Abstract
We report a case of therapy-related myelodysplastic syndrome (MDS), which developed 9 years after autologous peripheral blood stem cell transplantation (PBSCT) for peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS). A 65-year-old male was diagnosed with PTCL-NOS. After 6 cycles of the CHOP (cyclophosphamide [CPA], doxorubicin, vincristine, and prednisone) regimen, he achieved a first complete response (CR). He relapsed 33 months later and received salvage chemotherapy, which consisted of the CHASE regimen (CPA, high-dose cytarabine, dexamethasone, and etoposide). During the recovery phase of the first cycle of CHASE, his peripheral blood stem cells (PBSCs) were harvested and frozen in 2 bags. After 2 courses of CHASE, he underwent autologous PBSCT, which involved the use of the LEED preconditioning regimen (melphalan, CPA, etoposide, and dexamethasone) and one of the frozen bags. This resulted in a second CR. At 39 months after PBSCT, he relapsed with a tumor in his right arm. After it was resected, he received eight cycles of brentuximab vedotin and 45 Gy of involved-field irradiation concurrently and achieved a third CR. Nine years after autologous PBSCT, he was diagnosed with MDS with excess blasts 2 (MDS-EB-2). His disease progressed to acute myeloid leukemia after 2 courses of azacitidine therapy. He successfully underwent a second autologous PBSCT involving the busulfan and melphalan preconditioning regimen and the other frozen bag, which had been stored for 9 years. He has been in complete cytogenetic remission for 1 year since the second autologous PBSCT.
Keywords: peripheral T-cell lymphoma, not otherwise specified, autologous peripheral blood stem cell transplantation, therapy-related myelodysplastic syndrome
INTRODUCTION
Peripheral T-cell lymphomas (PTCL) are rare and heterogeneous forms of non-Hodgkin’s lymphoma, which, except for anaplastic large-cell lymphoma (ALCL), anaplastic lymphoma kinase (ALK)-positive subtype, are associated with poor clinical outcomes. The International PTCL Project showed that, around the world, the most common subtypes are PTCL, not otherwise specified (PTCL-NOS) (25.9%); angioimmunoblastic T-cell lymphoma (AITL) (18.5%); ALCL, ALK-positive (6.6%); and ALCL, ALK-negative (5.5%). The 5-year overall survival (OS) rates of these subtypes are as follows: PTCL-NOS: 32%; AITL: 32%; ALCL, ALK-positive: 70%; and ALCL, ALK-negative: 49%.1 The prospective International T-cell Project reported the survival of patients with primary refractory and relapsed PTCL. The 3-year OS rates for refractory and relapsed patients were 21% and 28%, respectively. Patients that did and did not undergo salvage bone marrow (BM) transplantation had 3-year survival rates of 48% and 18%, respectively.2
Myelodysplastic syndromes (MDS) are myeloid neoplasms characterized by peripheral cytopenia, morphological dysplasia, recurrent genetic abnormalities, and an increased risk of evolution to acute myeloid leukemia (AML).3 Therapy-related MDS (t-MDS) are included in the WHO category of therapy-related myeloid neoplasms (t-MNs), which occur as a late complication of cytotoxic chemotherapy and/or radiotherapy administered for a primary neoplasm or an autoimmune disease.4 The survival of patients with t-MNs is often poor, even with prompt diagnosis and treatment. Allogeneic hematopoietic cell transplantation (HCT) is the most effective curative treatment for t-MNs.
Here, we report a case of t-MDS, which developed 9 years after autologous peripheral blood stem cell transplantation (PBSCT) for PTCL-NOS. Although the patient did not undergo allogeneic HCT because of his age (78 years old), he underwent a successful transplantation of autologous peripheral blood stem cells (PBSCs) that had been frozen 9 years earlier.
CASE REPORT
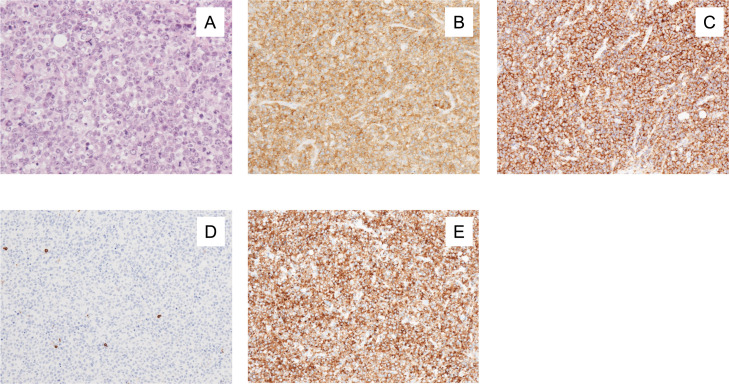
A 65-year-old male was referred to our hospital because of right axillary lymph node swelling, which had persisted for 2 months. He had been diagnosed with type 2 diabetes mellitus (DM) at the age of 50 and had been treated with subcutaneous insulin injections for the past 2 years. His Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 0. His serum lactate dehydrogenase (LDH) was 419 U/L (normal range: 124–222 U/L), and his serum soluble interleukin-2 receptor level was 675 U/mL (normal range: 190–650 U/mL). Although tests for both the hepatitis B surface and core antibodies (anti-HBs and anti-HBc) were positive, a test for hepatitis B virus (HBV)-DNA was negative. Computed tomography (CT) demonstrated swelling of the right axillary and mesenteric lymph nodes and thickening of the ileum wall. 18F-fluorodeoxyglucose positron emission tomography/CT (PET/CT) was not performed. A right axillary lymph node biopsy was carried out. Numerous large lymphoid cells that exhibited a diffuse growth pattern were seen. They contained scattered atypical mitoses and apoptotic bodies (Figure 1A). These cells were immunohistochemically positive for CD3, CD4, and CD30 (Figures 1B, 1C, and 1E), and negative for CD5, CD8, CD10, and CD20 (Figure 1D). The patient was diagnosed with PTCL-NOS. The disease was categorized as stage IV according to the Lugano classification.5 The patient was classified as low-intermediate risk according to the International Prognostic Index6 and as group 3 according to the Prognostic Index for PTCL-U.7 He was treated using the CHOP regimen, which consisted of cyclophosphamide (CPA), doxorubicin, vincristine, and prednisolone, every three weeks. After 6 cycles of CHOP, he achieved a first complete response (CR) (CR1), as shown by CT.
Fig. 1.
(A) An axillary lymph node biopsy showed the diffuse proliferation of atypical large lymphoid cells with eosinophilic cytoplasm. (B) CD3 staining. (C) CD4 staining. (D) CD8 staining. (E) CD30 staining.
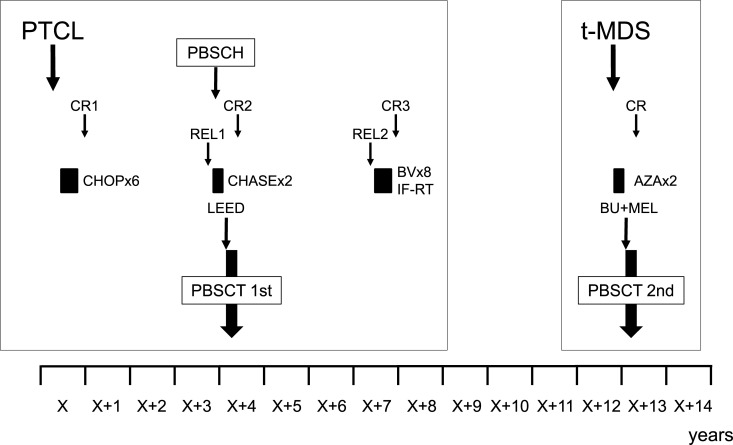
The patient relapsed 33 months later, which was confirmed by a right cervical lymph node biopsy. PET/CT demonstrated a hepatic tumor and abnormal uptake in the right cervical and mesenteric lymph nodes. He received salvage chemotherapy with the CHASE regimen, which consisted of CPA, high-dose cytarabine, dexamethasone, and etoposide.8 PBSCs were mobilized after the first cycle of CHASE by treating the patient with recombinant granulocyte colony–stimulating factor, and they were collected with the COBE Spectra (COBE BCT Inc., Lakewood, CO, USA). After mononuclear cells had been collected, they were cryopreserved with 20% human serum albumin and extracellular cryoprotectant solution (CP-1; Kyokuto Seiyaku Kogyo Co Ltd, Tokyo, Japan) with final concentrations of 3.2% albumin, 5% dimethyl sulfoxide, and 6% hydroxyethyl starch. The collected sample contained 20.14 x 106/kg CD34-positive cells and was separated into 2 bags (containing 100 mL and 76 mL of the sample, respectively) before being stored in a freezer at –152°C.9 After 2 cycles of CHASE, CT confirmed that the patient was showing a partial response. He underwent autologous PBSCT with the LEED preconditioning regimen, which consisted of 130 mg/m2 of melphalan for 1 day (day -1), 60 mg/kg of CPA for 2 days (day -4 to day -3), 500 mg/m2 of etoposide for 3 days (day -4 to day -2), and 40 mg/body of dexamethasone for 4 days (day -4 to day -1).10 Dexamethasone was not used to prevent the patient’s DM from worsening. His HCT-specific comorbidity index (HCT-CI) score was 1 due to his DM.11 The first bag containing PBSCs (the 100-mL bag), which included 11.44 x106/kg CD34-positive cells, was thawed, and the cells were infused on day 0. Neutrophil and platelet engraftment occurred on days 10 and 20, respectively. PET/CT performed on day 31 confirmed that the patient was exhibiting a second CR (CR2). At 39 months after PBSCT, he relapsed with a 1.7-cm tumor in his right arm. The tumor was completely resected and was confirmed to be a PTCL-NOS lesion. The patient received 8 cycles of brentuximab vedotin (BV) (1.8 mg/kg, every 3 weeks) and 45 Gy of concurrent involved-field radiotherapy (IF-RT), after which he achieved a third CR (CR3). His clinical course is shown in Figure 2.
Fig. 2.
A chart of the clinical course.
PTCL, peripheral T-cell lymphoma; t-MDS, therapy-related myelodysplastic syndrome; CR, complete response; CR1, first CR; CR2, second CR; CR3, third CR; REL1, first relapse; REL2, second relapse; PBSCH, peripheral blood stem cell harvest; PBSCT, peripheral blood stem cell transplantation; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CHASE, cyclophosphamide, high-dose cytarabine, dexamethasone, and etoposide; LEED, melphalan, cyclophosphamide, etoposide, dexamethasone; BV, brentuximab vedotin; IF-RT, involved-field radiotherapy; AZA, azacitidine; BU+MEL, busulfan and melphalan.
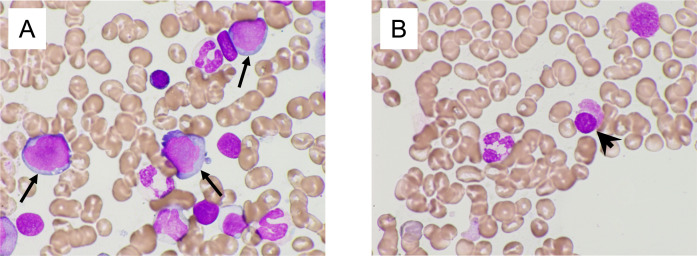
At 8 years and 10 months after PBSCT, his peripheral blood (PB) showed pancytopenia without symptoms. His laboratory findings demonstrated a white blood cell count of 1800/μL (blasts: 2%, polymorphonuclear leukocytes: 30%, eosinophils: 1%, monocytes: 1%, and lymphocytes: 66%), a hemoglobin level of 10.7 g/dL, a platelet count of 3.9x104/μL, a serum LDH level of 278 U/L (normal range: 110–210 U/L), and a WT-1 mRNA level of 1.1x104 copies/μgRNA (Table 1). BM aspiration showed a myeloblast frequency of 14.8%, degranulation of neutrophils, as well as micromegakaryocytes (Figures 3A and 3B). Hypo-segmented mature neutrophils (pseudo-Pelger-Huet anomaly) and ring sideroblasts were absent. Flow cytometry indicated that the myeloblasts were positive for CD13, CD33, CD34, CD117, and human leukocyte antigen-DR. G-banding analysis showed the +1, der(1;7)(q10;p10) abnormality in 19 of the 20 analyzed cells. The patient was diagnosed with MDS with excess blasts 2 (MDS-EB-2). The International Prognostic Scoring System (IPSS) classification of the MDS was high.12 The Revised IPSS classification of the MDS was very high.13 After 2 cycles of azacitidine (75 mg/m2 of azacitidine for 7 consecutive days, every 28 days), his BM showed a myeloblast frequency of 20.4%, and G-banding analysis showed an additional t(15;22)(q15;q13) translocation to +1, der(1;7)(q10;p10) in 15 of the 20 analyzed cells. The WT-1 mRNA level of his PB was 3.1x103 copies/μgRNA. His disease progressed to AML. It was difficult for him to undergo allogeneic HCT because of his age (78 years old). We planned to treat him with autologous PBSCT using the second frozen bag of PBSCs, which had been stored for 9 years. We first confirmed the viability of the PBSCs in the 1.5-mL dispenser tube stored in the same freezer. The viability of the CD34-positive cells in the tube was 79.0%, which was measured by 7-aminoactinomycin D (7-AAD) flow cytometry. The patient received an 80% reduced BU+MEL preconditioning regimen, which consisted of 3.2 mg/kg/day of intravenous busulfan for 3 days (day -6 to day -4) and 70 mg/m2 of melphalan for 2 days (day -3 to day -2). The patient’s HCT-CI score was 2 (DM and infection), and the second bag (the 76-mL bag), which had been frozen 9 years earlier, was thawed, and the cells were infused on day 0. The bag originally contained 8.70 x106/kg CD34-positive cells, and the percentage of viable CD34-positive cells was 82.3% according to flow cytometry. Neutrophil and platelet engraftment occurred on days 12 and 20, respectively. His BM revealed a cytogenetic complete remission involving a normal karyotype, and the WT-1 mRNA level of his PB was <50 copies/μgRNA on day 31. He has been in cytogenetic complete remission for 1 year since the second PBSCT. His HBV-DNA level has been checked regularly, and no reactivation has been observed so far.
Table 1. Laboratory findings at the onset of MDS.
| WBC | 1800 | /mL | TP | 6.8 | g/dL |
|---|---|---|---|---|---|
| blast | 2 | % | Alb | 3.8 | g/dL |
| seg | 30 | % | AST | 16 | U/L |
| eo | 1 | % | ALT | 11 | U/L |
| mo | 1 | % | ALP | 77 | U/L |
| lym | 66 | % | LDH | 278 | U/L |
| RBC | 313 | x104/mL | γ-GTP | 14 | U/L |
| Hb | 10.7 | g/dL | T.Bil | 0.4 | mg/dL |
| Ht | 32.9 | % | BUN | 25 | mg/dL |
| Plt | 3.9 | x104/mL | Cr | 1.22 | mg/dL |
| Ret | 11 | ‰ | UA | 5.9 | mg/dL |
| Na | 141 | mEq/L | |||
| PT-INR | 1.00 | K | 4.6 | mEq/L | |
| APTT | 30.1 | sec | Cl | 106 | mEq/L |
| Fbg | 526 | mg/dL | Ca | 9 | mg/dL |
| FDP | 5.1 | mg/mL | Glucose | 200 | mg/dL |
| DD | 1.6 | mg/mL | HbA1c | 6.9 | % |
| CRP | 2.16 | mg/dL | |||
| IgG | 995 | mg/dL | |||
| IgA | 406 | mg/dL | |||
| IgM | 81 | mg/dL | |||
| Ferritin | 137.3 | ng/mL | |||
| HBV-DNA | (-) | LogIU/mL | |||
| WT1 | 1.1x104 | copies/mgRNA |
Fig. 3.
A BM smear obtained at the diagnosis of MDS revealed an excess of blasts (arrow in A), degranulation of neutrophils, and micromegakaryoIcyte (arrowhead in B).
DISCUSSION
Patients with relapsed or refractory PTCL have dismal outcomes. The prospective International T-cell Project showed that after a late (>12 months) relapse patients who were able to undergo high-dose chemotherapy followed by HCT had the best chance of achieving long-term remission.2 In another study, the survival of patients with the three most common subtypes of PTCL (PTCL-NOS, AITL, and ALCL) who did not undergo transplantation after relapse or progression was investigated.14 The median time from the initial diagnosis to relapse or progression after primary therapy was 6.7 months, and the median OS and median progression-free survival (PFS) after relapse or progression (the second PFS) were 5.5 and 3.1 months, respectively. In addition, event-free survival (EFS) at 24 months (EFS24) and the subsequent OS were examined in large, multinational PTCL cohorts.15 Disease progression occurred within the first 24 months in 64% of patients, and they had a median OS of only 4.9 months (5-year OS, 11%). In contrast, the median OS after achieving EFS24 was not reached (5-year OS, 78%). Our patient relapsed 33 months after the completion of CHOP chemotherapy, and he underwent autologous PBSCT. Although he relapsed again 39 months after autologous HCT, the relapse site was local, and the tumor was treated with resection, BV therapy, and IF-RT. BV is an anti-CD30 antibody conjugated to monomethyl auristatin E (MMAE) and showed antitumor activity in patients with relapsed PTCL.16 With respect to his lymphoma, the patient is still exhibiting CR at 14 years after being diagnosed with PTCL-NOS. Although he experienced two episodes of relapse, the intervals between the last treatment and relapse were more than 24 months on both occasions. The slow speed of his tumor growth may have been related to the prolonged period of remission.
An increased risk of therapy-related MDS and AML (t-MDS/AML) has been described in adult patients with lymphoma who undergo autologous HCT. Kalaycio et al. analyzed 526 patients with lymphoma that were treated with autologous HCT, and 20 patients developed t-MDS/AML, resulting in an actuarial incidence of 6.8% at 10 years, and the median follow-up time for surviving patients was 69 months.17 They showed that prior exposure to radiotherapy, receiving four or more chemotherapy regimens, and more than 5 days of apheresis being needed to harvest enough stem cells were risk factors for t-MDS/AML. Yamasaki et al. analyzed 13,810 lymphoma patients in Japan who underwent autologous (n = 9,963) or allogeneic (n = 3,847) HCT.18 At a median OS of 52 and 46 months in the autologous and allogeneic HCT groups, respectively, the lymphoma patients that underwent autologous HCT (1.38% at 3 years after autologous HCT) were at significantly higher risk of developing t-MDS/AML than those who underwent allogeneic HCT (0.37% at 3 years after allogeneic HCT, P < 0.001). A high-stage risk at the time of the HCT, secondary malignancies, receiving cord-blood stem cells, and receiving IF-RT were reported to be significant risk factors for the development of t-MDS/AML after autologous or allogeneic HCT.
Two types of t-MNs are recognized clinically.4 The more common one occurs 5–10 years after exposure to alkylating agents and/or ionizing radiation. These cases often present with MDS combined with BM failure and cytopenia, and they are commonly associated with chromosome 5 and/or 7 abnormalities and complex karyotypes. The second subtype accounts for 20–30% of cases and occurs after treatment with agents that interact with DNA topoisomerase II (topoisomerase II inhibitors). It has a shorter latency period (1–5 years) and presents with overt acute leukemia without a myelodysplastic phase and is often associated with a balanced chromosomal translocation. The LEED preconditioning regimen used during treatment for lymphoma contains both alkylating agents (CPA and melphalan) and a topoisomerase II inhibitor (etoposide). Our patient progressed to MDS after 9 years of exposure to these drugs. He had a complex karyotype of +1, der(1;7)(q10;p10) at the diagnosis of MDS, and the t(15;22)(q15;q13) translocation appeared just before the second autologous PBSCT. His t-MDS was more likely to have been influenced by alkylating agents than by a topoisomerase II inhibitor. The unbalanced translocation der (1;7)(q10;p10) is a characteristic cytogenetic abnormality observed in MDS and other myeloid neoplasms, and has been reported at frequencies of 1–3% in MDS, 1–2% in AML, and 1% in myeloproliferative disorders.19 Sanada et al. analyzed 77 MDS cases involving der(1;7)(q10;p10). They were shown to have lower blast counts, higher hemoglobin concentrations at diagnosis, and to exhibit slower progression to AML than other MDS cases with monosomy 7 or 7q- (−7/7q-).20 Slovak et al. compared the clinicopathological features of 12 der(1;7) MDS patients with those of 51 MDS patients with del(7q) (n = 10) or -7 (n = 41) and reported that the 5-year survival rates for der(1;7), del(7q), and -7 (44.4, 32.0, and 23.6%, respectively) did not differ significantly.19
We used PBSCs stored for 9 years and confirmed that the viability of CD34-positive cells was 82.3%. Lisenko et al. analyzed the storage duration of PBSCs with multiple myeloma patients who received high-dose melphalan before autologous PBSCT. They showed the long-term (>60 months) cryopreservation of PBSCs products did not have a negative effect on hematopoietic recovery compared with the short-term (≤12 months) and the medium-term (>12 and ≤60 months).21 Our patient underwent a second autologous PBSCT involving the BU+MEL preconditioning regimen, which was reported to be effective when used before autologous HCT for relapsed acute promyelocytic leukemia.22 BU+MEL is a kind of myeloablative regimen and the concept of the autologous HCT of this case was the replacement of sick BM with healthy PBSCs; however, it was risky for a 78-year-old patient even if he had good PS and received 80% reduced regimen. We carefully decided on this transplantation during a meeting in which a wide variety of workers attended.
In conclusion, autologous HCT is useful for relapsed PTCL-NOS. Although our patient experienced a second relapse at a local site, he has experienced a prolonged CR3 since undergoing resection, BV therapy, and IF-RT. He developed t-MDS 9 years after undergoing autologous PBSCT for PTCL-NOS. He achieved cytogenetic complete remission after the second autologous PBSCT using PBSCs that had been frozen 9 years earlier; however, long-term follow-up will be needed to assess the outcomes of the second autologous HCT. t-MNs may arise after autologous HCT for lymphoma. It is difficult to cure t-MNs without allogeneic HCT; however, by using autologous PBSCs patients can “go back in time” to when they had a healthy BM. When we do not use all the harvested autologous PBSCs to treat a patient with lymphoma, we should keep the rest of them so that they can be utilized for a possible future transplantation.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008; 26: 4124-4130. [DOI] [PubMed] [Google Scholar]
- 2.Bellei M, Foss FM, Shustov AR, et al. The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, International T-Cell Project. Haematologica. 2018; 103: 1191-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cazzola M. Myelodysplastic Syndromes. N Engl J Med. 2020; 383: 1358-1374. [DOI] [PubMed] [Google Scholar]
- 4.Swerdlow SH. International Agency for Research on C: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed, Lyon, International Agency for Research on Cancer. 2017. [Google Scholar]
- 5.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32: 3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Non-Hodgkin’s Lymphoma Prognostic Factors Project . A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993; 329: 987-994. [DOI] [PubMed] [Google Scholar]
- 7.Gallamini A, Stelitano C, Calvi R, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004; 103: 2474-2479. [DOI] [PubMed] [Google Scholar]
- 8.Ogura M, Kagami Y, Taji H, et al. Pilot phase I/II study of new salvage therapy (CHASE) for refractory or relapsed malignant lymphoma. Int J Hematol. 2003; 77: 503-511. [DOI] [PubMed] [Google Scholar]
- 9.Maeda S, Kagami Y, Ogura M, et al. CD34+-selected autologous peripheral blood stem cell transplantation conditioned with total body irradiation for malignant lymphoma: increased risk of infectious complications. Int J Hematol. 2001; 74: 214-221. [DOI] [PubMed] [Google Scholar]
- 10.Ogura M, Yamamoto K, Morishima Y, et al. R‐High‐CHOP/CHASER/LEED with autologous stem cell transplantation in newly diagnosed mantle cell lymphoma: JCOG0406 STUDY. Cancer Sci. 2018; 109: 2830-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005; 106: 2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997; 89: 2079-2088. [PubMed] [Google Scholar]
- 13.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012; 120: 2454-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013; 31: 1970-1976. [DOI] [PubMed] [Google Scholar]
- 15.Maurer MJ, Ellin F, Srour L, et al. International assessment of event-free survival at 24 months and subsequent survival in peripheral T-cell lymphoma. J Clin Oncol. 2017; 35: 4019-4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014; 123: 3095-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalaycio M, Rybicki L, Pohlman B, et al. Risk factors before autologous stem-cell transplantation for lymphoma predict for secondary myelodysplasia and acute myelogenous leukemia. J Clin Oncol. 2006; 24: 3604-3610. [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki S, Suzuki R, Hatano K, et al. Therapy-related acute myeloid leukemia and myelodysplastic syndrome after hematopoietic cell transplantation for lymphoma. Bone Marrow Transplant. 2017; 52: 969-976. [DOI] [PubMed] [Google Scholar]
- 19.Slovak ML, O’Donnell M, Smith DD, Gaal K. Does MDS with der(1;7)(q10;p10) constitute a distinct risk group? A retrospective single institutional analysis of clinical/pathologic features compared to -7/del(7q) MDS. Cancer Genet Cytogenet. 2009; 193: 78-85. [DOI] [PubMed] [Google Scholar]
- 20.Sanada M, Uike N, Ohyashiki K, et al. Unbalanced translocation der(1;7)(q10;p10) defines a unique clinicopathological subgroup of myeloid neoplasms. Leukemia. 2007; 21: 992-997. [DOI] [PubMed] [Google Scholar]
- 21.Lisenko K, Pavel P, Kriegsmann M, et al. Storage duration of autologous stem cell preparations has no impact on hematopoietic recovery after transplantation. Biol Blood Marrow Transplant. 2017; 23: 684-690. [DOI] [PubMed] [Google Scholar]
- 22.Yanada M, Tsuzuki M, Fujita H, et al. Phase 2 study of arsenic trioxide followed by autologous hematopoietic cell transplantation for relapsed acute promyelocytic leukemia. Blood. 2013; 121: 3095-3102. [DOI] [PubMed] [Google Scholar]