Abstract
Background: Scar formation after trauma and surgery involves an inflammatory response and can lead to the development of chronic pain. Neurotropin ® (NTP) is a nonprotein extract of inflamed skin of rabbits inoculated with vaccinia virus. It has been widely used for the treatment of chronic pain. However, the in vivo effects of NTP on painful scar formation have not been determined. To investigate the molecular mechanisms underlying the effects of NTP on the inflammatory response, we evaluated gene expression in the scar tissues and dorsal root ganglions (DRGs) of mice administered NTP and control mice. Methods and results: Mice injected with saline or NTP were used as controls; other mice were subjected to surgery on the left hind paw to induce painful scar formation, and then injected with saline or NTP. Hind paw pain was evaluated by measuring the threshold for mechanical stimulation using the von Frey test. The paw withdrawal threshold gradually returned to pre-operative levels over 4 weeks post-operation; NTP-treated mice showed a significantly shortened recovery time of approximately 3 weeks, suggesting that NTP exerted an analgesic effect in this mouse model. Total RNA was extracted from the scarred hind paw tissues and DRGs were collected 1 week post-operation for a microarray analysis. Gene set enrichment analysis revealed that the expression of some gene sets related to inflammatory responses was activated or inhibited following surgery and NTP administration. Quantitative real-time reverse transcription-polymerase chain reaction analysis results for several genes were consistent with the microarray results. Conclusion: The administration of NTP to the hind paws of mice with painful scar formation following surgery diminished nociceptive pain and reduced the inflammatory response. NTP inhibited the expression of some genes involved in the response to surgery-induced inflammation. Therefore, NTP is a potential therapeutic option for painful scar associated with chronic pain.
Keywords: Chronic pain, gene expression, inflammatory response, neurotropin®, painful scar formation
Introduction
Surgery and trauma are major factors contributing to chronic pain in outpatient clinics. According to a survey of 5130 patients with chronic pain, 22.5% experienced pain following surgery, whereas 18.7% reported pain resulting from trauma. 1 Scar formation is initiated by the disruption of skin and soft tissues following trauma or surgery, which triggers an inflammatory response that may lead to the development of chronic pain.2,3 Developing effective treatment strategies to alleviate the chronic pain caused by surgery and trauma remains a critical challenge.
Neurotropin® (NTP) has been commonly used in Japan and China for over 50 years as an analgesic for various chronic pain conditions, including low back pain, osteoarthritis, postherpetic neuralgia, hyperesthesia of subacute myelo-optic neuropathy, and complex regional pain syndromes.4–7 NTP is a non-protein extract derived from inflamed skin of rabbits inoculated with vaccinia virus that exerts its antinociceptive effect by activating descending pain inhibitory systems, specifically the noradrenergic and serotonergic systems that project from the supraspinal regions to the spinal dorsal horn.8–11 NTP has no serious adverse effects and is well tolerated; therefore, it can be used even in patients receiving antiplatelet drugs. 4 In addition, NTP does not inhibit prostaglandin biosynthesis and the adverse effects of NTP, such as gastrointestinal disorders, are less severe than those of NSAIDs. Therefore, NTP can be administered for a prolonged period, which is advantageous. However, the application of NTP for alleviating painful scar formation and chronic pain remains unexplored. The aim of the present study was to determine the anti-allodynic effect of NTP on mice with painful scars and the underlying molecular mechanisms.
In our previous study, we established a mouse model to simulate painful scars by exfoliating the subcutaneous and tendon tissues of the plantar area of the hind paw. In this model, hind paw withdrawal caused by mechanical hyperalgesia persisted for 4 weeks 12 A comprehensive microarray analysis suggested that a multitude of genes are intricately involved and that they interact to induce the chronic pain and hyperalgesia associated with tissue fibrosis. 13 Therefore, we used this experimental mouse model in this study to investigate the molecular mechanisms underlying the effects of NTP.
Following surgery, painful scar tissue consistently induces an inflammatory response accompanied by hyperalgesia. 12 The inflammatory response triggers the release of inflammatory cytokines, the primary contributors to hyperalgesia or allodynia, 14 thereby directly activating or sensitizing nociceptive neurons and increasing their sensitivity to painful stimuli. 15 Neuroinflammation in the dorsal root ganglion (DRG) has been implicated in the initiation and maintenance of postoperative pain. 16 Therefore, our study focused on determining the changes in gene expression following surgery and NTP administration, with a specific emphasis on understanding inflammatory responses. Elucidating the effects of NTP could facilitate the discovery of novel therapeutic targets for chronic pain.
Materials and methods
Mice and treatments
A total of 64 C57BL/6JJmsSlc male mice (10 weeks old) were purchased from Japan SLC Co. (Shizuoka, Japan). Thirty-two mice (n = 32) were assigned to behavior monitoring and the remaining 32 were designated for cDNA microarray and Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR). Among the 32 mice, in the sham group, 16 were subjected to puncture using a 19-G needle without stripping. The mouse model was constructed following a previously described protocol, 12 a steel rod was inserted through the puncture hole exfoliating the subcutaneous and tendon tissues in the plantar area of the hind paw. Painful scar formation was induced in the left hind paws in the operation group (n = 16) by puncturing a hole at the proximal end of the sole and stripping the subcutaneous tissue of the entire sole. The mice were further divided into two groups (n = 8 per group): one group received an injection of saline (sham/saline and operation/saline), whereas the other received an injection of NTP (sham/NTP and operation/NTP). In all mice, the right hind paw was left untreated (Figure 1).
Figure 1.
Experimental system. Painful scars were generated in the left hind paws of mice by puncturing a hole at the proximal end of the sole and stripping the subcutaneous tissue of the entire sole (operation group n = 16). Mice subjected to puncture but no stripping served as controls (sham group n = 16). The mice were divided into two groups to receive injections of NTP (sham/NTP and operation/NTP) or saline (sham/saline and operation/saline) (n = 8 per group). The right hind paw was left untreated in all mice. NTP or saline was administered to all mice at a dose of 4 μL/g of body weight. The injections were scheduled at 2 h post-operation, every 24 h during the 1 week post-operation, and at 2, 3, and 4 weeks post-operation. NTP: Neurotropin®.
The mice were provided unlimited supply of water and food and housed under conditions of constant temperature (23 ± 1°C) and humidity (50% ± 15%) with a 12 h light-dark cycle. All experimental procedures were approved by the Experimental Animal Committee of Aichi Medical University (approval number: 2023-27).
NTP administration
NTP (20 NU/mL), a pale-yellow liquid with a small amount of suspension, was injected without dilution into the subcutaneous tissue at the proximal end of the instep of the left hind paw at a dose of 4 μL/g of body weight (Figure 1). Saline was injected in the same manner to mice in the control group. The injections were scheduled at 2 h post-operation, every 24 h during the first week post-operation and at 2, 3, and 4 weeks post-operation. To ensure blind testing, different researchers conducted injections and measurements. Experimental data were collected immediately before the injections.
Measurement of mouse body weight and hind paw circumference
Mouse body weight was measured before each injection to observe the effect of NTP. After surgery-induced swelling of the left hind paw was observed, a convenient measurement technique involving the measurement of the circumferential length of the hind paw.17,18 The circumference of the left hind paw was measured by surrounding it with a thin cotton thread 5 mm away from the heel and the changes in the appearance of the hind paws were documented through photographs. Notably, a manual measurement technique may introduce bias, as the method employed in this study can only the change in appearance and does not directly quantify the circumferential length of the hind paw. Before taking the measurements, we ensured that the researcher received no prior information about the group of mice.
Von Frey test
The threshold for mechanical stimulation causing hind limb withdrawal was measured to assess the hind paw pain. 19 We evaluated pain in the ipsilateral (operative side) and contralateral (nonoperative side) hind paws using the “up-down method” 20 by measuring the paw withdrawal threshold (PWT) to mechanical stimulation from von Frey filaments (0.04, 0.07, 0.16, 0.4, 0.6, 1, 1.4, and 2 g; Bioseb-In Vivo Research Instruments, Vitrolles, France). Mice were acclimatized on the metal mesh for at least 20 min to minimize disturbances from the external environment. Von Frey filaments were applied underneath the metal mesh to the proximal end of the toes on the hind paws to induce mechanical stimulation. The stimulus was applied at the lowest strength and then gradually increased, applying enough force to produce a slight bend and maintaining it for approximately 6–8 s. The threshold of mechanical stimulus was recorded when a filament successfully prompted consecutive paw withdrawal or rapid kicking responses. The von Frey test was also conducted in blinded manner before each injection.
Microarray analysis
Total RNA was extracted from the scarred hind paws and ipsilateral DRGs (L2–L5) in the first week post-operation. A cDNA microarray analysis was performed as described previously. 13 Briefly, equal amounts of total RNA from eight mice per group were pooled and used for cDNA synthesis. cRNA labeling with cyanine 3 dye was conducted using the Agilent Low Input Quick Amp Labeling Kit (Agilent Technologies, Santa Clara, CA, USA). The cyanine 3-labeled cRNA was purified, fragmented, and hybridized using a SurePrint G3 Mouse GE Microarray Kit 8 × 60k (Agilent Technologies). The representative constitutively expressed gene glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as an internal control for normalization. All samples were run on the microarray chip thrice to obtain an average value for comparison.
Quantitative real-time reverse transcription-polymerase chain reaction
We selected 25 genes that showed significant changes in expression after surgery and NTP administration, especially those associated with the inflammatory response, for quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) analyses to confirm the results of the microarray analysis. The primers for qRT-PCR were purchased from Hokkaido System Science Co., Ltd (Hokkaido, Japan). Primer sequences are listed in Supplemental Materials, Table S1. The cDNA Reverse Transcription Kit, qRT-PCR device, and cycling conditions are consistent with those used in a previous study. 13 The mean expression levels relative to Gapdh expression level were obtained from four independent PCR runs for each RNA sample (n = 4). The relative expression levels were compared with the expression levels in the sham/saline group (designated as 1.000).
Gene set enrichment analysis
The normalized microarray data were subjected to gene set enrichment analysis (GSEA) (gsea-msigdb.org), with the c2.cp.kegg. v2023.1.Hs. symbols database, including 186 gene sets. GSEA was used to determine the differential expression of gene sets after surgery (operation/saline vs sham/saline) and following NTP administration (operation/NTP vs operation/saline).
Results
Effects of NTP on body weight and hind paw inflammation
The body weight of mice increased during the experimental period (Figure 2). Statistically significant differences were not observed at any time point among the four groups, suggesting that surgery and NTP administration had no substantial effect on the general health of the mice.
Figure 2.
Body weight changes in mice. All mice were weighed before injection. Data are presented as mean values. No significant differences in weight among the four groups were determined using the Mann–Whitney U test.
Post-surgery, a local lesion, accompanied with skin cyanosis, was formed during the first week, followed by swelling and congestion that lasted approximately 3 weeks and finally led to the formation of a painful scar. The circumference of the operated hind paws increased until day 5 and declined to the pre-operation level after week three post-operation (Figure 3(a)). However, NTP administration inhibited swelling substantially, as evidenced by a decrease in the peak circumference and shift in the inflection point to day 2 post-operation. The final return to the pre-operation level occurred 2 weeks earlier than that in the operation/saline group. As shown Figure 3(b), on day 2 post-operation, NTP reduced the swelling of the operated paws and resulted in full recovery within the first week post-operation. These observations indicate that NTP has an anti-inflammatory effect in the acute-phase response to mechanical injury.
Figure 3.
Changes in the appearance of the operated heel in the mice. (a) The circumference was measured at 5 mm from the heel of the mice. Data are presented as mean values. To compare the circumference of the operated paw in each group more directly, the Part 2.5 mm below the ordinate was removed. Significant differences in operation/saline versus sham/saline (*: p < 0.05, **: p < 0.01) and operation/NTP versus operation/saline (+: p < 0.05, ++: p < 0.01) were determined using the Mann–Whitney U test. The measurement times were the same as those for body weight. (b) Changes in the appearance of the operated hind paw at 2 h, 2 days, and 1 week post-operation.
Mechanical stimulation-induced response: the von frey test
Pain in the hind paws was evaluated by measuring the threshold of mechanical stimulation that caused hind paw withdrawal. The test was started on day 1 pre-operation and continued until week four post-operation. In the operative side hind paws (Figure 4(a)), the PWT of the operation group (operation/saline and operation/NTP) declined rapidly 2 h post-operation. The PWT of the operation/saline group further decreased to the lowest value on day 5 post-operation and gradually returned to the pre-operation level at week four post-operation. Conversely, in the operation/NTP group, a decrease in PWT from day 4 post-operation was prevented, and PWT was significantly higher than that in mice receiving saline (Figure 4(a), operation/saline vs operation/NTP, p < 0.05). The PWT was almost normalized in the first week after surgery. NTP administration shortened the recovery time by approximately 3 weeks. Notably, as hind paw swelling started to recover following NTP administration, the PWT increased simultaneously (on day 3 post-operation). Mice in the sham group (Figure 4(a), sham/saline and sham/NTP) exhibited only a slight decrease in PWT during the first 4 days, which was not affected by NTP administration.
Figure 4.
Von Frey test. The von Frey test was used to measure the withdrawal threshold of the operative side hind paw (a) and nonoperative side hind paw (b) in response to mechanical stimuli. Each measurement was performed prior to all injections. Data are presented as mean values. Significant differences in operation/saline versus sham/saline (*: p < 0.05, **: p < 0.01) and operation/NTP versus operation/saline (+: p < 0.05, ++: p < 0.01) were determined using the Mann–Whitney U test.
Inflammatory mediators activate action potentials from the ipsilateral (injured) side. These signals are then transduced to nociceptor cell bodies within the DRG neurons, thereby transmitting the signals to the contralateral side via the nervous system of the DRG to cause pain-related responses.12,21 Therefore, we measured PWT in the nonoperative side of mice in the four groups (Figure 4(b)) and found that the changes in PWT were not as significant as those observed on the operative side; however, the trends in PWT closely resembled those on the operative side. These results show that NTP exerted an analgesic effect on both operated and non-operated sides in the painful scar formation mouse model.
Gene expression analysis
In the first week post-operation, the operation/NTP group showed the largest difference in PWT compared to the operation/saline group, and the PWT levels in the operation/NTP group were similar to pre-operation levels. Therefore, total RNA was extracted from the scarred hind paws and DRGs at this time point (Figure 4(a)).
The criterion for the upregulation and downregulation of gene expression in the microarray analysis was >2-fold or <0.5-fold changes; genes whose expression was upregulated or downregulated are listed in Table 1. The results offer a comprehensive overview of the effects of surgery and NTP treatment on gene expression. The qRT-PCR results were in good agreement with the microarray results. The consistency between qRT-PCR and microarray results of representative genes is shown in Supplemental Figure S1.
Table 1.
Number of upregulated or downregulated genes.
| Fold change in expression level* | Number of genes | |
|---|---|---|
| Paw | DRG | |
| Operation/saline vs. sham/saline >2-fold | 3,152 | 2,039 |
| Operation/NTP vs. operation/saline >2-fold | 1,094 | 1,319 |
| Operation /saline vs. sham/saline <0.5-fold | 370 | 453 |
| Operation /NTP vs. operation/saline <0.5-fold | 415 | 859 |
| Operation /saline vs. sham/saline >2-fold and operation/NTP vs. operation/saline >2-fold | 169 | 126 |
| Operation /saline vs. sham/saline <0.5-fold and operation/NTP vs. operation/saline <0.5-fold | 6 | 45 |
| Operation/saline vs. sham/saline >2-fold and operation/NTP vs. operation/saline <0.5-fold | 89 | 101 |
| Operation/saline vs. sham/saline <0.5-fold and operation/NTP vs. operation/saline >2-fold | 34 | 88 |
* Fold changes in the expression level in the different comparisons were calculated relative to levels in the sham/saline or operation/saline group (set to 1.00). NTP: Neurotropin®, DRG: dorsal root ganglion.
GSEA
The GSEA results were ranked based on the absolute normalized enrichment score (|NES|). When |NES| > 1 and false discovery rate (FDR) < 0.25, the results were considered significant. The top 20 hits are listed in Tables 2 and 3. Based on the enrichment results, the gene sets involved in the inflammatory response are highlighted in orange in Tables 2 and 3 We focused on the gene sets that appeared in both sets of comparisons, which are marked in red. The fold changes in expression levels for the core enriched genes in these gene sets are presented in Tables 4 and 5.
Table 2.
Top 20 hits in the GSEA of the paw.

|
Note: Operation/saline versus sham/saline enriched only three gene sets. Gene sets associated with pain or inflammation are marked in orange; gene sets that appeared simultaneously in both sets of comparisons (operation/saline vs sham/saline and operation/NTP vs operation/saline) are marked in red. NES: normalized enrichment score, FDR: false discovery rate.
Table 3.
Top 20 hits in the GSEA of the DRG.

|
Note: Gene sets associated with pain or inflammation are marked in orange; gene sets that appeared simultaneously in both sets of comparisons (operation/saline vs sham/saline and operation/NTP vs operation/saline) are marked in red. NES: normalized enrichment score, FDR: false discovery rate.
Table 4.
Fold change values for expression of the core enriched genes in the paw.
| Cytokine-cytokine receptor interaction | ||||||
|---|---|---|---|---|---|---|
| Gene name | Description | Sham/saline | Sham/NTP | Operation/saline | Operation/NTP | Raw (sham/saline) |
| Ccl19 | Chemokine (C-C motif) ligand 19 | 1.00 | 0.88 | 2.15 | 4.23 | 44.3 |
| Ccl2 | Chemokine (C-C motif) ligand 2 | 1.00 | 0.95 | 3.70 | 6.27 | 157.9 |
| Ccl8 | Chemokine (C-C motif) ligand 8 | 1.00 | 1.32 | 15.76 | 27.91 | 73.5 |
| Ccr6 | Chemokine (C-C motif) receptor 6 | 1.00 | 1.05 | 6.66 | 8.43 | 1.7 |
| Ccr9 | Chemokine (C-C motif) receptor 9 | 1.00 | 1.02 | 0.85 | 1.87 | 12.6 |
| Csf2ra | Colony stimulating factor 2 receptor, alpha | 1.00 | 1.23 | 4.81 | 6.52 | 448.8 |
| Cxcl16 | Chemokine (C-X-C motif) ligand 16 | 1.00 | 1.26 | 6.17 | 8.94 | 56.8 |
| Cxcl9 | Chemokine (C-X-C motif) ligand 9 | 1.00 | 1.00 | 1.17 | 5.05 | 46.2 |
| Cxcr4 | Chemokine (C-X-C motif) receptor 4 | 1.00 | 0.99 | 2.93 | 6.09 | 42.2 |
| Egfr | Epidermal growth factor receptor | 1.00 | 1.16 | 3.98 | 5.27 | 425.4 |
| Flt4 | FMS-like tyrosine kinase 4 | 1.00 | 1.01 | 2.74 | 2.57 | 4.5 |
| Hgf | Hepatocyte growth factor | 1.00 | 0.99 | 1.76 | 4.31 | 18.6 |
| Ifna5 | Interferon alpha 5 | 1.00 | 0.53 | 1.50 | 1.31 | 9.7 |
| Ifng | Interferon gamma | 1.00 | 1.01 | 2.58 | 1.17 | 42.0 |
| Il10 | Interleukin 10 | 1.00 | 0.93 | 0.88 | 3.19 | 71.8 |
| Il10ra | Interleukin 10 receptor, alpha | 1.00 | 1.02 | 1.39 | 3.01 | 156.4 |
| Il10rb | Interleukin 10 receptor, beta | 1.00 | 0.95 | 1.81 | 3.59 | 155.1 |
| Il11 | Interleukin 11 | 1.00 | 1.15 | 1.07 | 7.25 | 25.5 |
| Il11ra1 | Interleukin 11 receptor, alpha chain 1 | 1.00 | 1.11 | 2.31 | 2.68 | 8,786.9 |
| Il13 | Interleukin 13 | 1.00 | 0.96 | 1.05 | 3.47 | 38.1 |
| Il18 | Interleukin 18 | 1.00 | 1.13 | 2.66 | 1.67 | 30.4 |
| Il18r1 | Interleukin 18 receptor 1 | 1.00 | 1.28 | 3.26 | 7.82 | 3.9 |
| Il18rap | Interleukin 18 receptor accessory protein | 1.00 | 1.02 | 0.95 | 3.49 | 1.6 |
| Il1b | Interleukin 1 beta | 1.00 | 1.03 | 2.34 | 1.15 | 68.5 |
| Il1r2 | Interleukin 1 receptor, type II | 1.00 | 0.99 | 4.04 | 7.84 | 9.7 |
| Il1rap | Interleukin 1 receptor accessory protein | 1.00 | 1.36 | 1.61 | 2.36 | 13.3 |
| Il20rb | Interleukin 20 receptor beta | 1.00 | 0.94 | 1.03 | 2.18 | 146.2 |
| Il21r | Interleukin 21 receptor | 1.00 | 1.26 | 8.74 | 11.80 | 3.6 |
| Il2rg | Interleukin 2 receptor, gamma chain | 1.00 | 1.19 | 8.89 | 11.13 | 11.3 |
| Il4 | Interleukin 4 | 1.00 | 1.00 | 0.83 | 3.91 | 51.7 |
| Il4ra | Interleukin 4 receptor, alpha | 1.00 | 1.26 | 6.93 | 7.40 | 37.7 |
| Il5 | Interleukin 5 | 1.00 | 1.03 | 1.26 | 2.85 | 1.9 |
| Il6 | Interleukin 6 | 1.00 | 1.01 | 3.14 | 1.19 | 39.4 |
| Il6ra | Interleukin 6 receptor, alpha | 1.00 | 1.04 | 2.54 | 2.62 | 645.3 |
| Il7 | Interleukin 7 | 1.00 | 1.01 | 1.86 | 5.09 | 1.5 |
| Il7r | Interleukin 7 receptor | 1.00 | 1.35 | 5.64 | 14.12 | 11.3 |
| Kit | Kit oncogene | 1.00 | 1.17 | 4.20 | 5.03 | 27.3 |
| Lif | Leukemia inhibitory factor | 1.00 | 1.39 | 1.26 | 1.99 | 73.2 |
| Osm | Oncostatin M | 1.00 | 1.07 | 0.99 | 1.48 | 4.3 |
| Osmr | Oncostatin M receptor | 1.00 | 1.15 | 3.86 | 4.51 | 393.5 |
| Pdgfc | Platelet-derived growth factor, C polypeptide | 1.00 | 1.21 | 6.86 | 7.38 | 58.6 |
| Prlr | Prolactin receptor | 1.00 | 0.90 | 10.22 | 14.98 | 5.2 |
| Tgfb3 | Transforming growth factor, beta 3 | 1.00 | 1.11 | 2.78 | 3.00 | 1,884.7 |
| Tnfa | Tumor necrosis factor-alpha | 1.00 | 0.98 | 2.29 | 1.84 | 4,251.4 |
| Tnfrsf18 | Tumor necrosis factor receptor superfamily, member 18 | 1.00 | 1.13 | 4.81 | 5.94 | 4.2 |
| Tnfrsf1b | Tumor necrosis factor receptor superfamily, member 1b | 1.00 | 1.22 | 3.98 | 2.63 | 45.0 |
| Tnfrsf25 | Tumor necrosis factor receptor superfamily, member 25 | 1.00 | 1.18 | 3.49 | 3.27 | 131.3 |
| Tnfsf11 | Tumor necrosis factor (ligand) superfamily, member 11 | 1.00 | 1.04 | 8.52 | 10.97 | 54.6 |
| Tnfsf15 | Tumor necrosis factor (ligand) superfamily, member 15 | 1.00 | 1.23 | 3.10 | 4.39 | 64.1 |
| Tnfsf18 | Tumor necrosis factor (ligand) superfamily, member 18 | 1.00 | 1.01 | 3.34 | 4.40 | 51.5 |
| Tnfsf8 | Tumor necrosis factor (ligand) superfamily, member 8 | 1.00 | 1.22 | 3.18 | 4.71 | 41.8 |
| Vegfc | Vascular endothelial growth factor C | 1.00 | 1.03 | 2.41 | 2.75 | 114.3 |
Note: Fold change values were calculated relative to levels in the sham/saline group (set to 1.00).
Table 5.
Fold change values for expression of the core enriched genes in the DRG.
| TGF-β signaling pathway | ||||||
|---|---|---|---|---|---|---|
| Gene name | Description | Sham/ saline | Sham/ NTP | Operation/ saline | Operation/ NTP | Raw (sham/saline) |
| Acvr1c | Activin A receptor, type IC | 1.00 | 1.10 | 1.19 | 0.35 | 47.8 |
| Acvrl1 | Activin A receptor, type II-like 1 | 1.00 | 0.95 | 1.12 | 0.87 | 575.5 |
| Bmp2 | Bone morphogenetic protein 2 | 1.00 | 1.06 | 1.63 | 1.26 | 116.8 |
| Bmp4 | Bone morphogenetic protein 4 | 1.00 | 0.71 | 1.56 | 1.22 | 853.2 |
| Bmp5 | Bone morphogenetic protein 5 | 1.00 | 0.82 | 1.96 | 1.41 | 65.0 |
| Bmp6 | Bone morphogenetic protein 6 | 1.00 | 0.85 | 2.92 | 1.26 | 154.5 |
| Bmp7 | Bone morphogenetic protein 7 | 1.00 | 0.91 | 1.42 | 0.76 | 799.8 |
| Comp | Cartilage oligomeric matrix protein | 1.00 | 0.49 | 2.71 | 1.05 | 535.2 |
| Dcn | Decorin | 1.00 | 0.61 | 1.61 | 0.61 | 77,248.8 |
| E2f5 | E2F transcription factor 5 | 1.00 | 0.95 | 1.43 | 0.59 | 144.5 |
| Gdf5 | Growth differentiation factor 5 | 1.00 | 1.10 | 1.64 | 0.86 | 295.2 |
| Id1 | Inhibitor of DNA binding 1 | 1.00 | 1.11 | 1.53 | 1.08 | 1,619.2 |
| Id2 | Inhibitor of DNA binding 2 | 1.00 | 1.08 | 1.70 | 1.10 | 1,105.2 |
| Ifng | Interferon gamma | 1.00 | 1.02 | 1.54 | 1.24 | 331.6 |
| Inhba | Inhibin beta-A | 1.00 | 0.90 | 1.88 | 0.69 | 54.1 |
| Inhbb | Inhibin beta-B | 1.00 | 1.09 | 1.97 | 1.28 | 397.3 |
| Lefty2 | Left-right determination factor 2 | 1.00 | 1.10 | 5.22 | 1.04 | 74.6 |
| Ltbp1 | Latent transforming growth factor beta binding protein 1 | 1.00 | 0.76 | 1.47 | 0.66 | 515.4 |
| Rbl1 | Retinoblastoma-like 1 (p107) | 1.00 | 0.94 | 1.19 | 0.75 | 48.6 |
| Smad3 | SMAD family member 3 | 1.00 | 0.92 | 1.72 | 0.97 | 466.4 |
| Smad7 | SMAD family member 7 | 1.00 | 1.16 | 2.00 | 1.07 | 153.7 |
| Tgfb1 | Transforming growth factor, beta 1 | 1.00 | 1.06 | 1.54 | 1.45 | 392.4 |
| Tgfb2 | Transforming growth factor, beta 2 | 1.00 | 1.19 | 1.20 | 1.30 | 421.8 |
| Tgfb3 | Transforming growth factor, beta 3 | 1.00 | 1.09 | 1.25 | 1.20 | 1,250.0 |
| Thbs1 | Thrombospondin 1 | 1.00 | 0.57 | 3.56 | 0.57 | 418.2 |
| Thbs2 | Thrombospondin 2 | 1.00 | 0.92 | 1.25 | 0.91 | 103.0 |
| Thbs3 | Thrombospondin 3 | 1.00 | 1.00 | 1.48 | 1.06 | 880.9 |
| Thbs4 | Thrombospondin 4 | 1.00 | 0.95 | 1.59 | 0.96 | 2,548.1 |
| Tnfa | Tumor necrosis factor-alpha | 1.00 | 1.03 | 1.88 | 1.36 | 3,252.5 |
Note: Fold change values were calculated relative to levels in the sham/saline group (set to 1.00).
The GSEA demonstrated the activation of the cytokine–cytokine receptor interaction pathway in paw samples following both surgery and administration of NTP (Figure 5(a)). The expression of genes encoding proinflammatory cytokines such as tumor necrosis factor-α (Tnfa); interleukin-1β (Il1b); interleukin-6 (Il6); interleukin-18 (Il18); interferon-γ(Ifng); and chemokine (C–C motif) ligands 2, 8, and 19 (Ccl2, Ccl8, and Cc19) increased by more than 1.5-fold after the surgery but decreased following NTP administration. In contrast, the expression of genes encoding anti-inflammatory cytokines, such as interleukin-4 (Il4), interleukin-10 (Il10), interleukin-11 (Il11), and interleukin-13 (Il13) did not significantly differ between before and after surgery, but exhibited more than three-fold increase after NTP administration.
Figure 5.
GSEA of paw samples. Gene expression levels are presented as a gradient from high (red) to low (blue). Only core enriched genes in the gene sets are listed. (a) Cytokine–cytokine receptor interaction was activated by the surgery (operation/saline vs sham/saline) and NTP treatment (operation/NTP vs operation/saline). (b) Extracellular matrix receptor interaction was activated by the surgery (operation/saline vs sham/saline) but suppressed by NTP treatment (operation/NTP vs operation/saline). FDR: false discovery rate; NES: normalized enrichment score (|NES| > 1 and FDR <0.25 were considered significant).
The extracellular matrix (ECM) receptor interaction was activated by the surgery but was suppressed by NTP administration in both the paws and DRGs (Figure 5(b)). The core enriched genes that primarily contributed to ECM-receptor interactions were those encoding integrins and collagens. In the DRG samples, the expression of genes encoding integrins, such as Itga1, Itga2, Itga2b, Itga3, Itga5, Itga6, Itga9, Itga10, Itgb5, and Itgb7, was generally higher than that in the paws. In contrast, genes encoding collagen, including Col1a1, Col1a2, Col2a1, Col3a1, Col5a1, Col5a2, Col5a3, Col6a1, Col6a2, Col6a3, Col6a6, Col11a1, and Col11a2, typically displayed higher expression in paw samples than in DRGs.
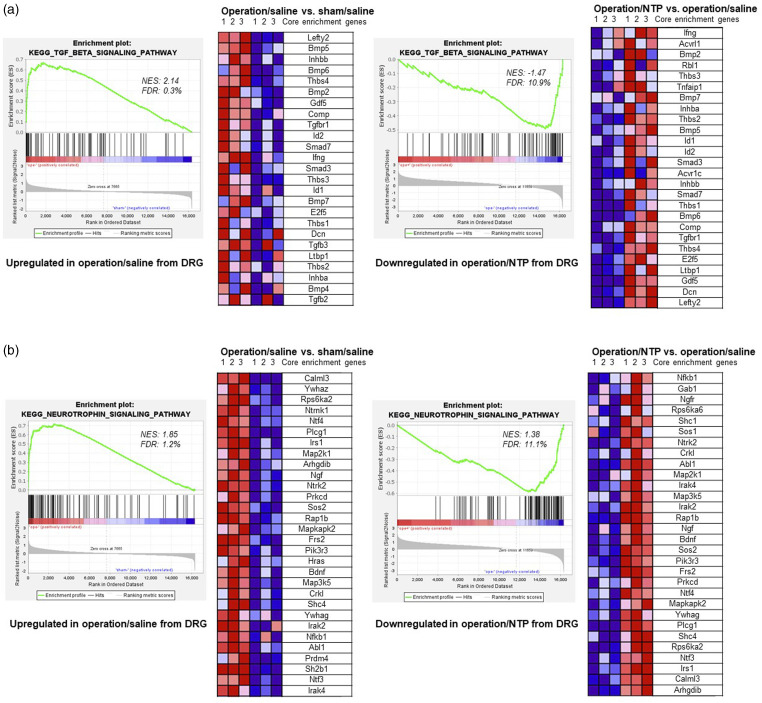
The transforming growth factor-β (TGF-β) signaling and neurotrophin (neurotrophins are a family of proteins that are distinct from the NTP) signaling pathways were significantly activated by the surgery but were suppressed by the administration of NTP in the DRGs. In the TGF-β signaling pathway (Figure 6(a)), the majority of the enriched genes encoded bone morphogenetic proteins (Bmp2, Bmp4, Bmp5, Bmp6, and Bmp7). The expression of genes encoding TGF-β (Tgfb1, Tgfb2, and Tgfb3) was slightly upregulated after the surgery; however, there was no significant change following NTP administration. In the neurotrophin signaling pathway (Figure 6(b)), the expression of genes encoding neurotrophins, including nerve growth factor (Ngf), brain-derived neurotrophic factor (Bdnf), neurotrophin 3 (Nt3), and neurotrophin 4 (Nt4), was also upregulated post-surgery. However, subsequent administration of NTP led to the downregulation of these genes in DRGs.
Figure 6.
GSEA of DRG samples. (a) The transforming growth factor-β signaling pathway was activated by the surgery (operation/saline vs sham/saline) but suppressed by NTP treatment (operation/NTP vs operation/saline) in the DRGs. (b) The neurotrophin (neurotrophins are a family of proteins that are distinct from NTP) signaling pathway was activated by the surgery (operation/saline vs sham/saline) but suppressed by NTP treatment (operation/NTP vs operation/saline) in the DRGs. FDR: false discovery rate; NES: normalized enrichment score (|NES| > 1 and FDR <0.25 were considered significant).
Other notable gene sets, such as those related to complement and coagulation cascades, chemokine signaling pathways, axon guidance, and mTOR signaling pathways, were not differentially expressed in either set of comparisons. Nonetheless, these gene sets identified in this analysis are crucial for understanding the effects of surgery and NTP administration.
Discussion
Mouse models of painful scars have demonstrated that tissue damage following surgery can lead to mechanical hyperalgesia. 12 Our study revealed that NTP administration directly to the site of injury effectively ameliorates the mechanical hyperalgesia in mice (Figure 4). Additionally, by measuring the circumference of the injured hind paw, we found that NTP alleviates local swelling and congestion resulting from the postoperative inflammatory response (Figure 3). The microarray analysis and GSEA showed that the analgesic effect of NTP could be because of the inhibition of the inflammatory response. Here, we discuss some critical genes relevant to inflammation identified using the GSEA. In particular, the gene sets enriched in both post-surgery and NTP treatment groups included those related to “cytokine–cytokine receptor interaction,” which was activated in paws by both surgery and NTP administration. “ECM receptor interaction” was activated by the surgery but inhibited upon NTP administration in both the paws and DRGs. The “TGF-β signaling pathway” and “neurotrophin signaling pathway” were activated after the surgery but inhibited by NTP administration in the DRGs.
Cytokines are secreted by various cells, and specific cytokines play crucial roles in the development of pathological pain and inflammation. 14 Previous studies have suggested that NTP has suppressive effects in various mouse models exhibiting inflammatory responses by inhibiting the expression of proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α.22,23 In our study, the expression of core enriched genes that encode proinflammatory cytokines, such as Tnfa, Il1b, Il6, Il18, Ifng, Ccl2, Ccl8, and Cc19 (Table 4 and Figure 5(a)), was upregulated within the paws following the surgery, and downregulated upon the administration of NTP. TNF-α could induce the expression of IL-1β and IL-6, which in turn contribute to the progression of inflammatory hyperalgesia. 24 In addition, anti-inflammatory cytokines can control the extent of proinflammatory and immune responses. 25 The expression of Il4, Il10, Il11, and Il13 increased markedly following NTP treatment in this study. Patients with chronic widespread pain have lower levels of anti-inflammatory cytokines, specifically IL-4 and IL-10. 26 Additionally, a single injection of IL-10 protein into the pathological site inhibits pain progression. 27 Indeed, NTP can potentially regulate the progression of chronic pain and inflammatory responses by either inhibiting the expression of proinflammatory cytokines or activating the expression of anti-inflammatory cytokines.
In the event of tissue trauma or inflammation, the ECM actively participates in various stages of wound healing. 28 The ECM undergoes synthesis and restoration processes to return to its original state, with collagen, a vital component, playing a critical role at every stage of the wound healing process. 28 In the present study, we observed that genes encoding collagen are upregulated in the paw tissues after the surgery (Table 4 and Figure 5(b)) and are downregulated in the NTP-treated group. Based on the alterations in the expression of genes encoding collagen and involved in the healing process in scarred paws following NTP treatment, NTP may expedite the progression of wound healing and mitigate the development of painful scars. Integrins, transmembrane receptors, play a vital role in cell adhesion to the ECM to maintain force balance 29 and are key contributors to the cellular signaling pathways that mediate nociceptive receptor sensitization in various inflammatory and neuropathic pain conditions. 30 In the present study, the expression of some genes encoding integrins in the DRGs (Table 5) was significantly higher than that in the paws following surgery. This finding implies that neuropathic pain and inflammation, mediated by integrins, may be more pronounced in sensory neurons in the DRGs. Notably, the expression of these surgically affected genes was upregulated and their levels returned to those in the untreated mouse group after NTP injection. Nevertheless, it is not clear whether NTP can mitigate inflammation and neuropathic pain in DRGs by inhibiting integrin expression.
The TGF-β signaling pathway regulates various important processes, such as cell growth and differentiation, apoptosis, ECM production, and immune responses. 31 Based on the GSEA, the enriched core genes primarily encoded bone morphogenetic proteins (BMPs), including BMP2, a potent osteoinductive cytokine. BMP2 not only induces inflammation but also triggers neuroinflammatory responses in the DRG, which can lower the threshold for pain.32,33 Noggin is a BMP inhibitor that prevents BMP from binding to its cell-surface receptors. 34 Additionally, the application of exogenous noggin greatly alleviates mechanical hyperalgesia. 35 In the microarray analysis (Table 5 and Figure 6(a)), the expression of the gene encoding noggin (Nog) was regulated in the direction opposite to that of Bmps in DRGs. Based on these findings, a noggin-like tissue response may explain the suppressive effects of NTP on BMPs and inhibition of neuroinflammatory reactions in the DRG.
Neuroinflammation affects neurotrophin signaling pathways. Neurotrophins are a family of proteins that include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3), and neurotrophin 4 (NT4). These neurotrophins play critical roles in the growth, maintenance, and apoptosis of neurons in the developing nervous system.36,37 NGF contributes to nociception and chronic pain by influencing the release of inflammatory mediators, modulating the activity of nociceptive ion channels or receptors, regulating nociceptive gene expression, and promoting local neuronal sprouting. 38
The inhibition of expression of NGF and its cognate receptor, tropomyosin-related kinase A (TrkA), has substantial analgesic effects in the treatment of chronic pain in both animals and humans. 39 BDNF also plays a major role in regulating inflammation and pain. In an animal model of inflammation-induced pain, BDNF upregulation in the DRG can potentially activate the presynaptic tropomyosin receptor kinase B (TrkB) receptor, which subsequently regulates synaptic excitability during pain transmission. 40 Inhibiting the binding of BDNF to full-length TrkB in the spinal cord after sciatic nerve ligation in mice suppressed thermal hyperalgesia. 41 Through a GSEA (Table 5 and Figure 6(b)), we identified Ngf and Bdnf as core genes with substantially higher expression in the DRGs than in paw tissues. The expression of Ngf, Bdnf, and their receptors Ntrk1 and Ntrk2 was upregulated in the DRGs following the surgery. However, NTP administration downregulated the expression of these genes. The expression of Nt3 and Nt4 in the DRGs were very low and therefore will not be discussed further. Given the pathological role of NGF and BDNF in pain, exploring the potential of small molecules such as nucleic acids, amino acids, and sugars present in NTP (unpublished observations) to block NGF-TrkA or BDNF-TrkB signaling is an intriguing avenue for future research.
This study has some limitations. All experiments were exclusively conducted using male mice, and as a result, the assumptions and conclusions drawn from this study are specific to male mice. Additionally, samples were collected from the ipsilateral scarred hind paws and DRGs. In future investigations, we will explore whether NTP exerts an analgesic effect on the contralateral side through similar molecular and cellular mechanisms. Scar formation is heterogeneous. Therefore, it is difficult to fully replicate the entire process of chronic pain and scar formation. This mouse model might be useful to analyze the mechanisms underlying chronic pain associated with scar formation. NTP administration may modulate the expression of critical genes and suppress the progression of chronic pain caused by inflammatory responses. Notably, the expression of these genes may not necessarily correspond to the levels of their respective proteins. In the future, we intend to examine the protein expression levels of the relevant genes within each pathway. These genes can be used to create genetically modified mice, to facilitate the identification of novel therapeutic targets for the amelioration of chronic pain.
Supplemental Material
Supplemental Material for Neurotropin® ameliorates chronic pain associated with scar formation in a mouse model: A gene expression analysis of the inflammatory response by Xuan Zhou, Hiroki Iida, Yuqiang Li, Akinobu Ota, Lisheng Zhuo, Reiko Nobuhara, Yuki Terajima, Mitsuru Naiki, A Hari Reddi, Koji Kimata, and Takahiro Ushida in Molecular Pain
Acknowledgements
The NTP used in this study was provided by Nippon Zoki Pharmaceutical Co., Ltd (Osaka, Japan). The authors would like to thank Editage (https://www.editage.com/) for English language editing.
Author contributions: T.U. and K.K. conceived the study and supervised the experimental procedures. A.O. offered technical guidance and assistance in the microarray process, and contributed to the analysis of the microarray data. A.H.R. and L.Z. reviewed the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Nippon Zoki Pharmaceutical Company Ltd., Japan.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Xuan Zhou https://orcid.org/0009-0006-8997-7863
Akinobu Ota https://orcid.org/0000-0002-6296-2921
Koji Kimata https://orcid.org/0000-0001-5304-3803
References
- 1.Crombie IK, Davies HTO, Macrae WA. Cut and thrust: Antecedent surgery and trauma among patients attending a chronic pain clinic. Pain 1998; 76: 167–171. [PubMed] [Google Scholar]
- 2.Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008; 101: 77–86. [DOI] [PubMed] [Google Scholar]
- 3.Abd-Elsayed A, Pope J, Mundey DA, Slavin KV, Falowski S, Chitneni A, Popielarski SR, John J, Grodofsky S, Vanetesse T, Fishman MA, Kim P. Diagnosis, treatment, and management of painful scar: a narrative review. J Pain Res 2022; 15: 925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eguchi Y, Aoki Y, Yamashita M, Fujimoto K, Sato T, Abe K, Sato M, Yamanaka H, Toyoguchi T, Shimizu K, Orita S, Inage K, Shiga Y, Ohtori S. Clinical efficacy of neurotropin for lumbar spinal stenosis with low back pain. Pain Ther 2023; 12: 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishiyama T, Matsukawa T, Yamashita K. Comparison between neurotropin and mepivacaine for stellate ganglion injection. J Anesth 2006; 20: 240–242. [DOI] [PubMed] [Google Scholar]
- 6.Okada M, Suzuki K, Hidaka T, Shinohara T, Kataharada K, Takada K, Tanaka H, Ohsuzu F. Complex regional pain syndrome type I induced by pacemaker implantation, with a good response to steroids and neurotropin. Intern Med 2002; 41: 498–501. [DOI] [PubMed] [Google Scholar]
- 7.Thiebauld C, Vandeput J, Lintermans J, Vandenbosch P. Action of neurotropin on cold-induced pain in normal volunteers: a double-blind placebo study. Fundam Clin Pharmacol 1990; 4: 141–146. [DOI] [PubMed] [Google Scholar]
- 8.Toda K, Muneshige H, Ikuta Y. Antinociceptive effects of neurotropin in a rat model of painful peripheral mononeuropathy. Life Sci 1998; 62: 913–921. [DOI] [PubMed] [Google Scholar]
- 9.Okazaki R, Namba H, Yoshida H, Okai H, Miura T, Kawamura M. The antiallodynic effect of Neurotropin is mediated via activation of descending pain inhibitory systems in rats with spinal nerve ligation. Anesth Analg 2008; 107: 1064–1069. [DOI] [PubMed] [Google Scholar]
- 10.Miura T, Okazaki R, Yoshida H, Namba H, Okai H, Kawamura M. Mechanisms of analgesic action of neurotropin on chronic pain in adjuvant-induced arthritic rat: roles of descending noradrenergic and serotonergic systems. J Pharmacol Sci 2005; 97: 429–436. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Li YH, Mashimo T. The antiallodynic and antihyperalgesic effects of neurotropin in mice with spinal nerve ligation. Anesth Analg 2005; 101: 793–799. [DOI] [PubMed] [Google Scholar]
- 12.Kajita Y, Suetomi K, Okada T, Ridwan H, Binti C, Sato J, Sato K, Ushida T. Animal model with painful scar: pain−related behavior and immunohistochemical study on the spinal dorsal horn and peripheral tissue. Pain Res 2010; 25: 135–144. [Google Scholar]
- 13.Li Y, Iida H, Kimata K, Zhuo L, Ota A, Kimura S, Yin X, Deie M, Ushida T. Establishment of a mouse model for injury-induced scar formation and the accompanying chronic pain: comprehensive microarray analysis of molecular expressions in fibrosis and hyperalgesia. Mol Pain 2019; 15: 174480691989238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin 2007; 45: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinho-Ribeiro FA, Verri WA, Chiu IM. Nociceptor sensory neuron–immune interactions in pain and inflammation. Trends Immunol 2017; 38: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noh MC, Mikler B, Joy T, Smith PA. Time course of inflammation in dorsal root ganglia correlates with differential reversibility of mechanical allodynia. Neuroscience 2020; 428: 199–216. [DOI] [PubMed] [Google Scholar]
- 17.Lee-Donaldson L, Witte MH, Bernas M, Witte CL, Way D, Stea B. Refinement of a rodent model of peripheral lymphedema. Lymphology 1999; 32: 111–117. [PubMed] [Google Scholar]
- 18.Oashi K, Furukawa H, Oyama A, Funayama E, Hayashi T, Saito A, Yamamoto Y. A new model of acquired lymphedema in the mouse hind limb: a preliminary report. Ann Plast Surg 2012; 69: 565–568. [DOI] [PubMed] [Google Scholar]
- 19.Ohmichi M, Ohmichi Y, Ohishi H, Yoshimoto T, Morimoto A, Li Y, Sakurai H, Nakano T, Sato J. Activated spinal astrocytes are involved in the maintenance of chronic widespread mechanical hyperalgesia after cast immobilization. Mol Pain 2014; 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 21.Du X, Hao H, Gigout S, Huang D, Yang Y, Li L, Wang C, Sundt D, Jaffe DB, Zhang H, Gamper N. Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain 2014; 155: 2306–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimoto S, Okada K, Tanaka H, Okamoto M, Fujisawa H, Okada T, Naiki M, Murase T, Yoshikawa H. Neurotropin attenuates local inflammatory response and inhibits demyelination induced by chronic constriction injury of the mouse sciatic nerve. Biologicals 2016; 44: 206. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Roh YS, Liang S, Liu C, Naiki M, Masuda K, Seki E. Neurotropin suppresses inflammatory cytokine expression and cell death through suppression of NF-κB and JNK in hepatocytes. PLoS One 2014; 9: e114071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol 1992; 107: 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opal SM, Depalo VA. Anti-inflammatory cytokines. Chest 2000; 117: 1162–1172. [DOI] [PubMed] [Google Scholar]
- 26.Üçeyler N, Valenza R, Stock M, Schedel R, Sprotte G, Sommer C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum 2006; 54: 2656–2664. [DOI] [PubMed] [Google Scholar]
- 27.Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O’Connor K, Martin D, Forsayeth JR, Maier SF, Johnson K, Chavez RA, Leinwand LA, Watkins LR. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 2005; 21: 2136–2148. [DOI] [PubMed] [Google Scholar]
- 28.Diller RB, Tabor AJ. The role of the extracellular matrix (ECM) in wound healing: a review. Biomimetics 2022; 7: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol 1998; 10: 232–239. [DOI] [PubMed] [Google Scholar]
- 30.Dina OA, Parada CA, Yeh J, Chen X, McCarter GC, Levine JD. Integrin signaling in inflammatory and neuropathic pain in the rat. Eur J Neurosci 2004; 19: 634–642. [DOI] [PubMed] [Google Scholar]
- 31.Moses HL, Roberts AB, Derynck R. The discovery and early days of TGF-β: a historical perspective. Cold Spring Harb Perspect Biol 2016; 8: a021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen V, Meyers CA, Yan N, Agarwal S, Levi B, James AW. BMP-2-induced bone formation and neural inflammation. J Orthop 2017; 14: 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell K, Shah JP, Dalgard CL, Tsytsikova LV, Tipton AC, Dmitriev AE, Symes AJ. Bone morphogenetic protein-2-mediated pain and inflammation in a rat model of posterolateral arthrodesis. BMC Neurosci 2016; 17: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkler DG, Yu C, Geoghegan JC, Ojala EW, Skonier JE, Shpektor D, Sutherland MK, Latham JA. Noggin and sclerostin bone morphogenetic protein antagonists form a mutually inhibitory complex. J Biol Chem 2004; 279: 36293–36298. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto M, Koda M, Furuya T, Murata A, Yamazaki M, Takahashi K. Intrathecal Noggin administration in rats temporally ameliorates mechanical allodynia induced by a chronic constriction injury. eNeurologicalSci 2016; 4: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceni C, Unsain N, Zeinieh MP, Barker PA. Neurotrophins in the regulation of cellular survival and death. In: Lewin GR, Carter BD. (eds). Neurotrophic factors. Berlin Heidelberg: Springer, 2014, pp. 193–221. [DOI] [PubMed] [Google Scholar]
- 37.Skaper SD. The neurotrophin family of neurotrophic factors: an overview. In: Skaper SD. (ed). Methods in Molecular Biology (Clifton, N.J.). Totowa, NJ: Humana Press, 2012, pp. 1–12. [DOI] [PubMed] [Google Scholar]
- 38.Barker PA, Mantyh P, Arendt-Nielsen L, Viktrup L, Tive L. Nerve growth factor signaling and its contribution to pain. J Pain Res 2020; 13: 1223–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL, Warner DS. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011; 115: 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin YT, Ro LS, Wang HL, Chen JC. Up-regulation of dorsal root ganglia BDNF and trkB receptor in inflammatory pain: an in vivo and in vitro study. J Neuroinflammation 2011; 8: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yajima Y, Narita M, Narita M, Matsumoto N, Suzuki T. Involvement of a spinal brain-derived neurotrophic factor/full-length TrkB pathway in the development of nerve injury-induced thermal hyperalgesia in mice. Brain Res 2002; 958: 338–346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Neurotropin® ameliorates chronic pain associated with scar formation in a mouse model: A gene expression analysis of the inflammatory response by Xuan Zhou, Hiroki Iida, Yuqiang Li, Akinobu Ota, Lisheng Zhuo, Reiko Nobuhara, Yuki Terajima, Mitsuru Naiki, A Hari Reddi, Koji Kimata, and Takahiro Ushida in Molecular Pain