Summary
Sodium is the main osmotically active ion in the extracellular fluid and its concentration goes hand in hand with fluid volume. Under physiological conditions, homeostasis of sodium and thus amount of fluid is regulated by neural and humoral interconnection of body tissues and organs. Both heart and kidneys are crucial in maintaining volume status. Proper kidney function is necessary to excrete regulated amount of water and solutes and adequate heart function is inevitable to sustain renal perfusion pressure, oxygen supply etc. As these organs are bidirectionally interconnected, injury of one leads to dysfunction of another. This condition is known as cardiorenal syndrome. It is divided into five subtypes regarding timeframe and pathophysiology of the onset. Hemodynamic effects include congestion, decreased cardiac output, but also production of natriuretic peptides. Renal congestion and hypoperfusion leads to kidney injury and maladaptive activation of renin-angiotensin-aldosterone system and sympathetic nervous system. In cardiorenal syndromes sodium and water excretion is impaired leading to volume overload and far-reaching negative consequences, including higher morbidity and mortality of these patients.
Keywords: Cardiorenal syndrome, Renocardiac syndrome, Volume overload, Sodium retention
Introduction
The function of the heart and kidneys is tightly, bidirectionally connected and both organs are crucial in maintaining water and electrolyte homeostasis. Because of this, damage to one organ often leads to injury of the other. This is mediated by different neurohormonal pathways, triggering a vicious cycle. This situation is called cardiorenal syndrome (CRS), where impaired cardiac function has negative effect on kidney functions and vice versa. CRS classification helps the medical professionals to understand the etiology and to direct appropriate therapy [1]. Moreover, patients with CRS also have worse prognosis than patients with single organ lesion. It has been classified into five subtypes (CRS 1–5, see Table 1) regarding pathophysiology and timeframe [2]. Heart and kidney dysfunction results in fluid retention, electrolyte disbalance etc.
Table 1.
Classification of cardiorenal syndromes (CRS) [1]
| Cardiorenal subtype | Description | Examples/Etiology |
|---|---|---|
| CRS Type 1 (acute CRS) | Rapid worsening of cardiac function leading to acute kidney injury | Acute myocardial infarction with cardiogenic shock, acute valvular insufficiency, acute decompensated heart failure |
| CRS Type 2 (chronic CRS) | Chronic abnormalities in cardiac function leading to chronic kidney disease | Chronic inflammation, long-term renin-angiotensin-aldosterone system and sympathetic nervous system activation, chronic hypoperfusion |
| CRS Type 3 (acute renocardiac syndrome) | Acute worsening of renal function leading to worsening cardiac function | Uremia causing impaired contractility, hyperkalemia causing arrythmias, volume overload causing pulmonary oedema |
| CRS Type 4 (chronic renocardiac syndrome) | Chronic worsening of renal function leading to worsening cardiac function | CKD leading to LVH, coronary disease and calcification, diastolic dysfunction, etc. |
| CRS Type 5 | Acute or chronic systemic disease leading to both cardiac and renal dysfunction | Sepsis, diabetes mellitus, amyloidosis, vasculitis |
Volume overload manifests as congestion and oedemas, with many negative effects on different organs [3]. In heart, volume overload induces dilation or hypertrophy of the heart chambers, which worsen heart function and predispose to heart failure, arrythmias etc. [4]. The consequence of gastrointestinal mucosal oedema is decreased absorption not only of nutrients, but also of medication, resulting in deficient effect of pharmacotherapy [5]. Pulmonary congestion and oedema manifest as dyspnea [6]. In systemic circulation, volume overload is characterized by increased central venous pressure that is transmitted into organs (including kidneys) and decrease the perfusion pressure. Specific responses of crucial organs will be described below.
Water and sodium homeostasis
Maintenance of water homeostasis is a complex process ensured by neural and humoral interconnection of body tissues and organs. Extracellular fluid volume is controlled by changes in blood pressure, blood osmolality and by variation of blood viscosity and velocities [7].
Kidneys
Adequate renal and heart functions are crucial for preservation of adequate volume status. Apart from water loss by breathing or sweating, the only possibility to excrete regulated volume of fluids is through kidneys. As about 150–200 l of primary urine is created daily, effective regulatory mechanisms are necessary [8]. Tubular system of the nephron passes through highly concentrated medullary interstitium, creating osmotic gradient which allows water reabsorption [9]. As the osmotic gradient wouldn’t be sufficient without adequate blood flow and filtration pressure, proper heart function is necessary. Under physiological conditions about 25 % of the resting cardiac output flows into the kidneys [10]. To prevent washout of the medullar concentration gradient, only 2 % of blood supply flow through deep medullar glomeruli and a mechanism called countercurrent exchange exists [11].
To maintain water homeostasis ability to create urine either more concentrated or more diluted than plasma is necessary. This is possible thanks to different permeability of nephron segments and specialized channels, especially by aquaporin channels (AQP) [10]. Seven types of AQP are present in specific regions of nephron’s tubular system, enabling transcellular water movement. Majority of these channels is present constitutively, but AQP 2 presence is administrated by vasopressin (mentioned below) and tightly regulate amount of excreted water [12,13]. Intercellular water and solutes passageway is controlled by tight junction proteins. Expression of some of these proteins is dependent directly on medullar tonicity changes [14].
Heart
Both cardiac atria and ventricles include different types of receptors. In atria, particularly in pulmonary venous and cavo-atrial junctions [15], there are two types of vagal afferent nerves (A and B) [16]. Type A receptors sense heart rate and as a response, they modulate systolic function. In higher heart rate they increase contractility and vice versa [17]. Type B receptors are stretch receptors sensing atrial volume [18]. As response to atrial distension heart rate and urine flow increases [19]. In situations when atrial pressure rises slowly, these receptors have ability to adapt [18]. Third type of atrial receptors include unmyelinated afferent fibers also sensitive to stretch. In case of atrial distension both cardiovascular and renal functions are affected. Distension especially of the left atrium causes tachycardia, hypotension and lowers the systemic vascular resistance [20]. In kidneys urine flow increases and discharge of renal sympathetic nerve decreases.
Ventricular reflexes are mediated by vagal and sympathetic afferent fibers. Two types of unmyelinated vagal fibers were described. The first, mechanoreceptors, are stimulated by increased end-diastolic volume and by increased end-diastolic and end-systolic pressure [21], but also have the ability to sense situations as ischemia etc., where myocardial motion is altered [22]. The second, chemoreceptors, react to hypoxia, probably thanks to released bradykinin and prostaglandins [23]. As a result, distension of ventricles, especially the left, leads to reflex vasodepression, peripheral vasodilation and bradycardia. In kidneys reflex renal vasodilation occurs [22]. Sympathetic ventricular afferent fibers are also both unmyelinated and myelinated. Myelinated fibers respond to blood pressure and coronary flow, especially in patients with heart failure [24]. Unmyelinated fibers are rapidly adapting, innervating mechano- and chemoreceptors sensitive to changes in intracardiac volume, wall motion, bradykinin, and prostaglandins [25, 26]. Their stimulation leads to excitatory or pressor reflex [27–29].
In congestive heart failure vagal afferents control plasmatic antidiuretic hormone level, renal nerve activity and plasma renin activity. As left atrial receptors have the ability of adaptation, in chronic state level of antidiuretic hormone remains inappropriately high, contributing to oedemas, ascites and hyponatremia [20].
Heart participates on maintaining fluid balance also by releasing atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) as a response to sensing myocardial stretching. Most important effects of natriuretic peptides include decrease in vascular tone and increase of natriuresis and water excretion via kidneys [30].
Humoral pathway
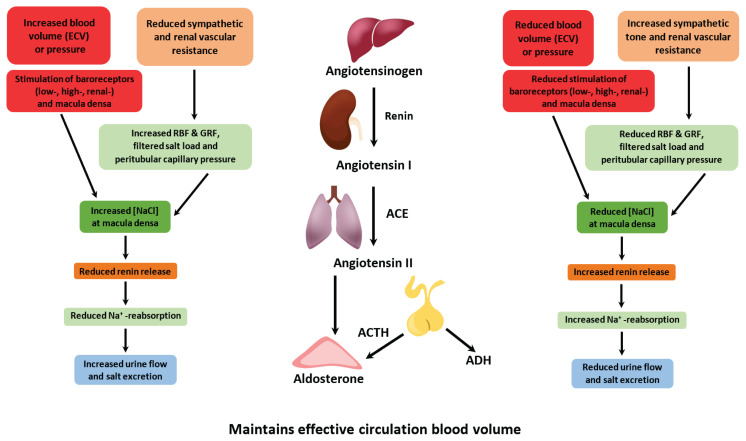
Important mechanism leading to sodium and water retention is triggering of renin-angiotensin-aldosterone system (RAAS), often activated in heart failure [31] (Fig. 1). This system participates in regulation of both intravascular volume and systemic vascular resistance but have also other effects e.g. pro-inflammatory, pro-apoptotic, etc. [32]. Decreased renal blood flow, decreased sodium content or beta-activation directly or indirectly result into renin flush out of specialized cells in afferent arterioles [33]. This triggers cascade of events as renin cleaves angiotensinogen produced in liver into inactive angiotensin I, which is converted into physiologically active angiotensin II. The last process takes place mostly in lungs and is catalyzed by angiotensin converting enzyme [34]. Angiotensin II has several effects including increased sodium reabsorption, vasoconstriction of systemic arterioles, potentiation of feeling of thirst, stimulation of neurohypophysis to vasopressin releasement and stimulation of aldosterone releasement from adrenal cortex leading to further sodium retention [35]. Acting in renal tubules, aldosterone increases reabsorption of both sodium and water by different transporters (e.g. Na+/Cl-symporter) [36]. Studies showed that increased levels of aldosterone have deleterious effect on kidney function and glomerular filtration rate [36,37]. Vasopressin, released from hypothalamus, regulates sodium concentration in serum by balancing renal water excretion and urine concentration. Under the vasopressin effect, AQP 2 is inserted into luminal membrane in kidney, letting water flow from the filtrate into medullary interstitium, creating more concentrated urine [38].
Fig. 1.
Blood volume and pressure control of renal water and sodium excretion. RBF - renal blood flow, GFR - glomerular filtration rate, ACE - angiotensin converting enzyme, ACTH - adrenocorticotropic hormone, ADH - antidiuretic hormone
ANP and BNP are parts of natriuretic peptide family secreted by the heart, acting as cardiac hormones [39]. In healthy organism proANP outbalances in healthy atrial tissue and is proteolytically processed into final bioactive α-ANP and side product β-ANP [40]. On the other side proBNP is processed within ventricular myocytes with active BNP and inactive N-terminal proBNP being final products [41]. Levels of all these proteins are increased in patients with heart failure. Their main effects include decrease in vascular tone and increase of natriuresis and thus also water excretion via kidneys, but antifibrotic and antihypertrophic effects are also present [42]. Both ANP and BNP decrease systemic blood pressure. They acutely act as vascular smooth muscle cells relaxants and in chronic state they also regulate endothelial permeability [30].
In some patients with advanced stages of kidney disease or HF the only possibility of treatment is organ transplantation. The changes in humoral pathways haven’t been clearly described in this group of patients, however few studies exist. In work of Issa et al. plasma renin activity and plasma aldosterone levels were measured five years after kidney transplantation, and their levels stayed in the normal range. In this study it was also proven, that blockage of angiotensin II is effective in the RAAS suppression [43]. Heart transplantation often leads to normalization of heart endocrine function, however levels of BNP remain high even in the good graft function and rather level change than absolute value should be considered [44].
Neural pathway
Apart from neural fibers mediating connection between tissues and organs as mentioned above, several brain areas contain specialized neurons able to sense plasma osmolality and volume change [45]. In situation of decreased plasma volume vasopressin is secreted from hypothalamus and the feeling of thirst arises [46,47].
As mentioned above, neural connections between tissues and organs, namely sympathetic nervous system (SNS) is overreactive in both HF and CKD [48]. Parasympathetic vagal nerve may inhibit SNS and norepinephrine release at presynaptic level. Under physiological conditions at rest parasympathetic tone predominates over SNS [49]. In both HF and kidney disease vagal activation is attenuated [50,51]. It was proven that regular dialysis halts parasympathetic damage [52].
Vagal nerve stimulation was tested on animal models with optimistic findings such as slower left ventricular dilation with improved ejection fraction [53], improvement of hemodynamic measures, decreased risk of arrythmias and death [54]. However, these positive effects were not proved in clinical studies [55]. On the other side, blunting SNS by betablockers showed as advantageous and is a basic part of HF treatment [56]. Trials with moxonidin (central sympatholytic agent) were prematurely terminated due to higher morbidity and mortality [57].
Kidneys play an important feedback role in in SNS activation and modulation of the axis of heart - brain - vasculature. In recent years the effect of renal denervation in patients with poorly controlled hypertension and HF was proven in several studies where ejection fraction and diastolic parameters improved, hypertrophy and maladaptive remodeling regressed [58–60].
Sodium and water shifts in the body
Body is composed by two main compartments, separated by semipermeable membrane, i.e., intracellular, and extracellular, which comprises both intravascular and interstitial compartment. Solute concentration in these compartments differs and creates concentration gradients and osmotic pressure, which is equilibrated by water movement through semipermeable membrane [61]. Sodium and water contents are responsible for cell volume. Specific situation occurs in brain to prevent brain oedema. Tight junctions in brain capillaries create blood barrier which sodium can’t pass. This is important as brain swelling or shrinkage may be a life-threatening condition [62].
As sodium is one of the main osmotically active ions in the extracellular fluid, its concentration goes hand in hand with fluid volume. Human body have precise osmoregulatory mechanisms, which under healthy conditions maintain sodium concentration within physiologic range [63]. Sodium shifts are primarily mediated by Na+/K+ATPase, an active transporter that exchange sodium from the cell for extracellular potassium (primarily intracellular cation) [64].
In study of Titze et al. it was proven that skin serves as “osmotically inactive” sodium storage by increasing glycosaminoglycan production and sulfatation. It was concluded that also monocyte phagocytes system contributes to the control of interstitial sodium and blood pressure homeostasis [65]. This mechanism was studied in patients with heart failure (HF), CKD and dialysis population and the levels of skin sodium in HF population was comparable to dialysis patients, and significantly higher than in CKD patients [66].
Other important mechanisms regulating water and sodium excretion as RAAS or role of hypothalamus are mentioned above.
Rapid sodium changes causing hyponatremia occur in case of excess water intake or other pathologic conditions, when kidneys are not capable adequately dilute urine and to excrete free water. Sodium concentration increases in case of insufficient urine concentration in large amount of sodium ingestion/infusion or if there is inadequate loss of electrolyte-free water [67]. Significantly worse ability of urine dilution or concentration was proven in patients with advanced CKD (e-GFR<60 ml/min) [68].
Cardiorenal syndromes
Cardiorenal syndrome type 1
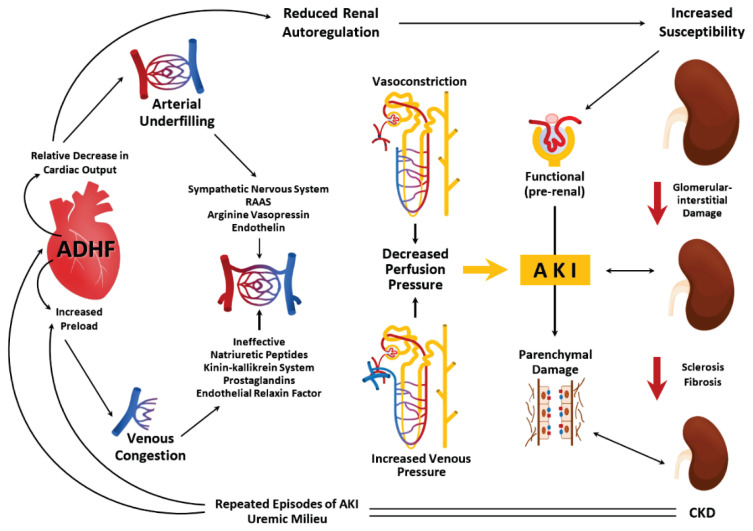
Acute heart failure (AHF), cause of CRS1, is a syndrome comprising several pathophysiologic entities, developing like de novo heart failure or as an acute decompensated chronic heart failure [69]. The most common AHF etiologies include acute coronary syndromes, severe arrhythmias, uncontrolled hypertension, severe valvular disease, pulmonary embolism etc. [70]. Symptoms may also vary depending on the predominant involvement of the left or right heart compartments. Acute failure of the left heart function is represented on one side by decreased cardiac output (CO) with peripheral hypoperfusion, on the other side by congestion, which transmits through the lung circulation, with the development of pulmonary congestion/oedema and pleural effusions, into the right heart. Since the right compartments are normally relatively low-pressure, an acute pressure overload leads to their failure with signs and symptoms of right heart failure [71]. This manifest as systemic congestion, including renal congestion, increased intra-abdominal pressure and in advanced stage also pleural effusions. Forward failure of the right heart results in underfilling of left ventricle with inadequate cardiac output or even in cardiogenic shock [72].
As about 25 % of the cardiac output flows into the kidneys to eliminate a sufficient amount of fluid, decrease in CO and decreased mean arterial pressure lower perfusion pressure [71], leading to kidney hypoperfusion [72]. On the contrary, renal congestion leads to distension of renal venules with their obliteration, further reducing renal perfusion pressure and causing fluid extravasation generating increased renal interstitial pressure. All these conditions cause hypoxic state of renal parenchyma with glomerular and tubular dysfunction and water retention. Symptoms as dyspnea, lower limb swelling, weight gain etc. occur [73]. Acute decrease in renal blood flow activates renin-angiotensin-aldosterone system (RAAS), SNS and reduces nitric oxide in the endothelium with releasement of inflammatory mediators [71] (Fig. 2).
Fig. 2.
Pathophysiology of cardiorenal syndrome type 1. ADHF - acutely decompensated heart failure, RAAS - renin-angiotensin-aldosterone system, AKI - acute kidney injury, CKD - chronic kidney disease
Cardiorenal syndrome type 2
CRS 2 is a syndrome where hemodynamic changes of chronic heart failure (CHF) cause impairment of kidney functions [74]. CHF affects about 1–2 % of adult population, but its’ prevalence in people over 70 years is more than 10 % [75]. It is characterized by elevated brain natriuretic peptides and by functional and/or structural changes of the heart accompanied by typical signs and symptoms of HF [76]. In patients with long-term stable CHF dependency between underlying CHF and worsening of renal function was proved [75, 77]. However, the reverse is also possible, and potential improvement of renal functions was proved after appropriate non-pharmacological therapy and pharmacological therapy, especially guideline-mediated therapy of HF, including gliflozins [78].
Among patients with CHF, the “backward” effects are much more common. They lead to venous congestion and increase of the renal venous pressure, which lowers the renal perfusion pressure. Chronic renal congestion induces structural kidney injury, including glomerulosclerosis and tubulointerstitial fibrosis [79]. Laboratory tests prove elevated renal injury biomarkers, mild to moderate proteinuria, and a progressive decline of glomerular filtration rate [80]. In “forward” HF, where cardiac output is decreased, the state of renal arteries underfilling leads to maladaptive activation of the RAAS and the SNS [81]. Angiotensin II, part of RAAS, has various effects, including vasoconstriction with increased systemic vascular resistance, increased venous tone, and leads to congestion. In kidneys, vasoconstriction affects predominantly the efferent arteriole. This exceeds glomerular filtration fraction and increases tubular sodium and water reabsorption. Further excess of body water is caused by its central effect increasing thirst [82] (Fig. 3).
Fig. 3.
Pathophysiology of cardiorenal syndrome type 2
Cardiorenal syndrome type 3
In CRS 3, acute kidney injury causes heart dysfunction and may be also described as acute renocardiac syndrome. Renal impairment may arise on different sites, i.e. prerenal, renal and postrenal, so proper fast evaluation of etiology and treatment is necessary [83]. Most common etiology, especially in elderly patients, is prerenal including hypovolemia or hypotension. About 50 % of causes are renal, caused by diseases of glomeruli, tubules or interstitium, and by vascular diseases. Nephrotoxic medication plays an important role and due to the availability of contrast examinations, the frequency of contrast-induced nephropathy increases [84]. Indirect effects of AKI are caused by different pathways including sodium and water retention, electrolyte imbalance, acidemia and uremic toxins [85]. Electrolyte imbalance may manifest itself as hyperkalemia, hyperphosphatemia, hypocalcemia etc. also varying due to primary cause of renal failure [86]. Hyperkalemia, as one of the most common, not only increases susceptibility to life-threatening arrythmias [87], but rapid changes in potassium levels result in muscle weakness, paralysis or change in mental status [88]. Decreased pH disturbs energy metabolism of cardiomyocytes and have negative inotropic effect, but also potentiate pulmonary vasoconstriction and increases afterload of the right ventricle [88]. Uremic toxins increase microvascular permeability, reduce cardiac contractility [89], and are associated with more frequent occurrence of myocardial infarction, or can cause pericarditis [90, 91]. All of this can trigger vicious circle, activation of SNS and RAAS with further organ dysfunction.
Cardiorenal syndrome type 4
This type of CRS, which may also by described as chronic renocardiac syndrome is caused by chronic kidney disease (CKD). CKD affects more than 13 % of worldwide population with rising tendency, especially due to rising prevalence of comorbidities as diabetes mellitus and hypertension, causing higher morbidity and mortality of afflicted patients [92]. It is a slowly progressing and irreversible syndrome reflecting functional and/or structural kidney changes. It is divided into five stages regarding glomerular filtration rate (GFR) and into three stages according to amount of albuminuria [93].
As kidneys lose their function, sodium and water excretion is impaired, and RAAS is activated [94]. Fluid overload often occurs, with far-reaching hemodynamic consequences. As kidneys’ blood flow is normally relatively high, approximately 400 ml/100 g of tissue per minute, and dependent on adequate intra- and transglomerular pressure [95], both appropriate inflow and outflow are inevitable. In these patients with systemic congestion and heart failure, renal functions further worsen due to hypoxia and ischemia. Ronco et al. described renal impairment as “Congestive kidney failure” and in this situation without adequate treatment vicious circle starts [96]. As mentioned, CKD is associated with increased SNS and RAAS activity, so not surprisingly angiotensin-convertin enzyme inhibitors, angiotensin receptor blockers or betablockers were proven profitable for this group of patients [97, 98]. Usage of gliflozins was also proven beneficial [99]. In patients with autosomal dominant polycystic kidney disease, tolvaptan was documented to prevent progression of CKD [100]. In end-stage kidney disease the use of hemodialysis is often necessary. Thanks to advances in technology the dialysis was proven to improve both mortality and morbidity [101]. Accelerated atherosclerosis in patients with decreased GFR results in coronary artery disease including myocardial infarctions with further hemodynamic impairment conditioned by calcifications in both vessels and heart, by increasing arterial stiffness and by decreased aortic compliance. Left ventricular hypertrophy develops or further worsens in advanced renal disease [102]. In addition, malnutrition and inflammation are often present [103] (Fig. 4).
Fig. 4.
Pathophysiology of cardiorenal syndrome 4. RAAS - renin-angiotensin-aldosterone system, LVH - left ventricle hypertrophy
Cardiorenal syndrome type 5
Pathophysiology of this type of CRS encounters different systemic disorders causing simultaneous impairment of heart and kidneys. It can also be caused by a chronic disease, for example liver cirrhosis. Common etiologies include particularly acute sepsis, but also connective tissues disorders such as lupus erythematosus or drug toxicity [104].
Pathophysiology of this syndrome varies by cause. In sepsis hemodynamic and microvascular changes lower flow velocities [105], leading to septic cardiac dysfunction caused by alteration in coronary blood flow, ischemia, inflammatory mediators and changes in myocardial metabolism [106]. Studies on septic kidney injury differ, however metanalyses showed that in about 30 % of studies renal blood flow (RBF) was preserved or increased. In this situation acute tubular apoptosis was main cause of renal injury [107]. In other studies RBF decreased, resulting into acute tubular necrosis. Other changes include changes in central nervous system in order to preserve blood supply to vital organs [108].
In all cardiorenal syndromes water and sodium homeostasis is impaired due to dysfunction of crucial organs - kidneys and heart. Although the pathophysiology of CRS types differs, without adequate treatment they all lead to vitious circle causing fluid retention with further consequences, including higher morbidity and mortality of these patients. Deleterious effects of heart failure include direct hemodynamic effects as congestion or decreased cardiac output, but also other effects connected with natriuretic peptides production described above. Renal congestion has a direct effect on tubules as tubular injury, apoptosis, or necrosis. Impaired renal functions have on one hand direct effect on kidney sodium and water excretion, on the other hand maladaptive activation of RAAS and SNS occurs.
Prevention
Type 2 diabetes mellitus (DM 2) is the most frequent cause of both CKD and heart failure with preserved ejection fraction (HFpEF) in developed countries [109]. Obesity is the predisposing factor to DM 2, therefore adequate management of obesity and diabetes is the most important measure for preventing CKD and HFpEF and therefore also the development of CRS. Other general influenceable risk factors include dyslipidemia, hypercholesterolemia, hypertension and smoking [110–112]. In all CRS types, the maintenance of normovolemia and avoidance of kidney ischemia is important and the usage of nephrotoxic and cardiotoxic medication should be minimized [113]. Regular medical check-ups with proper both non-pharmacological and pharmacological treatment are inevitable. In the prevention of CRS 2 positive effect of RAAS inhibitors, betablockers, statins and gliflozins was proved [113, 114], and so was the effect of pharmacological agents as RAAS blockers and gliflozins in CRS 4 [113,115,116]. Prevention of iodine contrast-induced nephropathy is important especially in patients with established CKD and included adequate hydration and, according to some, but not all studies also the administration of N-acetylcystein (CRS 3), especially if high dosed [116].
Gaps in the current knowledge and further research possibilities
There are still some blind spots giving us the opportunity for further research. In patients with ESRD on hemodialysis there is much unknown. The diagnosis of HFpEF in this group of patients remains the most frequently underdiagnosed entity in clinical practice of hemodialysis patients [117]. Hemodialysis induces a lot of changes, some of which are reversible, however we do not know how to avoid CKD- mineral and bone disorder, pathological hypertrophy of left ventricle etc. [118–121]. If arteriovenous access for hemodialysis is created, there is still the question of safe access flow [122].
In recent years, probably the biggest progress was in the study of biomarkers. It showed that NGAL may predict both renal and cardiovascular outcomes in myocardial infarction patients, but further research is needed [123, 124]. Newly also microRNA are used [125–128] and creation of multivariable panel of microRNAs in combination with conventional biomarkers is the next goal [128].
Another possibility for further research is represented by animal models. So far animal model usage in CRS study includes especially rat and mouse models [129, 130], however translation of these results to human is limited, so there is effort to use also larger animal models as canine, porcine or ovine models. Lately, dog models were used by Szczepankiewicz et al. while studied urine podocin/creatinine ratio which was higher in dogs with heart or kidney impairment [131]. Orieux et al. proved origin of CRS in porcine models with pulmonary hypertension [132]. Interesting option in recent years represents advanced in vitro models with control of cellular component and environment [133].
Acknowledgements
This study was supported by the Ministry of Health, Czech Republic-DRO (General University Hospital in Prague-VFN), 00064165.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Jalloh MB, Granger CB, Fonarow GC, Van Spall HGC. Multi-level implementation strategies to improve uptake of evidence-based therapies in heart failure. Eur Heart J. 2023;44:2055–2058. doi: 10.1093/eurheartj/ehad150. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C, House AA, Haapio M. Cardiorenal syndrome: refining the definition of a complex symbiosis gone wrong. Intensive Care Med. 2008;34:957–962. doi: 10.1007/s00134-008-1017-8. [DOI] [PubMed] [Google Scholar]
- 3.Miller WL. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail. 2016;9:e002922. doi: 10.1161/CIRCHEARTFAILURE.115.002922. [DOI] [PubMed] [Google Scholar]
- 4.Oikonomou E, Zografos T, Papamikroulis GA, Siasos G, Vogiatzi G, Theofilis P, Briasoulis A, Papaioannou S, Vavuranakis M, Gennimata V, Tousoulis D. Biomarkers in atrial fibrillation and heart failure. Curr Med Chem. 2019;26:873–887. doi: 10.2174/0929867324666170830100424. [DOI] [PubMed] [Google Scholar]
- 5.Valentova M, von Haehling S, Bauditz J, Doehner W, Ebner N, Bekfani T, Elsner S, Sliziuk V, Scherbakov N, Murín J, Anker SD, Sandek A. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J. 2016;37:1684–1691. doi: 10.1093/eurheartj/ehw008. [DOI] [PubMed] [Google Scholar]
- 6.Murray JF. Pulmonary edema: pathophysiology and diagnosis. Int J Tuberc Lung Dis. 2011;15(2):155–160. doi: 10.5588/ijtld.11.0324-2. [DOI] [PubMed] [Google Scholar]
- 7.Agnoli GC, Garutti C. Renal water-electrolyte excretion and its control mechanisms. Current status of knowledge. Minerva Med. 1976;67(56):3673–702. [PubMed] [Google Scholar]
- 8.Levey AS, Inker AL, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis. 2014;63(5):820–34. doi: 10.1053/j.ajkd.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knepper MA, Saidel GM, Hascall VC, Dwyer T. Concentration of solutes in the renal inner medulla: interstitial hyaluronan as a mechano-osmotic transducer [published correction appears in Am J Physiol Renal Physiol 2005 Jul;289(1):F225] Am J Physiol Renal Physiol. 2003;284:F433–F446. doi: 10.1152/ajprenal.00067.2002. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman DP, Basit H, Knohl SJ. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. Physiology, Glomerular Filtration Rate 2022. [PubMed] [Google Scholar]
- 11.Pallone TL, Turner MR, Edwards A, Jamison RL. Countercurrent exchange in the renal medulla. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1153–R1175. doi: 10.1152/ajpregu.00657.2002. [DOI] [PubMed] [Google Scholar]
- 12.Danziger J, Zeidel ML. Osmotic homeostasis. Clin J Am Soc Nephrol. 2015;10(5):852–862. doi: 10.2215/CJN.10741013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 14.Vermette D, Hu P, Canarie MF, Funaro M, Glover J, Pierce RW. Tight junction structure, function, and assessment in the critically ill: a systematic review. Intensive Care Med Exp. 2018;6:37. doi: 10.1186/s40635-018-0203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linden RJ. Function of cardiac receptors. Circulation. 1973;48:463–480. doi: 10.1161/01.CIR.48.3.463. [DOI] [PubMed] [Google Scholar]
- 16.Paintal AS. Vagal afferent fibres. Ergeb Physiol. 1963;52:74–156. doi: 10.1007/978-3-642-49896-1_3. [DOI] [PubMed] [Google Scholar]
- 17.Recordati G, Lombardi F, Bishop VS, Malliani A. Mechanical stimuli exciting type A atrial vagal receptors in the cat. Circ Res. 1976;38(5):397–403. doi: 10.1161/01.RES.38.5.397. [DOI] [PubMed] [Google Scholar]
- 18.Paintal AS. Natural stimulation of type B atrial receptors. J Physiol. 1963;169:116–136. doi: 10.1113/jphysiol.1963.sp007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hainsworth R. Sensory functions of the heart. Ann Acad Med Singap. 1994;23:546–551. [PubMed] [Google Scholar]
- 20.Longhurst JC. Cardiac receptors: their function in health and disease. Prog Cardiovasc Dis. 1984;27:201–222. doi: 10.1016/0033-0620(84)90005-7. [DOI] [PubMed] [Google Scholar]
- 21.Sleight P, Widdicombe JG. Action potentials in afferent fibres from pericardial mechanoreceptors in the dog. J Physiol. 1965;181:259–269. doi: 10.1113/jphysiol.1965.sp007758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberg B, Thorén P. Circulatory responses to stimulation of left ventricular receptors in the cat. Acta Physiol Scand. 1973;88(1):8–22. doi: 10.1111/j.1748-1716.1973.tb05429.x. [DOI] [PubMed] [Google Scholar]
- 23.Thorén PN. Atrial receptors with nonmedullated vagal afferents in the cat. Discharge frequency and pattern in relation to atrial pressure. Circ Res. 1976;38:357–362. doi: 10.1161/01.RES.38.5.357. [DOI] [PubMed] [Google Scholar]
- 24.Malliani A, Parks M, Tuckett RP, Brown AM. Reflex increases in heart rate elicited by stimulation of afferent cardiac sympathetic nerve fibers in the cat. Circ Res. 1973;32:9–14. [PubMed] [Google Scholar]
- 25.Ueda H, Uchida Y, Kamisaka K. Distribution and responses of the cardiac sympathetic receptors to mechanically induced circulatory changes. Jpn Heart J. 1969;10:70–81. doi: 10.1536/ihj.10.70. [DOI] [PubMed] [Google Scholar]
- 26.Coleridge HM, Coleridge JC. Cardiovascular afferents involved in regulation of peripheral vessels. Annu Rev Physiol. 1980;42:413–427. doi: 10.1146/annurev.ph.42.030180.002213. [DOI] [PubMed] [Google Scholar]
- 27.Malliani A, Recordati G, Schwartz PJ. Nervous activity of afferent cardiac sympathetic fibres with atrial and ventricular endings. J Physiol. 1973;229:457–469. doi: 10.1113/jphysiol.1973.sp010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson DF, Brown AM. Pressor reflexes produced by stimulation of afferent fibers in the cardiac sympathetic nerves of the cat. Circ Res. 1971;28(6):605–610. doi: 10.1161/01.RES.28.6.605. [DOI] [PubMed] [Google Scholar]
- 29.Pagani M, Schwartz PJ, Banks R, Lombardi F, Malliani A. Reflex responses of sympathetic preganglionic neurones initiated by different cardiovascular receptors in spinal animals. Brain Res. 1974;68:215–225. doi: 10.1016/0006-8993(74)90391-6. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa Y, Nishikimi T, Kuwahara K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides. 2019;111:18–25. doi: 10.1016/j.peptides.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Sayer G, Bhat G. The renin-angiotensin-aldosterone system and heart failure. Cardiol Clin. 2014;32(1):21–32. doi: 10.1016/j.ccl.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Laghlam D, Jozwiak M, Nguyen LS. Renin-Angiotensin-Aldosterone System and Immunomodulation: A State-of-the-Art Review. Cells. 2021:10. doi: 10.3390/cells10071767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nehme A, Zouein FA, Zayeri ZD, Zibara K. An Update on the Tissue Renin Angiotensin System and Its Role in Physiology and Pathology. J Cardiovasc Dev Dis. 2019;6:14. doi: 10.3390/jcdd6020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernstein KE, Khan Z, Giani JF, Cao DY, Bernstein EA, Shen XZ. Angiotensin-converting enzyme in innate and adaptive immunity. Nat Rev Nephrol. 2018;14(5):325–336. doi: 10.1038/nrneph.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fountain JH, Kaur J, Lappin SL. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. Mar 12, 2023. Physiology, Renin Angiotensin System. [PubMed] [Google Scholar]
- 36.Fourkiotis VG, Hanslik G, Hanusch F, Lepenies J, Quinkler M. Aldosterone and the kidney. Horm Metab Res. 2012;44:194–201. doi: 10.1055/s-0031-1295461. [DOI] [PubMed] [Google Scholar]
- 37.Hené RJ, Boer P, Koomans HA, Mees EJ. Plasma aldosterone concentrations in chronic renal disease. Kidney Int. 1982;21:98–101. doi: 10.1038/ki.1982.14. [DOI] [PubMed] [Google Scholar]
- 38.Bichet DG. Physiopathology of hereditary polyuric states: a molecular view of renal function. Swiss Med Wkly. 2012;142:w13613. doi: 10.4414/smw.2012.13613. [DOI] [PubMed] [Google Scholar]
- 39.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 40.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA. 2000;97(15):8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishikimi T, Nakagawa Y, Minamino N, Ikeda M, Tabei K, Fujishima A, Takayama K, Akimoto K, Yamada C, Nakao K, Minami T, Kuwabara Y, Kinoshita H, Tsutamoto T, Ishimitsu T, Kangawa K, Kuwahara K, Nakao K. Pro-B-type natriuretic peptide is cleaved intracellularly: impact of distance between O-glycosylation and cleavage sites. Am J Physiol Regul Integr Comp Physiol. 2015;309(6):R639–49. doi: 10.1152/ajpregu.00074.2015. [DOI] [PubMed] [Google Scholar]
- 42.Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69(2):318–328. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Issa N, Ortiz F, Reule SA, Kukla A, Kasiske BL, Mauer M, Jackson S, Matas AJ, Ibrahim HN, Najafian B. The renin-aldosterone axis in kidney transplant recipients and its association with allograft function and structure. Kidney Int. 2014;85(2):404–15. doi: 10.1038/ki.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talha S, Charloux A, Piquard F, Geny B. Brain natriuretic peptide and right heart dysfunction after heart transplantation. Clin Transplant. 2017;31(6) doi: 10.1111/ctr.12969. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Y, Wang D. Segmental Regulation of Sodium and Water Excretion by TRPV1 Activation in the Kidney. J Cardiovasc Pharmacol. 2008;51:437–42. doi: 10.1097/FJC.0b013e318168d120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9(7):519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- 47.Zimmerman CA, Leib DE, Knight ZA. Neural circuits underlying thirst and fluid homeostasis. Nat Rev Neurosci. 2017;18(8):459–469. doi: 10.1038/nrn.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 49.Yang T, Levy MN. The phase-dependency of the cardiac chronotropic responses to vagal stimulation as a factor in sympathetic-vagal interactions. Circulation Research. 1984;54:703–710. doi: 10.1161/01.RES.54.6.703. [DOI] [PubMed] [Google Scholar]
- 50.Kinugawa T, Dibner-Dunlap ME. Altered vagal and sympathetic control of heart rate in left ventricular dysfunction and heart failure. Am J Physiol. 1995;268:R310–16. doi: 10.1152/ajpregu.1995.268.2.R310. [DOI] [PubMed] [Google Scholar]
- 51.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118:863–871. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 52.Zoccali C, Ciccarelli M, Mallamaci F, Maggiore Q. Parasympathetic function in haemodialysis patients. Nephron. 1986;44:351–354. doi: 10.1159/000184018. [DOI] [PubMed] [Google Scholar]
- 53.Sabbah H, Imai M, Zaretsky A, Rastogi S, Wang M, Jiang A, Zacà V. Therapy with Vagus nerve electrical stimulation combined with beta-blockade improves left ventricular systolic function in dogs with heart failure beyond that seen with beta-blockade alone. Europ J Heart Failure Suppl. 2007;509:114–114. doi: 10.1016/S1567-4215(07)60316-6. [DOI] [Google Scholar]
- 54.Zheng C, Li M, Inagaki M, Kawada T, Sunagawa K, Sugimachi M. Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc. 2005;2005:7072–5. doi: 10.1109/IEMBS.2005.1616135. [DOI] [PubMed] [Google Scholar]
- 55.De Ferrari GM. Vagal stimulation in heart failure. J Cardiovasc Transl Res. 2014;7:310–320. doi: 10.1007/s12265-014-9540-1. [DOI] [PubMed] [Google Scholar]
- 56.Green L, Haddad H, Harkness K, Hernandez AF, Kouz S, LeBlanc MH, Masoudi FA, Ross HJ, Roussin A, Sussex B. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol. 2017;33:1342–1433. doi: 10.1016/j.cjca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 57.Cohn JN, Pfeffer MA, Rouleau J, Sharpe N, Swedberg K, Straub M, Wiltse C, Wright TJ MOXCON Investigators. Adverse mortality effect of central sympathetic inhibition with sustained-release moxonidine in patients with heart failure (MOXCON) Eur J Heart Fail. 2003;5:659–667. doi: 10.1016/S1388-9842(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 58.Fudim M, Sobotka PA, Piccini JP, Patel MR. Renal denervation for patients with heart failure: making a full circle. Circ Heart Fail. 2021;14:e008301. doi: 10.1161/CIRCHEARTFAILURE.121.008301. [DOI] [PubMed] [Google Scholar]
- 59.Nammas W, Koistinen J, Paana T, Karjalainen PP. Renal sympathetic denervation for treatment of patients with heart failure: summary of the available evidence. Ann Med. 2017;49:384–395. doi: 10.1080/07853890.2017.1282168. [DOI] [PubMed] [Google Scholar]
- 60.Kassab K, Soni R, Kassier A, Fischell TA. The Potential Role of Renal Denervation in the Management of Heart Failure. J Clin Med. 2022;11(14):4147. doi: 10.3390/jcm11144147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller M. Fluid and electrolyte homeostasis in the elderly: physiological changes of ageing and clinical consequences. Baillieres Clin Endocrinol Metab. 1997;11:367–387. doi: 10.1016/S0950-351X(97)80347-3. [DOI] [PubMed] [Google Scholar]
- 62.Abbott NJ, Friedman A. Overview and introduction: the blood-brain barrier in health and disease. Epilepsia. 2012;53(Suppl 6):1–6. doi: 10.1111/j.1528-1167.2012.03696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sterns RH. Disorders of plasma sodium--causes, consequences, and correction. N Engl J Med. 2015;372(1):55–65. doi: 10.1056/NEJMra1404489. [DOI] [PubMed] [Google Scholar]
- 64.Edelman IS, Leibman J, O’Mera MP, Birkenfeld LW. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236–1256. doi: 10.1172/JCI103712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Titze J, Dahlmann A, Lerchl K, et al. Spooky sodium balance. Kidney Int. 2014;85:759–767. doi: 10.1038/ki.2013.367. [DOI] [PubMed] [Google Scholar]
- 66.Lemoine S, Salermo F, Akbari A, McKelvie R, McIntyre C. Tissue Sodium Storage in Patients With Heart Failure: A New Therapeutic Target? Circulation: Cardiovascular Imaging. 2021;14(11):e012910. doi: 10.1161/CIRCIMAGING.121.012910. [DOI] [PubMed] [Google Scholar]
- 67.Sterns RH, Silver SM. Cerebral salt wasting versus SIADH: what difference? J Am Soc Nephrol. 2008;19:194–196. doi: 10.1681/ASN.2007101118. [DOI] [PubMed] [Google Scholar]
- 68.Pedersen EB, Thomsen IM, Lauridsen TG. Abnormal function of the vasopressin-cyclic-AMP-aquaporin2 axis during urine concentrating and diluting in patients with reduced renal function. A case control study. BMC Nephrol. 2010;11:26. doi: 10.1186/1471-2369-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137:352–360. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- 70.González-Pacheco H, Álvarez-Sangabriel A, Martínez-Sánchez C, Briseño-Cruz JL, Altamirano-Castillo A, Mendoza-García S, Manzur-Sandoval D, Amezcua-Guerra LM, Sandoval J, Bojalil R, Araiza-Garaygordobil D, Sierra-Lara D, Guiza-Sánchez CA, Gopar-Nieto R, Cruz-Rodríguez C, Valdivia-Nuño JJ, Salas-Teles B, Arias-Mendoza A. Clinical phenotypes, aetiologies, management, and mortality in acute heart failure: a single-institution study in Latin-America. ESC Heart Fail. 2021;8:423–437. doi: 10.1002/ehf2.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ismail Y, Kasmikha Z, Green HL, McCullough PA. Cardio-renal syndrome type 1: epidemiology, pathophysiology, and treatment. Semin Nephrol. 2012;32:18–25. doi: 10.1016/j.semnephrol.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 72.Kurmani S, Squire I. Acute Heart Failure: Definition, Classification and Epidemiology. Curr Heart Fail Rep. 2017;14:385–392. doi: 10.1007/s11897-017-0351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tabucanon T, Tang WHW. Right heart failure and cardiorenal syndrome. Cardiol Clin. 2020;8:185–202. doi: 10.1016/j.ccl.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J ADHERE Scientific Advisory Committee and Investigators. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 75.Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22:1342–1356. doi: 10.1002/ejhf.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 77.Al-Naher A, Wright D, Devonald MAJ, Pirmohamed M. Renal function monitoring in heart failure - what is the optimal frequency? A narrative review. Br J Clin Pharmacol. 2018;84(1):5–17. doi: 10.1111/bcp.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clark AL, Kalra PR, Petrie MC, Mark PB, Tomlinson LA, Tomson CR. Change in renal function associated with drug treatment in heart failure: national guidance. Heart. 2019;105(12):904–910. doi: 10.1136/heartjnl-2018-314158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.el Nahas AM, Muchaneta-Kubara EC, Essawy M, Soylemezoglu O. Renal fibrosis: insights into pathogenesis and treatment. Int J Biochem Cell Biol. 1997;29(1):55–62. doi: 10.1016/S1357-2725(96)00119-7. [DOI] [PubMed] [Google Scholar]
- 80.Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12(10):610–623. doi: 10.1038/nrneph.2016.113. [DOI] [PubMed] [Google Scholar]
- 81.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005;26(1):11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 82.Cruz DN, Schmidt-Ott KM, Vescovo G, House AA, Kellum JA, Ronco C, McCullough PA. Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI) Contrib Nephrol. 2013;182:117–136. doi: 10.1159/000349968. [DOI] [PubMed] [Google Scholar]
- 83.Patil VP, Salunke BG. Fluid Overload and Acute Kidney Injury. Indian J Crit Care Med. 2020;24(Suppl 3):S94–s97. doi: 10.5005/jp-journals-10071-23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malík J. In: Echokardiografie u pacientů s chronickým onemocněním ledvin a úvod do kardionefrologie. Maxdorf, editor. Praha: Jessenius; 2018. p. 93. [Google Scholar]
- 85.Singbartl K, Joannidis M. Short-term Effects of Acute Kidney Injury. Crit Care Clin. 2015;31:751–762. doi: 10.1016/j.ccc.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 86.Karet FE. Mechanisms in hyperkalemic renal tubular acidosis. J Am Soc Nephrol. 2009;20(2):251–4. doi: 10.1681/ASN.2008020166. [DOI] [PubMed] [Google Scholar]
- 87.Bagshaw SM, Cruz DN, Aspromonte N, Daliento L, Ronco F, Sheinfeld G, Anker SD, Anand I, Bellomo R, Berl T, Bobek I, Davenport A, Haapio M, Hillege H, House A, Katz N, Maisel A, Mankad S, McCullough P, Mebazaa A, Palazzuoli A, Ponikowski P, Shaw A, Soni S, Vescovo G, Zamperetti N, Zanco P, Ronco C Acute Dialysis Quality Initiative Consensus Group. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25(5):1406–1416. doi: 10.1093/ndt/gfq066. [DOI] [PubMed] [Google Scholar]
- 88.Forni LG, McKinnon W, Hilton PJ. Unmeasured anions in metabolic acidosis: unravelling the mystery. Critical Care. 2006;10(4):220. doi: 10.1186/cc4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harper S, Tomson C, Bates D. Human uremic plasma increases microvascular permeability to water and proteins in vivo. Kidney international. 2002;61:1416–22. doi: 10.1046/j.1523-1755.2002.00252.x. [DOI] [PubMed] [Google Scholar]
- 90.De Deyn PP, Vanholder R, D’Hooge R. Nitric oxide in uremia: effects of several potentially toxic guanidino compounds. Kidney Int Suppl. 2003;(84):S25–8. doi: 10.1046/j.1523-1755.63.s84.9.x. [DOI] [PubMed] [Google Scholar]
- 91.Scheuer J, Stezoski W. The effects of uremic compounds on cardiac function and metabolism. J Mol Cell Cardiol. 1973;5(3):287–300. doi: 10.1016/0022-2828(73)90068-0. [DOI] [PubMed] [Google Scholar]
- 92.Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. doi: 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 93.Ammirati AL. Chronic Kidney Disease. Rev Assoc Med Bras (1992) 2020;66(Suppl 1(Suppl 1)):03–09. doi: 10.1590/1806-9282.66.s1.3. [DOI] [PubMed] [Google Scholar]
- 94.Khan YH, Sarriff A, Adnan AS, Khan AH, Mallhi TH. Chronic Kidney Disease, Fluid Overload and Diuretics: A Complicated Triangle. PLoS One. 2016;11(7):e0159335. doi: 10.1371/journal.pone.0159335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matovinović MS. 1 Pathophysiology and Classification of Kidney Diseases. Ejifcc. 2009;20(1):2–11. [PMC free article] [PubMed] [Google Scholar]
- 96.Ronco C, Rinaldo B, Kellum J, Zaccaria R. In: Critical Care Nephrology. Third Edition. Claudio Ronco JAaZR, Bellomo Rinaldo, Kellum, editors. Vol. 3. Netherlands: Elsevier; 2019. [DOI] [Google Scholar]
- 97.Schmieder RE, Delles C, Mimran A, Fauvel JP, Ruilope LM. Impact of telmisartan versus ramipril on renal endothelial function in patients with hypertension and type 2 diabetes. Diabetes Care. 2007;30(6):1351–1356. doi: 10.2337/dc06-1551. [DOI] [PubMed] [Google Scholar]
- 98.Chonchol M, Benderly M, Goldbourt U. Beta-blockers for coronary heart disease in chronic kidney disease. Nephrol Dial Transplant. 2008;23(7):2274–9. doi: 10.1093/ndt/gfm950. [DOI] [PubMed] [Google Scholar]
- 99.Scheen AJ, Delanaye P. Inhibiteurs des SGLT2 chez les patients avec insuffisance rénale chronique : des essais contrôlés aux recommandations internationales et perspectives en pratique clinique [SGLT2 inhibitors in patients with chronic kidney disease : from clinical trials to guidelines and new prospects for clinical practice] Rev Med Liege. 2021;76(3):186–194. [PubMed] [Google Scholar]
- 100.Müller RU, Messchendorp AL, Birn H, Capasso G, Cornec-Le Gall E, Devuyst O, van Eerde A, Guirchoun P, Harris T, Hoorn EJ, Knoers NVAM, Korst U, Mekahli D, Le Meur Y, Nijenhuis T, Ong ACM, Sayer JA, Schaefer F, Servais A, Tesar V, Torra R, Walsh SB, Gansevoort RT. An update on the use of tolvaptan for autosomal dominant polycystic kidney disease: consensus statement on behalf of the ERA Working Group on Inherited Kidney Disorders, the European Rare Kidney Disease Reference Network and Polycystic Kidney Disease International. Nephrol Dial Transplant. 2022;37(5):825–839. doi: 10.1093/ndt/gfab312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clementi A, Virzì GM, Goh CY, et al. Cardiorenal syndrome type 4: a review. Cardiorenal Med. 2013;3(1):63–70. doi: 10.1159/000350397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foley RN, Curtis BM, Randell EW, Parfrey PS. Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol. 2010;5(5):805–813. doi: 10.2215/CJN.07761109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hung SC, Kuo KL, Peng CH, Wu CH, Lien YC, Wang YC, Tarng DC. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014;85:703–709. doi: 10.1038/ki.2013.336. [DOI] [PubMed] [Google Scholar]
- 104.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P Acute Dialysis Quality Initiative (ADQI) consensus group. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32(9):1825–1831. doi: 10.1097/01.CCM.0000138558.16257.3F. [DOI] [PubMed] [Google Scholar]
- 106.Levy RJ, Piel DA, Acton PD, Zhou R, Ferrari VA, Karp JS, Deutschman CS. Evidence of myocardial hibernation in the septic heart. Crit Care Med. 2005;33(12):2752–6. doi: 10.1097/01.CCM.0000189943.60945.77. [DOI] [PubMed] [Google Scholar]
- 107.Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006;69(11):1996–2002. doi: 10.1038/sj.ki.5000440. [DOI] [PubMed] [Google Scholar]
- 108.Papaioannou VE, Dragoumanis C, Theodorou V, Gargaretas C, Pneumatikos I. Relation of heart rate variability to serum levels of C-reactive protein, interleukin 6, and 10 in patients with sepsis and septic shock. J Crit Care. 2009;24(4):625.e1–625.e6257. doi: 10.1016/j.jcrc.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 109.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice [published correction appears in Eur Heart J 2022 Nov 7;43(42):4468] Eur Heart J. 2021;42(34):3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 110.Kondo T, Nakano Y, Adachi S, Murohara T. Effects of Tobacco Smoking on Cardiovascular Disease. Circ J. 2019;83(10):1980–1985. doi: 10.1253/circj.CJ-19-0323. [DOI] [PubMed] [Google Scholar]
- 111.Lakier JB. Smoking and cardiovascular disease. Am J Med. 1992;93(1a):8s–12s. doi: 10.1016/0002-9343(92)90620-Q. [DOI] [PubMed] [Google Scholar]
- 112.McCullough PA. Cardiorenal syndromes: pathophysiology to prevention. Int J Nephrol. 2010;2011:762590. doi: 10.4061/2011/762590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Vecchis R, Baldi C. Cardiorenal syndrome type 2: from diagnosis to optimal management. Ther Clin Risk Manag. 2014;10:949–961. doi: 10.2147/TCRM.S63255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 115.The EMPA-KIDNEY Collaborative Group. Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, Preiss D, Judge P, Mayne KJ, Ng SYA, Sammons E, Zhu D, Hill M, Stevens W, Wallendszus K, Brenner S, Cheung AK, Liu ZH, Li J, Hooi LS, Liu W, Kadowaki T, Nangaku M, Levin A, Cherney D, Maggioni AP, Pontremoli R, Deo R, Goto S, Rossello X, Tuttle KR, Steubl D, Petrini M, Massey D, Eilbracht J, Brueckmann M, Landray MJ, Baigent C, Haynes R. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117–127. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, De Metrio M, Galli S, Fabbiocchi F, Montorsi P, Veglia F, Bartorelli AL. N-Acetylcysteine and Contrast-Induced Nephropathy in Primary Angioplasty. New England Journal of Medicine. 2006;354(26):2773–2782. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 117.Malik J, Valerianova A, Pesickova SS, Michalickova K, Hladinova Z, Hruskova Z, Bednarova V, Rocinova K, Tothova M, Kratochvilova M, Kaiserova L, Buryskova Salajova K, Lejsek V, Sevcik M, Tesar V. Heart failure with preserved ejection fraction is the most frequent but commonly overlooked phenotype in patients on chronic hemodialysis. Front Cardiovasc Med. 2023;10:1130618. doi: 10.3389/fcvm.2023.1130618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cannata-Andía JB, Martín-Carro B, Martín-Vírgala J, Rodríguez-Carrio J, Bande-Fernández JJ, Alonso-Montes C, Carrillo-López N. Chronic Kidney Disease-Mineral and Bone Disorders: Pathogenesis and Management. Calcif Tissue Int. 2021;108(4):410–422. doi: 10.1007/s00223-020-00777-1. [DOI] [PubMed] [Google Scholar]
- 119.Hsu CY, Chen LR, Chen KH. Osteoporosis in patients with chronic kidney diseases: a systemic review. Int J Mol Sci. 2020;21(18):6846. doi: 10.3390/ijms21186846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nardi E, Mulè G, Giammanco A, Mattina A, Geraci G, Nardi C, Averna M. Left ventricular hypertrophy in chronic kidney disease: A diagnostic criteria comparison. Nutr Metab Cardiovasc Dis. 2021;31:137–144. doi: 10.1016/j.numecd.2020.08.028. [DOI] [PubMed] [Google Scholar]
- 121.McMahon LP, Roger SD, Levin A Slimheart Investigators Group. Development, prevention, and potential reversal of left ventricular hypertrophy in chronic kidney disease. J Am Soc Nephrol. 2004;15:1640–7. doi: 10.1097/01.ASN.0000130566.69170.5E. [DOI] [PubMed] [Google Scholar]
- 122.Malik J, Lomonte C, Rotmans J, Chytilova E, Roca-Tey R, Kusztal M, Grus T, Gallieni M. Hemodialysis vascular access affects heart function and outcomes: Tips for choosing the right access for the individual patient. The Journal of Vascular Access. 2021;22(1_suppl):32–41. doi: 10.1177/1129729820969314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zahler D, Merdler I, Banai A, Shusterman E, Feder O, Itach T, Robb L, Banai S, Shacham Y. predictive value of elevated neutrophil gelatinase-associated lipocalin (NGAL) levels for assessment of cardio-renal interactions among st-segment elevation myocardial infarction patients. J Clin Med. 2022;11:2162. doi: 10.3390/jcm11082162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Højagergaard MA, Beske RP, Hassager C, Holmvang L, Jensen LO, Shacham Y, Meyer MAS, Moeller JE, Helgestad OKL, Mark PD, Møgelvang R, Frydland M. Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Patients with ST-Elevation Myocardial Infarction and Its Association with Acute Kidney Injury and Mortality. J Clin Med. 2023;12(11):3681. doi: 10.3390/jcm12113681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gembillo G, Visconti L, Giusti MA, Siligato R, Gallo A, Santoro D, Mattina A. Cardiorenal Syndrome: New Pathways and Novel Biomarkers. Biomolecules. 2021;11(11):1581. doi: 10.3390/biom11111581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ishrat R, Ahmed MM, Tazyeen S, Alam A, Farooqui A, Ali R, Imam N, Tamkeen N, Ali S, Zubbair Malik M, Sultan A. In Silico Integrative Approach Revealed Key MicroRNAs and Associated Target Genes in Cardiorenal Syndrome. Bioinform Biol Insights. 2021;15:11779322211027396. doi: 10.1177/11779322211027396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Y, Liang Y, Zhao W, Fu G, Li Q, Min X, Guo Y. Circulating miRNA-21 as a diagnostic biomarker in elderly patients with type 2 cardiorenal syndrome. Sci Rep. 2020;10:4894. doi: 10.1038/s41598-020-61836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Siasos G, Bletsa E, Stampouloglou PK, Oikonomou E, Tsigkou V, Paschou SA, Vlasis K, Marinos G, Vavuranakis M, Stefanadis C, Tousoulis D. MicroRNAs in cardiovascular disease. Hellenic J Cardiol. 2020;61(3):165–173. doi: 10.1016/j.hjc.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 129.Liu S. Heart-kidney interactions: mechanistic insights from animal models. Am J Physiol Renal Physiol. 2019;316(5):F974–F985. doi: 10.1152/ajprenal.00624.2017. [DOI] [PubMed] [Google Scholar]
- 130.Martínez-Martínez E, Ibarrola J, Fernández-Celis A, Calvier L, Leroy C, Cachofeiro V, Rossignol P, López-Andrés N. Galectin-3 pharmacological inhibition attenuates early renal damage in spontaneously hypertensive rats. J Hypertens. 2018;36(2):368–376. doi: 10.1097/HJH.0000000000001545. [DOI] [PubMed] [Google Scholar]
- 131.Szczepankiewicz B, Paslawska U, Paslawski R, Gebarowski T, Zasada W, Michalek M, Noszczyk-Nowak A. The urine podocin/creatinine ratio as a novel biomarker of cardiorenal syndrome in dogs due to degenerative mitral valve disease. J Physiol Pharmacol. 2019;70 doi: 10.26402/jpp.2019.2.06. doi: 10.26402/jpp.2019.2.06. [DOI] [PubMed] [Google Scholar]
- 132.Orieux A, Samson C, Pieroni L, Drouin S, Dang Van S, Migeon T, Frere P, Brunet D, Buob D, Hadchouel J, Guihaire J, Mercier O, Galichon P. Pulmonary hypertension without heart failure causes cardiorenal syndrome in a porcine model. Sci Rep. 2023;13(1):9130. doi: 10.1038/s41598-023-36124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gabbin B, Meraviglia V, Mummery CL, Rabelink TJ, van Meer BJ, van den Berg CW, Bellin M. Toward Human Models of Cardiorenal Syndrome in vitro. Front Cardiovasc Med. 2022;9:889553. doi: 10.3389/fcvm.2022.889553. [DOI] [PMC free article] [PubMed] [Google Scholar]