Abstract
Objectives:
This study aimed to compare the analgesic effects of continuous femoral nerve block (FNB), femoral triangle block (FTB), and adductor canal block (ACB) following total knee arthroplasty (TKA). The goal was to identify the most effective nerve block technique among these.
Methods:
Patients undergoing TKA were randomly assigned to 1 of 3 groups: FNB, FTB, or ACB. Nerve blocks were administered preoperatively, with catheters placed for patient-controlled nerve analgesia (PCNA). The primary end point was the Numeric Rating Scale (NRS) score at movement at 24 hours postsurgery. Secondary end points included NRS scores at rest and movement, quadriceps strength, Timed Up and Go (TUG) test performance, range of motion, effective PCNA utilization, and opioid consumption at various postsurgery time points.
Results:
Of the 94 valid data sets analyzed (FNB: 31, FTB: 31, ACB: 32), significant differences were observed in the primary end point (H=7.003, P=0.03). Post hoc analysis with Bonferroni correction showed that the FNB group had a significantly lower median pain score (3 [2 to 4]) compared with the ACB group (4 [3 to 5], Bonferroni-adjusted P=0.03). Regarding secondary end points, both the FNB and FTB groups had significantly lower NRS scores than the ACB group at various time points after surgery. Quadriceps strength and TUG completion were better in the FTB and ACB groups. There were no statistically significant differences among the groups for the other end points.
Discussion:
Continuous FTB provides postoperative analgesia comparable to FNB but with the advantage of significantly less impact on quadriceps muscle strength, a benefit not seen with FNB. Both FTB and ACB are effective in preserving quadriceps strength postoperatively.
Key Words: total knee arthroplasty, analgesic effects, nerve blocks, postoperative pain, quadriceps strength
Total knee arthroplasty (TKA) is a widely recognized and effective treatment for severe knee diseases. Numerous studies have highlighted that postoperative pain, particularly prevalent within the first 24 hours following surgery, is a common challenge after TKA procedures.1,2 Inadequate pain control can lead to extended hospital stays, escalated medical costs, and an increased risk of postoperative complications.3–5 Consequently, effective pain management is vital for accelerating patient recovery, enhancing patient satisfaction, and minimizing complication risks.4 Nerve blocks, targeting the painful area directly, offer potent analgesia, can reduce systemic analgesic use, prevent drug addiction, and significantly contribute to the early recovery phases of TKA.6
The femoral nerve block (FNB) is a commonly employed technique for managing pain after TKA, achieved by blocking the femoral nerve (FN) at the level of the inguinal ligament (IL).1 While FNB provides effective analgesia, it often results in considerable quadriceps muscle weakness, impeding early patient mobilization and potentially increasing the risk of falls postoperatively.2,7 The preservation of quadriceps strength is of paramount importance for TKA patients, as it is essential for knee stabilization, mobility, and overall recovery. The existing literature underscores the necessity of evaluating pain management methods in TKA, aiming to strike a balance between effective pain control and the preservation of muscle function. This balance is key to optimizing recovery and reducing fall risk.7–9 To balance pain relief and muscle strength preservation, the saphenous nerve (SN), a purely sensory branch of the FN within the adductor canal (AC), has gained interest.10 This approach, known as adductor canal block (ACB), is preferred in many TKA-performing institutions.11,12 However, the AC has a clear anatomic location: the entrance of the AC, which forms the apex of the femoral triangle (FT), is defined by the junction of the medial sides of the sartorius muscle (SM) and the adductor longus muscle (ALM), and the adductor magnus hiatus is its exit.13 Preoperative AC catheterization is constrained by the precise anatomic location, impacting disinfection scope and surgical space. Consequently, it is frequently placed higher based on strict anatomic landmarks, possibly within the FT.13 The FT is demarcated by the IL, the medial side of the SM, and the medial side of the ALM; the FT’s floor is bounded laterally by the iliopsoas muscle and medially by the pectineus and AL muscles.14 Between the IL and the entrance of the AC, the branches of the FN, including the medial vastus nerve (MVN), SN, and medial femoral cutaneous nerve (MFCN), run through it.10,11,15
Previous studies have examined the effects of FN blocks or their branch blocks at various locations, yet determining the optimal nerve block location remains challenging due to differences in catheter insertion methods and locations.11,12,15–17 Furthermore, systematic studies comparing the differences in analgesic efficacy between FTB, ACB, and femoral triangle block (FTB) are limited. To address this, we conducted a randomized, controlled, double-blind clinical trial aiming to compare the analgesic effects of these methods.
METHODS
This clinical trial research received approval of the Ethics Committee of the First Affiliated Hospital, Chongqing Medical University (Ethics Number: 2022-K233). It was registered with the China Clinical Trial Registration Center on October 20, 2022 (Registration Number: ChiCTR2200064888), with the registration completed before the enrollment of any patients. The inclusion criteria of the trial were (1) elective unilateral TKA surgery; (2) ASA grade Ⅰ-Ⅲ; and (3) age 30 to 75 years. The exclusion criteria are (1) patients with cardiac function NYHA ≥Ⅲ grade; with COPD and lung function ≥Ⅲ grade; liver function Child-Pugh B, C grade; eGFR<60 mL/min); (2) prolonged use of opioid analgesics drugs for over 1 year; (3) with neuropsychiatric diseases who cannot cooperate; (4) with preoperative coagulation dysfunction (PT or APTT>1.5 times the normal value or INR>2.0, PLT <50×109/L); (5) with contraindications to local anesthetics. Written informed consent was obtained from all patients.
Patients were randomly divided into 3 groups (1-FNB group, 2-FTB group, and 3-ACB group) according to a random sequence (100 numbers). The patient numbers were assigned in the order of their operations by the nurse not involved in postoperative assessment. To maintain randomized assignment secrecy, sealed envelopes were used to inform the nerve block operator of the patients’ respective trial groups. Throughout the study, all relevant personnel remained blinded to group assignments, except for the nerve block operator. After catheter insertion, a large sterile occlusive dressing covered the area from below the AC entrance to the anterior superior iliac spine (ASIS) to conceal the catheter’s position, thus maintaining blinding. This manuscript adheres to the applicable CONSORT guidelines.
Nerve Block and Catheter Insertion Procedure
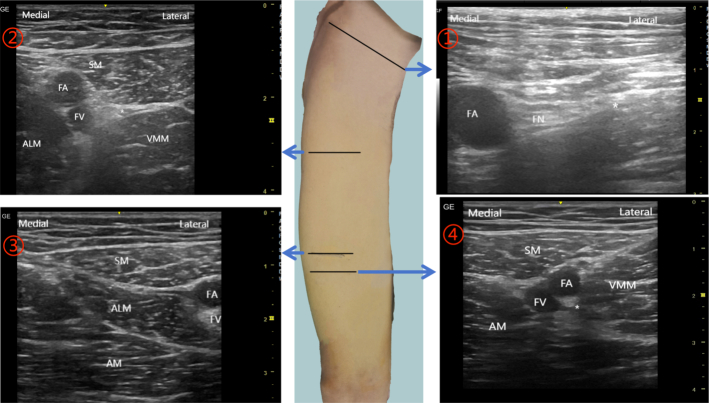
Patients were initially prepared in the anesthesia preparation area, with continuous monitoring of ECG, blood pressure, and oxygen saturation. Venous access was established, and mild sedation was administered, followed by oxygen delivery through a mask. An experienced anesthetist performed the nerve block, employing a real-time, ultrasound-guided in-plane approach while maintaining a strict aseptic technique. For the procedure, we used the Contiplex D needle-catheter system (18G×21/8” [1.3×55 mm], B. Braun, USA) and a high-frequency linear array probe 12L-RS (4.2-13Mhz, 192 array elements, GE Healthcare). Patients were positioned supine, with the knee joint slightly abducted and the leg externally rotated. The needle was inserted laterally at 30 degrees to 45 degrees. In the FNB group, the ultrasound probe targeted the middle third of the inguinal ligament’s transverse axis to locate the femoral artery and vein. The FN, identifiable by its high echogenicity, was found lateral to the femoral artery. The injection was administered either posteriorly or laterally to the FN. For the FTB and ACB groups, the initial step involved locating the apex of the FT, also the entrance to the AC. This was achieved by placing the ultrasound probe perpendicular to the thigh’s long axis and moving it to find the intersection of the medial borders of the ALM and SM. The target area for FTB was defined as the midpoint of the line connecting the apex of the FT and the ASIS, inside the midpoint of the FT’s outer side. To minimize the impact on surgical disinfection and operating range, the target area for ACB was set 3 cm below the AC entrance. After localization, 10 mL of 0.2% ropivacaine was injected laterally into the target nerve. We employed hydrodissection to create a liquid lacuna, ensuring effective anesthetic distribution. The anesthetic spread was closely monitored through ultrasound to confirm the nerve’s (mainly the FN, MVN, or SN) immersion in the solution. After confirming the nerve was immersed, the needle was withdrawn, and the catheter was inserted 3 to 5 cm into the lacuna under ultrasound guidance. Following catheter insertion, an additional 10 ml of local anesthetic was injected through the catheter under ultrasound guidance. This process was crucial for 2 reasons: firstly, it allowed us to observe the enlargement of the previously formed liquid lacuna, confirming effective anesthetic distribution. Secondly, the absence of resistance during the injection and no leakage of anesthetic at the puncture site were key indicators of the catheter’s correct positioning and patency within the established liquid space. The body surface locations and ultrasound images of each nerve block are shown in Figure 1.
FIGURE 1.
Location of catheter insertion and corresponding ultrasound images. ①, The location of FNB, the ultrasonic probe was placed in the middle one-third of the transverse axis of the inguinal ligament.②, The location of FTB, the midpoint of the line connecting the apex of FT and ASIS. ③, The location of the apex of the FT (also the entrance of the AC). ④, The location of ACB, 3 cm below the AC entrance. ALM indicates adductor longus muscle; AM, adductor magnus muscle; FA, femoral artery; FN, femoral nerve; FV, femoral vein; SM, sartorius muscle, VMM, vastus medialis muscle; *target area.
Before anesthesia induction, after ensuring there was no local anesthesia drug poisoning, we assessed the efficacy of the peripheral nerve block. This assessment, done through pinprick methods, focused on the surgical area and was compared with the contralateral knee to evaluate the effectiveness of the nerve block. The induction of anesthesia was standardized. Propofol was administered at 2 mg/kg combined with 2 mg of midazolam. Sufentanil was used at a dose of 4 ug/10 kg for induction, with an additional 10 ug given before the start of surgery and another 10 ug at skin closure. Remifentanil infusion, starting postintubation and continuing until the end of the surgery, was at 10 ug/kg/h. The depth of anesthesia and muscle relaxation were monitored using a Bispectral Index (BIS) monitor, with adjustments made to the propofol infusion rate and sevoflurane maintained at 0.5 MAC to keep the BIS value between 40% to 55%. Vecuronium was administered for neuromuscular blockade at 1 mg/10kg for induction, supplemented with a third of the induction dose before skin incision and adjusted as necessary to maintain E mg at 0%. In addition, 10 mg of dexamethasone was administered preoperatively. Before the final suturing of the skin incision and completion of the surgery, the surgeon performed an articular injection for local infiltration analgesia (LIA). This involved injecting a 20 ml solution, comprising 200 mg ropivacaine and a compound betamethasone formulation (5 mg betamethasone dipropionate and 2 mg betamethasone sodium phosphate), directly into the joint cavity through the surgical incision. And the patient-controlled nerve analgesia (PCNA) pump (the formula was 1% ropivacaine 500 mg + 0.9% normal saline 200 ml, configured as 0.2% ropivacaine 250 mL, the flow rate was 5 mL/h, self-controlled administration is 5 mL/time, and the minimum interval between self-controlled administration is 45 min) was connected through the continuous nerve blocking catheter at the end of surgery. Postoperative extubation in the PACU, performed by a professional anesthesiologist, was guided by BIS and E mg values to ensure no residual effects from neuromuscular blockers and that muscle strength had returned to normal. All patients received an intravenous infusion of flurbiprofen axetil 50 mg q12h for auxiliary analgesia after operation. If the resting NRS score is>3 points, patients can perform PCNA self-controlled administration. If pain persists or worsens within 45 minutes after self-control administration, the physician can administer oral or intravenous tramadol rescue analgesia (37.5 mg orally or 100 mg intravenous infusion, depending on the specific pain condition: oral for moderate pain (NRS 4-6) and IV for severe pain (NRS ≥7), all doses were converted to oMEDD for comparison). Within 24 hours postsurgery, patients received rehabilitation guidance from physician and were encouraged to walk with the assistance of a walker or under the supervision of rehabilitation professionals. The PCNA remained in place for 48 hours postoperatively.
Outcome Measurement
The primary end point of this study was the Numeric Rating Scale (NRS) score for pain during movement at 24 hours postoperation. Secondary end points included the quadriceps strength, NRS scores at rest and during movement(excluded 24 hours postoperation) at 2-, 6-, 12-, 24-, 48-, 72 hours postoperation and 10 minutes post-anesthesia recovery, knee joint range of motion (ROM) and the time spent on “up and go”(TUG) test at 24-, 48-, 72 hours postoperation, the times of effective PCNA pressed at 2-, 6-, 12-, 24-, 48 hours postoperation, dosage, and frequency of rescue analgesic medication within 72 hours postoperation and incidence of Postoperative Nausea and Vomiting(PONV), puncture site infection, postoperative fall, hematoma at the puncture site. The NRS is a subjective scale ranging from 0, denoting no pain, to 10, representing the most severe pain imaginable. Resting NRS scores were obtained while patients were at rest in a supine position. The NRS score during movement was measured during passive knee flexion at 60 degrees. The quadriceps strength is assessed using the Manual Muscle Testing (MMT) method as follows: Grade 0: No muscle contraction; Grade 1: Slight contraction, but cannot move the joint; Grade 2: The joint can move horizontally, but cannot resist gravity of the lower limb; Grade 3: Can resist gravity of the lower limb, but cannot resist resistance; Grade 4: Can resist gravity of the lower limb and some resistance; Grade 5: Can resist substantial resistance during movement. ROM was measured with the patient in a sitting position using a large goniometer. The goniometer was accurately aligned with the lateral epicondyle of the femur while flexing the knee joint to its maximum extent, and the angle between the moving arm and the fixed arm represents the joint’s range of motion. In the TUG test,18 the patient was seated in regular shoes on a chair. A colored strip was positioned on the floor, placed 3 m away from the chair. Upon receiving the “start” command, the patient stood up, walked forward 3 m using a standard walking aid, turned around at the designated point, walked back to the chair, and sat down. No physical assistance was provided during the test. The tester recorded the time (in sec). The assessment times for each outcome measure were as follows: anesthesia recovery, 10 minutes after patient awakening; postoperation 2 hours, 6 hours, and 12 hours with a measurement time fluctuation of±30 min; postoperation 24 hours, 48 hours, and 72 hours with a measurement time fluctuation of±1 h. Outcome measures were not collected between 11 PM and 6 AM. Preoperative baseline NRS scores and quadriceps muscle strength were established by the anesthesiologist between 3:00 PM and 8:00 PM on the day before surgery.
In determining the appropriate sample size for our study, we referenced the research by Alghadir et al.19 This study identified the minimum detectable change in NRS scores for knee joint pain in osteoarthritis as 1.33. We hypothesized that the postoperative 24-hour active NRS scores in the FTB group would be lower by 1.33 points compared with the ACB group, with no significant difference from the FNB group. We set the SD at 1.48, based on preliminary experiments. Using PASS software for a 2-sided α level of 0.05 and a power of 90%, we calculated a minimum sample size of 30 eligible cases per group. Considering a potential 10% loss to follow-up and postoperative exclusion rate, the study aimed to include 33 cases in each group. For our statistical analysis, we implemented a generalized mixed linear model to address potential confounders, considering baseline values, time effects, and group-dependent interaction time. Also, the Shapiro-Wilk tests were used to confirm the normality of the data distribution. One-way analysis of variance (ANOVA) and the Kruskal-Wallis test were employed to analyze parametric and nonparametric continuous variables, respectively. When there was a statistically significant difference among the overall data, Bonferroni (B) was used for post hoc comparisons to determine the specific group differences. For the comparison of categorical variables, Fisher exact test was used, and pairwise comparisons were performed using the α-splitting method. Continuous variables were presented as mean±SD or median with interquartile range (IQR). Categorical variables were expressed as numbers (%). Statistical analyses were conducted using SPSS V.25.0 (IBM Corp., Armonk, New York, USA). Statistical significance was set at 2-sided tests with (P)<0.05 (with α-splitting <0.017). We used a conservative approach for handling missing data in secondary outcomes, relying on available, nonmissing data points for specific statistical tests. For baseline characteristics like age, height, weight, BMI, and ASA Grade, we are now reporting standardized differences between groups. An absolute standardized difference exceeding 0.1 is considered indicative of any imbalance among the groups.
RESULTS
The trial spanned 6 months and enrolled 99 eligible patients from November 7, 2022 to April 18, 2023. Five patients were excluded from statistical analysis: 2 refused postsurgery outcome evaluation, 2 patients in the FNB group experienced catheter dislodgment within the first 24 hours, and 1 patient in the adductor canal block (ACB) group requested catheter removal due to experiencing chest tightness within the first 24 hours. Three patients did not have data included in the analysis for end points after and including 48 hours: 1 in the FTB group and 1 in the FNB group due to catheter dislodgment between 24 and 48 hours, and 1 ACB patient was discharged early within 48 hours. One patient refused to be assessed during an outing at 48 hours postoperatively. Patients discharged between 48 and 72 hours were contacted through video calls to obtain their NRS scores; quadriceps strength, ROM, and TUG were no longer collected. Ninety-four valid primary end points data were analyzed, including 31 cases from the FNB group, 31 cases from the FTB group, and 32 cases from the ACB group. No postoperative complications were observed in any of the 3 groups. The patient flow through the study is depicted in Figure 2.
FIGURE 2.
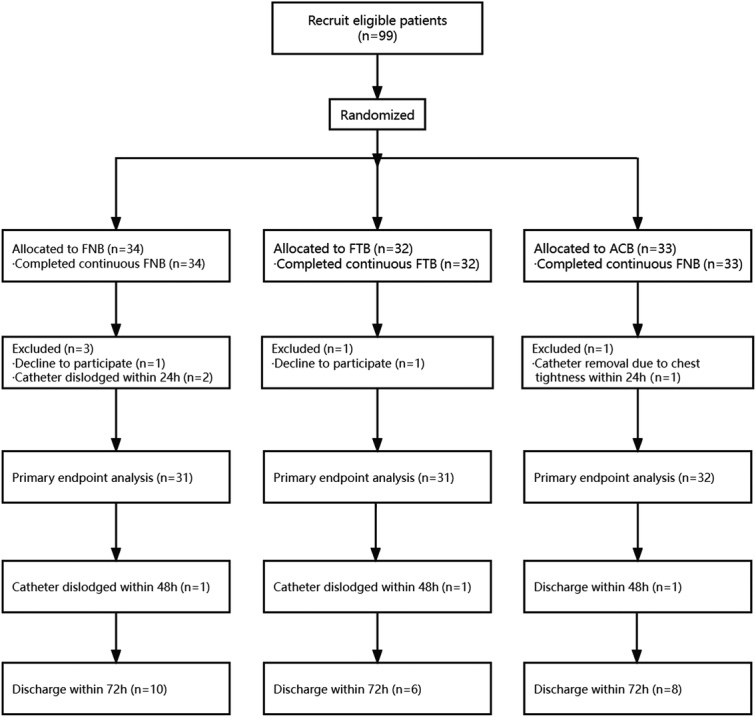
Consolidated standards of reporting trials flow diagram. ACB indicates adductor canal block; FNB, femoral nerve block; FTB, femoral triangle block.
Patient characteristics, operative data, and baseline assessments did not show statistically significant differences (Tables 1 and 2). Analysis utilizing the Generalized Mixed Linear Model indicated that most predictive variables, including group, time, and clinical factors, were not significant determinants of the NRS scores at rest. However, significant temporal effects on NRS scores during movement were observed at 24, 48, and 72 hours postoperation. Notably, at 48 and 72 hours postoperation, Group 3 (ACB) reported significantly higher NRS scores during movement than the control group (Group 1, FNB) as detailed in Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CJP/B107.
TABLE 1.
Patient Characteristics
| Comparison | Standardized difference | |
|---|---|---|
| Age | 1-FNB vs. 2-FTB | 0.059 |
| 1-FNB vs. 3-ACB | −0.088 | |
| 2-FTB vs. 3-ACB | −0.158 | |
| Height | 1-FNB vs. 2-FTB | −0.135 |
| 1-FNB vs. 3-ACB | −0.035 | |
| 2-FTB vs. 3-ACB | 0.088 | |
| Weight | 1-FNB vs. 2-FTB | −0.164 |
| 1-FNB vs. 3-ACB | −0.104 | |
| 2-FTB vs. 3-ACB | 0.051 | |
| BMI | 1-FNB vs. 2-FTB | −0.124 |
| 1-FNB vs. 3-ACB | −0.114 | |
| 2-FTB vs. 3-ACB | 0.006 | |
| ASA | 1-FNB vs. 2-FTB | −0.006 |
| 1-FNB vs. 3-ACB | 0.012 | |
| 2-FTB vs. 3-ACB | 0.006 |
An absolute SD higher than 0.1 is usually considered to indicate imbalance.ACB indicates adductor canal block; ASA, American Society of Anesthesiologists; BMI, body mass index; FNB, femoral nerve block; FTB, femoral triangle block.
TABLE 2.
The Baseline Values, Surgery, and Anesthesia Information
| Characteristics | 1∶ 2∶ 3 | 1-FNB | 2-FTB | 3-ACB | P |
|---|---|---|---|---|---|
| Operation time (min), mean±SD | 31∶ 31∶ 32 | 78.39±23.21 | 74.68±18.57 | 81.50±23.02 | 0.377 |
| Anesthesia time (min), mean±SD | 31∶ 31∶ 32 | 116.16±34.14 | 110.00±28.49 | 117.97±29.97 | 0.567 |
| Preoperative NRS score at rest M (P25, P75) | 31∶ 31∶ 32 | 1 (0, 2) | 0 (0, 1) | 0 (0, 1) | 0.447 |
| Preoperative NRS score at movement M (P25, P75) | 31∶ 31∶ 32 | 4 (2, 5) | 4 (3, 6) | 4 (4, 5) | 0.295 |
| Preoperative quadriceps strength M (P25, P75) | 31∶ 31∶ 32 | 4 (4, 4) | 4 (4, 4) | 4 (4, 4) | 0.350 |
| Dosage of propofol(mg), mean±SD | 31∶ 31∶ 32 | 307.82±115.14 | 287.90±82.68 | 266.77±51.07 | 0.180 |
| Dosage of vecuronium bromide (mg), mean±SD | 31∶ 31∶ 32 | 8.1±0.87 | 8.0±0.73 | 7.88±1.52 | 0.726 |
| Dosage of remifentanil (mg), mean±SD | 31∶ 31∶ 32 | 0.66±0.21 | 0.66±0.20 | 0.67±0.20 | 0.950 |
| Dosage of sufentanil (ug), mean±SD | 31∶ 31∶ 32 | 42.74±3.38 | 42.74±4.44 | 43.13±3.05 | 0.891 |
Values are presented as median (IQR) and mean±SD. 1-FNB; 2-FTB; and 3-ACB.
ACB indicates abductor canal block; FNB, femoral nerve block; FTB, femoral triangle block; NRS, numerical rating scale.
For the primary end point, we observed statistically significant differences between groups (H=7.003, P=0.03). Post hoc analysis with the Bonferroni correction indicated that the FNB group had a median pain score of 3 (2, 4), which was significantly lower than the 4 (3, 5) reported by the ACB group (Bonferroni-adjusted P=0.03). There were no significant differences between the FNB and FTB groups statistically (Bonferroni-adjusted P>0.99), nor between the ACB and FTB groups (Bonferroni-adjusted P=0.215), as shown in Figure 3.
FIGURE 3.
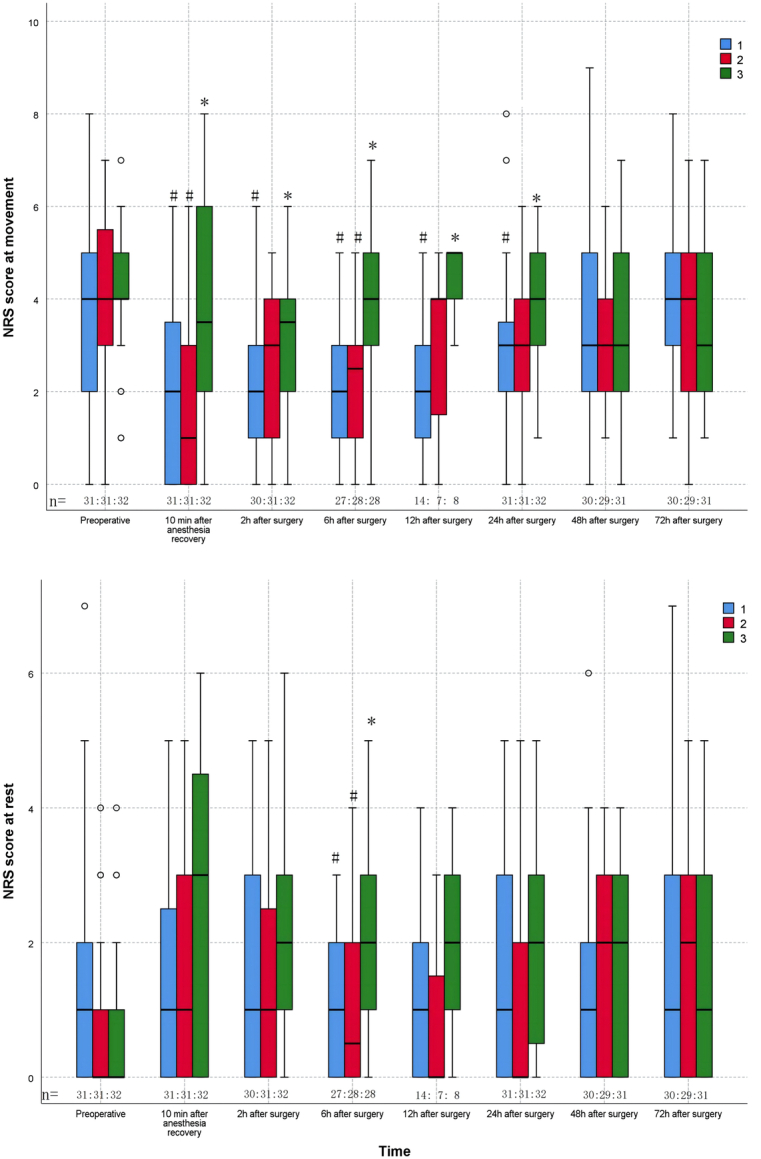
The NRS scores. Boxplot represents the median with the 25th/75th percentile. Whiskers reveal the minimum/maximum values, Points represent the outliers. 1-FNB; 2-FTB; 3-ACB.*P<0.05 between the FNB and the other groups in the post hoc analysis; # P<0.05 between the ACB and the other groups in the post hoc analysis. ACB indicates adductor canal block; FNB, femoral nerve block; FTB, femoral triangle block; NRS, numeric rating scale.
For the secondary end points (Table 3, Fig. 3), the FNB group had lower NRS scores than the ACB group at 6 hours (1[0, 2] vs. 2[1, 3], Bonferroni-adjusted P=0.016) postoperatively at rest, the FNB group had lower NRS scores than the ACB group at 10 minutes after anesthesia recovery (2 [0, 4] vs. 3.5 [2, 6], Bonferroni-adjusted P=0.014), 2 hours (2 [1, 3.25] vs 3.5 [2, 4], Bonferroni-adjusted P=0.03), 6 hours (2 [1, 3] vs. 4 [3, 5], Bonferroni-adjusted P<0.001), 12 hours (2 [1, 3] vs. 5 [4, 5], Bonferroni-adjusted P=0.003), 24 hours (3 [2, 4] vs. 4 [3, 5], Bonferroni-adjusted P=0.03) postoperatively at movement. The FTB group had lower NRS scores than the ACB group at 6 hours (0.5 [0, 2] vs. 2 [1, 3], Bonferroni-adjusted P=0.017) postoperatively at rest, 10 minutes after anesthesia recovery (1 [0, 3] vs. 3.5 [2, 6] Bonferroni-adjusted P=0.03) and 6 hours (2.5 [1, 3] vs. 4 [3, 5], Bonferroni-adjusted P=0.01) postoperatively at movement. There were nonsignificant statistical differences in NRS scores between the FNB group and the FTB group at any time point. At 6 hours (4 [3, 4] vs. 3 [2, 3], Bonferroni-adjusted P=0.001), 24 hours (4 [4,4] vs. 3 [2, 4], Bonferroni-adjusted P=0.00), and 48 hours (4 [4, 4] vs. 3 [2, 4], Bonferroni-adjusted P=0.01) postoperatively, quadriceps strength was higher in the FTB group compared with the FNB group, there were nonsignificant statistical differences between FTB and ACB group. At 24 hours postoperatively, the ACB group had higher quadriceps muscle strength than the FNB group (4 [3, 4] vs. 3 [2, 4], Bonferroni-adjusted P<0.001). There were nonsignificant statistical differences among the 3 groups in terms of the TUG and ROM results, times of effective PCNA pressed, the dosage and frequency of postoperative rescue analgesic drugs, or postoperative complications. However, at 24 hours postoperatively, the completion of TUG was better in the FTB (29 [93.5%] vs. 16 [51.6%], P<0.001) and ACB (26 [81.3%] vs. 16 [51.6%], P=0.012) groups compared with the FNB group (Supplemental Table 2, Supplemental Digital Content 2, http://links.lww.com/CJP/B108).
TABLE 3.
Quadriceps Strength, the Times of Effective PCNA Pressed, TUG, ROM, the Dosage and Frequency of Rescue Analgesic Drugs within 72 h Postoperatively, Postoperative Complications
| End points | 1∶ 2∶ 3 | 1-FNB | 2-FTB | 3-ACB | P |
|---|---|---|---|---|---|
| Quadriceps strength M (P25, P75) | |||||
| Preoperative | 31∶ 31∶ 32 | 4 (4, 4) | 4 (4, 4) | 4 (4, 4) | 0.350 |
| 10 min after anesthesia recovery | 31∶ 31∶ 32 | 2 (2, 3) | 3 (2, 4) | 3 (2, 4) | 0.05 |
| 2 h postoperatively | 30∶ 31∶ 32 | 3 (2, 3.25) | 3 (3, 4) | 3 (2, 4) | 0.165 |
| 6 h postoperatively | 27∶ 28∶ 28 | 3 (2, 3) | 4 (3, 4)* | 3 (2, 4) | 0.001 |
| 12 h postoperatively | 14∶ 7∶ 8 | 3 (2, 3.25) | 4 (3, 4) | 3.5 (3, 4) | 0.228 |
| 24 h postoperatively | 31∶ 31∶ 32 | 3 (2, 4)† | 4 (4, 4)* | 4 (3, 4)* | 0.001 |
| 48 h postoperatively | 30∶ 29∶ 31 | 3 (2, 4) | 4 (4, 4)* | 4 (3, 4) | 0.009 |
| 72 h postoperatively | 30∶ 29∶ 31 | 4 (3, 4) | 4 (4, 4) | 4 (3, 4) | 0.173 |
| The times of effective PCNA pressed M (P25, P75) | |||||
| 2 h postoperatively | 31∶ 31∶ 32 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.514 |
| 6 h postoperatively | 31∶ 31∶ 32 | 0 (0, 0) | 0 (0, 1) | 0 (0, 0) | 0.615 |
| 12 h postoperatively | 31∶ 31∶ 32 | 0 (0, 0) | 0 (0, 1) | 0 (0, 0) | 0.674 |
| 24 h postoperatively | 31∶ 31∶ 32 | 0 (0, 0) | 0 (0, 1) | 0 (0, 1) | 0.717 |
| 48 h postoperatively | 30∶ 30∶ 31 | 0 (0, 0) | 0 (0, 1) | 0 (0, 1) | 0.178 |
| TUG (s) mean±SD or M (P25, P75) | |||||
| 24 h postoperatively | 16∶ 29∶ 26 | 64.40 (38.55, 80.36) | 47.22 (37.69, 67.86) | 49.33 (41.30, 71.94) | 0.787 |
| 48 h postoperatively | 23∶ 29∶ 27 | 36.58 (30.30, 76.07) | 38.87 (30.52, 54.75) | 41,57 (31.39, 56.96) | 0.878 |
| 72 h postoperatively | 15∶ 22∶ 21 | 48.47 (25.21, 57.42) | 35.35 (25.03, 50.44) | 41.09 (31.98, 53.00) | 0.731 |
| ROM (°) Mean±SD | |||||
| 24 h postoperatively | 24∶ 30∶ 30 | 85.42±16.05 | 85.83±15.50 | 80.73±16.24 | 0.401 |
| 48 h postoperatively | 27∶ 29∶ 29 | 88.07±18.82 | 85.48±11.74 | 88.97±11.31 | 0.629 |
| 72 h postoperatively | 18∶ 23∶ 23 | 82.94±20.99 | 83.13±13.19 | 88.00±11.01 | 0.459 |
| The dosage of rescue analgesic drugs within 72 h postoperatively (mg) M (P25, P75) | 30∶ 30∶ 31 | 5.375 (0, 18.75) | 7.25 (0, 18.75) | 11.25 (0, 18.75) | 0.531 |
| The frequency of rescue analgesic drugs within 72 h postoperatively (times) M (P25, P75) | 30∶ 30∶ 31 | 1 (0, 5) | 2 (0, 5) | 2 (0, 5) | 0.865 |
| Postoperative Complications (%) | |||||
| Catheter Dislodgment | 34∶ 32∶ 33 | 3 (8.82) | 1 (3.13) | 0 (0) | 0.177 |
| PONV | 31∶ 31∶ 32 | 0 (0) | 0 (0) | 0 (0) | >0.99 |
| Puncture site infection | 31∶ 31∶ 32 | 0 (0) | 0 (0) | 0 (0) | >0.99 |
| puncture site hematoma | 31∶ 31∶ 32 | 0 (0) | 0 (0) | 0 (0) | >0.99 |
| Postoperative Fall | 31∶ 31∶ 32 | 0 (0) | 0 (0) | 0 (0) | >0.99 |
Values are presented as median (IQR), mean±SD, and percentages. 1-FNB; 2-FTB; 3-ACB.
P<0.05 between the FNB and the other groups in the post hoc analysis.
P<0.05 between the ACB and the other groups in the post hoc analysis.
ACB indicates abductor canal block; FNB, femoral nerve block; FTB, femoral triangle block; PCNA, patient-controlled nerve analgesia; ROM, range of motion; TUG, time up to go.
DISCUSSION
The blockade of the FN and its branches is critical in managing postoperative pain following TKA.2,11 Continuous nerve blocks, in particular, offer sustained and stable analgesia postoperatively.1,2 In this study, the Generalized Mixed Linear Model analysis revealed that most predictive variables, including group, time, and other clinical factors, did not significantly impact the NRS scores at rest. However, statistically significant time-related effects were observed on NRS scores during movement, especially at 24-, 48-, and 72 hours postoperation. This indicates that postoperative pain following TKA tends to be more pronounced during this period. Notably, Group 3 exhibited significantly higher NRS scores during movement compared with the control group (Group 1) at 48 and 72 hours, underscoring the need for targeted pain management strategies for certain patient groups at these specific postoperative periods. The overall significance of the model confirms its suitability for our data analysis. FNB provided excellent postoperative analgesia, outperforming the ACB at various time points after surgery. However, ACB showed superiority over FNB only in terms of postoperative 24-hour quadriceps strength and completion of the TUG test. There were nonsignificant statistical differences in NRS scores between the FNB group and the FTB group at any time point, while FTB significantly preserved muscle strength compared with the FNB, are similar to those of Jerome Guilley et al.20,21
The FN is a major sensory nerve in the thigh, supplying the anterior aspect of the thigh, the knee joint, and the anterior and medial aspects of the lower leg. FNB performed at the level of the IL can comprehensively block the FN and cover a wider area of nerve distribution, thus providing more comprehensive pain relief.2 In addition, the FN provides neural innervation to the quadriceps muscle group, playing a crucial role in knee extension and overall lower limb function. Blocking the FN can reduce the contraction of the quadriceps muscles, thereby alleviating pain during postoperative activities involving knee joint movement.22 However, this comes at the cost of a significant decrease in quadriceps muscle strength and an increased risk of falls. This can, to some extent, impact postoperative mobility and rehabilitation training, leading to delayed recovery after surgery.7,8,20
In the context of TKA, understanding the anatomy relevant to nerve blocks is crucial for effective pain management and minimizing complications. The branches of the FN occur ~3.1 cm distal to the inguinal ligament within the FT,14 the motor branches of the FN to the rectus femoris and vastus muscles originate in the Iliopectineal fossa. The apex of this region is demarcated by the intersection of the medial border of the SM and the lateral border of the AL. The FTB is a subsartorial injection anterolateral to the Femoral Artery, targeting the SN, the nerve to the Vastus Medialis, and the medial femoral cutaneous nerve; FTB does not completely block the FN,11,14,23,24 which may help mitigate issues related to muscle strength impairment after nerve blockade and reduce damage caused by the puncture.11,14,25 The mid-FTB can achieve similar analgesic effects to the FNB by blocking the MVN, the MFCN, and the SN, which play an important role in the domination of the knee joint.10,11,23 So FTB can offer effective postoperative analgesia while avoiding significant declines in quadriceps strength,15,26 a key consideration given that over 50% of TKA patients experience arthrogenic muscular inhibition (AMI).27 AMI arises from changes in articular sensory receptor discharge due to inflammation, joint laxity, and surgical trauma, affecting both spinal reflex and supraspinal pathways, irrespective of perineural catheter presence. Conversely, the primary target nerve for the ACB is the SN,11 the only nerve that is consistently observed in the AC. The medial genicular branch from the medial vastus nerve runs into a fascial tunnel proximal to the entrance of the AC between the MV and the AL outside the AC in 90% of human subjects.24 The posterior branch of the obturator nerve can also emerge in the AC, sometimes innervating the anteromedial knee capsule alongside the SN and the MVN.28 The MVN significantly contributes to the innervation of the knee joint capsule and the medial side subcutaneous tissues, whereas the SN plays a relatively minor role in knee joint innervation.10 A solitary SN block is often insufficient for substantial knee joint analgesia in major surgeries like TKA. Therefore, during ACB, particularly with catheter placement, only the SN is blocked within the AC, leading to potentially limited efficacy of the ACB.10,11,23
Theoretically, ACB should provide excellent muscle protection as it targets the SN without affecting other nerves that influence quadriceps strength, injecting a local anesthetic into the true AC along the vastoadductor membrane.23 However, this efficacy may be limited to small-dose injections within the AC. Because the AC is connected to the FT through the entrance of the AC, when using a continuous nerve block with a large volume of local anesthetic, the local anesthetic may spread upward to the FT, blocking other femoral nerve branches and causing a decline in quadriceps muscle strength. In addition, the AC maintains anatomic continuity with the popliteal fossa, so the downward spread of local anesthetics within the AC may also affect the sciatic nerve and, in turn, impact quadriceps muscle strength,29–32 and this impact could even be long-lasting.33 In addition, in the context of our study, the insertion position of the ACB was similar to that used in the study by Bora Lee,16 where continuous distal ACB (similar to our study) showed significant reductions in NRS scores during rest and early morning on postoperative day 2 (POD2) compared with continuous FTB. They proposed that administering local anesthetics continuously to the distal AC could enhance analgesic effects on posterior knee pain by spreading into the popliteal fossa. However, our study’s blocking position for FTB was higher than in their study, possibly providing a more effective blockade of the MVN and the MFCN.11 Moreover, the use of LIA in our protocol might have masked the advantages of ACB spreading to the popliteal fossa by affecting genicular nerves from the fibular and tibial nerves, although for a duration of less than 6 hours.2,31 Moreover, the analgesic effect of ACB is inferior to that of FTB and FNB, resulting in patients experiencing more pain when attempting forceful thigh lifting. Consequently, patients unconsciously reduce exertion, leading to a clinical manifestation of decreased quadriceps muscle strength.
In summary, although FNB can provide excellent postoperative analgesia, its adverse effects on quadriceps strength pose challenges for patients to actively engage in rehabilitation exercises and weight-bearing activities without the assistance of rehabilitation specialists, potentially prolonging hospital stays and increasing complication risks.3–5,7,8 Conversely, FTB offers comparable postoperative analgesia to FNB but significantly lessens the impact on quadriceps strength. This dual benefit makes FTB a more favorable option for pain relief and safer postoperative rehabilitation.11 In comparison to ACB, which necessitates precise anatomic localization and could pose technical challenges, FTB covers a wider area and is relatively simpler to administer. The entrance of the adductor canal, now understood to be closer to the lower third of the thigh,13 complicates ACB placement, potentially nearing the knee arthroplasty incision site. This lower insertion point may hinder dressing and catheter tunnel placement, affecting surgical disinfection and operative space. Therefore, in our opinion, continuous FTB emerges as a more suitable method for postoperative pain management in TKA. It effectively alleviates pain while preserving quadriceps strength and offers extensive nerve blockade without significantly interfering with surgical procedures.
This study has some limitations. Firstly, the use of subjective measures like NRS scores and MMT may introduce accuracy issues in the evaluation. Secondly, being a single-center randomized controlled study with participants from the same region and race, its findings may lack generalizability to different regions or races. Therefore, further studies involving diverse regions and ethnicities are necessary to validate the results. In addition, our choice of ACB location was strategically determined to minimize surgical interference, placing it 3 cm below the entrance of the AC rather than the middle section, which is traditionally considered optimal.10 This may increase the probability of local anesthetic spreading to the FT and decrease the chances of it spreading to the popliteal fossa. This could potentially impact postoperative analgesic effect and muscle strength since the spread of local anesthetic to the popliteal fossa may improve the blockage of sensory fibers involved in posterior knee capsule innervations.31,32,34 Moreover, our study did not adopt the AC catheter placement method aligned parallel to the saphenous nerve, which has been shown to reduce catheter migration rates and improve range of motion (ROM) and analgesic outcomes post-TKA.35
CONCLUSIONS
Continuous FTB can provide postoperative analgesia comparable to continuous FNB and is superior to ACB. Regarding muscle strength preservation, FNB significantly affects quadriceps muscle strength, while FTB and ACB effectively protect quadriceps muscle strength. There were no differences in opioid consumption among the 3 continuous catheter blocks.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the Department of Orthopedics-Joint Surgery, Nursing and Rehabilitation Team of the First Affiliated Hospital of Chongqing Medical University for their help during the trial.
Footnotes
This study was supported by the Chongqing Municipal Public Health Bureau, Chongqing People’s Municipal Government, 2022WSJK117, and the Future Medical Youth Innovation Team Development Support Program of Chongqing Medical University, W0004.
The authors declare no conflict of interest.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.clinicalpain.com.
Contributor Information
Minghe Tan, Email: tan17725021265@163.com.
Bozhou Chen, Email: 437069051@qq.com.
Qingshu Li, Email: 101773@cqmu.edu.cn.
Siqi Wang, Email: 375041474@qq.com.
Daiyu Chen, Email: chendaiyu_1996@163.com.
Maoji Zhao, Email: zhaomaoji34014@163.com.
Jun Cao, Email: caojun@hospital.cqmu.edu.cn.
REFERENCES
- 1. Fischer HBJ, Simanski CJP, Sharp C, et al. A procedure-specific systematic review and consensus recommendations for postoperative analgesia following total knee arthroplasty. Anaesthesia. 2008;63:1105–1123. [DOI] [PubMed] [Google Scholar]
- 2. Lavand’homme PM, Kehlet H, Rawal N, et al. Pain management after total knee arthroplasty: PROcedure SPEcific Postoperative Pain ManagemenT recommendations. Eur J Anaesthesiol. 2022;39:743–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lo LWT, Suh J, Chen JY, et al. Early postoperative pain after total knee arthroplasty is associated with subsequent poorer functional outcomes and lower satisfaction. J Arthroplasty. 2021;36:2466–2472. [DOI] [PubMed] [Google Scholar]
- 4. Grosu I, Lavand’homme P, Thienpont E. Pain after knee arthroplasty: an unresolved issue. Knee Surg Sports Traumatol Arthrosc. 2014;22:1744–1758. [DOI] [PubMed] [Google Scholar]
- 5. Bourne RB, Chesworth BM, Davis AM, et al. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elmallah RK, Cherian JJ, Pierce TP, et al. New and common perioperative pain management techniques in total knee arthroplasty. J Knee Surg. 2016;29:169–178. [DOI] [PubMed] [Google Scholar]
- 7. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block: a double-blinded randomized controlled study. Anesth Analg. 2016;122:1696–1703. [DOI] [PubMed] [Google Scholar]
- 8. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111:1552–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Angers M, Belzile EL, Vachon J, et al. Negative influence of femoral nerve block on quadriceps strength recovery following total knee replacement: a prospective randomized trial. Orthop Traumatol Surg Res. 2019;105:633–637. [DOI] [PubMed] [Google Scholar]
- 10. Burckett-St Laurant D, Peng P, Girón Arango L, et al. The nerves of the adductor canal and the innervation of the knee: an anatomic study. Reg Anesth Pain Med. 2016;41:321–327. [DOI] [PubMed] [Google Scholar]
- 11. Woodworth GE, Arner A, Nelsen S, et al. Pro and con: How important is the exact location of adductor canal and femoral triangle blocks? Anesth Analg. 2023;136:458–469. [DOI] [PubMed] [Google Scholar]
- 12. Kuang M-J, Xu L-Y, Ma J-X, et al. Adductor canal block versus continuous femoral nerve block in primary total knee arthroplasty: a meta-analysis. Int J Surg (London, England). 2016;31:17–24. [DOI] [PubMed] [Google Scholar]
- 13. Wong WY, Bjørn S, Strid JMC, et al. Defining the location of the adductor canal using ultrasound. Reg Anesth Pain Med. 2017;42:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lonchena TK, McFadden K, Orebaugh SL. Correlation of ultrasound appearance, gross anatomy, and histology of the femoral nerve at the femoral triangle. Surg Radiol Anat. 2016;38:115–122. [DOI] [PubMed] [Google Scholar]
- 15. Layera S, Aliste J, Bravo D, et al. Motor-sparing nerve blocks for total knee replacement: a scoping review. J Clin Anesth. 2021;68:110076. [DOI] [PubMed] [Google Scholar]
- 16. Lee B, Park SJ, Park KK, et al. Optimal location for continuous catheter analgesia among the femoral triangle, proximal, or distal adductor canal after total knee arthroplasty: a randomized double-blind controlled trial. Reg Anesth Pain Med. 2022;47:353–358. [DOI] [PubMed] [Google Scholar]
- 17. Chuan A, Lansdown A, Brick KL, et al. Adductor canal versus femoral triangle anatomical locations for continuous catheter analgesia after total knee arthroplasty: a multicentre randomised controlled study. Br J Anaesth. 2019;123:360–367. [DOI] [PubMed] [Google Scholar]
- 18. Yeung TSM, Wessel J, Stratford PW, et al. The timed up and go test for use on an inpatient orthopaedic rehabilitation ward. J Orthop Sports Phys Ther. 2008;38:410–417. [DOI] [PubMed] [Google Scholar]
- 19. Alghadir AH, Anwer S, Iqbal A, et al. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res. 2018;11:851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qin L, You D, Zhao G, et al. A comparison of analgesic techniques for total knee arthroplasty: a network meta-analysis. J Clin Anesth. 2021;71:110257. [DOI] [PubMed] [Google Scholar]
- 21. Guilley J, Besançon I, Hivert A, et al. Femoral nerve inguinal approach versus proximal femoral triangle ap proach for continuous regional analgesia in active rehabilitation after total knee arthroplasty: a prospective, randomised study. Anaesth Crit Care Pain Med. 2022;41:101043. [DOI] [PubMed] [Google Scholar]
- 22. Szczukowski MJ, Hines JA, Snell JA, et al. Femoral nerve block for total knee arthroplasty patients: a method to control postoperative pain. J Arthropl. 2004;19:720–725. [DOI] [PubMed] [Google Scholar]
- 23. Bendtsen TF, Moriggl B, Chan V, et al. The optimal analgesic block for total knee arthroplasty. Reg Anesth Pain Med. 2016:711–719. [DOI] [PubMed] [Google Scholar]
- 24. Andersen HL, Andersen SL, Tranum-Jensen J. The spread of injectate during saphenous nerve block at the adductor canal: a cadaver study. Acta Anaesthesiol Scand. 2015;59:238–245. [DOI] [PubMed] [Google Scholar]
- 25. Ishiguro S, Yokochi A, Yoshioka K, et al. Technical communication: anatomy and clinical implications of ultrasound-guided selective femoral nerve block. Anesth Analg. 2012;115:1467–1470. [DOI] [PubMed] [Google Scholar]
- 26. Ilfeld BM, McCartney CJL. Searching for the optimal pain management technique after knee arthroplasty: Analgesia is just the tip of the iceberg. Anesthesiology. 2017;126:768–770. [DOI] [PubMed] [Google Scholar]
- 27. Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum. 2010;40:250–266. [DOI] [PubMed] [Google Scholar]
- 28. Tran J, Giron Arango L, Peng P, et al. Evaluation of the proximal adductor canal block injectate spread: a cadaveric study. Reg Anesth Pain Med. 2019;44:689–694. [DOI] [PubMed] [Google Scholar]
- 29. Goffin P, Lecoq J-P, Ninane V, et al. Interfascial spread of injectate after adductor canal injection in fresh human cadavers. Anesth Analg. 2016;123:501–503. [DOI] [PubMed] [Google Scholar]
- 30. Gautier PE, Lecoq J-P, Vandepitte C, et al. Impairment of sciatic nerve function during adductor canal block. Reg Anesth Pain Med. 2015;40:85–89. [DOI] [PubMed] [Google Scholar]
- 31. Gautier PE, Hadzic A, Lecoq J-P, et al. Distribution of injectate and sensory-motor blockade after adductor canal block. Anesth Analg. 2016;122:279–282. [DOI] [PubMed] [Google Scholar]
- 32. Runge C, Moriggl B, Børglum J, et al. The spread of ultrasound-guided injectate from the adductor canal to the genicular branch of the posterior obturator nerve and the popliteal plexus: a cadaveric study. Reg Anesth Pain Med. 2017;42:725–730. [DOI] [PubMed] [Google Scholar]
- 33. Sengoku T, Nakase J, Mizuno Y, et al. Outcome comparison of femoral nerve block and adductor canal block during anterior cruciate ligament reconstruction: Adductor canal block may cause an unexpected decrease in knee flexor strength at 6 months postoperatively. Arch Orthop Trauma Surg. 2023;143:6305–6313. [DOI] [PubMed] [Google Scholar]
- 34. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126:923–937. [DOI] [PubMed] [Google Scholar]
- 35. Fujino T, Yoshida T, Kawagoe I, et al. Migration rate of proximal adductor canal block catheters placed parallel versus perpendicular to the nerve after total knee arthroplasty: a randomized controlled study. Reg Anesth Pain Med. 2023;48:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]