ABSTRACT
Diabetic retinopathy (DR) is a complication of diabetes with a complex pathophysiology and multiple factors involved. Recently, it has been found that the upregulation of the renin-angiotensin-aldosterone system (RAAS) leads to overexpression of angiotensin II (Ang II), which induces oxidative stress, inflammation, and angiogenesis in the retina. Therefore, RAAS may be a promising therapeutic target in DR. Notably, RAAS inhibitors are often used in the treatment of hypertension. Still, the potential role and mechanism of DR must be further studied. In this review, we discuss and summarize the pathology and potential therapeutic goals of RAAS in DR.
Keywords: Diabetic retinopathy, renin-angiotensin-aldosterone system, renin, prorenin
INTRODUCTION
Diabetic retinopathy (DR) is a potentially blinding ocular disease that is frequently observed in patients with long-term diabetes mellitus (1). The renin-aldosterone system (RAAS), one of the oldest studied hormone systems in the body, is well known for its roles in systemic vascular control and electrolyte homeostasis (2). One of the few tissues with local RAAS secretion is the retina, in which local production is suggested by a concentration of angiotensin II (Ang II) exceeding that of the circulation (2). The role of Ang II in the normal retina is most likely that of retinal homeostasis, including blood vessel constriction, regulation of glial cell function, and modulation of neuronal function (3). It is well established that all RAAS components are expressed in retinal cells (4), including (pro)renin, renin, angiotensinogen, angiotensin I (Ang I), Ang II, angiotensin-(1-7) (Ang-[1-7]), angiotensin-converting enzyme (ACE), ACE type 2 (ACE2), (pro)renin receptor ([P]RR), angiotensin II type 1 receptors (AT1R), angiotensin II type 2 receptors (AT2R), and Mas receptor (5–7). The discovery of ocular RAAS components provides evidence that RAAS is probably involved in the occurrence and development of DR. A Canadian study of longevity in patients with type 1 diabetes (T1DM) has also shown that RAAS activation is associated with DR (8). Therefore, RAAS may be a promising therapeutic target in DR treatment.
In this review, we aim to discuss the role of RAAS and the beneficial effects of its inhibitors in the progression of DR.
THE ROLE OF RAAS IN OCULAR PHYSIOLOGY
Local and circulating RAAS
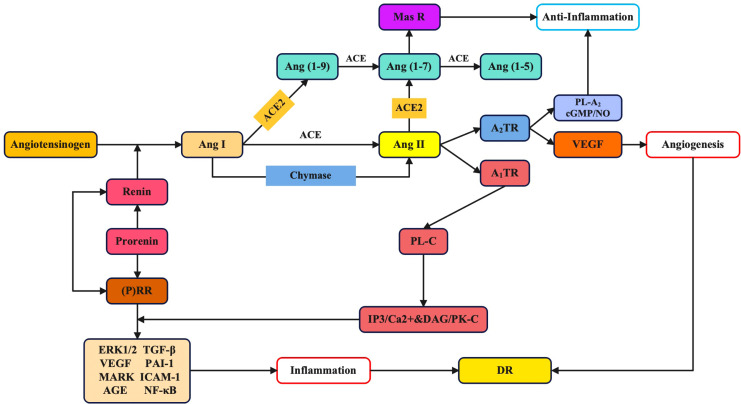
Prorenin is activated to form renin in the juxtaglomerular cells of the kidney (Figure 1). Renin then binds to liver-produced angiotensinogen to generate the decapeptide Ang I, which is then hydrolyzed by ACE present in circulation or locally within tissues to produce the oligopeptide Ang II (9). Additionally, Ang II adjusts multiple physiological effects by activating AT1R and AT2R to transmit signaling. In the retina, Ang II mainly elicits pathologic effects through AT1R (10), whereas the actions of AT2R may be regulated differently from those of AT1R and, possibly, be contrary to the vasodilatory effects of AT1R (the effects of AT2R remain unclear). Previous studies suggested that circulating RAAS components (such as Ang I, Ang II, and angiotensinogen in plasma) were unable to enter ocular tissues. However, after the discovery of renin mRNA in the eyes, the hypothesis that ocular RAAS components are synthesized locally was confirmed (11).
Figure 1. Involvement of the renin-angiotensin-aldosterone system in biochemical pathways and molecules in diabetic retinopathy.
Abbreviations: DR, diabetic retinopathy; RAAS, renin-angiotensin-aldosterone system; Ang I, angiotensin I, Ang II, angiotensin II; ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme type 2; (P)RR, (pro)renin receptor; AT1R, angiotensin II type 1 receptors; AT2R, angiotensin II type 2 receptors; ERK 1/2, extracellular signal-regulated kinase 1/2; MAPK, mitogen-activated protein kinase; VEGF, vascular endothelial growth factor; AGE, advanced glycation end product; TGF-β, transforming growth factor-β; ICAM-1, intracellular adhesion molecule-1; NF-κB, nuclear factor-kappa B; PAI-1, plasminogen activator inhibitor-1; DAG/PK-C, diacylglycerol/protein kinase-C; IP3, inositol-1,4,5-triphosphate; NO, nitric oxide; PL-A2, phospholipase A2; PL-C, phospholipase C; cGMP, cyclic guanosine monophosphate.
Renin expression in retinal pigment epithelium (RPE) has been shown to be decreased in mice after systemic administration of Ang II. Additionally, systemic administration of enalapril, an ACE inhibitor (ACEI), increases renin expression levels in the retina and kidney in mice; specifically, enalapril increased 20 times the renin expression level in RPE. The systemic infusion of losartan, an AT1R blocker, can prevent the Ang II-dependent downregulated expression of renin in RPE (12). These findings suggest that circulating RAAS may regulate ocular RAAS. Thus, local RAAS components may play a vital role in ocular diseases.
The three major RAAS signaling pathways
Notably, Ang II is mostly catalyzed by the enzyme ACE (i.e., the classical pathway), although it can also be catalyzed by other enzymes, including chymase, a gastrointestinal enzyme also expressed in ocular tissues (13,14). The signal transmission of Ang II has three pathways, including the ACE/Ang II/AT1R axis and chymase/Ang II axis, the ACE2/Ang-(1-7)/Mas axis, and the prorenin and (P)RR pathway.
ACE/Ang II/AT1R axis and chymase/Ang II axis
The ACE/Ang II/AT1R axis is known as the classical signaling pathway. The RPE cells express AT1R, transient-receptor-potential channel-V2 (TRPV2) and angiotensin-receptor-associated protein (Atrap). Specifically, AT1R and Atrap are located at the basolateral membrane of the RPE (15); AT1R is a Gq protein-associated G-protein coupled receptor, and Ang II bonds with AT1R to activate diacylglycerol-protein kinase C (DAG/PK-C) and inositol-1,4,5-triphosphate (IP3)/Ca2+ signaling cascades (16). The signaling cascades increase intracellular Ca2+ levels due to the transient release of Ca2+ via IP3 receptor and TRPV2 channels in the endoplasmic reticulum. In ocular diseases like DR and age-related macular degeneration (AMD), the signaling cascades enhance the accumulation of inflammatory and angiogenic cytokines, including extracellular signal-regulated kinase (ERK) (17), mitogen-activated protein kinase (MAPK) (18), vascular endothelial growth factor (VEGF) (19), intracellular adhesion molecule-1 (ICAM-1) (20), nuclear factor kappa B (NF-κB), transforming growth factor-β1 (TGF-β1) (21), reactive oxygen species (ROS) (22), advanced glycation end products (AGEs) (23), and nicotinamide adenine dinucleotide phosphate (NADP[H]). These cytokines disrupt cell growth and intracellular signaling. Thus, these findings support the hypothesis that the ACE/Ang II /AT1R axis regulates ocular pathophysiology through inflammation.
In addition, chymase can also induce the expression of Ang II from angiotensin-(1-12) (Ang-[1-12]), which is independent of the ACE pathway (24). Chymase can induce oxidative stress via chymase-enhanced Ang II to attack the pancreas in diabetic animal models. Furthermore, by inhibiting chymase, the production of Ang II can be reduced (25). Therefore, chymase may be a potential target in preventing DR progression.
ACE2/Ang-(1-7)/Mas axis
Notably, ACE2 – a homolog of ACE – has also been described in the human retina (26). It can transform Ang II into Ang-(1-7), which acts contrarily to Ang II through a novel angiotensin receptor (Mas). The Mas receptor is a G-protein coupled receptor that induces telangiectasis and antiproliferative, antiinflammatory, and antifibrotic actions and is active in body fluid homeostasis (27). This type of receptor has been found in the retina and ciliary body. The ACE2/Ang-(1-7)/Mas axis is considered a protective axis that offsets the negative cardiac and vascular effects induced by activation of the ACE/Ang II/AT1R axis (28). Additionally, the Mas receptor and AT2R have been found to be interdependent in stimulating nitric oxide (NO) (29).
The activation of ACE2 mitigates lipopolysaccharide-induced inflammatory action in human RPE cells (30). The Otsuka Long-Evans Tokushima Fatty (OLETF) rat with diabetes mellitus is an animal model for examination of the expression of amyloid β peptide (Aβ) in the diabetic retina. In the OLETF rat retina, the accumulation of Aβ peptide is enhanced by high plasma glucose concentration (31). In human RPE cells, the inflammation activated by Aβ can be improved via activation of ACE2/Ang-(1-7)/Mas axis (32). The increased expression of ACE2 modulates the local immune responses to lessen ocular inflammation in mice with experimental autoimmune uveitis. The effects are mediated by activating the Ang-(1-7)/Mas pathways and inhibiting the NF-κB, signal transducer and activator of transcription 3 (STAT3), and MAPK signaling pathways (33). Additionally, ACE2 has been proven to exert beneficial effects in mice with diabetes by upregulating adeno-associated virus (AAV)-mediated gene delivery (34). Intraocular administration of AAV-ACE2/Ang-(1-7) to diabetic rats and mice significantly reduces diabetic retinal vascular leakage, acellular capillaries, leukocyte infiltration, and oxidative damage. Therefore, experimental data suggest that RAAS overexpression in the eye is associated with DR.
Prorenin and (pro)renin receptor pathway
Apart from AT1R, (P)RR also plays an important physiological role in blood pressure regulation and cellular function, including inflammation, proliferation, angiogenesis, and activation of growth factors (35). Prorenin, the precursor of renin, circulates at high concentrations in plasma and was considered previously to be physiologically inert. Levels of prorenin are high in persons with diabetes. The high level of prorenin is now used as a biomarker to predict diabetic microvascular complications.
Prorenin and its receptor play a significant part in the RAAS because their binding is a rate-limiting step. Prorenin binds to the (P)RR and undergoes conformational structural change, exposing its active site and exhibiting enzymatic activity (36). The binding is not the conventional prorenin proteolysis, which triggers a signaling transduction pathway independent of Ang II (37). This is called a receptor-associated prorenin system (RAPS), which plays a significant part in DR (38). The binding of prorenin and (P)RR increases the formation of angiotensin in specific tissues and transduces signaling by the classic RAAS pathway. The nonproteolytic activation of prorenin selectively accelerates pathologic retinal neovascularization via inflammatory processes (39). Prorenin also increases the generation of Ang II by enhancing renin activity. In addition, the binding of prorenin and (P)RR also directly stimulates MAPKs of (P)RR, including phosphorylation of ERK 1/2 (40). Studies in mesangial cells indicate that inhibition of (P)RR may play an important role in preventing DR independent from Ang II blockade (41).
Handle region peptide (HRP), a peptide derived from the prosegment of prorenin, is a (P)RR inhibitor that has shown beneficial effects in ocular pathophysiology by suppressing ERK activation and generation of vascular endothelial growth factor (VEGF) in AT1R-deficient diabetic mice (42). In addition, independent of the progression of DR, (P)RR involves the production of VEGF and its tyrosine kinase receptors. Further, (P)RR initiates the expression of angiogenic cytokines, such as ERK1/2, TGF-β1, and VEGF/VEGF receptor-2 in the retina, which can be blocked via (P)RR/ERK signaling (43,44). Thus, prorenin and (P)RR, which take part in the upstream of RAAS to regulate physiological function, can be novel therapy targets in DR.
All in all, RAAS is an integral part of the physical function of the retina. All the possible signaling pathways discussed above play significant roles in regulating ocular physiology. The local RAAS function may be controlled by RAAS inhibitors, including renin inhibitors, AT1R blockers (AT1RBs), ACEI, and (P)RR blockers. The RAAS components affect the physiological function of the retina. The components also play a role in the progression of DR. These provide a promising therapeutic strategy in the pathologic progress of DR.
HYPERGLYCEMIA-INDUCES RAAS UPREGULATION
Hyperglycemia causes the upregulation of major glucose metabolic pathways, including the tricarboxylic acid cycle and glycolysis, as compensation for diabetic conditions. This compensatory mechanism increases the tricarboxylic acid cycle and leads to excessive accumulation of the intermediate succinate. The G protein-coupled receptor 91 (GPR91), also known as the succinate receptor, is expressed in the retinal ganglion cell layer. In retinal ganglion cells, succinate accumulates remarkably under ischemic conditions. GPR91 is activated via ERK1/2/cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) pathways to mediate VEGF-induced retinal vascular alteration. In DR, succinate activates GPR91 to induce VEGF expression (45). In addition, high glucose levels induce a paracrine mechanism involving GPR91 and succinate. Succinate binds to GPR91, initiating intracellular signaling transduction, including the release of intracellular Ca2+, activation of PGE2, and generation of NO. It directly activates the synthesis and release of renin (46). Hence, hyperglycemia increases renin release via the accumulation of succinate and GPR91 signaling pathways.
Nε-carboxymethyl lysine (CML) is a typical advanced glycation end product (AGE) that is formed as the result of the glycoxidation reaction of serine, lysine, and glucose (47). Hyperglycemia triggers the intracellular generation of CML. Further, CML induces NF-κB/p38/MAPK-dependent signaling transduction pathways, activating inducible nitric oxide synthase (iNOS) and, later, elevating NO formation (46).
Studies suggest that hyperglycemia elevates the amount of (P)RR in the plasma membrane of renal collecting duct cells. In M-1 cells treated with high glucose, (P)RRs are mainly localized at the surface of the plasma membrane, while in control rats, (P)RRs are located intracellularly. The augmented bind of (P)RR and prorenin increases the generation of renin (48). The hyperglycemia-induced increased (P)RR in the plasma membrane may be a novel mechanism to clarify the progression of DR. In conclusion, hyperglycemia activates RAAS overexpression in various ways, including through AGEs, GPR91, and (P)RR.
RAAS INVOLVEMENT IN DIABETIC RETINOPATHY
The pathophysiological mechanisms of DR involve various complex factors. The upregulation of RAAS, including overexpressed Ang II, induces alterations of multiple pathways in the progression of DR, such as oxidative stress, inflammation, and vascular proliferation. Moreover, aldosterone may play a role in DR, and the pathological pathways are interconnected. The detailed mechanisms are discussed below.
RAAS augments vascular proliferation and permeability
The RAAS upregulates the angiogenic cytokine VEGF probably via the Ang II/AT1R axis, leading to angiogenesis. In cultured mesenchymal stem cells, VEGF mRNA and VEGF protein levels increase after Ang II administration. The enhanced synthesis of VEGF has been speculated to occur via AT1R signaling transduction in ERK1/2 and protein kinase B (Akt) pathways (49). Further, Ang II-mediated VEGF induces angiogenesis (50) and increases retinal vascular permeability (51), increasing the possibility of hyperpermeability and neovascularization. Retinal neovascularization converts nonproliferative DR into proliferative DR, which expedites the DR progression. The blood-retinal barrier (BRB) is essential for a normal physiological function of the retinal microenvironment and low permeability (52). The BRB breakdown plays a critical role in the early pathogenesis of DR. In studies, the loss of tight junction proteins is induced by Ang II-mediated VEGF overexpression. Meanwhile, perindopril, an ACE inhibitor, can prevent the loss of tight junction proteins by blocking VEGFs. The recovery of the loss reduces the enhanced diabetic retina vessel permeability (53). Thus, RAAS increases angiogenesis and permeability through VEGF overexpression.
Apart from increasing angiogenesis, Ang II also plays a role in vessel remodeling. Pericyte loss is a major part of DR progression. In capillaries, pericyte cells regulate vessel tone and perfusion pressure, much like smooth muscle cells in larger vessels. The loss of pericytes decreases inner retina stability, making the inner retina vulnerable to hyperglycemia-induced pathological damage. In addition, the loss of pericyte triggers retinal vascular remodeling and retinal vasculature structure damage, results in abnormal revascularization, and further promotes the occurrence of DR (54). It has been reported that Ang II induces the apoptosis of pericyte cells via intracellular signaling of integrin α3 and β1 (55). In mouse retina, pericyte apoptosis is induced by increased Ang II under high glucose via integrin. By blocking integrin α3 and β1, the pericyte loss induced by Ang II is decreased. Hence, RAAS affects the revascularization of the retina via pericyte loss. Taken together, it seems that RAAS signaling pathways contribute heavily to pathological vessel events in DR.
RAAS induces oxidative stress
Notably, Ang II is one of the main components inducing oxidative stress and producing ROS. Further, ROS is well known in the progression of DR. In cultured retinal ganglion cells, AT1RB signaling is involved in oxidative stress-induced retinal neurodegeneration (56). This is considered a major pathophysiological event in DR. Additionally, NADP(H) oxidase is induced by Ang II, which proves the occurrence of a connection between RAAS and ROS. It is well known that AT1R signaling activates protein kinase C (PKC), especially its alpha isoform, which induces vascular NADP(H) oxidase to overexpress the superoxide radical (57). In addition, Ang II also mediates the overexpression of leukotriene B4 (LTB4), leading to NADP(H) oxidase activation (58). Activated NADP(H)-oxidase is necessary for diabetes-induced retinal leukostasis (59). Blockades of AT1R effectively block diabetes-induced inflammatory response and oxidative stress including AGEs (60), ICAM-1, NF-κB, and NADP(H) oxidase (61), and increase neuroprotective agents (22). Besides, in the human retinal pigment epithelial cell line-19 (ARPE-19), hyperglycemia increases VEGF expression and activation in an NADP(H) oxidase mechanism via prorenin receptor, which is independent of Ang II (62). Thus, oxidative stress can be induced by RAAS in both Ang II and prorenin pathways.
The apoptosis of retinal capillary pericytes is known as a crucial event in the breakdown of the inner BRB, which is a major step in the progression of DR. Further, NADP(H) oxidase-induced ROS produce much more damage than mitochondrial dysfunction-derived ROS under hyperglycemia condition in triggering caspase-3-induced apoptosis of retinal capillary pericytes. It has been proven that intracellular CML-modified proteins are produced by NADP(H) oxidase-derived ROS rather than mitochondria-derived ROS (63). Hence, the interrelationship of RAAS and NADP(H) oxidase-derived ROS is also connected with AGEs. The RPE cells are a crucial part of the outer BRB and are susceptible to hyperglycemia and ROS concentration (64). Therefore, Ang II enhances the activation of NADP(H) oxidase-induced ROS production, which may play a significant part in the pathogenesis of DR.
RAAS induces proinflammation in endothelial cells
Changes in inflammatory molecules have been detected in both diabetic animals and human retinas. These changes lead to increased BRB permeability and ischemia, driving angiogenesis (65). Further, Ang II activates NF-κB, and activated NF-κB induces the transcription of inflammatory mediators, including cellular adhesion molecules and VEGF (53). Increased levels of intracellular adhesion molecules indicate the occurrence of leukocyte recruitment and adhesion to the endothelium, and the subsequent fluid extravasation could damage the BRB. In animal models, increased Ang II concentration causes leukocytosis in the diabetic retina, which seems to be induced via overexpression of VEGF (66). The interaction of leukocytes and endothelial cells plays a significant part in the early stage of DR. Leukocytosis is a crucial step in endothelial cell dysfunction, so it may be possible that Ang II is involved in endothelial dysfunction.
Among adhesion molecules, ICAM-1 plays the most effective role in Ang II-induced retinal leukocytosis (67). Meanwhile, in the NF-κB signaling pathway, tumor necrosis factor-α (TNF-α) and interleukins are the most efficient proinflammatory mediators in retinal inflammation induced by Ang II. Interleukin-1β (IL-1β) also activates NF-κB, which increases the loss of pericytes in diabetic mouse retina (68). Therefore, Ang II-induced inflammation of endothelial cells leads to the breakdown of BRB in DR progression.
The role of aldosterone and endothelin-2 in ocular diseases
Aldosterone is a mineralocorticoid synthesized in the zona glomerulosa of the adrenal cortex (69). The enzyme that regulates aldosterone production is aldosterone synthase (cytochrome P450 family 11, subfamily B, member 2), which regulates gene expression by potassium and Ang II (70). Aldosterone plays an important role in maintaining sodium and water in the body by acting on mineralocorticoid receptors (MRs) in the distal renal tubules (71). Several studies have found that aldosterone synthase, 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), and MRs are expressed in Müller cells, retinal microvascular cells, and retinal ganglion cells (72,73). The MRs expressed in Müller cells are implicated in DR pathology.
Endothelin-2, a family member of potent vasoconstrictors and a possible mediator of Müller cells in monitoring photoreceptor damages (74), has been reported to be increased in serum in a mouse model of DR (75). Recent studies have found that inhibition of AT1R or MR reduces vascular leakage induced by intravitreal injection of endothelin-2 in mice, suggesting an association between RAAS and Müller cell dysfunction induced by endothelin-2 (76). Moreover, Ohashi and cols. examined the effects of high glucose conditions on MR in human retinal Müller cells in vitro. High-glucose treatment induces Müller cell swelling with increased expression of MR protein, which is suppressed by an MR antagonist (eplerenone) (77). A study by Zhao and cols. reported that MR is overexpressed in the retina of type 2 diabetes (T2DM) Goto-Kakizaki rats and humans (78); thus, local MR antagonism could be a novel therapeutic option for DR.
THE EFFECTIVE AND POTENTIAL THERAPEUTIC TARGETS OF RAAS
Clinical studies with ACEI/angiotensin II receptor blockers
Notably, ACEI and Ang II receptor blockers (ARBs) have shown effects on inhibiting DR progression. Preclinical studies have shown that ACEI and ARBs reduce retinal vascular leakage and VEGF levels as well as degeneration of retinal capillaries in diabetes (23,79). Both ACEI and ARBs have been shown to protect DR by reducing the overexpression of ocular VEGF/VEGF receptor-2 (80,81). Additionally, ARBs are relevant to the ACE2/Ang-(1-7)/Mas axis and can enhance the expressions of ACE2 and Ang-(1-7)/Mas and downregulate the expression of inflammatory cytokines, including interferon-c (IFN-c), interleukin-6 (IL-6), IL-1β, and TNF-α. Olmesartan, an ARB, blocks AGE-induced vascular cell adhesion molecule-1 (VCAM-1) gene expression by restoring the downregulated levels of ACE2 and stimulating the Ang-(1-7)/Mas receptor axis in renal mesangial cells (82). Telmisartan, an ACEI, may upregulate the ACE2/Ang-(1-7)/Mas axis to prevent apoptosis of retinal vascular endothelial cells (83).
Moreover, it has been found that AT1RBs can also recover the downregulation of glyoxalase-1 (GLO-1), a key regulator of AGE information, which is mediated by Ang II in retinal vascular cells (23). Retinal ganglion cell loss occurs in optic nerve inflammation. In an animal model of multiple sclerosis highly associated with retinal ganglion cell loss, the concentration of Ang II increases at an early stage, and after administration of candesartan, the retinal ganglion cell loss is reduced (84). Further, ACEI is effective in decreasing optic nerve inflammation. A meta-analysis of the effects of RAAS inhibitors in diabetic retinopathy has indicated that RAAS inhibitors could reduce the incidence and progression of DR and suggested that ACEI offers many more benefits than ARBs in DR (85). Despite effective results, RAAS is not blocked, due to a compensating feedback mechanism (86). Additionally, ACEI in low doses could not significantly slow down the progression of DR in patients with T2DM who had normal blood pressure, suggesting that the ACEI dose has an effect on ocular RAAS blockade (87). Thus, the conventional view that Ang II is a crucial therapeutic target must be reconsidered.
The Diabetic Retinopathy Candesartan Trials (DIRECT) failed to show benefits of candesartan, an ACEI, in preventing DR progression in patients with T1DM, while in those with T2DM, candesartan treatment resulted in a 34% regression of DR (88). Subsequent findings from DIRECT indicated that the presence of microaneurysms predicted an increased risk of DR progression in individuals with T1DM and T2DM, while ARB reduced the risk of microaneurysm progression (89). These results suggest a beneficial effect of candesartan in DR. Furthermore, a multicenter trial published by Mauer and cols. has found that retinopathy progression was reduced by 65% with enalapril (an ACE inhibitor) and by 70% with losartan (an AT1R blocker) (90). These findings confirm that AT1R blockade provides benefits for individuals with DR.
Clinical studies of renin and (P)RR
Direct renin inhibitors (DRIs), such as aliskiren, are also a potential treatment for DR and have the advantage of acting directly on renin compared with traditional RAAS inhibitors. In fact, DRIs are a limiting step in the activation of the signaling cytokine cascade and may be more effective in blocking RAAS. Wilkinson-Berka and cols. have shown that aliskiren offers similar or better protective effects on the retina in models of retinal disease (91). Additionally, aliskiren inhibits RAAS in RPE cells exposed to aliskiren (92). Endothelial progenitor cells (EPCs) are involved in the process of angiogenesis via VEGF and chemokine stromal cell-derived factor-1a (SDF-1a) (93), which can contribute to the repair of injured vessels (94). High glucose levels reduce EPC function (95), which may lead to the development of vascular complications in diabetes (96,97). Aliskiren improves the function of human EPCs in hyperglycemia status, which is related to upregulated VEGF/SDF-1a (98). This evidence supports the hypothesis that the upstream components of RAAS could be better targets in inhibiting RAAS function, although the mechanisms remain unclear. These findings indicate a novel and promising therapeutic target in preventing the development of DR.
Clinical studies of aldosterone/MR
The MR is an intracellular steroid hormone receptor and a member of the nuclear receptor superfamily of proteins. The development of the steroidal MR antagonists spironolactone and eplerenone led to convincing evidence from preclinical studies that MR blockade reduces tissue damage in various diseases, especially chronic kidney disease (99). Finerenone is a novel nonsteroidal MR antagonist. Results of the trials Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) and Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) have shown that finerenone significantly reduces kidney and cardiovascular outcomes (100,101), indicating that finerenone might have therapeutic benefit in other diabetic complications such as DR (102). A study by Jerome and cols. (103) reported that finerenone has beneficial effects on the retina of rodents with diabetes and ischemic retinopathy, reducing the hallmark features of vision-threatening vascular injury that includes a breakdown of the BRB and neovascularization, as well as a reduction in retinal inflammation. These findings suggest that finerenone might be a potential new oral treatment for patients with DR. The findings are consistent with a prior study in Goto-Kakizaki rats with T2DM that demonstrated that the intraocular administration of spironolactone reduced retinal vascular leakage and edema, although retinal VEGF levels were not reduced (78).
Clinical studies of the new pathway
Meanwhile, VEGF mediates the breakdown of the BRB to cause diabetic macula edema (DME) and promotes pre-retinal neovascularization, which leads to vitreous hemorrhage, tractional retinal detachment, and neovascular glaucoma (104). Therefore, intravitreal injection of anti-VEGF agents has become the standard treatment to improve visual acuity in DME (105). However, DME refractory to anti-VEGF treatment is reported to have a prevalence of approximately 40% in landmark clinical trials (106). A study using human retinal endothelial cells has shown that RAAS activation reduces the efficacy of anti-VEGF in reclosing the barrier, indicating that RAAS inhibitors may be a potential treatment modality for refractory DME (107).
Exploration of the angiopoietin (ANG) tyrosine kinase endothelial receptor (Tie) pathway has shown encouraging results for the management of AMD and DME (108). In adults, the ANG/Tie pathway is involved in the regulation of vascular homeostasis, modulation of vascular permeability, and neoangiogenic and proinflammatory processes (109). In detail, Tie-2 is a transmembrane receptor selectively located on endothelial cells in blood vessels and acts as a binding site for ANG1 and ANG2 (110). These latter molecules exert a different biological activity; ANG1 is a full Tie-2 agonist, inducing its phosphorylation and the activation of a downstream signaling process, which causes a positive effect on vascular stability and inhibits vessel permeability and leakage; in contrast, ANG2 acts as a partial agonist/antagonist of Tie-2. Therefore, its binding to the Tie-2 receptor prevents the activation of the entire pathway and leads to vascular leakage (111). Interestingly, preclinical studies have highlighted that the simultaneous dual inhibition of ANG2 and VEGF-A is superior to inhibition of VEGF-A or ANG2 alone (112).
Faricimab is the first bispecific antibody designed for intraocular use. Its antigen-binding fragments independently inhibit ANG2 and VEGF-A with high affinity and specificity. YOSEMITE (NCT03622580) and RHINE (NCT03622593) were two multicenter, randomized, double-masked, phase III clinical studies of DEM demonstrating that robust vision gains and anatomical improvements with faricimab were achieved with adjustable dosing up to every 16 weeks (113). These findings suggest that faricimab may be a potential new treatment for patients with DR.
In conclusion, overall, the retinal RAAS component has attracted the attention of scholars. Ocular RAAS has been proven to contribute to augmenting permeability, enhancing cell proliferation, and inducing proinflammation and oxidative stress during the development of DR. The inhibition of RAAS has shown some effects in clinical trials. Besides, the chymase-induced Ang II pathway is an independent endocrine pathway that could be a target for new therapeutic methods. The DRI and MR antagonists could also be new therapeutic targets in preventing the progression of DR. Meanwhile, more research about therapies for RAAS in DR is necessary, and so are more clinical trials to confirm the benefits and safety of RAAS inhibitors in DR. Therefore, we look forward to relevant studies like these in the future.
Acknowledgment
this work was supported by a grant from the Fund of Scientific Research Innovation of the First Affiliated Hospital of Harbin Medical University (grant number 2020M27, China)
REFERENCES
- 1.Simó R, Hernández C. Prevention and treatment of diabetic retinopathy: evidence from large, randomized trials. The emerging role of fenofibrate. Rev Recent Clin Trials. 2012 Feb;7(1):71–80. doi: 10.2174/157488712799363299. [DOI] [PubMed] [Google Scholar]
- 2.Phipps J, Dixon M, Jobling A, Wang AY, Greferath U, Vessey KA, et al. The renin-angiotensin system and the retinal neurovascular unit: A role in vascular regulation and disease. Exp Eye Res. 2019 Oct;187:107753–107753. doi: 10.1016/j.exer.2019.107753. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher E, Phipps J, Ward M, Vessey KA, Wilkinson-Berka JL. The renin-angiotensin system in retinal health and disease: Its influence on neurons, glia and the vasculature. Prog Retin Eye Res. 2010 Jul;29(4):284–311. doi: 10.1016/j.preteyeres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Choudhary R, Kapoor M, Singh A, Bodakhe S. Therapeutic targets of renin-angiotensin system in ocular disorders. J Curr Ophthalmol. 2016 Oct 20;29(1):7–16. doi: 10.1016/j.joco.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White A, Cheruvu S, Sarris M, Liyanage SS, Lumbers E, Chui J, et al. Expression of classical components of the renin-angiotensin system in the human eye. J Renin Angiotensin Aldosterone Syst. 2015 Mar;16(1):59–66. doi: 10.1177/1470320314549791. [DOI] [PubMed] [Google Scholar]
- 6.Holappa M, Valjakka J, Vaajanen A. Angiotensin(1-7) and ACE2, "The Hot Spots" of Renin-Angiotensin System, Detected in the Human Aqueous Humor. Open Ophthalmol J. 2015 Mar 31;9:28–32. doi: 10.2174/1874364101509010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad T, Verma A, Li Q. Expression and cellular localization of the Mas receptor in the adult and developing mouse retina. Mol Vis. 2014 Oct 17;20:1443–1455. [PMC free article] [PubMed] [Google Scholar]
- 8.Lovshin J, Lytvyn Y, Lovblom L, Katz A, Boulet G, Bjornstad P, et al. Retinopathy and RAAS Activation: Results From the Canadian Study of Longevity in Type 1 Diabetes. Diabetes Care. 2019 Feb;42(2):273–280. doi: 10.2337/dc18-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson-Berka J, Agrotis A, Deliyanti D. The retinal renin-angiotensin system: roles of angiotensin II and aldosterone. Peptides. 2012 Jul;36(1):142–150. doi: 10.1016/j.peptides.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Nagai N, Oike Y, Noda K, Urano T, Kubota Y, Ozawa Y, et al. Suppression of ocular inflammation in endotoxin-induced uveitis by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci. 2005 Aug;46(8):2925–2931. doi: 10.1167/iovs.04-1476. [DOI] [PubMed] [Google Scholar]
- 11.Brandt C, Pumfery A, Micales B, Bindley CD, Lyons GE, Sramek SJ, et al. Renin mRNA is synthesized locally in rat ocular tissues. Curr Eye Res. 1994 Oct;13(10):755–763. doi: 10.3109/02713689409047011. [DOI] [PubMed] [Google Scholar]
- 12.Milenkovic V, Brockmann M, Meyer C, Desch M, Schweda F, Kurtz A, et al. Regulation of the renin expression in the retinal pigment epithelium by systemic stimuli. Am J Physiol Renal Physiol. 2010 Aug;299(2):F396–F403. doi: 10.1152/ajprenal.00576.2009. [DOI] [PubMed] [Google Scholar]
- 13.Kramkowski K, Mogielnicki A, Buczko W. The physiological significance of the alternative pathways of angiotensin II production. J Physiol Pharmacol. 2006 Dec;57(4):529–539. [PubMed] [Google Scholar]
- 14.Maruichi M, Oku H, Takai S, Muramatsu M, Sugiyama T, Imamura Y, et al. Measurement of activities in two different angiotensin II generating systems, chymase and angiotensin-converting enzyme, in the vitreous fluid of vitreoretinal diseases: a possible involvement of chymase in the pathogenesis of macular hole patients. Curr Eye Res. 2004 Oct-Nov;29(4-5):321–325. doi: 10.1080/02713680490516161. [DOI] [PubMed] [Google Scholar]
- 15.Barro-Soria R, Stindl J, Müller C, Foeckler R, Todorov V, Castrop H, et al. Angiotensin-2-mediated Ca2+ signaling in the retinal pigment epithelium: role of angiotensin-receptor-associated-protein and TRPV2 channel. PLoS One. 2012;7(11):e49624. doi: 10.1371/journal.pone.0049624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarr J, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013 Jan 15;2013:343560–343560. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurihara T, Ozawa Y, Nagai N, Shinoda K, Noda K, Imamura Y, et al. Angiotensin II type 1 receptor signaling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina. Diabetes. 2008 Aug;57(8):2191–2198. doi: 10.2337/db07-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pons M, Cousins S, Alcazar O, Striker GE, Marin-Castaño ME. Angiotensin II-induced MMP-2 activity and MMP-14 and basigin protein expression are mediated via the angiotensin II receptor type 1-mitogen-activated protein kinase 1 pathway in retinal pigment epithelium: implications for age-related macular degeneration. Am J Pathol. 2011 Jun;178(6):2665–2681. doi: 10.1016/j.ajpath.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sjølie A, Chaturvedi N. The retinal renin-angiotensin system: implications for therapy in diabetic retinopathy. J Hum Hypertens. 2002 Aug;16(Suppl 3):S42–S46. doi: 10.1038/sj.jhh.1001438. [DOI] [PubMed] [Google Scholar]
- 20.Nagai N, Izumi-Nagai K, Oike Y, Koto T, Satofuka S, Ozawa Y, et al. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Invest Ophthalmol Vis Sci. 2007 Sep;48(9):4342–4350. doi: 10.1167/iovs.06-1473. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson-Berka J, Campbell D. (Pro)renin receptor: a treatment target for diabetic retinopathy? Diabetes. 2009 Jul;58(7):1485–1487. doi: 10.2337/db09-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ola M, Ahmed M, Abuohashish H, Al-Rejaie SS, Alhomida AS. Telmisartan ameliorates neurotrophic support and oxidative stress in the retina of streptozotocin-induced diabetic rats. Neurochem Res. 2013 Aug;38(8):1572–1579. doi: 10.1007/s11064-013-1058-4. [DOI] [PubMed] [Google Scholar]
- 23.Miller A, Tan G, Binger K, Pickering RJ, Thomas MC, Nagaraj RH, et al. Candesartan attenuates diabetic retinal vascular pathology by restoring glyoxalase-I function. Diabetes. 2010 Dec;59(12):3208–3215. doi: 10.2337/db10-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ola M, Alhomida A, Ferrario C, Ahmad S. Role of Tissue Renin-angiotensin System and the Chymase/angiotensin-(1-12) Axis in the Pathogenesis of Diabetic Retinopathy. Curr Med Chem. 2017;24(28):3104–3114. doi: 10.2174/0929867324666170407141955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takai S, Jin D, Miyazaki M. Chymase as an important target for preventing complications of metabolic syndrome. Curr Med Chem. 2010;17(28):3223–3229. doi: 10.2174/092986710792232003. [DOI] [PubMed] [Google Scholar]
- 26.Luhtala S, Vaajanen A, Oksala O, Valjakka J, Vapaatalo H. Activities of angiotensin-converting enzymes ACE1 and ACE2 and inhibition by bioactive peptides in porcine ocular tissues. J Ocul Pharmacol Ther. 2009 Feb;25(1):23–28. doi: 10.1089/jop.2008.0081. [DOI] [PubMed] [Google Scholar]
- 27.Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, et al. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005 Apr 12;111(14):1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- 28.Peña Silva RA, Kung DK, Mitchell IJ, Alenina N, Bader M, Santos RA, et al. Angiotensin 1-7 reduces mortality and rupture of intracranial aneurysms in mice. Hypertension. 2014 Aug;64(2):362–368. doi: 10.1161/HYPERTENSIONAHA.114.03415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel S, Ali Q, Samuel P, Steckelings UM, Hussain T. Angiotensin II Type 2 Receptor and Receptor Mas Are Colocalized and Functionally Interdependent in Obese Zucker Rat Kidney. Hypertension. 2017 Oct;70(4):831–838. doi: 10.1161/HYPERTENSIONAHA.117.09679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng C, Lei C, Chen Z, Zheng S, Yang H, Qiu Y, et al. Topical administration of diminazene aceturate decreases inflammation in endotoxin-induced uveitis. Mol Vis. 2015 Apr 10;21:403–411. [PMC free article] [PubMed] [Google Scholar]
- 31.Nagai N, Ito Y, Tanino T. Effect of high glucose levels on amyloid β production in retinas of spontaneous diabetes mellitus Otsuka Long-Evans Tokushima fatty rats. Biol Pharm Bull. 2015;38(4):601–610. doi: 10.1248/bpb.b14-00819. [DOI] [PubMed] [Google Scholar]
- 32.Fu X, Lin R, Qiu Y, Yu P, Lei B. Overexpression of Angiotensin-Converting Enzyme 2 Ameliorates Amyloid β-Induced Inflammatory Response in Human Primary Retinal Pigment Epithelium. Invest Ophthalmol Vis Sci. 2017 Jun 01;58(7):3018–3028. doi: 10.1167/iovs.17-21546. [DOI] [PubMed] [Google Scholar]
- 33.Qiu Y, Tao L, Zheng S, Lin R, Fu X, Chen Z, et al. AAV8-Mediated Angiotensin-Converting Enzyme 2 Gene Delivery Prevents Experimental Autoimmune Uveitis by Regulating MAPK, NF-κB and STAT3 Pathways. Sci Rep. 2016 Aug 25;6:31912–31912. doi: 10.1038/srep31912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sukumaran V, Veeraveedu P, Gurusamy N, Lakshmanan AP, Yamaguchi K, Ma M, et al. Telmisartan acts through the modulation of ACE-2/ANG 1-7/mas receptor in rats with dilated cardiomyopathy induced by experimental autoimmune myocarditis. Life Sci. 2012 Feb 13;90(7-8):289–300. doi: 10.1016/j.lfs.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson-Berka J. Prorenin and the (pro)renin receptor in ocular pathology. Am J Pathol. 2008 Dec;173(6):1591–1594. doi: 10.2353/ajpath.2008.080757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002 Jun;109(11):1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the "handle" region for nonproteolytic activation of prorenin. J Clin Invest. 2004 Oct;114(8):1128–1135. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanda A, Noda K, Saito W, Ishida S. Vitreous renin activity correlates with vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol. 2013 May;97(5):666–668. doi: 10.1136/bjophthalmol-2012-302680. [DOI] [PubMed] [Google Scholar]
- 39.Satofuka S, Ichihara A, Nagai N, Tsubota K, Itoh H, Ishida S. Pathologic roles of prorenin and (pro)renin receptor in the eye. Front Biosci. 2008 May 01;13:3884–3895. doi: 10.2741/2976. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, et al. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006 Jan;69(1):105–113. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- 41.Huang Y, Noble N, Zhang J, Xu C, Border WA. Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int. 2007 Jul;72(1):45–52. doi: 10.1038/sj.ki.5002243. [DOI] [PubMed] [Google Scholar]
- 42.Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, et al. (Pro)renin receptor-mediated signal transduction and tissue renin-angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes. 2009 Jul;58(7):1625–1633. doi: 10.2337/db08-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haque R, Hur E, Farrell A, Iuvone PM, Howell JC. MicroRNA-152 represses VEGF and TGFβ1 expressions through post-transcriptional inhibition of (Pro)renin receptor in human retinal endothelial cells. Mol Vis. 2015 Mar 07;21:224–235. [PMC free article] [PubMed] [Google Scholar]
- 44.Kanda A, Noda K, Saito W, Ishida S. (Pro)renin receptor is associated with angiogenic activity in proliferative diabetic retinopathy. Diabetologia. 2012 Nov;55(11):3104–3113. doi: 10.1007/s00125-012-2702-2. [DOI] [PubMed] [Google Scholar]
- 45.Li T, Hu J, Du S, Chen Y, Wang S, Wu Q. ERK1/2/COX-2/PGE2 signaling pathway mediates GPR91-dependent VEGF release in streptozotocin-induced diabetes. Mol Vis. 2014 Jul 31;20:1109–1121. [PMC free article] [PubMed] [Google Scholar]
- 46.Chang P, Chen T, Chang C, Hou CC, Chan P, Lee HM. Advanced glycosylation end products induce inducible nitric oxide synthase (iNOS) expression via a p38 MAPK-dependent pathway. Kidney Int. 2004 May;65(5):1664–1675. doi: 10.1111/j.1523-1755.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Han L, Fu Q, Li Y, Liang Z, Su J, et al. Formation and inhibition of Nε(carboxymethyl)lysine in saccharide-lysine model systems during microwave heating. Molecules. 2012 Oct 31;17(11):12758–12770. doi: 10.3390/molecules171112758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prieto M, Arita D, Bourgeois C, Satou R. Hyperglycemia increases prorenin receptor localization at the cell plasma membrane (1173.7) FASEB J. 2014;28(1) doi: 10.1096/fasebj.28.1_supplement.1173.7. [DOI] [Google Scholar]
- 49.Shi R, Wang J, Huang S, Wang XJ, Li QP. Angiotensin II induces vascular endothelial growth factor synthesis in mesenchymal stem cells. Exp Cell Res. 2009 Jan 01;315(1):10–15. doi: 10.1016/j.yexcr.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez L, Twickler J, Mead A. Neovascularization produced by angiotensin II. The J Lab Clin Med. 1985 Feb;105(2):141–145. doi: 10.1084/jem.161.6.1593. [DOI] [PubMed] [Google Scholar]
- 51.Aiello L, Bursell S, Clermont A, Duh E, Ishii H, Takagi C, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997 Sep;46(9):1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 52.Kim J, Kim J, Park J, Lee SW, Kim WJ, Yu YS, et al. Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol. 2006 Jul 31;39(4):339–345. doi: 10.5483/bmbrep.2006.39.4.339. [DOI] [PubMed] [Google Scholar]
- 53.Kim J, Kim J, Yu Y, Cho CS, Kim KW. Blockade of angiotensin II attenuates VEGF-mediated blood-retinal barrier breakdown in diabetic retinopathy. J Cereb Blood Flow Metab. 2009 Mar;29(3):621–628. doi: 10.1038/jcbfm.2008.154. [DOI] [PubMed] [Google Scholar]
- 54.Beltramo E, Porta M. Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem. 2013;20(26):3218–3225. doi: 10.2174/09298673113209990022. [DOI] [PubMed] [Google Scholar]
- 55.Park S, Yun J, Kim J, Kim KW, Cho CH, Kim JH. Angiopoietin 2 induces pericyte apoptosis via α3β1 integrin signaling in diabetic retinopathy. Diabetes. 2014 Sep;63(9):3057–3068. doi: 10.2337/db13-1942. [DOI] [PubMed] [Google Scholar]
- 56.Ozawa Y, Yuki K, Yamagishi R, Tsubota K, Aihara M. Renin-angiotensin system involvement in the oxidative stress-induced neurodegeneration of cultured retinal ganglion cells. Jpn J Ophthalmol. 2013 Jan;57(1):126–132. doi: 10.1007/s10384-012-0204-x. [DOI] [PubMed] [Google Scholar]
- 57.Geraldes P, King G. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010 Apr 30;106(8):1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brandes R, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005 Jan 01;65(1):16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Chen P, Guo A, Edwards P, Trick G, Scicli AG. Role of NADPH oxidase and ANG II in diabetes-induced retinal leukostasis. Am J Physiol Regul Integr Comp Physiol. 2007 Oct;293(4):R1619–R1629. doi: 10.1152/ajpregu.00290.2007. [DOI] [PubMed] [Google Scholar]
- 60.Sugiyama T, Okuno T, Fukuhara M, Oku H, Ikeda T, Obayashi H, et al. Angiotensin II receptor blocker inhibits abnormal accumulation of advanced glycation end products and retinal damage in a rat model of type 2 diabetes. Exp Eye Res. 2007 Sep;85(3):406–412. doi: 10.1016/j.exer.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 61.White A, Heller J, Leung J, Tassoni A, Martin KR. Retinal ganglion cell neuroprotection by an angiotensin II blocker in an ex vivo retinal explant model. J Renin Angiotensin Aldosterone Syst. 2015 Dec;16(4):1193–1201. doi: 10.1177/1470320314566018. [DOI] [PubMed] [Google Scholar]
- 62.Haque R, Iuvone P, He L, Hur EH, Chung Choi KS, Park D, et al. Prorenin receptor (PRR)-mediated NADPH oxidase (Nox) signaling regulates VEGF synthesis under hyperglycemic condition in ARPE-19 cells. J Recept Signal Transduct Res. 2017 Dec;37(6):560–568. doi: 10.1080/10799893.2017.1369120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mustapha N, Tarr J, Kohner E, Chibber R. NADPH Oxidase versus Mitochondria-Derived ROS in Glucose-Induced Apoptosis of Pericytes in Early Diabetic Retinopathy. J Ophthalmol. 2010;2010:746978–746978. doi: 10.1155/2010/746978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H, Han X, Wittchen E, Hartnett M. TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation. Mol Vis. 2016 Feb 03;22:116–128. [PMC free article] [PubMed] [Google Scholar]
- 65.Patel N. Targeting leukostasis for the treatment of early diabetic retinopathy. Cardiovasc Hematol Disord Drug Targets. 2009 Sep;9(3):222–229. doi: 10.2174/187152909789007052. [DOI] [PubMed] [Google Scholar]
- 66.Lai A, Lo A. Animal models of diabetic retinopathy: summary and comparison. J Diabetes Res. 2013;2013:106594–106594. doi: 10.1155/2013/106594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marin Garcia P, Marin-Castaño M. Angiotensin II-related hypertension and eye diseases. World J Cardiol. 2014 Sep 26;6(9):968–984. doi: 10.4330/wjc.v6.i9.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kowluru R, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004 Oct;88(10):1343–1347. doi: 10.1136/bjo.2003.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tait S, Tait J, Coghlan J. The discovery, isolation and identification of aldosterone: reflections on emerging regulation and function. Mol Cell Endocrinol. 2004 Mar 31;217(1-2):1–21. doi: 10.1016/j.mce.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 70.Connell J, Davies E. The new biology of aldosterone. J Endocrinol. 2005 Jul;186(1):1–20. doi: 10.1677/joe.1.06017. [DOI] [PubMed] [Google Scholar]
- 71.Funder J. Aldosterone and Mineralocorticoid Receptors-Physiology and Pathophysiology. Int J Mol Sci. 2017 May 11;18(5):1032–1032. doi: 10.3390/ijms18051032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao M, Valamanesh F, Celerier I, Savoldelli M, Jonet L, Jeanny JC, et al. The neuroretina is a novel mineralocorticoid target: aldosterone up-regulates ion and water channels in Müller glial cells. FASEB J. 2010 Sep;24(9):3405–3415. doi: 10.1096/fj.09-154344. [DOI] [PubMed] [Google Scholar]
- 73.Deliyanti D, Miller A, Tan G, Binger KJ, Samson AL, Wilkinson-Berka JL. Neovascularization is attenuated with aldosterone synthase inhibition in rats with retinopathy. Hypertension. 2012 Mar;59(3):607–613. doi: 10.1161/HYPERTENSIONAHA.111.188136. [DOI] [PubMed] [Google Scholar]
- 74.Rattner A, Nathans J. The genomic response to retinal disease and injury: evidence for endothelin signaling from photoreceptors to glia. J Neurosci. 2005 May 04;25(18):4540–4549. doi: 10.1523/JNEUROSCI.0492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Binz N, Rakoczy E, Ali Rahman I, Vagaja NN, Lai CM. Biomarkers for Diabetic Retinopathy - Could Endothelin 2 Be Part of the Answer? PLoS One. 2016 Aug 02;11(8):e0160442. doi: 10.1371/journal.pone.0160442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alrashdi S, Deliyanti D, Talia D, Wilkinson-Berka J. Endothelin-2 Injures the Blood-Retinal Barrier and Macroglial Müller Cells: Interactions with Angiotensin II, Aldosterone, and NADPH Oxidase. Am J Pathol. 2018 Mar;188(3):805–817. doi: 10.1016/j.ajpath.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Ohashi K, Hayashi T, Utsunomiya K, Nishimura R. The mineralocorticoid receptor signal could be a new molecular target for the treatment of diabetic retinal complication. Expert Opin Ther Targets. 2022 May;26(5):479–486. doi: 10.1080/14728222.2022.2072730. [DOI] [PubMed] [Google Scholar]
- 78.Zhao M, Gelize E, Levy R, Moulin A, Azan F, Berdugo M, et al. Mineralocorticoid Receptor Pathway and Its Antagonism in a Model of Diabetic Retinopathy. Diabetes. 2021 Nov;70(11):2668–2682. doi: 10.2337/db21-0099. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, Xi X, Gao L, Kern T. Captopril inhibits capillary degeneration in the early stages of diabetic retinopathy. Curr Eye Res. 2007 Oct;32(10):883–889. doi: 10.1080/02713680701584123. [DOI] [PubMed] [Google Scholar]
- 80.Zheng Z, Chen H, Ke G, Fan Y, Zou H, Sun X, et al. Protective effect of perindopril on diabetic retinopathy is associated with decreased vascular endothelial growth factor-to-pigment epithelium-derived factor ratio: involvement of a mitochondria-reactive oxygen species pathway. Diabetes. 2009 Apr;58(4):954–964. doi: 10.2337/db07-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moravski C, Kelly D, Cooper M, Gilbert RE, Bertram JF, Shahinfar S, et al. Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension. 2000 Dec;36(6):1099–1104. doi: 10.1161/01.hyp.36.6.1099. [DOI] [PubMed] [Google Scholar]
- 82.Ishibashi Y, Matsui T, Yamagishi S. Olmesartan blocks advanced glycation end products-induced vcam-1 gene expression in mesangial cells by restoring Angiotensin-converting enzyme 2 level. Horm Metab Res. 2014 Jun;46(6):379–383. doi: 10.1055/s-0033-1361114. [DOI] [PubMed] [Google Scholar]
- 83.Lin Z, Ni Y, Hou L, Song L, Wu Y, Hu H, et al. [Telmisartan reduces retina vessel endothelial cell apoptosis via upregulating retinal ACE2-Ang-(1-7)-Mas axis in spontaneous hypertensive rats] Zhonghua Xin Xue Guan Bing Za Zhi. 2015 Jul;43(7):625–630. [PubMed] [Google Scholar]
- 84.Guo X, Namekata K, Kimura A, Harada C, Harada T. The Renin-Angiotensin System Regulates Neurodegeneration in a Mouse Model of Optic Neuritis. Am J Pathol. 2017 Dec;187(12):2876–2885. doi: 10.1016/j.ajpath.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 85.Wang B, Wang F, Zhang Y, Zhao SH, Zhao WJ, Yan SL, et al. Effects of RAS inhibitors on diabetic retinopathy: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015 Apr;3(4):263–274. doi: 10.1016/S2213-8587(14)70256-6. [DOI] [PubMed] [Google Scholar]
- 86.Chen L, Kim S, Eisner C, Oppermann M, Huang Y, Mizel D, et al. Stimulation of renin secretion by angiotensin II blockade is Gsalpha-dependent. J Am Soc Nephrol. 2010 Jun;21(6):986–992. doi: 10.1681/ASN.2009030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pradhan R, Fong D, March C, Jack R, Rezapour G, Norris K, et al. Angiotensin-converting enzyme inhibition for the treatment of moderate to severe diabetic retinopathy in normotensive Type 2 diabetic patients. A pilot study. J Diabetes Complications. 2002 Nov-Dec;16(6):377–381. doi: 10.1016/s1056-8727(02)00188-5. [DOI] [PubMed] [Google Scholar]
- 88.Sjølie A, Klein R, Porta M, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008 Oct 18;372(9647):1385–1393. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 89.Sjølie A, Klein R, Porta M, Orchard T, Fuller J, Parving HH, et al. Retinal microaneurysm count predicts progression and regression of diabetic retinopathy. Post-hoc results from the DIRECT Programme. Diabet Med. 2011 Mar;28(3):345–351. doi: 10.1111/j.1464-5491.2010.03210.x. [DOI] [PubMed] [Google Scholar]
- 90.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009 Jul 02;361(1):40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilkinson-Berka J, Tan G, Binger K, Sutton L, McMaster K, Deliyanti D, et al. Aliskiren reduces vascular pathology in diabetic retinopathy and oxygen-induced retinopathy in the transgenic (mRen-2)27 rat. Diabetologia. 2011 Oct;54(10):2724–2735. doi: 10.1007/s00125-011-2239-9. [DOI] [PubMed] [Google Scholar]
- 92.Simão S, Santos D, Silva G. Aliskiren inhibits the renin-angiotensin system in retinal pigment epithelium cells. Eur J Pharm Sci. 2016 Sep 20;92:22–27. doi: 10.1016/j.ejps.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 93.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004 May 25;109(20):2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 94.Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006 Mar 17;98(5):697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 95.Chen Y, Lin S, Lin F, Wu TC, Tsao CR, Huang PH, et al. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007 Jun;56(6):1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- 96.Loomans C, de Koning E, Staal F, Rookmaaker MB, Verseyden C, de Boer HC, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004 Jan;53(1):195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 97.Tepper O, Galiano R, Capla J, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002 Nov 26;106(22):2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 98.Chang T, Wu T, Huang P, Chen JS, Lin LY, Lin SJ, et al. Aliskiren directly improves endothelial progenitor cell function from Type II diabetic patients. Eur J Clin Invest. 2016 Jun;46(6):544–554. doi: 10.1111/eci.12632. [DOI] [PubMed] [Google Scholar]
- 99.Barrera-Chimal J, Rocha L, Amador-Martínez I, Pérez-Villalva R, González R, Cortés-González C, et al. Delayed spironolactone administration prevents the transition from acute kidney injury to chronic kidney disease through improving renal inflammation. Nephrol Dial Transplant. 2019 May 01;34(5):794–801. doi: 10.1093/ndt/gfy246. [DOI] [PubMed] [Google Scholar]
- 100.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020 Dec 03;383(23):2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 101.Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N Engl J Med. 2021 Dec 09;385(24):2252–2263. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 102.Armanini D, Endres S, Kuhnle U, Weber PC. Parallel determination of mineralocorticoid and glucocorticoid receptors in T- and B-lymphocytes of human spleen. Acta Endocrinol (Copenh) 1988 Aug;118(4):479–482. doi: 10.1530/acta.0.1180479. [DOI] [PubMed] [Google Scholar]
- 103.Jerome JR, Deliyanti D, Suphapimol V, Kolkhof P, Wilkinson-Berka JL. Finerenone, a Non-Steroidal Mineralocorticoid Receptor Antagonist, Reduces Vascular Injury and Increases Regulatory T-Cells: Studies in Rodents with Diabetic and Neovascular Retinopathy. Int J Mol Sci. 2023 Jan 25;24(3):2334–2334. doi: 10.3390/ijms24032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Higashide T, Hirooka K, Kometani M, Sugiyama K. Aldosterone as a Possible Contributor to Eye Diseases. Endocrinology. 2022 Dec 19;164(2):bqac201–bqac201. doi: 10.1210/endocr/bqac201. [DOI] [PubMed] [Google Scholar]
- 105.Virgili G, Parravano M, Evans J, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2018 Oct 16;10(10) doi: 10.1002/14651858.CD007419.pub6. CD007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Madjedi K, Pereira A, Ballios B, Arjmand P, Kertes PJ, Brent M, et al. Switching between anti-VEGF agents in the management of refractory diabetic macular edema: A systematic review. Surv Ophthalmol. 2022 Sep-Oct;67(5):1364–1372. doi: 10.1016/j.survophthal.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 107.Li Y, Yan Z, Chaudhry K, Kazlauskas A. The Renin-Angiotensin-Aldosterone System (RAAS) Is One of the Effectors by Which Vascular Endothelial Growth Factor (VEGF)/Anti-VEGF Controls the Endothelial Cell Barrier. Am J Pathol. 2020 Sep;190(9):1971–1981. doi: 10.1016/j.ajpath.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov. 2017 Sep;16(9):635–661. doi: 10.1038/nrd.2016.278. [DOI] [PubMed] [Google Scholar]
- 109.Duran C, Borriello L, Karagiannis G, Entenberg D, Oktay MH, Condeelis JS. Targeting Tie2 in the Tumor Microenvironment: From Angiogenesis to Dissemination. Cancers (Basel) 2021 Nov 16;13(22):5730–5730. doi: 10.3390/cancers13225730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thurston G, Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb Perspect Med. 2012 Sep 01;2(9):a006550–a006550. doi: 10.1101/cshperspect.a006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parikh S. Angiopoietins and Tie2 in vascular inflammation. Curr Opin Hematol. 2017 Sep;24(5):432–438. doi: 10.1097/MOH.0000000000000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Foxton R, Uhles S, Grüner S, Revelant F, Ullmer C. Efficacy of simultaneous VEGF-A/ANG-2 neutralization in suppressing spontaneous choroidal neovascularization. EMBO Mol Med. 2019 May;11(5):e10204. doi: 10.15252/emmm.201810204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wykoff C, Abreu F, Adamis A, Basu K, Eichenbaum DA, Haskova Z, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022 Feb 19;399(10326):741–755. doi: 10.1016/S0140-6736(22)00018-6. [DOI] [PubMed] [Google Scholar]