Abstract
Aim:
In this study, we intend to evaluate the occurrence of small fiber neuropathy in patients with irritable bowel syndrome (IBS).
Background:
Small fiber neuropathy (SFN) is a sensory neuropathy that results from the degeneration of small Aδ and unmyelinated C fibers. SFN manifests positive symptoms, such as tingling, burning, prickling, and aching, and negative symptoms, including numbness, tightness, and coldness. The SFN coexistence with other comorbidities (e.g., fibromyalgia, inflammatory bowel disease, celiac disease) has been reported in previous studies.
Methods:
We conducted a cross-sectional study to assess the coexistence of SFN and IBS. Forty-two IBS patients and forty-three healthy individuals were asked to complete the Michigan Neuropathy Screening Instrument (MNSI) questionnaire. Results greater than three (>3) were considered positive. Participants with positive MNSI questionnaire results were examined for any neuropathy signs according to the Utah Early Neuropathy Scale (UENS) examination. The participants with positive results for the questionnaire and examination were checked for the sural and the superficial peroneal nerve conduction study (NCS). Normal NCS represented intact large fibers and the diagnosis of SFN.
Results:
Ten participants, 7 (16.7 %) in the IBS group and 3 (6.9 %) in the healthy group, had positive results for the questionnaire. Four participants were positive for the examination, with normal NCS, and were classified as SFN-positive. All four SFN diagnoses were from the IBS group. No one in the healthy group was diagnosed with SFN. We could find a significant statistical difference (p<0.05) between the IBS and healthy groups regarding the prevalence of SFN diagnosis.
Conclusion:
The co-occurrence of SFN and IBS suggests the possibility of a generalized neuropathy syndrome characterized by widespread neuronal impairment. Thus, any peripheral neuropathy symptom in IBS patients (and potentially other chronic pain disorders) should be evaluated for SFN since timely diagnosis and proper treatment result in a better quality of life for the patients.
Key Words: Small fiber neuropathy, Irritable bowel syndrome, Peripheral nervous system disease, Peripheral neuropathy
Introduction
Small fiber neuropathy (SFN) is a sensory neuropathy that mainly affects small-diameter peripheral nerve fibers (1, 2). These small-diameter peripheral nerve fibers include myelinated Aδ and unmyelinated C fibers, which carry sensory and autonomic information (3). SFN can be painful to the patients and troublesome for clinicians. Clinicians experience difficulties in the diagnosis and treatment of SFN (2). SFN is not a debilitating disease but causes a significant decrease in the patient’s quality of life by its throbbing or steady pain and autonomic symptoms (4). Electromyogram (EMG) and nerve conduction study (NCS), which is a proper diagnostic test for evaluating large fiber neuropathy, are mainly normal in SFN (1). SFN can be quantified by some specific tests such as sympathetic skin response, quantitative sensory testing (QST), quantitative sudomotor axon reflex test (QSART), Cardiovagal and adrenergic autonomic testing, and Epidermal nerve fiber analysis (2). However, clinical evaluations based on the patient’s reported symptoms without pathologic electrophysiological findings are the mainstay of diagnosis in routine clinical practice.
Few studies have addressed the prevalence and incidence of SFN. The incidence of the disease varies from 11.10 to 13.71 cases/100.000 per year, and the prevalence was reported at 52.95 cases/100.000 (5). Its clinical manifestations account for positive sensory symptoms, such as tingling, burning, prickling, shooting pain, or aching, that can interfere with sleep (2). Patients may also experience negative neuropathy symptoms, including numbness, tightness, and coldness (2). Increased or decreased sweating, facial flushing, skin discoloration, dry eyes and mouth, and changes in skin temperature are some of the autonomic nervous system symptoms of SFN (6). Erectile dysfunction can occur in up to 40% of male patients (6, 7).
SFN may be idiopathic (2) or secondary to other conditions such as diabetes mellitus, connective tissue diseases, dysthyroidism, Sjogren’s syndrome, vitamin B12 deficiency, human immunodeficiency virus, hepatitis C virus, celiac disease, restless legs syndrome, neurotoxic drug exposure, paraneoplastic syndrome (8), and hyperlipidemia (2).
SFN pathophysiology is still an unclear topic. Probable mechanisms are classified into three main categories: ischemia, cytokines, and oxidative stress. Previous studies have shown that small nerve fibers are more sensitive to ischemia than large fibers, and ischemia resulting from vasculitis or other conditions can cause SFN. Pharmacological and physiological studies have shown that cytokines like tumor necrosis factor-alpha (TNFα) play an important role in the pathophysiology of neuropathic pain. Oxidative stress is involved in the development of diabetic neuropathy. Nicotinamide adenine dinucleotide phosphatase (NADPH) is a defense agent against oxidative stress, and decreased amounts of NADPH have been reported in the erythrocytes of sarcoidosis patients. Thus, the significant prevalence of SFN among patients with sarcoidosis makes oxidative stress a probable pathophysiology for SFN (9).
Oaklander et al. and other investigators have reported the coexistence of SFN and Fibromyalgia Syndrome (FMS) (10, 11). Oaklander and his colleagues compared a group of individuals with diagnosed FMS with control subjects. They showed that 41% of the skin biopsies taken from participants in the FMS group compared to 3% of the controls’ skin biopsies were diagnostic for small fiber polyneuropathy (10). Besides, it has been informed that the number of IBS patients with FMS is significantly higher than controls, and the prevalence of FMS patients with concomitant IBS varies from 30 to 35% to 70% (12).
IBS patients experience altered visceral and somatic perceptions (13, 14). Also, concurrent FMS in these patients can enhance somatic perception but, conversely, mitigate visceral perception (15). Fatigue (16) and other sensory and autonomic symptoms (17) were reported in patients with IBS. Hence, we decided to perform this study to determine the SFN in patients with IBS.
Methods
This cross-sectional study comprised patients with a known history of IBS and healthy participants. We considered irritable bowel syndrome (IBS) an inclusion criterion for the IBS group. IBS and related comorbidities were diagnosed and recorded in a gastrointestinal (GI) specialty clinic (IBS diagnosis was made according to the ROME IV criteria (18)). All IBS patients were registered and visited regularly by expert gastroenterologists. Patients with known hyperlipidemia, hypothyroidism, obesity (BMI>30), diabetes mellitus, radicular neuropathies, and any other diseases associated with SFN (3) (such as fibromyalgia, Parkinson’s disease, and autoimmune disorders) were excluded from the study.
The second (healthy) group consisted of 43 non-IBS participants. So, our exclusion criteria for the healthy group were hyperlipidemia, hypothyroidism, obesity (BMI>30), diabetes mellitus, radicular neuropathies, other SFN-associated diseases (3), and irritable bowel syndrome. Also, patients who did not consent to perform the examination were excluded from the study. Written informed consent was obtained from the patients, and the Iranian national committee approved the study for Ethics in Biomedical Research.
Questionnaires and assessments
At first, IBS group participants were asked the Michigan neuropathy screening instrument (MNSI) questionnaire (19) questions. The patients with a score of over three were considered positive for the questionnaire (with a sensitivity of 40% and a specificity of 92% (19)) and were invited for the examination. The examination was done according to the Utah Early Neuropathy Scale (UENS) examination (20). In this scale, patients are assessed for neuropathy signs in five aspects: motor, pin sensation, allodynia/hyperesthesia, large fiber sensation, and deep tendon reflexes. They are scored from zero to a maximum score of 42. The large fiber sensation section in the UENS examination contains great toe vibration and position sensations. These two examinations determine both large and small fiber neuropathies. Therefore, the sural and superficial peroneal nerve conduction studies were performed to exclude large fiber neuropathies. A number 2 safety pin was used for the pin sensation test, and the great toe vibration was tested by holding a 128 Hz tuning fork against the dorsum of the great toe at the distal interphalangeal joint.
The severity of the IBS was evaluated by the Irritable Bowel Syndrome Severity Scoring System (IBS-SSS) (20). On this scale, <175, 175 to <300, and >300 represent mild, moderate, and severe diseases, respectively (21).
Healthy group members were non-IBS patients whose possible IBS diagnosis was ruled out by the Rome IV criteria. They were also asked the MNSI questionnaire questions. Then the participants with a score of over three were invited for the UENS examination. According to the UENS examination, any signs of neuropathy were detected.
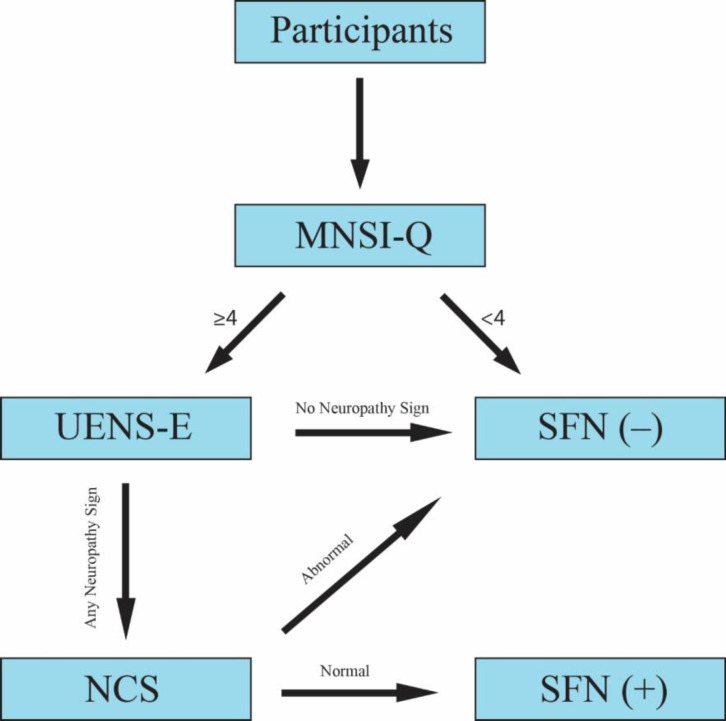
The sural and superficial peroneal nerve conduction studies were done according to the standard technique with surface electrodes. The maximum amplitude with supramaximal stimulation was measured. Tests were modified for all participants regarding temperature, technique, and the testing limb. NCS values higher than two standard deviations from the normal values (22) were considered abnormal. Both group participants with any signs of neuropathy in the examination (score > 0) were checked for nerve conduction study. If the patients’ NCS resulted in normal values, they were categorized as small fiber neuropathy-positive participants (Figure 1).
Figure 1.
Methodology flowchart MNSI-Q: Michigan Neuropathy Screening Instrument Questionnaire, UENS-E: Utah Early Neuropathy Scale Examination, SFN: Small Fiber Neuropathy, NCS: Nerve Conduction Study (the figure is created with the Adobe Illustrator 2023 application).
Statistical analysis
Both groups were analyzed for gender or age bias. The prevalence of SFN diagnoses was compared between two groups with the Chi-square test. We ran several correlation tests between the IBS severity (IBS-SSS scores) and the severity of neuropathy symptoms (MNSI questionnaire scores) among different subgroups of the participants. Also, a Chi-square test was done to see if the number of participants with MNSI scores over three was significantly higher in the IBS group than in the healthy group. Data normality was checked with the Shapiro-Wilk and Kolmogorov-Smirnov tests. All tests were performed using the IBM SPSS Statistics 27th edition application.
Results
Eighty-five participants were enrolled in the study (56 females (66%) and 29 (34%) males). There were 28 (67%) females and 14 (33%) males in the IBS group, 28 (65%) females and 15 (35%) males in the healthy group. There was no significant difference in the distribution of genders in the two groups (Chi-square, p=0.88). The median and interquartile range of age (IQR) for the IBS and healthy groups ages were 37 (IQR=10) and 35 (IQR=13), respectively. There was no significant difference between the ages of the two groups (Mann Whitney U test, P=0.66).
The MNSI Questionnaire (MNSI-Q) was positive (score >3) for 7 (16.7 %) participants in the IBS group and for 3 participants (6.9 %) in the healthy group (Table 1), which was not significant statistically (Chi-square, p=0.20). Four of the seven (9.5 %) participants with a positive MNSI-Q in IBS patients showed at least one clinical sign of neuropathy in the examination. NCS was in normal values, and these patients were classified as SFN patients. One out of three participants in the healthy group showed clinical signs of neuropathy in the examination. However, her sural NCS was in abnormal value, so she was not categorized as a positive participant for the SFN diagnosis (Table 1). According to statistical analysis, there is a significant difference between the proportion of SFN-positive participants in the IBS and healthy groups (Chi-square test, p<0.05).
Table 1.
Participants with MNSI scores ≥ 4. IBS-SSS: Irritable Bowel Syndrome Severity Scoring System, NCS: Nerve Conduction Study, GTV: Great Toe Vibration, DTR: Deep Tendon Reflex. The median MNSI score was 2 (IQR=2) for both IBS and healthy group participants. Statistical analysis showed no considerable difference between these scores (Mann Whitney U test, p=0.22).
| Participant number | Gender | Age | IBS-SSS Score | Questionnaire Score | Examination Score | Examination Abnormality | NCS |
|---|---|---|---|---|---|---|---|
| IBS Group | |||||||
| 28 | Female | 64 | 210 | 4 | 7 | Pin Sensation | Normal |
| 31 | Male | 32 | 210 | 4 | 8 | Pin Sensation | Normal |
| 35 | Male | 37 | 320 | 4 | 0 | None | - |
| 36 | Female | 62 | 200 | 5 | 0 | None | - |
| 37 | Male | 29 | 185 | 6 | 8 | Pin Sensation + Diminished GTV Sensation | Normal |
| 38 | Female | 41 | 300 | 4 | 0 | None | - |
| 39 | Female | 34 | 360 | 6 | 6 | Pin Sensation | Normal |
| Healthy Group | |||||||
| 2 | Female | 44 | 4 | 7 | Pin Sensation + Diminished DTR | Abnormal | |
| 8 | Male | 32 | 7 | 0 | None | - | |
| 31 | Female | 30 | 8 | 0 | None | - | |
Moreover, we ran several correlation tests between IBS-SSS scores and MNSI-Q scores. We could find a positive correlation between the IBS-SSS scores and the MNSI-Q scores in the IBS group (Spearman correlation coefficient, r=0.30, p<0.05). However, there was no significant correlation between the IBS-SSS scores and the MNSI-Q scores among other group subtypes. For instance, we could not find a considerable positive correlation among the patients with mild (r=0.01, p=0.96), moderate (r= -0.60, p=0.01), and severe (r=0.05, p=0.89) IBS, separately. Also, we tested the same correlation among male (r=0.30, p=0.29) and female (r=0.31, p=0.10) participants, and it did not turn out to be significant either.
Discussion
This cross-sectional study evaluated the prevalence of SFN among patients with IBS. There was a significant clinical and statistical difference in the prevalence of SFN in IBS and healthy controls. To our knowledge, this is the first study addressing SFN as a peripheral neurologic involvement of IBS. In our study, we noticed a link between IBS and peripheral neuropathy in the form of SFN. Peripheral neuropathy has previously been reported in other GI disorders, such as gastrointestinal motility disorders (23), inflammatory bowel disease (24), and celiac disease (25). Considering gastrointestinal motility disorders as a part of a more generalized neuropathy disorder involving peripheral and myenteric neurons is a hypothesis that has been described by Ohlsson et al. (23) recently. They have reported two cases of gastrointestinal dysmotility (due to Ehlers-Danlos syndrome and long-term GnRH agonist therapy) with complaints of autonomic and peripheral neuropathy and evidence of reduced intraepidermal nerve fiber density in histopathology evaluation of the skin.
Fibromyalgia syndrome (FMS) is a pain disorder causing widespread muscular tender points in patients (26). Üceyler et al. (11) evaluated the presence of SFN in patients with Fibromyalgia syndrome (FMS). They performed a case-control study and mainly used quantitative sensory testing and skin biopsy as measurement tests to diagnose SFN. Cold and warm detection thresholds were higher in FMS patients than in the control group. In skin biopsies, intraepidermal nerve fiber density and dermal unmyelinated nerve fiber bundles were reduced in the patients with FMS compared to those in the control group. These findings showed the involvement of small fibers in FMS. Thus, neuropathic pain in FMS can be the source of complaints in these patients. The overlap of FMS and IBS has been previously mentioned in the literature, suggesting a common pathophysiology (27). Approximately 60% of IBS patients develop FMS, and up to 70 % of FMS patients suffer from symptoms of IBS (27, 28). Therefore, a common cause for FMS, IBS, and SFN could be a proper explanation for the link between these disorders.
A study was conducted in 2005 investigating small fiber neuropathy in patients with celiac disease and neuropathy symptoms (25). They performed skin biopsies for eight patients with neuropathy symptoms who were diagnosed with celiac disease. Epidermal nerve fiber (ENF) density was reduced in 5 patients, and it resulted in lower limits of the normal range for the three other patients. Skin samples of the two patients with the low limits (within the normal range) of ENF density showed morphologic changes in axons. Five patients experienced an improvement in neuropathy symptoms after initiating a gluten-free diet.
Gondim et al. conducted a retrospective study evaluating the different types of peripheral neuropathy (PN) in inflammatory bowel disease (IBD) patients who were diagnosed with PN (24). They analyzed neurological examinations, clinical complaints, electrodiagnostic tests, and nerve biopsies to classify peripheral neuropathies. A small fiber neuropathy diagnosis was made by assessing the patient’s clinical history, physical examination, normal nerve conduction study, and skin biopsy. Thirty-three patients with IBD and PN were recruited. Among them, 18 patients had Crohn’s disease, and others had ulcerative colitis. 2 out of 18 patients with Crohn’s disease and 4 out of 15 patients with ulcerative colitis were diagnosed with small fiber neuropathy.
In addition to the above-mentioned GI diseases, a study was designed to calculate the prevalence of SFN among patients with refractory chronic pelvic pain (CPP) and CPP patients with comorbid pain syndromes (29). In this study, 64 % of participants had decreased epidermal nerve fiber density on immunofluorescence study of the skin biopsies indicative of small fiber polyneuropathy. Notably, two comorbidities with high prevalence among the participants were IBS (33%) and FMS (38%). Therefore, SFN has been presented in CPP (29), FMS (11), and IBS (our study), all classified as chronic pain disorders. Thus, exploring the hypothesis of small fiber neuropathy in chronic pain disorders is a worthwhile endeavor warranting further investigation that could lead to understanding the precise pathophysiology of these conditions.
In our study, we also found a positive correlation between peripheral neuropathy and IBS severities, i.e., the patients with more severe IBS symptoms suffered from more severe neuropathy symptoms. This positive correlation, along with the study's main finding (significantly higher SFN cases in the IBS group compared to the healthy group), further supports the idea of describing a generalized neuropathy syndrome. We calculated Cronbach’s alpha for the MNSI-Q among Iranian society, showing a reasonable internal consistency and reliability for the questionnaire (α=0.72).
This study has some potential limitations. The small sample size, because the main part of the study was performed during the SARS-CoV-2 infection pandemic, and several patients refused to participate in the UENS examination, might have impacted the results. Furthermore, more definite diagnosis methods (such as skin biopsy) may result in different outcomes for the study. Our findings suggest possible mutual pathophysiology for IBS and SFN; therefore, the hypothesis that IBS is a subset of a more generalized neural disorder is worth considering. We suggest more research to investigate small fiber neuropathy and other neural diseases in irritable bowel syndrome and chronic pain disorders. Research works with bigger sample sizes, and more accurate methodologies may result in a revolutionary discovery in neuropathy illnesses.
Conclusion
Small fiber neuropathy is a sensory peripheral nerve disorder that significantly affects the patient’s quality of life. Understanding the underlying mechanisms and pathophysiology of the disease can help us with proper treatment and, subsequently, a better prognosis for the patients. Abnormal neural sensation in irritable bowel syndrome patients can be due to comorbid SFN. Early SFN diagnosis in these patients results in earlier treatment and prevents multiple physician visits to investigate the symptoms. Studying the relationship between SFN and other disorders can help us understand the exact pathophysiology of this disease.
Ethical statement
Written informed consent was obtained from the patients. This study was approved by the Iranian national committee for ethics in biomedical research (IR.MUI.MED.REC.1400.230) and performed in accordance with the Helsinki Declaration of 1964 and its later amendment.
Conflict of interests
The authors declare no conflict of interest.
Acknowledgment
This study was supported by a grant from Isfahan University of Medical Sciences (grant number: 3400117). We thank the survey participants who helped us to do this study.
References
- 1.Zhou L. Small Fiber Neuropathy. Semin Neurol. 2019;39:570–7. doi: 10.1055/s-0039-1688977. [DOI] [PubMed] [Google Scholar]
- 2.Lacomis D. Small‐fiber neuropathy. Muscle Nerve. 2002;26:173–88. doi: 10.1002/mus.10181. [DOI] [PubMed] [Google Scholar]
- 3.Ghasemi M, Rajabally YA. Small fiber neuropathy in unexpected clinical settings: a review. Muscle Nerve. 2020;62:167–75. doi: 10.1002/mus.26808. [DOI] [PubMed] [Google Scholar]
- 4.Bakkers M, Faber CG, Hoeijmakers JG, Lauria G, Merkies IS. Small fibers, large impact: quality of life in small‐fiber neuropathy. Muscle Nerve. 2014;49:329–36. doi: 10.1002/mus.23910. [DOI] [PubMed] [Google Scholar]
- 5.Peters MJ, Bakkers M, Merkies IS, Hoeijmakers JG, van Raak EP, Faber CG. Incidence and prevalence of small-fiber neuropathy: a survey in the Netherlands. Neurology. 2013;81:1356–60. doi: 10.1212/WNL.0b013e3182a8236e. [DOI] [PubMed] [Google Scholar]
- 6.Novak V, Freimer M, Kissel J, Sahenk Z, Periquet I, Nash S, et al. Autonomic impairment in painful neuropathy. Neurology. 2001;56:861–8. doi: 10.1212/wnl.56.7.861. [DOI] [PubMed] [Google Scholar]
- 7.Stewart JD, Low PA, Fealey RD. Distal small fiber neuropathy: results of tests of sweating and autonomic cardiovascular reflexes. Muscle Nerve. 1992;15:661–5. doi: 10.1002/mus.880150605. [DOI] [PubMed] [Google Scholar]
- 8.Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med. 2009;76:297–305. doi: 10.3949/ccjm.76a.08070. [DOI] [PubMed] [Google Scholar]
- 9.Hoitsma E, Reulen J, De Baets M, Drent M, Spaans F, Faber C. Small fiber neuropathy: a common and important clinical disorder. J Neurol Sci. 2004;227:119–30. doi: 10.1016/j.jns.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain. 2013;154:2310–6. doi: 10.1016/j.pain.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Üçeyler N, Zeller D, Kahn A-K, Kewenig S, Kittel-Schneider S, Schmid A, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857–67. doi: 10.1093/brain/awt053. [DOI] [PubMed] [Google Scholar]
- 12.Sperber A, Atzmon Y, Neumann L, Weisberg I, Shalit Y, Abu-Shakrah M, et al. Fibromyalgia in the irritable bowel syndrome: studies of prevalence and clinical implications. Am J Gastroenterol. 1999;94:3541–6. doi: 10.1111/j.1572-0241.1999.01643.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang L. Brain responses to visceral and somatic stimuli in irritable bowel syndrome: a central nervous system disorder? Gastroenterol Clin North Am. 2005;34:271–9. doi: 10.1016/j.gtc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Verne NG, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 15.Tremolaterra F, Gallotta S, Morra Y, Lubrano E, Ciacci C, Iovino P. The severity of irritable bowel syndrome or the presence of fibromyalgia influencing the perception of visceral and somatic stimuli. BMC Gastroenterol. 2014;14:1–9. doi: 10.1186/1471-230X-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frändemark Å, Jakobsson Ung E, Törnblom H, Simrén M, Jakobsson S. Fatigue: a distressing symptom for patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017;29:12898. doi: 10.1111/nmo.12898. [DOI] [PubMed] [Google Scholar]
- 17.Heitkemper M, Burr RL, Jarrett M, Hertig V, Lustyk MK, Bond EF. Evidence for autonomic nervous system imbalance in women with irritable bowel syndrome. Dig Dis Sci. 1998;43:2093–8. doi: 10.1023/a:1018871617483. [DOI] [PubMed] [Google Scholar]
- 18.Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil. 2017;23:151. doi: 10.5056/jnm16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman W, Pop‐Busui R, Braffett B, Martin C, Cleary P, Albers J, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29:937–44. doi: 10.1111/j.1464-5491.2012.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singleton JR, Bixby B, Russell JW, Feldman EL, Peltier A, Goldstein J, et al. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst. 2008;13:218–27. doi: 10.1111/j.1529-8027.2008.00180.x. [DOI] [PubMed] [Google Scholar]
- 21.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 22.Javadian-Sarraf S, Moghtaderi A. Determining normal nerve conduction velocities in normal population of 20–40 years old. Zahedan University School of Medicine . 2002:160. [Google Scholar]
- 23.Ohlsson B, Dahlin LB, Englund E, Veress B. Autonomic and peripheral neuropathy with reduced intraepidermal nerve fiber density can be observed in patients with gastrointestinal dysmotility. Clin Case Rep. 2020;8:142–8. doi: 10.1002/ccr3.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gondim F, Brannagan III T, Sander H, Chin R, Latov N. Peripheral neuropathy in patients with inflammatory bowel disease. Brain. 2005;128:867–79. doi: 10.1093/brain/awh429. [DOI] [PubMed] [Google Scholar]
- 25.Brannagan TH, Hays AP, Chin SS, Sander HW, Chin RL, Magda P, et al. Small-fiber neuropathy/neuronopathy associated with celiac disease: skin biopsy findings. Arch Neurol. 2005;62:1574–8. doi: 10.1001/archneur.62.10.1574. [DOI] [PubMed] [Google Scholar]
- 26.Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 2016;338:114–29. doi: 10.1016/j.neuroscience.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valencia C, Fatima H, Nwankwo I, Anam M, Maharjan S, Amjad Z, et al. A correlation between the pathogenic processes of fibromyalgia and irritable bowel syndrome in the middle-aged population: a systematic review. Cureus. 2022:14. doi: 10.7759/cureus.29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperber AD, Dekel R. Irritable Bowel Syndrome and Co-morbid Gastrointestinal and Extra-gastrointestinal Functional Syndromes. J Neurogastroenterol Motil. 2010;16:113–9. doi: 10.5056/jnm.2010.16.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen A, De E, Argoff C. Small fiber polyneuropathy is prevalent in patients experiencing complex chronic pelvic pain. Pain Med. 2019;20:521–7. doi: 10.1093/pm/pny001. [DOI] [PubMed] [Google Scholar]