Abstract
Background
The subgenual cingulate cortex (SGC) has been identified as a key structure within multiple neural circuits whose dysfunction is implicated in the neurobiology of depression. Deep brain stimulation in the SGC is thought to reduce and normalize local metabolism, causing normalization of circuit behavior and an improvement in depressive symptoms. We hypothesized that nonablative stereotactic radiosurgery (SRS) to the SGC would reduce local metabolism and reduce the severity of depression in patients with treatment-resistant bipolar depression.
Methods
Under the FDA’s Humanitarian Device Exemption program, patients were screened for inclusion and exclusion criteria. Three volunteers meeting the criteria provided informed consent. Bilateral SGC targets were irradiated to a maximum dose of 75 Gy in one fraction. Subjects were followed for one year following the procedure with mood assessments (Hamilton Depression Rating Scale (HDRS), Clinical Global Impression-Improvement, Clinical Global Impression-Severity, and Young Mania Rating Scale), neurocognitive testing (Delis-Kaplan Executive Function System, Wechsler Adult Intelligence Scale III digit span, and California Verbal Learning Test II), and imaging. Further imaging was completed approximately two years after the procedure. Clinical improvement was defined as a ≥50% reduction in HDRS.
Results
Two of the three subjects showed clinical improvement in depressive symptoms during the follow-up period, while one subject showed no change in symptom severity. One of three subjects was hospitalized for the emergence of an episode of psychotic mania after discontinuing antipsychotic medications against medical advice but promptly recovered with the reinstitution of an antipsychotic. Sequential assessments did not reveal impairment in any cognitive domain assessed. For one of the three subjects, MRI imaging showed evidence of edema at 12 months post-SRS, which resolved at 22 months post-procedure. In a second of three patients, there was evidence of local edema at the target site at long-term follow-up. All imaging changes were asymptomatic.
Conclusion
Radiosurgical targeting of the SGC may be a noninvasive strategy for the reduction of severe depression in treatment-resistant bipolar disorder. Two out of three patients showed clinical improvement. While these results are promising, further study, including improvements in target selection and dosing considerations, is needed.
Keywords: bipolar depression, neuromodulation, radiosurgery, subcallosal cortex, treatment resistance
Introduction
Bipolar disorder has a progressive course broadly characterized by an early onset of illness, highly recurrent and chronic major depressive episodes (MDEs), and increasing disability. Bipolar 1 disorder is associated with euphoric manias early in the course, while over time these euphoric states are replaced by dysphoric or mixed manic states, rapid cycling, and severe, chronic MDEs that are difficult to treat [1-9]. Even with the appropriate use of mood stabilizers and adjunctive treatments, sustained remission occurs in a minority of patients.
Treatment-resistant bipolar depression (TRBD) has a notably higher risk of morbidity and mortality than unipolar depression [10-12]. While the manic phase defines the illness, over the course of time, patients are most symptomatic in the depressed phase, in which the risk of suicide is greatest [2]. The high prevalence of all bipolar disorders [13], the increased chronicity and disability primarily associated with depressive episodes over the illness course [14], and the large proportion of patients with treatment-resistant bipolar disorder make the development of new strategies and technologies for TRBD a high priority.
The brain circuits associated with bipolar disorder include the subgenual cingulate cortex (SGC). The SGC has been postulated to be an important nexus in a web of connected prefrontal, subcortical, temporal, and limbic structures in which dysregulation is thought to be a key feature of the pathophysiology of mood disorders [15,16]. Volumetric MRI studies have shown marked atrophy of SGC in bipolar disorder [15,17], which may be reversible [18]. Volumetric cell counts and cytoarchitectural studies suggest the volume loss in SGC is primarily due to the loss of glial cells rather than neurons [19]. The SGC has been reported to be hypermetabolic in the depressed state, which then normalizes in those who respond to antidepressant treatment [20], although the relationship between metabolism in the SGC and treatment is complex [21]. Mood disorders have been postulated to be disorders of circuit hyperconnectivity [22], a finding consistent with the presence of a strong bidirectional structural neuroanatomic pathway between the SGC and many limbic and prefrontal structures [23]. It has been reported that depression is associated with greater connectivity of the SGC to the default mode network [24]. Another study showed that over the course of electroconvulsive therapy (ECT) treatment, there was a significant reversal of hyperconnectivity between the SGC and the bilateral hippocampus, bilateral temporal pole, and ventromedial prefrontal cortex [25]. An open study of deep brain stimulation (DBS) in the SGC has been published for 17 unipolar and bipolar subjects with treatment-resistant depression [26]. These results and others have been encouraging, with many patients experiencing persisting improvement or remission [27]. DBS is relatively safe; however, the implantation is an open neurosurgical procedure and carries associated risks such as infection, blood loss, and neurological disability.
Stereotactic radiosurgery (SRS) has been used extensively for the treatment of trigeminal neuralgia by targeting either the cisternal or dorsal root entry zone of the nerve, with a low incidence of facial sensory loss as a result of treatment [28-31]. This is one piece of evidence that suggests that, at moderate doses, radiation can exert nondestructive effects on neural function. Studies in animal models and in humans show that delivery of low-dose radiation of up to 80 Gy does not appear to result in cell death in the Gasserian ganglion, although there is local destruction of the myelin sheath and clear neuronal death in doses above 100 Gy [32-34].
We hypothesized that targeting SGC with focused radiation could safely reduce local metabolic activity and, similar to DBS, reduce depressive symptoms in TRBD. The dose of radiation used in this study was based on published clinical studies for the treatment of pain with trigeminal neuralgia [35].
Materials and methods
This study was done as described in the FDA-approved Humanitarian Investigational Device Exemption (G090015/S010) titled “Radiosurgical Neuromodulation for Refractory Bipolar Depression.” Institutional IRB protocols were followed. Each subject was carefully screened and assessed, and a detailed discussion of the study and the potential risks and benefits ensued. This process was continued until the investigators were satisfied that the potential patient fully understood the study, the procedures, the potential risks, and the possible benefits of the radiosurgical procedure. The subjects were assessed by a psychiatrist, who was not otherwise involved in the study, to address the competence of the subject’s understanding of the risks and benefits of participation and to uncover special circumstances that might have created a context of undue influence on participation in the trial. Four subjects were consented to and screened for the study; one subject was excluded due to a preexisting structural abnormality on the subsequent screening MRI. The inclusion criteria for the study were as follows: primary diagnosis of bipolar depression per the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM IV-TR) criteria (study carried out between 2010 and 2012); duration of the current MDE of more than one year; Hamilton Depression Rating Scale-24 (HDRS-24) item greater than or equal to 20; treatment resistance: a history of failure to show clinical improvement after at least four different medication trials of adequate dose and duration; nonresponse or lack of sustained improvement after one course of ECT (six to 12 sessions); no viable treatment options at the time of enrollment; competent to understand the risks and potential benefits of the study; able to provide written informed consent for the full screening phase, as well as the treatment period of the protocol, including the baseline MRI, CT, and PET imaging; and signed consent form for participation in the study. The exclusion criteria were rapid cycling bipolar illness, history of schizophrenia, schizoaffective disorder, or psychosis; severe suicidal thoughts that may put the subject at risk of either attempted suicide or completed suicide for the duration of the trial, as determined by the investigator at the time of enrollment; current substance abuse or withdrawal from psychoactive substances, including alcohol, stimulants, or sedatives; previous whole-brain radiation; and brain-implanted devices such as DBS leads or aneurysm clips. The schedule of events and assessments is shown in Table 1. Demographic information, illness course, and medication history are shown in Table 2, Table 3, and Table 4 for the three subjects who completed the intervention phase of the protocol.
Table 1. Treatment protocol.
| Screen | Baseline | Week | Month | |||||
| 1 | 2 | 3 | 6 | 9 | 12 | |||
| Clinical scales | X | X | X | X | X | X | X | X |
| Cognitive tests | X | X | X | |||||
| MRI | X | X | X | X | X | X | ||
| PET/CT | X | X | ||||||
| Responsive neurostimulation treatment | X | |||||||
Table 2. Subject demographics and characteristics.
ADD, attention deficit disorder; BP, bipolar disorder; D, divorced; ECT, electroconvulsive therapy; ETOH, alcohol; MDD, major depressive disorder; PD, personality disorder; S, single
| Demographics | Subject 1 | Subject 2 | Subject 3 |
| Age at the time of study entry | 60 | 35 | 65 |
| Sex/race | M/White | F/Asian | M/White |
| Marital status | D | S | S |
| Age first received any psychiatric care | 37 | 12 | 20 |
| Diagnoses prior to BP diagnosis | MDD and conduct disorder | MDD and avoidant PD social anxiety disorder | Schizoid PD, ADD, MDD, and dysthymia |
| Substance abuse (last used) | Marijuana (6 months prior to study) | None | ETOH/cocaine (10 years) |
| Family history | |||
| Bipolar disorder | None | Two suspected: mother and maternal aunt | One suspected: maternal aunt |
| Other familial Axis 1 diagnoses | MDD in the mother, sister, and maternal uncle | Substance abuse by a maternal cousin | Postpartum depression in the maternal aunt and MDD in the maternal niece |
| Completed suicide | One maternal uncle | One maternal cousin | None |
| ECT | None | None | Maternal aunt |
Table 3. Illness course.
BP, bipolar disorder; CGI-S, Clinical Global Impression-Severity; GAF, Global Assessment of Functioning; MDE, major depressive episode; N/A, not applicable; STEP-BD, Systematic Treatment Enhancement Program for Bipolar Disorder
| Subject | 1 | 2 | 3 |
| Followed in the STEP-BD program since | 2003 | 2002 | N/A |
| Age at onset | Mid-30s | 16 | 15 |
| Age of diagnosis (BP1 or BP2) | 45 | 24 | 54 |
| Age of the first hypomanic or manic episode | 42 | 16 | 20 |
| Date of onset for the current MDE | 2005 (observed) | 2006 (observed) | 1997 (reported) |
| Longest period of observed improvement: (CGI-S scores ≤2 and GAF ≥60) | 10 months, 2004-2005 | 5 months, 2006 | N/A |
| Number of prior MDEs | 3 (chronic) | >25 | >5 |
| History of a psychotic mood episode | With mania | None | With MDE |
| History of rapid cycling | - | + | + |
| Last manic or hypomanic episode | 2004 | 2004 | 2009 |
| Number of hypomanic or manic episodes | 3 manic episodes | >5 | >3 |
| Hypomania and/or mania during the study | 1 manic episode | None | None |
| Number of past suicide attempts | 2 | 3 | None known |
| Number of past hospitalizations | 3 | >5 | 4 |
Table 4. Treatment history.
* The first acute/continuation ECT course was discontinued after 14 total treatments due to poor response and difficult-to-control paroxysmal atrial fibrillation with the last two ECT treatments. Two other acute courses were attempted in 2007 and 2009 but were discontinued after the first treatment due to the return of atrial fibrillation.
ARP, aripiprazole; BPN, bupropion; BRF, brief; CBT, cognitive behavioral therapy; CBZ, carbamazepine; CTP, citalopram; DBT, dialectical behavior therapy; DLPFC, dorsolateral prefrontal cortex; DLX, duloxetine; eCTP, escitalopram; FLX, fluoxetine; LT, long term; LTG, lamotrigine; MDF, modafinil; MRT, mirtazapine; MT, motor threshold; NFD, nefazodone; OFC, olanzapine/fluoxetine combination; OLZ, olanzapine; PD, psychodynamic; PRX, paroxetine; QTP, quetiapine; rMDF, armodafinil; RSP, risperidone; SRT, sertraline; ST, supportive; TCA, tricyclic antidepressant; TCN, tranylcypromine; TMS, transcranial magnetic stimulation; tSLG, transdermal selegiline; tx, treatments; VEN, venlafaxine; VPA, valproate; ZPN, ziprasidone
| Subject | 1 | 2 | 3 | |
| Treatment at study entry | Mood stabilizer | OLZ (OFC) (6 mg) | ZPN (10 mg) and clozapine (37.5 mg) | Lithium (300 mg) |
| Antidepressant | FLX (OFC) (50 mg), BPN (150 mg), and SRT (100 mg) | None | None | |
| At the end of the study | Mood stabilizer | OLZ (OFC) | ZPN and clozapine | Lithium |
| Antidepressant | FLX (OFC) | None | None | |
| ECT | 0 | 8 | 0 | |
| Lifetime | Mood stabilizers | Lithium, VPA, LTG, OLZ, ARP, ZPN, and RSP | Lithium, VPA, CBZ, LTG, OLZ, ARP, ZPN, QTP, and RSP | Lithium, LTG, VPA, QTP, RSP, and intolerance ARP |
| Antidepressants | TCN, tSLG, FLX, SRT, BPN, DLX, VEN, and MDF | PRX, VEN, FLX, CTP, MRT, BPN, and rMDF | eCTP, CTP, FLX, BPN, NFD, DLX, and >1 TCA | |
| Psychotherapy | LT: ST and PD; BRF: CBT and DBT | LT: ST and CBT; BRF: CBT and DBT | LT: ST and PD | |
| Prior ECT courses | 1 | 1-2 years maintenance | 3* | |
| Total number of ECT tx prior to study | 14 | 56 | None known | |
| Total number of ECT treatments during the study | 0 | 8 | 0 | |
| TMS 120% MT 10 Hz left DLPFC | 0 | 0 | One course of 25 treatments | |
All patients were interviewed using the Mini International Neuropsychiatric Interview (MINI) Structured Clinical Interview for DSM (SCID) to confirm the DSM-IV diagnosis of bipolar disorder in the depressive phase. Clinical rating scales used were the 17, 21, and 24-item HDRS (range 0-52), the Clinical Global Impression-Severity (CGI-S, range 1-7) and Clinical Global Impression-Improvement (CGI-I, range 1-7), and the Young Mania Rating Scale (YMRS, range 0-60). These clinical assessments were done at weeks 1, 2, and four weeks, and two, three, six, nine, and 12 months post-SRS. At baseline prior to the SRS procedure, patients completed a neuropsychiatric battery using the Delis-Kaplan Executive Function System (DKEFS), the Wechsler Adult Intelligence Scale III (WAIS-III) digit span, and the California Verbal Learning Test II (CVLT-II). These cognitive tests were repeated at the three- and 12-month follow-up visits. Adverse events were reviewed and logged with each visit.
SRS planning
A preprocedure CT of the head without contrast was obtained, as were T1-weighted, T2, and fluid-attenuated inversion recovery (FLAIR) sequence MRI data sets. The target location within the SGC was anatomically determined using a digital fusion of the CT data set with the T1-weighted MRI data set, and the accuracy of the fusion was verified by a neurosurgeon supervising the treatment. The target regions were then digitally demarcated by representing them as two targeted spheres (left and right), each 5 mm in diameter and 66 mm3 in volume. The 60 Gy was prescribed to the target volumes in the 80% isodose line by an automated beam planning process that used approximately 100 beams for each target; therefore, the maximum dose was 75 Gy.
SRS treatment
Radiation was administered to the two bilateral SGC targets on two consecutive days. During each procedure, the patient was supine on the treatment table with their head immobilized within a custom-fitted face mask. The patient was then registered within the stereotactic space using image-based registration. Once proper registration was confirmed, radiation delivery began in accordance with the treatment plan. Following each treatment, the patient was given 4 mg of dexamethasone to minimize the likelihood of potential nausea or edema associated with the treatment. The patient was then discharged home with follow-up instructions. A brief physical exam was conducted at the first follow-up appointment one week after the procedure by a neurosurgeon.
Imaging
Follow-up MRIs, which included T1, T2, and FLAIR sequences, were done at one week, three, six, nine, and 12 months in order to identify structural brain changes.
Results
Subjects had a mean decrease in HDRS-17 of 27% between baseline and one-year post-intervention follow-up.
Subject 1 met response criteria (≥50% reduction) on the HDRS-17 and HDRS-21 at six (54%) and 12 months (50%) post-intervention. He met response criteria on the HDRS-24 at three-, six-, and 12-months post-SRS. Subject 2 met response criteria at the two and three-month assessments and was remitted at six months on all HDRS scales. Subject 3 showed a pattern of decreasing severity of symptoms up to the three-month visit, but this was not sustained, with a return to baseline severity at 12 months. HDRS-17, HDRS-21, and HDRS-24 scores are found in Figure 1, Figure 2, and Figure 3.
Figure 1. HDRS-17 scores from screening through 12 months post-SRS.
HDRS, Hamilton Depression Rating Scale; SRS, stereotactic radiosurgery
Figure 2. HDRS-21 scores from screening through 12 months post-SRS.
HDRS, Hamilton Depression Rating Scale; SRS, stereotactic radiosurgery
Figure 3. HDRS-24 scores from screening through 12 months post-SRS.
HDRS, Hamilton Depression Rating Scale; SRS, stereotactic radiosurgery
Both Subjects 1 and 2 showed improvement on the CGI-S shown in Figure 4. Their scores decreased from 5 (severely depressed) to 2 (borderline mentally ill) at 12 months. Both subjects also showed marked improvement on the CGI-I, with both achieving a score of 1 (very much improved) at 12 months (not shown). Subject 1 had an elevation on the YMRS scale at six and nine months, corresponding to a switch into hypomania and then mania shortly after he self-discontinued the mood stabilizer olanzapine/fluoxetine combination. Although Subject 3 did not show an improvement on the depression scales, he did improve on both the CGI-S and CGI-I.
Figure 4. CGI-S scores from screening to the end of the study assessment.
CGI-S, Clinical Global Impression-Severity
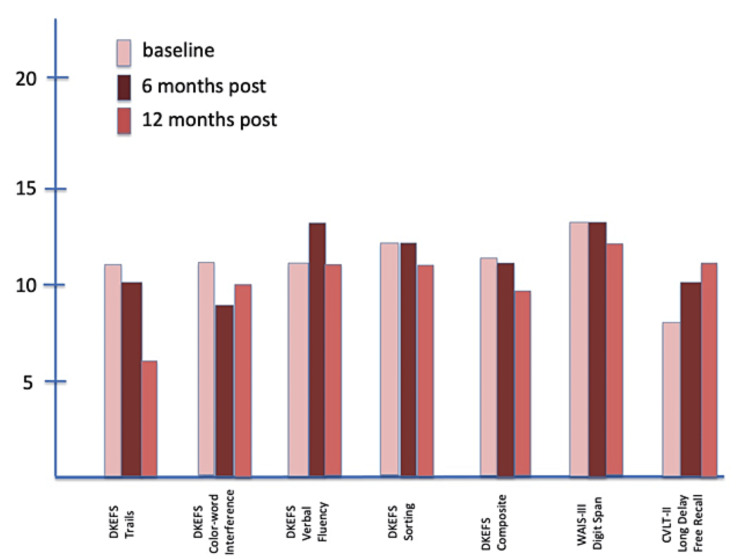
Neurocognitive outcomes are shown in Figure 5, Figure 6, and Figure 7. The DKEFS scores are composite scores for each type of assessment battery: trails, color-word interference, verbal fluency, sorting, and an overall composite score. The WAIS-III digit span and CVLT-II long delay-free recall outcomes follow the DKEFS scores. Serial testing at baseline, six, and 12 months showed improvement from baseline to the end of the study for Subject 1. Neither Subject 2 nor Subject 3 showed improvement or a decrease in performance on any cognitive domain tested.
Figure 5. Composite DKEFS CVLT-II scores at baseline, six months, and 12 months for Subject 1.
CVLT-II, California Verbal Learning Test II; DKEFS, Delis-Kaplan Executive Function System; WAIS-III, Wechsler Adult Intelligence Scale III
Figure 6. Composite DKEFS CVLT-II scores at baseline, six months, and 12 months for Subject 2.
CVLT-II, California Verbal Learning Test II; DKEFS, Delis-Kaplan Executive Function System; WAIS-III, Wechsler Adult Intelligence Scale III
Figure 7. Composite DKEFS CVLT-II scores at baseline, six months, and 12 months for Subject 3.
CVLT-II, California Verbal Learning Test II; DKEFS, Delis-Kaplan Executive Function System; WAIS-III, Wechsler Adult Intelligence Scale III
Clinical outcomes for both Subjects 1 and 2 over the 12-month follow-up period were complicated in each case by a circumstance that directly and negatively affected a consistent pattern of improvement seen over the first six months. Just after the six-month assessment, Subject 1 self-discontinued the olanzapine/fluoxetine combination (OFC) due to financial concerns. Four weeks later, he became hypomanic, and despite starting valproate at that point, within six weeks, he was hospitalized with a full manic episode. He was eventually restarted on OFC, along with lithium and lamotrigine. Shortly after restarting OFC, there was again a marked improvement in clinical assessments that continued until the 12-month time point.
Just before the two-month assessments post-SRS, Subject 2 experienced a family death (suicide). This loss had particular meaning for the subject, as she and this family member had made a pact with each other not to commit suicide. The subject’s resilience in the context of such a significant loss was supported by her own observation that she was grieving but did not feel “depressed” after the suicide. She did rate higher on the HDRS and CGI-S and lower on the CGI-I at the two-month assessment, but then regained earlier improvement by the next visit.
Near the nine-month time point, Subject 2 was hospitalized following decompensation due to another discrete and severely stressful precipitant. She received a course of ECT treatment (four acute and four at weekly intervals) and subsequently showed improvement; however, she did not achieve the level of sustained improvement she had experienced earlier in the trial.
Both Subjects 1 and 2 were part of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) study at Stanford. CGI-S scores (not shown) were available in the medical record for each clinic visit from 2003 for Subject 1 to 2002 for Subject 2. Subject 1 had a pattern of increasing severity and disability over the approximately eight years prior to SRS. Subject 2 had an illness course characterized by wide fluctuations in depressive severity and only five brief periods of improvement over the first four years of follow-up. Subject 2 then had a 33-month course of acute ECT followed by maintenance ECT (119 treatments total) prior to entering the study. Following the SRS procedure and during the study, the patient had a brief course of ECT following the stressful precipitant mentioned above. She has not required ECT over the nearly three years post-SRS.
Overall, all three subjects reported a subjective improvement after the SRS procedure. Subjects 1 and 2 have since requested retreatment. Subject 3 recalled having improved by the end of the study period (which is more consistent with the CGI scores than depression scales). He has since responded to acute and maintenance low-frequency TMS (1 Hz, 1600 pulses per session, over the right dorsolateral prefrontal cortex) over the last 13 months (88 treatments in total).
MRI studies are shown in Figure 8, Figure 9, and Figure 10. In Subject 1 (Figure 8), no abnormalities were visible up to 38 months post-SRS. In Subject 2, a signal abnormality on the FLAIR sequence MRI consistent with edema was observed at 32 months post-SRS; however, he remained neurologically intact. Subject 3 also showed a signal abnormality on the FLAIR sequence MRI consistent with edema at nine and 12 months post-SRS; however, at 22 months post-SRS, the majority of the edema had resolved. Despite these observed signal abnormalities, the subjects did not suffer any adverse effects.
Figure 8. Subject 1 MRI brain at (A) 12 and (B) 27 months post-SRS treatment.
The white arrows show the area of lesioning in the subgenual cingulate.
SRS, stereotactic radiosurgery
Figure 9. Subject 2 MRI brain at (A) 12 and (B) 32 months post-SRS.
The white arrows show the area of lesioning in the subgenual cingulate.
SRS, stereotactic radiosurgery
Figure 10. Subject 3 MRI brain at (A) six and (B) 21 months post-SRS.
The white arrows show the area of lesioning in the subgenual cingulate.
SRS, stereotactic radiosurgery
Discussion
In this paper, we present the results of a safety and feasibility study using SRS targeting SGC in patients with TRBD. These preliminary data suggest that SRS is a safe and effective procedure for the treatment of TRBD. There was an apparent clinical benefit in depression severity in two subjects following the SRS procedure that persisted over the 12-month study. Performance on serial neurocognitive tests and in the overall functional capacity of all three subjects did not reveal any negative effects of treatment. We documented mood improvement during the study period that occurred in the absence of lasting lesions on MRI or adverse effects. Given that mood improvement was modest and transient, we do not advocate treating additional patients with the same parameters. Local inflammation visible on FLAIR (not suggestive of necrosis) in one of three patients at 32 months post-SRS was the only durable change visible at the last follow-up.
These changes, seen at nine and 12 months, resolved to a punctate lesion 22 months after SRS. The appearance of MR signal abnormalities was not associated with a change in his depressive symptoms or performance on the 12-month cognitive battery, nor was there any change observed that would reflect new impairments in judgment or insight.
Of interest, Subject 3 had a course of high-frequency TMS prior to study entry without benefit. Two weeks after the 12-month SRS study was complete, he began a course of right-side low-frequency TMS treatments (1 Hz, 10 minutes, 600 pulses per session, 120% motor threshold) and completely remitted over five weeks. He sustained improvement over the last 15 months with maintenance treatment.
Of the two subjects who had longitudinal data from visits to the Stanford Bipolar Clinic, the CGI-S data clearly illustrated the chronicity, severity, and disability of their depression. The clinical response to ECT in both patients was limited and not sustained. In the context of this long-term historical data, the improvement in HDRS and CGI scores post-SRS appeared to show a clinical benefit of the procedure when contrasted with the overall trend in clinical status seen prior to the study. The clinical response to SRS, both in terms of severity of depression and duration of improvement, appears to be more robust than that previously seen with ECT treatment for both subjects.
The apparent lesion seen in the SGC in Subject 3 at nine and 12 months and Subject 2 at a 32-month follow-up scan was unexpected. It may be that the SGC was more vulnerable to radiation injury, perhaps related to its hypermetabolism during the depressed state, progressive atrophy, and a history of chronic cocaine and alcohol use in both subjects. Due to its neuroprotective and anti-apoptotic effects in bipolar disorder as well as neurodegenerative disorders [34], pretreatment with lithium may reduce the likelihood of a lesion arising in the targeted area.
Histologically lesion-free changes accompanied by bidirectional neuromodulation are seen in large animal models with PET [36] and with depth electrodes and visual evoked potential [37]. If target upregulation is a viable therapeutic strategy, it may be that treating at doses of 10-30 Gy, for example, may yield more favorable results.
It is possible that a lesion in the SGC may have a therapeutic outcome. Lesion-based neurosurgery is known to be effective; improvement in mood occurred before the destructive lesion was discovered [38]. Recent animal work shows evidence of nondestructive functional upregulation of focal gray matter at doses below 40 Gy and dose-dependent partial lesioning above that level [39]. Neurosurgical procedures such as anterior cingulotomy, subcaudate tractotomy, and stereotactic limbic leucotomy have been used to successfully treat refractory bipolar disorder and depression, as well as obsessive-compulsive disorder [39]. A study of healthy volunteers showed that connectivity reconstructions from diffusion tensor imaging tractography data suggested that the shared connectivity between these surgical approaches was through the subgenual region [40]. Such studies may provide some insight into how targeted radiation to the SGC may have therapeutic value for the most difficult-to-treat patients with bipolar disorder.
Limitations
The major limitation of this study is that only three patients were enrolled, with limited follow-up. Given the limited sample size without a control group, it is difficult to say definitively that SRS for TRBD is both safe and effective. However, it is hoped that the positive results presented here will inspire a future trial with a larger number of patients.
The positive results presented here should inspire future clinical and basic science investigations. In the realm of basic science, more molecular studies are needed to better understand the impact of focused radiation on neural tissues. On the clinical side, trials with a larger number of patients and sufficient controls are needed to adequately show the safety and effectiveness of SRS in the SGC for the treatment of TRBD.
Conclusions
These data from three subjects support the hypothesis that the SRS procedure does not seem detrimental and may provide some benefit for the treatment of TRBD. The radiation-related injury to SGC in Subjects 2 and 3 did not result in a worsening of depression or cognitive impairment. It is possible that a lower dose should be used in future trials. Overall, the procedure appears to have resulted in sustained clinical benefit in two subjects that exceeded the clinical outcomes seen with prior trials of medication and ECT for both subjects. This may be an indication that SRS could be a precisely targeted, noninvasive approach to the TRBD. More research is needed to further determine the safety and clinical efficacy of SRS for the treatment of this disease.
Acknowledgments
The authors would like to thank Dr. David J. Park and Dr. M. Bret Schneider for useful discussions and guidance during manuscript preparation.
Disclosures
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Stanford University School of Medicine issued approval 10186. IRB approval was obtained prior to study initiation.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: John R Adler is the CEO declare(s) employment from Zap Surgical.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Acquisition, analysis, or interpretation of data: Neelan J. Marianayagam, Hugh B. Solvason, Scott G. Soltys, Alan F. Schatzberg, Charles DeBattista, Terence Ketter, Po Wang, Steven D. Chang, David Spiegel, John R. Adler
Drafting of the manuscript: Neelan J. Marianayagam, Hugh B. Solvason, Scott G. Soltys, Alan F. Schatzberg, Charles DeBattista, Terence Ketter, Po Wang, Steven D. Chang, David Spiegel, John R. Adler
Critical review of the manuscript for important intellectual content: Neelan J. Marianayagam, Hugh B. Solvason, Scott G. Soltys, Alan F. Schatzberg, Charles DeBattista, Terence Ketter, Po Wang, Steven D. Chang, David Spiegel, John R. Adler
Concept and design: Hugh B. Solvason, John R. Adler
Supervision: Hugh B. Solvason, John R. Adler
References
- 1.Graphic representation of the life course of illness in patients with affective disorder. Post RM, Roy-Byrne PP, Uhde TW. https://europepmc.org/article/med/3381929. Am J Psychiatry. 1988;145:844–848. doi: 10.1176/ajp.145.7.844. [DOI] [PubMed] [Google Scholar]
- 2.Stage managing bipolar disorder. Berk M, Berk L, Dodd S, et al. https://onlinelibrary.wiley.com/doi/full/10.1111/bdi.12099. Bipolar Disord. 2014;16:471–477. doi: 10.1111/bdi.12099. [DOI] [PubMed] [Google Scholar]
- 3.Longitudinal course of bipolar I disorder: duration of mood episodes. Solomon DA, Leon AC, Coryell WH, et al. https://jamanetwork.com/journals/jamapsychiatry/article-abstract/210663. Arch Gen Psychiatry. 2010;67:339–347. doi: 10.1001/archgenpsychiatry.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterns of sensitisation in the course of affective illness: a life-charting study of treatment-refractory depressed patients. Ehnvall A, Agren H. https://jamanetwork.com/journals/jamapsychiatry/article-abstract/210663. J Affect Disord. 2002;70:67–75. doi: 10.1016/s0165-0327(01)00328-7. [DOI] [PubMed] [Google Scholar]
- 5.Long-term symptomatic status of bipolar I vs. bipolar II disorders. Judd LL, Schettler PJ, Akiskal HS, et al. https://academic.oup.com/ijnp/article/6/2/127/719854. Int J Neuropsychopharmacol. 2003;6:127–137. doi: 10.1017/S1461145703003341. [DOI] [PubMed] [Google Scholar]
- 6.Prevalence, chronicity, burden and borders of bipolar disorder. Fagiolini A, Forgione R, Maccari M, et al. https://www.sciencedirect.com/science/article/pii/S0165032713001146. J Affect Disord. 2013;148:161–169. doi: 10.1016/j.jad.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Do many patients with depression suffer from bipolar disorder? Angst J. https://journals.sagepub.com/doi/abs/10.1177/070674370605100102. Can J Psychiatry. 2006;51:3–5. doi: 10.1177/070674370605100102. [DOI] [PubMed] [Google Scholar]
- 8.The mood spectrum: improving the diagnosis of bipolar disorder. Angst J, Cassano G. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1399-5618.2005.00210.x. Bipolar Disord. 2005;7 Suppl 4:4–12. doi: 10.1111/j.1399-5618.2005.00210.x. [DOI] [PubMed] [Google Scholar]
- 9.Prevalence and characteristics of undiagnosed bipolar disorders in patients with a major depressive episode: the BRIDGE study. Angst J, Azorin JM, Bowden CL, Perugi G, Vieta E, Gamma A, Young AH. https://jamanetwork.com/journals/jamapsychiatry/article-abstract/1107421. Arch Gen Psychiatry. 2011;68:791–798. doi: 10.1001/archgenpsychiatry.2011.87. [DOI] [PubMed] [Google Scholar]
- 10.Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. Judd LL, Schettler PJ, Solomon DA, Maser JD, Coryell W, Endicott J, Akiskal HS. https://www.sciencedirect.com/science/article/pii/S0165032707003333. J Affect Disord. 2008;108:49–58. doi: 10.1016/j.jad.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Epidemiology of suicide in bipolar disorders: a systematic review of the literature. Pompili M, Gonda X, Serafini G, et al. https://onlinelibrary.wiley.com/doi/full/10.1111/bdi.12087. Bipolar Disord. 2013;15:457–490. doi: 10.1111/bdi.12087. [DOI] [PubMed] [Google Scholar]
- 12.Recent life events and completed suicide in bipolar affective disorder. A comparison with major depressive suicides. Isometsa E, Heikkinen M, Henriksson M, Aro H, Lonnq- vist J. https://www.sciencedirect.com/science/article/pii/016503279400079O. J Affect Disord. 1995;33:99–106. doi: 10.1016/0165-0327(94)00079-o. [DOI] [PubMed] [Google Scholar]
- 13.Epidemiology of bipolar disorders. Bauer M, Pfennig A. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1528-1167.2005.463003.x. Epilepsia. 2005;46 Suppl 4:8–13. doi: 10.1111/j.1528-1167.2005.463003.x. [DOI] [PubMed] [Google Scholar]
- 14.Mathers C, Fat DM, Boerma T. Geneva, Switzerland: World Health Organization; 2004. The Global Burden of Disease 2004 Update; p. 2004. [Google Scholar]
- 15.The subgenual anterior cingulate cortex in mood disorders. Drevets WC, Savitz J, Trimble M. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2729429/ CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limbic-frontal circuitry in major depression: a path modeling metanalysis. Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2729429/ Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Identification of atrophy of the subgenual anterior cingulate cortex, in particular the subcallosal area, as an effective auxiliary means of diagnosis for major depressive disorder. Niida A, Niida R, Matsuda H, Inada T, Motomura M, Uechi A. https://www.dovepress.com/identification-of-atrophy-of-the-subgenual-anterior-cingulate-cortex-i-peer-reviewed-fulltext-article-IJGM. Int J Gen Med. 2012;5:667–674. doi: 10.2147/IJGM.S34093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. Moore GJ, Cortese BM, Glitz DA, et al. https://www.psychiatrist.com/jcp/bipolar/longitudinal-study-effects-lithium-treatment-prefrontal/ J Clin Psychiatry. 2009;70:699–705. doi: 10.4088/JCP.07m03745. [DOI] [PubMed] [Google Scholar]
- 19.Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Rajkowska G. https://www.sciencedirect.com/science/article/pii/S0006322300009501. Biol Psychiatr. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 20.Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Kennedy SH, Evans KR, Krüger S, et al. https://ajp.psychiatryonline.org/doi/full/10.1176/appi.ajp.158.6.899. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 21.Relationship between regional brain metabolism, illness severity and age in depressed subjects. Konarski JZ, Kennedy SH, McIntyre RS, Rafi-Tari S, Soczynska JK, Mayberg HS. https://www.sciencedirect.com/science/article/pii/S0925492707000376. Psychiatry Res. 2007;155:203–210. doi: 10.1016/j.pscychresns.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Sheline YI, Price JL, Yan Z, Mintun MA. https://www.pnas.org/doi/abs/10.1073/pnas.1000446107. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The subcallosal cingulate gyrus in the context of major depression. Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. https://www.sciencedirect.com/science/article/pii/S0006322310010036. Biol Psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 24.Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Greicius MD, Flores BH, Menon V, et al. https://www.sciencedirect.com/science/article/pii/S0006322306011930. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subgenual cingulate cortical activity predicts the efficacy of electroconvulsive therapy. Argyelan M, Lencz T, Kaliora S, et al. https://www.nature.com/articles/tp201654. Transl Psychiatry. 2016;6:0. doi: 10.1038/tp.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Holtzheimer PE, Kelley ME, Gross RE, et al. https://jamanetwork.com/journals/jamapsychiatry/article-abstract/1107402. Arch Gen Psychiatry. 2012;69:150–158. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antidepressant effects after short-term and chronic stimulation of the subgenual cingulate gyrus in treatment-resistant depression. Merkl A, Schneider GH, Schönecker T, et al. https://jamanetwork.com/journals/jamapsychiatry/article-abstract/1107402. Exp Neurol. 2013;249:160–168. doi: 10.1016/j.expneurol.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 28.CyberKnife radiosurgery as a first treatment for idiopathic trigeminal neuralgia. Fariselli L, Marras C, De Santis M, Marchetti M, Milanesi I, Broggi G. https://journals.lww.com/neurosurgery/Fulltext/2009/02001/Image_guided_Robotic_Radiosurgery.00015.aspx. Neurosurgery. 2009;64:0–101. doi: 10.1227/01.NEU.0000341714.55023.8F. [DOI] [PubMed] [Google Scholar]
- 29.Cyberknife radiosurgery in treating trigeminal neuralgia. Lazzara BM, Ortiz O, Bordia R, Witten MR, Haas JA, Katz AJ, Brown JA. https://jnis.bmj.com/content/5/1/81.short. J Neurointerv Surg. 2013;5:81–85. doi: 10.1136/neurintsurg-2011-010125. [DOI] [PubMed] [Google Scholar]
- 30.CyberKnife stereotactic radiosurgical rhizotomy for refractory trigeminal neuralgia. Tang CT, Chang SD, Tseng KY, Liu MY, Ju DT. https://www.sciencedirect.com/science/article/pii/S0967586811002815. J Clin Neurosci. 2011;18:1449–1453. doi: 10.1016/j.jocn.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Salvage gamma knife stereotactic radiosurgery for surgically refractory trigeminal neuralgia. Little AS, Shetter AG, Shetter ME, Kakarla UK, Rogers CL. https://www.sciencedirect.com/science/article/pii/S0967586811002815. Int J Radiat Oncol Biol Phys. 2009;74:522–527. doi: 10.1016/j.ijrobp.2008.08.048. [DOI] [PubMed] [Google Scholar]
- 32.Dose-volume effects on brainstem dose tolerance in radiosurgery. Xue J, Goldman HW, Grimm J, LaCouture T, Chen Y, Hughes L, Yorke E. https://thejns.org/view/journals/j-neurosurg/117/Special_Suppl/article-p189.xml. J Neurosurg. 2012;117 Suppl:189–196. doi: 10.3171/2012.7.GKS12962. [DOI] [PubMed] [Google Scholar]
- 33.Histological effects of trigeminal nerve radiosurgery in a primate model: implications for trigeminal neuralgia radiosurgery. Kondziolka D, Lacomis D, Niranjan A, Mori Y, Maesawa S, Fellows W, Lunsford LD. https://journals.lww.com/neurosurgery/Fulltext/2000/04000/Histological_Effects_of_Trigeminal_Nerve.38.aspx. Neurosurgery. 2000;46:971–976. doi: 10.1097/00006123-200004000-00038. [DOI] [PubMed] [Google Scholar]
- 34.Tractographic analysis of historical lesion surgery for depression. Schoene-Bake JC, Parpaley Y, Weber B, Panksepp J, Hurwitz TA, Coenen VA. https://www.nature.com/articles/npp2010132. Neuropsychopharmacology. 2010;35:2553–2563. doi: 10.1038/npp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.A new look at an old drug: neuroprotective effects and therapeutic potentials of lithium salts. Dell'Osso L, Del Grande C, Gesi C, Carmassi C, Musetti L. https://journals.lww.com/neurosurgery/Fulltext/2000/04000/Histological_Effects_of_Trigeminal_Nerve.38.aspx. Neuropsychiatr Dis Treat. 2016;12:1687–1703. doi: 10.2147/NDT.S106479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reversal of insular and microstructural nerve abnormalities following effective surgical treatment for trigeminal neuralgia. DeSouza DD, Davis KD, Hodaie M. https://journals.lww.com/pain/FullText/2015/06000/Reversal_of_insular_and_microstructural_nerve.17.aspx. Pain. 2015;156:1112–1123. doi: 10.1097/j.pain.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 37.Effects of focal radiation on [18F]-fluoro-D-glucose positron emission tomography in the brains of miniature pigs: preliminary findings on local metabolism. Yeh CI, Cheng MF, Xiao F, et al. https://www.sciencedirect.com/science/article/pii/S1094715921061870. Neuromodulation. 2021;24:863–869. doi: 10.1111/ner.13147. [DOI] [PubMed] [Google Scholar]
- 38.Non-ablative doses of focal ionizing radiation alters function of central neural circuits. Zaer H, Fan W, Orlowski D, et al. https://www.sciencedirect.com/science/article/pii/S1935861X22000614. Brain Stimul. 2022;15:586–597. doi: 10.1016/j.brs.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Radiation-based neuromodulation: rationale and new directions. Schneider MB, Borchers D, Adler JR. Cureus. 2010;8 [Google Scholar]
- 40.Stereotactic lesions for the treatment of psychiatric disorders: a review. Leiphart JW, Valone FH 3rd. https://thejns.org/view/journals/j-neurosurg/113/6/article-p1204.xml. J Neurosurg. 2010;113:1204–1211. doi: 10.3171/2010.5.JNS091277. [DOI] [PubMed] [Google Scholar]