Abstract
Thiotepa/carmustine (TT-BCNU) is a commonly used autologous transplant (ASCT) conditioning regimen for primary DLBCL of the CNS (PCNSL). The total thiotepa dose varies among TT-BCNU recipients, with some centers administering a total dose of 20mg/kg, while others using 10mg/kg. We retrospectively assessed the impact of thiotepa dose intensity on ASCT outcomes in 218 adult PCNSL patients who underwent a first ASCT with TT-BCNU conditioning and received either a total thiotepa dose of 10mg/kg (TT-10 group; N=90), or 20mg/kg (TT-20 group; N=128). The median follow-up of survivors was 22 months. The cumulative incidence of 1-year non-relapse mortality (NRM) for TT-10 and TT-20 cohorts were 6% (95%CI=2–12%) vs. 4% (95%CI=1–8%), respectively (p=0.66). The 3-year cumulative incidence of relapse (15% vs. 13%; p=0.67), progression-free survival (PFS) (71% vs 80%; p=0.25) and overall survival (OS) (79% vs 83%; p=0.56) were similar in the TT-10 and TT-20 groups, respectively. On multivariate analysis compared to TT-10, the TT-20 cohort was not associated with significantly different risk of NRM (Hazard ration [HR]=0.77; p=0.64), relapse/progression (HR=0.87; p=0.74), PFS (HR=0.80; p=0.48) or OS (HR=1.10; p=0.80). In conclusion thiotepa dose-intensity in TT-BCNU conditioning does not impact ASCT outcomes of PCNSL patients.
Keywords: PCNSL, thiotepa, autologous transplant, dose-intensity
INTRODUCTION:
Primary diffuse large B-cell lymphoma (DLBCL) of the CNS (PCNSL) is an aggressive non-Hodgkin Lymphoma (NHL) with increasing incidence amongst patients older than 60 years. Current management in suitable subjects utilizes high-dose methotrexate (HD-MTX)-based regimens in the induction phase followed by consolidation through a variety of strategies.1–4 Consolidation therapy in PCNSL includes whole-brain radiotherapy (WBRT) and high-dose chemotherapy and autologous hematopoietic cell transplant (HDC-ASCT).5,6 WBRT carries potentially higher risk of decline in neurocognitive functioning, which has prompted renewed interest into HDC-ASCT as a first-line consolidation therapy for eligible patients newly diagnosed with PCNSL.3,7–9
We recently reported the superiority of thiotepa-containing conditioning regimens for ASCT in PCNSL, likely due to thiotepa’s ability to cross the blood-brain barrier. Furthermore, the two thiotepa-containing regimens we investigated, thiotepa/carmustine (TT-BCNU) and thiotepa/busulfan/cyclophosphoamide (TBC), provided comparable survival outcomes, but TT-BCNU was associated with lower risk of non-relapse mortality (NRM).10
In TT-BCNU conditioning regimen, the total thiotepa dose administered varies significantly across transplant programs, with some centers administering a total dose of 20mg/kg, while others 10mg/kg.3,11 To our knowledge, the impact of thiotepa dose-intensity on ASCT in PCNSL outcomes has not been investigated. We used the Center for International Blood and Marrow Transplant Research (CIBMTR) registry’s publicly available data to retrospectively examine the outcomes of patients receiving a total thiotepa dose of 10 mg/kg versus those receiving a dose of 20 mg/kg as part of TT-BCNU conditioning prior to ASCT in PCNSL patients.
METHODS
Data Source and Patients
The data used in this study were obtained from the publicly available datasets of the CIBMTR, for the previously published analysis was Scordo et al.10,12 The institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study. 218 PCNSL adult patients who underwent their first ASCT with TT-BCNU conditioning regimen as initial, or subsequent, consolidation between January 2011 and December 2018 were included. 90 patients received 10 mg/kg total thiotepa dose (TT-10 group), while 128 received the 20 mg/kg total thiotepa dose (TT-20 group). Autograft source was limited to peripheral blood. Patients were excluded if they had: a histologic subtype of NHL other than DLBCL, systemic NHL, HIV, received infrequently used or uncommon conditioning regimens, and/or were not in partial (PR) or complete remission (CR) prior to ASCT as previously described.10 Patients with missing thiotepa dose, or those receiving doses other than 10mg/kg or 20mg/kg were excluded.
Definitions and Endpoints
Disease response prior to ASCT was assessed using standard criteria.13 The primary outcome was progression-free survival (PFS), with treatment failure considered at lymphoma relapse, progression, or death from any cause. Secondary outcomes included time to neutrophil and platelet recovery, relapse/progression, NRM, and overall survival (OS). NRM was defined as death without evidence of PCNSL relapse/progression, with relapse considered a competing risk. Relapse/progression was defined as progressive lymphoma after ASCT, or lymphoma recurrence after a CR, with NRM considered a competing risk. Surviving patients were censored at the date of last follow-up. Neutrophil recovery was defined as the first of 3 successive days with an absolute neutrophil count of ≥500/μL after ASCT nadir. Platelet recovery was defined as the first of 3 consecutive days with a platelet count of ≥20,000/μL without platelet transfusion for 7 consecutive days. For neutrophil and platelet recovery, death without the event was considered a competing risk. PFS and OS probabilities were calculated using the Kaplan-Meier estimates.
Statistical Analysis
Baseline patient and transplant characteristics were compared using the Pearson chi-square test for discrete variables and the Kruskal-Wallis test for continuous variables. Cumulative incidences of hematopoietic recovery, relapse, and NRM were calculated to accommodate for competing risks. Cox proportional hazard analysis for PFS and OS and the proportional cause-specific hazards model for relapse and NRM were used to identify prognostic factors via forward stepwise selection. The proportional hazard assumption for each variable was examined by testing whether its coefficient is constant over time. The interaction between the conditioning regimen and significant covariates was also examined. Covariates with a P <0.05 were considered significant. The variables considered in the multivariable regression analysis included: total prescribed thiotepa dose (mg/kg), patient age (years), patient sex, Karnofsky Performance Score (KPS), patient race, remission status, time from diagnosis to transplant, rituximab used, and hematopoietic cell transplantation co-morbidity index (HCT-CI). A score test of homogeneity was used to check the center effect for each model.14 The adjusted cumulative incidence curve for NRM and the adjusted survival curves for OS and PFS were generated based on the final regression model.15,16 All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
A total of 218 patients undergoing ASCT for PCNSL with TT-BCNU conditioning were included in this study and were divided into two cohorts based on the total administered thiotepa dose of either 10 mg/kg (TT-10 group, n=90) or 20 mg/kg (TT-20 group, n=128). The median follow-up of survivors was 22.3 (5.1, 72.7) months. As summarized in Table 1, The two cohorts were comparable in terms of age, KPS, time from diagnosis to transplant, rituximab used in conditioning, HCT-CI, and number of prior therapy lines. There were more males in the TT-20 (n=79, 61.7%) than the TT-10 cohort (n=43, 47.8%) (P=0.04). More patients in the TT-10 group were in CR1 (n=59, 65.6%) or CR2+ (n=19, 21.1%) at HCT compared to the TT-20 group wherein 75 patients had a remission status of CR1 (58.6%) and 13 patients had a remission status of CR2+ (10.2%) (P<0.01).
Table 1.
Patient, disease and transplant characteristics
| Total prescribed thiotepa dose | ||||
|---|---|---|---|---|
| 10mg/kg (N=90) | 20mg/kg (N=128) | Total (N=218) | P-value | |
| Median patient age(years) | 62 (25–76) | 60 (23–78) | 61 (23–78) | 0.58 |
| ≥ 60 years | 49 (54.4%) | 64 (50.0%) | 113 (51.8%) | |
| Median (min-max) | ||||
| Male Sex, n (%) | 43 (47.8%) | 79 (61.7%) | 122 (56.0%) | 0.04 |
| Karnofsky performance score 90–100, n (%) | 37 (41.1%) | 54 (42.2%) | 91 (41.7%) | 0.14 |
| Missing | 6 (6.7%) | 2 (1.6%) | 8 (3.7%) | |
| Patient race, n (%) | 0.89 | |||
| Caucasian | 76 (84.4%) | 105 (82.0%) | 181 (83.0%) | |
| Non-Caucasian | 10 (11.1%) | 16 (12.5%) | 26 (11.9%) | |
| Missing | 4 (4.4%) | 7 (5.5%) | 11 (5.0%) | |
| Remission status, n (%) | <0.01 | |||
| CR1 | 59 (65.6%) | 75 (58.6%) | 134 (61.5%) | |
| CR2+ | 19 (21.1%) | 13 (10.2%) | 32 (14.7%) | |
| PR | 12 (13.3%) | 40 (31.3%) | 52 (23.9%) | |
| Time from diagnosis to transplant, n (%) | 0.98 | |||
| < 6 months | 36 (40.0%) | 51 (39.8%) | 87 (39.9%) | |
| ≥ 6 months | 54 (60.0%) | 77 (60.2%) | 131 (60.1%) | |
| Rituximab used in conditioning, n (%) | 10 (11.1%) | 8 (6.3%) | 18 (8.3%) | 0.20 |
| HCT Comorbidity index ≥3, n (%) | 46 (51.1%) | 61 (47.7%) | 107 (49.1%) | 0.84 |
| Median lines of therapy, n (%) | 1(1–3) | 1(1–3) | 1(1–3) | 0.37 |
| Year of HCT, n (%) | 0.42 | |||
| 2011 | 1 (1.1%) | 0 (0.0%) | 1 (0.5%) | |
| 2013 | 2 (2.2%) | 1 (0.8%) | 3 (1.4%) | |
| 2014 | 12 (13.3%) | 12 (9.4%) | 24 (11.0%) | |
| 2015 | 11 (12.2%) | 16 (12.5%) | 27 (12.4%) | |
| 2016 | 11 (12.2%) | 13 (10.2%) | 24 (11.0%) | |
| 2017 | 28 (31.1%) | 34 (26.6%) | 62 (28.4%) | |
| 2018 | 25 (27.8%) | 52 (40.6%) | 77 (35.3%) | |
Abbreviations:
CR, complete response; PR, partial response; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; Auto-HCT, autologous hematopoietic cell transplant.
Hematopoietic Recovery
The cumulative incidence of neutrophil recovery at day 28 was 99% for the TT-10 cohort (95% confidence interval [CI], 88–100%) and 99% for the TT-20 cohort (95% CI, 88–100%) (P =0.88; Table 2). The 28-day cumulative incidence of platelet recovery for the TT-10 and TT-20 cohorts were 81% (95% CI, 72–88%) and 83% (95% CI, 75–88%) respectively (P =0.77) (Table 2).
Table 2.
Transplantation outcomes
| Thiotepa 10mg/kg | Thiotepa 20mg/kg | ||||
|---|---|---|---|---|---|
| Outcomes | Number | Probability (95%CI) |
Number | Probability (95%CI) |
P Value |
| Neutrophil recovery | 90 | 124 | |||
| 28-day | 99(88, 100)% | 99 (88, 100)% | 0.88 | ||
| Platelet recovery | 90 | 124 | |||
| 28-day | 81 (72, 88)% | 83 (75, 88)% | 0.77 | ||
| Non-relapse mortality | 90 | 128 | 128 | ||
| 1-year | 6 (2, 12)% | 4 (1, 8)% | 0.66 | ||
| 3-year | 13 (6, 25)% | 7 (3, 14)% | 0.27 | ||
| Progression/relapse | 90 | 128 | |||
| 1-year | 8 (3, 15)% | 7 (4, 13)% | 0.85 | ||
| 3-year | 15 (7, 26)% | 13 (6, 21)% | 0.67 | ||
| Progression-free Survival | 90 | 128 | |||
| 1-year | 86 (77, 92)% | 89 (82, 93)% | 0.60 | ||
| 3-year | 71 (56, 82)% | 80 (70, 88)% | 0.25 | ||
| Overall survival | 90 | 128 | |||
| 1-year | 93 (86, 97)% | 93 (86, 96)% | 0.85 | ||
| 3-year | 79 (64, 88)% | 83 (71, 90)% | 0.56 | ||
Non-relapse Mortality and Relapse/Progression
The cumulative incidence of NRM at 1-year for the TT-10 cohort was 6% (95% CI, 2–12%) and was comparable to the TT-20 cohort which had an NRM of 4% (95% CI, 1–8%) (P=0.66; Figure 1A). Similarly, the cumulative incidence of NRM at 3-years for the TT-10 cohort was 13% (95%, CI 6–25%) and 7% for the TT-20 cohort (95% CI, 3–14%) (P=0.27). On multivariate analysis, the risk of NRM was comparable between the TT-10 and the TT-20 cohorts (HR=0.79; 95% CI, 0.28–2.17) (P=0.64) (Table 3, Figure 1A). Patient race and HCT-CI were independent predictors of NRM risk (details in Table 3).
Figure 1.
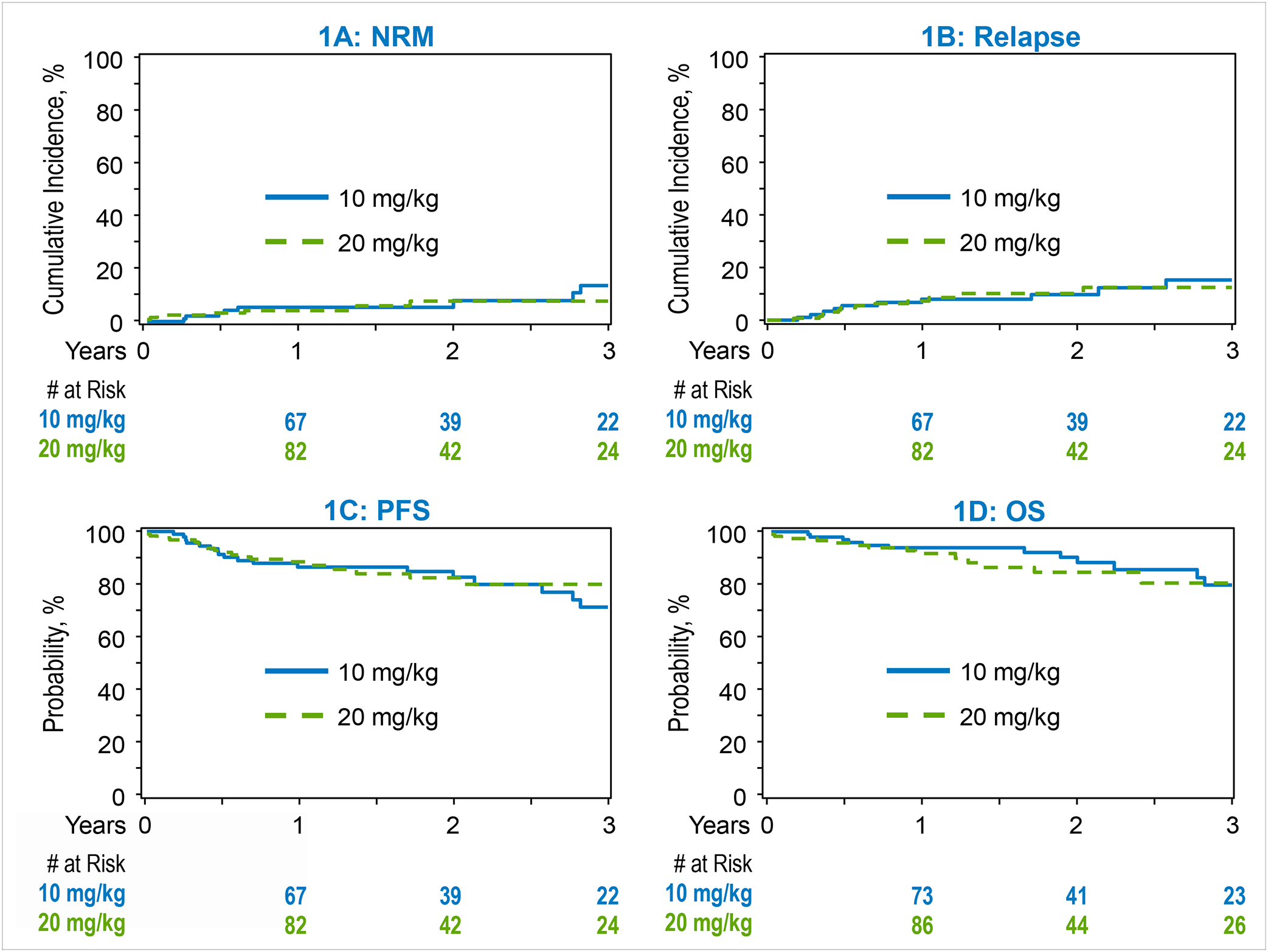
Transplantation outcomes. 1A: Non-relapse mortality. 1B: Relapse. 1C: Progression free survival. 1D: Overall Survival.
Table 3.
Multivariable regression analysis
| Non-relapse mortality | 95%CI | 95%CI | Overall | |||
|---|---|---|---|---|---|---|
| HR | Lower Limit | Upper Limit | p-value | p-value | N | |
| Main effect | ||||||
| Thiotepa 10mg/kg | 1.00 | 0.64 | 90 | |||
| Thiotepa 20mg/kg | 0.79 | 0.28 | 2.17 | 0.64 | 128 | |
| Patient race | ||||||
| Caucasian | 1.00 | 0.02 | 181 | |||
| Non Caucasian | 4.71 | 1.64 | 13.54 | 0.004 | 26 | |
| Missing | 2.46 | 0.30 | 20.08 | 0.40 | 11 | |
| HCT-Comorbidity index | ||||||
| 0 | 1.00 | 0.03 | 46 | |||
| 1&2 | 0.93 | 0.06 | 14.93 | 0.96 | 65 | |
| 3+ | 7.41 | 0.96 | 57.40 | 0.06 | 107 | |
| Contrast | ||||||
| HCT-CI: 1&2 vs 3+ | 0.12 | 0.02 | 0.96 | 0.0457 | ||
| Relapse | ||||||
| 95%CI | 95CI | Overall | ||||
| Main effect | HR | Lower Limit | Upper Limit | p-value | p-value | N |
| Thiotepa 10mg/kg | 1.00 | 0.75 | 90 | |||
| Thiotepa 20mg/kg | 0.87 | 0.38 | 1.98 | 0.75 | 128 | |
| Progression-free Survival | ||||||
| 95%CI | 95CI | Overall | ||||
| Main effect | HR | Lower Limit | Upper Limit | p-value | p-value | N |
| Thiotepa 10mg/kg | 1.00 | 0.48 | 90 | |||
| Thiotepa 20mg/kg | 0.80 | 0.42 | 1.50 | 0.48 | 128 | |
| HCT-Comorbidity index | ||||||
| 0 | 1.00 | 0.01 | 46 | |||
| 1&2 | 1.14 | 0.36 | 3.60 | 0.82 | 65 | |
| 3+ | 3.09 | 1.18 | 8.09 | 0.02 | 107 | |
| Overall Survival | ||||||
| 95%CI | 95CI | Overall | ||||
| Main effect | HR | Lower Limit | Upper Limit | p-value | p-value | N |
| Thiotepa 10mg/kg | 1.00 | 0.81 | 90 | |||
| Thiotepa 20mg/kg | 1.10 | 0.51 | 2.37 | 0.81 | 128 | |
| Patient Sex | ||||||
| Female | 1.00 | 0.03 | 122 | |||
| Male | 0.41 | 0.19 | 0.90 | 0.03 | 96 | |
| HCT-Comorbidity index | ||||||
| 0 | 1.00 | 0.01 | 46 | |||
| 1&2 | 0.66 | 0.15 | 2.94 | 0.58 | 65 | |
| 3+ | 3.39 | 1.15 | 9.96 | 0.03 | 107 |
Abbreviations:
HCT-CI, hematopoietic cell transplantation-specific comorbidity index; Auto-HCT, autologous hematopoietic cell transplant
The 3-year cumulative incidence of relapse/progression was 15% (95% CI, 7–26%) in the TT-10 cohort and 13% (95% CI, 6–21%) in the TT-20 cohort (P=0.67). On multivariate analysis, TT-20 was not associated with significantly lower risk of relapse compared with TT-10 (HR 0.87; 95% CI, 0.38–1.98) (P=0.75) (Table 3, Figure 1B).
Progression-free Survival and Overall Survival
The 3-year PFS for patients receiving TT-10 was 71% (95% CI, 56–82%) and 80% (95% CI, 70–88%) (P=0.25) for patients receiving TT-20 (Table 2, Figure 1C). On multivariate analysis, an HCT-CI of ≥3 was associated with inferior PFS (HR=3.09; 95% CI, 1.18–8.09) (P=0.02). Thiotepa dosing did not have a significant effect on PFS (HR=0.80; 95% CI, 0.42–1.50) (P=0.48; Table 3).
The 3-year OS for patients receiving TT-10 was 79% (95% CI, 64–88%) and 83% (95% CI, 71–90%) (P=0.56) for patients receiving TT-20 (Table 2, Figure 1D). On multivariate analysis, male sex was associated with a significantly lower mortality risk (HR=0.41; 95% CI, 0.19–0.90) (P=0.03), while an HCT-CI of ≥3 was associated with significantly higher mortality risk (HR=3.39; 95% CI, 1.15–9.96) (P=0.03). OS was not associated with thiotepa dosing (HR=1.10; 95% CI, 0.51–2.37) (P=0.81) (Table 3).
Analysis using age 65 years or older as a cutoff
Sixty-six patients included in this analysis were 65 years or older. Using age as categorical variable on multivariate analysis showed compared to patients < 65 years, age ≥ 65 years was associated with a significantly higher risk of NRM (HR=3.76; 95% CI, 1.29–11.01) (P=0.02), but there was no significant association with relapse risk (HR=0.20; 95% CI, 0.17–1.47) (P=0.21), PFS (HR=1.20; 95% CI, 0.62–2.35) (P=0.58) and OS (HR=1.46; 95% CI, 0.67–3.2) (P=0.34).
Causes of Death
At last follow-up, 14 patients each died in the TT-10 and TT-20 cohorts. PCNSL was the most common cause of death in both cohorts (11.1% for TT-10 and 7% for TT-20). Eight TT-10 cohort patients died after 1-year (causes of death include PCNSL=6; second malignancy=1, other=1), while 5 TT-20 died after 1-year, all due to relapsed PCNSL.
DISCUSSION
In this large registry analysis, we examined the impact of thiotepa dosing in PCNSL patients undergoing ASCT with TT-BCNU conditioning. We found comparable outcomes in terms of hematopoietic recovery, NRM, progression/relapse, PFS, and OS between the TT-10 and TT-20 cohorts. Among transplant centers using TT-BCNU conditioning for PCNSL patients, the total thiotepa dose administered varies significantly, with some programs administering a total dose of 20mg/kg, while others 10mg/kg.3,11 To our knowledge, no studies have compared the impact of thiotepa dose intensity in patients receiving TT-BCNU conditioning. The outcomes for TT-10 conditioning in our analysis are consistent with previously published data. Illerhaus et al used TT-BCNU in their study with a total thiotepa dose of 10 mg/kg. Of the 23 patients who received HDC-ASCT, 19 were in sustained CR (82.6%). Estimated survival probability at 3 years was 87%.17 Our findings are also comparable to TT-10 outcomes reported by the Mayo Clinic group.11
It is important to acknowledge that our data are retrospective and not a substitute for randomized comparison of the two thiotepa doses. However, in the current dataset, the administered thiotepa doses appeared to reflect a given transplant center’s practice, with no single center reporting using both TT-10 and TT-20 in different patients. Hence, it is unlikely that, based on a given patients fitness or disease characteristics, different thiotepa doses were administered. The two cohorts were balanced for patient age, KPS, race, HCT-CI, and number of prior therapy lines, but a higher proportion of the TT-20 cohort were in PR at the time of ASCT (Table 1). While the multivariate analysis adjusted for remission status at the time of ASCT, the possibility that remission status imbalance accounts for the efficacy difference between the two cohorts cannot be entirely ruled out. We also acknowledge that response assessment in PCNSL is technically challenging, and these data are not centrally reviewed and adjudicated in a registry dataset.
In the current analysis non-Caucasians had a higher rate of NRM and progression/relapse compared to Caucasians. Although Caucasians have a higher incidence rate of aggressive lymphomas in general than non-Caucasians, the latter group tends to have worse prognostic features than the former group. These prognostic features include higher disease burden, B symptoms, and extra nodal site involvement.18,19 A study conducted by Tao et al showed that though non-Caucasians had elevated mortality risk, after controlling for neighborhood socioeconomic status, racial/ethnic differences were not as pronounced.20 However, these data are not specific to a PCNSL population. HCT-CI ≥3 was also associated with inferior ASCT outcomes, in the current analysis. Our previous study comparing thiotepa-containing regimens to other chemotherapy combinations found a similar increase in mortality associated with an HCT-CI ≥ 3.10 In line with our observations, Curry et al reported that a Charlson comorbidity index ≥ 5 was associated it higher mortality risk factor for PCNSL (p=0.04).21 These observations underscore the impact of comorbid conditions on PCNSL outcomes.
Our data also indicated that male sex was associated with a significantly lower risk of mortality (Table 3). Biologically, supporting our observations is an analysis conducted by Roetzer et al that suggested differential sex-specific tumor-associated immune responses likely explains worse OS amongst female PCNSL patients, with presence of CD45RO+ and FoxP3+ tumor infiltrating lymphocytes predicting inferior survival in female but not male subjects.22
This study has several limitations, including its retrospective nature. The registry data do not capture adverse events, and we are not able to assess if lower doses are associated with a different toxicity profile (e.g. lower risk of thiotepa associated skin toxicity) or its impact of quality of life. Our analysis may be underpowered to detect small effect sizes. While thiotepa drug acquisition costs can be substantial, this study cannot assess the pharmacoeconomic impact of the two dosing strategies. Lastly, details of induction regimens used were not described in the publicly available dataset used for this analysis.
Nevertheless while acknowledging all the limitations discussed above, our report of similar outcomes in patients with PCNSL receiving higher versus lower intensity thiotepa dosing may be useful for clinicians in deciding on the level of intensity to be used for patients and provides justification for the use of either dosing strategy in clinical practice. Moreover, a detailed comparison of differences in early adverse events and lengths of hospital stay between the two groups would be of interest and requires future inquiry. A prospective, randomized study evaluating thiotepa dose intensity in PCNSL is warranted.
ACKNOWLEDGMENTS:
This dataset was collected by the Center for International Blood and Marrow Transplant Research which is supported primarily by the Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); 75R60222C00011 from the Health Resources and Services Administration (HRSA); N00014-21-1-2954 and N00014-23-1-2057 from the Office of Naval Research; the Medical College of Wisconsin and the National Marrow Donor Program.
Disclosure of conflict of interest:
Mehdi Hamadani reports Consultancy: Incyte Corporation, MorphoSys, SeaGen, Gamida Cell, Novartis, Legend Biotech, Kadmon, ADC Therapeutics; Omeros, CRISPR, Genmab, Kite, BMS, Caribou, Abbvie. Speaker’s Bureau: Sanofi Genzyme, AstraZeneca, BeiGene, ADC Therapeutics, Kite. DMC: Myeloid Therapeutics, Inc, Genentech.
Michael Scordo served as a paid consultant for McKinsey & Company, Angiocrine Bioscience, Inc., and Omeros Corporation; received research funding from Angiocrine Bioscience, Inc., and Omeros Corporation; served on ad hoc advisory boards for Kite – A Gilead Company; and received honoraria from i3Health and Medscape for CME-related activity.
Craig S Sauter has served as a paid consultant: Juno Therapeutics, Sanofi-Genzyme,
Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite/a Gilead Company,
Celgene/BMS, Gamida Cell, Karyopharm Therapeutics, Ono Pharmaceuticals, MorphoSys, CSL Behring, Syncopation Life Sciences, CRISPR Therapeutics and GSK. He has received research funds for clinical trials from: Juno Therapeutics, Celgene/BMS, Bristol-Myers Squibb, Precision Biosciences, Actinium Pharmaceuticals, Sanofi-Genzyme and NKARTA.
Farrukh T. Awan has provided consultancy to: Genentech, Astrazeneca, Abbvie, Janssen, Pharmacyclics, Gilead sciences, Kite pharma, Celgene, Karyopharm, MEI Pharma, Verastem, Incyte, Beigene, Johnson and Johnson, Dava Oncology, BMS, Merck, Cardinal Health, ADCT therapeutics, Epizyme, Caribou Biosciences, Cellecter Bisosciences, Loxo Oncology, Adaptive Biotechnologies, and received research funding from Pharmacyclics.
REFERENCES
- 1.Glass J, Won M, Schultz CJ, Brat D, Bartlett N, Suh J, et al. Phase I and II Study of Induction Chemotherapy With Methotrexate, Rituximab, and Temozolomide, Followed By Whole-Brain Radiotherapy and Postirradiation Temozolomide for Primary CNS Lymphoma: NRG Oncology RTOG 0227. J Clin Oncol. May 10 2016;34(14):1620–5. doi: 10.1200/jco.2015.64.8634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreri AJ, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi L, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. May 2016;3(5):e217–27. doi: 10.1016/s2352-3026(16)00036-3 [DOI] [PubMed] [Google Scholar]
- 3.Ferreri AJM, Cwynarski K, Pulczynski E, Fox C, Schorb E, Rosee P, et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. Nov 2017;4(11):e510–e523. doi: 10.1016/s2352-3026(17)30174-6 [DOI] [PubMed] [Google Scholar]
- 4.Ferreri AJM, Cwynarski K, Pulczynski E, Fox C, Schorb E, Celico C, et al. Long-term efficacy, safety and neurotolerability of MATRix regimen followed by autologous transplant in primary CNS lymphoma: 7-year results of the IELSG32 randomized trial. Leukemia. Jul 2022;36(7):1870–1878. doi: 10.1038/s41375-022-01582-5 [DOI] [PubMed] [Google Scholar]
- 5.Batchelor T, Giri S, Ruppert AS, Bartlett N, His E, Cheson B, et al. Myeloablative versus non-myeloablative consolidative chemotherapy for newly diagnosed primary central nervous system lymphoma: Results of CALGB 51101 (Alliance). Journal of Clinical Oncology. 2021;39(15_suppl):7506–7506. doi: 10.1200/JCO.2021.39.15_suppl.7506 [DOI] [Google Scholar]
- 6.Illerhaus G, Ferreri AJM, Binder M, Borchmann P, Hasenkamp J, Stilgenbauer S, et al. Effects on Survival of Non-Myeloablative Chemoimmunotherapy Compared to High-Dose Chemotherapy Followed By Autologous Stem Cell Transplantation (HDC-ASCT) As Consolidation Therapy in Patients with Primary CNS Lymphoma - Results of an International Randomized Phase III Trial (MATRix/IELSG43). Blood. 2022;140(Supplement 2):LBA-3–LBA-3. doi: 10.1182/blood-2022-171733 [DOI] [Google Scholar]
- 7.Steffanoni S, Calimeri T, Marktel S, Nitti R, Foppoli M, Ferreri AJM. Diagnosis and Treatment Using Autologous Stem-Cell Transplantation in Primary Central Nervous System Lymphoma: A Systematic Review. Cancers (Basel). Jan 15 2023;15(2)doi: 10.3390/cancers15020526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epperla N, Reljic T, Chowdhury SM, Ferreri AJM, Kumar A, Hamadani M. Autologous hematopoietic cell transplantation versus whole-brain radiotherapy consolidation in primary central nervous system lymphoma: A systematic review and meta-analysis. Hematol Oncol. Feb 2023;41(1):88–96. doi: 10.1002/hon.3083 [DOI] [PubMed] [Google Scholar]
- 9.Houillier C, Taillandier L, Dureau S, Lamy T, Laadhari M, Chinot O, et al. Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients 60 Years of Age and Younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J Clin Oncol. Apr 1 2019;37(10):823–833. doi: 10.1200/jco.18.00306 [DOI] [PubMed] [Google Scholar]
- 10.Scordo M, Wang TP, Ahn KW, Chen Y, Ahmed S, Awan F, et al. Outcomes Associated With Thiotepa-Based Conditioning in Patients With Primary Central Nervous System Lymphoma After Autologous Hematopoietic Cell Transplant. JAMA Oncol. Jul 1 2021;7(7):993–1003. doi: 10.1001/jamaoncol.2021.1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khurana A, Micallef IN, LaPlant BR, O’Neill B, Habermann T, Ansell S, et al. Outcomes of Autologous Stem Cell Transplant Consolidation in Primary Central Nervous System Lymphoma: A Mayo Clinic Experience. Biol Blood Marrow Transplant. Dec 2020;26(12):2217–2222. doi: 10.1016/j.bbmt.2020.08.012 [DOI] [PubMed] [Google Scholar]
- 12.Web address: https://cibmtr.org/Manuscript/a020h00001HH9E5AAL/P-5398 Last Assessed 6/29/2023.
- 13.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz L, Zucca E, et al. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. Journal of Clinical Oncology. 2014;32(27):3059–3067. doi: 10.1200/jco.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal. 1995;1(2):145–56; discussion 157–9. doi: 10.1007/bf00985764 [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. Nov 2007;88(2):95–101. doi: 10.1016/j.cmpb.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Zhang M-J. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Computer Methods and Programs in Biomedicine. 2011/01/01/ 2011;101(1):87–93. doi: 10.1016/j.cmpb.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Illerhaus G, Marks R, Ihorst G, Guttenberger R, Ostertag C, Derigs G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. Aug 20 2006;24(24):3865–70. doi: 10.1200/jco.2006.06.2117 [DOI] [PubMed] [Google Scholar]
- 18.Phillips AA, Smith DA. Health Disparities and the Global Landscape of Lymphoma Care Today. Am Soc Clin Oncol Educ Book. 2017;37:526–534. doi: 10.1200/edbk_175444 [DOI] [PubMed] [Google Scholar]
- 19.Shenoy PJ, Malik N, Nooka A, Sinha R, Ward K, Brawley O, et al. Racial differences in the presentation and outcomes of diffuse large B-cell lymphoma in the United States. Cancer. Jun 1 2011;117(11):2530–40. doi: 10.1002/cncr.25765 [DOI] [PubMed] [Google Scholar]
- 20.Tao L, Foran JM, Clarke CA, Gomez SL, Keegan TH. Socioeconomic disparities in mortality after diffuse large B-cell lymphoma in the modern treatment era. Blood. Jun 5 2014;123(23):3553–62. doi: 10.1182/blood-2013-07-517110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curry LD, Munker R, Li N, Yan D, Pryor P, Nozad S, et al. Performance status, comorbidities, and cycles of methotrexate exert the greatest influence on outcomes of primary and secondary CNS lymphomas: the Lexington experience. Ann Hematol. Jan 2023;102(1):141–154. doi: 10.1007/s00277-022-05018-z [DOI] [PubMed] [Google Scholar]
- 22.Roetzer T, Furtner J, Gesperger J, Seebrecht L, Bandke D, Brada M, et al. Sex-Specific Differences in Primary CNS Lymphoma. Cancers (Basel). Jun 16 2020;12(6)doi: 10.3390/cancers12061593 [DOI] [PMC free article] [PubMed] [Google Scholar]


