Abstract
Background:
Evobrutinib – an oral, central nervous system (CNS)-penetrant, and highly selective Bruton’s tyrosine kinase inhibitor – has shown efficacy in a 48-week, double-blind, Phase II trial in patients with relapsing MS.
Objective:
Report results of the Phase II open-label extension (OLE; up to week 192 from randomisation) and a cerebrospinal fluid (CSF) sub-study.
Methods:
In the 48-week double-blind period (DBP), patients received evobrutinib 25 mg once-daily, 75 mg once-daily, 75 mg twice-daily or placebo (switched to evobrutinib 25 mg once-daily after week 24). Patients could then enter the OLE, receiving evobrutinib 75 mg once-daily (mean (± standard deviation (SD)) duration = 50.6 weeks (±6.0)) before switching to 75 mg twice-daily.
Results:
Of 164 evobrutinib-treated patients who entered the OLE, 128 (78.0%) completed ⩾192 weeks of treatment. Patients receiving DBP evobrutinib 75 mg twice-daily: annualised relapse rate at week 48 (0.11 (95% confidence interval (CI) = 0.04-0.25)) was maintained with the OLE twice-daily dose up to week 192 (0.11 (0.05–0.22)); Expanded Disability Status Scale score remained stable; serum neurofilament light chain fell to levels like a non-MS population (Z-scores); T1 gadolinium-enhancing lesion numbers remained low. No new safety signals were identified. In the OLE, evobrutinib was detected in the CSF of all sub-study patients.
Conclusion:
Long-term evobrutinib treatment was well tolerated and associated with a sustained low level of disease activity. Evobrutinib was present in CSF at concentrations similar to plasma.
Keywords: Evobrutinib, Bruton’s tyrosine kinase, multiple sclerosis, open-label extension, cerebrospinal fluid
Introduction
Multiple sclerosis (MS) is an inflammatory, progressive neurodegenerative immune-mediated disease of the central nervous system (CNS).1–3 Neuroinflammation in MS is driven by both peripheral lymphocytes (B- and T-cells) and peripheral and CNS myeloid cells (macrophages and CNS-resident microglia). 1 Current MS treatments primarily target peripheral lymphocytes. Despite the effectiveness of available treatments at reducing acute inflammatory lesions and relapses,4–10 the more moderate effect on disability outcomes11,12 indicates there is an unmet need for additional treatment approaches. There is increasing evidence that chronic CNS-compartmentalised inflammation contributes to ongoing tissue loss and disability accumulation from the earliest stages of MS.13–16 CNS-compartmentalised inflammation associated with microglia activation has been detected by positron emission tomography imaging in normal appearing white matter of clinically isolated syndrome 17 patients and by magnetic resonance imaging (MRI) at the borders of slowly expanding lesions in relapsing multiple sclerosis (RMS) and progressive MS patients.14,15,18 In addition, infiltrating B- and T-cells that become trapped behind the blood–brain barrier also have a contributory role in CNS-compartmentalised inflammation. 19 Current treatments have only modest effects on CNS-compartmentalised inflammation.20,21
Bruton’s tyrosine kinase (BTK) is a member of the Tec family of non-receptor tyrosine kinases expressed in B-cells, macrophages and microglia.22,23 BTK plays an important signalling role in several immune processes relevant to the biological mechanisms underlying MS pathology, including B-cell proliferation, immunoglobulin and cytokine production, proinflammatory macrophage differentiation and inflammatory cytokine production by microglia. 24 Elevated levels of activated BTK have been detected in circulating B-cell sub-sets and microglia in lesions from patients.25–27 Modulation of BTK signalling may synergistically reduce both peripherally driven and CNS-compartmentalised inflammation,9,28,29 thereby halting neurodegeneration and demyelination to more effectively prevent disability accumulation in patients with MS.
Evobrutinib is an oral, CNS-penetrant, highly selective covalent BTK inhibitor.30,31 Evobrutinib decreases the activation, proliferation, migration and cytokine release of B-cells.26,32 Evobrutinib also inhibits proinflammatory macrophage differentiation 24 and inflammatory cytokine release by microglia, 24 enhances macrophage phagocytosis 24 and may change the polarisation of microglia/macrophages to a neuroprotective, reparative phenotype,24,33 demonstrating its potential to modulate both adaptive and innate immune processes.
The clinical efficacy of evobrutinib was first observed in a Phase II, double-blind, randomised controlled trial in patients with RMS, where evobrutinib 75 mg twice-daily (BID) had an annualised relapse rate (ARR) over 48 weeks of 0.11. Both evobrutinib 75 mg once-daily (QD) and BID significantly reduced T1 gadolinium-enhancing (Gd+) lesions versus placebo between weeks 12 and 24 (primary endpoint). 34 Evobrutinib was well tolerated with the most common adverse events of any grade being nasopharyngitis, increased levels of lipase and asymptomatic, reversible liver transaminase increases, which typically occurred within the first 90 days of treatment. 34 An integrated safety analysis of evobrutinib clinical trials in rheumatoid arthritis, systemic lupus erythematosus and MS demonstrated that, overall, evobrutinib was generally well tolerated across indications. 35
Here, we report the results from the open-label extension (OLE) of this Phase II trial up to 192 weeks from randomisation at the start of the original double-blind period (DBP). We also report the results of a sub-study investigating the ability of evobrutinib to cross the blood–brain barrier by measuring the concentration of evobrutinib in the cerebrospinal fluid (CSF) relative to plasma.
Patients and methods
Detailed descriptions of the protocol, methods and primary results have been published. 34 The trial is registered with ClinicalTrials.gov (NCT02975349) and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice Guidelines. Written informed consent was obtained from each patient before any trial-related activities were performed.
In brief, this Phase II, double-blind, randomised controlled trial with a parallel, open-label, dimethyl fumarate (DMF) reference group (Supplemental eTables 1 and 2), comprised a 48-week DBP followed by an OLE (Supplemental eFigure 1). Trial participants were 18–65 years of age, diagnosed with relapsing-remitting MS or secondary-progressive MS with superimposed relapses, had ⩾1 documented relapse(s) within 2 years prior to screening (either one relapse within 1 year prior to randomisation or ⩾1 T1 Gd+ MRI lesions within 6 months prior to randomisation), and had a baseline Expanded Disability Status Scale (EDSS) score of ⩽6.
In the 48-week DBP, patients were randomised 1:1:1:1:1 to receive either evobrutinib 25 mg QD, 75 mg QD, 75 mg BID, placebo (switched to evobrutinib 25 mg QD after week 24), or open-label DMF 240 mg BID (120 mg BID for 7 days then 240 mg BID). All evobrutinib and DMF doses were administered while fasting (i.e. taken >1 hour pre-meal or >2 hours post-meal). Patients could then enter the OLE. All patients were switched to evobrutinib 75 mg QD at OLE entry based on the original protocol. After an additional review of the DBP data from the primary analysis at 24 weeks and the blinded extension analysis at 48 weeks, which indicated that BID was superior to QD dosing across several clinical, imaging and biomarker endpoints, a protocol amendment was made to switch all patients in the OLE to evobrutinib 75 mg BID.
This analysis includes patients who completed ⩾192 weeks of treatment (i.e. 48 weeks in the DBP plus 144 weeks in the OLE) or discontinued (cut-off: 30 September 2021). For this publication, patient groups are referred to by the treatment they received in the DBP.
The following OLE endpoints were assessed in patients who had completed ⩾192 weeks of treatment: ARR, time to first qualified relapse (see Supplement), EDSS, T1 Gd+ and T2 lesions. Neurofilament light chain (NfL) levels were measured up to week 144 and reported as control-adjusted NfL Z-scores (i.e. a Z-score of +1.0 represents a mean NfL level in the patient population that is 1 standard deviation (SD) greater than the mean NfL level in a non-MS control population; a Z-score of −1.0 would be 1 SD less than the control mean; a Z-score of 0 represents the reference value of the non-MS control population; age- and body mass index(BMI)-adjusted). 36
Safety is reported from week 48/OLE baseline to ⩾week 192 or until discontinuation for all patients, assessed by the nature, severity and occurrence of treatment-emergent adverse events (TEAEs), as well as vital signs, electrocardiograms and clinical laboratory safety parameters (including liver transaminase levels). Safety for the DBP and 4-week safety follow-up period have been reported previously. 35
Statistical analyses
The statistical analyses are described in the Supplemental Material.
CSF sub-study
A separate sub-study to investigate the concentrations of evobrutinib in the CSF relative to plasma concentrations was also conducted (see Supplement for details).
Results
Clinical efficacy
Of the 164 DBP evobrutinib-treated patients who entered the OLE, 128 (78.0%) completed ⩾192 weeks of treatment with evobrutinib 75 mg QD and then 75 mg BID (evobrutinib-treated patients, mean (±SD) duration of exposure to evobrutinib 75 mg QD before switching to 75 mg BID: 50.6 (±6.0) weeks; Supplemental eFigure 2). Baseline characteristics of these patients are summarised in Table 1.
Table 1.
Demographics and baseline disease characteristics.
| Treatment received during the DBP | CSF sub-study populationa n = 9 (100%) | ||||
|---|---|---|---|---|---|
| Placebo + evobrutinib 25 mg QD n = 39 (100%) | Evobrutinib | ||||
| 25 mg QD n = 39 (100%) | 75 mg QD n = 42 (100%) | 75 mg BID n = 44 (100%) | |||
| Age, years | |||||
| Mean (±SD) | 42.0 (±11.1) | 42.7 (±9.6) | 43.5 (±9.9) | 44.1 (±11.3) | 49.3 ± 11.2 |
| Female, n (%) | 28 (71.8) | 22 (56.4) | 29 (69.0) | 30 (68.2) | 8 (88.9) |
| Type of MS, n (%) | |||||
| Relapsing-remitting | 34 (87.2) | 32 (82.1) | 35 (83.3) | 38 (86.4) | 9 (100.0%) |
| Secondary progressive | 5 (12.8) | 7 (17.9) | 7 (16.7) | 6 (13.6) | 0 (0.0%) |
| Time since MS onset, years | |||||
| Median (min; max) | 7.5 (0.1; 39.4) | 8.4 (1.6; 26.4) | 13.2 (0.4; 23.3) | 11.2 (0.2; 39.4) | 6.9 (0.3; 23.3) |
| Relapses in the previous 2 years, n (%) | |||||
| 1 relapse | 21 (53.8) | 25 (64.1) | 14 (33.3) | 22 (50.0) | – |
| ⩾2 relapses | 18 (46.2) | 14 (35.9) | 28 (66.7) | 22 (50.0) | – |
| Relapses in year prior to randomisation | |||||
| Mean (±SD) | 1.2 (±0.8) | 1.2 (±0.5) | 1.2 (±0.4) | 1.1 (±0.5) | – |
| Score on EDSS | |||||
| Mean (±SEM) | 3.4 (±0.3) | 3.3 (±0.2) | 3.6 (±0.2) | 3.5 (±0.2) | – |
| Median (min; max) | 3.5 (0.0; 6.0) | 3.0 (1.5; 6.0) | 3.5 (1.5; 6.0) | 3.3 (1.0; 6.0) | 3.0 (2.0; 5.0) |
| T1 Gd + lesions | |||||
| Patients with lesions, n (%) | 17 (43.6) | 12 (30.8) | 16 (38.1) | 16 (36.4) | – |
| Mean (±SEM) | 1.1 (±0.3) | 0.6 (±0.2) | 1.8 (±0.9) | 1.2 (±0.4) | – |
| T2 lesion volume, cm 3 | |||||
| Mean (±SEM) | 14.4 (±1.7) | 13.6 (±1.7) | 14.5 (±1.8) | 18.6 (±2.1) | – |
These patients had been in the OLE for 121 weeks on average and were treated with evobrutinib 75 mg BID during the OLE for 73 weeks on average. The patients had no confirmed relapses while on evobrutinib 75 mg BID during the OLE to the time of the CSF sub-study (cut-off 7 December 2020).
OLE analysis set.
BID: twice-daily; CSF: cerebrospinal fluid; EDSS: Expanded Disability Status Scale; Gd+: gadolinium-enhancing; MS: multiple sclerosis; n: number of patients; OLE: open-label extension; QD: once-daily; SD: standard deviation; SEM: standard error of the mean; %: proportion of patients.
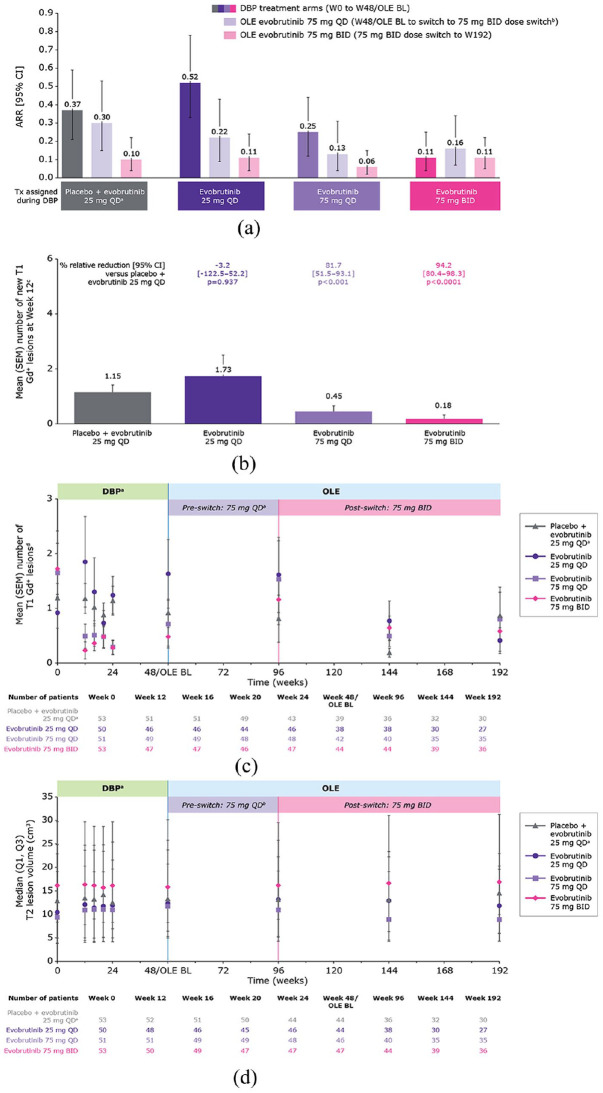
The mean number of relapses in the year prior to randomisation ranged from 1.1 to 1.2 across the original evobrutinib DBP treatment groups (Table 1). In patients receiving evobrutinib 75 mg BID in the DBP, the low ARR observed at week 48 (0.11, 95% confidence interval (CI) = (0.04–0.25)) increased when patients were switched to the OLE QD dosing regimen (0.16 (0.07–0.34)) reducing again once patients switched back to the BID dosing regimen (0.11 (0.05–0.22); Figure 1(a) and Table 2). Those patients treated with placebo/evobrutinib 25 mg QD, evobrutinib 25 mg QD and 75 mg QD during the DBP experienced a marked reduction in ARR during the OLE, especially after switching to evobrutinib 75 mg BID. Across all treatment groups, the ARR was the lowest once the patients had switched to the BID dosing regimen (range = 0.06–0.11) rather than the QD dosing regimen (range = 0.13–0.30) during the OLE.
Figure 1.
(a) Annualised relapse rate. (b) Mean (SEM) number of new T1 Gd+ lesions at week 12. (c) Mean (SEM) number of T1 Gd+ lesions over time. (d) Median T2 lesion volume over time.
ARR (95% CI) for the whole OLE period (week 48/OLE BL to week 192) was: placebo + evobrutinib 25 mg QD, 0.18 (0.10–0.28); evobrutinib 25 mg QD, 0.15 (0.08–0.26); evobrutinib 75 mg QD, 0.09 (0.04–0.16); evobrutinib 75 mg BID, 0.13 (0.07–0.22).
Cut-off 30 September 2021.
aPatients switched from placebo to evobrutinib 25 mg QD after 24 weeks in the DBP.
bEvobrutinib-treated patients (n = 151), mean (±SD) duration of exposure to evobrutinib 75 mg QD dose before the switch to 75 mg BID: 50.6 (±6.0) weeks.
cMean number of new T1 Gd+ lesions was estimated using negative binomial regression, with covariates for presence or absence of baseline T1 Gd+ lesions and treatment arm.
dT1 Gd+ lesion counts reported here were measured at individual time points (and do not represent annualised or cumulative values).
ARR: annualised relapse rate; BID: twice-daily; BL: baseline; CI: confidence interval; DBP: double-blind period; Gd+: gadolinium-enhancing; OLE: open-label extension; QD: once-daily; SD: standard deviation; SEM: standard error of mean; Tx: treatment; W: weeks.
Table 2.
MRI and clinical outcomes.
| Outcome | Treatment received during the DBP | |||
|---|---|---|---|---|
| Placebo + evobrutinib 25 mg QD |
Evobrutinib 25 mg QD |
Evobrutinib 75 mg QD |
Evobrutinib 75 mg BID |
|
| Treatment received during the OLE Evobrutinib 75 mg QD➔BID | ||||
| Unadjusted ARR (95% CI) | ||||
| Weeks 0–48 a | 0.37 (0.21–0.59) | 0.52 (0.33–0.78) | 0.25 (0.12–0.44) | 0.11 (0.04–0.25) |
| OLE evobrutinib 75 mg QD
c
(week 48/OLE BL to 75 mg BID dose switch b ) |
0.30 (0.15–0.53) | 0.22 (0.09–0.43) | 0.13 (0.04–0.31) | 0.16 (0.07–0.34) |
| OLE evobrutinib 75 mg BID
c
(75 mg BID dose switch to week 192 b ) |
0.10 (0.04–0.22) | 0.11 (0.04–0.24) | 0.06 (0.02–0.15) | 0.11 (0.05–0.22) |
| Week 48/OLE BL–week 192 c | 0.18 (0.10–0.28) | 0.15 (0.08–0.26) | 0.09 (0.04–0.16) | 0.13 (0.07–0.22) |
| Change from baseline (week 0) to week 192 in EDSS score | ||||
| Number of patients | 31 | 27 | 36 | 36 |
| Mean (±SEM) | 0.06 (±0.14) | 0.00 (±0.15) | 0.01 (±0.07) | 0.04 (±0.08) |
| Median (min; max) | 0.0 (-1.5; 3.0) | 0.0 (-1.5; 2.5) | 0.0 (-1.0; 1.5) | 0.0 (-0.5; 2.0) |
| Number of new T1 Gd + lesions at week 12 | ||||
| Number of patients | 52 | 48 | 51 | 50 |
| Mean (±SEM) | 1.15 (±0.27) | 1.73 (±0.78) | 0.45 (±0.21) | 0.18 (±0.14) |
| Median (min; max) | 0 (0; 9) | 0 (0; 34) | 0 (0; 10) | 0 (0; 7) |
| Number of T1 Gd + lesions at week 192 | ||||
| Number of patients | 30 | 27 | 35 | 36 |
| Mean (±SEM) | 0.87 (±0.43) | 0.41 (±0.23) | 0.80 (±0.59) | 0.58 (±0.19) |
| Median (min; max) | 0 (0; 11) | 0 (0; 6) | 0 (0; 20) | 0 (0; 5) |
| Change from baseline (week 0) to week 192 in T2 lesion volume, cm 3 | ||||
| Number of patients | 30 | 27 | 35 | 36 |
| Mean (±SEM) | 1.58 (±0.75) | 1.31 (±0.65) | 0.98 (±0.39) | 1.64 (±0.48) |
| Median (min; max) | 0.23 (-3.43; 18.34) | 0.06 (-1.86; 14.66) | 0.01 (-0.63; 10.67) | 0.74 (-2.23; 13.58) |
| NfL Z-scores at week 144 | ||||
| Number of patients | 30 | 29 | 33 | 37 |
| Median (95% CI) | 0.11 (0.67–0.84) | 0.03 (0.38–0.65) | -0.03 (0.85–0.73) | -0.13 (0.40–0.93) |
mITT analysis set: placebo + evobrutinib 25 mg QD/75 mg QD–BID, n = 53 (98%); evobrutinib 25 mg QD/75 mg QD–BID, n = 50 (96%); evobrutinib 75 mg QD/75 mg QD–BID, n = 51 (96%); evobrutinib 75 mg BID/75 mg QD–BID, n = 53 (98%).
Evobrutinib-treated patients (n = 151), mean (±SD) duration of exposure to evobrutinib 75 mg QD dose before the switch to 75 mg BID: 50.6 (± 6.0) weeks.
SAF-OLE analysis set: placebo + evobrutinib 25 mg QD/75 mg QD–BID, n = 39 (72%); evobrutinib 25 mg QD/75 mg QD–BID, n = 39 (75%); evobrutinib 75 mg QD/75 mg QD–BID, n = 42 (79%); evobrutinib 75 mg BID/75 mg QD–BID, n = 44 (82%).
The unadjusted ARR was defined as the number of relapses among patients divided by the number of patient-years of follow-up. For patients who discontinued the trial early, all relapses and follow-up through the safety follow-up visit were included. Mean number of new T1 Gd+ lesions were estimated using negative binomial regression, with covariates for presence or absence of baseline T1 Gd+ lesions and treatment arm.
ARR: annualised relapse rate; BID: twice-daily; CI: confidence interval; EDSS: Expanded Disability Status Scale; Gd+: gadolinium-enhancing; OLE: open-label extension; NE: not estimable; NfL: neurofilament light chain; QD: once-daily; SD: standard deviation; SEM: standard error of the mean.
Patients receiving evobrutinib 75 mg BID in the DBP had a >3-fold longer time to cumulative probability of 0.25 for a first qualified relapse than patients initiated on placebo/evobrutinib 25 mg QD (data not shown).
Mean EDSS scores remained stable week 0–192, regardless of the initial evobrutinib DBP treatment (Supplemental eFigure 3 and Table 2), with no change in median EDSS score from baseline to week 192 (Table 2). After reviewing EDSS in 176 patients, 160 (90.9%) patients did not have 12-week clinical disease progression by the end of the OLE phase.
MRI lesions
In the first 12 weeks of the DBP, evobrutinib 75 mg BID had a rapid onset of action and a large reduction in the mean number of new T1 Gd+ lesions versus placebo (relative reduction: 94.2%; Figure 1(b) and Table 2). In the long-term, the mean number of T1 Gd+ lesions remained low week 0–192 (Figure 1(c) and Table 2). During the OLE, the mean number of T1 Gd+ lesions fluctuated with dose changes. There was a temporary increase from week 48 to week 96, corresponding with evobrutinib 75 mg QD, with a subsequent decrease following the switch to evobrutinib 75 mg BID in the OLE. In all treatment groups, the switch to evobrutinib 75 mg BID during the OLE resulted in a reduction of T1 Gd+ lesions versus the 75 mg QD OLE treatment period.
Overall, T2 lesion volume remained stable across evobrutinib DBP treatment groups from baseline to week 192. Patients who received evobrutinib 75 mg BID had higher T2 lesion volume at baseline which remained stable during treatment. Patients who received QD dosing, however, had lower T2 lesion volume at baseline, and experienced a numerical decrease after switching to BID (Figure 1(c) and Table 2).
Serum biomarker – NfL
Evobrutinib reduced NfL levels in a dose-dependent manner during the DBP, with a rapid reduction within the first 12 weeks with evobrutinib 75 mg QD and BID. While at week 24, reductions in NfL levels compared with baseline levels were only observed in patients receiving evobrutinib 75 mg BID. In patients who received evobrutinib 75 mg BID during the 48-week DBP, an increase in NfL Z-score was observed while on evobrutinib 75 mg QD during the OLE, which decreased after switching back to evobrutinib 75 mg BID. The median NfL Z-score for all evobrutinib-treated patients approached zero, representing mean NfL values comparable with the age- and BMI-matched control group (Supplemental eFigure 4 and Table 2). 36 After switching from evobrutinib 75 mg QD to 75 mg BID in the OLE, NfL levels overall and within the original DBP treatment groups were further reduced and Z-scores dropped below zero at week 144.
Safety
From OLE initiation to week 192, no new safety signals were identified (Table 3). A total of 131/164 (80%) patients initiated on evobrutinib in the DBP had a TEAE during the OLE period. The majority of TEAEs during the OLE were mild/moderate (62%/48%; data not shown). The most common TEAEs reported across the evobrutinib-treatment groups included nasopharyngitis (15%) and lipase increase (12%) (Supplemental eTable 3). Serious TEAEs were reported by 24/164 (15%) patients initiated on evobrutinib in the DBP (Table 3 and Supplemental eTable 4). Seven patients had TEAEs leading to withdrawal (Table 3). Two fatal events occurred during the OLE reporting period in patients who had received evobrutinib in the DBP; according to the investigator, the fatal events were not deemed to be treatment related (COVID-19 pneumonia (unvaccinated, high BMI), and Escherichia coli sepsis with febrile state and acute tubulointerstitial nephritis). No clinically remarkable elevations in liver aminotransferases as seen in the DBP of the trial (data not shown; further information can be found in the original Phase II manuscript) 34 were observed in the OLE after patients switched from lower doses of evobrutinib to 75 mg BID, or after prolonged treatment.
Table 3.
TEAEs during the OLE period.
| Patients, n (%) | Treatment received during the DBP | |||
|---|---|---|---|---|
| Placebo + evobrutinib 25 mg QD |
Evobrutinib 25 mg QD |
Evobrutinib 75 mg QD |
Evobrutinib 75 mg BID |
|
| Treatment received during the OLE Evobrutinib 75 mg QD➔BID | ||||
| n = 39 | n = 39 | n = 42 | n = 44 | |
| Any TEAE | 30 (76.9) | 27 (69.2) | 40 (95.2) | 34 (77.3) |
| Any Grade 3 TEAE a | 4 (10.3) | 7 (17.9) | 7 (16.7) | 6 (13.6) |
| Any Grade 4 TEAE a | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.5) |
| TEAEs related to trial agent | 9 (23.1) | 5 (12.8) | 11 (26.2) | 13 (29.5) |
| Any serious TEAE | 6 (15.4) | 9 (23.1) | 5 (11.9) | 4 (9.1) |
| TEAEs leading to withdrawal of treatment b | 4 (10.3) | 2 (5.1) | 0 (0.0) | 1 (2.3) |
Includes all safety data from the OLE using a data cut-off of 30 September 2021.
According to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), Version 4.03.
Of these, four patients had events considered to be treatment related (placebo + evobrutinib 25 mg QD/75 mg QD–BID, n = 2; evobrutinib 25 mg QD/75 mg QD–BID, n = 1; evobrutinib 75 mg BID/75 mg QD–BID, n = 1).
Two fatal events occurred in patients receiving evobrutinib in the DBP, which were not deemed to be treatment related by the investigators (evobrutinib 25 mg QD (n = 1): COVID-19 pneumonia (unvaccinated); evobrutinib 75 mg BID (n = 1): E. coli sepsis with febrile state and acute tubulointerstitial nephritis).
BID: twice-daily; DBP: double-blind period; OLE: open-label extension; QD: once-daily; TEAEs: treatment-emergent adverse events.
CSF sub-study
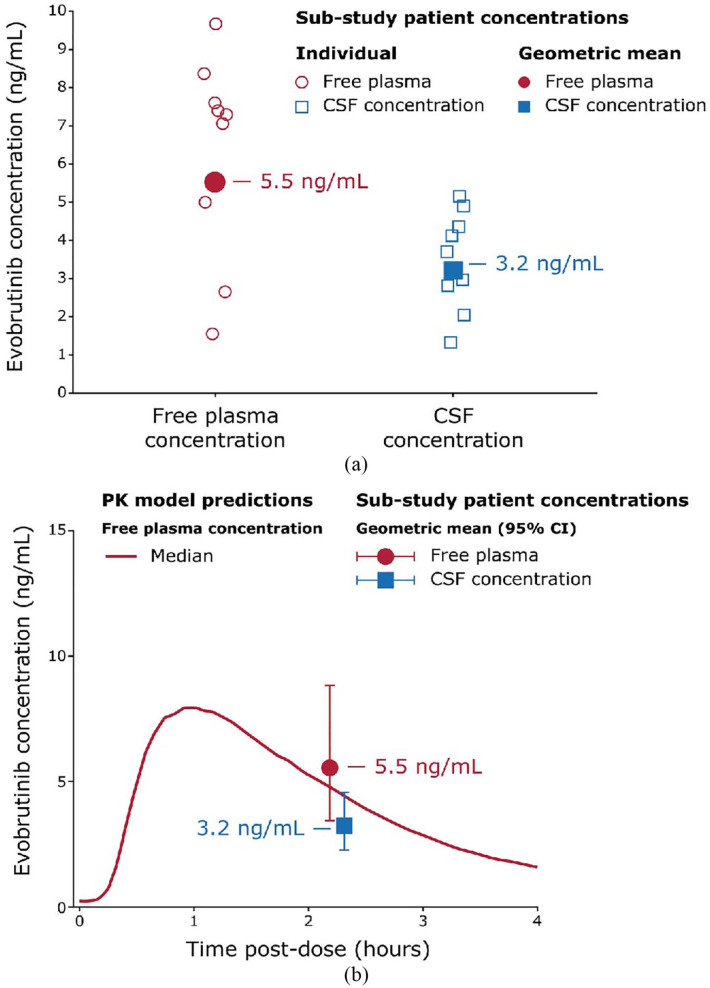
Nine patients administered with evobrutinib 75 mg BID for 73 weeks during the OLE were enrolled into the CSF sub-study (Table 1). These patients had no confirmed relapses while on evobrutinib 75 mg BID during the OLE to the time of the CSF sub-study (CSF sub-study cut-off: 7 December 2020; week 192 cut-off: 30 September 2021). Total and free plasma evobrutinib concentrations were consistent with the population pharmacokinetic (PK) model developed with data from all patients in the Phase II trial, demonstrating the CSF sub-study patients were representative of the entire trial population (Figure 2).37–40
Figure 2.
(a) Evobrutinib CSF and free plasma concentrations. (b) Evobrutinib CSF and free plasma concentrations relative to the population PK model.
CI: confidence interval; CSF: cerebrospinal fluid; PK: pharmacokinetic.
Approximately 2 hours after dosing, the geometric mean total evobrutinib concentration in plasma was 115.0 ng/mL (median (min; max): 152 ng/mL (32; 202)) and the geometric mean free plasma concentration was 5.5 ng/mL (7.3 ng/mL (1.5; 9.7) Figure 2). CSF samples were collected after blood sampling. Evobrutinib was detected in the CSF of all nine patients with a geometric mean concentration of 3.2 ng/mL (3.7 ng/mL (1.3–5.2)); this CSF concentration was 58% of the free plasma concentration. A PK/pharmacodynamic (PD) model developed with the Phase II data and previous studies predicted the concentrations measured in plasma and CSF would result in high and sustained Bruton’s tyrosine kinase occupancy (BTKO), an indicator of BTK inhibition. 40
Discussion
In this Phase II study, we report the efficacy and safety of evobrutinib over a period of >3.5 years. The low ARR observed in patients treated with evobrutinib 75 mg BID during the 48-week DBP was maintained in the OLE. The safety profile observed after an additional >2.5 years of treatment in the OLE was consistent with that observed during the 48-week DBP. Finally, this is the first report to measure BTK inhibitor concentrations in the CSF of patients with RMS, showing that at a therapeutic dose evobrutinib reached a concentration within the CSF, which is predicted by PK/PD modelling,37,39 to be sufficient to achieve high levels of BTKO. These results support the hypothesis that evobrutinib can target immune cells within the CNS compartment in addition to effects on peripheral immune cells.
Overall, the data support the choice of BID dosing over QD dosing of evobrutinib in the OLE and in Phase III evolutionRMS1 and evoutionRMS2 trials. In the DBP, numerical reductions on ARR and Gd+ lesions were the greatest with BID and the significant reductions in NfL seen at 12 weeks were maintained by BID dosing. In the OLE, following the switch from evobrutinib 75 mg QD to 75 mg BID, ARR, the number of T1 Gd+ lesions, and NfL levels were further reduced compared with both the DBP and the OLE pre-switch evobrutinib 75 mg QD dose. T2 lesion volume and EDSS remained stable throughout the OLE, regardless of the DBP treatment arm, despite the switch to QD and back to BID. This might reflect that EDSS is less sensitive to changes within a 1-year period compared with Gd+ lesions and ARR.
The reason for the reduced efficacy seen with QD dosing is likely due to the continuous turnover of endogenous BTK protein. Similar to other BTK inhibitors, evobrutinib is cleared from the system rapidly, plasma concentrations are <1% of Cmax within 8 hours, 41 and any newly synthesised BTK would not be bound until the next dose. BID dosing, therefore, reduces the time for newly synthesised BTK to remain unbound versus QD dosing. Exposure–response modelling of the DBP showed a significant relationship between daily evobrutinib exposure (area under the curve) and pre-dose BTK occupancy (BTKO), or BTK bound by evobrutinib (an indicator of BTK inhibition) in immune cells and the reduction in ARR and T1 Gd+ lesions/T2 lesion volume observed with evobrutinib. Based on PK and BTKO simulations, 75 mg BID dosing was predicted to maintain a high level of BTK inhibition (defined as >95% BTKO) in 92% of patients when measured just before their next dose, while less than half of patients (48%) maintained >95% BTKO with 75 mg QD dosing. 37 In this study, evobrutinib was taken with food, which increases the bioavailability by 49% compared with trials where evobrutinib is administered under fasting conditions. 40 Modelling predicted that an evobrutinib 45 mg BID dose taken with food would result in comparable evobrutinib exposure and BTKO (pre-dose BTKO > 95% in 93% of patients) to the 75 mg BID dose taken without food37,40 and was, therefore, the dose that was administered in the Phase III RMS trials (evolutionRMS 1 and 2). The combination of covalent binding, 31 BTK protein turnover 41 and BID dosing to achieve maximal sustained BTKO and daily exposure37,40 in the periphery and the CNS is likely the basis for the maintained efficacy profile of the evobrutinib BID dose over 192 weeks.
During the OLE, no new safety signals were identified with long-term evobrutinib treatment or exposure. As previously reported, transient liver aminotransferases elevations were observed in some patients treated with evobrutinib during the first 24 weeks of treatment in the DBP. 34 Elevations were not observed in the OLE after prolonged treatment or after the switch from lower doses of evobrutinib to 75 mg BID (Table 3). All patients receiving evobrutinib during the DBP had been on treatment for at least 56 weeks before entering the OLE; no clinically relevant liver enzyme elevations occurred in the OLE compared with what occurred at treatment initiation. In addition, the collected safety data in the DBP and OLE support the low potential for off-target inhibition and related adverse effects due to the high BTK selectivity of evobrutinib.30,31,42
The potential for evobrutinib to inhibit BTK in the CNS is supported by the CSF sub-study results, which demonstrated CSF concentrations of evobrutinib achieved with a therapeutic dose are in the range predicted to achieve high BTKO. Preclinical studies demonstrated that administration of evobrutinib at pharmacological doses resulted in high levels of BTKO in the periphery and the brain, persisting beyond evobrutinib plasma clearance as a result of covalent binding.41,43 Evobrutinib modulates B-cell function via inhibition of BTK signalling rather than B-cell depletion via cell lysis or apoptosis and also alters macrophage/microglia activity/function.30,32 Preclinical data have indicated that the effects of evobrutinib on B-cells and proinflammatory microglia/macrophages24,33 reduce neuroinflammation, demyelination and axonal pathology in the CNS. 44 In our study, levels of serum NfL, a biomarker of ongoing neuroaxonal damage, were reduced with evobrutinib 75 mg BID up to week 144 to levels similar to a non-MS control population. Importantly, the rapid reduction in NfL levels observed at week 12 was mirrored by a similar reduction in the mean number of new T1 Gd+ lesions also at week 12, suggesting that NfL can be used as a marker of disease activity and early treatment response. Indeed, NfL, rather than T1 Gd+ lesions, has been used as the primary endpoint in recent Phase II trials. 45 Furthermore, recent evidence that evobrutinib 75 mg BID reduces slowly expanding lesion volume indicates that evobrutinib affects brain lesions associated with chronic inflammation and tissue loss thought to be driven by microglia activity at the lesion edges. 46 Together, these data support the potential for evobrutinib to modulate both peripherally driven and CNS-compartmentalised inflammation.
Interpretation of these Phase II trial data should be done with due consideration of the open-label design of the extension phase and that the original DBP trial was powered for MRI disease activity rather than clinical endpoints. The small sample size may have also resulted in our study being underpowered to compare outcomes across dose levels of evobrutinib. Although the dropout rate could be seen as a potential source of bias, the dropout rate observed in this study is comparable to other OLE studies in MS. 47 Furthermore, the annual efficacy assessments conducted in this OLE study may have precluded the ability to observe short-term improvements in disease status. Finally, although encouraging, these data cannot be extrapolated to exposures longer than the >3.5 years of treatment reported here.
Conclusion
MS is a lifelong, progressive neurodegenerative disease characterised by brain tissue loss and disability accumulation. With current treatment options that primarily target peripheral immune cells, patients may experience ongoing CNS inflammation and disease progression, demonstrating the need for alternative therapeutic strategies. BTK inhibition is a novel mechanism for the treatment of MS and other autoimmune diseases. This Phase II OLE study provides evidence of a maintained efficacy and safety profile with CNS-penetrant evobrutinib 75 mg BID.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585241234783 for Efficacy and safety results after >3.5 years of treatment with the Bruton’s tyrosine kinase inhibitor evobrutinib in relapsing multiple sclerosis: Long-term follow-up of a Phase II randomised clinical trial with a cerebrospinal fluid sub-study by Xavier Montalban, Karolina Piasecka-Stryczynska, Jens Kuhle, Pascal Benkert, Douglas L Arnold, Martin S Weber, Andrea Seitzinger, Hans Guehring, Jamie Shaw, Davorka Tomic, Yann Hyvert, Danielle E Harlow, Martin Dyroff and Jerry S Wolinsky in Multiple Sclerosis Journal
Acknowledgments
The authors thank the patients and their families, as well as the investigators, co-investigators and the trial teams at each of the participating centres; Emily Martin and Roland Grenningloh (affiliation at the time the research was conducted: EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA) for providing input to the manuscript. Merck Healthcare KGaA, Darmstadt, Germany was involved in the trial design, collection, analysis and interpretation of the data and the development of this manuscript. Medical writing and editorial support were provided by Francesca Hemingway of Bioscript Group Ltd., Macclesfield, UK and supported by Merck Healthcare KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945). The authors have authorised the submission of the manuscript by Bioscript and approved any statements or declarations.
Footnotes
Data Availability Statement: Data are available upon reasonable request. Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck Healthcare KGaA, Darmstadt, Germany’s Data Sharing Policy. All requests should be submitted in writing to Merck Healthcare KGaA, Darmstadt, Germany’s data sharing portal https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck Healthcare KGaA, Darmstadt, Germany has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck Healthcare KGaA, Darmstadt, Germany will endeavour to gain agreement to share data in response to requests.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: X.M. has received speaking honoraria and/or travel expenses for participation in scientific meetings and/or has been a steering committee member of clinical trials and/or participated in advisory boards of clinical trials in the past years with Actelion; Alexion; Bayer; Biogen; Bristol Myers Squibb/Celgene; EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA; Genzyme; F. Hoffmann-La Roche; Immunic; Janssen Pharmaceuticals; MedDay; Merck Healthcare KGaA, Darmstadt, Germany; Mylan; Nervgen; Novartis; Sanofi Genzyme; Teva Pharmaceutical; TG Therapeutics; Excemed; MSIF and NMSS. K.P.-S. has received travel funding and/or speaker honoraria from EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA; Sanofi-Aventis; Biogen Idec; Teva Pharmaceutical; F. Hoffmann-La Roche; Bayer and Novartis and has served on scientific advisory boards for Sanofi-Aventis; Biogen Idec and EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA. J.K. received speaker fees, research support, travel support, and/or served on advisory boards by Swiss MS Society; Swiss National Research Foundation (320030_189140/1); University of Basel; Progressive MS Alliance; Alnylam; Bayer; Biogen; Bristol Myers Squibb; Celgene; Immunic; Merck Healthcare KGaA, Darmstadt, Germany; Neurogenesis; Novartis; Octave Bioscience; Quanterix; Roche; Sanofi and Stata DX. P.B. has nothing to disclose. D.L.A. has received personal fees for consulting from Albert Charitable Trust; Alexion; Biogen; Celgene; F. Hoffmann-La Roche; Frequency Therapeutics; Genentech; Med-Ex Learning; Merck Healthcare KGaA, Darmstadt, Germany; Novartis; Receptos and Sanofi-Aventis; grants from Biogen and Novartis and has an equity interest in NeuroRx Research. M.S.W. has received travel funding and/or speaker honoraria from Biogen Idec; EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA; Novartis; F. Hoffmann-La Roche; Teva Pharmaceutical; Bayer and Genzyme. Y.H., A.S. and H.G. are the employees of Merck Healthcare KGaA, Darmstadt, Germany. J.S., D.E.H. and M.D. are the employees of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA. D.T. is an employee of Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA, and received stock or an ownership interest from Novartis. J.S.W. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Cleveland Clinic Foundation; EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA; Novartis; Roche/Genentech; Sanofi Genzyme; The University of Alabama and Zenas BioPharma. Royalties have been received for out-licensed monoclonal antibodies through UTHealth from Millipore Corporation.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial was funded by Merck Healthcare KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945).
ORCID iDs: Pascal Benkert  https://orcid.org/0000-0001-6525-8174
https://orcid.org/0000-0001-6525-8174
Douglas L Arnold  https://orcid.org/0000-0003-4266-0106
https://orcid.org/0000-0003-4266-0106
Jerry S Wolinsky  https://orcid.org/0000-0002-8197-2762
https://orcid.org/0000-0002-8197-2762
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Xavier Montalban, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Hospital Universitario Vall d’Hebron, Barcelona, Spain.
Karolina Piasecka-Stryczynska, Department of Neurology, Poznan University of Medical Sciences, Poznan, Poland.
Jens Kuhle, Neurologic Clinic and Policlinic, MS Center and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB), University Hospital Basel, Basel, Switzerland; University of Basel, Basel, Switzerland.
Pascal Benkert, Clinical Trial Unit, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland.
Douglas L Arnold, Montreal Neurological Institute, McGill University, Montreal, QC, Canada; NeuroRx, Montreal, QC, Canada.
Martin S Weber, Institute of Neuropathology, Department of Neurology, University Medical Center, University of Göttingen, Göttingen, Germany; Fraunhofer-Institute for Translational Medicine and Pharmacology ITMP, Göttingen, Germany.
Andrea Seitzinger, Merck Healthcare KGaA, Darmstadt, Germany.
Hans Guehring, Merck Healthcare KGaA, Darmstadt, Germany.
Jamie Shaw, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA.
Davorka Tomic, Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA.
Yann Hyvert, Merck Healthcare KGaA, Darmstadt, Germany.
Danielle E Harlow, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA.
Martin Dyroff, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA.
Jerry S Wolinsky, McGovern Medical School, The University of Texas Health Science Center at Houston (UTHealth Houston), Houston, TX, USA.
References
- 1. Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers 2018; 4: 43. [DOI] [PubMed] [Google Scholar]
- 2. Dobson R, Giovannoni G. Multiple sclerosis – A review. Eur J Neurol 2019; 26: 27–40. [DOI] [PubMed] [Google Scholar]
- 3. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med 2018; 378: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ayzenberg I, Hoepner R, Kleiter I. Fingolimod for multiple sclerosis and emerging indications: Appropriate patient selection, safety precautions, and special considerations. Ther Clin Risk Manag 2016; 12: 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bar-Or A, Grove RA, Austin DJ, et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study. Neurology 2018; 90: e1805–e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buonomo AR, Zappulo E, Viceconte G, et al. Risk of opportunistic infections in patients treated with alemtuzumab for multiple sclerosis. Expert Opin Drug Saf 2018; 17(7): 709–717. [DOI] [PubMed] [Google Scholar]
- 7. Havrdova E, Cohen JA, Horakova D, et al. Understanding the positive benefit:risk profile of alemtuzumab in relapsing multiple sclerosis: Perspectives from the Alemtuzumab Clinical Development Program. Ther Clin Risk Manag 2017; 13: 1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mulero P, Midaglia L, Montalban X. Ocrelizumab: A new milestone in multiple sclerosis therapy. Ther Adv Neurol Disord 2018; 11: 1756286418773025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rotondi M, Molteni M, Leporati P, et al. Autoimmune thyroid diseases in patients treated with alemtuzumab for multiple sclerosis: An example of selective anti-TSH-receptor immune response. Front Endocrinol (Lausanne) 2017; 8: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singer BA. The role of natalizumab in the treatment of multiple sclerosis: Benefits and risks. Ther Adv Neurol Disord 2017; 10(9): 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parks NE, Pittock SJ, Mandrekar J, et al. Population-based study of ‘no evident disease activity’ in MS. Neurol Neuroimmunol Neuroinflamm 2018; 5(6): e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015; 72(2): 152–158. [DOI] [PubMed] [Google Scholar]
- 13. Kraemer M, Dabringhaus A, Gregori J, et al. Investigation of lesion volume dynamics in ms patients as detected by voxel-guided morphometry – A multi center study. Mult Scler 2020; 26(Suppl. 3): 403 (Abstract P0596). [Google Scholar]
- 14. Elliott C, Wolinsky JS, Hauser SL, et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler 2019; 25(14): 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elliott C, Arnold DL, Chen H, et al. Patterning chronic active demyelination in slowly expanding/evolving white matter MS lesions. AJNR Am J Neuroradiol 2020; 41(9): 1584–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calvi A, Tur C, Chard D, et al. Slowly expanding lesions relate to persisting black-holes and clinical outcomes in relapse-onset multiple sclerosis. Neuroimage Clin 2022; 35: 103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giannetti P, Politis M, Su P, et al. Increased PK11195-PET binding in normal-appearing white matter in clinically isolated syndrome. Brain 2015; 138(Pt 1): 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Preziosa P, Pagani E, Meani A, et al. Slowly expanding lesions predict 9-year multiple sclerosis disease progression. Neurol Neuroimmunol Neuroinflamm 2022; 9(2): e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Machado-Santos J, Saji E, Tröscher AR, et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018; 141: 2066–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Preziosa P, Pagani E, Moiola L, et al. Occurrence and microstructural features of slowly expanding lesions on fingolimod or natalizumab treatment in multiple sclerosis. Mult Scler 2021; 27: 1520–1532. [DOI] [PubMed] [Google Scholar]
- 21. Elliott C, Belachew S, Wolinsky JS, et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain 2019; 142: 2787–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. López-Herrera G, Vargas-Hernández A, González-Serrano ME, et al. Bruton’s tyrosine kinase – An integral protein of B cell development that also has an essential role in the innate immune system. J Leukoc Biol 2014; 95(2): 243–250. [DOI] [PubMed] [Google Scholar]
- 23. Bender AT, Gardberg A, Pereira A, et al. Ability of Bruton’s tyrosine kinase inhibitors to sequester Y551 and prevent phosphorylation determines potency for inhibition of Fc receptor but not B-cell receptor signaling. Mol Pharmacol 2017; 91: 208–219. [DOI] [PubMed] [Google Scholar]
- 24. Alankus Y, Grenningloh R, Haselmayer P, et al. BTK inhibition prevents inflammatory macrophage differentiation: A potential role in MS. Mult Scler 2018; 24(Suppl. 2): 264 (Abstract P557). [Google Scholar]
- 25. Gruber R, Dufault M, Chretien N, et al. Decoding Bruton’s tyrosine kinase signalling in neuroinflammation. Mult Scler 2020; 26(Suppl. 3): 270 (Abstract P0311). [Google Scholar]
- 26. Rijvers L, van Langelaar J, Bogers L, et al. Human T-bet+ B cell development is associated with BTK activity and suppressed by evobrutinib. JCI Insight 2022; 7: e160909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin E, Aigrot MS, Grenningloh R, et al. Bruton’s tyrosine kinase inhibition promotes myelin repair. Brain Plast 2020; 5: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mishra MK, Yong VW. Myeloid cells – Targets of medication in multiple sclerosis. Nat Rev Neurol 2016; 12(9): 539–551. [DOI] [PubMed] [Google Scholar]
- 29. Giovannoni G, Popescu V, Wuerfel J, et al. Smouldering multiple sclerosis: The ‘real MS’. Ther Adv Neurol Disord 2022; 15: 17562864211066751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haselmayer P, Camps M, Liu-Bujalski L, et al. Efficacy and pharmacodynamic modeling of the BTK inhibitor evobrutinib in autoimmune disease models. J Immunol 2019; 202: 2888–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caldwell RD, Qiu H, Askew BC, et al. Discovery of evobrutinib: An oral, potent, and highly selective, covalent Bruton’s tyrosine kinase (BTK) inhibitor for the treatment of immunological diseases. J Med Chem 2019; 62: 7643–7655. [DOI] [PubMed] [Google Scholar]
- 32. Torke S, Pretzsch R, Häusler D, et al. Inhibition of Bruton’s tyrosine kinase interferes with pathogenic B-cell development in inflammatory CNS demyelinating disease. Acta Neuropathol 2020; 140(4): 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geladaris A, Torke S, Weber M, et al. Targeting BTK in chronic CNS autoimmunity inhibits activation of microglia. Mult Scler 2021; 27(Suppl. 2): 790–791 (Abstract P971).32749910 [Google Scholar]
- 34. Montalban X, Arnold DL, Weber MS, et al. Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. N Engl J Med 2019; 380: 2406–2417. [DOI] [PubMed] [Google Scholar]
- 35. Montalban X, Wallace D, Genovese MC, et al. Characterisation of the safety profile of evobrutinib in over 1000 patients from phase II clinical trials in multiple sclerosis, rheumatoid arthritis and systemic lupus erythematosus: An integrated safety analysis. J Neurol Neurosurg Psychiatry 2023; 94(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol 2022; 21(3): 246–257. [DOI] [PubMed] [Google Scholar]
- 37. Papasouliotis O, Mitchell DY, Girard P, et al. Determination of a clinically effective evobrutinib dose: Exposure-response analyses of a phase II MS study. Eur J Neurol 2021; 28: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Papasouliotis O, Mitchell DY, Dyroff M, et al. Population pharmacokinetic model of evobrutinib, a Bruton’s tyrosine kinase inhibitor – An analysis of two phase I clinical trials in healthy subjects. Clin Pharmacol Ther 2020; 107: S94. [Google Scholar]
- 39. Papasouliotis O, Mitchell D, Girard P, et al. Population pharmacokinetic and pharmacodynamic modeling of evobrutinib in healthy adult participants. Clin Transl Sci 2022; 15(12): 2899–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Papasouliotis O, Mitchell D, Girard P, et al. Determination of a clinically effective evobrutinib dose: Exposure-response analyses of a phase II relapsing multiple sclerosis study. Clin Transl Sci 2022; 15(12): 2888–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Becker A, Martin EC, Mitchell DY, et al. Safety, tolerability, pharmacokinetics, target occupancy, and concentration-QT analysis of the novel BTK inhibitor evobrutinib in healthy volunteers. Clin Transl Sci 2020; 13(2): 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Estupiñán HY, Berglöf A, Zain R, et al. Comparative analysis of BTK inhibitors and mechanisms underlying adverse effects. Front Cell Dev Biol 2021; 9: 630942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boschert U, Crandall T, Pereira A, et al. T cell mediated experimental CNS autoimmunity induced by PLP in SJL mice is modulated by evobrutinib (M2951) a novel Bruton’s tyrosine kinase inhibitor. Mult Scler 2017; 23(Suppl. 3): 327 (Abstract P678). [Google Scholar]
- 44. Kebir H, Li C, May M, et al. Effectiveness of the Bruton’s tyrosine kinase inhibitor evobrutinib in a novel model for compartmentalized neuroinflammation in multiple sclerosis. Mult Scler 2022; 28(Suppl. 1): 20–214 (Abstract 748). [Google Scholar]
- 45. A study to evaluate the effect of SAR443820 on serum neurofilament levels in male and female adult participants with multiple sclerosis. ClinicalTrials.gov identifier: NCT05630547, 21 August 2023, https://beta.clinicaltrials.gov/study/NCT05630547?cond=multiple%20sclerosis&term=Neurofilament%20Light%20Chain&aggFilters=phase:2,status:act%20rec%20not&rank=1 (accessed 30 August 2023).
- 46. Arnold D, Elliott C, Montalban X, et al. Effects of evobrutinib, a Bruton’s tyrosine kinase inhibitor, on slowly expanding lesions: An emerging imaging marker of chronic tissue loss in multiple sclerosis. Mult Scler 2021; 27(Suppl. 2): 69 (Abstract 115). [Google Scholar]
- 47. Gold R, Radue EW, Giovannoni G, et al. Safety and efficacy of daclizumab in relapsing-remitting multiple sclerosis: 3-year results from the SELECTED open-label extension study. BMC Neurol 2016; 16: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585241234783 for Efficacy and safety results after >3.5 years of treatment with the Bruton’s tyrosine kinase inhibitor evobrutinib in relapsing multiple sclerosis: Long-term follow-up of a Phase II randomised clinical trial with a cerebrospinal fluid sub-study by Xavier Montalban, Karolina Piasecka-Stryczynska, Jens Kuhle, Pascal Benkert, Douglas L Arnold, Martin S Weber, Andrea Seitzinger, Hans Guehring, Jamie Shaw, Davorka Tomic, Yann Hyvert, Danielle E Harlow, Martin Dyroff and Jerry S Wolinsky in Multiple Sclerosis Journal