Abstract
Background
Hereditary adenomatous polyposis syndromes, including familial adenomatous polyposis and other rare adenomatous polyposis syndromes, increase the lifetime risk of colorectal and other cancers.
Methods
A team of 38 experts convened to update the 2008 European recommendations for the clinical management of patients with adenomatous polyposis syndromes. Additionally, other rare monogenic adenomatous polyposis syndromes were reviewed and added. Eighty-nine clinically relevant questions were answered after a systematic review of the existing literature with grading of the evidence according to Grading of Recommendations, Assessment, Development, and Evaluation methodology. Two levels of consensus were identified: consensus threshold (≥67% of voting guideline committee members voting either ‘Strongly agree’ or ‘Agree’ during the Delphi rounds) and high threshold (consensus ≥ 80%).
Results
One hundred and forty statements reached a high level of consensus concerning the management of hereditary adenomatous polyposis syndromes.
Conclusion
These updated guidelines provide current, comprehensive, and evidence-based practical recommendations for the management of surveillance and treatment of familial adenomatous polyposis patients, encompassing additionally MUTYH-associated polyposis, gastric adenocarcinoma and proximal polyposis of the stomach and other recently identified polyposis syndromes based on pathogenic variants in other genes than APC or MUTYH. Due to the rarity of these diseases, patients should be managed at specialized centres.
Background
Colorectal cancer (CRC) is one of the most frequently diagnosed cancers, accounting for 10% of cancer deaths worldwide1–3. There is a strong correlation between family history and CRC risk. Roughly 30% of CRC patients have at least one relative affected with CRC4–6. High-risk autosomal dominant or recessive hereditary syndromes account for 3–5% of CRCs and the chance of having a hereditary CRC syndrome is increased when CRC is diagnosed before the age of 50 years7,8. Patients with adenomatous polyposis syndromes are at high risk of developing gastrointestinal tumours, often accompanied by an increased risk of malignancies in other organs.
Genetic diversity and clinical characteristics of adenomatous polyposis syndromes
Familial adenomatous polyposis (FAP) is an autosomal dominant hereditary cancer syndrome that accounts for about 1% of all diagnosed CRCs. Heterozygous constitutional pathogenic variants (PVs) in the tumour suppressor gene APC cause FAP. APC encodes a negative regulator of the canonical WNT/β-catenin signalling pathway9. Whereas most PVs in APC are inherited, 15–30% arise de novo, and when occurring post-zygotically in embryonic tissues, they result in APC mosaicism. Somatic mosaicism may result in sporadic adenomatous polyposis patients, and is usually associated with milder phenotypes10–13. Longitudinal studies show that CRC risk in FAP is proportional to the colonic polyp burden. A very rare and specific phenotype named GAPPS (gastric adenocarcinoma and proximal polyposis of the stomach) is observed in individuals with specific heterozygous PVs affecting the APC promoter 1B14.
In patients with FAP who do not receive prophylactic treatment, the risk of CRC increases with age, reaching virtually 100% by 60 years. In addition, there is an increased risk of duodenal cancer, gastric cancer, thyroid cancer, desmoid tumours, pancreatic cancer, hepatoblastoma and a variety of non-cancerous manifestations including congenital hypertrophy of the retinal pigment epithelium (CHRPE), dermoid cysts, osteomas, and dental anomalies that are characteristic of the syndrome and may be the initial presenting features.
MUTYH-associated polyposis (MAP) is an autosomal recessive hereditary cancer syndrome and is the second most common cause of adenomatous polyposis, accounting for approximately 7% of patients with an adenomatous polyposis phenotype and 0.7% of CRCs15–18. As with FAP, there is a wide spectrum of phenotypes observed in the large bowel, from mild to profuse polyposis, and some may present with CRC without a polyposis phenotype19–22. Patients with MAP are at increased risk of developing extra-colonic malignancies, including non-melanoma skin cancer, duodenal cancer, ovarian and endometrial cancer or bladder cancer, among others19–23. Similar to FAP, MAP patients have a higher risk of developing CHRPE22. However, unlike FAP, MAP patients are not at higher risk of osteomas, desmoid tumours, or gastric cancer21,22. MAP is caused by biallelic PVs (that is the homozygous or compound heterozygous variants) in MUTYH, which encodes a glycosylase of the DNA base excision repair (BER) system17,18. Biallelic PVs in two other BER glycosylases, NTHL1 and MBD4, cause very rare autosomal recessive adenomatous polyposis and multiple organ cancer predisposition syndromes24–26.
Heterozygous germline PVs in POLE and POLD1 affecting the proofreading function of polymerases ε and δ (that is specific missense variants in their exonuclease domains) cause an autosomal dominant adenomatous polyposis and cancer predisposition syndrome called polymerase proofreading-associated polyposis (PPAP)27–29.
Adenomatous polyposis can be a feature of the rare multi-organ tumour condition, constitutional mismatch repair deficiency (CMMRD), which is caused by biallelic constitutional PVs in any of the Lynch syndrome associated DNA mismatch repair (MMR) genes MSH6, PMS2 or more rarely MLH1 or MSH230. Cases of adenomatous polyposis diagnosed in adulthood have been exceptionally associated with biallelic constitutional PVs in non-Lynch syndrome MMR genes, such as MSH3 and MLH331,32. Heterozygous PVs in AXIN2, another negative regulator of WNT signalling, have been associated with a predisposition to adenomatous polyposis and/or oligodontia33–35.
Methods
Guidelines working group
In 2008, guidelines for the clinical management of FAP were published by a group of European experts known as the Mallorca Group. The current revision was carried out by a working group formed by members of the European Hereditary Tumour Group (EHTG) and European Society of Coloproctology (ESCP), including many of the previous authors and extending the group with guideline- and/or topic-experienced society members. The Chair of EHTG (G.M.), appointed a guideline leader (G.Z.), who invited the authors to participate in the process of developing evidence-based guidelines including the literature search, grading and the Delphi process. A total of 39 experts from 13 countries, including surgeons, gastroenterologists, pathologists, clinical and molecular geneticists, gynaecologists, and a patient representative, were recruited. The topics to be addressed were divided into six subgroups (see Appendix S1): FAP lower gastrointestinal (GI) manifestations, FAP upper GI manifestations and GAPPS, FAP desmoid tumours, other extra-colonic manifestations in FAP, FAP chemoprevention, MAP and other rare adenomatous polyposis. The EHTG pursues the concept of dynamic guidance, implementing a continuous update process by the guideline members to address the gaps in recommendations or make changes when new evidence emerges in the literature.
Scope of updated guidelines
This updated revision of the FAP guidelines aims to investigate the latest evidence concerning the clinical issues covered in the previous FAP guidelines. Additionally, other adenomatous polyposis syndromes have been included. The guideline objective is to assess and explore additional facets related to FAP, MAP and other rare adenomatous polyposis, with a particular emphasis on identifying gaps in the existing literature within specific domains. The intention is to stimulate novel collaborative studies that address these knowledge gaps. The guidelines target both experts in the field of adenomatous polyposis syndrome and individuals who may not have extensive knowledge but who will encounter these conditions. In order to satisfy the different needs, we elaborated both a short version including the essentials of management and a more extensive version that discusses in depth the current literature.
Literature search
A PICO (Patients, Intervention, Comparison, Outcomes) model was created for each area of interest, based on questions previously developed by each subgroup (see Appendix S2). Expert librarians assisted in performing a systematic literature search using databases such as PubMed, the Cochrane Database of Systematic Reviews, Scopus, and Medline. Manual searches for relevant articles from January 2006 to July 2021 were conducted. Titles and abstracts were screened, and relevant English articles were reviewed independently by two members, with discrepancies resolved by a senior member (see Appendix S3). In cases where no articles meeting the criteria within the specified time frame were identified to adequately address the formulated research questions, earlier articles that were relevant but not within the designated period were included in the supplementary comments section for further reference and contextual information. The level of evidence was graded as high, moderate, low, or very low, following GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) criteria (http://www.gradeworkinggroup.org/). Additional manual searches were carried out in May 2023 to identify the latest relevant publications published between August 2021 and May 2023.
Voting process
Multiple anonymous Delphi procedures were conducted within the guideline committee to reach consensus. The level of agreement with statements was rated using a 5-point scale: ‘Strongly agree’ (SA), ‘Agree’ (A), ‘Neutral’ (N), ‘Disagree’ (D), and ‘Strongly disagree’ (SD). Participants had the option to opt out if the statement was not within their area of expertise. Participants were also asked to provide anonymous feedback on the clarity of statements and suggest improvements where needed. After the Delphi rounds, the statements were discussed and adjusted during online sessions, if necessary. Two levels of consensus were identified: consensus threshold (≥67% of voting guideline committee members voting either ‘Strongly agree’ or ‘Agree’ during the Delphi rounds) and high threshold (consensus ≥ 80%). The AGREE reporting checklist was used to guide the reporting of the process.
Results
A total of 140 recommendations for patients with FAP, MAP, GAPPS or other rare hereditary adenomatous polyposis syndromes were developed.
The guidelines were categorized into four topics: FAP, MAP, other hereditary adenomatous syndromes, and GAPPS. Where applicable, each topic was further divided into subsections. All the statements pertaining to each section are summarized in Tables 1–12.
Table 1.
Short version: statements pertaining to the lower gastrointestinal manifestations
| Statements | Level of evidence and agreement |
|---|---|
| Surveillance | |
| LGI.1A: Surveillance should begin at 12 years of age in asymptomatic patients with a germline PV in the APC gene (for FAP disease), or in asymptomatic patients with FDRs affected by classical FAP (if a genetic test is not available or if no PV is detected in the affected relative). | LE: low Agreement: 83% (SA 49%; A 34%; N 7%; D 10%) |
| LGI.1B: In symptomatic patients with germline PV in the APC gene (for FAP disease), or patients with FDRs affected by classical FAP if a genetic test is not available or if no PV is detected) colonoscopy should start at any age and as soon as possible. | LE: low Agreement: 90% (SA 56%; A 34%; N 3%; D 7%) |
| LGI.2A: Surveillance can start later but no later than 18–20 years of age in asymptomatic patients with a germline PV in the APC gene for attenuated FAP disease and an attenuated proband/family phenotype. Alternatively, surveillance should also begin in asymptomatic patients with first–degree relatives affected by attenuated FAP, if a genetic test is not available or if no known pathogenic mutations are detected. | LE: low Agreement: 69% (SA 31%; A 38%; N 17%; D 4%; SD 10%) |
| LGI.2B: Colonoscopy should start at any age and as soon as possible in symptomatic patients with a germline PV in the APC gene for a-FAP disease or in patients with FDRs affected by a-FAP (if a genetic test is not available or if no known pathogenic mutations are detected). | LE: low Agreement: 90% (SA 55%; A 35%; N 7%; D 3%) |
| LGI.3: The optimal modality for colorectal surveillance in classical FAP is high-definition white-light colonoscopy. Flexible sigmoidoscopy can be considered as an initial option, according to patient preference. If adenomas are identified high-definition white-light colonoscopy should be performed. | LE: low Agreement: 100% (SA 55%; A 45%) |
| LGI.4: The optimal modality for colorectal surveillance in a-FAP is high-definition white-light colonoscopy. | LE: low Agreement: 92% (SA 71%; A 21%; N 8%) |
| LGI.5A: Endoscopic surveillance of the colon should be adapted according to phenotype, genotype–phenotype and the severity of the disease. | LE: low Agreement: 90% (SA 40%; A 50%; N 7%; D 3%) |
LGI.5B: Repeat endoscopy should be performed within 1 year or less if at least one of the following criteria is present:
|
LE: low Agreement: 87% (SA 57%; A 30%; N 3%; D 10%) |
LG.5C: Repeat endoscopy may be performed at 2 years when the phenotype shows all of the following criteria:
|
LE: low Agreement: 70% (SA 20%; A 50%; N 20%; D 10%) |
| LGI.6A: White-light high-definition colonoscopy is sufficient for surveillance colonoscopy in FAP. | LE: low Agreement: 89% (SA 56%; A 33%; N 7%; D 4%) |
| LGI.6B: There are insufficient data to recommend the use of advanced imaging technology. | LE: low Agreement: 93% (SA 55%; A 38%; N 7%) |
| LGI.6C: White-light endoscopy is sufficient in most cases; virtual or dye-based chromoendoscopy could have advantages in discriminating between the clinical diagnosis of FAP versus a-FAP. | LE: low Agreement: 73% (SA 23%; A 50%; N 23%; D 4%) |
| Surgery | |
LGI.7: Absolute indications for immediate colorectal surgery in FAP are:
|
LE: low Agreement: 93% (SA 57%; A 36%; N 3.5%; D 3.5%) |
LGI.8A: IPAA may be offered to patients with either:
|
LE: low Agreement: 86% (SA 29%; A 57%; N 14%) |
| LG.8B: Patient preference about surgical choice should be considered. | LE: low Agreement: 100% (SA 44%; A 56%) |
| LGI.9: There is no conclusive evidence indicating a clear advantage or disadvantage in performing dissection with mesocolic/mesorectal excision. | LE: low Agreement: 96% (SA 31%; A 65%; SD 4%) |
| LGI.10: Routine diverting ileostomy is not mandatory when total proctocolectomy with IPAA is performed. | LE: low Agreement: 80% (SA 55%; A 25%; N 10%; D 5%; SD 5%) |
| LGI.11: When the rectum can be preserved, an ileo-sigmoid anastomosis could be considered to diminish the risk of anastomotic leak and improve functional outcome. | LE: low Agreement: 95% (SA 24%; A 71%; SD 5%) |
| Post-surgical management | |
| LGI.12A: The optimal modality for surveillance after an IRA is endoscopy. The surveillance interval should not exceed 2 years, starting from the colectomy, and should be individualized based on phenotype. | LE: low Agreement: 93% (SA 57%; A 36%; D 3.5%; SD 3.5%) |
| LGI.12B: All polyps >5 mm should be removed (endoscopically or with transanal excision). | LE: low Agreement: 86% (SA 41%; A 45%; N 7%; D 7%) |
| LGI.12C: Secondary proctectomy should be considered when polyposis is no longer conservatively manageable or in the presence of two or more polyps with HGD. | LE: low Agreement: 100% (SA 52%; A 48%) |
| LGI.13A: Endoscopic surveillance of an ileo-anal pouch should start 12 months after colectomy. | LE: low Agreement: 88% (A 88%; N 12%) |
| LGI.13B: Endoscopic surveillance of an ileo-anal pouch should be performed annually. | LE: low Agreement: 81% (SA 29%; A 52%; D 14%; SD 5%) |
| LGI.14A: Pouch adenomas may be managed endoscopically. | LE: low Agreement: 100% (SA 57%; A 43%) |
| LGI.14B: In the presence of HGD in/of complete polyp resection, the pouch should be surveilled within 6 months. | LE: low Agreement: 100% (SA 50%; A 50%) |
| LGI.14C In the presence of two or more polyps with HGD, surgery may be considered. | LE: low Agreement 100% (SA 52%; A 48%) |
| LGI.15: In the case of pouch carcinoma, pouchectomy/dismantling of the pouch is indicated. | LE: Expert opinion Agreement 100% (SA 58%; A 42%) |
| LGI.16: Expanding endoscopy to the more proximal small bowel should be performed during pouchoscopy in FAP patients after total proctocolectomy with IPAA. | LE: low Agreement 100% (SA 48%; A 52%) |
A, agree; a-FAP, attenuated familial adenomatous polyposis; D, disagree; FAP, familial adenomatous polyposis; FDR, first-degree relatives; HGD, high-grade dysplasia; HGIEN, high-grade intraepithelial neoplasia; IPAA, ileal pouch anal anastomosis; IRA, ileorectal anastomosis; LE, level of evidence; N, neutral; PV, pathogenic variant; SA, strongly agree; SD, strongly disagree.
Table 12.
Short version: statements pertaining to gastric adenocarcinoma and proximal polyposis of the stomach
| Statements | Level of evidence and agreement |
|---|---|
| GAPPS.1: Genetic testing should be offered to individuals with a clinical suspicion of GAPPS. | LE: Strong Agreement: 95% (SA 52%; A 43%; N 5%) |
| GAPPS.2A: The age to start upper GI surveillance in asymptomatic individuals at risk of gastric cancer should be evaluated on a case-by-case basis. The youngest age of gastric cancer in the family should be considered. | LE: Low Agreement: 82% (SA 25%; A 57%; N 11%; D 7%) |
| GAPPS.2B: Surveillance endoscopic intervals for GAPPS families should be flexible and decided on a case-by-case basis. | LE: Low Agreement: 93% (SA 24%; A 69%; N 3%; D 4%) |
| GAPPS.3: In GAPPS patients CRC surveillance may be considered, particularly when there is a family history of CRC. | LE: Low Agreement: 85% (SA 37%; A 48%; N 7%; D 8%) |
| GAPPS.4A: GAPPS results in a high risk of gastric cancer. Total gastrectomy should be considered in cases of high-grade dysplasia and progressive gastric polyposis. | LE: Low Agreement: 96% (SA 52%; A 44%; N 4%) |
| GAPPS.4B: There is not enough evidence to recommend an age for risk-reducing prophylactic gastrectomy: the decision should be individualized. | LE: Low Agreement: 96% (SA 52%; A 44%; N 4%) |
A, agree; CRC, colorectal cancer; D, disagree; GAPPS, gastric adenocarcinoma and proximal polyposis of the stomach; GI, gastrointestinal; LE, level of evidence; N, neutral; SA, strongly agree; SD, strongly disagree.
Table 2.
Short version: statements pertaining to upper gastrointestinal manifestations in familial adenomatous polyposis
| Statements | Level of evidence and agreement |
|---|---|
| Risk factors for upper GI neoplasia in FAP | |
| UGI.1: The risk factors most strongly associated with duodenal adenocarcinoma include Spigelman stage IV (either at first endoscopy or during surveillance), HGD in duodenal adenomas, duodenal adenomas >10 mm in diameter, and ageing. Additional risk factors have provided inconsistent evidence and need further evaluation. | LE: low Agreement: 93% (SA 55%; A 38%; N 3%; D 4%) |
| UGI.2A: The risk of papillary adenocarcinoma could increase with age. | LE: Very low Agreement: 89% (SA 43%; A 46%; N 6%; D 5%) |
UGI.2B:
|
LE: Very low Agreement: 90% (SA 38%; A 52%; N 3%; D 7%) |
| UGI.2C: The Spigelman classification could underestimate the risk of developing a papillary adenocarcinoma. | LE: Low Agreement: 90% (SA 38%; A 52%; N 3%; D 7%) |
| UGI.2D: Among the known pathogenic adenomatous polyposis coli gene variants, none have been identified as a risk factor for the development of papillary adenocarcinoma. | LE: Very low Agreement: 100% (SA 29%; A 71%) |
| Surveillance | |
| UGI.3: Endoscopic surveillance of the upper GI tract may start after the age of 18 years but no later than 30 years. | LE: Low Agreement: 89% (SA 35%; A 54%; N 4%; D 7%) |
| UGI.4A: Surveillance intervals depend on gastric, duodenal and neo-duodenal (post-surgical) endoscopic findings. The site with the most advanced stage should direct the surveillance interval. | LE: Low Agreement: 89% (SA 71%; A 18%; N 11%) |
| UGI.4B: Duodenal surveillance intervals should be based on the Spigelman stage and the appearance of the papilla. Surveillance recommendations are illustrated in Fig. 4. | LE: Low Agreement: 89% (SA 50%; A 39%; N 7%; D 4%) |
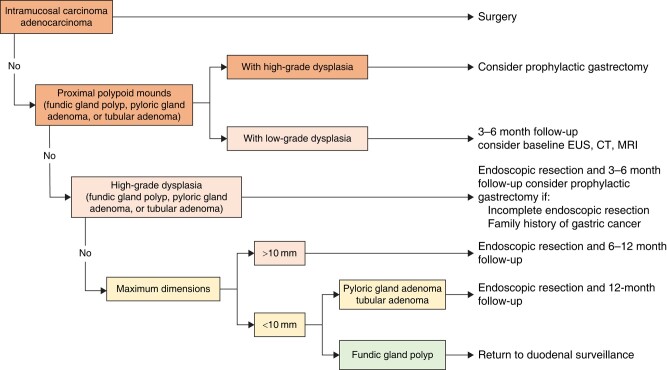
| UGI.4C: Gastric surveillance intervals should depend on the number, the dimensions and the histological characteristics of adenomas. Surveillance recommendations are illustrated in Fig. 5. | LE: Low Agreement: 89% (SA 45%; A 44%; N 7%; D 4%) |
| UGI.4D: Post-duodenal surgery surveillance intervals depend on the type of duodenal surgery performed. Surveillance recommendations are reported in Fig. 4. | LE: Low Agreement: 82% (SA 43%; A 39%; N 11%; D 4%; SD 3%) |
| UGI.5A: Duodenal and papillary surveillance could rely on cap-assisted forward-viewing endoscopy for complete visualization of the papilla. If the papilla is not adequately viewed, side-viewing endoscopy should be used. | LE: Moderate Agreement: 92% (SA 65%; A 27%; N 4%; D 4%) |
| UGI.5B: Chromoendoscopy, both digital and dye-chromoendoscopy, can be used to improve the visualization of duodenal, papillary and gastric adenomas. Narrow-band imaging could also improve the visualization of duodenal and papillary adenomas. | LE: Moderate Agreement: 81% (SA 39%; A 42%; N 15%; D 4%) |
| UGI.5C: Video-capsule endoscopy is not adequate for gastric, duodenal and papillary surveillance. | LE: Low Agreement: 96% (SA 65%; A 31%; N 4%) |
| UGI.5D: Endoscopic ultrasound and double-balloon enteroscopy are not part of routine endoscopic surveillance, but they could be useful as second-level diagnostic and/or therapeutic exams. | LE: Low Agreement: 85% (SA 54%; A 31%; N 15%) |
| UGI.6: No statement can be provided on the use of random duodenal biopsies. | LE: — Agreement: 87% (SA 54%; A 33%; N 13%) |
| UGI.7A: The impact of random biopsies on the prevention of papillary adenocarcinoma is unknown. Thus, no formal recommendations to adopt or not this strategy of systematic random papillary biopsies can be made. | LE: Very low Agreement: 86% (SA 38%; A 48%; N 14%) |
| UGI.7B: Taking random biopsies of the papilla improves the diagnosis of low-grade dysplasia. The benefit of random biopsies in macroscopically normal tissue to detect an HGD or an invasive adenocarcinoma of the papilla is very low, at least lower than 1% but not nil. |
LE: High Agreement: 86% (SA 52%; A 34%; N 10%; D 4%) |
| Spigelman staging system | |
| UGI.8: The Spigelman stage-based management provides the highest available level of evidence for duodenal cancer prevention. However, there are limitations to the Spigelman stage, which could be improved upon. | LE: Low Agreement: 97% (SA 59%; A 38%; N 3%) |
| UGI.9A: The average lifetime risk of duodenal cancer is estimated to be up to 30% for Sp-IV, 13% for Sp-III, 12% for Sp-II, and lower than 5% for Sp-I and Sp-0. | LE: Low Agreement: 89% (SA 22%; A 67%; N 11%) |
| UGI.9B: The estimated lifetime risk of duodenal cancer may be lowered after endoscopic or surgical downstaging. | LE: Low Agreement: 100% (SA 35%; A 65%) |
| Endoscopic treatment option: | |
| UGI.10A: Endoscopic downstaging should be personalized according to endoscopic findings. Ideally, Spigelman stage IV should be downstaged as much as possible. An attempt to downstage Spigelman stage III can be performed. | LE: Low Agreement: 85% (SA 31%; A 54%; N 8%; D 4%; SD 3%) |
| UGI.10B: All non-papillary duodenal lesions >10 mm should undergo endoscopic resection. | LE: Moderate Agreement: 93% (SA 52%; A 41%; D 7%) |
| UGI.10C: Non-papillary duodenal lesions measuring 5–10 mm in size could undergo either endoscopic resection or surveillance. | LE: Low Agreement: 82% (SA 21%; A 61%; N 11%; D 7%) |
| UGI.10D: All papillary adenomas should be candidates for endoscopic resection, but especially if harbouring HGD, villous histology, or if >10 mm in size. | LE: Low Agreement: 85% (SA 52%; A 33%; N 7%; D 8%) |
| UGI.10E: All gastric adenomas larger >5 mm should undergo endoscopic resection. | LE: Low Agreement: 83% (SA 28%; A 55%; N 10%; D 7%) |
| UGI.10F: All gastric, duodenal and ampullary histologically proven carcinomas with endoscopic features suggestive of invasive adenocarcinoma should undergo surgery with or without systemic therapy, rather than endoscopic resection. | LE: Strong Agreement: 93% (SA 72%; A 21%; D 7%) |
| Duodenal surgery versus endoscopic management | |
| UGI.11A: Curative surgical resection must be offered to surgically resectable, histologically proven duodenal and ampullary adenocarcinoma. | LE: Strong Agreement: 100% (SA 86%; A 14%) |
| UGI.11B: Prophylactic surgical resection could be considered for Spigelman stage IV duodenal polyposis. | LE: Moderate Agreement: 88% (SA 31%; A 57%; N 8%; D 4%) |
| UGI.11C: Prophylactic surgical resection could be considered for Spigelman stage II–III that is not endoscopically manageable. | LE: Low Agreement: 88% (SA 31%; A 57%; N 8%; D 4%) |
| UGI.11D: Papillary adenomas >10 mm or with HGD should undergo endoscopic resection, rather than surgical resection, if feasible. | LE: Low Agreement: 86% (SA 54%; A 32%; N 14%) |
| UGI.12A: All duodenal, papillary and gastric lesions with histologically proven invasive carcinoma should undergo surgery (if surgically completely resectable). | LE: Strong Agreement: 100% (SA 86%; A 14%) |
| UGI.12B: Spigelman stages III and IV duodenal polyposis without evidence of invasive tumour should undergo endoscopic treatment, if feasible, rather than surgical resection. However, there should be a low threshold to offer surgical resection once downstaging appears no longer manageable endoscopically. | LE: Low Agreement: 93% (SA 50%; A 43%; N 7%) |
| UGI.12C: Papillary and duodenal adenomas should undergo endoscopic resection, rather than surgery, if feasible. | LE: Low Agreement: 96% (SA 48%; A 48%; N 4%) |
| UGI.13: Pancreato-duodenectomy is the procedure of choice in case of suspected duodenal cancer. For prophylactic surgery, both pancreas-sparing duodenectomy and pancreatico-duodenectomy may be considered. | LE: Low Agreement: 100% (SA 35%; A 65%) |
| Management of gastric findings | |
| UGI.14A: Endoscopic resection of FGPs has not been demonstrated to reduce the risk of gastric adenocarcinoma. However, in cases of large or symptomatic FGPs, endoscopic resection may be considered after expert evaluation. | LE: Low Agreement: 100% (SA 35%; A 65%) |
| UGI.14B: Fundic gland polyposis may progress to gastric adenocarcinoma in patients with FAP. Such risk cannot be quantified up to now. | LE: Very low Agreement: 82% (SA 18%; A 64%; N 7%; D 11%) |
| UGI.15: Endoscopic resection may be a consideration for FGPs that are large or symptomatic, after expert evaluation. | LE: Low Agreement: 93% (SA 39%; A 54%; N 7%) |
| UGI.16: Suspected gastric adenomas should be removed, endoscopically if feasible. | LE: Low Agreement: 100% (SA 56%; A 44%) |
| Management of small intestinal findings including post UGI surgery | |
| UGI.17: After surgery, the neo-duodenum and jejunum should receive endoscopic surveillance. | LE: Low Agreement: 100% (SA 75%; A 25%) |
| UGI.18: Small bowel surveillance is not routinely indicated, but small bowel examination is recommended before duodenal surgical intervention. | LE: Low Agreement: 100% (SA 42%; A 58%) |
| UGI.19: When examination of the small bowel is indicated, video-capsule endoscopy is the method of choice. If positive, patients should undergo enteroscopy for diagnosis and therapy. | LE: Low Agreement: 95% (SA 16%; A 79%; D 5%) |
A, agree; D, disagree; FAP, familial adenomatous polyposis; FGP, fundic gland polyp; GI, gastrointestinal; HGD, high-grade dysplasia; LE, level of evidence; N, neutral; SA, strongly agree; SD, strongly disagree.
Table 4.
Short version: statements pertaining to desmoid tumours
| Statements | Level of evidence and agreement |
|---|---|
| Diagnosis and screening | |
| DTs.1: The different classifications can help in the choice of treatment; however, they must be strongly related to the clinical presentation and evaluation by the physician. | LE: low Agreement: 100% (SA 45%; A: 55%) |
| DTs.2: Preoperative screening for DT appears more relevant in patients who already had abdominal surgery as it might find a DT that can have impact on the surgical options choice. | LE: low Agreement: 90% (SA 28%; A: 62%; N 7%; D 3%) |
| DTs.3: There is no evidence in the literature that a screening programme for DT detection after abdominal surgery should be proposed. Moreover, with the actual possible treatment and the unpredictable evolution of DT such a screening programme might not be needed. | LE: low Agreement: 93% (SA 29%; A: 64%; N 3.5%; D 3.5%) |
| DTs.4: Confirmatory biopsies may be considered if there is a diagnostic dilemma or required to initiate medical therapy. | LE: very low Agreement: 92% (SA 71%; A: 21%; N 3.5%; D 4.5%) |
| DTs.5: In a patient with FTs without known FAP, screening of FAP (at least with colonoscopy and APC mutation testing if possible) should be performed. This is especially important among patients younger than 60 years, or with intra-abdominal desmoids or in the abdominal wall. | LE: Low Agreement: 89% (SA 46%; A: 43%; N 4%; D 7%) |
| Treatment | |
| DTs.6: Rapidly enlarging and life-threatening FT requires first-line aggressive treatment. Others should be surveyed in a watch-and-wait protocol. | LE: Low Agreement: 92% (SA 46%; A: 46%; N 8%) |
| DTs.7: Surgery should not be considered the ideal treatment for DTs, except in the case of DT complications, rapidly growing or life-threatening. | LE: Low Agreement: 89% (SA 30%; A: 59%; N 7%; D 4%) |
| DTs.8: There is currently no evidence to support the use of CP for high-risk patients undergoing surgery or in post-surgical care | LE: – Agreement: 88% (SA 35%; A: 53%; N 9%; D 3%) |
| Management for DTs identified during abdominal surgery | |
| DTs.9A: Continue with the intervention (proceeding with the surgical procedure) if technically feasible. | LE: Low Agreement: 92% (SA 21%; A: 71%; N 8%) |
| DTs.9B: Resection of mesenteric desmoid(s) should be avoided if it will result in sacrificing any small bowel. | LE: Low Agreement: 97% (SA 48.5%; A: 48.5%; N 3%) |
| DTs.10: Desmoid disease can potentially render restorative procedures technically challenging or impossible. In cases where it is feasible, restorative procedures should be cautiously considered and selectively recommended for patients with concomitant intra-abdominal DTs following prophylactic (procto)colectomy, taking into account the significant risk of desmoid recurrence and adhesion formation. In such circumstances, proctocolectomy with terminal ileostomy may represent the safest option. It is important to have a thorough discussion with the patients about the potential risks of compromised function and the possibility of requiring additional surgeries, ensuring that the choice is individualized to their specific situation. | LE: Low Agreement: 92% (SA 40%; A: 52%; N 8%) |
| DTs.11: The risk of DTs has not been evaluated on a systemic scale. When feasible, single-stage proctocolectomy is preferred for FAP patients in order to avoid DTs. | LE: — Agreement: 81% (SA 35%; A: 46%; N 12%; D 7%) |
A, agree; CP, chemoprevention; D, disagree; DT, desmoid tumour; FAP, familial adenomatous polyposis; LE, level of evidence; N, neutral; SA, strongly agree; SD, strongly disagree.
Table 5.
Short version: statements pertaining FAP-related other extra-colonic manifestations (OEM)
| Statements | Level of evidence and agreement |
|---|---|
| Thyroid | |
| OEM.1: The lifetime risk of thyroid cancer in FAP patients ranges between 1.5% and 12%. | LE: moderate Agreement: 89% (SA 41%; A: 48%; N 4%; D 7%) |
| OEM2.A: Thyroid surveillance, when performed, should include physical examination and thyroid ultrasound. | LE: low Agreement: 85% (SA 44%; A: 41%; N 7.5%; D 7.5%) |
| OEM2.B: Thyroid screening, if performed, can be initiated at the age of 16 in females and in adulthood in males. | LE: very low Agreement: 71% (SA 32%; A: 39%; N 7%; D 18%; SD 4%) |
| OEM2.C: When the baseline thyroid ultrasound is negative, we suggest a screening interval of 2–3 years. | LE: very low Agreement: 78% (SA 33%; A: 45%; N 11%; D 7%; SD 4%) |
OEM.3: Patients at higher risk for developing thyroid cancer include:
|
LE: low Agreement: 92% (SA 38%; A: 54%; N 8%) |
| OEM.4: The diagnosis of FAP should be considered in female patients younger than 35 years old, with a diagnosis of cribriform-morulae variant of papillary thyroid carcinoma. | LE: low Agreement: 89% (SA 41%; A: 48%; N 7%; D 4%) |
| Adrenal gland | |
| OEM.5: While adrenal mass incidence is 2–3 times higher in FAP patients compared to the general population, the development of adrenal gland cancer or pheochromocytomas is rare. | LE: low Agreement: 85% (SA 23%; A: 62%; N 7.5%; D 7.5%) |
| OEM.6: The reported proportion of patients with FAP who have adrenal incidentalomas ranges between 7% and 26%, which is 2–3 times higher than in the general population. | LE: low Agreement: 83% (SA 47%; A: 38%; N 13%; D 4%) |
| OEM.7A: The detection of an adrenal incidentaloma requires evaluation for both radiologically suspicious features and hyperfunction, regardless of patients’ characteristics but according to international guidelines for incidentaloma. | LE: low Agreement: 96% (SA 44%; A: 52%; D 4%) |
| OEM.7B: All patients with detected adrenal gland lesions should be referred to a specialized endocrinology clinic. | LE: low Agreement: 92% (SA 32%; A: 60%; D 8%) |
| Pancreas | |
| OEM.8: The lifetime risk of developing pancreatic cancer in FAP patients appears to be less than 2%. | LE: low Agreement: 88% (SA 21%; A: 67%; N 8%; D 4%) |
| Gallbladder | |
| OEM.9: The lifetime risk of the occurrence of gallbladder neoplasia (adenoma/carcinoma) has not been investigated to date. | LE: low Agreement: 85% (SA 27%; A: 58%; N 11%; D 4%) |
| Liver | |
| OEM.10: The lifetime risk of developing hepatoblastoma in FAP patients is approximately 2%, with the highest incidence occurring in the age group of 1–4 years. | LE: low Agreement: 100% (SA 50%; A: 50%) |
| OEM.11A: There are insufficient data to prove that hepatoblastoma screening increases survival. | LE: low Agreement: 92% (SA 46%; A: 46%; N 8%) |
| OEM.11B: If screening is performed it should start from birth and be performed every 6–12 months until the age of 5. | LE: low Agreement: 82% (SA 26%; A: 56%; N 8%; D 10%) |
| Brain | |
| OEM.12: There is insufficient evidence available to report on the lifetime risk of developing a brain tumour in FAP patients | LE: — Agreement: 81% (SA 31%; A: 50%; N 15%; D 4%) |
| Eyes | |
| OEM.13: People with multiple unilateral or bilateral lesions require germline testing for FAP. If germline testing is negative, a single colonoscopy should be considered in early adulthood. | LE: low Agreement: 90% (SA 55%; A: 35%; N 5%; D 5%) |
| Skin | |
| OEM.14: There is currently insufficient evidence to establish the cost-effectiveness of screening individuals with fibromas and epidermoid cysts for FAP. | LE: low Agreement: 88% (SA 28%; A: 60%; N 8%; D 4%) |
| Bones | |
| OEM.15: In patients with osteoma(s) FAP should be considered. | LE: low Agreement: 84% (SA 56%; A: 28%; N 13%; D 3%) |
| Gynaecological manifestations | |
| OEM.16: There are very limited data as to the incidence of gynaecological cancers in FAP carriers. Based on these limited data there does not seem to be an increased risk. | LE: low Agreement: 81% (SA 31%; A: 50%; N 15%; D 4%) |
| OEM.17A: There is no evidence to identify specific risk factors for the development of gynaecological cancers in FAP carriers. Women with FAP should be advised to maintain a healthy lifestyle and weight. | LE: low Agreement: 90% (SA 47%; A: 43%; N 7%; D 3%) |
| OEM.17B: Female FAP carriers seeking contraception should be advised as to the reduced colorectal cancer risk in those who use oestrogen-based contraceptives. | LE: low Agreement: 79% (SA 34%; A: 45%; N 14%; D 7%) |
| OEM.18A: Gynaecological cancer surveillance should be as for the general population in women with FAP. | LE: low Agreement: 83% (SA 55%; A: 28%; N 10%; D 3.5% SD 3.5%) |
OEM.18B: Women with FAP, like women generally, should report any abnormal symptoms suggestive of gynaecological cancer to their family doctor urgently. These symptoms include:
|
LE: low Agreement: 90% (SA 66%; A: 24%; N 10%) |
| OEM.19A: There is no evidence that FAP in and of itself leads to reduced female fertility. | LE: Low Agreement: 90% (SA 48%; A: 42%; N 7%; D 3%) |
| OEM.19B: Women of child-bearing age who are diagnosed with cancer should be referred to a fertility specialist to discuss their options in a timely manner. | LE: Low Agreement: 97% (SA 62%; A: 35%; N 3%) |
| OEM.19C: There is no convincing evidence showing different fertility outcomes between IPAA and IRA | LE: Low Agreement: 92% (SA 44%; A: 48%; N 4%; D 4%) |
| OEM.19D: Women who have undergone risk-reducing surgery and have not got pregnant within a year of trying should be referred to a fertility specialist. | LE: Low Agreement: 88% (SA 50%; A: 38%; N 12%) |
| OEM.20: The impact of childbirth in a patient with IPAA has not been evaluated so far. No risk can be assessed on the impact of childbirth. | LE: — Agreement: 96% (SA 46%;| A: 50%; N 4%) |
A, agree; D, disagree; FAP, familial adenomatous polyposis; IPAA, ileal pouch anal anastomosis; IRA, ileorectal anastomosis; LE, level of evidence; N, neutral; SA, strongly agree; SD, strongly disagree.
Table 6.
Short version: statements pertaining chemoprevention in familial adenomatous polyposis
| Statements | Level of evidence and agreement |
|---|---|
| CP.1: Currently, there are insufficient data to draw definitive conclusions regarding the effect of chemoprevention on the occurrence of colorectal cancer. | LE: — Agreement: 97% (SA 47%; A: 50%; N 3%) |
| CP.2: There is currently no available evidence indicating that chemoprevention prevents the occurrence or progression of small bowel cancer. | LE: — Agreement: 97% (SA 62%; A: 35%; N 3%) |
| CP.3: The effect of chemoprevention on the occurrence of gastric cancer has not been investigated. | LE: — Agreement: 97% (SA 55%; A: 42%; N 3%) |
| CP.4: Currently, there is insufficient evidence to support the recommendation of chemoprevention for reducing the number and/or size of colorectal polyps in clinical practice. The use of chemoprevention in this context can only be suggested within the framework of clinical trials. | LE: — Agreement: 90% (SA 55%; A: 35%; N 10%) |
| CP.5: There is currently insufficient evidence to support the recommendation of any chemopreventive agent for decreasing polyp size and number in the duodenum due to the lack of an acceptable risk/benefit ratio. Further trials with appropriate clinically meaningful endpoints are necessary. | LE: — Agreement: 94% (SA 48%; A: 46%; N 3%; D 3%) |
| CP.6: There is no evidence to support the role of chemoprevention in delaying or preventing colectomy in FAP patients. | LE: — Agreement: 97% (SA 58%; A: 39%; N 3%) |
| CP.7: Chemoprevention does not delay or prevent risk-reducing surgery in the upper GI tract. | LE: — Agreement: 90% (SA 67%; A: 23%; N 3%; D 7%) |
A, agree; D, disagree; FAP, familial adenomatous polyposis; GI, gastrointestinal; LE, level of evidence; N, neutral; SA, strongly agree; SD, strongly disagree.
Table 7.
Short version: statements pertaining lower gastrointestinal manifestations in MUTYH-associated polyposis
| Statements | Level of evidence and agreement |
|---|---|
| MAP.LGM.1: Lower-GI tract surveillance is recommended in individuals with biallelic MUTYH pathogenic variants. | LE: Low Agreement: 100% (SA 55%; A: 45%) |
| MAP.LGM.2: Colonoscopy surveillance, in the absence of symptoms, should generally start at the age of 18 years, but exceptionally may be started earlier, based upon family history. | LE: Low Agreement: 90% (SA 38%; A: 52%; N: 7%; D: 3%) |
| MAP.LGM.3: The surveillance interval should be 1–2 yearly but may be personalized according to phenotype (polyp burden). | LE: Low Agreement: 97% (SA 47%; A: 50%; D: 3%) |
| MAP.LGM.4A: Most MAP patients present with an a-FAP-like colorectal polyposis. For these patients, endoscopic resection of colorectal adenomas may be preferred over surgery. | LE: Low Agreement: 86% (SA 32%; A: 54%; N: 11%; D: 3%) |
| MAP.LGM.4B: If surgery is considered, it should be discussed in a multidisciplinary setting. The discussion must consider the polyp burden (colonic and rectal), age, co-morbidities, and the patient’s views, as well as their compliance with endoscopic surveillance. | LE: Low Agreement: 93% (SA 48%; A: 45%; N: 7%) |
| MAP.LGM.4C: The type of surgery depends on the rectal polyp burden. Consider colectomy with IRA as the first option. If there is dense rectal polyposis that cannot be managed endoscopically, consider proctocolectomy with IPAA. | LE: Low Agreement: 97% (SA 55%; A: 42%; N: 3%) |
| MAP.LGM.4D: Prophylactic surgery is not recommended in patients with pathogenic variants in MUTYH who have not developed colorectal polyps or cancer. | LE: Low Agreement: 96% (SA 57%; A: 39%; D: 4%) |
| MAP.LGM.5: MAP patients may benefit from a total colectomy instead of a segmental colectomy when they present with or without confirmed colorectal cancer. However, patients who have received thorough counselling may choose to undergo a segmental colectomy instead. | LE: Low Agreement: 92% (SA 38%; A: 54%; N: 8%) |
| MAP.LGM.6A: Lower-GI tract surveillance is recommended in MAP patients. The surveillance interval should be 1–2 yearly but may be personalized according to phenotype. | LE: Low Agreement: 100% (SA 53%; A: 47%) |
| MAP.LGM.6B: In patients having proctocolectomy with IPAA, endoscopic surveillance of the pouch is recommended post-surgery. | LE: Low Agreement: 97% (SA 55%; A: 42%; N: 3%) |
A, agree; a-FAP, attenuated familial adenomatous polyposis; D, disagree; FAP, familial adenomatous polyposis; GI, gastrointestinal; IPAA, ileal pouch anal anastomosis; IRA, ileorectal anastomosis; LE, level of evidence; MAP, MUTYH-associated polyposis; N, neutral; SA, strongly agree; SD, strongly disagree.
Table 8.
Short version: statements pertaining upper gastrointestinal manifestations in MUTYH-associated polyposis
| Statements | Level of evidence and agreement |
|---|---|
| MAP.UGM.1: Upper-GI tract surveillance is recommended in MAP patients. | LE: Low Agreement: 100% (SA 62%; A: 38%) |
| MAP.UGM.2: Upper GI surveillance by OGD should start from age 35 years. | LE: Low Agreement: 90% (SA 37%; A: 53%; 10%) |
| MAP.UGM.3: Upper GI surveillance in MAP should be adapted according to OGD findings, but not exceeding at interval 3 years. Polypectomy is recommended, regardless of polyp size. | LE: Low Agreement: 100% (SA 46%; A: 54%) |
A, agree; D, disagree; GI, gastrointestinal; LE, level of evidence; MAP, MUTYH-associated polyposis; N, neutral; OGD, oesophagogastro-duodenoscopy; SA, strongly agree; SD, strongly disagree.
Table 9.
Short version: statements pertaining to extra-gastrointestinal manifestations of MUTYH-associated polyposis
| Statements | Level of evidence and agreement |
|---|---|
| MAP.EIM.1: No surveillance for extra-intestinal cancers is recommended for MUTYH biallelic carriers. | LE: Low Agreement: 80% (SA 42%; A 38%; N 13%; SD 7%) |
A, agree; D, disagree; LE, level of evidence; N, neutral; SA, strongly agree; SD, strongly disagree.
If a high consensus was not achieved, the different opinions expressed were recorded, discussed within the working groups, and consequently elucidated in the comment sections. Each original article whose data contributed to the development of the statements was individually summarized. The summary of evidence from the referenced articles is provided in Appendix S4.
Short version of the guidelines
Both short and extended versions of the guidelines are provided below. The short versions provides an overview of the most clinically salient components of the guidelines with full description in the extended version.
Familial adenomatous polyposis
Section I: lower gastrointestinal manifestations (LGM)
Surveillance
It is well-established that children and adolescents at high risk of developing FAP or attenuated (a)-FAP (patients with a germline PV in the APC gene—for FAP disease—or patients with at least one first-degree relative affected by classical or a-FAP) should undergo regular surveillance36,37. Retrospective studies do not accurately reflect the true natural course of disease. A few studies have reported cases of exceptionally young age of cancer development38, which emphasizes the need to follow these patients proactively in surveillance programmes. As a result, surveillance in classical FAP patients is recommended to start at the age of 12. Historically, a-FAP has been used to describe a type of FAP with later onset of adenoma and CRC development39–41. This suggests that surveillance in a-FAP patients can theoretically safely begin later in life than in classical FAP. However, even between individuals with the same PV, the phenotype may vary in terms of the occurrence, severity and timing of manifestations42,43. Based on these considerations (age of adenoma onset typically between 35 and 45 years39 and median age of CRC diagnosis of approximately 55 years40,41), in a-FAP it is not safe to suggest starting surveillance later than 18–20 years of age.
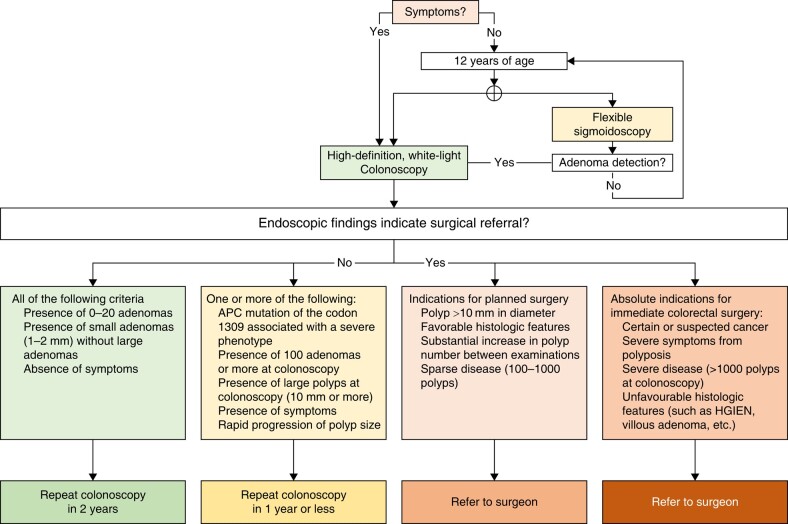
Alarm symptoms such as rectal bleeding, anaemia, increase in bowel movements and mucous discharge should prompt colonoscopy regardless of age, genotype and phenotype44,45. The role of colonoscopy surveillance among FAP patients is well established, demonstrating a reduction in the incidence of CRC and associated mortality46,47. Recent studies have highlighted the age-related absence of neoplasia in the rectosigmoidal segment, ranging from approximately 10% to 35%44,48. Therefore, a full colonoscopy is considered the safest modality for surveying the colon. Factors such as patient preference and the habits of the medical team, including routine sedation during the procedure, need to be taken into consideration. Taking the heterogeneity of polyp distribution into account39,40, the best modality of colonic surveillance in polyposis syndromes is a full colonoscopy. Despite chromoendoscopy showing greater detection of diminutive adenomas compared to white-light endoscopy in FAP colonoscopy49, it is unlikely that detection of these will in influence established management or affect the timing of surgery. There is a lack of evidence regarding the ideal interval for colonic surveillance. Different guidelines offer varying suggestions, ranging from yearly intervals36 to tailored intervals based on phenotypes37. A recent study found a correlation between polyp progression and the polyp count at the initial colonoscopy, particularly if the count is ≥100, or the patient has a PV in codon 130950. However, genotype alone is not sufficient to determine the timing of surveillance. These guidelines propose adjusting the interval of colonoscopy surveillance based on various factors, rather than relying on a fixed period. Certain critical factors, such as the presence of a PV in the codon 1309 of the APC gene, a high number of polyps, and the presence of large adenomas, may warrant a shorter surveillance interval (see Fig. 1).
Fig. 1.
Endoscopic surveillance and management for FAP patients
APC, adenomatous polyposis coli; FAP, familial adenomatous polyposis; HGIEN, high-grade intraepithelial neoplasia.
Surgery
Timing of surgery
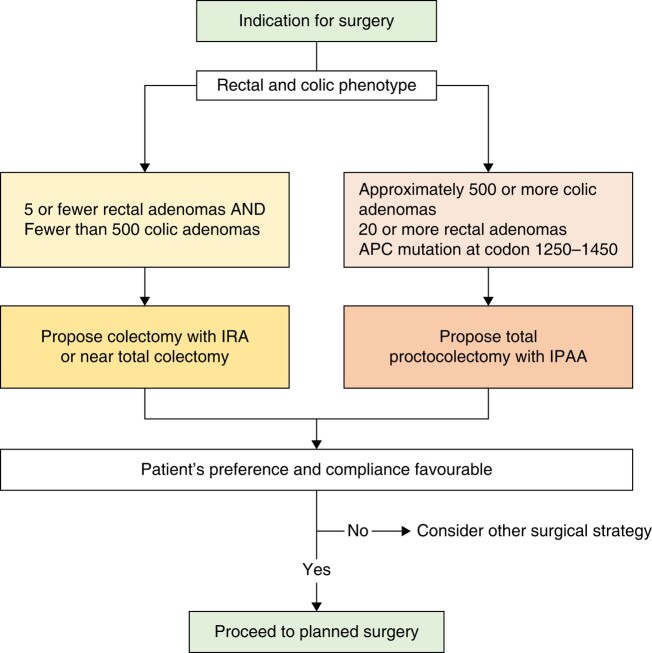
In order to determine the optimal timing for prophylactic surgery, different parameters must be assessed and weighed. Kobayashi et al. conducted a multicentre observational cohort study, compiling data from 303 patients who had colorectal surgery for FAP between 2000 and 2012 across 23 different institutions. Of these 303 surgical cases, 115 individuals (38%) were diagnosed with CRC. As expected, a significant correlation emerged between older age and various phenotypes. In the three distinct phenotypes—attenuated (<100 polyps), sparse (100–1000 polyps) and profuse polyposis (≥1000 polyps)—cancer was observed in 47.4%, 36.2% and 36.8% of patients respectively. Patients with CRC had mean ages of 50, 39 and 34 years for these phenotypes respectively, whereas patients without CRC had mean ages of 33, 31 and 31 years. The study aimed to pinpoint the optimal age threshold for predicting CRC development in individuals with attenuated, sparse, and profuse FAP, which were identified as 46, 31 and 27 years respectively51. Consequently, we propose that clinical management and the recommended timing for prophylactic surgery should be individually customized to match each phenotype. It is also known that patients with an APC pathogenic variant within codons 1250–154952 or codons 1250–146453 frequently present a severe phenotype and may benefit from earlier prophylactic surgery52. Some studies have associated the presence of symptoms, particularly rectal bleeding44, with the risk of dysplasia, suggesting the need for prompt intervention. A delay in performing prophylactic surgery could be acceptable in some situations54; however, there is a higher rate of malignant polyps in patients undergoing prophylactic surgery at higher ages at the time of surgery54. This finding reasonably suggests that surgeons and endoscopists/gastroenterologists should provide a clinical indication for prophylactic colectomy, allowing flexibility to schedule the surgery within the best window of time for patients. Prophylactic surgery can be planned at a time that is suitable for the patient, based on the risk of cancer as assessed by colonoscopy. The timing of surgery should consider social issues, family planning, emotional development of the patient in relation to age, and the likelihood of attending planned surveillance. The two main options for prophylactic removal of the large intestine are colectomy with ileorectal anastomosis (IRA) and proctocolectomy with ileal pouch–anal anastomosis (IPAA). In the IRA procedure, the rectum is preserved, whereas in the IPAA, a pelvic dissection is performed to create a pouch that mimics a reservoir. Each proposed surgery has its own advantages and disadvantages. Some patient characteristics can guide the choice (see Fig. 2):
Fig. 2.
Surgical strategy for FAP patients
FAP, familial adenomatous polyposis; IPAA, ileal pouch anal anastomosis; IRA, ileorectal anastomosis.
APC germline PV: patients with a severe genotype (codons 1250–1464) are good candidates for an IPAA, as they have the highest cumulative incidence of rectal cancer after rectal-sparing surgery53,55. On the other hand, indications for rectal-sparing surgery are APC mutation outside the codon 1250–1450, or in patients with a-FAP and mutations before codon 157 or beyond codon 1595, or, in the alternatively spliced part of exon 9, codons 312–412, who show a low risk of rectal cancer and secondary proctectomy after IRA53,56.
Polyp number in the rectum: the risk of proctectomy after IRA is reported to be zero if patients originally had fewer than 5 rectal adenomas and fewer than 1000 colonic adenomas. In patients with 5–20 preoperative rectal adenomas, the proctectomy rate is reported to be 13%, but when there were 20 or more rectal adenomas, the proctectomy rate increased to 54%57. The risk of developing cancer in the pouch, rectal cuff and anal transitional zone (ATZ) is low, but not zero. Carcinomas are detected more often in the rectal cuff/ATZ than in the pouch itself58. A higher incidence of adenomas in the ATZ (and rectal cuff) is documented in patients with remaining rectal epithelium58–60. It is recommended not leave the rectal cuff when performing an IPAA, or at least it should be as short as possible. However, the risk of further adenoma development and the possible worsening of faecal incontinence should be weighed against each other.
Choice of surgical procedure
Another major debate concerns which of the two surgical procedures is associated with a better quality of life. Historically, one of the main advantages of suggesting total colectomy with IRA is its good functional outcome. In a meta-analysis of 12 retrospective studies, patients undergoing IPAA had a higher (on average) number of bowel movements per day, with a higher rate of experiencing night-time defecation, wearing a pad, and 50.5% experiencing incontinence during a 24-hour period61. These considerations could lead to suggesting IRA, if compatible with an acceptably low risk of developing later rectal cancer. Another important aspect to consider is fertility issues, especially for women who wish to have children. Currently, there is no convincing evidence showing different fertility outcomes between IPAA and IRA procedures. However, there have been reports of reduced female fertility in IPAA compared to the IRA procedure62–65, which has led to suggestions of postponing or avoiding a colectomy with IPAA in young women who want to have children36. After an exhaustive review, including the distinction between IPAA performed for FAP or ulcerative colitis (UC), and considering the increased use of laparoscopic approaches, it was concluded that there is no convincing evidence showing different fertility outcomes between IPAA and IRA in female FAP patients (see OEM.19). In the largest retrospective study of 49 FAP patients after IRA and 51 after IPAA, no difference was detected in the rate of fertility problems (9/49 and 9/51 respectively)66. It is also worth noting that the use of the laparoscopic approach has been associated with a significantly higher subsequent pregnancy rate, making it the preferred method for most young women67,68.
Surgical technique
Extent of mesenteric resection
While mesocolic or mesorectal excision is well established in the oncological setting, its necessity in the prophylactic setting is less clear. In the prophylactic setting, an alternative to complete mesocolic and mesorectal excision is close rectal dissection (CRD). Studies have reported a higher rate of nerve injuries and diminished sexual function in patients undergoing total mesorectal excision for cancer (without IPAA). However, nerve injury leading to impotence can also occur during the anterolateral dissection of the rectum, and the rates are similar in both techniques69. Bartels et al.70 demonstrated that the increased rate of severe complications after a total mesorectal excisions may be related to steroid use, which is typically absent in FAP patients. Therefore, drawing conclusions for the FAP population becomes challenging.
Use of a diverting stoma in IPAA
Initially, a diverting stoma was always performed during restorative proctocolectomy, but surgical techniques have evolved. Nowadays, ileostomy is not mandatory, and an increasing number of publications have reported cohorts of patients having RPC without a stoma. This recommendation is applicable to each polyposis phenotype (including attenuated, classical and MAP) and regardless of the type of anastomosis (manual or stapled). Since 2006, four studies have been published on this matter by three teams71–74. Cases were mixed with UC patients, and it appears that several variables influence ileostomy omission, such as stapled anastomosis, no preoperative corticosteroid use, a FAP diagnosis, female sex and age <26 years72. Often, these variables apply to FAP patients. It has also been shown that there is no significant difference in postoperative morbidity, leakage rate or reoperation in patients with or without a stoma. In selected cases, a ghost ileostomy can be taken into account.
Total or subtotal colectomy
The main difference between a near-total colectomy (or subtotal colectomy) and a total colectomy consists of the preservation of the superior rectal artery, a branch of the inferior mesenteric artery, in subtotal colectomy. This is done to ensure adequate vascularization of the recto-sigmoid junction and the distal sigmoid. The difference in the level of anastomosis can have an impact on functional outcomes and quality of life. However, only one study has evaluated the short-term outcomes between the two surgical techniques56. In this study, the rate of reoperation was significantly lower in the group of patients who had an ileo-sigmoid anastomosis (0% versus 12.2%; P = 0.008), primarily due to a lower rate of anastomotic leakage (2% versus 10.8%, P = 0.0125)56. However, the number of adenomas developing per patient per year was significantly higher after ileo-sigmoid anastomosis (11 versus 6; P < 0.001)56. Patients who are considered for this surgery must be carefully selected based on criteria that exclude them from undergoing IPAA.
Post-surgical management
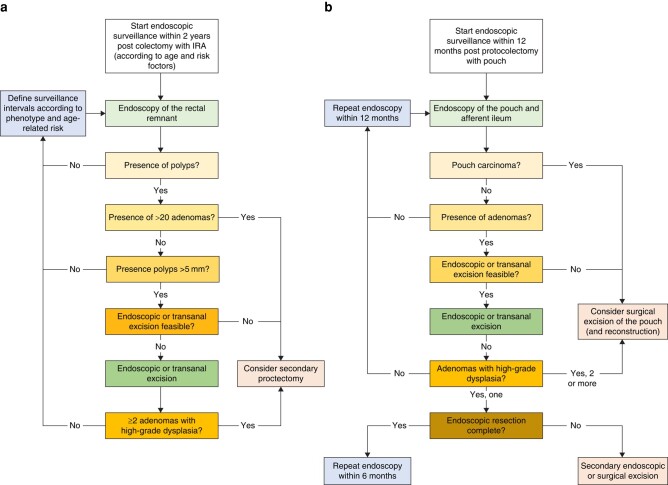
Data on surveillance post-IRA is inconsistent due to a mixed patient population (diagnosis based on phenotype versus APC mutation and surveillance for primary cancer versus metachronous), different surveillance protocols and different recommendations (see Fig. 3). Some recommendations suggest the removal of all polyps regardless of number and in the cases of diffuse polyposis, a multistep treatment has been proposed (with removal of all polyps regardless of number and reducing surveillance to 3 weeks until rectal polyp clearance)75, whereas others recommend annual endoscopy with polypectomy of all polyps >5 mm76. This is because the adenoma–carcinoma sequence after colectomy with IRA for FAP is similar to that of sporadic cancer. The cumulative risk of rectal cancer after IRA varies from 6% to 33%75,77–81, whereas the cumulative risk of dying from rectal cancer is between 9% and 12.5%77–80. Polyps in the rectal remnant can initially be treated endoscopically. If they are not manageable or meet the criteria indicating proctocolectomy (see LGI.8), a surgical approach with secondary proctectomy should be considered and discussed with the patient. After a proctocolectomy with ileo-anal pouch procedure, the incidence of adenomas in the pouch varies from 6.7% to 78%81–87. The incidence increases over time after surgery60,83,88 and seems to be related to the pouch age rather than the patient‘s age82. Adenomas are more frequent in stapled anastomoses than in handsewn ones89,90. Advanced neoplasia (polyp size >10 mm and/or high-grade intraepithelial neoplasia (HGIEN)) is found in 7% of pouches81.
Fig. 3.
Flowchart for post-surgical surveillance in IRA a and IPAA b
IPAA, ileal pouch anal anastomosis; IRA, ileorectal anastomosis.
Fortunately, the risk of developing pouch cancer seems to be low86, with an incidence of 1.9–3.8%84,89. Carcinomas are detected in the rectal cuff/ATZ more often than in the pouch itself58. Endoscopic surveillance of the pouch should start within 1 year after surgery91. The frequency of endoscopic surveillance of the pouch is mostly based on expert opinion and varies from every 6–12 months to biannual or life-long60,81–86,89,91–94. In case of high-grade dysplasia, polyps ≥ 10 mm, and/or a total polyp number ≥30, a 3–6-monthly endoscopic follow-up is advised82,83,91. Because duodenal and gastric adenoma have been identified as a risk factor in different studies87,92, particular attention should be paid to patients with these manifestations. Endoscopic resection of these polyps appears to be the first step for diagnosis, grading and treatment. However, there is a lack of evidence about the effects of any chemopreventive and curative treatments for these pouch adenomas. Tajika et al.95 demonstrated the feasibility of endoscopic surveillance and management of rectal polyposis and Patel et al.96 demonstrated the feasibility of endoscopic management, including in severe cases. Other guidelines also support the removal of all polyps larger than 5 mm during pouch surveillance97. However, once endoscopic resection is no longer effective or high-risk adenomas are found, other options must be discussed. Although there are no specific reports on such a situation, it appears that resection of the pouch is an option and that a new pouch can be created. When pouch carcinoma is present, pouch resection is indicated to ensure oncological margin resection. Pre-pouch examination should be included during pouchoscopy because it is important to ensure a comprehensive inspection of the pouch and to detect any potential pre-pouch adenomas and inflammation. The presence of adenoma, but not malignancy, in the inlet segment of small bowel proximal to the pouch has been reported87,88.
Section II: upper gastrointestinal manifestations (UGM)
Risk factors for upper gastrointestinal neoplasia in familial adenomatous polyposis
Several risk factors have been investigated for the development of duodenal and papillary adenocarcinoma, with some contributing to both types (Table 16). Risk factors for duodenal adenocarcinoma include ageing, stage IV Spigelman polyposis (OR 8.8, 95% c.i. 2.1–36.6), high-grade dysplasia in duodenal adenomas (OR 9.2, 95% c.i. 1.7–49.9), duodenal adenomas larger than 10 mm (OR 6.2, 95% c.i. 1.7–23.1), villous histology and high-grade dysplasia in the papilla98,99. The overall risk of developing papillary adenocarcinoma is lower compared to duodenal cancer100. Risk factors for papillary adenocarcinoma include ageing (without a specific age threshold), villous histology or high-grade dysplasia from papilla biopsies, ampulla size greater than 1 cm and the presence of an ampullary adenoma98–102. However, the Spigelman score alone poorly predicts the risk of papillary cancer98–100,103,104. Family history of colorectal or duodenal cancer may be a risk factor for duodenal cancer, but the evidence is currently limited98,99. Pregnancy and a personal history of extra-intestinal manifestations as risk factors are controversial105,106.
Table 16.
Duodenal adenocarcinoma risk factors
| Element | Interpretation | Data and study |
|---|---|---|
| Spigelman stage IV | Risk factor | ⇑ among duodenal cancer: 15.3% versus 7.1% (P = 0.003)98 OR, 8.8; 95% c.i., 2.1–36.698 |
| Duodenal adenoma HGD | Risk factor | ⇑ among duodenal cancer: 29.4% versus 5.9% (P = 0.003)98 OR, 9.2; 95% c.i., 1.7–49.998 |
| Duodenal adenoma >10 mm | Risk factor | ⇑ among duodenal cancer: 76.5% versus 47.1% (P = 0.027)98 OR, 6.2; 95% c.i., 1.7–23.198 |
| Duodenal adenoma with tubulovillous or villous histology | Not risk factor | Similar among cases and controls (P = 0.43)98 OR, 1.9; 95% c.i., 0.4–3.498 |
| Papilla TV/V histology | Risk factor | ⇑ among duodenal cancer: 83.3% versus 22.4% (P < 0.001)98 |
| Papilla HGD | Risk factor | ⇑ among duodenal cancer: 25.0% versus 3.5% (P = 0.02)98 |
| Spigelman stage, at first endoscopy | Risk factor | 33% for stage IV, 13% for stage III, 12% for stage II, 0% for stage I and 0104 |
| Family history of CRC | Risk factor | ⇑ among duodenal cancer: 58.8% versus 33.3% (P = 0.048)98 |
| Family history of duodenal polyposis | Not risk factor | Similar risk of duodenal cancer99 |
| Personal history CRC | Risk factor Not risk factor |
⇑ among duodenal cancer: 22.2% versus 4.7% (P = 0.012)98 Similar duodenal cancer risk: OR, 1.331 (P = 0.56)110 |
| Personal history of desmoids | Not risk factor | Similar risk of duodenal cancer99 |
| Personal history of thyroid cancer | Risk factor | ⇑ among duodenal polyposis (P = 0.031)121 |
| Personal history of gastric cancer | Risk factor | ⇑among duodenal polyposis (OR, 6.260; 95% c.i., 1.504, 26.056)121 |
| Type of colon surgery | Risk factor Not risk factor |
⇑ risk of stage IV Spigelman for ileoanal anastomosis versus ileorectal anastomosis (P = 0.0029)116. Similar risk of duodenal cancer99 |
| Sulindac | Protective | ⇓ among duodenal cancer: 11.1% versus 41.2% (P = 0.016)98 |
| Celecoxib | Protective | ⇓ among duodenal cancer: 5.6% versus 32.9% (P = 0.019)98 |
| Ageing | Risk factor | Progressive increase in Spigelman stage111 Score: +0.30 points/year Stage: +0.12/year |
| Gastric polyposis | Risk factor | ⇑ among duodenal polyposis: OR, 2.814 (P = 0.024)110 |
| Sex | Not risk factor Not risk factor |
Similar duodenal cancer risk110 Similar risk of duodenal cancer99 |
| Age at diagnosis of FAP | Not risk factor Not risk factor Risk factor Not risk factor |
OR 0.438 (P = 0.124)110 Similar risk of duodenal cancer99 ⇑among duodenal polyposis (OR, 0.963; 95% c.i., 0.937, 0.990)121 Similar risk of duodenal polyposis127 |
| COX-2 polymorphisms | Not risk factor | Similar distribution of Spigelman stages124 |
| UGT and GST polymorphisms | Not risk factor | Similar distribution of Spigelman stages334 |
| Pregnancy | Variable | ⇑ among polyposis stage III/IV, but only if APC mutations before codon 1020 (50% versus 0%, P = 0.005)105 |
| APC mutation site | Risk factor Not risk factor Not risk factor Not risk factor Not risk factor |
⇑ polyposis risk if at codon 3183–3187121 Similar duodenal cancer risk98 Similar duodenal cancer risk99 Similar duodenal cancer risk104 Similar risk of duodenal polyposis127 |
CRC, colorectal cancer; FAP, familial adenomatous polyposis; HGD, high-grade dysplasia; TV/V, tubulovillous or villous.
Surveillance
The decision to initiate surveillance should take into account the age-related risks of developing duodenal polyposis, advanced duodenal polyposis and duodenal cancer107,108. The lifetime risk of duodenal cancer in FAP is 18% (95% c.i. 8–28%) and increases steadily with age (3.2% at age 40, 7.6% at age 60, 34.0% at age 73)104,107–109. The median age at diagnosis of high-grade dysplasia is 73 years, but the risk also varies with age (5.7% at age 40, 15.2% at age 50, 23.2% at age 60)103,109. The cumulative lifetime risk of reaching stage IV Spigelman polyposis is 35%, with an age-dependent pattern (10% at age 50, 20% at age 57, 30% at age 70)104. Furthermore, 88% of FAP patients will develop duodenal polyposis (95% c.i. 84–93%), and the incidence of duodenal polyps increases with age (20% at age 37, 40% at age 45, 60% at age 55, 80% at age 65). Although most studies recommend starting upper GI surveillance between ages 25 and 45 years, it is important to note that by 45 years of age, the prevalence of duodenal polyposis likely exceeds 30%, with up to 10% already advanced, and rare cases present with cancer at the initial endoscopy (<2%)99,102,103,107,109–120. Therefore, the first endoscopic evaluation may begin after the age of 18 years. Patient preferences may be considered to some extent, but it should be emphasized that the risks of advanced duodenal polyposis and duodenal cancer become significant by the age of 35–40 years.
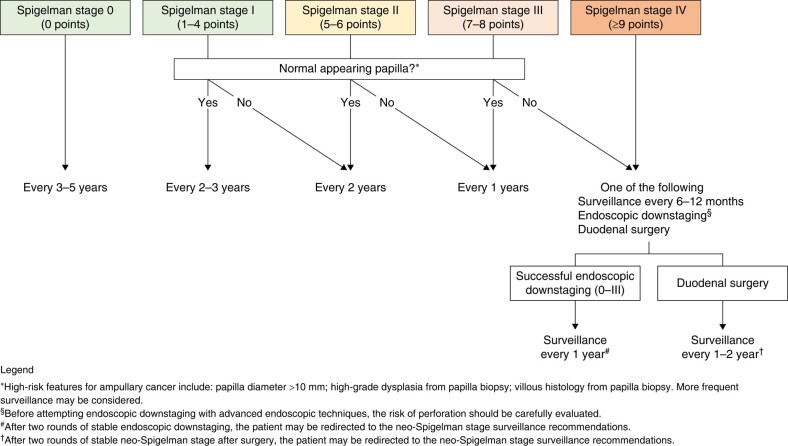
The optimal interval for endoscopic surveillance of the upper GI tract depends on the findings in the stomach, duodenum and jejunum, each of which confers different risks for developing gastric, duodenal and jejunal adenocarcinoma respectively. The surveillance interval should be determined based on the site with the highest-risk findings, which is typically the duodenum. The proposed surveillance algorithm for duodenal polyposis is illustrated in Fig. 4 and is based on the Spigelman stage (Table 3) and papillary endoscopic appearance. Figure 5 presents the proposed surveillance algorithm for gastric adenomas. The baseline Spigelman stage provides an estimation of the lifetime risk of duodenal cancer (33% for stage IV, 13% for stage III, 12% for stage II, 0% for stages I and 0)104, but the natural history of duodenal polyposis is to progress103,111,117. Endoscopic downstaging can reduce this risk,119,121,122 but the risk of re-progressing to stage IV within 1 year is as high as 50%115. Therefore, yearly surveillance is recommended even after downstaging115. In exceptional cases, patients stable in stages 0–II after downstaging may be considered for surveillance every 2 years119,121,122. Moreover, in the presence of factors that significantly increase the risk of papillary cancer (papilla diameter >10 mm, high-grade dysplasia or villous histology from papilla biopsy), more frequent surveillance endoscopy may be considered. Regarding the stomach, the risk of high-grade dysplasia in adenomas is proportional to the size of the adenoma (33% if >20 mm, 4% if ≤20 mm)123,124, but is rare in adenomas smaller than 5 mm124. Therefore, gastric adenomas larger than 5 mm should be resected en-bloc, and surveillance should depend on histological findings (Fig. 5).
Fig. 4.
Surveillance intervals according to duodenal findings
Table 3.
The Spigelman score
| 0 points | 1 point | 2 points | 3 points | |
|---|---|---|---|---|
| Number of polyps | 0 | 1–4 | 5–20 | >20 |
| Size of polyps (mm) | 0 | 1–4 | 5–10 | >10 |
| Histology | Normal | Tubular | Tubulovillous | Villous |
| Dysplasia | None | Low grade | High grade |
0 points = stage 0; 1–4 points = stage I; 5–6 points = stage II; 7–8 points = stage III; 9–12 points = stage IV.
Fig. 5.
Surveillance intervals according to gastric findings
EUS, endoscopic ultrasound.
After duodenal surgery, the neo-duodenum may develop adenomas in up to 59.4% of patients, at a median time of 55 months from surgery (range: 22–84 months)109,125. The evidence regarding the prevalence of jejunal adenomas is of low quality106,126, but suggests that 83.3–90% of patients with stage IV duodenal polyposis also have jejunal polyps. Therefore, small bowel enteroscopy may be offered to individuals with stage III/IV duodenal polyposis, but this should be done in a research setting. Endoscopic surveillance necessitates thorough and comprehensive visualization of the entire mucosal surface, including the papilla, to accurately count all duodenal polyps and assess the risk of adenomas or malignancy98,99,102–104,108,109,112,115,117,127. Forward-viewing endoscopy typically provides adequate visualization of the gastric and duodenal mucosa in almost all cases104,108,109,111. Cap-assisted endoscopy can visualize the papilla in up to 95–97% of cases128,129. In instances where the papilla cannot be visualized using a forward-viewing instrument, side-viewing endoscopy should be employed99,102,108,117. The use of chromoendoscopy and narrow-band imaging may double the number of adenomas detected per duodenum and improve visualization of larger ones, consequently leading to an increased Spigelman stage in approximately 10% of patients and, therefore, more intensive surveillance130–134. In the stomach, indigo and digital chromoendoscopy, as well as narrow-band imaging, can increase the median number of gastric adenomas detected per patient130,133,134. Video capsule endoscopy is only able to visualize the papilla in 10.4% of patients, making it unsuitable for surveillance135–137. Double-balloon enteroscopy and endoscopic ultrasound may have utility for diagnostic and therapeutic purposes but are not currently part of routine endoscopic surveillance101,138–140.
Performing random biopsies of macroscopically normal papillae may enhance the detection rate of adenomas98,141,142. Macroscopically normal ampullas may harbour abnormal histology in up to 44% of cases, including low-grade dysplasia (8–25%) and high-grade dysplasia (<0.5%)102,143,144. The risk of iatrogenic pancreatitis across studies was <1%, with no report of bleeding, perforation or stenosis143. However, there is a significant knowledge gap regarding the utility of random biopsies of duodenal polyps.
Spigelman staging system
The Spigelman stage is widely used to estimate the risk of duodenal cancer. However, it has certain limitations, including suboptimal sensitivity and specificity for predicting duodenal and ampullary cancer99,102–104,109,111,119,121,122. Stage IV Spigelman polyposis is associated with the highest 10-year risk of duodenal cancer (30%), and developing stage IV during surveillance increases the odds ratio of duodenal cancer by 8.898,99,102–104,107,111,115,118,121. However, a significant proportion of patients with duodenal (up to 53%) and papillary (up to 75%) adenocarcinomas do not have a prior history of stage IV duodenal polyposis98–100,103,145. Surgical case series also suggest that 10–30% of patients undergoing prophylactic duodenal surgery for stage IV have an unexpected duodenal cancer109,146,147. Additionally, not all components of the Spigelman system have equal predictive value for duodenal and papillary cancer, and there may be a need for future revision to assign different weights and include additional risk factors for papillary cancer98–100,103,119,145. Stages II and III Spigelman polyposis carry an intermediate lifetime risk of duodenal cancer (12% and 13% respectively)104. However, it may take several years for duodenal cancer to develop, as the 10-year risk is estimated to be 2% for both stages107. Interestingly, patients who are downstaged from stage IV to stages I, II or III have a significantly higher risk of duodenal cancer compared to patients who naturally progress to stages I, II or III112,115. However, the current Spigelman stage does not take this information into account102,115,119,148. Other concerns regarding the Spigelman stage include its application to the neo-duodenum after duodenal surgery109, its validity when chromoendoscopy significantly increases the duodenal polyp count and, consequently, the Spigelman stage122,149, and whether additional duodenal cancer risk factors should be included104,116,121,127.
Endoscopic treatment options and duodenal surgery versus endoscopic management
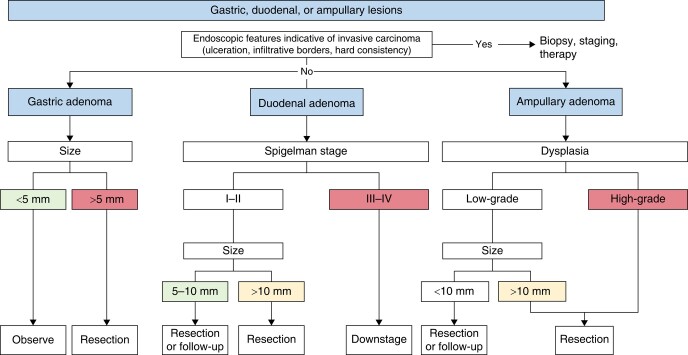
Upper GI surveillance aims to prevent gastric, duodenal and papillary adenocarcinoma98,114,150. Various risk factors contribute to the decision of whether to remove or observe gastric, duodenal, papillary or jejunoileal polyps, including the Spigelman stage, degree of dysplasia and polyp size (Fig. 6).
Fig. 6.
Proposed endoscopic management of gastric, duodenal, and papillary lesions
Gastric adenomas <5 mm rarely contain high-grade dysplasia (HGD), so it is recommended to resect all gastric adenomas >5 mm en-bloc124.
Patients with advanced duodenal polyposis (Spigelman stages III or IV) or advanced papillary lesions (>10 mm or with high-grade dysplasia) should undergo endoscopic downstaging119,121,122,151. Clinical practice and cohort studies suggest that duodenal polyps >10 mm should be removed, as lesions <10 mm rarely contain HGD or invasive carcinoma113,118. The survival of patients with treated adenomas >10 mm and those under surveillance with adenomas <10 mm did not differ significantly (7.13 versus 9.72 years, P = 0.08)113. During a median follow-up of 8.5 years, none of the duodenal adenomas under surveillance required treatment118,151. Stage IV duodenal polyposis has traditionally been treated with duodenal surgery, but patients may not develop cancer for several years and endoscopic downstaging can extend cancer-free surveillance (74% at 89 months)98,108,116,119. Duodenal surgery is associated with significant short-term mortality (about 5%) and morbidity (30–63%)142,152–155, so it should be offered to carefully selected patients141,156. Nevertheless, there should be a low threshold to escalate to duodenal surgery once the disease becomes no longer manageable endoscopically139,141,142,146,148,150,157,158. Several studies have reported a high risk (range 8–37%) of unsuspected duodenal cancers that are diagnosed only after histological review108,109,139,141,142,146–148,150,158.
There are no RCTs determining the ideal size threshold for endoscopic papillectomy145,159. Size is not a contraindication to endoscopic treatment per se, but high endoscopic expertise is needed159,160. The decision to intervene should not be based on the Spigelman stage of the rest of the duodenum, as it cannot predict papillary cancer100. Endoscopic features suggestive for invasive carcinoma include infiltrative border, ulceration and a hard consistency159,160. Such endoscopic findings should raise the suspicion of invasive carcinoma, which should be treated by radical surgery or systemic therapy.
Both pancreas-sparing duodenectomy and pancreato-duodenectomy have similar performance metrics in terms of safety and efficacy, including 10-year overall and disease-specific survival, 30-day mortality rate, morbidity rate and length of hospital stay146,152,153,158. However, there are practical reasons to prefer pancreato-duodenectomy over pancreas-sparing duodenectomy. First, most centres have greater experience with pancreato-duodenectomy161. Second, the allegedly lower incidence of diabetes and pancreatic exocrine insufficiency is not supported by data152,158. Third, there appears to be a higher risk of jejunal polyposis after pancreas-sparing duodenectomy125,142,155. Finally, a high percentage of patients have an unsuspected carcinoma based on preoperative assessment (8–37%)139,141,142,146,158 and if an unsuspected cancer is diagnosed after surgery, pancreas-sparing duodenectomy was not oncologically appropriate.
Management of gastric findings
Fundic gland polyps are common in patients with FAP (26–88%)162–167, even in children168 (Figures 5, 6). Limited evidence suggests that there may be a higher risk of gastric cancer in FAP patients with fundic gland polyposis123,163–165, with 50% of these polyps showing dysplasia or a second-hit APC gene mutation123,162,169,170. One surveillance protocol suggests endoscopic removal of multiple fundic gland polyps using a cold snare technique for larger pathology samples171. However, there is insufficient evidence to recommend different treatment modalities for fundic gland polyps in FAP patients compared to the general population172.
Gastric adenomas are becoming increasingly clinically significant in FAP, as reflected in the rising incidence of gastric adenocarcinoma in FAP123,163,173. However, gastric cancers often arise within a background of carpeting fundic gland polyposis123,163,166,167,173–175. Removal of suspicious gastric adenomas is crucial to prevent progression to adenocarcinoma174. Optical diagnosis of gastric adenoma is preferred, and routine biopsies are avoided to minimize the risk of fibrosis complicating definitive endoscopic resection. Studies suggest that polyps <5 mm are unlikely to exhibit HGD, making size a presumptive indicator of HGD risk124,166,173,176.
Management of small intestinal findings including post upper gastrointestinal surgery
After prophylactic duodenal surgery, adenomas can develop both in the duodenal bulb and in the remaining jejunum125,155,177 after a median of 55 months from surgery (range 22–84 months)125,177. Such polyps tend to be small and adenomatous126, but they may rarely develop into carcinoma (2.4%)177.
There is conflicting evidence on the extent and severity of jejuno-ileal polyposis in FAP106,117,126,137,178–183. Jejuno-ileal polyps are usually small (<5 mm), adenomatous and located in the proximal jejunum106,126,178–181. The presence and severity of duodenal adenomas generally predicts the presence and severity of small bowel polyps (P = 0.001)106,117,126,178,181,182. Capsule endoscopy is superior to MRI and barium studies in the identification of jejuno-ileal polyps106,137,178,180,181,183. There is no comparative study of video capsule endoscopy versus single-/double-balloon enteroscopy126,179. After colorectal surgery, capsule endoscopy is safe, provided that patients do not experience obstructive symptoms125,177. Nevertheless, a patency capsule may be considered as a safe precaution. There are not enough data to report on the efficacy and safety of capsule endoscopy after pancreato-duodenectomy.
Section III: desmoid tumours
Diagnosis and screening
The natural clinical course of desmoid tumours (DTs) can vary, including stable course, rapid progression, cycles of regression and growth and spontaneous regression184. This variability complicates the use of classifications for treatment decisions. To date, there are no studies that establish the utility of screening for DTs in patients with FAP. However, our panel of experts suggests it may be useful in patients who have already undergone abdominal surgery and require further interventions. When a DT is diagnosed, it is crucial to exclude FAP as the predisposing condition, because the prevalence among FAP patients is hugely increased compared to the general population185–188.
Treatment
Surgical management is often recommended for extra-abdominal DTs189, whereas non-surgical treatments are preferred for mesenteric DTs due to the high morbidity and recurrence rates associated with extensive intestinal resection. The clinical presentation, including complications arising from a DT, guides the need for treatment that is primarily focused on symptom management (see Fig. 7)190–192. Even in these scenarios, the most effective yet least invasive treatment should be considered the strategy of choice190,193,194. Surgical resection of mesenteric DTs could be considered for those resistant to medical strategies or radiation therapy or if they are rapidly growing or life-threatening195–199. When surgery is performed, efforts should be made to preserve function. The optimal treatment for DTs remains uncertain, and there are no RCTs comparing different treatments. Nevertheless, primary surgery can be considered for well-defined tumours of the abdominal wall that can be safely resected189,199–204. Diagnostic biopsies for suspected DT in patients with FAP are not routinely recommended, except when there is a differential diagnosis with carcinoma or adenopathy that cannot be solved through imaging198.
Fig. 7.
Management algorithm for intra-abdominal desmoids with air-fluid level; proposed by Bonvalot 190
Management for desmoid tumours identified during abdominal surgery
The management strategy for unexpected DTs (or precursor lesions, see Fig. 8) discovered during surgery for other indications is to proceed with the surgery, if feasible, even with technical modifications, while avoiding resection of mesenteric desmoids. On the other hand, the presence of a known DT may influence the decision regarding a proctocolectomy with ileal–anal pouch anastomosis205.
Fig. 8.
Desmoid precursor lesion
Section IV: other extra-colonic manifestations
Thyroid
The reported prevalence of thyroid cancer in FAP patients ranges from 1.5%206 to 12%207. In a review, the pooled prevalence of thyroid cancers was estimated to be 2.6% (95% c.i. 1.3–4.8)208. However, the authors noted that studies implementing a screening ultrasound programme and those published after 2002 reported a higher prevalence of thyroid cancer208. Several factors contribute to the increased lifetime risk of thyroid cancer among FAP patients: the improved life expectancy resulting from prophylactic proctocolectomy209, enhanced attention and screening for thyroid diseases among FAP patients208 and the overall rise in thyroid cancer incidence in the general population210. In conclusion, it can be inferred that FAP patients have a higher risk of developing thyroid cancer compared to the general population. However, currently, there is insufficient solid evidence supporting the effectiveness of a dedicated screening programme. In the light of this, the authors propose an approach that focuses on patient education. It is crucial to ensure that patients are aware of their increased risk of developing thyroid cancer compared to the general population. Furthermore, incorporating regular physical examinations, which incur minimal costs, into the surveillance routine for FAP patients is recommended. The age at which thyroid cancer is diagnosed in FAP patients varies across different studies, ranging from 26 to 42 years, depending on the specific population under examination206,211–215. Young age and female sex are recognized risk factors for developing papillary thyroid cancer211. Young patients with a negative thyroid ultrasound are unlikely to develop thyroid cancer within the next 4–5 years215. Therefore, it seems reasonable to reserve ultrasound investigations for specific patients and adjust the follow-up interval based on patient self-examination, symptoms and the results of the baseline screening. The cribriform-morulae variant (CMV) of papillary thyroid carcinoma (PTC) is a rare subtype that is associated with FAP. CMV accounts for approximately 0.16% of all PTC cases216, but its prevalence is higher in FAP patients, reaching up to 90% of PTC cases in some reports217. Therefore, the possible diagnosis of FAP (through genetic testing or colonoscopy) should be investigated in females <35 years old who have been diagnosed with CMV-PTC, particularly when multifocal tumours are present218,219.
Adrenal gland
The reported proportion of FAP patients with adrenal incidentalomas is 2–3 times higher than in the general population220. However, the development of adrenal gland cancer or pheochromocytomas in FAP patients appears to be rare221–225. Based on these findings, no surveillance strategy for adrenal cancer in FAP patients was recommended. Kallenberg et al. identified a prevalence of adrenal adenomas as high as 26% in unselected FAP patients226. There is no known association between the development of adrenal incidentalomas and factors such as gender, genotype or family history222. Incidentalomas in FAP patients require investigation when suspicious characteristics are present. According to European Society of Endocrinology (ESE)–European Network for the Study of Adrenal Tumours (ENSAT) guidelines, an adrenal mass is considered benign if appears homogeneous and lipid-rich, with a density ≤10 Hounsfield units (HU) at an unenhanced CT scan227. Reimondo et al. suggest no further imaging in patients >60 years of age with benign features228. If any suspicious findings are detected, it is essential to refer the patient to a specialist centre for appropriate management.
Liver
In a recent review, the rate of hepatoblastoma in children with FAP was reported to be approximately 2.5%229. The median age at diagnosis of hepatoblastoma has been reported to be 18 months in different studies38,207. Some studies indicate a relative risk of 847.0 and an absolute lifetime risk of 1.6% in FAP patients230. Some studies suggest that screening should strongly be considered229,231. The use of both sonography and α-fetoprotein measurement can help distinguish hepatoblastoma from other liver pathologies. However, it is important to note that frequent surveillance may be stressful, especially for the parents, and no risk factors have been identified so far to determine which children might benefit most from a screening programme. However, early identification may save a child’s life.
Eyes
Congenital hypertrophy of the retinal pigment epithelium (CHRPE) can be the earliest and most common extra-intestinal manifestation of FAP. The apparent prevalence of CHRPE varies based on the studies and populations analysed, but it is generally estimated to be around 80%232–236. CHRPE alone cannot be relied upon as a sole indicator for diagnosing FAP, even in individuals with a positive family history233. Instead, genetic testing is recommended in the event of coincidental observation. CHRPE is not suitable as a diagnostic tool to prove FAP. Currently, type B lesions, characterized as small round pigmented dots, appear to be the most frequently observed type of CHRPE associated with FAP. Other significant features include the presence of three or more lesions and bilateral lesions.
Bones
Due to the high incidence of osteomas in FAP patients38,237 and especially oral osteoma237 compared to the general population, genetic testing should be considered in those with oral or facial osteomas237,238.
Gynaecological manifestations
Incidence
There is a lack of evidence about gynaecological cancer incidence in women with FAP. No prospective studies were identified. Several studies have not detected a significant increase in germline pathogenic variant of the APC gene in endometrial cancer239–242. There is no literature on the risk factors for developing gynaecological cancer in FAP carriers. However, the risk factors for gynaecological cancers in non-FAP carriers are well established. There is no biological reason to hypothesize that the known risk factors for gynaecological cancers in the general population are not equally important in FAP carriers.
Colorectal cancer metastasis to ovary
FAP seems to be associated with CRC metastasis to the ovary. Crobach et al. found that 13% of women (4/30) with FAP-associated CRC had ovarian metastasis243. This is higher than expected and may suggest that the biology of FAP-related CRC predisposes to ovarian spread. However, this study only suggests an increased incidence of ovarian metastasis in FAP carriers and not an increased incidence of ovarian cancer.
Benefit of oestrogen therapy for familial adenomatous polyposis patients
Clinicians caring for women with FAP should be aware of the theoretical benefit of the use of oestrogen-based contraceptive. Systematic review and meta-analysis level data demonstrate a pooled relative risk reduction of 18% for CRC in women with a history of combined oral contraceptive use244. Oestrogen seems to be protective against CRC in FAP carriers245. Indeed, total polyp regression has been reported on the commencement of an oral contraceptive246. Therefore, female FAP carriers should be counselled as to the benefit of oestrogen-based contraception as a means to reduce their CRC risk.
Fertility implications
There is no evidence that being a carrier of FAP decreases rates of conception or live births247. Fertility in FAP carriers can be affected by the treatments they undergo. Women often undergo pelvic surgery during reproductive age. Additionally, those diagnosed with cancer may require systemic chemotherapy or pelvic radiotherapy, which can have a negative impact on fertility248. Therefore, before undergoing these interventions, patients should be fully informed of the potential impact on fertility and, when appropriate, be referred to a fertility expert. Women who have been diagnosed with CRC should be made aware of the increased rate of ovarian metastasis seen in FAP, which could influence their choice regarding ovarian preservation243. Women with FAP are often offered risk-reducing surgery such as total proctocolectomy (TPC) with IPAA or total colectomy with IRA. There is no strong evidence suggesting an impact on fertility after surgery (see LGI.8). The mechanism of infertility resulting from risk-reducing surgery is not clear; it has been suggested that it may be due to tubal occlusion63. This is significant because tubal disease can potentially be addressed through assisted reproductive techniques249. Therefore, women who have undergone risk-reducing surgery for FAP and have not conceived after 1 year of regular unprotected penetrative vaginal intercourse should be referred to a fertility specialist.
Section V: chemoprevention
Most chemoprevention (CP) trials in FAP patients have focused on polyp-related outcomes or measured the time to disease progression, as these outcomes can be evaluated over relatively short periods of time. Only a few studies have investigated the effects of CP on cancer development, especially CRC, due to the early surgical removal of the colon. In a study by Burke et al.250, the efficacy and safety of eflornithine and sulindac in combination versus each drug alone were evaluated for the prevention of disease progression in FAP patients. The results showed no significant difference in the proportion of patients experiencing disease progression among the treatment groups overall (32% in the combination group, 38% in the sulindac group and 40% in the eflornithine group). Furthermore, there was no significant difference in the mean times to the first event of disease progression between the combination therapy and monotherapy groups, as estimated by the Kaplan–Meier method at intention-to-treat analysis. The Children’s International Polyposis study evaluated the efficacy and safety of celecoxib (200–400 mg twice daily, depending on body weight) compared to placebo in children with FAP over a 5-year treatment period251. The number of patients meeting the primary outcome of disease progression was twice as high in the placebo arm compared to the celecoxib arm. However, it is important to note that the long-term impact of celecoxib on colorectal polyposis in children could not be evaluated due to the early termination of the trial. The trial was stopped prematurely because of the low occurrence of disease progression.
There have been a limited number of RCTs investigating the use of single drugs or combinations of drugs to achieve risk reduction in the small bowel250,252–254. However, the observation periods in these trials have been relatively short, and the primary or secondary endpoints have typically focused on polyp reduction in the duodenum as a surrogate marker for cancer risk. Consequently, there is insufficient evidence to support the recommendation of chemoprevention for the prevention of small bowel carcinoma in patients with FAP.
Because in FAP the sequence of adenoma–carcinoma is not accelerated but anticipated, reduction in the number and size of colorectal polyps has been a common outcome in CP clinical trials. Aspirin has been widely suggested as a chemopreventive agent against CRC. Unfortunately, large RCTs in FAP are lacking, and the few available studies have yielded contradictory results. In the largest trial, the CAPP-1 study, no difference was found between the aspirin group and the aspirin plus resistant starch group or the placebo group. However, two studies by Ishikawa et al., although limited by small sample size and adverse events (such as anaemia and aphthous and anastomotic ulcers), showed a reduction in the number and size of colorectal polyps in the aspirin group and a reduction in the recurrence of polyps larger than 5 mm255–257.
CP could provide an approach to reducing adenoma development and cancer risk, potentially delaying or avoiding the need for surgery. In an RCT testing the COX-2 inhibitor celecoxib at two different doses (100 mg or 400 mg twice daily) versus placebo for 6 months, the high dose showed a significant improvement in duodenal disease (P = 0.033)258. However, quantitative analysis comparing percentage change in areas of low- and high-density polyposis with placebo did not reach statistical significance. Another RCT combined celecoxib (400 mg BD) with ursodeoxycholic acid (UDCA, 1–2 g daily) and assessed the change in duodenal polyp density after 6 months as the primary outcome252. The control group receiving celecoxib/placebo showed a significant decrease in polyp density (P = 0.029). However, it is important to note that celecoxib, along with other selective COX-2 inhibitors, is associated with an increased risk of serious cardiovascular side effects. The European Medicines Agency’s Committee for Medicinal Products for Human Use concluded in 2011 that the benefit of celecoxib in FAP patients had not been sufficiently demonstrated and did not outweigh the increased risk of cardiovascular and GI side effects259. Additionally, celecoxib is no longer approved by the US FDA for polyp reduction in FAP patients260.
MUTYH-associated polyposis
Section I—lower gastrointestinal manifestations
MAP patients—that is, patients with biallelic MUTYH PVs—present with colorectal adenomatous polyposis, exhibiting considerable phenotypic variability. The majority of MAP patients present with <100 adenomas (77.4%)261. MAP patients are estimated to have a lifetime risk of CRC of approximately 50.5%, with an average age at CRC diagnosis of 47.85 years21,261–263. The earliest reported case of CRC in MAP patients occurred at the age of 22261. However, it should be noted that CRC can develop without an overt polyposis phenotype, underscoring the importance of lower GI surveillance. In two studies conducted by Nielsen et al.41,264, the mean ages at colorectal polyposis diagnosis were 45 and 47 years respectively, with age ranges of 12–68 and 30–70 years. Similarly, Aretz et al.265 reported a mean age of 45 years at colorectal polyposis diagnosis, with a range of 24–72 years. Based on these findings, it appears reasonable to commence surveillance at 18 years of age to include patients with more aggressive polyposis phenotypes. For most MAP individuals, the number of colonic polyps remains limited and periodic colonoscopic polypectomy is sufficient for CRC prevention. Consequently, colonoscopy is employed for the surveillance and prevention of CRC. The study by Nieuwenhuis et al. provided evidence for accelerated carcinogenesis, justifying the need for frequent screening every 1–2 years19. Endoscopic surveillance can be tailored according to the patient‘s phenotype. In patients with oligopolyposis or a mild, attenuated phenotype, CRC prevention might be achieved by 1–2-yearly surveillance colonoscopy with polypectomy. However, many patients will require surgery, particularly as around 50% present with a CRC.
The occurrence of CRC in MAP patients is mostly observed in the proximal colon (52%) or rectum (26%), but cancers may also appear in the distal colon (14%)264,266,267. According to two studies, the risk of metachronous CRC in MAP patients is significantly reduced by performing total colectomy with ileo-rectal anastomosis, with rectal preservation if this is feasible, based on the phenotype262,268.
Comparatively, the risk of metachronous CRC appears to be lower in patients treated with colectomy rather than segmental colon resection262. Considering these findings, annual colonoscopy would seem appropriate if colonoscopy surveillance is pursued. However, surgery may be a more suitable management strategy, taking into account factors such as age, co-morbidity, polyp burden, and expected functional outcome. Data from the St Mark’s Hospital Polyposis Registry revealed that among 108 MAP patients who underwent surgery as the primary management approach, 35 underwent segmental resection (34 for cancer and one for HGD). None of these patients had been diagnosed with MAP prior to surgery. Among these patients, 30 (86%) received postoperative surveillance of the remaining colon/rectum, but 5 (17%) developed another CRC while under surveillance. The remaining five patients who did not undergo postoperative surveillance developed another CRC (100%). Among the 47 patients who had a total colectomy, 2 (4%) developed metachronous cancer in the residual rectum. None of these patients had received postoperative surveillance of the rectum261. These data support the continuation of colorectal surveillance even after surgery.
Section II: upper gastrointestinal manifestations
The systematic review of the literature identified six studies that indicate that MAP patients may develop upper GI malignancies and premalignant neoplasms22,263,265,269–271. The highest risks correspond to duodenal adenomas and cancer22. The incidence and lifetime risk of duodenal cancer in MAP are unknown. Although estimated at around 4%22, the very small number of observations and lack of prospective data make this estimate unreliable. Duodenal polyposis occurs less frequently in MAP than FAP, affecting 20–35% of patients263,269 compared to 65–90% in FAP cohorts272. MAP patients may also develop duodenal cancer, although data are scarce. In particular, three studies reported eight MAP patients affected with duodenal cancer. The estimated average risk of duodenal cancer in MAP is 1.5% (range 1–2.17%). Statistically significant differences in the prevalence of duodenal polyposis depending on genotype were reported by Thomas et al.263, with higher risks for Y179C homozygous patients263.
Confirmatory studies with prospective follow-up data are required before genotype is considered in relation to stratification of surveillance. The efficacy of surveillance to prevent upper GI malignancies in MAP is still unclear. To date, four cases of gastric cancer and five cases of gastric adenomas have been reported in MAP patients22. A retrospective study that assessed the presence of extra-colonic manifestations in 150 MAP individuals who underwent oesophago-gastroduodenoscopy (OGD) identified gastric lesions in 17 (11%) patients22. In four of them (24%), gastric adenomas were described, and nine patients had fundic gland polyps only. Gastric cancer was observed three times; however, the incidence was not significantly increased compared to the general population (standardized incidence ratio (SIR): 4.2; 95% c.i.: 0.9–12).
The age at duodenal polyp diagnosis in the two largest studies reported to date263,269 ranged from 32 to 81 years. However, the actual age of onset of duodenal polyposis is difficult to estimate because the age at first OGD, when duodenal polyps are identified in most patients, depends on the age at MAP diagnosis. Thomas et al. reported that 14.5% of MAP patients have duodenal adenomas at their first endoscopy, and it has been estimated that the prevalence of duodenal adenomas reaches 18.2% (8/44) by the age of 40 years and 38.5% (15/39) by the age of 70 years. Moreover, 37.8% of adenomas (14/37) progressed to a higher Spigelman stage during follow-up263. A total of eight duodenal cancers have been reported in MAP263. Although the mean age at duodenal cancer diagnosis was 66.2 years, the youngest was diagnosed at age 47263. Previous guidelines recommended that the interval between upper GI endoscopies in MAP should be based on the Spigelman stage, as for FAP36,273,274. However, recent reports indicate that Spigelman stage is not a reliable predictor of cancer risk in MAP, because it fails to identify patients at risk of duodenal cancer263,269. The reported duodenal cancers developed without a recognized background of benign duodenal polyposis263. Based on this evidence, in the current guidelines, we do not recommend the use of Spigelman staging to determine the upper GI surveillance interval in MAP. In the largest study of duodenal adenomas and cancer reported to date263, three of four reported duodenal cancers were diagnosed within 12 months of a previous OGD, suggesting missed lesions and highlighting the need for high-quality endoscopy. The fact that HGD has been reported in subcentimetre MAP duodenal adenomas263, together with the differences in MAP and FAP-associated tumorigenesis (DNA repair defect versus signalling activation), which has also been identified in duodenal adenomas275, suggests biological differences between MAP and FAP duodenal adenomas that could mediate differences in natural history. Based on the available evidence, we recommend polypectomy regardless of polyp size or Spiegelman staging.
Section III: extra-gastrointestinal manifestations
The evidence of an increased risk for extra-GI cancers in biallelic MUTYH-mutation carriers is weak, and in most cases, controversial. Vogt et al.22 analysed 276 MUTYH patients from 181 unrelated families, and observed that 35 (13%) had at least one malignant extra-GI lesion. In MAP patients, the risk of developing extra-intestinal malignancies is nearly double that in the general population (SIR: 1.9; 95% c.i.: 1.4–2.5). The increased risk is particularly relevant for ovarian, bladder and skin cancers (SIR: 5.7, 7.2 and 2.8 respectively)22. The cumulative lifetime risk of developing extra-GI malignancies in MAP might reach 38%, with a median age of 51–6122. Other extra-GI features observed in MAP individuals resemble those of the FAP spectrum, including osteomas and CHRPE, but at a significantly lower rate21,276,277. Although more studies are needed, MAP patients might be at higher risk of developing lung, haematological, brain and skin cancers278–280. There may be some phenotypic overlap with Lynch syndrome, indicated by an increased risk of endometrial cancer, but the evidence is not conclusive thus far281. Unlike FAP, desmoids do not appear to belong to the spectrum of manifestations of MAP281. Despite the apparently increased cancer risks, there is no evidence of a cost-effectiveness or prognostic benefit from extra-intestinal screening in MAP patients.
Other rare adenomatous polyposis syndromes
The use of multi-gene panel testing (MGPT) has become standard in genetic diagnostics. This approach may use physical MGPT or virtual panels based on whole-exome or genome sequencing data. MGPT in patients with GI polyposis should include APC and MUTYH, which explain most identifiable inheritable forms of polyposis, as well as other genes relevant for adenomatous polyposis (MMR genes MLH1, MSH2, MSH6, PMS2, MSH3 and MLH3), POLE (exonuclease domain), POLD1 (exonuclease domain), NTHL1, MBD4 and AXIN2. In addition, it is recommended to include genes causing other polyposis syndromes such as STK11, BMPR1A, SMAD4, PTEN and RNF43, due to the phenotypic overlap (Table 11)24,26,27,31,32,282,283. Mosaic APC variants can be found in about 20–50% of the remaining unexplained polyposis cases11,265,284. When testing every patient referred to oncogenetic counselling (regardless of indication), the detection of MUTYH biallelic mutations is only about 0.2% (82/44 800)21. The total percentage of detected heritable polyposis syndromes will therefore likely not exceed 0.5% in a general oncological cohort21. Therefore, testing for polyposis genes in all patients undergoing germline oncogenetic testing should only be done as part of a broad gene panel. The observed decline in the mutation detection rate in patients receiving genetic testing for multiple colorectal polyps over time (due to more sensitive colonoscopies) and the very low frequency of patients with PVs in genes other than APC and MUTYH, especially those with 10–20 polyps and those above 60 years of age, suggest that the mutation detection rate in this group is likely <2–3%.
Table 11.
Extra-gastrointestinal manifestations in other rare adenomatous polyposis syndromes
| Genes | Extra-GI Manifestations/Cancer |
|---|---|
|
MLH1, MSH2, MSH6, PMS2 [Autosomal recessive] Constitutional MMR deficiency (CMMRD) |
Tumours of the CNS |
| Haematological malignancies | |
| Urinary tract cancer | |
| Breast cancer | |
| Endometrial cancer | |
| Ovarian cancer | |
| Embryonal and germ cell tumours | |
| Sarcomas | |
| Other cancers | |
| Café-au-lait macules (CALMs) and other skin manifestations | |
| Venous malformations | |
|
POLE, POLD1 [Autosomal dominant] Polymerase proofreading-associated polyposis (PPAP) |
Endometrial cancer |
| Breast cancer | |
| Ovarian cancer | |
| Tumours of the CNS | |
| Other cancers | |
|
NTHL1 [Autosomal recessive] NTHL1 tumour syndrome (NTS) |
Breast cancer |
| Endometrial cancer | |
| Tumours of the CNS | |
| Haematological malignancies | |
| Cancers of the urinary tract | |
| Head and neck cancers | |
| Skin cancer | |
| Other cancers | |
|
MBD4 [Autosomal recessive] MBD4-associated neoplasia syndrome (MANS) |
Acute myeloid leukaemia/myelodysplastic syndrome |
| Uveal melanoma | |
| Schwannomas | |
| MSH3 [Autosomal recessive] | Scarce data |
| MLH3 [Autosomal recessive] | Scarce data |
| AXIN2 [Autosomal dominant] | Ectodermal dysplasia |
| Oligodontia |
Including colorectal cancer diagnosed before 50 years of age in criteria for testing polyposis genes
Using stringent criteria based on polyp count will inevitably lead to some patients with a heritable form of polyposis being missed. Sutcliffe et al.21 showed that if only patients with >10–20 adenomatous polyps are tested, 10% of MUTYH biallelic patients will be missed. This was also shown by the study of Landon et al.285. Both studies suggest that including patients with <10 polyps but with CRC under age 50 will increase the detection rate of MAP and other clinically actionable hereditary CRC syndromes. Terlouw et al.286 reported that testing patients with adenomas above the age of 70 lead to a detection rate of PVs of about 1%. In considering patients older than 70 years, no MUTYH or APC variants were identified in patients with <20 adenomas (n = 82) and only one case of MAP was found among patients with >20 adenomas (1/90, 1.1%).
APC mosaic
APC mosaicism has been reported to be present in 25–50% of unexplained patients with >20 adenomas284. In most of these cases, the mosaicism was undetectable in leucocyte-derived DNA and required testing of DNA isolated from >2 adenomas. Tumour testing is still logistically challenging and is not performed in most diagnostic laboratories.
Concluding remarks
Testing individuals with >20 adenomas (aged below 70 years) seems widely accepted and is included in most previous guidelines. Testing individuals over age 70 or with <20 adenomas is indicated when additional features suggestive of a hereditary polyposis syndrome are present286,287. However, not all guidelines propose these age limits. Other indications (besides the presence of adenomatous polyps) for polyposis panel analysis are FAP-related extra-colonic manifestations, CRC aged < 50288, a somatic KRAS c.34G>T transversion, or a first-degree relative (FDR) with >10 adenomas286.
Whether testing for PVs identified in the index case should also be offered to FDRs depends on the mode of inheritance. For rare non-APC dominantly inherited syndromes (such as PPAP), testing should be offered to all FDRs with cascade testing. For recessively inherited syndromes (such as MUTYH- and NTHL1-associated polyposis), screening should be offered to siblings of index cases. Testing of offspring of index cases can be considered when PV allele frequencies in the relevant population are high—as for MUTYH in many geographical regions, the probability of inheriting two MUTYH pathogenic variants is around 1%. Other known recessive syndromes, such as NTHL1 and MSH3, have lower carrier rates (around 1 in 300)24,31; therefore, the risk of inheriting two pathogenic variants becomes very low (<1/600). If parents are related, the chances are obviously higher and testing should be considered. The French guidelines274 also advise complete MUTYH analysis in the unaffected partner of an index case as a possible strategy, particularly where there are a large number of offspring at risk289.
Germline variant interpretation and classification
The interpretation and classification of germline variants into five classes (pathogenic, likely pathogenic, unclear, likely benign, benign) should follow a standardized procedure, based on the American College of Medical Genetics and Genomics (ACMG)/Association for Molecular Pathology (AMP) guidelines for variant classification. For APC, gene-specific ACMG/AMP rules were developed recently by the subcommittee of InSiGHT/ClinGen Variant Curation Expert Panel (VCEP), which should replace the generic framework. In the near future, gene-specific ACMG/AMP modifications will be available for further actionable genes, which can be found on the ClinGen websites.
Currently, there are insufficient data to establish the appropriate age to initiate GI surveillance for each of the aforementioned genes. However, it is advisable to follow the guidelines provided for MAP, which recommend starting screening at 18 years of age. Additionally, consideration may be given to initiating screening 5 years earlier in patients with a highly aggressive familial phenotype. This is particularly relevant for heterozygous carriers of specific POLE or POLD1 variants associated with severe and early-onset phenotypes that present with a congenital mismatch repair deficiency (CMMRD)-like phenotype in childhood or adolescence290–292. In addition to the GI phenotypes, most of the rare adenomatous polyposis syndromes are associated with an increased risk of extra-GI tumours and other phenotypic manifestations (Table 10).
Table 10.
Short version: statements pertaining to other rare adenomatous polyposis syndrome
| Statements | Level of evidence and agreement |
|---|---|
| OAPS.1A. Germline multigene panel testing should be considered in patients with >20 cumulative colorectal adenomas. | LE: low Agreement: 97% (SA 60%; A 37%; N 3%) |
OAPS.1B. The threshold may be lowered to 10 cumulative adenomas if:
|
LE: low Agreement: 93% (SA 73%; A 20%; D 7%) |
| OAPS.1C. Germline multigene panel testing (for CRC and polyposis syndromes) should be undertaken in patients with gastrointestinal cancers presenting under the age of 50 years. | LE: low Agreement: 94% (SA 33%; A 61%; D 6%) |
| OAPS.1D. Somatic testing for APC mosaic mutations should be considered in unexplained polyposis patients fulfilling criteria from statements A and B. | LE: low Agreement: 94% (SA 38%; A 56%; D 6%) |
OAPS.2A. In the case of autosomal recessive hereditary polyposis syndromes, testing should always be offered to siblings. Children should be tested when:
|
LE: low Agreement: 90% (SA 57%; A 33%; N 10%) |
| OAPS.2B. In autosomal dominant polyposis syndromes, testing should be offered to all first-degree relatives followed by cascade testing. | LE: low Agreement: 97% (SA 72%; A 23%; N 3%) |
A, agree; CMMRD, congenital mismatch repair deficiency; CRC, colorectal cancer; D, disagree; LE, level of evidence; MMR, mismatch repair; N, neutral; SA, strongly agree; SD, strongly disagree.
Gastric adenocarcinoma and proximal polyposis of the stomach
GAPPS is an autosomal dominant hereditary gastric cancer syndrome with incomplete penetrance first identified in 2012. It is caused by germline point mutations at the promoter 1B of the APC gene14,293–302. GAPPS is distinct from a-FAP and presents with gastric cancer and extensive fundic gland polyposis sparing the antrum and lesser curvature, with limited evidence on the risk of CRC303–306. Before considering a diagnosis of GAPPS, clinicians should exclude the use of proton pump inhibitors and the presence of polyposis elsewhere in the GI tract307. Genetic testing can confirm the diagnosis, but not all gene panels include the 1B promoter294,295; therefore, clinicians should consult with their laboratory to ensure appropriate testing. The essential clinical criteria for GAPPS and for genetic testing are summarized in Table 13.
Table 13.
Clinical criteria for genetic testing
| Essential clinical criteria (both) | Supportive clinical criteria (any) |
|---|---|
| 1. Phenotypic features | A. Spectrum of other histological lesions: |
|
|
|
|
|
|
|
|
| 2. Proband or family member with either dysplastic fundic gland polyps or gastric adenocarcinoma | B. Family history (autosomal dominant pattern of inheritance) |
| 3. Mutation in the chr5:112043220_112043224 region of promoter 1B of the APC gene† |
*Exclusions include other heritable gastric polyposis syndromes and use of proton-pump inhibitors; in patients on proton-pump inhibitors, it is recommended to repeat the endoscopy off therapy. †The point mutations that segregate with GAPPS (c.-191T>C, c.-192A>G and c.-195A>C) are all positioned within the YY1 binding motif of the APC gene and confirm the diagnosis of GAPPS294,306
GAPPS is characterized by various microscopic features, including dysplastic fundic gland polyps, hyperproliferative aberrant pits (HPAP), hyperplastic polyps, gastric-type adenomas and adenocarcinomas. HPAP, unique to GAPPS, is considered the earliest marker of dysplasia300. Low-grade dysplasia can develop in gastric adenomas, gastric adenocarcinomas and multifocal ‘flat’ dysplasia in fundic gland polyposis301. Progression to dysplasia and cancer can occur rapidly14,293,295,298, even during endoscopic surveillance14,295.
The lifetime incidence of gastric adenocarcinoma ranges from 12% to 25%, with variable age of onset (22–75 years)14,293,295,298. Although there is no definitive age to begin gastric surveillance, FDRs of affected patients should be considered for endoscopy with biopsy sampling from the age of 15 years297,308. Gastroscopy should be of high quality, examining all gastric polyps and surrounding mucosa. Multiple biopsies should be taken from larger polyps and areas with vascular or structural irregularities to detect dysplasia or malignant transformation14,293,295,298,308. The interval of endoscopic surveillance should be individualized based on the limited available data and the heterogeneity of GAPPS patients308,309.
The risk of CRC in GAPPS is still inconclusive, as not all families develop CRC14,293,295,310. However, colonic polyps may be observed in both sexes as early as their third or fourth decade of life14,310. Somatic activating mutations in the β-catenin cascade are shared between gastric and colonic lesions, suggesting a common pathogenesis310. Although no specific studies on CRC surveillance exist, it may be prudent to consider colonoscopy surveillance from the age of 18 years to rule out colonic polyposis297. Repeat colonoscopies every 3 years may be considered if adenomas or serrated lesions are found308. Small bowel cancer has not been reported in GAPPS, but the presence of duodenal adenomas should raise suspicion of FAP rather than GAPPS308. Risk-reducing (prophylactic) total gastrectomy may be considered from the age of 30–35 years or 5 years before the youngest gastric cancer diagnosis in the family308. The timing of surgery may be individualized, taking into account patient preferences, while clearly explaining the risks of delay308. Women of child-bearing age should be reassured that prophylactic gastrectomy is compatible with successful childbirth and breastfeeding311. Both laparoscopic and robotic total gastrectomy may be appropriate309,312. Although the antrum is spared, there is no evidence for the long-term safety of proximal rather than total gastrectomy308. It is important to highlight that GAPPS is a relatively new entity and further research is required. Specifically, the potential risk of duodenal cancer cannot currently be excluded; therefore, gastric surgery should allow for subsequent prospective evaluation of the duodenum. Such surgical resections requiring specific reconstruction are more likely to be performed in very specialized centres.
Conclusion
These guidelines represent an updated and extensively revised version of the previous Mallorca group guidelines, originally published in 2008. The previous guidelines for FAP were primarily based on expert opinions derived from an extensive literature search. The objective of these updated guidelines is to provide current, comprehensive and evidence-based practical recommendations for the management of surveillance and treatment of FAP patients, encompassing additionally MAP, GAPPS and other recently identified Mendelian adenomatous polyposis syndromes.
To facilitate clear decision-making, well-defined flowcharts have been developed for both upper and lower GI tract surveillance and management. In the context of the upper GI tract, a critical analysis of the Spigelman classification has been conducted. For the lower GI tract, efforts have been made to establish more precise timing indications for surveillance and surgery. Although not all statements reached the predefined 80% agreement required to establish the high level of consensus, they have all been reported and, whenever possible, further information has been provided in the comments section to stimulate further discussion following each Delphi process. Some topics lack sufficient data for definitive recommendations, indicating the need for further investigation.
These guidelines also emphasize the importance of collaborative studies and international registries to facilitate updated evidence-based recommendations. Future research priorities for the European Hereditary Tumour Group (EHTG), in collaboration with other scientific societies, include assessing the actual impact of safe yet less-invasive colorectal strategies and exploring fertility and sexual health considerations for both males and females. Additionally, the re-evaluation of the true benefits of screening for extra-colonic manifestations such as thyroid cancer and the proposal of an alternative duodenal staging system to the Spigelman score will be addressed.
These guidelines highlight the roles of the discovery of recently identified pathogenic variants in genes other than APC and MUTYH that predispose to the development of adenomatous polyposis. Accurate monitoring and data collection are required to provide future recommendations regarding screening and surveillance in these newly described syndromes.
Recognizing the complexity of the polyposis syndromes, these guidelines aim to serve as a valuable tool for both patients and clinicians. Due to the rarity of these diseases and limited experience of them in many healthcare settings, it is crucial for patients to seek care at specialized centres with expertise in managing these conditions. The formulation of these guidelines involved a critical analysis of the most current available literature. In cases where data were lacking, expert opinions played a crucial role in formulating recommendations.
Extended version of the guidelines
The extended version of the guidelines aims to comment on each proposed statement with an extensive and comprehensive analysis of the available literature. Its purpose is to further elaborate on the comments previously provided in the short version of the guidelines (Tables 14–27).
Table 14.
Statements pertaining to the lower gastrointestinal tract—extended version
| Statements |
|---|
| Surveillance |
| LGI.1 At what age should surveillance commence in classical FAP? A: Surveillance should begin at 12 years of age in asymptomatic patients with a germline PV in the APC gene (for FAP disease), or in asymptomatic patients with first-degree relatives affected by classical FAP (if a genetic test is not available or if no PV is detected in the affected relative). B: In symptomatic patients with germline PV in the APC gene (for FAP disease), or patients with first-degree relatives affected by classical FAP if a genetic test is not available or if no PV is detected) colonoscopy should start at any age and as soon as possible. |
| LGI.2 At what age should surveillance commence in attenuated FAP? A: Surveillance can start later but no later than 18–20 years of age in asymptomatic patients with a germline PV in the APC gene for attenuated FAP disease and an attenuated proband/family phenotype. Alternatively, surveillance should also begin in asymptomatic patients with first-degree relatives affected by attenuated FAP, if a genetic test is not available or if no known pathogenic mutations are detected. B: Colonoscopy should start at any age and as soon as possible in symptomatic patients with a germline PV in the APC gene for attenuated FAP disease or in patients with first-degree relatives affected by attenuated FAP (if a genetic test is not available or if no known pathogenic mutations are detected). |
| LGI.3 What is the optimal modality for colorectal surveillance in classical FAP? The optimal modality for colorectal surveillance in classical FAP is high-definition white-light colonoscopy. Flexible sigmoidoscopy can be considered as an initial option, according to the patient’s preference. If adenomas are identified, then a high-definition white-light colonoscopy should be performed. |
| LGI.4 What is the optimal modality of colorectal surveillance in attenuated FAP? The optimal modality for colorectal surveillance in a-FAP is high-definition white-light colonoscopy. |
| LGI.5 What are the ideal intervals for endoscopic surveillance colonoscopy before prophylactic surgery in classical and attenuated FAP? A: Endoscopic surveillance of the colon should be adapted according to phenotype, genotype–phenotype, and the severity of the disease B: Repeat endoscopy should be performed within 1 year or less if at least one of the following criteria is present:
|
| LGI.6 Should screening colonoscopy routinely include advanced imaging technologies? A: White-light high-definition colonoscopy is sufficient for surveillance colonoscopy in FAP. B: There are insufficient data to recommend the use of advanced imaging technology. C: White-light endoscopy is sufficient in most cases; virtual or dye-based chromoendoscopy could have an advantage in discriminating between the clinical diagnosis of FAP versus a-FAP. |
| Surgery |
| LGI.7 When is prophylactic colorectal surgery indicated? Absolute indications for immediate colorectal surgery in FAP are:
|
| LGI.8 Which patient characteristics support restorative proctocolectomy over total or subtotal colectomy for prophylactic surgery? A: IPAA may be offered to patients with either:
|
| LGI.9 Does prophylactic surgery need to include mesocolic/mesorectal excision? There is no conclusive evidence indicating a clear advantage or disadvantage in performing dissection with mesocolic/mesorectal excision. |
| LGI.10 Should a diverting ileostomy be routinely performed in total proctocolectomy with ileal pouch anal anastomosis? Routine diverting ileostomy is not mandatory when total proctocolectomy with IPAA is performed. |
| LGI.11 Is subtotal colectomy superior to total colectomy? When the rectum can be preserved, an ileo-sigmoid anastomosis could be considered to diminish the risk of anastomotic leak and improve functional outcome. |
| Post-surgical management |
| LGI.12 What is the appropriate management for patients with ileo-rectal anastomosis? A: The optimal modality for surveillance after an IRA is endoscopy. The surveillance interval should not exceed 2 years, starting from the colectomy, and should be individualized based on phenotype. B: All polyps >5 mm should be removed (endoscopically or with transanal excision). C: Secondary proctectomy should be considered when polyposis is no longer conservatively manageable or in the presence of 2 or more polyps with HGD. |
| LGI.13 When and how frequently should the ileo-anal pouch be surveilled? A: Endoscopic surveillance of an ileo-anal pouch should start 12 months after colectomy. B: Endoscopic surveillance of an ileo-anal pouch should be performed annually. |
| LGI.14 In the case of pouch adenoma/multiple adenomas/polyposis, what is the recommended treatment? A: Pouch adenomas may be managed endoscopically. B: In the presence of HGD in/of complete polyp resection, the pouch should be surveilled within 6 months. C: In the presence of two or more polyps with HGD, surgery may be considered. |
| LGI.15 In the case of pouch carcinoma, is pouchectomy/dismantling of the pouch indicated? In the case of pouch carcinoma, pouchectomy/dismantling of the pouch is indicated. |
| LGI.16 Should pouchoscopy also include the ileum proximal to the pouch? Expanding endoscopy to the more proximal small bowel should be performed during pouchoscopy in FAP patients after total proctocolectomy with IPAA. |
a-FAP, attenuated familial adenomatous polyposis; FAP, familial adenomatous polyposis; HGD, high-grade dysplasia; HGIEN, high-grade intraepithelial neoplasia; IPAA, ileal pouch anal anastomosis; IRA, ileorectal anastomosis.
Table 27.
Statements pertaining to gastric adenocarcinoma and proximal polyposis of the stomach—extended version
| Statements |
|---|
| GAPPS.1 Regarding GAPPS, who should be offered genetic testing? Genetic testing should be offered to individuals with a clinical suspicion of GAPPS. |
| GAPPS.2 When should endoscopic surveillance of the upper-GI tract be performed in GAPPS syndrome? A: The age to start upper GI surveillance in asymptomatic individuals at risk of gastric cancer should be evaluated on a case-by-case basis. The youngest age of gastric cancer in the family should be considered. B: Surveillance endoscopic intervals for GAPPS families should be flexible and decided on a case-by-case basis. |
| GAPPS.3 Is surveillance of the colon and rectum indicated in GAPPS patients? In GAPPS patients CRC surveillance may be considered, particularly when there is a family history of CRC. |
| GAPPS.4 Which treatment modalities are available for GAPPS? A: GAPPS results in a high risk of gastric cancer. Total gastrectomy should be considered in cases of high-grade dysplasia and progressive gastric polyposis. B: There is not enough evidence to recommend an age for risk-reducing prophylactic gastrectomy: the decision should be individualized. |
CRC, colorectal cancer; GAPPS, gastric adenocarcinoma and proximal polyposis of the stomach; GI, gastrointestinal.
Familial adenomatous polyposis
Section I: lower gastrointestinal manifestations
LGI.1
It is now well-established, based on different guidelines, that children and adolescents predicted to develop FAP (patients with germline PV in the APC gene (for FAP) or patients with at least one FDR affected by classical FAP) should undergo surveillance (Table 14)36,37. Recent studies have not provided data on the true age distribution for the diagnosis of CRC. Older studies no longer reflect the current situation due to the impact of screening, adenoma removal, and prophylactic surgery. In a recent study by Kennedy et al., the median age of first adenoma detection was reported as 13.4 years38. They observed HGD in five patients (ages 13, 16, 17 and two at 20 years old) and invasive cancer in five patients (one at 19 years, two at 18 years and two at 17 years of age)38. Munk et al. reported a case of invasive cancer in an 8-year-old patient, but no information was provided regarding symptoms44. An overview of different European polyposis registries showed very low numbers of CRC cases in younger patients (0–10 years: none, 11–15 years: 2 patients, 16–20 years: 15 patients)36. The updated version of the guidelines still suggests starting the screening programme at the age of 12 in asymptomatic patients. Potential alarm symptoms, such as changes in bowel movements, rectal bleeding/anaemia, looser stool and mucous discharge, should indicate a colonoscopy in FAP patients44,45, as these might be indicators of more severe polyposis. Rectal bleeding, in particular, is a predictive factor for dysplasia44. In accordance with the previous guideline36 and in agreement with other international guidelines37, a colonoscopy should be performed in all symptomatic FAP patients, regardless of age.
LGI.2
As reported by Knudsen et al. in a review39, the age of adenoma onset in a-FAP patients is between 35 and 45 years, with a median age of a-FAP diagnosis at 43 years (ranging from 12 to 67). Although the benefits of surveillance are well established in these patients, there are no recent studies providing a definitive age of colonic cancer onset or the true incidence of CRC in this patient population. However, a recent survey established that the median age at the diagnosis of CRC in a-FAP patients was 55 years (ranging from 25 to 77), with 31% of patients being diagnosed before the age of 5040. These data were confirmed by Nielsen et al., who reported a median age of CRC diagnosis at 54 years41. Burt et al. established that there is a cumulative risk of 69% to develop CRC by the age of 80313. In previous decades, the prevalence of right-colonic adenomas was described39, but a recent study by Knudsen et al. suggested a more uniform distribution throughout the colon and rectum40. On the other hand, genotype–phenotype are not absolute, and patients with the same genotype mutation can exhibit different phenotypes and manifestations42. According to these considerations and the literature, regular surveillance can start later in patients affected by a-FAP with a proband/familial attenuated phenotype, but it is not safe to suggest starting surveillance later than at 18–20 years of age. The difficulty in achieving a high level of agreement arose due to two opposing views regarding the proposed age. According to some members, in line with the data presented above, the age to initiate the surveillance programme could be postponed at 18–20 years. On the other hand, according to other expert panel members, the age should be earlier, as there may exist an intrafamilial phenotypic discrepancy. Other guidelines do not differentiate between FAP and a-FAP, suggesting starting screening at the same age. Therefore, we believe that the decision should be made in agreement with the patient and their family. The possibility of postponing the start of screening compared to the usual age of 12 years for classical FAP should be strongly supported where there is a proven germline mutation of the APC gene associated with the attenuated form, along with a phenotype reflecting the attenuated form.
LGI.3
Nowadays, the role of colonoscopy surveillance among FAP patients is well established, demonstrating a reduction in the incidence of CRC and associated mortality46,47. The primary purpose of endoscopic surveillance in classical FAP is to detect neoplasia advancement early, providing the opportunity for prophylactic surgical management and reduction of cancer risk through the removal of adenomas314. However, without successful endoscopic management or surgical removal of bowel, the risk of CRC eventually reaches 100%. While chromoendoscopy has shown greater detection of diminutive adenomas compared to white-light endoscopy in FAP colonoscopy49, it is unlikely to alter the course of management or postpone surgery because most adenomas in FAP become large. There is no evidence supporting the routine use of chromoendoscopy in the surveillance of classical FAP. Recent studies have highlighted the detection of colonic polyps but their absence in the rectosigmoidal portion, ranging from 10% to approximately 35%44,48 in some patients, but no correlations based on genotype or familial phenotype were provided for these patients. In conclusion, a full colonoscopy is the preferred modality for surveying the colon, although sigmoidoscopy remains a valid initial option. Factors such as patients’ preference and the habits of the medical team, including routine sedation during the procedure, need to be taken into consideration.
LGI.4
As explained above (see LGI.2) there is variability in the distribution of colorectal adenomas39,40, leading to the conclusion that the best modality of colonic surveillance is a full colonoscopy. In the context of a-FAP, the usefulness of chromoendoscopy is still unclear (see LGI.5). In addition, other recent guidelines suggest performing high-definition white-light colonoscopy for surveillance in a-FAP315,316.
Please see Fig. 1 for a summary of endoscopic surveillance.
LGI.5
As prophylactic colorectal surgery remains the cornerstone of treatment for FAP and a-FAP patients, they should undergo regular colonoscopy surveillance to determine the appropriate timing of surgery and prevent the development of CRC. However, there is a lack of evidence regarding the ideal interval for colonic surveillance. Different guidelines offer varying suggestions, ranging from yearly intervals36 to tailored intervals based on phenotypes37. A recent study found a correlation between polyp progression and the polyp count at the initial colonoscopy, particularly if the count is ≥100, as well as a mutation in codon 130950. However, genotype alone is not sufficient to determine the timing of screening. The number of polyps at the first colonoscopy is also associated with a higher rate of polyp progression50. Furthermore, there is no evidence to support accelerated carcinogenesis in FAP patients. Considering these factors, we propose adjusting the interval of colonoscopy surveillance based on various factors, rather than relying on a fixed and inflexible period. The presence of symptoms should always prompt further investigation. Certain critical factors, such as the presence of a mutation in codon 1309 of the APC gene, a high number of polyps and the presence of large adenomas, may warrant a shorter surveillance interval.
LGI.6
In a single study comparing different forms of colonoscopic imaging techniques, 13 patients with FAP were evaluated using four different techniques: white-light colonoscopy, autofluorescence, narrow-band imaging and chromoendoscopy. Polyps were counted from pictures obtained at the same area and angle. Chromoendoscopy detected a significantly higher number of colon polyps (38.5–43.3) compared to white-light endoscopy (12.2–13.9, P ≥ 0.005). Chromoendoscopy was found to be superior to the other three techniques in detecting diminutive colorectal lesions49. Further studies are warranted to determine the role, if any, of chromoendoscopy and other advanced imaging techniques in this patient population. This conclusion is consistent with other guidelines287,317.
LGI.7
The gold standard therapy to prevent the development of CRC in FAP and a-FAP patients is the removal of the colon (and rectum). However, determining the appropriate timing of surgery remains a challenge for surgeons and clinicians. This section will primarily focus on indications based on clinical and screening findings, while acknowledging that other factors, particularly the patient‘s preference, will also influence the timing. Kobayashi et al. conducted a multicentre observational cohort study, compiling data from 303 patients who had colorectal surgery for FAP between 2000 and 2012 across 23 different institutions. Of these 303 surgical cases, 115 individuals (38%) were diagnosed with CRC. As expected, a significant correlation emerged between older age and various phenotypes. In the three distinct phenotypes—attenuated (<100 polyps), sparse (100–1000 polyps) and profuse polyposis (≥1000 polyps)—cancer was observed in 47.4%, 36.2% and 36.8% of patients, in that order. Patients with CRC had mean ages of 50, 39 and 34 years for these phenotypes respectively, whereas patients without CRC had mean ages of 33, 31 and 31 years. The study aimed to pinpoint the optimal age threshold for predicting CRC development in individuals with attenuated, sparse and profuse FAP, which were identified as 46, 31 and 27 years respectively51. Consequently, we propose that clinical management and the recommended timing for prophylactic surgery should be individually customized to match each phenotype. Newton et al.52 evaluated the correlation between genotype and phenotype, suggesting that patients with an APC mutation within codons 1250–1549 may benefit from earlier prophylactic surgery but should also have aggressive follow-up. It is also known that APC pathogenic variants within codons 1250–154952 or codons 1250–146453 are correlated with a severe phenotype, suggesting that endoscopic findings can guide the indication for surgery instead of relying solely on genotype. Some studies have correlated the presence of symptoms, particularly rectal bleeding44, with the risk of dysplasia, suggesting the need for prompt intervention. This is also in line with other consequences that may arise, such as anaemia, growth retardation and impacts on quality of life. Sarvepalli et al. showed that high polyp progression was the main indication for surgery in their cohort, highlighting the tendency of surgeons to base surgery decisions on the relative increase in polyp number rather than the absolute number50. Because establishing the correct timing for prophylactic surgery remains challenging, it is essential that FAP patients be referred to a dedicated centre to determine the appropriate timing. Some data suggest that a delay in performing prophylactic surgery could be acceptable54. However, it should be noted that this study, as suggested by the authors, included patients undergoing surgery for prophylactic purposes. They also found a higher rate of malignant polyps in the group with a higher median age at the time of surgery54. This finding reasonably suggests that surgeons and other clinicians should prioritize indication for prophylactic colectomy, allowing flexibility to schedule the surgery within the best window of time for patients. Prophylactic surgery can be planned at a time that is suitable for the patient, based on the risk of cancer as assessed by colonoscopy. The timing of surgery should consider social issues, family planning, emotional development of the patient in relation to age and the likelihood of attending planned surveillance. Sarvepalli et al.318 have developed a model based on measurable factors that can be used with patients and families to predict the likelihood of surgery within 2 and 5 years from the first colonoscopy. This can help patients and families with life planning. The model can be accessed at: http://app.calculoid.com/#/calculator/29638.
LGI.8
If finding the appropriate timing is complex in FAP and a-FAP patients, choosing the type of surgery that best suits the patient‘s needs while taking into account the associated potential risks and benefits becomes an even greater challenge for the surgeon. Therefore, it is imperative that patients seek advice from specialized centres where surgeons are equally proficient in all types of surgery. The two main options for prophylactic removal of the large intestine are colectomy with ileorectal anastomosis (IRA) and proctocolectomy with ileal pouch–anal anastomosis (IPAA). In the IRA procedure, the rectum is preserved, whereas in the IPAA, a pelvic dissection is performed to create a pouch that mimics a reservoir. Each proposed surgery has its own advantages and disadvantages. Dossa et al.319 attempted to assess surgeons’ preferences for prophylactic surgery in patients with familial adenomatous polyposis (regardless of the phenotype). Despite the varied choices, they concluded that the decision should be individualized based on the patient‘s future risk of cancer. However, when possible, colectomy with IRA was preferred for selected patients. Furthermore, high-volume surgeons attributed the lowest utility to a ‘poorly functioning ileoanal pouch’319. This emphasizes the importance of directing patients to specialized centres with experienced teams that excel in both surgical decision-making and procedures. In total colectomy with IRA, the rectum is preserved, but there is a real risk for patients to require secondary surgery after the initial prophylactic procedure. The main indications for secondary surgery are the development of rectal cancer, worsening adenoma burden or malignancy. A study has shown how the risk of secondary rectal excision after IRA can be predicted based on the APC mutation site. Patients with a severe genotype (codons 1250–1464) are good candidates for an IPAA, because they have the highest cumulative incidence of rectal cancer (8% in 15 years) and a secondary proctectomy rate of 74% at 20 years after rectal-sparing surgery53. Sinha et al. demonstrated that in a retrospective study of 427 patients with IRA, a rectal polyp count exceeding 20, APC mutation codon 1250–1450, colonic polyp count of 500 or more and age <25 years at the time of surgery are independent predictors of progressive rectal disease55. On the other hand, in a prospectively maintained database study of 191 patients with FAP, indications for rectal-sparing surgery were APC mutation outside the codon 1250–1450 or <500 total colonic polyps or <20 rectal polyps of less than 1 cm. In this cohort, none of the patients developed rectal cancer during the 5-year follow-up, suggesting that the preservation of the rectum can be feasible and safe if patients are well selected based on genotype and phenotype, even though the follow-up period was only 5 years56. This approach is further supported by another study, which assessed that the cumulative probability of rectal excision was 9.5% in patients with a-FAP (patients with mutations before codon 157, beyond codon 1595 and in the alternatively spliced site of exon 9—codons 312–412). The cumulative risk of rectal excision at 5 and 15 years of follow-up after surgery was 0% and 5.9% respectively53. The risk of proctectomy after IRA was zero if patients originally had <5 rectal adenomas and <1000 colonic adenomas. In patients with 5–20 preoperative rectal adenomas, the proctectomy rate was 13%, but when there were 20 or more rectal adenomas, the proctectomy rate was 54%57. A limit of 10 polyps in the rectum to spare the rectum has also been proposed320. In studies conducted before the IPAA era, the risk of rectal cancer and secondary proctectomy was high after colectomy and IRA. Rates of up to 37% for rectal cancers and 50% for secondary proctectomies have been reported. This may have been due to patients’ desire to avoid a permanent ileostomy at a time when IPAA was not yet available75,77,79,321–324.
One study has taken into consideration the time trend, considering the pre- and post-IPAA period. In this report, the incidence of rectal cancer after IRA was 10% during the pre-pouch period and 2% during the pouch period322. The risk of developing cancer in the pouch, rectal cuff and anal transitional zone (ATZ) is a rare event, but it is not zero (see LGI.12). As highlighted, carcinomas are detected more often in the rectal cuff/ATZ than in the pouch itself58. There are two different concepts that need to be highlighted: the rectal cuff and the ATZ. The ATZ is the area where the squamous and columnar epithelia from the rectum interchange close to the dentate line59. The rectal cuff is defined as the area that covers the region from the anastomosis to the ATZ, where rectal epithelium is present. Usually, the rectal cuff has a length ranging from 1.0 to 2.5 cm59. A rectal cuff is always present when the conventional stapling technique is performed, but it can also be present after a handsewn anastomosis. Better functional outcomes have been reported with the stapled anastomosis, but the handsewn anastomosis (if no rectal epithelium remains) should lead to a reduction in the risk of developing future polyps, dysplasia and cancer325. In both cases, follow-up of the pouch and rectal cuff is essential. A higher incidence of adenomas in the ATZ (and rectal cuff) is documented in patients with remaining rectal epithelium58–60. Due to the low incidence of cancer development in these patients, there are no reliable incidence data available325,326. Further studies need to address the best surgical strategies as well as options for endoscopic management. We suggest not leaving the rectal cuff when performing an IPAA, or at least it should be as short as possible. It should be noted that both surgical procedures (IRA and IPAA) are valid options. If we consider the risk of a secondary rectal excision, we should take genotype and phenotype into account. However, clear information needs to be provided to patients. If there is a high risk of secondary surgery or a need for screening and the patient‘s compliance is low, a single surgery (IPAA) should be offered as the first option. If patients are aware of that risk, willing to accept strict follow-up and concerned about the changes in their quality of life related to rectal excision, an IRA could be offered. Another major debate concerns which of the two surgical procedures is associated with a better quality of life. Historically, one of the main advantages for suggesting total colectomy with IRA is its good functional outcome. In a meta-analysis of 12 retrospective studies, which included 1002 FAP patients, 535 underwent IPAA and 467 underwent IRA. Patients undergoing IPAA had an average of 3.8–8 bowel movements per day, with 44% experiencing night-time defecation, 15% wearing a pad for 5 of 24 h in the day and 50.5% experiencing incontinence during a 24-h period. These results were all significantly worse than for IRA, with IRA patients having 2–6 bowel movements per day, 8.2% experiencing night-time defecation, 5% wearing a pad and 29.9% experiencing incontinence. Patients undergoing IPAA had lower rates of faecal urgency (14.2%) than patients undergoing IRA (39.1%)61. One study reported a mean number of stools per day of 4.4 after IRA and 5.5 after IPAA (P = 0.001). Faecal incontinence occurred in 14 patients (7.1%) in the IRA group versus 16 (17.4%) in the IPAA group (P = 0.03); 13 patients (6.6%) suffered from nocturnal leakage in the IRA group versus 20 (23.5%) in the IPAA group (P = 0.0001)75. These considerations could lead to suggesting a less-invasive intervention if compatible with the risk of developing cancer. The largest retrospective study did not identify a significant difference in cancer-free survival or overall survival after IPAA versus IRA in the modern surgical era when IPAA has been available as an option75,327. One propensity score-matched retrospective analysis of 340 IPAA and 585 IRA reported higher survival after IRA, but not significantly so in a multivariable model328. Patient characteristics related to survival outcomes comparing the two modes of surgery have not been reported. A meta-analysis comprising all studies published between 1991 and 2003, describing IRA for FAP and comparing it with IPAA, concluded that there was no difference in postoperative morbidity rates. More recent original studies have demonstrated the same; complications occurred in 28 patients (21%) after IRA and in 26 patients (30%) after IPAA. Severe complications (Clavien–Dindo ≥ 3b) occurred after IRA in 15 patients (11%) and after IPAA in 5 patients (6%)327. Another study reported postoperative morbidity rates in 29 patients (14.7%) of the IRA group and 15 patients (16.3%) of the IPAA group (P = 0.72; 95% c.i. 0.5–0.79)75. According to the data, there is not enough evidence to state that specific patient characteristics will predict survival or morbidity after IPAA versus IRA. Additionally, as suggested by Pasquer et al.75, the increasing use of colonoscopy strategies for adenoma management can support a more extensive use of IRA. However, considering in that case the even greater importance of adherence to follow-up, this choice must be shared and agreed with the patient. Another important aspect to consider is fertility issues, especially for women who wish to have children. Currently, there is no convincing evidence showing different fertility outcomes between IPAA and IRA procedures. However, there have been reports of reduced female fertility in IPAA compared to the IRA procedure62–65, which has led to suggestions of postponing or avoiding a colectomy with IPAA in young women who want to have children36. After an exhaustive review, including the distinction between IPAA performed for FAP or ulcerative colitis (UC), and considering the increased use of laparoscopic approaches, it was concluded that there is no convincing evidence showing different fertility outcomes between IPAA and IRA in female FAP patients (see OEM.19). In a systematic review and meta-analysis, six studies published before 2004 reporting fertility outcomes after IPAA versus IRA were included. The relative risk of infertility after IPAA was 3.91 (95% c.i. 2.06, 7.44)329. It should be noted that most patients included in the meta-analysis were UC patients, and the analysis is partly historical. In the largest retrospective study of 49 FAP patients after IRA and 51 after IPAA, no difference was detected in the rate of fertility problems (9/49 and 9/51 respectively)66. Telephone interview study of IPAA patients reported that 10 of 16 patients became pregnant, but there was no comparison group to IPAA in this study330. In addition, Olsen et al.62 suggest that a lesser effect on fertility in FAP compared to UC may be a result of the superior functional outcome of IPAA in FAP patients. It is also worth noting that the use of the laparoscopic approach has shown a significantly higher pregnancy rate, making it the preferred method in young women67,68.
Please see Fig. 2 for a summary of surgery indication.
LGI.9
While mesocolic or mesorectal excision is well established in the oncological setting, its necessity in the prophylactic setting is less clear. In the prophylactic setting, an alternative to complete mesocolic and mesorectal excision is close rectal dissection (CRD). The main difference between the two techniques lies in the plane of dissection. The former follows the avascular plane along the mesorectal and mesocolon fascia to the pelvic floor, also known as the ‘holy plane’ in the rectum. The difference between the two techniques is mainly seen in the posterior dissection in the rectum and pelvis. With CRD, the mesorectum is left in place, preserving the mesorectal fascia and the mesorectal fat, and the dissection proceeds in a non-anatomical plane. Studies have reported a higher rate of nerve injuries and diminished sexual function in patients undergoing total mesorectal excision for cancer (without IPAA). However, nerve injury leading to impotence can also occur during the anterolateral dissection of the rectum, and this is similar in both techniques69. Bartels et al.70 demonstrated that the increased rate of severe complications after total mesorectal excisions may be related to steroid use, which is typically absent in FAP patients. Therefore, drawing conclusions in the FAP subpopulation becomes challenging. Ambe et al.331 published the first case series of transanal total mesorectal excision (taTME) in FAP patients undergoing prophylactic restorative proctocolectomy with IPAA. They concluded that taTME is safe and effective in these patients. Collaborative prospective trials are encouraged to determine which procedure can provide the highest rate of benefit in FAP patients.
LGI.10
Since the description of restorative proctocolectomy (RPC), surgical techniques have evolved. Initially, a diverting stoma was always performed. However, in obese patients, such a stoma was not always possible and could cause severe complications. For these reasons, some obese patients could undergo RPC without a diverting stoma with good results. Ileostomy was then not mandatory, and an increasing number of publications reported cohorts of patients having RPC without a stoma. Even though this strategy is quite ancient, there is still no randomized controlled study demonstrating that such an option should be proposed systematically. This recommendation is applicable to each FAP phenotype (including attenuated, classical and MAP) and regardless of the type of anastomosis (manual or stapled). Since 2006, four studies have been published on this matter by three teams71–74. The first publication reports a retrospective comparison71. There was a higher rate of mortality in the group of patients with a stoma and none in the group without, and the rate of early complications was comparable. Late complications were less frequent in patients who had RPC without a diverting stoma. However, the groups were not comparable as ileostomy patients had a greater body surface area, were older, were more frequently male, required more blood transfusion and had longer operations. In addition, these results are not stratified for FAP patients. Of these patients, 96 in the ileostomy group and 48 in the no-ileostomy group had a diagnosis of FAP. The outcome in FAP patients was compared, and six cases of pelvic sepsis were registered in the ileostomy group compared to two in the no-ileostomy group. Obstruction and anastomotic stricture were observed in 21 and 14 cases in the ileostomy group and 8 and 2 cases in the no-ileostomy group respectively71. The second publication, also from the Cleveland Clinic in association with Saint Marks, focused on identifying variables that influence ileostomy omission72. The first part of the study confirmed the previous observations in 3733 patients undergoing RPC. In the analysis of the variables influencing ileostomy omission, it was observed that stapled anastomosis, no preoperative corticosteroid use, a familial adenomatous polyposis diagnosis, female sex and age <26 years were associated with ileostomy omission72. It appears, then, that ileostomy omission in FAP patients is a more relevant question. The third retrospective publication from the team of Saint-Antoine Hospital in Paris focused on the opportunity to avoid a diverting stoma while patients had a laparoscopic RPC and a handsewn anastomosis73. Among the 79 patients, 31 had FAP and only 3 had a diverting stoma. As no difference was observed in this series, it was concluded that in selected patients, a diverting stoma could be avoided73. The fourth retrospective publication from the team of Saint-Antoine Hospital in Paris included all patients operated between 2005 and 201774. The 388 patients were matched on a propensity score. Among these patients, 185 had FAP. After propensity score matching, there was no significant difference in postoperative morbidity rate, leakage rate or reoperation in patients with or without a stoma. These four publications seem to indicate that a diverting stoma may be avoided in selected patients, especially when performed for FAP, as the main criteria that lead to proctocolectomy are fulfilled by FAP patients. Additionally, another technique can be used in these patients as an alternative to the diverting ileostomy: a virtual ileostomy, also known as a ‘ghost ileostomy’. This technique involves creating a loop of the terminal ileum by passing a rubber tape through a mesenteric window in a predefined position (approximately 30 cm proximal to the inlet of the pouch) and exteriorizing it, anchoring it to the abdominal wall332. This allows for the exteriorization of the prepared ileal loop without the need for an abdominal incision332.
LGI.11
The main difference between a near-total colectomy (or subtotal colectomy) and a total colectomy consists of the preservation of the superior rectal artery, a branch of the inferior mesenteric artery, in subtotal colectomy. This is done to ensure adequate vascularization of the recto-sigmoid junction and the distal sigmoid. The difference in the level of anastomosis can have an impact on functional outcomes and quality of life. However, only one study has evaluated the short-term outcomes between the two surgical techniques56. In this study, the rate of reoperation was significantly lower in the group of patients who had an ileo-sigmoid anastomosis (0% versus 12.2%; P = 0.008), primarily due to a lower rate of anastomotic leakage (2% versus 10.8%, P = 0.0125)56. However, the adenoma count per patient per year was significantly higher after ileo-sigmoid anastomosis (11 versus 6; P < 0.001)56. Functional outcomes and quality of life were not compared in this study. Both strategies require endoscopic follow-up to monitor the possible development of adenomas. Further studies are needed to determine the optimal surgical option. Patients who are considered for this surgery must be carefully selected based on criteria that exclude them from undergoing IPAA (see LGI.8). The evaluation of phenotype and polyp location, along with the surgeon‘s experience and the informed choice of the patient, will guide the final surgical decision.
LGI.12
The cumulative risk of rectal cancer after IRA varies from 6% to 33%75,77–81, whereas the cumulative risk of dying from rectal cancer is between 9% and 12.5%77–80. The risk factors for rectal cancer post IRA include a rectal polyp count >20 or a colonic polyp count of 500 or more prior to colectomy, an APC mutation at codons 1250–1450, and age <25 years at the time of surgery55. Data on surveillance post IRA are inconsistent due to a mixed patient population (diagnosis based on phenotype versus APC mutation and surveillance for primary cancer versus metachronous), different surveillance protocols and recommendations. although some advise the removal of all polyps regardless of number and reducing surveillance to 3 weeks until rectal polyp clearance is achieved75, others recommend annual endoscopy76 with polypectomy of all polyps >5 mm. This is because the adenoma–carcinoma sequence after colectomy with IRA for FAP is similar to that of sporadic cancer. In some studies, surveillance data are mixed or missing. All studies are case series with no standard care controls. Polyps in the rectal remnant can initially be treated endoscopically. If they are not manageable or meet the criteria indicating proctocolectomy (see LGI.8), a surgical approach with secondary proctectomy should be considered and discussed with the patient.
LGI.13
Most knowledge about the development of (non-)advanced adenomas or carcinomas in an ileo-anal pouch is based on reviews and retrospective analysis in limited numbers of patients60,81–86,88,89,92,93. After a proctocolectomy with ileo-anal pouch procedure, the incidence of adenomas in the pouch varies from 6.7% to 78%81–87. The incidence increases over time, from 7% to 16% 5 years after surgery to about 82% 20 years after surgery60,83,88, and seems to be related to the pouch age itself rather than the patient’s age82. Adenomas are more frequent in stapled anastomosis than in handsewn89,90 and they are detected in the rectal cuff/ATZ as well as in the pouch itself59,326. Advanced neoplasia is found in 7% of the pouches81. Fortunately, the risk of developing a pouch cancer seems to be low86, with an incidence of 1.9–3.8%84,89. Carcinomas are detected in the rectal cuff/ATZ more often than in the pouch itself58. Endoscopic surveillance of the pouch should start 1 year after surgery91. The frequency of endoscopic surveillance of the pouch is mostly based on expert opinion and varies from every 6–12 months to biannual, and is life-long60,81–86,89,91–94. In case of high-grade dysplasia, polyps ≥10 mm and/or a total polyp number ≥30, a 3–6-monthly endoscopic follow-up is advised82,83,91. Because duodenal and gastric adenoma have been indicated as risk factors in different studies87,92, particular attention should be paid in patients with this manifestation.
LGI.14
Endoscopic resection of these polyps appears to be the first step for diagnosis, grading, and treatment. However, there is a lack of available publications studying the effects of any chemopreventive and curative treatments for these adenomas. Schulz et al.85 evaluated the use of local therapy with Clinoril as a treatment for patients with pouch adenoma, with a follow-up every three months. Their results showed regression after 6 months in 7 of 8 treated patients. However, the low level of evidence cannot support the use of local therapy as a first-line treatment. Currently, endoscopic resection appears to be the only available tool. Tajika et al.95 demonstrated the feasibility of endoscopic surveillance and management of rectal polyposis, and Patel et al.96 demonstrated the feasibility of endoscopic management also in severe cases. Other guidelines97 also support removing all polyps larger than 5 mm during pouch surveillance. However, once endoscopic resection is no longer effective or high-risk adenomas are found, other options must be discussed. Although there is no specific report on such a situation, it appears that resection of the pouch is an option and that a new pouch can be created.
LGI.15
The incidence of pouch carcinoma is very low (see LGI.13). Due to the rarity of this situation, there is a lack of literature. Only case reports have been published, collecting data from patients with different diagnoses who have undergone an ileo-anal pouch. Patel et al.333 reported pouch excision in two FAP patients with endoscopically unmanageable adenoma burden and the histopathological findings revealed unexpected cancer. When pouch carcinoma is present, pouchectomy is indicated to ensure oncological margin resection.
LGI.16
Our recommendation is based on expert opinion and the knowledge that adenomas can occur. Including a pre-pouch examination is important to ensure a comprehensive inspection of the pouch and to detect any potential pre-pouch adenomas and inflammation. This recommendation is supported by very low evidence based on two retrospective case series87,88. The first study by Tajika et al. included 26 patients with FAP and an IPAA, with a median follow-up of 21 years. Four patients (16.7%) developed pre-pouch adenomas, with a risk of 4.4% at 20 years and 36% at 30 years. All polyps were smaller than 4 mm and no pre-pouch cancers occurred88. In the study by Pommaret et al., 118 individuals with FAP after IPAA were followed for a median of 15 years after surgery. Afferent loop adenomas were detected in 9 (6.5%) patients, all of which were diminutive polyps (<5 mm) with low-grade dysplasia, and no cancer was detected87.
Please see Fig. 3 for a summary of post surgery management.
Section II: upper gastrointestinal manifestations
UGI.1
Several risk factors for the development of duodenal adenocarcinoma have been investigated (Tables 15 and 16). Some of these risk factors have been consistently associated with the risk of duodenal adenocarcinoma. There are inconsistent results for the following: personal history of CRC, type of colon surgery received, age at diagnosis of FAP, and the mutation site in the APC gene. Family history of colorectal or duodenal cancer may represent a risk factor for duodenal cancer, but the level of evidence is so far limited. Thus, family history cannot be considered a definite risk factor yet98,99. Pregnancy is a controversial risk factor105, as pregnancy does not increase the risk of duodenal cancer, but some evidence suggests that it could increase the risk of duodenal cancer among FAP patients with an APC mutation before codon 1020105. Some studies have suggested that different risk factors contribute to the risk of developing papillary and non-papillary adenocarcinoma. Risk factors for papillary adenocarcinoma include papilla biopsy with tubulo-villous/villous histology (80% versus 22.4%, P = 0.01), biopsies with high-grade dysplasia (0% versus 3.5%, P = 0.02)98 and having a papilla greater than 1 cm99. However, the development of papillary duodenal cancer did not correlate with the Spigelman stage or with any of the components of the Spigelman system98,99. Papillary cancers were associated with significantly fewer colon polyps than non-papillary cancers (496 versus 1322, P = 0.025)99. Risk factors for non-papillary adenocarcinoma instead include stage IV Spigelman polyposis (66.7% versus 15.3%, P < 0.001), high-grade dysplasia (4.4% versus 5.9%, P < 0.001), adenomas >10 mm (8.9% versus 47.1%, P = 0.031), papilla biopsy with tubulo-villous/villous histology (85.7% versus 22.4%, P = 0.001) and papilla biopsy with HGD (14.3% versus 3.5%, P = 0.28)98,99. FAP patients have a higher risk of duodenal adenocarcinoma than patients with MUTYH-associated polyposis. MAP patients tend to present with less-advanced duodenal polyposis stage275, but their duodenal polyps have a higher somatic mutational load compared to FAP patients275.
Table 15.
Statements pertaining to the upper gastrointestinal tract in familial adenomatous polyposis—extended version
| Statements |
|---|
| Risk factors for upper GI neoplasia in FAP |
| UGI.1 What are the risk factors of developing duodenal adenocarcinoma? The risk factors most strongly associated with duodenal adenocarcinoma include Spigelman stage IV (either at first endoscopy or during surveillance), high-grade dysplasia in duodenal adenomas, duodenal adenomas >10 mm in diameter, and ageing. Additional risk factors have provided inconsistent evidence and need further evaluation. |
| UGI.2 What are the risk factors of developing adenocarcinoma of the papilla? A: The risk of papillary adenocarcinoma could increase with age. B: Papillary adenoma could be a risk factor of papillary adenocarcinoma.
D: Among the known pathogenic adenomatous polyposis coli gene variants, none have been identified as a risk factor for the development of papillary adenocarcinoma. |
| Surveillance |
| UGI.3 From what age should endoscopic surveillance of the upper GI be performed? Endoscopic surveillance of the upper gastrointestinal tract may start after the age of 18 years but no later than 30 years. |
| UGI.4 What is the optimal interval of endoscopic surveillance of the upper GI tract? A: Surveillance intervals depend on gastric, duodenal and neo-duodenal (post-surgical) endoscopic findings. The site with the most advanced stage should direct the surveillance interval. B: Duodenal surveillance intervals should be based on the Spigelman stage and the appearance of the papilla. Surveillance recommendations are illustrated in Fig. 4. C: Gastric surveillance intervals should depend on the number, the dimensions, and the histological characteristics of adenomas. Surveillance recommendations are illustrated in Fig. 5. D: Post-duodenal surgery surveillance intervals depend on the type of duodenal surgery performed. Surveillance recommendations are reported in Fig. 4. |
| UGI.5 What are the optimal modalities of endoscopic surveillance of the duodenum and the papilla? A: Duodenal and papillary surveillance could rely on cap-assisted forward-viewing endoscopy for complete visualization of the papilla. If the papilla is not adequately viewed, side-viewing endoscopy should be used. B: Chromoendoscopy, both digital and dye-chromoendoscopy, could be used to improve the visualization of duodenal, papillary and gastric adenomas. Narrow-band imaging could also improve the visualization of duodenal and papillary adenomas. C: Video-capsule endoscopy is not adequate for gastric, duodenal and papillary surveillance. D: Endoscopic ultrasound and double-balloon enteroscopy are not part of routine endoscopic surveillance, but they could be useful as second-level diagnostic and/or therapeutic exams. |
| UGI.6: No statement can be provided on the use of random duodenal biopsies. |
| UGI.7 Do we need random biopsies of polyps in the duodenum for endoscopic surveillance? A: The impact of random biopsies on the prevention of papillary adenocarcinoma is unknown. Thus, no formal recommendations to adopt or not this strategy of systematic random papillary biopsies can be made. B: Taking random biopsies of the papilla improves the diagnosis of low-grade dysplasia. The benefit of random biopsies in macroscopically normal tissue to detect a high-grade dysplasia or an invasive adenocarcinoma of the papilla is very low, at least lower than 1% but not nil. |
| Spigelman staging system |
| UGI.8 Are current Spigelman staging-based surveillance and management recommendations optimal for prevention of duodenal cancer in FAP? Spigelman stage-based management provides the highest available level of evidence for duodenal cancer prevention. However, there are limitations to the Spigelman stage, which could be improved upon. |
| UGI.9 What is the duodenal cancer risk for each Spigelman stage? A: The average lifetime risk of duodenal cancer is estimated to be up to 30% for Sp-IV, 13% for Sp-III, 12% for Sp-II, and lower than 5% for Sp-I and Sp-0. B: The estimated lifetime risk of duodenal cancer may be lowered after endoscopic or surgical downstaging. |
| Endoscopic treatment option |
| UGI.10 When should endoscopic resection be considered? A: Endoscopic downstaging should be personalized according to endoscopic findings. Ideally, Spigelman stage IV should be downstaged as much as possible. An attempt to downstage Spigelman stage III could be done. B: All non-papillary duodenal lesions >10 mm should undergo endoscopic resection C: Non-papillary duodenal lesions measuring 5–10 mm in size could undergo either endoscopic resection or surveillance. D: All papillary adenomas should be candidates for endoscopic resection, but especially if harbouring high-grade dysplasia, villous histology, or if >10 mm in size. E: All gastric adenomas larger than 5 mm should undergo endoscopic resection. F: All gastric, duodenal and ampullary histologically proven carcinomas with endoscopic features suggestive of invasive adenocarcinoma should undergo surgery with or without systemic therapy rather than endoscopic resection. |
| Duodenal surgery versus endoscopic management |
| UGI.11 When should surgical resection be considered? A: Curative surgical resection must be offered to surgically resectable, histologically proven duodenal and ampullary adenocarcinoma. B: Prophylactic surgical resection could be considered for Spigelman stage IV duodenal polyposis. C: Prophylactic surgical resection could be considered for Spigelman stage II–III that is not endoscopically manageable. D: Papillary adenomas >10 mm or with high-grade dysplasia should undergo endoscopic resection, rather than surgical resection, if feasible. |
| UGI.12 When should endoscopic versus surgical resection be considered? A: All duodenal, papillary and gastric lesions with histologically proven invasive carcinoma should undergo surgery (if surgically completely resectable). B: Spigelman stage III and IV duodenal polyposis without evidence of invasive tumour should undergo endoscopic treatment, if feasible, rather than surgical resection. However, there should be a low threshold to offer surgical resection once downstaging appears no longer manageable endoscopically. C: Papillary and duodenal adenomas should undergo endoscopic resection, rather than surgery, if feasible. |
| UGI.13 Should pancreas-sparing duodenectomy or pancreatico-duodenectomy be preferred when prophylactic surgery is required? Pancreato-duodenectomy is the procedure of choice in case of suspected duodenal cancer. For prophylactic surgery, both pancreas-sparing duodenectomy and pancreatico-duodenectomy may be considered. |
| Management of gastric findings |
| UGI.14 Is treatment for fundic gland polyposis indicated? A: Endoscopic resection of fundic gland polyps has not been demonstrated to reduce the risk of gastric adenocarcinoma. However, in cases of large or symptomatic fundic gland polyps, endoscopic resection may be considered after expert evaluation. B: Fundic gland polyposis may progress to gastric adenocarcinoma in patients with FAP. Such risk cannot be quantified up to now. |
| UGI.15 What treatment modalities are available for fundic gland polyposis? Endoscopic resection may be a consideration for fundic gland polyps that are large or symptomatic, after expert evaluation. |
| UGI.16 Is treatment for gastric adenoma indicated? Suspected gastric adenomas should be removed, endoscopically if feasible. |
| Management of small intestinal findings including post upper GI surgery |
| UGI.17 Is endoscopic surveillance of the neo-duodenum and jejunum indicated after surgery? After surgery, the neo-duodenum and jejunum should receive endoscopic surveillance. |
| UGI.18 Is surveillance of the small bowel indicated? Small bowel surveillance is not routinely indicated, but small bowel examination is recommended before duodenal surgical intervention. |
| UGI.19 What modality should be preferred for small bowel surveillance: video-capsule endoscopy, single-/double-balloon enteroscopy, or small bowel MRI? When examination of the small bowel is indicated, video-capsule endoscopy is the method of choice. If positive, patients should undergo enteroscopy for diagnosis and therapy. |
FAP, familial adenomatous polyposis; GI, gastrointestinal.
UGI.2
The frequent pooling of non-papillary duodenal adenocarcinomas with papillary adenocarcinomas in the FAP literature impairs the identification of specific risk factors for papillary adenocarcinoma. We identified seven studies that report a specific risk factor analysis for papillary adenocarcinoma in FAP, without including non-papillary duodenal adenocarcinoma in the analysis98–100,102–104,106. Age was identified as being correlated with the risk of papillary HGD103. The rate of papillary endoscopic abnormalities increased from 18% at the first endoscopy to 47% at the fourth endoscopy103. This risk appears to increase progressively over the lifetime and without a threshold age effect. The presence of a papillary adenoma is a risk factor identified for the occurrence of papillary adenocarcinoma100,102. Personal history of extra-intestinal manifestations is associated with an increased risk of papillary adenoma106. In a cohort of 143 FAP patients with a papillary adenoma, only 2 patients (1.4%) developed a papillary adenocarcinoma after a mean follow-up of 8 years100. This result highlights that the risk for malignant progression of a papillary adenoma is limited. The rarity of this evolution underlines the difficulty of precisely identifying its risk factors. The risk of malignant progression is not homogeneous for all papillary adenomas. Male gender, personal history of cholecystectomy and personal history of extra-colonic malignancy are related with a higher risk of papillary adenoma progression100. Abnormal appearance of the papilla in endoscopy also increases this risk100, particularly when an increase >1 cm in papillary polyp size is observed101. A papillary polyp larger than 1 cm in one publication99 and larger than 3 cm in another101 is also associated with a higher risk of papillary adenocarcinoma. In contrast, 57–78% of papillary adenomas are identified only by routine biopsies despite the macroscopically normal appearance of the papilla on endoscopy100,102. These macroscopically normal papillary adenomas have a lower risk of progression99,100,103. The presence of HGD in a villous or tubular-villous component of the adenoma is associated with an increased risk of progression to adenocarcinoma98. In contrast, a high Spigelman score is not indicative of progression to papillary adenocarcinoma98–100,104. Finally, although the existence of familial clustering of papillary adenocarcinoma has been postulated99, no adenomatous polyposis coli gene pathogenic variants have been identified as being associated with an increased risk of papillary adenocarcinoma to date100.
UGI.3
The decision to start surveillance should consider the age-dependent risk of developing duodenal polyposis, the age-dependent risk of developing advanced duodenal polyposis, and the age-dependent risk of developing duodenal cancer107. Only age and endoscopic features have been consistently associated with the risk of developing duodenal polyposis, advanced polyposis and cancer108. No other risk factors have been consistently associated with the risk of duodenal cancer in FAP (see UGI.1 and UGI.2). Therefore, the age at first upper surveillance is based mainly on observations from cohort studies. Patients with FAP have a lifetime duodenal cancer risk of 18% (95% c.i., 8–28%) and such risk increases with age104,107–109. The cumulative incidence of duodenal cancer reaches 3.2% (s.e. 1.8%) at 40 years, 7.6% (s.e. 3.8%) at 60 years and 34.0% (s.e. 16.0%) at 75 years109. The median age at diagnosis of HGD is estimated at 73 years (95% c.i., 64–∞ y)103, but such risk is also age-dependent (5.7%, s.e. 2.3% at 40 years; 15.2%, s.e. 2.3% at 50 years; 23.2% s.e 5.9% at 60 years)109. Because duodenal cancer typically develops from a duodenal adenoma, the risk of duodenal cancer increases among those patients who have advanced duodenal polyposis107,108. In fact, the cumulative lifetime risk of stage IV Spigelman polyposis reaches 35% (95% c.i., 25–45)104, but it also increases with age (about 10% at age 50 years, 20% at age 57 years and 30% at age 70 years)104. Finally, FAP patients have a cumulative lifetime risk of duodenal polyposis of 88% (95% c.i., 84–93%), but such risk is also age-dependent (20% at age 37 years, 40% at age 45 years, 60% at age 55 years, and 80% at age 65 years). Most cohort studies have reported starting upper endoscopic surveillance from the age of 35–45 years. At first endoscopy, the prevalence of duodenal polyposis was variable across studies, generally ranging 30–40%, but it reached up to 70% in some studies102,107–118. Such polyposis was sometimes already advanced (up to 10% of patients across studies)99,102,107,113–117,119. Rarely, patients presented with duodenal or papillary cancers at index endoscopy (<2% across studies)113–118,120. In summary, OGD is a relatively invasive but safe procedure; therefore, it is appropriate to suggest the first endoscopic evaluation after the age of 18 years. Patient preference may be a consideration to delay the start of surveillance to some degree. However, the risks of advanced duodenal polyposis and duodenal cancer become significant at the age of 35–40 years. Therefore, the first endoscopic evaluation should not be delayed past the age of 35 years.
UGI.4
The optimal interval for endoscopic surveillance of the upper GI tract depends on the endoscopic findings in the stomach, the duodenum and the neo-jejunum. Findings from each of these three sites confer a higher or lower risk of developing gastric, duodenal and jejunal adenocarcinoma respectively. The surveillance interval should be determined by the anatomical site with the highest risk findings. Usually, the duodenal findings will dictate the frequency of endoscopic surveillance. The proposed surveillance algorithm for duodenal polyposis is shown in Fig. 4. The Spigelman stage categorizes the severity of duodenal disease based on four variables, which each contribute up to 3 points (Table 3). The two main determinants of the surveillance interval are Spigelman stage and papillary endoscopic appearance. The natural history of duodenal polyposis is to slowly progress: on average, healthy mucosa progresses to low-grade dysplasia in 29 months (95% c.i., 15–37 months) and to HGD in 261 months (95% c.i., 235–∞ months)103,117. The cumulative incidence of duodenal cancer is 3.2% (s.e. 1.8%) at 40 years, 7.6% (s.e. 3.8%) at 60 years and 34.0% (s.e. 16.0%) at 75 years109. The Spigelman score increases by +0.30 points per year, and the Spigelman stage by +0.12 per year111. The baseline Spigelman stage provides a guide to the lifetime risk of duodenal cancer (33% for stage IV, 13% for stage III, 12% for stage II, 0% for stage I and 0)104. Endoscopic downstaging is associated with a lower risk or delay in disease progression119,121,122. Where possible, stage IV polyposis should be downstaged, but because the risk of duodenal adenocarcinoma is still high115 and there is 50% chance of progression from stage III back to stage IV within 1 year115, yearly surveillance is recommended even when patients are downstaged115. Exceptionally, for patients who are stable in stages 0–II (after downstaging from stage IV), surveillance every 2 years may be considered119,121,122. In one study, the cumulative incidence of high-risk papillary findings (papilla >10 mm, HGD, villous histology, or cancer) reached 52.1% at 15 years, while performing more frequent surveillance reduced the risk of papillary progression (HR, 0.75, P = 0.027)100. However, the Spigelman score alone poorly predicts the risk of papillary adenocarcinoma/HGD99,103. All papillary adenocarcinomas, instead, occurred in papillae >10 mm in diameter99. Other risk factors for the progression of papillary findings include male sex (HR, 2.1; c.i., 1.02–4.16), prior cholecystectomy (HR, 2.5; c.i., 1.4–4.7), abnormal appearance of the papillae at first detection (HR, 2.1; c.i., 1.1–3.9), and personal history of extra-colonic malignancy (HR, 2.6; c.i., 1.2–5.7)100. Therefore, in the presence of features that significantly increase the risk of papillary cancer (papilla diameter >10 mm; HGD from papilla biopsy; villous histology from papilla biopsy), more frequent surveillance endoscopy may be considered on an individual basis. The incidence of gastric adenocarcinoma in FAP patients is rising123. The risk of HGD in gastric adenomas is proportional to the adenoma size (33% if >20 mm, 4% if ≤20 mm, P = 0.04)124, but adenomas smaller than 5 mm almost never demonstrate HGD124. Therefore, all gastric adenomas >5 mm should be resected en-bloc and their surveillance depends on the endoscopic and histologic findings. The proposed surveillance algorithm for gastric polyposis is shown in Fig. 5.
After surgical resection of the duodenum, the neo-duodenum may develop adenomas during follow-up109. Jejunal polyposis occurs in up to 59.4% of patients, after a median time of 55 months from surgery (range: 22–84)125. Therefore, given the risk of gastric cancer and the risk of small bowel carcinoma, surveillance should continue even after duodenal surgery (Fig. 4). Pancreas-sparing duodenectomy is associated with a higher risk of jejunal polyposis compared to pancreatico-duodenectomy (HR 4.0, c.i. 1.6–10.0) and segmental duodenectomy (P = 0.048)125. Therefore, patients receiving pancreas-sparing duodenectomy may receive more frequent surveillance. The evidence on the prevalence of jejunal adenomas is of low quality. Two studies offered small bowel enteroscopy to FAP patients with duodenal polyposis stages III or IV106,126. They reported that 83.3–90% of patients with stage IV polyposis had jejunal polyps, and 20% were greater than 20 mm. However, all jejunal polyps were adenomas with low-grade dysplasia. Therefore, small bowel enteroscopy may be offered to individuals with stage III/IV duodenal polyposis, but until further data are available, this should be in a research setting.
UGI.5
Endoscopic surveillance of the stomach, the duodenum and the papilla requires careful and complete visualization of their entire mucosal surface99,104,109,112,117,127. The duodenum and the papilla should be visualized entirely to allow precise count of all duodenal polyps and assess the risk of adenomas or malignancy98,99,102,103,108,115. Forward-viewing endoscopy provides adequate visualization of the gastric and duodenal mucosa in almost all circumstances104,108,109,111. Forward-viewing instruments can visualize the papilla as well, if supported by a cap128. Cap-assisted endoscopy can visualize the papilla in up to 95–97% of cases128,129 and, on average, it could visualize the papilla faster than regular endoscopy128. When the papilla cannot be visualized with a forward-viewing instrument, side-viewing endoscopy should be used99,102,108,117. There are techniques and technologies that improve the performances and the detection rate of endoscopic surveillance, including chromoendoscopy and narrow-band imaging. In the duodenum, indigo chromoendoscopy can increase the number of polyps detected per patient (13.5 versus 23, P < 0.0001)130,131, the median number of adenomas per patient (15 versus 21, P = 0.02)132, the number of patients with large adenomas >10 mm (12–19, P = 0.0391)130, the number of small adenomas131 and the largest median maximal size (8 versus 10 mm, P = 0.02)132. Therefore, indigo chromoendoscopy increases the Spigelman stage and allows a more strict surveillance132. Likewise, digital chromoendoscopy identified more adenomas than white-light endoscopy (13 versus 6, P = 0.03) and it was associated with a higher number of polyps detected per patient (8.7 versus 7.2, P < 0.001)133. Narrow-band imaging is also associated with a higher duodenal polyp detection rate (35.6%), resulting in an increased Spigelman stage in up to 11.1% of cases134. In the stomach, indigo chromoendoscopy increased the median number of gastric adenomas per patient (0 versus 0.5, P = 0.0025)130 compared to white-light endoscopy. Likewise, digital chromoendoscopy increases the median number of antral polyps (56 versus 24, P < 0.0001)133. However, digital chromoendoscopy did not improve the detection rate of fundic polyps133. Narrow-band imaging did not improve the diagnostic accuracy for gastric polyps134. Video-capsule endoscopy has a low sensitivity for duodenal and papillary lesions135. Video-capsule endoscopy may visualize the papilla in as few as 10.4% of patients136,137. Therefore a negative video-capsule exam does not substitute a proper endoscopic surveillance session135. Endoscopic ultrasound provides additional useful information for duodenal, papillary and gastric lesions that are considered for either surgical or endoscopic surveillance101,139. Endoscopic ultrasound could increase the accuracy of adenoma staging in up to 36% of cases138, but other studies could not reach similar results101. Therefore, endoscopic ultrasound is a second-level exam that is useful, but not part of routine endoscopic surveillance. Double-balloon enteroscopy could be useful for diagnostic and therapeutic purposes but should be reserved for patients with a high risk for or known significant jejunal polyposis140.
UGI.6
After an exhaustive review of the literature conducted in accordance with the methodology established for this collaborative effort to develop guidelines, no articles providing relevant answers to this question could be identified. Thus, it is not possible to establish recommendations based on the available literature to answer the question raised. This question is relevant in the management of patients with FAP and could be addressed in future investigations.
UGI.7
Adenomatous degeneration of the papilla in FAP patients is underdiagnosed by targeted biopsies of macroscopic adenomas performed during endoscopy98,141,142. The first hypothesis that could explain this is that targeted biopsies allow the analysis of a fragment of the adenoma only and not of all the adenoma. Another hypothesis is that dysplasia may exist in the papilla within a macroscopically normal mucosa that is not assessed by targeted biopsies. For this reason, it is legitimate to investigate whether random biopsies of the papilla could provide a better staging of papillary degeneration in FAP patients. We have identified three studies in the literature that report the histological findings of random papilla biopsies taking during endoscopic follow-up in patients with FAP102,143,144. Mehta et al. report the assessment of random papilla biopsies collected during 792 follow-up endoscopies of 273 patients with FAP143. The papilla appeared macroscopically normal in 546/792 (68.9%) of endoscopies. The random biopsies performed in these 546 patients showed normal papillary tissue without dysplasia in 503 patients (92%), low-grade dysplasia in 42 patients (7.9%) and HGD in 1 patient (0.1%). No invasive adenocarcinoma was found. Based on these results, the Spigelman stage increased in 15 (2.7%) patients due to the random biopsies: 9 patients were upstaged from I to II, 5 patients from II to III, and 1 patient from III to IV. In a second study reporting random biopsies in patients with FAP, 7 of the 24 patients biopsied (29%) had an abnormal histology with an adenomatous change despite the normal endoscopic aspect of the papilla102. Only low-grade dysplasia was found—none of these patients with macroscopically normal papilla showed HGD or carcinomatous degeneration. In a third study, Bertoni et al. noted abnormal histology in 44% (11/25) of random biopsies of the papilla from macroscopically normal tissue in a cohort of 25 patients with FAP144. These histological abnormalities were exclusively classified as low-grade dysplasia with no cases of HGD or invasive carcinoma. Concerning the morbidity rate of random biopsies of the papilla in the context of FAP, only one of the three studies cited above discussed this143. In this series of 792 papillary biopsies conducted in 273 patients with FAP, no immediate intraprocedural complications requiring hospitalization were observed and all patients were discharged home the day of the endoscopy. The only complication reported was acute pancreatitis, which affected 2 patients (0.73% of the cohort). No other complication, including no bleeding, perforation, or stenosis at the biopsy site, was reported. In conclusion, the rate of adenomatous change observed during random papillary biopsies in macroscopically healthy areas among patients with FAP ranged from 8% to 44% in the three studies identified98,141,142. For a total of 591 patients analysed in the three studies, only one case of HGD was identified, all other adenomatous changes observed were low-grade dysplasia. No cases of invasive adenocarcinoma were identified in these studies when the papilla was macroscopically normal. Nevertheless, this has already been reported in the literature98 emphasizing that, although the risk of papillary adenocarcinoma with a macroscopically normal papilla is minimal, it is not nil. No evidence that shows the benefit of random biopsies to detect these rare cases of papillary adenocarcinoma in papilla with a normal aspect by endoscopy has been published to date.
UGI.8
The Spigelman classification was originally proposed to objectively assess the burden of duodenal polyposis in FAP patients. It then become popular for several reasons, including the ease of computation, the replicability and the ability to monitor disease progression over time and after endoscopy therapy99,102–104,111,119,121,122. Therefore, there is a sufficiently large body of evidence to recommend its use in clinical practice104,118,122,148. However, it has some weaknesses that warrant careful scrutiny, including a suboptimal sensitivity and specificity for predicting the risk of duodenal and papillary cancer. The strengths and weaknesses of Spigelman staging are summarized in Table 17. Stage IV Spigelman polyposis significantly increases the risk of duodenal cancer99,104,111,115,121. However, up to 53% of patients developing duodenal (non-papillary) adenocarcinoma have no prior history of stage IV duodenal polyposis98,103. Likewise, up to 75% of patients with papillary cancer have no prior history of stage IV polyposis98–100,145. This implies a suboptimal sensitivity for duodenal and papillary cancer98. Moreover, the Spigelman stage uses four predictors that are weighted equally98. However, not all components of the Spigelman system are equally predictive of duodenal cancer, and perhaps different weightings should be used98,119. Some studies have reported that villous histology does not increase the risk of duodenal cancer significantly98. Likewise, the individual components of the Spigelman stage do not predict sufficiently well the risk of papillary adenocarcinoma and the need for ampullary resection/surgery99,100,103,145. Interestingly, patients downstaged from stage IV to stages I, II or III have a significantly higher risk of duodenal cancer than patients who naturally develop stages I, II or III112,115. This suggests a more aggressive course for patients who develop stage IV polyposis during their lifetime112,115. However, the Spigelman stage treats all patients with stages I, II or III equally, regardless of downstaging102,115,119,148. Moreover, it is unclear how the Spigelman stage should be applied to the neo-duodenum after duodenal surgery109. The Spigelman stage was originally developed with white-light endoscopy. Nonetheless, some studies now report that chromoendoscopy significantly increases the duodenal polyp count and, thus, the Spigelman stage122,149. Whether this finding should be incorporated in the Spigelman stage requires further evaluation149. One crucial observation is that Spigelman stage IV may sometimes harbour a carcinoma146,147. It is not uncommon for patients who undergo prophylactic duodenal surgery to have a duodenal cancer that was unsuspected before surgery109,146,147. Finally, the Spigelman stage only accounts for four risk factors104,116,121,127. There are additional duodenal cancer risk factors, including personal and family history of colorectal, duodenal and gastric cancer (see UGI.1).
Table 17.
Strengths and weaknesses of the Spigelman stage
| Strengths | Weaknesses |
|---|---|
| Practical, replicable, and easy to compute (four variables only) | Suboptimal sensitivity:
|
| Can measure progression/regression over time | Equal weight to all four components (villous histology has a weaker association with cancer than other components) |
| Developed with white-light endoscopy. Chromoendoscopy may increase the number of duodenal polyps. | |
| Fails to consider other risk factors for duodenal adenocarcinoma. | |
| Does not account for previous severity of polyposis |
UGI.9
The Spigelman stage is a well-known estimator of the risk of duodenal cancer. However, the risk of duodenal cancer for each Spigelman stage is predictable only partially104,109. In fact, the lack of controlled studies and the variability between studies imply that the precise risk of duodenal cancer can only be approximated102. The largest cohort studies to date have measured the risk of duodenal cancer based on the first endoscopic examination, not on subsequent surveillance endoscopic exams (Table 18)104,122. It is biologically plausible that the development of Stage IV polyposis during surveillance should carry a lifetime risk similar to that of having Stage IV at baseline108,109. In fact, time is a risk factor for both duodenal cancer and an increase in the Spigelman stages100,103,111,112,116. Stage IV Spigelman polyposis carries the highest risk of duodenal cancer. The lifetime risk and the 10-year risk of duodenal cancer for Spigelman stage IV at baseline reaches 30% at most102–104,107,118. The development of Spigelman Stage IV during surveillance has been associated with an odds ratio of duodenal cancer of 8.8 (95% c.i. 2.1–36.6)98. Interestingly, surgical case series suggest that 10–30% of patients receiving prophylactic duodenal surgery for Spigelman stage IV may also have an unsuspected duodenal cancer146,147. Stages III and II Spigelman polyposis carry an intermediate risk of duodenal cancer. The lifetime risk for duodenal cancer for patients with Sp-II and Sp-III is estimated at 12% and 13% respectively104. However, duodenal cancer may not develop for several years, because the 10-year risk of duodenal cancer is estimated at 2% for both107. Stages I and 0 Spigelman polyposis have the lowest risk of duodenal cancer, currently estimated at lower than 5%104,107,109. Interestingly, patients down staged from stage IV to stages I, II or III have a significantly higher risk of duodenal cancer than patients who naturally develop stages I, II or III115,118,121,122. Finally, the risk of papillary cancer cannot be predicted from Spigelman stage98–100,103,119,145.
Table 18.
Cancer risk by Spigelman stage
| Stage | Duodenal cancer risk |
|---|---|
| Spigelman stage 0 | Lifetime risk = 0% 10-year risk = 0% |
| Spigelman stage I | Lifetime risk = 0% 10-year risk = 0% |
| Spigelman stage II | Lifetime risk = 12% 10-year risk = 2% |
| Spigelman stage III | Lifetime risk = 13% 10-year risk = 2% |
| Spigelman stage IV | Lifetime risk = 33% 10-year risk = 36% |
UGI.10
Different risk factors contribute to the decision of remove or observe a gastric, duodenal, papillary or jejunoileal polyp, which include the Spigelman stage, the degree of dysplasia and the dimension of the polyp. Epidemiological factors have also been considered. The recommendations above are summarized in Fig. 6. The incidence of gastric adenocarcinoma in FAP patients is on the rise123. Adenoma size is the main risk factor of HGD (33% risk if >20 mm, 4% if ≤20 mm, P = 0.04)124. Adenomas < 5 mm almost never harbour HGD124. Therefore, all gastric adenomas >5 mm should be resected en-bloc and their surveillance depends on the endoscopic and histological findings. The baseline Spigelman stage offers an estimate of the lifetime risk of duodenal cancer (33% for stage IV, 13% for stage III)104,108. Therefore, whenever possible, stage IV polyposis should be downstaged, because polyposis downstaging is associated with a lower risk of disease progression119,121,122. The same rationale applies to stage III polyposis117,122. A few studies suggest that, once downstaged, the Spigelman stage remains at a lower stage119. However, one study reported that once duodenal polyposis reaches stage IV, the risk of duodenal adenocarcinoma remains high, even after downstaging115. There is no RCT on non-papillary duodenal polyps. Clinical practice and cohort studies suggest that a 10 mm cut-off provides the ideal cost–benefit cut-off113,118. In fact, lesions <10 mm almost never harbour HGD or invasive carcinoma. Lesions >10 mm may develop into a carcinoma and therefore justify the perforation risk of endoscopic mucosal resection113. The survival of patients with adenomas >10 mm (treated) and adenomas < 10 mm (surveillance only) did not differ significantly (7.13 years, range 4.59–8.57 versus 9.72 years, range 4.64–16.83, P = 0.08)113. During a median follow-up of 8.5 years, none of the duodenal adenomas under surveillance needed treatment and the survival rate was 96%118,151. There is no RCT on the ideal size for endoscopic ampullectomy. Adenomas >10 mm recur more commonly (76.9% versus 36.4%, P = 0.002) and earlier (95.8 ± 9.7 versus 34.7 ± 8.9 months, P = 0.04) than adenomas <10 mm145. This justifies a more conservative approach for smaller adenomas159. The decision to intervene should not be based on the Spigelman stage of the rest of the duodenum, which can predict the papillary disease only partially100. Finally, several case–control studies suggest that papillary adenomas in FAP are not substantially different from sporadic papillary adenomas113,160. In fact, the two groups do not differ in terms of safety and overall outcomes113,120,160. Endoscopic features suggestive for invasive carcinoma include infiltrative border, ulceration and a hard consistency159,160. Such endoscopic findings should raise the suspicion of invasive carcinoma, which should be treated by radical surgery or systemic therapy.
UGI.11
Upper GI surveillance aims to prevent duodenal and papillary adenocarcinoma98,114,150. Therefore, endoscopic downstaging of duodenal polyposis is the primary treatment strategy. When endoscopic treatment becomes no longer feasible, duodenal surgery may be considered for Spigelman stages III/IV148,150,157. Duodenal surgery has been traditionally offered to patients with stage IV duodenal polyposis, but this approach must be carefully considered98,139,141,142,146–148,150,152. Spigelman stage IV is one of the most important risk factors for duodenal cancer98,109, but patients may not develop cancer for several years and endoscopic downstaging prolongs cancer-free surveillance98,108,116. The rationale for endoscopic surveillance is supported by our most recent understanding of the adenoma–carcinoma sequence98,108,109,113,116. Duodenal surgery (both pancreas-sparing duodenectomy and pancreato-duodenectomy)152,153 are associated with considerable short-term morbidity and mortality142,152,154,155. On the other hand, duodenal cancer may also develop in the absence of Spigelman stage IV116. Moreover, several studies have reported that patients with duodenal polyposis may harbour unsuspected foci of duodenal cancer that are only apparent after duodenal surgery108,109,139,141,142,146–148,150,158. Papillary adenomas in FAP can be safely managed endoscopically113. Endoscopic therapy of papillary adenoma may provide a survival similar to matched patients without papillary adenoma (7.13 versus 9.72 years, P = 0.08)98,109,113. As previously stated in UGI.10, all papillary adenomas harbouring HGD, regardless of their size, or >10 mm in size should undergo endoscopic resection, if feasible. Therefore, an attempt should be made to downstage the duodenal disease as much as possible, especially for Spigelman stages III/IV. However, there should be a low threshold to escalate to duodenal surgery once the disease has become no longer endoscopically manageable.
UGI.12
Duodenal and papillary adenomas carry a significant risk of developing an invasive carcinoma. Patients with advanced duodenal polyposis (Spigelman stages III or IV) or advanced papillary lesions (>10 mm or carrying HGD) should undergo either surgical or endoscopic resection151. Endoscopic treatment should be the preferred option for benign lesions151. Endoscopic downstaging of duodenal polyposis interrupts the adenoma–carcinoma sequence and significantly prolongs cancer-free survival117,119,121,122. In fact, after endoscopic downstaging of Spigelman stage IV, duodenal polyposis may not reach stage IV for a median of 37 months119, although a risk of cancer persists even after downstaging115. The cancer-free survival after endoscopic treatment of advanced adenoma becomes similar to that of individuals without an advanced adenoma (7.13 years, range 4.59–8.57, versus 9.72 years, range 4.64–16.83, P = 0.08)113. Finally, the endoscopic treatment of duodenal and papillary adenomas offers a surgery–free and cancer–free survival of 74% at 89 months and of 71% at 71 months (for duodenal and papillary therapy respectively)119. Papillary adenomas in FAP can also be safely managed endoscopically160. Size is not a contraindication to endoscopic treatment per se, but high endoscopic expertise is needed159,160. Endoscopic therapy of papillary adenoma may provide a survival similar to matched patients without papillary adenoma (7.13 versus 9.72 years, P = 0.08). In one study, the risk of papillary adenoma recurrence was higher for those >10 mm (36.4% versus 76.9%, P = 0.002)145, but did not differ based on the endoscopic technique used (en-bloc versus piece-meal resections recurrence rate: 46.7% versus 77.8%, P = 0.29)145. On the other hand, endoscopic treatment is not adequate for adenocarcinomas. Irregular margins, hard consistency, and ulceration should raise the suspicion of invasive carcinoma, which should be treated by radical surgery or systemic therapy160,334. The involvement of the bile duct or the main pancreatic duct may not an absolute contraindication to endoscopic resection, but carcinoma should be ruled out first159. It should be emphasized that the degree of polyposis in Spigelman stages III/IV may hide some foci of invasive cancer146,147. Several studies have reported a high risk (range 8–37%) of unsuspected duodenal cancers that are diagnosed only after histological review108,109,139,141,142,146–148,150,158. Moreover, once FAP patients develop duodenal cancer, their overall survival may not differ substantially from FAP patients not under surveillance150. Interestingly, one study observed a lower all-cause and cancer-related five-year survival after prophylactic duodenal resection in patients with invasive tumours (n = 9) than in patients with Spigelman stages III or IV disease without invasive carcinoma (n = 29) (62.5% versus 81.6% [P = 0.325], 62.5% versus 88.5% [P = 0.116] respectively), although these differences did not reach statistical significance141. Therefore, duodenal cancer development should be considered a surveillance failure and there should be a low threshold to offer duodenal surgery if endoscopic control cannot be maintained. Duodenal surgery (both pancreas-preserving and pancreato-duodenectomy)152,153 is associated with significant short-term mortality (about 5%) and morbidity (30–63%), with possibly debilitating long-term consequences (insulin dependence 3–6%, exocrine insufficiency 30–60%)142,150,152,154,155,160,161. Therefore, it should be offered to carefully selected patients141,156. Nevertheless, in the long term, morbidity rate and quality of life after duodenal surgery do not differ significantly from matched FAP patients with no history of duodenal surgery157. This finding should reassure clinicians and patients of the carefully considered use of surgery157.
UGI.13
Duodenal prophylactic surgeries should only be performed in high-volume centres with a substantial expertise with such surgeries. Pancreas-sparing duodenectomy and pancreatico-duodenectomy have similar short- and long-term outcomes. There is no statistically significant difference between them in terms of 30-day mortality and morbidity rates and length of hospital stay146. Some studies reported that pancreas-sparing duodenectomy was a faster operation (391 versus 460 min, P = 0.002)152,153 with significantly less blood loss (428.1 ± 269.1 versus 550.3 ± 1222.6 ml, P = 0.0005)153, but other studies reported opposite results158. The 10-year overall survival and the 10-year disease-specific survival were not significantly different (74.7% versus 58.4%, P = 0.2925 and 77.7% versus 64.9%, P = 0.3837 respectively)146,153,158. The rationale for offering pancreas-sparing duodenectomy instead of pancreatico-duodenectomy is to limit the incidence of diabetes and pancreatic exocrine insufficiency. Most studies suggest that pancreas-sparing duodenectomy does not decrease the risk of diabetes (0% versus 12%, P = 0.107)152,158 compared to pancreato-duodenectomy. However, it may lower the risk of exocrine insufficiency (11% versus 30%; P = 0.03)152 and increase the risk of delayed acute pancreatitis (0% versus 16%; P = 0.012)152. Finally, the risk of jejunal polyposis seems higher after pancreas-sparing duodenectomy compared to pancreatico-duodenectomy (HR 4.0, 95% c.i. 1.6–10.0)125, reaching up to 31% at 71 months of follow-up142,155. Therefore, there is insufficient evidence to support a clinical benefit of pancreas-sparing duodenectomy over pancreato-duodenectomy. Finally, many studies reported that, after surgery, a high percentage of patients had a carcinoma that was unsuspected, based on preoperative assessment (8–37%)139,141,142,146,158. This finding highlights the importance of a scrupulous diagnostic workup before surgery. However, even a zealous workup may not rule out all cancers and it should be emphasized that pancreas-sparing duodenectomy does not comply with the lymphadenectomy requirements for duodenal cancer surgery. Therefore, the risk of performing an oncologically incomplete surgical resection should also be considered. There is not enough evidence to recommend pancreas-sparing duodenectomy over pancreato-duodenectomy. Both surgical options have similar performance metrics in terms of safety and efficacy. However, there are practical reasons to prefer pancreato-duodenectomy over pancreas-sparing duodenectomy. First, most centres have greater experience with pancreato-duodenectomy than with pancreas-sparing duodenectomy. Second, the alleged advantages of pancreas-sparing surgery have not been demonstrated thus far. Third, if an unsuspected cancer is diagnosed after surgery, pancreas-sparing duodenectomy is not oncologically appropriate.
UGI.14
Fundic gland polyps are defined as gastric polyps with an architectural disorder of the fundic gland. These lesions occur more frequently in FAP patients than in the general population, with a prevalence ranging from 26% to 88%162,165–167. Fundic gland polyps can develop early in FAP as a prevalence of up to 50% has been reported in a cohort of children with FAP168. The incidence of gastric adenocarcinoma is much lower than that of fundic gland polyps in patients with FAP, although there has been an apparent increase since 2017, to approximately 0.6–1.3%123,175. At the moment, the question of whether fundic gland polyps may constitute a pre-cancerous lesion has not received a definitive answer. There is biological and preliminary clinical evidence to suggest a risk of gastric cancer among FAP patients with fundic gland polyposis163,164. After a systematic literature search, we identified two clinical series assessing the risk of gastric adenocarcinoma. In one series of 26 gastric neoplasms diagnosed in 22 patients with FAP, 50% had fundic gland polyps165. In another series of 10 cases of gastric adenocarcinoma among patients with FAP, 8 had fundic gland polyps during endoscopic surveillance123. There is significant biological evidence to support an association between fundic gland polyps and gastric adenocarcinoma in FAP. The incidence of low- and high-grade dysplasia in fundic gland polyps ranges from 25% to 49%123,162,169,170. This risk of dysplasia increases with the size of the fundic gland polyps, with the stage of duodenal polyposis, and when an antral gastritis is associated102,162. Wu et al. reported that fundic gland polyps with dysplasia express higher levels of epithelial proliferation (measured by Ki-67 and p21 immunohistochemistry), although proliferative dysregulation was also found in fundic gland polyps without dysplasia169. This result supports the hypothesis that disturbances in cell proliferation within fundic gland polyps may occur prior to dysplastic morphological abnormalities. Moreover, Abraham et al. reported that FAP patients may harbour a somatic second-hit APC gene mutation in 50% of fundic gland polyps with dysplasia and 47% of fundic polyp glands without dysplasia170. In summary, no study has evaluated the direct impact of fundic gland polyp resection on the prevention of gastric adenocarcinoma. However, the endoscopic and histological data available in the literature to date support the possibility that fundic gland polyps may have potential to progress to adenocarcinoma, but the risk cannot be quantified. Therefore, endoscopic resection of fundic gland polyps may be suggested.
UGI.15
Fundic gland polyposis is a common finding in the stomach of patients with FAP162–164. Recent evidence indicates a rising incidence of gastric cancer in FAP patients123,165,176. The characteristics of fundic gland polyposis are discussed in UGI.14. In the longest (5.9 ± 3.4 years of follow-up) and largest study to date that reported longitudinal surveillance of fundic gland polyps in patients with FAP (35 FAP patients with 118 surveillance polypectomies), a novel endoscopic polypectomy surveillance protocol was proposed171. This suggested the endoscopic removal of multiple fundic gland polyps with a standard cold-snare technique. The longitudinal collection of large pathology samples suggests that fundic gland polyps may progress to dysplasia and cancer171. However, there is currently not enough evidence to recommend that the treatment modalities for fundic gland polyps should differ for patients with FAP compared to the general population172. Therefore, when fundic gland polyps need to be treated, this may be by conventional resection techniques, preferably endoscopically, if feasible.
UGI.16
The growing clinical awareness of gastric adenomas as a concern in FAP reflects the increasing incidence of gastric adenocarcinoma in FAP patients123,163,173. However, gastric adenomas in FAP patients often arise within a background of carpeting fundic gland polyposis123,163,173, making the detection and identification of gastric adenomas sometimes challenging166,167,174,175. Consequently, the prognosis of gastric cancer in FAP remains poor, primarily due to advanced disease at the time of diagnosis124,166,167,174,175. The prevalence of gastric adenomas in FAP reaches 14%124,166,173,176, with a median age of 47 years at the time of adenoma diagnosis124. Low-grade dysplasia develops in approximately 95% of adenomas, as evidenced by the largest available study that found low-grade dysplasia in 98 of 104 adenomas124. Additionally, about one-third of all patients (37/104) have multiple adenomas124. While adenomas generally follow a benign course, the most comprehensive study to date reported that 5% of FAP-associated adenomas develop HGD. Furthermore, the risk of HGD increases proportionately with the size of the adenoma (P = 0.04)124. For instance, 33% of adenomas larger than 20 mm exhibited HGD124. Therefore, the removal of suspicious gastric adenomas is expected to impede the progression to adenocarcinoma174. During post-polypectomy surveillance, metastatic gastric adenocarcinoma developed in less than 5% of FAP patients with a history of gastric adenomas (3/104), with a median age of 60 years (range 50–73) and a median time of 66 months from adenoma diagnosis to cancer. Of patients with adenoma, 83% also had fundic polyposis, highlighting the challenges posed by the development of gastric adenomas within the context of carpeting polyposis. Ideally, an optical diagnosis of gastric adenoma is preferred, and routine biopsies are avoided due to the potential risk of fibrosis, which could complicate definitive endoscopic resection. Although there are currently no validated optical diagnostic features for HGD, existing studies indicate that polyps smaller than 5 mm are unlikely to exhibit HGD124,166,173,176. Therefore, size may serve as a presumptive indicator of HGD risk and, whenever feasible, direct resection is preferred.
UGI.17
After prophylactic duodenal surgery, adenomas can develop both in the duodenal bulb (10.9% incidence) and in the remaining jejunum (51–59.4% incidence)125,155,177. The median time for the detection of jejunal polyps after surgery ranges 22–84 months125,177. Such polyps tend to be small and adenomatous126, but they may develop into carcinoma (2.4%)177. The risk of jejunal polyposis seems higher after pancreas-sparing duodenectomy compared to pancreatico-duodenectomy (HR 4.0, 95% c.i. 1.6–10.0) and segmental duodenectomy (P = 0.048)125. Some studies have reported that patients undergoing prophylactic duodenal surgery may already have an unsuspected duodenal adenocarcinoma139,142. This information reinforces the need for surveillance of the remaining jejunum. The detection of jejunal polyps after duodenal surgery may result from a combination of underestimation of jejunal involvement before and development after surgery. Jejunal surveillance after surgery should be considered.
UGI.18
The prevalence of small bowel polyps is estimated at 30.4–87%117,137,178–180, but there is conflicting evidence on the extent and severity of jejuno-ileal polyposis in FAP106,117,126,137,178–183. Jejuno-ileal polyps are usually small (<5 mm), adenomatous and located in the proximal jejunum106,126,178–181. The presence of duodenal adenomas has been found to predict the presence of small bowel polyps (P = 0.001)117,178, but not in all studies117,137,178–180. A more advanced duodenal polyposis stage (Spigelman III/IV) could be indicative of a higher risk of jejuno-ileal polyposis106,126,181,182, but not in all studies117,183. The prevalence of jejunal polyposis before duodenal surgery is estimated at 52–83%125,335–337. After surgery, adenomas can develop both in the duodenal bulb (10.9% incidence) and in the remaining jejunum (51–59.4% incidence)125,177. The median time to detection of jejunal polyps after surgery was 55 months (range 22–84). Such small bowel polyps are usually small and adenomatous, but they may also harbour a carcinoma (2.4%)177. The risk of jejunal polyposis seemed higher after pancreas-sparing duodenectomy compared to pancreatico-duodenectomy (HR 4.0, 95% c.i. 1.6–10.0) and segmental duodenectomy (P = 0.048)125. The detection of jejunal polyps after duodenal surgery may result from a combination of underestimation of jejunal involvement before and development after surgery. Therefore, jejunal assessment ahead of duodenal resection is mandatory for surgical planning, in order to decide on the length of adjacent jejunal segment inclusion. Moreover, the risk of small bowel polyps persists even after surgery specifically for the jejunum and therefore lifelong jejunal surveillance should be considered. Post-surgical surveillance is further explored in UGI.4.
UGI.19
Capsule endoscopy can accurately identify jejuno-ileal polyps and it can identify significantly more jejuno-ileal polyps than MRI and barium studies (29% versus 12%, P < 0.02)106,137,178,180,181,183. Capsule endoscopy is safe to perform after colorectal surgery, provided that patients do not experience obstructive symptoms125,177. Nevertheless, a patency capsule may be considered as a safe precaution, especially before the first capsule endoscopy. The use of contrast enhancement on capsule endoscopy does not increase the diagnostic yield significantly338. There are not enough data to report the efficacy and safety of capsule endoscopy after duodeno-pancreatectomy. Capsule endoscopy detects significantly fewer polyps than push enteroscopy for the first quarter of the bowel length (10.0, i.q.r., 5.0–19.0; versus 41.0, i.q.r., 19.0–64.0; P = 0.002)135. The combined use of push enteroscopy and capsule endoscopy increased the polyp count to 123.0 (i.q.r., 38.0–183.0, P < 0.001)135. There is no comparative study of video-capsule endoscopy versus single-/double-balloon enteroscopy. Single-balloon endoscopy for patients with Spigelman stage IV duodenal polyposis has a diagnostic yield of 90% for small jejunal polyps (20% of which are large >20 mm)126. Double-balloon chromoendoscopy revealed a 67% prevalence of jejunal polyps among FAP patients179. The sensitivity and widespread availability of the video capsules suggest that they can be used as the primary surveillance strategy for the small intestines, even after colorectal or duodenal surgery106,125,137,177,178,180,181,183. Moreover, capsule endoscopy can cover the intestinal areas that are beyond the reach of endoscopic exams135. However, capsule endoscopy with positive results should be coupled with an enteroscopic exam for both diagnostic and therapeutic purposes126,135,179.
Section III: desmoid tumours
DTs1
Classifications have been created to guide the treatment of DTs (Table 19). The first classification is anatomical:
Table 19.
Statements pertaining to desmoid tumours—extended version
| Statements |
|---|
| Diagnosis and screening |
| DTs.1 Value of classifications—can they help guide treatment? The different classifications can help in the choice of treatment; however, they must be strongly related to the clinical presentation and evaluation by the physician. |
| DTs.2 Should know FAP patients with high risk factors undergo abdominal desmoid screening (before surgery)? Preoperative screening for desmoid tumour appears more relevant in patients who already had abdominal surgery as it might find a DT that can have impact on the surgical options choice. |
| DTs.3 Should FAP patients undergo a post-colectomy screening programme for abdominal desmoid tumours? There is no evidence in the literature that a screening programme for desmoid tumour detection after abdominal surgery should be proposed. Moreover, with the actual possible treatment and the unpredictable evolution of DT, such a screening programme might not be needed. |
| DTs.4 Is a confirmatory biopsy required for the diagnosis of an intra-abdominal or abdominal wall desmoid in an FAP patient? Confirmatory biopsies may be considered if there is a diagnostic dilemma or required to initiate medical therapy. |
| DTs.5 Should the diagnosis of DT in a patient without known FAP mandate exclusion of FAP? In a patient with desmoid tumour/s without known FAP, screening of FAP (at least with colonoscopy and APC mutation testing if possible) should be performed. This is especially important among patients <60 years, or with intra-abdominal desmoids or in the abdominal wall. |
| Treatment |
| DTs.6 Which desmoid requires treatment? Rapidly enlarging and life-threatening desmoid tumour requires first-line aggressive treatment. Others should be surveyed in a watch-and-wait protocol. |
| DTs.7 Is surgery the ideal treatment for desmoid tumours? Surgery could not be considered the ideal treatment for desmoid tumours, except in the case of DT complications, rapidly growing or life-threatening. |
| DTs.8 Should patients at high risk for desmoids receive chemoprevention after colorectal surgery? There is currently no evidence to support the use of chemoprevention for high-risk patients undergoing surgery or in post-surgical care. |
| Management for DTs identified during abdominal surgery |
| DTs.9 What is the ideal strategy in patients with intraoperative findings of an unexpected desmoid tumour or precursor lesion(s)? A: We recommend continuing with the intervention (proceeding with the surgical procedure) if technically feasible. B: Resection of mesenteric desmoid(s) should be avoided if it will result in sacrificing any small bowel. |
| DTs.10 Can desmoid tumours modify the strategy of prophylactic (procto)colectomy? Desmoid disease can potentially render restorative procedures technically challenging or impossible. In cases where it is feasible, restorative procedures should be cautiously considered and selectively recommended for patients with concomitant intra-abdominal desmoid tumours following prophylactic (procto)colectomy, taking into account the significant risk of desmoid recurrence and adhesion formation. In such circumstances, proctocolectomy with terminal ileostomy may represent the safest option. It is important to have a thorough discussion with the patient about the potential risks of compromised function and the possibility of requiring additional surgeries, ensuring that the choice is individualized to their specific situation. |
| DTs.11 What is the incidence of desmoid tumours at the site of an ileostomy? The risk of desmoid tumours has not been evaluated on a systemic scale. When feasible, single-stage proctocolectomy is preferred for FAP patients in order to avoid desmoid tumours. |
DT, desmoid tumour; FAP, familial adenomatous polyposis.
extra-abdominal DTs
-
intra-abdominal DTs which can be further classified as:
-
–
mesenteric DTs
-
–
extra-mesenteric DTs
-
–
mixed DTs (with intra-abdominal plus abdominal wall)
The intra-abdominal DTs can be also classified according to Church’s classification339 as reported in Table 20.
Table 20.
Church classification according to table by Church et al.
| Stage | |
|---|---|
| I | Asymptomatic, <10 cm maximum diameter, and not growing |
| II | Mildly symptomatic (sensation of mass, pain, but no restriction), <10 cm maximum diameter, and not growing |
| III | Moderately symptomatic (sensation of mass, pain, restrictive but not hospitalized) or bowel/ureteric obstruction, or 10–20 cm, or slowly growing |
| IV | Severely symptomatic (sensation of mass, pain; restrictive and hospitalized), or >20 cm, or rapidly growing |
The clinical course of DT can be variable and unpredictable, with different patterns observed: stable course (50%), rapid progression (10%), cycles of regression and growth (30%), spontaneous regression (10%)184. This variability can complicate the use of classifications as a cornerstone in DT treatment decisions. However, a study by Inoue et al.189 suggests surgical management as the primary treatment for extra-abdominal disease. In this case, the anatomical classification can guide therapeutic choices. In the first staging classification proposed by Church et al., surgery is recommended as the first-line therapy for stage I DT (incidentally found during surgery) and also for stage II340. However, it is now known that trauma, including surgical procedures, can trigger tumour progression. Considering the high morbidity and mortality rates associated with extensive intestinal resection, non-surgical treatments remain the preferred approach for patients with mesenteric DT. The clinical presentation of DT can vary widely, and may even include life-threatening complications such as sepsis, perforation or haemorrhage, presenting a significant challenge for clinicians.
DTs.2
There are no reports on this matter; however, mesenteric DTs can pose a surgical challenge as they may impede an ileal pouch anastomosis. Mesenteric DTs are rare in patients who have not had surgery, but can be asymptomatic and are more frequent in patients who have undergone previous surgery341. Preoperative screening for patients who have never undergone surgery may seem irrelevant; however, in patients with previous abdominal surgery, such screening might aid in surgical planning. Additionally, it will help surgeons explain the risk of a permanent stoma to the patient. For instance, a patient who underwent an ileo-rectal anastomosis and subsequently develops rectal cancer may require a definitive ileostomy if a mesenteric DT restricts the small bowel from reaching the anus due to the insufficient length of the superior mesenteric artery as a result of mesenteric shortening caused by the DT.
DTs.3
DTs have an unpredictable evolution. While some may disappear, others can grow aggressively and become life-threatening. The objective of a screening programme should be to identify individuals at higher risk of developing DTs, enabling early diagnosis and treatment to reduce the progression of aggressive tumours. However, currently, there is no treatment with limited side effects available for early-stage DT. Treatments are typically offered when DT are considered aggressive and symptomatic. Furthermore, specific research on this matter is lacking342. Only a few retrospective analyses have been conducted to screen FAP patients after surgery aiming to identify asymptomatic DT development38,343. Retrospective analyses of patient cohorts have been published to determine the most effective radiological examination for detecting DTs344,345. In a limited series, the Saint Mark‘s group found that MRI detected two DT that were missed by CT scans345. Therefore, if a screening programme were to be tested, MRI should be considered as the screening modality.
DTs.4
There are no RCTs or prospective studies comparing FAP patients with a suspected DT who undergo diagnostic biopsy versus those who do not. Guidelines assessing diagnostic and surgical approaches for patients with suspected DTs have not specifically focused on FAP since 2022346. Our expert panel suggests that a diagnostic biopsy to confirm a DT should not be routinely performed in patients with a clinical and/or genetic diagnosis of FAP, especially if the biopsy does not serve a differential diagnostic purpose. This opinion is in line with the recent guideline publication by the French intergroup347. Similarly, Improta et al.198 recommend limiting biopsy to specific cases, such as when there is a differential diagnosis with carcinoma or adenopathy that cannot be resolved through imaging. In such cases, if feasible, a CT biopsy should be preferred over a surgical biopsy. In situations where planned surgery (for example, during prophylactic colectomy in FAP patients) reveals an incidental finding of an intraperitoneal or mesenteric mass highly suggestive of a DT, surgical biopsy should be avoided.
DTs.5
In the context of FAP, studies have shown that between 10% and 30% of patients will develop desmoid disease185–187. In contrast, the development of desmoid disease outside of FAP is rare, with an estimated incidence rate of 2–4 per million people per year188. Several studies have investigated the need for investigation for FAP in patients with apparently isolated DTs348–351. The incidence varies between studies, ranging from 1.8%348 to 15%349. Some predictive risk factors for FAP in patients with DTs have been reported, including younger age and the presence of intra-abdominal or abdominal wall tumours350–352. Although supported by limited evidence, there is a general consensus on the need for FAP screening among patients with apparently isolated desmoid-type fibromatosis. Because these patients carry a significant risk of underlying FAP, it is highly recommended to exclude this genetic disorder through APC mutation testing and colonoscopy, especially in patients with the aforementioned risk factors. Sequencing of apparently sporadic DTs for CTNNB1 somatic mutations may also help rule out FAP and potentially defer the need for colonoscopy.
DTs.6
Treatments for DTs have not been evaluated in RCTs, and there are mixed data available on the treatment of desmoids in both FAP and non-FAP patients. Bhandari et al. have proposed a flowchart for the treatment of DTs with air-fluid level, which outlines a possible management approach for patients with a specific type of desmoid, regardless of size or previous classifications353. This approach is particularly useful in urgent cases where patients present with symptoms related to the DT, rather than cases where the detection of a DT occurs during follow-up. In a review by Bonvalot et al.190, a stepwise approach is proposed (see Fig. 7), and the main indication for treatment is rapidly enlarging DTs or tumours located in critical anatomical areas (for example, neck, limb girdles). Additionally, Ophir et al.191, in their case series, reserved surgery for patients with complications related to DTs and demonstrated a high rate of recurrence among patients who underwent surgery. These findings were further supported by a case series conducted by Xhaja and Church192. The available evidence is limited, and based on expert opinions, management of most DTs with a watch-and-wait protocol is suggested, if feasible. However, a more aggressive approach may be warranted based on the clinical presentation and disease progression.
DTs.7
There are no RCTs in the literature about the ideal treatment of DTs354. Surgery is known to be associated with significant complications (such as haemorrhage, bowel perforation, short bowel syndrome and significant loss of function), morbidity, and mortality190,193,194. Moreover, the evolution and response of DTs after treatment are unpredictable, as a high percentage of cases reach a stabilization period but are prone to recurrence after surgery355. Therefore, surgery cannot be considered the ideal treatment for DTs193,356,357. Nevertheless, primary surgery could be considered for well-defined tumours of the abdominal wall that can be safely resected189,199–204. Surgical resection of mesenteric DTs could be considered for those resistant to medical strategies or radiation therapy or if they are rapidly growing or life-threatening195–199. Surgery is also indicated in cases of DT complications, such as bowel occlusion or perforation, ureteric obstruction, entero-cutaneous fistula, or mesenteric ischaemia200,358. When surgery is performed, efforts should be made to preserve function. When desmoid complications occur, if tumour excision would result in major small bowel sacrifice or other morbidity, it would be preferable to leave the tumour in place359.
DTs.8
To date, there are no studies suggesting the effectiveness of chemoprevention in the development of DTs in patients with FAP. Additionally, no study has evaluated the role of chemoprevention after colorectal surgery, even in high-risk patients such as female patients or those with a family history of DTs. Therefore, it is not possible to provide an answer to the question. Only future studies that compare the use of chemoprevention after colorectal surgery in high-risk patients with those who do not receive such therapy will be able to evaluate any potential benefits of chemoprevention in patients with FAP after colonic resection.
DTs.9
Unexpected DTs may be discovered during elective surgery for FAP. However, instead of investigating the optimal strategy for patients with an unexpected finding of DTs, the majority of researchers have focused on determining the best surgical procedure for patients with FAP who are at high risk of desmoid formation. It is also not uncommon to encounter precursor lesions during elective colorectal prophylactic surgery. These lesions are characterized by fibromatous, white-pearl-coloured plaques, primarily located in the mesentery. Elective proctocolectomy has been recommended for patients with FAP and a high risk of desmoid formation, based on the concern that future proctectomy may be hindered by the presence of DTs36. However, Church et al. conducted a retrospective analysis of 67 FAP patients at high risk for desmoid formation (39% in their cohort) who had previously undergone colectomy and were selected for proctectomy due to uncontrollable adenomas, cancer, or high-grade dysplasia360. Proctectomy was always feasible, although desmoid disease affected the surgical approach in 19% of cases and led to the interruption of IPAA in 10% of cases. They concluded that even in patients at high-risk for desmoid disease, the fear of an unresectable rectum or an impossible anastomosis should not be the sole indication for proctectomy. In a group of 140 FAP patients (without considering the risk of desmoid formation), <2% were unable to undergo proctectomy due to desmoid disease79. Kartheuser et al. proposed a different approach361. There is evidence that surgical trauma, especially at a young age, can induce the formation of desmoids. Therefore, in highly selected patients at risk of desmoid formation, such as those with a strong family history or with mutations after codon 1400 in the APC gene, elective colectomy may be postponed, and the disease can be managed through close surveillance and chemoprophylaxis until surgery becomes necessary45,361. In other words, delaying prophylactic surgery may be appropriate for compliant patients with attenuated FAP and risk factors for desmoid development. In conclusion, there is currently no solid published evidence suggesting the ideal strategy for patients with an intraoperative finding of unexpected desmoid disease. If, in consultation with the patient, it has been decided that surgery is the next step in FAP management, either due to symptoms or the extent and severity or nature of colorectal disease, it is reasonable to proceed with the intervention if technically feasible, even if modifications to the surgical approach are required. Although further investigation is needed, it appears that proctectomy may not be indicated solely based on the concern of an unresectable rectum in the future due to desmoid formation. Prophylactic surgery may be postponed for compliant patients with attenuated FAP and risk factors for desmoid development.
DTs.10
A recent systematic review found that up to 19% of patients may experience (re)recurrence following surgery for primary (17.7%) or recurrent (34%) desmoid disease, with the majority of recurrences occurring within the abdomen362. This information should be discussed with patients, and in cases where patients have concomitant intra-abdominal DTs, it may be more prudent to discourage restorative proctocolectomy due to the risk of recurrence and the higher risk of desmoid formation associated with ileal pouch construction205. Even a colectomy with ileo-rectal anastomosis, if technically feasible despite the desmoid, should be advocated with caution as the risk of recurrence and potential occurrence of rectal cancer are not eliminated. It has been reported that up to 38% of patients may have concomitant desmoids when the rectum needs to be removed after a colectomy360, and in 30% of cases, this could make subsequent pouch construction impossible186,360. Additionally, the potential for increased adhesion formation should be considered when deciding on restorative procedures in patients with desmoids. Studies have shown a correlation between the severity of adhesions and desmoid disease in patients with familial adenomatous polyposis, which could negatively impact function and complicate future surgeries. Therefore, a total proctocolectomy with terminal ileostomy may be the safest option. From a technical perspective, DTs in the mesentery can pose challenges during proctocolectomy and pouch construction. The presence of a DT may cause the small bowel to retract or make the mobilization of the superior mesenteric artery difficult. In such cases, restorative proctocolectomy may be impossible due to the inability to create a pouch that reaches the anus.
DTs.11
Two reports from the same team presented at a congress highlight a significant risk of DT development at the site of a diverting stoma created to protect an ileal pouch anastomosis363,364. Despite these reports, it is not possible to establish the true incidence of DTs at the site of an ileostomy. These findings provide further support for avoiding diverting stomas whenever possible. However, it should be emphasized that only one team has specifically reported the risk of developing DTs at the stoma site.
Section IV: Other extra-colonic manifestations
OEM.1
The exact incidence of thyroid cancer among FAP patients is still unclear, as different studies have assessed the prevalence of thyroid cancer among FAP patients and in various populations (Table 21)206–208,211–214,219,365–371. The reported prevalence ranges from 1.5%206 to 12%207. In a review by Chenbhanich et al.208, 12 studies206,211–213,217,367,371–376 were analysed, revealing a pooled prevalence of thyroid cancers of 2.6% (95% c.i. 1.3–4.8)208. However, the authors noted in the comments section that studies with a screening ultrasound programme and those published after 2002 reported a higher prevalence of thyroid cancer (5%, I2 = 83%) compared to studies published before or during 2002 (1%, I2 = 83.8%)208. This finding was also observed by Sada et al.214. Several factors likely contribute to the increasing lifetime risk of thyroid cancer among FAP patients:
Table 21.
Statements pertaining to familial adenomatous polyposis-related other extracolonic manifestations (OEM)—extended version
| Statements |
|---|
| Thyroid |
| OEM.1 What is the lifetime risk of thyroid cancer in FAP patients? The lifetime risk of thyroid cancer in FAP patients ranges between 1.5% and 12%. |
| OEM2 When and how should surveillance for the thyroid be performed? A: Thyroid surveillance, when performed, should include physical examination and thyroid ultrasound. B: Thyroid screening, if performed, can be initiated at the age of 16 in females and in adulthood in males. C: When the baseline thyroid ultrasound is negative, we suggest a screening interval of 2–3 years. |
| OEM.3 Which FAP patients are at a higher risk for developing thyroid neoplasia? Patients at higher risk for developing thyroid cancer include:
|
| OEM.4 When should we screen for FAP in a patient diagnosed with ‘papillary thyroid carcinoma’? The diagnosis of FAP should be considered in female patients <35 years old, with a diagnosis of cribriform-morulae variant of papillary thyroid carcinoma. |
| Adrenal gland |
| OEM.5 What is the lifetime risk of developing adrenal gland cancer in FAP patients? While adrenal mass incidence is 2–3 times higher in FAP patients compared to the general population, the development of adrenal gland cancer or pheochromocytomas is rare. |
| OEM.6 What is the risk of developing adrenal gland adenoma (incidentalomas), unilaterally or bilaterally, in FAP patients? The reported proportion of patients with FAP who have adrenal incidentalomas ranges between 7% and 26%, which is 2–3 times higher than in the general population. |
| OEM.7 Do adrenal lesion(s) require further diagnostic intervention? A: The detection of an adrenal incidentaloma requires evaluation for both radiologically suspicious features and hyperfunction, regardless of the patients’ characteristics but according to international guidelines for incidentaloma. B: All patients with detected adrenal gland lesions should be referred to a specialized endocrinology clinic. |
| Pancreas |
| OEM.8 What is the lifetime risk of developing a pancreatic neoplasia in FAP patients? The lifetime risk of developing pancreatic cancer in FAP patients could be less than 2%. |
| Gallbladder |
| OEM.9 What is the lifetime risk of developing gallbladder neoplasia in FAP patients? The lifetime risk of the occurrence of gallbladder neoplasia (adenoma/carcinoma) has not been investigated so far. |
| Liver |
| OEM.10 What is the lifetime risk of developing hepatoblastoma in FAP patients? The lifetime risk of developing hepatoblastoma in FAP patients is approximately 2%, with the highest incidence occurring in the age group of 1–4 years. |
| OEM.11 When and how should surveillance for hepatoblastoma in FAP patients be performed? A: There are insufficient data to prove that hepatoblastoma screening increases survival. B: If screening is performed it should start from birth and be performed every 6–12 months until the age of 5. |
| Brain |
| OEM.12 What is the lifetime risk of developing a brain tumour in FAP patients? There is insufficient evidence available to report on the lifetime risk of developing a brain tumour in FAP patients |
| Eyes |
| OEM.13 Should people with a diagnosis of CHRPE be investigated for FAP? People with multiple unilateral or bilateral lesions require germline testing for FAP. If germline testing is negative, a single colonoscopy should be considered in early adulthood. |
| Skin |
| OEM.14 When should screening for FAP be considered in a patient presenting with fibromas and epidermoid cysts? There is currently insufficient evidence to establish the cost-effectiveness of screening individuals with fibromas and epidermoid cysts for FAP. |
| Bones |
| OEM.15 Should patients with osteoma be screened for FAP? In patients with osteoma(s) FAP should be considered. |
| Gynaecological manifestations |
| OEM.16 What is the lifetime risk of developing gynaecological cancer in women with FAP? There are very limited data as to the incidence of gynaecological cancers in FAP carriers. Based on these limited data there does not seem to be an increased risk of gynaecological cancer in FAP carriers. |
| OEM.17 What are the risk factors for developing gynaecological cancer in FAP patients? A: There is no evidence as to identify specific risk factors for the development of gynaecological cancers in FAP carriers. Women with FAP should be advised to maintain a healthy lifestyle and weight. B: Female FAP carriers seeking contraception should be advised as to the reduced colorectal cancer risk in those who use oestrogen-based contraceptives. |
| OEM.18 Is there an effective form of gynaecological cancer surveillance for women with FAP? A: Gynaecological cancer surveillance should be as for the general population in women with FAP. B: Women with FAP, like women generally, should report any abnormal symptoms suggestive of gynaecological cancer to their family doctor urgently. These symptoms include:
|
| New-onset nausea and vomiting OEM.19 Does FAP have an impact on female fertility? A: There is no evidence that FAP in and of itself leads to reduced female fertility. B: Women of child-bearing age who are diagnosed with cancer should be referred to a fertility specialist to discuss their options in a timely manner. C: There is no convincing evidence showing different fertility outcomes between IPAA and IRA. D: Women who have undergone risk-reducing surgery and have not got pregnant within a year of trying should be referred to a fertility specialist. |
| OEM.20 What risk factors can impact on childbirth in a patient with IPAA? The impact of childbirth in a patient with IPAA has not been evaluated so far. No risk can be assessed on the impact of childbirth. |
CHRPE, congenital hypertrophy of the retinal pigmented epithelium; FAP, familial adenomatous polyposis; IPAA, ileal pouch anal anastomosis; IRA, ileorectal anastomosis.
The increased life expectancy of FAP patients following the introduction of prophylactic proctocolectomy209.
Enhanced attention and screening for thyroid diseases among FAP patients208.
The general population increase in thyroid cancer incidence210.
In conclusion, FAP patients appear to have a higher risk of developing thyroid cancer compared to the general population. However, there is no strong evidence to support a significant improvement in prognosis or the cost-effectiveness of a screening programme (see OEM.2 and OEM.3).
OEM.2
The impact of thyroid screening on the survival of FAP patients with thyroid cancer remains unclear206. However, it is evident that the lifetime risk of developing thyroid cancer is higher in FAP patients compared to the general population (see OEM.1). Nevertheless, there is currently no solid evidence supporting the effectiveness of an intensive screening programme. In light of this, the authors advocate a more appropriate approach, focusing on patient education. It is crucial to ensure that patients are aware of their increased risk of developing thyroid cancer compared to the general population. By fostering this awareness, patients can be vigilant and attentive to any signs or symptoms related to thyroid pathology. Furthermore, conducting physical examinations, which incur minimal costs, can be incorporated into the regular follow-up routine for FAP patients. Thyroid ultrasound and physical examinations are considered safe strategies for evaluating the presence of thyroid nodules and can be specifically tailored to patients with identified risk factors (see OEM.3). The age at which thyroid cancer is diagnosed in FAP patients varies across different studies, ranging from 26 to 42 years, depending on the specific population under examination206,211–215. Notably, Smith et al. observed thyroid cancers in patients as young as 17.2 and 19.8 years215. We suggest that if thyroid screening is performed, this should include physical examination and ultrasound scan. Furthermore, Smith et al. indicated that young patients with a negative thyroid ultrasound are unlikely to develop thyroid cancer within at least 4–5 years215. Although Herraiz et al. reported a median follow-up of 15 months and the third examination at 27 months206, it should be noted that their study was not prospective. Monachese et al. evaluated the outcomes of a 5-year thyroid ultrasound screening programme among 264 FAP patients and found that the only patients who developed cancer (2.3%) were those with baseline thyroid nodules366. They concluded that if ultrasound screening is performed, the interval could be extended to 2 years until nodules are detected. Taking this into account, it seems reasonable to reserve ultrasound investigations for specific patients, adjusting the follow-up interval based on symptoms and the results of the baseline screening, considering the factors mentioned above. In suspected cases of thyroid cancer, we recommend performing fine-needle aspiration biopsy (FNAB) in accordance with regional guidelines to confirm the diagnosis and exclude malignancy.
OEM.3
In recent years, several studies have focused on evaluating the risk factors for thyroid cancer in FAP patients. Steinhagen et al. reported a 4% rate of papillary thyroid carcinoma and identified women as being particularly at risk211. A multicentre study conducted in Japan, which included more than 280 patients, found an overall thyroid cancer rate of 6.4%, with rates of 1.4% in men (aged 39–57 years) and 11.4% in women (aged 17–41 years)214. In addition to gender, a young age at the time of FAP diagnosis (specifically, <33 years old in this study) was identified as an independent risk factor. Uchino et al. reported a thyroid cancer rate of 16% in women and 0% in men in a cohort of 129 FAP patients, most of whom had the cribriform-morula variant of papillary carcinoma219. An analysis of a Dutch registry comprising 582 APC mutation carriers found a thyroid cancer rate of 1.5%207. Among the nine patients with thyroid cancer, seven were female, representing 2.4% of all female patients, with a mean age of 33.5 at diagnosis. Kennedy et al. reported the diagnosis of papillary thyroid cancer in five patients, accounting for 3.1% of the study population. The average age at which these patients were diagnosed was 20.8 years, with a range of 19–23 years38. Monachese et al. undertook a 5-year thyroid ultrasound screening programme among 264 FAP patients and found that the only patients who developed cancer (2.3%) were those with baseline thyroid nodules366.
OEM.4
The CMV of PTC is a rare subtype that is associated with FAP. CMV accounts for approximately 0.16% of all PTC cases216, but its prevalence is higher in FAP patients, reaching up to 90% in some reports217. In an article by Levy et al., it was reported that nine patients with a diagnosis of CMV-PTC, but without a known diagnosis of FAP, were recommended to undergo screening. Among the six patients who underwent colonoscopy, one (17%) was diagnosed with FAP based on the presence of a polyposis phenotype and subsequent confirmation with genetic testing377. It has also been emphasized that CMV-PTC is more commonly found in younger female FAP patients, especially those who are 35 years old or younger219. A retrospective study by Park et al. revealed that all FAP-associated CMV-PTC patients had multifocal tumours218. Based on these findings, we recommend considering a diagnosis of FAP (through genetic testing or colonoscopy) in females <35 years old who have been diagnosed with CMV-PTC, particularly when multifocal tumours are present.
OEM.5
The reported proportion of FAP patients with adrenal adenomas is 2–3 times higher than in the general population220. However, the development of adrenal gland cancer or pheochromocytomas in FAP patients appears to be rare. In a historical cohort study by Kallenberg et al., 26% of FAP patients had adrenal lesions, but only one of them had uncertain malignant potential226. The study concluded that adrenal incidentalomas in FAP patients are common, benign and slowly progressive, and they do not exhibit hyperfunctionality. Sporadic cases of adrenocortical cancer and pheochromocytomas have been reported among the FAP population, but they are infrequent221–225. In a Canadian registry analysis of over 300 FAP patients, Shiroky et al. found a 16% rate of adrenal mass, with more than 96% of the cases being benign220. Only one patient had adrenocortical carcinoma, and another had an adrenal neuroblastoma220. Based on these findings, no surveillance strategy for adrenal cancer in FAP patients was recommended220.
OEM.6
Previous reports have indicated an incidence of 7–13% for adrenal incidentalomas in patients with FAP378. More recently, Kallenberg et al. found that 26% of FAP patients developed adrenal lesions226. In a Canadian registry analysis including more than 300 FAP patients, Shiroky et al. reported a 16% rate of adrenal mass220. Will et al. conducted a study involving 30 FAP patients and found no association between the development of adrenal incidentalomas and factors such as gender, genotype or family history222.
OEM.7
Adrenal incidentalomas in FAP patients require thorough investigation. Various management strategies have been proposed, primarily based on national and international guidelines222,379. However, the patient should undergo a comprehensive examination and evaluation using simple tests, including blood pressure assessment and the assessment of signs and symptoms that may indicate the presence of a pheochromocytoma, Cushing’s syndrome or Conn’s syndrome. Additionally, the collection of urinary catecholamines over a 24-hour period and blood tests are necessary. If any suspicious findings are detected in these tests or radiological images, it is essential to refer the patient to a specialist centre for appropriate management.
OEM.8
A slightly increased risk of pancreatic neoplasia has been suggested for patients with APC mutations380. The reported frequency of pancreatic tumours in FAP individuals is less than 2%. In a Dutch registry analysis involving 582 mutation carriers, the observed risk for pancreatic cancer was 0.3% compared to a pancreatic cancer risk of 0.01% in the general population381. A more recent study detected a significantly higher risk of pancreatic cancer in patients with FAP compared to controls (HR: 6.45, 95% c.i. 2.02–20.64, P = 0.002)382.
OEM.9
After conducting an extensive review of the literature following the established methodology for this collaborative effort, no articles addressing the lifetime risk of developing gallbladder neoplasia (adenoma/carcinoma) in FAP patients could be found. Unfortunately, there is a lack of available literature to provide relevant answers to this question. Therefore, it is not possible to establish evidence-based recommendations regarding the lifetime risk of gallbladder neoplasia in FAP patients. Further research in this area is warranted to gain a better understanding of the risk of gallbladder neoplasia in FAP patients.
OEM.10
In a recent review the incidence of hepatoblastoma in children with FAP was reported to be approximately 2.5%229. Other studies have reported varying rates of incidence, with some studies showing lower rates and others showing higher rates in certain cohorts207,231,383. The median age at diagnosis of hepatoblastoma has been reported to be 18 months in different studies38,207. Some studies indicate a relative risk of 847.0 and an absolute lifetime risk of 1.6% in FAP patients230.
OEM.11
While the correlation between FAP and the increased risk of developing hepatoblastoma is well described, the existence of an appropriate surveillance programme is still debated231. Paediatric guidelines do not recommend screening for hepatoblastoma in FAP patients37. In a recent review, the reported age at diagnosis of hepatoblastoma ranged from birth to 11.6 years of age, with a median age of 20 months. Three patients were diagnosed at or shortly after birth. Fourteen of 90 patients with hepatoblastoma had a known family history of FAP229. Trobaugh-Lotrario et al. suggest that screening should be strongly considered from birth by performing alpha-fetoprotein (α-FP) and abdominal ultrasound every 3 months229. Aretz et al.231 argue that screening can increase survival and reduce chemotherapy-related side effects. The use of both sonographic and α-FP examinations can help reduce the rate of false positives. However, it is important to note that frequent surveillance may be stressful and painful for children, and no risk factors have been identified so far to determine which population would benefit the most from the screening programme.
OEM.12
The existing studies38,207,368 on this topic have low quality and involve a small number of patients, which makes it challenging to draw reliable conclusions and provide evidence-based recommendations. Further research is needed to assess the lifetime risk of brain tumours in FAP patients.
OEM.13
CHRPE is a retinal pigmented lesion that can serve as the earliest and most common extra-intestinal manifestation of FAP. The prevalence of CHRPE can vary based on the studies and populations analysed, but it is generally estimated to be around 80%232–236. According to a systematic review conducted by Rehan and Aye233, CHRPE alone cannot be relied upon as a sole indicator for diagnosing FAP in individuals with a positive family history. Instead, a combined approach involving eye examinations, colonoscopy, and genetic testing is recommended. Currently, type B lesions, characterized as small round pigmented dots, appear to be the most frequently observed type of CHRPE associated with FAP. Other significant features include the presence of three or more lesions and bilateral lesion.
OEM.14
After conducting an extensive literature review using the established methodology for this collaborative effort, no articles addressing the cost-effectiveness of screening individuals with fibromas and epidermoid cysts for FAP were found. Regrettably, there is a scarcity of available literature that can provide relevant answers to this question. In a study by Burger et al.236, the prevalence of skin lesions in FAP patients was evaluated. The results revealed that approximately 48.2% of FAP patients had at least one FAP-associated skin lesion, while only 34.5% of the control group had such lesions. Despite the higher prevalence compared to the general population, it is insufficient to justify implementing a screening strategy for FAP based solely on the presence of skin lesions.
OEM.15
Due to the high incidence of osteomas in FAP patients38,237 and especially oral osteoma237 the diagnosis of FAP should be considered, when compared to the normal occurrence of osteoma in the healthy population, the diagnosis of FAP should be considered237,238.
OEM.16
There is a lack of evidence exploring the incidence of gynaecological cancer in women with FAP. No prospective studies were identified that could define the incidence of gynaecological cancer in FAP carriers. The Cancer Genome Atlas (TCGA) was a landmark series of studies that reported the results of genome-wide analysis for major gynaecological cancers (endometrial, ovarian, cervical cancers). In these cancers, APC mutations were rarely observed384–386. Only one germline pathogenetic variant of the APC gene was found in cervical cancer, but this is likely to be of no aetiological significance as cervical cancer is almost exclusively driven by the human papilloma virus (HPV) and not a heritable disease387. Similar work by Ring et al. found that of 381 unselected women with endometrial cancer, one had a pathogenetic variant of the APC gene239. In a Chinese cohort of endometrial cancer (n = 79), none harboured a germline pathogenetic variant of the APC gene240. These results are echoed by Lincoln et al. in that no germline pathogenetic variant of the APC gene were found in 118 ovarian cancers or 104 endometrial cancer241. A smaller study exploring hereditary risk in endometrial cancer found that of 156 unselected affected women, 5 had the APC I1307K polymorphism242. This would suggest a 3% incidence of carriers of this variant, which would be similar to the prevalence of Lynch syndrome in endometrial cancer, an inherited cancer predisposition closely associated with the disease388. However, although reported to be an unselected cohort of endometrial cancers, this study by Cadoo et al. has an abnormally high proportion of women with an Ashkenazi Jewish background (24%)242.The APC I1307K polymorphism is found in around 10% of people with Ashkenazi Jewish heritage389. Of the five women found to have the APC I1307K polymorphism, four were Ashkenazim. Furthermore, the APC I1307K polymorphism is thought to be only of moderate penetrance390. Therefore, it remains unclear if the finding reported in Cadoo et al. is an artefact or could represent an association between FAP and endometrial cancer. Of note, FAP does seem to be associated with CRC metastasis to the ovary. Work by Crobach et al. found 13% of women (4/30) with a CRC ovarian metastasis had FAP243. This is higher than expected and may suggest that the biology of FAP-related CRC predisposes it to ovarian spread. The authors of this study recommended that bilateral salpingo-oophorectomy is considered at the time of CRC resection. However, this study only suggests an increased incidence of ovarian metastasis in FAP carriers and not an increased incidence of ovarian cancer. In sum, there exist no prospective data sets by which to define the risk of gynaecological cancer in FAP. Large cohorts of women with gynaecological cancers who have undergone germline sequencing indicate that FAP is not consistently associated with gynaecological cancers. A large prospective database of FAP individuals is needed, however, before a robust estimate of gynaecological cancer lifetime risk in FAP can be made.
OEM.17
There is no literature on the risk factors for developing gynaecological cancer in FAP carriers. However, the risk factors for gynaecological cancers in non-FAP carriers are well established. Endometrial cancer is associated with unopposed oestrogen exposure391. This is commonly because of a raised BMI, which leads to the peripheral conversion of androgen precursors to oestrogen392. Indeed, endometrial cancer is the most strongly associated cancer with raised BMI393. Therefore maintaining a healthy BMI is vital when aiming to reduce an individual’s risk of endometrial cancer. Other modifiable risk factors include the use of hormonal-based contraception, breastfeeding, pregnancy, avoidance of tamoxifen and prevention of diabetes394–396. Modifiable risk factors for ovarian cancer in the general population include obesity, hormone replacement therapy and nulliparity397. As with endometrial cancer, the use of hormonal contraception is thought to be protective against ovarian cancer397. For cervical cancer, factors that reduce an individual’s exposure to HPV or increase their ability to clear HPV are seen as protective. The most important modifiable risk factor is smoking, which increases a woman’s risk of cervical cancer398. Other modifiable risk factors include poor attendance of screening programmes, immunosuppression, high numbers of sexual partners and lack of vaccination399. There is no clear biological reason to suppose these risk factors for gynaecological cancers are not also important in FAP carriers. Clinicians caring for women with FAP should be aware of the theoretical benefit of the use of oestrogen-based contraceptives. Systematic review and meta-analysis level data demonstrate a pooled relative risk reduction of 18% for CRC in women with a history of combined oral contraceptive use244. This benefit is also seen in randomized controlled data exploring the use of hormone replacement therapy (HRT) in postmenopausal women, with a hazard ratio of 0.63 for CRC400, although this needs to be balanced about the risks of combined HRT and should be commenced and monitored by an experienced clinician401. For those who have had a hysterectomy, oestrogen-only HRT can be prescribed, which avoids many of the risks of HRT402 yet still has the benefit of reducing CRC risk403. Oestrogen would seem to be protective against CRC in FAP carriers245. Indeed, total polyp regression has been reported on the commencement of oral contraceptives246. Therefore, female FAP carriers should be counselled as to the benefit of oestrogen-based contraception as a means to reduce their CRC risk.
OEM.18
As described in OEM.16, there is no clear evidence that there is a raised lifetime risk of developing gynaecological cancer in women with FAP. Other than cervical cancer, no gynaecological cancer surveillance programmes have been found to be of benefit in the general population404–406. Indeed, even in women with an established increased risk of gynaecological cancer, such as those with Lynch syndrome, gynaecological cancer surveillance remains controversial407. Therefore, women with FAP should be encouraged to report any concerning symptoms to their family doctor. In addition, they should attend cervical screening when advised to by their doctor or national programme.
OEM.19
Women with FAP may choose to avoid pregnancy, with a proportion of women choosing not to have children so as to prevent the transmission of FAP330. However, self-reported questionnaire-based data would suggest there is no evidence that being a carrier of FAP, in and of itself, decreases rates of conception or live births247. Fertility is affected by the treatments that carriers of FAP are subject to. Women often undergo pelvic surgery during reproductive age. In addition, those diagnosed with cancer may require systemic chemotherapy or pelvic radiotherapy, which can negatively impact an individual’s fertility248. Therefore, before undergoing these interventions, patients should be fully informed of the potential impact on fertility and, where appropriate, be referred to a fertility expert. In addition, where possible, these effects on fertility should be mitigated. Discussions should be had about transposition of the ovaries or ova/embryo freezing before treatment is commenced. These should be led by a fertility expert in conjunction with the oncology team. Those women who have been diagnosed with CRC should be made aware of the increased rate of ovarian metastasis seen in FAP, which could influence their choice around ovarian preservation243. Women with FAP are often offered risk-reducing surgery in the form of total proctocolectomy (TPC) with IPAA or total colectomy with ileo-rectal anastomosis (IRA). Systematic review and meta-analysis level data have concluded that IPAA has a negative impact on female fertility; the risk of infertility triples after the surgery248,329. However, the studies included in these analyses are of varying quality. Sexual function is modestly impacted by the IPAA. A self-reported questionnaire study of 75 sexually active women found 46% had sexual dysfunction after IPAA; the most common issue was dyspareunia330. There is less evidence regarding the impact of IRA on female fertility. A self-reported questionnaire study of 230 women found those who had undergone an IRA had similar levels of fecundity as the general population62. However, a study by Nieuwenhuis et al. in which 137 women were surveyed regarding fertility outcomes found there were similar rates of fecundity regardless of the type of surgery that was performed66. Of note, the authors concluded that the negative impact on fertility as a result of risk-reducing surgery was more pronounced the earlier in life it was performed. The mechanism of infertility following risk-reducing surgery is not clear; it would seem that surgeries that do not involve pelvic dissection do not lead to decreased fertility62. Cornish et al. have suggested it is as a result of tubal occlusion63. This is important, as tubular disease is potentially amenable to assisted reproductive techniques249. Therefore, women who have undergone risk-reducing surgery for FAP and have not conceived after one year of regular unprotected penetrative vaginal intercourse should be referred to a fertility specialist.
OEM.20
After an exhaustive literature review, no articles dealing specifically with the subject were found. To date, we have not been able to identify any risk factors concerning childbirth in patients with FAP undergoing colorectal surgery. Future multicentre studies are desirable to evaluate this topic.
Section V: chemoprevention
CP.1
The standard management of patients with FAP typically involves prophylactic surgical resection of the colon (Table 22). However, there may be a potential role for CP in delaying the need for colectomy, preventing cancer development in the upper GI tract (particularly the duodenum) and preventing cancer development in the retained rectum in patients who have undergone colectomy with ileo-rectal anastomosis. Most CP trials in FAP patients have focused on polyp-related outcomes or measured the time to disease progression, as these outcomes can be evaluated over relatively short periods of time. Only a few studies have investigated the effects of CP on cancer development, especially CRC, due to the early surgical removal of the colon. Two case reports have described the development of rectal carcinomas in FAP patients after starting courses of sulindac for CP. It is worth noting that both patients had previously undergone prophylactic colectomy408,409. In a study by Burke et al.250, the efficacy and safety of eflornithine and sulindac in combination versus each drug alone were evaluated for the prevention of disease progression in FAP patients. The primary endpoint, assessed using time-to-event analysis, was a composite outcome that included major surgery, endoscopic excision of advanced adenomas, diagnosis of HGD in the rectum or pouch, or progression of duodenal disease. The study included 171 patients who were randomized to receive daily eflornithine (750 mg), sulindac (150 mg), or a combination of both drugs for up to 48 months in a 1:1:1 ratio. Patients were stratified based on the anatomical site with the highest polyp burden and surgical status. The results showed no significant difference in the proportion of patients experiencing disease progression among the treatment groups overall (32% in the combination group, 38% in the sulindac group and 40% in the eflornithine group). Furthermore, there was no significant difference in the mean times to the first event of disease progression between the combination therapy and monotherapy groups, as estimated by the Kaplan–Meier method (ITT population). Importantly, no upper or lower GI cancers developed in any patient during the trial. The Children‘s International Polyposis (CHIP) study specifically included the occurrence of CRC as an outcome within a composite endpoint. Importantly, none of the patients in the trial developed colorectal malignancy251. The objective of this phase 3 RCT was to evaluate the efficacy and safety of celecoxib (200–400 mg twice daily, depending on body weight) compared to placebo in children with FAP over a 5-year treatment period. To be eligible for the study, patients were required to have fewer than 20 polyps larger than 2 mm in size at baseline colonoscopy, and these polyps had to be completely removed before starting the study. The primary endpoint was time to disease progression, defined as the duration from randomization to either the appearance of 20 or more polyps larger than 2 mm in size at any colonoscopy or the diagnosis of a colorectal malignancy. A total of 106 patients were enrolled in the study, with a median treatment duration of 23 months for the celecoxib group and 25.5 months for the placebo group. Among the intention-to-treat (ITT) population, 20 patients met the primary endpoint, all based on the development of polyps. The median time to disease progression was 2.1 years in the celecoxib group compared to 1.1 years in the placebo group. The number of patients meeting the primary outcome of disease progression was twice as high in the placebo arm compared to the celecoxib arm. However, it is important to note that the long-term impact of celecoxib on colorectal polyposis in children could not be evaluated due to the early termination of the trial. The trial was stopped prematurely because of the low occurrence of disease progression.
Table 22.
Statements pertaining chemoprevention in familial adenomatous polyposis—extended version
| Statements |
|---|
| CP.1 Does chemoprevention prevent the occurrence of colorectal cancer? Currently, there are insufficient data to draw definitive conclusions regarding the effect of chemoprevention on the occurrence of colorectal cancer. |
| CP.2 Does chemoprevention prevent the occurrence of small bowel cancer? There is currently no available evidence indicating that chemoprevention prevents the occurrence or progression of small bowel cancer. |
| CP.3 Does chemoprevention prevent the occurrence of gastric cancer? The effect of chemoprevention on the occurrence of gastric cancer has not been investigated so far. |
| CP.4 Is chemoprevention effective in decreasing/regressing the size and number of polyps in the colorectum? Currently, there is insufficient evidence to support the recommendation of chemoprevention for reducing the number and/or size of colorectal polyps in clinical practice. The use of chemoprevention in this context can only be suggested within the framework of clinical trials. |
| CP.5 Does chemoprevention lead to a decrease in polyp size and number in the duodenum? There is currently insufficient evidence to support the recommendation of any chemopreventive agent for decreasing polyp size and number in the duodenum due to the lack of an acceptable risk/benefit ratio. Further trials with appropriate clinically meaningful endpoints are necessary. |
| CP.6 Does chemoprevention delay or prevent colectomy in FAP patients? There is no evidence to support the role of chemoprevention in delaying or preventing colectomy in FAP patients. |
| CP.7 Does chemoprevention delay or prevent risk-reducing surgery in the upper GI tract (pancreas-sparing or pancreatico-duodenectomy) in FAP patients? Chemoprevention does not delay or prevent risk-reducing surgery in the upper GI tract. |
FAP, familial adenomatous polyposis; GI, gastrointestinal.
CP.2
Patients with FAP are at a significantly higher risk of developing small bowel carcinoma, particularly in the duodenum272. However, endoscopic monitoring and therapy for small bowel involvement in FAP can be complex, which highlights the potential importance of additional therapeutic approaches. One option in this regard is the preventive use of medication to reduce the risk. The risk of duodenal cancer is correlated with the severity of duodenal polyposis98,109. Therefore, polyp reduction is often used as a reliable surrogate endpoint when evaluating the effectiveness of CP. There have been a limited number of RCTs investigating the use of single drugs or combinations of drugs to achieve risk reduction in the small bowel250,252–254. However, the observation periods in these trials have been relatively short, and the primary or secondary endpoints have typically focused on polyp reduction in the duodenum as a surrogate marker for cancer risk. Only one study included duodenal carcinoma as a secondary endpoint, but it did not meet the predefined criteria. Consequently, there is currently no sufficient evidence to support the recommendation of CP for the prevention of small bowel carcinoma in patients with FAP.
CP.3
There have been emerging data regarding the risk of gastric cancer in recent years124,163,164. However, endoscopic monitoring and therapy for gastric cancer can be complex, highlighting the potential importance of additional therapeutic approaches. In this context, one option is the preventive use of a drug to reduce the risk. Despite conducting an exhaustive literature review following the established methodology for guideline development, no articles providing relevant answers to this question could be identified. Therefore, it is not possible to make recommendations based on the available literature regarding the preventive use of drugs for reducing gastric cancer risk. Given the emerging nature of gastric cancer risk, this question remains open for potential future investigations.
CP.4
Because in FAP the adenoma–carcinoma sequence is not accelerated but anticipated, the reduction in the number and size of colorectal polyps has been a common outcome in CP clinical trials. Aspirin has been widely suggested as a chemopreventive agent against CRC. Unfortunately, large RCTs in FAP are lacking, and the few available studies have yielded contradictory results. In the largest trial, the CAPP-1 study, no difference was found between the aspirin group and the aspirin plus resistant starch group or the placebo group. However, two studies by Ishikawa et al., although limited by small sample size and adverse events (such as anaemia, aphthae and anastomotic ulcers), showed a reduction in the number and size of colorectal polyps in the aspirin group and a reduction in the recurrence of polyps <5 mm255–257. The combination of sulindac and erlotinib has been tested in a post-hoc analysis of an RCT, which demonstrated a significant reduction in colorectal polyp burden after 6 months of treatment (69.4% net reduction in the treatment group compared to placebo, P = 0.009). Common adverse events included erlotinib-induced acneiform-like cutaneous eruption (68.3%), oral mucositis (32%), nausea (24%) and diarrhoea (24%). This study had several limitations, including a small sample size and early termination in the original study due to achievement of the primary endpoint410. Celecoxib was tested alone and in combination with eflornithine (also known as difluoromethylornitine, DFMO), an irreversible inhibitor of ornithine decarboxylase, in an RCT by Lynch et al. and no significant difference was found in polyp count between the two arms, but the authors observed a significant reduction in polyp burden, weighted by polyp diameter, in the celecoxib plus DFMO arm. Celecoxib was also tested in paediatric patients, resulting in a significant reduction in polyp number (44.2% in the 16 mg/kg/day arm, P = 0.001) with a good safety profile411. Enteric-coated eicosapentaenoic acid as a free fatty acid (EPA-FFA) was tested in FAP patients with ileo-rectal anastomosis for 6 months versus placebo. Through analysis of recorded videos of a specific tattooed area of the rectum, a statistically significant reduction in the number of polyps and polyp burden was reported in the treatment group412. Curcumin, in combination with quercetin to enhance absorption, was studied as a chemopreventive agent in FAP by Cruz-Correa et al. in a small pilot study with five patients, showing a reduction in polyp number and size413. The same authors designed an RCT in which 44 FAP patients were randomized to 100% pure curcumin versus placebo for 12 months, but no significant differences were found in mean polyp number and size414. Sirolimus (also known as rapamycin) was used as a chemopreventive agent in FAP in a pilot study by Roos et al.415. The number and size of colorectal polyps reduced, but only four patients were enrolled and significant adverse events occurred, including diarrhoea, fatigue, dyspnoea, sexual dysfunction and insomnia.
CP.5
As prophylactic colectomy has become the standard of care in managing patients with FAP, the main cause of cancer-related death has shifted to duodenal adenocarcinoma. The lifetime risk of duodenal adenomas approaches 100%, and approximately 4–18% of patients develop duodenal cancer166,416,417. Endoscopic surveillance with polyp resection and prophylactic duodenectomy may offer a prolonged disease-free interval, but the latter is associated with significant morbidity and mortality rates272,418. Therefore, CP could provide an approach to reducing adenoma development and cancer risk, potentially delaying or avoiding the need for surgery. In an RCT testing the COX-2 inhibitor celecoxib at two different doses (100 mg or 400 mg twice daily) versus placebo for 6 months, the high dose showed a significant improvement in duodenal disease (P = 0.033). The assessment was based on qualitative scoring of the full extent of duodenal polyposis258. However, quantitative analysis comparing percentage change in areas of low- and high-density polyposis with placebo did not reach statistical significance. Subset analysis of patients with greater than 5% coverage at baseline showed a marked reduction in duodenal polyposis with the high dose of celecoxib (P = 0.049). Another RCT combined celecoxib (400 mg BD) with ursodeoxycholic acid (UDCA, 1–2 g daily) and assessed the change in duodenal polyp density after 6 months as the primary outcome252. The control group receiving celecoxib/placebo showed a significant decrease in polyp density (P = 0.029), whereas the combination group showed an increase, indicating that UDCA counteracts any benefit of celecoxib. In another RCT, low-dose (10 mg/kg daily) UDCA alone versus placebo demonstrated no effect on the development of duodenal adenomas after 24 months of treatment, as measured by the Spigelman severity score254. However, it is important to note that celecoxib, along with other selective COX-2 inhibitors, is associated with an increased risk of serious cardiovascular side effects. The European Medicines Agency’s Committee for Medicinal Products for Human Use concluded in 2011 that the benefit of celecoxib in FAP patients had not been sufficiently demonstrated and did not outweigh the increased risk of cardiovascular and GI side effects259. Additionally, celecoxib is no longer approved by the US FDA for polyp reduction in FAP patients260.
The non-steroidal anti-inflammatory drugs (NSAIDs) sulindac (150 mg twice daily) and erlotinib (75 mg daily) have been used in combination to simultaneously inhibit COX and EGFR signalling in individuals with FAP253. The RCT, which involved 6 months of treatment, was stopped prematurely after randomization of 92 participants, because a preplanned interim analysis met the prespecified stopping rule for superiority; the change in total duodenal polyp burden, defined as the change in the median sum diameter of polyps, was significantly different between the placebo and sulindac–erlotinib groups at 6 months. Compared to baseline, there was an 8 mm median increase and 8.5 mm reduction in the placebo and combination group respectively (between-group difference, −19.0 mm (95% c.i., −32.0 to −10.9, P < 0.001)). The total duodenal polyp count was also decreased by a median of 2.8 polyps with combination treatment, whereas it increased by 4.3 polyps in the placebo group (between-group difference, −8.0 polyps (95% c.i., −12.2 to −4.7), P < 0.001). Adverse events may limit routine use of these drugs, at least at the doses employed in this trial, because grade 1 and 2 adverse events were more common in the sulindac–erlotinib group, with 87% participants experiencing an acne-like rash, compared to 20% in the placebo group (P < 0.001). Furthermore, 73% of participants in the combination group required dose reduction of erlotinib compared to 28% taking placebo. Dose reduction of sulindac was also more frequent in the treatment group compared to placebo (54% versus 28% of participants). Follow-up studies are needed to evaluate these findings in larger populations, investigate alternative dosing regimens to reduce adverse events and determine whether the observed effects will result in improved clinical outcomes. Sulindac has also been explored in combination with eflornithine, an irreversible inhibitor of the enzyme ornithine decarboxylase, which is involved in polyamine synthesis250. The efficacy of the combination, taken for up to 48 months, was compared with either agent alone and the primary endpoint was a time to event analysis with a composite endpoint to determine delay in progression (including duodenal disease) or major endoscopic or surgical procedures. Overall, the combination offered no significant improvement in disease progression compared to eflornithine or sulindac alone. Although results were reported for a number of parameters relating to duodenal disease progression across different surgical subgroups, comparisons cannot be made between treatment groups. The effect of Eviendep, a patented blend of phytoestrogens plus indigestible and insoluble fibre, on duodenal polyps has been investigated in FAP patients419. The underlying rationale for this small trial was that oestrogen receptor β plays a role in preventing malignant transformation of colon epithelial cells, and this explains the protective effect of oestrogens in cancer development. The study involved 11 patients with IPAA who all received Eviendep (5 mg twice daily) for 3 months after a baseline upper GI endoscopy to assess Spigelman score and remove polyps >10 mm. After 90 days, all patients showed a reduction in the number and size of duodenal polyps; the mean number pre-intervention (after polypectomy) was 25.7, compared with 8 at study end (P = 0.021), and the mean size was 7.6 mm versus 4.4 mm after taking Eviendep. The mean Spigelman score went from 6.4 to 6.6. An RCT by Wallace et al. in 2001 explored whether bile was involved in the pathogenesis of duodenal adenomas in patients with FAP420. Twenty-six individuals were randomized to the H2-receptor antagonist ranitidine (300 mg daily) or placebo for 6 months and the effect on duodenal polyp counts and DNA adduct levels (a form of DNA damage) associated with bile acid exposure were measured. There was no overall difference in appearance, number of duodenal polyps, or Spigelman classification seen between the two treatment groups. There was also no difference in the DNA adduct levels observed, indicating that acid suppression therapy does not improve duodenal polyposis.
CP.6
Colectomy is the gold standard of treatment in FAP, but it is often not well accepted by young patients and can be associated with significant morbidities and a reduced quality of life. Therefore, delaying colectomy to a more mature age could be a reasonable endpoint in order to increase compliance and disease consciousness and to improve quality of life. The only study addressing the delay of colectomy as a clinical outcome is a post-hoc analysis of a randomized phase 3 trial421. The combination of sulindac + eflornithine obtained a 80% risk reduction for disease progression compared to either drug alone, with a 100% risk reduction for major polypectomies (>10 mm), but there are several limitations, including the post-hoc design, the limited sample size and the smaller-than-expected event rate.
CP.7
Patients with FAP have a significantly increased risk of developing carcinoma of the papilla and duodenum272. There are also emerging data on gastric cancer risk in the last few years124,163,164. Endoscopic monitoring and therapy are complex, which is why additive therapy methods may become more important. In this context, one option is the preventive use of a drug to reduce the risk. The risk of duodenal cancer is associated with severity of duodenal polyposis98,109. When endoscopic treatment becomes no longer feasible, duodenal surgery may be considered for Spigelman stages III/IV150. Duodenal surgery is associated with high short-term morbidity and mortality rates153,157,422. Management of gastric cancer risk and risk stratification is still under investigation. In a few RCTs single or drug combinations were used to assess a possible role in risk reduction in the duodenum. There are no data on gastric involvement in chemoprevention trials. However, the observational time period was often short, and also the primary and secondary endpoints were often polyp reduction in the duodenum as a surrogate for need for surgery. Only in one study was risk-reducing surgery a secondary endpoint and this endpoint was not met. Consequently, there are no data to support a role of CP to prevent or delay the need for risk-reducing surgery in the upper GI tract.
MUTYH-associated polyposis
Section I: lower gastrointestinal manifestations
MAP.LGM.1
MAP patients present with colorectal adenomatous polyposis, but there is a significant variability in the polyposis expressivity (Table 23). Patients with MAP may present with a mild phenotype. Olschwang et al.261 reported that of 110 MAP patients, one-third had 5–14 colorectal adenomas, one-third had 15–99 adenomas and one-third had more than 100 adenomas. Sutcliffe and colleagues21 reported that 77.2% (61/79) of MAP patients presented with colorectal adenomas. Of these, approximately 10% had <10 colorectal adenomas, 75.4% had 10–99 and approximately 15% had >100. In another prospective study involving 134 MAP patients, 68 developed CRC and 80% had a median polyp count >100 at the time of colorectal surgery262. Among all the studies from the literature, the mean age at diagnosis of colorectal polyposis is 45–50 years, with the earliest diagnosis reported at the age of 22 years261. MAP patients have a lifetime risk of CRC that is estimated to be around 50.5%21,262,263, with a mean age at CRC diagnosis of 47.85 years21,261–263, and the earliest reported case of CRC at the age of 22 years.27 However, it must be highlighted that CRC may develop without an overt polyposis phenotype. In the article by Sutcliffe et al.21, 11 of 79 MAP patients (13.9%) developed CRC, with no adenomas found at colonoscopy. Similarly, Patel et al.262 reported that 5 of 134 MAP patients (5.7%) developed CRC with <10 colorectal adenomas.
Table 23.
Statements pertaining to MUTYH-associated polyposis-related colonic manifestations—extended version
| Statements |
|---|
| MAP.LGM.1 Is lower-GI tract surveillance recommended? Lower-GI tract surveillance is recommended in individuals with biallelic MUTYH pathogenic variants. |
| MAP.LGM.2 From what age should colonoscopy surveillance be performed? Colonoscopy surveillance, in the absence of symptoms, should generally start at the age of 18 years, but exceptionally may be started earlier, based upon family history. |
| MAP.LGM.3 What are the recommended intervals of colonoscopy/endoscopic surveillance? The surveillance interval should be 1–2 yearly but may be personalized according to phenotype (polyp burden). |
| MAP.LGM.4 Which patient characteristics determine the indication for prophylactic colonic resection? A: Most MAP patients present with an a-FAP-like colorectal polyposis. For these patients, endoscopic resection of colorectal adenomas may be preferred over surgery. B: If surgery is considered, it should be discussed in a multidisciplinary setting. The discussion must consider the polyp burden (colonic and rectal), age, co-morbidities, and the patient’s views, as well as their compliance with endoscopic surveillance. C: The type of surgery depends on the rectal polyp burden. Consider colectomy with IRA as the first option. If there is dense rectal polyposis that cannot be managed endoscopically, consider proctocolectomy with IPAA. D: Prophylactic surgery is not recommended in patients with pathogenic variants in MUTYH who have not developed colorectal polyps or cancer. |
| MAP.LGM.5 What is the recommended extent of resection/surgery according to the patient’s characteristics? MAP patients may benefit from a total colectomy instead of a segmental colectomy when they present with or without confirmed colorectal cancer. However, patients who have received thorough counselling may choose to undergo a segmental colectomy instead. |
| MAP.LGM.6 Is surveillance of the remaining lower-GI tract indicated after surgery? A: Lower-GI tract surveillance is recommended in MAP patients. The surveillance interval should be 1–2 yearly but may be personalized according to phenotype. B: In patients having proctocolectomy with IPAA, endoscopic surveillance of the pouch is recommended post-surgery. |
a-FAP, attenuated familial adenomatous polyposis; GI, gastrointestinal; IPAA, ileal pouch anal anastomosis; IRA, ileorectal anastomosis; MAP, MUTYH-associated polyposis.
MAP.LGM.2
After a systematic review of all available articles, the mean age at colorectal polyposis diagnosis in MAP patients is 47.85 years of age. In two studies by Nielsen and colleagues41,264, the mean ages at colorectal polyposis were 45 and 47 years of age (ranges: 12–68 and 30–70 years respectively). Similarly, Aretz et al. reported a mean age of 45 years at colorectal polyposis diagnosis (range: 24–72)265. In the study by Morak et al.423, 6 of 16 MAP patients developed colorectal polyposis before the age of 35 years, 3 of 16 at the age of 35–45 years, and 7 of 16 after the age of 45 years. Five studies commented on the age at CRC diagnosis in MAP patients. Patel et al. reported a mean age at CRC diagnosis of 47262. Similarly, Aretz and colleagues reported a mean age of 48 years (range: 29–72)265, and Morak et al. of 43 years (range: 29–64)423. In two studies by Nielsen and collaborators, the mean age at diagnosis was 48 and 50 years of age (ranges 21–70 and 39–70 years respectively)264,424.
MAP.LGM.3
For most individuals with MAP, colonic polyps are limited in number, and thus surveillance with periodic colonoscopic polypectomy is sufficient to prevent CRC. Nieuwenhuis et al.19 provided evidence for accelerated carcinogenesis in MAP, which was the basis for the surveillance interval of 1–2 years. According to the British guidelines287, colorectal surveillance should commence in individuals with MAP at the age of 18–20 years. If surgery is not performed, annual surveillance is recommended. Nieuwenhuis and colleagues19 reported that among MAP patients with a polyposis phenotype but without CRC, 9% developed CRC during 5 years of surveillance. If the initial presentation was with CRC, a 5-year risk of metachronous cancer of 11% was observed19. Based on these findings, annual colonoscopy appears appropriate for surveillance, but the choice of management strategy should consider factors such as age, co-morbidity, polyp burden, and expected functional outcome. The ASGE317 also advises close surveillance at 1- to 2-year intervals.
MAP.LGM.4
MAP patients have a lifetime risk of CRC that is estimated to be approximately 50.5% (43–63% by age 60)21,262,265, but it is believed that the lifetime risk may increase to 80–90% in absence of surveillance, with an elevated risk of metachronous CRC (23–27%)23,264,266,425,426. However, MAP patients with CRC had significantly better survival compared to matched CRC patients without MAP424. The development of CRC in MAP patients most commonly occurs in the proximal colon (52%) or the rectum (26%), but it can also occur in the distal colon (14%)264,266,267. The risk of metachronous CRC was assessed by Patel and colleagues262. Of 108 MAP patients, 35 (32.4%) underwent segmental colonic resection, and 10/35 (28.6%) later developed metachronous CRC (5 of these patients were under surveillance, 5 were not). Although comparing study groups may be challenging, two studies suggest that the risk of metachronous CRC may be lower after total colectomy with IRA. The same study by Patel et al. reports that of 108 MAP patients, 47 (43.5%) underwent colectomy with IRA, and only 2/47 (4.3%) developed rectal cancer outside of surveillance262. Similarly, Nascimbeni and colleagues268 reported a 0% rate of metachronous CRC during surveillance among 11 patients who underwent colectomy with IRA. However, it is important to note that all these patients underwent surveillance with proctoscopy and polypectomy after surgery.
MAP.LGI.5
Colonoscopy surveillance for MAP may have limited efficacy in reducing the risk of CRC, possibly due to an accelerated carcinogenesis19. Patients with MAP who have polyposis but no CRC at the initial endoscopy have a 5-year CRC risk of 5%, even in those with <10 adenomas262. On the other hand, MAP patients who present with CRC at the index endoscopy have a metachronous 5-year CRC risk of 11%19, and this risk may persist despite surveillance262. The risk of metachronous CRC appears to be lower among patients treated with colectomy compared to segmental colon resection262. Considering these findings, annual colonoscopy would seem appropriate if colonoscopy surveillance is pursued. However, it appears that surgery may be the more suitable management strategy, taking into account factors such as age, co-morbidity, polyp burden and expected functional outcome. It is worth noting that no cost–utility or cost–effectiveness analyses have been conducted on this matter. This recommendation aligns with that of the British Society of Gastroenterology (BSG), Association of Coloproctology of Great Britain and Ireland (ACPGBI), and United Kingdom Cancer Genetics Group (UKCGG)287. According to their guidelines, colonoscopy surveillance may not be effective in MAP, and an accelerated carcinogenesis has been suggested. Nieuwenhuis and colleagues19 reported that among individuals with a polyposis phenotype but without CRC, 9% developed CRC during 5 years of surveillance. For those who presented with CRC, a 5-year metachronous cancer risk of 11% was observed. In light of these data, surgery may be a more suitable management strategy.
MAP.LGI.6
These recommendations are based on the evidence gathered from the analysis of 134 MAP patients, 68 of whom had been diagnosed with CRC. The data were obtained from a prospectively maintained database, specifically the St Mark’s Hospital Polyposis Registry, and the study included 829 patient-years of follow-up261. Among the 108 MAP patients who underwent surgery as the primary management approach, 35 underwent segmental resection, with 34 for cancer and one for HGD. None of these patients had been diagnosed with MAP prior to surgery. Among these patients, 30 (86%) received postoperative surveillance of the remaining colon/rectum, but 5 (17%) developed another CRC while under surveillance. The remaining five patients who did not undergo postoperative surveillance also developed another CRC (100%). Among the 47 patients who had a total colectomy, 2 (4%) developed metachronous cancer in the residual rectum. None of these patients were receiving postoperative surveillance of the rectum. In total, 12 patients (17%) developed metachronous CRC: 10 after segmental resection and 2 after subtotal colectomy. Based on the data collected in this study, we recommend that all MAP patients who undergo segmental resection or total colectomy receive personalized postoperative surveillance of the remaining/residual colorectum at a frequency of 1–2 years. Additionally, for patients who underwent segmental resection due to a prior CRC diagnosis before the MAP diagnosis, surveillance of the remaining colon and discussion regarding surgical intervention should be personalized based on the individual‘s phenotype.
Section II: upper gastrointestinal manifestations
MAP.UGM.1
The systematic review of the literature revealed six studies that support the hypothesis that MAP patients may develop upper GI malignancies and premalignant neoplasms (Table 24)22,263,265,269–271. The most important risks in MAP appear to relate to duodenal adenomas and cancer, but gastric adenomas and cancer are also reported22. The incidence and lifetime risk of duodenal cancer in MAP is not established. Although estimated at around 4%22, the very small number of observations and lack of prospective data make this previous estimate imprecise. Duodenal polyposis occurs less frequently in MAP than FAP, affecting 20–35% of patients263,269 compared with 65–90% in FAP cohorts272. However, not all studies mentioned if the papilla was seen, or if a side-viewing endoscope was used. Only one patient from one study presented with papillary cancer at the age of 63 years269, and, most often, duodenal adenomas developed in the second duodenal portion269. MAP patients may also develop duodenal cancer. There have been eight reports of MAP patients developing duodenal cancer from three studies. The average risk is estimated to be 1.5% (range 1–2.17%). Statistically significant differences in the prevalence of duodenal polyposis were reported by Thomas et al.263, with higher risks for Y179C homozygotes263. Confirmatory studies with prospective follow-up data are required before genotype is considered in relation to stratification of surveillance. The efficacy of a surveillance protocol to prevent the occurrence of UGMs is still unclear. In one study, no cancer arose in patients undergoing endoscopic surveillance. Two patients underwent pancreas-sparing duodenectomy for stage IV duodenal polyposis (over 20 duodenal lesions) and eight patients received endoscopic therapy at a median age of 55 years (range: 38–62 years)269. Currently, there have been four reports of gastric cancer and five reports of gastric adenoma diagnoses in MAP patients22. In a retrospective study of extra-colonic manifestations of MAP by Vogt et al.22, of 150 patients who underwent esophagogastro-duodenoscopy, 17 (11%) had gastric lesions. In four of them (24%) gastric adenomas were described, and nine patients had fundic gland polyps only. Gastric cancer was observed three times; however, the incidence was not significantly increased compared to the general population (SIR: 4.2; 95% c.i.: 0.9–12).
Table 24.
Statements pertaining to MUTYH-associated polyposis-related upper-gastrointestinal manifestations—extended version
| Statements |
|---|
| MAP.UGM.1 Is upper-GI tract surveillance indicated? Upper-GI tract surveillance is recommended in MAP patients. |
| MAP.UGM.2 From what age should endoscopic surveillance of the upper-GI be performed? Upper GI surveillance by OGD should start from age 35 years. |
| MAP.UGM.3 What are the recommended modalities of endoscopic surveillance of the upper-GI tract? Upper gastrointestinal surveillance in MAP should be adapted according to OGD findings, but not exceeding at interval 3 years. Polypectomy is recommended, regardless of polyp size. |
GI, gastrointestinal; MAP, MUTYH-associated polyposis; OGD, oesophagogastro-duodenoscopy.
MAP.UGM.2
There are very limited data available on the natural history of duodenal polyposis in MAP and, as of 2023 only eight duodenal cancers had been reported in the literature. The relationship between age and development of duodenal adenomas is difficult to determine because the age at first OGD depends on age at MAP diagnosis and most patients already have polyps on first endoscopy. The age at duodenal polyp diagnosis in the two largest studies to date263,269 ranged from 32 to 81 years. Of eight reported duodenal cancers in patients with MAP, the earliest was diagnosed at 47 years of age263. In the largest study to date, Thomas et al.263 reported that 57 of 394 (14.5%) patients had adenomas (age: 37–81 years) at their first duodenoscopy, at a median age of 51 years (range 19–92 years). This prevalence was similar between males (31/197, 15.7%) and females (26/197, 13.2%, P = 0.45). In an earlier study of 92 patients with MAP reported by Walton et al.269 (with some overlap of patients in the study of Thomas et al.263), duodenal adenomas were detected in 31 patients (33.7%) at the median age of 50 years (range: 32–77 years)269. Likewise, Vogt and colleagues reported that the first OGD was performed at a mean age of 48 years (range: 14–70) and 26 of 150 patients (17.3%) already had duodenal polyps22. One patient in this study developed symptomatic gastric cancer at 17 years of age, suggesting additional causative factors22. It has been estimated that the prevalence of duodenal adenomas reaches 18.2% (8/44) by the age of 40 years and 38.5% (15/39) by the age of 70 years. Moreover, 37.8% of patients (14/37) progressed to a higher Spigelman stage during follow-up263. Of the eight reported cases of duodenal cancers that appear in the literature, the earliest was diagnosed at 47 years of age263. In the study by Thomas et al., the mean age at duodenal cancer diagnosis was 66.2 years (range: 63–83 years)263.
MAP.UGM.3
Previous guidelines have recommended that the interval between upper GI endoscopies in MAP should be based upon Spigelman stage, as for FAP36,273,274. However, recent reports indicate that Spigelman stage is not a reliable predictor of cancer risk in MAP, because it fails to identify patients at risk of duodenal cancer263,269. Duodenal cancers have arisen without a recognized background of benign polyposis263. Therefore, in these guidelines we do not recommend the use of Spigelman staging to determine the surveillance interval in MAP. In the largest study of duodenal adenomas and cancer to date263, three of four reported cancers were diagnosed within 12 months of a previous OGD, suggesting missed lesions and highlighting the need for high-quality endoscopy. An increased mutational burden has been reported in MAP duodenal adenomas compared to FAP duodenal adenomas275 and HGD has been reported in subcentimetre MAP duodenal adenomas263. This suggests biological differences between MAP and FAP duodenal adenomas that could mediate differences in natural history. Based on these data, we recommend polypectomy regardless of the polyp size or Spiegelman staging.
Section III: extra-intestinal manifestations in MUTYH-associated polyposis
The evidence of an increased risk for extra-intestinal cancers in biallelic MUTYH mutation carriers is weak, and in most cases controversial (Table 25). Vogt et al.22 analysed 276 biallelic carriers from 181 unrelated families and observed that 35 (13%) had at least one malignant extra-intestinal lesion. For MAP patients, the risk of developing extra-intestinal malignancies is nearly double that of the general population (SIR 1.9; 95% c.i. 1.4–2.5), particularly for ovarian, bladder and skin cancers (SIR: 5.7, 7.2 and 2.8 respectively)22. The cumulative lifetime risk of developing extra-intestinal malignancies can reach as high as 38%, with a median age of 51–6122. Other extra-intestinal features may resemble those of the FAP spectrum, including osteomas and CHRPE, but at a significantly lower rate21,276,277. Although more studies are needed, MAP patients may have a higher risk of developing lung, haematologic, brain and skin cancers278–280. There may be some phenotypic overlap with Lynch syndrome, indicated by an increased risk of endometrial cancer and Muir–Torre syndrome among MUTYH-biallelic carriers427, but the evidence is not conclusive thus far281. Unlike FAP, desmoids do not appear to belong to the spectrum of manifestations of MAP281. Finally, very preliminary data suggest that two MUTYH variants (rs3219476 and rs3219472) can increase the risk of developing cholangiocarcinomas when homozygous281.
Table 25.
Statements pertaining to MUTYH-associated polyposis-related extra-intestinal manifestations—extended version
| Statements |
|---|
| MAP.EIM.1 What is the appropriate surveillance strategy for extra-intestinal cancers? No surveillance for extra-intestinal cancers is recommended for MUTYH biallelic carriers. |
Despite the possibly increased extra-intestinal cancer risks, there is no evidence of a cost–effectiveness benefit from extra-intestinal screening in MAP patients.
Other rare adenomatous polyposis syndromes
OAPS.1
The use of MGPT has become standard in genetic diagnostics. This approach may use physical MGPT, or virtual panels based on whole-exome or genome-sequencing data. MGPT in patients with GI polyposis should include APC and MUTYH, which explain most identifiable inheritable forms of polyposis, as well as other genes relevant for adenomatous polyposis (MMR genes MLH1, MSH2, MSH6, PMS2, MSH3 and MLH3), POLE (exonuclease domain), POLD1 (exonuclease domain), NTHL1, MBD4 and AXIN2). In addition, it is recommended to include genes causing other polyposis syndromes such as STK11, BMPR1A, SMAD4, PTEN, and RNF43, due to the phenotypic overlap24,26,27,31,32,282,283. Mosaic APC variants can be found in about 20–50% of the remaining unexplained polyposis cases11,265,284. When testing every patient referred to oncogenetic counselling (regardless of indication), the detection of MUTYH biallelic mutations is only about 0.2% (82/44 800)21. The total percentage of detected heritable polyposis syndromes will therefore likely not exceed 0.5% in a general oncological cohort21. Therefore, testing for polyposis genes in all patients undergoing germline oncogenetic testing should only be done as part of a broad gene panel. The observed decline in the mutation detection rate in patients receiving genetic testing for multiple colorectal polyps over time (due to more sensitive colonoscopies) and the very low frequency of patients with PVs in genes other than APC and MUTYH, especially those with 10–20 polyps and those >60 years of age, suggest that the mutation detection rate in this group is likely less than 2–3%.
Including CRC diagnosed before 50 years of age in criteria for testing polyposis genes using stringent criteria based on polyp count will inevitably lead to some patients with a heritable form of polyposis being missed. Sutcliffe et al.21 demonstrated that if only patients with >10–20 adenomatous polyps are tested, 10% of MUTYH biallelic patients will be missed. This was also shown by the study of Landon et al.285. Both studies suggest that including patients with <10 polyps but with CRC under age 50 will increase the detection rate of MAP and other clinically actionable hereditary CRC syndrome. Terlouw et al.286 reported that testing patients with adenomas above the age of 70 lead to a detection rate of PVs of about 1%. In considering patients >70 years, no MUTYH or APC variants were identified in patients with <20 adenomas (n = 82) and only one case of MAP was found among patients with >20 adenomas (1/90, 1.1%).
APC mosaic
APC mosaicism has been reported to be present in 25–50% of unexplained patients with >20 adenomas284. In most of these cases, the mosaicism was undetectable in leucocyte-derived DNA and required testing of DNA isolated from >2 adenomas. Tumour testing is still logistically challenging and is not performed in most diagnostic laboratories.
Concluding remarks
Testing individuals with >20 adenomas (aged <70 years) seems widely accepted and is included in most guidelines. Testing individuals over age 70 or with <20 adenomas is indicated when more features suggestive of a hereditary polyposis syndrome are present286,287. Not all guidelines propose these age limits though. Other indications (besides the presence of adenomatous polyps) for polyposis panel analysis are FAP-related extra-colonic manifestations, CRC aged < 50288, a somatic KRAS c.34G>T transversion, or an FDR with >10 adenomas286.
OAPS.2
Whether testing for PVs identified in the index case should also be offered to FDRs depends on the mode of inheritance. For rare non-APC-dominantly inherited syndromes (such as PPAP), testing should be offered to all FDRs with cascade testing. For recessively inherited syndromes (such as MUTYH- and NTHL1-associated polyposis), screening should be offered to siblings. Testing the offspring of index cases can be considered when PV allele frequencies in the relevant population are high, as for MUTYH in many geographical regions, where the probability of inheriting a second MUTYH PV is around 1%. Other known recessive syndromes, such as NTHL1 and MSH3, have lower carriership rates (around 1 in 300)24,31; therefore, the risk of inheriting two PVs becomes very low (<1/600). If parents are related, the chances are higher, and testing should be considered for their offspring. The French guidelines274 also advise complete MUTYH analysis in the unaffected parent as a possible strategy, particularly where there are a large number of offspring at risk289.
Germline variant interpretation and classification
The interpretation and classification of germline variants into five classes (pathogenic, likely pathogenic, unclear, likely benign, benign) should follow a standardized procedure, based on the ACMG/AMP guidelines for variant classification. For APC, gene-specific ACMG/AMP rules were developed recently by the subcommittee of InSiGHT/ClinGen Variant Curation Expert Panel (VCEP), which should replace the generic framework. In the near future, gene-specific ACMG/AMP modifications will be available for further actionable genes, which can be found on the ClinGen websites.
Currently, there are insufficient data to establish the appropriate age to initiate GI surveillance for each of the aforementioned genes (Table 26). However, it is advisable to follow the guidelines provided for MAP, which recommend starting screening at 18 years of age. Additionally, consideration may be given to initiating screening 5 years earlier in patients with a highly aggressive familial phenotype. This is particularly relevant for heterozygous carriers of specific POLE or POLD1 variants associated with severe and early-onset phenotypes that present with a CMMRD-like phenotype in childhood or adolescence290–292. In addition to the GI phenotypes, most of the rare adenomatous polyposis syndromes are associated with an increased risk of extra-GI tumours and other phenotypic manifestations (Table 10).
Table 26.
Statements pertaining to other rare adenomatous polyposis syndromes (OAPS)—extended version
| Statements |
|---|
| OAPS.1 In which patients should germline screening for inherited types of adenomatous polyposis syndromes be performed? A. Germline multigene panel testing should be considered in patients with >20 cumulative colorectal adenomas. 1B. The threshold may be lowered to 10 cumulative adenomas if:
D. Somatic testing for APC mosaic mutations should be considered in unexplained polyposis patients fulfilling criteria from statements A and B. |
| OAPS.2 What is the best strategy for predictive testing in first-degree relatives? A. In the case of autosomal recessive hereditary polyposis syndromes, testing should always be offered to siblings. Children should be tested when:
|
CMMRD, congenital mismatch repair deficiency; CRC, colorectal cancer; GI, gastrointestinal; MMR, mismatch repair.
Gastric adenocarcinoma and proximal polyposis of the stomach
GAPPS.1
GAPPS is an autosomal dominant hereditary gastric cancer syndrome with incomplete penetrance that was identified in 201214,293–302 . It is characterized by extensive involvement of the fundus and body of the stomach with fundic gland polyps sparing the antrum and lesser curvature and predisposition for the development of gastric adenocarcinoma (Table 27)303–306. To consider a diagnosis of GAPPS, the use of proton pump inhibitors should be ruled out and the presence of polyposis elsewhere in the GI tract should be ruled out to exclude the possibility of (attenuated) FAP307. GAPPS families have been identified in Australia, North America, Europe and Japan14,293,295–302. The age of onset of gastric adenocarcinoma is variable, ranging from 22 to 75 years. The overall risk for gastric cancer in GAPPS is high with the estimated incidence ranging from 12% to 25%, although the only studies are case or family studies or small retrospective series14,297,306. However, the true risk may be much lower because most studies will be subjected to ascertainment bias. Although there is an inverse association between GAPPS and Helicobacter pylori infection, few data are available so far. In the setting of GAPPS, dysplasia (low grade) has been detected as early as 10 years of age. A range of microscopic features can be observed in GAPPS, including fundic gland polyps (FGPs), dysplastic FGPs, fundic gland–like polyps, HPAPs, hyperplastic polyps, gastric-type adenomas and adenocarcinomas (tubular/intestinal and mixed with a poorly cohesive component)300. Some lesions show a mixture of the above features301. The larger, dominant polyps tend to show foci of dysplasia or may be adenomatous301. HPAPs are unique lesions not described in other settings of gastric pathology and are considered the earliest marker of dysplasia14,293,295,298. Genetic testing has enabled confirmation of GAPPS since 2016, when three-point mutations within promoter 1B of the APC gene (positioned within the YY1 binding motif) were described in North American, Australian and European families with GAPPS294,306.
The essential and supportive clinical criteria for the diagnosis of gastric adenocarcinoma and proximal polyposis of the stomach and for genetic testing are shown in GAPPS.2
The age of onset among individuals with GAPPS is highly variable and is likely influenced by multiple factors, including genetics, environment and lifestyle298. It has been suggested that all FDRs of affected patients should undergo upper-GI endoscopy to identify the typical features of GAPPS, namely the lack of FGPs in the antrum, which can facilitate differential diagnosis to other GI polyposis syndromes297. Biopsy sampling for an accurate histopathological diagnosis of the encountered lesions is paramount. However, endoscopy may fail to sample polyps with malignant transformation14,295,297. These reports suggest that reliance on unchanged endoscopic appearance and histopathology of sampled polyps carries the risk of missing occult sites of malignant transformation and focal progression297. Gastroscopy should be performed with the best-quality endoscope available (high-definition endoscope, image-enhanced endoscopy (IEE) modalities with laser light sources, including blue laser imaging (BLI) or linked colour imaging (LCI)). Detailed inspection of gastric polyps and surrounding mucosa with multiple biopsies from the suspected areas (larger polyps and areas of vascular or structural irregularities) and exclusion of duodenal polyps should be a standard part of diagnostics308. Tacheci et al.308 suggested that upper-GI endoscopy in the FDRs of individuals with GAPPS with proven mutation of the 1B promoter of the APC gene should start at age 15. Because of the limited data available and the heterogeneity of GAPPS patients, recommendations on the surveillance of GAPPS families (interval of endoscopic surveillance) should be flexible and decided on a case-by-case basis308,309.
GAPPS.3
Non-gastric manifestations in GAPPS are poorly defined. Worthley et al.14 described a ‘mild colonic phenotype’ in a large family. Although no patients were reported to have colon cancer, 9 of 36 family members with a complete or a partial GAPPS phenotype were found to have adenomatous lesions involving either the left or right side of the colon. In contrast, colonoscopic screening in a family of Asian descent with GAPPS identified no colonic lesions293. Recently, McDuffie et al.310 observed that GAPPS patients were more frequently affected by colonic polyps than non-GAPPS family members within the same families (P = 0.007). The authors observed that colonic polyps shared immunohistochemical features seen in FGPs and gastric cancers, namely increased nuclear expression of β-catenin (both gastric and colonic lesions harboured activating somatic variants of β-catenin signalling). Colon cancer was described in some GAPPS families reported in the literature14,295,310. Small intestinal involvement has not been reported in GAPPS. The presence of duodenal adenomas makes a diagnosis of GAPPS unlikely and raises the possibility of FAP308. Colonic involvement in GAPPS may simply reflect the baseline risk of developing sporadic colonic adenomas in GAPPS families310. However, in GAPPS families, the occurrence of adenomatous polyps in patients in the third/fourth decades of life, the lack of a male gender preference as observed in sporadic colon polyps, the involvement of the right and left colon, and the increased nuclear expression of β-catenin/signalling-related genes in both gastric cancer and adenomatous colonic polyps may indicate a common pathogenesis at both organ sites310. There are no systematic studies on the impact of colorectal surveillance by colonoscopy or sigmoidoscopy on the incidence of colorectal polyps, adenomas or adenocarcinomas, or on their overall survival. Therefore, colonoscopy surveillance is prudent while risk estimates of colon and other cancer phenotypes possibly associated with GAPPS are awaiting clarification in larger cohorts297. A recent review paper suggested the following actions for the surveillance of colonic lesions in GAPPS: index colonoscopy in all cases of suspected GAPPS (from the age of 18 years) to exclude colonic polyposis; intervals for follow-up colonoscopy guided by initial findings; 3-year intervals in the presence of adenomas or serrated lesions as well as in patients younger than 40 years308.
GAPPS.4
The key goal of treatment of GAPPS is to prevent the development of gastric cancer, which is rarely diagnosed at an early stage (despite careful and adequate endoscopic examination) and often has a fatal prognosis308. Advanced adenocarcinoma in GAPPS is associated with poor prognosis and quality of life is seriously impaired by the gastric cancer itself and/or by the oncological treatments. The different phenotypes of GAPPS demand a tailored approach for each family member. Upper-GI endoscopy is useful for the early detection of GAPPS, and endoscopic biopsies at regular intervals may be considered to identify a progression to dysplasia or a malignant transformation. However, previous reports questioned prolonged endoscopic surveillance in patients with GAPPS, owing to the difficulty of evaluating the precise condition of gastric polyps. Cases of rapid progression to gastric adenocarcinoma and metastasis, despite endoscopic surveillance, have been reported14,295. The clinical management of GAPPS remains challenging. Although the antrum is spared in GAPPS and carpeting FGP is the typical presentation, there is no evidence for the long-term safety of proximal rather than total gastrectomy in these patients308. Risk-reducing (prophylactic) total gastrectomy has been suggested for GAPPS patients at either 30–35 years of age, or at 5 years before the age at which the youngest family member developed gastric cancer308. However, consideration should be given to the different phenotypes of GAPPS that demand an individual approach for each family member and it is essential to obtain detailed full information about the patient and his/her family. The timing of surgery may also vary according to the individual’s preferences308. Younger patients may often tend to postpone/refuse surgery. They should be made fully aware of the risks of delay and encouraged to undergo appropriate prophylactic surgery. In the case of the detection of gastric cancer and/or dysplasia, total gastrectomy is indicated without delay308. Annual gastroscopic surveillance should be performed in these patients and in patients who are not fully fit for surgery308. Women of child-bearing age should be assured of the real chance of uncomplicated pregnancy with successful childbirth and breastfeeding after prophylactic gastrectomy311. Both laparoscopic total gastrectomy309 and robotic total gastrectomy312 were recently used for GAPPS treatment. It should be highlighted that GAPPS has been known for only a decade; therefore, the evidence on the risk of duodenal cancer is still very limited. An increased risk of developing duodenal cancer cannot be excluded thus far; therefore, gastric surgery should allow for subsequent prospective evaluation of the duodenum.
Supplementary Material
Acknowledgements
The authors would like to express their gratitude to the British Journal of Surgery for their financial support of the project.
The authors would also like to thank the European Society of Coloproctology (ESCP) for their financial support and collaboration provided through Kleijnen Systematic Reviews Ltd.
Furthermore, the authors extend their thanks to Deborah Lepley, Agi Hajnal and Hayley Clark from the Library Services of East Suffolk and North Essex NHS Foundation Trust and Flavia Rampichini from the Central Library of Medicine and Surgery at the University of Milan for their contributions to the bibliographic research.
This research was supported (not financially) by the European Reference Network on Genetic Tumour Risk Syndromes (ERN GENTURIS)—project ID 739547; ERN GENTURIS is partly co-funded by the EU within the framework of the Third Health Programme ERN-2016—Framework Partnership Agreement 2017–2021.
Abbreviations
- APC
adenomatous polyposis coli
- A
agree
- a-FAP
attenuated familial adenomatous polyposis
- BER
base-excision repair
- CHRPE
congenital hypertrophy of the retinal pigmented epithelium
- CMMRD
congenital mismatch repair deficiency
- CMV
cribriform-morulae variant
- CP
chemoprevention
- CRC
colorectal cancer
- D
disagree
- DT
desmoid tumour
- EUS
endoscopic ultrasound
- FAP
familial adenomatous polyposis
- FDR
first-degree relatives
- GAPPS
gastric adenocarcinoma and proximal polyposis of the stomach
- GI
gastrointestinal
- HGIEN
high-grade intraepithelial neoplasia
- HPV
human papilloma virus
- IPAA
ileal pouch anal anastomosis
- IRA
ileorectal anastomosis
- ITT
intention to treat
- LE
level of evidence
- LGM
lower gastrointestinal manifestations
- MAP
MUTYH-associated polyposis
- MGPT
multi-gene panel testing
- MMR
mismatch repair
- N
neutral
- OGD
oesophagogastro-duodenoscopy
- PPAP
polymerase proofreading-associated polyposis
- PTC
papillary thyroid carcinoma
- PV
pathogenic variant
- SA
strongly agree
- SD
strongly disagree
- UDCA
ursodesoxycolic acid
- UGM
upper gastrointestinal manifestations
Contributor Information
Gloria Zaffaroni, Center for Hereditary Tumors, Bethesda Hospital, Duisburg, Germany; Faculty of Medicine and Surgery, University of Milan, Milan, Italy.
Alessandro Mannucci, Gastroenterology and Gastrointestinal Endoscopy Unit, Vita-Salute San Raffaele University, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Laura Koskenvuo, Department of Gastroenterological Surgery, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
Borja de Lacy, Department of Gastrointestinal Surgery, Hospital Clinic of Barcelona, Barcelona, Spain.
Anna Maffioli, Faculty of Medicine and Surgery, University of Milan, Milan, Italy; Department of General Surgery, Sacco Hospital, ASST Fatebenefratelli Sacco, Milan, Italy.
Tanya Bisseling, Department of Gastroenterology and Hepatology, Radboud University Medical Center, Nijmegen, The Netherlands.
Elizabeth Half, Cancer Prevention and Hereditary GI Cancer Unit, Rambam Health Care Campus, Haifa, Israel.
Giulia Martina Cavestro, Gastroenterology and Gastrointestinal Endoscopy Unit, Vita-Salute San Raffaele University, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Laura Valle, Hereditary Cancer Program, Catalan Institute of Oncology, Oncobell Program, IDIBELL, Barcelona, Spain; Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Madrid, Spain.
Neil Ryan, The College of Medicine and Veterinary Medicine, University of Edinburgh, Edinburgh, UK.
Stefan Aretz, Institute of Human, Genetics, Medical Faculty, University of Bonn and National Center for Hereditary Tumour Syndromes, University Hospital Bonn, Bonn, Germany.
Karen Brown, Leicester Cancer Research Centre, University of Leicester, Leicester, UK.
Francesco Buttitta, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy; IRCCS University Hospital of Bologna, Policlinico di Sant’Orsola, Bologna, Italy.
Fatima Carneiro, Faculty of Medicine of Porto University, Centro Hospitalar Universitário de São João, Ipatimup, Porto, Portugal.
Oonagh Claber, Department of Clinical Genetics, Northern Genetics Service, Newcastle upon Tyne, UK.
Ruth Blanco-Colino, Department of Gastrointestinal Surgery, Vall d’Hebron University Hospital, Barcelona, Spain; Universitat Autònoma de Barcelona, Barcelona, Spain.
Maxime Collard, Department of Digestive Surgery, Hôpital Saint-Antoine, Sorbonne University, APHP, Paris, France.
Emma Crosbie, Division of Cancer Sciences, University of Manchester, Manchester, UK.
Miguel Cunha, Department of Surgery, Algarve Universitary Hospital Center, Colorectal SurgeryGroup, Portimao, Portugal.
Triantafyllos Doulias, Department of Colorectal Surgery, Colchester Hospital, East Suffolk and North Essex NHS Foundation Trust, Colchester, UK; Colorectal Surgery Department, Kettering Hospital, University Hospitals of Northamptonshire, Kettering, Northamptonshire, UK; Department of Genetics and Genome Biology, Honorary Lecturer in the Leicester Cancer Research Centre, Leicester, UK.
Christina Fleming, Department of Colorectal Surgery, University Hospital Limerick, Limerick, Ireland.
Henriette Heinrich, Department for Gastroenterology and Hepatology, Clarunis Universitäres Bauchzentrum, Universitätsspital Basel, Basel, Switzerland.
Robert Hüneburg, Department of Internal Medicine I, University Hospital Bonn, Bonn, Germany; National Center for Hereditary Tumour Syndromes, University Hospital Bonn, Bonn, Germany.
Julie Metras, Department of Digestive Surgery, Hôpital Saint-Antoine, Sorbonne University, APHP, Paris, France.
Iris Nagtegaal, Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands.
Ionut Negoi, Department of General Surgery, Carol Davila University of Medicine and Pharmacy Bucharest, Emergency Hospital of Bucharest, Bucharest, Romania.
Maartje Nielsen, Clinical Genetics Department, Leiden University Medical Center, Leiden, The Netherlands.
Gianluca Pellino, Department of Gastrointestinal Surgery, Vall d’Hebron University Hospital, Barcelona, Spain; Universitat Autònoma de Barcelona, Barcelona, Spain; Department of Advanced Medical and Surgical Sciences, Università degli Studi della Campania ‘Luigi Vanvitelli’, Naples, Italy.
Luigi Ricciardiello, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy; IRCCS University Hospital of Bologna, Policlinico di Sant’Orsola, Bologna, Italy.
Abdurrahman Sagir, Center for Hereditary Tumors, Bethesda Hospital, Duisburg, Germany.
Luis Sánchez-Guillén, Department of Gastrointestinal Surgery, Elche General University Hospital, Elche, Alicante, Spain; Miguel Hernández University, Elche, Spain.
Toni T Seppälä, Department of Gastroenterological Surgery, Helsinki University Hospital and University of Helsinki, Helsinki, Finland; Applied Tumour Genomics Research Program, University of Helsinki, Helsinki, Finland; Faculty of Medicine and Health Technology, University of Tampere and TAYS Cancer Centre, Tampere, Finland; iCAN Precision Medicine Flagship, University of Helsinki, Helsinki, Finland.
Peter Siersema, Department of Gastroenterology and Hepatology, Radboud University Medical Center, Nijmegen, The Netherlands.
Benedikt Striebeck, Support Group Polyposis Coli e.V., FAP Patient, Germany.
Julian R Sampson, Institute of Medical Genetics, Division of Cancer and Genetics, Cardiff University School of Medicine, Cardiff, UK.
Andrew Latchford, Polyposis Registry, St Mark’s Hospital, Harrow, UK; Department of Surgery and Cancer, Imperial College, London, UK.
Yann Parc, Department of Digestive Surgery, Hôpital Saint-Antoine, Sorbonne University, APHP, Paris, France.
John Burn, Newcastle University Translational and Clinical Research Institute, Centre for Life, Newcastle upon Tyne, UK.
Gabriela Möslein, Center for Hereditary Tumors, Bethesda Hospital, Duisburg, Germany.
Funding
This research was supported financially by the British Journal of Surgery and by the ESCP (European Society of Coloproctology).
Disclosure
T.T.S. declares consultation fee from Amgen Finland. T.T.S is CEO and co-owner of Healthfund Finland and Clinical Advisory board member of LS Cancer Diag.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Not applicable.
Author contributions
Gloria Zaffaroni (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing—original draft), Alessandro Mannucci (Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft), Laura Koskenvuo (Investigation, Writing—original draft), F. Borja de Lacy (Investigation, Writing—original draft), Anna Maffioli (Investigation, Writing—original draft), Tanya Bisseling (Investigation, Writing—original draft), Elizabeth Half (Investigation, Writing—original draft), Giulia Cavestro (Investigation, Writing—original draft), Laura Valle (Investigation, Writing—original draft), Neil Ryan (Investigation, Writing—original draft), Stefan Aretz (Investigation, Writing—review & editing), Karen Brown (Investigation, Writing—review & editing), Francesco Buttitta (Investigation, Writing—review & editing), Fatima Carneiro (Investigation, Writing—review & editing), Oonagh Claber (Investigation, Writing—review & editing), Ruth Blanco-Colino (Investigation, Writing—review & editing), Maxime K. Collard (Investigation, Writing—review & editing), Emma J. Crosbie (Investigation, Writing—review & editing), Miguel Cunha (Investigation, Writing—review & editing), Doulias Triantafyllos (Investigation, Writing—review & editing), Christina Fleming (Investigation, Writing—review & editing), Henriette Heinrich (CRediT contribution not specified), Robert Hüneburg (Investigation, Writing—review & editing), Julie Metras (Investigation, Writing—review & editing), Iris Nagtegaal (Investigation, Writing—review & editing), Ionut Negoi (Investigation, Writing—review & editing), Maartje Nielsen (Investigation, Writing—review & editing), Gianluca Pellino (Investigation, Writing—review & editing), Luigi Ricciardiello (Investigation, Writing—review & editing), Abdurrahman Sagir (Writing—review & editing), Luis Sánchez-Guillén (Writing—review & editing), Toni Seppälä (Investigation, Writing—review & editing), Peter Siersema (Investigation, Writing—review & editing), Benedikt Striebeck (Writing—review & editing), Julian Sampson (Investigation, Writing—review & editing), Andrew Latchford (Investigation, Writing—review & editing), Yann Parc (Investigation, Writing—original draft, Writing—review & editing), John Burn (Investigation, Writing—review & editing) and Gabriela Moeslein (Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing—original draft, Writing—review & editing)
References
- 1. Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KAet al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020;159:335–349.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RRL, Laversanne M, Soerjomataram I, Jemal Aet al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249 [DOI] [PubMed] [Google Scholar]
- 3. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–691 [DOI] [PubMed] [Google Scholar]
- 4. Kastrinos F, Samadder NJ, Burt RW. Use of family history and genetic testing to determine risk of colorectal cancer. Gastroenterology 2020;158:389–403 [DOI] [PubMed] [Google Scholar]
- 5. Lowery JT, Ahnen DJ, Schroy PC, Hampel H, Baxter N, Boland CRet al. Understanding the contribution of family history to colorectal cancer risk and its clinical implications: a state-of-the-science review. Cancer 2016;122:2633–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Henrikson NB, Webber EM, Goddard KA, Scrol A, Piper M, Williams MSet al. Family history and the natural history of colorectal cancer: systematic review. Genet Med 2015;17:702–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mork ME, Rodriguez A, Bannon SA, Lynch PM, Rodriguez-Bigas MA, Thirumurthi Set al. Outcomes of disease-specific next-generation sequencing gene panel testing in adolescents and young adults with colorectal cancer. Cancer Genet 2019;235–236:77–83 [DOI] [PubMed] [Google Scholar]
- 8. Mork ME, You YN, Ying J, Bannon SA, Lynch PM, Rodriguez-Bigas MAet al. High prevalence of hereditary cancer syndromes in adolescents and young adults with colorectal cancer. J Clin Oncol 2015;33:3544–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedl W, Aretz S. Familial adenomatous polyposis: experience from a study of 1164 unrelated German polyposis patients. Hered Cancer Clin Pract 2005;3:95–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aretz S, Stienen D, Friedrichs N, Stemmler S, Uhlhaas S, Rahner Net al. Somatic APC mosaicism: a frequent cause of familial adenomatous polyposis (FAP). Hum Mutat 2007;28:985–992 [DOI] [PubMed] [Google Scholar]
- 11. Hes FJ, Nielsen M, Bik EC, Konvalinka D, Wijnen JT, Bakker Eet al. Somatic APC mosaicism: an underestimated cause of polyposis coli. Gut 2008;57:71–76 [DOI] [PubMed] [Google Scholar]
- 12. Spier I, Drichel D, Kerick M, Kirfel J, Horpaopan S, Laner Aet al. Low-level APC mutational mosaicism is the underlying cause in a substantial fraction of unexplained colorectal adenomatous polyposis cases. J Med Genet 2016;53:172–179 [DOI] [PubMed] [Google Scholar]
- 13. Rofes P, González S, Navarro M, Moreno-Cabrera JM, Solanes A, Darder Eet al. Paired somatic-germline testing of 15 polyposis and colorectal cancer–predisposing genes highlights the role of APC mosaicism in de novo familial adenomatous polyposis. J Mol Diagn 2021;23:1452–1459 [DOI] [PubMed] [Google Scholar]
- 14. Worthley DL, Phillips KD, Wayte N, Schrader KA, Healey S, Kaurah Pet al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): a new autosomal dominant syndrome. Gut 2012;61:774–779 [DOI] [PubMed] [Google Scholar]
- 15. Cleary SP, Cotterchio M, Jenkins MA, Kim H, Bristow R, Green Ret al. Germline MutY human homologue mutations and colorectal cancer: a multisite case–control study. Gastroenterology 2009;136:1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guarinos C, Juarez M, Egoavil C, Rodriguez-Soler M, Perez-Carbonell L, Salas Ret al. Prevalence and characteristics of MUTYH-associated polyposis in patients with multiple adenomatous and serrated polyps. Clin Cancer Res 2014;20:1158–1168 [DOI] [PubMed] [Google Scholar]
- 17. Oishi K, Hofmann S, Diaz GA, Brown T, Manwani D, Ng Let al. Biallelic germline mutations in MYH predispose to multiple colorectal adenoma and somatic G:C→T:A mutations. Hum Mol Genet 2002;11:2961–2967 [DOI] [PubMed] [Google Scholar]
- 18. Al-Tassan N, Chmiel NH, Maynard J, Fleming N, Livingston AL, Williams GTet al. Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors. Nat Genet 2002;30:227–232 [DOI] [PubMed] [Google Scholar]
- 19. Nieuwenhuis MH, Vogt S, Jones N, Nielsen M, Hes FJ, Sampson JRet al. Evidence for accelerated colorectal adenoma–carcinoma progression in MUTYH-associated polyposis? Gut 2012;61:734–738 [DOI] [PubMed] [Google Scholar]
- 20. Grover S, Kastrinos F, Steyerberg EW, Cook EF, Dewanwala A, Burbidge LAet al. Prevalence and phenotypes of APC and MUTYH mutations in patients with multiple colorectal adenomas. JAMA 2012;308:485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sutcliffe EG, Bartenbaker Thompson A, Stettner AR, Marshall ML, Roberts ME, Susswein LRet al. Multi-gene panel testing confirms phenotypic variability in MUTYH-associated polyposis. Fam Cancer 2019;18:203–209 [DOI] [PubMed] [Google Scholar]
- 22. Vogt S, Jones N, Christian D, Engel C, Nielsen M, Kaufmann Aet al. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology 2009;137:1976–1985.e10 [DOI] [PubMed] [Google Scholar]
- 23. Win AK, Dowty JG, Cleary SP, Kim H, Buchanan DD, Young JPet al. Risk of colorectal cancer for carriers of mutations in MUTYH, with and without a family history of cancer. Gastroenterology 2014;146:1208–1211.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weren RDAA, Ligtenberg MJLL, Kets CM, De Voer RM, Verwiel ETPP, Spruijt Let al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat Genet 2015;47:668–671 [DOI] [PubMed] [Google Scholar]
- 25. Grolleman JE, de Voer RM, Elsayed FA, Nielsen M, Weren RDA, Palles Cet al. Mutational signature analysis reveals NTHL1 deficiency to cause a multi-tumor phenotype. Cancer Cell 2019;35:256–266.e5 [DOI] [PubMed] [Google Scholar]
- 26. Palles C, West HD, Chew E, Galavotti S, Flensburg C, Grolleman JEet al. Germline MBD4 deficiency causes a multi-tumor predisposition syndrome. Am J Hum Genet 2022;109:953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, Broderick Pet al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet 2013;45:136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bellido F, Pineda M, Aiza G, Valdés-Mas R, Navarro M, Puente DAet al. POLE and POLD1 mutations in 529 kindred with familial colorectal cancer and/or polyposis: review of reported cases and recommendations for genetic testing and surveillance. Genet Med 2016;18:325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palles C, Martin L, Domingo E, Chegwidden L, McGuire J, Cuthill Vet al. The clinical features of polymerase proof-reading associated polyposis (PPAP) and recommendations for patient management. Fam Cancer 2022;21:197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aronson M, Colas C, Shuen A, Hampel H, Foulkes W, Baris Feldman Het al. Diagnostic criteria for constitutional mismatch repair deficiency (CMMRD): recommendations from the international consensus working group. J Med Genet 2022;59:318–327 [DOI] [PubMed] [Google Scholar]
- 31. Adam R, Spier I, Zhao B, Kloth M, Marquez J, Hinrichsen Iet al. Exome sequencing identifies biallelic MSH3 germline mutations as a recessive subtype of colorectal adenomatous polyposis. Am J Hum Genet 2016;99:337–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Olkinuora A, Nieminen TT, Mårtensson E, Rohlin A, Ristimäki A, Koskenvuo Let al. Biallelic germline nonsense variant of MLH3 underlies polyposis predisposition. Genet Med 2019;21:1868–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lammi L, Arte S, Somer M, Järvinen H, Lahermo P, Thesleff Iet al. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet 2004;74:1043–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leclerc J, Beaumont M, Vibert R, Pinson S, Vermaut C, Flament Cet al. AXIN2 germline testing in a French cohort validates pathogenic variants as a rare cause of predisposition to colorectal polyposis and cancer. Genes Chromosomes Cancer 2022;62:210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chan JM, Clendenning M, Joseland S, Georgeson P, Mahmood K, Walker Ret al. Rare germline variants in the AXIN2 gene in families with colonic polyposis and colorectal cancer. Fam Cancer 2022;21:399–413 [DOI] [PubMed] [Google Scholar]
- 36. Vasen HFA, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario Let al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008;57:704–713 [DOI] [PubMed] [Google Scholar]
- 37. Hyer W, Cohen S, Attard T, Vila-Miravet V, Pienar C, Auth Met al. Management of familial adenomatous polyposis in children and adolescents: position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr 2019;68:428–441 [DOI] [PubMed] [Google Scholar]
- 38. Kennedy RD, Potter DD, Moir CR, El-Youssef M. The natural history of familial adenomatous polyposis syndrome: a 24-year review of a single center experience in screening, diagnosis, and outcomes. J Pediatr Surg 2014;49:82–86 [DOI] [PubMed] [Google Scholar]
- 39. Knudsen AL, Bisgaard ML, Bulow S. Attenuated familial adenomatous polyposis (AFAP). A review of the literature. Fam Cancer 2003;2:43–55 [DOI] [PubMed] [Google Scholar]
- 40. Knudsen AL, Bülow S, Tomlinson I, Möslein G, Heinimann K, Christensen IJ. Attenuated familial adenomatous polyposis: results from an international collaborative study. Colorectal Disease 2010;12:e243–e249 [DOI] [PubMed] [Google Scholar]
- 41. Nielsen M, Hes FJ, Nagengast FM, Weiss MM, Mathus-Vliegen EM, Morreau Het al. Germline mutations in APC and MUTYH are responsible for the majority of families with attenuated familial adenomatous polyposis. Clin Genet 2007;71:427–433 [DOI] [PubMed] [Google Scholar]
- 42. Kerr SE, Thomas CB, Thibodeau SN, Ferber MJ, Halling KC. APC germline mutations in individuals being evaluated for familial adenomatous polyposis: A review of the Mayo Clinic experience with 1591 consecutive tests. J Mol Diagn 2013;15:31–43 [DOI] [PubMed] [Google Scholar]
- 43. Anele CC, Martin I, McGinty Duggan PM, Chauhan J, Clark SK, Faiz ODet al. Attenuated familial adenomatous polyposis: a phenotypic diagnosis but obsolete term? Dis Colon Rectum 2022;65:529–535 [DOI] [PubMed] [Google Scholar]
- 44. Munck A, Gargouri L, Alberti C, Viala J, Peuchmaur M, Lenaerts Cet al. Evaluation of guidelines for management of familial adenomatous polyposis in a multicenter pediatric cohort. J Pediatr Gastroenterol Nutr 2011;53:296–302 [DOI] [PubMed] [Google Scholar]
- 45. Friedl W, Caspari R, Sengteller M, Uhlhaas S, Lamberti C, Jungck Met al. Can APC mutation analysis contribute to therapeutic decisions in familial adenomatous polyposis? Experience from 680 FAP families. Gut 2001;48:515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barrow P, Khan M, Lalloo F, Evans DG, Hill J. Systematic review of the impact of registration and screening on colorectal cancer incidence and mortality in familial adenomatous polyposis and Lynch syndrome. Br J Surg 2013;100:1719–1731 [DOI] [PubMed] [Google Scholar]
- 47. Mallinson EKL, Newton KF, Bowen J, Lalloo F, Clancy T, Hill Jet al. The impact of screening and genetic registration on mortality and colorectal cancer incidence in familial adenomatous polyposis. Gut 2010;59:1378–1382 [DOI] [PubMed] [Google Scholar]
- 48. Anele CC, Xiang J, Martin I, Hawkins M, Clark SK, Faiz ODet al. Polyp progression in paediatric patients with familial adenomatous polyposis: a single-centre experience. J Pediatr Gastroenterol Nutr 2020;71:612–616 [DOI] [PubMed] [Google Scholar]
- 49. Matsumoto T, Esaki M, Fujisawa R, Nakamura S, Yao T, Iida M. Chromoendoscopy, narrow-band imaging colonoscopy, and autofluorescence colonoscopy for detection of diminutive colorectal neoplasia in familial adenomatous polyposis. Dis Colon Rectum 2009;52:1160–1165 [DOI] [PubMed] [Google Scholar]
- 50. Sarvepalli S, Burke CA, Monachese M, Leach BH, Laguardia L, O’Malley Met al. Natural history of colonic polyposis in young patients with familial adenomatous polyposis. Gastrointest Endosc 2018;88:726–733 [DOI] [PubMed] [Google Scholar]
- 51. Kobayashi H, Ishida H, Ueno H, Hinoi T, Inoue Y, Ishida Fet al. Association between the age and the development of colorectal cancer in patients with familial adenomatous polyposis: a multi-institutional study. Surg Today 2017;47:470–475 [DOI] [PubMed] [Google Scholar]
- 52. Newton KF, Mallinson EKL, Bowen J, Lalloo F, Clancy T, Hill Jet al. Genotype–phenotype correlation in colorectal polyposis. Clin Genet 2012;81:521–531. [DOI] [PubMed] [Google Scholar]
- 53. Nieuwenhuis MH, Mathus-Vliegen LM, Slors FJ, Griffioen G, Nagengast FM, Schouten WRet al. Genotype–phenotype correlations as a guide in the management of familial adenomatous polyposis. Clin Gastroenterol Hepatol 2007;5:374–378 [DOI] [PubMed] [Google Scholar]
- 54. Yamadera M, Ueno H, Kobayashi H, Konishi T, Ishida F, Yamaguchi Tet al. Current status of prophylactic surgical treatment for familial adenomatous polyposis in Japan. Surg Today 2017;47:690–696 [DOI] [PubMed] [Google Scholar]
- 55. Sinha A, Tekkis PP, Rashid S, Phillips RKS, Clark SK. Risk factors for secondary proctectomy in patients with familial adenomatous polyposis. Br J Surg 2010;97:1710–1715 [DOI] [PubMed] [Google Scholar]
- 56. Anele CC, Nachiappan S, Sinha A, Cuthill V, Jenkins JT, Clark SKet al. Safety and efficacy of laparoscopic near-total colectomy and ileo-distal sigmoid anastomosis as a modification of total colectomy and ileorectal anastomosis for prophylactic surgery in patients with adenomatous polyposis syndromes: a comparative study. Colorectal Dis 2020;22:799–805 [DOI] [PubMed] [Google Scholar]
- 57. Church J, Burke C, McGannon E, Pastean O, Clark B, Cohen Z. Predicting polyposis severity by proctoscopy: how reliable is it? Dis Colon Rectum 2001;44:1249–1252 [DOI] [PubMed] [Google Scholar]
- 58. Lee CHA, Kalady MF, Burke CA, Mankaney G, Ali Abbass M, Jia Xet al. Incidence and management of rectal cuff and anal transitional zone neoplasia in patients with familial adenomatous polyposis. Dis Colon Rectum 2021;64:977–985 [DOI] [PubMed] [Google Scholar]
- 59. Wasmuth HH, Tranø G, Myrvold HE, Aabakken L, Bakka A. Adenoma formation and malignancy after restorative proctocolectomy with or without mucosectomy in patients with familial adenomatous polyposis. Dis Colon Rectum 2013;56:288–294 [DOI] [PubMed] [Google Scholar]
- 60. Goldstein AL, Kariv R, Klausner JM, Tulchinsky H. Patterns of adenoma recurrence in familial adenomatous polyposis patients after ileal pouch–anal anastomosis. Dig Surg 2015;32:421–425 [DOI] [PubMed] [Google Scholar]
- 61. Aziz O, Athanasiou T, Fazio VW, Nicholls RJ, Darzi AW, Church Jet al. Meta-analysis of observational studies of ileorectal versus ileal pouch–anal anastomosis for familial adenomatous polyposis. Br J Surg 2006;93:407–417 [DOI] [PubMed] [Google Scholar]
- 62. Olsen K, Juul S, Bülow S, Järvinen HJ, Bakka A, Björk Jet al. Female fecundity before and after operation for familial adenomatous polyposis. Br J Surg 2003;90:227–231 [DOI] [PubMed] [Google Scholar]
- 63. Cornish JA, Tan E, Teare J, Teoh TG, Rai R, Darzi AWet al. The effect of restorative proctocolectomy on sexual function, urinary function, fertility, pregnancy and delivery: a systematic review. Dis Colon Rectum 2007;50:1128–1138 [DOI] [PubMed] [Google Scholar]
- 64. Öresland T, Palmablad S, Ellström M, Berndtsson I, Crona N, Hultén L. Gynaecological and sexual function related to anatomical changes in the female pelvis after restorative proctocolectomy. Int J Colorectal Dis 1994;9:77–81 [DOI] [PubMed] [Google Scholar]
- 65. Gorgun E, Remzi FH, Goldberg JM, Thornton J, Bast J, Hull TLet al. Fertility is reduced after restorative proctocolectomy with ileal pouch anal anastomosis: a study of 300 patients. Surgery 2004;136:795–803 [DOI] [PubMed] [Google Scholar]
- 66. Nieuwenhuis MH, Douma KF, Bleiker EM, Bemelman WA, Aaronson NK, Vasen HF. Female fertility after colorectal surgery for familial adenomatous polyposis: a nationwide cross-sectional study. Ann Surg 2010;252:341–344 [DOI] [PubMed] [Google Scholar]
- 67. Bartels SAL, D’Hoore A, Cuesta MA, Bensdorp AJ, Lucas C, Bemelman WA. Significantly increased pregnancy rates after laparoscopic restorative proctocolectomy: a cross-sectional study. Ann Surg 2012;256:1045–1048 [DOI] [PubMed] [Google Scholar]
- 68. Beyer-Berjot L, Maggiori L, Birnbaum D, Lefevre JH, Berdah S, Panis Y. A total laparoscopic approach reduces the infertility rate after ileal pouch–anal anastomosis: a 2-center study. Ann Surg 2013;258:275–282 [DOI] [PubMed] [Google Scholar]
- 69. Möslein G. Surgical considerations in FAP-related pouch surgery: could we do better? Fam Cancer 2016;15:457–466 [DOI] [PubMed] [Google Scholar]
- 70. Bartels SAL, Gardenbroek TJ, Aarts M, Ponsioen CY, Tanis PJ, Buskens CJet al. Short-term morbidity and quality of life from a randomized clinical trial of close rectal dissection and total mesorectal excision in ileal pouch–anal anastomosis. Br J Surg 2015;102:281–287 [DOI] [PubMed] [Google Scholar]
- 71. Remzi FH, Fazio VW, Gorgun E, Ooi BS, Hammel J, Preen Met al. The outcome after restorative proctocolectomy with or without defunctioning ileostomy. Dis Colon Rectum 2006;49:470–477 [DOI] [PubMed] [Google Scholar]
- 72. Lovegrove RE. To divert or not to divert. Arch Surg 2011;146:82. [DOI] [PubMed] [Google Scholar]
- 73. Hor T, Zalinski S, Lefevre JH, Shields C, Attal E, Tiret Eet al. Feasibility of laparoscopic restorative proctocolectomy without diverting stoma. Dig Liver Dis 2012;44:118–122 [DOI] [PubMed] [Google Scholar]
- 74. Ahmed O, Lefevre JH, Collard MK, Creavin B, Hor T, Debove Cet al. Is ileostomy mandatory for ileal pouch–anal anastomosis? A propensity matched analysis of 388 procedures. Surgery 2020;168:113–118 [DOI] [PubMed] [Google Scholar]
- 75. Pasquer A, Benech N, Pioche M, Breton A, Rivory J, Vinet Oet al. Prophylactic colectomy and rectal preservation in FAP: systematic endoscopic follow-up and adenoma destruction changes natural history of polyposis. Endosc Int Open 2021;9:E1014–E1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bülow C, Vasen H, Järvinen H, Björk J, Bisgaard ML, Bülow S. Ileorectal anastomosis is appropriate for a subset of patients with familial adenomatous polyposis. Gastroenterology 2000;119:1454–1460 [DOI] [PubMed] [Google Scholar]
- 77. Sasaki K, Nozawa H, Kawai K, Murono K, Emoto S, Kishikawa Jet al. Risk of extracolonic malignancies and metachronous rectal cancer after colectomy and ileorectal anastomosis in familial adenomatous polyposis. Asian J Surg 2022;45:396–400 [DOI] [PubMed] [Google Scholar]
- 78. Yamaguchi T, Yamamoto S, Fujita S, Akasu T, Moriya Y. Long-term outcome of metachronous rectal cancer following ileorectal anastomosis for familial adenomatous polyposis. J Gastrointest Surg 2010;14:500–505 [DOI] [PubMed] [Google Scholar]
- 79. Koskenvuo L, Renkonen-Sinisalo L, Järvinen HJ, Lepistö A. Risk of cancer and secondary proctectomy after colectomy and ileorectal anastomosis in familial adenomatous polyposis. Int J Colorectal Dis 2014;29:225–230 [DOI] [PubMed] [Google Scholar]
- 80. Church J, Burke C, McGannon E, Pastean O, Clark B. Risk of rectal cancer in patients after colectomy and ileorectal anastomosis for familial adenomatous polyposis: a function of available surgical options. Dis Colon Rectum 2003;46:1175–1181 [DOI] [PubMed] [Google Scholar]
- 81. Gleeson FC, Papachristou GI, Riegert-Johnson DL, Boller AM, Gostout CJ. Progression to advanced neoplasia is infrequent in post colectomy familial adenomatous polyposis patients under endoscopic surveillance. Fam Cancer 2009;8:33–38 [DOI] [PubMed] [Google Scholar]
- 82. Tajika M, Niwa Y, Bhatia V, Tanaka T, Ishihara M, Yamao K. Risk of ileal pouch neoplasms in patients with familial adenomatous polyposis. World J Gastroenterol 2013;19:6774–6783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Will OCC, Man RF, Phillips RKS, Tomlinson IP, Clark SK. Familial adenomatous polyposis and the small bowel: a loco-regional review and current management strategies. Pathol Res Pract 2008;204:449–458 [DOI] [PubMed] [Google Scholar]
- 84. M’koma AE, Herline AJ, Adunyah SE, M’koma AE. Subsequent adenomas of ileal pouch and anorectal segment after prophylactic surgery for familial adenomatous polyposis. World J Colorectal Surg 2013;3:art1. [PMC free article] [PubMed] [Google Scholar]
- 85. Schulz AC, Bojarski C, Buhr HJ, Kroesen AJ. Occurrence of adenomas in the pouch and small intestine of FAP patients after proctocolectomy with ileoanal pouch construction. Int J Colorectal Dis 2008;23:437–441 [DOI] [PubMed] [Google Scholar]
- 86. Tajika M, Nakamura T, Nakahara O, Kawai H, Komori K, Hirai Tet al. Prevalence of adenomas and carcinomas in the ileal pouch after proctocolectomy in patients with familial adenomatous polyposis. J Gastrointest Surg 2009;13:1266–1273 [DOI] [PubMed] [Google Scholar]
- 87. Pommaret E, Vienne A, Lefevre JH, Sogni P, Florent C, Desaint Bet al. Prevalence and risk factors for adenomas in the ileal pouch and the afferent loop after restorative proctocolectomy for patients with familial adenomatous polyposis. Surg Endosc 2013;27:3816–3822 [DOI] [PubMed] [Google Scholar]
- 88. Tajika M, Tanaka T, Ishihara M, Hirayama Y, Oonishi S, Mizuno Net al. Long-term outcomes of metachronous neoplasms in the ileal pouch and rectum after surgical treatment in patients with familial adenomatous polyposis. Endosc Int Open 2019;7:E691–E698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Friederich P, de Jong AE, Mathus-Vliegen LM, Dekker E, Krieken HH, Dees Jet al. Risk of developing adenomas and carcinomas in the ileal pouch in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008;6:1237–1242 [DOI] [PubMed] [Google Scholar]
- 90. Von Roon AC, Will OCC, Man RF, Neale KF, Phillips RKS, Nicholls RJet al. Mucosectomy with handsewn anastomosis reduces the risk of adenoma formation in the anorectal segment after restorative proctocolectomy for familial adenomatous polyposis. Ann Surg 2011;253:314–317 [DOI] [PubMed] [Google Scholar]
- 91. Hurlstone DP, Saunders BP, Church JM. Endoscopic surveillance of the ileoanal pouch following restorative proctocolectomy for familial adenomatous polyposis. Endoscopy 2008;40:437–442 [DOI] [PubMed] [Google Scholar]
- 92. Ganschow P, Trauth S, Hinz U, Schaible A, Büchler MW, Kadmon M. Risk factors associated with pouch adenomas in patients with familial adenomatous polyposis. Dis Colon Rectum 2018;61:1096–1101 [DOI] [PubMed] [Google Scholar]
- 93. Zahid A, Kumar S, Koorey D, Young CJ. Pouch adenomas in familial adenomatous polyposis after restorative proctocolectomy. Int J Surg 2015;13:133–136 [DOI] [PubMed] [Google Scholar]
- 94. Smith JC, Schäffer MW, Ballard BR, Smoot DT, Herline AJ, Adunyah SEet al. Adenocarcinomas after prophylactic surgery for familial adenomatous polyposis. J Cancer Ther 2013;4:260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tajika M, Tanaka T, Oonishi S, Yamada K, Kamiya T. Endoscopic management of adenomas in the ileal pouch and the rectal remnant after surgical treatment in familial adenomatous polyposis. J Clin Med 2022;11:3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Patel R V, Curtius K, Man R, Fletcher J, Cuthill V, Clark SKet al. Long-term outcomes of pouch surveillance and risk of neoplasia in familial adenomatous polyposis. Endoscopy 2023;55:836–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Van Leerdam ME, Roos VH, Van Hooft JE, Dekker E, Jover R, Kaminski MFet al. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2019;51:877–895 [DOI] [PubMed] [Google Scholar]
- 98. Thiruvengadam SS, Lopez R, O’Malley M, LaGuardia L, Church JM, Kalady Met al. Spigelman stage IV duodenal polyposis does not precede most duodenal cancer cases in patients with familial adenomatous polyposis. Gastrointest Endosc 2019;89:345–354.e2 [DOI] [PubMed] [Google Scholar]
- 99. Latchford AR, Neale KF, Spigelman AD, Phillips RKS, Clark SK. Features of duodenal cancer in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol 2009;7:659–663 [DOI] [PubMed] [Google Scholar]
- 100. Singh AD, Bhatt A, Joseph A, Lyu R, Heald B, Macaron Cet al. Natural history of ampullary adenomas in familial adenomatous polyposis: a long-term follow-up study. Gastrointest Endosc 2022;95:455–467.e3 [DOI] [PubMed] [Google Scholar]
- 101. Labib PL, Goodchild G, Turbett JP, Skipworth J, Shankar A, Johnson Get al. Endoscopic ultrasound in the assessment of advanced duodenal adenomatosis in familial adenomatous polyposis. BMJ Open Gastroenterol 2019;6:e000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cordero-Fernández C, Garzón-Benavides M, Pizarro-Moreno A, García-Lozano R, Márquez-Galán JL, López Ruiz Tet al. Gastroduodenal involvement in patients with familial adenomatous polyposis. Prospective study of the nature and evolution of polyps: evaluation of the treatment and surveillance methods applied. Eur J Gastroenterol Hepatol 2009;21:1161–1167 [DOI] [PubMed] [Google Scholar]
- 103. Sourrouille I, Lefèvre JH, Shields C, Colas C, Bellanger J, Desaint Bet al. Surveillance of duodenal polyposis in familial adenomatous polyposis: should the Spigelman score be modified? Dis Colon Rectum 2017;60:1137–1146 [DOI] [PubMed] [Google Scholar]
- 104. Bülow S, Christensen IJ, Højen H, Björk J, Elmberg M, Järvinen Het al. Duodenal surveillance improves the prognosis after duodenal cancer in familial adenomatous polyposis. Colorectal Dis 2012;14:947–952 [DOI] [PubMed] [Google Scholar]
- 105. Suraweera N, Latchford A, McCart A, Rogers P, Spain S, Sieber Oet al. Pregnancy does not influence colonic polyp multiplicity but may modulate upper gastrointestinal disease in patients with FAP. J Med Genet 2007;44:541–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sulbaran M, Campos F, Ribeiro U, Kishi H, Sakai P, de Moura Eet al. Risk factors for advanced duodenal and ampullary adenomatosis in familial adenomatous polyposis: a prospective, single-center study. Endosc Int Open 2018;6:E531–E540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Groves CJ, Saunders BP, Spigelman AD, Phillips RKS. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10-year prospective study. Gut 2002;50:636–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Serrano PE, Grant RC, Berk TC, Kim D, Al-Ali H, Cohen Zet al. Progression and management of duodenal neoplasia in familial adenomatous polyposis: a cohort study. Ann Surg 2015;261:1138–1144 [DOI] [PubMed] [Google Scholar]
- 109. Lepistö A, Kiviluoto T, Halttunen J, Järvinen HJ. Surveillance and treatment of duodenal adenomatosis in familial adenomatous polyposis. Endoscopy 2009;41:504–509 [DOI] [PubMed] [Google Scholar]
- 110. Park SY, Ryu JK, Park JH, Yoon H, Kim JY, Yoon YBet al. Prevalence of gastric and duodenal polyps and risk factors for duodenal neoplasm in Korean patients with familial adenomatous polyposis. Gut Liver 2011;5:46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mathus-Vliegen EMH, Boparai KS, Dekker E, Van Geloven N. Progression of duodenal adenomatosis in familial adenomatous polyposis: due to ageing of subjects and advances in technology. Fam Cancer 2011;10:491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Maehata Y, Esaki M, Hirahashi M, Kitazono T, Matsumoto T. Duodenal adenomatosis in Japanese patients with familial adenomatous polyposis. Dig Endosc 2014;26:30–34 [DOI] [PubMed] [Google Scholar]
- 113. Angsuwatcharakon P, Ahmed O, Lynch PM, Lum P, Gonzalez GN, Weston Bet al. Management of ampullary adenomas in familial adenomatous polyposis syndrome: 16 years of experience from a tertiary cancer center. Gastrointest Endosc 2020;92:323–330 [DOI] [PubMed] [Google Scholar]
- 114. Augustin T, Moslim MA, Cengiz TB, El-Hayek K, Simon R, Bhatt Aet al. Survival outcomes after surgical management of sporadic or familial adenomatous polyposis associated duodenal cancer. J Surg Oncol 2020;122:1132–1144 [DOI] [PubMed] [Google Scholar]
- 115. Balmforth DC, Phillips RKS, Clark SK. Advanced duodenal disease in familial adenomatous polyposis: how frequently should patients be followed up after successful therapy? Fam Cancer 2012;11:553–557 [DOI] [PubMed] [Google Scholar]
- 116. Biasco G, Nobili E, Calabrese C, Sassatelli R, Camellini L, Pantaleo MAet al. Impact of surgery on the development of duodenal cancer in patients with familial adenomatous polyposis. Dis Colon Rectum 2006;49:1860–1866 [DOI] [PubMed] [Google Scholar]
- 117. Campos FG, Martinez CAR, Sulbaran M, Bustamante-Lopez LA, Safatle-Ribeiro AV. Upper gastrointestinal neoplasia in familial adenomatous polyposis: prevalence, endoscopic features and management. J Gastrointest Oncol 2019;10:734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yachida T, Nakajima T, Nonaka S, Nakamura K, Suzuki H, Yoshinaga Set al. Characteristics and clinical outcomes of duodenal neoplasia in Japanese patients with familial adenomatous polyposis. J Clin Gastroenterol 2017;51:407–411 [DOI] [PubMed] [Google Scholar]
- 119. Roos VH, Bastiaansen BA, Kallenberg FGJ, Aelvoet AS, Bossuyt PMM, Fockens Pet al. Endoscopic management of duodenal adenomas in patients with familial adenomatous polyposis. Gastrointest Endosc 2021;93:457–466 [DOI] [PubMed] [Google Scholar]
- 120. Cecinato P, Parmeggiani F, Braglia L, Carlinfante G, Zecchini R, Decembrino Fet al. Endoscopic papillectomy for ampullary adenomas: different outcomes in sporadic tumors and those associated with familial adenomatous polyposis. J Gastrointest Surg 2021;25:457–466 [DOI] [PubMed] [Google Scholar]
- 121. Noh JH, Song EM, Ahn JY, Yang DH, Lee W, Hong Jet al. Prevalence and endoscopic treatment outcomes of upper gastrointestinal neoplasms in familial adenomatous polyposis. Surg Endosc 2022;36:1310–1319 [DOI] [PubMed] [Google Scholar]
- 122. Moussata D, Napoleon B, Lepilliez V, Klich A, Ecochard R, Lapalus MGet al. Endoscopic treatment of severe duodenal polyposis as an alternative to surgery for patients with familial adenomatous polyposis. Gastrointest Endosc 2014;80:817–825 [DOI] [PubMed] [Google Scholar]
- 123. Mankaney G, Leone P, Cruise M, LaGuardia L, O’Malley M, Bhatt Aet al. Gastric cancer in FAP: a concerning rise in incidence. Fam Cancer 2017;16:371–376 [DOI] [PubMed] [Google Scholar]
- 124. Martin I, Roos VH, Anele C, Walton SJ, Cuthill V, Suzuki Net al. Gastric adenomas and their management in familial adenomatous polyposis. Endoscopy 2021;53:795–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yoon JY, Mehta N, Burke CA, Augustin T, O’Malley M, LaGuardia Let al. The prevalence and significance of jejunal and duodenal bulb polyposis after duodenectomy in familial adenomatous polyposis retrospective cohort study. Ann Surg 2021;274:e1071–e1077 [DOI] [PubMed] [Google Scholar]
- 126. Alderlieste YA, Rauws EAJ, Mathus-Vliegen EMH, Fockens P, Dekker E. Prospective enteroscopic evaluation of jejunal polyposis in patients with familial adenomatous polyposis and advanced duodenal polyposis. Fam Cancer 2013;12:51–56 [DOI] [PubMed] [Google Scholar]
- 127. Sample DC, Samadder NJ, Pappas LM, Boucher KM, Samowitz WS, Berry Tet al. Variables affecting penetrance of gastric and duodenal phenotype in familial adenomatous polyposis patients. BMC Gastroenterol 2018;18:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Silva LC, Arruda RM, Botelho PFR, Taveira LN, Giardina KM, De Oliveira MAet al. Cap-assisted endoscopy increases ampulla of Vater visualization in high-risk patients. BMC Gastroenterol 2020;20:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kallenberg FGJ, Bastiaansen BAJ, Dekker E. Cap-assisted forward-viewing endoscopy to visualize the ampulla of Vater and the duodenum in patients with familial adenomatous polyposis. Endoscopy 2017;49:181–185 [DOI] [PubMed] [Google Scholar]
- 130. Hüneburg R, Heling D, Kaczmarek DJ, van Heteren P, Olthaus M, Fimmers Ret al. Dye chromoendoscopy leads to a higher adenoma detection in the duodenum and stomach in patients with familial adenomatous polyposis. Endosc Int Open 2020;08:E1308–E1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Picasso M, Filiberti R, Blanchi S, Conio M. The role of chromoendoscopy in the surveillance of the duodenum of patients with familial adenomatous polyposis. Dig Dis Sci 2007;52:1906–1909 [DOI] [PubMed] [Google Scholar]
- 132. Dekker E, Boparai KS, Poley JW, Mathus-Vliegen EMH, Offerhaus GJA, Kuipers EJet al. High resolution endoscopy and the additional value of chromoendoscopy in the evaluation of duodenal adenomatosis in patients with familial adenomatous polyposis. Endoscopy 2009;41:666–669 [DOI] [PubMed] [Google Scholar]
- 133. Lami G, Galli A, Biagini MR, Tarocchi M, Milani S, Polvani S. Gastric and duodenal polyps in familial adenomatous polyposis patients: conventional endoscopy vs virtual chromoendoscopy (fujinon intelligent color enhancement) in dysplasia evaluation. World J Clin Oncol 2017;8:168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lopez-Ceron M, Van Den Broek FJC, Mathus-Vliegen EM, Boparai KS, Van Eeden S, Fockens Pet al. The role of high-resolution endoscopy and narrow-band imaging in the evaluation of upper GI neoplasia in familial adenomatous polyposis. Gastrointest Endosc 2013;77:542–550 [DOI] [PubMed] [Google Scholar]
- 135. Wong RF, Tuteja AK, Haslem DS, Pappas L, Szabo A, Ogara MMet al. Video capsule endoscopy compared with standard endoscopy for the evaluation of small-bowel polyps in persons with familial adenomatous polyposis (with video). Gastrointest Endosc 2006;64:530–537 [DOI] [PubMed] [Google Scholar]
- 136. Clarke JO, Giday SA, Magno P, Shin EJ, Buscaglia JM, Jagannath SBet al. How good is capsule endoscopy for detection of periampullary lesions? Results of a tertiary-referral center. Gastrointest Endosc 2008;68:267–272 [DOI] [PubMed] [Google Scholar]
- 137. Mata A, Llach J, Castells A, Rovira JM, Pellisé M, Ginès Aet al. A prospective trial comparing wireless capsule endoscopy and barium contrast series for small-bowel surveillance in hereditary GI polyposis syndromes. Gastrointest Endosc 2005;61:721–725 [DOI] [PubMed] [Google Scholar]
- 138. Gluck N, Strul H, Rozner G, Leshno M, Santo E. Endoscopy and EUS are key for effective surveillance and management of duodenal adenomas in familial adenomatous polyposis. Gastrointest Endosc 2015;81:960–966 [DOI] [PubMed] [Google Scholar]
- 139. Drini M, Speer A, Dow C, Collier N, Bhathal P, Macrae FA. Management of duodenal adenomatosis in FAP: single centre experience. Fam Cancer 2012;11:167–173 [DOI] [PubMed] [Google Scholar]
- 140. Sekiya M, Sakamoto H, Yano T, Miyahara S, Nagayama M, Kobayashi Yet al. Double-balloon endoscopy facilitates efficient endoscopic resection of duodenal and jejunal polyps in patients with familial adenomatous polyposis. Endoscopy 2021;53:517–521 [DOI] [PubMed] [Google Scholar]
- 141. Skipworth JRA, Morkane C, Raptis DA, Vyas S, Olde Damink SW, Imber CJet al. Pancreaticoduodenectomy for advanced duodenal and ampullary adenomatosis in familial adenomatous polyposis. HPB 2011;13:342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Caillié F, Paye F, Desaint B, Bennis M, Lefèvre JH, Parc Yet al. Severe duodenal involvement in familial adenomatous polyposis treated by pylorus-preserving pancreaticoduodenectomy. Ann Surg Oncol 2012;19:2924–2931 [DOI] [PubMed] [Google Scholar]
- 143. Mehta NA, Shah RS, Yoon J, O’Malley M, LaGuardia L, Mankaney Get al. Risks, benefits, and effects on management for biopsy of the papilla in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol 2021;19:760–767 [DOI] [PubMed] [Google Scholar]
- 144. Bertoni G, Sassatelli R, Nigrisoli E, Pennazio M, Tansini P, Arrigoni Aet al. High prevalence of adenomas and microadenomas of the duodenal papilla and periampullary region in patients with familial adenomatous polyposis. Eur J Gastroenterol Hepatol 1996;8:1201–1206 [DOI] [PubMed] [Google Scholar]
- 145. Tianle M, Jang EJ, Zukerberg LR, Odze R, Gala MK, Kelsey PBet al. Recurrences are common after endoscopic ampullectomy for adenoma in the familial adenomatous polyposis (FAP) syndrome. Surg Endosc 2014;28:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Augustin T, Moslim MA, Tang A, Walsh RM. Tailored surgical treatment of duodenal polyposis in familial adenomatous polyposis syndrome. Surgery 2018;163:594–549 [DOI] [PubMed] [Google Scholar]
- 147. Watanabe Y, Ishida H, Baba H, Iwama T, Kudo A, Tanabe Met al. Pancreas-sparing total duodenectomy for Spigelman stage IV duodenal polyposis associated with familial adenomatous polyposis: experience of 10 cases at a single institution. Fam Cancer 2017;16:91–98 [DOI] [PubMed] [Google Scholar]
- 148. Greenblatt WH, Hur C, Knudsen AB, Evans JA, Chung DC, Gazelle GS. Cost-effectiveness of prophylactic surgery for duodenal cancer in familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev 2009;18:2677–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hurley JJ, Thomas LE, Walton SJ, Thomas-Gibson S, Haycock A, Suzuki Net al. The impact of chromoendoscopy for surveillance of the duodenum in patients with MUTYH-associated polyposis and familial adenomatous polyposis. Gastrointest Endosc 2018;88:665–673 [DOI] [PubMed] [Google Scholar]
- 150. Van Heumen BWH, Nieuwenhuis MH, Van Goor H, Mathus-Vliegen LMH, Dekker E, Gouma DJet al. Surgical management for advanced duodenal adenomatosis and duodenal cancer in Dutch patients with familial adenomatous polyposis: a nationwide retrospective cohort study. Surgery 2012;151:681–690 [DOI] [PubMed] [Google Scholar]
- 151. Jaganmohan S, Lynch PM, Raju RP, Ross WA, Lee JE, Raju GSet al. Endoscopic management of duodenal adenomas in familial adenomatous polyposis—a single-center experience. Dig Dis Sci 2012;57:732–737 [DOI] [PubMed] [Google Scholar]
- 152. Walsh RM, Augustin T, Aleassa EM, Simon R, El-Hayek KM, Moslim MAet al. Comparison of pancreas-sparing duodenectomy (PSD) and pancreatoduodenectomy (PD) for the management of duodenal polyposis syndromes. Surgery 2019;166:496–502 [DOI] [PubMed] [Google Scholar]
- 153. Ganschow P, Hackert T, Biegler M, Contin P, Hinz U, Büchler MWet al. Postoperative outcome and quality of life after surgery for FAP-associated duodenal adenomatosis. Langenbecks Arch Surg 2018;403:93–102 [DOI] [PubMed] [Google Scholar]
- 154. Parc Y, Mabrut JY, Shields C. Surgical management of the duodenal manifestations of familial adenomatous polyposis. Br J Surg 2011;98:480–484 [DOI] [PubMed] [Google Scholar]
- 155. Naples R, Simon R, Moslim M, Augustin T, Church J, Burke CAet al. Long-term outcomes of pancreas-sparing duodenectomy for duodenal polyposis in familial adenomatous polyposis syndrome. J Gastrointest Surg 2021;25:1233–1240 [DOI] [PubMed] [Google Scholar]
- 156. Alderlieste YA, Bastiaansen BA, Mathus-Vliegen EMH, Gouma DJ, Dekker E. High rate of recurrent adenomatosis during endoscopic surveillance after duodenectomy in patients with familial adenomatous polyposis. Fam Cancer 2013;12:699–706 [DOI] [PubMed] [Google Scholar]
- 157. Collard MK, Lefevre JH, Ahmed O, Voron T, Balladur P, Paye Fet al. Ten-year impact of pancreaticoduodenectomy on bowel function and quality of life of patients with ileal pouch–anal anastomosis for familial adenomatous polyposis 2020;22:1402–1410 [DOI] [PubMed] [Google Scholar]
- 158. De Castro SMM, Van Eijck CHJ, Rutten JP, Dejong CH, Van Goor H, Busch ORCet al. Pancreas-preserving total duodenectomy versus standard pancreatoduodenectomy for patients with familial adenomatous polyposis and polyps in the duodenum. Br J Surg 2008;95:1380–1386 [DOI] [PubMed] [Google Scholar]
- 159. Wong RF, DiSario JA. Endoscopic ampullectomy: management of periampullary/duodenal adenomas in familial adenomatous polyposis. Tech Gastrointest Endosc 2006;8:103–109 [Google Scholar]
- 160. Laleman W, Verreth A, Topal B, Aerts R, Komuta M, Roskams Tet al. Endoscopic resection of ampullary lesions: a single-center 8-year retrospective cohort study of 91 patients with long-term follow-up. Surg Endosc 2013;27:3865–3876 [DOI] [PubMed] [Google Scholar]
- 161. Cantalejo-Diáz M, Ramia-Ángel JM, Palomares-Cano A, Serradilla-Martín M. Pancreas-preserving total duodenectomy: a systematic review. Dig Surg 2021;38:186–197 [DOI] [PubMed] [Google Scholar]
- 162. Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008;6:180–185 [DOI] [PubMed] [Google Scholar]
- 163. Leone PJ, Mankaney G, Sarvapelli S, Abushamma S, Lopez R, Cruise Met al. Endoscopic and histologic features associated with gastric cancer in familial adenomatous polyposis. Gastrointest Endosc 2019;89:961–968 [DOI] [PubMed] [Google Scholar]
- 164. Mankaney GN, Cruise M, Sarvepalli S, Bhatt A, Arora Z, Baggot Bet al. Surveillance for pathology associated with cancer on endoscopy (SPACE): criteria to identify high-risk gastric polyps in familial adenomatous polyposis. Gastrointest Endosc 2020;92:755–762 [DOI] [PubMed] [Google Scholar]
- 165. Nakamura K, Nonaka S, Nakajima T, Yachida T, Abe S, Sakamoto Tet al. Clinical outcomes of gastric polyps and neoplasms in patients with familial adenomatous polyposis. Endosc Int Open 2017;05:E137–E145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Church JM, McGannon E, Hull-Boiner S, Sivak MV, Van Stolk R, Jagelman DGet al. Gastroduodenal polyps in patients with familial adenomatous polyposis. Dis Colon Rectum 1992;35:1170–1173 [DOI] [PubMed] [Google Scholar]
- 167. Sarre RG, Frost AG, Jagelman DG, Petras RE, Sivak MV, McGannon E. Gastric and duodenal polyps in familial adenomatous polyposis: a prospective study of the nature and prevalence of upper gastrointestinal polyps. Gut 1987;28:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Alkhouri N, Franciosi JP, Mamula P. Familial adenomatous polyposis in children and adolescents. J Pediatr Gastroenterol Nutr 2010;51:727–732 [DOI] [PubMed] [Google Scholar]
- 169. Wu TT, Kornacki S, Rashid A, Yardley JH, Hamilton SR. Dysplasia and dysregulation of proliferation in foveoral and surface epithelia of fundic gland polyps from patients with familial adenomatous polyposis. Am J Surg Pathol 1998;22:293–298 [DOI] [PubMed] [Google Scholar]
- 170. Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT. Fundic gland polyps in familial adenomatous polyposis: neoplasms with frequent somatic adenomatous polyposis coli gene alterations. Am J Pathol 2000;157:747–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wang KK, Kim K, Bancila L, Lew D, Larson BK, Kim Set al. Novel endoscopic polypectomy surveillance technique for fundic gland polyps in familial adenomatous polyposis can improve early detection of dysplasia and gastric cancer. Am J Gastroenterol 2022;117:1246–1254 [DOI] [PubMed] [Google Scholar]
- 172. Fatemi SR, Safaee A, Pasha S, Pourhoseingholi MA, Bahrainei R, Molaei M. Evaluation of endoscopic characteristics of upper gastrointestinal polyps in patients with familial adenomatous polyposis. Asian Pac J Cancer Prev 2014;15:6945–6948 [DOI] [PubMed] [Google Scholar]
- 173. Walton SJ, Frayling IM, Clark SK, Latchford A. Gastric tumours in FAP. Fam Cancer 2017;16:363–369 [DOI] [PubMed] [Google Scholar]
- 174. Wood LD, Salaria SN, Cruise MW, Giardiello FM, Montgomery EA. Upper GI tract lesions in familial adenomatous polyposis (FAP): enrichment of pyloric gland adenomas and other gastric and duodenal neoplasms. Am J Surg Pathol 2014;38:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Jagelman DG, Decosse JJ, Bussey HJR. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet 1988;331:1149–1151 [DOI] [PubMed] [Google Scholar]
- 176. Ngamruengphong S, Boardman LA, Heigh RI, Krishna M, Roberts ME, Riegert-Johnson DL. Gastric adenomas in familial adenomatous polyposis are common, but subtle, and have a benign course. Hered Cancer Clin Pract 2014;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Matsumoto M, Nakajima T, Kakugawa Y, Sakamoto T, Kuribayashi S, Otake Yet al. Surveillance using capsule endoscopy is safe in post-colectomy patients with familial adenomatous polyposis: a prospective Japanese study. Fam Cancer 2016;15:75–83 [DOI] [PubMed] [Google Scholar]
- 178. Iaquinto G, Fornasarig M, Quaia M, Giardullo N, D’Onofrio V, Iaquinto Set al. Capsule endoscopy is useful and safe for small-bowel surveillance in familial adenomatous polyposis. Gastrointest Endosc 2008;67:61–67 [DOI] [PubMed] [Google Scholar]
- 179. Mönkemüller K, Fry LC, Ebert M, Bellutti M, Venerito M, Knippig Cet al. Feasibility of double-balloon enteroscopy-assisted chromoendoscopy of the small bowel in patients with familial adenomatous polyposis. Endoscopy 2007;39:52–57 [DOI] [PubMed] [Google Scholar]
- 180. Günther U, Bojarski C, Buhr HJ, Zeitz M, Heller F. Capsule endoscopy in small-bowel surveillance of patients with hereditary polyposis syndromes. Int J Colorectal Dis 2010;25:1377–1382 [DOI] [PubMed] [Google Scholar]
- 181. Katsinelos P, Kountouras J, Chatzimavroudis G, Zavos C, Pilpilidis I, Fasoulas Ket al. Wireless capsule endoscopy in detecting small-intestinal polyps in familial adenomatous polyposis. World J Gastroenterol 2009;15:6075–6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Plum N, May A, Manner H, Eh C. Small-bowel diagnosis in patients with familial adenomatous polyposis: comparison of push enteroscopy, capsule endoscopy, ileoscopy, and enteroclysis. Z Gastroenterol 2009;47:339–346 [DOI] [PubMed] [Google Scholar]
- 183. Tescher P, Macrae FA, Speer T, Stella D, Gibson R, Tye-Din JAet al. Surveillance of FAP: a prospective blinded comparison of capsule endoscopy and other GI imaging to detect small bowel polyps. Hered Cancer Clin Pract 2010;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Tsukada K, Church JM, Jagelman DG, Fazio VW, McGannon E, George CRet al. Noncytotoxic drug therapy for intra-abdominal desmoid tumor in patients with familial adenomatous polyposis. Dis Colon Rectum 1992;35:29–33 [DOI] [PubMed] [Google Scholar]
- 185. Hartley JE, Church JM, Gupta S, McGannon E, Fazio VW, Phillips RKS. Significance of incidental desmoids identified during surgery for familial adenomatous polyposis. Dis Colon Rectum 2004;47:334–340 [DOI] [PubMed] [Google Scholar]
- 186. Nieuwenhuis MH, De Vos Tot Nederveen Cappel W, Botma A, Nagengast FM, Kleibeuker JH, Mathus-Vliegen EMHet al. Desmoid tumors in a Dutch cohort of patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008;6:215–219 [DOI] [PubMed] [Google Scholar]
- 187. Sturt NJH, Gallagher MC, Bassett P, Philp CR, Neale KF, Tomlinson IPMet al. Evidence for genetic predisposition to desmoid tumours in familial adenomatous polyposis independent of the germline APC mutation. Gut 2004;53:1832–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Reitamo JJ, Schelnin TM, Häyry P. The desmoid syndrome. New aspects in the cause, pathogenesis and treatment of the desmoid tumor. Am J Surg 1986;151:230–237 [DOI] [PubMed] [Google Scholar]
- 189. Inoue Y, Ishida H, Ueno H, Kobayashi H, Yamaguchi T, Konishi Tet al. The treatment of desmoid tumors associated with familial adenomatous polyposis: the results of a Japanese multicenter observational study. Surg Today 2017;47:1259–1267 [DOI] [PubMed] [Google Scholar]
- 190. Bonvalot S, Desai A, Coppola S, Le péchoux C, Terrier P, Dômont Jet al. The treatment of desmoid tumors: a stepwise clinical approach. Ann Oncol 2012;23:x158–x166 [DOI] [PubMed] [Google Scholar]
- 191. Ophir G, Sivan S, Hana S, Guy R, Nathan G, Naomi FIet al. Abdominal desmoid: course, severe outcomes, and unique genetic background in a large local series. Cancers (Basel) 2021;13:3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Xhaja X, Church J. Small bowel obstruction in patients with familial adenomatous polyposis related desmoid disease. Colorectal Dis 2013;15:1489–1492 [DOI] [PubMed] [Google Scholar]
- 193. Desurmont T, Lefèvre JH, Shields C, Colas C, Tiret E, Parc Y. Desmoid tumour in familial adenomatous polyposis patients: responses to treatments. Fam Cancer 2015;14:31–39 [DOI] [PubMed] [Google Scholar]
- 194. De Marchis ML, Tonelli F, Quaresmini D, Lovero D, Della-Morte D, Silvestris Fet al. Desmoid tumors in familial adenomatous polyposis. Anticancer Res 2017;37:3357–3366 [DOI] [PubMed] [Google Scholar]
- 195. Xiao J, Mao J, Li B. Clinical characteristics and treatment of intra-abdominal aggressive fibromatosis: a retrospective study of 16 patients. Front Med (Lausanne) 2020;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Melis M, Zager JS, Sondak VK. Multimodality management of desmoid tumors: how important is a negative surgical margin? J Surg Oncol 2008;98:594–602 [DOI] [PubMed] [Google Scholar]
- 197. Leal RF, Silva PVVT, Ayrizono MDLS, Fagundes JJ, Amstalden EMI, Coy CSR. Desmoid tumor in patients with familial adenomatous polyposis. Arq Gastroenterol 2010;47:373–378 [DOI] [PubMed] [Google Scholar]
- 198. Improta L, Tzanis D, Bouhadiba T, Abdelhafidh K, Bonvalot S. Desmoid tumours in the surveillance era: what are the remaining indications for surgery? Eur J Surg Oncol 2020;46:1310–1314 [DOI] [PubMed] [Google Scholar]
- 199. Sturt NJH, Clark SK. Current ideas in desmoid tumours. Fam Cancer 2006;5:275–285 [DOI] [PubMed] [Google Scholar]
- 200. Kalady MF, Church JM. Monitoring and management of desmoids and other extracolonic manifestations in familial adenomatous polyposis. Semin Colon Rectal Surg 2011;22:112–117 [Google Scholar]
- 201. Lev D, Kotilingam D, Wei C, Ballo MT, Zagars GK, Pisters PWTet al. Optimizing treatment of desmoid tumors. J Clin Oncol 2007;25:1785–1791 [DOI] [PubMed] [Google Scholar]
- 202. Okuno S. The enigma of desmoid tumors. Curr Treat Options Oncol 2006;7:438–443 [DOI] [PubMed] [Google Scholar]
- 203. Jung WB, Kim CW, Kim JC. Clinical characteristics and adequate treatment of familial adenomatous polyposis combined with desmoid tumors. Cancer Res Treat 2014;46:366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Church J. Management of desmoid disease. Semin Colon Rectal Surg 2018;29:111–115 [Google Scholar]
- 205. Sommovilla J, Liska D, Jia X, Kalady MF, Sklow B, Burke CAet al. IPAA is more ‘desmoidogenic’ than ileorectal anastomosis in familial adenomatous polyposis. Dis Colon Rectum 2022;65:1351–1361 [DOI] [PubMed] [Google Scholar]
- 206. Herraiz M, Barbesino G, Faquin W, Chan-Smutko G, Patel D, Shannon KMet al. Prevalence of thyroid cancer in familial adenomatous polyposis syndrome and the role of screening ultrasound examinations. Clin Gastroenterol Hepatol 2007;5:367–373 [DOI] [PubMed] [Google Scholar]
- 207. Ghorbanoghli Z, Bastiaansen BA, Langers AM, Nagengast FM, Poley JW, Hardwick JCet al. Extracolonic cancer risk in Dutch patients with APC (adenomatous polyposis coli)-associated polyposis. J Med Genet 2018;55:11–14 [DOI] [PubMed] [Google Scholar]
- 208. Chenbhanich J, Atsawarungruangkit A, Korpaisarn S, Phupitakphol T. Prevalence of thyroid diseases in familial adenomatous polyposis: a systematic review and meta-analysis. Fam Cancer 2018;18:53–62 [DOI] [PubMed] [Google Scholar]
- 209. Groen EJ, Roos A, Muntinghe FL, Enting RH, De Vries J, Kleibeuker JHet al. Extra-intestinal manifestations of familial adenomatous polyposis. Ann Surg Oncol 2008;15:2439–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006;295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 211. Steinhagen E, Hui VW, Levy RA, Markowitz AJ, Fish S, Wong RJet al. Results of a prospective thyroid ultrasound screening program in adenomatous polyposis patients. Am J Surg 2014;208:764–769 [DOI] [PubMed] [Google Scholar]
- 212. Steinhagen E, Guillem JG, Chang G, Salo-Mullen EE, Shia J, Fish Set al. The prevalence of thyroid cancer and benign thyroid disease in patients with familial adenomatous polyposis may be higher than previously recognized. Clin Colorectal Cancer 2012;11:304–308 [DOI] [PubMed] [Google Scholar]
- 213. Feng X, Milas M, Malley MO, Laguardia L, Berber E, Jin Jet al. Characteristics of benign and malignant thyroid and recommendations for disease surveillance. Thyroid 2015;25:325–332 [DOI] [PubMed] [Google Scholar]
- 214. Sada H, Hinoi T, Ueno H, Yamaguchi T, Inoue Y, Konishi Tet al. Prevalence of and risk factors for thyroid carcinoma in patients with familial adenomatous polyposis: results of a multicenter study in Japan and a systematic review. Surg Today 2019;49:72–81 [DOI] [PubMed] [Google Scholar]
- 215. Smith JR, Kamihara J, Church AJ, Asch E, Cherella CE, Fox VLet al. Thyroid nodules in children with familial adenomatous polyposis. Am J Gastroenterol 2022;117:1166–1168 [DOI] [PubMed] [Google Scholar]
- 216. Tomoda C, Miyauchi A, Uruno T, Takamura Y, Ito Y, Miya Aet al. Cribriform-morular variant of papillary thyroid carcinoma: clue to early detection of familial adenomatous polyposis-associated colon cancer. World J Surg 2004;28:886–889 [DOI] [PubMed] [Google Scholar]
- 217. Perrier ND, Van Heerden JA, Goellner JR, Williams ED, Gharib H, Marchesa Pet al. Thyroid cancer in patients with familial adenomatous polyposis. World J Surg 1998;22:738–743 [DOI] [PubMed] [Google Scholar]
- 218. Park J, Kim JW, Park H, Park SY, Kim TH, Kim SWet al. Multifocality in a patient with cribriform-morular variant of papillary thyroid carcinoma is an important clue for the diagnosis of familial adenomatous polyposis. Thyroid 2019 1;29:1606–1614 [DOI] [PubMed] [Google Scholar]
- 219. Uchino S, Ishikawa H, Miyauchi A, Hirokawa M, Noguchi S, Ushiama Met al. Age- and gender-specific risk of thyroid cancer in patients with familial adenomatous polyposis. J Clin Endocrinol Metab 2016;101:4611–4617 [DOI] [PubMed] [Google Scholar]
- 220. Shiroky JS, Lerner-Ellis JP, Govindarajan A, Urbach DR, Devon KM. Characteristics of adrenal masses in familial adenomatous polyposis. Dis Colon Rectum 2018;61:679–685 [DOI] [PubMed] [Google Scholar]
- 221. Marchesa P, Fazio VW, Church JM, McGannon E. Adrenal masses in patients with familial adenomatous polyposis. Dis Colon Rectum 1997;40:1023–1028 [DOI] [PubMed] [Google Scholar]
- 222. Will OCC, Hansmann A, Phillips RKS, Palazzo FF, Meeran K, Marshall Met al. Adrenal incidentaloma in familial adenomatous polyposis: a long-term follow-up study and schema for management. Dis Colon Rectum 2009;52:1637–1644 [DOI] [PubMed] [Google Scholar]
- 223. Terzolo M, Stigliano A, Chiodini I, Loli P, Furlani L, Arnaldi Get al. AME position statement on adrenal incidentaloma. Eur J Endocrinol 2011;164:851–870 [DOI] [PubMed] [Google Scholar]
- 224. Wakatsuki S, Sasano H, Matsui T, Nagashima K, Toyota T, Horii A. Adrenocortical tumor in a patient with familial adenomatous polyposis: a case associated with a complete inactivating mutation of the APC gene and unusual histological features. Hum Pathol 1998;29:302–306 [DOI] [PubMed] [Google Scholar]
- 225. Gaujoux S, Pinson S, Gimenez-Roqueplo AP, Amar L, Ragazzon B, Launay Pet al. Inactivation of the APC gene is constant in adrenocortical tumors from patients with familial adenomatous polyposis but not frequent in sporadic adrenocortical cancers. Clin Cancer Res 2010;16:5133–5141 [DOI] [PubMed] [Google Scholar]
- 226. Kallenberg FGJ, Bastiaansen BAJ, Nio CY, Soeters MR, Boermeester MA, Aalfs CMet al. Adrenal lesions in patients with (attenuated) familial adenomatous polyposis and MUTYH-associated polyposis. Dis Colon Rectum 2017;60:1057–1064 [DOI] [PubMed] [Google Scholar]
- 227. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev Aet al. Management of adrenal incidentalomas: European Society of Endocrinology clinical practice guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2016;175:G1–G34 [DOI] [PubMed] [Google Scholar]
- 228. Reimondo G, Muller A, Ingargiola E, Puglisi S, Terzolo M. Is follow-up of adrenal incidentalomas always mandatory? Endocrinol Metab 2020;35:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Trobaugh-Lotrario AD, López-Terrada D, Li P, Feusner JH. Hepatoblastoma in patients with molecularly proven familial adenomatous polyposis: clinical characteristics and rationale for surveillance screening. Pediatr Blood Cancer 2018;65:e27103. [DOI] [PubMed] [Google Scholar]
- 230. Galiatsatos P, Foulkes WD. Familial adenomatous polyposis. Am J Gastroenterol 2006;101:385–398 [DOI] [PubMed] [Google Scholar]
- 231. Aretz S, Koch A, Uhlhaas S, Friedl W, Propping P, von Schweinitz Det al. Should children at risk for familial adenomatous polyposis be screened for hepatoblastoma and children with apparently sporadic hepatoblastoma be screened for APC germline mutations? Pediatr Blood Cancer 2006;47:811–818 [DOI] [PubMed] [Google Scholar]
- 232. Chen CS, Phillips KD, Grist S, Bennet G, Craig JE, Muecke JSet al. Congenital hypertrophy of the retinal pigment epithelium (CHRPE) in familial colorectal cancer. Fam Cancer 2006;5:397–404 [DOI] [PubMed] [Google Scholar]
- 233. Rehan S, Aye K. In patients with a positive family history of familial adenomatous polyposis can the condition be diagnosed from the presence of congenital hypertrophy of the retinal pigment epithelium detected via an eye examination: a systematic review. Clin Exp Ophthalmol 2020;48:98–116 [DOI] [PubMed] [Google Scholar]
- 234. Mirinezhad SK, Mousavi F, Baghri M, Sepehri B, Ghavidel A, Ghojazadeh Met al. Congenital hypertrophy of retinal pigment epithelium for diagnosis of familial adenomatous polyposis—the first FAP registry in Iran. Asian Pac J Cancer Prev 2018;19:167–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Nusliha A, Dalpatadu U, Amarasinghe B, Chandrasinghe PC, Deen KI. Congenital hypertrophy of retinal pigment epithelium (CHRPE) in patients with familial adenomatous polyposis (FAP); a polyposis registry experience. BMC Res Notes 2014;7:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Burger B, Cattani N, Trueb S, Lorenzo R, Albertini M, Bontognali Eet al. Prevalence of skin lesions in familial adenomatous polyposis: a marker for presymptomatic diagnosis? Oncologist 2011;16:1698–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. D’Agostino S, Dell’Olio F, Tempesta A, Cervinara F, D’Amati A, Dolci Met al. Osteoma of the jaw as first clinical sign of Gardner’s syndrome: the experience of two Italian centers and review. J Clin Med 2023;12:1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Antohi C, Haba D, Caba L, Ciofu ML, Drug VL, Bărboi OBet al. Novel mutation in APC gene associated with multiple osteomas in a family and review of genotype–phenotype correlations of extracolonic manifestations in Gardner syndrome. Diagnostics (Basel) 2021;11:1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Ring KL, Bruegl AS, Allen BA, Elkin EP, Singh N, Hartman ARet al. Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod Pathol 2016;29:1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Wang Y, Yu M, Yang JX, Cao DY, Zhang Y, Zhou HMet al. Genomic comparison of endometrioid endometrial carcinoma and its precancerous lesions in Chinese patients by high-depth next generation sequencing. Front Oncol 2019;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Lincoln SE, Nussbaum RL, Kurian AW, Nielsen SM, Das K, Michalski Set al. Yield and utility of germline testing following tumor sequencing in patients with cancer. JAMA Netw Open 2020;3:e2019452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Cadoo KA, Mandelker DL, Mukherjee S, Stewart C, DeLair D, Ravichandran Vet al. Understanding inherited risk in unselected newly diagnosed patients with endometrial cancer. JCO Precis Oncol 2019;3:PO.18.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Crobach S, Van Wezel T, Vasen HF, Morreau H. Ovarian metastases of colorectal and duodenal cancer in familial adenomatous polyposis. Fam Cancer 2012;11:671–673 [DOI] [PubMed] [Google Scholar]
- 244. Fernandez E, Vecchia CLA, Balducci A, Chatenoud L, Franceschi S, Negri E. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer 2001;84:722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Stevanato Filho PR, Aguiar Júnior S, Begnami MD, Kuasne H, Spencer RM, Nakagawa WTet al. Oestrogen receptor beta isoform expression in sporadic colorectal cancer, familial adenomatous polyposis and progressive stages of colorectal cancer. BMC Cancer 2017;17:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Giardiello FM, Hylind LM, Trimbath JD, Hamilton SR, Romans KE, Cruz-Correa Met al. Oral contraceptives and polyp regression in familial adenomatous polyposis. Gastroenterology 2005;128:1077–1080 [DOI] [PubMed] [Google Scholar]
- 247. Johansen C, Bitsch M, Bülow S. Fertility and pregnancy in women with familial adenomatous polyposis. Int J Colorectal Dis 1990;5:203–206 [DOI] [PubMed] [Google Scholar]
- 248. Shandley LM, McKenzie LJ. Recent advances in fertility preservation and counseling for reproductive-aged women with colorectal cancer: a systematic review. Dis Colon Rectum 2019;62:762–771 [DOI] [PubMed] [Google Scholar]
- 249. Cornish JA, Tan E, Singh B, Bundock H, Mortensen N, Nicholls RJet al. Female infertility following restorative proctocolectomy. Colorectal Dis 2011;13:e339–e344 [DOI] [PubMed] [Google Scholar]
- 250. Burke CA, Dekker E, Lynch P, Samadder NJ, Balaguer F, Hüneburg Ret al. Eflornithine plus sulindac for prevention of progression in familial adenomatous polyposis. N Engl J Med 2020;383:1028–1039 [DOI] [PubMed] [Google Scholar]
- 251. Burke CA, Phillips R, Berger MF, Li C, Essex MN, Iorga Det al. Children’s International Polyposis (CHIP) study: a randomized, double-blind, placebo-controlled study of celecoxib in children with familial adenomatous polyposis. Clin Exp Gastroenterol 2017;10:177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Van Heumen BW, Roelofs HM, Vink-Börger ME, Dekker E, Mathus-Vliegen EM, Dees Jet al. Ursodeoxycholic acid counteracts celecoxib in reduction of duodenal polyps in patients with familial adenomatous polyposis: a multicentre, randomized controlled trial. Orphanet J Rare Dis 2013;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Samadder NJ, Neklason DW, Boucher KM, Byrne KR, Kanth P, Samowitz Wet al. Effect of sulindac and erlotinib vs placebo: on duodenal neoplasia in familial adenomatous polyposis: a randomized clinical trial. JAMA 2016;315:1266–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Parc Y, Desaint B, Fléjou JF, Lefèvre JH, Serfaty L, Vienne Aet al. The effect of ursodesoxycholic acid on duodenal adenomas in familial adenomatous polyposis: a prospective randomized placebo-control trial. Colorectal Dis 2012;14:854–860 [DOI] [PubMed] [Google Scholar]
- 255. Burn J, Bishop DT, Chapman PD, Elliott F, Bertario L, Dunlop MGet al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res (Phila) 2011;4:655–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Ishikawa H, Mutoh M, Sato Y, Doyama H, Tajika M, Tanaka Set al. Chemoprevention with low-dose aspirin, mesalazine, or both in patients with familial adenomatous polyposis without previous colectomy (J-FAPP Study IV): a multicentre, double-blind, randomised, two-by-two factorial design trial. Lancet Gastroenterol Hepatol 2021;6:474–481 [DOI] [PubMed] [Google Scholar]
- 257. Ishikawa H, Wakabayashi K, Suzuki S, Mutoh M, Hirata K, Nakamura Tet al. Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: double-blind, randomized clinical trial. Cancer Med 2013;2:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Phillips RK, Wallace MH, Lynch PM, Hawk E, Gordon GB, Saunders BPet al. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis familial adenomatous polyposis. Gut 2002;50:857–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. European Medicines Agency . European Medicines Agency Concludes on Use of Celecoxib in Familial Adenomatous Polyposis. 2011. https://www.ema.europa.eu/en/news/european-medicines-agency-concludes-use-celecoxib-familial-adenomatous-polyposis#:∼:text=The CHMP concluded that the, treatment used in FAP patients(accessed 1 November 2023)
- 260. Food and Drug Administration . Withdrawal of Approval of Familial Adenomatous Polyposis Indication for CELEBREX. 2012. https://www.federalregister.gov/documents/2012/06/08/2012-13900/pfizer-inc-withdrawal-of-approval-of-familial-adenomatous-polyposis-indication-for-celebrex(accessed 1 November 2023)
- 261. Olschwang S, Blanché H, De Moncuit C, Thomas G. Similar colorectal cancer risk in patients with monoallelic and biallelic mutations in the MYH gene identified in a population with adenomatous polyposis. Genet Test 2007;11:315–20 [DOI] [PubMed] [Google Scholar]
- 262. Patel R, McGinty P, Cuthill V, Hawkins M, Moorghen M, Clark SKet al. MUTYH-associated polyposis—colorectal phenotype and management. Colorectal Dis 2020;22:1271–1278 [DOI] [PubMed] [Google Scholar]
- 263. Thomas LE, Hurley JJ, Sanchez AA, Aznárez MR, Backman AS, Bjork Jet al. Duodenal adenomas and cancer in MUTYH-associated polyposis: an international cohort study. Gastroenterology 2021;160:952–954.e4 [DOI] [PubMed] [Google Scholar]
- 264. Nielsen M, Joerink—van de Beld MC, Jones N, Vogt S, Tops CM, Vasen HFAet al. Analysis of MUTYH genotypes and colorectal phenotypes in patients with MUTYH-associated polyposis. Gastroenterology 2009;136:471–476 [DOI] [PubMed] [Google Scholar]
- 265. Aretz S, Uhlhaas S, Goergens H, Siberg K, Vogel M, Pagenstecher Cet al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer 2006;119:807–814 [DOI] [PubMed] [Google Scholar]
- 266. Lipton L, Halford SE, Johnson V, Novelli MR, Jones A, Cummings Cet al. Carcinogenesis in MYH-associated polyposis follows a distinct genetic pathway. Cancer Res 2003;63:7595–7599 [PubMed] [Google Scholar]
- 267. O’Shea AM, Cleary SP, Croitoru MA, Kim H, Berk T, Monga Net al. Pathological features of colorectal carcinomas in MYH-associated polyposis. Histopathology 2008;53:184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Nascimbeni R, Pucciarelli S, Di Lorenzo D, Urso E, Casella C, Agostini Met al. Rectum-sparing surgery may be appropriate for biallelic MutYH-associated polyposis. Dis Colon Rectum 2010;53:1670–1675 [DOI] [PubMed] [Google Scholar]
- 269. Walton SJ, Kallenberg FGJ, Clark SK, Dekker E, Latchford A. Frequency and features of duodenal adenomas in patients with MUTYH-associated polyposis. Clin Gastroenterol Hepatol 2016;14:986–992 [DOI] [PubMed] [Google Scholar]
- 270. Bouguen G, Manfredi S, Blayau M, Dugast C, Buecher B, Bonneau Det al. Colorectal adenomatous polyposis associated with MYH mutations: genotype and phenotype characteristics. Dis Colon Rectum 2007;50:1612–1617 [DOI] [PubMed] [Google Scholar]
- 271. Lefevre JH, Parc Y, Svrcek M, Kernéis S, Colas C, Shields Cet al. APC, MYH, and the correlation genotype–phenotype in colorectal polyposis. Ann Surg Oncol 2009;16:871–877 [DOI] [PubMed] [Google Scholar]
- 272. Bülow S, Björk J, Christensen IJ, Fausa O, Järvinen H, Moesgaard Fet al. Duodenal adenomatosis in familial adenomatous polyposis. Gut 2004;53:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Stjepanovic N, Moreira L, Carneiro F, Balaguer F, Cervantes A, Balmaña Jet al. Hereditary gastrointestinal cancers: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:1558–1571 [DOI] [PubMed] [Google Scholar]
- 274. Colas C, Bonadona V, Baert-Desurmont S, Bonnet D, Coulet F, Dhooge Met al. MUTYH-associated polyposis: review and update of the French recommendations established in 2012 under the auspices of the National Cancer institute (INCa). Eur J Med Genet 2020;63:104078. [DOI] [PubMed] [Google Scholar]
- 275. Thomas LE, Hurley JJ, Meuser E, Jose S, Ashelford KE, Mort Met al. Burden and profile of somatic mutation in duodenal adenomas from patients with familial adenomatous- and MUTYH-associated polyposis. Clin Cancer Res 2017;23:6721–6732 [DOI] [PubMed] [Google Scholar]
- 276. Dunlop MG, Farrington SM. MUTYH-associated polyposis and colorectal cancer. Surg Oncol Clin N Am 2009;18:599–610 [DOI] [PubMed] [Google Scholar]
- 277. Poulsen MLM, Bisgaard ML. MUTYH associated polyposis (MAP). Curr Genomics 2008;9:420–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Torrezan GT, da Silva FC, Santos EM, Krepischi AC, Achatz MI, Jr ASet al. Mutational spectrum of the APC and MUTYH genes and genotype–phenotype correlations in Brazilian FAP, AFAP, and MAP patients. Orphanet J Rare Dis 2013;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Leoz ML, Carballal S, Moreira L, Ocaña T, Balaguer F. The genetic basis of familial adenomatous polyposis and its implications for clinical practice and risk management. Appl Clin Genet 2015;8:95–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Wasielewski M, Out AA, Vermeulen J, Nielsen M, Van Den Ouweland A, Tops CMJet al. Increased MUTYH mutation frequency among Dutch families with breast cancer and colorectal cancer. Breast Cancer Res Treat 2010;124:635–641 [DOI] [PubMed] [Google Scholar]
- 281. Sereno M, Merino M, López-Gómez M, Gómez-Raposo C, Zambrana Tébar F, Moreno Rubio Jet al. MYH polyposis syndrome: clinical findings, genetics issues and management. Clin Transl Oncol 2014;16:675–679 [DOI] [PubMed] [Google Scholar]
- 282. Lorca V, Rueda D, Martín-Morales L, Fernández-Aceñero MJ, Grolleman J, Poves Cet al. Contribution of new adenomatous polyposis predisposition genes in an unexplained attenuated Spanish cohort by multigene panel testing. Sci Rep 2019;9:9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Sanders MA, Chew E, Flensburg C, Zeilemaker A, Miller SE, Al Hinai ASet al. MBD4 guards against methylation damage and germ line deficiency predisposes to clonal hematopoiesis and early-onset AML. Blood 2018;132:1526–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Jansen AML, Crobach S, Geurts-Giele WRR, van den Akker BEWM, Garcia MV, Ruano Det al. Distinct patterns of somatic mosaicism in the APC gene in neoplasms from patients with unexplained adenomatous polyposis. Gastroenterology 2017;152:546–549.e3 [DOI] [PubMed] [Google Scholar]
- 285. Landon M, Ceulemans S, Saraiya DS, Strike B, Arnell C, Burbidge LAet al. Analysis of current testing practices for biallelic MUTYH mutations in MUTYH-associated polyposis. Clin Genet 2015;87:368–372 [DOI] [PubMed] [Google Scholar]
- 286. Terlouw D, Suerink M, Singh SS, Gille HJJP, Hes FJ, Langers AMJet al. Declining detection rates for APC and biallelic MUTYH variants in polyposis patients, implications for DNA testing policy. Eur J Hum Genet 2020;28:222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Monahan KJ, Bradshaw N, Dolwani S, Desouza B, Dunlop MG, East JEet al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020;69:411–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Cavestro GM, Mannucci A, Balaguer F, Hampel H, Kupfer SS, Repici Aet al. Delphi initiative for early-onset colorectal cancer (DIRECt) international management guidelines. Clin Gastroenterol Hepatol 2023;21:581–603.e33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Nielsen M, Hes FJ, Vasen HFA, van den Hout WB. Cost–utility analysis of genetic screening in families of patients with germline MUTYH mutations. BMC Med Genet 2007;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Sehested A, Meade J, Scheie D, Østrup O, Bertelsen B, Misiakou MAet al. Constitutional POLE variants causing a phenotype reminiscent of constitutional mismatch repair deficiency. Hum Mutat 2022;43:85–96 [DOI] [PubMed] [Google Scholar]
- 291. Wimmer K, Beilken A, Nustede R, Ripperger T, Lamottke B, Ure Bet al. A novel germline POLE mutation causes an early onset cancer prone syndrome mimicking constitutional mismatch repair deficiency. Fam Cancer 2017;16:67–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Lindsay H, Scollon S, Reuther J, Voicu H, Rednam SP, Lin FYet al. Germline POLE mutation in a child with hypermutated medulloblastoma and features of constitutional mismatch repair deficiency. Cold Spring Harb Mol Case Stud 2019;5:a004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Yanaru-Fujisawa R, Nakamura S, Moriyama T, Esaki M, Tsuchigame T, Gushima Met al. Familial fundic gland polyposis with gastric cancer. Gut 2012;61:1103–1104 [DOI] [PubMed] [Google Scholar]
- 294. Li J, Woods SL, Healey S, Beesley J, Chen X, Lee JSet al. Point mutations in Exon 1B of APC reveal gastric adenocarcinoma and proximal polyposis of the stomach as a familial adenomatous polyposis variant. Am J Hum Genet 2016;98:830–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Repak R, Kohoutova D, Podhola M, Rejchrt S, Minarik M, Benesova Let al. The first European family with gastric adenocarcinoma and proximal polyposis of the stomach: case report and review of the literature. Gastrointest Endosc 2016;84:718–725 [DOI] [PubMed] [Google Scholar]
- 296. Beer A, Streubel B, Asari R, Dejaco C, Oberhuber G. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS)—a rare recently described gastric polyposis syndrome—report of a case. Z Gastroenterol 2017;55:1131–1134 [DOI] [PubMed] [Google Scholar]
- 297. Rudloff U. Gastric adenocarcinoma and proximal polyposis of the stomach: diagnosis and clinical perspectives. Clin Exp Gastroenterol 2018;11:447–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Foretova L, Navratilova M, Svoboda M, Grell P, Nemec L, Sirotek Let al. GAPPS—gastric adenocarcinoma and proximal polyposis of the stomach syndrome in 8 families tested at Masaryk Memorial Cancer Institute—prevention and prophylactic gastrectomies. Klin Onkol 2019;32:109–117 [DOI] [PubMed] [Google Scholar]
- 299. Mala T, Førland DT, Vetti HH, Skagemo CU, Johannessen HO, Johnson E. Gastrisk adenokarsinom ogproksimal ventrikkelpolypose—ensjelden form for arvelig magesekkreft. Tidsskr Nor Laegeforen 2020;140:1–9 [DOI] [PubMed] [Google Scholar]
- 300. De Boer WB, Ee H, Kumarasinghe MP. Neoplastic lesions of gastric adenocarcinoma and proximal polyposis syndrome (GAPPS) are gastric phenotype. Am J Surg Pathol 2018;42:1–8 [DOI] [PubMed] [Google Scholar]
- 301. Grossman A, Colavito J, Levine J, Thomas KM, Greifer M. Filling in the “GAPPS”: an unusual presentation of a child with gastric adenocarcinoma and proximal polyposis of the stomach. Gastric Cancer 2022;25:468–472 [DOI] [PubMed] [Google Scholar]
- 302. Mitsui Y, Yokoyama R, Fujimoto S, Kagemoto K, Kitamura S, Okamoto Ket al. First report of an Asian family with gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS) revealed with the germline mutation of the APC exon 1B promoter region. Gastric Cancer 2018;21:1058–1063 [DOI] [PubMed] [Google Scholar]
- 303. Blair VR, McLeod M, Carneiro F, Coit DG, D’Addario JL, van Dieren JMet al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 2020;21:e386–e397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Capelle LG, Van Grieken NCT, Lingsma HF, Steyerberg EW, Klokman WJ, Bruno MJet al. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology 2010;138:487–492 [DOI] [PubMed] [Google Scholar]
- 305. Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro Het al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol 2015;1:23–32 [DOI] [PubMed] [Google Scholar]
- 306. Carneiro F, Chenevix-Trench G, de Boer W, Kumarasinghe M, Worthley D. GAPPS and other fundic gland polyposes. In: WHO Classification of Tumours Digestive System Tumours (5th edn). Lyon, France: 2019, 526–528 [Google Scholar]
- 307. Freeman HJ. Proton pump inhibitors and an emerging epidemic of gastric fundic gland polyposis. World J Gastroenterol 2008;14:1318–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Tacheci I, Repak R, Podhola M, Benesova L, Cyrany J, Bures Jet al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS)—a Helicobacter-opposite point. Best Pract Res Clin Gastroenterol 2021;50–51:101728. [DOI] [PubMed] [Google Scholar]
- 309. Matsumoto C, Iwatsuki M, Iwagami S, Morinaga T, Yamashita K, Nakamura Ket al. Prophylactic laparoscopic total gastrectomy for gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): the first report in Asia. Gastric Cancer 2022;25:473–478 [DOI] [PubMed] [Google Scholar]
- 310. McDuffie LA, Sabesan A, Allgäeuer M, Xin L, Koh C, Heller Tet al. β-Catenin activation in fundic gland polyps, gastric cancer and colonic polyps in families afflicted by ‘gastric adenocarcinoma and proximal polyposis of the stomach’ (GAPPS). J Clin Pathol 2016;69:826–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Higashizono K, Nomura S, Yagi K, Aikou S, Nishida M, Yamashita Het al. Pregnancy, delivery, and breastfeeding after total gastrectomy for gastric cancer: a case report. World J Surg Oncol 2018;16:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Iwakawa Y, Yoshikawa K, Okamoto K, Takayama T, Tokunaga T, Nakao Tet al. Four cases of gastric adenocarcinoma and proximal polyposis of the stomach treated by robotic total gastrectomy. Surg Case Rep 2022;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Burt RW, Leppert MF, Slattery ML, Samowitz WS, Spirio LN, Kerber RAet al. Genetic testing and phenotype in a large kindred with attenuated familial adenomatous polyposis. Gastroenterology 2004;127:444–451 [DOI] [PubMed] [Google Scholar]
- 314. Ishikawa H, Mutoh M, Iwama T, Suzuki S, Abe T, Takeuchi Yet al. Endoscopic management of familial adenomatous polyposis in patients refusing colectomy. Endoscopy 2016;48:51–55 [DOI] [PubMed] [Google Scholar]
- 315. Bisschops R, East JE, Hassan C, Hazewinkel Y, Kamiński MF, Neumann Het al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) guideline—update 2019. Endoscopy 2019;51:1155–1179 [DOI] [PubMed] [Google Scholar]
- 316. Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015;110:223–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Yang J, Gurudu SR, Koptiuch C, Agrawal D, Buxbaum JL, Abbas Fehmi SMet al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc 2020;91:963–982.e2 [DOI] [PubMed] [Google Scholar]
- 318. Sarvepalli S, Burke CA, Monachese M, Lopez R, Leach BH, Laguardia Let al. Web-based model for predicting time to surgery in young patients with familial adenomatous polyposis: an internally validated study. Am J Gastroenterol 2018;113:1881–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Dossa F, Morris AM, Wilson AR, Baxter NN. Life after surgery: surgeon assessments of quality of life among patients with familial adenomatous polyposis. Dis Colon Rectum 2018;61:1217–1222 [DOI] [PubMed] [Google Scholar]
- 320. Valanzano R, Ficari F, Curia MC, Aceto G, Veschi S, Cama Aet al. Balance between endoscopic and genetic information in the choice of ileorectal anastomosis for familial adenomatous polyposis. J Surg Oncol 2007;95:28–33 [DOI] [PubMed] [Google Scholar]
- 321. Maehata Y, Esaki M, Nakamura S, Hirahashi M, Ueki T, Iida Met al. Risk of cancer in the rectal remnant after ileorectal anastomosis in patients with familial adenomatous polyposis: single center experience. Dig Endosc 2015;27:471–478 [DOI] [PubMed] [Google Scholar]
- 322. Bülow S, Bülow C, Vasen H, Järvinen H, Björk J, Christensen IJ. Colectomy and ileorectal anastomosis is still an option for selected patients with familial adenomatous polyposis. Dis Colon Rectum 2008;51:1318–1323 [DOI] [PubMed] [Google Scholar]
- 323. Campos FG, Imperiale AR, Seid VE, Perez RO, Da Silva e Sousa AH, Kiss DRet al. Rectal and pouch recurrences after surgical treatment for familial adenomatous polyposis. J Gastrointest Surg 2009;13:129–136 [DOI] [PubMed] [Google Scholar]
- 324. Nieuwenhuis MH, Bülow S, Björk J, Järvinen HJ, Bülow C, Bisgaard MLet al. Genotype predicting phenotype in familial adenomatous polyposis: a practical application to the choice of surgery. Dis Colon Rectum 2009;52:1259–1263 [DOI] [PubMed] [Google Scholar]
- 325. Chambers WM, Mortensen NJMC. Should ileal pouch–anal anastomosis include mucosectomy? Colorectal Dis 2007;9:384–392 [DOI] [PubMed] [Google Scholar]
- 326. Ozdemir Y, Kalady MF, Aytac E, Kiran RP, Erem HH, Church JMet al. Anal transitional zone neoplasia in patients with familial adenomatous polyposis after restorative proctocolectomy and IPAA: incidence, management, and oncologic and functional outcomes. Dis Colon Rectum 2013;56:808–814 [DOI] [PubMed] [Google Scholar]
- 327. Koskenvuo L, Mustonen H, Renkonen-Sinisalo L, Järvinen HJ, Lepistö A. Comparison of proctocolectomy and ileal pouch–anal anastomosis to colectomy and ileorectal anastomosis in familial adenomatous polyposis. Fam Cancer 2015;14:221–227 [DOI] [PubMed] [Google Scholar]
- 328. Ardoino I, Signoroni S, Malvicini E, Ricci MT, Biganzoli EM, Bertario Let al. Long-term survival between total colectomy versus proctocolectomy in patients with FAP: a registry-based, observational cohort study. Tumori 2020;106:139–148 [DOI] [PubMed] [Google Scholar]
- 329. Rajaratnam SG, Eglinton TW, Hider P, Fearnhead NS. Impact of ileal pouch–anal anastomosis on female fertility: meta-analysis and systematic review. Int J Colorectal Dis 2011;26:1365–1374 [DOI] [PubMed] [Google Scholar]
- 330. Hor T, Lefevre JH, Shields C, Chafai N, Tiret E, Parc Y. Female sexual function and fertility after ileal pouch–anal anastomosis. Int J Colorectal Dis 2016;31:593–601 [DOI] [PubMed] [Google Scholar]
- 331. Ambe PC, Zirngibl H, Möslein G. Initial experience with taTME in patients undergoing laparoscopic restorative proctocolectomy for familial adenomatous polyposis. Tech Coloproctol 2017;21:971–974 [DOI] [PubMed] [Google Scholar]
- 332. Ambe PC, Zirngibl H, Möslein G. Routine virtual ileostomy following restorative proctocolectomy for familial adenomatous polyposis. World J Surg 2018;42:1867–1871 [DOI] [PubMed] [Google Scholar]
- 333. Patel R, Reza L, Worley G, Allison L, Evans S, Antoniou Aet al. Presentation, management and outcomes of ileoanal pouch cancer: a single centre experience. Colorectal Dis 2021;23:2041–2051 [DOI] [PubMed] [Google Scholar]
- 334. Inoki K, Nakajima T, Nonaka S, Abe S, Suzuki H, Yoshinaga Set al. Feasibility of endoscopic resection using bipolar snare for nonampullary duodenal tumours in familial adenomatous polyposis patients. Fam Cancer 2018;17:517–524 [DOI] [PubMed] [Google Scholar]
- 335. Burke CA, Santisi J, Church J, Levinthal G. The utility of capsule endoscopy small bowel surveillance in patients with polyposis. Am J Gastroenterol 2005;100:1498–1502 [DOI] [PubMed] [Google Scholar]
- 336. Matsumoto T, Esaki M, Yanaru-Fujisawa R, Moriyama T, Yada S, Nakamura Set al. Small-intestinal involvement in familial adenomatous polyposis: evaluation by double-balloon endoscopy and intraoperative enteroscopy. Gastrointest Endosc 2008;68:911–919 [DOI] [PubMed] [Google Scholar]
- 337. Saurin JC, Ligneau B, Ronchon T, Leprêtre J, Chavaillon A, Napoléon Bet al. The influence of mutation site and age on the severity of duodenal polyposis in patients with familial adenomatous polyposis. Gastrointest Endosc 2002;55:342–347 [DOI] [PubMed] [Google Scholar]
- 338. Hatogai K, Hosoe N, Imaeda H, Rey JF, Okada S, Ishibashi Yet al. Role of enhanced visibility in evaluating polyposis syndromes using a newly developed contrast image capsule endoscope. Gut Liver 2012;6:218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Church J, Lynch C, Neary P, LaGuardia L, Elayi E. A desmoid tumor-staging system separates patients with intra-abdominal, familial adenomatous polyposis-associated desmoid disease by behavior and prognosis. Dis Colon Rectum 2008;51:897–901 [DOI] [PubMed] [Google Scholar]
- 340. Church J, Berk T, Boman BM, Guillem J, Lynch C, Lynch Pet al. Staging intra-abdominal desmoid tumors in familial adenomatous polyposis: a search for a uniform approach to a troubling disease. Dis Colon Rectum 2005;48:1528–1534 [DOI] [PubMed] [Google Scholar]
- 341. Nieuwenhuis MH, Lefevre JH, Bülow S, Järvinen H, Bertario L, Kernéis Set al. Family history, surgery, and APC mutation are risk factors for desmoid tumors in familial adenomatous polyposis: an international cohort study. Dis Colon Rectum 2011;54:1229–1234 [DOI] [PubMed] [Google Scholar]
- 342. Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis 2009;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Sanchez-Mete L, Ferraresi V, Caterino M, Martayan A, Terrenato I, Mannisi Eet al. Desmoid tumors characteristics, clinical management, active surveillance, and description of our FAP case series. J Clin Med 2020;9:4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Bhandari S, Taylor NJ, Sinha A, Sonoda L, Sanghera B, Wong WLet al. Can combined 18F-FDG-PET and dynamic contrast-enhanced MRI predict behavior of desmoid tumors in patients with familial adenomatous polyposis? Dis Colon Rectum 2012;55:1032–1037 [DOI] [PubMed] [Google Scholar]
- 345. Sinha A, Hansmann A, Bhandari S, Gupta A, Burling D, Rana Set al. Imaging assessment of desmoid tumours in familial adenomatous polyposis: is state-of-the-art 1.5 T MRI better than 64-MDCT? Br J Radiol 2012;85:e254–e261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Kasper B, Baumgarten C, Garcia J, Bonvalot S, Haas R, Haller Fet al. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol 2017;28:2399–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Benech N, Bonvalot S, Dufresne A, Gangi A, Le Péchoux C, Lopez-Trabada-Ataz Det al. Desmoid tumors located in the abdomen or associated with adenomatous polyposis: French intergroup clinical practice guidelines for diagnosis, treatment, and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, SFR). Dig Liver Dis 2022;54:737–746 [DOI] [PubMed] [Google Scholar]
- 348. Cojocaru E, Gennatas S, Thway K, Fisher C, Smrke A, Strauss Det al. Approach to screening for familial adenomatous polyposis (FAP) in a cohort of 226 patients with desmoid-type fibromatosis (DF): experience of a specialist center in the UK. Fam Cancer 2022;21:69–74 [DOI] [PubMed] [Google Scholar]
- 349. Fallen T, Wilson M, Morlan B, Lindor NM. Desmoid tumors—a characterization of patients seen at Mayo Clinic 1976–1999. Fam Cancer 2006;5:191–194 [DOI] [PubMed] [Google Scholar]
- 350. Nieuwenhuis MH, Casparie M, Mathus-Vliegen LMH, Dekkers OM, Hogendoorn PCW, Vasen HFA. A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int J Cancer 2011;129:256–261 [DOI] [PubMed] [Google Scholar]
- 351. Koskenvuo L, Peltomäki P, Renkonen-Sinisalo L, Gylling A, Nieminen TT, Ristimäki Aet al. Desmoid tumor patients carry an elevated risk of familial adenomatous polyposis. J Surg Oncol 2016;113:209–212 [DOI] [PubMed] [Google Scholar]
- 352. van Houdt WJ, Wei IH, Kuk D, Qin LX, Jadeja B, Villano Aet al. Yield of colonoscopy in identification of newly diagnosed desmoid-type fibromatosis with underlying familial adenomatous polyposis. Ann Surg Oncol 2019;26:765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Bhandari S, Ranchod P, Sinha A, Gupta A, Clark SK, Phillips RKS. Familial adenomatous polyposis-related desmoids presenting with air-fluid level: a clinical review and management algorithm. Dis Colon Rectum 2012;55:810–814 [DOI] [PubMed] [Google Scholar]
- 354. Escobar C, Munker R, Thomas JO, Li BD, Burton GV. Update on desmoid tumors. Ann Oncol 2012;23:562–569 [DOI] [PubMed] [Google Scholar]
- 355. Garcia-Ortega DY, Martín-Tellez KS, Cuellar-Hubbe M, Martínez-Said H, Álvarez-Cano A, Brener-Chaoul Met al. Desmoid-type fibromatosis. Cancers (Basel) 2020;12:1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. de Bree E, Keus R, Mellissas J, Tsiftsis D, van Coevorden F. Desmoid tumors: need for an individualized approach. Expert Rev Anticancer Ther 2009;9:525–535 [DOI] [PubMed] [Google Scholar]
- 357. Quast DR, Schneider R, Burdzik E, Hoppe S, Möslein G. Long-term outcome of sporadic and FAP-associated desmoid tumors treated with high-dose selective estrogen receptor modulators and sulindac: a single-center long-term observational study in 134 patients. Fam Cancer 2016;15:31–40 [DOI] [PubMed] [Google Scholar]
- 358. Burtenshaw SM, Cannell AJ, McAlister ED, Siddique S, Kandel R, Blackstein MEet al. Toward observation as first-line management in abdominal desmoid tumors. Ann Surg Oncol 2016;23:2212–2219 [DOI] [PubMed] [Google Scholar]
- 359. Devata S, Chugh R. Desmoid tumors: a comprehensive review of the evolving biology, unpredictable behavior, and myriad of management options. Hematol Oncol Clin North Am 2013;27:989–1005 [DOI] [PubMed] [Google Scholar]
- 360. Church JM, Xhaja X, Warrier SK, Laguardia L, O’Malley M, Burke Cet al. Desmoid tumors do not prevent proctectomy following abdominal colectomy and ileorectal anastomosis in patients with familial adenomatous polyposis. Dis Colon Rectum 2014;57:343–347 [DOI] [PubMed] [Google Scholar]
- 361. Kartheuser A, Stangherlin P, Brandt D, Remue C, Sempoux C. Restorative proctocolectomy and ileal pouch–anal anastomosis for familial adenomatous polyposis revisited. Fam Cancer 2006;5:241–260 [DOI] [PubMed] [Google Scholar]
- 362. Moore D, Burns L, Creavin B, Ryan E, Conlon K, Kelly MEet al. Surgical management of abdominal desmoids: a systematic review and meta-analysis. Ir J Med Sci 2023;192:549–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Embase . Should Protective Ileostomies be Avoided at the Time of Proctocolectomy in FAP Patients Whenever Reasonably Safe?—Record Details—Embase. https://www-embase-com.pros2.lib.unimi.it/records?subaction=viewrecord&rid=1&page=1&id=L72057312 (accessed 1 January 2023)
- 364. Embase . Correlation Between Abdominal Wall Desmoids and Protective Ileostomies: Should a Routine-Ileostomy be Avoided in FAP Patients?—Record Details—Embase. https://www-embase-com.pros2.lib.unimi.it/records?subaction=viewrecord&rid=2&page=1&id=L71212000 (accessed 1 January 2023)
- 365. Babaya A, Yamano T, Matsubara T, Takenaka Y, Song J, Kimura K. Long-term clinical outcomes and follow-up status in Japanese patients with familial adenomatous polyposis after radical surgery: a descriptive, retrospective cohort study from a single institute. Int J Colorectal Dis 2020;35:675–684 [DOI] [PubMed] [Google Scholar]
- 366. Monachese M, Mankaney G, Lopez R, Malley MO, Laguardia L, Kalady MFet al. Outcome of thyroid ultrasound screening in FAP patients with a normal baseline exam. Fam Cancer 2019;18:75–82 [DOI] [PubMed] [Google Scholar]
- 367. Casellas-Cabrera N, Díaz-Algorri Y, Carlo-Chévere VJ, González-Pons M, Rodríguez-Mañón N, Pérez-Mayoral Jet al. Risk of thyroid cancer among Caribbean Hispanic patients with familial adenomatous polyposis. Fam Cancer 2016;15:267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. de Oliveira JC, Viana DV, Zanardo C, Santos EMM, de Paula AE, Palmero EIet al. Genotype–phenotype correlation in 99 familial adenomatous polyposis patients: a prospective prevention protocol. Cancer Med 2019;8:2114–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Chew MH, Quah H, Teh KL, Loi TTC, Eu KW, Tang CL. Twenty years of familial adenomatosis polyposis syndromes in the Singapore Polyposis Registry: an analysis of outcomes. Singapore Med J 2011;52:246–251 [PubMed] [Google Scholar]
- 370. Jarrar AM, Milas M, Mitchell J, Laguardia L, O’Malley M, Berber Eet al. Screening for thyroid cancer in patients with familial adenomatous polyposis. Ann Surg 2011;253:515–521 [DOI] [PubMed] [Google Scholar]
- 371. Martayan A, Sanchez-Mete L, Baldelli R, Falvo E, Barnabei A, Conti Let al. Gene variants associated to malignant thyroid disease in familial adenomatous polyposis: a novel APC germline mutation. J Endocrinol Invest 2010;33:603–606 [DOI] [PubMed] [Google Scholar]
- 372. Plail RO, Bussey HJR, Glazer G, Thomson JPS. Adenomatous polyposis: an association with carcinoma of the thyroid. Br J Surg 1987;74:377–380 [DOI] [PubMed] [Google Scholar]
- 373. Bülow C, Bülow S. Is screening for thyroid carcinoma indicated in familial adenomatous polyposis? The Leeds Castle Polyposis Group. Int J Colorectal Dis 1997;12:240–242 [DOI] [PubMed] [Google Scholar]
- 374. Van Der Linde K, Vasen HFA, Van Vliet ACM. Occurrence of thyroid carcinoma in Dutch patients with familial adenomatous polyposis. An epidemiological study and report of new cases. Eur J Gastroenterol Hepatol 1998;10:777–781 [DOI] [PubMed] [Google Scholar]
- 375. Ho JW, Chu KM, Tse CW, Yuen ST. Phenotype and management of patients with familial adenomatous polyposis in Hong Kong: perspective of the Hereditary Gastrointestinal Cancer Registry. Hong Kong Med J 2002;8:342–347 [PubMed] [Google Scholar]
- 376. Truta B, Allen BA, Conrad PG, Kim YS, Berk T, Gallinger Set al. Genotype and phenotype of patients with both familial adenomatous polyposis and thyroid carcinoma. Fam Cancer 2003;2:95–99 [DOI] [PubMed] [Google Scholar]
- 377. Levy RA, Hui VW, Sood R, Fish S, Markowitz AJ, Wong RJet al. Cribriform-morular variant of papillary thyroid carcinoma: an indication to screen for occult FAP. Fam Cancer 2014;13:547–551 [DOI] [PubMed] [Google Scholar]
- 378. Johnson Smith TGP, Clark SK, Katz DE, Reznek RH, Phillips RKS. Adrenal masses are associated with familial adenomatous polyposis. Dis Colon Rectum 2000;43:1739–1742 [DOI] [PubMed] [Google Scholar]
- 379. Ferrández A, Pho L, Solomon C, Samowitz WS, Kuwada SK, Knecht TPet al. An evidence-based, multidisciplinary approach to the clinical considerations, management, and surveillance of adrenal lesions in familial adenomatous polyposis: report of three cases. Dis Colon Rectum 2006;49:1781–1790 [DOI] [PubMed] [Google Scholar]
- 380. Giardiello FM, Offerhaus GJA, Lee DH, Krush AJ, Tersmette AC, Booker SVet al. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut 1993;34:1394–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. American Cancer Society . Key Statistics for Pancreatic Cancer. https://www.cancer.org/cancer/types/pancreatic-cancer/about/key-statistics.html (accessed 2 January 2023)
- 382. Karstensen JG, Bülow S, Højen H, Jelsig AM, Jespersen N, Andersen KKet al. Cancer in patients with familial adenomatous polyposis—a nationwide Danish cohort study with matched controls. Gastroenterology 2023;165:573–581.e3 [DOI] [PubMed] [Google Scholar]
- 383. Moore SW, Tshifularo N, Grobbelaar JJ. Lessons from the hepatoblastoma–familial polyposis connection. S Afr Med J 2012;102:888–889 [DOI] [PubMed] [Google Scholar]
- 384. Getz G, Gabriel SB, Cibulskis K, Lander E, Sivachenko A, Sougnez Cet al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Burk RD, Chen Z, Saller C, Tarvin K, Carvalho AL, Scapulatempo-Neto Cet al. Integrated genomic and molecular characterization of cervical cancer. Nature 2017;543:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Bell D, Berchuck A, Birrer M, Chien J, Cramer DW, Dao Fet al. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Wang X, Huang X, Zhang Y. Involvement of human papillomaviruses in cervical cancer. Front Microbiol 2018;9:2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Ryan NAJ, McMahon R, Tobi S, Snowsill T, Esquibel S, Wallace AJet al. The proportion of endometrial tumours associated with Lynch syndrome (PETALS): a prospective cross-sectional study. PLoS Med 2020;17:e1003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Boursi B, Sella T, Liberman E, Shapira S, David M, Kazanov Det al. The APC p.I1307K polymorphism is a significant risk factor for CRC in average risk Ashkenazi Jews. Eur J Cancer 2013;49:3680–3685 [DOI] [PubMed] [Google Scholar]
- 390. Liang J, Lin C, Hu F, Wang F, Zhu L, Yao Xet al. APC polymorphisms and the risk of colorectal neoplasia: a HuGE review and meta-analysis. Am J Epidemiol 2013;177:1169–1179 [DOI] [PubMed] [Google Scholar]
- 391. Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet 2022;399:1412–1428 [DOI] [PubMed] [Google Scholar]
- 392. Hazelwood E, Sanderson E, Tan VY, Ruth KS, Frayling TM, Dimou Net al. Identifying molecular mediators of the relationship between body mass index and endometrial cancer risk: a Mendelian randomization analysis. BMC Med 2022;20:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–578 [DOI] [PubMed] [Google Scholar]
- 394. Jordan SJ, Na R, Johnatty SE, Wise LA, Adami HO, Brinton LAet al. Breastfeeding and endometrial cancer risk: an analysis from the Epidemiology of Endometrial Cancer Consortium. Obstetrics and Gynecology 2017;129:1059–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Saed L, Varse F, Baradaran HR, Moradi Y, Khateri S, Friberg Eet al. The effect of diabetes on the risk of endometrial cancer: an updated a systematic review and meta-analysis. BMC Cancer 2019;19:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal Jet al. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer 2019;145:1719–1730 [DOI] [PubMed] [Google Scholar]
- 397. Whelan E, Kalliala I, Semertzidou A, Raglan O, Bowden S, Kechagias Ket al. Risk factors for ovarian cancer: an umbrella review of the literature. Cancers (Basel) 2022;14:2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Roura E, Castellsagué X, Pawlita M, Travier N, Waterboer T, Margall Net al. Smoking as a major risk factor for cervical cancer and pre-cancer: results from the EPIC cohort. Int J Cancer 2014;135:453–466 [DOI] [PubMed] [Google Scholar]
- 399. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet 2019;393:169–182 [DOI] [PubMed] [Google Scholar]
- 400. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick MLet al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–333 [DOI] [PubMed] [Google Scholar]
- 401. Vigneswaran K, Hamoda H. Hormone replacement therapy—current recommendations. Best Pract Res Clin Obstet Gynaecol 2022;81:8–21 [DOI] [PubMed] [Google Scholar]
- 402. Davis SR, Baber RJ. Treating menopause—MHT and beyond. Nat Rev Endocrinol 2022;18:490–502 [DOI] [PubMed] [Google Scholar]
- 403. Lin KJ, Cheung WY, Lai JYC, Giovannucci EL. The effect of estrogen vs. combined estrogen–progestogen therapy on the risk of colorectal cancer. Int J Cancer 2012;130:419–430 [DOI] [PubMed] [Google Scholar]
- 404. Menon U, Gentry-Maharaj A, Burnell M, Singh N, Ryan A, Karpinskyj Cet al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2021;397:2182–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Gentry-Maharaj A, Karpinskyj C. Current and future approaches to screening for endometrial cancer. Best Pract Res Clin Obstet Gynaecol 2020;65:79–97 [DOI] [PubMed] [Google Scholar]
- 406. Wentzensen N, Clarke MA. Cervical cancer screening-past, present, and future. Cancer Epidemiol Biomarkers Prev 2021;30:432–434 [DOI] [PubMed] [Google Scholar]
- 407. Ryan NAJ, Snowsill T, McKenzie E, Monahan KJ, Nebgen D. Should women with Lynch syndrome be offered gynaecological cancer surveillance? BMJ 2021;374:n2020. [DOI] [PubMed] [Google Scholar]
- 408. Niv Y, Fraser GM. Adenocarcinoma in the rectal segment in familial polyposis coli is not prevented by sulindac therapy. Gastroenterology 1994;107:854–857 [DOI] [PubMed] [Google Scholar]
- 409. Lynch HT, Thorson AG, Smyrk T. Rectal cancer after prolonged sulindac chemoprevention a case report. Cancer. 1995;75:936–938 [DOI] [PubMed] [Google Scholar]
- 410. Samadder NJ, Kuwada SK, Boucher KM, Byrne K, Kanth P, Samowitz Wet al. Association of sulindac and erlotinib vs placebo with colorectal neoplasia in familial adenomatous polyposis: secondary analysis of a randomized clinical trial. JAMA Oncol 2018;4:671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Lynch PM, Ayers GD, Hawk E, Richmond E, Eagle C, Woloj Met al. The safety and efficacy of celecoxib in children with familial adenomatous polyposis. Am J Gastroenterol 2010;105:1437–1443 [DOI] [PubMed] [Google Scholar]
- 412. West NJ, Clark SK, Phillips RKS, Hutchinson JM, Leicester RJ, Belluzzi Aet al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut 2010;59:918–925 [DOI] [PubMed] [Google Scholar]
- 413. Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SDet al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol 2006;4:1035–1038 [DOI] [PubMed] [Google Scholar]
- 414. Cruz-Correa M, Hylind LM, Marrero JH, Zahurak ML, Murray-Stewart T, Casero RAet al. Efficacy and safety of curcumin in treatment of intestinal adenomas in patients with familial adenomatous polyposis. Gastroenterology 2018;155:668–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Roos VH, Meijer BJ, Kallenberg FGJ, Bastiaansen BAJ, Koens L, Bemelman FJet al. Sirolimus for the treatment of polyposis of the rectal remnant and ileal pouch in four patients with familial adenomatous polyposis: a pilot study. BMJ Open Gastroenterol 2020;7:e000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Björk J, Åkerbrant H, Iselius L, Bergman A, Engwall Y, Wahlström Jet al. Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: cumulative risks and APC gene mutations. Gastroenterology 2001;121:1127–1135 [DOI] [PubMed] [Google Scholar]
- 417. Spigelman AD, Talbot IC, Williams CB, Domizio P, Phillips RKS. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet 1989;334:783–785 [DOI] [PubMed] [Google Scholar]
- 418. Heiskanen I, Kellokumpu I, Järvinen H. Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Endoscopy 1999;31:412–416 [DOI] [PubMed] [Google Scholar]
- 419. Calabrese C, Praticò C, Calafiore A, Coscia M, Gentilini L, Poggioli Get al. Eviendep® reduces number and size of duodenal polyps in familial adenomatous polyposis patients with ileal pouch–anal anastomosis. World J Gastroenterol 2013;19:5671–5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Wallace MH, Forbes A, Beveridge IG, Spigelman AD, Hewer A, Venitt Set al. Randomized, placebo-controlled trial of gastric acid-lowering therapy on duodenal polyposis and relative adduct labeling in familial adenomatous polyposis. Dis Colon Rectum 2001;44:1585–1589 [DOI] [PubMed] [Google Scholar]
- 421. Balaguer F, Stoffel EM, Burke CA, Dekker E, Samadder NJ, Van Cutsem Eet al. Combination of sulindac and eflornithine delays the need for lower gastrointestinal surgery in patients with familial adenomatous polyposis: post hoc analysis of a randomized clinical trial. Dis Colon Rectum 2022;65:536–545 [DOI] [PubMed] [Google Scholar]
- 422. Penna C, Bataille N, Balladur P, Tiret E, Parc R. Surgical treatment of severe duodenal polyposis in familial adenomatous polyposis. Br J Surg 1998;85:665–668 [DOI] [PubMed] [Google Scholar]
- 423. Morak M, Laner A, Bacher U, Keiling C, Holinski-Feder E. MUTYH-associated polyposis—variability of the clinical phenotype in patients with biallelic and monoallelic MUTYH mutations and report on novel mutations. Clin Genet 2010;78:353–363 [DOI] [PubMed] [Google Scholar]
- 424. Nielsen M, Van Steenbergen LN, Jones N, Vogt S, Vasen HFA, Morreau Het al. Survival of MUTYH-associated polyposis patients with colorectal cancer and matched control colorectal cancer patients. J Natl Cancer Inst 2010;102:1724–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Nielsen M, Infante E, Brand R. MUTYH Polyposis. GeneReviews®. Seattle: University of Washington, 1993 [Google Scholar]
- 426. Lubbe SJ, Di Bernardo MC, Chandler IP, Houlston RS. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J Clin Oncol 2009;27:3975–3980 [DOI] [PubMed] [Google Scholar]
- 427. Spurdle AB, Bowman MA, Shamsani J, Kirk J. Endometrial cancer gene panels: clinical diagnostic vs research germline DNA testing. Modern Pathol 2017;30:1048–1068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.