the theory of a U-shaped curve.
The confounding effect of smoking
In 6/10 of the studies finding heavy alcohol to have a negative effect on pulmonary function, smoking influenced the association between alcohol intake and pulmonary function [25, 31, 36, 37, 42, 46]. In the studies by Frantz et al. [36] and Ström et al. [25], no effect of alcohol on pulmonary function in never-smokers was found, which correlates with the studies by Choi et al. [39] and Wang et al. [40] that found a positive association between alcohol consumption and pulmonary function but only in nonsmoking subjects. The lack of a smoking effect in the study by Oleru et al. [22] could be attributed to the mean number pack-years being only 3.6. Sarić et al. [43] only found a decrease in FVC when correcting for age and smoking, whereas no effect was seen in terms of FEV1. The mean age was, however, only 37 years, so a possible effect may become evident in the years ahead.
In 3/6 studies that in the final analyses did not find an effect of heavy alcohol on pulmonary function, the effects of alcohol on pulmonary function seen in the crude analyses disappeared when adjusting for covariates such as smoking [24, 29, 47]. In the three other studies, study design and lack of smokers could be part of an explanation. In the study by Hoffstein et al. [21], all of the subjects were either current or former smokers. However, the study looked at improvement in pulmonary function following abstinence of alcohol for 4 weeks and such an improvement was not observed; as such, the study design did not align with any of the other included studies. In the case–control study by Lyons et al. [48], 23/27 participants were current or former smokers and the cases were matched on smoking history and pack-years. Both groups had impaired pulmonary function in 13 and 15 of the cases, probably related to smoking, but no association with alcohol intake was seen.
Only one study was conducted in nonsmokers and showed no effect of alcohol consumption on pulmonary function; however, only 10 subjects were included in this study [45].
As mentioned in the various studies, the examination of a nonsmoking subgroup has elucidated distinct findings regarding the impact of alcohol consumption on pulmonary function. Alcohol appears to have no effect on or a positive association with pulmonary function in nonsmokers in several studies [25, 36, 39, 40, 45]. In smokers, the beneficial effect of alcohol intake on pulmonary function appears to be counteracted by the detrimental effects of cigarette smoking. Notably, Lange et al. [23] reported a deleterious effect of heavy alcohol consumption on pulmonary function, particularly in nonsmokers. However, it is pertinent to note that this study lacks a distinct category for former smokers, raising the possibility that these individuals may have been included within the nonsmoking subgroup, potentially confounding the observe results.
Moreover, Emirgil et al. [42] highlighted the presence of COPD in 3/4 never-smoking women, suggesting a potential role of past alcoholism in the pathogenesis of COPD among female former alcoholics. Nevertheless, the statistical power of this study was low and crucial environmental factors influencing pulmonary health were not adequately described.
It is well established that smoking has a huge effect on the pulmonary function [49]. We know that the prevalence of respiratory disease in alcoholics is high, but as seen in this review the results regarding the effect of alcohol on pulmonary function are inconsistent and a major reason for this is the co-existing use of tobacco and alcohol. People with AUDs are two to three times more likely to smoke cigarettes than those without AUDs [50]. Taken together, there is a complex interplay between tobacco and alcohol use. Based on the current studies, it is not possible to dissect the effects of the two on pulmonary function. Future studies on alcohol's effect on pulmonary function should preferably be performed in nonsmokers or account for a very detailed smoking history.
Biological causes of the negative effects of alcohol
Besides tobacco smoking, we know that excessive alcohol consumption predisposes individuals to infectious diseases such as pneumonia [13], especially Klebsiella pneumoniae [51] and Streptococcus pneumoniae [52], as well as acute respiratory syndrome [14, 53]. Many factors may contribute to this. People who abuse alcohol are at greater risk of aspiration of gastric acid, impairment of mucociliary clearance [15, 54] and the effects of alcohol on the immune system [55–57]. The living conditions of heavy drinkers are often challenging, which may also predispose them to infections and liver damage [58]. In addition, high alcohol consumption is often associated with malnutrition [59], which can result in impaired immunity and an increased risk of community-acquired pneumonia [60]. However, this potential damage may not be significant in generally healthy free-standing populations.
Positive effects of light to moderate alcohol consumption on pulmonary function
Six studies found a positive link between low to moderate alcohol intake and pulmonary function when compared to nondrinkers [16, 27, 30, 33, 38, 40]. These studies contrast with studies that report negative or no effects. The six studies comprise larger studies with more subjects (ranging from 1333 to 149 773 subjects) and are more recent, with the oldest study from 2001 [27] and the most recent from 2022 [40]. There was no difference between the number of longitudinal and cross- sectional designs between the three groups of studies.
Makino et al. [16] conducted a large cross-sectional and longitudinal study in 2021 and found a positive association between alcohol intake and pulmonary function. These results are supported by the longitudinal study by Vasquez et al. [38]. After adjustment for various variables, as compared to never-drinkers, persistent drinkers had a slower decline of FVC and this trend was present independent of smoking status. The strength of the study by Vasquez et al. [38] is its large study population, encompassing drinking data collected over a 20-year follow-up period. Also, the subjects performed repeated pulmonary function tests in up to 11 surveys, which allows for an comprehensive assessment of the pulmonary function trajectory over time. A limitation is that they restricted analysis to light and moderate consumers of alcohol and, in turn, removed the influence of heavy drinking as subjects who reported heavy alcohol intake defined as more than 140 drinks·month−1 were excluded from primary analyses. The study by Sisson et al. [30] supports that alcohol can have a positive association with pulmonary function. Compared to “lifetime never-drinker” group, all categories of alcohol intake, except for the “former heavy drinker” group, were associated with a lower prevalence of pulmonary restriction. They found that “former heavy drinkers” appeared to have a higher prevalence of pulmonary obstruction. This can possibly be explained by smoking because the least common grouping “former heavy drinker” with “never-smoker” represented only 0.5% of the study population [30].
The positive effect of wine on pulmonary function
Schünemann et al. [28] and Siedlinski et al. [35] found a positive association between white wine and pulmonary function. Reverse causation could potentially be a concern in this context, as people with better health and pulmonary function might be more affluent and more likely to drink white wine. However, adjustments for factors known to be associated with better pulmonary function (e.g. higher socioeconomic status and other beneficial health factors) did not substantially change the effect estimates. Indeed, in vivo and in vitro studies have shown that polyphenolic compounds such as resveratrol and quercetin, found in red wine, have been shown to have antioxidant and anti-inflammatory effects [61–63]. This may explain the pronounced benefits of red wine on pulmonary function. The studies on red wine did not show a significant effect on pulmonary function, with the exception of Wang et al. [40]; whereas Cohen et al. [44] failed to find a protective effect of wine intake on pulmonary function whatsoever.
Biological causes of the positive effects of alcohol
Besides the possible beneficial effects of antioxidants, several other protective effects of alcohol have been suggested. A bronchodilator effect of alcohol has been shown in people with asthma, likely due to relaxation of bronchial smooth muscle [64, 65]. Other reasons could be that alcohol has been shown to lower inflammatory markers such as white blood cells and fibrinogen, indicating possible mechanisms and suggesting protection against inflammation at low intake but a poor anti-inflammatory response at high intake [66–68]. Along the same lines, one study suggested that while heavy alcohol exposure may impair mucociliary clearance, exposure to light to moderate alcohol may actually improve airway clearance [69]. Taken together, although evidence is still sparse, some studies indicate that a mild intake of alcohol, especially wine, may have a protective effect on pulmonary function.
U-shaped association between alcohol and pulmonary function
Two studies specifically studied the U-shaped association between alcohol consumption and pulmonary function and both studies found a U-shaped risk curve [4, 34]. This is in line with most of the results presented earlier in this review where we found that heavy alcohol consumption might have a negative effect on pulmonary function whereas a moderate to low intake might have a positive effect on pulmonary function. A limitation in the study of Siu et al. [34] is that the reference group consisted of nondrinkers, defined as someone who did not drink during the previous year, thereby failing to separate ex-drinkers from lifelong abstainers. The group could potentially contain people with alcohol-related or other medical problems and thereby make light to moderate drinkers spuriously appear healthier. They attempted to deal with the “sick quitter” problem by studying the subcohort with no evidence of cardiovascular and respiratory diseases. It remains to be clarified whether the beneficial effect of light alcohol consumption compared with not drinking alcohol regularly is caused by a direct effect of alcohol in the studies or by a confounding effect of ill health among ex-drinkers or by differences in other characteristics between the subjects in the different categories of alcohol consumption. Altogether, more studies investigating the U-shaped curve are needed to confirm the theory.
Association between alcohol intake and spirometric restriction versus obstruction
In this review we have examined various associations between light and heavy alcohol consumption and spirometric parameters indicative of restriction and obstruction. Studies conducted by Vasquez et al. [38], Sisson et al. [30] and Makino et al. [16] investigated pulmonary restriction, revealing a positive association with light to moderate alcohol consumption. However, Makino et al. [16] and Vasquez et al. [38] also noted a negative impact of alcohol on the FEV1/FVC ratio. This observation aligns with earlier findings indicating a detrimental effect of alcohol on obstructive parameters, particularly associated with heavy alcohol intake [23, 25, 29, 31, 36, 42, 46]. Sisson et al. [30] highlighted that individuals with a history of heavy alcohol consumption exhibited significantly increased odds of pulmonary obstruction compared to those who had never consumed alcohol [30]. Additionally, Makino et al. [16] demonstrated that the decline in FEV1/FVC ratio increased with increased total alcohol intake [16]. Conversely, Siedlinski et al. [35] found that the consumption of white wine was associated with a reduced risk of airway obstruction. Furthermore, Choi et al. [39] reported a significantly higher FEV1/FVC ratio among nonsmokers with higher AUDIT scores compared to those with a lower score. These findings indicate that alcohol intake may exert varying effects on restrictive and obstructive pulmonary function. Moreover, the quantity of alcohol consumed appears to influence these outcomes. Consequently, drawing definitive conclusions regarding the impact of alcohol on pulmonary function parameters remains challenging.
Limitations
There are limitations to this review. First and foremost, the studies did not use comparable measures for alcohol intake. Whereas pack-years for tobacco intake has become a generally accepted terminology, a similar quantification of alcohol intake does not exist [70]. Secondly, the studies have different stratifications for mild, moderate and heavy use, which is very consistent with the worldwide differences in recommendations for acceptable intake.
In most studies the recorded smoking history and alcohol consumption rely on self-reported data. This opens up the possibility of subjects either under- or over-reporting their smoking or alcohol habits and in several studies recall bias may also exist as people are asked to report previous use. Likewise, there may be a selection bias in the included studies as those who abstain from participation might be in poorer health and diverse factors contribute to the divergent outcomes observed in the studies. Individuals who formerly had high alcohol intake might have ceased due to health issues and symptoms, erroneously placing them in the nondrinking category instead of their prior excessive consumption.
There are some demographic challenges in the included studies. Gender distribution among participants varied, with many studies focusing solely on men or predominantly including men. Discrepancies in comorbidities exists among the studies, with some accounting for these factors while others omitting any mention. Different ethnicities also contribute to differences between the studies. Age distribution could also be a factor, because deterioration of pulmonary function in smokers is not immediate and most patients require years of consumption for changes in spirometry results to be observed. Thus, older people are more susceptible than younger adults. In addition, population size varied significant among studies, ranging from 10 participants to more than 100 000. The heterogeneity of the study designs and the poor power in many of the studies also makes them difficult to compare.
Only 10/30 studies described adjustments for socioeconomic status and/or education. Failing to account for these can introduce bias, confound the results and limit the generalisability of findings. Adjusting for these important factors is essential for obtaining accurate and reliable conclusions.
As presented earlier in the review, smoking is potentially a confounding factor in most of the included studies. In addition, there may be a dose–response confounder affecting the U-shaped curve theory, as most of the studies indicate that those who drink the most also have the largest tobacco consumption. However, our knowledge about actual tobacco consumption is limited, as the studies present the description of smoking differently and many studies do not quantify the number of cigarettes smoked.
One major limitation of this review is the lack of consensus on a generally accepted definition of one unit of alcohol, on the term “low-risk consumption” and how alcohol intake is measured. As seen in the 30 studies, there are huge differences in these factors making it almost impossible to compare them. Until we have a clear definition on how we describe alcohol intake it will be very difficult to reach a clear answer on how alcohol might affect pulmonary function.
Conclusion
The literature currently cannot confirm the hypothesis of the U-shaped curve, but there are strong indications of a negative effect of a heavy alcohol consumption and a positive effect of a low to moderate consumption. However, the available studies are diverse in quality and quantity and the quantification of alcohol intake is not comparable. To investigate this further, there is a need for more longitudinal and consistent international studies. Furthermore, smoking is thought to be a large confounder in most of these studies and it is almost impossible to separate the effects of smoking from the effects of alcohol.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0233-2023.SUPPLEMENT (99.2KB, pdf)
Acknowledgements
We want to thank Conni Skrubbeltrang, Master of Library and Information Science and Chief Librarian at Aalborg University Hospital (Aalborg, Denmark) for her contribution to the searches performed in the selected databases.
Provenance: Submitted article, peer reviewed.
Author contributions: L.B. Nielsen conceptualised the idea for the scoping review, led the protocol and methodology design and authored the initial draft of the manuscript. L.B. Nielsen, M.O. Johansen, S.J. Riddersholm and U.M. Weinreich contributed to the methodology design, designed the search strategy, and critically revised the manuscript. L.B. Nielsen, M.O. Johansen and U.M. Weinreich designed the data extraction form. All authors provided valuable inputs to the research questions and subject matter.
Conflict of interest: S.J. Riddersholm reports grants for post-doctoral employment from GE. U.M. Weinreich reports grants from Fisher & Paykel; lecture honoraria from AstraZeneca, GSK, ResMed, Fisher & Paykel, Orion Pharma, Boehringer Ingelheim and Pfizer; payment for expert testimony from GSK; travel support from Chiesi; advisory board participation for AstraZeneca, GSK, Novartis, TEVA and Boehringer Ingelheim; and leadership roles as Chairman of the Danish Respiratory Society, Chairman of the Lungeforeningens Research Grant, outside the submitted work. All other authors have nothing to disclose.
References
- 1.Hammer JH, Parent MC, Spiker DA. Global Status Report on Alcohol and Health 2018. World Health Organization, 2018. [Google Scholar]
- 2.Doll R, Peto R, Hall E, et al. . Mortality in relation to consumption of alcohol: 13 years’ observations on male British doctors. BMJ 1994; 309: 911–918. doi: 10.1136/bmj.309.6959.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Gaetano G, Costanzo S. Alcohol and health: praise of the J curves. J Am Coll Cardiol 2017; 70: 923–925. doi: 10.1016/j.jacc.2017.07.710 [DOI] [PubMed] [Google Scholar]
- 4.Tabak C, Smit HA, Räsänen L, et al. . Alcohol consumption in relation to 20-year COPD mortality and pulmonary function in middle-aged men from three European countries. Epidemiology 2001; 12: 239–245. doi: 10.1097/00001648-200103000-00018 [DOI] [PubMed] [Google Scholar]
- 5.Rehm J, Gmel GE, Gmel G, et al. . The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction 2017; 112: 968–1001. doi: 10.1111/add.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J, Stockwell T, Naimi T, et al. . Association between daily alcohol intake and risk of all-cause mortality: a systematic review and meta-analyses. JAMA Netw Open 2023; 6: E236185. doi: 10.1001/jamanetworkopen.2023.6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . No level of alcohol consumption is safe for our health. Date last accessed: 7 October 2023. Date last updated: 4 January 2023. www.who.int/europe/news/item/04-01-2023-no-level-of-alcohol-consumption-is-safe-for-our-health
- 8.Anderson BO, Berdzuli N, Ilbawi A, et al. . Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health 2023; 8: e6–e7. doi: 10.1016/S2468-2667(22)00317-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva, World Health Organization, 1993. [Google Scholar]
- 10.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th Edn. Washington, DC, American Psychiatric Association, 2013. [Google Scholar]
- 11.Saunders JB, Aasland OG, Babor TF, et al. . Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction 1993; 88: 791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 12.Kalinowski A, Humphreys K. Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction 2016; 111: 1293–1298. doi: 10.1111/add.13341 [DOI] [PubMed] [Google Scholar]
- 13.Brown LAS, Cook RT, Jerrells TR, et al. . Acute and chronic alcohol abuse modulate immunity. Alcohol Clin Exp Res 2006; 30: 1624–1631. doi: 10.1111/j.1530-0277.2006.00195.x [DOI] [PubMed] [Google Scholar]
- 14.Liang Y, Yeligar SM, Brown LAS. Chronic-alcohol-abuse-induced oxidative stress in the development of acute respiratory distress syndrome. Sci World J 2012; 2012: 740308. doi: 10.1100/2012/740308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyatt TA, Gentry-Nielsen MJ, Pavlik JA, et al. . Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcohol Clin Exp Res 2004; 28: 998–1004. doi: 10.1097/01.ALC.0000130805.75641.F4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makino K, Shimizu-Hirota R, Goda N, et al. . Unbiased, comprehensive analysis of Japanese health checkup data reveals a protective effect of light to moderate alcohol consumption on lung function. Sci Rep 2021; 11: 15954. doi: 10.1038/s41598-021-95515-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aromataris E, Munn Z, eds. Scoping reviews. In: JBI Manual for Evidence Synthesis. JBI. 2020. 10.46658/JBIMES-20-01 [DOI] [Google Scholar]
- 18.Nielsen LB, Johansen MO, Weinreich UM. The effect of alcohol consumption on pulmonary function – a scoping review. Date last accessed: 31 October 2023. https://vbn.aau.dk/da/publications/the-effect-of-alcohol-consumption-on-pulmonary-function-a-scoping
- 19.Tricco AC, Lillie E, Zarin W, et al. . Preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 11–12. doi: 10.7326/M18-0850.2 [DOI] [PubMed] [Google Scholar]
- 20.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. www.covidence.org [Google Scholar]
- 21.Hoffstein V, Carlen P, Thomas H, et al. . Pulmonary function in smokers after short-term cessation of alcohol ingestion. Chest 1987; 92: 86–89. doi: 10.1378/chest.92.1.86 [DOI] [PubMed] [Google Scholar]
- 22.Oleru UG. Pulmonary impairment in a cotton textile factory in Nigeria: is lifetime alcohol intake with low cigarette smoking a confounding factor? Arch Environ Health 1987; 42: 197–203. [PubMed] [Google Scholar]
- 23.Lange P, Groth S, Mortensen J, et al. . Pulmonary function is influenced by heavy alcohol consumption. Am Rev Respir Dis 1988; 137: 1119–1123. doi: 10.1164/ajrccm/137.5.1119 [DOI] [PubMed] [Google Scholar]
- 24.Garshick E, Segal MR, Worobec TG, et al. . Alcohol consumption and chronic obstructive pulmonary disease. Am Rev Respir Dis 1989; 140: 373–378. doi: 10.1164/ajrccm/140.2.373 [DOI] [PubMed] [Google Scholar]
- 25.Ström K, Janzon L, Hanson BS, et al. . Alcohol consumption modifies the total lung capacity in smokers. Respiration 1996; 63: 66–72. doi: 10.1159/000196520 [DOI] [PubMed] [Google Scholar]
- 26.Zureik M, Liard R, Kauffmann F, et al. . Alcohol consumption, gamma-glutamyl transpeptidase (GGT), and pulmonary function: a cross-sectional and longitudinal study in working men. Alcohol Clin Exp Res 1996; 20: 1507–1511. doi: 10.1111/j.1530-0277.1996.tb01691.x [DOI] [PubMed] [Google Scholar]
- 27.Tabak C, Smit HA, Heederik D, et al. . Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clin Exp Allergy 2001; 31: 747–755. doi: 10.1046/j.1365-2222.2001.01064.x [DOI] [PubMed] [Google Scholar]
- 28.Schünemann HJ, Grant BJB, Freudenheim JL, et al. . Beverage specific alcohol intake in a population-based study: evidence for a positive association between pulmonary function and wine intake. BMC Pulm Med 2002; 2: 3. doi: 10.1186/1471-2466-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin C, In KH, Shim JJ, et al. . Prevalence and correlates of airway obstruction in a community-based sample of adults. Chest 2003; 123: 1924–1931. doi: 10.1378/chest.123.6.1924 [DOI] [PubMed] [Google Scholar]
- 30.Sisson JH, Stoner JA, Romberger DJ, et al. . Alcohol intake is associated with altered pulmonary function. Alcohol 2005; 36: 19–30. doi: 10.1016/j.alcohol.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 31.Emirgil C, Sobol BJ, Heymann B, et al. . Pulmonary function in alcoholics. Am J Med 1974; 57: 69–77. doi: 10.1016/0002-9343(74)90770-0 [DOI] [PubMed] [Google Scholar]
- 32.Tang WK, Lum CM, Ungvari GS, et al. . Alcohol consumption, lung function, and quality of life in pneumoconiosis. Alcohol Clin Exp Res 2005; 29: 1230–1236. doi: 10.1097/01.alc.0000171939.49477.6b [DOI] [PubMed] [Google Scholar]
- 33.Hansel B, Thomas F, Pannier B, et al. . Relationship between alcohol intake, health and social status and cardiovascular risk factors in the urban Paris-Ile-de-France cohort: is the cardioprotective action of alcohol a myth? Eur J Clin Nutr 2010; 64: 561–568. doi: 10.1038/ejcn.2010.61 [DOI] [PubMed] [Google Scholar]
- 34.Siu ST, Udaltsova N, Iribarren C, et al. . Alcohol and lung airways function. Perm J 2010; 14: 11–18. doi: 10.7812/TPP/09-089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siedlinski M, Boer JMA, Smit HA, et al. . Dietary factors and lung function in the general population: wine and resveratrol intake. Eur Respir J 2012; 39: 385–391. doi: 10.1183/09031936.00184110 [DOI] [PubMed] [Google Scholar]
- 36.Frantz S, Wollmer P, Dencker M, et al. . Associations between lung function and alcohol consumption–assessed by both a questionnaire and a blood marker. Respir Med 2014; 108: 114–121. doi: 10.1016/j.rmed.2013.08.041 [DOI] [PubMed] [Google Scholar]
- 37.Sorli-Aguilar M, Martin-Lujan F, Flores-Mateo G, et al. . Dietary patterns are associated with lung function among Spanish smokers without respiratory disease. BMC Pulm Med 2016; 16: 162. doi: 10.1186/s12890-016-0326-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasquez MM, Sherrill DL, LeVan TD, et al. . Persistent light to moderate alcohol intake and lung function: a longitudinal study. Alcohol 2018; 67: 65–71. doi: 10.1016/j.alcohol.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi JW, Lee MH, Fujii T. Effect of high alcohol intake on heavy metal levels in the blood, urine cotinine metabolism, and pulmonary function according to the severity of smoking. Int J Clin Exp Med 2020; 13: 7700–7708. [Google Scholar]
- 40.Wang D, Cao L, Zhou M, et al. . Alcohol intake, beverage type, and lung function: a multicohort study of Chinese adults. Ann N Y Acad Sci 2022; 1511: 164–172. doi: 10.1111/nyas.14744 [DOI] [PubMed] [Google Scholar]
- 41.Zifodya JS, Ferguson TF, Siggins RW, et al. . Cross sectional analysis of the effect of alcohol on pulmonary function in a cohort of men and women living with HIV. Alcohol 2022; 101: 45–51. doi: 10.1016/j.alcohol.2022.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emirgil C, Sobol BJ. Pulmonary function in former alcoholics. Chest 1977; 72: 45–51. doi: 10.1378/chest.72.1.45 [DOI] [PubMed] [Google Scholar]
- 43.Sarić M, Lucić-Palaić S, Horton RJ. Chronic nonspecific lung disease and alcohol consumption. Environ Res 1977; 14: 14–21. doi: 10.1016/0013-9351(77)90061-5 [DOI] [PubMed] [Google Scholar]
- 44.Cohen BH, Celentano DD, Chase GA, et al. . Alcohol consumption and airway obstruction. Am Rev Respir Dis 1980; 121: 205–215. doi: 10.1164/arrd.1980.121.2.205 [DOI] [PubMed] [Google Scholar]
- 45.Sarkar TK, Gupta VK. Pulmonary function in nonsmoking chronic alcoholics. Postgrad Med 1980; 67: 96–106. doi: 10.1080/00325481.1980.11715450 [DOI] [PubMed] [Google Scholar]
- 46.Lebowitz MD. Respiratory symptoms and disease related to alcohol consumption. Am Rev Respir Dis 1981; 123: 16–19. doi: 10.1164/arrd.1981.123.1.16 [DOI] [PubMed] [Google Scholar]
- 47.Sparrow D, Rosner B, Cohen M, et al. . Alcohol consumption and pulmonary function. A cross-sectional and longitudinal study. Am Rev Respir Dis 1983; 127: 735–738. doi: 10.1164/arrd.1983.127.6.735 [DOI] [PubMed] [Google Scholar]
- 48.Lyons DJ, Howard SV, Milledge JS, et al. . Contribution of ethanol and cigarette smoking to pulmonary dysfunction in chronic alcoholics. Thorax 1986; 41: 197–202. doi: 10.1136/thx.41.3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977; 1: 1645–1648. doi: 10.1136/bmj.1.6077.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drobes DJ. Concurrent alcohol and tobacco dependence mechanisms and treatment. Alcohol Res Heal 2002; 26: 136–142. [Google Scholar]
- 51.Jong GM, Hsiue TR, Chen CR, et al. . Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest 1995; 107: 214–217. doi: 10.1378/chest.107.1.214 [DOI] [PubMed] [Google Scholar]
- 52.Bhatty M, Pruett SB, Swiatlo E, et al. . Alcohol abuse and Streptococcus pneumoniae infections: consideration of virulence factors and impaired immune responses. Alcohol 2011; 45: 523–539. doi: 10.1016/j.alcohol.2011.02.305.Alcohol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moss M, Bucher B, Moore FA, et al. . The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA 1996; 275: 50–54. [PubMed] [Google Scholar]
- 54.Simet SM, Pavlik JA, Sisson JH. Dietary antioxidants prevent alcohol-induced ciliary dysfunction. Alcohol 2013; 47: 629–635. doi: 10.1016/j.alcohol.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson S, Zhang P, Bagby G, et al. . Alcohol abuse, immunosuppression, and pulmonary infection. Curr Drug Abus Rev 2008; 1: 56–67. doi: 10.2174/1874473710801010056 [DOI] [PubMed] [Google Scholar]
- 56.Szabo G, Mandrekar P, Catalano D. Inhibition of superantigen-induced T cell proliferation and monocyte IL-1β, TNF-α, and IL-6 production by acute ethanol treatment. J Leukoc Biol 1995; 58: 342–350. doi: 10.1002/jlb.58.3.342 [DOI] [PubMed] [Google Scholar]
- 57.Heermans EH. Booze and blood: the effects of acute and chronic alcohol abuse on the hematopoietic system. Clin Lab Sci 1998; 11: 229–232. [PubMed] [Google Scholar]
- 58.Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res 2009; 33: 220–232. doi: 10.1111/j.1530-0277.2008.00842.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moss M. Epidemiology of sepsis: race, sex, and chronic alcohol abuse. Clin Infect Dis 2005; 41: Suppl. 7, S490–S497. doi: 10.1086/432003 [DOI] [PubMed] [Google Scholar]
- 60.McClain CJ, Barve SS, Barve A, et al. . Alcoholic liver disease and malnutrition. Alcohol Clin Exp Res 2011; 35: 815–820. doi: 10.1111/j.1530-0277.2010.01405.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hao HD, He LR. Mechanisms of cardiovascular protection by resveratrol. J Med Food 2004; 7: 290–298. doi: 10.1089/jmf.2004.7.290 [DOI] [PubMed] [Google Scholar]
- 62.Culpitt SV, Rogers DF, Fenwick PS, et al. . Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax 2003; 58: 942–946. doi: 10.1136/thorax.58.11.942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donnelly LE, Newton R, Kennedy GE, et al. . Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol 2004; 287: 774–783. doi: 10.1152/ajplung.00110.2004 [DOI] [PubMed] [Google Scholar]
- 64.Ayres J, Ancic P, Clark TJH. Airways responses to oral ethanol in normal subjects and in patients with asthma. J R Soc Med 1982; 75: 699–704. doi: 10.1177/014107688207500905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herxheimer H, Stresemann E. Ethanol and lung function in bronchial asthma. Arch Int Pharmacodyn Ther 1963; 144: 310–314. doi: 10.1016/j.alcohol.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 66.Imhof A, Froehlich M, Brenner H, et al. . Effect of alcohol consumption on systemic markers of inflammation. Lancet 2001; 357: 763–767. doi: 10.1016/S0140-6736(00)04170-2 [DOI] [PubMed] [Google Scholar]
- 67.Pai JK, Hankinson SE, Thadhani R, et al. . Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis 2006; 186: 113–120. doi: 10.1016/j.atherosclerosis.2005.06.037 [DOI] [PubMed] [Google Scholar]
- 68.Ström K. Alcohol, smoking and lung disease. Addict Biol 1999; 4: 17–22. doi: 10.1080/13556219971803 [DOI] [PubMed] [Google Scholar]
- 69.Venizelos PC, Gerrity TR, Yeates DB. Response of human mucociliary clearance to acute alcohol administration. Arch Environ Health 1981; 36: 194–201. doi: 10.1080/00039896.1981.10667625 [DOI] [PubMed] [Google Scholar]
- 70.Prignot J. Quantification and chemical markers of tobacco-exposure. Eur J Respir Dis 1987; 70: 1–7. [PubMed] [Google Scholar]
- 71.Page MJ, McKenzie JE, Bossuyt PM, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0233-2023.SUPPLEMENT (99.2KB, pdf)