Abstract
Aim:
The potency of Adenovector expressing Mda7-tLyp1 (Ad-Mda7-tLyp1) for death induction was evaluated on the breast (MCF7), liver (HepG2), and gastric (MKN45) cancer cell lines.
Background:
Mda-7 could be a possible complementary to traditional cancer therapy, and tethering to tumor-homing peptides (THPs) might improve its therapeutic efficacy.
Methods:
After the preparation of recombinant Ad-Mda7-tLyp1 and Ad-Mda7, the expression of recombinant proteins was analyzed by ELISA. Adenovectors were transduced (MOI=2-5) into Hep-G2, MCF7, MKN45, and normal skin fibroblast, then tumor-killing effect was measured by cytopathic effect (CPE) monitoring, MTT viability test, BAX gene expression analysis, and Caspase3/7 assay.
Results:
ELISA assay revealed a sustained level of recombinant protein secretion following Adenovector transduction. In CPE microscopy, all cancer cell lines showed a significant reduction (≥50%) in their normal phenotype after receiving Ad-Mda7-tLyp1 and Ad-Mda7. The viability was significantly lower compared to the control, indicating an anti-proliferating effect. In parallel, the viability test showed that Ad-Mda7 and Ad-Mda7-tLyp1 have a significant killing effect (≥50%) on MCF-7, Hep-G2, and MKN45 compared to normal fibroblast (P≤0.05). BAX gene expression analysis showed that both Ad-Mda7-tLyp1 and Ad-Mda7 vectors induced >2-fold increase of apoptosis (P<0.05), particularly in MCF7. Similarly, caspase3/7 activity showed a significant increase (P<0.05) following Ad-Mda7, and Ad-Mda7-tLyp1 transduction into cancer cell lines, but not in normal fibroblasts.
Conclusion:
The newly constructed Ad-Mda-tlyp1 showed a suitable tumor cell killing activity and enough specificity on studied cell lines.
Key Words: Adenovector, Mda-7, Cancer gene therapy, Tumor homing peptide, tLyp1, Cancer
Introduction
The use of viral vectors in gene therapy shows promise as an effective approach for managing cancer, since they possess high transduction efficiency and may stimulate immune responses. The user's text is empty. There are now several viral-based cancer gene therapy platforms approved by FDA. In 2015, talimogene laherparepvec which is a genetically modified Herpes simplex virus type 1 (HSV-1) could pass the clinical examination for approval, and very recently, in late 2022, the first FDA-approved Adenovector introduced for the unresponsive non-muscle invasive bladder cancer (2, 3). DNA viral vectors, such as Adenovirus vector, HSV-1, and Vaccinia virus are the most promising viral vectors for the elimination of malignant cells. These cells do not need to be integrated and may achieve a high level of transgene expression. Additionally, they are capable of inducing a substantial immune response, which is essential for eliminating tumors. Adenoviral vectors, the most popular vectors in cancer therapy, have advantages, such as the ability to infect dividing and non-dividing cells, the capacity to accommodate large transgenes, easy scaling-up or manipulation, outdoor stability, and high transduction efficiency. However, the toxicity of Adenovector would cause trouble during their application in gene therapy because of high immune stimulation when they were previously infected with adenovirus (4-6). Adenoviruses have double-stranded DNA and are so common that the large number of people develop life-long immunity to the most commonly seen type. During the construction of adenoviral vectors, regulatory genes that are essential for determining the appropriate size and application of the transgene were removed. Gendicine, an Adenovector cancer gene therapy platform, was registered for the first time in 2003 and it was E1 and E3 deleted Adenovector which expressed P53. Now there are E2, E3, and E4 deleted Adenovectors available which provide an additional 10 kbp of space for therapeutic gene insertion (7).
Melanoma differentiation-associated gene 7 (Mda-7), known as interleukin 24 (IL-24), is a unique cytokine with pro-inflammatory criteria (8, 9). It shows specific anti-tumor potency when over-expressed via an Adenoviral vector expressing system (10). The specific tumor-killing activity makes Mda-7 an attractive candidate for cancer-initiating cell elimination and inducing programmed cell death (11). Mda-7 protein, released by healthy cells, may disseminate to metastatic locations and induce programmed cell death in tumor cells that were not originally exposed to the therapeutic gene. This mechanism which is known as the bystander effect can be improved by tethering proteins to tumor-homing peptides (THPs) (12). THPs are extremely various which of most popular ones are RGD and Tat showed specificity, and efficiency for tumor penetration (13, 14). However, in a few studies, some attempts to improve Mda-7 activity by THPs have run into difficulties after peptide fusion (15). tLyp1 is a new tumor-homing peptide with some antitumor properties (16, 17). It (CGNKRTR) is a truncated form of tumor-homing peptide Lyp1 (CGNKRTRGC) which contains a CendR element and can penetrate tumor tissue via the neuropilin receptors (NRP1 and NRP2) dependent CendR internalization pathway (18). NRP1 and NRP2 are single-pass transmembrane glycoproteins that are overexpressed in the angiogenic vessels in most carcinomas (19, 20). tLyP-1 is a unique peptide because of its tumor penetrating ability as well as its inherent antitumor activity (21).
In general, incorporating an Adenovector as an expression platform for the secretory Mda-7 holds promise for its efficient, stable, and targeted production, thereby maximizing its therapeutic advantages. However, more study is necessary to fully understand and evaluate the advantages and practicality of using this method for tumor-killing secretory proteins like Mda-7. Adenoviral vectors expressing Mda-7 (Ad-Mda-7) and Mda-7-tLyp1 (Ad-Mda-7-tLyP1) were prepared, and then their killing potency was investigated on HepG2, MKN45 and MCF7 cell lines. This research is innovative since the tumor-killing potency of Adenovector expressing Mda-7-tLyp1 was not evaluated before.
Methods
Reagents and cell line
All the materials for DNA cloning including Pfu polymerase, T4 ligase, and restriction enzymes (PacI, PmeI, XhoI, Kpn1) were purchased from NEB (USA), Genabioscience (Germany), and Cinaclone Inc. (Iran). Plasmid, and DNA isolation/purification kits were purchased from MN (Germany).
Cell culture-related materials were purchased from BioIdea and Biosera Inc. (UK) which were stored in the recommended milieus. ELISA kit for the quantification of Human IL-24 (Mda-7) was purchased from R&D systems company (USA).
AD293, MCF7 (breast), HepG2 (hepatic), MKN45 (gastric), and foreskin fibroblast were prepared from the National Cell Bank of Iran or other labs. They were cultured in the Dulbecco’s modified Eagle’s medium (DMEM) or F12 supplemented with 100 IU/ml penicillin, 100 mg/ml streptomycin, 10% (v/v) fetal bovine serum, 20 mM HEPES, and non-essential amino acids.
Preparation of recombinant Adenoviral vectors
The pcDNA3.1 vectors expressing Mda-7 and Mda-tLyP1 were prepared to encode native and peptide-modified Mda-7 protein (22). The sequences were sub-cloned into a transfer vector (pAdtrack-CMV-GFP), as described before (23). To generate recombinant adenoviral plasmid expressing Mda-7 and Mda-7-tLyP1, PmeI enzyme is used to linearize pAdtrack-Mda-7 and pAdtrack-Mda-7-tLyp-1 transfer vector. Subsequently, the electro-competent E. coli BJ5183 cells were co-transformed with supercoiled pAdenovator E1/E3, which contains the backbone genome, in order to initiate homologous recombination. The proper recombinant vectors were screened by two different colony-PCRs using hexon-specific primers, and Pac-I digestion and gel electrophoresis.
Recombinant Adenovector particles expressing GFP (Ad-GFP), Mda-7 (Ad-Mda-7), and tLyP-1 (Ad-Mda-7-tLyP1) were prepared following linear plasmids transfection into AD-293 packaging cell-line with an incubation period of >5 days under 37oC and 5% CO2 atmosphere. The infectivity of these new vectors was evaluated on HEK-293 cells by observing the cytopathic effect (CPE), and GFP shining under fluorescent microscopy. Afterward, they were propagated on 15 T175 flasks in a 2% serum DMEM medium for 2 more days. The viral vector stocks underwent two consecutive 0.4 μm filtration steps, followed by Amicon® Ultra-15 centrifugal tangential filtration, to purify and concentrate the collected viruses. The titer of viral vectors was determined by doing the 50% tissue culture infectivity dose assay (TCID50). The endpoint was determined as the highest dilution in which the virus developed CPE on 50% of inoculated well, then proportional distance was calculated by the Reed-Muench method to add decimal to the number. Viral MOI (multiplicity of infection) was estimated by converting the TCID50 titer to the number of infectious particles/ml. Ultimately, MOI: 1–5 was used to assess the infectivity of the produced vectors on various normal and cancer cells. The process of MOIs determination for each line was initially screened by transduction rate assessment via fluorescent microscopy for each batch of viral vectors. The MOIs were then selected based on the lowest toxicity on normal cell, then by final transduction rate which was set up ≥50%.
Mda-7 ELISA assay
To quantify and normalize the expression level of vectors, they were transduced into Ad-293 cells. Then, the concentration of IL-24/Mda-7 in the supernatants was measured by a sandwich ELISA assay (DuoSet ELISA kit (R&D system Inc.). Besides, the pcDNA3.1 plasmid expressing Mda-7 was used as a positive control.
MTT Assay
MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) dye was used to measure the viability of test groups. Depending on the transduction sensitivity of cells, MOI=2-5 of Adenovector was applied on Hep-G2, MCF-7, MKN45, and skin fibroblast cells. Then, they were kept in 96 well plates for 48 hrs. Afterward, 100 μl of MTT dye was added to each well and tetrazolium crystals were dissolved in DMSO solvent. The absorbance intensity was measured by a microplate ELISA reader at 570 nm. The experiment was performed in a triplicate manner.
Quantitative real-time PCR of BCL2-associated X gene (BAX)
The total cellular RNA extraction from treated cells was performed using RNx plus solution (CinnaClone Inc.). Using gel electrophoresis and spectrophotometry, the concentration, purity, and integrity of RNAs were assessed by calculating the ratio of absorbance at 260 and 280 nm. Totally, one µg of extracted RNA was introduced to cDNA synthesis by ParsTous Kit. Quantitative real-time PCR (qPCR) was performed on QuantStudio Real-Time PCR System, using specific primers for the BAX gene (Table 1). Amplification was performed using the temperature cycling of a primary 95oC for 15 min, followed by 40 cycles of 95oC, 60oC, and 72oC for 20, 15, and 25 seconds, respectively. For the quality control and determining the specificity, melting curve analysis was performed and PGK gene (ID: 5230) was selected as a reference to normalize the gene expression data analysis. Finally, the 2-ΔΔCt method was used to compare the relative fold change in the expression of BAX gene among different groups.
Table 1.
The list of employed primers and relevant amplicons in the study
| Primer description | Sequence | Product length |
|---|---|---|
| BAX-forward | 5’-GCCCTTTTGCTTCAGGGTTTCA-3’ | 108bp |
| BAX-reverse | 5’- CAGCTTCTTGGTGGACGCAT-3’ | |
| PGK-forward | 5’-TAAAGCCGAGCCAGCCAAAA-3’ | |
| PGK-reverse | 5’-CTCCTACCATGGAGCTGTGG-3’ | |
| Mda7-forward | 5’-GGCTGTGAAAGACACTATGCAA-3’ | 210bp |
| Mda7-reverse | 5’-GGTTGCAGTTGTGACACGAT-3’ | |
| Hexon- forward | 5’-TGCAACATGACAAGACTCTGG-3’ | 126bp |
| Hexon-reverse | 5’-CTCATGGCTGACTCGGAATT-3 |
The Caspase3/7 activity assay
The enzymatic activity of caspase-3/7 was analyzed using a colorimetric assay (KIAzist Inc. Tehran). For this purpose, 100 µl of lysed cell supernatant was added to each well and the activity was measured based on standard cure absorbance under the wavelength 405 nm.
Statistical analysis
All data were analyzed by one-way ANOVA or Kruskal-Wallis of variance followed by post-test evaluation, using PRISM version 5 software (GraphPad Software, Inc., San Diego, CA). Values of p< 0.05 were considered as statistically significant.
Results
Adenoviral vectors characterization
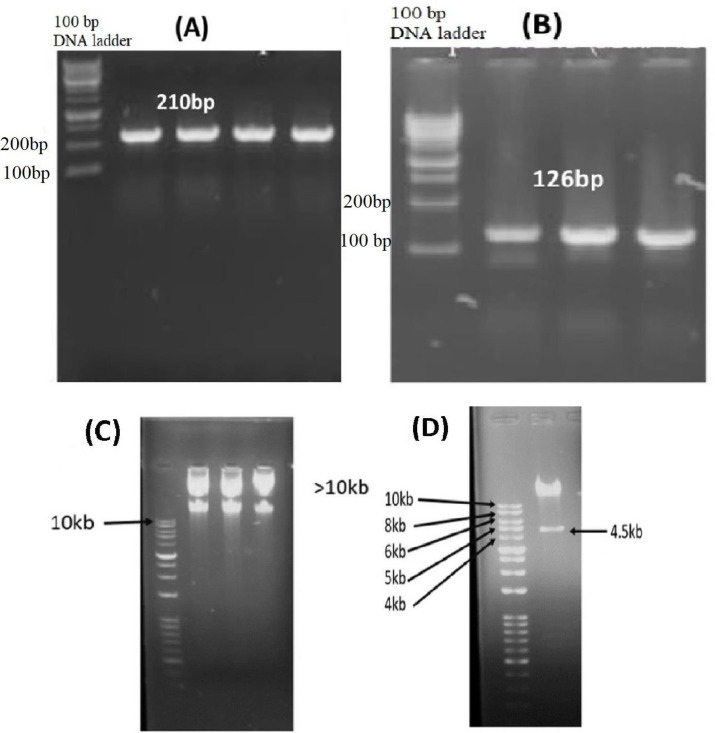
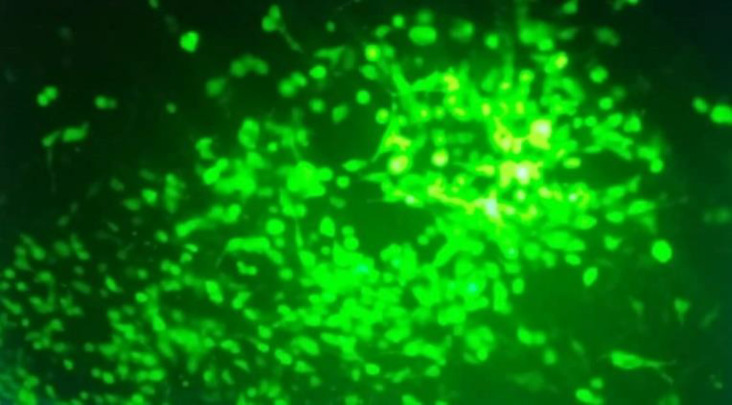
Mda-7 and Mda7-tLyp-1 genes were sub-cloned into shuttle vector correctly as confirmed through sequencing and digestion analysis. The accuracy of recombinant vectors was checked by Pac-1 restriction analysis, as well as hexon/Mda-7 PCR amplification (Figure 1). Pac-1 digestion analysis showed the presence of two fragments of 4.5 and >10 kbp which confirmed the integrity of Adenovector constructs, as shown in Figure 1. The titer of vectors was determined to be roughly 109 TCID50 particles/ml by TCID50 assay and transduction assay. The infectivity test on HEK-293 by recombinant vectors (MOI: 0.5) showed strong GFP foci spreading in plates and destroyed/rounded cells under fluorescent microscopy (Figure 2). The cancer cells transduction by Ad-Mda7 and Ad-Mda-tLyP1 showed ≥50% green signals in cell populations by selected MOIs, 1,2,5and 5, for HepG2, MCF7, MKN45, and normal fibroblast, respectively.
Figure 1.
Agarose gel electrophoresis results of Adenovectors constructing steps; (A) A 210 bp band indicated the insertion of Mda7 gene in transfer plasmid(B) A 126 bp band showed the presence of Hexon gene in main plasmid (C) Mini-preparation of recombinant Adenovector showed two bands with a significant distance to 10 kb ladder, revealing the presence of a big backbone construct, adenovector (D) Recombinant adenovector digestion by Pac1. The pattern showed 4.5 and >10 Kbp bands indicating the integrity of the new Ad-Mda7-tLyP1 construct
Figure 2.
Investigation of GFP expression by fluorescent microscopy. The replication of vector particles in packaging cells, and GFP expression analysis in HEK-293 cell-line evaluated after 48hr by fluorescent microscopy. A high percentage of infected HEK-293 cells showed GFP (around 50% of total cells).
Analysis of Mda-7 Expression
ELISA was used to assess the transgene expression of the recombinant viral vectors. The result showed a suitable expression level of Mda-7 after viral transduction into HEK-293 as well as MCF-7 cell lines. The expression level of Ad-Mda7-tLyP-1 and Ad-Mda7, was similar to the positive control plasmid, with a slight increase in the Ad-Mda7-tLyP-1 (P<0.05).
Cytopathic effects of viral vectors
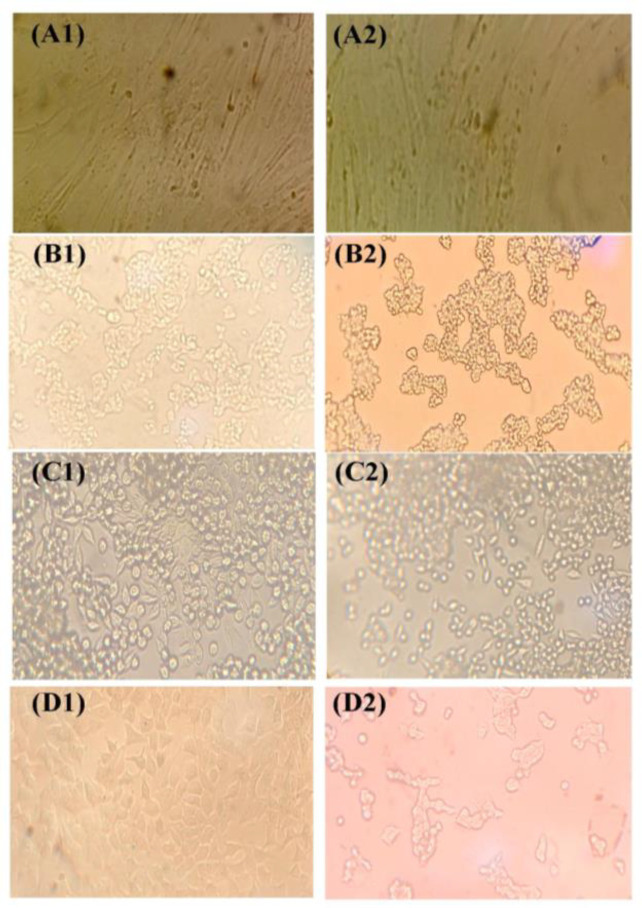
The treatment of equal MOI of Ad-Mda7-tLyP-1 showed a significant CPE on different cancer cells but not on normal skin fibroblast. The microscopy of Hep-G2, MKN45, and MCF7 cells showed a significant increase in CPE following Ad-Mda7 and Ad-Mda7-tLyP-1 treatment. The accumulation of cell granules, rounded cells, pycnotic nucleus and nuclear fragmentation and detached cells were significantly detectable in Adenovector-treated groups when compared to Ad-GFP group, as shown in Figure 3.
Figure 3.
The cytopathic effects of Adeno-GFP and Ad-Mda7-tLyP-1. (A) Normal Skin fibroblast (B) Hep-G2 cell line (C) MKN-45 cell line (D) MCF7 cell line. (1) Cell groups that were transduced with Adeno-GFP or (2) with Ad-Mda7-tLyP-1
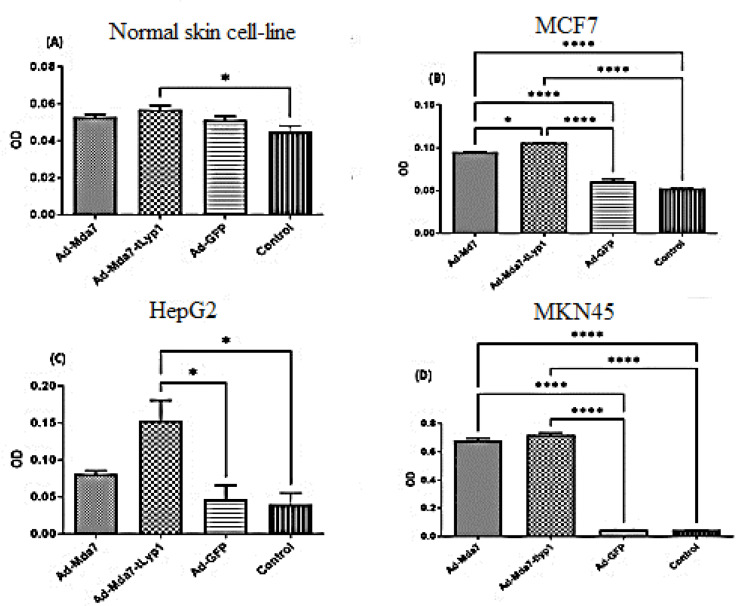
Cell viability and MTT analysis
MCF7, Hep-G2, MKN-45, and fibroblastic (skin) cell lines were transduced by different recombinant Adenovectors and control vectors (Ad-GFP).
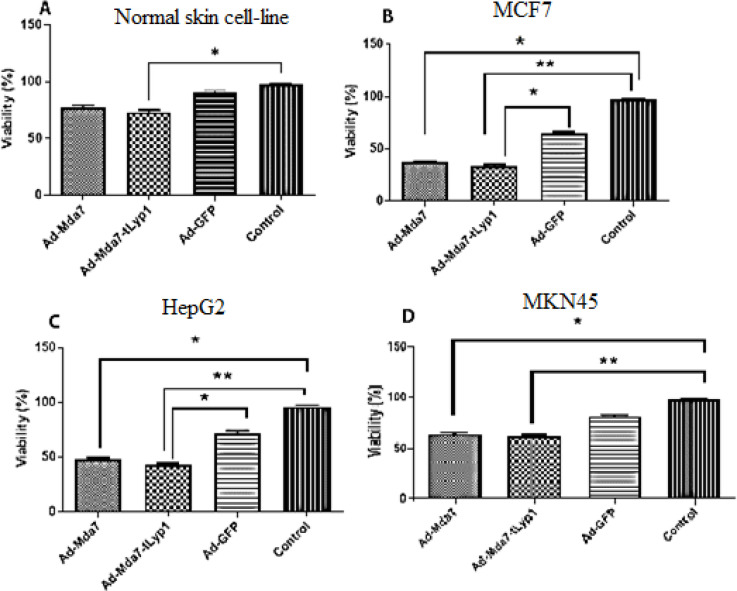
The viability analysis showed an increase in cell death in normal skin fibroblast when exposed to Ad-Mda7-tLyp1 (P value<0.05) (Figure 4). The results indicated a considerable variation in vector viability in the MCF7 cell line. Ad.Mda7 (37.1%), Ad.Mda7-tlyp1 (33.9%), and treated cells all had much lower viability rates than the negative control. Similar patterns were detected among HepG2 and MKN45, however, the rate of mortality in MKN45 was lower. In HepG2 cell lines, the result showed that the anti-proliferative activity of Ad-mda7, and Ad-Mda-tLyP1 were significantly higher than Ad-GFP control. However, in MKN45, the percentage of live cells was 63.9% and 61.7% following transduction by Ad-Mda7 and Ad-Mda-tLyP1. Moreover, in normal fibroblast, the viability of cells hardly dropped under 75% on average (Ad-mda7: 77.8% and Ad-Mda-tLyP1: 75% and control vector, Ad-GFP: 81%).
Figure 4.
Cell viability test. The cell viability results of Ad-Mda7 and Ad-Mda-tLyP1 on normal skin fibroblast (A), MCF7 (B), HepG2 (C), and MKN45 (D). Viral vectors cause varying percentages of cell cytotoxicity in tumor and normal cell lines. Every column is representative of the mean for 2-3 replicates
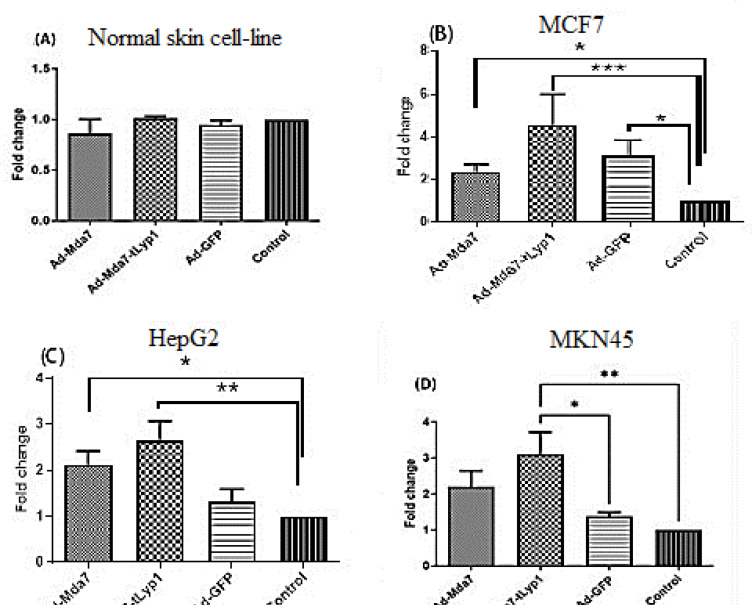
Effect of the Adenovector on the expression level of BAX gene
To find out the killing potency of recombinant Adenovectors on different cell lines the expression level of pro-apoptotic gene, BAX, was analyzed by RT-qPCR. The RT-qPCR data analysis showed no significant fold change in skin fibroblast cells. On the other hand, compared to Adeno-GFP, Ad-Mda7-tLyP1 dramatically increased the degree of BAX expression in the transduced cancer cell lines (Figure 5). The expression level of BAX by Ad-Mda7-tLyp1 was changed over 4 fold in MCF7, over 3 fold in MKN45, and nearly 3 fold in Hep-G2 cell line. The expression pattern of the BAX gene following transduction of Ad-Mda7 in cancer cell lines exhibits a lower upregulation (more than 2 times of the normal group) that was statistically different from control in MCF7 and HepG2 cell lines.
Figure 5.
The fold changes in the expression level of Bax gene. The expression level of Bax after transduction of different vectors in tumor (B and C and D), and normal skin fibroblast (A). Each column is representative of the mean for several samples
The Caspase 3/7 activity assay
To determine the potency of genes in the apoptosis induction, the activity of caspase 3/7 was analyzed by a colorimetric assay. The results indicated that the normal skin fibroblast as a negative control group exhibited apoptotic signs after transduction with different Ad-Mda7-tLyp1 (P value<0.05). However, Caspase 3/7 activity has remarkably risen in MCF7 and MKN45 after being exposed to Ad-Mda7-tLyp1 and Ad-Mda7 (P<0.0001). In the Hep-G2 cell line, only the Ad-Mda7-tLyp1 vector could increase the apoptosis activity of cells (P<0.05) but not Adeno-Mda7 (Figure 6).
Figure 6.
The apoptosis evaluation. (A)The activity of caspase3/7 in normal skin fibroblasts that were treated by vectors didn’t alter compared to negative control; (B) In MCF7, the activity of caspase3/7 has increased by Ad-mda7-tLyp1 and Ad-mda7 treatment; (C) In HepG2, activity of caspase3/7 has increased in the group of Ad-mda7-tLyp1; (D) In MKN-45, the activity of caspase3/7 has increased in Ad-mda7-tLyp1 treated group
Discussion
It is challenging to find more effective strategies to increase the killing power of candidates, even with the recent advancements in suicide cancer gene therapy. Previous studies showed that extraordinary expression of Mda-7/IL-24 induced cell death of cancerous cells by promoting ER stress and involvement of various signal transduction pathways (24). Moreover, fusing THPs to Mda-7 is expected to augment the antitumor effect by increasing the bystander effect, and destruction of neighboring cells. In our last attempt to enhance the potency, Mda-7 was fused with three different peptides in which pcDNA3.1-Mda-7-tLyp1 plasmid showed a significant killing effect on Huh-7 liver cancer cells (22). Herein, a new Adenovector (Ad-Mda7-tLyP1) was prepared and investigated on MCF-7 (breast), Hep-G2 (hepatic), MKN45 (gastric) cancer cells.
In conclusion, Ad-Mda7 and Ad-Mda7-tLyP1 significantly reduced the number of tumor cells in the examined cancer cells. Thus, there were no significant differences between the potential of these vectors following in vitro cell treatment; however, MCF7 cells showed greater sensitivity than other cell lines to killing by Ad-Mda7 and Ad-Mda7-tLyP1. As a suitable candidate, Mda-7 has been shown to harbor a significant killing effect on a wide spectrum of tumor cells albeit some are more susceptible than others (25-28).
Besides, the peptide modification of Mda-7 could enhance its potency if the design is done properly. Although the effect of Mda-7 was shown in the majority of cancer cells (7, 8, 9, 11), our results also emphasized its impact and retained its secretory potency even after fusing with tLyP1. The fusion of native Mda-7 to peptides had usually no effect on its functionality, whereas, some peptide-related modifications might destroy protein structure, and subsequently change killing activity. Furthermore, an examination of the expression levels of recombinant Ad-Mda7 and Ad-Mda7-tLyP1 indicated that the fusion of tLyp-1 to Mda-7 did not have an adverse impact on the protein concentration in the culture supernatant or the rate at which vectors transduced cells (data not shown). In our previous study which was conducted on other THPs, was shown that peptide modification of Mda-7 is pretty ineffective in changing the secretion pattern or expression level (29). Even more, the expression of Mda-7 in the bacterial expression system was not affected by the structural change of RGD-mutant for tumor-targeting purposes (30). However, our previous experiment showed the destructive effect of THP peptides on the expression level of IL-24.RGR and buf.IL-24 (22). On the other hand, it was reported that coupling RGD peptide could enhance the adhesion of Mda-7 and elevate apoptosis in various tumor cell lines (31, 32). In our case, the tLyp1 was chosen for its capability of adjusting into Mda-7 structure according to in-silico analysis and less destructive effect on protein structure as demonstrated previously (22).
The more sensitivity of MCF7 to the killing activity of Ad-Mda-7 and Mda-7-tLyP1 was an interesting result. Although the greater sensitivity of MCF7 to Mda-7 and Mda-7-tLyP1 might be in terms of different factors, the higher percentage of Mda-7 receptors (IL-20R/IL-22R, Sigma-1) and tLyP1 (neurophillin) on the cell surface would be a possible explanation. While endogenous interactions with cell proteins are more significant than the interaction of Mda-7 with surface receptors, the protein's secretory form may still have a critical role in initiating death signaling pathways. The recombinant Mda-7 protein has been shown to trigger different death pathways via interaction with IL-20/IL-22 surface receptor complexes and Sigma-1 (33). Zheng et al designed a mutant Mda-7 harboring RGD peptide and they showed the specific Mda-7 anti-cancer activity that induced via exogenous apoptosis pathways (32). Several reports indicated the importance of the attachment of Mda-7 protein to cell surface cognate receptors (34). In the way of activation of apoptosis from Bax up-regulation (35, 36) and interestingly by elongating the mda-7 mRNA half-life (37, 38). Besides, the bystander effect of secreted Mda-7 is possibly increased by this mechanism and leads new tLyP-1-modified protein to target neurophilin receptors surrounding the cells. Tumor invasion and progression are strongly correlated with the expression levels of NRP1 and NRP2 glycoproteins. Furthermore, because NRP1 is a marker on metastatic breast cancer cells but not on non-metastatic ones, competitive suppression of neurophilin signals might be advantageous in the management of metastatic lesions (39). Therefore, targeting these molecules on cancer cells could be a suitable way to combat malignant cell development.
Despite our expectation, Ad-Mda-7 and Mda-7-tLyP1 showed similar patterns of killing activity in different experiments on cell lines. Therefore, tethering tLyP1 to normal Mda7 has not demonstrated a superior activity due to some factors, such as the amount of tLyP1receptors on these cell lines or the blocking effect of peptide on the interaction with relevant protein candidates.
The current study showed that usage of mda7 with a coupled tLyP1 on normal skin fibroblast cells is enough safe to apply in a tumor milieu. In previous report also showed no significant side effects on several healthy cells which supports the recent finding (22). Numerous primary and immortalized normal cells, such as astrocytes, prostate epithelial cells, and melanocytes, have been studied to demonstrate their safety with long-lasting outcomes (40). However, based on our knowledge there isn’t any report regarding the effect of Mda-7 on normal skin fibroblast, in vitro.
The tLyP-1 peptide was used for delivering nanomaterials, and its fusion to proteins like Mda-7 was not considered, yet. However, it is expected to enhance the death induction potency of Mda7 via more efficient delivery of protein to cognate receptors and entry into cells. Moreover, tLyp-1 is a cancer-killer peptide when standing alone as a study has demonstrated that it crosses over cell membrane and affects genome content to trigger mitochondrial-dependent apoptosis (41). The present work was limited by the unavailability of more relevant normal cells and the omission of protein expression level investigation.
Conclusion
In conclusion, newly designed Adenoviral vector expressing Mda7-tLyp1 showed an encouraging cancer cell killing activity, as well as suitable specificity compared to Ad-Mda-7 but should be investigated more in vivo.
Ethical Statement
The study was designed and approved under the ethical approval of Shiraz University of Medical Sciences, IR.SUMS.1398.1100.
Conflict of interests
The authors declare no conflict of interest.
Acknowledgment
The authors have to thank staffs of central lab facility at School of Medicine for their assistance with qPCR and microscopy instruments. Each listed author confirms that their research is supported by Shiraz University of Medical Sciences that is primarily involved in education and research.
The study was supported by Shiraz University of Medical Sciences (Grant No. 20891, 23537 and 23716) as the MSc thesis by Fatemeh Vatanparast.
References
- 1.Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359:eaan4672. doi: 10.1126/science.aan4672. [DOI] [PubMed] [Google Scholar]
- 2.Conry RM, Westbrook B, McKee S, Norwood TG. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum. Vaccines Immunother. 2018;14:839–46. doi: 10.1080/21645515.2017.1412896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA approves first adenoviral vector-based gene therapy for high-risk Bacillus Calmette-Guérin unresponsive non-muscle invasive bladder cancer. FDA ; 2022. [Google Scholar]
- 4.Hutt JA, Assaf BT, Bolon B, Cavagnaro J, Galbreath E, Grubor B, et al. Scientific and Regulatory Policy Committee points to consider: nonclinical research and development of in vivo gene therapy products, emphasizing adeno-associated virus vectors. Toxicol Pathol. 2022;50:118–46. doi: 10.1177/01926233211041962. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Nagasato M, Yoshida T, Aoki K. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci. 2017;108:831–7. doi: 10.1111/cas.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goradel NH, Mohajel N, Malekshahi ZV, Jahangiri S, Najafi M, Farhood B, et al. Oncolytic adenovirus: A tool for cancer therapy in combination with other therapeutic approaches. J Cell Physiol. 2019;234:8636–46. doi: 10.1002/jcp.27850. [DOI] [PubMed] [Google Scholar]
- 7.Bezeljak U. Cancer gene therapy goes viral: viral vector platforms come of age. Radiol Oncol. 2022;56:1–13. doi: 10.2478/raon-2022-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menezes ME, Shen X-N, Das SK, Emdad L, Guo C, Yuan F, et al. MDA-7/IL-24 functions as a tumor suppressor gene in vivo in transgenic mouse models of breast cancer. Oncotarget. 2015;6:36928. doi: 10.18632/oncotarget.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menezes ME, Bhoopathi P, Pradhan AK, Emdad L, Das SK, Guo C, et al. Role of MDA-7/IL-24 a multifunction protein in human diseases. Adv Cancer Res. 2018;138:143–82. doi: 10.1016/bs.acr.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persaud L, De Jesus D, Brannigan O, Richiez-Paredes M, Huaman J, Alvarado G, et al. Mechanism of action and applications of interleukin 24 in immunotherapy. Int J Mol Sci. 2016;17:869. doi: 10.3390/ijms17060869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhutia SK, Das SK, Azab B, Menezes ME, Dent P, Wang XY, et al. Targeting breast cancer‐initiating/stem cells with melanoma differentiation‐associated gene‐7/interleukin‐24. Int J Cancer. 2013;133:2726–36. doi: 10.1002/ijc.28289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintarelli C, Savoldo B, Dotti G. Gene therapy to improve function of T cells for adoptive immunotherapy. Methods Mol Biol. 2010;651:119–30. doi: 10.1007/978-1-60761-786-0_8. [DOI] [PubMed] [Google Scholar]
- 13.Simón-Gracia L, Hunt H, Teesalu T. Peritoneal carcinomatosis targeting with tumor homing peptides. Molecules. 2018;23:1190. doi: 10.3390/molecules23051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morshed RA, Muroski ME, Dai Q, Wegscheid ML, Auffinger B, Yu D, et al. Cell-penetrating peptide-modified gold nanoparticles for the delivery of doxorubicin to brain metastatic breast cancer. Mol Pharm. 2016;13:1843–54. doi: 10.1021/acs.molpharmaceut.6b00004. [DOI] [PubMed] [Google Scholar]
- 15.Bina S, Shenavar F, Khodadad M, Haghshenas MR, Mortazavi M, Fattahi M-R, et al. Impact of RGD peptide tethering to IL24/mda-7 (Melanoma Differentiation Associated Gene-7) on apoptosis induction in hepatocellular carcinoma cells. Asian Pac J Cancer Prev. 2015;16:6073–80. doi: 10.7314/apjcp.2015.16.14.6073. [DOI] [PubMed] [Google Scholar]
- 16.Wu H-b, Wang Z, Wang Q-s, Han Y-j, Wang M, Zhou W-l, et al. Use of labelled tLyP-1 as a novel ligand targeting the NRP receptor to image glioma. PLoS One. 2015;10:0137676. doi: 10.1371/journal.pone.0137676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song N, Zhao L, Zhu M, Zhao J. Recent progress in LyP-1-based strategies for targeted imaging and therapy. Drug Deliv. 2019;26:363–75. doi: 10.1080/10717544.2019.1587047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth L, Agemy L, Kotamraju V, Braun G, Teesalu T, Sugahara K, et al. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene. 2012;31:3754–63. doi: 10.1038/onc.2011.537. [DOI] [PubMed] [Google Scholar]
- 19.Mercurio AM. VEGF/neuropilin signaling in cancer stem cells. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fantin A, Herzog B, Mahmoud M, Yamaji M, Plein A, Denti L, et al. Neuropilin 1 (NRP1) hypomorphism combined with defective VEGF-A binding reveals novel roles for NRP1 in developmental and pathological angiogenesis. Development. 2014;141:556–62. doi: 10.1242/dev.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larue L, Kenzhebayeva B, Al-Thiabat MG, Jouan–Hureaux V, Mohd–Gazzali A, Wahab HA, et al. tLyp–1: A peptide suitable to target NRP–1 receptor. Bioinorg Chem. 2023;130:106200. doi: 10.1016/j.bioorg.2022.106200. [DOI] [PubMed] [Google Scholar]
- 22.Rastegari M, Shiri A, Behzad-Behbahani A, Rasoolian M, Zare F, Rafiei G, et al. The evaluation of tLyP-1-bound Mda-7/IL-24 killing activity on a liver tumor cell line. Cancer Biother Radiopharm. 2021;36:827–36. doi: 10.1089/cbr.2019.3080. [DOI] [PubMed] [Google Scholar]
- 23.Hosseini SY, Sabahi F, Moazzeni SM, Modarressi MH, Firoozi MS, Ravanshad M. Construction and preparation of three recombinant adenoviruses expressing truncated NS3 and core genes of hepatitis C virus for vaccine purposes. Hepat Mon. 2012:12. doi: 10.5812/hepatmon.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasoolian M, Kheirollahi M, Hosseini SY. MDA-7/interleukin 24 (IL-24) in tumor gene therapy: application of tumor penetrating/homing peptides for improvement of the effects. Expert Opin Biol Ther. 2019;19:211–23. doi: 10.1080/14712598.2019.1566453. [DOI] [PubMed] [Google Scholar]
- 25.Deng L, Yang X, Ding Y, Fan J, Peng Y, Xu D, et al. Oncolytic therapy with vaccinia virus carrying IL-24 for hepatocellular carcinoma. Virol J. 2022;19:44. doi: 10.1186/s12985-022-01779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hambir S, Waghmare S, Kamble H, Andhale A, Asole S. Gene therapy for cancer: strategies, challenges, successes. Int Res J Modern Eng Technol Sci. 2022;3:1195–1204. [Google Scholar]
- 27.Emdad L, Bhoopathi P, Talukdar S, Pradhan AK, Sarkar D, Wang X-Y, et al. Semin Cancer Biol. Elsevier; 2020. Recent insights into apoptosis and toxic autophagy: The roles of MDA-7/IL-24, a multidimensional anti-cancer therapeutic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan S, Zhang H, Xie Y, Sheng W, Xiang J, Ye Z, et al. Recombinant human interleukin-24 suppresses gastric carcinoma cell growth in vitro and in vivo. Cancer Invest. 2010;28:85–93. doi: 10.3109/07357900903095672. [DOI] [PubMed] [Google Scholar]
- 29.Bina S, Hosseini SY, Shenavar F, Hosseini E, Mortazavi M. The effect of RGD/NGR peptide modification of melanoma differentiation-associated gene-7/interleukin-24 on its receptor attachment, an in silico analysis. Cancer Biother Radiopharm. 2017;32:205–14. doi: 10.1089/cbr.2017.2195. [DOI] [PubMed] [Google Scholar]
- 30.Liu J-J, Zhang B-F, Yin X-X, Pei D-S, Yang Z-X, Di J-H, et al. Expression, purification, and characterization of RGD-mda-7, a His-tagged mda-7/IL-24 mutant protein. J Immunoass Immunochem. 2012;33:352–68. doi: 10.1080/15321819.2012.659782. [DOI] [PubMed] [Google Scholar]
- 31.Zhang B-F, Liu J-J, Pei D-S, Yang Z-X, Di J-H, Chen F-F, et al. Potent antitumor effect elicited by RGD-mda-7, an mda-7/IL-24 mutant, via targeting the integrin receptor of tumor cells. Cancer Biother Radiopharm. 2011;26:647–55. doi: 10.1089/cbr.2011.0984. [DOI] [PubMed] [Google Scholar]
- 32.Pei D-S, Yang Z-X, Zhang B-F, Yin X-X, Li L-T, Li H-Z, et al. Enhanced apoptosis-inducing function of MDA-7/IL-24 RGD mutant via the increased adhesion to tumor cells. J Interferon Cytokine Res. 2012;32:66–73. doi: 10.1089/jir.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dent P, Yacoub A, Hamed HA, Park MA, Dash R, Bhutia SK, et al. MDA-7/IL-24 as a cancer therapeutic: from bench to bedside. Anticancer Drugs. 2010;21:725. doi: 10.1097/CAD.0b013e32833cfbe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang D-c, Gopalkrishnan RV, Lin L, Randolph A, Valerie K, Pestka S, et al. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene. 2004;23:1789–800. doi: 10.1038/sj.onc.1207300. [DOI] [PubMed] [Google Scholar]
- 35.Modi J, Roy A, Pradhan AK, Kumar A, Talukdar S, Bhoopathi P, et al. Insights into the Mechanisms of Action of MDA-7/IL-24: A Ubiquitous Cancer-Suppressing Protein. Int J Mol Sci. 2021;23:72. doi: 10.3390/ijms23010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebedeva IV, Emdad L, Su Z-Z, Gupta P, Sauane M, Sarkar D, et al. mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience. Int J Oncol. 2007;31:985–1007. [PubMed] [Google Scholar]
- 37.Madireddi MT, Dent P, Fisher PB. Regulation of mda-7 gene expression during human melanoma differentiation. Oncogene. 2000;19:1362–8. doi: 10.1038/sj.onc.1203424. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, et al. Melanoma differentiation associated gene-7 (mda-7)/IL-24: a ‘magic bullet’for cancer therapy? Expert Opin Biol Ther. 2007;7:577–86. doi: 10.1517/14712598.7.5.577. [DOI] [PubMed] [Google Scholar]
- 39.Liu SD, Zhong LP, He J, Zhao YX. Targeting neuropilin-1 interactions is a promising anti-tumor strategy. Chin Med J. 2020;134:508–517. doi: 10.1097/CM9.0000000000001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miri SM, Pourhossein B, Hosseini SY, Keshavarz M, Shahmahmoodi S, Zolfaghari MR, et al. Enhanced synergistic antitumor effect of a DNA vaccine with anticancer cytokine, MDA-7/IL-24, and immune checkpoint blockade. Virol J. 2022;19:106. doi: 10.1186/s12985-022-01842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Qu L, Gong L, Sun L, Gong R, Si J. Targeting and eradicating hepatic cancer cells with a cancer-specific vector carrying the Buforin II gene. Cancer Biother Radiopharm. 2013;28:623–30. doi: 10.1089/cbr.2012.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]