Abstract
Amplification of human immunoglobulin has many potential applications such as analysis of clonality, isolation of immunogenic antigens and antigen-specific immunotherapy. Here we describe a method for amplification of human immunoglobulin heavy and light chains from single B lymphocytes or plasma cells. Cells are isolated by FACS, and Ig is amplified by semi-nested RT–PCR. The method is versatile, sensitive and reliable: it provides appropriately paired heavy and light chains, requiring as little as 2 days to produce amplified Fab DNA from human tissues.
INTRODUCTION
Amplification of immunoglobulin to create reactive antibody or Fab (light chain plus Fd portion of heavy chain) is a common goal of much immunologic and clinical research, yet is not easily accomplished. Most existing methods for IgG amplification either do not yield correctly paired heavy and light chains, do not provide a source of affinity matured antibody, are laborious or unreliable, or some combination of the above. The primary difficulties encountered are low template amount, contamination of other sequences, variability in the 5′ variable region, lack of adequate controls to ensure pure single cell mRNA pools, difficulties in immortalization of B cells and difficulty of plasma cell isolation and analysis.
Combinatorial libraries created by RT–PCR of pooled lymphocytes or tissues provide a wealth of immunoglobulin sequences (reviewed in 1), but cannot be relied upon to provide correct in vivo pairings of heavy (VH) and light (VL) chain V regions. Even on panning in phage display or other format against an identified antigen source, which is both laborious and assumes availability of antigen, reactive Fab may not represent in vivo pairings, as light chain promiscuity may result in reactivity without the original, correct pairings (2). A single cell-based methodology is necessary to yield correctly paired VH + VL Fab.
Here, we describe a simple method for the amplification of correctly paired IgG κ or λ Fab from single human B cells or plasma cells that combines the best features of previous methods, and improves upon them by optimization and simplification. This method has the advantage of versatility, sensitivity and reliability, allowing amplification of correctly paired Fab from different cell types and sources. The method almost invariably provides pure mRNA extracts, and has built-in controls for the detection of template contamination.
MATERIALS AND METHODS
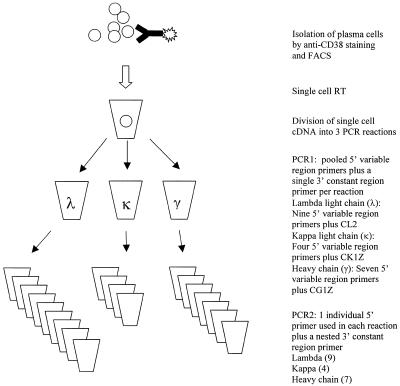
Isolation of single cells
Samples used in experiments in this report were human tonsil and a human medullary carcinoma of the breast. Solid tissue is first manually disaggregated in DMEM (Gibco, Rockville, MD). In this and all later steps, maintenance of low temperature, and minimal and gentle handling minimize cell lysis, a significant source of mRNA contamination of single cells. Both disaggregated tissues and blood samples are purified by centrifugation on a cool Ficoll gradient (Histopaque 1083, Sigma, St Louis, MO) for 20 min at 2500 r.p.m. and 4°C in a Sorvall benchtop centrifuge in order to enrich for plasma cells and lymphocytes. Samples can be stored at –80°C in 10% DMSO until use. Cells are washed once in cold PBS, and pelleted at 2000 r.p.m. for 2 min. Plasma cells are stained with FITC-conjugated mouse anti-human CD38 (Caltag, Burlingame, CA) at a 1:50 dilution in DMEM (Gibco) for 15 min at 4°C. IgG+ B cells are stained with FITC-conjugated mouse anti-human IgG, Fc-specific (Caltag). Cells are washed once with PBS, collected by a 2 min spin at 2000 r.p.m., resuspended in PBS and isolated by FACS (MOFLO cell sorter, Cytomation, Fort Collins, CO). FACS selection of the top 15% of CD38 expressing cells yields virtually pure plasma cells (3). Selection of B cells requires no such stringency, as no other cell types express surface Ig. Single cells are deposited into single wells of 96-well PCR plates (Costar Thermowell, Cambridge, MA) containing 10 µl of cold RT buffer A [6 µl DEPC H2O (Ambion, Austin, TX), 2 µl 5× first strand buffer (Gibco), 2 µl 0.1 M DTT (Gibco)]. Plates are immediately placed on dry ice following sorting. Sorted cells can be used immediately in RT reactions or stored at –80°C for later use.
Reverse transcription
Plates are spun briefly (30 s) to collect liquid and cells in the bottom of wells. Plates must be kept cooled in this and all subsequent steps. It is advisable to use a designated clean area and pipets for RNA work, separate from the PCR work area. To each well is added 5 µl cold RT buffer B [1 µl 0.1% Igepal CA-630 (Sigma), 1 µl oligo-d(T)16 (Perkin Elmer, Norwalk, CT), 0.25 µl DNase I-treated yeast tRNA (Boehringer Mannheim, Indianapolis, IN), 1 µl 5× first strand buffer, 0.5 µl of 40 U/µl RNAsin (Promega, Madison, WI), 1.5 µl DEPC H2O]. RT plates are heated to 65°C for 1 min, cooled to 55°C for 30 s, 45°C for 30 s, 35°C for 30 s, 23°C for 2 min, then 4°C in a PTC-100 Thermocycler (MJ Research Inc., Waltham, MA). An aliquot of 5 µl of cold buffer C [1 µl 10 mM dNTP mix (Gibco), 1 µl 5× first strand buffer, 1 µl Superscript II RnaseH-reverse transcriptase (Gibco), 2 µl DEPC H2O] is then added to each well, for a total reaction volume of 20 µl. RT is performed at 42°C for 90 min.
PCR1
Three PCR1 reactions are run per cell; one for lambda light chain (λ), one for kappa light chain (κ) and one for gamma heavy chain (γ). Ready-To-Go beads (Pharmacia, Piscataway, NJ) in 96-well plates are used for both PCR1 and PCR2. Each reaction uses 0.5 µl of each 20 µM 5′ primer, and 0.5 µl of a 20 µM 3′ constant region primer, H2O to 25 µl, and 5 µl single cell cDNA. Groups of 5′ primers are as described in Sblattero and Bradbury (4), to which terminal restriction sites were added for the purpose of cloning. 3′ constant region primers (CL2, CK1Z, CG1Z) are as described by Burton and Barbas (5). Primers are listed in Table 1. λ: 0.5 µl each VL1B, VL3B, VL38B, VL4B, VL7/8B, VL9B, VL11B, VL13B, VL15B; 0.5 µl CL2, 20 µl H2O, 5 µl cDNA. κ: 0.5 µl each VK1B, VK2B, VK9B, VK12B; 0.5 µl CK1Z, 22.5 µl H2O, 5 µl cDNA. γ: 0.5 µl each VH4B, VH5B, VH6B, VH10B, VH12B, VH14B, VH22B; 0.5 µl CG1Z, 21 µl H2O, 5 µl cDNA.
Table 1. IgG primers for single-cell PCR.
| Primer | Ig Template | |
|---|---|---|
| 5′ | ||
| CL2 | CGCCG(TCTAGA)ACTATGAACATTCTGTAG | λ constant |
| Lnest | GC(TCTAGA)ACTAATGCGTGACCTGGCAGCTGT | λ constant |
| VL1B | CCG(GAGCTC)CAGTCTGTSBTGACGCAGCCGCC | VL1 |
| VL3B | CCG(GAGCTC)TCCTATGWGCTGACWCAGCCAC | VL3 |
| VL38B | CCG(GAGCTC)TCCTATGAGCTGAYRCAGCYACC | VL3 |
| VL4B | CCG(GAGCTC)CAGCCTGTGCTGACTCARYC | VL1,4,5,9 |
| VL7/8B | CCG(GAGCTC)CAGDCTGTGGTGACYCAGGAGCC | VL7,8 |
| VL9B | CCG(GAGCTC)CAGCCWGKGCTGACTCAGCCMCC | VL1,5,9,10 |
| VL11B | CCG(GAGCTC)TCCTCTGAGCTGASTCAGGASCC | VL3 |
| VL13B | CCG(GAGCTC)CAGTCTGYYCTGAYTCAGCCT | VL2 |
| VL15B | CCG(GAGCTC)AATTTTATGCTGACTCAGCCCC | VL6 |
| CK1Z | GCGCCG(TCTAGA)ACTAACACTCTCCCCTGTTGAAGCTCTTTGTGACGGGCGATCTCA | κ constant |
| Knest | GC(TCTAGA)ACTAATGGGTGACTTCGCAGGCGTAGAC | κ constant |
| VK1B | CCG(GAGCTC)GACATCCRGDTGACCCAGTCTCC | VK1 |
| VK2B | CCG(GAGCTC)GAAATTGTRWTGACRCAGTCTCC | VK3,6 |
| VK9B | CCG(GAGCTC)GATATTGTGMTGACBCAGWCTCC | VK2,3,4,6 |
| VK12B | CCG(GAGCTC)GAAACGACACTCACGCAGTCTC | VK5 |
| CG1Z | GCATGT(ACTAGT)TTTGTCACAAGATTTGGG | IgG1 hinge |
| Hcnest | GG(ACTAGT)GTTGCAGATGTAGGTCTGGGTGC | IgG1 CH1 |
| VH4B | CCG(CTCGAG)CAGGTGCAGCTGCAGGAGTCSG | VH4 |
| VH5B | CCG(CTCGAG)CAGGTACAGCTGCAGCAGTCA | VH6 |
| VH6B | CCG(CTCGAG)CAGGTGCAGCTACAGCAGTGGG | VH4 |
| VH10B | CCG(CTCGAG)GAGGTGCAGCTGKTGGAGWCY | VH3 |
| VH12B | CCG(CTCGAG)CAGGTCCAGCTKGTRCAGTCTGG | VH1 |
| VH14B | CCG(CTCGAG)CAGRTCACCTTGAAGGAGTCTG | VH2 |
| VH22B | CCG(CTCGAG)CAGGTGCAGCTGGTGSARTCTGG | VH1,2,5,7 |
| CG2a | CTCGAC(ACTAGT)TTTGCGCTCAACTGTCTT | IgG2 hinge/CH1 |
| CG3a | TGTGTG(ACTAGT)GTCACCAAGTGGGGTTTT | IgG3 hinge |
| CG4a | GCATGA(ACTAGT)TGGGGGACCATATTTGGA | IgG4 hinge |
| CGu | GG(ACTAGT)CTGGGCAYSRTGGGCAY | IgG hinge (universal) |
| HcnestU | GG(ACTAGT)TCTTGTCCACCTTGGTGTTG | IgG CH1 (universal) |
The 5′ V region primers are from Sblattero and Bradbury (4), with added restriction sites for cloning indicated in parentheses. The preferred V family templates are specified, but there is significant cross-priming between families. For PCR1, the 3′ constant domain primers CL2, CK1Z and CG1Z (and CG2a, CG3a and CG4a) are from Burton and Barbas (5), including the added 5′ restriction sites for cloning. Only CG1Z of the heavy chain primers was employed in this study, which is γ1-specific. CL2 was reduced by 1 base at the 3′-end versus the published sequence (5) to suppress primer–dimer formation during PCR1 and improve amplification efficacy. For PCR2, the Hcnest was designed to be optimal for γ1 heavy chain. Although not specifically tested, Hcnest is likely to amplify all IgG subclasses, with mismatches of 1 (γ2, γ3) and 3 (γ4) bases. Single letter codes for nucleotide mixtures during primer synthesis: B = C+G+T; D = A+G+T; M = A+C; R = A+G; S = C+G; W = A+T; Y = C+T.
For broader applications, new consensus primers were designed after completion of these studies to capture all IgGs in single reactions. The 3′ CGu universal hinge domain degenerate primer is for amplifying γ1–γ4. (Alternatively, a mix of four separate primers for γ1–γ4 would achieve the same result.) The 3′ Hcnest universal nesting primer (HcnestU) has no mismatch for all γ1–γ4. These 3′ primers did not undergo the extensive testing of the γ1 primers used to generate the data of this report, but both γ-universal primers performed adequately in preliminary tests using a single γ1 RNA source (not shown). HcnestU may also be applied with the single specific γ subclass primers (CG2a, etc.), where class-specific amplification is sought, and, in particular, HcnestU may be preferable to Hcnest for γ4.
PCR1 reactions are run at 94°C for a 4 min initial hot start, followed by 35 cycles of 94°C for 1 min (denaturation), 55°C for 2 min (annealing) and 72°C for 3 min (elongation). A final 1 min elongation was performed at 72°C.
PCR2
PCR1 reactions are used as template for a second nested PCR (PCR2). It is not advisable to open PCR1 reactions for analysis prior to use as template for nested PCR, as this may result in contamination. Individual 5′ primers are used separately in individual reactions (Fig. 1), rather than being pooled, as in PCR1. Each reaction contains 0.5 µl 5′ variable region primer, 0.5 µl 3′ constant region primer, 24 µl of H2O, 1 µl of the appropriate PCR1 reaction and uses a PCR-Ready-To-Go bead. Nested 3′ primers for λ (Lnest), κ (Knest) and IgG heavy chain (Hcnest) are used in PCR2. (Amplifications represented in this report applied an Hcnest with a 1 base mismatch against all IgGs near the 3′-end of the primer. Hcnest in Table 1 is corrected to omit this mismatch, and performed adequately in separate tests not shown.)
Figure 1.
Single cell RT–PCR.
Amplification is as for PCR1, but with a 60°C annealing step.
Sequence analysis
An aliquot of 5 µl of each PCR2 product is analyzed by agarose gel electrophoresis. Contaminants may result in amplification of both κ and λ from one cell, while amplification of non-IgG plasma cells will result in amplification of light but not heavy chains. PCR2 products are purified (Qiagen Qiaquik, Valencia, CA) and directly sequenced using forward and reverse PCR primers (Perkin Elmer ABI Prism dye termination system). Multiple PCR products generated from different individual V region primers are sequenced from single cells for contamination quality control. These multiple sequences are aligned using CLUSTALV and MacVector, and a single resolved sequence is determined. Contamination results in considerable sequence variation between products from a single well, as contaminating templates will be preferentially amplified by the IgV gene family-specific primer corresponding to the family of the contaminant. Amplification of multiple transcripts even from a single IgV gene family within a single PCR reaction results in two visible templates in the reaction histogram, (Perkin Elmer ABI Prism system), and virtually unreadable sequence due to different (D)J germline gene usage. Further, PCR products are compared between cells. Although clonality results in extremely similar sequences, exact identity would likely indicate cross-contamination of samples. Germline gene usage was determined by comparison to the IMGT database of immunoglobulin sequences at http://imgt.cnusc.fr:8104 , using the DNAPLOT alignment tool.
RESULTS
The method in this report was used to amplify human IgG heavy and light chains from single tonsil plasma cells, tonsil B cells and tumor-infiltrating plasma cells. Paired VH and VL were successfully amplified, while template contamination was rare.
Meticulous analyses of single cell amplifications in our laboratory highlighted the difficulty of obtaining pure single cell mRNA pools from biological samples, and subsequently the difficulty of obtaining correctly paired Fab. mRNA from the lysis of adjacent cells has been determined to be a significant source of contamination, especially for any protocol with the exquisite sensitivity that is required for single cell amplification. Reducing such contaminations was a key feature of the success of this approach. We found that dilution was unsuitable for the isolation of plasma cells (B cells were not tested by this approach), and bead isolation was unsuitable for B cell and plasma cells. These methods almost invariably result in cell lysis and mRNA contamination even with extreme diligence. We have accordingly relied upon a FACS-based method to achieve clean cell isolates, as outlined in Materials and Methods.
Sensitivity and reliability
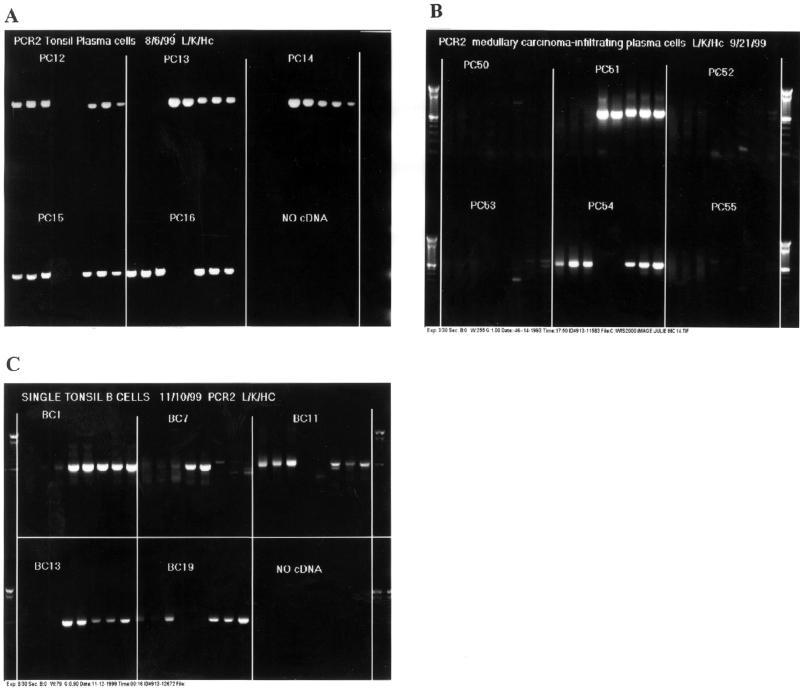
Plasma cells were isolated from tonsil specimens and sorted as single cells into single PCR plate wells to develop and test the single cell RT–PCR technique. The method is demonstrated with an IgG1-specific heavy chain primer, representing the dominant IgG subclass in humans. Light chains and IgG heavy chains were successfully amplified from tonsil plasma cells in 13 of 19 sorted wells, with an overall success rate of 68% (Fig. 2A). Of the six cells for which Fab was not amplified, three had light chains only amplified, possibly indicating a non-IgG isotype or an IgG subclass other than IgG1 (plasma cells are selected for CD38 rather than IgG isotype, in contrast to B cells, below). One well did not amplify, and one well contained multiple transcripts. Similarly, amplification of plasma cells isolated from a medullary carcinoma was demonstrated (Fig. 2B).
Figure 2.
Agarose gel electrophoresis of single cell RT–PCR of IgG. Shown are IgG Fab amplified from single cell. Blocks separated by lines represent multiple PCR amplifications of IgG Fab from single cDNA pools. The products of multiple reactions are shown for each cell in order from left to right: VL1B, VL3B, VL4B, VK1B, VK9B, VH5B, VH10B, VH22B. (A) Plasma cells from tonsil. Plasma cells PC12, PC15 and PC16 are λ positive, while PC13 and 14 are κ positive. (B) Plasma cells from medullary carcinoma. PC54 is λ positive, while PC51 is κ positive. (C) B cells from tonsil. B cells BC11 and BC19 are λ positive. BC1 and BC13 are κ positive. BC7 is κ positive, but there was no heavy chain amplification for this cell, indicating non-γ1 for the IgG + B cell heavy chain.
Because plasma cells are known to express up to 1000-fold more Ig mRNA per cell than B cells, a further, more stringent test of this technique was devised. IgG+ B cells were sorted from tonsil and single cell RT–PCR performed. Fab was amplified from 50% of the sorted wells (11/22) (Fig. 2C). Light chains amplified in three of the 22 wells, wherein heavy chains did not amplify, suggesting non-γ1 IgG subclass members among this minority of positive samples (3/14). Inasmuch as an independent test of the identity of cells in the wells was not performed, some wells may have contained non-B cells or no cells, and the approximate 50% recovery rate of VH + VL paired Fab may be regarded as a minimum efficiency.
In total, out of 131 wells with putative single B cells or plasma cells, paired IgG Fab was obtained for 44, with only one instance of multiple templates observed. The strategy to amplify both κ and λ from individual cells serves as an internal negative control with the non-expressed light chain, and demonstrates the low backgrounds typical of this method (Fig. 2).
Correct pairing and contamination controls
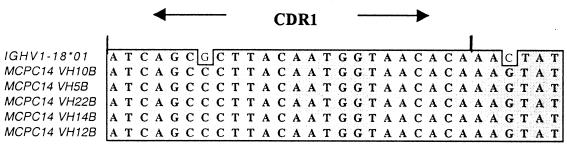
As seen in Figure 2, PCR2 amplification is almost invariably an ‘all or nothing’ phenomenon. There is either amplification with all primers within a group (λ, κ or γ), or none, regardless of the IgV gene family of the sequence being amplified, because of inter-V-family 5′ homologies. Contaminants, however, are preferentially amplified by the most homologous IgV gene family primer (data not shown). Without contaminations, the internal sequences of the amplified products from all primers are identical (Fig. 3), indicating template purity and sequence quality. Lack of contamination of individual cells and subsequent correct Fab pairing is demonstrated both by homogeneity of amplified PCR products from a single cell, and the diversity of sequences amplified between cells (Table 2). No tonsil plasma cell sequences from this sample were clonally related.
Figure 3.
Alignment of sequences from a single plasma cell. Sequences shown are a segment from separate PCR amplifications of a single plasma cell cDNA pool, utilizing different individual IgVH gene family primers (VH10B, VH5B, VH22B, VH14B, VH12B). All VH primer PCR sequences (including regions not shown) were identical and are shown aligned with the parental germline VH gene IGHV1-18*01 for this segment. Two somatic mutations are evident in this segment versus germline (G>C and C>G).
Table 2. Germline gene usage of single tonsil plasma cells.
| Plasma cell | Heavy chain | Light chain | |||
|---|---|---|---|---|---|
| V | D | J | V | J | |
| PC12 | IGHV1-18*01 | IGHD2-15*01 | IGHJ5*02 | IGLV1-40*02 | IGLJ3*02 |
| PC5 | IGHV1-46*03 | IGHD2-08*02 | IGHJ5*02 | IGKV4-01*01 | IGKJ5*01 |
| PC18 | IGHV1-69*01 | IGHD3-16*01 | IGHJ6*03 | IGKV3-20*01 | IGKJ1*01 |
| PC2 | IGHV2-05*07 | IGHD5-18*01 | IGHJ6*03 | IGKV3-15*01 | IGKJ4*01 |
| PC15 | IGHV2-70*01 | IGHD2-21*02 | IGHJ3*02 | IGLV1-47*01 | IGLJ3*02 |
| PC14 | IGHV3-15*02 | IGHD7-27*01 | IGHJ4*02 | IGKV22D-28*01 | IGKJ2*01 |
| PC9 | IGHV3-21*02 | IGHD3-16*01 | IGHJ5*02 | IGKV1-12*01/1D-12 | IGKJ4*01 |
| PC16 | IGHV3-21*02 | IGHD6-13*01 | IGHJ6*03 | IGLV1-47*01 | IGLJ3*02 |
| PC17 | IGHV3-21*02 | IGHD6-13*01 | IGHJ6*03 | IGLV1-40*02 | IGLJ3*01 |
| PC7 | IGHV3-49*01 | IGHD4-11*01/inv | IGHJ4*02 | IGKV2D-28*01 | IGKJ4*01 |
| PC4 | IGHV4-34*08 | IGHD4-17*01 | IGHJ3*02 | IGLV3-19*01 | IGLJ3*02 |
| PC1 | IGHV4-39*06 | IGHD6*01 | IGHJ4*02 | IGKV3-20*01 | IGKJ1*01 |
| PC13 | IGHV5-51*03 | IGHD3-22*01/inv | IGHJ3*02 | IGKV1D-39*01 | IGKJ2*01 |
Sequences are grouped by common heavy chain germline V gene usage.
In practice, the ‘all or nothing’ amplification in PCR2 means that the second round of PCR may frequently be simplified by employing as few as two 5′ V region primers rather than the full set. (A minimum of two primers is recommended as a control for contaminations.)
DISCUSSION
The advent of rapid, accurate single cell FACS sorting, prefabricated PCR reagents and inexpensive, rapid automated sequencing has facilitated this simple method for the amplification of paired IgG heavy and light chains from single cells. By taking advantage of these technologies, researchers can recreate in vivo pairings of VH and VL from large numbers of cells in a short period of time. The method described in this report represents a significant advance over prior methods in that it is rapid, less laborious, describes amplification of κ, λ and IgG heavy chains, and provides controls to avoid creation of incorrectly paired Fab. Further, the method is versatile, and can be used with a variety of human cells and tissue sources.
Because of the sensitivity of this single cell RT–PCR protocol, even minute contaminations can result in artifactual amplifications. FACS was found to be a superior method of single cell isolation in comparison to other methods in this regard. Both serial dilution of cells and bead isolation regularly resulted in varying levels of mRNA contamination in our laboratory. Amplification of contaminants in droplets with no cells was RT-dependent and titratable (data not shown), leading us to conclude that cell lysis was the source of mRNA contamination. Plasma cells are particularly problematic with these alternative methods, as plasma cells rapidly undergo apoptosis ex vivo and contain large amounts of Ig mRNA. Cell lysis is avoidable by maintaining low temperatures, and by employing rapid, gentle handling methods such as brief centrifugations with no vortexing. FACS isolation also minimizes contamination because it is rapid, surprisingly gentle and deposits cells with minimal volumes of accompanying solution.
Low template amount per cell necessitates optimal PCR amplification conditions and freedom from contamination. Given the small amounts of cDNA from single cells, even modest contamination can overwhelm amplifications. Prepackaged PCR reagents such as PCR-Ready-To-Go beads minimize opportunities for contamination. Use of prepackaged reagents also requires less handling time and allows the processing of large numbers of cells more efficiently. The long extension times (3 min) and low annealing temperatures (55 or 60°C) employed in our PCR method maximize amplification, but lengthen PCR cycling times. However, we would caution in the use of newer rapid cycling machines such as the Perkin Elmer Robocycler in PCR1 that may occasionally sacrifice yields of amplified products relative to the more ‘primitive’ units with longer ramping times.
While some researchers have employed IgV gene leader region primers, we have preferred to use individual family consensus primers for sensitivity, for convenience in subsequent cloning and bacterial expression (where eukaryotic leader sequences must be removed), and for detection of contaminating template. While contamination is not common with our current protocol, it is important to detect, as misidentified Fab will fail to provide accurate information, and will be unlikely to have affinity for appropriate antigen. The primary disadvantage of V family primers is that the extreme 5′ end of the framework 1 of the PCR product is not genuine V gene sequence, but only closely-homologous primer sequence, and likely minor divergences in this small domain will not be apparent. However, this very limited ambiguity does not affect positive identification of the appropriate V family. A system based on leader sequences would require a full reoptimization of conditions to obtain single cell results equivalent to what is shown here.
Other approaches to VH + VL identification have been described. In vivo pairings have been recreated from B cells by monoclonal culture, followed by RT–PCR or PCR of immunoglobulins, which is not a single cell technique and which is potentially susceptible to additional V gene modifications during the expansion (6,7). Furthermore, the B cell isolation and immortalization or expansion is laborious, and frequently unreliable. Others have amplified immunoglobulins by single cell genomic DNA PCR with murine B cells or hybridomas (8,9). Genomic DNA PCR methods have the disadvantage of requiring modification of PCR products to remove intronic DNA before expression of functional Fab in bacterial expression systems. Methodologies based on B cells potentially yield antibody that is naïve, not affinity matured or irrelevant to the antigen of interest. On the other hand, plasma cells, which are, by contrast, derived from antigen-activated B cells and affinity-matured, have a very short survival ex vivo, making handling and culture difficult. Our validated FACS-based procedure for contamination-free single plasma cell and B cell isolation are thus an important component of the presently described method.
Single cell RT–PCR of immunoglobulins creates correctly paired Fab, and is well suited for use with plasma cells, which contain high levels of Ig mRNA, but was also successful with B cells with substantially lower amounts of Ig mRNA per cell. There are numerous examples of single cell RT–PCR experiments putatively amplifying one or more transcripts from single cells (10–12). Many of these examples amplified transcripts for which there is no sequence diversity from cell to cell such as actin or IL2. Consequently, it is impossible to determine whether multiple amplification products are truly from one cell inasmuch as the transcripts are identical molecules regardless of the cell of origin. In contrast, because of the somatic recombination and mutation of the immunoglobulin locus, each unrelated B cell or plasma cell has a unique genetic identity.
To our knowledge, there is only one published method for amplification of both heavy and light chains by single cell RT–PCR, specific to rodent antibody V genes (13). There has been no general RT–PCR method for the routine amplification of human heavy and light chain V regions from single B cells or plasma cells, which we have shown required its own optimization, with distinct amplification conditions. Our demonstration of this method focussed on the human IgG isotype, and specifically IgG1. IgG1 is the dominant subclass of IgG, also reflected in our random sample of IgG+ B cells (11/14), and is the predominant antibody responding to T-dependent antigens. Primers for other IgG subclasses are listed in Table 1, where specific interests dictate a different or broader IgG representation. Going beyond IgG to other classes, however, would require an appropriate design and testing of constant region and nested primers, with which the method may equivalently be adapted to any heavy chain isotype for selective immunoglobulin class amplification.
In this report, we have described amplification of paired IgG Fab from tonsil plasma cells, tonsil B cells and tumor-infiltrating plasma cells. This method can thus be used for a variety of clinical and research purposes. For example, this is a simple and definitive method to establish clonality in the diagnosis of lymphoma, multiple myeloma or other lymphoid malignancies by comparing independent single cells. It can also be used in the analysis of response to vaccination or other immune challenge and the molecular maturation of the humoral response. Finally, this method may be used to retrieve reactive, appropriately paired VH + VL from lymphoplasmacytic infiltrated tumor specimens, in which the eliciting antigen is not generally known (J.A.Coronella et al., in preparation). Such Igs have a multitude of potential therapeutic and investigative uses. This method is a powerful tool for creating antigen-specific Fabs and other immunoglobulins to supply the current resurgence of interest in antibody immunotherapies.
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported by postdoctoral fellowships from the National Institutes of Health (J.A.C.), from the US Army (P.T.), and from the Ernst Schering Research Foundation (F.Y.) and grants to R.P.J. from the US Army and the Massachusetts Department of Public Health.
REFERENCES
- 1.Marks C. and Marks,J.D. (1996) New Engl. J. Med., 335, 730–733. [DOI] [PubMed] [Google Scholar]
- 2.Kang A.S., Jones,T.M. and Burton,D.R. (1991) Proc. Natl Acad. Sci. USA, 88, 11120–11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merville P., Dechanet,J., Desmouliere,A., Durnad,I., de Boutellier,O., Garrone,P., Bancherau,J. and Liu,Y.-J. (1996) J. Exp. Med., 183, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sblaattero D. and Bradbury,A. (1998) Immunotechnology, 3, 271–278. [DOI] [PubMed] [Google Scholar]
- 5.Burton D.R. and Barbas,C.F.,III. (1994) Adv. Immunol., 59, 191–280. [DOI] [PubMed] [Google Scholar]
- 6.De Wildt R.M.T., Steenbakkers,P.G., Pennings,A.H.M., van den Hoogen,F.H.J., van Venrooij,W.J. and Hoet,R.M.A. (1997) J. Immunol. Methods, 207, 61–67. [DOI] [PubMed] [Google Scholar]
- 7.Lagerkvist A.C., Furebring,C. and Borrebaek,C.A.K. (1995) Biotechniques, 18, 862–869. [PubMed] [Google Scholar]
- 8.Kantor A.B., Merrill,C.E., MacKenzie,J.D., Herzenberg,L.A. and Hillson,J.L. (1995) Ann. NY Acad. Sci., 764, 224–227. [DOI] [PubMed] [Google Scholar]
- 9.Liu A.H., Creadon,G. and Wysocki,L.J. (1992) Proc. Natl Acad. Sci. USA, 89, 7610–7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong J., Bendahhou,S., Chen,H. and Agnew,W.S. (1994) Nucleic Acids Res., 22, 3253–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toeller K.-M., Scheel-Toellner,D., Seitzer,U., Sprenger,R., Trumper,L., Schluter,C., Flad,H.-D. and Gertes,J. (1996) J. Immunol. Methods, 191, 71–75. [DOI] [PubMed] [Google Scholar]
- 12.Kelso A., Groves,P., Ramm,L. and Doyle,A.G. (1999) Int. Immunol., 11, 617–621. [DOI] [PubMed] [Google Scholar]
- 13.Babcook J.S., Leslie,K.B., Olsen,O.A., Salmon,R.A. and Schraeder,J.W. (1996) Proc. Natl Acad. Sci. USA, 93, 7843–7848. [DOI] [PMC free article] [PubMed] [Google Scholar]