Abstract
Parasites are responsible for the most neglected tropical diseases, affecting over a billion people worldwide (WHO, 2015) and accounting for billions of cases a year and responsible for several millions of deaths. Research on extracellular vesicles (EVs) has increased in recent years and demonstrated that EVs shed by pathogenic parasites interact with host cells playing an important role in the parasite's survival, such as facilitation of infection, immunomodulation, parasite adaptation to the host environment and the transfer of drug resistance factors. Thus, EVs released by parasites mediate parasite‐parasite and parasite‐host intercellular communication. In addition, they are being explored as biomarkers of asymptomatic infections and disease prognosis after drug treatment. However, most current protocols used for the isolation, size determination, quantification and characterization of molecular cargo of EVs lack greater rigor, standardization, and adequate quality controls to certify the enrichment or purity of the ensuing bioproducts. We are now initiating major guidelines based on the evolution of collective knowledge in recent years. The main points covered in this position paper are methods for the isolation and molecular characterization of EVs obtained from parasite‐infected cell cultures, experimental animals, and patients. The guideline also includes a discussion of suggested protocols and functional assays in host cells
Keywords: EVs methodology, extracellular vesicles, helminth, host‐parasite interaction, infection, protocols, protozoan parasites
1. INTRODUCTION
1.1. Extracellular vesicles and parasites
The study of extracellular vesicles (EV) has become incredibly important due to their ability to mediate the signaling and transfer of biomolecules between cells. This EV‐mediated communication eliminates the need for cell‐cell contact and allows cells to deliver messages to remote sites (Campos et al., 2015; Colombo et al., 2014; Marcilla et al., 2014, 2012; Théry et al., 2006, 2002, 2009, 2018; Torrecilhas et al., 2020; Witwer et al., 2013). RNA, DNA, Proteins, RNA, DNA, glycoconjugates, lipids and metabolites are found in EVs and can be transported from one cell to another. These molecules have been shown to be involved in drug resistance, regulation of cell growth, regulation or activation/modulation of cells of the immune system, modulation of cellular development and differentiation, neurotransmission, and so forth (Abou Andre et al., 2001; Araldi et al., 2012; Colombo et al., 2014; Dekel et al., 2021; Kalra et al., 2016; Karam et al., 2022; Mantel et al., 2016, 2013; Ofir‐Birin et al., 2021; Raposo & Stoorvogel, 2013; Regev‐Rudzki et al., 2013; Sisquella et al., 2017; Théry et al., 2006, 2018; Wolfers et al., 2001). Upon fusion, physiological processes such as blood coagulation, cell differentiation and inflammation, as well as pathological processes can lead to the development of cancer, neurological alterations, cardiovascular and infectious diseases (Andre et al., 2001; Araldi et al., 2012; Campos et al., 2015; Colombo et al., 2014; Raposo & Stoorvogel, 2013; Torrecilhas et al., 2020; Wolfers et al., 2001).
EV‐mediated intercellular communication in parasites has been observed both between the same species as well as between different species (Marcilla et al., 2014; Torrecilhas et al., 2020). EVs released by pathogenic parasites were found to play an important role in the establishment of the infection by promoting survival or by regulating infection and modifying host immunomodulatory processes. In some cases, EVs transfer information that allow drug resistance (Regev‐Rudzki et al., 2013). EVs can also mediate parasite‐parasite communication (Torrecilhas et al., 2012, 2020; Trocoli Torrecilhas et al., 2009; Vasconcelos et al., 2021). Furthermore, the fact that EVs have been found in most biological fluids (Yáñez‐Mó et al., 2015) makes them an important target for the identification of new biomarkers in parasitic infections (Marcilla et al., 2014). All together the date reviewed above, illustrates the wide spectrum of physiological functions elicited by EVs in parasitic diseases (Figure 1).
FIGURE 1.
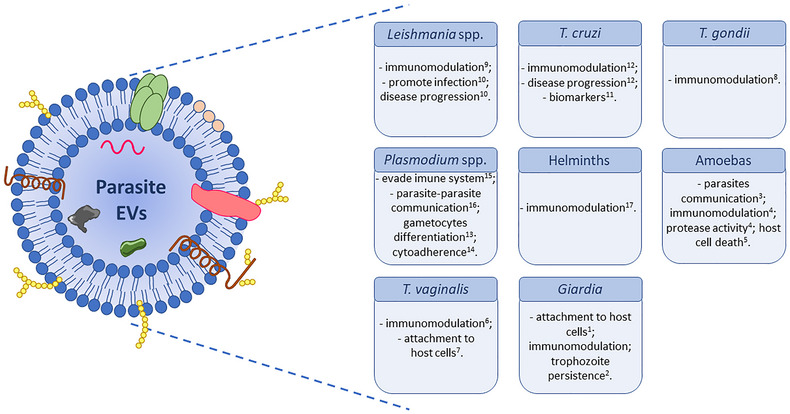
EVs from distinct pathogenic parasites (different forms and stages of the life cycle). Parasite EVs are composed of a lipid bilayer and a several molecules derived from the plasma membrane and/or cytosol. EVs can participate in the parasite‐host relationship since they on carry proteins, lipids, and nucleic acids distributed inside, outside or merged in the lipid bilayer (a). EVs from pathogenic parasites functions. Parasite EVs participate in the parasite‐parasite and parasite‐host relationship. EVs have different roles depending on the species or the evolutive form. They can induce an immune response (activating or suppressing the immune cells), facilitate the parasite attachment to the host cells, participate in parasite‐parasite communication, and contribute to the parasite‐parasite communications, among other functions (b). (Evans‐Osses et al., 2017)1 and 2; (Ma'ayeh et al., 2017)−3; (Sharma et al., 2020)−4; (Costa et al., 2021)−5; (Goncalves et al., 2018)−6; (Olmos‐Ortiz et al., 2017)−7; (Twu et al., 2013)−8; (Silva et al., 2018)−9; (Atayde et al., 2016; Silverman et al., 2010b)−10; (Madeira et al., 2021; Torrecilhas et al., 2012; Trocoli Torrecilhas et al., 2009)−11 and 12; (Mantel & Marti, 2014)−13; (Toda et al., 2020)−14; (Sisquella et al., 2017)–15; (Regev‐Rudzki et al., 2013)–16; (Marcilla et al., 2014)−17.
A workshop was organized in 2016 in Brazil to discuss the nature and origin of EVs in different pathogenic organisms and on how EVs could be characterized in each one. Furthermore, the members of the International Society for EVs (ISEV) organized a satellite meeting (Cross‐Organism Communication by Extracellular Vesicles: Hosts, Microbes, Parasites) that included mainly infectious diseases. The major goal of the ISEV workshop was to promote a high‐level scientific discussion of modes of EV‐mediated communication between hosts and pathogens or commensals. The major raised questions were about the EV structure and composition in different kingdoms and how these EVs can be characterized and studied in different and unrelated species. The discussion resulted in a meeting report (Soares et al., 2017). In November 2018, a second ISEV workshop (EVs in Clinical Theranostic) occurred in Guangzhou, China, and focused on advances in analytical technologies to provide guidance considering the use of EVs in diagnostic and therapeutic applications. The meeting pointed out the major challenges and requirements for separation of EVs from different cell types and body fluids to allow adequate clinical usage (Soekmadji et al., 2020). It was consensus that it is still necessary to develop and improve protocols and techniques for purification and characterization of EVs isolated from the pathogenic organism. This conclusion underscores the need to establish guidelines to explore the EVs isolated from parasites. Unique characteristics of each organism, however, require to be treated separately. Moreover, variations in the size, quantity, and intrinsic composition of EVs from each parasite (and even from different life‐cycle stages and strains of the same parasite) often make it difficult to obtain consistently enriched and homogenous preparations, thus requiring specific methods for isolation and molecular characterization.
Here, we propose a set of guidelines based on the methods already developed for the best purification and characterization of released EVs from parasites, together with biological assays, to understand their function. An important point to consider is that ascribing a specific function for the ensuing biomaterial may include subtypes of EVs, eventually containing contaminants that could vary in each batch. This guideline article additionally includes technical tables and steps to follow and to document specific functional activities of parasite derived EVs. We also provide a checklist to improve each step of the protocols and summaries of the key points for the isolation, purification and molecular and functional characterization of EVs from parasites.
Many different methods are available for the isolation, characterization and purification of EVs. Therefore, it is crucial to report all experimental procedures to maximize the number of known and reportable parameters, allowing for their standardization, quality control and comparative analysis. Our aim is to give the reader the possibility to assess critical technical issues observed in the field of parasite derived EVs, specifically regarding (1) EV isolation and purification; (2) analysis of proteins, lipids, glycoconjugates (glycoproteins, glycolipids, glycolipoproteins) and nucleic acids; (3) the level of enrichment and homogeneity of EV preparations and (4) the need to improve and share purification and separation protocols.
1.2. Trypanosomatidae: Kinetoplastida
1.2.1. Trypanosoma cruzi
Trypanosoma cruzi is the causative agent of Chagas disease or American trypanosomiasis, a neglected tropical disease (NTD). Approximately, 6–8 million people are already chronically infected in the Latin America, and 100 million are at risk of acquiring the disease in North America, Europe and other nonendemic regions (Bern, 2015; Coura & Viñas, 2010; Dias et al., 2002). Transmission of the parasitic protozoan to humans occurs through contaminated feces of insect vectors (triatomines), oral transmission (contaminated food and juices), blood transfusion, organ transplantation, congenital contagion and laboratory accidents. During the natural infection, T. cruzi is transmitted through the excreta of the hematophagous triatomine bug, which contains the metacyclic trypomastigote (MT) forms. These forms gain access to the mammalian host through the insect bite wound or through exposed (oral or ocular) mucosal membranes, where the parasites invade nucleated cells. The parasites transform into amastigote forms, which multiply in the cytosol. Then, they transform into trypomastigotes and rupture the host‐cell plasma membrane, reaching the extracellular matrix and eventually, the bloodstream. The trypomastigote surface membrane is composed mainly of glycoconjugates (glycoproteins and glycolipids) such as glycosylphosphatidylinositol (GPI)‐anchored mucin‐like glycoproteins (tGPI‐mucins or TcMUC II), trans‐sialidases (TS), Tc85, mucin‐associated surface proteins (MASP), and glycoinositolphospholipids (GIPLs) (Acosta‐Serrano et al., 2007; Almeida et al., 1994; Buscaglia et al., 2004, 2006; de Lederkremer & Agusti, 2009; de Pablos Torró et al., 2018; Schenkman & Eichinger, 1993; Schenkman et al., 1994, 1993, 1992; Soprano et al., 2018; Uehara et al., 2012). Gonçalves and cols. (1991), Torrecilhas and cols. (2009), and Osuna and cols. (De Pablos et al., 2016; Díaz Lozano et al., 2017) have shown that T. cruzi trypomastigotes derived from infected mammalian cells release vesicles into the culture medium. Proteomic analysis of this fraction showed that about 30% of the hits correspond to proteins of the Tc85/TS family of T. cruzi (Ribeiro et al., 2018), indicating that these EVs contain large amounts of surface membrane components. Several pieces of evidence indicate that these T. cruzi‐derived EVs participate in the interaction with host cells during infection (Cronemberger‐Andrade et al., 2014, 2020; Ramirez et al., 2017), including delivery of cargo (virulence factors and antigens) into host cells, modulation of host innate and adaptive immune responses and immune evasion (Bayer‐Santos et al., 2013; Cestari et al., 2012; Torrecilhas et al., 2012, 2020).
1.2.2. Leishmania spp
Leishmania comprises several species of a unicellular protozoan parasite, which is responsible for different clinical forms of leishmaniasis, an NTD that causes major health problems in tropical and subtropical areas (Scott & Novais, 2016). These parasites have a digenetic life cycle: they live as highly motile promastigote forms in the sand fly vector and differentiate into a less motile amastigote forms in the mammalian host macrophages (Belo et al., 2017). EVs released by Leishmania spp. can contribute to the establishment of infection and host immunomodulation (Barbosa et al., 2018). The study of the biology of Leishmania EVs began in 2008 (Silverman et al., 2010a) and, since then, tremendous effort has been devoted to characterizing and understanding their biological roles and pathophysiological impact in the host. These efforts showed that Leishmania can release EVs enriched with major virulence factors at different life cycle stages. These EVs can alter host cell signaling and microbicidal functions (Atayde et al., 2015, 2019b; Hassani et al., 2014; Silverman & Reiner, 2011; Silverman et al., 2010a, 2010b). The group lead by Reiner provided the first clear demonstration of Leishmania EV release (Silverman et al., 2010a), while the Olivier team provided the first evidence of GP63 clustering within Leishmania EVs (Gomez et al., 2009). They further showed that knocking out this important virulence factor almost completely abolished the ability of these EVs to modulate host immune response in comparison with its wild‐type counterparts (Gomez & Olivier, 2010; Hassani et al., 2014). These are among the first clear demonstrations that Leishmania parasites can release small vesicles as well as the fact that almost all Leishmania exosomal proteins lack a signal peptide (Hassani et al., 2011). One of the most studied characteristics of Leishmania EVs is their effect on cytokine production in host immune cells. For instance, L. donovani vesicles induce IFN‐γ secretion in human monocytes and increase immunosuppressive cytokine production by murine CD4+ lymphocytes (Silverman et al., 2010b). The findings to date all support the important roles of Leishmania EVs to trigger major immunomodulatory actions in favor of facilitating the infection process and survival of the parasites in their hosts, as recently reviewed (Torrecilhas et al., 2020).
1.3. Apicomplexa
1.3.1. Toxoplasma gondii
Toxoplasma gondii is an apicomplexan parasitic protozoan, the causative agent of toxoplasmosis that is widely distributed (Ybanez et al., 2020). T. gondii is one of the best adapted parasites, being able to infect numerous species of animals and different types of cells. These parasites can persist for long periods in their hosts, probably for a lifetime. The course of the infection and pathogenicity depends on the doses of inoculated parasites, parasite genetic background, host genetics and immunological status (Habegger de Sorrentino et al., 2005; Hunter & Sibley, 2012). T. gondii life cycle comprises definitive hosts represented by members of the Felidae family, including domestic cats (de Barros et al., 2022). They are infected when ingesting meat containing tissue cysts or tachyzoites. Felids shed with feces the environmentally resistant oocysts produced after T. gondii sexual cycle. Once in contact with the atmosphere, the oocysts sporulate to form sporozoites, which infective other definitive or intermediate hosts (Dubey, 1998, 2008). Human and animal infections are mostly acquired by ingesting food or water contaminated with sporulated oocysts, undercooked meat infected with latent cysts and via fecal matter (de Barros et al., 2022; Montoya & Liesenfeld, 2004).
Toxoplasmosis prevalence in humans is highly variable since it is influenced by socioeconomic and environmental conditions, as well as cultural habits (de Barros et al., 2022; Mareze et al., 2019). Other ways of infection include congenital transmission (during pregnancy); ingestion of tissue cysts from infected animal tissues; ingestion of oocysts from contaminated water, soil or food; blood transfusion and organ transplantation (Dubey et al., 2021; Montoya & Liesenfeld, 2004). The infection is normally asymptomatic or subclinical; however, primary infection during pregnancy can cause damage to the fetus and even produce abortion. In addition, primary infection, or recrudescence of parasitemia in immunocompromised humans and animals lead to severe neurological and ocular clinical signs, causing significant impacts on public health and animal production (Dubey, 2008; Pereira‐Chioccola et al., 2009).
T. gondii antigen secretion is essential to stimulate the T‐ and B‐cell responses to develop lifelong protective immunity against reinfection (Carruthers, 2002; Dubey et al., 2012; Hill et al., 2005). Most of these antigens are excretory/secretory antigens (ESA) that have been shown as important components in the invasion and replication of T. gondii within host cells. This process is rapid and dynamic and relies on the secretion of numerous secretory proteins from micronemes, rhoptries, and dense granules. ESA plays an important role in toxoplasmosis pathogenesis since they are highly immunogenic (Carruthers, 2002; Meira et al., 2008). The number of studies of EVs produced by T. gondii has increased in recent years, and several protocols have been used to produce and characterize their EVs. As T. gondii is an obligate intracellular parasite, the choice of tissue‐culture lineage to be used is a very important parameter to consider in obtaining and evaluating EVs from this parasite.
1.3.2. Plasmodium spp
Malaria, caused by the genus Plasmodium belonging to the phylum Apicomplexa, is one of the most debilitating and life‐threatening infectious diseases. In fact, almost half of the world's population is exposed to the risk of malaria infection. The most recent World Health Organization (WHO) malaria report estimated 247 million cases in 84 malaria endemic countries and 619,000 deaths in 2021 (WHO 2022; 2015, World malaria report 2022). From the nearly 120 Plasmodium species that infect mammals, birds and reptiles, only five species can infect humans: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae and Plasmodium knowlesi (Graham, 1966; Singh et al., 2004). Among them, P. falciparum is known to be the most prevalent and virulent malaria parasite (Weiss et al., 2019), followed by P. vivax, which was responsible for 3.3% of all estimated cases 7 million clinical cases in 2019 (Battle et al., 2019).
The human malaria life cycle is a complex process that comprehends different stages of the parasite in several cells and tissues between the two hosts, human and Anopheles mosquito (responsible for the transmission). The ability to sense extracellular signals and to coordinate with other cells is especially important for pathogens, as they constantly face the hostile environment of their host. Indeed, EVs play a crucial role in the complex life cycle of many parasites, including malaria parasites.
In P. falciparum (Pf), the deadliest malaria‐causing species in humans, several studies concerning the role of Pf ‐derived EVs have shown that they are involved in diverse processes during the life cycle of the parasite (Mantel et al., 2016, 2013; Regev‐Rudzki et al., 2013; Sisquella et al., 2017), targeted at different host cells. One of the initial studies, showed that circulating Pf‐derived EVs were correlated with cerebral malaria disease severity (Nantakomol et al., 2011). On the other hand, EVs released to the conditioned medium by infected red blood cells (iRBCs) are transferred between iRBCs promoting sexual differentiation and providing the parasite with an escape route from the hostile environment of their host to the mosquito vector (Mantel et al., 2013; Regev‐Rudzki et al., 2013). Furthermore, it was shown that Pf‐derived EVs are involved in altering host cells; the functional 20S proteasome cargo, for example, was found to modulate the mechanical properties of human RBCs, by altering membrane stiffness, thus priming naïve host RBCs for parasite invasion (Dekel et al., 2021). EVs also target the immune (Mantel et al., 2013; Sisquella et al., 2017; Ye et al., 2018) and endothelial cells (Mantel et al., 2016), both of which play an important role in malaria pathogenesis. Pf genomic DNA present in EVs leads to the activation of a Type‐I interferon response in recipient monocytes (Sisquella et al., 2017), while human microRNAs packed inside the EVs are involved in promoting endothelial activation, leakage and parasite sequestration (Mantel et al., 2016).
In P. vivax, as there is still no in vitro culture, most of the studies have been done using plasma samples from infected patients. A pioneer field study in Brazil showed the indirect association of circulating platelet MVs and disease severity during vivax infections (Campos et al., 2010). A year later, a field study in Thailand investigating RBC‐derived MVs in P. falciparum, P. vivax and P. malariae infections, confirmed increase levels of MVs during infections and its indirect association with severity (Nantakomol et al., 2011).
Only recently, a proteomic study has also demonstrated the presence of P. vivax proteins associated to plasma‐derived EVs from vivax malaria patients isolated by size‐exclusion chromatography (SEC) (Toda et al., 2020). Of notice, the signal and number of proteins have been increased when direct immuno‐affinity capture (DIC) technique, using anti‐CD71antibody, have been implemented to enrich circulating EVs derived from plasma of P. vivax infected patients (Aparici‐Herraiz et al., 2021). These studies represent a significant advance in identifying new antigens for vaccination against P. vivax malaria parasite. Moreover, using a P. vivax infected liver‐humanized mouse model, it has been identified parasite proteins in circulating EVs, provided a new insight into the research of biomarkers for vivax liver infection (Gualdron‐Lopez et al., 2021).
The physiological role of EVs during vivax infections has remained unexplored until very recently. Toda et al., have demonstrated that plasma‐derived EVs isolated from P. vivax patients (PvEVs) interact with human spleen fibroblasts, inducing expression of ICAM‐1 via NF‐kB nuclear translocation and facilitating parasite cytoadhesion (Toda et al., 2020). Another recent study has employed PvEVs to analyze through in vitro studies, the interaction between human spleen cells and plasma derived PvEVs. The results showed an increased proportion of T cells, monocytes, B cells and erythrocytes interacting with PvEVs as compared to plasma‐derived EVs from healthy donors (hEVs) (Gualdron‐Lopez et al., 2021). Together, these data provide novel EVs‐based insights into the mechanisms of P. vivax pathology and support the existence of cytoadherence of P. vivax in the spleen.
1.4. Pathogenic ameboid protists
The Ameboid protist gathers a broad group of protozoa with few species considered human pathogens. Among these, only the intestinal amoebae Entamoeba histolytica and E. dispar are considered sensu stricto parasites. Both shares the same morphology, but only the former can invade intestinal mucosa. Other pathogenic amoebae are Naegleria fowleri, Balamuthia mandrillaris and Acanthamoeba spp., which are primarily free‐living organisms that occasionally cause infections in humans. Currently, E. histolytica, Acanthamoeba and Naegleria have been investigated on EVs topics, but the field is still in its infancy considering the low number of publications reported so far.
1.4.1. Entamoeba histolytica
Entamoeba histolytica is the etiologic agent of amoebiasis, an infection whose invasive forms may result in severe dysentery or extra‐intestinal disease. Amoebiasis still persists as an endemic health problem in developing countries, causing 40,000–100,000 deaths per year worldwide (Carrero et al., 2020). The life cycle of E. histolytica includes the cystic infective stages excreted with feces, and the vegetative trophozoites, which proliferate in the colon and can invade the mucosa and submucosa, initiating the pathogenic process (Betanzos et al., 2018). The first step in this process is the attachment to colonic cells mediated by adhesins and lectins, as the galactose‐and N‐acetyl‐d‐galactosamine (Gal/GalNAc) and 220 kDa lectins and 112 kDa adhesin (Aguirre Garcia et al., 2015). Cytolysis, phagocytosis and degradation of ingested cells follow on with the participation of other components such as cysteine proteases and pore‐forming peptides (Betanzos et al., 2019).
1.4.2. Acanthamoeba spp
The free‐living amoebas of the genus Acanthamoeba are involved in a corneal infection termed Acanthamoeba keratitis (AK), which the contact lens wears as the main risk group. The protozoan can also cause granulomatous amoebic encephalitis (GAE), a highly lethal disease that affects predominantly immunocompromised patients (Khan, 2006). AK is usually characterized by eye redness, tearing, photophobia, epithelial alterations, corneal opacity and stromal infiltration. The infection can evolve to blindness if not treated (Khan, 2006). Amoebas reach the cornea by contact lens or other objects that accidentally injure the tissue. Amoebic adhesion in the cornea is favored by microtrauma as those naturally caused by the lens or by other external causes. These events upregulate the expression of host cells mannosylated glycoproteins, which are not only recognized by mannose‐binding receptors on the trophozoite membrane, initiating the amoeba‐host cell interaction, but also stimulate the release of cytopathic‐factors as a 133 kDa mannose‐induced protease (MIP 133) and other proteases (Neelam & Niederkorn, 2017). In GAE, amoebas use skin wounds or the respiratory treat as the main entry routes, and hematogenous dissemination lead them to the brain. Trespassing of the blood‐brain barrier also involves amoebic mannose‐binding proteins, proteases and other hydrolytic enzymes, which together host immune response elements, resulting in the typical granulomatous lesions (Siddiqui et al., 2011). The disease progresses with neurological symptoms simulating bacterial or viral meningitis, and death occurs in 1 or 2 months (Kalra et al., 2020).
1.4.3. Naegleria fowleri
The amoeboflagellate Naegleria fowleri is the causative agent of primary amoebic meningoencephalitis (PAM), a fulminant disease with lethality over to 95% (Jahangeer et al., 2020). Differently from encephalitis by Acanthamoeba, N. fowleri infection has no association with immunocompromise (Visvesvara et al., 2007). As a thermophilic species, N. fowleri supports temperatures up to 45°C, proliferating preferentially in warm water. In most cases, patients affected by PAM had a history of recreative activities in water, which propitiate the contact with amoebas with the nasal mucosa. The protozoan reaches the olfactory neuroepithelium and progresses through the cribriform plate, penetrating the brain parenchyma. N. fowleri trophozoites engulf host cells using food‐cup structures or amoebostomes, and along with other cytopathic factors such as phospholipases, neuraminidase, metalloproteases and perforin‐like proteins, determine tissue damage and a picture of hemorrhagic meningoencephalitis (Jahangeer et al., 2020; Visvesvara et al., 2007). The initial symptoms are like bacterial meningoencephalitis, with an onset of headaches, fever, nuchal rigidity, nausea and vomiting, followed by other neurological symptoms, which usually evolve to death in 3–7 days if not treated promptly (Jahangeer et al., 2020; Visvesvara et al., 2007).
1.5. Non‐trypanosomatid flagellates
1.5.1. Giardia intestinalis
The flagellate protozoan Giardia intestinalis (syn. G. lamblia, G. duodenalis) is a globally distributed intestinal pathogen that causes diarrhea and can affect above 30% of the population in developing countries. The infective stages, the cysts, can contaminate water and food when excreted with the feces of from both humans and other animals. Therefore, giardiasis is considered a waterborne, foodborne and zoonotic infection, with several outbreaks reported worldwide (Feng & Xiao, 2011; Leung et al., 2019). The ingested cysts hatch on the duodenum releasing an intermediary forms (exozoites) that divides and originate pear‐shaped, binucleate, octa‐flagellated trophozoites, in a process stimulated by the low pH of the stomach and a subsequent exposition to bile salts and trypsin in the small intestine (Ankarklev et al., 2010). Trophozoites adhere to the intestinal microvilli using a typical suctorial ventral disc, causing epithelial lesions that result in diarrhea associated or not to malabsorption syndrome in symptomatic patients (Ankarklev et al., 2010). The inverse process, the encystation, involves the detachment of trophozoites from epithelia, cell rounding and cyst wall production (Einarsson et al., 2016).
The knowledge about the formation of vesicular structures by Giardia dates to the 80s and 90s, with the description of peripheral vesicles (PVs) and encystation‐specific vesicles (ESVs) (Feely & Dyer, 1987; Reiner et al., 1990). The formers, associated with nutrient uptake, underlie the plasma membrane on the dorsal surface and in some regions of the trophozoite suctorial disc, representing an endosomal‐lysosomal complex concentrated in a single system (Lanfredi‐Rangel et al., 1998). ESVs emerge as soon as the encystation stimulus occurs and are later externalized in the plasma membrane, releasing proteins to make up the cystic wall (Reiner et al., 1990). ESVs externalization occurs through an incomplete process of exocytosis, in which the membranous segments are disrupted but can reseal, forming empty vesicles (Nievas et al., 2018).
Although PVs and ESVs were previously known to be involved in endocytic pathways, they do not typically represent shed EVs. However, recent findings indicated that PVs exhibit characteristics of the multivesicular bodies (MVBs) (Midlej et al., 2019), which in turn are involved in the biogenesis of exosomes (Théry et al., 2009, 2002, 2018). Consistent with these findings, more recently, Grajeda et al. (Grajeda et al., 2022) demonstrated that G. lamblia trophozoites release two types of bona fide EVs: exosomal‐like nanoparticles (<100 nm), named small vesicles (SVs), and microvesicle‐like nanoparticles, named large vesicles (LVs, 100–400 nm). Proteomic analysis of the giardial SVs and LVs revealed they were enriched in potential virulence factors. Treatment of trophozoite forms with lipid‐raft disruptors, such as nystatin and oseltamivir, led to considerable changes (mostly downregulation) in the differential expression of virulence factors in the giardial SVs in comparison with control parasites. Finally, treatment of C57BL/6 mice with oseltamivir resulted in a significant reduction of parasite burden, suggesting a role of secreted giardial EVs in the establishment and dissemination of the infection.
1.5.2. Trichomonas vaginalis
Considered the most common non‐viral agent of sexually transmitted infections, Trichomonas vaginalis is a flagellated protozoan that inhabits the urogenital tract of men and women. Although a treatable infection, trichomoniasis has an estimated of 276.4 million cases per year worldwide (World Health Organization, 2012). It causes vaginitis and cervicitis in the female genital tract, while in males the infection tends to be asymptomatic or cause mild symptoms (Ryan et al., 2011). The infection has been also associated to the acquisition of other health conditions such as cervical and prostate cancer, infertility, prematurity in pregnancy and HIV infection (Bala & Chhonker, 2018).
Trophozoites are the only stage in the life cycle, presenting and ovoid shape, with four anterior flagella, a single posterior flagellum forming an undulant membrane and an axostyl passing through the cell until the extracellular environment (Edwards et al., 2016). During the contact with the urogenital area, the parasite assumes an ameboid shape, interacting with epithelial cells through primary cytoadherence mediators as surface lipophosphoglycan, adhesins and glyceralde‐hyde‐3‐phosphate dehydrogenase (GAPDH). The process results in inflammatory response with neutrophilic infiltration, which contributes to damage the epithelia, while other released factors as CP30 cysteine proteases and polyamine compounds are involved in epithelial cell cytotoxicity and apoptosis (Edwards et al., 2016).
1.6. Helminths
Helminthiases are parasitic diseases with a high prevalence. It has been reported that at least 1.5 billion people worldwide, which represents at least one‐sixth of the estimated human population (8 billion, as for November 2022), are infected by parasitic helminths (Hotez et al., 2008; WHO, 2020). The most common helminthiases are caused by infection with intestinal helminths (i.e., ascariasis, trichuriasis and diseases caused by hookworms), followed by schistosomiasis and filariasis (Hotez et al., 2008). These diseases can produce mild to severe symptoms, and at times can be deadly.
Helminths include flatworms (Trematodes and Cestodes) and roundworms (Nematodes), which exhibit great diversity in their biology and life cycles, including different intermediate and definitive hosts. Of the many species that exist in these groups, about half are parasitic and some are important human pathogens. Helminths establish long‐term infections presumably by modulating the host immune response for survival, releasing excretory‐secretory products containing EVs (Drurey & Maizels, 2021).
The first description of “small membrane‐limited vesicles” by helminths dates to the 1960s, where some of these structures were observed by TEM in Fasciola hepatica and Schistosoma mansoni (Threadgold, 1968). In 1989, Andresen and collaborators coined the term ‘extracellular vesicles’ to refer to structures apparently secreted by Echinostoma caproni (Andresen et al., 1989). However, it was not until 2012 when Marcilla and co‐workers isolated and characterized these vesicles following protocols developed for mammalian EVs, which include ultracentrifugation, TEM, immunogold labelling and proteomics, identifying an important mechanism for protein transport in trematodes (Marcilla et al., 2012). Later, the same group also identified, for the first time, the presence of miRNA in helminth EVs (Bernal et al., 2014). Since then, EVs have been characterized from many species of parasitic helminths, and considerable evidence supports important functions for their cargo (proteins and miRNA) in host‐parasite relationships (Drurey & Maizels, 2021; Sanchez‐Lopez et al., 2021; Sotillo et al., 2020; Tritten & Geary, 2018). A very recent review has outlined best practices for those investigating the biology of helminth derived EVs to complement the MISEV guidelines (White et al., 2023).
2. SPECIFIC METHODOLOGIES FOR ISOLATION, CHARACTERIZATION, AND PURIFICATION OF EVS FROM PARASITES
2.1. Protocols to obtain the EVs isolated from parasites
2.1.1. Species, parasites forms, sample types and experimental conditions
Different protocols are available for the isolation, characterization and purification of EVs from nonpathogenic and pathogenic parasites. Therefore, it is crucial to report all experimental procedures to maximize the number of known and reportable parameters, allowing for their standardization and comparison (Théry et al., 2018). Different life‐cycle stages of parasites release EVs in many other hosts. The protocols to isolate and characterize EVs should be efficient, rapid and reproducible. The gold standard protocol should be able to enrich the EVs and avoid contamination with non‐vesicle components, such as soluble molecules released by the parasite and host cells, fetal bovine serum proteins, and host biofluid‐derived proteins. Many techniques are used to isolate and purify EVs from isolated parasites, biological fluids and in vitro infected cell cultures. These techniques include differential centrifugation/ultracentrifugation, affinity‐based capturing (e.g., antibody‐immobilized magnetic beads or resins), ultrafiltration (UF), size‐exclusion chromatography (SEC) and asymmetrical flow field‐flow fractionation (AF4) of EVs. The literature is incipient in standardized methods and protocols by which highly enriched or purified EVs can be obtained from pathogens. The great challenge is the complex nature of EVs and their various biochemical and biophysical properties, which should be considered for their use in research and clinical applications, for example, as biomarkers of diseases. The general techniques for obtaining and characterizing EVs released by parasites are described below.
2.1.2. Isolation and purification of EVs
Isolation and purification of parasites EVs bear the same considerations as those for collecting. EVs from other eukaryotic sources such as cell‐ and tissue‐culture samples and human and experimental animal specimens (Torrecilhas et al., 2012, 2020). One significant advantage of working with parasites is that they can often be cultured in large quantities in the laboratory; therefore, EV collection can be scaled up more easily. However, there is also a significant challenge in isolating EVs from field strains of protozoan parasites and some helminths that are difficult to grow using laboratory conditions. Furthermore, the quantity of EVs released by different parasite species, strains and/or life‐cycle stages can vary greatly, limiting the available number of isolation protocols that can be used for low‐abundance and/or low‐producing EV parasites (and their strains and developmental forms) isolated from invertebrate and vertebrate hosts. Parasite viability must also be considered when using a culture medium with little or no exogenous amino acids, proteins, growth factors, carbohydrates, lipids and other components required for cell growth and maintenance to collect EVs.
2.1.3. Centrifugation and ultracentrifugation
Centrifugation (C), ultracentrifugation (UC) and differential ultracentrifugation (DC) are the primary methods used by many research groups in the field of parasite EVs. The methodology is simple and the first choice for collecting and enriching EVs from parasites. These protocols have many steps until the enrichment or purification of parasite particles. The first step is eliminating debris from parasite‐infected and uninfected cells, considering that usually not all host cells are infected; even if they are, the process is often asynchronous. Initial low‐speed spin centrifugation is recommended for EV samples from parasites and/or infected cells, depending on the type of organism. In the case of helminths, this step is essential to either remove or collect the eggs released by the parasite during the incubation (removal of eggs from helminths, 400 x g for 15 min). The next step is to remove the larger‐size vesicles and additional debris (from infected and uninfected cells and parasites) from the supernatant. The final high‐speed spin is applied at 100,000–120,000 × g for 2–24 h to precipitate all EVs, including small‐ and large‐particle sizes from pathogenic parasites. One more step, additional centrifugation at 10,000 × g for 3 h, can be added after the initial debris removal, which allows for the recovery of large vesicles (Baek et al., 2016; Sidik et al., 2016). This additional step increases the enrichment of the isolated microparticles (MPs) or microvesicles (MVs) but does not reduce the isolation time. Some purification protocols based on differential centrifugation are coupled to membrane filtration to eliminate large contaminants from parasites before the ultracentrifugation step. In our experience centrifugation protocols of different organisms should be checked to avoid contamination with soluble glycoproteins, lipoproteins, nucleic acids and other ‘sticky’ or charged macromolecules (e.g., host‐derived ribosomal proteins, histones and proteoglycans, and parasite‐derived glycolipoconjugates and polysaccharides) that may bind nonspecifically and/or specifically to the MPs/MVs. Therefore, to improve the purification of MPs/MVs from parasites, it is necessary to use a further step of sucrose‐gradient centrifugation. However, it demands (i) availability of ultracentrifuge; (ii) technical expertise; (iii) additional purification steps and, thus, potential EV loss; (iv) contamination with soluble molecules. Alternatively, it is possible to perform SEC or iodixanol gradient, which forms isosmotic solutions and provides preservation of EVs (Kuipers et al., 2022).
2.1.4. Size‐exclusion chromatography (SEC)
The SEC principle is protein separation and fractionation of particles according to size. The column used in SEC contains a stationary matrix made of Sepharose. Inside the column, big particles elute faster than smaller ones, which are slowed down by entering the pores of the polymer. Over the last years, SEC technique has gained popularity in the field of EVs, the advantage of this procedure is reproducibility, speed and simplicity to perform, mainly with small volumes (1 mL with 108–109 particles/mL) of EVs samples obtained from supernatants of parasites, infected cell cultures or fluids isolated from patients. In addition, it maintains the particle properties and integrity (Bayer‐Santos et al., 2013; Cortes‐Serra et al., 2022, 2020; Ribeiro et al., 2018; Trocoli Torrecilhas et al., 2009). Usually, EVs are larger than soluble contaminants from culture media, or body fluids such as serum components. It can also fractionate different EVs populations that can be further recognized by the presence of specific antigens (Ribeiro et al., 2018; Trocoli Torrecilhas et al., 2009). In malaria, has been recently used to purified EVs directly from plasma of P. vivax‐infected patients or from liver‐humanized mouse models (Gualdron‐Lopez et al., 2022, 2018; Toda et al., 2020).
It is also possible to use immunocapture methods with specific antibodies, for example, recognizing specific parasite molecules (Atayde et al., 2019a, 2016; Dong et al., 2021; Nogueira et al., 2015; Ramirez et al., 2018; Ribeiro et al., 2018). For example, T. cruzi EVs is enriched with molecules modified by α‐galactosyl residues that are recognized by specific antibodies developed by Chagasic patients. Alternatively, fractions can be characterized by proteomic techniques (Bayer‐Santos et al., 2013; Ribeiro et al., 2018), which allows detection of additional components that can be present either in the lumen or in the membrane of EVs after additional fractionation.
2.1.5. Ultrafiltration (UF)
The UF techniques using membranes that allow passage of proteins with sizes smaller than EVs is also an efficient method to concentrate and purify MPs particles from parasites. It uses membranes with different pore sizes attached to centrifuge cartridges. Microparticles larger than the pore size (30–100 kDa) is retained with the passage of small components and soluble proteins. Therefore, it can be employed to obtain different populations of EVs. It does not remove non‐vesicle aggregates or large proteins like the previous methods. The UF method can be combined with SEC or ultracentrifugation to generate highly enriched or purified EVs from parasites or infected host cells (Ofir‐Birin et al., 2021).
2.1.6. Asymmetrical flow field‐flow fractionation (AF4)
The AF4 method fractionates EVs from parasites and other cells based on their hydrodynamic sizes (Abou Karam et al., 2022; Zhang & Lyden, 2019). The loading sample containing EVs collected from parasites is injected into a small chamber with two semipermeable membranes. The separation occurs through the interaction of the EVs with two distinct flows (a cross flow and a parabolic flow) (Zhang & Lyden, 2019). The AF4 method is an efficient method to recover EVs, and it is reproducible and preserves the membrane integrity. Since there is no unspecific membrane adherence in the AF4, a lower loss of EVs than in the SEC or UF approach is observed. Nevertheless, it is vital to start with a partially purified total population of EVs, previously isolated by other procedures (i.e., SEC, UC or UF), to guarantee a longer life of the membrane of the AF4 equipment.
2.1.7. Affinity capture of EVs
Immobilized molecules that bind to known components of the EVs surface can be additionally employed to isolate and/or purify particular populations of EVs. It is recommended to use immune‐capture methods with specific antibodies to parasite ligands (Atayde et al., 2015, 2019b; Dong et al., 2021; Nogueira et al., 2015; Ribeiro et al., 2018). However, this depends on the availability of specific antibodies and known parasite ligands, which are only sometimes possible when working with parasites. The antibody or ligand is covalently coupled to a resin (i.e., agarose, Sepharose, etc.) or magnetic beads. The EV sample is then incubated with the immobilized antibody or ligand. The resin or magnetic beads is/are extensively washed with a physiological neutral buffer (e.g., PBS, Hepes) to remove non‐specific interactors. Finally, the resin/magnetic beads is/are eluted with a mild acidic solution (e.g., 50 mM citric acid, pH 2.8). This approach has been recently adapted to purify EVs derived from P. vivax‐infected reticulocytes using the transferrin receptor (CD71) as one of the primary surface markers presented in reticulocyte‐derived EVs (Aparici‐Herraiz et al., 2021). This method can be adapted to small sample volumes and complex biological fluids. EV populations devoid of the specific ligand are found in the unbound fractions and further purified by the previously described methods.
2.1.8. EVs derived from in vitro infection of host cells
The host cells, such as macrophages, red blood cells, and epithelial and endothelial cells infected with parasites, can release EVs containing molecules originating from the host and/or parasites (Cronemberger‐Andrade et al., 2020; Diaz‐Varela et al., 2018; Hassani & Olivier, 2013) that can stimulate other host cells of the immune system (Cronemberger‐Andrade et al., 2014). For example, considering Leishmania spp., T. cruzi and Plasmodium are intracellular parasites, studying these EVs is essential to better understand the pathogenesis of protozoan parasites. However, the maintenance of these cultures and the obtention of these EVs must be performed very carefully. Host cells must be cultured with medium‐free vesicles to avoid artifacts and/or exogenous EVs (e.g., derived from fetal bovine serum or AB serum). It is critical to assess whether commercial EV‐depleted FBS or human serum will be prepared in‐house. These sera can be obtained after an ultracentrifugation process to remove the EVs, considering the methods established by MISEV 2018 (Théry et al., 2018). Other factors that should also be considered are cell viability, the absence of microbial contamination (including routine mycoplasma testing), confluence and cell passage for immortalized cells (Ramirez et al., 2018).
When isolating EVs from cultured parasites, it is essential to use fetal serum free of vesicles or serum‐free medium. It is also necessary to check for the parasite viability to avoid membrane fragments derived from damaged cells and employ homogeneous cultures containing a determined life cycle stage to prevent secondary contamination. In the case of parasites directly derived from host cells, washing the parasites is necessary to ensure that host EVs are not present. Moreover, cells in culture must be PBS‐washed after infection to remove non‐internalized parasites that can release vesicles into the extracellular medium and contaminate the released parasites. On the other hand, EVs can be isolated from parasite‐infected cells. This can be achieved by several methodologies, as described above. In this case, it is crucial to avoid the presence of extracellular parasites. These methodologies have advantages and disadvantages and must be used and chosen depending on the experimental model, the kind of EVs, and the intended functional studies.
2.1.9. EVs derived from in vitro protozoan cultures
Considering the possibility that some parasites can grow independently of host cells, two main approaches can be envisioned to obtain EVs. The first and more commonly used involves the discontinuous growth of the parasites. This approach requires the culture of the parasite to the desired growth phase in a standard medium and then performing transient sub‐cultivation of the parasites for a defined time frame in a minimum media and recovering the minimum spent media for the analysis. Another possibility is a continuous approach in which the parasites are grown in a defined media (or cultivated with an FBS‐depleted medium or human AB‐blood type serum). Then the spent medium is recovered for analysis. The discontinuous approach requires washing the parasites to remove any medium contaminants, cellular debris, and naturally produced EVs during the parasite's growth. A continuous approach maintains the parasite in the medium of interest without change. The discontinuous approach is the most employed. It has been used with success to evaluate the effect of temperature (26°C–37°C) and pH in EVs production, to compare different species, strains and mutants, and different stages of growth (Gomez & Olivier, 2010; Gomez et al., 2009; Hassani & Olivier, 2013; Hassani et al., 2014; Nogueira et al., 2015; Paranaiba et al., 2019; Silverman & Reiner, 2011; Silverman et al., 2010b). This approach is the most adequate for evaluating de novo EV production in specific conditions. Still, the most significant limitation to this approach is that the parasites will respond to the environment change (pH, osmolality) associated with the washes and the new media adaptation, leading to a stress response that might cause a specific EV profile. The temperature increases, pH decreases and drug‐induced stress can lead to the rise of EVs production, suggesting that EVs release is an environmentally coordinated process (Hassani et al., 2011; Perez‐Cabezas et al., 2019). Consequently, different EV populations might be under or overrepresented during the media change. There are already reports that suggest the existence of distinct vesicle populations with varying sizes after flotation in a sucrose gradient in L. infantum (Perez‐Cabezas et al., 2019). Further studies to address the relevance of these populations are required. A continuous approach presents different limitations and advantages. The main advantage is that the EVs produced are representative of the parasite's expected growth, enabling more informed stage‐specific comparisons without the shock of media change. The main caveat in this approach is that debris from dead parasites will interfere with the analysis depending on the cultivation time. In 2013, a study compared the effect of the two approaches on EVs recovery using logarithmic and stationary L. infantum (Esteves et al., 2022; Santarém et al., 2013). Significantly, most differences were not qualitative but quantitative, with very different relative proteomic compositions. This fact, compiled with the possibility of distinct EV populations existing, must be considered for the experimental setup avoiding a ‘one approach fits all question’. Considering this, several aspects related to the recovery should be reported, the medium used before and during collection, the specific culture conditions, flask size, the volume of medium per flask, time of culture, initial inoculum, number of passages of the parasites and elapsed time of washes, the interval before conditioned media harvest. Accurately defining all these parameters is essential for establishing a solid scientific baseline and better interpreting the generated data. The recovery process from conditioned media is simple and not much different than for other approaches; if the conditioned medium does not contain FBS or is fully defined, the samples recovered will be purer and easier to purify. Standard ultracentrifugation/ultrafiltration or SEC can be used to obtain EV preparations. Complementary approaches like that must always address the purity of the preparations and will be further characterized by protein quantification methods, bead‐based assay, NTA, western blot and TEM. The purity and quality of the preparations must always be accessed even if using established protocols. Finally, it is important to consider whether the in vitro conditions represent the natural environment of the parasite.
2.1.10. EVs derived from animal models
Rodent models, mouse, and hamster models specifically, are commonly used to study parasite infection in vivo (Dupin et al., 2021; Torrecilhas et al., 2012, 2020; Trocoli Torrecilhas et al., 2009). Both host cells and host EVs can be collected from different biological fluids following distinct routes of infection to assess host immune response to parasite infection. This approach has some limitations. For example, in the case of Leishmaniasis, host cells from rodent spleens, lymph nodes and lesions can be collected when using a dermis infection model, but the isolation of EVs from such models has so far been unsuccessful. In these cases, it is more practical to collect and culture immune cells from these tissues and perform an ex vivo infection with the parasite. The collection of parasite EVs from in vivo infection models is also very challenging, as it remains practically impossible to collect parasite EVs in significant quantities compared to host EVs and the methods to separate parasite EVs from host EVs are also currently out of reach. To Leishmania parasites, efforts have been made to identify specific protozoa parasite EV biomarkers, but such results are still untested in terms of practical use (Torrecilhas et al., 2020). Once such biomarkers are confidently identified and tested, high resolution flow cytometry will prove invaluable to investigating the action of protozoa parasite EVs within the animal model.
The identification of biomarkers along with a greater understanding of parasite EV biogenesis can also be used to tag parasite EVs, providing an alternative way to track parasite EVs within the host. One confounding factor is that host macrophage EVs often express parasite proteins once infected, which can obscure such methods. Another example consists of CD, in which one of the in vivo models consists of intraperitoneally infecting of mice. In the acute phase, the blood of infected animals contains parasites that can be used to recover parasites or blood extracellular vesicles. These materials can be used to understand how EVs participate in the interaction with the mammalian host and if they can act in the promotion and/or progression of the disease (Trocoli Torrecilhas et al., 2009). Otherwise, if no parasites can be recovered after the resolution of the acute infection, the presence of EVs in blood becomes an experimental challenge. One of the major remaining questions is the actual role and presence of parasite released EVs in the hosts. Otherwise, it is accepted that infection induces the host to release EVs, that might or might not contain parasite components. In most cases, this remains to be shown and sensitive methodologies to detect their presence should be established.
In the particular case of malaria infection, studies based on the use of reticulocyte‐prone malaria rodent models such as P. yoelii demonstrated the presence of parasite proteins in plasma‐derived EVs and showed that reticulocyte‐derived exosomes from infected mice protected immunized animals against lethal P. yoelii XL infections (Martin‐Jaular et al., 2011). Additionally, this protection was spleen‐dependent and involved CD8+ T cell mediated immune response (Martin‐Jaular et al., 2016). In these studies, EVs were purified directly from plasma of infected mice by differential centrifugation, followed by a density gradient in 30% sucrose. For some applications, EVs were also obtained from ex vivo parasite cultures enriched through a percoll gradient and maintained in EV‐depleted medium for 24 h. After this period, EVs were isolated following the same methodology described above.
Recently, studies involving liver‐humanized mouse models, which can sustain P. vivax infections, have enabled the study of PvEVs. Thus, using this liver humanized mouse model bearing P. vivax pre‐erythrocytic stages, parasite proteins were identified in EVs coming from plasma of these infected liver humanized mouse model, which represents new avenues for the research of biomarkers for vivax liver infection, including hypnozoites (Gualdron‐Lopez et al., 2022, 2018). In contrast to previous studies, EVs were purified by SEC using 300–500 μL of plasma from malaria‐infected liver‐chimeric humanized mice.
2.2. EV characterization
EVs are complex membranous structures that contain several biomolecules of interest such as lipids, nucleic acids, proteins and metabolites (Colombo et al., 2014; Théry et al., 2009; Torrecilhas et al., 2020). These molecules have been exploited in the context of several models in EVs research to generate information about biological functions and interactions and in the context of biomarker development (Madeira et al., 2021). EV characterization should include multiple, complementary techniques to assess the results of separation methods and to prove that biomarkers or functions are associated with EVs and no other co‐isolated materials (Cortes‐Serra et al., 2022; Madeira et al., 2022; Théry et al., 2018). The characterization of EVs can be performed considering the following aspects: quantification; general characterization, including electron microscopy, content (protein, lipid, metabolites, nucleic acid); at the level of the entire population or at in single EVs.
2.2.1. Quantification of EVs
As mentioned in MISEV 2018, both the source of EVs and the EV preparation must be described quantitatively. This includes descriptions of the parasite number/concentration, culture volume, culture media, experimental conditions, initial and final volume of EVs collected. EVs themselves can be further quantified based on the number of particles, their total protein and lipid amount and their DNA/RNA content. This quantification can be used to assess the purity of the EV sample by analyzing their components and ratios (Théry et al., 2018). It is also recommended to describe the time and the temperature of EVs storage before such analyses.
2.2.2. Particle quantity and size
Parasite EVs are most quantified using nanoparticle tracking analysis (NTA), a light‐based technique that calculates vesicle quantity and size using Brownian motion (Vucetic et al., 2020). NTA is also compatible with fluorescent tags, allowing identification of EV subpopulations based on specific biomarkers (Thane et al., 2019). Tunable resistive pulse sensing (TRPS) is an alternative to NTA that boasts higher dynamic range, using a voltage‐gated pinhole to physically count passing particles (Blundell et al., 2015). However, it cannot be used to separate EVs subpopulations. High‐resolution flow cytometry using instruments specifically designed for nano‐sized can also be used in the context of parasites. However, there is a general lack of markers that can be confidently used to distinguish parasite EVs. Although TEM can be used to determine particle size, this is often labor‐intensive and heavily reliant on the expertise of the user. Additionally, visual artifacts resulting from sample preparation can negatively impact results (Koritzinsky et al., 2017).
2.2.3. Total protein and lipid amount
Protein concentration can be measured by any standard colorimetric assay, though assay specificity can be a major factor in choosing the type of assay. Samples can be lysed using detergent, though diluting EV samples in ultrapure water achieves sufficient EV lysis for an accurate measurement. It should be noted that, depending on the efficacy of sample washes, co‐contaminants from the culture media may be present, especially when only ultracentrifugation is used. Lipid quantification can be achieved using sulfovanilin assay, fluorescent dyes that incorporate into membrane bilayers or Fourier transform infrared spectroscopy, but such assays are still limited in scope and accessibility. They are most useful for determining and discriminating protein: lipid ratios (Skotland et al., 2019, 2020).
To demonstrate the presence of lipid bilayer, at least one transmembrane, or GPI‐anchored protein must be detected. For example, presence of known membrane proteins, indicates a membranous nature of the particle (Atayde et al., 2015, 2019a; Théry et al., 2018). To demonstrate that parasite EVs do not contain more than open cell fragments at least one cytoplasmic protein with lipid or membrane protein‐binding ability must also be shown (Théry et al., 2018). Cytoplasmic proteins reported so far are heat shock protein (Marcilla et al., 2014, 2012), actin or tubulin (Silverman & Reiner, 2011), demonstrating that the EVs preparation enclose intracellular material. These proteins, which are associated to the membrane must be released after particles solubilization by detergents. Purity controls are needed and include proteins found in most common co‐isolated contaminants, for example, HGPRT, apolipoproteins and albumin (Atayde et al., 2019b). Non‐protein markers, for example, phospholipids, RNA, DNA (with strictly nuclear RNA or DNA as negative control) (Mu et al., 2021) can be used to better characterize the parasite EVs.
2.2.4. Proteomics
Most research related to the systematic identification of biomolecules in parasite EVs is made by proteomic characterization. EVs can carry known virulence factors (Douanne et al., 2020; Ribeiro et al., 2018), which are detected by proteomic studies and that might be enriched in more virulent parasites (Nogueira et al., 2015). Interestingly, although a generic conserved core set of proteins is consistently detected, further research is required to define a set of proteins that might be specific to an EVs subpopulation as a measure of EVs purity (Sotillo, 2022). Most proteomic approaches involve the extraction of proteins from the isolated EVs, the separation of the extracted proteins, and their digestion before MS analysis. Proteins will be usually extracted using a detergent or non‐detergent lysis buffer. Quantitative MS‐based on label and label‐free approaches can be used depending on the scientific question. Most concerns related to the generation of proteomics data sets are related to the EVs purification process and storage of the sample, thus it is essential to define, characterize and standardize the pre‐analytical conditions such as freezing and storage in comparative studies on EVs to obtain consistent data. Since parasites are made up of complex, heterogenic subpopulations, EVs' proteomic profiles can show significant fluctuations in both richness and diversity. For this reason, it is strongly recommended to consider the inclusion of several replicates per biological condition or many EVs proteomes to gain biological significance (Sotillo et al., 2020). On the other hand, it is important to guarantee that the peptides detected are derived from the EVs and not from free protein contaminating the preparation (e.g., from cell culture media). While not perfect, different ratios such as the number of particles per microgram of protein could help when assessing the amount of non‐EV derived free protein (Bayer‐Santos et al., 2013; Forrest et al., 2020; Nakayasu et al., 2012; Sotillo, 2022).
2.2.5. Western blot or enzyme‐linked immunosorbent assay (ELISA)
The most widely used methods to demonstrate the presence of a particular protein in EVs are Western blot and ELISA. In western blot, proteins are separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a membrane for further immunoblotting of specific EV‐related proteins, such as tetraspanins or specific EV‐parasite proteins (Toda et al., 2020). Similarly, in the ELISA technique, EV samples are tested using antibodies against classical EV markers. These techniques require a large sample volume, extensive processing and specialized instrumentation (Nogueira et al., 2015; Ribeiro et al., 2018; Trocoli Torrecilhas et al., 2009).
2.2.6. DNA/RNA characterization
The characterization of RNA content in EVs is a hot subject, considering the known capacity of exosomes to deliver their cargo and induce phenotypic changes in target recipient cells. In distinct protozoan parasites, the RNA molecules present in EVs have been characterized. In Leishmania, EVs were reported to contain mostly non‐coding RNAs, detected in two distinct species (Lambertz et al., 2015). In T. cruzi, the EVs also presented a variety of small RNAs derived from rRNA, tRNA, CDS, snoRNA, pseudogene and snRNA fragments (Fernandez‐Calero et al., 2015). In malaria, has been reported several studies demonstrating the presence of small non‐coding RNA in EVs (Babatunde et al., 2018; Mantel et al., 2016, 2013), as well as parasite gDNA (Sisquella et al., 2017). In T. vaginalis, tRNA‐derived fragments consisting of small RNAs were the most abundant RNAs present in EVs (Artuyants et al., 2020). The potential of RNAs to be involved in host‐pathogen interactions is real and further studies involving their characterization will clarify their possible roles in parasite biology, infection and disease. A crucial step to characterize the RNA content inside EVs is to perform DNAse and RNase treatment before RNA extraction to prevent the identification of molecules outside the EVs. The RNA can be extracted using a variety of kits commercially available kits. It is worth noting that as most of the molecules present in EVs are smaller than 200 nt, it is important to use a kit that includes this size of RNAs in the extraction. As the yield of RNA obtained can be very low (picograms), it is necessary to quantify the RNA by sensitive methods such as Qubit miRNA assay or a bioanalyzer high sensitivity kits. The most frequent downstream analysis of the EV‐RNA characterization is the qPCR or Next‐generation sequencing. Notably, the characterization of EV type‐specific RNA cargo remains highly challenging and strongly depends on the purification method (Cucher et al., 2021; Sotillo et al., 2020).
2.2.7. Lipidomics and metabolomics
Lipids are the primary components of EVs, though, the systematic evaluation of these components is limited. The knowledge obtained from lipidomic on parasite EVs can contribute to defining specific EV subsets and might also contribute to finding new biomarkers of infection and new therapeutic approaches. The main recommendations for lipidomics involve the accurate reporting of pre‐analytical parameters for proper interpretation of the results. So far, the impact of different pre‐analytical variables in the EV lipidome has not been thoroughly evaluated. The capacity of EVs with unique lipid composition to bind and induce specific molecules in immune cells demonstrates that lipids from EVs are relevant players in host‐pathogen interactions (Cucher et al., 2021; Sotillo et al., 2020).
Molecules with size below 2 kDa are considered biologically relevant metabolites (Williams et al., 2019). Metabolomics is the newest of the omics technologies and it has been fundamental in the discovery and validation of pathways in the parasitic protozoa. Unsurprisingly, its application to the study of EVs is continuously growing in relevance (Skotland et al., 2020; Williams et al., 2019). Interestingly, the metabolite systematic characterization can be applied alongside proteomics to evaluate functional enzymes in the EVs and address the metabolite composition of EVs under specific stimuli. The field of metabolomics contains the promise of delivering the most basic explanations for complex biological phenomena. In fact, research areas like immunometabolism are now of growing interest. Metabolomic studies of EVs are still limited and the impact of different pre‐analytical variables has not been evaluated (Skotland et al., 2020; Williams et al., 2019). Such accurate reporting is recommended for the establishment of solid foundations for this nascent field.
2.2.8. Electron microscopy
Parasites EVs are most imaged using TEM using a simple negative stain technique (Vucetic et al., 2020) or by cryo‐electron microscopy (cryo‐EM). EVs should demonstrate a double lipid bilayer. EVs derived from infected host cells can additionally be immunogold‐labeled. Parasite EVs can be labeled as well once parasite EV protein markers are confidently identified (Chuo et al., 2018). Protozoa parasites can be sliced and imaged by TEM to observe EV release at the cellular level (Atayde et al., 2019b). Most parasites, including, protozoa, can also be imaged by scanning electron microscopy (SEM) to observe EV release from the parasite surface (Nogueira et al., 2015; Ribeiro et al., 2018). The TEM images of the EVs show nanoscale resolution. The produced images can be further used for measuring particle size. TEM and cryo‐EM are techniques that can be used to characterize parasite releasing EVs, or EVs already isolated from parasite‐infected cells (Gualdron‐Lopez et al., 2018; Sisquella et al., 2017). TEM and cryo‐EM have a lot of advantages, sing these approaches, the morphology of the EVs can be kept intact maintaining the ultrastructure. The images from microscopy are the best approaches to verify spherical and lipid bilayer membranes of EVs.
2.2.9. Flow cytometry (FC)
Flow cytometry (FC) has been widely used for the characterization of EV surface markers and is one of the more frequently used techniques. Size and morphology are also measured through flow cytometry. Flow cytometers capture the scattering and fluorescence signal generated by individual particles when these are illuminated by a laser beam while passing through a nozzle. Unfortunately, most of the conventional flow cytometers cannot measure small particles since the detection limit is around 300 nm, they are within the electronic noise of the instrument (van der Pol et al., 2018). Thus, the most widely used solution is the coupling of EVs to beads (latex) of a bigger size that later will be recognized by antibodies fluorescently labelled against specific surface EV‐markers and further analyzed by the cytometer (Cortes‐Serra et al., 2020; Toda et al., 2020). In 2020 a guideline has been proposed by a MISEV group as the Minimum Information about a FC experiment (MIFlowCyt) to improve the capacity to analyze and interpreter FC data (Welsh et al., 2020). It describes standardization assays to support the development of new instruments for EV studies.
Recently, a new flow cytometer (Cytoflex from Beckman Coulter) with a higher sensitivity of forward scatter detection, fluorescent amplification, and high‐resolution imaging have been developed to distinguish stained exosomes from background contaminants (Brittain GC 4th et al., 2019). In addition, the Nano‐flow Cytometer (NanFCM) has been developed to detect particles from 7 to 1000 nm. Cytoflex detects particles below 300 nm, because of its increased sensitivity, which cannot be seen by conventional flow cytometry. The scattering provides information about the size of different populations of EVs, quantification and the expression of parasite molecules present on the surface of EVs isolated from the parasite, infected cells markers, cellular debris, parental cells and beads. Labelling with fluorescent molecules that intercalate in the lipid bilayer or fluorescent ligands can be used to increase specificity and sensibility of analysis. The NanoFCM also combines fluorescence detection and side scatter at high speed, which allows detect of several types of nanoparticles (Caputo et al., 2021).
An alternative to detect, fluorescently labelled sub‐micron size is to use Imaging Flow Cytometry (IFC) (Droste et al., 2021; Erdbrugger et al., 2014; Gorgens et al., 2019; Headland et al., 2014; Mastoridis et al., 2018; Ofir‐Birin et al., 2018; Tertel et al., 2020). IFC combines the information‐rich microscopy images with the high‐throughput quantification of flow cytometry. The most common imaging flow cytometer is ImageStream (Amnis Corp., part of Luminex, Seattle, WA). Cells pass, in a single file, through the flow cell, where they are illuminated by a set of LEDs and lasers. The emitted light passes through a set of lenses and is collected by a sensitive CCD camera in time‐delay integration (TDI) mode, which allows the acquisition of high‐resolution images at a rate of up to 5000 cells per second. Up to 12 channels can be acquired simultaneously, two bright‐field channels and up to 10 fluorescent channels, allowing the usage of most commercially available fluorophores. As triggering is done from all channels, an object can be detected based only on its fluorescence. This allows the detection of EVs, which are smaller than the detection limit in the bright‐field image. In addition, IFC allows the rapid, high‐throughput, uniform acquisition needed to record up to 100,000 objects within a few minutes. Since accurate quantification is challenging, inclusion of proper controls is essential for the analysis of EVs. First, unlabelled EVs should be run to determine the background levels. Additionally, as the number of events is dependent on the triggering of the instrument, unlabelled EVs will not necessarily appear on the bright‐field image, and without fluorescence, they will not be recorded as an event. For this reason, it is recommended to acquire the sample not according to event number but according to volume/time.
Another important control is to verify that the acquired objects are indeed EVs and not dye aggregates, which some dyes are known to create and are within the size range of EVs. To address this, a sample should be prepared in the same manner as the EVs but only with dye. Another way of distinguishing between dye aggregates or other debris and EVs is treatment with a detergent. This should eliminate the EV signal while dye aggregates will remain intact.
A common issue in EV detection in FC is the detection of several particles as one object, as EV particles are smaller than the laser beam (known as the swarm effect). To verify that indeed individual EVs are recorded, serial dilutions of EVs should be performed and analyzed. The percentage or number of positive events should decrease with each dilution, but the intensity should remain the same. If the intensity decreases as well, this will indicate that several EVs are detected simultaneously. To avoid this, the concentration should be decreased accordingly, until individual EVs are detected.
2.2.10. Atomic force microscopy imaging
In the amplitude modulation mode, the phase change of the cantilever oscillation is affected by the mechanical interactions between the tip and the sample, reflecting the combined outcome of various sample properties (i.e., elasticity, viscoelasticity, and adhesion). Therefore, the interpretation of the phase image is not (Zhong et al., 1993; Magonov et al., 1997). Phase imaging has been used to investigate structural changes in EVs. The observed AFM phase contrast indicated a heterogeneous structure, attributed to variable constitutive elements (lipid, protein, RNA ratio) (Sharma et al., 2010). Similarly, to the amplitude modulation mode, in the off‐resonance mode, the tip approaches and recedes from the surface. The force‐distance curves affected by the tip‐sample interaction are recorded in each measured pixel. From the force‐distance curves, the topography and nano mechanical properties, such as adhesiveness and elasticity, can be calculated and mapped simultaneously (Xu et al., 2018). For example, Young's modulus can be calculated from the stiffness and contact area calculated from the tip shape and the indentation depth) under reversible deformation, by using contact mechanics models (Oliver & Pharr, 1992; Rosenhek‐Goldian & Cohen, 2020). The elastic properties of EVs were successfully calculated from AFM force‐indentation experiments, indicating changes associated with pathological conditions such as cancer (Sharma et al., 2014) and malaria‐infection (LeClaire et al., 2021; Sorkin et al., 2018). To enable reliable AFM measurements, the EVs must be well attached to a surface. Yet, the strength of the interaction of the EVs with the surface may deform the adsorbed vesicle, leading to variations in its aspect ratio (height vs. length). Ridolfi et.al. have shown that the mechanical stiffness of individual EVs can be estimated by the way they are deformed on the surface, from the vesicle‐surface contact angle (Ridolfi et al., 2020). Proper design of the scanning conditions is essential for obtaining good results. For example, the presence of impurities in the EV solution may significantly influence the images obtained. Since EVs are purified from biological solutions, even traces of proteins may compete with the adsorption of the EVs to the surface and influence the image's quality. Extra attention should be given to the force applied to the EVs, as EVs are soft vesicles prone to rupture during AFM measurements (Vorselen et al., 2018).
2.2.11. EV bioinformatic analysis
Omics‐based technologies have emerged as invaluable tools in the quest to discover and develop innovative approaches against several parasites. Large‐scale omics studies of EVs are essential to define molecular profiles that might be associated with specific biological roles. In fact, several studies have already shown that in vitro recovered EVs can have relevant immunomodulatory properties (Coakley et al., 2016; Drurey & Maizels, 2021; Hoffmann et al., 2020; Montaner et al., 2014; Sanchez‐Lopez et al., 2021; Tritten & Geary, 2018; Zakeri et al., 2018) and potentially participate to the drug resistance, diagnostic (Douanne et al., 2022; Khosravi et al., 2020; Mu et al., 2021) and disease prevention (Drurey et al., 2020; Khosravi et al., 2020; Maizels, 2021). Thus, characterization of EVs biomolecule composition should be a priority and availability in databases should be implemented. For example, a seminal work in 2015 reported that promastigote‐derived exosomes recovered from infected sand flies are like those produced in vitro (Atayde et al., 2015). This supports the notion that studies of in vitro produced exosomes will produce biologically relevant data. Overall, the generation of information on EVs content will be essential to clarify the biogenesis mechanism, to define the natural biological role of EVs, to help understand relevant phenomena associated to infection host‐pathogen interactions and to be a source of relevant biomarkers for disease management. The generated data and associated methodologic details should be submitted to EV‐TRACK (evtrack.org) and to EV‐specific databases (e.g., EVpedia, Vesiclepedia, exRNA atlas).
2.2.12. Methodological approaches to study biogenesis of the EVs of the parasites
Although EVs have been characterized and shown to play important roles in the establishment of infection, specific processes involved in the control of EVs release and whether there are different EVs populations acting at variable levels are poorly understood in most parasites. This is particularly important and might help to understand the disease process, evolution, and to provide new therapeutic strategies.
In eukaryotic organisms, EVs can be generated after formation of endosomal membranes in organelles defined as multicellular vesicular bodies (MVB). Usually, these structures fuse with lysosomes and are degraded. On the other hand, MVB fuses with the plasma membrane and release vesicles described as exosomes, which the size between 50 and 150 nm (Colombo et al., 2014). In some, but not all cases, MVB formation depends on endosomal sorting complex required for transport (ESCRT) complexes, key accessory components that bind to the membrane surface and help in the invagination and evagination of endosomal membranes from several organelles. Essentially, the ESCRT‐0, ‐I and ‐II complexes recognize and sequester ubiquitinated membrane proteins at the MVB delimiting membrane, whereas the ESCRT‐III complex is responsible for endosomal membrane budding and actual scission of the intraluminal vesicles (ILVs) (Raiborg & Stenmark, 2009). Alternatively, lipids (ceramide and phosphatidic acid), and tetraspanins (CD9, CD63 and CD81) can play a similar role in the MVBs and ILVs formation independently of the ESCRT complex (Colombo et al., 2014). Among the ESCRT proteins, VPS4 is responsible for recycling ESCRT subunits from the MVB (Braulke & Bonifacino, 2009). It was reported that VPS4 mediate the final step of MVB pathway, disassembling terminal ESCRT III complexes, and providing the only known energy input for ESCRT‐driven vesicle budding and scission.
However, in some cells endosomal organelles can fuse with the cell's plasma membrane and be released in the extracellular medium. These are denominated microvesicles or ectosomes and do not display size restriction, being large as 1 μm or as small as 100 nm. They also have variable composition, depending on their origin. Thus, different microvesicles subpopulations coexist, with some being designed for the degradation pathway (merging with lysosomes), while others are fated for cell release (Cocucci & Meldolesi, 2015). Nevertheless, microvesicles/ectosomes release also requires a protein called vacuolar protein sorting 4A (VPS4), a member of the ATPase associated with diverse cellular activities (AAA) (Shestakova et al., 2010).
It was shown that VPS4 by RNAi in T. brucei significantly enhanced lysosomal degradation and induced the accumulation of VPS23 at the late endosomes, consistent with the blocking of final ESCRT disassembly (Silverman et al., 2013). In L. major, it was reported that the negative VPS4 mutant (VPS4E235Q) accumulated the mutated protein around vesicular structures of the endocytic system, showing deficiency in further transport to the multivesicular tubule‐lysosome (Besteiro et al., 2006). In this work, it was also suggested that L. major VPS4 is relevant for the MVB composition, and VPS4 associated with the MVB compartment is significant for culture differentiation and resistance to starvation. Consequently, affecting VPS4 activity is a robust tool to analyze ESCRT function in EV production, since other ESCRT components might play redundant roles. Not all subunits and complexes necessarily participate in all ESCRT‐driven processes. Another relevant component of the ESCRT‐I complex is VPS23, which in mammalian cells is referred to as TSG101. The depletion of TSG101, reduced exosome secretion in HeLa‐CIITA, MCF‐7, and in immortalized RPE1 epithelial cells (Colombo et al., 2014). For instance, TbVPS23 affected T. brucei exocytosis (Besteiro et al., 2006). VPS36, an ESCRT‐II component, can also be considered a target to prevent secretion of exosomes present in MVB, changing EV release and modifying the parasites social motility behavior (Eliaz et al., 2017).
In addition to the proteins involved in the invagination/evagination of membranes, components that bring together the different organelles are also required for the endocytic processes and consequently, the EVs formation and release. These are the RabGTPases, regulators of the interaction with the cytoskeleton and of the endosomal tethering. For instance, Rab11 has been directly shown to be required for exosome secretion and in the particular case of T. cruzi, it was demonstrated that it affects TS release (Niyogi & Docampo, 2015) Although non‐essential in some cases, Rab7, 35 and 27 also participate in EV production. Similarly, another class of proteins are involved in membrane fusion and EV release, such as the SNAREs formed by soluble N‐ethylmaleimide‐sensitive fusion attachment protein (SNAP).
In eukaryotic cells, ubiquitination, as mentioned above, plays an important role in the sorting of proteins into exosomes (Moreno‐Gonzalo et al., 2014). Ubiquitin modification of target proteins increases the structure of proteins changing their activation state, subcellular localization, stability and capacity to interact with other proteins (Mevissen & Komander, 2017). Ubiquitin is a small compact globular protein of 76 amino acids with an exposed C‐terminal tail that can be covalently linked to lysine residues of a protein substrate. The coordinated activity of ubiquitin‐activating (E1), ubiquitin conjugating (E2) and ubiquitin‐ligating (E3) enzymes leads to the activation and transfer of ubiquitin to specific target proteins: a process known as ubiquitination (Haglund & Dikic, 2012). It can be counterbalanced by deubiquitinating enzymes (DUBs), which remove ubiquitin from the targeted proteins (Drurey et al., 2020; Khosravi et al., 2020; Maizels, 2021; Mevissen & Komander, 2017). It has been described that ubiquitination is important for sorting proteins into ILVs destined for degradation by fusion of the MVB with lysosomes via the endosomal ESCRT complex (Raiborg & Stenmark, 2009). ESCRT complex recognizes ubiquitinated proteins and catalyzes the abscission of endosomal invagination generating ILVs with the sorted cargo. The subcomplexes ESCRT‐0, ‐I and ‐II contain Ubiquitin‐Binding Domains (UBDs) that interact with ubiquitinated proteins. These complexes are involved in capturing and sorting ubiquitylated cargo into the ILVs of MVEs (Tanno & Komada, 2013). The ESCRT‐III complex completes ILV biogenesis by membrane scission. However, before protein translocation into the ILV of the MVE, ubiquitin must be removed from the protein by DUBs. This process ensures that ubiquitin molecules are recycled to maintain cellular ubiquitin levels and regulates protein turnover (Clague et al., 2019).
There are several techniques that can be successfully employed to understand the secretory pathways in different eukaryotic parasites. RNAi has been successfully used to silence specific proteins in some Trypanosomatids, (Alsford & Horn, 2008), Entamoeba (Bettadapur et al., 2021), Acanthamoeba (Lorenzo‐Morales et al., 2005), Giardia (Marcial‐Quino et al., 2017), Plasmodium (Mueller et al., 2014) and Ascaris larvae (Xu et al., 2010). It can be directly used to evaluate changes in the number, size and composition of produced EVs by these organisms. It is important to choose adequate targets and consider whether their decreased expression is not largely affecting cell viability and physiology. Gene knockout techniques are also suitable to investigate the components involved in EV production and/or secretion. Replacement of genes can be achieved by insertion of antibiotic resistance markers through homologous recombination followed by drug selection. In haploid parasite stages such as Toxoplasma and Plasmodium DNA transfection results in efficient depletion of non‐essential genes (Donald & Roos, 1994; Kim et al., 1993; Sultan et al., 2001), and it was used to reveal that ESCRTIII is relevant for EVs production (Avalos‐Padilla et al., 2021). Similar approach can also be used for non‐haploid organisms. In these cases, partial deletion of essential genes can be achieved and shown significant phenotypes. The development of CRISPR/Cas9 technologies, particularly for RNAi non‐competent species such T. cruzi and Leishmania has largely facilitated the process (Costa et al., 2018; Lander et al., 2015; Peng et al., 2014; Sollelis et al., 2015; Zhang et al., 2017). A wide CRISPR/Cas9 genomic approach can additionally be employed to select components that affect EV production (Sidik et al., 2016). This approach has been used in certain stages of helminth parasites. In all cases, important controls excluding major changes in viabilit, physiology should be discarded and preferentially re‐introduction of the depleted gene should be used to recover the observed phenotype.
3. METHODOLOGIES TO ISOLATE AND CHARACTERIZE EVS PURIFICATION FROM SPECIFIC PARASITES
3.1. Trypanosoma cruzi EVs
3.1.1. EVs generated in cellular cultures
The first step to obtain EVs from T. cruzi stages corresponding to insect‐growing forms or parasite forms found in the mammalian hosts should be devoid of Mycoplasma or another microorganisms’ contamination. Below we illustrate the procedure to obtain EVs from trypomastigotes released by infected kidney epithelial cells (LLCMK2), which can be adapted to any other culture system.
The infected cells can be maintained in culture by weekly passages in DMEM medium supplemented with 10% FCS (without EVs from serum) and antibiotics, in CO2 atmosphere at 37°C. Culture supernatants, rich in trypomastigote forms can be collected 5–9 days after a 24 h incubation of parasites with sub‐confluent cell monolayers. The suspension containing parasites are subjected to centrifugation (2000 × g for 10 min), washed and resuspended in appropriate conditions at 1 × 108 parasites/mL as initially described (Gonçalves et al., 1991; Trocoli Torrecilhas et al., 2009). Briefly, the parasites previously washed are incubated from 2 to 3 h at 37°C for EV release. One part of the initial suspension is spared, and parasites collected by centrifugation and kept frozen at −70°C for further analysis. When analyzing forms that proliferate without cells in serum‐free media such as epimastigotes, EVs can be collected in the culture supernatants (2000 × g for 10 min). After the release step, parasites are subjected to a new centrifugation at 2000 × g for 10 min, thereafter, the supernatant is filtered through the 0.45 μm membrane and used as rough EVs.
The rough EV samples can be fractionated on the SEC gel‐filtration column (40 × 1 cm). The column is previously washed with the equivalent of 10 volumes of 100 mM ammonium acetate pH 6.5. Then, the supernatant (1–2 mL), prepared on the same day, is applied to the column at a flow rate (4 mL/h) with the aid of a peristaltic pump. The column is eluted with 100 mM ammonium acetate pH 6.5 and up to 80 fractions (1 mL each) are collected. The T. cruzi EVs can be detected for their reactivity in chemiluminescent (CL‐ELISA) with specific antibodies such as the anti‐α‐Gal Abs found in the sera of CD patients, which recognize terminal nonreducing α‐galactosyl residues on the highly abundant tGPI‐mucins (Almeida et al., 1994, 1991). Trypomastigote EVs are highly reactive with these antibodies (Nogueira et al., 2015; Trocoli Torrecilhas et al., 2009). The most convenient way to detect these antigens is to coat polypropylene 96‐well plates directly with 0.1 mL of the fractions eluted from the column and perform conventional ELISA or CL‐ELISA.
T. cruzi EVs contain glycoconjugates expressed on the surface of different developmental forms of the parasite in the mammalian and insect‐vector hosts. Thus, the characterization and functional assays (Cronemberger‐Andrade et al., 2020; Gutierrez et al., 2022; Nogueira et al., 2015) and the detection of biomarkers (Madeira et al., 2021, 2022) can be performed. Osuna group (Prescilla‐Ledezma et al., 2022) suggested that EVs isolated from tissue‐culture derived trypomastigotes can be characterized using AFM‐based single molecule‐force spectroscopy as a suitable technique for the detection and location of functional components on the surface of EVs. To detect trans‐sialidase (TS) and other specific genes in EVs, it is possible to use specific monoclonal antibodies. For example, it has been shown the presence of TS using coupled antibodies to sensors (Prescilla‐Ledezma et al., 2022). The components present in T. cruzi EVs obtained with different methodologies, their characterization, and the biological function are summarized in Table 1.
TABLE 1.
Studies on Trypanosoma cruzi EVs.
| Reference | Vesicle cellular origin | Cell amounts (parasites/mL) | Vesiculation general conditions | Cell viability at time of EV harvest | Pre‐isolation/cell debris remotion | EV isolation and/or purification | EV characterization | EVs yield per batch (size/concentration) | In vitro/in vivo experiments using EVs | Markers |
|---|---|---|---|---|---|---|---|---|---|---|
| da Silveira et al., 1979 | EVs from epimastigotes (Y strain) | 1‐5 × 109 par/mL |
37°C, 1 h, LIT medium |
NR |
C: 4000 × g, 10 min, 4°C UC: 100,000 × g, 1 h, 4°C Purification at SEC |
UC: 105,000 × g, no time reported | Radioactivity determination and EM | NR | pH (base/acid) | NR |
| Abuin et al., 1996a, 1989, 1996b | EVs observed from trypomastigotes of the Y and YuYu strains |
5 × 107par./mL |
Cells cultured in LIT, TAU or DMEM with 5% FCS |
NR |
Fixation: 60 min in glutaraldehyde/4% formaldehyde, 0.1 M phosphate buffer, pH 7.2, Blocking: Incubation in NH4Cl H1A10 MAb (anti‐gp85) |
Treatment with formvar and carbon‐coated gold grids 0.1% polylysine | TEM | NR | Detection of parasite antigens | GP85 |
| Gonçalves et al., 1991 |
Exosomes secreted by trypomastigotes from Y, CA1, RA and YuYu strains obtained from infected LLC‐MK2 cells |
5 × 107par./mL |
Strains cultured for 4 h, 37°C, in DMEM w/5 % dialyzed FCS |
PBS with 500 μCi Na131I, 20 μg iodo‐gen, 4°C, 10 min |
C: 2500 × g, 5 min Filtration: 0.22 μm |
SEC | EM |
20–80 nm Conc.: NR |
IP | GP85 |
| Bayer‐Santos et al., 2013 | EVs obtained from Dm28c clone of epimastigotes and metacyclic trypomastigote forms |
1 × 08par./mL |
Strain cultured in LIT and TAU3AAG at 28°C | FC: propidium iodide |
C: 3000 × g, 10 min, 4°C → F: 0.45 μm |
UC: 100,000 × g, 2 h, 4°C 100,000 × g, 16 h, 4°C |
NTA, TEM, proteomics (2D LC−MS/MS) |
74–143 nm (mean) Conc.: NR |
WB; IIF |
FCaBP, GP82, GP35/50 (35‐50 kDa mucins) |
| Garcia‐Silva et al., 2014 | EVs obtained from Dm28c clone epimastigotes and trypomastigotes | 1 × 1011 par./mL | LIT and RPMI 1640 with 10% FBS | FC: propidium iodide | C: 2000 × g, 15 min → 2000 × g, 30 min 15,000 × g, 30 min |
UC: 110,000 × g, 4°C 70 min → 110,000 × g, 1 h, 4°C |
TEM | NR | Exchange of tsRNAs; TcPIWItryp protein Small RNA deep sequencing; electroporation sequencing | NR |
| Fernandez‐Calero et al., 2015 | EVs obtained from the Dm28c clone epimastigotes | 1 × 1010 par./mL | LITd with 10% FBS at 28°C | FC | C ‐ 2000 × g for 15 min/C ‐ 15,000 × g at 4°C for 30 min | UC at 110,000 × g at 4°C for 70 min. The pellet was washed twice in PBS and UC at 110,000 × g for 1 h. | TEM and Bradford | 1.2 μg per 1 × 1010 parasites | RNA Analyse | NR |
| De Pablos et al., 2016 | Trypomastigotes EVs from mice infected with trypomastigotes of PAN4 strain | 5 × 107 par./mL |
RPMI 1640 + 10% IFCS UC: 100,000 × g, 1 h |
NR | C: 3000 × g, 15 min, → 17,000 × g, 20 min → Filtration: 0.2 μm | UC: 100,000 × g, 1 h, 2 × | TEM |
90–300 nm (mean: 154 nm) |
IEM | Clathrin |
| Díaz Lozano et al., 2017 | Trypomastigotes (PAN4 strain) was isolated from a patient; cultured amastigotes and trypomastigote forms were obtained from infected Vero cells culture | NR |
RPMI 1640 with 25 mM HEPES pH 7.4 and 10% EV‐free IFCS |
NR |
C: 3000 × g, 15 min → 17,000 × g, 20 min → F: 0.2 μm |
UC: 100,000 × g, 1 h (twice) |
SEM TEM CM |
50–100 nm | WB and ELISA | NR |
| Lovo‐Martins et al., 2018 |
EVs obtained from Y strain trypomastigotes |
1 × 108 par./mL | Incubation in RPMI without FBS for 2 h at 37°C | NR | C ‐ 2600 × g, 10 min | Filtration (0.45 μm); total exosome isolation from cell‐culture media reagent | NTA | 136.33 nm | Experimental model | NR |
| Ribeiro et al., 2018 | EVs from Y and YuYu strains trypomastigotes | 1 × 109 par./mL | DMEM 2% glucose at 37°C, 5% CO2 | NR | C at 1000 × g for 10 min; filtration | SEC and UC–100,000 × g for 16 h at 4°C |
SEM NTA |
YuYu:1.0 × 107– 9.7 × 109 particles/mL; Y: 2.7 × 106– 8.9 × 108 particles/mL |
ELISA. WB, and PA, invasion assay | Mucin, GP85 and TS |
| Paranaiba et al., 2019 | EVs obtained from epimastigotes | 1 × 105 par./mL | LIT incubation at 28°C for 2 h | NR | NR | Filtration (0.22 μm) and ultracentrifugati on (100,000 × g, 2 h, 4°C) |
NTA TEM |
223.1 nm, (D10 = 143.6; D50 = 245.5 and D90 = 264.7); 6.84 × 107 particles/mL | Functional studies | NR |
| Retana Moreira et al., 2019 | EVs from trypomastigotes forms from Pan4 strain derived from Vero and 3T3 cells cultures | NR | RPMI medium buffered with 25 mM HEPES at 7.2 and supplemented with 10% exosome‐free IFBS at 37°C | Trypan blue |
Centrifugation: 3500 × g for 15 min 17,000 × g for 30 min at 4°C Filtration through a 0.22 μm pore filter |
UC‐100,000 × g for 16–18 h. |
NTA TEM DLS |
70.7 nm/5 × 1010 p/mL | WB; invasion assays | NR |
| Cronemberger‐Andrade et al., 2020 |
EVs from THP‐1 cells infected and with trypomastigotes from Y strain |
NR | Trypomastigotes EVs were obtained by incubation of parasites for 2 h in DMEM containing 2% glucose at 37°C. THP‐1 EVs were obtained incubating trypomastigotes for 4 h at 37°C/5% CO2 in RPMI | NR | C—10 min at 500 × g, 10 min at 3000 × g, 15 min at 8000 × g | UC‐ 100,000 × g for 1 h |
NTA SEM |
50–200 nm/105‐ 106 particles/mL |
WB; RT‐PCR |
Mucin, GP85 and TS |
| Retana Moreira et al., 2021 | EVs from epimastigotes and trypomastigotes forms from Pan4 strain derived from Vero cell cultures | 5 × 107 |
MEM with 10% FBS maintained at 37°C LIT with 10% FBS serum, at 28°C. |
Trypan blue |
UC ‐ 100,000 × g 16–18 h C‐3500 × g for 15 min. Filtration ‐ 0.22 μm |
UC‐ 100,000 × g 16–18 h |
NTA TEM DLS |
143–259 nm | PA, WB | NR |
| Vasconcelos et al., 2021 |
EVs obtained from Y strain trypomastigotes |
1 × 107 | DMEM with FBS or 5% glucose, temperatures and pH levels, and/or in the presence of metabolic inhibitors and nitroxidative compounds (NaN3 and NaNO2) and methylβcyclodextrin (CBD). | PrestoBlue | C ‐ 1000 × g for 15 min | C‐ 10,000 × g for 15 min. Then, the supernatant was filtered (0.22 μm) and ultracentrifuged at 100,000 × g, for 1 h, at 4°C |
SEM NTA |
150 nm |
EV labeling (Fluorescent cell dye), macrophage interaction, gene expression by qRT‐PCR |
NR |
| Cestari et al., 2012 |
EVs from epimastigote, MT and tissue culture trypomastigote |
5 × 106 |
Cells were incubated in RPMI at 37°C for 1 h and then 5 min on ice |
NR | NR |
DC (5 min, 1603 × g; 2 × 3 30 min, 4000 × g; and 90 min, 100,000 × g) |
TEM | NR | Invasion assay, cleavage assay, kinetic studies, WB, ELISA, in vivo model | NR |
| Trocoli Torrecilhas et al., 2009 | EVs from trypomastigotes | NR | Cells were incubated in RPMI at 37°C for 2 h | NR | C ‐ 2500 × g for 5 min and filtration 0.22 μm | SEC | EM | NR |
WB In vivo model |
TS/gp85 Cruzipain and mucin |
| Cortes‐Serra et al., 2020 | Plasma‐derived EVs from chronic Chagas disease patients | 1 mL of plasma from blood collected in sodium citrate | NR | NR | 2000 × g for 10 min at RT (2×) | SEC (iZON columns) | BBA, BCA, NTA, proteomic analysis | For proteomic analysis: 100 mL of SEC fractions (710) pooled | Identification of potential biomarkers of chronic Chagas disease in plasma‐derived EVs |
CD5L CD9 CD63 |
| Madeira et al., 2021, 2022 | Plasma‐derived EVs from chronic Chagas disease patients | 3 mL of plasma from blood collected in sodium citrate/EDTA or serum | NR | NR | 2000 × g for 10 min at RT |
UC SEC |
NTA BCA |
NR | Identification of potential biomarkers of chronic Chagas disease in plasma‐derived EVs | CD9,CD81, TS and total parasites antigens |
Abbreviations: NR, not reported; C, centrifugation; UC, ultracentrifugation; FC, flow cytometry; DLS, dynamic light scattering; NTA, Nanoparticle tracking analysis; PA, proteomic analysis; SEM, scanning electronic microscopy; TEM, transmission electronic microscopy.
3.1.2. EV obtained from T. cruzi‐infected patients
The pre‐analytical phase is an important step to consider, as it can be a source of variability and contribute to artifacts. Currently, plasma is the preferred source of EVs, as additional vesicles are released during the clot formation when preparing serum (Coumans et al., 2017). Nowadays, all published studies working with EVs isolated from CD patients use plasma as the primary source. Blood is usually collected in sodium EDTA tubes (Chowdhury et al., 2017; Madeira et al., 2021), but it can also be collected in BD vacutainers containing sodium citrate (Cortes‐Serra et al., 2020) or heparin (Ramirez et al., 2018). To obtain plasma, the blood can be kept at room temperature for 4 h and then stored at 4°C overnight (Madeira et al., 2021, 2022). Alternatively, the collection tubes can be kept at 4°C just after blood extraction and samples processed directly by performing a two‐step centrifugation (one at 500 × g for 10 min at 4°C, and a second step at 2000 x g for 15 min at 4°C) (Cortes‐Serra et al., 2020). One single centrifugation at 2000 x g for 15 min at 4°C (Ramirez et al., 2018) or two steps centrifugation of 20 min each at 270 and 1000 x g (Cestari et al., 2012) are among the common's methods used to separate plasma before EV enrichment.
It is known that the methodology used for EVs isolation is crucial and will affect the results obtained in further experiments. As all methods present advantages and limitations, the selection of the methodology should be based on downstream analysis requirements (Coumans et al., 2017). Ultracentrifugation is one of the most popular methods, also to isolate EVs from CD patients. Plasma samples are centrifuged at 100,000 x g for 1 h, and pellets resuspended in filtered PBS (Madeira et al., 2021). Other researchers performed three series of centrifugation at 15,000 x g for 15 min to isolate MVs, which can be stored at −80°C (Chowdhury et al., 2017).
For proteomic analysis, SEC has also been used to isolate EVs from plasma of CD patients. This technique has gained popularity in the field of EVs as it is user friendly and provides high purity of EVs, removing most of the soluble plasma proteins and maintaining the major EVs' characteristics, including vesicular structure and content. Sepharose CL‐2B can be packed in 10 or 1 mL plastic syringes, which are equilibrated with phosphate‐buffered saline (PBS). Columns are prepared in sterile conditions one day before the isolation and kept at 4°C ON. Commercial Sepharose CL‐2B 10 or 1 mL columns can also be used (iZon Sciences). In any case, frozen plasma samples are thawed on ice, centrifuged at 2000 x g for 15 min at 4°C and the sample is loaded on the Sepharose columns. Immediately, 14 fractions of 0.5 mL each or 10 fractions of 0.1 mL (for 10 or 1 mL columns, respectively) are collected with PBS as the elution buffer (Cortes‐Serra et al., 2020).
3.2. EVs obtained from Leishmania that causes visceral or cutaneous infections
3.2.1. EVs from infected host fluids
The study of Leishmania EVs or infection associated EVs in the context of complex sources like biological fluids can be challenging. In the case of Leishmania, the most common biological sources can be either naturally infected hosts, like dogs and humans, or from experimental models of infection (mice and hamsters).
For a while only studies in vitro and in vivo models were used to study leishmaniasis, but in 2014 the first study using human samples was carried out (Oliveira et al., 2014). They assessed renal damage related in patients with American cutaneous leishmaniasis. To achieve their objectives, urine samples were collected from patients after a 12 h water deprivation and intensively vortexed in the presence of protease inhibitors. Then a series of differential centrifugation steps were conducted until a low‐density membrane pellet is obtained. The membrane pellet was characterized by electrophoresis and immunoblotting for the expression of proteins related to tubular dysfunction. Even though considered by Oliveira and colleagues to be exosomes, no characterization using electron microscopy, nanoparticle tracking analysis and protein markers enriched in vesicles were assessed.
Simple pre‐analytical variables like collection and storage of biological fluid can interfere with the nature and quantity of the recovered EVs. Among others, the tube used to collect blood, the anticoagulant used, the time between the collection and processing of the sample, the volume of sample collected, degree of hemolysis, the conditions in which the samples are transported, and storage conditions, are all aspects to have in consideration and should be described in all experiments (Baek et al., 2016; Yuana et al., 2015). Although several biological fluids such as urine or cerebrospinal fluid might be of interest in the context of Leishmania infection, most EVs will be recovered from blood. Thus, most generic guidelines reported in the MISEV2018 should be complied (Théry et al., 2018). When working with blood plasma should be the main biological fluid of interest. Plasma is the physiological medium in which EVs exist in the blood, thus, to recover biologically relevant EVs it would be more appropriate to use plasma. If serum is used, platelet derived EVs will be released during the process of clotting, these can contribute to an increase of 50% of the number of EVs in the biological fluid (Witwer et al., 2013). The choice of anticoagulant should be based on the intended downstream application for the EVs and should be maintained for all the samples. When working with hospital samples it is feasible to standardize the sample collection, the main issue is the time until sample processing. Experimental data supports that EVs are stable at 30 min after collection but after this frame it is expected to have an increase in EVs level (Jayachandran et al., 2012). Thus, it is relevant to define this frame or describe this information for accurate reporting. After recovery of the biological fluid EV recovery should follow, but this is not always possible and biobanking of biological samples is a necessity for most researchers. Thus, it is important to report storage conditions. Storing the biological samples in single use aliquots would be highly recommendable to avoid freeze and thaw cycles. The approach for recovery of EVs from biological samples should be adjusted to the goal. Some approaches enable pure EVs preparations, like SEC or high. If the goal is to study specific sub‐types of EVs populations, the combination of approaches or the use of density gradients are recommended. Considering the complexity of the starting biological sample, the use of ultrafiltration combined with SEC originates EVs that are sufficiently pure for downstream applications, like omics. The combination with high molecular weight filtration devices can be used to increase purity although the binding to the filter can be an issue. There is no, one approach fits all ends. The recovery approach must be adjusted to the end goal of balancing yield and purity. The recovered EVs should be then characterized and stored at −80°C in low binding tubes to avoid the adsorption of molecules to the Eppendorf's surface. At this temperature, the samples can be stored for a long time without losing their characteristics. Freeze and thaw cycles should also be avoided.
In the context of experimental Leishmania infections it is easy to have full standardization of all the experimental variables. The same is not true for human and canine samples, in this context several variables are at stake and contribute to experimental variability that might be relevant depending on the intended goal. For example, in the context of biomarker discovery it will be important that the biomarkers are robust and not detected in very specific conditions, while for basic characterization of infection associated EVs and functional studies it is more important to have samples that reflect as possible the natural environment that they were collected, in these situations it is paramount to describe, at least, donor status if available (age, sex, food/water intake, collection time, disease, medication, other); the tube used to collect blood, the anticoagulant used, the time between the collection and processing of the sample, the volume of sample collected, degree of hemolysis, the conditions in which the samples are transported, and storage conditions until sample recovery; volume used for EV isolation (if pooled from several donors).
3.2.2. Collection of EVs from sandflies
Leishmania are transmitted through the bite of a sand fly vector, and it is now well established that the parasite can release EVs within the insect midgut compartment while dividing actively, and to be co‐transmitted with Leishmania promastigotes to the mammalian host during a blood meal (Atayde et al., 2015). The collection of Leishmania EVs within its insect vector is one of the most challenging. The vector being quite small, hundreds of Leishmania‐infected sand flies are needed to collect up to 200 μL of midgut lavage containing Leishmania promastigotes and EVs in suspension. Midguts are rinsed by dissecting the sandflies and cutting open the midgut in a droplet of PBS to wash the luminal cavity. Because of the low amount of starting material especially after differential centrifugation, EV purification methods with high yield must be used, often leaving only commercial kits available for use like SBI's ExoQuick‐TC and Invitrogen's Total Exosome Isolation Reagent (Skotland et al., 2020). Inoculum can be collected during feeding using chicken skin as reported (Rogers et al., 2004) and the resulting material can be differentially centrifuged and EVs can be purified by high yield methods. Although the isolation of Leishmania EVs in its sand fly vector is challenging, with the difficulty depending on the vector size, it provides valuable insight into vector‐parasite interaction and host infection. Leishmania EVs and different methodologies are summarized in Table 2.
TABLE 2.
Studies on Leishmania EVs.
| Reference | Vesicle cellular origin | Cell amounts | Vesiculation general conditions | Cell viability at time of EV harvest | Pre‐isolation/cell debris remotion | EV isolation and/or purification | EV characterization | EVs yield per batch (size/concentration) | In vitro/in vivo experiments using EVs | Markers |
|---|---|---|---|---|---|---|---|---|---|---|
| Silverman et al., 2010b | Stationary L. donovani promastigotes | NR | 24 h incubation in FBS‐free RPMI at 26/37°C and 7.5/5.5 pH | 95% at 26°C 92% at 37°C | C – 300 × g for 10 min 700 × g for 30 min 15,000 × g for 45 min | UC – 110,000 × g for 60 min, 110,000 × g for 90 min, 0.252.5 M sucrose gradient at 200,000 × g for 20 h | BCA, TEM with gold immunolabelling, proteomic analysis, and WB | NR | Murine macrophage treatment and infection | HSP70 HSP90 EF1α |
| Silverman et al., 2010a | Stationary L. donovani and L. major promastigotes | NR | 24 h incubation in FBS‐free RPMI at 37°C and 5.5 pH | NR | C – 300 × g for 10 min, 700 × g for 30 min, 15,000 × g for 45 min, and 0.2 μm filtration | UC – 110,000 × g with 100 kDa ultrafiltration and 1 M sucrose cushion | BCA, Sucrose gradient, WB and PA | NR | Treatment of monocytes and dendritic cells, C57/BL6 mice vaccination and infection | HSP70 HSP90 EF1α |
| Hassani et al., 2011 | Stationary L. mexicana promastigotes | NR | 2/4 h incubation in FBS‐free RPMI at 26/37°C | NR | C – 10,000 × g for 35 min | UC ‐ 100,000 × g for 1 h | SEM | NR | Treatment of murine B10R macrophage with exoproteome | NR |
| Hassani & Olivier, 2013 | Murine J774 macrophages infected with stationary L. mexicana | NR | 24 h culture in exosome depleted RPMI at 37°C | NR | C at 4000 × g for 20 min and 0.45 μm filtration | UC at 100,000 × g for 1 h, 0.22 μm filtration, 100,000 × g for 1 h, 0‐2 M SG | Silver staining, BCA, TEM, PA, and WB | NR | Stimulation of murine J774 macrophages | GP63 PABP |
| Cronemberger‐Andrade et al., 2014 | BALB/c BMDMs infected with stationary L. amazonensis | 20,000 macrophages | 9‐day culture in exosome‐depleted RPMI at 37°C | 35% uninfected, 8% infected after 9 days | C – 500 × g for 10 min, 1500 × g for 10 min, 8000 × g for 5 min | UC – 100,000 × g for 45 min | BCA, FC, TEM | 5 × 107 EVs by FC | Treatment of BALB/c peritoneal macrophages | NR |
| Lambertz et al., 2015 | L. donovani and L. braziliensis axenic amastigotes | 2‐4 × 1010 amastigotes | 24 h incubation in FBS‐free RPMI at 37/34°C and 5.5 pH | NR | C – 300 × g for 10 min, 700 × g for 30 min, 15,000 × g for 45 min, and 0.2 μm filtration | UC‐ 110,000 × g with 100 kDa ultrafiltration and 1 M sucrose cushion, 100,000 × g for 60 min | BCA, NTA, RNA analysis | NR | Treatment of THP‐1 cells with SYTO‐labelled L. donovani axenic amastigote EVs | NR |
| Atayde et al., 2015 | Sandflies infected with L. major and L. infantum | NR | Midguts dissected in the drop of PBS | NR | C‐ 3000 rpm, 1000 rpm | ExoQuick following manufacturer protocol | BCA, TEM, PA and WB | 10 μg | Co‐injection of BALB/c footpads with L. major EVs and parasite | GP63 |
| Barbosa et al., 2018 | Stationary L. amazonensis promastigotes | 1 × 108 promastigotes | 1/2/4/24 h incubation in FBSfree RPMI at 26/34/37°C | 97–10% viability from 1–24 h incubation | 0.45 μm filtration and C – 500 × g for 10 min, 1500 × g for 10 min, 10,000 × g for 10 min | UC‐100,000 × g for 1 h twice and size exclusion chromatography | BCA, SEM, NTA, Dot blots, ELISA | NR | Treatment of BALB/c BMDMs and B1 cells, BALB/c footpad coinjection | NR |
| Marshall et al., 2018 | Stationary, logarithmic, and metacyclic L. infantum promastigotes | NR | Overnight incubation in FCSfree at 26°C | NR | Centrifugation at 1200 × g for 10 min and 0.2 μM filtration | 100 kDa ultrafiltration and ultracentrifugation at 110,000 × g for 4 h and 16 h, 0.25‐2 M sucrose gradient | BCA. TEM, WB and PA | NR | NR | NR |
| Atayde et al., 2019 | Stationary L. guyanensis, L. panamensis, L. mexicana, L. braziliensis promastigotes | NR | 4 h incubation in FBS‐free RPMI at 37°C | NR | C‐ 2555 × g, 8500 × g and 0.45 μm, 0.2 μm filtration | UC‐100,000 × g for 1 h, 0–2 M sucrose gradient at 100,000 × g for 1.5 h | BCA, TEM, SEM, NTA, WB, PA, qRTPCR, RNase protection assay | NR | Co‐injection of BALB/c footpads, transfer assay | HSP83 HSP70 α‐tubulin GP63 |
| Douanne et al., 2020 | Stationary WT and drug resistant L. infantum promastigotes | 1.25–2.5 × 109 promastigotes | 4 h incubation in FBS‐free RPMI at 37°C | Greater than 95% | C‐ 3000 × g for 10 min, 8500 × g for 10 min, 0.45 and 0.2 μm filtration | UC‐ 100,000 × g for 1 h | BCA, TEM, NTA, PA | 7.69 × 109‐2.98 × 1010 particles/μg protein | NR | Common exosomal proteins |
| Forrest et al., 2020 | Logarithmic and stationary L. chagasi promastigotes | NR | 24 h incubation in TPB‐M199 or exosome‐depleted original M199 at 25°C | NR | Centrifugation at 300 × g for 10 min, 2000 × g for 10 min, 10,000 × g for 30 min | Ultracentrifugation at 100,000 × g for 1 h | Pierce 600 nm colorimetric assay, proteomic analysis | NR | NR | Common exosomal proteins |
| Nogueira et al., 2020 | Stationary L. infantum, L. braziliensis, L. amazonensis promastigotes | NR | 2 h incubation in FBS‐free M199 at 37°C | Greater than 90% | 0.22 μm filtration | UC −100,000 × g for 2 h | BCA, TEM, SEM, NTA | NR | C57BL/6 peritoneal macrophages | NR |
| Reis et al., 2020 | Stationary L. amazonensis promastigotes | 1 × 108 promastigotes per microtube | 4 h incubation in FBS‐free M199 at 26°C | NR | 0.45 μm filtration and centrifugation at 500 × g for 10 min, 1500 × g for 10 min, 10,000 × g for 10 min | UC 100,000 × g for 1 h twice | BCA, NTA | NR | BALB/c intraperitoneal co‐injection and B‐1 cell enrichment | NR |
| Gioseffi et al., 2020 | RAW264.7 macrophages infected with L. donovani | NR | 72 h infection in exosome depleted DMEM at 37°C | NR | C‐ 3000 × g for 10 min, 16,000 × g for 30 min and 0.22 μm filtration | UC‐ 100,000 × g for 3 h, 100,000 × g for 18 h | NTA, SEM, TEM with gold immunolabelling, PA and WB | 1.0‐1.5 × 105 particles/cell | Treatment of MDAMB‐231 and HUVEC epithelial cells | CD9 CD63 Annexin A3 |
| Das et al., 2021 | GAPDH mutant L. major promastigotes | 2 × 108 promastigotes | Cultured in exosome depleted M199 at 22°C | NR | C – 300 × g 10 min, 2000 × g 15 min, 10,000 × g 30 min and 0.22 μm filtration | UC – 100,000 × g for 90 min with 30% sucrose cushion, 100,000 × g for 90 min | NTA, AFM and WB | NR | Treatment of RAW264.7 macrophages | GP63 |
| da Silva Lira Filho et al., 2021 | Stationary WT and GP63 mutant L. amazonensis promastigotes | NR | 4 h incubation in FBS‐free RPMI at 37°C | NR | C‐ 300 × g for 5 min, 2000 × g for 10 min, 0.45 and 0.22 μm filtration | UC 100,000 × g for 1 h twice | BCA, NTA, TEM and PA | NR | Treatment of B10R macrophages and BALB/c footpad coinjection | NR |
| Douanne et al., 2022 | Stationary L. infantum and L. major promastigotes. MRPA mutant L. infantum promastigotes. L. infantum antimony‐, miltefosine‐ and amphotericin Bresistant promastigotes. L. major methotrexateresistant promastigotes. | 2.5−5.0 × 108 promastigotes/mL | 4 h incubation in FBS‐free RPMI at 37 ⁰C | Greater than 95% | C‐ 3000 × g for 10 min, 8500 × g for 10 min, 0.45 and 0.2 μm filtration | UC‐ 100,000 × g for 1 h | BCA, TEM, NTA, proteomic and DNAcontent analysis | >1011 particles/μg protein | Intra and interspecific horizontal gene transfer assays. In vitro fitness gain using EVs derived from drug resistant parasites | NR |
| Shokouhy et al., 2022 | L. tarentolae GFP+ (ATCC 30267) and L. major GFP+ (MRHO/ IR/75/ER) | 1 × 107 promastigote per microtube | Serum‐free media at 26°C and 37°C for L. tarentolae GFP+ and L. major GFP+, respectively. | L. tarentolae GFP+ 98% L. majorGFP + 95% | C ‐ 1800 × g, the supernatants were filtered through 0.45 μm and subjected to serial centrifugation at 4°C as follows: 500 × g for 10 min, 1500 × g for 10 min, and 10,000 × g for 10 min | Concentrated using Amicon Ultra‐15‐3 K and passed through a 0.5‐mL qEV SEC (Izon) | BCA, NTA, TEM, SEM and Dot blot | L. tarentolae GFP+ 29.6 × 106 particles/μg L. major GFP+ 23.6 × 106 particles/μg | Human macrophage treatment (THP‐1) and infection | GP63 GFP |
| Santarém et al., 2013 | Logarithmic and stationary L. infantum promastigotes | NR | 24 h growth for logarithmic parasites and 96 h for stationary promastigotes | >94% at 27°C | C‐1800 × g for 10 min and 0.45 μm filtration Centrifugation at 10,000 × g for 10 min, 4°C | UC‐ 100,000 × g for 4 h, 4°C | TEM, PA, SDS‐PAGE and DLS | NR | NR | NR |
| Belo et al., 2017 | Stationary promastigotes of L. infantum | Start: 1 × 106/mL promastigotes End: 1.4 × 107/mL promastigotes | 96 h for stationary promastigotes | NR | C‐ 1800 × g for 10 min and 0.45 μm filtration | 100 kDa ultrafiltration and UC −100,000 × g, 4°C for 16 h | NR | NR | iNKT expansion and activation assays and effect on CD1d binding | NR |
| Perez‐Cabezas et al., 2019 | Stationary promastigotes of L. infantum | Start: 2 × 106/mL promastigotes End: 1 × 107/mL promastigotes | 96 h for stationary promastigotes | NR | C‐ 1800 × g for 10 min and 0.45 μm filtration | 100 kDa ultrafiltration and UC‐ 100,000 × g, 4°C for 14 h | TEM, DLS and TA | 1.84 × 1012/ mL for a final concentration of EVs produced by 1 × 107 promastigotes | In vitro: effect on BDM and DC activation, capacity to secondary stimulus (LPS) and infection. In vivo: Cell recruitment in air pouch model and infection | NR |
Abbreviations: NR, not reported; C, centrifugation; UC, ultracentrifugation; FC, flow cytometry; DLS, dynamic light scattering; NTA, nanoparticle tracking analysis; PA, proteomic analysis; SEM, scanning electronic microscopy; SG, sucrose gradient; TEM, transmission electronic microscopy.
3.3. Toxoplasma gondii
3.3.1. EVs‐derived from T. gondii infected cultured cells
The study of EVs is this parasite has increased along the past decades and several techniques have been used. Since, this protozoan is an obligate intracellular parasite, it is very important to consider the cell host used for assessing EV production. Most of pioneer studies have used RH strain of T. gondii. Particles production by this protozoan was early reported by in T. gondii infected macrophages (Sibley et al., 1986). A two‐step centrifugation protocol can be employed to purify a so‐called intraphagosomal membrane network (IMP). This network composed of EVs should be disrupted, followed by centrifugation at 10,000 x g and 100,000 g. Also, the presence of network‐derived EVs may be confirmed by TEM. Only recently, other strains were included such as ME 49. However, no consensus on cell type has been reached and several cell lines have been used, including THP‐1 cells, L6 rat myoblast cells, DC2.4 cells and human foreskin fibroblast (HFF). Also, using defined medium such as RPMI and DMEM has been extensively employed in promoting higher purity for EVs. Particles derived from human THP‐1 cells infected with T. gondii functionally induced TNF‐α production (Bhatnagar et al., 2007). EVs purification may be achieved by a series of centrifugation steps, including a sucrose‐gradient purification from 0.25 to 2 M sucrose for 15 h at 100,000 g and characterized by TEM and FC. The presence of mRNA and miRNA can be detected after Trizol extraction (Pope & Lasser, 2013).
Many recent contributions to T. gondii EVs have come from Asian investigators in China and Korea, and more recently, from Brazil. Infected L6 rat myoblasts grown in Dulbecco's modified Eagle's medium (DMEM) depleted of exosomes were used in this study (Kim et al., 2016). Another study from Brazil used human foreskin fibroblast (HFF) infected with T. gondii (Wowk et al., 2017), showing that EVs can be obtained from emerged parasites from those cultures. A 2‐h incubation should be performed to allow EVs to release from tachyzoites, and viability must be checked. These studies should follow MISEV 2014 (Lotvall et al., 2014) and perform vital controls to ensure EVs purify, including NTA and TEM. Two landmark studies from China (Li et al., 2018a, 2018b) have assessed exosomes in the ME 49 strain of T. gondii. Those papers suggest using a specific exosome purification kit, Exo Easy Maxi Kit (Qiagen) and Viva Cell for concentrating. Immunoblotting can confirm exosomes in the preps by specific T. gondii surface markers, including HSP70 and P30 (SAG1).
More recently, one Brazilian group has published several articles on T. gondii EVs, bringing new information on the host‐parasite interaction (Maia et al., 2021a, 2021b; Silva et al., 2018). EVs released from T. gondii can be purified by using a basic SEC previously used for T. cruzi (Trocoli Torrecilhas et al., 2009). It is important to mention that it should attend MISEV (2018) criteria (Théry et al., 2018). Later, the same group (Maia et al., 2021a) assessed EVs in the sera from mice immunized with EVs from T. gondii obtained in the previous study (Silva et al., 2018). This group has also explored the release of EVs in different T. gondii strains (Types I, II and III) (Quiarim et al., 2021). Those included RH, ME‐49 and VEG. Regardless of the strain, all of them could be successfully purified by gel‐exclusion chromatography using the same procedure as before (Silva et al., 2018). Again, the quality of EVs followed MISEV 2018 guidelines and used NTA, SEM and TEM. A proteomic approach can be successfully applied to T. gondii EVs by a Mexican group (Ramirez‐Flores et al., 2019). In this study, the authors performed a basic two‐step ultracentrifugation protocol, SEM/TEM and Western blot (WB) to analyze EVs. The main markers to evaluate EVs by WB were ROP1 and ROP2, GRA1‐GRA6, MIC1‐MIC4, SAG 1 and α‐tubulin.
In conclusion, the number of studies in T. gondii EVs and/or T. gondii‐infected EVs has been increasingly reported in the past years. Ultracentrifugation has been far from the most used method for EVs purification in this model, but gradient and SEC have been recently included. Although the most used strain is RH, comparative studies also include ME‐49 and VEG. They all successfully release EVs in quality and amounts enough for different molecular, immunological and biochemical approaches. However, there is also the need to standardize the cell model for cultivating the parasite since it successfully infects other cells. We do not know to which extent the cell type will affect parasite release and consequently the EV release by T. gondii. It is worth mentioning that over the years the methods and techniques to quantify and characterize EVs from T. gondii have evolved and become more sophisticated, summarized in Table 3.
TABLE 3.
Studies on Toxoplasma gondii EVs.
| Reference | Vesicle cellular origin | Cell amounts | Vesiculation general conditions | Cell viability at time of EV harvest | Pre‐isolation/cell debris remotion | EV isolation and/or purification | EV characterization | EVs yield per batch (size and concentration) | In vitro/in vivo experiments using EVs | Markers |
|---|---|---|---|---|---|---|---|---|---|---|
| Aline et al., 2004 | Exosomes excreted by DCs infected with tachyzoites | 1 × 106 DC cells/mL | DCs cultured for 18 h and supernatant recovered | NR |
C ‐ 3000 × g/30 min 10,000 × g/30 min |
UC‐ 100,000 × g/60 min Pellets resuspended in PBS (200 μL) containing protease inhibitors. |
TEM Protein concentration |
NR | Immunization | NR |
| Beauvillain et al., 2007 | Exosomes excreted by DCs treated with tachyzoites antigen | NR | DC supernatant recovered | NR |
C ‐ 3000 × g/30 min 10,000 × g/30 min |
UC −100,000 × g/60 min |
TEM Protein concentration |
Exosomes‐10 μg | Immunization | NR |
| Beauvillain et al., 2009 | Exosomes excreted by DCs infected with tachyzoites | NR | DC supernatant recovered | NR |
C ‐ 3000 × g/30 min 10,000 × g/30 min |
UC 100,000 × g/ 60 min |
TEM Protein concentration |
NR | Immunization | NR |
| Pope & Lasser, 2013 |
HFF infected with tachyzoites in DMEM 10% FBS |
NR |
Tachyzoites in FBS free DMEM used to induce exosome in incubation period of 24 and 72‐h |
NR |
C (4°C). 300 × g/10 min 16,500 × g/20 min |
UC 140,000 × g/45 min. EVs resuspended in 200 μL PBS |
TEM | NR | Microarray miRNA isolation | NR |
| Kim et al., 2016 | L6 cells infected with tachyzoites | 5 × 105 L6 cells infected with tachyzoites | Infected L6 cells supernatant recovered after 12 h | NR |
C 300 × g/10 min 2000 × g/10 min 10,000 × g/30 min |
UC in 2 steps of 100,000 × g/70 min. Pellet resuspended in 300 μL of PBS. |
FC | NR | miRNA microarray | NR |
| Wowk et al., 2017 | HFF cells in DMEM infected with tachyzoites | HFF cells infected with tachyzoites (MOI 10) |
Incubation of parasites in FBS free DMEM 2 h/37°C EV‐containing supernatants filtrated in 0.45 μm sterile cartridges |
Observation of tachyzoites viability and concentration | C to remove parasites | Total Exosome Isolation kit (Invitrogen) |
NTA TEM Protein concentrations in Quibit fluorometric quantification |
NR | PA | NR |
| Silva et al., 2018 | VERO cells in DMEM 10% FBS infected with tachyzoites | NR |
Tachyzoites in FBS free DMEM (1 mL) incubated at 37°C in different times (1, 3, 6, 12, 20 and 24 h). EV‐containing supernatants filtrated in 0.22‐μm sterile cartridges |
In TEM | C – 3500 × g/10 min. | SEC |
NTA TEM SEM Protein concentration |
NR |
EV proteins analysis and immunoblotting miRNA isolation Cytokine assay |
NR |
| Li et al., 2018b | HFF infected with tachyzoites | 100 mL of supernatant containing tachyzoites |
Tachyzoites maintaining in FBS free DMEM (100 mL) for 24 h EV‐containing supernatants filtered in 0.22 μm sterile cartridges and concentrated in a filter unit of Vivaflow 100 kDa MWCO PES (EMD Millipore, Billerica, MA, USA) |
TEM | C ‐ 5000 × g/10 min | ExoEasy Maxi Kit (Qiagen, Hilden, Germany). |
NTA TEM FC |
80 μg of EVs from × in 8 106 tachyzoites DMEM (100 mL) |
Macrophage cytokine assay Immunization and challenge Uptake of T. gondii exosomes by macrophages |
CD63 P30 HSP70 |
| Li et al., 2018a |
DC2.4 in 10% EVdepleted FBS RPMI infected with tachyzoites |
DC2.4 cells infected with tachyzoites (MOI 3) for 28 h |
Supernatants collected for exosome isolation. EV‐containing supernatants filtrated with a 0.22 μm sterile cartridges |
In TEM |
C ‐ 2000 × g/20 min 10,000 × g/30 min |
UC (4°C) 100,000 × g/60 min Pellet resuspended in PBS (0.1 mL) |
NTA TEM Exosomal proteins Immunoblotting |
NR |
Total and small RNA extraction RNA sequencing miRNA sequencing Analysis of the differential exosomal miRNAs |
CD63 TSG101 |
| Ramirez‐Flores et al., 2019 | MDCK cells cultured in coverslips with DMEM and infected with tachyzoites | 3 × 108 tachyzoites/mL |
Tachyzoites in sterile PBS incubated at 4 h (37°C) EV‐containing supernatants filtrated with a 0.22 μm sterile cartridges |
Tachyzoites viability determined by the exclusion of SYTOX‐acid green nucleic stain |
C at 4°C 200 × g/10 min 10,000 × g/10 min |
UC 150,000 × g at 4°C twice |
NTA TEM Protein concentration Immunoblotting |
NR |
Molecular and morphological identification TEM SEM Ultrastructure EVs: Immune‐recognition Self‐assembled tubular structures |
Lamin B1 α‐Tubulin TgARO H3 β‐Tubulin |
| da Cruz et al., 2020 | Human EVs purified of CSF and sera from patients with toxoplasmosis |
CSF: 600 μL Serum:300 μL |
NR | TEM |
C 13,500 × g/15 min |
UC 100,000 × g/60 min at 25°C. Pellets, containing EVs, resuspended in 100 μL of filtered PBS. |
NTA TEM Protein concentration Immunoblotting |
Toxoplasmosis patients: mean of 2.4 ×1010 EVs/mL Seronegative individuals: mean of 5.9 × 109 EVs/mL |
Human miRNA expression |
CD63 CD9 |
| Maia et al., 2021a | VERO cells in DMEM 10% FBS infected with tachyzoites | NR |
Tachyzoites in FBS free RPMI 1640 (1 mL) incubated for 2 h/37°C. EV containing supernatants filtrated with a 0.22 μm sterile cartridges. |
TEM |
C (5x) 2500 × g/10 min After vesiculation: Centrifugation 3500 × g/10 min Murine sera centrifugation 13,500 × g/15 min |
SEC Murine sera (1 mL) Ultracentrifugation 100,000 × g/60 min (25°C) |
NTA TEM Protein concentration Immunoblotting |
Mice: pooled serumderived EVs (1 mL): Normal: 2.22 × 1010 EVs/mL Immunized: 8.40 × 1010 EVs/mL) Infected: 8.92 × 1010 EVs/mL |
Murine miRNA expression Murine cytokine determination |
CD63 CD9 |
| Maia et al., 2021b | VERO cells in DMEM 10% FBS infected with tachyzoites | 1 × 109 tachyzoites |
Tachyzoites in FBS free RPMI 1640 (1 mL) incubated for 2 h/37°C. EV containing supernatants filtrated with a 0.22 μm sterile cartridges |
TEM |
C (5x) 2500 × g/10 min After vesiculation: Centrifugation 3500 × g/10 min |
SEC |
NTA BCA TEM Protein concentration Immunoblotting |
2.55 × 109 EVs/mL |
Immunizations and challenge Stimulation of cellular and humoral responses |
NR |
| Quiarim et al., 2021 | VERO cells in DMEM 10% FBS infected with 3 T. gondii strains tachyzoites | NR |
Tachyzoites, of each T. gondii strain, in FBS free DMEM (1 mL) incubated for 2 h/37°C. EV containing supernatants filtrated with a 0.22 μm sterile cartridges |
TEM | ‐T. gondii EVs from culture supernatants were washed 5 times (2500 × g/5 min) and filtered. | SEC |
NTA TEM Immunoblotting SEM |
EV released in 2 h for each strain: 8.0 × 108 (RH), 1.9 × 108.(ME49), 4.8 × 108 (VEG) EV released in 24 h for the 3 strain: around 1.2 × 108 |
miRNA isolation by reverse transcription and quantitative realtime. Murine cytokine determination Virulence in mice |
NR |
Abbreviations: NR, not reported; C, centrifugation; UC, ultracentrifugation; FC, flow cytometry; NTA, Nanoparticle tracking analysis; PA, proteomic analysis; SEM, scanning electronic microscopy; TEM, transmission electronic microscopy.
3.3.2. EVS obtained from infected hosts and patients with toxoplasmosis
EVs can be obtained from sera of mice with toxoplasmosis by ultracentrifugation procedures and then quantified by NTA. Functional assays in vitro using splenocytes showed up‐regulated IFN‐γ, TNF‐α and IL‐17 compared with EVs obtained from control mice. miRNA composition analysis of these EVs showed higher amounts of miR‐155‐5p, miR‐29c‐3p and miR‐125b‐5p when compared to control mice (Meira‐Strejevitch et al., 2020). EVs isolated from toxoplasmosis patients’ samples were only described in 2020 (da Cruz et al., 2020). To study toxoplasmosis patients, EVs from serum or cerebral spinal fluid (CS) can be obtained by centrifugation at 13,500 x g for 15 min, and the supernatants ultracentrifuged at 100,000 x g for 60 min at 25°C (Pereira et al., 2020). Higher particles/mL can be observed in the serum of pregnant women with gestational toxoplasmosis and CSF patients with cerebral toxoplasmosis/HIV co‐infection. The presence of exosomes can be confirmed by Western blotting with antibodies against CD63 and CD9 and by TEM.
3.4. Neospora caninum
There is only one article on Neospora caninum (NC‐1 strain) EVs. Similar to T. gondii, it is also an obligate intracellular parasite that can easily grow in Vero cells in RPMI. EVs can be isolated from free tachyzoites incubated for 24 h in an exosome‐depleted medium. Then, a two‐step ultracentrifugation protocol (10,000 x g and 100,000 x g) can be employed. It is essential to mention that in that paper, the authors checked possible endotoxin contamination using the LAL Assay. Different from previous studies in T. gondii, checking endotoxin contamination should be a requirement for functional studies with exosomes from pathogens in innate immune protocols. Proteomic analysis showed the presence of 705 proteins with eight top 50 enriched ones. Three of them could be detected by immunoblotting, including 14‐3‐3, enolase and HSP70 (Li et al., 2021)
3.5. Plasmodium spp.
3.5.1. EVs obtained from red blood cell cultures
EV field research in malaria has achieved significant thanks to the continuous in vitro culture of P. falciparum (Pf) blood‐stages, which has allowed to investigate the role of Pf iRBCs‐EVs (Sampaio et al., 2017). Typically, EVs are purified from the conditioned medium supplemented with Albumax or EV‐depleted human serum. To avoid cell lysis, it is also important to keep a healthy growing culture, with parasitemia between 5% and 10%. Although not strictly necessary, keeping the culture in gentle agitation can help increase the yield of EV production (Mbagwu et al., 2017). In most of the studies, EVs are enriched by a combination of sequential rounds of centrifugation at increasing speed to remove contaminating cells and debris. The EVs are further purified by ultracentrifugation at 100,000 x g on a continuous 10%−30% OptiPrep (Axis‐Shield) gradient (Regev‐Rudzki et al., 2013) or by performing one step 60% sucrose gradient and spun at 100,000 x g for 16 h (Mantel et al., 2013). More recently, other methods, such as size exclusion chromatography has been applied for EV enrichment of culture supernatants (Avalos‐Padilla et al., 2021). In many cases, given the fact that it is necessary to use high concentration of EVs for functional assays, large volumes of culture medium are used. For this reason, the culture supernatants are filtered through a 0.45‐μm filter and concentrated down using a VivaCell 100,000 MWCO PES (Sartorious Stedium) (Dekel et al., 2021). Last year, has been applied a very sophisticated biophysical technique to isolate different EV subpopulations produced by P. falciparum iRBCs (Abou Karam et al., 2022). Using AF4, the authors identified two distinct EV subpopulations that differ in size and protein content. Large EVs are enriched in proteasome subunits and small EVs in complement‐system proteins.
Classically, NTA, WB, electron microscopy or FC have been used to quantify and characterized Pf iRBCs‐EVs. But recently, atomic force microscopy (AFM) has been applied to determine the size and morphology of Pf‐derived EVs (Abou Karam et al., 2022). Similarly, IFC is a high‐throughput instrument that allows rapid multispectral imaging of EVs. IFC enabled us before to fluorescently detect various EV cargo components, including proteins, DNA and RNA following their uptake to recipient host cells (Alfandari et al., 2021; Ofir‐Birin et al., 2018).
3.5.2. Plasma‐derived EVs from malaria patients
Many EV studies in the malaria field involved biological samples from malaria‐infected patients as a source of EVs isolation. In that sense, experimental design and pre‐analytical variables are factors to be considered. General and clinical data of patients (such as patient's location, age, biological sex, pregnancy, fasting status, time of sample collection and relevant clinical data related to malaria infection) are essential data to be collected to know the physiological status at the sample collection moment, which can strongly affect EVs composition. Gathering such data can result in post‐analysis statistical associations.
Blood sample collection can be influenced by several factors affecting the quality of plasma for the subsequent EVs isolation. These include needle gauge, collection tubes type, handling, the temperature of processing platelets depletion and storage. Those are essential parameters to be considered following MISEV 2018 guidelines (Théry et al., 2018).
Anticoagulant is required for the collection of blood samples for the isolation of plasma derived EVs. The selection of the anticoagulant reagent will depend on the downstream applications used for EVs analysis (nucleic acids analysis, etc.). The most common procedure used for blood sample collection for microparticles isolation from malaria patients include the use of tubes with anticoagulant trisodium citrate (Campos et al., 2010; Nantakomol et al., 2011; Sahu et al., 2013b) followed by use of EDTA vacutainer (Antwi‐Baffour et al., 2016; Moro et al., 2016) and acid‐citrate‐dextrose (Antwi‐Baffour et al., 2016). For medium and small‐size EVs, anticoagulants used included sodium citrate (Gualdron‐Lopez et al., 2021; Toda et al., 2020).
Platelet separation is required for EVs isolation from plasma. In the vast majority of reports, the isolation of malaria patient's plasma microparticles involved two centrifugation steps (1500 x g for 15 min, followed by 13,000 x g for 2, 3 or 10 min) (Campos et al., 2010; Nantakomol et al., 2011; Sahu et al., 2013b). For medium and small EVs, platelet depletion has been reported to be achieved by two sequential centrifugation steps (400 x g for 10 min at RT followed by 2000 x g for 10 min at 4°C) (Gualdron‐Lopez et al., 2021; Toda et al., 2020) generating the starting plasma for the subsequent EVs purification. After obtaining platelet‐free plasma, samples can be either processed directly for EVs isolation or can be aliquoted and stored for posterior analysis at −80°C in cryovials.
For EV isolation, the first studies reporting EVs from plasma of malaria patients focused on the microparticle analysis. These are typically obtained by diluting plasma samples in a standard PBS, or a washing buffer containing HEPES before its processing by ultracentrifugation. Diluted platelet‐free plasma is ultracentrifuged (different speed and centrifugation times has been reported going from 14,000 x g for 90 min (Campos et al., 2010), 19,000 x g for 130 min (Antwi‐Baffour et al., 2016) up to 100,000 x g for 30, 60 and 70 min (Moro et al., 2016; Sahu et al., 2013a, 2013b), respectively. Other study has also processed two‐fold diluted plasma by sonication using a sonicating water bath (5 × 1 min) before performing differential centrifugation to separate medium size EVs (15,000 x g for 40 min) and small EVs (100,000 x g for 90 min) (Antwi‐Baffour et al., 2020). Importantly, plasma‐derived microparticles and EVs obtained by ultracentrifugation require extensive washes in standard PBS, sodium citrate‐PBS or wash buffer to remove abundant contaminating plasma proteins. Recent studies have also reported the use of SEC (using commercial qEV (iZon Science) 10 mL Sepharose CL‐2B columns or in‐house‐packaged Sepharose CL‐2B (Sigma‐Aldrich) as a convenient method for the isolation of plasma‐derived EVs from P. vivax malaria patients (Gualdron‐Lopez et al., 2021; Toda et al., 2020). Important considerations related to the EV's downstream applications (Ex. functional assays, proteomics, NTA, etc.) need to be considered before employing this technique. For available analysis, in‐house packed Sepharose columns should be prepared under sterile conditions. Notably, the columns type must be prepared and stored O/N at 4°C to allow Sepharose resin stacking. Columns and equilibrating buffers must be kept at room temperature during EVs purification to guarantee appropriate mobility of particles according to their size in the resin matrix. After column equilibration with at least a 5‐column volume of equilibrating buffer, plasma samples are carefully loaded into the columns with an open flow and allowed to enter the resin matrix completely. Usually, 15 fractions of 0.5 mL are collected using sterile low protein binding tubes. SEC fractions enriched in EVs (usually 7–9) can be used directly in downstream applications or aliquoted and stored at −80°C. More recently, using plasma of P. vivax‐infected patients it has been implemented the direct immune‐affinity capture (DIC) technique for the isolation and enrichment of CD71+‐EVs coming from reticulocytes with the final objective of determined their protein composition by mass spectrometry‐based proteomics and to identify parasite antigens associated with circulating EVs in natural infections. Once purified, EV protein content can be quantified using several methodologies employing beads (flow cytometry), light scattering technologies (NTA) or commercial kits such as BCA.
For characterization of large size EVs, such as microparticles, can be performed a direct flow cytometry analysis using reference beads or immune‐phenotyped using antibodies against surface markers from their cell of origin. This strategy has been largely used in the initial studies of microparticles from malaria patients. Commonly, conjugated Annexin V antibody is used to perform the characterization of these EVs together with other conjugated antibodies to detect markers of their cell of origin such as CD41a, CD144, CD235a, CD45, CD14, CD31, PSG1, CD3, CD11b, CD105 and CD51 (Campos et al., 2010; Nantakomol et al., 2011; Sahu et al., 2013a, 2013b) (Moro et al., 2016). Several types of beads are employed in these characterization assays, some studies have reported the use of commercial synthetic 0.7–0.9 μm SPHERO™ Amino Fluorescent Particles (Antwi‐Baffour et al., 2016; Campos et al., 2010), while others used fluorescent beads (TrueCount™) (Sahu et al., 2013a, 2013b).
Additionally, fluorescent calibration microspheres of diameters 0.2 and 1 μm (Invitrogen Molecular Probes) are utilized in the publication of Moro (Moro et al., 2016), while FlowcountH beads are employed by Tiberti et al. (2013). employed flow count beads. The next step is incubating the beads with the relevant antibodies before data acquisition on the flow cytometer for further quantification. The bead‐based assay methodology is used for the characterization of small EVs. It is necessary to couple EVs to beads of several micrometers in size because small EV size is under the limit of detection with commonly used flow cytometers. This methodology has only been reported for the isolation of EVs from P. vivax. Once the EVs are obtained coupling step to 4 μm‐aldehyde/sulfate‐latex beads (Invitrogen) is performed for 15 min at RT. Beads are resuspended in 1 mL of bead‐coupling buffer (BCB) and incubated O/N at RT on rotation. EV‐coated beads are then centrifuged at 2000 x g for 10 min at RT and washed once with BCB before incubation with primary antibodies (Gualdron‐Lopez et al., 2021; Toda et al., 2020).
The use of TEM methodology has become a valuable option in the field of EVs for its capability to detect and characterize single EV as well as to give information regarding biochemical properties of EV surface proteins once employed immune‐gold labeling (Cizmar & Yuana, 2017). The only study in which TEM methodology was reported to be employed for EVs characterization was done by Antwi‐Baffour (Antwi‐Baffour et al., 2020). Additional information on methods and findings in Malaria EV‐field is summarized in Table 4.
TABLE 4.
Studies on Plasmodium EVs.
| Reference | Vesicle cellular origin/Biofluid | Cell amounts/volume | Vesiculation general conditions | Cell viability at time of EV harvest | Preisolation/cell debris remotion | EV isolation and/or purification | EV characterization |
EVs yield per batch (size and concentration) |
In vitro/in vivo experiments using EVs/ main findings |
Markers |
|---|---|---|---|---|---|---|---|---|---|---|
| Combes et al., 2004 | Plasma‐derived microparticles (MPs) from P. falciparum infected patients | 0.5 mL citrated blood | NR | NR | 1500 × g/15 min |
Differential centrifugation Supernatant from 13,000 × g/2 min |
FC | NR | MPs present in infected individuals | CD51 |
| Combes et al., 2005 |
Plasma‐derived MPs from P. berghei ANKA infected mice |
0.4 mL citrated blood | NR | NR | 1500 × g/15 min |
Differential centrifugation supernatant from 13,000 × g/2 min |
FC using markers of different human cells | NR | MPs involved in cerebral malaria | CD51, CD14, CD41 |
| Faille et al., 2009 | Platelet‐derived MPs from healthy donors | 1 × 109/mL platelets in HEPES buffer |
Platelets were incubated for 45 min/37°C in calcium ionophore |
NR |
2000 × g/6 min (2×) 13,000 × g/60 min |
Differential centrifugation 20,800 × g/2 min |
FC using markers of different human cells | NR |
Binding assay/Platelet MPs are involved in iRBC cytoadhesion to human brain endothelial cells |
Annexin V |
| Campos et al., 2010 |
Plasma‐derived MPs from P. vivax infected patients |
0.5 mL citrated blood | NR | NR |
1500 × g/15 min 13,000 × g/3 min to obtain platelet free plasma |
Differential centrifugation 14,000 × g/90 min at 15°C |
FC using markers of different human cells | NR | MPs in infected individuals are associated with malaria disease severity | Annexin V, CD41a, CD144, CD235a, CD45, CD14 |
| Pankoui Mfonkeu et al., 2010 | Plasma‐derived MPs from P. falciparum infected patients | 2.0 mL trisodium citrated blood | NR | NR | 1500 × g/15 min |
Differential centrifugation supernatant from 13,000 × g/2 min |
NR | NR | MPs in infected individuals are associated with cerebral malaria | Annexin V, CD41, CD63, CD11b, CD105, CD235a |
| Nantakomol et al., 2011 | Plasma‐derived MPs from P. falciparum, P. vivax and P. malariae infected patients | 0.5 mL trisodium citrated blood | NR | NR | 1500 × g/15 min |
Differential centrifugation 13,000 × g/2 min |
FC using markers of different human cells | NR | MPs are released from iRBC during active infection | Annexin V, glycophorin A, |
| Martin‐Jaular et al., 2011 | Plasma‐derived exosomes from P. yoelii infected mice and from ex‐vivo culture of infected reticulocytes (iRet) |
0.5 mL citrated blood or 3.6 × 108 iRet |
iRet were cultured for 24 h at 37°C in DMEM supplemented 5% FCS EV‐depleted |
NR | 500 × g/30 min |
Differential centrifugation 15,000 × g/45 min 100,000 × g/2 h at 4°C or centrifuge on density gradient 30% sucrose |
Bead based assay by FC, TEM, proteomic analysis | 5 mg exosomes/immunization | Immunizations/iRBC release exosomes with parasite antigens; exosomes can be used to immunize naive mice |
Lamp1 Tfr Itga4 CD36 MHCII CD41 |
| Sahu et al., 2013a, 2013b | Plasma‐derived MPs from P. falciparum infected patients | Venous blood was collected in 0.129 mol/of trisodium citrate/liter | NR | NR | 1,500 × g for 15 min at 20°C to make platelet rich plasma (PRP) |
Differential centrifugation 13,000 × g for 10 min at 20°C |
FC using markers of different human cells | NR | MPs in infected individuals are associated with severe malaria and TNF levels |
Annexin‐V Glycophorin A CD31 for CD41a |
| Regev‐Rudzki et al., 2013 |
Exosome‐like vesicles derived from P. falciparum in vitro culture |
2 mL supernatant (SN) of P. falciparum culture |
iRBCs were maintained in in RPMI‐HEPES supplemented with 0.5% (w/v) Albumax at 2–4% hamatocrit | NR | NR |
Ultracentrifugation 250,000 × g for 18 h at 4°C through a continuous 10%–30% OptiPrep gradient. Fractions (1 mL) were collected from the top of the gradient |
Atomic Force Microscopy, negative staining and cryo‐TEM |
NR |
Transwell experiments/ Exosomes used for intraparasitic communication, inducing gametocytogenesis |
NR |
| Mantel et al., 2013 | Microvesicles (MVs) derived from P. falciparum in vitro culture | 1 L of SN mixed culture |
iRBCs were maintained in in RPMI‐HEPES supplemented with 10% heat inactivated human serum depleted of EVs |
NR |
600 × g, 1600 × g, 3600 × g and finally 10,000 × g for 15 min each. The SN was concentrated by filtration through a Vivacell 100 kDa filter |
concentrated supernatant was pelleted at 100,000 × g, pellet resuspended in PBS and layered on top of 60% sucrose cushion and spun at 100,000 × g for 16 h |
ImageStream and flow cytometry, electron microscopy, BCA, NTA, WB, immunoblot, RNA analysis, proteomic analysis |
50 μg/100,000 macrophages 1 μg/100,000 neutrophils 50 μg/RBCs (2% HCT/5% parasitemia) |
RMV internalization and activation of monocyte‐derived human macrophages; neutrophil activation; internalization assays into RBCs/MVs derived from P. falciparum iRBCs mediate cellular communication within the parasites and with the host immune system. |
Stomatin and band 3 (human markers); RESA, and SBP1 (parasite markers) |
| El‐Assaad et al., 2014 |
MPs derived from plasma of P. bergheiANKA infected mice |
venous blood in 0.129 mol/L sodium citrate (ratio of blood to anticoagulant 4:1) |
NR | NR | 1500 × g for 15 min at 20°C |
Differential centrifugation 18,000 × g for 4 min at 20°C (2×) or 18,000 × g for 1 h |
FC using human markers |
400 × 103 MP in 200 mL of PBS/mouse |
Biodistribution of labelled MPs in recipient mice/ MPs localize to brain during infection, and can directly induce pathology |
Annexin‐V |
| Tiberti et al., 2016 |
MPs derived from plasma of P. bergheiANKA infected mice and plasma from P. falciparum infected patients |
Venous blood in 3.2% sodium citrate |
NR | NR | 1500 × g for 15 min at 20°C |
Differential centrifugation 18,000 × g for 3 min at 20°C followed of 18,000 × g for 45 min at 15°C |
FC using human markers, SEM, proteomic analysis |
NR |
Proteomic characterization of MPs in cerebral malaria |
Annexin‐V |
| Moro et al., 2016 |
Plasma‐derived MPs from P. falciparum or HIV infected patients |
NR | NR | NR | 1000 × g for 10 min at 20°C |
Differential centrifugation 10,000 × g and ultracentrifugation at 100,000 × g 30 min at 4°C |
FC using human markers, electron microscopy, qPCR | MPs from 200 mL of plasma |
Analysis of placental microRNA content by qPCR; in vitro stimulation of dendritic cells with MPs/ Description of placental MPs and microRNAs in pregnant women with P. falciparum or HIV infection |
Annexin‐V Glycoprotein1 (PSG1, trophoblast marker) |
| Abdi et al., 2017 | EVs from a Kenyan P. falciparum clinical isolate adapted to in vitro culture | 100 mL of SN of P. falciparum in vitro culture |
6 flasks, each containing 500 μL packed cells at 5%−10% parasitemia cultured in RPMI with AlbumaxII |
NR |
440 × g for 5 min (2×) 2000 × g for 10 min (2×) |
Differential centrifugation 3600 × g for 10 min 15,000 × g 30 min. SN was filtered 0.2 mm and centrifuged at 150,000 × g for 2 h. Pellet washed in PBS and spun at 150,000 × g for 2 h. Final pellet was loaded onto OptiPrep™ density gradient and centrifuged at 250,000 × g /18 h |
TEM, proteomic analysis | NR | Proteomic analysis of EVs from P. falciparum Kenyan clinical isolate | NR |
| Antwi‐Baffour et al., 2016 | MPs derived from plasma of P. falciparum infected patients |
3 mL of EDTA‐ anticoagulated blood |
NR | NR |
160 × g for 5 min 4000 × g for 60 min |
Differential centrifugation 19,000 × g for 120 min |
FC, PA, WB | NR | Proteomic analysis of MP derived from P. falciparum infected patients | Annexin‐V |
| Sisquella et al., 2017 | EVs from P. falciparum in vitro culture | NR | Parasites were maintained in RPMI medium/HEPES/A lbumaxII. | NR | Differential centrifugation at 400 × g, 1900 × g, and 17,000 × g |
SN concentrated using a Vivaflow 100,000 MWCO PES and centrifuged at 150,000 × g to pellet nanovesicles followed of OptiPrep density gradient |
Cryo‐TEM, NTA, WB, DNA and RNA isolation |
Vesicles intake: ∼5 × 106 EVs per 1 × 106 cells for 5 min at 37°C/ 5% CO2 |
Vesicle intake by THP‐1 cells or PBMCs/Ring‐stage P. falciparum‐iRBC (PfiRBCs) EVs contain parasite gDNA; ring stage Pf‐EVs activate immune gene induction in monocytes | Actin STING pIRF3 pTBK1 |
| Sampaio et al., 2017 | EVs from P. falciparum in vitro culture | NR | Parasites or uRBC were grown in 0.5% AlbumaxII for 12 h | NR |
sequential centrifugation at 300 × g for 5 min and 3000 × g for 10 min. SN through 0.2‐μm filter |
Ultracentrifugation at 120,000 × g overnight at 4°C | BCA for protein concentration, WB, proteomic analysis | PBMCs were stimulated with 1, 5 or 10 μg/mL EVs for 12 h at 37°C in 5% CO2 |
Monocyte stimulation with parasite‐derived EVs/Parasite‐derived EVs contain PfEMP1 and induce transcriptional changes in human monocytes |
SR1 PfEMP1 Pf Aldolase Pf ADF1 |
| Babatunde et al., 2018 | EVs derived from P. falciparum in vitro culture | 1 L of SN mixed culture |
iRBCs were maintained in in RPMI‐HEPES supplemented with 10 % heat inactivated human serum depleted of EVs |
NR |
600 × g, 1600 × g, 3600 × g and finally 10,000 × g for 15 min each. The SN was concentrated by filtration through a Vivacell 100 kDa filter |
100,000 × g, the pellet resuspended in PBS and layered on top of a 60% sucrose and spun at 100,000 × g for 16 h. Interphase was collected and washed with PBS (2×) at 100,000 × g for 1 h |
Isolation of RNA (Bioanalyzer), TEM, WB, NTA |
NR |
RNA seq from EVs Taken up experiments by endothelial cells/Pf‐iRBCs release smallRNAs through EVs |
RESA Stomatin Bip |
| Gualdron‐Lopez et al., 2018 |
Plasma‐derived exosomes from liver‐chimeric humanized mice infected with P. vivax |
0.3–0.5 mL of plasma (obtained from heparin blood) |
NR | NR | 2000 × g for 5 min at 4°C (2×) | Size exclusion chromatography (SEC) (iZON columns) following commercial instructions |
Bead‐Based Flow Cytometry assay (BBA), cryo‐EM, NTA, WB, BCA for protein concentration, PA |
NR |
Proteomic analysis/ First proteomic characterization of plasma‐derived EVs from P. vivax‐infected liver chimeric humanized mice |
CD9 CD5L |
| Demarta‐Gatsi et al., 2019 | EVs from supernatants of cultured P. berghei iRBCs | 50 mL of SN |
iRBCs obtained from mice were cultured for 12–16 h on a shaker at 37°C in 50 mL of RPMI1640 medium |
NR |
400 × g for 10 min at 4°C 2000 × g for 10 min at 4°C |
Sequential ultracentifugation (UC) at 15,000 × g for 2 h at 4°C to get rid of microvesicles, and EVs pelleted at 150,000 × g for 3 h at 4°C, washed in PBS and UC 150,000 × g for 2 h at 4°C |
NTA, Atomic force microscopy, TRPS analysis (qNano), WB, EV labelling and Amnis ImageStreamX analysis, PA | NR |
P. berghei iRBC‐derived EVs inhibit antigen‐specific T cell proliferation |
EF‐1a |
| Toda et al., 2020 | Plasma‐derived EVs from P. vivax infected patients |
1 mL of plasma from blood collected in sodium citrate |
NR | NR | 2000 × g for 10 min at RT (2×) | SEC (iZON columns) |
BBA, BCA, NTA, WB, STED microscopy, proteomic analysis |
For binding assay: 2.5 × 104 cells/well were incubated for 24 or 48 h with 50 μL of EVs. For NF‐kB inhibition assay: 20 μg/mL of EVs |
In vivo distribution of EVs in mice; EVs uptake by human spleen fibroblast; NF‐kB nuclear translocation/ Plasma‐derived EVs from P. vivax patients contain parasite proteins and facilitate parasite cytoadherence to spleen fibroblasts |
CD9 CD63 CD81 Gal3 CD5L CD71 |
| Ofir‐Birin et al., 2021 | EVs derived from P. falciparum in vitro culture | NR | Parasites were maintained in RPMI medium/HEPES/A lbumaxII. | NR | Differential centrifugation at 400 × g, 1900 × g, and 17,000 × g |
SN concentrated using a Vivaflow 100,000 MWCO PES and centrifuged at 150,000 × g to pellet nanovesicles followed of OptiPrep density gradient |
NTA, WB |
1 × 106 THP1 cells/mL were treated with Pfderived EVs, at a final concentration of 1 k EVs/cell |
Uptake of Pf‐derived EVs by monocytes/Pf‐derived EVs inhibit the translation of CXCL10. |
CXCL‐10 HSP90 |
| Dekel et al., 2021 | EVs derived from P. falciparum‐iRBCs | 200–400 mL of a parasite growth medium | Pf‐iRBC at ∼8% parasitemia and 4% hematocrit (highly synchronized), were cultured 24 h post invasion into the RBC | NR | 413 × g for 5 min, 1650 × g for 10 min followed by centrifugation at 15,180 × g for 1 h |
Supernatant was filtered through a 0.45‐μm, concentrated using a VivaCell 100,000 MWCO PES and ultracentrifuged at 150,000 × g for 18 h to pellet EVs or by OptiPrep gradient fractionation. |
NTA, WB |
100 μL suspension of EVs yields a typical concentration of 1 × 1012 particles per ml |
Parasite growth assay/EVs derived from Pf‐iRBCs promote parasite growth in human RBCs; EVs secrete assembled and functional 20S proteasome complexes |
HSP90 SR1 PSMA1 PSMD1 |
| Avalos‐Padilla et al., 2021 |
EVs derived from P. falciparum‐iRBCs |
25 mL of culture SN |
Medium was collected at 40 hpi from pRBC cultures with an initial parasitemia of 3% |
NR |
400 × g for 10 min 2000 × g for 10 min |
25 mL SN was concentrated to 1 mL using Amicon Ultra‐15 centrifugal filter (100 kDa cut‐off). EVs were purified by SEC using a 10 mL homemade Sepharos e CL‐4B column |
Dynamic light scattering (DLS), EVs imaging by super‐resolution microscopy, dot blot | NR |
ESCRT‐III machinery participates in the production of EVs and protein export during Pf infection |
GPA PfVps32 PfVps60 PfBro1 |
| Aparici‐Herraiz et al., 2021 |
Plasma‐derived EVs from P. vivax infected patients |
1 mL of plasma from blood collected in sodium citrate |
NR | NR |
400 × g for 10 min 2000 × g for 10 min at RT |
1 mL of plasma was diluted 1:10 in cold PBS and subjected to UC at 120,000 × g for 4 h. Pellet resuspended in 300 μL of PBS and subjected to direct immune‐affinity capture (DIC) using anti‐CD71 |
BCA, WB, Proteomic analysis |
NR | Circulating CD71+ EVs obtained from natural infections contain diverse parasite proteins | CD71 |
| Gualdron‐Lopez et al., 2022 |
Plasma‐derived EVs from liver‐chimeric humanized mice infected with P. vivax |
0.3‐0.5 mL of plasma (obtained from heparin blood) | NR | NR | 2000 × g for 5 min at 4°C (2×) |
Size exclusion chromatography (SEC) (iZON columns) following commercial instructions |
Bead‐Based Flow Cytometry assay (BBA), NTA, WB, BCA for protein concentration, PA |
NR |
P. vivax Hypnozoite Biomarker Discovery |
CD9 CD5L |
| Abou Karam et al., 2022 |
EVs derived from P. falciparum‐iRBCs |
NR | Media of high parasitemia (≥5%) Pf‐iRBC cultures was collected. | NR |
Differential centrifugation at 413 × g for 5 min, 1900 × g for 10 min and 18,000 × g for 1 h |
SN concentrated using a Vivaflow 100,000 MWCO PES and centrifuged at 150,000 × g for 16 h at 4°C. Pellet resuspended in PBS and loaded on 2 mL 20% sucrose solution and UC at 100,000 × g for 4 h at 4°C. Asymmetric flow fieldflow fractionation (AF4) |
NTA, Atomic force microscopy, CryoTEM, PA, WB | NR |
P. falciparum parasites release EV subpopulations with signatures of different destinations |
NR |
| Alfandari et al., 2021 |
EVs derived from P. falciparum‐iRBCs |
NR | Media of high parasitemia (≥5%) Pf‐trophozoite blood stage culture was collected | NR | 413 × g for 5 min (2×), 1650 × g for 10 min followed by centrifugation at 15,180 × g for 1 h | Supernatant was filtered through a 0.45 μm, concentrated using a VivaCell 100,000 MWCO PES and ultracentrifuged at 150,000 × g for 18 h to pellet EVs | NTA, staining of RNA cargo | NR | Pf‐derived EV uptake by THP‐1 cells and macrophages/To study the distribution dynamics of the vesicle cargo post uptake to different types of cells. | NR |
Abbreviations: NR, not reported; C, centrifugation; UC, ultracentrifugation; ESCRT, Endosomal Sorting Complex Required for Transport; FC, flow cytometry; DLS, dynamic light scattering; NTA, Nanoparticle tracking analysis; PA, proteomic analysis; SEM, scanning electronic microscopy; TEM, transmission electronic microscopy.
3.6. Pathogenic ameboid protists
3.6.1. Entamoeba histolytica
E. histolytica trophozoites can be cultivated axenically in non‐defined media, mainly TYI‐S‐33 or YI‐S, at a strict physiologic temperature and pH 6.8. These media have components such as trypticase, yeast extract, glucose and vitamins, and 10% adult bovine serum must be added. Trophozoites grow under microaerophilic conditions using high medium column in regular culture tubes in an inclined position (Clark & Diamond, 2002). In the first report on E. histolytica EVs, serum‐free TYI‐S‐33 was used for vesiculation of the reference strain HM1‐IMSS, incubated for 16 h in an anaerobic chamber (Sharma et al., 2020). Based on this study, E. histolytica supernatants obtained by centrifugation (1000 x g) and passage through a 0.22 μm filters, contain EVs that can be ultracentrifuged (100,000 × g, for 12 h) and further purified by Total Exosome Isolation Reagent (Invitrogen). NTA and TEM should be used to characterize the produced EVs, which in the above work, comprised a predominant, homogeneous population of 125 nm. The authors comment that a possible drawback in this EVs isolation technique is the contamination with non‐EV proteins. Indeed, this problem usually arises with the use of non‐defined medium, which forms protein aggregates due to heterogeneous components as trypticase and yeast extract, that can hinder EVs analysis.
Covering this issue, a recent study on E. histolytica EVs reported the vesiculation of HM1‐IMSS strain in 3 mL of free‐serum RPMI‐1640 medium for 1 h at 37°C (Diaz‐Godinez et al., 2022). The authors also filtrate free‐cell supernatant in 0.22 μm membranes and used Total Exosome Isolation Reagent (Invitrogen), but they did not adopt the intermediary step of ultracentrifugation reported in the Sharma et al. study (Sharma et al., 2020). A predominant peak of 167 nm was identified by NTA, but other populations of 141, 230, 348 and 483 nm were found, characterizing a more heterogeneous EVs profile in this preparation. Since a defined medium was unavailable for E. histolytica, the defined medium RPMI is a suitable alternative for vesiculation. Future studies should include a careful assessment of the trophozoites' viability during the process. Of note, both studies showed cell surface galactose/N‐acetylgalactosamine‐binding lectin, and several typical exosome markers in E. histolytica EVs, but not tetraspanins. The use of antibodies targeting Lgl, and beta‐actin are potential markers to identify E. histolytica EVs. Additional information on methods used in E. histolytica EVs studies are described in Table 5.
TABLE 5.
Studies on Protista EVs.
| Reference | Vesicle cellular origin | Cell amounts | Vesiculation general conditions | Cell viability at time of EV harvest | Pre‐isolation/cell debris remotion | EV isolation and/or purification | EV characterization | EVs yield per batch (size and concentration) | In vitro/in vivo experiments using EVs | Markers |
|---|---|---|---|---|---|---|---|---|---|---|
| Sharma et al., 2020 |
Entamoeba histolytica trophozoites |
NR |
16 h‐incubation at 35.5°C/anaerobiosis in serum‐free TYI‐S‐33 a (10 mL) |
NR | C (2000 rpm) and filtration (0.22 mm) | Total Exosome Isolation reagent |
TEM, NTA, Small RNA cargo analysis |
125 nm (NTA) Concentration NR |
PA EVs signalizing in encystation | Lgl |
| Díaz‐Godínez et al., 2022 |
Entamoeba histolytica trophozoites |
2.5 × 105/mL |
1 h‐incubation at 37°C in RPMI (3 mL) |
NR | C (1400 rpm, 5 min) and filtration (0.22 mm) | Total Exosome Isolation reagent | TEM, NTA, Lipid staining (DiO dye) |
167 nm (NTA) Concentration NR |
PA Neutrophil uptake Neutrophil NETs and ROS assays | NR |
| Galindo et al., 2022 |
Entamoeba histolytica trophozoites |
NR | NR | NR | C (10,000 rpm, 30 min) | UC (100,000 × g 70 min) | TEM |
68‐110 nm (TEM) Concentration NR |
Labelling with antiVps23 antibody | EhVps23 |
| Costa et al., 2021 | Acanthamoeba trophozoites | 5 × 106/mL | 2 h‐incubation at 37oC, 180 rpm in serum free RPMI (8 mL) | >93% | C (700 × g, 10 min; 2000 × g, 20 min) and filtration (0.22 mm) | UC (100,000 × g 1 h) | TEM, SEM, NTA |
101‐150 nm (NTA) 1.2 x 109 to 8.6 × 1011/mL |
Zymography Murine macrophages stimulation (cytokines production) |
NR |
| Lin et al., 2020 | Acanthamoeba trophozoites | NR |
48 h‐ incubation at 28°C in PAS b (volume NR) |
NR | C (2000 × g 30 min; 10,000 × g, 30 min) and filtration (0.22 mm) | Total Exosome Isolation reagent | TEM, NTA |
118 nm (NTA) 2.83 × 1010/mL |
PA Rat glial C6 cells uptake and cytotoxicity THP‐1 cells stimulation (cytokines transcription) Aminopeptidase activity |
NR |
| Retana‐Moreira et al., 2019 | Acanthamoeba trophozoites | 4 × 106 in the total |
24 h‐incubation at 28°C and 37°C in PYG c (volume NR) |
>99% | C (3500 × g, 15 min; 17,000 × g, 15 min) and filtration (0.22 mm) | UC (100,000 × g 18 h) | DLS, AFM |
184 nm (28°C) 111 nm (37°C) Concentration NR |
Zymography | NR |
| Gonçalves et al., 2018 | Acanthamoeba trophozoites | NR |
48 h‐incubation at 28°C in PYG c and glucose medium d (volume NR) |
NR |
Concentration in 10 KDa cut‐off membrane, C (1100 × g, 10 min) and filtration (0.8 mm) |
UC (100,000 × g 1 h) | TEM, SEM, DLS |
117 nm (TEM) 150‐235 nm (DLS) Concentration NR |
PA Lipidomic (GC‐MS) Zymography CHO and T98G cells uptake and cytotoxicity |
NR |
| Lertjuthaporn et al., 2022 | Naegleria fowleri trophozoites | NR |
24 h‐incubation at 37°C in PAS b (volume NR) |
75 ± 9.41% | C (1500 × g, 15 min) and filtration (1.2 mm) |
C (21,000 × g, 70 min), fil‐tration (0.22 mm) and UC (110,000 × g 1.5 h) |
TEM, NTA |
∼158 nm‐ NfMP ∼141 nm—NfExo (NTA) ∼4.6 x 108/mL |
Uptake by THP‐1 macrophages THP‐1 macrophages stimulation (costimulatory molecules expression and inflammatory cytokines production) |
NR |
| Retana Moreira et al., 2022 | Naegleria fowleri trophozoites | 5 × 106/mL | 5 h‐incubation at 37°C in serum‐free 2% casein hydrolysate medium (10 mL) | 97.5% | C (3500 × g, 15 min; 17,000 × g, 15 min) and filtration (0.22 mm) | UC (120,000 × g 2.5 h) |
Protein Micro BCA assay, NTA, TEM, AFM, DLS |
206 nm (NTA) 4.96 × 1010/mL |
PA Zymography Immunoproteomic (using polyclonal antiN. fowleri antibodies) |
NR |
Abbreviations: NR, not reported; C, centrifugation; UC, ultracentrifugation; AFM, atomic force microscopy; NTA, Nanoparticle tracking analysis; DLS, dynamic light scattering; PA, proteomic analysis; SEM, scanning electronic microscopy; TEM, transmission electronic microscopy.
Trypticase, yeast‐extract, glucose, ascorbic acid, cysteine, ferric ammonium citrate, vitamins, salts and bovine serum, pH 6.8.
Pageʼs modified Neffʼs amoeba saline (only salts).
Proteose‐peptone, yeast extract, glucose, and salts, pH 6.5.
Glucose and salts (Proteose‐peptone‐free and yeast extract‐free PYG).
3.6.2. Acanthamoeba spp
Acanthamoeba can be cultivated regularly in vitro, but unlike E. histolytica, it supports aerophilic conditions and temperatures ranging from ambient to 37°C. Although defined media are already described for trophozoite cultures, the widely used is PYG (Proteose Peptone, yeast extract, glucose), within most of the strains can grow abundantly at around 30°C (Khan, 2006; Schuster, 2002). The pioneer study on Acanthamoeba EVs described a comparative analysis of vesiculation in PYG and in the same medium without peptone/yeast extract (termed glucose medium) (Goncalves et al., 2018). They showed that after 48 h of incubation at 28°C, cell debris could be separated by centrifugation (1100 g for 10 min), and an ultrafiltration system (10 KDa cut‐off membrane) could be used to reduce the 1 L of supernatant volume. Also using PYG, another study showed that vesiculation for 24–48 h in distinct temperatures (28°C and 37°C) preserves trophozoites viability in about 99% (Retana Moreira et al., 2020). A piece of evidence that Acanthamoeba tolerates well the switching to a defined medium is provided in Costa et al. investigation, which suggested vesiculation time of 2 h in RPMI as it recorded 95% of cell viability (Costa et al., 2021). Page's saline, a salt solution usually used for washing amoebas, is also an alternative for vesiculation (Lin et al., 2019). As the defined medium appears to work well to support Acanthamoeba, it should be preferable, especially in studies of functional assays that require EV purity, such as immune stimulation assays. Cell viability check is recommended.
In summary, after collecting the culture supernatant, cellular debris can be removed by two cycles of centrifugation followed by filtration through 0.22 μm membranes as suggested (Costa et al., 2021; Lin et al., 2019; Retana Moreira et al., 2020). Acanthamoeba EVs can be retrieved by ultracentrifugation (100,000 g) during times of 1, 2 or 18 h (Costa et al., 2021; Goncalves et al., 2018; Retana Moreira et al., 2020) or using the Total Exosome Isolation Reagent (Life Technologies) (Lin et al., 2019). The techniques applied to characterize Acanthamoeba EVs after purification could include TEM, SEM, DLS and AFM (Costa et al., 2021; Gonçalves et al., 2018; Lin et al. 2020; Retana Moreira et al., 2019). TEM allows to determine the vesicle size which can be extended to the entire population by DLS analysis (Goncalves et al., 2018). Vesiculation parameters as volume, number of cells per batch, and cell viability at the time of EV harvest can be found in detail in Table 5.
3.6.3. Naegleria fowleri
Only two studies reported the characterization of N. fowleri EVs so far (Lertjuthaporn et al., 2022; Retana Moreira et al., 2022). The authors used non‐defined formulation containing 2% casein hydrolysate or liver hydrolysate (Nelson´s medium) complemented with fetal bovine serum (FBS) to grow trophozoites at 37°C. In one of these studies, EVs production was induced in the casein hydrolysate medium without FBS and antibiotics for 5 h at the same temperature, in which the cell viability was 97.5% (Retana Moreira et al., 2022). To remove trophozoites and larger vesicles, the authors recommended centrifugation sequentially at 2500 × g for 15 min, and then at 17,000 × g for 30 min, both at 4°C. The 0.22 μm filtered supernatant can be ultracentrifuged once to pellet EVs and twice for washing in PBS, at 120,000 × g for 150 min. Besides TEM, AFM, DLS and NTA should be applied to characterize EVs. Retana‐Moreira et al.. performed zeta potential measurements with the obtained EVs. The negative charges in EVs indicate they could be efficiently incorporated and release their contents within the host cells (Retana Moreira et al., 2022). The authors complemented the study with functional assays to identify immunogenic proteins with polyclonal serum anti‐N. fowleri antibodies. They also performed complete proteomic analysis and determined protease activity by zymography.
To perform immune stimulation assays, vesiculation was conducted in 0.22 μm‐filtrated Page's amoeba saline without supplement for 24 h at 37°C. In this vesiculation protocol, trophozoites can reach 75′% viability a lower value compared to the previous work, in which the vesiculation was in Nelson´s medium (Lertjuthaporn et al., 2022). For immune assays, after cell debris separation (1500 × g, 15 min), the collected supernatants can be stored at −20°C for a posterior multi‐step differential centrifugation. The thawed supernatant is filtered through a 1.2 μm membrane and centrifuged twice (21,000 × g, 70 min) to pellet one fraction of EVs resuspended in PBS. Another fraction is separated by filtering the remaining supernatant in a 0.22 μm membrane, followed by two cycles of ultracentrifugation (110,000 × g for 90 min, 4°C), the last suspending the pellet in PBS.
Overall, the studies reported so far on amoebic protists EVs present variation in methods, mainly regarding the medium type and time of vesiculation, which had impact on cell viability. Considering the increasing interest on amoeba EVs topics, studies aiming to standardize vesiculation conditions could assure reproducibility and comparability in subsequent research in the area.
3.7. Non‐trypanosomatid flagellates
3.7.1. Giardia intestinalis
The first evaluation of Giardia EVs released in extracellular medium was reported by Evans‐Osses et al. (2017) which induced vesiculation in serum‐free, modified version of TYI‐S‐33. This medium, described to cultivate E. histolytica, is modified to support Giardia trophozoites in axenic culture, with the addition of bovine bile and increased amount of cysteine. Vesiculation can be performed for 1 h at 37°C, in 1 mL of this serum‐free medium containing 1 mM CaCl2, with 1 × 106 trophozoites. Two step‐centrifugation (2500 × g, 5 min; 4000 × g, 30 min) is adopted to remove cell debris, and then ultracentrifugation (100,000 × g for 2 h) to pellet the EVs. The suspensions can be dried using speed vacuum for storage and shipping. Therefore, it is recommended to conduct vesiculation in serum‐free/bile‐free TYI‐S‐33 for 1–4 h, at 37°C and with a defined amount of trophozoites. Viability should be checked after the vesiculation period. Cell debris should be removed by centrifugation (∼1500 × g for 15 min) and by filtration with 0.22 μm filter. EVs can be pelleted by ultracentrifugation (100,000 × g form 4 h). Further fractionation by sucrose gradient can be used to separate different fractions, and all collected by 200,000 × g for 1 h performed (Moyano et al., 2019). Possible separation of large (LEV) and small extracellular vesicles (SEV) can be alternatively achieved (Gavinho et al., 2020). A first centrifugation at 15,000 × g for 1 h separated a pellet of LEV, while the remaining supernatant was ultracentrifuged for 100,000 × g for 4 h to isolate SEV. The presence of 12.5% exosome‐depleted fetal bovine serum can be included and in this case the presence of additional CaCl2 might be omitted although longer vesiculation time may be required. Of note, a concern exists about the viability when incubations are made in absence of serum, which can result in cell stress and death, when long vesiculation times are required. Possible media changes as performed (Ma'ayeh et al., 2017) to obtain the secretome of G. intestinalis, incubating the parasites for 2 and 6 h. This report did not focus on EVs characterization but was devoted to investigating effects of the whole Giardia secretome on host cells by proteomic and functional analysis. However, it included methodological procedures to prove EVs release using ExoQuick‐TC Kit and TEM. The authors recommended a viability evaluation after incubation and showed that it reaches 98%. These findings indicated that, although the use of exosome‐free serum and other purification strategies could be used to obtain EVs from Giardia, employing a defined medium can be a feasible way to keep parasites viability. Summary of the current literature with the techniques for obtaining EVs from Giardia and their biological activities found are described in Table 6.
TABLE 6.
Studies on Giardia EVs.
| Reference | Cell amounts | Vesiculation general conditions | Cell viability at time of EV harvest | Pre‐isolation/cell debris remotion | EV isolation and/or purification | EV characterization | EVs yield per batch (size and concentration) | In vitro/in vivo experiments using EVs | Markers |
|---|---|---|---|---|---|---|---|---|---|
| (Evans‐Osses et al., 2017) | 1 × 106 trophozoites/mL | 1 h‐incubation at 37°C in serum free YISa +1 mM CaCl2 (1 mL) | NR | C (2500 × g, 5 min; 4000 × g, 30 min) | UC (100,000 × g, 1.5 h) | NTA, TEM, FC |
∼201 nm (NTA) 5.77 × 108/mL |
PA Attachment to Caco‐2 cells Immature Dendritic cells (iDCs) stimulation Uptake by iDCs Lipid raft dependence assays |
NR |
| (Ma'ayeh et al., 2017) | NR |
2 h‐incubation and 6 h at 37°C in RPMI (50 mL) |
∼98% | C (930 × g, 10 min) and filtration (0.22 um), protease inhibitor treatment |
Concentration with Amicon Ultra 3 kDa cutoff AlbuminOUT kit (serum residue elimination) ExoQuick TC Kit |
TEM |
NR (Focus on whole secretory products, not in EVs) |
Uptake by intestinal epithelial cells in vitro | NR |
| (Moyano et al., 2019) | 14 × 107 trophozoites in total |
4 h‐incubation at 37°C in Bovine bile free/serum free TYI‐S‐33b |
NR | C (1455 × g, 15 min) and filtration (0.11 mm) |
UC (100,000 × g, 3.3 h) SG (1.03−1.25 g/cm3) separation UC of SG fractions (200,000 × g, 1 h) |
DLS, TEM |
50‐100 nm (DLS) 80 nm in a SG enriched fraction (TEM) Concentration NR |
Biogenesis investigation (detection of ESCRT‐ associated proteins by CLSM and TEM) |
actin, tubulin, g14‐3‐3, gQa1, PDI2 |
| (Gavinho et al., 2020) | 1 × 106 trophozoites/mL |
1 h‐incubation at 37°C in serum free TYIS‐33b +1 mM CaCl2 (1 mL) |
NR | C (600 × g, 5 min; 4000 × g, 30 min) |
C (15,000 × g, 1 h)—Large extracellular vesicles (LEV) UC (100,000 × g, 4 h)—Small extracellular vesicles (SEV) |
Protein Micro BCA assay, NTA |
187.6 nm—LEV 67.7 nm—SEV ∼1 × 109/mL—LEV ∼1 × 108/mL‐ SEV |
PA EVs inhibition assays (Cl‐amine and Cannabidiol) Effect of EVs on Caco‐2 cells/parasite adherence Uptake by Caco‐2 cells |
NR |
| (Zhao et al., 2021) | 1 × 106 trophozoites/mL |
12 h‐incubation at 37°C in TYI‐S‐33b with exosome‐depleted serum (1 mL) |
NR |
C (2000 × g, 10 min; 10,000 × g 45 min) and filtration (0.22 mm) |
UC (100,000 × g, 1 h) | Protein Micro BCA assay, NTA, TEM |
143.5 nm (NTA) 4,7 × 1010/mL |
PA Uptake by murine peritoneal macrophages (MPM) Stimulation of MPM (qPCR and ELISA to evaluate cytokines transcription and secretion) |
NR |
| (Grajeda et al., 2022) | 1 × 107 trophozoites in total |
3 h‐incubation at 37°C in PBS with 5 mM L‐cysteine, 5 mM glucose, and 1 mM CaCl2, pH 7.1 |
>99% | C (600 × g, 5 min; 4000 × g, 30 min) |
C (15,000 × g, 1 h)—Large vesicles UC (100,000 × g, 4 h)—Small vesicles |
NTA, TEM |
100‐400 nm—large vesicles <100 nm—small vesicles Concentration NR |
PA Treatment with giardial lipid raft (gLR) disruptors |
NR |
Abbreviations: NR, not reported; C, centrifugation; UC, ultracentrifugation; CLSM, confocal laser scanning microscopy; ESCRT, Endosomal Sorting Complex Required for Transport; FC, flux cytometry; DLS, dynamic light scattering; NTA, Nanoparticle tracking analysis; PA, proteomic analysis; SEM, scanning electronic microscopy; SG, sucrose gradient; TEM transmission electronic microscopy.
eYeast extract, glucose, ascorbic acid, cysteine, ferric ammonium citrate, vitamins, salts and bovine serum pH 6.8.
fTrypticase, yeast‐extract, glucose, ascorbic acid, cysteine, ferric ammonium citrate, vitamins, salts and bovine serum, pH 6.8.
3.7.2. Trichomonas vaginalis
Two media widely used for axenic cultivation of T. vaginalis are TYI‐S‐33, the same used for E. histolytica, and TYM (trypticase, yeast extract, maltose) (Clark & Diamond, 2002). They must be complemented respectively with bovine and horse serum to support T. vaginalis growth, therefore, most of the current protocols include serum‐free version of these medium for vesiculate T. vaginalis. The first work on T. vaginalis was reported by Twu et al. (2013), which isolated EVs from serum‐free TYM supernatant containing 106 parasites/mL after 4 h of incubation. After centrifugation and filtration (0.22 mm filter), EVs can be collected by an initial ultracentrifugation (100,000 × g for 75 min), followed by a treatment with protease inhibitor and another ultracentrifugation step. The EVs can be further purified by floatation on a linear sucrose gradient.
Subsequent reports described similar procedures for vesiculation adopting the same incubation time (4 h) and serum‐free TYM containing 106 trophozoites/mL (Artuyants et al., 2020; Nievas et al., 2018; Rai & Johnson, 2019; Salas et al., 2021) or TYI‐S‐33 medium (Olmos‐Ortiz et al., 2017). Vesiculation can also be performed occurred in a yeast extract and iron‐serum media (YI‐S) without serum, also for 4 h at 37o (Ong et al., 2022). Addition of 1 mM CaCl2 (Nievas et al., 2018) and incubation at a higher parasite density (107/mL) (Olmos‐Ortiz et al., 2017) may improve the process. Cell debris remotion can be performed with steps of centrifugation and filtration, while ultracentrifugation is used to concentrate EVs (Artuyants et al., 2020; Nievas et al., 2018; Olmos‐Ortiz et al., 2017; Salas et al., 2021). If necessary further purification by sucrose floatation may be included (Ong et al., 2022; Rai & Johnson, 2019). NTA and TEM are recommended for EVs characterization (Artuyants et al., 2020) following MISEV2018. In T. vaginalis the mechanism of secretion can be studied by comparing EVs produced by wild type and transfected parasites (Salas et al., 2021). With this approach, VPS32, a member of ESCRT‐III, is shown to have a role in the biogenesis and cargo sorting of T. vaginalis EVs. However, Trichomonas viability during EVs collection has to be considered (Table 7), as serum depletion can affect trophozoites (Govender et al., 2020). Therefore, it is recommended to conduct release at a low cellular density (4 × 105 parasites/mL) and long time (24 h) and use medium without parasites as control in all assays, including functional analysis of immune activation. In this case, it is suggested to use Total Exosome Isolation Reagent instead of ultra‐centrifugation to obtain EVs with an additional purification step with Exosome Spin Columns.
TABLE 7.
Studies on Trichomonas EVs.
| Reference | Vesicle cellular origin | Cell amounts | Vesiculation general conditions | Cell viability at time of EV harvest | Pre‐isolation/cell debris remotion | EV isolation and/or purification | EV characterization | EVs yield per batch (size and concentration) | In vitro/in vivo experiments using EVs | Markers |
|---|---|---|---|---|---|---|---|---|---|---|
| (Twu et al., 2013) |
Serum‐free TYM medium parasite culture with 10% horse serum) |
∼1 × 106 cells/mL | 4 h/37°C | NR |
One step centrifugation (500 × g) Filtration (0.22 mm) Concentration with Vivaflow 200 100,000 MWCO PES |
UC (100,000 × g, 75 min) + Ressupension in 2 mL cold PBS+1 × HALT protease inhibitors + UC at 100,000 × g for 70 min + putification on sucrose gradient |
NTA BCA Kit TEM |
25 × 1014 |
‐ mRNA by Agilent 2000 Bioanalyzer ‐ Annexin V‐FITC in FC PKH‐67 |
NR |
| (Olmos‐Ortiz et al., 2017) |
TYI‐S‐33 medium without serum (Parasite culture with 6% bovine serum) |
1 × 107/mL | 4 h /37°C | NR | According Two et al. with modification One step centrifugation (500 × g) Filtration (0.22 mm) | UC ‐ 100,000 × g, 2 h |
TEM Comparative Electrophoresis Bradford |
NR |
‐NO dosage ‐Expression cytokines in vivo after EVs stimulation in mice ‐Inflammation (edema) in vivo after EVs application |
NR |
| (Nievas et al., 2018) |
Serum‐free TYM medium +1 mM CaCl2 (a test with and without 1 mM CaCl2 was performed) |
106 cells/mL |
37°C for 30 min to verify 1 M Ca Cl2 effect by tEM 37°C for 4 h to obtain MVs |
PI was used to show shedding of LVs (large vesicles >1 mm, nonapoptotic) |
C – 500 × g Filtration 0.8 mm Filtration 0.2 mm (0.2 filter also washed to recover MVs) |
UC 100,000 × g, 90 min |
SEM TEM DLS (380 and 63 nm) |
NR |
LC/MS Immunolocalization anti‐HA tag antibody PKH67 |
NR |
| (Rai & Johnson, 2019) |
Serum‐free TYM médium parasite culture with 10% horse serum) |
106 cells/mL | = Two et al. | NR | = Twu et al. | = Twu et al. | NR (focus in functional assays) | NR | ‐Cell uptake with EVs CFSE‐labeled and R18labled (Octadecyl Rhodamine B Chloride). Se of inhibitors to decipher mechanism | NR |
| (Artuyants et al., 2020) | Serum‐free Diamonds´ medium = TYM | 2‐4 L/ 106 cells/mL | 4 h/37°C | Reported (0.95%) only before vesiculation | = Twu et al. |
UC ‐ 100,000 × g, 75 min = Two et al, without sucrose step |
NTA—105 nm TEM |
2.74 × 1012/mL |
‐ RNA and DNA analysis → small RNA in EVs cargo (tested with and without triton X‐100) ‐RNA sequencing |
NR |
| (Govender et al., 2020) |
Diamond's medium = TYM 10% horse serum) They did not deplete the medium because of suboptimal growth. |
100 mL calculated from Mat & Met 4 × 105 /mL |
24 h/35°C |
Growth curve to evaluate depleted x complete medium |
C ‐ 2000 × g for 30 min) and filtration (0.22 μm filter) |
Total Exosome Isolation Reagent for cell culture medium Exosome Spin Columns (remotion of low molecular weight contaminants (MW < 3000) |
‐ ZetaView® Particle Metrix, by translational diffusion size distribution. ‐ NTA (76.6– 106.9 nm) ‐ TEM using CD63 |
1.1 × 109−1.65 × 1011/mL |
‐ EVs stimulation NF‐kB Activity in endocervical cells ‐ EVs stimulation IL‐8, IL‐6, IL‐10, and TNF‐a in PBMC ‐ Endocervical cells and PBMC viability ‐ EVs proteomic EVS FROM VIRUS POSITIVE Trichomonas HAVE IMMUNOSUPRESSIVE EFFECTS |
NR |
Abbreviations: NR, not reported; UC, ultracentrifugation; DLS, dynamic light scattering; NTA, Nanoparticle tracking analysis; PA, proteomic analysis; SEM, scanning electronic microscopy; SG, sucrose gradient; TEM, transmission electronic microscopy.
Overall, the need for complex, non‐defined and serum‐supplemented media represents a major challenge to obtain EVs from Giardia and Trichomonas. Some strategies can be adopted to improve the purity of EVs recovered from these protozoa and ensure the reproducibility of their functional effects; for instance, it is recommended to use of the medium alone as a control, adoption of shorter vesiculation times in defined media, supplementation with exosome‐depleted serum or additional purification techniques. In all cases, parasite viability should be a concern in future studies, considering that culture or cellular condition can influence protein expression and other functional parameters of EVs.
3.8. Helminths
Helminths include a great diversity of species, many of them parasites. EVs have emerged as a ubiquitous component of helminth excretory‐secretory products. To obtain EVs from most helminth parasites it is required to maintain them in their host, which makes the EV isolation and purification difficult. Therefore, in most cases, EVs are purified form parasites incubated in vitro and ex vivo, checking the viability of the parasites before and after the incubation. There are several reviews describing different methods to purify and characterize parasitic helminth EVs (Drurey & Maizels, 2021; Galiano et al., 2020; Sanchez‐Lopez et al., 2021) and very recently a review addressing general recommendations for methodologies and reporting in the helminth EV field have been published (White et al., 2023). In this position paper, a summary of the community‐agreed standards for studying EVs derived from helminths was produced with recommendations that would be of great help for those who are (or plan) working not only in helminths, but also in other non‐model organisms. EV research in the last years has demonstrated that it is important to choose the appropriate EV isolation method, which depends on the research question and the parasite source of EVs. Here, we only present some examples of EV isolation from trematodes, cestodes and nematodes, which could be helpful when starting to work with helminths (Table 8). This is not an exhaustive list of the published studies, and we apologize to the authors whose studies have not been included due to space restrictions.
TABLE 8.
Examples of studies on Helminth EVs.
| Reference | Vesicle cellular origin | Cell amounts | Vesiculation general conditions | Cell viability at time of EV harvest | Pre‐isolation/cell debris remotion | EV isolation and/or purification | EV characterization | In vitro/in vivo experiments using EVs | Markers |
|---|---|---|---|---|---|---|---|---|---|
| TREMATODES | |||||||||
|
Fasciola hepatica Echinostoma caproni (Marcilla et al., 2012) |
20‐30 mL/serum free RPMI1640 + protease inhibitor cocktail |
2 adults (F. hepatica)/mL 10 adults (E. caproni)/mL |
5 h/37°C | 100% |
Three steps centrifugation at 4°C (300 × g, 10 min; 700 × g, 30 min; 15,000 × g, 45 min) and filtration (0.22 μm) |
UC (120,000 × g, 1 h) |
TEM: 30‐100 nm |
PA TEM immunogold Uptake to intestinal cells (IEC‐18) FM4‐64 dye |
Enolase; Leucine aminopeptidase (LAP) |
| F. hepatica (Davis et al., 2019) | DMEM | 1 adult/mL | 5 h/37°C | 100% | Two steps centrifugation at 4°C (300 × g, 10 min; 700 × g, 30 min) |
UC (100,000 × g, 80 min) ‐concentration Amicon 10 KDa, centrifugation 4000 × g, 20 min and size exclusion chromatography (SEC) in qEV columns (Izon) |
TEM: 95‐505 nm (DC/UC) 76‐285 nm (SEC) |
PA |
fatty acid binding protein V; glutathione transferase (GST) sigma I; cathepsin L1 |
| (Murphy et al., 2020) | 200 mL RPMI | 1 adult/2 mL | 5 h/37°C | 100% | Two steps centrifugation at 4°C (300 × g, 10 min; 700 × g, 30 min) | Dialysis in 1000 kDa, then gravity flow and filtration 0.22 μm | TEM: 30–200 nm | PA Uptake dendritic cells |
Thioredoxin; LAP; HSP70; Annexins |
| (Trelis et al., 2022) | RPMI1640 + protease inhibitor cocktail | 20‐30 juveniles/mL |
4 h/37°C (juveniles) |
>90% |
Two steps centrifugation at 4°C (3000 × g, 10 min; 15,000 × g, 30 min) 2 h/37°C (eggs/miracidia) |
Filtration 0.22 μm and concentration Amicon 100 kDa (3200 × g, 20 min) and SEC in home‐ |
NTA: 160 nm (eggs); 200 nm (juveniles) |
PA made columns (Sepharose‐CL2B) |
Eggs: Thioredoxin; PEPCK; TEM: 100 nm Juveniles: Tubulin; HSP70; Enolase; 14‐3‐3 |
| Dicrocoelium dendriticum (Bernal et al., 2014) | RPMI1640 + protease inhibitor cocktail | 25 adults/mL | 5 h/37°C | 100% |
Three steps centrifugation at 4°C (300 × g, 10 min; 700 × g, 30 min; 15,000 × g, 45 min) and filtration (0,22 μm) |
UC (120,000 × g, 1 h) |
TEM: 30‐100 nm |
PA Transcriptomics (miRNAs) |
GAPDH; Enolase; HSP70; Annexins Let‐7 miR‐71 miR‐190 |
|
Schistosoma japonicum (Liu et al., 2018) |
RPMI1640 | 5 adult pairs/mL | 2 h/37°C | NR | Centrifugation at 4°C (2000 × g, 30 min) |
Filtration 0.22 μm and dyalisis. Exosome isolation kit (ThermoFisher Scientific) |
Zetasizer: 100‐400 nm TEM |
WB Transcriptomics (miRNAs) Uptake macrophages In vivo assays |
GAPDH; HSP70; HSP90 miR‐125b bantam miR‐61 |
| Schistosoma mansoni (Nowacki et al., 2015) | DMEM | 7500 schistosomula/mL | 72 h/37°C | 95% | Two steps centrifugation at 4°C (500 × g, 2 min; 700 × g, 20 min) | UC (120,000 × g, 80 min) | TEM: 30‐100 nm |
Proteomics Transcriptomics (miRNAs) |
Tetraspanins HSPs Annexins miR‐215 miR‐271 |
| (Sotillo et al., 2016) | serum‐free medium Basch | 50 adult pairs/4 mL |
7 days/37°C (sample recovered every 24 h) |
NR |
Several steps of centrifugation at 4°C (500 × g, 20 min; 2000 × g, 20 min; 4000 g, 20 min; 12,000 × g, 45 min) |
UC (120,000 × g, 3 h) followed by discontinuous Iodixanol gradient UC (60%−5%), 120,000 × g, 18 h |
NTA: 77‐98 nm; 8 × 109‐1.8 × 1010 TEM: 50‐100 nm |
WB and PA |
TSP‐2 HSP70 Enolase GST |
| (Kuipers et al., 2022) | DMEM |
7500 schistosomula/mL 10 adults/mL |
72 h/37°C 48 h/37°C |
NR |
Three steps centrifugation at 4°C (200 × g, 10 min × 2; 500 × g, 10 min × 2; 5000 × g, 30 min) |
DUC (100,000 × g, 65 min) Iodixanol gradient (60%−10% optiprep) ultracentrifugation (166‐169 kg, 15–16 h) SEC qEV (Izon) Ultrafiltration after gradient using Amicon 3 or 10 kDa (from 1100 worms) |
Cryo‐TEM: <100 nm | WB | TSP‐2 |
| CESTODES | |||||||||
|
Taenia crassiceps Mesocestoides corti Echinococcus multilocularis/ (Ancarola et al., 2017) |
10‐55 mL DMEM |
Cysticerci ND Tetrathiridia ND Metacestodes ND |
1‐4 days/37°C | 100% |
Three steps centrifugation at 10°C (500 × g, 10 min; 2000 × g, 20 min; 10,000 × g, 30 min) |
UC (100,000 × g, 70 min) | TEM: 30–200 nm |
PA Transcriptomics (RT‐PCR) (miRNAs) |
HSP70 Enolase Annexins Let‐7 |
|
Echinococcus granulosus (Zhou et al., 2019) |
3000 Protoescoleces/well 24 |
3 days/37°C | NR | Filter 0.8 μm |
exoEasy Maxi Kit (Qiagen) |
TEM: < 200 nm NTA: 30‐200 nm |
PA Uptake T cells |
Proteasome Annexin 6 |
|
| NEMATODES | |||||||||
|
Heligmosomoides bakeri (Buck et al., 2014) |
15 mL Serum free RPMI1640 |
1000 adults/15 mL | 3 days/37°C | NR | Filtration 0.22 μm, three centrifugation steps (300 × g, 10 min; 2000 × g, 10 min; 10,000 × g, 30 min) | UC −100,000 × g, 2 h. Concentration with Vivaspin 6 5 kDa (Thermo) | TEM: 50‐100 nm | PA Transcriptomics (miRNAs) In vivo assays |
TSP11 HSP70 Alix miR71 Bantam PKH67 label |
| (Chow et al., 2019) | 15 mL Serum free RPMI1640 | 1000 adults/15 mL | 24‐96 h/37°C | NR |
Centrifugation 400 × g, 10 min; filtration 0.22 μm; |
UC‐ 100,000 × g, 2 h and then in sucrose gradient (192,000 × g, 18‐20 h) |
TEM: 100 nm NTA: 2 × 1010 particles in 1.16 g/cm3 fraction |
WB ex‐WAGO (Argonaute protein) Transcriptomics (siRNAs) |
exWAGO |
|
Heligmosomoides bakeri Trichuris muris (White et al., 2020) |
15 mL Serum free RPMI1640 | 1000 adults/15 mL | 3 days/37°C | NR | Filtration 0.22 μm, three centrifugation steps (300 × g, 10 min; 2000 × g, 10 min; 10,000 × g, 30 min) |
Concentration Vivaspin 5 kDa; SEC in Superdex 200 10/300 GL column FPLC |
NTA: 90‐180 nm (qNano Gold‐Izon) | Transcriptomics (miRNAs) |
Let‐7 miR71 |
|
Ascaris suum (Hansen et al., 2019) |
30 mL Serum free RPMI | NR |
3 days/37°C (sample recovered every 24 h) |
NR | Filtration 0.22 μm, three centrifugation steps (300 × g, 10 min; 2000 × g, 10 min; 10,000 × g, 30 min) |
UC‐ 100,000 × g, 2 h SEC in qEV columns (Izon) |
TEM: 80‐200 nm; NTA: 210‐260 nm (SEC and UC); 6 × 1011 particles (SEC samples); 2,84 × 1013 (UC samples) |
PA (adults and larvae) Transcriptomics (miRNA) (adults and larvae) |
HSP70 LAP‐1 miR‐71 miR‐100 |
Abbreviations: NR, not reported; C, centrifugation; UC, ultracentrifugation; CLSM, confocal laser scanning microscopy; ESCRT, Endosomal Sorting Complex Required for Transport; FC, flow cytomet ry; DLS, dynamic light scattering; NTA, Nanoparticle tracking analysis; PA, proteomic analysis; SEM, scanning electronic microscopy; SG, sucrose gradient; TEM, transmission electronic microscopy.
Abbreviations in Tables: Scanning Electron Microscopy (SEM); Transmission Electron Microscopy (TEM); Confocal Microscopy (CM); Zetasizer Nanorange System (ZNS); Ultracentrifugation (UC); Centrifugation (C); Sucrose gradient (SG); Sepharose Chromatography Column (SEC); Flow Cytometer (FC); Immuno Electron Microscopy (IEM); Proteomic analysis (PA); Dynamic Light Scattering (DLS); Dulbecco's modified Eagle's médium (DMEM); Fetal Bovine Serum (FBS); Liver Infusion Tryptose (LIT); Triatomine Artificial Urine (TAU); Roswell Park Memorial Institute (RPMI); N‐2‐hydroxyethylpiperazine‐N'−2‐ethanesulfonic acid (HEPES); Fetal Calf Serum (FCS); Iron Fortified Calf Serum (IFCS); Enzyme Linked Immunosorbent Assay (ELISA); Nanoparticle tracking analysis (NTA); Western Blotting (WT) and Electronic Microscopy (EM); Atomic force microscopy (AFM); Biological T. gondii form living in indeterminate host; # experiments performed in intermediate host (humans and mice); Dendritic cells (DC); human foreskin fibroblasts (HFF); African green monkey kidney cells (VERO): Madin‐Darby Canine Kidney epithelial cells (MDCK); cerebrospinal fluid (CSF).
The first isolation and characterization of helminth EVs were described in F. hepatica and E. caproni (Marcilla et al., 2012). EVs were isolated from excretory–secretory products (ESP) obtained by incubating worms (collected from infected cows from abattoirs or experimentally‐infected mice, respectively) in RPMI‐1640 culture medium containing antibiotics. The culture medium containing protease inhibitors was used to obtain the EVs. Three step‐centrifugation (300 × g, 10 min; 700 × g, 30 min, and 15,000 x g, 45 min) was carried out to remove eggs and cell debris, and after membrane filtration, the EVs were purified by UC (100,000 x g, 1.5 h). Since then, hundreds of studies have appeared on flatworms and nematodes EVs, as reviewed by Sotillo et al. (Sotillo et al., 2020), using procedures based on this differential ultracentrifugation protocol. For example, the isolation of EVs from the trematode Dicrocoelium dendriticum was carried out following the same UC protocol (Bernal et al., 2014), and it is the only report characterizing the EV protein and miRNAs of this parasite. Differential centrifugation has characterized two subpopulations of EVs that differ according to size, cargo molecules and site of release from F. hepatica and S. mansoni. Large vesicles are obtained after the centrifugation at 15,000 × g for 45 min and a population of smaller vesicles are obtained after centrifuging at 120,000 × g for 1 h (Cwiklinski et al., 2015; Kifle et al., 2020).
In 2019, Davis and collaborators demonstrated, using F. hepatica as a model pathogenic helminth, that DC and SEC methods are not equivalent, proposing the use of SEC as a purification method to obtain a higher EV purity and avoid more free proteins and tegumental artifacts (Davis et al., 2019). In that study, two‐step centrifugation (300 × g, 10 min; 700 × g, 30 min) is employed to remove large particles. The sample was concentrated using a 10‐kDa MWCO Amicon filter before SEC using qEVoriginal SEC columns (IZON science). More recently, the use of SEC to isolate F. hepatica EVs has shown that they exhibit a wide range of morphologies, suggesting that each EV subpopulation may have specific functions (Sanchez‐Lopez et al., 2020). However, with the technical capacity to isolate the different subpopulations, it is easier to assess a particular function of each EV type.
Other isolation protocols for F. hepatica include the density gradient ultracentrifugation (Eichenberger et al., 2018; Sotillo et al., 2016) and gravity flow methods (Murphy et al., 2020). The latter isolation technique consists of the mentioned two‐step centrifugation of the ESP obtained before applying hydrostatic pressure to force the EV‐containing solution through a dialysis membrane with a molecular weight cut‐off (MWCO) of 1000 kDa. Once concentrated, the solution is filtered through a 0.2‐μm filter (Murphy et al., 2020).
Several methods have been reported for EV isolation for other trematodes, such as schistosomes. In the first studies characterizing EVs from adult Schistosoma japonicum and S. mansoni schistosomula, EVs are purified using a protocol based on centrifugation coupled to a membrane filtration (Nowacki et al., 2015; Wang et al., 2015). However, Liu et al. presented an alternative method for isolating EVs from adult S. japonicum, collected from infected rabbits and cultivated for 2 h in vitro. This short culture time minimized the stress to which parasites are exposed. The EVs can be isolated using centrifugation (2000 x g, 30 min) combined with dialysis (molecular weight cut‐off: 3.5 kDa), ultrafiltration, and a commercial exosome isolation kit (Liu et al., 2018, 2020). In the first description of the EVs from S. mansoni adult worms, EVs were purified using other methods, such as density gradient UC (Marcilla et al., 2012; Sotillo et al., 2016). The ESP is subjected to differential centrifugation (500 x g, 2000 x g and 4000 x g for 20 min each), and the supernatant is concentrated and UC (12,000 x g, 45 min; 120,000 x g, 3 h). The resultant pellet should be solubilized in PBS and subjected to Optiprep® discontinuous gradient (ODG) separation, using 40%, 20%, 10% and 5% iodixanol solutions centrifuging at 120,000 x g for 18 h. Recently, a similar approach has been used to develop an optimized protocol for isolating EVs from S. mansoni schistosomula and adult worms. Compared with methods that separate vesicles based on sizes, such as differential UC and SEC, the iodixanol density gradient ultracentrifugation is the best method to obtain high‐purity EV preparations from the different S. mansoni life stages, including the separation of EVs from non‐EV contaminants (Kuipers et al., 2022).
Most studies on trematode EVs have focused on the adult stage, but it has been demonstrated that other developmental stages can secrete EVs. For example, EVs have been observed in eggs from S. japonicum, embryonated eggs, and newly excited juvenile worms (NEJs) from F. hepatica (Sanchez‐Lopez et al., 2020; Trelis et al., 2022; Zhu et al., 2016). S. japonicum eggs can be isolated from the livers of infected rabbits 6–7 weeks post‐infection. The ExoQuick‐TC Exosome Precipitation Kit (SBI, Mountain View, CA, USA) should be employed to isolate the EVs (Zhu et al., 2016). F. hepatica eggs are collected by centrifugation of the culture medium (750 g for 10 min) obtained after incubating the adult parasites and embryonated in mineral water at 24°C for 28 days. To obtain F. hepatica NEJs, metacercaria is excysted, and NEJs maintained RPMI‐1640 culture medium supplemented with 50% chicken serum. To prevent contamination with serum EVs, washing the NEJs before incubating in RPMI‐1640 serum‐depleted medium is very important. Obtaining EVs from NEJS is challenging, first because NEJs are very labile and require efficient handling, and second, because of the low amount of secreted EVs. The ESP was centrifuged, filtered and concentrated with Amicon Ultra‐4 filter devices (Merck Millipore) at 3200 g for 20 min to purify the EVs by SEC using handmade columns of Sepharose‐CL2B (Sigma‐Aldrich) (Sanchez‐Lopez et al., 2020; Trelis et al., 2022).
The information on EV secretion in cestode parasites was limited to ultrastructural studies until 2017, when Ancarola et al. determined the presence of EVs by analyzing the metacestode stages of the model cestodes Taenia crassiceps and Mesocestoides corti and the zoonotic species Echinococcus multilocularis, obtained from experimental infections. Parasites are usually washed with PBS and filtered through a 150 μm pore mesh to remove murine cells or debris, and then, parasites are incubated for 1–4 days in DMEM without serum. Differential centrifugation was used to purify the EVs. Culture media are collected and centrifuged at 10°C (2000 x g, 20 min: 10,000 x g, 30 min). The resulting supernatants are ultracentrifuged for 70 min at 100,000 x g at 4°C, washed with PBS, and ultracentrifuged again (279).
In parasitic infections, few studies focus on host‐derived EVs, which could provide excellent new biomarkers for the disease and check for the effectiveness of treatments. There are exciting publications focused on the EVs isolated from the liquid of E. granulosus hydatid cysts from human patients and from protoscolex culture supernatant, as well as from mice infected with E. granulosus at different stages. The EVs can be isolated and purified from fresh hydatid cyst fluid and protoscolex culture supernatant using an ExoEasy Maxi Kit (Qiagen, Germany), a membrane‐based affinity binding. Previously, the samples were filtered to exclude particles larger than 0.8 μm (Zhou et al., 2019).
Nematodes are highly abundant animals, and many species have a parasitic lifestyle. One of the more representative publications on nematodes described the role of the EVs from the gastrointestinal nematode Heligmosomoides polygyrus (Buck et al., 2014). This work identified a specific set of miRNAs and full‐length Y RNAs contained in EVs that stabilize these RNAs against degradation. To purify EVs from H. polygyrus, using differential centrifugation, the parasites can be collected from the small intestine of experimentally infected CF1 mice and then kept in serum‐free media in vitro. The ESP can be collected every 3 days for 1 week. After removing the eggs, the ESP should be filtered through a 0.2‐μm filter and centrifuged at 100,000 x g for 2 h. The EVs can be washed twice with PBS and ultracentrifuged again. To concentrate the supernatant, it is possible to use a Vivaspin 6 5000 MWCO tube (Fisher) spin at 5000 x g and rewash it with PBS. More recently, the H. polygyrus EV isolation in the same laboratory was carried out by adding one additional step of ultracentrifugation using a linear sucrose gradient (2.0−0.4 M sucrose) at 192,000 x g for 18–20 h for RNA extraction. The two fractions with densities of 1.16−1.18 g/cm3 (measured by refractometry) were pooled, diluted 10 times in PBS, and centrifuged again at 192,000 x g for 90 min (Chow et al., 2019).
In most of the publications mentioned above, the goal of the studies was to identify the presence of EVs in the helminth excretory‐secretory products, to demonstrate their uptake by host cells, and to carry out proteomic and transcriptomic analysis to determine the EV cargoes. RNAs are one type of cargo molecule that can explain some of the EV functions. The comparison of the EV sRNAs in the gastrointestinal nematode
H. polygyrus with those in EVs from the distantly related gastrointestinal nematode Trichuris muris has shown that EV purification methods introduce slight variation in the detected sRNAs. In contrast, variations in library preparation methods yielded more considerable differences. T. muris EVs can be purified by ultracentrifugation, and samples were concentrated using a Vivaspin 6 spin 5‐kDa MWCO column. Heligmosomoides bakeri ES products were concentrated in a Vivaspin 20 MWCO 3‐kDa concentrator (Sartorius, Göttingen, Germany) before isolating EVs by SEC, using a Superdex 200 10/300 GL column with an AKTA basic FPLC system (GE Healthcare) (White et al., 2020).
In the first in‐depth characterization of EVs from different developmental stages and body parts of the porcine nematode A. suum, EVs were purified by differential centrifugation, including two final ultracentrifugation steps at 100,000 g and SEC. This works describes in detail how to collect the different samples from
A. suum could help study its human counterpart, A. lumbricoides (Hansen et al., 2019).
In other nematodes, EV release has also been observed from all intra‐mammalian life stages of Brugia malayi (microfilariae, L3, L4) and Dirofilaria immitis (Harischandra et al., 2018) and from Trichinella spiralis muscle larvae (Kosanovic et al., 2019). EVs can be obtained using differential centrifugation. Most of the investigations of parasitic helminth EVs mentioned above include TEM to detect the presence and quality of EVs and NTA to determine the size and concentration of EVs.
We have summarized some methods used in the last decade to purify EVs from helminth parasites. The referred publications and many others have described the importance of helminth‐derived EVs in host‐parasite interactions using different ‐omic approaches (proteomics, transcriptomics, lipidomics, glycomics, genomics and metabolomics), as well as immunodetection and ELISA analysis, describing proteins and RNA molecules with diverse immunomodulatory properties. For these studies, obtaining pure EV populations by combining different fractionation techniques is imperative. Our understanding of helminth secreted EVs has significantly advanced mainly due to the molecular characterization of EV proteomes (Montano et al., 2021). A protocol to analyze the proteomic content of helminth‐derived EVs independently of the source has been recently published (Sotillo, 2022). This method can differentiate transmembrane from cargo proteins, which can help select proteins with essential functions in the interaction between the parasite and the host and select proteins for diagnostic and vaccination purposes.
Extracellular RNAs have been described in parasitic helminths from nematodes, trematodes, and cestodes. miRNAs are the most thoroughly characterized RNA biotype in helminth EVs, where they are protected from degradation (Cucher et al., 2021) and constitute potential biomarkers of many diseases. Several approaches and methodologies are used for miRNA sequencing or detection (Sotillo, 2022). There is a growing number of reports on helminth EVs and their cargo due to the diversity and multifunctionality of EVs. Increasing evidence suggests that EV characterization will provide biomarkers and identify molecules employed in diagnostic and therapeutic approaches, as well as in vaccines.
4. PARASITE EVS AND VACCINES
Pioneering studies with EVs secreted by Epstein‐Barr virus (EBV)‐transformed B‐cells demonstrated stimulation of T‐cell in an antigen‐specific manner (Raposo et al., 1996). Two years later, the same research group showed that dendritic cells (DCs), responsible for generating specific immunity, secreted exosomes with MHC class I molecules coupled to peptides thus generating anti‐tumoral CD8 T‐cytotoxic responses (Zitvogel et al., 1998). Together, these publications laid the molecular basis that EVs act in intercellular communication in the immune system and paved the way for the potential use of exosomes as new vaccines against tumors.
Because of their ability to modulate the immune response, exosomes are also being explored as novel therapeutic agents against infectious diseases. Pioneering studies demonstrated that macrophages infected with Mycobacterium bovis secreted exosomes inducing bacterial‐specific pro‐inflammatory activity (Bhatnagar et al., 2007). Similar results were obtained with exosomes from macrophages infected with Mycobacterium tuberculosis and this response was also evident in other infectious diseases caused by intracellular pathogens (Marcilla et al., 2014).
EVs carry biologically active and antigenic molecules from the parasites that can modulate host immune responses. These characteristics make EVs an alternative model for studying to the development of protective or therapeutic vaccines. Thus, different groups have evaluated the possibility of using these particles in immunization protocols. Although, vaccination studies with parasite‐EVs are still in infancy, some advances have already been achieved. Exploratory studies using EVs released by trypanosomatids in immunization protocols have been carried out. T. cruzi EVs stimulate macrophage responses and interact with TLR2‐type receptors (Cronemberger‐Andrade et al., 2020), making it interesting to study their protective potential as therapeutic or protective vaccines.
In the case of Leishmania, initial investigations showed that the treatment of mice with EVs released by L. donovani or L. major before the parasite challenge exacerbated the infection (Dong et al., 2019, 2021). However, some evidence suggests that changes in the content of EVs may impact the immune response and disease progression. Studies performed with genetically modified parasites showed that in a mouse model of air pouch formation (Murine Air Pouch Injection), EVs derived from
L. major GP63 knockout (KO) (L. major GP63‐/‐) induced greater recruitment of inflammatory cells compared to EVs derived from wild parasites (Hassani et al., 2014). In addition, EVs derived from L. donovani HSP100 KO induced a pro‐inflammatory response and did not exacerbate the disease (Dong et al., 2019, 2021). Therefore, EV from attenuated, avirulent, or non‐pathogenic Leishmania can provide precious tools for various purposes to control leishmaniasis (Barbosa et al., 2018; Dupin et al., 2021). It is worth to mention that L. tarentolae as nonpathogenic to human, could manipulated genetically to express different proteins for further utilization in vaccination, immunotherapy and even delivering different immunomodulators (Abdossamadi et al., 2017; Rafati et al., 2006, 2008; Saljoughian et al., 2013; Shokouhy et al., 2022). To this end, EVs derived from parasites with different profiles could have different applications and will induce distinct immune responses in an experimental immunization model (Dupin et al., 2021). Therefore, the use of methodologies that addresses these components in Leishmania EVs contribute to the development of EVs‐vaccines by uncovering their content. Thus, vaccines based on EVs (Dong et al., 2019, 2021; Torrecilhas et al., 2020) undergo characterization of the molecules present in these particles using proteomics, lipidomics, metabolomics, among other methodologies. In addition to studies involving the delivery of immunogenic antigens by EVs released by Leishmania, it is also important to evaluate their association with adjuvants. Although several studies have shown that EVs released by bacteria contain molecules capable of acting as adjuvants activating innate immunity receptors these approaches are not yet clear for Leishmania. Some in vitro studies have shown activation of toll‐like receptors (TLRs) by EVs of some Leishmania species. Other works have also demonstrated the activation of macrophages and other immune cells by Leishmania EVs (Belo et al., 2017; Silverman & Reiner, 2011). However, it remains to be investigated whether this stimulation is sufficient for these vesicles to have an effect as a potential adjuvant. Currently, studies addressing EVs for developing effective vaccines for leishmaniasis and their use in immunotherapy is still a field to be explored.
Similar studies have been carried out with non‐trypanosomatids protozoan parasites. Immunization with EVs released by tachyzoites of T. gondii reduced the parasitemia and increased the survival index in mice susceptible to T. gondii infection (Maia et al., 2021b). In addition, mice immunized with EVs derived from dendritic cells pulsed with T. gondii also triggered humoral and mucosal immune responses against T. gondii infection (Beauvillain et al., 2007). In the same way, studies addressing T. gondii EVs as potential for vaccines have been studied. EV‐immunization could induce immune protection, eliciting high production of IgG1, IFN‐γ, IL‐10 and TNF‐α and had a reduction on parasitemia after challenged with 100 tachyzoites with a high virulent T. gondii strain (Maia et al., 2021a, 2021b).
In its strict sense, exosomes were first described in malaria using the Plasmodium yoelii 17X‐BALB/c mouse model (Martin‐Jaular et al., 2011). In this non‐lethal malaria murine model, the parasites present a tropism for reticulocytes, resembling the human malaria infection by P. vivax. Of relevance, reticulocyte‐derived exosomes (REX) from infected mice contained parasite proteins and animals immunized with REX in the presence of CpG showed long lasting protection against lethal infection induced by P. yoelii XL (Martin‐Jaular et al., 2011). Moreover, such protection was spleen‐dependent and involved the presence of CD4 + and CD8 + T cells mediated immune response (Martin‐Jaular et al., 2016). To extrapolate these data to human infections, it was later shown that reticulocyte‐derived exosomes obtained from in vitro cultures of human reticulocytes contain MHC class I antigens and were specifically uptake by mature dendritic cells (Diaz‐Varela et al., 2018). In addition, immunocapture of circulating EVs in natural P. vivax infections using antibodies against the transferrin receptor (CD71) followed by mass spectrometry identified novel antigens for vaccination (Aparici Herraiz et al., 2022). Altogether, these data support the research and development of EVs as a novel vaccination approach against malaria.
In experimental malaria, immunization studies with EVs derived from infected reticulocytes have been performed. Animals immunized with reticulocyte‐derived exosomes (rex) in the presence of CpG showed long lasting protection against lethal infection induced by Plasmodium yoelii, an increase in survival, and the presence of CD4 + and CD8 + T cells effector memory phenotype (Martin‐Jaular et al., 2016, 2011). Proteomic studies demonstrated that rex contains antigenic proteins derived from the parasite, suggesting that these particles may indeed have potential for use in immunization protocols (Martin‐Jaular et al., 2011).
EVs released by helminth parasites at different life stages have also demonstrated a protective role in experimental immunization models. Studies conducted with EVs of T.muris (Shears et al., 2018), S. mansoni (Mossallam et al., 2021), H. polygyrus (Coakley et al., 2017), E. caproni (Trelis et al., 2016) and Opisthorchis viverrini (Chaiyadet et al., 2019) have shown the protective role of these EVs and their potential application in vaccine development. Many of the first experimental vaccines in different helminth models have used the ESP released by the parasite to modify their environment and down‐regulate the host immune system, achieving high levels of protection. Another approach is the use of multicomponent vaccines using a combination of recombinant antigens. Most recently, a new strategy in developing helminth vaccines has emerged based on the discovery of EVs. These can function as a source of antigens, acting on the host immune system to induce immunogenic or tolerogenic responses, depending on each specific parasite (Maizels, 2021).
There are several examples of vaccination with helminth EVs in murine models. In one of the first studies, immunization against E. caproni using EVs reduced symptom severity and increased survival upon infection, suggesting that parasitic EVs may hold therapeutic potential in a wide range of helminth infections (Trelis et al., 2016). Mice vaccinated with EVs from H. polygyrus generated protective immunity against larval challenge, inhibiting the expression of the IL‐33 receptor that is required to initiate the type 2 immune response (Coakley et al., 2017). More recent studies reported that immunization with EVs from the trematode Opisthorchis viverrini (Chaiyadet et al., 2019), and the nematode T. muris (Shears et al., 2018), induce a protective immune response. It is possible that EVs contain homologous antigens conserved in several species due to the complexity of the EV cargo, so creating a vaccine that targets multiple helminth species might be possible (Maizels, 2021).
There is an urgent need for human and veterinary vaccines that would confer immunity against infections with parasites. Some initiatives, in the veterinary area, have led to the development of vaccines using irradiated infective larvae or vaccines based on purified/recombinant antigens. However, no effective vaccines against most helminth parasites infecting humans have been successfully developed.
5. CONCLUSIONS AND PERSPECTIVES
The MISEV 2018 suggests the Extracellular Vesicle as the generic term to describe particles released by cells. Thus, we recommend maintaining this term to refer to particles released by parasites as research on the biogenesis of particles released by pathogens still needs to be done. In this guide, we describe the main findings in the EVs released by the parasites, as well as the functions, physical and biochemical characteristics, and/or culture conditions to obtain the particles from infected cells, in vivo models or from patient samples. There are several separation techniques, and their combination increases the chance of getting pure particles with quality to perform functional assays. This is required to allow reliable reproducibility among laboratories, without generating artifacts and/or misinterpretations (Figure 2).
FIGURE 2.
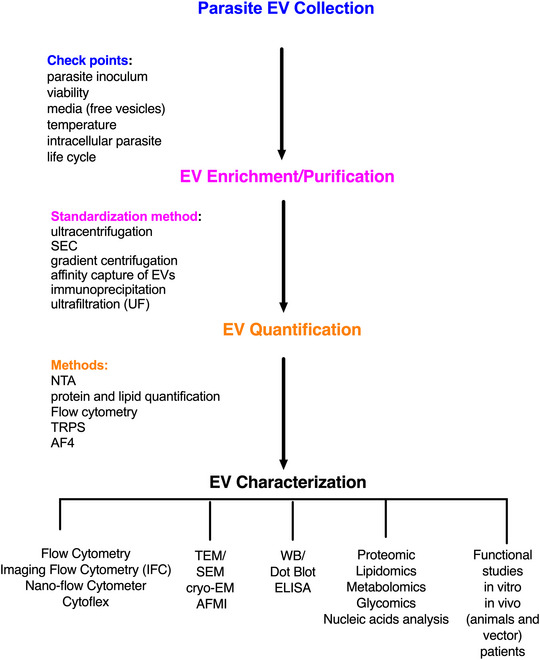
Flowchart to obtain, characterize and perform functional studies with parasite extracellular vesicles. The different methods suggested in each step are highlighted to the left of the directional arrows.
We included tables showing the main guidelines for better characterization of EVs of each parasite with protein, glycoconjugate, and lipid markers because they help demonstrate the presence, purity, and type of EV in each case. Significantly, markers expressed in the parasites' EVs differ from host cell markers. It should be considered the parasite's development and the host type (invertebrate or vertebrate). A significant challenge in the EVs field of parasites is whether the components associated with EVs are specifically associated with the particles and what their topology is in the membrane.
The field of parasite EVs had significant advances in recent years. New techniques for separating EVs combined with new genetic tools such as CRISP‐CAS9 are available and promise further understanding of the mechanisms of particle release and, consequently, disease control. Identifying novel biomarkers enriched in EVs and their use as new vaccines may impact the diagnosis and prevention of infectious diseases caused by parasites.
AUTHOR CONTRIBUTIONS
Carmen Fernandez‐Becerra: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; visualization; writing—original draft; writing—review and editing. Patrícia Xander: Conceptualization; formal analysis; investigation; methodology; project administration; resources; supervision; validation; visualization; writing—original draft; writing—review and editing. Daniel Alfandari: Formal analysis; methodology; project administration; writing—original draft; writing—review and editing; George Dong: Formal analysis; methodology; visualization; writing—original draft; writing—review and editing. Iris Aparici‐Herraiz: Methodology; writing—original draft; writing—review and editing. Irit Rosenhek‐Goldian: Formal analysis; methodology; project administration; supervision; writing—original draft; writing—review and editing. Mehrdad Shokouhy: Methodology; project administration; writing—original draft; writing—review and editing. Melisa Gualdron‐Lopez: Investigation; methodology; project administration; visualization; writing—original draft; writing—review and editing. Nicholy Lozano: Methodology; project administration; validation; writing—original draft; writing—review and editing. Nuria Cortes‐Serra: Investigation; methodology; supervision; writing—original draft; writing—review and editing. Paula Abou Karam: Formal analysis; methodology; visualization; writing—original draft; writing—review and editing. Paula Meneghetti: Investigation; methodology; writing—original draft; writing—review and editing. Rafael Pedro Madeira: Methodology; validation; writing—original draft; writing—review and editing. Ziv Porat: Data curation; formal analysis; methodology; project administration; supervision; validation; visualization; writing—original draft; writing—review and editing. Rodrigo Pedro Soares: Formal analysis; validation; visualization; writing—original draft; writing—review and editing. Adriana Oliveira Costa: Investigation; methodology; project administration; supervision; validation; visualization; writing—original draft; writing—review and editing. Sima Rafati: Data curation; formal analysis; investigation; methodology; supervision; validation; visualization; writing—original draft; writing—review and editing. Anabela‐Cordeiro da Silva: Formal analysis; methodology; project administration; supervision; validation; visualization; writing—original draft; writing—review and editing. Nuno Santarém: Methodology; validation; visualization; writing—original draft; writing—review and editing. Christopher Fernandez‐Prada: Formal analysis; investigation; methodology; project administration; validation; visualization; writing—original draft; writing—review and editing. Marcel I. Ramirez: Conceptualization; formal analysis; funding acquisition; investigation; methodology; supervision; validation; visualization; writing—original draft; writing—review and editing. Dolores Bernal: Formal analysis; investigation; methodology; project administration; software; supervision; validation; writing—original draft; writing—review and editing. Antonio Marcilla: Conceptualization; formal analysis; investigation; methodology; project administration; supervision; writing—original draft; writing—review and editing. Vera Lucia Pereira‐Chioccola: Methodology; validation; visualization; writing—original draft; writing—review and editing. Lysangela Ronalte Alves: Methodology; supervision; validation; writing—original draft; writing—review and editing. Hernando Del Portillo: Formal analysis; investigation; methodology; supervision; validation; visualization; writing—original draft; writing—review and editing. Neta Regev‐Rudzki: Formal analysis; investigation; methodology; project administration; supervision; validation; visualization; writing—original draft; writing—review and editing. Igor Correia de Almeida: Investigation; methodology; project administration; supervision; validation; visualization; writing—original draft; writing—review and editing. Sergio Schenkman: Formal analysis; investigation; methodology; project administration; supervision; validation; visualization; writing—original draft; writing—review and editing. Martin Olivier: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; supervision; visualization; writing—original draft; writing—review and editing. Ana Claudia Torrecilhas: Conceptualization; formal analysis; funding acquisition; methodology; project administration; resources; supervision; validation; writing—original draft; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
No potential conflicts of interest were reported by the authors.
ACKNOWLEDGEMENTS
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grant 2019/15909‐0 to A.C.T., N.R.‐R. and S.S.), and FAPESP 2020/07870‐4 to A.C.T. and S.S., FAPESP 2019/21614‐3 to P.X. FAPESP 2021/02217‐3 to V.L.P‐.C. Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq): grant 408186/2018‐6 to ACT; grant 303788/2020‐8 and CNPq‐INCTV to SS; grant 303566/2021‐3 to V.L.P‐.C. Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) to A.C.T. and V.L.P‐.C. M.O. research is supported by the Canadian Institute of Health Research (CIHR; Grant PJT‐159765) and the Natural Sciences and Engineering Research Council of Canada (NSERC; Discovery Grant RGPIN‐ 2018–03849). Research on extracellular vesicles in the laboratory of C.F.‐B. and H.A.P. is funded by Fundació La Marató de TV3 (reference 566/U/2018); CIBER‐Consorcio Centro de Investigación Biomédica en Red (CB 2021), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, and Unión Europea–NextGenerationEU; Ministerio de Ciencia e Innovación (PID2019‐111795RB‐I00). We acknowledge support from the Spanish Ministry of Science and Innovation through the Centro de Excelencia Severo Ochoa 2019–2023 Program (CEX2018‐ 000806‐S), and support from the Generalitat de Catalunya through the CERCA Program. Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia under the project PTDC/CVT‐CVT/6798/2020; NS is an assistant researcher funded by national funds through FCT and co‐funded through the European Social Fund within the Human Potential Operating Programme 2021. 04285.CEECIND. D.B. and A.M. are supported by the Agencia Estatal de Investigación, Ministerio de Ciencia e Innovación, Spain (Grant number PID2019‐105713GB‐I00/AEI/10.13039/501100011033), and Conselleria d'Educació, Cultura i Esports, Generalitat Valenciana, Valencia, Spain (Grant PROMETEO/2020/071). I.C.A. is funded by the National Institutes of Health (NIH) grants # U01AI129783 (to ICA) and U54MD007592 (to Robert A. Kirken). The authors are thankful for the Biomolecule Analysis and Omics Unit (BAOU) at BBRC/UTEP, supported by the NIH grants 2G12MD007592 and U54MD007592 (to Robert A. Kirken) for the full access to the LC‐MS/MS instruments used in several of our publications cited in these guidelines.
Fernandez‐Becerra, C. , Xander, P. , Alfandari, D. , Dong, G. , Aparici‐Herraiz, I. , Rosenhek‐Goldian, I. , Shokouhy, M. , Gualdron‐Lopez, M. , Lozano, N. , Cortes‐Serra, N. , Karam, P. A. , Meneghetti, P. , Madeira, R. P. , Porat, Z. , Soares, R. P. , Costa, A. O. , Rafati, S. , da Silva, A.‐C. , Santarém, N. , … Torrecilhas, A. C. (2023). Guidelines for the Purification and Characterization of Extracellular Vesicles of Parasites. Journal of Extracellular Biology, 2, e117. 10.1002/jex2.117
Present address
Melisa Gualdron‐Lopez, School of Public Health, University of Alberta, Edmonton, AB, Canada
Contributor Information
Carmen Fernandez‐Becerra, Email: carmen.fernandez@isglobal.org.
Martin Olivier, Email: martin.olivier@mcgill.ca.
Ana Claudia Torrecilhas, Email: ana.torrecilhas@unifesp.br.
REFERENCES
- Abdi, A. I. , Hodgson, S. H. , Muthui, M. K. , Kivisi, C. A. , Kamuyu, G. , Kimani, D. , Hoffman, S. L. , Juma, E. , Ogutu, B. , Draper, S. J. , Osier, F. , Bejon, P. , Marsh, K. , & Bull, P. C. (2017). Plasmodium falciparum malaria parasite var gene expression is modified by host antibodies: Longitudinal evidence from controlled infections of Kenyan adults with varying natural exposure. BMC Infectious Diseases [Electronic Resource], 17, 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdossamadi, Z. , Taheri, T. , Seyed, N. , Montakhab‐Yeganeh, H. , Zahedifard, F. , Taslimi, Y. , Habibzadeh, S. , Gholami, E. , Gharibzadeh, S. , & Rafati, S. (2017). Live Leishmania tarentolae secreting HNP1 as an immunotherapeutic tool against Leishmania infection in BALB/c mice. Immunotherapy, 9, 1089–1102. [DOI] [PubMed] [Google Scholar]
- Abou Karam, P. , Rosenhek‐Goldian, I. , Ziv, T. , Ben Ami Pilo, H. , Azuri, I. , Rivkin, A. , Kiper, E. , Rotkopf, R. , Cohen, S. R. , Torrecilhas, A. C. , Avinoam, O. , Rojas, A. , Morandi, M. I. , & Regev‐Rudzki, N. (2022). Malaria parasites release vesicle subpopulations with signatures of different destinations. Embo Reports, 23, e54755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuin, G. , Colli, W. , & Alves, M. J. (1996a). Turnover and shedding of the Tc‐85 surface glycoprotein of Trypanosoma cruzi trypomastigotes. Brazilian Journal of Medical and Biological Research, 29, 335–341. [PubMed] [Google Scholar]
- Abuin, G. , Colli, W. , de Souza, W. , & Alves, M. J. (1989). A surface antigen of Trypanosoma cruzi involved in cell invasion (Tc‐85) is heterogeneous in expression and molecular constitution. Molecular and Biochemical Parasitology, 35, 229–237. [DOI] [PubMed] [Google Scholar]
- Abuin, G. , Couto, A. S. , de Lederkremer, R. M. , Casal, O. L. , Galli, C. , Colli, W. , & Alves, M. J. (1996b). Trypanosoma cruzi: The Tc‐85 surface glycoprotein shed by trypomastigotes bears a modified glycosylphosphatidylinositol anchor. Experimental Parasitology, 82, 290–297. [DOI] [PubMed] [Google Scholar]
- Acosta‐Serrano, A. , Hutchinson, C. , Nakayasu, E. S. , Almeida, I. C. , & Carrington, M. (2007). Comparison and evolution of the surface architecture of trypanosomatid parasites. In Barry J. D., Mottram J. C., McCulloch R., & Acosta‐Serrano A. (Eds.), Trypanosomes: After the genome (pp. 319–337). Horizon Scientific Press. [Google Scholar]
- Aguirre Garcia, M. , Gutierrez‐Kobeh, L. , & Lopez Vancell, R. (2015). Entamoeba histolytica: Adhesins and lectins in the trophozoite surface. Molecules (Basel, Switzerland), 20, 2802–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfandari, D. , Ben Ami Pilo, H. , Abou Karam, P. , Dagan, O. , Joubran, C. , Rotkopf, R. , Regev‐Rudzki, N. , & Porat, Z. (2021). Monitoring distribution dynamics of EV RNA cargo within recipient monocytes and macrophages. Frontiers in Cellular and Infection Microbiology, 11, 739628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aline, F. , Bout, D. , Amigorena, S. , Roingeard, P. , & Dimier‐Poisson, I. (2004). Toxoplasma gondii antigen‐pulsed‐dendritic cell‐derived exosomes induce a protective immune response against T. gondii infection. Infection and Immunity, 72, 4127–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, I. C. , Ferguson, M. A. , Schenkman, S. , & Travassos, L. R. (1994). Lytic anti‐alpha‐galactosyl antibodies from patients with chronic Chagas' disease recognize novel O‐linked oligosaccharides on mucin‐like glycosyl‐phosphatidylinositol‐anchored glycoproteins of Trypanosoma cruzi . Biochemical Journal, 304, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, I. C. , Milani, S. R. , Gorin, P. A. , & Travassos, L. R. (1991). Complement‐mediated lysis of Trypanosoma cruzi trypomastigotes by human anti‐alpha‐galactosyl antibodies. Journal of Immunology, 146, 2394–2400. [PubMed] [Google Scholar]
- Alsford, S. , & Horn, D. (2008). Single‐locus targeting constructs for reliable regulated RNAi and transgene expression in Trypanosoma brucei . Molecular and Biochemical Parasitology, 161, 76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancarola, M. E. , Marcilla, A. , Herz, M. , Macchiaroli, N. , Pérez, M. , Asurmendi, S. , Brehm, K. , Poncini, C. , Rosenzvit, M. , & Cucher, M. (2017). Cestode parasites release extracellular vesicles with microRNAs and immunodiagnostic protein cargo. International Journal for Parasitology, 47, 675–686. [DOI] [PubMed] [Google Scholar]
- Andre, F. , Andersen, M. , Wolfers, J. , Lozier, A. , Raposo, G. , Serra, V. , Ruegg, C. , Flament, C. , Angevin, E. , Amigorena, S. , & Zitvogel, L. (2001). Exosomes in cancer immunotherapy: Preclinical data. Advances in Experimental Medicine and Biology, 495, 349–354. [DOI] [PubMed] [Google Scholar]
- Andresen, K. , Simonsen, P. E. , Andersen, B. J. , & Birch‐Andersen, A. (1989). Echinostoma caproni in mice: Shedding of antigens from the surface of an intestinal trematode. International Journal for Parasitology, 19, 111–118. [DOI] [PubMed] [Google Scholar]
- Ankarklev, J. , Jerlstrom‐Hultqvist, J. , Ringqvist, E. , Troell, K. , & Svard, S. G. (2010). Behind the smile: Cell biology and disease mechanisms of Giardia species. Nature Reviews Microbiology, 8, 413–422. [DOI] [PubMed] [Google Scholar]
- Antwi‐Baffour, S. , Adjei, J. K. , Agyemang‐Yeboah, F. , Annani‐Akollor, M. , Kyeremeh, R. , Asare, G. A. , & Gyan, B. (2016). Proteomic analysis of microparticles isolated from malaria positive blood samples. Proteome Science, 15, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antwi‐Baffour, S. , Malibha‐Pinchbeck, M. , Stratton, D. , Jorfi, S. , Lange, S. , & Inal, J. (2020). Plasma mEV levels in Ghanain malaria patients with low parasitaemia are higher than those of healthy controls, raising the potential for parasite markers in mEVs as diagnostic targets. Journal of Extracellular Vesicles, 9, 1697124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparici‐Herraiz, I. , Gualdron‐Lopez, M. , Castro‐Cavadia, C. J. , Carmona‐Fonseca, J. , Yasnot, M. F. , Fernandez‐Becerra, C. , & Del Portillo, H. A. (2021). Antigen discovery in circulating extracellular vesicles from plasmodium vivax patients. Frontiers in Cellular and Infection Microbiology, 11, 811390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi, E. , Krämer‐Albers, E. M. , Hoen, E. N. , Peinado, H. , Psonka‐Antonczyk, K. M. , Rao, P. , van Niel, G. , Yáñez‐Mó, M. , & Nazarenko, I. (2012). International Society for Extracellular Vesicles: First annual meeting, April 17–21, 2012: ISEV‐2012. Journal of Extracellular Vesicles, 1, 19995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artuyants, A. , Campos, T. L. , Rai, A. K. , Johnson, P. J. , Dauros‐Singorenko, P. , Phillips, A. , & Simoes‐Barbosa, A. (2020). Extracellular vesicles produced by the protozoan parasite Trichomonas vaginalis contain a preferential cargo of tRNA‐derived small RNAs. International Journal for Parasitology, 50, 1145–1155. [DOI] [PubMed] [Google Scholar]
- Atayde, V. D. , Aslan, H. , Townsend, S. , Hassani, K. , Kamhawi, S. , & Olivier, M. (2015). Exosome secretion by the parasitic protozoan leishmania within the sand fly midgut. Cell Reports, 13, 957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atayde, V. D. , da Silva Lira Filho, A. , Chaparro, V. , Zimmermann, A. , Martel, C. , Jaramillo, M. , & Olivier, M. (2019a). Exploitation of the Leishmania exosomal pathway by Leishmania RNA virus 1. Nature Microbiology, 4, 714–723. [DOI] [PubMed] [Google Scholar]
- Atayde, V. D. , da Silva Lira Filho, A. , Chaparro, V. , Zimmermann, A. , Martel, C. , Jaramillo, M. , & Olivier, M. (2019b). Publisher correction: Exploitation of the Leishmania exosomal pathway by Leishmania RNA virus 1. Nature Microbiology, 4, 724. [DOI] [PubMed] [Google Scholar]
- Atayde, V. D. , Hassani, K. , da Silva Lira Filho, A. , Borges, A. R. , Adhikari, A. , Martel, C. , & Olivier, M. (2016). Leishmania exosomes and other virulence factors: Impact on innate immune response and macrophage functions. Cellular Immunology, 309, 7–18. [DOI] [PubMed] [Google Scholar]
- Avalos‐Padilla, Y. , Georgiev, V. N. , Lantero, E. , Pujals, S. , Verhoef, R. , N Borgheti‐Cardoso, L. , Albertazzi, L. , Dimova, R. , & Fernandez‐Busquets, X. (2021). The ESCRT‐III machinery participates in the production of extracellular vesicles and protein export during Plasmodium falciparum infection. Plos Pathogens, 17, e1009455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babatunde, K. A. , Mbagwu, S. , Hernandez‐Castaneda, M. A. , Adapa, S. R. , Walch, M. , Filgueira, L. , Falquet, L. , Jiang, R. H. Y. , Ghiran, I. , & Mantel, P. Y. (2018). Malaria infected red blood cells release small regulatory RNAs through extracellular vesicles. Scientific Reports, 8, 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek, R. , Sondergaard, E. K. , Varming, K. , & Jorgensen, M. M. (2016). The impact of various preanalytical treatments on the phenotype of small extracellular vesicles in blood analyzed by protein microarray. Journal of Immunological Methods, 438, 11–20. [DOI] [PubMed] [Google Scholar]
- Bala, V. , & Chhonker, Y. S. (2018). Recent developments in anti‐Trichomonas research: An update review. European Journal of Medicinal Chemistry, 143, 232–243. [DOI] [PubMed] [Google Scholar]
- Barbosa, F. M. C. , Dupin, T. V. , Toledo, M. D. S. , Reis, N. , Ribeiro, K. , Cronemberger‐Andrade, A. , Rugani, J. N. , De Lorenzo, B. H. P. , Novaes, E. B. R. R. , Soares, R. P. , Torrecilhas, A. C. , & Xander, P. (2018). Extracellular vesicles released by Leishmania (Leishmania) amazonensis promote disease progression and induce the production of different cytokines in macrophages and B‐1 cells. Frontiers in Microbiology, 9, 3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer‐Santos, E. , Aguilar‐Bonavides, C. , Rodrigues, S. P. , Cordero, E. M. , Marques, A. F. , Varela‐Ramirez, A. , Choi, H. , Yoshida, N. , da Silveira, J. F. , & Almeida, I. C. (2013). Proteomic analysis of Trypanosoma cruzi secretome: Characterization of two populations of extracellular vesicles and soluble proteins. Journal of Proteome Research, 12, 883–897. [DOI] [PubMed] [Google Scholar]
- Battle, K. E. , Lucas, T. C. D. , Nguyen, M. , Howes, R. E. , Nandi, A. K. , Twohig, K. A. , Pfeffer, D. A. , Cameron, E. , Rao, P. C. , Casey, D. , Gibson, H. S. , Rozier, J. A. , Dalrymple, U. , Keddie, S. H. , Collins, E. L. , Harris, J. R. , Guerra, C. A. , Thorn, M. P. , Bisanzio, D. , … Gething, P. W. (2019). Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000‐17: a spatial and temporal modelling study. Lancet, 394(10195), 332–343. 10.1016/S0140-6736(19)31096-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvillain, C. , Juste, M. O. , Dion, S. , Pierre, J. , & Dimier‐Poisson, I. (2009). Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine, 27, 1750–1757. [DOI] [PubMed] [Google Scholar]
- Beauvillain, C. , Ruiz, S. , Guiton, R. , Bout, D. , & Dimier‐Poisson, I. (2007). A vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic mice. Microbes and Infection, 9, 1614–1622. [DOI] [PubMed] [Google Scholar]
- Belo, R. , Santarem, N. , Pereira, C. , Perez‐Cabezas, B. , Macedo, F. , Leite‐de‐Moraes, M. , & Cordeiro‐da‐Silva, A. (2017). Leishmania infantum exoproducts inhibit human invariant NKT cell expansion and activation. Frontiers in Immunology, 8, 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern, C. (2015). Chagas' disease. New England Journal of Medicine, 373, 456–466. [DOI] [PubMed] [Google Scholar]
- Bernal, D. , Trelis, M. , Montaner, S. , Cantalapiedra, F. , Galiano, A. , Hackenberg, M. , & Marcilla, A. (2014). Surface analysis of Dicrocoelium dendriticum. The molecular characterization of exosomes reveals the presence of miRNAs. Journal of Proteomics, 105, 232–241. [DOI] [PubMed] [Google Scholar]
- Besteiro, S. , Williams, R. A. , Morrison, L. S. , Coombs, G. H. , & Mottram, J. C. (2006). Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. Journal of Biological Chemistry, 281, 11384–11396. [DOI] [PubMed] [Google Scholar]
- Betanzos, A. , Banuelos, C. , & Orozco, E. (2019). Host invasion by pathogenic amoebae: epithelial disruption by parasite proteins. Genes (Basel), 10(8), 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betanzos, A. , Zanatta, D. , Banuelos, C. , Hernandez‐Nava, E. , Cuellar, P. , & Orozco, E. (2018). Epithelial cells expressing EhADH, an entamoeba histolytica adhesin, exhibit increased tight junction proteins. Frontiers in Cellular and Infection Microbiology, 8, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettadapur, A. , Hunter, S. S. , Suleiman, R. L. , Ruyechan, M. C. , Huang, W. , Barbieri, C. G. , Miller, H. W. , Tam, T. S. Y. , Settles, M. L. , & Ralston, K. S. (2021). Establishment of quantitative RNAi‐based forward genetics in Entamoeba histolytica and identification of genes required for growth. Plos Pathogens, 17, e1010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar, S. , Shinagawa, K. , Castellino, F. J. , & Schorey, J. S. (2007). Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood, 110, 3234–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell, E. L. C. J. , Mayne, L. J. , Billinge, E. R. , & Platt, M. (2015). Emergence of tunable resistive pulse sensing as a biosensor. Analytical Methods, 7, 7055–7066. [Google Scholar]
- Braulke, T. , & Bonifacino, J. S. (2009). Sorting of lysosomal proteins. Biochimica Et Biophysica Acta, 1793, 605–614. [DOI] [PubMed] [Google Scholar]
- Brittain IV, G. C. , Chen, Y. Q. , Martinez, E. , Tang, V. A. , Renner, T. M. , Langlois, M. A. , & Gulnik, S. (2019). A novel semiconductor‐based flow cytometer with enhanced light‐scatter. Sensitivity for the analysis of biological nanoparticles. Scientific Reports, 9(1), 16039. 10.1038/s41598-019-52366-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, A. H. , Coakley, G. , Simbari, F. , McSorley, H. J. , Quintana, J. F. , Bihan, T. L. e , Kumar, S. , Abreu‐Goodger, C. , Lear, M. , Harcus, Y. , Ceroni, A. , Babayan, S. A. , Blaxter, M. , Ivens, A. , & Maizels, R. M. (2014). Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nature Communications, 5, 5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscaglia, C. A. , Campo, V. A. , Di Noia, J. M. , Torrecilhas, A. C. , De Marchi, C. R. , Ferguson, M. A. , Frasch, A. C. , & Almeida, I. C. (2004). The surface coat of the mammal‐dwelling infective trypomastigote stage of Trypanosoma cruzi is formed by highly diverse immunogenic mucins. Journal of Biological Chemistry, 279, 15860–15869. [DOI] [PubMed] [Google Scholar]
- Buscaglia, C. A. , Campo, V. A. , Frasch, A. C. , & Di Noia, J. M. (2006). Trypanosoma cruzi surface mucins: Host‐dependent coat diversity. Nature Reviews Microbiology, 4, 229–236. [DOI] [PubMed] [Google Scholar]
- Campos, F. M. , Franklin, B. S. , Teixeira‐Carvalho, A. , Filho, A. L. , de Paula, S. C. , Fontes, C. J. , Brito, C. F. , & Carvalho, L. H. (2010). Augmented plasma microparticles during acute Plasmodium vivax infection. Malaria Journal, 9, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos, J. H. , Soares, R. P. , Ribeiro, K. , Andrade, A. C. , Batista, W. L. , & Torrecilhas, A. C. (2015). Extracellular vesicles: Role in inflammatory responses and potential uses in vaccination in cancer and infectious diseases. Journal of Immunology Research, 2015, 832057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo, F. , Vogel, R. , Savage, J. , Vella, G. , Law, A. , Camera, G. D. , Hannon, G. , Peacock, B. , Mehn, D. , Ponti, J. , Geiss, O. , Aubert, D. , Prina‐Mello, A. , & Calzolai, L. (2021). Measuring particle size distribution and mass concentration of nanoplastics and microplastics: Addressing some analytical challenges in the sub‐micron size range. Journal of Colloid & Interface Science, 588, 401–417. [DOI] [PubMed] [Google Scholar]
- Carrero, J. C. , Reyes‐Lopez, M. , Serrano‐Luna, J. , Shibayama, M. , Unzueta, J. , Leon‐Sicairos, N. , & de la Garza, M. (2020). Intestinal amoebiasis: 160 years of its first detection and still remains as a health problem in developing countries. International Journal of Medical Microbiology, 310, 151358. [DOI] [PubMed] [Google Scholar]
- Carruthers, V. B. (2002). Host cell invasion by the opportunistic pathogen Toxoplasma gondii. Acta Tropica, 81, 111–122. [DOI] [PubMed] [Google Scholar]
- Cestari, I. , Ansa‐Addo, E. , Deolindo, P. , Inal, J. M. , & Ramirez, M. I. (2012). Trypanosoma cruzi immune evasion mediated by host cell‐derived microvesicles. Journal of Immunology, 188, 1942–1952. [DOI] [PubMed] [Google Scholar]
- Chaiyadet, S. , Sotillo, J. , Krueajampa, W. , Thongsen, S. , Brindley, P. J. , Sripa, B. , Loukas, A. , & Laha, T. (2019). Vaccination of hamsters with Opisthorchis viverrini extracellular vesicles and vesicle‐derived recombinant tetraspanins induces antibodies that block vesicle uptake by cholangiocytes and reduce parasite burden after challenge infection. PLOS Neglected Tropical Diseases, 13, e0007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, F. W. , Koutsovoulos, G. , Ovando‐Vazquez, C. , Neophytou, K. , Bermudez‐Barrientos, J. R. , Laetsch, D. R. , Robertson, E. , Kumar, S. , Claycomb, J. M. , Blaxter, M. , Abreu‐Goodger, C. , & Buck, A. H. (2019). Secretion of an Argonaute protein by a parasitic nematode and the evolution of its siRNA guides. Nucleic Acids Res., 47, 3594–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, I. H. , Koo, S. J. , Gupta, S. , Liang, L. Y. , Bahar, B. , Silla, L. , Nunez‐Burgos, J. , Barrientos, N. , Zago, M. P. , & Garg, N. J. (2017). Gene expression profiling and functional characterization of macrophages in response to circulatory microparticles produced during Trypanosoma cruzi Infection and Chagas disease. Journal of Innate Immunity, 9, 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuo, S. T. , Chien, J. C. , & Lai, C. P. (2018). Imaging extracellular vesicles: Current and emerging methods. Journal of Biomedical Science, 25, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizmar, P. , & Yuana, Y. (2017). Detection and characterization of extracellular vesicles by transmission and cryo‐transmission electron microscopy. Methods in Molecular Biology, 1660, 221–232. 10.1007/978-1-4939-7253-1_18 [DOI] [PubMed] [Google Scholar]
- Clague, M. J. , Urbe, S. , & Komander, D. (2019). Breaking the chains: Deubiquitylating enzyme specificity begets function. Nature Reviews Molecular Cell Biology, 20(6), 338–352. [DOI] [PubMed] [Google Scholar]
- Clark, C. G. , & Diamond, L. S. (2002). Methods for cultivation of luminal parasitic protists of clinical importance. Clinical Microbiology Reviews, 15, 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakley, G. , Buck, A. H. , & Maizels, R. M. (2016). Host parasite communications‐messages from helminths for the immune system: Parasite communication and cell‐cell interactions. Molecular and Biochemical Parasitology, 208, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakley, G. , McCaskill, J. L. , Borger, J. G. , Simbari, F. , Robertson, E. , Millar, M. , Harcus, Y. , McSorley, H. J. , Maizels, R. M. , & Buck, A. H. (2017). Extracellular vesicles from a helminth parasite suppress macrophage activation and constitute an effective vaccine for protective immunity. Cell Reports, 19, 1545–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci, E. , & Meldolesi, J. (2015). Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends in Cell Biology, 25, 364–372. [DOI] [PubMed] [Google Scholar]
- Colombo, M. , Raposo, G. , & Thery, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology, 30, 255–289. [DOI] [PubMed] [Google Scholar]
- Combes, V. , Coltel, N. , Alibert, M. , van Eck, M. , Raymond, C. , Juhan‐Vague, I. , Grau, G. E. , & Chimini, G. (2005). ABCA1 gene deletion protects against cerebral malaria: Potential pathogenic role of microparticles in neuropathology. American Journal of Pathology, 166, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes, V. , Taylor, T. E. , Juhan‐Vague, I. , Mege, J. L. , Mwenechanya, J. , Tembo, M. , Grau, G. E. , & Molyneux, M. E. (2004). Circulating endothelial microparticles in Malawian children with severe falciparum malaria complicated with coma. Jama, 291, 2542–2544. [DOI] [PubMed] [Google Scholar]
- Cortes‐Serra, N. , Gualdron‐Lopez, M. , Pinazo, M. J. , Torrecilhas, A. C. , & Fernandez‐Becerra, C. (2022). Extracellular vesicles in Trypanosoma cruzi infection: Immunomodulatory effects and future perspectives as potential control tools against Chagas disease. Journal of Immunology Research, 2022, 5230603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes‐Serra, N. , Mendes, M. T. , Mazagatos, C. , Segui‐Barber, J. , Ellis, C. C. , Ballart, C. , Garcia‐Alvarez, A. , Gállego, M. , Gascon, J. , Almeida, I. C. , Pinazo, M. J. , & Fernandez‐Becerra, C. (2020). Plasma‐derived extracellular vesicles as potential biomarkers in heart transplant patient with chronic Chagas disease. Emerging Infectious Diseases, 26, 1846–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, A. O. , Chagas, I. A. R. , de Menezes‐Neto, A. , Rego, F. D. , Nogueira, P. M. , Torrecilhas, A. C. , Furst, C. , Fux, B. , & Soares, R. P. (2021). Distinct immunomodulatory properties of extracellular vesicles released by different strains of Acanthamoeba. Cell Biology International, 45, 1060–1071. [DOI] [PubMed] [Google Scholar]
- Costa, F. C. , Francisco, A. F. , Jayawardhana, S. , Calderano, S. G. , Lewis, M. D. , Olmo, F. , Beneke, T. , Gluenz, E. , Sunter, J. , Dean, S. , Kelly, J. M. , & Taylor, M. C. (2018). Expanding the toolbox for Trypanosoma cruzi: A parasite line incorporating a bioluminescence‐fluorescence dual reporter and streamlined CRISPR/Cas9 functionality for rapid in vivo localisation and phenotyping. PLOS Neglected Tropical Diseases, 12, e0006388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumans, F. A. W. , Brisson, A. R. , Buzas, E. I. , Dignat‐George, F. , Drees, E. E. E. , El‐Andaloussi, S. , Emanueli, C. , Gasecka, A. , Hendrix, A. , Hill, A. F. , Lacroix, R. , Lee, Y. , van Leeuwen, T. G. , Mackman, N. , Mager, I. , Nolan, J. P. , van der Pol, E. , Pegtel, D. M. , Sahoo, S. , … Nieuwland, R. (2017). Methodological guidelines to study extracellular vesicles. Circulation Research, 120, 1632–1648. [DOI] [PubMed] [Google Scholar]
- Coura, J. R. , & Viñas, P. A. (2010). Chagas disease: A new worldwide challenge. Nature, 465, S6–S7. [DOI] [PubMed] [Google Scholar]
- Cronemberger‐Andrade, A. , Aragao‐Franca, L. , de Araujo, C. F. , Rocha, V. J. , Borges‐Silva Mda, C. , Figueira, C. P. , Oliveira, P. R. , de Freitas, L. A. , Veras, P. S. , & Pontes‐de‐Carvalho, L. (2014). Extracellular vesicles from Leishmania‐infected macrophages confer an anti‐infection cytokine‐production profile to naive macrophages. PLOS Neglected Tropical Diseases, 8, e3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronemberger‐Andrade, A. , Xander, P. , Soares, R. P. , Pessoa, N. L. , Campos, M. A. , Ellis, C. C. , Grajeda, B. , Ofir‐Birin, Y. , Almeida, I. C. , Regev‐Rudzki, N. , & Torrecilhas, A. C. (2020). Trypanosoma cruzi‐infected human macrophages shed proinflammatory extracellular vesicles that enhance host‐cell invasion via toll‐like receptor 2. Frontiers in Cellular and Infection Microbiology, 10, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucher, M. A. , Ancarola, M. E. , & Kamenetzky, L. (2021). The challenging world of extracellular RNAs of helminth parasites. Molecular Immunology, 134, 150–160. [DOI] [PubMed] [Google Scholar]
- Cwiklinski, K. , de la Torre‐Escudero, E. , Trelis, M. , Bernal, D. , Dufresne, P. J. , Brennan, G. P. , O'Neill, S. , Tort, J. , Paterson, S. , Marcilla, A. , Dalton, J. P. , & Robinson, M. W. (2015). The extracellular vesicles of the helminth pathogen, Fasciola hepatica: Biogenesis pathways and cargo molecules involved in parasite pathogenesis. Molecular & Cellular Proteomics, 14, 3258–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz, A. B. , Maia, M. M. , Pereira, I. S. , Taniwaki, N. N. , Namiyama, G. M. , Telles, J. P. M. , Vidal, J. E. , Spegiorin, L. , Brandao de Mattos, C. C. , Mattos, L. C. , Meira‐Strejevitch, C. D. S. , & Pereira‐Chioccola, V. L. (2020). Human extracellular vesicles and correlation with two clinical forms of toxoplasmosis. PLoS ONE, 15, e0229602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, P. , Mukherjee, A. , & Adak, S. (2021). Glyceraldehyde‐3‐phosphate dehydrogenase present in extracellular vesicles from Leishmania major suppresses host TNF‐alpha expression. Journal of Biological Chemistry, 297, 101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Lira Filho, A. , Fajardo, E. F. , Chang, K. P. , Clement, P. , & Olivier, M. (2021). Leishmania exosomes/extracellular vesicles containing GP63 are essential for enhance cutaneous leishmaniasis development upon co‐inoculation of Leishmania amazonensis and its exosomes. Frontiers in Cellular and Infection Microbiology, 11, 709258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silveira, J. F. , Abrahamsohn, P. A. , & Colli, W. (1979). Plasma membrane vesicles isolated from epimastigote forms of Trypanosoma cruzi . Biochimica Et Biophysica Acta, 550, 222–232. [DOI] [PubMed] [Google Scholar]
- Davis, C. N. , Phillips, H. , Tomes, J. J. , Swain, M. T. , Wilkinson, T. J. , Brophy, P. M. , & Morphew, R. M. (2019). The importance of extracellular vesicle purification for downstream analysis: A comparison of differential centrifugation and size exclusion chromatography for helminth pathogens. PLOS Neglected Tropical Diseases, 13, e0007191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Barros, R. A. M. , Torrecilhas, A. C. , Marciano, M. A. M. , Mazuz, M. L. , Pereira‐Chioccola, V. L. , & Fux, B. (2022). Toxoplasmosis in human and animals around the world. Diagnosis and perspectives in the one health approach. Acta Tropica, 231, 106432. [DOI] [PubMed] [Google Scholar]
- Dekel, E. , Yaffe, D. , Rosenhek‐Goldian, I. , Ben‐Nissan, G. , Ofir‐Birin, Y. , Morandi, M. I. , Ziv, T. , Sisquella, X. , Pimentel, M. A. , Nebl, T. , Kapp, E. , Ohana Daniel, Y. , Karam, P. A. , Alfandari, D. , Rotkopf, R. , Malihi, S. , Temin, T. B. , Mullick, D. , Revach, O. Y. , … Regev‐Rudzki, N. (2021). 20S proteasomes secreted by the malaria parasite promote its growth. Nature Communications, 12, 1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lederkremer, R. M. , & Agusti, R. (2009). Glycobiology of Trypanosoma cruzi. Advances in Carbohydrate Chemistry and Biochemistry, 62, 311–366. [DOI] [PubMed] [Google Scholar]
- Demarta‐Gatsi, C. , Rivkin, A. , Di Bartolo, V. , Peronet, R. , Ding, S. , Commere, P. H. , Guillonneau, F. , Bellalou, J. , Brule, S. , Abou Karam, P. , Cohen, S. R. , Lagache, T. , Janse, C. J. , Regev‐Rudzki, N. , & Mecheri, S. (2019). Histamine releasing factor and elongation factor 1 alpha secreted via malaria parasites extracellular vesicles promote immune evasion by inhibiting specific T cell responses. Cellular Microbiology, 21, e13021. [DOI] [PubMed] [Google Scholar]
- De Pablos, L. M. , Díaz Lozano, I. M. , Jercic, M. I. , Quinzada, M. , Giménez, M. J. , Calabuig, E. , Espino, A. M. , Schijman, A. G. , Zulantay, I. , Apt, W. , & Osuna, A. (2016). The C‐terminal region of Trypanosoma cruzi MASPs is antigenic and secreted via exovesicles. Scientific Reports, 6, 27293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pablos Torró, L. M. , Moreira, L. R. , & Osuna, A. (2018). Extracellular vesicles in chagas disease: A new passenger for an old disease. Frontiers in Microbiology, 9, 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, J. C. , Silveira, A. C. , & Schofield, C. J. (2002). The impact of Chagas disease control in Latin America: A review. Memorias Do Instituto Oswaldo Cruz, 97, 603–612. [DOI] [PubMed] [Google Scholar]
- Diaz‐Godinez, C. , Rios‐Valencia, D. G. , Garcia‐Aguirre, S. , Martinez‐Calvillo, S. , & Carrero, J. C. (2022). Immunomodulatory effect of extracellular vesicles from Entamoeba histolytica trophozoites: Regulation of NETs and respiratory burst during confrontation with human neutrophils. Frontiers in Cellular and Infection Microbiology, 12, 1018314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz‐Varela, M. , de Menezes‐Neto, A. , Perez‐Zsolt, D. , Gamez‐Valero, A. , Segui‐Barber, J. , Izquierdo‐Useros, N. , Martinez‐Picado, J. , Fernandez‐Becerra, C. , & Del Portillo, H. A. (2018). Proteomics study of human cord blood reticulocyte‐derived exosomes. Scientific Reports, 8, 14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald, R. G. , & Roos, D. S. (1994). Homologous recombination and gene replacement at the dihydrofolate reductase‐thymidylate synthase locus in Toxoplasma gondii. Molecular and Biochemical Parasitology, 63, 243–253. [DOI] [PubMed] [Google Scholar]
- Dong, G. , Filho, A. L. , & Olivier, M. (2019). Modulation of host‐pathogen communication by extracellular vesicles (EVs) of the protozoan parasite leishmania. Frontiers in Cellular and Infection Microbiology, 9, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G. , Wagner, V. , Minguez‐Menendez, A. , Fernandez‐Prada, C. , & Olivier, M. (2021). Extracellular vesicles and leishmaniasis: Current knowledge and promising avenues for future development. Molecular Immunology, 135, 73–83. [DOI] [PubMed] [Google Scholar]
- Douanne, N. , Dong, G. , Amin, A. , Bernardo, L. , Blanchette, M. , Langlais, D. , Olivier, M. , & Fernandez‐Prada, C. (2022). Leishmania parasites exchange drug‐resistance genes through extracellular vesicles. Cell Reports, 40, 111121. [DOI] [PubMed] [Google Scholar]
- Douanne, N. , Dong, G. , Douanne, M. , Olivier, M. , & Fernandez‐Prada, C. (2020). Unravelling the proteomic signature of extracellular vesicles released by drug‐resistant Leishmania infantum parasites. PLOS Neglected Tropical Diseases, 14, e0008439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste, M. , Tertel, T. , Jeruschke, S. , Dittrich, R. , Kontopoulou, E. , Walkenfort, B. , Borger, V. , Hoyer, P. F. , Buscher, A. K. , Thakur, B. K. , & Giebel, B. (2021). Single extracellular vesicle analysis performed by imaging flow cytometry and nanoparticle tracking analysis evaluate the accuracy of urinary extracellular vesicle preparation techniques differently. International Journal of Molecular Sciences, 22, 12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drurey, C. , Coakley, G. , & Maizels, R. M. (2020). Extracellular vesicles: New targets for vaccines against helminth parasites. International Journal for Parasitology, 50, 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drurey, C. , & Maizels, R. M. (2021). Helminth extracellular vesicles: Interactions with the host immune system. Molecular Immunology, 137, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey, J. P. (1998). Advances in the life cycle of Toxoplasma gondii. International Journal for Parasitology, 28, 1019–1024. [DOI] [PubMed] [Google Scholar]
- Dubey, J. P. (2008). The history of Toxoplasma gondii–the first 100 years. Journal of Eukaryotic Microbiology, 55, 467–475. [DOI] [PubMed] [Google Scholar]
- Dubey, J. P. , Ferreira, L. R. , Martins, J. , & McLeod, R. (2012). Oral oocyst‐induced mouse model of toxoplasmosis: Effect of infection with Toxoplasma gondii strains of different genotypes, dose, and mouse strains (transgenic, out‐bred, in‐bred) on pathogenesis and mortality. Parasitology, 139, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey, J. P. , Murata, F. H. A. , Cerqueira‐Cezar, C. K. , Kwok, O. C. H. , & Villena, I. (2021). Congenital toxoplasmosis in humans: An update of worldwide rate of congenital infections—CORRIGENDUM. Parasitology, 148, 1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin, T. V. , Reis, N. F. C. , Perez, E. C. , Soares, R. P. , Torrecilhas, A. C. , & Xander, P. (2021). Long‐term in vitro passaging had a negligible effect on extracellular vesicles released by leishmania amazonensis and induced protective immune response in BALB/c mice. Journal of Immunology Research, 2021, 7809637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, T. , Burke, P. , Smalley, H. , & Hobbs, G. (2016). Trichomonas vaginalis: Clinical relevance, pathogenicity and diagnosis. Critical Reviews in Microbiology, 42, 406–417. [DOI] [PubMed] [Google Scholar]
- Eichenberger, R. M. , Talukder, M. H. , Field, M. A. , Wangchuk, P. , Giacomin, P. , Loukas, A. , & Sotillo, J. (2018). Characterization of Trichuris muris secreted proteins and extracellular vesicles provides new insights into host‐parasite communication. Journal of Extracellular Vesicles, 7, 1428004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsson, E. , Ma'ayeh, S. , & Svard, S. G. (2016). An up‐date on giardia and giardiasis. Current Opinion in Microbiology, 34, 47–52. [DOI] [PubMed] [Google Scholar]
- El‐Assaad, F. , Wheway, J. , Hunt, N. H. , Grau, G. E. , & Combes, V. (2014). Production, fate and pathogenicity of plasma microparticles in murine cerebral malaria. Plos Pathogens, 10, e1003839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliaz, D. , Kannan, S. , Shaked, H. , Arvatz, G. , Tkacz, I. D. , Binder, L. , Waldman Ben‐Asher, H. , Okalang, U. , Chikne, V. , Cohen‐Chalamish, S. , & Michaeli, S. (2017). Exosome secretion affects social motility in Trypanosoma brucei . PLoS Pathogens, 13, e1006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdbrugger, U. , Rudy, C. K. , Etter, M. E. , Dryden, K. A. , Yeager, M. , Klibanov, A. L. , & Lannigan, J. (2014). Imaging flow cytometry elucidates limitations of microparticle analysis by conventional flow cytometry. Cytometry Part A: The Journal of the International Society for Analytical Cytology, 85, 756–770. [DOI] [PubMed] [Google Scholar]
- Esteves, S. , Costa, I. , Luelmo, S. , Santarem, N. , & Cordeiro‐da‐Silva, A. (2022). Leishmania vesicle‐depleted exoproteome: What, why, and how? Microorganisms, 10(12), 2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans‐Osses, I. , Mojoli, A. , Monguió‐Tortajada, M. , Marcilla, A. , Aran, V. , Amorim, M. , Inal, J. , Borràs, F. E. , & Ramirez, M. I. (2017). Microvesicles released from Giardia intestinalis disturb host‐pathogen response in vitro. European Journal of Cell Biology, 96, 131–142. [DOI] [PubMed] [Google Scholar]
- Faille, D. , Combes, V. , Mitchell, A. J. , Fontaine, A. , Juhan‐Vague, I. , Alessi, M. C. , Chimini, G. , Fusai, T. , & Grau, G. E. (2009). Platelet microparticles: A new player in malaria parasite cytoadherence to human brain endothelium. Faseb Journal, 23, 3449–3458. [DOI] [PubMed] [Google Scholar]
- Feely, D. E. , & Dyer, J. K. (1987). Localization of acid phosphatase activity in Giardia lamblia and Giardia muris trophozoites. Journal of Protozoology, 34, 80–83. [DOI] [PubMed] [Google Scholar]
- Feng, Y. , & Xiao, L. (2011). Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clinical Microbiology Reviews, 24, 110–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Calero, T. , Garcia‐Silva, R. , Pena, A. , Robello, C. , Persson, H. , Rovira, C. , Naya, H. , & Cayota, A. (2015). Profiling of small RNA cargo of extracellular vesicles shed by Trypanosoma cruzi reveals a specific extracellular signature. Molecular and Biochemical Parasitology, 199, 19–28. [DOI] [PubMed] [Google Scholar]
- Forrest, D. M. , Batista, M. , Marchini, F. K. , Tempone, A. J. , & Traub‐Cseko, Y. M. (2020). Proteomic analysis of exosomes derived from procyclic and metacyclic‐like cultured Leishmania infantum chagasi. Journal of Proteomics, 227, 103902. [DOI] [PubMed] [Google Scholar]
- Galiano, A. , Minguez, M. T. , Sanchez, C. M. , & Marcilla, A. (2020). Isolation and analysis of Fasciola hepatica extracellular vesicles. Methods in Molecular Biology, 2137, 37–50. [DOI] [PubMed] [Google Scholar]
- Galindo, A. , Javier‐Reyna, R. , Garcia‐Rivera, G. , Banuelos, C. , Chavez‐Munguia, B. , Salazar‐Villatoro, L. , & Orozco, E. (2022). EhVps23, an ESCRT‐I member, is a key factor in secretion, motility, phagocytosis and tissue invasion by Entamoeba histolytica. Frontiers in Cellular and Infection Microbiology, 12, 835654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Silva, M. R. , das Neves, R. F. , Cabrera‐Cabrera, F. , Sanguinetti, J. , Medeiros, L. C. , Robello, C. , Naya, H. , Fernandez‐Calero, T. , Souto‐Padron, T. , de Souza, W. , & Cayota, A. (2014). Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitology Research, 113, 285–304. [DOI] [PubMed] [Google Scholar]
- Gavinho, B. , Sabatke, B. , Feijoli, V. , Rossi, I. V. , da Silva, J. M. , Evans‐Osses, I. , Palmisano, G. , Lange, S. , & Ramirez, M. I. (2020). Peptidylarginine deiminase inhibition abolishes the production of large extracellular vesicles from giardia intestinalis, affecting host‐pathogen interactions by hindering adhesion to host cells. Frontiers in Cellular and Infection Microbiology, 10, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioseffi, A. , Hamerly, T. , Van, K. , Zhang, N. , Dinglasan, R. R. , Yates, P. A. , & Kima, P. E. (2020). Leishmania‐infected macrophages release extracellular vesicles that can promote lesion development. Life Science Alliance, 3(12), e202000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, M. A. , Contreras, I. , Halle, M. , Tremblay, M. L. , McMaster, R. W. , & Olivier, M. (2009). Leishmania GP63 alters host signaling through cleavage‐activated protein tyrosine phosphatases. Science Signaling, 2, ra58. [DOI] [PubMed] [Google Scholar]
- Gomez, M. A. , & Olivier, M. (2010). Proteases and phosphatases during Leishmania‐macrophage interaction: Paving the road for pathogenesis. Virulence, 1, 314–318. [DOI] [PubMed] [Google Scholar]
- Goncalves, D. S. , Ferreira, M. D. S. , Liedke, S. C. , Gomes, K. X. , de Oliveira, G. A. , Leao, P. E. L. , Cesar, G. V. , Seabra, S. H. , Cortines, J. R. , Casadevall, A. , Nimrichter, L. , Domont, G. B. , Junqueira, M. R. , Peralta, J. M. , & Guimaraes, A. J. (2018). Extracellular vesicles and vesicle‐free secretome of the protozoa Acanthamoeba castellanii under homeostasis and nutritional stress and their damaging potential to host cells. Virulence, 9, 818–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves, M. F. , Umezawa, E. S. , Katzin, A. M. , de Souza, W. , Alves, M. J. , Zingales, B. , & Colli, W. (1991). Trypanosoma cruzi: Shedding of surface antigens as membrane vesicles. Experimental Parasitology, 72, 43–53. [DOI] [PubMed] [Google Scholar]
- Gorgens, A. , Bremer, M. , Ferrer‐Tur, R. , Murke, F. , Tertel, T. , Horn, P. A. , Thalmann, S. , Welsh, J. A. , Probst, C. , Guerin, C. , Boulanger, C. M. , Jones, J. C. , Hanenberg, H. , Erdbrugger, U. , Lannigan, J. , Ricklefs, F. L. , El‐Andaloussi, S. , & Giebel, B. (2019). Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using fluorescence‐tagged vesicles as biological reference material. Journal of Extracellular Vesicles, 8, 1587567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govender, Y. , Chan, T. , Yamamoto, H. S. , Budnik, B. , & Fichorova, R. N. (2020). The role of small extracellular vesicles in viral‐protozoan symbiosis: Lessons from trichomonasvirus in an isogenic host parasite model. Frontiers in Cellular and Infection Microbiology, 10, 591172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grajeda, B. I. , De Chatterjee, A. , Villalobos, C. M. , Pence, B. C. , Ellis, C. C. , Enriquez, V. , Roy, S. , Roychowdhury, S. , Neumann, A. K. , Almeida, I. C. , Patterson, S. E. , & Das, S. (2022). Giardial lipid rafts share virulence factors with secreted vesicles and participate in parasitic infection in mice. Frontiers in Cellular and Infection Microbiology, 12, 974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualdron‐Lopez, M. , Diaz‐Varela, M. , Toda, H. , Aparici‐Herraiz, I. , Pedro‐Cos, L. , Lauzurica, R. , Lacerda, M. V. G. , Fernandez‐Sanmartin, M. A. , Fernandez‐Becerra, C. , & Del Portillo, H. A. (2021). Multiparameter flow cytometry analysis of the human spleen applied to studies of plasma‐derived EVs from Plasmodium vivax patients. Frontiers in Cellular and Infection Microbiology, 11, 596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualdron‐Lopez, M. , Diaz‐Varela, M. , Zanghi, G. , Aparici‐Herraiz, I. , Steel, R. W. J. , Schafer, C. , Cusco, P. , Chuenchob, V. , Kangwangransan, N. , Billman, Z. P. , Olsen, T. M. , Gonzalez, J. R. , Roobsoong, W. , Sattabongkot, J. , Murphy, S. C. , Mikolajczak, S. A. , Borras, E. , Sabido, E. , Fernandez‐Becerra, C. , … Del Portillo, H. A. (2022). Mass spectrometry identification of biomarkers in extracellular vesicles from Plasmodium vivax liver hypnozoite infections. Molecular & Cellular Proteomics, 21, 100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualdron‐Lopez, M. , Flannery, E. L. , Kangwanrangsan, N. , Chuenchob, V. , Fernandez‐Orth, D. , Segui‐Barber, J. , Royo, F. , Falcon‐Perez, J. M. , Fernandez‐Becerra, C. , Lacerda, M. V. G. , Kappe, S. H. I. , Sattabongkot, J. , Gonzalez, J. R. , Mikolajczak, S. A. , & Del Portillo, H. A. (2018). Characterization of Plasmodium vivax proteins in plasma‐derived exosomes from malaria‐infected liver‐chimeric humanized mice. Frontiers in Microbiology, 9, 1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, B. C. , Ancarola, M. E. , Volpato‐Rossi, I. , Marcilla, A. , Ramirez, M. I. , Rosenzvit, M. C. , Cucher, M. , & Poncini, C. V. (2022). Extracellular vesicles from Trypanosoma cruzi‐dendritic cell interaction show modulatory properties and confer resistance to lethal infection as a cell‐free based therapy strategy. Frontiers in Cellular and Infection Microbiology, 12, 980817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habegger de Sorrentino, A. , Lopez, R. , Motta, P. , Marinic, K. , Sorrentino, A. , Iliovich, E. , Rubio, A. E. , Quarleri, J. , & Salomon, H. (2005). HLA class II involvement in HIV‐associated Toxoplasmic encephalitis development. Clinical Immunology, 115, 133–137. [DOI] [PubMed] [Google Scholar]
- Haglund, K. , & Dikic, I. (2012). The role of ubiquitylation in receptor endocytosis and endosomal sorting. Journal of Cell Science, 125, 265–275. [DOI] [PubMed] [Google Scholar]
- Hansen, E. P. , Fromm, B. , Andersen, S. D. , Marcilla, A. , Andersen, K. L. , Borup, A. , Williams, A. R. , Jex, A. R. , Gasser, R. B. , Young, N. D. , Hall, R. S. , Stensballe, A. , Ovchinnikov, V. , Yan, Y. , Fredholm, M. , Thamsborg, S. M. , & Nejsum, P. (2019). Exploration of extracellular vesicles from Ascaris suum provides evidence of parasite‐host cross talk. Journal of Extracellular Vesicles, 8, 1578116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harischandra, H. , Yuan, W. , Loghry, H. J. , Zamanian, M. , & Kimber, M. J. (2018). Profiling extracellular vesicle release by the filarial nematode Brugia malayi reveals sex‐specific differences in cargo and a sensitivity to ivermectin. PLOS Neglected Tropical Diseases, 12, e0006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani, K. , Antoniak, E. , Jardim, A. , & Olivier, M. (2011). Temperature‐induced protein secretion by Leishmania mexicana modulates macrophage signalling and function. PLoS ONE, 6, e18724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani, K. , & Olivier, M. (2013). Immunomodulatory impact of leishmania‐induced macrophage exosomes: A comparative proteomic and functional analysis. PLOS Neglected Tropical Diseases, 7, e2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani, K. , Shio, M. T. , Martel, C. , Faubert, D. , & Olivier, M. (2014). Absence of metalloprotease GP63 alters the protein content of Leishmania exosomes. PLoS ONE, 9, e95007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headland, S. E. , Jones, H. R. , D'Sa, A. S. , Perretti, M. , & Norling, L. V. (2014). Cutting‐edge analysis of extracellular microparticles using ImageStream(X) imaging flow cytometry. Scientific Reports, 4, 5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herraiz, A. , I., Caires, H. R. , Castillo‐Fernandez, O. , Sima, N. , Mendez‐Mora, L. , Risueno, R. M. , Sattabongkot, J. , Roobsoong, W. , Hernandez‐Machado, A. , Fernandez‐Becerra, C. , Barrias, C. C. , & Del Portillo, H. A. (2022). Advancing key gaps in the knowledge of Plasmodium vivax cryptic infections using humanized mouse models and organs‐on‐chips. Frontiers in Cellular and Infection Microbiology, 12, 920204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, D. E. , Chirukandoth, S. , & Dubey, J. P. (2005). Biology and epidemiology of Toxoplasma gondii in man and animals. Animal Health Research Reviews, 6, 41–61. [DOI] [PubMed] [Google Scholar]
- Hoffmann, K. F. , Hokke, C. H. , Loukas, A. , & Buck, A. H. (2020). Helminth extracellular vesicles: Great balls of wonder. International Journal for Parasitology, 50, 621–622. [DOI] [PubMed] [Google Scholar]
- WHO . (2015). Investing to overcome the global impact of neglected tropical diseases: WHO third report on neglected tropical diseases. In Holmes P. (Ed.). WHO. [Google Scholar]
- WHO and malaria . (2022). https://www.who.int/news‐room/fact‐sheets/detail/malaria
- Hotez, P. J. , Brindley, P. J. , Bethony, J. M. , King, C. H. , Pearce, E. J. , & Jacobson, J. (2008). Helminth infections: The great neglected tropical diseases. Journal of Clinical Investigation, 118, 1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, C. A. , & Sibley, L. D. (2012). Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nature Reviews Microbiology, 10, 766–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangeer, M. , Mahmood, Z. , Munir, N. , Waraich, U. E. , Tahir, I. M. , Akram, M. , Ali Shah, S. M. , Zulfqar, A. , & Zainab, R. (2020). Naegleria fowleri: Sources of infection, pathophysiology, diagnosis, and management; a review. Clinical and Experimental Pharmacology & Physiology, 47, 199–212. [DOI] [PubMed] [Google Scholar]
- Jayachandran, M. , Miller, V. M. , Heit, J. A. , & Owen, W. G. (2012). Methodology for isolation, identification and characterization of microvesicles in peripheral blood. Journal of Immunological Methods, 375, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra, H. , Drummen, G. P. , & Mathivanan, S. (2016). Focus on extracellular vesicles: Introducing the next small big thing. International Journal of Molecular Sciences, 17, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra, S. K. , Sharma, P. , Shyam, K. , Tejan, N. , & Ghoshal, U. (2020). Acanthamoeba and its pathogenic role in granulomatous amebic encephalitis. Experimental Parasitology, 208, 107788. [DOI] [PubMed] [Google Scholar]
- Khan, N. A. (2006). Acanthamoeba: Biology and increasing importance in human health. Fems Microbiology Review, 30, 564–595. [DOI] [PubMed] [Google Scholar]
- Khosravi, M. , Mirsamadi, E. S. , Mirjalali, H. , & Zali, M. R. (2020). Isolation and functions of extracellular vesicles derived from parasites: The promise of a new era in immunotherapy, vaccination, and diagnosis. International Journal of Nanomedicine, 15, 2957–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kifle, D. W. , Pearson, M. S. , Becker, L. , Pickering, D. , Loukas, A. , & Sotillo, J. (2020). Proteomic analysis of two populations of Schistosoma mansoni‐derived extracellular vesicles: 15k pellet and 120k pellet vesicles. Molecular and Biochemical Parasitology, 236, 111264. [DOI] [PubMed] [Google Scholar]
- Kim, K. , Soldati, D. , & Boothroyd, J. C. (1993). Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science, 262, 911–914. [DOI] [PubMed] [Google Scholar]
- Kim, M. J. , Jung, B. K. , Cho, J. , Song, H. , Pyo, K. H. , Lee, J. M. , Kim, M. K. , & Chai, J. Y. (2016). Exosomes secreted by Toxoplasma gondii‐infected L6 cells: Their effects on host cell proliferation and cell cycle changes. Korean Journal of Parasitology, 54, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koritzinsky, E. H. , Street, J. M. , Star, R. A. , & Yuen, P. S. (2017). Quantification of exosomes. Journal of Cellular Physiology, 232, 1587–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosanovic, M. , Cvetkovic, J. , Gruden‐Movsesijan, A. , Vasilev, S. , Svetlana, M. , Ilic, N. , & Sofronic‐Milosavljevic, L. (2019). Trichinella spiralis muscle larvae release extracellular vesicles with immunomodulatory properties. Parasite Immunology, 41, e12665. [DOI] [PubMed] [Google Scholar]
- Kuipers, M. E. , Koning, R. I. , Bos, E. , Hokke, C. H. , Smits, H. H. , & Nolte‐’t Hoen, E. N. M. (2022). Optimized protocol for the isolation of extracellular vesicles from the parasitic worm Schistosoma mansoni with improved purity, concentration, and yield. Journal of Immunology Research, 2022, 5473763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertz, U. , Oviedo Ovando, M. E. , Vasconcelos, E. J. , Unrau, P. J. , Myler, P. J. , & Reiner, N. E. (2015). Small RNAs derived from tRNAs and rRNAs are highly enriched in exosomes from both old and new world Leishmania providing evidence for conserved exosomal RNA Packaging. BMC Genomics [Electronic Resource], 16, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander, N. , Li, Z. H. , Niyogi, S. , & Docampo, R. (2015). CRISPR/Cas9‐induced disruption of paraflagellar rod protein 1 and 2 genes in Trypanosoma cruzi reveals their role in flagellar attachment. MBio, 6, e01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfredi‐Rangel, A. , Attias, M. , de Carvalho, T. M. , Kattenbach, W. M. , & De Souza, W. (1998). The peripheral vesicles of trophozoites of the primitive protozoan Giardia lamblia may correspond to early and late endosomes and to lysosomes. Journal of Structural Biology, 123, 225–235. [DOI] [PubMed] [Google Scholar]
- LeClaire, M. , Gimzewski, J. , & Sharma, S. (2021). A review of the biomechanical properties of single extracellular vesicles. Nano Select, 2, 1–15. [Google Scholar]
- Lertjuthaporn, S. , Somkird, J. , Lekmanee, K. , Atipimonpat, A. , Sukapirom, K. , Sawasdipokin, H. , Tiewcharoen, S. , Pattanapanyasat, K. , & Khowawisetsut, L. (2022). Extracellular vesicles from Naegleria fowleri induce IL‐8 response in THP‐1 macrophage. Pathogens, 11(6), 11. 10.3390/pathogens11060632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, A. K. C. , Leung, A. A. M. , Wong, A. H. C. , Sergi, C. M. , & Kam, J. K. M. (2019). Giardiasis: An overview. Recent Patents on Inflammation & Allergy Drug Discovery, 13, 134–143. [DOI] [PubMed] [Google Scholar]
- Li, S. , Gong, P. , Tai, L. , Li, X. , Wang, X. , Zhao, C. , Zhang, X. , Yang, Z. , Yang, J. , Li, J. , & Zhang, X. (2018a). Extracellular vesicles secreted by Neospora caninum Are Recognized by toll‐like receptor 2 and modulate host cell innate immunity through the MAPK signaling pathway. Frontiers in Immunology, 9, 1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Zhang, N. , Liu, S. , Li, J. , Liu, L. , Wang, X. , Li, X. , Gong, P. , & Zhang, X. (2021). Protective immunity against Neospora caninum infection induced by 14‐3‐3 protein in mice. Frontiers in Veterinary Science, 8, 638173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Liu, Y. , Xiu, F. , Wang, J. , Cong, H. , He, S. , Shi, Y. , Wang, X. , Li, X. , & Zhou, H. (2018b). Characterization of exosomes derived from Toxoplasma gondii and their functions in modulating immune responses. International Journal of Nanomedicine, 13, 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Xiu, F. , Mou, Z. , Xue, Z. , Du, H. , Zhou, C. , Li, Y. , Shi, Y. , He, S. , & Zhou, H. (2018b). Exosomes derived from Toxoplasma gondii stimulate an inflammatory response through JNK signaling pathway. Nanomedicine (Lond), 13(10), 1157–1168. 10.2217/nnm-2018-0035 [DOI] [PubMed] [Google Scholar]
- Lin, W. C. , Tsai, C. Y. , Huang, J. M. , Wu, S. R. , Chu, L. J. , & Huang, K. Y. (2019). Quantitative proteomic analysis and functional characterization of Acanthamoeba castellanii exosome‐like vesicles. Parasit Vectors, 12, 467. 10.1186/s13071-019-3725-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Zhu, L. , Wang, L. , Chen, Y. , Giri, B. R. , Li, J. , & Cheng, G. (2018). Isolation and characterization of extracellular vesicles from adult Schistosoma japonicum. Journal of Visualized Experiments: JoVE, 135, 57514. 10.3791/57514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M. , Zhang, Z. , Ding, C. , Wang, T. , Kelly, B. , & Wang, P. (2020). Transcriptomic analysis of extracellular RNA governed by the endocytic adaptor protein Cin1 of Cryptococcus deneoformans . Frontiers in Cellular and Infection Microbiology, 10, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo‐Morales, J. , Ortega‐Rivas, A. , Foronda, P. , Abreu‐Acosta, N. , Ballart, D. , Martinez, E. , & Valladares, B. (2005). RNA interference (RNAi) for the silencing of extracellular serine proteases genes in Acanthamoeba: Molecular analysis and effect on pathogenecity. Molecular and Biochemical Parasitology, 144, 10–15. [DOI] [PubMed] [Google Scholar]
- Lotvall, J. , Hill, A. F. , Hochberg, F. , Buzas, E. I. , Di Vizio, D. , Gardiner, C. , Gho, Y. S. , Kurochkin, I. V. , Mathivanan, S. , Quesenberry, P. , Sahoo, S. , Tahara, H. , Wauben, M. H. , Witwer, K. W. , & Thery, C. (2014). Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. Journal of Extracellular Vesicles, 3, 26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovo‐Martins, M. I. , Malvezi, A. D. , Zanluqui, N. G. , Lucchetti, B. F. C. , Tatakihara, V. L. H. , Morking, P. A. , de Oliveira, A. G. , Goldenberg, S. , Wowk, P. F. , & PingeFilho, P. (2018). Extracellular vesicles shed by Trypanosoma cruzi potentiate infection and elicit lipid body formation and PGE2 production in murine macrophages. Frontiers in Immunology, 9, 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano, D. , I.M., Pablos, L. M. D. e , Longhi, S. A. , Zago, M. P. , Schijman, A. G. , & Osuna, A. (2017). Immune complexes in chronic Chagas disease patients are formed by exovesicles from Trypanosoma cruzi carrying the conserved MASP N‐terminal region. Scientific Reports, 7, 44451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma'ayeh, S. Y. , Liu, J. , Peirasmaki, D. , Hornaeus, K. , Bergstrom Lind, S. , Grabherr, M. , Bergquist, J. , & Svard, S. G. (2017). Characterization of the Giardia intestinalis secretome during interaction with human intestinal epithelial cells: The impact on host cells. PLOS Neglected Tropical Diseases, 11, e0006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira, R. P. , Dal'Mas Romera, L. M. , de Cássia Buck, P. , Mady, C. , Ianni, B. M. , & Torrecilhas, A. C. (2021). New biomarker in Chagas disease: Extracellular vesicles isolated from peripheral blood in chronic Chagas disease patients modulate the human immune response. Journal of Immunology Research, 2021, 6650670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira, R. P. , Meneghetti, P. , de Barros, L. A. , de Cassia Buck, P. , Mady, C. , Ianni, B. M. , Fernandez‐Becerra, C. , & Torrecilhas, A. C. (2022). Isolation and molecular characterization of circulating extracellular vesicles from blood of chronic Chagas disease patients. Cell Biology International, 46, 883–894. [DOI] [PubMed] [Google Scholar]
- Magonov, S. N. , Elings, V. , & Whangbo, M. H. (1997). Phase imaging and stiffness in tapping‐mode atomic force microscopy. Surface Science, 375, L385–L391. [Google Scholar]
- Maia, M. M. , da Cruz, A. B. , Pereira, I. S. , Taniwaki, N. N. , Namiyama, G. M. , & Pereira‐Chioccola, V. L. (2021a). Characterization of murine extracellular vesicles and Toxoplasma gondii infection. Parasite Immunology, 43, e12869. [DOI] [PubMed] [Google Scholar]
- Maia, M. M. , da Cruz, A. B. , Taniwaki, N. N. , Namiyama, G. M. , Gava, R. , Gomes, A. H. S. , Kanamura, C. T. , Barbo, M. L. P. , & Pereira‐Chioccola, V. L. (2021b). Immunization with extracellular vesicles excreted by Toxoplasma gondii confers protection in murine infection, activating cellular and humoral responses. International Journal for Parasitology, 51, 559–569. [DOI] [PubMed] [Google Scholar]
- Maizels, R. M. (2021). Identifying novel candidates and configurations for human helminth vaccines. Expert Review of Vaccines, 20, 1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel, P. Y. , Hjelmqvist, D. , Walch, M. , Kharoubi‐Hess, S. , Nilsson, S. , Ravel, D. , Ribeiro, M. , Gruring, C. , Ma, S. , Padmanabhan, P. , Trachtenberg, A. , Ankarklev, J. , Brancucci, N. M. , Huttenhower, C. , Duraisingh, M. T. , Ghiran, I. , Kuo, W. P. , Filgueira, L. , Martinelli, R. , & Marti, M. (2016). Infected erythrocyte‐derived extracellular vesicles alter vascular function via regulatory Ago2‐miRNA complexes in malaria. Nature Communications, 7, 12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel, P. Y. , Hoang, A. N. , Goldowitz, I. , Potashnikova, D. , Hamza, B. , Vorobjev, I. , Ghiran, I. , Toner, M. , Irimia, D. , Ivanov, A. R. , Barteneva, N. , & Marti, M. (2013). Malaria‐infected erythrocyte‐derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host & Microbe, 13, 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel, P. Y. , & Marti, M. (2014). The role of extracellular vesicles in plasmodium and other protozoan parasites. Cellular Microbiology, 16, 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcial‐Quino, J. , Gomez‐Manzo, S. , Fierro, F. , Rufino‐Gonzalez, Y. , Ortega‐Cuellar, D. , Sierra‐Palacios, E. , Vanoye‐Carlo, A. , Gonzalez‐Valdez, A. , Torres‐Arroyo, A. , Oria‐Hernandez, J. , & Reyes‐Vivas, H. (2017). RNAi‐mediated specific gene silencing as a tool for the discovery of new drug targets in Giardia lamblia. Evaluation using the NADH oxidase gene. Genes (Basel), 8(11), 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcilla, A. , Martin‐Jaular, L. , Trelis, M. , de Menezes‐Neto, A. , Osuna, A. , Bernal, D. , Fernandez‐Becerra, C. , Almeida, I. C. , & Del Portillo, H. A. (2014). Extracellular vesicles in parasitic diseases. Journal of Extracellular Vesicles, 3, 25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcilla, A. , Trelis, M. , Cortes, A. , Sotillo, J. , Cantalapiedra, F. , Minguez, M. T. , Valero, M. L. , Sanchez del Pino, M. M. , Munoz‐Antoli, C. , Toledo, R. , & Bernal, D. (2012). Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS ONE, 7, e45974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareze, M. , Benitez, A. D. N. , Brandao, A. P. D. , Pinto‐Ferreira, F. , Miura, A. C. , Martins, F. D. C. , Caldart, E. T. , Biondo, A. W. , Freire, R. L. , Mitsuka‐Bregano, R. , & Navarro, I. T. (2019). Socioeconomic vulnerability associated to Toxoplasma gondii exposure in southern Brazil. PLoS ONE, 14, e0212375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, S. , Kelly, P. H. , Singh, B. K. , Pope, R. M. , Kim, P. , Zhanbolat, B. , Wilson, M. E. , & Yao, C. (2018). Extracellular release of virulence factor major surface protease via exosomes in Leishmania infantum promastigotes. Parasit Vectors, 11, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Jaular, L. , de Menezes‐Neto, A. , Monguio‐Tortajada, M. , Elizalde‐Torrent, A. , Diaz‐Varela, M. , Fernandez‐Becerra, C. , Borras, F. E. , Montoya, M. , & Del Portillo, H. A. (2016). Spleen‐dependent immune protection elicited by CpG adjuvanted reticulocyte‐derived exosomes from malaria infection is associated with changes in T cell subsets' distribution. Frontiers in Cell and Developmental Biology, 4, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Jaular, L. , Nakayasu, E. S. , Ferrer, M. , Almeida, I. C. , & Del Portillo, H. A. (2011). Exosomes from Plasmodium yoelii‐infected reticulocytes protect mice from lethal infections. PLoS ONE, 6, e26588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastoridis, S. , Bertolino, G. M. , Whitehouse, G. , Dazzi, F. , Sanchez‐Fueyo, A. , & Martinez‐Llordella, M. (2018). Multiparametric analysis of circulating exosomes and other small extracellular vesicles by advanced imaging flow cytometry. Frontiers in Immunology, 9, 1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbagwu, S. , Walch, M. , Filgueira, L. , & Mantel, P. Y. (2017). Production and characterization of extracellular vesicles in malaria. Methods in Molecular Biology, 1660, 377–388. [DOI] [PubMed] [Google Scholar]
- Meira, C. S. , Costa‐Silva, T. A. , Vidal, J. E. , Ferreira, I. M. R. , Hiramoto, R. M. , & Pereira‐Chioccola, V. L. (2008). Use of the serum reactivity against Toxoplasma gondii excreted‐secreted antigens in cerebral toxoplasmosis diagnosis in human immunodeficiency virus‐infected patients. Journal of Medical Microbiology, 57, 845–850. [DOI] [PubMed] [Google Scholar]
- Meira‐Strejevitch, C. S. , Pereira, I. S. , Hippolito, D. D. C. , Maia, M. M. , Cruz, A. B. , Gava, R. , Brandao de Mattos, C. C. , Frederico, F. B. , Siqueira, R. C. , Mattos, L. C. , & Pereira‐Chioccola, V. L. (2020). Ocular toxoplasmosis associated with up‐regulation of miR‐155‐5p/miR‐29c‐3p and down‐regulation of miR‐21‐5p/miR‐125b‐5p. Cytokine, 127, 154990. [DOI] [PubMed] [Google Scholar]
- Mevissen, T. E. T. , & Komander, D. (2017). Mechanisms of Deubiquitinase Specificity and Regulation. Annual Review of Biochemistry, 86, 159–192. [DOI] [PubMed] [Google Scholar]
- Midlej, V. , de Souza, W. , & Benchimol, M. (2019). The peripheral vesicles gather multivesicular bodies with different behavior during the Giardia intestinalis life cycle. Journal of Structural Biology, 207, 301–311. [DOI] [PubMed] [Google Scholar]
- Montaner, S. , Galiano, A. , Trelis, M. , Martin‐Jaular, L. , Del Portillo, H. A. , Bernal, D. , & Marcilla, A. (2014). The role of extracellular vesicles in modulating the host immune response during parasitic infections. Frontiers in Immunology, 5, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano, K. J. , Loukas, A. , & Sotillo, J. (2021). Proteomic approaches to drive advances in helminth extracellular vesicle research. Molecular Immunology, 131, 1–5. [DOI] [PubMed] [Google Scholar]
- Montoya, J. G. , & Liesenfeld, O. (2004). Toxoplasmosis. Lancet, 363, 1965–1976. [DOI] [PubMed] [Google Scholar]
- Moreira, L. R. , Prescilla‐Ledezma, A. , Cornet‐Gomez, A. , Linares, F. , Jodar‐Reyes, A. B. , Fernandez, J. , Ibarrola Vannucci, A. K. , De Pablos, L. M. , & Osuna, A. (2021). Biophysical and biochemical comparison of extracellular vesicles produced by infective and non‐infective stages of Trypanosoma cruzi . International Journal of Molecular Sciences, 22(10), 5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, L. R. , Serrano, F. R. , & Osuna, A. (2019). Extracellular vesicles of Trypanosoma cruzi tissue‐culture cell‐derived trypomastigotes: Induction of physiological changes in non‐parasitized culture cells. PLOS Neglected Tropical Diseases, 13(2), e0007163. 10.1371/journal.pntd.0007163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno‐Gonzalo, O. , Villarroya‐Beltri, C. , & Sanchez‐Madrid, F. (2014). Post‐translational modifications of exosomal proteins. Frontiers in Immunology, 5, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro, L. , Bardaji, A. , Macete, E. , Barrios, D. , Morales‐Prieto, D. M. , Espana, C. , Mandomando, I. , Sigauque, B. , Dobano, C. , Markert, U. R. , Benitez‐Ribas, D. , Alonso, P. L. , Menendez, C. , & Mayor, A. (2016). Placental microparticles and MicroRNAs in pregnant women with Plasmodium falciparum or HIV infection. PLoS ONE, 11, e0146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossallam, S. F. , Abou‐El‐Naga, I. F. , Abdel Bary, A. , Elmorsy, E. A. , & Diab, R. G. (2021). Schistosoma mansoni egg‐derived extracellular vesicles: A promising vaccine candidate against murine schistosomiasis. PLOS Neglected Tropical Diseases, 15, e0009866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano, S. , Musso, J. , Feliziani, C. , Zamponi, N. , Frontera, L. S. , Ropolo, A. S. , Lanfredi‐Rangel, A. , Lalle, M. , & Touz, M. (2019). Exosome biogenesis in the protozoa parasite Giardia lamblia: A model of reduced interorganellar crosstalk. Cells, 8(12), 1600. 10.3390/cells8121600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, Y. , McManus, D. P. , Gordon, C. A. , & Cai, P. (2021). Parasitic helminth‐derived microRNAs and extracellular vesicle cargos as biomarkers for helminthic infections. Frontiers in Cellular and Infection Microbiology, 11, 708952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, A. K. , Hammerschmidt‐Kamper, C. , & Kaiser, A. (2014). RNAi in plasmodium. Current Pharmaceutical Design, 20, 278–283. [DOI] [PubMed] [Google Scholar]
- Murphy, A. , Cwiklinski, K. , Lalor, R. , O'Connell, B. , Robinson, M. W. , Gerlach, J. , Joshi, L. , Kilcoyne, M. , Dalton, J. P. , & O'Neill, S. M. (2020). Fasciola hepatica extracellular vesicles isolated from excretory‐secretory products using a gravity flow method modulate dendritic cell phenotype and activity. PLOS Neglected Tropical Diseases, 14, e0008626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayasu, E. S. , Sobreira, T. J. , Torres R., Jr. , Ganiko, L. , Oliveira, P. S. , Marques, A. F. , & Almeida, I. C. (2012). Improved proteomic approach for the discovery of potential vaccine targets in Trypanosoma cruzi. Journal of Proteome Research, 11, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantakomol, D. , Dondorp, A. M. , Krudsood, S. , Udomsangpetch, R. , Pattanapanyasat, K. , Combes, V. , Grau, G. E. , White, N. J. , Viriyavejakul, P. , Day, N. P. , & Chotivanich, K. (2011). Circulating red cell‐derived microparticles in human malaria. Journal of Infectious Diseases, 203, 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelam, S. , & Niederkorn, J. Y. (2017). Pathobiology and immunobiology of acanthamoeba keratitis: Insights from animal models. The Yale Journal of Biology and Medicine, 90, 261–268. [PMC free article] [PubMed] [Google Scholar]
- Nievas, Y. R. , Coceres, V. M. , Midlej, V. , de Souza, W. , Benchimol, M. , Pereira‐Neves, A. , Vashisht, A. A. , Wohlschlegel, J. A. , Johnson, P. J. , & de Miguel, N. (2018). Membrane‐shed vesicles from the parasite Trichomonas vaginalis: Characterization and their association with cell interaction. Cellular and Molecular Life Sciences, 75, 2211–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi, S. , & Docampo, R. (2015). A novel role of Rab11 in trafficking GPI‐anchored trans‐sialidase to the plasma membrane of Trypanosoma cruzi . Small GTPases, 6, 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira, P. M. , de Menezes‐Neto, A. , Borges, V. M. , Descoteaux, A. , Torrecilhas, A. C. , Xander, P. , Revach, O. Y. , Regev‐Rudzki, N. , & Soares, R. P. (2020). Immunomodulatory properties of Leishmania extracellular vesicles during host‐parasite interaction: Differential activation of TLRs and NF‐kappaB translocation by dermotropic and viscerotropic species. Frontiers in Cellular and Infection Microbiology, 10, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira, P. M. , Ribeiro, K. , Silveira, A. C. , Campos, J. H. , Martins‐Filho, O. A. , Bela, S. R. , Campos, M. A. , Pessoa, N. L. , Colli, W. , Alves, M. J. , Soares, R. P. , & Torrecilhas, A. C. (2015). Vesicles from different Trypanosoma cruzi strains trigger differential innate and chronic immune responses. Journal of Extracellular Vesicles, 4, 28734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacki, F. C. , Swain, M. T. , Klychnikov, O. I. , Niazi, U. , Ivens, A. , Quintana, J. F. , Hensbergen, P. J. , Hokke, C. H. , Buck, A. H. , & Hoffmann, K. F. (2015). Protein and small non‐coding RNA‐enriched extracellular vesicles are released by the pathogenic blood fluke Schistosoma mansoni. Journal of Extracellular Vesicles, 4, 28665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir‐Birin, Y. , Abou Karam, P. , Rudik, A. , Giladi, T. , Porat, Z. , & Regev‐Rudzki, N. (2018). Monitoring extracellular vesicle cargo active uptake by imaging flow cytometry. Frontiers in Immunology, 9, 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir‐Birin, Y. , Ben Ami Pilo, H. , Cruz Camacho, A. , Rudik, A. , Rivkin, A. , Revach, O. Y. , Nir, N. , Block Tamin, T. , Abou Karam, P. , Kiper, E. , Peleg, Y. , Nevo, R. , Solomon, A. , Havkin‐Solomon, T. , Rojas, A. , Rotkopf, R. , Porat, Z. , Avni, D. , Schwartz, E. , … Regev‐Rudzki, N. (2021). Malaria parasites both repress host CXCL10 and use it as a cue for growth acceleration. Nature Communications, 12, 4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, I. A. , Freire‐de‐Lima, L. , Penha, L. L. , Dias, W. B. , & Todeschini, A. R. (2014). Trypanosoma cruzi Trans‐sialidase: Structural features and biological implications. Sub‐Cellular Biochemistry, 74, 181–201. [DOI] [PubMed] [Google Scholar]
- Oliver, W. C. , & Pharr, G. M. (1992). An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. Journal of Materials Research, 7, 1564–1583. [Google Scholar]
- Olmos‐Ortiz, L. M. , Barajas‐Mendiola, M. A. , Barrios‐Rodiles, M. , Castellano, L. E. , Arias‐Negrete, S. , Avila, E. E. , & Cuéllar‐Mata, P. (2017). Trichomonas vaginalis exosome‐like vesicles modify the cytokine profile and reduce inflammation in parasite‐infected mice. Parasite Immunology, 39(6), e12426. [DOI] [PubMed] [Google Scholar]
- Ong, S. C. , Cheng, W. H. , Ku, F. M. , Tsai, C. Y. , Huang, P. J. , Lee, C. C. , Yeh, Y. M. , Rada, P. , Hrdy, I. , Narayanasamy, R. K. , Smutna, T. , Lin, R. , Luo, H. W. , Chiu, C. H. , Tachezy, J. , & Tang, P. (2022). Identification of endosymbiotic virus in small extracellular vesicles derived from Trichomonas vaginalis. Genes (Basel), 13(3), 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankoui Mfonkeu, J. B. , Gouado, I. , Fotso Kuate, H. , Zambou, O. , Amvam Zollo, P. H. , Grau, G. E. , & Combes, V. (2010). Elevated cell‐specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS ONE, 5, e13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranaiba, L. F. , Guarneri, A. A. , Torrecilhas, A. C. , Melo, M. N. , & Soares, R. P. (2019). Extracellular vesicles isolated from Trypanosoma cruzi affect early parasite migration in the gut of Rhodnius prolixus but not in Triatoma infestans. Memorias Do Instituto Oswaldo Cruz, 114, e190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, D. , Kurup, S. P. , Yao, P. Y. , Minning, T. A. , & Tarleton, R. L. (2014). CRISPR‐Cas9‐mediated single‐gene and gene family disruption in Trypanosoma cruzi . MBio, 6(1), e02097.–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, I. S. , Maia, M. M. , da Cruz, A. B. , Telles, J. P. M. , Vidal, J. E. , Gava, R. , Meira‐Strejevitch, C. S. , & Pereira‐Chioccola, V. L. (2020). Plasma extracellular microRNAs are related to AIDS/cerebral toxoplasmosis co‐infection. Parasite Immunology, 42, e12696. [DOI] [PubMed] [Google Scholar]
- Pereira‐Chioccola, V. L. , Vidal, J. E. , & Su, C. (2009). Toxoplasma gondii infection and cerebral toxoplasmosis in HIV‐infected patients. Future Microbiology, 4, 1363–1379. [DOI] [PubMed] [Google Scholar]
- Perez‐Cabezas, B. , Santarem, N. , Cecilio, P. , Silva, C. , Silvestre, R. , A M Catita, J. , & Cordeiro da Silva, A. (2019). More than just exosomes: Distinct Leishmania infantum extracellular products potentiate the establishment of infection. Journal of Extracellular Vesicles, 8, 1541708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope, S. M. , & Lasser, C. (2013). Toxoplasma gondii infection of fibroblasts causes the production of exosome‐like vesicles containing a unique array of mRNA and miRNA transcripts compared to serum starvation. Journal of Extracellular Vesicles, 2(1), 00–00. 10.3402/jev.v2i0.22484.eCollection [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescilla‐Ledezma, A. , Linares, F. , Ortega‐Munoz, M. , Retana Moreira, L. , Jodar‐Reyes, A. B. , Hernandez‐Mateo, F. , Santoyo‐Gonzalez, F. , & Osuna, A. (2022). Molecular recognition of surface trans‐sialidases in extracellular vesicles of the parasite Trypanosoma cruzi using atomic force microscopy (AFM). International Journal of Molecular Sciences, 23(13), 7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiarim, T. M. , Maia, M. M. , da Cruz, A. B. , Taniwaki, N. N. , Namiyama, G. M. , & Pereira‐Chioccola, V. L. (2021). Characterization of extracellular vesicles isolated from types I, II and III strains of Toxoplasma gondii. Acta Tropica, 219, 105915. [DOI] [PubMed] [Google Scholar]
- Rafati, S. , Ghaemimanesh, F. , & Zahedifard, F. (2006). Comparison of potential protection induced by three vaccination strategies (DNA/DNA, Protein/Protein and DNA/Protein) against Leishmania major infection using Signal Peptidase type I in BALB/c mice. Vaccine, 24, 3290–3297. [DOI] [PubMed] [Google Scholar]
- Rafati, S. , Zahedifard, F. , Azari, M. K. , Taslimi, Y. , & Taheri, T. (2008). Leishmania infantum: Prime boost vaccination with C‐terminal extension of cysteine proteinase type I displays both type 1 and 2 immune signatures in BALB/c mice. Experimental Parasitology, 118, 393–401. [DOI] [PubMed] [Google Scholar]
- Rai, A. K. , & Johnson, P. J. (2019). Trichomonas vaginalis extracellular vesicles are internalized by host cells using proteoglycans and caveolin‐dependent endocytosis. PNAS, 116, 21354–21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg, C. , & Stenmark, H. (2009). The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature, 458, 445–452. [DOI] [PubMed] [Google Scholar]
- Ramirez, M. I. , Amorim, M. G. , Gadelha, C. , Milic, I. , Welsh, J. A. , Freitas, V. M. , Nawaz, M. , Akbar, N. , Couch, Y. , Makin, L. , Cooke, F. , Vettore, A. L. , Batista, P. X. , Freezor, R. , Pezuk, J. A. , Rosa‐Fernandes, L. , Carreira, A. C. O. , Devitt, A. , Jacobs, L. , … Dias‐Neto, E. (2018). Technical challenges of working with extracellular vesicles. Nanoscale, 10, 881–906. [DOI] [PubMed] [Google Scholar]
- Ramirez, M. I. , Deolindo, P. , de Messias‐Reason, I. J. , Arigi, E. A. , Choi, H. , Almeida, I. C. , & Evans‐Osses, I. (2017). Dynamic flux of microvesicles modulate parasite‐host cell interaction of Trypanosoma cruzi in eukaryotic cells. Cellular Microbiology, 19(4), e12672. [DOI] [PubMed] [Google Scholar]
- Ramirez‐Flores, C. J. , Cruz‐Miron, R. , Mondragon‐Castelan, M. E. , Gonzalez‐Pozos, S. , Rios‐Castro, E. , & Mondragon‐Flores, R. (2019). Proteomic and structural characterization of self‐assembled vesicles from excretion/secretion products of Toxoplasma gondii. Journal of Proteomics, 208, 103490. [DOI] [PubMed] [Google Scholar]
- Raposo, G. , Nijman, H. W. , Stoorvogel, W. , Liejendekker, R. , Harding, C. V. , Melief, C. J. , & Geuze, H. J. (1996). B lymphocytes secrete antigen‐presenting vesicles. Journal of Experimental Medicine, 183, 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo, G. , & Stoorvogel, W. (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. Journal of Cell Biology, 200, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev‐Rudzki, N. , Wilson, D. W. , Carvalho, T. G. , Sisquella, X. , Coleman, B. M. , Rug, M. , Bursac, D. , Angrisano, F. , Gee, M. , Hill, A. F. , Baum, J. , & Cowman, A. F. (2013). Cell‐cell communication between malaria‐infected red blood cells via exosome‐like vesicles. Cell, 153, 1120–1133. [DOI] [PubMed] [Google Scholar]
- Reiner, D. S. , McCaffery, M. , & Gillin, F. D. (1990). Sorting of cyst wall proteins to a regulated secretory pathway during differentiation of the primitive eukaryote, Giardia lamblia. European Journal of Cell Biology, 53, 142–153. [PubMed] [Google Scholar]
- Reis, N. F. C. , Dupin, T. V. , Costa, C. R. , Toledo, M. D. S. , de Oliveira, V. C. , Popi, A. F. , Torrecilhas, A. C. , & Xander, P. (2020). Promastigotes or extracellular vesicles modulate B‐1 cell activation and differentiation. Frontiers in Cellular and Infection Microbiology, 10, 573813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retana Moreira, L. , Ramirez, D. V. , Linares, F. , Prescilla Ledezma, A. , Vaglio Garro, A. , Osuna, A. , Lorenzo Morales, J. , & Abrahams Sandi, E. (2020). Isolation of acanthamoeba T5 from water: Characterization of its pathogenic potential, including the production of extracellular vesicles. Pathogens, 9(2), 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retana Moreira, L. , Serrano, F. R. , & Osuna, A. (2019). Extracellular vesicles of Trypanosoma cruzi tissue‐culture cell‐derived trypomastigotes: Induction of physiological changes in non‐parasitized culture cells. PLOS Neglected Tropical Diseases, 13(2), e0007163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retana Moreira, L. , Steller Espinoza, M. F. , Camacho, N. C. , Cornet‐Gomez, A. , Saenz‐Arce, G. , Osuna, A. , Lomonte, B. , & Sandi, E. A. (2022). Characterization of extracellular vesicles secreted by a clinical isolate of Naegleria fowleri and identification of immunogenic components within their protein cargo. Biology (Basel), 11(7), 983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, K. S. , Vasconcellos, C. I. , Soares, R. P. , Mendes, M. T. , Ellis, C. C. , Aguilera‐Flores, M. , de Almeida, I. C. , Schenkman, S. , Iwai, L. K. , & Torrecilhas, A. C. (2018). Proteomic analysis reveals different composition of extracellular vesicles released by two. Journal of Extracellular Vesicles, 7, 1463779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridolfi, A. , Brucale, M. , Montis, C. , Caselli, L. , Paolini, L. , Borup, A. , Boysen, A. T. , Loria, F. , van Herwijnen, M. J. C. , Kleinjan, M. , Nejsum, P. , Zarovni, N. , Wauben, M. H. M. , Berti, D. , Bergese, P. , & Valle, F. (2020). AFM‐based high‐throughput nanomechanical screening of single extracellular vesicles. Analytical Chemistry, 92, 10274–10282. [DOI] [PubMed] [Google Scholar]
- Rogers, M. E. , Ilg, T. , Nikolaev, A. V. , Ferguson, M. A. , & Bates, P. A. (2004). Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature, 430, 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhek‐Goldian, I. , & Cohen, S. R. (2020). Nanomechanics of biomaterials—from cells to shells. Israel Journal of Chemistry, 7, 6–5–621. [Google Scholar]
- Ryan, C. M. , de Miguel, N. , & Johnson, P. J. (2011). Trichomonas vaginalis: Current understanding of host‐parasite interactions. Essays in Biochemistry, 51, 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu, U. , Mohapatra, B. N. , Kar, S. K. , & Ranjit, M. (2013a). Promoter polymorphisms in the ATP binding cassette transporter gene influence production of cell‐derived microparticles and are highly associated with susceptibility to severe malaria in humans. Infection and Immunity, 81, 1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu, U. , Sahoo, P. K. , Kar, S. K. , Mohapatra, B. N. , & Ranjit, M. (2013b). Association of TNF level with production of circulating cellular microparticles during clinical manifestation of human cerebral malaria. Human Immunology, 74, 713–721. [DOI] [PubMed] [Google Scholar]
- Salas, N. , Coceres, V. M. , Melo, T. D. S. , Pereira‐Neves, A. , Maguire, V. G. , Rodriguez, T. M. , Sabatke, B. , Ramirez, M. I. , Sha, J. , Wohlschlegel, J. A. , & de Miguel, N. (2021). VPS32, a member of the ESCRT complex, modulates adherence to host cells in the parasite Trichomonas vaginalis by affecting biogenesis and cargo sorting of released extracellular vesicles. Cellular and Molecular Life Sciences, 79, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saljoughian, N. , Taheri, T. , Zahedifard, F. , Taslimi, Y. , Doustdari, F. , Bolhassani, A. , Doroud, D. , Azizi, H. , Heidari, K. , Vasei, M. , Namvar Asl, N. , Papadopoulou, B. , & Rafati, S. (2013). Development of novel prime‐boost strategies based on a tri‐gene fusion recombinant L. tarentolae vaccine against experimental murine visceral leishmaniasis. PLOS Neglected Tropical Diseases, 7, e2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio, N. G. , Cheng, L. , & Eriksson, E. M. (2017). The role of extracellular vesicles in malaria biology and pathogenesis. Malaria Journal, 16, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Lopez, C. M. , Trelis, M. , Bernal, D. , & Marcilla, A. (2021). Overview of the interaction of helminth extracellular vesicles with the host and their potential functions and biological applications. Molecular Immunology, 134, 228–235. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Lopez, C. M. , Trelis, M. , Jara, L. , Cantalapiedra, F. , Marcilla, A. , & Bernal, D. (2020). Diversity of extracellular vesicles from different developmental stages of Fasciola hepatica. International Journal for Parasitology, 50, 663–669. [DOI] [PubMed] [Google Scholar]
- Santarém, N. , Racine, G. , Silvestre, R. , Cordeiro‐da‐Silva, A. , & Ouellette, M. (2013). Exoproteome dynamics in Leishmania infantum. Journal of Proteomics, 84, 106–118. [DOI] [PubMed] [Google Scholar]
- Schenkman, S. , & Eichinger, D. (1993). Trypanosoma cruzi trans‐sialidase and cell invasion. Parasitology Today, 9, 218–222. [DOI] [PubMed] [Google Scholar]
- Schenkman, S. , Eichinger, D. , Pereira, M. E. , & Nussenzweig, V. (1994). Structural and functional properties of Trypanosoma trans‐sialidase. Annual Review of Microbiology, 48, 499–523. [DOI] [PubMed] [Google Scholar]
- Schenkman, S. , Ferguson, M. A. , Heise, N. , de Almeida, M. L. , Mortara, R. A. , & Yoshida, N. (1993). Mucin‐like glycoproteins linked to the membrane by glycosylphosphatidylinositol anchor are the major acceptors of sialic acid in a reaction catalyzed by trans‐sialidase in metacyclic forms of Trypanosoma cruzi. Molecular and Biochemical Parasitology, 59, 293–303. [DOI] [PubMed] [Google Scholar]
- Schenkman, S. , Kurosaki, T. , Ravetch, J. V. , & Nussenzweig, V. (1992). Evidence for the participation of the Ssp‐3 antigen in the invasion of nonphagocytic mammalian cells by Trypanosoma cruzi. Journal of Experimental Medicine, 175, 1635–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster, F. L. (2002). Cultivation of pathogenic and opportunistic free‐living amebas. Clinical Microbiology Reviews, 15, 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, P. , & Novais, F. O. (2016). Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nature Reviews Immunology, 16, 581–592. [DOI] [PubMed] [Google Scholar]
- Sharma, M. , Morgado, P. , Zhang, H. , Ehrenkaufer, G. , Manna, D. , & Singh, U. (2020). Characterization of extracellular vesicles from Entamoeba histolytica identifies roles in intercellular communication that regulates parasite growth and development. Infection and Immunity, 88(10), e00349.–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. , Das, K. , Woo, J. , & Gimzewski, J. K. (2014). Nanofilaments on glioblastoma exosomes revealed by peak force microscopy. Journal of the Royal Society, Interface, 11, 20131150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. , Rasool, H. I. , Palanisamy, V. , Mathisen, C. , Schmidt, M. , Wong, D. T. , & Gimzewski, J. K. (2010). Structural‐mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS Nano, 4, 1921–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears, R. K. , Bancroft, A. J. , Hughes, G. W. , Grencis, R. K. , & Thornton, D. J. (2018). Extracellular vesicles induce protective immunity against Trichuris muris. Parasite Immunology, 40, e12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestakova, A. , Hanono, A. , Drosner, S. , Curtiss, M. , Davies, B. A. , Katzmann, D. J. , & Babst, M. (2010). Assembly of the AAA ATPase Vps4 on ESCRT‐III. Molecular Biology of the Cell, 21, 1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokouhy, M. , Sarvnaz, H. , Taslimi, Y. , Lajevardi, M. S. , Habibzadeh, S. , Mizbani, A. , Shekari, F. , Behbahani, M. , Torrecilhas, A. C. , & Rafati, S. (2022). Isolation, characterization, and functional study of extracellular vesicles derived from Leishmania tarentolae. Frontiers in Cellular and Infection Microbiology, 12, 921410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley, L. D. , Krahenbuhl, J. L. , Adams, G. M. , & Weidner, E. (1986). Toxoplasma modifies macrophage phagosomes by secretion of a vesicular network rich in surface proteins. Journal of Cell Biology, 103, 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui, R. , Emes, R. , Elsheikha, H. , & Khan, N. A. (2011). Area 51: How do Acanthamoeba invade the central nervous system? Trends in Parasitology, 27, 185–189. [DOI] [PubMed] [Google Scholar]
- Sidik, S. M. , Huet, D. , Ganesan, S. M. , Huynh, M. H. , Wang, T. , Nasamu, A. S. , Thiru, P. , Saeij, J. P. , Carruthers, V. B. , Niles, J. C. , & Lourido, S. (2016). A genome‐wide CRISPR screen in toxoplasma identifies essential apicomplexan genes. Cell, 166, 1423–1435 e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, V. O. , Maia, M. M. , Torrecilhas, A. C. , Taniwaki, N. N. , Namiyama, G. M. , Oliveira, K. C. , Ribeiro, K. S. , Toledo, M. D. S. , Xander, P. , & Pereira‐Chioccola, V. L. (2018). Extracellular vesicles isolated from Toxoplasma gondii induce host immune response. Parasite Immunology, 40, e12571. [DOI] [PubMed] [Google Scholar]
- Silverman, J. M. , Clos, J. , de'Oliveira, C. C. , Shirvani, O. , Fang, Y. , Wang, C. , Foster, L. J. , & Reiner, N. E. (2010a). An exosome‐based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. Journal of Cell Science, 123, 842–852. [DOI] [PubMed] [Google Scholar]
- Silverman, J. M. , Clos, J. , Horakova, E. , Wang, A. Y. , Wiesgigl, M. , Kelly, I. , Lynn, M. A. , McMaster, W. R. , Foster, L. J. , Levings, M. K. , & Reiner, N. E. (2010b). Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. Journal of Immunology, 185, 5011–5022. [DOI] [PubMed] [Google Scholar]
- Silverman, J. M. , & Reiner, N. E. (2011). Exosomes and other microvesicles in infection biology: Organelles with unanticipated phenotypes. Cellular Microbiology, 13, 1–9. [DOI] [PubMed] [Google Scholar]
- Silverman, J. S. , Muratore, K. A. , & Bangs, J. D. (2013). Characterization of the late endosomal ESCRT machinery in Trypanosoma brucei. Traffic (Copenhagen, Denmark), 14, 1078–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, N. , Kataria, O. , & Singh, M. P. (2004). The changing dynamics of Plasmodium vivax and P. falciparum in central India: trends over a 27‐year period (1975–2002). Vector Borne and Zoonotic Diseases (Larchmont, N.Y.), 4(3), 239–248. 10.1089/vbz.2004.4.239 [DOI] [PubMed] [Google Scholar]
- Sisquella, X. , Ofir‐Birin, Y. , Pimentel, M. A. , Cheng, L. , Abou Karam, P. , Sampaio, N. G. , Penington, J. S. , Connolly, D. , Giladi, T. , Scicluna, B. J. , Sharples, R. A. , Waltmann, A. , Avni, D. , Schwartz, E. , Schofield, L. , Porat, Z. , Hansen, D. S. , Papenfuss, A. T. , Eriksson, E. M. , … Regev‐Rudzki, N. (2017). Malaria parasite DNA‐harbouring vesicles activate cytosolic immune sensors. Nature Communications, 8, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotland, T. , Hessvik, N. P. , Sandvig, K. , & Llorente, A. (2019). Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. Journal of Lipid Research, 60, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotland, T. , Sagini, K. , Sandvig, K. , & Llorente, A. (2020). An emerging focus on lipids in extracellular vesicles. Advanced Drug Delivery Reviews, 159, 308–321. [DOI] [PubMed] [Google Scholar]
- Soares, R. P. , Xander, P. , Costa, A. O. , Marcilla, A. , Menezes‐Neto, A. , del Portillo, H. , Witwer, K. , Wauben, M. , Nolte‐T Hoen, E. , Olivier, M. , Criado, M. F. , da Silva, L. L. P. , Abdel Baqui, M. M. , Schenkman, S. , Colli, W. , Alves, M. J. M. , Ferreira, K. S. , Puccia, R. , … Torrecilhas, A. C. (2017). Highlights of the São Paulo ISEV workshop on extracellular vesicles in cross‐kingdom communication. Journal of Extracellular Vesicles, 6, 1407213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soekmadji, C. , Li, B. , Huang, Y. , Wang, H. , An, T. , Liu, C. , Pan, W. , Chen, J. , Cheung, L. , Falcon‐Perez, J. M. , Gho, Y. S. , Holthofer, H. B. , Le, M. T. N. , Marcilla, A. , O'Driscoll, L. , Shekari, F. , Shen, T. L. , Torrecilhas, A. C. , Yan, X. , … Zheng, L. (2020). The future of extracellular vesicles as theranostics—an ISEV meeting report. Journal of Extracellular Vesicles, 9, 1809766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollelis, L. , Ghorbal, M. , MacPherson, C. R. , Martins, R. M. , Kuk, N. , Crobu, L. , Bastien, P. , Scherf, A. , Lopez‐Rubio, J. J. , & Sterkers, Y. (2015). First efficient CRISPR‐Cas9‐mediated genome editing in Leishmania parasites. Cellular Microbiology, 17, 1405–1412. [DOI] [PubMed] [Google Scholar]
- Soprano, L. L. , Parente, J. E. , Landoni, M. , Couto, A. S. , & Duschak, V. G. (2018). Trypanosoma cruzi serinecarboxipeptidase is a sulfated glycoprotein and a minor antigen in human Chagas disease infection. Medical Microbiology and Immunology, 207, 117–128. [DOI] [PubMed] [Google Scholar]
- Sorkin, R. , Huisjes, R. , Boskovic, F. , Vorselen, D. , Pignatelli, S. , Ofir‐Birin, Y. , Freitas Leal, J. K. , Schiller, J. , Mullick, D. , Roos, W. H. , Bosman, G. , Regev‐Rudzki, N. , Schiffelers, R. M. , & Wuite, G. J. L. (2018). Nanomechanics of extracellular vesicles reveals vesiculation pathways. Small, 14, e1801650. [DOI] [PubMed] [Google Scholar]
- Sotillo, J. (2022). MS‐based extracellular vesicle (EVs) analysis: An application to helminth‐secreted EVs. Methods in Molecular Biology, 2420, 11–20. [DOI] [PubMed] [Google Scholar]
- Sotillo, J. , Pearson, M. , Potriquet, J. , Becker, L. , Pickering, D. , Mulvenna, J. , & Loukas, A. (2016). Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates. International Journal for Parasitology, 46, 1–5. [DOI] [PubMed] [Google Scholar]
- Sotillo, J. , Robinson, M. W. , Kimber, M. J. , Cucher, M. , Ancarola, M. E. , Nejsum, P. , Marcilla, A. , Eichenberger, R. M. , & Tritten, L. (2020). The protein and microRNA cargo of extracellular vesicles from parasitic helminths—current status and research priorities. International Journal for Parasitology, 50, 635–645. [DOI] [PubMed] [Google Scholar]
- Sultan, A. A. , Thathy, V. , de Koning‐Ward, T. F. , & Nussenzweig, V. (2001). Complementation of Plasmodium berghei TRAP knockout parasites using human dihydrofolate reductase gene as a selectable marker. Molecular and Biochemical Parasitology, 113, 151–156. [DOI] [PubMed] [Google Scholar]
- Tanno, H. , & Komada, M. (2013). The ubiquitin code and its decoding machinery in the endocytic pathway. Journal of Biochemistry, 153, 497–504. [DOI] [PubMed] [Google Scholar]
- Tertel, T. , Gorgens, A. , & Giebel, B. (2020). Analysis of individual extracellular vesicles by imaging flow cytometry. Methods in Enzymology, 645, 55–78. [DOI] [PubMed] [Google Scholar]
- Thane, K. E. , Davis, A. M. , & Hoffman, A. M. (2019). Improved methods for fluorescent labeling and detection of single extracellular vesicles using nanoparticle tracking analysis. Scientific Reports, 9, 12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry, C. , Amigorena, S. , Raposo, G. , & Clayton, A. (2006). Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current Protocols in Cell Biology, 3, 322. [DOI] [PubMed] [Google Scholar]
- Théry, C. , Ostrowski, M. , & Segura, E. (2009). Membrane vesicles as conveyors of immune responses. Nature Reviews Immunology, 9, 581–593. [DOI] [PubMed] [Google Scholar]
- Théry, C. , Witwer, K. W. , Aikawa, E. , Alcaraz, M. J. , Anderson, J. D. , Andriantsitohaina, R. , Antoniou, A. , Arab, T. , Archer, F. , Atkin‐Smith, G. K. , Ayre, D. C. , Bach, J. , Bachurski, D. , Baharvand, H. , Balaj, L. , Baldacchino, S. , Bauer, N. N. , Baxter, A. A. , Bebawy, M. , … & Zuba‐Surma, E. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7, 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry, C. , Zitvogel, L. , & Amigorena, S. (2002). Exosomes: Composition, biogenesis and function. Nature Reviews Immunology, 2, 569–579. [DOI] [PubMed] [Google Scholar]
- Threadgold, L. T. (1968). The tegument and associated structures of Haplometra cylindracea. Parasitology, 58, 1–7. [DOI] [PubMed] [Google Scholar]
- Tiberti, N. , Latham, S. L. , Bush, S. , Cohen, A. , Opoka, R. O. , John, C. C. , Juillard, A. , Grau, G. E. , & Combes, V. (2016). Exploring experimental cerebral malaria pathogenesis through the characterisation of host‐derived plasma microparticle protein content. Scientific Reports, 6, 37871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda, H. , Diaz‐Varela, M. , Segui‐Barber, J. , Roobsoong, W. , Baro, B. , Garcia‐Silva, S. , Galiano, A. , Gualdron‐Lopez, M. , Almeida, A. C. G. , Brito, M. A. M. , de Melo, G. C. , Aparici‐Herraiz, I. , Castro‐Cavadia, C. , Monteiro, W. M. , Borras, E. , Sabido, E. , Almeida, I. C. , Chojnacki, J. , Martinez‐Picado, J. , … Del Portillo, H. A. (2020). Plasma‐derived extracellular vesicles from Plasmodium vivax patients signal spleen fibroblasts via NF‐kB facilitating parasite cytoadherence. Nature Communications, 11, 2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilhas, A. C. , Schumacher, R. I. , Alves, M. J. , & Colli, W. (2012). Vesicles as carriers of virulence factors in parasitic protozoan diseases. Microbes and Infection, 14, 1465–1474. [DOI] [PubMed] [Google Scholar]
- Torrecilhas, A. C. , Soares, R. P. , Schenkman, S. , Fernández‐Prada, C. , & Olivier, M. (2020). Extracellular vesicles in trypanosomatids: Host cell communication. Frontiers in Cellular and Infection Microbiology, 10, 602502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelis, M. , Galiano, A. , Bolado, A. , Toledo, R. , Marcilla, A. , & Bernal, D. (2016). Subcutaneous injection of exosomes reduces symptom severity and mortality induced by Echinostoma caproni infection in BALB/c mice. International Journal for Parasitology, 46, 799–808. [DOI] [PubMed] [Google Scholar]
- Trelis, M. , Sanchez‐Lopez, C. M. , Sanchez‐Palencia, L. F. , Ramirez‐Toledo, V. , Marcilla, A. , & Bernal, D. (2022). Proteomic analysis of extracellular vesicles from Fasciola hepatica hatching eggs and juveniles in culture. Frontiers in Cellular and Infection Microbiology, 12, 903602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritten, L. , & Geary, T. G. (2018). Helminth extracellular vesicles in host‐parasite interactions. Current Opinion in Microbiology, 46, 73–79. [DOI] [PubMed] [Google Scholar]
- Trocoli Torrecilhas, A. C. , Tonelli, R. R. , Pavanelli, W. R. , da Silva, J. S. , Schumacher, R. I. , de Souza, W. , Silva, N. C. E. , de Almeida Abrahamsohn, I. , Colli, W. , & Manso Alves, M. J. (2009). Trypanosoma cruzi: Parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes and Infection, 11, 29–39. [DOI] [PubMed] [Google Scholar]
- Twu, O. , de Miguel, N. , Lustig, G. , Stevens, G. C. , Vashisht, A. A. , Wohlschlegel, J. A. , & Johnson, P. J. (2013). Trichomonas vaginalis exosomes deliver cargo to host cells and mediate hostratioparasite interactions. Plos Pathogens, 9, e1003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara, L. A. , Moreira, O. C. , Oliveira, A. C. , Azambuja, P. , Lima, A. P. , Britto, C. , dos Santos, A. L. , Branquinha, M. H. , & d'Avila‐Levy, C. M. (2012). Cruzipain promotes Trypanosoma cruzi adhesion to Rhodnius prolixus midgut. PLOS Neglected Tropical Diseases, 6, e1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pol, E. , Sturk, A. , van Leeuwen, T. , Nieuwland, R. , Coumans, F. , & group, I.‐S.‐V. W. (2018). Standardization of extracellular vesicle measurements by flow cytometry through vesicle diameter approximation. Journal of Thrombosis and Haemostasis, 16, 1236–1245. [DOI] [PubMed] [Google Scholar]
- Vasconcelos, C. I. , Cronemberger‐Andrade, A. , Souza‐Melo, N. , Maricato, J. T. , Xander, P. , Batista, W. L. , Soares, R. P. , Schenkman, S. , & Torrecilhas, A. C. (2021). Stress induces release of extracellular vesicles by Trypanosoma cruzi trypomastigotes. Journal of Immunology Research, 2021, 2939693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvesvara, G. S. , Moura, H. , & Schuster, F. L. (2007). Pathogenic and opportunistic free‐living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. Fems Immunology and Medical Microbiology, 50, 1–26. [DOI] [PubMed] [Google Scholar]
- Vorselen, D. , Marchetti, M. , Lopez‐Iglesias, C. , Peters, P. J. , Roos, W. H. , & Wuite, G. J. L. (2018). Multilamellar nanovesicles show distinct mechanical properties depending on their degree of lamellarity. Nanoscale, 10, 5318–5324. [DOI] [PubMed] [Google Scholar]
- Vucetic, A. , Filho, A. , Dong, G. , & Olivier, M. (2020). Isolation of extracellular vesicles from Leishmania spp. Methods in Molecular Biology, 2116, 555–574. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Li, Z. , Shen, J. , Liu, Z. , Liang, J. , Wu, X. , Sun, X. , & Wu, Z. (2015). Exosome‐like vesicles derived by Schistosoma japonicum adult worms mediates M1 type immune‐ activity of macrophage. Parasitology Research, 114, 1865–1873. [DOI] [PubMed] [Google Scholar]
- Weiss, D. J. , Lucas, T. C. D. , Nguyen, M. , Nandi, A. K. , Bisanzio, D. , Battle, K. E. , Cameron, E. , Twohig, K. A. , Pfeffer, D. A. , Rozier, J. A. , Gibson, H. S. , Rao, P. C. , Casey, D. , Bertozzi‐Villa, A. , Collins, E. L. , Dalrymple, U. , Gray, N. , Harris, J. R. , Howes, R. E. , … Gething, P. W. (2019). Mapping the global prevalence, incidence, and mortality of Plasmodium falciparum, 2000‐17: A spatial and temporal modelling study. Lancet, 394(10195), 322–331. 10.1016/S0140-6736(19)31097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh, J. A. , Van Der Pol, E. , Arkesteijn, G. J. A. , Bremer, M. , Brisson, A. , Coumans, F. , Dignat‐George, F. , Duggan, E. , Ghiran, I. , Giebel, B. , Görgens, A. , Hendrix, A. , Lacroix, R. , Lannigan, J. , Libregts, S. F. W. M. , Lozano‐Andrés, E. , Morales‐Kastresana, A. , Robert, S. , … Jones, J. C. (2020). MIFlowCyt‐EV: A framework for standardized reporting of extracellular vesicle flow cytometry experiments. Journal of Extracellular Vesicles, 9, 1713526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, R. , Kumar, S. , Chow, F. W. , Robertson, E. , Hayes, K. S. , Grencis, R. K. , Duque‐Correa, M. A. , & Buck, A. H. (2020). Extracellular vesicles from Heligmosomoides bakeri and Trichuris muris contain distinct microRNA families and small RNAs that could underpin different functions in the host. International Journal for Parasitology, 50, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, R. , Sotillo, J. , Ancarola, M. E. , Borup, A. , Boysen, A. T. , Brindley, P. J. , Buzas, E. I. , Cavallero, S. , Chaiyadet, S. , Chalmers, I. W. , Cucher, M. A. , Dagenais, M. , Davis, C. N. , Devaney, E. , Duque‐Correa, M. A. , Eichenberger, R. M. , Fontenla, S. , Gasan, T. A. , Hokke, C. H. , … Hoffmann, K. F. (2023). Special considerations for studies of extracellular vesicles from parasitic helminths: A community‐led roadmap to increase rigour and reproducibility. Journal of Extracellular Vesicles, 12, e12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, C. , Palviainen, M. , Reichardt, N. C. , Siljander, P. R. , & Falcon‐Perez, J. M. (2019). Metabolomics applied to the study of extracellular vesicles. Metabolites, 9(11), 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer, K. W. , Buzas, E. I. , Bemis, L. T. , Bora, A. , Lasser, C. , Lotvall, J. , Nolte‐’t Hoen, E. N. , Piper, M. G. , Sivaraman, S. , Skog, J. , Thery, C. , Wauben, M. H. , & Hochberg, F. (2013). Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. Journal of Extracellular Vesicles, 2(1), 00–00. 10.3402/jev.v2i0.20360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers, J. , Lozier, A. , Raposo, G. , Regnault, A. , Théry, C. , Masurier, C. , Flament, C. , Pouzieux, S. , Faure, F. , Tursz, T. , Angevin, E. , Amigorena, S. , & Zitvogel, L. (2001). Tumor‐derived exosomes are a source of shared tumor rejection antigens for CTL cross‐priming. Nature Medicine, 7, 297–303. [DOI] [PubMed] [Google Scholar]
- Wowk, P. F. , Zardo, M. L. , Miot, H. T. , Goldenberg, S. , Carvalho, P. C. , & Morking, P. A. (2017). Proteomic profiling of extracellular vesicles secreted from Toxoplasma gondii. Proteomics, 17(15–16), 1600477. [DOI] [PubMed] [Google Scholar]
- Xu, K. , Sun, W. , Shao, Y. , Wei, F. , Zhang, X. , Wang, W. , & Li, P. (2018). Recent development of PeakForce Tapping mode atomic force microscopy and its applications on nanoscience. Nanotechnology Reviews, 7, 605–621. [Google Scholar]
- Xu, M. J. , Chen, N. , Song, H. Q. , Lin, R. Q. , Huang, C. Q. , Yuan, Z. G. , & Zhu, X. Q. (2010). RNAi‐mediated silencing of a novel Ascaris suum gene expression in infective larvae. Parasitology Research, 107, 1499–1503. [DOI] [PubMed] [Google Scholar]
- Yáñez‐Mó, M. , Siljander, P. R. , Andreu, Z. , Zavec, A. B. , Borràs, F. E. , Buzas, E. I. , Buzas, K. , Casal, E. , Cappello, F. , Carvalho, J. , Colás, E. , Cordeiro‐da Silva, A. , Fais, S. , Falcon‐Perez, J. M. , Ghobrial, I. M. , Giebel, B. , Gimona, M. , Graner, M. , Gursel, I. , , … De Wever, O. (2015). Biological properties of extracellular vesicles and their physiological functions. Journal of Extracellular Vesicles, 4, 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybanez, R. H. D. , Ybanez, A. P. , & Nishikawa, Y. (2020). Review on the current trends of toxoplasmosis serodiagnosis in humans. Frontiers in Cellular and Infection Microbiology, 10, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, W. , Chew, M. , Hou, J. , Lai, F. , Leopold, S. J. , Loo, H. L. , Ghose, A. , Dutta, A. K. , Chen, Q. , Ooi, E. E. , White, N. J. , Dondorp, A. M. , Preiser, P. , & Chen, J. (2018). Microvesicles from malaria‐infected red blood cells activate natural killer cells via MDA5 pathway. Plos Pathogens, 14, e1007298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuana, Y. , Boing, A. N. , Grootemaat, A. E. , van der Pol, E. , Hau, C. M. , Cizmar, P. , Buhr, E. , Sturk, A. , & Nieuwland, R. (2015). Handling and storage of human body fluids for analysis of extracellular vesicles. Journal of Extracellular Vesicles, 4, 29260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakeri, A. , Hansen, E. P. , Andersen, S. D. , Williams, A. R. , & Nejsum, P. (2018). Immunomodulation by Helminths: Intracellular pathways and extracellular vesicles. Frontiers in Immunology, 9, 2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , & Lyden, D. (2019). Asymmetric‐flow field‐flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nature Protocols, 14, 1027–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. W. , Lypaczewski, P. , & Matlashewski, G. (2017). Optimized CRISPR‐Cas9 genome editing for leishmania and its use to target a multigene family, induce chromosomal translocation, and study DNA break repair mechanisms. mSphere., 2(1), e00340.–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, P. , Cao, L. , Wang, X. , Dong, J. , Zhang, N. , Li, X. , Li, J. , Zhang, X. , & Gong, P. (2021). Extracellular vesicles secreted by Giardia duodenalis regulate host cell innate immunity via TLR2 and NLRP3 inflammasome signaling pathways. PLOS Neglected Tropical Diseases, 15, e0009304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, Q. , Inniss, D. , Kjoller, K. , & Elings, V. B. (1993). Fractured polymer/silica fiber surface studied by tapping mode atomic force microscopy. Surface Science, 290, L688–L692. [Google Scholar]
- Zhou, X. , Wang, W. , Cui, F. , Shi, C. , Ma, Y. , Yu, Y. , Zhao, W. , & Zhao, J. (2019). Extracellular vesicles derived from Echinococcus granulosus hydatid cyst fluid from patients: Isolation, characterization and evaluation of immunomodulatory functions on T cells. International Journal for Parasitology, 49, 1029–1037. [DOI] [PubMed] [Google Scholar]
- Zhu, S. , Wang, S. , Lin, Y. , Jiang, P. , Cui, X. , Wang, X. , Zhang, Y. , & Pan, W. (2016). Release of extracellular vesicles containing small RNAs from the eggs of Schistosoma japonicum. Parasit Vectors, 9, 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel, L. , Regnault, A. , Lozier, A. , Wolfers, J. , Flament, C. , Tenza, D. , Ricciardi‐Castagnoli, P. , Raposo, G. , & Amigorena, S. (1998). Eradication of established murine tumors using a novel cell‐free vaccine: Dendritic cell‐derived exosomes. Nature Medicine, 4, 594–600. [DOI] [PubMed] [Google Scholar]


