Abstract
Preweaning mortality is a widespread problem in laboratory mouse breeding, particularly in the case of fragile mouse models. While numerous studies explore alternative care methods to increase the survivability of common mouse strains, there remains a paucity of research into the care of mice with fragile health conditions that result from induced or natural genetic mutations. In this study, standard husbandry practices were enhanced by the addition of a softened diet, a nutritionally fortified dietary supplement, soft bedding, gentle handling techniques, decreased handling, lengthened weaning age, and dam productivity tracking. This alternative care plan was shown to increase the survival of a fragile recessive dystrophic epidermolysis bullosa mouse model, and some aspects could be used in developing a care plan for other fragile mouse strains.
Abbreviation and Acronym: RDEB, recessive dystrophic epidermolysis bullosa
Introduction
Preweaning mortality of laboratory mice is a prevalent issue for many breeding programs.3,20 For one of the most common inbred lines in biomedical research, C57BL/6J, reported preweaning mortality rates reach up to 50%.15,28 Single pups and entire litters can be lost, significantly contributing to reduced breeding efficiency. To make up for the loss of pups, more litters must be produced. This can place excess strain on dams who must bear additional litters or require the use of more animals for breeding. Despite ongoing efforts to reduce the number of animals used in research and improve their welfare according to the 3Rs principle (replacement, reduction, and refinement) originally proposed in 1959,22 high preweaning mortality rates persist, with the underlying causes not fully understood.
As an altricial species, mouse pups are entirely dependent on their mother for nutrition and thermoregulation early in life; thus, maternal behavior is key to offspring survival.26,27 In general, a dam’s stress has been shown to negatively affect pup survivability, robustness, and litter size.17,23,24 Maternal stress has also been shown to reduce oocyte implantation rates and development potential and is associated with reduced pup weight and survivability.15,17,18,28,30 Because of these effects, stress in female laboratory mice during pregnancy and after parturition can lead to smaller litter sizes, directly affecting the efficiency of research breeding programs.
Several publications have looked into common stressors present in the vivarium setting because of the great effect that stress, in addition to dam age, litter size, and overlapping litters, can have on the productivity of laboratory-breeding mice.20 Excess noise has been shown to increase stress in laboratory mice and is associated with decreased breeding efficiency.2,21,25 The handling of an animal’s cage during cage changes and transportation to a different room can also elicit a stress response.1 From these findings, several recommendations have come about to reduce the amount of stress animals are exposed to and therefore improve their productivity. It is often recommended to decrease the amount of noise a vivarium is exposed to and adjust the arrangement of animal rooms so that they are furthest from unpreventable noises such as cage washing.
Another common recommendation made to laboratory and animal care staff is to be sparing with the handling of cages and animals. Despite these recommendations and research showing that handling can elevate anxiety levels in laboratory mice, few alternative methods have been explored.13 This is particularly true in the case of sensitive mouse models, where alternative handling and care may be necessary. The assessment of a few handling techniques including padded-tip forceps, gloved hands, and various indirect methods such as tubes, scoops, and enrichment devices has been well documented.6 One publication even directly investigated the effects of nonaversive (“tunnel-handled”) and standard (tail-lift with forceps) handling on breeding productivity in C57BL/6J mice.12 However, these studies use relatively robust, common, mouse strains. To date, there are few publications that explore how handling methods impact preweaning mortality and breeding productivity of mouse strains with sensitive health conditions that result from induced or natural genetic mutations.
With the fragility of a particularly sensitive mouse model comes the ethical and legal obligation to reduce or eliminate the animal’s pain and distress as much as possible. Often, this comes in the form of daily monitoring and handling of animals and their cages to assess the animal for signs of pain or distress. This is the case for our laboratory, where all animal models of recessive dystrophic epidermolysis bullosa (RDEB) are monitored daily for common signs of pain or distress. RDEB is a rare mucocutaneous skin disorder caused by mutations of the Col7a1 gene. These mutations result in a lack of normal collagen type VII development, a vital protein that anchors the epidermis and dermis.4 This lack of functional collagen type VII results in mechanical fragility of the skin, causing blisters, debilitating chronic wounding, inflammation, pruritus, and scarring.5 To date, there are several mouse models that imitate the various RDEB mutations, although many of these fragile strains face the same limitation, preweaning pup mortality. For example, a commonly used hypomorphic collagen VII expressing mouse model of RDEB (Col7a1flNeo/flNeo) reports greater than 60% mortality by 28 d.8 Using the same hypomorphic RDEB mouse model, our laboratory saw an even higher mortality rate of 92% by 21 d when the standard housing and handling conditions for nonsensitive strains were employed. This report details our approach to the development of an altered care plan, including handling, which focuses on reducing dam stress while catering to the needs of a sensitive mouse strain. We hypothesized that this alternative care plan would increase the survivability of (Col7a1flNeo/flNeo) mice.
Materials and Methods
Standard care protocol.
All data were collected from previously established colonies at the University of Minnesota. Research was approved by the IACUC at the University of Minnesota. As per University IACUC guidelines, all mice were housed in standard small animal research conditions with a 12:12-h light:dark cycle, unlimited access to food and water, temperatures ranging from 22 to 24 °C, and humidity levels ranging from 30% to 32%. Mice were given unlimited access to Teklad 2919 irradiated chow (Envigo, Madison, WI) as well as clean, untreated, tap water via bottle. The cages were housed on the rack furthest from the door and hood, with all cages on the side of the rack facing the wall. All procedures requiring the opening of the cage were performed within a laminar flow hood. Data were collected from studies using a previously established type VII collagen hypomorphic mouse model (Col7a1flNeo/flNeo) kept on a C57BL/6:129sv background obtained from Dr. Alexander Nystrom (University of Freiburg, Freiburg, Germany).8
All animals were housed in static Allentown Mouse 75Jag Cages (Allentown, Allentown, NJ) with ALPHA-Dri PLUS bedding and Enviro-dri nesting material (Shepherd Specialty Papers, Amherst, MA). For enrichment, all mice were provided steam-sterilized cardboard glove boxes, cut in half. Cage changes were performed weekly by various University of Minnesota Research Animal Resources staff. Daily health monitoring was performed by both these individuals and laboratory staff members. Cages were opened twice daily to collect survivability data, check for the presence of new litters, and monitor for signs of pain or distress. For dams with litters, the nest was removed from around the pups, the pups were examined, and the nest was placed back around the pups. No special efforts were made to reduce noise during cage changes or daily monitoring, and standard tail-base handling was used for all animals.
Breeding pairs consisted of heterozygous (Col7a1flNeo/WT) males and females. The age of mice when enrolled in the breeding program varied from 9 to 30 wk. The date of set-up, the date of birth of each litter, and litter sizes for each were recorded for every breeding pair. Breeder pairs were typically replaced once they reached one year of age. All pups were separated by sex after removal from the mother at 21 d of age. For survivorship analysis, litters were checked daily to establish survival data, with genotypes confirmed at the time of death via PCR using a previously established protocol.8
Health surveillance for mouse pathogens was conducted quarterly via PCR and/or serology using dirty bedding sentinels. The following agents are tested for and excluded in this facility: mouse parvovirus, minute virus of mice, murine rotavirus, mouse theilovirus, ectromelia virus, murine cytomegalovirus, murine polyomavirus, lymphocytic choriomeningitis virus, reovirus, pneumonia virus of mice, Sendai virus, Filobacterium rodentium, pinworms (Aspiculuris tetraptera, Syphacia obvelata, and Syphacia muris) mycoptes, and fur mites (Myobia musculi, Mycoptes musculinus, and Radfordia affinis).
Altered care protocol.
The animals were fed a pelleted diet as described above. In addition to traditional pellet food, a soft mash composed of finely ground-up pellet feed and water in an approximately 1:1 ratio was provided to all Col7a1flNeo/flNeo pups beginning between days 10 and 14 of life in combination with a sterile nutritionally fortified dietary supplement with an animal (milk) protein source (DietGel 76A, Westbrook, ME) until the time of natural death. The soft mash was held in a sterile culture dish on the floor for easy access, and the dietary supplement was placed in an angled magnetic holder (Marine Ecological Habitats, Biddeford, ME) for ease of access and prevention of contamination from bedding material. The soft mash was replaced daily, and the dietary supplement was replaced 2 times a week. All animals were provided Cellu-nest bedding (Shepherd Specialty Papers, Amherst, MA) in addition to the enrichment described above. Handling of both mice and their cages was limited to 2 laboratory staff members with additional training in gentle handling techniques. No other laboratory staff members were allowed access to these animals. The status of all animals was monitored daily by laboratory staff members, and the majority of health reports were made by these individuals. For health concerns that required medical intervention, veterinary staff partnered with the trained laboratory members in the handling and treatment of the animals.
The trained laboratory staff were responsible for all care surrounding these animals, including cage changes. Decreasing the subjective measurement noise that animals were exposed to during the changing of their cages was prioritized. Laboratory staff were gentle when setting the cage in the hood, setting the lid down once it was removed, placing the lid back on once finished, and placing the cage back on the rack. In addition to reducing the amount of subjective noise produced during cage changes, the handling of mice was made as gentle as possible. Adult breeders were picked up by gently grasping the base of the tail with one hand and sliding another hand under their body for support. The animal was then lifted up using the hand under the body, with the one grasping the tail preventing them from jumping off during the transfer. The hand the animal was sitting on was placed at the bottom of the cage, and the mouse was allowed to walk off into the cage of their own accord. For young pups, cage changes were delayed until they were at least 5 d old. When moving pups to a clean cage, dirty bedding was rubbed on the gloved hands, and the entire nest was picked up and moved over to the new cage. If a pup had left the nest before it was moved over to the new cage, the gloved hands were dirtied, and the pup was scooped up with the bedding surrounding it and placed in the transferred nest. This same method of scooping up the animal with the surrounding bedding to buffer the friction from the gloves was also performed for all adult Col7a1flNeo/flNeo animals as well.
Mice were not handled by trained staff outside of cage changes unless experimentally necessary, and cages were handled only once daily to collect survivability data, check for the presence of new litters, and monitor for signs of pain or distress. For breeders with litters suspected soon, daily cage handling involved slowly and gently moving the cage slightly off the rack so that all sides of the cage could be inspected for the presence of pups. Once pups were visualized, they would be counted from outside the cage to minimize disturbances. The cage was then gently slid back into place. For breeders with known litters, daily cage handling involved slowly moving the cage slightly off the rack until the litter was visible. The pups were counted from outside the cage. If the same number of pups as the day before were not able to be visualized, the cage would be gently removed from the rack and carefully walked to the hood where it was then opened. The number of pups was counted again. If this number still did not match the number from the day before, gloved hands were dirtied using urine-soaked bedding from the cage, and singular pieces of bedding were removed from the nest until all animals could be visualized. No mice were ever handled in this process. If the number of pups observed still did not match, it was assumed a pup had died and a carcass was attempted to be recovered and used for genotyping. After this process was completed, the cage was gently closed, minimizing noise, and placed back on the rack.
The age of the mice when enrolled in the breeding program varied from 10 to 12 wk. If a litter had not occurred within 6 wk of set-up or within 6 wk of the previous litter’s birth, the animals were removed from the breeding program. Replacement of animals also occurred if litter size was less than 4 pups, or greater than 11 pups, for 2 or more litters. After all breeder pairs reached 9 mo of age, they were replaced with younger animals.
Col7a1flNeo/flNeo pups were identified via PCR of a tail snip obtained between 10 and 14 d of age. Handling techniques of pups during tail clipping involved placing a hand warmer inside the glove of the hand that the animal would rest in, dirtying gloves with soiled bedding, and applying gentle pressure to stimulate a warm nesting environment while the snip was obtained from the distal end of the tail. For identification, straight lines were drawn around the proximal part of the tail. Briefly, the number of lines drawn around the proximal part of the tail indicated the identification number of that pup within that litter. For example, pup number 4 would have 4 lines around its tail. This was done just before the snip was obtained. After genotypes were confirmed, heterozygous and wild-type littermates were removed until there were only 4 pups left in the litter. Col7a1flNeo/flNeo pups were separated by sex after removal from the mother at day 42 of age.
Survivorship statistics.
Survivorship analysis of interventional compared with control groups was performed using log-rank (Mantel-Cox) tests for significance with a significance threshold of P < 0.05. The log-rank test calculates a log-rank statistic which is equivalent to a χ2 value. Thus, the critical P value is then determined using the χ2 distribution. Standard assumptions of a log-rank test were analyzed and held true for this analysis. Censoring was independent of prognosis, survival probabilities were similar for those included early in the study when compared with late as the study took place at once, and the events occurred at the stated intervals. The assumption of proportional hazard held for this analysis. Kaplan-Meier curves were used for the visualization of log-rank survival analysis. Relative risk was calculated using a defined calculation comparing the probability of mortality in the intervention group by the probability of mortality in the control group by 21-d-old. Confidence intervals were calculated via Koopman asymptotic score. Hazard ratios (HRS) of interventional compared with control group mortality were calculated using Mantel-Haenszel analysis methods. A χ2 test of independence was performed for analysis of the interventional group compared with the control group survival to wean (21 d). All statistical analyses were performed in GraphPad Prism v 10.0.2 (GraphPad Software, Boston, MA).
Results
Temporal inclusion of care changes.
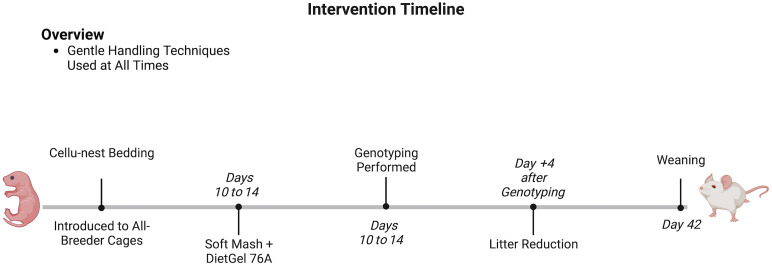
In total, 45 Col7a1flNeo/flNeo mice, among 45 litters, were observed from birth to time of natural death between both the pre- and postcare protocol change periods. Twenty-six of these animals were from the precare protocol change group, and 19 were from the postcare protocol change group. Figure 1 demonstrates the temporal inclusion of care changes in the experimental group.
Figure 1.
Timeline of interventions from birth to weaning (42 d) of RDEB mice. An interventional timeline outlining the age (in days) at which interventional methods were implemented to improve the survivability of RDEB mice. Figure was created with BioRender.com.
Evaluation of alterations on survival.
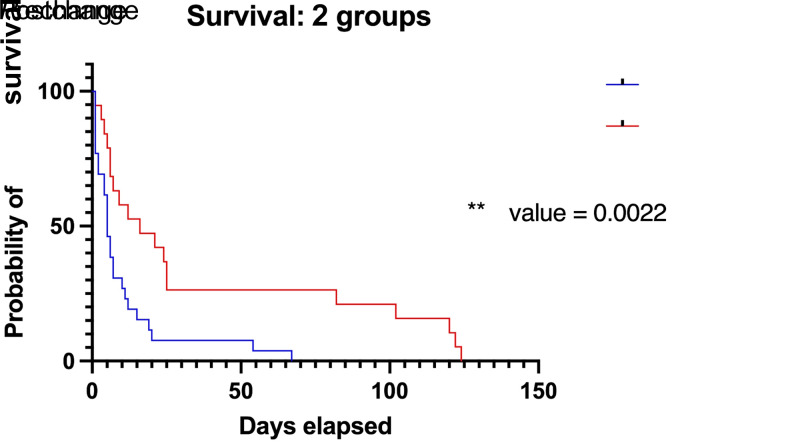
To assess the effect of these changes on survival, log-rank survivorship analysis was performed, showing a median survival of 5 d for the precare protocol change group and 16 d for the postcare protocol change group (Figure 2). A χ2 value of 9.357 demonstrated a statistically significantly different group survival in the postcare protocol change group compared with the precare protocol change group (P = 0.0022). Hazard ratio analysis elucidated increased hazard of censored death for the precare protocol change group (HR: 2.89; 95% confidence interval [CI]: 1.458 to 5.605) compared with the postcare protocol change group (HR: 0.35; 95% CI: 0.1784 to 0.6857).
Figure 2.
Alterations to the standard care protocol increased survival of RDEB pups. A Kaplan-Meier survivorship curve of RDEB pups from the prechange care protocol period (n = 26) compared with the postchange period (n = 19). Mice in the postchange period have a statistically significantly different probability of survival. Significance was determined via log-rank (Mantel-Cox) survivorship analysis P ≤ 0.05. The postchange period involved the enhancement of standard husbandry practices with the addition of a softened diet, a nutritionally fortified dietary supplement, soft bedding, gentle handling techniques, decreased handling, lengthened weaning age, and dam productivity tracking.
Evaluation of alterations on survival to 21 d.
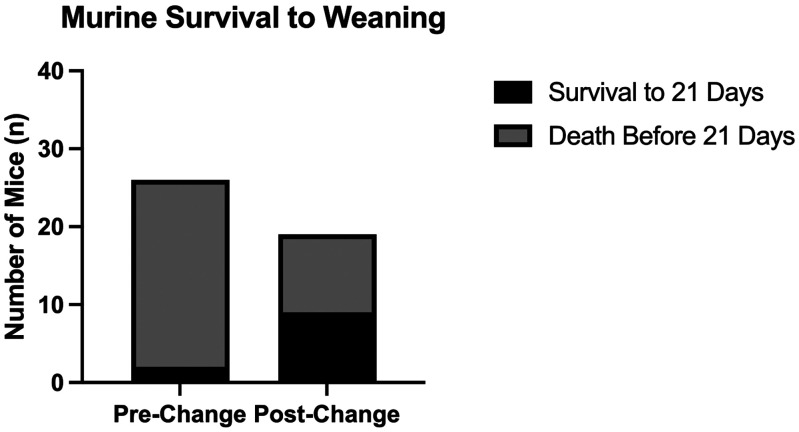
The effect of care protocol changes was evaluated on survival to 21 d (Figure 3). Of the 26 mice included in the precare protocol change group, 2 mice (7.69%) survived to 21 d, compared with 9 mice (47.37%) of the 19 total in the postcare protocol changes. A χ2 test of independence demonstrated a statistically significant difference in survival to 21 d (χ2 = 9.357; P = 0.0022). The relative risk associated with the postcare protocol changes demonstrated a protective effect (RR: 0.1624; 95% CI: 0.0424 to 0.5755).
Figure 3.
Alterations to the standard care protocol increased the survival to wean (21 d) of RDEB pups. Distribution showing the number of RDEB mice in the prechange (n = 26) and postchange (n = 19) groups that did or did not survive to 21 d. The postchange period was characterized by enhancement of standard husbandry practices with the addition of a softened diet, a nutritionally fortified dietary supplement, soft bedding, gentle handling techniques, decreased handling, lengthened weaning age, and dam productivity tracking.
Discussion
The survival rate to the age of 21 d jumped from 7.69 to 47.37% for Col7a1flNeo/flNeo mice after the care protocol for these animals was altered. As demonstrated by the protective nature of the relative risk, the interventional group experienced statistically significantly fewer instances of preweaning mortality. Hazard ratio analysis revealed a greater than 2-fold increase in mortality hazard during the overall lifespan of the control group. In our laboratory, the previous standard of care included a pelleted diet and clean water provided ad libitum with ALPHA-Dri PLUS bedding and Enviro-dri nesting material (Shepherd Specialty Papers, Amherst, MA) in Allentown Mouse 75Jag Cages (Allentown, Allentown, NJ). No special efforts were made to decrease the amount of handling and noise animals were exposed to during cage changes or daily counting of pups for survivability data. Rather, the cages were opened, and the nest was disturbed daily. After noticing poor survivability of these animals, ideas were discussed, and numerous changes were made.
To formulate a care plan for this sensitive model, the potential health problems were carefully considered. This hypomorphic mouse model is characterized by mutations to the Col7a1 gene that result in a lack of collagen type VII. This lack of functional collagen type VII results in mechanically fragile skin, eventually causing cutaneous blisters. Consistent with previous publications, we observed visible blisters on pups within 24 h of birth on the paws, upper trunk, mouth, and ears of the hypomorphic mouse model used in this study.8 Any sort of friction to the skin of these animals can be debilitating in the future. Thus, a softer cellulose fiber bedding was used to prevent as much friction as possible. Nest scoring was not performed with this project, but caregiver observations indicated that animals with the cellulose fiber bedding seemed to form more robust nests when compared with breeders with the standard bedding. Nest building is often regarded as a good indicator of animal welfare and offers benefits for heat retention of pups as well as increased survivability, feed efficiency, and weaning weight.9,10,15,16,27 In the future, we would like to further investigate nest building and bedding preference between the 2 materials used in this study. In addition to a reduction in the normal amount of collagen type VII in the epidermal-dermal junction of the skin, Col7a1flNeo/flNeo pups also had reduced collagen VII present in the mucosal epithelium of the esophagus.8 In humans, this can present clinically as esophageal strictures, which can severely limit the intake of food or liquids.19,29 Hypothesizing that this could be affecting the ability of our animals to intake food, and result in a negative impact on their survivability, we decided to provide the respective diets required by an unrelated concurrent study in the form of a soft mash in an approximately 1:1 ratio of clean water to finely ground pelleted feed. The ground-up pellet feed allowed for more control over the consistency of the mash compared with traditional methods of moistening solid pellets with water, while still maintaining the standard dietary requirements of the animals. In addition to the soft mash, a gelatinous dietary supplement (DietGel 76A) with a high moisture content was also provided. Both were started between days 10 and 14 of the pup’s life and continued until the time of natural death. Typically, mouse pups start consuming solid foods around the time their eyes open (observed between days 12 to 14 in our colony) and will primarily consume solid foods by 21-d-old.7,11 The diet of a weaning mouse is largely dependent on the mother, with pups often preferring the same diet the dam consumes.26 This, with the additional knowledge that mice can be neophobic toward food, is why we decided to introduce the diet earlier than the typical weaning date of pups.14
In addition to altering the care of these animals to meet the specific needs of this strain, other factors known to increase stress and therefore affect pup survivability and dam efficiency were taken into account. Due to the increase in stress excess noise can cause, it is often recommended to reduce the amount of noise a mouse colony is exposed to.2,25 Excessive noise has also been shown to decrease reproductive efficiency and live birth rates in mice.21 Because of these findings, special efforts were made to reduce the amount of noise that animals were exposed to. First, within the facility, the furthest room from cage washing was chosen to house these animals. This reduced the overall noise and foot traffic from outside the room. Within the room where they were housed, the animals were moved onto the rack furthest away from both the door and biologic safety cabinet to reduce their exposure to the noise from activity surrounding both. The door to the colony was also gently closed when entering and exiting to avoid the sound produced when it was allowed to slam shut of its own accord. Research Animal Resources and laboratory staff members were also encouraged to keep loud talking to a minimum and to not listen to music. In addition to the reduction of noise, we also took into consideration the known effects of excess handling on the stress levels of animals and its subsequent impact on a dam’s reproductive ability.17,23,24 While the handling of animals and cages could not be entirely eliminated, the adapted methods allowed for gentle daily interactions.
Evaluation of the breeders also allowed for more precise control over the colony’s total reproductive efficiency. It is known that advanced dam age and both small (n = 4) and large (n = 11) litter sizes can affect the survivability of pups.20 Therefore, we chose to remove breeders that were older than 9 mo or produced litters smaller than 4 pups or larger than 11 pups for 2 or more litters.
The changes outlined in this paper can potentially reduce the number of animals needed for certain studies. Before our changes, it took an average of 13 litters to produce a single Col7a1flNeo/flNeo mouse that lived to 21 d. With this average, a large number of breeders would need to be maintained to achieve an adequate amount of mutant (Col7a1flNeo/flNeo) animals for experimentation. In addition, due to the nature of recessive disorders, a large number of unaffected pups would be born and culled in this scenario. Alternatively, the average number needed to produce a Col7a1flNeo/flNeo mouse that lived to 21 d dropped to 2.3 litters after our changes were employed. Significantly fewer breeders would be needed to achieve the same number of mutant animals for experimentation. Less unaffected, and likely not experimentally necessary, pups would be produced as well. By enacting these changes, we can reduce the number of intermediate animals used and produced for experiments. In turn, these changes support the proposed 3Rs principle.22 With a reduction in the number of intermediate animals used and produced for experiments, our changes also have the potential to reduce total per-diem charges for animal care. In addition to supporting the 3Rs principle, the changes outlined in this paper also uphold the ethical and legal obligation to care for animals with induced deleterious health conditions. By providing specialized supplemental care, we were able to increase the lifespan of (Col7a1flNeo/flNeo) mice and therefore reduce the distress and suffering often seen with early death.
The small scale and nature of this study present a few limitations. To accommodate the wide and diverse range of human disease mutations, the number of frail mouse models was vast. Our study focused on a small sample size of a singular fragile mouse model. Therefore, some of the changes made to accommodate the needs of this disease model may not prove helpful for other strains. Knowing this, we included the thought processes behind each of our changes so that one may curate a care plan that best suits the needs of their colony. In addition to the small scale of this study, the inclusion of several care plan alterations limits our ability to dissect outwhich practices might have been the most effective. While we certainly speculate that specific enhancements had the most impact based on prior research and experience, we are not able to definitively determine this. One of the ways to alleviate this would have been through the inclusion of individual necropsies to determine the exact causation of death. An added benefit of a necropsy is that it would allow for the determination of health issues that a specific mouse strain faces and then the selection of treatment options to address those health issues. Another way this study could have been improved would be by including nest building scores and bedding preference tests to further quantify the differences between bedding materials. Finally, there are often slight variances between vivariums such as temperature, humidity, and level of noise that may impact the effectiveness of our changes.
The alternative care plan curated for our RDEB mouse model introduced a softened diet, a nutritionally fortified dietary supplement, Cellu-nest bedding, gentle handling techniques, decreased handling, lengthened weaning age, and dam productivity tracking. These adaptations proved successful in increasing the lifespan of fragile RDEB mice. Components of this care plan could prove useful in curating a routine that works well for other fragile mouse strains. Future studies should address the care of other fragile mouse models.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Funding
This research was conducted with funding support from NIH NIAMS grant R01 AR063070 and research grants from Cure EB and DebRA (JT). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 2.Baldwin AL, Schwartz GE, Hopp DH. 2007. Are investigators aware of environmental noise in animal facilities and that this noise may affect experimental data? J Am Assoc Lab Anim Sci 46:45–51. [PubMed] [Google Scholar]
- 3.Brajon S, Morello GM, Capas-Peneda S, Hultgren J, Gilbert C, Olsson A. 2021. All the pups we cannot see: Cannibalism masks perinatal death in laboratory mouse breeding but infanticide is rare. Animals (Basel) 11:2327. 10.3390/ani11082327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung HJ, Uitto J. 2010. Type VII collagen: The anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol Clin 28:93–105. 10.1016/j.det.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cianfarani F, Zambruno G, Castiglia D, Odorisio T. 2017. Pathomechanisms of altered wound healing in recessive dystrophic epidermolysis bullosa. Am J Pathol 187:1445–1453. 10.1016/j.ajpath.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Doerning CM, Thurston SE, Villano JS, Kaska CL, Vozheiko TD, Soleimanpour SA, Lofgren JL. 2019. Assessment of mouse handling techniques during cage changing. J Am Assoc Lab Anim Sci 58:767–773. 10.30802/AALAS-JAALAS-19-000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duysen EG, Fry DL, Lockridge O. 2002. Early weaning and culling eradicated Helicobacter hepaticus from an acetylcholinesterase knockout 129S6/SvEvTac mouse colony. Comp Med 52:461–466. [PubMed] [Google Scholar]
- 8.Fritsch A, Loeckermann S, Kern JS, Braun A, Bosl MR, Bley TA, Schumann H, et al. 2008. A hypomorphic mouse model of dystrophic epidermolysis bullosa reveals mechanisms of disease and response to fibroblast therapy. J Clin Invest 118:1669–1679. 10.1172/JCI34292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaskill BN, Karas AZ, Garner JP, Pritchett-Corning KR. 2013. Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp 85:51012. 10.3791/51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaskill BN, Pritchett-Corning KR, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. 2013. Energy reallocation to breeding performance through improved nest building in laboratory mice. PLoS One 8:e74153. 10.1371/journal.pone.0074153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hickman DL, Swan MP. 2011. Effects of age of pups and removal of existing litter on pup survival during cross-fostering between multiparous outbred mice. J Am Assoc Lab Anim Sci 50:641–646. [PMC free article] [PubMed] [Google Scholar]
- 12.Hull MA, Reynolds PS, Nunamaker EA. 2022. Effects of non-aversive versus tail-lift handling on breeding productivity in a C57BL/6J mouse colony. PLoS One 17:e0263192. 10.1371/journal.pone.0263192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurst JL, West RS. 2010. Taming anxiety in laboratory mice. Nat Methods 7:825–826. 10.1038/nmeth.1500. [DOI] [PubMed] [Google Scholar]
- 14.Kronenberger JP, Medioni J. 1985. Food neophobia in wild and laboratory mice (Mus musculus domesticus). Behav Processes 11:53–59. 10.1016/0376-6357(85)90102-0. [DOI] [PubMed] [Google Scholar]
- 15.Lecker J, Froberg-Fejko K. 2016. Using environmental enrichment and nutritional supplementation to improve breeding success in rodents. Lab Anim (NY) 45:406–407. 10.1038/laban.1114. [DOI] [PubMed] [Google Scholar]
- 16.Leidinger CS, Thone-Reineke C, Baumgart N, Baumgart J. 2019. Environmental enrichment prevents pup mortality in laboratory mice. Lab Anim 53:53–62. 10.1177/0023677218777536. [DOI] [PubMed] [Google Scholar]
- 17.Mann MA, Kinsley C, Broida J, Svare B. 1983. Infanticide exhibited by female mice: Genetic, developmental and hormonal influences. Physiol Behav 30:697–702. 10.1016/0031-9384(83)90165-8. [DOI] [PubMed] [Google Scholar]
- 18.Marchlewska-Koj A. 1997. Sociogenic stress and rodent reproduction. Neurosci Biobehav Rev 21:699–703. 10.1016/S0149-7634(96)00021-8. [DOI] [PubMed] [Google Scholar]
- 19.Michalak A, Cichoz-Lach H, Prozorow-Krol B, Buk L, Dzida M. 2018. A rare case of skin blistering and esophageal stenosis in the course of epidermolysis bullosa - Case report and literature review. BMC Gastroenterol 18:47. 10.1186/s12876-018-0771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morello GM, Hultgren J, Capas-Peneda S, Wiltshire M, Thomas A, Wardle-Jones H, Brajon S, Gilbert C, Olsson IAS. 2020. High laboratory mouse pre-weaning mortality associated with litter overlap, advanced dam age, small and large litters. PLoS One 15:e0236290. 10.1371/journal.pone.0236290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmussen S, Glickman G, Norinsky R, Quimby FW, Tolwani RJ. 2009. Construction noise decreases reproductive efficiency in mice. J Am Assoc Lab Anim Sci 48:363–370. [PMC free article] [PubMed] [Google Scholar]
- 22.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. London (UK): Methuen. [Google Scholar]
- 23.Sanderson AE, Multari HM, Lohmiller JJ, Boutin SR. 2010. Effect of cage-change frequency on rodent breeding performance. Lab Anim (NY) 39:177–182. 10.1038/laban0610-177. [DOI] [PubMed] [Google Scholar]
- 24.Sternberg WF, Ridgway CG. 2003. Effects of gestational stress and neonatal handling on pain, analgesia, and stress behavior of adult mice. Physiol Behav 78:375–383. 10.1016/S0031-9384(03)00015-5. [DOI] [PubMed] [Google Scholar]
- 25.Turner JG, Parrish JL, Hughes LF, Toth LA, Caspary DM. 2005. Hearing in laboratory animals: Strain differences and nonauditory effects of noise. Comp Med 55:12–23. [PMC free article] [PubMed] [Google Scholar]
- 26.Valsecchi P, Moles A, Mainardi M. 1993. Transfer of food preferences in mice (Mus domesticus) at weaning: The role of maternal diet. Boll Zool 60:297–300. 10.1080/11250009309355827. [DOI] [Google Scholar]
- 27.Weber EM, Olsson IAS. 2008. Maternal behaviour in Mus musculus sp.: An ethological review. Appl Anim Behav Sci 114:1–22. 10.1016/j.applanim.2008.06.006. [DOI] [Google Scholar]
- 28.Whitaker J, Moy SS, Godfrey V, Nielsen J, Bellinger D, Bradfield J. 2009. Effects of cage size and enrichment on reproductive performance and behavior in C57BL/6Tac mice. Lab Anim (NY) 38:24–34. 10.1038/laban0109-24. [DOI] [PubMed] [Google Scholar]
- 29.Xu Z, Huang T, Pan M, Huang Y, Jiang Y. 2022. Case report: Recessive dystrophic epidermolysis bullosa with severe esophageal stenosis: A case report and literature review. Br J Biomed Sci 79:10200. 10.3389/bjbs.2022.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang SY, Wang JZ, Li JJ, Wei DL, Sui HS, Zhang ZH, Zhou P, Tan JH. 2011. Maternal restraint stress diminishes the developmental potential of oocytes. Biol Reprod 84:672–681. 10.1095/biolreprod.110.087890. [DOI] [PubMed] [Google Scholar]