Abstract
Physiologically based biopharmaceutics modeling (PBBM) is used to elevate drug product quality by providing a more accurate and holistic understanding of how drugs interact with the human body. These models are based on the integration of physiological, pharmacological, and pharmaceutical data to simulate and predict drug behavior in vivo. Effective utilization of PBBM requires a consistent approach to model development, verification, validation, and application. Currently, only one country has a draft guidance document for PBBM, whereas other major regulatory authorities have had limited experience with the review of PBBM. To address this gap, industry submitted confidential PBBM case studies to be reviewed by the regulatory agencies; software companies committed to training. PBBM cases were independently and collaboratively discussed by regulators, and academic colleagues participated in some of the discussions. Successful bioequivalence “safe space” industry case examples are also presented. Overall, six regulatory agencies were involved in the case study exercises, including ANVISA, FDA, Health Canada, MHRA, PMDA, and EMA (experts from Belgium, Germany, Norway, Portugal, Spain, and Sweden), and we believe this is the first time such a collaboration has taken place. The outcomes were presented at this workshop, together with a participant survey on the utility and experience with PBBM submissions, to discuss the best scientific practices for developing, validating, and applying PBBMs. The PBBM case studies enabled industry to receive constructive feedback from global regulators and highlighted clear direction for future PBBM submissions for regulatory consideration.
Keywords: PBBM (physiologically based biopharmaceutics model(s)(ing), PBPK (physiologically based pharmacokinetics), MIDD (model informed drug development), patient-centric drug product quality standards, clinically relevant dissolution specifications (CRDS), drug product performance, drug product quality, biopredictive dissolution, virtual bioequivalence (VBE), safe space
1. Introduction
Physiology based biopharmaceutics modeling (PBBM), the application of PBPK for biopharmaceutics applications, is an evolving tool that can be used throughout drug product development (model informed drug development), regulatory approval, and life cycle management. PBBM focuses on providing a mechanistic understanding and quantifying the interaction of drug product quality attributes with physiology influencing in vivo drug performance. The results of a PBBM application can play an important role in the development of drug products, and therefore, the assessment of the PBBM can constitute a key component for regulatory approval. This is justified by the impact of PBBM on clinically relevant specifications and continued drug safety and efficacy characterization throughout the product life cycle. PBBM can be used to visualize the use of mechanistic absorption modeling in drug product development when assessing bioavailability (i.e., rate and extent of absorption), and the pharmacokinetics (PK) process that formulation development can influence which has a direct impact on in vivo drug product performance. Applications for PBBM in oral drug product development are highlighted in Figure 1.
Figure 1.
Uses of PBBM in oral formulation development. Adapted with permission from Yuvaneshwari et al.1 Copyright 2022 Elsevier.
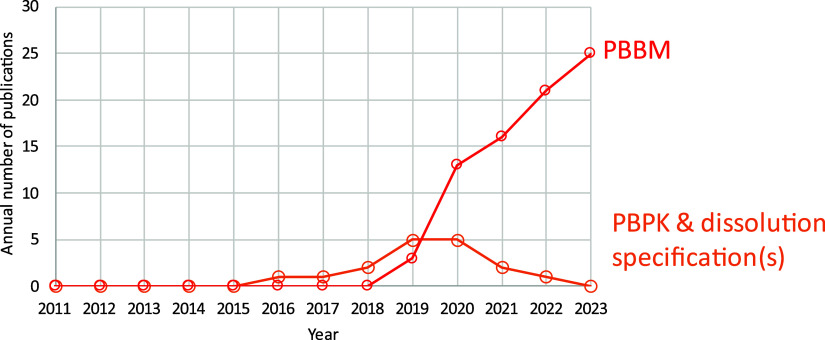
The use of PBBM is gaining momentum and importance if we take a brief look back through the development and progression in both dialogue and exchanges of information. In 2017, the workshop “Dissolution and Translational Modeling Strategies Enabling Patient-Centric Drug Product Development” was organized by the University of Maryland Center of Excellence in Regulatory Science and Innovation (M-CERSI). This laid the foundation for a rational application for dissolution testing and its link to both in vivo performance and to set clinically relevant dissolution specifications for drug products.2−4
In 2019, the workshop “Current State and Future Expectations of Translational Modeling Strategies to Support Drug Product Development, Manufacturing Changes and Controls” sponsored by the US Food and Drug Administration (FDA) in collaboration with M-CERSI was the place where the term PBBM was collectively defined.5 This 3 day workshop was an opportunity to discuss the current science around model development and use and covered in vitro biopredictive methods,6 best practices in developing and validating models,7 and model applications.8 The need for a specified subcategory (i.e., physiologically based pharmacokinetics, PBPK) of PBBM was identified at the 2019 workshop, where analogies were made much in the way clinicians have specialty roles (e.g., oncologists, endocrinologists, cardiologists) to gain efficiencies and enhance patient care. Since the 2019 workshop,5 there has been a steady increase in the number of publications in the literature adopting the term PBBM (Figure 2). In addition, by its introduction in the 2020 draft FDA guidance,9 the usage/utility of the terminology seems to have gained traction by both regulators and industry alike, which suggests the need for the PBPK subcategory. PBBM includes biopharmaceutics of both orally administered drug where absorption through the GI tract is required and locally acting drugs which are administered using oral or nonoral routes of administration.9
Figure 2.
Growth in the number of publications including the term PBBM. Pubmed (https://www.ncbi.nlm.nih.gov/pmc/) was searched between 2011 and 2023 using the following 2 queries: PBBM = (“PBBM”) AND ((“biopharmaceutic”) OR (“biopharmaceutics”) OR (“biopharmaceutical”)); PBPK AND dissolution specification(s) = ((“specification”) OR (“specifications”)) AND (“PBPK”) AND (“dissolution”) NOT (“PBBM”)
Although the utility of PBBM is recognized as promising in support of patient-centric drug product development, the gaps in optimal model parametrization and validation, with a focus on any significant risk to patients, were highlighted as major themes for future focus by the scientific community. These aspects remain challenging as, first, both critical drug substance characteristics and mechanistic elements of drug release/dissolution from the drug product relevant to any interactions with physiological conditions need to be described, parametrized, and visualized to be able to explain (simulate) the observed in vivo PK. Second, the depth and breadth of data sets needed and technical acceptance criteria for the verification and validation of the PBBM needs to be discussed in light of the model influence, risk, and regulatory impact (i.e., low, medium, high; Musuamba et al.,10 Skottheim et al.,11 Kuemmel et al.).12 These aspects highlight the need for further dialogue to facilitate clearer and harmonized understanding on the applicability of PBBM, together with the determined model influence, risk, and regulatory impact (i.e., low, medium, high; Musuamba et al.,10 Skottheim et al.,11 Kuemmel et al.).12 More examples which can increase the understanding of where models can be successful and fail should be discussed, together with a broad-based level of understanding between industry and regulatory scientists on how to pave the way to a more routine use of PBBM.5
In 2021 a group of scientists from the International Consortium for Innovation & Quality (IQ) in Pharmaceutical Development started discussing the need to engage the scientific community with worldwide regulators on the content of PBBM, validation strategies, and their utilization to support the regulatory approval of clinically relevant specifications and continued quality assurance throughout the product life cycle. There was a desire to harmonize the views of the scientific community, whether from the pharmaceutical industry, regulatory agencies, academic institutions, software developers or contract research organizations around the current gaps in the science and best practices for PBBM development, validation, and utilization. There was a sense that as PBBM is a promising new concept, fostering scientific collaboration could drive expertise building across the board, derive a common language, increase regulatory interactions, and ultimately improve the use of PBBM to support a sound understanding of product quality and support the waiving of unnecessary animal and human studies. The idea was conceived to ask IQ industry partners and global regulators to participate in a case study review of PBBMs, where sponsors would provide models to answer specific questions and regulators would individually and jointly review these models.
Both industry and global regulators agreed this would be a good step forward to advance the science of PBBM and increase scientific understanding. The IQ members companies which submitted PBBM case studies for oral drug products were Amgen, AstraZeneca, EMD (Merck KGaA), Janssen, Merck & Co., Inc. Rahway, NJ, USA and Pfizer. The regulatory agencies which discussed the PBBM case studies were ANVISA, EMA, FDA, Health Canada, MHRA and PMDA. The summary of these discussions would be shared at a workshop open to the public.
The workshop entitled “Physiologically Based Biopharmaceutics Modeling, PBBM Best Scientific Practices for Drug Product Quality: Regulatory and Industry Perspectives” sponsored by FDA in collaboration with M-CERSI (University of Maryland Center of Excellence in Regulatory Science and Innovation) was held on 29–31 August 2023 at the Universities at Shady Grove, Rockville, hosted by the M-CERSI program https://www.pharmacy.umaryland.edu/centers/cersievents/PBBM2023/.
This 3 day workshop brought together 187 delegates across academia, industry, regulatory agencies, and software providers to discuss the best scientific practices for developing PBBMs for orally administered, systemically active drug products and how these models can be leveraged to streamline pharmaceutical drug product development and support manufacturing changes and controls with a focus on the following:
challenges and considerations in the development of biorelevant/biopredictive inputs such as solubility, dissolution, permeability, etc., for PBBM development
scientific considerations for establishing verification and validation strategies for PBBM for their intended purpose of application
regulatory strategy and applications of PBBM during clinical development, marketing application, and post approval change
considerations for when to extrapolate outside the safe space and data needed
discussions around a framework for reporting a PBBM
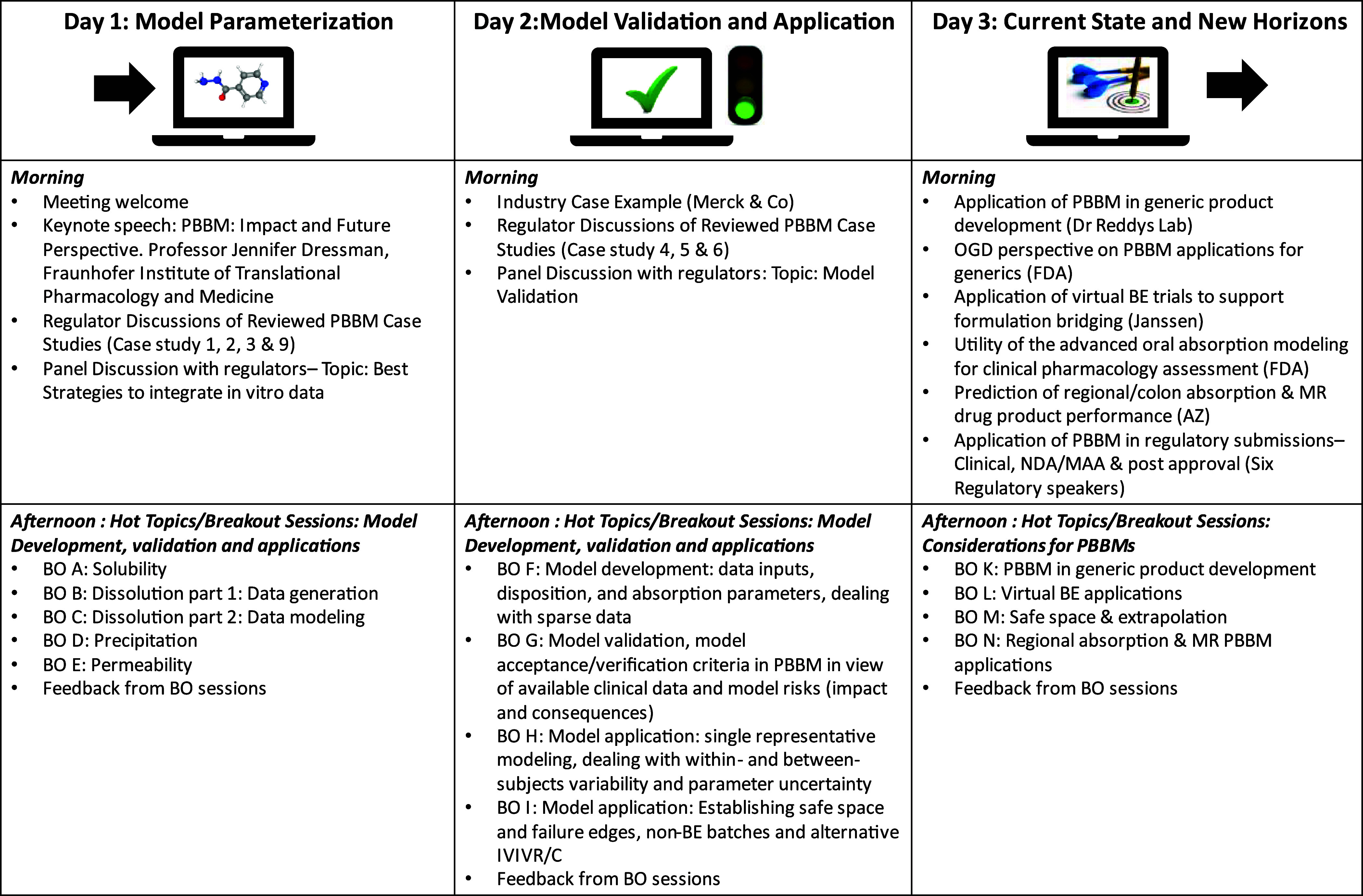
Each day of the workshop had a dedicated theme and was split into two main sessions. In the morning session on each of the days, participants heard from industry presentations and/or regulatory agencies on two to three of the submitted PBBM cases and participated in panel discussions on specified topics. The afternoon sessions were dedicated to breakout sessions (BO) covering the hot topics associated with that day’s theme (see Figure 3). At the end of each day, the breakout sessions relayed back to the meeting participants, including major topics discussed, suggested frameworks, and decision trees (work in progress). For more details on the breakout session topics please refer to https://www.pharmacy.umaryland.edu/centers/cersievents/PBBM2023/.
Figure 3.
Presentations and discussion during the 2023 PBBM workshop. https://cersi.umd.edu/physiologically-based-biopharmaceutics-modeling-pbbm-best-practices-drug-productquality
In this workshop summary report, we present highlights and an overview of the 3 days, report out on a survey which was conducted among meeting attendees, and share the major scientific and regulatory findings from the meeting. More detailed descriptions of the proceedings for each day of the workshop will be published in three separate reports. The team will finalize the paper series with the publication of an industry led PBBM template, which will cover the major fields of a PBBM submission (e.g., executive summary, model questions/context of use, model risk and decision consequence, model build rationale, input data and associated details, clinical data sets used to validate the models, and finally model application).
2. Workshop Summary Structure
The 3 day workshop structure, including presentations, panel discussions, and breakout sessions , is presented in Figure 3.
3. Day Summaries
The following day summaries highlight the main messages from the podium presentations, regulatory discussion of the reviewed PBBM case studies, the panel discussions with regulators, and the breakout sessions from each day. The case studies presented by each of the regulators represent the views of the respective speaker and do not necessarily represent the view of the lead agency for the regulator’s discussion of the case studies or that of other agencies present. A link to the presentations shared during the workshop is provided at https://cersi.umd.edu/physiologically-based-biopharmaceutics-modeling-pbbm-best-practices-drug-product-qualityDay.
3.1. Day 1: Model Parameterization
On Day 1, the morning sessions included a workshop introduction, the keynote lecture, and discussion on four of the regulatory reviewed PBBM cases, followed by a roundtable discussion with the regulatory authorities to share their experiences regarding PBBM and model parametrization. In the afternoon, breakout sessions covered key PBBM inputs such as solubility, permeability, dissolution, and precipitation.
3.1.1. Workshop Introduction and Keynote Lecture
Bhagwant Rege (FDA) introduced the workshop by giving a presentation highlighting the role and importance of PBBM in the USA to support patient centric quality standards (PCQS) for drug products and the strong involvement of the US FDA in the development of regulatory guidelines to support biopharmaceutics applications of PBPK tools during drug development.
Jennifer Dressman (Fraunhofer Institute of Translational Medicine and Pharmacology) gave a keynote speech that articulated what PBBM stood for. The main message is that there are still gaps in our knowledge for oral and nonoral route applications of PBBM. Developers and end users must balance the requirements of model flexibility for regulatory applications, with the flexibility to be able to incorporate novel scientific development or refinements in how the physiology is described. This flexibility is particularly important for new administration routes, although it is also recognized that gaps still exist in how the oral route is described. Some suggested examples of areas of improvement for oral route PBBMs were stomach emptying phases, lower GI permeability, impact of excipients on drug dissolution, and precipitation.
3.1.2. Regulatory Discussion of Reviewed PBBM Case Studies
Case Study 1 was presented by Shereeni Veerasingham and Arthur Okumu, Health Canada, and summarized the assessment of drug X from Amgen. Case Study 1 described a dissolution safe space established using PBBM for an immediate-release tablet containing a Biopharmaceutics Classification System (BCS) Class II drug. This PBBM approach provided an understanding of absorption mechanisms and established an in vitro–in vivo relationship. The intended use of the model was to widen the dissolution specifications for the oral tablet while ensuring bioequivalent in vivo performance. The model was developed based on physicochemical and biopharmaceutical properties, and intravenous and oral pharmacokinetic data from five clinical studies. Initial simulations predicted the pharmacokinetic profile for the oral solution well; however, the model needed to be refined for tablets considering that due to a common ion effect, aqueous solubility of the drug (HCl salt) decreases in the presence of chloride ions. In addition, a mechanistic model based on classical nucleation theory was used to account for drug precipitation. Validation of the model employing single simulations for three independent data sets demonstrated adequate predictive performance for the intended use. PBBM-based virtual bioequivalence (VBE) trials compared pharmacokinetic parameters for simulations of theoretical dissolution profiles to those of the reference tablet to establish a dissolution safe-space. Regulatory perspectives on the case study are discussed. The overall assessment considers the model risk, which was considered low per the credibility assessment framework, and the model was accepted for its intended purpose.
Case Study 2 was presented by Anders Lindahl, Swedish Medical Products Agency, Sweden, and Flora Musuamba Tshinanu, Belgian Federal Agency for Medicines and Health Products (FAMHP), Belgium, and summarized the EMA assessment of the PBBM provided by AstraZeneca on Lesinurad immediate release tablets.
The purpose of the PBBM work was to support the proposed in vitro dissolution specification limit Q 80% at 30 min for the drug product. In the EMA region, the PBBM was not submitted as part of a marketing authorization application, and the in vitro dissolution specification limit was accepted based on the in vitro dissolution of several pivotal batches and two nonbioequivalent batches. The regulatory impact of the model is therefore considered low. However, the model assessment exercise was performed irrespective of this consideration in the context of the preparation for the workshop, and several issues were identified. A top-down data driven approach was used to create individual models with subject-specific gastric emptying rates (lag-time) and effective permeability (Peff). In vitro dissolution data were fitted to a product particle size distribution (P-PSD) that would match observed in vitro dissolution per batch by using the quality control method. The P-PSD was then used as input in GastroPlus and the formulation was switched to delayed release enteric coated tablet in the model to ensure no release in the stomach. This led to uncertainties in values of Peff and gastric emptying (fitted values), fluid volumes in GI tract, as well as issues with formulation switch and the lower variability in the simulated virtual population compared to in the clinical studies. In conclusion, the model would have not been accepted to justify an extended in vitro dissolution safe space beyond the Q of 80% in 30 min.
Case Study 3 was presented by Rebecca Moody (FDA) and summarized an assessment of the PBBM provided by AstraZeneca on acalabrutinib immediate release capsules. The purpose of the submitted PBBM was to evaluate if two acalabrutinib product batches, W026394 and L0505009, which failed f2 similarity factor at certain pH conditions within the physiological pH range, could be declared bioequivalent using PBBM. The PBBM strategy involved modeling of individual subject pharmacokinetic data and then validating if that population was able to reproduce the observed mean Cmax and AUC from several different clinical scenarios. In vitro dissolution was incorporated into the model mechanistically through the product particle size distribution (P-PSD) approach.14 The model was validated by evaluating the accuracy of the 8 subject population in simulating acalabrutinib exposure from 16 different clinical scenarios. Overall, considering the totality of evidence, the risk of bioinequivalence due to dissimilar dissolution at high pH (i.e., pH 4.5 and above) was low. However, the future applicability of the PBBM is limited due to uncertainties in model parametrization and applicability to the general population (i.e., beyond the 8 subject population used for model validation).
Case Study 9 was presented by Øyvind Holte (Norwegian Medicines Agency) and summarized the assessment of the PBBM provided by Pfizer on fluconazole. One of the purposes of the model was to demonstrate the BE between a series of solid oral formulations compared to the commercial formulation to justify a potential widening of the dissolution space. The data included in this case study was selected from a wide body of data that exists for fluconazole: different strengths of tablets and capsules, oral suspension, and an intravenous formulation. The results of several clinical PK studies, performed between 1983 and 2019, were available for development and verification of the model. A total of 28 simulations were performed. Separate data sets were used for model validation, model verification and model application. It was acknowledged that for the purpose of this case study, all relevant details were not available. Nevertheless, based on the data presented, the conclusions made by the company could not be fully endorsed by the EMA. There were uncertainties regarding the model’s ability to predict the PK of fluconazole. Several of the simulations were overestimating Cmax and/or AUC. Apparently, the initial model was neither adjusted nor corrected based on these observations.
VBE trials were performed, based on the model, to capitalize on the observed results from the available BE studies. Some of the VBE studies demonstrated better results than the original BE studies without reasonable explanation. In conclusion, based on the data provided with the case study, the PBBM represented a limited value and would probably not be considered sufficient as a substitute for clinical data in a regulatory setting by the EMA.
3.1.3. PBBM Roundtable Discussions by Regulatory Agencies: Topic: Model Parameterization
The Day 1 roundtable discussion brought together representatives from various regulatory agencies, including the FDA, ANVISA, MHRA, Health Canada, EMA, and PMDA to discuss their perspectives on model parametrization. The following three areas were highlighted.
3.1.3.1. PBBM: A (Growing) Regulatory Tool
Apart from the USA, other regions have not issued their own guidance regarding the development, validation, and utilization of PBBM; however, all the regulators present at the workshop stated they would consider PBBM as part of the totality of the data to support proposed specifications or waivers. The totality of data represents, for example, all data related to product dissolution (method, discrimination ability, performance of clinical and commercial batches), stability, release mechanism, excipient composition, and evolution thereof during development, as well as PK, clinical efficacy, and safety data. PBBM is considered in the context of the totality of data to come to a conclusion.
3.1.3.2. Utilization of PBBM
PBBM could be used pre- or postapproval for innovator and generic companies alike to support specifications or changes thereof and reduce the need for animal or human evaluation. All regulators present at the workshop indicated that they had reviewed a growing number of PBBM cases over the last 2 years.
3.1.3.3. Format and Content of PBBMs
During this exercise of mock submission, it emerged that the history of the model development was important, especially for regulators who had access to previous model submissions which were initially rejected. In the future, a versioning of the PBBM or unique identifier could be important together with explanation of differences in the models. There was an additional request to summarize the model development steps: What were the optimized parameters, the magnitude of the changes operated, and the rationale for running this optimization? Overall, the regulators welcomed the initiative of the industry to come up with a proposed draft PBBM template to set the expectations in terms of format and content and required context for each step of model development, validation, and use.
3.1.4. Breakout Session Topics
3.1.4.1. Solubility
The thermodynamic drug solubility as a function of pH is a fundamental drug parameter to measure and be compared to an expected theoretical profile, analyzing potential deviations. However, depending on the type of drug substance or formulation, conducting other solubility measurements is needed to predict in vivo drug dissolution and absorption. For amorphous drugs, polymer excipients not only can increase solubility but also delay and slow precipitation or increase permeation through the formation of drug-polymer colloids. The solubility at the surface of the solid drug should be measured and predicted to be able to understand in vitro and in vivo dissolution. This can be affected by the presence of excipients too, and during the workshop, some best practices to measure solubility for various formulations were outlined.
3.1.4.2. Biopredictive Dissolution Methods
There is no one size fits all for the biopredictive dissolution method, and it depends on the type of formulation, release mechanism, and in vivo limitation to drug absorption. Flow-through cells and transfer models are useful for dynamic dissolution protocols. Small volumes and low buffer concentrations should be considered to mimic the physiological environments in the GI tract. Enteric coated formulations should be developed by using carbonate buffers or suitable surrogates. Biphasic dissolution is an important tool to mimic the GI environment, with dissolution and absorption occurring in parallel. Lipid dissolution is a promising approach to assess excipient effects of lymphotropic drugs.
3.1.4.3. Modeling In Vitro Dissolution
Modeling in vitro dissolution with a mechanistic model (e.g., z-factor or P-PSD) is recommended for immediate release products when the in vitro dissolution is highly sensitive to pH or surfactants or when the model aims to predict in vivo dissolution across different physiologies. Nonmechanistic modeling approaches can still be used assuming that only the formulation controls the drug release and that the in vitro release method is biopredictive. Since most models do not consider in vivo disintegration, this should be reintegrated in the modeling strategy by either keeping the formulation in the stomach or delaying the drug release in vivo. The different modeling approaches can be compared in terms of the fit, observed versus predicted in different conditions, and the impact on in vivo PK prediction. To this effect, the use of model prediction performance indicators such as the average fold error and absolute average fold errors is recommended to compare dissolution modeling options. The choice of dissolution model should be justified in the PBBM report, and a decision tree and checklist are proposed as part of this workshop.
3.1.4.4. Precipitation
Supersaturation may lead to precipitation in vitro and in vivo, but it remains hard to predict in vivo precipitation from in vitro experiments. The in vitro experiments can be used as a screening tool to rule out the presence of in vivo precipitation, since they can reproduce harsh conditions of rapid drug transfer with no sink conditions in the receiver compartment. The impact of excipients and physiological buffers was also highlighted during the presentations and discussions on this topic. Whenever high-quality human PK data are available, searches for signs of in vivo precipitation should be made if suspected. It is proposed that in vivo human data are used to fit precipitation models and verify their applicability during model validation. A decision tree was proposed to summarize the approach.
3.1.4.5. Permeability
Permeability can occur through passive, transcellular, or paracellular and carrier mediated processes. Permeability can be predicted using a variety of in vitro, in vivo, and in silico tools. Understanding the regional permeability of a drug candidate is key to understanding its developability, especially when a modified release formulation is required. The rat perfusion technique seems to be the best in vivo nonclinical technique to assess the regional permeability or the impact of excipients on drug permeability, compared to cell lines which tend to be more sensitive to the excipients effect.
3.1.4.6. Conclusions
The discussions during Day 1 highlighted that care should be taken to obtain relevant and accurate measurements of solubility, dissolution, precipitation, and permeability and that the type of formulation and excipients can affect all of these measurements. In addition, certain excipients can also impact the physiology depending on the administration route, which should also be considered during model validation. This may be a current gap of the PBBM tools since they rely heavily on the properties of the drug rather than the properties of the formulation (drug and excipients). Another important aspect of the measurement of input parameters was the physiological relevance of the method or medium utilized to predict the in vivo situation. For example, the use of bicarbonate, a physiological buffer, is suggested if an acid–base reaction occurs between the drug and or excipients as it better represents the environment in the intestine. This is true for the dissolution and precipitation measurements. In all cases, input parameters such as solubility, dissolution, precipitation, and permeability should be justified based on sound science, and assumptions should be made clearly and verified ideally with orthogonal measurements. Further details on best practices and decision trees from the breakout sessions will be shared in the Day 1 manuscript.
3.2. Day 2: Model Validation and Application Steps
On Day 2, four case studies were discussed, one from industry and three from the regulatory reviewed PBBM cases. This was followed by a roundtable discussion with the regulatory authorities to share their experience with PBBM model validation. In the afternoon, breakout sessions covered model development, model validation, and model applications.
3.2.1. Industry and Regulatory Discussion of Reviewed PBBM Case Studies
An industry case study presented by Tycho Heimbach, for fevipiprant, a low molecular weight, BCS class IV drug substance, has been tested in Phase 3 trials. PBBM was used to aid in setting dissolution specifications.15 The model included clinical pharmacokinetic data for two doses with bioequivalence (BE) and clinically observed non-BE data. IV microdosing data were used to describe disposition parameters. Dissolution data were modeled by using Weibull functions and z-factor models. The use of PBBM allowed for the successful definition of the fevipiprant BE safe space for the quality control (QC) dissolution method. A second case study described the use of PBBM for safe space analysis of molnupiravir capsules. These two case studies were not reviewed by regulators as part of this workshop, but they have interesting data to share with the scientific community.
Case Study 4 was presented by Luiza Borges (ANVISA) and summarized the assessment of EMD Compound A (provided by Merck Healthcare KGaA). It involved the use of PBBM to assess the impact of drug substance particle size distribution (DS-PSD) on absorption and pharmacokinetics to support D10, D50, and D90 specifications for an IR tablet. EMD Compound A, a BCS IV hydrochloride salt, was used as an example. Microdose intravenous (IV) and solution data were used to parametrize a two-compartment disposition model. In vitro solubility was measured in different media added with 100 mM sodium chloride to account for decreased drug solubility due to the chloride common ion effect. Some input parameters were fitted to the observed data such as pKa and fraction unbound in enterocytes. Other input parameters were estimated by various methods and supported by parameter sensitivity analysis (PSA) such as the Peff and precipitation time. Solid oral formulations were considered for model validation, although the in vitro dissolution data was not integrated into the model. The conclusion was that this PBBM would not support a regulatory decision making on DS PSD specification and possible approaches for model refinement were suggested.
Case Study 5 was presented by Maria Malamatari and Susan Cole (MHRA) and summarized the assessment of the PBBM provided by Janssen to investigate the impact of not meeting the QC dissolution specification on drug exposure. A mechanistic absorption model was developed using compound-specific parameters in GastroPlus. Physiology-based dissolution testing (PBDT) was established as a two-phase dissolution approach using biorelevant media to mimic the fed state. The z-factor fitted to the PBDT profiles was used to integrate dissolution into the model. The distribution, metabolism, and excretion of the drug were simulated using a compartmental model. The mechanistic absorption model was validated using a relative bioavailability study. VBE trials were performed comparing stability batches with out-of-specification (OOS) dissolution results to a reference batch. It was predicted that the OOS batches were bioequivalent to the reference batch for both Cmax and AUC0–72h under the fed conditions, indicating no impact on drug exposure.
Case Study 6, presented by Shinichi Kijima (PMDA), summarized the assessment of the PBBM provided by Janssen to justify the presence of a polymorphic impurity with slower dissolution in the drug product. A validated PBBM model was applied to assess the impact of the polymorphic impurity on in vivo exposure. PK predictions for different percentages of the polymorphic impurity were conducted using virtual bioequivalence trials. The presenter stated that the acceptability of a certain percentage of the impurity depended on the evaluation of the virtual bioequivalence trial, and a more conservative value may need to be chosen if the variability setting is assessed as uncertain.
3.2.2. PBBM Roundtable Discussions by Regulatory Agencies: Topic: Model Validation
The Day 2 roundtable discussion brought together representatives from various regulatory agencies, including the FDA, ANVISA, MHRA, Health Canada, FAMHP, and PMDA. Seven key topics were discussed addressing:
Nonavailability of IV data: The participants agreed that the PBBM community is continuously learning and evolving. It was recognized that IV data are often not available for PBBM as some companies may not generate IV data unless intravenous dosing is one of the intended dosing routes. The use of IV PK data is usually preferred to build confidence in verification of systemic disposition parameters before building the absorption model, although other approaches to building a verifiable model can be viable. Other suitable approaches may include the use of clinical PK data from a low dose oral solution study with high bioavailability and absorption or newer preclinical scaling methods. The participants agreed that it would be helpful to compile a decision tree to estimate or generate human IV data suitable for PBBM.
Determining the minimum number of data sets required for model validation was recognized as a context-dependent decision. Factors such as model risk analysis, regulatory implications, and uncertainty in the modeling context should be considered when determining data set requirements.
The discussion on the importance of non-BE batches for model validation highlighted the need to introduce “bad or wacky batches” to assess the model’s robustness. At the same time, it was emphasized that the introduction of non-BE batches should be guided by meaningful changes in the formulation, particle size, or process parameters. It was also acknowledged that formulating non-BE batches may present practical challenges, and the focus should be on selecting batches that exhibit significant differences between the in vitro release profiles.
Regulatory agencies expressed their commitment to promoting PBBM and MIDD. Recommendations included proactive engagement by companies to present modeling approaches before drug development projects, publication of additional guidance documents by regulatory agencies, bridging the gap between clinical pharmacology and CMC teams, continuous education for regulatory staff, the adoption of a credibility assessment, framework,10−12 transparency, and clarity in communication, and early regulatory interactions (e.g., EMA scientific advice and presubmission meetings to align expectations).
The possibility to utilize animal data for model validation and input parameter assessment was discussed. The acceptability of using animal data would be determined on a case-by-case basis, and the feasibility of using a single model for multiple purposes would depend on the specific model context of use, model risks, and questions to be answered.
The establishment of predefined acceptance criteria was deemed important in the modeling and validation process and individual pharmaceutical companies and the IQ consortium have previously provided comments to the PBPK draft guidance for oral biopharmaceutics applications on acceptance criteria.13 The criteria would be tailored to the specific application (PK, PD, and tox) and guided by the context and goals of the modeling endeavor. However, the final evaluation and decision-making process should consider the totality of evidence to ensure a comprehensive assessment of the model’s performance.
Overall, the discussions highlighted the commitment of regulatory agencies to promote PBBM and MIDD and provided valuable insights into various aspects of PBBM.
3.2.3. Breakout Session Topics
3.2.3.1. IV Data Considerations for Model Development
There is no stringent regulatory requirement to routinely generate IV data for oral products. The decision to conduct IV studies is driven by factors such as the clinical use of the IV formulation, the need to understand drug–drug interactions (DDI), and the formulation purpose, particularly for drugs with low bioavailability.
A draft decision tree for the utilization of IV data in PBBM was presented. The decision tree aimed to provide guidance on when and how to incorporate IV data into PBBM modeling efforts. Further details will be included in the day 2 manuscript.
Model validation and acceptance/verification criteria in PBBM should consider available clinical data, model regulatory impact, and model risks. Model credibility, which refers to the trust in the predictive ability of the model, should be based on demonstrated evidence. Model regulatory impact outlines the influence the model will have on the final decision as well as what the current evidence standard is for answering the question(s) of interest.10,11 Model risk, which refers to the possibility of incorrect decisions and adverse outcomes, should be categorized into low, medium, and high based on the evidence available. Model verification relates to the underlying mathematical model, and model validation is the process of determining how accurately a model represents the real world compared to real data, as presented by Min Li at the workshop.
The FDA’s PBBM validation guidance is risk-based and fit for purpose. It recommends demonstrating the model’s predictive performance based on PK data from batches exhibiting unacceptable bioavailability (BA). Challenges in the validation of PBBM assumptions include the lack of complete understanding of the interaction between the drug product and the gastrointestinal (GI) tract, the effect of excipients on in vivo dissolution, and the gap between in vitro and in vivo dissolution. Validation and verification of models in the context of pharmaceutical bioequivalence require careful consideration of credibility and risk, accuracy of predictions compared with real-world data, and justification of model assumptions, as presented by Min Li at the workshop. It was also mentioned during the breakout session that an overarching MIDD guideline is being drafted at the ICH level (M15) that will include the principles of credibility assessment and should be available for public consultation within the coming months.
3.2.3.2. Variability and VBE
Variability should be considered in VBE studies as reference and test products may have different in vivo variability. Within-subject variability (WSV) can be modeled by propagating the known variability of physiology through population-based PBPK analysis. However, more data and studies are needed to understand the variability of physiological parameters and narrow the sources of variability. Apparent between-subject variability (BSV) is a hybrid measure of between-subject and within-subject variability when a single sampling from each individual, as presented by Prof. Amin Rostami at the workshop.
In VBE studies, the sample size estimation should match the clinical setting and be based on the power needed for a real clinical study. Monte Carlo simulation can be used to test different sample sizes until the desired passing rate is achieved. The choice between WSV and BSV variabilities depends on the specific case, as presented by Dr. Viera Lukacova at the workshop.
3.2.3.3. Safe Spaces
The concept of safe spaces in PBBM refers to defining edges of bioequivalence failure based on PK bioequivalence or exposure-response relationships. Safe spaces can be expanded based on exposure-response analyses for efficacy and safety. Case studies presented at the workshop demonstrated the use of safe spaces in PBBM for different drugs and formulations, including the consideration of dissolution profiles, WSV, and formulation bridging.
3.3. Day 3: Current State and New Horizons
On Day 3, podium presentations highlighted the utility of PBBM for various applications in the field of innovator and generic drug product development. This was followed by a roundtable discussion with the regulatory authorities to share their experience in PBBM submissions spanning the Clinical, NDA/MAA, and Post Approval stages. In the afternoon, breakout sessions covered the utilization of PBBM for generics application, the conduct of virtual bioequivalence studies, the definition of safe space, and the application of PBBM to support modified release formulation development.
3.3.1. Podium Presentations
Sivacharan Kollipara (Dr. Reddy’s Lab) presented on applications of PBBM in generic product development highlighting that similar modeling principles can be utilized for both innovative drugs/NCEs and generic drugs. It was recognized that PBBM has been increasingly used in generic drug applications and can impact regulatory decision making for generic drug products. Examples of applications of PBBM for generic product development include formulation development support, biopharmaceutics risk assessment, bioequivalence assessment including study design, between generic and reference product, establishment of dissolution safe space, justification of dissolution dissimilarity, and justification for biowaiver of lower strengths. In addition, three case studies were presented describing the utility of PBBM in generic applications.
Fang Wu (FDA) presented “OGD Perspectives on PBBM Applications for Generics” where a few case examples of recent models impacting regulatory decision making from the Office of Generic Drugs (OGD), FDA related to risk assessment and biowaiver, were presented. For example, PBPK absorption modeling was used for evaluating the impact of PSD on BE and support setting a clinically relevant three tier PSD specification. PBPK modeling was also used to evaluate the impact of noncomparable dissolution profile of lower strength on in vivo BE and support biowaiver and help identify biopredictive dissolution and support BE evaluation for a locally acting GI product.
Claire Mackie (Janssen) presented on the application of VBE trials to support formulation bridging. Two examples were discussed to demonstrate how VBE simulations have been used in drug product decision making: 1) PBBM to assess the impact of drug product changes on the exposure of JNJ-X. Based on this work, a switch from drug product 1 to drug product 2 would have a very low risk from an absorption perspective. 2) PBBM to assess the impact of changes in DS polymorph on the exposure of JNJ-Y. VBE simulations demonstrated that when comparing form 1 with form 2 no change in the oral bioavailability was expected. To close, some points for consideration were discussed: 1) How many independent data sets do we need to validate a PBBM? 2) Appropriate population size for the VBE trial. 3) Number of trials to be simulated. 4) Inclusion of WSV and BSV. 5) When could VBE simulations help in a project? This included timing for internal project decision making and timing if the strategy was to be discussed with regulatory agencies.
Miyoung Yoon (FDA) provided a presentation on the current and future perspectives on the “Utility of the advanced oral absorption PBPK modeling in clinical pharmacology assessment” sharing that advanced oral absorption modeling/PBBM is important for clinical pharmacology assessment when mechanistic characterization of complex oral absorption matters arising from interplay among drug physicochemical properties, formulation characteristics, and physiological factors is needed. It was emphasized that regulatory experience is being built in the PBPK advanced absorption model applications to clinical pharmacology assessments. The presented case examples provided insights into potential opportunities and areas for further improvement of PBPK/PBBM including, but not limited to, 1) the ability to prospectively predict the effects of drug product formulation on in vivo drug PK, 2) the in vitro to in vivo extrapolation of key absorption related parameters, 3) the understanding of age-related changes in GI absorption physiology (especially younger than age 2), and 4) the impact and potential interactions with drug and/or formulation excipients on absorption.
Christer Tannergren (AstraZeneca) presented on the use of PBBM to predict regional/colon absorption and the in vivo performance of modified release (MR) drug products. It was highlighted that prediction of colon absorption is a biopharmaceutics modeling and simulation capability gap. Recently published investigations demonstrating the merits, gaps, and opportunities of the current models in the prediction of colon absorption and in vivo performance of MR drug products were presented. It was concluded that a priori PBBM approaches are sufficiently accurate to enable the current models to be used to predict the in vivo performance of MR products in candidate drug risk assessment, product design, and early development. It was also concluded that there is now an available approach providing an opportunity for highly accurate PBBMs to be developed suitable for predictions for commercial and regulatory applications for MR products. More details will be shared in the Day 3 manuscript.
3.3.2. Regulatory Agency Perspective on Applications of PBBM in Regulatory Submissions
To close out the morning session, six regulatory speakers presented on “Applications of PBBM in regulatory submissions: Clinical, NDA/MAA and Post Approval”. Kimberly Raines (FDA) presented that to date, the FDA has received approximately 50 A/NDA and IND submissions, of which 48% were found acceptable. The applications for these models were to support dissolution method acceptance criteria, clinically relevant specifications of critical material attributes (CMA) and critical process parameters (CPP), and scale-up and postapproval changes (SUPAC)/risk assessment. Since publication of the draft “Guidance for Industry: The Use of Physiologically Based Pharmacokinetic Analyses – Biopharmaceutics Applications for Oral Drug Product Development, Manufacturing Changes, and Controls”,13 the submissions for SUPAC changes have increased from 5% to 36%. FDA identified deficiencies within the PBBM submissions related to inadequate model development, inappropriate model validation, and drug product specific anomalies. Luiza Borges (ANVISA) shared that between 2020 and 2022, six PBBM cases were submitted to ANVISA for NME and generic drug products. Applications were presented in both registration and postapproval stages aiming to support dissolution specifications, BE risk assessment for CMC changes, and biowaiver of different strengths than the one tested in the BE study. After regulatory review, 2 cases were approved, 2 were denied, 1 was withdrawn, and 1 was considered as informative data. In general, major issues observed on applications included incomplete understanding of product CMA, CPP, critical quality attributes (CQA) relationships, uncertainties on drug disposition due to lack of IV data, and uncertainties on within- and between-subject variability incorporation on simulations. Maria Malamatari (MHRA) shared key considerations regarding development regulatory submissions of PBBM, which included clearly stated model objective(s), high quality experimental data, justification of predicted parameters and selected method for dissolution data input, the need of in vivo data for model validation as well as the need to use prespecified acceptance criteria. Shereeni Veerasingham (Health Canada) presented that PBBM is increasingly employed in drug submissions filed with Health Canada as alternative approaches to establishing bioequivalence and to support risk assessments for drug product quality. Since August 2022 Health Canada has seen a 2-fold increase in the number of submissions that included biopharmaceutic modeling methods. This reflects the recent growth in the regulatory applications of PBBM and the perception of regulatory preparedness. Deficiencies noted during evaluation of PBBM included undefined/unclear model objectives, inadequate justification of input parameter or dissolution data input methods, inappropriate model validation data sets or acceptance criteria, and uncertainty in parameter estimates. Often, variability was not mechanistically or adequately incorporated in the virtual trial population used for simulations, and model risk and feasibility of alternative approaches were not discussed. In addition, Evangelos Kotzagiorgis (EMA) presented the high-level expectations for submission of PBBM documentation in regulatory submissions and suggested where and how PBBM information should appear in the eCTD. Some common deficiencies on PBBM application were described, which largely overlap with those presented by previous speakers. He reiterated the need for a framework for modeling in regulatory reviews, where the data quality requirements and the regulatory scrutiny will depend on the intended use and ultimately on the regulatory impact of the model. Finally, he emphasized the value of early engagement of sponsors with regulators. Hiroyuki Tsuji presented PMDA perspectives on applications of PBBM in regulatory submissions.
3.3.3. Breakout Session Topics
3.3.3.1. Applications of PBBM in Generic Drug Product Development
The main themes of the discussions were the latest advances and challenges of utilizing PBBM and virtual simulation for generic drug development purposes to facilitate formulation design and risk assessment and to provide scientific evidence and justification to support biowaivers. Special emphasis was put on the use of discriminatory and biopredictive dissolution methods, integration of in vitro dissolution data into the models, predicting the effects of excipient substitution, and establishment of PBBM safe space for generic drugs.
3.3.3.2. Virtual Bioequivalence Applications
The breakout session on virtual bioequivalence trials acknowledged their increasing use as surrogates for clinical BE studies to minimize the number of PK studies. The key outcomes of the breakout session discussion included the following: 1) The objective of the VBE trial should be clearly stated. 2) The number of subjects used in VBE trial should be the same as that used in any prior clinical BA/BE study. 3) Most attendees expressed that 10 VBE trials were reasonable; however, regulatory agencies have at times requested to conduct 100–200 VBE trials. 4) Selection of the virtual population should be driven by the intended use of the product and therapeutic area (e.g., male or female participants only). 5) The availability of non-BE data to verify the model should not be a requirement due to technical and logistical challenges; however, if such data are available, then they must be used in model verification. 6) Regulatory agencies are requested to provide specific positive feedback if VBE models were found to be adequate.
3.3.3.3. PBBM Defined Dissolution Safe Space and Extrapolation
It was acknowledged that establishing a safe space necessitates incorporation of quality by design (QbD) principles, risk assessments, and prior knowledge to understand and identify failure modes. The participants believed formulations containing modifying/functional excipients require understanding the underlying mechanisms in the interaction between the DS, excipient, and the biologic system for defining the safe space. The discussions addressed the critical question of the application of safe space to various failure modes not assessed in PK studies. Overcoming these challenges involves compiling identified CMAs, CPPs, and CFVs and conducting multivariate dissolution testing on proposed design spaces. The group indicated preference of using Phase 3 clinical batch formulation(s) as the target/reference to define the safe space; however, it acknowledged that leveraging PK data from multiple studies, if available, supports a well-informed dissolution safe space with reduced bias.
A decision tree for when to extrapolate outside the safe space was proposed and will be presented and discussed in the Day 3 manuscript. The participants believe that although the risk is low for safe space extrapolation in BCS 1/IR drug products with justification, a deep understanding of excipient impact is crucial for BCS 3/IR drug products, with successful extrapolation hinging on demonstrated dissolution method correlation with in vivo performance. Exploring exposure–response relationships, safety/efficacy profiles, and innovative data sources challenges the industry to redefine boundaries for safe space and its extrapolation. Ultimately, ensuring efficacy and safety beyond the safe space demands a combination of scientific and regulatory expertise, innovative thinking, and meticulous risk assessment.
3.3.3.4. Regional Absorption and Modified Release Formulation PBBM Applications
PBBM of MR drug products was acknowledged to be challenging despite the intention to link the in vitro release rate with PK as regional differences in absorption and bioavailability, especially in the colon, need to be accounted for in the model. Examples of applications of PBBM for MR product development include candidate drug risk assessment, product design, and development support; however, the use of PBBM for regulatory purpose is in some cases hindered by lack of mechanistic models for release mechanisms and a lack of understanding regarding the in vivo behavior of the functional excipients. The key outcomes of the breakout session discussions were that it was recognized that there is an increased use of physiologically based IVIVCs when traditional IVIVCs are unsuccessful. In addition, it was recognized that PBBM can be used to assess the risk for alcohol-induced dose dumping as well as food effects for MR drug products.
4. Utility of PBBM, Experience with PBBM Submissions, and Risk Based Approach: Participant Survey
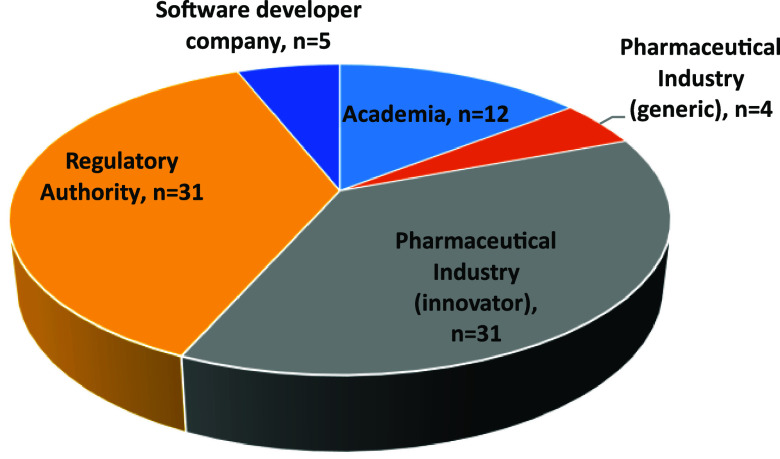
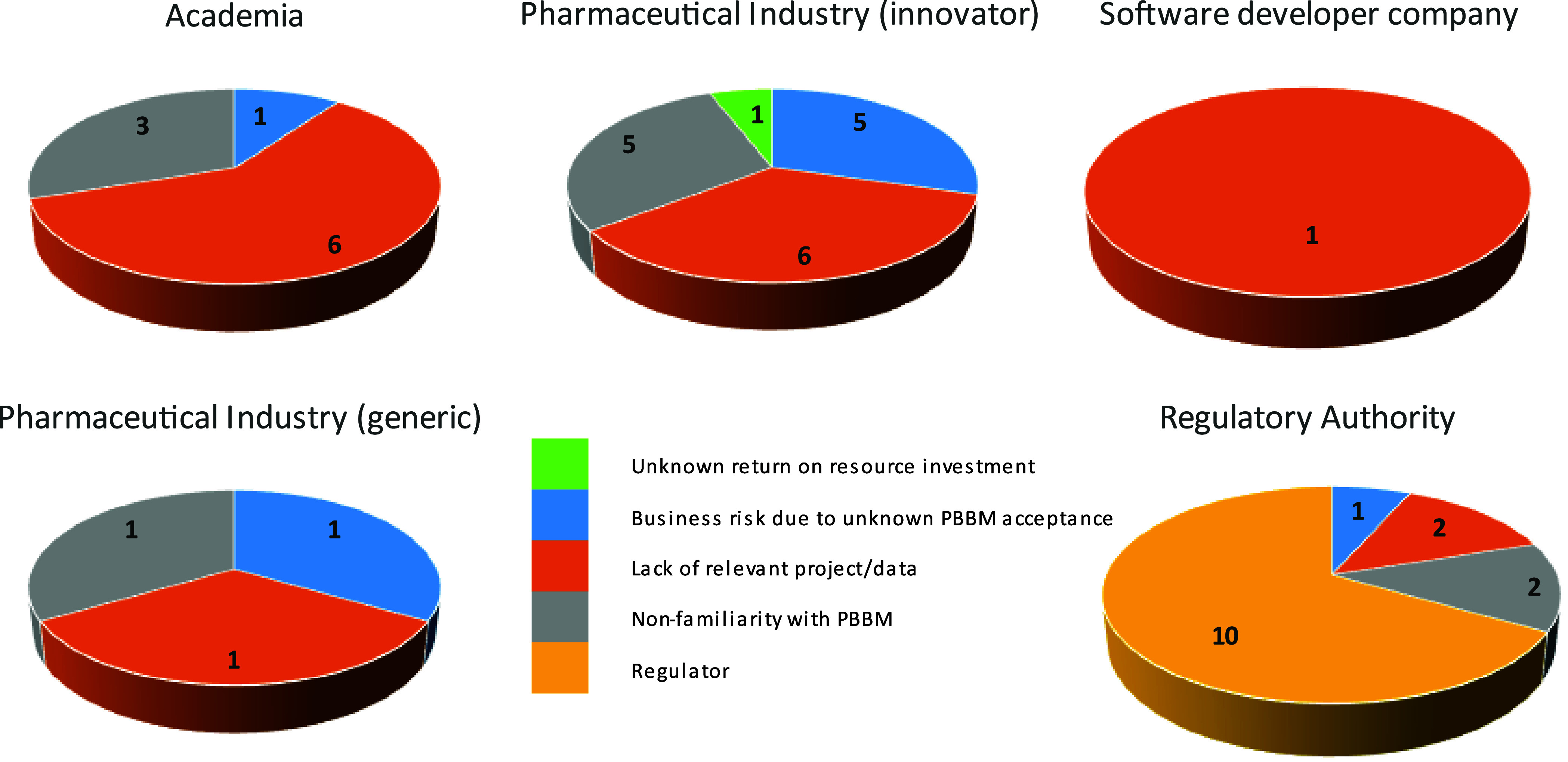
These discussions were supported by a Microsoft Forms survey conducted during the workshop. A Microsoft Forms survey was distributed to the in-person and remote attendees of the PBBM workshop. The survey consisted of 13 questions, elaborated by the OC of the workshop and inspired by previous questions asked during the 2019 workshop. The responses to the survey were collected during the 3 days of the workshop. A total of 83 respondents participated in the survey. The responses were anonymous; however, the respondents were asked to which institutions they belonged. Oral feedback from certain participants highlighted that some institutions were forgotten, such as contract research organizations. There was a good balance in the survey participation between pharmaceutical industry and regulators (Figure 4).
Figure 4.
Question 1: Please indicate the institution to which you are affiliated.
The respondents were asked about their experience with either a submission or the review of a PBBM for regulatory application. On average, the percentage of respondents with no experience in either submitting or reviewing a PBBM was 69% (range 17–80%), which suggests PBBM, even though interest is rising, still has room for increased uptake. The pharmaceutical industry participants and regulators participating in the workshop had a similar level of experience (∼30%). Academics participating in the workshop had the least experience (17%), while participants from software developer companies had the greatest experience (80%). Overall, an aim of the workshop was to familiarize participants in the parametrization, validation, and utilization of PBBM, and this result was therefore expected.
When participants who responded to question 2 with a “no previous experience in submission” were asked through question 3 about the reasons why they did not submit a model (Figure 5), they responded that they did not have the opportunity (i.e., relevant project or data to do this simulation) and, in the second position, that they were not familiar with the tools. Some respondents, especially in the pharmaceutical industry also pointed out to the business risk due to the unknown PBBM acceptance by regulators. Regulators were also asked question 3, but it was ill-formulated for them since by definition, regulators do not submit PBBMs. We can assume that if the question included a “why did you not review” a PBBM, most of the answers would have been that they did not receive a submission including PBBM, since the number of submissions comprising PBBMs is still limited.
Figure 5.
Question 3: If you did not submit any PBBM, what were the common reasons?
The majority of PBBM submissions covered oral immediate release products of all BCS classes (70%). PBBMs dealing with nonoral products were a minority (5%). Modified release formulations comprised approximately one-third of the oral product PBBM submissions. The respondents were then asked how the submitted PBBM were applied (i.e., what were the main quality questions that the models were trying to answer (Figure 6)).
Figure 6.
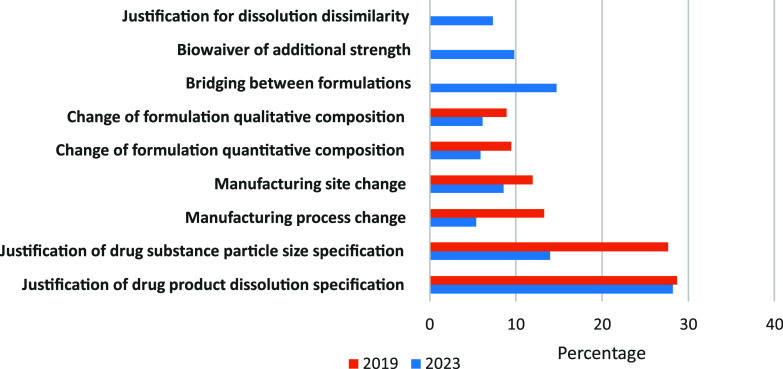
Question 5: Quality questions answered by the PBBM? 2019 data from Pepin et al.5
The majority of the PBBMs reported during the 2023 survey concern the justification of drug product dissolution specifications. This percentage is very close to what was observed in 2019.5 The second main application of PBBM corresponds to the justification of drug substance particle size specifications. New utilizations specific to the generic industry or late clinical stages for innovator industry such as formulation bridging, waivers of strength, or justification of dissolution dissimilarity appear as new important uses in 2023. The level of PBBM submission rejections has remained very constant over the last 5 years, and almost two-thirds of the submissions are rejected. The reasons for PBBM rejection are illustrated in Figure 7.
Figure 7.
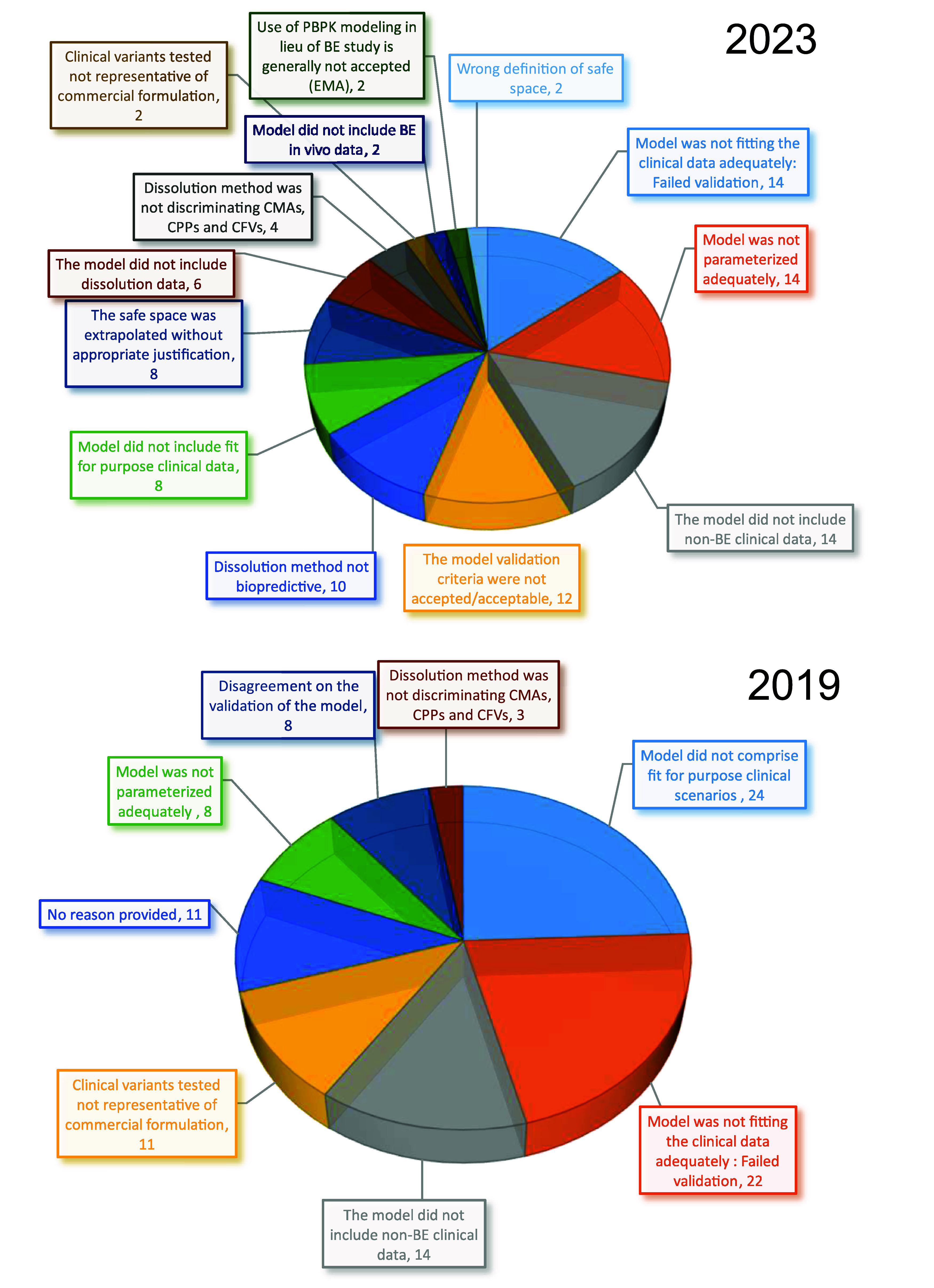
Question 7: What were the main reasons for the PBBM rejection? 2019 data from Pepin et al.5 Data are expressed as a percentage of the total rejection reasons.
The reasons for PBBM submission rejections as reported in this 2023 survey have slightly changed compared to those reported in 2019.5 In 2019, 24% of the model rejections were because the clinical scenarios on which the models were validated were not considered “fit for purpose”, i.e., including clinical batch variants which comprised variations of the quality attribute that was specified by the PBBM. In 2023 this reason has dropped to 8% of the rejection causes, which is considered an improvement as the regulatory expectations in terms of model validation are better understood. Another improvement between 2019 and 2023 is the percent rejection due to the lack of representation of the commercial formulation for clinical variants used for model validation. In 2019, this rejection cause represented 11%, while in 2023, it represents only 2%. The second main rejection reason in 2019 which is the first rejection reason in 2023 is the lack of adequate in vivo data fitting. In 22% of the rejection cases in 2019 and 14% of the cases in 2023, the model was considered to have failed validation. In 2023, it is apparent that this validation failure is also supported by a disagreement between the industry and regulators of what constitutes acceptable validation criteria since this reason represents 12% of the rejection causes. This information reinforces the need for the discussions on workshop Day 2. Without a common understanding and expectation in terms of model validation indicators and acceptance criteria, PBBM submissions cannot be made with confidence.
A constant rejection reason between 2019 and 2023 is the absence of nonbioequivalent (i.e., relative bioavailability was dissimilar) batch to support PBBM validation. This rejection reason represents 14% of the cases in both surveys. This topic was also heavily discussed during this workshop. A rejection reason which has increased significantly between 2019 and 2023 is the inadequate model parametrization which jumped from 8% to 14% over 5 years. Model parametrization is the topic of this workshop discussion on day 1, and this increase in rejection level justifies why harmonization and training are needed to understand best practices to measure and integrate solubility, dissolution, precipitation, and permeability.
The use of PBBM to support various applications is not without risk to the patient should the decision be based on the wrong model. The respondents were asked to rank the risk to the patient when using PBBM to inform internal or regulatory decision making for various purposes (Figure 8). Risks were in three categories: low, medium, and high. An average risk score was calculated by multiplying the fractional votes in the low, medium, and high risk categories by the values 0, 50, and 100 respectively and summing up these categories into a single value ranging from 0 for lowest risk to 100 for highest risk. According to the 2023 survey respondents, the equal highest risk to the patient was to use PBBM to waive clinical studies to bridge different drug products either during or after phase 3 or when the f2 similarity criterion fails. This could result in a drug product with a reduced exposure potentially influencing efficacy or an increased exposure potentially influencing safety. The two lowest reported risks to the patient were when PBBM was used to support the development of a biopredictive dissolution method or to obtain regulatory flexibility when proposing changes to the product specifications within an established safe space. This question was geared toward understanding and ranking model consequence as defined by Kuemmel et al. in their credibility assessment framework.12
Figure 8.
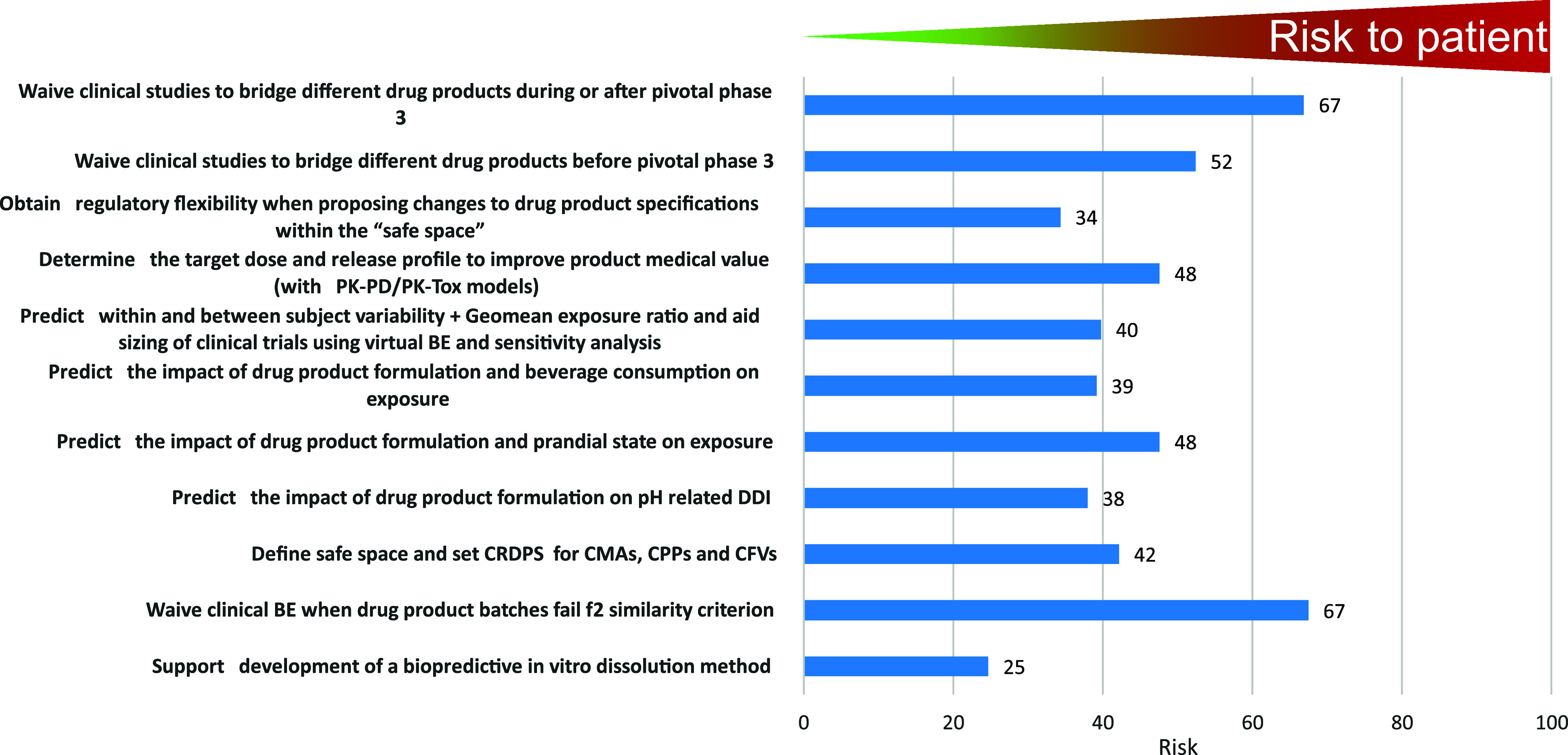
Question 8: How do you rank the risk associated with the patient when using PBBM to inform internal or regulatory decision making for the following purposes?
Interestingly, question 9, which used the same categories of PBBM applications but probed at the perceived difficulty in gaining regulatory acceptance on the submitted PBBM, ranked very similarly to risk perceived to the patient (Figure 9).
Figure 9.
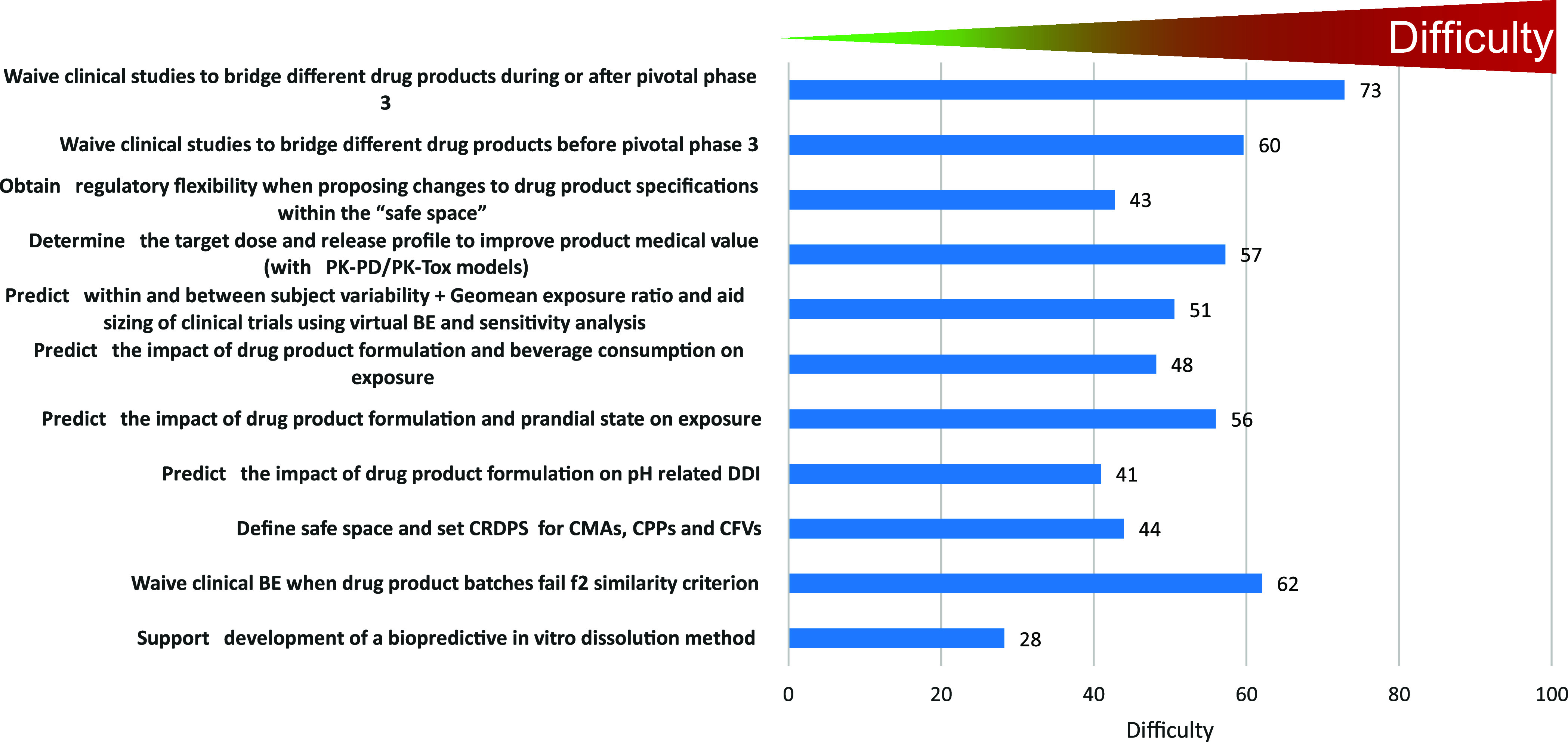
Question 9: What is in your opinion the likelihood of regulatory acceptance of state-of-the-art PBBM for the following purposes?.
This result was somewhat surprising since the likelihood of a PBBM acceptance should depend on the quality of the data available for model parametrization, development, and validation within the context of the intended use. However, the regulatory hurdles, such as the credibility requirements for accepting a model, depend on the regulatory impact and model influence, which are considered for defining model risk. To mitigate this risk, predictions should always be analyzed considering the totality of data, including clinical data that have evaluated/confirmed scenarios close to the predicted ones, the properties of the drug product and drug substance, and how well the control strategy proposed by the sponsor can ensure reproducible quality to the product.
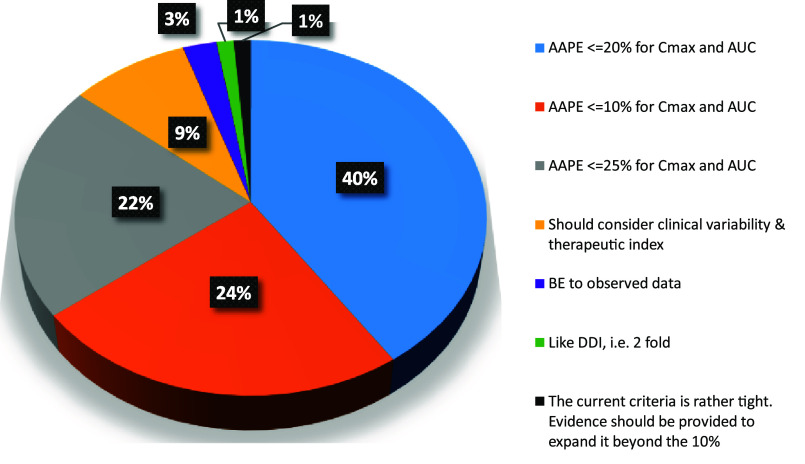
Question 10 was probing the current criteria for the model performance indicator applied to PBBM validation (Figure 10). Although no definitive criteria for model validation are proposed by the current FDA draft “Guidance for Industry: The Use of Physiologically Based Pharmacokinetic Analyses – Biopharmaceutics Applications for Oral Drug Product Development, Manufacturing Changes, and Controls”,13 the guideline mentions that “...model validation acceptance criteria should be established a priori and the criteria should be appropriate for the specified application...”. For instance, the acceptance criteria for a mechanistic IVIVC model to support a biowaiver should comply with the criteria provided in the “Guidance for Industry Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations”.14 PBBMs are rarely developed solely on crossover IVIVC type clinical trials and validated using unit impulse responses individually fitted to a reference formulation. In most instances, the PBBM leverage PK data from healthy volunteers and/or patients, using rich PK data obtained during the clinical pharmacology studies which supported the product development, ranging from single ascending dose studies, multiple ascending dose studies, food effect studies, and pH-related DDI studies. For PBBM validation, fit for purpose clinical trials are generated using commercially representative formulation variants that assess the quality attribute of the drug product or drug substance that the PBBM will specify. The populations of these clinical trials will therefore be different in number and physiology, and the distribution and drug elimination will not be reproducible from one study to another. Sometimes, validation will also cover different types of formulation and therefore there is an argument that the criteria for model validation cannot be as strict as those applied for the IVIVC studies conducted for modified release formulations.14
Figure 10.
Question 10: Assuming full PBPK model and PBBM validated on all rich PK data during development (e.g., 1 SAD, 1 MAD, 1 food effect study, 1 ARA study, 1 fit for purpose study with 3 variants), what should the average absolute prediction error (AAPE) for PBBM validation be?
Question 10 asked in the scenario described above (i.e., a PBBM based on all rich PK data available for a drug) what the best criterion to apply to model validation would be (Figure 10).
Only 24% of the respondents would consider that the current 10% absolute average prediction error (AAPE) criterion in the draft FDA guidance is adapted to the most standard PBBM validation scenario; 63% of the respondents considered that the AAPE criterion should be increased. Interestingly, 9% pointed out that the therapeutic index and clinical variability should be considered in setting the acceptance criteria. Most of the votes went for NMT 20% AAPE to support model validation. Question 11 went a step further to probe model validation, and most respondents (80%) agreed with the statement that AAPE could be different for AUC and Cmax with justification. Similarly, many of the respondents (84%) agreed with the need to consider clinical within subject variability, when setting the AAPE acceptance criterion for model validation.
The responses to the final question (question 13) were almost a unanimous vote (96%) in favor of the development of a PBBM report template by the industry to help with getting things right when submitting PBBM to the regulatory agencies in the future. Industry members plan to develop a template, as the final paper in the series from this PBBM workshop, to cover the major fields of a PBBM submission (e.g., executive summary, model questions/context of use, model risk and decision consequence, model build rationale, input data and associated details, clinical data sets used to validate the models, and finally model application).
5. Summary and Next Steps
The workshop provided opportunities for industrial, academic, regulatory scientists, and software companies to further discuss the topic of PBBM best practices and applications for drug product quality, including regulatory and industry perspectives, with the goal of increasing patient benefit through scientific collaboration.
In this partnership, industry including the software companies submitted PBBM case studies for discussion by participating regulatory agencies to ensure consistent review. PBBM cases were independently and collaboratively discussed by regulators, and academic colleagues participated in some of the discussions. Overall, six regulatory agencies were involved in the case study exercise, ANVISA, FDA, Health Canada, MHRA, PMDA, and EMA (experts from Belgium, Germany, Norway, Portugal, Spain and Sweden), in what we believe was the first time such a collaboration has taken place.
The PBBM case studies enabled the industry to receive constructive feedback from global regulators. The case study review presentations on Days 1 and 2, along with the application of PBBM presentations on Day 3 by the participating agencies, highlighted clear direction for future PBBM submissions for regulatory consideration. Cross-agency feedback from the Day 3 panel discussion included the following themes and key messages:
outline the PBBM question of interest and context of use
consider an interdisciplinary approach for model summary
identify and describe model assumptions clearly
provide hyperlinks to appropriate sections of the eCTD where available
provide detailed PBBM reports describing the drug substance and drug product characteristics, which will ensure all relevant information is shared with regulators
engage regulators early in drug development when approaching PBBM (e.g., EMA scientific and qualification procedures, presubmission meetings, FDA and MIDD paired meeting program)
Although it is clear from the presentations, panel discussions, and breakout sessions that we continue to make significant progress in the field of PBBM, there is still the need to continue the momentum and dialogue between industry and regulators. Next steps will include the following:
manuscripts covering detailed proceedings for each of the 3 days
a manuscript on an industry led PBBM template including the Credibility Assessment Framework which regulators have agreed to review
a plan for future workshops in different locations
encouragement for the industry to take up the earlier regulatory interaction and discussion
Acknowledgments
The meeting organizers are extremely grateful to Keiasia Robinson and Dana Hammell (University of Maryland, Baltimore) and the FDA Office of Regulatory Science and Innovation (ORSI) for significantly contributing to the success of the workshop. This research was supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award 5U01FD005946 totaling $5,000 with 100% funded by FDA/HHS. The collaboration work was supported by the IQ consortium.
Glossary
Acronyms/Abbreviations
- AAPE
absolute average prediction error
- ANVISA
Brazilian Health Regulatory Agency
- ANDA
amended new drug application
- ARA
acid reducing agent
- AUC
area under the curve
- BA
bioavailability
- BCS
biopharmaceutical classification system
- BE
bioequivalence or bioequivalent
- BO
breakout session
- BSV
between subject variability
- CFV
critical formulation variant
- CMA
critical material attribute
- Cmax
maximum concentration
- CMC
chemical and manufacturing controls
- CPP
critical process parameter
- CQA
critical quality attribute
- CRDS
clinically relevant dissolution specification
- DDI
drug–drug interaction
- DP
drug product
- DS
drug substance
- EMA
European Medicines Agency
- eCTD
electronic Common Technical Document
- EU
European Union
- FDA
Food and Drug Administration
- FAMHP
Belgian Federal Agency for Medicines and Health Products
- HCl
hydrochloric acid
- GI
gastrointestinal
- IND
investigational new drug
- IR
immediate release
- IQ
International Consortium for Innovation & Quality in Pharmaceutical Development
- IV
intravenous
- IVIVC
in vitro in vivo correlation
- MAA
marketing authorization application
- MAD
multiple ascending dose
- MCERSI
Maryland Center of Excellence in Regulatory Science and Innovation
- MHRA
Medicine and Healthcare products Regulatory Agency, UK
- MIDD
model informed drug development
- MR
modified release
- MS
Microsoft
- NCE
new chemical entity
- NDA
new drug application
- NME
new molecular entity
- NMT
not more than
- OC
organizing committee
- OGD
Office of Generic Drugs
- OOS
out of specification
- Peff
effective permeability
- PBDT
physiologically based dissolution testing
- PBBM
physiologically based biopharmaceutics model(s), physiologically based biopharmaceutics modeling
- PBPK
physiologically based pharmacokinetics
- PD
pharmacodynamics
- PK
pharmacokinetics
- PMDA
Pharmaceuticals and Medical Devices Agency, Japan
- P-PSD
product particle size distribution
- PSA
parameter sensitivity analysis
- PSD
particle size distribution
- QbD
quality by design
- QC
quality control
- SAD
single ascending dose
- SUPAC
scale up and post approval changes
- Tox
toxicology
- VBE
virtual bioequivalence
- WSV
within subject variation
- US/USA
United States of America
- z-factor
option to simulate the dissolution/release from solid oral dosage forms
This article reflects the views of the individual authors and should not be construed to represent the views, policies or nomenclature of their organizations, including ANVISA, FAMHP, EMA, FDA, Health Canada, MHRA, Norwegian Medicines Agency, Swedish MPA, PMDA.
The authors declare the following competing financial interest(s): C. Mackie, S. Arora, X. Pepin, T. Heimbach, C. Tannergren, A. Mitra, S. Suarez-Sharp, and G. Rullo are employees of their respective companies and have ownership, options, and/or interests in their respective stock.
Special Issue
Published as part of Molecular Pharmaceuticsvirtual special issue “2023 PBBM Workshop for Drug Product Quality”.
References
- K Y.; Kollipara S.; Ahmed T.; Chachad S. Applications of PBPK/PBBM modeling in generic product development: An industry perspective. J. Drug Delivery Sci. Technol. 2022, 69, 103152. 10.1016/j.jddst.2022.103152. [DOI] [Google Scholar]
- Abend A.; Heimbach T.; Cohen M.; Kesisoglou F.; Pepin X.; Suarez-Sharp S. Dissolution and Translational Modeling Strategies Enabling Patient-Centric Drug Product Development: the M-CERSI Workshop Summary Report. AAPS J. 2018, 20 (3), 60. 10.1208/s12248-018-0213-x. [DOI] [PubMed] [Google Scholar]
- Suarez-Sharp S.; Cohen M.; Kesisoglou F.; Abend A.; Marroum P.; Delvadia P.; Kotzagiorgis E.; Li M.; Nordmark A.; Bandi N. Applications of Clinically Relevant Dissolution Testing: Workshop Summary Report. AAPS J. 2018, 20 (6), 93. 10.1208/s12248-018-0252-3. [DOI] [PubMed] [Google Scholar]
- Heimbach T.; Suarez-Sharp S.; Kakhi M.; Holmstock N.; Olivares-Morales A.; Pepin X.; Sjögren E.; Tsakalozou E.; Seo P.; Li M. Dissolution and Translational Modeling Strategies Toward Establishing an In Vitro-In Vivo Link–a Workshop Summary Report. AAPS J. 2019, 21 (2), 29 10.1208/s12248-019-0298-x. [DOI] [PubMed] [Google Scholar]
- Pepin X. J. H.; Parrott N.; Dressman J.; Delvadia P.; Mitra A.; Zhang X.; Babiskin A.; Kolhatkar V.; Suarez-Sharp S. Current State and Future Expectations of Translational Modeling Strategies to Support Drug Product Development, Manufacturing Changes and Controls: A Workshop Summary Report. J. Pharm. Sci. 2021, 110, 555–566. 10.1016/j.xphs.2020.04.021. [DOI] [PubMed] [Google Scholar]
- Pepin X.; Dressman J.; Parrott N.; Delvadia P.; Mitra A.; Zhang X.; Babiskin A.; Kolhatkar V.; Seo P.; Taylor L.; et al. In Vitro Biopredictive Methods: A Workshop Summary Report. Journal of pharmaceutical sciences 2021, 110, 567–583. 10.1016/j.xphs.2020.09.021. [DOI] [PubMed] [Google Scholar]
- Parrott N.; Suarez-Sharp S.; Kesisoglou F.; Pathak S. M.; Good D.; Wagner C.; Dallmann A.; Mullin J.; Patel N.; Riedmaier A. E.; et al. Best Practices in the Development and Validation of Physiologically Based Biopharmaceutics Modeling. A Workshop Summary Report. J. Pharm. Sci. 2021, 110, 584–593. 10.1016/j.xphs.2020.09.058. [DOI] [PubMed] [Google Scholar]
- Mitra A.; Suarez-Sharp S.; Pepin X. J. H.; Flanagan T.; Zhao Y.; Kotzagiorgis E.; Parrott N.; Sharan S.; Tistaert C.; Heimbach T.; et al. Applications of Physiologically Based Biopharmaceutics Modeling (PBBM) to support Drug Product Quality: A Workshop Summary Report. J. Pharm. Sci. 2021, 110, 594–609. 10.1016/j.xphs.2020.10.059. [DOI] [PubMed] [Google Scholar]
- Anand O.; Pepin X. J. H.; Kolhatkar V.; Seo P. The Use of Physiologically Based Pharmacokinetic Analyses—in Biopharmaceutics Applications -Regulatory and Industry Perspectives. Pharm. Res. 2022, 39, 1681. 10.1007/s11095-022-03280-4. [DOI] [PubMed] [Google Scholar]
- Musuamba F. T.; Skottheim Rusten I.; Lesage R.; Russo G.; Bursi R.; Emili L.; Wangorsch G.; Manolis E.; Karlsson K. E.; Kulesza A.; et al. Scientific and regulatory evaluation of mechanistic in silico drug and disease models in drug development: Building model credibility. CPT Pharmacometrics Syst. Pharmacol 2021, 10 (8), 804–825. 10.1002/psp4.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skottheim Rusten I.; Musuamba F. T. Scientific and regulatory evaluation of empirical pharmacometric models: An application of the risk informed credibility assessment framework. CPT Pharmacometrics Syst. Pharmacol 2021, 10 (11), 1281–1296. 10.1002/psp4.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuemmel C.; Yang Y.; Zhang X.; Florian J.; Zhu H.; Tegenge M.; Huang S.-M.; Wang Y.; Morrison T.; Zineh I. Consideration of a Credibility Assessment Framework in Model-Informed Drug Development: Potential Application to Physiologically-Based Pharmacokinetic Modeling and Simulation. CPT: Pharmacometrics & Systems Pharmacology 2020, 9 (1), 21–28. 10.1002/psp4.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Use of Physiologically Based Pharmacokinetic Analyses–Biopharmaceutics Applications for Oral Drug Product Development, Manufacturing Changes, and Controls; FDA-2020-D-1517; FDA, Center for Drug Evaluation and Research, 2020.
- Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations Guidance for Industry; FDA, Center for Drug Evaluation and Research, 1997. [DOI] [PubMed]
- Kourentas A.; Gajewska M.; Lin W.; Dhareshwar S. S.; Steib-Lauer C.; Kulkarni S.; Hirsch S.; Heimbach T.; Mueller-Zsigmondy M. Establishing the Safe Space via Physiologically Based Biopharmaceutics Modeling. Case Study: Fevipiprant/QAW039. AAPS J. 2023, 25, 25. 10.1208/s12248-023-00787-5. [DOI] [PubMed] [Google Scholar]