Abstract
Nerve growth factor is a member of the neurotrophin family of trophic factors that have been reported to be essential for the survival and development of sympathetic neurons and a subset of sensory neurons. Nerve growth factor exerts its effects mainly by interaction with the specific receptor TrkA, which leads to the activation of several intracellular signaling pathways. Once activated, TrkA also allows for a rapid and moderate increase in intracellular calcium levels, which would contribute to the effects triggered by nerve growth factor in neurons. In this report, we analyzed the relationship of calcium to the activation of the Ras/extracellular signal-regulated kinase pathway in PC12 cells. We observed that calcium and calmodulin are both necessary for the acute activation of extracellular signal-regulated kinases after TrkA stimulation. We analyzed the elements of the pathway that lead to this activation, and we observed that calmodulin antagonists completely block the initial Raf-1 activation without affecting the function of upstream elements, such as Ras, Grb2, Shc, and Trk. We have broadened our study to other stimuli that activate extracellular signal-regulated kinases through tyrosine kinase receptors, and we have observed that calmodulin also modulates the activation of such kinases after epidermal growth factor receptor stimulation in PC12 cells and after TrkB stimulation in cultured chicken embryo motoneurons. Calmodulin seems to regulate the full activation of Raf-1 after Ras activation, since functional Ras is necessary for Raf-1 activation after nerve growth factor stimulation and calmodulin-Sepharose is able to precipitate Raf-1 in a calcium-dependent manner.
Neurotrophins (NTs) are neurotrophic factors involved in the development, maintenance, and repair of the nervous system (reviewed in reference 60). This family is composed of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 and neurotrophin 4/5. NGF was the first NT described and has been shown to be essential for the survival and development of sympathetic neurons, some sensory neurons, and a population of cholinergic cells located at the basal forebrain (14, 39, 94). Each of these NTs exhibits trophic effects on a specific, although partially overlapping, subset of neuronal populations in either the central or the peripheral nervous system both in vivo and in vitro (6, 15). NTs bind to two types of receptors, p75LNTR and the Trk family of tyrosine kinases. All NTs bind to p75LNTR. However, they show a high degree of specificity for Trk receptors. TrkA is the preferential receptor for NGF, TrkB is that for BDNF and neurotrophin 4/5, and TrkC is that for neurotrophin 3 (5). In the last few years, much attention has been focused on ascertaining the molecular mechanism by which Trk signaling mediates the effects of NTs.
The paradigm for studying the intracellular signaling pathways underlying TrkA activation has been the stimulation of this receptor with NGF in the PC12 cell line (38). Once phosphorylated, TrkA becomes a scaffolding structure that recruits several adapter proteins and enzymes that ultimately propagate the NGF signal. Among these proteins, the adapter protein Shc and phospholipase Cγ have been involved in the activation of extracellular signal-regulated kinases (ERKs) (96). Shc protein allows the interaction of TrkA with the Src homology 2 (SH2) domain of Grb2, which subsequently activates Ras through the Ras GTP exchange factor (GEF) Sos (25, 61, 62, 77, 90, 93). Activated Ras interacts with several proteins related to intracellular signaling pathways (reviewed in reference 51). One of these pathways is the cascade of kinases of the ERK–mitogen-activated protein (MAP) kinase pathway. The first kinase in the cascade is the serine-threonine kinase Raf, which phosphorylates and activates MAP/ERK kinase 1 (MEK1) and MEK2 (43, 56, 63) which, in turn, phosphorylate and activate ERK1 and ERK2 (108, 113). ERK proteins translocate to the nucleus, where they can phosphorylate transcription factors that regulate gene expression (for a review, see reference 87).
The mechanism by which Ras activates Raf is not completely understood, although it seems that the translocation of Raf from the cytosol to the plasma membrane upon Ras activation is essential (reviewed in reference 73). Moreover, full activation of Raf-1 requires its phosphorylation on residues S338 and Y341 in the amino-terminal region of the catalytic domain (7, 17, 19, 46, 69). This phenomenon has been demonstrated to be Ras GTP dependent (66). However, the kinases responsible for Raf phosphorylation on amino acid residues S338 and Y341 are under study. It seems that p21-activated protein kinase Pak3 phosphorylates Raf-1 on S338 both in vitro and in vivo (52). The kinase that phosphorylates Y341 is unknown.
B-Raf is highly expressed in PC12 cells and is also activated following NGF treatment (45, 71, 106). However, the regulation of B-Raf activation seems to be different from that of Raf-1. First, Raf-1 activation after NGF stimulation is transient, whereas B-Raf activation is sustained (106, 112). Second, Raf-1 activation is dependent on Ras, whereas B-Raf activation can be mediated either by Ras (107, 115) or by a different small GTPase, named Rap-1, depending on the stimuli used (112). Third, Rap-1 is activated by Trk receptors upon NGF stimulation through a series of adapter proteins different from Ras. These include FRS, CRK, and the GTP-GDP exchanger C3G (71, 112). It has been postulated that sustained activation of the ERK-MAP kinase pathway is responsible for the neuronal phenotype induced by NGF in PC12 cells (67, 83).
When phospholipase Cγ becomes phosphorylated and activated by TrkA, it triggers the release of Ca2+ from the endoplasmic reticulum and increases the degree of formation of diacylglycerol through the cleavage of phosphatidylinositol 4,5-bisphosphate (78). The role of the intracellular Ca2+ concentration ([Ca2+]i) in mediating or modulating the effects of NGF has not been directly established. However, it has been reported that NGF and epidermal growth factor (EGF) treatments cause a rapid and small increase in [Ca2+]i (57, 65, 80, 92; reviewed in reference 47). In the cytosol, Ca2+ encounters a variety of proteins that can mediate its functional effects. Central among them is calmodulin (CaM), a small Ca2+-binding protein that is able to regulate the activity of many different proteins, including protein kinases. Recently, it has been reported that Ca2+ and CaM can regulate some of the signaling pathways that are activated by Trk. Ca2+ and CaM are able to bind to and modulate phosphatidylinositol 3-kinase (PI 3-kinase) (35, 49). Moreover, it has been reported that CaM can also activate Akt, a downstream effector of PI 3-kinase, in a CaM-dependent protein kinase (CaM-K) manner (111). Increases in [Ca2+]i due to Ca2+ influx have been shown to activate ERK-MAP kinase activity (reviewed in reference 34). Most of these mechanisms activate the ERK-MAP kinase pathway at the level of Ras or upstream of it (88), although the mechanisms involved are not completely understood. We have previously reported that Ca2+ influx after membrane depolarization activates ERK-MAP kinases by a Ca2+- and CaM-dependent mechanism downstream of Ras (27, 95).
The relevance of [Ca2+]i in the modulation of the signaling pathways activated by physiological stimuli such as the ligands of tyrosine kinase receptors remains to be elucidated. Since the elevation of [Ca2+]i after TrkA stimulation has been observed concomitant with the activation of TrkA-triggered signaling pathways (55, 75), we wanted to know whether Ca2+ and CaM modulate the activation of these signaling pathways. The results presented here demonstrate that Ca2+ and CaM are both necessary for the activation of ERK-MAP kinase after NGF stimulation. The involvement of CaM is restricted to the high, rapid, and initial activation of ERKs. CaM modulation occurs at the level of the kinase(s) that phosphorylates MEK, since CaM inhibitors abolish Raf activity without affecting Ras activity. We also present evidence that this modulation occurs when the pathway is stimulated by other tyrosine kinase receptors, such as the EGF receptor in PC12 cells or TrkB in cultures of primary neurons, such as motoneurons (MTNs), when stimulated with BDNF.
MATERIALS AND METHODS
Cell culturing, cell lysates, and cell transfection.
PC12 and M-M17-26 cells were cultured as described previously (26). For experiments, cells were allowed to proliferate in polyornithine-precoated 60-mm tissue culture dishes (Corning) until they reached 80% confluence. Before stimulation, cells were serum starved for 12 to 15 h. During the last hour of serum starvation, cultures were exposed to different drugs: the Ca2+ chelator BAPTA-AM (Molecular Probes, Eugene, Oreg.); the CaM inhibitors calmidazolium chloride and trifluoperazine dimaleate (Calbiochem-Novabiochem Corp., San Diego, Calif.); the CaM inhibitors W5, W7, W12, and W13; the L-type voltage-gated Ca2+ channel inhibitor nifedipine; the PI 3-kinase inhibitor LY294002; and the MEK inhibitor PD098059 (Calbiochem-Novabiochem Corp.). Unless otherwise indicated, W12 and W13 were used at a 70 μM final concentration. At the end of treatments, PC12 cells were stimulated for various times with serum-free medium containing NGF (100 ng/ml), EGF (2.5 ng/ml), KCl (75 mM), or ionomycin (10 μM) plus freshly added drug. When needed, stimulation was performed in the presence of 5 mM EGTA in the culture media.
MTNs were purified from 5.5-day-old chick embryos according to Comella et al. (13) with minor modifications described elsewhere (20). BDNF stimulation (50 ng/ml) was performed as described previously (20).
For total cell lysates, cells were rinsed rapidly twice in ice-cold phosphate-buffered saline (PBS) at pH 7.2, solubilized with boiling 2% sodium dodecyl sulfate (SDS)–125 mM Tris (pH 6.8), and sonicated. Alternatively, for immunoprecipitation Ras activity, or CaM precipitation studies, cells were solubilized at 4°C in the corresponding lysis buffer (see below). After 15 min of incubation on ice, cells were scraped from the dishes and cell lysates were orbitally rotated for 30 min at 4°C. Nuclei and cellular debris were removed by centrifugation at 10,000 × g and 4°C for 15 min.
Protein concentrations in cell lysates were quantified by a modified Lowry assay as described by the provider (Bio-Rad Dc protein assay; Bio-Rad, Hercules, Calif.).
PC12 cells were cotransfected by electroporation with the Glu217-Glu221 MAPKK1 mutant and EGFP constructs (Clontech, Palo Alto, Calif.). After 24 h of transfection, PC12 cells containing green fluorescent protein were sorted, reaching a population of 90 to 95% of positive cells. After sorting, cells were cultured for an additional 24 h before treatments.
Western blotting.
Western blotting was performed with immunoprecipitates or cell lysates by resolving the proteins in SDS-polyacrylamide gel electrophoresis (PAGE) gels. The proteins were transferred to polyvinylidine difluoride Immobilon-P membrane filters (Millipore, Bedford, Mass.) using a semidry Trans-Blot apparatus (Amersham Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer's instructions. Antiphosphorylation (anti-phospho) antibodies against cyclic AMP response element-binding protein (CREB), ERK1/2, MEK1/2, and Akt and anti-MEK and anti-CREB antibodies were purchased from New England Biolabs, Inc. (Beverly, Mass.). Anti-phospho-CaM-KII antibody was obtained from Promega (Madison, Wis.). Anti-pan-ERK, anti-Raf-1, and anti-Shc antibodies were purchased from Transduction Laboratories (Lexington, Ky.). Anti-Akt (C-20), anti-B-Raf, and anti-A-Raf antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.). Antiphosphotyrosine [anti-Tyr(P); 4G10] antibody and anti-c-Fos antibody were purchased from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Anti-α-tubulin antibody was obtained from Sigma (St. Louis, Mo.). All the antibodies were used according to supplier instructions. After incubation with specific peroxidase-conjugated secondary antibodies, membranes were developed with an enhanced chemiluminescence Western blotting detection system (Pierce Chemical Co., Rockford, Ill.).
Immunoprecipitation.
Immunoprecipitation of Shc, Raf-1, B-Raf, and A-Raf proteins was performed with specific antibodies according to supplier instructions (see above). To detect Grb2 association in Shc immunoprecipitates, anti-Grb2 antibody was used as previously described (26). TrkA immunoprecipitation was performed with anti-pan-Trk antibody 203 as previously described (8, 27).
Raf kinase activity assay.
Raf proteins were immunoprecipitated from lysates of PC12 cells with specific anti-Raf-1, anti-B-Raf, or anti-A-Raf antibodies. Raf kinase activity in the immunoprecipitates was measured with recombinant MEK (Santa Cruz Biotechnology Inc.) as a substrate and [γ-32P]ATP (Amersham Pharmacia Biotech) as described previously (26). Reactions were stopped with 5× sample buffer, and products were separated by SDS-PAGE. After the gels were dried, the phosphorylation signal was quantified on a phosphorimager (Boehringer GmbH, Mannheim, Germany). Radioactive spots were also detected by autoradiography by exposing the dried gels to Fuji medical X-ray film (Fuji Photo Film Co. Ltd., Tokyo, Japan) overnight at −70°C.
PI 3-kinase activity assay.
PI 3-kinase was immunoprecipitated from Nonidet P-40 lysates of PC12 cells with anti-Tyr(P) monoclonal antibody 4G10. PI 3-kinase activity in the immunoprecipitates was measured with l-α-phosphatidylinositol and [γ-32P]ATP (Amersham Pharmacia Biotech) as substrates as described elsewhere (26). When needed, a 10 μM final concentration of the PI 3-kinase inhibitor LY294002 was added to the kinase assay buffer. Phosphorylated lipids were then extracted and resolved by thin-layer chromatography, and radioactive spots were detected by autoradiography by exposing the thin-layer chromatography plates to Fuji medical X-ray film overnight at −70°C.
Ras activity assay.
Ras activity was determined by GTP loading by using immunoprecipitates of Ras obtained with antibody Y13-259 (Oncogene Research Products, Cambridge, Mass.) from protein extracts of PC12 cells metabolically labeled with [32P]H3PO4 as described previously (82). Results were quantitated as the percentage of GTP in the immunoprecipitates according to the expression (GTP counts/3)/[GTP counts/3) + (GDP counts/2)] × 100.
Ras activity was also measured by a nonradioactive method as described previously (18). Briefly, treated cells were solubilized for 15 min in lysis buffer containing 25 mM Tris (pH 7.5), 5 mM EGTA, 15 mM NaCl, 5 mM MgCl2, 1% Triton X-100, 1% N-octyl-β-d-glucopyranoside, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, 2 mM benzamidine, and 20 μg of leupeptin per ml. Fifty micrograms of recombinant glutathione S-transferase–Ras-binding domain of Raf-1 (GST-RBD) previously coupled to glutathione-Sepharose (Amersham Pharmacia Biotech) was added to approximately 750 μg of protein extract. Protein complexes were allowed to form for 2 h at 4°C. Precipitates were washed three times with lysis buffer without N-octyl-β-d-glucopyranoside and once with PBS. Finally, precipitates were resuspended in 5× sample buffer, and denatured proteins were analyzed by SDS–12% PAGE. Immunodetection was done with an anti–pan-Ras antibody (Oncogene Research Products) and anti-mouse immunoglobulin G coupled to horseradish peroxidase as a secondary antibody. Blots were developed with the enhanced chemiluminescence Western blotting detection system described above.
CaM-Sepharose precipitation.
CaM-Sepharose precipitation was performed by use of human recombinant CaM conjugated to Sepharose. After stimulation, cells were solubilized for 15 min in lysis buffer containing 1% Triton X-100, 150 mM NaCl, 20 mM Tris (pH 7.5), 50 mM β-glycerophosphate, 25 mM NaF, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, 2 mM benzamidine, and 20 μg of leupeptin per ml. After the removal of cellular debris, protein extracts containing 400 μg of protein were supplemented either with 1 mM EGTA or with 0.1 mM CaCl2 (final concentrations). Then, 40 μl (vol/vol) of CaM-Sepharose previously blocked with 1% bovine serum albumin and equilibrated with the corresponding lysis buffer (containing either 1 mM EGTA or 0.1 mM CaCl2) was added to the corresponding lysate. After 2 h at 4°C, complexes were precipitated and washed three times with lysis buffer containing 1 mM EGTA or 0.1 mM CaCl2 and twice with PBS containing 1 mM EGTA or 0.1 mM CaCl2. When needed, precipitates were washed with lysis buffer containing 5 mM EGTA. Complexes were analyzed by Western blotting with anti-Raf-1 and anti-B-Raf antibodies as described above.
Statistical analysis.
All the experiments were performed at least three times. For statistical analysis of data, Student's t test was used. Values are expressed as mean ± standard error of the mean. Data were considered statistically different at a P value of <0.01.
Materials.
W13, W12, W7, W5, and the rest of the biochemicals were obtained from Sigma. Anti-Grb2 was a gift from J. Ureña (Universitat de Barcelona, Barcelona, Spain), anti-pan-Ras and CaM-Sepharose were from O. Bachs and N. Agell (Universitat de Barcelona, Barcelona, Spain), EGF was from G. Capellà and C. García (Hospital Sant Pau, Barcelona, Spain), and anti-pan-Trk (antibody 203) was from D. Martin-Zanca (Consejo Superior de Investigaciones Cientificas [CSIC]-Universidad de Salamanca, Salamanca, Spain). The GST-RBD construct was obtained from F. McKenzie (State University of New York, Stony Brook) through O. Bachs and N. Agell. The PC12 subline M-M17-26, kindly provided by G. M. Cooper (Harvard Medical School, Boston, Mass.) through A. Aranda (CSIC, Madrid, Spain), was obtained after transfection with the dominant negative Ha-ras mutant (Asn-17). The Glu217-Glu221 MAPKK1 mutant construct was kindly provided by C. E. Marshall (Institute of Cancer Research, London, United Kingdom) through A. López-Rivas (CSIC, Granada, Spain). NGF (7S) was prepared in our laboratory from salivary glands as described previously (72).
RESULTS
NGF requires mobilization of Ca2+ to activate ERK-MAP kinases.
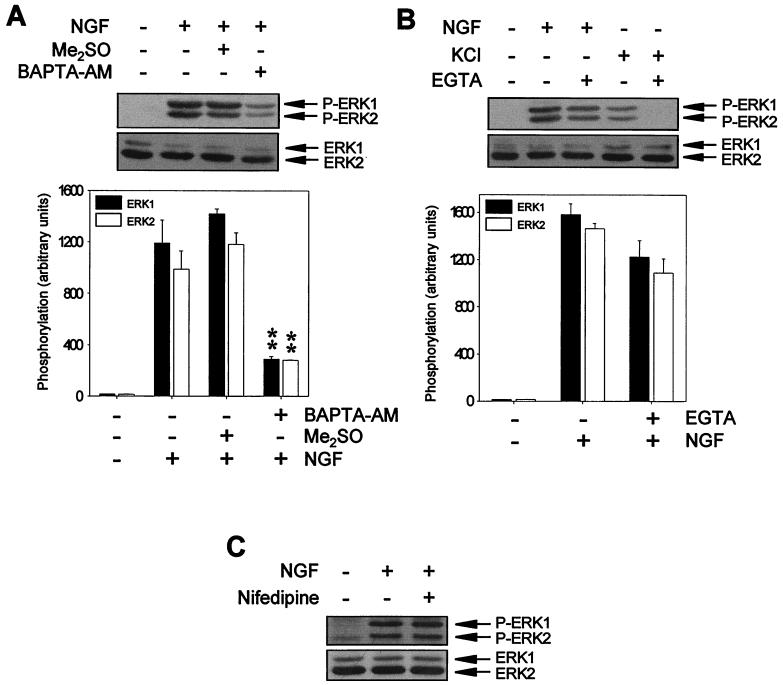
It has been reported that NGF induces a transient increase in [Ca2+]i in PC12 cells (reviewed in reference 47). Previous results showed that [Ca2+]i increases due to Ca2+ influx were able to activate ERK-MAP kinases (34). We analyzed whether the increases in [Ca2+]i induced by NGF are relevant for TrkA-induced ERK activation. ERK activity was monitored with specific antibodies. Phosphorylation of ERKs on both threonine and tyrosine residues has been shown to be a good indicator of the kinase activity of these proteins (10, 27, 85). Pretreatment of cultures with the extracellular Ca2+ chelator EGTA slightly reduced the ERK phosphorylation induced by NGF stimulation (Fig. 1B). In accordance with these results, the use of specific voltage-gated Ca2+ channel inhibitors, such as nifedipine, did not prevent the phosphorylation of ERKs after NGF stimulation (Fig. 1C). However, when cells were pretreated with an intracellular Ca2+ chelator, such as BAPTA-AM, NGF-induced ERK phosphorylation was strongly and significantly reduced (Fig. 1A). As a control of the effectiveness of EGTA and nifedipine at the concentrations used, they were shown to be able to completely prevent the activation of ERKs in PC12 cells upon Ca2+ influx due to membrane depolarization (Fig. 1B; see also reference 27). Taken together, these results indicate that NGF requires Ca2+ to activate ERKs. Moreover, they suggest that TrkA preferentially uses intracellular Ca2+ to activate these kinases.
FIG. 1.
Ca2+ chelators block NGF-induced ERK phosphorylation. (A) PC12 cells were pretreated (+) or not pretreated (−) for 1 h with 50 μM BAPTA-AM or with vehicle (Me2SO) and then stimulated (+) or not stimulated (−) for 5 min with NGF. (B) PC12 cells were stimulated (+) or not stimulated (−) for 5 min with NGF or KCl in the presence (+) or in the absence (−) of 5 mM EGTA. (C) PC12 cells were pretreated (+) or not pretreated (−) for 1 h with 5 μM nifedipine and then stimulated (+) or not stimulated (−) for 5 min with NGF. After treatments, cells were lysed, and protein extracts were analyzed by Western blotting with an anti-phospho-ERK antibody (upper panels) and stripped and reprobed with an anti-pan-ERK antibody (lower panels) as a control for the protein content per lane. Graphs in panels A and B show the average ERK phosphorylation from three independent experiments. ∗∗, P value of <0.01, as determined by Student's t test. Arrows labeled P-ERK1 and P-ERK2 or ERK1 and ERK2 indicate the positions of phosphorylated and total ERK1 and ERK2 proteins, respectively.
Functional inhibitors of CaM prevent the activation of the ERK-MAP kinases induced by NGF.
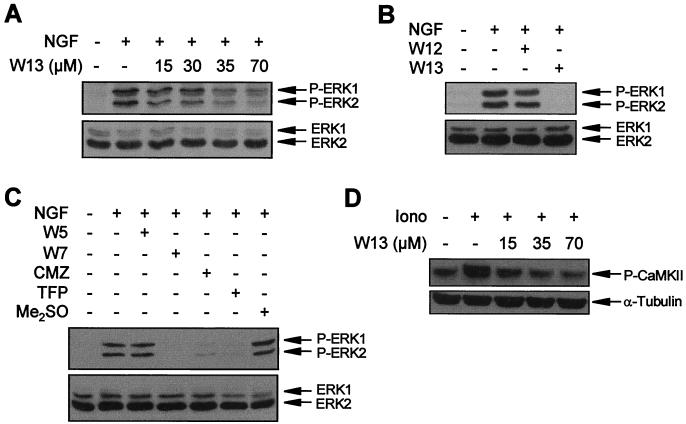
To test the involvement of CaM in the NGF-induced activation of ERK-MAP kinases, we functionally blocked CaM using different specific antagonists. PC12 cultures were pretreated with W13 for 1 h and then stimulated with NGF. Protein extracts were analyzed with an anti-phospho-ERK antibody. W13 blocked the phosphorylation of the ERK-MAP kinases induced by NGF in a dose-dependent manner (Fig. 2A). At 70 μM, the phosphorylation of ERKs was almost completely inhibited (Fig. 2A). As a control for the specificity of the W13 effect, we used its structural analogue W12, which is five times less potent than W13 (41, 42). W12 used at the same concentrations did not show any significant effect on ERK phosphorylation (Fig. 2B). Other CaM inhibitors, such W7 (41, 99), calmidazolium (36, 44), or trifluoperazine (70, 100), produced results similar to those observed with W13 (Fig. 2C). W5, the structural analogue of W7 without functional activity on CaM, had no effect on ERK phosphorylation (Fig. 2C).
FIG. 2.
CaM inhibitors block NGF-induced ERK phosphorylation. (A) PC12 cells were pretreated or not pretreated (−) for 1 h with the indicated concentrations of the CaM inhibitor W13 and then stimulated (+) or not stimulated (−) for 5 min with NGF. (B) PC12 cells were pretreated (+) or not pretreated (−) for 1 h with W12 or W13 and then stimulated (+) or not stimulated (−) for 5 min with NGF. (C) PC12 cells were pretreated (+) or not pretreated (−) for 1 h with 100 μM W5 or W7, 25 μM calmidazolium (CMZ), 50 μM trifluoperazine (TFP), or vehicle (Me2SO) and then stimulated (+) or not stimulated (−) for 5 min with NGF. After treatments, cells were lysed and ERK phosphorylation was analyzed as described in the legend to Fig. 1. (D) PC12 cells were pretreated (+) or not pretreated (−) for 1 h with the indicated concentrations of CaM inhibitor W13 and then stimulated (+) or not stimulated (−) for 30 s with ionomycin (Iono). Lysates were probed with an antibody to phosphorylated CaMKII T286 (upper panel) and reprobed with an anti–α-tubulin antibody to assess comparable loading of lanes (lower panel).
Finally, we tested whether the doses of W13 used to inhibit NGF-induced ERK activation were comparable to those needed to inhibit a well-known CaM-dependent phenomenon in PC12 cells. For these cells, it has been reported previously that ionomycin is able to activate CaM-KII, a kinase that requires CaM to be activated (64). Thus, we have monitored the activation of CaM-KII induced by ionomycin in the presence of different W13 concentrations. When CaM interacts with CaM-KII, the kinase activity of the enzyme becomes activated and the enzyme phosphorylates itself at Thr286 (12). Using a specific antibody to phosphorylated CaM-KII Thr286, we have observed that W13 blocks in a dose-dependent manner the phosphorylation of this enzyme (Fig. 2D, upper panel).
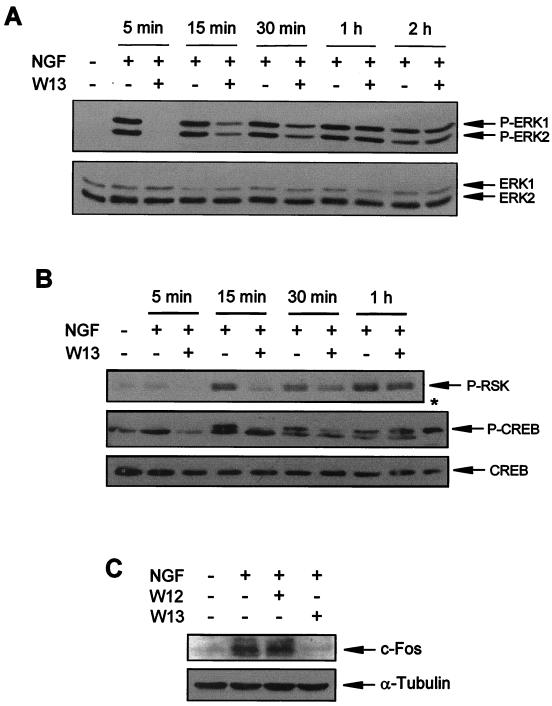
The effects of CaM inhibitors on NGF-induced ERK activation over time were also investigated. W13-pretreated PC12 cells were stimulated with NGF, and ERK phosphorylation was analyzed after different times. Interestingly, W13 only prevented the activation of ERKs at 5 min after NGF stimulation, whereas at 15 and 30 min, the levels of ERK phosphorylation steadily increased (Fig. 3A). After 1 h, the levels of ERK phosphorylation observed in drug-treated and non-drug-treated cultures were comparable (Fig. 3A). A similar effect was observed with other CaM antagonists, such as W7 (data not shown). These results suggest that CaM is required only for the rapid and initial activation of ERKs.
FIG. 3.
CaM antagonists have a transient inhibitory effect on the phosphorylation of ERKs induced by NGF. (A) PC12 cells were pretreated (+) or not pretreated (−) for 1 h with W13 and then stimulated (+) or not stimulated (−) with NGF for the indicated times. After treatment, cells were lysed and protein extracts were analyzed as described in the legend to Fig. 1 to detect ERK phosphorylation. (B) PC12 cells were treated as described in panel A for the indicated times, and protein extracts were analyzed by Western blotting with an anti-phospho-RSK antibody (P-RSK) and an anti-phospho-CREB antibody (P-CREB) and stripped and reprobed with an anti-CREB antibody (CREB) as a control for the protein content per lane. (C) PC12 cells were pretreated (+) or not pretreated (−) for 1 h with W12 or W13 and then stimulated (+) or not stimulated (−) for 1 h with NGF. After treatment, cells were lysed and protein extracts were analyzed by Western blotting with an anti-c-Fos antibody (upper panel) and stripped and reprobed with an anti-α-tubulin antibody (lower panel) as a control for the protein content per lane. ∗, positive control for CREB phosphorylation obtained from the antibody supplier (total cell extracts from SK-N-MC cells treated with Forskolin).
We also investigated if the effects of W13 were functionally important at the transcriptional level. For this investigation, we have monitored the state of phosphorylation of S133 of the transcription factor CREB. S133 of CREB has been shown to be one of the most relevant residues regulating the activity of CREB after NGF induction (109, 110). NGF phosphorylation of CREB after ERK-MAP kinase pathway stimulation is mediated by pp90 ribosomal S6 kinase (109). CREB activation results in an increase in c-fos transcription due to the binding of CREB to the cyclic AMP response element of the c-fos upstream regulatory region (2). Figure 3B shows that NGF is able to phosphorylate and thus activate pp90 ribosomal S6 kinase and CREB. According to the results of the ERK-MAP kinase activity blockade, CaM inhibitor W13 was able to transiently prevent the phosphorylation of both proteins (Fig. 3B). These changes were also observed in c-Fos expression, as measured by the increase in the amount of c-Fos protein after 1 h of NGF stimulation. NGF was also able to increase the levels of this transcription factor, and W13 completely blocked the effects of NGF at the time point analyzed (1 h) (Fig. 3C). None of the changes described was found in cells treated with W12 (Fig. 3C).
CaM modulates neither the tyrosine phosphorylation of TrkA and Shc nor the association of Grb2 with Shc.
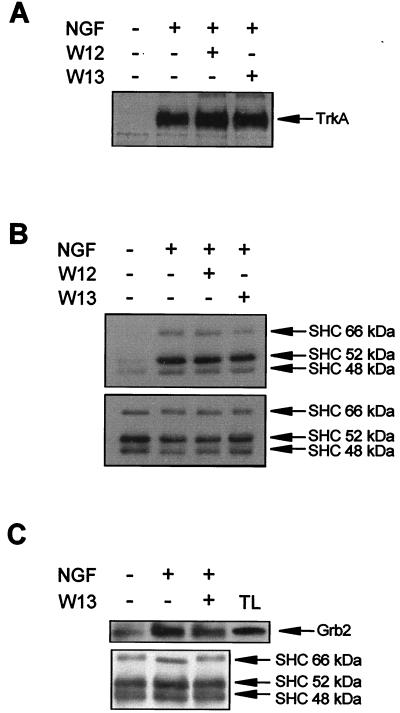
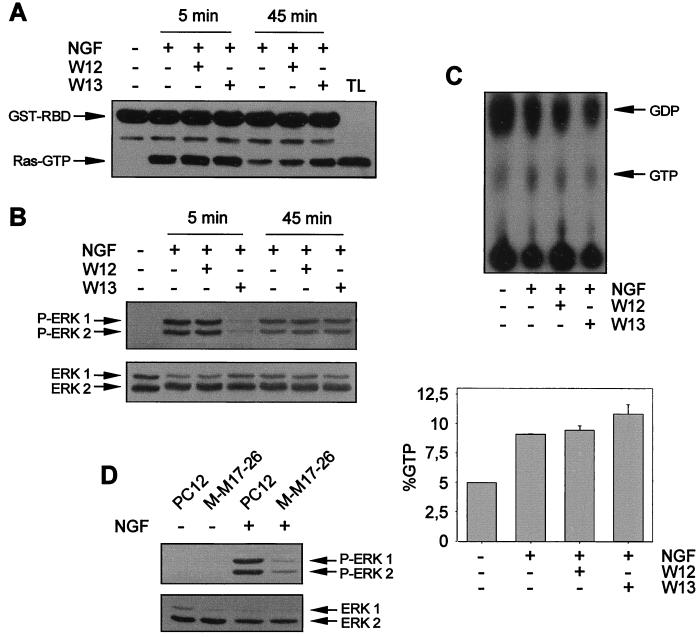
We next investigated whether the effect of CaM on ERK phosphorylation could be explained by the modulation of TrkA activity and/or Shc function. PC12 cells were pretreated with W13 or W12 and stimulated with NGF, and cell lysates were subjected to TrkA immunoprecipitation with an anti-pan-Trk antibody. Trk phosphorylation was analyzed with anti-Tyr(P) antibody. As shown in Fig. 4A, the contents of phosphorylated tyrosines of TrkA were very similar for W13- and W12-treated cultures or NGF-stimulated cultures without drug pretreatment. This result indicates that W13 does not affect the tyrosine kinase activity of the receptor or the interaction of NGF with TrkA.
FIG. 4.
CaM inhibitors do not modify the tyrosine phosphorylation of TrkA and Shc or the association of Grb2 with Shc upon NGF stimulation. PC12 cells were pretreated (+) or not pretreated (−) for 1 h with W12 or W13 and then stimulated (+) or not stimulated (−) for 5 min with NGF. After treatment, cells were lysed and protein extracts were obtained. (A) Protein extracts were subjected to immunoprecipitation with an anti-pan-Trk antibody, and immunoprecipitates were analyzed by Western blotting with the anti-Tyr(P) antibody 4G10. (B and C) Alternatively, protein extracts were subjected to immunoprecipitation with an anti-Shc antibody, and immunoprecipitates were analyzed by Western blotting with the anti-Tyr(P) antibody 4G10 (B, upper panel) or with an anti-Grb2 antibody (C, upper panel) and stripped and reprobed with an anti-Shc antibody (B and C, lower panels) as a control for the amount of immunoprecipitated protein per lane. Arrows indicate the positions of the three isoforms of Shc proteins. TL, total cell extracts from PC12 cells.
The second step in the activation of ERKs by TrkA is the recruitment of Shc to the plasma membrane and its subsequent tyrosine phosphorylation by Trk (77, 96). Phosphorylated Shc serves as a docking protein for Grb2 being translocated to the plasma membrane (90). To analyze whether CaM had the ability to modulate Shc function, the tyrosine phosphorylation of Shc proteins and their association with Grb2 after NGF stimulation were studied. PC12 cells were pretreated with W12 and W13 as described above and then stimulated with NGF. Protein extracts were subjected to immunoprecipitation with a specific antibody that recognizes the 66-, 52-, and 46-kDa isoforms of Shc. Immunoprecipitates were subjected to analysis with the anti-Tyr(P) antibody or, alternatively, with an anti-Grb2 antibody. NGF stimulation increased the tyrosine phosphorylation of Shc proteins (Fig. 4B) and the amount of coimmunoprecipitated Grb2 (Fig. 4C). However, W13 did not modify the tyrosine phosphorylation of Shc or the amount of Grb2 coimmunoprecipitated (Fig. 4B and C, respectively). Reprobing the membranes with an anti-Shc antibody showed that there were no significant differences in the amounts of immunoprecipitated Shc proteins in both analyses (Fig. 4B and C). In parallel blots of the same samples, we observed that W13 completely abolished ERK phosphorylation, whereas W12 did not (data not shown). It seems, therefore, that W13 modulation of NGF-induced ERK activity was not due to an alteration in the tyrosine kinase activity of Trk, to a diminution of the tyrosine phosphorylation level of Shc, or to a disruption of the association of Grb2 with Shc.
CaM modulates NGF-induced ERK activity downstream of p21ras.
p21ras has been reported to be essential for the ERK-MAP kinase activation induced by NGF in PC12 cells (86, 101, 102, 107). Recently, it has been demonstrated that Ca2+ and CaM can activate Ras through Ras GEF proteins (24, 33). Moreover, some CaM-binding Ras-like GTPases have been described, even though their functional activity on MAP kinase activation has not yet been tested (58, 105). In this context, we wanted to know whether the inhibitory effects of CaM inhibitor W13 on ERK activity could be mediated through the modulation of Ras function. We monitored the activation of p21ras using the radioactive method of GTP-GDP Ras loading and the nonradioactive method described by de Rooij and Bos (18) (see Materials and Methods). Using these two techniques, we observed that Ras activity increased after 5 min of NGF stimulation and was maintained after 45 min of NGF stimulation (Fig. 5A and C). However, W13 treatment did not modify significantly the state of Ras activity after 5 min of NGF stimulation (Fig. 5A and C). Interestingly, after 45 min of NGF stimulation, there was a slight increase in Ras GTP levels in W13-treated cultures compared to W12-treated or non-drug-treated ones (Fig. 5A). For the same cells, the W13 pretreatment was able to completely block ERK phosphorylation due to NGF stimulation, as measured with the anti-phospho-ERK antibody (Fig. 5B). These results indicate that W13 modulation of NGF-induced ERK activation does not depend on an effect of CaM inhibitors on Ras.
FIG. 5.
CaM inhibitors do not modify the profile of p21ras activation after NGF stimulation. PC12 cells were pretreated (+) or not pretreated (−) for 1 h with W12 or W13 and then stimulated (+) or not stimulated (−) for 5 or 45 min with NGF. After treatment, cells were lysed and protein extracts were obtained. (A) Protein extracts were subjected to precipitation with 50 μg of recombinant GST-RBD precoupled to gluthatione-Sepharose (see Materials and Methods). Precipitates were analyzed by Western blotting with an anti-pan-Ras antibody. Arrows indicate the positions of the proteins. TL, total cell extracts from PC12 cells. (B) Protein extracts from cell lysates in panel A were analyzed for ERK phosphorylation as described in the legend to Fig. 1. (C) PC12 cells were metabolically labeled with [32P]H3PO4, pretreated (+) or not pretreated (−) for 1 h with W12 or W13, and then stimulated (+) or not stimulated (−) for 5 min with NGF. Protein extracts were subjected to a Ras GTP-GDP loading assay as described in Materials and Methods (upper panel). The lower panel (graph) shows the average Ras activity from three independent experiments expressed as a percentage of GTP normalized by phosphorus according to the expression (GTP counts/3)/[(GTP counts/3) + (GDP counts/2)] × 100. (D) PC12 and M-M17-26 cells, which constitutively express the dominant negative Ha-ras mutant (Asn-17), were stimulated (+) or not stimulated (−) for 5 min with NGF. After treatment, cells were lysed and ERK phosphorylation was analyzed as described in the legend to Fig. 1.
Even though Ras activity was not modulated by W13, it seems clear from previous reports that Ras is necessary to stimulate ERK after NGF stimulation of TrkA (86, 101, 102, 107). We have directly approached this hypothesis by using a PC12 subline (M-M17-26) that constitutively expresses a dominant negative Ha-ras mutant (Asn-17) (97). We determined the level of ERK activation after NGF stimulation using the anti-phospho-ERK antibody. ERK activation was almost completely prevented after NGF stimulation in the M-M17-26 subline compared to wild-type PC12 cells (Fig. 5D).
Taken together, these results suggest that Ras and CaM are both necessary to signal the rapid and high activation of ERKs after NGF stimulation and that CaM seems to modulate the pathway downstream of Ras.
PI 3-kinase is not required for the early activation of the ERK-MAP kinases after NGF stimulation.
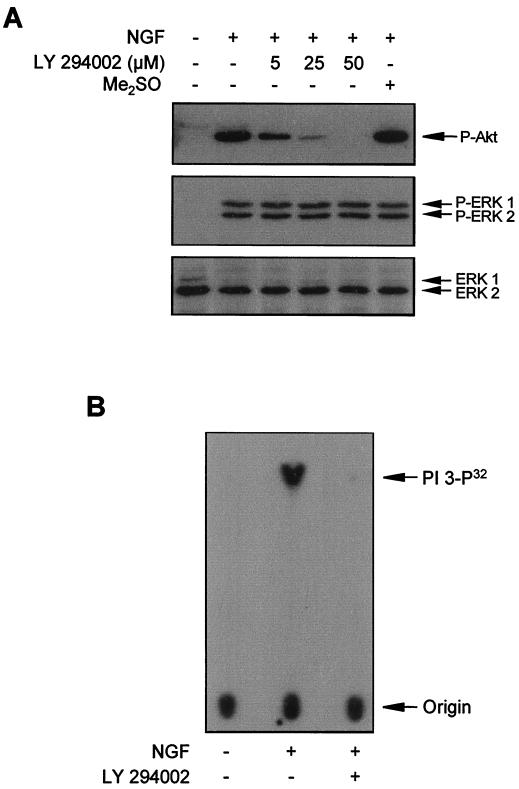
Another protein that becomes tyrosine phosphorylated and activated after TrkA stimulation is PI 3-kinase (79, 84). It has been suggested that PI 3-kinase can be involved in the regulation of the ERK-MAP kinase pathway, although this seems to be cell and ligand specific (22, 37, 50, 53, 54). Moreover, recent studies showed that CaM was able to bind and modulate the activity of the PI 3-kinase (35, 49). However, the contribution of PI 3-kinase to ERK activation after NGF stimulation in PC12 cells remains to be elucidated. We pretreated PC12 cells with LY295002, a PI 3-kinase-specific inhibitor (104), and then stimulated cells with NGF. Figure 6A shows that LY294002 was unable to modify the ERK-MAP kinase phosphorylation induced by NGF. However, the phosphorylation of Akt, a well-known downstream element of PI 3-kinase (for reviews, see references 3, 21, and 40), was almost completely blocked by 25 μM LY294002, thus demonstrating that the drug was effective in inhibiting PI 3-kinase activity (Fig. 6A). Furthermore, the drug was very active in blocking PI 3-kinase activity itself, as directly assessed by a PI 3-kinase assay (Fig. 6B). These results demonstrate that the rapid activation of ERK-MAP kinases after NGF stimulation is not dependent on PI 3-kinase in PC12 cells.
FIG. 6.
PI 3-kinase activity does not contribute to the activation of ERKs after NGF stimulation. (A) PC12 cells were pretreated (+) or not pretreated (−) for 30 min with the indicated concentrations of the PI 3-kinase inhibitor LY294002 or with vehicle (Me2SO) and then stimulated (+) or not stimulated (−) for 5 min with NGF. After treatment, cells were lysed and protein extracts were analyzed by Western blotting with an anti-phospho-Akt antibody (upper panel) or an anti-phospho-ERK antibody (middle panel) and stripped and reprobed with an anti-pan-ERK antibody (lower panel) as a control for the protein content per lane. (B) PC12 cells were stimulated (+) or not stimulated (−) for 1 min with NGF. After treatment, cells were lysed and protein extracts were subjected to immunoprecipitation with the anti-Tyr(P) antibody 4G10. PI 3-kinase activity was assayed in the immunoprecipitates in the presence (+) or in the absence (−) of 10 μM LY294002. Arrows labeled PI 3-P32 and Origin indicate the positions of in vitro radiolabeled l-α-phosphatidylinositol and the sample application, respectively.
NGF-induced MEK phosphorylation is inhibited by CaM antagonists.
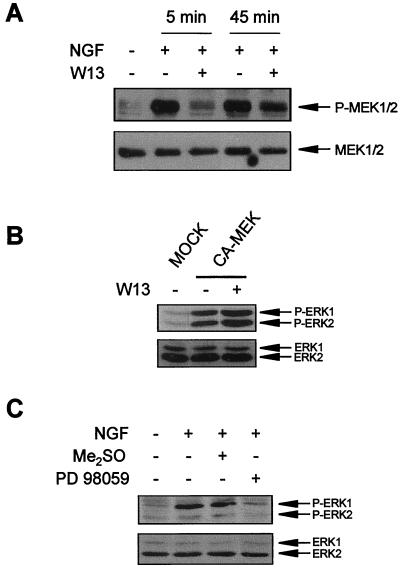
ERKs are activated by phosphorylation on threonine and tyrosine residues by MEK1 and MEK2 (67, 87). MEK itself is activated by phosphorylation on specific serine residues in response to trophic factor stimulation. This phosphorylation is generally attributed to Raf kinases (for a review, see reference 11). We analyzed the effect of CaM inhibitors on MEK activation using an anti-phospho-MEK1/2 antibody that recognizes phosphorylated Ser217 and Ser221 residues. The phosphorylation of these residues correlates with its functional activation (4, 26, 113, 114). NGF-induced MEK phosphorylation was prevented by W13 pretreatment, but only during the initial phase of NGF stimulation (Fig. 7A), following a pattern similar to that found for the inhibition of ERK phosphorylation (Fig. 3A). This correlation suggests that the effect of CaM antagonists on ERK activity was due to an inhibitory effect on MEK activation after NGF stimulation. However, the lack of MEK phosphorylation in the W13-treated cultures indicates that CaM does not directly modulate MEK activity but probably modulates the function of an upstream kinase. In fact, when PC12 cells were transiently transfected with a constitutively active form of MEK, ERK was found to be phosphorylated in a W13-independent manner (Fig. 7B). Finally, the selective MEK inhibitor PD098059 (23, 81) was able to prevent ERK phosphorylation by NGF stimulation (Fig. 7C), indicating that ERK phosphorylation upon NGF stimulation of PC12 cells is dependent on a functional MEK.
FIG. 7.
CaM inhibitor W13 prevents ERK activation upstream of MEK. (A) PC12 cells were pretreated (+) or not pretreated (−) for 1 h with W13 and then stimulated (+) or not stimulated (−) for 5 and 45 min with NGF. After treatment, cells were lysed and protein extracts were analyzed by Western blotting with an anti-phospho-MEK1/2 antibody (upper panel) and stripped and reprobed with an anti-MEK1/2 antibody (lower panel) as a control for the protein content per lane. (B) PC12 cells were transfected with a constitutive form of MEK1 (CA-MEK) or with a plasmid control (MOCK) (see Materials and Methods). After 48 h of transfection, cells were treated (+) or not treated (−) with W13. After treatment, protein extracts were analyzed by Western blotting for ERK phosphorylation as described in the legend to Fig. 1. (C) PC12 cells were pretreated (+) or not pretreated (−) for 1 h with 25 μM PD098059 or with 0.1% Me2SO as a vehicle and then were stimulated (+) or not stimulated (−) for 5 min with NGF. After treatment, cells were lysed and protein extracts were analyzed by Western blotting for ERK phosphorylation as described in the legend to Fig. 1.
NGF-induced Raf kinase activation is prevented by CaM antagonists.
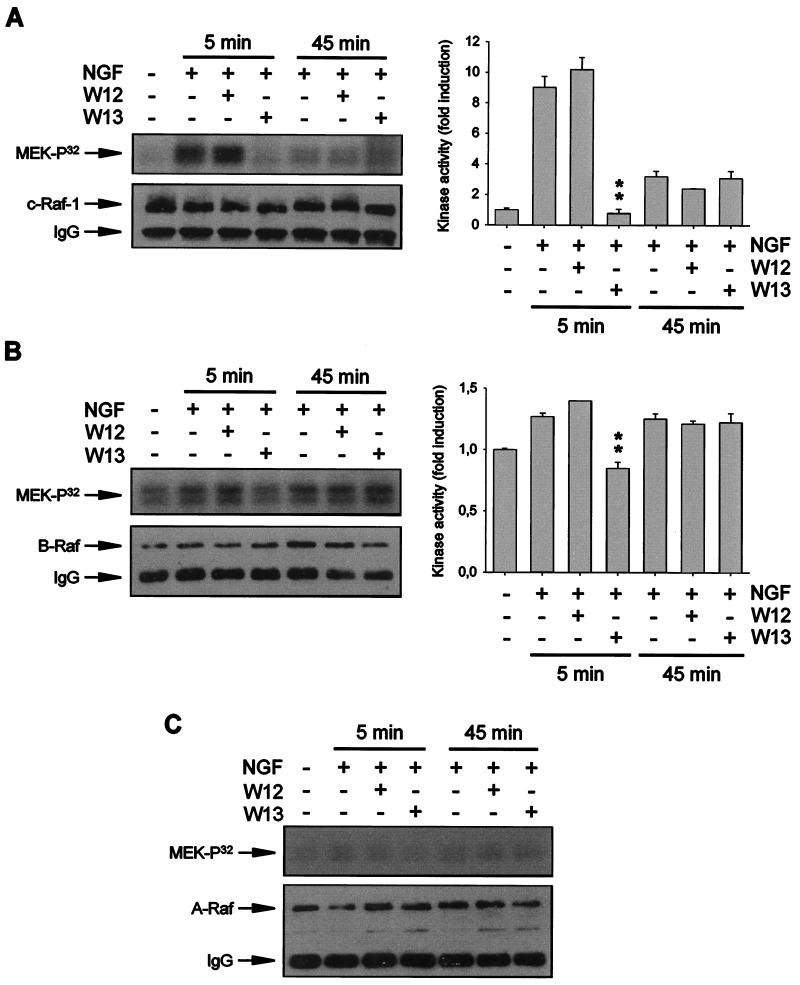
The results reported above indicate the existence of a MEK kinase activity, stimulated by Ras, that would be modulated directly or indirectly by Ca2+ and CaM. The best-characterized MEK kinases belong to the Raf family and include Raf-1, B-Raf, and A-Raf (for reviews, see references 32 and 87). In PC12 cells, initial NGF-induced ERK activation correlates with the activation of Raf-1, whereas sustained ERK activation correlates with the prolonged activation of A-Raf and B-Raf (106). The transient modulation of ERK activity by CaM antagonists suggests that CaM could modulate Raf-1 activity. In order to test this possibility, we analyzed whether W13 was able to inhibit NGF-induced Raf kinase activation. Kinase assays were performed with immunoprecipitates obtained by use of specific antibodies against Raf-1, B-Raf, and A-Raf. As shown in Fig. 8A, Raf-1 activity increased ∼9- to 11-fold after 5 min of NGF stimulation and was nearly undetectable after 45 min of NGF treatment. However, W13 pretreatment completely prevented the increase in Raf-1 activity observed after 5 min of NGF stimulation. When the effects of W13 on B-Raf activation were tested, similar results were obtained. After 5 min of NGF stimulation, B-Raf kinase activity measured by MEK phosphorylation increased ∼1.3-fold compared to that in untreated cultures (Fig. 8B). These increases were completely prevented by pretreatment with W13 (Fig. 8B). NGF was able to maintain the long-term activation of B-Raf kinase activity. After 45 min of NGF treatment, MEK phosphorylation due to B-Raf kinase was found to be ∼1.2 times that found in untreated cells (Fig. 8B). However, W13 did not have any effect on B-Raf kinase activity (Fig. 8B). A-Raf kinase activity was not quantifiable at any of the times tested and seemed to be less relevant for ERK activation (Fig. 8C). W12 had no effect in any of the Raf assays, and the amounts of Raf immunoprecipitated by the specific antibodies were comparable among the different experimental conditions (Fig. 8, lower panels). In all of these assays, W13 efficiently blocked ERK phosphorylation induced by NGF at 5 min but not at 45 min (data not shown).
FIG. 8.
CaM inhibitors prevent the acute activation of Raf kinases after NGF stimulation. PC12 cells were pretreated (+) or not pretreated (−) for 1 h with W12 or W13 and stimulated (+) or not stimulated (−) for 5 or 45 min with NGF. After treatment, cells were lysed and protein extracts were subjected to immunoprecipitation with specific antibodies against Raf-1 (A), B-Raf (B), or A-Raf (C). Immunoprecipitates were used to determine kinase activity with wild-type MEK1 as a substrate (upper panels) and analyzed by Western blotting with the same antibody used in the immunoprecipitation step as a control for the enzyme content in the immunoprecipitates (lower panels). Graphs in panels A and B show the average Raf activity (expressed as fold induction over the kinase activity obtained in untreated cultures) from three independent experiments. ∗∗, P value of <0.01, as determined by Student's t test. A-Raf activity was not quantifiable in any of the experimental conditions. IgG, immunoglobulin G.
We have assayed the ability of W13 to directly block the kinase activities of the different Raf proteins. For these assays, immunoprecipitates obtained with specific antibodies were obtained from PC12 cells treated for 5 min with NGF in a manner similar to that described for the above experiments. Then, W13 or W12 at various concentrations and up to the dose that completely blocks c-Raf, B-Raf, or ERK activation in the cells (i.e., 70 μM) was added to the immunoprecipitates, and kinase activity was measured. None of the concentrations of W12 or W13 tested was able to significantly modify the kinase activity of Raf-1 or B-Raf in these in vitro assays. PC12 cells treated for 5 min with NGF increased their Raf-1 kinase activity 12-fold (12 ± 0.08). Raf-1 kinase activity in lysates from NGF-stimulated cells in which 70 μM W13 was included in the reaction medium was found to be 11 ± 0.39 times that in the nonstimulated control. Comparable results were obtained with B-Raf (data not shown). These results suggest that CaM inhibitors do not exert their effects through a direct inhibition of the kinase activity of Raf-1 or B-Raf but rather through a functional blockade of a Ca2+- or CaM-dependent step that is relevant for Raf-1 and B-Raf activation at early times of NGF stimulation.
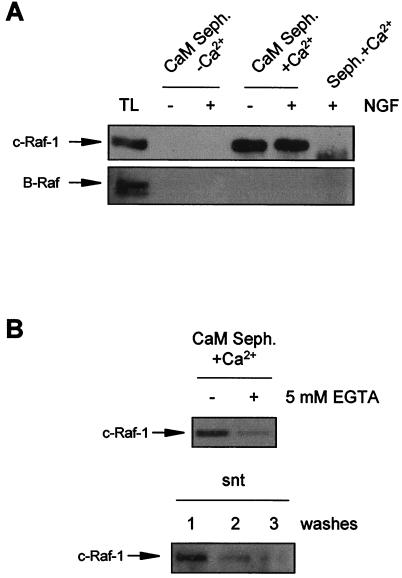
In order to further explore the mechanism by which CaM regulates Raf activity, we analyzed whether or not CaM could interact with Raf kinases. For this purpose, we used CaM coupled to Sepharose beads to precipitate CaM-binding proteins from cell lysates. Then, we used specific antibodies to analyze the presence of the Raf isoforms in the CaM-Sepharose precipitates. Using this approach, we found that Raf-1 was able to precipitate with CaM-Sepharose only when the cell lysate was supplemented with Ca2+ (Fig. 9A, upper panel). Raf-1 was not recovered in the precipitates either when EGTA was added to the lysates (Fig. 9A, upper panel) or when precipitates were obtained with plain Sepharose beads without bound CaM (Fig. 9A, upper panel). Moreover, when CaM-Sepharose precipitates in the presence of Ca2+ were washed with buffers containing 5 mM EGTA, Raf-1 was significantly eluted from CaM-Sepharose beads and was recovered in the supernatants (Fig. 9B). Interestingly, the CaM–Raf-1 interaction was not dependent on the state of Raf-1 activation, since we were unable to observe any significant differences in the amounts of precipitated Raf-1 from NGF-stimulated or nonstimulated PC12 cells (Fig. 9A, upper panel). Finally, we were unable to observe similar behavior for B-Raf despite the fact that NGF-induced enzyme activity was also modulated by W13 (Fig. 9A, lower panel).
FIG. 9.
c-Raf interacts with CaM. (A) PC12 cells were stimulated (+) or not stimulated (−) with NGF. After treatment, cells were lysed and protein extracts were subjected to precipitation with CaM-Sepharose (CaM Seph.) in the presence of 0.1 mM CaCl2 without EGTA (+Ca2+) or in the presence of 1 mM EGTA (−Ca2+) (see also Materials and Methods). Seph.+Ca2+, precipitates obtained with Sepharose without CaM in the presence of Ca2+. Precipitates were analyzed by Western blotting with an anti-Raf-1 antibody (upper panel) or with an anti-B-Raf antibody (lower panel). TL, total cell extracts from PC12 cells. (B) CaM precipitates obtained from nonstimulated cells in the presence of Ca2+ were washed (+) or not washed (−) with lysis buffer containing 5 mM EGTA. Precipitates (upper panel) and supernatants (snt, lower panel) from three consecutive washes were analyzed by Western blotting with an anti-Raf-1 antibody.
CaM inhibitors prevent the activation of the ERK-MAP kinases mediated by other tyrosine kinase receptors.
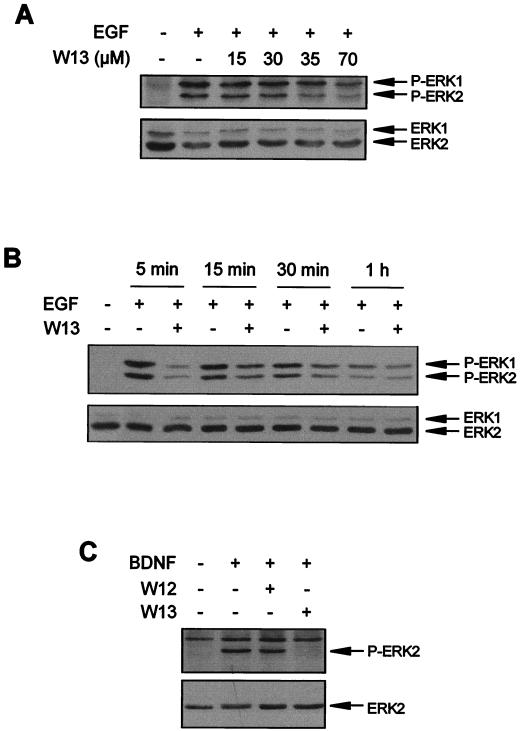
In order to determine the relevance of CaM modulation for the ERK pathway, we used other stimuli (EGF or BDNF) and other cellular systems (primary cultures of neurons) to verify similar dependencies on CaM. Thus, the ability of CaM to modulate EGF-induced ERK activation in PC12 cells was assessed. W13 at doses similar to those used in the NGF experiments was able to inhibit EGF-induced ERK phosphorylation (Fig. 10A). This effect was specific, since W12 had no effect on the modulation of ERK phosphorylation (data not shown). We also observed that the effects of W13 on EGF-induced ERK phosphorylation were transient and comparable to those observed for NGF (Fig. 10B).
FIG. 10.
CaM inhibitors abolish the activation of ERKs induced by EGF in PC12 cells and by BDNF in chicken MTNs. (A) PC12 cells were pretreated or not pretreated (−) for 1 h with the indicated concentrations of the CaM inhibitor W13 and then stimulated (+) or not stimulated (−) for 5 min with EGF. (B) PC12 cells were pretreated (+) or not pretreated (−) with W13 for 1 h and then stimulated (+) or not stimulated (−) for the indicated times with EGF. (C) MTNs were pretreated (+) or not pretreated (−) for 1 h with W12 or W13 and then stimulated (+) or not stimulated (−) for 5 min with BDNF. ERK phosphorylation was determined as described in the legend to Fig. 1.
We finally analyzed the involvement of CaM in the activation of ERK-MAP kinases mediated by BDNF in MTNs. We have previously demonstrated that chicken embryo MTNs express functional TrkB receptors that are able to activate the ERK-MAP kinase and PI 3-kinase signaling pathways after BDNF stimulation (8, 20). On the basis of these observations, we wanted to know whether CaM antagonists were able to block the phosphorylation of ERKs induced by BDNF in cultured MTNs. W13, but not W12, pretreatment completely prevented the phosphorylation of ERKs induced by BDNF (Fig. 10C).
Taken together, these results suggest that CaM plays a key role in the early and high activation of ERK-MAP kinases induced by several tyrosine kinase receptors once these receptors are activated by their specific ligands.
DISCUSSION
The activation of the ERK-MAP kinase pathway by NGF is one of the best-characterized intracellular signaling pathways in PC12 cells (see reference 98). However, the mechanisms involved in the regulation of this pathway are not completely understood. In this context, the results presented here show that Ca2+ and CaM are both necessary for the high and rapid activation of ERKs upon TrkA stimulation in PC12 cells. We observed that CaM inhibitors block Raf-1, B-Raf, MEK, and ERK kinase activities without affecting the function of upstream elements involved in the activation of the ERK-MAP kinase cascade, such as Ras, Grb2, Shc, or Trk. Finally, we show that CaM is also involved in the activation of the ERK-MAP kinases after EGF stimulation in PC12 cells and after BDNF stimulation in primary neurons, such as MTNs.
Most of the studies demonstrating that increases in [Ca2+]i are able to activate ERKs have been performed with excitable cells (i.e., neurons and rat aortic vascular smooth muscle cells). In those studies, the increase in cytosolic Ca2+ levels was accomplished by depolarizing the cells or by using Ca2+ ionophores or agents that are able to release Ca2+ from the intracellular stores (e.g., thapsigargin) (reviewed in reference 34). However, some reports have indicated that NGF and EGF are able to induce a small and rapid increase in [Ca2+]i (57, 65, 80, 92; reviewed in reference 47). More recent results have reported that NGF-induced increases in [Ca2+]i seem to arise from both intracellular stores and extracellular spaces being mediated by the Trk receptor (16, 48, 75). However, in those reports, the functional relevance of Ca2+ mobilization after NGF stimulation was not clear. When functional studies were included, the authors related the Ca2+ increases with the regulation of neurotransmitter release, and no correlation was established with ERK activation (76). Therefore, the results presented here indicate an additional and important role for the Ca2+ increase after ligand-induced tyrosine kinase receptor stimulation (Trk or EGF receptor), since we demonstrate that intracellular Ca2+, through a CaM-dependent mechanism, is required for ERK-MAP kinase activation.
Much attention has been focused in recent years on ascertaining the detailed mechanisms by which Ca2+ is able to activate ERKs. CaM has been involved in the activation of the ERK-MAP kinases after membrane depolarization in MTNs and in PC12 cells (27, 95). Similar results involving CaM in ERK-MAP kinase activation have been described with other experimental approaches (1, 29, 30). Interestingly, most of these Ca2+-dependent mechanisms appear to act at the level of Ras or further upstream (34, 88), including the involvement of protein kinases, such as PYK2 and Src, or the transactivation of EGFR (28, 59, 74, 89, 91, 116). At the level of Ras, other molecules have been described as potential Ca2+ and CaM regulators of the ERK-MAP kinase pathway. Among them are Ras GEF proteins such as Ras GRF or Ras GRP (24, 33). However, in our system, CaM seems to regulate NGF-stimulated ERK activation downstream of Ras, at the level of Raf, because we have not seen major differences in Ras activation in cultures treated with or without CaM inhibitors. Nonetheless, functional Ras is necessary for NGF to activate the ERK-MAP kinase pathway. A relevant observation in these experiments is that at the later times analyzed (45 min), there was a slight increase in the amount of active Ras in W13-treated cultures (Fig. 5A). Other authors have previously described increases in Ras and MAP kinase activity as a consequence of treatment of the cells with the CaM inhibitor W13 (9). These authors postulate that CaM normally inhibits ERK pathway activation at the level of Ras and that the blockade of CaM with W13 results in an increase in Ras activity, as measured by the GST-RBD method (9).
Another part of the present work analyzed the possible involvement of PI 3-kinase in the CaM regulation of ERK activation. CaM has been reported to interact with and modulate PI 3-kinase (35, 49). Moreover, several examples demonstrate that PI 3-kinase can modulate the activation of ERK-MAP kinases by different stimuli. Interestingly, most evidence suggests that the PI 3-kinase-dependent pathway modulates ERK activity at some step downstream of Ras (37, 50, 53, 54). However, although NGF is able to activate PI 3-kinase, we failed to observe any significant effect of functional PI 3-kinase inhibitors on the activation of ERKs after Trk stimulation, suggesting that PI 3-kinase does not contribute to this process. We obtained similar results with PC12 cells when ERKs were stimulated by Ca2+ influx due to membrane depolarization (26). It has been reported that the involvement of PI 3-kinase in the activation of ERKs is a cell- and ligand-dependent phenomenon (22). Thus, it may be possible that the rapid and high activation of ERKs in PC12 cells does not require PI 3-kinase, at least after Trk and Ca2+ influx stimulation.
We have observed that CaM antagonists inhibit only rapid and high NGF-induced ERK-MAP kinase activation. The time course of ERK inhibition observed for cultures pretreated with CaM antagonists prior to NGF or EGF stimulation correlates with the time course of the [Ca2+]i increase, which is maximal after 5 min of NGF treatment and gradually decreases even in the continued presence of NGF (55, 75). From these observations, we hypothesize that CaM could be necessary for regulating the pathway when Ca2+ levels increase in the cytosol.
Another possibility to explain the transient effect of CaM antagonists is that the elements of the pathway involved in the rapid and initial activation of ERK-MAP kinases after NGF stimulation are different from those involved in persistent activation. In this regard, it has been suggested that the initial activation of ERK-MAP kinases by NGF is mediated by the Grb2-SOS complex and requires the small G protein Ras, whereas the sustained activation is mediated by the CRK-C3G complex and requires the small G protein Rap1 (68, 71, 112). These results indicate the possibility that CaM can regulate the downstream effectors of the Grb2-SOS-Ras pathway but not those of the CRK-C3G-Rap1 pathway. The main downstream effector of Ras is Raf-1, which would be responsible for the initial and transient activation of the ERK-MAP kinase pathway after NGF stimulation of PC12 cells (71, 106, 112). It has been proposed that sustained activation of the ERK-MAP kinases is due to B-Raf through a Rap-1-dependent mechanism (71, 106, 112). However, all these results seem controversial, since Zwartkruis et al. (115) reported that acute activation of ERK due to EGF stimulation could be mediated by both Rap1 and Ras, whereas acute activation of ERK due to NGF stimulation was exclusively mediated by Ras (i.e., the authors did not detect Rap1 activation). Furthermore, sustained activation due to NGF stimulation seemed to be mediated by a Ras-dependent, Rap1-independent mechanism, since Rap1 did not seem to be activated by NGF (115). Furthermore, NGF needed functional Ras to stimulate ERKs, at either acute or sustained phases. These experiments were performed with PC12 cells stably transfected with a dominant negative form of Ras under the control of dexamethasone (107). Further information related to these complex regulatory mechanisms includes activation by Trk of these two pathways in response to NGF stimulation of PC12 cells by different initial adapters. Specifically, Meakin et al. (71) have suggested that Ras and Rap1 would be activated through the engagement of Shc and FRS2 proteins, respectively. Moreover, the authors reported that both adapters would compete for the same phosphorylated tyrosine residues of the Trk receptor (Y499 in rat Trk) (71). Our results show that the most prominent effects of CaM inhibitors were observed in blocking the initial activation of the pathway, a phenomenon that is mainly due to Ras and Raf-1 activation.
The mechanisms that control the activation of Raf-1 remain incompletely understood (reviewed in reference 73). It seems clear that an initial and important step for Raf-1 activation is its binding to Ras. This is a necessary but not sufficient step to activate Raf-1 (66). The main function of the Ras–Raf-1 interaction seems to be to bring Raf-1 to the plasma membrane, where it will become phosphorylated at different amino acid residues of the N-terminal part of the catalytic domain. Phosphorylation of two specific residues (S338 and Y341) seems important for the full activation of Raf-1 (17, 19, 46, 69). On the other hand, the regulation of B-Raf activity is different. For example, among other differences, B-Raf does not have a phosphorylatable residue equivalent to residue Y341 of Raf-1 (69). There is little information about the kinases responsible for Raf-1 phosphorylation. Recently, it has been described that the p21-activated protein kinase Pak3 phosphorylates Raf-1 at residue S338, both in vivo and in vitro (52). There is no information about the kinase(s) that phosphorylates Raf-1 at the Y341 residue.
More important in the present context is the absence of information about the Ca2+ and CaM regulatability of these Raf kinases. Our present results suggest that CaM could be one of the important elements regulating the kinase involved in the phosphorylation that activates Raf-1. In fact, it has been reported that a CaM-Ks cascade (CaM-KK and CaM-KIV) is able to modulate the activation of MAPK kinases, mainly p38 and Jun kinase (JNK) and, to a lesser extent, ERK (31). However, in PC12 cells, CaM-KIV is expressed in limited amounts, suggesting that another CaM-K, such as CaM-KII, may mediate these effects (31). We have approached the possibility that CaM-KII could mediate the effects of CaM antagonists on the activation of ERKs induced by NGF. We have used the specific CaM-KII inhibitor KN-62 and have observed that it does not modify significantly the activation of ERKs induced by NGF (data not shown). In accordance with this result, we were not able to detect any significant activation of CaM-KII in NGF-stimulated cells (data not shown). These results correlate with those recently published by Vaillant et al. (103) showing that KN-62 treatment does not affect the activation of ERKs induced by NGF in sympathetic neurons. However, these results do not negate the possibility that other CaM-regulated kinases are involved in Raf-1 phosphorylation and activation. Further work should be performed in order to confirm or deny this possibility.
Until now, no direct interaction and/or regulation by CaM and Raf-1 has been reported. Nevertheless, we show that CaM is able to specifically interact with Raf-1 in a Ca2+-dependent manner. This binding seems relevant, since other members of the Raf family, such as B-Raf, were not able to bind CaM. Whether this interaction is responsible for the modulation of Raf-1 activity by CaM or CaM-regulated kinases remains to be elucidated.
ACKNOWLEDGMENTS
This work was funded by the Comisión Interministerial de Ciencia y Tecnologia through the Plan Nacional de Salud y Farmacia (contract no. 97-0094), Telemarató de TV3 (Edició 1997: Malalties Degeneratives Hereditàries), EU Biotech Program (contract no. BIO4-CT96-0433), and Ajuntament de Lleida. J. Egea is a predoctoral fellow of the Generalitat de Catalunya. S. Peiró is a predoctoral fellow of the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS).
We thank colleagues in our laboratory for criticism and technical support. The assistance of Dionisio Martin-Zanca, Martí Aldea, and Carme Gallego in many aspects of our work is especially acknowledged. We thank the indicated persons for the generous gifts of the following antibodies: anti-Grb2 (J. Ureña), anti–pan-Ras (O. Bachs and N. Agell), and anti–pan-Trk (203) (D. Martin-Zanca). We thank G. Capellà and C. García for the generous gift of EGF. We also thank F. McKenzie, O. Bachs, and N. Agell for the generous gift of the prokaryotic expression vector containing the GST-RBD construct; G. M. Cooper and A. Aranda for the M-M17-26 cells; and C. E. Marshall and A. López-Rivas for the Glu217-Glu221 MAKK1 mutant construct. We are grateful to J. Fibla for purification of NGF. We thank Isabel Sánchez and Roser Pané for expert technical assistance and A. Porras for helpful technical comments in the PI 3-kinase and Ras GTP loading assays.
REFERENCES
- 1.Abraham S T, Benscoter H A, Schworer C M, Singer H A. A role for Ca2+/calmodulin-dependent protein kinase II in the mitogen-activated protein kinase signaling cascade of cultured rat aortic vascular smooth muscle cells. Circ Res. 1997;81:575–584. doi: 10.1161/01.res.81.4.575. [DOI] [PubMed] [Google Scholar]
- 2.Ahn S, Olive M, Aggarwal S, Krylov D, Ginty D D, Vinson C. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol. 1998;18:967–977. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1998;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 4.Alessi D R, Saito Y, Campbell D G, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall C J, Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 6.Barde Y-A, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnard D, Diaz B, Clawson D, Marshall M. Oncogenes, growth factors and phorbol esters regulate Raf-1 through common mechanisms. Oncogene. 1998;17:1539–1547. doi: 10.1038/sj.onc.1202061. [DOI] [PubMed] [Google Scholar]
- 8.Becker E, Soler R M, Yuste V J, Gine E, Sanz-Rodriguez C, Egea J, Martin-Zanca D, Comella J X. Development of survival responsiveness to brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5, but not to nerve growth factor, in cultured motoneurons from chick embryo spinal cord. J Neurosci. 1998;18:7903–7911. doi: 10.1523/JNEUROSCI.18-19-07903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch M, Gil J, Bachs O, Agell N. Calmodulin inhibitor W13 induces sustained activation of ERK2 and expression of p21cip1. J Biol Chem. 1998;273:22145–22150. doi: 10.1074/jbc.273.34.22145. [DOI] [PubMed] [Google Scholar]
- 10.Boulton T G, Nye S H, Robbins D J, Ip N Y, Radziejewska E, Morgenbesser S D, Depino R A, Panayotos N, Cobb M H, Yancopoulos G D. ERKs: a family of protin-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 11.Cobb M H, Goldsmith E J. How MAP kinases are regulated. J Cancer Res. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 12.Colbran R J. Regulation and role of brain calcium/calmodulin-dependent protein kinase II. Neurochem Int. 1992;21:469–497. doi: 10.1016/0197-0186(92)90080-b. [DOI] [PubMed] [Google Scholar]
- 13.Comella J X, Sanz-Rodriguez C, Aldea M, Esquerda J E. Skeletal muscle-derived trophic factors prevent motoneurons from entering an active cell death program in vitro. J Neurosci. 1994;14:2674–2686. doi: 10.1523/JNEUROSCI.14-05-02674.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowley C, Spencer S D, Nishimura M C, Chen K S, Pitts-Meek S, Armanini M P, Ling L H, MacMahon S B, Shelton D L, Levinson A D, Phillips H S. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 15.Davies A M. The role of neurotrophins in the developing nervous system. J Neurobiol. 1994;25:1334–1348. doi: 10.1002/neu.480251103. [DOI] [PubMed] [Google Scholar]
- 16.De Bernardi M A, Rabin S J, Colangelo A M, Brooker G, Mocchetti I. TrkA mediates the nerve growth factor-induced intracellular calcium accumulation. J Biol Chem. 1996;271:6092–6098. doi: 10.1074/jbc.271.11.6092. [DOI] [PubMed] [Google Scholar]
- 17.Dent P, Reardon D B, Morrison D K, Sturgill T W. Regulation of Raf-1 and Raf-1 mutants by Ras-dependent and Ras-independent mechanisms in vitro. Mol Cell Biol. 1995;15:4125–4235. doi: 10.1128/mcb.15.8.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Rooij J, Bos J L. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- 19.Diaz B, Barnard D, Filson A, MacDonald S, King A, Marshall M. Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol Cell Biol. 1997;17:4509–4516. doi: 10.1128/mcb.17.8.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolcet X, Egea J, Soler R M, Martin-Zanca D, Comella J X. Activation of phosphatidylinositol 3-kinase, but not extracellular-regulated kinases, is necessary to mediate brain-derived neurotrophic factor-induced motoneuron survival. J Neurochem. 1999;73:521–531. doi: 10.1046/j.1471-4159.1999.0730521.x. [DOI] [PubMed] [Google Scholar]
- 21.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 22.Duckworth B C, Cantley L C. Conditional inhibition of the mitogen-activated protein kinase cascade by wortmannin. J Biol Chem. 1997;272:27665–27670. doi: 10.1074/jbc.272.44.27665. [DOI] [PubMed] [Google Scholar]
- 23.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebinu J O, Bottorff D A, Chan E Y, Stang S L, Dunn R J, Stone J C. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 25.Egan S E, Giddings B W, Brooks M W, Buday L, Sizeland A M, Weinberg R A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993;363:45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- 26.Egea J, Espinet C, Comella J X. Calcium influx activates extracellular-regulated kinase/mitogen-activated protein kinase pathway through a calmodulin-sensitive mechanism in PC12 cells. J Biol Chem. 1999;274:75–85. doi: 10.1074/jbc.274.1.75. [DOI] [PubMed] [Google Scholar]
- 27.Egea J, Espinet C, Comella J X. Calmodulin modulates mitogen-activated protein kinase activation in response to membrane depolarization in PC12 cells. J Neurochem. 1998;70:2554–2564. doi: 10.1046/j.1471-4159.1998.70062554.x. [DOI] [PubMed] [Google Scholar]
- 28.Eguchi S, Iwasaki H, Inagami T, Numaguchi K, Yamakawa T, Motley E D, Owada K M, Marumo F, Hirata Y. Involvement of PYK2 in angiotensin II signaling of vascular smooth muscle cells. Hypertension. 1999;33:201–206. doi: 10.1161/01.hyp.33.1.201. [DOI] [PubMed] [Google Scholar]
- 29.Eguchi S, Matsumoto T, Motley E D, Utsunomiya H, Inagami T. Identification of an essential signaling cascade for mitogen-activated protein kinase activation by angiotensin II in cultured rat vascular smooth muscle cells—possible requirement of G(q)-mediated p21(ras) activation coupled to a Ca2+/calmodulin-sensitive tyrosine kinase. J Cancer Res. 1996;271:14169–14175. doi: 10.1074/jbc.271.24.14169. [DOI] [PubMed] [Google Scholar]
- 30.Elliott R C, Inturrisi C E, Black I B, Dreyfus C F. An improved method detects differential NGF and BDNF gene expression in response to depolarization in cultured hippocampal neurons. Mol Brain Res. 1994;26:81–88. doi: 10.1016/0169-328x(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 31.Enslen H, Tokumitsu H, Stork P J S, Davis R J, Soderling T R. Regulation of mitogen-activated protein kinases by a calcium/calmodulin-dependent protein kinase cascade. Proc Natl Acad Sci USA. 1996;93:10803–10808. doi: 10.1073/pnas.93.20.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fanger G R, Gerwins P, Widmann C, Jarpe M B, Johnson G L. MEKKs, GCKs, MLKs, PAKs, TAKs, and tpls: upstream regulators of the c-Jun amino-terminal kinases? Curr Opin Genet Dev. 1997;7:67–74. doi: 10.1016/s0959-437x(97)80111-6. [DOI] [PubMed] [Google Scholar]
- 33.Farnsworth C L, Freshney N W, Rosen L B, Ghosh A, Greenberg M E, Felg L A. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 34.Finkbeiner S, Greenberg M E. Ca(2+)-dependent routes to Ras: mechanisms for neuronal survival, differentiation, and plasticity? Neuron. 1996;16:233–236. doi: 10.1016/s0896-6273(00)80040-9. [DOI] [PubMed] [Google Scholar]
- 35.Fischer R, Julsgart J, Berchtold M W. High affinity calmodulin target sequence in the signaling molecule PI 3-kinase. FEBS Lett. 1998;425:175–177. doi: 10.1016/s0014-5793(98)00225-7. [DOI] [PubMed] [Google Scholar]
- 36.Gietzen K, Xu Y H, Galla H J, Bader H. Multimers of anionic amphiphiles mimic calmodulin stimulation of cyclic nucleotide phosphodiesterase. Biochem J. 1982;207:637–640. doi: 10.1042/bj2070637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grammer T C, Blenis J. Evidence for MEK-independent pathways regulating the prolonged activation of the ERK-MAP kinases. Oncogene. 1997;14:1635–1642. doi: 10.1038/sj.onc.1201000. [DOI] [PubMed] [Google Scholar]
- 38.Greene L, Tischler A. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamburger V, Levi-Montalcini R. Proliferation, differentiation and degeneration in the spinal ganglia of the chick embryo and the normal and experimental conditions. J Exp Zool. 1949;111:457–501. doi: 10.1002/jez.1401110308. [DOI] [PubMed] [Google Scholar]
- 40.Hemmings B A. Ptdlns(3,4,5)P3 gets its message across. Science. 1997;277:534. doi: 10.1126/science.277.5325.534. [DOI] [PubMed] [Google Scholar]
- 41.Hidaka H, Sasaki Y, Tanaka T, Endo T, Ohno S, Fujii H, Nagata N-(6-Aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci USA. 1981;78:4354–4357. doi: 10.1073/pnas.78.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hidaka H, Tanaka T. Naphthalenesulfonamides as calmodulin antagonists. Methods Enzymol. 1983;102:185–194. doi: 10.1016/s0076-6879(83)02019-4. [DOI] [PubMed] [Google Scholar]
- 43.Howe L R, Leevers S J, Gomez N, Nakielny S, Cohen P, Marshall C J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992;71:335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- 44.Hu J, el-Fakahany E E. The calmodulin antagonist calmidazolium stimulates release of nitric oxide in neuroblastoma N1E-115 cells. NeuroReport. 1993;4:198–200. doi: 10.1097/00001756-199302000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Jaiswal R K, Moodie S A, Wolfman A, Landreth G E. The mitogen-activated protein kinase cascade is activated by B-Raf in response to nerve growth factor through interaction with p21ras. Mol Cell Biol. 1994;14:6944–6953. doi: 10.1128/mcb.14.10.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jenilek T, Dent P, Sturgill T W, Weber M J. Ras-induced activation of Raf-1 is dependent on tyrosine phosphorylation. Mol Cell Biol. 1996;16:1027–1034. doi: 10.1128/mcb.16.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang H, Guroff G. Actions of the neurotrophins on calcium uptake. J Neurosci Res. 1997;50:355–360. doi: 10.1002/(SICI)1097-4547(19971101)50:3<355::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 48.Jiang H, Ulme D S, Dickens G, Chabuk A, Lavarreda M, Lazarovici P, Guroff G. Both p140(trk) and p75(NGFR) nerve growth factor receptors mediate nerve growth factor-stimulated calcium uptake. J Biol Chem. 1997;272:6835–6837. doi: 10.1074/jbc.272.11.6835. [DOI] [PubMed] [Google Scholar]
- 49.Joyal J L, Burks D J, Pons S, Matter W F, Vlahos C J, White M F, Sacks D B. Calmodulin activates phosphatidylinositol 3-kinase. J Biol Chem. 1997;272:28183–28186. doi: 10.1074/jbc.272.45.28183. [DOI] [PubMed] [Google Scholar]
- 50.Karnitz L M, Burns L A, Sutor S L, Blenis J, Abraham R T. Interleukin-2 triggers a novel phosphatidylinositol 3-kinase-dependent MEK activation pathway. Mol Cell Biol. 1995;15:3049–3057. doi: 10.1128/mcb.15.6.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katz M E, McCormick F. Signal transduction from multiple Ras effectors. Curr Opin Genet Dev. 1997;7:75–79. doi: 10.1016/s0959-437x(97)80112-8. [DOI] [PubMed] [Google Scholar]
- 52.King A J, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall M S. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- 53.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Brugge J S. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knall C, Young S, Nick J A, Buhl A M, Worthen G S, Johnson G L. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem. 1996;271:2832–2838. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- 55.Kozak A, Nikodijevic D, Yavin E, Guroff G. Intracellular calcium levels regulate the actions of nerve growth factor on calcium uptake in PC12 cells. J Neurosci Res. 1992;33:30–36. doi: 10.1002/jnr.490330105. [DOI] [PubMed] [Google Scholar]
- 56.Kyriakis J M, App H, Zhang X F, Banerjee P, Brautigan D L, Rapp U, Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992;358:417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- 57.Lazarovici P, Levi B Z, Lelkes P, Koizumi S, Fujita K, Matsuda Y, Ozato K, Guroff G. K-252a inhibits the increase in c-fos transcription and the increase in intracellular calcium produced by nerve growth factor in PC12 cells. J Neurosci Res. 1989;23:1–8. doi: 10.1002/jnr.490230102. [DOI] [PubMed] [Google Scholar]
- 58.Lee C H J, Della N G, Chew C E, Zack D J. Rin, a neuron-specific and calmodulin-binding small G-protein, and Rit define a novel subfamily of Ras proteins. J Neurosci. 1996;16:6784–6794. doi: 10.1523/JNEUROSCI.16-21-06784.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 60.Lewin G R, Barde Y A. Physiology of neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 61.Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar-Sagi D, Margolis B, Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993;363:85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- 62.Lowenstein E J, Daly R J, Batzer A G, Li W, Margolis B, Lammers R, Ullrich A, Skolnik E Y, Bar-Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 63.MacDonald S G, Crews C M, Wu L, Driller J, Clark R, Erikson R L, McCormick F. Reconstitution of the Raf1-MEK-ERK signal transduction pathway in vitro. Mol Cell Biol. 1993;13:6615–6620. doi: 10.1128/mcb.13.11.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacNicol M, Schulman H. Multiple Ca2+ signaling pathways converge on CaM kinase in PC12 cells. FEBS Lett. 1992;304:237–240. doi: 10.1016/0014-5793(92)80627-s. [DOI] [PubMed] [Google Scholar]
- 65.Magni M, Meldolesi J, Pandiella A. Ionic events induced by epidermal growth factor. Evidence that hyperpolarization and stimulated cation influx play a role in the stimulation of cell growth. J Biol Chem. 1991;266:6329–6335. [PubMed] [Google Scholar]
- 66.Marais R, Light Y, Paterson H F, Marshall C J. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 68.Marshall C J. Taking the Rap. Nature. 1998;392:553–554. doi: 10.1038/33293. [DOI] [PubMed] [Google Scholar]
- 69.Mason C S, Springer C J, Cooper R G, Superti-Furga G, Marshall C J, Marais R. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 1999;18:2137–2148. doi: 10.1093/emboj/18.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masson L, Lee H, Jarrett H W. Trifluoperazine binding to porcine brain calmodulin and skeletal muscle troponin C. Biochemistry. 1990;29:671–681. doi: 10.1021/bi00455a012. [DOI] [PubMed] [Google Scholar]
- 71.Meakin S O, MacDonald J L, Gryz E A, Kubu C J, Verdi J M. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J Biol Chem. 1999;274:9861–9870. doi: 10.1074/jbc.274.14.9861. [DOI] [PubMed] [Google Scholar]
- 72.Mobley W C, Schenker A, Shooter E M. Characterization and isolation of proteolytically modified nerve growth factor. Biochemistry. 1976;15:5543–5552. doi: 10.1021/bi00670a019. [DOI] [PubMed] [Google Scholar]
- 73.Morrison D K, Cutler R E., Jr The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- 74.Murasawa S, Mori Y, Nozawa Y, Masaki H, Maruyama K, Tsutsumi Y, Moriguchi Y, Shibasaki Y, Tanaka Y, Iwasaka T, Inada M, Matsubara H. Role of calcium-sensitive tyrosine kinase Pyk2/CAKbeta/RAFTK in angiotensin II induced Ras/ERK signaling. Hypertension. 1998;32:668–675. doi: 10.1161/01.hyp.32.4.668. [DOI] [PubMed] [Google Scholar]
- 75.Nikodijevic B, Guroff G. Nerve growth factor-induced increase in calcium uptake by PC12 cells. J Neurosci Res. 1991;28:192–199. doi: 10.1002/jnr.490280206. [DOI] [PubMed] [Google Scholar]
- 76.Nikodijevic B, Guroff G. Nerve growth factor and K-252a increase catecholamine release from PC12 cells. J Neurosci Res. 1990;26:288–295. doi: 10.1002/jnr.490260304. [DOI] [PubMed] [Google Scholar]
- 77.Obermeier A, Lammers R, Wiesmuller K, Jung G, Schlessinger J, Ullrich A. Identification of Trk binding sites for SHC and phosphatidylinositol 3′-kinase and formation of a multimeric signaling complex. J Biol Chem. 1993;268:22963–22966. [PubMed] [Google Scholar]
- 78.Obermeier A, Tinhofer I, Grunicke H H, Ullrich A. Transforming potentials of epidermal growth factor and nerve growth factor receptors inversely correlate with their phospholipase C gamma affinity and signal activation. EMBO J. 1996;15:73–82. [PMC free article] [PubMed] [Google Scholar]
- 79.Ohmichi M, Decker S J, Saltiel A R. Activation of phosphatidylinositol-3 kinase by nerve growth factor involves indirect coupling of the trk proto-oncogene with src homology 2 domains. Neuron. 1992;9:769–777. doi: 10.1016/0896-6273(92)90039-g. [DOI] [PubMed] [Google Scholar]
- 80.Pandiella-Alonso A, Malgaroli A, Vicentini L M, Meldolesi J. Early rise of cytosolic Ca2+ induced by NGF in PC12 cells. FEBS Lett. 1986;208:48–51. doi: 10.1016/0014-5793(86)81529-0. [DOI] [PubMed] [Google Scholar]
- 81.Pang L, Sawada T, Decker S J, Saltiel A R. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 82.Porras A, Nebreda A R, Benito M, Santos E. Activation of Ras by insulin in 3T3-L1 cells does not involve GTPase-activating protein phosphorylation. J Biol Chem. 1992;267:21124–21131. [PubMed] [Google Scholar]
- 83.Rabin S J, Cleghon V, Kaplan D R. SNT, a differentiation-specific target of neurotrophic factor-induced tyrosine kinase activity in neurons and PC12 cells. Mol Cell Biol. 1993;13:2203–2213. doi: 10.1128/mcb.13.4.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raffioni S, Bradshaw R A. Activation of phosphatidylinositol 3-kinase by epidermal growth factor, basic fibroblast growth factor, and nerve growth factor in PC12 pheochromocytoma cells. Proc Natl Acad Sci USA. 1992;89:9121–9125. doi: 10.1073/pnas.89.19.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ray L B, Sturgill T W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci USA. 1988;85:3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robbins D J, Cheng M G, Zhen E Z, Vanderbilt C A, Feig L A, Cobb M H. Evidence for a Ras-dependent extracellular signal-regulated protein kinase (ERK) cascade. Proc Natl Acad Sci USA. 1992;89:6924–6928. doi: 10.1073/pnas.89.15.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 88.Rosen L B, Ginty D D, Weber M J, Greenberg M E. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron. 1994;12:1207–1221. doi: 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 89.Rosen L B, Greenberg M E. Stimulation of growth factor receptor signal transduction by activation of voltage-sensitive calcium channels. Proc Natl Acad Sci USA. 1996;93:1113–1118. doi: 10.1073/pnas.93.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rozakis-Adcock A, McGlade J, Mbamalu G, Pellici G, Daly R, Li W, Batzer A, Thomas S, Dugge J, Pellici P G, et al. Association of the SHC and Grb2/sem-5 SH2 containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992;360:689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- 91.Rusanescu G, Qi H, Thomas S M, Brugge J S, Halegoua S. Calcium influx induces neurite growth through a Src-Ras signalling cassette. Neuron. 1995;15:1415–1425. doi: 10.1016/0896-6273(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 92.Schubert D, LeCorbiere M, Whitlock C, Stallcup W. Alterations in the surface properties of cells responsive to nerve growth factor. Nature. 1978;273:718–723. doi: 10.1038/273718a0. [DOI] [PubMed] [Google Scholar]
- 93.Simon M A, Dodson G S, Rubin G M. An SH3-SH2-SH3 protein is required for p21ras1 activation and binds to Sevenless and Sos proteins in vitro. Cell. 1993;73:169–177. doi: 10.1016/0092-8674(93)90169-q. [DOI] [PubMed] [Google Scholar]
- 94.Smeyne R J, Klein R, Schnapp A, Long L K, Bryant S, Lewin A, Lira S A, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 95.Soler R M, Egea J, Mintenig G M, Sanz-Rodriguez C, Iglesias M, Comella J X. Calmodulin is involved in membrane depolarization-mediated survival of motoneurons by phosphatidylinositol-3 kinase- and MAPK-independent pathways. J Neurosci. 1998;18:1230–1239. doi: 10.1523/JNEUROSCI.18-04-01230.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stephens R M, Loeb D M, Copeland T D, Pawson T, Greene L A, Kaplan D R. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma 1 to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 97.Szeberenyi J, Cai H, Cooper G M. Effect of a dominant inhibitory Ha-ras mutation on neuronal differentiation of PC12 cells. Mol Cell Biol. 1990;10:5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Szeberenyi J, Erhardt P. Cellular components of nerve growth factor signaling. Biochim Biophys Acta. 1994;1222:187–202. doi: 10.1016/0167-4889(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 99.Tanaka T, Hidaka H. Hydrophobic regions function in calmodulin-enzyme(s) interactions. J Biol Chem. 1980;255:11078–11080. [PubMed] [Google Scholar]
- 100.Tatsuta M, Iishi H, Baba M, Yano H, Uehara H, Nakaizumi A, Iseki K. Inhibition by the calmodulin antagonist trifluoperazine of experimental hepatocarcinogenesis induced by N-nitrosomorpholine in Sprague-Dawley rats. Cancer Lett. 1996;107:179–185. doi: 10.1016/0304-3835(96)04353-4. [DOI] [PubMed] [Google Scholar]
- 101.Thomas S M, DeMarco M, D'Arcangelo G, Halegoua S, Brugge J S. Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell. 1992;68:1031–1040. doi: 10.1016/0092-8674(92)90075-n. [DOI] [PubMed] [Google Scholar]
- 102.Troppmair J, Bruder J T, App H, Hong C, Liptak L, Szeberenyi J, Cooper G M, Rapp U R. Ras controls coupling of growth factor receptors and protein kinase-C in the membrane to Raf-1 and B-Raf protein serine kinases in the cytosol. Oncogene. 1992;7:1867–1873. [PubMed] [Google Scholar]
- 103.Vaillant A R, Mazzoni I, Tudan C, Boudreau M, Kaplan D R, Miller F D. Depolarization and neurotrophins converge on the phosphatidylinositol 3-kinase-Akt pathway to synergistically regulate neuronal survival. J Cell Biol. 1999;146:955–966. doi: 10.1083/jcb.146.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 105.Wes P D, Yu M J, Montell C. RIC, a calmodulin-binding Ras-like GTPase. EMBO J. 1996;15:5839–5848. [PMC free article] [PubMed] [Google Scholar]
- 106.Wixler V, Smola U, Schuler M, Rapp U. Differential regulation of Raf isozymes by growth versus differentiation inducing factors in PC12 pheochromocytoma cells. FEBS Lett. 1996;385:131–137. doi: 10.1016/0014-5793(96)00363-8. [DOI] [PubMed] [Google Scholar]
- 107.Wood K W, Sarnecki C, Roberts T M, Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992;68:1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- 108.Wu J, Harrison J K, Vincent L A, Haystead C, Haystead T A J, Michel H, Hunt D F, Lynch K R, Sturgill T W. Molecular structure of a protein-tyrosine/threonine kinase activating p42 mitogen-activated protein (MAP) kinase:MAP kinase kinase. Proc Natl Acad Sci USA. 1993;90:173–177. doi: 10.1073/pnas.90.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xing J, Ginty D D, Greenberg M E. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 110.Xing J, Kornhauser J M, Xia Z, Thiele E A, Greenberg M E. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol. 1998;18:1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yano S, Tokumitsu H, Soderling T R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 112.York R D, Yao H, Dillon T, Ellig C L, Eckert S P, McCleskey E W, Stork P J S. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 113.Zheng C-F, Guan K-L. Cloning and characterization of two distinct human extracellular signal-regulated kinase activator kinases, MEK1 and MEK2. J Biol Chem. 1993;268:11435–11439. [PubMed] [Google Scholar]
- 114.Zheng C-F, Guan K-L. Activation of MEK family kinases requires phosphorylation on two conserved Ser/Thr residues. EMBO J. 1994;13:1123–1131. doi: 10.1002/j.1460-2075.1994.tb06361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zwartkruis F J T, Wolthuis R M F, Nabben N M J M, Franke B, Bos J L. Extracellular signal-regulated activation of Rap1 fails to interfere in Ras effector signalling. EMBO J. 1998;17:5905–5912. doi: 10.1093/emboj/17.20.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zwick E, Daub H, Naohito A, Yamaguchi-Aoki Y, Tinhofer I, Maly K, Ullrich A. Critical role of calcium-dependent epidermal growth factor receptor transactivation in PC12 cell membrane depolarization and bradykinin signaling. J Biol Chem. 1997;272:24767–24770. doi: 10.1074/jbc.272.40.24767. [DOI] [PubMed] [Google Scholar]