Abstract
BACKGROUND:
Heart failure triggers a shift in myocardial metabolic substrate utilization, favoring the ketone body 3-hydroxybutyrate as energy source. We hypothesized that 14-day treatment with ketone ester (KE) would improve resting and exercise hemodynamics and exercise capacity in patients with heart failure with reduced ejection fraction.
METHODS:
In a randomized, double-blind cross-over study, nondiabetic patients with heart failure with reduced ejection fraction received 14-day KE and 14-day isocaloric non-KE comparator regimens of 4 daily doses separated by a 14-day washout period. After each treatment period, participants underwent right heart catheterization, echocardiography, and blood sampling at plasma trough levels and after dosing. Participants underwent an exercise hemodynamic assessment after a second dosing. The primary end point was resting cardiac output (CO). Secondary end points included resting and exercise pulmonary capillary wedge pressure and peak exercise CO and metabolic equivalents.
RESULTS:
We included 24 patients with heart failure with reduced ejection fraction (17 men; 65±9 years of age; all White). Resting CO at trough levels was higher after KE compared with isocaloric comparator (5.2±1.1 L/min versus 5.0±1.1 L/min; difference, 0.3 L/min [95% CI, 0.1–0.5), and pulmonary capillary wedge pressure was lower (8±3 mm Hg versus 11±3 mm Hg; difference, −2 mm Hg [95% CI, −4 to −1]). These changes were amplified after KE dosing. Across all exercise intensities, KE treatment was associated with lower mean exercise pulmonary capillary wedge pressure (−3 mm Hg [95% CI, −5 to −1] ) and higher mean CO (0.5 L/min [95% CI, 0.1–0.8]), significantly different at low to moderate steady-state exercise but not at peak. Metabolic equivalents remained similar between treatments. In exploratory analyses, KE treatment was associated with 18% lower NT-proBNP (N-terminal pro-B-type natriuretic peptide; difference, −98 ng/L [95% CI, −185 to −23]), higher left ventricular ejection fraction (37±5 versus 34±5%; P=0.01), and lower left atrial and ventricular volumes.
CONCLUSIONS:
KE treatment for 14 days was associated with higher CO at rest and lower filling pressures, cardiac volumes, and NT-proBNP levels compared with isocaloric comparator. These changes persisted during exercise and were achieved on top of optimal medical therapy. Sustained modulation of circulating ketone bodies is a potential treatment principle in patients with heart failure with reduced ejection fraction.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT05161650.
Keywords: cardiac output, echocardiography, heart failure, hemodynamics, ketone bodies, metabolism
Clinical Perspective.
What Is New?
Treatment with ketone ester for 14 days was associated with more favorable measures of selected hemodynamics at rest and during exercise in patients with heart failure with reduced ejection fraction.
Improvement in selected hemodynamic parameters at rest was evident at predose 3-hydroxybutyrate levels, demonstrated by increased cardiac output and elevated left ventricular ejection fraction.
This coincided with reduced measures of congestion, including pulmonary capillary wedge pressure, left atrial and ventricular volumes, and natriuretic peptides.
The cardiovascular effects were further amplified after ketone ester dosing at rest and persisted during incremental exercise.
What Are the Clinical Implications?
The results of this study demonstrate favorable cardiovascular effects of 14-day ketone ester treatment in patients with stable heart failure with reduced ejection fraction, achieved on top of optimal medical heart failure therapy. It highlights sustained modulation of circulating ketone bodies as a novel treatment principle.
These results prompt the need for larger long-term trials to assess the clinical benefits of ketone ester administration in patients with HFrEF.
Editorial, see p 1490
As a metabolic omnivore, the healthy heart seamlessly transitions between various metabolic substrates to sustain energy production and maintain contractile function.1 However, a hallmark of heart failure (HF) is significant metabolic remodeling with increasing reliance on alternative substrates such as ketone bodies.2,3 Recent evidence indicates that the ketone body 3-hydroxybutyrate (3-OHB) plays a pivotal role as an alternative metabolic substrate in the failing heart.4 In patients with advanced HF with reduced ejection fraction (HFrEF), myocardial ketolytic enzymes and utilization of 3-OHB are increased.5 Conversely, inducing overexpression of genes essential for myocardial 3-OHB utilization confers resistance against contractile dysfunction.6 Indeed, ketones are readily taken up by the myocardium and oxidized proportionally to circulating levels.7,8 Given the favorable energetic characteristics of 3-OHB and the adaptive response of the failing heart to increased ketone utilization,5,9 elevating circulating 3-OHB through persistent administration may provide cardiovascular benefits and improve cardiac oxidative metabolism, thereby potentially enhancing cardiac function.2
Acute infusion with 3-OHB significantly increases cardiac output (CO) and left ventricular (LV) ejection fraction (LVEF) in patients with HFrEF.10 Furthermore, a single dose of oral ketone ester (KE) induces favorable hemodynamic changes in patients with more advanced HF in cardiogenic shock.11 To date, no study has evaluated the hemodynamic effects of 3-OHB treatment beyond short-term treatment. We hypothesized that acute effects might persist with sustained treatment with KE over a period of 14 days, even at pharmacological trough levels, and during the stress of exercise. Therefore, we aimed to investigate the effects of a 14-day treatment regimen with KE on resting hemodynamics before and after short-term administration of KE, exercise hemodynamics and capacity, clinical symptoms, and safety parameters.
METHODS
This trial adhered to the principles of the Declaration of Helsinki with respect to design, execution, and reporting. The trial was approved by the local Danish ethics committee (1-10-72-362-18) and was conducted in accordance with good clinical practice guidelines. Written informed consent was obtained from all participating patients. The study is registered with ClinicalTrials.gov (NCT05161650).
Patients
We included patients with stable HFrEF (≥18 years of age) with LVEF ≤40% who were in New York Heart Association functional class II or III on optimal guideline-recommended HFrEF treatment from the outpatient HF clinic at Aarhus University Hospital, Denmark. Key exclusion criteria comprised history of diabetes, pregnancy, significant cardiac valve disease, severe stable angina pectoris, severe kidney disease (estimated glomerular filtration rate <20 mL·min−1·1.73 m−2 and/or current dialysis) or liver disease, severe comorbidity as judged by the investigator, and inability to give informed consent (Supplemental Material).
Design
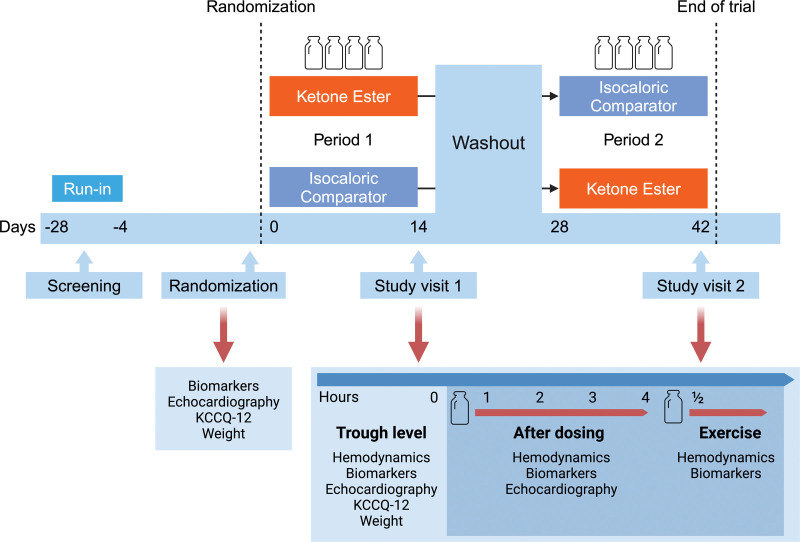
In this randomized, double-blind, comparator-controlled, cross-over trial, participants were randomized to oral KE drink (25 g; KE4 pro, Ketone Aid Inc) 4 times daily or an isocaloric, isovolumic, taste-matched non-KE comparator (IC; containing 1:3 kcal from carbohydrates and 2:3 kcal from fat; Table S1) 4 times daily for a 14-day period, followed by a 14-day washout period and cross-over to the other intervention. The 4-times-daily regimen aimed to sustain safe ketosis for at least 16 hours daily, considering an elimination half-life of 0.8 to 3.1 hours.12–14 Participants attended a study visit after each 14-day treatment period, during which they were evaluated with right heart catheterization, transthoracic echocardiography, venous blood samples, the Kansas City Cardiomyopathy Questionnaire, 12-item version (KCCQ-12), and exercise hemodynamics. Thus, each participant attended a total of 4 visits, including screening, randomization, and study visits 1 and 2 (Figure 1). A 1-day run-in period for each intervention was scheduled at the screening visit to ensure tolerability before definitive study enrollment (Figure S1). To uphold the blinding of interventions, it was concealed within identical plastic bottles placed inside an opaque, sealed container. Randomization was overseen by an independent, unblinded research team member without direct patient involvement. Each identical container received a unique randomization number linked to an identifier key accessible only to the unblinded research team member. Compliance with the intervention regimen was evaluated through a bottle count, a log form, and twice-weekly telephone contact; compliance >80% was deemed sufficient.15
Figure 1.
Study design. A randomized, double-blind, isocaloric comparator–controlled cross-over trial in 24 stable patients with heart failure with reduced ejection fraction. Participants underwent a 14-day treatment with oral ketone ester and an isocaloric comparator, after which they were evaluated with right-sided heart catheterization, echocardiography, blood sampling, Kansas City Cardiomyopathy Questionnaire (KCCQ-12), and exercise hemodynamics. Study periods were separated by a 14-day washout period. A 1-day run-in period of each intervention after screening was planned to ensure tolerability before enrollment.
Study Visits
During the randomization visit, after an overnight fast, participants underwent resting echocardiography, noninvasive blood pressure measurement, venous blood sampling, body weight recording and a KCCQ-12 was completed. Next, participants received 14-day study intervention to be initiated 1±4 days after randomization. After completing each 14-day treatment period, participants were examined on day 15 after an overnight fast and avoidance of strenuous physical activity for 48 hours. Participants were instructed to refrain from taking the study intervention medication on the day of their scheduled attendance while continuing their regular medical background therapy as prescribed. They arrived at 7 am, had their body weight measured, and completed a KCCQ-12. Next, baseline venous blood samples were collected. Then, echocardiography, noninvasive blood pressure measurements, and right heart catheterization measurements were performed at rest. After trough measurements, the allocated intervention was consumed, and hemodynamics, echocardiography, biomarkers, and 3-OHB were assessed at hourly intervals for a duration of 4 hours to assess pharmacodynamic effects after dosing. Finally, 30 minutes after administration of a second allocated intervention, a hemodynamic cardiopulmonary exercise test was conducted with the right heart catheter in situ.
Right Heart Catheterization
After local anesthesia, a 7.5F triple-lumen fluid-filled Swan-Ganz catheter was inserted through the right internal jugular vein under ultrasound guidance and advanced into the pulmonary artery. The correct wedge position was verified by fluoroscopy and characteristic waveforms. Pressure transducers were zeroed at the midaxillary level. Right atrial pressure (RAP), systolic pulmonary arterial pressure, mean pulmonary arterial pressure (mPAP), and pulmonary capillary wedge pressure (PCWP) were measured during end expiration with participants lying in supine position. Mixed venous saturation (Svo2) was measured (ABL800 FLEX) in blood gas collected from the pulmonary artery. CO was determined with the bolus thermodilution technique (averaged over ≥3 consecutive measurements within <10% variation) and indexed to body surface area (cardiac index). Systolic blood pressure (sBP), mean arterial pressure (MAP), and heart rate (HR) were monitored by sphygmomanometry; rate-pressure product was calculated (RPP=sBP×HR). Stroke volume (SV=CO/HR), systemic vascular resistance [SVR=(MAP−RAP)×80/CO], pulmonary vascular resistance [PVR=(mPAP−PCWP)×80/CO], and effective arterial elastance (Ea=0.9×sBP/SV) were calculated.16 End-systolic elastance (Ees) was estimated by the modified single-beat method,17 and the systolic vascular-ventricular coupling ratio (Ea/Ees) was calculated. Preload recruitable stroke work was calculated by the single-beat method,18 and LV stroke work was estimated and normalized to LV end-diastolic volume (LVEDV).19 Arteriovenous oxygen difference (AVo2 difference) was measured as the contrast between oxygen contents in systemic and pulmonary arterial blood. Plasma volume was estimated using the following formula: (1−hematocrit)×[α+(β×weight)].20 For women, the values were α=864 and β=47.9. The corresponding values for men were α=1530 and β=41.
Transthoracic Echocardiography
Two- and 3-dimensional echocardiography was conducted with a 3.5-MHz transducer on a Vivid E95 (General Electric) and stored for subsequent assessor-blinded analysis (EchoPAC version 204, General Electric). All echocardiographic parameters were analyzed as the mean of ≥3 cardiac cycles. LVEDV and LV end-systolic volume were assessed by 2-dimensional (ie, Simpson biplane) and 3-dimensional acquisition; LVEF was acquired. Other parameters included global longitudinal strain (absolute value), peak excursion velocity for systolic mitral plane (LV S’max), early (E) and late (A) diastolic mitral inflow velocities, E deceleration time and slope, early (e´) and late (a´) diastolic mitral plane tissue velocity, and tricuspid annular peak systolic excursion.21 Left atrial (LA) maximal volume and strain were attained as previously described.22
End-Diastolic Pressure-Volume Relationship
The LV end-diastolic pressure-volume relationship (EDPVR) was estimated at rest by the single-beat estimation technique. LV end-diastolic pressure (LVEDP) was approximated with PCWP, and LVEDV was determined by echocardiography. Unstressed LVEDV (V0), predicted LVEDV at LVEDP of 30 mm Hg (V30), and individual constants for diastolic curve fitting (α) and diastolic stiffness (β) were calculated (Supplemental Material).23 Then, the EDPVR was estimated by forcing the curve trough predefined points on the EDPVR (ie, LVEDP of 0, 2.5, 5, 10, 15, 20, 25, and 30 mm Hg), and the predicted V0 and V30 was calculated as LVEDP=α×LVEDV.
Blood Samples
Immediately after venous blood sampling, we analyzed hemoglobin, hematocrit, electrolytes, lactate, HCO3−, and pH (ABL800 FLEX). The remaining samples were stored at −80° C for subsequent batch analysis. NT-proBNP (N-terminal pro-B-type natriuretic peptide) was measured on a chemiluminescent microparticle immunoassay (Cobas 8000). Circulating 3-OHB levels were measured at randomization and on each study day during fasting, before dose (trough), after dosing, and during cardiopulmonary exercise test and quantified by hydrophilic interaction liquid chromatography tandem mass spectrometry. Estimated glomerular filtration rate was calculated by assessing cystatin C (turbidimetric assay; Atellica CH).24 Alanine transaminase was assessed by absorption photometry (Atellica CH). High-sensitivity cardiac troponin I was assessed with an immunoassay kit (Atellica IM).
Cardiopulmonary Exercise Test
The hemodynamic cardiopulmonary exercise test was performed using a semisupine bicycle (eBike L Ergometer, General Electric). Patients began at 0 W and incrementally increased the workload by 25 W every 3 minutes. If the investigator anticipated a maximal exercise capacity exceeding 100 W, 50-W increments were used. Participants followed the same protocol during both study visits. They maintained a pedal speed at 65±5 rounds/min until a predefined Borg scale rating of 18. Continuous 12-lead electrocardiography was applied. Oxygen uptake (Vo2) was assessed through breath-by-breath technique (JAEGER Vyntus CPX, CareFusion). At rest and peak exercise, mixed venous 3-OHB was measured as described. Peak oxygen uptake (peak Vo2/kg) was calculated as the highest 10-second averaged values within the final 30 seconds of the test. During semisupine rest and 90 seconds into each workload increment, the following parameters were measured: RAP, mPAP, PCWP, CO (by bolus thermodilution technique), pulse oximetry, MAP, HR, lactate, and Svo2. Furthermore, peak CO was estimated by the direct Fick method (CO=peak Vo2/AVo2 difference).
End Points
Given that persistent 3-OHB treatment for 14 days might restore myocardial energetic function,2,4,25 we hypothesized that such treatment would improve cardiac performance at rest. Thus, the primary end point was CO at rest after 14-day treatment with KE compared with IC. This measure is reliable and consistent26 and less influenced by the variability of patient effort or physical condition. Secondary end points included resting and exercise PCWP and peak exercise CO and metabolic equivalents. Exploratory end points included resting LVEF, NT-proBNP levels, peak Vo2, and KCCQ-12.
Power Calculation and Statistics
The coefficient of variation for CO at rest was expected to be 4%.26 By enrolling 24 patients, assuming a mean CO of 5.0 L/min, we expected to identify a relative difference of 9% in the primary end point with a power of 80% and a 2-sided significance level of 5%. The power analysis was based on a paired analysis adopting a mixed effects model fitting for comparisons within individuals and accommodating a within-participants correlation.
Data normality was tested by qq-plots and histograms. For continuous data, normally and nonnormally distributed data are presented as mean±SD and median (interquartile range), respectively, unless otherwise stated. Categorical data are displayed as count and frequency. Temporal effects are displayed as mean±SEM. A linear mixed model was exploited to analyze treatment effects of KE compared with IC, incorporating treatment, period, and treatment sequence as fixed effects and participants as random effects. Mixed-model residual normality and homoscedasticity were inspected. Logarithmic transformations were applied when appropriate and presented with geometric means and reverse-transformed geometric mean ratios. For repeated measures after intervention dosing at rest and during exercise, a treatment-by-time or treatment-by-workload interaction was added as a fixed effect to the model; period nested within participants was added as a random effect to allow individual variations in baseline values and variations in the impact of period among participants. Treatment effects are reported as pairwise difference (95% CI). Post hoc subgroup analyses were performed and are reported as mean between-treatment difference and 95% CI within each subgroup strata; the P value indicates subgroup interactions. Statistical significance was defined as 2-sided values of P<0.05. A detailed description of the statistical analysis plan is available in the Supplemental Material.
Data analysis and figures were generated with R software (version 2022.02.3). The primary investigator has access to all the data and takes responsibility for data integrity and the data analysis. Data and research materials will not be provided to other researchers with the explicit aim of reproducing the findings or duplicating the procedure independently. However, on reasonable request anonymized data may be shared with other researchers.
RESULTS
Baseline Characteristics
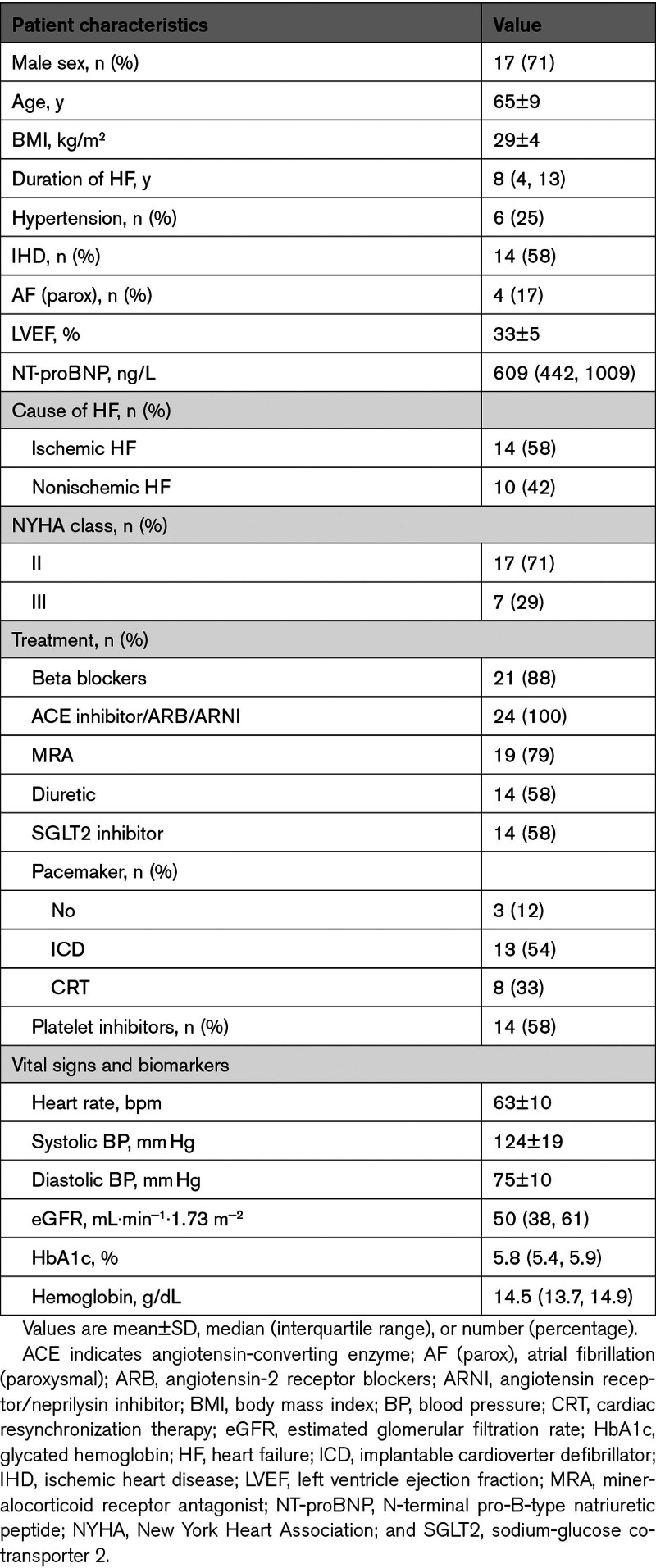
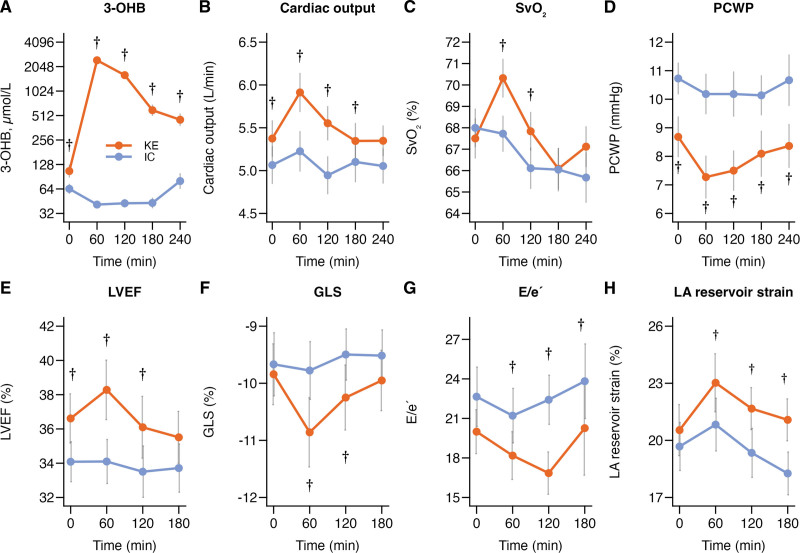
A total of 80 patients were screened for eligibility between January 2022 and March 2023. Among these, 35 were invited to screening, and 26 participants were randomized (Figure S1). Two participants were subsequently withdrawn from the study: 1 due to a procedure-related intermittent third-degree atrioventricular block during right heart catheterization and another due to pathogen-verified infectious gastroenteritis with symptom onset before initial supplementation intake. Thus, the final analysis included 24 patients who successfully completed the study. The mean age was 65±9 years, and 71% were male (Table 1). All participants were White. The median duration of HF was 8 years (interquartile range, 4–13 years); 58% had ischemic HF, and 42% had nonischemic HF. Guideline-recommended HF treatment was highly prevalent among the participants; 58% were treated with sodium-glucose cotransporter 2 (SGLT2) inhibitors. The median NT-proBNP level was 609 pmol/L (interquartile range, 442–1009 pmol/L); the mean LVEF was 33±5%. Among the participants, 71% and 31% were in New York Heart Association class II and III, respectively.
Table 1.
Baseline Characteristics (N=24)
Effect of KE on Trough Resting Hemodynamic Parameters
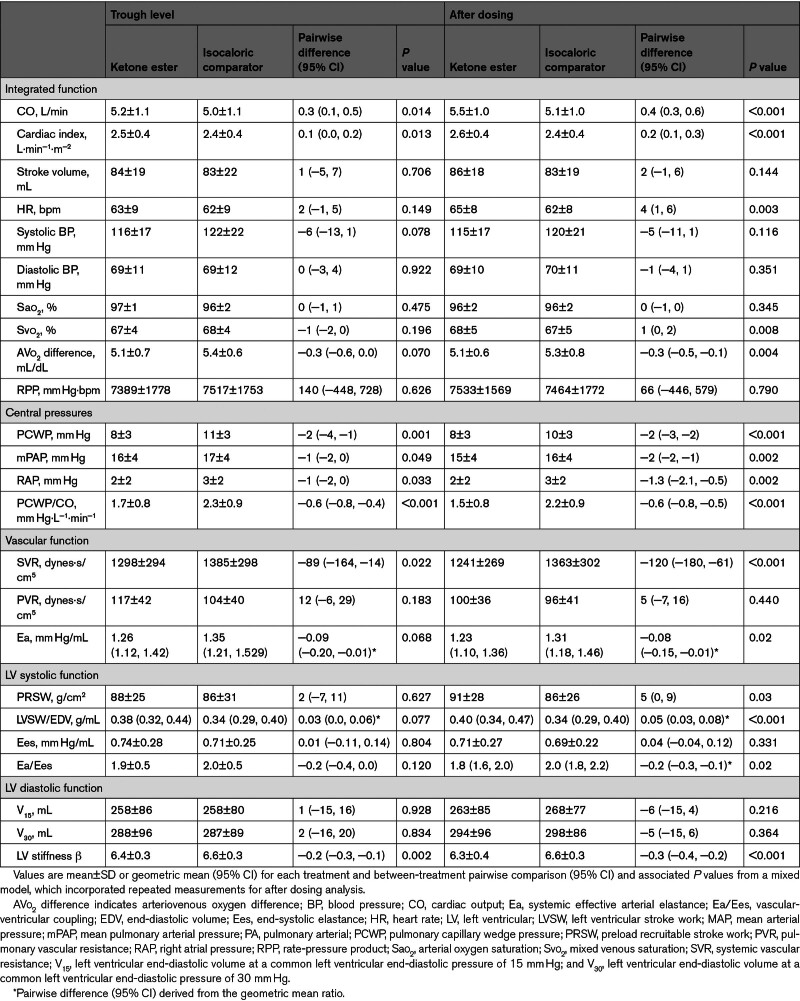
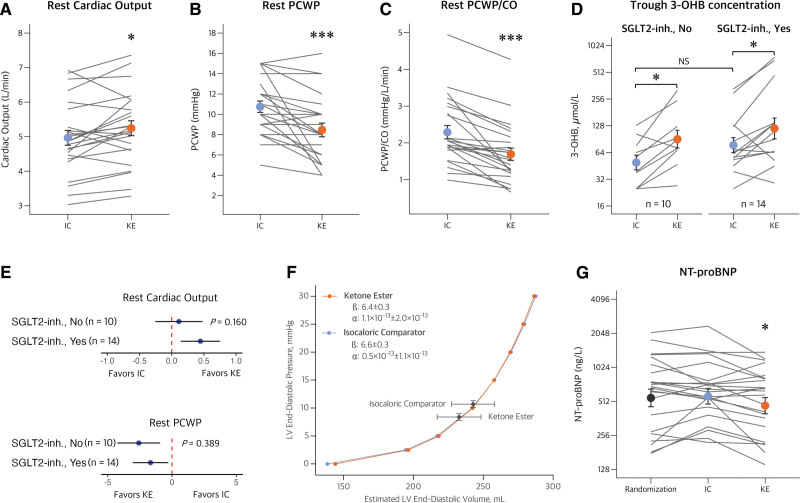
Resting CO was higher after 14-day treatment with KE compared with the IC drink (5.2±1.1 L/min versus 5.0±1.1 L/min; difference, 0.3 L/min [95% CI, 0.1–0.5]; P=0.01; Table 2; Figure 2A).
Table 2.
Resting Hemodynamic Parameters at Trough Level and After Dosing
Figure 2.
Hemodynamic parameters during KE and IC treatment. Mean or geometric mean with bars indicating SE. A, Cardiac output (CO; P=0.014) was higher, and pulmonary capillary wedge pressure (PCWP; P=0.001; B) and PCWP/CO (C; P<0.001) were lower after 14-day treatment with ketone ester (KE) compared with the isocaloric comparator (IC). D, Circulating trough levels of 3-hydroxybutyrate (3-OHB) were similarly higher after 14-day KE treatment in patients with and without ongoing sodium-glucose cotransporter 2 inhibitor (SGLT2-inh) treatment. E, Forest plot displays the between-treatment pairwise comparisons (coefficients) and 95% CI in each subgroup, and P values indicate subgroup comparisons for interaction testing. There were no significant interactions between treatment with SGLT2 inhibitors and treatment response to KE on CO or PCWP at rest. F, The left ventricular (LV) end-diastolic pressure-volume relationship remained similar during each treatment, whereas KE improved LV unloading by reducing LV end-diastolic pressure, volume, and LV stiffness. G, Cardiac unloading was supported by a reduction in N-terminal pro-B-type natriuretic-peptide (NT-proBNP) during KE treatment compared with IC and measurements at randomization (P=0.010). NS indicates not statistically different. * P<0.05 vs IC; ***P<0.001 vs IC.
KE treatment was associated with a lower trough PCWP (8±3 mm Hg versus 11±3 mm Hg; difference, −2 mm Hg [95% CI, −4 to −1]; P=0.001) and PCWP/CO ratio (P<0.001) compared with IC (Figure 2B and 2C). RAP and mPAP were also lower (Table 2). Lower SVR and a trend toward lower Ea (P=0.068) were observed. Resting HR, RPP, and load-independent measures of LV systolic function, including preload recruitable stroke work, LV stroke work normalized to end-diastolic volume, and Ees, did not differ between treatments during the trough assessment. There were no significant interactions between treatment sequence and between-treatment differences on hemodynamic parameters (Table S2; Figure S2). Patients with ischemic HF showed a greater between-treatment difference in CO during trough levels compared with patients with nonischemic HF (Pinteraction=0.03).
Fasting circulating 3-OHB was 2-fold higher after the 14-day KE treatment period (85 µmol/L [95% CI, 54–131]) compared with randomization values (43 µmol/L [95% CI, 32–57]) and the IC period (42 µmol/L [95% CI, 33–55]; P<0.001; Table 3). The 3-OHB levels were not influenced by body mass index (Pinteraction=0.711) or SGLT2 inhibitor treatment (Pinteraction=0.550; Figure 2D). The impact of KE treatment on CO and PCWP remained consistent across exploratory subgroups, including SGLT2 inhibitor treatment (Figure 2E), sex, age, body mass index, blood pressure, and disease severity (Figure S3). In addition, hemodynamics were not affected by the change in trough 3-OHB levels (Table S3).
Table 3.
Biomarkers, Quality of Life, and Echocardiographic Parameters
Effect of KE on Hemodynamics After Dosing
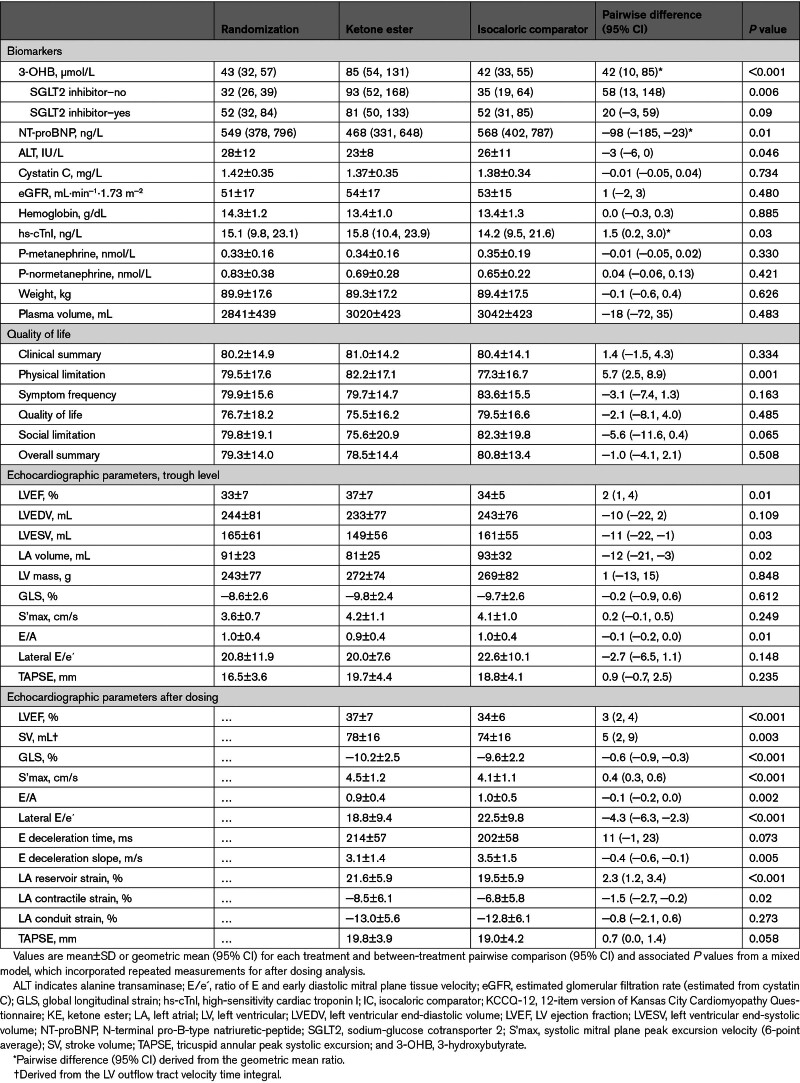
KE dosing increased circulating 3-OHB from 104 µmol/L (95% CI, 74–146) to 2419 µmol/L (95% CI, 1859–3169 µmol/L) within the first hour (P<0.001 versus IC; Figure 3A). Four hours after intake, the mean levels were 437 µmol/L (95% CI, 315–607; P<0.001 versus IC). CO remained higher, with a 4-hour mean value of 5.5±1.0 L/min compared with 5.1±1.0 L/min during IC (P<0.001; Table 2); the greatest difference occurred 1 hour after KE ingestion (Figure 3B). Svo2 increased after KE intake, consistent with the increase in O2 delivery and CO (Figure 3C).
Figure 3.
Between-treatment difference in circulating 3-OHB, hemodynamic, and echocardiographic parameters at trough levels and after dosing. Measurements after 14-day treatment with ketone ester (KE) and isocaloric comparator (IC) at trough levels (time=0) and after dosing. A, The 14-day KE treatment increased circulating 3-hydroxybutyrate (3-OHB) both at trough levels and after dosing. B, Trough cardiac output was higher and increased further after KE dosing (P<0.001). C, Mixed venous saturation (Svo2) increased after KE dosing (P=0.008). D, KE treatment was associated with lower pulmonary capillary wedge pressure (PCWP) at trough levels and further after KE dosing (P<0.001). E, Trough left ventricular ejection fraction (LVEF) was higher and remained elevated after KE dosing (P<0.001). F through H, Global longitudinal strain (GLS), ratio of E and early diastolic mitral plane tissue velocity (E/e´), and left atrial (LA) reservoir strain improved after KE dosing. Data are shown as mean or geometric mean, with bars indicating SEM. †P<0.05 vs IC.
RAP, mPAP, PCWP, and PCWP/CO remained significantly lower after KE dosing up to 4 hours after intake, with maximal effects observed after 1 hour (Table 2; Figure 3; Figure S4). In addition, Ea and SVR were lower after KE dosing, whereas HR was slightly higher compared with IC (65±8 bpm versus 62±8 bpm; difference, 4 bpm [95% CI, 1–6]; P=0.003). Meanwhile, RPP remained similar between treatments. LV contractility assessed by preload recruitable stroke work and LV stroke work normalized to end-diastolic volume was higher during KE dosing compared with IC, whereas Ees remained similar between treatments; Ea/Ees was slightly reduced. No correlation was observed between peak circulating 3-OHB levels and between-treatment difference in CO (Table S3).
Effect of KE on Cardiac Structure and LVEF
LVEF was higher after 14-day KE treatment compared with IC (37±7% versus 34±5%; difference, 2% [95% CI, 1%–4%]; P=0.01; Table 3; Figure 3E). LVEDV and LV end-systolic volume were lower compared with the IC period. These findings were further supported by 3-dimensional echocardiography (available for 67% of participants; Table S4). LA volume was also lower (81±25 mL versus 93±32 mL; difference, −12 mL [95% CI, −21 to −3 mL]; P=0.02), accompanied by lower E/A. Global longitudinal strain, S’max, LV mass, E/e´, and tricuspid annular peak systolic excursion did not differ at trough levels. Treatment sequence did not affect echocardiographic parameters (Table S5; Figure S2).
The LV EDPVR was similar after 14-day treatment with KE and IC (Table 2; Figure 2F). Even so, KE treatment was associated with lower LV stiffness β compared with IC. Of importance, the relationship between LVEDV and estimated LVEDP shifted to a lower point on the EDPVR after 14-day KE treatment, in contrast to IC (Figure 2F).
After KE dosing, LVEF, SV, global longitudinal strain, and S’max improved compared with IC (Table 3; Figure 3). Moreover, diastolic parameters improved, evident by lower E/A, E/e´, and E deceleration slope, whereas LA reservoir and contractile strain, e´, and a´ were enhanced (Table 3; Figure 3; Table S4).
Effect of KE on Biomarkers
NT-proBNP was ≈18% lower after KE treatment compared with IC (pairwise difference, −98 ng/L [95% CI, −185 to −23 ng/L]; P=0.01; Figure 2G). Markers of kidney function, hemoglobin, hematocrit, and platelets were similar in both treatment periods, whereas alanine transaminase was slightly lower after KE treatment (Table 3). Methoxyadrenalin and methoxynoradrenalin remained similar between treatments. Trough bicarbonate levels and pH were slightly higher after KE treatment compared with IC. These parameters initially decreased after acute KE intake and returned to similar levels as IC during the 4-hour observation period (Table S6; Figure S5). Furthermore, potassium and lactate were slightly higher after KE dosing compared with IC. Body weight and estimated plasma volume did not differ between both treatment periods (Table 3).
Quality of Life
The KCCQ-12 clinical summary score remained similar between the treatments (P=0.334; Table 3), whereas the physical limitation score (P=0.001) was higher after KE treatment. A trend toward lower social limitation was observed during KE treatment (Table 3).
Effect of KE on Hemodynamic Parameters During Exercise
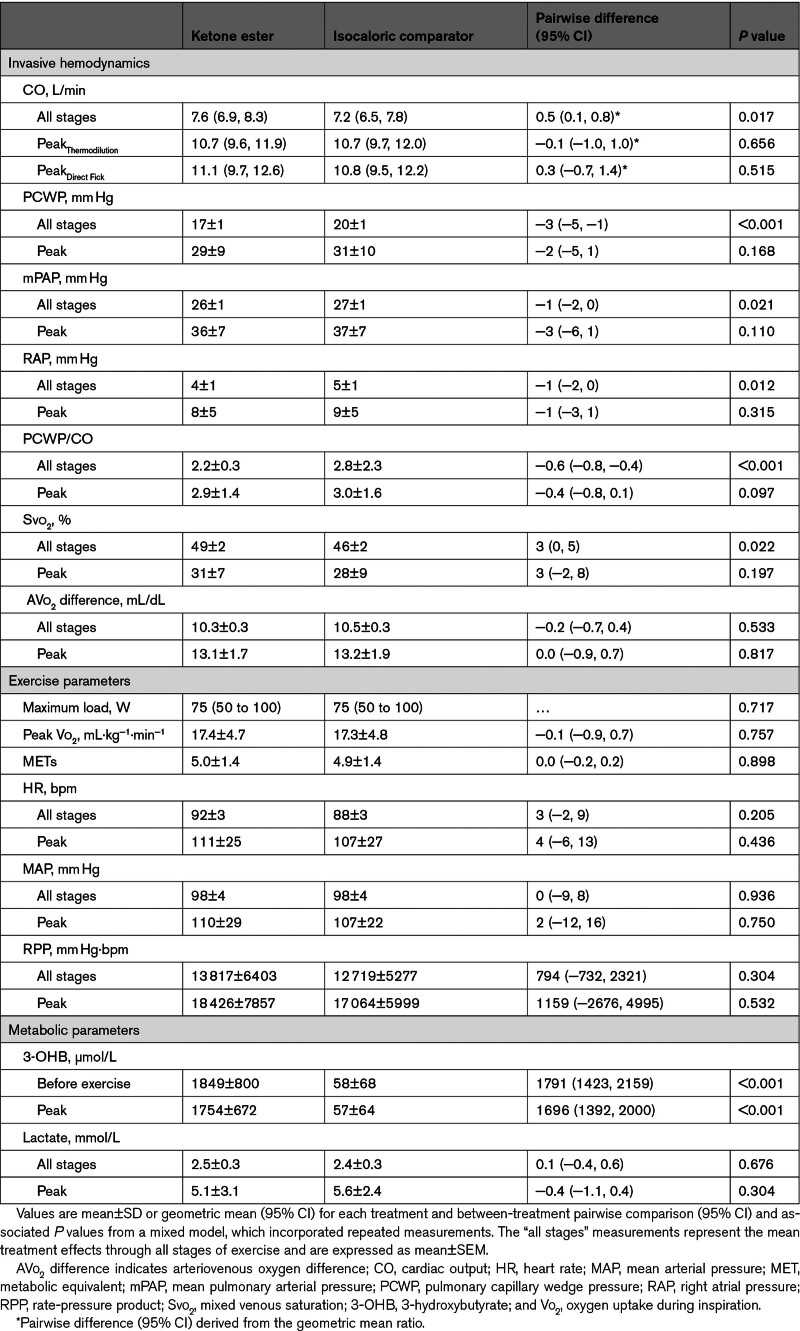
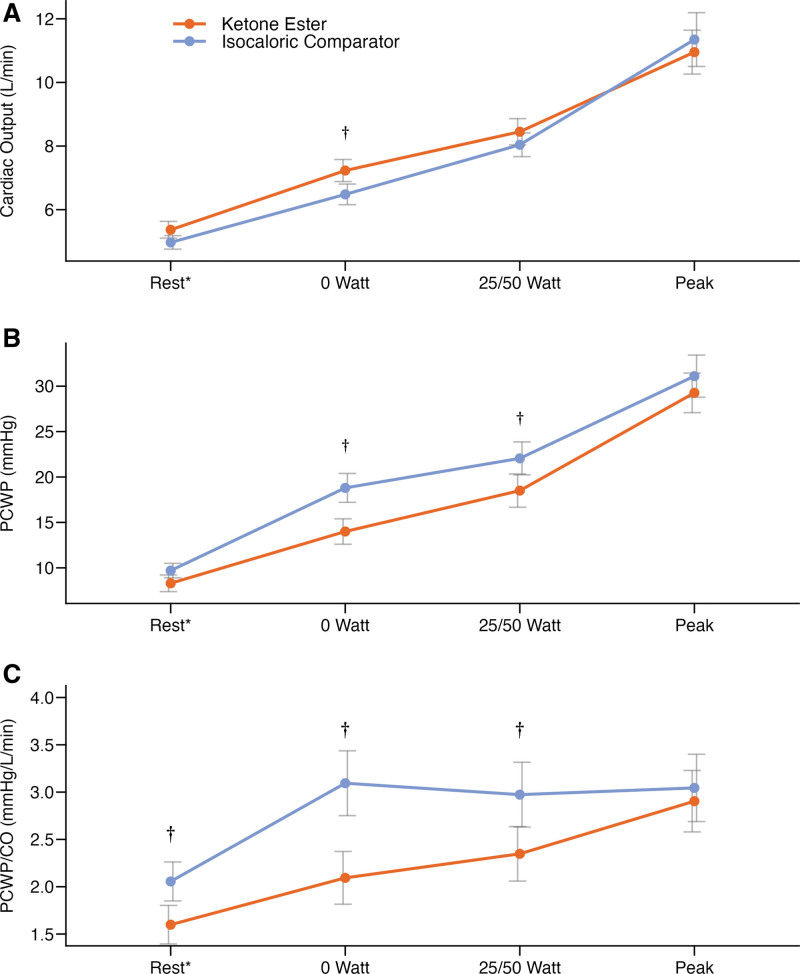
Thirty minutes after an oral KE bolus, circulating 3-OHB increased to 1849±800 µmol/L and remained elevated throughout peak exercise (1754±627 µmol/L); in contrast, circulating 3-OHB remained low at 58±68 µmol/L after IC (P<0.001; Table 4). Low steady-state CO was higher after KE treatment compared with IC, whereas low and moderate steady-state PCWP and PCWP/CO ratio were lower (Figure 4; Table S7). Mean exercise values for RAP and mPAP were also lower after KE compared with IC (Table 4). Hemodynamic and physiologic parameters remained similar between treatments at submaximal and peak exercise (Table 4; Table S7).
Table 4.
End-Point Parameters During Exercise
Figure 4.
Between-treatment differences in hemodynamic parameters during exercise testing from rest to peak. Mean with bars indicating SEM. Treatment with ketone ester increased mean exercise cardiac output (CO; A) and reduced mean exercise pulmonary capillary wedge pressure (PCWP; P<0.001; B) and PCWP/CO (P<0.001; C). *Semisupine rest just before exercise initiation. †P<0.05 vs isocaloric comparator.
Safety and Compliance
Compliance was good during both study periods (Table S8). KE treatment was well tolerated, and no serious adverse events were recorded throughout the study period (Table S8). During treatment with KE, more episodes of mild fatigue (4 versus 0), headache (3 versus 0), abdominal pain (2 versus 0), and reduced appetite (2 versus 0) occurred. IC treatment was associated with more episodes of diarrhea (4 versus 2) and cough (2 versus 0). High-sensitivity cardiac troponin I was higher after KE treatment compared with IC (difference, 1.5 ng/L [95% CI, 0.2–3.0 ng/L]; P=0.03), although it was not different from randomization values (P=0.394; Table 3).
DISCUSSION
This study is the first randomized controlled trial to evaluate the cardiovascular effects of prolonged KE treatment in patients with stable HFrEF. Our findings demonstrated favorable effects of a 14-day KE treatment regimen on hemodynamic measurements both at rest and during exercise. First, resting CO and LVEF were higher after KE treatment, accompanied by lower measures of LV congestion that drive symptoms and disease severity in HFrEF, including NT-proBNP, PCWP, and both LA and LV volumes, highlighting the beneficial unloading effect of KE. These salutary effects of KE were also maintained during exercise; patients reported less physical limitations during the 14-day KE treatment period. KE administration proved to be safe and well tolerated among participants, with only minor adverse events being reported. These data call for larger trials testing the efficacy and safety of long-term KE administration.
Therapeutic Potential of Ketone Bodies in HF
Elevated circulating levels of 3-OHB have been associated with a poorer prognosis in patients with both acute and chronic cardiovascular conditions.27,28 Furthermore, human studies have unveiled a parallel upregulation in key cardiac ketone-utilizing enzymes in severely failing hearts.5,9 Compelling evidence emphasizes a metabolic shift toward augmented ketone utilization as a possible adaptive feature in HF pathophysiology.3 In addition, a growing body of evidence suggests that ketone treatment exerts beneficial effects on cardiac function.29 Our group has previously demonstrated that short-term administration of 3-OHB increases CO and LVEF in a dose-dependent and reproducible manner in patients with HFrEF,10,30 cardiogenic shock,11 and various types of pulmonary hypertension.31
Until now, the therapeutic role of ketone bodies has been studied only during short-term administration in clinical human trials.10,32,33 We performed a detailed hemodynamic assessment at plasma trough levels, revealing that a 14-day KE treatment compared with IC associated with improved cardiac function independently of changes in trough 3-OHB levels. This was accompanied by beneficial effects on markers of LV unloading and reverse remodeling, which occurred even in the presence of optimal guideline-recommended HFrEF treatment. In acute studies, similar hemodynamic results were present at much higher levels of 3-OHB (≈700 µmol/L versus ≈100 µmol/L in the present study). Therefore, the enhanced cardiovascular function at trough levels is likely a result of the 14-day treatment period, indicating improved cardiac function and decongestion, rather than being directly related to the circulating levels of 3-OHB. These effects were even more substantial than those observed after 12-week treatment with SGLT2 inhibitors.34 An important point is that we observed consistent response to KE in patients with or without chronic treatment with SGLT2 inhibitors. KE dosing intensified the between-treatment difference in hemodynamic parameters, mirroring elevated 3-OHB levels, and caused improvement in load-independent measures of LV contractility. Furthermore, KE dosing was associated with improved echocardiographic indices of both systolic and diastolic function, including lowered LA function and volume, which are associated with long-term survival.35 Our findings indicate that 2-week KE treatment added to guideline-recommended HF therapy leads to sustained hemodynamic improvements that extend beyond the acute ketosis phase. Similar magnitudes of LV reverse remodeling are consistently correlated to improved clinical outcomes.36 Thus, our findings could have implications for future treatment in patients with cardiac dysfunction.
We demonstrated a hemodynamic benefit of KE treatment during exercise. Filling pressures remained lower as exercise intensity increased, along with an improved PCWP/CO ratio, which encompasses the primary components of HFrEF pathophysiology. Similar to SGLT2 inhibitor treatment,34 the unchanged peak CO during KE treatment suggests no enhancement of maximal cardiac contractile reserve. However, in line with previous reports on the hemodynamic response to short-term compared with long-term HF treatment,37 trials with longer follow-up may be necessary to fully capture significant myocardial structural changes to affect contractile reserve. Meanwhile, the hemodynamic effects were particularly pronounced during low to moderate exercise. Consistent with improved exercise hemodynamics, participants reported improvement in self-reported physical performance. KE treatment reduced NT-proBNP levels in just 14 days, a noteworthy finding linked to improved long-term prognosis.38 Therefore, we have provided compelling evidence that 2-week KE treatment has beneficial effects on several end points known to affect HF outcomes. The present study extends previous results of an increased cardiac performance during ketone supplementation beyond acute intake. Larger, long-term studies and ascending-dose trials with more patient-centered outcomes are warranted to further investigate the therapeutic potential of KE treatment in patients with HFrEF.
Mechanisms of Action
Ketone bodies exert a wide range of pleiotropic effects in the heart and noncardiac tissues.25 Preclinical experiments demonstrated that 3-OHB administration leads to both systemic vasorelaxation and enhanced cardiac contractility.39 Each of these factors may contribute to the observed increase in CO. We observed a reduction in SVR, Ea, and ventricular volumes during the KE treatment period, supporting a potential direct vasorelaxant effect. In addition, indirect measures of contractile function improved (eg, preload recruitable stroke work and LV stroke work normalized to end-diastolic volume ratio), arguing against a pure vasorelaxant mechanism of effect.40 Although KE dosing resulted in a minor increase in HR, it did not affect the RPP, suggesting myocardial oxygen demand remained unchanged. Of importance, our documented hemodynamic effects after 14-day KE treatment occurred without any significant difference in circulating metanephrines and even before KE dosing at low 3-OHB levels. There was no interaction between baseline MAP and KE for effects on CO or PCWP in the post hoc subgroup analysis (Figure S3). Thus, prolonged KE treatment seems to increase cardiac performance beyond the effects of acute ketosis-mediated vasorelaxation.
In patients with HFrEF, 3-OHB augments contractility in isolated perfused rat hearts and increases myocardial oxygen consumption, whereas cardiac efficiency remains unaltered.10,39 However, these effects have been studied primarily in the acute state of ketosis. Therefore, prolonged KE treatment may plausibly restore myocardial adenosine triphosphate levels, as observed in animal studies,41 thereby improving contractile function without compromising myocardial energetics. It is intriguing that patients with ischemic HF demonstrated greater response on CO at trough levels compared with patients with nonischemic HF, which needs to be addressed in future trials. The pleiotropic vasorelaxant effects unload the heart, reducing myocardial oxygen demand, thus aiding cardiac function and vascular-ventricular coupling. Indeed, the last parameter was slightly reduced.
Prolonged ketosis may play a pivotal role in enhancing myocardial energetics by serving as an efficient alternative metabolic substrate for the failing heart.2,42 Indeed, when 3-OHB levels are raised through KE administration, it becomes a major source for cardiac oxidative phosphorylation, and this effect is particularly pronounced in patients with HFrEF.7 In the present study, we administered 4 daily doses of KE, determined through our dosing experiments to yield an anticipated mean 3-OHB level of ≈1 to 2 mmol/L during both rest and exercise for at least 16 hours each day.12 Thus, 3-OHB could become a major substrate for cardiac oxidative phosphorylation for at least ≈70% of the 14-day treatment period, and the observed cardiac enhancement may stem from myocardial metabolic recovery and improved mechanoenergetic coupling.
Safety
Few mild adverse events were reported. Two participants withdrew from the trial because of adverse events judged to be unrelated to the study treatment. Compliance was deemed to be excellent. Although there was a significant statistical difference in high-sensitivity cardiac troponin I levels between the KE and IC groups, the change from baseline within the KE group was minimal and not statistically significant. This safety issue obviously requires careful assessment during subsequent studies. Finally, the acid-base profile of sustained ketosis demonstrated only minor fluctuations after dosing and even slight alkalosis at trough levels, which may be an adaptive phenomenon.
Limitations
One of the major limitations of this trial is not having baseline hemodynamic data against which to assess changes. Individual differences could potentially influence the acclimation to the invasive assessments, and serial hemodynamics can vary over time without intervention.43 To diminish these effects, we used a randomized cross-over design. A 14-day washout period was implemented between the treatment periods, representing >100 elimination half-lives, which should ensure no carryover or legacy effect.14 Moreover, we adopted a mixed-effects model, adjusting for period and treatment sequence to adjust for acclimation effects that may occur between visits, and this model detected no significant cross-over effects on the study end points. Next, resting CO was chosen as the primary measure of effect, although exercise CO is closer associated with symptom burden and functional limitation in patients with CHF. Exercise CO was a predefined secondary end point.
A taste-matched non-KE IC served as a control to standardize caloric intake effects. We chose this as IC because a peroral meal intake is known to increase cardiac function per se.44 Although this approach allowed a direct comparison of hemodynamic responses to KE, it did not account for a true placebo effect. The study intervention was not weight adjusted, but KE dosing induced significant ketosis in all participants, underscoring its high bioavailability. Furthermore, hemodynamic responses did not significantly differ between participants with low and high body mass index. Taste-matched interventions with added stevia ensured palatability, and a 4-dose regimen was used for sustained ketosis.12–14
Resting and exercise hemodynamics were assessed in the supine position and semisupine position, respectively. It is important to note that PCWP is position dependent and may rise during supine exercise because of increased venous return, and supine exercise assessments might increase sensitivity.45 However, the paired comparisons in our cross-over design possibly limit bias, and the agreement between supine and semisupine results bolsters confidence in our findings. Next, although 2 patients had LVEF >40% at randomization, this may be attributed to test-retest variability and day-to-day variations within the up to 4-week interval between screening and randomization.46
We excluded patients with diabetes because of the complex relationship among hyperglycemia, insulin resistance, and myocardial metabolic remodeling.47 Diabetes is linked to reduced cardiac ketone utilization,48,49 a crucial aspect of our investigation. By concentrating on patients with HFrEF without diabetes, we aimed to reduce confounding effects and gain clarity on the impact of KE on cardiovascular function in this specific population. Future trials should broaden inclusion criteria to encompass patients with diabetes for a more comprehensive understanding and broader applicability. Similar to previous trials, 20% declined participation after being briefed on study procedures (Figure S1).34 Our baseline characteristics match those in prior HFrEF trials, supporting the relevance and broad applicability of our findings despite potential underrepresentation of frailer individuals.34,50 Finally, all participants were White, and future trials should emphasize racial diversity.
Conclusions
Treatment with KE for 14 days was safe and led to significant and lasting benefits for patients with HFrEF, including improved cardiac function at rest in parallel with cardiac decongestion evidenced by hemodynamics, cardiac volumes, and NT-proBNP levels. These effects persisted during incremental exercise, and perceived physical limitations improved. This was achieved on top of optimal medical HF therapy and highlights sustained modulation of circulating ketone bodies as a promising therapeutic strategy for patients with HFrEF.
ARTICLE INFORMATION
Acknowledgments
The authors thank Susanne Sørensen, Lene Trudsø, Lone Kvist, Maria Louise Flink Schwartz, and Eva Mølgaard Jensen, Department of Endocrinology and Metabolism, Aarhus University Hospital, for excellent technical assistance throughout the study day. Figure 1 was created with Biorender.com.
Sources of Funding
This study was supported by the Independent Research Fund Denmark (0134-00043B), the Danish Heart Foundation (19-R135-A9280-22126), the Novo Nordisk Foundation (NNF17OC0028230), and Ellen and Ivan Osiiers Legat.
Disclosures
Dr Wiggers has been the principal investigator or a subinvestigator in studies involving the following pharmaceutical companies: MSD, Bayer, Daiichi-Sankyo, Novartis, Novo Nordisk, Sanofi-Aventis, and Pfizer. Dr Borlaug receives research support from the National Institutes of Health and the US Department of Defense, as well as research grant funding from AstraZeneca, Axon, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, Rivus, and Tenax Therapeutics. Dr Borlaug has served as a consultant for Actelion, Amgen, Aria, Axon Therapies, BD, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, NGM, NXT, and VADovations, and is named inventor (US patent No. 10,307,179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure. The other authors report no conflicts.
Supplemental Material
Eligibility criteria
Supplemental equations
Tables S1–S8
Supplemental Figures S1–S5
Statistical analysis plan
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 3-OHB
- 3-hydroxybutyrate
- AVo2 difference
- arteriovenous oxygen difference
- CO
- cardiac output
- Ea
- effective arterial elastance
- EDPVR
- left ventricular end-diastolic pressure-volume relationship
- Ees
- end-systolic elastance
- HF
- heart failure
- HFrEF
- heart failure with reduced ejection fraction
- HR
- heart rate
- IC
- isocaloric comparator
- KCCQ-12
- Kansas City Cardiomyopathy Questionnaire, 12-item version
- KE
- ketone ester
- LA
- left atrial
- LV
- left ventricular
- LVEDP
- left ventricular end-diastolic pressure
- LVEDV
- left ventricular end-diastolic volume
- LVEF
- left ventricular ejection fraction
- MAP
- mean arterial pressure
- mPAP
- mean pulmonary arterial pressure
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- PCWP
- pulmonary capillary wedge pressure
- RAP
- right atrial pressure
- RPP
- rate-pressure product
- sBP
- systolic blood pressure
- SGLT2
- sodium-glucose cotransporter 2
- SV
- stroke volume
- SVR
- systemic vascular resistance
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.123.067971.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
For Sources of Funding and Disclosures, see page 1488.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Kristian Hylleberg Christensen, Email: krstchri@rm.dk.
Bertil Ladefoged, Email: berlad@rm.dk.
Mads Jønsson Andersen, Email: Andersen.Mads@rm.dk.
Barry A. Borlaug, Email: borlaug.barry@mayo.edu.
Roni Nielsen, Email: bent.nils@midt.rm.dk.
Niels Møller, Email: nielsem@dadlnet.dk.
REFERENCES
- 1.Taegtmeyer H, Golfman L, Sharma S, Razeghi P, van Arsdall M. Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Ann N Y Acad Sci. 2004;1015:202–213. doi: 10.1196/annals.1302.017 [DOI] [PubMed] [Google Scholar]
- 2.Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res. 2021;128:1487–1513. doi: 10.1161/circresaha.121.318241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, Rabinowitz JD, Frankel DS, Arany Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370:364–368. doi: 10.1126/science.abc8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yurista SR, Nguyen CT, Rosenzweig A, Boer RAD, Westenbrink BD. Endocrinology & metabolism ketone bodies for the failing heart: fuels that can fix the engine? Trends Endocrinol Metab. 2021;1:13. doi: 10.1016/j.tem.2021.07.006 [DOI] [PubMed] [Google Scholar]
- 5.Bedi KC, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–716. doi: 10.1161/CIRCULATIONAHA.115.017545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchihashi M, Hoshino A, Okawa Y, Ariyoshi M, Kaimoto S, Tateishi S, Ono K, Yamanaka R, Hato D, Fushimura Y, et al. Cardiac-specific Bdh1 overexpression ameliorates oxidative stress and cardiac remodeling in pressure overload-induced heart failure. Circ Heart Fail. 2017;10:1–11. doi: 10.1161/CIRCHEARTFAILURE.117.004417 [DOI] [PubMed] [Google Scholar]
- 7.Monzo L, Sedlacek K, Hromanikova K, Tomanova L, Borlaug BA, Jabor A, Kautzner J, Melenovsky V. Myocardial ketone body utilization in patients with heart failure: the impact of oral ketone ester. Metabolism. 2021;115:154452. doi: 10.1016/j.metabol.2020.154452 [DOI] [PubMed] [Google Scholar]
- 8.Luong TV, Nielsen EN, Falborg L, Kjærulff MLG, Tolbod LP, Søndergaard E, Møller N, Munk OL, Gormsen LC. Intravenous and oral whole body ketone dosimetry, biodistribution, metabolite correction and kinetics studied by (R)-[1-11C]β-hydroxybutyrate ([11C]OHB) PET in healthy humans. EJNMMI Radiopharm Chem. 2023;8:12. doi: 10.1186/s41181-023-00198-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705. doi: 10.1161/CIRCULATIONAHA.115.017355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frøkiær J, Eiskjaer H, Jespersen NR, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139:2129–2141. doi: 10.1161/CIRCULATIONAHA.118.036459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg-Hansen K, Christensen KH, Gopalasingam N, Nielsen R, Eiskjær H, Møller N, Birkelund T, Christensen S, Wiggers H. Beneficial effects of ketone ester in patients with cardiogenic shock: a randomized, controlled, double-blind trial. JACC Heart Fail. 2023;11:1337–1347. doi: 10.1016/j.jchf.2023.05.029 [DOI] [PubMed] [Google Scholar]
- 12.Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, Magor-Elliott S, Hiyama S, Stirling M, Clarke K. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:1–13. doi: 10.3389/fphys.2017.00848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soto-Mota A, Vansant H, Evans RD, Clarke K. Safety and tolerability of sustained exogenous ketosis using ketone monoester drinks for 28 days in healthy adults. Regul Toxicol Pharmacol. 2019;109:104506–104509. doi: 10.1016/j.yrtph.2019.104506 [DOI] [PubMed] [Google Scholar]
- 14.Clarke K, Tchabanenko K, Pawlosky R, Carter E, Todd King M, Musa-Veloso K, Ho M, Roberts A, Robertson J, VanItallie TB, et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol. 2012;63:401–408. doi: 10.1016/j.yrtph.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 16.Lam CSP, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PYA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/S0735-1097(01)01651-5 [DOI] [PubMed] [Google Scholar]
- 18.Lee WS, Huang WP, Yu WC, Chiou KR, Yu-An Ding P, Chen CH. Estimation of preload recruitable stroke work relationship by a single-beat technique in humans. Am J Physiol Heart Circ Physiol. 2003;284:744–750. doi: 10.1152/ajpheart.00455.2002 [DOI] [PubMed] [Google Scholar]
- 19.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2015;66:1672–1682. doi: 10.1016/j.jacc.2015.07.067 [DOI] [PubMed] [Google Scholar]
- 20.Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, Velmurugan S, Mendonca M, Rashid M, Kang S, et al. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail. 2015;17:35–43. doi: 10.1002/ejhf.193 [DOI] [PubMed] [Google Scholar]
- 21.Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 22.Pathan F, Elia ND, Nolan MT, Marwick TH. Normal ranges of left atrial strain by speckle-tracking echocardiography: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2022;30:59–70.e8. doi: 10.1016/j.echo.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 23.Klotz S, Hay I, Dickstein ML, Yi GH, Wang J, Maurer MS, Kass DA, Burkhoff D. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol. 2006;291:H403–H412. doi: 10.1152/ajpheart.01240.2005 [DOI] [PubMed] [Google Scholar]
- 24.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, et al. ; CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation. 2020;141:1800–1812. doi: 10.1161/CIRCULATIONAHA.119.045033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroon M, Groeneveld ABJ, Smulders YM. Cardiac output measurement by pulse dye densitometry: comparison with pulmonary artery thermodilution in post-cardiac surgery patients. J Clin Monit Comput. 2005;19:395–399. doi: 10.1007/s10877-005-6865-y [DOI] [PubMed] [Google Scholar]
- 27.Lommi J, Kupari M, Koskinen P, Näveri H, Leinonen H, Pulkki K, Härkönen M. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol. 1996;28:665–672. doi: 10.1016/0735-1097(96)00214-8 [DOI] [PubMed] [Google Scholar]
- 28.de Koning MSLY, Westenbrink BD, Assa S, Garcia E, Connelly MA, van Veldhuisen DJ, Dullaart RPF, Lipsic E, van der Harst P. Association of circulating ketone bodies with functional outcomes after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2021;78:1421–1432. doi: 10.1016/j.jacc.2021.07.054 [DOI] [PubMed] [Google Scholar]
- 29.Yurista SR, Chong C-R, Badimon JJ, Kelly DP, de Boer RA, Westenbrink BD. Therapeutic potential of ketone bodies for patients with cardiovascular disease. J Am Coll Cardiol. 2021;77:1660–1669. doi: 10.1016/j.jacc.2020.12.065 [DOI] [PubMed] [Google Scholar]
- 30.Gopalasingam N, Christensen KH, Berg Hansen K, Nielsen R, Johannsen M, Gormsen LC, Boedtkjer E, Nørregaard R, Møller N, Wiggers H. Stimulation of the hydroxycarboxylic acid receptor 2 with the ketone body 3-hydroxybutyrate and niacin in patients with chronic heart failure: hemodynamic and metabolic effects. J Am Heart Assoc. 2023;12:1–13. doi: 10.1161/jaha.123.029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen R, Christensen KH, Gopalasingam N, Berg-Hansen K, Seefeldt J, Homilius C, Boedtkjer E, Andersen MJ, Wiggers H, Møller N, et al. Hemodynamic effects of ketone bodies in patients with pulmonary hypertension. J Am Heart Assoc. 2023;12:e028232. doi: 10.1161/JAHA.122.028232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svart M, Gormsen LC, Hansen J, Zeidler D, Gejl M, Vang K, Aanerud J, Moeller N. Regional cerebral effects of ketone body infusion with 3-hydroxybutyrate in humans: reduced glucose uptake, unchanged oxygen consumption and increased blood flow by positron emission tomography: a randomized, controlled trial. PLoS One. 2018;13:e0190556. doi: 10.1371/journal.pone.0190556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gormsen LC, Svart M, Thomsen HH, Søndergaard E, Vendelbo MH, Christensen N, Tolbod LP, Harms HJ, Nielsen R, Wiggers H, et al. Ketone body infusion with 3-hydroxybutyrate reduces myocardial glucose uptake and increases blood flow in humans: a positron emission tomography study. J Am Heart Assoc. 2017;6:1–11. doi: 10.1161/JAHA.116.005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omar M, Jensen J, Frederiksen PH, Kistorp C, Videbæk L, Poulsen MK, Möller S, Ali M, Gustafsson F, Køber L, et al. Effect of empagliflozin on hemodynamics in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2020;76:2740–2751. doi: 10.1016/j.jacc.2020.10.005 [DOI] [PubMed] [Google Scholar]
- 35.Triposkiadis F, Pieske B, Butler J, Parissis J, Giamouzis G, Skoularigis J, Brutsaert D, Boudoulas H. Global left atrial failure in heart failure. Eur J Heart Fail. 2016;18:1307–1320. doi: 10.1002/ejhf.645 [DOI] [PubMed] [Google Scholar]
- 36.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 37.Franciosa JA. Isosorbide dinitrate and exercise performance in patients with congestive heart failure. Am Heart J. 1985;110:245–250. doi: 10.1016/0002-8703(85)90494-6 [DOI] [PubMed] [Google Scholar]
- 38.Lam CSP, Gamble GD, Ling LH, Sim D, Leong KTG, Yeo PSD, Ong HY, Jaufeerally F, Ng TP, Cameron VA, et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi-ethnic cohort study. Eur Heart J. 2018;39:1770–1780. doi: 10.1093/eurheartj/ehy005 [DOI] [PubMed] [Google Scholar]
- 39.Homilius C, Seefeldt JM, Axelsen JS, Pedersen TM, Sørensen TM, Nielsen R, Wiggers H, Hansen J, Matchkov VV, Bøtker HE, et al. Ketone body 3-hydroxybutyrate elevates cardiac output through peripheral vasorelaxation and enhanced cardiac contractility. Basic Res Cardiol. 2023;118:37. doi: 10.1007/s00395-023-01008-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam CSP, Shah AM, Borlaug BA, Cheng S, Verma A, Izzo J, Oparil S, Aurigemma GP, Thomas JD, Pitt B, et al. Effect of antihypertensive therapy on ventricular-arterial mechanics, coupling, and efficiency. Eur Heart J. 2013;34:676–683. doi: 10.1093/eurheartj/ehs299 [DOI] [PubMed] [Google Scholar]
- 41.Yurista SR, Matsuura TR, Silljé HHW, Nijholt KT, Mcdaid KS, Shewale SV, Leone TC, Newman JC, Verdin E, Van Veldhuisen DJ, et al. Ketone ester treatment improves cardiac function and reduces pathologic remodeling in preclinical models of heart failure. Circ Hear Fail. 2021;14:112–124. doi: 10.1161/CIRCHEARTFAILURE.120.007684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neubauer S. The failing heart: an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052 [DOI] [PubMed] [Google Scholar]
- 43.Packer M, Medina N, Yushak M. Hemodynamic changes mimicking a vasodilator drug response in the absence of drug therapy after right heart catheterization in patients with chronic heart failure. Circulation. 1985;71:761–766. doi: 10.1161/01.cir.71.4.761 [DOI] [PubMed] [Google Scholar]
- 44.Watson WD, Green PG, Lewis AJM, Arvidsson P, De Maria GL, Arheden H, Heiberg E, Clarke WT, Rodgers CT, Valkovič L, et al. Retained metabolic flexibility of the failing human heart. Circulation. 2023;148:109–123. doi: 10.1161/CIRCULATIONAHA.122.062166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizumi S, Goda A, Takeuchi K, Kikuchi H, Inami T, Soejima K, Satoh T. Effects of body position during cardiopulmonary exercise testing with right heart catheterization. Physiol Rep. 2018;6:e13945–e13949. doi: 10.14814/phy2.13945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorstensen A, Dalen H, Amundsen BH, Aase SA, Stoylen A. Reproducibility in echocardiographic assessment of the left ventricular global and regional function, the HUNT study. Eur J Echocardiogr. 2010;11:149–156. doi: 10.1093/ejechocard/jep188 [DOI] [PubMed] [Google Scholar]
- 47.Rider OJ, Apps A, Miller JJJJ, Lau JYC, Lewis AJM, Peterzan MA, Dodd MS, Lau AZ, Trumper C, Gallagher FA, et al. Noninvasive in vivo assessment of cardiac metabolism in the healthy and diabetic human heart using hyperpolarized 13C MRI. Circ Res. 2020;725:736. doi: 10.1161/CIRCRESAHA.119.316260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brahma MK, Ha CM, Pepin ME, Mia S, Sun Z, Chatham JC, Habegger KM, Abel ED, Paterson AJ, Young ME, et al. Increased glucose availability attenuates myocardial ketone body utilization. J Am Heart Assoc. 2020;9:1–18. doi: 10.1161/JAHA.119.013039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, Dyck JE, Uddin GM, Oudit GY, Mayoux E, et al. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci. 2018;3:575–587. doi: 10.1016/j.jacbts.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]