Abstract
Introduction
Ultrasound has been nicknamed “the surgeon’s stethoscope”. The advantages of laparoscopic ultrasound beyond a substitute for the sense of touch are considerable, especially for robotic surgery. Being able to see through parenchyma and into vascular structures enables to avoid unnecessary dissection by providing a thorough assessment at every stage without the need for contrast media or ionising radiation. The limitations of restricted angulation and access within the abdominal cavity during laparoscopy can be overcome by robotic handling of miniaturised ultrasound probes and the use of various and specific frequencies will meet tissue- and organ-specific characteristics. The aim of this systematic review was to assess the reported applications of intraoperative ultrasound-guided robotic surgery and to outline future perspectives.
Methods
The study adhered to the PRISMA guidelines. PubMed, Google Scholar, ScienceDirect and ClinicalTrials.gov were searched up to October 2023. Manuscripts reporting data on ultrasound-guided robotic procedures were included in the qualitative analysis.
Results
20 studies met the inclusion criteria. The majority (53%) were related to the field of general surgery during liver, pancreas, spleen, gallbladder/bile duct, vascular and rectal surgery. This was followed by other fields of oncological surgery (42%) including urology, lung surgery, and retroperitoneal lymphadenectomy for metastases. Among the studies, ten (53%) focused on locating tumoral lesions and defining resection margins, four (15%) were designed to test the feasibility of robotic ultrasound-guided surgery, while two (10.5%) aimed to compare robotic and laparoscopic ultrasound probes. Additionally two studies (10.5%) evaluated the robotic drop-in probe one (5%) assessed the hepatic tissue consistency and another one (5%) aimed to visualize the blood flow in the splenic artery.
Conclusion
The advantages of robotic instrumentation, including ergonomics, dexterity, and precision of movements, are of relevance for robotic intraoperative ultrasound (RIOUS). The present systematic review demonstrates the virtue of RIOUS to support surgeons and potentially reduce minimally invasive procedure times.
Keywords: Image-guided surgery, Robotic-assisted surgery, Ultrasound, Digital surgery, Artificial intelligence, New technologies
In the last few decades, there has been a rapid succession of technological advances, marking a radical shift from the open to the minimally invasive surgical (MIS) approach [1]. The advantages of laparoscopy over laparotomy are now widely acknowledged [2]. Over the past twenty-five years, robotic surgery has experienced a raise and today, with the availability of several platforms alongside the continuously leading da Vinci systems (Intuitive Surgical Inc., Sunnyvale, CA, USA), robotic approaches are playing an increasingly crucial role [3, 4]. Despite observed advantages for certain patient characteristics (e.g., BMI > 30), challenges such as the lack of dedicated reimbursement, high costs and often longer operating times still limit the widespread use of robotic platforms worldwide [5]. Open surgery provides direct visual and tactile information of the explored regions. In contrast, MIS comes at the cost of predominantly two-dimensional view and limited tactile assessment. Intraoperative ultrasound (IOUS) is commonly utilized during open surgery with linear or finger probes, particularly in the hepatobiliary (HPB) and urological fields [6, 7]. In laparoscopic setting, ultrasound probes for guidance in MIS are more challenging to handle [8]. To overcome this limitation, innovative approaches for robotic platforms integrate ultrasound imaging to facilitate its use in MIS [9]. Image-guided robotic approaches, particularly those based on three-dimensional (3D) imaging, augmented reality (AR), and machine learning algorithms, offer advantages in the era of digital surgery [10]. Real-time, non-invasive, cost-effective and dynamic intraoperative imaging of complex anatomy are the main benefits of computer-assisted surgery. In this context, IOUS has emerged as the imaging modality of choice facilitated by the introduction of articulated robotic instruments to handle ultrasound probes [11]. The augmentation and fusion of imaging modalities are especially beneficial for delineating healthy and neoplastic tissue in oncological surgery [12]. The navigation of drop-in ultrasound probes manoeuvred by articulated robotic graspers provides access to anatomical spaces and angles that are inconvenient for relatively rigid laparoscopic probes. While initial reports of applications of intraoperative ultrasound during robotic surgery (RIOUS) have been published in the fields traditionally managed by open surgery, with encouraging results., pooled data are lacking [6, 13]. Therefore, the aim of this systematic review is to assess the reported applications of intraoperative ultrasound-guided robotic surgery and to outline future perspectives.
Materials and methods
Search strategy
The systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14] and registered with the International Prospective Register of Systematic Reviews PROSPERO (n CRD42023494430) prior to data extraction. Articles were obtained by querying the PubMed database, Google Scholar, ScienceDirect and ClinicalTrial.gov filtered by the English language up to October 2023 without additional restrictions. The database was retrieved through title and abstract screening using the following search terms: “intraoperative”, “robotic”, “surgery”, “ultrasound”, “laparoscopic”, “probe”.
Data extraction
After removing duplicate publications, titles, abstracts, and keywords were independently reviewed by M.P and E.T. for inclusion, followed by full text review of eligible articles. In case of discrepancies, a consensus was reached through agreement with a third author (M.G.). The inclusion criterion was the description of ultrasound-assisted robotic surgical procedures. Excluded were articles without robotic use of the probe, as well as abstracts, reviews, meta-analyses, letters, and editorials. Studies reporting robotic ultrasound imaging independently of a surgical procedure, or those focused on percutaneous ultrasound-guided techniques and biopsies were also excluded. Data about the authors, surgical procedures, probes specifics and ultrasound-assisted robotic procedures were extracted for further analysis.
Results
The search strategy identified studies reporting intraoperative ultrasound imaging during robotic surgery. Initially, 781 studies were identified, and 68 full texts were selected through title and abstract screening. Finally, 20 studies met the inclusion criteria for the systematic review (Fig. 1). Due to the low number of reports, a qualitative analysis was performed [6–8, 11, 13, 15–28].
Fig. 1.
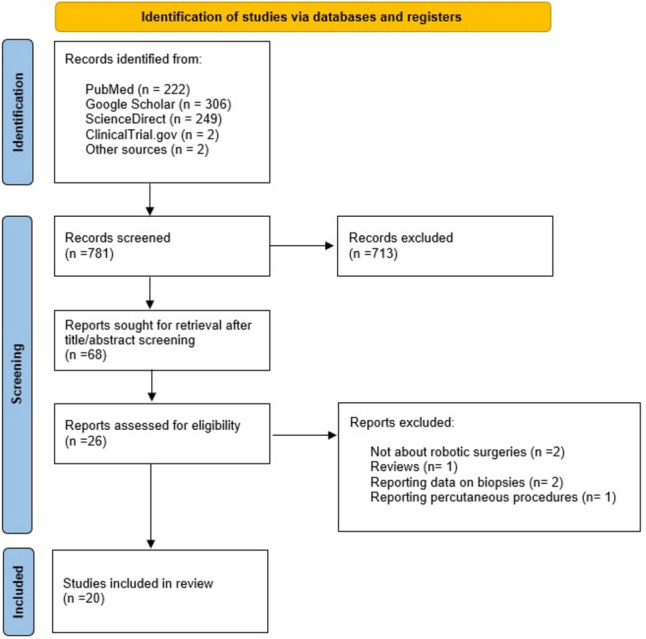
PRISMA flow diagram of study selection
Among the included studies, two were prospective (10%), fifteen teen were retrospective (75%), three were experimental (15%), involving laboratory tests in vivo (porcine models) or on ex vivo phantoms. The studies were mainly (53%) from the field of general surgery during liver, pancreas, spleen, gallbladder/bile duct, vascular and rectal surgery [6, 8, 11, 16–22, 28]. The remaining studies(42%) covered other fields of oncological surgery including urology [7, 13, 23–26], lung surgery [27], and retroperitoneal lymphadenectomy for metastases [15].
Ten studies (53%) were focused on locating tumoral lesions and defining resection margins [7, 11, 13, 18–20, 22, 25, 27, 29]. Additionally, four studies (15%) were designed to assess the feasibility of robotic ultrasound-guided surgery [8, 15, 23], two (10.5%) aimed to compare robotic and laparoscopic ultrasound probes [6, 24], another two (10.5%) were conducted to evaluate the robotic drop-in probe [13, 16], one study (5%) focused on assessing hepatic tissue consistency [17] and another (5%) aimed to visualize the blood flow in the splenic artery [21].
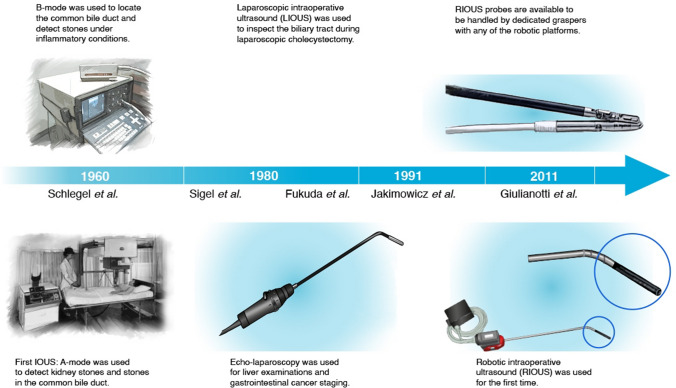
In eleven articles (55%), a miniaturized linear drop-in probe was used [6, 7, 11, 13, 15, 16, 23–25, 27, 28]. These probes can be introduced via a 10–12 mm accessory trocar and steered from the surgeon’s console using robotic graspers (Fig. 2). Five manuscripts reported the use of rigid probes, which can be docked to the robotic arm (12 mm trocar) as prototypes corresponding to da Vinci robotic instruments (Fig. 2) [8, 21]. Alternatively, a laparoscopic articulated probe can be used during robotic surgery, introduced via the 10 mm accessory port and manipulated by the bedside assistant (Fig. 2). The ultrasound frequencies of the probes used in the included studies ranged from 3 to 13 MHz. All reported procedures were performed with the da Vinci robotic platforms. Table 1 summarizes the characteristics of the included articles, and details about ultrasound probes and surgical applications. No clinical trials on the use of RIOUS were registered at the timepoint of the database query. Due to the heterogeneity of data concerning probes, frequencies, procedures and study outcomes, a quantitative analysis of the results was deemed inappropriate.
Fig. 2.
Timeline of intraoperative ultrasound techniques
Table 1.
Study details and ultrasound
| Year | Author | Country | Design | Sample Size | Ultrasound Target | Purpose | Probe docking | Probe Type | MHz | Platform |
|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | Giulianotti et al. [1] | Italy | Case Series | 6 | Splenic Artery | Vascular blood flow | Robotic arm | NA | NA | Da Vinci |
| 2012 | Yakoubi et al. [2] | USA | Feasibility Test | In vivo porcine model | Kidney | To evaluate a novel ultrasound probe specifically developed for robotic surgery by determining its efficiency in identifying renal tumors | Robotic arm | BK Drop-In 8826 | 5–12 MHz | Da Vinci S |
| 2012 | Schneider et al. [3] | USA | Feasibility Test | In vivo animal model/Phantom Liver | Liver | In vivo porcine hepatic visualization and probe manipulation, lesion detection accuracy, and biopsy precision | Robotic arm | Rigid Robotic Laparoscopic (RLUS) probe | 7.5 MHz | Da Vinci |
| 2012 | Kaczmarek et al. [4] | USA | Case Series | 22 | Kidney | Accurate tumor identification during partial nephrectomy | Robotic arm assisted | Dro-in Hitachi-Aloka | 4–13 MHz | Da Vinci |
| 2012 | Billings et al. [5] | USA | Feasibility Test | Phantom tissue experiment | NA | Tissue hardness by elastography | Robotic arm | Rigid Robotic Laparoscopic (RLUS) probe | NA | Da Vinci Si |
| 2013 | Kaczmarek et al. [6] | USA | Retrospective | 75 | Kidney | To evaluate and compare perioperative outcomes of robotic partial nephrectomy (RPN) using robotic and laparoscopic ultrasound probe for tumor identification | Robotic arm | Drop-in Hitachi-Aloka | 4–13 MHz | Da Vinci |
| 2015 | Pessaux et al. [7] | France | Case series | 3 | Liver | The explore the potential of AR navigation as a tool to improve safety of the surgical dissection is outlined for robotic hepatectomy | Robotic arm | NA | NA | Da Vinci |
| 2015 | Guerra et al. [8] | Italy | Case Series | 10 | Liver | To evaluate the feasibility and reliability of robotically integrated ultrasound to guide resection of malignant hepatic tumors | Robotic arm assisted | BK Drop-In 8826 | 5–12 MHz | Da Vinci Si |
| 2015 | Liu et al. [9] | China | Case Series | 7 | Pancreas | To seek for previously undetected lesions and to determine the accurate surgical resection margins in pancreatic tumors | Accessory trocar in robotic surgery | Rigid UST-5410 miniprobe (Aloka Alpha 7 Hitachi) | 4–13 MHz | Da Vinci S |
| 2016 | Gunelli et al.[10] | Italy | Prospective | 22 | Kidney | Renal tumor enucleation | Robotic arm assisted | BK Drop-In 8826 | 5–12 MHz | Da Vinci |
| 2017 | Zhou et al. [11] | China | Prospective | 17 | Lung | To investigate the efficacy of intraoperative ultrasonographic localization during da Vinci thoracic surgery | Robotic arm assisted | BK Drop-In 8826 | 5–12 MHz | Da Vinci |
| 2018 | Araujo et al. [12] | Brazil | Case Series | 2 | Liver | Liver lesions identification | Robotic arm assisted | NA | NA | NA |
| 2019 | Zhang K et al. [13] | China | Case Series | 1 | Lymph node | To describe the robot-assisted laparoscopic management of post-chemotherapy retroperitoneal metastasis | Robotic arm assisted | BK Drop-In 8826 | 5–12 MHz | Da Vinci Si |
| 2020 | Sun et al. [14] | China | Retrospective | 38 | Kidney | To introduce the role and use of intraoperative ultrasound (IOUS) performed in robotic-assisted renal partial nephrectomy (RAPN) for endophytic renal tumors | Robotic arm assisted | BK Drop-In 8826 | 5–12 MHz | Da Vinci |
| 2020 | Chang et al. [15] | Taiwan | Prospective | 93 | Tongue | Explore soft laringeal tissues | Accessory trocar in Robotic surgery | Rigid UST-533 miniprobe (Aloka Alpha 7 Hitachi) | 4–13 MHz | Da Vinci Si |
| 2020 | Zhang Y et al. [16] | China | Retrospective | 29 | Kidney | To report the experience in treating endophytic renal tumor by robot-assisted partial nephrectomy with a standard laparoscopic ultrasound probe | Accessory trocar in robotic surgery | Rigid UST-5550 (Hitachi Aloka Medical, Japan) | 4–10 MHz | Da Vinci Si |
| 2023 | Di Mitri et al. [17] | Italy | Case Series | 1 | Spleen | Splenic cyst identification | Accessory trocar in Robotic surgery | 2D rigid LPS US | NA | Da Vinci |
| 2023 | Glaysher et al. [18] | UK | Case Series | NA | Cholecystectomy | Delineate superiority of robotic-assisted US to laparoscopic US and IOC for the anatomy of the porta hepatis, and accurate measurements of the biliary tree and any ductal stones in choledocholithiasis | Robotic arm assisted | Drop-in L51K Hitachi-Aloka | 3–15 MHz | Da Vinci Xi |
| 2023 | Otani et al. [19] | Japan | Case Series | 3 | Rectum | To demonstrate the usefulness of intraoperative sonography (IOUS) for detecting the rectal tumor site in robotic surgery | Robotic arm assisted | Drop-in L43K Hitachi-Aloka | 2–12 MHz | Da Vinci Si/Xi |
| 2023 | Maertens et al. [20] | UK | Retrospective | 32 | Rectum | To evaluate the aid of IOUS for safe vessels dissection during complete mesocolic excision | Robotic arm assisted | Drop-in L51K Hitachi-Aloka | 3–15 MHz | Da Vinci Xi |
Discussion
In this systematic review, we present a a comprehensive analysis that sheds light on the current state of intraoperative ultrasound for guidance in robotic procedures.
Summary of main results
Despite the high quality level of evidence supporting laparoscopic ultrasound in various thoraco-abdominal pathologies [30] and the desire to implement RIOUS for over two decades [31], the literature still reflects limited evidence regarding ultrasound guidance during robotic surgery, with relatively small cohort sizes.
All included studies, however, consistently report satisfactory performance of RIOUS. To facilitate the widespread adoption of RIOUS, there is a need for increased adoption of robotic surgical procedures and training for surgeons in IOUS. The utilization of computer assistance for image acquisition and interpretation, through the development and training of machine learning algorithms, could contribute to overcoming operator dependency in ultrasound examinations [32]. In line with the findings of this analysis on RIOUS, image guidance for identifying resection margins by differentiating between healthy and neoplastic tissues has proven particularly useful in oncological diseases [33]. The foremost beneficiary of (R)IOUS thus far is the hepatobiliary field, particularly for the comprehensive anatomical assessment of the biliary and vascular trees [34]. In liver surgery, IOUS plays a well-established role as an intraoperative guidance tool in combination with preoperative CT and MRI imaging. Surgical radicality depends on the detectability of lesions in the different imaging modalities. Techniques such as image fusion of CT/MRI and US, multimodal registration of 2D and 3D imaging modalities as well as (contrast-enhanced) ultrasound contribute to identifying known and preoperatively undetected lesions in order to intraoperatively tailor the surgical strategy [35, 36]. Furthermore, RIOUS was demonstrated to have superior performance compared to conventional LIOUS with a success rate exceeding the one of LIOUS in liver surface exploration (85% vs. 73%, P = 0.030) and tool manipulation (79% vs. 57%, P = 0.028) [8]. Post-task questionnaires completed by participating surgeons revealed that robotic ultrasound significantly improved probe positioning (80%), reduced fatigue (90%), and was overall more useful than LIOUS (90%) [8]. Facilitating precise probe positioning in RIOUS not only enhances surgical precision but also reduces the physical strain on surgeons during complex procedures [6]. An even more significant benefit is the opportunity to identify otherwise undetected lesions, such as in pancreatic lesions [18]. In benign disease of the biliary tract, IOUS has demonstrated comparable efficacy with intraoperative cholangiography in diagnosing choledocholithiasis, surpassing it in terms of speed and completion rates. This is achieved without the need for a contrast agent, with reduced invasiveness and a decreased risk of infection.The comprehensive assessment of the intra- and extrahepatic biliary tree can be accomplished in an average time of 164.1 s using RIOUS and can be complemented by Doppler ultrasound for assessing the porta hepatis. Precise measurements of the biliary tree and ductal stones enable intraoperative decision-making and management of ductal pathologies, including hybrid approaches [6, 37].
Similarly, rectal tumours were successfully detected using RIOUS, showing its effectiveness in determining the optimal transection line for rectal surgeries, especially in cases where tumours are too high for transanal palpation [16]. Furthermore, in obese patient with rectal cancer RIOUS has been proved to be useful to safely guide vascular dissection [28]. Nephron-sparing surgery, as an alternative to radical nephrectomy, is gaining support as an oncologically equivalent procedure while preserving renal functional capacity [9]. The evolution of robot-assisted partial nephrectomy techniques has ushered in a progressive refinement of tools aiding surgeons in the identification of masses and their vascular networks. A remarkable 100% success rate was demonstrated in identifying kidney lesions with RIOUS [25], optimizing tumour identification, enhancing renal tissue preservation through partial nephrectomy, and ensuring oncological safety [6, 9, 37]. In transoral robotic tongue base resection for obstructive sleep apnoea RIOUS has emerged as an invaluable tool for locating the lingual artery and assessing laryngeal tissues. The integration of RIOUS significantly enhances efficiency by substantially reducing the risk of detrimental intraoperative bleeding complications [29].
Despite the numerous advantages observed across various surgical domains, the integration of intraoperative ultrasound in the robotic field remains underused due to costs consideration, lack of expertise, and the necessity for highly skilled minimally invasive surgeons trained in both robotics and ultrasound techniques [11]. Moreover, although rigid prototypes compatible with robotic arms have been developed [8, 20], they are barely due to cost and the absence of a significant advantages over rigid laparoscopic probes, [6, 38]. In contrast, the adaptability of drop-in probes to all multi- and single-port robotic platforms offers high scalability in clinical applications [6, 8, 20, 38].
Results in the context of published literature
Applications of IOUS originated in 1960 for the identification of kidney stones in A-mode [34, 39]. Since 1980s, rapid innovations have progressed with applications in hepato-pancreato-biliary and gastrointestinal surgery [40, 41]. In the 1990s, attempts were made to extend the benefits of IOUS to minimally invasive surgery by creating dedicated probes for laparoscopic ultrasound [42]. When used in the robotic setting, these probes were operated by the bedside assistant. However, laparoscopic probes lack the flexibility of IOUS in open surgery (Fig. 2). As robotic platforms do not yet provide integrated ultrasound probes, a specific transducer known as the “drop-in-probe" was recently introduced for robotic surgery. This probe, with a dorsal fin to be grasped with a robotic instrument, can be steered from the console. The small transducer attached to a highly flexible cable, coupled with the motion range of the articulating instrument, facilitates access to anatomical areas that are hard to reach with standard laparoscopic probes.
Furthermore, dedicated robotic console software, such as TilePro (Intuitive Surgical Inc., Sunnyvale, CA, USA), enables the surgeon to create an in-console split-view with side-by-side intraoperative and ultrasound images, or switch between the minimally invasive 3D camera and ultrasound view directly from the console [22]. On platforms with open consoles, surgeons can switch from the integrated robotic display to the external ultrasound screen ideally positioned close to the console surgeon [43]. One of the known limitations of laparoscopy, and even more so in robotic surgery, is the reduced/absent tactile feedback, requiring considerable training is needed to learn to replace haptic with visual information. Consequently, the availability of additional information via RIOUS is particularly relevant in oncology, where achieving zero residual tumour is a major prognostic factor [44–48]. Exploration of the abdominal cavity with LIUOS can detect malignant deposits preventing conversions to open surgery when remaining disease can be excluded [44–47, 49]. Fertility-sparing surgery can be enhanced by IOUS assistance by discriminating healthy from cancerous tissues and to spare ovarian parenchyma [46]. Image-guided organ exploration during surgery could also impact the detection of undiagnosed masses, especially in pancreatic and splenic diseases [11, 18, 19]. Therefore, margin assessment and mapping resection guidance with IOUS are highly relevant in conservative oncologic surgery [50, 51]. However, large-scale future randomized controlled trials (RCTs) are necessary to demonstrate the utility of IOUS in assessing oncological outcomes.
Although the limited number of publications and the presence of heterogeneity among the included studies, mostly consisting of case reports and case series which have been included to report comprehensively the literature evidence, this systematic review on RIOUS procedures highlights the relevance of the technical advances in robotic surgery which underline its expected impact in the field of image-guided surgery.
Implications for practice and future research
In recent years, an increasing number of robotic platforms has entered the marketplace, a trend expected to persist with decreasing costs and user-friendly platforms for a variety of procedures [3]. However, as the integration of advanced technology based on artificial intelligence and augmented reality is not yet fully automated, making the inclusion of real-time 3D image information into MIS a crucial step in advancing surgical care [10]. Ultrasound-assisted procedures are poised to play a pivotal role in filling this technological gap and are anticipated to grow in parallel with ongoing advancements.
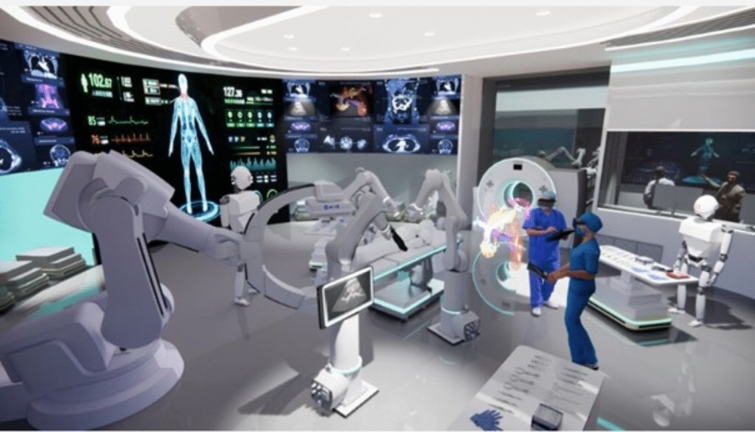
Beyond 3D macroscopic guidance, there is a growing demand for real-time intraoperative tissue analysis, particularly for tailoring the radicality of resection in oncological diseases. In vivo 3D tissue analysis would be ideal for guiding surgery intraoperatively. A variety of intraoperative optical imaging techniques are currently under assessment to complement or potentially replace extemporaneous histopathological analysis [23, 52]. For in vivo tissue, 3D high resolution ultrasound represents a significant step forward in intraoperative analysis within the anatomical context, aiding decision-making on whether resection is required, such as in lymph node metastasis [50]. High (up to 70 MHz) and ultra-high (up to 100 MHz) frequency probes are considered candidates to achieve a resolution of 30 µm, similar to histopathology [53]. An immediate ex vivo imaging system that does not require dedicated sample preparation is full-field optical coherence tomography (FF-OCT), showing a rapid learning curve and analysis of tissue sections similar to [54, 55]. On resected specimens, whole-slide imaging can be used for digital reconstruction as a 3D volume preventing missed lesions for skipped depth slide [56]. In the era of digital surgery, robotic platforms represent computer interfaces capable of integrating multiple modalities of real-time data analysis [10] (Fig. 3). The integration of surgical and imaging sciences will need interdisciplinary training and specific core curricula such as the Master in Image-Guided Surgery, teaching surgeons to perform IOUS, particularly in MIS [57]. Moreover, ongoing studies in deep learning applied to new diagnostic technologies will address the need for standardised IOUS performance and data interpretation by surgeons who may lack adequate radiological expertise [58–60].
Fig. 3.
The next-generation hybrid operating room integrating artificial intelligence and robotics for diagnostic imaging, procedure planning and execution: the operating room of the future is envisioned as the centre of a technology ecosystem. Illustrated technology include advanced interactive digital displays with real-time connectivity and AI analytics, mixed-reality environments, and robotic applications for various interventions, imaging (ultrasound, cone-beam CT, intraoperative CT/MRI, etc.), nursing assistance and sterile instrument management, as well as a predictive logistics supply system with Automatic Guided Vehicles [61]
(Copyright Barbara Seeliger/ Carlos Amato; Chengyuan Yang; Niloofar Badihi; IHU Strasbourg and Cannon Design USA)
Conclusions
Robotic surgery has become increasingly common in routine clinical practice. Recent technological advancements have paved the way for new tools and equipment in robotic and image-guided surgery. The advantages of robotic instrumentation, including ergonomics, dexterity, and precision of movements, are particularly relevant for robotic intraoperative ultrasound. This systematic review demonstrates the virtue of RIOUS to support intraoperative decision-making and potentially reduce minimally invasive procedure times. Prospective studies, however, are needed to better understand its potential, including disciplines like gynaecologic oncology, where these procedures are not yet commonly performed.
Acknowledgements
The authors are grateful to Catherine Cers-Meunier for illustrating the surgical procedures.
Author contributions
MP, BS, AT and DQ contributed to the study design. MP, ET and MG performed the literature research. MP, BS, ET, MG, NB drafted the manuscript. LL, CA, AF, JM, GS, ACT and DQ are responsible for the critical revision of the manuscript and for important intellectual content. All Authors have read and commented on the working versions and approved the final manuscript before submission.
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. This work was supported by French state funds managed within the “Plan Investissements d’Avenir” and by the ANR (reference ANR-10-IAHU-02).
Data availability
All data generated or analysed in this review are included in this article and/or its figures. Further enquiries can be directed to the corresponding author.
Declarations
Disclosures
Barbara Seeliger has a research and education consultant agreement with CMR Surgical and Intuitive Surgical. Jacques Marescaux is the President and Founder of the IRCAD Institute, which is partly funded by Karl Storz, Medtronic and Intuitive Surgical. Matteo Pavone, Elena Teodorico, Marta Goglia, Nicolo` Bizzarri, Cristina Taliento, Antonello Forgione Giovanni Scambia, Antonia Carla Testa and Denis Querleu have no relevant conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gueli Alletti S, Rosati A, Capozzi VA, Pavone M, Gioè A, Cianci S, et al. Use of laparoscopic and laparotomic J-plasma handpiece in gynecological malignancies: results from a pilot study in a tertiary care center. Front Oncol. 2022;12:868930. doi: 10.3389/fonc.2022.868930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gueli Alletti S, Petrillo M, Vizzielli G, Bottoni C, Nardelli F, Costantini B, et al. Minimally invasive versus standard laparotomic interval debulking surgery in ovarian neoplasm: a single-institution retrospective case-control study. Gynecol Oncol. 2016;143(3):516–520. doi: 10.1016/j.ygyno.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Pavone M, Marescaux J, Seeliger B (2023) Current status of robotic abdominopelvic surgery. 秀傳醫學雜誌;(預刊文章):1–15
- 4.Pavone M, Seeliger B, Alesi MV, Goglia M, Marescaux J, Scambia G, et al. Initial experience of robotically assisted endometriosis surgery with a novel robotic system: first case series in a tertiary care center. Updates Surg. 2023 doi: 10.1007/s13304-023-01724-z. [DOI] [PubMed] [Google Scholar]
- 5.Monterossi G, Pedone Anchora L, Gueli Alletti S, Fagotti A, Fanfani F, Scambia G. The first European gynaecological procedure with the new surgical robot HugoTM RAS. A total hysterectomy and salpingo-oophorectomy in a woman affected by BRCA-1 mutation. Facts Views Vis Obgyn. 2022;14(1):91–4. doi: 10.52054/FVVO.14.1.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glaysher MA, Beable R, Ball C, Carter NC, Knight BC, Pucher PH, et al. Intra-operative ultrasound assessment of the biliary tree during robotic cholecystectomy. J Robot Surg. 2023;17(6):2611–2615. doi: 10.1007/s11701-023-01701-z. [DOI] [PubMed] [Google Scholar]
- 7.Kaczmarek BF, Sukumar S, Petros F, Trinh QD, Mander N, Chen R, et al. Robotic ultrasound probe for tumor identification in robotic partial nephrectomy: initial series and outcomes. Int J Urol. 2013;20(2):172–176. doi: 10.1111/j.1442-2042.2012.03127.x. [DOI] [PubMed] [Google Scholar]
- 8.Schneider CM, Peng PD, Taylor RH, Dachs GW, Hasser CJ, DiMaio SP, et al. Robot-assisted laparoscopic ultrasonography for hepatic surgery. Surgery. 2012;151(5):756–762. doi: 10.1016/j.surg.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Di Cosmo G, Verzotti E, Silvestri T, Lissiani A, Knez R, Pavan N, et al. Intraoperative ultrasound in robot-assisted partial nephrectomy: state of the art. Arch Ital Urol Androl. 2018;90(3):195–198. doi: 10.4081/aiua.2018.3.195. [DOI] [PubMed] [Google Scholar]
- 10.Lecointre L, Verde J, Goffin L, Venkatasamy A, Seeliger B, Lodi M, et al. Robotically assisted augmented reality system for identification of targeted lymph nodes in laparoscopic gynecological surgery: a first step toward the identification of sentinel node. Surg Endosc. 2022;36(12):9224–9233. doi: 10.1007/s00464-022-09409-1. [DOI] [PubMed] [Google Scholar]
- 11.Guerra F, Amore Bonapasta S, Annecchiarico M, Bongiolatti S, Coratti A. Robot-integrated intraoperative ultrasound: initial experience with hepatic malignancies. Minim Invas Ther Allied Technol. 2015;24(6):345–349. doi: 10.3109/13645706.2015.1022558. [DOI] [PubMed] [Google Scholar]
- 12.Sokolenko A, Preobrazhenskaya E, Marchetti C, Piermattei A, Zagrebin F, Kuligina E, et al. Origin of residual tumor masses in BRCA1/2-driven ovarian carcinomas treated by neoadjuvant chemotherapy: selection of preexisting BRCA1/2-proficient tumor cells but not the gain of second ORF-restoring mutation. Pathobiology. 2023 doi: 10.1159/000533591. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Wang W, Zhang Q, Zhao X, Xu L, Guo H. Intraoperative ultrasound: technique and clinical experience in robotic-assisted renal partial nephrectomy for endophytic renal tumors. Int Urol Nephrol. 2021;53(3):455–463. doi: 10.1007/s11255-020-02664-y. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2006;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K, Zhu G, Liu X, Tian J, Gu Y, Zhai M, et al. Robot-assisted laparoscopic retroperitoneal lymph node dissection with concomitant inferior vena cava thrombectomy for metastatic mixed testicular germ cell cancer: a case report. J Med Case Rep. 2019;13(1):272. doi: 10.1186/s13256-019-2200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otani K, Kiyomatsu T, Ishimaru K, Kataoka A, Hayashi Y, Gohda Y. Usefulness of real-time navigation using intraoperative ultrasonography for rectal cancer resection. Asian J Endosc Surg. 2023;16(4):819–821. doi: 10.1111/ases.13242. [DOI] [PubMed] [Google Scholar]
- 17.Giulianotti PC, Buchs NC, Coratti A, Sbrana F, Lombardi A, Felicioni L, et al. Robot-assisted treatment of splenic artery aneurysms. Ann Vasc Surg. 2011;25(3):377–383. doi: 10.1016/j.avsg.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Ji WB, Wang HG, Luo Y, Wang XQ, Lv SC, et al. Robotic spleen-preserving laparoscopic distal pancreatectomy: a single-centered Chinese experience. World J Surg Oncol. 2015;13(1):275. doi: 10.1186/s12957-015-0671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Araujo RLC, de Castro LA, Fellipe FEC, Burgardt D, Wohnrath DR. Robotic left lateral sectionectomy as stepwise approach for cirrhotic liver. J Robot Surg. 2018;12(3):549–552. doi: 10.1007/s11701-017-0730-0. [DOI] [PubMed] [Google Scholar]
- 20.Di Mitri M, Thomas E, Di Carmine A, Manghi I, Cravano SM, Bisanti C, et al. Intraoperative ultrasound in minimally invasive laparoscopic and robotic pediatric surgery: our experiences and literature review. Children (Basel) 2023;10(7):1153. doi: 10.3390/children10071153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billings S, Deshmukh N, Kang HJ, Taylor R, Boctor EM (2012) System for robot-assisted real-time laparoscopic ultrasound elastography. In: Medical Imaging 2012: Image-Guided Procedures, Robotic Interventions, and Modeling. SPIE, pp 589–596. 10.1117/12.911086. Accessed 1 Oct 2023
- 22.Pessaux P, Diana M, Soler L, Piardi T, Mutter D, Marescaux J. Towards cybernetic surgery: robotic and augmented reality-assisted liver segmentectomy. Langenbecks Arch Surg. 2015;400(3):381–385. doi: 10.1007/s00423-014-1256-9. [DOI] [PubMed] [Google Scholar]
- 23.Yakoubi R, Autorino R, Laydner H, Guillotreau J, White MA, Hillyer S, et al. Initial laboratory experience with a novel ultrasound probe for standard and single-port robotic kidney surgery: increasing console surgeon autonomy and minimizing instrument clashing. Int J Med Robot. 2012;8(2):201–205. doi: 10.1002/rcs.452. [DOI] [PubMed] [Google Scholar]
- 24.Kaczmarek BF, Sukumar S, Kumar RK, Desa N, Jost K, Diaz M, et al. Comparison of robotic and laparoscopic ultrasound probes for robotic partial nephrectomy. J Endourol. 2013;27(9):1137–1140. doi: 10.1089/end.2012.0528. [DOI] [PubMed] [Google Scholar]
- 25.Gunelli R, Fiori M, Salaris C, Salomone U, Urbinati M, Vici A, et al. The role of intraoperative ultrasound in small renal mass robotic enucleation. Arch Ital Urol Androl. 2016;88(4):311–313. doi: 10.4081/aiua.2016.4.311. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Ouyang W, Wu B, Pokhrel G, Ding B, Xu H, et al. Robot-assisted partial nephrectomy with a standard laparoscopic ultrasound probe in treating endophytic renal tumor. Asian J Surg. 2020;43(2):423–427. doi: 10.1016/j.asjsur.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z, Wang Z, Zheng Z, Cao J, Zhang C, He Z, et al. An ‘alternative finger’ in robotic-assisted thoracic surgery: intraoperative ultrasound localization of pulmonary nodules. Med Ultrason. 2017;19(4):374–379. doi: 10.11152/mu-1053. [DOI] [PubMed] [Google Scholar]
- 28.Maertens V, Stefan S, Mykoniatis I, Siddiqi N, David G, Khan JS. Robotic CME in obese patients: advantage of robotic ultrasound scan for vascular dissection. J Robot Surg. 2023;17(1):155–161. doi: 10.1007/s11701-022-01398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang CC, Wu JL, Hsiao JR, Lin CY. Real-time, intraoperative, ultrasound-assisted transoral robotic surgery for obstructive sleep apnea. Laryngoscope. 2021;131(4):E1383–E1390. doi: 10.1002/lio2.615. [DOI] [PubMed] [Google Scholar]
- 30.Jamal KN, Smith H, Ratnasingham K, Siddiqui MR, McLachlan G, Belgaumkar AP. Meta-analysis of the diagnostic accuracy of laparoscopic ultrasonography and intraoperative cholangiography in detection of common bile duct stones. Ann R Coll Surg Engl. 2016;98(4):244–249. doi: 10.1308/rcsann.2016.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angelini L, Papaspyropoulos V. Robotics and telecommunication systems to provide better access to ultrasound expertise in the OR. Minim Invas Ther Allied Technol. 2000;9(3–4):219–224. doi: 10.1080/13645700009169651. [DOI] [PubMed] [Google Scholar]
- 32.Avesani G, Tran HE, Cammarata G, Botta F, Raimondi S, Russo L, et al. CT-based radiomics and deep learning for BRCA mutation and progression-free survival prediction in ovarian cancer using a multicentric dataset. Cancers (Basel) 2022;14(11):2739. doi: 10.3390/cancers14112739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sena G, Paglione D, Gallo G, Goglia M, Osso M, Nardo B. Surgical resection of a recurrent hepatocellular carcinoma with portal vein thrombosis: is it a good treatment option? A case report and systematic review of the literature. J Clin Med. 2022;11(18):5287. doi: 10.3390/jcm11185287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlegel JU, Diggdon P, Cuellar J. The use of ultrasound for localizing renal calculi. J Urol. 1961;86(4):367–369. doi: 10.1016/S0022-5347(17)65180-2. [DOI] [PubMed] [Google Scholar]
- 35.Jung EM, Clevert DA. Contrast-enhanced ultrasound (CEUS) and image fusion for procedures of liver interventions. Radiologe. 2018;58(6):538–544. doi: 10.1007/s00117-018-0411-7. [DOI] [PubMed] [Google Scholar]
- 36.Torzilli G. Contrast-enhanced intraoperative ultrasonography in surgery for liver tumors. Eur J Radiol. 2004;51(Suppl):S25–29. doi: 10.1016/j.ejrad.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Dietrich CF, Braden B, Burmeister S, Aabakken L, Arciadacono PG, Bhutani MS, et al. How to perform EUS-guided biliary drainage. Endosc Ultrasound. 2022;11(5):342–354. doi: 10.4103/EUS-D-21-00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leven J, Burschka D, Kumar R, Zhang G, Blumenkranz S, Dai XD, et al. DaVinci canvas: a telerobotic surgical system with integrated, robot-assisted, laparoscopic ultrasound capability. Med Image Comput Comput Assist Interv. 2005;8(Pt 1):811–818. doi: 10.1007/11566465_100. [DOI] [PubMed] [Google Scholar]
- 39.Knight PR, Newell JA. Operative use of ultrasonics in Cholelithiasis. Lancet. 1963;281(7289):1023–1025. doi: 10.1016/S0140-6736(63)92427-9. [DOI] [PubMed] [Google Scholar]
- 40.Sigel B, Coelho JC, Spigos DG, Donahue PE, Renigers SA, Capek V, et al. Real-time ultrasonography during biliary surgery. Radiology. 1980;137(2):531–533. doi: 10.1148/radiology.137.2.7433687. [DOI] [PubMed] [Google Scholar]
- 41.Fukuda M. Studies on echolaparoscopy. Scan J Gastroenterol. 1982;17(Suppl 78):186. [Google Scholar]
- 42.Jakimowicz JJ, Ruers TJM. Ultrasound-assisted laparoscopic cholecystectomy: preliminary experience. Dig Surg. 2008;8(2):114–117. doi: 10.1159/000172013. [DOI] [Google Scholar]
- 43.Pavone M, Goglia M, Campolo F, Scambia G, Ianieri MM. En-block butterfly excision of posterior compartment deep endometriosis: the first experience with the new surgical robot Hugo™ RAS. Facts Views Vis Obgyn. 2023;15(4):359–362. doi: 10.52054/FVVO.14.5.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Blasis I, Tortorella L, Macchi C, Arciuolo D, Scambia G, Testa AC. Intraoperative ultrasound diagnosis of metastatic lymph node in serous borderline ovarian tumor. Ultrasound Obstet Gynecol. 2019;54(4):562–563. doi: 10.1002/uog.20234. [DOI] [PubMed] [Google Scholar]
- 45.Mascilini F, Quagliozzi L, Moro F, Moruzzi MC, Gallotta V, Alletti SG, et al. Role of intraoperative ultrasound to extend the application of minimally invasive surgery for treatment of recurrent gynecologic cancer. J Minim Invas Gynecol. 2018;25(5):848–854. doi: 10.1016/j.jmig.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 46.Mascilini F, Quagliozzi L, Bolomini G, Scambia G, Testa AC, Fagotti A. Intraoperative ultrasound through laparoscopic probe in fertility-sparing surgery for borderline ovarian tumor recurrence. Ultrasound Obstet Gynecol. 2019;54(2):280–282. doi: 10.1002/uog.20138. [DOI] [PubMed] [Google Scholar]
- 47.Moro F, Uccella S, Testa AC, Scambia G, Fagotti A. Intraoperative ultrasound-guided excision of cardiophrenic lymph nodes in an advanced ovarian cancer patient. Int J Gynecol Cancer. 2018;28(9):1672–1675. doi: 10.1097/IGC.0000000000001363. [DOI] [PubMed] [Google Scholar]
- 48.Marchetti C, Rosati A, De Felice F, Boccia SM, Vertechy L, Pavone M, et al. Optimizing the number of cycles of neoadjuvant chemotherapy in advanced epithelial ovarian carcinoma: a propensity-score matching analysis. Gynecol Oncol. 2021;163(1):29–35. doi: 10.1016/j.ygyno.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 49.Jones BP, Saso S, Farren J, El-Bahrawy M, Ghaem-Maghami S, Smith JR, et al. Ultrasound-guided laparoscopic ovarian wedge resection in recurrent serous borderline ovarian tumours. Int J Gynecol Cancer. 2017;27(9):1813–1818. doi: 10.1097/IGC.0000000000001096. [DOI] [PubMed] [Google Scholar]
- 50.Ferrucci M, Milardi F, Passeri D, Mpungu LF, Francavilla A, Cagol M, et al. Intraoperative ultrasound-guided conserving surgery for breast cancer: no more time for blind surgery. Ann Surg Oncol. 2023;30(10):6201–6214. doi: 10.1245/s10434-023-13900-x. [DOI] [PubMed] [Google Scholar]
- 51.Juvekar P, Torio E, Bi WL, Bastos DCDA, Golby AJ, Frisken SF. Mapping resection progress by tool-tip tracking during brain tumor surgery for real-time estimation of residual tumor. Cancers (Basel) 2023;15(3):825. doi: 10.3390/cancers15030825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mascagni P, Padoy N. OR black box and surgical control tower: recording and streaming data and analytics to improve surgical care. J Visc Surg. 2021;158(3S):S18–25. doi: 10.1016/j.jviscsurg.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Izzetti R, Vitali S, Aringhieri G, Nisi M, Oranges T, Dini V, et al. Ultra-high frequency ultrasound, a promising diagnostic technique: review of the literature and single-center experience. Can Assoc Radiol J. 2021;72(3):418–431. doi: 10.1177/0846537120940684. [DOI] [PubMed] [Google Scholar]
- 54.Yang H, Zhang S, Liu P, Cheng L, Tong F, Liu H, et al. Use of high-resolution full-field optical coherence tomography and dynamic cell imaging for rapid intraoperative diagnosis during breast cancer surgery. Cancer. 2020;126(S16):3847–3856. doi: 10.1002/cncr.32838. [DOI] [PubMed] [Google Scholar]
- 55.Pavone M, Spiridon IA, Lecointre L, Seeliger B, Scambia G, Venkatasamy A, et al. Full-field optical coherence tomography imaging for intraoperative microscopic extemporaneous lymph node assessment. Int J Gynecol Cancer. 2023 doi: 10.1136/ijgc-2023-005050. [DOI] [PubMed] [Google Scholar]
- 56.Seeliger B, Spiridon IA (2023) Towards optimisation in surgical pathology–the potential of artificial intelligence. BJS Academy. 10.58974/bjss/azbc011
- 57.Petter Frühling MD, Seeliger B, Rivera AKU, Freedman J, Giménez M, Digests HPB. Image-guided ablation for liver tumours–an addition to the armamentarium of multidisciplinary oncological and surgical approaches. HPB. 2023 doi: 10.58974/bjss/azbc025. [DOI] [Google Scholar]
- 58.Ho C, Calderon-Delgado M, Chan C, Lin M, Tjiu J, Huang S, et al. Detecting mouse squamous cell carcinoma from submicron full-field optical coherence tomography images by deep learning. J Biophotonics. 2021;14(1):e202000271. doi: 10.1002/jbio.202000271. [DOI] [PubMed] [Google Scholar]
- 59.Mandache D, Dalimier E, Durkin JR, Boceara C, Olivo-Marin JC, Meas-Yedid V (2018) Basal cell carcinoma detection in full field OCT images using convolutional neural networks. In: 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), pp 784–787. https://ieeexplore.ieee.org/abstract/document/8363689
- 60.Scholler J, Mandache D, Mathieu MC, Lakhdar AB, Darche M, Monfort T, et al. Automatic diagnosis and classification of breast surgical samples with dynamic full-field OCT and machine learning. J Med Imaging (Bellingham) 2023;10(3):034504. doi: 10.1117/1.JMI.10.3.034504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seeliger B, Karagyris A, Mutter D (2023) The role of artificial intelligence in diagnostic medical imaging and next steps for guiding surgical procedures. BJS Academy. https://www.bjsacademy.com/the-role-of-artificial-intelligence-in-diagnostic-medical-imaging-and-next-steps-for-guiding-surgical-procedures. Accessed 17 Nov 2023
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed in this review are included in this article and/or its figures. Further enquiries can be directed to the corresponding author.