Abstract
Background
Congenital pseudoarthrosis of the tibia (CPT) is a rare pathological disease associated with neurofibromatosis type 1 (NF1). It presents with tibial bowing and can progress into a nonhealing fracture. Treatment options include conservative approaches such as serial bracing or various surgical options.
Surgically, the aims are to achieve long-term bone union, prevent limb length discrepancies (LLDs), and avoid mechanical axis deviation, soft tissue lesions, nearby joint stiffness, and pathological fracture.
The purpose of our study is to highlight our experience with both the conservative approach and the use of vascularized free fibula reconstruction of these deformities, including the challenges encountered with a long-term follow-up until skeletal maturity.
Methods
We present a retrospective analysis of a total of nine (9) patients consisting of three (3) girls and six (6) boys. Six (6) children were treated with a vascularized fibula flap, and the other three (3) were treated conservatively. Outcomes measured included fractures, LLD, ankle valgus deformity, donor site morbidity, and number of surgical corrections.
Results
All patients had flap survival. Three (3) of six children had a previous failed surgery with intramedullary nail and bone graft prior to performing a vascularized free fibula reconstruction. The follow-up period ranged from 8 months to 200 months. The complications included stress fractures (50%), LLD (66.6%), and ankle valgus (33.3%). During growth phases, these children required multiple corrective surgeries.
Conclusions
Fibula free flap is a good treatment option for CPT even in patients with prior surgical failures with variable results.
Level of Evidence - Level 4 - Case series Therapeutic Studies—Investigating the Results of Treatment.
Keywords: Congenital Pseudoarthrosis of Tibia, Neurofibromatosis, Fibular flap
Introduction
Pediatric pseudoarthrosis of the tibia or congenital pseudoarthrosis of the tibia (CPT) is a rare condition with an incidence of 1:140000 to 1:250000 worldwide.1 It is usually associated with neurofibromatosis type 1 (NF1). The condition is usually detected within the first year of birth by clinical examination of the apparent deformity and a radiograph, but in some cases, it may remain undetected until early adolescence.
The association between NF1 and CPT was confirmed in 1950 by Aegerter et al,2 but the exact pathophysiology is not clearly defined. NF1 is an autosomal dominant condition that includes skeletal abnormalities with a broad range of clinical manifestations. Tibial dysplasia is an infrequent but serious skeletal manifestation of this condition. This osseous dysplasia manifests as an anterolateral bowing due to segmental weakness of the tibia and leads to reduced growth of the distal tibial epiphysis, subsequently leading to shortening of the affected leg.3
Various treatment modalities have been described, with all having the common goal of achieving symmetry in length and alignment, while facilitating growth and avoiding fractures and deformities.
In this study, we highlighted our experience in using vascularized fibula flap for treating this very difficult condition as well as a conservative approach for milder forms. The long follow-up period in this study allowed us to document the challenges faced in treating these children from the time of presentation until completion of growth.
Patients and methods
A single-center retrospective analysis was performed of nine children between 2004 and 2021 after obtaining necessary approvals from the Institutional Review Board. All children who presented with CPT were included in the study. Six children were treated surgically, and three were treated conservatively. A single experienced senior surgeon performed the plastic microsurgical interventions on all the six operated children. Clinical examination and radiographs were used to evaluate the patients. The children were referred to pediatric orthopedics for follow-up with regards to the sequelae of skeletal development.
Surgical procedure
The surgery performed was a resection of the dysplasia followed by reconstruction using a free vascularized fibular osteocutaneous flap from the contralateral limb (Image A, Image B, Image C, Image D, Image E, Image F, Image G, Image H, Image I).
Image A.
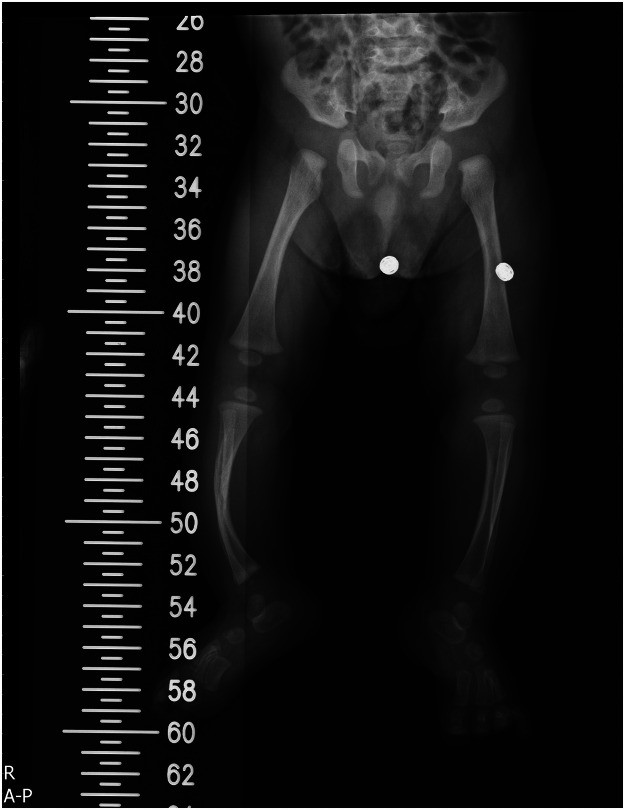
Preoperative x-ray of a child with CPT.
Image B.
Preoperative photograph showing the affected right leg with obvious limb length discrepancy
Image C.
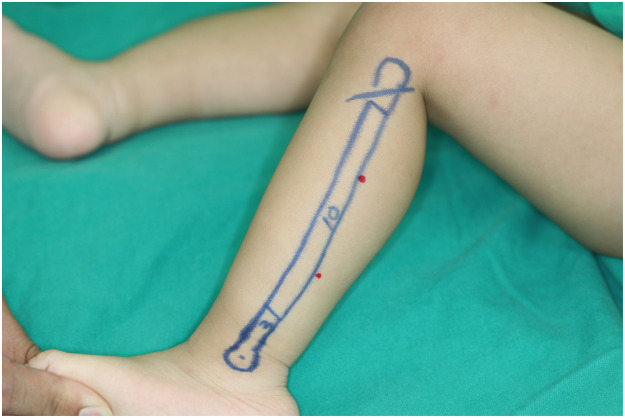
Preoperative planning of fibula flap removal from the left leg
Image D.
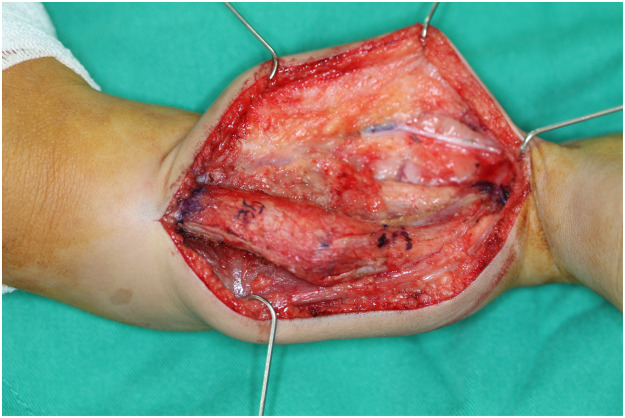
Intraoperative photograph after exposure of the pseudoarthosis of the right tibia
Image E.
Specimen after excision of the diseased segment
Image F.
Image of the fibula flap after harvest with the buoy flap for monitoring
Image G.
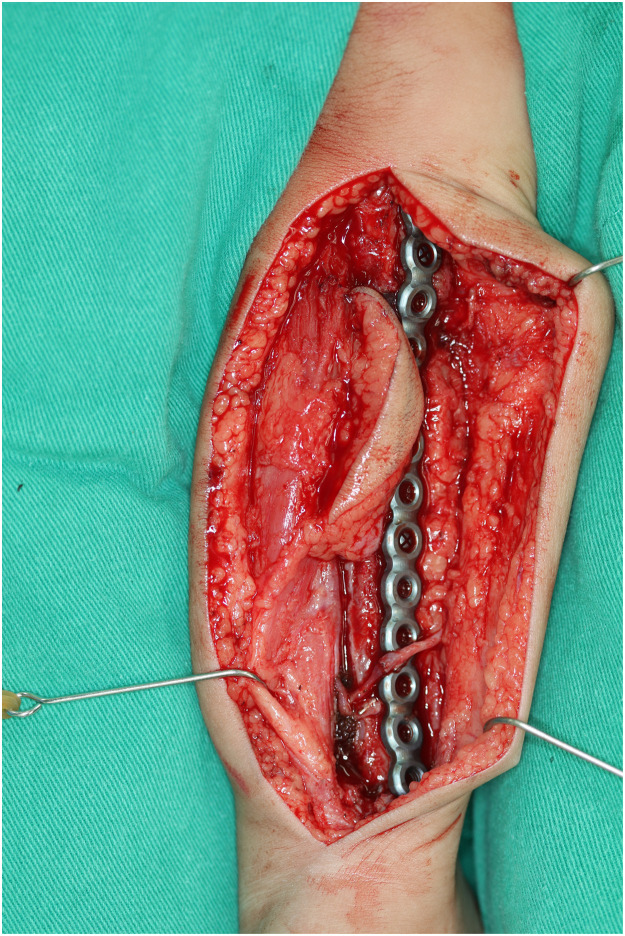
Vascularized fibula flap inset into the defect and internally fixed with Recon plate and screw
Image H.
Postoperative image of the child with limb length discrepancy corrected
Image I.
Postoperative x-ray of the child after the fibula flap procedure.
Under general anesthesia and bilateral thigh tourniquets, the fibular flap was harvested using the posterior approach. A strip of skin paddle less than 2 cm wide was included as a buoy flap for monitoring. Because of the tiny caliber of the vessels in children, a microscope was used to identify the skin paddle perforators and dissection of the peroneal artery and veins. Osteotomies were performed leaving the proximal and distal 3-4 cm (proportional to the length of fibula available).
Intraoperatively, the diseased tibia segment was excised with a 1-cm clear margin into visible healthy bone marrow, guided by plain film radiograph. The fibula was shaped and pegged into the tibial medullary cavity and fixed with plates and screws. The peroneal vessels of the flap were anastomosed to the anterior tibial vessels.
The patients were regularly followed up by pediatric orthopedics for the measurement of limb length and monitoring for bilateral ankle valgus using plain radiographs and scanograph. Timely secondary procedures were performed as required during the follow-up period.
Results
Patient demographics and clinical characteristics
The series comprised three girls and six boys with an age of presentation between one and three years. All nine children had features of NF1. Three children did not present with a pathological fracture and were treated conservatively. Six presented with pathological fracture that necessitated surgical intervention. Of this operative group, three had failed previous intramedullary nailing with allograft. All six children underwent reconstruction using vascularized free fibular flap from the contralateral leg. The age range was one year nine months to five years nine months at the time of operative intervention. All of the peroneal vessels of the flap were anastomosed to the anterior tibial vessels. The fibular struts were internally fixed with screws and plates in all cases. The postoperative follow-up period ranged between 8 months and 200 months for the most recent to the earliest patient, respectively (Table 1).
Table 1.
Children treated by surgical method
| Patient No. | Age at diagnosis | Current Age | M/F | Laterality and Crawford grade | Age at first Surgery | Number of operations so far | Surgeries prior to Free Fibula | Type of Fibula Flap | Residual Limb Length discrepancy | Residual Valgus deformity | Corrective Osteotomy | Donor site problems | Other Sequelae | Clavien Dindo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | <1 year | 19 | F | Left and Grade 4 | 2y3m | 6 | Failed Bone graft + IM Nail | Single Barrel | Y 40mm | Y about 30 degrees | Yes, twice | No | Distal fibula flap nonunion–bone grafted thrice | 3b |
| 2 | 1 year | 19 | M | Right and Grade 4 | 5y9m | 4 | Failed Bone graft + IM Nail | Single Barrel | Y 55mm | N | N | No | Stress fracture–at 14 months after surgery–Treated with splinting | 1 |
| 3 | 3 years | 17 | F | Right and Grade 4 | 4 | 7 | Failed Bone graft + IM Nail | Single Barrel | Y 17mm | Y–DTFJ Fusion + Corrective Osteotomy | Yes for both donor and recipient | Yes, Valgus Ankle | - | 3b |
| 4 | 3 years | 14 | M | Right and Grade 4 | 4 | 6 | Nil | Double Barrel | 17mm | N | Yes for both donor and recipient | Yes, Valgus Ankle | Fracture at distal tibia–fibula flap junction | 3b |
| 5 | 2 years | 9 | M | Left and Grade 4 | 2y6m | 6 | Nil | Single Barrel | Negligible (7mm) | N | Yes for both donor and recipient | Yes, Valgus Ankle | Overgrowth discrepancy treated by removal of tethering physis and DTFJ fusion | 3b |
| 6 | 1 year | 3 | M | Right and Grade 4 | 2 | 1 | Nil | Single Barrel | N Equal | N | N | No | None | n/a |
IM nail - Intramedullary Nail, Y - Yes, N - No, DTFJ - Distal Tibiofibular Joint.
Analysis of postoperative course and follow-up period
Limb length was the most important indicator measured serially during the course of follow-up and growth. Three children (#1-3) who had primarily undergone intramedullary nail and tibial allografts followed by secondary reconstruction with fibula free flap demonstrated limb length shortening with discrepancy of 17 mm to 55 mm after 84 to 144 months follow-up. The other three children (#4-6) who underwent primary fibula flap for pathologic fracture did not have stress fracture and presented limb length discrepancy (LLD) of 17, 7, and 0 mm, respectively, as shown in Table 1. Child number 5 had an overgrowth discrepancy, which was treated by distal tibiofibular joint fusion.
In one of the children (#1), a fibular flap distal osteosynthesis nonunion was noted, which was managed by conventional bone grafting three times in the following 3 years. Two children had stress fractures (#2,4) that occurred after reconstruction and were treated by splinting.
Residual ankle valgus deformity on the side of the CPT was noted in two children (33%). One child had corrective osteotomies for treating this deformity and Illizarov bone distraction 108 months after fibular flap reconstruction. The other patient underwent corrective osteotomy for valgus and 1 cm overgrowth at the 84-month follow-up.
Except for the child who recently underwent the free fibular flap procedure (9 months ago), the other five children who underwent microsurgical procedures between 81 months and 200 months had to undergo multiple other corrective procedures. These children underwent between three to six procedures with a mean of five procedures. The secondary procedures performed included removal of metal work, removal of broken metal work, corrective osteotomies, and in one case, correction of LLD.
Fifty percent of the children who underwent surgery had ankle valgus deformity of the donor site, which required a corrective osteotomy procedure.
Analysis of Conservative Management
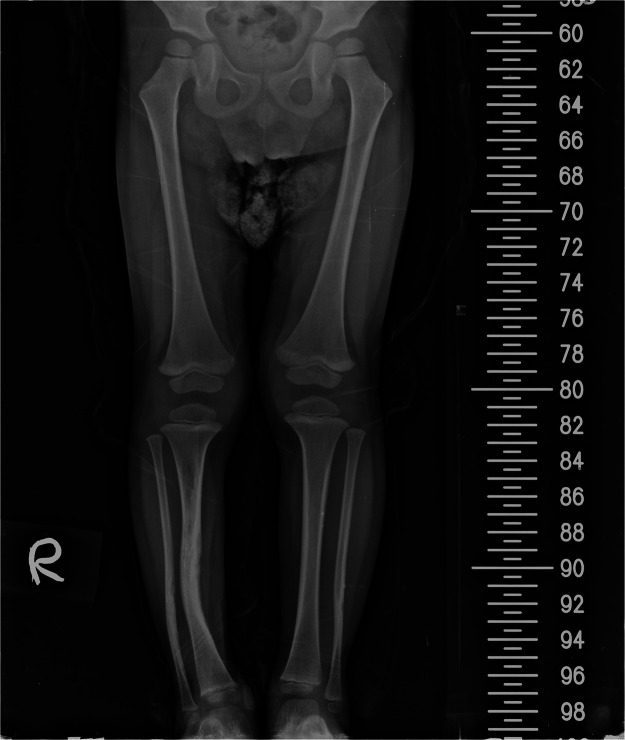
Table 2 shows the features of the children treated by conservative measures. Three children with Crawford Grade 1 who were diagnosed below the age of one were treated conservatively. These children presented with only deformity and no fracture, and thus, serial bracing was the treatment option chosen. They had regular 6-month clinical follow-ups with radiographic assessments each time for a period of 3 to 13 years. The outcomes of serial x-rays revealed the LLD was less than 1 cm in the conservative group. One child underwent a distal ankle fusion due to medial malleolar tethering and contralateral knee guided growth to correct the discrepancy (Figure 1, Figure 2, Figure 3).
Table 2.
Children treated by conservative method.
| Patient No. | Age at diagnosis | Current Age | M/F | Laterality and Crawford grade | Age at first Surgery | Number of operations so far | Surgeries prior to Free Fibula | Type of Fibula Flap | Residual Limb Length discrepancy | Residual Valgus deformity | Corrective Osteotomy | Donor site problems | Other Sequelae |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 year | 5years | M | Right and Grade 1 | N/A | N/A | N/A - splint | Conservative | Y 1 cm | N | N/A | N/A | Mild angulation |
| 2 | 5 months | 6 years | M | Grade 1 | N/A | N/A | N/A - splint | Conservative | Negligible (5mm) | N | N/A | N/A | N/A |
| 3 | 2 months | 12years | F | Grade1 | 6 years | 1 | DTFJ Fusion at the age of 6 years | Conservative | N | N | N/A | N/A | N/A |
Figure 1.
Initial radiograph of a child who underwent conservative management.
Figure 2.
Serial x-ray taken 3 years later of the same child who underwent conservative management—no obvious fracture was observed, and the deformity was manageable.
Figure 3.
Serial x-ray taken 4 years after the initial x-ray of the same child—no fracture and the deformity was manageable.
Discussion
Congenital pseudoarthrosis of the tibia is a complex problem usually presenting as a unilateral condition, with bilateral presentations regarded as extremely rare.1
Consistent with that of previous studies, our cohort at time of presentation ranged between the ages of 2-3, with four children presenting earlier.4 The pathology presents between the junction of the middle and distal 1/3 of the tibia with associated fibula involvement in 30-50% of cases.4
An association with NF1 has been extensively described1,2,4,5 and was present in all patients in our cohort.
With unclear pathophysiology, various hypotheses proposed include genetic, vascular, and mechanical etiologies or a combination. The most commonly proposed theory considers a combination of mechanical and vascular factors including a “fibrous hamartoma” and a “defective periosteum.” Fibrous hamartoma acts as a mechanical barrier, and defective periosteum causes inadequate vascularization of the bone.6 Fifty percent (50%) of CPT cases manifest features of NF1, with theories proposing defective osteoblastic activity due to inactive RAS-MAPK with an overexpression of RAS pathway causing increased activity of osteoclasts.6
Histopathologically, a fibroblast4 and fibrocartilage-rich “fibrous hamartoma” has been identified at the site of the pseudoarthosis. There is also increased osteoclastic activity with giant osteoclasts and resorption lacunae noted at the bone-hamartoma junction, which suggests a bone remodeling defect as well.6,7 This provides a possible explanation for one of the patients who underwent vascularized fibula on lay grafting on the fractured tibia, the tibial bone showed progressive resorption, which resulted in angulation of the involved limb.
There have been various classifications mentioned to describe the degree of the CPT such as the Crawford,8 Boyd,9 and Apoli 210 classifications. None of the classifications are useful to monitor progression of the condition. The Crawford classification is the most frequently used (Table 3) and was therefore adopted for this cohort.
Table 3.
Crawford Classification
| Type | Description |
|---|---|
| 1 | Anterior bowing with an increase in cortical density and a narrow medulla |
| 2 | Anterior bowing with narrow, sclerotic medulla |
| 3 | Anterior bowing associated with a cyst or signs of a pre-fracture |
| 4 | Anterior bowing and a clear fracture with pseudarthrosis often associating the tibia and fibula |
All the six children undergoing surgical treatment were type IV, and three non-fractured cases undergoing conservative management were type I.
In terms of monitoring, the standard is via normal radiography, which we adopted; however, newer modalities such as MR imaging can be considered and used.11
Our main goals of treatment are12
-
•
Correction of alignment
-
•
Achieving bony union of the fractured tibia
-
•
Equalizing the LLD
-
•
Correction of any deformities during the course of growth
-
•
Maintaining the above by regular monitoring and timely correction till bony union and walking.
Optimum treatment age
The optimal treatment age is an area of contention within the European Pediatric Orthopedic Society,13 which proposes that children be treated after the age of three both for surgical reasons as well as for biological reasons, as evidenced by studies conducted by Boyd on importance of osteolytic activity at the site in these children. The optimal results for vascularized fibula flap have been seen between 3.5–7.5 years,14 whereas for the Illizarov technique, it is between 4-9 years.15 The children in our series had the vascularized fibula flap between 3 and 6 years of age in keeping with the European guidelines as well as the data for best outcome via the vascularized fibula flap.
Treatment
Use of the vascularized free fibula flap in treating CPT was first mentioned in 1978 by Gilbert and Judet.16 The key concept is removal of the diseased bone/hamartoma through a wide resection until healthy tibial bone forms and replacement with healthy vascularized fibula bone occurs. There are reports of using the ipsilateral fibula as a pedicled transfer17 as a non-microvascular option. The fibula has also been used in a single-barrel or a double-barrel configuration. There are various studies describing results with primary union rates of more than 70%.14 Various fixation techniques described include internal fixation with plates and screws, external fixation with devices such as an Illizarov frame, impelling the fibula into the medullary cavity of the tibia, or any combination of these.14
Aside from use of vascularized fibula, other techniques utilized are:
Risks and complications
The postsurgical course of CPT is not free of sequelae and complications.9 The commonest problems expected during the course of treatment are fractures, valgus deformity of the ankle (45% incidence) as per Fragniere et al,20 LLD, nonunion, pseudoarthrosis, asymmetric growth, and ankle stiffness.5
The complications encountered in our series were LLD in four children, residual ankle valgus in two children, stress fractures in two children and nonunion of the distal fibula flap osteosynthesis in one child. The results are summarized in Table 1 and include Clavien-Dindo scores. Of note, none of the children treated conservatively required any surgical interventions, but this can be explained by the very strict selection criteria for the method of treatment chosen. In terms of the surgically treated cohort, the indication for initial surgical treatment was absolute (nonhealing stress fracture and failed intramedullary nail), and the complications encountered are well documented in the literature and are to be expected with the method chosen. The parents of the surgically treated cohort were counseled of these possible sequelae prior to surgical treatment, and they understood that there would be possible multiple corrective procedures necessary following the initial vascularized fibula operation.
Various treatment modalities have been described to counter LLD such as Illizarov lengthening18 and distal tibiofibular joint fusion20 with corrective osteotomies being advocated for valgus deformities.21 There is a risk of stress fracture at the site of the tibia-fibular flap junction, which is only resolved when the bone hypertrophies at the site. These fractures result in multiple operations with the possible need for bone grafts.
Donor site complications of harvested free fibula can result in progressive ankle valgus, with 50% of children in our series having to undergo corrective procedures. Tibiofibular metaphyseal synostosis (Langenskiöld procedure) has been shown to delay this problem but does not completely prevent it.21
Follow-up and monitoring
An objective method of follow-up evaluation has been described by Johnston et al22 and was used in our study. Our study had a long follow-up period of 200 months for the oldest patient to 8 months for the most recent one. This has given us a valuable perspective into the varying degrees of outcomes and the journey that is involved from diagnosis until completed bone growth.
These children do require long-term follow-up with regular monitoring with radiographs till they attain skeletal maturity.
Conservative treatment
In our series, three children underwent a conservative approach of treatment with serial bracing. Our recommendation is that this is only suitable for a narrow subset of cases of CPT with Crawford grade 1 type with no previous fractures. These patients require close monitoring with regular (at least 6-12 monthly) radiographs and limb length and ankle valgus measurements. The indications to transition to surgical treatment after discussions with parents are:
From our experience and a long-term follow-up of these children, these milder forms can be treated with conservative measures without significant problems of growth or mobility. This technique is controversial, as it is widely believed that the natural progression of CPT is worsening angulation, shortening, and fracture, and hence surgery is the only mode of management.25
Study design
Due to its retrospective nature, the study design had its inherent flaws of recall bias and observer bias. We mitigated this by including objective limb length measurements and serial radiography measurements as outcomes. We also limited physician-to-patient recall bias by only including patients from a single senior surgeon. Because this condition is rare, large numbers of cases with long-term follow-up are not readily available, and the study cannot be done prospectively as long-term follow-up is required until skeletal maturity.
Conclusion
CPT is a complex problem that needs a multidisciplinary approach. Early intervention in children with severe grades using free fibula flap reconstruction gives good results, although multiple corrective surgeries will be necessary until growth is completed. Regular monitoring through clinical and x-ray assessments is necessary to avoid severe postsurgical deformities such as valgus, varus, shortening, or lengthening.
We also recommend that regular monitoring of the donor site is necessary, as during the course of growth, it may show valgus deformity, which may need corrective surgery. Further exploration of whether any other procedure in addition to the fusion of the distal tibiofibular joint at the time of harvest of the free fibula flap could completely prevent this future deformity is required.
Conservative measures such as protective serial bracing for milder forms of CPT should be studied further to support or debunk the popularly held belief that surgery is the only treatment option.
Conflict of interest
None declared.
Acknowledgments
Funding
None.
Ethical approval
None required.
Contributor Information
Quillan Young Sing, Email: qyoungsing@gmail.com.
Ashwin Alke Pai, Email: drashwinpai@gmail.com.
Maxim GEEROMS, Email: maximgeeroms@gmail.com.
Soo-Min Cha, Email: csm9857@hanmail.net.
Chih-Hung Lin, Email: chihhung@cgmh.org.tw.
References
- 1.Zhu G., Zheng Y., Liu Y., et al. Identification and characterization of NF1 and non-NF1 congenital pseudarthrosis of the tibia based on germline NF1 variants: genetic and clinical analysis of 75 patients. Orphanet Journal of Rare Dis. 2019;14:221. doi: 10.1186/s13023-019-1196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aegerter E.E. The possible relationship of neurofibromatosis, congenital pseudarthrosis, and fibrous dysplasia. J Bone Joint Surg. 1950;32–A:618–626. [PubMed] [Google Scholar]
- 3.Andersen K.S. Radiological classification of congenital pseudarthrosis of the tibia. Acta Orthop Scand. 1973;44:719–727. doi: 10.3109/17453677308989112. [DOI] [PubMed] [Google Scholar]
- 4.Hefti F., Bollini G., Dungl P., Fixsen J., Grill F., Ippolito E., et al. Congenital pseudarthrosis of the tibia: history, etiology, classification, and epidemiologic data. J Pediatr Orthop. 2000;9:11–15. doi: 10.1097/01202412-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Sakamoto A., Yoshida T., Yamamoto H., Oda Y., Tsuneyoshi M., Iwamoto Y. Congenital pseudarthrosis of the tibia: analysis of the histology and the NF1 gene. J Orthop Sci. 2017;12:361–365. doi: 10.1007/s00776-007-1142-1. [DOI] [PubMed] [Google Scholar]
- 6.Cho T.J., Seo J.B., Lee H.R., Yoo W.J., Chung C.Y., Choi I.H. Biologic characteristics of fibrous hamartoma from congenital pseudarthrosis of the tibia associated with neurofibromatosis type 1. J Bone Joint Surg. 2008;90–A:2735–2744. doi: 10.2106/JBJS.H.00014. [DOI] [PubMed] [Google Scholar]
- 7.Hermanns-Sachweh B., Senderek J., Alfer J., Klosterhalfen B., Buttner R., Fuzesi L., et al. Vascular changes in the periosteum of congenital pseudarthrosis of the tibia. Pathol Res Pract. 2005;201:305–312. doi: 10.1016/j.prp.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Crawford A.H., Jr., Bagamery N. Osseous manifestations of neurofibromatosis in childhood. J Pediatr Orthop. 1986;6:72–88. doi: 10.1097/01241398-198601000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Boyd H.B. Pathology and natural history of congenital pseudarthrosis of the tibia. Clin Orthop. 1982;166:5–13. [PubMed] [Google Scholar]
- 10.Apoil A. Les pseudarthroses congénitales de jambe. À propos de 13 observations. Rev Chir Orthop. 1970;56:120–138. [PubMed] [Google Scholar]
- 11.Mahnken H., Staatz G., Hermanns B., Gunther R.W., Weber M. Congenital pseudarthrosis of the tibia in pediatric patients. American Journal of Roentgenology. 2001;177:1025–1029. doi: 10.2214/ajr.177.5.1771025. [DOI] [PubMed] [Google Scholar]
- 12.Pannier S. Congenital pseudarthrosis of the tibia. Orthopaedics & Traumatology: Surgery & Research. 2011;97:750–761. doi: 10.1016/j.otsr.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Grill F., Bollini G., Dungl P., Fixsen J., Hefti F., Ippolito E., et al. Treatment approaches for congenital pseudarthrosis of tibia: results of the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS) J Pediatr Orthop. 2000;9:75–89. doi: 10.1097/01202412-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert A., Brockman R. Congenital pseudarthrosis of the tibia. Long-term followup of 29 cases treated by microvascular bone transfer. Clin Orthop. 1995;314:37–44. [PubMed] [Google Scholar]
- 15.Damsin J.P., Ghanem I., Carlioz H. Apport du matériel d'Ilizarov dans le traitement de la pseudarthrose congénitale de jambe. Rev Chir Orthop. 1996;82:34–41. [PubMed] [Google Scholar]
- 16.J. Judet, A. Gilbert, H. Judet, Servan, Menard. Apport de la microchirurgie à la chirurgie osseuse Chirurgie, 104 (1978), pp. 921-924. [PubMed]
- 17.Coleman S.S., Coleman D.A. Congenital pseudarthrosis of the tibia: treatment by transfer of the ipsilateral fibula with vascular pedicle. J Pediatr Orthop. 1994;14:156–160. doi: 10.1097/01241398-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Ilizarov G.A., Gracheva V.I. Bloodless treatment of congenital pseudarthrosis of the crus with simultaneous elimination of shortening using dosed distraction. Ortop Travmatol Protez. 1971;32:42–46. [PubMed] [Google Scholar]
- 19.Charnley J. Congenital pseudarthrosis of the tibia treated by intramedullary nail. J Bone Joint Surg Am. 1956;38–A:283–290. [PubMed] [Google Scholar]
- 20.Fragniere B., Wicart P., Mascard E., Dubousset J. Prevention of ankle valgus after vascularized fibular grafts in children. Clin Orthop. 2003;408:245–251. doi: 10.1097/00003086-200303000-00032. [DOI] [PubMed] [Google Scholar]
- 21.Kanaya K., Wada T., Kura H., Yamashita T., Usui M., Ishii S. Valgus deformity of the ankle following harvesting of a vascularized fibular graft in children. J Reconstr Microsurg. 2002;18:91–96. doi: 10.1055/s-2002-19888. [DOI] [PubMed] [Google Scholar]
- 22.Johnston C.E. Congenital pseudarthrosis of the tibia: results of technical variations in the Charnley-Williams procedure. J Bone Joint Surg Am. 2002;84–A:1799–1810. [PubMed] [Google Scholar]
- 23.Kliegman R.M. Nelson Textbook of Pediatrics; 2020. Leg-Length Discrepancy. [Google Scholar]
- 24.Beals R.K., Shea M. Correlation of chronological age and bone age with the correction of ankle valgus by surface epiphysiodesis of the distal medial tibial physis. J Pediatr Orthop B. 2005;14:436–438. doi: 10.1097/01202412-200511000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Rogez J.M. Courbures et pseudarthroses congénitale de jambe. Cahiers d'enseignement de la SOFCOT, L'Expansion Scientifique éd., Paris. 1989;34:237–244. [Google Scholar]