INTRODUCTION
Normal wound healing of the face and neck is a commonly expected and assumed outcome from patients. However, when postsurgical wound complications occur, disfigurement and compromise of quality of life are clearly apparent for all to see with public scrutiny. The goals of this review article are to identify and understand common factors that contribute to poor surgical healing. By understanding these factors, one can help reduce surgical wound complications. Early recognition and clinical modification of variables that contribute to poor healing can assist in improved surgical wound outcomes.
OVERVIEW OF NORMAL WOUND HEALING
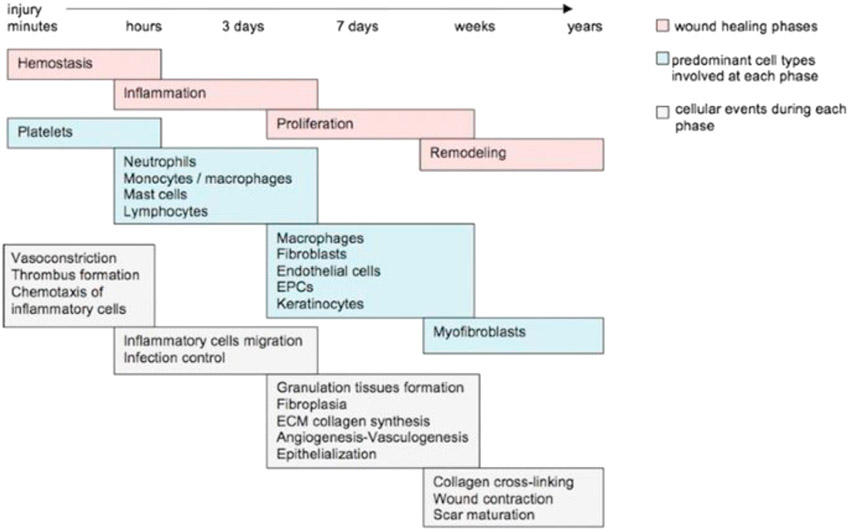
The wound healing process is made up of a highly orchestrated biological cascade of cell signaling and repair mechanisms via cytokines, growth factors, various cell types, and structural elements. This process is divided into four main overlapping phases: hemostasis, inflammation, proliferation, and maturation or remodeling. Any delay or interruption in the phases of acute healing ultimately results in prolonged healing and an increased risk of developing a chronic or nonhealing wound. The four phases of normal wound healing are briefly described in the following section and are illustrated in Fig. 1.
Fig. 1.
Depiction of overlapping phases of repair in wound healing. Any disruption in the natural cascade of healing will ultimately delay healing and potentially lead to poor surgical healing, chronic wounds, and scarring. ECM, extracellular matrix; EPCs, endothelial progenitor cells. (From Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and Treatment of Impaired Wound Healing in Diabetes Mellitus: New Insights. Adv Ther. 2014;31(8):817-836. https://doi.org/10.1007/s12325-014-0140-x with permission.)
Hemostasis
The first stage of wound healing is the hemostatic phase that begins within seconds to minutes of acute tissue injury. This is marked by vasoconstriction that reduces blood loss and the initiation of the extrinsic clotting cascade. Thrombocytes are activated by exposed collagen, and activated platelets adhere to the collagen and release cell signaling molecules resulting in the formation of a fibrin-platelet matrix.1 The matrix serves a role in clot formation and acts as a scaffold for growth factors and cytokines to signal the initiation of the healing process.
Inflammation
The inflammatory phase begins within 30 minutes and usually persists for several days. As a result of platelet degranulation and cytokine cascade, this phase is marked by 2 to 3 days of capillary vasodilation, which facilitates the migration of the characteristic inflammatory infiltrate to the wound.2 Neutrophils are the initial cell type that phagocytose debris, kill bacteria, and secrete proteolytic enzymes. Monocytes then appear and transition to macrophages which are key for proper wound healing which phagocytize remaining debris and produce key cytokines that stimulate fibroblasts and regulate the production of collagen. A key point is that a wound remains in the inflammatory phase as long there is a persistent bacterial burden or other inflammatory nidus present such as a reactive foreign body. This is important to recognize as wounds that exhibit signs of persistent inflammation for more than 7 days are prolonging the inflammatory phase, a common initial sign of poor or delayed wound healing.1
Proliferation
The proliferative phase is marked by fibroplasia, neovascularization, and reepithelialization. Fibroblasts migrate to the wound and proliferate forming disorganized type III collagen during the formation of granulation tissue. Neovascularization allows new blood vessels to form to provide nutrients and oxygen for cells to sustain wound healing. After adequate granulation tissue has developed, reepithelialization initiated by keratinocytes and wound contraction by myofibroblasts should begin to occur within 2 to 3 weeks to complete the proliferative phase.
Maturation and Remodeling
The final stage of wound healing is the maturation or remodeling phase during which type III collagen is remodeled and replaced by stronger, more organized type I collagen. The remodeling phase begins at about 3 weeks and continues approximately 1 year. Although the maximum amount of collagen is present in the wound around 3 to 6 weeks, collagen is continuously remodeled by macrophages that secrete proteases and collagenases forming mature collagen and increasing the wound’s tensile strength which plateaus at 80% of its original strength 3 months following injury.2,3 The final appearance of the scar occurs 6 to 12 months after injury.
PREOPERATIVE CONSIDERATIONS AND PATIENT CHARACTERISTICS
Reducing the risk of poor surgical wound healing ultimately begins during the preoperative consultation when interviewing patients about their medical history and comorbidities that may affect their surgical outcome. This requires a thorough history and exam to look for extrinsic and intrinsic factors (Table 1) that may adversely impact wound healing. During this process, common conditions that affect tissue oxygenation such as pulmonary diseases, congestive heart failure, anemia, and cigarette smoking can be elicited. In addition, comorbid conditions such as malnutrition, diabetes, hypothyroidism, alcoholism, malignancy, and chronic conditions requiring certain medications can usually be identified during routine history and physical. In certain circumstances, additional workup may be indicated to diagnose or monitor chronic conditions including a complete blood count, metabolic panel, renal panel, serum glucose, hemoglobin A1c, nutritional panel, and/or thyroid stimulating hormone level.
Table 1.
Common factors contributing to poor surgical wound healing
| Extrinsic Factors |
Intrinsic Factors |
|---|---|
| Previous irradiation | Malnutrition |
| Chronic steroid use | Obesity |
| Cigarette smoking | Diabetes |
| Vaping | Hypothyroidism |
| Medicationsa | Aging |
| Ischemic conditions (ie, anemia, COPD, CHF, vascular disease, and renal failure) | |
| Alcoholism | |
| Immunodeficiency |
Medications listed below.
Surgical wounds on the face and neck generally are known to heal rapidly with few complications due to the robust blood supply in this area. As a result, when poor wound healing does occur, it can often be attributed to one of the following underlying extrinsic or intrinsic factors that impede healing.
EXTRINSIC FACTORS CONTRIBUTING TO POOR SURGICAL HEALING
Previous irradiation
Radiotherapy is a common modality used in the management of certain head and neck cancers either as primary treatment, adjuvant treatment (in addition to surgical resection with or without chemotherapy), or palliative therapy. Along with damaging tumor cells, however, radiotherapy also damages surrounding healthy tissue leading to complications such as skin atrophy, soft tissue fibrosis, desquamation, epithelial ulceration, and microvascular damage that overall contribute to poor healing of wounds.4 Short-term reactions from radiotherapy occur in almost all patients due to the acute radiation injury to healthy epithelial cells resulting in radiation-induced DNA mutations, microvascular damage, and fibrosis. As consecutive radiation doses are applied, the cycle of normal cell regeneration is interrupted which may lead to delayed or nonhealing radiation ulcers. Cell damage is progressive and may continue for several years even after the course of radiotherapy resulting in long-term complications such as necrosis, atrophy, fibrosis, vascular damage, and carcinogenesis. Because of these detrimental effects on wound healing, subsequent surgical procedures on tissues that were previously irradiated heal poorly resulting in increased rates of delayed healing and complications such as flap failures, dehiscence, fistulas, wound necrosis, and infection.
Current strategies to reduce the risk of poor surgical healing in irradiated tissues are still very limited. Radiation oncologists are focusing on more precise targeting of radiotherapy to limit the dose of radiation to surrounding healthy, noncancerous cells and subsequent radiation-induced damage. For the surgeon, preoperative planning and a preventive approach to optimize conditions for wound healing and minimize potential complications are critical. The timing of radiotherapy in reference to surgery can also significantly affect the healing process. In patients receiving postoperative radiation, radiation can be initiated as early as 3 weeks following surgery without significantly impeding wound healing. According to Payne and colleagues,5 wound healing is most affected when surgery is performed 6 months or more after completion of radiotherapy. Therefore in patients receiving preoperative radiation, the optimal time for surgery is 3 weeks to 3 months following completion of radiation therapy.6 In cases with similar oncologic outcomes in patients treated with preoperative versus postoperative radiation therapy, postoperative radiation is preferred due to fewer wound healing complications.5 Other proposed strategies to reduce the risk of surgical wound healing complications in irradiated head and neck cancer patients include adjunctive wound care modalities to reduce bacterial colonization, promote a moist environment, and stimulate granulation in addition to microvascular free tissue transfer.7 Hyperbaric oxygen therapy (HBO) has also been used clinically in patients with wound healing complications after radiotherapy.
Chronic steroid use
Molecular studies have shown that corticosteroids affect all major phases of the wound-healing process. For example, during the inflammatory phase, treatment with dexamethasone has been shown to decrease the expression of important cytokines such as TGF-beta1, platelet-derived growth factor, tumor necrosis factor, and interleukin-1alpha thereby limiting the chemotactic and mitogenic stimulus for other inflammatory cells.8,9 Dexamethasone use also causes decreased expression of intercellular adhesion molecules resulting in impaired cellular adhesion and migration.10 Corticosteroids affect the second stage or proliferative phase of wound healing by reducing fibroblast proliferation and reepithelialization. Furthermore, during the final stage of wound healing, the collagen maturation process is adversely affected due to reduced wound tensile strength. A review of animal studies and the effect of steroids on wound strength shows an approximately 30% reduction in wound tensile strength at cortisone doses of 15 to 40 mg/kg/d (equivalent to 200 to 560 mg/d of prednisone in a 70-kg human).11 In addition, patients chronically exposed to high levels of adrenocorticoids such as those with Cushing syndrome show the closest evidence that corticosteroid excess in humans decreases cutaneous wound tensile strength in a similar fashion by approximately 40%.
Although there are no formal recommendations regarding preoperative chronic steroid use in facial plastic surgery, data from other surgical specialties suggest that patients taking prednisone for at least 30 days preoperatively at doses of 40 mg/d or higher, may have increased wound complication rates by approximately two to five times compared with patients not taking corticosteroids.11 In addition, a recent retrospective study of more than 94,000 plastic surgery cases from the American College of Surgeons National Surgical Quality Improvement Program database showed that chronic steroid users making up 1.8% of the study population were 1.25 times more likely to develop surgical complications and 1.77 times more likely to develop medical complications on multivariate analysis.12 Limitations to the previously mentioned study include the inability to determine the dose or duration of chronic steroid use and the indication for which it was used; however, this information is still helpful in preoperative counseling of patients on steroid therapy.
Vitamin A plays an important role in wound healing due to its ability to stimulate epithelial growth, fibroblasts, granulation tissue, angiogenesis, collagen synthesis, epithelialization, and fibroplasia.13 As a result, Vitamin A has been used to aid wound healing in steroid patients. In fact, studies have shown that dexamethasone significantly impairs the healing of both tracheal anastomoses and intestinal anastomoses in rats and rabbits, respectively, and Vitamin A has been used to counteract this inhibitory effect.14,15 Unfortunately, the evidence for Vitamin A supplementation is limited due to the lack of human clinical trials; however, in wound patients, short-term vitamin A supplementation of 10,000 to 25,000 IU/day has been recommended.16 Vitamin a toxicity can be a serious issue and even result in death; therefore, the use of vitamin A for counteracting corticosteroid use and optimization of wound healing should be carefully weighed against potential risks and side effects.
Medications
Certain medications are associated with poor wound healing and such medications should be elicited during the preoperative assessment. The patient should be informed that the risk of slow or poor surgical healing can occur with concurrent use of these medications (Box 1).
Box 1. Common medications associated with poor wound healing.
Steroids
Chemotherapeutic medications
Aspirin
NSAIDs (ibuprofen)
Penicillamine
Colchicine
Antirheumatic drugs (methotrexate)
Vasoconstrictive drugs (nicotine, adrenaline, and cocaine)
Anticoagulants (heparin and warfarin)
Smoking
The detrimental effects of cigarette smoke on wound healing were first reported in the late 1970s by Mosely and colleagues17 and it is now widely accepted that smoking cessation may reduce the risk of postoperative wound complications. Although cigarette smoke contains more than 4,000 ingredients, nicotine, carbon monoxide, hydrogen cyanide, and nitric oxide are the main culprits for impaired wound healing. This involves multiple mechanisms working synergistically to decrease oxygen delivery to tissues, cause endothelial injury, reduce oxygen utilization, increase thrombogenesis, and impair the means of cellular repair. Nicotine, one of the most commonly studied cigarette components, is believed to cause local tissue ischemia due to its vasoconstrictive activity. One study showed that smoking one cigarette reduces tissue perfusion by 22% to 48% in 30 minutes.18 Interestingly, gum containing nicotine did not affect tissue oxygenation with less nicotine serum peak levels compared with smoking.
Cigarette smoking also negatively impacts the inflammatory and proliferative phases of wound healing. For example, a randomized controlled trial in which smokers were randomized to continuous smoking or abstinence with a nicotine or placebo patch showed attenuated inflammation and fibroblast proliferation in smokers and restoration of inflammation following abstinence.19 Smoking has also been associated with higher rates of wound infections and randomized trials have shown reduced incidences of infections following 4 weeks of smoking cessation.20
Facial plastics literature suggests that cigarette use is associated with increased severe surgical complications. Retrospective studies have shown increased nasal recovery time and increased rates of development of septal perforation in smokers following septoplasty compared with non-smokers21 and almost three times the rate of skin slough in smokers following facelift procedures.22 Parikh and Jacono23 showed that a deep plan face-lifting technique can be used to decrease rates of skin slough even in smokers. Nevertheless, due to the electiveness of these procedures, some facial plastic surgeons have recommended mandatory smoking cessation before consideration of performing certain surgical procedures.
Although the evidence is clear that smoking adversely affects wound healing, established guidelines to guide surgeons in reducing perioperative risk among smokers are lacking. The exact duration of perioperative abstinence before elective surgery is unknown. Studies suggest that at least 4 weeks is ideal; however, longer durations of smoking cessation, approximately 6 weeks have been shown to significantly reduce perioperative risk.24 Nevertheless, all patients should be counseled on smoking cessation preoperatively with a goal of abstinence for at least 4 to 6 weeks before elective surgery.
Vaping (e-cigarettes)
Vaping is believed to be a detrimental risk factor for poor surgical wound healing, akin to cigarette smoking; however, the exact mechanism of tissue damage remains unknown. One study sought to identify the underlying molecular mechanism and found that both cigarette smoking and vaping were associated with decreased vascular endothelial growth factor expression, decreased microvessel density, and decreased areas of fibrosis in flap tissue of rodents exposed to vaping or cigarettes compared with controls.25 Another animal study found that regular vaping within a month before surgery increases the risk of flap necrosis and that smoking and vaping were equally detrimental to wound healing.26 As such, vaping should not be seen as a better alternative to cigarette smoking in the context of surgical wound healing and preoperative cessation should also be encouraged.
INTRINSIC FACTORS CONTRIBUTING TO POOR SURGICAL HEALING
Malnutrition
Adequate nutritional status is fundamental to the wound healing process. Malnutrition occurs when there is an imbalance between the nutrients that the body receives and the energy needed for the body to function properly. This imbalance may be undernutrition or overnutrition. For the purposes of this review, we focus on undernutrition and how nutrient deficiencies affect the wound healing process.
Undernutrition can be divided into macronutrient and micronutrient deficiencies. Macronutrients include proteins, fats, and carbohydrates. Protein malnutrition affects all four stages of wound healing. For example, inflammatory cell production depends on available protein and a deficiency results in an impaired immune response, which in turn will delay progression from the inflammatory to the proliferative phase. Protein deficiency also decreases fibroblast activity in the proliferative and remodeling phases, resulting in reduced angiogenesis and collagen formation.27 Protein deficiency is associated with weight loss and decreased lean body mass. Although the body favors wound healing in patients who have lost approximately 20% lean body mass, this process is delayed while the body restores lean mass in patients who lose 30% lean body mass or more.28 In addition, both carbohydrates and fats are important to support the inflammatory process, cellular formation and activity, angiogenesis, and collagen deposition. Specifically, carbohydrates are necessary for key events such as fibroblast production, leukocyte activity, and hormone and growth factor secretion. A depleted carbohydrate state also leads to protein catabolism which in turn depletes protein reserves essential to healing.6 Fats are essential in building cell membranes and are also precursors to prostaglandins which are mediators of cellular inflammation and metabolism. Despite this evidence, formal recommendations for macronutrient supplementation are not well established.
Micronutrients involved in wound healing include amino acids, vitamins, and minerals. Arginine and glutamine are the key amino acids that play a role in the wound healing process and are involved in the inflammatory phase, collagen synthesis, antioxidation, and other essential enzymatic reactions. Kirk and colleagues29 showed significantly higher amounts of total protein and improved reparative collagen synthesis in elderly patients treated with arginine supplementation for 2 weeks suggesting that arginine supplementation may enhance wound healing and immune responses in this patient population. Similarly vitamins such as vitamins A and C and B vitamins all significantly impact wound healing. Deficiencies in vitamin A result in impaired stimulation of fibroblasts, granulation tissue, angiogenesis, collagen synthesis, epithelialization, and fibroplasia.13 Local (topical) and systemic supplementation with vitamin A has been proven to increase dermal collagen deposition and vitamin A has been shown to counteract delayed wound healing due to corticosteroid use. Patients with vitamin A deficiency should receive 25,000 IU/day of vitamin A supplementation.5 Vitamin C and B1 (thiamine) are essential for cross-linking during collagen synthesis and deficiencies result in decreased wound strength.6,30 Vitamin C is also involved in cell migration and transformation into macrophages during the inflammatory process and has key antioxidant properties that counteract the production of free radicals in damaged cells.27 The current recommendation for vitamin C supplementation to reduce the risk of poor healing in noncomplicated wounds is 500 mg/d.28 Supplementation should be increased to 1 to 2 g/d in patients with severe wounds. Chronic alcohol consumption is a known risk factor for thiamine deficiency. Although thiamine deficiency is rare in other well-developed populations, it may also be seen in patients with a history of bariatric surgery, Crohn’s disease, anorexia, kidney disease, or restrictive diets and should be corrected.
Zinc is an essential trace element in the human body and an important cofactor in numerous processes including cellular DNA replication, activation of lymphocytes, formation of antibodies, auto-debridement via matrix metalloproteinases, and stimulation of collagen production, fibroblast proliferation, and epithelialization. As a result, some experts recommend zinc supplementation in the perioperative period especially in high-risk populations such as head and neck cancer patients.6 Current data support the use of oral zinc supplementation in zinc-deficient chronic leg ulcer patients31; however, the benefit of supplementation in non-zinc-deficient patients and surgical patients remains controversial. Topical zinc administration to surgical wounds and application of zinc-containing surgical bandages to surgical wounds, however, has been shown to improve the wound healing process.31,32
Malnourishment in surgical patients may be more common than one expects. One study showed that 12% of non-cancer, vascular, and abdominal surgery patients had preexisting malnutrition that adversely affected their postoperative outcome and increased their hospital stay.33 Owing to the high incidence and significant implications of malnourishment on surgical healing, nutritional status should be considered during preoperative evaluation. Signs of malnutrition such as cachexia, muscle wasting, history of >20% weight loss, and gastrointestinal malabsorption (ie, history of gastric bypass or Crohn’s disease) should be identified and corrected before surgery if possible. In addition, malnutrition can be shown in patients with low serum albumin (<3.0 mg/dL), prealbumin (<15 mg/dL), or transferrin (<200 mg/dL).1 Common risk factors for malnutrition include low income, chronic illnesses, advanced age, and pediatric populations. Preoperative optimization of nutritional status with protein/multi-nutrient supplementation as early as possible is recommended for patients with preexisting malnutrition to reduce the risk of poor surgical healing. For those with poor nutritional status and decreased oral intake, enteral nutrition has been shown to significantly improve postoperative healing.5
Obesity
Obesity, referring to a body mass index (BMI) ≥ 30 kg/m2, is a well-established risk factor for poor surgical outcomes, particularly poor wound healing; however, the exact mechanisms responsible are not well understood. Postulated mechanisms of obesity-associated impairments in wound healing are related to reduced perfusion of adipose tissue due to decreased capillary density and impaired angiogenesis and chronic aberrant low-grade inflammation which inhibits normal inflammatory responses that facilitate wound healing.34 Animal studies have shown impaired reepithelialization, decreased fibroblast activity, reduced wound strength, and scar formation in rodents fed high-fat diets compared with nonobese control mice.35,36
Diabetes
Hyperglycemia (glucose >140 mg/dL) is a known independent risk factor for poor surgical outcomes including delayed wound healing, increased rates of infection, prolonged hospital stay, and higher postoperative mortality. The mechanisms of impaired wound healing are related to vascular dysfunction and neurologic impairment in addition to impaired inflammatory and cellular signaling processes affecting the inflammatory, proliferative, and remodeling phases of the healing cascade.37 In diabetic patients, a detailed history of their medical condition including type of diabetes, anti-diabetic medications, current glycemic control, and related complications should be obtained during preoperative evaluation. In addition, a glycosylated hemoglobin A1c (HbA1c) should be checked on all patients if one has not already been obtained within the last 3 months as data suggests that the risk of wound healing complications is four times higher in diabetic patients with HbA1c greater than 7.8% compared with diabetic patients with HbA1c levels less than 7.8%.38 As a result, surgeons should carefully weigh this risk and consider postponing elective or aesthetic surgeries until a patient’s blood glucose levels are better controlled.
Aging
As we age, several age-related changes occur in normal skin, which negatively impact wound healing. These changes include decreased collagen density, fewer fibroblasts, macrophages, and mast cells, increased elastin fragmentation, and slower wound contraction.6,39 Other notable changes in elderly skin are decreased epidermal growth rate, flattening of the dermal-epidermal junction, and reduced dermal cellularity and vascularity.39,40 Morphologic alterations in elderly skin ultimately affect all phases in the wound healing process resulting in functional impairment and temporal delay in wound healing; however, the quality of healing may be unaffected.41 Similar to obese patients, delayed wound healing in the elderly is associated with an altered inflammatory response including increased secretion of inflammatory mediators, delayed infiltration of macrophages and lymphocytes, impaired macrophage function, and decreased secretion of growth factors. Other age-related alterations in the healing process are enhanced platelet aggregation, delayed reepithelialization, delayed angiogenesis and collagen deposition, reduced collagen remodeling, and decreased wound strength.42 In addition, elderly patients have a higher susceptibility to comorbid chronic conditions, medication usage, and infection which may further impede wound healing. Aside from optimizing comorbid conditions and known risk factors, exercise has been reported to decrease the level of pro-inflammatory cytokines in healing tissue and improve wound healing in aged mice and humans.43-45
Chronic Conditions
Chronic medical conditions such as chronic obstructive pulmonary disease, congestive heart failure, peripheral vascular disease, and anemia impair tissue oxygenation placing patients at higher risk for poor surgical healing. In addition, hypothyroidism is an important factor for poor or delayed wound healing due to decreased collagen formation. Chronic kidney disease (CKD) is also known to affect wound healing and animal studies suggest that CKD impacts wound healing through disruption of normal reepithelialization and granulation tissue deposition rates, reduced cellular proliferation and angiogenesis, and a chronic dysfunctional inflammatory state.46 Uremia from renal failure has also been associated with wound healing impairment. It is important to screen for these systemic comorbidities ideally before surgical intervention as these conditions can be optimized in collaboration with a primary medical physician to improve surgical wound outcomes.
GENERAL APPROACH TO DELAYED SURGICAL HEALING
Poorly healing wounds of the face and neck can be especially debilitating, both functionally and cosmetically, thus it is important that facial plastic surgeons not only be familiar with risk factors and prevention of poor surgical healing but also be well-informed about the signs and symptoms of at-risk wounds, treatment of delayed and chronic wounds, and adjuvant techniques to improve healing. Wound care nurses can also be very instructive in suggesting contemporary wound care protocols and dressings available for slow-healing wounds.
A generalized approach to treating poorly healing wounds should be based on the principles of proper wound debridement, infection control, maintaining a moist environment while avoiding excessive exudates, and adequate wound edge reepithelialization. When wound healing is not proceeding normally over 2 to 4 weeks, the clinician must distinguish between an acute healing wound that is delayed versus a chronic healing wound based on physical characteristics such as size and depth of the wound, increased inflammatory changes, excessive exudate, purulence, necrotic tissue, wound edge epithelialization, and moisture balance.47
Clinical signs of a decline in wound healing are increased odor, pain, exudate, dehiscence, and tissue necrosis. If the wound is not healing, one can ask:
What is the most likely cause for poor healing?
What are the extrinsic or intrinsic factors contributing?
Is appropriate wound care being given?
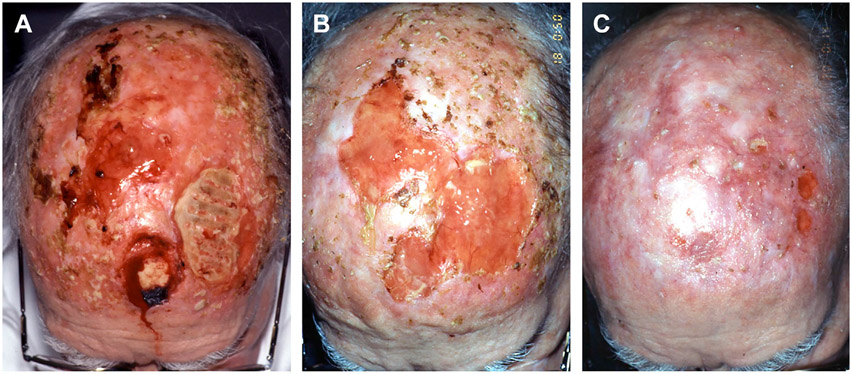
A delayed healing wound usually responds to clinical intervention quicker than a chronic healing wound; however, if a delayed healing wound persists, it can transform into a chronic healing wound. Chronic healing wounds require a more comprehensive strategic approach involving wound bed preparation to achieve successful wound healing (Fig. 2).
Fig. 2.
(A) An 82-year-old man with a history of scalp postoperative radiation treatment 10 years previously and multiple excisions of squamous cell cancers on the scalp. He had a persistent 2 year history of an open nonhealing scalp wound down to calvarial bone. Multiple biopsies showed no evidence of neoplastic recurrence and CT scan showed no evidence of osteomyelitis. Over 2 years he saw multiple physician providers and despite multiple different aggressive daily dressings and antibiotic trials, no clinical healing or granulation tissue occurred. (B) He required “jump starting” this chronic wound to get it back to the acute wound healing state by burring down the outer calvarium to the diploic layer to stimulate granulation tissue growth along with moisture retentive dressings. Other possible options for treating this wound would be a vacuum-assisted closure (VAC) device or a synthetic dermal substitute, (ex. Integra, Integra Life Sciences, Plainsboro, NJ)to stimulate granulation tissue on the bone. (C) After adequate granulation tissue formation, a split thickness skin graft successfully covered most of the open wound so that he no longer required aggressive daily dressing changes. The chronic wound was transformed into an acute healing wound.
If a wound fails to heal despite initial intervention, reevaluation of the current management and identification of the contributing factors that may be causing the poor healing should be reassessed. Wounds should be evaluated for the presence of foreign bodies, infection, tissue ischemia, and venous insufficiency. In addition, for a persistent nonhealing wound, a skin biopsy should be considered to rule out neoplasia. At the systemic level, metabolic conditions such as undiagnosed diabetes, anemia, or malignancy need to be ruled out. In addition, some patients may have concurrent factors playing a role in slower healing. One example is a poorly controlled insulin-dependent diabetic who is chronically on steroids for asthma. In this particular instance, as healing occurs over time, management of these comorbid conditions will have to be modified.48
Once the local and systemic tissue factors that contribute to poor wound healing are identified, the next step is to “jump-start” the tissues back into the acutely healing phases by removing necrotic tissue and eschar with effective debridement (Table 2).
Table 2.
Methods of tissue debridement
| Types of Debridement |
Description |
|---|---|
| Surgical debridement | Removes necrotic tissues via sharp surgical excision, typically with cold instruments either at bedside or in the operating room. Most effective form of debridement but results in most underlying tissue damage. |
| Mechanical debridement | Removes debris by physical force. Most common type is wet-to-dry dressings that should also include wound irrigation with either normal saline or antibiotic irrigations. Results in moderate amount of tissue damage. |
| Autolytic debridement | Relies on innate proteolytic enzymes to break down and liquefy necrotic debris by placing an occlusive or semiocclusive dressing over the wound for 2 to 3 d while the enzymatic process takes place. Less effective at wound debridement, but is the gentlest method for underlying tissues. This method is not appropriate for highly exudative, infected or deep wounds. |
| Enzymatic debridement | Exogenous enzymes such as papain-urea cream or collagenase ointment are applied topically to digest and break down necrotic debris. This method is slightly harsher than autolytic debridement, but debrides tissues slightly faster. |
Once initial debridement is performed, the next step is recognizing and treating the infection. Infected wounds prolong the inflammatory phase and typically present with increased erythema, warmth, and induration that persists more than 5 to 7 days after the initial insult. When infection is present, wounds should be opened to allow an oxygenated environment to eliminate obligate anaerobic bacteria. Wound cultures should also be obtained so that antibiotic treatment can be tailored to culture results. Topical antibiotics and skin cleansing solutions are also beneficial during dressing changes during the acute period.
Moist environments enhance the rate of epithelial migration and promote wound healing. As such, moisture balance in the wound should be maintained by using moisture-retaining ointment and hydrogels to avoid desiccation while also preventing excessive fluid which may cause maceration and lead to poor wound healing.
Adjunctive treatments such as hyperbaric oxygen and vacuum-assisted closure devices, can be considered in the treatment of unsatisfactory surgical healing that is refractory to standard treatment and wound-bed preparation.
SUMMARY
Common risk factors for poor surgical healing on the face and neck can be divided into extrinsic and intrinsic factors. Extrinsic factors include prior irradiation, chronic steroid use, cigarette use, and vaping. Intrinsic factors include comorbid medical conditions that affect tissue oxygenation and vascularity, malnutrition, diabetes, obesity, and normal aging. These risk factors should be identified and optimized ideally before surgical intervention to improve surgical healing outcomes. The facial plastic surgeon should be cognizant of the signs and symptoms of poor wound healing so that at-risk wounds can be recognized quickly, local and systemic factors affecting proper wound healing can be identified, and early intervention can be made. The principles of treating a delayed or chronic healing wound include effective debridement, recognizing and treating infection, adequate moisture balance, and wound edge reepithelialization. Chronic wounds may require more comprehensive wound bed preparation in addition to adjunctive treatments such as HBO, vacuum-assisted closure devices, and consultation with wound care nurses for additional treatments.
KEY POINTS.
Poor surgical wound healing of the face and neck can lead to significant morbidity and dissatisfaction for patients.
Preoperative evaluation should include a detailed history of chronic medical conditions, previous irradiation, diabetes, chronic steroid use, malnutrition, and smoking or e-cigarette use.
Persistent inflammation for longer than 7 days is a common initial sign of delayed or poor surgical healing. If delayed healing persists, the wound can transform into a chronic healing wound.
Clinical signs of decline in wound healing are increased odor, increased pain, increased exudate, dehiscence, and tissue necrosis.
When poor wound healing does occur in this area, the primary goal is the identification of extrinsic and intrinsic factors contributing to poor wound healing and timely intervention.
CLINICS CARE POINTS.
The risk of poor surgical wound healing can be reduced preoperatively by optimizing chronic comorbid medical conditions (i.e. diabetes, ect.), ensuring adequate nutritional status, and encouraging weight loss in addition to smoking or vaping cessation.
Postoperative radiation can be initiated as early as 3 weeks following surgery and is typically preferred over pre-operative radiation due to fewer wound healing complications.
Patients with a history of irradiation may develop chronic nonhealing wounds that require extensive wound bed preparation to “jump start” the wound back into the acute healing process, in addition to adjunctive therapies such as HBO, dermal substitutes, specialized dressings including vacuum assisted closure devices, and/or even free flap reconstruction.
Once a delayed healing wound is identified, appropriate care consists of identifying and removing risk factors, tissue debridement, infection control, maintaining a moist environment, and wound edge reepithelialization.
Footnotes
DISCLOSURE
The authors have nothing to disclose.
REFERENCES
- 1.Houlton JJ, Hom DB. Approaching Delayed-Healing Wounds on the Face and Neck. Facial Plast Surg Clin North Am 2013;21(1):81–93. [DOI] [PubMed] [Google Scholar]
- 2.Ramirez AR, Mabrie DC, Maas CS, et al. Wound Management and Suturing Manual; 2001, American Academy of Facial Plastic and Reconstructive Surgery, VA, USA. [Google Scholar]
- 3.Levenson SM, Geever EF, Chowley LV, et al. The Healing of Rat Skin Wounds. Ann Surg 1965;161(2):293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dormand E-L, Banwell PE, Goodacre TE. Radiotherapy and wound healing. Int Wound J 2005;2(2):112–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne WG, Naidu DK, Wheeler CK, et al. Wound healing in patients with cancer. Eplasty 2008;8:e9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18264518. [PMC free article] [PubMed] [Google Scholar]
- 6.Gantwerker EA, Hom DB. Skin: Histology and Physiology of Wound Healing. Facial Plast Surg Clin North Am 2011;19(3):441–53. [DOI] [PubMed] [Google Scholar]
- 7.Kwon D, Genden EM, de Bree R, et al. Overcoming wound complications in head and neck salvage surgery. Auris Nasus Larynx 2018;45(6):1135–42. [DOI] [PubMed] [Google Scholar]
- 8.Hübner G, Brauchle M, Smola H, et al. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoidtreated mice. Cytokine 1996;8(7):548–56. [DOI] [PubMed] [Google Scholar]
- 9.Beer H-D, Longaker MT, Werner S. Reduced Expression of PDGF and PDGF Receptors During Impaired Wound Healing. J Invest Dermatol 1997;109(2):132–8. [DOI] [PubMed] [Google Scholar]
- 10.Cronstein BN, Kimmel SC, Levin RI, et al. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci 1992;89(21):9991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg 2013;206(3):410–7. [DOI] [PubMed] [Google Scholar]
- 12.Barcha CP, Ranzer MJ. Impact of Chronic Steroid Use on Plastic Surgery Outcomes. Plast Reconstr Surg 2018;142(5):770e–9e. [DOI] [PubMed] [Google Scholar]
- 13.Zinder R, Cooley R, Vlad LG, et al. Vitamin A and Wound Healing. Nutr Clin Pract 2019;34(6):839–49. [DOI] [PubMed] [Google Scholar]
- 14.Talas DU, Nayci A, Atis S, et al. The effects of corticosteroids and vitamin A on the healing of tracheal anastomoses. Int J Pediatr Otorhinolaryngol 2003;67(2):109–16. [DOI] [PubMed] [Google Scholar]
- 15.Phillips JD, Kim CS, Fonkalsrud EW, et al. Effects of chronic corticosteroids and vitamin a on the healing of intestinal anastomoses. Am J Surg 1992;163(1):71–7. [DOI] [PubMed] [Google Scholar]
- 16.Hunt TK, Ehrlich HP, Garcia JA, et al. Effect of Vitamin A on Reversing the Inhibitory Effect of Cortisone on Healing of Open Wounds in Animals and Man. Ann Surg 1969;170(4):633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosely L, Finseth F. Cigarette smoking: impairment of digital blood flow and wound healing in the hand. Hand 1977;9(2):97–101. [DOI] [PubMed] [Google Scholar]
- 18.Jensen JA. Cigarette Smoking Decreases Tissue Oxygen. Arch Surg 1991;126(9):1131. [DOI] [PubMed] [Google Scholar]
- 19.Sørensen LT, Toft B, Rygaard J, et al. Smoking attenuates wound inflammation and proliferation while smoking cessation restores inflammation but not proliferation. Wound Repair Regen 2010;18(2):186–92. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen LT, Karlsmark T, Gottrup F. Abstinence From Smoking Reduces Incisional Wound Infection. Ann Surg 2003;238(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cetiner H, Cavusoglu I, Duzer S. The Effect of Smoking on Perforation Development and Healing after Septoplasty. Am J Rhinol Allergy 2017;31(1):63–5. [DOI] [PubMed] [Google Scholar]
- 22.Rees TD, Liverett DM, Guy CL. The Effect of Cigarette Smoking on Skin-Flap Survival in the Face Lift Patient. Plast Reconstr Surg 1984;73(6):911–5. [DOI] [PubMed] [Google Scholar]
- 23.Sachin Parikh, Jacono A. Deep-Plane Face-life as an Alternative in the Smoking Patinet. Arch Facial Plast Surg 2011;13(4):283–5. [DOI] [PubMed] [Google Scholar]
- 24.Rinker B. The Evils of Nicotine. Ann Plast Surg 2013;70(5):599–605. [DOI] [PubMed] [Google Scholar]
- 25.Jaleel Z, Blasberg E, Troiano C, et al. Association of vaping with decreased vascular endothelial growth factor expression and decreased microvessel density in cutaneous wound healing tissue in rats. Wound Repair Regen 2021;29(6):1024–34. [DOI] [PubMed] [Google Scholar]
- 26.Troiano C, Jaleel Z, Spiegel JH. Association of Electronic Cigarette Vaping and Cigarette Smoking With Decreased Random Flap Viability in Rats. JAMA Facial Plast Surg 2019;21(1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barchitta M, Maugeri A, Favara G, et al. Nutrition and Wound Healing: An Overview Focusing on the Beneficial Effects of Curcumin. Int J Mol Sci 2019;20(5):1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molnar JA, Underdown MJ, Clark WA. Nutrition and Chronic Wounds. Adv Wound Care 2014;3(11):663–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirk SJ, Hurson M, Regan MC, et al. Arginine stimulates wound healing and immune function in elderly human beings. Surgery 1993;114(2):155–9. discussion 160. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8342121. [PubMed] [Google Scholar]
- 30.ALVAREZ OM, GILBREATH RL. Effect of Dietary Thiamine on Intermolecular Collagen Cross-linking during Wound Repair. J Trauma Inj Infect Crit Care 1982;22(1):20–4. [DOI] [PubMed] [Google Scholar]
- 31.Enoch S, Grey JE, Harding KG. Non-surgical and drug treatments. BMJ 2006;332(7546):900–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lansdown ABG, Mirastschijski U, Stubbs N, et al. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen 2007;15(1):2–16. [DOI] [PubMed] [Google Scholar]
- 33.WARNOLD I, LUNDHOLM K. Clinical Significance of Preoperative Nutritional Status in 215 Noncancer Patients. Ann Surg 1984;199(3):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierpont YN, Dinh TP, Salas RE, et al. Obesity and Surgical Wound Healing: A Current Review. ISRN Obes 2014;2014:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nascimento AP, Costa AMA. Overweight induced by high-fat diet delays rat cutaneous wound healing. Br J Nutr 2006;96(6):1069–77. [DOI] [PubMed] [Google Scholar]
- 36.Biondo-Simões M, Zammar GR, Fernandes RS, et al. Obesity and abdominal wound healing in rats. Acta Cir Bras 2010;25(1):86–92. [DOI] [PubMed] [Google Scholar]
- 37.Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and Treatment of Impaired Wound Healing in Diabetes Mellitus: New Insights. Adv Ther 2014;31(8):817–36. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham DJ, Baumgartner RE, Federer AE, et al. Elevated Preoperative Hemoglobin A1c Associated with Increased Wound Complications in Diabetic Patients Undergoing Primary, Open Carpal Tunnel Release. Plast Reconstr Surg 2019;144(4):632e–8e. [DOI] [PubMed] [Google Scholar]
- 39.Sgonc R, Gruber J. Age-Related Aspects of Cutaneous Wound Healing: A Mini-Review. Gerontology 2013;59(2):159–64. [DOI] [PubMed] [Google Scholar]
- 40.Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol 1986;15(4):571–85. [DOI] [PubMed] [Google Scholar]
- 41.Gosain A, DiPietro LA. Aging and Wound Healing. World J Surg 2004;28(3):321–6. [DOI] [PubMed] [Google Scholar]
- 42.Guo S, DiPietro LA. Factors Affecting Wound Healing. J Dent Res 2010;89(3):219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keylock KT, Vieira VJ, Wallig MA, et al. Exercise accelerates cutaneous wound healing and decreases wound inflammation in aged mice. Am J Physiol Integr Comp Physiol 2008;294(1):R179–84. [DOI] [PubMed] [Google Scholar]
- 44.Emery CF, Kiecolt-Glaser JK, Glaser R, et al. Exercise Accelerates Wound Healing Among Healthy Older Adults: A Preliminary Investigation. Journals Gerontol Ser A Biol Sci Med Sci 2005;60(11):1432–6. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien J, Finlayson K, Kerr G, et al. Evaluating the effectiveness of a self-management exercise intervention on wound healing, functional ability and health-related quality of life outcomes in adults with venous leg ulcers: a randomised controlled trial. Int Wound J 2017;14(1):130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seth AK, De la Garza M, Fang RC, et al. Excisional wound healing is delayed in a murine model of chronic kidney disease. PLoS One 2013;8(3):e59979. Sen U, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rijswijk L. Wound assessment and documentation. In: Chronic wound care: a clinical source book for healthcare professionals. Malvern, PA: HMP Communications; 2001. p. 101–15. [Google Scholar]
- 48.Hom DB, Dresner H. General approach to a poor healing wound- a practical overview. In: Essential tissue healing of the face and neck. Cary, NC: PMPH USA, Ltd; 2009. p. 317–29. [Google Scholar]