Abstract
Cannabidiol (CBD) has been used as a pharmacological treatment for psychiatric disorders in many studies, but few of good quality at the moment. Our objective was to assess the effect of CBD in mono/add-on therapy on symptom severity in psychiatric disorders. We performed a systematic review of clinical trials and randomized controlled trials that used CBD as treatment for psychiatric disorders. PRISMA criteria have been used for methodological purposes. Two assessors individually examined the results based on title and abstract, and decided which papers warranted full read. We included studies in English that measured disease severity as primary outcome. Out of 226 studies returned from the search, 9 warranted full read. There were 4 studies using CBD in schizophrenia, 3 studies of substance use disorder and 2 studies of social anxiety. CBD has a good safety profile even in higher doses, but results are inconclusive regarding improvements in disease severity.
Keywords: Anxiety, cannabidiol, CBD, schizophrenia, substance use
Main Points
CBD is safe to use, even in higher doses, in patients with psychiatric disorders such as schizophrenia, substance use disorders, and anxiety disorders.
The efficacy of CBD on disease severity is doubtful at the moment due to a low number of high-quality studies and lack of common ground regarding mean dose to be used and means of administration.
CBD could be useful in treating some symptoms of psychiatric disorders due to its effects on the ECS, but its effect on disease severity is limited.
Introduction
Cannabidiol (CBD), a cannabis derivative with low psychoactive activity, was revealed in 19401 and its structure fully explained in 1963,2 yet its mechanisms of action are still not fully understood. Since the isolation of Δ9-tetrahydrocannabinol (THC) as the main compound of cannabis in 1964,3 no further advances were made for 24 years until the first cannabinoid receptor (CB1) was discovered.4
The role of the endocannabinoid system (ECS) in the human brain is to influence synaptic communication between neurons and also to control other processes such as eating, anxiety, learning and memory, reproduction, metabolism, growth, and development.5 The ECS is known to regulate physiological appetite and energy metabolism, by increasing food intake through agonistic effects6 while affecting appetitive and consummatory aspects.7-9 The ECS also has a role in weight gain, independent from its effect on appetite,10 by inducing lipogenesis and increasing insulin resistance. One of the other roles of the ECS is to reduce both neuropathic and inflammatory pain.11,12
The 2 receptors, the cannabinoid receptor 1 (CB1R) and cannabinoid receptor 2 (CB2R), together with their primary ligands, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and the primary catabolic enzymes for these ligands, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), comprise the ECS.13 CBD is one of the many cannabinoids isolated from Cannabis sativa.14 Unlike its other major component, delta-9-tetrahydrocannabinol (THC), it does not cause psychotomimetic effects.15 What is more, preclinical studies have proven that CBD may present antipsychotic proprieties,16 currently believed to be through FAAH inhibition.17
Besides the 2 cannabinoid receptors (CB1 and CB2), there is the highly intricate endogenous cannabinoid system in the human brain which contains other receptors such as GPR55, GPR18 and GPR119, TRPA, TRPM, TRPC, and PPAR.18 These receptors decrease intracellular levels of calcium ions, either indirectly through coupling with G-proteins or directly through ionic channels. The endpoint of this cascade is the inhibition of release of neurotransmitters, such as GABA and glutamate. Besides these effects, it has been shown in preclinical studies that it may exhibit neuroprotective effects by inhibiting nitric oxide (NO) production, increasing brain-derived neurotrophic factor, and reducing zinc mobilization.20-22
The ECS has a neuroprotective effect in case of neuronal injury, both in acute (e.g., stroke) and chronic (e.g., Alzheimer’s disease) conditions.23 The mechanism behind this effect is still under debate. It is speculated that the deposition of beta-amyloid could induce the release of endocannabinoids, thus creating an imbalance between its neuroprotective effects (mediated by action on CB1R) and proinflammatory effects (mediated by action on CB2R). The ECS has also been linked to movement disorders, via its inhibitory effects on the CB1R, which is abundant in the basal ganglia.24 It is ventured that an imbalance in the ECS could be responsible for the movement deficit in Parkinson’s disease, Huntington’s disease, Gilles de la Tourette’s syndrome, tardive dyskinesia, and dystonia.6 There is also an endocannabinoid hypothesis in the etiology of schizophrenia which postulates that overactivity of the ECS could lead to an increase in dopamine and lowering in glutamate, which leads to the development of positive symptoms.25
CBD has been proposed as a pharmacological intervention for epilepsy, anxiety disorders, psychotic disorders, dementia, and other psychiatric diseases and neurodegenerative disorders.26
Initial evidence of the effect of CBD on anxiety came from studies on healthy volunteers that presented increased anxiety after the use of THC,27 and studies that showed increased use of cannabis in persons with anxiety as a form of “self-medication,”28 which lead to believe that there are both anxiogenic and anxiolytic compounds in cannabis. Yet, the mechanisms behind these effects are not fully understood.
There is growing interest in expanding treatment options where no new drugs with novel mechanisms of action have been introduced. Several studies suggest that CBD could be efficient in treating certain psychiatric disorders. Therefore, the purpose of our systematic review is to assess the effect of CBD as a pharmacological intervention in psychiatric disorders and its impact on disease severity.
Methods
The present systematic review was conducted in line with the PRISMA guidelines and reporting criteria.29
We searched multiple databases (PubMed—MEDLINE, ClinicalTrials.gov, Web of Science All Databases) for articles that focused on the use of CBD as monotherapy or add-on therapy in psychiatric patients. In MEDLINE, we performed a search using the phrasing “(CBD [MeSH Terms]) AND (psychiatry and psychology category[MeSH Terms])” and “CBD AND psychiatry” on Web of Science, and in ClinicalTrials.gov, due to differences in search input format and applied filters for English language, Randomized Controlled Trials and Clinical Trials. The PubMed search yielded 81 results between 1975 and 2020. The ClinicalTrials.gov database returned 86 studies, with 28 completed studies, out of which 9 presented results. The Web of Science All Databases search returned 59 clinical trials during the same time period.
We included in the systematic review only the studies that assessed the effect of CBD as monotherapy or add-on therapy on disease severity as primary outcome in psychiatric disorders, and only studies published in English. All studies were screened by title and abstract in order to further select the studies that met the eligibility criteria.
After the screening process, all the remaining articles were reviewed and the following data were extracted: author, year of publication, psychiatric disorder, sample size, drugs administered and dosage, and primary outcome measure used.
Results
There were a total of 226 studies screened for eligibility (see selection diagram for detailed process). Of the 81 studies from PubMed, 73 were excluded because they did not use only CBD in the intervention group (there was a combination of CBD and THC), did not address psychiatric patients (but healthy volunteers or neurologic patients), or did not measure disease severity as primary outcome. Only 9 studies were read in full, out of which 4 were schizophrenia trials,17,30-32 3 addressed substance use disorders,33,34 and 2 addressed anxiety disorders35,36 (see Table 1 for details about each study included).
Table 1.
Studies Included in the Review
| Author/Year | Psychiatric Disorder | Duration | Sample Size | CBD Dosage | Monotherapy/Add-On | Comparator | Primary Outcome Measure | Risk of Bias45 |
|---|---|---|---|---|---|---|---|---|
| Zuardi et al.32 2006 | Treatment-resistant Schizophrenia | 5 weeks | 3 | 40-1280 mg per day | Monotherapy | None (case–control design) | BPRS | - |
| Leweke et al.17 2012 | Schizophrenia | 4 weeks | 39 | 200-800 mg/day | Monotherapy | Amisulpride | BPRS and PANSS | Low |
| McGuire et al.30 2017 | Schizophrenia | 6 weeks | 88 | 1000 mg/day | Add-on | Placebo | PANSS, SANS, CGI, BACS | Low |
| Boggs et al.31 2018 | Schizophrenia | 6 weeks | 36 | 600 mg/day | Add-on | Placebo | MCCB, PANSS | High |
| Morgan et al.34 2013 | Tobacco addiction | 1 week | 24 | 400 μg aerosol solution/day | Monotherapy | Placebo | Number of cigarettes smoked per week | High |
| Hurd et al.33 2019 | Heroin use disorder | 1 week | 42 | 400 or 800 mg/day | Monotherapy | Placebo | VAS-C/VAS-A | High |
| Hill37 2017 | Cannabis use disorder | 6 weeks | 10 | Up to 800 mg/day | Monotherapy | Placebo | Self-report Cannabis Use | High |
| Crippa et al.36 2011 | Social anxiety | 140 min | 10 | Single 400 mg dose | Monotherapy | Placebo | VAMS | High |
| Bergamaschi et al.35 2011 | Social anxiety | 2.30 min | 36 | Single 600 mg dose | Monotherapy | Placebo and healthy controls | VAMS, SSPS-N | High |
BPRS, brief psychiatric rating scale; PANSS, positive and negative symptoms scale; SANS, scale for the assessment of negative symptoms; CGI, clinical global impression scale; BACS, brief assessment of cognition in schizophrenia; MCCB, matrics consensus cognitive battery; VAS-C/A, visual analog scale for craving/anxiety; VAMS, visual analog mood scale; SSPS-N, negative self-statement scale.
Out of all 9 completed studies with results presented from ClinicalTrials.gov, 8 were excluded on the grounds of being doubles, not including psychiatric patients, or not using CBD alone in the intervention group. Only 1 study was included for full read and it addressed patients with cannabis use disorder.37
All the Web of Science results were doubles of the PubMed results and none were included for full read. In total there were 9 studies warranting full read. The full diagram of study selection is presented in Figure 1.
Discussion
CBD in Schizophrenia
All the studies included examined the effect of CBD on patients with schizophrenia or schizophrenia spectrum disorders. Only one of the studies had a case–control design,32 the rest being randomized controlled trials. The case control study evaluated the potential effect of CBD in treatment-resistant schizophrenia. Although the study had negative results, it emphasized the good tolerability and safety profiles of high-dose CBD (max 1280 mg/day).
One17 study focused on the effect of CBD on levels of AEA, an endocannabinoid transmitter, believed to be involved in modulation of pain, mood, and cognition.38 This study aimed to alleviate psychotic symptoms in patients with schizophrenia and schizophreniform psychosis by raising AEA signaling through administration of CBD (200-800 mg/day), using amisulpride (200-800 mg/day) as comparator. Both groups had less severe symptoms at 28 days, as measured by PANSS, but the study could not prove non-inferiority of the CBD group (P = .27). Their results suggest that CBD could be an effective alternative to improve psychotic symptoms, with good tolerance and safety profile, although sample size and follow-up duration were rather small, warranting replication studies with larger sample sizes.
Two studies used CBD as adjunctive therapy30,31 in patients with schizophrenia, with conflicting results. Boggs et al.31 showed that both the CBD (600 mg/day) and the placebo group showed a decrease in PANSS scores over a period of 6 weeks but concluded that the improvement was not attributable to CBD. The same results were observed for cognitive improvement. Furthermore, a post-hoc analysis revealed that only the placebo group showed cognitive improvements over time, despite the similar baseline cognitive functioning. In comparison, McGuire et al.30 showed no statistically significant improvements in PANSS total, general, or negative scores in the CBD (1000 mg/day) group compared to placebo at study endpoint (8 weeks), but there were significant improvements (P = .019) after 8 weeks in the CBD group on the PANSS positive symptoms subscale. What is more, the CBD group showed improved motor speed but not executive functioning, thus showing some benefits of CBD on cognition. One important finding by McGuire et al.30 was the presence of improvements in CGI-S and CGI-I in the CBD group, meaning that even though the improvements according to scale scores were modest, they were clinically meaningful.
It seems that CBD is safe to use in schizophrenia patients but the results are mixed when assessing its effects on disease severity. The results are even more difficult to interpret in the case of studies where clinicians declared improvements in severity even though the objective scales showed no change.
More so, the later improvements in positive symptoms shown in some studies warrants replication due to possible confounding factors. Improvements could be due to longer duration of antipsychotic treatment or the natural course of illness. On the other hand, the neuromodulation effect induced by CBD through the ECS may only become apparent after a longer period of time.
CBD in Substance Use Disorders
There were 3 studies assessing the use of CBD in substance use disorders.33,34,37 The use of CBD in these disorders is due to the involvement of the ECS in the reward and reinforcement circuits of the brain,39 which is common ground with substance use disorders. CBD has been shown34 to significantly reduce the number of cigarettes smoked in individuals with tobacco addiction, but at the same time had no impact on cigarette craving. Thus, the mechanism behind its possible efficiency remains unclear.34 It is speculated that, from a neurobiological point of view, its effects are directly related to the action of CBD on the CB1R. Another piece of evidence comes from preclinical studies on rats where it has been shown that inhibiting FAAH by CBD administration17 causes a decrease in the reinforcing and neurochemical effects of nicotine.40 Smoking reduction could occur due to modulation of salience cues determined by CBD, also shown in preclinical studies.41 Further clinical studies designed to evaluate the effect of CBD on cigarette addiction,34 as performed in the case of CBD for heroin addiction,33 are required to assess these preclinical findings. Hurd et al.33 found that administering either 400 mg or 800 mg/day of CBD significantly reduced heroin craving compared to placebo. Craving was slightly lower in the 800 mg/day group but the intragroup differences did not reach statistical significance. After 1 week since the last CBD dose, craving was significantly reduced in the 800 mg group while at the same time increased in the control group. Challenged with video drug cues, all groups showed an increase in anxiety compared to baseline assessment; furthermore, participants receiving CBD at 800 mg/day had the lowest baseline-adjusted increase in anxiety. While there was no difference in cognitive performance between the groups, statistically significant benefits for physiological measures were present in the CBD group, with lower heart-rates, temperature, and salivary cortisol levels when exposed to drug cues, compared to placebo. This shows promising results for the use of CBD in reducing drug-cue-induced craving and anxiety in opioid use disorder, and could be an important addition to currently available addiction medication.
One completed trial for the use of CBD in cannabis use disorder was identified,37 rendering negative results. The use of 800 mg/day CBD did not decrease the number of cannabis inhalations per day while the placebo group showed significantly reduced daily consumption. The results from this study cannot be used to draw firm conclusions in regard to the use of CBD in cannabis use disorder due to its low patient number (N = 9) and fewer inhalations of cannabis per day present at baseline in the placebo group.
CBD could be used to reduce symptoms of anxiety related to substance abuse (both in abstinent and currently dependent patients) and the number of drug administrations. These effects seem to be different depending on the type of substance the person is addicted to and the phase in which it is administered, with better results in presently abstinent patients compared to active users. There were no severe side effects in this trial.
CBD in Anxiety Disorders
Both studies assessing single-dose administration of CBD in anxiety disorders focused on its effects on social anxiety.35,36 Participants who received CBD in 400 mg and 600 mg/day reported significantly lower levels of subjective anxiety compared to controls. This anxiolytic effect has been shown in fMRI studies to be related to reduced activity in limbic and paralimbic brain areas, both known to be associated with anxiety.42,43 From the neurobiological perspective, CBD could exert its anxiogenic effects through 5-HT1A receptor agonism.44 Due its safety and tolerability, CBD could be considered a viable option for treating social phobia, with current results demanding further research for its use in other anxiety disorders as well.
Limitations
Our review depicts the current results of using CBD for reducing disease severity in psychiatric diseases. One possible limitation is the language barrier, in including only papers written in English. The limited data available should also raise caution in interpreting the results. Moreover, there were studies assessed as having a high risk of bias. This warrants improvements in study design and methodology to be used in future studies in order to increase the validity of results.
Conclusion
Despite the general interest in CBD treatment of psychiatric diseases, there is an extremely low number of quality studies in this domain. CBD has been proven to be safe to use even in higher doses in social phobia, schizophrenia, and substance use disorders, as monotherapy or add-on therapy, but the evidence regarding its effects on disease severity is contradictory. It seems that the use of CBD in psychiatric disorders is still a gray area and there is uncertainty regarding its efficacy in this field.
Figure 1.
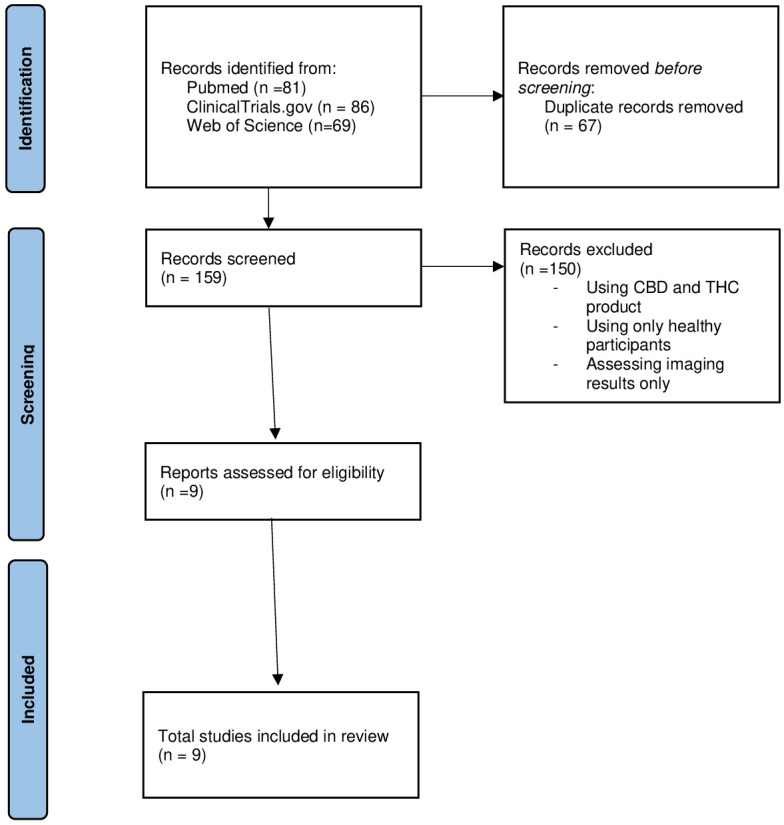
PRISMA flow diagram.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Peer Review: Externally peer-reviewed.
Author Contributions: Design - A.P.; Writing - A.P., R.P.; Critical Reviews - V.M.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1. Adams R, Hunt M, Clark JH. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I. J Am Chem Soc. 1940;62:196 200. 10.1021/ja01858a058 [DOI] [Google Scholar]
- 2. Mechoulam R, Shvo Y. Hashish—I. Tetrahedron. 1963;19:2073 2078. 10.1016/0040-4020(63)85022-X [DOI] [PubMed] [Google Scholar]
- 3. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646 1647. 10.1021/ja01062a046 [DOI] [Google Scholar]
- 4. Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605 613. [PubMed] [Google Scholar]
- 5. Skaper SD, Di Marzo V. Endocannabinoids in nervous system health and disease: the big picture in a nutshell. Philos Trans R Soc B Biol Sci. 2012;367:3193 3200. 10.1098/rstb.2012.0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sierra H, Cordova M, Chen C-SJ, Rajadhyaksha M. Confocal imaging–guided laser ablation of basal cell carcinomas: an ex vivo study. J Invest Dermatol. 2015;135:612 615. 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaperon F, Soubrié P, Puech AJ, Thiébot MH. Involvement of central cannabinoid (CB 1 ) receptors in the establishment of place conditioning in rats. Psychopharmacology. 1998;135:324 332. 10.1007/s002130050518 [DOI] [PubMed] [Google Scholar]
- 8. Thornton-Jones ZD, Vickers SP, Clifton PG. The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacol (Berl). 2005;179:452 460. 10.1007/s00213-004-2047-8 [DOI] [PubMed] [Google Scholar]
- 9. Hungund BL. Endocannabinoid system: a newer molecular target for the treatment of alcohol-related behaviors. World J Pharmacol. 2012;1:44. 10.5497/wjp.v1.i3.44 [DOI] [Google Scholar]
- 10. Greenberg I, Kuehnle J, Mendelson JH, Bernstein JG. Effects of marihuana use on body weight and caloric intake in humans. Psychopharmacol (Berl). 1976;49:79 84. 10.1007/BF00427475 [DOI] [PubMed] [Google Scholar]
- 11. Guindon J, De Léan A, Beaulieu P. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain. 2006;121:85 93. 10.1016/j.pain.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 12. Guindon J, LoVerme J, De Léan A, Piomelli D, Beaulieu P. Synergistic antinociceptive effects of anandamide, an endocannabinoid, and nonsteroidal anti-inflammatory drugs in peripheral tissue: a role for endogenous fatty-acid ethanolamides? Eur J Pharmacol. 2006;550:68 77. 10.1016/j.ejphar.2006.08.045 [DOI] [PubMed] [Google Scholar]
- 13. Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21 47. 10.1146/annurev-psych-113011-143739 [DOI] [PubMed] [Google Scholar]
- 14. ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A. Phytochemistry of cannabis sativa L. In: progress in the chemistry of organic natural products [internet]. 2017. Berlin; Springer:1 36. [DOI] [PubMed] [Google Scholar]
- 15. Zuardi AW, Guimarães FS, Moreira AC. Effect of cannabidiol on plasma prolactin, growth hormone and cortisol in human volunteers. Braz J Med Biol Res. 1993;26:213 217. [PubMed] [Google Scholar]
- 16. Gomes FV, Llorente R, Del Bel EA, et al. Decreased glial reactivity could be involved in the antipsychotic-like effect of cannabidiol. Schizophr Res. 2015. May;164:155 163. 10.1016/j.schres.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 17. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94 e94. 10.1038/tp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dewey WL. Cannabinoid pharmacology. J Ethnopharmacol. 1987;20:293 294. 10.1016/0378-8741(87)90061-4 [DOI] [Google Scholar]
- 19. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19:833. 10.3390/ijms19030833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SH, Won SJ, Mao XO, Jin K, Greenberg DA. Molecular mechanisms of cannabinoid protection from neuronal excitotoxicity. Mol Pharmacol. 2006;69:691 696. 10.1124/mol.105.016428 [DOI] [PubMed] [Google Scholar]
- 21. Khaspekov LG, Brenz Verca MS, Frumkina LE, et al. Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. Eur J Neurosci. 2004;19:1691 1698. 10.1111/j.1460-9568.2004.03285.x [DOI] [PubMed] [Google Scholar]
- 22. Sánchez-Blázquez P, Rodríguez-Muñoz M, Vicente-Sánchez A, Garzón J. Cannabinoid receptors couple to NMDA receptors to reduce the production of NO and the mobilization of zinc induced by glutamate. Antioxid Redox Signal. 2013;19:1766 1782. 10.1089/ars.2012.5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramos JA, González S, Sagredo O, Gómez-Ruiz M, Fernández-Ruiz J. Therapeutic potential of the endocannabinoid system in the brain. Mini Rev Med Chem. 2005;5:609 617. 10.2174/1389557054368817 [DOI] [PubMed] [Google Scholar]
- 24. Sañudo-Peña MC, Tsou K, Walker JM. Motor actions of cannabinoids in the basal ganglia output nuclei. Life Sci. 1999;65 703 713. 10.1016/s0024-3205(99)00293-3 [DOI] [PubMed] [Google Scholar]
- 25. Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci. 2006;63:1597 1613. 10.1007/s00018-006-6027-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huestis MA, Solimini R, Pichini S, et al. Cannabidiol adverse effects and toxicity. Curr Neuropharmacol. 2019;17:974 989. 10.2174/1570159X17666190603171901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515 523. 10.1002/hup.1048 [DOI] [PubMed] [Google Scholar]
- 28. Buckner JD, Schmidt NB, Lang AR, et al. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. J Psychiatr Res. 2008;42:230 239. 10.1016/j.jpsychires.2007.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med. 2009;6:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175:225 231. 10.1176/appi.ajp.2017.17030325 [DOI] [PubMed] [Google Scholar]
- 31. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol (Berl). 2018;235:1923 1932. 10.1007/s00213-018-4885-9 [DOI] [PubMed] [Google Scholar]
- 32. Zuardi AW, Hallak JEC, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20:683 686. 10.1177/0269881106060967 [DOI] [PubMed] [Google Scholar]
- 33. Hurd YL, Spriggs S, Alishayev J, et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals With heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019;176:911 922. 10.1176/appi.ajp.2019.18101191 [DOI] [PubMed] [Google Scholar]
- 34. Morgan CJA, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav. 2013;38:2433 2436. 10.1016/j.addbeh.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 35. Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36:1219 1226. 10.1038/npp.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crippa JAS, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25:121 130. 10.1177/0269881110379283 [DOI] [PubMed] [Google Scholar]
- 37. Hill K. Cannabidiol pharmacotherapy for adults With cannabis use disorder. Full Text View ClinicalTrials.gov 2016:1 20. [Google Scholar]
- 38. Di Marzo V, Petrosino S. Endocannabinoids and the regulation of their levels in health and disease. Curr Opin Lipidol. 2007;18(2):129 140. 10.1097/MOL.0b013e32803dbdec [DOI] [PubMed] [Google Scholar]
- 39. Serrano A, Parsons LH. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Ther. 2011;132:215 241. 10.1016/j.pharmthera.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. González S, Grazia Cascio M, Fernández-Ruiz J, et al. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954(1):73 81. 10.1016/S0006-8993(02)03344-9 [DOI] [PubMed] [Google Scholar]
- 41. Ren Y, Whittard J, Higuera-Matas A, Morris C V., Hurd YL. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci. 2009;29:14764 14769. 10.1523/JNEUROSCI.4291-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fusar-Poli P, Allen P, Bhattacharyya S, et al. Modulation of effective connectivity during emotional processing by Δ9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol. 2010;13:421-432. 10.1017/S1461145709990617 [DOI] [PubMed] [Google Scholar]
- 43. Fusar-Poli P, Crippa JA, Bhattacharyya S, et al. Distinct effects of Δ9-tetrahydrocannabinol and cannabidiol on neural activation During emotional processing. Arch Gen Psychiatry. 2009;66:95-105. 10.1001/archgenpsychiatry.2008.519 [DOI] [PubMed] [Google Scholar]
- 44. Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1A receptors. Neurochem Res. 2005;30:1037 1043. 10.1007/s11064-005-6978-1 [DOI] [PubMed] [Google Scholar]
- 45. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a