Abstract
Background and Aims
Anemia has been a common comorbidity in most chronic diseases, but has not been well monitored in type 2 diabetes mellitus (T2DM) patients. In this study, we investigated the prevalence of anemia and its nexus with iron stores among T2DM patients in health facilities in the Ashanti Region of Ghana.
Methods
This multicenter cross‐sectional study recruited 213 T2DM out‐patients attending the diabetic clinics at the Kumasi South Hospital and St. Michaels Hospital, Jachie Pramso, Ghana, for routine check‐ups. Self‐reported questionnaires were used to collect sociodemographic, lifestyle, and clinical data from study participants. Blood samples were collected to estimate hematological parameters and iron stores. Mann–Whitney U test was used to assess the difference in hematological parameters and iron stores between anemic and nonanemic patients. All p < 0.05 were considered statistically significant.
Results
Of the 213 T2DM participants, the prevalence of anemia was 31.9%. More females 145 (68.1%) were registered than males 68 (31.9%). Anemic patients had significantly lower levels of mean cell volume [79.30/fL vs. 82.60/fL, p = 0.001], mean cell hemoglobin [26.60/pg vs. 27.90/pg, p < 0.0001], and mean cell hemoglobin concentration [33.10/g/dL) vs. 33.80/g/dL, p < 0.0001] than those without anemia. Serum levels of ferritin (p = 0.1140), transferrin (p = 0.5070), iron (p = 0.7950), and total iron binding capacity (p = 0.4610) did not differ significantly between T2DM patients with or without anemia.
Conclusion
Despite the high prevalence of anemia among the T2DM patients in our cohort, patients present with apparently normal iron stores. This unrecognized mild anemia must be frequently monitored among T2DM patients.
Keywords: anemia, ferritin, iron stores, total iron binding capacity, transferrin, type 2 diabetes mellitus
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM), one of the major diseases of the 21st century, is reported to affect about 463 million of the world's population according to the International Diabetes Federation. 1 One in 11 adults presently have type 2 diabetes, and by 2040, 642 million people worldwide are estimated to have the disease. 2 19.4 million adults in Africa are thought to have undiagnosed diabetes. 3 T2DM is a complicated, heterogeneous, and multisystemic metabolic syndrome defined by chronic hyperglycemia brought on by either absolute or relative insulin resistance or both. 4 It is linked to chronic long‐term micro‐ and macro‐vascular problems, acute life‐threatening metabolic abnormalities, and hematological dyscrasias such as anemia. 5 , 6 Many people with diabetes often experience anemia, which is a common blood‐related problem. 7 It mostly occur in people with diabetes who also have renal impairment. 8 , 9 Research findings suggest that the presence of anemia in individuals with type 2 diabetes is usually linked to the kidneys' inability to produce sufficient erythropoietin. 10 , 11
Anemia (decreased blood hemoglobin) is one of the major public health issues recently facing the world. 12 Almost 1.6 billion people are estimated to be anemic worldwide. 13 Every stage of life is affected by anemia, but children, women, and people from low‐income, resource‐limited regions of the world are disproportionately affected. Anemia is a silent disease that frequently remains misdiagnosed and unmanaged, impairing adults' physical performance and productivity. 14 Its incidence is higher in patients with T2DM. 15 Research from numerous nations has revealed that between 14% and 45% of diabetic people are anemic. 15 , 16
Anemia has long been thought to be a common and undetected comorbidity of T2DM. 17 Reduced erythropoietin responsiveness has been linked to an apparent diabetes‐specific risk of anemia. 18 Furthermore, one major cause of anemia in type 2 diabetes patients is a decrease in iron stores, which can occur due to a variety of factors such as poor nutrition, inflammation, and blood loss. Iron is essential for the production of red blood cells, and a deficiency can lead to anemia. However, there are other factors, such as, oxidative stress, advanced glycation, microangiopathy, male hypogonadism, and metformin‐associated decreased serum vitamin B12 concentrations, that may be involved in the depressed erythrocyte production and accelerated destruction in diabetes. 18 These patients have normal or high serum ferritin levels but low serum transferrin saturation, which measures the amount of circulating iron. 19
Anemia can cause low glycated hemoglobin (HBA1c) values, which may lead to inadequate management of hyperglycemia, which in turn will speed up the development of both microvascular and macrovascular diabetes complications. 20 Except in cases where patients are admitted to the hospital suddenly due to complications or other unrelated incidents, anemia is not often measured at annual evaluations. Yet, it is interesting to note that a growing percentage of T2DM patients have been identified as anemic without underlying chronic kidney disease (CKD) symptoms. 21 Therefore, we investigated the prevalence of anemia and its nexus with iron stores among T2DM patients in multicenter health facilities in the Ashanti region of Ghana.
2. MATERIALS AND METHODS
2.1. Study design and sites
This was a multicenter cross‐sectional study conducted between February 2021 and December 2022 at Kumasi South Hospital, St. Michaels Hospital, Jachie Pramso, in the Ashanti region of Ghana.
These hospitals serve as referral hospitals for towns and villages surrounding the region. These hospitals have about 2000 registered Diabetes patients each and about 500 of these visit the diabetic clinic monthly for care. Some patients depending on severity of diabetes and complications, visit the hospital weekly or biweekly.
2.2. Study population, inclusion/exclusion criteria
The study included diabetic outpatients seeking care at the diabetic unit at either Kumasi South Hospital or St Michael Hospital, Jachie Pramso who had been diagnosed with T2DM at least 1 year earlier. However, the study excluded patients with known type 1 diabetes mellitus and all inpatients and finally patients who disagreed to participate in the study.
The diagnosis of T2DM was made based American Diabetes Association (ADA) criteria based on defective progressive insulin secretory on the background of insulin resistance using fasting blood sugar ≥7.0 mmol/L and glycated hemoglobin ≥6.5%. 22
2.3. Sample size estimation
The sample size was obtained using the formula; , where n = sample size, z = z‐value at confident level of 95% (1.96), P = prevalence diabetes, 6.4% 23 in urban areas in Ghana, Q = (1‐P) and d = margin of error. Substituting these parameters in the formula, . The minimum number of participants required for the study was 92. To increase statistical power and account for missing data, 213 participants were recruited for the study.
2.4. Data collection
A well‐structured questionnaire was used to collect sociodemographic, lifestyle, and clinical data from the study participants. Data obtained were cross‐verified by comparing it with the information present in the patient's case file. Sociodemographic data included age, sex, occupation, and marital status. Lifestyle characteristics included exercise, number of meals per day, stress at workplace, and alcohol intake. Clinical data included family history of DM and hypertension history as well as duration of T2DM.
2.5. Blood sample collection and biochemical assay
Six milliliters of fasting venous blood were taken aseptically from the antecubital fossa region with participants seated. Fasting blood glucose was determined immediately using a Accu‐Check Aviva Plus glucometer (Roche Diabetes care Inc) while 3 mL of the blood was dispensed into tri‐potassium ethylenediamine tetra‐acetic acid (K3‐EDTA) tube for hemoglobin (Hb), red cell indices assessments namely: mean cell volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC) using the five (5)‐part differential Sysmex FBC analyser (Sysmex Inc.); and glycated haemoglobin (HbA1c) assessment using multipurpose Finecare hormonal analyser (Wondfo Biotech Co. Ltd). The remaining 3 mL blood was dispensed into serum separator tube, centrifuged at 3000 rpm for 15 min, and stored at –80°C until laboratory analyses. Commercial kits (QuickAuto Neo Fe and QuickAuto Neo UIBC, Shino‐Test) were used to measure the levels of serum iron and unsaturated iron‐binding capacity (UIBC) respectively. Serum ferritin concentrations were measured by a chemiluminescent immunoassay method (ADVIA Centaur, Siemens). To calculate transferrin saturation, the formula 100 × iron/(total iron‐binding capacity) was used.
2.6. Definition of anemia
Women with hemoglobin (Hb) level less than 12 g/dL and men with Hb less than 13 g/dL were defined as anemic per the World Health Organization guidelines. 15
2.7. Data analysis
Data entry was done using Microsoft Excel 2016 and analysis was performed using SPSS version 26.0 (SPSS Inc.) and GraphPad Prism 8.0.1 (GraphPad LLC). Categorical data were presented as frequency (percentages). The normality of continuous variables was done using Kolmogorov–Smirnov test. Nonparametric data were presented as median (interquartile range), compared between anemia groups using Mann–Whitney U test whereas parametric data were presented as mean ± standard deviation and compared using independent t test. The χ 2 or Fischer's exact tests were used to assess the association between categorical variables and anemia. Multivariate logistic regression was used to determine the odds of association of the sociodemographic variables with anemia among the T2DM cohort. All p < 0.05 were considered statistically significant.
3. RESULTS
3.1. Sociodemographic, lifestyle, and clinical characteristics of the study participants
Table 1 displays the sociodemographic, lifestyle, and clinical characteristics of study participants.
Table 1.
Sociodemographic, lifestyle, and clinical characteristics of the study participants.
| Variable | Frequency (N = 213) | Percentage (%) |
|---|---|---|
| Age category (years) | ||
| 29–49 | 123 | 57.7 |
| 50–67 | 90 | 42.3 |
| Sex | ||
| Females | 145 | 68.1 |
| Males | 68 | 31.9 |
| Marital status | ||
| Single | 11 | 5.2 |
| Married | 139 | 65.3 |
| Widowed | 37 | 17.4 |
| Divorced | 26 | 12.2 |
| Level of education | ||
| Unschooled | 51 | 23.9 |
| Primary | 46 | 21.6 |
| Secondary | 94 | 44.1 |
| Tertiary | 22 | 10.3 |
| Occupation | ||
| Unemployed | 94 | 44.1 |
| Self‐employed | 87 | 40.8 |
| Government | 32 | 15 |
| Number of eating times/day | ||
| Two times | 53 | 24.9 |
| Three times | 156 | 73.2 |
| Four times | 4 | 1.9 |
| Exercise | ||
| No | 111 | 52.1 |
| Yes | 102 | 47.9 |
| Alcoholic beverages | 47.9 | |
| No | 168 | 78.9 |
| Yes | 45 | 21.1 |
| Stress at workplace | ||
| No | 157 | 73.7 |
| Yes | 56 | 26.3 |
| Family history of T2DM | ||
| No | 99 | 46.5 |
| Yes | 114 | 53.5 |
| Hypertension history | ||
| No | 99 | 46.5 |
| Yes | 114 | 53.5 |
| Age diagnosed with T2DM | ||
| 4–6 years | 36 | 16.9 |
| 7–10 years | 177 | 83.1 |
| Duration of T2DM | ||
| 1–3 years | 74 | 34.7 |
| 4–6 years | 57 | 26.8 |
| 7–10 years | 40 | 18.8 |
| 11 years and above | 42 | 19.7 |
| Hemoglobin (g/dL) (mean ± SD) | 12.71 ± 1.33 | |
| FBG (mmol/L) [median (IQR)] | 7.70 (6.56–10.45) | |
| HBA1c (%) [median (IQR)] | 7.00 (6.50–8.60) |
Abbreviations: FBG, fasting blood glucose; HBA1c, glycated haemoglobin; IQR, interquartile range; N, number; T2DM, type 2 diabetes mellitus.
Of the 213 participants studied, more than half (57.7%) were 29–49 years and the remaining (42.3%) were 50–67 years old. Likewise, more than half of the subjects (68.1%) were females, married (65.3%), and unemployed (54.2%). Almost one‐fifth (19.7%) had their T2DM duration lasted for more than 11 years. Concerning participants' lifestyle, more than half of the participants (52.1%) did not engage in any exercise. Majority of them consumed no alcoholic beverage (78.9%), ate three times daily (73.2%), or experienced no stress at work (73.7%). Concerning clinical characteristics, more than half of the participants had history of hypertension and a family history of T2DM (53.5% respectively). Mean hemoglobin of the study participants was 12.71 g/dL whereas the median fasting blood glucose (FBG) and glycated hemoglobin (HBA1c) were 7.7 mmol/L and 7.0% respectively.
3.2. Prevalence of anemia among the study participants
Figure 1 displays the proportion of anemia among T2DM patients. Out of 213 patients the prevalence of anemia was found to be 31.9% (n = 145).
Figure 1.
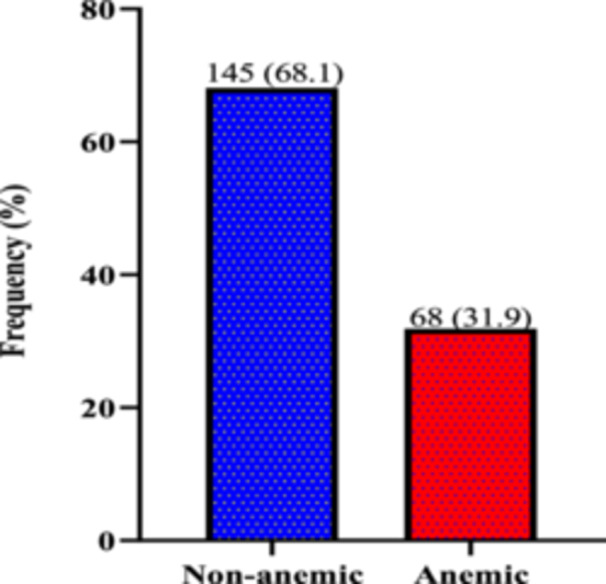
Prevalence of anemia among patients with type 2 diabetes mellitus.
3.3. Iron stores and red cell indices among type‐2 diabetes patients with or without anemia
The T2DM patients with anemia recorded significantly lower levels of mean cell volume [79.30 (75.25–84.50)/fL vs. 82.60 (79.35–86.00)/fL, p = 0.001], mean cell hemoglobin [26.60 (24.95–28.35)/pg vs. 27.90 (79.35–86.00)/pg, p < 0.0001] and mean cell hemoglobin concentration [33.10 (31.63–34.20)/g/dL) vs. 33.80 (33.00–34.40)/g/dL, p < 0.001] compared to patients without anemia. However, serum levels of ferritin (p = 0.1140), transferrin (p = 0.5070), iron (p = 0.7950), and total iron binding capacity (p = 0.4610) did not differ significantly between T2DM patients with or without anemia (Figure 2).
Figure 2.
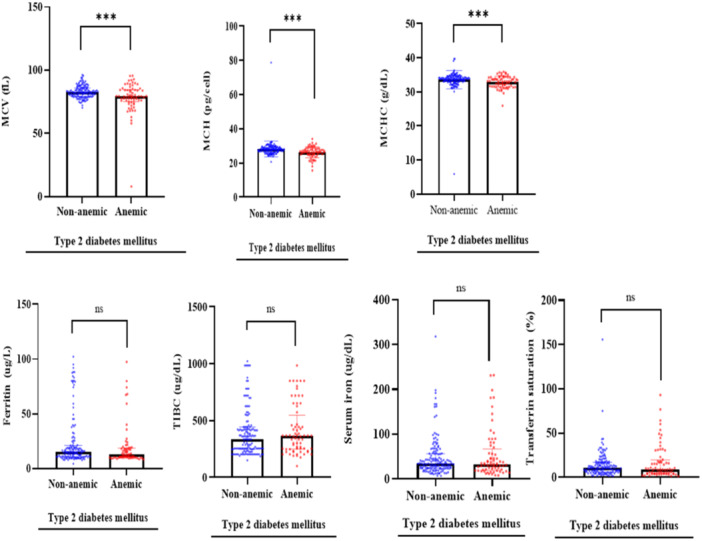
Iron stores and red cell indices among patients with type 2 diabetes mellitus.
3.4. Sociodemographic, lifestyle, and clinical characteristics associated with anemia
Table 2 displays the sociodemographic, lifestyle, and clinical characteristics associated with anemia. Out of the 68 anemic participants, 39 (57.4%) were females aged between 29 and 49 years. Furthermore, 42 of the participants (61.8%) were married, and a majority of 51 participants (75.0%) reported eating three times per day. Sex (p = 0.022) and occupation (p = 0.002) were significantly associated with anemia. However, age category, marital status, levels of education, duration of diabetes, number of eating times, eating late at night, exercising, taking in alcoholic beverages, stress at work, and age diagnosed with diabetes did not significantly differ between the two groups (anemia vs. nonanemia) (all p > 0.05).
Table 2.
Sociodemographic, lifestyle, and clinical characteristics associated with anemia.
| Variables | Nonanemic (N = 145) | Anemic (N = 68) | p Value |
|---|---|---|---|
| Age category (years) | 0.416 | ||
| 29–49 | 81 (55.9) | 42 (61.8) | |
| 50–67 | 64 (44.1) | 26 (38.2) | |
| Sex | 0.022 | ||
| Females | 106 (73.1) | 39 (57.4) | |
| Males | 39 (26.9) | 29 (42.6) | |
| Marital status | 0.859 | ||
| Single | 8 (5.5) | 3 (4.4) | |
| Married | 92 (63.4) | 47 (69.1) | |
| Widowed | 27 (18.6) | 10 (14.7) | |
| Divorced | 18 (12.4) | 8 (11.8) | |
| Level of education | 0.416 | ||
| Unschooled | 33 (22.8) | 18 (26.5) | |
| Primary | 33 (22.8) | 13 (19.1) | |
| Secondary | 67 (46.2) | 27 (39.7) | |
| Tertiary | 12 (8.3) | 10 (14.7) | |
| Occupation | 0.002 | ||
| Unemployed | 63 (43.4) | 31 (45.6) | |
| Self‐employed | 68 (46.9) | 19 (27.9) | |
| Government | 14 (9.7) | 18 (26.5) | |
| Duration of T2DM | 0.102 | ||
| 1–3 years | 57 (39.3) | 17 (25.0) | |
| 4–6 years | 38 (26.2) | 19 (27.9) | |
| 7–10 years | 27 (18.6) | 13 (19.1) | |
| 11 years and above | 23 (15.9) | 19 (27.9) | |
| Number of eating times/day | 0.903 | ||
| Two times | 37 (25.5) | 16 (23.5) | |
| Three times | 105 (72.4) | 51 (75.0) | |
| Four times | 3 (2.1) | 1 (1.5) | |
| Eating late at night | 0.084 | ||
| No | 22 (15.2) | 17 (25.0) | |
| Yes | 123 (84.8) | 51 (75.0) | |
| Exercise | 0.192 | ||
| No | 80 (55.2) | 31 (45.6) | |
| Yes | 65 (44.8) | 37 (54.4) | |
| Alcoholic beverages | 0.556 | ||
| No | 116 (80.0) | 52 (76.5) | |
| Yes | 29 (20.0) | 16 (23.5) | |
| Stress at workplace | 0.479 | ||
| No | 109 (75.2) | 48 (70.6) | |
| Yes | 36 (24.8) | 20 (29.4) | |
| Age diagnosed with diabetes | 0.171 | ||
| 4–6 years | 28 (19.3) | 8 (11.8) | |
| 7–10 years | 117 (80.7) | 60 (88.2) |
Note: Data are presented as frequency (proportion); compared using χ 2 test or Fisher's exact test. p < 0.05 was considered significant for T2DM patients with and without anemia. Bold value indicates the statistically significant p Values.
Abbreviations: N, number; T2DM, type 2 diabetes mellitus.
3.5. Sociodemographic, lifestyle, and clinical predictors of anemia among study participants
After adjusting for possible confounders in multivariate logistic regression analysis, unemployed (aOR = 0.34, 95% Cl (0.141–0.795), p = 0.013) and self‐ employed (aOR = 0.20, 95% Cl (0.081–0.504); p = 0.001) subjects had a significant 66% and 80% decreased odds of being anemic compared to government workers accordingly. However, participants who had been diagnosed with diabetes for more than 11 years (aOR = 2.605 95% Cl (1.119–6.064); p = 0.026) were 2.6 times more likely of being anemic compared to participants whose diabetes had lasted 1–3 years (Table 3).
Table 3.
Sociodemographic, lifestyle, and clinical predictors of anemia.
| Variable | cOR (95% Cl) | p Value | aOR (95% Cl) | p Value |
|---|---|---|---|---|
| Age category (years) | ||||
| 29–49 | 1.00 (Ref) | ‐ | ‐ | ‐ |
| 50–67 | 0.70 (0.332–1.462) | 0.339 | ‐ | ‐ |
| Marital status | ||||
| Single | 1.00 | ‐ | ||
| Married | 1.34 (0.206–8.686) | 0.76 | ‐ | ‐ |
| Widowed | 0.67 (0.091–4.919) | 0.692 | ‐ | ‐ |
| Divorced | 0.53 (0.063–4.533) | 0.564 | ‐ | ‐ |
| Levels of education | ||||
| Unschooled | 2.01 (0.474–8.475) | 0.344 | ‐ | ‐ |
| Primary | 0.91 (0.219–3.781) | 0.894 | ‐ | ‐ |
| Secondary | 0.97 (0.265–3.551) | 0.963 | ‐ | ‐ |
| Tertiary | 1.00 | ‐ | ‐ | ‐ |
| Occupation | ||||
| Unemployed | 0.24 (0.063–0.884) | 0.032 | 0.34 (0.141–0.795) | 0.013 |
| Self‐employed | 0.17 (0.046–0.621) | 0.007 | 0.20 (0.081–0.504) | 0.001 |
| Government | 1.00 | ‐ | ‐ | ‐ |
| Duration of T2DM | ||||
| 1–3 years | 1.00 | ‐ | ‐ | ‐ |
| 4–6 years | 2.24 (0.924–5.417) | 0.074 | 2.00 (0.892–4.430) | 0.093 |
| 7–10 years | 1.42 (0.519–3.886) | 0.494 | 1.29 (0.521–3.170) | 0.586 |
| 11 years and above | 3.96 (1.413–11.125) | 0.009 | 2.60 (1.119–6.064) | 0.026 |
| Number of eating times | ||||
| Two times | 1.00 | ‐ | ‐ | |
| Three times | 0.75 (0.062–9.075) | 0.821 | ‐ | ‐ |
| Four times | 0.80 (0.071–9.043) | 0.857 | ‐ | ‐ |
| Eating late at night | ||||
| Yes | 1.29 (0.399–4.187) | 0.668 | ‐ | ‐ |
| No | 1.00 | ‐ | ‐ | ‐ |
| Exercise | ||||
| No | 1.00 | ‐ | ‐ | ‐ |
| Yes | 1.49 (0.719–3.097) | 0.283 | ‐ | ‐ |
| Alcoholic beverages | ||||
| No | 1.00 | ‐ | ‐ | ‐ |
| Yes | 0.53 (0.179–1.556) | 0.247 | ‐ | ‐ |
| Stress at workplace | ||||
| No | 1.00 | ‐ | ‐ | ‐ |
| Yes | 2.05 (0.889–4.731) | 4.731 | ‐ | ‐ |
| Age diagnosed with diabetes | ||||
| 4–6 years | 1.00 | ‐ | ‐ | ‐ |
| 7–10 years | 0.78 (0.207–2.957) | 0.718 | ‐ | ‐ |
| Family history of diabetes | ||||
| No | 1.00 | ‐ | ‐ | ‐ |
| Yes | 0.76 (0.385–1.507) | 0.435 | ‐ | |
| Hypertension | ||||
| No | 1.00 | ‐ | ‐ | ‐ |
| Yes | 0.60 (0.279–1.266) | 0.178 | ‐ | ‐ |
Note: Compared using univariate and multivariate logistic regression; p < 0.05 was considered significant. Bold value indicates the statistically significant p Values.
Abbreviations: aOR: Adjusted odds ratio; cOR, crude odds ratio; T2DM, type 2 diabetes mellitus.
4. DISCUSSION
T2DM is increasing throughout the world and has been linked to chronic long‐term micro‐ and macro‐vascular problems and hematological dyscrasias such as anemia. Anemia (decreased blood hemoglobin), is one of the major public health issues recently facing the world. Though anemia has long been thought to be a common and undetected comorbidity in most chronic diseases, it is interesting to note that T2DM patients are mostly not monitored for anemia. 21 Thus, in this study, we investigated the prevalence of anemia and its nexus with iron stores in T2DM patients in two‐selected health facilities in Ghana.
In the current study, we reported prevalence of anemia of 31.9% among the T2DM patients, meaning 3 out of 10 T2DM adults had anemia. This prevalence is slightly higher than that reported by Salma and colleagues (29.4%), among Kuwait T2DM patients. 24 Also, Hisham and colleagues in Saudi Arabia reported a much lower prevalence (22%) of anemia in T2DM patients compared to our study. 25 According to the findings of the Solomon et al. 26 the prevalence of anemia among adult diabetes mellitus patients was 18.1%, which is much lower compared to our study. Disparities in the previous and current findings in anemia prevalence could be due to differences in sample population and ethnicity While the present study included only T2DM individuals, Solomon et al. 26 sampled both type 1 and type 2 diabetes which might have accounted for the observed disparity. In addition to this, greatly different life styles of the two populations also matters. On the other hand, the prevalence of anemia in our study is slightly lower compared with the findings of the study conducted among patients attending public hospitals in the Harari region of Ethiopia, 27 which was 34.8% and the similarity in representation might be because of the inclusion of only T2DM patients in the study. Despite these disparities presented above, all the studies indicate that the prevalence of anemia is high among T2DM patients. Knowing that anemia is associated with mortality in diabetes, anemia assessment must be frequently monitored among patients with T2DM.
Interestingly in this study, anemia was accompanied by statistically significant reduction of red cell indices. Although MCV, MCH and MCHC were reduced, they were still within the normal reference ranges suggesting that the anemia was mild and wasn't due to defective erythropoiesis such as iron deficiency anemia (IDA). 28 The reduction of red cell size and altered shape may be due to slight hyperglycemia which may increase blood tonicity and subsequent loss of fluid from red cells to the plasma.
Again, we did not observe changes in iron stores between patients with or without anemia in the current study. Serum levels of ferritin, transferrin, iron and total iron binding capacity (TIBC) did not differ significantly between T2DM patients with or without anemia. The iron stores give an overview of possible cause of anemia and alterations in their levels is usually used to predict IDA. Iron is an essential trace element in the human body and its metabolism at the cellular level and the whole systemic level are tightly regulated. Balance of iron homeostasis depends on the expression levels and activities of iron carriers, iron transporters, and iron regulatory and storage proteins. After absorption of nonheme iron from the diet and subsequent export of iron into the circulation, transferrin then carries iron to various tissues and cells. Iron enters the cell's cytoplasm after binding to transferrin receptor 1. Free iron is either utilized in metabolic processes, such as synthesis of hemoglobin and Fe–S cluster, or sequestered in the cytosolic ferritin, serving as a cellular iron store. Iron metabolism favoring adequate and steady iron is important to prevent anemia. It is known that diabetes medications such as metformin are known to alter gut absorption of iron and therefore the entire iron metabolism. 29 , 30 It is important to note that, in the current study, the serum iron stores were apparently normal and did not differ significantly between patients with or without anemia. These findings together again support our suggestions that anemia among T2DM patients is mild and not as a result of defective iron metabolism or altered erythropoiesis. The observed anemia may be due to mild crenation and shorter lifespan in hyperglycemic environment. Our findings could be confirmed by peripheral blood film comments and bone marrow hemosiderin and ferrokinetics to rule out IDA. Collectively, we show that anemia is common among T2DM patients but they have apparently normal iron stores. Our findings contradict that of Praveen and colleagues, who explored anemia in patients with T2DM without nephropathy among the Indian population. 31 They found significantly decreased serum ferritin and iron in T2DM patients with anemia compared to those without anemia. Moreover, in this same study, TIBC and serum iron were significantly reduced in patients with total iron deficiency. Possible reason for the observed disparity between the two studies could be due to sample population differences. Whiles our current study did not exclude diabetic nephropathy patients, the former study excluded these patients. This might have accounted for the observed differences in the level of significance.
This study explored the factors associated with anemia among T2DM patients. In the χ 2 analysis, we found a significant association between occupation type and anemia., In the multivariate logistic regression model, we found that participants who were unemployed and self‐employed were associated with lesser chances of being anemic compared to government workers. Similar findings have been reported by Kafiyalew et al. 32 and Kassa et al. 33 These findings may suggest that, individuals who are unemployed or self‐employed may have more flexibility in their daily routines, which could allow them to prioritize their health and adhere to a healthy diet. This could contribute to a better balance of essential nutrients, including iron, leading to a reduced chance of anemia. On the other hand, individuals with government employment often face demanding schedules and may end up eating late at home, potentially affecting the nutritional adequacy of their diets and thereby increasing their susceptibility to anemia. Additionally, individuals who are employed may have more work‐related stress, which could negatively impact their overall health and increase their risk of anemia. The findings of this study suggest that employment status may be an important factor to consider when identifying individuals at risk for anemia among patients with T2DM. However, more research is needed to fully understand the relationship between employment status and anemia and to identify potential interventions to address this issue.
In the current study, the duration of T2DM presented one of the independent risk factors associated with the presence of anemia. It was observed that compared with patients with 1–3 years duration of T2DM, the odds ratio of developing anemia in individuals with ≥11 years was approximately three times. This finding is in agreement with the previous studies in Australia, 34 Korea, 35 and India. 36 The reason for this increased chance of anemia development with an increasing duration of DM may be due to the chronic effects of hyperglycemia. Diabetes‐related chronic hyperglycemia can cause a chronic hypoxic milieu in the renal interstitium and disturbance of the interstitial organization or vascular architecture, atypical cell growth and collagen proliferation in tubular cells and peritubular fibroblasts, which cause impaired synthesis of erythropoietin by the peritubular fibroblasts. 37 In addition, in patients with prolonged hyperglycemic conditions, the erythrocyte precursors cells in the bone marrow might be exposed to prolonged direct glucose toxicity leading to disturbances in the erythrocyte production. 38
Our study had few limitations. First the study was conducted with a relatively low sample size and we could not run peripheral blood firm comments and further tests to confirm the sources or types of anemia observed. Second, the study participants were sampled within a short sampling frame which did not allow researcher include the annual finding of the study area. It is important to note that we reveal unrealized high prevalence of anemia among T2DM patients which was associated with normal iron stores. Future studies should comprehensively address oral hypoglycemic usage and consider longer sampling durations to augment our understanding of anemia in type 2 diabetics.
In conclusion, the prevalence of anemia is high among T2DM patients in our cohort. Despite this high prevalence, patients present with apparently normal iron stores. The findings from our study suggest that this unrecognized mild anemia must be frequently monitored among T2DM patients to prevent the condition from worsening.
AUTHOR CONTRIBUTIONS
Wina I. O. Boadu: Conceptualization; data curation; investigation; methodology; project administration; supervision; writing—review & editing. William K. B. A. Owiredu: Investigation; methodology; supervision; writing—review & editing. Emmanuel Timmy Donkoh: Data curation; investigation; methodology; writing—review & editing. Kwame O. Boadu: Conceptualization; data curation; investigation; methodology; supervision; writing—review & editing. Afia A. Kwayie: Conceptualization; formal analysis; investigation; methodology; writing—original draft; writing—review & editing. Joseph Frimpong: Formal analysis; investigation; methodology; writing—original draft; writing—review & editing. Enoch O. Anto: Investigation; methodology; supervision; writing—review & editing. Christian Obirikorang: Methodology; supervision; writing—review & editing. Emmanuel E. Korsah: Formal analysis; investigation; writing—review & editing. Ezekiel Ansah: Formal analysis; investigation; software; writing—review & editing. Michael Nyantakyi: Investigation; software; visualization; writing—review & editing. Stephen Opoku: Formal analysis; methodology; writing—review & editing. Ebenezer Senu: Formal analysis; methodology; resources; writing—review & editing. Elizabeth Aboagye: Investigation; methodology; writing—review & editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Ethical approval was sought from the Ethics Committee of the respective hospitals and the Committee on Human Research Publication and Ethics, School of Medical Sciences, Kwame Nkrumah University of Science and Technology (CHRPE/AP/058/16). Written and informed consent was sought from both the hospitals administration and study participants before the research was conducted.
TRANSPARENCY STATEMENT
The lead author Wina Ivy Ofori Boadu affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
The authors would like to thank the study participants, hospital personnel, research assistants, and volunteers who all helped make the study a success. The authors did not receive any external funding.
Boadu WIO, Owiredu WKBA, Donkoh TE, et al. Association of body iron stores and anemia in a Ghanaian type‐2 diabetes mellitus population: a multicentered cross‐sectional study. Health Sci Rep. 2024;7:e2059. 10.1002/hsr2.2059
DATA AVAILABILITY STATEMENT
All authors have read and approved the final version of the manuscript (corresponding author or manuscript guarantor) had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1. IDF . IDF Diabetes Atlas. 2013. International Diabetes Federation; 2019. 10.1289/image [DOI] [Google Scholar]
- 2. Gatimu SM, Milimo BW, Sebastian MS. Prevalence and determinants of diabetes among older adults in Ghana. BMC Public Health. 2016;16(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asmelash D, Asmelash Y. The burden of undiagnosed diabetes mellitus in adult African population: a systematic review and meta‐analysis. J Diabetes Res. 2019;2019:4134937. 10.1155/2019/4134937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riddle MC, Bakris G, Blonde L, et al. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Suppl 1):S1‐S2. [DOI] [PubMed] [Google Scholar]
- 5. Harcourt BE, Penfold SA, Forbes JM. Coming full circle in diabetes mellitus: from complications to initiation. Nat Rev Endocrinol. 2013;9(2):113‐123. [DOI] [PubMed] [Google Scholar]
- 6. Chang J‐T, Dou HY, Yen CL, et al. Effect of type 2 diabetes mellitus on the clinical severity and treatment outcome in patients with pulmonary tuberculosis: a potential role in the emergence of multidrug‐resistance. J Formos Med Assoc = Taiwan yi zhi. 2011;110(6):372‐381. [DOI] [PubMed] [Google Scholar]
- 7. Thomas MC, MacIsaac RJ, Tsalamandris C, et al. The burden of anaemia in type 2 diabetes and the role of nephropathy: a cross‐sectional audit. Nephrol Dial Transplant. 2004;19(7):1792‐1797. [DOI] [PubMed] [Google Scholar]
- 8. El‐Achkar TM, Ohmit SE, Mccullough PA, et al. Higher prevalence of anemia with diabetes mellitus in moderate kidney insufficiency: The Kidney Early Evaluation Program. Kidney Int. 2005;67(4):1483‐1488. [DOI] [PubMed] [Google Scholar]
- 9. Mahjoub AR, Patel E, Ali S, et al. Anemia in Diabetic Patients Without Underlying Nephropathy. A Retrospective Cohort Study. American Society of Hematology Washington, DC; 2016. [Google Scholar]
- 10. Thomas MC, Cooper ME, Rossing K, Parving HH. Anaemia in diabetes: is there a rationale to TREAT? Diabetologia. 2006;49:1151‐1157. [DOI] [PubMed] [Google Scholar]
- 11. Thomas MC. The high prevalence of anemia in diabetes is linked to functional erythropoietin deficiency. Sem Nephrol. 2006;26(4):275‐282. [DOI] [PubMed] [Google Scholar]
- 12. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993‐2005. Public Health Nutr. 2009;12(4):444‐454. [DOI] [PubMed] [Google Scholar]
- 13. Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. WHO, U.J.G., WHO, UNU . Iron Deficiency Anaemia: Assessment, Prevention, and Control. WHO, U.J.G., WHO, UNU; 2001. [Google Scholar]
- 15. Ezenwaka CE, Jones‐LeCointe A, Nwagbara E, et al. Anaemia and kidney dysfunction in Caribbean type 2 diabetic patients. Cardiovasc Diabetol. 2008;7(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kebede SA, Tusa BS, Weldesenbet AB. Prevalence of anaemia and its associated factors among type 2 diabetes mellitus patients in University of Gondar comprehensive specialized hospital. Anemia. 2021;2021:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Angelousi A, Larger E. Anaemia, a common but often unrecognized risk in diabetic patients: a review. Diabetes Metab. 2015;41(1):18‐27. [DOI] [PubMed] [Google Scholar]
- 18. Singh DK, Winocour P, Farrington K. Erythropoietic stress and anemia in diabetes mellitus. Nat Rev Endocrinol. 2009;5(4):204‐210. [DOI] [PubMed] [Google Scholar]
- 19. Ford BA, Coyne DW, Eby CS, Scott MG. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney Int. 2009;75(1):104‐110. [DOI] [PubMed] [Google Scholar]
- 20. Camargo JL, Gross JL. Conditions associated with very low values of glycohaemoglobin measured by an HPLC method. J Clin Pathol. 2004;57(4):346‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas MC, MacIsaac RJ, Tsalamandris C, Power D, Jerums G. Unrecognized anemia in patients with diabetes. Diabetes Care. 2003;26(4):1164‐1169. [DOI] [PubMed] [Google Scholar]
- 22. Hu F, Zhang TJIJoGM. Study on risk factors of diabetic nephropathy in obese patients with type 2 diabetes mellitus. Int J Gen Med. 2020;13:351‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asamoah‐Boaheng M, Sarfo‐Kantanka O, Tuffour AB, Eghan B, Mbanya JC. Prevalence and risk factors for diabetes mellitus among adults in Ghana: a systematic review and meta‐analysis. Int Health. 2019;11(2):83‐92. [DOI] [PubMed] [Google Scholar]
- 24. AlDallal SM, Jena N. Prevalence of anemia in type 2 diabetic patients. J Hematol. 2018;7(2):57‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waggiallah H, Alzohairy M. The effect of oxidative stress on human red cells glutathione peroxidase, glutathione reductase level, and prevalence of anemia among diabetics. N Am J Med Sci. 2011;3(7):344‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Solomon D, Bekele K, Atlaw D, et al. Prevalence of anemia and associated factors among adult diabetic patients attending Bale zone hospitals, South‐East Ethiopia. PLoS One. 2022;17(2):e0264007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bekele A, Teji Roba K, Egata G, Gebremichael B. Anemia and associated factors among type‐2 diabetes mellitus patients attending public hospitals in Harari Region, Eastern Ethiopia. PLoS One. 2019;14(12):e0225725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ida S, Kaneko R, Imataka K, Murata K. Relationship between frailty and mortality, hospitalization, and cardiovascular diseases in diabetes: a systematic review and meta‐analysis. Cardiovasc Diabetol. 2019;18(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahmed H, Fadl N, Kotob S. Impact of long term metformin therapy on hepcidin and iron status in type II diabetic patients. Int J Pharm Clin Res. 2015;7(3):185‐193. [Google Scholar]
- 30. Yang J, Zhou Y, Xie S, et al. Metformin induces Ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J Exp Clin Cancer Res. 2021;40(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Praveen M, Jain N, Raizada N, Sharma S, Narang S, Madhu SV. Anaemia in patients with type 2 diabetes mellitus without nephropathy is related to iron deficiency. Diabetes Metab Syndr: Clinical Res Rev. 2020;14(6):1837‐1840. [DOI] [PubMed] [Google Scholar]
- 32. Kefiyalew F, Zemene E, Asres Y, Gedefaw L. Anemia among pregnant women in Southeast Ethiopia: prevalence, severity and associated risk factors. BMC Res Notes. 2014;7(1):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kassa GM, Muche AA, Berhe AK, Fekadu GA. Prevalence and determinants of anemia among pregnant women in Ethiopia; a systematic review and meta‐analysis. BMC Hematol. 2017;17(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomas M, Tsalamandris C, MacIsaac R, Jerums G. Anaemia in diabetes: an emerging complication of microvascular disease. Curr Diabetes Rev. 2005;1(1):107‐126. [DOI] [PubMed] [Google Scholar]
- 35. Chung JO, Cho DH, Chung DJ, Chung MY. Associations between hemoglobin concentrations and the clinical characteristics of patients with type 2 diabetes. Korean J Intern Med. 2012;27(3):285‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ranil PK, Raman R, Rachepalli SR, et al. Anemia and diabetic retinopathy in type 2 diabetes mellitus. J Assoc Physicians India. 2010;58:91‐94. [PubMed] [Google Scholar]
- 37. Antwi‐Bafour S, Hammond S, Adjei JK, Kyeremeh R, Martin‐Odoom A, Ekem I. A case‐control study of prevalence of anemia among patients with type 2 diabetes. J Med Case Reports. 2016;10(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Samuel TR, Tejaswi N, Kumar P, Prudhvi K, Sravani NS, Govardhini B. Clinical significance of screening for anaemia in diabetic patients. Artic Int J Pharm Sci Rev Res. 2018;48(2):20‐24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors have read and approved the final version of the manuscript (corresponding author or manuscript guarantor) had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.


