Abstract
Due to the phaseout of methyl bromide (MeBr), there is a need for broad-spectrum soil fumigation alternatives for pest management. Little is known about the impact of fumigation alternatives on foodborne pathogens, such as Salmonella, in agricultural soils. This study investigated the effect of MeBr alternative fumigants on Salmonella reduction in soil. Sandy loam soil was collected from a conventional farmed vegetable field and inoculated with either Salmonella Newport J1892 or Typhimurium ATCC 14028 (5.9 ± 0.3 log10 colony-forming unit [CFU]/g). Each of the four fumigants labeled for pest management (1,3-dichloropropene, chloropicrin, dimethyl disulfide, and metam sodium) was applied at labeled maximum application field levels to soil in pots and stored for a 2-week period. Sterile water was used as a control. Following the 2-week period, Salmonella concentrations in soil samples were enumerated at 1, 7, 14, and 21 days postfumigation. The mean concentration of Salmonella Newport was significantly higher than that of Salmonella Typhimurium 1 day after fumigation (p = 0.015). Fumigation using 1,3-dichloropropene or dimethyl disulfide significantly reduced Salmonella Newport and Salmonella Typhimurium concentrations, compared with the sterile water control. The rate of Salmonella reduction in soil treated with dimethyl disulfide was higher (0.17 ± 0.02 log10 CFU/g/day), compared with soil treated with the other fumigants (0.10–0.12 log10 CFU/g/day). Due to the reduction of Salmonella, alternative fumigation treatments may mitigate potential Salmonella contamination in soil within farm environments.
Keywords: Salmonella, fumigation, soil, methyl bromide, preharvest, food safety
Introduction
Salmonella is a bacterial pathogen of public health concern as it is one of the leading causes of foodborne illnesses, hospitalizations, and deaths in the United States annually (CDC, 2018; Dhaliwal et al., 2021; Scallan et al., 2011). Over the last decade, there has been an increasing number of Salmonella outbreaks associated with fresh produce (Bell et al., 2015; Carstens et al., 2019; Lynch et al., 2009). Since fruits and vegetables are often consumed raw, mitigating contamination early in the supply chain is a priority for the industry. Previous research has shown that Salmonella can be introduced in the preharvest environment by a variety of routes, including from soil, water (e.g., irrigation, application of pesticide sprays), and/or wild animal intrusion in production fields (FDA 2015; Franz and Van Bruggen, 2008; Honjoh et al., 2014; Stine et al., 2011; Suslow, 2010).
When contaminated water or soil amendments are applied to fields, it may directly or indirectly contaminate fresh produce (FDA, 2015; Natvig et al., 2002; Sallach et al., 2015; You et al., 2006). Salmonella has frequently been isolated from waterways and agricultural soil in the Mid-Atlantic region, with Salmonella Newport and Salmonella Typhimurium among the predominant serovars isolated (Bell et al., 2015; Gu et al., 2019; Marine et al., 2015; Micallef et al., 2012; Murphy et al., 2023; Sharma et al., 2020). In addition, soil composition (e.g., silt, sand, clay), management practices, temperature, and moisture can affect the survival of Salmonella in soil (Bardsley et al., 2021; Chandler and Craven, 1980; Gu et al., 2013b; Holley et al., 2006; Natvig et al., 2002).
Chemical fumigation of agricultural soil has been shown to be effective against plant pests of concern (plant pathogens, insects and weeds), and has been proposed as a possible antimicrobial strategy against foodborne pathogens to optimize existing preharvest practices (Miller et al., 2022). Methyl bromide (MeBr) was a commonly used broad-spectrum fumigant and has been suggested as an effective mitigation method to reduce Escherichia coli O157:H7 concentration in soil, and transfer from soil to lettuce leaf (Ibekwe et al., 2007). However, MeBr was classified as a class I ozone-depleting substance due to its capability to deplete the ozone layer (United States Environmental Protection Agency [US EPA], 2021). As such, the use of MeBr as a soil fumigant has been slowly phased out by the EPA since 2005, in accordance with the Montreal Protocol (US EPA, 2022; US EPA, 2011).
Researchers and growers have spent the last two decades searching for fumigation alternatives to MeBr that are effective on production of pests faced in produce fields. Therefore, there is a need to evaluate the synergistic and antagonistic effects on food safety hazards when using MeBr alternatives, specifically the impact of these fumigants on Salmonella concentrations in preharvest agricultural soils.
In the Mid-Atlantic region, MeBr alternative fumigants have successfully provided weed, plant disease, and nematode control (Kuhar et al., 2020). Registered and commonly suggested fumigants include chloropicrin, 1,3-dichloropropene, metam sodium, and dimethyl disulfide (McAvoy and Freeman, 2013; Shi et al., 2022). 1,3-Dichloropropene is a colorless liquid organochlorine compound, which is widely used in the United States as a pesticide, applied specifically as a preplant fumigant (US EPA, 2000). Currently, registered in many states, as well as globally, dimethyl disulfide is an organic chemical applied preplant to fields, and is commonly used during the production of berries, cucurbit vegetable, fruiting vegetable (e.g., tomato, pepper), field-grown ornamental, and forest tree nursery crops (US EPA, 2012). Chloropicrin is a liquid chemical compound used as a preplant soil fumigant to manage a broad spectrum of fungi, bacteria, insects, and other harmful pests. It is commonly used in combination/coformulation with 1,3-dichloropropene (US EPA, 2014). Metam sodium is the sodium salt of methyl-dithiocarbamate, which is a nonselective soil fumigant with fungicidal, herbicidal, insecticidal, and nematicidal properties. As one of the most widely used agricultural soil fumigants in the United States, metam sodium is currently labeled for use on most food, feed, and fiber crops. It is used preplant on turfgrass to control invading plant roots and in drains and sewers (US EPA, 2013). Thus, the objective of this study was to evaluate the effect of four MeBr fumigant alternatives on the concentration of Salmonella serovars Typhimurium and Newport in inoculated agricultural soil during and postfumigation.
Materials and Methods
Salmonella inoculum preparation
Similar to previous research in the Mid-Atlantic region (Han and Micallef, 2014), Salmonella serovars Typhimurium and Newport were chosen with the purpose of addressing the gap in knowledge surrounding these two pertinent serovars. Salmonella enterica serovar Newport strain J1892 (Salmonella Newport; associated with a previous tomato-borne outbreak) was obtained from the U.S. Centers for Disease Control and Prevention (CDC, Atlanta, GA). Salmonella enterica serovar Typhimurium strain ATCC 14028 (Salmonella Typhimurium) was obtained from the American Type Culture Collection (Manassas, VA).
Both bacterial cultures were stored in Luria-Bertani (LB) broth containing 20% glycerol at −80°C until used in this study. Before each experiment, cultures were reinoculated into LB broth and incubated at 37°C for 20 h. Each overnight culture was centrifuged at 4000 rpm for 15 min at 22°C, and the pellets were suspended in 100 mL of sterile water to an optical density (600 nm) of 0.3 (∼8 log10 colony-forming unit [CFU]/mL) for use as inoculum in this study.
Soil collection
Sandy loam soil was obtained from vegetable production fields at the Virginia Tech's Eastern Shore Agricultural Research and Extension Center (ESAREC) in Painter, VA. Plastic containers with 3.07 L capacity (Glad, Amherst, VA) were used to collect 4 kg soil samples. A total of 36 soil samples were collected and transported to a biological safety level 2 greenhouse at the ESAREC. Soil collected for this study had average nitrogen (N), phosphorus (P), and potassium (K) amounts of 320, 136, and 103 mg/kg, respectively, and an average pH of 6.2. Soil was tested and negative for Salmonella.
Salmonella inoculation and soil fumigation
This study consisted of two independent trials organized in a randomized complete block design in the study. Of the 36 soil samples, 15 were inoculated with Salmonella Newport and 15 with Salmonella Typhimurium in plastic containers (3.07 L) by mixing each soil sample with 40 mL of the corresponding Salmonella suspension to reach a target initial concentration of ∼6 log10 CFU/g. To ensure homogeneity, each container was subjected to 5 min of shaking. Six soil samples (without inoculation and fumigation) were used as negative controls.
Fumigants used in this study included 1,3-dichloropropene (Telone II; Teleos Ag Solutions, Pinehurst, NC), chloropicrin (Chloropicrin 100; Cardinal Professional Product, Gilroy, CA), dimethyl disulfide (Paladin; Arkema, King of Prussia, PA), and metam sodium (Vapam HL; AMVAC Chemical Corporation, Newport Beach, CA). Immediately following inoculation, each of the four fumigants were applied to three Salmonella Newport and three Salmonella Typhimurium inoculated soil samples, respectively, at equivalent maximum application levels in fields (Table 1). Sterile tap water was applied to control samples. Each tested fumigant was mixed with sterile water to a final volume of 1 mL and evenly drop-applied (50 μL per drop) onto the surface of each soil sample. After applying the fumigant to the soil sample, each container with the soil sample was then covered with a 0.03-mm-thick Blockade impermeable plastic mulch (Berry Plastics Corp., Evansville, IN) and sealed with transparent Scotch tape (3M, Saint Paul, MN).
Table 1.
List of Fumigants and Application Doses
| Fumigants | L/haa | mL/4 kgb |
|---|---|---|
| 1,3-Dichloropropene | 168,000 | 0.156 |
| Chloropicrin | 132,400 | 0.123 |
| Dimethyl disulfide | 442,400 | 0.411 |
| Metam sodium | 700,000 | 0.65 |
| Control | N/Ac | 1 |
Application amount in fields.
Equivalent application amount in study.
Sterile tap water: none applied in field.
Sealed containers with treated soils were placed in a biological safety level 2 greenhouse equipped with ridge vents, a cooling air conditioning unit, and a gas heater at ESAREC for a 2-week fumigation period at 24°C ± 3°C. Soil pH data were collected for each sample after fumigation using an Orion 5-Star Benchtop Multiparameter Meter (Thermo Fisher Scientific). To maintain sandy loam soils within the typical moisture range (10–12%), the soil moisture was adjusted and maintained at 10% ± 2% during the study by manually adding sterile tap water (Bardsley et al., 2021; Rawls and Brakensiek, 1982).
Soil sampling and Salmonella detection
Three soil samples (4 g each) were removed immediately after inoculation of soil. Salmonella was enumerated in soil samples as described below and was recorded as the starting concentration. Following the 2-week fumigation period, soil samples (4 g each) were also collected at 1, 7, 14, and 21 days (n = 3/time point) and Salmonella concentrations were enumerated. Specifically, each 4 g soil sample was diluted in 40 mL of sterile water. Serial dilutions in sterile water were performed and 0.1 mL of each dilution plated directly onto xylose-lysine-tergitol 4 (XLT-4; Thermo Scientific, Waltham, MA) agar plates using an Eddy Jet 2 spiral plater (IUL Instruments, Barcelona, Spain), and incubated at 37°C for 24 h.
After enumeration, presumptive Salmonella colonies with characteristic colony formation (black or black-centered colonies with a yellow or pink periphery) were quantified using a Neutec Flash & Go automated colony counter (Neutec Group, Inc., Farmingdale, NY). From each plate, up to three colonies were restreaked onto XLT-4 plates and confirmed as Salmonella by polymerase chain reaction for the invA gene, as previously described (Luo et al., 2014).
Statistical analyses
All the statistical analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC). Salmonella concentrations were converted to log10 CFU/g, and reductions in Salmonella concentrations for each fumigation treatment were determined for each time point. The effects of fumigation application on soil samples (concentration reduction) were examined by analysis of variance (ANOVA; p ≤ 0.05). The rates of decline and intercept of Salmonella concentration density in soil samples were calculated by fitting log10-transformed data to the linear model, as previously described (Gu et al., 2013a). Estimated values of the parameters were analyzed by multivariate analysis of variance (MANOVA; p ≤ 0.05). Soil pH values of each sample before and after fumigation, and among treatments, were compared by t-test and ANOVA, respectively (p ≤ 0.05).
Results
Reduction of Salmonella in soil due to fumigation
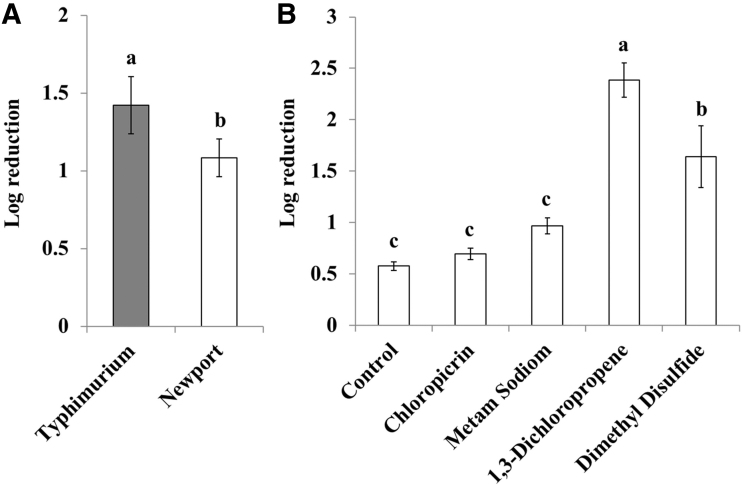
The average initial concentration of Salmonella in inoculated soil samples before fumigation was 5.9 ± 0.3 log10 CFU/g. Regardless of the fumigant used, log10 reductions were significantly higher in Salmonella Typhimurium (1.4 ± 0.2 log10 CFU/g) compared with Salmonella Newport (1.1 ± 0.1 log10 CFU/g) immediately postfumigation (p = 0.015; Fig. 1A). For individual fumigates, irrespective of serovar, 1,3-dichloropropene showed the greatest log10 reduction of Salmonella immediately postfumigation (2.4 ± 0.1 log10 CFU/g), compared with all other treatments (p < 0.05, Fig. 1B). Furthermore, Salmonella reductions in dimethyl disulfide (1.6 ± 0.3 log10 CFU/g) treatments were significantly greater, compared with the control (0.6 ± 0.1 log10 CFU/g), chloropicrin (0.7 ± 0.1 log10 CFU/g), and metam sodium (1.0 ± 0.1 log10 CFU/g) treatments (p < 0.05; Fig. 1B).
FIG. 1.
Log10 reduction of Salmonella in inoculated soils 1 day after the 2-week fumigation. (A) Log10 reduction of Salmonella by serovar. (B) Log10 reduction of Salmonella by fumigant treatment. Different letters (a–c) denote significant differences between variables (p < 0.05). Bars represent standard errors of the reduction levels.
Soil pH was not significantly different before and after fumigation, or among different fumigant treatments (p > 0.05; data not shown). No Salmonella was detected from negative control samples.
Salmonella die-off in soil after fumigation
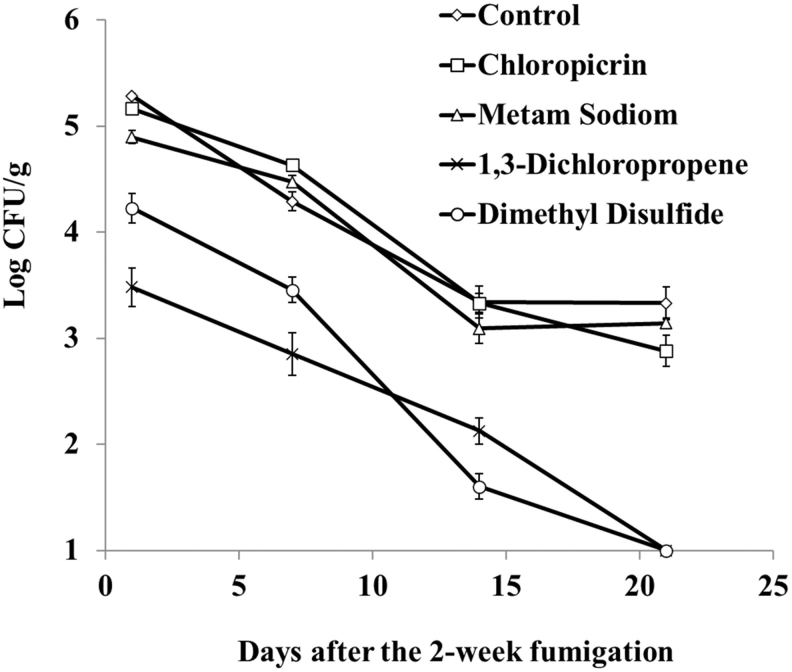
Postfumigation (day 1), the concentration of Salmonella was significantly higher in the control soils (5.29 ± 0.02 log10 CFU/g) and soils treated with chloropicrin (5.17 ± 0.18 log10 CFU/g) compared with metam sodium (4.90 ± 0.05 log10 CFU/g)-, 1,3-dichloropropene (3.48 ± 0.16 log10 CFU/g)-, and dimethyl disulfide (4.23 ± 0.13 log10 CFU/g)-treated soils (Table 2). Salmonella concentrations in soil samples with 1,3-dichloropropene and dimethyl disulfide treatment fell below the limit of detection (<1.0 log10 CFU/g) by day 21 postfumigation (Table 2). Salmonella concentrations significantly decreased between 1 and 21 days postfumigation, regardless of the fumigation treatment applied (p < 0.05; Table 2 and Fig. 2). Due to the fact that Salmonella concentrations were significantly different by treatment postfumigation (day 1; Table 2), a log10 linear model was used to describe the reduction rate (log10 CFU/g/day; Table 3).
Table 2.
Mean and Standard Deviation of Salmonella Concentrations (log10 CFU/g) in Soils After Treatment with Fumigation
| Time (days) | 1,3-Dichloropropene | Chloropicrin | Dimethyl disulfide | Metam sodium | Control |
|---|---|---|---|---|---|
| 1 | 3.48 ± 0.16 | 5.17 ± 0.18 | 4.23 ± 0.13 | 4.90 ± 0.05 | 5.29 ± 0.02 |
| 7 | 2.85 ± 0.10 | 4.63 ± 0.10 | 3.45 ± 0.22 | 4.48 ± 0.16 | 4.29 ± 0.16 |
| 14 | 2.13 ± 0.03 | 3.33 ± 0.19 | 1.60 ± 0.26 | 3.09 ± 0.21 | 3.34 ± 0.26 |
| 21 | <1.00a | 2.88 ± 0.13 | <1.00a | 3.14 ± 0.27 | 3.33 ± 0.24 |
Data for both Salmonella serovars combined.
Concentrations fell below the limit of detection (<1.0 log10 CFU/g).
CFU, colony-forming unit.
FIG. 2.
Survival of Salmonella in soils postfumigation period (1–21 days after the 2-week fumigation). Bars represent standard error of Salmonella concentration densities at each sampling point. CFU, colony-forming unit.
Table 3.
Parameters of the log10 Linear Modela Describing the Reduction Rate (Mean ± Standard Deviation) and Intercept (Mean ± Standard Deviation) Salmonella Concentrations in Soils After Treatment with Fumigation
| Fumigant | Reduction rate (log10 CFU/g/day) | Intercept (log10 CFU/g) |
|---|---|---|
| 1,3-Dichloropropene | 0.12 ± 0.05Ab | 3.48 ± 1.11B |
| Chloropicrin | 0.12 ± 0.03A | 5.31 ± 0.13A |
| Dimethyl disulfide | 0.17 ± 0.02B | 4.41 ± 0.39B |
| Metam sodium | 0.10 ± 0.06A | 5.00 ± 0.19A |
| Control | 0.10 ± 0.02A | 5.14 ± 0.22A |
Coefficient of variation (R2) of 0.94.
Different letters denote significant differences within a column (p ≤ 0.05).
CFU, colony-forming unit.
The linear model used to describe Salmonella concentrations captured the majority of variance with a coefficient of variation (R2) of 0.94 (Table 3). In addition, since the die-off rates by Salmonella serovars were not statistically significant (p = 0.18), data in Table 3 and Figure 2 reflect data for the serovars combined. The observed rates (log10 CFU/g/day) of Salmonella concentration reduction in inoculated soil samples were significantly different between the fumigation treatments (Wilk's Lambda significance = 0.0001). The rates of Salmonella reduction in inoculated soil samples treated with dimethyl disulfide (0.17 ± 0.02 log10 CFU/g/day) treatment was significantly faster, compared with all other treatments as well as the control (p < 0.05; Table 3). No significant differences were observed when comparing the rate of Salmonella reduction in soil samples treated with 1,3-dichloropropene, chloropicrin, metam sodium, and control with die-off rates of 0.10–0.12 log10 CFU/g/day (p > 0.05).
Discussion
Several salmonellosis foodborne outbreaks associated with fresh produce have been attributed to serovars Newport or Typhimurium (Bell et al., 2015; Callejón et al., 2015; Jackson et al., 2013). Furthermore, soil, including soils with biological amendments, has been reported to be one of the primary sources of Salmonella contamination in produce (FDA, 2015; Honjoh et al., 2014; Natvig et al., 2002; You et al., 2006). Previous studies evaluating the prevalence of Salmonella serovars in biological soil amendments and survival in soil after the addition of an amendment have reported Newport and Typhimurium to be among the most commonly identified serovars (Gu et al., 2019; Gu et al., 2018; Murphy et al., 2022). In this study, immediately following the 2-week fumigation treatments, recovered concentrations of Salmonella Newport were significantly higher than those of Salmonella Typhimurium, suggesting that Salmonella Newport may exhibit a greater tolerance to fumigation (at least initially).
Salmonella survival was not observed to be significantly different between serovars postfumigation treatment (up to 21 days). Further research on the bactericidal effect on other serovars of Salmonella commonly implicated in fresh produce-related outbreaks could provide additional insight into the synergistic effects or dynamics of integrated pest management strategies with fumigation on Salmonella concentrations in soil.
This study demonstrated that there was a significant die-off in Salmonella concentration after 21 days in the soil. The total reduction of Salmonella due to chloropicrin (2.29 log10 CFU/g) and metam sodium (1.76 log10 CFU/g) treatments was not significantly different from the control (1.96 log10 CFU/g) treatment. This suggested that these two fumigants had a minimal additional antimicrobial effect on reducing Salmonella in soil. Chloropicrin works as a broad-spectrum fumigant by penetrating the bacterial cell wall and membrane, disrupting functions such as bacterial replication and cell division (Ajwa et al., 2010; Gray et al., 2013). Metam sodium affects bacteria by producing reactive substances that damage cells, inhibiting their metabolism and energy production (Li et al., 2022). It is important to note that these two fumigants, which have varying modes of action, did not facilitate enhanced Salmonella survival, and thus did not increase the food safety risk.
On the contrary, the total reduction of Salmonella concentrations after 21 days in the soil treated with 1,3-dichloropropene (>2.48 log10 CFU/g) or dimethyl disulfide (>3.23 log10 CFU/g) was significantly different from that of the control (1.96 log10 CFU/g). Salmonella concentrations in those soil samples were reduced below the limit of detection. The effect was comparable with the total reduction of the foodborne pathogen E. coli 0157:H7 in soil by MeBr, as previously reported (Ibekwe et al., 2007). Both 1,3-dichloropropene and dimethyl disulfide have the same mode of action that works by inhibiting bacterial virulence and related gene expression by interfering with bacterial enzyme systems that are involved in energy production (e.g., respiration and electron transport) (Ajwa et al., 2010; Antunes et al., 2010).
In addition, the Salmonella reduction rate after dimethyl disulfide treatment was significantly higher during the 3 weeks postfumigation when compared with control and other fumigant treatments. Overall, the significant reduction in Salmonella concentrations both immediately after fumigation and up to 21 days post-treatment suggested that the use of these four fumigants does not enhance Salmonella survival, compared with control samples.
It is well-established that fumigants impact the agricultural production environment beyond plant pathogen and pest targets (Castellano-Hinojosa et al., 2021; Dangi et al., 2017; De Neve et al., 2004; Ibekwe et al., 2001; Pietri and Brookes, 2008; Zhang et al., 2019). Chloropicrin and 1,3-dichloropropene have been reported to lower soil pH (Cheng et al., 2020), which may negatively impact nitrification rates in the amended soil and result in decreased soil fertility. However, this study showed that none of the fumigants used significantly impacted soil pH, suggesting that the use of these products with the intent to minimize foodborne pathogens in biologically amended soil does not directly negatively impact the primary purpose of the initial amendment. This study indicated that fumigation using 1,3-dichloropropene or dimethyl disulfide, which are labeled for broad-spectrum plant pest management, may also minimize the potential risks associated with Salmonella contamination in sandy loam soils.
However, a limitation of this study is that only one soil type was investigated with four sampling time points, thus understanding the reduction of Salmonella in fumigate-treated soils with a variety of compositions and moisture contents with more intensive sampling is of interest. Furthermore, similar to previous studies (Gu et al., 2019; Lee et al., 2019; Shah et al., 2019), XLT4 was used to enumerate Salmonella populations in soil samples; however, further research with the application of solid agar overlay may benefit the recovery of injured cells (Kang and Siragusa, 1999). Future research is also needed to evaluate the efficacy of other MeBr alternative chemicals or the combination of fumigants, such as both 1,3-dichloropropene and dimethyl disulfide, on the reduction of Salmonella in agricultural soil to reduce potential contamination risks.
Acknowledgments
The authors would like to thank Christine Waldenmaier for greenhouse and laboratory assistance. They are also grateful to Dr. Steven Pao for insightful comments during the preparation of the article.
Authors' Contributions
G.G.: Data collection, data analysis, article writing, and article revisions. C.M.M.: Article writing. J.Z.: Experimental design and article revisions. X.N.: Article revisions. S.L.R.: Funding, experimental design, data analysis, and article revisions. L.K.S.: Funding, data analysis, article writing, and article revisions.
Disclosure Statement
No competing financial interests exist.
Funding Information
This research was funded by Virginia Department of Agriculture and Consumer Services, the U.S. Food and Drug Administration, and an integrated, internal competitive grant from Virginia Agricultural Experiment Station, Virginia Cooperative Extension, and College of Agriculture and Life Science at Virginia Tech.
References
- Ajwa H, Ntow WJ, Qin R, et al. Properties of soil fumigants and their fate in the environment. In: Hayes' Handbook of Pesticide Toxicology. Academic Press: Cambridge, MA; 2010; pp. 315–330. [Google Scholar]
- Antunes LCM, Buckner MM, Auweter SD, et al. Inhibition of Salmonella host cell invasion by dimethyl sulfide. Appl Environ Microbiol 2010;76(15):5300–5304; doi: 10.1128/AEM.00851-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley CA, Weller DL, Ingram DT, et al. Strain, soil-type, irrigation regimen, and poultry litter influence Salmonella survival and die-off in agricultural soils. Front Microbiol 2021;12:590303; doi: 10.3389/fmicb.2021.590303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Zheng J, Burrows E, et al. Ecological prevalence, genetic diversity, and epidemiological aspects of Salmonella isolated from tomato agricultural regions of the Virginia Eastern Shore. Front Microbiol 2015;6:415; doi: 10.3389/fmicb.2015.00415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejón RM, Rodríguez-Naranjo MI, Ubeda C, et al. Reported foodborne outbreaks due to fresh produce in the United States and European Union: Trends and causes. Foodborne Pathog Dis 2015;12(1):32–38; doi: 10.1089/fpd.2014.1821 [DOI] [PubMed] [Google Scholar]
- Carstens C, Salazar JK, Darkoh C. Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010–2017. Front Microbiol 2019;10:2667; doi: 10.3389/fmicb.2019.02667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano-Hinojosa A, Boyd NS, Strauss SL. Impact of fumigants on non-target soil microorganisms: A review. J Hazard Mater 2021;427:128149; doi: 10.1016/j.jhazmat.2021.128149 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Estimates of foodborne illness in the United States. 2018. Available from: https://www.cdc.gov/foodborneburden/index.html [Last accessed: February 9, 2023].
- Chandler D, Craven J. Relationship of soil moisture to survival of Escherichia coli and Salmonella typhimurium in soils. Aust J Agric Res 1980;31(3):547–555; doi: 10.1071/AR9800547 [DOI] [Google Scholar]
- Cheng H, Zhang D, Huang B, et al. Organic fertilizer improves soil fertility and restores the bacterial community after 1, 3-dichloropropene fumigation. Sci Total Environ 2020;738:140345; doi: 10.1016/j.scitotenv.2020.140345 [DOI] [PubMed] [Google Scholar]
- Dangi SR, Tirado-Corbalá R, Gerik J, et al. Effect of long-term continuous fumigation on soil microbial communities. Agron 2017;7(2):37; doi: 10.3390/agronomy7020037 [DOI] [Google Scholar]
- De Neve S, Csitári G, Salomez J, et al. Quantification of the effect of fumigation on short-and long-term nitrogen mineralization and nitrification in different soils. J Environ Qual 2004;33(5):1647–1652; doi: 10.2134/jeq2004.1647 [DOI] [PubMed] [Google Scholar]
- Dhaliwal S, Hoffmann S, White A, et al. Cost of hospitalizations for leading foodborne pathogens in the United States: Identification by international classification of disease coding and variation by pathogen. Foodborne Pathog Dis 2021;18(11):812–821; doi: 10.1089/fpd.2021.0028 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA). “Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption.” US Food and Drug Administration. Silver Spring, MD; 2015. [Google Scholar]
- Franz E, Van Bruggen AH. Ecology of E. coli O157: H7 and Salmonella enterica in the primary vegetable production chain. Crit Rev Microbiol 2008;34(3–4):143–161; doi: 10.1080/10408410802357432 [DOI] [PubMed] [Google Scholar]
- Gray MJ, Wholey WY, Jakob U. Bacterial responses to reactive chlorine species. Annu Rev Microbiol 2013;8,67:141–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Cevallos-Cevallos JM, Bruggen AH. Ingress of Salmonella enterica Typhimurium into tomato leaves through hydathodes. PLoS One 2013a;8(1):53470; doi: 10.1371/journal.pone.0053470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Cevallos-Cevallos JM, Vallad GE, et al. Organically managed soils reduce internal colonization of tomato plants by Salmonella enterica serovar Typhimurium. Phytopathology 2013b;103(4):381–388; doi: 10.1094/PHYTO-04-12-0072-FI [DOI] [PubMed] [Google Scholar]
- Gu G, Strawn LK, Oryang DO, et al. Agricultural practices influence Salmonella contamination and survival in pre-harvest tomato production. Front Microbiol 2018;9:2451; doi: 10.3389/fmicb.2018.02451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Strawn LK, Zheng J, et al. Diversity and dynamics of Salmonella enterica in water sources, poultry litters, and field soils amended with poultry litter in a major agricultural area of Virginia. Front Microbiol 2019;10:2868. doi: 10.3389/fmicb.2019.02868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Micallef SA. Salmonella Newport and Typhimurium colonization of fruit differs from leaves in various tomato cultivars. J Food Prot 2014;7(11):1844–1850; doi: 10.4315/0362-028X [DOI] [PubMed] [Google Scholar]
- Holley RA, Arrus KM, Ominski KH, et al. Salmonella survival in manure-treated soils during simulated seasonal temperature exposure. J Environ Qual 2006;35(4):1170–1180; doi: 10.2134/jeq2005.0449 [DOI] [PubMed] [Google Scholar]
- Honjoh KI, Mishima T, Kido N, et al. Investigation of routes of Salmonella contamination via soils and the use of mulch for contamination control during lettuce cultivation. Food Sci Technol Res 2014;20(5):961–969; doi: 10.3136/fstr.20.961 [DOI] [Google Scholar]
- Ibekwe AM, Grieve CM, Yang CH. Survival of Escherichia coli O157:H7 in soil and on lettuce after soil fumigation. Can J Microbiol 2007;53(5):623–635; doi: 10.1139/W07-003 [DOI] [PubMed] [Google Scholar]
- Ibekwe AM, Papiernik SK, Gan J, et al. Impact of fumigants on soil microbial communities. Appl Environ Microbiol 2001;67(7):3245–3257; doi: 10.1128/AEM.67.7.3245-3257.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BR, Griffin PM, Cole D, et al. Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998–2008. Emerg Infect Dis 2013;19(8):1239; doi: 10.3201/eid1908.121511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DH, Siragusa GR. Agar underlay method for recovery of sublethally heat-injured bacteria. Appl Environ Microbiol 1999:65:5334–5337; doi: 10.1128/aem.65.12.5334-5337.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar TP, Hastings PD, Hamilton GC, et al. 2020–2021 Mid-Atlantic Commercial Vegetable Recommendations. 2020. Available from: https://extension.umd.edu/programs/agriculture-food-systems/program-areas/fruit-vegetable-production/maryland-vegetables/mid-atlantic-commercial-vegetable-production-recommendations [Last accessed: February 9, 2023].
- Lee D, Tertuliano M, Harris C, et al. Salmonella survival in soil and transfer onto produce via splash events. J Food Prot 2019:82:2023–2037; doi: 10.4315/0362-028X.JFP-19-066 [DOI] [PubMed] [Google Scholar]
- Li X, Skillman V, Dung J, et al. Legacy effects of fumigation on soil bacterial and fungal communities and their response to metam sodium application. Environ Microbiome 2022;17(1):59; doi: 10.1186/s40793-022-00454-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Gu G, Giurcanu MC, et al. Development of a novel cross-streaking method for isolation, confirmation, and enumeration of Salmonella from irrigation ponds. J Microbiol Methods 2014;101:86–92; doi: 10.1016/j.mimet.2014.03.012 [DOI] [PubMed] [Google Scholar]
- Lynch MF, Tauxe RV, Hedberg CW. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol Infect 2009;137(3):307–315; doi: 0.1017/S0950268808001969 [DOI] [PubMed] [Google Scholar]
- Marine SC, Pagadala S, Wang F, et al. The growing season, but not the farming system, is a food safety risk determinant for leafy greens in the mid-Atlantic region of the United States. Appl Environ Microbiol 2015;81(7):2395–2407; doi: 10.1128/AEM.00051-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy T, Freeman JH. Yellow nutsedge (Cyperus esculentus) control with reduced rates of dimethyl disulfide in combination with totally impermeable film. Weed Technol 2013;27(3):515–519; doi: 10.1614/WT-D-12-00128.1 [DOI] [Google Scholar]
- Micallef SA, Goldstein RER, George A, et al. Occurrence and antibiotic resistance of multiple Salmonella serotypes recovered from water, sediment and soil on mid-Atlantic tomato farms. Environ Res 2012;114:31–39; doi: 10.1016/j.envres.2012.02.005 [DOI] [PubMed] [Google Scholar]
- Miller SA, Ferreira JP, LeJeune JT. Antimicrobial use and resistance in plant agriculture: A one health perspective. Agriculture 2022;12(2):289; doi: 10.3390/agriculture12020289 [DOI] [Google Scholar]
- Murphy CM, Weller DL, Reiter MS, et al. Anaerobic soil disinfestation, amendment-type, and irrigation regimen influence Salmonella survival and die-off in agricultural soils. J Appl Microbiol 2022;132(3):2342–2354; doi: 10.1111/jam.15324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CM, Weller DL, Strawn LK. Salmonella prevalence is strongly associated with spatial factors while Listeria monocytogenes prevalence is strongly associated with temporal factors on Virginia produce farms. Appl Environ Microbiol 2023;89(2):01529; doi: 10.1128/aem.01529-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natvig EE, Ingham SC, Ingham BH, et al. Salmonella enterica serovar Typhimurium and Escherichia coli contamination of root and leaf vegetables grown in soils with incorporated bovine manure. Appl Environ Microbiol 2002;68(6):2737–2744; doi: 10.1128/AEM.68.6.2737-2744.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri JA, Brookes P. Relationships between soil pH and microbial properties in a UK arable soil. Soil Biol Biochem 2008;40(7):1856–1861; doi: 10.1016/j.soilbio.2008.03.020 [DOI] [Google Scholar]
- Rawls WJ, Brakensiek DL. Estimating soil water retention from soil properties. J Irrig Drain 1982;108(2);166–171; doi: 10.1061/JRCEA4.0001383 [DOI] [Google Scholar]
- Sallach JB, Zhang Y, Hodges L, et al. Concomitant uptake of antimicrobials and Salmonella in soil and into lettuce following wastewater irrigation. Environ Pollut 2015;197:269–277; doi: 10.1016/j.envpol.2014.11.018 [DOI] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—Major pathogens. Emerg Infect Dis 2011;17(1):7; doi: 10.3201/eid1701.P21101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MK, Bradshaw R, Nyarko E, et al. Salmonella enterica in soils amended with heat-treated poultry pellets survived longer than bacteria in unamended soils and more readily transferred to and persisted on spinach. Appl Environ Microbiol 2019;85:e00334-19; doi: 10.1128/AEM.00334-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Handy ET, East CL, et al. Prevalence of Salmonella and Listeria monocytogenes in non-traditional irrigation waters in the Mid-Atlantic United States is affected by water type, season, and recovery method. PLoS One 2020;15(3):e0229365; doi: 10.1371/journal.pone.0229365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Zhu J, Wu J, et al. Effects of chloropicrin, dimethyl disulfide and metham sodium applied simultaneously on soil-born bacteria and fungi. Agriculture 2022;12(12):1982; doi: 10.3390/agriculture12121982 [DOI] [Google Scholar]
- Stine SW, Song I, Choi CY, et al. Application of pesticide sprays to fresh produce: a risk assessment for hepatitis A and Salmonella. Food Environ Virol 2011;3:86–91; doi: 10.1007/s12560-011-9061-x [DOI] [Google Scholar]
- Suslow T. Standards for irrigation and foliar contact water. In Produce safety project issue brief. 2010. Available from: https://www.pewtrusts.org/~/media/Assets/2009/PSPWaterSuslow1pdf.pdf [Last accessed: February 9, 2023].
- United States Environmental Protection Agency (US EPA). 1,3-Dichloropropene: Hazard Summary. 2000. Available from: https://www.epa.gov/sites/default/files/2016-09/documents/1-3-dichloropropene.pdf [Last accessed: February 9, 2023].
- United States Environmental Protection Agency (US EPA). Cancellation Order for Registration Amendments to Terminate Certain Soil Uses: Methyl Bromide. 2011. Available from: www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2005-0123-0754 [Last accessed: February 9, 2023].
- United States Environmental Protection Agency (US EPA). US EPA, Pesticide Product Label, PALADIN PIC-21. 2012. Available from: https://www3.epa.gov/pesticides/chem_search/ppls/055050-00007-20160614.pdf [Last accessed: February 9 2023].
- United States Environmental Protection Agency (US EPA). EPA label for Metam CLR. 2013. Available from: https://www3.epa.gov/pesticides/chem_search/ppls/045728-00016-20130814.pdf [Last accessed: February 9, 2023].
- United States Environmental Protection Agency (US EPA). Soil Fumigant Labels—Chloropicrin. 2014. Available from: https://www.epa.gov/soil-fumigants/soil-fumigant-labels-chloropicrin [Last accessed: February 9, 2023].
- United States Environmental Protection Agency (US EPA). International Treaties and Cooperation about the Protection of the Stratospheric Ozone Layer. 2021. Available from: https://www.epa.gov/ozone-layer-protection/international-treaties-and-cooperation-about-protection-stratospheric-ozone [Last accessed: February 9, 2023].
- United States Environmental Protection Agency (US EPA). Phaseout of Class I Ozone-Depleting Substances. 2022. Available from: https://www.epa.gov/ods-phaseout/phaseout-class-i-ozone-depleting-substances#:~:text=Section%20604%20of%20the%20Clean,several%20exemptions%20from%20the%20phaseout [Last accessed: February 9, 2023].
- You Y, Rankin SC, Aceto HW, et al. Survival of Salmonella enterica serovar Newport in manure and manure-amended soils. Appl Environ Microbiol 2006;72(9):5777–5783; doi: 10.1128/AEM.00791-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WW, Chong W, Rui X, et al. Effects of salinity on the soil microbial community and soil fertility. J Integr Agric 2019;18(6):1360–1368; doi: 10.1016/S2095-3119(18)62077-5 [DOI] [Google Scholar]