Abstract
Cell culture‐conditioned medium (CCM) is a valuable source of extracellular vesicles (EVs) for basic scientific, therapeutic and diagnostic applications. Cell culturing parameters affect the biochemical composition, release and possibly the function of CCM‐derived EVs (CCM‐EV). The CCM‐EV task force of the Rigor and Standardization Subcommittee of the International Society for Extracellular Vesicles aims to identify relevant cell culturing parameters, describe their effects based on current knowledge, recommend reporting parameters and identify outstanding questions. While some recommendations are valid for all cell types, cell‐specific recommendations may need to be established for non‐mammalian sources, such as bacteria, yeast and plant cells. Current progress towards these goals is summarized in this perspective paper, along with a checklist to facilitate transparent reporting of cell culturing parameters to improve the reproducibility of CCM‐EV research.
Keywords: cell culture‐conditioned medium, cellular therapy, ectosomes, exosomes, extracellular vesicles, in vitro, reproducibility, rigor, standardization
1. INTRODUCTION
Extracellular vesicles (EVs) are heterogenous membrane‐delimited particles that are released from cells in physiological and pathological states (Buzas, 2023; György et al., 2011; Witwer & Théry, 2019) and include endosome‐origin exosomes and plasma membrane‐shed ectosomes or microvesicles. EVs are composed of luminal cargo, a surrounding phospholipid membrane and membrane‐embedded and ‐attached macromolecular entities. A more loosely associated bio‐corona may also be of critical importance (Buzas, 2022; Wolf et al., 2022; Yerneni et al., 2022). In vivo, EVs are present in biological fluids such as blood, cerebrospinal fluid, saliva, tears and urine and in solid tissues. In vitro, EVs can be prepared from cell culture‐conditioned media (CCM; Figure 1a). EVs in CCM are released by the cultured cells but may also originate from supplements used for cell growth and/or differentiation. Since cells and medium supplements also release or contain non‐EV particles, it is often necessary to separate EVs from non‐EV components to identify EV‐specific contents and functions (Figure 1b).
FIGURE 1.
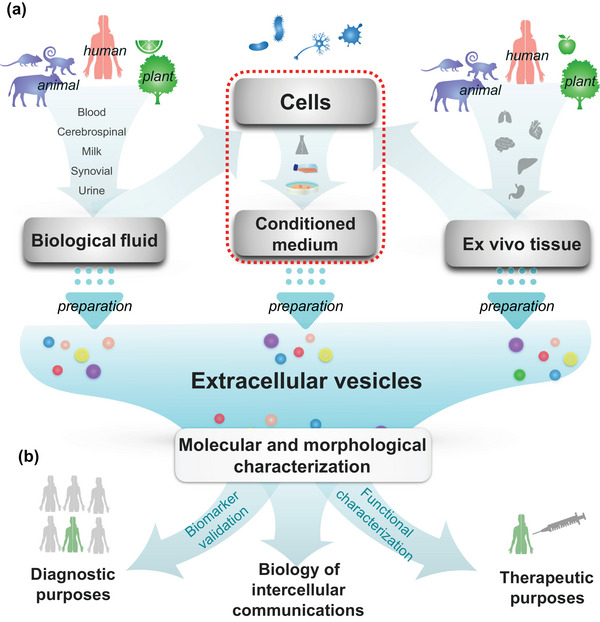
Source and application of extracellular vesicles. (a) Extracellular vesicles (EVs) can be prepared from biological fluids, cell culture‐conditioned medium (CCM), or ex vivo tissue. (b) Following molecular and morphological characterization, EVs can be used to study intercellular communication biology, to diagnose diseases (biomarker research), or as therapeutic agents (directly or as drug delivery vehicles). The dotted box indicates the focus of the CCM‐derived EV (CCM‐EV) task force on cell culturing parameters and their effects on EV release, content, and biological function.
Among the different sources of EVs, CCM is especially important because CCM‐derived EVs (CCM‐EV), whether native or engineered, can exert therapeutic functions. CCM‐EV have been administered to animals and patients to assess their therapeutic potential for several pathological conditions (Elahi et al., 2020; Kordelas et al., 2014; Kwon et al., 2020; Morse et al., 2005; Nassar et al., 2016; Park et al., 2021; Sengupta et al., 2020; Shekari et al., 2021; Warnecke et al., 2021). Table S1 gives an overview of clinical trials which have used or are currently using CCM‐EV from cellular sources such as dendritic cells, mesenchymal stromal cells (MSCs), tumor cells, T cells and plant cells. Producing cells, cell culture components, culture conditions and CCM collection and processing will impact the biochemical composition of EVs, association with other entities such as a co‐purifying bio‐corona, and biological function. However, there are currently no reporting guidelines for CCM‐derived EV.
To maximize the reliability and reproducibility of CCM‐EV research, transparent reporting is needed for the production and characterization of CCM‐EV. There are several guidelines on good practices for general cell and tissue culture for cellular therapy (Coecke et al., 2005; Pamies, 2018; Pamies et al., 2017). Furthermore, minimal criteria for reporting EV studies were proposed in MISEV2014 (Lötvall et al., 2014) and MISEV2018 (Théry et al., 2018), and a systematic assessment of articles published from 2012 to 2020 showed a clear association of study quality with citation of MISEV (Poupardin et al., 2021). In addition, working groups including members of the International Society for Extracellular Vesicles (ISEV), the International Society for Cell and Gene Therapy (ISCT), and the Society for Clinical, Research and Translation of Extracellular Vesicles Singapore (SOCRATES) have published best practice recommendations to produce MSC‐EVs and assays for therapeutic applications (Gimona et al., 2021; Pachler et al., 2017; Rohde et al., 2019; Witwer et al., 2019).
The ISEV Rigor and Standardization (R&S) Subcommittee (Nieuwland et al., 2020) aims to improve reproducibility of EV research (Börger et al., 2020; Clayton et al., 2019; Erdbrügger et al., 2021; Welsh et al., 2020). Although MISEV2018 provides mandatory reporting regarding CCM (Théry et al., 2018), and the importance of culturing parameters for the production of EVs is commonly recognized (Hill et al., 2013; Gudbergsson et al., 2016; Gurunathan et al., 2021; Patel et al., 2017, 2018; Staubach et al., 2021), standardized reporting of culturing parameters has not been implemented in most publications. Therefore, the R&S subcommittee established a task force on CCM‐EV to focus on cell culturing parameters and their effects on EVs (Figure 1a, red dotted box). Here, the CCM‐EV task force focuses on selected cell monoculture parameters that may affect EV characteristics, gives recommendations for transparent reporting, and identifies open questions. Our goals are to increase awareness and promote reproducibility in EV research when CCM is the starting material.
2. CELL CULTURE PARAMETERS
2.1. Cells (source cells)
2.1.1. Cell identity
The identity of the donor/parental cell is a major determinant of EV yield, phenotype and biological function. Different cells may have different basal rates of EV production. For example, EV yield and function may differ between MSCs derived from different donors and tissue sources (Almeria et al., 2022; Fafián‐Labora et al., 2017; Kang et al., 2016; Komaki et al., 2017; Mendt et al., 2018; Merckx et al., 2020; Nakamura et al., 2015; Rosenberger et al., 2019; Teng et al., 2015; Tracy et al., 2019). Also, different cancer cell lines produce different numbers of EVs (Charoenviriyakul et al., 2017; Hurwitz et al., 2016; Salomon et al., 2014). For cell lines, regular short tandem repeat (STR) profiling can confirm identity of cells and detect cellular cross‐contamination (American Type Culture Collection Standards Development Organization Workgroup ASN‐000, 2010; Barallon et al., 2010; Masters et al., 2001; Reid et al., 2004).
2.1.1.1. Recommendations
Report the identity of EV‐producing cells according to consensus in the research communities that regularly use such cells. For example, ISCT criteria define multipotent MSCs by positive and negative marker expression and differentiation potential (Dominici et al., 2006; Viswanathan et al., 2019; Witwer et al., 2019). Criteria for clinical‐grade human induced pluripotent stem cell lines have also been established (Sullivan et al., 2018). Additional information regarding specific cell lines can be found at https://www.atcc.org/, and cell‐line specific molecular information is available at https://depmap.org/portal.
If downstream EV analysis focuses on a particular marker, confirm that the EV marker of interest is also present in/on the cultured cells and thus likely originates from those cells and not from another source.
2.1.1.2. Outstanding questions
In some cell monocultures, multiple populations of cells are nevertheless present (Costa et al., 2021; Sato et al., 2016; Wang et al., 2021) (i.e., cells with genetic, epigenetic, morphologic or functional differences), which contributes to the heterogeneity of EVs and makes it more difficult to interpret from which cell types the detected EVs are derived. How can we reduce or control the heterogeneity of source cells?
2.1.2. Characteristics of the cell/tissue donor
The potency of primary cells may be affected by characteristics of the cell donor, including overall health status, age (Choudhery et al., 2014; Dorronsoro et al., 2021; Gruber et al., 2012; Katsara et al., 2011; Payr et al., 2020; Sarkar et al., 2018; Siegel et al., 2013), biological sex (Katsara et al., 2011; Sammour et al., 2016; Siegel et al., 2013), pregnancy (Li et al., 2017; Mathew & Bhonde, 2017; Xia et al., 2007; Zhu et al., 2020), medication intake, nutritional status, infection and microbiota. In turn, these factors may influence the release, composition and function of EVs. For example, functional comparison of EVs from young and old donors revealed donor age dependencies (Agarwal et al., 2017; Sun et al., 2020; Umezu et al., 2017). The biochemical composition of EVs derived from different human donors also varies, possibly due to donor characteristics (Kim et al., 2016; Kordelas et al., 2014).
2.1.2.1. Recommendations
Report (if available) known donor characteristics, including but not limited to biological sex, age, chronic diseases, infection status, medication and pregnancy/complications.
Screen cells for the presence of infectious agents, especially if clinical applications are expected. Note that some viruses can integrate into the genome.
Report pre‐culture processing of the cells.
2.1.3. Initial seeding density
The initial concentration, or the number of cells per volume or surface area in the culture flask or plate, affects cell growth and differentiation, as well as the release of EVs. This dependence was shown for MSCs and cancer cells (Ludwig et al., 2019; Patel et al., 2017).
2.1.3.1. Recommendations
Report the initial seeding density of cells, ideally as cells per cm2 (for adherent cells) or cells per mL (for suspension cells).
Reduce variation by using the same seeding concentration and cell splitting intervals across experiments.
Optimal plating density has been reported for specific cells and culturing conditions (Sotiropoulou et al., 2006); otherwise, optimize seeding density.
2.1.4. Cell density or confluency of cells at the time of CCM collection
The density of cells affects the cell physiology (Balint et al., 2015; Bereiter‐Hahn et al., 1998; Jacobs et al., 2016; Ren et al., 2015; Sekiya et al., 2002; Trajkovic et al., 2019), thereby affecting the production rate, biochemical composition and function of released EVs (Cao et al., 2020; Gao et al., 2020; Guerreiro et al., 2018; Gurunathan et al., 2021; Haraszti et al., 2018; Hayes et al., 2005; Mitchell et al., 2008; Palviainen et al., 2019; Rocha et al., 2019; Sekiya et al., 2002; Steinman et al., 2003; Thippabhotla et al., 2019; Yan & Wu, 2020; Yang et al., 2020). In over‐confluent cell cultures, the therapeutic potential of MSCs may change due to contact‐inhibited growth or contact‐induced senescence (Balint et al., 2015; Ho et al., 2011; Sekiya et al., 2002). For 2D cultures, the percentage of surface area covered can be reported, but this metric may be inadequate, as cells are not equally large.
2.1.4.1. Recommendations
Count cell numbers in a standardized procedure (e.g., with technical replicates) before seeding and after final harvesting at a given passage.
Report cell density at the time of CCM collection (cells/cm2 or cells/mL). For 3D cultures of spheroids or organoids, the reporting parameter might be the diameter of the spheroids or another appropriate measure.
2.1.4.2. Outstanding questions
In 2D cultures, the term ‘confluency’ is often used. However, this remains a largely ‘qualitative’ parameter, and measurements may differ between individual researchers and laboratories due to different equipment and procedures. More objective measurements may be achieved by preparing and following detailed standard operating procedures that help to improve reproducibility.
2.1.5. Cell sub‐culturing, population doubling and passage number
Transferring cultured cells from high density to low density for propagation (‘sub‐culturing’, ‘passaging’, ‘splitting’ or ‘re‐plating’) can influence cellular properties including the expression of cell surface markers, senescence and genetic stability (Kassem et al., 1997; Meza‐Zepeda et al., 2008; Wagner et al., 2008; Yang et al., 2018). Suspension cells are either simply diluted into fresh culture medium, or, in some cases, the whole medium is replaced by fresh medium, following a low‐speed centrifugation step. However, adherent cells regularly must be detached from vessel of carrier surfaces with the help of enzymes and/or mechanical intervention before passaging, and detachment methods may affect the cells and their membrane. For example, human embryonic stem cells experienced genetic instability based on the sub‐culturing method (Bai et al., 2015; Garitaonandia et al., 2015). The passage time is directly related to the population doubling time of a given cell culture. If primary cells are used, senescence is dictated by the total achievable population doubling number (Hayflick limit) (Shay & Wright, 2000) and depends on cell type, donors and culture conditions (Meza‐Zepeda et al., 2008). EVs from different passage numbers of the same cells may differ in size, concentration and functions (Beer et al., 2015; Boulestreau et al., 2020; Dorronsoro et al., 2021; Fafián‐Labora et al., 2017; Lehmann et al., 2008; Lei et al., 2017; Patel et al., 2017, 2018; Sarkar et al., 2018; Takahashi et al., 2017; Takasugi et al., 2017; Venugopal et al., 2017).
2.1.5.1. Recommendations
Report passage number, starting from the original stock and consider Master and Working Cell Bank passage numbers. It is recommended to consider the maximum number of passages that primary cells or cancer cells can undergo without changing their genotype and/or phenotype.
For adherent cells, indicate the method of passaging with details necessary for exact replication, including but not limited to the type of treatment (e.g., trypsin or EDTA with any commercial name, mechanical, or any other methods), the concentration of any reagents, time of treatment (in minutes), temperature of incubation and if/how any enzymatic treatments are stopped.
Report recovery time, if any, between sub‐culturing and the start of EV collection.
Consider avoiding the application of undefined animal‐derived products during sub‐culturing processes, the use of recombinant enzymes is preferable.
2.1.5.2. Outstanding questions
To what extent does the effect of passage number and senescence differ between culture conditions, including static versus bioreactor, and adherent versus free‐floating?
2.1.6. Cell viability
In contrast to healthy cells, dying cells may preferentially release EV subtypes such as apoptotic bodies and necroptotic bodies, although these may also have therapeutic activity (Atkin‐Smith et al., 2015; Battistelli & Falcieri, 2020; Baxter et al., 2019; Brock et al., 2019; Caruso & Poon, 2018; Crescitelli et al., 2013; Dieudé et al., 2015; Galluzzi et al., 2018; Gregory & Dransfield, 2018; Kakarla et al., 2020; Lázaro‐Ibáñez et al., 2014; Li et al., 2020; Liu et al., 2020; Park et al., 2018; Phan et al., 2020; Poon et al., 2019; Shlomovitz et al., 2021; Théry et al., 2001; Zheng et al., 2021). Since the proportion of live and dying/dead cells affects the proportion of EV subtypes in CCM, it is important to assess the viability of the cells as well as the contribution of dying or dead cells at the time of CCM collection. Cell death is commonly estimated using membrane permeability‐based stains or metabolic assays. In bioreactors, metabolic readouts such as glucose and lactate provide insight throughout the cell culture (Mendt et al., 2018). However, only living cells contribute to metabolism, and these methods do not indicate the percentage of apoptotic or dying cells. An early ISEV position paper recommended that cell death percentage in culture should be less than 5% (Witwer et al., 2013); however, achieving 95% viability is not always possible—for example, in drug response studies—and assessing viability is also not always immediately possible, such as with some types of bioreactors and in multiple‐ or continuous‐harvest 2D systems.
2.1.6.1. Recommendations
Wherever possible, report the percentage of viable cells at the time of CCM collection.
Wherever possible, document cellular morphology by taking representative images when harvesting.
2.1.6.2. Outstanding questions
Can we understand the contribution of dying cell‐derived EVs to the overall biological and potential therapeutic activity in a given EV preparation?
Can we develop monitoring systems, for example, glucose consumption or lactate production, for 2D and 3D cell expansion systems?
3. EV PRODUCTION MEDIUM
Basal cell culture medium contains nutrients, especially glucose (as the main source of energy), and amino acids, and can be supplemented with serum or other components. The EV production medium (the medium used for EV isolation) may be different from the basal cell culture medium.
3.1. Basal medium, glucose and amino acids
Commonly used basal media for mammalian cell cultures, such as Roswell Park Memorial Institute (RPMI), Dulbecco's Modified Eagle Medium (DMEM), and alpha‐modified Minimal Essential Medium (MEM), contain inorganic salts (sodium, ferric, magnesium and potassium salts), and micronutrients (vitamins and minerals). Any of these components may affect cell growth and differentiation, and EVs (Arigony et al., 2013; Arodin Selenius et al., 2019; Bhat et al., 2021; Kawakami et al., 2016; Watchrarat et al., 2017; Wu et al., 2009; Zhu et al., 2021).
D‐glucose is the major carbon source in the cell culture growth medium. Increased release of EVs has been observed in the presence of both elevated and reduced glucose levels, and this effect seems cell type‐dependent (Burger et al., 2017; Garcia et al., 2015; Rice et al., 2015; Thom et al., 2017). Several reports have shown a change in the biochemical composition or biological function of EVs when producing cells cultured in a medium containing high concentrations of glucose (Davidson et al., 2018; De Jong et al., 2012; Huang et al., 2020; Lin et al., 2019; Thom et al., 2017; Wu et al., 2017; Zhu et al., 2019; Zhou et al., 2021).
Amino acids and proteins are required for cell growth. The effects of glutamine and leucine on cell proliferation and EV biogenesis have been documented (Dai et al., 2015; Fan et al., 2020; Kim et al., 2017; Rubin, 2019; Zhao et al., 2021). If stabilized versions of amino acids are used as supplements, a different concentration of this amino acid in the medium over time will be maintained than when using the native version. Moreover, free amino acids present in the CM may become incorporated into EVs.
3.1.1. Recommendations
Report the basal medium used, including the catalog number.
Report the nature and concentration of additives added during cell culture.
3.2. Antibiotics and antimycotics
Antibiotics affect cell characteristics (marker expression) and function, and may also change the biochemical composition and function of EVs (Cohen et al., 2006; Llobet et al., 2015; Ryu et al., 2017; Skubis et al., 2017). Similarly, antimycotics may also change the production and composition of EVs (Németh et al., 2017).
3.2.1. Recommendations
Report the use of antibiotics and antimycotics, including if they are used during pre‐conditioning and/or conditioning steps.
3.2.2. Outstanding questions
What are the effects of antibiotics and antimycotics on the biochemical composition and function of EVs?
If packaged into or associated with EVs, how do antibiotics and antimycotics affect the therapeutic function of EVs?
3.3. Complex biological supplements
The most commonly used complex biological supplements are serum and platelet lysate (PL). Serum, often fetal bovine serum (FBS), also known as fetal calf serum (FCS), provides essential components such as growth factors for cell proliferation and differentiation. However, FBS also contains (bovine‐derived) EVs and non‐EV particles such as lipoproteins (Brunner, 2010; Fisher et al., 1958; Gstraunthaler, 2003; Palviainen et al., 2020; Subbiahanadar Chelladurai et al., 2021).
Platelet lysate (PL) has effects comparable or superior to serum in some cell cultures (Hemeda et al., 2014), and, if harvested from donors of the same species as the cultured cells, PL may be used as a xenogeneic‐free serum substitute (Becherucci et al., 2018; Bianchetti et al., 2021; Capelli et al., 2007; De Almeida Fuzeta et al., 2020; Gottipamula et al., 2012; Griffiths et al., 2013; Hemeda et al., 2014; Johansson et al., 2003; Juhl et al., 2016; Menard et al., 2013; Oeller et al., 2021). Like serum, also PL is rich in growth factors and may contain pathogens like viruses, and may exhibit donor‐to‐donor variability (Burnouf, 2018; Guiotto et al., 2020; Hawkes, 2015; Henschler et al., 2019; Hemeda et al., 2014; Schallmoser et al., 2020). Pathogen inactivation or depletion by irradiation, with or without the addition of photoactive chemicals or filtration (Oeller et al., 2021; Organization, 2004; Schallmoser et al., 2020; Viau et al., 2019) is required for regulatory compliance. PL also contains components secreted from platelets such as EVs, coagulation proteins and growth factors, which may contribute to its proliferation‐supporting activity (Torreggiani et al., 2014) (reviewed in Antich‐Rosselló et al., 2021; Kerris et al., 2020; Melki et al., 2017).
A position statement from the working group on cellular therapies of the International Society of Blood Transfusion (ISBT) discussed human PL (hPL) production, manufacturing, and quality management (Schallmoser et al., 2020), and the barriers to the translational use of hPL have been discussed in a joint publication of the Association for the Advancement of Blood and Biotherapies (AABB) and ISCT (Bieback et al., 2019). The use of hPL reduces the problem of animal components, although the use of PL for the manufacturing of therapeutic EVs requires specific precautions and pre‐processing steps.
The presence of coagulation factors and fibrinogen in PL can lead to fibrin precipitates during the cell expansion process, which may be incompatible with processes such as filtration. Addition of heparin to the growth medium inhibits fibrin formation. Alternatively, addition of calcium chloride to PL may trigger coagulation and fibrin formation (Staubach et al., 2021), and the fibrin clot that is formed can be removed by centrifugation prior to use.
3.3.1. Recommendations
Report the percentage, producing company (city, country), and catalog number of complex biological additives including sera.
Report the source and percentage of PL used at the various steps of cell expansion and CCM production.
Report the process of PL production, including fibrin depletion methods and pathogen inactivation.
Report the concentration of heparin present in the growth medium, and consider the potential adverse effects of heparin on separation and downstream analysis of EVs (Atai et al., 2013; Beutler et al., 1990).
3.4. EV depletion
Serum and PL contain EVs, DNA fragments, non‐EV particles such as protein aggregates and lipoproteins, and micronutrients (Arigony et al., 2013; Lehrich et al., 2021; Urzì et al., 2022), which may complicate the isolation, assessment, and interpretation of the biochemical composition and function of CCM EVs. For example, FBS‐derived EVs or particles may co‐isolate with EVs produced by cultured cells (Lehrich et al., 2021). Various methods have been described to deplete EVs from the serum (Driedonks et al., 2019; Kim et al., 2021; Kornilov et al., 2018; Lehrich et al., 2018; Liao et al., 2019, 2017; Mannerstrom et al., 2019). However, these methods do not remove all or exclusively EVs (Kornilov et al., 2018; Lehrich et al., 2018; Shelke et al., 2014; Tosar et al., 2017), and most studies do not report the degree of depletion. The biochemical composition and function of EVs released by cells cultured in the presence of EV‐depleted serum may not be identical to those released in the presence of EV‐containing serum. This is possibly due to the fact that serum‐derived EVs themselves have multiple effects on cultured cells (Beninson & Fleshner, 2015; Cavallari et al., 2017; Gu et al., 2018; Ochieng et al., 2009; Urzì et al., 2022).
3.4.1. Recommendations
When serum is diluted prior to removal of EVs, indicate whether, how (fold dilution), and with what (buffer, medium) the serum was diluted.
For ultracentrifugation‐based depletion of EVs, report the centrifugation speed and time, rotor specifications (K value, angle, tube volume), and temperature.
For tangential flow filtration‐based depletion of EVs, report the details including membrane/device manufacturer, material type, pore size, filtration surface area, flow rate, and temperature.
Monitor and report the level of EV depletion by comparing pre‐and post‐depletion material
3.5. Serum‐free culturing
Considering the complex nature and important functions of serum in cell culture media, any manipulation including replacing the medium with a serum‐free medium, or heat treatment to inactivate the complement system, may alter the physiology of the cultured cells and derivative EVs (Alcolea et al., 2016; Angelini et al., 2016; Aswad et al., 2016; Bhat et al., 2021; Cavallari et al., 2017; Clabaut et al., 2015; Eitan et al., 2015; Frigerio et al., 2021; Lehrich et al., 2021, 2018; Li et al., 2015; Liao et al., 2017; Mehta et al., 2010; Obrochta et al., 2015; Pham et al., 2021; Urzì et al., 2023). Some reports showed that serum‐free or chemically defined medium enhance EV production (Biadglegne et al., 2021; Bost et al., 2022; Faruqu et al., 2021; Figuero, 2021).
3.5.1. Recommendations
In the case of serum‐free media, report if no replacement supplement was added (e.g., in ‘starvation’ experiments), or if replacement supplements were added that are nutritionally complete.
Report the time duration of cell culture without serum or other complex additives.
Document any observed change in cellular morphology or characteristics.
Quantify the number of particles in non‐conditioned medium using the same process as for conditioned medium at least once.
3.5.2. Outstanding questions
EV depletion from serum may result in the removal of non‐EV components such as lipids and proteins. Does EV‐depleted growth medium support cell proliferation to the same extent as a non‐EV depleted serum‐containing medium?
The efficiency of EV depletion methods varies. Monitoring the efficiency of EV depletion is difficult because none of the currently available EV detection methods can detect all EVs. Performing a procedural control, that is, comparing the negative control of the same volume of complete, unconditioned medium processed the same way as CCM, is a functional alternative, but will not fully answer the question of depletion efficiency. How can we best report the efficiency of EV depletion?
3.6. Cell (pre)conditioning
Other factors used to promote proliferation, differentiation, silencing, or priming of cells, and/or to otherwise manipulate cells and their functions, may influence the release of EVs (Bai et al., 2020; Emam et al., 2018; Martens‐Uzunova et al., 2021; Pecan et al., 2020; Savina et al., 2003; Segura et al., 2005; Soekmadji et al., 2017; Taylor et al., 2017; Wang et al., 2020) or their therapeutic potential (Akbar et al., 2017; Cossetti et al., 2014; De Jong et al., 2012; Hosseinkhani et al., 2017, 2020; Lopatina et al., 2014; Patel et al., 2019; Peltzer et al., 2020; Sutter et al., 2021; Varkouhi et al., 2019; Yang et al., 2020; Zhang et al., 2018, 2020).
3.6.1. Recommendations
Report the concentration, company (city, country), and catalog number of small molecules, cytokines/chemokines, or other RNA/protein/lipid‐based cell‐modulatory reagents. Also indicate if they are used during pre‐conditioning and/or medium‐conditioning periods.
3.6.2. Outstanding questions
Do cell‐modifying factors associate with EVs or non‐EV particles, and if so, what are their possible effects?
4. CULTURE CONDITIONS
Changes in biophysical and biochemical cell culture conditions may affect cell growth and the release and biochemical composition of EVs. External culturing conditions include the concentration of gasses, 2D or 3D culturing, pH, temperature, physical stimulus, the composition of culture vessels/substrates, and in‐experiment manipulations of the cell culture medium.
4.1. Gas conditioning
The effect of oxygen concentration on the biochemical composition, function, and release of EVs is well studied (Agarwal et al., 2017; Alharbi et al., 2021; Almeria et al., 2019; Davidson et al., 2018; De Jong et al., 2012; Dougherty et al., 2020; Dutta et al., 2020; King et al., 2012; Kucharzewska et al., 2013; Kumar & Deep, 2020; Salomon, Kobayashi et al., 2013; Salomon, Ryan et al., 2013; Xue et al., 2018; Zhang et al., 2017). So‐called ‘normoxia’, that is, 21% O2 or ‘atmospheric’ oxygen, is in fact hyperoxia for most mammalian cell types. Even in lung alveoli, the oxygen level is 14.5%, and at the venous end of circulation, oxygenation is around 6.5% (Mckeown, 2014). Depending on the exact tissue type and anatomy, physiological hypoxia may be in the range of 2%−6% oxygen, and pathological hypoxia may be even lower, ranging from 0.3% to 4.2% oxygen (Mckeown, 2014). Cells cultured under atmospheric conditions have thus been exposed or even adapted to higher‐than‐physiologic oxygen concentrations. To reach true normoxia, specialized culture chambers are required.
4.1.1. Recommendation
Report gas concentrations used during cell culture, especially of oxygen, but where applicable also of CO2, and nitrogen, as well as any interruptions in the gas conditions during the culture process.
4.2. 2D or 3D cultures: vessels, coatings, matrices
Some reports have shown that cells cultured in 3D systems typically produce more EVs compared with conventional 2D cultures or adherent cultures (Cao et al., 2020; De Almeida Fuzeta et al., 2020; Grangier et al., 2021; Guo et al., 2021; Haraszti et al., 2018; Kim et al., 2021; Patel et al., 2018; Phan et al., 2019, 2018; Rocha et al., 2019; Watson et al., 2016; Yan & Wu, 2019; Yan et al., 2018; Yang et al., 2020; Zhang et al., 2021). On the other hand, 3D system of cultures can lead to different biochemical compositions and functions of EVs compared with those obtained with 2D systems (Cao et al., 2020; Gao et al., 2020; Haraszti et al., 2018; Jarmalavičiūtė et al., 2015; Kim et al., 2021; Miceli et al., 2019; Rocha et al., 2019; Thippabhotla et al., 2019; Yan & Wu, 2019, 2020; Yang et al., 2019). The materials from which cell culture vessels (e.g., flasks or plates) are, as well as any support matrices, such as bead substrates and vessel coatings, may affect EV characteristics and should be reported accordingly (Jangamreddy et al., 2018; Park et al., 2020; Sutter et al., 2021; Szvicsek et al., 2019; Wu et al., 2021).
4.2.1. Recommendations
Report all culturing conditions, including, but not limited to, surface area, volume, and preconditioning with buffers.
Report the composition of any support matrix, including, for example, substrate beads and vessel coatings.
Indicate the procedure used to prepare and apply any support matrix.
4.3. pH
The pH of the medium affects release, uptake and biochemical composition of EVs (Ban et al., 2015; Boussadia et al., 2018; Cheng et al., 2019; Logozzi et al., 2018; Parolini et al., 2009; Perut et al., 2019; Nakase et al., 2021). Medium pH affects the stability of released EVs (Cheng et al., 2019). Although we are not aware of reports on the effects of pH on generation of therapeutic EVs, changes in pH influence stem cell reprogramming or differentiation (Fliefel et al., 2016; Kim, 2021; Massa et al., 2017).
4.3.1. Recommendations
Monitor and report the pH of medium.
Report if cells were grown using a pH buffering agent, and, if so, its concentration.
4.3.2. Outstanding questions
How does pH affect the production and composition of EVs?
4.4. Physical stimulation
Applying physical stimuli such as electrical stimulation, acoustic irradiation, and mechanical forces (including shear and stretching of the scaffold) may increase the production and/or change the biochemical composition of EVs (Ambattu et al., 2020; Eichholz et al., 2020; Fukuta et al., 2020; Guo et al., 2021; Hergenreider et al., 2012; Letsiou et al., 2015; Maeshige et al., 2021; Morrell et al., 2018; Ridger et al., 2017; Vion et al., 2013; Xia et al., 2020; Yang et al., 2017; Zeng et al., 2019; Zhao et al., 2020). Moreover, for some cell types, temperature may affect EV release and uptake dynamics and may also change EV function (Mahmood et al., 2023; Otsuka et al., 2020).
4.4.1. Recommendation
Report all applied physical stimuli in detail, including, but not limited to, intensity, voltage, time and temperature.
4.5. Replacement of medium
Medium replacement during cell culturing keeps the cells healthy by providing fresh nutrients and eliminating waste products. However, during medium replacement, the secretome of cells including EVs is removed, and cells start to secrete new EVs (Patel et al., 2017; Vis et al., 2020).
4.5.1. Recommendations
Report the medium replacement protocol, including what percentage of the volume is replaced, at what interval (in hours) the medium is changed or replenished, intermediate washing steps (if any), changes to the medium composition, and continuous‐flow feeding (with recirculation or not).
In some cases, different media formulations are used at various stages of an experiment. If so, indicate when and how, as well as the details of each formulation and why each specific formulation was used.
4.5.2. Outstanding questions
What are the effects of maintaining cells in the same medium for extended durations and of medium replacement on EV release, biochemical composition, and function?
5. COLLECTION (HARVEST) OF CCM
Duration and frequency of CCM collection may affect the yield and surface protein expression of EVs (Mendt et al., 2018; Patel et al., 2017). There are three main approaches to CCM harvest: single harvest, multiple harvests, and continuous harvest. The duration of conditioning differs between studies and assays from hours to days. During conditioning, cells continuously release and uptake EVs; therefore, the time interval during which cells are cultured affects the biochemical composition, function, and release of EVs due to changes in the release and possible re‐uptake of EVs, along with cell growth and differentiation (; Davidson et al., 2018; Flores‐Bellver et al., 2021; Kim et al., 2016; Li et al., 2015).
5.1. Recommendations
For a single harvest: report the total time (duration) of conditioning.
For multiple harvests: report the number of harvests, duration of conditioning for each, and whether all or some fraction of medium is collected (i.e., complete or partial medium replacement), specifying the fraction where applicable.
Report how many (if any) of the multiple harvests are pooled.
Report on any pre‐pooling analyses and inclusion criteria.
For continuous harvest, report the duration of continuous collection and harvest rate (i.e., volume per unit time).
Report the duration of conditioning and keep it constant across experimental repeats.
5.2. Outstanding questions
How do continuous harvest and multiple harvests affect EV release, biochemical composition and function?
Since cells release EVs into and simultaneously uptake EVs from CCM, cultures may reach an equilibrium between EV release and uptake, and this may occur at different times for different cultures and conditions. What are the effects of conditioning time on EV release, biochemical composition and function?
6. MICROBIAL CONTAMINANTS OF CCM
CCM may be contaminated with viruses, bacteria, fungi, and other unwanted bioactive components such as endotoxins. Active bacterial and fungal contaminations are often apparent in culture and in stored EV samples by visual examination or aided by light microscopy, in which case such materials can be discarded. However, many contaminations are not easily detected. The presence of Mycoplasma, for example, may not be readily apparent, and Mycoplasma species can release EVs into CCM (Gaurivaud et al., 2018). Mycoplasma‐infected cells release EVs that differ in function from EVs released by non‐infected cells (Cronemberger‐Andrade et al., 2020; Quah & O'neill, 2007; Yang et al., 2012). Bacterial or fungal‐derived EVs and other bioactive components such as endotoxins may also be introduced into CCM from raw materials or culture vessels even if actively replicating organisms are not present. Viruses and viral components may also be present in cells and/or culture components and may cause unintended effects (Barone et al., 2020; Merten, 2002). Contaminants such as Mycoplasma and viruses may not be eliminated by 0.22‐micron filtration of culture medium, conventionally used to eliminate active bacterial contamination.
6.1. Recommendations
Report if any screening of Mycoplasma or other contaminants in parental cell lines/cultures was done and the results thereof. Report details of any antimicrobial treatment.
Especially if EVs are administered to animals or humans, measure and report the level of endotoxins in prepared EVs according to international pharmacopoeia (USP<85>, EP 2.6.14, JP 4.01) (Commission, E.P., E.D.f.t.Q.o 2010; Revision, U.S.P.C.C.o 2008; The Japanese pharmacopoeia 1996), and provide evidence of sterility of prepared EVs according to international pharmacopoeia (USP<71>, EP 2.6.1, JP 4.06) (Commission, E.P., E.D.f.t.Q.o 2010; Revision, U.S.P.C.C.o 2008; The Japanese pharmacopoeia 1996).
7. DISCUSSION
In this perspective paper, we aim to raise awareness of the effects of cell culturing parameters (Figure 2a) that may affect EV release (Figure 2b), biochemical composition, and/or function (Figure 2c). Cells can ingest components that are present in the media and re‐package them into or onto EVs (Figure 2d). Moreover, cell culturing parameters can influence cellular processes other than EV biogenesis that indirectly affect EVs (Figure 2e). We have compiled a checklist of these parameters in Table 1. The aim of the checklist is to collect as much information as possible to improve reproducibility. The information can be summarized in the Methods section, and the checklist can be added as supplementary information to the manuscript. We are aware that some recommendations may be harder to implement than others. For instance, measuring pH of a culture medium at different stages of the culture is not a routine process; thus, it may not become readily or routinely implemented. At present, multiple task forces and working groups have developed or are developing checklists, and the goal of ISEV is to develop on online repository, either as a stand‐alone or combined and integrated with an already existing platform such as EV‐TRACK. Such an online repository will be useful to re‐evaluate collected data and can be used to update future recommendations accordingly.
FIGURE 2.
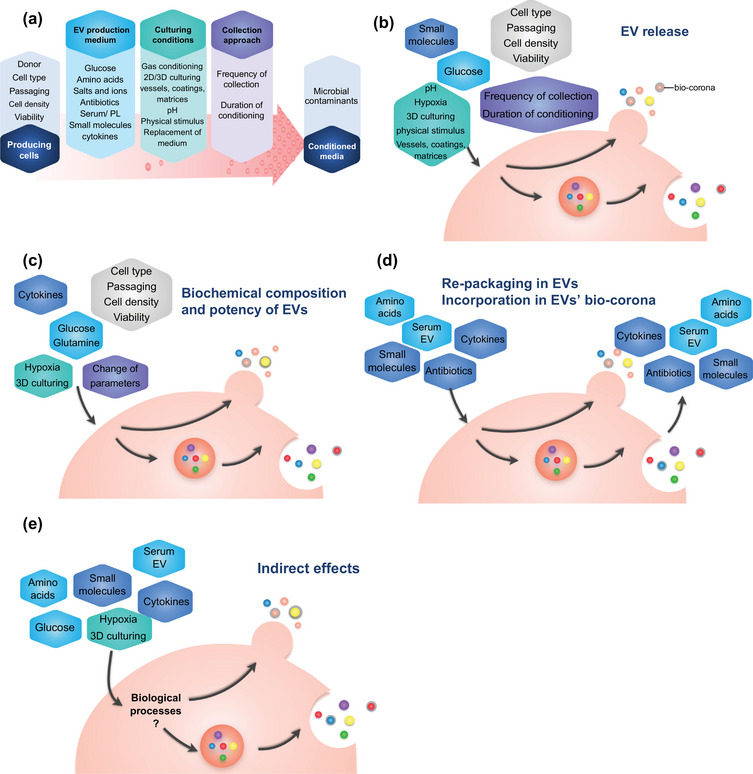
Cell culture parameters and their effects on production of extracellular vesicles. (a) All parameters affect cells during conditioning and release of extracellular vesicles (EVs). (b) Parameters influencing release of EVs, and (c) biochemical composition of EVs. (d) Cells might also re‐package cell culture medium supplement components into/onto released EVs. (e) Cell culturing parameters may affect processes that indirectly affect EVs.
TABLE 1.
Cell culturing parameter checklist.
| Cell culturing parameters | Items | ||
|---|---|---|---|
| 1. Producing cells (source cells) | 1.1 Cell identity |
Name of cell Origin (e.g. donor or cell bank) Cell type identification |
|
| 1.2 Cell donor characteristics |
Biological sex Age Health status Pre‐culture processing of the cells |
||
| 1.3 Initial seeding density | Initial seeding number per surface area or volume | …………………. | |
| 1.4 Cell density or confluency of cells at the time of CCM collection |
Percentage of surface area covered (2D cultures) Estimated number of cells per volume (suspension cultures) The diameter of the spheroids (3D cultures) |
……… % …………………. …………………. |
|
| 1.5 Cell sub‐culturing, population doubling, and passage number |
Passage number Passaging method Recovery time |
…………………. …………………. …………………. |
|
| 1.6 Cell viability |
Percentage of viable cells Method of viability assessment |
……… % …………………. |
|
| 2. EV production medium | 2.1 Basal medium, glucose, and amino acids |
Name of basal culturing medium Company, and catalog number Additions Salts (name, concentration) Glucose (D/L glucose, concentration) Amino acids (name, concentration) |
…………………. …………………. …………….. , …… mM …………….. , …… mM …………….. , …… mM |
| 2.2 Antibiotic |
Name Concentration During conditioning or pre‐conditioning or both |
……… ……… ……… ……… mM ……… mM ……… mM …………………. |
|
| 2.3 Complex biological supplements |
If the EV production medium contains serum/PL Percentage Producing company, and catalog number of sera Process of PL production, including fibrin depletion methods and pathogen inactivation Concentration of heparin present in the growth medium If the EV production medium contains EV‐depleted serum/PL Indicate whether, how (fold dilution), and with what (buffer, medium) the serum was diluted before EV depletion Method of EV depletion Checking the efficiency of EV depletion Result of checking the efficiency of EV depletion If there are washing steps (before changing to EV depleted medium), write down the details Source, and catalog number of commercial EV‐depleted serum If serum/PL free media is used indicate time duration If using a replacement supplement Percentage Producing company, and catalog number of replacement supplement |
…………………. …………………. …………………. …………………. …………………. …………………. …………………. …………………. …………………. …………………. …………………. …………………. …………………. |
|
| 2.4 Cell (pre) conditioning |
Name of factor Company, and catalog number Concentration Conditioning or pre‐conditioning |
…………………. ……… ……… ……… ……… mM ……… mM ……… mM …………………. |
|
| 3. Culturing conditions | 3.1 Gas conditioning |
Name (e.g oxygen, nitrogen, etc) Percentage |
……… ……… % |
| 3.2 2D or 3D cultures: vessels, coatings, matrices |
Type (2D, 3D, or spheroid) Vessels (e.g. flasks or plates) Coating materials 3D support matrix (type, details) Preconditioning with buffers Volume of medium (at the time of CCM harvest for EV preparation) Details of spheroid formation |
…………………. …………………. …………………. …………………. …………………. …………………. …………………. |
|
| 3.3 pH |
pH of medium If a pH buffering agent is used indicate its concentration |
…………………. …………………. |
|
| 3.4 Physical stimulus |
Type (such as acoustic irradiation, and mechanical forces including shear and stretching of the scaffold) Details Culture temperature |
…………………. …………………. …… °C |
|
| 3.5 Replacement of medium |
What percentage of the volume is replaced At what interval (in hours) the medium is changed or replenished Intermediate washing steps (if any) Continuous‐flow feeding (with recirculation or not) Are different media formulations used at various stages of an experiment? |
…………………. …………………. …………………. …………………. …………………. |
|
| 4. Collection (harvest) of CCM | Duration and frequency of CCM collection |
Single harvest: Duration of conditioning (in hours) Multiple harvests: Number of harvests Duration of conditioning (in hours) Whether all or some fraction of medium is collected (i.e., complete or partial medium replacement), specifying the fraction where applicable. How many of the multiple harvests are pooled (if any) Continuous harvest: Duration of collection (in hours) Harvest rate (i.e. volume per unit time) |
…………………. …………………. …………………. …………………. ………………. ………………… .………………… |
| 5. Microbial contaminants of CCM | Viruses, bacteria, fungi, and any unwanted microbial components |
Presence of viruses Measurement method When is checked? Outcome Presence of bacteria (e.g. mycoplasma) Measurement method When is checked? Outcome Presence of fungi Measurement method When is checked? Outcome Presence of other unwanted microbial components (e.g. endotoxin) Measurement method When is checked? Outcome |
yes no …………………. in producing cells in CCM in both …………………. yes no………………….in producing cells in CCM in both …………………. yes no …………………. in producing cells in CCM in both …………………. yes no…………………. in producing cells in CCM in both …………………. |
Abbreviations: CCM, cell culture‐conditioned medium; EV, extracellular vesicle; PL, platelet lysate.
There is hardly any discussion on the mechanisms by which changes in cell culture impact EVs, but these should no longer be overlooked. Cellular processes such as autophagy can affect EV production both directly (Xing et al., 2021; Xu et al., 2018) and indirectly (Moruno et al., 2012; Wang et al., 2021), influencing the number and type of released EVs. Comparing autophagy levels in cultures used for preparing EV batches may explain inter‐experiment / batch differences in EVs. Since hypotonic dialysis can be used to load drugs into EVs (Mehryab et al., 2020), allowing therapeutic agents to cross the membrane (Xie et al., 2021), the osmolarity of the culture medium may affect EV cargo. Given the effects of amino acids on the mammalian target of rapamycin (mTOR) signaling (Jewell et al., 2013) and the connection between mTOR and EV release (Zou et al., 2019), it is reasonable to assume that amino acid concentrations will impact EV production. Finally, oxygen concentration modulates cellular senescence, which may impact EVs, as mentioned in the cellular sub‐culturing section (Seno et al., 2018; Welford & Giaccia, 2011; You et al., 2019).
Of all cell culture supplements, serum and PL are among the most ubiquitous and challenging. They are rich in EVs, their EVs support cell growth and function in ways that cannot easily be achieved with a ‘defined’ medium, and EV depletion from these supplements is time‐consuming and/or inefficient (Lehrich et al., 2021). In addition, they may indirectly influence cells and provide cells with materials that can be re‐packaged into newly produced EVs or incorporated as a bio‐corona (Figure 2d). Hence, more investigations are needed to critically evaluate the consequences of serum/PL removal or EV depletion on each cell type.
In summary, the effects of cell culture parameters on EVs are complex, and our current understanding of these influences is far from complete. A distinction should be made between culturing cells for basic EV research versus therapeutic applications since the latter involved additional considerations around safety and regulation. Missing and vague information about manufacturing and processing parameters can lead to misinterpretations and false conclusions and hampers study reproduction. We therefore emphasize the importance of rigorous reporting of all cell culture parameters to enable researchers to better compare experimental in vitro and in vivo data and pre‐clinical and clinical outcomes. Only with full reporting can we achieve our common goal of comprehensively understanding the biological functions of EVs. We hope that these suggestions (Table 1) will be a useful starting point for further discussions and that they will promote good reporting practices in CCM‐EV research.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
The achievement of the "CCM‐EV" task force was made attainable through the involvement of all the authors. The task force received support from the International Society for Extracellular Vesicles (ISEV), and the authors extend their gratitude to ISEV.
Shekari, F. , Alibhai, F. J. , Baharvand, H. , Börger, V. , Bruno, S. , Davies, O. , Giebel, B. , Salekdeh, G. H. , Martin‐Jaular, L. , Mathivanan, S. , Nelissen, I. , Nolte‐’t Hoen, E. , O'Driscoll, L. , Perut, F. , Pluchino, S. , Pocsfalvi, G. , Salomon, C. , Soekmadji, C. , Staubach, S. , … Nieuwland, R. (2023). Cell culture‐derived extracellular vesicles: Considerations for reporting cell culturing parameters. Journal of Extracellular Biology, 2, e115. 10.1002/jex2.115
REFERENCES
- Agarwal, U. , George, A. , Bhutani, S. , Ghosh‐Choudhary, S. , Maxwell, J. T. , Brown, M. E. , Mehta, Y. , Platt, M. O. , Liang, Y. , Sahoo, S. , & Davis, M. E. (2017). Experimental, systems, and computational approaches to understanding the microRNA‐mediated reparative potential of cardiac progenitor cell‐derived exosomes from pediatric patients. Circulation Research, 120(4), 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antich‐Rosselló, M. , Forteza‐Genestra, M. A. , Monjo, M. , & Ramis, J. M. (2021). Platelet‐derived extracellular vesicles for regenerative medicine. International Journal of Molecular Sciences, 22(16), 8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arigony, A. L. , de Oliveira, I. M. , Machado, M. , Bordin, D. L. , Bergter, L. , Prá, D. , & Henriques, J. A. (2013). The influence of micronutrients in cell culture: A reflection on viability and genomic stability. BioMed Research International, 2013, 597282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar, N. , Digby, J. E. , Cahill, T. J. , Tavare, A. N. , Corbin, A. L. , Saluja, S. , Dawkins, S. , Edgar, L. , Rawlings, N. , Ziberna, K. , Mcneill, E. , Johnson, E. , Aljabali, A. A. , Dragovic, R. A. , Rohling, M. , Belgard, T. G. , Udalova, I. A. , Greaves, D. R. , Channon, K. M. , … Choudhury, R. P. (2017). Endothelium‐derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction. JCI Insight, 2(17), e93344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcolea, J. , Alonso, A. , Moreno‐Izquierdo, M. A. , Degayón, M. A. , Moreno, I. , & Larraga, V. (2016). Serum removal from culture induces growth arrest, ploidy alteration, decrease in infectivity and differential expression of crucial genes in leishmania infantum promastigotes. PLoS ONE, 11(3), e0150172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi, M. , Lai, A. , Sharma, S. , Kalita‐De Croft, P. , Godbole, N. , Campos, A. , Guanzon, D. , Salas‐Burgos, A. , Carrion, F. , Zuñiga, F. A. , Perrin, L. , He, Y. , Pejovic, T. , Winters, C. , Morgan, T. , Hooper, J. D. , Rice, G. E. , & Salomon, C. (2021). Extracellular vesicle transmission of chemoresistance to ovarian cancer cells is associated with hypoxia‐induced expression of glycolytic pathway proteins, and prediction of epithelial ovarian cancer disease recurrence. Cancers (Basel), 13(14), 3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeria, C. , Weiss, R. , Roy, M. , Tripisciano, C. , Kasper, C. , Weber, V. , & Egger, D. (2019). Hypoxia conditioned mesenchymal stem cell‐derived extracellular vesicles induce increased vascular tube formation in vitro. Frontiers in Bioengineering and Biotechnology, 7, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeria, C. , Kreß, S. , Weber, V. , Egger, D. , & Kasper, C. (2022). Heterogeneity of mesenchymal stem cell‐derived extracellular vesicles is highly impacted by the tissue/cell source and culture conditions. Cell & Bioscience, 12(1), 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambattu, L. A. , Ramesan, S. , Dekiwadia, C. , Hanssen, E. , Li, H. , & Yeo, L. Y. (2020). High frequency acoustic cell stimulation promotes exosome generation regulated by a calcium‐dependent mechanism. Communications Biology, 3(1), 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Type Culture Collection Standards Development Organization Workgroup ASN‐0002 . (2010). Cell line misidentification: The beginning of the end. Nature Reviews Cancer, 10(6), 441–448. [DOI] [PubMed] [Google Scholar]
- Angelini, F. , Ionta, V. , Rossi, F. , Miraldi, F. , Messina, E. , & Giacomello, A. (2016). Foetal bovine serum‐derived exosomes affect yield and phenotype of human cardiac progenitor cell culture. Bioimpacts, 6(1), 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arodin Selenius, L. , Wallenberg Lundgren, M. , Jawad, R. , Danielsson, O. , & Björnstedt, M. (2019). The cell culture medium affects growth, phenotype expression and the response to selenium cytotoxicity in A549 and HepG2 cells. Antioxidants (Basel), 8(5), 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswad, H. , Jalabert, A. , & Rome, S. (2016). Depleting extracellular vesicles from fetal bovine serum alters proliferation and differentiation of skeletal muscle cells in vitro. BMC Biotechnology [Electronic Resource], 16, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin‐Smith, G. K. , Tixeira, R. , Paone, S. , Mathivanan, S. , Collins, C. , Liem, M. , Goodall, K. J. , Ravichandran, K. S. , Hulett, M. D. , & Poon, I. K. H. (2015). A novel mechanism of generating extracellular vesicles during apoptosis via a beads‐on‐a‐string membrane structure. Nature Communications, 6, 7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atai, N. A. , Balaj, L. , Van Veen, H. , Breakefield, X. O. , Jarzyna, A. , Van Noorden, C. J. F. , Skog, J. , & Maguire, C. A. (2013). Heparin blocks transfer of extracellular vesicles between donor and recipient cells. Journal of Neuro‐Oncology, 115(3), 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Q. , Ramirez, J.‐M. , Becker, F. , Pantesco, V. , Lavabre‐Bertrand, T. , Hovatta, O. , Lemaître, J.‐M. , Pellestor, F. , & De Vos, J. (2015). Temporal analysis of genome alterations induced by single‐cell passaging in human embryonic stem cells. Stem Cells and Development, 24(5), 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, G. , Matsuba, T. , Niki, T. , & Hattori, T. (2020). Stimulation of THP‐1 macrophages with lps increased the production of osteopontin‐encapsulating exosome. International Journal of Molecular Sciences, 21(22), 8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint, R. , Richardson, S. M. , & Cartmell, S. H. (2015). Low‐density subculture: A technical note on the importance of avoiding cell‐to‐cell contact during mesenchymal stromal cell expansion. Journal of Tissue Engineering and Regenarative Medicine, 9(10), 1200–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistelli, M. , & Falcieri, E. (2020). Apoptotic bodies: Particular extracellular vesicles involved in intercellular communication. Biology, 9(1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban, J.‐J. , Lee, M. , Im, W. , & Kim, M. (2015). Low pH increases the yield of exosome isolation. Biochemical and Biophysical Research Communications, 461(1), 76–79. [DOI] [PubMed] [Google Scholar]
- Barone, W. , Wiebe, M. E. , Leung, J. C. , Hussein, I. T. M. , Keumurian, F. J. , Bouressa, J. , Brussel, A. , Chen, D. , Chong, M. , Dehghani, H. , Gerentes, L. , Gilbert, J. , Gold, D. , Kiss, R. , Kreil, T. R. , Labatut, R. , Li, Y. , Müllberg, J. , Mallet, L. , … Springs, S. L. (2020). Viral contamination in biologic manufacture and implications for emerging therapies. Nature Biotechnology, 38(5), 563–572. [DOI] [PubMed] [Google Scholar]
- Barallon, R. , Bauer, S. R. , Butler, J. , Capes‐Davis, A. , Dirks, W. G. , Elmore, E. , Furtado, M. , Kline, M. C. , Kohara, A. , Los, G. V. , MacLeod, R. A. , Masters, J. R. , Nardone, M. , Nardone, R. M. , Nims, R. W. , Price, J. , Reid, Y. A. , Shewale, J. , Sykes, G. , … Kerrigan, L. (2010). Recommendation of short tandem repeat profiling for authenticating human cell lines, stem cells, and tissues. In Vitro Cellular & Developmental Biology‐Animal, 46(9), 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter, A. A. , Phan, T. K. , Hanssen, E. , Liem, M. , Hulett, M. D. , Mathivanan, S. , & Poon, I. K. H. (2019). Analysis of extracellular vesicles generated from monocytes under conditions of lytic cell death. Scientific Reports, 9(1), 7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherucci, V. , Piccini, L. , Casamassima, S. , Bisin, S. , Gori, V. , Gentile, F. , Ceccantini, R. , De Rienzo, E. , Bindi, B. , Pavan, P. , Cunial, V. , Allegro, E. , Ermini, S. , Brugnolo, F. , Astori, G. , & Bambi, F. (2018). Human platelet lysate in mesenchymal stromal cell expansion according to a GMP grade protocol: A cell factory experience. Stem Cell Research & Therapy, 9(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer, L. , Zimmermann, M. , Mitterbauer, A. , Ellinger, A. , Gruber, F. , Narzt, M. S. , Zellner, M. , Gyöngyösi, M. , Madlener, S. , Simader, E. , Gabriel, C. , Mildner, M. , & Ankersmit, H. J. (2015). Analysis of the secretome of apoptotic peripheral blood mononuclear cells: Impact of released proteins and exosomes for tissue regeneration. Scientific Reports, 5, 16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninson, L. A. , & Fleshner, M. (2015). Exosomes in fetal bovine serum dampen primary macrophage IL‐1beta response to lipopolysaccharide (LPS) challenge. Immunology Letters, 163(2), 187–192. [DOI] [PubMed] [Google Scholar]
- Bhat, S. , Viswanathan, P. , Chandanala, S. , Prasanna, S. J. , & Seetharam, R. N. (2021). Expansion and characterization of bone marrow derived human mesenchymal stromal cells in serum‐free conditions. Scientific Reports, 11(1), 3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter‐Hahn, J. , Münnich, A. , & Woiteneck, P. (1998). Dependence of energy metabolism on the density of cells in culture. Cell Structure and Function, 23(2), 85–93. [DOI] [PubMed] [Google Scholar]
- Beutler, E. , Gelbart, T. , & Kuhl, W. (1990). Interference of heparin with the polymerase chain reaction. Biotechniques, 9(2), 166. [PubMed] [Google Scholar]
- Biadglegne, F. , Rademacher, P. , De Sulbaran, Y. G. J. , König, B. , Rodloff, A. C. , Zedler, U. , Dorhoi, A. , & Sack, U. (2021). Exosomes in serum‑free cultures of THP‑1 macrophages infected with Mycobacterium tuberculosis. Molecular Medicine Reports, 24(5), 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchetti, A. , Chinello, C. , Guindani, M. , Braga, S. , Neva, A. , Verardi, R. , Piovani, G. , Pagani, L. , Lisignoli, G. , Magni, F. , Russo, D. , & Almici, C. (2021). A blood bank standardized production of human platelet lysate for mesenchymal stromal cell expansion: proteomic characterization and biological effects. Frontiers in Cell and Developmental Biology, 9, 650490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieback, K. , Fernandez‐Muñoz, B. , Pati, S. , & Schäfer, R. (2019). Gaps in the knowledge of human platelet lysate as a cell culture supplement for cell therapy: A joint publication from the AABB and the International Society for Cell & Gene Therapy. Cytotherapy, 21(9), 911–924. [DOI] [PubMed] [Google Scholar]
- Börger, V. , Weiss, D. J. , Anderson, J. D. , Borràs, F. E. , Bussolati, B. , Carter, D. R. F. , Dominici, M. , Falcón‐Pérez, J. M. , Gimona, M. , Hill, A. F. , Hoffman, A. M. , de Kleijn, D. , Levine, B. L. , Lim, R. , Lötvall, J. , Mitsialis, S. A. , Monguió‐Tortajada, M. , Muraca, M. , Nieuwland, R. , … Giebel, B. (2020). International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: Considerations for potential therapeutic agents to suppress coronavirus disease‐19. Cytotherapy, 22(9), 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulestreau, J. , Maumus, M. , Rozier, P. , Jorgensen, C. , & Noël, D. (2020). Mesenchymal stem cell derived extracellular vesicles in aging. Frontiers in Cell and Developmental Biology, 8, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost, J. P. , Saher, O. , Hagey, D. , Mamand, D. R. , Liang, X. , Zheng, W. , Corso, G. , Gustafsson, O. , Görgens, A. , Smith, C. E. , Zain, R. , El Andaloussi, S. , & Gupta, D. (2022). Growth media conditions influence the secretion route and release levels of engineered extracellular vesicles. Advanced Healthcare Materials, 11(5), 2101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussadia, Z. , Lamberti, J. , Mattei, F. , Pizzi, E. , Puglisi, R. , Zanetti, C. , Pasquini, L. , Fratini, F. , Fantozzi, L. , Felicetti, F. , Fecchi, K. , Raggi, C. , Sanchez, M. , D'atri, S. , Carè, A. , Sargiacomo, M. , & Parolini, I. (2018). Acidic microenvironment plays a key role in human melanoma progression through a sustained exosome mediated transfer of clinically relevant metastatic molecules. Journal of Experimental & Clinical Cancer Research, 37(1), 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock, C. K. , Wallin, S. T. , Ruiz, O. E. , Samms, K. M. , Mandal, A. , Sumner, E. A. , & Eisenhoffer, G. T. (2019). Stem cell proliferation is induced by apoptotic bodies from dying cells during epithelial tissue maintenance. Nature Communications, 10(1), 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger, D. , Turner, M. , Xiao, F. , Munkonda, M. N. , Akbari, S. , & Burns, K. D. (2017). High glucose increases the formation and pro‐oxidative activity of endothelial microparticles. Diabetologia, 60(9), 1791–1800. [DOI] [PubMed] [Google Scholar]
- Brunner, D. (2010). Serum‐free cell culture: The serum‐free media interactive online database. Altex, 27(1), 53–62. [DOI] [PubMed] [Google Scholar]
- Burnouf, T. (2018). Multifaceted regenerative lives of ‘expired’ platelets. ISBT Science Series, 13(3), 323–330. [Google Scholar]
- Buzas, E. I. (2022). Opportunities and challenges in studying the extracellular vesicle corona. Nature Cell Biology, 24(9), 1322–1325. [DOI] [PubMed] [Google Scholar]
- Buzas, E. I. (2023). The roles of extracellular vesicles in the immune system. Nature Reviews Immunology, 23(4), 236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari, C. , Ranghino, A. , Tapparo, M. , Cedrino, M. , Figliolini, F. , Grange, C. , Giannachi, V. , Garneri, P. , Deregibus, M. C. , Collino, F. , Rispoli, P. , Camussi, G. , & Brizzi, M. F. (2017). Serum‐derived extracellular vesicles (EVs) impact on vascular remodeling and prevent muscle damage in acute hind limb ischemia. Scientific Reports, 7(1), 8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, J. , Wang, B. , Tang, T. , Lv, L. , Ding, Z. , Li, Z. , Hu, R. , Wei, Q. , Shen, A. , Fu, Y. , & Liu, B. (2020). Three‐dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin‐induced acute kidney injury. Stem Cell Research Therapy, 11(1), 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelli, C. , Domenghini, M. , Borleri, G. , Bellavita, P. , Poma, R. , Carobbio, A. , Micò, C. , Rambaldi, A. , Golay, J. , & Introna, M. (2007). Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. Bone Marrow Transplantation, 40(8), 785–791. [DOI] [PubMed] [Google Scholar]
- Caruso, S. , & Poon, I. K. H. (2018). Apoptotic cell‐derived extracellular vesicles: More than just debris. Frontiers in Immunology, 9, 1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clabaut, A. , Grare, C. , Léger, T. , Hardouin, P. , & Broux, O. (2015). Variations of secretome profiles according to conditioned medium preparation: The example of human mesenchymal stem cell‐derived adipocytes. Electrophoresis, 36(20), 2587–2593. [DOI] [PubMed] [Google Scholar]
- Crescitelli, R. , Lässer, C. , Szabó, T. G. , Kittel, A. , Eldh, M. , Dianzani, I. , Buzás, E. I. , & Lötvall, J. (2013). Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. Journal of Extracellular Vesicles, 2(1), 20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenviriyakul, C. , Takahashi, Y. , Morishita, M. , Matsumoto, A. , Nishikawa, M. , & Takakura, Y. (2017). Cell type‐specific and common characteristics of exosomes derived from mouse cell lines: Yield, physicochemical properties, and pharmacokinetics. European Journal of Pharmaceutical Sciences, 96, 316–322. [DOI] [PubMed] [Google Scholar]
- Cheng, Y. , Zeng, Q. , Han, Q. , & Xia, W. (2019). Effect of pH, temperature and freezing‐thawing on quantity changes and cellular uptake of exosomes. Protein Cell, 10(4), 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhery, M. S. , Badowski, M. , Muise, A. , Pierce, J. , & Harris, D. T. (2014). Donor age negatively impacts adipose tissue‐derived mesenchymal stem cell expansion and differentiation. Journal of Translational Medicine, 12, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, A. , Boilard, E. , Buzas, E. I. , Cheng, L. , Falcón‐Perez, J. M. , Gardiner, C. , Gustafson, D. , Gualerzi, A. , Hendrix, A. , Hoffman, A. , Jones, J. , Lässer, C. , Lawson, C. , Lenassi, M. , Nazarenko, I. , O'driscoll, L. , Pink, R. , Siljander, R.‐M. , Soekmadji, C. , … Nieuwland, R. (2019). Considerations towards a roadmap for collection, handling and storage of blood extracellular vesicles. Journal of Extracellular Vesicles, 8(1), 1647027–1647027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coecke, S. , Balls, M. , Bowe, G. , Davis, J. , Gstraunthaler, G. , Hartung, T. , Hay, R. , Merten, O.‐W. , Price, A. , Schechtman, L. , Stacey, G. , & Stokes, W. (2005). Guidance on good cell culture practice. A report of the second ECVAM task force on good cell culture practice. Alternatives to Laboratory Animals, 33(3), 261–287. [DOI] [PubMed] [Google Scholar]
- Cohen, S. , Samadikuchaksaraei, A. , Polak, J. M. , & Bishop, A. E. (2006). Antibiotics reduce the growth rate and differentiation of embryonic stem cell cultures. Tissue Engineering, 12(7), 2025–2030. [DOI] [PubMed] [Google Scholar]
- Commission, E.P., E.D.f.t.Q.o . (2010). Medicines, and Healthcare, European pharmacopoeia., Vol. 1. Council of Europe. [Google Scholar]
- Cossetti, C. , Iraci, N. , Mercer, T. R. , Leonardi, T. , Alpi, E. , Drago, D. , Alfaro‐Cervello, C. , Saini, H. K. , Davis, M. P. , Schaeffer, J. , Vega, B. , Stefanini, M. , Zhao, C. , Muller, W. , Garcia‐Verdugo, J. M. , Mathivanan, S. , Bachi, A. , Enright, A. J. , Mattick, J. S. , & Pluchino, S. (2014). Extracellular vesicles from neural stem cells transfer IFN‐gamma via Ifngr1 to activate Stat1 signaling in target cells. Molecular Cell, 56(2), 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, L. A. , Eiro, N. , Fraile, M. , Gonzalez, L. O. , Saá, J. , Garcia‐Portabella, P. , Vega, B. , Schneider, J. , & Vizoso, F. J. (2021). Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: Implications for further clinical uses. Cellular and Molecular Life Sciences, 78(2), 447–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronemberger‐Andrade, A. , Xander, P. , Soares, R. P. , Pessoa, N. L. , Campos, M. A. , Ellis, C. C. , Grajeda, B. , Ofir‐Birin, Y. , Almeida, I. C. , Regev‐Rudzki, N. , & Torrecilhas, A. C. (2020). Trypanosoma cruzi‐infected human macrophages shed proinflammatory extracellular vesicles that enhance host‐cell invasion via toll‐like receptor 2. Frontiers in Cellular and Infection Microbiology, 10, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, J.‐M. , Yu, M.‐X. , Shen, Z.‐Y. , Guo, C.‐Y. , Zhuang, S.‐Q. , & Qiu, X.‐S. (2015). Leucine promotes proliferation and differentiation of primary preterm rat satellite cells in part through mTORC1 signaling pathway. Nutrients, 7(5), 3387–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, S. M. , Riquelme, J. A. , Zheng, Y. , Vicencio, J. M. , Lavandero, S. , & Yellon, D. M. (2018a). Endothelial cells release cardioprotective exosomes that may contribute to ischaemic preconditioning. Scientific Reports, 8(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, S. M. , Riquelme, J. A. , Takov, K. , Vicencio, J. M. , Boi‐Doku, C. , Khoo, V. , Doreth, C. , Radenkovic, D. , Lavandero, S. , & Yellon, D. M. (2018b). Cardioprotection mediated by exosomes is impaired in the setting of type II diabetes but can be rescued by the use of non‐diabetic exosomes in vitro. Journal of Cellular and Molecular Medicine, 22(1), 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida Fuzeta, M. , Bernardes, N. , Oliveira, F. D. , Costa, A. C. , Fernandes‐Platzgummer, A. , Farinha, J. P. , Rodrigues, C. A. V. , Jung, S. , Tseng, R.‐J. , Milligan, W. , Lee, B. , Castanho, M. A. R. B. , Gaspar, D. , Cabral, J. M. S. , & Da Silva, C. L. (2020). Scalable production of human mesenchymal stromal cell‐derived extracellular vesicles under serum‐/xeno‐free conditions in a microcarrier‐based bioreactor culture system. Frontiers in Cell and Developmental Biology, 8, 553444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong, O. G. , Verhaar, M. C. , Chen, Y. , Vader, P. , Gremmels, H. , Posthuma, G. , Schiffelers, R. M. , Gucek, M. , & Van Balkom, B. W. M. (2012). Cellular stress conditions are reflected in the protein and RNA content of endothelial cell‐derived exosomes. Journal of Extracellular Vesicles, 1, 18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudé, M. , Bell, C. , Turgeon, J. , Beillevaire, D. , Pomerleau, L. , Yang, B. , Hamelin, K. , Qi, S. , Pallet, N. , Béland, C. , Dhahri, W. , Cailhier, J.‐F. , Rousseau, M. , Duchez, A.‐C. , Lévesque, T. , Lau, A. , Rondeau, C. , Gingras, D. , Muruve, D. , … Hébert, M.‐J. (2015). The 20S proteasome core, active within apoptotic exosome‐like vesicles, induces autoantibody production and accelerates rejection. Science Translational Medicine, 7(318), 318ra200. [DOI] [PubMed] [Google Scholar]
- Dominici, M. , Le Blanc, K. , Mueller, I. , Slaper‐Cortenbach, I. , Marini, F. C. , Krause, D. S. , Deans, R. J. , Keating, A. , Prockop, D. J. , & Horwitz, E. M. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317. [DOI] [PubMed] [Google Scholar]
- Dorronsoro, A. , Santiago, F. E. , Grassi, D. , Zhang, T. , Lai, R. C. , Mcgowan, S. J. , Angelini, L. , Lavasani, M. , Corbo, L. , Lu, A. , Brooks, R. W. , Garcia‐Contreras, M. , Stolz, D. B. , Amelio, A. , Boregowda, S. V. , Fallahi, M. , Reich, A. , Ricordi, C. , Phinney, D. G. , … Robbins, D. (2021). Mesenchymal stem cell‐derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. Aging Cell, 20(4), e13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty, J. A. , Patel, N. , Kumar, N. , Rao, S. G. , Angelos, M. G. , Singh, H. , Cai, C. , & Khan, M. (2020). Human cardiac progenitor cells enhance exosome release and promote angiogenesis under physoxia. Frontiers in Cell and Developmental Biology, 8, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driedonks, T. A. P. , Nijen Twilhaar, M. K. , & Nolte‐'t Hoen, E. N. M. (2019). Technical approaches to reduce interference of Fetal calf serum derived RNA in the analysis of extracellular vesicle RNA from cultured cells. Journal of Extracellular Vesicles, 8(1), 1552059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, S. , Lai, A. , Scholz‐Romero, K. , Shiddiky, M. J. A. , Yamauchi, Y. , Mishra, J. S. , Rice, G. E. , Hyett, J. , Kumar, S. , & Salomon, C. (2020). Hypoxia‐induced small extracellular vesicle proteins regulate proinflammatory cytokines and systemic blood pressure in pregnant rats. Clinical Science (London, England: 1979), 134(6), 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichholz, K. F. , Woods, I. , Riffault, M. , Johnson, G. P. , Corrigan, M. , Lowry, M. C. , Shen, N. , Labour, M.‐N. , Wynne, K. , O'driscoll, L. , & Hoey, D. A. (2020). Human bone marrow stem/stromal cell osteogenesis is regulated via mechanically activated osteocyte‐derived extracellular vesicles. Stem Cells Translational Medicine, 9(11), 1431–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan, E. , Zhang, S. , Witwer, K. W. , & Mattson, M. P. (2015). Extracellular vesicle‐depleted fetal bovine and human sera have reduced capacity to support cell growth. Journal of Extracellular Vesicles, 4, 26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi, F. M. , Farwell, D. G. , Nolta, J. A. , & Anderson, J. D. (2020). Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells, 38(1), 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdbrügger, U. , Blijdorp, C. J. , Bijnsdorp, I. V. , Borràs, F. E. , Burger, D. , Bussolati, B. , Byrd, J. B. , Clayton, A. , Dear, J. W. , Falcón‐Pérez, J. M. , Grange, C. , Hill, A. F. , Holthöfer, H. , Hoorn, E. J. , Jenster, G. , Jimenez, C. R. , Junker, K. , Klein, J. , Knepper, M. A. , … Martens‐Uzunova, E. S. (2021). Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. Journal of Extracellular Vesicles, 10(7), e12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emam, S. E. , Ando, H. , Abu Lila, A. S. , Shimizu, T. , Ukawa, M. , Okuhira, K. , Ishima, Y. , Mahdy, M. A. , Ghazy, F.‐E. S. , & Ishida, T. (2018). A novel strategy to increase the yield of exosomes (extracellular vesicles) for an expansion of basic research. Biological & Pharmaceutical Bulletin, 41(5), 733–742. [DOI] [PubMed] [Google Scholar]
- Fafián‐Labora, J. , Lesende‐Rodriguez, I. , Fernández‐Pernas, P. , Sangiao‐Alvarellos, S. , Monserrat, L. , Arntz, O. J. , Van De Loo, F. A. J. , Mateos, J. , & Arufe, M. C. (2017). Effect of age on pro‐inflammatory miRNAs contained in mesenchymal stem cell‐derived extracellular vesicles. Scientific Reports, 7, 43923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, S.‐J. , Kroeger, B. , Marie, P. , Bridges, E. M. , Mason, J. D. , Mccormick, K. , Zois, C. E. , Sheldon, H. , Khalid Alham, N. , Johnson, E. , Ellis, M. , Stefana, M. I. , Mendes, C. C. , Wainwright, S. M. , Cunningham, C. , Hamdy, F. C. , Morris, J. F. , Harris, A. L. , Wilson, C. , & Goberdhan, D. C. (2020). Glutamine deprivation alters the origin and function of cancer cell exosomes. Embo Journal, 39(16), e103009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruqu, F. N. , Liam‐Or, R. , Zhou, S. , Nip, R. , & Al‐Jamal, K. T. (2021). Defined serum‐free three‐dimensional culture of umbilical cord‐derived mesenchymal stem cells yields exosomes that promote fibroblast proliferation and migration in vitro. The FASEB Journal, 35(1), e21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa‐Valdés, A. I. , De La Fuente, C. , Hidalgo, Y. , Vega‐Letter, A. M. , Tapia‐Limonchi, R. , Khoury, M. , & Alcayaga‐Miranda, F. (2021). A chemically defined, xeno‐ and blood‐free culture medium sustains increased production of small extracellular vesicles from mesenchymal stem cells. Frontiers in Bioengineering and Biotechnology, 9, 619930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, H. W. , Puck, T. T. , & Sato, G. (1958). Molecular growth requirements of single mammalian cells: The action of fetuin in promoting cell attachment to glass. PNAS, 44(1), 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliefel, R. , Popov, C. , Tröltzsch, M. , Kühnisch, J. , Ehrenfeld, M. , & Otto, S. (2016). Mesenchymal stem cell proliferation and mineralization but not osteogenic differentiation are strongly affected by extracellular pH. Journal of Cranio‐Maxillofacial Surgery, 44(6), 715–724. [DOI] [PubMed] [Google Scholar]
- Flores‐Bellver, M. , Mighty, J. , Aparicio‐Domingo, S. , Li, K. V. , Shi, C. , Zhou, J. , Cobb, H. , Mcgrath, P. , Michelis, G. , Lenhart, P. , Bilousova, G. , Heissel, S. , Rudy, M. J. , Coughlan, C. , Goodspeed, A. E. , Becerra, S. P. , Redenti, S. , & Canto‐Soler, M. V. (2021). Extracellular vesicles released by human retinal pigment epithelium mediate increased polarised secretion of drusen proteins in response to AMD stressors. Journal of Extracellular Vesicles, 10(13), e12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, R. , Musicò, A. , Brucale, M. , Ridolfi, A. , Galbiati, S. , Vago, R. , Bergamaschi, G. , Ferretti, A. M. , Chiari, M. , Valle, F. , Gori, A. , & Cretich, M. (2021). Extracellular vesicles analysis in the COVID‐19 era: Insights on serum inactivation protocols towards downstream isolation and analysis. Cells, 10(3), 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta, T. , Nishikawa, A. , & Kogure, K. (2020). Low level electricity increases the secretion of extracellular vesicles from cultured cells. Biochemistry and Biophysics Report, 21, 100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaurivaud, P. , Ganter, S. , Villard, A. , Manso‐Silvan, L. , Chevret, D. , Boulé, C. , Monnet, V. , & Tardy, F. (2018). Mycoplasmas are no exception to extracellular vesicles release: Revisiting old concepts. PLoS ONE, 13(11), e0208160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, N. A. , Ontoria‐Oviedo, I. , González‐King, H. , Diez‐Juan, A. , & Sepúlveda, P. (2015). Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS ONE, 10(9), e0138849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona, M. , Brizzi, M. F. , Choo, A. B. H. , Dominici, M. , Davidson, S. M. , Grillari, J. , Hermann, D. M. , Hill, A. F. , De Kleijn, D. , Lai, R. C. , Lai, C. P. , Lim, R. , Monguió‐Tortajada, M. , Muraca, M. , Ochiya, T. , Ortiz, L. A. , Toh, W. S. , Yi, Y. W. , Witwer, K. W. , … Lim, S. K. (2021). Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell‐derived small extracellular vesicles. Cytotherapy, 23(5), 373–380. [DOI] [PubMed] [Google Scholar]
- Gottipamula, S. , Sharma, A. , Krishnamurthy, S. , Majumdar, A. S. , & Seetharam, R. N. (2012). Human platelet lysate is an alternative to fetal bovine serum for large‐scale expansion of bone marrow‐derived mesenchymal stromal cells. Biotechnology Letters, 34(7), 1367–1374. [DOI] [PubMed] [Google Scholar]
- Grangier, A. , Branchu, J. , Volatron, J. , Piffoux, M. , Gazeau, F. , Wilhelm, C. , & Silva, A. K. A. (2021). Technological advances towards extracellular vesicles mass production. Advanced Drug Delivery Reviews, 176, 113843. [DOI] [PubMed] [Google Scholar]
- Griffiths, S. , Baraniak, R. , Copland, I. B. , Nerem, R. M. , & Mcdevitt, T. C. (2013). Human platelet lysate stimulates high‐passage and senescent human multipotent mesenchymal stromal cell growth and rejuvenation in vitro. Cytotherapy, 15(12), 1469–1483. [DOI] [PubMed] [Google Scholar]
- Gregory, C. D. , & Dransfield, I. (2018). Apoptotic tumor cell‐derived extracellular vesicles as important regulators of the onco‐regenerative niche. Frontiers in Immunology, 9, 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, H. E. , Somayaji, S. , Riley, F. , Hoelscher, G. L. , Norton, H. J. , Ingram, J. , & Hanley, E. N. Jr (2012). Human adipose‐derived mesenchymal stem cells: Serial passaging, doubling time and cell senescence. Biotechnic & Histochemistry, 87(4), 303–311. [DOI] [PubMed] [Google Scholar]
- Galluzzi, L. , Vitale, I. , Aaronson, S. A. , Abrams, J. M. , Adam, D. , Agostinis, P. , Alnemri, E. S. , Altucci, L. , Amelio, I. , Andrews, D. W. , Annicchiarico‐Petruzzelli, M. , Antonov, A. V. , Arama, E. , Baehrecke, E. H. , Barlev, N. A. , Bazan, N. G. , Bernassola, F. , Bertrand, M. J. M. , Bianchi, K. , … Kroemer, G. (2018). Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death & Differentiation, 25(3), 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, W. , Liang, T. , He, R. , Ren, J. , Yao, H. , Wang, K. , Zhu, L. , & Xu, Y. (2020). Exosomes from 3D culture of marrow stem cells enhances endothelial cell proliferation, migration, and angiogenesis via activation of the HMGB1/AKT pathway. Stem Cell Research, 50, 102122. [DOI] [PubMed] [Google Scholar]
- Garitaonandia, I. , Amir, H. , Boscolo, F. S. , Wambua, G. K. , Schultheisz, H. L. , Sabatini, K. , Morey, R. , Waltz, S. , Wang, Y.‐C. , Tran, H. , Leonardo, T. R. , Nazor, K. , Slavin, I. , Lynch, C. , Li, Y. , Coleman, R. , Gallego Romero, I. , Altun, G. , Reynolds, D. , … Laurent, L. C. (2015). Increased risk of genetic and epigenetic instability in human embryonic stem cells associated with specific culture conditions. PLoS ONE, 10(2), e0118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gstraunthaler, G. (2003). Alternatives to the use of fetal bovine serum: Serum‐free cell culture. Altex, 20(4), 275–281. [PubMed] [Google Scholar]
- Gu, H. , Liu, Z. , Li, Y. , Xie, Y. , Yao, J. , Zhu, Y. , Xu, J. , Dai, Q. , Zhong, C. , Zhu, H. , Ding, S. , & Zhou, L. (2018). Serum‐Derived Extracellular Vesicles Protect Against Acute Myocardial Infarction by Regulating miR‐21/PDCD4 Signaling Pathway. Frontiers in Physiology, 9, 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbergsson, J. M. , Johnsen, K. B. , Skov, M. N. , & Duroux, M. (2016). Systematic review of factors influencing extracellular vesicle yield from cell cultures. Cytotechnology, 68(4), 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro, E. M. , Vestad, B. , Steffensen, L. A. , Aass, H. C. D. , Saeed, M. , Øvstebø, R. , Costea, D. E. , Galtung, H. K. , & Søland, T. M. (2018). Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size‐exclusion chromatography. PLoS ONE, 13(9), e0204276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan, S. , Kang, M.‐H. , & Kim, J.‐H. (2021). A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. International Journal of Nanomedicine, 16, 1281–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiotto, M. , Raffoul, W. , Hart, A. M. , Riehle, M. O. , & Di Summa, G. (2020). Human platelet lysate to substitute fetal bovine serum in hMSC expansion for translational applications: A systematic review. Journal of Translational Medicine, 18(1), 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, S. , Debbi, L. , Zohar, B. , Samuel, R. , Arzi, R. S. , Fried, A. I. , Carmon, T. , Shevach, D. , Redenski, I. , Schlachet, I. , Sosnik, A. , & Levenberg, S. (2021). Stimulating extracellular vesicles production from engineered tissues by mechanical forces. Nano Letters, 21(6), 2497–2504. [DOI] [PubMed] [Google Scholar]
- György, B. , Szabó, T. G. , Pásztói, M. , Pál, Z. , Misják, P. , Aradi, B. , László, V. , Pállinger, É. , Pap, E. , Kittel, Á. , Nagy, G. , Falus, A. , & Buzás, E. I. (2011). Membrane vesicles, current state‐of‐the‐art: Emerging role of extracellular vesicles. Cellular and Molecular Life Sciences, 68(16), 2667–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraszti, R. A. , Miller, R. , Stoppato, M. , Sere, Y. Y. , Coles, A. , Didiot, M.‐C. , Wollacott, R. , Sapp, E. , Dubuke, M. L. , Li, X. , Shaffer, S. A. , Difiglia, M. , Wang, Y. , Aronin, N. , & Khvorova, A. (2018). Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Molecular Therapy, 26(12), 2838–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, O. , Ramos, B. , Rodríguez, L. L. , Aguilar, A. , Badía, T. , & Castro, F. O. (2005). Cell confluency is as efficient as serum starvation for inducing arrest in the G0/G1 phase of the cell cycle in granulosa and fibroblast cells of cattle. Animal Reproduction Science, 87(3‐4), 181–192. [DOI] [PubMed] [Google Scholar]
- Hawkes, W. (2015). Fetal bovine serum: Geographic origin and regulatory relevance of viral contamination. Bioresources and Bioprocessing, 2(1), 34. [Google Scholar]
- Hemeda, H. , Giebel, B. , & Wagner, W. (2014). Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy, 16(2), 170–180. [DOI] [PubMed] [Google Scholar]
- Henschler, R. , Gabriel, C. , Schallmoser, K. , Burnouf, T. , & Koh, M. B. C. (2019). Human platelet lysate current standards and future developments. Transfusion, 59(4), 1407–1413. [DOI] [PubMed] [Google Scholar]
- Hergenreider, E. , Heydt, S. , Tréguer, K. , Boettger, T. , Horrevoets, A. J. G. , Zeiher, A. M. , Scheffer, M. P. , Frangakis, A. S. , Yin, X. , Mayr, M. , Braun, T. , Urbich, C. , Boon, R. A. , & Dimmeler, S. (2012). Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature Cell Biology, 14(3), 249–256. [DOI] [PubMed] [Google Scholar]
- Hill, A. F. , Pegtel, D. M. , Lambertz, U. , Leonardi, T. , O'driscoll, L. , Pluchino, S. , Ter‐Ovanesyan, D. , & Nolte‐‘T Hoen, E. N. M. (2013). ISEV position paper: Extracellular vesicle RNA analysis and bioinformatics. Journal of Extracellular Vesicles, 2, 22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, J. H. , Chen, Y.‐F. , Ma, W.‐H. , Tseng, T.‐C. , Chen, M.‐H. , & Lee, O. K. (2011). Cell contact accelerates replicative senescence of human mesenchymal stem cells independent of telomere shortening and p53 activation: Roles of Ras and oxidative stress. Cell Transplantation, 20(8), 1209–1220. [DOI] [PubMed] [Google Scholar]
- Hosseinkhani, B. , Van Den Akker, N. , D'haen, J. , Gagliardi, M. , Struys, T. , Lambrichts, I. , Waltenberger, J. , Nelissen, I. , Hooyberghs, J. , Molin, D. G. M. , & Michiels, L. (2017). Direct detection of nano‐scale extracellular vesicles derived from inflammation‐triggered endothelial cells using surface plasmon resonance. Nanomedicine, 13(5), 1663–1671. [DOI] [PubMed] [Google Scholar]
- Hosseinkhani, B. , Van Den Akker, N. M. S. , Molin, D. G. M. , & Michiels, L. (2020). (Sub)populations of extracellular vesicles released by TNF‐α ‐triggered human endothelial cells promote vascular inflammation and monocyte migration. Journal of Extracellular Vesicles, 9(1), 1801153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Li, R. , Zhang, L. , Chen, Y. , Dong, W. , Zhao, X. , Yang, H. , Zhang, S. , Xie, Z. , Ye, Z. , Wang, W. , Li, C. , Li, Z. , Liu, S. , Dong, Z. , Yu, X. , & Liang, X. (2020). Extracellular vesicles from high glucose‐treated podocytes induce apoptosis of proximal tubular epithelial cells. Frontiers in Physiology, 11, 579296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz, S. N. , Conlon, M. M. , Rider, M. A. , Brownstein, N. C. , & Meckes, D. G. (2016). Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis. Journal of Extracellular Vesicles, 5(1), 31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, K. , Zambelli, F. , Mertzanidou, A. , Smolders, I. , Geens, M. , Nguyen, H. T. , Barbé, L. , Sermon, K. , & Spits, C. (2016). Higher‐density culture in human embryonic stem cells results in DNA damage and genome instability. Stem Cell Reports, 6(3), 330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangamreddy, J. R. , Haagdorens, M. K. C. , Mirazul Islam, M. , Lewis, P. , Samanta, A. , Fagerholm, P. , Liszka, A. , Ljunggren, M. K. , Buznyk, O. , Alarcon, E. I. , Zakaria, N. , Meek, K. M. , & Griffith, M. (2018). Short peptide analogs as alternatives to collagen in pro‐regenerative corneal implants. Acta Biomaterialia, 69, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarmalavičiūtė, A. , Tunaitis, V. , Pivoraitė, U. , Venalis, A. , & Pivoriūnas, A. (2015). Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6‐hydroxy‐dopamine‐induced apoptosis. Cytotherapy, 17(7), 932–939. [DOI] [PubMed] [Google Scholar]
- Jewell, J. L. , Russell, R. C. , & Guan, K.‐L. (2013). Amino acid signalling upstream of mTOR. Nature Reviews Molecular Cell Biology, 14(3), 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, L. , Klinth, J. , Holmqvist, O. , & Ohlson, S. (2003). Platelet lysate: A replacement for fetal bovine serum in animal cell culture? Cytotechnology, 42(2), 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhl, M. , Tratwal, J. , Follin, B. , Søndergaard, R. H. , Kirchhoff, M. , Ekblond, A. , Kastrup, J. , & Haack‐Sørensen, M. (2016). Comparison of clinical grade human platelet lysates for cultivation of mesenchymal stromal cells from bone marrow and adipose tissue. Scandinavian Journal of Clinical and Laboratory Investigation, 76(2), 93–104. [DOI] [PubMed] [Google Scholar]
- Kakarla, R. , Hur, J. , Kim, Y. J. , Kim, J. , & Chwae, Y. J. (2020). Apoptotic cell‐derived exosomes: Messages from dying cells. Experimental & Molecular Medicine, 52(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, T. , Jones, T. M. , Naddell, C. , Bacanamwo, M. , Calvert, J. W. , Thompson, W. E. , Bond, V. C. , Chen, Y. E. , & Liu, D. (2016). Adipose‐derived stem cells induce angiogenesis via microvesicle transport of miRNA‐31. Stem Cells Translational Medicine, 5(4), 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami, T. , Kawamura, K. , Fujimori, K. , Koike, A. , & Amano, F. (2016). Influence of the culture medium on the production of nitric oxide and expression of inducible nitric oxide synthase by activated macrophages in vitro. Biochemistry and Biophysics Report, 5, 328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsara, O. , Mahaira, L. G. , Iliopoulou, E. G. , Moustaki, A. , Antsaklis, A. , Loutradis, D. , Stefanidis, K. , Baxevanis, C. N. , Papamichail, M. , & Perez, S. A. (2011). Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow‐derived mesenchymal stem cells. Stem Cells and Development, 20(9), 1549–1561. [DOI] [PubMed] [Google Scholar]
- Kassem, M. , Ankersen, L. , Eriksen, E. F. , Clark, B. F. C. , & Rattan, S. I. S. (1997). Demonstration of cellular aging and senescence in serially passaged long‐term cultures of human trabecular osteoblasts. Osteoporosis International, 7(6), 514–524. [DOI] [PubMed] [Google Scholar]
- Kerris, E. W. J. , Hoptay, C. , Calderon, T. , & Freishtat, R. J. (2020). Platelets and platelet extracellular vesicles in hemostasis and sepsis. Journal of Investigative Medicine, 68(4), 813–820. [DOI] [PubMed] [Google Scholar]
- Kim, N. (2021). pH variation impacts molecular pathways associated with somatic cell reprogramming and differentiation of pluripotent stem cells. Reproductive Medicine and Biology, 20(1), 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D.‐K. , Nishida, H. , An, S. Y. , Shetty, A. K. , Bartosh, T. J. , & Prockop, D. J. (2016). Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. PNAS, 113(1), 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B. , Li, J. , Jang, C. , & Arany, Z. (2017). Glutamine fuels proliferation but not migration of endothelial cells. Embo Journal, 36(16), 2321–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. H. , Chung, C. , Oh, M. H. , Jun, J. H. , Ko, Y. , & Lee, J. H. (2021). Extracellular vesicles from a three‐dimensional culture of perivascular cells accelerate skin wound healing in a rat. Aesthetic Plastic Surgery, 45, 2437–2446. [DOI] [PubMed] [Google Scholar]
- Kim, J. Y. , Rhim, W.‐K. , Yoo, Y.‐I. , Kim, D.‐S. , Ko, K.‐W. , Heo, Y. , Park, C. G. , & Han, D. K. (2021). Defined MSC exosome with high yield and purity to improve regenerative activity. Journal of Tissue Engineering, 12, 204173142110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, H. W. , Michael, M. Z. , & Gleadle, J. M. (2012). Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer, 12, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaki, M. , Numata, Y. , Morioka, C. , Honda, I. , Tooi, M. , Yokoyama, N. , Ayame, H. , Iwasaki, K. , Taki, A. , Oshima, N. , & Morita, I. (2017). Exosomes of human placenta‐derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Research & Therapy, 8(1), 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordelas, L. , Rebmann, V. , Ludwig, A.‐K. , Radtke, S. , Ruesing, J. , Doeppner, T. R. , Epple, M. , Horn, A. , Beelen, D. W. , & Giebel, B. (2014). MSC‐derived exosomes: A novel tool to treat therapy‐refractory graft‐versus‐host disease. Leukemia, 28(4), 970–973. [DOI] [PubMed] [Google Scholar]
- Kornilov, R. , Puhka, M. , Mannerström, B. , Hiidenmaa, H. , Peltoniemi, H. , Siljander, P. , Seppänen‐Kaijansinkko, R. , & Kaur, S. (2018). Efficient ultrafiltration‐based protocol to deplete extracellular vesicles from fetal bovine serum. Journal of Extracellular Vesicles, 7(1), 1422674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzewska, P. , Christianson, H. C. , Welch, J. E. , Svensson, K. J. , Fredlund, E. , Ringnér, M. , Mörgelin, M. , Bourseau‐Guilmain, E. , Bengzon, J. , & Belting, M. (2013). Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia‐dependent activation of vascular cells during tumor development. PNAS, 110(18), 7312–7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. , & Deep, G. (2020). Hypoxia in tumor microenvironment regulates exosome biogenesis: Molecular mechanisms and translational opportunities. Cancer Letters, 479, 23–30. [DOI] [PubMed] [Google Scholar]
- Kwon, H. , Yang, S. , Lee, J. , Park, B. , Park, K. , Jung, J. , Bae, Y. , & Park, G. (2020). Combination treatment with human adipose tissue stem cell‐derived exosomes and fractional CO2 laser for acne scars: A 12‐week prospective, double‐blind, randomized, split‐face study. Acta Dermato‐Venereologica, 100(18), adv00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázaro‐Ibáñez, E. , Sanz‐Garcia, A. , Visakorpi, T. , Escobedo‐Lucea, C. , Siljander, P. , Ayuso‐Sacido, Á. , & Yliperttula, M. (2014). Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: Apoptotic bodies, microvesicles, and exosomes. The Prostate, 74(14), 1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, Z. , Jaular, L. M. , Soueidi, E. , Jouve, M. , Muth, D. C. , Schøyen, T. H. , Seale, T. , Haughey, N. J. , Ostrowski, M. , Théry, C. , & Witwer, K. W. (2019). Acetylcholinesterase is not a generic marker of extracellular vesicles. Journal of Extracellular Vesicles, 8(1), 1628592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X. , Li, S. , Wang, Y.‐J. , Wang, Y. I. , Zhong, J.‐Y. , He, J.‐Y. , Cui, X.‐J. , Zhan, J.‐K. , & Liu, Y.‐S. (2019). Exosomal Notch3 from high glucose‐stimulated endothelial cells regulates vascular smooth muscle cells calcification/aging. Life Sciences, 232, 116582. [DOI] [PubMed] [Google Scholar]
- Lehrich, B. M. , Liang, Y. , & Fiandaca, M. S. (2021). Foetal bovine serum influence on in vitro extracellular vesicle analyses. Journal of Extracellular Vesicles, 10(3), e12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, Q. , Liu, T. , Gao, F. , Xie, H. , Sun, L. , Zhao, A. , Ren, W. , Guo, H. , Zhang, L. , Wang, H. , Chen, Z. , Guo, A.‐Y. , & Li, Q. (2017). Microvesicles as potential biomarkers for the identification of senescence in human mesenchymal stem cells. Theranostics, 7(10), 2673–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, B. D. , Paine, M. S. , Brooks, A. M. , Mccubrey, J. A. , Renegar, R. H. , Wang, R. , & Terrian, D. M. (2008). Senescence‐associated exosome release from human prostate cancer cells. Cancer Research, 68(19), 7864–7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrich, B. , Liang, Y. , Khosravi, P. , Federoff, H. , & Fiandaca, M. (2018). Fetal bovine serum‐derived extracellular vesicles persist within vesicle‐depleted culture media. International Journal of Molecular Sciences, 19(11), 3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsiou, E. , Sammani, S. , Zhang, W. , Zhou, T. , Quijada, H. , Moreno‐Vinasco, L. , Dudek, S. M. , & Garcia, J. G. N. (2015). Pathologic mechanical stress and endotoxin exposure increases lung endothelial microparticle shedding. American Journal of Respiratory Cell and MolecularBiology, 52(2), 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Lee, Y. , Johansson, H. J. , Mäger, I. , Vader, P. , Nordin, J. Z. , Wiklander, O. P. B. , Lehtiö, J. , Wood, M. J. A. , & Andaloussi, S. E. (2015). Serum‐free culture alters the quantity and protein composition of neuroblastoma‐derived extracellular vesicles. Journal of Extracellular Vesicles, 4, 26883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Yawno, T. , Sutherland, A. , Loose, J. , Nitsos, I. , Allison, B. J. , Bischof, R. , Mcdonald, C. A. , Jenkin, G. , & Miller, S. L. (2017). Term vs. preterm cord blood cells for the prevention of preterm brain injury. Pediatric Research, 82(6), 1030–1038. [DOI] [PubMed] [Google Scholar]
- Li, M. , Liao, L. , & Tian, W. (2020). Extracellular vesicles derived from apoptotic cells: An essential link between death and regeneration. Frontiers in Cell and Developmental Biology, 8, 573511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Qiu, X. , Lv, Y. , Zheng, C. , Dong, Y. , Dou, G. , Zhu, B. , Liu, A. , Wang, W. , Zhou, J. , Liu, S. , Liu, S. , Gao, B. , & Jin, Y. (2020). Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Research & Therapy, 11(1), 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, Z. , Muth, D. C. , Eitan, E. , Travers, M. , Learman, L. N. , Lehrmann, E. , & Witwer, K. W. (2017). Serum extracellular vesicle depletion processes affect release and infectivity of HIV‐1 in culture. Scientific Reports, 7(1), 2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötvall, J. , Hill, A. F. , Hochberg, F. , Buzás, E. I. , Di Vizio, D. , Gardiner, C. , Gho, Y. S. , Kurochkin, I. V. , Mathivanan, S. , Quesenberry, P. , Sahoo, S. , Tahara, H. , Wauben, M. H. , Witwer, K. W. , & Théry, C. (2014). Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. Journal of Extracellular Vesicles, 3, 26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logozzi, M. , Mizzoni, D. , Angelini, D. , Di Raimo, R. , Falchi, M. , Battistini, L. , & Fais, S. (2018). Microenvironmental pH and exosome levels interplay in human cancer cell lines of different histotypes. Cancers (Basel), 10(10), 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatina, T. , Bruno, S. , Tetta, C. , Kalinina, N. , Porta, M. , & Camussi, G. (2014). Platelet‐derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Communication and Signaling, 12, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobet, L. , Montoya, J. , López‐Gallardo, E. , & Ruiz‐Pesini, E. (2015). Side effects of culture media antibiotics on cell differentiation. Tissue Engineering Part C Methods, 21(11), 1143–1147. [DOI] [PubMed] [Google Scholar]
- Ludwig, N. , Razzo, B. M. , Yerneni, S. S. , & Whiteside, T. L. (2019). Optimization of cell culture conditions for exosome isolation using mini‐size exclusion chromatography (mini‐SEC). Experimental Cell Research, 378(2), 149–157. [DOI] [PubMed] [Google Scholar]
- Maeshige, N. , Langston, K. , Yuan, Z.‐M. , Kondo, H. , & Fujino, H. (2021). High‐intensity ultrasound irradiation promotes the release of extracellular vesicles from C2C12 myotubes. Ultrasonics, 110, 106243. [DOI] [PubMed] [Google Scholar]
- Masters, J. R. , Thomson, J. A. , Daly‐Burns, B. , Reid, Y. A. , Dirks, W. G. , Packer, P. , Toji, L. H. , Ohno, T. , Tanabe, H. , Arlett, C. F. , Kelland, L. R. , Harrison, M. , Virmani, A. , Ward, T. H. , Ayres, K. L. , & Debenham, G. (2001). Short tandem repeat profiling provides an international reference standard for human cell lines. Proceedings of the National Academy of Sciences, 98(14), 8012–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa, A. , Perut, F. , Chano, T. , Woloszyk, A. , Mitsiadis, T. , Avnet, S. , & Baldini, N. (2017). The effect of extracellular acidosis on the behaviour of mesenchymal stem cells in vitro. European Cells & Materials [Electronic Resource], 33, 252–267. [DOI] [PubMed] [Google Scholar]
- Mahmood, A. , Otruba, Z. , Weisgerber, A. W. , Palay, M. D. , Nguyen, M. T. , Bills, B. L. , & Knowles, M. K. (2023). Exosome secretion kinetics are controlled by temperature. Biophysical Journal, 122(7), 1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannerstrom, B. , Paananen, R. O. , Abu‐Shahba, A. G. , Moilanen, J. , Seppänen‐Kaijansinkko, R. , & Kaur, S. (2019). Extracellular small non‐coding RNA contaminants in fetal bovine serum and serum‐free media. Scientific Reports, 9(1), 5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens‐Uzunova, E. S. , Kusuma, G. D. , Crucitta, S. , Lim, H. K. , Cooper, C. , Riches, J. E. , Azad, A. , Ochiya, T. , Boyle, G. M. , Southey, M. C. , Del Re, M. , Lim, R. , Ramm, G. A. , Jenster, G. W. , & Soekmadji, C. (2021). Androgens alter the heterogeneity of small extracellular vesicles and the small RNA cargo in prostate cancer. Journal of Extracellular Vesicles, 10(10), e12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew, S. A. , & Bhonde, R. (2017). Mesenchymal stromal cells isolated from gestationally diabetic human placenta exhibit insulin resistance, decreased clonogenicity and angiogenesis. Placenta, 59, 1–8. [DOI] [PubMed] [Google Scholar]
- Mckeown, S. R. (2014). Defining normoxia, physoxia and hypoxia in tumours‐implications for treatment response. The British Journal of Radiology, 87(1035), 20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehryab, F. , Rabbani, S. , Shahhosseini, S. , Shekari, F. , Fatahi, Y. , Baharvand, H. , & Haeri, A. (2020). Exosomes as a next‐generation drug delivery system: An update on drug loading approaches, characterization, and clinical application challenges. Acta Biomaterialia, 113, 42–62. [DOI] [PubMed] [Google Scholar]
- Melki, I. , Tessandier, N. , Zufferey, A. , & Boilard, E. (2017). Platelet microvesicles in health and disease. Platelets, 28(3), 214–221. [DOI] [PubMed] [Google Scholar]
- Menard, C. , Pacelli, L. , Bassi, G. , Dulong, J. , Bifari, F. , Bezier, I. , Zanoncello, J. , Ricciardi, M. , Latour, M. , Bourin, P. , Schrezenmeier, H. , Sensebé, L. , Tarte, K. , & Krampera, M. (2013). Clinical‐grade mesenchymal stromal cells produced under various good manufacturing practice processes differ in their immunomodulatory properties: Standardization of immune quality controls. Stem Cells and Development, 22(12), 1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, I. S. , Amira, M. , Harvey, A. J. , & Bridger, J. M. (2010). Rapid chromosome territory relocation by nuclear motor activity in response to serum removal in primary human fibroblasts. Genome Biology, 11(1), R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendt, M. , Kamerkar, S. , Sugimoto, H. , Mcandrews, K. M. , Wu, C.‐C. , Gagea, M. , Yang, S. , Blanko, E. V. R. , Peng, Q. , Ma, X. , Marszalek, J. R. , Maitra, A. , Yee, C. , Rezvani, K. , Shpall, E. , Lebleu, V. S. , & Kalluri, R. (2018). Generation and testing of clinical‐grade exosomes for pancreatic cancer. JCI Insight, 3(8), e99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten, O.‐W. (2002). Virus contaminations of cell cultures–a biotechnological view. Cytotechnology, 39(2), 91–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merckx, G. , Hosseinkhani, B. , Kuypers, S. , Deville, S. , Irobi, J. , Nelissen, I. , Michiels, L. , Lambrichts, I. , & Bronckaers, A. (2020). Angiogenic effects of human dental pulp and bone marrow‐derived mesenchymal stromal cells and their extracellular vesicles. Cells, 9(2), 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza‐Zepeda, L. A. , Noer, A. , Dahl, J. A. , Micci, F. , Myklebost, O. , & Collas, P. (2008). High‐resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. Journal of Cellular and Molecular Medicine, 12(2), 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, J. P. , Court, J. , Mason, M. D. , Tabi, Z. , & Clayton, A. (2008). Increased exosome production from tumour cell cultures using the Integra CELLine Culture System. Journal of Immunological Methods, 335(1–2), 98–105. [DOI] [PubMed] [Google Scholar]
- Miceli, V. , Pampalone, M. , Vella, S. , Carreca, A. P. , Amico, G. , & Conaldi, G. (2019). Comparison of immunosuppressive and angiogenic properties of human amnion‐derived mesenchymal stem cells between 2D and 3D culture systems. Stem Cells International, 2019, 7486279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell, A. E. , Brown, G. N. , Robinson, S. T. , Sattler, R. L. , Baik, A. D. , Zhen, G. , Cao, X. , Bonewald, L. F. , Jin, W. , Kam, L. C. , & Guo, X. E. (2018). Mechanically induced Ca(2+) oscillations in osteocytes release extracellular vesicles and enhance bone formation. Bone Research, 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse, M. A. , Garst, J. , Osada, T. , Khan, S. , Hobeika, A. , Clay, T. M. , Valente, N. , Shreeniwas, R. , Sutton, M. , Delcayre, A. , Hsu, D.‐H. , Le Pecq, J.‐B. , & Lyerly, H. K. (2005). A phase I study of dexosome immunotherapy in patients with advanced non‐small cell lung cancer. Journal of Translational Medicine, 3(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruno, F. , Pérez‐Jiménez, E. , & Knecht, E. (2012). Regulation of autophagy by glucose in Mammalian cells. Cells, 1(3), 372–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, Y. , Miyaki, S. , Ishitobi, H. , Matsuyama, S. , Nakasa, T. , Kamei, N. , Akimoto, T. , Higashi, Y. , & Ochi, M. (2015). Mesenchymal‐stem‐cell‐derived exosomes accelerate skeletal muscle regeneration. Febs Letters, 589(11), 1257–1265. [DOI] [PubMed] [Google Scholar]
- Nakase, I. , Ueno, N. , Matsuzawa, M. , Noguchi, K. , Hirano, M. , Omura, M. , Takenaka, T. , Sugiyama, A. , Bailey Kobayashi, N. , Hashimoto, T. , Takatani‐Nakase, T. , Yuba, E. , Fujii, I. , Futaki, S. , & Yoshida, T. (2021). Environmental pH stress influences cellular secretion and uptake of extracellular vesicles. FEBS Open Bio, 11(3), 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar, W. , El‐Ansary, M. , Sabry, D. , Mostafa, M. A. , Fayad, T. , Kotb, E. , Temraz, M. , Saad, A.‐N. , Essa, W. , & Adel, H. (2016). Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomaterials Research, 20, 21–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh, A. , Orgovan, N. , Sódar, B. W. , Osteikoetxea, X. , Pálóczi, K. , Szabó‐Taylor, K. É. , Vukman, K. V. , Kittel, Á. , Turiák, L. , Wiener, Z. , Tóth, S. , Drahos, L. , Vékey, K. , Horvath, R. , & Buzás, E. I. (2017). Antibiotic‐induced release of small extracellular vesicles (exosomes) with surface‐associated DNA. Scientific Reports, 7(1), 8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwland, R. , Falcón‐Pérez, J. M. , Théry, C. , & Witwer, K. W. (2020). Rigor and standardization of extracellular vesicle research: Paving the road towards robustness. Journal of Extracellular Vesicles, 10(2), e12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrochta, K. M. , Krois, C. R. , Campos, B. , & Napoli, J. L. (2015). Insulin regulates retinol dehydrogenase expression and all‐trans‐retinoic acid biosynthesis through FoxO1. Journal of Biological Chemistry, 290(11), 7259–7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochieng, J. , Pratap, S. , Khatua, A. K. , & Sakwe, A. M. (2009). Anchorage‐independent growth of breast carcinoma cells is mediated by serum exosomes. Experimental Cell Research, 315(11), 1875–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeller, M. , Laner‐Plamberger, S. , Krisch, L. , Rohde, E. , Strunk, D. , & Schallmoser, K. (2021). Human platelet lysate for good manufacturing practice‐compliant cell production. International Journal of Molecular Sciences, 22(10), 5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W. H. (2004). Guidelines on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products. WHO Technical Report, Series, 924, 150–224. [Google Scholar]
- Otsuka, K. , Yamamoto, Y. , & Ochiya, T. (2020). Uncovering temperature‐dependent extracellular vesicle secretion in breast cancer. Journal of Extracellular Vesicles, 10(2), e12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachler, K. , Lener, T. , Streif, D. , Dunai, Z. A. , Desgeorges, A. , Feichtner, M. , Öller, M. , Schallmoser, K. , Rohde, E. , & Gimona, M. (2017). A Good Manufacturing Practice‐grade standard protocol for exclusively human mesenchymal stromal cell‐derived extracellular vesicles. Cytotherapy, 19(4), 458–472. [DOI] [PubMed] [Google Scholar]
- Palviainen, M. , Saari, H. , Kärkkäinen, O. , Pekkinen, J. , Auriola, S. , Yliperttula, M. , Puhka, M. , Hanhineva, K. , & Siljander, R.‐M. (2019). Metabolic signature of extracellular vesicles depends on the cell culture conditions. Journal of Extracellular Vesicles, 8(1), 1596669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palviainen, M. , Saraswat, M. , Varga, Z. , Kitka, D. , Neuvonen, M. , Puhka, M. , Joenväärä, S. , Renkonen, R. , Nieuwland, R. , Takatalo, M. , & Siljander, R. M. (2020). Extracellular vesicles from human plasma and serum are carriers of extravesicular cargo—Implications for biomarker discovery. PLoS ONE, 15(8), e0236439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamies, D. , Bal‐Price, A. , Simeonov, A. , Tagle, D. , Allen, D. , Gerhold, D. , Yin, D. , Pistollato, F. , Inutsuka, T. , Sullivan, K. , Stacey, G. , Salem, H. , Leist, M. , Daneshian, M. , Vemuri, M. C. , McFarland, R. , Coecke, S. , Fitzpatrick, S. C. , Lakshmipathy, U. , … Hartung, T. (2017). Good cell culture practice for stem cells and stem‐cell‐derived models. Altex, 34(1), 95–132. [DOI] [PubMed] [Google Scholar]
- Pamies, D. (2018). Advanced good cell culture practice for human primary, stem cell‐derived and organoid models as well as microphysiological systems. Altex, 35(3), 353–378. [DOI] [PubMed] [Google Scholar]
- Park, S. J. , Kim, J. M. , Kim, J. , Hur, J. , Park, S. , Kim, K. , Shin, H.‐J. , & Chwae, Y.‐J. (2018). Molecular mechanisms of biogenesis of apoptotic exosome‐like vesicles and their roles as damage‐associated molecular patterns. Proceedings of the National Academy of Sciences, 115(50), E11721–E11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, D. J. , Yun, W. S. , Kim, W. C. , Park, J.‐E. , Lee, S. H. , Ha, S. , Choi, J. S. , Key, J. , & Seo, Y. J. (2020). Improvement of stem cell‐derived exosome release efficiency by surface‐modified nanoparticles. Journal of Nanobiotechnology, 18(1), 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, K. Y. , Han, H. S. , Park, J. W. , Kwon, H. H. , Park, G. H. , & Seo, S. J. (2021). Exosomes derived from human adipose tissue‐derived mesenchymal stem cells for the treatment of dupilumab‐related facial redness in patients with atopic dermatitis: A report of two cases. Journal of Cosmetic Dermatology, 21(2), 844–849. [DOI] [PubMed] [Google Scholar]
- Parolini, I. , Federici, C. , Raggi, C. , Lugini, L. , Palleschi, S. , De Milito, A. , Coscia, C. , Iessi, E. , Logozzi, M. , Molinari, A. , Colone, M. , Tatti, M. , Sargiacomo, M. , & Fais, S. (2009). Microenvironmental pH is a key factor for exosome traffic in tumor cells. Journal of Biological Chemistry, 284(49), 34211–34222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, D. B. , Gray, K. M. , Santharam, Y. , Lamichhane, T. N. , Stroka, K. M. , & Jay, S. M. (2017). Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell‐derived extracellular vesicles. Bioengineering and Translational Medicine, 2(2), 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, D. B. , Santoro, M. , Born, L. J. , Fisher, J. P. , & Jay, S. M. (2018). Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: Impact of the bioproduction microenvironment. Biotechnology Advances, 36(8), 2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, D. B. , Luthers, C. R. , Lerman, M. J. , Fisher, J. P. , & Jay, S. M. (2019). Enhanced extracellular vesicle production and ethanol‐mediated vascularization bioactivity via a 3D‐printed scaffold‐perfusion bioreactor system. Acta Biomaterialia, 95, 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payr, S. , Schuseil, T. , Unger, M. , Seeliger, C. , Tiefenboeck, T. , Balmayor, E. R. , & Van Griensven, M. (2020). Effect of donor age and 3D‐cultivation on osteogenic differentiation capacity of adipose‐derived mesenchymal stem cells. Scientific Reports, 10(1), 10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon, I. K. H. , Parkes, M. A. F. , Jiang, L. , Atkin‐Smith, G. K. , Tixeira, R. , Gregory, C. D. , Ozkocak, D. C. , Rutter, S. F. , Caruso, S. , Santavanond, J. P. , Paone, S. , Shi, B. , Hodge, A. L. , Hulett, M. D. , Chow, J. D. Y. , Phan, T. K. , & Baxter, A. A. (2019). Moving beyond size and phosphatidylserine exposure: Evidence for a diversity of apoptotic cell‐derived extracellular vesicles in vitro. Journal of Extracellular Vesicles, 8(1), 1608786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecan, P. , Hambalkó, S. , Ha, V. T. , Nagy, C. T. , Pelyhe, C. , Lainšček, D. , Kenyeres, B. , Brenner, G. B. , Görbe, A. , Kittel, Á. , Barteková, M. , Ferdinandy, P. , Manček‐Keber, M. , & Giricz, Z. (2020). calcium ionophore‐induced extracellular vesicles mediate cytoprotection against simulated ischemia/reperfusion injury in cardiomyocyte‐derived cell lines by inducing heme oxygenase 1. International Journal of Molecular Sciences, 21(20), 7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer, J. , Lund, K. , Goriot, M. E. , Grosbot, M. , Lataillade, J. J. , Mauduit, P. , & Banzet, S. (2020). Interferon‐γ and hypoxia priming have limited effect on the miRNA landscape of human mesenchymal stromal cells‐derived extracellular vesicles. Frontiers in Cell and Developmental Biology, 8(1434), 581436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perut, F. , Roncuzzi, L. , Zini, N. , Massa, A. , & Baldini, N. (2019). Extracellular nanovesicles secreted by human osteosarcoma cells promote angiogenesis. Cancers (Basel), 11(6), 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, C. V. , Midge, S. , Barua, H. , Zhang, Y. , Ngoc‐Gia Nguyen, T. , Barrero, R. A. , Duan, A. , Yin, W. , Jiang, G. , Hou, Y. , Zhou, S. , Wang, Y. , Xie, X. , Tran, H. L. , Xiang, D. , & Duan, W. (2021). Bovine extracellular vesicles contaminate human extracellular vesicles produced in cell culture conditioned medium when ‘exosome‐depleted serum’ is utilised. Archives of Biochemistry and Biophysics, 708, 108963. [DOI] [PubMed] [Google Scholar]
- Phan, J. , Kumar, P. , Hao, D. , Gao, K. , Farmer, D. , & Wang, A. (2018). Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell‐free therapy. Journal of Extracellular Vesicles, 7(1), 1522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupardin, R. , Wolf, M. , & Strunk, D. (2021). Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Advanced Drug Delivery Reviews, 176, 113872. [DOI] [PubMed] [Google Scholar]
- Phan, T. K. , Ozkocak, D. C. , & Poon, I. K. H. (2020). Unleashing the therapeutic potential of apoptotic bodies. Biochemical Society Transactions, 48(5), 2079–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quah, B. J. C. , & O'neill, H. C. (2007). Mycoplasma contaminants present in exosome preparations induce polyclonal B cell responses. Journal of Leukoc Biology, 82(5), 1070–1082. [DOI] [PubMed] [Google Scholar]
- Reid, Y. , Storts, D. , Riss, T. , & Minor, L. (2004). Authentication of human cell lines by STR DNA profiling analysis. In Markossian S. (Ed.), et al., Assay guidance manual. Bethesda (MD). [Google Scholar]
- Revision, U.S.P.C.C.o . (2008). United States Pharmacopeia, the National Formulary. United States Pharmacopeial Convention. Incorporated. [Google Scholar]
- Ren, J. , Wang, H. , Tran, K. , Civini, S. , Jin, P. , Castiello, L. , Feng, J. , Kuznetsov, S. A. , Robey, G. , Sabatino, M. , & Stroncek, D. F. (2015). Human bone marrow stromal cell confluence: Effects on cell characteristics and methods of assessment. Cytotherapy, 17(7), 897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, G. E. , Scholz‐Romero, K. , Sweeney, E. , Peiris, H. , Kobayashi, M. , Duncombe, G. , Mitchell, M. D. , & Salomon, C. (2015). The effect of glucose on the release and bioactivity of exosomes from first trimester trophoblast cells. Journal of Clinical Endocrinology and Metabolism, 100(10), E1280–E1288. [DOI] [PubMed] [Google Scholar]
- Ridger, V. C. , Boulanger, C. M. , Angelillo‐Scherrer, A. , Badimon, L. , Blanc‐Brude, O. , Bochaton‐Piallat, M. L. , Boilard, E. , Buzas, E. I. , Caporali, A. , Dignat‐George, F. , Evans, C. , Lacroix, R. , Lutgens, E. , Ketelhuth, D. F. J. , Nieuwland, R. , Toti, F. , Tunon, J. , Weber, C. , & Hoefer, I. E. (2017). Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb Haemost, 117(7), 1296–1316. [DOI] [PubMed] [Google Scholar]
- Rocha, S. , Carvalho, J. , Oliveira, P. , Voglstaetter, M. , Schvartz, D. , Thomsen, A. R. , Walter, N. , Khanduri, R. , Sanchez, J.‐C. , Keller, A. , Oliveira, C. , & Nazarenko, I. (2019). 3D cellular architecture affects microRNA and protein cargo of extracellular vesicles. Advanced Science (Weinh), 6(4), 1800948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde, E. , Pachler, K. , & Gimona, M. (2019). Manufacturing and characterization of extracellular vesicles from umbilical cord‐derived mesenchymal stromal cells for clinical testing. Cytotherapy, 21(6), 581–592. [DOI] [PubMed] [Google Scholar]
- Rosenberger, L. , Ezquer, M. , Lillo‐Vera, F. , Pedraza, L. , Ortúzar, M. I. , González, L. , Figueroa‐Valdés, A. I. , Cuenca, J. , Ezquer, F. , Khoury, M. , & Alcayaga‐Miranda, F. (2019). Stem cell exosomes inhibit angiogenesis and tumor growth of oral squamous cell carcinoma. Scientific Reports, 9(1), 663–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, H. (2019). Deprivation of glutamine in cell culture reveals its potential for treating cancer. Proceedings of the National Academy of Sciences, 116(14), 6964–6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, A. H. , Eckalbar, W. L. , Kreimer, A. , Yosef, N. , & Ahituv, N. (2017). Use antibiotics in cell culture with caution: Genome‐wide identification of antibiotic‐induced changes in gene expression and regulation. Scientific Reports, 7(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, C. , Kobayashi, M. , Ashman, K. , Sobrevia, L. , Mitchell, M. D. , & Rice, G. E. (2013). Hypoxia‐induced changes in the bioactivity of cytotrophoblast‐derived exosomes. PLoS ONE, 8(11), e79636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, C. , Ryan, J. , Sobrevia, L. , Kobayashi, M. , Ashman, K. , Mitchell, M. , & Rice, G. E. (2013). Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS ONE, 8(7), e68451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, C. , Yee, S. , Scholz‐Romero, K. , Kobayashi, M. , Vaswani, K. , Kvaskoff, D. , Illanes, S. E. , Mitchell, M. D. , & Rice, G. E. (2014). Extravillous trophoblast cells‐derived exosomes promote vascular smooth muscle cell migration. Frontiers in Pharmacology, 5, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammour, I. , Somashekar, S. , Huang, J. , Batlahally, S. , Breton, M. , Valasaki, K. , Khan, A. , Wu, S. , & Young, K. C. (2016). The effect of gender on mesenchymal stem cell (MSC) efficacy in neonatal hyperoxia‐induced lung injury. PLoS ONE, 11(10), e0164269–e0164269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, P. , Redondo, J. , Kemp, K. , Ginty, M. , Wilkins, A. , Scolding, N. J. , & Rice, C. M. (2018). Reduced neuroprotective potential of the mesenchymal stromal cell secretome with ex vivo expansion, age and progressive multiple sclerosis. Cytotherapy, 20(1), 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, S. , Rancourt, A. , Sato, Y. , & Satoh, M. S. (2016). Single‐cell lineage tracking analysis reveals that an established cell line comprises putative cancer stem cells and their heterogeneous progeny. Scientific Reports, 6(1), 23328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina, A. , Furlán, M. , Vidal, M. , & Colombo, M. I. (2003). Exosome release is regulated by a calcium‐dependent mechanism in K562 cells. Journal of Biological Chemistry, 278(22), 20083–20090. [DOI] [PubMed] [Google Scholar]
- Schallmoser, K. , Henschler, R. , Gabriel, C. , Koh, M. B. C. , & Burnouf, T. (2020). Production and quality requirements of human platelet lysate: A position statement from the working party on cellular therapies of the international society of blood transfusion. Trends in Biotechnology, 38(1), 13–23. [DOI] [PubMed] [Google Scholar]
- Segura, E. , Nicco, C. , Lombard, B. , Véron, P. , Raposo, G. , Batteux, F. , Amigorena, S. , & Théry, C. (2005). ICAM‐1 on exosomes from mature dendritic cells is critical for efficient naive T‐cell priming. Blood, 106(1), 216–223. [DOI] [PubMed] [Google Scholar]
- Sekiya, I. , Larson, B. L. , Smith, J. R. , Pochampally, R. , Cui, J.‐G. , & Prockop, D. J. (2002). Expansion of human adult stem cells from bone marrow stroma: Conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells, 20(6), 530–541. [DOI] [PubMed] [Google Scholar]
- Sengupta, V. , Sengupta, S. , Lazo, A. , Woods, P. , Nolan, A. , & Bremer, N. (2020). Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID‐19. Stem Cells and Development, 29(12), 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno, K. , Tanikawa, N. , Takahashi, H. , Ohkuchi, A. , Suzuki, H. , Matsubara, S. , Iwata, H. , Kuwayama, T. , & Shirasuna, K. (2018). Oxygen concentration modulates cellular senescence and autophagy in human trophoblast cells. American Journal of Reproductive Immunology, 79(6), e12826. [DOI] [PubMed] [Google Scholar]
- Shlomovitz, I. , Erlich, Z. , Arad, G. , Edry‐Botzer, L. , Zargarian, S. , Cohen, H. , Manko, T. , Ofir‐Birin, Y. , Cooks, T. , Regev‐Rudzki, N. , & Gerlic, M. (2021). Proteomic analysis of necroptotic extracellular vesicles. Cell Death & Disease, 12(11), 1059–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay, J. W. , & Wright, W. E. (2000). Hayflick, his limit, and cellular ageing. Nature Reviews Molecular Cell Biology, 1(1), 72–76. [DOI] [PubMed] [Google Scholar]
- Shekari, F. , Nazari, A. , Assar Kashani, S. , Hajizadeh‐Saffar, E. , Lim, R. , & Baharvand, H. (2021). Pre‐clinical investigation of mesenchymal stromal cell‐derived extracellular vesicles: A systematic review. Cytotherapy, 23(4), 277–284. [DOI] [PubMed] [Google Scholar]
- Shelke, G. V. , Lässer, C. , Gho, Y. S. , & Lötvall, J. (2014). Importance of exosome depletion protocols to eliminate functional and RNA‐containing extracellular vesicles from fetal bovine serum. Journal of Extracellular Vesicles, 3, 24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, G. , Kluba, T. , Hermanutz‐Klein, U. , Bieback, K. , Northoff, H. , & Schäfer, R. (2013). Phenotype, donor age and gender affect function of human bone marrow‐derived mesenchymal stromal cells. BMC Medicine, 11, 146–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubis, A. , Gola, J. , Sikora, B. , Hybiak, J. , Paul‐Samojedny, M. , Mazurek, U. , & Łos, M. (2017). Impact of antibiotics on the proliferation and differentiation of human adipose‐derived mesenchymal stem cells. International Journal of Molecular Sciences, 18(12), 2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soekmadji, C. , Riches, J. D. , Russell, J. , Ruelcke, J. E. , Mcpherson, S. , Wang, C. , Hovens, C. M. , Corcoran, N. M. , Hill, M. M. , & Nelson, C. C. (2017). Modulation of paracrine signaling by CD9 positive small extracellular vesicles mediates cellular growth of androgen deprived prostate cancer. Oncotarget, 8(32), 52237–52255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulou, A. , Perez, S. A. , Salagianni, M. , Baxevanis, C. N. , & Papamichail, M. (2006). Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells, 24(2), 462–471. [DOI] [PubMed] [Google Scholar]
- Staubach, S. , Bauer, F. N. , Tertel, T. , Börger, V. , Stambouli, O. , Salzig, D. , & Giebel, B. (2021). Scaled preparation of extracellular vesicles from conditioned media. Advanced Drug Delivery Reviews, 177, 113940. [DOI] [PubMed] [Google Scholar]
- Steinman, R. A. , Wentzel, A. , Lu, Y. , Stehle, C. , & Grandis, J. R. (2003). Activation of Stat3 by cell confluence reveals negative regulation of Stat3 by cdk2. Oncogene, 22(23), 3608–3615. [DOI] [PubMed] [Google Scholar]
- Subbiahanadar Chelladurai, K. , Selvan Christyraj, J. D. , Rajagopalan, K. , Yesudhason, B. V. , Venkatachalam, S. , Mohan, M. , Chellathurai Vasantha, N. , & Selvan Christyraj, J. R. S. (2021). Alternative to FBS in animal cell culture—An overview and future perspective. Heliyon, 7(8), e07686–e07686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, S. , Stacey, G. N. , Akazawa, C. , Aoyama, N. , Baptista, R. , Bedford, P. , Bennaceur Griscelli, A. , Chandra, A. , Elwood, N. , Girard, M. , Kawamata, S. , Hanatani, T. , Latsis, T. , Lin, S. , Ludwig, T. E. , Malygina, T. , Mack, A. , Mountford, J. C. , Noggle, S. , … Song, J. (2018). Quality control guidelines for clinical‐grade human induced pluripotent stem cell lines. Regenerative Medicine, 13(7), 859–866. [DOI] [PubMed] [Google Scholar]
- Sun, L. , Zhu, W. , Zhao, P. , Zhang, J. , Lu, Y. , Zhu, Y. , Zhao, W. , Liu, Y. , Chen, Q. , & Zhang, F. (2020). Down‐regulated exosomal microRNA‐221‐3p derived from senescent mesenchymal stem cells impairs heart repair. Frontiers in Cell and Developmental Biology, 8(263), 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter, A. , Rouillard, M. E. , Alshawi, S. A. , & Crocker, S. J. (2021). Extracellular matrix influences astrocytic extracellular vesicle function in wound repair. Brain Research, 1763, 147462. [DOI] [PubMed] [Google Scholar]
- Szvicsek, Z. , Oszvald, Á. , Szabó, L. , Sándor, G. O. , Kelemen, A. , Soós, A. Á. , Pálóczi, K. , Harsányi, L. , Tölgyes, T. , Dede, K. , Bursics, A. , Buzás, E. I. , Zeöld, A. , & Wiener, Z. (2019). Extracellular vesicle release from intestinal organoids is modulated by Apc mutation and other colorectal cancer progression factors. Cellular and Molecular Life Sciences, 76(12), 2463–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, A. , Okada, R. , Nagao, K. , Kawamata, Y. , Hanyu, A. , Yoshimoto, S. , Takasugi, M. , Watanabe, S. , Kanemaki, M. T. , Obuse, C. , & Hara, E. (2017). Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nature Communications, 8, 15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi, M. , Okada, R. , Takahashi, A. , Virya Chen, D. , Watanabe, S. , & Hara, E. (2017). Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nature Communications, 8, 15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J. , Jaiswal, R. , & Bebawy, M. (2017). Calcium‐calpain dependent pathways regulate vesiculation in malignant breast cells. Current Cancer Drug Targets, 17(5), 486–494. [DOI] [PubMed] [Google Scholar]
- Teng, X. , Chen, L. , Chen, W. , Yang, J. , Yang, Z. , & Shen, Z. (2015). Mesenchymal stem cell‐derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti‐inflammation. Cellular Physiology and Biochemistry, 37(6), 2415–2424. [DOI] [PubMed] [Google Scholar]
- The Japanese pharmacopoeia . (1996). 1996: Thirteenth edition. Tokyo : Society of Japanese Pharmacopoeia : Distributed by Yakuji Nippo.
- Théry, C. , Witwer, K. W. , Aikawa, E. , Alcaraz, M. J. , Anderson, J. D. , Andriantsitohaina, R. , Antoniou, A. , Arab, T. , Archer, F. , Atkin‐Smith, G. K. , Ayre, D. C. , Bach, J.‐M. , Bachurski, D. , Baharvand, H. , Balaj, L. , Baldacchino, S. , Bauer, N. N. , Baxter, A. A. , Bebawy, M. , … Zuba‐Surma, E. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7(1), 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thippabhotla, S. , Zhong, C. , & He, M. (2019). 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Scientific Reports, 9(1), 13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom, S. R. , Bhopale, V. M. , Yu, K. , Huang, W. , Kane, M. A. , & Margolis, D. J. (2017). Neutrophil microparticle production and inflammasome activation by hyperglycemia due to cytoskeletal instability. Journal of Biological Chemistry, 292(44), 18312–18324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosar, J. P. , Cayota, A. , Eitan, E. , Halushka, M. K. , & Witwer, K. W. (2017). Ribonucleic artefacts: Are some extracellular RNA discoveries driven by cell culture medium components? Journal of Extracellular Vesicles, 6(1), 1272832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy, S. A. , Ahmed, A. , Tigges, J. C. , Ericsson, M. , Pal, A. K. , Zurakowski, D. , & Fauza, D. O. (2019). A comparison of clinically relevant sources of mesenchymal stem cell‐derived exosomes: Bone marrow and amniotic fluid. Journal of Pediatric Surgery, 54(1), 86–90. [DOI] [PubMed] [Google Scholar]
- Trajkovic, K. , Valdez, C. , Ysselstein, D. , & Krainc, D. (2019). Fluctuations in cell density alter protein markers of multiple cellular compartments, confounding experimental outcomes. PLoS ONE, 14(2), e0211727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry, C. , Boussac, M. , Véron, P. , Ricciardi‐Castagnoli, P. , Raposo, G. , Garin, J. , & Amigorena, S. (2001). Proteomic analysis of dendritic cell‐derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. Journal of Immunology, 166(12), 7309–7318. [DOI] [PubMed] [Google Scholar]
- Torreggiani, E. , Perut, F. , Roncuzzi, L. , Zini, N. , Baglìo, S. , & Baldini, N. (2014). Exosomes: Novel effectors of human platelet lysate activity. European Cells & Materials [Electronic Resource], 28, 137–151. discussion 137. [DOI] [PubMed] [Google Scholar]
- Urzì, O. , Bagge, R. O. , & Crescitelli, R. (2022). The dark side of foetal bovine serum in extracellular vesicle studies. Journal of Extracellular Vesicles, 11(10), 12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal, C. , Shamir, C. , Senthilkumar, S. , Babu, J. V. , Sonu, K. , Nishtha, K. J. , Rai, K. S. , & Dhanushkodi, A. (2017). Dosage and passage dependent neuroprotective effects of exosomes derived from rat bone marrow mesenchymal stem cells: An in vitro analysis. Current Gene Therapy, 17(5), 379–390. [DOI] [PubMed] [Google Scholar]
- Vion, A.‐C. , Ramkhelawon, B. , Loyer, X. , Chironi, G. , Devue, C. , Loirand, G. , Tedgui, A. , Lehoux, S. , & Boulanger, C. M. (2013). Shear stress regulates endothelial microparticle release. Circulation Research, 112(10), 1323–1333. [DOI] [PubMed] [Google Scholar]
- Viswanathan, S. , Shi, Y. , Galipeau, J. , Krampera, M. , Leblanc, K. , Martin, I. , Nolta, J. , Phinney, D. G. , & Sensebe, L. (2019). Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy, 21(10), 1019–1024. [DOI] [PubMed] [Google Scholar]
- Vis, M. A. M. , Ito, K. , & Hofmann, S. (2020). Impact of culture medium on cellular interactions in in vitro co‐culture systems. Frontiers in Bioengineering and Biotechnology, 8, 911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.‐H. , Tao, Y. C. , Wu, D. B. , Wang, M. L. , Tang, H. , & Chen, E. Q. (2021). Cell heterogeneity, rather than the cell storage solution, affects the behavior of mesenchymal stem cells in vitro and in vivo. Stem Cell Research & Therapy, 12(1), 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B. , Shi, Y. , Chen, J. , Shao, Z. , Ni, L. , Lin, Y. , Wu, Y. , Tian, N. , Zhou, Y. , Sun, L. , Wu, A. , Hong, Z. , Wang, X. , & Zhang, X. (2021). High glucose suppresses autophagy through the AMPK pathway while it induces autophagy via oxidative stress in chondrocytes. Cell Death & Disease, 12(6), 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke, A. , Prenzler, N. , Harre, J. , Köhl, U. , Gärtner, L. , Lenarz, T. , Laner‐Plamberger, S. , Wietzorrek, G. , Staecker, H. , Lassacher, T. , Hollerweger, J. , Gimona, M. , & Rohde, E. (2021). First‐in‐human intracochlear application of human stromal cell‐derived extracellular vesicles. Journal of Extracellular Vesicles, 10(8), e12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer, K. W. , & Théry, C. (2019). Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. Journal of Extracellular Vesicles, 8(1), 1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer, K. W. , Van Balkom, B. W. M. , Bruno, S. , Choo, A. , Dominici, M. , Gimona, M. , Hill, A. F. , De Kleijn, D. , Koh, M. , Lai, R. C. , Mitsialis, S. A. , Ortiz, L. A. , Rohde, E. , Asada, T. , Toh, W. S. , Weiss, D. J. , Zheng, L. , Giebel, B. , & Lim, S. K. (2019). Defining mesenchymal stromal cell (MSC)‐derived small extracellular vesicles for therapeutic applications. Journal of Extracellular Vesicles, 8(1), 1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh, J. A. , Van Der Pol, E. , Bettin, B. A. , Carter, D. R. F. , Hendrix, A. N. , Lenassi, M. , Langlois, M.‐A. , Llorente, A. , Van De Nes, A. S. , Nieuwland, R. , Tang, V. , Wang, L. , Witwer, K. W. , & Jones, J. C. (2020). Towards defining reference materials for measuring extracellular vesicle refractive index, epitope abundance, size and concentration. Journal of Extracellular Vesicles, 9(1), 1816641–1816641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford, S. M. , & Giaccia, A. J. (2011). Hypoxia and senescence: The impact of oxygenation on tumor suppression. Molecular Cancer Research, 9(5), 538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, M. , Poupardin, R. W. , Ebner‐Peking, P. , Andrade, A. C. , Blöchl, C. , Obermayer, A. , Gomes, F. G. , Vari, B. , Maeding, N. , Eminger, E. , Binder, H.‐M. , Raninger, A. M. , Hochmann, S. , Brachtl, G. , Spittler, A. , Heuser, T. , Ofir, R. , Huber, C. G. , Aberman, Z. , … Strunk, D. (2022). A functional corona around extracellular vesicles enhances angiogenesis, skin regeneration and immunomodulation. Journal of Extracellular Vesicles, 11(4), e12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, B. , Gao, J. , Li, S. , Huang, L. , Zhu, L. , Ma, T. , Zhao, L. , Yang, Y. , Luo, K. , Xiaowei, Shi, Mei, L. , Zhang, H. , Zheng, Y. , Lu, L. , Luo, Z. , & Huang, J. (2020). Mechanical stimulation of Schwann cells promote peripheral nerve regeneration via extracellular vesicle‐mediated transfer of microRNA 23b‐3p. Theranostics, 10(20), 8974–8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, H. , Tan, J. , Miao, Y. , Lv, Y. , & Zhang, Q. (2021). Crosstalk between exosomes and autophagy: A review of molecular mechanisms and therapies. Journal of Cellular and Molecular Medicine, 25(5), 2297–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Camfield, R. , & Gorski, S. M. (2018). The interplay between exosomes and autophagy–partners in crime. Journal of Cell Science, 131(15), jcs215210. [DOI] [PubMed] [Google Scholar]
- Xue, C. , Shen, Y. , Li, X. , Li, B. , Zhao, S. , Gu, J. , Chen, Y. , Ma, B. , Wei, J. , Han, Q. , & Zhao, R. C. (2018). Exosomes derived from hypoxia‐treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cells and Development, 27(7), 456–465. [DOI] [PubMed] [Google Scholar]
- Yang, C. , Chalasani, G. , Ng, Y.‐H. , & Robbins, D. (2012). Exosomes released from Mycoplasma infected tumor cells activate inhibitory B cells. PLoS ONE, 7(4), e36138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Q. , Nanayakkara, G. K. , Drummer, C. , Sun, Y. , Johnson, C. , Cueto, R. , Fu, H. , Shao, Y. , Wang, L. , Yang, W. Y. , Tang, P. , Liu, L.‐W. , Ge, S. , Zhou, X.‐D. , Khan, M. , Wang, H. , & Yang, X. (2017). Low‐intensity ultrasound‐induced anti‐inflammatory effects are mediated by several new mechanisms including gene induction, immunosuppressor cell promotion, and enhancement of exosome biogenesis and docking. Frontiers in Physiology, 8, 818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerneni, S. S. , Solomon, T. , Smith, J. , & Campbell, G. (2022). Radioiodination of extravesicular surface constituents to study the biocorona, cell trafficking and storage stability of extracellular vesicles. Biochimica et Biophysica Acta General Subjects, 1866(2), 130069. [DOI] [PubMed] [Google Scholar]
- You, K. , Parikh, P. , Khandalavala, K. , Wicher, S. A. , Manlove, L. , Yang, B. , Roesler, A. , Roos, B. B. , Teske, J. J. , Britt, R. D. , Pabelick, C. M. , & Prakash, Y. S. (2019). Moderate hyperoxia induces senescence in developing human lung fibroblasts. American Journal of Physiology. Lung Cellular and Molecular Physiology, 317(5), L525–L536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu, T. , Imanishi, S. , Azuma, K. , Kobayashi, C. , Yoshizawa, S. , Ohyashiki, K. , & Ohyashiki, J. H. (2017). Replenishing exosomes from older bone marrow stromal cells with miR‐340 inhibits myeloma‐related angiogenesis. Blood Advances, 1(13), 812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzì, O. , Moschetti, M. , Lässer, C. , Johansson, J. , D'Arrigo, D. , Bagge, R. O. , & Crescitelli, R. (2023). Heat inactivation of foetal bovine serum causes protein contamination of extracellular vesicles. BioRxiv, 2023.03.01.530627. [Google Scholar]
- Varkouhi, A. K. , Jerkic, M. , Ormesher, L. , Gagnon, S. , Goyal, S. , Rabani, R. , Masterson, C. , Spring, C. , Chen, Z. , Gu, F. X. , Dos Santos, C. C. , Curley, G. F. , & Laffey, J. G. (2019). Extracellular vesicles from interferon‐γ–primed human umbilical cord mesenchymal stromal cells reduce Escherichia coli–induced acute lung injury in rats. Anesthesiology, 130(5), 778–790. [DOI] [PubMed] [Google Scholar]
- Viau, S. , Eap, S. , Chabrand, L. , Lagrange, A. , & Delorme, B. (2019). Viral inactivation of human platelet lysate by gamma irradiation preserves its optimal efficiency in the expansion of human bone marrow mesenchymal stromal cells. Transfusion, 59(3), 1069–1079. [DOI] [PubMed] [Google Scholar]
- Wagner, W. , Horn, P. , Castoldi, M. , Diehlmann, A. , Bork, S. , Saffrich, R. , Benes, V. , Blake, J. , Pfister, S. , Eckstein, V. , & Ho, A. D. (2008). Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS ONE, 3(5), e2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Bonacquisti, E. E. , Brown, A. D. , & Nguyen, J. (2020). Boosting the biogenesis and secretion of mesenchymal stem cell‐derived exosomes. Cells, 9(3), 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchrarat, K. , Korchunjit, W. , Buranasinsup, S. , Taylor, J. , Ritruechai, P. , & Wongtawan, T. (2017). MEM α promotes cell proliferation and expression of bone marrow derived equine mesenchymal stem cell gene markers but depresses differentiation gene markers. Journal of Equine Veterinary Science, 50, 8–14. [Google Scholar]
- Watson, D. C. , Bayik, D. , Srivatsan, A. , Bergamaschi, C. , Valentin, A. , Niu, G. , Bear, J. , Monninger, M. , Sun, M. , Morales‐Kastresana, A. , Jones, J. C. , Felber, B. K. , Chen, X. , Gursel, I. , & Pavlakis, G. N. (2016). Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials, 105, 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer, K. W. , Buzás, E. I. , Bemis, L. T. , Bora, A. , Lässer, C. , Lötvall, J. , Nolte‐‘T Hoen, E. N. , Piper, M. G. , Sivaraman, S. , Skog, J. , Théry, C. , Wauben, M. H. , & Hochberg, F. (2013). Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. Journal of Extracellular Vesicles, 2(1), 20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , Lin, M. , Li, Y. , Zhao, X. , & Yan, F. (2009). Effects of DMEM and RPMI 1640 on the biological behavior of dog periosteum‐derived cells. Cytotechnology, 59(2), 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , Gao, Y. , Xu, L. , Dang, W. , Yan, H. , Zou, D. , Zhu, Z. , Luo, L. , Tian, N. , Wang, X. , Tong, Y. , & Han, Z. (2017). Exosomes from high glucose‐treated glomerular endothelial cells trigger the epithelial‐mesenchymal transition and dysfunction of podocytes. Scientific Reports, 7(1), 9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. , He, D. , & Li, H. (2021). Bioglass enhances the production of exosomes and improves their capability of promoting vascularization. Bioact Mater, 6(3), 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, L. , Zhou, X. P. , Zhu, J. H. , Xie, X. D. , Zhang, H. , Wang, X. X. , Chen, J. Z. , & Jian, S. (2007). Decrease and dysfunction of endothelial progenitor cells in umbilical cord blood with maternal pre‐eclampsia. Journal of Obstetrics and Gynaecology Research, 33(4), 465–474. [DOI] [PubMed] [Google Scholar]
- Xie, M. , Wu, D. , Li, G. , Yang, J. , & Zhang, Y. S. (2021). Exosomes targeted towards applications in regenerative medicine. Nano Select, 2(5), 880–908. [Google Scholar]
- Yan, L. , & Wu, X. (2019). Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow‐fiber bioreactor show improved osteochondral regeneration activity. Cell Biology and Toxicology, 36(2), 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L. , & Wu, X. (2020). Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow‐fiber bioreactor show improved osteochondral regeneration activity. Cell Biology and Toxicology, 36(2), 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, I. K. , Shukla, N. , Borrelli, D. A. , & Patel, T. (2018). Use of a hollow fiber bioreactor to collect extracellular vesicles from cells in culture. Methods in Molecular Biology, 1740, 35–41. [DOI] [PubMed] [Google Scholar]
- Yang, Y.‐H. K. , Ogando, C. R. , Wang See, C. , Chang, T.‐Y. , & Barabino, G. A. (2018). Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Research Therapy, 9(1), 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L. , Zhai, Y. , Hao, Y. , Zhu, Z. , & Cheng, G. (2020). The regulatory functionality of exosomes derived from hUMSCs in 3D culture for alzheimer's disease therapy. Small, 16(3), e1906273. [DOI] [PubMed] [Google Scholar]
- Yang, L. , Zhai, Y. , Hao, Y. , Zhu, Z. , & Cheng, G. (2019). The regulatory functionality of exosomes derived from hUMSCs in 3D culture for Alzheimer's disease therapy. Small, p. e1906273. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Knight, R. , Stephens, P. , & Zhang, Y. (2020). Three‐dimensional culture of oral progenitor cells: Effects on small extracellular vesicles production and proliferative function. Journal of Oral Pathology & Medicine, 49(4), 342–349. [DOI] [PubMed] [Google Scholar]
- Yang, R. , Huang, H. , Cui, S. , Zhou, Y. , Zhang, T. , & Zhou, Y. (2020). IFN‐γ promoted exosomes from mesenchymal stem cells to attenuate colitis via miR‐125a and miR‐125b. Cell Death & Disease, 11(7), 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Q. , Hong, S. , Wang, X. , Cheng, Y. , Sun, J. , & Xia, W. (2019). Regulation of exosomes secretion by low‐intensity pulsed ultrasound in lung cancer cells. Experimental Cell Research, 383(1), 111448. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Zhou, X. , Yao, Q. , Liu, Y. , Zhang, H. , & Dong, Z. (2017). HIF‐1‐mediated production of exosomes during hypoxia is protective in renal tubular cells. American Journal of Physiology. Renal Physiology, 313(4), F906–F913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Fu, L. , Liang, Y. , Guo, Z. , Wang, L. , Ma, C. , & Wang, H. (2018). Exosomes originating from MSCs stimulated with TGF‐β and IFN‐γ promote Treg differentiation. Journal of Cellular Physiology, 233(9), 6832–6840. [DOI] [PubMed] [Google Scholar]
- Zhang, G. , Zhu, Z. , Wang, H. , Yu, Y. , Chen, W. , Waqas, A. , Wang, Y. , & Chen, L. (2020). Exosomes derived from human neural stem cells stimulated by interferon gamma improve therapeutic ability in ischemic stroke model. Journal of Advanced Research, 24, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Wang, N. , Huang, Y. , Li, Y. , Li, G. , Lin, Y. , Atala, A. J. , Hou, J. , & Zhao, W. (2021). Extracellular vesicles from three dimensional culture of human placental mesenchymal stem cells ameliorated renal ischemia/reperfusion injury. The International Journal of Artificial Organs, p. 391398820986809. [DOI] [PubMed] [Google Scholar]
- Zhao, Z. , Qu, L. , Shuang, T. , Wu, S. , Su, Y. , Lu, F. , Wang, D. , Chen, B. , & Hao, Q. (2020). Low‐intensity ultrasound radiation increases exosome yield for efficient drug delivery. Journal of Drug Delivery Science and Technology, 57. [Google Scholar]
- Zhao, Y. , Albrecht, E. , Stange, K. , Li, Z. , Schregel, J. , Sciascia, Q. L. , Metges, C. C. , & Maak, S. (2021). Glutamine supplementation stimulates cell proliferation in skeletal muscle and cultivated myogenic cells of low birth weight piglets. Scientific Reports, 11(1), 13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, C. , Sui, B. , Zhang, X. , Hu, J. , Chen, J. , Liu, J. , Wu, D. , Ye, Q. , Xiang, L. , Qiu, X. , Liu, S. , Deng, Z. , Zhou, J. , Liu, S. , Shi, S. , & Jin, Y. (2021). Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. Journal of Extracellular Vesicles, 10(7), e12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, S. , Fang, J. , Hu, M. , Pan, S. , Liu, D. , Xing, G. , & Liu, Z. (2021). Determining the influence of high glucose on exosomal lncRNAs, mRNAs, circRNAs and miRNAs derived from human renal tubular epithelial cells. Aging (Albany NY), 13(6), 8467–8480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Q.‐J. , Zhu, M. , Xu, X.‐X. , Meng, X.‐M. , & Wu, Y.‐G. (2019). Exosomes from high glucose‐treated macrophages activate glomerular mesangial cells via TGF‐beta1/Smad3 pathway in vivo and in vitro. Faseb Journal, 33(8), 9279–9290. [DOI] [PubMed] [Google Scholar]
- Zhu, D. , Kusuma, G. D. , Schwab, R. , Chan, S. T. , Tan, J. , Saad, M. I. , Leeman, K. T. , Kim, C. , Wallace, E. M. , & Lim, R. (2020). Prematurity negatively affects regenerative properties of human amniotic epithelial cells in the context of lung repair. Clinical Science (London, England: 1979), 134(20), 2665–2679. [DOI] [PubMed] [Google Scholar]
- Zhu, D. , Fang, H. , Kusuma, G. D. , Schwab, R. , Barabadi, M. , Chan, S. T. , Mcdonald, H. , Leong, C. M. , Wallace, E. M. , Greening, D. W. , & Lim, R. (2021). Impact of chemically defined culture media formulations on extracellular vesicle production by amniotic epithelial cells. Proteomics, 21, e2000080. [DOI] [PubMed] [Google Scholar]
- Zou, W. , Lai, M. , Zhang, Y. , Zheng, L. , Xing, Z. , Li, T. , Zou, Z. , Song, Q. , Zhao, X. , Xia, L. , Yang, J. , Liu, A. , Zhang, H. , Cui, Z.‐K. , Jiang, Y. , & Bai, X. (2019). Exosome Release Is Regulated by mTORC1. Advanced Science (Weinh), 6(3), 1801313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


