Abstract
Heart failure with preserved ejection fraction (HFpEF) patients have an increased ventilatory demand. Whether their ventilatory capacity can meet this increased demand is unknown, especially in those with obesity. Body composition (DXA) and pulmonary function were measured in 20 patients with HFpEF (69 ± 6 yr;9 M/11 W). Cardiorespiratory responses, breathing mechanics, and ratings of perceived breathlessness (RPB, 0–10) were measured at rest, 20 W, and peak exercise. FVC correlated with %body fat (R2 =0.51,P = 0.0006), VO2peak (% predicted,R2 =0.32,P = 0.001), and RPB (R2 =0.58,P = 0.0004). %Body fat correlated with end-expiratory lung volume at rest (R2 =0.76,P < 0.001), 20 W (R2 =0.72,P < 0.001), and peak exercise (R2 =0.74,P < 0.001). Patients were then divided into two groups: those with lower ventilatory reserve (FVC<3 L,2 M/10 W) and those with higher ventilatory reserve (FVC>3.8 L,7 M/1 W). VO2peak was ~22% less (p < 0.05) and RPB was twice as high at 20 W (p < 0.01) in patients with lower ventilatory reserve. Ventilatory reserves are limited in patients with HFpEF and obesity; indeed, the margin between ventilatory demand and capacity is so narrow that exercise capacity could be ventilatory limited in many patients.
Keywords: Exercise capacity, Pulmonary function, Operational lung volumes, Breathing mechanics, Breathlessness, Exercise intolerance, Aging
1. Introduction
In patients with heart failure with preserved ejection fraction (HFpEF), dyspnea on exertion (DOE) and exercise intolerance are hallmark symptoms (Obokata et al., 2018). An important contributor to these symptoms is an increased ventilatory demand during exercise (i.e., increased ventilation [VE] to carbon dioxide output [VCO2] ratio) (Smith et al., 2019a; Van Iterson et al., 2017; Smith et al., 2019b). However, very little focus has been placed on the patient’s ability to meet an increased ventilatory demand (i.e., mechanical ventilatory reserves).
Ventilatory reserves could be very limited in patients with HFpEF. First, mechanical ventilatory capacity is lower in older adults due to the age-related decline in forced vital capacity (FVC) (Smith et al., 2019a), which limits tidal volume (VT), and in maximal expiratory flow (MEF) (Knudson et al., 1983, 1977, 1976), which restricts breathing frequency (Fb) (Babb and Rodarte, 2000; DeLorey and Babb, 1999; Babb, 1999). Also, older adults have an increased ventilatory demand (VE/VCO2) during exercise compared with younger adults (DeLorey and Babb, 1999) (e.g., increased physiological dead space), further increasing ventilatory demands in older HFpEF patients. Another limitation to ventilatory capacity in older patients with HFpEF is obesity (Haass et al., 2011; Shah et al., 2013; Carbone et al., 2016). Obesity reduces ventilatory capacity, decreases pulmonary function, alters operational lung volumes, and increases the work of breathing (Babb, 1999; Carbone et al., 2016; Sarma et al., 2020; Lorenzo and Babb, 2013). Obesity can also provoke DOE and exercise intolerance (Babb et al., 2008, 2011; Salome et al., 2010; Parameswaran et al., 2006; Ray et al., 1983). Lastly, there are reductions in pulmonary function in patients with HFpEF independent of the effects of aging and obesity (Smith et al., 2019b; Singh et al., 2019a, 2019b; Villarraga et al., 2022). However, the origins of these HFpEF-related changes in lung function are poorly defined but are consistent among patients. Thus, since aging, obesity, and HFpEF all could potentially decrease FVC (i.e., potentially limiting VT augmentation), it is possible that patients with the smallest FVC could experience the greatest ventilatory limitations. We propose that ventilatory reserves will be limited in older patients with HFpEF and obesity due to age- and obesity-related mechanical ventilatory constraints, which could be associated with DOE and exercise intolerance.
2. Methods
We evaluated the first 20 patients with HFpEF who were enrolled in a larger ongoing study investigating mechanisms of exertional dyspnea and exercise intolerance in patients with HFpEF (Clinical Trials number: NCT04068844). Patients were eligible to participate in the larger study if they had signs and symptoms of heart failure based on Framingham criteria (Lofstrom et al., 2019), an ejection fraction ≥ 50%, and evidence of volume overload (e.g., elevated wedge pressure during exercise) which we confirmed by a pulmonary capillary wedge pressure (PCWP) of ≥ 25 mmHg at peak exercise or an increase in PCWP> 15 mmHg from rest to peak exercise (Sarma et al., 2023). Participants were excluded if they had severe valvular heart disease, congenital heart disease, left bundle branch block, known restrictive or infiltrative cardiomyopathy, acute myocarditis, NYHA Class IV chronic heart failure or chronic heart failure that cannot be stabilized on medical therapy, a prior ejection fraction < 50%, manifest/provocable ischemic heart disease, chronic kidney disease stage IV or greater, significant obstructive lung disease (i.e., forced expiratory volume in 1 s [FEV1] <40% predicted), or regularly used phosphodiesterase inhibitors (e.g., sildenafil and tadalafil). Prior to all testing, written and informed consent was obtained. The study was approved by the UT Southwestern IRB (STU2019–0617) and written informed consent was obtained.
During the first visit, the patients underwent health screening, body composition measurements, pulmonary function tests, and a maximal exercise test to determine participant eligibility (i.e., ability to perform exercise, no significant ischemic disease or arrythmias; data not reported). Body composition was determined by DXA scans (GE). Standard pulmonary function testing was performed according to ATS/ERS guidelines (Graham et al., 2019; Stanojevic et al., 2022). The modified Medical Research Council (mMRC) and Dyspnea-12 questionnaires were administered.
During the second visit, the patients undertook resting measurements, and performed a six-minute constant-load cycling test at 20 W and a maximal incremental cycling test with pulmonary artery and radial artery catheterizations (Balmain et al., 2022a, 2022b). The patients had a Swan-Ganz catheter placed in the pulmonary artery via brachial or antecubital vein access under fluoroscopic guidance. The patients also underwent radial artery catheterization. PaCO2, PaCO2, hemoglobin O2 saturation (HbO2%), and lactate (mmol/L) were measured.
While sitting upright on the semi-recumbent cycle ergometer (Lode BV), heart rate (HR) and rhythm were monitored (12-leadECG), and blood pressure was monitored via arterial waveform tracings. Invasive central hemodynamic measurements and mixed venous (data not presented) and radial arterial blood gases were measured. Pulmonary gas exchange was measured using a customized breath-by-breath system (Beck Integrative Physiological System, USA).
Following rest, patients cycled at 20 W for 6 min. HR and rhythm, pulmonary gas exchange, breathing mechanics, invasive central hemodynamic measurements (data not reported), mixed venous (data not reported), and radial arterial blood gases were measured. Ratings of perceived exertion (RPE, Borg 6–20 scale), breathlessness (RPB, Borg scale 0–10), and unpleasantness (RPU, Borg scale 0–10) were collected during the last minute of the exercise. Patients then performed a maximal incremental test after a brief rest period. The work rate was increased by 5 or 10 W/min until the participant reached volitional exhaustion.
Breathing mechanics including basic spirometry and operational lung volumes were measured at rest and during exercise. Flow was measured using an inspiratory pneumotachograph and a heated expiratory pneumotachograph connected to Hans Rudolph valves via large bore tubing. Flow signals were combined into a single bidirectional flow signal and digitally integrated to yield volume. Inspiratory capacity (IC) was measured at rest, during submaximal exercise, and during each stage of the maximal incremental cycling test to determine placement of tidal flow-volume loops within the maximal flow-volume loop (Babb, 1999; Babb and Rodarte, 1993). End-expiratory lung volume (EELV) was estimated from IC and TLC measured during body plethysmography (EELV=TLC-IC, L or %TLC). End-inspiratory lung volume (EILV) was calculated as the sum of EELV and VT (L or %TLC). Maximal flow-volume loops were determined at rest using the same system. Expiratory flow limitation (EFL) was defined as the percentage of VT where tidal expiratory flow impinged on maximal expiratory flow (MEF). Variables reported from the dual pneumotach breathing mechanics system will be denoted by ‘mech’ (e.g., VE mech), since these variables may be measured at a different time than the gas exchange system values.
All data were analyzed using SAS 9.4 (SAS Institute, Inc., Cary, NC). Relationships between variables were assessed using Pearson’s Correlation Coefficient analyses.
3. Results
Participant characteristics and pulmonary function data are displayed in Table 1. None of the patients were currently smoking. The range in lung function was rather large and four patients had at least one or more pulmonary function variables below the lower limit of normal. Two of these patients had documented restrictive pattern (one of the two had obstruction as well). Four patients had a prior history of smoking (range 5–29 pack years with a mean of 16 and SD of 12).
Table 1.
Participant characteristics (N = 20).
| Mean ± SD | Range | |
|---|---|---|
| Demographics | ||
| Age (y) | 69 ± 6 | 59–79 |
| Gender (Men/Women) | 9/11 | - |
| Height (m) | 1.70 ± 0.10 | 1.55–1.83 |
| Weight (kg) | 110 ± 18 | 76–154 |
| BMI (kg/m2) | 38 ± 7 | 25–50 |
| % Body Fat (%) | 46 ± 9 | 27–57 |
| VAT (kg) | 3.3 ± 1.5 | 0.8–6.7 |
| Trunk Fat (%Total Fat) | 52 ± 8 | 32–62 |
| Pulmonary function | ||
| FVC (%predicted) | 88 ± 17 | 49–122 |
| FEV1 (%predicted) | 87 ± 18 | 41–128 |
| FEV1/FVC (%) | 75 ± 10 | 42–88 |
| MVV (%Predicted) | 87 ± 19 | 43–124 |
| TLC (%predicted) | 96 ± 16 | 71–133 |
| DLCO (%predicted) | 75 ± 18 | 52–113 |
| DLCO/VA (%predicted) | 107 ± 26 | 45–154 |
| mMRC | 1.4 ± 0.8 | 0–3 |
Data are mean ± SD and range. BMI, body mass index; VAT, visceral adipose tissue; FVC, forced vital capacity; FEV1, forced expired volume in one second; MVV, maximal voluntary ventilation; TLC, total lung capacity; DLCO, diffusing capacity of the lung for carbon monoxide; VA, alveolar volume; mMRC, modified Medical Research Council. Prediction equations: Spirometry, (Hankinson et al., 1999), Lung volumes, (Goldman and Becklake, 1959), Diffusing capacity, (Burrows et al., 1961).
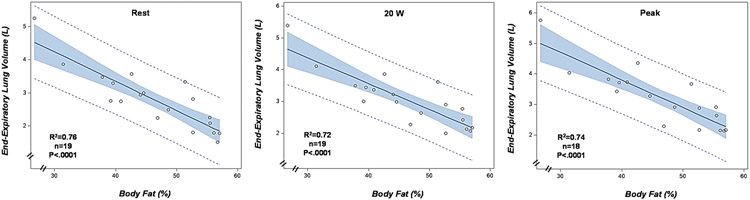
There was a significant association between FVC (L) and %body fat (R2 =0.51, P < 0.001, N = 20) (Fig. 1). %Body fat was also strongly correlated with EELV (L) at rest (R2 =0.76, P < 0.001), during exercise at 20 W (R2 =0.72, P < 0.001), and at peak exercise (R2 =0.74, P < 0.001) (Fig. 2). FVC was also significantly correlated with %predicted peak VO2 (R2 =0.32, P = 0.01) (Fig. 3) and with RPB measured during exercise at 20 W (R2 =0.58, P = 0.004, N = 17). Resting IC had a significant association with peak VO2 (%predicted, R2 =0.48, P = 0.0007) and RPB during exercise at 20 W (R2 =0.64, P < 0.0001) (Fig. 4).
Fig. 1.
Relationship between forced vital capacity (FVC) in liters and total body fat (%) (N = 19).
Fig. 2.
Relationship between total body fat (%) and end-expiratory lung volume (EELV, L) at rest, during exercise at 20 W, and during peak exercise.
Fig. 3.
Relationship between forced vital capacity (FVC) in liters and peak oxygen uptake (%predicted) (N = 20).
Fig. 4.
Relationships between inspiratory capacity (IC) and peak oxygen uptake (%predicted) and rating of perceived breathlessness (RPB, Borg scale 0–10) during exercise at 20 W.
To investigate the effects of a small FVC on exercise capacity and DOE (based upon the close association of FVC and IC with exercise capacity and breathlessness, the importance of FVC size in patients with HFpEF (Villarraga et al., 2022), and the distribution of FVC) (Fig. 5), the patients were divided into two groups: those with lower ventilatory reserves (FVC<3 L, 2 M/10 W) and those with higher ventilatory reserves (FVC>3.8 L, 7 M/1 W). Patient characteristics, cardiorespiratory parameters, breathing mechanics, breathlessness, and arterial blood gas data at rest, submaximal exercise, and peak exercise were compared using independent t-tests. Comparisons were not made between conditions (i.e., rest, 20 W, and peak exercise). Statistical significance was defined as p < 0.05 and all data are presented as means ± SD.
Fig. 5.
Individual data (N = 20) for forced vital capacity (FVC) in liters and percent predicted.
Patient characteristics for the two groups are shown in Table 2. % Body fat was greater in the FVC< 3 L group (P < 0.01). Most pulmonary function variables were lower in the FVC< 3 L group.
Table 2.
Group Characteristics.
| FVC < 3 L | FVC > 3.8 L | |
|---|---|---|
| N = 12 | N = 8 | |
| Demographics | ||
| Age (y) | 69 ± 6 | 69 ± 5 |
| Gender (Men/Women) | 2/10 | 7/1 |
| Height (m) | 1.65 ± 0.09 | 1.78 ± 0.04* * |
| Weight (kg) | 109 ± 14 | 110 ± 24 |
| BMI (kg/m2) | 40 ± 7 | 35 ± 7 |
| %Body Fat (%) | 51 ± 6 | 38 ± 6 * * |
| Fat Mass (kg) | 55.6 ± 11.2 | 39.8 ± 12 |
| Trunk Fat (%body fat) | 55 ± 6 | 45 ± 7 |
| VAT (kg) | 3.0 ± 0.9 | 3.9 ± 2.1 |
| FFM (kg) | 52.4 ± 7.6 | 63.4 ± 7.4 |
| Waist Circumference (cm) | 124 ± 14 | 121 ± 18 |
| Hip Circumference (cm) | 134 ± 14 | 114 ± 9* * |
| Pulmonary function | ||
| FVC (L) | 2.39 ± 0.33 | 4.37 ± 0.48 * * |
| FVC (%predicted) | 79 ± 14 | 100 ± 13 * * |
| FEV1 (L) | 1.84 ± 0.32 | 3.11 ± 0.70 * * |
| FEV1 (%predicted) | 81 ± 17 | 95 ± 18 * * |
| FEV1/FVC (%) | 77 ± 8 | 71 ± 12 |
| MVV (L/min) | 70 ± 14 | 119 ± 26 * * |
| MVV (%Predicted) | 82 ± 20 | 95 ± 16 |
| TLC (L) | 4.47 ± 0.70 | 7.03 ± 0.86 * * |
| TLC (%predicted) | 88 ± 11 | 108 ± 15 * * |
| RV (L) | 1.97 ± 0.61 | 2.47 ± 0.42 |
| RV (%predicted) | 92 ± 19 | 98 ± 17 |
| DLco (%predicted) | 64 ± 10 | 91 ± 17 * * |
| DLco/VA (%predicted) | 121 ± 20 | 86 ± 18 * * |
| mMRC | 1.7 ± 0.6 | 1.1 ± 0.8 |
| Dyspnea-12 Sensory | 7.0 ± 5.6 | 5.4 ± 6.3 |
| Dyspnea-12 Affective | 4.0 ± 4.3 | 1.8 ± 2.5 |
Data are mean ± SD. BMI, body mass index; VAT, visceral adipose tissue; FFM, fat free mass; FVC, forced vital capacity; FEV1, forced expired volume in one second; MVV, maximal voluntary ventilation; TLC, total lung capacity; RV, residual volume; DLCO, diffusing capacity of the lung for carbon monoxide; VA, alveolar volume; mMRC, modified Medical Research Council. * *P < 0.01.
Cardiorespiratory responses, breathing mechanics, breathlessness, and blood gas data at rest, during submaximal exercise at 20 W, and during peak exercise are shown in Table 3. Ventilatory demand (VE/VCO2) was elevated equally in both groups. However, VT was smaller and breathing frequency (Fb) greater in the FVC< 3 L group (P < 0.05) even though VE was similar between the two groups. At peak exercise, work rate was almost twice as high (P < 0.01) in the FVC> 3.8 L group. Thus, VE, VT, VO2, VCO2, and [La] were all greater in the FVC> 3.8 L group due to a greater exercise capacity (potentially partially due to sex differences). However, peak VO2 in relative terms (i.e., %predicted based upon an estimated ideal body weight (Younes, 1984)) was significantly lower in the FVC< 3 L group (P < 0.05). Also, the lower respiratory exchange ratio (RER) in the FVC< 3 L group could have represented their inability to increase VE/VCO2 further due to ventilatory constraints (see blood gas results below) since they demonstrated a genuine maximal effort at peak exercise.
Table 3.
Cardiorespiratory responses, breathing mechanics, and blood gas data at rest, during submaximal exercise, and during peak exercise in patients with an FVC< 3 L (N = 12) and an FVC> 3.8 L (N = 8).
| Rest | Submaximal | Peak | ||||
|---|---|---|---|---|---|---|
| FVC < 3 L | FVC > 3.8 | FVC < 3 L | FVC > 3.8 | FVC < 3 L | FVC > 3.8 | |
| Cardiorespiratory | ||||||
| Work Rate (W) | 0 | 0 | 20 | 20 | 64 ± 16 | 113 ± 38 * * |
| HR (beats/min) | 75 ± 20 | 74 ± 14 | 96 ± 18 | 86 ± 11 | 117 ± 20 | 124 ± 18 |
| HR (%Peak) | 63 ± 9 n=11 | 60 ± 12 | 82 ± 7 | 70 ± 10 * * | - | - |
| HR (%Predicted max) | - | - | - | - | 76 ± 11 | 81 ± 10 |
| VE (L/min) | 10.62 ± 1.72 | 13.70 ± 3.00 * * | 29.48 ± 5.70 | 30.21 ± 3.63 | 50.55 ± 11.13 | 83.24 ± 23.36** |
| VT (L) | 0.64 ± 0.05 | 0.90 ± 0.21 * | 1.01 ± 0.09 | 1.38 ± 0.31 * | 1.18 ± 0.22 | 2.10 ± 0.55 * * |
| Fb (bpm) | 16.5 ± 3.0 | 15.9 ± 5.0 | 29.4 ± 6.7 | 22.6 ± 4.6 * | 44.2 ± 10.5 | 38.0 ± 8.7 |
| VO2 (L/min) | 0.21 ± 0.05 | 0.31 ± 0.08 * * | 0.78 ± 0.15 | 0.87 ± 0.20 | 1.18 ± 0.20 | 1.79 ± 0.60 * |
| VO2 (ml/min/kg) | - | - | - | - | 10.70 ± 1.97 | 15.86 ± 4.43 * |
| VO2 (%Peak) | 18 ± 3 | 18 ± 5 | 67 ± 11 | 53 ± 14 * | - | - |
| VO2 (%Predicted) | - | - | - | - | 69 ± 9 | 91 ± 23 * |
| VCO2 (L/min) | 0.17 ± 0.05 | 0.23 ± 0.06 * | 0.67 ± 0.14 | 0.72 ± 0.16 | 1.18 ± 0.30 | 2.00 ± 0.70 * |
| VE/VCO2 (ratio) | 65.2 ± 12.1 | 60.5 ± 13.9 | 44.3 ± 4.0 | 43.2 ± 7.4 | 44.1 ± 5.5 | 41.2 ± 7.9 |
| RER (ratio) | 0.79 ± 0.05 | 0.76 ± 0.07 | 0.86 ± 0.07 | 0.82 ± 0.03 | 1.01 ± 0.12 | 1.12 ± 0.07 * |
| Lactate (mmol) | 1.0 ± 0.5 | 1.6 ± 0.7 * | 2.1 ± 0.9 | 1.9 ± 0.5 | 5.4 ± 1.9 | 8.2 ± 2.7 * |
| RPE (Borg 6–20) | - | - | 11.6 ± 1.9 | 10.1 ± 2.5 | 17.8 ± 1.2 | 17.9 ± 1.1 |
| Breathing Mechanics | ||||||
| EELV (%TLC) | 52 ± 7 | 50 ± 9 | 59 ± 6 | 53 ± 8 | 60 ± 6 n=11 | 58 ± 8 |
| EILV (%TLC) | 69 ± 6 | 64 ± 8 | 82 ± 6 | 73 ± 8 * * | 87 ± 3 n=11 | 88 ± 4 |
| IRV (%FVC) | 58 ± 6 | 58 ± 12 | 33 ± 9 | 43 ± 11 * | 23 ± 5 n=11 | 19 ± 5 |
| ERV (%FVC) | 12 ± 6 | 20 ± 12 | 23 ± 6 | 25 ± 12 | 28 ± 6 n=11 | 32 ± 11 |
| IC (L) | 2.09 ± 0.27 | 3.52 ± 0.78 * * | 1.83 ± 0.32 | 3.31 ± 0.73 * * | 1.75 ± 0.30 | 2.98 ± 0.68 * * |
| EELV (L) | 2.38 ± 0.64 | 3.51 ± 0.81 * * | 2.56 ± 0.54 | 3.72 ± 0.78 * * | 2.70 ± 0.60 n=11 | 4.04 ± 0.77 * * |
| EILV (L) | 3.10 ± 0.66 | 4.47 ± 0.83 * * | 3.58 ± 0.59 | 5.15 ± 0.93 * * | 3.88 ± 0.64 n=11 | 6.22 ± 0.87 * * |
| IRV (L) | 1.38 ± 0.24 | 2.56 ± 0.70 * * | 0.79 ± 0.29 | 1.87 ± 0.56 * * | 0.56 ± 0.16 n=11 | 0.81 ± 0.24 * |
| ERV (L) | 0.29 ± 0.17 | 0.85 ± 0.52 * | 0.55 ± 0.15 | 1.06 ± 0.48 * | 0.66 ± 0.14 | 1.39 ± 0.45 * * |
| VE mech (L/min) | 12.12 ± 2.90 | 15.52 ± 3.87 * | 30.48 ± 5.14 | 30.87 ± 4.70 | 53.27 ± 11.92 | 82.29 ± 20.14 * * |
| Fb mech (bpm) | 17.0 ± 3.8 | 16.5 ± 4.6 | 29.9 ± 6.6 | 22.4 ± 4.7 * | 46.0 ± 12.0 | 39.1 ± 8.0 |
| VT mech (L) | 0.72 ± 0.07 | 0.96 ± 0.20 | 1.04 ± 0.12 | 1.44 ± 0.37 * | 1.18 ± 0.21 | 2.17 ± .62 * * |
| VT mech (%FVC) | 30 ± 4 | 22 ± 4 * * | 44 ± 6 | 33 ± 6 * * | 50 ± 5 | 49 ± 12 |
| VT/TI (L/s) | 0.49 ± 0.12 | 0.63 ± 0.13 * | 1.19 ± 0.18 | 1.20 ± 0.21 | 1.96 ± 0.36 | 2.94 ± 0.74 * * |
| VT/TE (L/s) | 0.34 ± 0.09 | 0.44 ± 0.12 | 0.89 ± 0.18 | 0.92 ± 0.17 | 1.63 ± 0.44 | 2.57 ± 0.62 * * |
| EFL (%VT) | 76 ± 10 n=9 | 65 ± 34 n=6 | 63 ± 21 n=8 | 61 ± 12 n=5 | 54 ± 29 n=10 | 57 ± 20 n=8 |
| VE mech/MVV(%) | 18 ± 5 | 14 ± 4 * | 45 ± 8 | 27 ± 6 * * | 77 ± 12 | 71 ± 10 |
| RPB (Borg 0–10) | - | - | 3.8 ± 1.1 n=9 | 1.9 ± 1.3 * * | 8.6 ± 1.3 | 7.9 ± 2.0 |
| RPU (Borg 0–10) | - | - | 3.2 ± 1.7 n=9 | 1.4 ± 1.4 * | 7.8 ± 1.6 | 6.5 ± 2.4 |
| Blood Gases (n = 19) | ||||||
| PaO2 (mmHg) | 84 ± 6 n=12 | 84 ± 6 n=7 | 85 ± 14 n=11 | 89 ± 9 n=7 | 87 ± 15 n=11 | 99 ± 8 n=7 |
| PAO2 (mmHg) | 100 ± 5 | 103 ± 7 | 103 ± 6 | 105 ± 5 | 110 ± 6 | 118 ± 3 * |
| PaCO2 (mmHg) | 42 ± 3 | 38 ± 4 * | 42 ± 4 | 39 ± 4 | 40 ± 4 | 35 ± 3 * |
| PETCO2 (mmHg) | 45 ± 4 | 41 ± 6 | 45 ± 5 | 43 ± 5 | 40 ± 7 | 37 ± 4 |
| A-aO2 Diff (mmHg) | 16 ± 7 | 19 ± 4 | 19 ± 10 | 16 ± 5 | 24 ± 10 | 21 ± 7 |
| VD/VT (ratio) | 0.39 ± 0.07 | 0.40 ± 0.06 | 0.35 ± 0.05 | 0.34 ± 0.05 | 0.34 ± 0.05 | 0.30 ± 0.07 |
| HbO2 (%) | 95 ± 2 | 95 ± 2 | 94 ± 4 | 96 ± 1 | 94 ± 4 | 96 ± 1 |
| pH | 7.42 ± 0.02 | 7.41 ± 0.02 | 7.40 ± 0.02 n=11 | 7.41 ± 0.01 | 7.40 ± 0.03 | 7.39 ± 0.02 |
Data are mean ± SD. HR, heart rate; VE, minute ventilation; VT, tidal volume; Fb, breathing frequency, VO2, oxygen consumption; VCO2, carbon dioxide elimination; RER, respiratory exchange ratio; RPE, ratings of perceived exertion; EELV, end-expiratory lung volume; EILV, end-inspiratory lung volume; IRV, inspiratory reserve volume; ERV, expiratory reserve volume; ‘mech’, denotes variables reported from the breathing mechanics system, which may not be collected simultaneously with the cardiopulmonary variables; TI, inspiratory time; TE, expiratory time; EFL, expiratory flow limitation; RPB, ratings of perceived breathlessness; RPU, ratings of perceived unpleasantness of breathlessness; PaO2, partial pressure of arterial oxygen; PAO2, partial pressure of alveolar oxygen, PaCO2, partial pressure of arterial carbon dioxide; PETCO2, end-tidal partial pressure of carbon dioxide; A-aO2 Diff, alveolar-arterial difference in PO2; and HbO2, arterial oxygen saturation. Predicted peak VO2 Jones (1988). In cases of missing data, the n is denoted as superscript (for blood gas data, this was due to unsuccessful arterial cannulation and for EFL, there was no EFL present. Significant differences between groups: *P < 0.05; **P < 0.01. Comparisons were not made between conditions (rest, 20 W, Peak).
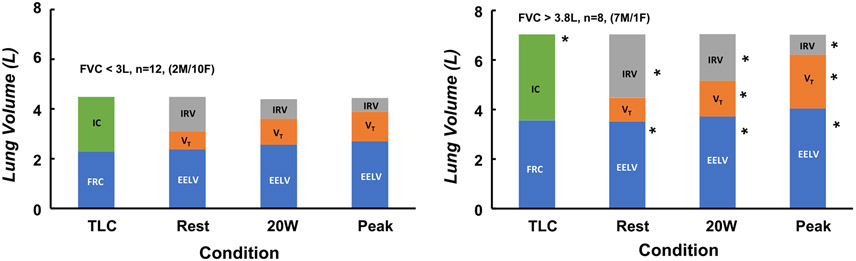
Lung volume subdivisions were similar between the two groups as a percent of TLC at rest but different in liters (see Breathing Mechanics Table 3). Absolute values are presented in Table 3, and especially in Fig. 6, since they best demonstrate the actual mechanical ventilatory reserves available for VT expansion and the magnitude of encroachment on TLC during exercise [i.e., as EELV increases, IC decreases (IC = VT + IRV), and EILV (VT + EELV) approaches TLC]. On average, the FVC< 3 L group had the potential to increase VT by 1.67 L (ERV+IRV) compared with the FVC> 3.8 L group’s potential to increase VT by 3.41 L (ERV+IRV) from rest to peak exercise. VE mech was larger in the FVC> 3.8 L group at rest, while VT (%FVC) and VE mech / MVV ratio (%) were higher in the FVC< 3 L group (P < 0.05). Many of the patients in both groups had EFL at rest.
Fig. 6.
Demonstrates the constraints on tidal volume (VT) during exercise in patients with an FVC< 3 L and an FVC> 3.8 L (FVC=forced vital capacity). Left bar, lung volume subdivisions measured during pulmonary function testing (TLC, total lung capacity; FRC, functional residual capacity; IC, inspiratory capacity). EELV, end-expiratory lung volume; VT, tidal volume; IRV, inspiratory lung volume measured at rest on the cycle ergometer, during exercise cycling at 20 W, and during peak exercise. Note: IC = VT + IRV and progressively declines with exercise reducing potential for VT augmentation and increasing the encroachment on TLC. End-inspiratory lung volume (EILV) = VT + EELV. As EILV approaches TLC 1) work of breathing increases remarkably, 2) VT cannot increase further, and 3) patients usually experience increased breathlessness. Both groups display marked mechanical ventilatory constraints during exercise. *Significant differences between groups within conditions, P < 0.05. Comparisons were not made between conditions.
Dynamic hyperinflation is demonstrated by the increase in EELV and the decrease in IC in both groups at 20 W (Table 3 and Fig. 6). While EELV (%TLC) was not different between groups, EILV (%TLC) was greater at 20 W in the FVC< 3 L group despite a lower absolute VT (L). However, the lower VT used a greater proportion of FVC (i.e., VT as % FVC; P < 0.01) in the FVC< 3 L group. While VE was not different between groups, the FVC< 3 L group had to utilize a greater Fb to generate their VE mech (P < 0.01). Also, VE mech/MVV (%) was much greater in the FVC< 3 L group as was RPB and RPU (P < 0.05). EFL (%VT) was not different between groups but the FVC< 3 L group was experiencing greater mechanical ventilatory limitations regarding operational lung volumes and breathing pattern.
At peak exercise, EILV was approaching the limits of TLC (near 90% of TLC), indicating that both groups had used most of their ventilatory reserves (Fig. 6). That is, VT (%FVC) could not increase further in either group because the augmentation in VT was limited to the volume reserve available between the rise in EELV (%TLC) and the limit on EILV (%TLC) by TLC (Fig. 6). Even at a VE mech of 53 L/min, the FVC< 3 L group was only able to lower PaCO2 by ~2 torr at peak exercise, which is evidence of an inadequate ventilatory response again suggesting limited ventilatory reserves at peak exercise (also the inability to increase RER at peak exercise). Fig. 7 shows flow-volume limitations for two typical patients, one from each group. Both groups maximally utilized their ventilatory reserves. EFL was greater than 50% (%VT) at peak exercise, again suggesting limited ventilatory reserves. Ventilatory demand (VE/VCO2) was not different between the groups, although it was elevated in both groups (Sun et al., 2002). RPB and RPU were similar at peak exercise as expected for maximal exercise.
Fig. 7.
Maximal and tidal flow-volume loops from typical subject with forced vital capacity less than 3 liters (FVC< 3 L) and typical subject with forced vital capacity greater than 3.8 liters (FVC> 3.8 L). Black maximal flow-volume loop from pulmonary function testing (mouth flow vs. mouth volume) and the blue curve is maximal flow-volume loop corrected for gas compression artifact (mouth flow vs. box volume). Tidal flow-volume loops measured during rest, at 20 W, and during peak exercise as marked.
4. Discussion
Our major finding is that ventilatory reserves are limited in patients with HFpEF and obesity. In many patients, the reserves at peak exercise are so small that exercise capacity may be ventilatory limited. This novel finding is particularly true for patients who have a small or reduced FVC, and/or a smaller IC. A small FVC in combination with an increasing EELV due to EFL, and an EILV encroaching on TLC leaves little reserve for VT expansion. While some patients increase Fb to meet the increased ventilatory demand of HFpEF, this option is limited since MEF is also low due to the age-related decline in MEF combined with the lower EELV due to obesity (i.e., EFL over 50% of VT pushes EELV upward as obesity pushes resting EELV downward). Obesity can also contribute to a decrease in FVC and TLC, which limits IC and the potential to increase VT during exercise even more. These ventilatory limitations are evident at a work rate as low as 20 W, which may explain in part why these patients often experience breathlessness and exercise intolerance at low levels of physical activity (e.g., activities of daily living). Consideration of ventilatory limitations in patients with HFpEF and obesity is critical to establishing whether they can meet the challenges of an increased ventilatory demand, which is characteristic of HFpEF, and whether these limitations may contribute to DOE and exercise tolerance in older patients with HFpEF and obesity. Indeed, the margin between ventilatory demand and ventilatory reserves is so narrow that exercise capacity could be ventilatory limited in many patients.
4.1. Effects of obesity
Obesity is certainly a major contributor to ventilatory constraints, not only in its effects on FVC and EELV, but also its effects on exertional metabolic costs, respiratory work to breathe, and increased ventilatory demands (Salome et al., 2010; Ray et al., 1983; Villarraga et al., 2022; Jones and Nzekwu, 2006). This is good news in some respects because there are treatment options for obesity unlike that for HFpEF. Also, both exercise training and weight loss have significant effects on obesity-related DOE and exercise capacity (Bernhardt and Babb, 2014; Bernhardt et al., 2016, 2019). Based upon our findings, pulmonary function should be assessed in all patients with HFpEF and obesity. Further, operational lung volumes (including IC), EFL, and mechanical constraints should be carefully considered during exercise and quantified as they may contribute to reduced ventilatory reserves, increased DOE, and decreased exercise tolerance in patients with HFpEF and obesity, especially if they also have additional reductions in lung function (e.g., lung disease).
4.2. Effects of sex
Our findings suggest that sex differences may also play a role in the hallmark symptoms of HFpEF. Most of the patients in the low ventilatory reserves group (FVC<3 L) were women, and women have lower ventilatory reserves relative to men (Bhammar et al., 2022; Dominelli et al., 2019; Molgat-Seon et al., 2018; Sheel, 2016). Thus, ventilatory limitations may be present in female patients at lower levels of exercise and could have more effect on peak exercise capacity (%predicted). This could be why women report symptoms that may be indicative of HFpEF, which is why patients seek treatment, get evaluated, and eventually diagnosed with HFpEF. Thus, this is possibly a reason why HFpEF is appears more prevalent in women (Sotomi et al., 2021).
4.3. Effects of HFpEF
Our findings of increased ventilatory demand (Smith et al., 2019a; Van Iterson et al., 2017; Smith et al., 2019b), decreased lung function (Smith et al., 2019a; Van Iterson et al., 2017; Smith et al., 2019b; Singh et al., 2019a, 2019b; Villarraga et al., 2022; Bhammar et al., 2022) reduced exercise capacity (Smith et al., 2019a; Van Iterson et al., 2017; Smith et al., 2019b), increased DOE (Obokata et al., 2018; Olson et al., 2016), and the presence of obesity (Carbone et al., 2016) agree with prior publications on HFpEF patients. The exact mechanism of the reduced FVC in the low ventilatory reserves group is unclear. This is true of many studies on HFpEF patients where pulmonary function results are reported. One actually points out the reduction in lung capacity (Villarraga et al., 2022). Some of the patients had normal values while others had values below 80% of predicted (Fig. 5). Obesity could play a role in the lower FVC as there was a moderate association between FVC (L) and %body fat (R2 =0.51, p < 0.001, Fig. 1). There was also a very strong association between EELV (L) and %body fat at rest and during exercise (see below and Fig. 2). Sex also played a significant role since many in the low reserves group were women. Nevertheless, if the FVC is small, regardless of whether the FVC is within normal limits or not (% predicted), FVC and IC become important factors in determining ventilatory capacity (Babb, 1999; Babb and Rodarte, 1993). The FVC dictates the potential size of VT during exercise, which usually reaches 50–60% of FVC. This was true for both groups at peak exercise, although VT in the low ventilatory reserves group was about half of that in the higher ventilatory reserves group (FVC>3.8 L) (Table 3 & Fig. 6). Lastly, FVC and IC were both significantly correlated with peak VO2 as a % predicted (Figs. 3 & 4) and breathlessness at 20 W. The stronger association of IC with peak VO2 (%predicted) and RPB may suggest that FVC may be less important than where patients breathe within their FVC (i.e., the capacity to augment VT and encroachment on TLC). While peak VO2 (L/min) would be expected to be smaller in women, it would not be expected to be a lower percentage of predicted. The low ventilatory reserves group had a 20% lower peak VO2 (%predicted). However, this relationship is also influenced by other factors that could reduce exercise capacity such as severity of heart failure, lung disease, and regular participation in physical activity.
4.4. Effects of FVC and operational lung volumes
The combination of a low FVC, small IC, and limited MEF reserves also play a major role in determining ventilatory reserves and their effects on operational lung volumes, the magnitude of VT expansion during exercise, and the encroachment on TLC (Babb, 1999; Babb and Rodarte, 1993; Stickland et al., 2022; O’Donnell et al., 2021). While EELV (%TLC) was not different between groups, absolute EELV (L) was lower and highly correlated with %body fat at rest and all levels of exercise (Fig. 2). Thus, we suggest that weight on the thorax is important to the EELV (L) adopted at rest and during exercise. That is, obesity is pushing EELV downward while EFL is pushing it upward and may play a role in EELV regulation during exercise. Thus, obesity causes low lung volume breathing where MEF is low and creates extensive EFL yielding greater dynamic hyperinflation (Babb, 1999, 2013; Johnson and Babb, 2011; Pellegrino et al., 1993) (i.e., decrease in IC and encroachment on TLC). All patients showed a rise in EELV (%TLC) with exercise at 20 W due to EFL over a large portion of VT. This rise in EELV was much more costly to the low ventilatory reserves group (FVC<3 L) leaving them little room to increase VT. As a result, VT changed very little in the low reserves group from 20 W to peak exercise in contrast with the high ventilatory reserves group (FVC>3.8 L) (Table 3 and Fig. 6). In fact, there was a significant correlation between FVC (L) and VT (L) at peak exercise as one might expect (R2 = 0.76, p < 0.001; data not shown). While some patients increased Fb to meet the increased ventilatory demand of HFpEF, this option was limited by MEF as most patients had EFL over 50% of VT. However, as small as the increase in VT was in the low reserves group, EILV (%TLC) still approached TLC as much as in the high reserves group. While both groups approached important ventilatory constraints at peak exercise, they appeared to be greater in the low ventilatory reserves group. In fact, the ventilatory response to exercise did not appear to be large enough in the low reserves group to decrease PaCO2 at peak exercise as much as in the high ventilatory reserves group (FVC>3.8 L) or to achieve an RER much over 1.01 despite maximal effort and a high rating of breathing discomfort (Table 3), suggesting exercise capacity could be more ventilatory limited in the low ventilatory reserve group (Wasserman, 1997). However, in both groups, exercise capacity may have been ventilatory limited. If pulmonary capillary wedge pressures are not directly responsible for exercise limitation as recently suggested (Sarma et al., 2023), then ventilatory limitations may be a reasonable candidate (Villarraga et al., 2022). The effects of an increased ventilatory capacity via some type of mechanical unloading would be of interest relative to improving exercise capacity, breathlessness, and breathing limitations.
4.5. Need for measurements of breathing mechanics
Almost all these ventilatory constraints could have been overlooked if it were not for the pulmonary function and breathing mechanics measurements. Furthermore, the magnitude of these limitations would have been missed if only the VE/MVV (%) ratio had been measured (Figs. 6 and 7). The VE/MVV ratio is imperfect in its ability to accurately assess ventilatory limitations, which has been shown before in patients with mild chronic airflow limitation (Babb and Rodarte, 1993; Babb, 2005; Babb and Rodarte, 1992; Babb et al., 1991). Thus, in these patients, the VE/MVV (%) ratio drastically underestimates mechanical ventilatory constraints and as such masks the severity of low ventilatory capacity and we therefore do not recommend it’s use.
5. Conclusion
In conclusion, ventilatory reserves are limited in patients with HFpEF and obesity; indeed, the margin between ventilatory demand and capacity is so narrow that exercise capacity could be ventilatory limited in many patients. This may be particularly true for patients who have a small or reduced FVC and/or a smaller IC. We suggest that quantifying ventilatory constraints in individual patients is critical to establishing whether they can meet the challenges of an increased ventilatory demand and whether these limitations may play a role in the hallmark symptoms of exertional dyspnea and exercise intolerance in older patients with HFpEF and obesity.
Acknowledgments
The authors wish to thank Raksa B. Moran, Jessica N. Alcala, Margot Morris, Marcus Payne, Mitchell Samels, Cindi Foulk, Janet Delatte, and Rebekah Summerall Woodward for their help with data collection and processing for this project.
Funding
National Institutes of Health (1P01HL137630 [BDL] and K99HL164957 [BNB]), King Foundation, Cain Foundation, and Texas Health Presbyterian Hospital Dallas.
Footnotes
Declaration of Competing Interest
No conflicts of interest, financial or otherwise, are declared by the authors.
CRediT authorship contributions statement
Tony G. Babb, Bryce N. Balmain, Andrew R. Tomlinson, Linda S. Hynan, Benjamin D. Levine, James P. MacNamara, and Satyam Sarma had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Each of the authors also contributed substantially to the study design, data collection, data analysis, interpretation, and writing of the manuscript.
Notation of prior abstract publication
APS annual meeting April 2023.
References
- Babb TG, 1999. Mechanical ventilatory constraints in aging, lung disease, and obesity: perspectives and brief review. Med Sci. Sports Exerc 31 (1), S12–S22. [DOI] [PubMed] [Google Scholar]
- Babb TG, 2005. Estimation of mechanical ventilatory limitation. J. Qinghai Med. Coll 26, 145–152. [Google Scholar]
- Babb TG, 2013. Exercise ventilatory limitation: the role of expiratory flow limitation. Exerc. Sport Sci. Rev 41 (1), 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TG, Rodarte JR, 1992. Exercise capacity and breathing mechanics in patients with airflow limitation. Med Sci. Sports Exerc 24, 967–974. [PubMed] [Google Scholar]
- Babb TG, Rodarte JR, 1993. Estimation of ventilatory capacity during submaximal exercise. J. Appl. Physiol 74, 2016–2022. [DOI] [PubMed] [Google Scholar]
- Babb TG, Rodarte JR, 2000. Mechanism of reduced maximal expiratory flow with aging. J. Appl. Physiol 89, 505–511. [DOI] [PubMed] [Google Scholar]
- Babb TG, Viggiano R, Hurley B, Staats BA, Rodarte JR, 1991. Effect of mild to moderate airflow limitation on exercise capacity. J. Appl. Physiol 70, 223–230. [DOI] [PubMed] [Google Scholar]
- Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B, 2008. Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am. J. Respir. Crit. Care Med 178, 116–123. [DOI] [PubMed] [Google Scholar]
- Babb TG, Wyrick BL, Chase PJ, et al. , 2011. Weight loss via diet and exercise improves exercise breathing mechanics in obese men. Chest 140, 454–460. [DOI] [PubMed] [Google Scholar]
- Balmain BN, Tomlinson AR, MacNamara JP, et al. , 2022a. Alveolar dead space is augmented during exercise in patients with heart failure with preserved ejection fraction. Chest 162 (6), 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmain BN, Tomlinson AR, MacNamara JP, et al. , 2022b. Physiological dead space during exercise in patients with heart failure with preserved ejection fraction. J. Appl. Physiol. (1985) 132 (3), 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Babb TG, 2014. Weight loss reduces dyspnea on exertion in obese women. Respir. Physiol. Neurobiol 204, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Stickford JL, Bhammar DM, Babb TG, 2016. Aerobic exercise training without weight loss reduces dyspnea on exertion in obese women. Respir. Physiol. Neurobiol 221, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Bhammar DM, Marines-Price R, Babb TG, 2019. Weight loss reduces dyspnea on exertion and unpleasantness of dyspnea in obese men. Respir. Physiol. Neurobiol 261, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhammar DM, Balmain BN, Babb TG, Bernhardt V, 2022. Sex differences in the ventilatory responses to exercise in mild to moderate obesity. Exp. Physiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows B, Kasik JE, Niden AH, Barclay WR, 1961. Clinical usefulness of the single-breath pulmonary diffusing capacity test. Am. Rev. Respir. Dis 84, 789–806. [DOI] [PubMed] [Google Scholar]
- Carbone S, Canada JM, Buckley LF, et al. , 2016. Obesity contributes to exercise intolerance in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol 68 (22), 2487–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey DS, Babb TG, 1999. Progressive mechanical ventilatory constraints with aging. Am. J. Respir. Crit. Care Med 160, 169–177. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Molgat-Seon Y, Sheel AW, 2019. Sex Differences in the Pulmonary System Influence the Integrative Response to Exercise. Exerc Sport Sci. Rev 47 (3), 142–150. [DOI] [PubMed] [Google Scholar]
- Goldman HI, Becklake MR, 1959. Respiratory function tests: normal values at median altitudes and the prediction of normal results. Am. Rev. Tube 457–467. [DOI] [PubMed] [Google Scholar]
- Graham BL, Steenbruggen I, Miller MR, et al. , 2019. Standardization of Spirometry 2019 Update. American Thoracic Society, 200 (8), e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass M, Kitzman DW, Anand IS, et al. , 2011. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction. Circ. Heart Fail 4 (3), 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB, 1999. Spirometric reference values from a sample of the general u.s. population. Am. J. Respir. Crit. Care Med 159, 179–187. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Babb TG, 2011. Is obesity deflating? J. Appl. Physiol Ill (1), 2–4. [DOI] [PubMed] [Google Scholar]
- Jones NL, 1988. Clinical Exercise Testing, 3 ed..,. Saunders WB,, Philadelphia. [Google Scholar]
- Jones RL, Nzekwu MM, 2006. The effects of body mass index on lung volumes. Chest 130 (3), 827–833. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Slatin RC, Lebowitz MD, Burrows B, 1976. The maximal expiratory flow-volume curve: Normal standards, variability and effects of age. Am. Rev. Respir. Dis 113, 587–600. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Clark DF, Kennedy TC, Knudson DE, 1977. Effect of aging alone on mechanical properties of the normal adult human lung. J. Appl. Physiol 43, 1054–1062. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Lebowitz MD, Holberg J, Burrows B, 1983. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am. Rev. Respir. Dis 127, 725–734. [DOI] [PubMed] [Google Scholar]
- Lofstrom U, Hage C, Savarese G, et al. , 2019. Prognostic impact of Framingham heart failure criteria in heart failure with preserved ejection fraction. ESC Heart Fail 6 (4), 830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo S, Babb TG, 2013. Exercise physiology in obese subjects: respiratory function. In: Hansen D (Ed.), Exercise Therapy in Adult Individuals with Obesity. Nova Science Publishers, Inc, New York, pp. 87–99. [Google Scholar]
- Molgat-Seon Y, Dominelli PB, Ramsook AH, et al. , 2018. The effects of age and sex on mechanical ventilatory constraint and dyspnea during exercise in healthy humans. J. Appl. Physiol. (1985) 124 (4), 1092–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA, 2018. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur. Heart J 39 (30), 2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell DE, Laveneziana P, Neder JA, 2021. Editorial: clinical cardiopulmonary exercise testing. Front Physiol. 12, 711505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TP, Johnson BD, Borlaug BA, 2016. Impaired pulmonary diffusion in heart failure with preserved ejection fraction. JACC Heart Fail 4 (6), 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran K, Todd DC, Soth M, 2006. Altered respiratory physiology in obesity. Can. Respir. J 13, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino R, Brusasco V, Rodarte JR, Babb TG, 1993. Expiratory flow limitation and regulation of end-expiratory lung volume during exercise. J. Appl. Physiol 74 (5), 2552–2558. [DOI] [PubMed] [Google Scholar]
- Ray CS, Sue DY, Bray GA, Hansen JE, Wasserman K, 1983. Effects of obesity on respiratory function. Am. Rev. Respir. Dis 128, 501–506. [DOI] [PubMed] [Google Scholar]
- Salome CM, King GG, Berend N, 2010. Physiology of obesity and effects on lung function. J. Appl. Physiol 108, 206–211. [DOI] [PubMed] [Google Scholar]
- Sarma S, MacNamara J, Livingston S, et al. , 2020. Impact of severe obesity on exercise performance in heart failure with preserved ejection fraction. Physiol. Rep 8 (22), e14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma S, MacNamara JP, Balmain BN, et al. , 2023. Challenging the Hemodynamic Hypothesis in Heart Failure With Preserved Ejection Fraction: Is Exercise Capacity Limited by Elevated Pulmonary Capillary Wedge Pressure? Circulation 147 (5), 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SJ, Heitner JF, Sweitzer NK, et al. , 2013. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ. Heart Fail 6 (2), 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel AW, 2016. Sex differences in the physiology of exercise: an integrative perspective. Exp. Physiol 101 (2), 211–212. [DOI] [PubMed] [Google Scholar]
- Singh I, Rahaghi FN, Naeije R, Oliveira RKF, Systrom DM, Waxman AB, 2019a. Right ventricular-arterial uncoupling during exercise in heart failure with preserved ejection fraction: role of pulmonary vascular dysfunction. Chest 156 (5), 933–943. [DOI] [PubMed] [Google Scholar]
- Singh I, Oliveira RKF, Naeije R, et al. , 2019b. Pulmonary vascular distensibility and early pulmonary vascular remodeling in pulmonary hypertension. Chest 156 (4), 724–732. [DOI] [PubMed] [Google Scholar]
- Smith JR, Borlaug BA, Olson TP, 2019a. Exercise ventilatory efficiency in older and younger heart failure patients with preserved ejection fraction. J. Card. Fail 25 (4), 278–285. [DOI] [PubMed] [Google Scholar]
- Smith JR, Van Iterson EH, Johnson BD, Borlaug BA, Olson TP, 2019b. Exercise ventilatory inefficiency in heart failure and chronic obstructive pulmonary disease. Int J. Cardiol 274, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomi Y, Hikoso S, Nakatani D, et al. , 2021. Sex differences in heart failure with preserved ejection fraction. J. Am. Heart Assoc 10 (5), e018574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanojevic S, Kaminsky DA, Miller MR, et al. , 2022. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J 60 (1). [DOI] [PubMed] [Google Scholar]
- Stickland MK, Neder JA, Guenette JA, O’Donnell DE, Jensen D, 2022. Using cardiopulmonary exercise testing to understand dyspnea and exercise intolerance in respiratory disease. Chest 161 (6), 1505–1516. [DOI] [PubMed] [Google Scholar]
- Sun XG, Hansen JE, Garatachea N, Storer TW, Wasserman K, 2002. Ventilatory efficiency during exercise in healthy subjects. Am. J. Respir. Crit. Care Med 166 (11), 1443–1448. [DOI] [PubMed] [Google Scholar]
- Van Iterson EH, Johnson BD, Borlaug BA, Olson TP, 2017. Physiological dead space and arterial carbon dioxide contributions to exercise ventilatory inefficiency in patients with reduced or preserved ejection fraction heart failure. Eur. J. Heart Fail 19 (12), 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarraga N, Warner B, Bruhn EJ, et al. , 2022. Higher work of breathing during exercise in heart failure with preserved ejection fraction. Chest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman K., 1997. Diagnosing cardiovascular and lung pathophysiology from exercise gas exchange. Chest 112, 1091–1101. [DOI] [PubMed] [Google Scholar]
- Younes M., 1984. Interpretation of clinical exercise testing in respiratory disease. Clin. Chest Med 5, 189–206. [PubMed] [Google Scholar]