Abstract
Early language delay (ELD) is one of the earliest indicators of autism spectrum disorder (ASD), and predicts later cognitive and behavioral outcomes. We aimed to determine the neural correlates of ELD in autism, and examine the relationships between gray matter (GM), age of first word/phrase, and core ASD symptoms. We used voxel-based morphometry to examine whole-brain differences in GM in 8–13 year old children with autism (n = 13 ELD; n = 22 non-ELD) and 35 age-matched typically developing (TD) children. Multiple regression analyses examined the relationships between GM, age of first word/phrase, and autism diagnostic observation schedule (ADOS) scores. Composite age of first word/phrase negatively correlated with GM throughout the cerebellum. Both ASD groups (ELD and non-ELD) had reduced GM in right cerebellar Crus I/II when compared to TD children. Left cerebellar Crus I/II was the only region in the brain that differentiated ELD and non-ELD children, with ELD children showing reduced GM relative to both non-ELD and TD groups. Group×score interactions converged in left Crus I/II, such that the non-ELD group showed poorer ADOS scores with increasing GM, whereas the ELD group showed poorer ADOS scores as GM decreased. Reduced GM in right cerebellar Crus I/I was related ASD diagnosis, while children with ELD showed additional reduced GM in left Crus I/II. These findings highlight the importance of specific cerebellar networks in both ASD and early language development, and suggest that bilateral disruption in cerebellar regions that interconnect with fronto-parietal networks could impact language acquisition in ASD.
Keywords: autism, cerebellum, early language delay, voxel-based morphometry, ADOS, imaging
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by persistent social interaction and communication deficits and stereotyped or repetitive behaviors and interests [American Psychiatric Association, 2013]. Because a wide range of symptoms and severities fall under the ASD diagnosis, research investigating the neurobiological bases of ASD is often complicated by the large phenotypic heterogeneity within the population.
One area of particular heterogeneity is language development. There are clear differences within ASD regarding early language delay (ELD), typically defined as the absence of phrase speech before approximately 36 months of age [American Psychiatric Association, 1994]. ELD is one of the first identifiable signs of ASD and might serve as an endophenotype in ASD [Alarcón et al., 2005]—a heritable trait associated with the condition which is present in non-affected family members at higher rates than in the general population [Spencer et al., 2011]. Non-affected siblings of individuals with autism have poorer receptive and expressive language, social communication, and pragmatic language abilities than the general population, even when broader academic skills are intact [Piven & Palmer, 1997; Yirmiya & Shaked, 2005; Landa & Garrett-Mayer, 2006; Toth et al., 2007; Ben-Yizhak et al., 2011]. Under the DSM-IV, ELD differentiated a diagnosis of Asperger’s disorder from a diagnosis of autism, yet this categorization was eliminated in the DSM-5 [American Psychiatric Association, 2013]: both ELD and non-ELD children show poor language outcomes even when language development milestones are in the typical range, providing insufficient behavioral evidence that Asperger’s is a separate syndrome [Howlin, 2003]. Nonetheless, recent studies of language delay in ASD suggest that history of language delay in childhood can result in suboptimal language outcomes such as reduced verbal IQ and poorer word generation abilities in adulthood [Lai et al., 2014].
In addition, previous neuroimaging studies suggest that though behaviorally similar, there may be underlying neural differences between autistic individuals with ELD and those without. Compared to typically developing (TD) groups, individuals with high functioning autism (HFA) show decreased gray matter (GM) in right Crus I and II of the cerebellum, cingulate cortex, and frontostriatal areas, and increased GM in the temporal lobe [McKelvey et al., 1995; Oktem et al., 2001; McAlonan et al., 2002; Kwon et al., 2004; Lotspeich et al., 2004]. In comparison, individuals with Asperger’s (ASP) had decreased GM in the caudate, thalamus, and posterior cerebellar vermis [McKelvey et al., 1995; Oktem et al., 2001; McAlonan et al., 2002; Kwon et al., 2004; Lotspeich et al., 20041. Though rare, direct comparisons of HFA and ASP children find decreased GM in the cerebellar vermis in ASP children relative to their HFA counterparts [McAlonan et al., 2008, 2009]. Differences have also been reported in HFA and ASP groups regarding the relationship between GM and age of language acquisition: decreased GM in left frontal regions correlated with delay in language acquisition in HFA children, but in the ASP children decreased right frontal GM did not correlate with language onset [McAlonan et al., 2008, 2009]. One recent study in adult males with autism found that language delay in childhood was associated with smaller insula, basal ganglia, and temporal lobe GM volumes, but larger GM volumes in brainstem structures such as the pons and medulla, when compared to ASD adults without language delay [Lai et al., 2014]. When comparing both ELD and non-ELD adults to TD adults, both ASD groups had overlapping decreased GM in right Crus I of the cerebellum [Lai et al., 2014]. Despite these neural differences between ASD adults with and without language delay, no significant behavioral differences in autism symptoms were noted [Lai et al., 2014].
Although neural differences have been found between ELD and non-ELD groups, very few studies have specifically examined the neural correlates of early language delay in children with ASD [Kwon et al., 2004; Lotspeich et al., 2004; McAlonan et al., 2008, 2009; Lai et al., 2014], and results are often inconsistent and confounded by the previous DSM criteria for classification of ELD within autism. Further, to our knowledge, no study has examined interactions between brain structure, core ASD symptoms, and early language delay in ASD children. Heterogeneity within ASD populations might contribute to the inconsistent results seen in neuroimaging studies of ASD. The present study used voxel-based morphometry (VBM) to examine the neural underpinnings of ELD in ASD and to relate structural differences to core ASD symptoms. We analyzed the whole brain to investigate GM differences between ASD children with a history of ELD and those without a history of ELD (non-ELD), and age-matched TD children. To determine regions where the relationship between behavioral scores and GM differed between the ELD and non-ELD groups, we then examined interactions between regional GM and scores on autism diagnostic measures.
We predicted that there would be neural differences between the ELD and non-ELD group in areas related to language and language acquisition, and that both ASD subgroups would show neural differences in regions previously shown to be related to “core” ASD symptomology. We further predicted that GM in regions differentiating the ELD and non-ELD groups would correlate with severity of impairment in communication skills.
Methods and Materials
Participants
Seventy children aged 8–13 years participated in this study: 35 children with ASD and 35 age-matched TD children. These ASD and TD children also participated in a previous study [D’Mello et al., 2015]. Participants were selected from an original ASD sample of n = 64. We eliminated participants with IQ < 80 (n = 2). Then we used strict quality control criteria to eliminate scans with motion artifact, ensuring proper management of false positives that can arise from motion artifact in VBM [Reuter et al., 2015]. Approximately 44% (n = 27) of the remaining ASD sample had visibly detectable motion artifact (ringing or blurring) and were eliminated from further analyses, leaving n = 35 ASD participants. This data loss is consistent with previous studies in developmental populations [e.g. Koldewyn et al., 2014]. To ensure that there were no significant behavioral differences between the included and excluded participants (leading to a potential sampling bias), we compared the behavioral characteristics of the groups using two-sample t-tests. There were no significant differences between the 35 ASD subjects selected for this study and the excluded ASD subjects on any of the autism diagnostic observation schedule (ADOS), autism diagnostic interview (ADI), or Weschler intelligence scale for children (WISC-IV) subscales (see Supporting Information Table S1). Within the final ASD group, 22 children had no history of early language delay (non-ELD) and 13 children had a history of early language delay (ELD).
Out of an original sample of n = 94, TD participants were age-matched to ASD participants by closest age. When there were multiple exact or closest-aged TD participants, participants were matched by sex and optimal scan quality. After quality control and age- and gender-matching, the remaining TD participants were excluded from further analysis (n = 59).
Participants were recruited as part of an ongoing study conducted by the Center for Neurodevelopmental and Imaging Research (CNIR) at the Kennedy Krieger Institute. This study was approved by the Johns Hopkins Medical Institutional Review Board. Written consent was obtained from a parent/guardian and assent was obtained from the participating child. None of the children had intellectual disability, seizure disorder, neurological disorder, any severe chronic medical disorder, diagnosed genetic disorder, or psychotic disorder. In the TD group, additional exclusions included any psychiatric disorder, speech or language disorder, broader autism phenotype effects [Piven & Palmer, 1997], or a family history of first-degree relatives with ASD. Intellectual ability was assessed by the WISC-IV [Wechsler, 2003]. All TD children and 33 out of the 35 ASD subjects had a full scale IQ (FSIQ) of 80 or above. In line with recommendations to individualize measures best suited for estimation of cognitive abilities in children with ASD [Mottron, 2004], two ASD subjects with a FSIQ below 80 were included due to Verbal Comprehension Index (VCI) and perceptual reasoning index (PRI) scores ≥85. FSIQ was not included as a covariate as it can produce spurious effects in imaging analyses in neurodevelopmental populations [Dennis et al., 2009].
ASD Diagnosis
To confirm ASD diagnosis, the autism diagnostic interview-revised (ADI-R) [Lord et al., 19941 and the autism diagnostic observation schedule–generic module 3 (ADOS-G) [Lord et al., 2000] were administered by a master’s level or higher research-reliable psychologist. All subjects met DSM-IV criteria for ASD based on the ADOS-G or ADI-R and the clinical impression of the investigators. Early language delay was assessed by the ADI-R age of first word and age of first phrase subscales or parental recollection of age of first word and phrase. A child was given a diagnosis of early language delay if they were either (a) older than 24 months when they spoke their first word or (b) older than 33 months when they spoke their first phrase. If neither of these were true, a child was categorized as non-ELD.
Image Acquisition
A high-resolution T1-weighted MP-RAGE was acquired for each subject on a Philips 3T Achieva MRI scanner (Best, Netherlands) using an eight-channel head coil (TR = 7.99 ms, TE = 3.76 ms, flip angle 8°, voxel size = 1 mm3 isotropic).
Image Processing
Voxel-based morphometry (VBM) was used to identify differences in GM volume between the ELD, non-ELD and TD groups using SPM8 implemented in Matlab 2012b (Mathworks, Natick, MA). T1 anatomical images were pre-processed using optimized VBM [Good et al., 2001; Mechelli et al., 2005], including: (a) examining each image for gross anatomical abnormalities, poor GM/WM differentiation, and ringing around the edges of the images due to motion; (b) setting the origin to the anterior commissure; (c) segmenting images into GM, WM, and CSF using new segment; (d) creating a study-specific template by importing parameter files produced during segmentation into DARTEL; (e) affine transformation of segmented tissues into MNI space; and (f) standard smoothing with an 8 mm FWHM Gaussian kernel. Smoothed, modulated, and normalized data was entered into the statistical analyses. Modulation was added so that final VBM statistics reflected “volume” rather than “concentration” differences in GM [Good et al., 2001; Mechelli et al., 2005].
Statistical Analyses
Comparisons of total intracranial volume.
Total intracranial volume (TIV) was calculated by summing the total GM, WM, and CSF volumes. Group differences in total GM, total WM, total CSF, and TIV were assessed in a one-way ANOVA.
Behavioral comparisons.
Two-tailed t-tests were used to compare WISC-IV measures (FSIQ; verbal comprehension, perceptual reasoning, processing speed and working memory indices), ADI-R (age of first word, age of first phrase, walking age), and ADOS scores (ADOS social interaction, ADOS communication, ADOS communication + social interaction, ADOS stereotyped behaviors and restricted interests, and ADOS total) between the ELD and non-ELD group. Due to missing data in three non-ELD subjects, ADOS behavioral data were available for 19 non-ELD and 13 ELD participants.
Voxel-based morphometry.
Regional differences in GM between groups (ELD, non-ELD, and TD) were assessed using a general linear model (GLM) in SPM8. An absolute threshold mask of 0.2 was used to avoid edge effects at the borders of GM and WM. Smoothed, modulated, and normalized GM images were entered into a one-way ANOVA. Results were thresholded at an uncorrected voxel-level P < 0.001 with a cluster size of 145 voxels, corresponding to a corrected cluster-level threshold of P < 0.001 (AlphaSim implemented in the REST toolbox) [Song et al., 2011]. Post hoc t-test contrasts were defined within the ANOVA contrast manager in SPM8 to test for the direction of GM differences between groups in the regions that were statistically significant in the ANOVA (P < 0.001, k = 25).
Multiple regression analysis was used to examine the relationship between regional GM and a composite score of age of first word and age of first phrase [(age of first word + age of first phrase)/2] in ASD. This language composite score was created due to the high correlation between age of first word and age of first phrase measures (Pearson’s r = 0.790, P < 0.001). Results were thresholded at P < 0.001 with a cluster threshold of 145 (cluster Pcorr < 0.001). Only data from subjects with both ADI age of first word and ADI age of first phrase measures were included in these analyses. Age of first phrase data was not available for 10 participants, and so these composite scores were calculated for the remaining 25 ASD participants.
Interaction analysis.
A full factorial model was used to identify interactions between ASD group membership (ELD vs. non-ELD) and ADOS scores to reveal regions where GM volumes were differentially related to ADOS scores depending on group affiliation. Results were thresholded at a voxel-level P < 0.001 with an extent threshold of 145 voxels (cluster Pcorr < 0.001). GM volume was adjusted for the effects of TIV (“adjusted GM volume”) (x-axis, Fig. 3) and was extracted using SPM8.
Figure 3.
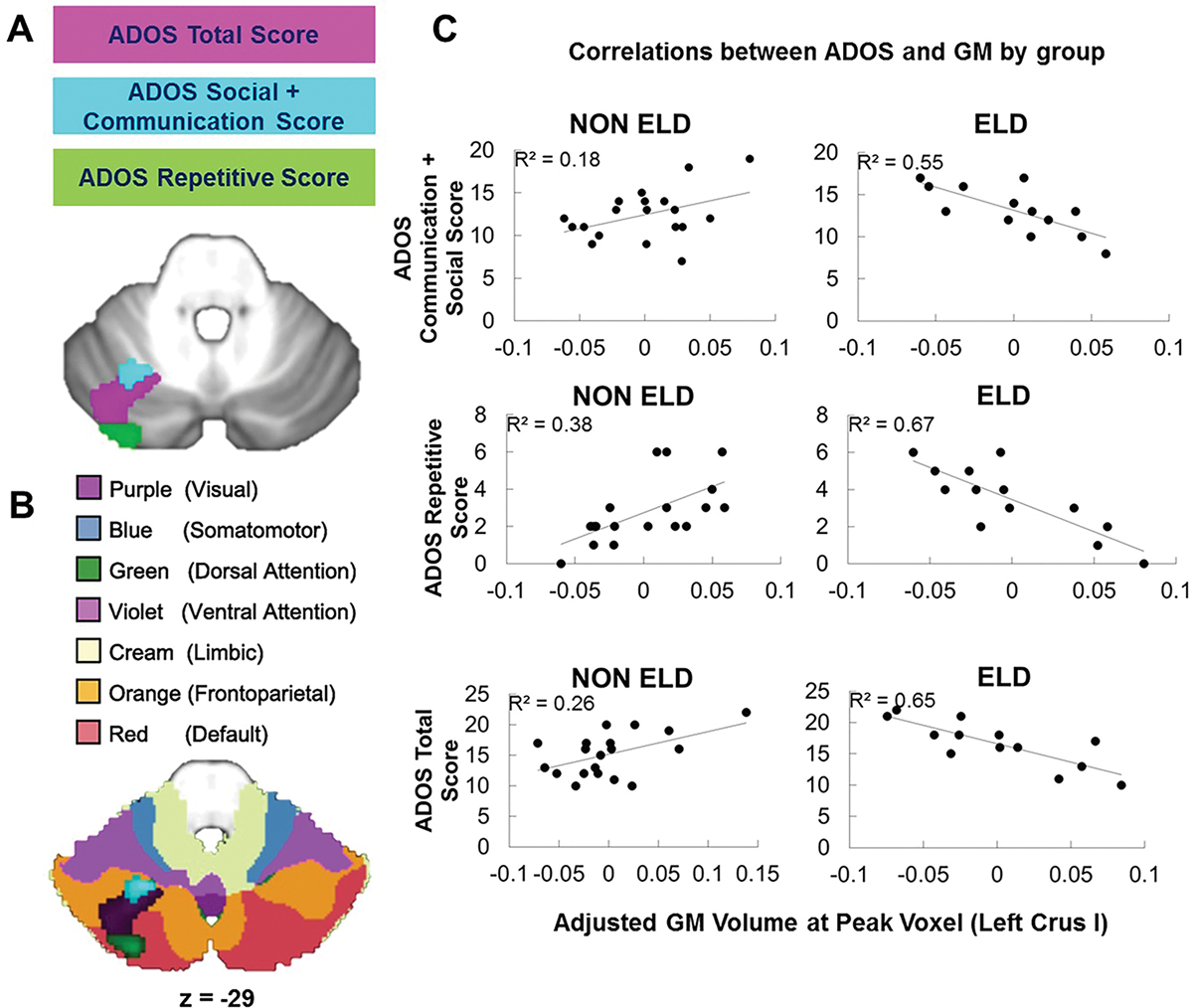
Left Crus I is differentially related to core ASD symptoms. (A) Group × ADOS score interactions converged on left lobule VI/Crus I. (B) Group × ADOS score interactions converged on a region of the cerebellum functionally connected to default mode and fronto-parietal networks; the clusters are shown overlaid onto functional connectivity maps from Buckner and colleagues available in the SUIT atlas (62). (C) Direction of correlations between GM and ADOS scores differed by ELD group. More impaired scores were associated with increased GM in the non-ELD group but decreased GM in the ELD group. GM volume (x-axis) is adjusted for the effects of total intracranial volume (TIV).
Results
Brain Volumes
Total GM, WM, and CSF for ELD, non-ELD, and TD groups were entered into a one-way ANOVA in SPSS (Table 1). As this analysis revealed significant group × GM (P = 0.028), group × WM (P = 0.029), and group × CSF (P = 0.007) interactions, total intracranial volume (TIV) was entered as a covariate in all subsequent analyses.
Table 1.
Total GM, WM, CSF, and TIV Volumes for Each Group
| Tissue type | TD | ELD | Non-ELD | ANOVA P value |
|---|---|---|---|---|
|
| ||||
| GM | 0.745077 | 0.799985 | 0.751718 | 0.028 |
| WM | 0.48466 | 0.522723 | 0.491105 | 0.029 |
| CSF | 0.282074 | 0.309808 | 0.287741 | 0.007 |
| TIV | 1.511811 | 1.632515 | 1.530564 | 0.017 |
Behavioral Scores
There were no significant differences between the ELD group and the non-ELD group in age, WISC-IV scores, or performance on any of the ADOS subscales (Table 2). ADI-R motor milestones (walking age) did not differ between the groups (Table 2).
Table 2.
Behavioral Scores in TD, ELD, and Non-ELD Children With ASD
| Diagnostic subtest | ASD with ELD mean ± SD | ASD Non-ELD mean ± SD | TD mean ± SD | P value |
|---|---|---|---|---|
|
| ||||
| Gender | 13 M | 18 M | 21 M | |
| Handedness (Edinburgh handedness inventory) | 2 L | 1 L | 2 L, 1 Mixed | |
| Age | 10.23 ± 1.23 years | 11.01 ± 1.60 years | 10.36 ± 1.52 years | 0.122 (t-test: ELD vs. non-ELD) |
| Language composite score | 35.59 ± 10.52 | 18.50 ± 5.40 | n/a | 0.0002 (t-test: ELD vs. non-ELD |
| (ADI-R age of first word + age of first phrase/2) | Range (24–63) | Range (8–27) | ||
| ADI-R motor milestones (walking age) | 13.54 ± 2.79 | 12.6 ± 2.39 | n/a | 0.329 (t-test: ELD vs. non-ELD) |
| WISC-IV verbal comprehension | 108.31 ± 15.05 | 110.09 ± 14.53 | 117.74 ± 11.05 | 0.736 (t-test: ELD vs. non-ELD) |
| WISC-IV perceptual reasoning | 109.19 ± 12.62 | 110.38 ± 15.90 | 114 ± 8.96 | 0.821 (t-test: ELD vs. non-ELD) |
| WISC-IV processing speed | 82.92 ± 25.81 | 86.67 ± 13.42 | 102.90 ± 10.30 | 0.646 (t-test: ELD vs. non-ELD) |
| WISC-IV working memory | 98.25 ± 11.46 | 99.90 ± 15.24 | 107.48 ± 11.52 | 0.727 (t-test: ELD vs. non-ELD) |
| FSIQ | 103.17 ± 12.99 | 103.95 ± 11.95 | 115.26 ± 8.15 | 0.865 (t-test: ELD vs. non-ELD) |
| <0.001 (AN0VA; post hoc TD > ELD, TD > non-ELD) | ||||
| ADOS social interaction | 9.38 ± 2.50 | 8.36 ± 2.14 | n/a | 0.244 |
| ADOS communication | 3.77 ± 0.60 | 4.05 ± 1.13 | n/a | 0.365 |
| ADOS repetitive | 3.46 ± 1.85 | 2.74 ± 1.69 | n/a | 0.272 |
| ADOS communication + social | 13.15 ± 2.82 | 12.42 ± 2.95 | n/a | 0.485 |
| ADOS total | 16.62 ± 3.71 | 15.16 ± 3.58 | n/a | 0.279 |
Voxel-Based Morphometry
Significant differences in GM were found in left Crus I/II (F(2, 66) = 10.22, MNI = −27 – 88 – 30) and right Crus I/II (F(2, 66) 10.15, MNI = 26 – 72 – 38) of the cerebellum between the ELD, non-ELD and TD groups. These cerebellar clusters were the only significant results in the whole brain (Fig. 1A). Post hoc t-tests revealed that, when compared to TD children, ELD children showed reduced GM bilaterally in Crus I/II whereas non-ELD children showed reduced GM in only right Crus I/II. Within the ASD group, ELD children showed reduced GM in left Crus I/II when compared to non-ELD children (Fig. 1B and Table 3). Our sample included five left-handed participants (two typically developing children, two ELD ASD, one non-ELD ASD children). Because handedness may inform language lateralization, we re-ran the analyses excluding left-handers (n = 5). While our power to detect results was reduced due to the decreased sample size (results thresholded at P < 0.005 with a cluster threshold of 245 voxels estimating a cluster corrected threshold of P < 0.01), the patterns of cerebellar results remained the same (see Supporting Information Fig. S3 and Tables S2 and S3 for discussion): right Crus I showed significant GM reduction in both ASD groups, whereas left Crus I differentiated the ELD and non-ELD groups from one another. In this analysis, the ANOVA revealed an additional cluster of GM difference in the lingual gyrus. This cluster showed reduced GM in both ASD groups when compared to the TD children, but did not distinguish the two ASD sub-groups.
Figure 1.
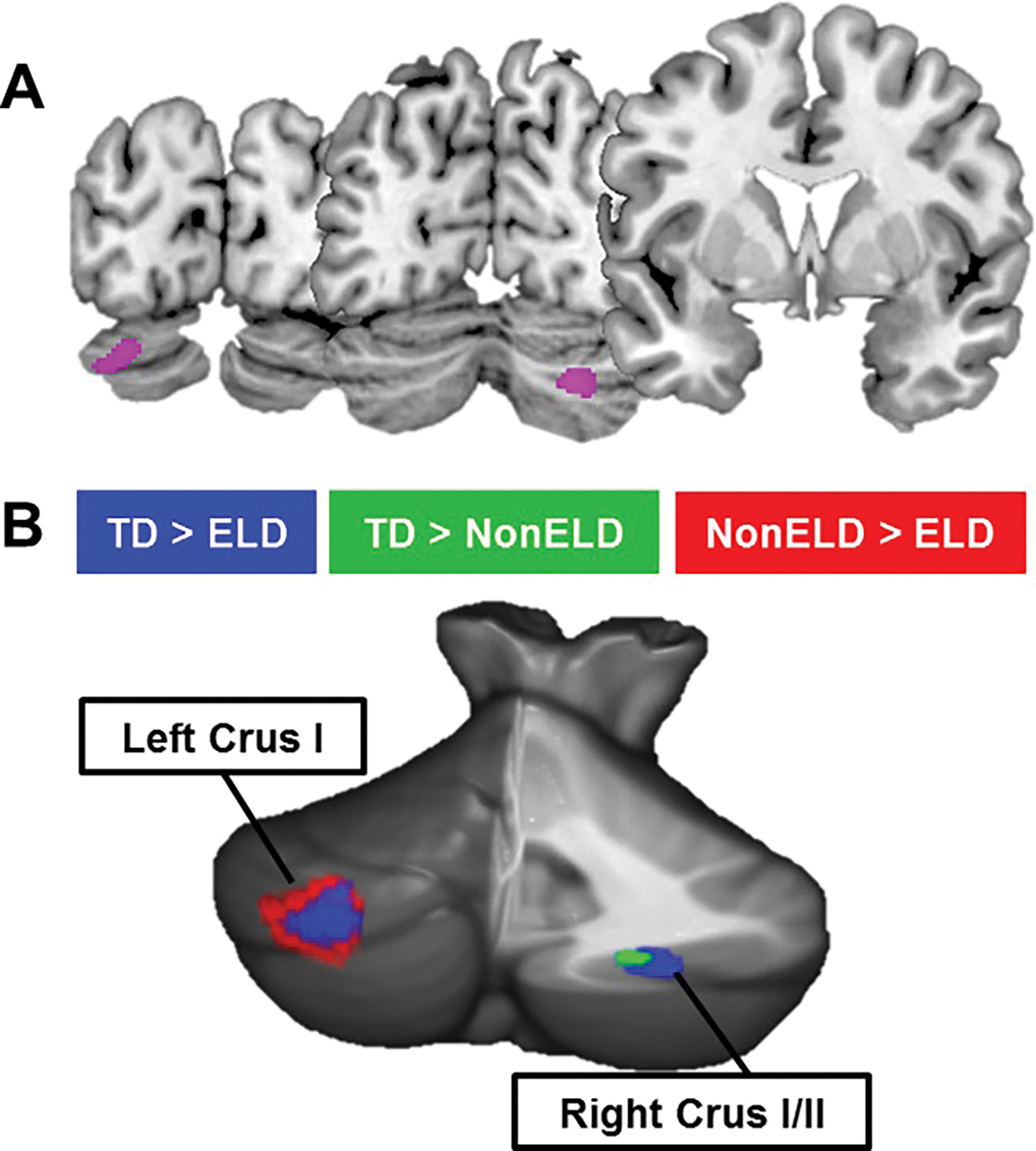
Cerebellar GM differentiates subgroups of ASD. (A) ANOVA comparing TD, ELD, and non-ELD children revealed differences in right Crus I/II and left Crus I/II. These were the only significant differences in the whole brain (Pcorr < 0.001). (B) Post hoc analyses showed reductions in right Crus I/II in both ELD and non-ELD children compared to TD children. Decreased GM in left Crus I/II differentiated ELD and non-ELD children.
Table 3.
Post Hoc t-tests for Directionality of ANOVA Results
| Contrast | Location | Cluster size | Max T | P value | MNI coordinates |
|---|---|---|---|---|---|
|
| |||||
| TD > non-ELD | Right Crus I/II | 42 | 3.58 | 0.0003 | 24 −70 −39 |
| TD > ELD | Left Crus I/II | 1142 | 4.29 | 0.00003 | −26 −88 −30 |
| Right Crus I/II | 630 | 4.03 | 0.00007 | 29 −76 −38 | |
| Non-ELD>ELD | Left Crus I/II | 516 | 4.23 | 0.00004 | −27 −87 −29 |
P-values are uncorrected, voxel-level p-values.
GM and Age of First Word/Phrase
Whole-brain multiple regression analyses revealed significant associations between cerebellar GM and age of first word/phrase (Fig. 2A). GM decreases in right lobules V, VI, Crus I, Crus II, VllB, VIIIA and VIIIB and left lobules V, VI, Crus I, Crus II, VIIIA and VIIIB were associated with later age of first word/phrase (Fig. 2B and Table 4). The cerebellum was the only region in the brain where GM volume significantly correlated with age of first word/phrase.
Figure 2.
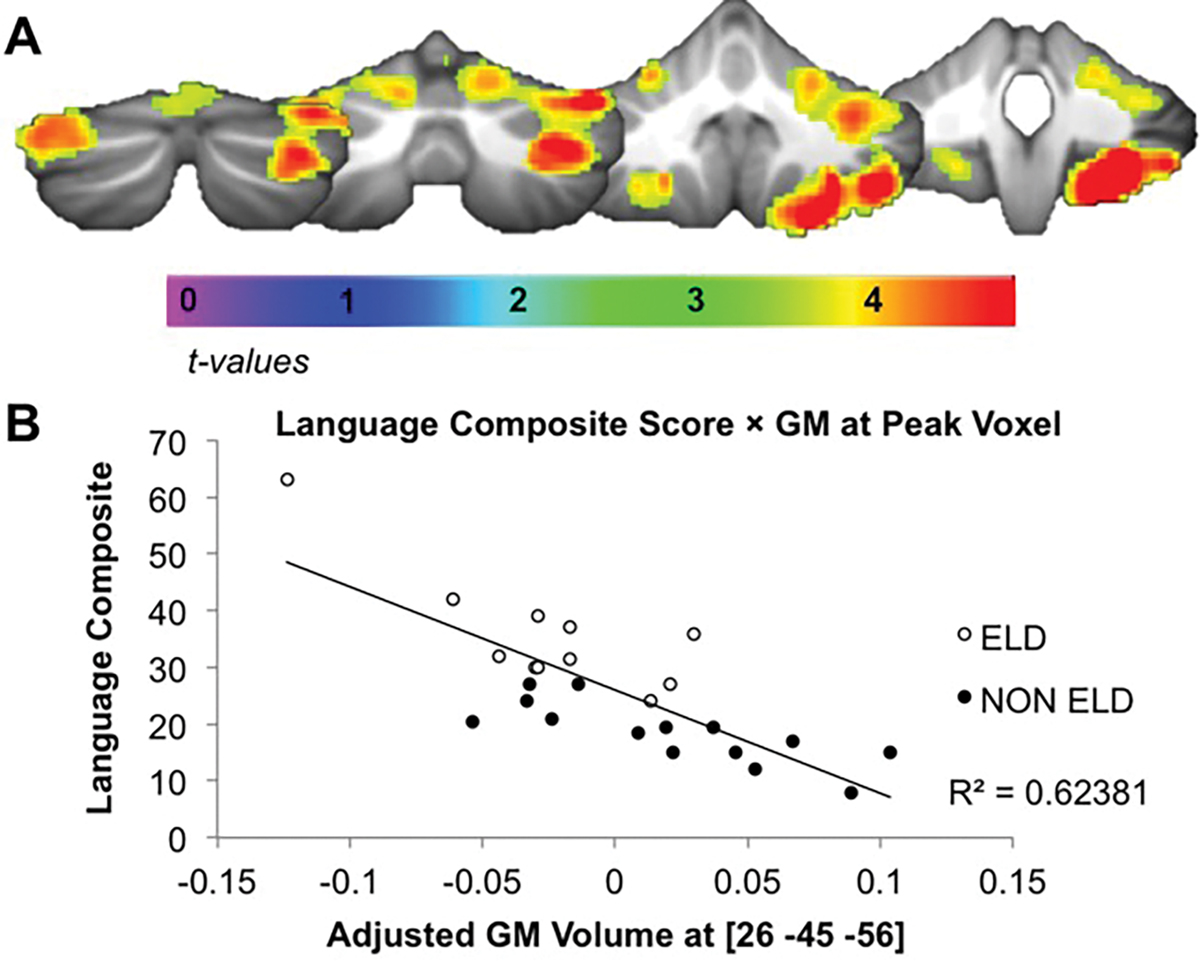
Cerebellar GM volume correlates with age of language acquisition. (A) Reduced GM in the cerebellum correlated with age of first word and phrase composite score (average of age of first word + age of first phrase; Pcorr < 0.001). (B) Relationship between GM volume and age of first word and phrase (in months) composite score at peak voxel.
Table 4.
Correlation Between GM and Language Composite Measure
| Location | Cluster size (voxels) | Max T | P value | MNI coordinates |
|---|---|---|---|---|
|
| ||||
| Right V/VI/Crus I/Crus II/VllB/VIIIA/VIIIB | 10630 | 6.74 | 4.46 × 10−7a | 26 −45 −56 |
| Left V/VI/Crus I/Crus II/VIIIA/VIIIB | 414 | 4.80 | 4.29 × 10−5 | −27 −39 −47 |
Cluster met FWE cluster threshold (P = 3.12 × 10 14), P values are uncorrected, voxel-level P values.
GM and Motor Milestones
To ensure that our findings were not related to early gross motor delays, we compared ELD and non-ELD children on age of walking, as measured by the ADI-R. There were no significant differences between groups in age of walking (P = 0.33, Table 2). To determine whether our cerebellar findings were related more to early motor development than to language acquisition, we also conducted additional multiple regression analyses to examine the relationship between age of walking and whole-brain GM. We found no significant relationships between age of walking and GM.
Behavioral Interactions
We next examined whether the relationship between cerebellar GM and ADOS scores differed in the ELD and non-ELD groups (group × behavioral score interactions). There were significant group × behavioral score interactions for ADOS communication + social interaction, ADOS repetitive, and ADOS total scores in left lobule VI/Crus I (Fig. 3A). For all subscales, decreased GM was associated with more impaired scores in the ELD group while increased GM was associated with more impaired scores in the non-ELD group (Fig. 3C and Table 5).
Table 5.
Correlation Between ADOS Score and GM in Left Lobule VI/Crus I
| Behavioral, scale | Location | Cluster size | Max T | P value | MNI coordinates |
|---|---|---|---|---|---|
|
| |||||
| ADOS communication + social | Left Vl/Crus I | 164 | 3.77 | 0.0004 | −24 −60 −30 |
| ADOS repetitive | Left Vl/Crus I | 834 | 5.30 | 0.000007 | −27 −85 −24 |
| ADOS total | Left Vl/Crus I | 1108 | 4.62 | 0.00004 | −35 −70 −24 |
P values are uncorrected, voxel-level P values.
Discussion
Our goal was to examine the relationship between neural structure and early language delay in children with ASD. Previous studies examined differences between high functioning autism and Asperger’s groups [Kwon et al., 2004; Lotspeich et al., 2004; McAlonan et al., 2008; McAlonan et al., 2009; Toal et al., 2009; Lai et al., 2014], which by definition incorporate a difference in early language skills; however, only one previous study has specifically examined structural correlates of ELD in adults [Lai et al., 2014]. To our knowledge, this is the first study to directly compare structural correlates of ELD and non-ELD in children with autism and to also examine interactions between GM and core ASD symptoms.
When comparing ELD, non-ELD, and TD groups, GM differences in bilateral Crus I/II of the cerebellum were the only significant findings in the whole brain. In post hoc comparisons, the ELD group showed decreased GM compared to TD children in both right and left Crus I/II, whereas the non-ELD group showed decreased GM only in right Crus I/II. Even when excluding left-handed participants from the analyses, the cerebellum was the only region in the brain that differentiated ELD and non-ELD groups. Further, the cerebellum was the only region in the whole brain where GM correlated with age of first word and phrase. Lastly, associations with behavioral measures of core autism features revealed different patterns within ASD, with autism severity associated with decreased left Crus I/II GM in the ELD group, but with increased GM in the non-ELD group. Our analyses revealed a dissociation between the cerebellar regions related to “core” ASD and the regions associated with ELD. Both ASD groups showed reduced GM in right Crus I/II, suggesting this region was related to autism diagnosis, whereas reduced GM in left Crus I/II was specifically related to language delay in autism.
Cerebellar differences in ELD vs. non-ELD populations are of particular interest in light of the long-standing speculation regarding the cerebellar contributions to the pathophysiology of autism [Fatemi et al., 2012; Becker & Stoodley, 2013; Rogers et al., 2013; D’Mello and Stoodley, 2015]. Our understanding of the cerebellum has broadened, with increased recognition of its crucial contributions not only to motor control, but also to cognitive (including language) and affective functions. The localization of these findings to the posterior cerebellum is consistent with the functional topography of the cerebellum, whereby anterior regions are structurally and functionally connected to sensorimotor areas of the cerebral cortex, while the posterolateral cerebellum—including Crus I and II—is structurally and functionally connected to supratentorial regions involved in language, executive function, and general cognition [Stoodley & Schmahmann, 2010]. The posterior cerebellum, specifically lobules Crus I and II, is functionally and anatomically connected to contralateral cerebral language regions, including the inferior frontal gyrus and temporal association areas [Jansen et al., 2005; Booth et al., 2007; Lidzba et al., 2008; Ackermann, 2013; Berl et al., 2014; Verly et al., 2014], and Crus I and II are engaged during a variety of language tasks, including verb generation, semantic processing, and verbal fluency [Stoodley, 2012]. Damage to the posterior cerebellum can lead to mutism, agrammatism, and expressive and receptive language impairments [Schmahmann & Sherman, 1998; Limperopoulos et al., 2007; Tavano et al., 2007; Bolduc & Limperopoulos, 2009; Bolduc et al., 2012], and damage to the left cerebellum has been associated with impaired processing of specific aspects of language, such as verb tense [Mangano et al., 2014].
Further, consistent with the concept of a cerebellar role in skill acquisition, recent evidence suggests that the cerebellum may be involved in typical language development (see Mariën et al. [2014] for review). Voxel-based morphometry studies show that GM concentration in the right posterior cerebellum at 7 months predicts receptive language abilities at 12 months [Deniz Can et al., 2013], and increased right lateralization in the cerebellum is associated with stronger language skills [Berl et al., 2014]. It is thought that the cerebellum modulates language performance, and that cerebro-cerebellar connections may play an important role in the development of cerebral cortical regions important in language [Knickmeyer et al., 2008]. Increased recruitment of the cerebellum might be a crucial underlying factor in the expansion of language skills seen in the second year of life [Redcay et al., 2008]. Consistent with a potential role in language learning, functional MRI studies find increased activation bilaterally in Crus I/II during language learning [Paulesu et al., 2009] and second language learning [Mueller et al., 2014], and structural neuroimaging studies have found that increased GM in bilateral Crus I/II is associated with second language learning in healthy adults [Pliatsikas et al., 2014]. These findings suggest that the cerebellum might support the acquisition of language skills as well as broader language processing. In ASD, abnormal cerebellar structure and activation are related to language impairments. Communication impairments have been associated with reduced GM in right Crus I/II [Rojas et al., 2006; Riva et al., 2013; D’Mello et al., 2015], and very young children with ASD show reduced activation when listening to speech in right lobules IV-V and left VI of the cerebellum [Redcay et al., 2008].
It is possible that these cerebellar correlates of early language are related to the cerebellum’s role in speech motor control and motor learning [Mariën et al., 2014]. That said, the cerebellar regions typically associated with motoric aspects of speech, such as articulation, are located in the anterior lobe of the cerebellum [Frings et al., 2006; Stoodley & Schmahmann, 2015], consistent with the representation of the articulatory muscles for speech and articulation in medial lobule VI. In contrast, the group differences identified here were found in regions that are more often associated with cognitive rather than motoric aspects of language [Frings et al., 2006; Stoodley & Schmahmann, 2015 for review]. Nonetheless, correlations between GM and age of first word/phrase in the current study suggest that, in addition to posterior regions of the cerebellum, decreased GM in anterior regions involved in articulation and speech production are also associated with later age of first word and phrase.
Our findings both support and extend previous research reporting structural and functional abnormalities in right cerebellar Crus I/II in ASD [Stoodley, 2014; D’Mello et al., 2015]. A recent study examining language delay in ASD adults found that TD adults have greater GM in the right cerebellum (Crus I/II) compared with ASD adults both with and without ELD [Lai et al., 2014]. Supporting these findings, our previous research using the same group of children with ASD suggests that when comparing a heterogeneous ASD group (including both ELD and non-ELD individuals) to TD individuals, reduced GM in right Crus I/II was one of the most significant findings in the whole brain [D’Mello et al., 2015]. Consistent with this, aberrant functional connectivity between Crus I/II of the cerebellum and language areas of the cerebral cortex is related to worse ASD symptom severity [Verly et al., 2014]. Support for a role of left Crus I/II in language in ASD comes from studies reporting that reduced GM in left Crus II was associated with more impaired communication scores in individuals with ASD [Rojas et al., 2006; Riva et al., 2013].
We did not find GM differences in “canonical” language regions of the brain. Though unexpected, this is not unprecedented. A study of ELD in adult males with ASD found GM reductions in areas such as the cingulate, thalamus, basal ganglia, and cerebellum, but not expected left-lateralized language regions such as Broca’s area [Lai et al., 2014]. While our cerebellar findings replicate those of Lai et al. [2014], gray matter differences in other brain regions reported by Lai and colleagues may reflect the effects of age, gender, or compensatory mechanisms. Consistent with these findings, functional connectivity studies of language in ASD have shown preserved functional connectivity between classic language centers such as Broca’s area and Wernicke’s area, but a loss of functional connectivity between right Crus I/II of the cerebellum and these cerebral language regions [Verly et al., 2014]. These findings suggest that the cerebellum might play a special role in language development and delay in ASD.
Relationships Between GM and Behavior
The relationship between ADOS scores and GM differed in each ELD group, such that increased GM in left lobule VI/Crus I/II was associated with more impaired scores in the non-ELD group while decreased GM in this region was associated with more impaired scores in the ELD group. Significant group × score interactions converged on left cerebellar lobule VI/Crus I/Crus II for ADOS social + communication scores, ADOS repetitive scores, and ADOS total scores, overlapping with the region that distinguished ELD from non-ELD children in our VBM group analysis. Our previous research in this group of children revealed correlations between reduced GM and more severe ASD symptoms that converged on right Crus I/II in the combined group (ELD and non-ELD) [D’Mello et al., 2015]. On the other hand, the current study aimed to find regions that distinguished ELD and non-ELD, and found that the relationship between GM volumes and behavior differed by group in left Crus I/II. Previous studies comparing subtypes of ASD also report different relationships between gray matter and behavioral scores. Lotspeich et al. [2004] found that both verbal and performance IQ (VIQ; PIQ) were negatively correlated with cerebral cortical gray matter in the high functioning ASD (HFA) group, but positively correlated with cerebral GM in the Asperger’s group. In addition, McAlonan et al. [2008] reported a significant negative correlation between GM in the left inferior frontal gyrus (BA44) and age of first word in only their HFA group, but not in their Asperger’s participants. Lastly, in studies of ASD compared to TD [Rojas et al., 2006], regional cerebellar GM correlated with ADI-R scales in both positive and negative directions (i.e., positive correlation between ADI-R social + communication scores, but a negative correlation between ADI-R repetitive and stereotyped behaviors). Our findings are therefore consistent with previous literature suggesting that patterns of brain-behavior relationships differ in subtypes of ASD that are defined by age of language acquisition. In addition, these findings suggest that both increased and decreased GM in the cerebellum are atypical and are related to increased core autism symptomology and language delays. These findings further support the interpretation that the organization of the brain might differ in ELD and non-ELD children with ASD.
In terms of anatomical specificity and functional relevance, left lobules VI and Crus I are thought to participate in the default mode and frontoparietal cerebrocortical networks [Buckner et al., 2011], and reduced functional connectivity in these networks is related to more impaired symptoms in ASD [Assaf et al., 2010; Redcay et al., 2013; Washington et al., 2013]. Further, when viewing moving stimuli, healthy individuals with increased left Crus I activation were more likely to describe the motion in social terms rather than purely motion-related terms [Jack & Pelphrey, 2014]. In fact, differences in language acquisition might be related to other core symptoms of ASD. Both ADOS communication + social interaction and ADOS repetitive behavior scores trended towards a significant positive correlation with the language composite score (Pearson’s r = 0.375 and 0.387; P = 0.065 and 0.056, respectively). It is conceivable that delays in language acquisition might be related to impairments in social interaction and motor behaviors. Previous studies in typically developing individuals have reported that motor deficiencies are predictive of social/communication impairments as well as other ASD symptoms [e.g. Linkenauger et al., 2012; Travers et al., 2012; Leonard et al., 2013], and motor therapies have been used to improve social and language outcomes in ASD [see McCleery et al., 2013]. Therefore, abnormalities in this region might be related to core ASD symptoms in movement and social processing. These findings imply that right Crus I/II is related to core ASD symptoms, but regions distinguishing heterogeneous groups within ASD (such as left Crus I/II) may have different relationships with behavior depending on the group characteristics.
Lateralization in Early Language Delay
Typically, individuals show agreement in lateralization between the cerebellum and cerebral cortex [Berl et al., 2014]; language is lateralized to the left cerebrum and right cerebellum in most individuals [Gelinas et al., 2014]. Consistent with this, in TD children stronger core language skills are associated with increased right lateralization in the cerebellum [Berl et al., 2014]. Cerebellar lateralization over the course of development mirrors maturation patterns in the inferior frontal gyrus, and the contralateral cerebellum is co-activated with frontal language regions during language tasks [Schlösser et al., 1998; Berl et al., 2014]. These findings suggest that the contralateral connections between the right cerebellum and left language areas are important during language development.
Neuroimaging studies have reported that autism is associated with abnormal or reversed language lateralization in the cerebral cortex, as well as bilateral representation in cerebellar lobules VI/Crus I/Crus II [Redcay et al., 2008; Knaus et al., 2010; Nielsen et al., 2014]. However, while right language lateralization in the cerebral cortex in autism is atypical, it might not be maladaptive. Recent research has shown that increased rightward asymmetry of language areas such as the pars opercularis was associated with earlier language onset and better language skills in children with ASD [Joseph et al., 2014]. While left hemisphere language areas rely on normal language input to specialize, right hemisphere homologues of language areas typically mature earlier and faster, and might therefore be less disrupted by abnormalities in brain development than left hemisphere regions [Joseph et al., 2014]. Current research suggests that early cerebellar injury or abnormal cerebellar development leads to significant alterations in cortical organization and might contribute to the etiology of autism [Limperopoulos et al., 2010; Wang et al., 2014]. Crucially, injury to the cerebellum earlier in life, but not in adulthood, is related to long-term cognitive deficits and ASD symptoms [Wang et al., 2014]. These data suggest that the cerebellum might be an “upstream” driver that shapes cerebral cortical development [Wang et al., 2014]. Therefore, disruptions to distinct cerebellar regions could affect maturation and specialization of developing cerebral neural circuits for language [D’Mello and Stoodley, 2015].
This theoretical construct is supported by the finding that cerebellar injury is the second highest risk factor for ASD, carries considerably more risk than having an autistic fraternal twin, and is superseded only by having an identical twin with autism [Wang et al., 2014]. In addition, genes related to language delay in ASD, such as CNTNAP2, are associated with decreased functional connectivity between areas such as the medial prefrontal cortex and cerebellum [Alarcón et al., 2005; Scott-Van Zeeland et al., 2010]. Variations within this gene were also related to variability in age of first word in ASD individuals, and homozygotes for the CNTNAP2 risk allele showed reduced GM in left lobule VI, and bilateral Crus I/II of the cerebellum [Alarcón et al., 2008; Tan et al., 20101, consistent with the localization of our cerebellar GM differences in ELD and non-ELD children.
Proposed model.
One model supported by our data suggests that GM reductions in the right and left cerebellum (specifically Crus I/II) might lead to long-lasting interruptions of bilateral cerebro-cerebellar circuits, disrupting cortical specialization of language in the left cerebral cortex and also impacting any compensatory right-lateralization (Fig. 4). In this case, we would expect a “typical” ASD profile, including delayed language onset. On the other hand, normal left cerebellar volume in Crus I/II in the context of decreased right cerebellar volume might allow for compensatory right-lateralization of language in the cerebral cortex in ASD. In this case, we might expect a typical onset of language, albeit with residual language problems. This model would predict that right Crus I/II GM reductions might generally relate to autism diagnosis and are thus present in both individuals with early language delay and those without. Reductions in left Crus I/II GM might further predict early language delays due to inability to compensate by using right cerebral homologues. Future studies will be necessary to determine the direction and nature of the relationship between cerebellar dysfunction and cortical specialization of language in ASD.
Figure 4.
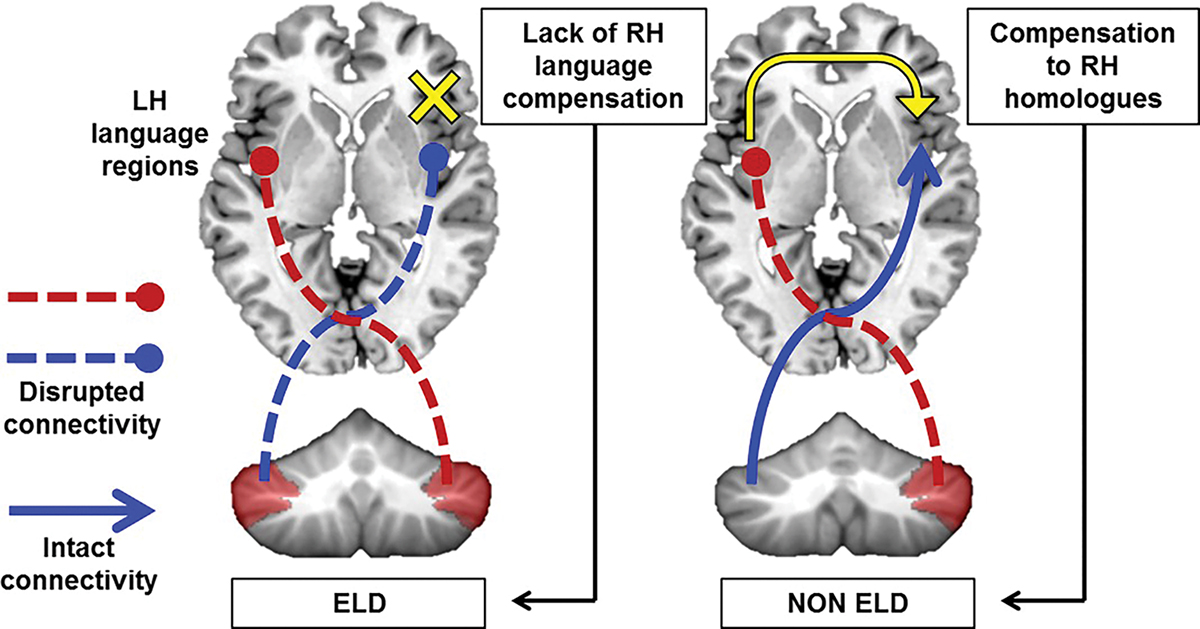
Model of cerebellar involvement in ELD. Left, GM reductions in bilateral Crus I/II might impair cerebro-cerebellar connectivity with left hemisphere language regions as well as any compensatory right lateralization, leading to ELD. Right, reductions in right Crus I/II only might allow for compensation to right language homologues and more typical onset of language.
Limitations.
Although the reported GM differences were robust, the study had several limitations. First, sample size was limited by the age of language acquisition and the quality of available T1 scans, leading to uneven, relatively small group sizes. Due to strict quality control criteria, about 50% of the available sample of scans was eliminated. However, results were highly statistically significant and controlled for multiple comparisons. In addition, there were no behavioral differences in ADOS, ADI, or WISC-IV scores between the 35 selected ASD individuals and the rest of the available sample. Second, given that a majority of both right- and left-handers lateralize language to the left hemisphere [e.g. Knecht et al., 2000; Szaflarski et al., 2012], we did not exclude left-handed participants, which could potentially influence the lateralization of results. To ensure that this was not the case, we conducted these analyses including handedness as a covariate. We found no difference in the results when covarying for handedness. Further, when excluding the left-handed participants (n = 5) from our sample, we found the same pattern of results in the cerebellum (see Supporting Information for discussion). Third, because the studied population was between the ages of 8 and 13 years, it was not possible to determine whether cerebellar GM differences directly cause ELD. However, many previous studies have documented abnormal lateralization patterns, aberrant activation, and GM differences in the cerebella of infants and children with ASD before language onset [e.g. Redcay et al., 2008]. Lastly, the ADOS is not specified for language, and the age of first word measures are based on parental reports as acquired through the ADI-R, which might be inaccurate. Future studies will aim to use language measures that more specifically assess receptive language, expressive language, and language acquisition in these cohorts. In addition, future studies should include a non-ASD language delayed group to determine whether these neural correlates of language delay are specific to ASD.
Implications for ASD neuroimaging.
Our findings suggest that failing to account for heterogeneity might obscure neural differences and explain inconsistent results in ASD [see Waterhouse & Gillberg, 2014]. Research incorporating endophenotypes in autism has become one way to manage the large variability in symptoms, severity, and impairments. However, further neuroimaging work examining the links between brain structure and endophenotypes such as early language delay is needed [Buxbaum et al., 2001], and may provide an improved approach to examining structure-function relationships in ASD.
Conclusions
Reduced GM in right Crus I/II is consistently associated with ASD diagnosis, whereas we found reduced GM in left Crus I/II to be specifically associated with ELD. Typical GM volumes in left Crus I/II may enable the age-appropriate onset of language in children with ASD. The proposed model provides a testable hypothesis of the role of the cerebellum in early language delay in autism.
Supplementary Material
Table S1. Comparisons between included and excluded participants
Table S2. ANOVA comparing ELD, non-ELD, and TD participants, excluding left handed participants
Table S3. Post-hoc t-tests to assess directionality of ANOVA results, excluding left handed participants.
Figure S2. GM correlations with FSIQ. Multiple regression analyses were conducted to determine regions of the brain where GM correlated with FSIQ (p<0.001, k=145). (A) In the combined ASD + TD group, there was a positive correlation between GM and FSIQ in the left putamen. (B) In TD children, FSIQ was negatively correlated with GM in the left angular gyrus. (C) In the ASD group, FSIQ was positively correlated with GM volume in the right superior temporal gyrus. There were no regions of the cerebellum where GM correlated with FSIQ.
Figure S1. SPM8 output for main results. Thresholded image files were saved in SPM8 and were used as overlays on the normalized templates available in MRI-Cron for visualization purposes. Reported p-values and cluster sizes were taken from SPM8 output tables. (A) SPM8 output for ANOVA comparing typically developing children, ASD children with early language delay, and ASD children without early delay (voxel threshold at p<0.001 with a minimum cluster size set at k=145); and (B) SPM8 output showing the regression between grey matter and the composite age of first word/phrase scores (p<0.001, k=145).
Figure S3. Analyses without left-handed participants. (A) ANOVA results without left-handed individuals. Cerebellar results remained the same (right and left Crus I/II). There was an additional cluster in the right lingual gyrus. As power was reduced due to smaller sample size, threshold is p<0.005, k=245 (corrected cluster p<0.01). (B) Post-hoc tests revealed that only the cerebellar clusters differentiated the ASD groups. The lingual gyrus cluster was reduced in both non-ELD and ELD children compared to TD children. Red=TD>ELD, Green-non-ELD>ELD, Blue=TD>non-ELD.
Acknowledgments
This work was supported by NIH/NINDS ROI NS048527–08, NIH/NCATS grants UL1 TR 000424–06 and P41 EB015909–13, and the Autism Speaks Foundation grants #2506, #2384, and #1739. The authors would like to thank Dr. Lauren McGrath for her contributions to the manuscript.
Footnotes
Conflict of Interest
The authors declare no biomedical financial interests or potential conflicts of interest.
Contributor Information
Anila M. D’Mello, Developmental Neuroscience Lab, Department of Psychology, and Center for Behavioral Neuroscience, American University, Washington, DC
Dorothea M. Moore, Developmental Neuroscience Lab, Department of Psychology, and Center for Behavioral Neuroscience, American University, Washington, DC
Deana Crocetti, Center for Neurodevelopmental and Imaging Research (CNIR), Kennedy Krieger Institute, Baltimore, Maryland.
Stewart H. Mostofsky, Center for Neurodevelopmental and Imaging Research (CNIR), Kennedy Krieger Institute, Baltimore, Maryland Department of Neurology, Johns Hopkins University School of Medicine, Johns Hopkins University, Baltimore, Maryland; Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Catherine J. Stoodley, Developmental Neuroscience Lab, Department of Psychology, and Center for Behavioral Neuroscience, American University, Washington, DC
References
- Ackermann H (2013). The contribution of the cerebellum to speech and language. Brain Language, 127, 315–316. [DOI] [PubMed] [Google Scholar]
- Alarcón M, Yonan AL, Gilliam TC, Cantor RM, & Geschwind DH (2005). Quantitative genome scan and ordered-subsets analysis of autism endophenotypes support language QTLs. Molecular Psychiatry, 10, 747–757. [DOI] [PubMed] [Google Scholar]
- Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, et al. (2008). Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. American Journal of Human Genetics, 82, 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders, 4th ed., Arlington, VA: American Psychiatric Press. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders, 5th ed., Arlington, VA: American Psychiatric Press. [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, sahl R, O’Boyle JG, Schultz RT, & Pearlson GD (2010). Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuro-Image, 53, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker EBE, & Stoodley CJ (2013). Autism spectrum disorder and the cerebellum. International Review of Neurobiology, 113, 1–34. [DOI] [PubMed] [Google Scholar]
- Ben-Yizhak N, Yirmiya N, Seidman I, Alon R, Lord C, & Sigman M (2011). Pragmatic language and school related linguistic abilities in siblings of children with autism. Journal of Autism and Developmental Disorders, 41, 750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl MM, Mayo J, Parks EN, Rosenberger LR, VanMeter J, Ratner NB, Vaidya CJ, & Gaillard WD (2014). Regional differences in the developmental trajectory of lateralization of the language network. Human Brain Mapping, 35, 270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc M-E, & Limperopoulos C (2009). Neurodevelopmental outcomes in children with cerebellar malformations: a systematic review. Developmental Medicine & Child Neurology, 51, 256–267. [DOI] [PubMed] [Google Scholar]
- Bolduc M-E, du Plessis AJ, Sullivan N, Guizard N, Zhang X, Robertson RL, & Limperopoulos C (2012). Regional cerebellar volumes predict functional outcome in children with cerebellar malformations. Cerebellum, 11, 531–542. [DOI] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, & Bitan T (2007). The role of the basal ganglia and cerebellum in language processing. Brain Research, 1133, 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, & Yeo BTT (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, Smith CJ, Kilifarski M, Reichert J, Hollander E, Lawlor BA, Fitzgerald M, Greenberg DA, & Davis KL (2001). Evidence for a susceptibility gene for autism on chromosome 2 and for genetic heterogeneity. American Journal of Human Genetics, 68, 1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniz Can D, Richards T, & Kuhl PK (2013). Early gray-matter and white-matter concentration in infancy predict later language skills: a whole brain voxel-based morphometry study. Brain Language, 124, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, & Fletcher JM (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello AM, and Stoodley CJ (2015). Cerebro-cerebellar circuits in autism spectrum disorder. Frontiers in Neuroscience, 9, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello AM, Crocetti D, Mostofsky SH, & Stoodley CJ (2015). Cerebellar gray matter and lobular volumes correlate with core autism symptoms. NeuroImage Clinical, 7, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, Chauhan V, Dager SR, Dickson PE, et al. (2012). Consensus paper: pathological role of the cerebellum in autism. Cerebellum, 11, 777–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings M, Dimitrova A, Schorn CF, Elles HG, Hein-Kropp C, Gizewski ER, et al. (2006). Cerebellar involvement in verb generation: an fMRI study. Neuroscience Letters, 409, 19–23. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Fitzpatrick KPV, Kim HC, & Bjornson BH (2014). Cerebellar language mapping and cerebral language dominance in pediatric epilepsy surgery patients. NeuroImage Clinical, 6, 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, & Frackowiak RS (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14, 21–36. [DOI] [PubMed] [Google Scholar]
- Howlin P (2003). Outcome in high-functioning adults with autism with and without early language delays: implications for the differentiation between autism and Asperger syndrome. Journal of Autism and Developmental Disorders, 33, 3–13. [DOI] [PubMed] [Google Scholar]
- Jack A, & Pelphrey KA (2014). Neural correlates of animacy attribution include neocerebellum in healthy adults. Cerebral Cortex, 25, 4240–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Flöel A, Van Randenborgh J, Konrad C, Rotte M, Förster A-F, Deppe M, & Knecht S (2005). Crossed cerebro–cerebellar language dominance. Human Brain Mapping, 24, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Fricker Z, Fenoglio A, Lindgren KA, Knaus TA, & Tager-Flusberg H (2014). Structural asymmetries of language-related gray and white matter and their relationship to language function in young children with ASD. Brain Imaging and Behavior, 8, 60–72. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Kennedy M, Lindgren KA, Dominick KC, Siegel J, & Tager-Flusberg H (2010). Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain Language, 112, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein EB, & Henningsen H (2000). Handedness and hemispheric language dominance in healthy humans. Brain, 123, 2512–2518. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, & Gilmore JH (2008). A structural MRI study of human brain development from birth to 2 years. Journal of Neuroscience, 28, 12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Yendiki A, Weigelt S, Gweon H, Julian J, Richardson H, Malloy C, Saxe R, Fischl B, & Kanwisher N (2014). Differences in the right inferior longitudinal fasciculus but no general disruption of white matter tracts in children with autism spectrum disorder. Proceedings of the National Academy of Sciences, 111, 1981–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H, Ow AW, Pedatella KE, Lotspeich LJ, & Reiss AL (2004). Voxel-based morphometry elucidates structural neuroanatomy of high-functioning autism and Asperger syndrome. Developmental Medicine & Child Neurology, 46, 760–764. [DOI] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Ecker C, Chakrabarti B, Suckling J, Bullmore ET, & Happé F, MRC AIMS Consortium, Murphy DGM, Baron-Cohen S (2014). Neuroanatomy of individual differences in language in adult males with autism. Cerebral Cortex, 25, 3613–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, & Garrett-Mayer E (2006). Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psychology and Psychiatry 47, 629–638. [DOI] [PubMed] [Google Scholar]
- Leonard HC, Bedford R, Charman T, Elsabbagh M, Johnson MH, Hill EL, & The BASIS Team. (2013). Motor development in children at risk of autism: a follow-up study of infant siblings. Autism 18, 281–291. [DOI] [PubMed] [Google Scholar]
- Lidzba K, Wilke M, Staudt M, Krägeloh-Mann I, & Grodd W (2008). Reorganization of the cerebro-cerebellar network of language production in patients with congenital left-hemispheric brain lesions. Brain Language 106, 204–210. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Sullivan NR, Benson CB, Avery L, Stewart J, Md JSS, Ringer SA, et al. (2007). Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors?. Pediatrics, 120, 584–593. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Chilingaryan G, Guizard N, Robertson RL, & Du Plessis AJ (2010). Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatric Research, 68, 145–150. [DOI] [PubMed] [Google Scholar]
- Linkenauger SA, Lerner MD, Ramenzoni VC, & Proffitt DR (2012). A perceptual-motor deficit predicts social and communicative impairments in individuals with autism spectrum disorders. Autism Research 5, 352–362. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, Pickles A, & Rutter M (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. [PubMed] [Google Scholar]
- Lotspeich LJ, Kwon H, Schumann CM, Fryer SL, Goodlin-Jones BL, Buonocore MH, Lammers CR, Amaral DG, & Reiss AL (2004). Investigation of neuroanatomical differences between autism and Asperger syndrome. Archives of General Psychiatry, 61, 291–298. [DOI] [PubMed] [Google Scholar]
- Mangano GR, Turriziani P, Bonni S, Caltagirone C, & Oliveri M (2014). Processing past tense in the left cerebellum. Neurocase, 21, 185–189. [DOI] [PubMed] [Google Scholar]
- Mariën P, Ackermann H, Adamaszek M, Barwood CHS, Beaton A, Desmond J, De Witte E, Fawcett AJ, Hertrich I, Küper M, et al. (2014). Consensus paper: language and the cerebellum: an ongoing enigma. Cerebellum, 13, 386–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, Simmons A, Sigmundsson T, Greenwood K, Russell A, et al. (2002). Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain 125, 1594–1606. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Suckling J, Wong N, Cheung V, Lienenkaemper N, Cheung C, & Chua SE (2008). Distinct patterns of grey matter abnormality in high-functioning autism and Asperger’s syndrome. J. Child Psychology and Psychiatry, 49, 1287–1295. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung C, Cheung V, Wong N, Suckling J, & Chua SE (2009). Differential effects on white-matter systems in high-functioning autism and Asperger’s syndrome. Psychological Medicine, 39, 1885–1893. [DOI] [PubMed] [Google Scholar]
- McCleery JP, Elliott NA, Sampanis DS, & Stefanidou CA (2013). Motor development and motor resonance difficulties in autism: relevance to early intervention for language and communication skills. Frontiers in Integrative Neuroscience, 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey JR, Lambert R, Mottron L, & Shevell MI (1995). Right-hemisphere dysfunction in Asperger’s syndrome. Journal of Child Neurology, 10, 310–314. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, & Ashburner J (2005). Voxel-based morphometry of the human brain: methods and applications. Current Medical Imaging Reviews, 1, 105–113. [Google Scholar]
- Mottron L (2004). Matching strategies in cognitive research with individuals with high-functioning autism: current practices, instrument biases, and recommendations. Journal of Autism and Developmental Disorders, 34, 19–27. [DOI] [PubMed] [Google Scholar]
- Mueller JL, Rueschemeyer S-A, Ono K, Sugiura M, Sadato N, & Nakamura A (2014). Neural networks involved in learning lexical-semantic and syntactic information in a second language. Language Science, 5, 1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JA, Zielinski BA, Fletcher PT, Alexander AL, Lange N, Bigler ED, Lainhart JE, & Anderson JS (2014). Abnormal lateralization of functional connectivity between language and default mode regions in autism. Molecular Autism, 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktem F, Diren B, Karaagaoglu E, & Anlar B (2001). Functional magnetic resonance imaging in children with Asperger’s syndrome. Journal of Child Neurology, 16, 253–256. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Vallar G, Berlingeri M, Signorini M, Vitali P, Burani C, Perani D, & Fazio F (2009). Supercalifragilisticexpialidocious: how the brain learns words never heard before. NeuroImage 45, 1368–1377. [DOI] [PubMed] [Google Scholar]
- Piven J, & Palmer P (1997). Cognitive deficits in parents from multiple-incidence autism families. Journal of Child Psychology and Psychiatry, 38, 1011–1021. [DOI] [PubMed] [Google Scholar]
- Pliatsikas C, Johnstone T, & Marinis T (2014). Grey matter volume in the cerebellum is related to the processing of grammatical rules in a second language: a structural voxel-based morphometry study. Cerebellum, 13, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Haist F, & Courchesne E (2008). Functional neuroimaging of speech perception during a pivotal period in language acquisition. Developmental Science, 11, 237–252. [DOI] [PubMed] [Google Scholar]
- Redcay E, Moran JM, Mavros PL, Tager-Flusberg H, Gabrieli JDE, & Whitfield-Gabrieli S (2013). Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Frontiers in Human Neuroscience, 7, 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Tisdall MD, Qureshi A, Buckner RL, van der Kouwe AJW, & Fischl B (2015). Head motion during MRI acquisition reduces gray matter volume and thickness estimates. NeuroImage 107, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D, Annunziata S, Contarino V, Erbetta A, Aquino D, & Bulgheroni S (2013). Gray matter reduction in the Vermis and CRUS-II is associated with social and interaction deficits in low-functioning children with autistic spectrum disorders: a VBM-DARTEL study. Cerebellum, 12, 676–685. [DOI] [PubMed] [Google Scholar]
- Rogers TD, McKimm E, Dickson PE, Goldowitz D, Blaha CD, & Mittleman G (2013). Is autism a disease of the cerebellum? An integration of clinical and pre-clinical research. Frontiers in Systems Neuroscience, 7, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, & Tregellas JR (2006). Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry, 6, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlösser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Dewey SL, & Brodie JD (1998). Functional magnetic resonance imaging of human brain activity in a verbal fluency task, Journal of Neurology, Neurosurgery & Psychiatry, 64, 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, & Sherman JC (1998). The cerebellar cognitive affective syndrome. Brain, 121, 561–579. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, McNealy K, Wang AT, Sigman M, Bookheimer SY, & Dapretto M (2010). No neural evidence of statistical learning during exposure to artificial languages in children with autism spectrum disorders. Biological Psychiatry, 68, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- song X-W, Dong Z−Y, Long X-Y, Li S-F, Zuo X-N, Zhu C-Z, He Y, Yan C-G, & Zang Y-F (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLOS ONE, 6, e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer MD, Holt RJ, Chura LR, Suckling J, Calder AJ, Bullmore ET, & Baron-Cohen S (2011). A novel functional brain imaging endophenotype of autism: the neural response to facial expression of emotion. Translational Psychiatry, 1, e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ (2012). The cerebellum and cognition: evidence from functional imaging studies. Cerebellum, 11, 352–365. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ (2014). Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Frontiers in Systems Neuroscience, 8, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, & Schmahmann JD (2010). Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex, 46, 831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, & Schmahmann JD (2015). Functional linguistic topography of the cerebellum, in The Linguistic Cerebellum, Marien P and Manto M, eds., Waltham, MA: Academic Press, pp. 315–335. [Google Scholar]
- Szaflarski JP, Rajagopal A, Altaye M, Byars AW, Jacola L, Schmithorst VJ, et al. (2012). Left-handedness and language lateralization in children. Brain Research, 1433C, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GCY, Doke TF, Ashburner J, Wood NW, & Frackowiak RSJ (2010). Normal variation in fronto-occipital circuitry and cerebellar structure with an autism-associated polymorphism of CNTNAP2. NeuroImage 53, 1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, & Borgatti R (2007). Disorders of cognitive and affective development in cerebellar malformations. Brain, 130, 2646–2660. [DOI] [PubMed] [Google Scholar]
- Toal F, Bloemen OJN, Deeley Q, Tunstall N, Daly EM, Page L, Brammer MJ, Murphy KC, & Murphy DGM (2009). Psychosis and autism: magnetic resonance imaging study of brain anatomy. British Journal of Psychiatry, 194, 418–425. [DOI] [PubMed] [Google Scholar]
- Toth K, Dawson G, Meltzoff AN, Greenson J, & Fein D (2007). Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. Journal of Autism and Developmental Disorders, 37, 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Powell P.s., Klinger LG, & Klinger MR (2012). Motor difficulties in autism spectrum disorder: linking symptom severity and postural stability. Journal of Autism and Developmental Disorders, 43, 1568–1583. [DOI] [PubMed] [Google Scholar]
- Verly M, Verhoeven J, Zink I, Mantini D, Peeters R, Deprez S, Emsell L, Boets B, Noens, 1., Steyaert, J., et al. (2014). Altered functional connectivity of the language network in ASD: role of classical language areas and cerebellum. NeuroImage Clinical, 4, 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS-H, Kloth AD, & Badura A (2014). The cerebellum, sensitive periods, and autism. Neuron, 83, 518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington SD, Gordon EM, Brar J, Warburton S, Sawyer AT, Wolfe A, Mease-Ference ER, Girton L, Hailu A, Mbwana J, et al. (2013). Dysmaturation of the default mode network in autism. Human Brain Mapping, 35, 1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse L, & Gillberg C (2014). Why autism must be taken apart. Journal of Autism and Developmental Disorders, 44, 1788–1792. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2003), Wechsler intelligence scale for children - fourth edition (WISC-IV), San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Yirmiya N, & Shaked M (2005). Psychiatric disorders in parents of children with autism: a meta-analysis. Journal of Child Psychology and Psychiatry, 46, 69–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparisons between included and excluded participants
Table S2. ANOVA comparing ELD, non-ELD, and TD participants, excluding left handed participants
Table S3. Post-hoc t-tests to assess directionality of ANOVA results, excluding left handed participants.
Figure S2. GM correlations with FSIQ. Multiple regression analyses were conducted to determine regions of the brain where GM correlated with FSIQ (p<0.001, k=145). (A) In the combined ASD + TD group, there was a positive correlation between GM and FSIQ in the left putamen. (B) In TD children, FSIQ was negatively correlated with GM in the left angular gyrus. (C) In the ASD group, FSIQ was positively correlated with GM volume in the right superior temporal gyrus. There were no regions of the cerebellum where GM correlated with FSIQ.
Figure S1. SPM8 output for main results. Thresholded image files were saved in SPM8 and were used as overlays on the normalized templates available in MRI-Cron for visualization purposes. Reported p-values and cluster sizes were taken from SPM8 output tables. (A) SPM8 output for ANOVA comparing typically developing children, ASD children with early language delay, and ASD children without early delay (voxel threshold at p<0.001 with a minimum cluster size set at k=145); and (B) SPM8 output showing the regression between grey matter and the composite age of first word/phrase scores (p<0.001, k=145).
Figure S3. Analyses without left-handed participants. (A) ANOVA results without left-handed individuals. Cerebellar results remained the same (right and left Crus I/II). There was an additional cluster in the right lingual gyrus. As power was reduced due to smaller sample size, threshold is p<0.005, k=245 (corrected cluster p<0.01). (B) Post-hoc tests revealed that only the cerebellar clusters differentiated the ASD groups. The lingual gyrus cluster was reduced in both non-ELD and ELD children compared to TD children. Red=TD>ELD, Green-non-ELD>ELD, Blue=TD>non-ELD.


