Abstract
Malvaceae family, also known as the Mallow family, is a family of flowering plants containing Hibiscus rosa-sinensis and other plants of high medicinal value. This study focuses on the challenges associated with high-quality RNA extraction from Hibiscus rosa-sinensis and its related plants characterized by high levels of mucilage and phenolic compounds in their tissues. High mucilage and secondary metabolite content pose obstacles in obtaining high-quality RNA, negatively impacting downstream applications, such as gene expression analysis. Our research aimed to develop an efficient RNA extraction method tailored to the unique characteristics of Malvaceae family plants especially Hibiscus rosa-sinensis. Through the substitution of NaCl with KCl, a crucial component of the CTAB buffer, our methodology successfully addressed the challenges posed by high mucilage and phenolic compound levels. This modification led to a significant reduction in sample viscosity, which is because of the high mucilage in these plants. Our modified CTAB extraction method yielded significantly more RNA with higher purity than the conventional CTAB methods alone. The extracted RNA was largely intact, as indicated by 28S/18S ratios and RIN values, yielding high-quality RNA with improved purity suggested by the 260/280 and 260/230 ratios. The proposed approach not only serves as a solution to the specific challenges encountered in Hibiscus rosa-sinensis but also holds promise for broader applications across different plants within the family.
Introduction
The Malvaceae family comprises a diverse group of plants with approximately 243 genera and more than 4225 species that are widely distributed across different geographic regions. The family includes wild as well as ornamental, e.g., Hibiscus rosa-sinensis, Malvaviscus arboreus, Altheae roseae, Abutilon indicum, Sida acuta, etc.1 species. These plants are known for their unique features, including the presence of high content of mucilage,2 and phenolic compounds3 in their leaf tissues.
Mucilage, a complex polysaccharide made of sugars and uronic acid that is found in various parts of the plant, including leaves, stems, roots, and seeds, is highly acidic and proteinaceous.4 Mucilage is highly viscous and gelatinous in nature. Its primary function is to retain water, protect the plant from desiccation, provide a barrier against pathogens, and ionic balance of plant cells.5 On the other hand, phenolic compounds are a class of secondary metabolites having a hydroxyl group attached to an aromatic ring. These are also associated with a range of biological functions, including plant defense against pathogens and herbivores. The subclasses flavanols and flavones with additional OH groups at the C-8 A ring and/or the C-5′ B ring positions are characteristic features of the family Malvaceae.1
However, the presence of high levels of mucilage and secondary metabolites can pose significant challenges in RNA extraction from the leaf tissue of Malvaceae family members as mucilage binds to secondary metabolites like phenolic compounds and coprecipitates with nucleic acid.6,7 This affects downstream applications like gene expression analysis negatively.8 Mucilage, because of its gelatinous nature causes high viscosity9 that also interferes with RNA extraction by hindering the homogenization and disruption of cells, clogging up the extraction columns or filters, making it difficult to extract RNA, leading to poor RNA quality and low yields.
Therefore, the development of efficient RNA extraction methods for Malvaceae family members is essential to overcoming these challenges. A large number of RNA extraction protocols have been developed using phenol/SDS or guanidinium thiocyanate,10 and TRIZOL reagent11 but none of them are found to be suitable for plants rich in mucilage,4 oils, polysaccharides, and other secondary metabolites.7,12 Several protocols have been developed using different concentrations of salts but the most popular of them is using 1.4 M NaCl, however, high concentrations are suggested for plants rich in polysaccharides.13,14
In this study, the purpose of our research was to devise a more efficient RNA extraction method suitable for tissues with high mucilage and phenolic compound levels. We have modified the famous CTAB method originally described by.15 By replacing NaCl with KCl, a critical component of the CTAB buffer, we succeeded in achieving this goal. Our methodology resulted in a substantial decrease in the viscosity of the crushed samples. As a result, we were able to obtain high-quality RNA with good purity and yield. This approach could potentially be applied to other Malvaceae species and thus will be significant in downstream applications including gene expression analysis and transcriptome sequencing.
Materials and Methods
Plant Materials
Fresh leaves of Hibiscus rosa-sinensis, Althaea rosea, Malvaviscus arboreus, Abutilon indicum, and Sida acuta were collected from the Campus of Jamia Hamdard, New Delhi, were used for RNA extraction.
Reagents
Five different extraction buffers (EBs) were made for the isolation of RNA whose constituents are listed in Table 1, and other reagents that were used in the experiment are listed in Table 2. All solutions were made with autoclaved distilled water. All of the mortars and pestles were used after being washed, dried, treated with 0.1% DEPC overnight, and then autoclaved.
Table 1. Reagents Utilized in the Preparation of Different Kinds of Extraction Buffers.
| s. no. | extraction buffer (EB) | CTAB (%) | Tris–HCl pH 8 (M) | salt types (2.0 M) | EDTA (pH 8) (mM) | PVP (%) |
|---|---|---|---|---|---|---|
| 1. | EBI | 2 | 0.1 | NaCl | 20 | 3 |
| 2. | EBII | 2 | 0.1 | KCl | 20 | 3 |
| 3. | EBIII | 2 | 0.1 | MgCl2 | 20 | 3 |
| 4. | EBIV | 2 | 0.1 | CaCl2 | 20 | 3 |
| 5. | EBV | 2 | 0.1 | LiCl | 20 | 3 |
Table 2. List of Other Reagents Utilized for Extraction of RNA.
| s. no. | name of reagents |
|---|---|
| 1. | DEPC-treated and autoclaved distilled water |
| 2. | chloroform: isoamyl alcohol (24:1) |
| 3. | 7.5 M LiCl (made with autoclaved distilled water) |
| 4. | β-mercaptoethanol (1%) |
| 5. | 80% ethanol (made with autoclaved distilled water) |
| 6. | 50X TAE (made with autoclaved distilled water) |
| 7. | fermantas DNA gel loading dye, 6X, Thermo Scientific |
pH Measurements
One gram of the sample was ground in 10 mL of extraction buffer and centrifuged for 2 min at 10,000 rpm (at room temperature), and the pH was measured for the supernatant obtained using a pH meter (Thermo Scientific, Eutech Instruments pH700).
Viscosity Measurement
One gram of the leaf sample was crushed in 10 mL of extraction buffer and the viscosity was measured by using an MCR 101 rheometer (Anton Paar, Germany) attached to a water bath having a thermostatically controlled circulating system, using a cone and plate (CP-50-1) and plate and plate geometry (PP-2-5) operated by Rheoplus software.16
Isolation of Total RNA
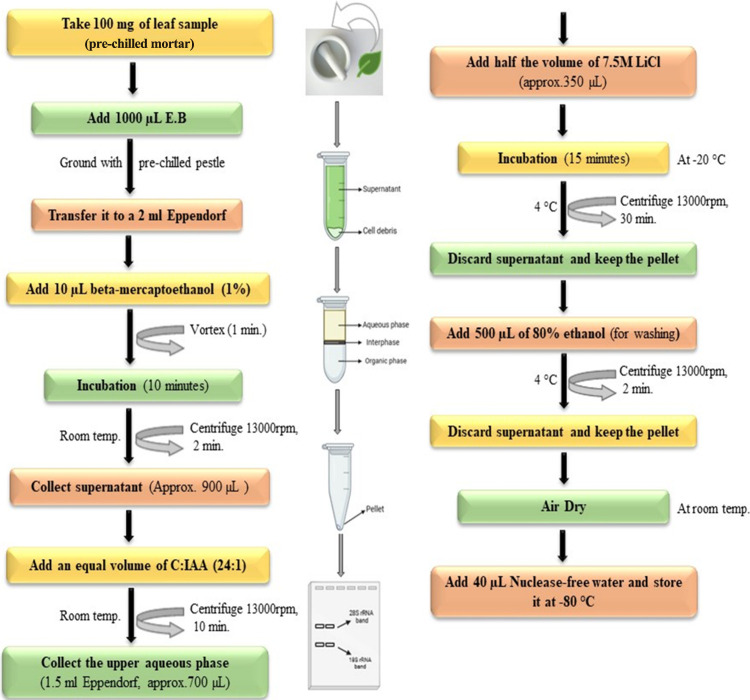
A 100 mg portion of the leaf sample was taken and crushed in 1000 μL of prewarmed (at 65 °C) extraction buffer (Table 1) in prechilled mortar pestles. The crushed sample was transferred to 2 mL Eppendorf tubes, and 10 μL β-mercaptoethanol was added and vortexed for 1 min. Then, it was incubated at 65 °C in a water bath for 10 min and centrifuged at 13000 rpm for 2–4 min at room temperature. The supernatant (approximately 600–1000 μL) was taken to another Eppendorf tube and an equal volume of chloroform–isoamyl alcohol (24:1) was added. It was vortexed for 1 min and centrifuged at 13000 rpm for 10–15 min (at room temperature). Again, the supernatant was obtained (approximately 500–900 μL), to which half the amount of lithium chloride (7.5M) was added, and stored at −20 °C for 15–20 min. The stored samples were centrifuged at 13000 rpm for 30–40 min at 4 °C. The supernatant was decanted, and the pellet was kept and washed with 500 μL of 80% ethanol at 13000 rpm for 2–5 min. Again, the tube was decanted, and the pellet was kept and air-dried for approximately 30–40 min. At last, the pellet was dissolved in 30–50 μL of nuclease-free water Figure 1.
Figure 1.
Schematic diagram describing the major steps involved in the total RNA extraction.
Electrophoretic Analyses of Isolated RNA
To check the integrity of RNA, 5 μL of total RNA mixed with 1 μL of gel loading dye was loaded in 1.5% agarose gel having 1 μL of 10 mg/mL ethidium bromide. The gel was made and run in 1X TAE [from a stock 50X TAE] at 100 V for 30–40 min and was observed in a UV Trans illuminator and photographed by AlphaDigiDocTM.
Spectrophotometric Analyses of Isolated RNA
The quantity and quality of isolated RNA were assessed by measuring the optical density (OD) at 260 and 280 nm. Purity (260/280 ratio) and contaminants (260/230 ratio) were checked using a Thermo Scientific, Nanodrop 2000c UV Spectrophotometer.
RIN Value Measurements
The quality of the RNA was also assessed with Agilent 4150 TapeStation System and Qubit 3.0 which is designed for analyzing Eukaryote and Prokaryote RNA molecules from 50–6000 nts (nucleotides) to calculate the RINe values, following the manufacturer’s instructions17 (Agilent Technologies, Inc. 2013–2014, 2015, Agilent Technologies Waldbronn, Germany).
cDNA Synthesis and PCR Gene Amplification
The RNA (1 μg) isolated was used to synthesize cDNA using Thermo Scientific, RevertAid, First strand cDNA Synthesis Kit according to the manufacturer’s instructions. For polymerase chain reaction (PCR) amplification of the Actin gene was done using primers ActinF- (5′-ATCCAAGCTGTTCTCTCTTTG-3′), ActinR- (5′-GAAGGTGCTGAGTGATGCC-3′)18 for one reaction and ActinF- (5′-GCATAGAGGGAAAGCACAGC-3′), ActinR- (5′-TGGTAGGTATGGGCCAGAAA-3′)19 for another reaction. One μL of cDNA samples of total RNA extracted from the leaf tissue of Hibiscus rosa-sinensis was added to a 10 μL PCR reaction mix containing GeneDireX OnePCRTM that has 2X concentrated solution of Taq DNA polymerase, dNTPs, and all other components required for PCR except for primers and template. The amplification was carried out in a thermal cycler (T100 Thermal Cycler, Bio-Rad Inc., Hercules, CA, USA) with PCR conditions of initial denaturation at 95 °C for 2 min, then 35 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, extension at 72 °C for 40 s, then final extension at 72 °C for 5 min and hold at 12 °C for infinite time. The transcripts were visualized in an ethidium bromide-stained 1% TAE-agarose gel.
Results and Discussion
Standardization of Protocol
Using Different Extraction Buffers Having Different Salts
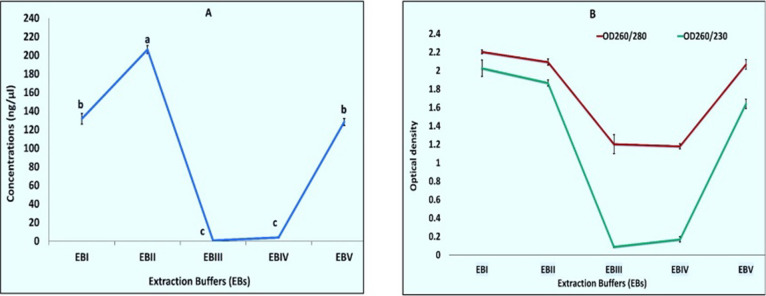
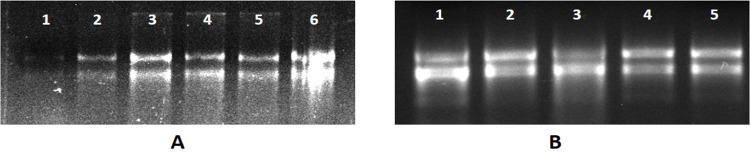
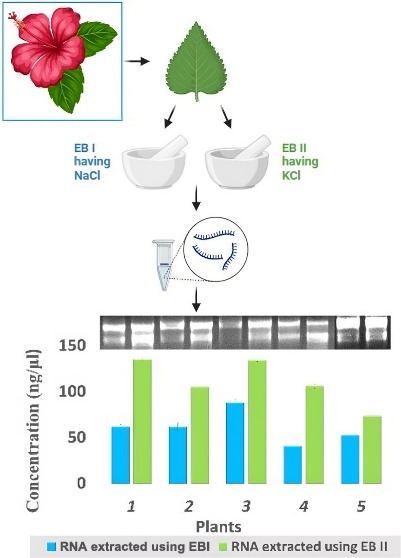
When RNA extraction was done using Extraction Buffer I, II, III, IV, and V (Table 1) results were found to be positive in the cases of EBI, EBII, and EBIII (Figure 2A). RNA extracted using EBV had only one band of 28SrRNA, and RNA isolated with EB1 had both bands but the 18SrRNA band faded i.e., less concentration as compared to the bands which we got in the case of EBII (Table S1). RNA isolated with EBII had both the bands intact and better quality and quantity (Table S1 and Figure 3). Although all buffers were made with s-block elements’ salts, the results were found to be positive in the case of buffers made with salts NaCl, KCl, and LiCl and negative in the case of buffers made with MgCl2 and CaCl2. This can be explained by the fact that Na, K, and Li belong to the same group known as alkali metals having similar properties according to the periodic table, and Mg and Ca belong to different groups known as alkaline earth metals having different similar properties. Further comparing first NaCl and KCl and then KCl and LiCl, KCl, has better ionic strength so a better reagent while making an extraction buffer.
Figure 2.
(A) Agarose gel electrophoresis of total RNA from Hibiscus using different extraction buffers (EBs). (A) Lane 1 shows RNA isolated with EBI, lane 2 shows RNA isolated with EBII, lane 3 shows EBIII, lane 4 shows EBIV, and lane 5 shows RNA isolated with EBV. (B) Total RNA extracted with EBII, where different concentrations of potassium chloride (KCl) were used. Lane 1 contains RNA extracted without KCl, lane 2 contains 0.5 M KCl, lane 3 contains 1.0 M KCl, lane 4 contains 1.5 M KCl, and lane 5 contains 2.0 M KCl.
Figure 3.
Graphical representation (A) concentrations and (B) OD when RNA extracted using various extraction buffers (EBs). Values are means ± standard deviation of at least three replicates; within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT.
Using Different Salt Concentrations
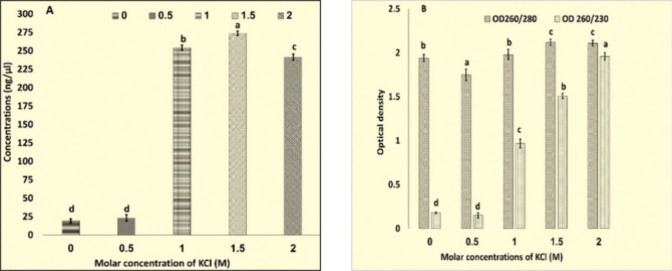
Extraction Buffer II having KCl in place of NaCl further experimented with different concentrations, viz. 0, 0.5, 1.0, 1.5, and 2 M (Figure 2B). At different ionic strengths, CTAB works differently.14 The ionic strength required for CTAB to precipitate polysaccharides is provided by salt concentrations greater than 0.5 M.20,21 Although a 1.4 M concentration of NaCI has been indicated in numerous protocols, larger concentrations of NaCI and/or CTAB have been advised in protocols established for eliminating polysaccharides.14 The RNA here was successfully extracted with an Extraction Buffer having KCl with concentrations of 1, 1.5, and 2.0 M. However, maximum concentrations and best O.D. (Table S2, Figure 4) was observed in RNA extracted with a buffer having 2.0 M KCl.
Figure 4.
Graphical representation (A) concentrations and (B) OD260/280, OD260/230 for RNA extracted using extraction buffer having different molarity of KCl. Values are means ± standard deviation of at least three replicates; within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT.
Using Different Time Spans for Precipitation at the Last Step Using 7.5 M LiCl
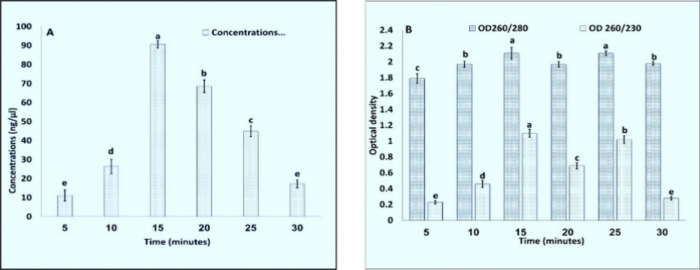
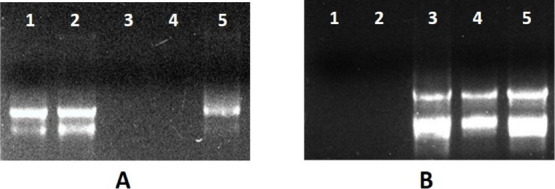
For precipitation at the last step with LiCl, incubation at −20 °C was done for 5, 10, 15, 20, 25, and 30 min (Figure 5A). Several protocols used high amounts and overnight or hours of incubation15,22,23 for precipitation. In the RNA extraction protocol13 the incubation time required for precipitation is reduced to 15 min at −80 °C but in the modified protocol, the incubation time for precipitation was 15 min at −20 °C (Table S3, Figure 6). This reduced the overall time for the extraction process of RNA, i.e., only 2–3 h.
Figure 5.
Agarose gel electrophoresis of the total RNA. (A) Extracted and subsequently precipitated with LiCl for varying durations. Lane 1 for 5 min, Lane 2 for 10 min, Lane 3 for 15 min, Lane 4 for 20 min, Lane 5 for 25 min, Lane 6 for 30 min, (B) extracted from different species within the Malvaceae family. Lane 1 contains RNA from Hibiscus rosa-sinensis, Lane 2 from Althaea rosea, Lane 3 from Malvaviscus arboreus, Lane 4 from Abutilon indicum, and Lane 5 from Sida acuta.
Figure 6.
Graphical representation (A) concentrations and (B) OD260/280, OD260/230 for RNA extracted using various times for precipitation with LiCl (7.5M). Values are means ± standard deviation of at least three replicates; within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT.
RNA Isolated from Different Plants with a New Protocol
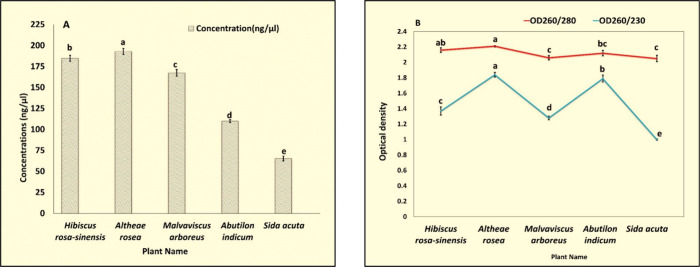
A large number of protocols and commercial kits have been discovered for RNA extraction13,24,25 but found incompatible with a majority of medicinal plants rich in mucilage. RNA from five different species of the family Malvaceae named Hibiscus rosa-sinensis, Althaea rosea, Malvaviscus arboreus, Abutilon indicum, and Sida acuta was successfully extracted with our CTAB modified protocol (Figure 5B). All of them have good concentrations; OD260/280 ranges from 2.00 to 2.20 (Table S4, Figure 7) suggesting high purity. OD260/230 for the RNA extracted are more than 1.00 which suggests more removal of contaminating proteins.
Figure 7.
Graphical representation (A) concentrations and (B) OD260/280, and OD260/230 for RNA extracted using EBII from different plants of the family Malvaceae. Values are means ± standard deviation of at least three replicates; within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT.
Comparison of RNA Extracted Using a Modified Protocol with the Standard Protocol
A further experiment was done using Extraction Buffer II having 2.0 M KCl and compared for quality, quantity, pH, viscosity, RIN values, cDNA synthesis, and PCR amplification with RNA isolated with Extraction Buffer I having 2.0 M NaCl used by a previously reported method.13
Quality and Quantity
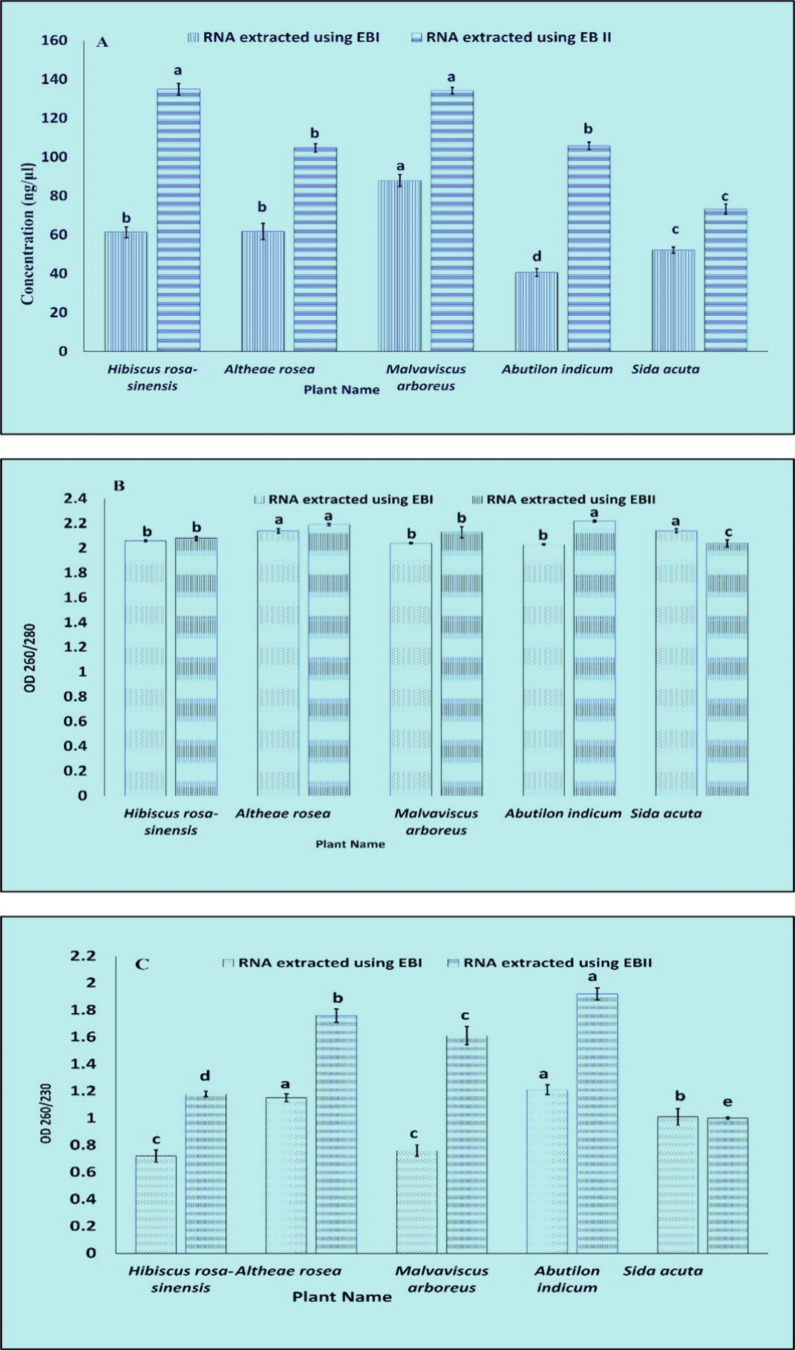
The concentrations and spectroscopic analysis (Table S5, Figure 9) as compared have shown that modified protocol can be a better choice for extracting RNA from plants having high mucilage, i.e., plants of the family Malvaceae, that can also be evaluated from the gel picture (Figure 8), concentrations, OD260/280 and OD260/230 that increases for every plant when the modified protocol was used, suggesting more removal of proteins, polyphenols, and polysaccharides.
Figure 9.
Graphical representation, comparison (A) concentrations, (B) OD260/280, (C) OD260/230 for RNA extracted using EBI and EBII for different plants of the family Malvaceae. Values are means ± standard deviation of at least three replicates; within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT.
Figure 8.
Agarose gel electrophoresis was used to analyze total RNA extracted from various plant species, with the extractions differentiated by two methods, EBI and EBII. RNA from Hibiscus rosa-sinensis was analyzed in Lanes 1 (EBI) and 2 (EBII). For Althaea rosea, the RNA extractions were placed in Lanes 3 (EBI) and 4 (EBII). Malvaviscus arboreus RNA samples were observed in Lanes 5 (EBI) and 6 (EBII), while Abutilon indicum RNA was observed in Lanes 7 (EBI) and 8 (EBII). RNA from Sida acuta was shown in Lanes 9 (EBI) and 10 (EBII).
pH Comparison Analysis
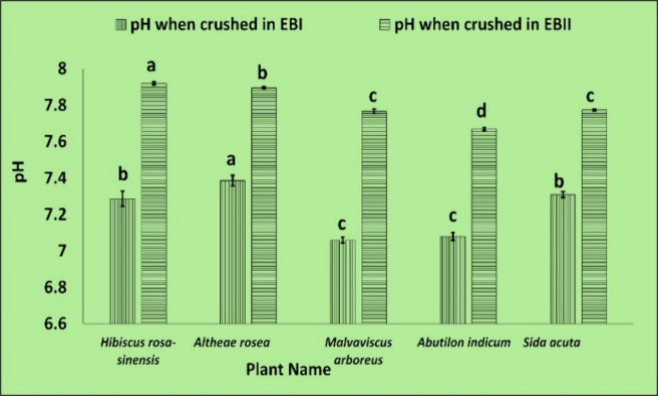
The EBI and EBII had pH of 7.75 ± 0.02 and 8.15 ± 0.02, respectively. However, once the plant material was crushed in it, the pH decreased to −0.5 (Table S6 and Figure 10), which improved the environment for isolating nucleic acids, since the ideal pH range for nucleic acid extraction is between 7.5 and 8.5.14 Generally, RNA gathers in pH 6–7, and DNA in pH 8–9 because proteins and DNA stay in the lower liquid phase of the lysate in acidic environments, but RNA stays in the upper liquid phase either in the lower organic phase or interphase. The protocol was developed22 on cinnamon as it is inherently sticky, tested the CTAB buffer’s pH, and found it to be approximately 8. As a result, they have adjusted the buffer pH 6–6.5 by adding copious HCl to keep RNA in the upper aqueous phase. Reducing the pH level resulted in a decrease in the supernatant’s viscosity as well as significant DNA contamination. But here, using our procedure, RNA was successfully extracted without the need to alter pH with the aid of KCl substitution, and viscosity was also significantly reduced (Table S7), facilitating higher-quality RNA extraction from the plants with high mucilage content. RNA can be successfully isolated even at alkaline pH using the modified protocol.
Figure 10.
pH changes when leaf samples from different plants of the family Malvaceae are crushed using EBI and EBII. Values are means ± standard deviation of at least three replicates; within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT.
Viscosity Comparison
Comparing the viscosity results of plant material crushed in EBI and EBII individually (Table S7 and Figure 11) reveals that EBII reduces the crushed sample’s viscosity by a factor of 10–1000. There is a presence of high mucilage in the family Malvaceae2 that causes high viscosity in these plants9 while extracting RNA and interferes with the process6,23 so decreased viscosity while extracting RNA with the modified buffer, i.e., EBII can be a reason that gives better results (Table S5).
Figure 11.
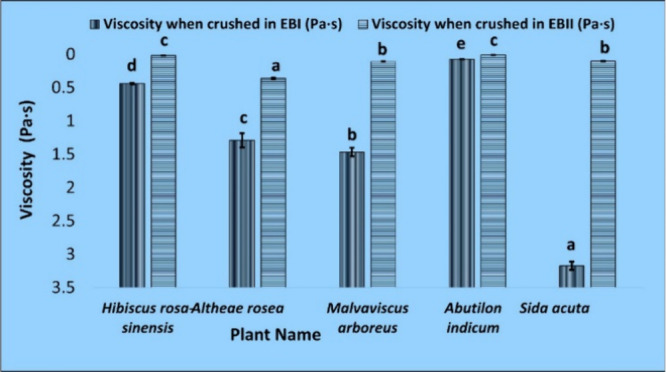
Viscosity changes when leaf samples from different plants of the family Malvaceae are crushed using EBI and EBII. Values are means ± standard deviation of at least three replicates; within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT.
Comparison RIN Values
The RIN values of RNA isolated from Hibiscus rosa-sinensis using EBI and EBII are 8.8 and 8.7, respectively. The RNA integrity number (RINe) assigned by the software determines the integrity of the RNA. RINe readings ranged from 1 to 10, with 1 being the lowest and 10 being the highest. RINe values less than 5 indicate total degradation, 5–7 suggest moderately degraded RNA, and values greater than 8 indicate good-quality RNA can be utilized for a variety of purposes, including NGS techniques.
cDNA Synthesis and PCR Gene Amplification
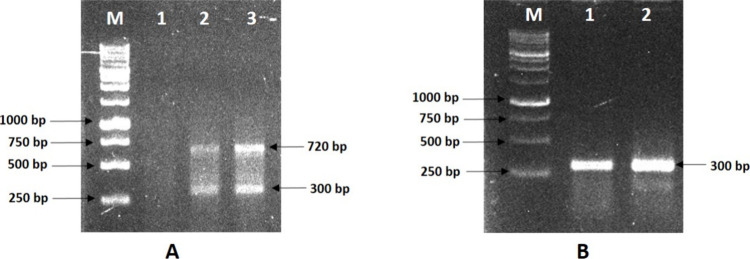
The clear and sharp fragment for both primers of actin having amplification sizes 300 and 720 bp, respectively (Figure 12A,B), during RT-PCR analysis indicates successful cDNA synthesis, and RNA is acceptable for downstream applications. Also, the bands when compared have better intensity in the case of RNA extracted using EBII.
Figure 12.
Agarose gel electrophoresis of the PCR-amplified actin gene products. (A) A duplex RT-PCR showing the amplification of 720 and 300 bp of actin gene derived from RNA extracted with two different buffers, EBI and EBII, shown in lanes 2 and 3, respectively. Water as a negative control is shown in Lane 1. (B) RT-PCR showing amplification of a 300-bp fragment of actin gene derived from RNA extracted with extinction buffer EBI and EBII, shown in lanes 1 and 2, respectively. One kb DNA ladder is presented in Lane M.
Conclusions
This study aimed to develop an efficient, simple, swift, and cost-effective RNA extraction protocol for the plants of the Malvaceae family that are rich in mucilage and cause high viscosity when crushed in an extraction buffer. The modified CTAB-based RNA extraction method gave good results with high-quality RNA from all five plants of the family Malvaceae that were used in the experiment. Extraction Buffer II containing potassium chloride in place of sodium chloride can be used from a range of 1.0–2.0 M concentrations. The RNA can be extracted in just 2–3 h by using this protocol. To the best of our knowledge, no research has made a CTAB-modified Extraction Buffer containing KCl in place of NaCl so far.
To summarize, we have optimized a robust and reliable method for the RNA extraction of high-mucilage-containing plants that can be used for almost all downstream applications.
Acknowledgments
The University Grant Commission (UGC), Government of India, New Delhi, is greatly acknowledged for providing a Junior Research Fellowship (JRF) to Nikita.
Glossary
Abbreviations
- CTAB
cetyltrimethylammonium bromide
- EB
extraction buffer
- OD
optical density
- SDS
sodium dodecyl sulfate
- DEPC
diethyl pyrocarbonate
- TAE
tris-acetate-EDTA
- RT-PCR
reverse transcription—polymerase chain reaction
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c00877.
Concentrations and spectroscopic analysis for RNA extracted using different extraction buffers; concentrations and spectroscopic analysis for RNA extracted using different salt concentrations of EBII; concentrations and spectroscopic analysis for RNA extracted using different time spans for precipitation at the last step with LiCl (7.5 M); concentrations and spectroscopic analysis for RNA extracted from different plants; spectroscopic and concentration comparison of RNA extracted using the modified protocol with the standard protocol; pH comparison when Malvaceae family plants crushed in buffers EBI and EBII; viscosity comparison when Malvaceae family plants crushed in buffers EBI and EBII (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Vadivel V.; Sriram S.; Brindha P. Distribution of flavonoids among Malvaceae family members—A review. Int. J. Green Pharm. 2016, 10 (1), S34. [Google Scholar]
- Bayer C.; et al. Support for an expanded family concept of Malvaceae within a recircumscribed order Malvales: a combined analysis of plastid atpB and rbcL DNA sequences. Botanical Journal of the Linnean Society 1999, 129 (4), 267–303. 10.1111/j.1095-8339.1999.tb00505.x. [DOI] [Google Scholar]
- de Oliveira A. M. Total Phenolic Content and Antioxidant Activity of Some Malvaceae Family Species. Antioxidants 2012, 1 (1), 33–43. 10.3390/antiox1010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen A. M.; Phillips G. O.; Williams P. A.. Food Polysaccharides and Their Applications; CRC/Taylor & Francis, 2006. [Google Scholar]
- Albuquerque P. B. S.; Coelho L. C. B. B.; Teixeira J. A.; Carneiro-Da-Cunha M. G.. Approaches in Biotechnological Applications of Natural Polymers; AIMS Press, 2016, doi: 10.3934/molsci.2016.3.386. [DOI] [Google Scholar]
- Samanta P.; Sadhukhan S.; Das S.; Joshi A.; Sen S. K.; Basu A. Isolation of RNA from Field-Grown Jute (Corchorus capsularis) Plant in Different Developmental Stages for Effective Downstream Molecular Analysis. Mol. Biotechnol 2011, 49 (2), 109–115. 10.1007/s12033-011-9376-8. [DOI] [PubMed] [Google Scholar]
- Choudhary S. B.; et al. An efficient and cost effective method of RNA extraction from mucilage, phenol and secondary metabolite rich bark tissue of tossa jute (C. olitorius L.) actively developing phloem fiber,. 3 Biotech 2016, 6 (1), 100. 10.1007/s13205-016-0415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood N.; Ahmed R.; Azam M. S.; Khan H. A Simple and Swift Method for Isolating High Quality RNA from Jute (Corchorus spp.). Plant Tissue Cult. Biotechnol. 2011, 21 (2), 207–211. 10.3329/ptcb.v21i2.10244. [DOI] [Google Scholar]
- Vignesh R. M.; Nair B. R. Extraction and characterisation of mucilage from the leaves of Hibiscus rosa-sinensis Linn. (Malvaceae). Int. J. Pharm. Sci. Res. 2018, 9 (7), 2883. 10.13040/IJPSR.0975-8232.9(7).2883-90. [DOI] [Google Scholar]
- Tan S. C.; Yiap B. C. DNA, RNA, and Protein Extraction: The Past and The Present. J. Biomed Biotechnol. 2009, 2009, 574398 10.1155/2009/574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgin D. D.; DeLucia E. H.; Clough S. J. A robust plant RNA isolation method suitable for Affymetrix GeneChip analysis and quantitative real-time RT-PCR. Nat. Protoc 2009, 4 (3), 333–340. 10.1038/nprot.2008.249. [DOI] [PubMed] [Google Scholar]
- Ghawana S.; et al. An RNA isolation system for plant tissues rich in secondary metabolites. BMC Res. Notes 2011, 4 (1), 85. 10.1186/1756-0500-4-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorani M. S.; Awasthi P.; Sharma M. P.; Ram R.; Zaidi A. A.; Hallan V. Simultaneous detection and identification of four cherry viruses by two step multiplex RT-PCR with an internal control of plant nad5 mRNA. J. Virol Methods 2013, 193 (1), 103–107. 10.1016/j.jviromet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Heikrujam J.; Kishor R.; Mazumder P. B. The chemistry behind plant DNA isolation protocols. Biochem. Anal. Tools–Methods Bio-Molecules Stud. 2020, 8, 131–141. 10.5772/intechopen.92206. [DOI] [Google Scholar]
- Chang S.; Puryear J.; Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Report 1993, 11 (2), 113–116. 10.1007/BF02670468. [DOI] [Google Scholar]
- Hasan N.; et al. Formulation and development of novel lipid-based combinatorial advanced nanoformulation for effective treatment of non-melanoma skin cancer. Int. J. Pharm. 2023, 632, 122580 10.1016/j.ijpharm.2022.122580. [DOI] [PubMed] [Google Scholar]
- “https://www.agilent.com/cs/library/usermanuals/public/ScreenTape_RNA_QG.pdf.”
- Yang G.; Zhou R.; Tang T.; Shi S. Simple and Efficient Isolation of High-Quality Total RNA from Hibiscus tiliaceus, a Mangrove Associate and Its Relatives. Prep Biochem Biotechnol 2008, 38 (3), 257–264. 10.1080/10826060802164991. [DOI] [PubMed] [Google Scholar]
- Khan Z. A.; Abdin M. Z.; Khan J. A. Functional Characterization of a Strong Bi-directional Constitutive Plant Promoter Isolated from Cotton Leaf Curl Burewala Virus. PLoS One 2015, 10 (3), 1–17. 10.1371/journal.pone.0121656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G.; Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8 (19), 4321–4326. 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A. H.; Brubaker C. L.; Wendel J. F. A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Mol. Biol. Report 1993, 11 (2), 122–127. 10.1007/BF02670470. [DOI] [Google Scholar]
- Liyanage N. M.; Chandrasekara B. C.; Bandaranayake P. C. A CTAB protocol for obtaining high-quality total RNA from cinnamon (Cinnamomum zeylanicum Blume). 3 Biotech 2021, 11 (4), 201. 10.1007/s13205-021-02756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman P.; Choudhary A. K.; Geeta R. A modified protocol yields high-quality RNA from highly mucilaginous Dioscorea tubers. 3 Biotech 2017, 7 (2), 150. 10.1007/s13205-017-0775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal S. M. S. Abundant high-quality RNA from medicinal plants for molecular applications. Journal of Medicinal Plants Research 2012, 6 (39), 5214–5221. 10.5897/JMPR11.1660. [DOI] [Google Scholar]
- George A. Simple and efficient method for functional RNA extraction from tropical medicinal plants rich in secondary metabolites. Tropical Plant Research 2018, 5 (1), 8–13. 10.22271/tpr.2018.v5.i1.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.