Abstract
Plethysmography is employed in nonhuman primates (NHPs) to calculate respiratory minute volume and determine the exposure time required to deliver an aerosol at the target dose. Anesthetic drugs can impact breathing parameters like steady-state minute volume (SSMV) central to aerosol dosing. Alfaxalone-midazolam mixtures (AM) provide superior parameters for plethysmography in cynomolgus macaques. An obstacle to the use of AM is the volume required to anesthetize via intramuscular injection. A more concentrated formulation of alfaxalone will reduce injection volumes and refine AM protocols. The purpose of this study was to compare AM using the Indexed 10-mg/mL (AM10) formulation compared with an investigational 40-mg/mL (AM40) formulation for IM administration in cynomolgus macaques undergoing plethysmography. We hypothesized that AM10 and AM40 would show no difference in quality of anesthesia (QA), duration of anesthesia, SSMV, accumulated minute volume (AMV), and side effects. We also hypothesized that female macaques would have a longer duration of anesthesia compared with males using both formulations. The study used 15 cynomolgus macaques comprised of 8 females and 7 males. NHPs were compared between 2 separate and randomized anesthetic events no less than one week apart. Each animal served as its own control and animals were randomized by random number generation. Anesthetized NHPs were placed in a sealed plethysmography chamber, and minute volume measurements were calculated every 10 s to determine SSMV. Once SSMV was achieved for 20 min, the trial ended. There were no statistically significant differences between AM10 and AM40 for duration of anesthesia, SSMV, AMV, side effects, or QA. AM40 had a significantly smaller injection volume. Females did not show a significantly longer median duration of anesthesia using either of the alfaxalone formulations. Overall, AM40 offers a more humane anesthetic than AM10 for plethysmography in cynomolgus macaques.
Abbreviations and Acronyms: AM, alfaxalone (5 mg/kg)-midazolam (0.3 mg/kg) combination anesthetic protocol; AM10, 10 mg/mL alfaxalone combined with midazolam; AM40, 40 mg/mL alfaxalone combined with midazolam; AMV, accumulated minute volume; QA, quality of anesthesia; SSMV, steady-state minute volume
Introduction
At the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) the primary mission is to protect military personnel from biologic threats. For many infectious biologic agents, inhalation is a common route of exposure (either natural or experimental), and nonhuman primates (NHPs) provide reasonable models for many infectious disease processes in humans. This route of exposure is important for Venezuelan equine encephalitis virus, pneumonic plague (Yersinia pestis), anthrax (Bacillus anthracis), and monkeypox virus in cynomolgus macaques (Macaca fascicularis).2,7,10,18,19,23,25,26,29,30 It is critical for military and civilian researchers to have the ability to model aerosolized agents to produce effective countermeasures. In the study of infectious diseases using animal models, it is crucial to simulate exposure as naturally as possible to produce the most accurate and useful results.
To simulate the aerosol route of exposure in NHPs, inoculation of infectious agents is often performed in a system where the NHP’s head is placed in an aerosol chamber, and an aerosolized agent is delivered directly to the chamber at a controlled rate.15 To ensure that an accurate dose is delivered, it is necessary to have an accurate measurement of the animal’s respiratory parameters, which is often done by whole-body or head-out plethysmography.6 During head-out plethysmography, the NHP’s chest movements are measured by a pressure transducer for a defined amount of time and the computer software uses this information to measure respiratory parameters such as tidal volume (TV) and frequency (F) to calculate minute volume (MV) (TV × F = MV). The average MV is used in the calculation to determine the aerosol duration needed to present a target dose. Once an NHP is transferred to the head-only aerosol exposure system, the MV cannot be measured or adjusted. Because the MV cannot be adjusted, consistent anesthesia is essential to sustain a steady MV for the correct dosing of inhaled agents.3,15
There are a variety of anesthetics available for NHP anesthesia, each with varying effects on physiologic parameters as well as varying duration of action. Ideal characteristics for NHP plethysmography anesthesia protocols include the following: subcutaneous or intramuscular administration to permit cage-side anesthesia, a minimum 45-min duration of action, and rapid steady-state minute volume (SSMV) achievement (defined as no more than a ±10% change) with a greater accumulated minute volume (AMV), thus decreasing the amount of time required in the aerosol chamber.3,15 A recent 5-mo study performed at USAMRIID found that an alfaxalone-midazolam (AM) combination outperformed tiletamine-zolazepam and ketamine-acepromazine, the most common anesthetics currently used in NHPs for plethysmography.11 Although AM provided a superior quality of anesthesia, the major drawback was the large injection volumes required to achieve anesthesia.11 To limit intramuscular injections to no more than 3 mL per site, NHPs weighing greater than 5.35 kg required 2 separate injections to receive a full dose. This was unavoidable due to only a single concentration of alfaxalone being available as a Center for Veterinary Medicine (CVM)/FDA Indexed product for NHPs. Recently, the opportunity became available to test a more concentrated formulation of alfaxalone. This investigational formulation contains 4 times as much alfaxalone and cyclodextrin (vehicle) but is adjusted to the same pH of 7. Both the Indexed commercial 10-mg/mL formulation (AM10) and the investigational 40-mg/mL formulation (AM40) have the same preservative system. The investigational formulation is also a ready-to-use solution but is more viscous than the AM10 counterpart. By using the more concentrated solution of alfaxalone, smaller injection volumes can be administered resulting in less pain and distress to the animal, while providing the same level of anesthesia. Currently, the concentrated version of alfaxalone has been tested in combination with other drugs in both bighorn sheep and elk and found to be an efficacious anesthetic drug.8,16
Since the concentrated version of alfaxalone has not yet been tested in cynomolgus macaques, it is reasonable to compare the 2 formulations (AM10 compared with AM40) to ensure the drug acts as expected and maintains the level of anesthesia required for plethysmography studies. If found to be equivalent to the currently Indexed product, this study would confirm the efficacy of AM40 for future plethysmography studies, thus enhancing animal welfare while maintaining scientific integrity. We compared AM at a 5 and 0.3 mg/kg dose using 2 separate concentrations of alfaxalone, the Indexed 10-mg/mL concentration and the investigational 40-mg/mL concentration. Quality of anesthesia, respiratory parameters, duration of anesthesia, and side effects were examined and compared between the 2 sedative protocols in cynomolgus macaques. In addition, injection sites were monitored for local reactions at 24 h, 7 d, and 14 to 21 d postinjection. Each syringe containing an AM mixture was observed for miscibility before anesthetic administration to an NHP. Miscibility of both alfaxalone formulations with the same formulation of midazolam was observed for each anesthetic episode to observe for mixing reactions. Differences in anesthetic parameters between sexes were explored, as sex differences in clearance of alfaxalone, duration of anesthesia, and blood pressure have been shown to exist in rats but have not yet been explored for cynomolgus macaques.27,28
The primary purpose of our study was to compare the required injection volumes, duration of anesthesia, anesthetic quality (QA), AMV, SSMV, and observable side effects based on the safety of the animal and disruption to the study of 2 different alfaxalone formulations when mixed with the same midazolam formulation (AM10 and AM40) to determine if a more concentrated product (40 mg/mL alfaxalone) can be used for NHPs undergoing aerosol exposure studies. Using a more concentrated formulation of alfaxalone will result in a lower injection volume to achieve the same dosage, thereby resulting in improved welfare for NHPs. We hypothesized that the 2 different alfaxalone formulations, when used at the same dosage, in combination with midazolam, would show no difference in QA, duration of anesthesia, SSMV, AMV, and side effects. Based on previously published studies in rats, we formed a secondary hypothesis that female macaques would experience a longer duration of anesthesia as compared with male macaques at the same anesthetic dose.
Materials and Methods
Animals.
The animal research conducted for this study was performed under the oversight of the USAMRIID IACUC following a comprehensive protocol review and approval process. All animals were handled in accordance with the AALAS Position Statement on the Humane Care and Use of Laboratory Animals, the Animal Welfare Act, Public Health Service Policy, and other Federal statutes and regulations concerning the care of animals used in experimental research. Animals were housed in AAALAC International-accredited facilities that strictly adhere to the standards of the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011. The study group consisted of 15 cynomolgus macaques (Macaca fascicularis) with mixed Cambodian (11 total, 6 females, and 5 males) and Chinese (4 total, 2 females, and 2 males) origins. Animals were selected from USAMRIID’s NHP colony based on availability and evenly distributed across the weight and sex of the animals as closely as possible. At the beginning of the study, the average age of female NHPs was 6 y (range: 5.2 to 7 y) and the average age of male NHPs was 9.7 y (range: 5.4 to 18.3 y). The average female body weight throughout the trial was 4.79 kg (range: 3.53 to 6.57 kg), and the average male body weight was 8.39 kg (6.15 to 10.09 kg). The authors expected that males would be larger than females on average due to sexual dimorphism in cynomolgus macaques. The body condition score (BCS) ranged from 2 to 5 (lean to underweight) to 5 to 5 (overweight). No significant health issues or immunologic impairments were identified on preassignment physical examinations, recent lab work, and health record reviews of the animals. No animals were the product of genetic modification or alterations of any type. All anesthetic events were performed in ABSL2 laboratory space. Animals assigned to the study were fed twice daily (Teklad 2050; Envigo, Madison, WI). Animals were observed at least twice daily. Dietary enrichment and manipulanda were provided for each animal daily. All animals were housed in modular primate caging appropriate for their size (Lab Products, Seaford, DE) and were fully, or partially, paired for social interaction in accordance with standards set forth in the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act. NHPs were housed in ABSL2 rooms at 64 to 84 °F (17.8 to 28.9 °C), 30% to 70% relative humidity, and 12:12-h light/dark cycle. All animals used for the study had acclimated within the NHP colony at the institute for more than 90 d.
Anesthesia and monitoring.
Each NHP assigned to the study underwent 2 separate anesthetic events, once with 10 mg/mL alfaxalone (Alfaxan Multidose; Jurox [a part of Zoetis], North Kansas City, MO) and 5 mg/mL midazolam (Heritage Pharmaceuticals, East Brunswick, NJ) and once with 40 mg/mL alfaxalone investigational formulation, Jurox [a part of Zoetis], Rutherford, NSW, Australia) and midazolam 5 mg/mL. The dose of the alfaxalone-midazolam combinations was the same in each trial at 5 mg/kg alfaxalone and 0.3 mg/kg midazolam. The only difference was the formulation of alfaxalone administered, with the anesthetic events using the 10 mg/mL concentration designated as the control group. All anesthetic combinations were administered IM with no single injection exceeding 3.0 mL. The total injection volumes varied between 0.45 and 5.3 mL. For total volumes exceeding 3.0 mL, 2 separate injection sites were administered in rapid succession, and injection sites were documented. Upon loss of consciousness, the injection sites were clipper shaved, marked, and photodocumented. The injection sites were then monitored for reactions at 24 h, 7 d, and 14 to 21 d. Throughout each anesthetic event, all vital signs were collected via a veterinary patient monitor (Bionet, Tustin, CA) and a detached pulse oximetry monitor (Edan, San Diego, CA). NHP heart rates, respiratory rates, oxygen saturation of hemoglobin (SpO2), and body temperature via esophageal probe were monitored. Parameters were set to measure potential anesthetic side effects and were defined as follows: tachycardia was a heart rate greater than or equal to 200 beats per minute (bpm) lasting for 5 min or more14; hypothermia was a body temperature less than 94 °F (34.4 °C)4,24 that was unresponsive to warming efforts for 15 min; hypoxia was a pulse oximetry reading (SpO2) of less than 85% lasting for 5 min or more;5,13 and apnea was an observed cessation in breathing for more than 30 s.5 Anesthetic monitoring equipment was placed on the animal before sealing the chamber for plethysmography. Anesthetized animals were sealed in the plethysmograph chamber in a supine or lateral supine position. Animals exceeding 6.5 kg in weight with a BCS of 3/5 or more were rotated into a 45° right lateral supine orientation to prevent apnea and reduce respiratory depression in overweight animals. This adjustment was made based on previous studies documenting apnea with alfaxalone use in cynomolgus macaques, research in monkeys as a model for sleep apnea, and the decreased severity of apnea in overweight human patients when placed in lateral recumbency.1,11,17,21, Vital signs were recorded every 5 min on standardized forms. Adverse events during anesthesia were addressed with appropriate interventions and recorded on standard forms then tabulated as side effects data for the study. Body temperatures were measured approximately every 15 min in accordance with USAMRIID standard operating procedures (SOPs) via an esophageal thermometer. If esophageal core body temperature dropped below 94 °F for more than 15 min, external warming was implemented. If after another 15 min the temperature did not rise above 94 °F, the trial was immediately terminated, and the animal recovered from anesthesia. At the completion of each anesthetic episode, the NHP was returned to its home cage for recovery and observation. An NHP was considered recovered when it regained a righting reflex and could maintain an upright position. Anesthetic events took place on 6 different days with 5 animals anesthetized on each of those days. For each of the 15 NHPs, the order of the anesthetic event and formulation administered was randomized into 2 iterations consisting of AM10 dosing and AM40 dosing. There was at least one week between anesthetic events for each animal. The shortest interval between anesthetic events for an individual animal was 10 d and the longest was 35 d.
Miscibility of alfaxalone formulations.
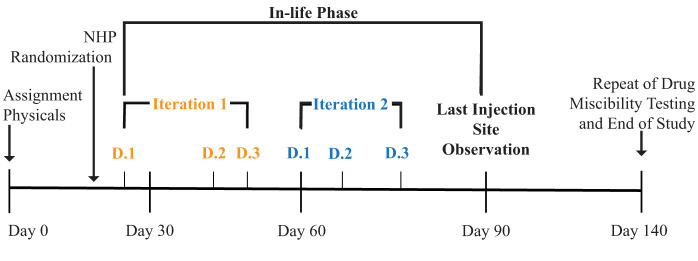
Immediately prior to the start of anesthetic events on each of the 6 study days, the AM10 or AM40 drug mixtures were prepared in syringes before injection. Once prepared, the syringes were labeled and placed in brown paper bags to protect them from light prior to administration. Video of the preparation was recorded and used to observe the miscibility of each mixture. A general description of any reactions or changes in the physical properties of the respective AM10 and AM40 mixtures within each syringe was recorded on a study-specific form created to document observations on AM mixing. The purpose of this description was to document any physical or chemical changes upon mixing the solutions for injection, such as particulate formation. Formation of a “cloudy” mixture occurred in 10 out of 15 of the AM10 mixtures during the in-life phase of the study. In-life phase refers to the phase of the experiment following administration of the test agents in live animals (Figure 1). The mixing of the same drug combinations was repeated in a separate study after the in-life phase of the study was completed. During this separate study, the mixtures were observed in each syringe under a running clock with seconds as the unit of measurement. The values for time intervals for the disappearance of the visual changes and the volumes of midazolam and alfaxalone in each of the respective syringes were recorded in a spreadsheet (Microsoft Excel 2023, Redmond, WA). The mean, median, and range of times to dissolution were calculated in the spreadsheet for all syringes that had visual signs of separation.
Figure 1.
Timeline for the study by day with visual representation of data iterations and the in-life phase. NHP randomization broke the study population into 2 trial iterations where each NHP received AM40 or AM10. On the following iteration, each NHP received the alternate AM formulation. Randomization also allocated 5 NHPs to 3 plethysmography data collection days (D.1 to D.3) within each iteration. The end of the study is marked by the end of the repeat of drug miscibility testing.
Plethysmography.
Once NHPs were safely anesthetized, they were taken from their home cages and placed into a head-out plethysmography system. Two sizes of plethysmography chambers (large coffin type: width at shoulders (WS) = 10.75 in, width at feet (WF) = 6.75 in., L = 33 in., and H = 8 in.; extralarge coffin type: WS = 14.75 in., WF = 10.75 in., L = 36 in., and H = 8 in.) were employed throughout the study. The extralarge chamber was used for animals weighing greater than 8 kg. Both chambers were calibrated daily, using a calibrated syringe (model 5510; Hans Rudolph, Shawnee, KS), with a predetermined volume of air in accordance with the plethysmography software manual and USAMRIID SOPs. In both chamber types, the NHP’s torso and extremities were contained inside the plethysmography chamber and the head extended through a hole in the chamber oriented toward the anesthesia monitoring equipment. A piece of dental dam with a hole cut in the center was used to seal the chamber by going over the animal’s head and fitting tightly around the neck. Next, a closed-cell silicone rubber dam (mousepad with a hole cut in the center), foam, and an acrylic faceplate were secured around the animal’s neck and latched into place to form the final seal on the chamber. The NHP’s respirations were checked for excess upper airway noise to ensure the seal was not too tight, and a folded towel was provided to support the head in a manner that maintained the animal’s airway. Movement of the NHP’s chest created a pressure differential within the chamber that was measured by a pressure transducer (TRD 5700; Data Sciences International [DSI], St. Paul, MN). The transducer was connected to a preamplifier (QT digital preamplifier; Data Sciences International [DSI]), which was connected to the computer with the plethysmography software (FinePointe v2.3.1.6; Data Sciences International [DSI]) that measured respiratory parameters including TV and F. The plethysmography system generated a calculated MV reading every 10 s. These readings were transcribed real time into an Excel spreadsheet (Microsoft) on a separate laptop computer that calculated the average of 6 MV readings recorded over each minute of the trial. Percent change in MV was calculated between each minute in succession using the programmed spreadsheet. The NHP was determined to have reached SSMV when the percent change between minutes did not vary by ±10% for 5 min. After the NHP attained 20 consecutive minutes of SSMV, the trial was completed. If the change between minutes varied by more than ±10% before the completion of 20 min, then the streak of SSMV was broken. A second consecutive attempt was then made with the NHP in the chamber to achieve SSMV for 20 min. If the second attempt failed, the trial was completed. If the NHP showed signs of recovery, such as voluntary movement, or the total length of the trial reached 60 min, the trial was ended. Upon completion or termination of the trial, NHPs were transported to their respective home cages and monitored until recovered.
Data and statistical analysis.
Head-to-head comparison of anesthetic drug protocols included examination of the following parameters: total anesthetic injection volume, SSMV, AMV, duration of anesthesia, duration of anesthesia for each sex, QA, and side effects. Additional variables that were analyzed included the following: miscibility of the drug mixtures, time to start of anesthesia, and BCS. The values for injection volumes were the total volume of both midazolam and the respective alfaxalone formulation for each anesthetic event. For AM10, several of the volumes were administered as 2 injections due to limitations on single-site injection volumes under USAMRIID SOPs. The value for SSMV was calculated as the average MV between all periods qualifying as SSMV during a trial. AMV was defined as the summation of all MV averages throughout periods qualifying as SSMV for a given trial. The value for the duration of anesthesia was the number of minutes between the injection of the respective anesthetic protocol and the time to NHP recovery in its home cage. Time to start was measured as the time from injection of the anesthetic protocol to the recording of the first set of vital signs to start the trial in the plethysmography chamber. QA was scored based on categorical criteria from 1 to 4, with 4 being the highest or best quality. A score of 1 was assigned if the NHP did not reach SSMV at all during the trial. A score of 2 was assigned if the NHP reached SSMV during the first attempt but failed to maintain it for a minimum of 20 min and did not reach SSMV again during the second attempt. A score of 3 was assigned if the NHP reached SSMV twice. A score of 4 was assigned if the NHP reached SSMV once and maintained it for 20 consecutive minutes. Side effects were scored on a categorical 4-point scale. A score of 0 indicated that there were no side effects noted throughout the anesthetic period. A score of 1 consisted of tremors or muscle fasciculations with no disruption to data collection. A score of 2 consisted of variation in vital signs prompting intervention and patient manipulation without disrupting the trial (limited tachycardia, hypothermia, hypoxemia). A score of 3 required the postponement of data collection and immediate intervention (apnea, prolonged and severe physiologic disturbance).
This study was designed for a sample size of 15 total NHPs, and all NHPs were randomized to receive either the AM10 or AM40 anesthetic protocol in the first iteration. After a washout period of no less than 7 d, each NHP underwent trials in the second iteration with the alternate AM formulation used in the first iteration. In this way, each NHP served as their own control. The number of NHPs required for the experiment was based on a combination of the observed performance of AM10 in NHPs in a previous study to form the null hypothesis and a reasonable alternative hypothesis for improved performance by AM40 via a smaller injection volume.11 Statistical power was developed using measurements for anesthetic events with an estimated correlation between observed and hypothesized anesthesia quality results of 0.829, achieving approximately 96.5% power to detect a difference of 0.02 between the null hypothesis median difference of 0.0 and an actual median difference of 0.02 at the 0.05 significance level (α) using a 2-sided Wilcoxon signed-rank test. These results were based on 2,000 simulated samples from the null distribution of multinomial distribution (6.67%, 0%, 13.33%, and 80%) and the alternative distribution of multinomial (0%, 0%, 13.33%, and 86.67%). Randomization was used to allocate animals and provide a time order for animals within experimental and control groups and the order of anesthetic events within groups. The randomization of experimental and control groups was completed in SAS/STAT version 9.4 (TS 1M5; SAS Institute, Cary, NC) per the scheme below.20 NHPs were randomized by iteration with one anesthesia episode per NHP. Five NHPs were assigned to be tested each day. NHPs were divided as close as mathematically possible to being even between sexes. For the first iteration, approximately equal numbers of NHPs received anesthesia at each of the AM mixtures. To obtain comparable measurements for each NHP, the AM mixtures provided to each NHP in the second iteration were the alternative to the AM mixture received in the first iteration. With the same constraints as the first iteration, the NHPs were randomized to different days and a different order within the day for the second iteration. A timeline for the study with a depiction of data iterations is included in Figure 1. The research team was not blinded as it would have required a separate team of another veterinarian and a veterinary technician to prepare drug mixtures. Furthermore, the 40-mg/mL alfaxalone formulation is investigational, and little information was available on potential safety issues with the formulation. The veterinary staff supporting the study remained aware when the investigational 40 mg/mL formulation was in use.
Upon completion of data collection, results were analyzed using SAS/STAT version 9.4. The 2-sided signed-rank test for analysis of paired nonparametric data was used to test for statistically significant differences between AM mixtures concerning total injection volume, SSMV, duration of anesthesia, and AMV.9 The 2-sided signed-rank test was used to analyze side effects and QA paired data for statistically significant differences between AM mixtures. A Hahn and Meeker 95% confidence interval was used to summarize data further for all the aforementioned variables (Table 1).12 In addition, since no one-sided test was available for the signed-rank test, this confidence interval was used in conjunction with the signed-rank test to determine the appropriate P value. Since both sides of the confidence interval are below zero, we know that the confidence interval indicates that the total injection volume for AM40 is less than AM10 (AM40 − AM10 < 0). Since we also know that the signed-rank tests have a 2-sided P value less than 0.0001, the P value for AM40 less than AM10 is P less than or equal to 0.0001(lower P values are rounded to this number). Differences between AM mixtures and sexes for quality (2-sided) and duration of anesthesia (one-sided) were analyzed using the Wilcoxon exact rank sum test. These comparisons were described further using a Hodges-Lehman 95% confidence interval (Table 2).12 In all cases, P values less than or equal to 0.05 were statistically significant.
Table 1.
Key variables for comparison of alfaxalone-midazolam 10- and 40-mg/mL mixtures with variation in differences displayed by confidence intervals
| Variable | Median and Hans and Meeker 2-sided 95% CI | ||||
|---|---|---|---|---|---|
| Test type | P value | LCL | Median | UCL | |
| Total volume injected (mL) | Sign rank | <0.0001 | −3.08 | −2.25 | −1.77 |
| Quality (quality score) | Sign | 0.4531 | 0 | 0 | 1 |
| SSMV (mL/min) | Sign rank | 0.5416 | −50.24 | −4.005 | 36.33 |
| AMV (mL) | Sign rank | 1.0000 | −1,000 | 160 | 2,100 |
| Side effects | Sign | 0.2891 | −1 | 0 | 0 |
| Duration of anesthesia (min) | Sign rank | 0.3998 | −33 | −10 | 26 |
The use of bold formatting is meant to highlight that the P value for total volume injected was statistically significant. LCL, lower confidence limit; UCL, upper confidence limit.
Table 2.
P values listed for quality scoring describe significant differences between sexes for respective alfaxalone-midazolam 10-mg/mL (AM10) and 40-mg/mL (AM40) protocols
| Variable | P value | Alternative hypothesis | Hodges-Lehman 2-sided 95% confidence interval | ||
|---|---|---|---|---|---|
| LCL | Median | UCL | |||
| Quality score AM10 | 0.6280 | F ≠ M | −2 | −0.5 | 1 |
| Quality score AM40 | 0.5692 | F ≠ M | |||
| Duration of anesthesia AM10 (min) | 0.1043 | F > M | −64 | −6 | 52 |
| Duration of anesthesia AM40 (min) | * | F > M | −65 | −35.5 | −6 |
The P values for duration of anesthesia describe the probability that females have a longer duration of anesthesia than males. Confidence intervals are provided for reference and the summary data is depicted in Table 4. LCL, lower confidence limit; UCL, upper confidence limit.
P value could not be calculated as the data suggested that females have a shorter duration of anesthesia using AM40.
Statistically significant differences between drug mixtures occurred when P values were less than or equal to 0.05 for total volume of AM mixture injected, or when both the upper and lower bounds of the Hahn and Meeker 2-sided 95% confidence interval were less than or greater than zero. Medians were used in place of mean values due to relatively small sample sizes and the unknown probability distributions of the data sets. There was no loss of information if any of these data followed the normal distribution. for the normal distribution, the observed mean and the median are approximately equal.
Results
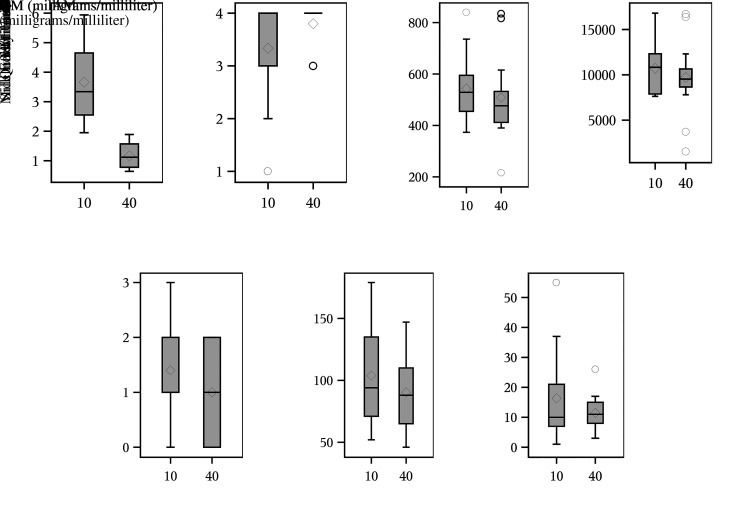
The total injection volumes were compared between AM40 and AM10 mixtures (Table 1, Figure 2A) demonstrating that AM40 required a smaller injection volume than AM10 for the study (P ≤ 0.0001). The Hahn and Meeker 2-sided 95% confidence interval for the difference in injection volume had a median of −2.25 mL (95% CI: −3.08 to −1.77 mL). Since both sides of the confidence limit are negative numbers and the 2-sided signed-rank test has P < 0.0001, the AM40 < AM10 difference in injection volume is statistically significant. AM40 had a median injection volume of 1.12 mL compared with a median volume of 3.34 mL for AM10 for an overall decrease in injection volume of 66.47% across the study (Table 3). For AM40, the reduction in volume allowed for all 15 NHPs to be dosed with a single IM injection compared with only 8 out of 15 NHPs dosed with a single AM10 injection. AM40 dosing had a standard deviation of 0.4297 mL compared with 1.362 mL for AM10.
Figure 2.
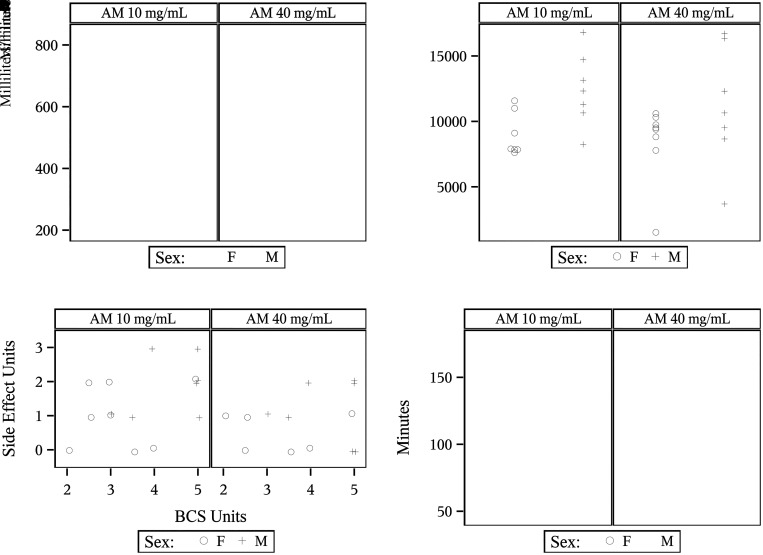
Paired comparison box plots for alfaxalone-midazolam (AM) mixtures by (A) injection volume; (B) distribution of quality scoring; (C) steady-state minute volume averages (SSMV); (D) accumulated minute volume (AMV); (E) side effects scores; (F) duration of anesthesia; (G) time to start after administering anesthesia. Symbols in the box plots are a line across the middle indicating the median value and a diamond representing the mean; upper and lower ends of the box are 25th and 75th percentiles, respectively; upper and lower whiskers represent the highest and lowest expectation for observed values; and circles represent extreme values greater than expectation.22
Table 3.
Summary statistics for paired test results comparing alfaxalone-midazolam mixtures across key variables and covariables
| Differences between AM mixtures | AM40 | AM10 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Min | Median | Max | SD | Min | Median | Max | SD | Min | Median | Max | SD |
| Total volume injected (mL) | −4.05 | −2.25 | −1.31 | 0.94087 | 0.64 | 1.12 | 1.89 | 0.4297 | 1.95 | 3.34 | 5.94 | 1.362 |
| Quality score | −1 | 0 | 3 | 1.19 | 3 | 4 | 4 | 0.414 | 1 | 4 | 4 | 1.11 |
| SSMV (mL/min) | −334 | −4.005 | 160 | 126.18 | 217 | 476.5 | 835 | 156.06 | 373 | 528.8 | 840 | 133.21 |
| Duration of SSMV (min) | −15.5 | 0 | 11.5 | 6.854 | 4.5 | 20 | 20 | 4.9917 | 8.5 | 20 | 20 | 3.7228 |
| AMV (mL) | −9,000 | 160 | 3,000 | 3,970 | 2,000 | 9,500 | 20,000 | 3,880 | 8,000 | 11,000 | 20,000 | 2,850 |
| Side effects score | −2 | 0 | 1 | 0.91 | 0 | 1 | 2 | 0.845 | 0 | 1 | 3 | 0.986 |
| Duration of anesthesia (min) | −97 | −10 | 52 | 38.596 | 46 | 88 | 147 | 29.28 | 52 | 94 | 179 | 39.846 |
| Time to start (min) | −50 | −3 | 20 | 17.7 | 3 | 11 | 30 | 5.84 | 1 | 10 | 60 | 15.1 |
Min, minimum; Max, maximum; AM10, alfaxalone-midazolam 10 mg/mL; AM40, alfaxalone-midazolam 40 mg/mL; SSMV, steady-state minute volume; AMV, accumulated minute volume.
QA was compared between AM10 and AM40 (Table 1 and Figure 2B), and no statistically significant difference was detected (P = 0.4531) between AM mixtures. QA was compared between sexes and respective AM mixtures under the assumption that female quality scores would be equivalent to males with both mixtures (Table 2). For AM10 and AM40, no statistically significant difference was found between sexes (AM10: P = 0.6280 and AM40: P = 0.5692). For both sexes and mixtures, all had median and maximum scores of 4 with the majority of animals reaching and maintaining SSMV for 20 min on the first attempt (Table 4). The minimum AM40 score of 3 was attained for both sexes with AM40 animals reaching SSMV twice during the trials. For AM10, the female minimum score was 1 with the NHP failing to reach SSMV during the trial while the male minimum score of 2 indicates that SSMV was reached once and broken. The female AM10 group had the highest standard deviation in QA (1.36) compared with the lowest standard deviation in female AM40 NHPs (0.354) with a range in variability in QA of 1.01 across the entire study.
Table 4.
Summary statistics by variable, alfaxalone-midazolam mixture, and sex
| Variable | Sex | AM10 | AM40 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Median | Max | SD | Min | Median | Max | SD | ||
| Total volume injected (mL) | F | 1.95 | 2.72 | 3.84 | 0.69557 | 0.64 | 0.835 | 1.23 | 0.21461 |
| M | 2.99 | 4.65 | 5.94 | 1.0551 | 1.12 | 1.57 | 1.89 | 0.30569 | |
| Quality score | F | 1 | 4 | 4 | 1.36 | 3 | 4 | 4 | 0.354 |
| M | 2 | 4 | 4 | 0.787 | 3 | 4 | 4 | 0.488 | |
| SSMV (mL/min) | F | 393 | 508.3 | 580 | 74.34 | 217 | 473.6 | 530 | 100.24 |
| M | 373 | 594.9 | 840 | 159.14 | 410 | 532.3 | 835 | 179.23 | |
| AMV (mL) | F | 7,620 | 7,886 | 11,600 | 1,663.5 | 1,520 | 9,471 | 10,600 | 2,940 |
| M | 8,240 | 1,320 | 16,800 | 2,795.3 | 3,690 | 10,650 | 16,700 | 4,545 | |
| Side effects score | F | 0 | 1 | 2 | 0.926 | 0 | 1 | 2 | 0.835 |
| M | 1 | 2 | 3 | 0.9 | 0 | 1 | 2 | 0.9 | |
| Duration of anesthesia (min) | F | 52 | 71 | 179 | 50.951 | 46 | 66.5 | 124 | 25.854 |
| M | 81 | 107 | 157 | 26.456 | 84 | 102 | 147 | 23.155 | |
| Time to start (min) | F | 1 | 9 | 60 | 19.1 | 7 | 9.5 | 20 | 3.29 |
| M | 5 | 11 | 40 | 11.2 | 3 | 12 | 30 | 8.06 | |
AM10, alfaxalone-midazolam 10 mg/mL; AM40, alfaxalone-midazolam 40 mg/mL; AMV, accumulated minute volume; Max, maximum; Min, minimum; SSMV, steady-state minute volume.
SSMV was compared between AM mixtures (Table 1 and Figure 2C). There was no statistically significant difference (P = 0.5416) between AM10 and AM40 mixtures. The median AM40 SSMV (476.5 mL/min) remained relatively close to the median AM10 SSMV (528.8 mL/min) with a median difference in SSMV between mixtures of −4.005 mL/min throughout the entire study (Table 3). SSMV values are broken down by sex and mixture (Table 4). Median values for SSMV remained relatively consistent, with AM10 males having the highest median SSMV (594.9 mL/min) and AM40 females having the lowest median SSMV (473.6 mL/min) with a difference of only 121.3 mL/min throughout the trial. Male NHPs in both mixture groups achieved maximum SSMV values over 800 mL/min compared with SSMV values below 600 mL/min for female NHPs across both mixtures (Figure 3A).
Figure 3.
Outcome parameters by sex and alfaxalone-midazolam (AM) mixtures: (A) steady-state minute volume (SSMV); (B) accumulated minute volume (AMV) by sex and alfaxalone-midazolam (AM) mixture; (C) side effects scores and body condition scores (BCS); (D) Duration of anesthesia. Scatter plot values have been displaced slightly or “jittered” to make the entries more visible to the reader.
AMV was compared between AM10 and AM40 groups (Table 1 and Figure 2D), and no statistically significant difference (P = 1.000) was found between AM mixtures. The AMV median values for AM40 (9,500 mL) and AM10 (11,000 mL) were relatively close with a difference of 1,500 between the listed median values and a median difference of only 160 mL for the entire study (Table 3). AM40 had a standard deviation (3,880 mL) that was 36.14% higher than AM10 (2,850 mL). The maximum AMV values for AM10 and AM40 were the same (20,000 mL), with a maximum difference of 3,000 mL across the data set for the entire study. Table 4 demonstrates that AMV minimum values for AM40 males (3,960 mL) and females (1,520 mL) differ from AM10 males (8,240 mL) and females (7,620 mL) by 4,280 mL and 6,100 mL, respectively. The standard deviation for AM40 (3,880 mL) was 136.1% that of the AM10 standard deviation (2,850 mL) for the entire study. Across sexes and mixtures, AM40 males had the highest standard deviation (4,545 mL) compared with the AM10 females with the lowest standard deviation (1,663.5 mL) for a range in variability of 2,881.5 mL. The heightened variability for AM40 is due to a male and female NHP that had AMV values far below the median within the group. Both animals had QA scores of 3 denoting 2 entries into SSMV for their respective trials. The female had a mean duration of SSMV that was only 4.5 min and an average SSMV value of 217.05 mL/min. The male had an average SSMV duration that lasted only 10 min reducing his total AMV value far below the median for the group. Figure 3B shows the distribution of AMV values by AM mixture and sex for the entire study.
Side effects scoring was also compared between the AM40 and AM10 anesthetic groupings (Table 1 and Figure 2E). There was no statistically significant difference (P = 0.2891) in side effects between the 2 anesthetic groups. Both mixture groups had a median side effects score of 1 (Table 3). The AM10 maximum side effects score rose to 3 while the maximum side effects score for the AM40 group was 2. The standard deviation in side effects scores was 16.7% higher for the AM10 group (0.986) compared with AM40 (0.845). Side effects scores by sex and mixture are shown in Table 4. AM10 males were the only anesthetic group to reach a maximum side effects score of 3 while all other anesthetic groupings had maximum side effects scores of 2. AM10 females had the highest variability in side effects scoring with a standard deviation of 0.926.
Side effects are further broken down by type of side effect and score by individual animal (Table 5). Side effects scores for each animal under respective AM mixtures were assigned based on the highest ranked side effect noted for each animal per trial. No side effects were observed among 33.3% of NHPs in the AM40 groupings and 20% of NHPs in the AM10 group. Side effects scoring was applied per the discussion in Data and Analysis in Materials and Methods. The only noted side effects in score category one were tremors and muscle fasciculations (46.67%). This side effect was observed in 40% of NHPs receiving AM40 and 53.3% receiving AM10. Across all anesthetic trials, 33.3% of animals overall and in the AM40 and AM10 groups, respectively, were assigned a score of 2. In this category, the most common side effect was stertor or stridor (AM40 20%; AM10 26.6%). The second most frequent was a split between hypoxemia (SpO2 less than 85%; AM40: 13.3%; AM10: 20%) and tachycardia (> 200 bpm; AM40: 13.3%; AM10: 20%). In the AM10 groupings, 13.3% of animals had bronchospasms (defined by coughing under anesthesia) and 6.7% of animals developed transient hypothermia (< 34 °C [94 °F]). In the AM40 groupings, hypothermia was also observed in 6.7% of animals, but no animals were observed with bronchospasms.
Table 5.
Side effects scoring by scoring interval and side effect type compared between the alfaxalone-midazolam 10- and 40-mg/mL mixtures
| Side effects score | Side effect type | AM10 | AM40 |
|---|---|---|---|
| Score 0: no side effects | None | 3 | 5 |
| Score 1: no impact on data collection | Tremors/fasciculations | 8 | 6 |
| Score 2: adjustments to supportive care | Hypothermia | 1 | 1 |
| Hypoxemia | 3 | 2 | |
| Tachycardia | 3 | 2 | |
| Stertor/stridor | 4 | 3 | |
| Bronchospasms | 2 | 0 | |
| Score 3: disruption to data collection | Apnea | 2 | 0 |
Side effects scores were assigned to an animal based on the highest-ranked side effect. Many animals displayed multiple side effects across score levels depicted below. AM10, alfaxalone-midazolam 10 mg/mL; AM40, alfaxalone-midazolam 40 mg/mL.
The only side effect noted in category 3 was apnea (cessation in respirations for greater than 30 s). AM10 males were the only group represented in this category with 28.6% of animals experiencing apnea during positioning in the plethysmography chamber. Note that apnea occurred before the attachment of anesthetic monitoring devices and the start of the trials preventing the measurement of other concurrent side effects such as hypoxemia, tachycardia, and hypothermia during the apneic episode. Since BCS was taken into consideration for the risk of apnea, the side effects data is displayed for the entire study with AM mixture, side effects scores, and BCS (Figure 3C). Note that both NHPs with a side effect score of 3 were males under AM10 with a BCS of 4/5 and 5/5, respectively.
Duration of anesthesia was compared between AM mixtures (Table 1 and Figure 2F). There was no statistically significant difference (P = 0.3998) in duration of anesthesia between the AM40 and AM10 groups. The AM10 group had a median duration of anesthesia (94 min) 6.8% higher than the AM40 group (88 min), and the median difference between mixtures showed that AM40 trials were 10 min shorter in duration overall. AM10 also had a standard deviation in duration of anesthesia (39.846 min) that was 36.09% higher than the standard deviation for AM40 (29.28 min) (Table 4).
Duration of anesthesia was compared based on the assumption that females would have longer anesthetic trials than males when administered AM mixtures (Table 2 and Figure 3D). No statistically significant increase in duration of anesthesia was detected for females (P = 0.1043) in the AM10 group. A P value could not be produced for the sex comparisons within the AM40 group; however, the data suggested that females in this group had a shorter duration of anesthesia. A Hodges-Lehman 2-sided confidence interval is included for the sex comparisons within the AM40 group with a median of 35.5 min (95% CI: −65 to −6 min). For AM40, females had a median duration of anesthesia (66.5 min) that was 34.8% shorter in duration than males (102 min). For AM10, females had a median duration of anesthesia (71 min) that was 33.6% shorter than males (107 min). The longest anesthetic trial was an AM10 female at 179 min compared with the shortest anesthetic trial of 46 min in an AM40 female with a range in anesthetic duration of 133 min across the entire study. AM10 females had the greatest standard deviation in duration of anesthesia (50.951 min) compared with the AM40 males who had the shortest (23.155 min).
Time to start was also compared between the AM40 and AM10 mixtures to provide information about the onset of anesthesia and consistency of the timing of anesthetic procedures (Table 3 and Figure 2G). Median time-to-start values for AM10 (10 min) and AM40 (11 min) differed by a small amount. The median difference showed that AM40 trials started 3 min sooner than AM10. The standard deviation for time to start under AM10 (15.1 min) was 258.6% more than AM40 (5.84 min). Table 4 shows sex comparisons in time to start between AM mixtures. While the median values remain relatively consistent across all groups, the standard deviation for AM10 females (19.1 min) had the greatest variability compared with the lowest standard deviation in AM40 females (3.29 min) with a range of variability of 15.81 min across the entire study.
During the original mixing of AM10 mixtures, 10 out of the 15 AM10 doses prepared had noted separation of the midazolam and alfaxalone forming 2 layers in the syringe before administration. The separations were resolved before administration during the study. Following completion of the in-life study, an additional in vitro mixing study was performed assessing the optical changes to each of the drug mixtures (that is, AM10 and AM40) when mixed in a syringe. Upon the second iteration of mixing of the AM10 reactors during the in-life phase of the study, 9 of the original 10 AM10 reactors displayed the 2-phase separation reaction. The range of time to dissolution was 22 to 1,448 s (mean: 343.9 s; median: 248.5 s). None of the NHPs administered the AM10 or the AM40 injections had injection site reactions at any of the rechecks indicating that the syringes with separation reactions did not cause adverse events in the animals.
The following items are documented exceptions from data collection. On the first day of iteration one, the plethysmography computer malfunctioned, and a lapse in data collection for an NHP under AM40 occurred during the fifth minute of SSMV. To adjust for this complication, the time trial was extended an additional minute to obtain another MV reading. The impact of this additional measurement was included in the calculated 20 min of SSMV for this entry. During the third day of iteration 1 of data collection, the plethysmography computer malfunctioned and a lapse in data collection for an NHP under AM40 occurred during 20 s of the 18th minute of SSMV. To adjust for this complication, the 20 s immediately following the malfunction was recorded in place of the missing data. The impact of this additional measurement was included in the calculated 20 min of SSMV for this entry. On the third day of iteration 2, a female NHP under AM10 failed to achieve the loss of voluntary movement. No anesthetic measurements could be taken as the NHP could not be safely removed from her enclosure. The NHP was monitored until fully recovered without complication. The AM10 event was incorporated into data for QA, side effects, and total volume injected as these values could still be observed. QA was assessed with a score of 1 unit since the NHP did not attain anesthesia and therefore failed to reach SSMV. The AM10 event was omitted from AMV, duration of anesthesia, and SSMV data as none of the measurements used to calculate these values could be safely and accurately obtained.
Discussion
This study compared a novel AM40 anesthetic drug protocol against the AM10 protocol already established as a superior option for cynomolgus macaques undergoing plethysmography.11 The AM40 protocol uses a significantly smaller injection volume than AM10. The primary purpose of the study was to establish whether this higher concentration investigational alfaxalone formulation could improve the ease of drug administration and animal welfare via a smaller intramuscular injection volume. We hypothesized that the AM40 drug mixture would show no significant differences in duration of anesthesia, SSMV, AMV, side effects, and QA when compared with AM10. The results of the study were consistent with the primary hypothesis and there was no statistically significant difference between the AM anesthetic drug mixtures for duration of anesthesia, SSMV, AMV, side effects, and QA. Furthermore, we formed a secondary hypothesis that females would have a longer duration of anesthesia across AM mixtures than males. The secondary hypothesis was not supported by the results of the study, and there was no statistically significant difference in duration of anesthesia for females for both AM10 and AM40 anesthetic drug mixtures.
While none of the studied variables other than injection volume had statistically significant differences between AM mixtures, it is arguably more practical to compare the 2 mixtures in terms of the ideal injectable anesthetic for plethysmography. As noted earlier, the ideal injectable anesthetic drug protocol would meet the following criteria: be administered as a single IM injection, have an anesthetic duration of at least 45 min, rapidly achieve an anesthetic plane and maintain SSMV, maintain a consistent AMV, and produce minimal side effects influencing patient safety and data collection during the study. As we mentioned previously, AM40 mixtures produced a significantly smaller injection volume than AM10, but more importantly, all AM40 doses were able to be administered as a single IM injection with a median injection volume of 1.12 mL, which is 66.47% less than AM10 (Table 3 and Figure 2A). Furthermore, none of the AM40 dose mixtures had an observed phase separation reaction compared with 60% of the AM10 doses that had separation reactions with an average time to dissolution of 5.73 min. The cause of the separations in AM10 on mixing is unclear as both the 10- and 40-mg/mL alfaxalone formulations have the same alfaxalone: cyclodextrin ratio (weight:weight) and identical preservative systems. More research is required to understand the reason(s) for the differences in the time to mix. However, this point is likely inconsequential since the AM10 mixtures had no significant difference from AM40 mixtures outside of injection volume. In addition, the AM10 mixture, like the AM40 mixture, did not cause any injection site reactions in the NHPs. When assessing the benchmark of anesthetic duration of at least 45 min, both minimum values for AM10 and AM40 achieved this criterion (Table 3 and Figure 2F). While achieving a minimum duration of 45 min allows for adequate dosing with aerosol agents under plethysmography, longer anesthetic durations can also cause problems with prolonged recoveries within biocontainment spaces.
When assessing the rapidity of onset and the quality of SSMV, we examined the values for time to start and quality scoring. Both drug protocols had near equivalent median time-to-start values (AM10:10 min, AM40:11 min), but the standard deviation in time to start was 258.6% greater in the AM10 group compared with the AM40 group (Table 3 and Figure 2G). This increased variability in onset of anesthesia in AM10 affected the flow of the experiment and caused increased waiting times between AM10 trials within study day groupings. The median quality score for SSMV for both mixtures was 4 denoting a full 20 min of SSMV on the first attempt; however, there was an AM10 female who failed to achieve anesthesia (QA score = 1) while every animal under AM40 attained SSMV at least twice (QA score = 3). While the standard deviation in quality scoring for the AM10 group was 384% higher than the AM40 group, the standard deviation was skewed due to the small sample size and the score of 1 for the single female AM10 NHP that failed to lose consciousness after injection (Table 4 and Figure 2B). Average SSMV values were also examined and remained relatively consistent between AM mixture groups with a median difference of only −4.005 mL/min across the study (Table 3 and Figure 2C). Average SSMV is a primary variable in the calculation to determine the aerosol duration required to present a target dose. For this reason, it is ideal to have a predictable and consistent average SSMV.
A large AMV relies on the input of 2 variables, the duration an animal remains in SSMV and the average volume of SSMV throughout the trial. Due to the correlation of AMV to SSMV duration and volume, the direct comparison of AMV for AM10 and AM40 is useful in verifying the similarities in SSMV between the 2 anesthetic mixtures. The AMV median values for AM40 and AM10 were relatively close with a difference of 1,500 mL and a median difference of only 160 mL for the entire study (Table 3 and Figure 2D). AM40 had an AMV standard deviation that was 36.14% higher than AM10. This increased AMV variability for AM40 is likely due to the 2 data point outliers depicted in Figures 2D and 3D. A single AM40 female had a reduced AMV due to a low SSMV value of 217.05 mL/min and a reduced average SSMV duration of 4.5 min, despite having a QA score of 3. An AM40 male had only a 10-min average duration of SSMV, despite having a QA score of 3, lowering his total AMV value for the trial. It is also noteworthy that one of the AM10 females did not lose consciousness after AM10 administration and so AMV could not be calculated for that animal and that data point is not considered in the data for AMV for the study. When these outliers are accounted for, the overall AMV between AM mixtures is similar.
Finally, the ideal anesthetic drug protocol would have minimal side effects that threaten harm to the animal or disruption to data collection. A scoring system, seen in Table 5, was applied to the data following the completion of anesthetic trials based on the possibility of an adverse anesthetic event and the threat to disrupt data collection during the study. Side effects scores were assigned based on the highest-ranked-side effects observed for a trial. AM40 had 33.33% of animals listed as having no side effects or adverse events with a side effects score of 0 compared with AM10 which only had 20% of animals within this category. The most observed side effects across the study were tremors and muscle fasciculations. This side effect was observed as a single side effect in 46.67% of animals across the study. This was the only side effect qualifying as a score category of 1 for the trial. Both AM40 and AM10 had 33.33% of animals with a side effects score of 1 with fasciculations and tremors scored as the highest-ranked side effect. A score of 2 was assigned for episodes of transient hypothermia, tachycardia, hypoxemia, upper airway stertor or stridor, bronchospasms (coughing under anesthesia), or any combination of these side effects. Both mixtures had 33.33% of animals assigned a side effects score of 2. The most common side effect was stertor or stridor. The second most frequent was a split between hypoxemia and tachycardia. For AM10, 13.3% of animals had bronchospasms and 6.7% had transient hypothermia (<34 °C [94 °F]). For AM40, hypothermia was also observed in 6.7% of animals, but no animals were observed as having bronchospasms (Table 5).
The only observed side effect that qualified as a score of 3 was positional apnea in 2 AM10 males, which represents 28.86% of that group. Both NHPs experienced a cessation in breathing lasting more than 30 s during placement and positioning into the plethysmography chamber. No monitoring equipment had been connected at the time of the observed apnea. These male NHPs were perceived to be at a higher risk due to their larger size and BCS assignments in the overweight categories of 4/4 and 5/5 (Figure 3C). Both cases of apnea resolved with minimal intervention in the form of chest compressions and rotation into a 45° right lateral supine position, but both cases resulted in delays to the start of trials lasting 2 to 10 min. Overall, AM40 had fewer total side effects for scores 1 to 3, had no animals receive a side effects score of 3, and had more animals score 0 (no side effects) than the AM10 group.
The primary limitation to the study is the small population size of 15 NHPs. The authors recommend a reassessment for the study measures with a larger sample evaluated for statistical significance for the chosen study values. Another limitation in this study was that evaluators and study participants were not blinded to the AM mixtures administered for each anesthetic event, primarily due to staffing limitations and NHP safety in anesthetic dosing. Lack of blinding may have contributed to bias in the assessment of more subjective study measures such as observed side effects. Based on the results of this study, the authors suggest the blinding of future studies using investigational 40-mg/mL alfaxalone in cynomolgus macaques as the drug appears to be safe and efficacious. Another potential area of bias is our application of side effects scoring measures and the determination of side effects scoring measures following the completion of anesthetic trials. Ideally, predetermined values and criteria for assessing side effects would be established before the start of the study. For our study, we assigned these measures following anesthetic trials, except for the hypothermia criterion (<94 °F) which was assigned before the start of the study. Patients meeting body weight and BCS criteria were rotated into lateral positioning for both trials. There were no controls established for data comparison in relation to patient positioning to avoid apnea. The data underwent systematic reviews between the authors and the USAMRIID internal review process to minimize potential bias within the data and analysis. Another area for consideration is the effect of additional anesthetic administration on the duration and quality of anesthesia. Often, researchers and veterinarians may need to extend an anesthetic event to provide care to an animal or complete an additional procedure. Additional data on the efficacy of a second dose of AM mixtures could provide useful clinical insights. Also worth consideration is the adjustment of the total anesthetic dose to match the purpose and duration of anesthesia. Finally, the authors recommend a study assessing the effective concentration of alfaxalone in the blood stream producing anesthesia in cynomolgus macaques. Body composition may or may not affect the effective concentration. This type of study is beyond the scope of this manuscript.
Overall, this study supports the use of AM40 to replace AM10 as an anesthetic protocol to perform plethysmography in cynomolgus macaques. Values for QA, AMV, side effects, duration of anesthesia, and SSMV were not statistically different between the AM40 and AM10 anesthetic protocol groups. AM40 also has the advantage of a much smaller injection volume than the AM10 protocol. Females cynomolgus macaques did not demonstrate significantly longer anesthetic durations on AM protocols. AM40 may present many additional advantages over AM10 as a primary anesthetic protocol for plethysmography, but more data with larger sample sizes is needed to make that determination. Further research in pharmacokinetic studies, and broad applications of AM10 and AM40 protocols are recommended by the authors.
Acknowledgments
The authors thank the USAMRIID leadership, IACUC, and research review team for their support on the study and continuing research within the institute, and to James Writer and William Discher who provided technical writing assistance in formatting and editing of the manuscript. A special thanks to Stephanie Bellanca, Steeven Brussot, and Kenneth Aiello within the USAMRIID Veterinary Medicine Division for their technical contributions to conducting plethysmography for the study.
Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army. The use of either trade or manufacturers’ names in this report does not constitute an official endorsement of any commercial products. This report may not be cited for purposes of advertisement.
Conflicts of Interest
Dr. Kirby Pasloske is listed as an author of the paper due to his technical assistance in the procurement of the investigational 40-mg/mL alfaxalone mixture for use within the study. He provided critical technical and regulatory guidance. Dr. Pasloske is employed by Jurox Pty Limited (a part of Zoetis) who manufactures alfaxalone as a veterinary pharmaceutical and for research purposes.
Funding
This work was internally funded.
References
- 1.Álvarez D, Arroyo CA, de Frutos JF, Crespo A, Cerezo-Hernández A, Gutiérrez-Tobal GC, Vaquerizo-Villar F, et al. 2020. Assessment of nocturnal autonomic cardiac imbalance in positional obstructive sleep apnea. A multiscale nonlinear approach. Entropy (Basel) 22:1404. 10.3390/e22121404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnewall RE, Fisher DA, Robertson AB, Vales PA, Knostman KA, Bigger JE. 2012. Inhalational monkeypox virus infection in cynomolgus macaques. Front Cell Infect Microbiol 2:117. 10.3389/fcimb.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besch TK, Ruble DL, Gibbs PH, Pitt ML. 1996. Steady-state minute volume determination by body-only plethysmography in juvenile rhesus monkeys. Lab Anim Sci 46:539–544. [PubMed] [Google Scholar]
- 4.Bohm RP, Gilbert MH. 2012. Emergency medicine and critical care for nonhuman primates, p 373–374. In: Nonhuman primates in biomedical research: Biology and management, vol 1. Waltham (MA): Elsevier. [Google Scholar]
- 5.Flecknell P. 2015. Anesthesia and monitoring, p 77–108. In: Laboratory animal anaesthesia, 4th ed. New York: Academic Press. [Google Scholar]
- 6.Foster CD, Hunter TC, Gibbs PH, Leffel EK. 2008. Whole-body plethysmography in African green monkeys (Chlorocebus aethiops) with and without jackets. J Am Assoc Lab Anim Sci 47:52–55. [PMC free article] [PubMed] [Google Scholar]
- 7.Hartman AL, Nambulli S, McMillen CM, White AG, Tilston-Lunel NL, Albe JR, Cottle E, et al. 2020. SARS-CoV-2 infection of African green monkeys results in mild respiratory disease discernible by PET/CT imaging and shedding of infectious virus from both respiratory and gastrointestinal tracts. PLoS Pathog 16:e1008903. 10.1371/journal.ppat.1008903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hector RC, Mama KR, Fisher MC, Green SA, Pasloske K, Wolfe LL. 2021. Evaluation of two medetomidine-azaperone-alfaxalone combinations in captive rocky mountain elk (Cervus elaphus nelsoni). J Zoo Wildl Med 51:825–833. 10.1638/2020-0028. [DOI] [PubMed] [Google Scholar]
- 9.Hollander M, Wolfe DA, Chicken E. 2014. Nonparametric statistical methods, 3rd ed. Hoboken (NJ): Wiley. [Google Scholar]
- 10.Layton RC, Brasel T, Gigliotti A, Barr E, Storch S, Myers L, Hobbs C, Koster F. 2011. Primary pneumonic plague in the African Green monkey as a model for treatment efficacy evaluation. J Med Primatol 40:6–17. 10.1111/j.1600-0684.2010.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marion BM, Ghering JM, Dixon BC, Casselman AM, Astleford SM, White CE, Bowling PA. 2022. Comparison of alfaxalone-midazolam, tiletamine-zolazepam, and ketamineacepromazine anesthesia during plethysmography in cynomolgus macaques (Macaca fascicularis) and rhesus macaques (Macaca mulatta). Comp Med 72:248–256. 10.30802/AALAS-CM-22-000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meeker WQ, Hahn GJ, Escobar LA. 2017. Statistical intervals: A guide for practitioners and researchers. Hoboken (NJ): Wiley. 10.1002/9781118594841. [DOI] [Google Scholar]
- 13.Murphy KL, Baxter MG, Flecknell PA. 2012. Anesthesia and analgesia in nonhuman primates, p 403–433. In: Abee CR, Mansfield K, Morris T, Tardif S, editors. Nonhuman primates in biomedical research, vol 1. New York: Academic Press. [Google Scholar]
- 14.Nakayama S, Koie H, Pai C, Ito-Fujishiro Y, Kanayama K, Sankai T, Yasutomi Y, Ageyama N. 2020. Echocardiographic evaluation of cardiac function in cynomolgus monkeys over a wide age range. Exp Anim 69:336–344. 10.1538/expanim.19-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obot Akata CJ, Blair LF, Barr EB, Storch S, Vigil G, Campen MJ. 2007. Development of a head-out plethysmograph system for non-human primates in an Animal Biosafety Level 3 facility. J Pharmacol Toxicol Methods 55:96–102. 10.1016/j.vascn.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Patterson M, Caulkett N, Neuhaus P, Pasloske K, Ruckstuhl K. 2021. The utility of a novel formulation of alfaxalone in a remote delivery system. Vet Anaesth Analg 48:537–540. 10.1016/j.vaa.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Philip P, Gross CE, Taillard J, Bioulac B, Guilleminault C. 2005. An animal model of a spontaneously reversible obstructive sleep apnea syndrome in the monkey. Neurobiol Dis 20:428–431. 10.1016/j.nbd.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Reed DS, Lind CM, Sullivan LJ, Pratt WD, Parker MD. 2004. Aerosol infection of cynomolgus macaques with enzootic strains of venezuelan equine encephalitis viruses. J Infect Dis 189:1013–1017. 10.1086/382281. [DOI] [PubMed] [Google Scholar]
- 19.Rossi CA, Ulrich M, Norris S, Reed DS, Pitt LM, Leffel EK. 2008. Identification of a surrogate marker for infection in the African green monkey model of inhalation anthrax. Infect Immun 76:5790–5801. 10.1128/IAI.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.S1. Software for statistical analysis. Cary (NC): SAS Institute Inc. [Google Scholar]
- 21.Szollosi I, Roebuck T, Thompson B, Naughton MT. 2006. Lateral sleeping position reduces severity of central sleep apnea/Cheyne-Stokes respiration. Sleep 29:1045–1051. 10.1093/sleep/29.8.1045. [DOI] [PubMed] [Google Scholar]
- 22.Tufte ER. 1983. The visual display of quantitative information. Cheshire (CT): Graphics Press. [Google Scholar]
- 23.Twenhafel NA, Alves DA, Purcell BK. 2009. Pathology of inhalational Francisella tularensis spp. tularensis SCHU S4 infection in African green monkeys (Chlorocebus aethiops). Vet Pathol 46:698–706. 10.1354/vp.08-VP-0302-T-AM. [DOI] [PubMed] [Google Scholar]
- 24.Unwin S. Anaesthesia, p 277. In: Bullock G, Petrusz P, editors. The laboratory primate. San Diego (CA): Elsevier Academic Press. [Google Scholar]
- 25.Vasconcelos D, Barnewall R, Babin M, Hunt R, Estep J, Nielsen C, Carnes R, Carney J. 2003. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis). Lab Invest 83:1201–1209. 10.1097/01.LAB.0000080599.43791.01. [DOI] [PubMed] [Google Scholar]
- 26.Warren R, Lockman H, Barnewall R, Krile R, Blanco OB, Vasconcelos D, Price J, House RV, Bolanowksi MA, Fellows P. 2011. Cynomolgus macaque model for pneumonic plague. Microb Pathog 50:12–22. 10.1016/j.micpath.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 27.West SE, Lee JC, Johns TN, Nunamaker EA. 2020. Intraperitoneal alfaxalone and alfaxalone-dexmedetomidine anesthesia in sprague-dawley rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 59:531–538. 10.30802/AALAS-JAALAS-19-000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White KL, Paine S, Harris J. 2017. A clinical evaluation of the pharmacokinetics and pharmacodynamics of intravenous alfaxalone in cyclodextrin in male and female rats following a loading dose and constant rate infusion. Vet Anaesth Analg 44:865–875. 10.1016/j.vaa.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Yingst SL, Facemire P, Chuvala L, Norwood D, Wolcott M, Alves DA. 2014. Pathological findings and diagnostic implications of a rhesus macaque (Macaca mulatta) model of aerosol-exposure melioidosis (Burkholderia pseudomallei). J Med Microbiol 63:118–128. 10.1099/jmm.0.059063-0. [DOI] [PubMed] [Google Scholar]
- 30.Yingst SL, Huzella LM, Chuvala L, Wolcott M. 2010. A rhesus macaque (Macaca mulatta) model of aerosol-exposure brucellosis (Brucella suis): Pathology and diagnostic implications. J Med Microbiol 59:724–730. 10.1099/jmm.0.017285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]