Abstract
A central goal of photoprotective energy dissipation processes is the regulation of singlet oxygen (1O2*) and reactive oxygen species in the photosynthetic apparatus. Despite the involvement of 1O2* in photodamage and cell signaling, few studies directly correlate 1O2* formation to nonphotochemical quenching (NPQ) or lack thereof. Here, we combine spin-trapping electron paramagnetic resonance (EPR) and time-resolved fluorescence spectroscopies to track in real time the involvement of 1O2* during photoprotection in plant thylakoid membranes. The EPR spin-trapping method for detection of 1O2* was first optimized for photosensitization in dye-based chemical systems and then used to establish methods for monitoring the temporal dynamics of 1O2* in chlorophyll-containing photosynthetic membranes. We find that the apparent 1O2* concentration in membranes changes throughout a 1 h period of continuous illumination. During an initial response to high light intensity, the concentration of 1O2* decreased in parallel with a decrease in the chlorophyll fluorescence lifetime via NPQ. Treatment of membranes with nigericin, an uncoupler of the transmembrane proton gradient, delayed the activation of NPQ and the associated quenching of 1O2* during high light. Upon saturation of NPQ, the concentration of 1O2* increased in both untreated and nigericin-treated membranes, reflecting the utility of excess energy dissipation in mitigating photooxidative stress in the short term (i.e., the initial ∼10 min of high light).
Introduction
As sessile organisms, plants must acclimate to a broad variety of environmental conditions, especially in response to rapid changes in incident light intensity reaching the pigment–protein complexes housed in the thylakoid membrane. In the natural environment, plants regularly encounter high light (HL) intensities, leading to the closure of reaction centers and subsequent accumulation of excited states of chlorophyll (Chl) pigments throughout the light-harvesting antenna of photosystem II (PSII). Under HL stress, singlet oxygen (1O2*), the electronically excited state of molecular oxygen, can be produced via photosensitization,1 a process in which a light-absorbing molecule (e.g., Chl) transfers excitation energy to ground state triplet 3O2.2,3 Once produced, 1O2* is an electrophilic species that is highly reactive for a wide array of biomolecules, including the D1 protein of PSII,4−7 photosynthetic antenna proteins,8,9 amino acid residues of other proteins,10 DNA,11 and lipids.12,13 All photosynthetic organisms thus employ a range of strategies to carefully regulate the photophysics and photochemistry of Chl excited states and the accompanying production of reactive oxygen species (ROS) such as 1O2*. For reviews on the roles of 1O2*, ROS, and oxidative stress in photosynthesis, see refs (14−20).
One important regulatory process is nonphotochemical quenching (NPQ),21,22 which harmlessly dissipates excess excitation energy in the light-harvesting antenna as thermal energy and therefore protects PSII against damage. Despite the known importance of NPQ-related dissipation to plant fitness and survival,23 many of its underlying molecular mechanisms remain controversial. Additionally, while the necessity of NPQ is frequently framed in terms of preventing the buildup of ROS, such as 1O2*, this has not been explicitly demonstrated, and more recent thinking posits that ROS play essential roles in plant signaling and stress responses.19,24,25 There remains much to be learned about the production, reactivity, and regulation of ROS inside intact photosynthetic systems, such as thylakoid membranes, chloroplasts, and plant cells.26
One open area for advancing our understanding of dynamic photooxidative chemistry occurring in the photosynthetic apparatus is the quantitative detection of 1O2* and changes in its steady state concentration during illumination. Deciphering the participation of 1O2* in in situ functioning of photosynthetic light harvesting regulation is a longstanding challenge in the field. In principle, detection of luminescence (1Δg → 3∑g–) provides direct evidence for the existence of the 1O2* excited state. However, 1O2* emission occurs in the near-IR spectral region and its detection is complicated by spectral congestion, a short lifetime, and quenching in an aqueous solution.27 More commonly, exogenous fluorophores are utilized to selectively detect 1O2* in photosynthesis. However, the bulky and nonpolar molecular structures of abiotic fluorophores can lead to difficulties in their localization at the site of 1O2* generation. Commercially available probes, such as singlet oxygen sensor green (SOSG),28 are also known to undergo “self” photosensitization upon visible light illumination.29−32 To overcome these experimental difficulties, we set out to develop an alternative method for monitoring the concentration of 1O2* in intact photosynthetic systems and its temporal dynamics during illumination.
Electron paramagnetic resonance (EPR) spectroscopy is a well-established technique that is capable of detecting unpaired electrons with high sensitivity. The spin trap 2,2,6,6-tetramethylpiperidine (TEMP) reacts with 1O2* to form EPR-active nitroxide radical species 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO).33 In 1994, Hideg et al. demonstrated spin-trapping EPR for probing the involvement of 1O2* during photoinhibition in plant thylakoid membrane suspensions.34 However, the connection between 1O2* photosensitization and NPQ and the temporal changes in the concentration of 1O2* were left uninvestigated. Here, we build on the method of Hideg et al. with the goal of tracking the real-time involvement of reactive 1O2* during photoprotection. In control experiments using dye-based systems, we defined the correlation of EPR spin probe signals to illumination conditions. We then extend the snapshot EPR methodology to thylakoid membranes for gaining insight into the 1O2* stress response during excess light exposure. Our results support the conventional model of NPQ in regulating the amount of 1O2* produced in the photosynthetic apparatus during short-term photooxidative stress.
Materials and Methods
Thylakoid Membrane Preparation
Preparation of thylakoid membranes was performed in a dark room under green light. Baby spinach leaves (280–450 g, store-bought) were washed and dark-adapted overnight at 4 °C. Thylakoid membranes were purified according to the protocol of Utschig et al.35 Destemmed leaves were blended (Sunbeam, 1.5 L) in 300–500 mL batches of ice-cold grinding buffer with 3–5 short pulses of 1 s duration, followed by filtration through a Hamilton Beach Big Mouth juice extractor. The grinding buffer (pH 7.5) contained 0.4 M NaCl, 20 mM HEPES, 4 mM MgCl2, 5 mM EDTA, and 1 mg/mL fatty acid-free BSA. The spinach solution was then transferred to centrifuge bottles and spun in a Beckman Coulter Avanti J-26 XP centrifuge set to 4 °C for 6 min at 6500g. The supernatant was discarded, and the pellet was gently resuspended in wash buffer (pH 6.5) containing 0.15 M NaCl, 20 mM HEPES, 4 mM MgCl2, 1 mM EDTA, and 1 mg/mL fatty acid-free BSA. Resuspended thylakoids were centrifuged for 1 min at 500g. The supernatant was collected and centrifuged for 6 min at 6000g. Pellets were resuspended using ∼30 mL suspension buffer and centrifuged for 8 min at 12,000g. The suspension buffer (pH 6.0) contained 15 mM NaCl, 20 mM HEPES, 5 mM MgCl2, 1 mM EDTA, 1 mg/mL fatty acid-free BSA, and 20% glycerol. Pellets were then resuspended in 30 mL suspension buffer and centrifuged for 8 min at 16,000g. Finally, pellets were resuspended in 6–8 mL suspension buffer and stored on ice until use.
The approximate chlorophyll concentration of each thylakoid preparation was measured in cold 80% acetone using a Beckman DU 800 spectrophotometer and quantified using the Arnon equations.36 The oxygen evolution activity of isolated thylakoids was confirmed using a Unisense Oxy-NP probe (Figure S1). Thylakoid samples were either used fresh by diluting the stock directly into buffer or frozen at −80 °C until use.
EPR Spin-Trapping Measurements
Continuous-wave (cw) X-band (9.5 GHz) EPR spectra and time traces (kinetics) were measured using a Bruker ELEXSYS II E500 EPR spectrometer (Bruker Biospin Corp, Ettlingen, Germany) equipped with a TE102 rectangular EPR resonator (Bruker ER 4102ST). Samples were measured at room temperature in glass capillary tubes with 1 mm inner diameter. O2-saturated and O2-depleted samples were produced by bubbling the dye stock solutions and equilibrating the EPR capillary tube and syringe with a steady stream of oxygen or nitrogen gas, respectively. All EPR spectra were acquired using field modulation (100 kHz) with an amplitude modulation of 2 G and phase-sensitive lock-in detection, leading to first derivative-type spectra. Unless otherwise specified, spectra were acquired using 12.6 mW microwave power (12 dB attenuation).
To illuminate samples for photosensitization of singlet oxygen, a daylight white LED (SOLIS-3C, Thorlabs) was focused into the EPR cavity with illumination covering the entire visible spectral region from 400 to 800 nm (LED spectrum shown in Figure S2). The photosynthetic photon flux density (PPFD, units of μmol m–2 s–1) was adjusted by altering the brightness of the LED with approximate values specified in each figure caption. The spin traps (2,2,6,6-tetramethylpiperidine (TEMP) and 4-hydroxy-2,2,6,6-tetramethylpiperidine (TEMP-OH)) and spin label (TEMPO) were purchased from Sigma-Aldrich and used as received.
EPR “Snapshot” Measurements of Singlet Oxygen in Thylakoid Membranes
Purified thylakoid membranes (∼2.8 mg Chl mL–1) were diluted to a final concentration of ∼250 μg Chl mL–1 in glycerol resuspension buffer (15 mM NaCl, 20 mM MES, and 20% glycerol in Milli-Q water at pH 6) in the presence of 0.5 mM ATP. Where specified, 2 μL of nigericin (50 mM stock prepared in ethanol) was also added at a final concentration of 100 μM. The thylakoid suspension was stirred and preilluminated with 1000 μmol m–2 s–1 of white light for a set duration. Following the preillumination period, 50 μL of TEMP (1 M stock prepared in acetonitrile) was added to reach a final concentration of 50 mM and incubated for 1 min with continued stirring. As a control experiment, in place of TEMP, 50 μL of the TEMPO nitroxide radical (600 μM stock prepared in acetonitrile) was added to reach a final concentration of 30 μM to assess the stability of TEMPO and any degradation processes leading to destruction of the EPR signal.
To avoid the HL-induced decrease in the TEMPO nitroxide radical signal that was observed during extended illumination of thylakoid membranes in the EPR cavity, the illumination period in the presence of TEMP was limited to 1 min, corresponding to the approximate time for generating a maximal TEMPO signal. Following 1 min illumination in the presence of TEMP, aliquots were transferred to a microcentrifuge tube and immediately flash-frozen with liquid nitrogen, a process that took 15–30 s in total. Frozen samples were stored in the dark at −80 °C until measurement. All EPR experiments were performed within 10 days of freezing the thylakoid sample.
Prior to EPR measurements, each sample was thawed and promptly transferred to an EPR capillary tube (1 mm inner diameter), a process that took 1–2 min in total. Care was taken to minimize illumination of the sample during this process. A cw X-band EPR spectrum was recorded for each sample at room temperature. Spin quantification was performed using the Xepr Spin Count function to quantify the concentration of TEMPO in each sample. This function relies on double integration of the first derivative-type cw EPR spectra and takes the quality factor of the resonator and instrument parameters into account. As a secondary quantification, the relative intensities of the low-field (≈3358 G) or midfield EPR peaks (≈3375 G) of the nitroxide were compared to estimate the changes in TEMPO signal, yielding similar results.
Fluorescence Lifetime Measurements
Prior to florescence lifetime measurements, the thylakoid suspension was diluted in glycerol resuspension buffer (pH 6) with 50 μM methyl viologen as a secondary electron acceptor and 0.5 mM ATP to mediate ATP hydrolysis. Thylakoid membrane samples were placed in a 1 cm quartz cuvette with a microstir bar. To induce NPQ in the membranes, thylakoids were illuminated with 1000 μmol m–2 s–1 of white light provided by an LED (SOLIS-3C, Thorlabs). At specified time points, a 300 μL aliquot of the sample was transferred to a 1 mm cuvette (21-Q-1, Starna Cells) and measured by time-correlated single-photon counting, where the combined transfer and measurement process took ∼30 s.
Excitation was provided by a 405 nm laser diode
(PC-405B, Picoquant) at a repetition rate of 20 MHz with a power of
360 μW at the sample. Chl a fluorescence emission
was collected using a 40 mm focal length collection lens, directed
to a monochromator (SP2300, Acton) set to 680 nm with a slit width
of 25 μm, and detected using an avalanche photodiode (PDM, OptoElectronics
Corp.). The photon arrival time histogram bin width was 25 ps, and
emission was integrated for 4 s per snapshot. Fluorescence lifetimes
were fit using an exponential decay function. The extent of quenching
was calculated as 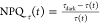 , where τdark is the original
fluorescence lifetime prior to illumination and τ(t) is the fluorescence lifetime at time point t during
the 30 min HL illumination. Unlike fluorescence yield measurements,
fluorescence lifetimes are not impacted by nonquenching processes,
such as pigment bleaching. Under most experimental conditions, the
long fluorescence lifetimes in the dark (>1 ns) indicate closure
of
PSII reaction centers (see discussion in refs (37) and (38)), eliminating contributions
of photochemical quenching. As a control experiment, the addition
of DCBQ, an artificial electron acceptor for PSII, resulted in a substantially
shortened fluorescence lifetime due to increased activity of reaction
centers, presumably because DCBQ replenishes the depleted plastoquinone
pool (Figure S3).
, where τdark is the original
fluorescence lifetime prior to illumination and τ(t) is the fluorescence lifetime at time point t during
the 30 min HL illumination. Unlike fluorescence yield measurements,
fluorescence lifetimes are not impacted by nonquenching processes,
such as pigment bleaching. Under most experimental conditions, the
long fluorescence lifetimes in the dark (>1 ns) indicate closure
of
PSII reaction centers (see discussion in refs (37) and (38)), eliminating contributions
of photochemical quenching. As a control experiment, the addition
of DCBQ, an artificial electron acceptor for PSII, resulted in a substantially
shortened fluorescence lifetime due to increased activity of reaction
centers, presumably because DCBQ replenishes the depleted plastoquinone
pool (Figure S3).
Results and Discussion
EPR Spin-Trapping Method for Detection of Singlet Oxygen Photosensitization
We selected two commercially available dyes that are commonly used for photosensitization of 1O2*, toluidine blue (TB)33,34,39 and rose bengal (RB),40−42 to validate the EPR spin-trapping method for detection of 1O2* in a system significantly less complicated than that of the thylakoid membrane. Following excitation of the dyes to the S1 singlet excited states (UV–visible absorption spectra shown in Figure S4), a nanosecond-timescale intersystem crossing to the longer-lived triplet state (S1 → T1) can lead to triplet–triplet energy transfer in which the T1 state of the chromophore is quenched by the ground state of molecular oxygen. The 1O2* product of this photosensitization process can react with the spin trap TEMP, yielding the TEMPO nitroxide radical (Figure 1A), which can be detected by EPR. In lieu of the lipophilic TEMP, a more hydrophilic version of the spin trap (TEMP-OH with a hydroxyl group at the 4′ position) was used for detection of 1O2* in aqueous solutions.
Figure 1.

(A) Schematic for the detection of photosensitized 1O2* using TEMP as a spin trap. The reaction of 1O2* with the spin trap (TEMP, non-EPR-active) produces the EPR-active nitroxide radical TEMPO, detectable as a triplet in the EPR spectrum. For monitoring 1O2* in an aqueous solution, 4-hydroxy-2,2,6,6-tetramethylpiperidine (TEMP-OH) was employed in place of TEMP, which yields an identical EPR spectrum. (B) Spectra of RB + TEMP-OH measured before and after illumination. The vertical line depicts the midfield peak (3373 G) used for monitoring the kinetics of 1O2* photosensitization. (C) Time traces show comparison of toluidine blue (TB) and rose bengal (RB) as photosensitizers for production of 1O2*. The LED was turned ON at 2 min (see upright arrow) at a PPFD of ∼1780 μmol m–2 s–1. (A) 15 μM TEMPO in acetonitrile; (B, C) 0.2 mM RB or TB and 10 mM TEMP-OH. All EPR spectra and traces were measured at room temperature with 2G modulation amplitude and 12 dB attenuation.
As expected, upon illumination of an aqueous solution of RB and TEMP-OH in the EPR cavity with white light at an environmentally relevant PPFD level of 1000 μmol m–2 s–1, we observed an increase in the EPR signal, confirming the conversion of the EPR-silent spin trap to the EPR-active TEMPO nitroxide radical (Figure 1B). The rate of the signal increase was highly dependent on the light intensity: at low PPFD (∼400 μmol m–2 s–1), the signal continued to increase for at least 10 min, while at higher PPFD (∼4000 μmol m–2 s–1), the maximal signal was reached more quickly, even though the overall amount of signal was identical (Figure S5). In either case, prolonged illumination resulted in decreasing EPR signal with a rate that again depended on illumination intensity (higher light intensity resulted in faster decay). Illumination of an aqueous solution of RB and TEMP generated a much larger TEMPO signal compared to TB (Figure 1C). This difference can be explained by the higher quantum yield of intersystem crossing (ΦISC) for RB relative to TB, likely enabled by the presence of multiple heavy atoms (halogen substituents) in RB.42,43 It is also consistent with a reported ∼75% singlet oxygen quantum yield (ΦΔ) for RB compared to ∼50% for TB at neutral pH.39,44,45 Given its high ΦISC and ΦΔ leading to a significant TEMPO signal, RB was chosen as the optimal dye for systematic investigation of the effects of light intensity and dissolved O2 concentration on 1O2* photosensitization.
In addition, we illuminated an aqueous solution of 0.2 mM RB and 10 mM TEMP-OH directly in the EPR cavity. Upon exposure to low PPFD (220 μmol m–2 s–1), we observed an increase in EPR signal at 3358 G corresponding to the TEMPO product (Figure 2). Upon increasing the PPFD to 1780 μmol m–2 s–1, the slope of the signal increased, and a subsequent increase in the PPFD to greater than 8000 μmol m–2 s–1 resulted in an even larger increase in signal. Compared to an ambient solution, the oxygen-saturated solution of RB and TEMP-OH showed much larger increases in signal at all three PPFDs, likely due to the availability of more O2 to react with the triplet excited state of RB. Conversely, as a control experiment, an oxygen-depleted solution of RB and TEMP-OH showed no increase in signal due to the absence of O2 precluding energy transfer between RB and molecular O2.
Figure 2.
Kinetics of singlet oxygen photosensitization by RB (0.2 mM), detected by TEMP-OH (10 mM) in water. (A) Time traces for the EPR signal monitored at 3358 G for ambient, O2-saturated (+O2), and O2-depleted (+N2) samples with direct illumination in the EPR cavity. The LED intensity was increased at time points denoted by upward-facing arrows, and the LED was switched off at 25 min. Inset: enlargement of the first 10 min. (B) Relative rates of singlet oxygen photosensitization for each illumination intensity, taken as the initial slope of the EPR signal increase at 3358 G during the first 100 s of each illumination condition. Dark, low, medium, and high light levels correspond to 0, 220, 1780, and 8000 μmol m–2 s–1, respectively.
To conclude, RB is an excellent photosensitizer for production of 1O2* in an aqueous solution, resulting in a roughly ∼17-fold higher EPR signal than TB under similar conditions (Figure 1C). Both dyes exhibit photosensitization of 1O2* with rates of TEMPO generation that scale with increasing PPFD. As expected, the rate of signal increase depends on the dissolved O2 content, with larger TEMPO signals being observed for an O2-saturated solution. Longer illumination eventually results in destruction of the nitroxide radical TEMPO signal, a process that is also dependent on the PPFD.
EPR Detection of Singlet Oxygen Production in Thylakoids during Illumination
To assess the effect of thylakoid membrane illumination on the detectable EPR signal of TEMPO, we incubated thylakoids with TEMP and recorded the X-band EPR spectrum in the dark and after illumination. The initial TEMPO signal recorded before illumination (Figure 3A) represents typical impurity in the TEMP spin trap on the order 0.1%. A fraction of this species may also be generated by redox reactions under ambient conditions in a complex redox-active biological system like thylakoid membranes. Upon light illumination of the TEMP-containing thylakoids, we observed complicated kinetics of the TEMPO EPR signal, which depend upon light intensity and duration of the illumination (Figure 3B). It is important to note that the initial growth of the TEMPO EPR signal is due to the reaction of 1O2* with the spin trap TEMP. Control experiments discussed below and shown in Figure 4 confirm that illumination of TEMPO alone cannot lead to an increase in the TEMPO EPR signal but only to a decrease in the signal.
Figure 3.

EPR spectral and kinetic analysis of 1O2* in purified thylakoid membranes. (A) EPR spectra before illumination in the dark (black) and after prolonged illumination (red). The spin trap TEMP (2,2,6,6-tetramethypiperidine) was used at a concentration of 50 mM in glycerol resuspension buffer (pH 6) with 0.5 mM ATP. The dashed vertical line corresponds to the low-field peak (3358 G) used for tracking illumination-induced kinetics. (B) Time trace of the EPR signal at 3358 G for the same thylakoid membrane sample exposed to changes in illumination intensity inside the EPR cavity. Upward- and downward-facing arrows indicate the timing for light intensity changes (values specify PPFD in μmol photons m–2 s–1). The thylakoid concentration was approximately 500 μg Chl/mL. Additional thylakoid membrane aliquots exposed to different light sequences are shown in Figure S6.
Figure 4.

Light-intensity dependence of the nitroxide radical concentration. Time traces for the EPR signal at 3358 G for (A) 15 μM TEMPO in acetonitrile, (B) 15 μM TEMPO in the presence of a photosensitizer (RB) in acetonitrile, and (C) 300 μM TEMPO in a thylakoid membrane suspension at 500 μg Chl/mL with 0.5 mM ATP dissolved in glycerol resuspension buffer (pH 6). There was no TEMP present; hence, no increase in EPR signal is expected and each trace is instead plotted as the fraction of the original signal remaining. The starting signal intensity at 3358 G was ∼0.3 for 15 μM TEMPO in acetonitrile (A, B) and ∼14.5 for 300 μM in thylakoid suspension (C). Upward- and downward-facing arrows indicate the timing for light intensity changes where labels specify the approximate light intensity (μmol photons m–2 s–1).
The light dependence of the EPR signal of the nitroxide TEMPO (Figure 3B) demonstrates that TEMP is capable of monitoring 1O2* in the thylakoid membrane. The observed decay of the TEMPO signal during prolonged illumination is likely due to a combination of 1O2* and the highly redox active environment of the thylakoid membrane, which has previously been shown to cause reduction of TEMPO, leading to a loss of EPR signal.46 Kinetic analysis of the low-field EPR peak at 3358 G showed that the magnitude of the light-induced decrease in signal scaled with the PPFD (Figure S6). ATP was included in the thylakoids to mediate ATP hydrolysis.47 Control experiments show no effect of ATP on nitroxide EPR signals (Figure S7).
To gain further insight into the origin of the decreasing TEMPO signal during illumination of thylakoid membranes, the intensity of the low-field peak at 3358 G (Figure 3A) was tracked for several samples in the presence of high concentrations of the TEMPO nitroxide radical. A sample of 15 μM TEMPO without dye in acetonitrile showed no change in TEMPO signal (Figure 4A), indicating that the nitroxide radical is stable during illumination. In contrast, 15 μM TEMPO in the presence of 0.1 mM RB showed a sizable decrease in signal during illumination (Figure 4B), demonstrating the effect of 1O2* on the decay of TEMPO. Likewise, thylakoid membranes incubated with 300 μM TEMPO showed a very large decrease in signal during illumination, with the signal entirely consumed within 15 min of illumination at PPFDs less than 2000 μmol m–2 s–1 (Figure 4C). Therefore, it was concluded that illumination of the TEMPO nitroxide radical itself is not directly responsible for the decrease in EPR signal. Instead, such a decrease only occurs in the presence of a light-absorbing molecule, either dye or Chl, and the rate of the decrease is faster for higher PPFD. This suggests that an interaction between the excited states of the photosensitizer molecules and TEMPO may occur during continuous illumination. Additionally, chemical reactions leading to TEMPO degradation, such as those documented for TEMPO derivatives exposed to hydroxyl radicals,48 might contribute to the observed decrease in EPR signal during prolonged illumination.
The possibility of a secondary light-dependent reaction resulting in reduction of EPR-active TEMPO to an EPR-silent hydroxylamine implies that EPR detection of 1O2* is an underestimate of the real 1O2* concentration.49 Such a reaction resulting in the disappearance of EPR signal during illumination has been observed in isolated LHCII proteins, with a rate that depended on the PPFD.9 Therefore, side reactions of TEMPO pose a challenge for EPR-based detection of 1O2* in plant systems due to the high concentration of redox-active equivalents generated during illumination of thylakoids. One possible solution is to attempt to reoxidize the diamagnetic TEMPO species back to the radical form via aeration in the presence of lead oxide, followed by extraction into ethyl acetate.9,34 Separately, a decrease in the TEMPO EPR signal has been previously observed in thylakoid membrane systems, which was suggested to be due to the formation of a nonbilayer phase of the thylakoid membrane lipids.50 It has also been suggested that a temporary burst of singlet oxygen produced by free and solubilized Chl may occur at the beginning of illumination,8 although the mechanism remains undetermined.
EPR “Snapshot” Spectroscopic Studies of Photoprotection in Thylakoids
To track the relative levels of 1O2* in thylakoid membranes during photoprotection, we took advantage of the time interval associated with the maximal TEMPO signal during HL (see Figure 3B). By limiting the illumination of the thylakoids in the presence of TEMP to exactly 1 min, we hypothesized that we could minimize the decrease in TEMPO signal that occurs during extended periods of illumination and gain insights into the temporal dynamics of 1O2* production and its regulation in thylakoid membranes. Following 1 min of illumination at 1000 μmol photons m–2 s–1, the TEMPO concentration in a representative thylakoid aliquot increased by 46% as a result of production of 1O2* and its subsequent trapping by TEMP (Figure S8). The increase in TEMPO signal indicates that a 1 min illumination of the thylakoids in the presence of TEMP followed by EPR measurement is well suited for detecting 1O2* produced by HL-exposed thylakoid membranes, consistent with the EPR time traces shown in Figure 3B. We confirmed that the spin concentration remained identical when reacquiring a spectrum for the same sample after rapid freezing and thawing of the capillary, indicating that the process of freezing and thawing a preilluminated thylakoid sample does not alter the measurable concentration of TEMPO (Figure S8).
We thus designed an experimental protocol for reliable detection of 1O2*: (1) expose thylakoid membranes to HL for a set length of time, (2) add TEMP and perform the final minute of HL to capture the 1O2* generated during the HL time period, and (3) freeze-quench the sample for later EPR analysis (see Figure 5A for the experimental design). We confirmed that rapid freeze quenching of the sample in the dark terminates side reactions involved in TEMPO decay, preserving the EPR-active product. Each thylakoid aliquot thus provides a “snapshot” for the amount of 1O2* produced by the membrane at various time points during a total duration of 1 h of HL exposure. We note that a similar experimental approach of storing preilluminated membrane samples in liquid nitrogen until measurement of the EPR spectrum at room temperature has been successfully employed for PSII-containing membranes.46,51 Measurement of 1O2* produced by thylakoid membranes has also been demonstrated using LC/MS-based detection of TEMPO, yielding comparable TEMPO concentrations to the EPR method.52
Figure 5.

“Snapshot” EPR experiment method for monitoring 1O2* in thylakoid membranes (∼250 μg Chl mL–1) during HL exposure. (A) Schematic showing the experimental design for assessing light-induced changes in 1O2* concentration in thylakoid membranes. Thylakoid aliquots were illuminated at 1000 μmol photons m–2 s–1 in separate cuvettes with stirring. The spin trap TEMP was added to each cuvette at a concentration of 50 mM for the final minute of illumination. After the 1 min HL incubation period, the sample was immediately flash-frozen, a process that took 15–30 s. Thylakoid aliquots were stored in the dark at −80 °C and used within 10 days. For measurement, samples were thawed and immediately transferred to an EPR capillary tube. (B) EPR snapshot measurements of 1O2* formed in untreated thylakoid membranes or (C) membranes treated with 100 μM nigericin as a function of the total duration of HL treatment. Individual replicates are presented as gray markers, while the average of replicates and standard error of mean (n = 3) are indicated in black. The TEMPO concentration in each thylakoid aliquot was quantified by double integration of each EPR spectrum. The concentration of TEMPO formed in each HL-exposed thylakoid aliquot (ΔTEMPO) was estimated by subtracting the baseline TEMPO concentration measured in control thylakoid samples that were incubated with TEMP for 1 min in the dark (TEMPO concentration: 34 ± 4 μM, n = 4 replicates). The uncorrected data are shown in Figure S9 along with snapshots for 1 min incubations with TEMPO.
Using our “snapshot” EPR approach, we successfully observed changes in the apparent concentration of 1O2* in thylakoid membrane samples during HL exposure (Figure 5B,C). A significant decrease in the concentration of TEMPO occurred during the initial stage of illumination, with the lowest concentrations observed near the 5 min time point of illumination (Figure 5B). This represents an ∼65% decrease in the rate of 1O2* production by the thylakoid membrane during the first few minutes of HL exposure. Following the initial decrease, the concentration of TEMPO gradually increased, eventually returning to the initial value at 60 min of illumination.
We further investigated how the transmembrane proton gradient alters the dynamics of 1O2* in the thylakoid membrane. Treatment with the chemical uncoupler nigericin dissipates the transthylakoid pH gradient,53 preventing the conversion of violaxanthin to zeaxanthin and hindering the activation of NPQ. Like the untreated sample, thylakoids treated with nigericin showed a sizable decrease in TEMPO during the initial illumination period (Figure 5C). However, this decrease in signal was delayed by ∼5 min relative to the untreated sample, with the minimum TEMPO signal in the presence of nigericin observed around 10 min of illumination. Similarly, the timescale associated with the decrease in TEMPO signal was faster in the absence of nigericin (Figure S10), suggesting a possible dependence of 1O2* kinetics on ΔpH.
To elucidate the connection between 1O2* production and NPQ under HL, we compared our snapshot EPR measurements of TEMPO production with Chl fluorescence lifetimes measured on thylakoid samples under similar conditions (Figure 6A–C). Both the untreated and nigericin-treated thylakoid samples exhibited similar unquenched lifetimes prior to illumination (∼1.8 ns). The laser power, integration time, and long fluorescence lifetimes indicate successful closure of PSII reaction centers and minimal contributions of photochemical quenching in the measurements. Upon high-light exposure, the untreated sample showed a rapid quenching response, reaching a lifetime of ∼1.2 ns within 5 min of illumination due to NPQ. In contrast, the nigericin-treated sample showed less overall quenching (Figure 6C) and a delayed onset of NPQ, requiring >10 min of illumination to reach the same 1.2 ns lifetime (Figure 6B), likely due to a slower formation of ΔpH in the light.
Figure 6.
Correlation of nonphotochemical quenching kinetics and apparent 1O2* concentration in thylakoid membranes treated with various concentrations of nigericin. (A) Fluorescence decay curves for untreated and nigericin-treated thylakoids corresponding to 0, 10, and 30 min of illumination. (B) Average fluorescence lifetime and (C) calculated NPQτ values for each duration of illumination. (D–F) Comparison of fluorescence lifetime (black) and TEMPO concentration (blue) at specified intervals of illumination at 1000 μmol m–2 s–1. Untreated thylakoids (D) and thylakoids in the presence of 1 μM (E) or 100 μM (F) nigericin. Fluorescence lifetimes were measured in glycerol resuspension buffer (pH 6) with 50 μM methyl viologen and 0.5 mM ATP. TEMPO concentrations were measured as specified in Figure 5 and in Materials and Methods.
The comparison of the EPR and time-resolved fluorescence spectroscopy data suggests two distinct stages in the production of 1O2* in thylakoid membranes. During the first stage, representing the membrane’s short-term response to HL, defined here as the first 10 min of illumination, the TEMPO signal is correlated to the average Chl fluorescence lifetime. As NPQ processes switch on, the shortening of the 1Chl* excited state lifetime leads to less 1O2* production (Figure 6D), detectable as decreased levels of TEMPO. The correlation between the trends in TEMPO concentration and dynamics of Chl fluorescence quenching is further supported by the nigericin-treated thylakoid sample, which exhibited an ∼5 min delay to reach the minimum TEMPO concentration (Figure 6F) alongside slower activation of NPQ.
Although the observed trends in TEMPO concentration correlated well with the dynamics of Chl fluorescence quenching for short illumination periods (Figure 6D–F), differences become apparent upon prolonged exposure to HL. After 15 min of illumination, the thylakoid samples show only relatively small changes in fluorescence lifetime due to nearly complete saturation of NPQ of the isolated membranes. Yet, a marked increase in TEMPO signal is observed, with the relative TEMPO concentration eventually matching the starting concentration (i.e., prior to the induction of any NPQ) following 30 min of HL (Figure 6D). Thus, increased amounts of 1O2* are produced upon saturation of all NPQ processes in thylakoid membranes.
We propose that the biphasic temporal trends in 1O2* can be understood in terms of two distinct mechanisms involved in regulation of ROS during HL stress (Figure 7). In the initial regime, the activation of NPQ limits the amount of 1O2* due to a decrease in the average lifetime of the Chl excited state, a response that is the strongest during initial exposure to HL. The correlation between NPQ activation and the decreased production of 1O2* supports the canonical view that the rapid activation of NPQ-related energy dissipation provides crucial regulation during short-term light stress21,23 by limiting the production of 1O2* and thus protecting the photosynthetic apparatus from ROS-induced photooxidative stress. Other than NPQ, on longer timescales, 1O2* levels are controlled by biochemical sinks for ROS removal via antioxidants. These sinks, including carotenoids,54,55 lipids,13 and proteins,10 are generally lipophilic molecules that are capable of chemical and physical scavenging of 1O2* molecules.22,56 The combined interaction of ROS and antioxidants in the photosynthetic apparatus creates a delicate balance of redox active species, which may participate (or provide input) into signaling cascades that can affect the system-wide plant function through gene regulation.57,58 We speculate that saturation of the antioxidant capacity for scavenging and removal of 1O2*—combined with the inability of isolated membranes to activate longer-term processes, such as modulation of gene expression—underlies the observed increase in 1O2* during phase II of the thylakoid’s HL response (Figure 7).
Figure 7.
Proposed biphasic model for 1O2* production in photosynthetic systems during HL stress. Upon HL exposure, the activation of NPQ is one of the initial processes limiting the formation of 1O2*. The decreased Chl excited lifetime results in less 1O2* and therefore decreases the TEMPO signal during the first ∼10 min of illumination of the thylakoid membranes (phase I). On a longer timescale, biochemical regulatory processes, including scavenging of 1O2* by antioxidant molecules (carotenoids, lipids, proteins, etc.) combined with various signaling cascades in the cell, continue to modulate 1O2* levels. In our measurements of isolated thylakoid membranes, continued illumination saturates the antioxidant capacity, leading to increased production of 1O2* during phase II of HL exposure.
The precise cause of the increased 1O2* levels during prolonged HL exposure of thylakoid membranes awaits further investigation. In a fully intact photosynthetic system, longer-term regulatory mechanisms (sustained quenching, photoinhibition, PSII repair, etc.)22 together with stress signaling processes25,59 control in vivo ROS levels, enabling plant survival during extended periods of abiotic stress. The ongoing development of techniques for sensitive and specific measurements of ROS and other redox-active species will reveal new insights into the complex photooxidative chemistry occurring in the photosynthetic apparatus on timescales relevant to environmental perturbations.
Conclusions
Singlet oxygen, an excited state of molecular oxygen, is an unavoidable byproduct of photosynthetic light-harvesting especially under HL conditions. While it has crucial roles in both damage and signaling, the temporal evolution of 1O2* in the photosynthetic apparatus during HL stress is largely unknown. In benchmark studies combining spin-trapping EPR and time-resolved fluorescence spectroscopies, we reveal the complex temporal dynamics of 1O2* during the first hour of HL illumination of spinach thylakoid membranes. We observe an interference effect on the nitroxide radical EPR signal that arises from direct illumination of chromophores, both in dye-based and natural Chl-based systems, which implies an underestimation of 1O2* levels by the spin trapping method. Nonetheless, we successfully demonstrate that the apparent 1O2* concentration in thylakoid membranes changes throughout different stages of HL illumination and offer new insights into the complicated photooxidative chemistry occurring in the photosynthetic apparatus. During the first regime, corresponding to the membrane’s initial response to HL, the relative concentration of 1O2* decreases concurrently with quenching of the Chl fluorescence lifetime. Afterward, upon saturation of NPQ, the concentration of 1O2* rises, reflecting the utility of NPQ in mitigating photooxidative stress in the short term. These findings support previous hypotheses that the physiological role of photoprotective quenching is to reduce the amount of singlet oxygen produced in the photosynthetic apparatus.
Acknowledgments
The authors thank David Tiede, Karen Mulfort, Lin Chen, and Emily Sprague-Klein for useful conversations and scientific resources. Setsuko Wakao and Krishna Niyogi provided valuable feedback on the manuscript. Work performed in the Solar Energy Conversion Group at Argonne National Laboratory (J.N., O.G.P., and L.M.U.) was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences under contract no. DE-AC02-06CH11357. Work performed at the Center for Nanoscale Materials, a U.S. Department of Energy Office of Science User Facility, was supported by the U.S. DOE, Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357. Work performed in Berkeley (C.J.S. and G.R.F.) was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division under Field Work Proposal No. 449B. This material is based upon work supported by the U.S. Department of Energy, Office of Science, Office of Workforce Development for Teachers and Scientists, Office of Science Graduate Student Research (SCGSR) program. The SCGSR program is administered by the Oak Ridge Institute for Science and Education (ORISE) for the DOE. ORISE is managed by ORAU under contract number DE-SC0014664. All opinions expressed in this paper are the authors' and do not necessarily reflect the policies and views of DOE, ORAU, or ORISE.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.biochem.4c00028.
O2 evolution characterization of thylakoids (Figure S1), spectrum of LED light source (Figure S2), fluorescence lifetime measurements of thylakoids with MV and DCBQ (Figure S3), absorption spectra of photosensitizers (TB and RB) (Figure S4), light intensity dependence of 1O2* photosensitization by TB (Figure S5), additional replicates for the kinetics of 1O2* in thylakoids (Figure S6), EPR control measurements with ATP (Figure S7), freeze–thaw effect on the TEMPO EPR signal (Figure S8), spin concentrations measured in snapshot EPR spectroscopy during illumination of thylakoid membranes (Figure S9), and kinetic analysis of changes in TEMPO concentration during illumination (Figure S10) (PDF)
Author Contributions
This manuscript is based on work performed by C.J.S. at Argonne National Laboratory in collaboration with L.M.U., O.G.P., and J.N. as part of a Department of Energy Office of Science Graduate Student Research (SCGSR) award. C.J.S. designed and performed experiments, analyzed data, and wrote the paper. All authors commented on the final version of the paper.
The authors declare no competing financial interest.
Supplementary Material
References
- Kasha M.; Brabham D. E. Singlet Oxygen Electronic Structure and Photosensitization. Singlet Oxygen 1979, 1–33. [Google Scholar]
- Foote C. S. Mechanisms of Photosensitized Oxidation. Science 1968, 162 (3857), 963–970. 10.1126/science.162.3857.963. [DOI] [PubMed] [Google Scholar]
- Ogilby P. R. Singlet Oxygen: There Is Indeed Something New under the Sun. Chem. Soc. Rev. 2010, 39 (8), 3181. 10.1039/b926014p. [DOI] [PubMed] [Google Scholar]
- Barber J.; Andersson B. Too Much of a Good Thing: Light Can Be Bad for Photosynthesis. Trends Biochem. Sci. 1992, 17 (2), 61–66. 10.1016/0968-0004(92)90503-2. [DOI] [PubMed] [Google Scholar]
- Telfer A.; Bishop S. M.; Phillips D.; Barber J. Isolated Photosynthetic Reaction Center of Photosystem II as a Sensitizer for the Formation of Singlet Oxygen. Detection and Quantum Yield Determination Using a Chemical Trapping Technique. J. Biol. Chem. 1994, 269 (18), 13244–13253. 10.1016/S0021-9258(17)36825-4. [DOI] [PubMed] [Google Scholar]
- Weisz D. A.; Gross M. L.; Pakrasi H. B. Reactive oxygen species leave a damage trail that reveals water channels in Photosystem II. Sci. Adv. 2017, 3 (11), eaao3013 10.1126/sciadv.aao3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale R.; Hebert A. E.; Frankel L. K.; Sallans L.; Bricker T. M.; Pospíšil P. Amino Acid Oxidation of the D1 and D2 Proteins by Oxygen Radicals during Photoinhibition of Photosystem II. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (11), 2988–2993. 10.1073/pnas.1618922114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla L.; Rinalducci S. Involvement of Active Oxygen Species in Degradation of Light-Harvesting Proteins under Light Stresses. Biochemistry 2002, 41 (48), 14391–14402. 10.1021/bi0265776. [DOI] [PubMed] [Google Scholar]
- Rinalducci S.; Pedersen J. Z.; Zolla L. Formation of Radicals from Singlet Oxygen Produced during Photoinhibition of Isolated Light-Harvesting Proteins of Photosystem II. Biochim. Biophys. Acta - Bioenerg. 2004, 1608 (1), 63–73. 10.1016/j.bbabio.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Davies M. J. Reactive Species Formed on Proteins Exposed to Singlet Oxygen. Photochem. Photobiol. Sci. 2004, 3 (1), 17. 10.1039/b307576c. [DOI] [PubMed] [Google Scholar]
- Martinez G. R.; Loureiro A. P. M.; Marques S. A.; Miyamoto S.; Yamaguchi L. F.; Onuki J.; Almeida E. A.; Garcia C. C. M.; Barbosa L. F.; Medeiros M. H. G.; Di Mascio P. Oxidative and Alkylating Damage in DNA. Mutat. Res. - Rev. Mutat. Res. 2003, 544 (2–3), 115–127. 10.1016/j.mrrev.2003.05.005. [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C.; Krischke M.; Hoeberichts F. A.; Ksas B.; Gresser G.; Havaux M.; Van Breusegem F.; Mueller M. J. Singlet Oxygen Is the Major Reactive Oxygen Species Involved in Photooxidative Damage to Plants. Plant Physiol. 2008, 148 (2), 960–968. 10.1104/pp.108.125690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M.Review of Lipid Biomarkers and Signals of Photooxidative Stress in Plants. In Plant Abiotic Stress Signaling, Methods in Molecular Biology; Humana: New York, NY, 2023; Vol. 2642, pp 111–128. [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A. Singlet Oxygen Production in Photosynthesis. J. Exp. Bot. 2004, 56 (411), 337–346. 10.1093/jxb/erh237. [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A.; Fufezan C.; Trebst A. Singlet Oxygen Production in Photosystem II and Related Protection Mechanism. Photosynth. Res. 2008, 98 (1–3), 551–564. 10.1007/s11120-008-9349-3. [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C.; Havaux M. Singlet Oxygen in Plants: Production, Detoxification and Signaling. Trends Plant Sci. 2009, 14 (4), 219–228. 10.1016/j.tplants.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Pospíšil P. Production of Reactive Oxygen Species by Photosystem II. Biochim. Biophys. Acta - Bioenerg. 2009, 1787 (10), 1151–1160. 10.1016/j.bbabio.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Pospíšil P. Production of Reactive Oxygen Species by Photosystem II as a Response to Light and Temperature Stress. Front. Plant Sci. 2016, 7, 1950. 10.3389/fpls.2016.01950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C. H.; Ruban A. V.; Noctor G. Viewing Oxidative Stress through the Lens of Oxidative Signalling Rather than Damage. Biochem. J. 2017, 474 (6), 877–883. 10.1042/BCJ20160814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorobrykh S.; Havurinne V.; Mattila H.; Tyystjärvi E. Oxygen and ROS in Photosynthesis. Plants 2020, 9 (1), 91. 10.3390/plants9010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. I. G.; Amarnath K.; Park S.; Steen C. J.; Morris J. M.; Fleming G. R. Models and Mechanisms of the Rapidly Reversible Regulation of Photosynthetic Light Harvesting. Open Biol. 2019, 9 (4), 190043 10.1098/rsob.190043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi R.; Dall’Osto L. Dissipation of Light Energy Absorbed in Excess: The Molecular Mechanisms. Annu. Rev. Plant Biol. 2021, 72 (1), 47–76. 10.1146/annurev-arplant-071720-015522. [DOI] [PubMed] [Google Scholar]
- Külheim C.; Agren J.; Jansson S. Rapid Regulation of Light Harvesting and Plant Fitness in the Field. Science 2002, 297 (5578), 91–93. 10.1126/science.1072359. [DOI] [PubMed] [Google Scholar]
- Dogra V.; Kim C. Singlet Oxygen Metabolism: From Genesis to Signaling. Front. Plant Sci. 2020, 10, 1640. 10.3389/fpls.2019.01640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R.; Zandalinas S. I.; Fichman Y.; Van Breusegem F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. 10.1038/s41580-022-00499-2. [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A.; Shimakawa G. Regulation of the Generation of Reactive Oxygen Species during Photosynthetic Electron Transport. Biochem. Soc. Trans. 2022, 50 (2), 1025–1034. 10.1042/BST20211246. [DOI] [PubMed] [Google Scholar]
- Li H.; Melø T. B.; Arellano J. B.; Razi Naqvi K. Temporal Profile of the Singlet Oxygen Emission Endogenously Produced by Photosystem II Reaction Centre in an Aqueous Buffer. Photosynth. Res. 2012, 112 (1), 75–79. 10.1007/s11120-012-9739-4. [DOI] [PubMed] [Google Scholar]
- Flors C.; Fryer M. J.; Waring J.; Reeder B.; Bechtold U.; Mullineaux P. M.; Nonell S.; Wilson M. T.; Baker N. R. Imaging the Production of Singlet Oxygen in Vivo Using a New Fluorescent Sensor, Singlet Oxygen Sensor Green(R). J. Exp. Bot. 2006, 57 (8), 1725–1734. 10.1093/jxb/erj181. [DOI] [PubMed] [Google Scholar]
- Kim S.; Fujitsuka M.; Majima T. Photochemistry of Singlet Oxygen Sensor Green. J. Phys. Chem. B 2013, 117 (45), 13985–13992. 10.1021/jp406638g. [DOI] [PubMed] [Google Scholar]
- Prasad A.; Sedlářová M.; Pospíšil P. Singlet Oxygen Imaging Using Fluorescent Probe Singlet Oxygen Sensor Green in Photosynthetic Organisms. Sci. Rep. 2018, 8 (1), 13685. 10.1038/s41598-018-31638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragàs X.; Jiménez-Banzo A.; Sánchez-García D.; Batllori X.; Nonell S. Singlet Oxygen Photosensitisation by the Fluorescent Probe Singlet Oxygen Sensor Green®. Chem. Commun. 2009, 20, 2920. 10.1039/b822776d. [DOI] [PubMed] [Google Scholar]
- Gollmer A.; Arnbjerg J.; Blaikie F. H.; Pedersen B. W.; Breitenbach T.; Daasbjerg K.; Glasius M.; Ogilby P. R. Singlet Oxygen Sensor Green®: Photochemical Behavior in Solution and in a Mammalian Cell. Photochem. Photobiol. 2011, 87 (3), 671–679. 10.1111/j.1751-1097.2011.00900.x. [DOI] [PubMed] [Google Scholar]
- Lion Y.; Delmelle M.; van de Vorst A. New Method of Detecting Singlet Oxygen Production. Nature 1976, 263 (5576), 442–443. 10.1038/263442a0. [DOI] [PubMed] [Google Scholar]
- Hideg E.; Spetea C.; Vass I. Singlet Oxygen Production in Thylakoid Membranes during Photoinhibition as Detected by EPR Spectroscopy. Photosynth. Res. 1994, 39 (2), 191–199. 10.1007/BF00029386. [DOI] [PubMed] [Google Scholar]
- Utschig L. M.; Soltau S. R.; Mulfort K. L.; Niklas J.; Poluektov O. G. Z-Scheme Solar Water Splitting via Self-Assembly of Photosystem I-Catalyst Hybrids in Thylakoid Membranes. Chem. Sci. 2018, 9 (45), 8504–8512. 10.1039/C8SC02841A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra R. J.; Thompson W. A.; Kriedemann P. E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta - Bioenerg. 1989, 975 (3), 384–394. 10.1016/S0005-2728(89)80347-0. [DOI] [Google Scholar]
- Miloslavina Y.; Szczepaniak M.; Müller M. G.; Sander J.; Nowaczyk M.; Rögner M.; Holzwarth A. R. Charge Separation Kinetics in Intact Photosystem II Core Particles Is Trap-Limited. A Picosecond Fluorescence Study. Biochemistry 2006, 45 (7), 2436–2442. 10.1021/bi052248c. [DOI] [PubMed] [Google Scholar]
- Sylak-Glassman E. J.; Zaks J.; Amarnath K.; Leuenberger M.; Fleming G. R. Characterizing Non-Photochemical Quenching in Leaves through Fluorescence Lifetime Snapshots. Photosynth. Res. 2016, 127 (1), 69–76. 10.1007/s11120-015-0104-2. [DOI] [PubMed] [Google Scholar]
- Pottier R.; Bonneau R.; Joussot-Dubien J. PH Dependence of Singlet Oxygen Production in Aqueous Solutions Using Toluidine Blue as a Photosensitzer. Photochem. Photobiol. 1975, 22 (1–2), 59–61. 10.1111/j.1751-1097.1975.tb06722.x. [DOI] [PubMed] [Google Scholar]
- Ledford H. K.; Chin B. L.; Niyogi K. K. Acclimation to Singlet Oxygen Stress in Chlamydomonas Reinhardtii. Eukaryot. Cell 2007, 6 (6), 919–930. 10.1128/EC.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P.-T.; Khan S.; Frei H. Time-Resolved O2 1Δg⃗3Σg– Chemiluminescence upon UV Laser Photolysis of an Aromatic Endoperoxide in Aqueous Solution. Chem. Phys. Lett. 1986, 129 (5), 463–467. 10.1016/0009-2614(86)80229-9. [DOI] [Google Scholar]
- Stiel H.; Teuchner K.; Paul A.; Leupold D.; Kochevar I. E. Quantitative Comparison of Excited State Properties and Intensity-Dependent Photosensitization by Rose Bengal. J. Photochem. Photobiol. B Biol. 1996, 33 (3), 245–254. 10.1016/1011-1344(95)07248-9. [DOI] [PubMed] [Google Scholar]
- Murasecco-Suardi P.; Gassmann E.; Braun A. M.; Oliveros E. Determination of the Quantum Yield of Intersystem Crossing of Rose Bengal. Helv. Chim. Acta 1987, 70 (7), 1760–1773. 10.1002/hlca.19870700712. [DOI] [Google Scholar]
- Redmond R. W.; Gamlin J. N. A Compilation of Singlet Oxygen Yields from Biologically Relevant Molecules. Photochem. Photobiol. 1999, 70 (4), 391–475. 10.1111/j.1751-1097.1999.tb08240.x. [DOI] [PubMed] [Google Scholar]
- Kochevar I. E.; Redmond R. W.. Photosensitized Production of Singlet Oxygen. In Methods in Enzymology; Academic Press, 2000; Vol. 319, pp 20–28. [DOI] [PubMed] [Google Scholar]
- Yadav D. K.; Pospíšil P. Evidence on the Formation of Singlet Oxygen in the Donor Side Photoinhibition of Photosystem II: EPR Spin-Trapping Study. PLoS One 2012, 7 (9), e45883 10.1371/journal.pone.0045883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore A. M.; Shinkarev V. P.; Hazlett T. L.; Govindjee Quantitative Analysis of the Effects of Intrathylakoid PH and Xanthophyll Cycle Pigments on Chlorophyll a Fluorescence Lifetime Distributions and Intensity in Thylakoids. Biochemistry 1998, 37 (39), 13582–13593. 10.1021/bi981384x. [DOI] [PubMed] [Google Scholar]
- Marshall D. L.; Christian M. L.; Gryn’ova G.; Coote M. L.; Barker P. J.; Blanksby S. J. Oxidation of 4-Substituted TEMPO Derivatives Reveals Modifications at the 1- and 4-Positions. Org. Biomol. Chem. 2011, 9 (13), 4936–4947. 10.1039/c1ob05037k. [DOI] [PubMed] [Google Scholar]
- Hideg É.; Vass I.; Kálai T.; Hideg K.. Singlet Oxygen Detection with Sterically Hindered Amine Derivatives in Plants under Light Stress. In Methods in Enzymology; Academic Press, 2000; Vol. 319, pp 77–85. [DOI] [PubMed] [Google Scholar]
- Kóta Z.; Horváth L. I.; Droppa M.; Horváth G.; Farkas T.; Páli T. Protein Assembly and Heat Stability in Developing Thylakoid Membranes during Greening. Proc. Natl. Acad. Sci. U. S. A. 2002, 99 (19), 12149–12154. 10.1073/pnas.192463899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D. K.; Kruk J.; Sinha R. K.; Pospíšil P. Singlet Oxygen Scavenging Activity of Plastoquinol in Photosystem II of Higher Plants: Electron Paramagnetic Resonance Spin-Trapping Study. Biochim. Biophys. Acta - Bioenerg. 2010, 1797 (11), 1807–1811. 10.1016/j.bbabio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Karonen M.; Mattila H.; Huang P.; Mamedov F.; Styring S.; Tyystjärvi E. A Tandem Mass Spectrometric Method for Singlet Oxygen Measurement. Photochem. Photobiol. 2014, 90 (5), 965–971. 10.1111/php.12291. [DOI] [PubMed] [Google Scholar]
- Rees D.; Noctor G.; Ruban A. V.; Crofts J.; Young A.; Horton P. PH Dependent Chlorophyll Fluorescence Quenching in Spinach Thylakoids from Light Treated or Dark Adapted Leaves. Photosynth. Res. 1992, 31 (1), 11–19. 10.1007/BF00049532. [DOI] [PubMed] [Google Scholar]
- Ramel F.; Birtic S.; Cuiné S.; Triantaphylidès C.; Ravanat J.-L.; Havaux M. Chemical Quenching of Singlet Oxygen by Carotenoids in Plants. Plant Physiol. 2012, 158 (3), 1267–1278. 10.1104/pp.111.182394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widomska J.; Welc R.; Gruszecki W. I. The Effect of Carotenoids on the Concentration of Singlet Oxygen in Lipid Membranes. Biochim. Biophys. Acta - Biomembr. 2019, 1861 (4), 845–851. 10.1016/j.bbamem.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Kruk J.; Szymańska R. Singlet Oxygen Oxidation Products of Carotenoids, Fatty Acids and Phenolic Prenyllipids. J. Photochem. Photobiol. B Biol. 2021, 216, 112148 10.1016/j.jphotobiol.2021.112148. [DOI] [PubMed] [Google Scholar]
- Noctor G.; Reichheld J.-P.; Foyer C. H. ROS-Related Redox Regulation and Signaling in Plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. 10.1016/j.semcdb.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B.; Polutchko S. K.; Adams W. W. III Structure-Function-Environment Relationship of the Isomers Zeaxanthin and Lutein. Photochem 2022, 2 (2), 308–325. 10.3390/photochem2020022. [DOI] [Google Scholar]
- Woodson J. D. Chloroplast Stress Signals: Regulation of Cellular Degradation and Chloroplast Turnover. Current Opinion in Plant Biology 2019, 52, 30–37. 10.1016/j.pbi.2019.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






