Abstract
DNA polymerase ɛ (Polɛ) of Saccharomyces cerevisiae is purified as a complex of four polypeptides with molecular masses of >250, 80, 34 (and 31) and 29 kDa as determined by SDS–PAGE. The genes POL2, DPB2 and DPB3, encoding the catalytic Pol2p, the second (Dpb2p) and the third largest subunits (Dpb3p) of the complex, respectively, were previously cloned and characterised. This paper reports the partial amino acid sequence of the fourth subunit (Dpb4p) of Polɛ. This protein sequence matches parts of the predicted amino acid sequence from the YDR121w open reading frame on S.cerevisiae chromosome IV. Thus, YDR121w was renamed DPB4. A deletion mutant of DPB4 (Δdpb4) is not lethal, but chromosomal DNA replication is slightly disturbed in this mutant. A double mutant haploid strain carrying the Δdpb4 deletion and either pol2-11 or dpb11-1 is lethal at all temperatures tested. Furthermore, the restrictive temperature of double mutants carrying Δdpb4 and dpb2-1, rad53-1 or rad53-21 is lower than in the corresponding single mutants. These results strongly suggest that Dpb4p plays an important role in maintaining the complex structure of Polɛ in S.cerevisiae, even if it is not essential for cell growth. Structural homologues of DPB4 are present in other eukaryotic genomes, suggesting that the complex structure of S.cerevisiae Polɛ is conserved in eukaryotes.
INTRODUCTION
In Saccharomyces cerevisiae, three DNA polymerases, α, δ and ɛ, participate in chromosomal DNA replication (see 1 for a review). DNA polymerase α (Polα) consists of four subunits [Pol1p (Cdc17p), Pol12p, Pri1p and Pri2p] and its primary role is in the initiation of DNA replication and priming of Okazaki fragments. DNA polymerases δ and ɛ (Polδ and Polɛ) are required to complete synthesis of both the leading and lagging strands. Consistent with this notion, it has been shown by chromatin immunoprecipitation assay (2,3) that both Polδ and Polɛ bind at/or near replication origins and move with the replication fork. However, the precise functions performed by Polδ and Polɛ on leading and lagging strands have not yet been defined (1,4,5).
Saccharomyces cerevisiae Polδ is a multi-subunit complex that contains the three subunits Pol3 (Cdc2) (5), Hys2 (Pol31) (6) and Pol32 (6,7), which are homologues of Schizosaccharomyces pombe Pol3, Cdc1 and Cdc27, respectively (8). Polδ requires additional replication factors, PCNA and RF–C complex, to catalyse processive DNA synthesis, suggesting that Polδ is the leading strand polymerase (see 5 for a review). Polδ also has a 3′→5′ exonuclease activity that acts as an editing function during DNA synthesis (9,10).
Polɛ is also a multi-subunit complex consisting of Pol2p, Dpb2p, Dpb3p and Dpb4p (1,11,12). Although Polɛ itself is a highly processive enzyme, it also requires PCNA and RF–C complex to catalyse processive DNA synthesis under certain conditions (11,13,14). The Pol2p protein is the catalytic subunit and is encoded by the POL2 gene (15). It is a class B polymerase, characterised by a series of conserved domains, called domains I–VI, containing the 3′→5′ exonuclease subdomains and the DNA polymerase active site residues in the N-terminal half of the protein (16). Yeast strains with the pol2-9 or pol2-18 mutations within the polymerase domain in POL2 are temperature-sensitive for chromosomal DNA replication (17).
POL2 also includes a large region that is conserved in Polɛ from all organisms but is not found in any other class B polymerase. The C-terminus of POL2 has dual roles in DNA replication and the response to DNA damage during S phase (18), as demonstrated by the fact that the C-terminal pol2-11 and pol2-12 mutants are deficient in both processes (18–20). In addition, suppressor studies, synthetic lethal tests and purification of various Polɛ subassemblies from yeast (21–23) have shown that the C-terminal half of POL2 is important in the assembly of the Polɛ holoenzyme [PolII* (11)].
The second subunit of Polɛ (Dpb2), encoded by the DPB2 gene, is essential for yeast cell growth and required for chromosomal DNA replication (12). The DPB3 gene encodes the third subunit of Polɛ; this subunit is not essential for yeast cell growth, although it plays an important role in stabilising the subunit structure of the polymerase (21).
It was recently shown that Polɛ along with its interacting proteins, Dpb11p and Sld2p, plays an important role bringing other polymerase complexes and replication proteins to replication origins before elongation of DNA strands (3,23). More recent studies have shown that although Polɛ may play an enzymatic role during DNA replication, this function does not appear essential, whereas the essential function of Polɛ is provided by its non-catalytic C-terminal domain (24,25). Nonetheless, Polɛ, like Polδ, has the 3′→5′ exonuclease activity that provides editing function during DNA synthesis. Both Polδ and Polɛ 3′→5′ exonuclease-deficient yeast strains are spontaneous mutators (see 16 for a review), strongly suggesting that both polymerases are involved in normal chromosomal DNA replication. The subunit structure of Polɛ in other eukaryotic systems including the fission yeast S.pombe (26) is not well characterised yet, although it is known that calf thymus Polɛ also includes at least the second subunit (27).
In this paper, the gene encoding the fourth subunit of Polɛ is identified and characterised for the first time. The fourth subunit (Dpb4p) of Polɛ is encoded by the YDR121w open reading frame on the S.cerevisiae chromosome IV. Although the gene is not essential for cell growth, its gene product plays an important function not only in maintaining the complex structure of Polɛ, but also in interactions between Polɛ and other replication proteins in the cell.
MATERIALS AND METHODS
Yeast strains
Yeast S.cerevisiae strains used in this study are shown in Table 1. Yeast cells were grown in YPD medium (28) unless otherwise noted. In order to maintain selectable plasmids, yeast cells were grown in SD complete medium (28) minus the selectable amino acid(s) or uracil.
Table 1. Yeast Strains.
| Strain | Genotype | Source |
|---|---|---|
| W303-1A | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3 | R. Rothstein |
| W303-1B | MATα ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3 | R. Rothstein |
| W303-1A/B | MATa/MATα diploid, cross of W303-1A and W303-1B | R. Rothstein |
| CG379 | MATα ade5-1 his7-2 leu2-3, 112 trp1-289 ura3-52 | A. Morrison |
| YTO101 | CG379 dpb4::URA3 | This study |
| YTO102 | W303-1A dpb4::URA3 | This study |
| YTO103 | W303-1A dpb4::TRP1 | This study |
| YTO104 | W303-1A pol2-11 | This study |
| YTO105 | W303-1A dpb2-1 | This study |
| YTO106 | W303-1A dpb11-1 | This study |
| YTO107 | W303-1A rad53-1 | This study |
| YTO108 | W303-1A rad53-21 | This study |
| YHA7 | MATa can1 dpb11-1 his3-Δ200 ura3-52 trp1-289 | H. Araki |
| YK9 | MATa leu2-3, 112 trp1-289 ura3-52 pep4::TRP1 | This study |
| YNN101 | W303-1A hys2-2 | N. Nakashima |
| YNN102 | W303-1A Δpol32::URA3 | N. Nakashima |
Expression of Dpb4 protein in Escherichia coli
The NcoI–BamHI DNA fragment containing the YDR121w gene was inserted into pET15b plasmid (Novagen), resulting in the pET15b-DPB4 plasmid. Escherichia coli BL21 (DE3) cells transformed with pET15b-DPB4 plasmid DNA were grown at 37°C in LB + 0.1 mg/ml ampicillin (900 ml) to 8 × 108 cells/ml and 0.5 mM IPTG was added into the cell culture to induce protein overexpression. After 3 h incubation, cells were collected by centrifugation, suspended in 10 ml buffer A (lacking PMSF) and sonicated at 0°C. The cell lysate was centrifuged at 8000 r.p.m. for 20 min in a Beckman JA20 rotor. The supernatant in the sample buffer was heated at 95°C for 3 min and subjected to SDS–PAGE. The 29-kDa polypeptide was eluted from the gel and identified as Dpb4 protein by protein sequencing. The protein eluted from the gel was used to immunise rabbits as reported previously (6).
Purification of Polɛ holoenzyme
More than 90% pure Polɛ holoenzyme [PolII* (11)] (~500 µg) was purified from 2 kg of yeast CB001 cells as described previously (11,14).
Protein sequencing
Polɛ holoenzyme (200 µg) was fractionated by electrophoresis in a 5% SDS–polyacrylamide gel. After staining the gel with Coomassie brilliant blue, the 29-kDa band (Dpb4p) was excised and electroeluted. The eluted polypeptide (∼20 µg) was digested in 0.2 M Tris–HCl (pH 9.5) containing 5 pmol of lysylendopeptidase (Wako) at 37°C overnight. To terminate the proteolysis, 1/10 vol of 10% trifluoroacetic acid was added and the supernatant was collected. Oligopeptides were separated by reverse-phase high-pressure liquid chromatography and were subjected to amino acid sequencing using a PSQ-10 protein sequencer (Shimadzu) as described (6).
Construction of Δdpb4 mutant
The 5′ non-coding region (∼500 bp) of DPB4 was amplified by PCR using primers containing EcoRI and BamHI sites. Similarly, the 3′ non-coding region was amplified by PCR using two primers containing BamHI and HindIII sites, respectively. The amplified DNA fragments were treated with BamHI and ligated together by DNA ligase. The resultant DNA fragments were treated with EcoRI and HindIII and inserted into pUC119 pretreated with the same enzymes. The recombinant plasmid DNA was cut with BamHI, mixed with URA3 excised from YDp-U (29) by BamHI, and ligated together to generate pUC119-Δdpb4::URA3. Similarly, pUC119-Δdpb4::TRP1 was constructed using YDp-W (29). Either Δdpb4::URA3 or Δdpb4::TRP1 was cut out and used to transform yeast and to replace chromosomal DPB4 with Δdpb4::URA3 or Δdpb4::TRP1.
Synchronisation of yeast cells
Either W303-1A bar1 or its derivatives were grown in YPD at 25°C to 1 × 107 cells/ml, transferred to YPD containing 30 ng/ml α factor and incubated for 2 h. Cells were harvested by centrifugation and resuspended in YPD containing 0.1 mg/ml Actinase E (Kaken Seiyaku) as described (23). After α-factor release, cells were incubated at either 25 or 37°C.
Fluorescence activated cell sorting (FACS) analysis
FACS analysis was performed as described previously (30) after yeast cells were fixed with ethanol and stained with propidium iodide.
Antibodies
Rabbit antiserum against yeast Polɛ complex was as described previously (21). Rabbit antiserum against Dpb4p expressed and purified from E.coli was raised as described previously (6).
Other methods
Western blotting, SDS–PAGE and other methods used in this study were as previously described (6,14,23).
RESULTS
Identification of the gene encoding the 29-kDa polypeptide (Dpb4) co-purified with Polɛ activity
Amino acid sequences of two oligopeptides (LDEYQAAVEQR and EHDEIEEQGDALQDVEESSE, where an amino acid is indicated by a single letter) were obtained from the 29-kDa polypeptide (the fourth subunit of Polɛ) co-purified with Polɛ activity. These peptide sequences were used to search Stanford Saccharomyces Genome databases (http://genome-www.stanford.edu/Saccharomyces/ ). A completely identical sequence was found in the polypeptide predicted from the open reading frame YDR121w. The sequence match is located at residues 115–126 and 157–177 of the predicted polypeptide from YDR121w.
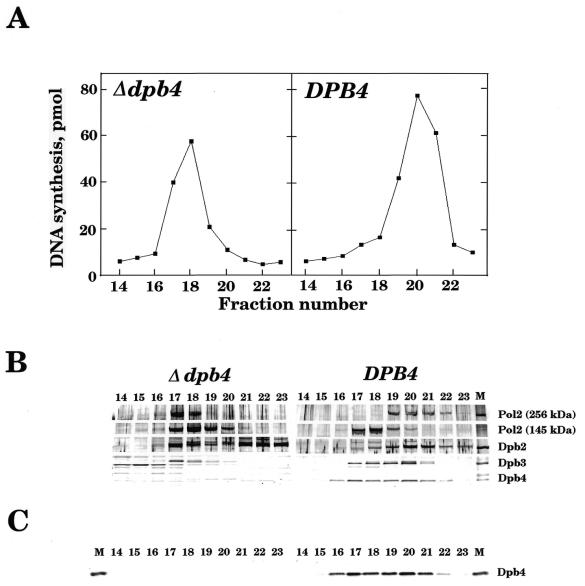
In order to prove that YDR121w encodes the 29-kDa polypeptide (Dpb4), a yeast genomic DNA fragment containing YDR121w was cloned and expressed in E.coli as described in Materials and Methods. The expressed polypeptide was used as an antigen to raise rabbit antiserum. As shown in Figure 1, this antiserum specifically recognised the 29-kDa polypeptide that co-purified with Pol2p (>200 kDa polypeptide, the catalytic subunit of Polɛ) in both partially and highly purified Polɛ fractions. As described below, a deletion mutant (Δdpb4) of DPB4 did not express this 29-kDa polypeptide (Fig. 1). These results indicate that the open reading frame YDR121w encodes Dpb4p. We therefore rename it DPB4.
Figure 1.
Partial purification of Polɛ complex from wild-type and Δdpb4 mutant yeast cells. Yeast cell extracts were prepared, applied to an S–Sepharose column and the proteins retained on the column were eluted with buffer A containing 0.5 M NaCl as described previously (11). The eluate was dialysed against buffer A, 0.1 M NaCl, applied to a Mono Q column equilibrated with the same buffer, and Polɛ was eluted with a linear gradient from 0.1 to 0.5 M NaCl in buffer A (11). Assays for the activity of Polɛ were carried out using oligo(dT)10/poly(dA)>500 (1:50) as a template-primer (A) and the fractions were analysed by SDS–PAGE and western blotting (6). Filters were probed with rabbit antibodies against Polɛ complex (21) (B) and Dpb4p (C). The purified Polɛ complex (11) (20 ng) was also used as a control (M) in (B) and (C). The numbers shown in the Figure are the fraction numbers after Mono Q column chromatography.
DPB4 is not essential for yeast cell growth
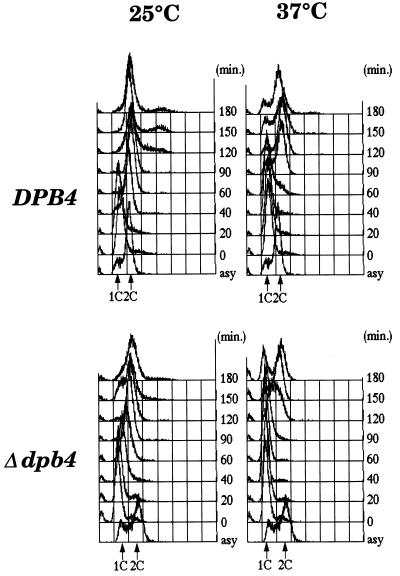
To investigate whether DPB4 is essential for cell growth, the open reading frame of the chromosomal gene was replaced with either URA3 or TRP1 by the one step replacement technique. Haploid strains with the deleted DPB4 allele were viable and readily obtained (data not shown), indicating that the DPB4 gene is not essential for cell growth in yeast. The obtained Δdpb4 haploid strains were tested for their sensitivity to UV light, X-rays, methylmethane sulfonate and hydroxyurea. The results showed that they were not sensitive to any of those treatments (data not shown). However, a slight growth defect was observed in a Δdpb4 haploid strain at both 25 and 37°C. The generation times of a Δdpb4 haploid strain at 25 and 37°C in YPD medium were 210 and 250 min, respectively, while those of a parental wild-type strain at both temperatures were 180 and 140 min, respectively. Logarithmically growing Δdpb4 mutant and wild-type cells were synchronised by α factor (23) and released into fresh YPD medium at either 25 or 37°C. After incubation at either 25 or 37°C, cells were harvested, fixed with ethanol, stained with propidium iodide and analysed by a FACScan. As shown in Figure 2, a slightly prolonged S phase was observed in Δdpb4 mutant cells at 25°C. This prolonged cell cycle progression in S phase was enhanced at 37°C. A majority of Δdpb4 mutant cells were still in G1/S phase 90 min after α factor release at 37°C, when wild-type cells were already in G2/M phase (Fig. 2). These results suggest that although the DPB4 gene is not essential for yeast cell growth, its gene product plays an important role in chromosomal DNA replication.
Figure 2.
Δdpb4 mutant has a defect in S phase progression. Yeast strains [W303-1A (DPB4) and congenic W303-1A Δdpb4 strain (Δdpb4)] grown at 25°C in YPD medium to 1 × 106 cells/ml were synchronised with α factor as described in Materials and Methods and separated into two equal portions. One was further incubated at 25°C and the other portion was incubated at 37°C. At various times, an aliquot was withdrawn, cells were collected by centrifugation, fixed with ethanol, stained with propidium iodide and analysed by FACScan as published (23). 1C and 2C, relative DNA content per cell; asy, before synchronisation.
Polɛ complex is less stable in Δdpb4 mutant cells than in wild-type cells
To prove that Dpb4p is missing in Δdpb4 mutant cells, Polɛ activity was partially purified from Δdpb4 mutant cells by MonoQ column chromatography as described (11). As shown in Figure 1A, Polɛ activity was detected in fractions different from those of wild-type Polɛ. Furthermore, the total amount of Polɛ from Δdpb4 mutant cells was ~50% of Polɛ from wild-type cells. The fractions containing the activity were subjected to western blot, and probed with Polɛ complex and Dpb4p antibodies. As shown in Figure 1B and C, no Dpb4p was detected in the fractions where a degraded form of Polɛ and Polɛ activities eluted. Furthermore, an intact form of Polɛ (which contains >250 kDa Pol2 polypeptide) could not be separated from a degraded form of Polɛ (~145 kDa polypeptide) by MonoQ column chromatography, which does not occur in wild-type cells (11,14). In addition, both Dpb2p and Dpb3p dissociate more easily from the Pol2 polypeptide after MonoQ chromatography in extracts from Δdpb4 mutant cells, resulting in a much smaller amount of Dpb3p rather than wild-type Dpb3p being associated with Pol2p polypeptide (Fig. 1B). Therefore, these results suggest that Polɛ complex is much less stable in Δdpb4 mutant cells than in wild-type cells. This observation is consistent with the notion that Dpb4 plays an important role in maintaining the Polɛ complex in the cell.
Biochemical properties of Polɛ from Δdpb4 mutant cells
To investigate whether Dpb4p plays a role in an in vitro DNA synthesis catalysed by Polɛ, we compared biochemical properties of Polɛ from Δdpb4 mutant cells with those from wild-type cells using various templates/primers including an activated calf thymus DNA, oligo(dT)10/poly(dA)>500 (1:50) and singly-primed φX174 viral single-stranded DNA as previously published (11,14). Since we could not separate a degraded form of Polɛ from Polɛ complex in mutant cell extracts, we had to use the mixture of the two forms. However, the mixture exhibited the same optimal pH, optimal MgCl2 concentration, salt sensitivity, Km for dNTPs and processivity as those of wild-type Polɛ complex (data not shown). Therefore, we concluded that Dpb4p does not have any positive role for in vitro DNA synthesis catalysed by Polɛ.
Genetic interactions between the Δdpb4 mutant and other DNA replication mutants
As shown in Figure 2, the Δdpb4 mutant exhibits a slight delay in S phase progression, suggesting that it has a slight defect in chromosomal DNA replication. This prompted us to investigate genetic interactions between Δdpb4 and other DNA replication mutants. A Δdpb4 mutant was genetically crossed with other DNA replication mutants. The resultant diploids were sporulated and the tetrads were micro-dissected, germinated at 25°C for 4 days and each of the spores was analysed genetically. When Δdpb4 was crossed with a temperature-sensitive pol2-11 mutant, no spores containing both Δdpb4 and pol2-11 were recovered from more than 40 tetrads (data not shown). On the other hand, a haploid spore containing both mutations could be easily recovered from the tetrads, if a single copy plasmid containing either POL2 or DPB4 was introduced into the strain before crossing (data not shown). Thus, these results indicate that a haploid strain with Δdpb4 and pol2-11 is not viable (synthetic lethal) at 25°C. Similar synthetic lethality was also observed between Δdpb4 and pol2-12 and Δdpb4 and dpb11-1 (Table 2 and data not shown). In contrast, double mutant haploid strains were viable with Δdpb4 and either rad53-1, rad53-21, dpb2-1, pol3-12, hys2-2 or Δpol32. The growth of these double mutant strains (and the control single mutant strains) was tested on a YPD plate at various temperatures (31). As shown in Table 2, the double mutants Δdpb4 rad53-1, Δdpb4 rad53-21, Δdpb4 dpb2-1, Δdpb4 hys2-2 and Δdpb4 Δpol32 had significantly lower restrictive temperatures than the corresponding single mutant strains. In particular, Δdpb4 rad53-1 and Δdpb4 rad53-21 double mutants exhibited a very poor growth phenotype at 25°C. Significantly, a Δdpb3 Δdpb4 deletion mutant had the same restrictive temperature as the single deletion mutant Δdpb3 or Δdpb4. Nevertheless, these results indicate a wide variety of genetic interactions between the DPB4 and other DNA replication genes, which suggests that Dpb4p plays an important function as the subunit of Polɛ during chromosomal DNA replication in S.cerevisiae.
Table 2. Genetic interaction between Δdpb4 and various mutations.
| Mutation | Temperature | ||||||
|---|---|---|---|---|---|---|---|
| 25°C | 30°C | 32°C | 33°C | 34°C | 35°C | 37°C | |
| DPB4 | +++ | +++ | +++ | +++ | +++ | +++ | ++ |
| Δdpb4 | +++ | +++ | +++ | +++ | +++ | +++ | ++ |
| rad53-1 | +++ | +++ | +++ | +++ | ++ | + | – |
| rad53-1 Δdpb4 | + | + | + | – | – | – | – |
| rad53-21 | +++ | +++ | +++ | +++ | ++ | + | – |
| rad53-21 Δdpb4 | + | + | + | + | – | – | – |
| pol1-17 | +++ | +++ | + | – | – | – | – |
| pol1-17 Δdpb4 | +++ | +++ | + | – | – | – | – |
| dpb2-1 | +++ | +++ | ++ | ++ | + | – | – |
| dpb2-1 Δdpb4 | +++ | +++ | ++ | – | – | – | – |
| hys2-2 | +++ | +++ | ++ | + | + | – | – |
| hys2-2 Δdpb4 | +++ | +++ | ++ | – | – | – | – |
| Δpol32 | +++ | +++ | +++ | +++ | +++ | ++ | + |
| Δpol32 Δdpb4 | +++ | +++ | +++ | ++ | + | – | – |
| Δdpb3 | +++ | +++ | +++ | +++ | +++ | +++ | ++ |
| Δdpb3 Δdpb4 | +++ | +++ | +++ | +++ | +++ | +++ | ++ |
| pol3-12 | +++ | ++ | – | – | nt | nt | – |
| pol3-12 Δdpb4 | +++ | ++ | – | – | nt | nt | – |
| pol2-11 | +++ | +++ | +++ | ++ | + | – | – |
| pol2-11 Δdpb4 | lethal | ||||||
| dpb11-1 | +++ | +++ | +++ | ++ | + | – | – |
| dpb11-1 Δdpb4 | lethal | ||||||
+++, Normal growth; ++, slightly slow growth; +, poor growth, –, no growth; nt, not tested.
Dpb4p contains the histone-fold motif
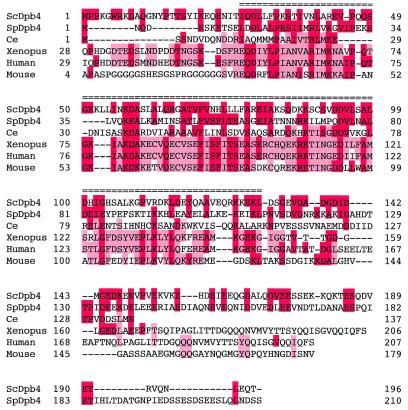
The predicted amino acid sequence of DPB4 is similar to several other predicted proteins in the databases: DPB4 has 30% identity and 55% similarity to a putative DNA binding protein of S.pombe (DDBJ/EMBL/GenBank accession no. CAB09118); 23% identity and 51% similarity to Caenorhabditis elegans putative DNA binding protein (DDBJ/EMBL/GenBank accession no. AAC77511); 26% identity and 50% similarity to Xenopus and human nuclear transcription factor Y (DDBJ/EMBL/GenBank accession nos AAC82336 and NP-006157, respectively); and 26% identity and 50% similarity to mouse CCAAT-binding transcription factor subunit A (CBF-A) (DDBJ/EMBL/GenBank accession no. P25209). These six proteins were aligned using the program ClustalW 1.7 (32) and the result is shown in Figure 3. The block Maker program identified one conserved region at the N-terminal end of the protein that contains a histone-fold motif (shown by a double-line in the figure). The histone-fold motif resembles an extended helix–strand–helix motif first identified in the core histone proteins as primarily responsible for dimerisation of the H2A/H2B and H3/H4 histone pairs (33). Recently, this motif has been found in a number of proteins involved in protein–protein and protein–DNA interactions (34).
Figure 3.
Amino acid alignment of S.cerevisiae Dpb4p with homologues from other organisms. Amino acid sequence comparison between S.cerevisiae Dpb4p, S.pombe polypeptide (SpDpb4, accession no. CAB09118), C.elegans putative DNA binding protein (Ce, accession no. AAC77511), mouse CBF-A (Mouse, accession no. P25209), Xenopus nuclear transcription factor Y (Xenopus, accession no. AAC82336) and human nuclear transcription factor Y (Human, accession no. NP-006157) were carried out using the program ClustalW1.7 (32). Residues are shaded in red boxes to indicate sequence identity between either the S.cerevisiae Dpb4p or S.pombe Dpb4p homologue. Residues are shaded in light red to indicate sequence identity between other Dpb4 homologues. Dashes denote gaps in the sequence introduced to maximise the alignment. The conserved histone-fold motif is double overlined.
DISCUSSION
This paper presents evidence that the YDR121w open reading frame on S.cerevisiae chromosome IV encodes the fourth subunit of Polɛ complex. Therefore, YDR121w has been renamed DPB4. In addition, it is shown that Dpb4p (Fig. 3) and Dpb3p (our unpublished results) contain the histone-fold motif that is also present in many other proteins. The histone-fold motifs of Dpb4p and Dpb3p are related to those of subunits A and C, respectively, of human CCAAT binding factor CBF. An essential function of the histone-fold motifs of the CBF subunits is to create a protein–protein interaction surface for binding to the histone acetyltransferase enzymes such as GCN5 and p/CAF (35). These histone acetyltransferases can activate CBF transactivation in vivo, possibly by acetylation of the N-terminal lysine residues of histones which disrupts local chromatin structure, thereby facilitating CBF access to CCAAT promoter sites. At this time there is no evidence that Dpb3p and Dpb4p are part of the CCAAT binding factor; however, Dpb3p and Dpb4p may also create a protein–protein interaction surface allowing the Polɛ complex to interact with other proteins, such as the Dpb11p–Sld2p complex (23), which could also modify chromatin structure. Consistent with this, Δdpb4 genetically interacts with many other replication mutants (Table 2). Among those genetic interactions, the interaction between Δdpb4 and dpb11-1 is very strong. This strong interaction suggests that two proteins physically interact inside the cell. In fact, previous works showed that Dpb11p physically interacts with Polɛ complex during S phase (23). The interaction between Δdpb4 and pol2 is allelic specific. Only pol2-11 and pol2-12 mutations, but neither pol2-9, pol2-18 or pol2-16, showed synthetic lethality with Δdpb4 mutation (Table 2 and our unpublished results). Thus, these results strongly suggest that Dpb4p physically interacts with the C-terminal portion of Polɛ polypeptide. Other weak interactions observed between Δdpb4 and other DNA replication mutants may reflect their indirect interactions. Interestingly, no physical or genetic interaction has been detected between Dpb3p and Dpb4p (Table 2 and our unpublished results). Therefore, it is possible that Dpb3p and Dpb4p function independently.
Compared to the extensive knowledge of the influence of histone acetylation on transcription, little is known of its influence on chromatin structure in replication. It is possible that local disruption of chromatin structure by histone acetylation might facilitate access of replication factors to nucleosomal DNA. Thus, more information on the possible interaction between Polɛ subunits and proteins including histone acetyltransferases will provide insight into chromosomal DNA replication and the role of Polɛ in that process.
Recently Li et al. identified and cloned the two small subunits (p17 and p12 polypeptides) of HeLa Polɛ and found that they are homologues of S.cerevisiae Dpb4p and Dpb3p, respectively (36). Furthermore, we detected two smaller subunits similar to those two subunits of human Polɛ in Xenopus egg Polɛ (T.Ohya, S.Waga and A.Sugino, unpublished results). Thus, it is highly possible that the subunit structure of Polɛ is well conserved from lower eukaryotes to higher eukaryotes including human cells.
In summary, this study shows that the fourth subunit of S.cerevisiae Polɛ complex, and the overall subunit structure of Polɛ, is conserved in eukaryotes. In addition, the results indicate that while Dpb4p is not essential for cell viability, it plays a crucial role, along with Dpb3p, in maintaining the subunit structure of Polɛ and may facilitate interaction of the Polɛ complex with other replication proteins.
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported by Grants-in-Aid for Scientific Research on Priority Area from the Ministry of Education, Science, Sports and Culture of Japan and by a grant from the Mitsubishi Foundation to A.S.
REFERENCES
- 1.Sugino A. (1995) Trends Biochem. Sci., 20, 319–323. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio O.M., Weinstein,D.M. and Bell,S.P. (1997) Cell, 91, 59–69. [DOI] [PubMed] [Google Scholar]
- 3.Masumoto H., Sugino,A. and Araki,H. (2000) Mol. Cell. Biol., 20, 2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waga S. and Stillman,B. (1998) Annu. Rev. Biochem., 67, 721–751. [DOI] [PubMed] [Google Scholar]
- 5.Burgers P.M. (1998) Chromosoma, 107, 218–227. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto K., Nakashima,N., Ohara,T., Maki,S. and Sugino,A. (1998) Nucleic Acids Res., 26, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerik K.J., Li,X., Pautz,A. and Burgers,P.M. (1998) J. Biol. Chem., 273, 19747–19755. [DOI] [PubMed] [Google Scholar]
- 8.Zuo S., Bermudez,V., Zhang,G., Kelman,Z. and Hurwitz,J. (2000) J. Biol. Chem., 275, 5153–5162. [DOI] [PubMed] [Google Scholar]
- 9.Morrison A., Bell,J., Kunkel,T.A. and Sugino,A. (1991) Proc. Natl Acad. Sci. USA, 88, 9473–9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon M., Giot,L. and Faye,G. (1991) EMBO J., 10, 2165–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamatake R.K., Hasegawa,H., Clark,A.B., Bebenek,K., Kunkel,T.A. and Sugino,A. (1990) J. Biol. Chem., 265, 4072–4083. [PubMed] [Google Scholar]
- 12.Araki H., Hamatake,R.K., Johnston,L.H. and Sugino,A. (1991) Proc. Natl Acad. Sci. USA, 88, 4601–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgers P.M.J. (1991) J. Biol. Chem., 266, 22698–22706. [PubMed] [Google Scholar]
- 14.Maki S., Hashimoto,K., Ohara,T. and Sugino,A. (1998) J. Biol. Chem., 273, 21332–21341. [DOI] [PubMed] [Google Scholar]
- 15.Morrison A., Araki,H., Clark,A.B., Hamatake,R.K. and Sugino,A. (1990) Cell, 62, 1143–1151. [DOI] [PubMed] [Google Scholar]
- 16.Morrison A. and Sugino,A. (1994) Mol. Gen. Genet., 242, 289–296. [DOI] [PubMed] [Google Scholar]
- 17.Araki H., Ropp,P.A., Johnson,A.L., Johnston,L.H., Morrison,A. and Sugino,A. (1992) EMBO J., 11, 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navas T.A., Zhou,Z. and Elledge,S.J. (1995) Cell, 80, 29–39. [DOI] [PubMed] [Google Scholar]
- 19.Navas T.A., Sanchez,Y. and Elledge,S.J. (1996) Genes Dev., 10, 2632–2643. [DOI] [PubMed] [Google Scholar]
- 20.Budd M.E. and Campbell,J.L. (1993) Mol. Cell. Biol., 13, 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araki H., Hamatake,R.K., Morrison,A., Johnson,A.L., Johnston,L.H. and Sugino,A. (1991) Nucleic Acids Res., 19, 4867–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araki H., Leem,S.-H., Phongdara,A. and Sugino,A. (1995) Proc. Natl Acad. Sci. USA, 92, 11791–11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamimura Y., Masumoto,H., Sugino,A. and Araki,H. (1998) Mol. Cell. Biol., 18, 6102–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kesti T., Flick,K., Keranen,S., Syvaoja,J.E. and Wittenberg,C. (1999) Mol. Cell, 3, 679–685. [DOI] [PubMed] [Google Scholar]
- 25.Dua R., Levy,D.L. and Campbell,J.L. (1999) J. Biol. Chem., 274, 22283–22288. [DOI] [PubMed] [Google Scholar]
- 26.Sugino A., Ohara,T., Sebastian,J., Nakashima,N. and Araki,H. (1998) Genes Cells, 3, 99–110. [DOI] [PubMed] [Google Scholar]
- 27.Hubscher U., Nasheuer,I. and Syvaoja,J.E. (2000) Trends Biochem. Sci., 25, 143–147. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser C., Michaelis,S. and Mitchell,A. (1994) Methods in Yeast Genetics. A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Berben G., Duont,J., Gilliquet,V., Bolle,P.A. and Hilger,F. (1991) Yeast, 7, 475–477. [DOI] [PubMed] [Google Scholar]
- 30.Paulovich A.G., Margulies,R.U., Garvik,B.M. and Hartwell,L.H. (1997) Genetics, 145, 45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitada K., Johnston,L.H., Sugino,T. and Sugino,A. (1992) Genetics, 131, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson J.D., Higgins,D.G. and Gibson,T.J. (1994) Comput. Appl. Biosci., 10, 19–29. [DOI] [PubMed] [Google Scholar]
- 33.Arents G. and Moudrianakis,E.N. (1993) Proc. Natl Acad. Sci. USA, 90, 10489–10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baxevanis A.D., Arents,G., Moudrianakis,E.N. and Landsman,D. (1995) Nucleic Acids Res., 23, 2685–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Currie R.A. (1998) J. Biol. Chem., 273, 1430–1434. [DOI] [PubMed] [Google Scholar]
- 36.Li Y., Pursell,Z. and Linn,S. (2000) J. Biol. Chem., 275, 23247–23252. [DOI] [PubMed] [Google Scholar]