Summary
Lifestyle intervention encompassing nutrition and physical activity are effective strategies to prevent progressive lipid deposition in the liver. This study aimed to explore the effect of dietary change, and/or high-intensity interval training (HIIT) on hepatic lipid accumulation in high fat diet (HFD)-induced obese rats. We divided lean rats into lean control (LC) or HIIT groups (LH), and obese rats into obese normal chow diet (ND) control (ONC) or HIIT groups (ONH) and obese HFD control (OHC) or HIIT groups (OHH). We found that dietary or HIIT intervention significantly decreased body weight and the risk of dyslipidemia, prevented hepatic lipid accumulation. HIIT significantly improved mitochondrial fatty acid oxidation through upregulating mitochondrial enzyme activities, mitochondrial function and AMPK/PPARα/CPT1α pathway, as well as inhibiting hepatic de novo lipogenesis in obese HFD rats. These findings indicate that dietary alone or HIIT intervention powerfully improve intrahepatic storage of fat in diet induced obese rats.
Keywords: Obesity, Exercise, Diet, Mitochondrial function, Lipid deposition
Introduction
Nutrient overload is a major cause of lipid accumulation in fat tissue, skeletal muscle, and the liver [1]. Intrahepatic lipid accumulation results from lipid metabolism abnormalities, such as increased liver FFA uptake, VLDL synthesis, whole body lipolysis as well as reduced FFA oxidation and triglyceride (TG) export [2]. Fatty acids are catabolized in the liver by β-oxidation, which is therefore highly dependent on mitochondrial metabolism. Although several therapeutic approaches can be used in the context of fatty liver disease, optimizing nutrition and increasing physical activity, are still the most effective strategies [3,4].
Mice fed with obesogenic diets are a well-established experimental model of liver steatosis [5,6]. Liver lipid accumulation, fatty acids (FA) composition and inflammation were modulated by the dietary composition of lipids or carbohydrates. In research studies, the FA composition of the diet influences hepatic lipogenesis by regulating metabolic pathways [7], mainly through modulating the activity of specific mitochondrial fatty acid oxidation enzymes, as well as the mitochondrial function or gene expression levels affecting fat oxidation [8]. In addition to diet, an increase in caloric expenditure through exercise can significantly affect the progression of intrahepatic lipid accumulation. Numerous studies have reported the effects of interval training in patients with fatty liver disease [9–12]. Oh et al. compared the effects of 12-week HIIT, moderate-intensity continuous exercise training, and resistance training, and found that an improvement in hepatic stiffness was only observed with HIIT [10]. Since HIIT has been recognized as a novel exercise modality that can reduce fat mass, visceral adipose tissue, and intrahepatic lipid levels and improve hepatic fitness [4,13,14], its underlying mechanism has not yet been elucidated. Therefore, this study explored the effect of HIIT and dietary intervention on hepatic lipid metabolism and related molecular events involved in mitochondrial adaptation in HFD-induced obese rats.
Materials and Methods
Animals and protocol
Sixty 4-week-old male Sprague-Dawley rats were purchased from Qinglongshan Animal Breeding Farm (Nanjing, China). The animals weighted 68.4±2.4 g at the beginning of the experiment. All of the animals were reared in a laboratory (at a temperature of 24 °C–25 °C, and a humidity of 70 %–75 %, under a 12 h light/dark lighting regimen), and with free access to water and food. The normal chow diet (15.1 % fat, 21 % protein and 63.9 % carbohydrate)consisted of pellets manufactured by Shanghai SLAC Laboratory Animal Co., Ltd. The HFD was a commercially available rat pellet diet from Research Diets Inc (D12451: 45 % fat, 20 % protein and 35 % carbohydrate).
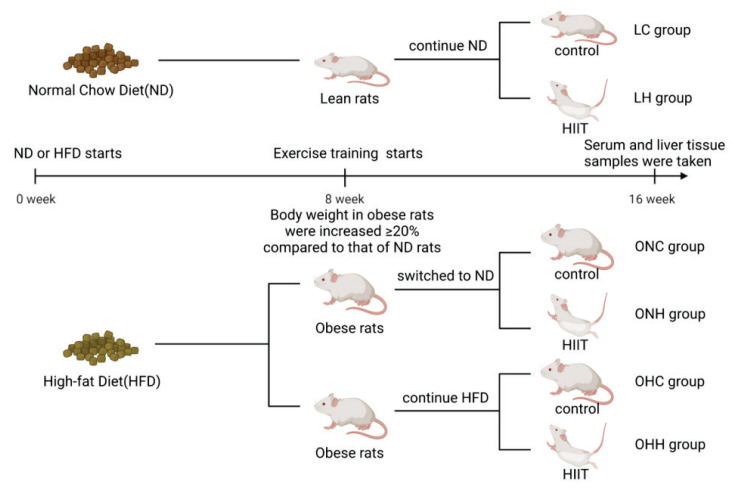
Rats were fed with a ND or a HFD for 8 weeks without exercise regimens. Body weight was monitored once weekly during the study. After these periods, obese rats were defined as those with a ≥ 20 % increase in body weight compared to that of ND rats. Following the confirmation of obesity, ND lean rats, still fed with a normal diet, were divided into the lean control group (LC group, n=10) and the lean HIIT group (LH group, n=10). Meanwhile, half of the obese rats switched to a ND, and were included in the obese ND control group (ONC group, n=10) and obese ND HIIT group (ONH group, n=10). The other half of the obese rats continued to receive a HFD and were divided into the obese HFD control group (OHC group, n=10) and obese HFD HIIT group (OHH group, n=10) (Fig. 1).
Fig. 1.
Overall design of this study.
High-intensity interval training
Swimming training groups performed 5–14 sets of swimming training protocols per day, for 5 days a week, for 8 weeks [15,16]. The swimming training was conducted in an individual glass chamber (60-cm high × 30-cm diameter) with a controlled water temperature of 31±1 °C. The load intensity was attached to the rat’s tail and was individually adjusted in each exercise session according to the rat’s body mass. Regarding load intensity, a previous study indicated that the lactate threshold was achieved with loads between 5 % and 6 % of a rat’s body mass [17]. Therefore, the interval protocol that we used consisted of high intensity (load between 5 %–16 % of body mass). Table 1 shows the swimming training protocols used in this study. Serum and liver tissue samples were taken 24 h after the rats’ last training sessions.
Table 1.
High intensity interval training protocol
| Week | Set | Time | Rest | Load(% body weight) |
|---|---|---|---|---|
| 1 | 5 | 1 min | 1 min | 0–5 % |
| 2 | 5 | 1 min | 1 min | 7 % |
| 3 | 5 | 1 min | 1 min | 8 % |
| 4 | 5 | 1 min | 1 min | 10 % |
| 5 | 14 | 20 s | 10 s | 13 % |
| 6 | 14 | 20 s | 10 s | 14 % |
| 7 | 14 | 20 s | 10 s | 15 % |
| 8 | 14 | 20 s | 10 s | 16 % |
Histological analysis
Liver tissue was harvested, cut into small pieces, fixed in 4 % paraformaldehyde, and embedded in the pre-cooled optimal cutting compound (OCT) for cryostat sectioning. The OCT-embedded samples were serially sectioned at 6 μm and stained with Oil Red-O to visualize the accumulation of hepatic lipid droplets. The samples were observed and imaged under a microscope (IX3-AN, Olympus, Japan). To estimate the adipogenesis of the liver, after removing the staining solution, Oil Red-O was extracted by isopropanol and its optical density was monitored spectrophotometrically at 492 nm via a microplate reader for quantitative analysis with Image Pro Plus software.
Blood biochemical analyses and liver lipid’s extract for triglyceride (TG)
After 8 weeks, all rats were deprived of food for 12 h, serum was collected from retro-orbital blood samples after centrifugation at 1500×g for 20 min, and kept at −20 °C to be processed for biochemical analysis. Serum total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) and glucose were measured via a microplate assay (Tecan 2000, Japan) from Nanjing Jiancheng Bioengineering Institute (China). The Liver TG content was determined by an enzyme-reaction kit (Nanjing Jiancheng Bioengineering Institute, China), and the TG levels were normalized to the protein concentration of each sample.
Enzyme-linked immunosorbent assays (ELISAs)
The activities of hepatic enzymes, including carnitine palmitoyl transferase 1α (CPT-1α), β-hydroxyacyl-CoA dehydrogenase (β-HAD), acetyl-CoA carboxylase (ACC), and citrate synthase (CS), were measured using ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Ltd.). All of the measurements were performed according to the manufacturers’ instructions.
Flow cytometric analysis
Freshly isolated mixed liver tissue was minced, and homogenized. The mitochondria were fractionated by performing differential centrifugation, as described previously [18]. To analyze the changes in mitochondrial membrane potential, 50 μg purified mitochondria were loaded with 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine (JC-1) fluorescent probe (Beyotime, C2003S, China), and the fluorescence intensity was detected by microplate reader (TECAN, Austria) (emission spectra at 490 and 525 nm and excitation spectra at 530 and 590 nm). The data were collected and analyzed using i-control 1.10 (for infinite reader) software. The positive control treated with carbonyl cyanide m-chlorophenylhydrazone (CCCP) at 20 μM was used as the reference gate to detect mitochondrial membrane potential.
Western blotting
Protein contents were extracted either from nuclear or whole muscle lysates from liver tissue. Whole muscle protein was isolated by lysing in RIPA Lysis buffer (Beyotime) supplemented with phosphatase and protease inhibitor cocktails (Beyotime), and the supernatant was collected. The concentrations of nuclear and whole-muscle proteins in the acquired supernatants were determined via a BCA assay kit (Thermo Fisher Scientific). Proteins were separated by SDS-PAGE using a 7.5 % (PGC-1α and Histone, H3) polyacrylamide gel, and proteins were subsequently transferred onto a PVDF membrane. PGC-1α (1:1000, Proteintech), H3(1:10000, Ruiying Biological), p-AMPK(1:1000, Bioworld), PPARα(1:500, SANTA), CPT1α(1:4000, Proteintech), ChREBP(1:1000, Cell Signaling), SREBP(1:800, SANTA) were used to detect proteins. Blots were quantified using a GelDoc Go Gel Imaging System and Image Lab software (BIO-RAD, USA).
Statistical analyses
Values are given as the mean ± standard deviation (SD) for all variables. Data are expressed as the means ± SD and were analyzed using GraphPad Prism software, version 6.0 (Graph-Pad Software Inc., San Diego, CA). One-way analysis of variance (ANOVA) was used as appropriate. Differences were considered statistically significant at p<0.05.
Results
Dietary or HIIT reduced body weight, liver weight, liver fat content, and adipose tissue weight in obese rats.
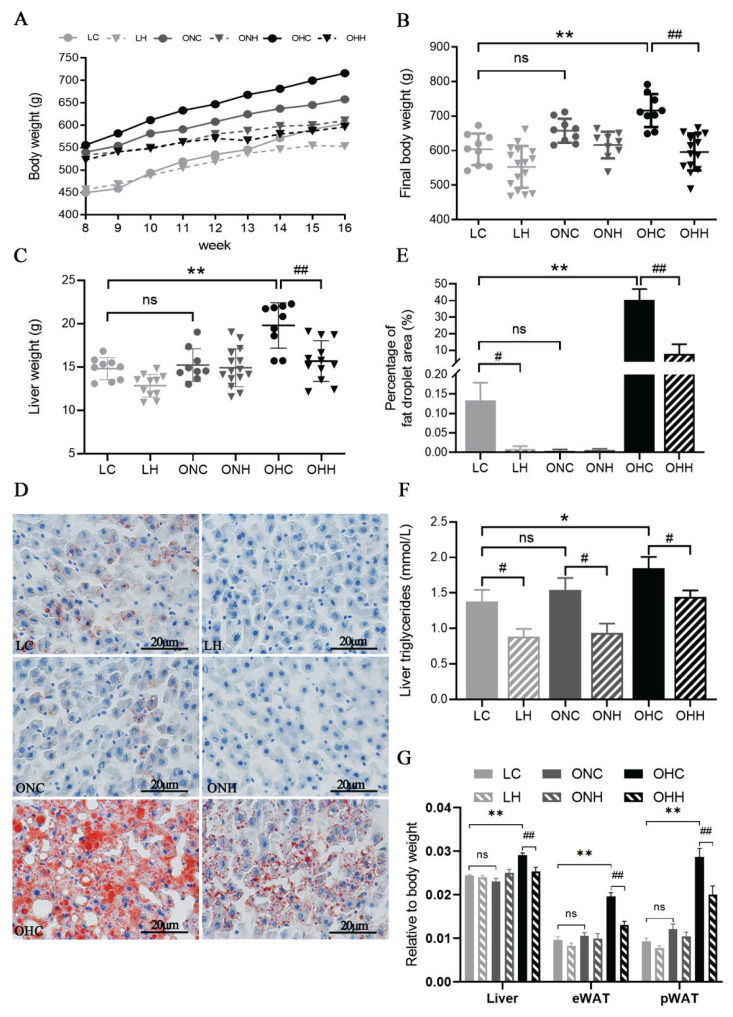
The body weight of animals were monitored weekly, and their liver tissue were collected after sacrificing. Body weight and liver weight in obese HFD rats were significantly increased compared to lean rats (P<0.05) (Fig. 1A, 1B). However, the 8-week HIIT training significantly reduced the body weight and liver weight of obese rats (P<0.05) to the same level as the lean rats in the LC group. Additionally, the trend of increased body weight and liver weight in the ONC group was effectively inhibited by treating with 8-week normal diets in obese rats. HIIT decreased the body weight and liver weight in both lean and obese rats, although the magnitude of the declines was smaller in the lean and obese ND rats compared to obese HFD rats. Oil red O staining and TG content reflected the lipid deposition status in the liver. Representative images of oil red O-stained liver tissues showed that the amount of hepatic lipid droplets and triglycerides was much greater in the obese HFD diet rats than that of lean rats or obese rats swished to normal diet (Fig. 2D,E) (P<0.05).
Fig. 2.
Effects of HIIT and dietary changes on rat body, liver weight and insulin level. (A) Body weights from week 1 to week 8, (B) body weight at the final week, (C) liver weight, (D) Oil Red-O staining of liver tissue, (E) Percentage of fat droplet area (%), (F) liver TG levels and (G) liver and different adipose tissues relative to body weight: liver weight/body weight, epididymal adipose tissue (eWAT) weight/body weight, and perigonadal adipose tissue (pWAT) weight/body weight. Values are presented as the mean ± SD (n=8–10 per group). *P<0.05, **P<0.01; #P<0.05, ##P<0.01.
In addition, 8-week HIIT intervention clearly reduced the accumulation of lipid droplets and TG content in hepatocytes both in lean and obese rats (P<0.05) (Fig. 2F). We still observed that the effect of HIIT on liver lipid deposition was even more obvious when combined with dietary changes. In addition, liver index, eWAT and pWAT weight were significantly increased in obese HFD rats (P<0.05), but was unchanged in ONC group compared to the LC group (Fig. 2G). The results revealed that the effect of HIIT intervention on reducing liver weight and adipose tissue weight were only found in obese HFD rats.
Dietary or HIIT intervention improved abnormal lipid metabolism induced by HFD diet.
To test lipid metabolism in different groups, the serum was taken at the time of execution. Compared to the rats in LC group, HFD treatment markedly increased the serum levels of total HDL-C, TG and TC in OHC rats (P<0.05), while decreased levels of total HDL-C, TG and TC were found after 8-week HIIT intervention (Table 2). HIIT also significantly reduced the level of serum TG in lean rats (P<0.05), but showed no significant changes in ONC rats. In addition, insulin level and insulin resistance index were significantly increased in obese HFD rats (P<0.05), but was unchanged in ONC group compared to the LC group (Table 2). These results suggest that changing diet and HIIT can effectively prevent dyslipidemia induced by HFD.
Table 2.
Changes in the serum lipids of rats in each group
| LC | LH | ONC | ONH | OHC | OHH | |
|---|---|---|---|---|---|---|
| Glucose (mmol/L) | 8.10±0.56 | 9.11±2.19 | 8.08±1.66 | 10.28±2.42 | 10.90±1.72 | 9.20±1.66 |
| TG (mmol/L) | 0.34±0.04 | 0.25±0.02* | 0.39±0.07 | 0.36±0.04 | 0.50±0.06* | 0.40±0.06# |
| TC (mmol/L) | 1.11±0.15 | 1.06±0.09 | 1.26±0.11 | 1.30±0.16 | 1.64±0.15* | 1.29±0.12# |
| HDL-C (mmol/L) | 0.21±0.02 | 0.27±0.02 | 0.25±0.03 | 0.28±0.03 | 0.35±0.04* | 0.29±0.05 |
| Insulin (pmol/ml) | 194.76±51.66 | 200.27±90.55 | 172.98±72.34ns | 131.02±46.46 | 369.32±137.28** | 253.83±107.50 |
| Insulin resistance index | 71.31±18.35 | 58.45±34.86 | 63.35±25.77ns | 57.71±15.91 | 178.06±70.00** | 100.33±30.63## |
Notes: Values are presented as the mean ± SD (n=8–10 per group).
P<0.05 vs. LC group,
P<0.05 vs OHC group.
Insulin resistance index, fasting insulin * fasting blood glucose/22.5
HIIT upregulated mitochondrial function and lipid metabolic enzymatic activity in obese rats.
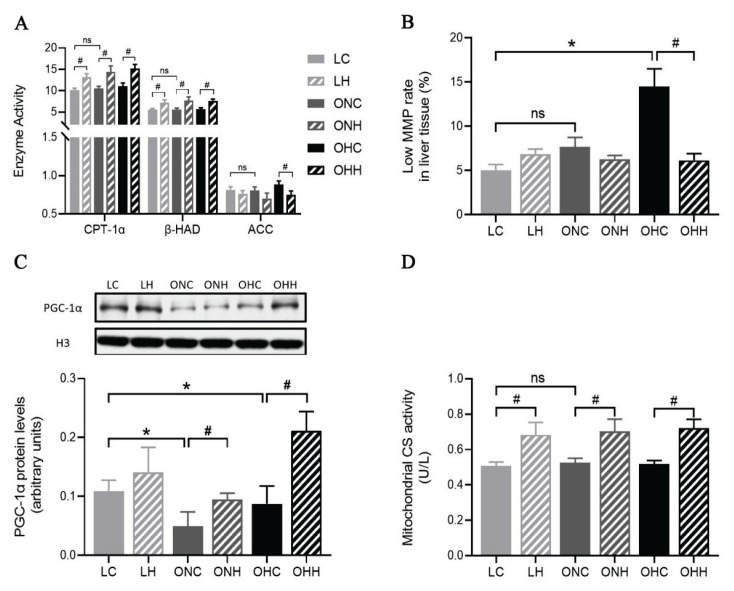
ELISA were used to measure the activities of mitochondrial lipid oxidative metabolic enzymes. The results showed that CPT-1α and β-HAD were markedly increased in both lean and obese groups following HIIT (P<0.05), but no significant change was observed under diet charge. We also detected the activity of ACC, compared to the OHC group, a significantly lower level of ACC activity was found in the OHH group (P<0.05) (Fig. 3A). Liver mitochondrial function and oxidative stress were evaluated to investigate the protective influence of exercise or diet on HFD induced liver tissue damage. As shown in Figures 3B, mitochondria isolated from the OHC group showed a higher proportion of lower mitochondrial membrane potential (MMP) compared to the LC group. Nevertheless, the 8-week HIIT training significantly inhibited lower MMP (P<0.05). Furthermore, to determine whether mitochondrial biogenesis and lipid metabolic enzymatic activities were influenced by diet or exercise intervention, we evaluated the lever of nuclear peroxisome proliferator-activated receptor gamma coactivator-1-α (PGC-1α) expression and citrate synthase (CS) enzyme activity. The results showed that the observed decreased level of nuclear PGC-1α expression in obese rats were significantly elevated by 8 weeks HIIT intervention (P<0.05). In addition, HIIT markedly increased the CS enzyme activity in both lean and obese rats (P<0.05). (Fig. 3C,D).
Fig. 3.
Effects of HIIT and dietary changes on hepatic mitochondrial function. (A) Alterations in mitochondrial CPT-1α, β-HAD, and ACC activities. (B) Low MMP rate in liver tissue. (C) Changes in nuclear PGC-1α (91 kDa) protein content and (D) mitochondrial maximal citrate synthase activity. Values are presented as the mean ± SD (n=8–10 per group). *P<0.05, **P<0.01; #P<0.05, ##P<0.01. Low MMP rate, the proportion of the mitochondrial number at the low membrane potential.
HIIT regulated hepatic mitochondrial lipid oxidation and de novo lipogenesis genes expression in lean and obese rats.
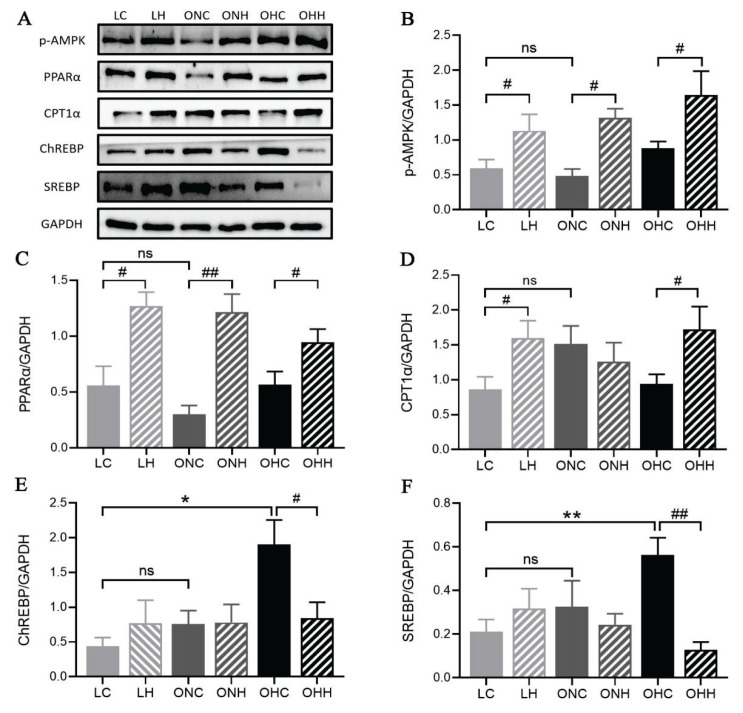
AMPK/PPARα/CPT1α is a hallmark of hepatic fat oxidation. Although the phosphorylation level of AMPK and the expression of PPARα and CPT1α did not change significantly with the dietary intervention among LC, ONC and OHC groups, HIIT markedly increased the AMPK phosphorylation level and PPARα expression in both lean and obese rats (P<0.05), regardless of whether they were feed by normal diet or HFD (Fig. 4A–D). Moreover, the CPT1α expression levels had significantly increased in lean and obese HFD rats after 8 weeks HIIT intervention (P<0.05). However, the CPT1α protein expression levels in ONH rats were unchanged compared with ONC rats (Fig. 4D). Next, we explored the gene expression levels of regulating hepatic DNL. The expression levels of regulatory element-binding protein (ChREBP) and sterol regulatory element-binding protein (SREBP) in the OHC rats were much greater than that of LC rats, whereas 8-week HIIT intervention but not diet change significantly blunted the increase induced by the HFD (P<0.05) (Fig. 4E,F). Altogether, our data support that HIIT improved mitochondrial lipid oxidation by modulating the AMPK/PPARα/CPT1α pathway and suppressed the gene expression necessary for de-novo lipogenesis to overcome the detrimental effects of obesity and ameliorates hepatic lipid accumulation.
Fig. 4.
HIIT regulated hepatic protein expressions. (A) Blots for p-AMPK, PPARα, CPT1α, ChREBP, SREBP. (B–F) Protein expressions of p-AMPK, PPARα, CPT1α, ChREBP, SREBP. Values are presented as the mean ± SD (n=8–10 per group). *P<0.05, **P<0.01; #P<0.05, ##P<0.01.
Discussion
The liver is a key metabolic organ that regulates lipid and glucose metabolism and perturbations in intrahepatic processes have potential to impact widely on metabolic disease risk. In health, free fatty acids (FFA) originate from lipolysis of triglycerides (TG) in adipose tissue leading to circulating non-esterified fatty acids, (NEFA, ~60 %), from dietary fatty acids (~15 %), and from dietary sugars undergoing de novo lipogenesis (DNL, ~25 %) mediated by several proteins. Hepatic lipid accumulation occurs as a result of increased delivery of fatty acids from adipose tissue, which is accompanied by an imbalance between lipid degradation and de novo lipid synthesis in the liver. Nonalcoholic fatty liver disease (NAFLD) has emerged as the most common chronic liver disease worldwide. As there are no proven pharmacological therapies for the effective treatment of NAFLD, lifestyle modification (exercise and diet) is currently the cornerstone for economically effective interventions [19–21]. Therefore, the aim of the current investigation was to evaluate the effect of HIIT exercise training and rational diet intervention on hepatic lipid accumulation and its underlying mechanisms.
In this work, we employed HFD to induce overweight/obesity in rats and applied HIIT or diet change for 8-week intervention. Our results found that the body weight, liver weight and insulin resistance of obese rats were not significantly different from the lean rats after switching to normal diet, but were further increased after 8-week HFD diet. Furthermore, 8 weeks of HIIT reversed the HFD-induced body weight gain, liver weight gain and insulin resistance in obese HFD rats, however, the intervention of exercise training had no significant effect on the lean rats and obese ND rats. Our finding are reminiscent of a previous report in a clinical research showing that dietary and exercise interventions are effective in body weight reduction [19–21]. In terms of the overall lipoprotein profile, compared to lean rats, obese-HFD rats exhibited typical hepatic TG abnormalities and dyslipidemia, consisting of increased TG, and TC. Previous researches have reported that obesity and NAFLD could affect lipid metabolism and contribute to hypercholesterolemia and hypertrigly-ceridemia [23,24], which occurs in part due to increased FFA fluxes to the liver. Liver FFAs arising from plasma FFAs either enter mitochondria to undergo β-oxidation or are esterified and stored as TGs. Several studies have suggested that exercise [25] can partially lower serum TG and LDL-C levels, decreased hepatic TG [26] and increase HDL-C levels [27]. Biochemical analyses revealed that the higher level of LDL-C, TG and TC, hepatic TG could be evidently reduced by 8-week HIIT intervention in obese HFD rats. However, the effect of HIIT on the HDL level did not meet the criteria of effectiveness, possibly due to the differences in the duration and intensity of the exercise protocol [27] or the complex composition, shape, and size of plasma HDL [28]. Moreover, the level of serum lipids in obese ND rats were similar to those of lean rats, which suggested that the diet seems to be an important intervention in the control of blood lipid metabolism. These results are in accordance with previous studies, which showed that the HFD caused dyslipidemia, but low intakes of fats decrease the risk of dyslipidemia [29]. Therefore, our data confirm the beneficial effects of rational diet and HIIT on serum lipoprotein profile.
Although aerobic exercise is believed to be important in the management of metabolic dysfunction associated fatty liver disease [13], several studies have demonstrated that HIIT is more favorable at preventing liver lipid accumulation. This occurs by restoring the mRNA levels of genes involved in hepatic lipogenesis and β-oxidation (PPARα, CPT1α, β-HAD) or by easing liver inflammation, and fibrosis, without any changes in body weight [30,31]. ACC catalyzes the rate-limiting step of DNL and regulates fatty acid β-oxidation in hepatocytes. In the present study, we observed an increase in CPT-1 and β-HAD enzyme activities with HIIT training in both lean and obese rats, regardless of dietary change. We also demonstrated that although the ACC activity showed no alteration between lean and obese rats, 8 weeks of HIIT intervention reduced the ACC activity in obese HFD rats, with no significant change in other groups. These results suggest that, compared to dietary change, HIIT exhibited a stronger effect on mitochondrial β-oxidation by regulating the activities of key enzymes in lipid metabolism.
It has been reported that HIIT contribute a more favorable regulation of metabolic dysfunctions in diet-induced obesity mice compared with moderate-intensity continuous training (MICT) [31–33]. To understand the mechanism of HIIT and dietary intervention in hepatic mitochondrial adaptation, mitochondrial biogenesis and function were analyzed. The MMP is a critical parameter for understanding mitochondrial function. Here, we demonstrated that HIIT intervention and dietary change showed significantly protective influence against HFD induced impaired MMP in obese rats. This result is in accordance with other findings in the literature showing that hepatocyte mitochondria from obese animals are associated with an increased production of hydrogen peroxide and ROS [34–36]. Additionally, our data indicate that dietary change positively influences mitochondrial function in obese rats, by increasing the expression levels of mitochondrial lipid metabolism genes, associated with decreases in body weight and lipid deposition. Furthermore, HIIT elicits stronger beneficial effects on the nuclear content of PGC-1α in obese rats. This is in agreement with data presented in Layne et al., in which increased physical activity implemented using a wheel running rodent model enhanced PGC-1α expression in the liver [37]. In line with these findings, the improvement in hepatic mitochondrial function after the HIIT protocol, is a novel finding, which resulted in easing of liver fat deposition in HFD induced obese rats.
Besides mitochondrial fat oxidation, de novo fatty acid synthesis in liver is another important factor affecting lipid content in liver [38]. AMPK has been implicated as a key regulator of physiological energy dynamics. Activation of hepatic AMPK leads to increased fatty acid oxidation and simultaneously inhibition of hepatic lipogenesis [39]. Here we found an increase in the activation of AMPK and PPAR-α, an AMPK downstream target [40,41] in the exercised groups. Moreover, although no effects of dietary intervention in phosphorylated AMPK and PPAR-α expression level were observed, we found increased levels of its target genes CPT1α in exercised groups. Thereby, we hypothesized this outcome is related with the activation AMPK-PPAR-α signaling and PPAR-γ upregulation in liver. Considering HIIT exercise mode relies more on glycometabolism, we assume that the significant downregulation of SREBP1c and ChREBP in obese HFD exercised rats could due to the large amount of carbohydrate consumed which inhibition of glucose-induced lipogenesis. Moreover, HIIT reduces oxidative stress and hepatic gluconeogenesis [2], which is also a possible mechanism for the effect of HIIT on NAFLD. Therefore, HIIT intervention can promote a fine-tuning regulation of fatty-acid β-oxidation and de-novo lipogenesis in hepatocytes.
In summary, the data from this experimental study demonstrated that in chronic HFD induced obesity, HIIT and dietary intervention had preventive and therapeutic effects on fat accumulation in the liver, this inhibited disease progression in obese rats through mitochondrial adaptations, related to enhance mitochondrial fatty acid oxidation and function.
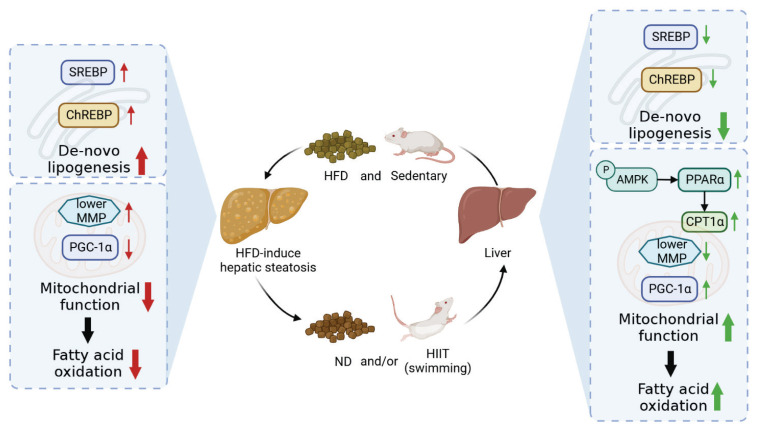
Ameliorating mechanisms of HIIT on HFD-induced hepatic steatosis are the upregulation of AMPK/PPARα/CPT1α pathway and diminishment of hepatic DNL related genes expression (Fig. 5). Additionally, in terms of non-obese rats, HIIT intervention alone also significantly decreased liver fat content mainly through upregulated gene expression level that involved in fat catabolism.
Fig. 5.
Effect of dietary and HIIT on HFD-induced hepatic steatosis. HFD increases DNL and impairs mitochondrial function, which results in hepatic steatosis. Dietary change and HIIT intervention significantly relieve hepatic lipid deposition. HIIT inhibits hepatic de novo lipogenesis and activate AMPK/PPARα/CPT1α pathway, thereby promoting fatty-acid oxidation. Red arrows indicate the effect of HFD. Green arrows indicate the effect of HIIT.
There may be some possible limitations in this study. Although currently data indicated that HIIT or diet changes alleviates lipid accumulation in hepatocytes accompanied by improvement in hepatic mitochondrial function, especially the upregulation of AMPK/PPARα/CPT1α pathway and increased activities of key enzymes in fatty-acid β-oxidation. It is unclear whether this result is related to liver glycogen content and gluconeogenesis levels, given the significant effect of HIIT exercise on liver glucose metabolism. In addition, currently, we have not been able to isolate mitochondria to measure mitochondrial fatty acid oxidation ability, future studies will try to use more accurate technique.
Acknowledgements
This work was supported by the Youth Project of National Natural Science Foundation of China (32000839), The National Key R&D Program of China (No. 2020YFC2007002), Graduate Research and Innovation Projects of Jiangsu Province (KYCX22_2247 and KYCX22_2256), Qing Lan Project of Jiangsu Province of China ([2021]11), The Innovation and Entrepreneurship Training Program for Undergraduates of Jiangsu Province of China (202210330009Z).
The authors would like to thank Jiao Lu for his assistance in morphological analysis and thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Abbreviations
- ACC
acetyl-CoA carboxylase
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- CPT-1α
carnitine palmitoyl transferase 1α
- CS
citrate synthase
- EGTA
ethylene glycol tetraacetic acid
- ELISAs
enzyme-linked immunosorbent assays
- FFA
free fatty acid
- FITC
fluoresceine isothiocyanate
- β-HAD
β-hydroxyacyl-CoA dehydrogenase
- HDL-C
high-density lipoprotein cholesterol
- HFD
high fat diet
- HIIT
high-intensity interval training
- IL-β
interleukin-1β
- JC-1
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine
- LDL-C
low-density lipoprotein cholesterol
- MCP-1
macrophage chemoattractant protein-1
- MIT
moderate-intensity continuous training
- MMP
mitochondrial membrane potential
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- ND
normal chow diet
- OCT
optimal cutting compound
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator-1-α
- PUFA
n-3 polyunsaturated fatty acids
- PVDF
polyvinylidene difluoride
- ROS
reactive oxygen species
- TC
total cholesterol
- TG
triglyceride
- TNF-α
tumor necrosis factor-α
- VLDL
very low density lipoprotein
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi H, Kotani K, Tanaka K, Egucih Y, Anzai K. Therapeutic approaches to nonalcoholic fatty liver disease: exercise intervention and related mechanisms. Front Endocrinol (Lausanne) 2018;9:588. doi: 10.3389/fendo.2018.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Strijcker D, Lapauw B, Ouwens DM, Van de Velde D, Hansen D, Petrovic M, Cuvelier C, et al. High intensity interval training is associated with greater impact on physical fitness, insulin sensitivity and muscle mitochondrial content in males with overweight/obesity, as opposed to continuous endurance training: a randomized controlled trial. J Musculoskelet Neuronal Interact. 2018;18(2):215–226. [PMC free article] [PubMed] [Google Scholar]
- 4.Marcinko K, Sikkema SR, Samaan MC, Kemp BE, Fullerton MD, Steinberg GR. High intensity interval training improves liver and adipose tissue insulin sensitivity. Mol Metab. 2015;4(12):903–915. doi: 10.1016/j.molmet.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araújo EP, De Souza CT, Ueno M, Cintra DE, Bertolo MB, Carvalheira JB, Saad MJ, et al. Infliximab restores glucose homeostasis in an animal model of diet-induced obesity and diabetes. Endocrinology. 2007;148(12):5991–5997. doi: 10.1210/en.2007-0132. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes-Lima F, Monte TL, Nascimento FA, Gregório BM. Short exposure to a high-sucrose diet and the first ‘hit’ of nonalcoholic fatty liver disease in mice. Cells Tissues Organs. 2015;201(6):464–472. doi: 10.1159/000446514. [DOI] [PubMed] [Google Scholar]
- 7.Riazi K, Raman M, Taylor L, Swain MG, Shaheen AA. Dietary Patterns and Components in Nonalcoholic Fatty Liver Disease (NAFLD): What key messages can health care providers offer? Nutrients. 2019;11(12):2878. doi: 10.3390/nu11122878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simões ICM, Fontes A, Pinton P, Zischka H, Wieckowski MR. Mitochondria in non-alcoholic fatty liver disease. Int J Biochem Cell Biol. 2018;95:93–99. doi: 10.1016/j.biocel.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Hallsworth K, Thoma C, Hollingsworth KG, Cassidy S, Anstee QM, Day CP, Trenell MI. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci (Lond) 2015;129(12):1097–1105. doi: 10.1042/CS20150308. [DOI] [PubMed] [Google Scholar]
- 10.Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, Isobe T, et al. High-intensity aerobic exercise improves both hepatic fat content and stiffness in sedentary obese men with nonalcoholic fatty liver disease. Sci Rep. 2017;7:43029. doi: 10.1038/srep43029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winn NC, Liu Y, Rector RS, Parks EJ, Ibdah JA, Kanaley JA. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity - A randomized trial. Metabolism. 2018;78:128–140. doi: 10.1016/j.metabol.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Soliman GS. A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine (Baltimore) 2019;98(12):e14918. doi: 10.1097/MD.0000000000014918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winn NC, Liu Y, Rector RS, Parks EJ, Ibdah JA, Kanaley JA. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity - A randomized trial. Metabolism. 2018;78:128–140. doi: 10.1016/j.metabol.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Hamasaki H. Perspectives on interval exercise interventions for non-alcoholic fatty liver disease. Medicines (Basel) 2019;6(3):83. doi: 10.3390/medicines6030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terada S, Tabata I, Higuchi M. Effect of high-intensity intermittent swimming training on fatty acid oxidation enzyme activity in rat skeletal muscle. Jpn J Physiol. 2004;54(1):47–52. doi: 10.2170/jjphysiol.54.47. [DOI] [PubMed] [Google Scholar]
- 16.Carnevali LC, Jr, Eder R, Lira FS, Lima WP, Gonçalves DC, Zanchi NE, Nicastro H, et al. Effects of high-intensity intermittent training on carnitine palmitoyl transferase activity in the gastrocnemius muscle of rats. Braz J Med Biol Res. 2012;45(8):777–783. doi: 10.1590/S0100-879X2012007500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gobatto CA, de Mello MA, Sibuya CY, de Azevedo JR, dos Santos LA, Kokubun E. Maximal lactate steady state in rats submitted to swimming exercise. Comp Biochem Physiol A Mol Integr Physiol. 2001;130(1):21–27. doi: 10.1016/S1095-6433(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 18.Hynes J, Swiss RL, Will Y. High-Throughput Analysis of Mitochondrial Oxygen Consumption. Methods Mol Biol. 2018;1782:71–87. doi: 10.1007/978-1-4939-7831-1_4. [DOI] [PubMed] [Google Scholar]
- 19.Saeed N, Nadeau B, Shannon C, Tincopa M. Evaluation of dietary approaches for the treatment of non-alcoholic fatty liver disease: a systematic review. Nutrients. 2019;11(12):3064. doi: 10.3390/nu11123064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saigo Y, Sasase T, Tohma M, Uno K, Shinozaki Y, Maekawa T, Sano R, et al. High-cholesterol diet in combination with hydroxypropyl-beta-cyclodextrin induces NASH-like disorders in the liver of rats. Physiol Res. 2023;72(3):371–382. doi: 10.33549/physiolres.934981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golabi P, Gerber L, Paik JM, Deshpande R, de Avila L, Younossi ZM. Contribution of sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Rep. 2020;2(6):100171. doi: 10.1016/j.jhepr.2020.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naimimohasses S, O’Gorman P, Wright C, Ni Fhloinn D, Holden D, Conlon N, Monaghan A, et al. Differential effects of dietary versus exercise intervention on intrahepatic MAIT cells and histological features of NAFLD. Nutrients. 2022;14(11):2198. doi: 10.3390/nu14112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toriniwa Y, Muramatsu M, Ishii Y, Riya E, Miyajima K, Ohshida S, Kitatani K, et al. Pathophysiological characteristics of non-alcoholic steatohepatitis-like changes in cholesterol-loaded type 2 diabetic rats. Physiol Res. 2018;67(4):601–612. doi: 10.33549/physiolres.933784. [DOI] [PubMed] [Google Scholar]
- 24.Vekic J, Zeljkovic A, Stefanovic A, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V. Obesity and dyslipidemia. Metabolism. 2019;92:71–81. doi: 10.1016/j.metabol.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Fikenzer K, Fikenzer S, Laufs U, Werner C. Effects of endurance training on serum lipids. Vascul Pharmacol. 2018;101:9–20. doi: 10.1016/j.vph.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Zhang W, Zeng LQ, Bai H, Li J, Zhou J, Zhou GY, et al. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 2020;36:101635. doi: 10.1016/j.redox.2020.101635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muscella A, Stefàno E, Marsigliante S. The effects of exercise training on lipid metabolism and coronary heart disease. Am J Physiol Heart Circ Physiol. 2020;319(1):H76–H88. doi: 10.1152/ajpheart.00708.2019. [DOI] [PubMed] [Google Scholar]
- 28.Zannis VI, Chroni A, Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med (Berl) 2006;84(4):276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 29.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 30.Fredrickson G, Barrow F, Dietsche K, Parthiban P, Khan S, Robert S, Demirchian M, et al. Exercise of high intensity ameliorates hepatic inflammation and the progression of NASH. Mol Metab. 2021;53:101270. doi: 10.1016/j.molmet.2021.101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang N, Liu Y, Ma Y, Wen D. High-intensity interval versus moderate-intensity continuous training: Superior metabolic benefits in diet-induced obesity mice. Life Sci. 2017;191:122–131. doi: 10.1016/j.lfs.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Banitalebi E, Faramarzi M, Nasiri S, Mardaniyan M, Rabiee V. Effects of different exercise modalities on novel hepatic steatosis indices in overweight women with type 2 diabetes. Clin Mol Hepatol. 2019;25(3):294–304. doi: 10.3350/cmh.2018.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan Z, Xiao-Wei L, Juan W, Xiu-Juan L, Nian-Yun Z, Lei S. HIIT and MICT attenuate high-fat diet-induced hepatic lipid accumulation and ER stress via the PERK-ATF4-CHOP signaling pathway. J Physiol Biochem. 2022;78(3):641–652. doi: 10.1007/s13105-022-00884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satapati S, Kucejova B, Duarte JA, Fletcher JA, Reynolds L, Sunny NE, He T, et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest. 2015;125(12):4447–4462. doi: 10.1172/JCI82204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aharoni-Simon M, Hann-Obercyger M, Pen S, Madar Z, Tirosh O. Fatty liver is associated with impaired activity of PPARγ-coactivator 1α (PGC1α) and mitochondrial biogenesis in mice. Lab Invest. 2011;91(7):1018–1028. doi: 10.1038/labinvest.2011.55. [DOI] [PubMed] [Google Scholar]
- 36.Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21(5):739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, Booth FW, et al. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol. 1985 doi: 10.1152/japplphysiol.91186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foulds CE, Treviño LS, York B, Walker CL. Endocrine-disrupting chemicals and fatty liver disease. Nat Rev Endocrinol. 2017;13(8):445–457. doi: 10.1038/nrendo.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia D, Hellberg K, Chaix A, Wallace M, Herzig S, Badur MG, Lin T, et al. Genetic liver-specific AMPK activation protects against diet-induced obesity and NAFLD. Cell Rep. 2019;26(1):192–208.e6. doi: 10.1016/j.celrep.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diniz TA, de Lima EA, Junior, Teixeira AA, Biondo LA, da Rocha LAF, Valadão IC, Silveira LS, et al. Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 2021;266:118868. doi: 10.1016/j.lfs.2020.118868. [DOI] [PubMed] [Google Scholar]
- 41.Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]