Abstract
Ebolaviruses and Marburgviruses (family Filoviridae) are among the most virulent pathogens for humans and great apes causing severe haemorrhagic fever and death within a matter of days. This group of viruses is characterized by a linear, non-segmented, single-stranded RNA genome of negative polarity. The overall burden of filovirus infections is minimal and negligible compared to the devastation caused by malnutrition and other infectious diseases prevalent in Africa such as malaria, dengue or tuberculosis. In this paper, we review the knowledge gained on the eco/epidemiology, the pathogenesis and the disease control measures for Marburg and Ebola viruses developed over the last 15 years. The overall progress is promising given the little attention that these pathogen have achieved in the past; however, more is to come over the next decade given the more recent interest in these pathogens as potential public and animal health concerns. Licensing of therapeutic and prophylactic options may be achievable over the next 5–10 years.
1. Introduction
Filoviruses are part of the order Mononegavirales together with the Rhabdoviridae, the Paramyxoviridae and the Bornaviridae families. In this order, viruses are characterized by a lipid envelope and a non-segmented single-stranded RNA (ssRNA) genome of negative polarity [1–3]. Filovirus particles are long filaments shaped in several different forms such as the number ‘6’, the letter ‘U’ or a circle, which give them a rather unique morphology in the viral world (Fig. 1). Viruses of the Ebolavirus, Marburgvirus, and Cuevavirus genera are the only representatives of the Filoviridae family. The Ebolavirus and Marburgvirus genomes are about 19,000 nucleotides long and are transcribed into eight major subgenomic mRNAs. These mRNAs encode seven structural proteins, nucleoprotein (NP), virion protein 35 (VP35), VP40, glycoprotein (GP), VP30, VP24, and RNA-dependent RNA polymerase (L), as well as two nonstructural proteins, soluble (sGP) and small soluble glycoprotein (ssGP) (Fig. 1A–B). Nowadays, seven species of filoviruses have been identified and classified [4]. Specifically, the genus Ebolavirus is composed of five recognized species: Tai Forest ebolavirus (Tai Forest virus, TAFV), Reston ebolavirus (Reston virus, RESTV), Sudan ebolavirus (Sudan virus, SUDV), Zaire ebolavirus (Ebola virus, EBOV), and Bundibugyo virus (Bundibugyio virus, BDBV) (Table 1). The genus Marburgvirus consists of only one species, Marburg margburgvirus, which includes two viruses with approximately 20% genetic divergence: Marburg virus (MARV) and Ravn virus (RAVV). Finally, the genus Cuevavirus is composed of one species called Lloviu cuevavirus (Lloviu virus, LLOV) (Table 1).
Fig. 1.
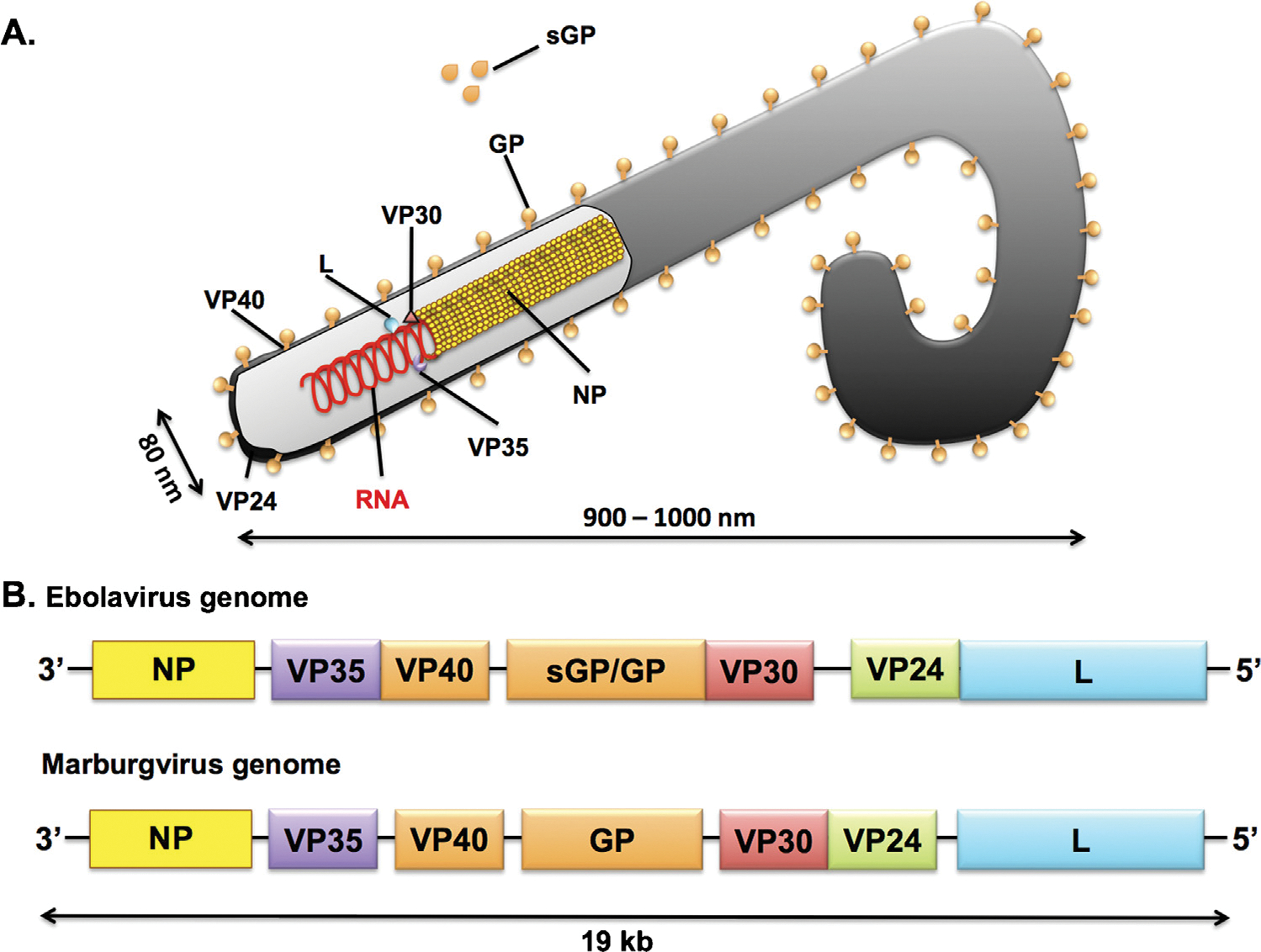
(A) A schematic illustration of a filovirus particle is presented. Four proteins are involved in the formation of the ribonucleoprotein complex: polymerase or large protein (L), nucleoprotein (NP), virion structural protein 30 (VP30), VP35. The glycoprotein (GP) is a type I trans membrane protein and is anchored with the carboxy-terminal part in the virion membrane. The soluble GP (sGP) is a non-structural glycoprotein secreted from infected cells and is only secreted by Ebolaviruses. VP40 and VP24 are membrane-associated proteins. (B) Schematic representation of Ebola virus (EBOV) and Marburg virus (MARV) genomes.
Table 1.
The country of origin, infected hosts, number of cases reported, and case fatality rates are presented.
| Family | Genus | Species (virus) | Country | Infected hosts | Human reported cases | Case mortality (%) |
|---|---|---|---|---|---|---|
|
| ||||||
| Filoviridae | Ebolavirus | Tai forest ebolavirus (TAFV) | Ivory coast | Human | 1 | 100 |
| - | - | Reston ebolavirus (RESTV) | Philippines | Non-human primates, bats and pigs | 141 | 0 |
| - | - | Sudan ebolavirus (SUDV) | Sudan, Uganda | Human and non-human primates | 761 | 42–65 |
| Zaire ebolavirus (EBOV) | Democratic Republic of Congo, Republic of Congo, Gabon, Guiena, Sierra Leonne, Liberia | Human, non-human primates and bats | 23541 | 40–90 | ||
| - | - | Bundibugyo virus (BDBV) | Uganda | Human and non-human primates | 149 | 25 |
| - | Marburgvirus | Marburg margburgvirus | Uganda, Republic Democratic of Congo, Angola, Gabon | Human, non-human primates and bats | 594 | 23–88 |
| - | Cuevavirus | Lloviu cuevavirus (LLOV) | Spain | Insectivorous bats | NA | NA |
Among the seven species of filoviruses, four (SUDV, EBOV, BDBV and MARV) are responsible for fatal outbreaks of haemorrhagic fever in Africa, for which there is no effective treatment. These viruses are also responsible for several outbreaks that contributed to ecological disasters in terms of population density decreases of chimpanzees and gorillas, especially in Gabon, Uganda, and the Republic of Congo. The case fatality in human populations due to EBOV is the highest, with about 90% [2,5]. The other species have lower case fatality rates, with 42–65% for SUDV [6] and between 34% and 44% for BDBV [7–9]. Marburgvirus infections lead to death in about 24–88% of cases depending on the outbreak [10,11]. Only one other species, for which unknown virulence has been observed, has been reported in Africa, the TAFV. Indeed, this TAFV has only been responsible of one non-fatal human case [12]. The two other species not responsible for outbreaks in human populations have been identified in Asia and Europe. RESTV, originating from the Philippines, has been responsible for infections and death in nonhuman primates. Finally, more recently LLOV, an Ebola-like filovirus, has been identified in insectivorous bats in Spain [13]. In contrast to MARV and EBOV that have been reported to asymptomically circulate in bats (evolution through avirulence), several observations suggested that LLOV might be pathogenic forbats [13].
Over the past 45 years, the global public health burden of filoviruses has been limited despite their high virulence and impact on African ecosystems. The extreme virulence and the communicable nature of these viruses together with the lack of countermeasures made them biothreat pathogens in the post-Cold war era [14–16]. Since 15 years, research in biology, ecology, evolution, epidemiology and pathophysiology has remarkably advanced leading to a better understanding of virus biology and development of therapeutic/antiviral strategies and candidate vaccines [17–22]. In this paper, we review achievements in the eco-epidemiology (outbreak history and description), pathogenesis, diagnostic, prophylaxis, and therapy.
2. Filovirus eco-epidemiology
2.1. Advances in eco-epidemiology, outbreaks history
Marburgviruses and ebolaviruses have caused only a few documented outbreaks since their discovery 45 years ago. Until 2014, outbreaks have been responsible for 2989 clinical cases of which 2068 have died (Tables 1 and 2, and Fig. 2). The overall burden of filoviruses is low in comparison to others diseases such as malaria or malnutrition.
Table 2.
Filoviruses outbreaks including date, location, source of infection, number of clinical cases, and case fatality rates since discovery in 1967.
| Genus | Species (virus) | Date | Country | Number of cases | Number of deaths | Fatality (%) |
|---|---|---|---|---|---|---|
|
| ||||||
| Ebolavirus | Zaire ebolavirus (EBOV) | 1976 | Democratic Republic of Congo | 318 | 280 | 88 |
| - | - | 1977 | Democratic Republic of Congo | 1 | 1 | 100 |
| - | - | 1994 | Gabon | 52 | 31 | 60 |
| - | - | 1995 | Democratic Republic of Congo | 315 | 254 | 82 |
| - | - | 1996–1997 | Gabon | 91 | 66 | 73 |
| - | - | South Africa (ex-Gabon) | 1 | 1 | 100 | |
| - | - | 2001–2002 | Gabon | 65 | 53 | 82 |
| - | - | Congo | 59 | 44 | 75 | |
| - | - | 2003 | Congo (Nov-Dec) | 35 | 29 | 83 |
| Congo (Jan-Apr) | 143 | 128 | 90 | |||
| - | - | 2005 | Congo | 12 | 10 | 83 |
| - | - | 2007 | Democratic Republic of Congo | 264 | 187 | 71 |
| - | - | 2008 | Democratic Republic of Congo | 32 | 14 | 44 |
| - | - | 2011 | Uganda | 1 | 1 | 100 |
| - | - | 2012 | Uganda | 7 | 4 | 57 |
| - | - | 2014 | Liberia, Sierra Leonne, Guinea | 22057 | 8795 | 40 |
| - | Sudan ebolavirus (SUDV) | 1976 | Sudan | 284 | 151 | 53 |
| - | - | 1979 | Sudan | 34 | 22 | 65 |
| - | - | 2000 | Uganda | 425 | 224 | 53 |
| - | - | 2004 | Sudan | 17 | 7 | 42 |
| - | - | 2011 | Sudan | 1 | 1 | 100 |
| - | Tai Forest ebolavirus (TAFV) | 1994 | Ivory Coast | 1 | 0 | 0 |
| - | Bundibugyo virus (BDBV) | 2007 | Uganda | 149 | 37 | 25 |
| Marburgvirus | Marburg marburgvirus (MARV) | 1967 | Germany-Yugoslavia | 31 | 7 | 23 |
| - | - | 1975 | South Africa | 3 | 1 | 33 |
| - | - | 1980 | Kenya | 2 | 1 | 50 |
| - | - | 1987 | Kenya | 1 | 1 | 100 |
| - | - | 1998–2000 | Democratic Republic of Congo | 154 | 128 | 83 |
| - | - | 2005 | Angola | 374 | 329 | 88 |
| - | - | 2007 | Uganda | 4 | 2 | 20 |
| - | - | 2008 | Uganda | 2 | 1 | 50 |
| - | - | 2012 | Uganda | 23 | 15 | 65 |
Fig. 2.
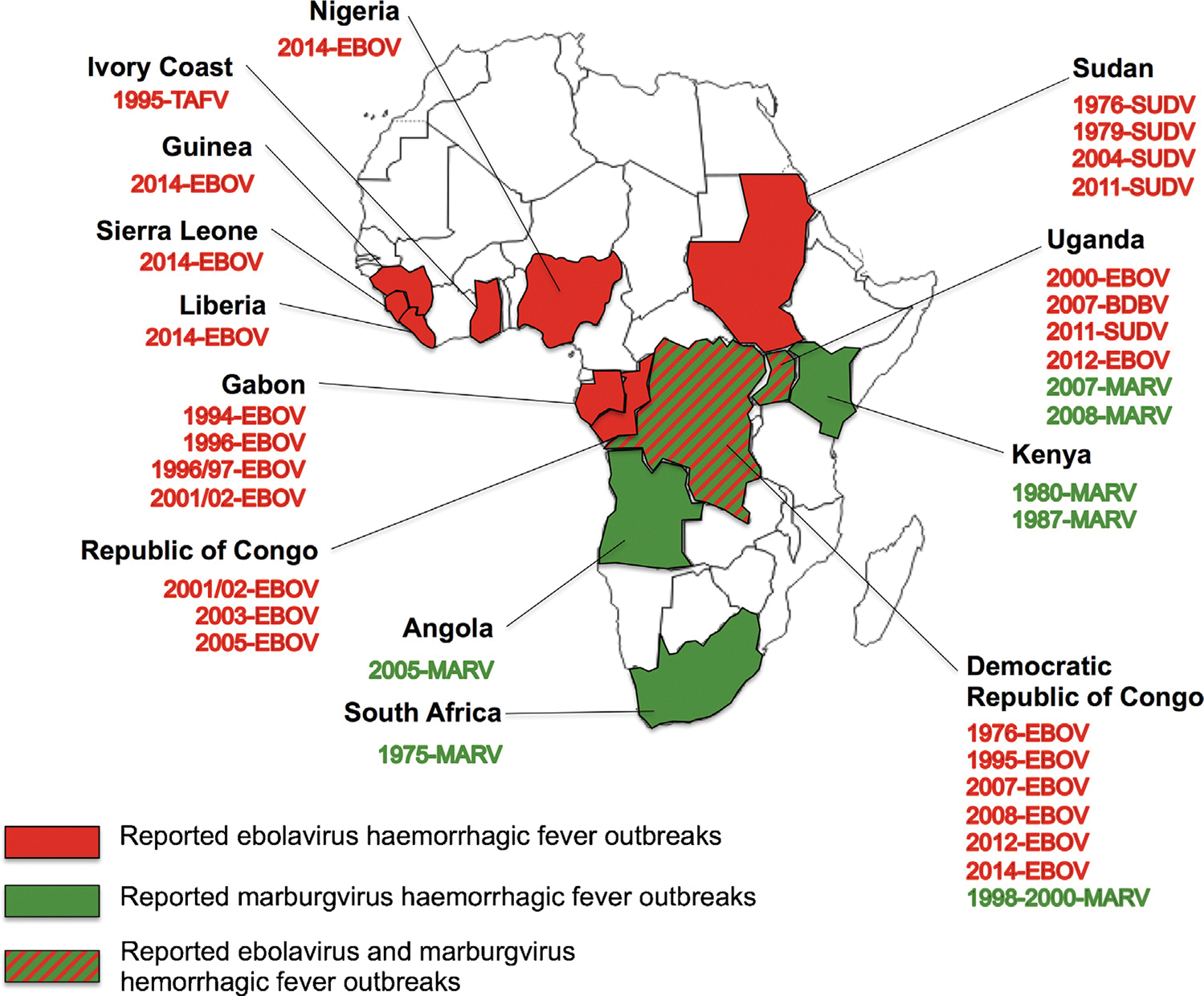
Reported outbreaks or isolated cases of haemorrhagic fever caused by marburgviruses (MARV, represented in green) and Ebolaviruses (EBOV, TAFV, SUDV, and BDBV, represented in red) in Africa.
2.1.2. Marburgviruses outbreaks
The first outbreak of filoviral haemorrhagic fever happened in 1967 simultaneous in Germany (Marburg and Frankfurt) and Yugoslavia (Belgrade). A total of 32 laboratory workers got infected after contact with tissue and blood from African green monkeys (Cercopithecus aethiops) imported from Uganda; seven of these cases were fatal [23–26]. Since the discovery of this new virus, named Marburg virus, only a few sporadic incidences with low numbers of infected persons have been reported: three in South Africa in 1975, two and then one in Kenya in 1980 and 1987 (Table 2). As the case fatalities of these outbreaks/incidences were lower than those observed for EBOV outbreaks, MARV was thought to be less threatening. However, following a decade with no reported cases, MARV was responsible of two large outbreaks characterized by high fatality rates: one in 1998–2000 in DRC (Durba), which was connected to people working in underground gold mines (128 deaths; case fatality rate 83%) [27], and the largest one in 2004–2005 in Angola (Uige province, 329 deaths; case fatality rate 88%) [11] (Table 2). Since these two dramatic episodes, six clinical cases have been recorded among workers in lead and gold mines in Uganda between 2007 and 2008. The last outbreak occurred in 2012, in southwestern Uganda and led to 23 clinical cases and 15 deaths (65% case fatality rate).
2.1.3. Ebolaviruses outbreaks
In 1976, the first ebolaviruses were discovered during two nearby simultaneous outbreaks in Sub-Saharan Africa [28–30]. One outbreak occurred in Sudan with a case fatality rate of 53% (Table 2). The Ebolavirus species responsible of this first outbreak has been identified and named Sudan ebolavirus (SUDV). The other outbreak occurred in DRC and was caused by the species EBOV resulting in a case fatality rate of 88% (Table 2). These two filoviruses were isolated from patients in both countries and have been shown to be morphologically similar to but serologically distinct from MARV [28–31]. In 1979, SUDV re-emerged in Sudan with a case fatality rate of 64.7%. Until 1994, no cases were reported over 15 years.
From 1994 to 1997, EBOV re-emerged three times in northern Gabon and once in DRC. It first occurred in 1995, in and around the town of Kikwit, south DRC, with a case fatality rate of 81% [32]. Despite the deployment of sophisticated scientific and medical resources, this outbreak was similar in terms of size as the one in 1976, in Yambuku [33]. In Gabon, the first episode occurred in 1994 in gold-mining camps located in the heart of the forest (Mekouka outbreak). The second outbreak occurred in 1996 in the village of Mayibout and affected mainly children, who carried and butchered a chimpanzee carcass discovered in the forest. The last outbreak occurred in 1997 in Booue. Imported cases have been reported from Libreville and South Africa [34–36]. The same period marked the discovery and isolation of the third Ebola species. TAFV was isolated from a surviving ethnologist, who got infected by necropsying a dead chimpanzee found in the Tai Forest National Park, Ivory Coast [37,38] (Fig. 2). After three years without any cases, the largest Ebola outbreak occurred in 2000 in Uganda caused by SUDV [39,40]. From 2001 to 2005, four EBOV outbreaks hit Gabon, RC and DRC, and two SUDV episodes occurred in Uganda and Sudan. An EBOV episode occurred in 2001–2002 along the border between northeast Gabon and northeast RC. In 2003, RC was affected twice by EBOV and once in 2005. Concerning SUDV outbreaks, the largest occurred in Uganda in 2000 in three different foci, causing 173 deaths and another one happened in Sudan with 7 fatal cases reported. The latest Ebola species was discovered in 2007 in Uganda. Bundibugyo Ebola virus (BDBV) has been responsible for a large outbreak causing 116 cases and 30 deaths [41] (Fig. 2). In 2011, two sporadic fatal cases have been reported, one SUDV infection in Sudan and one EBOV infection in Uganda [42]. In 2012 and 2013, three outbreaks caused by SUDV occurred: two in Uganda causing 18 cases and 8 deaths and one in DRC leading to 77 cases and 34 deaths (Table 2). Finally, beginning 2014, Guinea has been touched for the first time in its history by an outbreak caused by EBOV. In January 2015, this unprecedented epidemic, characterized by a magnitude never observed before across three districts, killed 1910 out of 2917 patients. Other cases imported from people who travelled in Guinea weeks ago, have then been reported in Sierra Leonne and Liberia. Within the next months, the outbreak spread rapidly and caused 19140 clinical cases and 6885 deaths (WHO report of the 27th of January 2015).
3. Filovirus infection clinical outcome, pathogenesis and immune responses
3.1. Advances in basic virology, mechanisms of pathogenesis
Even if the scientific advances considerably increased our knowledge of filovirus, we are still far from understanding clearly the pathogenesis and immune responses of infections with these pathogens. The four pathogenic filovirus species are known to cause the most severe haemorrhagic fever syndromes in human and non-human primates, with case fatality rates in humans of up to 90% [8,11,25,26,30,32,43,44] (Table 2). Following an incubation period of 2 to 21 days (mean of 4–9 days), human EBOV and MARV infections show an abrupt disease onset with high fever finally resulting in haemorrhages and multi-organ failure. The disease appears first with non-specific symptoms such as fever, headache, nausea, and muscle pain. This is rapidly followed in fatal cases with multi-organ involvement including gastrointestinal problems (stomach pain, anorexia, vomiting, nausea), respiratory symptoms (throat and chest pain, shortness of breath, cough,) and neurological manifestations (prostration, confusion, delirium, seizure). Haemorrhagic manifestations vary in severity and location, and appear only in 1/3 of patients during the peak of the illness. This fatal stage is often associated with skin rash, nose bleeding, melena, hematemesis, and bleeding at venipuncture sites. Patients die in the context of tachypnea, coma, convulsions, severe metabolic disturbance and shock. In non-fatal disease, fever is present for about 5 to 9 days. Improvements appear rapidly and coincide with the detection of an antibody response (days 7–11) resulting in clearance of viremia. Prolonged convalescence is frequent with episodic non-specific symptoms such as asthenia, myalgia, and fever. More specific signs associated with transient persistence of the virus in immune privileged sites, such as the eyes and testicles, have been reported [43,45].
Disease is associated with striking virus replication and immune and vascular dysregulation. Exposure/infection occurs through close contact with skin and secretory products from infected animals/humans (Fig. 3). Subsequently, virus particles enter the body through lymphatic and/or blood vessels. Dendritic cells, macrophages and monocytes are the first cells infected by filoviruses [18,46–48]. Because of their extensive distribution among the different organs and tissues, and their migratory capacity after activation through blood and lymphatic systems, dendritic cells and macrophages are probably responsible for viral dissemination and systemic infection [18]. After the infection and replication in primary target cells, viral particles spread to hepatocytes, endothelial cells, fibroblasts and epithelial cells [48,49]. Intensive viral replication then occurs in secondary lymphoid organs, spleen, and liver [1].
Fig. 3.
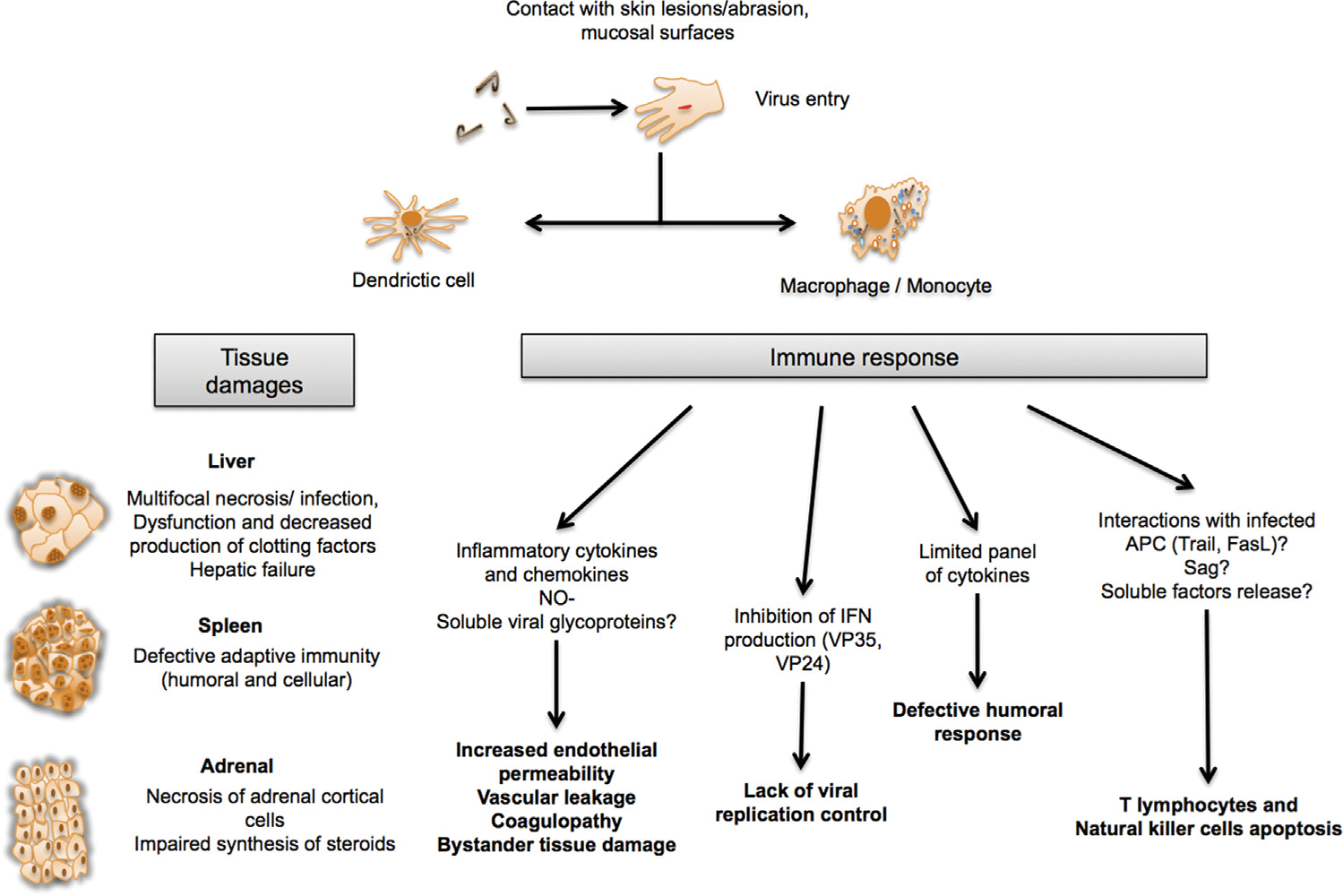
Pathogenesis model, based on findings with EBOV (Zaire ebolavirus). Viruses enter the body through lymphatic and/or blood vessels. Dendritic cells and macrophages are the first cells to be infected by filoviruses. The virus spreads from the initial site of infection to secondary lymphoid organs and liver where intense replication takes place in other cells of the host organisms (hepatocytes, endothelial cells, fibroblasts and epithelial cells). The extensive infection of antigen-presenting cells (APC) leads to altered inflammatory responses and uncontrolled release of mediators. This contributes to the pathogenesis by attracting inflammatory responses followed by further and uncontrolled release of mediators.
Fatal filovirus infections are characterized by a dysregulation of the innate immune response and the adaptive immunity (Fig. 3). First, infected macrophages and monocytes lead to aberrant release of soluble inflammatory mediators such as MIP-1α and MCP-1. These cytokines are known to be involved in the recruitment of additional macrophages to infected areas. Infected macrophages and monocytes also lead to an increase of Tumor Necrosis Factor-α (TNF-α), interleukin (IL)-1β, IL-6, IL-8, IL-15, IL-16, IL-1 receptor antagonist, soluble TNF receptor, IL10, NO-, growth regulated oncogene-α, CCL3, CCL4, CXCL10, monocyte chemotactic protein-1, and eotaxin [1,35,50–56]. This systemic immune dysregulation (cytokine storm) is particularly prominent in the terminal stages of the infection resulting in three major biological consequences: (i) vascular leakage mediated through TNF-α, NO and other vasoactive compounds that increase endothelial permeability, decrease of vascular tone and altered endothelial cell functions [1,57–59]; (ii) bystander tissue damage mediated through MCP-1 and IL-8 mediators that induce the expression of adhesion molecules at the surface of endothelial cells and thus allow the invasion of infection sites by neutrophils and monocytes [60]; (iii) finally, disseminated intravascular coagulation induced through infected macrophages expressing abundant tissue factor [52,56,58–64] (Fig. 3). In addition, the massive infection of antigen-presenting cells results in lymphoid depletion in spleen, lymph node and thymus as well as T lymphocytes and natural killer cells apoptosis [18,52,56,59,61,62] (Fig. 3). Since lymphocytes are not infected by EBOV, T cell death could not be linked to a direct viral cytopathic effect, but would rather result from interactions with infected antigen-presenting cells and soluble mediators (such as Fas/FasL, TNF/TRAIL) and/or from apoptotic soluble mediators and/or from superantigenic activity of viral proteins [18,52,56,63]. The early infection of dendritic cells, the increased expression of TNF-related apoptosis-inducing ligand TRAIL and bystander apoptosis of lymphocytes could explain the observation of lymphoid apoplasia and the absence of detectable adaptive immune responses in fatal infections [18,46,64,65]. This is in line with the lack of specific IgG and barely detectable IgM in fatal cases [33,52]. Specifically, it has been shown that infected dendritic cells produce only a limited panel of cytokines without full activation and maturation, which may have an impact on their ability to trigger adaptive immunity [18,46,64,65]. Furthermore, specific filoviral proteins have the ability to neutralize innate immune responses by counteracting cellular antiviral defenses (Fig. 3). Specifically, VP35 blocks IFN-α/β synthesis by preventing the activation of interferon regulatory factors (IFR) IFR-3 and IFR-7 [66–69]. VP35 interferes with the dsRNA- dependent protein kinase activation [70], and VP30 and VP40 are suppressors of RNA silencing [71]. Moreover, VP24 inhibits the nuclear accumulation of the tyrosine-phosphorylated signal transducer and activator of transcription I and thereby neutralizes IFN-α/β and IFN-γ signaling [72,73]. In this context, the inhibition of IFN-1 synthesis seems to be a crucial element in EBOV virulence. Finally, the glycoprotein GP seems to be involved in immune suppression by inactivation of neutrophils [74–76] and in the activation of endothelial cells [18,77]. Furthermore, full length GP synthesis was associated with the induction of cytotoxic effects in human endothelial cells [78].
In opposite, non-fatal and asymptomatic infections are associated with an early and moderate inflammatory response [53,61]. This response probably assures the control of the infection and leads to specific immunity. The survival of patients is strongly associated with an early humoral immune response [52]. T-cell responses seem to be crucial for controlling the infection. Asymptomatic individuals are characterized by a lack of T cell apoptosis and by the circulation of activated T cells probably including cytotoxic T lymphocytes [55]. This has been linked with decreased viremia and recovery from clinical symptoms [52].
The extremely different outcomes of EBOV infections are not well understood and may depend on the route of infection, the dose of infection, the cell types initially infected, potentially previously acquired immunity, heterologous immunity or MHC status [56,63,79]. Studies showed no genetic differences in the coding sequences of virus isolates from survivors, fatal cases or cases characterized by different clinical symptoms [34,80] suggesting a limited role of genetic variation on outcome of infection.
4. Diagnostic tests for filoviruses
4.1. Advances in clinical virology: advances in diagnostics and bedside tests
Since rapid confirmation of a filovirus infection through laboratory diagnosis is fundamental, fast, sensitive and specific tests have been developed. Moreover, confirmatory tests are recommended to avoid any misdiagnosis and any potential critical social consequences of it. Blood and tissue samples suspected for filoviruses infections must be handled in biosafety level 4 laboratory.
Laboratory diagnosis is separated into direct and indirect detection assays. Among the direct detection assays, five techniques are commonly used: (i) the most widely applied test is based on reverse transcription polymerase chain reaction (RT-PCR) assay, which allows the detection of viral RNA in tissues and fluids. This technique has been adapted to the field [44]. Initial conventional RT-PCR assays needed confirmation by sequencing of PCR amplicons. Nowadays, real time RT-PCR assays have been implemented for filoviruses detection with species identification [81] and are superior due to their higher sensitivity and rapid acquisition of results; (ii) The detection of viral antigen by enzyme linked immunosorbent assay (ELISA) is still used as the primary diagnostic assay for EBOV infections and the most widely used confirmatory assay. This test is reasonably sensitive, highly specific and could be easily applied during the viremic stage of the infection; (iii) the third way to identify filoviruses is by virus isolation, but it requires several days for tissue culture and complementary tests for species identification. Inoculation is more efficient from blood or liver samples than from mucosal swabs or other body fluids. The most commonly used cell lines for virus isolation and propagation of filoviruses are Vero or Vero E6 cells (Cercopithecus aethiops, African green monkey kidney). It has to be noticed that the isolation of SUDV and RESTV needs several passages in cell cultures [31]; (iv) viral particles can be demonstrated by electron microscopic analysis of blood, tissue and culture supernatants [82,83]. Filovirus particles are easy to identify because of their appearance as long filamentous particles up to 10,000 nm (in average); the diameter of the particles is uniform with 80 nm (Fig. 1). Further technologies have to be applied to confirm a positive EM diagnosis; (v) finally, immunohistochemistry allows the detection of filoviral antigens in clinical material using specific antibodies [84].
Indirect technics are based on antibody detection and their application is limited to late stages of infection or convalescence: (i) indirect immunofluorescence assay (IFA) has been the test of choice in the past for epidemiological and seroprevalence studies [85,86]. New IFI tests have been developed based on recombinant expressed antigens that seem to be more sensitive and specific than the original IFI using inactivated infected cell lysates [87]; (ii) nowadays antibody detection methods are based on ELISA technology. Quantification of IgM by immunocapture ELISA technology is applied to identify suspect cases in acute infection and early convalescence. IgM antibodies appear as early as two to four days after onset of symptoms, are never very high and usually undetectable 2 months after infection. In Gabon, studies showed that one third of fatal cases presented specific IgM antibodies [52,54,55]; (iii) the detection of IgG antibodies is done using classic ELISA technology. This tests showed that IgG antibodies appear around 8 to 10 days after onset of symptoms and are still detectable 2 years after infection [88,89]. It has to be noticed that IgG antibodies are rarely observed in fatal cases. Thus, IgG ELISA should only be applied for diagnostics in late stage infections or more commonly during or following convalescence.
Currently, three main methods are used during outbreak investigations: (i) RT-PCR for rapid and sensitive detection of viral nucleic acid (RNA); (ii) antigen capture ELISA for the detection of viral antigens during acute phase of the infection; (iii) and antibody ELISA for identification of IgM and IgG responses during infection and convalescence, respectively.
5. Treatment and prevention
5.1. Advances in prophylaxis and therapy: vaccine and antivirals
Currently no specific therapy exists for filovirus infections [90,91]. Supportive care is adopted consisting of rehydration, nutritional supplementation and psychosocial support [92]. Intravenous fluid replacement to maintain blood volume, blood pressure and electrolyte balance, as well as analgesics and standard barrier nursing are critical. In the past, convalescent serums, extracorporal blood treatment with haemosorbent and dialysis, and IFN were used to treat human filovirus cases, but their efficacy is uncertain [93,94]. Recently, passive transfer of polyclonal and monoclonal antibodies showed efficacy in rodents, guinea pigs [76,95,96] and macaques models [97,98] and are promising avenues for future application. One promising approach is to manipulate the coagulation system by inhibiting the tissue factor pathway with a factor VIIa/tissue factor inhibitor [18] or by activating the natural coagulant protein C pathway with recombinant activated protein C [99]. However, the survival benefit is still limited. A last approach would be to target viral replication with small interfering RNA or antisense oligonucleotides [20,100].
Considering vaccination against Ebola and Marburg viruses, numerous approaches have been taken (Table 3); however, no licensed vaccine is currently available. The main challenge is to develop a vaccine conferring cross-protection against heterologous virus species, including emerging strains and ideally after a single dose injection. Several vaccine candidates have been tested and showed good efficiency in non-human primates over the past ten years. The use of virus-like particles to produce EBOV GP as the immunogen is a promising approach, providing largely sterilizing immunity after a single dose [101–105]. The first breakthrough came in 2000 and was based on a boost of DNA and an adenovirus vector vaccine, which showed 80% protection against EBOV infection in macaques [106]. One limitation of the adenovirus platform is pre-existing immunity in the human population. These vectors do not replicate in target cells and to induce protective immunity high doses for vaccination are required [107]. To address this issue, a new adenoviral vector, characterized by a higher expression rate of the immunogen through codon-optimization, has been tested in the mouse model and revealed protection using 100 times lower doses [108]. Rhabdovirus vector vaccines offered a very good platform to generate specific immunity as well as cross-protective immunity for both filoviruses and rhabdoviruses [109,110]. Attenuated recombinant vesicular stomatitis virus (rVSV) vectors expressing the filovirus glycoprotein instead of the VSV glycoprotein have shown promising results [103]. These vectors conferred complete protection to macaques infected with homologous virus strains and partially also against challenge with heterologous strains [102,111,112]. This candidate vaccine confers substantial post-exposure protection when administrated very rapidly to non-human primates [111,112]. The major advantages of these vector-based vaccine platforms are the strong immune response to a replication-competent attenuated vector and very limited pre-existing immunity against VSV in the human population [113]. Paramyxovirus-based vaccine vectors expressing filovirus glycoproteins have also shown promising results. A human parainfluenza virus type 3, (HPIV3) based vector expressing the EBOV GP administered as an aerosol resulted in complete protection against EBOV challenge of non-human primates [114]. Even in primates with pre-existing immunity against HPIV3, this vector still replicated and induced immune response [114]. However, even if this vaccine vector could theoretically protect populations at 100%, the live-attenuated nature of this vector may constitute a safety risk that needs to be further evaluated. One solution would be to use of paramyxovirus vectors that are only known as animal pathogens or show less pathogenicity for humans. Along these lines, a new vector has been designed based on the Newcastle disease virus (NDV), an avian paramyxovirus that infects the respiratory tract of birds. Tests, based on the administration of recombinant (by insertion of a gene cassette encoding the hemagglutinin neuraminidase protein of HPIV3) version of NDV in non-human primates, revealed that NDV was highly attenuated due to host restriction processes [114]. This makes of NDV a promising vector for the development of vaccines for emerging human diseases. In this context, vaccine efficacy tests with EBOV challenge in non-human primates would be interesting to be performed. Finally, virus-like particles (VLPs) are a safe alternative platform for vaccination. VLPs are non-replicating particles that carry certain filoviral immunogens but lack filoviral genetic material and thus are replication-deficient. VLP based vaccination approaches have been evaluated for immunogenicity and protective efficacy in rodent as well as non-human primate models against homologous EBOV challenge and even heterologous challenge with other Ebola species [105,115].
Table 3.
Promising vaccines candidates effective in non-human primates. The name and the type of vaccine, as well as the protective efficacies are shown.
| Vaccine | Type | Species tested | Efficiency |
References | ||||
|---|---|---|---|---|---|---|---|---|
| EBOV | MARV | SUDV | TAFV | BDBV | ||||
|
| ||||||||
| DNA vaccine | DNA type | Macaques | 100% | 100% | 100% | - | - | Swenson et al., [119] |
| rVSV-GP | Rhabdovbirus-based | Macaques | 100% | 100% | 100% | 100% | 75% | Geisbert et al., [112] |
| HPIV3 | Paramyxovirus-based | Macaques | 100% | - | - | - | - | Bukreyev et al., [120] |
| VLP | Non-replicative virus particule | Macaques | 100% | 100% | - | - | - | Warfield et al., [121] |
Overall, there has been quite some advance in vaccine development for filoviruses over the past 10 years. However, several concerns must be dealt with prior to human use, such as safety of viral vectors, possible pre-existing immunity against certain vectors and formulation of a single-dose cross-protective vaccine [63].
Today, the only way to limit transmission during outbreaks is patient isolation and basic supportive therapy. The lack of countermeasures against filovirus infections is not only due to the difficulties to manipulate filoviruses in biosafety level 4 facilities, but also to the lack of interest by industry because of a missing market due to limited number of affected people. The more recent increased frequency of EBOV outbreaks [116–118], the discovery of ebolavirus in a livestock species, the recent discovery of several new filoviruses (LLOV and BDBV) [12,13,44] and the biothreat potential presented by filoviruses have increased the attention to these viruses and will drive research into countermeasure development in the near future.
Supplementary Material
Funding
We thank the Gabonese Government, Total Gabon, the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 593 and 1021 (Germany) and the Intramural Research Program of NIAID, NIH (U.S.A) for financial support.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2015.01.014.
References
- [1].Feldmann H, Klenk HD, Marburg and Ebola viruses, Adv. Virus Res. 47 (1996) 1–52. [DOI] [PubMed] [Google Scholar]
- [2].Sanchez A, Geisbert TW, Feldmann H, in: Knipe DM, Howley PM (Eds.), Filoviruses, Lippincott Williams, Wilkins, Philadelphia, 2007, pp. 1409–1448. [Google Scholar]
- [3].Feldmann H, Geisbert TW, Ebola haemorrhagic fever, Lancet 377 (2011) 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kuhn JH, Radoshitzky SR, Bavari S, Jahrling PB, The international code of virus classification and nomenclature (ICVCN): proposal to delete Rule 3.41, Arch. Virol. 158 (2013) 297–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hoenen T, Groseth A, Falzarano D, Feldmann H, Ebola virus: unravelling pathogenesis to combat a deadly disease, Trends Mol. Med. 12 (2006) 206–215. [DOI] [PubMed] [Google Scholar]
- [6].Baron RC, McCormick JB, Zubeir OA, Ebola virus disease in southern Sudan: hospital dissemination and intrafamilial spread, Bull. World Health Org. 61 (1983) 997–1003. [PMC free article] [PubMed] [Google Scholar]
- [7].MacNeil A, Farnon EC, Wamala J, Okware S, Cannon DL, Reed Z, Proportion of deaths and clinical features in Bundibugyo Ebola virus infection, Uganda, Emerg. Infect. Dis. 16 (2010) 1969–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Towner JS, Sealy TK, Khristova ML, Albariño CG, Conlan S, Reeder SA, Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda, PLoS Pathog. 4 (2008) e1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wamala JF, Lukwago L, Malimbo M, Nguku P, Yoti Z, Musenero M, Ebola hemorrhagic fever associated with novel virus strain, Uganda, 2007–2008, Emerg. Infect. Dis. 16 (2010) 1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bausch DG, Borchert M, Grein T, Roth C, Swanepoel R, Libande ML, Risk factors for Marburg hemorrhagic fever, Democratic Republic of the Congo, Emerg. Infect. Dis. 9 (2003) 1531–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Towner JS, Khristova ML, Sealy TK, Vincent MJ, Erickson BR, Bawiec DA, Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola, J. Virol. 80 (2006) 6497–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Le Guenno B, Formenty P, Formentry P, Wyers M, Gounon P, Walker F, Isolation and partial characterisation of a new strain of Ebola virus, Lancet 345 (1995) 1271–1274. [DOI] [PubMed] [Google Scholar]
- [13].Negredo A, Palacios G, Vázquez-Morón S, González F, Dopazo H, Molero F, Discovery of an ebolavirus-like filovirus in europe, PLoS Pathog. 7 (2011) e1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bray M, Murphy FA, Filovirus research: knowledge expands to meet a growing threat, J. Infect. Dis. 196 (Suppl. 2) (2007) S438–443. [DOI] [PubMed] [Google Scholar]
- [15].Kuhn JH, Filoviruses–a compendium of 40 years of epidemiological, clinical, and laboratory studies, Arch. Virol. Suppl. 20 (2008) 13–360. [PubMed] [Google Scholar]
- [16].Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM, Public health assessment of potential biological terrorism agents, Emerg. Infect. Dis. 8 (2002) 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Feldmann H, Jones S, Klenk H-D, Schnittler H-J, Ebola virus: from discovery to vaccine, Nat. Rev. Immunol. 3 (2003) 677–685. [DOI] [PubMed] [Google Scholar]
- [18].Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection, Am. J. Pathol. 163 (2003) 2347–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hensley LE, Jones SM, Feldmann H, Jahrling PB, Geisbert TW, Ebola and Marburg viruses: pathogenesis and development of countermeasures, Curr. Mol. Med. 5 (2005) 761–772. [DOI] [PubMed] [Google Scholar]
- [20].Warfield KL, Swenson DL, Demmin G, Bavari S, Filovirus-like particles as vaccines and discovery tools, Expert Rev. Vacc. 4 (2005) 429–440. [DOI] [PubMed] [Google Scholar]
- [21].Paragas J, Geisbert TW, Development of treatment strategies to combat Ebola and Marburg viruses, Expert Rev. Anti. Infect. Ther. 4 (2006) 67–76. [DOI] [PubMed] [Google Scholar]
- [22].Bausch DG, Feldmann H, Geisbert TW, Bray M, Sprecher AG, Boumandouki P, Outbreaks of filovirus hemorrhagic fever: time to refocus on the patient, J. Infect. Dis. 196 (Suppl. 2) (2007) S136–141. [DOI] [PubMed] [Google Scholar]
- [23].Stille W, Böhle E, Helm E, van Rey W, Siede W, [On an infectious disease transmitted by Cercopithecus aethiops: (Green monkey disease)], Dtsch Med Wochenschr 93 (1968) 572–582. [DOI] [PubMed] [Google Scholar]
- [24].Smith CE, Simpson DI, Bowen ET, Zlotnik I, Fatal human disease from vervet monkeys, Lancet 2 (1967) 1119–1121. [DOI] [PubMed] [Google Scholar]
- [25].Martini GA, Marburg agent disease: in man, Trans. R. Soc. Trop. Med. Hyg. 63 (1969) 295–302. [DOI] [PubMed] [Google Scholar]
- [26].Martini GA, Siegert R Marburg virus disease. New York: 1971. [Google Scholar]
- [27].Bausch DG, Nichol ST, Muyembe-Tamfum JJ, Borchert M, Rollin PE, Sleurs H, Marburg hemorrhagic fever associated with multiple genetic lineages of virus, N. Engl. J. Med. 355 (2006) 909–919. [DOI] [PubMed] [Google Scholar]
- [28].Bres P, The epidemic of Ebola haemorrhagic fever in Sudan and Zaire, 1976: introductory note] (1978) 245. [PMC free article] [PubMed] [Google Scholar]
- [29].Johnson KM, Ebola haemorrhagic fever in Zaire, 1976 (1978) 271–293. [PMC free article] [PubMed] [Google Scholar]
- [30].Smith DIH, Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team; (1978) 247–270. [PMC free article] [PubMed] [Google Scholar]
- [31].Webb PAJ, K.M.; Wulff H, Lange JV. Some observations on the properties of Ebola virus, in Ebola virus haemorrhagic fever. Amsterdam: 1978. [Google Scholar]
- [32].Bwaka MA, Bonnet MJ, Calain P, Colebunders R, De Roo A, Guimard Y, Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients, J. Infect. Dis. 179 (Suppl. 1) (1999) S1–7. [DOI] [PubMed] [Google Scholar]
- [33].Khan AS, Tshioko FK, Heymann DL, Le Guenno B, Nabeth P, Kerstiëns B, The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidëmies à Kikwit, J. Infect. Dis. 179 (Suppl. 1) (1999) S76–S86. [DOI] [PubMed] [Google Scholar]
- [34].Georges AJ, Leroy EM, Renaut AA, Benissan CT, Nabias RJ, Ngoc MT, Ebola hemorrhagic fever outbreaks in Gabon, 1994–1997: epidemiologic and health control issues, J. Infect. Dis. 179 (Suppl. 1) (1999) S65–75. [DOI] [PubMed] [Google Scholar]
- [35].Amblard J, Obiang P, Edzang S, Prehaud C, Bouloy M, Guenno BL, Identification of the Ebola virus in Gabon in 1994, Lancet 349 (1997) 181–182. [DOI] [PubMed] [Google Scholar]
- [36].Georges-Courbot MC, Sanchez A, Lu CY, Baize S, Leroy E, Lansout-Soukate J, Isolation and phylogenetic characterization of Ebola viruses causing different outbreaks in Gabon, Emerg. Infect. Dis. 3 (1997) 59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lamunu M, Lutwama JJ, Kamugisha J, Opio A, Nambooze J, Ndayimirije N, et al. Containing a haemorrhagic fever epidemic: the Ebola experience in Uganda (October 2000–January 2001) 2004:27–37. [DOI] [PubMed] [Google Scholar]
- [38].Okware SI, Omaswa FG, Zaramba S, Opio A, Lutwama JJ, Kamugisha J, et al. An outbreak of Ebola in Uganda, (2002), 1068–75. [DOI] [PubMed] [Google Scholar]
- [39].Lamunu M, Lutwama JJ, Kamugisha J, Opio A, Nambooze J, Ndayimirije N, Containing a haemorrhagic fever epidemic: the Ebola experience in Uganda (October 2000–January 2001), Int. J. Infect. Dis. 8 (2004) 27–37. [DOI] [PubMed] [Google Scholar]
- [40].Okware SI, Omaswa FG, Zaramba S, Opio A, Lutwama JJ, Kamugisha J, An outbreak of Ebola in Uganda, Trop. Med. Int. Health 7 (2002) 1068–1075. [DOI] [PubMed] [Google Scholar]
- [41].Towner JS, Sealy TK, Khristova ML, Albarino CG, Conlan S, Reeder SA, et al. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda (2008):e1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shoemaker T, MacNeil A, Balinandi S, Campbell S, Wamala JF, McMullan LK, Reemerging Sudan Ebola virus disease in Uganda, 2011, Emerg. Infect. Dis. 18 (2012) 1480–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Smith DH, Johnson BK, Isaacson M, Swanapoel R, Johnson KM, Killey M, Marburg-virus disease in Kenya, Lancet 1 (1982) 816–820. [DOI] [PubMed] [Google Scholar]
- [44].Towner JS, Rollin PE, Bausch DG, Sanchez A, Crary SM, Vincent M, Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome, J. Virol. 78 (2004) 4330–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rowe AK, Bertolli J, Khan AS, Mukunu R, Muyembe-Tamfum JJ, Bressler D, Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidémies à Kikwit, J. Infect. Dis. 179 (Suppl. 1) (1999) S28–S35. [DOI] [PubMed] [Google Scholar]
- [46].Mahanty S, Gupta M, Paragas J, Bray M, Ahmed R, Rollin PE, Protection from lethal infection is determined by innate immune responses in a mouse model of Ebola virus infection, Virology 312 (2003) 415–424. [DOI] [PubMed] [Google Scholar]
- [47].Geisbert TW, Jahrling PB, Hanes MA, Zack PM, Association of Ebola-related Reston virus particles and antigen with tissue lesions of monkeys imported to the United States, J. Comp. Pathol. 106 (1992) 137–152. [DOI] [PubMed] [Google Scholar]
- [48].Ryabchikova EI, Kolesnikova LV, Luchko SV, An analysis of features of pathogenesis in two animal models of Ebola virus infection, J. Infect. Dis. 179 (Suppl. 1) (1999) S199–202. [DOI] [PubMed] [Google Scholar]
- [49].Baskerville A, Fisher-Hoch SP, Neild GH, Dowsett AB, Ultrastructural pathology of experimental Ebola haemorrhagic fever virus infection, J. Pathol. 147 (1985) 199–209. [DOI] [PubMed] [Google Scholar]
- [50].Stroher U, West E, Bugany H, Klenk HD, Schnittler HJ, Feldmann H, Infection and activation of monocytes by Marburg and Ebola viruses (2001); 11025–11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Leroy EM, Baize S, Lu CY, McCormick JB, Georges AJ, Georges-Courbot MC, et al. , Diagnosis of Ebola haemorrhagic fever by RT-PCR in an epidemic setting, J. Med. Virol. 60 (2000) 463–467. [PubMed] [Google Scholar]
- [52].Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debré P, et al. , Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients, Nat. Med. 5 (1999) 423–426. [DOI] [PubMed] [Google Scholar]
- [53].Baize S, Leroy EM, Georges AJ, Georges-Courbot MC, Capron M, Bedjabaga I, et al. Inflammatory responses in Ebola virus-infected patients (2002):163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gupta M, Mahanty S, Bray M, Ahmed R, Rollin PE, Passive transfer of antibodies protects immunocompetent and imunodeficient mice against lethal Ebola virus infection without complete inhibition of viral replication, J. Virol. 75 (2001) 4649–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Leroy EM, Baize S, Debre P, Lansoud-Soukate J, Mavoungou E, Early immune responses accompanying human asymptomatic Ebola infections, Clin. Exp. Immunol. 124 (2001) 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM, Human fatal zaire ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis, PLoS Negl. Trop. Dis. 4 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Stroher U, West E, Bugany H, Klenk HD, Schnittler HJ, Feldmann H, Infection and activation of monocytes by Marburg and Ebola viruses (2001); 11025–11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schnittler HJ, Feldmann H, Marburg and Ebola hemorrhagic fevers: does the primary course of infection depend on the accessibility of organ-specific macrophages? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 27 (1998) 404–406. [DOI] [PubMed] [Google Scholar]
- [59].Hensley LE, Young HA, Jahrling PB, Geisbert TW, Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily, Immunol. Lett. 80 (2002) 169–179. [DOI] [PubMed] [Google Scholar]
- [60].Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA Jr, et al. , MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions, Nature 398 (1999) 718–723. [DOI] [PubMed] [Google Scholar]
- [61].Baize S, Leroy EM, Mavoungou E, Fisher-Hoch SP, Apoptosis in fatal Ebola infection. Does the virus toll the bell for immune system? Apoptosis Int. J. Program Cell Death 5 (2000) 5–7. [DOI] [PubMed] [Google Scholar]
- [62].Reed DS, Hensley LE, Geisbert JB, Jahrling PB, Geisbert TW, Depletion of peripheral blood T lymphocytes and NK cells during the course of ebola hemorrhagic fever in cynomolgus macaques, Viral Immunol. 17 (2004) 390–400. [DOI] [PubMed] [Google Scholar]
- [63].Leroy E, Baize S, Gonzalez JP, [Ebola and Marburg hemorrhagic fever viruses: update on filoviruses], Méd. Trop. Rev. Corps. Santé Colon 71 (2011) 111–121. [PubMed] [Google Scholar]
- [64].Bosio CM, Aman MJ, Grogan C, Hogan R, Ruthel G, Negley D, et al. , Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation, J. Infect. Dis. 188 (2003) 1630–1638. [DOI] [PubMed] [Google Scholar]
- [65].Jin H, Yan Z, Prabhakar BS, Feng Z, Ma Y, Verpooten D, et al. , The VP35 protein of Ebola virus impairs dendritic cell maturation induced by virus and lipopolysaccharide, J. Gen. Virol. 91 (2010) 352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Basler CF, Mikulasova A, Martinez-Sobrido L, Paragas J, Mühlberger E, Bray M, et al. , The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3, J. Virol. 77 (2003) 7945–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cardenas WB, Loo YM, Gale M Jr, Hartman AL, Kimberlin CR, Martinez-Sobrido L, et al. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling (2006); 5168–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Prins KC, Cárdenas WB, Basler CF, Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKepsilon and TBK-1, J. Virol. 83 (2009) 3069–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Basler CF, Wang X, Mühlberger E, Volchkov V, Paragas J, Klenk HD, et al. , The Ebola virus VP35 protein functions as a type I IFN antagonist, Proc. Natl. Acad. Sci. U. S. A. 97 (2000) 12289–12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Feng Z, Cerveny M, Yan Z, He B, The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR, J. Virol. 81 (2007) 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Haasnoot J, de Vries W, Geutjes E-J, Prins M, de Haan P, Berkhout B, The Ebola virus VP35 protein is a suppressor of RNA silencing, PLoS Pathog. 3 (2007) e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation, J. Virol. 80 (2006) 5156–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Reid SP, Valmas C, Martinez O, Sanchez FM, Basler CF, Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin alpha proteins with activated STAT1, J. Virol. 81 (2007) 13469–13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yang Z, Delgado R, Xu L, Todd RF, Nabel EG, Sanchez A, Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins, Science 279 (1998) 1034–1037. [DOI] [PubMed] [Google Scholar]
- [75].Sui J, Marasco WA, Evidence against Ebola virus sGP binding to human neutrophils by a specific receptor, Virology 303 (2002) 9–14. [DOI] [PubMed] [Google Scholar]
- [76].Maruyama T, Parren PW, Sanchez A, Rensink I, Rodriguez LL, Khan AS, Recombinant human monoclonal antibodies to Ebola virus, J. Infect. Dis. 179 (Suppl. 1) (1999) S235–239. [DOI] [PubMed] [Google Scholar]
- [77].Wahl-Jensen VM, Afanasieva TA, Seebach J, Ströher U, Feldmann H, Schnittler H-J, Effects of Ebola virus glycoproteins on endothelial cell activation and barrier function, J. Virol. 79 (2005) 10442–10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yang ZY, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ, Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury, Nat. Med. 6 (2000) 886–889. [DOI] [PubMed] [Google Scholar]
- [79].Wauquier N, Becquart P, Gasquet C, Leroy EM, Immunoglobulin G in Ebola outbreak survivors, Gabon, Emerg. Infect. Dis. 15 (2009) 1136–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Leroy EM, Souquière S, Rouquet P, Drevet D, Re-emergence of ebola haemorrhagic fever in Gabon, Lancet 359 (2002) 712. [DOI] [PubMed] [Google Scholar]
- [81].Drosten C, Göttig S, Schilling S, Asper M, Panning M, Schmitz H, Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR, J. Clin. Microbiol. 40 (2002) 2323–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Geisbert TW, Jahrling PB, Use of immunoelectron microscopy to show Ebola virus during the 1989 United States epizootic, J. Clin. Pathol. 43 (1990) 813–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Geisbert TW, Rhoderick JB, Jahrling PB, Rapid identification of Ebola virus and related filoviruses in fluid specimens using indirect immunoelectron microscopy, J. Clin. Pathol. 44 (1991) 521–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zaki SR, Shieh WJ, Greer PW, Goldsmith CS, Ferebee T, Katshitshi J, A novel immunohistochemical assay for the detection of Ebola virus in skin: implications for diagnosis, spread, and surveillance of Ebola hemorrhagic fever. Commission de Lutte contre les Epidémies à Kikwit, J. Infect. Dis. 179 (Suppl. 1) (1999) S36–S47. [DOI] [PubMed] [Google Scholar]
- [85].Van der Groen G, Kurata T, Mets C, Modifications to indirect immunofluorescence tests on Lassa, Marburg, and Ebola material, Lancet 1 (1983) 654. [DOI] [PubMed] [Google Scholar]
- [86].Johnson KM, Elliott LH, Heymann DL, Preparation of polyvalent viral immunofluorescent intracellular antigens and use in human serosurveys, J. Clin. Microbiol. 14 (1981) 527–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Saijo M, Niikura M, Morikawa S, Kurane I, Immunofluorescence method for detection of Ebola virus immunoglobulin g, using HeLa cells which express recombinant nucleoprotein, J. Clin. Microbiol. 39 (2001) 776–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ksiazek TG, West CP, Rollin PE, Jahrling PB, Peters CJ, ELISA for the detection of antibodies to Ebola viruses, J. Infect. Dis. 179 (Suppl. 1) (1999) S192–198. [DOI] [PubMed] [Google Scholar]
- [89].Ksiazek TG, Rollin PE, Williams AJ, Bressler DS, Martin ML, Swanepoel R, Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995, J. Infect. Dis. 179 (Suppl. 1) (1999) S177–187. [DOI] [PubMed] [Google Scholar]
- [90].Sanchez A, Ksiazek TG, Rollin PE, Miranda ME, Trappier SG, Khan AS, Detection and molecular characterization of Ebola viruses causing disease in human and nonhuman primates, J.Infect. Dis. 179 (Suppl. 1) (1999) S164–169. [DOI] [PubMed] [Google Scholar]
- [91].Slenczka WG, The Marburg virus outbreak of 1967 and subsequent episodes, Curr. Top. Microbiol. Immunol. 235 (1999) 49–75. [DOI] [PubMed] [Google Scholar]
- [92].Roddy P, Howard N, Van Kerkhove MD, Lutwama J, Wamala J, Yoti Z, Clinical manifestations and case management of Ebola haemorrhagic fever caused by a newly identified virus strain, Bundibugyo, Uganda, 2007–2008, PLoS ONE 7 (2012) e52986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bray M, Raymond JL, Geisbert T, Baker RO, 3-deazaneplanocin A induces massively increased interferon-alpha production in Ebola virus-infected mice, Antiviral Res. 55 (2002) 151–159. [DOI] [PubMed] [Google Scholar]
- [94].Friedrich BM, Trefry JC, Biggins JE, Hensley LE, Honko AN, Smith DR, Potential vaccines and post-exposure treatments for filovirus infections, Viruses 4 (2012) 1619–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Parren PWHI, Geisbert TW, Maruyama T, Jahrling PB, Burton DR, Pre- and postexposure prophylaxis of Ebola virus infection in an animal model by passive transfer of a neutralizing human antibody, J. Virol. 76 (2002) 6408–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wilson JA, Hevey M, Bakken R, Guest S, Bray M, Schmaljohn AL, Epitopes involved in antibody-mediated protection from Ebola virus, Science 287 (2000) 1664–1666. [DOI] [PubMed] [Google Scholar]
- [97].Marzi A, Yoshida R, Miyamoto H, Ishijima M, Suzuki Y, Higuchi M, Protective efficacy of neutralizing monoclonal antibodies in a nonhuman primate model of Ebola hemorrhagic fever, PLoS ONE 7 (2012) e36192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Qiu X, Audet J, Wong G, Pillet S, Bello A, Cabral T, Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies, Sci. Transl. Med. 4 (2012) 138ra81. [DOI] [PubMed] [Google Scholar]
- [99].Hensley LE, Stevens EL, Yan SB, Geisbert JB, Macias WL, Larsen T, Recombinant human activated protein C for the postexposure treatment of Ebola hemorrhagic fever, J. Infect. Dis. 196 (Suppl. 2) (2007) S390–399. [DOI] [PubMed] [Google Scholar]
- [100].Geisbert TW, Bausch DG, Feldmann H, Prospects for immunisation against Marburg and Ebola viruses, Rev. Med. Virol. 20 (2010) 344–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bukreyev A, Rollin PE, Tate MK, Yang L, Zaki SR, Shieh W-J, Successful topical respiratory tract immunization of primates against Ebola virus, J. Virol. 81 (2007) 6379–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, Grolla A, Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus, J. Virol. 83 (2009) 7296–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Jones SM, Feldmann H, Ströher U, Geisbert JB, Fernando L, Grolla A, Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses, Nat. Med. 11 (2005) 786–790. [DOI] [PubMed] [Google Scholar]
- [104].Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang Z-Y, Roederer M, Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates, Nature 424 (2003) 681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S, Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge, J. Infect. Dis. 196 (Suppl. 2) (2007) S430–437. [DOI] [PubMed] [Google Scholar]
- [106].Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ, Development of a preventive vaccine for Ebola virus infection in primates, Nature 408 (2000) 605–609. [DOI] [PubMed] [Google Scholar]
- [107].Martin JE, Sullivan NJ, Enama ME, Gordon IJ, Roederer M, Koup RA, et al. , A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial, Clin. Vaccine Immunol. 13 (2006) 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Richardson JS, Yao MK, Tran KN, Croyle MA, Strong JE, Feldmann H, Enhanced protection against Ebola virus mediated by an improved adenovirus-based vaccine, PLoS ONE 4 (2009) e5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Blaney JE, Marzi A, Willet M, Papaneri AB, Wirblich C, Feldmann F, Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine, PLoS Pathog. 9 (2013) e1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Blaney JE, Wirblich C, Papaneri AB, Johnson RF, Myers CJ, Juelich TL, Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses, J. Virol. 85 (2011) 10605–10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Feldmann H, Jones SM, Daddario-DiCaprio KM, Geisbert JB, Ströher U, Grolla A, Effective post-exposure treatment of Ebola infection, PLoS Pathog. 3 (2007) e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, Reed DS, Feldmann F, Grolla A, Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses, Vaccine 26 (2008) 6894–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Qiu X, Fernando L, Alimonti JB, Melito PL, Feldmann F, Dick D, Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses, PLoS ONE 4 (2009) e5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bukreyev A, Marzi A, Feldmann F, Zhang L, Yang L, Ward JM, Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge, Virology 383 (2009) 348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Warfield KL, Bosio CM, Welcher BC, Deal EM, Mohamadzadeh M, Schmaljohn A, Ebola virus-like particles protect from lethal Ebola virus infection, Proc. Natl. Acad. Sci. U. S. A. 100 (2003) 15889–15894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Fruit bats as reservoirs of Ebola virus, Nature 438 (2005) 575–576. [DOI] [PubMed] [Google Scholar]
- [117].Rouquet P, Froment J-M, Bermejo M, Kilbourn A, Karesh W, Reed, Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001–2003, Emerg. Infect. Dis. 11 (2005) 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bermejo M, Rodríguez-Teijeiro JD, Illera G, Barroso A, Vilà C, Walsh PD, Ebola outbreak killed 5000 gorillas, Science 314 (2006) 1564. [DOI] [PubMed] [Google Scholar]
- [119].Swenson DL, Wang D, Luo M, Warfield KL, Woraratanadharm J, Holman DH, et al. , Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections, Clin. Vaccine Immunol. (2008) 460–467 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18216185 [DOI] [PMC free article] [PubMed]
- [120].Bukreyev A, Marzi A, Feldmann F, Zhang L, Yang L, Ward JM, et al. , Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge, Virology 383 (2009) 348–361, 10.1016/j.virol.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S, Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge, J. Infect. Dis. (2007) S430–S437 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17940980 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


