Abstract
Introduction:Metabolic dysfunction-associated steatotic liver disease (MASLD) is an entity with a growing incidence but only a few pharmacological options. In Romania, the prevalence of MASLD has been increasing, while that of viral hepatitis has been decreasing. The purpose of this study is to compare two supplements for the treatment of MASLD.
Methods:Between January 2020 and May 2022, 90 patients with MASLD were randomized to receive either silymarin 150 mg b.i.d (45 subjects) or essential phospholipids (EPLs) 825 mg b.i.d. (45 subjects) for six months. All study participants received recommendations for lifestyle and diet modifications. Assessment of the severity of steatosis and liver fibrosis was performed using FibroScan® with controlled attenuated parameter (CAP) at the beginning and end of treatment.
Results:A total of 68 patients completed the trial. The two groups were statistically comparable in terms of clinical, biological and FibroScanR parameters. Aspartate transferase (AST) decreased from a median of 40 to 28 IU/L in the EPL arm (compared to 25→¨25.5 IU/L in the silymarin arm) (p-value=0.11) and alanine transaminase (ALT) decreased from 46 to 37.5 IU/L (compared to 31→30 IU/L) (p-value = 0.38). Plasma cholesterol levels also decreased significantly in the EPL group (218→189.5 mg/dL) compared to the silymarin arm (217→209 mg/dL) (p = 0.01). At the end of treatment, liver stiffness decreased by 0.7 KPa (6.9→6.2 KPa) in the EPL group but increased by 2.3 KPa (7.2→9.5 KPa) in the silymarin group (p = 0.1). The reduction in hepatic steatosis was comparable between the two groups: it decreased by 5% of the initial value.
Conclusion:In our study, a six-month treatment with EPLs was superior to silymarin in MASLD patients because it succeeded in improving both laboratory parameters and liver fibrosis, as estimated by FibroScan®.
Keywords:sylimarin, essential phospholipids, metabolic associated steatotic liver disease (MASLD), prospective trial.
INTRODUCTION
Nowadays, we are witnessing an important shift in the pathogenesis of chronic liver diseases. The prevalence of hepatitis B and C has lowered due to highly successful therapies. Meanwhile, there is an increasingly elevated risk of metabolic liver diseases (1, 2).
Metabolic dysfunction-associated steatotic liver disease (MASLD, formerly NAFLD) is a complex multifactorial disease that associates hepatic steatosis with at least one of the following: abdominal obesity, high blood pressure, impaired glucose metabolism, hypertriglyceridemia, low high density lipoprotein (HDL)-cholesterol (3). The imbalance between calorie intake and calorie expenditure, coupled with abnormal fatty acid oxidation, increased liver lipogenesis, insulin resistance and local inflammation, mainly constitute the pathogenesis mechanism in steatotic liver diseases (4).
The global prevalence of MASLD is increasing and it is estimated around 30% in the USA and 20-30% in Europe and Asian countries (5). While specific prevalence data for liver steatosis in Romania may not be readily available, the similarity of risk factors and lifestyle trends across European countries can provide a rough reference point.
Nowadays, we are witnessing an important shift in the pathogenesis of chronic liver diseases. The prevalence of hepatitis B and C has lowered due to highly successful therapies. Meanwhile, there is an increasingly elevated risk of metabolic liver diseases (1, 2).
Metabolic dysfunction-associated steatohepatitis (MASH) has the potential for evolution towards cirrhosis and hepatocellular carcinoma (6).
Lifestyle interventions like reduced calorie intake and increased regular physical activity are the main recommendations for people diagnosed with MASLD. However, due to low compliance or comorbidities (especially cardiovascular problems), which alter adherence to such interventions, only 10% of patients can adhere to diet and exercise chart (7). A few treatment lines have been explored, including pioglitazone or vitamin E, but their use can be limited due to side effects (8).
Silymarin is a mixture of flavonolignans that has been used for the therapy of liver diseases for more than 2000 years. It is a combination of four substances, including silibinin, isosilibinin, silidianin and silichristine, of which the first one is considered to be the most important one. The active ingredients are extracted with aqueous alcohol from the seeds of milk thistle. The standardized silymarin extract can be administered as a sugar-coated tablet with a 20-50% absorption rate after oral administration. The bioavailability of silymarin can be increased by adding solubilizers and by ingestion of other substances such as phenol derivates, proteins and fats (9).
Experimental studies with silymarin administered in a model of acute liver injury caused by carbon tetrachloride (CCl4) have shown decreased fibrogenesis in rats treated with CCl4 (10). Many other clinical and experimental trials demonstrated an important anti-oxidant effect of silymarin, reflected through a low level of malondialdehyde (MDA) as a marker of lipid peroxidation and low levels of oxidized glutathione (11, 12).
Several clinical trials proved the important hepatoprotective effect of silymarin in MASLD patients, with a significant decrease of transaminases and improvements in lipid profile and insulin sensitivity (13, 14).
Essential phospholipids (EPLs) are nutritional supplements also used in chronic liver diseases, but with a shorter history than silymarin. Phospholipids (PLs) are key components of all cellular membranes, with phosphatidylcholine and phosphatidylethanolamine being the most abundant. As the cellular membrane delimits the inner from the outer cell environment, membrane PLs are crucial for the fluidity of the structure, cell-to-cell communication, differentiation and proliferation (15).
Essential phospholipids are purified soybean extracts with a high, standardized content of 72-96% of 3-sn-phosphatidylcholine. After ingestion, their absorption rate is very high (90%) and most of EPLs are driven to the liver. Other organs might also benefit, such as the gastrointestinal (GI) tract, spleen, kidneys and brain (16, 17).
Similar to silymarin, EPLs have also anti-oxidant, anti-inflammatory, anti-apoptotic and antifibrogenic properties (18). Recent clinical trials demonstrate the hepatoprotective properties of EPLs, as shown by decreased transaminase levels, improved lipid profile and ultrasonography of the liver.
Measurement of liver stiffness is a valuable diagnostic tool for estimating the severity of steatosis and fibrosis in MASLD (19, 20).
Even though MASLD patients are always prescribed a hypocaloric diet and aerobic exercise, compliance with lifestyle changes is not always optimal, and hepatoprotective molecules should be evaluated in terms of efficiency and safety of administration. Current EASL guidelines recommend that pharmacotherapy should be reserved for patients with MASH, particularly if there is significant fibrosis (21). For the majority of MASLD patients (the remaining 70-80%), diet, lifestyle changes and a nutraceutical approach are recommended (22).
MATERIAL AND METHODS
This was an open-label, interventional, prospective, non-controlled, randomized clinical study which aimed to evaluate the efficacy and safety of silymarin 150 mg twice daily versus ELPs 825 mg (Fortifikat forte) (23), twice daily, in patients diagnosed with MASLD after six months of treatment.
All subjects in the silymarin arm received the same product containing a standardized dose of 150 mg of silymarin, while those in the EPL group were given a standardized essential phospholipid product containing 76% phosphatidylcholine.
Between January 2020 – May 2022 we included 90 patients who had been diagnosed with MASLD using Fibroscan® with controlled attenuated parameter (CAP). Patients were then randomized to receive either silymarin 150 mg twice daily (45 subjects) or EPLs 825 mg twice daily (45 subjects) for six months.
Diagnosis of MASLD using Fibroscan® with a CAP defined by CAP > 302 dB/m (19) was the inclusion criterium. Exclusion criteria comprised alcoholic liver disease (recent consumption of more than 20 g or 10 g of pure ethanol per day by males or females, respectively); chronic infection with HBV and/or HCV; recent administration (within the last three months) of silymarin and/or phospholipids; chronic pancreatitis; concomitant therapy with ursodeoxicholic acid, pentoxifylline, vitamin E, pioglitazone and rosiglitazone; lack of informed consent. Concomitant therapy for comorbidities was recorded and maintained throughout the six-month period.
All patients signed an informed consent which stated that their clinical and demographic data could be used for scientific purposes. The present study was approved by the Ethics Committee of Fundeni Clinic Institute.
The following clinical and laboratory parameters were assessed at the inclusion in the study and after six months of therapy: sex, BMI, AST, ALT, ALKP, GGT, total bilirubin, cholesterol and triglycerides. All subjects received an individualized low-calorie and hypolipidemic diet and the recommendation to do progressive aerobic training 2-3 times per week in sessions of 30-60 minutes. Severity of steatosis and liver fibrosis was performed using FibroScan® with CAP at the beginning and end of treatment.
The accuracy of FibroScanR Vibration-Controlled Transient Elastography with CAP in assessing steatosis in non-alcoholic liver disease is very good, according to the literature data: CAP identified patients with steatosis with an area under the receiver operator characteristic curve (AUROC) of 0.87 for S≥S1, 0.77 for S≥S2 and 0.70 (95% CI 0.64.0.75) for S=S3. Regarding the diagnostic value of FibroScanR liver stiffness measurement (LSM) in estimating the severity of fibrosis in MASLD, LSM identified patients with fibrosis with AUROCs of 0.77 for F≥F2, 0.80 for F≥F3 and 0.89 for F=F4 (19, 20).
Statistical analysis
For data analysis, IBM Corporation's SPSS statistical software (version 20.0, Armonk, NY, USA) was used. The normality of data was assessed using the Kolmogorov-Smirnov test. Descriptive statistics for quantitative variables with a parametric distribution were presented as mean and standard deviation (SD), while variables with a non-parametric distribution were summarized as median with minimum and maximum values. The independent sample t-test was employed to compare normally distributed data, whereas the Mann-Whitney U test was utilized for non-normally distributed data. Categorical variables were expressed as percentages and compared using Fisher's exact test. Two-sided hypothesis testing was applied, with a p-value less than 0.05 considered statistically significant.
RESULTS
Initially, 90 subjects signed informed consent and entered the study. A total of 22 patients (18 from the silymarin group and four from the EPL group) were not compliant with the study procedures and were excluded from the statistical analysis (six declared they could not swallow the capsules, six discontinued treatment and 10 did not come for the six-month evaluation).
A total of 68 subjects completed the six-month protocol, 62% of whom were males, with a median age of 53 years (36%72 years). Three out of 68 patients (4.4%) had a normal BMI, 36% overweight and 47% obesity (with three having morbid obesity). Comorbidities were found in 70% of patients, including different types of hyperlipidemia (70%), type 2 diabetes (18%) and high blood pressure (44%). Overall, 70% of patients had severe steatosis according to FibroScanR with CAP (value over 337 dB/m); 17% of subjects had moderate to severe fibrosis, including F3 (10%) and F4 (7%), with F3 being defined as liver stiffness measurement (LSM) between 9.7 and 13.5 KPa, and F4 as LSM≥13.6 KPa. The majority of patients had lower fibrosis stages: F0 (10%), F1 (53%) and F2 (20%).
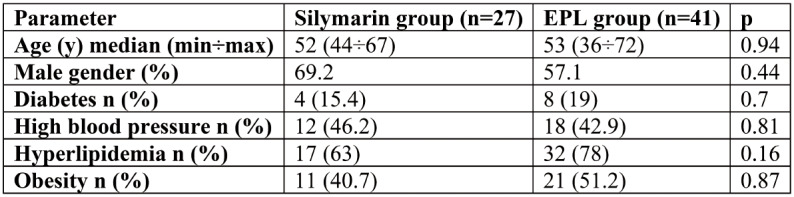
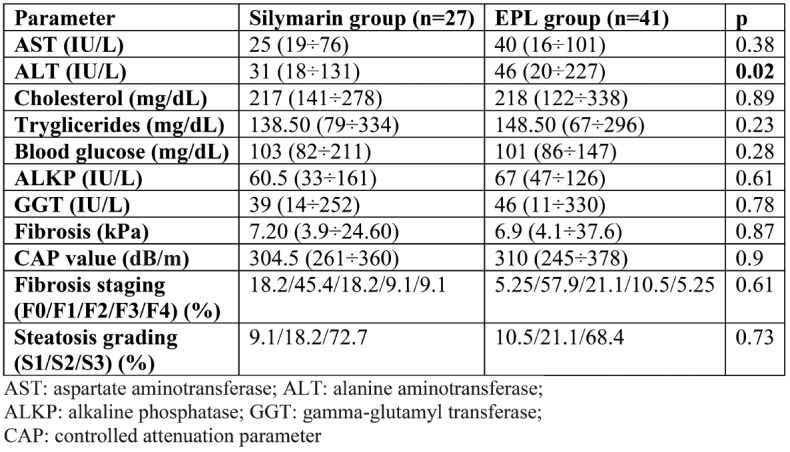
Tables 1 and 2 summarize the clinical and laboratory characteristics of the two groups of patients at inclusion. Groups had comparable parameters, except for ALT values, which were significantly higher in the EPL group.
First of all, we followed the evolution of BMI over time. Thus, BMI decreased by 0.7 kg/m² (2.4% of the initial value) in patients treated with silymarin and by 2.3 kg/m² in those who received EPLs (7.5%). However, this difference was not statistically significant and multiple factors could be involved in this result.
At inclusion in the study, 35% of all participants had increased values of AST and/or ALT, suggesting a diagnosis of MASH. The shift in ALT values during the 24 weeks of treatment is showed in Figure 1: the median value of ALT decreased by 9 IU/L in the EPL group compared to 1 IU/L in the silymarin group. Despite this tendency, the p value was not significant. Moreover, the group treated with EPL had a more important decrease in the median value of AST (12 IU/L) compared to the silymarin group, without reaching the threshold of statistical significance (Figure 2).
Median values of serum cholesterol at 24 weeks of treatment were significantly decreased by 28.5 mg/dL in the EPL group, compared to 8 mg/dL in the silymarin group (p=0.01). Triglyceride level decreased by 18.5 mg/dL in subjects treated with phospholipids and by 12 mg/dL in the silymarin group (p=NS).
Figure 3 illustrates CAP variations using FibroScan® during the six-month treatment regimen. The median CAP value decreased by 12 dB/m in the phospholipid group but by only 1 dB/m in the silymarin group (p=NS).
The evolution of liver fibrosis at the end of treatment was another important target of our study (Figure 4). After 24 weeks, LSM decreased by 0.7 kPa in patients treated with phospholipids and increased by 2.3 kPa in those who received silymarin, but statistical significance was not obtained (p=0.13). The favorable evolution of liver stiffness in subjects treated with phospholipids is also illustrated in the evolution of proportions.
In our study, both silymarin and EPL showed an excellent safety profile, with no reported side effects.
DISCUSSION
This is the first prospective head-to-head comparative study of silymarin versus EPL in the literature, evaluating the effect of the two supplements over 24 weeks. In this limited timeframe, we observed an improvement in biological parameters among patients treated with both supplements. Moreover, patients in the EPL group had a greater BMI decrease, which could in part explain the better values of their biological parameters than those measured in subjects of the silymarin group.
Silymarin has multiple beneficial effects and is being studied for its effectiveness in treating MASLD. There is significant heterogeneity among the existing trials of silymarin in MASLD, with daily doses ranging from 100 mg to 700 mg and therapy duration between 12 to 48 weeks (24). We chose a dose of 300 mg/day since it is the standard recommended dose, but also due to the accessibility of the product, as silymarin is not reimbursed in Romania.
Also, the improvement of liver enzymes observed in our study was similar to the data in the literature (24-28).
Despite the excellent safety profiles shared by both supplements, we encountered compliance issues in the silymarin arm, which led to a smaller number of patients who completed the study. This could be due to either the fact that the type of pill used by us was much more difficult to swallow or because our study started when we were dealing with COVID infection.
In our patients, there was no significant weight loss in the silymarin arm nor a significant decrease in elastography parameters. In contrast, a 2012 study reported that weight loss of more than 5% combined with silymarin (dose of 210 mg b.i.d.) led to a significant decrease in the severity of liver fibrosis estimated by transient elastography (from an average of 6.9 kPa to 5.57 kPa in six months) (27). Whether these are independent or derivative factors is yet to be determined.
Essential phospholipid preparations have a very good oral bioavailability and are incorporated into the hepatocyte membranes affected by hepatic steatosis, resulting in improvement of liver functions associated with the membrane (detoxification, improvement of oxidative stress, reduction of apoptosis, restoration of membrane traffic, improvement of glucose and lipid metabolism) (23, 30).
Several published studies investigated the effectiveness of EPLs in MASLD, with daily doses varying between 1.05 and 1.8 g/day for time periods ranging from four weeks to more than six months. All those studies described a significant improvement in liver enzymes and other liver function tests (total bilirubin, alkaline phosphatase and ã-GT) (32-36). Their findings are similar to our results. Serum cholesterol, triglycerides and low-density lipoprotein (LDL)-cholesterol significantly decreased in the EPL group, which was similar to previously published studies (33, 35). Additionally, we observed a notable decrease in liver enzyme levels in the EPL group, although statistical significance was not reached. We attributed this to the small number of subjects enrolled in the study.
The use of phospholipids improved liver ultrasound appearance in three studies (32, 34, 36), while other authors reported a significant improvement of the fatty liver estimated through either computed tomography (CT) scan or liver biopsy (37, 38).
A study published by Dajani et al in 2015, which included 320 patients with MASLD treated with 1800 mg EPLs per day for 24 weeks, reported an improvement of liver stiffness in 21% of subjects, with a mean reduction of liver stiffness of 3.1 kPa (36). We observed an improvement in liver fibrosis among patients who received EPL (0.7 kPa). In contrast, those treated with silymarin progressed with 2.3 kPa in six months. Our results underline a strong tendency, even though the significance threshold was not met.
A combination of the two hepatoprotective agents could be more effective than EPL alone. In a randomized open controlled study, the combination of essential phospholipids with silybin, glucuronolactone and vitamin B complex in patients with fatty liver were more effective than ELPs alone (39).
Our findings regarding adverse reactions are in line with the previous data reported. Both silymarin and EPL are well tolerated. No adverse reactions were reported. However, compliance to silymarin was lower and more patients discontinued treatment. One hypothesis is that patients were more diligent with the EPL treatment and rather more compliant with lifestyle recommendations.
There are some study limitations, which mainly comprise the small number of selected patients and the difficulty in assessing compliance to lifestyle changes which could act as a co-factor in the improvement of biological parameters. However, there is a strong signal towards an added benefit of EPL in the management of MASLD, which should warrant further studies.
CONCLUSION
In conclusion, administration of silymarin 300 mg per day or EPL 1650 mg/day (Fortifikat forte) (23) for six months shows comparable benefits in the treatment of non-alcoholic fatty liver disease, with a slight superiority for EPL regarding AST, ALT and triglyceride levels. Essential phospholipids were shown to lead not only to improvements in patients with severe liver fibrosis but also to a statistically significant decrease in serum cholesterol (with 28.5 mg/dL). These findings could also suggest that higher daily doses of silymarin (450-600 mg/day) are needed in the treatment of MASLD. Different combination treatments and weight loss in patients with MASLD should be further explored.
Conflicts of interest: Teodora Manuc, Doina Istratescu, Razvan Cerban, Carmen Ester, Corina Gabriela Meianu, Raluca Ioana Alecu, Claudiu Mihai Ciuciureanu, Alexandra Ioana Marin, Letitia Tugui, Tudor Gheorghe Stroie, Cristian Tieranu, Mircea Manuc have nothing to disclose. Carmen Monica Preda, Liana Gheorghe, Mircea Diculescu received honorary or grants from Terapia company.
This study was supported by Terapia company.
Authors’ contribution: all authors read and approved the final manuscript.
TABLE 1.
Clinical characteristics of patients at inclusion
TABLE 2.
Biological characteristics of patients at inclusion in the study. Values are reported as median (min÷max). Fibrosis staging and steatosis grading are reported as percentages
FIGURE 1.
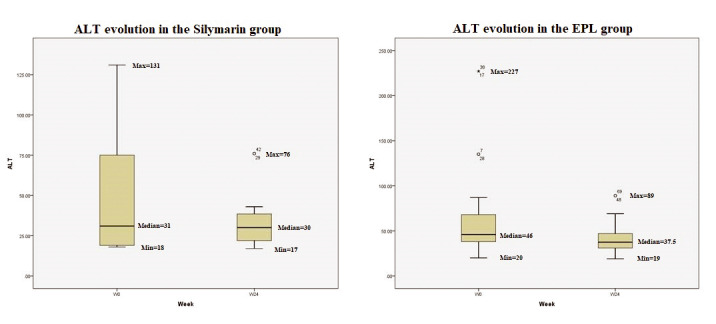
Evolution of ALT in the two groups during the 24 weeks of treatment
FIGURE 2.
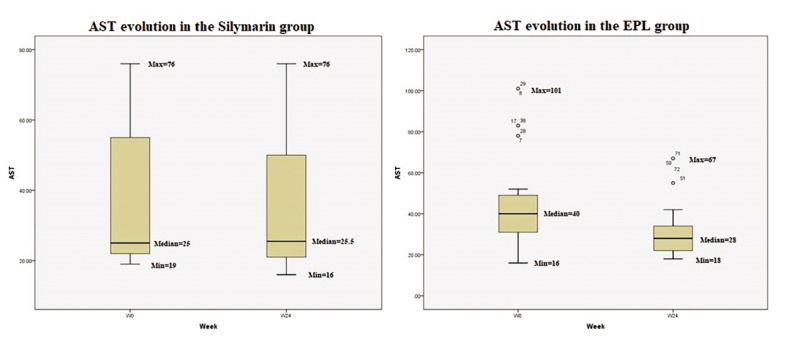
Evolution of AST in the two groups during the 24 weeks of treatment
FIGURE 3.
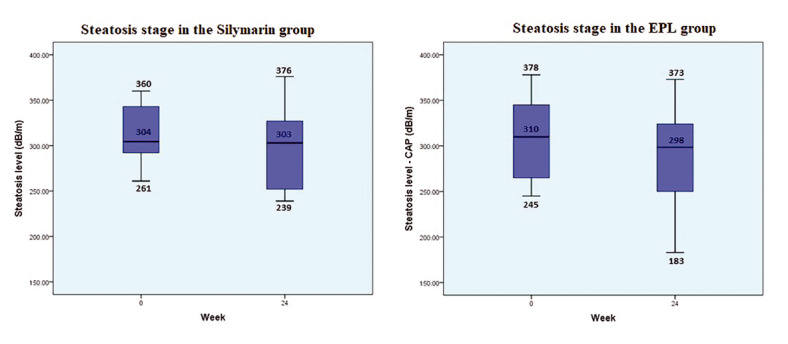
Time evolution of controlled attenuated parameter in the two treatment groups
FIGURE 4.
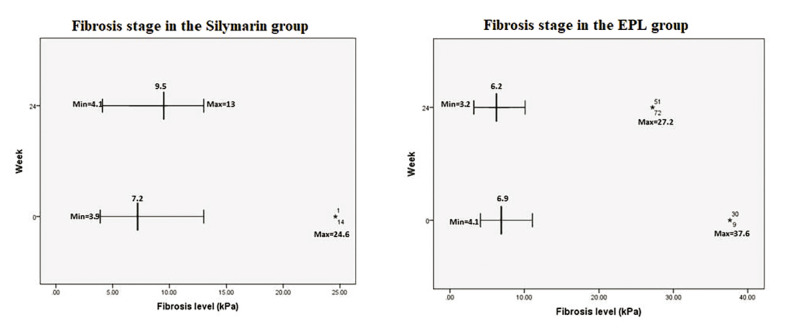
A comparative dynamics of liver stiffness estimated by FibroScan in the two groups of patients
Contributor Information
Teodora MANUC, ”Carol Davila” University of Medicine and Pharmacy, Gastroenterology and Hepatology Department, Bucharest, Romania; Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
Carmen Monica PREDA, ”Carol Davila” University of Medicine and Pharmacy, Gastroenterology and Hepatology Department, Bucharest, Romania; Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
Doina ISTRATESCU, ”Carol Davila” University of Medicine and Pharmacy, Gastroenterology and Hepatology Department, Bucharest, Romania; Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
Liana GHEORGHE, ”Carol Davila” University of Medicine and Pharmacy, Gastroenterology and Hepatology Department, Bucharest, Romania; Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
Razvan CERBAN, ”Carol Davila” University of Medicine and Pharmacy, Gastroenterology and Hepatology Department, Bucharest, Romania; Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
Carmen ESTER, ”Carol Davila” University of Medicine and Pharmacy, Gastroenterology and Hepatology Department, Bucharest, Romania; Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
Tudor Gheorghe STROIE, ”Carol Davila” University of Medicine and Pharmacy, Gastroenterology and Hepatology Department, Bucharest, Romania; Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
Raluca Ioana ALECU, Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
Claudiu Mihai CIUCIUREANU, Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
Alexandra Ioana MARIN, Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
Letitia TUGUI, Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
Cristian TIERANU, Fundeni Clinical Institute, Department of Surgery, Bucharest, Romania.
Sorin Lucian ANDREI, Elias Emergency Hospital, Gastroenterology & Hepatology Department, Bucharest, Romania.
Mircea DICULESCU, ”Carol Davila” University of Medicine and Pharmacy, Gastroenterology and Hepatology Department, Bucharest, Romania; Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
Mircea MANUC, ”Carol Davila” University of Medicine and Pharmacy, Gastroenterology and Hepatology Department, Bucharest, Romania; Fundeni Clinical Institute, Department of Gastroenterology, Bucharest, Romania.
References
- 1.Petrescu M, Vlaicu SI, Ciumărnean L, et al. Chronic Inflammation-A Link between Nonalcoholic Fatty Liver Disease (NAFLD) and Dysfunctional Adipose Tissue. Medicina (Kaunas) 2022;58:641. doi: 10.3390/medicina58050641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abenavoli L. Non-alcoholic fatty liver disease and cardiovascular disease: a close relationship. J Gastrointestin Liver Dis. 2021;30:183–184. doi: 10.15403/jgld-3698. [DOI] [PubMed] [Google Scholar]
- 3.Abenavoli L. Multinational liver societies announce new ‘Fatty’ liver disease nomenclature that is affirmative and non-stigmatising - EASL-The Home of Hepatology. Available: https://easl.eu/news/new_fatty_liver_disease_nomenclature-2/ Accessed: Feb. 03, 2024.
- 4.Tanase DM, Gosav EM, Costea CF, et al. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res. 2020;2020:3920196. doi: 10.1155/2020/3920196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 6.Ugonabo O, Udoh US, Rajan PK, et al. The Current Status of the Liver Liquid Biopsy in MASH Related HCC: Overview and Future Directions. Biomolecules. 2023;13:1369. doi: 10.3390/biom13091369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dijk AM, Schattenberg JM, Holleboom AG, Tushuizen ME. Referral care paths for non-alcoholic fatty liver disease-Gearing up for an ever more prevalent and severe liver disease. United European Gastroenterol J. 2021;9:903–909. doi: 10.1002/ueg2.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Issa D, Patel V, Sanyal AJ. Future therapy for non-alcoholic fatty liver disease. Liver Int. 2018;38:56–63. doi: 10.1111/liv.13676. [DOI] [PubMed] [Google Scholar]
- 9.Abenavoli L, Izzo AA, Milić N, et al. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother Res. 2018;32:2202–2213. doi: 10.1002/ptr.6171. [DOI] [PubMed] [Google Scholar]
- 10.Clichici S, Olteanu D, Nagy AL, et al. Silymarin inhibits the progression of fibrosis in the early stages of liver injury in CCl₄-treated rats. J Med Food. 2015;18:290–298. doi: 10.1089/jmf.2013.0179. [DOI] [PubMed] [Google Scholar]
- 12.Saller R, Melzer J, Reichling J, et al. An updated systematic review of the pharmacology of silymarin. Forsch Komplementmed. 2007;14:70–80. doi: 10.1159/000100581. [DOI] [PubMed] [Google Scholar]
- 13.Zhong S, Fan Y, Yan Q, et al. The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease: A meta-analysis (PRISMA) of randomized control trials. Medicine (Baltimore), 2017. [DOI] [PMC free article] [PubMed]
- 14.Castera L. Diagnosis of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: Non-invasive tests are enough. Liver Int. 2018;38:67–70. doi: 10.1111/liv.13658. [DOI] [PubMed] [Google Scholar]
- 15.Lüchtenborg C, Niederhaus B, Brügger B, et al. Lipid Profiles of Five Essential Phospholipid Preparations for the Treatment of Nonalcoholic Fatty Liver Disease: A Comparative Study. Lipids. 2020;55:271–278. doi: 10.1002/lipd.12236. [DOI] [PubMed] [Google Scholar]
- 16.Biswas S, Mukherjee PK, Kar A, et al. Optimized piperine-phospholipid complex with enhanced bioavailability and hepatoprotective activity. Pharm Dev Technol. 2021;26:69–80. doi: 10.1080/10837450.2020.1835956. [DOI] [PubMed] [Google Scholar]
- 17.Niederau C, Strohmeyer G, Heintges T, et al. Polyunsaturated phosphatidyl-choline and interferon alpha for treatment of chronic hepatitis B and C: a multi-center, randomized, double-blind, placebo-controlled trial. Leich Study Group. Hepatogastroenterology. 1998;45:797–804. [PubMed] [Google Scholar]
- 18.Gordon BR, Parker TS, Levine DM, et al. Neutralization of endotoxin by a phospholipid emulsion in healthy volunteers. J Infect Dis. 2005;1:1515–1522. doi: 10.1086/428908. [DOI] [PubMed] [Google Scholar]
- 19.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717–1730. doi: 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Constantinescu C, Sandulescu L, Saftoiu A. The Role of Elastography in Non-Alcoholic Fatty Liver Disease. Curr Health Sci J. 2020;46:255–269. doi: 10.12865/CHSJ.46.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Rusu E, Enache G, Jinga M, et al. Medical nutrition therapy in non-alcoholic fatty liver disease-a review of literature. J Med Life. 2015;8:258–262. [PMC free article] [PubMed] [Google Scholar]
- 24.Yang K, Chen J, Zhang T, et al. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front Immunol. 2022;13:949746. doi: 10.3389/fimmu.2022.949746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anushiravani A, Haddadi N, Pourfarmanbar M, Mohammadkarimi V. Treatment options for nonalcoholic fatty liver disease: a double-blinded randomized placebo-controlled trial. Eur J Gastroenterol Hepatol. 2019;31:613–617. doi: 10.1097/MEG.0000000000001369. [DOI] [PubMed] [Google Scholar]
- 26.Hajiaghamohammadi AA, Ziaee A, Oveisi S, Masroor H. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic Fatty liver disease: a randomized controlled pilot study. Hepat Mon. 2012;12:e6099. doi: 10.5812/hepatmon.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumitru E, Condur L, Alexandrescu L, et al. Triple Antioxidant Therapy, an Alternative for Patients with Chronic Liver Disease - A Prospective Multicenter Interventional Study. Maedica (Bucur) 2020. [DOI] [PMC free article] [PubMed]
- 28.Colletta C, Colletta A, Placentino G. Lifestyle and silymarin: a fight against liver damage in NAFLD associated - prediabetic disease. J Diabetes Metab Disord. 2020;19:883–894. doi: 10.1007/s40200-020-00576-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizzo M, Colletti A, Penson PE, et al. International Lipid Expert Panel (ILEP). Nutraceutical approaches to non-alcoholic fatty liver disease (NAFLD): A position paper from the International Lipid Expert Panel (ILEP). Pharmacol Res. 2023;189:106679. doi: 10.1016/j.phrs.2023.106679. [DOI] [PubMed] [Google Scholar]
- 30.Gundermann KJ, Gundermann S, Drozdzik M, Mohan Prasad VG. Essential phospholipids in fatty liver: a scientific update. Clin Exp Gastroenterol. 2016;9:105–117. doi: 10.2147/CEG.S96362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gundermann KJ, Kuenker A, Kuntz E, Drozdzik M. Activity of essential phospholipids (EPL) from soybean in liver diseases. Pharmacol Rep. 2011;63:643–659. doi: 10.1016/s1734-1140(11)70576-x. [DOI] [PubMed] [Google Scholar]
- 32.Poongothai S, Karkuzhali K, Siva Prakash G. Effect of essentiale in diabetic subjects with non-alcoholic fatty liver. Int J Diab Dev Ctries. 2005;25:12–19. [Google Scholar]
- 33.Ohbayashi H, Fujimoto M, Yamase H, Ito M. Improvement of NASH with two-year treatment with oral polyenephosphatidylcholine. J Rural Med. 2007;1:67–73. [Google Scholar]
- 34.Sas E, Grinevich V, Efimov O, Shcherbina N. Beneficial influence of polyunsaturated phosphatidylcholine enhances functional liver condition and liver structure in patients with nonalcoholic steatohepatitis accompanied by diabetes type 2. Results of prolonged randomized blinded prospective clinical study (abstract). J Hepatol, 2013.
- 35.Arvind N, Savaikar P, Rajkumar JS. Therapy for NAFLD – comparative study of essential phospholipids vs ursodeoxycholic acid. Ind J Clin Pract, 2006.
- 36.Dajani AI, Abu Hammour AM, Zakaria MA, et al. Essential phospholipids as a supportive adjunct in the management of patients with NAFLD. Arab J Gastroenterol. 2015;16:99–104. doi: 10.1016/j.ajg.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Li JH, Chen XY, Zhong CF, Min J. A randomized controlled study of essential phospholipids (Essentiale capsules) in the treatment of fatty liver. Infect Dis Info. 2000;13:180–181. [Google Scholar]
- 38.Ohbayashi H, Fujimoto M, Yoshida M, et al. The therapeutic effect of polyenephosphatidylcholine (EPL) on NASH. Liver Bile Pancreas. 2006;52:637–642. [Google Scholar]
- 39.Zhou SY, Sun ZY. Therapeutic efficacy of polyunsaturated phosphatidylcholine on fatty liver disease. J Clin Hepatol. 2010;26:286–287. [Google Scholar]


