Abstract
The current restrictive criteria for gasotransmitters exclude oxygen (O2) as a gasotransmitter in vertebrates. In this manuscript, I propose a revision of gasotransmitter criteria to include O2 per se as a signaling molecule and 'essential gasotransmitter' for vertebrates. This revision would enable us to search for protein‐based O2‐binding sensors (gasoreceptors) in all cells in the brain or other tissues rather than specialized tissues such as the carotid body or gills. If microorganisms have protein‐based O2‐binding sensors or gasoreceptors such as DosP or FixL or FNR with diverse signaling domains, then eukaryotic cells must also have O2‐binding sensors or gasoreceptors. Just as there are protein‐based receptor(s) for nitric oxide (GUCY1A, GUCY1B, CLOCK, NR1D2) in cells of diverse tissues, it is reasonable to consider that there are protein‐based receptors for O2 in cells of diverse tissues as well. In mammals, O2 must be acting as a gasotransmitter or gaseous signaling molecule via protein‐based gasoreceptors such as androglobin that very likely mediate acute sensing of O2. Accepting O2 as an essential gasotransmitter will enable us to search for gasoreceptors not only for O2 but also for other nonessential gasotransmitters such as hydrogen sulfide, ammonia, methane, and ethylene. It will also allow us to investigate the role of environment‐derived metal ions in acute gas (or solute) sensing within and between organisms. Finally, accepting O2 per se as a signaling molecule acting via gasoreceptors will open up the field of gasocrinology.
Keywords: essential gasotransmitter, gasocrine, gasoreceptor, gasocrinology
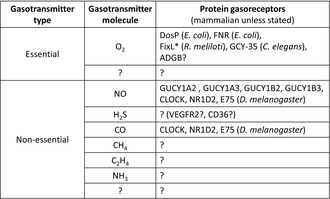
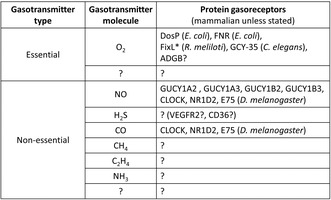
A revision to gasotransmitter classification has been proposed to include oxygen as an 'essential gasotransmitter'. The revision may enable the investigation of the identity and role of protein‐based gas (solute)‐sensing gasoreceptors in all the cells. Few examples of gasotransmitters and their counterpart gasoreceptors are listed.
In biochemistry textbooks, amino acids that are derived from the environment and that cannot be synthesized by cells are classified as essential amino acids. 1 However, according to current criteria for gasotransmitters, oxygen (O2) is excluded as one of the gasotransmitters and is not even mentioned as a potential candidate for gasotransmitters. 2 , 3 Such a restrictive criterion has not been applied for the classification of essential amino acids. If we were to apply the same restrictive criterion used for gasotransmitters to amino acids, essential amino acids might not even be considered amino acids. They might instead be referred to by alternative names, such as “small amino molecules.” I propose that for any organism whose cellular physiology, signaling, metabolism, or behavior requires gasotransmitters that they do not synthesize, such gasotransmitters must be considered as “essential gasotransmitters” or “essential gaseous signaling molecules” for those organisms. This approach would allow us to consider and investigate the role of O2 and other environment‐only‐derived gases as essential gasotransmitters or signaling molecules. 2 , 3
Another ongoing debate revolves around the general applicability of the term “gasotransmitters.” 4 , 5 In my opinion, the use of different terms such as “gasotransmitters” (gaseous transmitter) or “gaseous signaling molecules” tends to divide researchers rather than unite them. Ultimately, if there is a receptor involved, whether it is considered a transmitter or signaling molecule becomes less relevant. Therefore, it may be beneficial to agree on a unifying terminology such as “receptor” or “gasoreceptor” for gas‐ or gasotransmitter‐sensing proteins that directly interact with gas (or solute). 6 Proteins whose structures can be altered depending on the interaction state with gasotransmitters or gaseous signaling molecules (directly or via cofactors such as heme or iron–sulfur cluster or metal ions) and trigger a cellular signaling event via its additional domains (e.g., histidine kinase or phosphodiesterase or guanylate cyclase or DNA binding or RNA binding or protease) are very likely gasoreceptors. 6 , 7 , 8 An example of such structural changes is the reported nitric oxide (NO)–cysteine interaction or the repositioning of the β H‐NOX (heme nitric oxide/oxygen) protein domain, which can regulate soluble guanylate cyclase activity, an NO receptor or gasoreceptor. 9 , 10 For instance, oxytocin is a neuropeptide‐based neurotransmitter. 11 Nevertheless, we have a unified terminology for oxytocin‐sensing protein, which is a G‐protein‐coupled receptor, commonly referred to as an “oxytocin receptor.” Both the neurotransmitter research community and the endocrinology research community consistently refer to the oxytocin‐sensing protein as an oxytocin receptor. Even in nonvertebrate organisms, oxytocin orthologue–sensing proteins are referred to as receptors. 12 We don’t see the neurotransmitter community referring to them as sensors and the endocrinology community referring to them as receptors. This unity has led to a comprehensive understanding of the identity and the role of oxytocin receptors and their orthologues in both non‐mammalian and mammalian model organisms. 13 However, in the gasotransmitter versus gas‐sensing research community, it appears that this unity is lacking. 7 , 14 The gasotransmitter community refers to the NO‐sensing protein‐soluble guanylate cyclase as a “receptor,” whereas soluble guanylate cyclase involved in O2 sensing in Caenorhabditis elegans is referred to as “O2 sensor.” 14 , 15 We could argue whether the lack of unity is an issue and whether it makes any difference to call a gas‐sensing protein a sensor or a receptor. In my opinion, it matters, especially if it can unite researchers across diverse research fields, as the implications are about not only human health but also the loss of valuable resources due to incomplete scientific knowledge propagated by partial and biased scientific manuscripts that largely ignores knowledge from research on microorganisms. For instance, if there is a protein‐based receptor for NO in cells, then there must also be a protein‐based receptor for O2 in cells. However, the majority of recent scientific literature on O2‐sensing mechanisms (on plants or mammals), including notable announcements such as the 2019 Nobel Prize award in the field of physiology or medicine, does not mention a protein‐based receptor for O2. 8 , 16 , 17 , 18 , 19 , 20 This scenario reminds me of Plato’s allegory of the cave, and we are still tied down by the weight and prestige of such awards and scientific journals. If bacteria have O2‐sensing protein receptors such as DosP (direct sensor of O2, an O2‐binding heme‐based phosphodiesterase) or FixL* (truncated sensor protein FixL, an O2‐binding heme‐based kinase), or FNR (fumarate and nitrate reductase, an O2‐binding iron–sulfur cluster‐based transcriptional activator), then it is very likely that other organisms also possess O2‐binding protein receptors with diverse signaling domains and/or DNA‐binding transcriptional factors. 21 , 22 , 23
It is important to explicitly mention “protein‐based receptors or gasoreceptors” for O2 in literature reviews to enable the addition of O2‐binding protein‐based receptors in Wikipedia page on O2 sensing. This is essential for raising awareness about the role of O2 receptors, not only among scientists but also among students who switch to nonlibrary‐based sources of scientific information. 24 In both developed and developing countries, assignments to students are increasingly being completed using AI‐based tools like ChatGPT, which also depend on information from Wikipedia pages. 25 Failing to acknowledge protein‐based O2‐sensing receptors in literature reviews delays not only the dissemination of knowledge but also progression of the oxygen‐sensing research field in vertebrates and plants. 25
Another issue is the overlooked role of O2 per se as a signaling molecule in vertebrates. 26 , 27 The majority of the O2‐based developmental and disease animal model studies focus on aerobic respiration, hypoxia, ROS (reactive oxygen species)–induced oxidative stress, or ROS as a signaling molecule. 17 , 28 , 29 , 30 However, O2 per se also acts as a signaling molecule, as evidenced by the presence of O2‐sensing protein gasoreceptors with diverse functions in various organisms. 5 , 6 , 8 , 18 Despite evidence suggesting the signaling role of O2, its lack of explicit classification as a gasotransmitter or even as a candidate gasotransmitter is perplexing. 2 , 3 This ambiguity hinders the challenge, validation, refutation, or further study of O2's role in gasocrine signaling. 6 “A gasocrine signaling occurs when a gasotransmitter or gaseous signaling molecule can bind to a protein‐based gasoreceptor (or sensor protein or chemoreceptor protein) in its molecular state (or as solute) and trigger a cellular signal or response.” 6
A systematic investigation of O2 as a signaling molecule will facilitate the search for the identity and role of all O2 gasoreceptors, similar to the research that identified soluble guanylate cyclase as one of the receptor for the mammalian nonessential gasotransmitter, NO. 9 In eukaryotic organisms, due to the importance of tightly regulated extracellular and intracellular O2 levels, gasoreceptors for O2 are likely expressed in nearly every cell that O2 can diffuse into rather than being restricted to specialized tissues such as the carotid body. 31 , 32 In my opinion, O2‐binding proteins such as androglobin, known as a spermatogenesis‐inducing factor, and whose expression appears not to be affected by hypoxia, are among the candidate gasoreceptors for O2. 33 , 34 I also wonder if cytochrome C oxidase could serve as an O2 gasoreceptor, considering cytochrome C oxidase assembly appears to be regulated by O2 in yeast and isolated mitochondria. 35 This mechanism is reminiscent of the role of O2 in FNR activity, a bacterial O2 gasoreceptor or sensor, which is regulated by dimerization states due to direct O2 binding. 22
If we accept gasoreceptors for O2 or O2 as an essential gasotransmitter, then we must also reconsider gasoreceptor‐focused experiments where O2 has not been excluded as a ligand (Table 1). This includes experiments conducted primarily under conditions that did not test the effect of O2. 36 , 37 For instance, NO/CO gasoreceptor‐based circadian regulators such as Drosophila E75 (ecdysone‐induced protein 75) and mammalian CLOCK (Clock Circadian Regulator) do not appear to function as O2 gasoreceptor. 38 , 39 , 40 However, it remains unclear whether NO/CO gasoreceptor NR1D2 (nuclear receptor subfamily 1, group D, member 2, also known as REV‐ERBβ) acts as a gasoreceptor for O2 or not (personal communication with Stephen W. Ragsdale, University of Michigan, USA and Keith Pardee, University of Toronto, Canada). 36 , 37 Accepting O2 as an essential gasotransmitter or essential gaseous signaling molecule and recognizing O2‐binding sensor proteins as protein gasoreceptors would unite diverse researchers across different research fields. This acceptance would enable us to explore the identity and role of gasoreceptors not only for O2 but also for other gaseous signaling molecules or nonessential gasotransmitters such as H2S (hydrogen sulfide), ammonia, methane, and ethylene in animals. 36 , 37 , 41 , 42 , 43 , 44 , 45 , 46 This approach would also facilitate consideration of the ethical implications of engineering cow's microbiome to produce altered levels of methane without a full understanding of the identity and the role of methane gasoreceptors. 47 Investigating the identity and role of gasoreceptors in gasocrine signaling will open up the field of gasocrinology, which encompasses not only gasocrine interactions within organisms but also between different organisms and/or man‐made machine‐derived gases. 48 , 49 It may also allow us to better understand the fundamental mechanisms and general principles underlying disease ontogeny, animal behavior, and to develop better drugs, or to better understand the reasons why drugs fail in clinical trials, or why cells would require environment‐derived metal ions to sense gases, or if there are gases that can be sensed without the need for metal ions. Additionally, it could help us appreciate the need for animal model‐based curiosity‐driven basic science research to understand the role of gasocrine signaling in development, behavior, and disease ontology. 50 Finally, if O2 is a gasocrine signaling molecule between organisms, then it is essential to identify all the factors that can intefere with O2‐mediated gasocrine signaling. 51 It is also essential to identify and investigate the role of all the other gasocrine signaling molecules within and between organisms acting via protein gasoreceptors.
TABLE 1.
List of essential and non‐essential gasotransmitters and protein gasoreceptors that can sense such signaling molecules and trigger a cellular response.
AUTHOR CONTRIBUTIONS
Savani Anbalagan: conceptualization, writing of the original draft, and review and editing of the manuscript.
FUNDING INFORMATION
The author was supported by grants from the National Science Centre (SONATA‐BIS 2020/38/E/NZ3/00090 and SONATA 2021/43/D/NZ3/01798). The funding agency and the institution the author is affiliated with were not involved in the contents of the manuscript. The author thank the Institute of Molecular Biology and Biotechnology and the Faculty of Biology at the Adam Mickiewicz University, Poznań for their unconditional support.
CONFLICT OF INTEREST STATEMENT
The author is the creator of the terms and concepts of 'gasoreceptor', 'gasocrine signaling' and 'gasocrinology'.
DISCLOSURES
The author employed ChatGPT for correcting the scientific English. The author takes full responsibility for the content of this manuscript.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
The author thanks Zofia Szweykowska‐Kulinska (Institute of Molecular Biology and Biotechnology, Faculty of Biology, Adam Mickiewicz University, Poznan, Poland) for allowing him to attend her inspiring lectures on molecular evolution. The author also thanks Agnieszka Chacinska (past affiliation: Centre of New Technologies, University of Warsaw, Regenerative Mechanisms for Health—International Research Agendas Programme; current affiliation: International Institute of Molecular Machines and Mechanisms, Polish Academy of Science, Warsaw, Poland) for suggesting him to attend the 44th FEBS Congress meeting. The author also thanks José López Barneo (University of Seville, Spain) and James Imlay (University of Illinois, Urbana‐Champaign, Illinois, USA) for active e‐discussions on oxygen sensing.
Anbalagan S. Oxygen is an essential gasotransmitter directly sensed via protein gasoreceptors. Anim Models Exp Med. 2024;7:189‐193. doi: 10.1002/ame2.12400
REFERENCES
- 1. Berg JM, Stryer L, Tymoczko JL, Gatto GJ. Biochemistry. Macmillan Learning; 2015. [Google Scholar]
- 2. Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792‐1798. doi: 10.1096/fj.02-0211hyp [DOI] [PubMed] [Google Scholar]
- 3. Wang R. Gasotransmitters: growing pains and joys. Trends Biochem Sci. 2014;39:227‐232. doi: 10.1016/j.tibs.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 4. Wareham LK, Southam HM, Poole RK. Do nitric oxide, carbon monoxide and hydrogen sulfide really qualify as “gasotransmitters” in bacteria? Biochem Soc Trans. 2018;46:1107‐1118. doi: 10.1042/BST20170311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fukuto JM, Carrington SJ, Tantillo DJ, et al. Small molecule signaling agents: the integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem Res Toxicol. 2012;25:769‐793. doi: 10.1021/tx2005234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anbalagan S. Heme‐based oxygen gasoreceptors. Am J Physiol Endocrinol Metab. 2024;326:E178‐E181. doi: 10.1152/ajpendo.00004.2024 [DOI] [PubMed] [Google Scholar]
- 7. Hou S, Freitas T, Larsen RW, et al. Globin‐coupled sensors: a class of heme‐containing sensors in archaea and bacteria. Proc Natl Acad Sci. 2001;98:9353‐9358. doi: 10.1073/pnas.161185598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Lima TM, Nery LEM, Maciel FE, Ngo‐Vu H, Kozma MT, Derby CD. Oxygen sensing in crustaceans: functions and mechanisms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2021;207:1‐15. doi: 10.1007/s00359-020-01457-z [DOI] [PubMed] [Google Scholar]
- 9. Horst BG, Yokom AL, Rosenberg DJ, et al. Allosteric activation of the nitric oxide receptor soluble guanylate cyclase mapped by cryo‐electron microscopy. eLife. 2019;8:e50634. doi: 10.7554/eLife.50634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernhoff NB, Derbyshire ER, Marletta MA. A nitric oxide/cysteine interaction mediates the activation of soluble guanylate cyclase. Proc Natl Acad Sci USA. 2009;106:21602‐21607. doi: 10.1073/pnas.0911083106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leng G, Leng RI. Oxytocin: a citation network analysis of 10 000 papers. J Neuroendocrinol. 2021;33:e13014. doi: 10.1111/jne.13014 [DOI] [PubMed] [Google Scholar]
- 12. Koehbach J, Stockner T, Bergmayr C, Muttenthaler M, Gruber CW. Insights into the molecular evolution of oxytocin receptor ligand binding. Biochem Soc Trans. 2013;41:197‐204. doi: 10.1042/BST20120256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leng G, Leng RI, Ludwig M. Oxytocin—a social peptide? Deconstructing the evidence. Philos Trans R Soc Lond Ser B Biol Sci. 2022;377:20210055. doi: 10.1098/rstb.2021.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ignarro LJ, Freeman B. Nitric Oxide: Biology and Pathobiology. Academic Press; 2017. [Google Scholar]
- 15. Gray JM, Karow DS, Lu H, et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317‐322. doi: 10.1038/nature02714 [DOI] [PubMed] [Google Scholar]
- 16. Batie M, Fasanya T, Kenneth NS, Rocha S. Oxygen‐regulated post‐translation modifications as master signalling pathway in cells. EMBO Rep. 2023;24:e57849. doi: 10.15252/embr.202357849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson JW, Shakir D, Batie M, Frost M, Rocha S. Oxygen‐sensing mechanisms in cells. FEBS J. 2020;287:3888‐3906. doi: 10.1111/febs.15374 [DOI] [PubMed] [Google Scholar]
- 18. Weits DA, van Dongen JT, Licausi F. Molecular oxygen as a signaling component in plant development. New Phytol. 2021;229:24‐35. doi: 10.1111/nph.16424 [DOI] [PubMed] [Google Scholar]
- 19. Jiang Y, Duan L‐J, Fong G‐H. Oxygen‐sensing mechanisms in development and tissue repair. Development. 2021;148:dev200030. doi: 10.1242/dev.200030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The Nobel Prize in Physiology or Medicine 2019 NobelPrize.org. https://www.nobelprize.org/prizes/medicine/2019/summary/
- 21. Delgado‐Nixon VM, Gonzalez G, Gilles‐Gonzalez MA. Dos, a heme‐binding PAS protein from Escherichia coli, is a direct oxygen sensor. Biochemistry. 2000;39:2685‐2691. doi: 10.1021/bi991911s [DOI] [PubMed] [Google Scholar]
- 22. Jervis AJ, Crack JC, White G, et al. The O2 sensitivity of the transcription factor FNR is controlled by Ser24 modulating the kinetics of [4Fe‐4S] to [2Fe‐2S] conversion. Proc Natl Acad Sci USA. 2009;106:4659‐4664. doi: 10.1073/pnas.0804943106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Monson EK, Weinstein M, Ditta GS, Helinski DR. The FixL protein of Rhizobium meliloti can be separated into a heme‐binding oxygen‐sensing domain and a functional C‐terminal kinase domain. Proc Natl Acad Sci USA. 1992;89:4280‐4284. doi: 10.1073/pnas.89.10.4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adetayo AJ. Post Covid‐19 pandemic and library users' education: impact on examination and survey. J Acad Librariansh. 2023;49:102695. doi: 10.1016/j.acalib.2023.102695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Memarian B, Doleck T. ChatGPT in education: methods, potentials, and limitations. Comput Human Behav Artif Hum. 2023;1:100022. doi: 10.1016/j.chbah.2023.100022 [DOI] [Google Scholar]
- 26. Zhu H, Bunn HF. Oxygen sensing and signaling: impact on the regulation of physiologically important genes. Respir Physiol. 1999;115:239‐247. doi: 10.1016/s0034-5687(99)00024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsia CCW, Schmitz A, Lambertz M, Perry SF, Maina JN. Evolution of air breathing: oxygen homeostasis and the transitions from water to land and sky. Compr Physiol. 2013;3:849‐915. doi: 10.1002/cphy.c120003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363‐383. doi: 10.1038/s41580-020-0230-3 [DOI] [PubMed] [Google Scholar]
- 29. Sies H, Belousov VV, Chandel NS, et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol. 2022;23:499‐515. doi: 10.1038/s41580-022-00456-z [DOI] [PubMed] [Google Scholar]
- 30. Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453‐R462. doi: 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao L, Ortega‐Sáenz P, Moreno‐Domínguez A, López‐Barneo J. Mitochondrial redox signaling in O2‐sensing chemoreceptor cells. Antioxid Redox Signal. 2022;37:274‐289. doi: 10.1089/ars.2021.0255 [DOI] [PubMed] [Google Scholar]
- 32. Hu H, Sosnovsky G, Swartz HM. Simultaneous measurements of the intra‐ and extra‐cellular oxygen concentration in viable cells. Biochim Biophys Acta Biomembr. 1992;1112:161‐166. doi: 10.1016/0005-2736(92)90387-2 [DOI] [PubMed] [Google Scholar]
- 33. Hoogewijs D, Ebner B, Germani F, et al. Androglobin: a chimeric globin in metazoans that is preferentially expressed in mammalian testes. Mol Biol Evol. 2012;29:1105‐1114. doi: 10.1093/molbev/msr246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keppner A, Correia M, Santambrogio S, et al. Androglobin, a chimeric mammalian globin, is required for male fertility. eLife. 2022;11:e72374. doi: 10.7554/eLife.72374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Woodrow G, Schatz G. The role of oxygen in the biosynthesis of cytochrome c oxidase of yeast mitochondria. J Biol Chem. 1979;254:6088‐6093. doi: 10.1016/S0021-9258(18)50522-6 [DOI] [PubMed] [Google Scholar]
- 36. Pardee KI, Xu X, Reinking J, et al. The structural basis of gas‐responsive transcription by the human nuclear hormone receptor REV‐ERBbeta. PLoS Biol. 2009;7:e43. doi: 10.1371/journal.pbio.1000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sarkar A, Carter EL, Harland JB, Speelman AL, Lehnert N, Ragsdale SW. Ferric heme as a CO/NO sensor in the nuclear receptor Rev‐Erbß by coupling gas binding to electron transfer. Proc Natl Acad Sci USA. 2021;118:e2016717118. doi: 10.1073/pnas.2016717118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reinking J, Lam MMS, Pardee K, et al. The Drosophila nuclear receptor E75 contains heme and is gas responsive. Cell. 2005;122:195‐207. doi: 10.1016/j.cell.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 39. Freeman SL, Kwon H, Portolano N, et al. Heme binding to human CLOCK affects interactions with the E‐box. Proc Natl Acad Sci. 2019;116:19911‐19916. doi: 10.1073/pnas.1905216116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lukat‐Rodgers GS, Correia C, Botuyan MV, Mer G, Rodgers KR. Heme‐based sensing by the mammalian circadian protein CLOCK. Inorg Chem. 2010;49:6349‐6365. doi: 10.1021/ic902388q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bond JH, Engel RR, Levitt MD. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane‐producing colonic bacteria. J Exp Med. 1971;133:572‐588. doi: 10.1084/jem.133.3.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ernst L, Steinfeld B, Barayeu U, et al. Methane formation driven by reactive oxygen species across all living organisms. Nature. 2022;603:482‐487. doi: 10.1038/s41586-022-04511-9 [DOI] [PubMed] [Google Scholar]
- 43. Lieberman M, Hochstein P. Ethylene formation in rat liver microsomes. Science. 1966;152:213‐214. doi: 10.1126/science.152.3719.213 [DOI] [PubMed] [Google Scholar]
- 44. Paardekooper LM, van den Bogaart G, Kox M, et al. Ethylene, an early marker of systemic inflammation in humans. Sci Rep. 2017;7:6889. doi: 10.1038/s41598-017-05930-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aono S. Gas Sensing in Cells. Royal Society of Chemistry; 2017. [Google Scholar]
- 46. Ganesh I, Gwon D‐A, Lee JW. Gas‐sensing transcriptional regulators. Biotechnol J. 2020;15:e1900345. doi: 10.1002/biot.201900345 [DOI] [PubMed] [Google Scholar]
- 47. Kahn J. Crispr pioneer Jennifer Doudna has the guts to take on the microbiome. Wired; 2023. https://www.wired.com/story/crispr‐jennifer‐doudna‐microbiome/ [Google Scholar]
- 48. Anbalagan S. Gasocrinology. Zenodo. 2024. doi: 10.5281/zenodo.10668718 [DOI] [Google Scholar]
- 49. Anbalagan S. Impact of gasocrine signaling. OSF Preprints; 2024. 10.31219/osf.io/nwby2 [DOI] [Google Scholar]
- 50. Anbalagan S. “Blind men and an elephant”: the need for animals in research, drug safety studies, and understanding civilizational diseases. Animal Model Exp Med. 2023;6(6):627‐633. doi: 10.1002/ame2.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tetu SG, Sarker I, Schrameyer V, et al. Plastic leachates impair growth and oxygen production in prochlorococcus, the ocean's most abundant photosynthetic bacteria. Commun Biol. 2019;14(2):184. doi: 10.1038/s42003-019-0410-x [DOI] [PMC free article] [PubMed] [Google Scholar]


