Abstract
Peritoneal lymphomatosis (PL) is a rare lymphoma-associated condition defined as the dissemination of lymphoma cells in the peritoneum. An 82-year-old man presented with abdominal pain, heartburn, and high fever. Radiological findings, including positron emission tomography-computed tomography (PET-CT), and gastrointestinal fiberscopy, showed diffuse thickening of the peritoneum, omentum, and mesentery; however, no lymphadenopathy, hepatosplenomegaly, or gastrointestinal lesions were observed. Under suspicion of peritonitis carcinomatosa of unknown origin, exploratory laparoscopy was performed that revealed multiple white nodules and masses on the surfaces of the peritoneum, mesentery, and intestinal serosa. The histopathological and cytogenetic findings of the peritoneum revealed high-grade B-cell lymphoma, not otherwise specified, and a gain of MYC by fluorescence in-situ hybridization. The patient was treated with two cycles of R-CHOP therapy, followed by six cycles of dose-adjusted EPOCH-R therapy, and a complete metabolic response was confirmed by PET-CT. Since there are no specific radiological findings to confirm the diagnosis of PL, a histopathological diagnosis is usually required. Most PL exhibit an aggressive lymphoma phenotype and can be cured by appropriate chemotherapy. Therefore, early diagnosis and treatment are desirable.
Keywords: peritoneal lymphomatosis, high-grade B-cell lymphoma, MYC, DA-EPOCH-R
INTRODUCTION
Peritoneal lymphomatosis (PL) refers to the dissemination of lymphoma cells in the peritoneum.1 Although PL may rarely be observed in the advanced stage of lymphoma, development of PL at initial diagnosis is very rare, reportedly occurring in 0.75% of all lymphoma cases.2 As radiological findings of PL resemble those of peritonitis carcinomatosa (PC), diagnosis of PL without histopathological findings is difficult.3–5 The prognosis of PC is generally poor, whereas PL can be cured by appropriate chemotherapy. Thus, early and accurate diagnosis of PL is crucial.
High-grade B-cell lymphoma (HGBL), not otherwise specified (NOS), is a rare aggressive mature B-cell lymphoma accounting for 1–2% of non-Hodgkin’s lymphoma.6 This diagnostic category was first introduced in the 2016 revised fourth edition of the World Health Organization (WHO) classification, and retained in the 2022 fifth edition of the WHO classification.7,8 HGBL, NOS, lymphoma cells have Burkitt-like or blastoid morphology and do not carry concurrent MYC and BCL2 and/or BCL6 rearrangements.7–9 Single MYC rearrangements are observed in 8–45% of HGBL, NOS, cases, whereas MYC amplification is observed in 11–32% of cases.6,9,10
Here, we present a PL case, in which the histopathological diagnosis of HGBL, NOS, accompanied by MYC alteration was determined from a laparoscopic biopsy specimen. Subsequent intensive chemotherapy induced complete remission of the disease.
CASE PRESENTATION
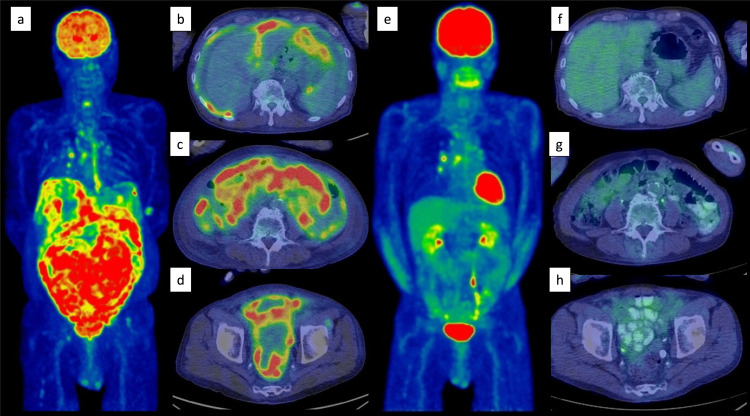
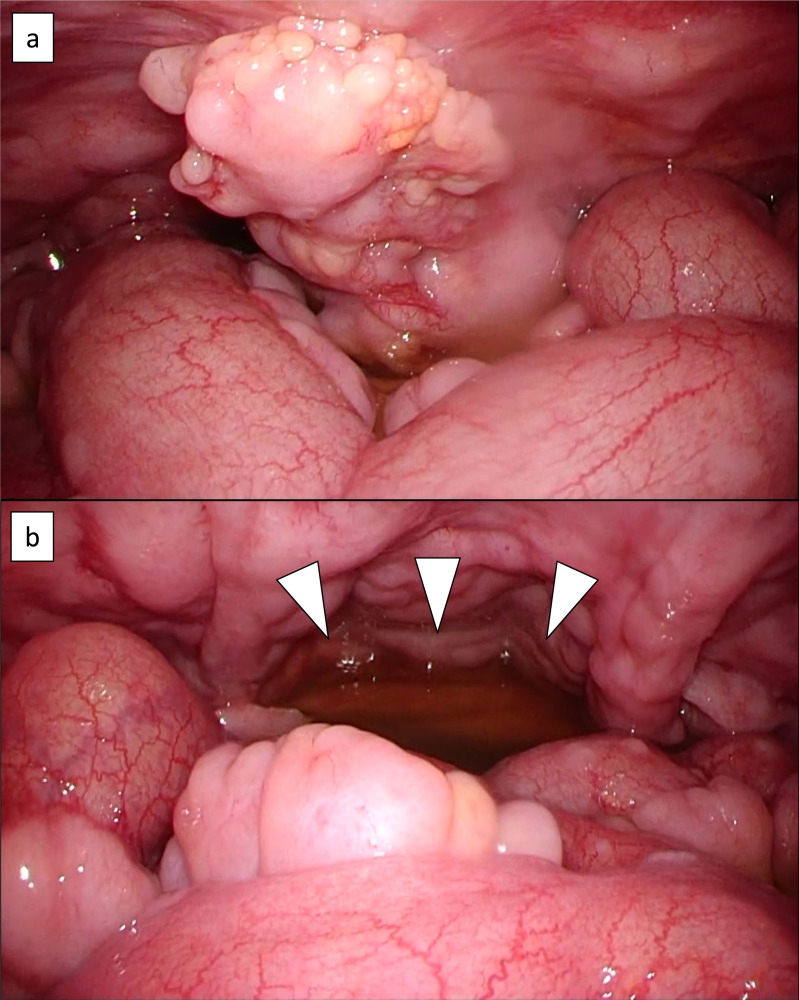
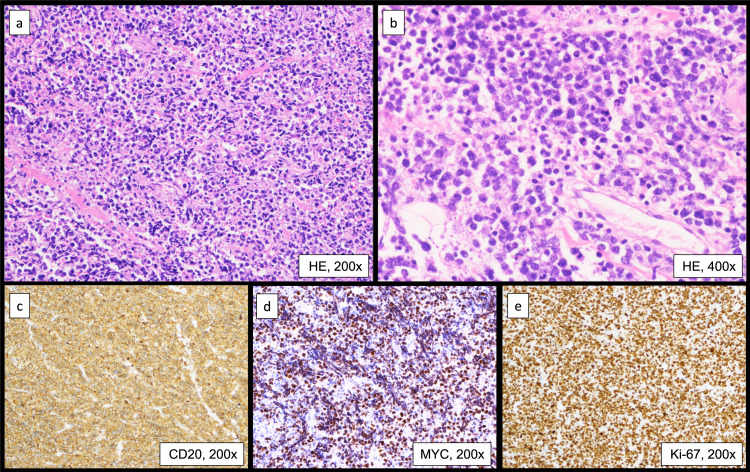
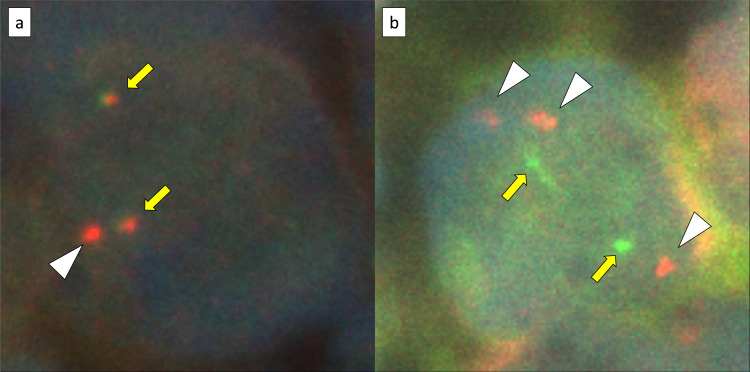
An 82-year-old man presented with a one-month history of abdominal distension and heartburn. The patient had a medical history of distal gastrectomy for a gastric ulcer at the age of 35 years and had been treated for hypertension, dyslipidemia, hyperuricemia, and post-cerebral infarction without paresis. Esophagogastroduodenoscopy showed hiatal herniation and gastritis; therefore, a proton pump inhibitor was prescribed. However, high fever and abdominal pain emerged after 2 weeks, leading to hospitalization. Laboratory data on admission were as follows: normal complete blood count, lactate dehydrogenase 333 U/L, serum creatinine 2.22 mg/dL, soluble interleukin-2 receptor 6,240 U/mL (reference range 157–474 U/mL), and CA125 435.5 U/mL (reference range <35 U/mL). Positron emission tomography-computed tomography (PET-CT) showed diffuse thickening of the peritoneum, omentum, and mesentery, with a maximum standardized uptake value (SUVmax) of 9.8 for 18F-fluorodeoxyglucose (FDG) accumulation (Figure 1a–d). A small amount of ascites was also observed; however, pleural effusion, lymphadenopathy, hepatosplenomegaly, or solid organ lesions were not detected (Figure 1a–d). Mild FDG accumulation in the mediastinal lymph nodes (SUVmax = 5.1) was also detected, suggesting reactive lymphadenopathy (Figure 1a). Total colonoscopy, including that of the terminal ileum, revealed no abnormalities. Approximately 2 weeks after admission, exploratory laparoscopy was performed under the suspicion of PC of unknown origin. Laparoscopy revealed multiple white nodules and masses on the surfaces of the peritoneum, mesentery, and intestinal serosa (Figure 2a, b). Ascites was also observed in the pelvic cavity (Figure 2b). Histopathological examination of the peritoneal biopsy specimen revealed diffuse proliferation of medium-sized cells with an increase in the nuclear: cytoplasmic ratio. The cells were relatively uniform in size and had hyperchromatic nuclei, without conspicuous nucleoli. Coarse nuclear chromatin and marked nuclear pleomorphism, which are often observed in typical DLBCL, were not prominent (Figure 3a, b). Immunohistochemically, these cells stained positive for CD10, 20, BCL6, and MYC and negative for CD3, CD5, CD23, CD30, BCL2, MUM1, and CyclinD1 (Figure 3c, d). The Ki-67 index of the tumor cells was approximately 95% (Figure 3e). EBV encode small RNA in-situ hybridization (ISH) was negative. Chromosomal analysis by G-banding was not performed. The histopathological diagnosis was HGBL, and required confirmation via cytogenetic analysis. Fluorescence ISH (FISH) of formalin-fixed paraffin-embedded tissues was used to detect MYC, BCL2, and BCL6 gene rearrangements. The MYC gene status was analyzed using the Vysis LSI-MYC dual color break-apart rearrangement probe (Abbott Laboratories, Des Plaines, IL, USA) and Vysis LSI IGH/MYC dual fusion probe (Abbott). The MYC break-apart probe kit contains the LSI-MYC SpectrumOrange and LSI-MYC SpectrumGreen probes. The LSI-MYC SpectrumOrange probe begins 119 kb centromeric to the 5’ of the MYC gene and extends 260 kb towards the centromere. The LSI-MYC SpectrumGreen probe starts approximately 1.5 Mb telomeric to the 3’ of MYC gene and extends towards the telomere for about 400 kb. The Vysis LSI IGH/MYC dual fusion probe kit contains an approximately 821 kb SpectrumOrange probe and an approximately 1.6 Mb SpectrumGreen probe, which cover the MYC and IGH regions, respectively. The MYC break-apart probe showed an extra red signal besides two sets of red and green fusion signals, in 84.0% of the cells (Figure 4a). The IGH-MYC dual-fusion probe showed no fusion signal; however, three isolated red signals and two green signals were observed, in most of the analyzed cells (Figure 4b). FISH analyses of both BCL2 and BCL6 rearrangements yielded negative results. Analyses of MYC-IGK and MYC-IGL translocations could not be performed at our institution. These results indicated a gain of the MYC gene accompanied by a green signal loss. Owing to the diagnosis of aggressive B-cell lymphoma, two cycles of rituximab plus miniCHOP (R-miniCHOP) therapy were administered, which resulted in lymphoma remission. Based on the additional cytogenetic analysis, we diagnosed the patient with HGBL, NOS, according to the revised fourth edition of the WHO classification, and the treatment regimen was switched to dose-adjusted (DA)-EPOCH-R therapy. Finally, the patient completed six courses of DA-EPOCH-R therapy, and complete metabolic remission was confirmed using PET-CT (Figure 1e–h). Currently, the patient remains without recurrence for two years after completing chemotherapy.
Fig. 1.
Positron emission tomography-computed tomography (PET-CT) findings: (a–d) at the initial diagnosis and (e–h) after DA-EPOCH-R therapy completion. PET-CT at the initial diagnosis shows diffuse accumulation of 18F-fluorodeoxyglucose (FDG) in the thickened peritoneum, omentum, and mesentery with a maximum standardized uptake value of 9.8. No lymphadenopathy, hepatosplenomegaly, or solid organ lesions are observed (a–d). Mild FDG accumulation detected in the mediastinal lymph node (SUV max = 5.1), suggestive of reactive lymphadenopathy (a). Increased 18F-FDG uptake in the lower esophagus is also visible, compatible with esophagitis (a). All abnormal findings associated with PL are resolved (e–h).
Fig. 2.
Laparoscopy findings: Multiple white nodules and masses on the surfaces of the peritoneum, mesentery, and intestinal serosa are observed (a, b). A small amount of ascites is also visible (white arrow head) (b).
Fig. 3.
Histopathological examinations of a peritoneal biopsy specimen: (a) hematoxylin and eosin staining (H&E) ×200, (b) H&E ×400, (c) CD20 ×200, (d) MYC ×200, (e) Ki-67 labeling index ×200. Diffuse proliferation of medium-sized cells with an increase in the nuclear: cytoplasmic ratio is observed. These cells are relatively uniform in size and have hyperchromatic nuclei without conspicuous nucleoli. Coarse nuclear chromatin and marked nuclear pleomorphism, which are often observed in typical DLBCL, are not prominent (a, b). Tumor cells are positive for CD20 and MYC (c, d). The Ki-67 labeling index is approximately 95% (e).
Fig. 4.
MYC gene status analysis by fluorescence in situ hybridization using formalin-fixed paraffin-embedded tissues: (a) the Vysis LSI-MYC dual color break-apart rearrangement probe (Abbott Laboratories), which contains LSI-MYC SpectrumOrange and LSI-MYC SpectrumGreen probes. The LSI-MYC SpectrumOrange probe begins 119 kb centromeric to the 5’ of the MYC gene and extends 260 kb towards the centromere. The LSI-MYC SpectrumGreen probe starts approximately 1.5 Mb telomeric to the 3’ of MYC gene and extends towards the telomere for about 400 kb. (b) the Vysis LSI IGH/MYC dual fusion probe (Abbott Laboratories), which contains an approximately 821 kb of the SpectrumOrange probe and an approximately 1.6 Mb of the SpectrumGreen probe, which cover the MYC and IGH regions, respectively. The MYC break-apart probe shows an extra red signal (white arrow head) besides two sets of red and green fusion signals (yellow arrow) in 84.0% of the cells (a). The IGH-MYC dual-fusion probe shows no fusion signal, however, three isolated red signals (white arrow head) and two green signals (yellow arrow) are observed in most analyzed cells (b).
DISCUSSION
In the present case, because radiological findings, including PET-CT, provided only a suspected diagnosis of PC of unknown origin, exploratory laparoscopy was performed immediately. Consequently, a diagnosis of PL was made, and the patient received appropriate chemotherapy, which resulted in complete remission. Although no specific radiological findings to confirm the diagnosis of PL existed, characteristic CT findings of PL have been reported.11,12 The frequencies of the peritoneal, omental, and mesenteric thickness in PL cases were 91–94%, 67–95%, and 67%, respectively.11,12 In addition, lymphadenopathy, hepatosplenomegaly, digestive tract wall thickening, ascites, pleural effusion, and solid organ involvement were detected in 63–77%, 27–69%, 67%, 75–82%, 64%, and 56% of PL cases, respectively.11,12 Regarding a differential diagnosis based upon the above radiological findings, the following disorders should be considered: PL, PC, peritoneal sarcomatosis, tuberculosis peritonitis, pseudomyxoma peritonei, and mesothelioma.3,4,13 As PC is the most common, some helpful features for distinguishing PL from PC have been reported.5,12 First, peritoneal lesions of PL are larger and more homogenous on contrast-enhanced CT than those of PC.5 Second, PL often shows diffuse lymphadenopathy, whereas those of PC are usually located around the primary tumor.5 Finally, splenomegaly is noted more frequently in PL patients (27%) than that in PC patients (2%).12 In the present case, although diffuse thickening of the peritoneum, omentum, and mesentery was observed, neither lymphadenopathy or splenomegaly were not noted. That is, no typical CT findings suggestive of PL were noted, which required a diagnosis based on histopathological findings.
Since 2001, 45 PL cases have been reported in detail in the English-language literature. A summary of the 46 cases, including this case, is presented in Table 1.14–52 The median age at disease onset was 55 years, with a male predominance. The major histopathological types included DLBCL in 20 cases, Burkitt’s lymphoma (BL) in 11, follicular lymphoma in 3, and BL-like lymphoma in 2. Approximately 83% of cases (38/46) manifested an aggressive phenotype. Lymphadenopathy was observed in 16 cases (34.8%) and was primarily restricted to the intraperitoneal cavity (14 cases; 30.4%). Moreover, digestive tract infiltration was observed in 12 cases (26.1%): 6 in the stomach, 6 in the small intestine, and 1 in the colon. Splenomegaly was observed in only 7 cases (15.2%) (Table 1).
Table 1. A summary of reported cases of peritoneal lymphomatosis since 2001.
| n | % | |
|---|---|---|
| Case number | 46 | 100 |
| Age, years | ||
| Median | 55 | |
| Min-max | 4–89 | |
| Gender | ||
| Man | 35 | 76.0 |
| Woman | 11 | 24.0 |
| Pathology | ||
| Diffuse large B-cell lymphoma | 20 | 43.5 |
| Burkitt lymphoma | 11 | 23.9 |
| Burkitt-like lymphoma | 2 | 4.3 |
| High-grade B-cell lymphoma, NOS | 1 | 2.2 |
| High-grade T-cell lymphoma | 1 | 2.2 |
| Plasmablastic lymphoma | 1 | 2.2 |
| B lymphoblastic lymphoma | 1 | 2.2 |
| T lymphoblastic lymphoma | 1 | 2.2 |
| Follicular lymphoma | 3 | 6.5 |
| B-cell lymphoma | 3 | 6.5 |
| T-cell lymphoma | 2 | 4.3 |
| Sites of involvement | ||
| Lymphadenopathy | 16 | 34.8 |
| Intraperitoneal lymphadenopathy | 14 | 30.4 |
| Splenomegaly | 7 | 15.2 |
| Digestive tract | 12 | 26.1 |
| Stomach | 6 | 13.0 |
| Small intestine | 6 | 13.0 |
| Large intestine | 1 | 2.2 |
The rarity of PL may be contributed to by the absence of lymphoid tissues in the peritoneum.4 Regarding the primary site of PL, a substantial number of cases may originate from the gastrointestinal epithelium. As described above, 26.1% of PL cases exhibit digestive wall infiltration (Table 1), and a previous report described that approximately two-thirds of PL cases exhibit bowel wall thickening.11 The pathway by which lymphoma cells invade the peritoneum has not clearly elucidated. In cases of gastrointestinal lymphoma, possible pathways have been presumed, including the gastrocolic ligament, transverse mesocolon, and visceral peritoneal surface.4,5 Notably, in the present case, lymphadenopathy, hepatosplenomegaly, gastrointestinal lesions, or a solid organ mass, which could be the origin of the PL, were not identified by detailed examinations using PET-CT and gastrointestinal fiberscopy. Thus, isolated peritoneal, omental, and mesenteric lesions were the only and primary lesions of PL. Among the 46 cases listed in Table 1, 13 presented with no lymphoma lesions other than PL.16,24,27,29,33,37,38,43–45,49 All 13 cases, including the present case, are listed in Table 2. However, it should be considered that not all of the 13 cases underwent sufficient examinations, probably due to their poor general condition or regional differences (Table 2). In particular, PET-CT was performed in only four cases, and gastrointestinal fiberscopy results have not been described, except in the present case (Table 2). All patients exhibited an aggressive phenotype (Table 2). Eight patients received CHOP-based therapy and one patient received EPOCH therapy as induction therapy; however, lymphoma remission was observed in only six patients (Table 2). Six of the 13 patients died due to disease progression or tumor lysis syndrome (Table 2). The poor prognosis of primary PL may be attributed to its aggressive clinical course and difficulty in diagnosis owing to the absence of lymphadenopathy, hepatosplenomegaly, gastrointestinal lesions, and solid organ involvement. Reports regarding the origin of the lymphoma cells in patients with primary PL are lacking. It is speculated that in such cases, the primary lesion may spontaneously regress before PL development, or that physicians may fail to detect the primary lesion owing to insufficient examinations.
Table 2. Reported cases of primary peritoneal lymphomatosis.
| Case | Age | Sex | Pathological Diagnosis | PET-CT | Gastrointestinal Fiber Scope | Diagnostic Procedure | Treatment | Outcome | Reported year | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | Male | High grade T-cell lymphoma | ND | ND | Autopsy | Not done | Dead by lymphoma | 2009 | 16 |
| 2 | 38 | Male | Burkitt-like lymphoma | Done | ND | Omentum, biopsy, US-guided | Unknown | Unknown | 2012 | 24 |
| 3 | 12 | Male | Burkitt lymphoma | Done | ND | Biopsy | Chemotherapy | Remission | 2013 | 27 |
| 4 | 57 | Female | DLBCL | ND | ND | Peritoneum, biopsy, laparoscopy | Not done | Dead by lymphoma | 2014 | 29 |
| 5 | 67 | Female | DLBCL | ND | ND | Peritoneum, biopsy, CT-guided | R-CHOP/MA | Remission | 2016 | 33 |
| 6 | 46 | Male | DLBCL | ND | ND | Peritoneum, biopsy, CT-guided | R-CHOP/MA | Remission | 2016 | 33 |
| 7 | 61 | Male | DLBCL | ND | ND | Omentum/Peritoneum, biopsy, US-guided | R-CHOP/DA-EPOCH-R | Dead by lymphoma | 2019 | 37 |
| 8 | 62 | Male | DLBCL | ND | ND | Omentum, biopsy, US-guided | R-CHOP | Remission | 2019 | 38 |
| 9 | 16 | Male | DLBCL | ND | ND | Omentum, biopsy, laparoscopy | R-CHOP | Dead by lymphoma | 2021 | 43 |
| 10 | 74 | Male | DLBCL | ND | ND | Peritoneum, biopsy, US-guided | R-CHOP | Dead by TLS | 2022 | 44 |
| 11 | 63 | Male | DLBCL | Done | ND | Chest wall, biopsy | EPOCH-R | Remission | 2022 | 45 |
| 12 | 16 | Male | Burkitt lymphoma | ND | ND | Omentum/Peritoneum, biopsy, laparoscopy | CHOP | Dead by lymphoma | 2022 | 49 |
| 13 | 82 | Male | HGBL, NOS | Done | Done | Peritoneum, biopsy, laparoscopy | R-CHOP/DA-EPOCH-R | Remission | Current case | |
• Primary peritoneal lymphomatosis indicates that the absence of lymphoma lesions other than peritoneal lymphomatosis.
• Abbreviations: DLBCL, diffuse large B-cell lymphoma; HGBL, high-grade B-cell lymphoma; NOS, not otherwise specified; PET-CT, Positron emission tomography-computed tomography; ND, not described; US, ultrasonography; CT, computed tomography; MA, methotrexate and cytarabine; TLS, tumor lysis syndrome; Ref, reference.
In the present case, FISH analysis demonstrated an increase in MYC copy number, whereas BCL2 and BCL6 rearrangements were not detected. As the histopathological findings indicated HGBL rather than DLBCL or BL, we diagnosed the patient with HGBL, NOS, accompanied by a gain of the MYC gene. The diagnosis of HGBL, NOS, is based on the diagnostic category of the revised fourth edition of the WHO classification, and this diagnosis is also applied in the fifth edition of the WHO classification published in 2022.7,8 Regarding the FISH analysis of the present case, the break-apart rearrangement probe for MYC gene showed an unbalanced pattern of an extra red signal, in addition to, two fusion signals. This pattern denotes gain of the region covered by the red probe and deletion of the telomeric region covered by the green probe. Collinge et al. analyzed the 531 DLBCL and HGBL cases in which FISH MYC break-apart probe showed unbalanced patterns.53 In that report, they detected the breakpoint locations and MYC-rearranged partners by using whole genome or capture sequencing.53 In nine cases of loss of green signal, the breakpoint location was telomeric to MYC in all of the cases, namely, the MYC gene was located on the derivative chromosome containing the red signal.53 In addition, 64% of them had various MYC-rearrangement partners, including PAX5 or BCL6 (29%), IGL or IGK (14%), IGH (7%), and other non-recurrent partners (14%).53 Thus, the present case had an extra copy of the MYC gene in the region covered by the red probe, and the amplified MYC gene might have rearranged to some genes other than IGH.
Two cycles of R-miniCHOP therapy were initiated based on the initial histopathological diagnosis of aggressive B-cell lymphoma. Furthermore, based on the histopathological and cytogenic analysis results, the treatment regimen was changed to more intensive DA-EPOCH-R therapy. Currently, DA-EPOCH-R therapy is utilized in elderly patients with BL, with promising outcomes.54 In addition, this regimen induced durable remission in 53 patients with DLBCL and HGBL with MYC rearrangement, in a prospective multicenter phase 2 study.55 Recently, Zayac et al. reported a multi-institutional retrospective study of 160 patients with HGBL, NOS, in which MYC rearrangement, and MYC extra copies were observed in 28%, and 11% of the patients, respectively.6 DA-EPOCH-R therapy was used in up to 43% of the patients as a remission induction therapy.6 The prognosis of the patients with MYC rearrangement or MYC extra copies was not statistically inferior to that in patients without MYC alteration, irrespective of treatment regimens, including DA-EPOCH-R.6 In the present case, as the histopathological diagnosis is HGBL, NOS, and the tumor had a possibility of MYC rearrangement, indication of DA-EPOCH-R therapy was appropriate. DA-EPOCH-R therapy was effective and well tolerated, resulting in long-term remission of the lymphoma.
The prognosis of PC is generally poor, whereas remission can be induced in PL with appropriate chemotherapy because most PL manifest as aggressive phenotypes. Several PL case reports described initial misdiagnosis of PC rather than PL, which could be a risk factor for delay in initiating chemotherapy, leading to treatment failure.14,20,26,32,43 In particular, as described above, the diagnosis of primary PL might be more difficult and all case reports had aggressive phenotypes. PL should be considered in the differential diagnosis of peritoneal lesions, even in the absence of other lymphoma lesions. Furthermore, subsequent prompt histopathological examinations and appropriate chemotherapy, such as DA-EPOCH-R therapy, lead to better patient outcomes.
ACKNOWLEDGMENTS
None.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Runyon BA, Hoefs JC. Peritoneal lymphomatosis with ascites. A characterization. Arch Intern Med. 1986; 146: 887-888. [PubMed] [Google Scholar]
- 2.Glazer HS, Lee JK, Balfe DM, et al. Non-Hodgkin lymphoma: computed tomographic demonstration of unusual extranodal involvement. Radiology. 1983; 149: 211-217. [DOI] [PubMed] [Google Scholar]
- 3.Diop AD, Fontarensky M, Montoriol PF, Da Ines D. CT imaging of peritoneal carcinomatosis and its mimics. Diagn Interv Imaging. 2014; 95: 861-872. [DOI] [PubMed] [Google Scholar]
- 4.Vicens RA, Patnana M, Le O, et al. Multimodality imaging of common and uncommon peritoneal diseases: a review for radiologists. Abdom Imaging. 2015; 40: 436-456. [DOI] [PubMed] [Google Scholar]
- 5.Cabral FC, Krajewski KM, Kim KW, Ramaiya NH, Jagannathan JP. Peritoneal lymphomatosis: CT and PET/CT findings and how to differentiate between carcinomatosis and sarcomatosis. Cancer Imaging. 2013; 13: 162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zayac AS, Landsburg DJ, Hughes ME, et al. High-grade B-cell lymphoma, not otherwise specified: a multi-institutional retrospective study. Blood Adv. 2023; 7: 6381-6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kluin PM, Harris NL, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed, Lyon, IARC. 2017; pp. 335-341. [Google Scholar]
- 8.Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022; 36: 1720-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olszewski AJ, Kurt H, Evens AM. Defining and treating high-grade B-cell lymphoma, NOS. Blood. 2022; 140: 943-954. [DOI] [PubMed] [Google Scholar]
- 10.Moore EM, Aggarwal N, Surti U, Swerdlow SH. Further exploration of the complexities of large B-cell lymphomas with MYC abnormalities and the importance of a blastoid morphology. Am J Surg Pathol. 2017; 41: 1155-1166. [DOI] [PubMed] [Google Scholar]
- 11.Karaosmanoglu D, Karcaaltincaba M, Oguz B, et al. CT findings of lymphoma with peritoneal, omental and mesenteric involvement: peritoneal lymphomatosis. Eur J Radiol. 2009; 71: 313-317. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill AC, Shinagare AB, Rosenthal MH, et al. Differences in CT features of peritoneal carcinomatosis, sarcomatosis, and lymphomatosis: retrospective analysis of 122 cases at a tertiary cancer institution. Clin Radiol. 2014; 69: 1219-1227. [DOI] [PubMed] [Google Scholar]
- 13.Cho JH, Kim SS. Peritoneal carcinomatosis and its mimics: review of CT findings for differential diagnosis. J Belg Soc Radiol. 2020; 104: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horger M, Müller-Schimpfle M, Yirkin I, Wehrmann M, Claussen CD. Extensive peritoneal and omental lymphomatosis with raised CA 125 mimicking carcinomatosis: CT and intraoperative findings. Br J Radiol. 2004; 77: 71-73. [DOI] [PubMed] [Google Scholar]
- 15.Weng SC, Wu CY. Lymphoma presenting as peritoneal lymphomatosis with ascites. J Chin Med Assoc. 2008; 71: 646-650. [DOI] [PubMed] [Google Scholar]
- 16.Aslam MB. Peritoneal lymphomatosis, a morphological look alike to peritoneal carcinomatosis: an autopsy report. J Clin Pathol. 2009; 62: 480. [DOI] [PubMed] [Google Scholar]
- 17.Wong S, Sanchez TRS, Swischuk LE, Huang FS. Diffuse peritoneal lymphomatosis: atypical presentation of Burkitt lymphoma. Pediatr Radiol. 2009; 39: 274-276. [DOI] [PubMed] [Google Scholar]
- 18.Park EK, Lee SR, Kim YC, Oh SY, Choe JG. Peritoneal lymphomatosis imaged by F-18 FDG PET/CT. Nucl Med Mol Imaging. 2010; 44: 159-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu SJ, Sinha AK. Peritoneal lymphomatosis shown on positron emission tomography/computed tomography scanning. Br J Haematol. 2011; 153: 290. [DOI] [PubMed] [Google Scholar]
- 20.Kim YG, Baek JY, Kim SY, et al. Peritoneal lymphomatosis confounded by prior history of colon cancer: a case report. BMC Cancer. 2011; 11: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharifah MI, Zamzami NA, Rafeah TN. Diffuse peritoneal lymphomatosis simulating peritoneal carcinomatosis. Med J Malaysia. 2011; 66: 270-272. [PubMed] [Google Scholar]
- 22.Kaneko K, Masunari S, Yoshida T, Omagari J. FDG-PET/CT findings of peritoneal lymphomatosis. Clin Nucl Med. 2012; 37: 1117-1119. [DOI] [PubMed] [Google Scholar]
- 23.Ridolfini MP, Caprino P, Berardi S, et al. A very advanced case of a T cell peritoneal lymphomatosis. Ann Ital Chir. 2012; 83: 71-73. [PubMed] [Google Scholar]
- 24.Yapar AF, Reyhan M. 18F-FDG uptake in diffuse peritoneal lymphomatosis. Clin Nucl Med. 2012; 37: e176-e177. [DOI] [PubMed] [Google Scholar]
- 25.van Rheenen RWJ, Bongaerts AHH, Glaudemans AWJM. Peritoneal lymphomatosis found on 18F‐FDG PET/CT. Eur J Haematol. 2012; 89: 503-504. [DOI] [PubMed] [Google Scholar]
- 26.Sia DSY, Kapur J, Thian YL. Peritoneal lymphomatosis mimicking peritoneal carcinomatosis: important imaging clues for correct diagnosis. Singapore Med J. 2013; 54: e93-e96. [DOI] [PubMed] [Google Scholar]
- 27.Uslu L, Sen F, Sager S, Halaç M. Extensive peritoneal and pleural lymphomatosis in a patient with Burkitt lymphoma revealed with 18F-FDG PET/CT. Nuklearmedizin. 2013; 52: N56-N57. [PubMed] [Google Scholar]
- 28.Tiwari P, Madan K, Jain D, et al. Pleuro-peritoneal lymphomatosis with concurrent tonsillar involvement in T-cell nonHodgkin’s lymphoma: Clinical presentation mimicking disseminated tuberculosis. Lung India. 2014; 31: 380-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curakova E, Genadieva-Dimitrova M, Misevski J, et al. Non-Hodgkin’s lymphoma with peritoneal localization. Case Rep Gastrointest Med. 2014; 2014: 723473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunningham N, Ffrench-Constant S, Planche K, Gillmore R. Peritoneal lymphomatosis: a rare presentation of follicular lymphoma mimicking peritoneal carcinomatosis. BMJ Case Rep. 2015; 2015: bcr2014207136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olofson AM, Loo EY, Hill PA, Liu X. Plasmablastic lymphoma mimicking carcinomatosis: A case report and review of the literature. Diagn Cytopathol. 2017; 45: 243-246. [DOI] [PubMed] [Google Scholar]
- 32.Choi WY, Kim JH, Choi SJ, et al. Peritoneal lymphomatosis confused with peritoneal carcinomatosis due to the previous history of gastric cancer: a case report. Clin Imaging. 2016; 40: 837-839. [DOI] [PubMed] [Google Scholar]
- 33.Flores E, Aydin N, Vu D, Misra S. A Case Series of diffuse large B-cell lymphoma and burkitt lymphoma presenting with peritoneal lymphomatosis. Int J Surg Case Rep. 2016; 28: 262-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fei N, Shah N. Adult sporadic Burkitt’s lymphoma presenting with rapid development of peritoneal lymphomatosis. Case Rep Oncol Med. 2017; 2017: 4789706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravindranath A, Srivastava A, Seetharaman J, et al. Peritoneal lymphomatosis masquerading as pyoperitoneum in a teenage Boy. ACG Case Rep J. 2019; 6: e00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senthil R, Gangadharan VP, Nair Visakh AR, Mahadevan P, Pratap T. Peritoneal lymphomatosis mimicking peritoneal carcinomatosis from ovarian malignancy on F-18 fluorodeoxyglucose positron emission tomography/computed tomography. Indian J Nucl Med. 2019; 34: 147-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kareff S, Yin C, Feigert J. High-grade B-cell lymphoma masquerading as peritoneal lymphomatosis. BMJ Case Rep. 2019; 12: e231238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HB, Hong R, Na YS, et al. Isolated peritoneal lymphomatosis defined as post-transplant lymphoproliferative disorder after a liver transplant: A case report. World J Clin Cases. 2019; 7: 4299-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benameur Y, Touil S, Sahel OA, et al. Peritoneal super scan on 18F-FDG PET/CT in two patients with lymphoma. Asia Ocean J Nucl Med Biol. 2020; 8: 149-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sachpekidis C, Exadaktylou P, Katsampoukas D, Moralidis E, Arsos G. 18F-FDG PET/CT in treatment response evaluation of Burkitt lymphoma: complete remission of a peritoneal super scan. Hell J Nucl Med. 2020; 23: 76-78. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe-Okochi N, Imai Y, Kimura H, et al. Intestinal T-cell lymphoma, NOS, presenting with sole peritoneal and mucosal lymphomatosis throughout abdominal cavity. J Clin Exp Hematop. 2020; 60: 117-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaikh DH, Gongati S, Salman SH, Reyes OA, Chilimuri S. Peritoneal lymphomatosis: the great mimicker. Cureus. 2021; 13: e14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu M, Wu Z, Yang Z, et al. Non-Hodgkin’s lymphoma presenting as isolated peritoneal lymphomatosis: A case report and literature review. Front Oncol. 2021; 11: 719554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chic Acevedo C, Ruiz Molina I, Contreras De Miguel E, Solís García E. Peritoneal lymphomatosis. A case report. Hematol Transfus Cell Ther. 2022; 44: 433-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ichikawa S, Fukuhara N, Saito K, et al. Diffuse large b-cell lymphoma presenting as peritoneal lymphomatosis: A Case Report and Literature Review. Intern Med. 2022; 61: 2057-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thirunavukkarasu B, Samanta J, Bhatia P, Bal A. De novo double-hit B-cell precursor leukemia/lymphoma - an unusual presentation as peritoneal lymphomatosis. Autops Case Rep. 2021; 11: e2021278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bisegna ML, Noccioli N, Della Starza I, et al. Utility of flow cytometry ascitic fluid analysis for rapid diagnosis of B-cell peritoneal lymphomatosis. Mediterr J Hematol Infect Dis. 2022; 14: e2022068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asghar N, Ahmad W, Hassan A. Searing abdomen: Infrequent presentation of peritoneal lymphomatosis in diffuse large B-Cell lymphoma on 18F-FDG PET-CT scan. J Pak Med Assoc. 2022; 72: 1670-1671. [DOI] [PubMed] [Google Scholar]
- 49.Sabbah M, Nakhli A, Bellil N, et al. Sporadic Burkitt lymphoma presenting with peritoneal lymphomatosis. Clin Case Rep. 2022; 10: e6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujioka M, Yabunaka K, Shirai Y, Noguchi K, Inoue M. A case of peritoneal lymphomatosis diagnosed with ultrasound imaging. J Med Ultrasound. 2022; 30: 306-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yılmaz F, Önner H. A rare involvement of diffuse large B-cell lymphoma: Peritoneal lymphomatosis with a peritoneal super-scan appearance on 18F-FDG PET/CT. Hell J Nucl Med. 2022; 25: 103-105. [DOI] [PubMed] [Google Scholar]
- 52.Lin PC, Chen SH, Wei CH, Kong SS, Chen SY. Peritoneal lymphomatosis presented with acute intermittent abdominal pain in a child. Pediatr Neonatol. 2023; 64: 362-363. [DOI] [PubMed] [Google Scholar]
- 53.Collinge B, Ben-Neriah S, Hilton LK, et al. Do unbalanced MYC break-apart FISH patterns indicate the presence of a MYC rearrangement? Blood. 2022; 140: 9244-9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roschewski M, Dunleavy K, Abramson JS, et al. Multicenter study of risk-adapted therapy with dose-adjusted EPOCH-R in adults with untreated Burkitt lymphoma. J Clin Oncol. 2020; 38: 2519-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018; 5: e609-e617. [DOI] [PMC free article] [PubMed] [Google Scholar]