Abstract
Arboviruses can be difficult to detect in the field due to relatively low prevalence in mosquito populations. The discovery that infected mosquitoes can release viruses in both their saliva and excreta gave rise to low-cost methods for the detection of arboviruses during entomological surveillance. We implemented both saliva and excreta-based entomological surveillance during the emergence of Zika virus (ZIKV) in French Guiana in 2016 by trapping mosquitoes around households of symptomatic cases with confirmed ZIKV infection. ZIKV was detected in mosquito excreta and not in mosquito saliva in 1 trap collection out of 85 (1.2%). One female Ae. aegypti L. (Diptera: Culicidae) was found with a ZIKV systemic infection in the corresponding trap. The lag time between symptom onset in a ZIKV-infected individual living near the trap site and ZIKV detection in this mosquito was 1 wk. These results highlight the potential of detection in excreta from trapped mosquitoes as a sensitive and cost-effective method to non invasively detect arbovirus circulation.
Keywords: arbovirus surveillance, Zika virus, detection, mosquito excreta
Graphical Abstract
Graphical Abstract.
Background
Growing global trade and travel activities are responsible for the global spread of arboviruses and their mosquito vectors, thereby increasing the threat of mosquito-borne diseases on human health worldwide (Wilder-Smith et al. 2017). The emergence of Zika virus (ZIKV) is the most recent example of how fast arboviruses can spread outside their endemic area to other regions where mosquito vectors are already present and environmental conditions are suitable (Fauci and Morens 2016). ZIKV was detected in South America in 2015 (Zanluca et al. 2015), with a first reported case in French Guiana in November 2015 (CIRE Antilles Guyane 2016). Although most ZIKV infections are asymptomatic or cause a mild self-limiting illness, the virus can be linked to neurological disorders (Brasil et al. 2016), severe congenital abnormalities, and human birth defects (Cauchemez et al. 2016, Rasmussen et al. 2016), leading the World Health Organization to declare a Public Health Emergency of International Concern (World Health Organization [WHO] 2016). ZIKV is transmitted to humans by mosquitoes, primarily Aedes (Stegomyia ) aegypti L. (Diptera: Culicidae) (Epelboin et al. 2017), but several recent studies have also highlighted that ZIKV can be transmitted between humans through sexual contact or from mother to fetus (Calvet et al. 2016, D’Ortenzio et al. 2016, de Laval et al. 2017).
The early detection of virus transmission can help in the implementation of adapted protective measures in areas where the risk of exposure is the highest. Current surveillance methods mainly involve case detection and seroprevalence studies in humans (Flamand et al. 2019) or detection of the virus in mosquito populations (i.e., entomological surveillance). An innovative entomological surveillance technique exploits the observation that infectious mosquitoes expectorate viruses in their saliva during sugar feeding (van den Hurk et al. 2007, 2014, Hall-Mendelin et al. 2010, Ibáñez-Justicia et al. 2012, Girod et al. 2016, Melanson et al. 2017, Kurucz et al. 2019, Wipf et al. 2019) and in their excreta (Fontaine et al. 2016, Ramírez et al. 2018, L’Ambert et al. 2023). The idea of surveillance based on these sample types is to attract mosquitoes to traps where they can feed and collectively expel virus particles in saliva or excreta onto paper filter cards which are treated with proprietary chemicals to preserve nucleic acids. Saliva and excreta-based methods have the benefit of making the processing of large quantities of trapped mosquitoes optional. Virus screening in mosquitoes can thus be undertaken only in traps where the virus has been detected in saliva or excreta, which greatly reduces cost and logistics compared with processing all captured mosquitoes.
Here, we implemented both saliva and excreta-based entomological surveillance during the emergence of ZIKV in French Guiana in 2016 by trapping mosquitoes around households of confirmed ZIKV symptomatic cases. Our results further extend the proof-of-concept of sugar feeding and excreta-based xenomonitoring to ZIKV.
Materials and Methods
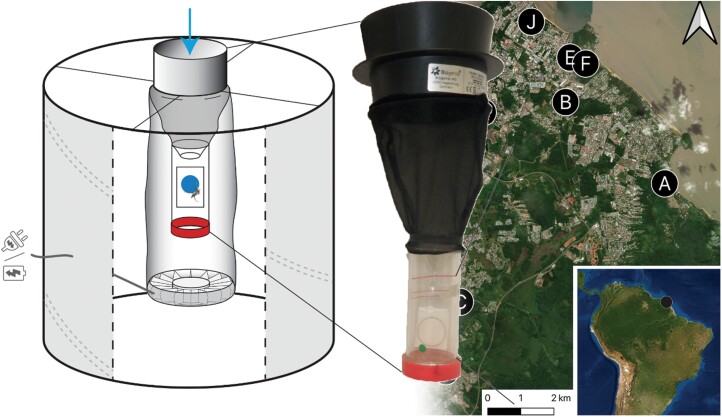
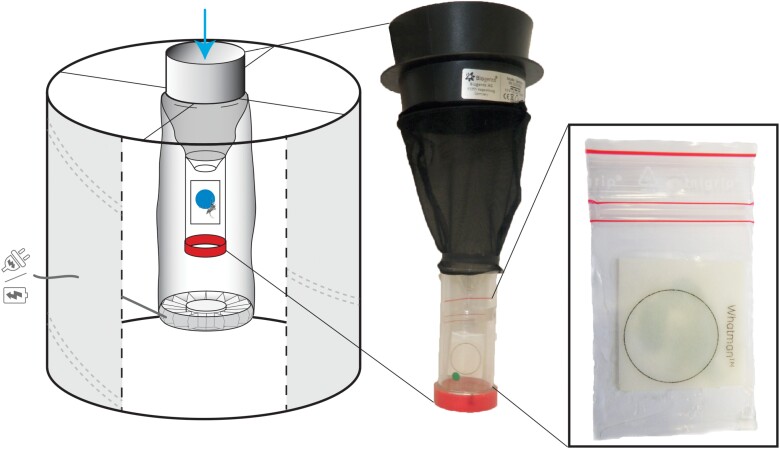
BG-Sentinel (BG, Biogents AG, Regensburg, Germany) traps were modified to replace the original catch bag with lodging for trapped mosquitoes (Fig. 1). The lodging was made of a transparent 70 × 30 mm polypropylene screw cap tube (40 mL) (Dutscher, France, ref 688252) from which the bottom had been cut off. The tube was inverted with its open-end (cut section) directed upward and attached beneath the intake funnel. A colored honey-impregnated FTA (Flinders Technology Associates) card (Whatman, GE Healthcare, Florham Park, NJ, USA), placed in a small plastic bag with an opening at its center, was positioned on the tube wall to collect saliva during sugar feeding. An untreated filter paper (UFP) (Whatman, grade 3, ref. 1003-917) was placed at the bottom, inside the screwed cap, to collect mosquito excreta. Blue food coloring was added to honey to easily spot mosquito excreta on the UFP surface after sugar digestion. A BG-Lure (Biogents) attractant was placed in each trap.
Fig. 1.
Schematic of the modified BG-Sentinel trap (Biogents). The trap was modified to incorporate a lodging for trapped mosquitoes with permanent access to colored honey impregnated in an FTA filter paper card to collect saliva, and a UFP placed at the bottom of the device to collect mosquito excreta.
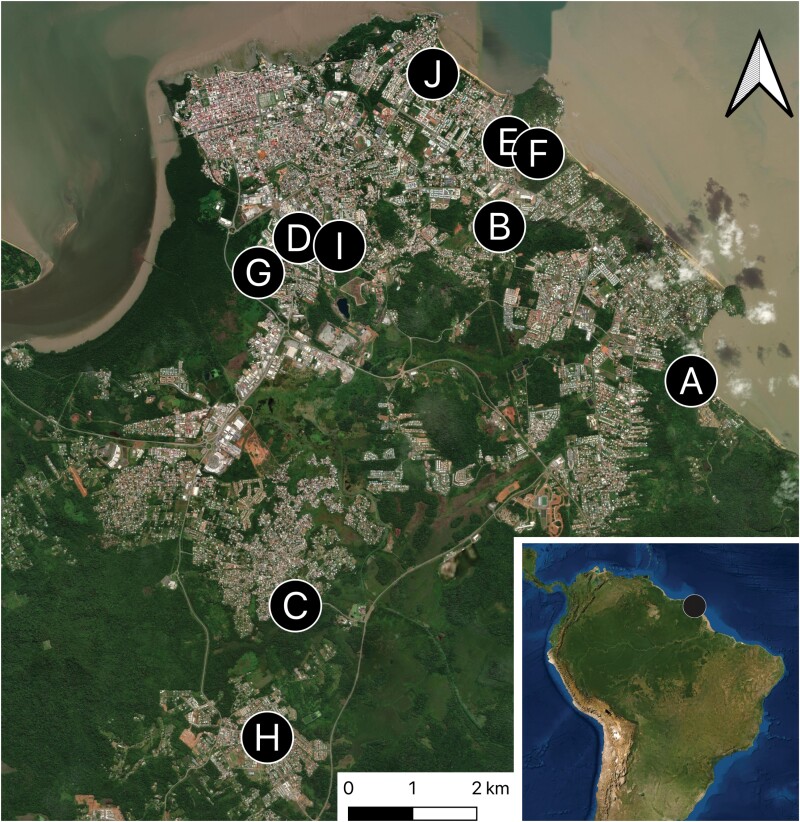
Modified BG traps were placed in 10 households of confirmed ZIKV-infected patients in Cayenne, Rémire-Montjoly, and Matoury cities (Fig. 2). The ZIKV-infected patients, who were already enrolled in a clinical study under the agreement number ID RCB: 2016-A00394-47 (de Laval et al. 2018), provided authorization for the placement of traps. A minimum of 2 traps per house, placed indoors and outdoors, were set during May and June 2016. After 3–7 consecutive days, the lodgings containing mosquitoes, FTA, and UFP cards were replaced. A total of 29 traps were monitored, and the procedure was repeated in each study site over a period of 4 wk. On the collection date, mosquitoes and cards from each site were transported to laboratory.
Fig. 2.
Study map. Modified BG-Sentinel traps (Biogents) localization in 10 distinct households of confirmed Zika virus cases on the French Guiana coastline. The map was created using the free and open source QGIS (Geographic Information System using satellite imagery) from the Environmental Systems Research Institute.
All mosquitoes were sorted by sex and identified at the species level based on the taxonomic keys of local species. The head and thorax of each mosquito were removed and placed individually into vials, while abdomens were pooled in 1.5 mL tubes to a maximum of 25 samples by species, site, and collection date. RNA extraction, reverse transcription, cDNA preamplification, and high-throughput real-time PCR were first performed following Moutailler et al. (2019) protocols on pools of mosquito abdomens. When ZIKV was detected in pools of abdomens, the cDNA of head/thorax of individual mosquitoes composing each pool was generated and screened for arboviruses by real-time PCR on a LightCycler 480 (LC480) (Roche Applied Science, Penzberg, Germany) (Moutailler et al. 2019). Sequencing was performed using the S5 Ion torrent technology (Thermo Fisher Scientific, Waltham, MA, USA) following manufacturer’s instructions.
FTA and UFP cards, exposed to trapped mosquitoes (i.e., potentially containing infected saliva), were cut and placed in 1.5 mL tubes containing 200 µL of DMEM (Dulbecco’s Modified Eagle Medium). After grinding with a sterile pestle, RNA was extracted from FTA and UFP homogenates using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Detection of ZIKV genomic RNA was performed by amplifying a 77 bp genomic region coding for the envelope protein with a one-step reverse transcription real-time quantitative polymerase chain reaction (RT-qPCR) assay using the SuperScript III Platinum one-step RT-qPCR mix (Invitrogen, Cergy Pontoise, France). Reactions contained 0.5 µL of SuperScript III RT/Platinum Taq Mix, 12.5 µL of 2× Reaction Mix, 0.5 µL of ZIKV forward (5ʹ-CCGCTGCCCAACACAAG-3ʹ, primer 1086) and reverse (5ʹ-CCACTA ACGTTCTTTTGCAGACAT-3ʹ, primer 1162c) primers from Lanciotti et al. (2008) at 50 µM and 0.5 µL of a slightly modified probe (5ʹ-FAM-CCTACCTTGACAAGCARTCAGACAC-3ʹBHQ-1) at 10 µM, 5.5 µL of RNase-free water, and 5 μL of RNA in a final volume of 20 μL. Amplifications were performed on a StepOnePlus Real-Time PCR System (Applied Biosystems, Gaithersburg, MD, USA) using the following cycling protocol: 50 °C for 30 min, 95 °C for 2 min, 45 cycles at 95 °C for 15 s, 60 °C for 1 min.
Results
A total of 1,379 mosquitoes were collected during the ZIKV epidemic, which affected French Guiana in 2016. Out of these mosquitoes, 521 (38%) were Ae. aegypti. Engorged Ae. aegypti females with a blue abdomen were detected in each site except for site A (Table 1). ZIKV was detected in 1 pool of abdomens out of 53 (2%) grouped by species, site, and collection date. This pool originated from mosquitoes trapped in Cayenne in site C on 9 June 2016. Heads and thoraxes from each mosquito corresponding to this pool were then individually screened for the presence of ZIKV. The virus was detected in the head and thorax of 1 female Ae. aegypti with a Cycle threshold (Ct) of 14. The full-length genome of this virus was sequenced (GenBank Accession number MN185326) and identified as the Asian genotype which is consistent with the strain circulating during the epidemic. This Ae. aegypti female was found dead in the trap and had a blue abdomen, indicative of a recent sugar meal. The time between symptom onset declared in the ZIKV-infected individual living in this site, and ZIKV detection in this mosquito was 1 wk. A total of 858 mosquitoes that were not Ae. aegypti species were also collected in this study. No ZIKV was detected in pooled abdomens (N = 80) from these mosquitoes (Supplementary Table S1).
Table 1.
Summary of the entomological surveillance data of Aedes aegypti mosquitoes. Mosquito captures and collections of excreta and saliva cards were carried out in 10 houses from 12 May 2016 to 28 June 2016 spread across 3 cities where the Zika virus (ZIKV) was circulating. For each city, the total sample number (No.) of Ae. aegypti collections is indicated. The number of mosquitoes with blue abdomens (males and females combined) is also listed, indicating the ingestion of previously dyed blue honey on the filter paper card and, thus, the associated saliva deposition. The presence or absence of the ZIKV is also indicated in the abdomen pools of females, in saliva collection FTA filter paper cards, and in excreta collection UFP cards, with tested positive samples being indicated in bold in the table
| Cities | No. of houses of mosquitoes collection (No. of traps) |

|

|
||||
|---|---|---|---|---|---|---|---|
| Field indication of mosquito salivation on cards | Screening viruses on females Ae. Aegypti | Screening ZIKV in cards | |||||
| Total No. of Ae. Aegypti | Total No. of Ae. Aegypti with blue abdomen (percent density) | Total No. of females (percent density) | No. of abdomens pools with ZIKV/No. of pools of females tested | No. of cards with ZIKV in saliva/FTA cards processed | No. of cards with ZIKV in excreta/UFP cards processed | ||
| Cayenne | 6 (18) | 379 | 40 (11%) | 183 (48%) | 1/32 | 0/52 | 1/52 |
| Rémire-Montjoly | 2 (5) | 60 | 5 (8%) | 32 (53%) | 0/7 | 0/13 | 0/12 |
| Matoury | 2 (6) | 82 | 16 (20%) | 54 (66%) | 0/14 | 0/19 | 0/18 |
| Total | 10 (29) | 521 | 61 (12%) | 269 (52%) | 1/53 | 0/84 | 1/82 |
A total of 166 cards out of the 226 cards collected during the study were processed after excluding cards from traps that did not contain mosquitoes. The virus was not detected in any of the 84 salivae FTA cards. ZIKV was detected in 1 excreta card out of the 82 excreta UFP cards with a Ct of 37. This card corresponded to the same trap in which the female Ae. aegypti infected with ZIKV was detected.
Discussion
Although entomological surveillance aims to detect pathogens in the environment before symptomatic human cases emerge, its implementation faces challenges due to the need to process a substantial number of mosquitoes, the majority of which are uninfected, resulting in high costs and logistical constraints. Indeed, we observed a low virus detection rate even though traps were specifically placed in proximity to symptomatic human cases. Similar to previous studies (van den Hurk et al. 2007, 2014, Hall-Mendelin et al. 2010, Flies et al. 2015, Fontaine et al. 2016, Melanson et al. 2017, Ramírez et al. 2018, 2019, Meyer et al. 2019, L’Ambert et al. 2023), we have demonstrated that surveillance methods which exploit saliva or excreta can be used as an initial screen and whole mosquitoes processed only from traps which are positive.
Detecting viruses in the saliva is reflective of the presence of infectious vectors at a given place and date, thereby excluding any virus detection in digested blood or mosquitoes that are noninfectious or not yet infectious. In this study, the trapped female Ae aegypti infected with ZIKV had a blue abdomen, which was evidence of a recent sugar feeding on the colored FTA card. However, ZIKV was not detected on the FTA card but was detected in excreta. This could reflect the higher quantity of virus in excreta compared with that in saliva (Ramírez et al. 2018), or it was too early in the extrinsic incubation period for transmission to occur.
While detection of virus in excreta cannot be distinguished from virus present in species capable of transmission, it is indicative of the circulation of the pathogen in a specific location. In the future, DNA meta-barcoding strategies or high-throughput sequencing could be applied to excreta samples to provide information on the mosquito, vertebrate, and viral community diversity (Birnberg et al. 2020, Ramírez et al. 2020, L’Ambert et al. 2023). Overall, the detection of virus in trapped mosquito excreta is a promising tool in the development of a sustainable entomological surveillance system to prevent or limit the spread of arbovirus in a human population.
Supplementary Material
Supplementary material is available at Journal of Medical Entomology online.
Acknowledgments
We would like to thank the inhabitants of French Guiana who allowed access to their property for mosquito and card collections. We are also grateful to Jean-Bernard Duchemin for proofreading the manuscript.
Contributor Information
Amandine Guidez, Unité d’Entomologie Médicale, Institut Pasteur de la Guyane, Cayenne, French Guiana.
Albin Fontaine, Unité Parasitologie et Entomologie, Département de Microbiologie et Maladies Infectieuses, Institut de Recherche Biomédicale des Armées (IRBA), 19-21 Boulevard Jean Moulin,13005 Marseille, France; Aix Marseille Univ, IRD, SSA, AP-HM, UMR Vecteurs – Infections Tropicales et Méditerranéennes (VITROME), Marseille, France; Institut Hospitalo-Universitaire (IHU)–Méditerranée Infection, Marseille, France.
Léna Yousfi, ANSES, INRAE, Ecole Nationale Vétérinaire d’Alfort, UMR BIPAR, Laboratoire de Santé Animale, Maisons-Alfort F-94700, France.
Sara Moutailler, ANSES, INRAE, Ecole Nationale Vétérinaire d’Alfort, UMR BIPAR, Laboratoire de Santé Animale, Maisons-Alfort F-94700, France.
Romuald Carinci, Unité d’Entomologie Médicale, Institut Pasteur de la Guyane, Cayenne, French Guiana.
Jean Issaly, Unité d’Entomologie Médicale, Institut Pasteur de la Guyane, Cayenne, French Guiana.
Pascal Gaborit, Unité d’Entomologie Médicale, Institut Pasteur de la Guyane, Cayenne, French Guiana.
Arnaud Cannet, CNEV, IRD, 34000 Montpellier, France.
Franck de Laval, French Army Center for Epidemiology and Public Health (CESPA), Marseille, France.
Séverine Matheus, Centre National de Référence des Arbovirus, laboratoire associé, Institut Pasteur de la Guyane, Cayenne, French Guiana.
Dominique Rousset, Centre National de Référence des Arbovirus, laboratoire associé, Institut Pasteur de la Guyane, Cayenne, French Guiana.
Isabelle Dusfour, MIVEGEC, UMR IRD 224-CNRS 5290, Université de Montpellier, Montpellier, France; Département de Santé Globale, Institut Pasteur, Paris, France.
Romain Girod, Unité d’Entomologie Médicale, Institut Pasteur de la Guyane, Cayenne, French Guiana.
Sébastien Briolant, Unité Parasitologie et Entomologie, Département de Microbiologie et Maladies Infectieuses, Institut de Recherche Biomédicale des Armées (IRBA), 19-21 Boulevard Jean Moulin,13005 Marseille, France; Aix Marseille Univ, IRD, SSA, AP-HM, UMR Vecteurs – Infections Tropicales et Méditerranéennes (VITROME), Marseille, France; Institut Hospitalo-Universitaire (IHU)–Méditerranée Infection, Marseille, France.
Funding
This study received funding from Regional Health Agency of French Guiana (Conventions n° FIR/ARS/2014/91, n° FIR/ARS/2015/68) and was supported by the European Union’s Horizon 2020 Research and Innovation Programme under ZIKAlliance Grant Agreement no. 734548.
Ethics Approval
The ZIKV-infected patients were enrolled in a clinical study under the agreement number ID RCB: 2016-A00394-47. Ethical clearance was obtained on 29 February 2016 from the Comité de protection des Personnes Sud Méditerranée I (internal No. 1631).
Author Contributions
Amandine Guidez (Data curation [equal], Formal analysis [equal], Investigation [equal], Methodology [equal], Resources [equal], Validation [equal], Writing—original draft [lead], Writing—review & editing [lead]), Albin Fontaine (Data curation [equal], Formal analysis [equal], Resources [equal], Validation [equal], Visualization [lead], Writing—original draft [lead], Writing—review & editing [lead]), Léna Yousfi (Data curation [equal], Investigation [equal], Resources [equal], Writing—review & editing [equal]), Sara Moutailler (Data curation [equal], Investigation [equal], Resources [equal], Writing—review & editing [equal]), Romuald Carinci (Data curation [equal], Investigation [equal], Resources [equal], Writing—review & editing [equal]), Jean Issaly (Data curation [equal], Investigation [equal], Resources [equal]), Pascal Gaborit (Data curation [equal], Investigation [equal], Resources [equal], Writing—review & editing [equal]), Arnaud Cannet (Data curation [equal], Investigation [equal], Resources [equal], Writing—review & editing [equal]), Franck de Laval (Funding acquisition [equal], Writing—review & editing [equal]), Severine Matheus (Funding acquisition [equal], Writing—review & editing [equal]), Dominique Rousset (Funding acquisition [equal], Writing—review & editing [equal]), Isabelle Dusfour (Conceptualization [equal], Funding acquisition [equal], Investigation [equal], Methodology [equal], Project administration [equal], Supervision [equal], Validation [equal], Writing—review & editing [equal]), Romain Girod (Conceptualization [equal], Funding acquisition [equal], Investigation [equal], Methodology [equal], Project administration [equal], Supervision [equal], Validation [equal], Writing—review & editing [equal]), and Sebastien Briolant (Conceptualization [lead], Data curation [equal], Formal analysis [equal], Funding acquisition [lead], Investigation [equal], Methodology [equal], Project administration [lead], Resources [equal], Supervision [lead], Validation [equal], Writing—original draft [lead], Writing—review & editing [lead])
Data Availability
ZIKV genome is accessible under the GenBank accession number MN185326.
References
- Birnberg L, Temmam S, Aranda C, Correa-Fiz F, Talavera S, Bigot T, Eloit M, Busquets N.. Viromics on honey-baited FTA cards as a new tool for the detection of circulating viruses in mosquitoes. Viruses. 2020:12(3):274. 10.3390/v12030274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, Siqueira AM, de Mendonca MCL, Nogueira RMR, de Filippis AMB, et al. Guillain-Barré syndrome associated with Zika virus infection. Lancet (London, England). 2016:387(10026):1482. 10.1016/S0140-6736(16)30058-7 [DOI] [PubMed] [Google Scholar]
- Calvet G, Aguiar RS, Melo ASO, Sampaio SA, de Filippis I, Fabri A, Araujo ESM, de Sequeira PC, de Mendonça MCL, de Oliveira L, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016:16(6):653–660. 10.1016/S1473-3099(16)00095-5 [DOI] [PubMed] [Google Scholar]
- Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016:387(10033):2125–2132. 10.1016/s0140-6736(16)00651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRE Antilles Guyane. Surveillance du virus Zika aux Antilles Guyane. Point épidémiologique du 06 octobre 2016 - N° 38/ 2016. 2016.
- D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Leparc-Goffart I.. Evidence of sexual transmission of Zika virus. N Engl J Med. 2016:374(22):2195–2198. 10.1056/NEJMc1604449 [DOI] [PubMed] [Google Scholar]
- de Laval F, d’Aubigny H, Mathéus S, Labrousse T, Ensargueix AL, Lorenzi EM, Le Flem FX, André N, Belleoud D, Leparc-Goffart I, et al. Evolution of symptoms and quality of life during Zika virus infection: a 1-year prospective cohort study. J Clin Virol. 2018:109:57–62. 10.1016/j.jcv.2018.09.015 [DOI] [PubMed] [Google Scholar]
- de Laval F, Matheus S, Labrousse T, Enfissi A, Rousset D, Briolant S.. Kinetics of Zika viral load in semen. N Engl J Med. 2017:377(7):697–699. 10.1056/NEJMc1612600 [DOI] [PubMed] [Google Scholar]
- Epelboin Y, Talaga S, Epelboin L, Dusfour I.. Zika virus: an updated review of competent or naturally infected mosquitoes. PLoS Negl Trop Dis. 2017:11(11):e0005933. 10.1371/journal.pntd.0005933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Morens DM.. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med. 2016:374(7):601–604. 10.1056/NEJMp1600297 [DOI] [PubMed] [Google Scholar]
- Flamand C, Bailly S, Fritzell C, Berthelot L, Vanhomwegen J, Salje H, Paireau J, Matheus S, Enfissi A, Fernandes-Pellerin S, et al. Impact of Zika virus emergence in French Guiana: a large general population seroprevalence survey. J Infect Dis. 2019:220(12):1915–1925. 10.1093/infdis/jiz396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flies EJ, Toi C, Weinstein P, Doggett SL, Williams CR.. Converting mosquito surveillance to arbovirus surveillance with honey-baited nucleic acid preservation cards. Vector Borne Zoonotic Dis (Larchmont, N.Y.). 2015:15(7):397–403. 10.1089/vbz.2014.1759 [DOI] [PubMed] [Google Scholar]
- Fontaine A, Jiolle D, Moltini-Conclois I, Lequime S, Lambrechts L.. Excretion of dengue virus RNA by Aedes aegypti allows non-destructive monitoring of viral dissemination in individual mosquitoes. Sci Rep. 2016:6(1):24885. 10.1038/srep24885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod R, Guidez A, Carinci R, Issaly J, Gaborit P, Ferrero E, Ardillon V, Fontaine A, Dusfour I, Briolant S.. Detection of Chikungunya virus circulation using sugar-baited traps during a major outbreak in French Guiana. PLoS Negl Trop Dis. 2016:10(9):e0004876. 10.1371/journal.pntd.0004876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Mendelin S, Ritchie SA, Johansen CA, Zborowski P, Cortis G, Dandridge S, Hall RA, van den Hurk AF.. Exploiting mosquito sugar feeding to detect mosquito-borne pathogens. Proc Natl Acad Sci U S A. 2010:107(25):11255–11259. 10.1073/pnas.1002040107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Justicia A, Scholte E-J, Reusken C, de Vries A, Dik M, Braks M.. An additional tool for arbovirus surveillance in The Netherlands: the use of honey-baited cards to detect circulating mosquito-borne viruses. Proc Dutch Entomol Soc. 2012:23: 63–71. [Google Scholar]
- Kurucz N, Minney-Smith CA, Johansen CA.. Arbovirus surveillance using FTATM cards in modified CO2-baited encephalitis virus surveillance traps in the Northern Territory, Australia. J Vector Ecol. 2019:44(1):187–194. 10.1111/jvec.12343 [DOI] [PubMed] [Google Scholar]
- L’Ambert G, Gendrot M, Briolant S, Nguyen A, Pages S, Bosio L, Palomo V, Gomez N, Benoit N, Savini H, et al. Analysis of trapped mosquito excreta as a noninvasive method to reveal biodiversity and arbovirus circulation. Mol Ecol Resour. 2023:23(2):410–423. 10.1111/1755-0998.13716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR.. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008:14(8):1232–1239. 10.3201/eid1408.080287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanson V, Jochim R, Yarnell M, Ferlez K, Shashikumar S, Richardson J.. Improving vector-borne pathogen surveillance: a laboratory-based study exploring the potential to detect dengue virus and malaria parasites in mosquito saliva. J Vector Borne Dis. 2017:54(4):301. 10.4103/0972-9062.225834 [DOI] [PubMed] [Google Scholar]
- Meyer DB, Ramirez AL, van den Hurk AF, Kurucz N, Ritchie SA.. Development and field evaluation of a system to collect mosquito excreta for the detection of arboviruses. J Med Entomol. 2019:56(4):1116–1121. 10.1093/jme/tjz031 [DOI] [PubMed] [Google Scholar]
- Moutailler S, Yousfi L, Mousson L, Devillers E, Vazeille M, Vega-Rúa A, Perrin Y, Jourdain F, Chandre F, Cannet A, et al. A new high-throughput tool to screen mosquito-borne viruses in Zika virus endemic/epidemic areas. Viruses. 2019:11(10):904. 10.3390/v11100904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez AL, Colmant AMG, Warrilow D, Huang B, Pyke AT, McMahon JL, Meyer DB, Graham RMA, Jennison AV, Ritchie SA, et al. Metagenomic analysis of the virome of mosquito excreta. mSphere. 2020:5(5):e00587–e00520. 10.1128/mSphere.00587-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez AL, Hall-Mendelin S, Doggett SL, Hewitson GR, McMahon JL, Ritchie SA, van den Hurk AF.. Mosquito excreta: a sample type with many potential applications for the investigation of Ross River virus and West Nile virus ecology. PLoS Negl Trop Dis. 2018:12(8):e0006771. 10.1371/journal.pntd.0006771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez AL, van den Hurk AF, Mackay IM, Yang ASP, Hewitson GR, McMahon JL, Boddey JA, Ritchie SA, Erickson SM.. Malaria surveillance from both ends: concurrent detection of Plasmodium falciparum in saliva and excreta harvested from Anopheles mosquitoes. Parasit Vectors. 2019:12(1):355. 10.1186/s13071-019-3610-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR.. Zika Virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016:374(20):1981–1987. 10.1056/NEJMsr1604338 [DOI] [PubMed] [Google Scholar]
- van den Hurk AF, Hall-Mendelin S, Townsend M, Kurucz N, Edwards J, Ehlers G, Rodwell C, Moore FA, McMahon JL, Northill JA, et al. Applications of a sugar-based surveillance system to track arboviruses in wild mosquito populations. Vector Borne Zoonotic Dis (Larchmont, N.Y.). 2014:14(1):66–73. 10.1089/vbz.2013.1373 [DOI] [PubMed] [Google Scholar]
- van den Hurk AF, Johnson PH, Hall-Mendelin S, Northill JA, Simmons RJ, Jansen CC, Frances SP, Smith GA, Ritchie SA.. Expectoration of Flaviviruses during sugar feeding by mosquitoes (Diptera: Culicidae). J Med Entomol. 2007:44(5):845–850. 10.1603/0022-2585(2007)44[845:eofdsf]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW.. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017:17(3):e101–e106. 10.1016/S1473-3099(16)30518-7 [DOI] [PubMed] [Google Scholar]
- Wipf NC, Guidi V, Tonolla M, Ruinelli M, Müller P, Engler O.. Evaluation of honey-baited FTA cards in combination with different mosquito traps in an area of low arbovirus prevalence. Parasit Vectors. 2019:12(1):554. 10.1186/s13071-019-3798-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. Geneva: WHO; 2016[accessed 2023 Nov 11]. https://www.who.int/news/item/01-02-2016-who-statement-on-the-first-meeting-of-the-international-health-regulations-(2005)-(ihr-2005)-emergency-committee-on-zika-virus-and-observed-increase-in-neurological-disorders-and-neonatal-malformations [Google Scholar]
- Zanluca C, Melo VCA de, Mosimann ALP, Santos GIV, Santos CND, Luz K.. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015:110(4):569–572. 10.1590/0074-02760150192 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
ZIKV genome is accessible under the GenBank accession number MN185326.