Abstract
Liver cancer is the 6th leading cause of cancer related deaths in the US even though it ranks 14th in incidence. More men are diagnosed with liver cancer than women, and the number of projected deaths among men (20,020) is almost double that among women (10,140) in the US. Infections like hepatitis and metabolic conditions like obesity are believed to be major risk factors for the onset of liver cancer. Hepatocellular carcinoma (HCC), the most common type of liver cancer, accounts for 75% of all cases. Chemotherapy has not been effective in treating HCC. Targeted therapies are being used in advanced HCC patients due to a better survival and less side effects when compared to traditional chemotherapy. Therapeutic agents targeting the regulators of growth factor signaling pathways and the mediators of downstream signaling—for example, inhibitors of the tyrosine kinase receptor—are used as targeted molecular therapies. Kinase inhibitors that modulate growth signals, such as sorafenib and lenvatinib, are commonly employed in targeted molecular therapy for HCC patients. This review covers these agents, highlighting modes of action and providing details on clinical trials.
Keywords: Hepatocellular carcinoma, targeted molecular therapy, sorafenib, lenvatinib
I. INTRODUCTION
Liver cancer is a leading cause of cancer-related deaths worldwide.1 It is also one of the cancers showing a recent increase in incidence and number of cancer-related deaths in the US.2 At time of diagnosis, liver cancer may be categorized as primary (e.g., hepatocellular carcinoma, HCC; cholangio-carcinoma; sarcoma), originally arising from liver cells, or secondary, arising from metastases to the liver.1,3 In the US and Europe, secondary liver cancer is more common than HCC whereas the opposite is true in Asia and Africa.
Several risk factors are associated with HCC, and their contribution to incidence varies based on educational, environmental, and genetic differences among various populations. In Asia, where the disease is endemic, vertical transmission of hepatitis B virus (HBV) and aflatoxin exposure are the most common causes of HCC development.4-7 Further, Asian males are more affected than Asian females, showing earlier disease onset. In contrast, risk factors in Western countries include hepatitis C virus (HCV), cirrhosis, alcoholism, nonalcoholic fatty liver disease, obesity, and smoking.5,6 Genetic disorders, such as tyrosinemia, hereditary hemochromatosis, and glycogen storage disease type Ia are also implicated in HCC development and progression.1,3,8
Because of variations in risk factor prevalence among populations, global incidence of HCC is also heterogeneous. In 2018, worldwide incidence was 841,080, with rates highest in East Asia and sub-Saharan Africa due to the previously listed endemic causes; incidence was lowest in the Americas.1,9 Incidence in East Asia and sub-Saharan Africa is close to 20 per 100,000, while in the US it is 6.6 per 100,000.1,2 Despite a global increase in HBV vaccination rates, aflatoxin exposure prevention, and general health education, the overall global incidence of HCC is expected to continue to increase.1,2,10 In particular, the increase in the US correlates to an increase in environmental factors, such as HCV among adults born between 1945 and 1965, nonalcoholic fatty liver disease, and obesity. When factoring in ethnic variations, males have up to a threefold higher incidence than females.2,10 Among different ethnicities, American Indians and Alaskan Natives have the highest incidence. Hispanics and blacks show an increasing incidence compared to non-Hispanic whites, who continue to have the lowest rates, partly due to greater awareness of what constitutes high-risk behaviors for HCC.11-13
The prognosis for HCC is dependent on disease stage, demographics, and racial factors. The stage of HCC is a reliable predictor of patient prognosis, with earlier stages (e.g., 0, A) correlating with higher survival rates. Table 1 provides correlations between staging/Child-Pugh classification for liver cirrhosis (detailed further in Table 2) and associated survival rates. Moreover, variations in demographics and race are correlated with prognosis among US populations. For example, some studies report that younger patients have a poorer survival rate than older patients due to a greater tumor burden and cancer aggressiveness at diagnosis, and that men have a poorer prognosis than women.14-16 Blacks are more likely to be younger at presentation and with a more advanced stage than non-Hispanic whites due to higher rates of viral hepatitis in blacks.15,17,18
TABLE 1:
Hepatocellular carcinoma staging, classification, and survival rate
| Stage | Lesions | Child-Pugh class | Survival rate with therapy | Ref. |
|---|---|---|---|---|
| 0 | Single lesion, < 2 cm | A or B | 86% (5 yr) | 20 |
| A | Single lesion or ≤ 3 lesions ≤ 3 cm | A or B | 69% (5 yr) | 25 |
| B | Multinodular | A or B | 50% (5 yr) | 55 |
| C | Vascular invasion and hepatic spread | — | 13% (3 yr) | 56 |
| D | Extrahepatic and vascular spread | C | 8% (3 yr) | 57 |
TABLE 2:
Child-Pugh classification system for liver cirrhosis (based on the results of Clarke and Hurwitz58)
| Criteria | 1 point | 2 points | 3 points |
|---|---|---|---|
| Albumin (g/dL) | > 3.5 | 2.8–3.5 | < 2.8 |
| Ascites | None | Mild-moderate; diuretic responsive | Severe; diuretic refractory |
| Bilirubin (mg/dL) | < 2 | 2–3 | > 3 |
| Encephalopathy | None | Grade 1–2 | Grade 3–4 |
| INR | < 1.7 | 1.7–2.3 | > 2.3 |
| Total Points | 5–6 = Class A | 7–9 = Class B | 10–15 = Class C |
Although HCC is the third most common cause of cancer-related deaths worldwide, HCC prognosis and survival continue to improve because of increasing avoidance of high-risk behaviors, accessibility of treatment, and treatment better tailored to genetic causes.19,20 Depending on disease stage and other patient related factors, treatment options include complete resection via surgery, liver transplant, local ablation, systemic chemotherapy, and targeted therapy. Of these, oral targeted therapy can be used in many ways, such as in conjunction with local therapies for localized or locally advanced HCC, as a stand-alone therapy for metastatic HCC, and for a variety of patient populations.21-23
Despite increasing treatment options, racial and ethnic disparities remain an issue due to cost of treatment, access to care and lack of resources.24
II. MOLECULAR TARGET THERAPIES
As previously mentioned, using targeted molecular drugs in HCC therapy is advantageous for many reasons. Targeted therapy may be used in conjunction with other therapies. It can also treat patient populations with unresectable cancers. Many such therapies target the molecular pathways that are involved in HCC development and eventual metastasis.21-23 Examples of these pathways are those that regulate growth factor signaling and downstream signaling mediators of the receptor tyrosine kinase.24,25
A. Regulation of Growth Factor Signaling
Cells control proliferation and temporary senescence via a variety of growth factor pathways. These pathways are generally stimulated by growth factors that bind to tyrosine kinase receptors, such as vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth (PDGFR) receptor, among others.25 In malignant cells, regulation of these pathways at the receptor level is often lost, allowing for continuous cell proliferation. For example, constitutive activation of VEGFR encourages angiogenesis; an increase in the release of growth factors may stimulate other growth factor receptors.25 Thus, it is advantageous to include molecular target therapy that directly inhibits receptors (Table 3).
TABLE 3:
Targets of HCC molecular therapy
| Agent | Molecular targets | Efficacy compared to sorafenib |
Status | Refs. |
|---|---|---|---|---|
| Sorafenib | VEGFR, PDGFR, c-KIT, RET, Ras/Raf, MEK/ERK | — | Approved 1st line | 23,26,27,33,59 |
| Lenvatinib | VEGFR, PDFGFR, FGFR-4, RET | Equivalent | Approved 1st line | 58 |
| Cabozantinib | VEGFR, KIT, MET AXL | Superior (compared to placebo) | Approved later lines (after sorafenib) | 58 |
| Regorafenib | VEGFR, PDGFR, FGFR | Superior (compared to placebo) | Approved later lines (after sorafenib) | 51 |
| Brivanib | VEGFR2, FGFR1 | Inferior | Completed clinical trial 2013 | 22,36 |
| Linifanib | PDFGF-Rs, VEGF-R | Inferior | Completed clinical trial 2012 | 37 |
B. Downstream Tyrosine Kinase Receptor Signaling Mediators
Activation of tyrosine kinase receptors stimulates downstream pathways that include Ras, Raf, mitogen-activated protein kinase (MAPK), and extracellular signal–regulated kinase (ERK). The receptors activate Ras, which in turn phosphorylates Raf serine threonine kinase. Raf eventually activates MEK (MAPK/ERK) to initiate inappropriate cell growth and angiogenesis while inhibiting the apoptosis pathway.22,26,27 In addition, activation of the tyrosine kinase receptors may stimulate the PI3k/Akt/mTOR downstream pathways. In particular, the activated receptors work with phosphoinositide-3 kinase (PI3K) to activate phosphatidylinositol 4,5-bisphosphate (PIP2), which activates phosphatidylinositol 3,4,5-triphosphate (PIP3), which is negatively regulated by PTEN (phosphatase and tensin homolog detected on chromosome). PIP3 may then activate serine threonine kinase (Akt), which activates mTOR, a serine/threonine kinase. Ultimately, mTOR modifies gene transcription to favor cell proliferation and inhibits apoptosis. Because products of the PI3k/Akt/mTOR pathway participate in other regulation pathways, HCC targeted therapies designed to inhibit this pathway may be extremely efficacious.23,28-30
III. CURRENT TARGETED THERAPIES
Surgery for complete resection is the treatment of choice for patients with good liver function and/or localized tumors without metastasis or vascular expansion. Liver transplant is the ideal treatment to remove the tumor as well as the underlying liver disease.29-32 For patients who cannot receive surgery, transplants or other therapies, local ablation (e.g., transarterial chemoembolization, TACE), and targeted molecular therapy are appropriate.20 Here we focus on first-line targeted molecular therapies.
A. Sorafenib
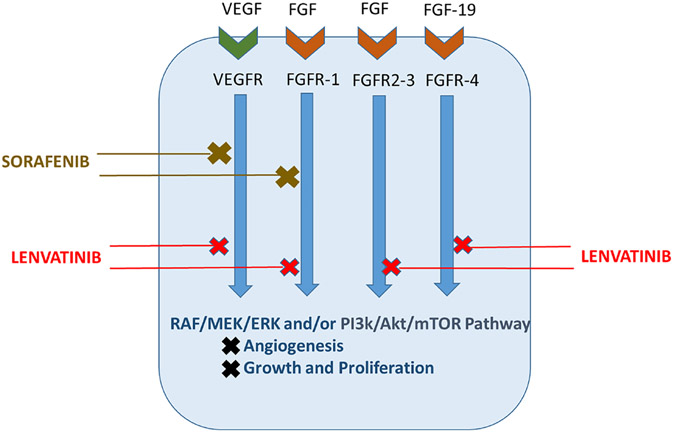
Sorafenib is the first oral multikinase inhibitor to be used in the treatment of HCC. It is especially suitable for patients for whom localized therapy is not an option but who have advanced or intermediate HCC and preserved liver function. Its mechanism targets many crucial pathways involved in HCC tumorigenesis, including the targeted tyrosine kinase receptors VEGFR, PDGFR, and FGFR and downstream serine threonine kinases in the Raf/MEK/ERK pathways (Fig. 1). According to the double-blind, placebo-controlled SHARP trial, patients receiving sorafenib for systemic treatment of HCC had a 3-month increase in median survival rate (7.9 months to 10.7 months). A trial focusing on the Asia-Pacific region showed similar rates in the studied populations.25,33-35
FIG. 1:
Mechanism of action for first-line HCC targeted molecular therapies. Current targeted therapies affect molecular pathways important in HCC development and metastatic ability. Shown are sorafenib, which competitively inhibits VEGFR and FGFR1 signaling at the receptor level and at their respective downstream signaling pathways, and lenvatinib, a preferred first-line targeted therapy due to its inhibition at multiple receptors and their associated downstream signal cascades. A primary target of lenvatinib is the receptor FGFR4, which has been shown to decrease the apoptotic ability of sorafenib if overexpressed.
B. Sorafenib versus Other Drugs
Monotherapy agents like brivanib, sunitinib, and linifanib have been studied and compared to sorafenib, but have not proved to be as efficacious. Brivanib, which inhibits VEGFR and FGFR, was compared to sorafenib in the BRISK-FL study. In this phase III trial, brivanib’s overall survival (OS) did not meet its primary end point when compared to sorafenib (9.5 versus 9.9 months, respectively).36 Similarly, a phase III study comparing sunitinib to sorafenib, in which the median OS was 7.9 months for sorafenib and 10.2 months for sunitinib, showed that sunitinib was neither equivalent nor superior to sorafenib. Sunitinib side effects and toxicity led to early termination of the trial.33
A phase III trial of linifanib versus sorafenib yielded the same conclusion as the previous ones: linifanib did not meet predetermined superiority or noninferiority OS boundaries. The median OS was 9.1 for linifanib and 9.8 for sorafenib.37
C. Combinations with Sorafenib
Erlotinib has been studied as conjunctive therapy with sorafenib. In a placebo-controlled trial, median OS between sorafenib plus erlotinib and sorafenib plus placebo was 9.5 and 8.5 months, respectively. Overall survival did not improve with erlotinib in patients with advanced HCC.38 Doxorubicin has been used in combination with sorafenib as well, but the addition of doxorubicin did not significantly improve OS and had higher toxicity than sorafenib alone.39
Selective internal radiation therapy (SIRT) with yittrium-90 has been studied compared with sorafenib in patients with locally advanced or intermediate HCC. Median OS was 8 months and 9.9 months for SIRT and sorafenib, respectively, which was not considered a significant difference.40
IV. LENVATINIB AS FIRST-LINE THERAPY
Lenvatinib is an inhibitor of multiple proteins involved in angiogenesis and tumor growth. It competitively inhibits tyrosine kinase receptors VEGFR, FGFR, KIT, and RET, and thus their respective downstream pathways.41 Of note is lenvatinib’s action on FGFR-4, which binds fibroblast growth factor 19. Overexpression of this ligand-receptor complex has been implicated in decreased formation of reactive oxygen species and apoptosis by sorafenib.55,56 Lenvatinib’s inhibition of signals generated by the FGF-19/FGFR4 complex is an important feature of its antitumor activity, as it overcomes a mechanism that leads to sorafenib’s failure in slowing the development and spread of HCC (Fig. 1).42,43
Lenvatinib was studied in phase I trials in patients with advanced HCC and Child-Pugh A and B. Optimal doses were determined to be 12 mg for Child-Pugh A and 8 mg for Child-Pugh B, with a tolerable side effect profile.44 In a phase II study, Asian patients with advanced HCC not eligible for local therapy were given 12 mg of lenvatinib once a day in 28-day cycles.45 Median time to progression was 7.4 months and median OS was 18.7 months. Lenvatinib was used in these patients for a median of 7.3 months, and the most common adverse event leading to discontinuation was proteinuria. Further analysis of these patients showed that 12 mg a day was optimal for Child-Pugh A patients weighing 60 kg or more and 8 mg a day for those weighing less than 60 kg.45,46 In a phase III trial, patients with unresectable HCC were recruited from Asia-Pacific, Europe, and North America and randomly assigned to either lenvatinib or sorafenib treatments. Median survival for the lenvatinib group was 13.6 months, which was noninferior to that of sorafenib at 12.3 months.47
In addition to being as efficacious as sorafenib, lenvatinib may have an advantage in cost; it has been found to provide a 0.27 life year increase with a 0.23 quality improvement at a negative incremental cost compared to sorafenib.47
V. CONCLUSION
Various targeted therapies have been investigated for treatment of HCC. The most successful of these has been sorafenib, which is a multi–receptor kinase inhibitor. This standard-of-care treatment was one of the first to be approved as a first-line therapy to improve survival in patients with advanced disease. New first-line and second-line treatments have since been approved. Most of them (e.g., regorafenib, cabozantinib) are receptor tyrosine kinase inhibitors with different inhibition profiles. Cabozantinib has a profile that is partly similar to that of sorafenib, and it inhibits receptor tyrosine kinases including MET and AXL.48 It is approved for use in patients who have previously been treated with sorafenib. In the CELESTIAL (Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma) phase III clinical trial involving HCC patients with advanced disease who previously received sorafenib, cabozantinib treatment increased both overall and progression-free survival compared to placebo treatment.49
Another drug, regorafenib, was the first to be approved for patients whose cancer had progressed on sorafenib.50 Although structurally similar to sorafenib, it has a potent and distinct activity profile. Its approval as a second-line treatment was based on the outcome of the multinational phase III RESORCE (Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment) trial.51 Regorafenib treatment resulted in disease stabilization, which significantly improved overall and progression free survivals compared to placebo.51,52
VI. FUTURE DIRECTIONS
A tremendous increase in genetic and immunological understanding of HCC has recently gained momentum. Treatment options are rapidly expanding, with several agents undergoing clinical trials. Immunotherapy trials have had mixed results. A phase III trial (KEYNOTE-240, NCT02702401) with pembrolizumab, a single-agent immune checkpoint inhibitor, did not find this drug to be superior to placebo treatment in patients treated previously with sorafenib.53 However, based on the overall response rate of 31% and the duration of response in a Check-Mate 040 trial in March of 2020 the FDA granted accelerated approval for the combination of immunotherapy drugs nivolumab and ipilimumab for patients with HCC who have been previously treated with sorafenib. There is optimism as well regarding other checkpoint inhibitors and therapy that combine these immune checkpoint inhibitors with receptor tyrosine kinases or antiangiogenic therapies (ClinicalTrials.gov; NCT03347292, NCT03755791, NCT03434379).54 In addition to research focused on elucidating the molecular mechanisms of HCC, research is under way to better understand the association of environmental and other contributors in these populations. The results are urgently needed to address the disparities in HCC survival rates among different population groups.
ACKNOWLEDGMENTS
RB and UTS are supported by NIMHD (Grant No. 2U54 MD006882-06) and NCI (Grant No. 1P20CA233355-01).
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. Nov;68(6):394–424. Epub 2018/09/13. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020. Jan;70(1):7–30. Epub 2020/01/09. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001. Sep;2(9):533–43. Epub 2002/03/22. [DOI] [PubMed] [Google Scholar]
- 4.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology. 2004. Nov;127(5 Suppl 1):S5–S16. Epub 2004/10/28. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011. Sep 22;365(12):1118–27. Epub 2011/10/14. [DOI] [PubMed] [Google Scholar]
- 6.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology. 2010. May;51(5):1820–32. Epub 2010/05/01. [DOI] [PubMed] [Google Scholar]
- 7.Valla DC. The diagnosis and management of the Budd-Chiari syndrome: Consensus and controversies. Hepatology. 2003. Oct;38(4):793–803. Epub 2003/09/27. [DOI] [PubMed] [Google Scholar]
- 8.Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Epub 2017/07/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: Consider the population. J Clin Gastroenterol. 2013. Jul;47(Suppl):S2–S6. Epub 2013/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ami Intern Med. 2014. Mar 4;160(5):293–300. Epub 2014/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Island F, Miller KD, Siegel RL, Fedewa SA, Ward EM, Jemal A. Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J Clin. 2017. Jul 8;67(4):273–89. Epub 2017/06/07. [DOI] [PubMed] [Google Scholar]
- 12.Salvatore M, Jeon J, Meza R. Changing trends in liver cancer incidence by race/ethnicity and sex in the US: 1992–2016. Cancer Causes Control 2019. Dec;30(12):1377–88. Epub 2019/10/14. [DOI] [PubMed] [Google Scholar]
- 13.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010. Aug;7(8):448–58. Epub 2010/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimada S, Kamiyama T, Yokoo H, Wakayama K, Tsuruga Y, Kakisaka T, Kamachi H, Taketomi A. Clinicopathological characteristics and prognostic factors in young patients after hepatectomy for hepatocellular carcinoma. World J Surg Oncol. 2013. Mar 2;11:52. Epub 2013/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D, Hanna DL, Usher J, LoCoco J, Chaudhari P, Lenz HJ, Setiawan VW, El-Khoueiry A. Impact of sex on the survival of patients with hepatocellular carcinoma: A surveillance, epidemiology, and end results analysis. Cancer. 2014. Dec 1;120(23):3707–16. Epub 2014/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho SJ, Yoon JH, Hwang SS, Lee HS. Do young hepatocellular carcinoma patients with relatively good liver function have poorer outcomes than elderly patients? J Gastroenterol Hepatol. 2007. Aug;22(8):1226–31. Epub 2007/05/15. [DOI] [PubMed] [Google Scholar]
- 17.Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D, Sakellariou S, Kykalos S, Tsourouflis G, Garmpi A, Delladetsima I, Kontzoglou K, Kouraklis G. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol. 2017. Aug 7;23(29):5282–94. Epub 2017/08/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and geographic disparities in hepatocellular carcinoma outcomes. Am J Prev Med. 2018. Nov;55(5 Suppl 1):S40–S8. Epub 2019/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010. Dec 15;127(12):2893–917. Epub 2011/02/26. [DOI] [PubMed] [Google Scholar]
- 20.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003. Dec 6;362(9399):1907–17. Epub 2003/12/12. [DOI] [PubMed] [Google Scholar]
- 21.Gholam P. The role of sorafenib in hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015. Apr;ll(4):253–5. Epub 2016/04/22. [PMC free article] [PubMed] [Google Scholar]
- 22.Kudo M. Signaling pathway and molecular-targeted therapy for hepatocellular carcinoma. Dig Dis. 2011;29(3):289–302. Epub 2011/08/11. [DOI] [PubMed] [Google Scholar]
- 23.Kudo M. Targeted therapy for liver cancer: Updated review in 2012. Curr Cancer Drug Targets. 2012. Nov 1;12(9):1062–72. Epub 2012/08/28. [PubMed] [Google Scholar]
- 24.Frenette CT. Increasing awareness on racial disparities in liver transplantation for hepatocellular carcinoma in the United States. Hepatol Coimnun. 2019. Jan;3(1):5–7. Epub 2019/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: Current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012. May 8;4:19–37. Epub 2012/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: Potential targeting for therapeutic intervention. Leukemia. 2003. Jul;17(7):1263–93. Epub 2003/07/02. [DOI] [PubMed] [Google Scholar]
- 27.Kudo M. Signaling pathway/molecular targets and new targeted agents under development in hepatocellular carcinoma. World J Gastroenterol. 2012. Nov 14;18(42):6005–17. Epub 2012/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker S, Wankell M, Ho V, White R, Deo N, Devine C, Dewdney B, Bhathal P, Govaere O, Roskams T, Qiao L, George J, Hebbard L. Targeting mTOR and Src restricts hepatocellular carcinoma growth in a novel murine liver cancer model. PLoS One. 2019;14(2):e0212860. Epub 2019/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimri M, Satyanarayana A. Molecular signaling pathways and therapeutic targets in hepatocellular carcinoma. Cancers (Basel). 2020. Feb 20;12(2). Epub 2020/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang S, Liu G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol Lett. 2017. Mar;13(3):1041–7. Epub 2017/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore). 2017. Mar;96(9):e5904. Epub 2017/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: Analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002. Oct;8(10):873–83. Epub 2002/10/03. [DOI] [PubMed] [Google Scholar]
- 33.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009. Jan;10(1):25–34. Epub 2008/12/20. [DOI] [PubMed] [Google Scholar]
- 34.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, Taylor I, Moscovici M, Saltz LB. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006. Sep 10;24(26):4293–300. Epub 2006/08/16. [DOI] [PubMed] [Google Scholar]
- 35.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008. Oct;7(10):3129–40. Epub 2008/10/15. [DOI] [PubMed] [Google Scholar]
- 36.Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J, Lu L, Chao Y, Boucher E, Han KH, Paik SW, Robles-Avina J, Kudo M, Yan L, Sobhonslidsuk A, Komov D, Decaens T, Tak WY, Jeng LB, Liu D, Ezzeddine R, Walters I, Cheng AL. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013. Oct 1;31(28):3517–24. Epub 2013/08/28. [DOI] [PubMed] [Google Scholar]
- 37.Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, Kudo M, Kang YK, Chen PJ, Toh HC, Gorbunova V, Eskens FA, Qian J, McKee MD, Ricker JL, Carlson DM, El-Nowiem S. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2015. Jan 10;33(2):172–9. Epub 2014/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, Bruix J, Qin S, Thuluvath PJ, Llovet JM, Leberre MA, Jensen M, Meinhardt G, Kang YK. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015. Feb 20;33(6):559–66. Epub 2014/12/31. [DOI] [PubMed] [Google Scholar]
- 39.Abou-Alfa GK, Shi Q, Knox JJ, Kaubisch A, Niedzwiecki D, Posey J, Tan BR Jr, Kavan P, Goel R, Lammers PE, Bekaii-Saab TS, Tam VC, Rajdev L, Kelley RK, El Dika I, Zemla T, Potaracke RI, Balletti J, El-Khoueiry AB, Harding JH, Suga JM, Schwartz LH, Goldberg RM, Bertagnolli MM, Meyerhardt J, O‘Reilly EM, Venook AP. Assessment of treatment with sorafenib plus doxorubicin vs. sorafenib alone in patients with advanced hepatocellular carcinoma: Phase 3 CALGB 80802 randomized clinical trial. JAMA Oncol. 2019. Sep 5. Epub 2019/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, Sibert A, Bouattour M, Lebtahi R, Allaham W, Barraud H, Laurent V, Mathias E, Bronowicki JP, Tasu JP, Perdrisot R, Silvain C, Gerolami R, Mundler O, Seitz JF, Vidal V, Aube C, Oberti F, Couturier O, Brenot-Rossi I, Raoul JL, Sarran A, Costentin C, Itti E, Luciani A, Adam R, Lewin M, Samuel D, Ronot M, Dinut A, Castera L, Chatellier G, Group ST. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017. Dec;18(12):1624–36. Epub 2017/11/07. [DOI] [PubMed] [Google Scholar]
- 41.Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, Okamoto K, Minoshima Y, Iwata M, Funahashi Y. Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. Epub 2014/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao L, Wang X, Tang Y, Huang S, Hu CA, Teng Y. FGF19/FGFR4 signaling contributes to the resistance of hepatocellular carcinoma to sorafenib. J Exp Clin Cancer Res. 2017. Jan 9;36(1):8. Epub 2017/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kudo M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver Cancer. 2018. Mar;7(1)4–19. Epub 2018/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikeda M, Okusaka T, Mitsunaga S, Ueno H, Tamai T, Suzuki T, Hayato S, Kadowaki T, Okita K, Kumada H. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2016. Mar 15;22(6):1385–94. Epub 2015/10/27. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda M, Okusaka T, Tamai T, Suzuki T, Hisai T, Hayato S, Okita K, Kumada H. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017. Apr;52(4):512–9. Epub 2016/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamai T, Hayato S, Hojo S, Suzuki T, Okusaka T, Ikeda K, Kumada H. Dose finding of lenvatinib in subjects with advanced hepatocellular carcinoma based on population pharmacokinetic and exposure-response analyses. J Clin Pharmacol. 2017. Sep;57(9):1138–47. Epub 2017/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018. Mar 24;391(10126):1163–73. Epub 2018/02/13. [DOI] [PubMed] [Google Scholar]
- 48.Deeks ED. Cabozantinib: A review in advanced hepatocellular carcinoma. Target Oncol. 2019. Feb;14(1):107–13. Epub 2019/02/16. [DOI] [PubMed] [Google Scholar]
- 49.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klumpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018. Jul 5;379(1):54–63. Epub 2018/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rey JB, Launay-Vacher V, Tournigand C. Regorafenib as a single agent in the treatment of patients with gastrointestinal tumors: An overview for pharmacists. Target Oncol. 2015. Jun;10(2):199–213. Epub 2014/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G, RESORCE investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017. Jan 7;389(10064):56–66. Epub 2016/12/10. [DOI] [PubMed] [Google Scholar]
- 52.Heo YA, Syed YY. Regorafenib: A review in hepatocellular carcinoma. Drugs. 2018. Jun;78(9):951–8. Epub 2018/06/20. [DOI] [PubMed] [Google Scholar]
- 53.Film RS, Ryoo B-Y, Merle P, Kudo M, Bouattour M, Lim H-Y, Breder VV, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox JJ, Daniele B, Ebbinghaus S, Chen E, Siegel AB, Zhu AX, Cheng A-L, KEYNOTE investigators. Results of KEYNOTE-240: Phase 3 study of pembrolizumab (Pembro) vs. best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2019;37(15 Suppl):4004. [Google Scholar]
- 54.Xu W, Liu K, Chen M, Sun JY, McCaughan GW, Lu XJ, Ji J. Immunotherapy for hepatocellular carcinoma: Recent advances and future perspectives. Ther Adv Med Oncol. 2019;11:1758835919862692. Epub 2019/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford). 2005;7(1):35–41. Epub 2008/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinn DH, Cho JY, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Different survival of Barcelona clinic liver cancer stage C hepatocellular carcinoma patients by the extent of portal vein invasion and the type of extrahepatic spread. PLoS One. 2015;10(4):e0124434. Epub 2015/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsilimigras DI, Bagante F, Sahara K, Moris D, Hyer JM, Wu L, Ratti F, Marques HP, Soubrane O, Paredes AZ, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Pawlik TM. Prognosis after resection of Barcelona clinic liver cancer (BCLC) stage 0, A, and B hepatocellular carcinoma: A comprehensive assessment of the current BCLC classification. Ann Surg Oncol. 2019. Oct;26(11):3693–700. Epub 2019/07/04. [DOI] [PubMed] [Google Scholar]
- 58.Clarke JM, Hurwitz HI. Targeted inhibition of VEGF receptor 2: An update on ramucirumab. Expert Opin Biol Ther. 2013. Aug;13(8):1187–96. Epub 2013/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G, Omata M, Pitman Lowenthal S, Lanzalone S, Yang L, Lechuga MJ, Raymond E. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J Clin Oncol. 2013. Nov 10;31(32):4067–75. Epub 2013/10/02. [DOI] [PubMed] [Google Scholar]