Abstract
Background
Prenatal ultrasound is widely used to screen for structural anomalies before birth. While this is traditionally done in the second trimester, there is an increasing use of first‐trimester ultrasound for early detection of lethal and certain severe structural anomalies.
Objectives
To evaluate the diagnostic accuracy of ultrasound in detecting fetal structural anomalies before 14 and 24 weeks’ gestation in low‐risk and unselected pregnant women and to compare the current two main prenatal screening approaches: a single second‐trimester scan (single‐stage screening) and a first‐ and second‐trimester scan combined (two‐stage screening) in terms of anomaly detection before 24 weeks’ gestation.
Search methods
We searched MEDLINE, EMBASE, Science Citation Index Expanded (Web of Science), Social Sciences Citation Index (Web of Science), Arts & Humanities Citation Index and Emerging Sources Citation Index (Web of Science) from 1 January 1997 to 22 July 2022. We limited our search to studies published after 1997 and excluded animal studies, reviews and case reports. No further restrictions were applied. We also screened reference lists and citing articles of each of the included studies.
Selection criteria
Studies were eligible if they included low‐risk or unselected pregnant women undergoing a first‐ and/or second‐trimester fetal anomaly scan, conducted at 11 to 14 or 18 to 24 weeks’ gestation, respectively. The reference standard was detection of anomalies at birth or postmortem.
Data collection and analysis
Two review authors independently undertook study selection, quality assessment (QUADAS‐2), data extraction and evaluation of the certainty of evidence (GRADE approach). We used univariate random‐effects logistic regression models for the meta‐analysis of sensitivity and specificity.
Main results
Eighty‐seven studies covering 7,057,859 fetuses (including 25,202 with structural anomalies) were included. No study was deemed low risk across all QUADAS‐2 domains. Main methodological concerns included risk of bias in the reference standard domain and risk of partial verification. Applicability concerns were common in studies evaluating first‐trimester scans and two‐stage screening in terms of patient selection due to frequent recruitment from single tertiary centres without exclusion of referrals.
We reported ultrasound accuracy for fetal structural anomalies overall, by severity, affected organ system and for 46 specific anomalies. Detection rates varied widely across categories, with the highest estimates of sensitivity for thoracic and abdominal wall anomalies and the lowest for gastrointestinal anomalies across all tests.
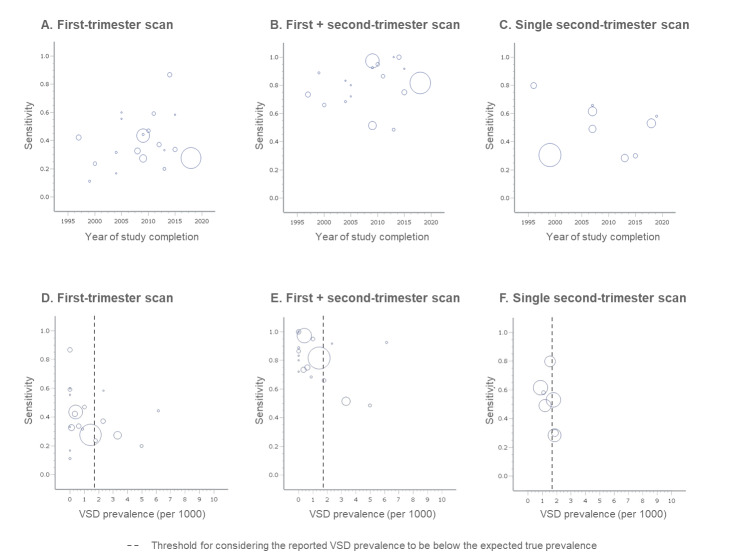
The summary sensitivity of a first‐trimester scan was 37.5% for detection of structural anomalies overall (95% confidence interval (CI) 31.1 to 44.3; low‐certainty evidence) and 91.3% for lethal anomalies (95% CI 83.9 to 95.5; moderate‐certainty evidence), with an overall specificity of 99.9% (95% CI 99.9 to 100; low‐certainty evidence).
Two‐stage screening had a combined sensitivity of 83.8% (95% CI 74.7 to 90.1; low‐certainty evidence), while single‐stage screening had a sensitivity of 50.5% (95% CI 38.5 to 62.4; very low‐certainty evidence).
The specificity of two‐stage screening was 99.9% (95% CI 99.7 to 100; low‐certainty evidence) and for single‐stage screening, it was 99.8% (95% CI 99.2 to 100; moderate‐certainty evidence).
Indirect comparisons suggested superiority of two‐stage screening across all analyses regarding sensitivity, with no significant difference in specificity. However, the certainty of the evidence is very low due to the absence of direct comparisons.
Authors' conclusions
A first‐trimester scan has the potential to detect lethal and certain severe anomalies with high accuracy before 14 weeks’ gestation, despite its limited overall sensitivity. Conversely, two‐stage screening shows high accuracy in detecting most fetal structural anomalies before 24 weeks’ gestation with high sensitivity and specificity.
In a hypothetical cohort of 100,000 fetuses, the first‐trimester scan is expected to correctly identify 113 out of 124 fetuses with lethal anomalies (91.3%) and 665 out of 1776 fetuses with any anomaly (37.5%). However, 79 false‐positive diagnoses are anticipated among 98,224 fetuses (0.08%). Two‐stage screening is expected to correctly identify 1448 out of 1776 cases of structural anomalies overall (83.8%), with 118 false positives (0.1%).
In contrast, single‐stage screening is expected to correctly identify 896 out of 1776 cases before 24 weeks’ gestation (50.5%), with 205 false‐positive diagnoses (0.2%). This represents a difference of 592 fewer correct identifications and 88 more false positives compared to two‐stage screening.
However, it is crucial to acknowledge the uncertainty surrounding the additional benefits of two‐stage versus single‐stage screening, as there are no studies directly comparing them. Moreover, the evidence supporting the accuracy of first‐trimester ultrasound and two‐stage screening approaches primarily originates from studies conducted in single tertiary care facilities, which restricts the generalisability of the results of this meta‐analysis to the broader population.
Keywords: Female; Humans; Pregnancy; Bias; Congenital Abnormalities; Congenital Abnormalities/diagnostic imaging; Pregnancy Trimester, First; Pregnancy Trimester, Second; Sensitivity and Specificity; Ultrasonography, Prenatal; Ultrasonography, Prenatal/statistics & numerical data
Plain language summary
Accuracy of a first‐ and second‐trimester ultrasound scan for identifying fetal anomalies in low‐risk and unselected pregnancies
Key messages
Prenatal ultrasound is commonly used in the first and second trimesters of pregnancy to identify potential issues with a developing baby (fetus). In this study, we analysed 87 studies covering over 7 million fetuses. While both first‐ and second‐trimester scans confirm normal development well (high specificity), their ability to detect issues (sensitivity) varied. Women with both scans seemed to have more anomalies detected before 24 weeks compared to those with only a second‐trimester scan. However, differences might be due to study setup variations rather than a real difference in detection.
What are fetal anomalies?
Fetal anomalies are abnormalities that can affect the baby's organs or body parts, which develop during pregnancy. These anomalies can range from severe conditions incompatible with life to less significant ones, some of which may be considered normal variations.
How are fetal anomalies detected?
Fetal anomalies are primarily detected by ultrasound, which uses sound waves to create detailed images of the baby’s internal organs. Most countries offer one ultrasound scan during pregnancy to check for fetal anomalies, typically conducted between 18 and 24 weeks of pregnancy (second‐trimester scan). Some countries also offer an early anomaly scan to identify some major anomalies at an earlier stage. This scan is typically performed at 11 to 14 weeks (first‐trimester scan).
What did we want to find out?
The goal was to understand how accurate ultrasound scans are in detecting structural anomalies in low‐risk and unselected pregnant women when conducted in the first and second trimesters. The study also aimed to compare the accuracy of two different approaches: a single‐stage screening approach involving only a second‐trimester scan and a two‐stage approach involving both first‐ and second‐trimester scans.
What did we do?
We reviewed 87 studies, covering over 7 million fetuses. These studies focused on low‐risk pregnant and unselected women who had undergone first‐ and/or second‐trimester ultrasound scans as part of routine prenatal care. We assessed the quality of the studies, extracted relevant data and used statistical methods to analyse the accuracy of the ultrasound scans.
What did we find?
A first‐trimester scan appears accurate in early detection of lethal and some severe fetal anomalies. However, its overall ability to detect anomalies is limited. In a hypothetical group of 100,000 fetuses, this scan is expected to correctly identify 113 out of 124 fetuses with lethal anomalies (91.3%) and 665 out of 1776 fetuses with any anomaly (37.5%). Unfortunately, about 79 out of 98,224 healthy fetuses (0.08%) might mistakenly receive a diagnosis of a fetal anomaly when, in reality, there isn't one (false‐positive diagnosis). Although the chance of receiving a false‐positive diagnosis is very low, in the cases where this occurs, this can lead to unnecessary anxiety and investigations.
The combination of a first‐ and second‐trimester scan seems highly sensitive, expecting to identify 1448 out of 1776 cases (83.8%) before 24 weeks in a hypothetical group of 100,000 pregnancies. However, around 118 out of 98,224 healthy fetuses (0.1%) may receive a false‐positive diagnosis.
Fewer fetal anomalies seem to be identified before 24 weeks in groups of women undergoing only a second‐trimester scan (single‐stage screening) compared to those also undergoing a first‐trimester scan (two‐stage screening). A single second‐trimester scan is expected to detect 896 out of 1776 cases (50.5%, 592 fewer than two‐stage screening), potentially resulting in false‐positive diagnoses for around 205 out of 98,224 healthy fetuses (0.2%, 88 more than two‐stage screening).
However, studies solely focusing on the second‐trimester scan were designed differently. Women generally entered these studies after the first trimester. Easily detectable anomalies might have been identified before study entry through other investigations, leaving only the more subtle cases in the study populations. This difference may have led to underestimation of overall anomaly detection in studies assessing the accuracy of a single second‐trimester scan.
It is crucial to note varying anomaly detection rates across organ systems. Abdominal wall anomalies had the highest detection rates: 95.6% (first‐trimester scan), 99.0% (first‐ and second‐trimester scan combined) and 90.8% (single second‐trimester scan). Digestive tract anomalies had the lowest rates: 8.3%, 46.5% and 33.3%, respectively.
What are the limitations of the evidence?
The results of the included studies varied widely, making it challenging to draw consistent conclusions. Additionally, none of the studies were entirely free from potential issues in how they were conducted. Concerns mainly focused on confirming normal and abnormal prenatal findings after birth and how well the results applied to the general population, as most studies were conducted in major university hospitals. Lastly, no studies directly compared detection rates between groups receiving both scans and those with only a second‐trimester scan. Although the results of the review indicate that the combination of a first‐ and second‐trimester scan might be better at detecting anomalies before 24 weeks of pregnancy than a single second‐trimester scan, this difference could be due to variations in study designs and entry times.
How up‐to‐date is this evidence?
The search for evidence was conducted up to 22 July 2022.
Summary of findings
Summary of findings 1. Accuracy of a first‐trimester scan in detecting fetal structural anomalies overall, and of varying severity, before 14 weeks' gestation.
| Accuracy of a first‐trimester scan in detecting fetal structural anomalies overall, and of varying severity, before 14 weeks' gestation | |||||||||||||
| Sensitivity | Specificity | Test performance per 100,000 pregnancies | |||||||||||
| Studies | Fetuses (cases) | Sensitivity (95% CI) | Certainty (GRADE) | Studies | Fetuses (cases) | Specificity (95% CI) | Certainty (GRADE) | Median prevalence (%) | Cases detected (TP) | Cases missed (FN) | True negatives (TN) |
False positives (FP) | |
| I. Overall accuracy of ultrasound in detecting fetal structural anomalies | |||||||||||||
| Fetuses affected | 20 | 195,264 (3293) |
37.5 (31.1 to 44.3) |
Lowa,b | 10 | 23,037 (341) |
99.9 (99.9 to 100) |
Lowb,c | 1.8** | 665 | 1111 | 98,145 | 79 |
| Anomalies* | 20 | 246,130 (5417) |
39.2 (32.5 to 46.2) |
Lowa,b | ‐ | ‐ | ‐ | ‐ | 2.0** | 798 | 1241 | ‐ | ‐ |
| *No specificity was determined as the number of true‐negative test results could not be determined by the number of anomalies. **Test performance per 100,000 pregnancies was assessed using the median prevalence of structural anomalies from all studies included in our meta‐analysis on the accuracy of a first‐trimester scan. | |||||||||||||
| II. Subgroup analysis of ultrasound accuracy in detecting anomalies that are less susceptible to under‐reporting due to incomplete postnatal identification (i.e. anomalies that are externally visible, symptomatic at birth or considered to be lethal) | |||||||||||||
| Fetuses affected | 20 | 195,264 (1668) |
52.8 (45.7 to 59.8) | Moderateb | 10 | 23,037 (173) |
100 (99.9 to 100) | Lowb,c | 0.9** | 456 | 408 | 99,116 | 20 |
| III. Accuracy of ultrasound in detecting anomalies of varying severity | |||||||||||||
| Major anomalies | 33 | 303,319 (5449) |
45.6 (37.1 to 52.3) | Very lowa,b,d | 15 | 56,327 (842) |
100 (99.9 to 100) | Moderatec | 1.4** | 615 | 764 | 98,591 | 30 |
|
29 | 301,863 (529) |
91.3 (83.9 to 95.5) | Moderateb | 13 | 55,793 (65) |
N/A* Range: 100 to 100 | N/A* | 0.1** | 113 | 11 | N/A* | N/A* |
|
33 | 303,319 (4922) |
37.7 (30.6 to 45.3) | Very lowa,b,d | 15 | 56,327 (778) |
100 (99.9 to 100) | Moderatec | 1.3** | 495 | 818 | 98,658 | 30 |
| Minor anomalies | 15 | 236,383 (550) | 4.3 (1.0 to 16.5) | Very lowa,b,e | 8 | 16,947 (64) |
100 (99.9 to 100) | Moderatec | 0.4** | 16 | 362 | 99,612 | 10 |
| *Meta‐analysis was not possible because of failure of the random‐effects models to converge due to a lack of variation between studies. **Test performance per 100,000 pregnancies was assessed using the median prevalence of structural anomalies from all 20 studies included in our meta‐analysis on the accuracy of a first‐trimester scan. | |||||||||||||
| CI: confidence interval; FN: false negative; FP: false positive; N/A: not applicable; TN: true negative; TP: true positive | |||||||||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||||||||
|
aContributing studies had design limitations (‐1), including risk of bias in the reference standard domain (‐0.75) and risk of bias in the flow and timing domain (‐0.25). bIndirectness in terms of patient selection (‐1). cContributing studies had design limitations, including risk of partial verification bias of pregnancies due to incomplete verification of ultrasound findings in pregnancies with an adverse outcome (‐1). dUnexplained heterogeneity of study results (‐1). eWide 95% CI, deviating more than 10% from the summary estimate (‐1). | |||||||||||||
Summary of findings 2. Accuracy of a first + second‐trimester scan in detecting fetal structural anomalies overall, and of varying severity, before 24 weeks' gestation.
| Accuracy of a first + second‐trimester scan in detecting fetal structural anomalies overall, and of varying severity, before 24 weeks' gestation | |||||||||||||
| Sensitivity | Specificity | Test performance per 100,000 pregnancies | |||||||||||
| Studies | Fetuses (cases) | Sensitivity (95% CI) |
Certainty (GRADE) | Studies | Fetuses (cases) | Specificity (95% CI) | Certainty (GRADE) | Median prevalence (%) | Cases detected (TP) | Cases missed (FN) | True negatives (TN) |
False positives (FP) | |
| I. Overall accuracy of ultrasound in detecting fetal structural anomalies | |||||||||||||
| Fetuses affected | 18 | 181,614 (3051) | 83.8 (74.7 to 90.1) |
Lowa,b | 10 | 23,037 (341) |
99.9 (99.7 to 100) |
Lowb,c | 1.8** | 1488 | 288 | 98,106 | 118 |
| Anomalies* | 17 | 229,274 (4975) | 84.5 (75.6 to 90.5) |
Lowa,b | ‐ | ‐ | ‐ | ‐ | 2.0** | 1696 | 311 | ‐ | ‐ |
| *No specificity was determined as the number of true‐negative test results could not be determined by the number of anomalies. **Test performance per 100,000 pregnancies was assessed using the median prevalence of structural anomalies from all studies included in our meta‐analysis on the accuracy of a first + second‐trimester scan. | |||||||||||||
| II. Subgroup analysis of ultrasound accuracy in detecting anomalies that are less susceptible to under‐reporting due to incomplete postnatal identification (i.e. anomalies that are externally visible, symptomatic at birth or considered to be lethal) | |||||||||||||
| Fetuses affected | 18 | 181,614 (1534) |
86.7 (78.8 to 92.0) | Moderateb | 10 | 23,037 (173) |
100 (99.9 to 100) | Lowb,c | 0.9** | 749 | 115 | 99,106 | 30 |
| III. Accuracy of ultrasound in detecting anomalies of varying severity | |||||||||||||
| Major anomalies | 28 | 265,132 (4816) |
85.2 (77.9 to 90.4) | Lowa,b | 12 | 31,790 (432) |
99.9 (99.9 to 100) | Moderatec | 1.3** | 1128 | 196 | 98,607 | 69 |
|
24 | 263,676 (494) |
100 (97.4 to 100) | Moderatea | 10 | 31,256 (43) |
N/A* Range: 100 to 100 | N/A* | 0.1** | 114 | 0 | N/A* | N/A* |
|
28 | 265,132 (4324) |
82.6 (74.4 to 88.6) | Lowa,b | 12 | 31,790 (390) |
99.9 (99.9 to 100) | Moderatec | 1.2** | 965 | 203 | 98,763 | 69 |
| Minor anomalies | 12 | 219,512 (491) |
48.1 (20.1 to 77.3) | Very lowa,b,d | 7 | 13,726 (24) |
100 (99.3 to 100) | Moderatec | 0.3** | 160 | 173 | 99,657 | 10 |
| *Meta‐analysis was not possible because of failure of the random‐effects models to converge due to a lack of variation between studies. **Test performance per 100,000 pregnancies was assessed using the median prevalence of structural anomalies from all studies included in our meta‐analysis on the accuracy of a first + second‐trimester scan. | |||||||||||||
| CI: confidence interval; FN: false negative; FP: false positive; N/A: not applicable; TN: true negative; TP: true positive | |||||||||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||||||||
|
aContributing studies had design limitations (‐1), including risk of bias in the reference standard domain (‐0.75) and risk of bias in the flow and timing domain (‐0.25). bIndirectness in terms of patient selection (‐1). cContributing studies had design limitations, including risk of partial verification bias of pregnancies due to a missing reference standard (autopsy) in pregnancies with an adverse outcome (‐1). dWide 95% CI, deviating more than 20% from the summary estimate (‐2). | |||||||||||||
Summary of findings 3. Accuracy of a single second‐trimester scan in detecting fetal structural anomalies overall, and of varying severity, before 24 weeks' gestation.
| Accuracy of a single second‐trimester scan in detecting fetal structural anomalies overall, and of varying severity, before 24 weeks' gestation | |||||||||||||
| Sensitivity | Specificity | Test performance per 100,000 pregnancies | |||||||||||
| Studies | Fetuses (cases) | Sensitivity (95% CI) |
Certainty (GRADE) | Studies | Fetuses (cases) | Specificity (95% CI) |
Certainty (GRADE) | Median prevalence (%) | Cases detected (TP) | Cases missed (FN) | True negatives (TN) |
False positives (FP) | |
| I. Overall accuracy of ultrasound in detecting fetal structural anomalies | |||||||||||||
| Fetuses affected | 9 | 158,767 (3168) |
50.5 (38.5 to 62.4) |
Very lowa,b,c | 5 | 33,120 (593) |
99.8 (99.2 to 100) |
Moderated | 2.2** | 1115 | 1094 | 97,585 | 205 |
| Anomalies* | 3 | 17,845 (340) |
52.2 (33.2 to 70.7) |
Very lowa,b,c | ‐ | ‐ | ‐ | ‐ | 4.2** | 2194 | 2006 | ‐ | ‐ |
| *No specificity was determined as the number of true negative test results could not be determined by the number of anomalies. **Test performance per 100,000 pregnancies was assessed using the median prevalence of structural anomalies from all studies included in our meta‐analysis on the accuracy of a single second‐trimester scan. | |||||||||||||
| II. Subgroup analysis of ultrasound accuracy in detecting anomalies that are less susceptible to under‐reporting due to incomplete postnatal identification (i.e. anomalies that are externally visible, symptomatic at birth or considered to be lethal) | |||||||||||||
| Fetuses affected | 7 | 58,014 (559) |
59.2 (45.7 to 71.5) | Lowb,c | 4 | 32,120 (286) |
99.9 (99.6 to 100) |
Moderated | 1.0** | 579 | 400 | 98,952 | 69 |
| III. Accuracy of ultrasound in detecting anomalies of varying severity | |||||||||||||
| Major anomalies | 9 | 75,042 (1312) |
47.9 (33.3 to 62.8) | Very lowa,b,c | 5 | 35,124 (469) |
99.9 (99.6 to 100) | Moderated | 1.3** | 625 | 681 | 98,635 | 59 |
|
7 | 71,109 (73) |
N/A* Range: 80.0 to 100 | N/A* | 3 | 31,191 (20) |
N/A* Range: 100 to 100 | N/A* | 0.1** | N/A* | N/A* | N/A* | N/A* |
|
8 | 72,038 (1214) |
48.6 (33.0 to 64.5) | Very lowa,b,c | 4 | 32,120 (424) |
99.9 (99.3 to 100) | Moderated | 1.5** | 713 | 753 | 98,425 | 108 |
| Minor anomalies | 7 | 58,015 (96) |
24.0 (6.1 to 60.9) | Very lowa,b,e | 4 | 32,120 (82) |
N/A* Range: 100 to 100 | Moderated | 0.3** | 79 | 251 | N/A* | N/A* |
| *Meta‐analysis was not possible because of failure of the random‐effects models to converge due to a lack of variation between studies. **Test performance per 100,000 pregnancies was assessed using the median prevalence of structural anomalies from all studies included in our meta‐analysis on the accuracy of a single second‐trimester scan. | |||||||||||||
| CI: confidence interval; FN: false negative; FP: false positive; N/A: not applicable; TN: true negative; TP: true positive | |||||||||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||||||||
|
aContributing studies had design limitations (‐1), including risk of bias in the reference standard domain (‐0.75) and risk of bias in the flow and timing domain (‐0.25). bUnexplained heterogeneity of study results (‐1). cWide 95% CI, deviating more than 10% from the summary estimate (‐1). dContributing studies had design limitations, including risk of partial verification bias of pregnancies due to incomplete verification of ultrasound findings in pregnancies with an adverse outcome (‐1). eWide 95% CI, deviating more than 20% from the summary estimate (‐2). | |||||||||||||
Summary of findings 4. Accuracy of first‐ and second‐trimester ultrasound in detecting anomalies of individual organ systems and 46 selected structural anomalies.
| Accuracy of first‐ and second‐trimester ultrasound in detecting anomalies of individual organ systems and 46 selected structural anomalies | |||||||||
| Two‐stage screening approach | Single‐stage screening approach | ||||||||
| First‐trimester scan (detection < 14 weeks' gestation) |
First + second‐trimester scan (total detection < 24 weeks' gestation) |
Single second‐trimester scan (detection < 24 weeks' gestation) |
|||||||
| Studies | Fetuses (cases) | Sensitivity (95% CI) |
Studies | Fetuses (cases) |
Sensitivity (95% CI) |
Studies | Fetuses (cases) |
Sensitivity (95% CI) |
|
| Central nervous system | |||||||||
| Overall (any type) | 23 | 260,409 (843) | 56.6 (47.6 to 65.2) | 19 | 240,786 (779) | 92.9 (85.9 to 96.6) | 10 | 159,170 (663) | 77.5 (65.5 to 86.2) |
| Anencephaly | 30 | 548,041 (445) | 99.5 (90.9 to 100) | 24 | 317,464 (312) | N/A* Range: 0.0 (Taipale 2004) to 100 (remaining 23 studies) |
23 | 1,284,752 (716) |
95.6 (90.6 to 98.0) |
| Spina bifida | 23 | 480,116 (286) | 35.1 (22.0 to 51.1) | 18 | 252,802 (147) | 97.9 (90.0 to 99.6) | 25 | 1,897,018 (946) | 76.3 (65.7 to 84.4) |
| Holoprosencephaly | 15 | 270,372 (99) | 92.4 (71.6 to 98.3) | 11 | 229,181 (90) | N/A* Range: 100 to 100 |
3 | 162,338 (13) | N/A* Range: 77.8 to 100 |
| Hydrocephalus | 14 | 253,796 (99) | 4.2 (0.7 to 21.3) | 10 | 218,872 (81) | 75.4 (53.4 to 89.2) | 8 | 61,812 (154) | 76.0 (45.0 to 92.5) |
| Encephalocele | 15 | 390,064 (84) | 96.3 (62.8 to 99.8) | 12 | 189,661 (47) | N/A* Range: 94.7 to 100 | 5 | 168,263 (37) | 81.8 (49.7 to 95.4) |
| Respiratory | |||||||||
| Overall (any type) | 13 | 239,670 (158) | 14.6 (9.7 to 21.6) | 10 | 222,798 (146) | 83.8 (72.2 to 91.2) | 8 | 157,767 (63) | 83.8 (67.1 to 92.9) |
| CDH | 17 | 2,106,233 (520) |
22.2 (10.1 to 42.2) | 13 | 2,071,309 (513) |
75.8 (44.7 to 92.4) | 9 | 689,973 (243) | 62.9 (56.7 to 68.7) |
| CPAM | 7 | 207,768 (66) | 1.4 (0.1 to 15.6) | 5 | 195,687 (63) | N/A* Range: 92.9 to 100 |
7 | 352,922 (42) | N/A* Range: 50.0 to 100 |
| Cardiac | |||||||||
| Overall (any type) | 28 | 2,719,925 (1543) | 36.7 (27.7 to 46.6) | 24 | 254,349 (1433) | 81.5 (69.8 to 89.4) | 17 | 800,716 (6917) | 33.8 (24.7 to 44.2) |
| Septal defecta | 34 | 301,645 (476) | 32.7 (20.5 to 47.9) | 28 | 275,487 (453) | 73.3 (50.7 to 87.9) | 11 | 1,056,215 (671) | 36.7 (16.0 to 63.9) |
| Valvular anomaly (biventricular heart)b | 17 | 247,073 (99) | 26.8 (11.6 to 50.5) | 14 | 229,673 (85) | 99.7 (10.5 to 100) | 10 | 1,056,169 (173) | 38.6 (30.8 to 47.0) |
| Venous return anomalyc | 6 | 74,351 (27) | N/A*
Range: 0.0 to 100;
0.0 (Becker 2012; Kenkhuis 2018); 57.1 to 100 (remaining 4 studies) |
4 | 66,388 (25) | N/A* Range: 0.0 to 100; 0.0 (Becker 2012; Kenkhuis 2018); 85.7 to 100 (Gireada 2022; Liao 2021) | 2 | 723,265 (49) | N/A* Range: 25.0 (Ingale 2017) to 33.3 (van Velzen 2016) |
| Aortic arch anomalyd | 18 | 242,677 (185) | 24.8 (16.1 to 36.2) | 13 | 219,782 (167) | 69.4 (38.8 to 89.1) | 8 | 1,047,246 (263) |
31.6 (26.1 to 37.6) |
| Conotruncal anomalye | 31 | 291,090 (284) | 34.5 (21.1 to 50.9) | 25 | 264,931 (255) | 90.0 (76.5 to 96.2) | 12 | 1,066,551 (606) | 66.4 (38.8 to 86.1) |
| HRHSf | 7 | 86,642 (25) | 92.7 (0.0 to 100) | 5 | 72,992 (23) | N/A* Range: 0.0 to 100; 0.0 (Gireada 2022); 100 (remaining 4 studies) | 5 | 1,009,708 (40) | 55.7 (35.3 to 74.4) |
| HLHSg | 24 | 260,805 (155) | 79.0 (45.2 to 94.5) | 21 | 247,473 (147) | 98.2 (75.8 to 99.9) | 12 | 1,579,434 (392) | 87.5 (79.1 to 92.8) |
| Other single ventricle defectsh | 14 | 242,246 (67) | 100 (58.8 to 100) | 12 | 232,136 (63) | N/A* Range: 0 (Hernadi 1997) to 100 (remaining 11 studies) | 7 | 1,328,100 (255) |
85.6 (79.6 to 90.1) |
| Complex defects with atrial isomerismi | 10 | 207,098 (28) | N/A* Range: 0.0 to 100; 0.0 (Vellamkondu 2017); 57.1 to 100 (remaining 9 studies) | 8 | 203,174 (26) | N/A* Range: 91.7 to 100 | 4 | 1,011,866 (130) | 75.1 (62.0 to 84.7) |
| Miscellaneous major cardiac defectsj | 18 | 249,653 (166) | 20.1 (2.1 to 75.1) | 12 | 212,983 (74) | 87.9 (69.6 to 95.8) | 10 | 1,391,739 (978) | 38.8 (23.3 to 56.9) |
| Minor cardiac defectsk | 11 | 89,679 (198) | 9.7 (2.9 to 27.8) | 10 | 87,776 (181) | 83.8 (52.8 to 96.0) | 3 | 24,043 (78) | N/A* Range: 0 to 4.2 |
| Thoracic and abdominal wall | |||||||||
| Overall (any type) | 17 | 251,923 (358) | 95.6 (89.1 to 98.3) | 14 | 235,051 (335) | 99.0 (87.3 to 99.9) | 7 | 58,014 (31) | 90.8 (54.7 to 98.8) |
| Gastroschisis | 17 | 276,233 (99) | 100 (74.8 to 100) | 13 | 241,309 (91) | 100 (99.1 to 100) | 10 | 811,149 (368) | N/A* Range: 0.0 to 100; 0.0 (Ingale 2017); 93.5 to 100 (remaining 9 studies) |
| Omphalocele | 23 | 288,522 (273) | 98.0 (90.4 to 99.6) | 18 | 250,335 (247) | 99.2 (98.5 to 99.6) | 9 | 827,508 (330) | N/A* Range: 79.3 to 100 |
| Gastrointestinal | |||||||||
| Overall (any type) | 13 | 238,447 (155) | 8.3 (4.9 to 13.8) | 10 | 221,575 (138) | 46.5 (39.0 to 54.1) | 9 | 157,923 (197) | 33.3 (14.8 to 59.0) |
| Oesophageal atresia | 8 | 202,606 (26) | N/A* Range: 0.0 to 50.0; 0.0 (7 studies); 50.0 (Iliescu 2013) | 5 | 170,904 (18) | N/A* Range: 0.0 to 50.0; 0.0 to 0.25 (4 studies); 50.0 (Syngelaki 2019) | 6 | 63,913 (19) |
N/A* Range: 0.0 to 100; 0.0 to 16.7 (5 studies); 100 (Drukker 2020) |
| Duodenal atresia | 7 | 202,562 (23) | N/A* Range: 0.0 to 12.5 | 5 | 194,508 (21) | 98.2 (0.0 to 100) | 6 | 47,284 (45) | 52.3 (18.2 to 84.4) |
| Small bowel obstruction | 3 | 165,978 (18) | N/A* Range: 0.0 to 0.0 | 2 | 147,926 (12) | N/A* Range: 0.0 (Syngelaki 2019) to 33.3 (Liao 2021) | 2 | 19,528 (3) | N/A* Range: 0.0 (Rydberg 2017) to 50.0 (Jacobsen 2011) |
| Urinary tract | |||||||||
| Overall (any type) | 19 | 253,523 (906) | 23.5 (17.0 to 31.6) | 16 | 236,649 (843) | 87.1 (67.9 to 95.6) | 9 | 158,415 (972) | 60.6 (30.9 to 84.0) |
| Renal agenesis | 15 | 210,855 (46) | N/A*
Range: 0.0 to 100;
0.0 (10 studies); 15.3 to 50.0 (4 studies); 100 (Sainz 2020) |
11 | 175,931 (39) | 90.3 (45.5 to 99.1) | 8 | 72,038 (31) | N/A* Range: 66.7 (Drukker 2020) to 100 (7 remaining studies) |
|
7 | 74,458 (17) | N/A* Range: 0.0 (6 studies) to 100 (Brown 2021) | 5 | 62,377 (15) | 73.4 (38.0 to 92.5) | 4 | 35,300 (16) | N/A* Range: 50.0 (Drukker 2020) to 100 (remaining 3 studies) |
|
12 | 203,506 (30) | 16.6 (6.5 to 36.2) | 8 | 168,582 (24) | 100 (57.8 to 100) | 9 | 698,237 (103) | 91.4 (68.9 to 98.1) |
| MCDK | 15 | 238,157 (129) | 6.8 (0.0 to 36.2) | 13 | 230,144 (124) | 80.3 (54.5 to 93.3) | 5 | 38,486 (39) | 82.1 (34.1 to 97.6) |
|
6 | 153,637 (79) | N/A* Range: 0.0 (5 studies) to 100 (Whitlow 1999) | 5 | 148,846 (78) | 88.4 (79.6 to 93.7) | 0 | ‐ | ‐ |
|
5 | 149,834 (10) | N/A* Range: 0.0 to 0.0 | 4 | 145,044 (9) | N/A* Range: 100 to 100 | 0 | ‐ | ‐ |
| Other renal dysplasias | 12 | 252,838 (87) | 16.2 (9.9 to 25.4) | 10 | 225,927 (77) | N/A* Range: 78.6 to 100 | 4 | 34,481 (18) | 71.7 (16.9 to 96.9) |
|
1 | 13,639 (2) | N/A* Range: 0.0 to 0.0 (Grande 2012) | 1 | 13,639 (2) | N/A* Range: 100 to 100 (Grande 2012) | 2 | 23,216 (11) | N/A* Range: 20.0 (Hildebrand 2010) to 100 (Jacobsen 2011) |
|
8 | 222,602 (40) | 22.4 (11.5 to 39.3) | 8 | 222,602 (40) | N/A* Range: 78.6 to 100 | 2 | 10,121 (2) | N/A* Range: 100 to 100 (Johnson 1997; Leiroz 2021) |
| Upper urinary tract obstruction | 17 | 268,687 (225) | 0.9 (0.0 to 10.8) | 13 | 233.763 (188) | 68.2 (38.7 to 87.9) | 5 | 40,990 (85) | 33.9 (2.3 to 91.8) |
| Lower urinary tract obstruction | 18 | 262,135 (167) | 94.0 (82.6 to 98.1) | 16 | 250,018 (157) | 99.0 (70.0 to 100) | 7 | 194,823 (27) | N/A* Range: 83.3 to 100 |
| Genital | |||||||||
| Overall (any type) | 8 | 177,556 (145) | N/A* Range: 0.0 to 0.0 | 6 | 169,543 (137) | NA* Range: 0.0 to 8.1 | 6 | 149,642 (107) | N/A* Range: 0.0 to 33.3; 0.0 to 3.0 (5 studies); 33.3 (Chen 2009) |
| Ambiguous genitalia | 2 | 99,504 (6) | N/A* Range: 0.0 to 0.0 (Iliescu 2013; Syngelaki 2019) | 1 | 94,713 (5) | N/A* Range: 80.0 to 80.0 (Syngelaki 2019) | 1 | 13,516 (1) | N/A* Range: 0.0 to 0.0 (Drukker 2020) |
| Musculoskeletal | |||||||||
| Overall (any type) | 21 | 257,229 (696) | 25.4 (18.2 to 34.3) | 18 | 240,357 (635) | 77.5 (59.9 to 88.8) | 10 | 158,988 (536) | 43.8 (29.3 to 59.5) |
| Skeletal dysplasias | 16 | 269,236 (83) | 54.1 (39.9 to 67.7) | 14 | 246,393 (77) | N/A* Range: 50.0 to 100 | 8 | 670,352 (102) | NA* Range: 0.0 to 100; 0.0 (Jacobsen 2011); 50.0 to 100 (remaining 7 studies) |
|
10 | 229,873 (32) | 56.5 (30.7 to 79.2) | 9 | 224,819 (30) | N/A* Range: 100 to 100 | 4 | 189,617 (26) | N/A* Range: 75.0 to 100 |
|
7 | 146,455 (26) | 37.2 (9.8 to 76.3) | 5 | 123,612 (20) | N/A* Range: 0.0 to 100; 0 (Taipale 2004); 50.0 to 100 (remaining 4 studies) | 4 | 40,383 (9) |
N/A* Range: 0.0 to 66.7; 0.0 to 25.0 (3 studies); 66.7 (Stefos 1999) |
| Limb reduction defect | 18 | 273,864 (144) |
62.0 (43.3 to 77.7) | 13 | 233,886 (107) | 100 (67.4 to 100) | 9 | 211,781 (66) | 46.3 (24.7 to 96.4) |
| Talipes | 17 | 244,832 (266) | 6.0 (2.2 to 15.6) | 13 | 222,906 (241) | 72.1 (46.4 to 88.5) | 8 | 72,151 (133) | 42.6 (24.0 to 63.5) |
| Ear, face and neck | |||||||||
| Overall (any type) | 16 | 247,420 (334) | NA* Range: 0.0 to 50.0; 0.0 to 22.2 (14 studies); 50.0 (Becker 2012; Iliescu 2013) | 14 | 233,770 (321) | 59.6 (37.8 to 78.1) | 7 | 144,251 (117) | 32.3 (14.8 to 56.7) |
| Orofacial clefts | 19 | 276,776 (264) | 13.5 (5.1 to 31.4) | 15 | 241,811 (216) | N/A*
Range: 0.0 to 100;
0.0 (Carvalho 2002; Hernadi 1997; Souka 2006); 57.1 to 100 (remaining 12 studies) |
12 | 737,082 (786) | 44.1 (26.9 to 62.9) |
|
9 | 181,324 (48) | 0.7 (0.0 to 8.1) | 8 | 176,533 (47) | 68.4 (40.9 to 87.1) | 5 | 109,843 (43) | 76.7 (65.1 to 85.3) |
|
8 | 176,319 (126) | 31.7 (24.4 to 40.1) | 6 | 162,669 (118) | 100 (81.5 to 100) | 8 | 324,854 (261) | 81.9 (77.7 to 85.5) |
|
4 | 170,424 (26) | N/A* Range: 0.0 to 0.0 | 3 | 161,565 (23) | 11.7 (1.8 to 49.4) | 5 | 93,865 (34) | 2.9 (0.1 to 53.4) |
| Multisystem anomalies | |||||||||
| Overall (any type) | 21 | 256,603 (413) | 69.6 (59.0 to 78.5) | 18 | 239,731 (386) | 94.7 (85.2 to 98.2) | 7 | 58,014 (73) | 40.6 (4.5 to 90.9) |
| Fetal hydrops | 20 | 252,415 (196) | 72.4 (38.7 to 91.6) | 18 | 240,293 (188) | 96.6 (76.1 to 99.6) | 3 | 26,856 (8) | N/A* Range: 0.0 to 100; 0.0 (Rydberg 2017); 80.0 (Drukker 2020); 100 (Lee 1998) |
| MCA or syndrome | 28 | 298,117 (268) | 70.4 (53.0 to 83.3) | 23 | 259,930 (232) | 96.0 (82.3 to 99.2) | 9 | 301,085 (1119) | 69.6 (24.9 to 94.0) |
| *N/A: meta‐analysis was not possible because of failure of the random‐effects models to converge due to a lack of variation between studies, or was not performed due to a lack of data (i.e. two or fewer studies available). | |||||||||
|
a‐kCardiac anomalies were grouped according to their anatomical classification as proposed by van Velzen 2016.
Examples of anomalies that were included in each category: aSeptal defects: e.g. major VSD, balanced AVSD. bValvular anomalies (biventricular heart): e.g. significant pulmonary or aortic valve stenosis, Ebstein’s anomaly, significant tricuspid dysplasia or regurgitation. cVenous return anomalies: e.g. total anomalous pulmonary venous return, partial anomalous pulmonary venous return. dAortic arch anomalies: e.g. aortic coarctation, hypoplastic or interrupted aortic arch, double aortic arch, right aortic arch with left ductus arteriosus. eConotruncal anomalies: e.g. Tetralogy of Fallot, double outlet right ventricle, transposition of the great arteries, truncus arteriosus, pulmonary atresia with VSD. fHypoplastic right heart syndrome (HRHS): HRHS or hypoplastic right heart, pulmonary atresia with intact ventricular septum, critical pulmonary valve stenosis with right ventricular hypoplasia. gHypoplastic left heart syndrome (HLHS): HLHS or hypoplastic left heart, aortic valve atresia or critical aortic valve stenosis with left ventricular hypoplasia. hOther single ventricle defects: e.g. unbalanced AVSD, tricuspid atresia, pulmonary atresia without VSD, unspecified single ventricle defect. iComplex defects with atrial isomerism: e.g. left or right atrial isomerism, heterotaxy syndromes. jMiscellaneous major cardiac defects: e.g. unspecified major or complex heart defects, myocardial abnormalities, rhabdomyomas. kMinor cardiac defects: e.g. minor or insignificant septal defects, minor or insignificant isolated valvular anomalies, isolated right aortic arch with right ductus arteriosus, isolated persistent left vena cava superior. | |||||||||
| AVSD: atrioventricular septal defect; CI: confidence interval; CDH: congenital diaphragmatic hernia; CPAM: congenital pulmonary airway malformation; FN: false negative; FP: false positive; HRHS: hypoplastic right heart syndrome; HLHS: hypoplastic left heart syndrome; MCA: multiple congenital anomalies; MCDK: multicystic dysplastic kidney; TN: true negative; TP: true positive; VSD: ventricular septal defect | |||||||||
Summary of findings 5. Comparative accuracy of single‐ and two‐stage screening approaches in detecting fetal structural anomalies before 24 weeks' gestation.
| Comparative accuracy of single‐ and two‐stage screening approaches in detecting fetal structural anomalies before 24 weeks' gestation | ||||||||||||
|
First + second‐trimester scan (two‐stage screening approach) |
Single second‐trimester scan (single‐stage screening approach) | Comparison | ||||||||||
| Sensitivity | Specificity | |||||||||||
| Studies | Fetuses (cases) | Sensitivity (95% CI) | Specificity (95% CI) | Studies | Fetuses (cases) | Sensitivity (95% CI) | Specificity (95% CI) | P value | Certainty (GRADE) | P value | Certainty (GRADE) | |
| I. Overall accuracy of ultrasound in detecting fetal structural anomalies | ||||||||||||
| Fetuses affected | 18 | 181,614 (3051) | 83.8% (74.7 to 90.1) |
99.9 (99.7 to 100) |
9 | 158,767 (3168) | 50.5 (38.5 to 62.4) | 99.8 (99.2 to 100) |
< 0.001 | Very lowa,b,c | 0.350 | Lowc,e |
| Anomalies* | 17 | 229,274 (4975) | 84.5 (75.6 to 90.5) |
‐ | 3 | 17,845 (340) | 52.2 (33.2 to 70.7) | ‐ | 0.012 | Very lowa,b,c | 0.692 | Lowc,e |
| Implications** |
|
|||||||||||
| II. Subgroup analysis of ultrasound accuracy in detecting anomalies that are less susceptible to under‐reporting due to incomplete postnatal identification (i.e. anomalies that are externally visible, symptomatic at birth or considered to be lethal) | ||||||||||||
| Fetuses affected | 18 | 181,614 (1534) |
86.7 (78.8 to 92.0) | 100 (99.9 to 100) | 7 | 58,014 (559) |
59.2 (45.7 to 71.5) | 99.9 (99.6 to 100) |
< 0.001 | Very lowb,c,d | 0.170 | Lowc,e |
| Implications** |
|
|||||||||||
|
CI: confidence interval *No specificity was determined as the number of true‐negative test results could not be determined by the number of anomalies. **To allow accurate comparison of the effectiveness of single‐ and two‐stage screening approaches, all calculations are based on the median prevalence of structural anomalies reported across studies that evaluated a first + second‐trimester scan combined (Table 2). | ||||||||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||||||||
|
aContributing studies of both groups had design limitations (‐1), including risk of bias in the reference standard domain (‐0.75) and risk of bias in the flow and timing domain (‐0.25). bUnexplained heterogeneity of results among studies included in the single second‐trimester scan group (‐1). cIndirectness in terms of the comparison between single‐ and two‐stage screening strategies (‐1). dWide 95% CI, deviating more than 10% from the summary estimate of the single second‐trimester scan studies (‐1). eContributing studies had design limitations, including risk of partial verification bias of pregnancies due to incomplete verification of ultrasound findings in pregnancies with an adverse outcome (‐1). | ||||||||||||
Background
Prenatal ultrasound allows for the visualisation of fetal anatomy in pregnancy, and is widely used to detect structural anomalies before birth. Each year, nearly four million infants worldwide — three percent of all live births — are affected by congenital anomalies (Moorthie 2018; World Fertility Data 2008). Congenital anomalies comprise a broad spectrum of anomalies of varying severity, which can cause severe long‐term morbidity, disability, or, in the very worst cases, be fatal (March Of Dimes 2006). In high‐income countries, an estimated 20% to 29% of deaths under the age of five result from congenital abnormalities (Boyle 2018; Liu 2016; WHO 2021), more than half of which occur during the neonatal period (WHO 2021). Anatomical defects can result from chromosomal or genetic disorders, exposure to certain environmental factors such as teratogenic agents (e.g. isotretinoin, barbiturates or radiation), nutrient deficiencies (e.g. folic acid deficiency), metabolic factors (e.g. uncontrolled diabetes), infections (e.g. toxoplasmosis, rubella) or due to mechanical problems (e.g. amniotic band constrictions, severe oligohydramnios) (Brent 2004; March Of Dimes 2006). However, in the majority of cases, severe structural anomalies occur in pregnancies without known or pre‐existing risk factors, which highlights the important role of population‐based prenatal ultrasound screening to improve pre‐ and postnatal care for individuals affected by congenital anomalies (Brent 2004; Donofrio 2014; March Of Dimes 2006; Olde Scholtenhuis 2003). Identification of anomalies through ultrasound imaging requires systematic visualisation of fetal anatomical structures, using standardised imaging planes. This type of ultrasound examination is commonly referred to as a “fetal anomaly scan”, which is still considered the gold standard for detecting structural anomalies before birth and is performed in the second trimester of pregnancy.
Although routine ultrasound screening for fetal anomalies in the second trimester is considered standard of care in most countries, screening protocols, ultrasound technology and screening approaches are continuously evolving. With improved ultrasound resolution, routine first‐trimester ultrasound assessment of fetal anatomy is feasible. First trimester anatomy screening is increasingly being implemented as part of national prenatal screening programmes in some countries (e.g. France, Croatia, Spain and Switzerland (Boyd 2010), and the Netherlands, albeit in a research setting (RIVM 2021)). The main advantages of a routine first‐trimester scan include earlier identification of pregnancies with congenital anomalies and the option of confirmatory testing. This allows more time for decision‐making about treatment options and possible interventions, including termination of pregnancy. However, since several structural anomalies are known to become detectable only at later gestations (e.g. duodenal atresia, brain anomalies such as hydrocephalus and microcephaly, and fetal tumours), a first‐trimester scan is considered an add‐on screening test, rather than a potential replacement of the traditional second‐trimester fetal anomaly scan (Salomon 2013; Syngelaki 2019). Consequently, a first‐trimester anomaly scan is generally only offered in combination with a follow‐up second‐trimester scan. As a result of the increasing but still heterogeneous use of first‐trimester ultrasound as part of population‐based prenatal screening, there currently are two main approaches to ultrasound screening for fetal anomalies in women without known risk factors: a single scan, performed either during the first or second trimester of pregnancy; or two scans, performed during the first and then second trimesters of pregnancy. In the following sections, we will refer to these screening strategies as single‐ and two‐stage approaches, respectively.
Implementation of routine first‐trimester screening for fetal anomalies may have an impact on overall screening performance with regard to the total number of anomalies detected before birth, and the number of false‐positive diagnoses. The accuracy of first‐trimester ultrasound in detecting fetal structural anomalies was evaluated in 2017 by Karim et al., who estimated that the sensitivity of first‐trimester ultrasound in detecting fetal structural anomalies overall was 32.4% in low‐risk and unselected pregnant women (Karim 2017). However, the role of a first‐trimester scan in a two‐stage screening approach and its impact on overall effectiveness of ultrasound screening for fetal structural anomalies has not been determined. Furthermore, the evidence on the accuracy of a second‐trimester scan in a single‐stage screening approach is in need of an update. It was last reviewed in a Health Technology Assessment (HTA) Review by Bricker et al. (Bricker 2000), and was updated by the National Institute for Health and Care Excellence (NICE) in 2008 (NICE 2008). Since these publications, several factors have changed that may have an impact on the detection of fetal anomalies (e.g. changes to anatomical screening protocols (Everwijn 2018), and widespread availability of modern real‐time ultrasound equipment with high‐resolution transabdominal and transducers, harmonic imaging and colour flow Doppler (Campbell 2013)), necessitating an updated review of the literature.
In this systematic review, we evaluate the diagnostic accuracy of first‐ and second‐trimester ultrasound screening in low‐risk and unselected pregnant women for detection of fetal structural anomalies by way of a single‐ and two‐stage screening approach. Furthermore, we provide a comprehensive overview of all available evidence on each screening approach in terms of the accuracy of ultrasound in detecting fetal structural anomalies overall, as well as in detecting subgroups of anomalies of varying severity, and of anomalies of individual organ systems and selected anomalies individually (Buijtendijk 2021). We compare the overall accuracy of a single‐ and two‐stage screening approach in detecting fetal structural anomalies before 24 weeks’ gestation. Lastly, within a two‐stage screening approach, we distinguish between anomalies detected before 14 weeks’ gestation by the first‐trimester scan and overall detection of fetal structural anomalies by both scans combined.
Target condition being diagnosed
The target conditions of interest are fetal structural anomalies identifiable on ultrasound imaging, excluding soft markers for chromosomal abnormalities. Structural anomalies can occur in any organ system and are defined as anatomical malformations resulting from abnormal development of organs or body parts, which can result in the structure not being formed, being partially formed or being formed in an abnormal fashion. The clinical and ultrasound characteristics and spectrum of severity are highly variable among individual structural anomalies. Structural anomalies are commonly categorised according to their clinical consequences as major or minor anomalies.
Major anomalies are defined as structural anomalies that have serious medical or cosmetic consequences. About 75% of major structural anomalies are isolated (i.e. a single defect). The remaining 25% are associated with other anomalies (i.e. multiple defects) (WHO/CDC/ICBDSR 2014). In some cases, a group of defects may form part of a well‐described association, sequence or syndrome. However, the majority of cases occur without a known relation to one another. When a fetus or infant is diagnosed with two or apparently unrelated more major anomalies that involve multiple organ systems, this is commonly referred to as “multiple congenital anomaly”. For practicality, we did not distinguish between groups of anomalies that occur with or without a known association with one another. However, because fetuses with multiple defects may be identified more easily than those with a single defect, we did distinguish between cases with multiple structural anomalies and cases with an isolated structural anomaly.
For practical reasons, evaluations of prenatal ultrasound often focus on detection of major anomalies, which are the target conditions of interest of prenatal ultrasound screening programmes. However, when evaluating the performance of prenatal screening – or when considering implementation of an additional screening test such as a routine first‐trimester scan – it is important to also consider incidental findings such as anomalies considered to be minor. A finding of a minor structural anomaly may cause parental distress and requires careful counselling. Furthermore, the impact of a given anomaly for an individual cannot be fully objectified. Therefore, we chose not to exclude minor anomalies from our analyses.
We did not evaluate detection of chromosomal abnormalities or of minor anomalies that may indicate an underlying chromosomal abnormality, known as soft markers for chromosomal aneuploidy. For a review of screening for common aneuploidies, we refer to the reviews by Alldred 2017a, Alldred 2017b and Badeau 2017.
Index test(s)
We examined the diagnostic accuracy of routine first‐ and second‐trimester ultrasound screening for fetal structural anomalies. A routine ultrasound scan during which the fetal anatomy is evaluated to screen for anomalies, is typically referred to as a fetal anomaly scan.
It is important to distinguish between a routine fetal anomaly scan and a targeted or detailed ultrasound examination. A routine fetal anomaly scan is usually performed by an obstetrician or (obstetric) sonographer in a screening setting and is generally offered to all pregnant women as part of routine prenatal care. Women who receive this test have no known risk factors for fetal structural anomalies.
A targeted or detailed ultrasound examination is used to evaluate women who are identified in advance as being at increased risk for fetal anomalies (Wax 2014). It may also be used as a diagnostic follow‐up test in case of an anomaly suspected or detected during a routine ultrasound examination (Public Health England 2018). Detailed or targeted ultrasound examinations typically use high‐quality equipment and are performed by a fetal medicine specialist or a specially trained sonographer, and they include a more extensive examination of fetal anatomical structures (Wax 2014).
In this review we only evaluated the accuracy of routine ultrasound examination in a screening setting. We only considered ultrasound screening programmes that involved a complete survey of the fetal anatomy for inclusion in the review (i.e. fetal anomaly scans). From this section onwards, we will refer to fetal anomaly scans as “scans”.
We defined the first‐trimester scan as a routine ultrasound scan performed from 11 weeks and 0 days' gestation (11 + 0) to 13 weeks and six days' gestation (13 + 6), which included a complete examination of the fetal anatomy in accordance with international guidelines for the performance of first‐trimester fetal anomaly screening (Audibert 2017; Boyd 2010; Salomon 2013; van den Hof 2019).
The second‐trimester scan was defined as a routine fetal anomaly scan performed between 18 weeks and 0 days' (18 + 0) and 23 weeks and six days' (23 + 6) gestation. For this review, we have chosen to set the upper limit of the second‐trimester scan at 23 + 6 weeks' gestation as this gestation is widely regarded as the limit of viability and the legal limit for pregnancy termination in countries where termination of pregnancy is restricted (Boyd 2010; Lavelanet 2018).
Clinical pathway
Prior test(s)
During their first visit to an antenatal clinic, women are classified as being at high or low risk for fetal anomalies, based on assessment of their medical and obstetric history and other risk factors for fetal anomalies such as exposure to teratogens or a fetal anomaly being diagnosed in a previous pregnancy. Further risk stratification may occur if women choose to enrol in a fetal aneuploidy screening programme, which aims to identify women at increased risk of carrying a fetus with Down’s syndrome (trisomy 21), Edwards' syndrome (trisomy 18) or Patau syndrome (trisomy 13). The main screening tests for these conditions include the first‐trimester combined test (which relies on measurement of nuchal translucency (NT) by ultrasound scan and serum protein markers at 11 to 13 + 6 weeks' gestation), and non‐invasive prenatal testing (NIPT) involving analysis of fetal DNA fragments in maternal blood from 10 weeks' gestation (RCOG 2014). Fetuses with an increased NT may also be detected at a routine first‐trimester anomaly scan. An increased NT is considered a marker for chromosomal as well as structural abnormalities, in particular cardiac defects (Bardi 2019; Souka 2005). Increased NT is therefore considered an important indication for further detailed anatomical examination, which may include referral for targeted fetal echocardiography (AIUM 2013; Wax 2014).
Role of index test(s)
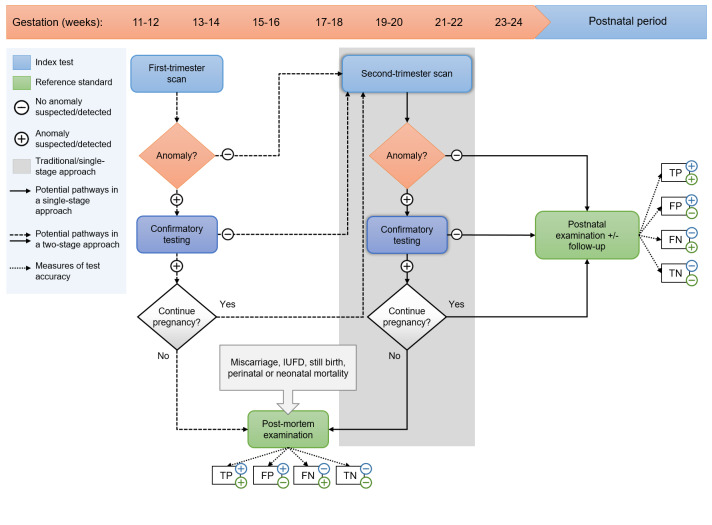
The potential pathways and roles of the index tests in the context of a single‐ and two‐stage screening approach are illustrated in Figure 1.
1.
Clinical pathways in single‐ and two‐stage screening approaches. The horizontal arrow indicates the timeline with pregnancy duration in gestational weeks and the postnatal period, respectively. In a single‐stage approach (grey shaded area, solid black arrows), women are offered a fetal anomaly scan during the second trimester of pregnancy (at 18 to 22 weeks' gestation). In a two‐stage approach, women are offered an early anomaly scan (performed at 11 to 14 weeks' gestation) in addition to a routine second‐trimester anomaly scan (at 18 to 22 weeks' gestation). IUFD: intra‐uterine fetal demise; TP: true‐positive test result (anomaly suspected/detected on ultrasound imaging and confirmed after birth); FP: false‐positive test result (anomaly suspected/detected on ultrasound imaging, but not confirmed after birth); TN: true‐negative test result (no anomaly suspected/detected on ultrasound imaging and no anomaly found after birth); FN: false‐negative test result (an anomaly was found after birth, which was not detected on ultrasound imaging).
Second‐trimester screening for fetal structural anomalies: single‐stage approach
In a single‐stage approach, a single second‐trimester scan is the primary screening test used to detect fetal structural anomalies (Figure 1 – grey shaded area, solid black arrows). Generally, this scan is performed between 18 and 22 weeks' gestation. If scan results are abnormal (i.e. one or more anomalies are suspected or detected), women are referred to a specialist centre for further investigations. This may include an advanced ultrasound scan to re‐evaluate or confirm the suspected anomaly, parental blood testing, invasive prenatal testing, or a combination of these (Public Health England 2018). Depending on the final diagnosis, parents may opt to continue or terminate the pregnancy. When parents choose to continue the pregnancy, prenatal, intrapartum and postnatal care will be provided according to the usual obstetric standards. Postnatal care may involve additional diagnostic testing to confirm or further specify the antenatally suspected condition. If parents choose to terminate the pregnancy, a postmortem examination (autopsy) may be offered to confirm or provide further information about the diagnosis. However, postmortem examination may not be offered routinely at all institutions and, when offered, acceptance of this investigation is at the discretion of the parents.
If the second‐trimester scan results are normal, the pregnancy will follow its natural course and most pregnancies will result in a live birth. All neonates undergo a postnatal physical examination to check the infant's well‐being and to screen for congenital anomalies that might necessitate medical intervention or follow‐up. However, not all anomalies may present immediately after birth and may only become apparent during the late neonatal period or in childhood. In case of an adverse outcome (i.e. perinatal mortality), parents are often offered the option of a postmortem examination to determine the cause of death.
First‐ and second‐trimester screening for fetal structural anomalies: two‐stage approach
In a two‐stage approach, women receive an early anomaly scan (performed at 11 to 14 weeks' gestation) aiming to detect gross anomalies during the first trimester of pregnancy. This early scan is offered in addition to a routine second‐trimester anomaly scan (Figure 1). If a structural anomaly is detected or suspected at a first‐trimester scan, women are usually referred to a specialist centre for further anatomical evaluation and follow‐up testing. If the first‐trimester scan is normal, women receive another routine scan during the second trimester of pregnancy. At this point, anomalies may still be identified as not all anomalies are detectable during the first trimester of pregnancy and some may develop at later gestations (Syngelaki 2019).
Alternative test(s)
Currently, there are no alternative tests available to screen for fetal structural anomalies during pregnancy.
Rationale
Ultrasound screening policies for fetal structural anomalies vary widely. There is currently no international consensus on whether population‐based prenatal screening programmes should include a first‐trimester scan, in addition to the routine second‐trimester scan traditionally offered. Screening for fetal structural anomalies at 18 to 22 weeks' gestation is thought to allow for an optimal balance between maximising detection rates, while leaving sufficient time for counselling, further investigations and decision‐making prior to 24 weeks’ gestation (Salomon 2011; Ward 2011) ‐ the legal limit for termination of pregnancy in most countries (although exceptions may be made in case of lethal conditions or when a continued pregnancy threatens the mother’s physical or mental health) (Lavelanet 2018). However, the psychological cost following a second‐trimester pregnancy termination may be substantial. Long‐term follow‐up of women after termination of pregnancy has shown that advanced gestational age was associated with higher levels of grief, stress and post‐traumatic symptoms, with a substantial number of women developing pathological scores for post‐traumatic stress (Korenromp 2005). Long‐term psychological morbidity after early termination of pregnancy (before 14 weeks’ gestation) was, however, rare (Korenromp 2005). An offer of a first‐trimester scan, as suggested with a two‐stage screening approach, intends to detect some gross anomalies for which termination of pregnancy might be considered early in pregnancy. First‐trimester detection of fetal anomalies further offers the advantage of allowing more time for further investigations, counselling and discussions of possible interventions. However, any screening programme has the potential of false‐positive diagnoses, which can cause anxiety throughout the remaining weeks of pregnancy. The potential for false‐positive diagnoses therefore needs to be evaluated when assessing the performance of potential prenatal screening approaches (NICE 2008).
Objectives
The main objectives of this review were to evaluate the accuracy of first‐ and second‐trimester fetal anomaly screening in low‐risk pregnant women, and to compare the overall accuracy of single‐ and two‐stage screening approaches with regard to the number of cases detected before birth, as well as the proportion of false‐positive diagnoses.
Our main outcome was the accuracy of ultrasound screening in detecting fetuses affected by one or more structural anomalies overall. In addition to overall accuracy, we evaluated the accuracy of ultrasound screening for detection of subgroups of anomalies based on their severity and the organ system they occurred in, and determined sensitivity and specificity for selected individual anomalies within each organ system.
Secondary objectives
Our secondary objective was to identify potential sources of heterogeneity between studies by investigating the effect of clinical factors (i.e. prior testing, factors related to the conduct of the ultrasound examination, and healthcare setting of the study) and methodological factors (i.e. related to the study design, such as methods used for postnatal identification of cases) on screening performance.
Methods
Criteria for considering studies for this review
Types of studies
A major challenge in evaluating the accuracy of ultrasound screening for fetal structural anomalies is the prevalence of individual anomalies. Whereas structural anomalies are relatively common, the individual anomalies that constitute this group are rare. In order to include a sufficient number of cases to generate more accurate estimates of test accuracy of individual anomalies, we considered reports of ultrasound detection of selected anomalies in addition to general reports of ultrasound accuracy for inclusion in the review.
We included studies with the following study designs:
Randomised controlled studies in which pregnant women were randomised to different screening strategies for the detection of fetal structural anomalies.
Prospective and retrospective cohort studies reporting on the diagnostic accuracy of ultrasound screening for fetal structural anomalies.
Registry‐based cohort studies reporting on the number of prenatally and postnatally diagnosed fetal structural anomalies over a period in which a population‐based ultrasound screening programme was implemented.
Additional inclusion criteria were as follows:
The study used a low‐risk or unselected population.
The ultrasound scan included a complete examination of the fetal anatomy that was offered as part of routine prenatal care (i.e. offered to all women, without referral).
The scan was performed between 11 + 0 and 13 + 6 and/or 18 + 0 and 23 + 6 weeks' gestation.
The reference standard used by the study is described and included, at a minimum, postnatal examination or postnatal follow‐up (or both) of all fetuses that received the index test.
Data could be extracted or derived for constructing a 2 x 2 table of the number of true‐positive, false‐positive, false‐negative and true‐negative test results for the target conditions of interest at the level of the organ system(s) evaluated or for each type of anomaly individually.
Although we strived to extract data for construction of the full 2 x 2 tables, we anticipated that it would not be possible to extract the number of false‐positive test results from the majority of studies. Therefore, we also included studies from which it was only possible to determine the number of true positives and false negatives. These studies were only included for meta‐analysis of sensitivity of ultrasound screening. Studies from which it was possible to derive the full 2 x 2 table were analysed separately for the determination of both sensitivity and specificity of ultrasound screening.
Exclusion criteria
Studies that evaluate the diagnostic accuracy of targeted ultrasound examination of a specific organ system (e.g. targeted fetal echocardiography or dedicated fetal neurosonography).
Case‐control study designs (i.e. studies in which the accuracy of sonographic markers for diagnosing a specific anomaly is retrospectively evaluated between cases with and without a known specific anomaly).
Literature reviews, case reports, conference abstracts.
Restriction of publication year
Since the introduction of routine ultrasound screening for fetal anomalies in the 1970s, significant changes to both the content and conduct of this examination have taken place. An important addition to fetal anatomy assessment has been the introduction of the three vessel view (Yoo 1997). The introduction of this view to standard ultrasound screening protocols has had a major impact on the detection of congenital heart defects (Everwijn 2018) and is now considered a standard screening view for evaluating the fetal heart. Given its impact on the detection of congenital heart defects, and the relatively high prevalence of cardiac outflow tract anomalies, we have decided to exclude literature preceding the introduction of this view. For a review of studies published before 1997, we refer to the HTA by Bricker and colleagues (Bricker 2000) and the NICE guideline (NICE 2008) on this topic.
Participants
We included data from studies that used a low‐risk or unselected population from any country and healthcare setting. A low‐risk population was defined as a study population without known risk factors for fetal anomalies at the time of inclusion in the study. An unselected population was defined as a study population that included all pregnant women attending a healthcare centre for routine prenatal care during the study period.
Index tests
We evaluated the following tests/approaches to ultrasound screening (Figure 1):
First‐trimester scan; i.e. ultrasound detection of fetal structural anomalies before 14 + 0 weeks’ gestation by first‐trimester ultrasound in a two‐stage screening approach.
First + second‐trimester scan; i.e. total ultrasound detection of fetal structural anomalies before 24 + 0 weeks’ gestation by first‐ and second‐trimester ultrasound combined in a two‐stage screening approach.
Single second‐trimester scan; i.e. ultrasound detection of fetal structural anomalies before 24 + 0 weeks’ gestation by a single second‐trimester scan, when no prior first‐trimester scan is offered.
For the analysis of a single second‐trimester scan, we only included cohorts of women that did not receive a first‐trimester anomaly scan prior to the second‐trimester fetal anomaly scan. We did not exclude studies from this analysis based on other types of prior testing.
Target conditions
We considered all fetal structural anomalies potentially identifiable on ultrasound imaging, excluding soft markers for chromosomal aneuploidies as defined by the American College of Obstetricians and Gynecologists (ACOG 2016). The anomalies (soft markers) listed below were excluded from our analyses:
First‐trimester soft markers
Increased NT thickness
Cystic hygroma
Second‐trimester soft markers
Mild or moderate ventriculomegaly (defined as an atrial diameter of less than 15 mm)
Choroid plexus cysts
Echogenic intracardiac foci
Echogenic bowel
Mild hydronephrosis or pyelectasis (antero‐posterior pelvic diameter measuring 4 mm to 7 mm)
Short femur length (measurement at 2.5 centile or below for the gestational age)
Thickened nuchal fold (nuchal fold measurement of 6 mm or greater)
Reference standards
We considered several reference standards, depending on the pregnancy outcome: live birth or adverse outcome. For the purpose of this review, we considered any type of fetal or perinatal mortality (i.e. miscarriage, pregnancy termination, intrauterine death, stillbirth, perinatal mortality) as an adverse pregnancy outcome. In live births, the reference standards we considered included postnatal examination and postnatal follow‐up. In cases of an adverse pregnancy outcome, we considered postmortem examination as the reference standard.
Search methods for identification of studies
Electronic searches
We searched MEDLINE (Ovid MEDLINE(R) ALL 1946 to 22 July 2022), Embase (Ovid Embase Classic and Embase 1947 to 22 July 2022), Science Citation Index Expanded (Web of Science), Social Sciences Citation Index (Web of Science), Arts & Humanities Citation Index and Emerging Sources Citation Index (Web of Science) from 1 January 1997 to 22 July 2022, using thesaurus terms like MeSH terms and free‐text words. We searched for structural anomalies in general, as well as for prespecified specific diagnoses (Buijtendijk 2021), combined with terms related to ultrasonography, including nuchal translucency and echocardiography, complemented with general terms like scan and prenatal diagnosis, as not all papers may mention the imaging method used. Mild or transient congenital anomalies that are mainly confined to the third trimester of pregnancy (e.g. hydronephrosis or pyelectasis) were combined with a filter for the first and second trimester of pregnancy to restrict these conditions to the pregnancy trimesters of interest as third‐trimester screening for fetal anomalies is beyond the scope of this review. Next, we combined the search with a broad filter for the prenatal period. We limited our search to studies published after 1997 and excluded animal studies, reviews and case reports (Appendix 1; Appendix 2; Appendix 3). No further restrictions were applied. We imported identified records into EndNote and removed duplicate records.
Searching other resources
We cross‐checked reference lists and citing articles of each of the included studies and other identified relevant papers, including published systematic reviews for additional relevant studies, using Science Citation Index Expanded (Web of Science), Social Sciences Citation Index (Web of Science), Arts & Humanities Citation Index and Emerging Sources Citation Index (Web of Science).
Data collection and analysis
We applied the methods described in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (methods.cochrane.org/sdt/handbook-dta-reviews). To ensure consistent and reproducible conduct of the study selection, assessment of methodological quality and data extraction components of this review, we developed standardised forms. We selected five publications from the identified studies to pilot our forms and to ensure the criteria were applied consistently.
Selection of studies
Screening of the titles and abstracts was performed using the systematic review software EPPI Reviewer (www.eppi.ioe.ac.uk). All studies identified by the search strategy were randomly divided over a total of 13 review authors (MFJB, BBB, MMGL, HS, TR, TCDG, DD, GMMT, YD, MAL, BB, MvdH, BSB). Review authors were instructed to be over‐inclusive during the initial screening phase. We conducted a pilot using 100 randomly selected articles to trial the screening tool and to ensure criteria were applied consistently. We obtained full‐text versions of studies considered potentially relevant. Two review authors (MFJB and BBB) independently evaluated the full‐text papers for inclusion by using the above‐mentioned inclusion and exclusion criteria, using a full‐text assessment tool developed in EPPI Reviewer. We excluded studies that did not meet the inclusion criteria and recorded the reason for exclusion. In case of insufficient information to judge the eligibility of a potentially relevant study, we contacted the study authors if we suspected that the missing information might be available. If multiple publications resulted from the same study cohort, only the results from the most comprehensive and relevant publication were included. Discrepancies in selection of studies between review authors were resolved by consensus or consultation of a third review author (EP or MMGL).
Data extraction and management
Data extraction was performed in two parts. Two review authors (TR and TCDG) independently extracted data for the first part of the data extraction using the piloted data extraction tool developed in EPPI Reviewer. For the first part of the data extraction, we extracted the following data from each study:
Study characteristics (e.g. reference details allowing for identification of the study, language and study design).
Population characteristics (e.g. sample size, recruitment methods, in‐ and exclusion criteria, prior testing, study period, healthcare setting, country and geographic region where the study was conducted).
Target conditions evaluated and definitions used for major and minor anomalies.
Features of the index test (e.g. type of professional performing the scan, experience level of the operator, mode of examination, ultrasound screening protocol).
Features of the reference standard (e.g. postnatal and postmortem examination, postnatal follow‐up duration, additional postnatal verification methods).
Another two review authors (MFJB and BBB) independently extracted data for constructing 2 x 2 tables of true‐positive, false‐positive, false‐negative and true‐negative test results or summary statistics from which these data can be derived using a separate data collection form (Excel® format). We extracted data for constructing 2 x 2 tables for each reported structural anomaly that met our selection criteria. We excluded the following anomalies from our analyses: soft markers for chromosomal anomalies, structural anomalies associated with a chromosomal abnormality or intra‐uterine infection, anomalies considered benign normal variants (e.g. aberrant right subclavian artery; intrathymic brachiocephalic vein), non‐structural anomalies (e.g. cardiac arrhythmias), anomalies considered not detectable during the fetal period (e.g. persistent ductus arteriosus; undescended testes) and growth abnormalities.
We cross‐checked all extracted data and reported data and resolved discrepancies by discussion and iteration until consensus was reached. If required, we consulted a third author with senior clinical (EP) or methodological expertise (MMGL). We contacted study authors if crucial information was missing for correct classification and interpretation of the reported data. If a study presented study results for high‐risk and low‐risk pregnant women, or a pre‐screening and a screening period, we only considered the low‐risk or screening subgroup of the cohort, respectively.
Assessment of methodological quality
We used a modified version of the revised QUality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool for assessment of methodological quality of included studies (Whiting 2011), tailored to our review question (Appendix 4). We made the following main adjustments:
In the reference standard domain, we included individual signalling questions to evaluate the ability of the reference standard to correctly classify anomalies that are externally visible (e.g. cleft lip), that present with clinically relevant symptoms shortly after birth (e.g. oesophageal atresia), or that are considered to be lethal/incompatible with life (e.g. bilateral renal agenesis), and for internal anomalies that may remain asymptomatic during the immediate postnatal period (e.g. certain cardiac defects and renal anomalies). We chose to distinguish between these groups of anomalies when evaluating the quality of the reference standard because we anticipated that incomplete postnatal identification of internal anomalies of the latter group would be a key methodological issue in studies that followed pregnancies and infants only until birth or until discharge from hospital. We considered a postnatal follow‐up period one month or longer to be adequate to detect the majority of clinically relevant anomalies that may be missed during the immediate postnatal period.
We anticipated that another important methodological concern was the potential for partial verification bias arising from low acceptance rates of postmortem examination (Lewis 2018). We expected to find bias when a postmortem examination was not performed following unexplained fetal death, miscarriage or still birth, as this could potentially influence false negative and true negative rates. Additionally – though less likely – a missing reference standard could also influence false positive and true positive rates when postmortem examination was not performed following elective pregnancy termination for a suspected fetal anomaly. We chose to code this issue as originating from the flow and timing domain and included separate signalling questions for evaluation of patient flow in pregnancies that resulted in a live birth and in pregnancies that had an adverse outcome.
Lastly, we omitted the QUADAS‐2 item assessing the methodological quality of the study according to the time interval between the index test and reference standard as clinically relevant structural fetal anomalies are expected to be either present or absent.
We answered each signalling question with “yes”, “no” or “unclear”. “Unclear” was only used if insufficient information was available. If a study was recorded as “yes” on all signalling questions related to risk of bias, the overall judgement of risk of bias was considered low. If one or more signalling questions in a domain were answered with “no” or “unclear”, we judged the study as having “high” or “unclear” risk of bias in that domain if we suspected that it would lead to potential bias. Exceptions where answering “no” to one of the signalling questions did not lead to a “high risk of bias” judgement included the following:
Lack of blinding of index test results when interpreting the reference standard. In a clinical setting, prenatal ultrasound results will always be communicated to the team responsible for the peri‐ and postnatal care of newborn infants and their mothers. As all relevant studies were conducted in a clinical setting, they all lacked blinding in the reference standard domain. However, in most cases of structural anomalies, the anomaly is either present or absent and knowledge of prenatal ultrasound findings is unlikely to change the postnatal diagnosis. Exceptions may exist. However, since these will affect only a minority of very specific cases, we did not regard lack of blinding as a source of bias in the overall assessment of methodological quality of studies.
The use of a different reference standard for live‐born infants with and without prenatally suspected anomalies. Different reference standards (e.g. postnatal echocardiography, abdominal X‐ray, etc.) may be used to confirm and further classify a suspected condition based on prenatal ultrasound findings or postnatal signs and symptoms that require further investigation. As the use of different reference standards only occurs when an anomaly has already been suspected, this will most likely not affect the number of cases identified in studies. Therefore, we did not regard this as a source of bias.
Two review authors (MFJB and BBB) independently used the modified QUADAS‐2 tool to evaluate the methodological quality of each study. Disagreements were resolved by discussion and consensus. If necessary, a third author was consulted (EP or MMGL).
Statistical analysis and data synthesis
The unit of analysis was the fetus that was screened for the presence of structural anomalies. We evaluated the performance of a first‐trimester scan, of a first + second‐trimester scan combined, and of a single second‐trimester scan. We evaluated the performance of each scan or combination of scans by the number of fetuses affected as well as the total number of anomalies detected. Because fetal structural anomalies comprise a heterogeneous group of conditions with extensive differences in treatment options, prognoses and sonographic markers associated with the defect, and likelihood of complete postnatal identification, we performed subgroup analyses on the organ system affected.
First, we distinguished fetuses with anomalies that are less susceptible to under‐reporting due to incomplete postnatal identification of all other anomalies. This subgroup included anomalies that are externally visible, present with clinically relevant symptoms at or shortly after birth, or that are considered lethal (i.e. those anomalies that would generally be identified immediately after birth in all cases). Anomalies that may be acquired or develop at later gestations (e.g. hydrocephalus, fetal hydrops, tumours, etc.) were excluded from this subgroup analysis because we were unable to verify if the anomaly was missed at the ultrasound scan (false negative), or developed only after the examination was undertaken (true negative at the time of the ultrasound examination).
Second, we distinguished groups of anomalies based on the severity of the condition, modified after a proposal by The Royal College of Obstetricians and Gynaecologists (RCOG 1997) and Saltvedt 2006. We recognised the following two main subgroups:
1. Major anomalies (conditions with serious medical implications).
This category includes all anomalies that are lethal, that require immediate postnatal evaluation and/or intervention, or that are associated with short‐ or long‐term morbidity/handicap of at least moderate severity. Within the group of major anomalies, we analysed lethal anomalies (i.e. anomalies that are incompatible with life) separately from all other anomalies considered major.
2. Minor anomalies (conditions of minor functional, social or cosmetic significance).
Because the distinction between major and minor anomalies leaves considerable room for interpretation, we referred to the guidelines of two congenital anomaly registers that published lists of anomalies considered minor: the Metropolitan Atlanta Congenital Defects Programme (MACDP) and the European Registration of Congenital Anomalies and Twins register (EUROCAT). Discrepancies between guidelines were resolved by consultation of a clinical expert (EP). If the classification of an anomaly depended on the size or extent of the defect and this information was not included in the report (e.g. ventricular septal defect), we followed the classification provided by the study authors. If the authors did not distinguish between major and minor anomalies in their report, we classified such anomalies as major by default.
Lastly, we distinguished groups of anomalies based on the anatomical system they occurred in and determined the accuracy of ultrasound screening for the 46 preselected anomalies (Buijtendijk 2021). Only studies from which we were able to extract data for 2 x 2 tables of all structural anomalies were included in the analysis of overall detection of fetal anomalies. Studies that only provided data for subgroups of anomalies were included for the meta‐analyses of the relevant subgroups.
Sensitivity could be obtained from all studies. However, specificity could only be derived from a subgroup of studies because many failed to measure or report the number of false‐positive results. Because specificity was close to 100% across studies that did allow for its calculation, we assumed that a strong correlation between sensitivity and specificity was unlikely. Therefore, we removed the correlation parameter from the bivariate model and thus performed separate meta‐analyses of sensitivity and specificity using a univariate random‐effects logistic regression model. In cases where there were fewer than three studies in the meta‐analysis, or where all studies (or all except one) reported a sensitivity of 100%, or when the random‐effects models failed to converge due to other reasons, we refrained from performing the meta‐analysis and reported the results descriptively.
All meta‐analyses were performed using the NLMIXED procedure in the SAS software (SAS version 9.4, SAS Institute Inc. 2013).
Coding of reported anomalies
When evaluating the accuracy of ultrasound screening for fetal anomalies, data can be reported as the number of anomalies detected or as the number of fetuses affected (i.e. the number of fetuses with one or more anomalies) (Bricker 2000; Karim 2017). We used the number of fetuses affected as the unit of analysis for our main outcome and subgroup analyses, including the analyses of selected anomalies and detection within individual organ systems. If a fetus had more than one major abnormality involving multiple organ systems, we coded the condition as “multiple congenital anomaly or syndrome” and only included the fetus for this category. If a fetus had multiple anomalies within the same organ system, e.g. multiple cardiac anomalies, we only extracted the data of the most severe defect (i.e. the defect that would require treatment first).
From studies reporting on all anomalies that occurred in their cohort, we extracted the number of anomalies detected in addition to the number of affected fetuses. Using this number, we determined a second estimate of overall detection of fetal anomalies, as both the number of fetuses affected and the number of anomalies detected provide important information. We calculated the number of anomalies detected following the methods proposed by Karim and colleagues (Karim 2017). First, all bilateral anomalies were considered as two anomalies. Second, a fetus diagnosed with multiple abnormalities that are part of a known sequence, association or syndrome was counted as having one abnormality (i.e. one syndrome). On the other hand, if a fetus was diagnosed with two or more unrelated major abnormalities, each anomaly was counted individually. Anomalies that may develop secondarily to a structural defect such as hydrops or hydrocephalus as well as multiple unrelated anomalies within the same organ system, were also counted individually if their individual counts could be extracted from the report.
We considered any suspicion of a structural abnormality found at a routine screening scan as a true‐positive test result if the fetus was confirmed to have one or more structural anomalies after birth. We recognised that the main purpose of prenatal ultrasound screening is to distinguish between fetuses at increased risk of having a structural abnormality and related complications from those that are at low risk. Therefore, we considered cases in which the fetus was confirmed to have an abnormality after birth, but in whom the exact diagnosis changed, as a true‐positive test result. Only if the incorrect classification at the initial screening scan led to misdiagnosis of a defect considered lethal or incompatible with life did we consider the misclassification as a false‐positive or false‐negative result.
Any structural anomaly that was diagnosed after 14 + 0 weeks’ gestation or after 24 + 0 weeks’ gestation was considered a false‐negative test result for a first‐trimester anomaly scan or second‐trimester anomaly scan, respectively. All prenatally suspected anomalies that were not confirmed after birth were considered false‐positive results. False‐positive diagnoses made before 14 + 0 weeks’ gestation were considered false positives for the first‐trimester scan, whereas false‐positive diagnoses made before 24 + 0 weeks’ were considered false‐positive results for the second‐trimester scan.
Presentation of data
For each analysis, we used Review Manager (RevMan 2024) to generate coupled forest plots of sensitivity and specificity, along with their 95% confidence intervals (CIs). From studies that provided insufficient data to construct full 2 x 2 tables (missing false‐positive rates), we presented data for sensitivity in the forest plots. Because specificity was near 100% across all studies that provided data for estimation of specificity, we decided to present results only as forest plots and did not generate Summary Receiver Operating Characteristic (SROC) plots.
Comparison between tests
We compared the overall screening performance of single‐ and two‐stage screening approaches with regard to overall accuracy by the number of fetuses affected, as well as by the number of anomalies detected. We also compared the accuracy between single‐ and two‐stage screening approaches in detecting the subgroup of anomalies that are less susceptible to under‐reporting due to incomplete postnatal identification (i.e. anomalies that are externally visible, present with clinically relevant symptoms at or shortly after birth, or that are considered lethal) to eliminate potential differences in postnatal identification rates as a confounding factor. Comparative meta‐analyses were done by adding a covariate for the test type to the univariate random‐effects meta‐regression models.
Investigations of heterogeneity
We investigated potential sources of heterogeneity relating to the main outcome (overall detection of fetuses affected by structural abnormalities by ultrasound screening). Based on previous systematic reviews, we expected to find extensive heterogeneity in the sensitivity of prenatal ultrasound. We predefined factors that we expected to lead to heterogeneity between studies. If sufficient data were available for meta‐regression, we fitted each factor as a covariate in a hierarchical model to identify factors associated with the diagnostic accuracy of ultrasound screening for overall detection of fetuses affected by structural anomalies for each index test.
We investigated the effects of the following:
Study period (defined by the year of study completion). We investigated the impact of the study period on the accuracy of ultrasound screening by adding the year in which the study period ended as a continuous variable to the meta‐regression model. If the study period was not reported, we assumed the end of the study period to be one year before publication of the study. To facilitate interpretation of the meta‐regression results, instead of only presenting the beta‐coefficient or the estimated change in sensitivity per year, we presented the results of the meta‐regression analysis as the estimated sensitivity for three time points: 1999, 2009, 2019 for the first‐trimester scan and the first + second‐trimester scan, and 1997, 2007 and 2017 for the single second‐trimester scan. These time points were chosen to cover most of the time range covered by the included studies.
Completeness of postnatal identification of structural anomalies. Reports of accuracy of ultrasound screening depend on the thoroughness of postnatal identification, which may not always be complete. In particular, anomalies that are not externally visible, such as cardiac septal defects, are prone to under‐reporting. Therefore, we used this number as a means of identifying reports where poor postnatal identification of anomalies may have occurred, resulting in potential overestimation of sensitivity of ultrasound screening (Bricker 2000; Crane 1994). Under the assumption that isolated ventricular septal defects (VSDs) have a comparable true prevalence in most cohorts, we compared the estimated accuracy between studies with a reported VSD prevalence at or above 1.7 per 1000, and those below. We chose to set the threshold slightly lower than the expected value of about 3 per 1000 (Crane 1994; Bricker 2000; van der Linde 2011) to allow some chance variation in the prevalence of VSDs because several studies had a population of around 1000 participants. We selected the threshold based on the third percentile of reported VSD prevalence amongst the studies included for each of the tests. We then selected the lowest value to use as the threshold for all tests to allow for better comparison between them.
The level of detail of the anatomical screening protocol, excluding evaluation of the heart. We classified the screening protocol as “basic” or “detailed”, following the classification proposed by Karim 2017.
The minimum requirements for cardiac screening, specifically, as stated in the anatomical screening protocol. We classified cardiac screening as “basic” if the four‐chamber view was the only cardiac screening view that was mandatory to obtain during the examination. If it was mandatory to obtain additional cardiac screening views, which, at a minimum, included evaluation of the cardiac outflow tracts, we classified cardiac screening as “extended” cardiac screening.
The income level of the country where the study was conducted. We classified countries as high‐ or low‐income based on the World Bank income classification. We included upper‐middle‐income countries in the high‐income group and lower‐middle‐income countries in the low‐income group.
Sensitivity analyses
We performed sensitivity analyses to evaluate the effect of excluding studies with methodological concerns on the summary estimates of test accuracy. We performed the sensitivity analyses on studies included for the main outcome of each index test (overall accuracy in detecting fetal structural anomalies by the number of fetuses affected).
We investigated the effect of excluding studies with the following characteristics:
Studies with “high” or “unclear” applicability concerns according to the modified QUADAS‐2 assessment tool.
Studies at “high” or “unclear” risk of bias according to the modified QUADAS‐2 assessment tool.
Studies that reported incomplete 2 x 2 tables of true‐positive, false‐positive, false‐negative and true‐negative test results.
Studies with fewer than 10 fetuses with structural anomalies in the studied cohort.
Studies with a loss to follow‐up rate of 10% or more.
Studies that used data obtained from birth defect registries.
Assessment of reporting bias
We did not include a formal assessment of reporting bias in our review due to the existing controversy regarding the assessment and interpretation of reporting bias in test accuracy reviews, especially in the presence of heterogeneity (Deeks 2005; Macaskill 2010).
Assessment of the certainty of the evidence
Two review authors (MFJB and BBB) independently evaluated the overall certainty of evidence using the GRADE approach as outlined in the GRADE handbook. We evaluated the certainty of the body of evidence based on assessment of four domains (risk of bias, inconsistency, indirectness and imprecision) relating to the following outcomes:
Overall detection of fetal structural anomalies (we distinguished between sensitivity and specificity as defined by the number of fetuses affected and by the number of anomalies).
Anomalies that are likely to be identified before postnatal discharge.
Major anomalies overall (we distinguished between lethal anomalies and all other anomalies considered major).
Minor anomalies.
We downgraded for risk of bias by one (‐1) or two points (‐2) if a majority of studies in the analysis had high risk of bias in one or more domains based on the assessment of methodological quality using the modified QUADAS‐2 tool (Appendix 4).
For evaluation of inconsistency, we visually inspected the spread of the estimates of sensitivity and specificity of studies, taking into account possible chance variation when studies had only a few cases. We downgraded by one point (‐1) if there were some important outliers. We downgraded for inconsistency by two points (‐2) if there was no overlap in estimates of accuracy between studies.
For non‐comparative outcomes, we downgraded for indirectness of the evidence if there were concerns regarding the applicability of included studies to our review questions in one (‐1) or more (‐2) of the QUADAS‐2 domains. For grading of indirectness in the comparison of accuracy of single‐ and two‐stage screening approaches, we downgraded for indirect comparison.
We downgraded for imprecision by one point (‐1) for a width of 10.0% to 19.9% on either side of the summary estimate. If the width of the confidence interval exceeded 20.0% on either side, we downgraded the certainty of evidence level by two points (‐2).
Results
Results of the search
We found a total of 52,912 publications through our electronic searches from January 1997 to July 2022 (see PRISMA study flow diagram in Figure 2). A total of 15,323 papers were identified through MEDLINE, 15,279 through Embase and 22,310 through Web of Science. After removal of duplicates, 32,840 titles and abstracts were screened by a total of 13 review authors. Of the 32,840 records, 31,406 were considered irrelevant to our review question. We sought to retrieve 1434 full‐text reports, of which 1403 were retrieved and 31 were unavailable. Two review authors independently assessed the eligibility of the 1403 remaining studies. After resolving disagreements between review authors and contacting the authors of potentially eligible studies for additional information, we included 87 studies. Of the 1316 reports that were not included in the review, 26 were excluded during data extraction. The reasons for excluding these reports are detailed in the Characteristics of excluded studies table. This table also offers explanations for excluding studies considered ineligible after contact with study authors was attempted to obtain crucial missing data, or upon identifying overlap in study cohorts. In addition, this table specifies the reasons for excluding studies that were included in a prior systematic review on the same topic but did not meet the criteria for inclusion in the present one. No additional relevant studies were identified through cross‐checking of reference lists and citing articles of identified relevant papers, including published systematic reviews, using Web of Science. Two studies presented data for multiple cohorts of pregnant women, from which we extracted 12 (Boyd 2000‐1; Boyd 2000‐2; Boyd 2000‐3; Boyd 2000‐4; Boyd 2000‐5; Boyd 2000‐6; Boyd 2000‐7; Boyd 2000‐8; Boyd 2000‐9; Boyd 2000‐10; Boyd 2000‐11; Boyd 2000‐12) and four (Yu 2011‐1; Yu 2011‐2; Yu 2011‐3; Yu 2011‐4) reports, respectively. With the additional reports extracted from these two studies, a total 101 reports were included in the meta‐analysis of 7,057,859 fetuses (including 25,202 fetuses with structural anomalies) screened by a first and/or second trimester fetal anomaly scan.
2.
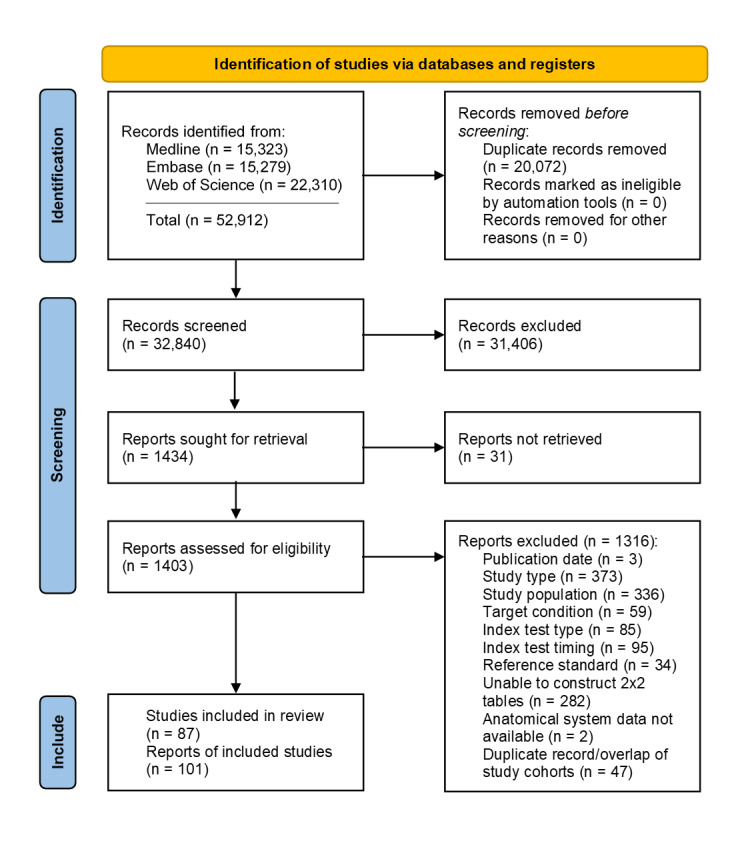
PRISMA flow diagram for selection of studies from January 1997 to July 2022.
Main characteristics of included studies
Characteristics of the included studies are described in the Characteristics of included studies table.
Study population
Seventy‐nine studies (90.8%) enrolled unselected pregnant women (Abu‐Rustum 2010; Andrew 2015; Bardi 2022; Becker 2012; Bister 2011; Biswas 2013; Bodin 2018; Boyd 2000‐1; Boyd 2000‐2; Boyd 2000‐3; Boyd 2000‐4; Boyd 2000‐5; Boyd 2000‐6; Boyd 2000‐7; Boyd 2000‐8; Boyd 2000‐9; Boyd 2000‐10; Boyd 2000‐11; Boyd 2000‐12; Boyd 2011; Brown 2021; Carvalho 2002; Chelli 2009; Choudry 2009; Dane 2007; de Robertis 2017; Drukker 2020; Drysdale 2002; Ebrashy 2019; Erenel 2019; Fleurke‐Rozema 2014; Fleurke‐Rozema 2016; Fleurke‐Rozema 2017; Galindo 2011; Gallot 2007; Gireada 2022; Gornall 2003; Grande 2012; Grandjean 1999; Gu 2011; Hafner 2003; Hartge 2011; Hautala 2019; He 2016; Hernadi 1997; Hildebrand 2010; Iliescu 2013; Ingale 2017; Jacobsen 2011; Johnson 1997; Kenkhuis 2018; Krapp 2011; Lee 1998; Leiroz 2021; Li 2008; Liao 2021; McAuliffe 2005; Novotna 2012; Orlandi 2014; Oztekin 2009; Petousis 2020; Pilalis 2012; Rydberg 2017; Sainz 2020; Saltvedt 2006; Salvador 2005; Sepulveda 2013; Smrcek 2006; Souka 2006; Srisupundit 2006; Stefos 1999; Syngelaki 2011; Syngelaki 2019; Taipale 2004; Takita 2016; Tiechl 2021; Timbolschi 2015; Tudorache 2016; Tydeman 2013; van Velzen 2016; Vayna 2018; Vellamkondu 2017; Vimpelli 2006; Waern 2021; Wang 2013; Wayne 2002; Whitlow 1999; Wu 2009; Zhang 2021; Zheng 2014). Three studies (3.4%) only included pregnant women without risk factors for fetal abnormalities (Chen 2009; Del Bianco 2006; Ogge 2006) and another three studies (3.4%) reported data for low‐risk and high‐risk pregnant women separately (Maarse 2011; Natu 2014; Yu 2011‐1; Yu 2011‐2; Yu 2011‐3; Yu 2011‐4). From these studies, we only included the low‐risk cohort and considered the study population as low‐risk for the purpose of the review. Two studies (2.3%) did not specify the type of study population that was used (Batra 2014; Zhang 2020).
Study design
Thirty‐five studies (40.2%) were prospective observational studies (Batra 2014; Becker 2012; Biswas 2013; Carvalho 2002; Chelli 2009; de Robertis 2017; Drysdale 2002; Ebrashy 2019; Erenel 2019; Grandjean 1999; Hernadi 1997; Iliescu 2013; Kenkhuis 2018; Leiroz 2021; Maarse 2011; McAuliffe 2005; Natu 2014; Novotna 2012; Ogge 2006; Orlandi 2014; Petousis 2020; Sainz 2020; Souka 2006; Srisupundit 2006; Stefos 1999; Taipale 2004; Takita 2016; Tiechl 2021; Tudorache 2016; Vellamkondu 2017; Wang 2013; Wayne 2002; Whitlow 1999; Wu 2009; Yu 2011‐1; Yu 2011‐2; Yu 2011‐3; Yu 2011‐4), 37 (42.5%) were retrospective observational studies (Abu‐Rustum 2010; Andrew 2015; Bister 2011; Brown 2021; Chen 2009; Choudry 2009; Dane 2007; Dane 2007; Drukker 2020; Galindo 2011; Gireada 2022; Grande 2012; Gu 2011; Hafner 2003; Hartge 2011; He 2016; Hildebrand 2010; Ingale 2017; Jacobsen 2011; Johnson 1997; Krapp 2011; Lee 1998; Li 2008; Liao 2021; Oztekin 2009; Pilalis 2012; Rydberg 2017; Sepulveda 2013; Smrcek 2006; Syngelaki 2011; Syngelaki 2019; Tydeman 2013; van Velzen 2016; Vayna 2018; Waern 2021; Zhang 2020; Zheng 2014), 13 (14.9%) were population‐based retrospective cohort studies using data obtained from congenital anomaly registers (Bardi 2022; Bodin 2018; Boyd 2000‐1; Boyd 2000‐2; Boyd 2000‐3; Boyd 2000‐4; Boyd 2000‐5; Boyd 2000‐6; Boyd 2000‐7; Boyd 2000‐8; Boyd 2000‐9; Boyd 2000‐10; Boyd 2000‐11; Boyd 2000‐12; Boyd 2011; Fleurke‐Rozema 2014; Fleurke‐Rozema 2016; Fleurke‐Rozema 2017; Gallot 2007; Gornall 2003; Hautala 2019; Salvador 2005; Timbolschi 2015; Zhang 2021), one study (1.2%) was described as a cross‐sectional study (Vimpelli 2006) and one study (1.2%) involved a randomised controlled trial comparing the effectiveness of a single routine scan at 11 to 14 weeks’ gestation to a single scan at 15 to 22 weeks’ gestation (Saltvedt 2006).
Study setting
Fifty‐eight studies (66.7%) were single‐centre studies. Of these, 49 (84.5%) were performed at a tertiary care facility (Andrew 2015; Becker 2012; Bister 2011; Biswas 2013; Brown 2021; Carvalho 2002; Chelli 2009; Chen 2009; Choudry 2009; Dane 2007; de Robertis 2017; Del Bianco 2006; Ebrashy 2019; Erenel 2019; Grande 2012; Gu 2011; Hafner 2003; Hartge 2011; He 2016; Ingale 2017; Jacobsen 2011; Krapp 2011; Liao 2021; Lee 1998; Leiroz 2021; Li 2008; McAuliffe 2005; Natu 2014; Novotna 2012; Orlandi 2014; Oztekin 2009; Petousis 2020; Pilalis 2012; Sainz 2020; Sepulveda 2013; Smrcek 2006; Srisupundit 2006; Stefos 1999; Takita 2016; Tudorache 2016; Vayna 2018; Vellamkondu 2017; Vimpelli 2006; Wang 2013; Wayne 2002; Whitlow 1999; Wu 2009; Yu 2011‐1; Yu 2011‐2; Yu 2011‐3; Yu 2011‐4; Zheng 2014), six (10.3%) at a secondary care facility (Drysdale 2002; Hernadi 1997; Rydberg 2017; Souka 2006; Taipale 2004; Tydeman 2013) and three (5.2%) at a private hospital or clinic (Abu‐Rustum 2010; Batra 2014; Gireada 2022). None of the single‐centre studies were performed at a primary care facility.
The remaining 29 studies (33.3%) used data obtained from multiple centres. Of these, 16 (55.2%) were multicentre studies (Drukker 2020; Galindo 2011; Grandjean 1999; Hildebrand 2010; Iliescu 2013; Johnson 1997; Kenkhuis 2018; Maarse 2011; Ogge 2006; Saltvedt 2006; Syngelaki 2011; Syngelaki 2019; Tiechl 2021; van Velzen 2016; Waern 2021; Zhang 2020). The number of participating centres ranged from two (Drukker 2020; Iliescu 2013; Syngelaki 2011; Syngelaki 2019; Tiechl 2021; Zhang 2020) to 61 (Grandjean 1999). The remaining 13 studies (44.8%) used data obtained from regional or national population‐based birth registers that received data from all facilities offering prenatal ultrasound in the covered region (Bardi 2022; Bodin 2018; Boyd 2000‐1; Boyd 2000‐2; Boyd 2000‐3; Boyd 2000‐4; Boyd 2000‐5; Boyd 2000‐6; Boyd 2000‐7; Boyd 2000‐8; Boyd 2000‐9; Boyd 2000‐10; Boyd 2000‐11; Boyd 2000‐12; Boyd 2011; Fleurke‐Rozema 2014; Fleurke‐Rozema 2016; Fleurke‐Rozema 2017; Gallot 2007; Gornall 2003; Hautala 2019; Salvador 2005; Timbolschi 2015; Zhang 2021).
Methodological quality of included studies
Risk of bias
Figure 3, Figure 4 and Figure 5 show the risk of bias and applicability concerns for each study included for the analysis of fetal structural anomalies detected by a first‐trimester scan, a first + second‐trimester scan combined, or a single second‐trimester scan, respectively. Figure 6 summarises the quality assessment results of all studies. No study was considered as being at low risk of bias across all four domains (Figure 3; Figure 4; Figure 5).
3.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each study included in the meta‐analyses of test accuracy of a first‐trimester scan.
4.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each study included in the meta‐analyses of test accuracy of a first + second‐trimester scan combined in a two‐stage screening approach.
5.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each study included in the meta‐analyses of test accuracy of a single second‐trimester scan in a single‐stage screening approach.
6.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented for the 101 reports that were extracted from 87 included studies. Multiple reports were extracted from Boyd 2000 (n = 12) and Yu 2011 (n = 4), both of which reported on the accuracy of a single second‐trimester scan.
Patient selection
In the patient selection domain, we considered 75 studies (86.2%) as being at low risk of bias. We considered five studies (5.7%) as being at high risk of bias, which arose from inappropriate exclusions in four. The exclusions were mainly due to an inability to visualise the anatomical structures of interest during the examination. We judged one study as being at high risk of bias for the patient selection domain due to the use of a convenience sample. The remaining seven studies (8.1%) had an unclear risk of bias (Figure 6).
Index test
In the index test domain, we considered the risk of bias to be low in all studies, independent of which index test was assessed (Figure 6).
Reference standard
All studies used a reference standard likely to correctly identify anomalies that are externally visible, considered to be lethal, or that are present with clinically relevant symptoms shortly after birth. Thirty‐two studies (36.8%) used a reference standard likely to correctly classify anomalies that may present after hospital discharge and we judged them as being at low risk of bias. Another 31 studies (35.6%) used a reference standard that would be unable to correctly classify such anomalies. We considered these studies at high risk of bias. In the remaining 24 studies (27.6%) the follow‐up duration was not specified, and we judged the risk of bias as unclear (Figure 6).
Flow and timing
We considered 22 studies (25.3%) to be at high risk of bias because of a missing reference standard in the cases of pregnancy termination for a prenatally diagnosed fetal abnormality or following spontaneous fetal demise after the index test. We considered five studies (5.7%) at low risk of bias. We judged the remaining 60 studies (69.0%) as at unclear risk of bias (Figure 6). In 53 of the 60 studies judged as at unclear risk of bias, information on performance of a reference standard in all cases of fetal demise was missing. In seven studies, we judged the risk of bias as unclear due to either insufficient information on patient flow or a number lost to follow‐up of 5% to 20%. We considered numbers lost to follow‐up of 5% or less as most likely leading to little bias, whereas we considered studies with > 20% lost to follow‐up as being at high risk of bias, and we deemed studies with a number lost to follow‐up of 5% to 20% to be at unclear risk of bias.
Applicability concerns
Patient selection
We considered a study cohort representative of the general population if the study was conducted in a primary or secondary care facility, if the study evaluated the accuracy of ultrasound screening across an entire geographic region, if the study only included low‐risk pregnancies or if referrals from other centres were excluded from a study conducted in a tertiary care facility. Concerns regarding applicability arose most frequently in the patient selection domain (Figure 6). Thirty‐eight studies (43.7%) had high applicability concerns because they included unselected pregnant women recruited from a single tertiary care facility where referrals from other centres for tertiary level care were not excluded from the cohort. This raised concerns about whether the cohort of pregnant women used in these studies was representative of the general population. In 43 studies (49.4%), we considered the applicability concerns low. In the remaining six studies (6.9%), it was unclear if the studied cohort was representative of the general population and matched our review question. In four studies, unselected pregnant women were recruited from a private care facility (Abu‐Rustum 2010; Batra 2014; Gireada 2022; Pilalis 2012). The remaining two were multicentre studies (Johnson 1997; Kenkhuis 2018). More than half of the cohort in one of these studies consisted of unselected pregnant women who were screened in a tertiary level care facility on self‐referral (Johnson 1997). In the other study, unselected pregnant women who opted for the combined test or who had an increased a priori risk of fetal anomalies were offered a first‐trimester fetal anomaly scan (Kenkhuis 2018).
Index tests
A total of 51 studies evaluated the accuracy of a first‐trimester scan, of which 20 studies evaluated detection of all fetal anomalies, and 31 studies of selected conditions. We judged 39 studies (76.5%) to be of low applicability concern in the index test domain as they matched our review question. In nine studies (17.6%) we rated the applicability concern as high (Figure 6). In five of these studies, this judgement was due to a single highly experienced operator performing all scans (Abu‐Rustum 2010; Becker 2012; Krapp 2011; Oztekin 2009; Vimpelli 2006). Two studies did not include evaluation of the heart in the routine first‐trimester examination (Hernadi 1997; Pilalis 2012). In one study, the first‐trimester scan most likely only included NT measurement (Drysdale 2002). Lastly, one study prospectively evaluated the accuracy of measurements of the brainstem diameter and brainstem‐to‐occipital‐bone distance in detecting open spina bifida, which were performed in addition to the routine first‐trimester scan (Tiechl 2021). In the remaining three studies (5.9%), we considered applicability concerns to be unclear. In two studies, it was unclear if cardiac evaluation was performed in all screened fetuses or only in those with an increased NT or other risk factors (Carvalho 2002; Novotna 2012). In one study that evaluated the accuracy of first trimester ultrasound in detecting fetal cardiac anomalies, the heart was targeted before other segments of fetal anatomy and sonographers completed a questionnaire on the cardiac examination during the ultrasound scan (Tudorache 2016). We considered the impact on the conduct and the interpretation of the scan to be unclear.
Forty‐two studies assessed the accuracy of a first + second‐trimester scan combined. Among the 42 studies, 18 evaluated accuracy for all fetal anomalies and 24 for selected conditions. Thirty‐eight of the 42 studies (90.5%) evaluating the accuracy of a first + second‐trimester scan matched our review question and were considered to be of low applicability concern. We considered four studies (9.5%) to be of high applicability concern (Figure 6). In three of these, both the first and second‐trimester examinations were performed by a single operator (Abu‐Rustum 2010; Becker 2012; Krapp 2011). In one study, there was a high suspicion that the first‐trimester scan only involved NT evaluation and not a complete anatomy survey in all cases (Drysdale 2002).
Thirty‐six studies reported on the accuracy of a single second‐trimester scan, nine of which evaluated the accuracy of detection of all fetal anomalies, and 27 for selected conditions. Thirty‐two of the 36 studies (88.9%) matched our review question. In one study (2.8%), applicability concerns were high due to a single operator performing all scans (Del Bianco 2006). In the remaining four studies (11.1%), we judged applicability concerns as unclear (Figure 6). In two multicentre studies, not all centres participating in the study routinely examined the fetal heart (Galindo 2011; Hildebrand 2010). In one study, it was unclear whether a single‐ or two‐stage screening strategy was applied according to our definitions. This uncertainty arose because a first‐trimester scan was performed, but its purpose and protocol were not specified, and no data were reported for the first‐trimester scan (Zhang 2021). The authors were contacted to clarify the nature and purpose of the first‐trimester scan, but did not respond. Given that only detection at the second‐trimester scan was provided, the study was included for the analysis of a single second‐trimester scan, but as the first‐trimester scan may have included a complete fetal anatomy survey, we considered the study as being of unclear applicability concern.
Reference standard
In the domain of the reference standard, all studies matched our review question, and we considered them to be of low applicability concern.
Findings
Among the 87 studies that were included in the review, 31 reported on overall detection of fetal anomalies by a first‐ and/or second‐trimester scan and were included for the analyses of overall detection of fetal anomalies by the relevant tests (Abu‐Rustum 2010; Andrew 2015; Biswas 2013; Brown 2021; Carvalho 2002; Chelli 2009; Chen 2009; Drukker 2020; Drysdale 2002; Grande 2012; Gu 2011; Iliescu 2013; Ingale 2017; Jacobsen 2011; Kenkhuis 2018; Liao 2021; Leiroz 2021; McAuliffe 2005; Natu 2014; Novotna 2012; Rydberg 2017; Salvador 2005; Souka 2006; Srisupundit 2006; Stefos 1999; Syngelaki 2011; Syngelaki 2019; Takita 2016; Vellamkondu 2017; Wang 2013; Whitlow 1999).
The remaining 56 studies provided data on selected anomalies (e.g. major fetal structural anomalies, neural tube defects, cardiac anomalies, etc.). These studies were included in the meta‐analyses of only those conditions or subgroups of anomalies for which data were provided (Bardi 2022; Batra 2014; Becker 2012; Bister 2011; Bodin 2018; Boyd 2000‐1; Boyd 2000‐2; Boyd 2000‐3; Boyd 2000‐4; Boyd 2000‐5; Boyd 2000‐6; Boyd 2000‐7; Boyd 2000‐8; Boyd 2000‐9; Boyd 2000‐10; Boyd 2000‐11; Boyd 2000‐12; Boyd 2011; Choudry 2009; Dane 2007; Del Bianco 2006; de Robertis 2017; Ebrashy 2019; Erenel 2019; Fleurke‐Rozema 2014; Fleurke‐Rozema 2016; Fleurke‐Rozema 2017; Galindo 2011; Gallot 2007; Gireada 2022; Gornall 2003; Grandjean 1999; Hafner 2003; Hartge 2011; Hautala 2019; He 2016; Hernadi 1997; Hildebrand 2010; Johnson 1997; Krapp 2011; Lee 1998; Li 2008; Maarse 2011; Ogge 2006; Orlandi 2014; Oztekin 2009; Petousis 2020; Pilalis 2012; Sainz 2020; Saltvedt 2006; Sepulveda 2013; Smrcek 2006; Taipale 2004; Tiechl 2021; Timbolschi 2015; Tudorache 2016; Tydeman 2013; van Velzen 2016; Vayna 2018; Vimpelli 2006; Waern 2021; Wayne 2002; Wu 2009; Yu 2011‐1; Yu 2011‐2; Yu 2011‐3; Yu 2011‐4; Zhang 2020; Zhang 2021; Zheng 2014).
Results are presented for detection of fetal structural anomalies overall (Table 1; Table 2; Table 3), for subgroups of anomalies of varying severity (Table 1; Table 2; Table 3), for individual organ systems (Figure 6; Figure 7; Figure 8; Table 4), and for 46 selected structural anomalies individually (Table 4). Results of comparative accuracy of single‐ and two‐stage screening approaches are summarised in Table 5.
7.
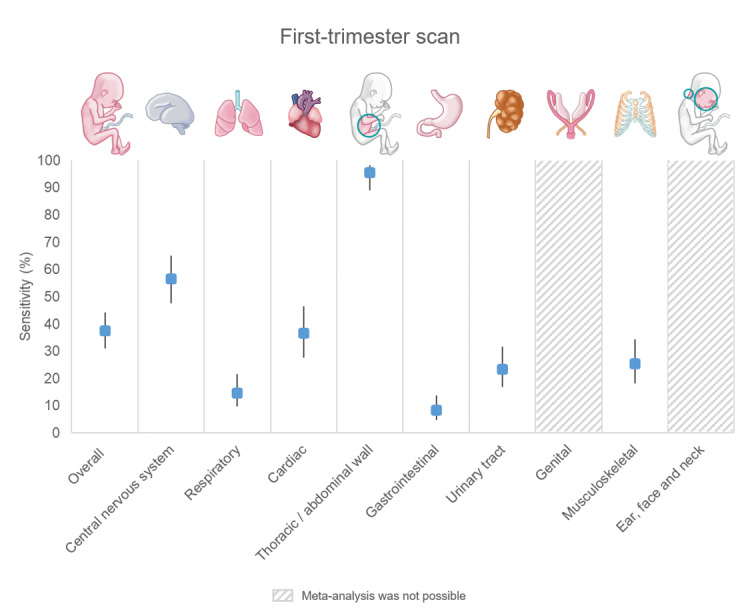
Sensitivity of a first‐trimester scan in detecting fetal structural anomalies overall, and within individual organ systems, before 14 weeks' gestation. The width of the 95% confidence intervals is indicated by the vertical black lines. Meta‐analysis of sensitivity was not possible for genital and ear, face and neck anomalies due to a lack of variation between studies. Sensitivity of a first‐trimester scan in detecting genital anomalies could be derived from eight studies and ranged from 0.0% to 0.0%. Sensitivity of a first‐trimester scan in detecting ear, face and neck anomalies could be derived from 16 studies and ranged from 0.0% (14 studies) to 50.0% (two studies).
8.
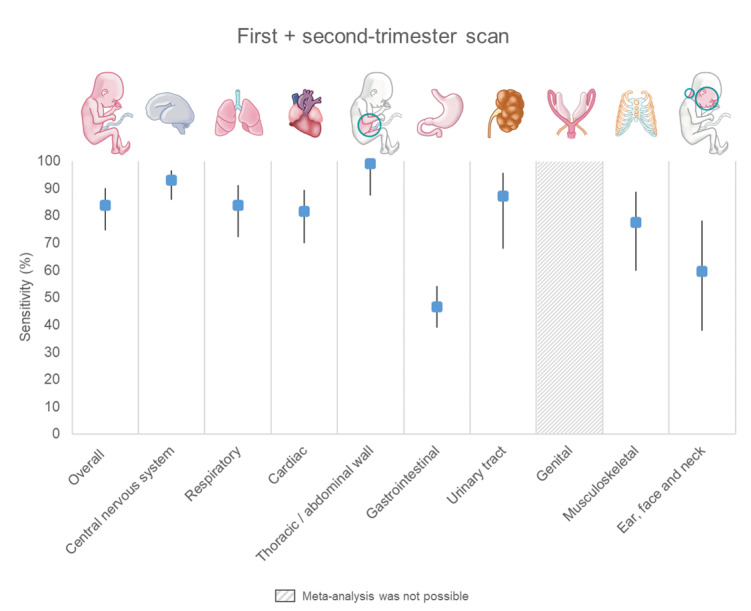
Sensitivity of a first + second‐trimester combined scan in detecting fetal structural anomalies overall, and within individual organ systems, before 24 weeks' gestation. The width of the 95% confidence intervals is indicated by the vertical black lines. Meta‐analysis of sensitivity was not possible for genital anomalies due to a lack of variation between studies. Sensitivity of a first + second‐trimester scan combined in detecting genital anomalies could be derived from six studies and ranged from 0.0% to 8.1%.
We did not determine the specificity of ultrasound in detecting anomalies in individual organ systems or of the 46 selected anomalies because all tests had near‐perfect specificity in the analyses of overall accuracy, as well as in the subgroups of anomalies based on their severity (Table 1; Table 2; Table 3).
1. Two‐stage screening approach
1.1. First‐trimester scan
1.1.1. Overall sensitivity of ultrasound in detecting fetal structural anomalies
Twenty studies reported on the overall sensitivity of a first‐trimester scan by the number of fetuses affected (Abu‐Rustum 2010; Andrew 2015; Carvalho 2002; Chelli 2009; Drysdale 2002; Grande 2012; Gu 2011; Iliescu 2013; Kenkhuis 2018; McAuliffe 2005; Natu 2014; Novotna 2012; Souka 2006; Srisupundit 2006; Syngelaki 2011; Syngelaki 2019; Takita 2016; Vellamkondu 2017; Wang 2013; Whitlow 1999). These included 195,264 fetuses, of whom 3293 had one or more structural anomalies. The median prevalence of fetuses with one or more structural anomalies was 1.8% (interquartile range (IQR) 1.0 to 2.6). The summary sensitivity was 37.5% (95% confidence interval (CI) 31.1 to 44.3; low‐certainty evidence, downgraded due to risk of bias and indirectness; Table 1).
The sensitivity based on the number of anomalies was derived from 20 studies, reporting on 246,130 fetuses, among whom there were 5417 structural anomalies (Abu‐Rustum 2010; Andrew 2015; Brown 2021; Carvalho 2002; Chelli 2009; Drysdale 2002; Grande 2012; Gu 2011; Iliescu 2013; Liao 2021; McAuliffe 2005; Novotna 2012; Souka 2006; Srisupundit 2006; Syngelaki 2011; Syngelaki 2019; Takita 2016; Vellamkondu 2017; Wang 2013; Whitlow 1999). The median prevalence of structural anomalies was 2.0% (IQR 1.4 to 3.4). The summary sensitivity was 39.2% (95% CI 32.5 to 46.2; low‐certainty evidence, downgraded due to risk of bias and indirectness – Table 1).
1.1.2. Overall specificity of ultrasound in detecting fetal structural anomalies
It was possible to estimate specificity for 10 studies (Abu‐Rustum 2010; Drysdale 2002; Gu 2011; Kenkhuis 2018; McAuliffe 2005; Natu 2014; Souka 2006; Takita 2016; Wang 2013; Whitlow 1999). These included 23,037 fetuses, of whom 341 had one or more known structural anomalies (median prevalence: 1.7%, IQR 0.8 to 2.0). The specificity was estimated at 99.9% (95% CI 99.9 to 100; low‐certainty evidence, downgraded due to risk of bias and indirectness; Table 1).
1.1.3. Subgroup analysis of anomalies that are externally visible, symptomatic at birth, or considered to be lethal
Among the 195,264 fetuses that were included in the meta‐analysis of overall sensitivity of a first‐trimester scan, 1668 had a structural anomaly that was externally visible, expected to be symptomatic at or shortly after birth, or that was considered lethal. We judged the reference standards used by all studies (i.e. a minimum of postnatal physical examination and postnatal follow‐up until hospital discharge) to be able to correctly classify the anomalies included in this subgroup as being present or absent. Consequently, we expected that the estimated accuracy of ultrasound in detecting this subgroup of anomalies would be less susceptible to differences in postnatal identification rates between studies. For this subgroup of anomalies, the sensitivity was estimated at 52.8% (95% CI 45.7 to 59.8; moderate‐certainty evidence, downgraded due to indirectness). The specificity was estimated at 100% (95% CI 99.9 to 100; 23,037 fetuses, 173 with the target condition; low certainty‐evidence, downgraded due to risk of bias and indirectness; Table 1).
1.1.4. Detection of major structural anomalies
Thirty‐three studies reported data on detection of major fetal structural anomalies by a first‐trimester scan. These included 303,319 fetuses, of whom 5449 had one or more structural anomalies. The summary sensitivity of a first‐trimester scan in detecting major fetal structural anomalies overall was 45.6 (95% CI 37.1 to 52.3; very low‐certainty evidence, downgraded due to risk of bias, indirectness and inconsistency). The sensitivity in detecting lethal anomalies was estimated at 91.3% (95% CI 83.9 to 95.5; moderate‐certainty evidence, downgraded due to indirectness), whereas that of detecting severe and moderate anomalies was estimated at 37.7% (95% CI 30.6 to 45.3; very low‐certainty evidence, downgraded due to risk of bias, indirectness and inconsistency; Table 1).
1.1.5. Detection of minor structural anomalies
Fifteen studies were included for the analysis of sensitivity of a first‐trimester scan in detecting minor structural anomalies. These included 236,383 fetuses, of whom 550 had minor structural anomalies. The sensitivity was estimated at 4.3% (95% CI 1.0 to 16.5; very low‐certainty evidence, downgraded due to risk of bias, indirectness and imprecision; Table 1).
1.1.6. Organ system‐specific detection of selected structural anomalies
Fifty‐one studies were included for one or more meta‐analyses of organ system‐specific detection of selected structural anomalies by a first‐trimester scan. Results for sensitivity are summarised in Figure 7 and Table 4.
The sensitivity of a first‐trimester scan varied considerably between different organ systems (Figure 7). The summary estimates of sensitivity (95% CI) were: central nervous system, 56.6% (47.6 to 65.2); respiratory system, 14.6% (9.7 to 21.6); cardiac, 36.7% (27.7 to 46.6); thoracic and abdominal wall, 95.6% (89.1 to 98.3); gastrointestinal tract, 8.3% (4.9 to 13.8); urinary tract, 23.5% (17.0 to 31.6); musculoskeletal, 25.4% (18.2 to 34.3). Sensitivity of a first‐trimester scan in detecting multisystem anomalies was 69.6% (59.0 to 78.5). Meta‐analysis was not possible for genital and ear, face and neck anomalies due to a lack of variation between studies. The results for sensitivity are presented descriptively in Table 4.
The sensitivity of a first‐trimester scan in detecting gross structural anomalies (such as anencephaly, abdominal wall defects and megacystis), and in detecting individual anomalies that are considered to be lethal, was generally high. The highest detection rates were found for anencephaly, 99.5% (90.9 to 100); holoprosencephaly, 92.4% (71.6 to 98.3); encephalocele, 96.3% (62.8 to 99.8); gastroschisis, 100% (74.8 to 100); and omphalocele, 98.0% (90.4 to 99.6). Exceptions were lethal renal anomalies, such as bilateral renal agenesis and bilateral multicystic dysplastic kidneys (MCDK), which had very low first‐trimester detection rates. The estimated sensitivity of a first‐trimester scan in detecting bilateral renal agenesis was 16.6% (6.5 to 36.2). Meta‐analysis of bilateral MCDK was not possible due to a lack of variation between studies. However, all five studies from which these data could be obtained (149,834 fetuses, of whom 10 had bilateral MCDK) reported an estimated sensitivity of 0.0% (Table 4).
First‐trimester detection of cardiac anomalies varied widely between different types of cardiac anomalies and within individual diagnoses, resulting in wide 95% CIs. The highest summary estimates of sensitivity among cardiac anomalies were found for single ventricle heart defects such as hypoplastic left heart syndrome (HLHS), 79.0% (45.2 to 94.5); hypoplastic right heart syndrome (HRHS), 92.7% (0.0 to 100); and other single ventricle defects, 100% (58.8 to 100). The lowest detecting rates among major cardiac anomalies were found for valvular anomalies, 26.8 (11.6 to 50.5); aortic arch anomalies, 24.8% (16.1 to 36.2); conotruncal anomalies, 34.5% (21.1 to 50.9); and for miscellaneous major cardiac defects; 20.1% (2.1 to 75.1) (Table 4).
1.2. First + second‐trimester scan
1.2.1. Overall sensitivity of ultrasound in detecting fetal structural anomalies
Eighteen studies reported data on overall sensitivity of a first + second‐trimester scan by the number of fetuses affected (Abu‐Rustum 2010; Andrew 2015; Carvalho 2002; Chelli 2009; Drysdale 2002; Grande 2012; Gu 2011; Kenkhuis 2018; McAuliffe 2005; Natu 2014; Souka 2006; Srisupundit 2006; Syngelaki 2011; Syngelaki 2019; Takita 2016; Vellamkondu 2017; Wang 2013; Whitlow 1999). These included 181,614 fetuses, of whom 3051 had one or more structural anomalies (median prevalence: 1.8%, IQR 0.9 to 2.6). The summary sensitivity was 83.8% (95% CI 74.7 to 90.1; low‐certainty evidence, downgraded due to risk of bias and indirectness; Table 2).
The sensitivity based on the number of anomalies was derived from 17 studies, reporting on 229,274 fetuses, among whom there were 4975 structural anomalies (Abu‐Rustum 2010; Andrew 2015; Carvalho 2002; Chelli 2009; Drysdale 2002; Grande 2012; Gu 2011; Liao 2021; McAuliffe 2005; Souka 2006; Srisupundit 2006; Syngelaki 2011; Syngelaki 2019; Takita 2016; Vellamkondu 2017; Wang 2013; Whitlow 1999). The median prevalence of structural anomalies was 2.0% (IQR 1.3 to 3.0). The summary sensitivity was 84.5% (95% CI 75.6 to 90.5; low‐certainty evidence, downgraded due to risk of bias and indirectness; Table 2).
1.2.2. Overall specificity of ultrasound in detecting fetal structural anomalies
It was possible to estimate specificity for 10 studies (Abu‐Rustum 2010; Drysdale 2002; Gu 2011; Kenkhuis 2018; McAuliffe 2005; Natu 2014; Souka 2006; Takita 2016; Wang 2013; Whitlow 1999). These included 23,037 fetuses, of whom 341 had one or more structural anomalies (median prevalence: 1.7%, IQR 0.8 to 2.0). The specificity was estimated at 99.9% (95% CI 99.7 to 100; low‐certainty evidence, downgraded due to risk of bias and indirectness; Table 2).
1.2.3. Subgroup analysis of anomalies that are externally visible, symptomatic at birth, or considered to be lethal
Among the 181,614 fetuses that were included in the meta‐analysis of overall sensitivity of a first + second‐trimester scan combined, 1534 had a structural anomaly that was externally visible, symptomatic at birth, or considered to be lethal. For this subgroup of anomalies, the sensitivity was estimated at 86.7% (CI 78.8 to 92.0; moderate‐certainty evidence, downgraded due to indirectness). The specificity was estimated at 100% (95% CI 99.9 to 100; 10 studies; 23,037 fetuses, 173 of whom had the target condition; low‐certainty evidence, downgraded due to risk of bias; Table 2).
1.2.4. Detection of major structural anomalies
Twenty‐eight studies reported data on detection of major fetal structural anomalies by a first + second‐trimester scan combined. These included 265,132 fetuses, of whom 4816 had one or more structural anomalies. The summary sensitivity of a first‐trimester scan in detecting major fetal structural anomalies overall was 85.2% (95% CI 77.9 to 90.4; low‐certainty evidence, downgraded due to risk of bias and indirectness). The sensitivity in detecting lethal anomalies was estimated at 100% (95% CI 97.4 to 100; moderate‐certainty evidence, downgraded due to indirectness), whereas that of detecting severe and moderate anomalies was estimated at 82.6% (95% CI 74.4 to 88.6; low‐certainty evidence, downgraded due to risk of bias and indirectness; Table 2).
1.2.5. Detection of minor structural anomalies
Twelve studies were included for the analysis of the sensitivity of a first + second‐trimester scan combined in detecting minor structural anomalies. These included 219,512 fetuses, of whom 491 had minor structural anomalies. The sensitivity was estimated at 48.1% (95% CI 20.1 to 77.3; very low‐certainty evidence, downgraded due to risk of bias, indirectness and imprecision; Table 2).
1.2.6. Organ system‐specific detection of selected structural anomalies
Forty‐one studies were included for one or more meta‐analyses of organ system‐specific detection of selected structural anomalies by a first + second‐trimester scan combined. The results are summarised in Figure 8 and Table 4.
The sensitivity of a first + second‐trimester scan combined was relatively high for all organ systems, except for gastrointestinal and ear, face and neck anomalies, which had lower sensitivities (Figure 8). The summary estimates of sensitivity (95% CI) were: central nervous system, 92.9% (85.9 to 96.6); respiratory system, 83.8% (72.2 to 91.2); cardiac, 81.5% (69.8 to 89.4); thoracic and abdominal wall, 99.0% (87.3 to 99.9); gastrointestinal, 46.5% (39.0 to 54.1); urinary tract, 87.1% (67.9 to 95.6); musculoskeletal, 77.5% (59.9 to 88.8); ear, face and neck, 59.6% (37.8 to 78.1). Sensitivity of a first + second‐trimester scan in detecting multisystem anomalies was 94.7% (85.2 to 98.2). Meta‐analysis was not possible for genital anomalies due to a lack of variation between studies (Table 4).
The highest summary estimates of sensitivity (95% CI) were found for spina bifida, 97.9% (90.0 to 96.6); gastroschisis, 100% (99.1 to 100); omphalocele, 99.2% (98.5 to 99.6); cleft lip and palate 100% (81.5 to 100); multiple congenital anomalies (MCA) or syndromes, 96.0% (82.3 to 99.2). A first + second‐trimester scan combined appears to be able to detect most lethal anomalies, including lethal renal anomalies, with near 100% sensitivity, albeit the detection of bilateral renal agenesis varied widely between studies as reflected by the wide 95% CI (57.8 to 100). Meta‐analysis of bilateral MCDK was not possible, but all four studies from which this information could be obtained reported 100% sensitivity in detecting MCDK before 24 weeks’ gestation (Table 4).
Detection of cardiac anomalies by a first + second‐trimester scan combined was generally high. Summary estimates of sensitivity (95% CI) ranged from 69.4% (38.8 to 89.1) for aortic arch anomalies, to over 90% for severe cardiac anomalies such as HLHS, 98.2% (75.8 to 99.9), valvular anomalies, 99.7% (10.5 to 100) and conotruncal anomalies, 90.0% (76.5 to 96.2) (Table 4).
2. Single‐stage screening approach
2.1. Second‐trimester scan
2.1.1. Overall sensitivity of ultrasound in detecting fetal structural anomalies
Nine studies reported data on the overall sensitivity of a single second‐trimester scan by the number of fetuses affected (Biswas 2013; Chen 2009; Drukker 2020; Ingale 2017; Jacobsen 2011; Leiroz 2021; Rydberg 2017; Salvador 2005; Stefos 1999). These included 158,767 fetuses, of whom 3168 had one or more structural anomalies (median prevalence: 2.2%, IQR 1.7 to 3.8). The summary sensitivity was 50.5% (95% CI 38.5 to 62.4; very low‐certainty evidence, downgraded due to risk of bias, inconsistency and imprecision; Table 3).
The sensitivity based on the number of anomalies was derived from three studies, reporting on 17,845 fetuses, among whom there were 340 structural anomalies (Biswas 2013; Chen 2009; Ingale 2017). The median prevalence of structural anomalies was 4.2% (IQR 1.2 to 4.4). The summary sensitivity was 52.2% (95% CI 33.2 to 70.7; very low‐certainty evidence, downgraded due to risk of bias, inconsistency and imprecision; Table 3).
2.1.2. Overall specificity of ultrasound in detecting fetal structural anomalies
Five studies provided complete 2 x 2 tables, allowing for estimation of specificity (Biswas 2013; Chen 2009; Leiroz 2021; Rydberg 2017; Stefos 1999). These included 33,120 fetuses, of whom 593 had one or more structural anomalies (median prevalence: 2.2%, IQR 1.6 to 3.8). The summary specificity was 99.8% (95% CI 99.2 to 100; moderate‐certainty evidence, downgraded due to risk of bias; Table 3).
2.1.3. Subgroup analysis of anomalies that are externally visible, symptomatic at birth, or considered to be lethal
Data on detection of structural anomalies that are externally visible, symptomatic at birth, or considered to be lethal could be derived from seven studies, reporting on 58,014 fetuses (Chen 2009; Drukker 2020; Ingale 2017; Jacobsen 2011; Leiroz 2021; Rydberg 2017; Stefos 1999). Among these, 559 had an anomaly that was eligible for inclusion in this subgroup analysis. The summary sensitivity was 59.2% (95% CI 45.7 to 71.5; low‐certainty evidence, downgraded due to inconsistency and imprecision). The specificity was estimated at 99.9% (4 studies; 32,120 fetuses, among whom 286 had the target condition; 95% CI 99.6 to 100; moderate‐certainty evidence, downgraded due to risk of bias; Table 3).
2.1.4. Detection of major structural anomalies
Nine studies reported data on detection of major fetal structural anomalies by a single second‐trimester scan. These included 75,042 fetuses, of whom 1312 had one or more structural anomalies. The summary sensitivity of a single second‐trimester scan in detecting major fetal structural anomalies overall was 47.9% (95% CI 33.3 to 62.8; very low‐certainty evidence, downgraded due to risk of bias, inconsistency and imprecision). Meta‐analysis of sensitivity in detecting lethal anomalies was not possible, but ranged from 80.0% to 100% among the seven studies from which this information could be obtained. The sensitivity in detecting severe and moderate anomalies was estimated at 48.6% (95% CI 33.0 to 64.5; very low‐certainty evidence, downgraded due to risk of bias, inconsistency and imprecision; Table 3).
2.1.5. Detection of minor structural anomalies
Seven studies were included for the analysis of the sensitivity of a single second‐trimester scan in detecting minor structural anomalies. These included 58,015 fetuses, of which 96 had minor structural anomalies. The sensitivity was estimated at 24.0% (95% CI 6.1 to 60.9; very low‐certainty evidence, downgraded due to risk of bias, inconsistency and imprecision; Table 3).
2.1.6. Organ system‐specific detection of selected structural anomalies
Thirty‐six studies were included for one or more meta‐analyses of organ system‐specific detection of selected structural anomalies by a single second‐trimester scan. The results are summarised in Figure 9 and Table 4.
9.
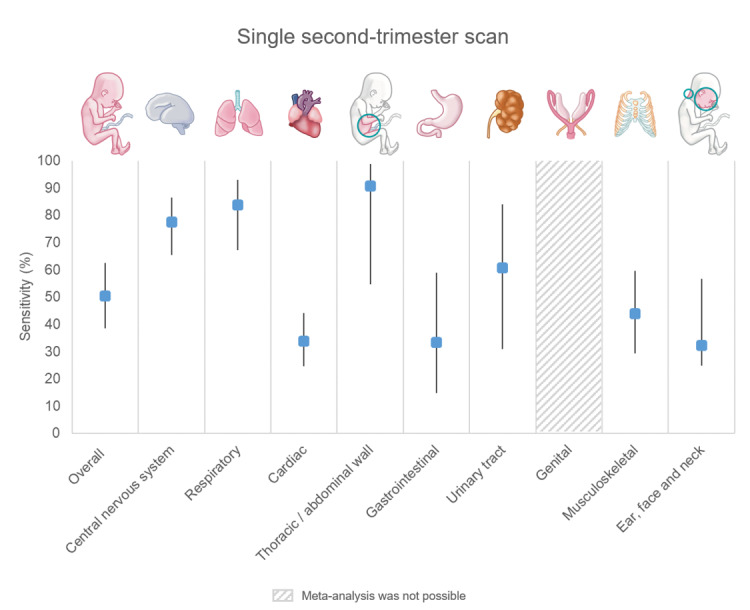
Sensitivity of a single second‐trimester combined scan in detecting fetal structural anomalies overall, and within individual organ systems, before 24 weeks' gestation. The width of the 95% confidence intervals is indicated by the vertical black lines. Meta‐analysis of sensitivity was not possible for genital anomalies due to a lack of variation between studies. Sensitivity of a single second‐trimester scan in detecting genital anomalies could be derived from six studies and ranged from 0.0% (five studies) to 33.3% (one study).
The sensitivity of a single second‐trimester scan varied considerably between organ systems (Figure 9). The summary estimates of sensitivity (95% CI) were: central nervous system, 77.5% (65.5 to 86.2); respiratory system, 83.8% (67.1 to 92.9); cardiac, 33.8% (24.7 to 44.2); thoracic and abdominal wall, 90.8% (54.7 to 98.8); gastrointestinal, 33.3% (14.8 to 59.0); urinary tract, 60.6% (30.9 to 84.0); musculoskeletal, 43.8% (29.3 to 59.5); ear, face and neck, 32.3% (14.8 to 56.7). The sensitivity of a single second‐trimester scan in detecting multisystem anomalies was 40.6% (4.5 to 90.9). Meta‐analysis was not possible for genital anomalies due to a lack of variation between studies (Table 4).
Also, among individual anomalies, summary estimates of sensitivity varied considerably and frequently had wide 95% CIs. Those with summary estimates of sensitivity (95% CI) of 80% or above included anencephaly 95.6% (90.6 to 98.0), encephalocele 81.8% (49.7 to 95.4), HLHS 87.5% (79.1 to 92.8), other single ventricle cardiac defects 85.6% (79.6 to 90.1), bilateral renal agenesis 91.4% (68.9 to 98.1), MCDK overall 82.1% (34.1 to 97.6), and cleft lip and palate 81.9% (77.7 to 85.5) (Table 4).
Anomalies that had summary estimates of sensitivity below 50% included: cardiac septal defects 36.7% (16.0 to 63.9), valvular anomalies 38.6% (30.8 to 47.0), miscellaneous major cardiac anomalies 38.8% (23.3 to 56.9), upper urinary tract obstruction 33.9% (2.3 to 91.8), limb reduction defects 46.3% (24.7 to 96.4), talipes 42.6% (24.0 to 63.5), orofacial clefts overall 44.1% (26.9 to 62.9) and isolated cleft palate 2.9% (0.1 to 53.4). Summary estimates of sensitivity of the remaining anomalies were between 50% and 70%, or meta‐analysis could not be performed due to lack of data, or lack of variation between studies (Table 4).
3. Comparison of single‐ and two‐stage screening approaches
No studies provided direct comparison of a single‐ and a two‐stage screening strategy. An indirect comparison of the nine single second‐trimester scan studies (single‐stage screening approach) and the 18 studies that evaluated a first + second‐trimester scan combined (two‐stage screening approach) showed a significant difference in sensitivity in detection of fetal structural anomalies before 24 weeks’ gestation in favour of a two‐stage screening approach (Table 5). This difference was observed in overall estimates of sensitivity (both by the number of fetuses affected and by the number of anomalies), as well as for the subgroup of anomalies that are assumed to be less susceptible to differences in completeness of postnatal identification between studies (i.e. anomalies that are externally visible, symptomatic at birth, or that are considered to be lethal). The certainty of evidence for the observed differences in sensitivity of ultrasound between single‐ and two‐stage screening approaches was rated as very low for all three measures of sensitivity and was downgraded due to risk of bias, inconsistency and indirectness. There were no differences in specificity between single‐ and two‐stage screening approaches (low‐certainty evidence, downgraded due to risk of bias and indirectness) (Table 5).
4. Investigation of heterogeneity
The first‐trimester scan and first + second‐trimester scan included a sufficient number of studies to allow for investigation of the impact of the study period, completeness of postnatal identification, level of detail of the anatomical screening protocol, cardiac screening specifically and the income level of the country where the study was conducted. For the single second‐trimester scan, sufficient data were only available to evaluate the impact of the study period and completeness of postnatal identification. No heterogeneity was observed in the specificities of all three index tests. Therefore, potential sources of heterogeneity were explored for sensitivity of ultrasound screening only. The results of the exploration of potential sources of heterogeneity in sensitivity are presented in Table 6, Table 7, Table 8 and Figure 10.
1. Impact of potential sources of heterogeneity on the sensitivity of a first‐trimester scan in detecting fetal structural anomalies.
| Impact of potential sources of heterogeneity on the sensitivity of a first‐trimester scan in detecting fetal structural anomalies | ||||
| Covariate | First‐trimester scan | |||
| Sensitivity estimate (95% CI) | P value | |||
| Fetuses affected | Anomalies | Fetuses affected | Anomalies | |
| 1. Study period* | ||||
| 1999 | 31.6 (19.2 to 44.0) | 29.5 (18.1 to 40.9) | ‐ | ‐ |
| 2009 | 37.6 (30.8 to 44.3) | 38.7 (32.2 to 45.2) | ‐ | ‐ |
| 2019 | 44.0 (29.1 to 58.9) | 48.7 (35.7 to 61.8) | ‐ | ‐ |
| Beta | 0.3 (‐0.03 to 0.08) | 0.04 (‐0.01 to 0.09) | 0.311 | 0.08 |
| 2. Reported prevalence of isolated ventricular septal defects | ||||
| ≤ 1.7 per 1000 | 41.0 (32.8 to 48.1) | 41.3 (32.6 to 50.0) | 0.195 | 0.429 |
| < 1.7 per 1000 | 31.9 (22.3 to 41.6) |
35.7 (25.2 to 46.2) | ||
| 3. Anatomical screening protocol | ||||
| Basic | 39.4 (36.2 to 27.5) | 39.7 (27.8 to 51.6) | 0.915 (basic vs detailed) 0.810 (basic vs not reported) |
0.845 (basic vs detailed) 0.896 (basic vs not reported) |
| Detailed | 36.2 (27.5 to 58.3) | 38.2 (28.7 to 47.7) | ||
| Not reported | 36.4 (27.5 to 45.0) | 41.1 (23.7 to 58.5) | ||
| 4. Cardiac screening protocol | ||||
| Basic | 39.5 (30.5 to 48.5) | 39.1 (29.0 to 49.2) | 0.915 (basic vs extended) 0.277 (basic vs not reported) | 0.567 (basic vs extended) 0.591 (basic vs not reported) |
| Extended | 38.7 (26.7 to 50.7) | 44.0 (30.5 to 57.4) | ||
| Not reported | 30.9 18.7 to 43.1) | 34.7 (22.5 to 46.8) | ||
| 5. Country | ||||
| LIC | 67.7 (49.7 to 85.8) | 67.9 (48.5 to 87.3) | 0.002** | 0.006** |
| HIC | 34.4 (29.2 to 39.6) | 36.5 (30.5 to 42.5) | ||
| CI: confidence interval; LIC: low‐ or lower‐middle‐income country; HIC: high‐ or upper‐middle‐income country | ||||
|
*Study period was defined by the year in which the study period ended. The impact of the study period on the estimated sensitivity was investigated as a continuous variable through meta‐regression. **P < 0.05 was considered statistically significant. | ||||
2. Impact of potential sources of heterogeneity on the sensitivity of a first + second‐trimester scan combined in detecting fetal structural anomalies.
| Impact of potential sources of heterogeneity on the sensitivity of a first + second‐trimester scan combined in detecting fetal structural anomalies | ||||
| Covariate | Sensitivity estimate (95% CI) | P value | ||
| Fetuses affected | Anomalies | Fetuses affected | Anomalies | |
| 1. Study period* | ||||
| 1999 | 82.7 (63.2 to 100) | 79.9 (64.1 to 79.9) | ‐ | ‐ |
| 2009 | 84.9 (76.5 to 91.5) | 84.8 (77.6 to 92.0) | ‐ | ‐ |
| 2019 | 86.9 (71.4 to 100) |
88.8 (77.5 to 100) | ‐ | ‐ |
| Beta | 0.02 (‐0.1 to 0.14) | 0.03 (‐0.06 to 0.12) | 0.789 | 0.452 |
| 2. Reported prevalence of isolated ventricular septal defects | ||||
| ≤ 1.7 per 1000 | 87.4 (63.2 to 100) | 87.9 (81.5 to 94.2) | 0.055 | 0.067 |
| > 1.7 per 1000 | 71.3 (53.5 to 89.1) |
73.1 (56.0 to 90.1) | ||
| 3. Anatomical screening protocol | ||||
| Basic | 86.7 (76.3 to 97.2) | 86.2 (75.3 to 97.1) | 0.751 (basic vs detailed) 0.378 (basic vs not reported) |
0.993 (basic vs detailed) 0.879 (basic vs not reported) |
| Detailed | 79.5 (67.5 to 91.5) | 80.6 (69.4 to 91.9) | ||
| Not reported | 89.8 (75.1 to 100) | 91.9 (80.2 to 100) | ||
| 4. Cardiac screening protocol | ||||
| Basic | 84.3 (74.5 to 94.1) | 84.2 (74.3 to 94.1) | 0.920 (basic vs extended) 0.893 (basic vs not reported) |
0.879 (basic vs extended) 0.889 (basic vs not reported) |
| Extended | 83.0 (67.2 to 98.9) | 84.2 (69.3 to 99.0) | ||
| Not reported | 83.3 (65.9 to 100) | 85.7 (70.4 to 100) | ||
| 5. Country | ||||
| LIC | 95.5 (77.0 to 99.3) | 95.7 (77.1 to 99.3) |
0.106 | 0.118 |
| HIC | 81.2 (71.0 to 88.4) | 82.2 (72.5 to 89.0) | ||
| CI: confidence interval; LIC: low‐ or lower‐middle‐income country; HIC: high‐ or upper‐middle‐income country | ||||
|
*Study period was defined by the year in which the study period ended. The impact of the study period on sensitivity was investigated as a continuous variable through meta‐regression. **P < 0.05 was considered statistically significant. | ||||
3. Impact of potential sources of heterogeneity on the sensitivity of a single second‐trimester scan combined in detecting fetal structural anomalies.
| Impact of potential sources of heterogeneity on the sensitivity of a single second‐trimester scan combined in detecting fetal structural anomalies | ||||
| Covariate | Sensitivity estimate (95% CI) | P value | ||
| Fetuses affected | Anomalies* | Fetuses affected | Anomalies* | |
| 1. Study period** | ||||
| 1997 | 59.4 (38.4 to 80.4) | ‐ | ‐ | ‐ |
| 2007 | 51.9 (39.9 to 64.0) | ‐ | ‐ | ‐ |
| 2017 | 44.4 (27.6 to 61.1) | ‐ | ‐ | ‐ |
| Beta | ‐0.03 (‐0.09 to 0.03) | ‐ | 0.332 | ‐ |
| 2. Reported prevalence of isolated ventricular septal defects | ||||
| ≤ 1.7 per 1000 | 63.0 (51.3 to 74.8) | ‐ | 0.006 | ‐ |
| > 1.7 per 1000 | 36.8 (23.5 to 50.1) | ‐ | ‐ | |
| CI: confidence interval | ||||
|
*Anomalies: insufficient data available to investigate the impact of potential sources of heterogeneity on the overall sensitivity of a single second‐trimester scan by the number of anomalies. **Study period was defined by the year in which the study period ended. The impact of the study period on sensitivity was investigated as a continuous variable through meta‐regression. P < 0.05 was considered statistically significant. | ||||
CI: confidence interval
10.
A to C Plots of sensitivity against year of study completion of studies included in the analysis of the accuracy of a first‐trimester scan (A), a first + second‐trimester scan combined (B) and a single second‐trimester scan (C) in detecting fetuses affected by structural anomalies overall. There was an increase in the estimated sensitivity of a first‐trimester scan and a first + second‐trimester scan over time, whereas the sensitivity of a single second‐trimester scan decreased. D to F Plots of sensitivity against prevalence of isolated ventricular septal defects (VSDs) in the reported cohorts. Studies that found a VSD prevalence below 1.7 per 1000 fetuses among their cohort had a higher sensitivity than those that found a VSD prevalence of ≥ 1.7 per 1000 fetuses. A low VSD prevalence in a reported cohort was assumed to be indicative of under‐reporting of internal anomalies that are asymptomatic at birth (such as isolated VSDs), resulting in an overestimation of sensitivity in these studies.
4.1. Study period
The estimated sensitivity of both the first‐trimester scan and the first + second‐trimester scan increased over time based on the year of study completion. However, this increase was not statistically significant, and there was evident overlap in the confidence intervals of the estimates at each time point (1999, 2009, 2019). According to meta‐regression analysis of the year of study completion, the sensitivity of a first‐trimester scan increased from 31.6% (95% CI 19.2 to 44.0) in 1999, to 44.0% (95% CI 29.1 to 58.9) in 2019 (Figure 10, panel A; Table 6). The estimated sensitivity of a first + second‐trimester scan combined increased from 82.7% (95% CI 63.2 to 100) in 1999, to 86.9% (95% CI 71.4 to 100) in 2019 (Figure 10, panel B; Table 7). In contrast, the sensitivity of a single second‐trimester scan decreased from 59.4% (95% CI 38.4 to 80.4) in 1997 to 44.4% (95% CI 27.6 to 61.1) in 2017 (Figure 10, panel C Table 8). Importantly, the observed decrease in estimated sensitivity of a single‐second trimester scan was not statistically significant and the confidence intervals at each of the time point estimates (1997, 2007, 2017) clearly overlapped.
4.2. Completeness of postnatal identification of structural anomalies
Among all tests, the estimated sensitivity was higher for studies that reported a prevalence of isolated VSDs below the selected threshold of 1.7 per 1000 fetuses (Table 6; Table 7; Table 8), in keeping with the assumption that a low VSD prevalence in a reported cohort may be indicative of under‐reporting of internal anomalies that are asymptomatic at birth (such as isolated VSDs), resulting in a potential overestimation of sensitivity in these studies. Of note, the reported prevalence of isolated VSDs varied widely among first‐ and first + second‐trimester scan studies and was below 1.7 per 1000 fetuses in 70.0% and 72.2% of these studies, compared to 57.1% of single second‐trimester scan studies. Moreover, 35.0% and 38.9% of first‐ and first + second‐trimester scan studies respectively reported a prevalence of 0 per 1000 fetuses among their cohort (Figure 10, panel D and E), whereas this did not occur among single second‐trimester scan studies (Figure 10, panel F).
4.3. Level of detail of the anatomical and minimum requirements of cardiac screening
The level of detail of the anatomical screening protocol, or the cardiac screening views that were used, did not have a significant effect on the estimated sensitivity of the tests evaluated (Table 6; Table 7; Table 8).
4.4. Income level of the country where the study was conducted
The estimated sensitivity of a first‐trimester scan differed significantly between low‐ and high‐income countries (Table 6), whereas the income status of the country where the study was conducted had less of an impact on the estimated sensitivity of a first + second‐trimester scan combined (Table 7) and that of a single second‐trimester scan (Table 8). The sensitivity of a first‐trimester scan among studies conducted in low‐income countries was estimated at 67.7% (95% CI 49.7 to 85.8), whereas among studies conducted in high‐income countries, the sensitivity was estimated at 34.4% (95% CI 29.2 to 39.6). However, there were few data available for the evaluation of the accuracy of a first‐trimester scan in low‐income countries. The subgroup of studies conducted in a low‐income country included four studies and 5961 fetuses, of whom 35 were structurally abnormal (Andrew 2015; Chelli 2009; Natu 2014; Vellamkondu 2017), three of which reported on a study population of fewer than 600 fetuses (Chelli 2009; Natu 2014; Vellamkondu 2017). No clear methodological differences were identified between the studies conducted in high‐ and low‐income countries, although missing information was more frequently encountered in studies conducted in low‐income countries. Of note, one of the studies that was included in the subgroup of low‐income countries reported an exceptionally high sensitivity of the first‐trimester scan of 86.7% and a sensitivity of 100% for the first + second‐trimester scan combined (Andrew 2015), compared to a median sensitivity of 35.5% and a range of 11.1% to 60% among the other 19 first‐trimester scan studies. In the same study, there were concerns that postnatal identification of cases with normal ultrasound findings may have been incomplete, which may be reflected by the exceptionally low prevalence of fetuses with structural anomalies of 0.3%, compared to a median prevalence of 1.8% among first‐trimester scan studies.
4.5. Spectrum of anomalies among studies that used a single‐ or two‐stage screening approach
Because of the large difference in estimated sensitivity between a single‐ and two‐stage screening approach, we explored potential differences in the spectrum of anomalies that were present at the time of ultrasound screening to investigate whether there may have been differences in the types of anomalies that were present at the time of ultrasound screening, which may have had an impact on the results. We descriptively explored this by plotting the average proportion of anomalies that was accounted for by eight gross structural anomalies that have high first‐trimester detection rates (anencephaly, holoprosencephaly, encephalocele, HLHS, HRHS, abdominal wall defects, lethal skeletal dysplasias and megacystis) for each index test (Figure 11). We found that for five of the eight gross anomalies that we evaluated, their relative contribution to the total number of anomalies was two to five times higher among the first + second‐trimester scan studies (two‐stage screening approach), than among the single second‐trimester scan studies (single‐stage screening approach), suggestive of a difference in the spectrum of anomalies between these groups of studies.
11.
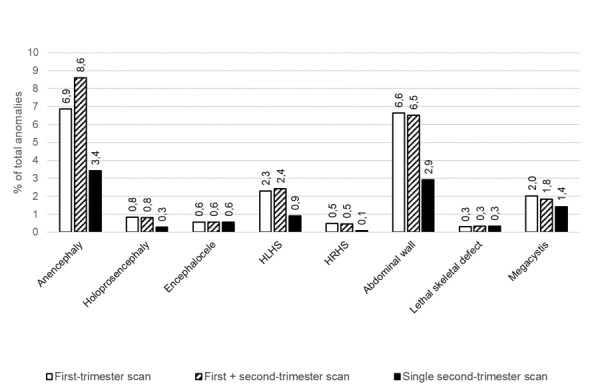
The proportion of the total number of structural anomalies that was accounted for by gross structural anomalies across study cohorts. The graph shows that for five of the eight gross anomalies for which we determined their relative contribution to the total number of anomalies, their relative contribution was two to five times higher among the first‐ and second‐trimester scan studies (two‐stage screening approach), than among the single second‐trimester scan studies (single‐stage screening approach), suggestive of a difference in the spectrum of anomalies between these groups of studies.
5. Sensitivity analyses
Results of the sensitivity analyses are presented in Appendix 5. We did not perform sensitivity analyses of specificity due to lack of variation in estimates of specificity.
There was no relevant difference in the estimated sensitivity of the index tests when studies were excluded based on their risk of bias, applicability concerns, the use of data obtained from birth defect registries, low number of cases, high loss to follow‐up rate or missing data for construction of 2 x 2 tables among the sensitivity analyses that we were able to perform. Other sensitivity analyses could not be performed due to a lack of studies that were available for the analyses.
Discussion
Summary of main results
We aimed to evaluate the diagnostic accuracy of first‐ and second‐trimester ultrasound screening in low‐risk and unselected pregnant women for detection of fetal structural anomalies in a single‐ and two‐stage screening approach. The review included 87 studies of 7,057,859 fetuses (including 25,202 fetuses with structural anomalies). We assessed a large number of studies (20 and 18 studies, respectively) that evaluated the accuracy of a single first‐trimester scan and a first + second‐trimester scan combined to screen for fetal structural anomalies. The number of studies that evaluated the accuracy of a single second‐trimester scan overall was limited (nine studies). However, up to 25 studies were available for the analyses of subgroups of anomalies. No studies provided direct comparisons of a single‐ versus a two‐stage screening strategy.
Both a first‐trimester scan and a single second‐trimester scan seem to have high specificity for identifying fetal structural anomalies, but poor sensitivity, whereas the combination of a first + second‐trimester scan had both a high specificity and sensitivity. The difference in sensitivity of a single second‐trimester scan compared to a first + second‐trimester scan combined was an unexpected finding (Table 5). We expected to find earlier detection of some anomalies in a two‐stage screening approach, but we did not expect that the addition of an early fetal anomaly scan would significantly impact the overall sensitivity in detecting fetal structural anomalies before 24 weeks’ gestation. Furthermore, in the analyses of overall detection of anomalies in different organ systems and of the 46 selected anomalies that we analysed individually, the combination of a first + second‐trimester scan outperformed the single second‐trimester scan in terms of sensitivity (Table 4).
However, caution is warranted when interpreting these results due to the indirectness of the comparison and the limited number of studies available for evaluating the single second‐trimester scan. Notable differences exist between studies using a single‐stage screening approach and those employing a two‐stage screening approach, potentially impacting the results. In particular, we found a difference in the spectrum of anomalies that were found in the study populations. This difference was most marked for structural anomalies that are amenable to detection in the first trimester, such as anencephaly and abdominal wall defects (Figure 11). Detection of these anomalies in the first trimester in pregnancies that may have resulted in a spontaneous miscarriage or fetal death prior to the second trimester may partly explain the differences in sensitivity. However, selective exclusion of gross structural anomalies through incidental findings at prior (screening) tests (e.g. first‐trimester combined test, NIPT and dating scans), form a more likely explanation. Unmeasured effects of prior testing may also partly explain the unexpected finding of a decrease in sensitivity of a single second‐trimester scan (Figure 10, panel C). It is possible that both increased uptake of first‐trimester tests, including the widespread adoption of NIPT, as well as increased experience in identification of fetal anomalies on first‐trimester ultrasound, have led to increased selective exclusion of gross anomalies from single second‐trimester scan study cohorts. In keeping with this, the sensitivity of the first‐trimester scan and of the first + second‐trimester scan combined both increased over time (Figure 10, panels A and B). Nevertheless, it is crucial to emphasise that the discerned trends in sensitivity over time lacked statistical significance, evident in the distinct overlap in confidence intervals at each assessed time point. Consequently, drawing robust conclusions from these findings is not possible.
In addition to differences in the spectrum of anomalies, we also noted that the overall prevalence of fetuses with structural anomalies and the prevalence of isolated ventricular septal defects was higher among single second‐trimester scan studies than those that evaluated a first + second‐trimester scan combined. This may reflect a real difference between the study populations or indicate differences in postnatal identification rates. In turn, this may result in an overestimation of sensitivity, particularly for anomalies that are prone to under‐reporting, such as cardiac septal defects and anomalies that may be asymptomatic at birth. However, we found a similar difference in estimated sensitivity for the subgroup of anomalies that are less susceptible to incomplete postnatal identification (i.e. anomalies that are externally visible, symptomatic at birth, or lethal ‐ Table 5). Therefore, there may be other factors such as differences in patient selection and healthcare settings, which have a more significant effect on the estimated sensitivity differences between single‐ and two‐stage screening studies. Whereas 77.8% of the single‐stage screening studies matched our review question in the patient selection domain, this was the case for only 11.1% of two‐stage screening studies. Concerns regarding applicability among two‐stage screening studies mostly arose from patients being recruited from a single tertiary care facility, without exclusion of referrals from other centres. This may have led to referral bias and overestimation of the sensitivity of a two‐stage screening approach. Furthermore, three of the four studies that contributed most to the summary estimate of sensitivity of a single‐stage approach were conducted in a non‐tertiary setting, which may have further contributed to the observed differences in the performance of the two screening strategies. Sonographers operating in tertiary care facilities are more likely to have access to high‐quality equipment, are generally highly trained in prenatal diagnosis of (rare) fetal anomalies and work in close collaboration with fetal medicine specialists. In primary and secondary care facilities, resources may be more limited.
In conclusion, it is difficult to determine the true size of the difference in sensitivity between single‐ and two‐stage screening approaches based on the available evidence. However, the results presented indicate that unreported prior testing may have an important impact on the estimated sensitivity of a single second‐trimester scan and may mask improvements in sensitivity over time. It may therefore be useful to include results of prior screening tests as a variable when evaluating the accuracy of population‐based ultrasound screening.
A final, but important, general observation is that the extensive variation in estimates of sensitivity of ultrasound is most likely due to chance variation, which complicates meta‐analysis of ultrasound sensitivity. Even estimates of ultrasound sensitivity in detecting fetal structural anomalies overall are susceptible to this, as they depend on detection of individual anomalies, most of which are rare. We observed that among most studies, there were several types of anomalies of which there were only one or two cases in the cohort, resulting in a detection rate of 0.0%, 50% or 100%, with a high likelihood of the results being skewed by chance. This is best illustrated by the estimated sensitivity of a first + second‐trimester scan combined in detecting duodenal atresia (Table 4). The summary sensitivity was 98.2%, with a 95% CI of 0.0 to 100%. This analysis included five studies. Two of these included a single case of duodenal atresia and reported a sensitivity of 0.0 and 100%, respectively (Pilalis 2012; Abu‐Rustum 2010). One study included two cases and also reported a sensitivity of 100% (Syngelaki 2011). The remaining two studies included nine and eight cases (Liao 2021; Syngelaki 2019), respectively, and reported a sensitivity of 11.1% and 100%. The reason for the difference in these last two studies cannot be explained based on the reported data as both included a routine third‐trimester scan, which should have detected cases missed on the second‐trimester scan in the study that found a sensitivity of 11.1%. As duodenal atresia is generally considered to be a third‐trimester diagnosis, the finding of 100% sensitivity by Liao 2021 seems to be an outlier. However, due to the low number of cases in the majority of the studies included in the analysis, this can not be verified.
Strengths and weaknesses of the review
Strengths
This review provides a comprehensive overview of first‐ and second‐trimester ultrasound screening for fetal anomalies in low‐risk and unselected populations. We examined publications from around the world, covering a wide cross‐section of pregnant women receiving ultrasound screening for fetal anomalies. We performed various subgroup analyses to determine the accuracy of ultrasound screening in detecting 46 selected anomalies individually, such as spina bifida, different types of congenital heart defects and congenital diaphragmatic hernia, as well as detection of anomalies in different organ systems overall, and of subgroups of anomalies of varying severity, ranging from lethal to minor.
A major challenge in evaluating the accuracy of ultrasound in detecting fetal structural anomalies is the prevalence of individual conditions. Despite a relatively high prevalence of structural anomalies overall, the anomalies that comprise this group are quite rare individually. To maximise the number of cases available for the analysis of the selected structural anomalies, we searched for papers that reported on those conditions specifically in addition to general reports of fetal ultrasound screening. This allowed us to pool data for more cases and determine more accurate estimates of test accuracy in detecting rare anomalies. A clear example is congenital diaphragmatic hernia (CDH). We obtained a total of 75 cases from general reports, whereas an additional 688 cases were identified from studies reporting on CDH specifically.
Further strengths relate to the review methodology, which was established through collaboration between researchers with methodological and clinical expertise. Our review methods were transparent. We published the full review protocol in the Cochrane Library (Buijtendijk 2021). Evaluation of full‐text papers for inclusion in the review, assessment of methodological quality of included studies, data extraction and evaluation of the certainty of the evidence using the GRADE approach were performed by two review authors independently. Authors of potentially eligible studies from which essential data for application of the selection criteria were missing (such details on the timing of ultrasound screening and gestational age at prenatal diagnosis) were contacted multiple times to ensure that no study was wrongfully excluded. Authors from included papers were contacted to provide missing data that was essential for correct interpretation and classification of the reported results. We put in considerable efforts to reduce heterogeneity in study results arising from the use of different classification systems and performed extraction of 2 x 2 tables from studies on a case‐by‐case basis to ensure the same classification system was applied. In an effort to minimise confounding of the results through prior testing, we excluded all cases of structural anomalies that were associated with an underlying condition such as chromosomal anomalies and intra‐uterine infections, as the structural anomalies may have been diagnosed through follow‐up investigations for the underlying condition, rather than being identified through ultrasound screening.
Weaknesses
Despite our efforts to ensure the quality of included studies, there were several factors that complicated the meta‐analysis of the data. We have, however, tried to identify and adapt to the most important issues to allow for comparison of the data presented in different studies.
There are many different screening protocols used to screen for fetal anomalies during pregnancy. This means that direct comparison is more difficult than if all studies used the same screening protocol to screen for fetal anomalies.
Sensitivity of ultrasound screening is dependent on the thoroughness of postnatal identification of anomalies, especially for those anomalies that are not externally visible and that present in the first few weeks or months after birth, such as certain cardiac and renal anomalies. We selected studies upon a minimum reference standard of neonatal examination. In studies that included a longer follow‐up period, we expected to find a lower sensitivity for internal anomalies that were likely to be asymptomatic at birth. Unfortunately, we were unable to test this suspicion as there was an insufficient number of studies reporting on our main outcome that included a follow‐up period of more than a month. Therefore, we tested whether the prevalence of isolated VSDs in the reported cohort was correlated with the sensitivity found by studies. We found that studies that reported a VSD prevalence that was closer to the expected value of 3 per 1000 fetuses (van der Linde 2011) had a lower sensitivity than those that found a lower prevalence. However, as this is an indirect measure of completeness of postnatal identification, it is not possible to infer absolute conclusions about which estimates of sensitivity may be closer to the truth, as unmeasured confounders cannot be excluded.
A limitation of the bivariate model is that it requires sufficient variation in study results in order for the models to converge and to accurately perform the analysis. There were several subgroups of anomalies in which the bivariate model did not converge, possibly due to studies only having a single case that was either detected or missed during ultrasound screening, resulting in an estimated sensitivity of 0 or 100%. This issue occurred most frequently in the analyses of the single second‐trimester scan, as there were fewer studies available that reported on the accuracy of this screening approach. In cases where meta‐analysis was not possible, we presented the data descriptively.
We assumed no correlation between sensitivity and specificity, considering that specificity was consistently close to 100% in studies allowing its calculation. The validity of this assumption depends on the ability to correctly identify anomalies when present and the potential for false identification when absent. Our assumption should not pose problems with estimates of ultrasound accuracy in detecting gross structural anomalies such as anencephaly, as these are rarely falsely identified in a structurally normal fetus. For more subtle anomalies, like cardiac defects and digestive tract issues, theoretically, this assumption may have been problematic. Nevertheless, the low prevalence of these anomalies and the resulting high number of true‐negative test results should mitigate any significant impact of our decision to remove the correlation parameter from our analyses on the estimates of specificity. Therefore, we believe that while sensitivity varies, specificity consistently approaches 100% for all structural anomalies evaluated in this review.
Based on descriptive exploration of differences in the spectrum of anomalies among study cohorts, a suspicion arose that gross structural anomalies accounted for a larger proportion of anomalies in studies that evaluated a first‐trimester scan and a first + second‐trimester scan combined, than in studies that evaluated a single second‐trimester scan. However, this suspicion was not formally explored and its contribution to the observed differences in sensitivity remains to be determined.
Recently, the .kf field tag was introduced to the Embase database within Ovid. However, as this field tag was not available at the time of the search design, the .kw field tag was used. As the.kf field tag is more sensitive, implementation of this field tag should be explored in any future review update.
Applicability of findings to the review question
We aimed to determine the accuracy of first‐ and second‐trimester ultrasound screening in the general population. However, the vast majority of studies that reported on our main outcome for a first‐trimester scan, or a first + second‐trimester scan combined, recruited patients from tertiary referral centres and did not exclude patients referred from other centres, which could lead to referral bias. This limits the applicability of the findings of the meta‐analyses of a first‐trimester scan and a first + second‐trimester scan combined to our review question in terms of the performance of these tests in the general population. We chose not to exclude studies with these methodological concerns as we anticipated that a paucity of studies would remain if we applied such strict selection criteria. We planned to investigate the impact of including studies with applicability concerns in terms of patient selection. However, this was not possible due to a lack of studies considered to have low concerns regarding applicability in terms of patient selection.
We also aimed to compare the accuracy of single‐ and two‐stage screening approaches in detecting fetal structural anomalies before 24 weeks’ gestation. No papers made direct comparisons between single‐ and two‐stage screening strategies, making it difficult to determine whether there is a real difference between these strategies. It is not certain whether the differences that we detected are related to the screening approach itself or to confounding factors. Furthermore, we were unable to account for the effects of prior testing as this information was not reported by studies. It is possible that gross structural anomalies were selectively excluded from single second‐trimester scan studies as the patients were either no longer pregnant or no longer considered low‐risk at the time of the second‐trimester scan due to an incidental finding of a fetal anomaly before the second‐trimester scan was undertaken. Together, these factors warrant caution when interpreting these findings.
In addition to the factors mentioned above, there were important methodological issues in all studies that limited the applicability of the results to the review question. The main methodological concerns included completeness of postnatal identification of anomalies that were asymptomatic at birth, and verification of ultrasound findings in pregnancies with an adverse outcome. Although we are aware that partial verification of pregnancies with an adverse outcome can generally not be avoided due to ethical considerations, it should be noted that studies evaluating the accuracy of prenatal ultrasound are prone to bias as a result of this limitation.
Authors' conclusions
Implications for practice.
A first‐trimester scan appears to be an accurate prenatal screening test to detect lethal structural anomalies and some severe anomalies at an early stage of pregnancy (before 14 weeks’ gestation) with a low overall false‐positive rate. This means that in a hypothetical cohort of 100,000 fetuses, a total of 124 cases of lethal anomalies are expected to occur, of which 113 would be detected by a first‐trimester scan. Seventy‐nine of 98,224 fetuses without structural anomalies (0.08%) are expected to receive a false‐positive diagnosis by a first‐trimester scan. However, it should be noted that the sensitivity of a first‐trimester scan in detecting fetal structural anomalies overall appears to be poor.
The combination of a first + second‐trimester scan appears to be able to detect most structural anomalies before 24 weeks’ gestation with high sensitivity and specificity. In a hypothetical cohort of 100,000 pregnancies, 1448 of 1776 cases with structural anomalies are expected to be correctly identified before 24 weeks’ gestation by a first + second‐trimester scan combined, whereas a total of 118 of 98,224 fetuses without structural anomalies (0.1%) are expected to receive a false‐positive diagnosis. However, the evidence for the accuracy of both a first‐trimester scan and a first + second‐trimester scan combined is currently mainly based on studies conducted in a single tertiary care facility. Therefore, the results of this systematic review may not be generalisable to population‐based screening.
A single second‐trimester scan appears to perform worse than a first + second‐trimester scan combined in detecting fetal structural anomalies overall, with considerably more variation in sensitivity across organ systems. At the same prevalence of fetuses affected by structural anomalies, a single second‐trimester scan is expected to correctly identify 896 cases (552 fewer than a first + second‐trimester scan combined is expected to find) and 205 of 98,224 fetuses without structural anomalies (0.2%) would receive a false‐positive diagnosis (88 more than a first + second‐trimester scan would be expected to find). While a two‐stage screening approach appears to have the potential to identify a higher number of anomalies without increasing false‐positive diagnoses based on this review, the certainty of evidence for this incremental benefit is very low, mainly due to the scarcity of studies directly comparing the screening strategies. Therefore, the results of this meta‐analysis cannot straightforwardly be used to estimate the potential impact of introducing an additional first‐trimester scan to a population‐based prenatal screening programme.
In conclusion, this review underscores the substantial variation in detection of fetal structural anomalies through first‐ and second‐trimester ultrasound screening. We have conducted a comprehensive review of the available evidence and presented estimates of accuracy of first‐ and second trimester ultrasound in detecting different subgroups of anomalies and selected individual diagnoses. This information is crucial for counselling expectant parents about the limitations of ultrasound screening and interpreting the results. However, limited evidence for specific diagnoses, especially rare anomalies, emphasises the ongoing need for continuous efforts to evaluate and enhance the accuracy of prenatal ultrasound screening. Ideally, quality control mechanisms and auditing systems should actively encourage the reporting of anomalies to regional birth defect registries, facilitating the monitoring of both changes in the prevalence of (fetal) structural anomalies and accuracy of prenatal screening. These efforts also play a pivotal role in identifying specific areas for improvement, guiding targeted initiatives to enhance sonographer training and educational programmes.
Implications for research.
In this systematic review, we have presented estimates of sensitivity and specificity for first‐ and second‐trimester ultrasound in detecting fetal structural anomalies. These findings provide crucial information for future studies that evaluate other vital aspects of incorporating first‐trimester ultrasound into a prenatal screening programme, including cost‐effectiveness, women's and their partner's wishes with regard to this scan, and the potential influence on their decision‐making regarding pregnancy continuation when a fetal anomaly is suspected or diagnosed. Researchers interested in using the data presented in this review for further analyses are encouraged to submit a data request to MMGL.
We have also identified that there is a scarcity of studies evaluating first‐trimester ultrasound in non‐tertiary settings. As routine first‐trimester ultrasound is increasingly implemented in population‐based prenatal screening programmes, it becomes imperative to conduct studies that evaluate the accuracy of this screening in population‐based settings to provide more robust evidence on its accuracy, limitations and added benefits. We also observed that most studies on ultrasound screening have a high risk of bias in the reference standard domain. To mitigate risk of bias in the reference standard and prevent overestimation of sensitivity due to incomplete postnatal identification, we recommend a minimum postnatal follow‐up duration of four weeks after birth, as this will allow for identification of infants with clinically relevant anomalies that were asymptomatic at birth, such as cardiac defects with associated left‐to‐right shunts (Gao 2010; King 2007). Alternatively, cross‐referencing with clinical records of relevant paediatric and surgical departments and birth defect registries may be used to complete the lists of postnatally diagnosed cases (Fleurke‐Rozema 2014; Fleurke‐Rozema 2016; Fleurke‐Rozema 2017; Saltvedt 2006; van Velzen 2016; Waern 2021; Wayne 2002).
History
Protocol first published: Issue 7, 2021
| Date | Event | Description |
|---|---|---|
| 3 August 2021 | Amended | Edited methods/types of studies to include 'false negative' in the composition of the 2 x 2 data table. This category had been omitted in error. |
Acknowledgements
We thank the Editorial Staff of Cochrane Pregnancy and Childbirth and the Editorial Team for Cochrane Diagnostic Test Accuracy Reviews for their support.
Editorial and peer reviewer contributions
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Nai Ming Lai, School of Medicine, Faculty of Health and Medical Sciences at Taylor’s University, Malaysia
Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article): Liz Bickerdike, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks, collated peer reviewer comments and supported the editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy editing and production): Jenny Bellorini, Cochrane Central Production Service
Peer reviewers (provided comments and recommended an editorial decision): Robin Turner (statistics), Gianni Virgili (general), Julie Glanville (search), A/Prof Lisa Hui, Department of Obstetrics, Gynaecology and Newborn Medicine, University of Melbourne; Reproductive Epidemiology Group, Murdoch Children's Research Institute, Parkville VIC Australia (clinical) and Munzer Naima (consumer).
As part of the prepublication editorial process, the review has been commented on by two peers from Cochrane Pregnancy and Childbirth (an editor and one referee who is external to the editorial team). The authors are grateful to Jim Thornton, Prof OBGYN at the University of Nottingham, UK, for his time and comments. As part of the Cochrane Diagnostic Test Accuracy Editorial Team (DTA‐ET) process, the protocol was commented on by four peers (an editor and three peer referees).
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Lastly, we thank Jianan Wang for his help in translating Chinese papers, Sally‐Ann Clur for her advice on classification of cardiac anomalies for the meta‐analysis, and the Dutch Top Sector Life Sciences & Health Consortium for financially supporting the research of Marieke Buijtendijk (project number: 2018‐22484).
Appendices
Appendix 1. Search strategy MEDLINE
| Database(s): Ovid MEDLINE(R) ALL 1946 to July 22, 2022 Search strategy: 22 July 2022 | ||
| # | Searches | Results |
| 1 | congenital abnormalities/ or fetal diseases/cn or (fetus/ and ab.fs.) | 38314 |
| 2 | abnormalities, severe teratoid/ | 1564 |
| 3 | cardiovascular abnormalities/ or heart defects, congenital/ or aortic coarctation/ or truncus arteriosus/ or crisscross heart/ or ebstein anomaly/ or heart septal defects/ or aortopulmonary septal defect/ or truncus arteriosus, persistent/ or endocardial cushion defects/ or heart septal defects, ventricular/ or double outlet right ventricle/ or heterotaxy syndrome/ or situs inversus/ or dextrocardia/ or levocardia/ or hypoplastic left heart syndrome/ or "tetralogy of fallot"/ or "transposition of great vessels"/ or univentricular heart/ or vascular malformations/ or scimitar syndrome/ or vascular ring/ or aortic arch syndromes/ or aortic valve stenosis/ or pulmonary atresia/ or pulmonary valve stenosis/ or tricuspid atresia/ or tricuspid valve insufficiency/ or tricuspid valve stenosis/ or heart/ab or fetal heart/ab or heart septum/ab or endocardial cushions/ab or heart ventricles/ab or exp heart valves/ab or exp aorta/ab or pulmonary veins/ab or exp heart valve diseases/cn | 151494 |
| 4 | nervous system malformations/ or holoprosencephaly/ or neural tube defects/ or anencephaly/ or encephalocele/ or spinal dysraphism/ or meningocele/ or meningomyelocele/ or hydrocephalus/ or central nervous system/ab or neural tube/ab or spine/ab | 51920 |
| 5 | musculoskeletal abnormalities/ or craniofacial abnormalities/ or maxillofacial abnormalities/ or cleft palate/ or limb deformities, congenital/ or ectromelia/ or lower extremity deformities, congenital/ or foot deformities, congenital/ or talipes/ or clubfoot/ or upper extremity deformities, congenital/ or hand deformities, congenital/ or bone diseases, developmental/ or dwarfism/ or achondroplasia/ or thanatophoric dysplasia/ or osteochondrodysplasias/ or exp bone malalignment/ or stomatognathic system abnormalities/ or craniofacial abnormalities/ or maxillofacial abnormalities/ or mouth abnormalities/ or cleft lip/ or cleft palate/ or face/ab or lip/ab [zonder dysostosis] | 70785 |
| 6 | abdominal wall/ab or gastroschisis/ or hernia, umbilical/ or hernia abdominal/ | 7480 |
| 7 | digestive system abnormalities/ or esophageal atresia/ or intestinal atresia/ or intestinal obstruction/ or duodenal obstruction/ or intestinal volvulus/ or esophageal fistula/ or tracheoesophageal fistula/ | 47081 |
| 8 | respiratory system abnormalities/ or tracheal stenosis/ or "cystic adenomatoid malformation of lung, congenital"/ or hernias, diaphragmatic, congenital/ | 13024 |
| 9 | "disorders of sex development"/ or genitalia/ab | 9924 |
| 10 | urogenital abnormalities/ or kidney diseases, cystic/ or multicystic dysplastic kidney/ or polycystic kidney diseases/ or kidney diseases/cn | 16760 |
| 11 | (urinary tract/ab or urethra/ab or ureter/ab or kidney/ab or urinary bladder/ab or (hydronephros* or hydro‐nephros* or pyelectas* or (uret* adj3 (obstruct* or defect*1 or anomal* or malformat* or abnormalit* or deformit* or d#smorph* or aplas* or d#splas* or atres* or atret* or agenes*))).mp.) and (trimester, first/ or trimester, second/ or ((("10" or ten or tenth or "11" or 11th or eleven* or "12" or 12th or twelv* or twelfth or "13" or 13th or thirteen* or "14" or 14th or fourteen* or "15" or 15th or fifteen* or "16" or 16th or sixteen* or "17" or 17th or seventeen* or "18" or 18th or eighteen* or "19" or 19th or nineteen* or "20" or 20th or twentieth or twenty or "21" or 21st or "22" or 22nd or "23" or 23rd or "24" or 24th) adj5 (week* or wk or wks)) or ((early or ((first or 1st) adj2 half) or mid or midterm) adj2 (pregnan* or gestat* or gravidit*)) or ((mid or first or 1st or second or 2nd) adj3 trimester*) or midtrimester or midpregnan* or midgestat*).tw,kf.) | 1454 |
| 12 | hydrops fetalis/ | 2372 |
| 13 | (congenit* adj6 (disorder* or diseas* or abnormal* or anomal* or malform* or deform* or defect*1)).tw,kf. | 137230 |
| 14 | birth defect*.tw,kf. | 11125 |
| 15 | ((severe or major) adj3 (anomal* or abnormalit* or malformat* or defect*1 or deformit*)).tw,kf. | 31136 |
| 16 | ((structural or structure*1 or non‐chromosom* or nonchromosom* or anatom* or morphological* or isolated) adj6 (anomal* or abnormalit* or malform* or deformit* or defect* or distort* or contort* or misshap*)).tw,kf. | 70481 |
| 17 | (f?etal adj3 (anomal* or malform* or defect*1 or deformit*)).tw,kf. | 8403 |
| 18 | f?etal abnormalit*.tw,kf. | 2037 |
| 19 | (f?etus* adj (anomal* or abnormalit* or malform* or defect*1 or deform*)).tw,kf. | 105 |
| 20 | (((sever* or major) adj3 teratoid*) or ((abnormalit* or anomal* or defect*1) adj3 terato*) or monster or monsters or monstrui*).tw,kf. | 2487 |
| 21 | ((congen* adj2 (heart or cardiac or valvul*) adj2 (dis* or fail*)) or ((cardiac or intracard* or heart or cardiothor* or cardio‐thor* or cardiovasc* or cardio‐vasc* or (great* adj2 vessel*) or valvul* or valve*1) adj3 (anomal* or deformit* or defect*1 or abnormalit* or malformat* or atres* or atret* or d#splas* or d#smorph* or aplas* or agenes*))).tw,kf. | 82783 |
| 22 | (((major or sever* or critical or f?etal or f?etus* or prenat* or antenat*) adj3 CHD) or ((CHD or MCHD* or CCHD*) and congenital*)).tw,kf. | 8440 |
| 23 | (Fallot* or Ebstein* or coarct* or (CoA and aort*)).tw,kf. | 24039 |
| 24 | ((aort* adj1 arch* adj4 (hypoplas* or obstruct* or interrupt* or double or right or isolat*)) or (RAA and aort*) or ((vascular or aort*) adj2 (ring* or sling* or annul*)) or arter* lusor*).tw,kf. | 15873 |
| 25 | ((aort* adj4 (anomal* or abnormal* or deformit* or malformat* or defect* or stenos* or stenot* or atres* or atret* or d#splas* or anomal* or d#smorph* or aplas* or agenes*)) or aortosteno*).tw,kf. | 33315 |
| 26 | (double outlet* or DORV).tw,kf. | 2100 |
| 27 | ((hypoplas* adj4 (((left or right) adj3 (heart or ventric* or card* or chamber* or cavit*)) or RV* or LV*)) or ventric* hypoplas* or HLHS* or HLV* or HRHS*).tw,kf. | 4998 |
| 28 | (univentric* or monoventric* or ((single or mono or uni or triloculare bia*) adj2 (ventric* or cor))).tw,kf. | 7079 |
| 29 | ((outflow tract or OFT or conotrunc* or cono‐trunc*) adj6 (anomal* or abnormalit* or malformat* or defect*1 or deformit*)).tw,kf. | 1947 |
| 30 | ((transpos* adj6 (((great or large or main) adj2 (arter* or vessel*)) or congenital* or dextr*)) or TGA or TGAs or TGA's or CCTGA or CCTGAs or CTGA or CTGA's or DTGA or DTGAs or DTGA's).tw,kf. | 20979 |
| 31 | ((common arterial or arterios*) adj2 (trunk or truncus)).tw,kf. | 1904 |
| 32 | ((tricusp* or tri‐cusp* or TV or right AV or right atrioventr* or ((aort* or pulm*) adj2 valv*)) adj6 (steno* or regurg* or re‐gurg* or insuf* or incompet* or d#sfunct* or impair* or atres* or atret* or d#splas* or anomal* or malformat* or abnormalit* or deformit* or d#smorph* or aplas* or agenes*)).tw,kf. | 30631 |
| 33 | (pulmonar* adj3 (atres* or atret*)).tw,kf. | 3513 |
| 34 | (((septal or septum or septation or ((endocard* or AV or atri*ventr* or atri*‐ventr*) adj2 cushion*)) adj3 defect*) or ((heart or intracard* or intra‐card*) adj2 shunt*) or VSD or (comm* adj3 (sept* or AV or atri*ventr* or atri*‐ventr*) adj3 (canal* or osti*)) or canal defect* or AVSD* or CAVC* or (aort* adj2 window*) or APW or crisscross or criss‐cross).tw,kf. | 32388 |
| 35 | (scimitar* or (anomal* adj4 pulmon* ve?n*4 adj2 (return* or connect*)) or TAPVC* or PAPVC*).tw,kf. | 3272 |
| 36 | (heterotax* or isomerism* or (situs adj2 inver*) or ((lateral* or axis) adj2 defect*) or (malpos* adj2 complex*) or l*vocard* or dextrocard* or ((card* or heart) adj2 (dextrovers* or dextro‐vers* or dextrorotat* or dextro‐rotat* or dextroposit* or dextro‐posit*))).tw,kf. | 8482 |
| 37 | (((atrioventric* or atrio‐ventr* or AV) adj3 defect*) or ((ventric* or atrio* or VA or AV) adj3 discord*)).tw,kf. | 3091 |
| 38 | ((vascular or pulmonar* ve?n*) adj3 malformat*).tw,kf. | 8947 |
| 39 | ((non‐cardia* or noncardia* or extracard* or extra‐card* or nervous‐system or CNS or brain or cerebell* or cerebra* or neural‐tube* or craniospin* or cranio‐spin* or musc*skelet* or skelet* or spondyloepiphys* or epiphys* or bone or bones or osseous or spine or spinal or limb or limbs or extremit* or foot or feet or hand or hands or radial or radius or tibia* or ulna* or fibula* or orofac* or stomato* or craniofac* or maxill* or facial* or face or midfac* or oral or palat* or mouth* or lip or lips or (digest* adj2 (system or tract*)) or GI or gastrointest* or intestin* or bowel or gut or midgut or duoden* or ileum or ileal or ile*jejun* or jejun* or esophag* or oesophag* or trach* or abdom* or thoraco* or thoracic or thorax or (body adj3 (wall or stalk)) or bodystalk* or bodywall or respirator* or airway* or pulmonar* or lung or lungs or diaphragm* or hemidiaphragm* or sex or sexual or genit* or urogenit* or kidney* or renal or bladder) adj5 (defect*1 or anomal* or malform* or abnormalit* or deform* or d*smorph* or aplas* or d*splas* or atres* or atret* or agenes* or cleft* or misalign* or malalign* or malpos* or malrot* or ((mis or mal or wrong or incorrect or incomplet*) adj2 (rotat* or align* or position*)))).tw,kf. | 290498 |
| 40 | (encephalo*c*l* or cephaloc*el* or meningoencephaloc*el* or notoencephaloc*le or craniac*el* or ((cereb* or mening*) adj2 (hernia* or protru* or bulg*)) or exencephal* or anencephal* or acrani* or aprosencephal* or ((spin* or cranium or crania) adj2 (bifid* or open or cleft*)) or d#srap* or schistor*ach* or rachischis* or crani*‐schis* or crani*schis* or neur*‐schis* or neur*schis* or myeloc*le* or mening*myeloc* or hydrocephal* or hydro‐cephal* or holoprosencephal* or holo‐prosencephal* or arhinencephal*).tw,kf. | 47364 |
| 41 | ((developmental adj3 (bone or osseous) adj3 dis*) or ((bone or bones or osseous or limb or limbs) adj3 (reduct* or absent or absenc* or deficien* or missing or shorten* or very small* or lack* or deficit*)) or dwarfism* or achondroplasi* or achondrogenes* or hypochondrogenes* or thanatophor* or osteochondrod*splas* or osteod*splast* or osteod*d*stroph* or chondrod*splas* or chondrod*stroph* or radial ray* or talipes or equinovar* or equino‐var* or clubfoot or club‐foot or clubfeet or club‐feet or micromel* or d*smeli* or ectromel* or phocomel* or tetraphocomel* or amelia or hemimel* or palatolabio*chi* or labio*chi* or cheilo* or palat*chi* or harelip* or hare‐lip* or schi*oprosop*).tw,kf. | 36925 |
| 42 | (gastro*chis* or gastro‐schis* or ((umbil* or abdomin*) adj2 (hernia* or protru* or bulg*)) or omphaloc*el* or laparoc*el* or exomphal*).tw,kf. | 10180 |
| 43 | (((trach*sophag* or oesophag* or esophag*) adj4 fistula*) or ((bowel or intestin* or gastrointest* or GI or mid‐gut or midgut or duoden* or ileum or ileal or ile*jejun* or jejun*) adj3 obstruct*) or double bubble or volvulus).tw,kf. | 47049 |
| 44 | ((diaphragm* adj2 (hernia* or protru* or bulg*)) or (cystic adj2 adenomat* adj2 malformat*) or CCAM or CPAM or CAML).tw,kf. | 13418 |
| 45 | ((sex* adj2 (development or differentiat*) adj2 (diseas* or disorder* or defect*)) or intersex* or inter‐sex* or ((genital* or sex*) adj3 ambigu*)).tw,kf. | 7416 |
| 46 | (((cystic or polycyst* or multicyst*) adj2 (kidney* or (renal adj2 (diseas* or disorder*)))) or PKD or MCKD or (hyperechogen* adj2 kidney*) or megacystis).tw,kf. | 14464 |
| 47 | (hydrops adj3 f?etalis).tw,kf. | 2491 |
| 48 | or/1‐47 [ congenital defects; fetal (structural) anomalies ] | 884328 |
| 49 | ultrasonography/ or ultrasonography, prenatal/ | 221772 |
| 50 | nuchal translucency measurement/ | 1345 |
| 51 | echocardiography/ | 93134 |
| 52 | prenatal diagnosis/ | 38345 |
| 53 | early diagnosis/ or diagnostic tests, routine/ or diagnostic screening programs/ | 42357 |
| 54 | (ultraso* or ultra‐so* or sonogra* or echo‐gra* or echogra* or TVUS* or TV‐US*).tw,kf. | 450253 |
| 55 | (((anatom* or screen* or routin* or two‐step* or 2‐step* or two‐stage* or 2‐stag* or two‐phase* or 2‐phase* or obstetric or obstetrical or transvagin* or trans‐vagin* or transabdom* or trans‐abdom* or prenat* or pre‐nat* or antenat* or ante‐nat* or f?etal or f?etus* or twodimension* or two‐dimension* or 2‐dimension* or 2d) adj2 (US or USS)) or 2dUS or 2dUSS).tw,kf. | 3421 |
| 56 | (standard* adj (US or USS)).tw,kf. | 316 |
| 57 | (echo not ((spin or gradient or T2 or T2W) adj3 echo)).tw,kf. | 32882 |
| 58 | (scan or scans or scanning or scanned).tw,kf. | 567176 |
| 59 | ((transabdom* or trans‐abdom* or transvag* or trans‐vag* or anatom* or anomal*) adj3 imag*).tw,kf. | 9956 |
| 60 | (nuchal adj3 translucen*).tw,kf. | 2404 |
| 61 | (echocard* or echo‐card* or ((cardiac or cardiovascul* or heart) adj3 (view or views))).tw,kf. | 165059 |
| 62 | ((((("4" or "four") adj chamber*) or outflow tract* or (("3" or "three") adj vessel*)) adj6 (view* or plane or planes or asses* or visual* or screen* or survey* or imag* or US or USS)) or 4CV or 4‐CV or 3VV or 3‐VV or 3VTV or 3V‐TV).tw,kf. [4 chamber‐, outflow tract‐ or 3 vessel‐view] | 3263 |
| 63 | ((prenat* or pre‐nat* or antenat* or ante‐nat* or f?etal or f?etus*) adj3 (diagnos* or detect* or screen*)).tw,kf. | 51955 |
| 64 | ((detect* or screen* or diagnos* or PD) adj3 (rate or rates)).tw,kf. | 61639 |
| 65 | (("over time" or trend) adj6 (diagn* or detect*)).tw,kf. | 8021 |
| 66 | (timing adj6 (detect* or diagnos* or screen*)).tw,kf. | 3658 |
| 67 | (routin* adj3 (screening or imaging or diagnostic test*)).tw,kf. | 19754 |
| 68 | (screening adj3 (national* or nationwide or nation‐wide or state‐wide or statewide)).tw,kf. | 6105 |
| 69 | anomaly screen* program*.tw,kf. | 18 |
| 70 | or/49‐69 [ ultrasonography incl echocardiography & nuchal translucency /scan /prenatal diagnosis ] | 1394040 |
| 71 | 48 and 70 [ ultrasound (prenatal detection) of congenital structural defects ] | 133353 |
| 72 | fetus/ or fetal heart/ or fetal diseases/ or exp ultrasonography, prenatal/ or prenatal diagnosis/ or prenatal care/ or pregnancy trimester, first/ or pregnancy trimester, second/ or (f?etal or f?etus* or prenat* or pre‐nat* or antenat* or ante‐nat* or (nuchal adj3 translucen*) or (early adj (pregnan* or gestation* or gravidit*)) or ((first or 1st) adj2 half adj3 (pregnan* or gestat* or gravidit*)) or ((mid or first or 1st or second or 2nd) adj3 trimester*) or midtrimester* or midpregnan* or mid‐pregnan* or midgestat* or mid‐gestat*).tw,kf. or ((pregnancy or pregnant or gestation or gravidit*) and (("10" or 10th or ten or tenth or "11" or 11th or eleven* or "12" or 12th or twelve or twelfth or "13" or 13th or thirteen* or "14" or 14th or fourteen* or "18" or 18th or eighteen* or ((before or prior) adj2 ("20" or 20th or twenty or twentieth or "21" or 21st or twentyfirst or "22" or 22nd or twentysecond or "23" or 23rd or twentythird or "24" or 24th or twentyfourth))) adj5 (week* or wk or wks))).tw,kf. [prenatal filter] | 533394 |
| 73 | 71 and 72 [ ultrasound/prenatal detection of congenital structural defects+ prenatal filter ] | 36343 |
| 74 | ((exp animals/ or exp animal experimentation/ or models, animal/ or disease models, animal/) not humans/) or (animal model* or mouse model* or sheep model* or ovine model* or large animal* or animal experiment*).ti,kf. or ((sheep or lamb or lambs or ovine or ewe or ewes or sow or sows) not human*).ti. [EXCLUSION ANIMALS] | 4934345 |
| 75 | editorial/ or "systematic review"/ or cochrane.ti,jw. or (editorial or reply or comment).ti. or ((review/ or (review or overview).ti. or case report*.mp,jw.) not (cohort studies/ or prospective studies/ or retrospective studies/ or "cross‐sectional studies"/ or exp clinical trial/ or trial.ti,ot,kf. or (cohort* or retrospectiv* or prospectiv* or crosssection* or cross‐section*).tw,kf.)) [EXCLUSION case reports/reviews/editorials] | 5719350 |
| 76 | 74 or 75 [EXCLUSION ANIMALS/REVIEWS/CASE REPORTS] | 10435047 |
| 77 | 73 not 76 [ ultrasound/prenatal detection of congenital structural defects + prenatal filter ‐ original human studies ] | 20108 |
| 78 | limit 77 to yr="1997 ‐Current" [ ultrasound/prenatal detection of congenital structural defects + prenatal filter ‐ original human studies > 1997] | 14456 |
| 79 | from 78 keep 1‐6000 | 6000 |
| 80 | remove duplicates from 79 | 5995 |
| 81 | from 78 keep 6001‐12000 | 6000 |
| 82 | remove duplicates from 81 | 6000 |
| 83 | from 78 keep 12001‐14456 | 2456 |
| 84 | remove duplicates from 83 | 2456 |
| 85 | 80 or 82 or 84 [ ultrasound/prenatal detection of congenital structural defects + prenatal filter ‐ original human studies > 1997 ‐duplicates removed] | 14451 |
Appendix 2. Search strategy Embase
| Database(s): Embase Classic and Embase 1947 to 22 July 2022 Search strategy: 22 July 2022 | ||
| # | Searches | Results |
| 1 | congenital malformation/ or fetus malformation/ or fetus disease/cn or birth defect/ [some papers wrongly indexed with birth defect/ instead of congenital malformation/] | 187988 |
| 2 | severe teratoid abnormality/ | 245 |
| 3 | cardiovascular malformation/ or congenital heart disease/ or congenital heart malformation/ or ebstein anomaly/ or tricuspid valve atresia/ or heart septum defect/ or atrioventricular septal defect/ or criss cross atrioventricular relationship/ or endocardial cushion defect/ or Fallot tetralogy/ or heart right ventricle double outlet/ or great vessels transposition/ or heart right ventricle double outlet/ or heart ventricle septum defect/ or heart single ventricle/ or heterotaxy syndrome/ or heterotaxy syndrome/ or levocardia/ or dextrocardia/ or situs inversus/ or hypoplastic left heart syndrome/ or aorta anomaly/ or aortic arch anomaly/ or aortic arch interruption/ or aortic coarctation/ or aortopulmonary septal defect/ or vascular ring/ or arterial trunk/ or congenital blood vessel malformation/ or scimitar syndrome/ or pulmonary valve atresia/ or aortic stenosis/ or aortic valve stenosis/ or pulmonary valve stenosis/ or tricuspid valve stenosis/ or tricuspid valve regurgitation/ or exp valvular heart disease/cn | 223099 |
| 4 | nervous system malformation/ or central nervous system malformation/ or brain malformation/ or anencephalus/ or congenital hydrocephalus/ or encephalocele/ or encephalomeningocele/ or encephalomyelocele/ or exencephaly/ or holoprosencephaly/ or neural tube defect/ or spinal dysraphism/ or myelocystocele/ or meningocele/ or spina bifida/ or acrania/ [ some papers wrongly indexed with spina bifida/ or acrania/ ] | 56468 |
| 5 | musculoskeletal system malformation/ or limb malformation/ or arm malformation/ or hand malformation/ or leg malformation/ or foot malformation/ or clubfoot/ or limb absence/ or limb reduction defect/ or limb defect/ or bone malformation/ or congenital bone disease/ or skeleton malformation/ or spine malformation/ or bone dysplasia/ or micromelia/ or phocomelia/ or thanatophoric dwarfism/ or achondroplasia/ or dwarfism/ or chondrodysplasia/ or face deformity/ or face malformation/ or cleft face/ or craniofacial malformation/ or mouth malformation/ or exp "cleft lip with or without cleft palate"/ or lip malformation/ or palate malformation/ or cleft lip face palate/ or cleft palate/ or face disorder/cn or mouth disease/cn or lip disease/cn | 129536 |
| 6 | abdominal wall defect/ or umbilical hernia/ or abdominal wall hernia/ or gastroschisis/ or omphalocele/ | 25252 |
| 7 | digestive system malformation/ or esophagus malformation/ or esophagus atresia/ or esophagus fistula/ or tracheoesophageal fistula/ or gastrointestinal malformation/ or intestine malformation/ or intestine atresia/ or duodenum atresia/ or intestine malrotation/ or malrotation syndrome/ or gastrointestinal obstruction/ or intestine obstruction/ or intestine stenosis/ or intestine volvulus/ or small intestine obstruction/ or duodenum stenosis/ or duodenum obstruction/ or intestinal obstruct*.dq. | 86657 |
| 8 | respiratory tract malformation/ or trachea stenosis/ or congenital lung disease/ or lung malformation/ or cystic adenomatoid malformation/ or lung agenesis/ or congenital diaphragm hernia/ or diaphragm hernia/ | 30482 |
| 9 | genital malformation/ or ambiguous genitalia/ or female genital tract malformation/ or male genital tract malformation/ or "disorder of sex development"/ or hermaphroditism/ or intersex/ | 13542 |
| 10 | urogenital tract malformation/ or urinary tract malformation/ or bladder malformation/ or kidney malformation/ or kidney polycystic disease/ or kidney agenesis/ or kidney dysplasia/ or multicystic dysplastic kidney/ | 39626 |
| 11 | (hydronephros* or hydro‐nephros* or pyelectas* or (uret* adj3 (obstruct* or defect*1 or anomal* or malformat* or abnormalit* or deformit* or d#smorph* or aplas* or d#splas* or atres* or atret* or agenes*))).mp. and (first trimester pregnancy/ or second trimester pregnancy/ or ((("10" or ten or tenth or "11" or 11th or eleven* or "12" or 12th or twelv* or twelfth or "13" or 13th or thirteen* or "14" or 14th or fourteen* or "15" or 15th or fifteen* or "16" or 16th or sixteen* or "17" or 17th or seventeen* or "18" or 18th or eighteen* or "19" or 19th or nineteen* or "20" or 20th or twentieth or twenty or "21" or 21st or "22" or 22nd or "23" or 23rd or "24" or 24th) adj5 (week* or wk or wks)) or ((early or ((first or 1st) adj2 half) or mid or midterm) adj2 (pregnan* or gestat* or gravidit*)) or ((mid or first or 1st or second or 2nd) adj3 trimester*) or midtrimester or midpregnan* or midgestat*).tw,kw.) | 2058 |
| 12 | fetus hydrops/ | 5865 |
| 13 | (congenit* adj6 (disorder* or diseas* or abnormal* or anomal* or malform* or deform* or defect*1)).tw,kw. | 199525 |
| 14 | birth defect*.tw,kw. | 15747 |
| 15 | ((severe or major) adj3 (anomal* or abnormalit* or malformat* or defect*1 or deformit*)).tw,kw. | 45303 |
| 16 | ((structural or structure*1 or non‐chromosom* or nonchromosom* or anatom* or morphological* or isolated) adj6 (anomal* or abnormalit* or malform* or deformit* or defect* or distort* or contort* or misshap*)).tw,kw. | 97719 |
| 17 | (f?etal adj3 (anomal* or malform* or defect*1 or deformit*)).tw,kw. | 13675 |
| 18 | f?etal abnormalit*.tw,kw. | 3185 |
| 19 | (f?etus* adj (anomal* or abnormalit* or malform* or defect*1 or deform*)).tw,kw. | 377 |
| 20 | (((sever* or major) adj3 teratoid*) or ((abnormalit* or anomal* or defect*1) adj3 terato*) or monster or monsters or monstrui*).tw,kw. | 2569 |
| 21 | ((congen* adj2 (heart or cardiac or valvul*) adj2 (dis* or fail*)) or ((cardiac or intracard* or heart or cardiothor* or cardio‐thor* or cardiovasc* or cardio‐vasc* or (great* adj2 vessel*) or valvul* or valve*1) adj3 (anomal* or deformit* or defect*1 or abnormalit* or malformat* or atres* or atret* or d#splas* or d#smorph* or aplas* or agenes*))).tw,kw. | 116735 |
| 22 | (((major or sever* or critical or f?etal or f?etus* or prenat* or antenat*) adj3 CHD) or ((CHD or MCHD* or CCHD*) and congenital*)).tw,kw. | 15507 |
| 23 | (Fallot* or Ebstein* or coarct* or (CoA and aort*)).tw,kw. | 35586 |
| 24 | ((aort* adj1 arch* adj4 (hypoplas* or obstruct* or interrupt* or double or right or isolat*)) or (RAA and aort*) or ((vascular or aort*) adj2 (ring* or sling* or annul*)) or arter* lusor*).tw,kw. | 23326 |
| 25 | ((aort* adj4 (anomal* or abnormal* or deformit* or malformat* or defect* or stenos* or stenot* or atres* or atret* or d#splas* or anomal* or d#smorph* or aplas* or agenes*)) or aortosteno*).tw,kw. | 53945 |
| 26 | (double outlet* or DORV).tw,kw. | 3271 |
| 27 | ((hypoplas* adj4 (((left or right) adj3 (heart or ventric* or card* or chamber* or cavit*)) or RV* or LV*)) or ventric* hypoplas* or HLHS* or HLV* or HRHS*).tw,kw. | 8044 |
| 28 | (univentric* or monoventric* or ((single or mono or uni or triloculare bia*) adj2 (ventric* or cor))).tw,kw. | 11519 |
| 29 | ((outflow tract or OFT or conotrunc* or cono‐trunc*) adj6 (anomal* or abnormalit* or malformat* or defect*1 or deformit*)).tw,kw. | 2679 |
| 30 | ((transpos* adj6 (((great or large or main) adj2 (arter* or vessel*)) or congenital* or dextr*)) or TGA or TGAs or TGA's or CCTGA or CCTGAs or CTGA or CTGA's or DTGA or DTGAs or DTGA's).tw,kw. | 28387 |
| 31 | ((common arterial or arterios*) adj2 (trunk or truncus)).tw,kw. | 2853 |
| 32 | ((tricusp* or tri‐cusp* or TV or right AV or right atrioventr* or ((aort* or pulm*) adj2 valv*)) adj6 (steno* or regurg* or re‐gurg* or insuf* or incompet* or d#sfunct* or impair* or atres* or atret* or d#splas* or anomal* or malformat* or abnormalit* or deformit* or d#smorph* or aplas* or agenes*)).tw,kw. | 48596 |
| 33 | (pulmonar* adj3 (atres* or atret*)).tw,kw. | 5443 |
| 34 | (((septal or septum or septation or ((endocard* or AV or atri*ventr* or atri*‐ventr*) adj2 cushion*)) adj3 defect*) or ((heart or intracard* or intra‐card*) adj2 shunt*) or VSD or (comm* adj3 (sept* or AV or atri*ventr* or atri*‐ventr*) adj3 (canal* or osti*)) or canal defect* or AVSD* or CAVC* or (aort* adj2 window*) or APW or crisscross or criss‐cross).tw,kw. | 49367 |
| 35 | (scimitar* or (anomal* adj4 pulmon* ve?n*4 adj2 (return* or connect*)) or TAPVC* or PAPVC*).tw,kw. | 4737 |
| 36 | (heterotax* or isomerism* or (situs adj2 inver*) or ((lateral* or axis) adj2 defect*) or (malpos* adj2 complex*) or l*vocard* or dextrocard* or ((card* or heart) adj2 (dextrovers* or dextro‐vers* or dextrorotat* or dextro‐rotat* or dextroposit* or dextro‐posit*))).tw,kw. | 11273 |
| 37 | (((atrioventric* or atrio‐ventr* or AV) adj3 defect*) or ((ventric* or atrio* or VA or AV) adj3 discord*)).tw,kw. | 4530 |
| 38 | ((vascular or pulmonar* ve?n*) adj3 malformat*).tw,kw. | 13037 |
| 39 | ((non‐cardia* or noncardia* or extracard* or extra‐card* or nervous‐system or CNS or brain or cerebell* or cerebra* or neural‐tube* or craniospin* or cranio‐spin* or musc*skelet* or skelet* or spondyloepiphys* or epiphys* or bone or bones or osseous or spine or spinal or limb or limbs or extremit* or foot or feet or hand or hands or radial or radius or tibia* or ulna* or fibula* or orofac* or stomato* or craniofac* or maxill* or facial* or face or midfac* or oral or palat* or mouth* or lip or lips or (digest* adj2 (system or tract*)) or GI or gastrointest* or intestin* or bowel or gut or midgut or duoden* or ileum or ileal or ile*jejun* or jejun* or esophag* or oesophag* or trach* or abdom* or thoraco* or thoracic or thorax or (body adj3 (wall or stalk)) or bodystalk* or bodywall or respirator* or airway* or pulmonar* or lung or lungs or diaphragm* or hemidiaphragm* or sex or sexual or genit* or urogenit* or kidney* or renal or bladder) adj5 (defect*1 or anomal* or malform* or abnormalit* or deform* or d*smorph* or aplas* or d*splas* or atres* or atret* or agenes* or cleft* or misalign* or malalign* or malpos* or malrot* or ((mis or mal or wrong or incorrect or incomplet*) adj2 (rotat* or align* or position*)))).tw,kw. | 412700 |
| 40 | (encephalo*c*l* or cephaloc*el* or meningoencephaloc*el* or notoencephaloc*le or craniac*el* or ((cereb* or mening*) adj2 (hernia* or protru* or bulg*)) or exencephal* or anencephal* or acrani* or aprosencephal* or ((spin* or cranium or crania) adj2 (bifid* or open or cleft*)) or d#srap* or schistor*ach* or rachischis* or crani*‐schis* or crani*schis* or neur*‐schis* or neur*schis* or myeloc*le* or mening*myeloc* or hydrocephal* or hydro‐cephal* or holoprosencephal* or holo‐prosencephal* or arhinencephal*).tw,kw. | 70235 |
| 41 | ((developmental adj3 (bone or osseous) adj3 dis*) or ((bone or bones or osseous or limb or limbs) adj3 (reduct* or absent or absenc* or deficien* or missing or shorten* or very small* or lack* or deficit*)) or dwarfism* or achondroplasi* or achondrogenes* or hypochondrogenes* or thanatophor* or osteochondrod*splas* or osteod*splast* or osteod*d*stroph* or chondrod*splas* or chondrod*stroph* or radial ray* or talipes or equinovar* or equino‐var* or clubfoot or club‐foot or clubfeet or club‐feet or micromel* or d*smeli* or ectromel* or phocomel* or tetraphocomel* or amelia or hemimel* or palatolabio*chi* or labio*chi* or cheilo* or palat*chi* or harelip* or hare‐lip* or schi*oprosop*).tw,kw. | 52277 |
| 42 | (gastro*chis* or gastro‐schis* or ((umbil* or abdomin*) adj2 (hernia* or protru* or bulg*)) or omphaloc*el* or laparoc*el* or exomphal*).tw,kw. | 14795 |
| 43 | (((trach*sophag* or oesophag* or esophag*) adj4 fistula*) or ((bowel or intestin* or gastrointest* or GI or mid‐gut or midgut or duoden* or ileum or ileal or ile*jejun* or jejun*) adj3 obstruct*) or double bubble or volvulus).tw,kw. | 64043 |
| 44 | ((diaphragm* adj2 (hernia* or protru* or bulg*)) or (cystic adj2 adenomat* adj2 malformat*) or CCAM or CPAM or CAML).tw,kw. | 15314 |
| 45 | ((sex* adj2 (development or differentiat*) adj2 (diseas* or disorder* or defect*)) or intersex* or inter‐sex* or ((genital* or sex*) adj3 ambigu*)).tw,kw. | 10146 |
| 46 | (((cystic or polycyst* or multicyst*) adj2 (kidney* or (renal adj2 (diseas* or disorder*)))) or PKD or MCKD or (hyperechogen* adj2 kidney*) or megacystis).tw,kw. | 20399 |
| 47 | (hydrops adj3 f?etalis).tw,kw. | 3463 |
| 48 | or/1‐47 [ congenital defects; fetal (structural) anomalies ] | 1330673 |
| 49 | echography/ or echocardiography/ or exp doppler echocardiography/ or exp fetus echography/ or real time echography/ or transvaginal echography/ | 670611 |
| 50 | ultrasound scanner/ or echograph/ or exp cardiovascular ultrasound system/ or real time ultrasound scanner/ or ultrasound transducer/ | 41471 |
| 51 | prenatal screening/ or prenatal diagnosis/ | 69567 |
| 52 | early diagnosis/ | 128471 |
| 53 | (ultraso* or ultra‐so* or sonogra* or echo‐gra* or echogra* or TVUS* or TV‐US*).tw,kw. | 696324 |
| 54 | (((anatom* or screen* or routin* or two‐step* or 2‐step* or two‐stage* or 2‐stag* or two‐phase* or 2‐phase* or obstetric or obstetrical or transvagin* or trans‐vagin* or transabdom* or trans‐abdom* or prenat* or pre‐nat* or antenat* or ante‐nat* or f?etal or f?etus* or twodimension* or two‐dimension* or 2‐dimension* or 2d) adj2 (US or USS)) or 2dUS or 2dUSS).tw,kw. | 5909 |
| 55 | (standard* adj (US or USS)).tw,kw. | 560 |
| 56 | (echo not ((spin or gradient or T2 or T2W) adj3 echo)).tw,kw. | 61352 |
| 57 | (scan or scans or scanning or scanned).tw,kw. | 817219 |
| 58 | ((transabdom* or trans‐abdom* or transvag* or trans‐vag* or anatom* or anomal*) adj3 imag*).tw,kw. | 15939 |
| 59 | (nuchal adj3 translucen*).tw,kw. | 3515 |
| 60 | (echocard* or echo‐card* or ((cardiac or cardiovascul* or heart) adj3 (view or views))).tw,kw. | 302926 |
| 61 | ((((("4" or "four") adj chamber*) or outflow tract* or (("3" or "three") adj vessel*)) adj6 (view* or plane or planes or asses* or visual* or screen* or survey* or imag* or US or USS)) or 4CV or 4‐CV or 3VV or 3‐VV or 3VTV or 3V‐TV).tw,kw. [4 chamber‐, outflow tract‐ or 3 vessel‐view] | 7379 |
| 62 | ((prenat* or pre‐nat* or antenat* or ante‐nat* or f?etal or f?etus*) adj3 (diagnos* or detect* or screen*)).tw,kw. | 72729 |
| 63 | ((detect* or screen* or diagnos* or PD) adj3 (rate or rates)).tw,kw. | 104465 |
| 64 | (("over time" or trend) adj6 (diagn* or detect*)).tw,kw. | 13439 |
| 65 | (timing adj6 (detect* or diagnos* or screen*)).tw,kw. | 6052 |
| 66 | (routin* adj3 (screening or imaging or diagnostic test*)).tw,kw. | 31762 |
| 67 | (screening adj3 (national* or nationwide or nation‐wide or state‐wide or statewide)).tw,kw. | 10513 |
| 68 | anomaly screen* program*.tw,kw. | 67 |
| 69 | or/49‐68 [ ultrasonography incl echocardiography & nuchal translucency /scan /prenatal diagnosis ] | 2272632 |
| 70 | 48 and 69 [ I ultrasound (prenatal detection) of congenital structural defects ] | 246752 |
| 71 | fetus/ or "fetus (anatomy)"/ or fetus brain/ or fetus heart/ or fetus kidney/ or fetus lung/ or fetus myocardium/ or fetus disease/ or prenatal disorder/ or fetus malformation/ or exp fetus echography/ or prenatal care/ or prenatal diagnosis/ or prenatal screening/ or prenatal period/ or first trimester pregnancy/ or second trimester pregnancy/ or (f?etal or f?etus* or prenat* or pre‐nat* or antenat* or ante‐nat* or (nuchal adj3 translucen*) or (early adj (pregnan* or gestation* or gravidit*)) or ((first or 1st) adj2 half adj3 (pregnan* or gestat* or gravidit*)) or ((mid or first or 1st or second or 2nd) adj3 trimester*) or midtrimester* or midpregnan* or mid‐pregnan* or midgestat* or mid‐gestat*).tw,kw. or ((pregnancy or pregnant or gestation or gravidit*) and (("10" or 10th or ten or tenth or "11" or 11th or eleven* or "12" or 12th or twelve or twelfth or "13" or 13th or thirteen* or "14" or 14th or fourteen* or "18" or 18th or eighteen* or ((before or prior) adj2 ("20" or 20th or twenty or twentieth or "21" or 21st or twentyfirst or "22" or 22nd or twentysecond or "23" or 23rd or twentythird or "24" or 24th or twentyfourth))) adj5 (week* or wk or wks))).tw,kw. [prenatal filter] | 768165 |
| 72 | 70 and 71 [ I II ultrasound/prenatal detection of congenital structural defects + prenatal filter ] | 58252 |
| 73 | ((exp animal/ or animal experiment/ or exp animal model/ or exp experimental animal/ or exp female animal/ or exp veterinary medicine/) not human/) or (animal model* or mouse model* or sheep model* or ovine model* or large animal* or animal experiment*).ti,kw. or ((sheep or lamb or lambs or ovine or ewe or ewes or sow or sows) not human*).ti. [EXCLUSION ANIMALS] | 6311659 |
| 74 | editorial/ or "systematic review"/ or (editorial or conference abstract or conference review or note).pt. or (editorial or reply).ti. or cochrane.ti,jw. or ((review.pt. or review/ or (review or overview).ti. or case report*.mp,jw.) not (cohort analysis/ or longitudinal study/ or prospective study/ or retrospective study/ or exp clinical trial/ or trial.ti,ot,kw. or (cohort* or retrospectiv* or prospectiv* or crosssection* or cross‐section*).tw,kw.)) [EXCLUSION case reports/reviews/editorials/conference abstracts] | 11739567 |
| 75 | 73 or 74 [EXCLUSION ANIMALS/REVIEWS/CASE REPORTS/CONFERENCE ABSTRACT] | 17356775 |
| 76 | 72 not 75 [ I II ultrasound/prenatal detection of congenital structural defects ‐ original human studies ] | 24535 |
| 77 | limit 76 to yr="1997 ‐Current" [ I II ultrasound/prenatal detection of congenital structural defects + prenatal filter ‐ original human studies > 1997] | 18350 |
| 78 | 77 not medline.cr. [ I II ultrasound/prenatal detection of congenital structural defects + prenatal filter ‐ original human studies > 1997 embase records only ] | 15279 |
Appendix 3. Search strategy Science Citation Index Expanded (Web of Science), Social Sciences Citation Index (Web of Science), Arts & Humanities Citation Index and Emerging Sources Citation Index (Web of Science)
Database(s): Science Citation Index Expanded (Web of Science), Social Sciences Citation Index (Web of Science), Arts & Humanities Citation Index and Emerging Sources Citation Index (Web of Science) from 1 January 1997 to 22 July 2022 Search strategy: 22 July 2022
(TS=(((congenit* NEAR/6 (disorder* or diseas* or abnormal* or anomal* or malform* or deform* or defect or defects)) or birth‐defect* or ((severe or major) NEAR/3 (anomal* or abnormalit* or malformat* or defect or defects or deformit*)) or ((structural or structure OR structures or non‐chromosom* or nonchromosom* or anatom* or morphological* or isolated) NEAR/6 (anomal* or abnormalit* or malform* or deformit* or defect or defects or distort* or contort* or misshap*)) or ((fetal or foetal) NEAR/3 (anomal* or malform* or defect or defects or deformit*)) or fetal‐abnormalit* or foetal‐abnormalit* or fetus*‐anomal* or fetus*‐abnormalit* or fetus*‐malform* or fetus*‐defect or fetus*‐defects or fetus*‐deform* OR foetus*‐anomal* or foetus*‐abnormalit* or foetus*‐malform* or foetus*‐defect or foetus*‐defects or foetus*‐deform* or ((sever* or major) NEAR/3 teratoid*) or ((abnormalit* or anomal* or defect or defects) NEAR/3 terato*) or monster or monsters or monstrui* or (congen* NEAR/2 (heart or cardiac or valvul*) NEAR/2 (diseas* or disorder* or fail*)) or ((cardiac or intracard* or heart or cardiothor* or cardio‐thor* or cardiovasc* or cardio‐vasc* or (great* NEAR/2 vessel*) or valvul* or valve or valves) NEAR/3 (anomal* or deformit* or defect or defects or abnormalit* or malformat* or atres* or atret* or dysplas* or displas* or dysmorph* or dismorph* or aplas* or agenes*)) or ((major or sever* or critical or fetal or fetus* or foetal or foetus* or prenat* or antenat*) NEAR/3 CHD) or ((CHD or MCHD* or CCHD*) and congenital*) or Fallot* or Ebstein* or coarct* or (CoA and aort*) or (aort*‐arch* NEAR/4 (hypoplas* or obstruct* or interrupt* or double or right or isolat*)) or (RAA and aort*) or ((vascular or aort*) NEAR/2 (ring* or sling* or annul*)) or arter*‐lusor* or (aort* NEAR/4 (anomal* or abnormal* or deformit* or malformat* or defect* or stenos* or stenot* or atres* or atret* or dysplas* or displas* or anomal* or dysmorph* or dismorph or aplas* or agenes*)) or aortosteno* or double‐outlet* or DORV or (hypoplas* NEAR/4 (((left or right) NEAR/3 (heart or ventric* or card* or chamber* or cavit*)) or RV or LV)) or ventric*‐hypoplas* or HLHS* or HLV* or HRHS* or univentric* or monoventric* or ((single or mono or uni or triloculare‐bia*) NEAR/2 (ventric* or cor)) or ((outflow‐tract or OFT or conotrunc* or cono‐trunc*) NEAR/6 (anomal* or abnormalit* or malformat* or defect or defects or deformit*)) or (transpos* NEAR/6 (((great or large or main) NEAR/2 (arter* or vessel*)) or congenital* or dextr*)) or TGA or TGAs or TGA's or CCTGA or CCTGAs or CTGA or CTGA's or DTGA or DTGAs or DTGA's or ((common‐arterial or arterios*) NEAR/2 (trunk or truncus)) or ((tricusp* or tri‐cusp* or TV or right‐AV or right‐atrioventr* or ((aort* or pulm*) NEAR/2 valv*)) NEAR/6 (steno* or regurg* or re‐gurg* or insuf* or incompet* or dysfunct* or disfunct* or impair* or atres* or atret* or dysplas* or displas* or anomal* or malformat* or abnormalit* or deformit* or dysmorph* or dismorph* or aplas* or agenes*)) or ((septal or septum or septation or ((endocard* or AV or atri*ventr* or atri*‐ventr*) NEAR/2 cushion*)) NEAR/3 defect*) or ((heart or intracard* or intra‐card*) NEAR/2 shunt*) or VSD or (comm* NEAR/3 (sept* or AV or atri*ventr* or atri*‐ventr*) NEAR/3 (canal* or osti*)) or canal‐defect* or AVSD* or CAVC* or (aort* NEAR/2 window*) or APW or crisscross or criss‐cross or scimitar* or (anomal* NEAR/4 pulmon*‐ve* NEAR/2 (return* or connect*)) or TAPVC* or PAPVC* or heterotax* or isomerism* or (situs NEAR/2 inver*) or ((lateral* or axis) NEAR/2 defect*) or (malpos* NEAR/2 complex*) or l*vocard* or dextrocard* or ((card* or heart) NEAR/2 (dextrovers* or dextro‐vers* or dextrorotat* or dextro‐rotat* or dextroposit* or dextro‐posit*)) or ((atrioventric* or atrio‐ventr* or AV) NEAR/3 defect*) or ((ventric* or atrio* or VA or AV) NEAR/3 discord*) or ((vascular or pulmon*‐ve*) NEAR/3 malformat*) or ((non‐cardia* or noncardia* or extracard* or extra‐card* or nervous‐system or CNS or brain or cerebell* or cerebra* or neural‐tube* or craniospin* or cranio‐spin* or musc*skelet* or skelet* or spondyloepiphys* or epiphys* or bone or bones or osseous or spine or spinal or limb or limbs or extremit* or foot or feet or hand or hands or radial or radius or tibia* or ulna* or fibula* or orofac* or stomato* or craniofac* or maxill* or facial* or face or midfac* or oral or palat* or mouth* or lip or lips or (digest* NEAR/2 (system or tract*)) or GI or gastrointest* or intestin* or bowel or gut or midgut or duoden* or ileum or ileal or ile*jejun* or jejun* or esophag* or oesophag* or trach* or abdom* or thoraco* or thoracic or thorax or (body NEAR/3 (wall or stalk)) or bodystalk* or bodywall or respirator* or airway* or pulmonar* or lung or lungs or diaphragm* or hemidiaphragm* or sex or sexual or genit* or urogenit* or kidney* or renal or bladder) NEAR/5 (defect or defects or anomal* or malform* or abnormalit* or deform* or d*smorph* or aplas* or d*splas* or atres* or atret* or agenes* or cleft* or misalign* or malalign* or malpos* or malrot* or ((mis or mal or wrong or incorrect or incomplet*) NEAR/2 (rotat* or align* or position*)))) or encephalo*c*l* or cephaloc*el* or meningoencephaloc*el* or notoencephaloc*le or craniac*el* or ((cereb* or mening*) NEAR/2 (hernia* or protru* or bulg*)) or exencephal* or anencephal* or acrani* or aprosencephal* or ((spin* or cranium or crania) NEAR/2 (bifid* or open or cleft*)) or dysrap* or disrap* or schistor*ach* or rachischis* or crani*‐schis* or crani*schis* or neur*‐schis* or neur*schis* or myeloc*le* or mening*myeloc* or hydrocephal* or hydro‐cephal* or holoprosencephal* or holo‐prosencephal* or arhinencephal* or (developmental NEAR/3 (bone or osseous) NEAR/3 dis*) or ((bone or bones or osseous or limb or limbs) NEAR/3 (reduct* or absent or absenc* or deficien* or missing or shorten* or very‐small* or lack* or deficit*)) or dwarfism* or achondroplasi* or achondrogenes* or hypochondrogenes* or thanatophor* or osteochondrod*splas* or osteod*splast* or osteod*d*stroph* or chondrod*splas* or chondrod*stroph* or radial‐ray* or talipes or equinovar* or equino‐var* or clubfoot or club‐foot or clubfeet or club‐feet or micromel* or d*smeli* or ectromel* or phocomel* or tetraphocomel* or amelia or hemimel* or palatolabio*chi* or labio*chi* or cheilo* or palat*chi* or harelip* or hare‐lip* or schi*oprosop* or gastro*chis* or gastro‐schis* or ((umbil* or abdomin*) NEAR/2 (hernia* or protru* or bulg*)) or omphaloc*el* or laparoc*el* or exomphal* or ((trach*sophag* or oesophag* or esophag*) NEAR/4 fistula*) or ((bowel or intestin* or gastrointest* or GI or mid‐gut or midgut or duoden* or ileum or ileal or ile*jejun* or jejun*) NEAR/3 obstruct*) or double‐bubble or volvulus or (diaphragm* NEAR/2 (hernia* or protru* or bulg*)) or (cystic NEAR/2 adenomat* NEAR/2 malformat*) or CCAM or CPAM or CAML or (sex* NEAR/2 (development or differentiat*) NEAR/2 (diseas* or disorder* or defect*)) or intersex* or inter‐sex* or ((genital* or sex*) NEAR/3 ambigu*) or ((cystic or polycyst* or multicyst*) NEAR/2 (kidney* or (renal NEAR/2 (diseas* or disorder*)))) or PKD or MCKD or (hyperechogen* NEAR/2 kidney*) or megacystis OR (hydrops NEAR/3 (fetalis or foetalis)) or ((hydronephros* or hydro‐nephros* or pyelectas* or (uret* NEAR/3 (obstruct* or defect or defects or anomal* or malformat* or abnormalit* or deformit* or dismorph* or dysmorph* or aplas* or dysplas* or displas* or atres* or atret* or agenes*))) and ((("10" or ten or tenth or "11" or 11th or eleven* or "12" or 12th or twelv* or twelfth or "13" or 13th or thirteen* or "14" or 14th or fourteen* or "15" or 15th or fifteen* or "16" or 16th or sixteen* or "17" or 17th or seventeen* or "18" or 18th or eighteen* or "19" or 19th or nineteen* or "20" or 20th or twentieth or twenty or "21" or 21st or "22" or 22nd or "23" or 23rd or "24" or 24th) NEAR/5 (week* or wk or wks)) or ((early or ((first or 1st) NEAR/2 half) or mid or midterm) NEAR/2 (pregnan* or gestat* or gravidit*)) or ((mid or first or 1st or second or 2nd) NEAR/3 trimester*) or midtrimester or midpregnan* or midgestat*))) AND (ultraso* or ultra‐so* or sonogra* or echo‐gra* or echogra* or TVUS* or TV‐US* or ((anatom* or screen* or routin* or two‐step* or 2‐step* or two‐stage* or 2‐stag* or two‐phase* or 2‐phase* or obstetric or obstetrical or transvagin* or trans‐vagin* or transabdom* or trans‐abdom* or prenat* or pre‐nat* or antenat* or ante‐nat* or fetal or foetal or fetus* or foetus* or twodimension* or two‐dimension* or 2‐dimension* or 2d) NEAR/2 (US or USS)) or 2dUS or 2dUSS or standard*‐US or standard*‐USS or (echo not ((spin or gradient or T2 or T2W) NEAR/3 echo)) or scan or scans or scanning or scanned or ((transabdom* or trans‐abdom* or transvag* or trans‐vag* or anatom* or anomal*) NEAR/3 imag*) or (nuchal NEAR/3 translucen*) or echocard* or echo‐card* or ((cardiac or cardiovascul* or heart) NEAR/3 (view or views)) or ((4‐chamber* or four‐chamber* or outflow‐tract* or 3‐vessel* or three‐vessel*) NEAR/6 (view* or plane or planes or asses* or visual* or screen* or survey* or imag* or US or USS)) or 4CV or 4‐CV or 3VV or 3‐VV or 3VTV or 3V‐TV or ((prenat* or pre‐nat* or antenat* or ante‐nat* or fetal or fetus* or foetal or foetus*) NEAR/3 (diagnos* or detect* or screen*)) or ((detect* or screen* or diagnos* or PD) NEAR/3 (rate or rates)) OR (("over‐time" or trend) NEAR/6 (diagn* or detect*)) or (timing NEAR/6 (detect* or diagnos* or screen*)) or (routin* NEAR/3 (screening or imaging or diagnostic‐test*)) or (screening NEAR/3 (national* or nationwide or nation‐wide or state‐wide or statewide)) or anomaly‐screen*‐program*)) AND TS=(fetal or foetal or fetus* or foetus* or prenat* or pre‐nat* or antenat* or ante‐nat* or (nuchal NEAR/3 translucen*) or early‐pregnan* or early‐gestation* or early‐gravidit* or ((first or 1st) NEAR/2 half NEAR/3 (pregnan* or gestat* or gravidit*)) or ((mid or first or 1st or second or 2nd) NEAR/3 trimester*) or midtrimester* or midpregnan* or mid‐pregnan* or midgestat* or mid‐gestat* or ((pregnancy or pregnant or gestation or gravidit*) and (("10" or 10th or ten or tenth or "11" or 11th or eleven* or "12" or 12th or twelve or twelfth or "13" or 13th or thirteen* or "14" or 14th or fourteen* or "18" or 18th or eighteen* or ((before or prior) NEAR/2 ("20" or 20th or twenty or twentieth or "21" or 21st or twentyfirst or "22" or 22nd or twentysecond or "23" or 23rd or twentythird or "24" or 24th or twentyfourth))) NEAR/5 (week* or wk or wks))))) NOT (TI=(((animal* or pig or pigs or goat or goats* or sheep or lamb or lambs or ovine or ewe or ewes or sow or sows or ovine or rodent* or rabbit* or mice or mouse or murine* or rat or rats or dog or dogs) NOT human*) or editorial or reply or comment or review or overview OR case‐reports) OR TS=(case‐report OR conferenc* OR congres*))
Appendix 4. Modified QUADAS‐2 tool
|
Signalling question ↓ |
Signalling question ↓ |
Signalling question ↓ |
Risk of bias ↓ |
Applicability ↓ |
| Domain 1. Patient selection | ||||
| Was a consecutive or random sample of patients enrolled? | Was a case‐control design avoided? | Did the study avoid inappropriate exclusions? | Could the selection of patients have introduced bias? | Are there any concerns that the included patients may not represent an unselected or low‐risk population? |
| “Yes” if patients were enrolled consecutively, if all eligible patients were enrolled, or if a random sample of patients were enrolled in the study. “No” if patients were selected from those eligible. “Unclear” if patient selection was not clear from the report |
“Yes” if a case‐control study design was avoided. “No” if a case‐control study design was used. “Unclear” if the study design could not be determined based on the report. |
“Yes” if the study avoided inappropriate exclusions (e.g. only excluding multiple pregnancies, high‐risk pregnancies, aneuploid fetuses). “No” if participants were excluded inappropriately (e.g. based on expected difficulties in visualising fetal structures). “Unclear” if appropriateness of exclusion from the study could not be derived from the report. |
“Low risk” if all signalling questions were answered with “yes”. “High” or “unclear risk” if “no” or “unclear” was answered to one or more of the signalling questions. |
“Low concern” if the sample of included patients represented an unselected or low‐risk population. “High concern” if the sample of women represent a selected or high‐risk population. “Unclear concern” if patient selection methods may or may not have resulted in a patient population that differs from our review question, or if insufficient information was available. |
| Domain 2. Index test | ||||
| Were index test results interpreted without knowledge of the results of the reference standard? | If a threshold was used, was it prespecified? | Could the conduct or interpretation of the index test have introduced bias? | Are there concerns that the index test, its conduct, or its interpretation differ from the review question? | |
| “Yes” if the ultrasound results were interpreted without knowledge of the pregnancy or postnatal outcome. “No” if the ultrasound results were interpreted with knowledge of the pregnancy or postnatal outcome. “Unclear” if this was not clear from the report. |
“Yes” if criteria for a positive test were prespecified. “No” if the criteria for a positive test were not prespecified. “Unclear” if this was not clear from the report. |
“Low risk” if all signalling questions were answered with “yes”. “High” or “unclear risk” if “no” or “unclear” was answered to one or more of the signalling questions. |
“Low concern” if the aim of the ultrasound scan included evaluation of fetal anatomy to screen for structural anomalies. “High concern” if the primary purpose of the ultrasound scan differs from our review question. “Unclear concern” if insufficient information was available. |
|
| Domain 3. Reference standard | ||||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Were the reference standard results interpreted without knowledge of the results of the index test? | Could the reference standard, its conduct, or interpretation have introduced bias? | Are there concerns that the target condition as defined by the reference standard does not match the review question? |
| “Yes” if an acceptable reference standard was used (e.g. postnatal examination, postnatal follow‐up, postmortem examination). “No” if no acceptable reference standard was used. “Unclear” if this was not clear from the report. |
“Yes” if the reference standard (e.g. postnatal follow‐up) is likely to detect cases presenting after discharge from postnatal care. “No” if the reference standard is unlikely to detect cases presenting after discharge from postnatal care. “Unclear” if this was not clear from the report. |
“Yes” if the diagnosis of presence or absence of structural abnormalities was made without the knowledge of the results of the ultrasound scan. “No” if the diagnosis of the presence or absence of structural abnormalities was made with knowledge of the ultrasound scan. “Unclear” if this was not clear from the report. |
“Low risk” if all signalling questions were answered with “yes”. “High” or “unclear risk” if “no” or “unclear” was answered to one or more of the signalling questions. |
“Low concern” if an acceptable reference standard was used and was interpreted without knowledge of the index test results. “High concern” if no reference standard was used or if the reference standard was used with knowledge of the index test results. “Unclear concern” if insufficient information was available. |
| Domain 4. Flow and timing | ||||
| Did all live‐born infants receive a reference standard? | Did all live‐born infants receive the same reference standard? | Did all cases of fetal or perinatal loss receive the reference standard (including miscarriage, termination of pregnancy, intra‐uterine death, still birth, perinatal mortality)? | Were all patients included in the analysis? | Could the patient flow have introduced bias? |
| “Yes” if all live‐born infants received a reference standard. “No” if not all live‐born infants received a reference standard. “Unclear” if this was not clear from the report. |
“Yes” if all live‐born infants received the same reference standard. “No” if not all live‐born infants received the same reference standard. “Unclear” if this was not clear from the report. |
“Yes” if all cases of fetal or perinatal loss received the reference standard. “No” if not all cases of fetal or perinatal loss received the reference standard. “Unclear” if this was not clear from the report. |
“Yes” if all patients recruited to participate in the study were included in the final analysis. “No” if not all participants were included in the final analysis. “Unclear” if this was not clear from the report. |
“Low risk” if all signalling questions were answered with “yes”. “High” or “unclear risk” if “no” or “unclear” was answered to one or more of the signalling questions. |
Appendix 5. Sensitivity analyses
| Sensitivity analyses | ||||
| Fetuses affected | Anomalies | |||
| Studies | Sensitivity (95% CI) | Studies | Sensitivity (95% CI) | |
| First‐trimester scan | ||||
| Summary estimate of all included studies | 20 | 37.5 (31.1 to 44.3) | 20 | 39.2 (32.5 to 46.2) |
| Summary estimate of studies that reported complete 2 x 2 tables | 10 | 35.5 (27.0 to 45.0) | 9 | 36.7 (26.2 to 48.6) |
| Summary estimate of studies at low risk of bias ‐ patient selection | 17 | 35.1 (28.6 to 42.2) |
17 | 37.4 (30.1 to 45.3) |
| Summary estimate of studies with low applicability concerns ‐ patient selection | 4 | N/A* | 3 | N/A* |
| Summary estimate of studies at low risk of bias ‐ index test | 20 | 37.5 (31.1 to 44.3) |
20 | 39.2 (32.5 to 46.2) |
| Summary estimate of studies with low applicability concerns ‐ index test | 16 | 40.3 (32.9 to 48.2) |
16 | 42.4 (35.3 to 49.9) |
| Summary estimate of studies at low risk of bias ‐ reference standard | 2 | N/A* | 2 | N/A* |
| Summary estimate of studies with low applicability concerns ‐ reference standard | 20 | 37.5 (31.1 to 44.3) | 20 | 39.2 (32.5 to 46.2) |
| Summary estimate of studies at low risk of bias ‐ flow and timing | 1 | N/A* | 1 | N/A* |
| Summary estimate, excluding studies that used data obtained from birth defect registries | 20 | 37.5 (31.1 to 44.3) | 20 | 39.2 (32.5 to 46.2) |
| Summary estimate, excluding studies with fewer than 10 cases that had the target condition | 17 | 37.6 (30.9 to 41.6) | 18 | 39.3 (32.4 to 46.5) |
| Summary estimate, excluding studies with ≥ 10% lost to follow‐up | 15 | 36.6 (29.1 to 44.7) | 15 | 36.9 (29.0 to 45.5) |
| First + second‐trimester scan | ||||
| Summary estimate of all included studies | 18 | 83.8 (74.7 to 90.1) | 17 | 84.5 (75.8 to 90.5) |
| Summary estimate of studies that reported complete 2 x 2 tables | 10 | 82.6 (71.2 to 90.1) | 8 | 84.0 (71.9 to 91.6) |
| Summary estimate of studies at low risk of bias ‐ patient selection | 15 | 82.4 (71.7 to 89.7) | 14 | 83.0 (72.7 to 90.0) |
| Summary estimate of studies with low applicability concerns ‐ patient selection | 4 | N/A* | 3 | N/A* |
| Summary estimate of studies at low risk of bias ‐ index test | 18 | 83.8 (74.7 to 90.1) | 17 | 84.5 (75.8 to 90.5) |
| Summary estimate of studies with low applicability concerns ‐ index test | 16 | 82.6 (72.3 to 89.7) | 15 | 83.3 (73.3 to 90.1) |
| Summary estimate of studies at low risk of bias ‐ reference standard | 2 | N/A* | 2 | N/A* |
| Summary estimate of studies with low applicability concerns ‐ reference standard | 18 | 83.8 (74.7 to 90.1) | 17 | 84.5 (75.8 to 90.5) |
| Summary estimate of studies at low risk of bias ‐ flow and timing | 1 | N/A* | 1 | N/A* |
| Summary estimate, excluding studies that used data obtained from birth defect registries | 18 | 83.8 (74.7 to 90.1) | 17 | 84.5 (75.8 to 90.5) |
| Summary estimate, excluding studies with fewer than 10 cases that had the target condition | 15 | 83.4 (73.3 to 90.2) | 15 | 84.4 (74.9 to 90.8) |
| Summary estimate, excluding studies with ≥ 10% lost to follow‐up | 14 | 84.3 (74.5 to 90.8) | 14 | 85.2 (76.0 to 91.3) |
| Single second‐trimester scan | ||||
| Summary estimate | 9 | 50.5 (38.5 to 62.4) | 3 | 52.2 (33.2 to 70.7) |
| Summary estimate of studies that reported complete 2 x 2 tables | 5 | 59.3 (42.4 to 74.2) | N/A* | N/A* |
| Summary estimate of studies at low risk of bias ‐ patient selection | 7 | 52.5 (38.6 to 66.1) | N/A* | N/A* |
| Summary estimate of studies with low applicability concerns ‐ patient selection | 8 | 53.2 (40.8 to 65.2) | N/A* | N/A* |
| Summary estimate of studies at low risk of bias ‐ index test | 9 | 50.5 (38.5 to 62.4) | N/A* | N/A* |
| Summary estimate of studies with low applicability concerns ‐ index test | 9 | 50.5 (38.5 to 62.4) | N/A* | N/A* |
| Summary estimate of studies at low risk of bias ‐ reference standard | 4 | N/A* | N/A* | N/A* |
| Summary estimate of studies with low applicability concerns ‐ reference standard | 9 | 50.5 (38.5 to 62.4) | N/A* | N/A* |
| Summary estimate of studies at low risk of bias ‐ flow and timing | 1 | N/A* | N/A* | N/A* |
| Summary estimate, excluding studies that used data obtained from birth defect registries | 8 | 52.2 (33.2 to 70.6) | N/A* | N/A* |
| Summary estimate, excluding studies with fewer than 10 cases that had the target condition | 9 | 50.5 (38.5 to 62.4) | N/A* | N/A* |
| Summary estimate, excluding studies with ≥ 10% lost to follow‐up | 9 | 50.5 (38.5 to 62.4) | N/A* | N/A* |
| *N/A: sensitivity analysis was not performed if four or fewer studies were available. | ||||
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.

First‐trimester scan: fetuses affected
2. Test.

First‐trimester scan: anomalies
3. Test.

First‐trimester scan: subgroup analysis*
5. Test.

First‐trimester scan: major anomalies
6. Test.

First‐trimester scan: lethal anomalies
7. Test.

First‐trimester scan: severe or moderate anomalies
8. Test.

First‐trimester scan: minor anomalies
9. Test.

First‐trimester scan: central nevous system overall
10. Test.

First‐trimester scan: anencephaly
11. Test.

First‐trimester scan: spina bifida
12. Test.

First‐trimester scan: holoprosencephaly
13. Test.

First‐trimester scan: hydrocephalus
14. Test.

First‐trimester scan: encephalocele
15. Test.

First‐trimester scan: central nervous system misc
16. Test.

First‐trimester scan: respiratory overall
17. Test.

First‐trimester scan: CDH
18. Test.

First‐trimester scan: CPAM
19. Test.

First‐trimester scan: respiratory misc
20. Test.

First‐trimester scan: cardiac overall
21. Test.

First‐trimester scan: septal defect
22. Test.

First‐trimester scan: valvular anomaly (biventricular heart)
23. Test.

First‐trimester scan: venous return anomaly
24. Test.

First‐trimester scan: aortic arch anomaly
25. Test.

First‐trimester scan: conotruncal anomaly
26. Test.

First‐trimester scan: HRHS
27. Test.

First‐trimester scan: HLHS
28. Test.

First‐trimester scan: other single ventricle defect
29. Test.

First‐trimester scan: complex defect with atrial isomerism
30. Test.

First‐trimester scan: miscellaneous and unspecified major cardiac defects
31. Test.

First‐trimester scan: minor cardiac defects
32. Test.

First‐trimester scan: thoracic and abdominal wall overall
33. Test.

First‐trimester scan: gastroschisis
34. Test.

First‐trimester scan: omphalocele
35. Test.

First‐trimester scan: thoracic and abdominal wall misc
36. Test.

First‐trimester scan: gastrointestinal overall
37. Test.

First‐trimester scan: oesophageal atresia
38. Test.

First‐trimester scan: duodenal atresia
39. Test.

First‐trimester scan: small bowel atresia
40. Test.

First‐trimester scan: gastrointestinal misc
41. Test.

First‐trimester scan: urinary tract overall
42. Test.

First‐trimester scan: renal agenesis overall
43. Test.

First‐trimester scan: renal agenesis unilateral
44. Test.

First‐trimester scan: renal agenesis bilateral
45. Test.

First‐trimester scan: MCDK overall
46. Test.

First‐trimester scan: MCDK unilateral
47. Test.

First‐trimester scan: MCDK bilateral
48. Test.

First‐trimester scan: other renal dysplasias overall
49. Test.

First‐trimester scan: other renal dysplasias unilateral
50. Test.

First‐trimester scan: other renal dysplasias bilateral
51. Test.

First‐trimester scan: upper urinary tract obstruction
52. Test.

First‐trimester scan: lower urinary tract obstruction
53. Test.

First‐trimester scan: urinary tract misc.
54. Test.

First‐trimester scan: genital overall
55. Test.

First‐trimester scan: ambiguous genitalia
56. Test.

First‐trimester scan: genital misc
57. Test.

First‐trimester scan: musculoskeletal overall
58. Test.

First‐trimester scan: skeletal dysplasias overall
59. Test.

First‐trimester scan: lethal skeletal dysplasia
60. Test.

First‐trimester scan: non‐lethal skeletal dysplasia
61. Test.

First‐trimester scan: limb reduction defect
62. Test.

First‐trimester scan: talipes
63. Test.

First‐trimester scan: musculoskeletal misc.
64. Test.

First‐trimester scan: ear face and neck overall
65. Test.

First‐trimester scan: orofacial clefts overall
66. Test.

First‐trimester scan: cleft lip
67. Test.

First‐trimester scan: cleft lip and cleft palate
68. Test.

First‐trimester scan: cleft palate
69. Test.

First‐trimester scan: ear face and neck misc
70. Test.

First‐trimester scan: multisystem anomalies overall
71. Test.

First‐trimester scan: fetal hydrops
72. Test.

First‐trimester scan: MCA or syndrome
73. Test.

First‐trimester scan: other
74. Test.

First + second‐trimester scan: fetuses affected
75. Test.

First + second‐trimester scan: anomalies
76. Test.

First + second‐trimester scan: subgroup analysis*
78. Test.

First + second‐trimester scan: major anomalies
79. Test.

First + second‐trimester scan: lethal anomalies
80. Test.

First + second‐trimester scan: severe or moderate anomalies
81. Test.

First + second‐trimester scan: minor anomalies
82. Test.

First + second‐trimester scan: central nervous system overall
83. Test.

First + second‐trimester scan: anencephaly
84. Test.

First + second‐trimester scan: spina bifida
85. Test.

First + second‐trimester scan: holoprosencephaly
86. Test.

First + second‐trimester scan: hydrocephalus
87. Test.

First + second‐trimester scan: encephalocele
88. Test.

First + second‐trimester scan: central nervous system misc
89. Test.

First + second‐trimester scan: respiratory overall
90. Test.

First + second‐trimester scan: CDH
91. Test.

First + second‐trimester scan: CPAM
92. Test.

First + second‐trimester scan: respiratory misc
93. Test.

First + second‐trimester scan: cardiac overall
94. Test.

First + second‐trimester scan: septal defect
95. Test.

First + second‐trimester scan: valvular anomaly (biventricular heart)
96. Test.

First + second‐trimester scan: venous return anomaly
97. Test.

First + second‐trimester scan: aortic arch anomaly
98. Test.

First + second‐trimester scan: conotruncal anomaly
99. Test.

First + second‐trimester scan: HRHS
TST-100. Test.

First + second‐trimester scan:: HLHS
TST-101. Test.

First + second‐trimester scan: other single ventricle defect
TST-102. Test.

First + second‐trimester scan: complex defect with atrial isomerism
TST-103. Test.

First + second‐trimester scan: miscellaneous cardiac defects
TST-104. Test.

First + second‐trimester scan: minor cardiac defects
TST-105. Test.

First + second‐trimester scan: thoracic and abdominal wall overall
TST-106. Test.

First + second‐trimester scan: gastroschisis
TST-107. Test.

First + second‐trimester scan: omphalocele
TST-108. Test.

First + second‐trimester scan: thoracic and abdominal wall misc
TST-109. Test.

First + second‐trimester scan: gastrointestinal overall
TST-110. Test.

First + second‐trimester scan: oesophageal atresia
TST-111. Test.

First + second‐trimester scan: duodenal atresia
TST-112. Test.

First + second‐trimester scan: small bowel atresia
TST-113. Test.

First + second‐trimester scan: gastrointestinal misc
TST-114. Test.

First + second‐trimester scan: urinary tract overall
TST-115. Test.

First + second‐trimester scan: renal agenesis overall
TST-116. Test.

First + second‐trimester scan: renal agenesis unilateral
TST-117. Test.

First + second‐trimester scan: renal agenesis bilateral
TST-118. Test.

First + second‐trimester scan: MCDK overall
TST-119. Test.

First + second‐trimester scan: MCDK unilateral
TST-120. Test.

First + second‐trimester scan: MCDK bilateral
TST-121. Test.

First + second‐trimester scan: other renal dysplasias overall
TST-122. Test.

First + second‐trimester scan: other renal dysplasias unilateral
TST-123. Test.

First + second‐trimester scan: other renal dysplasias bilateral
TST-124. Test.

First + second‐trimester scan: upper urinary tract obstruction
TST-125. Test.

First + second‐trimester scan: lower urinary tract obstruction
TST-126. Test.

First + second‐trimester scan: urinary tract misc.
TST-127. Test.

First + second‐trimester scan: genital overall
TST-128. Test.

First + second‐trimester scan: ambiguous genitalia
TST-129. Test.

First + second‐trimester scan: genital misc
TST-130. Test.

First + second‐trimester scan: musculoskeletal overall
TST-131. Test.

First + second‐trimester scan: skeletal dysplasia overall
TST-132. Test.

First + second‐trimester scan: lethal skeletal dysplasia
TST-133. Test.

First + second‐trimester scan: non‐lethal skeletal dysplasia
TST-134. Test.

First + second‐trimester scan: limb reduction defect
TST-135. Test.

First + second‐trimester scan: talipes
TST-136. Test.

First + second‐trimester scan: musculoskeletal misc
TST-137. Test.

First + second‐trimester scan: ear face and neck overall
TST-138. Test.

First + second‐trimester scan: orofacial clefts overall
TST-139. Test.

First + second‐trimester scan: cleft lip
TST-140. Test.

First + second‐trimester scan: cleft lip and palate
TST-141. Test.

First + second‐trimester scan: cleft palate
TST-142. Test.

First + second‐trimester scan: ear face and neck misc.
TST-143. Test.

First + second‐trimester scan: multisystem anomalies overall
TST-144. Test.

First + second‐trimester scan: fetal hydrops
TST-145. Test.

First + second‐trimester scan: MCA or syndrome
TST-146. Test.

First + second‐trimester scan: other
TST-147. Test.

Single second‐trimester scan: fetuses affected
TST-148. Test.

Single second‐trimester scan: anomalies
TST-149. Test.

Single second‐trimester scan: subgroup analysis*
TST-151. Test.

Single second‐trimester scan: major anomalies
TST-152. Test.

Single second‐trimester scan: lethal anomalies
TST-153. Test.

Single second‐trimester scan: severe or moderate anomalies
TST-154. Test.

Single second‐trimester scan: minor abnormalities
TST-155. Test.

Single second‐trimester scan: central nervous system overall
TST-156. Test.

Single second‐trimester scan: anencephaly
TST-157. Test.

Single second‐trimester scan: spina bifida
TST-158. Test.

Single second‐trimester scan: holoprosencephaly
TST-159. Test.

Single second‐trimester scan: hydrocephalus
TST-160. Test.

Single second‐trimester scan: encephalocele
TST-161. Test.

Single second‐trimester scan: central nervous system misc
TST-162. Test.

Single second‐trimester scan: respiratory overall
TST-163. Test.

Single second‐trimester scan: CDH
TST-164. Test.

Single second‐trimester scan: CPAM
TST-165. Test.

Single second‐trimester scan: respiratory misc
TST-166. Test.

Single second‐trimester scan: cardiac overall
TST-167. Test.

Single second‐trimester scan: septal defect
TST-168. Test.

Single second‐trimester scan: valvular anomaly (biventricular heart)
TST-169. Test.

Single second‐trimester scan: venous return anomaly
TST-170. Test.

Single second‐trimester scan: aortic arch anomaly
TST-171. Test.

Single second‐trimester scan: conotruncal anomaly
TST-172. Test.

Single second‐trimester scan: HRHS
TST-173. Test.

Single second‐trimester scan: HLHS
TST-174. Test.

Single second‐trimester scan: other single ventricle defect
TST-175. Test.

Single second‐trimester scan: complex defects with atrial isomerism
TST-176. Test.

Single second‐trimester scan: miscellaneous and unspecified major cardiac defects
TST-177. Test.

Single second‐trimester scan: minor cardiac defects
TST-178. Test.

Single second‐trimester scan: thoracic and abdominal wall overall
TST-179. Test.

Single second‐trimester scan: gastroschisis
TST-180. Test.

Single second‐trimester scan: omphalocele
TST-181. Test.

Single second‐trimester scan: thoracic and abdominal wall misc
TST-182. Test.

Single second‐trimester scan: gastrointestinal overall
TST-183. Test.

Single second‐trimester scan: oesophageal atresia
TST-184. Test.

Single second‐trimester scan: duodenal atresia
TST-185. Test.

Single second‐trimester scan: small bowel atresia
TST-186. Test.

Single second‐trimester scan: gastrointestinal misc
TST-187. Test.

Single second‐trimester scan: urinary tract overall
TST-188. Test.

Single second‐trimester scan: renal agenesis overall
TST-189. Test.

Single second‐trimester scan: renal agenesis unilateral
TST-190. Test.

Single second‐trimester scan: renal agenesis bilateral
TST-191. Test.

Single second‐trimester scan: MCDK overall
TST-192. Test.

Single second‐trimester scan: MCDK unilateral
TST-193. Test.

Single second‐trimester scan: MCDK bilateral
TST-194. Test.

Single second‐trimester scan: other renal dysplasias overall
TST-195. Test.

Single second‐trimester scan: other renal dysplasias unilateral
TST-196. Test.

Single second‐trimester scan: other renal dysplasias bilateral
TST-197. Test.

Single second‐trimester scan: upper urinary tract obstruction
TST-198. Test.

Single second‐trimester scan: lower urinary tract obstruction
TST-199. Test.

Single second‐trimester scan: urinary tract misc
TST-200. Test.

Single second‐trimester scan: genital overall
TST-201. Test.

Single second‐trimester scan: ambiguous genitalia
TST-202. Test.

Single second‐trimester scan: genital misc
TST-203. Test.

Single second‐trimester scan: musculoskeletal overall
TST-204. Test.

Single second‐trimester scan: skeletal dyplasia overall
TST-205. Test.

Single second‐trimester scan: lethal skeletal dysplasia
TST-206. Test.

Single second‐trimester scan: non‐lethal skeletal dysplasia
TST-207. Test.

Single second‐trimester scan: limb reduction defect
TST-208. Test.

Single second‐trimester scan: talipes
TST-209. Test.

Single second‐trimester scan: musculoskeletal misc
TST-210. Test.

Single second‐trimester scan: ear face and neck overall
TST-211. Test.

Single second‐trimester scan: orofacial clefts overall
TST-212. Test.

Single second‐trimester scan: cleft lip
TST-213. Test.

Single second‐trimester scan: cleft lip and cleft palate
TST-214. Test.

Single second‐trimester scan: cleft palate
TST-215. Test.

Single second‐trimester scan: ear face and neck misc
TST-216. Test.

Single second‐trimester scan: multisystem anomalies overall
TST-217. Test.

Single second‐trimester scan: fetal hydrops
TST-218. Test.

Single second‐trimester scan: MCA or syndrome
TST-219. Test.

Single second‐trimester scan: other
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abu‐Rustum 2010.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of all pregnant women attending the study centre for first‐trimester screening during the study period were reviewed Study start and end date: March 2002 to February 2009 |
||
| Patient characteristics and setting | Setting: private practice (Center for Advanced Fetal Care, Najah Center) Region(s) and country/countries from which participants were recruited: Tripoli, Lebanon Sample size: 1304 Study eligibility criteria: all patients having first‐trimester screening during the study period were included. Isolated sonographic findings of choroid plexus cysts and echogenic intracardiac foci were excluded. Number of participants with the target condition: 27 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 weeks to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: basic Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: single Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation Second‐trimester anomaly scan: Timing (weeks and days gestation): 20 to 23 weeks' gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: single Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: major was defined as lethal anomalies and those requiring immediate surgical intervention at birth Reference standard (live birth): neonatal examination by a paediatrician and when clinically indicated echocardiography and neonatal imaging were performed Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: until neonatal examination |
||
| Flow and timing | Eligible patients: 1370 Exclusions (study investigator): 30 excluded (14 spontaneous fetal death before index test, 16 fetuses with echogenic intracardiac foci or choroid plexus cysts), 27 lost to follow‐up (2.0%) Exclusions (review team): 9 (soft markers for chromosomal abnormalities) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Andrew 2015.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of all pregnant women attending the study centre for a first trimester scan during the study period were reviewed Study start and end date: January 2014 to December 2014 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Fetal Medicine Division of the Sri Ramachandra Medical College) Region(s) and country/countries from which participants were recruited: Porur, India Sample size: 4397 Study eligibility criteria: pregnant women with at least one viable fetus with a crown‐rump length between 44 and 84 mm Number of participants with the target condition: 15 Population type: unselected population Prior testing: combined screening for chromosomal abnormalities using nuchal translucency measurement and serum biochemistry (beta‐HCG and PAPP‐A) at the time of the first‐trimester anomaly scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks' gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple (4) Staff qualification and/or operator experience level: doctors trained and certified in nuchal translucency measurement Second‐trimester scan: Timing (weeks and days gestation): 18 to 20 weeks' gestation Ultrasound scanning protocol: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy outcomes of cases determined to be abnormal were obtained from the institution database and from the patients telephonically if they chose to deliver at another institution Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 4421 Exclusions (study investigator): 16 excluded (11 cases of spontaneous fetal death before index test, 1 scar pregnancy, 4 cases with gynaecological problems), 1 lost to follow‐up (< 0.1%) Exclusions (review team): 7 (soft markers for chromosomal abnormalities: increased nuchal translucency) |
||
| Comparative | |||
| Notes | Funding source: the authors declared that no financial or other competing interests were present. The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Unclear | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Bardi 2022.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins Northern Netherlands (EUROCAT‐NNL) database Recruitment: all cases of live births, fetal deaths and terminated pregnancies with congenital anomalies in the region are registered in the database after parental consent Study start and end date: January 2010 to December 2019 |
||
| Patient characteristics and setting | Setting: regional congenital anomaly registry Region(s) and country/countries from which participants were recruited: the 3 northern provinces of the Netherlands (Groningen, Friesland and Drenthe) Sample size: 139,630 (based on approximate number of births during the study period in the covered region) Study eligibility criteria: all cases of fetuses and children with congenital anomalies are registered in the database. There is no lower limit for gestational age and data are continuously updated in the register until the completed 10th year of age of the child. Only when parents actively refuse registration are cases excluded. Number of participants with the target condition: 377 Population type: unselected population Prior testing: combined test (2007 onwards); non‐invasive prenatal testing (2017 onwards) |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: second trimester Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): selected major fetal structural anomalies; anencephaly, encephalocele, spina bifida, holoprosencephaly, tricuspid/pulmonary valve atresia, hypoplastic left heart, abdominal wall and limb reduction defects, lethal skeletal dysplasia, megacystis, multiple congenital anomalies Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): postnatal patient records, pregnancy outcome and delivery reports and cytogenetic laboratory investigations Reference standard (fetal or neonatal demise): cytogenetic laboratory investigations and postmortem examinations Postnatal follow‐up duration: cases are registered and updated until the completed 10th year of the child |
||
| Flow and timing | Eligible patients: 155,000 (approximate number of births during the study period in the covered region) Exclusions (study investigator): 15,269 excluded (6.6%/10,230 actively refused registration, 5039 did not meet inclusion criteria) Exclusions (review team): 101 (59 abnormal karyotype; 42 cases of selected anomalies within the larger categories of other single ventricle heart defects and conotruncal anomalies, respectively) |
||
| Comparative | |||
| Notes | Structural abnormalities detected during the first trimester of pregnancy were included as true positive test results in the 2 x 2 tables of index test 3 Funding source: the authors received no specific funding for this work |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Batra 2014.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: unclear Study start and end date: April 2012 to April 2013 |
||
| Patient characteristics and setting | Setting: private hospital/fertility clinic (CIMAR Fertility Centre) Region(s) and country/countries from which participants were recruited: Cochin, India Sample size: 353 Study eligibility criteria: pregnant women were enrolled after taking informed consent Number of participants with the target condition: 5 Population type: not reported Prior testing: nuchal translucency measurement at the time of the first trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 weeks to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Mode of examination: transabdominal and transvaginal Cardiac screening: extended Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported Second‐trimester scan: Timing: 18 weeks to 22 weeks of gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): follow‐up at delivery Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: until delivery |
||
| Flow and timing | Eligible patients: 355 Exclusions (study investigator): none reported Exclusions (review team): 2 (1 abnormal karyotype, 1 lost to follow‐up after the second trimester anomaly scan ‐ excluded on no reference standard) |
||
| Comparative | |||
| Notes | Funding source: CIMAR Fertility Centre, Kochi, Kerala, India | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Becker 2012.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: first‐trimester anomaly screening was performed in 7023 consecutive pregnant women attending the study centre for prenatal care Study start and end date: July 1997 to December 2009 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Center for Prenatal Diagnostics and Human Genetics, Charité) Region(s) and country/countries from which participants were recruited: Berlin, Germany Sample size: 6507 Study eligibility criteria: fetuses with a crown‐rump length of 45 to 84 mm and/or a gestational age of 11 weeks to 13 weeks and 6 days, independent of the nuchal translucency measurement Number of participants with the target condition: 74 Population type: unselected population, fetuses with a normal nuchal translucency measurement were analysed separately Prior testing: nuchal translucency measurement at the time of the first trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 weeks and 0 days to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: single Staff qualification and/or operator experience level: 10 years of experience at the beginning of the observations Second‐trimester scan: Timing (weeks and days gestation): 20 weeks to 23 weeks' gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: single operator Staff qualification and/or operator experience level: 10 years of experience at the beginning of the observations |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: major was defined as an anomaly present at conception (genetic disorder) or developing during the first trimester of pregnancy that – at least without treatment – is not compatible with life or will lead to severe handicap. Reference standard (live birth): neonatal examination by a paediatrician directly after birth and at 10 days after delivery. In case of anomalies, follow‐up was obtained by medical reports. Reference standard (fetal or neonatal demise): by autopsy report, if performed, or by ultrasound report of at least one second examiner Postnatal follow‐up duration: 10 days after birth |
||
| Flow and timing | Eligible patients: 7023 Exclusions (study investigator): 21 excluded (6 with acquired anomalies, 15 with familial/monogenetic conditions without structural anomalies), 144 lost to follow‐up (2.1%) Exclusions (review team): we excluded the increased nuchal translucency subgroup (n = 344). From the normal nuchal translucency subgroup, a further 37 cases were excluded (abnormal karyotype). |
||
| Comparative | |||
| Notes | Funding source: the authors declared that no funding was received for this work | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Bister 2011.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: pre‐ and postnatal hospital records from the obstetric ultrasound department, regional Craniofacial Unit and Perinatal Pathology department were reviewed to identify fetuses and neonates with pre‐ or postnatally diagnosed orofacial clefts Study start and end date: 1993 to 1997 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Addenbrooke’s Hospital), servicing the Cambridge area Region(s) and country/countries from which participants were recruited: Cambridge, United Kingdom Sample size: 23,573 Study eligibility criteria: ultrasound examination at 18 to 20 weeks of gestation Number of participants with the target condition: 26 Population type: unselected population Prior testing: dating scan |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 weeks to 20 weeks of gestation Ultrasound scanning protocol: not reported Cardiac screening: not applicable Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: trained sonographers, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): orofacial clefts Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcomes from hospital records Reference standard (fetal or neonatal demise): review of autopsy records of fetuses over 16 weeks’ gestation Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 23,577 Exclusions (study investigator): 4 excluded (1 considered not detectable by antenatal ultrasound, 1 no screening received until third trimester, 2 spontaneous fetal death) Exclusions (review team): none |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Biswas 2013.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: 1000 consecutive pregnant women were included Study start and end date: September 2006 to August 2007 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Vivekanada Institute of Medical Sciences) Region(s) and country/countries from which participants were recruited: West Bengal, India Sample size: 1000 Study eligibility criteria: gestational age at the time of the anomaly scan between 18 and 22 weeks' gestation Number of participants with the target condition: 38 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 22 weeks' gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: radiologists, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: European Registration of Congenital Anomalies and Twins (EUROCAT) classification system Reference standard (live birth): neonatal examination and follow‐up investigations (not further specified) Reference standard (fetal or neonatal demise): autopsy was performed in all cases after termination of pregnancy Postnatal follow‐up duration: until birth (no postnatal investigations required) or up to 7 days after birth (postnatal investigations required) |
||
| Flow and timing | Eligible patients: 1000 Exclusions (study investigator): none reported Exclusions (review team): none |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bodin 2018.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the Danish Fetal Medicine Database (DFMD) Recruitment: data from all obstetric departments in Denmark are registered in the database Study start and end date: January 2008 to December 2015 |
||
| Patient characteristics and setting | Setting: national fetal malformation registry Region(s) and country/countries from which participants were recruited: data included from all obstetric departments in Denmark Sample size: 443,465 Study eligibility criteria: all pregnancies that underwent a second‐trimester ultrasound scan and had a pre‐ or postnatally registered diagnosis of open spina bifida, meningocele or a lipomatous malformation with neurological deficit of a skin‐covered spina bifida, were included. Babies born and having had primary surgery outside of Denmark were excluded. All cases with spina bifida occulta, lipomatous malformations or tethered cord without neurological deficits, as well as suspected but not confirmed spina bifida cases, were excluded. Number of participants with the target condition: 234 Population type: unselected population Prior testing: 11 to 14 weeks ultrasound scan (scanning primarily for chromosomal abnormalities) |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 weeks to 21 weeks Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): spina bifida, including meningocele and lipomatous malformations with neurological deficits of the skin‐covered spina bifida Definitions used for major and minor congenital abnormalities: European Registration of Congenital Anomalies and Twins (EUROCAT) classification system Reference standard (live birth): data were linked to postnatal outcomes from the Danish Cytogenetic Register, the National Patient Register and the National Birth Register Reference standard (fetal and neonatal demise): medical records on all infants and mothers in whom a termination of pregnancy was performed were evaluated Postnatal follow‐up duration: 1 year after birth |
||
| Flow and timing | Eligible patients: 475,679 Exclusions (study investigator): 32,203 excluded (32,062 no prenatal scan, 6 duplicates, 108 no spina bifida history, 23 spina bifida occulta or tethered cord, 4 other) Exclusions (review team): 11 (6 cases of spina bifida who had no prenatal scan, 5 cases of spina bifida with no information on prenatal screening) |
||
| Comparative | |||
| Notes | Funding source: this work received financial support from Region Midts Forskningsfond, Jascha Fonden, Fonden af 1870, NFOG | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2000‐1.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins (EUROCAT) database from 18 congenital malformation registers from 11 European countries Recruitment: all live births, still births and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth are registered in the database Study start and end date: duration ranging between 9 and 30 months, not further specified |
||
| Patient characteristics and setting | Setting: congenital malformation registry Region(s) and country/countries from which participants were recruited: Zagreb, Croatia Sample size: 10,718 Study eligibility criteria: congenital malformations from all live births, still birth and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth Number of participants with the target condition: 7 Population type: unselected population Prior testing: first‐trimester dating scan; routine alpha‐fetoprotein screening |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: around 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: trained operators, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly, spina bifida Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): multiple sources of ascertainment and active case finding, following the EUROCAT guidelines Reference standard (fetal or neonatal demise): pathology or autopsy records, following EUROCAT guidelines Postnatal follow‐up duration: 7 days after birth |
||
| Flow and timing | Eligible patients: 10718 (Zagreb, Croatia); 670,766 (total cohort) Exclusions (study investigator): none reported Exclusions (review team): no cases excluded from Zagreb, Croatia; 128,539 cases excluded from total cohort (no routine prenatal screening in registry area: 8788 from Odense, Denmark, 34,085 from Northern Netherlands; 47,895 from South Western Netherlands; registry not population‐based: 8745 from Leipzig, Germany; 29,026 from Styria, Austria) |
||
| Comparative | |||
| Notes | Funding source: the Euroscan Study has received support from the European Union Biomed 2 Concerted Action | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2000‐10.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins (EUROCAT) database from 18 congenital malformation registers from 11 European countries Recruitment: all live births, still births and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth are registered in the database Study start and end date: duration ranging between 9 and 30 months, period not further specified |
||
| Patient characteristics and setting | Setting: congenital malformation registry Region(s) and country/countries from which participants were recruited: Vinnista, Ukraine Sample size: 44,761 Study eligibility criteria: congenital malformations from all live births, still birth and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth Number of participants with the target condition: 33 Population type: unselected population Prior testing: first‐trimester dating scan |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: around 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: trained operators, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly, spina bifida Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): multiple sources of ascertainment and active case finding, following the EUROCAT guidelines Reference standard (fetal or neonatal demise): pathology or autopsy records, following EUROCAT guidelines Postnatal follow‐up duration: 7 days after birth |
||
| Flow and timing | Eligible patients: 44,761 (Vinnista, Ukraine); 670,766 (total cohort) Exclusions (study investigator): none reported Exclusions (review team): no cases excluded from Vinnista, Ukraine; 128,539 cases excluded from total cohort (no routine prenatal screening in registry area: 8788 from Odense, Denmark, 34,085 from Northern Netherlands; 47,895 from South Western Netherlands; registry not population‐based: 8745 from Leipzig, Germany; 29,026 from Styria, Austria) |
||
| Comparative | |||
| Notes | Funding source: the Euroscan Study has received support from the European Union Biomed 2 Concerted Action | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2000‐11.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins (EUROCAT) database from 18 congenital malformation registers from 11 European countries Recruitment: all live births, still births and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth are registered in the database Study start and end date: duration ranging between 9 and 30 months, period not further specified |
||
| Patient characteristics and setting | Setting: congenital malformation registry Region(s) and country/countries from which participants were recruited: Oxford, United Kingdom Sample size: 13,136 Study eligibility criteria: congenital malformations from all live births, still birth and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth Number of participants with the target condition: 11 Population type: unselected population Prior testing: routine alpha‐fetoprotein screening |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: around 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: trained operators, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly, spina bifida Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): multiple sources of ascertainment and active case finding, following the EUROCAT guidelines Reference standard (fetal or neonatal demise): pathology or autopsy records, following EUROCAT guidelines Postnatal follow‐up duration: 7 days after birth |
||
| Flow and timing | Eligible patients: 13,136 (Oxford, United Kingdom); 670,766 (total cohort) Exclusions (study investigator): none reported Exclusions (review team): no cases excluded from Oxford, United Kingdom; 128,539 cases excluded from total cohort (no routine prenatal screening in registry area: 8788 from Odense, Denmark, 34,085 from Northern Netherlands; 47,895 from South Western Netherlands; registry not population‐based: 8745 from Leipzig, Germany; 29,026 from Styria, Austria) |
||
| Comparative | |||
| Notes | Funding source: the Euroscan Study has received support from the European Union Biomed 2 Concerted Action | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2000‐12.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins (EUROCAT) database from 18 congenital malformation registers from 11 European countries Recruitment: all live births, still births and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth are registered in the database Study start and end date: duration ranging between 9 and 30 months, period not further specified |
||
| Patient characteristics and setting | Setting: congenital malformation registry Region(s) and country/countries from which participants were recruited: Wessex, United Kingdom Sample size: 65,559 Study eligibility criteria: congenital malformations from all live births, still birth and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth Number of participants with the target condition: 56 Population type: unselected population Prior testing: first‐trimester dating scan |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: around 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: trained operators, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly, spina bifida Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): multiple sources of ascertainment and active case finding, following the EUROCAT guidelines Reference standard (fetal or neonatal demise): pathology or autopsy records, following EUROCAT guidelines Postnatal follow‐up duration: 7 days after birth |
||
| Flow and timing | Eligible patients: 65,559 (Wessex, United Kingdom); 670,766 (total cohort) Exclusions (study investigator): none reported Exclusions (review team): no cases excluded from Wessex, United Kingdom; 128,539 cases excluded from total cohort (no routine prenatal screening in registry area: 8788 from Odense, Denmark, 34,085 from Northern Netherlands; 47,895 from South Western Netherlands; registry not population‐based: 8745 from Leipzig, Germany; 29,026 from Styria, Austria) |
||
| Comparative | |||
| Notes | Funding source: the Euroscan Study has received support from the European Union Biomed 2 Concerted Action | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2000‐2.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins (EUROCAT) database from 18 congenital malformation registers from 11 European countries Recruitment: all live births, still births and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth are registered in the database Study start and end date: duration ranging between 9 and 30 months, period not further specified |
||
| Patient characteristics and setting | Setting: congenital malformation registry Region(s) and country/countries from which participants were recruited: Paris, France Sample size: 27,550 Study eligibility criteria: congenital malformations from all live births, still birth and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth Number of participants with the target condition: 18 Population type: unselected population Prior testing: first‐trimester dating scan; alpha‐fetoprotein screening |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: around 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: trained operators, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly, spina bifida Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): multiple sources of ascertainment and active case finding, following the EUROCAT guidelines Reference standard (fetal or neonatal demise): pathology or autopsy records, following EUROCAT guidelines Postnatal follow‐up duration: 7 days after birth |
||
| Flow and timing | Eligible patients: 27,550 (Paris, France); 670,766 (total cohort) Exclusions (study investigator): none reported Exclusions (review team): no cases excluded from Paris, France; 128,539 cases excluded from total cohort (no routine prenatal screening in registry area: 8788 from Odense, Denmark, 34,085 from Northern Netherlands; 47,895 from South Western Netherlands; registry not population‐based: 8745 from Leipzig, Germany; 29,026 from Styria, Austria) |
||
| Comparative | |||
| Notes | Funding source: the Euroscan Study has received support from the European Union Biomed 2 Concerted Action | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2000‐3.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins (EUROCAT) database from 18 congenital malformation registers from 11 European countries Recruitment: all live births, still births and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth are registered in the database Study start and end date: duration ranging between 9 and 30 months, period not further specified |
||
| Patient characteristics and setting | Setting: congenital malformation registry Region(s) and country/countries from which participants were recruited: Strasbourg, France Sample size: 33,155 Study eligibility criteria: congenital malformations from all live births, still birth and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth Number of participants with the target condition: 19 Population type: unselected population Prior testing: first‐trimester dating scan; alpha‐fetoprotein screening; triple test introduced during study |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: around 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: trained operators, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly, spina bifida Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): multiple sources of ascertainment and active case finding, following the EUROCAT guidelines Reference standard (fetal or neonatal demise): pathology or autopsy records, following EUROCAT guidelines Postnatal follow‐up duration: 7 days after birth |
||
| Flow and timing | Eligible patients: 33,155 (Strasbourg, France); 670,766 (total cohort) Exclusions (study investigator): none reported Exclusions (review team): no cases excluded from Strasbourg, France; 128,539 cases excluded from total cohort (no routine prenatal screening in registry area: 8788 from Odense, Denmark, 34,085 from Northern Netherlands; 47,895 from South Western Netherlands; registry not population‐based: 8745 from Leipzig, Germany; 29,026 from Styria, Austria) |
||
| Comparative | |||
| Notes | Funding source: the Euroscan Study has received support from the European Union Biomed 2 Concerted Action | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2000‐4.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins (EUROCAT) database from 18 congenital malformation registers from 11 European countries Recruitment: all live births, still births and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth are registered in the database Study start and end date: duration ranging between 9 and 30 months, period not further specified |
||
| Patient characteristics and setting | Setting: congenital malformation registry Region(s) and country/countries from which participants were recruited: Mainz, Germany Sample size: 9535 Study eligibility criteria: congenital malformations from all live births, still birth and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth Number of participants with the target condition: 16 Population type: unselected population Prior testing: first‐trimester dating scan; alpha‐fetoprotein screening |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: around 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: trained operators, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly, spina bifida Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): multiple sources of ascertainment and active case finding, following the EUROCAT guidelines Reference standard (fetal or neonatal demise): pathology or autopsy records, following EUROCAT guidelines Postnatal follow‐up duration: 7 days after birth |
||
| Flow and timing | Eligible patients: 9535 (Mainz, Germany); 670,766 (total cohort) Exclusions (study investigator): none reported Exclusions (review team): no cases excluded from Mainz, Germany; 128,539 cases excluded from total cohort (no routine prenatal screening in registry area: 8788 from Odense, Denmark, 34,085 from Northern Netherlands; 47,895 from South Western Netherlands; registry not population‐based: 8745 from Leipzig, Germany; 29,026 from Styria, Austria) |
||
| Comparative | |||
| Notes | Funding source: the Euroscan Study has received support from the European Union Biomed 2 Concerted Action | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2000‐5.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins (EUROCAT) database from 18 congenital malformation registers from 11 European countries Recruitment: all live births, still births and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth are registered in the database Study start and end date: duration ranging between 9 and 30 months, period not further specified |
||
| Patient characteristics and setting | Setting: congenital malformation registry Region(s) and country/countries from which participants were recruited: north‐eastern Italy Sample size: 111,719 Study eligibility criteria: congenital malformations from all live births, still birth and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth Number of participants with the target condition: 36 Population type: unselected population Prior testing: first‐trimester dating scan; alpha‐fetoprotein screening |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: around 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: trained operators, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly, spina bifida Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): multiple sources of ascertainment and active case finding, following the EUROCAT guidelines Reference standard (fetal or neonatal demise): pathology or autopsy records, following EUROCAT guidelines Postnatal follow‐up duration: 7 days after birth |
||
| Flow and timing | Eligible patients: 111,719 (North‐eastern Italy); 670,766 (total cohort) Exclusions (study investigator): none reported Exclusions (review team): no cases excluded from north‐eastern Italy; 128,539 cases excluded from total cohort (no routine prenatal screening in registry area: 8788 from Odense, Denmark, 34,085 from Northern Netherlands; 47,895 from South Western Netherlands; registry not population‐based: 8745 from Leipzig, Germany; 29,026 from Styria, Austria) |
||
| Comparative | |||
| Notes | Funding source: the Euroscan Study has received support from the European Union Biomed 2 Concerted Action | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2000‐6.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins (EUROCAT) database from 18 congenital malformation registers from 11 European countries Recruitment: all live births, still births and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth are registered in the database Study start and end date: duration ranging between 9 and 30 months, period not further specified |
||
| Patient characteristics and setting | Setting: congenital malformation registry Region(s) and country/countries from which participants were recruited: Tuscany, Italy Sample size: 67,120 Study eligibility criteria: congenital malformations from all live births, still birth and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth Number of participants with the target condition: 28 Population type: unselected population Prior testing: first‐trimester dating scan; alpha‐fetoprotein screening |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: around 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: trained operators, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly, spina bifida Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): multiple sources of ascertainment and active case finding, following the EUROCAT guidelines Reference standard (fetal or neonatal demise): pathology or autopsy records, following EUROCAT guidelines Postnatal follow‐up duration: 7 days after birth |
||
| Flow and timing | Eligible patients: 67,120 (Tuscany, Italy); 670,766 (total cohort) Exclusions (study investigator): none reported Exclusions (review team): no cases excluded from Tuscany, Italy; 128,539 cases excluded from total cohort (no routine prenatal screening in registry area: 8788 from Odense, Denmark, 34,085 from Northern Netherlands; 47,895 from South Western Netherlands; registry not population‐based: 8745 from Leipzig, Germany; 29,026 from Styria, Austria) |
||
| Comparative | |||
| Notes | Funding source: the Euroscan Study has received support from the European Union Biomed 2 Concerted Action | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2000‐7.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins (EUROCAT) database from 18 congenital malformation registers from 11 European countries Recruitment: all live births, still births and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth are registered in the database Study start and end date: duration ranging between 9 and 30 months, period not further specified |
||
| Patient characteristics and setting | Setting: congenital malformation registry Region(s) and country/countries from which participants were recruited: Sicily, Italy Sample size: 25,339 Study eligibility criteria: congenital malformations from all live births, still birth and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth Number of participants with the target condition: 11 Population type: unselected population Prior testing: first‐trimester dating scan; alpha‐fetoprotein screening |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: around 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: trained operators, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly, spina bifida Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): multiple sources of ascertainment and active case finding, following the EUROCAT guidelines Reference standard (fetal or neonatal demise): pathology or autopsy records, following EUROCAT guidelines Postnatal follow‐up duration: 7 days after birth |
||
| Flow and timing | Eligible patients: 25,339 (Sicily, Italy); 670,766 (total cohort) Exclusions (study investigator): none reported Exclusions (review team): no cases excluded from Sicily, Italy; 128,539 cases excluded from total cohort (no routine prenatal screening in registry area: 8788 from Odense, Denmark, 34,085 from Northern Netherlands; 47,895 from South Western Netherlands; registry not population‐based: 8745 from Leipzig, Germany; 29,026 from Styria, Austria) |
||
| Comparative | |||
| Notes | Funding source: the Euroscan Study has received support from the European Union Biomed 2 Concerted Action | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2000‐8.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins (EUROCAT) database from 18 congenital malformation registers from 11 European countries Recruitment: all live births, still births and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth are registered in the database Study start and end date: duration ranging between 9 and 30 months, period not further specified |
||
| Patient characteristics and setting | Setting: congenital malformation registry Region(s) and country/countries from which participants were recruited: Basque Country, Spain Sample size: 32,429 Study eligibility criteria: congenital malformations from all live births, still birth and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth Number of participants with the target condition: 19 Population type: unselected population Prior testing: first‐trimester dating scan |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: around 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: trained operators, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly, spina bifida Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): multiple sources of ascertainment and active case finding, following the EUROCAT guidelines Reference standard (fetal or neonatal demise): pathology or autopsy records, following EUROCAT guidelines Postnatal follow‐up duration: 7 days after birth |
||
| Flow and timing | Eligible patients: 32,429 (Basque Country, Spain); 670,766 (total cohort) Exclusions (study investigator): none reported Exclusions (review team): no cases excluded from Basque Country, Spain; 128,539 cases excluded from total cohort (no routine prenatal screening in registry area: 8788 from Odense, Denmark, 34,085 from Northern Netherlands; 47,895 from South Western Netherlands; registry not population‐based: 8745 from Leipzig, Germany; 29,026 from Styria, Austria) |
||
| Comparative | |||
| Notes | Funding source: the Euroscan Study has received support from the European Union Biomed 2 Concerted Action | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2000‐9.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the European Registration of Congenital Anomalies and Twins (EUROCAT) database from 18 congenital malformation registers from 11 European countries Recruitment: all live births, still births and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth are registered in the database Study start and end date: duration ranging between 9 and 30 months, period not further specified |
||
| Patient characteristics and setting | Setting: congenital malformation registry Region(s) and country/countries from which participants were recruited: El Valles, Spain Sample size: 5737 Study eligibility criteria: congenital malformations from all live births, still birth and pregnancies that result in termination after a structural or chromosomal abnormality has been detected prenatally by ultrasound scan or diagnosed within 7 days of birth Number of participants with the target condition: 7 Population type: unselected population Prior testing: first‐trimester dating scan |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: around 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: trained operators, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly, spina bifida Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): multiple sources of ascertainment and active case finding, following the EUROCAT guidelines Reference standard (fetal or neonatal demise): pathology or autopsy records, following EUROCAT guidelines Postnatal follow‐up duration: 7 days after birth |
||
| Flow and timing | Eligible patients: 5737 (El Valles, Spain); 670,766 (total cohort) Exclusions (study investigator): none reported Exclusions (review team): no cases excluded from El Valles, Spain; 128,539 cases excluded from total cohort (no routine prenatal screening in registry area: 8788 from Odense, Denmark, 34,085 from Northern Netherlands; 47,895 from South Western Netherlands; registry not population‐based: 8745 from Leipzig, Germany; 29,026 from Styria, Austria) |
||
| Comparative | |||
| Notes | Funding source: the Euroscan Study has received support from the European Union Biomed 2 Concerted Action | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Boyd 2011.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study based on data from the British Isles Network of Congenital Anomaly Registers (BINOCAR) database Recruitment: BINOCAR is a network of regional and disease‐specific population‐based registers across the British Isles. All births in Wales and 43% of births in England are covered by the regional congenital anomaly registers. Cases included all fetuses and infants with the target condition of interest notified within 1 year with a date of delivery/termination during the study period. Study start and end date: January 2005 to December 2006 |
||
| Patient characteristics and setting | Setting: congenital anomaly registry Region(s) and country/countries from which participants were recruited: United Kingdom (South Central, East Midlands & South Yorkshire, South West, North East & North Cumbria, West Midlands and Wales) Sample size: 601,500 Study eligibility criteria: cases included all affected fetuses and infants with 1 of the 9 major structural anomaly groups selected in discussion with the Fetal Anomaly Screening Programme (FASP), notified within 1 year with a date of delivery/termination Number of participants with the target condition: 2839 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing (weeks and days gestation): 18 weeks and 0 days to 20 weeks and 6 days of pregnancy Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): 9 selected major anomaly groups: anencephaly, spina bifida, serious cardiac anomalies, diaphragmatic hernia, gastroschisis, exomphalos, bilateral renal agenesis, lethal/severe skeletal dysplasias, cleft lip with or without palate Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): to achieve high levels of ascertainment and completeness, BINOCAR registers identify cases using multiple source notifications prospectively throughout pregnancy and confirmation or otherwise at delivery. These sources include laboratories, parents, ultrasound departments, delivery suites, obstetric notes, neonatal units, child health systems, inpatient administration systems and paediatric notes. Reference standard (fetal or neonatal demise): post‐mortem reports Postnatal follow‐up duration: anomalies are included in the registry when diagnosed up to the age of 5 years |
||
| Flow and timing | Eligible patients: 601,545 Exclusions (study investigator): none reported Exclusions (review team): 45 (unknown gestation at time of prenatal diagnosis) |
||
| Comparative | |||
| Notes | Funding source: this paper reports on an independent study which is funded/part‐funded by the Policy Research Programme in the Department of Health and the Healthcare Quality Improvement Partnership (HQIP). Part funding is also from local primary care trusts and the Welsh Assembly Government. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Brown 2021.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of all pregnant women undergoing a pre‐cell‐free DNA (cfDNA) ultrasound scan at the study centre were reviewed Study start and end date: January 2017 to December 2018 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Monash Ultrasound for Women) Region(s) and country/countries from which participants were recruited: Melbourne, Australia Sample size: 3222 Study eligibility criteria: pregnancies with a viable fetus with a crown‐rump length less than 45 mm at pre‐cfDNA ultrasound who also had at least one subsequent ultrasound examination performed at the centre were eligible for inclusion. Women were considered ineligible if they were under 18 years of age at the time of pre‐cfDNA ultrasound scan. Number of participants with the target condition: 149 fetal abnormalities Population type: unselected population Prior testing: pre‐cfDNA ultrasound scan and cf‐DNA screening (maternal plasma) |
||
| Index tests |
Type: two‐stage screening (data were only reported for the first‐trimester scan) First‐trimester scan: Timing (weeks and days gestation): 11 weeks to 14 weeks of gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: transvaginal alone Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported Second‐trimester scan: Data to construct 2 x 2 tables could not be extracted or derived from the report |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: major and minor were defined upon the severity of the structural abnormality and the likely impact on future quality of life and on consensus among the authors Reference standard (live birth): postnatal findings obtained from hospital records Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 6208 Exclusions (study investigator): 1611 excluded (175 spontaneous fetal death before index test, 1435 crown rump length of 45 mm or greater, 1 patient under 18 years of age), 1264 (27.5%) lost to follow‐up Exclusions (review team): 111 (92 soft markers for chromosomal abnormalities, 1 placental anomaly, 18 aberrant subclavian artery) |
||
| Comparative | |||
| Notes | Data for 2 x 2 tables reported as fetal abnormalities Funding source: the authors declared that no funding was required for this study |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Carvalho 2002.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: all pregnant women attending the study centre for prenatal care were offered first‐trimester screening Study start and end date: October 1995 to July 2000 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Clinical Hospital of São Paulo University) Region(s) and country/countries from which participants were recruited: São Paulo, Brazil Sample size: 2832 Study eligibility criteria: pregnant women receiving screening for chromosomal and fetal abnormalities by ultrasound scan between 11 and 14 weeks Number of participants with the target condition: 106 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 weeks to 14 weeks of gestation Ultrasound scanning protocol: basic Cardiac screening: detailed early fetal echocardiography was performed in fetuses with an increased nuchal translucency thickness. It is unclear whether routine cardiac examination was performed in fetuses with normal nuchal translucency thickness. Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple (8) Staff qualification and/or operator experience level: fetal medicine specialists (2), fetal echocardiography specialist (1), fetal medicine fellows with more than 2 years of experience (5) Second‐trimester scan: Timing: 18 weeks to 24 weeks of gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: major anomalies were defined as lethal, incurable or curable severe abnormalities with high risk of residual handicap Reference standard (live birth): pregnancy outcomes from hospital records and patients themselves Reference standard (fetal or neonatal demise): pathological examination Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 2853 Exclusions (study investigator): only the number of patients with complete follow‐up data is reported Exclusions (review team): 21 (17 soft markers for chromosomal abnormalities, 4 anomalies considered not detectable by prenatal ultrasound) |
||
| Comparative | |||
| Notes | Funding source: this project was supported by a grant acquired from FAPESP, São Paulo, Brazil (number 97/05581‐7) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Chelli 2009.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: hospital records of all pregnant women attending the study centre for first‐trimester screening during the study period were reviewed Study start and end date: January 2005 to December 2005 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Maternity and Neonatology Center of Tunisia, La Rabta Hospital) Region(s) and country/countries from which participants were recruited: Tunisia Sample size: 587 Study eligibility criteria: all women who underwent a first‐trimester ultrasound between 11 and 14 weeks' gestation during the study period Number of participants with the target condition: 5 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 13 weeks' gestation Ultrasound scanning protocol: the scan is described as a mini‐morphological examination; scanning protocol not reported Mode of examination: not reported Cardiac screening: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: senior ultrasonographers with training and diplomas in ultrasound Second‐trimester scan: Timing (weeks and days gestation): 22 to 24 weeks' gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: senior ultrasonographers with training and diplomas in ultrasound |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): live born infants were followed, complete neonatal examination at birth by a paediatrician Reference standard (fetal and neonatal demise): not reported Postnatal follow‐up duration: 3 years (until the end of 2008) |
||
| Flow and timing | Eligible patients: 597 Exclusions (study investigator): none reported Exclusions (review team): 10 (6 soft markers for chromosomal abnormalities, 2 abnormal karyotype, 2 abnormal biometry) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Chen 2009.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective cohort study Recruitment: hospital records of all women who attended the study centre for prenatal care during the study period were reviewed Study start and end date: March 2000 to May 2007 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Prince of Wales Hospital, the Chinese University of Hong Kong) Region(s) and country/countries from which participants were recruited: Hong Kong, China Sample size: 13,659 Study eligibility criteria: women with risk factors for fetal anomalies, such as pre‐existing diabetes, advanced maternal age unless they had negative result for an optional first‐ or second‐trimester screening test, and multiple pregnancies were excluded from the study Number of participants with the target condition: 156 Population type: low‐risk population Prior testing: optional first‐ or second‐trimester screening in the case of advanced maternal age (not further specified) |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 23 weeks' gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: transabdominal alone Single or multiple operators: multiple Staff qualification and/or operator experience level: certified by American Registry of Diagnostic Medical Sonographers (ARDMS) |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: minor deformities: nose‐, ear‐ and face anomalies, congenital dislocation of hip, inguinal hernia, undescended testes, hydrocele, phimosis, isolated skin lesions, hypospadias, functional cardiac murmurs, spinal dysraphism, alveolar mass, congenital hypodontia and facial palsy. Soft markers were analysed separately. Reference standard (live birth): all newborns were examined according to a standard protocol by an obstetrician or neonatologist. Women that delivered elsewhere were contacted by telephone or mail. Reference standard (fetal or neonatal demise): post‐mortem examination was offered to all women who had termination of pregnancy, stillbirth or neonatal death Postnatal follow‐up duration: first few days after delivery |
||
| Flow and timing | Eligible patients: 13,882 Exclusions (study investigator): 189 lost to follow‐up/no complete outcome (1.36%) Exclusions (review team): 34 (7 soft markers for chromosomal abnormalities, 18 abnormal karyotype, 7 anomalies considered not detectable by prenatal ultrasound, 2 non‐structural anomalies) |
||
| Comparative | |||
| Notes | Funding source: the authors declared that no conflicts of interest were present. The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Choudry 2009.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records were reviewed of all pregnant women attending the study centre for prenatal ultrasound during the study period. In addition, hospital records of all neonates treated at the neonatal surgical department during the study period were reviewed to identify postnatally diagnosed cases. Cases were ascertained from the local congenital anomaly registry. Study start and end date: January 1995 to December 2004 |
||
| Patient characteristics and setting | Setting: tertiary care facility (John Radcliffe Hospital) Region(s) and county/countries from which participants were recruited: Oxford, United Kingdom Sample size: not reported Study eligibility criteria: prenatal suspicion or a postnatal diagnosis of isolated or non‐isolated duodenal atresia Number of participants with the target condition: 26 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: approximately 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: sonographer, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): duodenal atresia Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcomes obtained from hospital records, the obstetric, paediatric and neonatal surgical databases; case ascertainment from the local congenital anomaly registry Reference standard (fetal or neonatal demise): postmortem examination records Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 61 participants with the target condition Exclusions (study investigator): none reported Exclusions (review team): 35 (cases with associated major anomalies or abnormal karyotype) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Dane 2007.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: all pregnant women attending the study centre for first‐trimester ultrasound during the study period Study start and end date: not reported |
||
| Patient characteristics and setting | Setting: tertiary care facility (Haseki Hospital) Region(s) and country/countries from which participants were recruited: Istanbul, Turkey Sample size: 1284 Study eligibility criteria: referred cases with known or suspected anomalies. Cases with minor anomalies were not included in the analyses. Isolated increased nuchal translucency was not considered an anomaly. Number of participants with the target condition: 18 Population type: unselected population Prior testing: routine nuchal translucency measurement and maternal serum biochemistry (free beta‐HCG, PAPP‐A), unclear timing |
||
| Index tests |
Type: two‐stage screening First‐trimester anomaly: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: basic Cardiac screening: not reported Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple (2) Staff qualification and/or operator experience level: 6 and 2 years of experience in ultrasound screening Second‐trimester anomaly: Timing: 22 to 24 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (2) Staff qualification and/or operator experience level: 6 and 2 years of experience in ultrasound screening |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: minor anomalies: mild hydronephrosis, choroid plexus cysts, mild ventriculomegaly, digital anomalies and cardiac defects not requiring treatment Reference standard (live birth): pregnancy outcomes were obtained from the hospital records and from the patients themselves Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 1326 Exclusions (study investigator): 36 lost to follow‐up (2.7%) Exclusions (review team): 6 (2 soft markers for chromosomal abnormalities, 4 abnormal karyotype) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
de Robertis 2017.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: consecutive pregnant women undergoing routine first‐trimester screening for chromosomal abnormalities at the study centre were enrolled in the study Study start and end date: September 2013 to September 2015 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Di Venere‐Sarcone Hospital) Region(s) and country/countries from which participants were recruited: Bari, Italy Sample size: 5319 Study eligibility criteria: pregnant women with a viable singleton pregnancy and follow‐up data available. Cases of pregnancy termination or spontaneous fetal death where autopsy results were unavailable were excluded. Number of participants with the target condition: 33 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: two‐stage screening (data were only reported for first‐trimester scan): First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation Second‐trimester scan: Data to construct 2 x 2 tables could not be extracted or derived from the report |
||
| Target condition and reference standard(s) | Target condition(s): major cardiac anomalies Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): confirmation by fetal cardiologist and/or surgery Reference standard (fetal or neonatal demise): postmortem autopsy Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 6350 Exclusions (study investigator): 24 excluded (autopsy unavailable), 1007 lost to follow‐up (15.9%) Exclusions (review team): none excluded |
||
| Comparative | |||
| Notes | Funding source: the authors declared that no funding was received for this work | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Del Bianco 2006.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: all consecutive low‐risk pregnant women were included in the study Study start and end date: January 2004 to December 2004 |
||
| Patient characteristics and setting | Setting: tertiary care facility (University Hospital of Foggia) Region(s) and country/countries from which participants were recruited: Foggia, Italy Sample size: 2847 Study eligibility criteria: singleton low‐risk pregnancies with confirmed dates by first trimester scan having full antenatal care and delivery at the study centre. Women with risk factors for congenital heart defects or other congenital anomalies were excluded. Number of participants with the target condition: 26 Population type: low‐risk population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 20 to 24 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: single Staff qualification and/or operator experience level: obstetrician/gynaecologist |
||
| Target condition and reference standard(s) | Target condition(s): major cardiac anomalies Definitions used for major and minor congenital abnormalities: minor cardiac anomalies which were excluded from the study were small ventricular septal and isolated atrial septal defects and non‐patent ductus were not included Reference standard (live birth): neonatal examination by a neonatologist Reference standard (fetal or neonatal demise): autopsy Postnatal follow‐up duration: directly after birth |
||
| Flow and timing | Eligible patients: 2847 Exclusions (study investigator): none reported Exclusions (review team): none excluded |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Drukker 2020.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of all pregnant women attending the study centre for prenatal care during the study period were reviewed Study start and end date: October 2016 to September 2018 |
||
| Patient characteristics and setting | Setting: multicentre (2) (John Radcliffe Hospital ‐ tertiary care facility; Horton General Hospital ‐ secondary care facility) Region(s) and country/countries from which participants were recruited: Oxford and Banbury, United Kingdom Sample size: 13,516 Study eligibility criteria: viable singleton pregnancies attending their hospitals for routine pregnancy care. Women referred to the study centre from other institutions for tertiary fetal medicine assessment were excluded. Number of participants with the target condition: 302 Population type: unselected population Prior testing: nuchal translucency measurement and biochemical screening for fetal aneuploidies |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 weeks to 22 weeks of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: scans were performed by accredited sonographers or clinical fellows |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: European Registration of Congenital Anomalies and Twins (EUROCAT) classification system Reference standard (live birth): hospital records, neonatal care records, radiology and laboratory records, and the National Congenital Anomaly and rare Disease Registration service (NCARDRS) Reference standard (fetal or neonatal demise): hospital records, radiology and laboratory records, and NCARDSRS Postnatal follow‐up duration: 6 months after birth |
||
| Flow and timing | Eligible patients: 15,244 Exclusions (study investigator): 200 excluded (33 spontaneous fetal death before index test, 167 no anomaly scan), 1358 no follow‐up (90%) Exclusions (review team): 170 (68 soft markers for chromosomal abnormalities, 96 abnormal karyotype, 4 genetic conditions not associated with structural defects, 2 abnormalities secondary to intra‐uterine infection) |
||
| Comparative | |||
| Notes | Funding Source: Lior Drukker, the primary author, and Aris T. Papageorghiou, one of the two co‐senior authors, receive support from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Drysdale 2002.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: all women presenting before 12 weeks' gestation were asked to participate in the study Study start and end date: May 1998 to May 1999 |
||
| Patient characteristics and setting | Setting: secondary care facility (St Mary’s Hospital) Region(s) and country/countries from which participants were recruited: Isle of Wight, United Kingdom Sample size: 947 Study eligibility criteria: gestational age of 12 weeks or less at first presentation Number of participants with the target condition: 18 Population type: unselected population Prior testing: booking scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 12 to 14 weeks’ gestation Ultrasound scanning protocol: not reported. The test is described as an early pregnancy scan, including nuchal translucency measurement with or without calculation of risk for trisomy 21. No further specification of views used to screen for fetal abnormalities is given. Cardiac screening: not reported Mode of examination: primary transabdominal Single or multiple operators: multiple (2) Staff qualification and/or operator experience level: certification for first trimester ultrasound and screening for fetal chromosomal abnormalities (Fetal Medicine Foundation) Second‐trimester scan: Timing: 20 to 21 weeks’ gestation Ultrasound scanning protocol: not reported, described as an anomaly scan Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): neonatal examination Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: until neonatal examination |
||
| Flow and timing | Eligible patients: 991 Exclusions (study investigator): 31 excluded (24 spontaneous fetal death before index test, 7 declined examination) Exclusions (review team): 13 (9 soft markers for chromosomal abnormalities, 3 abnormal karyotype, 1 non‐structural anomaly) |
||
| Comparative | |||
| Notes | Funding source: financial funding for this study was provided by the Isle of Wight Healthcare NHS Trust Strategic Development Fund | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Ebrashy 2019.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: all women referred for a first‐trimester anomaly scan to the study centre were enrolled in the study Study start and end date: December 2013 to December 2018 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Cairo University Hospital) Region(s) and country/countries from which participants were recruited: Cairo, Egypt Sample size: 3239 Study eligibility criteria: crown‐rump length between 45 and 84 mm at the time of the first‐trimester anomaly scan Number of participants with the target condition: 95 Population type: unselected population, 25% women with BMI > 30 kg/m2 Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 weeks and 0 days to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation, senior ultrasonographers with more than 15 years of experience Second‐trimester scan: Timing: 20 to 24 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation, senior ultrasonographers with more than 15 years of experience |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): neonatal examination and if indicated neonatal echocardiogram Reference standard (fetal or neonatal demise): autopsy and pathological examination were offered Postnatal follow‐up duration: until neonatal examination |
||
| Flow and timing | Eligible patients: 3400 Exclusions (study investigator): 160 lost to follow‐up (4.7%) Exclusions (review team): 18 (1 anomaly considered normal variant) |
||
| Comparative | |||
| Notes | Funding source: the authors declare that the funding organisation(s) played no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the report for publication. The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Erenel 2019.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women attending the clinic for first‐trimester screening for aneuploidies Study start and end date: August 2016 to February 2018 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Istanbul University Hospital) Region(s) and country/countries from which participants were recruited: Istanbul, Turkey Sample size: 704 Study eligibility criteria: pregnant women with vital pregnancy between 11 and 14 weeks’ gestation, based on crown‐rump length between 45 and 84 mm or last menstrual period at the time of the first‐trimester scan Number of participants with the target condition: 7 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple (4) Staff qualification and/or operator experience level: experienced, not further specified Second‐trimester scan: Timing: 18 to 23 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (4) Staff qualification and/or operator experience level: experienced, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy outcome was obtained from hospital records or by telephonic follow‐up to identify missed cases Reference standard (fetal or neonatal demise): autopsy in case of termination of pregnancy Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 707 Exclusions (study investigator): none reported Exclusions (review team): 3 (abnormal karyotype) |
||
| Comparative | |||
| Notes | Funding source: the study was supported by the Scientific Research Projects of İstanbul University (project no: 50825; May 29, 2015) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Fleurke‐Rozema 2014.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study Recruitment: cases with open spina bifida were identified from regional screening databases and the Dutch division of the European Registration of Congenital Anomalies and Twins (EUROCAT) database Study start and end date: January 2003 to December 2011 |
||
| Patient characteristics and setting | Setting: geographical regions covered by 2 tertiary care facilities (Academic Medical Center and University Medical Center of Groningen) Region(s) and country/countries from which participants were recruited: north‐western and north‐eastern regions of the Netherlands Sample size: 168,000 (based on estimated annual birthrate in the covered regions) Study eligibility criteria: pre‐ or postnatal diagnosis of open spina bifida with or without additional congenital anomalies Number of participants with the target condition: 85 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing (weeks and days gestation): 18 to 24 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): open spina bifida Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): postnatal diagnoses identified from regional registries, completed by paediatric spina bifida teams and data from the EUROCAT congenital anomaly registry Reference standard (fetal or neonatal demise): postnatal diagnoses identified from regional registries, completed by paediatric spina bifida teams and data from the EUROCAT congenital anomaly registry Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 336,000 Exclusions (study investigator): none reported Exclusions (review team): 168,000 (cases born in the period before implementation of the routine mid‐trimester scan in the Netherlands) |
||
| Comparative | |||
| Notes | Funding source: this study was funded by the Netherlands Organization of Health Research and Development (ZonMw, project number 200320012) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Fleurke‐Rozema 2016.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study Recruitment: pre‐ and postnatally diagnosed cases of cleft lip with or without cleft palate were identified from the Perinatal Registry and Dutch Association for Cleft Palate and Craniofacial Anomalies register Study start and end date: January 2008 to December 2012 |
||
| Patient characteristics and setting | Setting: geographical regions covered by 2 tertiary care facilities (Academic Medical Center and University Medical Center of Groningen) Region(s) and country/countries from which participants were recruited: north‐western and north‐eastern region of the Netherlands Sample size: 221,863 Study eligibility criteria: all isolated and non‐isolated cases with a pre‐ or postnatal diagnosis of cleft lip with or without cleft palate were included in the study Number of participants with the target condition: 227 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing (weeks and days gestation): 18 to 23 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple Staff qualification and/or operator experience level: certified by the Dutch medical authorities |
||
| Target condition and reference standard(s) | Target condition(s): cleft lip with or without cleft palate Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcomes from hospital records, data from paediatric surgeons' lists, the Dutch Association for Cleft Palate and Craniofacial Anomalies registry and The Netherlands Perinatal Registry Reference standard (fetal or neonatal demise): birth records from 16 weeks’ gestation were available in The Netherlands Perinatal Registry Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 222,000 Exclusions (study investigator): none reported Exclusions (review team): 137 (103 cases with associated major anomalies, 34 cases who did not receive the index test) |
||
| Comparative | |||
| Notes | Funding source: this study was funded by the Netherlands Organization of Health Research and Development (ZonMw, project number 200320012) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Fleurke‐Rozema 2017.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study Recruitment: cases with omphalocele or gastroschisis were identified from regional screening databases, paediatric surgeons' lists and the Dutch division of the European Registration of Congenital Anomalies and Twins (EUROCAT) database Study start and end date: January 2009 to December 2013 |
||
| Patient characteristics and setting | Setting: geographical regions covered by 2 tertiary care facilities (Academic Medical Center and University Medical Center of Groningen) Region(s) and country/countries from which participants were recruited: north‐western and north‐eastern regions of the Netherlands Sample size: not reported Study eligibility criteria: pre‐ or postnatal diagnosis of omphalocele or gastroschisis with or without additional congenital anomalies Number of participants with the target condition: 63 Population type: unselected population Prior testing: dating scan, combined test (optional) |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing (weeks and days gestation): 20 weeks’ scan, not further specified Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): omphalocele, gastroschisis Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcomes from hospital records, data from paediatric surgeons' lists, validated using the Netherlands Perinatal Registry and the EUROCAT registry Reference standard (fetal or neonatal demise): pregnancy and neonatal outcomes from hospital records, data from paediatric surgeons' lists, validated using the Netherlands Perinatal Registry and the EUROCAT registry Postnatal follow‐up duration: 1 year after birth |
||
| Flow and timing | Eligible patients: 185 cases with the target condition Exclusions (study investigator): none reported Exclusions (review team): 124 (122 non‐isolated cases, 2 cases who did not receive the index test) |
||
| Comparative | |||
| Notes | Funding source: the authors declared that no funding was received for this work | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Galindo 2011.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: prenatal screening centres in Spain were invited to participate in a survey study Study start and end date: 2004 to 2006 |
||
| Patient characteristics and setting | Setting: multicentre (33) (set of hospital centres which make prenatal diagnosis by ultrasound) Region(s) and country/countries from which participants were recruited: Spain Sample size: 264,612 Study eligibility criteria: the criteria for selecting the centres was that all provinces be represented (n = 50) by at least 1 centre, preferably from the public network, and/or a minimally significant activity volume set at at least 500 births/year. Centres were excluded if they did not respond to the survey or if the authors considered the response invalid. Responses were considered invalid if no data were provided, if prenatal but not postnatal data were provided, and if there was no differentiation between cases detected in the reference population and cases detected following referral from other health areas. Number of participants with the target condition: 1060 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: second trimester of pregnancy Ultrasound scanning protocol: 68% of centres used a detailed protocol; the remaining centres did not use a standardised protocol Cardiac screening: 68% of centres used extended cardiac screening; the remaining centres did not use a standardised protocol. In 11% of centres, cardiac screening was not included in the routine second‐trimester anomaly scan, which accounted for 67% of births in the survey. Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: in the majority of the centres, this consisted of obstetricians with more than 5 years of experience |
||
| Target condition and reference standard(s) | Target condition(s): major cardiac anomalies Definitions used for major and minor congenital abnormalities: major or severe congenital cardiac anomalies were defined as those which have the potential to be life‐threatening and are likely to require intervention within the first year of life Reference standard (live birth): anomalies detected before birth and which were totally or partially confirmed after birth by imaging studies and surgeries were considered true positive. False‐negative cases were those diagnosed primarily after birth. This information was obtained by the collaboration of paediatricians and paediatric cardiologists from the original and referral centres. Parents were contacted when necessary. Reference standard (fetal or neonatal demise): by necropsy reports from the original and referral centre Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 495,382 Exclusions (study investigator): 230,770 excluded (no or invalid response to survey) Exclusions (review team): none excluded |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Gallot 2007.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study Recruitment: malformations in neonates, still births and fetuses following termination of pregnancy in the region are registered in the database based on data collected from maternity wards, paediatric units, cytogenetic laboratories, pathology laboratories, departments of medical genetics and birth certificates Study start and end date: January 1986 to December 2003 |
||
| Patient characteristics and setting | Setting: Central‐Eastern France Registry database Region(s) and country/countries from which participants were recruited: Central‐Eastern France Sample size: 1,834,972 Study eligibility criteria: registered infants aged 1 year or younger with a verified diagnosis of congenital diaphragmatic hernia Number of participants with the target condition: 451 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): at the end of the first trimester Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: not reported Second‐trimester scan: Timing: 22 to 24 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based) Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): congenital diaphragmatic hernia Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): data on postnatal diagnostic findings collected from maternity wards and paediatric units. Other sources of information include cytogenetic laboratories, pathology laboratories, departments of medical genetics and birth certificates. Reference standard (fetal or neonatal demise): if available, pathology reports Postnatal follow‐up duration: infants with malformations are registered after discharge up to the age of 1 year |
||
| Flow and timing | Eligible patients: 1,835,022 Exclusions (study investigator): none reported Exclusions (review team): 50 (missing information on gestational age at time of prenatal diagnosis based ‐ data supplied by authors) |
||
| Comparative | |||
| Notes | Funding source: the ‘ARDEMO’ team was supported by the French Research Department (JE2447). The primary author, D. Gallot, was supported by a grant from the Société Française de Médecine Périnatale and from the Collège National des Gynécologues et Obstétriciens Français. V. Sapin was supported by an INSERM grant (Contrat d’Interface). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Gireada 2022.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of unselected consecutive patients attending the study centre for a routine anomaly scan during the study period were reviewed Study start and end date: 2019 to 2021 |
||
| Patient characteristics and setting | Setting: private setting Region(s) and country/countries from which participants were recruited: Romania Sample size: 1597 Study eligibility criteria: pregnant women presenting for a routine fetal anomaly scan, with a known pregnancy outcome, who were scanned in the second or third trimester of pregnancy, or who were only scanned in the first trimester due to early termination of pregnancy Number of participants with the target condition: 36 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple (3) Staff qualification and/or operator experience level: specifically trained, not further specified Second‐trimester scan: Timing: 20 to 24 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: transabdominal and transvaginal Single or multiple operators: multiple (3) Staff qualification and/or operator experience level: specifically trained, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: cardiac anomalies were considered major if they could require immediate care after birth Reference standard (live birth): all live‐born babies were examined by the neonatologist in the first 3 days of life Reference standard (fetal or neonatal demise): confirmed by transvaginal ultrasound (first trimester termination), confirmed by autopsy (second trimester termination) Postnatal follow‐up duration: 1 month after birth |
||
| Flow and timing | Eligible patients: 1608 Exclusions (study investigator): none reported Exclusions (review team): 11 (2 anomalies considered not detectable by prenatal ultrasound, 9 anomalies considered normal variants) |
||
| Comparative | |||
| Notes | Funding source: the authors declared that this research received no external funding | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Gornall 2003.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study Recruitment: all cases diagnosed with congenital cystic adenomatoid malformation in the region that were notified to the registry during the study period were extracted from the database Study start and end date: January 1997 to December 2001 |
||
| Patient characteristics and setting | Setting: Trent Congenital Anomalies Register (CAR) Region(s) and country/countries from which participants were recruited: East Midlands of England, United Kingdom Sample size: 284,999 Study eligibility criteria: all suspected cases of congenital cystic adenomatoid malformation that were notified to the Trent CAR Number of participants with the target condition: 30 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing (weeks and days gestation): 18 to 22 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): congenital cystic adenomatoid malformation Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): for all cases, a data collection form was sent to the units where they were managed, including timing of detection of the anomaly and how it was detected, ultrasound appearances, other investigations and procedures performed during the prenatal period, associated anomalies, gestation at delivery, neonatal management including diagnostic tools used, whether surgery was performed and whether the child was found to have any resulting respiratory problems Reference standard (fetal or neonatal demise): where the fetus or infant had died, data were collected from post‐mortem results Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 285,000 Exclusions (study investigator): none reported Exclusions (review team): 1 (missing gestational age at prenatal diagnosis) |
||
| Comparative | |||
| Notes | Funding source: during the period of this research, the Trent Congenital Anomalies Register was funded by Trent Executive NHS R&D and Elizabeth Draper was funded by Leicestershire Health Authority. Jennifer Kurinczuk, senior author, is funded by a National Public Health Career Scientist Award from the DH and NHS R&D (PHCS 022). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Grande 2012.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of all pregnant women who received prenatal ultrasound screening between 11 and 14 weeks' gestation during the study period were reviewed Study start and end date: January 2002 to December 2009 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Department of Maternal‐Fetal Medicine, Hospital Clinic Barcelona) Region(s) and country/countries from which participants were recruited: Barcelona, Spain Sample size: 13,639 Study eligibility criteria: chromosomally normal singleton fetuses Number of participants with the target condition: 355 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan in all pregnancies, combined test in pregnancies with increased risk for fetal trisomies |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: basic Mode of examination: transabdominal and transvaginal Single or multiple operators: multiple (19) Staff qualification and/or operator experience level: obstetricians (no further specification) Second‐trimester scan: Timing: 20 to 22 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: European Registration of Congenital Anomalies and Twins (EUROCAT) classification system Reference standard (live birth): hospital records on pregnancy outcome and neonatal follow‐up, or obstetricians or the women themselves were contacted by telephone Reference standard (fetal or neonatal demise): postmortem examination was systematically performed at fetal demise after 10 weeks’ gestation Postnatal follow‐up duration: until neonatal discharge |
||
| Flow and timing | Eligible patients: 16301 Exclusions (study investigator): excluded (312 abnormal karyotype, 103 single gene disorders, 230 fetal death before index test), 1933 lost to follow‐up (12.3%) Exclusions (review team): 84 (80 soft markers for chromosomal abnormalities, 4 non‐structural anomalies) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Grandjean 1999.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: all ultrasonographic diagnoses of malformations and outcomes of the fetuses, including all cases of malformation diagnosed after abortion or after birth were recorded in 61 European obstetric units Study start and end date: January 1990 to June 1993 |
||
| Patient characteristics and setting | Setting: multicentre (61 centres participating in the Eurofetus Study) Region(s) and country/countries from which participants were recruited: Austria, Belgium, Croatia, Finland, France, Hungary, Italy, Luxembourg, Norway, Poland, Portugal, Spain, Sweden, United Kingdom Sample size: total number of screened fetuses is not reported Study eligibility criteria: all cases with structural anomalies diagnosed during pregnancy by routine ultrasound or defect found at birth or abortion Number of participants with the target condition: 1675 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 22 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: qualified as “level 2” |
||
| Target condition and reference standard(s) | Target condition(s): isolated fetal structural abnormalities Definitions used for major and minor congenital abnormalities: major was defined as lethal abnormalities, incurable abnormalities, those requiring major surgery or leading to handicap. The remaining were defined as minor. Excluded were abnormalities without serious medical consequences. Reference standard (live birth): pregnancy and neonatal outcomes were obtained from medical records Reference standard (fetal or neonatal demise): outcomes after abortion were obtained from medical records Postnatal follow‐up duration: 6 days after birth |
||
| Flow and timing | Eligible patients: 3685 with structural anomalies, of which 1675 had isolated malformations. Rate of diagnosis < 24 weeks' gestation was only specified for the latter group Exclusions (study investigator): none reported Exclusions (review team): 2010 (rate of diagnosis < 24 weeks' gestation unspecified) |
||
| Comparative | |||
| Notes | Funding source: the Eurofetus Study has received support from the European Union | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Gu 2011.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of 2844 pregnant women who received ultrasound screening at the facility during the study period were reviewed Study start and end date: February 2007 to July 2010 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Nanjing Drum Tower Hospital) Region(s) and country/countries from which participants were recruited: Nanjing, China Sample size: 3008 Study eligibility criteria: data were collected from pregnant women who had ultrasound screening during the first and second trimester in the study centre Number of participants with the target condition: 98 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 13 weeks and 6 days of gestation Ultrasound scanning protocol: basic Cardiac screening: basic Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported Second‐trimester scan: Type: single‐stage screening (index test 3) Timing: 18 to 24 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported Second‐trimester anomaly scan: Timing (weeks and days gestation): 18 to 24 weeks of gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): follow‐up visits were performed for all newborns Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 3135 Exclusions (study investigator): none reported Exclusions (review team): 127 (75 fetal death before index test, 32 soft markers for chromosomal abnormalities, 14 unspecified umbilical cord abnormalities, 5 fetal growth abnormalities, 1 anomaly considered not detectable by prenatal ultrasound) |
||
| Comparative | |||
| Notes | Funding source: National Project of Scientific and Technical Supporting Programs of China during the 11th Five‐year Plan Period (Grant No 2006BAl05A04) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Hafner 2003.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of all pregnant women who booked for delivery at the hospital in the first trimester of pregnancy during the study period were reviewed Study start and end date: January 1994 to December 2000 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Donauspital) Region(s) and country/countries from which participants were recruited: Vienna, Austria Sample size: 12,978 Study eligibility criteria: pregnant women with a viable fetus and who had a nuchal translucency measurement performed at the study centre. Criteria for exclusion were aneuploidy and cases who were scanned at the study centre following referral for increased fetal nuchal translucency. Number of participants with the target condition: 27 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing (weeks and days gestation): 22 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): major cardiac anomalies Definitions used for major and minor congenital abnormalities: all cardiac anomalies were defined as major, except for small muscular ventricular septal defects (VSDs), atrial septal defects II (ASDII) and patent ductus arteriosus Reference standard (live birth): neonatal examination by an experienced paediatrician. Echocardiography was performed when there was a suspect finding in the prenatal scanning or when a cardiac murmur or other irregularity was observed. When CHD was detected after the newborn was discharged, the department that made the diagnosis informed the study centre. Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: until neonatal examination |
||
| Flow and timing | Eligible patients: 13,716 Exclusions (study investigator): 117 (89 aneuploidy, 28 spontaneous fetal death before index test), 621 lost to follow‐up (45%) Exclusions (review team): none excluded |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Hartge 2011.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of 3221 pregnant women who attended the study centre for prenatal care during the study period were reviewed Study start and end date: 1993 to 2007 |
||
| Patient characteristics and setting | Setting: tertiary care facility (University Hospital of Schleswig‐Holstein) Region(s) and country/countries from which participants were recruited: Luebeck, Germany Sample size: 3171 Study eligibility criteria: pregnant women with a viable singleton pregnancy, with an early anomaly scan including fetal echocardiography Number of participants with the target condition: 29 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 weeks and 0 days to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported Second‐trimester scan: Timing (weeks and days gestation): second trimester Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): major cardiac anomalies Definitions used for major and minor congenital abnormalities: isolated intracardiac shunt malformations were not classified as a major congenital cardiac defect and were excluded from the final analysis Reference standard (live birth): medical records, including reports, prints and videotapes of the ultrasound examinations, and neonatal records were evaluated Reference standard (fetal or neonatal demise): autopsy reports if available Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 3221 Exclusions (study investigator): none reported Exclusions (review team): 50 (abnormal karyotype) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Hautala 2019.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study Recruitment: study data were collected from national registers containing information on all births, stillbirths and pregnancy terminations due to fetal anomaly, including all prenatally and postnatally diagnosed cases of univentricular heart and transposition of the great arteries Study start and end date: 2004 to 2014 |
||
| Patient characteristics and setting | Setting: multiple population‐based national registers: 1) the National Register of Paediatric Cardiac Surgery; (2) the Finnish Register of Congenital Malformations; (3) the Register of Induced Abortions; (4) the Medical Birth Register; and (5) the Cause‐of‐Death Register Region(s) and country/countries from which participants were recruited: Finland Sample size: 299,559 Study eligibility criteria: cases with univentricular heart and transposition of great arteries Number of participants with the target condition: 232 Population type: unselected population Prior testing: nuchal translucency measurement, maternal serum biochemistry |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing (weeks and days gestation): 18 weeks and 0 days to 21 weeks and 6 days of gestation Ultrasound scanning protocol: 2004 to 2009: no standardised national screening programme, 2010 to 2014: detailed Cardiac screening: 2004 to 2009: no standardised national screening programme, 2010 to 2014: extended Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: examinations are mainly performed by trained midwives, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): selected cardiac anomalies; univentricular heart and transposition of the great arteries Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): data were cross‐checked with the mothers’ and live‐born infants’ identification numbers and all information was verified from hospital records, including prenatal and postnatal reports, karyotype. One paediatric and fetal cardiologist confirmed all cardiac diagnoses and one clinical geneticist confirmed all extra‐cardiac malformations. Reference standard (fetal or neonatal demise): data were verified using autopsy reports Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 651,969 Exclusions (study investigator): none reported Exclusions (review team): 352,410 (352,332 cases born during the pre‐screening period (2004 to 2009), 78 cases of a selected anomaly within the larger category of conotruncal anomalies) |
||
| Comparative | |||
| Notes | Funding source: all authors declare no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
He 2016.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of pregnant women undergoing prenatal care at the study centre were reviewed Study start and end date: October 2013 to April 2016 |
||
| Patient characteristics and setting | Setting: tertiary care facility (People’s Hospital of Xinjiang) Region(s) and country/countries from which participants were recruited: Xinjiang, China Sample size: 7309 Study eligibility criteria: pregnant women who underwent prenatal care and delivery at the study centre were included Number of participants with the target condition: 38 Population type: unselected population Prior testing: nuchal translucency measurement |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 24 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): limb anomalies Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): cases detected after birth are reported, methods of postnatal verification not further specified Reference standard (fetal or neonatal demise): cases detected after elective pregnancy termination are reported, methods of post‐mortem verification not further specified Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 7328 Exclusions (study investigator): none reported Exclusions (review team): 19 (cases of a selected anomaly within the larger categories of miscellaneous musculoskeletal defects, multiple congenital anomalies or syndrome, and skeletal dysplasias, respectively) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Unclear | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Hernadi 1997.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: all pregnant women attending the department for prenatal care were offered a routine transvaginal ultrasound scan Study start and end date: 3‐year period, starting in February 1992 |
||
| Patient characteristics and setting | Setting: secondary care facility (Markhot F County Hospital) Region(s) and country/countries from which participants were recruited: Eger, Hungary Sample size: 3969 Study eligibility criteria: pregnant women in the 12th week of pregnancy. Cases with missed abortion or incomplete follow‐up data were excluded. Number of participants with the target condition: 42 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): in the 12th week of gestation Ultrasound scanning protocol: basic Cardiac screening: cardiac anatomy was not examined Mode of examination: transabdominal and transvaginal Single or multiple operators: multiple (2) Staff qualification and/or operator experience level: 1 obstetrician and 1 sonographer with more than 6 years of experience Second‐trimester scan: Timing: in the 18th week of gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): neonatal examination by a paediatrician Reference standard (fetal or neonatal demise): autopsy in cases of termination of pregnancy Postnatal follow‐up duration: 5 days after birth |
||
| Flow and timing | Eligible patients: 4164 Exclusions (study investigator): 139 excluded (19 spontaneous fetal death before index test, 120 fetuses on gestational age), 34 lost to follow‐up (0.8%) Exclusions (review team): 22 (soft markers for chromosomal abnormalities) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Hildebrand 2010.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of pregnant women attending one of the participating hospitals for a second‐trimester anomaly scan during the study period were reviewed Study start and end date: September 2001 to August 2004 |
||
| Patient characteristics and setting | Setting: multicentre (5) (4 secondary care facilities ‐ units from Norrköping, Motala, Jönköping and Värnamo; 1 tertiary care facility ‐ University Hospital in Linköping) Region(s) and country/countries from which participants were recruited: South‐eastern Sweden; Linköping, Norrköping, Motala, Jönköping and Värnamo Sample size: 14,024 Study eligibility criteria: pregnant women undergoing a second‐trimester anomaly scan with complete follow‐up data available. Infants/fetuses with minor anomalies were excluded from the analyses. Number of participants with the target condition: 264 Population type: unselected population Prior testing: none |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: second trimester of pregnancy (Motala, Jönköping, Norrköping, Värnamo) Ultrasound scanning protocol: basic in 3 of the 4 participating centres offering a routine second‐trimester scan (Jönköping, Norrköping, Värnamo); in Motala, no standardised checklist for fetal anatomy was used Cardiac screening: basic in 3 of the 4 participating centres (Jönköping, Norrköping, Värnamo); in Linköping, no standardised checklist for the fetal heart was used Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: specially trained midwives, training consisted of 2 compulsory courses as well as practical training with an experienced midwife Notes: At the fifth unit (Linköping), the routine scan was performed at 11 to 14 weeks' gestation and no routine second‐trimester scan was offered. The 11‐ to 14‐week scan did not meet our inclusion criteria because the scan did not include a routine anatomical survey. |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: fetal anomaly diagnoses were grouped according to their likely clinical consequences: major anomalies (lethal), anomalies associated with long‐term handicap, anomalies potentially amenable to intrauterine treatment and fetal conditions that required postnatal investigation and/or treatment (as proposed by Royal College of Obstetricians and Gynaecologists in 1997) Reference standard (live birth): pregnancy and neonatal outcomes from hospital records, all structural anomalies diagnosed or suspected were obtained from each department; the Swedish maternal personal identification number was used to match obstetric and neonatal data Reference standard (fetal or neonatal demise): if available, pathology or autopsy records Postnatal follow‐up duration: first week after birth |
||
| Flow and timing | Eligible patients: 23,935 Exclusions (study investigator): 450 excluded (fetuses/infants with minor anomalies), 2746 incomplete follow‐up (11.7%) Exclusions (review team): 6692 (no routine second trimester anomaly scan); from the cases who did receive a routine second trimester scan, a further 23 cases were excluded (1 soft marker for chromosomal abnormalities, 16 abnormal karyotype, 6 anomalies considered minor by the review team) |
||
| Comparative | |||
| Notes | Funding source: FORSS, the Southeast Region medical research council, financially supported the study | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Iliescu 2013.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: an extended first‐trimester ultrasound scan was performed in 5472 consecutive women attending the participating study centres Study start and end date: January 2010 to March 2012 |
||
| Patient characteristics and setting | Setting: multicentre (2) (University of Craiova ‐ tertiary care facility; University of Athens ‐ tertiary care facility) Region(s) and country/countries from which participants were recruited: Craiova, Romania; Athens, Greece Sample size: 4791 Study eligibility criteria: written informed consent before ultrasound examination Number of participants with the target condition: 129 Population type: unselected population Prior testing: nuchal translucency measurement |
||
| Index tests |
Type: two‐stage screening (data were only included for the first‐trimester scan): First‐trimester scan: Timing (weeks and days gestation): 12 gestational weeks to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple Staff qualification and/or operator experience level: trained and certified obstetricians with more than 5 years of experience Second‐trimester scan: Data for the second‐trimester scan were excluded on index test timing (16 to 26 weeks' gestation) |
||
| Target condition and reference standard(s) | Target condition(s): structural anomalies Definitions used for major and minor congenital abnormalities: major was defined as lethal anomalies or anomalies with severe immediate or long‐term morbidity. Moderate or minor was defined as anomalies with short‐ or long‐term morbidity of minor or moderate severity. Reference standard (live birth): neonatal examination by a paediatrician Reference standard (fetal or neonatal demise): following termination of pregnancy or intrauterine demise the case was examined by an interdisciplinary team of pathologists and obstetricians Postnatal follow‐up duration: 3 days after birth |
||
| Flow and timing | Eligible patients: 5021 Exclusions (study investigator): 645 lost to follow‐up (11.8%) Exclusions (review team): 36 (9 soft markers for chromosomal abnormalities, 17 abnormal karyotype, 2 anomalies considered not detectable by prenatal ultrasound, 5 non‐structural anomalies, 1 normal variant, 1 anomalies secondary to intra‐uterine infection, 1 abnormal biometric measurements) |
||
| Comparative | |||
| Notes | First‐trimester false‐positive diagnoses are reported overall, but not per anomaly. As these data may include false‐positive diagnoses for soft markers for chromosomal abnormalities, we have decided not to extract the overall numbers of false‐positive diagnoses for the meta‐analysis. Funding source: not reported |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Ingale 2017.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of pregnant women who received prenatal ultrasound at the facility during the study period were considered Study start and end date: not reported |
||
| Patient characteristics and setting | Setting: tertiary care facility (Krishna Institute of Medical Sciences) Region(s) and country/countries from which participants were recruited: Karad, India Sample size: 3186 Study eligibility criteria: antenatal scan at around 20 weeks' gestation Number of participants with the target condition: 140 Population type: unselected Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): anomalies detected postnatally Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 3200 Exclusions (study investigator): none reported Exclusions (review team): 14 (6 soft markers for chromosomal abnormalities, 7 anomalies considered not detectable by prenatal ultrasound, 1 anomaly we were unable to classify due to the use of an unspecified and uncommon abbreviation) |
||
| Comparative | |||
| Notes | Funding source: the authors declared that no financial or other competing interests were present. The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Jacobsen 2011.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: all pregnant women who planned to deliver at the study centre and who chose to have the 11‐ to 14‐week as well as the 18‐ to 20‐week scan were included Study start and end date: January 2003 to June 2007 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Copenhagen University Hospital) Region(s) and country/countries from which participants were recruited: Copenhagen, Denmark Sample size: 9192 Study eligibility criteria: missed miscarriages diagnosed at the 11‐ to 14‐week scan were excluded. Second‐trimester miscarriages were included if the mother had the 18‐ to 20‐week scan. Women referred from other hospitals were excluded. Number of participants with the target condition: 155 Population type: unselected population Prior testing: first‐trimester nuchal translucency scan |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing (weeks and days gestation): 18 to 20 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: basic Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: nurses trained and certified in nuchal translucency measurement according to the Fetal Medicine Foundation performed all examinations. Unclear if additional certification was required for the performance of the 18‐ to 20‐week scan. |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: malformations were classified as proposed by the Royal College of Obstetrics and Gynecology in 1997 Reference standard (live birth): pregnancy and neonatal outcome data obtained from the neonatology database, concerning all children with a malformation born during the study period Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: until neonatal discharge |
||
| Flow and timing | Eligible patients: 9324 Exclusions (study investigator): 127 excluded (pyelectasis) Exclusions (review team): 5 (2 abnormal karyotype, 3 anomalies considered not detectable by prenatal ultrasound) |
||
| Comparative | |||
| Notes | Funding source: the authors declared that no specific funding was received for this work | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Johnson 1997.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of pregnant women who underwent ultrasound screening as part of routine antenatal care or on self‐referral were reviewed Study start and end date: September 1992 to January 1996 |
||
| Patient characteristics and setting | Setting: multicentre (8) (7 secondary care facilities; 1 tertiary care facility) Region(s) and country/countries from which participants were recruited: Basildon, Ascot, London, Camberley, Haywards Heath, Sidcup and Chertsey, United Kingdom Sample size: 55,237 Study eligibility criteria: pregnant women with live fetuses at 10 to 14 weeks’ gestation Number of participants with the target condition: 47 Population type: mixed population of unselected women screened as part of routine prenatal at one of the 7 secondary care facilities (n = 28,891) and unselected self‐referred women screened at the tertiary care facility (n = 26,346) Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 10 to 14 weeks’ gestation Ultrasound scanning protocol: basic Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: (obstetric) sonographer, not further specified Second‐trimester scan: Timing: 18 to 22 weeks’ gestation Ultrasound scanning protocol: examination is described as detailed, protocol not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: (obstetric) sonographer, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): anencephaly Definitions used for major and minor congenital abnormalities: not applicable Reference standard (live birth): pregnancy outcomes from hospital records or patients themselves Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 55,237 Exclusions (study investigator): none reported Exclusions (review team): none excluded |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Kenkhuis 2018.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: all pregnant women who chose to have a combined test during the study period were asked to participate in the study Study start and end date: November 2012 to December 2015 |
||
| Patient characteristics and setting | Setting: multicentre (8) (6 ultrasound practices ‐ primary care facilities; Isala Hospital ‐ secondary care facility; University Medical Center Groningen ‐ tertiary care facility) Region(s) and country/countries from which participants were recruited: Groningen and Overrijsel, the Netherlands Sample size: 5021 Study eligibility criteria: high‐ and low‐risk pregnant women opting for the combined test Number of participants with the target condition: 68 Population type: pregnant women opting for the combined test Prior testing: combined test at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 weeks and 0 days to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple Staff qualification and/or operator experience level: sonographers, certified for the performance of nuchal translucency measurement Second‐trimester scan: Timing: in the 20th week of gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcomes from hospital records, follow‐up forms provided to participating practices and hospitals Reference standard (fetal or neonatal demise): if available, pathology records Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 5534 Exclusions (study investigator): 495 lost to follow‐up (8.9%) Exclusions (review team): 18 (1 soft marker for chromosomal abnormalities, 17 non‐structural anomalies) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Krapp 2011.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of all fetuses screened at the study centre during the study period were reviewed Study start and end date: October 2007 to June 2009 |
||
| Patient characteristics and setting | Setting: tertiary care facility Region(s) and country/countries from which participants were recruited: Hamburg, Germany Sample size: 609 Study eligibility criteria: all fetuses with crown‐rump length between 45 and 84 mm were included Number of participants with the target condition: 6 Population type: mixed population of unselected women referred for first‐trimester screening (n = 617; 89.4%), high‐risk women referred on increased nuchal translucency (n = 54; 7.8%) and unknown (n = 19; 2.8%). A total of 17 cases were diagnosed with a congenital heart defect during the study, of which 15 were referred because of abnormal nuchal translucency. Prior testing: nuchal translucency measurement at the time of, or prior to, the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): first trimester of the pregnancy Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: single Staff qualification and/or operator experience level: not reported Second‐trimester scan: Timing: second trimester of pregnancy Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): participants received a questionnaire for postnatal evaluation. In case of no response, the referring physician was contacted. Reference standard (fetal or neonatal demise): if available, autopsy report Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 690 Exclusions (study investigator): none reported Exclusions (review team): 81 (16 aneuploidy; 65 no reference standard or lost to follow‐up (9.4%)) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Lee 1998.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of all women who delivered at the study centre during the study period were reviewed for eligibility Study start and end date: 1990 to 1994 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Yongdong Severance Hospital, Yonsei University College of Medicine) Region(s) and country/countries from which participants were recruited: Seoul, Korea Sample size: 3004 Study eligibility criteria: women with singleton pregnancies who were delivered at the study centre and had at least one ultrasound scan during pregnancy. Ultrasound scans that were done on referred patients were analysed separately from those done on a routine basis. Number of participants with the target condition: 23 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing (weeks and days gestation): 18 to 20 weeks' gestation Ultrasound scanning protocol: detailed Cardiac screening: basic Mode of examination: not reported Single or multiple operators: unclear Staff qualification and/or operator experience level: a trained obstetric fellow |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: European Registration of Congenital Anomalies and Twins (EUROCAT) classification system Reference standard (live birth): clinical newborn examinations, chromosomal analyses, radiologic studies, operative findings Reference standard (fetal or neonatal demise): autopsy findings when available Postnatal follow‐up duration: 2 years after birth |
||
| Flow and timing | Eligible patients: 5544 Exclusions (study investigator): 725 (no ultrasound scan during pregnancy) Exclusions (review team): 1815 (referred cases) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Leiroz 2021.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: convenience sample of pregnant women undergoing prenatal ultrasound screening for fetal anomalies at the facility Study start and end date: July 2016 to December 2019 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Caliper Imaging School) Region(s) and country/countries from which participants were recruited: Salvador, Brazil Sample size: 929 Study eligibility criteria: pregnant women who did not have an ultrasound scan before 20 weeks' gestation, who were referred because of suspicious or confirmed fetal anomalies and who revoked their consent at any time during follow‐up were excluded Number of participants with the target condition: 74 Population type: unselected population Prior testing: dating scan |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 20 to 24 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: transabdominal and transvaginal Single or multiple operators: multiple (11) Staff qualification and/or operator experience level: all medical specialists had more than 2 years of experience, the majority more than 5 years |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural and chromosomal anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): participants were called 6 months after the ultrasound scan to reaffirm their willingness to remain enrolled in the study. Then, they informed the health service where the delivery occurred and their registration numbers. The examiners collected data from maternal and neonatal records regarding the general and segmental neonatal physical assessment recommended by the Brazilian Ministry of Health and additional tests performed during hospitalisation when indicated. Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: 6 months after ultrasound (~2 months after birth) |
||
| Flow and timing | Eligible patients: 967 Exclusions (study investigator): none reported Exclusions (review team): 38 (1 with missing neonatal outcome data, 37 soft markers for chromosomal abnormalities) |
||
| Comparative | |||
| Notes | Funding source: the authors received financial support for this work from the National Council for Scientific and Technological Development (CNPq) for the master’s scholarship grant no. 405842/2016−3 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Li 2008.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: 2288 consecutive pregnant women were evaluated during the routine 11‐ to 14‐week ultrasound scan Study start and end date: a 3‐year period, not further specified |
||
| Patient characteristics and setting | Setting: tertiary care facility (Guangzhou Maternal and Neonatal Hospital) Region(s) and country/countries from which participants were recruited: Guangdong, China Sample size: 2228 Study eligibility criteria: consecutive unselected pregnant women were evaluated during the routine ultrasound scan Number of participants with the target condition: 22 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: protocol not reported Cardiac screening: not reported Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple (2) Staff qualification and/or operator experience level: both operators had more than 8 years of experience Second‐trimester scan: Timing (weeks and days gestation): 20 to 24 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (2) Staff qualification and/or operator experience level: both operators had more than 8 years of experience |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): cases detected after birth are reported, methods of postnatal verification not specified Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 2288 Exclusions (study investigator): 56 lost to follow‐up (2.4%) Exclusions (review team): 4 (soft markers for chromosomal abnormalities) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Unclear | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Liao 2021.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: data from all pregnant women that attended the study centre for a routine first‐trimester scan were evaluated Study start and end date: October 2008 to December 2015 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Shenzhen Maternity & Child Healthcare Hospital) Region(s) and country/countries from which participants were recruited: Shenzhen, China Sample size: 53,213 Study eligibility criteria: singleton pregnancies with a live fetus between 11 and 13 + 6 weeks and complete follow‐up data were included. Unexplained miscarriages and fetal deaths were excluded. Number of participants with the target condition: 1920 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple Staff qualification and/or operator experience level: all sonographers underwent a rigorous 6‐month training Second‐trimester scan: Timing: 18 to 24 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: extended Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: all sonographers underwent a rigorous 6 months' training |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: cardiac anomalies considered minor: rhabdomyoma, ventricular septal defects, persistent left superior vena cava and vascular rings. No further definitions for major and minor congenital anomalies given. Reference standard (live birth): data on postnatal findings were obtained from Shenzhen Birth Defects Surveillance Database and Chinese Birth Defects Monitoring Network. In addition, data from hospital records and telephone calls to patients themselves were collected. Reference standard (fetal or neonatal demise): results of genetic tests and autopsies were extracted from medical records if available Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 59063 Exclusions (study investigator): 420 excluded (miscarriage or spontaneous fetal death), 5294 lost to follow‐up (9.0%) Exclusions (review team): 136 (105 anomalies associated with holoprosencephaly that were reported separately in addition to the main diagnosis, 19 anomalies considered not detectable by prenatal ultrasound, 12 physiological midgut herniations) |
||
| Comparative | |||
| Notes | Funding source: the study was supported by the National Key Research and Development Program of China (2018YFC1002202), the National Nature Science Foundation of China (81771598,81571758) and the Shenzhen Science and Technology project (JCYJ20170307091013214) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Maarse 2011.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: all screened pregnant women in the geographic region were included Study start and end date: January 2007 to December 2008 |
||
| Patient characteristics and setting | Setting: multicentre; 25 non‐profit ultrasound screening centres (primary/secondary care facilities) belonging to the Wilhelmina's Children's Hospital referral network (tertiary care facility) Region(s) and country/countries from which participants were recruited: Utrecht, the Netherlands Sample size: 35,924 Study eligibility criteria: pregnant women undergoing ultrasound screening in 25 centres, separated into a low‐risk and high‐risk population based on positive family history of orofacial clefts or other risk factors for fetal abnormalities Number of participants with the target condition: 54 Population type: low‐risk population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 22 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: trained midwives, sonographers and obstetricians, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): orofacial clefts Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcome were obtained from medical records Reference standard (fetal or neonatal demise): autopsy was recommended to verify the prenatal diagnosis, no further specification on uptake of autopsy following fetal demise Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 38,760 Exclusions (study investigator): none reported Exclusions (review team): 2836 (high‐risk pregnant women) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
McAuliffe 2005.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: all pregnant women undergoing first‐trimester ultrasound screening for fetal abnormalities at the study centre were asked to participate in the study Study start and end date: July 2003 to January 2004 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Center of Excellence in Obstetric Ultrasound, Mount Sinai Hospital) Region(s) and country/countries from which participants were recruited: Toronto, Canada Sample size: 299 Study eligibility criteria: pregnant women with a single live fetus at 11 to 14 weeks gestation and maternal informed consent Number of participants with the target condition: 6 Population type: unselected population Prior testing: screening for chromosomal abnormalities using nuchal translucency measurement and serum biochemistry at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: basic Cardiac screening: basic Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple (8) Staff qualification and/or operator experience level: certified in nuchal translucency scanning according to the Fetal Medicine Foundation and trained in first‐trimester anatomy screening Second‐trimester scan: Timing (weeks and days gestation): 18 to 22 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: major was defined as lethal, incurable or curable severe abnormalities with a high risk of residual handicap. Minor was defined as other defects less severe or benign and of no functional or cosmetic significance. Reference standard (live birth): neonatal examination by a paediatrician, pregnancy outcome and postnatal findings from hospital records, chromosome analysis when indicated Reference standard (fetal or neonatal demise): autopsy Postnatal follow‐up duration: until neonatal examination |
||
| Flow and timing | Eligible patients: 325 Exclusions (study investigator): 25 lost to follow‐up (7.7%) Exclusions (review team): 1 (umbilical cord anomaly) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Natu 2014.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women attending the facility for prenatal care were informed and enrolled after informed consent Study start and end date: December 2011 to October 2013 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Sri Aurobindo Institute of Medical Sciences) Region(s) and country/countries from which participants were recruited: Indore, India Sample size: 548 Study eligibility criteria: pregnant women with a viable pregnancy before 14 weeks’ gestation were included. Missed abortions and molar pregnancies were excluded. Patients were divided into two groups based on risk of having a fetus affected by a congenital anomaly. High‐risk pregnancy was defined as any one of the following risk factors: maternal age > 30 years, previous history of any congenital anomaly or pregnancy loss, family history of structural defects, pregnancy after assisted reproductive techniques, maternal diseases like diabetes mellitus or epilepsy, multiple pregnancy, history of smoking or alcohol consumption in the antenatal period, previous affected child with chromosomal aberration. Number of participants with the target condition: 3 Population type (cohort included for meta‐analysis): low‐risk Prior testing: not reported |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: basic Cardiac screening: basic Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported Second‐trimester scan: Timing: 18 to 22 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): neonatal examination by a paediatrician Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: until neonatal examination |
||
| Flow and timing | Eligible patients: 1500 Exclusions (study investigator): 323 excluded (321 on exclusion criteria, 2 unspecified exclusions), 130 lost to follow‐up (11.0%) Exclusions (review team): 499 (496 high‐risk pregnancies, 3 soft markers for chromosomal abnormalities from the low‐risk cohort) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Novotna 2012.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women attending the study centre for prenatal screening Study start and end date: 2003 to 2008 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Charles University) Region(s) and country/countries from which participants were recruited: Prague, Czech Republic Sample size: 8859 Study eligibility criteria: pregnant women with a viable pregnancy and a crown‐rump length of 45 to 84 mm at the first‐trimester scan with at least one of the examinations in the second or third trimester Number of participants with the target condition: 113 Population type: unselected population, including referrals Prior testing: first‐trimester serum screening of free beta‐HCG (human chorionic gonadotropin) and PAPP‐A (pregnancy‐associated plasma protein A) prior to ultrasound screening (preferably around 10 weeks' gestation) |
||
| Index tests |
Type: two‐stage screening (data were only reported for the first‐trimester scan): First‐trimester scan: Timing (weeks and days gestation): 11 weeks and 1 day to 14 weeks and 0 days of gestation Ultrasound scanning protocol: basic Cardiac screening: unclear if morphological evaluation of the heart was part of the routine first trimester scan Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple Staff qualification and/or operator experience level: trained according to the standards of the Fetal Medicine Foundation and at least 2 years of experience Second‐trimester scan: Data to construct 2 x 2 tables could not be extracted or derived from the report |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: soft markers for chromosomal abnormalities (isolated borderline ventriculomegaly, mild hydronephrosis, choroid plexus cysts and isolated increased nuchal translucency) were considered minor. No further definition of anomalies considered minor was reported. Reference standard (live birth): pregnancy and neonatal outcomes from all patients delivering in the hospital were retrieved from hospital records. The information about postpartum detected malformations was obtained from the neonatal database. Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 9150 Exclusions (study investigator): 91 excluded (terminated pregnancies), 170 lost to follow‐up (1.9%) Exclusions (review team): 30 (soft markers for chromosomal abnormalities) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Ogge 2006.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: all (17) obstetric units of the Piedmont Region equipped with an echographic service that had participated in a previous prospective multicentre study to evaluate the accuracy of the four‐chamber view were invited to take part in the study, of which 15 agreed to participate. All women attending one of the participating centres during the study period for a routine ultrasound examination after 18 weeks’ gestation were eligible for inclusion. Study start and end date: January 2002 to December 2004 |
||
| Patient characteristics and setting | Setting: multicentre (15); secondary and tertiary care facilities Region(s) and country/countries from which participants were recruited: Piedmont region, Italy Sample size: 4873 Study eligibility criteria: low‐risk pregnant women after 18 weeks’ gestation undergoing routine second trimester screening. Women with risk factors for congenital heart defects were excluded. Number of participants with the target condition: 39 Population type: low‐risk population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 24 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: obstetrician/gynaecologist with 2‐week training course and 1 day of training every 4 months thereafter |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcomes were obtained from hospital records Reference standard (fetal or neonatal demise): postmortem examination was performed in all cases of termination of pregnancy, spontaneous miscarriage or perinatal death Postnatal follow‐up duration: until discharge from hospital |
||
| Flow and timing | Eligible patients: 7041 Exclusions (study investigator): 673 lost to follow‐up (9.6%) Exclusions (review team): 1495 (1454 ultrasound screening performed > 24 weeks' gestation, 41 missing information on timing of ultrasound scan) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Orlandi 2014.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: unselected pregnant women referred to the study centre during the study period for first‐trimester screening of aneuploidies were eligible for inclusion Study start and end date: January 2009 to December 2011 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Centre for Prenatal Diagnosis of Palermo) Region(s) and country/countries from which participants were recruited: Palermo, Italy Sample size: 4021 Study eligibility criteria: pregnant women with a singleton pregnancy and crown‐rump length of 38 to 84 mm were included in the study. Cases lost to second‐trimester follow‐up or at delivery were excluded. Additionally, cases were excluded if cardiac morphology could not be visualised appropriately, both with a transabdominal and transvaginal approach. Number of participants with the target condition: 23 Population type: unselected population Prior testing: genetic screening at the time of the first‐trimester scan based on maternal age, levels of maternal serum free beta human chorionic gonadotrophin (hCG) and pregnancy‐associated plasma protein A (PAPP‐A), nuchal translucency measurement and presence of fetal nasal bone |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple (3) Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation Second‐trimester scan: Timing (weeks and days gestation): 20 to 22 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: major was defined as the anomaly being either lethal or required surgery or interventional cardiac catheterisation during the first year of life in the newborn. Minor was defined as anomalies that are generally asymptomatic. Reference standard (live birth): neonatal examination by a neonatologist. Additional information provided by the mothers, or their gynaecologists. In cases where the infant underwent surgery, pertinent information was provided by the paediatric surgeon. Reference standard (fetal or neonatal demise): post‐mortem examination in cases in which the patients decided for termination of pregnancy was not available Postnatal follow‐up duration: follow‐up at 6 and 12 months |
||
| Flow and timing | Eligible patients: 4820 Exclusions (study investigator): 141 excluded (failure to visualise cardiac morphology properly at the first trimester scan), 649 lost to follow‐up (13.9%) Exclusions (review team): 9 (abnormal karyotype) |
||
| Comparative | |||
| Notes | False‐positive diagnoses were only reported for major cardiac anomalies; there were no false‐positive diagnoses among major cardiac anomalies Funding source: the authors declared that no funding was received for this work |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Oztekin 2009.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: ultrasound screening was performed at 11 to 14 weeks in all pregnant women who attended the ultrasound unit during the study period Study start and end date: 2003 to 2007 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Bozyaka Training and Research Hospital) Region(s) and country/countries from which participants were recruited: Izmir, Turkey Sample size: 1041 Study eligibility criteria: all pregnant women undergoing first‐trimester ultrasound screening at the study centre were included Number of participants with the target condition: 18 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: single Staff qualification and/or operator experience level: experienced radiologist, not further specified Second‐trimester scan: Timing: 18 to 22 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: major was defined as lethal, incurable or curable severe abnormalities with a high risk of residual handicap. Less severe or benign abnormalities constituted the group of minor structural abnormalities. Reference standard (live birth): pregnancy and neonatal outcomes from hospital records, from contacting clinicians and from patients themselves Reference standard (fetal or neonatal demise): unclear; results mention whether suspected anomalies were confirmed in terminated pregnancies; methods for confirmation of the anomaly are not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 1085 Exclusions (study investigator): 41 lost to follow‐up (3.8%) Exclusions (review team): 3 (2 cases with isolated soft markers for chromosomal abnormalities, 1 case with a soft marker and an anomaly considered minor by the review team) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Petousis 2020.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: all pregnant women attending the study centre for a routine first trimester scan during the study period were eligible for inclusion in the study Study start and end date: June 2013 to May 2017 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Aristotle University of Thessaloniki) Region(s) and country/countries from which participants were recruited: Thessaloniki, Greece Sample size: 3263 Study eligibility criteria: pregnant women with a singleton pregnancy who attended the study centre for a first‐trimester ultrasound between 11 and 14 weeks, a second trimester scan between 20 and 24 weeks and a known pregnancy outcome, were included in the study. Only cases with a confirmed or apparently normal karyotype were included in the analysis. Number of participants with the target condition: 57 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening (data were only reported for the first‐trimester scan): First‐trimester scan: Timing (weeks and days gestation): 11 weeks and 0 days to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: basic Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation Second‐trimester scan: Data to construct 2 x 2 tables could not be extracted or derived from the report |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: European Registration of Congenital Anomalies and Twins (EUROCAT) classification system Reference standard (live birth): neonatal examination by a paediatrician Reference standard (fetal or neonatal demise): postmortem examination if available Postnatal follow‐up duration: until neonatal examination |
||
| Flow and timing | Eligible patients: 3378 Exclusions (study investigator): 17 excluded (abnormal karyotype), 98 lost to follow‐up or miscarried after normal index test (29%) Exclusions (review team): none excluded |
||
| Comparative | |||
| Notes | Funding source: no potential conflict of interest was reported by the authors. The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Pilalis 2012.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of pregnant women who attended the study centre for prenatal care were reviewed Study start and end date: not reported |
||
| Patient characteristics and setting | Setting: private maternity hospital (Leto Maternity Hospital) Region(s) and country/countries from which participants were recruited: Athens, Greece Sample size: 3889 Study eligibility criteria: pregnant women with a viable singleton pregnancy who completed the two‐step ultrasound screening protocol, who delivered at the study centre and with a known pregnancy outcome were included Number of participants with the target condition: 48 Population type: unselected Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: not routinely examined Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation, with more than 2 years of experience Second‐trimester scan: Timing: 20 to 24 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation, with more than 2 years of experience |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: major anomalies were defined as requiring medical and/or surgical treatment or causing mental handicap Reference standard (live birth): all neonates were examined by a paediatrician. Pregnancy outcomes were obtained from hospital records and contact with obstetricians. Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: until neonatal examination (up to 48 hours after birth) |
||
| Flow and timing | Eligible patients: 3902 Exclusions (study investigator): none reported Exclusions (review team): 13 (1 soft marker for chromosomal abnormalities, 10 abnormal karyotype, 1 anomaly considered not detectable by prenatal ultrasound, 1 non‐structural anomaly) |
||
| Comparative | |||
| Notes | Funding source: the authors reported no conflicts of interest. The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Rydberg 2017.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of fetuses screened at the study centre during the study period were reviewed Study start and end date: January 2006 to December 2013 |
||
| Patient characteristics and setting | Setting: secondary care facility (Östersund County Hospital) Region(s) and country/countries from which participants were recruited: Östersund, Sweden Sample size: 10,336 Study eligibility criteria: pregnant women undergoing ultrasound screening before 22 weeks’ gestation. All babies who were delivered at the study centre, babies who delivered elsewhere as a consequence of prenatal diagnosis of fetal abnormalities detected at the study centre. Fetuses lost by miscarriages, termination of pregnancy or intrauterine fetal death with fetal abnormalities were included. Number of participants with the target condition: 166 Population type: unselected population Prior testing: in cases of increased risk for chromosomal abnormalities: amniocentesis (2006 to 2009) or combined test (> 2009) |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 22 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: 2006 to 2009: basic, 2010 to 2013: extended Mode of examination: transabdominal alone Single or multiple operators: multiple (9) Staff qualification and/or operator experience level: specially trained midwives (6) and obstetricians (3), experiences ranging from 1 month to 17 years |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: following guidelines of the Royal College of Obstetrics and Gynaecology Reference standard (live birth): neonatal examination by paediatrician, pregnancy outcome from hospital records Reference standard (fetal or neonatal demise): if available, postmortem examination Postnatal follow‐up duration: until neonatal discharge |
||
| Flow and timing | Eligible patients: 10,414 Exclusions (study investigator): none reported Exclusions (review team): 22 (21 soft markers for chromosomal abnormalities, 1 anomaly considered not detectable by prenatal ultrasound) |
||
| Comparative | |||
| Notes | Funding source: the authors have stated explicitly that there are no conflicts of interest in connection with this article. No special funding was received. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Sainz 2020.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women belonging to the south of Seville, Spain were included in the study following informed consent. Recruitment methods not further specified. Study start and end date: not reported |
||
| Patient characteristics and setting | Setting: tertiary care facility (Valme’s Hospital) Region(s) and country/countries from which participants were recruited: Seville, Spain Sample size: 493 Study eligibility criteria: viable singleton pregnancy with a known pregnancy outcome Number of participants with the target condition: 13 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: transabdominal alone Single or multiple operators: multiple (2) Staff qualification and/or operator experience level: 1 sonographer with more than 5 years of experience, 1 sonographer with standardised training but less than 1 year of experience Second‐trimester scan: Timing (weeks and days gestation): 18 to 20 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: European Registration of Congenital Anomalies and Twins (EUROCAT) classification system Reference standard (live birth): neonatal examination at 24 and 48 hours after delivery Reference standard (fetal or neonatal demise): pathological assessment Postnatal follow‐up duration: up to 48 hours after delivery |
||
| Flow and timing | Eligible patients: 512 Exclusions (study investigator): 2 excluded (miscarriage), 6 lost to follow‐up (1.2%) Exclusions (review team): 11 (aneuploidy) |
||
| Comparative | |||
| Notes | Funding source: no potential conflict of interest was reported by the authors. The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Saltvedt 2006.
| Study characteristics | |||
| Patient Sampling | Study design: randomised controlled trial Recruitment: pregnant women were recruited at the obstetric care units of the participating centres early in pregnancy and were offered written and oral information about the trial. Women who agreed to participate after written and oral information were randomised to one routine scan either at 12 to 14 gestational weeks or at 15 to 22 gestational weeks. Study start and end date: March 1999 to October 2002 |
||
| Patient characteristics and setting | Setting: multicentre (8); primary and secondary care facilities Region(s) and country/countries from which participants were recruited: Stockholm, Lund and Uppsala, Sweden Sample size: 18,052 (fetuses included in 2 x 2 tables) Study eligibility criteria: ability to understand the information about the trial and gestational age at booking before or at 13 weeks and 2 days gestation according to the last menstrual period. Cases with chromosomal abnormality, incomplete follow‐up data and with malformations classified as minor were excluded from the analyses. For the 12‐ to 14‐week scan group specifically: if the scanning midwife foresaw difficulties with evaluating the fetal anatomy at 14 gestational weeks, e.g. in case of maternal obesity, the woman was rescheduled for a scan at 18 gestational weeks instead. Number of participants with the target condition: 249 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first trimester scan |
||
| Index tests |
Type: single‐stage screening during the first or second trimester of pregnancy. Data were only included for the first‐trimester scanning group. First‐trimester scan: Timing (weeks and days gestation): 12 to 14 weeks’ gestation, preferably at 13 weeks Ultrasound scanning protocol: basic Cardiac screening: basic Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple (46) Staff qualification and/or operator experience level: midwives with 5 to 17 years of experience, certified by the Fetal Medicine Foundation Second‐trimester scan: Data for the second trimester scanning group were excluded on index test timing (15 to 22 weeks' gestation) |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: modified after a proposal by the Royal College of Obstetrics and Gynaecology in 1997. Anomalies were grouped into the following categories according to their likely clinical consequences: 1. Lethal malformations, 2. Severe malformations (i.e. malformations associated with possible survival and severe immediate or long‐term morbidity), 3. Malformations of moderate severity (i.e. associated with short‐ or long‐term morbidity of moderate severity), 4. Minor malformations (i.e. malformations of minor morbidity or only occasional long‐term morbidity). Reference standard (live birth): pregnancy and neonatal outcomes from hospital records (departments of neonatology, cardiology, paediatric and plastic surgery). Data from the Swedish Registry of Congenital Malformations. Questionnaire about pregnancy outcome was given to all participants. Reference standard (fetal or neonatal demise): pathology and autopsy reports after termination of pregnancy, miscarriage or stillbirth Postnatal follow‐up duration: directly after birth until dismissed from postnatal care. For congenital heart defects up to 12 months after birth. |
||
| Flow and timing | Eligible patients: 19,796 (pregnant women allocated to the 11‐ to 14‐week scan group) Exclusions (study investigator): 1918 pregnant women were excluded (1579 failed to turn up for scan, 244 spontaneous fetal death before index test, 95 abnormal karyotype), 118 women lost to follow‐up (0.6%) Exclusions (review team): 1 (anomaly considered not detectable by prenatal ultrasound) |
||
| Comparative | |||
| Notes | Funding source: this trial was supported by grants from the Stockholm County Council Public Health and Medical Services Committee Research and Development departments; Landstingsfinansierad regional forskning, Region Skåne (a governmental grant); Uppsala County Council and Karolinska Institute South hospital. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Salvador 2005.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study Recruitment: all registered cases of newborns (live‐borns, intrauterine fetal demises from 20 weeks onwards and early neonatal deaths) and fetuses from pregnancy terminations, showing at least either 1 major or 2 minor defects were included Study start and end date: 1992 to 1999 |
||
| Patient characteristics and setting | Setting: population‐based registry of birth defects of Barcelona (Registre de Defectes Congènits de la Ciutat de Barcelona; REDCB) Region(s) and country/countries from which participants were recruited: Barcelona, Spain Sample size: 99,753 Study eligibility criteria: newborns (live‐borns, intrauterine fetal demises from 20 weeks onwards, early neonatal deaths) and pregnancy terminations with at least either 1 major or 2 minor structural anomalies Number of participants with the target condition: 1976 Population type: unselected population Prior testing: more than 3 ultrasound scans were performed in 73% of pregnancies; timing and type of scan unspecified |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: second trimester of pregnancy, before 23 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based study) Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): neonatal exam after delivery, data from hospital records and by contacting mothers after delivery Reference standard (fetal or neonatal demise): termination of pregnancies, intrauterine death and neonatal deaths were registered, data from hospital records and by contacting mothers after the termination Postnatal follow‐up duration: 2 to 3 days after delivery |
||
| Flow and timing | Eligible patients: 99,753 Exclusions (study investigator): none reported Exclusions (review team): none |
||
| Comparative | |||
| Notes | Funding source: Fondo de Investigaciones Sanitarias (FIS #PI021514 and RTIC G03/123), Health Ministry of Spain | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Sepulveda 2013.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of patients who were diagnosed with holoprosencephaly pre‐ or postnatally at the study centre during the study period were reviewed Study start and end date: January 2004 to December 2012 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Fetal Medicine Center of Clinica Las Condes) Region(s) and country/countries from which participants were recruited: Santiago, Chile Sample size: 11,058 Study eligibility criteria: cases of holoprosencephaly that had sonographic evaluation in the first trimester in the study centre Number of participants with the target condition: 1 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening (data were only reported for the first‐trimester scan) First‐trimester scan: Timing (weeks and days gestation): 11 to 13 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: not reported Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported Second‐trimester scan: Data to construct 2 x 2 tables could not be extracted or derived from the report |
||
| Target condition and reference standard(s) | Target condition(s): holoprosencephaly Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcomes were obtained from hospital records, contacting the referring obstetrician or patients themselves in cases delivering in another institution Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 11,068 Exclusions (study investigator): none reported Exclusions (review team): 10 (abnormal karyotype) |
||
| Comparative | |||
| Notes | Funding source: this work was supported by an unrestricted research grant from the Sociedad Profesional de Medicina Fetal ‘Fetalmed’, Chile | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Smrcek 2006.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of pregnant women attending the study centre for detailed ultrasonography during the study period were reviewed Study start and end date: 1997 to 2003 |
||
| Patient characteristics and setting | Setting: tertiary care facility (University Hospital Schleswig‐Holstein) Region(s) and country/countries from which participants were recruited: Lübeck, Germany Sample size: 2139 Study eligibility criteria: pregnant women with a viable singleton pregnancy undergoing detailed fetal echocardiography in first trimester Number of participants with the target condition: 20 Population type: unselected population of mixed high‐ and low‐risk patients. Women were referred for maternal indications (n = 840; 38.8%), nuchal translucency screening (n = 810; 37.4%), anamnestic indications such as complications in previous pregnancy and positive family history (n = 433; 20.0%) and miscellaneous (n = 82; 3.8%). Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 13 weeks and 6 days Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: transabdominal and transvaginal Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported Second‐trimester scan: Timing: second trimester of pregnancy Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): neonatal examination by a paediatrician immediately after birth and at 3 to 10 days after birth Reference standard (fetal or neonatal demise): if available, postmortem examination or histopathologic workup Postnatal follow‐up duration: 3 to 10 days after birth |
||
| Flow and timing | Eligible patients: 2269 Exclusions (study investigator): 104 lost to follow‐up (4.6%) Exclusions (review team): 26 (abnormal karyotype) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Souka 2006.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: all pregnant women booking for delivery at the study centre were offered an ultrasound examination at 11 to 14 weeks Study start and end date: since May 2002 (end date not specified) |
||
| Patient characteristics and setting | Setting: secondary care facility (Alexandra Maternity Hospital) Region(s) and country/countries from which participants were recruited: Athens, Greece Sample size: 1136 Study eligibility criteria: all pregnant women with a viable singleton pregnancy between 11 and 14 weeks' gestation and known pregnancy outcome Number of participants with the target condition: 19 Population type: unselected population Prior testing: screening for chromosomal abnormalities using nuchal translucency measurement and maternal serum biochemistry (free b‐hCG and PAPP‐A) was offered at 11 to 14 weeks' gestation |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: transabdominal and transvaginal Single or multiple operators: multiple (3) Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation Second‐trimester scan: Timing: 22 to 24 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: major was defined as requiring medical and/or surgical treatment or causing mental handicap. Minor structural defects included mild hydronephrosis, choroid plexus cysts, digital anomalies, cryptorchidism and cardiac defects not requiring treatment. Reference standard (live birth): pregnancy outcome was obtained from hospital records, contact with obstetricians or patients themselves. All women were contacted to confirm normal development of the child. Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: 3 months after birth |
||
| Flow and timing | Eligible patients: 1148 Exclusions (study investigator): only the number of pregnancies with complete outcome data is reported. Number excluded/lost to follow‐up is unclear. Exclusions (review team): 12 (4 terminations of pregnancy for non‐structural abnormalities, 4 fetal death following normal first trimester scan, 2 abnormal karyotype, 2 anomalies considered not detectable by prenatal ultrasound) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Srisupundit 2006.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: 597 pregnant women attending the antenatal care clinic were recruited and written informed consent was obtained; no further information on patient sampling Study start and end date: July 2003 and July 2005 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Maharaj Nakorn Chiang Mai Hospital) Region(s) and country/countries from which participants were recruited: Chiang Mai, Thailand Sample size: 591 Study eligibility criteria: pregnant women with a viable singleton pregnancy between 11 and 14 weeks’ gestation, with voluntary informed consent and complete follow‐up data available Number of participants with the target condition: 18 Population type: unselected population Prior testing: none reported |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: basic Cardiac screening: basic Mode of examination: transabdominal alone Single or multiple operators: multiple (3) Staff qualification and/or operator experience level: experienced perinatologists, not further specified Second‐trimester scan: Timing: second trimester Ultrasound scanning protocol: basic Cardiac screening: basic Mode of examination: transabdominal alone Single or multiple operators: multiple (3) Staff qualification and/or operator experience level: experienced perinatologists, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): neonatal examination by a paediatrician Reference standard (fetal or neonatal demise): postnatal examination by a pathologist Postnatal follow‐up duration: until neonatal examination |
||
| Flow and timing | Eligible patients: 597 Exclusions (study investigator): none reported Exclusions (review team): 6 (3 soft markers for chromosomal abnormalities, 3 abnormal karyotype) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Stefos 1999.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women attending the study centre for prenatal care Study start and end date: 1990 to 1996 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Ioannina University) Region(s) and country/countries from which participants were recruited: Ioannina, Greece Sample size: 7196 Study eligibility criteria: prenatal care and delivery at the study centre Number of participants with the target condition: 654 Population type: unselected population Prior testing: dating scan |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing (weeks and days gestation): 18 to 22 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (2) Staff qualification and/or operator experience level: experienced obstetricians, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcomes from hospital records. The recordings were used in matching the prenatal and postnatal findings. Reference standard (fetal or neonatal demise): examination of pathologists Postnatal follow‐up duration: 3 months |
||
| Flow and timing | Eligible patients: 7236 Exclusions (study investigator): none reported Exclusions (review team): 40 (22 soft markers for chromosomal abnormalities, 18 abnormal karyotype) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Syngelaki 2011.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of pregnant women who received an 11‐ to 13‐week ultrasound scan at the participating centres were reviewed Study start and end date: March 2006 to September 2009 |
||
| Patient characteristics and setting | Setting: multicentre (2) (Medway Maritime Hospital ‐ secondary care facility; King’s College Hospital ‐ tertiary care facility) Region(s) and country/countries from which participants were recruited: London and Gillingham, United Kingdom Sample size: 41,389 Study eligibility criteria: pregnant women with a viable singleton pregnancy, undergoing ultrasound screening at 11 to 13 weeks, excluding the cases with aneuploidies or no follow‐up Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 weeks and 0 days to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: basic Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple (165) Staff qualification and/or operator experience level: (obstetric) sonographers certified by the Fetal Medicine Foundation Second‐trimester scan: Timing: 20 weeks and 0 days to 23 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: transabdominal alone Single or multiple operators: multiple (165) Staff qualification and/or operator experience level: (obstetric) sonographers certified by the Fetal Medicine Foundation |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy outcome from hospital records, neonatal examination by a paediatrician Reference standard (fetal or neonatal demise): postmortem examination (not in all cases) Postnatal follow‐up duration: until neonatal discharge |
||
| Flow and timing | Eligible patients: 45,191 Exclusions (study investigator): 332 excluded (aneuploidy), 3466 lost to follow‐up (2739 no follow‐up, 116 pregnancy terminations after normal first trimester scan, 611 fetal death after normal index test results) (7.7%) Exclusions (review team): 4 (unilateral short long bone) |
||
| Comparative | |||
| Notes | Funding source: this study was supported by a grant from the Fetal Medicine Foundation (Charity No: 1037116) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Syngelaki 2019.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: pregnant women attending one of the study centres for routine prenatal care Study start and end date: October 2009 to July 2018 |
||
| Patient characteristics and setting | Setting: multicentre (2) (Medway Maritime Hospital ‐ secondary care facility; King’s College Hospital ‐ tertiary care facility) Region(s) and country/countries from which participants were recruited: London and Gillingham, United Kingdom Sample size: 94,713 Study eligibility criteria: pregnant women with a viable singleton pregnancy, visiting between 11 + 0 and 13 + 6 weeks’ gestation Number of participants with the target condition: 1715 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 weeks and 0 days to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple Staff qualification and/or operator experience level: sonographers certified by the Fetal Medicine Foundation Second‐trimester scan: Timing (weeks and days gestation): 18 to 24 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple operators Staff qualification and/or operator experience level: sonographers certified by the Fetal Medicine Foundation |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): neonatal examination by a paediatrician Reference standard (fetal or neonatal demise): the findings of the last ultrasound examination in cases of pregnancy termination, miscarriage or stillbirth because postmortem examination was not performed systematically Postnatal follow‐up duration: until neonatal discharge |
||
| Flow and timing | Eligible patients: 101,793 Exclusions (study investigator): 796 excluded (aneuploidy), 6279 lost to follow‐up (221 pregnancy termination after normal index test, 1256 fetal death after normal index test, 4802 no follow‐up) (6.2%) Exclusions (review team): 5 (2 anomalies considered not detectable by prenatal ultrasound, 3 non‐structural anomalies) |
||
| Comparative | |||
| Notes | Funding source: this study was supported by a grant from The Fetal Medicine Foundation (Charity No: 1037116) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Taipale 2004.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: consecutive pregnant women who agreed to have 2 screening examinations by ultrasonography at the study centre Study start and end date: November 1994 to May 1996 |
||
| Patient characteristics and setting | Setting: secondary care facility (Jorvi Hospital) Region(s) and country/countries from which participants were recruited: western part of greater Helsinki, Finland Sample size: 4784 Study eligibility criteria: singleton pregnancies with 2 screening examinations Number of participants with the target condition: 76 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 13 to 14 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: transvaginal alone Single or multiple operators: multiple (6) Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation Second‐trimester scan: Timing (weeks and days gestation): 18 to 22 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: transabdominal alone Single or multiple operators: multiple (5) Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural abnormalities Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy outcome was obtained from hospital records. Obstetric and paediatric records were reviewed for all those coded with a diagnosis of malformation, including newborns referred to neonatal or paediatric units. The data were supplemented by information from the Finnish national birth and malformation registries, to which all structural and chromosomal anomalies up to 1 year of age are reported. Reference standard (fetal or neonatal demise): autopsy reports, supplemented by information from the Finnish national birth and malformation registries Postnatal follow‐up duration: 1 year after birth |
||
| Flow and timing | Eligible patients: 4856 Exclusions (study investigator): 58 excluded (3 pregnancy terminations for social reasons, 35 spontaneous abortion, 20 on gestational age of > 23 weeks' gestation at first attendance), 9 lost to follow‐up (0.19%) Exclusions (review team): 5 (4 abnormal karyotype, 1 anomaly considered minor by the review team) |
||
| Comparative | |||
| Notes | Funding source: the primary author, Pekka Taipale, received grants from the Jorvi Hospital Foundation, the Maud Kuistila Foundation and the Finnish Cultural Foundation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Takita 2016.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women attending the study centre for first‐ and second‐trimester prenatal screening Study start and end date: February 2011 to September 2013 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Showa University Hospital) Region(s) and country/countries from which participants were recruited: Shinagawa‐Ku, Tokyo, Japan Sample size: 2010 Study eligibility criteria: singleton pregnancy, delivery at the study centre. Patients referred for an ultrasound diagnosis of morphological abnormalities or other complications were excluded. Number of participants with the target condition: 35 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 13 weeks and 6 days of gestation Ultrasound scanning protocol: basic Cardiac screening: basic Mode of examination: transabdominal alone Single or multiple operators: multiple (6) Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation Second‐trimester scan: Timing (weeks and days gestation): 18 to 20 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: transabdominal alone Single or multiple operators: multiple (6) Staff qualification and/or operator experience level: certified by the Fetal Medicine Foundation |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): after delivery, neonatal congenital abnormalities were reviewed and compared with the ultrasound findings obtained in the first and second trimesters Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: until neonatal examination |
||
| Flow and timing | Eligible patients: 2028 Exclusions (study investigator): none reported Exclusions (review team): 18 (6 soft markers for chromosomal abnormalities, 12 abnormal karyotype) |
||
| Comparative | |||
| Notes | Funding source: the authors declared that there are no financial or other relations that could lead to a conflict of interest. The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Tiechl 2021.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: 4755 women presenting for their first‐trimester assessment at the participating centres during the study period were included following informed consent Study start and end date: April 2011 to July 2015 |
||
| Patient characteristics and setting | Setting: multicentre (2); tertiary care facilities (Medical University Hospital Innsbruck and the Ambulatorium fur Fetalmedizin Feldkirch) Region(s) and country/countries from which participants were recruited: Innsbruck and Feldkirch, Austria Sample size: 4658 Study eligibility criteria: viable fetus with a crown‐rump length between 45 and 84 mm at the time of the first‐trimester scan and complete follow‐up data Number of participants with the target condition: 7 Population type: unselected population Prior testing: dating scan, nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: detailed, in addition to the routine first trimester scan, specific investigations were performed to screen for open spina bifida and evaluate the accuracy of these proposed markers for open spina bifida screening Cardiac screening: not reported Mode of examination: transabdominal alone Single or multiple operators: multiple Staff qualification and/or operator experience level: doctors certified by the Fetal Medicine Foundation Second‐trimester scan: Timing: 20 to 23 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): open spina bifida Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcomes were obtained from hospital records and standardised phone interviews with the patients themselves Reference standard (fetal or neonatal demise): pathological examination after termination of pregnancy, miscarriage or intrauterine death Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 4949 Exclusions (study investigator): 291 no pregnancy outcome data (5.9%) Exclusions (review team): none excluded |
||
| Comparative | |||
| Notes | Funding source: the authors declared that there are no conflicts of interest. The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Timbolschi 2015.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study Recruitment: infants born to mothers living and giving birth in the Départements of Bas‐Rhin and Haut‐Rhin of the Alsace region are registered in the database. The registry focuses on congenital malformations and/or chromosomal anomalies among live births, stillbirths after 20 weeks’ gestation only and terminations of pregnancy whatever the gestational age. The ascertainment of cases is actively carried out from multiple sources of information to improve the completeness and the quality of data. Cases with neural tube defects born during the study period were identified from the database. Study start and end date: January 1995 to December 2009 |
||
| Patient characteristics and setting | Setting: registry of Congenital Malformations of Alsace, part of the European Registration of Congenital Anomalies and Twins (EUROCAT) network and the International Clearinghouse for Birth Defects Surveillance and Research Region(s) and country/countries from which participants were recruited: Alsace, France Sample size: 192,390 Study eligibility criteria: cases with neural tube defects, whose mothers lived and gave birth in Bas‐Rhin. All neural tube defects were included among live births (anomaly detected during the pregnancy or after birth up to the 24th month of life), stillbirths after 22 weeks of amenorrhoea and terminations of pregnancy (whatever the term of pregnancy). Stillbirths with neural tube defects before 22 weeks spontaneous abortions were not included as recommended by EUROCAT. Number of participants with the target condition: 271 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: two‐stage screening (data were only reported for the first trimester scan): First‐trimester scan: Timing (weeks and days gestation): 11 weeks to 14 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple (population‐based study) Staff qualification and/or operator experience level: not reported Second‐trimester scan: Data to construct 2 x 2 tables could not be extracted or derived from the report |
||
| Target condition and reference standard(s) | Target condition(s): neural tube defects, defined as: (1) anencephaly/exencephaly, defined as an absent calvarium with total or partial absence of the brain and including cases of craniorachischisis; (2) cephalocele, defined as a herniation of meninges (meningocele) and/or brain tissue (meningoencephalocele) through a defect in the calvarium, and (3) spina bifida, defined as a bony defect of the spine with exposure of meninges and/or neural tissue, and encompassing several subgroups of defects, the most serious of them being myelomeningocele Definitions used for major and minor congenital abnormalities: EUROCAT classification system Reference standard (live birth): pregnancy and neonatal outcomes from hospital records of obstetrical, paediatric surgery, medical genetic units Reference standard (fetal or neonatal demise): outcomes from the pathological laboratories and performed autopsies Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 192,391 Exclusions (study investigator): none reported Exclusions (review team): 1 (ultrasound screening performed after 24 weeks' gestation) |
||
| Comparative | |||
| Notes | Funding source: the authors declared that there are no conflicts of interest. The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Tudorache 2016.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women attending the study centre for first‐trimester aneuploidy screening were recruited consecutively. A complete follow‐up programme was offered to all participants. Study start and end date: January 2011 to January 2015 |
||
| Patient characteristics and setting | Setting: tertiary care facility (County Emergency University Hospital Craiova) Region(s) and country/countries from which participants were recruited: Craiova, Romania Sample size: 2908 Study eligibility criteria: pregnant women with a viable pregnancy, fetal crown‐rump length of 44 to 74 mm, available proof from at least a standard test and known pregnancy outcome Number of participants with the target condition: 37 Population type: unselected population. Approximately 20% of the study group were referrals from primary or secondary centres. Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 weeks and 2 days to 13 weeks and 4 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended. The heart was targeted before other segments of the fetal anatomy. During the examination, the operator completed a study form on the cardiac examination. Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: not reported Second‐trimester scan: Timing: second trimester Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): neonatal examination and pulse oximetry after birth. Postnatal follow‐up by a general practitioner. Feedback was obtained at 6 months and 1 year of age. Reference standard (fetal or neonatal demise): pathology results from autopsy if available Postnatal follow‐up duration: 1 year after birth |
||
| Flow and timing | Eligible patients: 3240 Exclusions (study investigator): 332 lost to follow‐up (10.2%) Exclusions (review team): no further exclusions |
||
| Comparative | |||
| Notes | Funding source: the authors declared that there are no conflicts of interest. The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Tydeman 2013.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: data from prenatally diagnosed fetal anomalies were collected from the fetal medicine reporting and audit system. Those cases not diagnosed prenatally were reported by a variety of means: from paediatric departments, fetal and neonatal pathology, cytogenetic labs, the Scottish congenital heart disease register (Heartsuite) and other sources. All women had been offered a fetal anomaly scan since the early 1990s. Study start and end date: 2003 to 2009 |
||
| Patient characteristics and setting | Setting: secondary care facility (Victoria Hospital) Region(s) and country/countries from which participants were recruited: Fife, United Kingdom Sample size: 25,627 Study eligibility criteria: all prenatally diagnosed fetal anomalies from the reporting and audit system and postnatally diagnosed cases in the same period Number of participants with the target condition: 125 Population type: unselected population Prior testing: dating scan, nuchal translucency measurement, maternal serum biochemistry |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 weeks and 0 days to 20 weeks and 6 days Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: multiple (2) Staff qualification and/or operator experience level: midwives specialised in fetal medicine, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): 11 selected conditions: anencephaly, open spina bifida, severe heart defects, bilateral renal agenesis, lethal skeletal dysplasia, diaphragmatic hernia, exomphalos, gastroschisis, cleft lip, trisomy 18 and trisomy 13 Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcomes were obtained from hospital records, the Scottish Congenital Heart Disease Register and other sources Reference standard (fetal or neonatal demise): data obtained from fetal and neonatal pathology departments Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 25,644 Exclusions (study investigator): none reported Exclusions (review team): 17 (abnormal karyotype) |
||
| Comparative | |||
| Notes | Funding source: the authors declared that no funding was received for this work | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
van Velzen 2016.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: all cases with a prenatal or postnatal diagnosis of severe congenital heart defects within the studied region were identified using the prenatal ultrasound databases (prenatal cases) and the paediatric cardiology departmental databases and cross‐checked with catheterisation schedules, operating schedules and emergency admissions (postnatal cases) of the tertiary care centres servicing the region Study start and end date: 2007 to 2011 |
||
| Patient characteristics and setting | Setting: multicentre; 3 tertiary care facilities (Academic Medical Centre Amsterdam, VU Medical Centre Amsterdam and Leiden University Medical Centre Leiden) responsible for the care of children with congenital heart defects in the studied region and all affiliated primary and secondary healthcare centres offering prenatal screening Region(s) and country/countries from which participants were recruited: north‐west region of the Netherlands Sample size: 720,079 Study eligibility criteria: cases with a pre‐ or postnatal diagnosis of a severe congenital heart defect irrespective of the presence or absence of additional congenital anomalies were included. For inclusion, the mother of the fetus or the infant had to be resident within the study region at the time of birth. Number of participants with the target condition: 992 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing (weeks and days gestation): 18 to 22 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: certified according to Fetal Medicine foundation criteria |
||
| Target condition and reference standard(s) | Target condition(s): severe congenital heart defects Definitions used for major and minor congenital abnormalities: severe congenital heart defects were defined as being potentially life‐threatening and requiring surgery or intervention within the first year of life Reference standard (live birth): pregnancy and neonatal outcomes were obtained from hospital records. The case list was complemented with all postnatally diagnosed infants with a congenital heart defect who needed surgery or therapeutic cardiac catheterisation or who died within the first year of life. The postnatal echocardiography, surgical report or magnetic resonance imaging determined the definitive diagnosis in the analysis of the data. Reference standard (fetal or neonatal demise): the postmortem databases of the departments of pathology were studied for cardiac anomalies Postnatal follow‐up duration: first year of life |
||
| Flow and timing | Eligible patients: 720,138 Exclusions (study investigator): 53 excluded (date of birth in 2012 or postnatal cases with the first cardiac surgery after the age of 12 months), 6 lost to follow‐up (< 0.1% of total cohort, 0.4% of cases with eligible congenital heart defects) Exclusions (review team): no further exclusions |
||
| Comparative | |||
| Notes | Funding source: the authors declared that no financial support was obtained for this study | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Vayna 2018.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: hospital records of pregnant women who attended the study centre for ultrasound examination were reviewed Study start and end date: October 2009 to December 2016 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Filantropia Hospital) Region(s) and country/countries from which participants were recruited: Bucharest, Romania Sample size: 6110 Study eligibility criteria: pregnant women undergoing ultrasound examination at 11 to 14 weeks' gestation Number of participants with the target condition: 115 Population type: unselected population Prior testing: screening for aneuploidies at the time of the first‐trimester scan using a combination of data on maternal age, NT thickness, fetal heart rate, fetal nasal bone, tricuspid flow, ductus venous flow, free β‐human chorionic gonadotropin level in maternal serum, and pregnancy‐associated plasma protein A level in maternal serum |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: transabdominal and transvaginal Single or multiple operators: multiple (8) Staff qualification and/or operator experience level: fetal medicine specialists, certified by the Fetal Medicine Foundation Second‐trimester scan: Timing: 20 to 24 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple (8) Staff qualification and/or operator experience level: fetal medicine specialists, certified by the Fetal Medicine Foundation |
||
| Target condition and reference standard(s) | Target condition(s): major fetal structural anomalies Definitions used for major and minor congenital abnormalities: major anomalies were defined as lethal anomalies or anomalies associated with short or long‐term severe morbidity. Uncomplicated pelvic kidney and uncomplicated single kidney were not considered major structural anomalies. Reference standard (live birth): pregnancy and neonatal outcomes were obtained from hospital records or patients themselves Reference standard (fetal or neonatal demise): postmortem examination, if available. Without postmortem examination, the ultrasound diagnosis was assumed to be correct. Postnatal follow‐up duration: until hospital discharge (at least 2 days after birth) |
||
| Flow and timing | Eligible patients: 7576 Exclusions (study investigator): 1462 lost to follow‐up (19.3%) Exclusions (review team): 4 (normal variant) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Vellamkondu 2017.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: 500 pregnant women undergoing combined screening, receiving information and signing informed consent; no further information on patient sampling is given Study start and end date: 2‐year period |
||
| Patient characteristics and setting | Setting: tertiary care facility (Kasturba Medical College of Manipal University) Region(s) and country/countries from which participants were recruited: Karnataka, India Sample size: 429 Study eligibility criteria: pregnant women with a singleton viable pregnancy between 11 and 14 weeks, who underwent combined screening and with available follow‐up data Number of participants with the target condition: 12 Population type: population opting for combined screening for aneuploidy Prior testing: combined test at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: transabdominal and transvaginal Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported Second‐trimester scan: Timing: 18 to 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy outcomes were recorded by following them until delivery Reference standard (fetal or neonatal demise): confirmed after termination of pregnancy, not further specified Postnatal follow‐up duration: until delivery |
||
| Flow and timing | Eligible patients: 500 Exclusions (study investigator): 60 lost to follow‐up (12.0%) Exclusions (review team): 11 (10 soft markers for chromosomal abnormalities, 1 anomaly secondary to intra‐uterine infection) |
||
| Comparative | |||
| Notes | Funding source: the authors declared that there are no conflicts of interest for any author (financial or otherwise). The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Vimpelli 2006.
| Study characteristics | |||
| Patient Sampling | Study design: cross‐sectional Recruitment: unselected pregnant women attending the study centre for routine first‐trimester ultrasound screening were enrolled in the study following written informed consent Study start and end date: not reported |
||
| Patient characteristics and setting | Setting: tertiary care facility (Health Center of Tampere) Region(s) and country/countries from which participants were recruited: Tampere, Finland Sample size: 584 Study eligibility criteria: viable intrauterine pregnancies at 11 weeks and 0 days to 13 weeks and 6 days gestation were included. Cases with a contraindication to transvaginal ultrasonography or inability to measure the crown‐rump length or nuchal translucency within the allocated time were excluded. Number of participants with the target condition: 6 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 weeks and 0 days to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: transvaginal alone Single or multiple operators: single Staff qualification and/or operator experience level: obstetrician, not further specified Second‐trimester scan: Timing: 18 to 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: major cardiac anomalies were defined as defects leading to in utero demise, termination of pregnancy, or surgical intervention Reference standard (live birth): pregnancy and neonatal outcomes were recorded in all cases Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 611 Exclusions (study investigator): 27 excluded (inability to measure crown rump length or nuchal translucency within allocated time) Exclusions (review team): no further exclusions |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Waern 2021.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: data on all pregnant women referred from the region to fetal cardiologists in the study centre were retrieved. To determine prenatal detection rate, all neonates who underwent surgery or catheter intervention for a critical congenital heart defect born during the study period were included. Study start and end date: January 2013 to June 2017 |
||
| Patient characteristics and setting | Setting: multicentre (4); 1 tertiary care facility (Sahlgrenska University Hospital) and 3 secondary care facilities which offer routine ultrasound screening in the region. All fetuses with a suspected congenital heart in the region are referred to Sahlgrenska University Hospital. Region(s) and country/countries from which participants were recruited: Västra Götaland region, Sweden Sample size: 88,145 Study eligibility criteria: all pregnant women with a fetal diagnosis of a congenital heart defect were included and neonates born with complex congenital heart defects, irrespective of the presence or absence of additional congenital anomalies. Cases of isolated fetal arrhythmias were excluded. For the subgroup analysis to determine the detection rate of critical congenital heart defects, all neonates born with a critical congenital heart defect during the study period were also included. Number of participants with the target condition: 23 Population type: unselected population Prior testing: nuchal translucency measurement |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 20 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: specially trained midwives and obstetricians, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): critical congenital heart defects (conditions for which prenatal detection rate was determined) Definitions used for major and minor congenital abnormalities: congenital heart defects were considered major if they were potentially lethal and/or likely to require surgery or catheter intervention before 12 months of age, and minor when no intervention was likely to be required during the first year. Within the group of major heart defects, a subgroup of critical congenital heart defects were identified. Critical congenital heart defects were defined as requiring surgery and/or catheter intervention within the first 2 months of life in order to avoid serious complications and/or death. Reference standard (live birth): for the subgroup analysis to determine the detection rate of critical congenital heart defects, all neonates with a critical congenital heart defect born during the study period were added to the prenatally diagnosed cases with an estimated due date or actual delivery during the same period Reference standard (fetal or neonatal demise): if available, autopsy reports Postnatal follow‐up duration: not reported for the total screened cohort |
||
| Flow and timing | Eligible patients: 88,230 Exclusions (study investigator): none reported Exclusions (review team): 85 (selected anomaly within the larger categories of aortic arch anomalies, conotruncal anomalies and other single ventricle defects, respectively) |
||
| Comparative | |||
| Notes | Funding source: this work was supported by Frimurarfonden Barnhusdirektionen Foundation (senior author, Ylva Carlsson, GLS 7000991), Wilhelm and Martina Lundgren foundation (senior author, Ylva Carlsson, 2019–2941), Linnea and Josef Carlsson Foundation (senior author, Ylva Carlsson, 2019). The study was also financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (senior author, Ylva Carlsson, ALFGBG‐77860 and 75710). Mats Mellander was supported by the Swedish Heart and Lung Foundation. Open Access funding provided by University of Gothenburg. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Wang 2013.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women who received an 11‐ to 14‐week ultrasound scan at the study centre during the study period Study start and end date: September 2008 to March 2011 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Beijing Obsetrics and Gynaecology Hospital Capital Medical University) Region(s) and country/countries from which participants were recruited: Beijing, China Sample size: 2821 Study eligibility criteria: pregnant women with a viable pregnancy between 11 and 14 weeks and complete follow‐up Number of participants with the target condition: 22 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: two‐stage screening First‐trimester scan: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: basic Cardiac screening: basic Mode of examination: transabdominal alone Single or multiple operators: multiple (5) Staff qualification and/or operator experience level: experienced obstetric sonographers, not further specified Second‐trimester scan: Timing: 20 to 24 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcome from hospital records Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 3134 Exclusions (study investigator): 312 lost to follow‐up (10.0%) Exclusions (review team): 1 (abnormal karyotype) |
||
| Comparative | |||
| Notes | Funding source: this study was supported by grants from the National Natural Science Foundation of China (No. 81071159), Beijing Municipal Science and Technology Commission Key Project (No. D101100050010039) and Capital Medicine Development Science Research Foundation (No. 2009‐3131) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Wayne 2002.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: all pregnant women undergoing routine anomaly scans at the study centre during the study period were enrolled in the study Study start and end date: January 1996 and December 2000 |
||
| Patient characteristics and setting | Setting: tertiary care facility (St George’s Hospital Medical School) Region(s) and country/countries from which participants were recruited: London, United Kingdom Sample size: 17,523 Study eligibility criteria: all locally seen and referred patients for isolated facial clefts were included, cases with associated multiple malformations or abnormal karyotypes were excluded. Referred and non‐referred cases were analysed separately. Number of participants with the target condition: 12 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 23 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): isolated facial clefts Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): data obtained from from birth registers, hospital and neonatal surgery records Reference standard (fetal or neonatal demise): data from birth registers and postmortem records Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 17,551 Exclusions (study investigator): 10 excluded (associated multiple malformations or abnormal karyotype) Exclusions (review team): 18 (referred cases) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Whitlow 1999.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study (cross‐sectional) Recruitment: pregnant women who booked at the study facility for prenatal care were offered a first‐trimester scan, information was given and consent obtained Study start and end date: not reported |
||
| Patient characteristics and setting | Setting: tertiary care facility (Royal Free Hospital) Region(s) and country/countries from which participants were recruited: London, United Kingdom Sample size: 5943 Study eligibility criteria: pregnant women with a viable early pregnancy and who provided verbal consent for participation in the study Number of participants with the target condition: 45 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: two‐stage screening First‐trimester anomaly: Timing (weeks and days gestation): 11 to 14 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: basic Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple (10) Staff qualification and/or operator experience level: clinicians trained in first trimester ultrasound screening (6), qualified ultrasonographers (4) Second‐trimester scan: Timing: 18 to 20 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): fetal structural anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcomes from hospital records, patient administration system database or patient enquiry by telephone Reference standard (fetal or neonatal demise): if available, postmortem reports Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 6719 Exclusions (study investigator): 173 excluded (spontaneous fetal death before index test), 599 lost to follow‐up (9.2%) Exclusions (review team): 4 (soft markers for chromosomal abnormalities) |
||
| Comparative | |||
| Notes | Funding source: this study was funded by WellBeing | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Wu 2009.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women attending the study centre during the study period for routine fetal sonographic screening were eligible for inclusion Study start and end date: January 2007 to December 2007 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Beijing Obstetrics and Gynaecology Hospital, Capital Medical University) Region(s) and country/countries from which participants were recruited: Beijing, China Sample size: 8021 Study eligibility criteria: singleton pregnancies without known risk factors who were scheduled for routine fetal sonographic screening at 20 to 24 weeks’ gestation and subsequently delivered in the study centre were included in the study. Referrals from other hospitals were excluded. Number of participants with the target condition: 24 Population type: unselected population Prior testing: not reported |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 20 to 24 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: multiple (4) Staff qualification and/or operator experience level: specifically trained obstetric sonographers, not further specified |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): neonatal examination by a paediatrician Reference standard (fetal or neonatal demise): autopsy reports Postnatal follow‐up duration: 6 months after birth |
||
| Flow and timing | Eligible patients: 8025 Exclusions (study investigator): none reported Exclusions (review team): 4 (abnormal karyotype) |
||
| Comparative | |||
| Notes | Funding source: this project was funded by grant D0906005000091 from the Beijing Municipal Science and Technology Commission Foundation, grant KZ200610025014 from the Beijing Natural Science Foundation Program and Scientific Research Key Program of the Beijing Municipal Commission of Education, and grant 20050025002 from the Research Fund for the Doctoral Program of Higher Education of China | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Yu 2011‐1.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women who received ultrasonography to screen for congenital heart defects at the study centre during the study period Study start and end date: January 2006 to December 2006 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Nanjing Maternal and Child Health Hospital, Nanjing Medical University) Region(s) and country/countries from which participants were recruited: Nanjing, China Sample size: 7726 Study eligibility criteria: pregnant women with at least one prenatal ultrasonographic examination to screen for congenital heart defects and with available follow‐up data were included in the study. Fetuses with and without risk factors for congenital heart defects were analysed separately. Number of participants with the target condition: 82 Population type: low‐risk population Prior testing: fetal aneuploidy screening, not further specified |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 22 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): all cases underwent follow‐up (not further specified), and pre‐ and postnatal diagnoses of cardiac abnormalities were correlated Reference standard (fetal or neonatal demise): autopsy reports Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 8859 (low‐risk 2006 cohort) Exclusions (study investigator): 1133 lost to follow‐up from the 2006 cohort (12.8%) Exclusions (review team): no further exclusions from the low‐risk 2006 cohort. We excluded the 2006 high‐risk cohort (334 cases). |
||
| Comparative | |||
| Notes | Funding source: this work was supported by a grant from the National Natural Science Foundation of China (No. 30973213 and 81070500) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Yu 2011‐2.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women who received ultrasonography to screen for congenital heart defects at the study centre during the study period Study start and end date: January 2007 to December 2007 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Nanjing Maternal and Child Health Hospital, Nanjing Medical University) Region(s) and country/countries from which participants were recruited: Nanjing, China Sample size: 8722 Study eligibility criteria: pregnant women with at least one prenatal ultrasonographic examination to screen for congenital heart defects and with available follow‐up data were included in the study. Fetuses with and without risk factors for congenital heart defects were analysed separately. Number of participants with the target condition: 92 Population type: low‐risk population Prior testing: fetal aneuploidy screening, not further specified |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 22 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): all cases underwent follow‐up (not further specified), and pre‐ and postnatal diagnoses of cardiac abnormalities were correlated Reference standard (fetal or neonatal demise): autopsy reports Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 9352 (low‐risk 2007 cohort) Exclusions (study investigator): 630 lost to follow‐up from the 2007 cohort (7.1%) Exclusions (review team): no further exclusions from the low‐risk 2007 cohort. We excluded the high‐risk 2007 cohort (394 cases). |
||
| Comparative | |||
| Notes | Funding source: this work was supported by a grant from the National Natural Science Foundation of China (No. 30973213 and 81070500) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Yu 2011‐3.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women who received ultrasonography to screen for congenital heart defects at the study centre during the study period Study start and end date: January 2008 to December 2008 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Nanjing Maternal and Child Health Hospital, Nanjing Medical University) Region(s) and country/countries from which participants were recruited: Nanjing, China Sample size: 8860 Study eligibility criteria: pregnant women with at least one prenatal ultrasonographic examination to screen for congenital heart defects and with available follow‐up data were included in the study. Fetuses with and without risk factors for congenital heart defects were analysed separately. Number of participants with the target condition: 140 Population type: low‐risk population Prior testing: fetal aneuploidy screening, not further specified |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 22 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): all cases underwent follow‐up (not further specified), and pre‐ and postnatal diagnoses of cardiac abnormalities were correlated Reference standard (fetal or neonatal demise): autopsy reports Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 9438 (low‐risk 2008 cohort) Exclusions (study investigator): 578 lost to follow‐up from the 2008 cohort (6.1%) Exclusions (review team): no further exclusions from the low‐risk 2008 cohort. We excluded the high‐risk 2008 cohort (682 cases). |
||
| Comparative | |||
| Notes | Funding source: this work was supported by a grant from the National Natural Science Foundation of China (No. 30973213 and 81070500) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Yu 2011‐4.
| Study characteristics | |||
| Patient Sampling | Study design: prospective observational study Recruitment: pregnant women who received ultrasonography to screen for congenital heart defects at the study centre during the study period Study start and end date: January 2009 to December 2009 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Nanjing Maternal and Child Health Hospital, Nanjing Medical University) Region(s) and country/countries from which participants were recruited: Nanjing, China Sample size: 8763 Study eligibility criteria: pregnant women with at least one prenatal ultrasonographic examination to screen for congenital heart defects and with available follow‐up data were included in the study. Fetuses with and without risk factors for congenital heart defects were analysed separately. Number of participants with the target condition: 209 Population type: low‐risk population Prior testing: fetal aneuploidy screening, not further specified |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 18 to 22 weeks’ gestation Ultrasound scanning protocol: detailed Cardiac screening: extended Mode of examination: not reported Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): all cases underwent follow‐up (not further specified), and pre‐ and postnatal diagnoses of cardiac abnormalities were correlated Reference standard (fetal or neonatal demise): autopsy reports Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 9143 (low‐risk 2009 cohort) Exclusions (study investigator): 380 lost to follow‐up from the 2009 cohort (4.2%) Exclusions (review team): no further exclusions from the low‐risk 2009 cohort. We excluded the high‐risk 2009 cohort (136 cases). |
||
| Comparative | |||
| Notes | Funding source: this work was supported by a grant from the National Natural Science Foundation of China (No. 30973213 and 81070500) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zhang 2020.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: pregnant women who were examined at the study centre, recruitment methods not specified Study start and end date: November 2012 to November 2016 |
||
| Patient characteristics and setting | Setting: multicentre (2) (Jilin Province FAW General Hospital; secondary care facility and The First Hospital of Jilin University; tertiary care facility) Region(s) and country/countries from which participants were recruited: Jilin Province, China Sample size: 2751 Study eligibility criteria: pregnant women with singleton pregnancy or multiple pregnancy, and transabdominal ultrasound examination of crown‐rump length of 45 to 84 mm Number of participants with the target condition: 14 Population type: not specified Prior testing: nuchal translucency measurement at the time of the first‐trimester scan |
||
| Index tests |
Type: two‐stage screening (data were only reported for the first‐trimester scan): First‐trimester scan: Timing (weeks and days gestation): 11 to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: not reported Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: not reported Staff qualification and/or operator experience level: not reported Second‐trimester scan: Data to construct 2 x 2 tables could not be extracted or derived from the report |
||
| Target condition and reference standard(s) | Target condition(s): central nervous sytem anomalies (any type) Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): neonatal examination. For test‐positive cases, the final diagnosis was obtained by telephone and letter follow‐up. Reference standard (fetal or neonatal demise): if available, pathology reports Postnatal follow‐up duration: until neonatal examination (test negative cases) |
||
| Flow and timing | Eligible patients: 2751 Exclusions (study investigator): none reported Exclusions (review team): none excluded |
||
| Comparative | |||
| Notes | Funding source: the authors declared that there is no commercial or financial conflict of interest. The funding source for this work was not specified. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zhang 2021.
| Study characteristics | |||
| Patient Sampling | Study design: population‐based retrospective cohort study Recruitment: data obtained from the birth defect surveillance system in Beijing. A birth defect registration card was used by medical staff for data collection in the surveillance hospitals, and the information for women and their babies, birth defect diagnosis and pregnancy test results were obtained from clinical records, all of which were available online. Study start and end date: 2018 to 2020 |
||
| Patient characteristics and setting | Setting: the birth defect surveillance system in Beijing, covering all regions of Beijing and including all midwifery hospitals in the region Region(s) and country/countries from which participants were recruited: Beijing, China Sample size: 594,860 Study eligibility criteria: all cases with congenital heart defects reported to the birth defect registry Number of participants with the target condition: 5501 Population type: unselected population Prior testing: a first‐trimester scan was performed at 11 to 13 weeks' gestation; purpose and protocol for the first‐trimester scan not specified |
||
| Index tests |
Type: single‐stage screening Second‐trimester scan: Timing: 20 to 24 weeks’ gestation Ultrasound scanning protocol: not reported Cardiac screening: not reported Mode of examination: not reported Single or multiple operators: multiple Staff qualification and/or operator experience level: not reported |
||
| Target condition and reference standard(s) | Target condition(s): cardiac anomalies (any type) Definitions used for major and minor congenital abnormalities: cardiac anomalies were classified as simple or critical congenital heart defects. Simple congenital heart defects refer to a single defect that generally does not cause haemodynamic changes. This category included patent ductus arteriosus > 3 mm and atrial septal defects > 3 mm. Critical congenital heart defects were defined according to the Centers for Disease Control and Prevention standards, namely persistent truncus arteriosus, double‐outlet right ventricle, d transposition of the great vessels, single ventricle, tetralogy of Fallot, pulmonary valve atresia, Ebstein malformation, tricuspid atresia, hypoplastic left heart syndrome, coarctation of the aorta, interrupted aortic arch and total anomalous pulmonary venous return. Reference standard (live birth): pregnancy and neonatal outcome were obtained from hospital records Reference standard (fetal or neonatal demise): not reported Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 594,860 Exclusions (study investigator): none reported Exclusions (review team): 383 (cases with a congenital heart defect who did not receive 20‐ to 24‐week ultrasound screening) |
||
| Comparative | |||
| Notes | A first‐trimester scan was performed at 11 to 13 weeks' gestation. Purpose and scanning protocol were not reported. We classified the study as having a single‐stage screening approach under the assumption that the 11‐ to 13‐week scan did not include complete examination of the fetal anatomy. Funding source: this work was supported by the National Key R&D Program of China (2018YFC1002304) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zheng 2014.
| Study characteristics | |||
| Patient Sampling | Study design: retrospective observational study Recruitment: pregnant women attending the study centre for nuchal translucency measurement Study start and end date: January 2011 to March 2013 |
||
| Patient characteristics and setting | Setting: tertiary care facility (Shenzhen People’s Hospital, Jinan University) Region(s) and country/countries from which participants were recruited: Shenzhen, China Sample size: 5054 Study eligibility criteria: pregnant women with a singleton or multiple pregnancy Number of participants with the target condition: 45 Population type: unselected population Prior testing: nuchal translucency measurement at the time of the first trimester scan |
||
| Index tests |
Type: two‐stage screening (data were only reported for the first‐trimester scan): First‐trimester scan: Timing (weeks and days gestation): 11 to 13 weeks and 6 days of gestation Ultrasound scanning protocol: detailed Cardiac screening: not reported Mode of examination: primary transabdominal, transvaginal if necessary Single or multiple operators: multiple Staff qualification and/or operator experience level: more than 3 years of experience Second‐trimester scan: Data to construct 2 x 2 tables could not be extracted or derived from the report |
||
| Target condition and reference standard(s) | Target condition(s): limb anomalies Definitions used for major and minor congenital abnormalities: not reported Reference standard (live birth): pregnancy and neonatal outcomes were obtained from hospital records and in case of delivery elsewhere, patients were contacted by telephone Reference standard (fetal or neonatal demise): postmortem examination Postnatal follow‐up duration: not reported |
||
| Flow and timing | Eligible patients: 8346 Exclusions (study investigator): 3291 lost to follow‐up (39.4%) Exclusions (review team): 1 (anomaly classified as a syndrome) |
||
| Comparative | |||
| Notes | Funding source: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (First‐trimester scan) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (First + second‐trimester scan) | |||
| DOMAIN 2: Index Test (Single second‐trimester scan) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standard likely to correctly classify anomalies that are externally visible, present with clinically relevant symptoms shortly after birth, or that are considered to be lethal/incompatible with life? | Yes | ||
| Is the reference standard likely to correctly classify anomalies that may present after discharge from postnatal care? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Did all live‐born infants receive a reference standard? | Yes | ||
| Did all live‐born infants receive the same reference standard? | No | ||
| Did all cases of fetal or perinatal loss receive the reference standard (including termination of pregnancy, intra‐uterine death, stillbirth, perinatal mortality)? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
BMI: body mass index CHD: congenital heart defect NT: nuchal translucency
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abu‐Rustum 2010a | Overlap of study cohorts between Abu‐Rustum 2010 and Abu‐Rustum 2010a. Abu‐Rustum 2010 contains the more complete record and was selected for inclusion in the review. |
| Anumba 2005 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Authors were contacted to provide missing data. After initial contact, no further response. |
| Becker 2005 | Overlap of study cohorts between Becker 2012, Becker 2005 and Becker 2006. Becker 2012 contains the more complete record and was selected for inclusion in the review. |
| Becker 2006 | Overlap of study cohorts between Becker 2012, Becker 2005 and Becker 2006. Becker 2012 contains the more complete record and was selected for inclusion in the review. |
| Boyd 1998 | Time of diagnosis not specified for prenatally detected anomalies The total number of fetuses with a prenatal diagnosis < 24 weeks' gestation is reported. Authors were contacted to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for detection < 24 weeks' gestation at the level of the organ system and for individual anomalies. Data not available. |
| Boyd 2011a | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| Boyd 2012 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| Brantberg 2017 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Braz 2018 | Unable to extract or derive data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. |
| Brenes 2003 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Bullen 2001 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No valid email address identified. |
| Byrne 2018 | Study population. Only live‐born infants were included in the study, causing selection bias. |
| Campana 2010 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Cash 2001 | Duplicate record: Bister 2011. |
| Cedergren 2006 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data available. However, study was excluded on the index test type during data extraction as it did not meet the criteria for a routine first trimester fetal anomaly scan. |
| Chatzipapas 1999 | Overlap of study cohorts between Whitlow 1999, Economides 1998 and Michailidis 2001. Whitlow 1999 contains the more complete record and was selected for inclusion in the review. |
| Chelemen 2011 | Overlap of study cohorts between Syngelaki 2011 and Chelemen 211. Syngelaki 2011 contains the more complete record and was selected for inclusion in the review. |
| Chen 2019 | Reference standard. Results of the first‐trimester scan are compared with results of the second‐trimester scan. Postnatal confirmation of the index test is only reported for cases with pathological prenatal findings. |
| Chu 2017 | Index test timing. Routine ultrasound screening was performed at 18 to 28 weeks' gestation. |
| Chua 2000 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Clausen 2022 | Target condition. Evaluation of the prenatal detection of a single type of cardiac septal defect. |
| Clementi 2000 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Colosi 2015 | Included for contacting authors to specify the reference standard. No response. |
| Delmas 2017 | Overlap of study cohorts between Laurichesse 2017 and Delmas 2017. Laurichesse 2017 contains the more complete record and was selected for inclusion for contacting authors. |
| Duta 2021 | Overlap of study cohorts with Vayna 2018. The latter study contains the more complete record and was selected for inclusion in the review. |
| Economides 1998 | Overlap of study cohorts between Whitlow 1999, Economides 1998 and Michailidis 2001. Whitlow 1999 contains the more complete record and was selected for inclusion in the review. |
| Eleftheriades 2012 | Study population. Only fetuses that survived until the third trimester of pregnancy were included in the study, causing selection bias. |
| Ensing 2014 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| Eurenius 1999 | Ultrasound screening was performed at 15 to 22 weeks' gestation; excluded on index test timing. |
| Everwijn 2018 | Target condition. Evaluation of the prenatal detection of two specific types of conotruncal anomalies. |
| Ficara 2020 | Study population. Only fetuses that survived until the third trimester of pregnancy were included in the study, causing selection bias. |
| Forci 2020 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data available. However, study was excluded as there was no routine ultrasound screening programme during the study period. |
| Fricke 2021 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Garne 2001 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| Garne 2002 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| Grandjean 1998 | Overlap of study cohorts between Grandjean 1999 and Grandjean 1998. Grandjean 1999 contains the more complete record and was selected for inclusion in the review. |
| Haeusler 2002 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Hafner 1998 | Overlap of study cohorts between Hafner 2003 and Hafner 1998. Hafner 2003 contains the more complete record and was selected for inclusion in the review. |
| Hunter 2000 | Unable to extract or derive data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index test of interest. |
| Iliescu 2014 | Overlap of study cohorts between Iliescu 2013 and Iliescu 2014. Iliescu 2013 contains the more complete record and was selected for inclusion in the review. |
| Itsukaichi 2018 | Included for contacting authors to obtain data to specify the index text type. Contacting authors was attempted, but no valid email address identified. |
| Johnson 2012 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Jorgensen 2015 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| Kansal 2022 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No valid email address identified. |
| Khoo 2008 | Unable to extract or derive data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index test of interest. |
| Laurichesse 2017 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Levi 2003 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No valid email address identified. |
| Li 2019 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Liao 2016 | Overlap of study cohorts between Liao 2021a and Liao 2021b. Liao 2021b contains the more complete record and larger cohort and was therefore selected for inclusion in the review over Liao 2016 and Liao 2021a. |
| Liao 2021a | Overlap of study cohorts between Liao 2016 and Liao 2021b. Liao 2021b contains the more complete record and larger cohort and was selected for inclusion in the review over Liao 2016 and Liao 2021a. |
| Liou 2011 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Long 1998 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No valid email address identified. |
| Ma 2018 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Magripes 1998 | Ultrasound screening was performed at 16 to 19 weeks' gestation; excluded on index test timing. |
| Marek 2011 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| Michailidis 2001 | Overlap of study cohorts between Whitlow 1999, Economides 1998 and Michailidis 2001. Whitlow 1999 contains the more complete record and was selected for inclusion in the review. |
| Minnella 2020 | Overlap of study cohorts between Syngelaki 2019 and Minella 2020. Syngelaki 2019 contains the more complete record and was selected for inclusion in the review. |
| Nakling 2005 | Unclear upper and lower limit of midtrimester ultrasound scan and second trimester diagnoses. Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Nikkila 2006 | Time of diagnosis not specified for prenatally detected anomalies The total number of fetuses with a prenatal diagnosis < 24 weeks' gestation is reported. Authors were contacted to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for detection < 24 weeks' gestation at the level of the organ system and for individual anomalies. No valid email address identified. |
| Ou 2022 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Pavlicek 2020 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Pitukkijronnakorn 2009 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Poggenpoel 2012 | Anatomical system data not available. |
| Queisser‐Luft 1998 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No valid email address identified. |
| Sainz 2015 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Stoll 1998 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Stoll 2000a | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Stoll 2000b | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Stoll 2001 | Target condition. Evaluation of prenatal diagnosis of associated congenital heart defects by ultrasound screening. Isolated cases are not reported in the present study. |
| Stoll 2002 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. No response. |
| Tabor 2003 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| Taipale 2003 | Overlap of study cohorts between Taipale 2004 and Taipale 2003. Taipale 2003 contains the more complete record and was selected for inclusion in the review. |
| Tegnander 2006 | Included for contacting authors to obtain data to specify the upper and lower limit of the "18 week cohort". Scanning was performed at 16 + 0 ‐ 22 + 6 weeks' gestation. Study excluded on index test timing. |
| Tegnander 2006a | Overlap of study cohorts Tegnander 2006 and Tegnander 2006a. Tegnander 2006a contains the more complete record and was selected for contacting authors. |
| Todros 1997 | Unable to extract or derive data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index test of interest. |
| Trines 2013 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| Tudorache 2016a | Target condition. Evaluation of the prenatal detection of abnormalities of the fetal kidneys specifically. Anomalies of the urinary tract other than renal malformations are not reported. |
| Tudorache 2016b | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| van Allen 2006 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| van Dorsten 1998 | Ultrasound screening was performed at 15 to 22 weeks' gestation; excluded on index test timing. |
| van Velzen 2015 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| Vavilala 2011 | Anatomical system data not available. Since anatomical systems were presented for prenatal diagnoses, but not for postnatal ones, authors were contacted to provide the missing information as we suspected this may be available. No response after multiple attempts. |
| Westin 2006 | Overlap of study cohorts between Saltvedt 2006 and Westin 2006. Saltvedt 2006 contains the more complete record and was selected for inclusion in the review. |
| Wiesel 2005 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
| Wong 2004 | Included for contacting authors to obtain data to construct 2 x 2 tables of TP, FP, FN and TN test results for the index tests of interest. Data not available. |
FN: false negative; FP: false positive; TN: true negative; TP: true positive
Differences between protocol and review
We adapted the search approach published in the protocol for this review (Buijtendijk 2021) for the final review. The protocol mentioned that we planned to search the Cochrane Central Register of Controlled Trials (CENTRAL) in addition to OVID MEDLINE, OVID Embase and Web of Science. However, we did not apply the search to CENTRAL as we did not expect that this would identify additional relevant publications. Furthermore, key papers identified by stakeholders and members of the review team after publication of the protocol were not captured by the proposed search approach, resulting in a need to adapt the search strategy. As we adapted part I of the search strategy, it became clear through testing that the new search was so sensitive that adding the second component of the search as initially planned did not add relevant studies but did add a lot of noise. Therefore, the second component of the search, as mentioned in the protocol, was omitted.
In the Criteria for considering studies for this review section of the methods, we have refined the selection criteria to ensure that the included studies matched our review question. We excluded studies that reported on detection of fetal abnormalities by a prenatal ultrasound scan that only involved biometric measurements or markers for chromosomal abnormalities. In addition, we had planned that two review authors would independently screen all titles and abstracts. However, this was not feasible due to the high number of papers identified. Therefore, we decided that each title and abstract would be evaluated by a single review author only. To minimise the risk of subjective selection bias, we randomly divided studies over a total of 13 review authors, who screened the papers in an over‐inclusive manner.
In the Statistical analysis and data synthesis section, we made changes to the prespecified subgroups of anomalies that we intended to analyse. We narrowed subgroup 1, evaluating the accuracy of ultrasound screening for conditions that are likely to be detected either immediately after birth or before hospital discharge, to only include abnormalities that are truly of congenital origin. Anomalies that may be acquired (e.g. hydrocephalus, fetal hydrops, tumours, etc.) were excluded from this subgroup as they may or may not have been present at the time of the ultrasound examination.
For the subgroup analysis of anomalies according to their likely clinical consequences, we decided to merge the severity subgroups “severe” and “moderate severity” because insufficient information was available from papers to distinguish anomalies that are considered to be severe from those considered to be of moderate severity. For the analyses of organ system‐specific data and selected conditions, we chose to omit the “miscellaneous” categories for each organ system because this category provided little additional information to the estimates of “overall detection” within organ systems. For the analysis of cardiac anomalies, we decided to keep the category of “miscellaneous major cardiac anomalies” and to add another category for the analysis of minor cardiac anomalies. We added the latter category in order to distinguish between miscellaneous severe cardiac anomalies (e.g. unspecified complex heart defects) and those considered to be insignificant or minor.
We planned to investigate the impact of likely missing false‐negative test results by conducting sensitivity analyses where we inflated the number of false‐negative results in studies where there was a high number of cases with a missing reference standard, as proposed by Mol 1999 and Alldred 2012. However, we decided not to perform this analysis given the high number of studies included for many of the index tests and because the number of true and false negatives varied greatly between studies to begin with, which may have arisen from a number of factors.
Lastly, we noticed that in the modified QUADAS‐2 tool that was presented in Appendix 4 of the published protocol, the signalling question “Did all live‐born infants receive the same reference standard?” in domain 4 was missing. This signalling question was included in the modified QUADAS‐2 tool that we used for methodological quality assessment of the included studies.
Contributions of authors
MFJB and HS initiated this review project. MFJB recruited all authors and co‐ordinated the production of this review.
MFJB and CEJML drafted the protocol. MMGL, HS, YD, MAL, EP, MJBvdH and BSdB critically appraised the protocol and provided their comments.
CEJML designed and applied the search strategy in collaboration with MFJB.
MFJB, BBB, MMGL, HS, TR, TCDG, DD, GMMT, YD, MAL, BB, MJBvdH and BSdB screened titles and abstracts of publications identified by the search. MFJB, BBB, TR and TCDG obtained full‐text versions of potentially relevant publications. MFJB and BBB applied the selection criteria and evaluated the methodological quality of the included studies. BBB contacted authors of potentially eligible studies to provide missing information. MFJB contacted the authors of included studies.
MFJB, BBB, TR and TCDG extracted data. DD and GMMT checked the data extraction. MMGL undertook the statistical analyses.
MFJB wrote the draft review. BBB, MMGL, TR, HS, YD, MAL, EP, MJBvdH and BSdB revised and commented on the manuscript. MMGL and EP provided methodological and clinical perspectives, respectively.
The final manuscript was approved by all authors.
Sources of support
Internal sources
-
Amsterdam University Medical Centers, Netherlands
Malou Lugthart and Bo Bet receive their salaries from the Amsterdam University Medical Centers.
-
Amsterdam University Medical Centers, Netherlands
The senior authors, namely Mariska Leeflang, Eva Pajkrt, Maurice van den Hoff and Bernadette de Bakker, receive their salaries from the Amsterdam University Medical Centers.
-
University of Amsterdam, Netherlands
Yousif Dawood is financially supported by an AMC PhD Scholarship (University of Amsterdam).
-
Imperial College London, UK
Harsha Shah receives salary from Imperial College London.
External sources
-
TKI‐LSH Dutch Top Sector Life & Science & Health consortium grant, Netherlands
Marieke Buijtendijk is financially supported by a PhD fellowship funded by a TKI‐LSH Dutch Top Sector Life Sciences & Health consortium grant, where Samsung Medison South Korea was a contributor to the pool of funds supporting the fellowship.
Declarations of interest
Marieke Buijtendijk was supported by a PhD fellowship funded by a TKI‐LSH Dutch Top Sector Life Sciences & Health consortium grant, where Samsung Medison South Korea was a contributor to the pool of funds supporting the fellowship. Samsung loans ultrasound equipment to the department for conducting pre‐clinical research projects. Additionally, she was invited to present during a webinar on embryology and first‐trimester 3D ultrasound imaging organised by the American Institute for Ultrasound in Medicine (AIUM) in collaboration with NeuroLogica, a subsidiary of Samsung Electronics, for which the department received payment.
Bo Bet and Mariska Leeflang received no funding and report no conflicts of interest.
Harsha Shah has received funding from Samsung Medison South Korea to support travel costs for research in three‐dimensional ultrasound. She received payments for lectures/presentations from NeuroLogica Corporation first‐trimester 3D ultrasound imaging.
Tom Reuvekamp, Timothy Goring, Daniël Docter and Melanie Timmerman received no funding and report no conflicts of interest.
Yousif Dawood received funding from the AMC PhD scholarship (University of Amsterdam) for four years for the conception of a microfocus computed tomography (micro‐CT) based three‐dimensional atlas of human fetal development. There is no conflict of interest.
Malou Lugthart, Bente Berends and Jacqueline Limpens do not receive funding. There is no conflict of interest.
Eva Pajkrt received funding from ZonMW for the Pessary or Cerclage to prevent preterm delivery in women with short cervical length and a history of preterm birth (PC) study (study number: 837002406; Koullali 2017) and receives money from ZonMW for the Expectant Management versus Induction of Labor (PROMEXIL) Follow‐up trial (study number: 843002826; de Ruigh 2021). She was appointed the Minister of Health, Welfare and Sport and the Minister of Security and Justice to the role of chairperson of the review commission for late pregnancy termination and termination of life of newborns (appointed on 27 January 2016) and has been reimbursed expenses for attending meetings.
Maurice van den Hoff obtained funding for a PhD fellowship through a TKI‐LSH Dutch Top Sector Life Sciences & Health consortium grant, where Samsung was a contributor to the pool of funds supporting the fellowship. Samsung loans ultrasound equipment to the department for conducting pre‐clinical research projects. Dr. van den Hoff is Past‐Chair of the European Society of Cardiology (ESC) Working Group Development, Anatomy and Pathology. From September 2012 onward, he held various roles, including board member, treasurer, vice chair and chair. During this time, he received support for travel expenses to attend ESC meetings.
Bernadette de Bakker declares no conflicts of interests.
New
References
References to studies included in this review
Abu‐Rustum 2010 {published data only}
- Abu-Rustum RS, Daou L, Abu-Rustum SE. Role of first-trimester sonography in the diagnosis of aneuploidy and structural fetal anomalies. Journal of Ultrasound in Medicine 2010;29(10):1445-52. [DOI] [PubMed] [Google Scholar]
Andrew 2015 {published data only}
- Andrew C, Gopal S, Ramachandran H, Suvika M. First trimester ultrasound: addition of anatomical screening adds value to the examination: a retrospective case series. Journal of Evolution of Medical and Dental Sciences 2015;4(16):2690-9. [Google Scholar]
Bardi 2022 {published data only}
- Bardi F, Bergman JEH, Siemensma-Muhlenberg N, Walle HEK, Bakker MK. Prenatal diagnosis and pregnancy outcome of major structural anomalies detectable in the first trimester: a population-based cohort study in the Netherlands. Paediatric and Perinatal Epidemiology 2022;6(36):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batra 2014 {published data only}
- Batra M, Gopinathan KK, Balakrishnan B, Gopinath P, Kuriakose R, Gupta J. The detection rate of cardiac anomalies at 11-13+6 week scan using four chamber view and three vessel view. Journal of Fetal Medicine 2014;1(2):95-8. [Google Scholar]
Becker 2012 {published data only}
- Becker R, Schmitz L, Kilavuz S, Stumm M, Wegner R D, Bittner U. 'Normal' nuchal translucency: a justification to refrain from detailed scan? Analysis of 6858 cases with special reference to ethical aspects. Prenatal Diagnosis 2012;32(6):550-6. [DOI] [PubMed] [Google Scholar]
Bister 2011 {published data only}
- Bister D, Set P, Cash C, Coleman N, Fanshawe T. Incidence of facial clefts in Cambridge, United Kingdom. European Journal of Orthodontics 2011;33(4):372-6. [DOI] [PubMed] [Google Scholar]
Biswas 2013 {published data only}
- Biswas J, De K, Dasgupta S, Ghosh B, Sanyal P, Mukherjee A. Prenatal ultrasound screening to diagnose congenital anomalies among one thousand unselected women and their pregnancy outcome. Journal of Evolution of Medical and Dental Sciences 2013;2(13):1966-71. [Google Scholar]
Bodin 2018 {published data only}
- Bodin CR, Rasmussen MM, Tabor A, Westbom L, Tiblad E, Ekelund CK, et al. Ultrasound in prenatal diagnostics and its impact on the epidemiology of spina bifida in a national cohort from Denmark with a comparison to Sweden. BioMed Research International 2018;2018:9203985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyd 2000‐1 {published data only}
- Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. Journal of Medical Screening 2000;7(4):169-74. [DOI] [PubMed] [Google Scholar]
Boyd 2000‐10 {published data only}
- Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. Journal of Medical Screening 2000;7(4):169-74. [DOI] [PubMed] [Google Scholar]
Boyd 2000‐11 {published data only}
- Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. Journal of Medical Screening 2000;7(4):169-74. [DOI] [PubMed] [Google Scholar]
Boyd 2000‐12 {published data only}
- Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. Journal of Medical Screening 2000;7(4):169-74. [DOI] [PubMed] [Google Scholar]
Boyd 2000‐2 {published data only}
- Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. Journal of Medical Screening 2000;7(4):169-74. [DOI] [PubMed] [Google Scholar]
Boyd 2000‐3 {published data only}
- Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. Journal of Medical Screening 2000;7(4):169-74. [DOI] [PubMed] [Google Scholar]
Boyd 2000‐4 {published data only}
- Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. Journal of Medical Screening 2000;7(4):169-74. [DOI] [PubMed] [Google Scholar]
Boyd 2000‐5 {published data only}
- Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. Journal of Medical Screening 2000;7(4):169-74. [DOI] [PubMed] [Google Scholar]
Boyd 2000‐6 {published data only}
- Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. Journal of Medical Screening 2000;7(4):169-74. [DOI] [PubMed] [Google Scholar]
Boyd 2000‐7 {published data only}
- Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. Journal of Medical Screening 2000;7(4):169-74. [DOI] [PubMed] [Google Scholar]
Boyd 2000‐8 {published data only}
- Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. Journal of Medical Screening 2000;7(4):169-74. [DOI] [PubMed] [Google Scholar]
Boyd 2000‐9 {published data only}
- Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centres across Europe. Journal of Medical Screening 2000;7(4):169-74. [DOI] [PubMed] [Google Scholar]
Boyd 2011 {published data only}
- Boyd PA, Tonks AM, Rankin J, Rounding C, Wellesley D, Draper ES. Monitoring the prenatal detection of structural fetal congenital anomalies in England and Wales: register-based study. Journal of Medical Screening 2011;18(1):2-7. [DOI] [PubMed] [Google Scholar]
Brown 2021 {published data only}
- Brown I, Rolnik DL, Fernando S, Menezes M, Ramkrishna J, Costa FD, et al. Ultrasound findings and detection of fetal abnormalities before 11 weeks of gestation. Prenatal Diagnosis 2021;41(13):1675-84. [DOI] [PubMed] [Google Scholar]
Carvalho 2002 {published data only}
- Carvalho MH, Brizot M L, Lopes LM, Chiba CH, Miyadahira S, Zugaib M. Detection of fetal structural abnormalities at the 11-14 week ultrasound scan. Prenatal Diagnosis 2002;22(1):1-4. [DOI] [PubMed] [Google Scholar]
Chelli 2009 {published data only}
- Chelli D, Gaddour I, Najar I, Boudaya F, Zouaoui B, Sfar E, et al. First trimester ultrasound: an early screening tool for fetal structural and chromosomic abnormalities. [French]. Tunisie Medicale 2009;87(12):857-62. [PubMed] [Google Scholar]
Chen 2009 {published data only}
- Chen M, Leung TY, Sahota DS, Fung TY, Chan LW, Law LW, et al. Ultrasound screening for fetal structural abnormalities performed by trained midwives in the second trimester in a low-risk population--an appraisal. Acta Obstetricia et Gynecologica Scandinavica 2009;88(6):713-9. [DOI] [PubMed] [Google Scholar]
Choudry 2009 {published data only}
- Choudhry MS, Rahman N, Boyd P, Lakhoo K. Duodenal atresia: associated anomalies, prenatal diagnosis and outcome. Pediatric Surgery International 2009;25(8):727-30. [DOI] [PubMed] [Google Scholar]
Dane 2007 {published data only}
- Dane B, Dane C, Sivri D, Kiray M, Cetin A, Yayla M. Ultrasound screening for fetal major abnormalities at 11-14 weeks. Acta Obstetricia et Gynecologica Scandinavica 2007;86(6):666-70. [DOI] [PubMed] [Google Scholar]
Del Bianco 2006 {published data only}
- Del Bianco A, Russo S, Lacerenza N, Rinaldi M, Rinaldi G, Nappi L, et al. Four chamber view plus three-vessel and trachea view for a complete evaluation of the fetal heart during the second trimester. Journal of Perinatal Medicine 2006;34(4):309-12. [DOI] [PubMed] [Google Scholar]
de Robertis 2017 {published data only}
- De Robertis V, Rembouskos G, Fanelli T, Volpe G, Muto B, Volpe P. The three-vessel and trachea view (3VTV) in the first trimester of pregnancy: an additional tool in screening for congenital heart defects (CHD) in an unselected population. Prenatal Diagnosis 2017;37(7):693-8. [DOI] [PubMed] [Google Scholar]
Drukker 2020 {published data only}
- Drukker L, Cavallaro A, Salim I, Ioannou C, Impey L, Papageorghiou A T. How often do we incidentally find a fetal abnormality at the routine third-trimester growth scan? A population-based study. American Journal of Obstetrics & Gynecology 2020;223(6):919.e1-13. [DOI] [PubMed] [Google Scholar]
Drysdale 2002 {published data only}
- Drysdale K, Ridley D, Walker K, Higgins B, Dean T. First-trimester pregnancy scanning as a screening tool for high-risk and abnormal pregnancies in a district general hospital setting. Journal of Obstetrics & Gynaecology 2002;22(2):159-65. [DOI] [PubMed] [Google Scholar]
Ebrashy 2019 {published data only}
- Ebrashy A, Aboulghar M, Elhodiby M, El-Dessouky S H, Elsirgany S, Gaafar H M, et al. Fetal heart examination at the time of 13 weeks scan: a 5 years' prospective study. Journal of Perinatal Medicine 2019;47(8):871-8. [DOI] [PubMed] [Google Scholar]
Erenel 2019 {published data only}
- Erenel H, Karslı MF, Özel A, Korkmaz SO, Arısoy R, Saltık L, et al. The importance of four-chamber and three-vessel (3-V) views in the screening of fetal cardiac anomalies in the first trimester. Perinatal Journal 2019;27(3):169-75. [Google Scholar]
Fleurke‐Rozema 2014 {published data only}
- Fleurke-Rozema JH, Vogel TA, Voskamp BJ, Pajkrt E, den Berg PP, Beekhuis JR, et al. Impact of introduction of mid-trimester scan on pregnancy outcome of open spina bifida in The Netherlands. Ultrasound in Obstetrics & Gynecology 2014;43(5):553-6. [DOI] [PubMed] [Google Scholar]
Fleurke‐Rozema 2016 {published data only}
- Fleurke-Rozema JH, de Kamp K, Bakker MK, Pajkrt E, Bilardo CM, Snijders RJ. Prevalence, diagnosis and outcome of cleft lip with or without cleft palate in The Netherlands. Ultrasound in Obstetrics & Gynecology 2016;48(4):458-63. [DOI] [PubMed] [Google Scholar]
Fleurke‐Rozema 2017 {published data only}
- Fleurke-Rozema H, de Kamp K, Bakker M, Pajkrt E, Bilardo C, Snijders R. Prevalence, timing of diagnosis and pregnancy outcome of abdominal wall defects after the introduction of a national prenatal screening program. Prenatal Diagnosis 2017;37(4):383-8. [DOI] [PubMed] [Google Scholar]
Galindo 2011 {published data only}
- Galindo A, Herraiz I, Escribano D, Lora D, Melchor JC, la Cruz J. Prenatal detection of congenital heart defects: a survey on clinical practice in Spain. Fetal Diagnosis & Therapy 2011;29(4):287-95. [DOI] [PubMed] [Google Scholar]
Gallot 2007 {published data only}
- Gallot D, Boda C, Ughetto S, Perthus I, Robert-Gnansia E, Francannet C, et al. Prenatal detection and outcome of congenital diaphragmatic hernia: a French registry-based study. Ultrasound in Obstetrics & Gynecology 2007;29(3):276-83. [DOI] [PubMed] [Google Scholar]
Gireada 2022 {published data only}
- Gireada R, Socolov D, Mihalceanu E, Matasariu R, Ursache A, Akad M, et al. The additional role of the 3-vessels and trachea view in screening for congenital heart disease. Medicina 2022;58(2):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gornall 2003 {published data only}
- Gornall AS, Budd JL, Draper ES, Konje JC, Kurinczuk JJ. Congenital cystic adenomatoid malformation: accuracy of prenatal diagnosis, prevalence and outcome in a general population. Prenatal Diagnosis 2003;23(12):997-1002. [DOI] [PubMed] [Google Scholar]
Grande 2012 {published data only}
- Grande M, Arigita M, Borobio V, Jimenez J M, Fernandez S, Borrell A. First-trimester detection of structural abnormalities and the role of aneuploidy markers. Ultrasound in Obstetrics & Gynecology 2012;39(2):157-63. [DOI] [PubMed] [Google Scholar]
Grandjean 1999 {published data only}
- Grandjean H, Larroque D, Levi S. The performance of routine ultrasonographic screening of pregnancies in the Eurofetus Study. American Journal of Obstetrics & Gynecology 1999;181(2):446-54. [DOI] [PubMed] [Google Scholar]
Gu 2011 {published data only}
- Gu Y, Hu YL, Ru T, Yang Y, Dai CY, Yang LJ. Combination prenatal ultrasound screening in early and middle stage of pregnancy for detecting fetal abnormalities. [Chinese]. Chinese Journal of Medical Imaging Technology 2011;27(10):2087-90. [Google Scholar]
Hafner 2003 {published data only}
- Hafner E, Schuller T, Metzenbauer M, Schuchter K, Philipp K. Increased nuchal translucency and congenital heart defects in a low-risk population. Prenatal Diagnosis 2003;23(12):985-9. [DOI] [PubMed] [Google Scholar]
Hartge 2011 {published data only}
- Hartge DR, Weichert J, Krapp M, Germer U, Gembruch U, Axt-Fliedner R. Results of early foetal echocardiography and cumulative detection rate of congenital heart disease. Cardiology in the Young 2011;21(5):505-17. [DOI] [PubMed] [Google Scholar]
Hautala 2019 {published data only}
- Hautala J, Gissler M, Ritvanen A, Tekay A, Pitkanen-Argillander O, Stefanovic V, et al. The implementation of a nationwide anomaly screening programme improves prenatal detection of major cardiac defects: an 11-year national population-based cohort study. British Journal of Obstetrics & Gynaecology 2019;126(7):864-73. [DOI] [PubMed] [Google Scholar]
He 2016 {published data only}
- He H, Wan J, Yu L, Sui S, Guo W. Three-dimensional ultrasound in diagnosis of fetal limb deformities. [Chinese]. Chinese Journal of Medical Imaging Technology 2016;32(11):1714-8. [Google Scholar]
Hernadi 1997 {published data only}
- Hernadi L, Torocsik M. Screening for fetal anomalies in the 12th week of pregnancy by transvaginal sonography in an unselected population. Prenatal Diagnosis 1997;17(8):753-9. [DOI] [PubMed] [Google Scholar]
Hildebrand 2010 {published data only}
- Hildebrand E, Selbing A, Blomberg M. Comparison of first and second trimester ultrasound screening for fetal anomalies in the southeast region of Sweden. Acta Obstetricia et Gynecologica Scandinavica 2010;89(11):1412-9. [DOI] [PubMed] [Google Scholar]
Iliescu 2013 {published data only}
- Iliescu D, Tudorache S, Comanescu A, Antsaklis P, Cotarcea S, Novac L, et al. Improved detection rate of structural abnormalities in the first trimester using an extended examination protocol. Ultrasound in Obstetrics & Gynecology 2013;42(3):300-9. [DOI] [PubMed] [Google Scholar]
Ingale 2017 {published data only}
- Ingale SY, Menon SS. To correlate the incidence of congenital anomalies on antenatal scan and those detected in postnatal period in Krishna Institute of Medical Sciences, Karad. Journal of Evolution of Medical and Dental Sciences 2017;6(5):395-7. [Google Scholar]
Jacobsen 2011 {published data only}
- Jakobsen TR, Sogaard K, Tabor A. Implications of a first trimester Down syndrome screening program on timing of malformation detection. Acta Obstetricia et Gynecologica Scandinavica 2011;90(7):728-36. [DOI] [PubMed] [Google Scholar]
Johnson 1997 {published data only}
- Johnson SP, Sebire NJ, Snijders RJ, Tunkel S, Nicolaides KH. Ultrasound screening for anencephaly at 10-14 weeks of gestation. Ultrasound in Obstetrics & Gynecology 1997;9(1):14-6. [DOI] [PubMed] [Google Scholar]
Kenkhuis 2018 {published data only}
- Kenkhuis MJA, Bakker M, Bardi F, Fontanella F, Bakker MK, Fleurke-Rozema JH, et al. Effectiveness of 12-13-week scan for early diagnosis of fetal congenital anomalies in the cell-free DNA era. Ultrasound in Obstetrics & Gynecology 2018;51(4):463-9. [DOI] [PubMed] [Google Scholar]
Krapp 2011 {published data only}
- Krapp M, Ludwig A, Axt-Fliedner R, Kreiselmaier P. First trimester fetal echocardiography: which planes and defects can be displayed during the daily routine in a prenatal medicine unit? Ultraschall in der Medizin 2011;32(4):362-6. [DOI] [PubMed] [Google Scholar]
Lee 1998 {published data only}
- Lee K, Kim SY, Choi SM, Kim JS, Lee BS, Seo K, et al. Effectiveness of prenatal ultrasonography in detecting fetal anomalies and perinatal outcome of anomalous fetuses. Yonsei Medical Journal 1998;39(4):372-82. [DOI] [PubMed] [Google Scholar]
Leiroz 2021 {published data only}
- Leiroz R, Aquino MA, Santos KP, Monteiro MDC, Aires TSF, Araujo Junior E, et al. Accuracy of the mid-trimester ultrasound scan in the detection of fetal congenital anomalies in a reference center in Northeastern Brazil. Journal of Gynecology Obstetrics and Human Reproduction 2021;50(10):102225. [DOI] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li Q, Guan B, Li D. Detection of fetal structural abnormalities by early pregnancy ultrasound in China. International Journal of Gynaecology & Obstetrics 2008;100(3):277-8. [DOI] [PubMed] [Google Scholar]
Liao 2021 {published data only}
- Liao Y, Wen H, Ouyang S, Yuan Y, Bi J, Guan Y, et al. Routine first-trimester ultrasound screening using a standardized anatomical protocol. American Journal of Obstetrics & Gynecology 2021;224(4):396.e1-396.e15. [DOI] [PubMed] [Google Scholar]
Maarse 2011 {published data only}
- Maarse W, Pistorius LR, Van Eeten WK, Breugem CC, Kon M, Van den Boogaard MJ, et al. Prenatal ultrasound screening for orofacial clefts. Ultrasound in Obstetrics & Gynecology 2011;38(4):434-9. [DOI] [PubMed] [Google Scholar]
McAuliffe 2005 {published data only}
- McAuliffe FM, Fong KW, Toi A, Chitayat D, Keating S, Johnson JA. Ultrasound detection of fetal anomalies in conjunction with first-trimester nuchal translucency screening: a feasibility study. American Journal of Obstetrics & Gynecology 2005;193(3 Pt 2):1260-5. [DOI] [PubMed] [Google Scholar]
Natu 2014 {published data only}
- Natu N, Wadnere N, Yadav S, Kumar R. Utility of first trimester anomaly scan in screening of congenital abnormalities in low and high risk pregnancies. JK Science 2014;16(4):151-5. [Google Scholar]
Novotna 2012 {published data only}
- Novotna M, Haslik L, Svabik K, Zizka Z, Belosovicova H, Brestak M, et al. Detection of fetal major structural anomalies at the 11-14 ultrasound scan in an unselected population. Ceska Gynekologie 2012;77(4):330-5. [PubMed] [Google Scholar]
Ogge 2006 {published data only}
- Ogge G, Gaglioti P, Maccanti S, Faggiano F, Todros T. Prenatal screening for congenital heart disease with four-chamber and outflow-tract views: a multicenter study. Ultrasound in Obstetrics & Gynecology 2006;28(6):779-84. [DOI] [PubMed] [Google Scholar]
Orlandi 2014 {published data only}
- Orlandi E, Rossi C, Perino A, Musico G, Orlandi F. Simplified first-trimester fetal cardiac screening (four chamber view and ventricular outflow tracts) in a low-risk population. Prenatal Diagnosis 2014;34(6):558-63. [DOI] [PubMed] [Google Scholar]
Oztekin 2009 {published data only}
- Oztekin O, Oztekin D, Tinar S, Adibelli Z. Ultrasonographic diagnosis of fetal structural abnormalities in prenatal screening at 11-14 weeks. Diagnostic & Interventional Radiology 2009;15(3):221-5. [PubMed] [Google Scholar]
Petousis 2020 {published data only}
- Petousis S, Sotiriadis A, Margioula-Siarkou C, Tsakiridis I, Christidis P, Kyriakakis M, et al. Detection of structural abnormalities in fetuses with normal karyotype at 11-13 weeks using the anatomic examination protocol of the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG). Journal of Maternal-Fetal & Neonatal Medicine 2020;33(15):2581-7. [DOI] [PubMed] [Google Scholar]
Pilalis 2012 {published data only}
- Pilalis A, Basagiannis C, Eleftheriades M, Faros E, Troukis E, Armelidou E, et al. Evaluation of a two-step ultrasound examination protocol for the detection of major fetal structural defects. Journal of Maternal-Fetal & Neonatal Medicine 2012;25(9):1814-7. [DOI] [PubMed] [Google Scholar]
Rydberg 2017 {published data only}
- Rydberg C, Tunon K. Detection of fetal abnormalities by second-trimester ultrasound screening in a non-selected population. Acta Obstetricia et Gynecologica Scandinavica 2017;96(2):176-82. [DOI] [PubMed] [Google Scholar]
Sainz 2020 {published data only}
- Sainz JA, Gutierrez L, Garcia-Mejido J, Ramos Z, Bonomi MJ, Fernandez-Palacin A, et al. Early fetal morphological evaluation (11-13 + 6 weeks) accomplished exclusively by transabdominal imaging and following routine midtrimester fetal ultrasound scan recommendations. Since when can it be performed? Journal of Maternal-Fetal & Neonatal Medicine 2020;33(7):1140-50. [DOI] [PubMed] [Google Scholar]
Saltvedt 2006 {published data only}
- Saltvedt S, Almstrom H, Kublickas M, Valentin L, Grunewald C. Detection of malformations in chromosomally normal fetuses by routine ultrasound at 12 or 18 weeks of gestation-a randomised controlled trial in 39,572 pregnancies. British Journal of Obstetrics & Gynaecology 2006;113(6):664-74. [DOI] [PubMed] [Google Scholar]
Salvador 2005 {published data only}
- Salvador J, Borrell A, Lladonosa A. Increasing detection rates of birth defects by prenatal ultrasound leading to apparent increasing prevalence. Lessons learned from the population-based registry of birth defects of Barcelona. Prenatal Diagnosis 2005;25(11):991-6. [DOI] [PubMed] [Google Scholar]
Sepulveda 2013 {published data only}
- Sepulveda W, Wong AE. First trimester screening for holoprosencephaly with choroid plexus morphology ('butterfly' sign) and biparietal diameter. Prenatal Diagnosis 2013;33(13):1233-7. [DOI] [PubMed] [Google Scholar]
Smrcek 2006 {published data only}
- Smrcek JM, Berg C, Geipel A, Fimmers R, Axt-Fliedner R, Diedrich K, et al. Detection rate of early fetal echocardiography and in utero development of congenital heart defects. Journal of Ultrasound in Medicine 2006;25(2):187-96. [DOI] [PubMed] [Google Scholar]
Souka 2006 {published data only}
- Souka AP, Pilalis A, Kavalakis I, Antsaklis P, Papantoniou N, Mesogitis S, et al. Screening for major structural abnormalities at the 11- to 14-week ultrasound scan. American Journal of Obstetrics & Gynecology 2006;194(2):393-6. [DOI] [PubMed] [Google Scholar]
Srisupundit 2006 {published data only}
- Srisupundit K, Tongsong T, Sirichotiyakul S, Chanprapaph P. Fetal structural anomaly screening at 11-14 weeks of gestation at Maharaj Nakorn Chiang Mai Hospital. Journal of the Medical Association of Thailand 2006;89(5):588-93. [PubMed] [Google Scholar]
Stefos 1999 {published data only}
- Stefos T, Plachouras N, Sotiriadis A, Papadimitriou D, Almoussa N, Navrozoglou I, et al. Routine obstetrical ultrasound at 18-22 weeks: our experience on 7,236 fetuses. Journal of Maternal-Fetal Medicine 1999;8(2):64-9. [DOI] [PubMed] [Google Scholar]
Syngelaki 2011 {published data only}
- Syngelaki A, Chelemen T, Dagklis T, Allan L, Nicolaides K H. Challenges in the diagnosis of fetal non-chromosomal abnormalities at 11-13 weeks. Prenatal Diagnosis 2011;31(1):90-102. [DOI] [PubMed] [Google Scholar]
Syngelaki 2019 {published data only}
- Syngelaki A, Hammami A, Bower S, Zidere V, Akolekar R, Nicolaides K H. Diagnosis of fetal non-chromosomal abnormalities on routine ultrasound examination at 11-13 weeks' gestation. Ultrasound in Obstetrics & Gynecology 2019;54(4):468-76. [DOI] [PubMed] [Google Scholar]
Taipale 2004 {published data only}
- Taipale P, Ammala M, Salonen R, Hiilesmaa V. Two-stage ultrasonography in screening for fetal anomalies at 13-14 and 18-22 weeks of gestation. Acta Obstetricia et Gynecologica Scandinavica 2004;83(12):1141-6. [DOI] [PubMed] [Google Scholar]
Takita 2016 {published data only}
- Takita H, Hasegawa J, Arakaki T, Nakamura M, Hamada S, Tokunaka M, et al. Usefulness of antenatal ultrasound fetal morphological assessments in the first and second trimester: a study at a single Japanese university hospital. Journal of Medical Ultrasonics 2016;43(1):57-62. [DOI] [PubMed] [Google Scholar]
Tiechl 2021 {published data only}
- Tiechl J, Abdel Azim S, Leitner K, Berger A, Mutz-Dehbalaie I, Goebel G, et al. Screening for open spina bifida in a routine clinical setting at the first-trimester scan: a prospective multicentre cohort study. Fetal Diagnosis & Therapy 2021;48(2):96-102. [DOI] [PubMed] [Google Scholar]
Timbolschi 2015 {published data only}
- Timbolschi D, Schaefer E, Monga B, Fattori D, Dott B, Favre R, et al. Neural tube defects: the experience of the registry of congenital malformations of Alsace, France, 1995-2009. Fetal Diagnosis & Therapy 2015;37(1):6-17. [DOI] [PubMed] [Google Scholar]
Tudorache 2016 {published data only}
- Tudorache S, Ungureanu A, Dragusin R C, Sorop FM, Cara ML, Iliescu DG. First trimester diagnostic accuracy of a two-dimensional simplified ultrasound technique in congenital heart diseases and great arteries anomalies. Obstetrica si Ginecologie 2016;64(3):165-76. [Google Scholar]
Tydeman 2013 {published data only}
- Tydeman G, Clegg I, Brown S. Detection rates of the Fetal Anomaly Screening Programme (FASP) 11 key conditions in one unit: is the recommended annual audit of any value? Prenatal Diagnosis 2013;33(9):910-2. [DOI] [PubMed] [Google Scholar]
van Velzen 2016 {published data only}
- Velzen CL, Clur SA, Rijlaarsdam ME, Bax CJ, Pajkrt E, Heymans MW, et al. Prenatal detection of congenital heart disease--results of a national screening programme. British Journal of Obstetrics & Gynaecology 2016;123(3):400-7. [DOI] [PubMed] [Google Scholar]
Vayna 2018 {published data only}
- Vayna AM, Veduta A, Duta S, Panaitescu A M, Stoica S, Buinoiu N, et al. Diagnosis of fetal structural anomalies at 11 to 14 weeks. Journal of Ultrasound in Medicine 2018;37(8):2063-73. [DOI] [PubMed] [Google Scholar]
Vellamkondu 2017 {published data only}
- Vellamkondu A, Vasudeva A, Bhat RG, Kamath A, Amin SV, Rai L, et al. Risk assessment at 11-14-week antenatal visit: a tertiary referral center experience from South India. Journal of Obstetrics & Gynaecology of India 2017;67(6):421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vimpelli 2006 {published data only}
- Vimpelli T, Huhtala H, Acharya G. Fetal echocardiography during routine first-trimester screening: a feasibility study in an unselected population. Prenatal Diagnosis 2006;26(5):475-82. [DOI] [PubMed] [Google Scholar]
Waern 2021 {published data only}
- Waern M, Mellander M, Berg A, Carlsson Y. Prenatal detection of congenital heart disease - results of a Swedish screening program 2013-2017. BMC Pregnancy & Childbirth 2021;21(1):579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang L, Wu QQ, Chen Y, Ma YQ, Yao L, Li M. Ultrasound screening of fetal structural abnormalities by standard ultrasound views during the first trimester. Chinese Medical Journal 2013;126(5):986-7. [PubMed] [Google Scholar]
Wayne 2002 {published data only}
- Wayne C, Cook K, Sairam S, Hollis B, Thilaganathan B. Sensitivity and accuracy of routine antenatal ultrasound screening for isolated facial clefts. British Journal of Radiology 2002;75(895):584-9. [DOI] [PubMed] [Google Scholar]
Whitlow 1999 {published data only}
- Whitlow BJ, Chatzipapas IK, Lazanakis ML, Kadir RA, Economides DL. The value of sonography in early pregnancy for the detection of fetal abnormalities in an unselected population. British Journal of Obstetrics & Gynaecology 1999;106(9):929-36. [DOI] [PubMed] [Google Scholar]
Wu 2009 {published data only}
- Wu Q, Li M, Ju L, Zhang W, Yang X, Yan Y, et al. Application of the 3-vessel view in routine prenatal sonographic screening for congenital heart disease. Journal of Ultrasound in Medicine 2009;28(10):1319-24. [DOI] [PubMed] [Google Scholar]
Yu 2011‐1 {published data only}
- Yu Z, Xi Y, Ding W, Han S, Cao L, Zhu C, et al. Congenital heart disease in a Chinese hospital: pre- and postnatal detection, incidence, clinical characteristics and outcomes. Pediatrics International 2011;53(6):1059-65. [DOI] [PubMed] [Google Scholar]
Yu 2011‐2 {published data only}
- Yu Z, Xi Y, Ding W, Han S, Cao L, Zhu C, et al. Congenital heart disease in a Chinese hospital: pre- and postnatal detection, incidence, clinical characteristics and outcomes. Pediatrics International 2011;53(6):1059-65. [DOI] [PubMed] [Google Scholar]
Yu 2011‐3 {published data only}
- Yu Z, Xi Y, Ding W, Han S, Cao L, Zhu C, et al. Congenital heart disease in a Chinese hospital: pre- and postnatal detection, incidence, clinical characteristics and outcomes. Pediatrics International 2011;53(6):1059-65. [DOI] [PubMed] [Google Scholar]
Yu 2011‐4 {published data only}
- Yu Z, Xi Y, Ding W, Han S, Cao L, Zhu C, et al. Congenital heart disease in a Chinese hospital: pre- and postnatal detection, incidence, clinical characteristics and outcomes. Pediatrics International 2011;53(6):1059-65. [DOI] [PubMed] [Google Scholar]
Zhang 2020 {published data only}
- Zhang N, Dong H, Wang P, Wang Z, Wang Y, Guo Z. The value of obstetric ultrasound in screening fetal nervous system malformation. World Neurosurgery 2020;138:645-53. [DOI] [PubMed] [Google Scholar]
Zhang 2021 {published data only}
- Zhang Y, Zhang W, Xu H, Liu K. Epidemiological aspects, prenatal screening and diagnosis of congenital heart defects in Beijing. Frontiers in Cardiovascular Medicine 2021;8:777899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2014 {published data only}
- Zheng J, Jiao Y, Wang HF, Xiong Y, Lin Q, Xu FH, et al. Ultrasonographic diagnosis of fetal limb abnormalities at 11-13 weeks of gestation. [Chinese]. Chinese Journal of Medical Imaging Technology 2014;30(8):1230-3. [Google Scholar]
References to studies excluded from this review
Abu‐Rustum 2010a {published data only}
- Abu-Rustum RS, Daou L, Abu-Rustum SE. Role of ultrasonography in early gestation in the diagnosis of congenital heart defects. Journal of Ultrasound in Medicine 2010;29(5):817-21. [DOI] [PubMed] [Google Scholar]
Anumba 2005 {published data only}
- Anumba DO, Scott JE, Plant ND, Robson SC. Diagnosis and outcome of fetal lower urinary tract obstruction in the northern region of England. Prenatal Diagnosis 2005;25(1):7-13. [DOI] [PubMed] [Google Scholar]
Becker 2005 {published data only}
- Becker R, Albig M, Gasiorek-Wiens A, Entezami M, Knoll T, Wegner R D. The potential of first trimester anomaly scan and first trimester fetal echocardiography as screening procedures in a medium risk population. Journal of Obstetrics and Gynecology of India 2005;55(3):228-30. [Google Scholar]
Becker 2006 {published data only}
- Becker R, Wegner RD. Detailed screening for fetal anomalies and cardiac defects at the 11-13-week scan. Ultrasound in Obstetrics & Gynecology 2006;27(6):613-8. [DOI] [PubMed] [Google Scholar]
Boyd 1998 {published data only}
- Boyd PA, Chamberlain P, Hicks NR. 6-year experience of prenatal diagnosis in an unselected population in Oxford, UK. Lancet 1998;352(9140):1577-81. [DOI] [PubMed] [Google Scholar]
Boyd 2011a {published data only}
- Boyd PA, Tonks AM, Rankin J, Rounding C, Wellesley D, Draper ES, et al. Monitoring the prenatal detection of structural fetal congenital anomalies in England and Wales: register-based study. Journal of Medical Screening 2011;18(1):2-7. [DOI] [PubMed] [Google Scholar]
Boyd 2012 {published data only}
- Boyd PA, Rounding C, Chamberlain P, Wellesley D, Kurinczuk JJ. The evolution of prenatal screening and diagnosis and its impact on an unselected population over an 18-year period. British Journal of Obstetrics & Gynaecology 2012;119(9):1131-40. [DOI] [PubMed] [Google Scholar]
Brantberg 2017 {published data only}
- Brantberg A, Blaas HG, Haugen SE, Eik-Nes SH. Esophageal obstruction-prenatal detection rate and outcome. Ultrasound in Obstetrics & Gynecology 2007;30(2):180-7. [DOI] [PubMed] [Google Scholar]
Braz 2018 {published data only}
- Braz P, Machado A, Matias Dias, C. The impact of prenatal diagnosis on congenital anomaly outcomes: Data from 1997 to 2016. European Journal of Medical Genetics 2018;61(9):508-12. [DOI] [PubMed] [Google Scholar]
Brenes 2003 {published data only}
- Brenes J, Asenjo JE, Gomez M, Costales C, Santos J Ga, De Marco E, et al. Evaluation of prenatal diagnosis of fetal cardiopathies. [Spanish]. Toko-Ginecologia Practica 2003;62(668):188-95. [Google Scholar]
Bullen 2001 {published data only}
- Bullen PJ, Rankin JM, Robson SC. Investigation of the epidemiology and prenatal diagnosis of holoprosencephaly in the North of England. American Journal of Obstetrics & Gynecology 2001;184(6):1256-62. [DOI] [PubMed] [Google Scholar]
Byrne 2018 {published data only}
- Byrne JJ, Morgan JL, Twickler DM, McIntire DD, Dashe JS. Utility of follow-up standard sonography for fetal anomaly detection. American Journal of Obstetrics & Gynecology 2020;222(6):615.e1-9. [DOI] [PubMed] [Google Scholar]
Campana 2010 {published data only}
- Campana H, Ermini M, Aiello HA, Krupitzki H, Castilla EE, Lopez-Camelo JS. Prenatal sonographic detection of birth defects in 18 hospitals from South America. Journal of Ultrasound in Medicine 2010;29(2):203-12. [DOI] [PubMed] [Google Scholar]
Cash 2001 {published data only}
- Cash C, Set P, Coleman N. The accuracy of antenatal ultrasound in the detection of facial clefts in a low-risk screening population. Ultrasound in Obstetrics & Gynecology 2001;18(5):432-6. [DOI] [PubMed] [Google Scholar]
Cedergren 2006 {published data only}
- Cedergren M, Selbing A. Detection of fetal structural abnormalities by an 11-14-week ultrasound dating scan in an unselected Swedish population. Acta Obstetricia et Gynecologica Scandinavica 2006;85(8):912-5. [DOI] [PubMed] [Google Scholar]
Chatzipapas 1999 {published data only}
- Chatzipapas IK, Whitlow BJ, Economides DL. The 'Mickey Mouse' sign and the diagnosis of anencephaly in early pregnancy. Ultrasound in Obstetrics & Gynecology 1999;13(3):196-9. [DOI] [PubMed] [Google Scholar]
Chelemen 2011 {published data only}
- Chelemen T, Syngelaki A, Maiz N, Allan L, Nicolaides K H. Contribution of ductus venosus Doppler in first-trimester screening for major cardiac defects. Fetal Diagnosis & Therapy 2011;29(2):127-34. [DOI] [PubMed] [Google Scholar]
Chen 2019 {published data only}
- Chen FC, Bacovsky A, Entezami M, Henrich W. Nearly half of all severe fetal anomalies can be detected by first-trimester screening in experts' hands. Journal of Perinatal Medicine 2019;47(6):619-24. [DOI] [PubMed] [Google Scholar]
Chu 2017 {published data only}
- Chu C, Yan Y, Ren Y, Li X, Gui Y. Prenatal diagnosis of congenital heart diseases by fetal echocardiography in second trimester: a Chinese multicenter study. Acta Obstetricia et Gynecologica Scandinavica 2017;96(4):454-63. [DOI] [PubMed] [Google Scholar]
Chua 2000 {published data only}
- Chua LK, Rose DH. The detection rate of major and common foetal heart abnormalities in routine prenatal ultrasonography. Clinical Radiology 2000;55(11):897. [Google Scholar]
Clausen 2022 {published data only}
- Clausen AB, Garne E, Damkjaer M. Live birth prevalence of atrioventricular septal defect after the implementation of new prenatal screening guidelines. Danish Medical Journal 2022;69(2):13. [PubMed] [Google Scholar]
Clementi 2000 {published data only}
- Clementi M, Tenconi R, Bianchi F, Stoll C. Evaluation of prenatal diagnosis of cleft lip with or without cleft palate and cleft palate by ultrasound: experience from 20 European registries. EUROSCAN study group. Prenatal Diagnosis 2000;20(11):870-5. [PubMed] [Google Scholar]
Colosi 2015 {published data only}
- Colosi E, Musone R, Filardi G, Fabbo A. First trimester fetal anatomy study and identification of major anomalies using 10 standardized scans. Journal of Prenatal Medicine 2015;9(3-4):24-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delmas 2017 {published data only}
- Delmas HL, Kohler M, Doray B, Lemery D, Francannet C, Quistrebert J, et al. Congenital unilateral renal agenesis: prevalence, prenatal diagnosis, associated anomalies. Data from two birth-defect registries. Birth Defects Research 2017;109(15):1204-11. [DOI] [PubMed] [Google Scholar]
Duta 2021 {published data only}
- Duta S, Veduta A, Vayna AM, Panaitescu A, Nedelea F, Peltecu G. The outcome of structural heart defects diagnosed in the first trimester of pregnancy. Journal of Maternal-Fetal & Neonatal Medicine 2021;34(9):1389-94. [DOI] [PubMed] [Google Scholar]
Economides 1998 {published data only}
- Economides DL, Braithwaite JM. First trimester ultrasonographic diagnosis of fetal structural abnormalities in a low risk population. British Journal of Obstetrics & Gynaecology 1998;105(1):53-7. [DOI] [PubMed] [Google Scholar]
Eleftheriades 2012 {published data only}
- Eleftheriades M, Tsapakis E, Sotiriadis A, Manolakos E, Hassiakos D, Botsis D. Detection of congenital heart defects throughout pregnancy; impact of first trimester ultrasound screening for cardiac abnormalities. Journal of Maternal-Fetal & Neonatal Medicine 2012;25(12):2546-50. [DOI] [PubMed] [Google Scholar]
Ensing 2014 {published data only}
- Ensing S, Kleinrouweler CE, Maas SM, Bilardo CM, Van der Horst CM, Pajkrt E. Influence of the 20-week anomaly scan on prenatal diagnosis and management of fetal facial clefts. Ultrasound in Obstetrics & Gynecology 2014;44(2):154-9. [DOI] [PubMed] [Google Scholar]
Eurenius 1999 {published data only}
- Eurenius K, Axelsson O, Cnattingius S, Eriksson L, Norsted T. Second trimester ultrasound screening performed by midwives; sensitivity for detection of fetal anomalies. Acta Obstetricia et Gynecologica Scandinavica 1999;78(2):98-104. [PubMed] [Google Scholar]
Everwijn 2018 {published data only}
- Everwijn SMP, Nisselrooij AEL, Rozendaal L, Clur SAB, Pajkrt E, Hruda J, et al. The effect of the introduction of the three-vessel view on the detection rate of transposition of the great arteries and tetralogy of Fallot. Prenatal Diagnosis 2018;38(12):951-7. [DOI] [PubMed] [Google Scholar]
Ficara 2020 {published data only}
- Ficara A, Syngelaki A, Hammami A, Akolekar R, Nicolaides K H. Value of routine ultrasound examination at 35-37 weeks' gestation in diagnosis of fetal abnormalities. Ultrasound in Obstetrics & Gynecology 2020;55(1):75-80. [DOI] [PubMed] [Google Scholar]
Forci 2020 {published data only}
- Forci K, Alami MH, Bouaiti E, Slaoui M, Alaoui AM, Izgua AT. Prevalence of congenital malformations at the "les Orangers" maternity and reproductive health Hospital of Rabat: descriptive study of 470 anomalies. BMC Pediatrics 2020;20(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fricke 2021 {published data only}
- Fricke K, Bhat M, Avdikos V, Asp A, Brodszki J, Thurn L. High prenatal detection rates of complex congenital heart defects (CHD). Lakartidningen 2021;118(11):17. [PubMed] [Google Scholar]
Garne 2001 {published data only}
- Garne E, Eurocat Working Group. Prenatal diagnosis of six major cardiac malformations in Europe--a population based study. Acta Obstetricia et Gynecologica Scandinavica 2001;80(3):224-8. [PubMed] [Google Scholar]
Garne 2002 {published data only}
- Garne E, Haeusler M, Barisic I, Gjergja R, Stoll C, Clementi M, et al. Congenital diaphragmatic hernia: evaluation of prenatal diagnosis in 20 European regions. Ultrasound in Obstetrics & Gynecology 2002;19(4):329-33. [DOI] [PubMed] [Google Scholar]
Grandjean 1998 {published data only}
- Grandjean H, Larroque D, Levi S, Eurofetus Team. Sensitivity of routine ultrasound screening of pregnancies in the Eurofetus database. In: Levi S, Chervenak F A, editors(s). Ultrasound Screening for Fetal Anomalies: Is It Worth It?: Screening Revisited after the Eurofetus Data. 847 edition. New York: New York Acad Sciences, 1998:118-24. [DOI] [PubMed] [Google Scholar]
Haeusler 2002 {published data only}
- Haeusler MC, Berghold A, Stoll C, Barisic I, Clementi M, Group Euroscan Study. Prenatal ultrasonographic detection of gastrointestinal obstruction: results from 18 European congenital anomaly registries. Prenatal Diagnosis 2002;22(7):616-23. [DOI] [PubMed] [Google Scholar]
Hafner 1998 {published data only}
- Hafner E, Scholler J, Schuchter K, Sterniste W, Philipp K. Detection of fetal congenital heart disease in a low-risk population. Prenatal Diagnosis 1998;18(8):808-15. [DOI] [PubMed] [Google Scholar]
Hunter 2000 {published data only}
- Hunter S, Heads A, Wyllie J, Robson S. Prenatal diagnosis of congenital heart disease in the northern region of England: benefits of a training programme for obstetric ultrasonographers. Heart 2000;84(3):294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iliescu 2014 {published data only}
- Iliescu DG, Cara ML, Tudorache S, Antsaklis P, Novac LV, Antsaklis A, et al. Agenesis of ductus venosus in sequential first and second trimester screening. Prenatal Diagnosis 2014;34(11):1099-105. [DOI] [PubMed] [Google Scholar]
Itsukaichi 2018 {published data only}
- Itsukaichi M, Serikawa T, Yoshihara K, Suzuki H, Haino K, Yamaguchi M, et al. Effectiveness of fetal cardiac screening for congenital heart disease using a combination of the four-chamber view and three-vessel view during the second trimester scan. Journal of Obstetrics & Gynaecology Research 2018;44(1):49-53. [DOI] [PubMed] [Google Scholar]
Johnson 2012 {published data only}
- Johnson N, Bishop K, Trotman H, Reid M. Congenital abnormalities at a tertiary center in Jamaica: an 18-month maternal-fetal medicine experience. Journal of Maternal-Fetal & Neonatal Medicine 2012;25(6):687-91. [DOI] [PubMed] [Google Scholar]
Jorgensen 2015 {published data only}
- Jorgensen DE, Vejlstrup N, Jorgensen C, Maroun LL, Steensberg J, Hessellund A, et al. Prenatal detection of congenital heart disease in a low risk population undergoing first and second trimester screening. Prenatal Diagnosis 2015;35(4):325-30. [DOI] [PubMed] [Google Scholar]
Kansal 2022 {published data only}
- Kansal K, Mittal M, Gupta A. Detection of congenital structural anomalies by mid trimester ultrasonography. Journal of Cardiovascular Disease Research 2022;13(1):1671-7. [Google Scholar]
Khoo 2008 {published data only}
- Khoo NS, Van Essen P, Richardson M, Robertson T. Effectiveness of prenatal diagnosis of congenital heart defects in South Australia: a population analysis 1999-2003. Australian & New Zealand Journal of Obstetrics & Gynaecology 2008;48(6):559-63. [DOI] [PubMed] [Google Scholar]
Laurichesse 2017 {published data only}
- Laurichesse Delmas H, Kohler M, Doray B, Lemery D, Francannet C, et al. Congenital unilateral renal agenesis: prevalence, prenatal diagnosis, associated anomalies. Data from two birth-defect registries. Birth Defects Research 2017;109(15):1204-11. [DOI] [PubMed] [Google Scholar]
Levi 2003 {published data only}
- Levi S, Zhang WH, Alexander S, Viart P, Grandjean H. Short-term outcome of isolated and associated congenital heart defects in relation to antenatal ultrasound screening. Ultrasound in Obstetrics and Gynecology 2003;21(6):532-8. [DOI] [PubMed] [Google Scholar]
Li 2019 {published data only}
- Li ZY, Chen YM, Qiu LQ, Chen DQ, Hu CG, Xu JY, et al. Prevalence, types, and malformations in congenital anomalies of the kidney and urinary tract in newborns: a retrospective hospital-based study. Italian Journal of Pediatrics 2019;45(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2016 {published data only}
- Liao YM, Li SL, Luo GY, Wen HX, Ouyang SY, Chen CY, et al. Routine screening for fetal limb abnormalities in the first trimester. Prenatal Diagnosis 2016;36(2):117-26. [DOI] [PubMed] [Google Scholar]
Liao 2021a {published data only}
- Liao Y, Wen H, Luo G, Ouyang S, Bi J, Yuan Y, et al. Fetal open and closed spina bifida on a routine scan at 11 weeks to 13 weeks 6 days. Journal of Ultrasound in Medicine 2021;40(2):237-47. [DOI] [PubMed] [Google Scholar]
Liou 2011 {published data only}
- Liou JD, Huang YH, Hung TH, Hsieh CL, Hsieh TT, Lo LM. Prenatal diagnostic rates and postnatal outcomes of fetal orofacial clefts in a Taiwanese population. International Journal of Gynaecology & Obstetrics 2011;113(3):211-4. [DOI] [PubMed] [Google Scholar]
Long 1998 {published data only}
- Long G, Sprigg A. A comparative study of routine versus selective fetal anomaly ultrasound scanning. Journal of Medical Screening 1998;5(1):6-10. [DOI] [PubMed] [Google Scholar]
Ma 2018 {published data only}
- Ma Y, Zhang J, Yang X. Clinical application of standardized prenatal ultrasound screening during 11-13 weeks gestation. [Chinese]. Chinese Journal of Medical Imaging Technology 2018;34(1):86-9. [Google Scholar]
Magripes 1998 {published data only}
- Magriples U, Copel JA. Accurate detection of anomalies by routine ultrasonography in an indigent clinic population. American Journal of Obstetrics & Gynecology 1998;179(4):978-81. [DOI] [PubMed] [Google Scholar]
Marek 2011 {published data only}
- Marek J, Tomek V, Skovranek J, Povysilova V, Samanek M. Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21-year experience. Heart 2011;97(2):124-30. [DOI] [PubMed] [Google Scholar]
Michailidis 2001 {published data only}
- Michailidis GD, Economides DL. Nuchal translucency measurement and pregnancy outcome in karyotypically normal fetuses. Ultrasound in Obstetrics & Gynecology 2001;17(2):102-5. [DOI] [PubMed] [Google Scholar]
Minnella 2020 {published data only}
- Minnella GP, Crupano FM, Syngelaki A, Zidere V, Akolekar R, Nicolaides KH. Diagnosis of major heart defects by routine first-trimester ultrasound examination: association with increased nuchal translucency, tricuspid regurgitation and abnormal flow in ductus venosus. Ultrasound in Obstetrics & Gynecology 2020;55(5):637-44. [DOI] [PubMed] [Google Scholar]
Nakling 2005 {published data only}
- Nakling J, Backe B. Routine ultrasound screening and detection of congenital anomalies outside a university setting. Acta Obstetricia et Gynecologica Scandinavica 2005;84(11):1042-8. [DOI] [PubMed] [Google Scholar]
Nikkila 2006 {published data only}
- Nikkila A, Rydhstroem H, Kallen B, Jorgensen C. Ultrasound screening for fetal anomalies in southern Sweden: a population-based study. Acta Obstetricia et Gynecologica Scandinavica 2006;85(6):688-93. [DOI] [PubMed] [Google Scholar]
Ou 2022 {published data only}
- Ou Y, Bloom MS, Mai J, Francois M, Pan W, Xiao X, et al. Prenatal detection of congenital heart diseases using echocardiography: 12-year results of an improving program with 9782 cases. Frontiers in Public Health 2022;10:886262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pavlicek 2020 {published data only}
- Pavlicek J, Piegzova A, Klaskova E, Kapralova S, Palatova A, Spacek R, et al. Development, effectiveness, and current possibilities in prenatal detection of congenital heart defects. Cor Et Vasa 2020;62(1):21-8. [Google Scholar]
Pitukkijronnakorn 2009 {published data only}
- Pitukkijronnakorn S, Chittacharoen A, Jetsawangsri T, Panburana P, Jaovisidha A, Roungsipragarn R, et al. The value of mid-trimester routine ultrasonographic screening in antenatal detection of congenital malformations. Journal of the Medical Association of Thailand 2009;92(6):748-53. [PubMed] [Google Scholar]
Poggenpoel 2012 {published data only}
- Poggenpoel EJ, Geerts LT, Theron GB. The value of adding a universal booking scan to an existing protocol of routine mid-gestation ultrasound scan. International Journal of Gynecology & Obstetrics 2012;116(3):201-5. [DOI] [PubMed] [Google Scholar]
Queisser‐Luft 1998 {published data only}
- Queisser-Luft A, Stopfkuchen H, Stolz G, Schlaefer K, Merz E. Prenatal diagnosis of major malformations: quality control of routine ultrasound examinations based on a five-year study of 20,248 newborn fetuses and infants. Prenatal Diagnosis 1998;18(6):567-76. [PubMed] [Google Scholar]
Sainz 2015 {published data only}
- Sainz JA, Zurita MJ, Guillen I, Borrero C, Garcia-Mejido J, Almeida C, et al. Prenatal screening of congenital heart defects in population at low risk of congenital defects. A reality today. Anales de Pediatria 2015;82(1):27-34. [DOI] [PubMed] [Google Scholar]
Stoll 1998 {published data only}
- Stoll C, Alembik Y, Dott B, Meyer MJ, Pennerath A, Peter MO, et al. Evaluation of prenatal diagnosis of congenital heart disease. Prenatal Diagnosis 1998;18(8):801-7. [PubMed] [Google Scholar]
Stoll 2000a {published data only}
- Stoll C, Dott B, Alembik Y, Roth M. Evaluation of prenatal diagnosis of cleft lip/palate by foetal ultrasonographic examination. Annales de Genetique 2000;43(1):11-4. [DOI] [PubMed] [Google Scholar]
Stoll 2000b {published data only}
- Stoll C, Wiesel A, Queisser-Luft A, Froster U, Bianca S, Clementi M. Evaluation of the prenatal diagnosis of limb reduction deficiencies. EUROSCAN Study Group. Prenatal Diagnosis 2000;20(10):811-8. [DOI] [PubMed] [Google Scholar]
Stoll 2001 {published data only}
- Stoll C, Garne E, Clementi M, Group Euroscan Study. Evaluation of prenatal diagnosis of associated congenital heart diseases by fetal ultrasonographic examination in Europe. Prenatal Diagnosis 2001;21(4):243-52. [DOI] [PubMed] [Google Scholar]
Stoll 2002 {published data only}
- Stoll C, Dott B, Alembik Y, De Geeter B. Evaluation and evolution during time of prenatal diagnosis of congenital heart diseases by routine fetal ultrasonographic examination. Annales de Genetique 2002;45(1):21-7. [DOI] [PubMed] [Google Scholar]
Tabor 2003 {published data only}
- Tabor A, Zdravkovic M, Perslev A, Moller LK, Pedersen BL. Screening for congenital malformations by ultrasonography in the general population of pregnant women: factors affecting the efficacy. Acta Obstetricia et Gynecologica Scandinavica 2003;82(12):1092-8. [DOI] [PubMed] [Google Scholar]
Taipale 2003 {published data only}
- Taipale P, Ammala M, Salonen R, Hiilesmaa V. Learning curve in ultrasonographic screening for selected fetal structural anomalies in early pregnancy. Obstetrics & Gynecology 2003;101(2):273-8. [DOI] [PubMed] [Google Scholar]
Tegnander 2006 {published data only}
- Tegnander E, Eik-Nes SH. The examiner's ultrasound experience has a significant impact on the detection rate of congenital heart defects at the second-trimester fetal examination. Ultrasound in Obstetrics & Gynecology 2006;28(1):8-14. [DOI] [PubMed] [Google Scholar]
Tegnander 2006a {published data only}
- Tegnander E, Williams W, Johansen OJ, Blaas HG, Eik-Nes SH. Prenatal detection of heart defects in a non-selected population of 30,149 fetuses--detection rates and outcome. Ultrasound in Obstetrics & Gynecology 2006;27(3):252-65. [DOI] [PubMed] [Google Scholar]
Todros 1997 {published data only}
- Todros T, Faggiano F, Chiappa E, Gaglioti P, Mitola B, Sciarrone A. Accuracy of routine ultrasonography in screening heart disease prenatally. Gruppo Piemontese for Prenatal Screening of Congenital Heart Disease. Prenatal Diagnosis 1997;17(10):901-6. [PubMed] [Google Scholar]
Trines 2013 {published data only}
- Trines J, Fruitman D, Zuo KJ, Smallhorn JF, Hornberger LK, Mackie AS. Effectiveness of prenatal screening for congenital heart disease: assessment in a jurisdiction with universal access to health care. Canadian Journal of Cardiology 2013;29(7):879-85. [DOI] [PubMed] [Google Scholar]
Tudorache 2016a {published data only}
- Tudorache S, Cara M, Iliescu DG, Stoica A, Simionescu C, Novac LV, et al. Fetal kidneys ultrasound appearance in the first trimester - clinical significance and limits of counseling. Current Health Sciences Journal 2016;42(1):19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tudorache 2016b {published data only}
- Tudorache S, Cara ML, Burada F, Simionescu C, Dragoescu AN, Iliescu DG. First trimester diagnostic accuracy of two-dimensional and four-dimensional ultrasound in major congenital heart diseases. Obstetrica si Ginecologie 2016;64(4):221-32. [Google Scholar]
van Allen 2006 {published data only}
- Van Allen MI, Boyle E, Thiessen P, McFadden D, Cochrane D, Chambers GK, et al. The impact of prenatal diagnosis on neural tube defect (NTD) pregnancy versus birth incidence in British Columbia. Journal of Applied Genetics 2006;47(2):151-8. [DOI] [PubMed] [Google Scholar]
van Dorsten 1998 {published data only}
- VanDorsten JP, Hulsey TC, Newman RB, Menard MK. Fetal anomaly detection by second-trimester ultrasonography in a tertiary center. American Journal of Obstetrics & Gynecology 1998;178(4):742-9. [DOI] [PubMed] [Google Scholar]
van Velzen 2015 {published data only}
- Velzen CL, Haak MC, Reijnders G, Rijlaarsdam ME, Bax CJ, Pajkrt E, et al. Prenatal detection of transposition of the great arteries reduces mortality and morbidity. Ultrasound in Obstetrics & Gynecology 2015;45(3):320-5. [DOI] [PubMed] [Google Scholar]
Vavilala 2011 {published data only}
- Vavilala S, Geeta K. The 11-13 weeks scan: where do we stand? A 5-year review at Fernandez Hospital. International Journal of Infertility and Fetal Medicine 2011;2(2):65-9. [Google Scholar]
Westin 2006 {published data only}
- Westin M, Saltvedt S, Bergman G, Kublickas M, Almstrom H, Grunewald C, et al. Routine ultrasound examination at 12 or 18 gestational weeks for prenatal detection of major congenital heart malformations? A randomised controlled trial comprising 36,299 fetuses. British Journal of Obstetrics & Gynaecology 2006;113(6):675-82. [DOI] [PubMed] [Google Scholar]
Wiesel 2005 {published data only}
- Wiesel A, Queisser-Luft A, Clementi M, Bianca S, Stoll C, Group Euroscan Study. Prenatal detection of congenital renal malformations by fetal ultrasonographic examination: an analysis of 709,030 births in 12 European countries. European Journal of Medical Genetics 2005;48(2):131-44. [DOI] [PubMed] [Google Scholar]
Wong 2004 {published data only}
- Wong SF, Welsh A, Chan FY. Outcome of a routine ultrasound screening program in a tertiary center in Australia. International Journal of Gynaecology & Obstetrics 2004;87(2):153-4. [DOI] [PubMed] [Google Scholar]
Additional references
ACOG 2016
AIUM 2013
- American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of fetal echocardiography. Journal of Ultrasound in Medicine 2013;32:1067-82. [DOI] [PubMed] [Google Scholar]
Alldred 2012
- Alldred SK, Deeks JJ, Guo B, Neilson JP, Alfirevic Z. Second trimester serum tests for Down's Syndrome screening. Cochrane Database of Systematic Reviews 2012, Issue 6. Art. No: CD009925. [DOI: 10.1002/14651858.CD009925] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alldred 2017a
- Alldred SK, Takwoingi Y, Guo B, Pennant M, Deeks JJ, Neilson JP, et al. First and second trimester serum tests with and without first trimester ultrasound tests for Down's syndrome screening. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD012599. [DOI: 10.1002/14651858.CD012599] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alldred 2017b
- Alldred SK, Takwoingi Y, Guo B, Pennant M, Deeks JJ, Neilson JP, et al. First trimester ultrasound tests alone or in combination with first trimester serum tests for Down's syndrome screening. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD012600. [DOI: 10.1002/14651858.CD012600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Audibert 2017
- Audibert F, De Bie I, Johnson J-A, Okun N, Wilson DR, Armour C, et al. No. 348-joint SOGC-CCMG guideline: update on prenatal screening for fetal aneuploidy, fetal anomalies, and adverse pregnancy outcomes. Journal of Obstetrics and Gynaecology Canada 2017;39(9):805-17. [DOI] [PubMed] [Google Scholar]
Badeau 2017
- Badeau M, Lindsay C, Blais J, Nshimyumukiza L, Takwoingi Y, Langlois S, et al. Genomics‐based non‐invasive prenatal testing for detection of fetal chromosomal aneuploidy in pregnant women. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD011767. [DOI: 10.1002/14651858.CD011767.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bardi 2019
- Bardi F, Bosschieter P, Verheij J, Go A, Haak M, Bekker M, et al. Is there still a role for nuchal translucency measurement in the changing paradigm of first trimester screening? Prenatal Diagnosis 2019;40:197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyd 2010
- Boyd P, Garne E, Cariati E, Strigini F, Bianchi F, Pierini A. EUROCAT (European Surveillance of Congenital Anomalies) Special Report: Prenatal Screening Policies in Europe. EUROCAT Central Registry, Newtownabbey: University of Ulster 2010.
Boyle 2018
- Boyle B, Addor MC, Arriola L, Barisic I, Bianchi F, Csáky-Szunyogh M, et al. Estimating global burden of disease due to congenital anomaly: an analysis of European data. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018;103:F22-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brent 2004
- Brent RL. Environmental causes of human congenital malformations: the pediatrician’s role in dealing with these complex clinical problems caused by a multiplicity of environmental and genetic factors. Pediatrics 2004;113:957-68. [PubMed] [Google Scholar]
Bricker 2000
- Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al. Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views. Health Technology Assessment 2000;4:7-28. [PubMed]
Buijtendijk 2021
- Buijtendijk M, Shah H, Lugthart MA, Dawood Y, Limpens J, Bakker BS, et al. Diagnostic accuracy of ultrasound screening for fetal structural abnormalities during the first and second trimester of pregnancy in low‐risk and unselected populations. Cochrane Database of Systematic Reviews 2021, Issue 7. Art. No: CD014715. [DOI: 10.1002/14651858.CD014715] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2013
- Campbell S. A short history of sonography in obstetrics and gynaecology. Facts Views & Vision in ObGyn 2013;5(3):213-29. [PMC free article] [PubMed] [Google Scholar]
Crane 1994
- Crane JP, LeFevre ML, Winborn RC, Evans JK, Ewigman BG, Bain RP, et al. A randomized trial of prenatal ultrasonographic screening: impact on the detection, management, and outcome of anomalous fetuses. The RADIUS Study Group. American Journal of Obstetrics & Gynecology 1994;171(2):392-9. [DOI] [PubMed] [Google Scholar]
de Ruigh 2021
- Ruigh AA, Simons NE, Van 't Hooft J, Wassenaer-Leemhuis AG, Aarnoudse-Moens CSH, Wely M, et al. Child outcomes after induction of labour or expectant management in women with preterm prelabour rupture of membranes between 34 and 37 weeks of gestation: study protocol of the PPROMEXIL Follow-up trial. A long-term follow-up study of the randomised controlled trials PPROMEXIL and PPROMEXIL-2. BMJ Open 2021;11(6):e046046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58:882‐93. [DOI] [PubMed] [Google Scholar]
Donofrio 2014
- Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, SklanskyMS, Abuhamad A, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation 2014;129:2183–242. [DOI] [PubMed] [Google Scholar]
Everwijn 2018
- Everwijn SMP, Nisselrooij AEL, Rozendaal L, Clur SAB, Hrunda J, Linskens IH, et al. The effect of the introduction of the three‐vessel view on the detection rate of transposition of the great arteries and tetralogy of Fallot. Prenatal Diagnosis 2018;38:951-7. [DOI] [PubMed] [Google Scholar]
Gao 2010
- Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiological Reviews 2010;90(4):1291-335. [DOI] [PubMed] [Google Scholar]
Karim 2017
- Karim JN, Roberts NW, Salomon LJ, Papageorghiou AT. Systematic review of first-trimester ultrasound screening for detection of fetal structural anomalies and factors that affect screening performance. Ultrasound in Obstetrics & Gynecology 2017;50:429–41. [DOI] [PubMed] [Google Scholar]
King 2007
- King TC. 7 - Cardiovascular Pathology. In: King TC, editors(s). Elsevier's Integrated Pathology. Philadelphia: Mosby, 2007:169-95. [Google Scholar]
Korenromp 2005
- Korenromp MJ, Christiaens GC, den Bout J, Mulder EJ, Hunfeld JA, Bilardo CM, et al. Long‐term psychological consequences of pregnancy termination for fetal abnormality: a cross‐sectional study. Prenatal Diagnosis 2005;25:253-60. [DOI] [PubMed] [Google Scholar]
Koullali 2017
- Koullali B, Kempen LEM, Zijl MD, Naaktgeboren CA, Schuit E, Bekedam DJ, et al. A multi-centre, non-inferiority, randomised controlled trial to compare a cervical pessary with a cervical cerclage in the prevention of preterm delivery in women with short cervical length and a history of preterm birth – PC study. BMC Pregnancy and Childbirth 2017;17(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavelanet 2018
- Lavelanet AF, Schlitt S, Johnson BR Jr, Ganatra B. Global Abortion Policies Database: a descriptive analysis of the legal categories of lawful abortion. BMC International Health and Human Rights 2018;18(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2018
- Lewis C, Hill M, Arthurs OJ, Hutchinson C, Chitty LS, Sebire NJ. Factors affecting uptake of postmortem examination in the prenatal, perinatal and paediatric setting. British Journal of Obstetrics & Gynaecology 2018;125:172-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2016
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016;388:3027-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and Presenting Results. In: Deeks JJ, Bossuyt PM, Gatsonis C (editors), Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2010. Available from methods.cochrane.org/sdt/handbook-dta-reviews.
March Of Dimes 2006
- Christianson A, Howson CP, Modell B. March of Dimes: global report on birth defects. www.marchofdimes.org (accessed 12 March 2020).
Mol 1999
- Mol BW, Lijmer JG, Meulen J, Pajkrt E, Bilardo CM, Bossuyt PM. Effect of study design on the association between nuchal translucency measurement and Down syndrome. Obstetrics and Gynecology 1999;94(5 Pt 2):864-9. [DOI] [PubMed] [Google Scholar]
Moorthie 2018
- Moorthie S, Blencowe H, Darlison MW, Lawn J, Morris JK, Modell B, et al. Estimating the birth prevalence and pregnancy outcomes of congenital malformations worldwide. Journal of Community Genetics 2018;9:387-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Care Excellence (UK). Antenatal care: routine care for the healthy pregnant woman (NICE Clinical Guidelines, No. 62). London: RCOG Press, 2008. [PubMed] [Google Scholar]
Olde Scholtenhuis 2003
- Olde Scholtenhuis MA, Cohen-Overbeek TE, Offringa M, Barth PG, Stoutenbeek P, Gooskens RH, et al. Audit of prenatal and postnatal diagnosis of isolated open spina bifida in three university hospitals in The Netherlands. Ultrasound in Obstetrics & Gynecology 2003;21:48-52. [DOI] [PubMed] [Google Scholar]
Public Health England 2018
- Public Health England. NHS Fetal Anomaly Screening Programme Handbook. https://www.gov.uk/government/publications/fetal-anomaly-screening-programme-handbook 2018.
RCOG 1997
- The Royal College of Obstetricians and Gynaecologists. Ultrasound screening for fetal abnormalities. Report of the RCOG Working Party. London: RCOG Press, 1997. [Google Scholar]
RCOG 2014
- PW Soothill, Lo YMD, on behalf of the Royal College of Obstricians and Gynaecologists. Scientific Impact Paper No. 15: Non-invasive prenatal testing for chromosomal abnormality using maternal plasma DNA. The Obstetrician & Gynaecologist 2014;16(2):148-148. [Google Scholar]
RevMan 2024 [Computer program]
- Review Manager (RevMan). Version 7.2.0. The Cochrane Collaboration, 2024. Available at revman.cochrane.org.
Salomon 2011
- Salomon LJ, Alfirevic Z, Berghella V, Bilardo C, Hernandez-Andrade E, Johnsen SL, et al. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound in Obstetrics & Gynecology 2011;37(1):116-26. [DOI] [PubMed] [Google Scholar]
Salomon 2013
- Salomon LJ, Alfirevic Z, Bilardo CM, Chalouhi GE, Ghi T, Kagan KO, et al. ISUOG practice guidelines: performance of first-trimester fetal ultrasound scan. Ultrasound in Obstetrics & Gynecology 2013;41(1):102-13. [DOI] [PubMed] [Google Scholar]
Saltvedt 2006
- Saltvedt S, Almström H, Kublickas M, Valentin L, Grunewald C. Detection of malformations in chromosomally normal fetuses by routine ultrasound at 12 or 18 weeks of gestation—a randomised controlled trial in 39 572 pregnancies. British Journal of Obstetrics & Gynaecology 2006;113:664–74. [DOI] [PubMed] [Google Scholar]
Souka 2005
- Souka AP, Von Kaisenberg CS, Hyett JA, Sonek JD, Nicolaides KH. Increased nuchal translucency with normal karyotype. American Journal of Obstetrics and Gynecology 2005;192:1005–21. [DOI] [PubMed] [Google Scholar]
Syngelaki 2019
- Syngelaki A, Hammami A, Bower S, Zidere V, Akolekar R, Nicolaides KH. Diagnosis of fetal non-chromosomal abnormalities on routine ultrasound examination at 11-13 weeks’ gestation. Ultrasound in Obstetrics & Gynecology 2019;54:468-76. [DOI] [PubMed] [Google Scholar]
van den Hof 2019
- Van den Hof MC, Smithies M, Nevo O, Oullet A. No. 375- clinical practice guideline on the use of first trimester ultrasound. Journal of Obstetrics and Gynaecology Canada 2019;41(3):388-95. [DOI] [PubMed] [Google Scholar]
van der Linde 2011
- Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide. Journal of the American College of Cardiology 2011;58(21):2241-7. [DOI] [PubMed] [Google Scholar]
Ward 2011
- Ward P, Soothill P. Fetal anomaly ultrasound scanning: the development of a national programme for England. The Obstetrician & Gynaecologist 2011;13:211-17. [Google Scholar]
Wax 2014
- Wax J, Minkoff H, Johnson A, Coleman B, Levine D, Helfgott A, et al. Consensus report on the detailed fetal anatomic ultrasound examination: indications, components, and qualifications. Journal of Ultrasound in Medicine 2014;33:189-95. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. Quadas-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155:529-36. [DOI] [PubMed] [Google Scholar]
WHO 2021
- Global Health Observatory (GHO) data: causes of child death. https://apps.who.int/gho/data/node.main.COCD?lang=en (accessed 6 July 2021).
WHO/CDC/ICBDSR 2014
- International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR), National Center on Birth Defects and Developmental Disabilities from the United States Centers for Disease Control and Prevention (CDC), World Health Organization (WHO). Birth Defects Surveillance: A Manual for Programme Managers. Geneva: World Health Organization, 2014. [Google Scholar]
World Bank
- Country Classifications by Income: FY 2021-2022. World Development Indicators - The World by Income and Region (worldbank.org) 2023.
World Fertility Data 2008
- United Nations, Department of Economic and Social Affairs, Population Division. Annual number of births and crude birth rate the 2008 revision of World Fertility Data. www.un.org/en/development/desa/population/publications/dataset/fertility/data.asp (accessed 6 July 2021).
Yoo 1997
- Yoo S J, Lee YH, Kim ES, Ryu HM, Kim MY, Choi HK, et al. Three-vessel view of the fetal upper mediastinum: an easy means of detecting abnormalities of the ventricular outflow tracts and great arteries during obstetric screening. Ultrasound in Obstetrics & Gynecology 1997;9(3):173-82. [DOI] [PubMed] [Google Scholar]