INTRODUCTION
HBV exposure perinatally or in early life is likely to result in chronic infection, while exposure during adulthood is less likely to do so. Many individuals living with chronic HBV are unaware. Alterations to immune function can lead to HBV reactivation (HBVr), which can result in acute hepatitis and less commonly, acute liver failure. Prophylaxis against HBVr with antiviral medications is warranted when the risk of HBVr outweighs the risks of prophylactic treatment. The US Centers for Disease Control and Prevention (CDC) recommends universal one-time screening for HBV for all adults. Screening for HBV is recommended before the initiation of immunosuppressive drug therapy with HBsAg and HBcAb, with or without HBsAb, by the American Association for the Study of Liver Diseases (AASLD), American Gastroenterological Association (AGA), and American Society for Clinical Oncology (ASCO). The addition of HBsAb to the above two tests is recommended by the European Association for the Study of the Liver (EASL), and the Asian Pacific Association for the Study of the Liver (APASL) recommends both HBsAb and fibrosis assessment to inform risk stratification for HBVr.1–5
DEFINING HBVr
If evidence of HBV exposure is identified upon screening, the infection must be further characterized as acute (HBsAg-positive for <6 mo), chronic (HBsAg-positive for ≥6 mo), or resolved (HBsAg-negative, HBcAb positive, and undetectable HBV DNA). The loss of immune control of HBV can lead to reactivation in chronic or resolved HBV infection. Reactivation in chronic inactive or chronic occult HBV is defined as the reappearance or rapid rise in HBV DNA to at least 100 times the baseline DNA level, while reactivation in resolved HBV infection is defined as either conversion from HBsAg-negative to HBsAg-positive or reappearance of HBV DNA (Table 1).
TABLE 1.
Identifying HBV reactivation
| Original serologies | Original state | Change in laboratory studies indicating reactivation |
|---|---|---|
| HBsAg-positive | Inactive chronic HBV | HBV DNA undetectable → HBV DNA detectable or HBV DNA increase by 100-fold |
| HBV DNA variable | Occult chronic HBV | |
| HBsAg-negative Anti-HBc–positive |
Resolved HBV | HBsAg-negative → HBsAg-positive or HBV DNA undetectable → HBV DNA detectable |
TREATMENTS ASSOCIATED WITH HBVr
Antineoplastic agents
B-cell–depleting therapies (eg, rituximab) are commonly used in the treatment of lymphoma, lymphocytic leukemias, and other neurologic, dermatologic, and rheumatologic conditions, and are associated with the highest risk of HBVr.1 Chemotherapies used in solid malignancies including anthracyclines, platinum, taxanes, and associated combination regimens (eg, FOLFOX or FOLFIRI) are also associated with HBVr.6 Immune checkpoint inhibitors (eg, pembrolizumab) and tyrosine kinase inhibitors (eg, imatinib) both also have established associations with HBVr.7 The risk of HBVr for individuals who are HBsAg-positive undergoing immune checkpoint inhibitor therapy is high (at least 10%), whereas HBVr risk is low (<1%) in individuals who are HBsAg-negative/HBcAb-positive receiving these therapies.
There is less experience evaluating the risk of HBVr associated with T-cell–depleting agents (eg, abatacept) and CAR T-cell immunotherapy. Smaller studies have demonstrated moderate (1%–10%) HBVr risk among individuals who are HBsAg-positive treated with T-cell–depleting agents targeting CD80/CD86/CD52 (low risk among HBsAg−/HBcAb+) and CAR T-cell immunotherapy (both HBsAg+ and HBsAg−/HBcAb+).7
Immunosuppressants and immunomodulators
Corticosteroids may be used to suppress inflammation in various medical conditions. The risk of HBVr likely varies depending on the HBV serologic status of the patient and the dose and duration of steroids.7 Doses of 20 mg or more of prednisone (or its equivalent) for at least 4 weeks carry moderate (1%–10%) risk of HBVr regardless of the HBsAg status. The risk of HBVr in persons treated with anti-TNF therapy (eg, infliximab) is estimated at around 1%. Cytokine inhibitors other than anti-TNF agents (eg, ustekinumab) were associated with high HBVr risk (35.5%) among persons who are HBsAg-positive not receiving prophylaxis and moderate HBVr risk among persons with HBsAg−/HBcAb+ serology.7
Limited data are available to define the risk of HBVr in the context of treatment with antiproliferative medications (eg, methotrexate, 6-mercaptopurine, azathioprine, and mycophenolic acid) and novel immunotherapies and calcineurin inhibitors (eg, tacrolimus), mTOR inhibitors (eg, sirolimus), and Janus kinase inhibitors (eg, tofacitinib).7 Calcineurin inhibitors appear to carry low HBVr risk among persons with HBsAg−/HBcAb+ serology, while the risk of HBVr is not well described with the use of mTOR inhibitors and Janus kinase inhibitors and may be high risk for HBsAg−/HBcAb+. Additional data are needed to more clearly define HBVr risk with novel immunotherapy regimens to guide recommendations on pre-emptive antiviral therapy.
Treatment of HCV coinfection
Treatment of HCV infection with direct-acting antivirals (DAAs) is associated with HBVr, and is hypothesized to be triggered by a loss of immune break of HCV infection with DAA-associated viral suppression in patients with active chronic HBV or resolved HBV.8,9 Outcomes from HBVr can be very serious: in a case series of 29 adverse event reports of HBVr associated with DAA treatment for HCV, 2 affected individuals died and 1 underwent liver transplantation.10
Unmet needs in predicting and managing HBVr
Beyond further characterizing HBVr risk with the treatments listed above, there are several unmet needs in preventing and managing HBVr. Many individuals who require immunosuppressing treatments may be unaware of their HBV history. For example, 1 study of patients with new cancer diagnoses in the United States found that 42% of patients with chronic HBV were newly diagnosed through the study and about half of these patients had no identifiable risk factors for HBV.11 The implementation of HBV screening guidelines (Table 2) is a key step to prevent HBVr in practice. The presence of HBsAb is associated with protection against HBVr, prompting further evaluation of the role of HBsAb in risk stratifying patients for HBVr and the potential benefit of immunization against HBV to prevent HBVr.13 The duration of HBV prophylaxis after completion of immunosuppressive therapy is an area of controversy and HBVr after prophylactic treatment completion should be compared across settings adhering to variable duration guidelines (Table 3).
TABLE 2.
Who to screen for HBV12
| People who… …were born in countries with a high (≥2% HbsAg-positive) prevalence of HBV infection …were not previously vaccinated and have parents born in regions with high (≥8%) prevalence of HBsAg …have behaviors that increase the risk of HBV exposure (eg, high-risk sex or injection drug use) …have household or sexual contact with someone who is HBsAg-positive or at increased risk of HBV infection …are undergoing HCV treatment with DAAs …have abnormal liver function tests of unknown etiology …are immunosuppressed including patients who have HIV, are on dialysis, are status post organ transplantation, and are receiving chemotherapy and immunosuppressive therapy …are at least 18 years of age (CDC only) |
Abbreviations: CDC, Centers for Disease Control and Prevention; DAA, direct-acting antiviral.
TABLE 3.
Recommended duration of antiviral prophylaxis against HBV reactivation
| Guideline | Recommended duration |
|---|---|
| AASLD | 6 mo after completion of immunosuppressive therapy |
| AGA | ≥12 mo after completion of B-cell–depleting therapies ≥6 mo after completion of all other immunosuppressive therapies |
| ASCO | 12 mo after completion of B-cell–depleting therapies 6 mo after completion of other chemotherapies |
| EASL | 18 mo after completion of B-cell–depleting therapies 12 mo after completion of other immunosuppressive therapies |
| APASL | Consider termination 6 mo after completion depending on HBV serologies and degree of underlying liver fibrosis |
Abbreviations: AGA, American Gastroenterological Association; APASL, Asian Pacific Association for the Study of the Liver; ASCO, American Society for Clinical Oncology (ASCO); EASL, European Association for the Study of the Liver.
PREVENTING AND MANAGING HBVr
The AASLD recommends prophylactic treatment for all patients who are HBsAg-positive at the onset of treatment for cancer or immunosuppression, while the AGA and EASL recommend treatment for those with moderate (1%–10%) or high (≥10%) risk of HBVr. The APASL guidelines use the presence of advanced fibrosis or cirrhosis to guide prophylactic treatment in persons with negative HBsAg receiving therapies that carry a moderate risk of HBVr and persons receiving therapies carrying a low risk of HBVr.
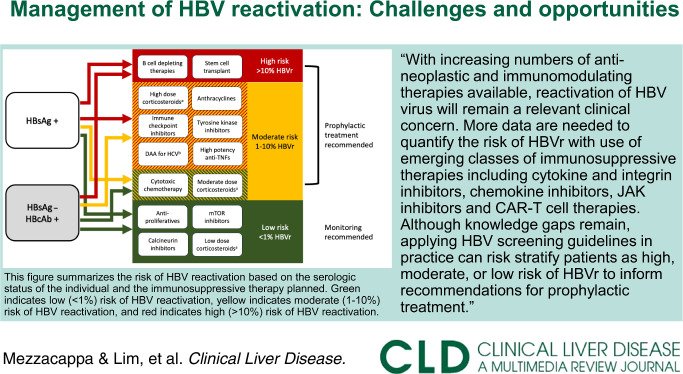
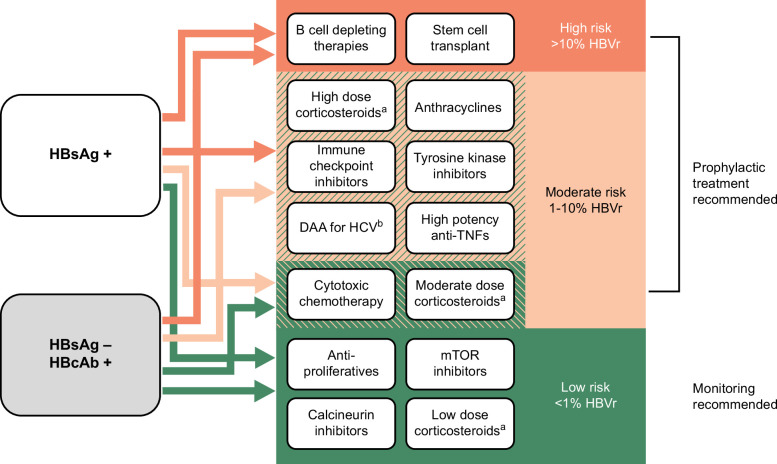
Patients at high risk of HBVr include all patients with either positive HBsAg or positive HBcAb receiving B-cell–depleting therapies or stem cell transplant and patients with positive HBsAg receiving high-dose corticosteroids, anthracyclines, immune checkpoint inhibitors, tyrosine kinase inhibitors, DAA therapy for HCV, and high potency anti-TNF therapies. Patients at moderate risk of HBVr include those with positive HBsAg receiving cytotoxic chemotherapy or moderate dose corticosteroids and those with negative HBsAg but positive HBcAb receiving the other high-risk treatments listed above. A simplified summary abstracted from available guidelines and meta-analyses is provided in Figure 1.
FIGURE 1.
Risk of HBV reactivation by baseline HBV status and planned treatment. aHigh dose: ≥20 mg/d for ≥4 weeks, moderate dose: 10–20 mg/d for ≥4 weeks, low dose: <10 mg/d. bExcept in persons without cirrhosis and with HBsAg<10 IU/mL. This figure summarizes the risk of HBV reactivation based on the serologic status of the individual and the immunosuppressive therapy planned. Green indicates a low (<1%) risk of HBV reactivation, light orange indicates a moderate (1%–10%) risk of HBV reactivation, and orange indicates a high (>10%) risk of HBV reactivation. The risk of HBV reactivation differs according to the serologic status of the individual for some treatments, which are shown in areas with 2 overlapping colors. The color of the arrow originating from the boxes describing the patient’s serologic status (HBsAg+ or HBsAg−/HBcAb+) indicates the risk level (high, moderate, or low) for an individual with a given serologic status receiving a given immunosuppressive treatment. For example, a person with HBsAg+ serology receiving cytotoxic chemotherapy is at moderate risk of HBV reactivation (light orange arrow), while a person with HBsAg−/HBcAb+ serology receiving cytotoxic chemotherapy is at low risk of HBV reactivation (green arrow).
The third-generation nucleoside/nucleotide analogs entecavir or tenofovir are recommended for prophylaxis over earlier agents like lamivudine due to lower rates of resistance. Tenofovir alafenamide has a safer side effect profile than its predecessor tenofovir disoproxil fumarate, and has been demonstrated to be safe and effective in prophylaxis against HBVr.14 Prophylactic treatment should ideally begin 2–4 weeks before immunosuppressive drug therapy or as soon as possible following initiation. Treatment should continue for a minimum of 6 months after the completion of immunosuppressive therapy, but recommended durations vary by society guidelines and are summarized in Table 3.
In patients who do not meet the criteria for prophylactic treatment, monitoring for HBVr is recommended by measuring serum hepatic transaminases, HBV DNA, and HBsAg (if negative at treatment start) every 1–3 months while on immunosuppressive treatment and for 6–12 months after the completion of the immunosuppressive treatment. If HBVr is detected by conversion from HBsAg-negative to HBsAg-positive or by reappearance or rapid rise in HBV DNA, HBVr should be treated with entecavir or tenofovir as described above.
CONCLUSIONS
Chronic HBV infection remains common in high-prevalence geographies. With increasing numbers of antineoplastic and immunomodulating therapies available, the reactivation of HBV virus will remain a relevant clinical concern. More data are needed to quantify the risk of HBVr with the use of emerging classes of immunosuppressive therapies including cytokine and integrin inhibitors, chemokine inhibitors, JAK inhibitors, and chimeric antigen receptor T-cell therapies. Although knowledge gaps remain, applying HBV screening guidelines in practice can risk stratify patients as high, moderate, or low risk of HBVr to inform recommendations for prophylactic treatment. The role of HBsAb in risk stratification and duration of prophylactic treatment require further study.
Footnotes
Abbreviations: AGA, American Gastroenterological Association; ASCO, American Society for Clinical Oncology; APASL, Asian Pacific Association for the Study of the Liver; CDC, Centers for Disease Control and Prevention; DAA, direct-acting antiviral; EASL, European Association for the Study of the Liver; HBVr, HBV reactivation.
Contributor Information
Catherine Mezzacappa, Email: catherine.mezzacappa@yale.edu.
Joseph K. Lim, Email: joseph.lim@yale.edu.
CONFLICTS OF INTEREST
Joseph K. Lim received institutional grants from Gilead, Intercept, Inventiva, Novo Nordisk, Pfizer, and Viking. The remaining author has no conflicts to report.
REFERENCES
- 1. Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT. American Gastroenterological Association I. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215–219; quiz e216–217. [DOI] [PubMed] [Google Scholar]
- 2. Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49:S156–S165. [DOI] [PubMed] [Google Scholar]
- 3. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: Current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L . EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. [DOI] [PubMed] [Google Scholar]
- 5. Lau G, Yu ML, Wong G, Thompson A, Ghazinian H, Hou JL, et al. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol Int. 2021;15:1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paul S, Saxena A, Terrin N, Viveiros K, Balk EM, Wong JB. Hepatitis B virus reactivation and prophylaxis during solid tumor chemotherapy: A systematic review and meta-analysis. Ann Intern Med. 2016;164:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papatheodoridis GV, Lekakis V, Voulgaris T, Lampertico P, Berg T, Chan HLY, et al. Hepatitis B virus reactivation associated with new classes of immunosuppressants and immunomodulators: A systematic review, meta-analysis, and expert opinion. J Hepatol. 2022;77:1670–1689. [DOI] [PubMed] [Google Scholar]
- 8. Oh JH, Park DA, Ko MJ, Yoo JJ, Yim SY, Ahn JH, et al. Direct-acting antivirals and the risk of hepatitis B reactivation in hepatitis B and C co-infected patients: A systematic review and meta-analysis. J Pers Med. 2022;12:1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu CJ, Sheen IS, Chen CY, Chuang WL, Wang HY, Tseng KC, et al. Ledipasvir/sofosbuvir for patients coinfected with chronic hepatitis C and hepatitis B in Taiwan: Follow-up at 108 weeks posttreatment. Clin Infect Dis. 2022;75:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, et al. Virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: A review of cases reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann Intern Med. 2017;166:792–798. [DOI] [PubMed] [Google Scholar]
- 11. Ramsey SD, Unger JM, Baker LH, Little RF, Loomba R, Hwang JP, et al. Prevalence of hepatitis B virus, hepatitis C virus, and HIV infection among patients with newly diagnosed cancer from academic and community oncology practices. JAMA Oncol. 2019;5:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paul S, Dickstein A, Saxena A, Terrin N, Viveiros K, Balk EM, et al. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: A meta-analysis. Hepatology. 2017;66:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suda G, Baba M, Yamamoto Y, Sho T, Ogawa K, Kimura M, et al. Prophylactic tenofovir alafenamide for hepatitis B virus reactivation and reactivation-related hepatitis. J Med Virol. 2023;95:e28452. [DOI] [PubMed] [Google Scholar]