To the Editor
Studies have assessed the risk of adverse outcomes in pregnant women with COVID-19 infection compared with either pregnant women without COVID-19 infection, as in Villar et al,1 or women with COVID-19 infection who were not pregnant.2 Finding an appropriate reference group is a challenge for both study types. In Villar et al,1 COVID-19 diagnosis was defined as laboratory confirmation of infection or the presence of symptoms. The reference group was pregnant women without a COVID-19 diagnosis; a negative test result was not required because most countries did not perform universal testing in pregnancy. As the authors noted, this definition may result in exposure misclassification, which tends to bias results toward the null.
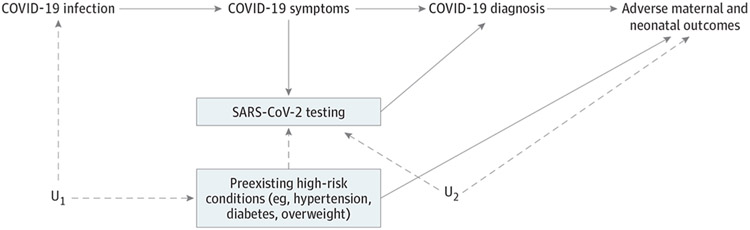
However, requiring a test result for asymptomatic exposed participants but not asymptomatic unexposed participants may bias the effect estimates upward. Test selection bias can occur when risk factors for the outcome of interest also increase the likelihood of testing and when testing is required for a participant to be classified as exposed or unexposed (Figure).3 Outside of population-based screening, reasons for SARS-CoV-2 testing in pregnancy include symptoms of infection and, in asymptomatic women, the presence of factors associated with risk, such as preeclampsia symptoms. If a positive test result is required for inclusion of asymptomatic pregnant women as exposed, women with these risk factors are preferentially selected in the exposed group. In Villar et al,1 44% of women with positive test results were asymptomatic and likely had other reasons for being tested. Prevalence of preexisting conditions, such as overweight, diabetes, and hypertension, was higher in the exposed group. The observed differences could reflect selective testing of asymptomatic women with these risk factors, true differences in rates of overall or symptomatic infection, or a combination of these. The authors adjusted for certain preexisting high-risk conditions, but residual test selection bias could exist if unknown or unmeasured shared risk factors for testing and adverse outcomes were present (Figure). In particular, the reported association between asymptomatic COVID-19 infection and preeclampsia could be partially explained by testing of women with gestational hypertension.
Figure. Directed Acyclic Graph for Test Selection Bias.
Requiring SARS-CoV-2 testing opens a noncausal path from COVID-19 diagnosis to adverse maternal outcomes through U2, unknown or unmeasured risk factors for adverse pregnancy outcomes that also increase the likelihood of testing. A similar noncausal path through preexisting high-risk maternal conditions can be blocked by adjusting for these variables. HTN indicates hypertension; U1, unknown or unmeasured common causes of COVID-19 infection and preexisting high-risk conditions; U2, unknown or unmeasured common reasons for SARS-CoV-2 testing and adverse maternal and neonatal outcomes.
If a negative test result had been required for classifying asymptomatic pregnant women as unexposed, women with risk factors also associated with testing would be selected preferentially in the reference group, resulting in downward bias of the effect estimate. To avoid test selection bias, future prospective studies could enroll a random population-based asymptomatic negative-test-confirmed reference group from the same study population and at the same gestational age as participants with confirmed COVID-19 infection. Otherwise, studies need to adjust for risk factors and reasons for testing.
Footnotes
Conflict of Interest Disclosures: Dr McElrath reports research support from the National Institutes of Health and NxPrenatal Inc; he has been compensated for serving on the scientific advisory boards of Mirvie Inc, Hoffman-LaRoche Ltd, and Momenta Pharmaceuticals, Inc; and he is a paid consultant for Comanche Biopharma, Inc. No other disclosures were reported.
Contributor Information
Chelsea J. Messinger, Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, Massachusetts.
Sonia Hernàndez-Díaz, Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, Massachusetts.
Thomas McElrath, Department of Obstetrics and Gynecology, Brigham and Women’s Hospital, Boston, Massachusetts McElrath)..
References
- 1.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID Multinational Cohort Study. JAMA Pediatr. Published online April 22, 2021. doi: 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zambrano LD, Ellington S, Strid P, et al. ; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–1647. doi: 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messinger CJ, Lipsitch M, Bateman BT, et al. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr. 2020;174(12):1159–1167. doi: 10.1001/jamapediatrics.2020.3009 [DOI] [PMC free article] [PubMed] [Google Scholar]