Abstract
Chagas cardiomyopathy caused by infection with the intracellular parasite Trypanosoma cruzi is the most common and severe expression of human Chagas disease. Heart failure, systemic and pulmonary thromboembolism, arrhythmia, and sudden cardiac death are the principal clinical manifestations of Chagas cardiomyopathy. Ventricular arrhythmias contribute significantly to morbidity and mortality and are the major cause of sudden cardiac death. Significant gaps still exist in the understanding of the pathogenesis mechanisms underlying the arrhythmogenic manifestations of Chagas cardiomyopathy. This article will review the data from experimental studies and translate those findings to draw hypotheses about clinical observations. Human- and animal-based studies at molecular, cellular, tissue, and organ levels suggest 5 main pillars of remodeling caused by the interaction of host and parasite: immunologic, electrical, autonomic, microvascular, and contractile. Integrating these 5 remodeling processes will bring insights into the current knowledge in the field, highlighting some key features for future management of this arrhythmogenic disease.
Keywords: arrhythmias, cardiac; cardiovascular system; Chagas cardiomyopathy; Chagas disease; myocytes, cardiac; Trypanosoma cruzi; ventricular remodeling
Chagas disease (CD), a parasitic infection caused by the intracellular protozoan Trypanosoma cruzi (T. cruzi),1 is endemic in regions of Mexico, Central America, and South America, affecting ≈6 to 7 million people.2 In the United States, it is estimated that 288 000 individuals, predominantly immigrants, are living with CD, which they acquired primarily in Latin American countries.3 Domestic transmission within the United States through contact with triatomine bugs, the disease’s primary vector, is relatively rare.4
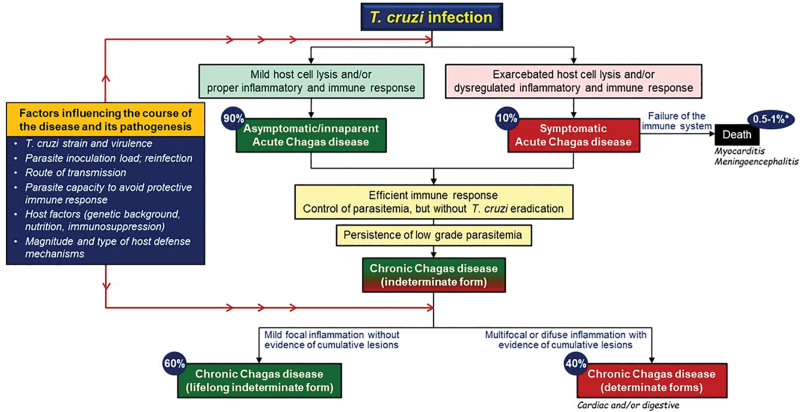
CD has 2 phases: acute and chronic (Figure 1).5 During the acute phase, 90% of individuals are asymptomatic or have mild symptoms, while the remaining 10% can exhibit more severe symptoms, which may lead to death from complications such as myocarditis or meningoencephalitis in case the immune system initial reaction fails. If the infection is not treated and cleared, it enters a chronic phase. Approximately 60% of these chronic cases remain with the indeterminate form, without symptoms throughout the individual’s life, whereas 40% evolve to determinate chronic CD. This latter group can suffer from significant cardiac and digestive problems due to ongoing inflammation and tissue damage. Both the acute and chronic phases of CD are influenced by various factors, including the virulence of the T. cruzi strain, the initial load of the parasite, the route of transmission, the parasite’s ability to evade the immune system, the host’s genetic background, nutritional status, and immune response.2 The most common and severe manifestation of chronic CD is Chagas cardiomyopathy (CCM), appearing in nearly one-third of individuals with CD. Notably, CCM has a poor prognosis, with an estimated overall annual mortality of 4%.6 Despite its prevalence and morbidity, the pathophysiologic understanding of CCM remains to be fully elucidated.
Figure 1.
The clinical course of Trypanosoma cruzi (T. cruzi) infection and influential factors in the pathogenesis and progression of Chagas disease. *The overall mortality rate for acute Chagas disease, encompassing both asymptomatic and symptomatic cases, ranges from 1 in 100 to 1 in 200 cases.
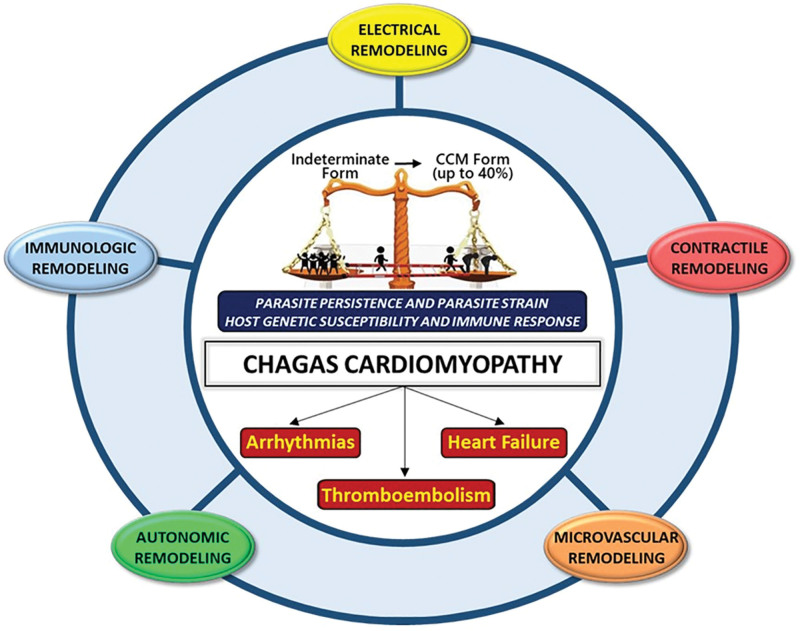
In this review, we will focus on the concept of remodeling that occurs in the cardiovascular system during the progression of CCM. The distinct but intermingled immunologic, electrical, autonomic, microvascular, and contractile remodeling mechanisms all contribute to the phenotypic manifestations of CCM (Figure 2). We propose that the cumulative effects of such structural and functional remodeling of the cardiovascular system evolve into an integrated manner to develop clinically apparent CCM following the long-standing indeterminate form of CD.
Figure 2.
Chagas cardiomyopathy (CCM): a quintet of interrelated remodeling processes. The progression of Chagas disease from the indeterminate (asymptomatic) form to CCM, which occurs in up to 40% of infected individuals, is guided by 5 intimately linked remodeling processes. These processes collectively shape the pathophysiological landscape of the disease. Central to the pathogenesis of CCM is the interplay among ongoing parasite infection, Trypanosoma cruzi strain, the patient’s genetic vulnerability, and their immune system’s reaction, influencing the clinical progression of the disease into its major syndromes: arrhythmias, heart failure, and thromboembolism.
CLINICAL ARRHYTHMOGENIC MANIFESTATIONS OF CHAGAS CARDIOMYOPATHY
CCM is often regarded as an essentially arrhythmogenic cardiomyopathy, and nearly, all rhythm disturbances may occur, including sinus node dysfunction, atrioventricular blocks, supraventricular arrhythmias, and most commonly, a variety of ventricular arrhythmias, such as isolated or coupled premature ventricular contractions, nonsustained ventricular tachycardia (VT), sustained monomorphic VT, polymorphic VT, and ventricular fibrillation.7 The frequency and severity of ventricular arrhythmias generally correlate with the degree of left ventricular (LV) dysfunction; however, a hallmark of CD consists in the fact that patients with preserved global ventricular function, presenting only with segmental abnormalities, may also experience ominous ventricular arrhythmias and sudden cardiac death (SCD).8 Not infrequently, both ventricular arrhythmias and conduction system alterations coexist in the same patient.7
SCD is the primary cause of death, accounting for 55% to 65% of all cardiovascular deaths in CCM.9 Sudden death is usually associated with VT or ventricular fibrillation or more rarely with asystole or complete atrioventricular block. Because the clinical course of CCM varies widely, it can be difficult to predict who is at higher risk of SCD. For example, some patients may remain asymptomatic throughout their life span, exhibiting only mild ECG alterations; others have conduction defects, ventricular arrhythmias, and only segmental wall motion abnormalities; others even develop global ventricular dysfunction with only mild symptoms of heart failure or show multiple disturbances of rhythm, thromboembolic phenomena, and severe symptoms of heart failure; and a nonnegligible number of individuals die suddenly with or without previous cardiac symptoms.10
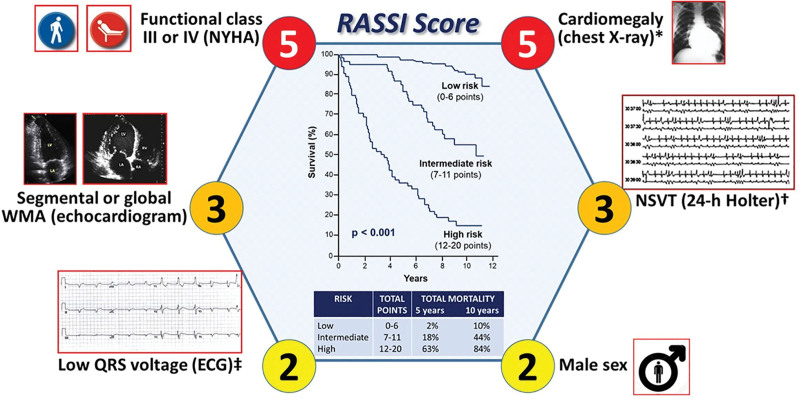
Given the dominant arrhythmic profile of CCM and its pivotal relationship with CCM mortality, there has been considerable effort towards risk prediction. In 2006, Rassi et al6 developed and validated a risk score to predict all-cause mortality and cardiovascular and SCD in CCM. Six independent predictors of mortality were identified, as illustrated in Figure 3. Apart from the incorporation of ventricular arrhythmia (nonsustained VT) in the RASSI score, other electrical indicators of adverse prognosis have been proposed by other authors, including increased T-wave amplitude variability, abnormal T-wave axis deviation, QT interval dispersion, signal-averaged ECG changes (spectral turbulence and filtered QRS), decreased heart rate variability, and increased QRS complex duration.2 However, these additional factors still lack external validation by independent cohort studies.
Figure 3.
RASSI score. This illustration presents the RASSI score, comprising 6 independent all-cause mortality predictors, with their associated points inside the circles. It shows the division into 3 risk categories and displays both the 5- and 10-year overall mortality rates, alongside the Kaplan-Meier survival curves for each risk subgroup. Of note, all these variables were also strong predictors of the risk of cardiovascular deaths and sudden cardiac deaths, except for the male sex, which was of borderline significance for the prediction of cardiovascular death, and low QRS voltage, which was of borderline significance for the prediction of sudden cardiac death. HR indicates heart rate; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; and WMA, wall motion abnormality. *Cardiothoracic ratio >0.50. †≥3 beats and duration <30 s (HR >100 bpm). ‡≤ 0.5 mV in all limb leads.
CCM outcomes are strongly dependent on the relationship between the host and the parasite, which occurs in an inflammatory milieu.11 Herein, we present experimental data suggesting the mechanistic underpinnings of the arrhythmogenic remodeling observed in CCM. While we present the data as 5 main mechanistic pillars, it is important to acknowledge that all these mechanisms likely work simultaneously and are interdependent.
IMMUNOLOGIC REMODELING IN CCM
Excellent reviews detailing the complex and intricate host immune response following T. cruzi infection are available.12–14 Here, we will provide a synthetic overview of this phenomenon that is fundamental to understand how the remodeling of the host immune system is able to trigger a remodeling at a cellular scale in cardiomyocytes and contributes to the global electrical and mechanical remodeling in CCM.
The variability in the clinical manifestation of CCM can be partly attributed to the heterogeneity of T. cruzi strains, encompassing a broad spectrum of genotypes and phenotypes.14,15 However, the precise nature of this relationship remains incompletely understood. The surface of T. cruzi is rich in pathogen-associated molecular patterns,16 while host tissue injury releases damage-associated molecular patterns.17 Together, these molecular signatures serve as key triggers for activating the host’s innate and adaptive immune responses.
This activation plays a critical role in the body’s defense mechanisms against the pathogen and influences the course of the disease. Initially, pathogen-associated molecular patterns and damage-associated molecular patterns activate macrophages and dendritic cells to produce IL (interleukin)-12, which, in turn, stimulates natural killer cells to produce IFN-γ (interferon gamma). IFN-γ is a key molecule in this process, as it activates macrophages to produce iNOS (inducible nitric oxide synthase) and NO production, both of which are essential in effectively targeting and combating the T. cruzi.18
Additionally, another cytokine, TNF-α (tumor necrosis factor-alpha), complements this response by signaling IFN-γ-activated macrophages and T. cruzi-infected cardiomyocytes to increase NO synthesis, enhancing the antiparasitic response. This confluence of IFN-γ and TNF-α induces a cytokine storm that prompts infected macrophages to escalate iNOS expression and NO production.19 Importantly, iNOS and NO play relevant roles in altering potassium and calcium currents within infected cardiomyocytes, thus impacting the heart’s electrical signaling.
Following T. cruzi infection, macrophages stimulated by proinflammatory cytokines also increase the production of reactive oxygen species (ROS). These molecules can help in controlling the infection by causing oxidative stress to the parasite. However, similar to NO, high levels of ROS can exacerbate oxidative stress in host tissues, contributing to cell damage, inflammation, and the chronic manifestations of CD.12,13,17
It is important to highlight that cytokine production in response to T. cruzi infection is not limited to immune cells. Cardiac fibroblasts, endothelial cells, and cardiomyocytes themselves contribute to a wider immunologic response. Cytokines such as IFN-γ, TNF-α, IL-1β, TGF-β (transforming growth factor-beta), and IL-10 are upregulated in heart cells cultured with T. cruzi, influencing electrical and contractile functions of the heart muscle and driving the remodeling process that characterizes CCM.19
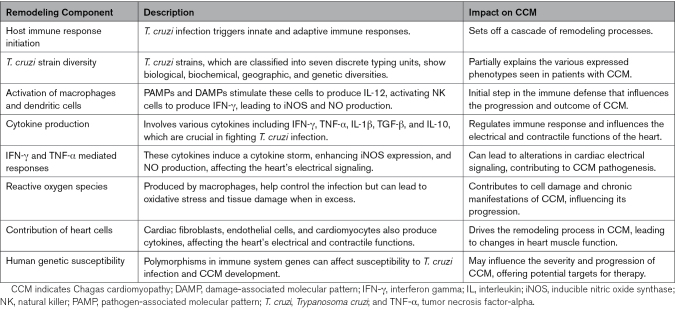
The significance of immune system remodeling to the pathogenesis of CCM is further highlighted by several lines of evidence showing that polymorphisms in genes involved in the structure and function of the immune system are associated with susceptibility to T. cruzi infection and development of CCM.20 For example, a reduction of inflammation and iNOS expression occurs in IL-18-deficient mice infected with T. cruzi, suggesting that the deficiency of IL-18 is cardioprotective during the infection.21 Table 1 summarizes the principal features of the immunologic remodeling in CCM.
Table 1.
Summary of Immunologic Remodeling in CCM
ELECTRICAL REMODELING IN CCM
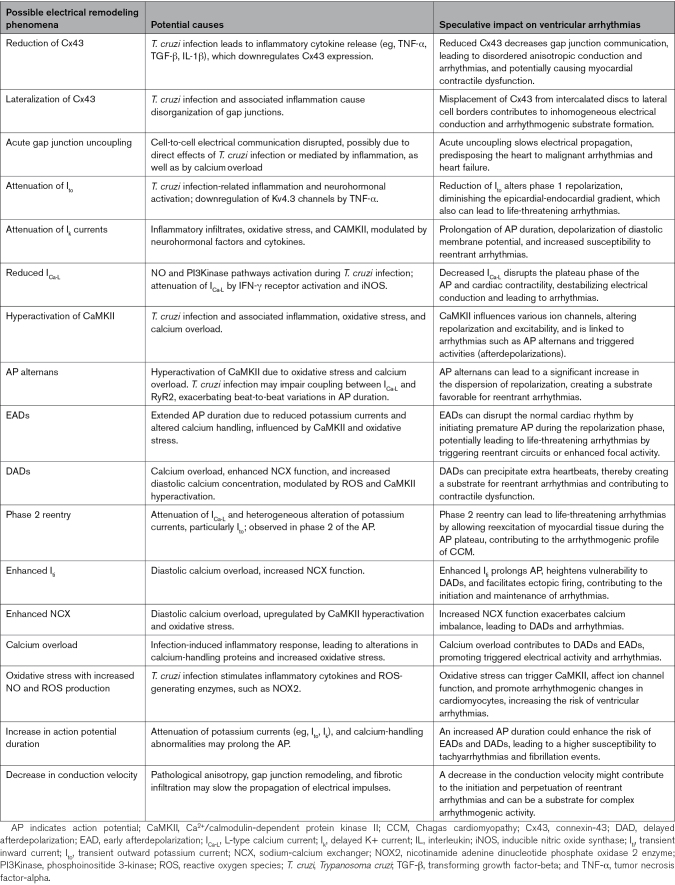
The current knowledge of the cellular basis of the observed arrhythmias derives mostly from investigations in experimental murine models of CCM and, to a lesser extent, from the heart tissue of patients with CCM. Both animal and human models suggest that myocytes infected with T. cruzi exhibit disruptions in several key components of intrinsic cardiac electrophysiology, which includes abnormalities in gap junctions involving Cx43 (connexin-43), potassium channels, and calcium-handling mechanisms. These key features of the cellular machinery related to the electrical signaling remodeling are summarized in Table 2 and illustrated in Figure 4.
Table 2.
Summary of the Various Possible Mechanisms of Electrical Remodeling Observed in CCM, Their Potential Causes, and Their Speculative Implications for Ventricular Arrhythmias
Figure 4.
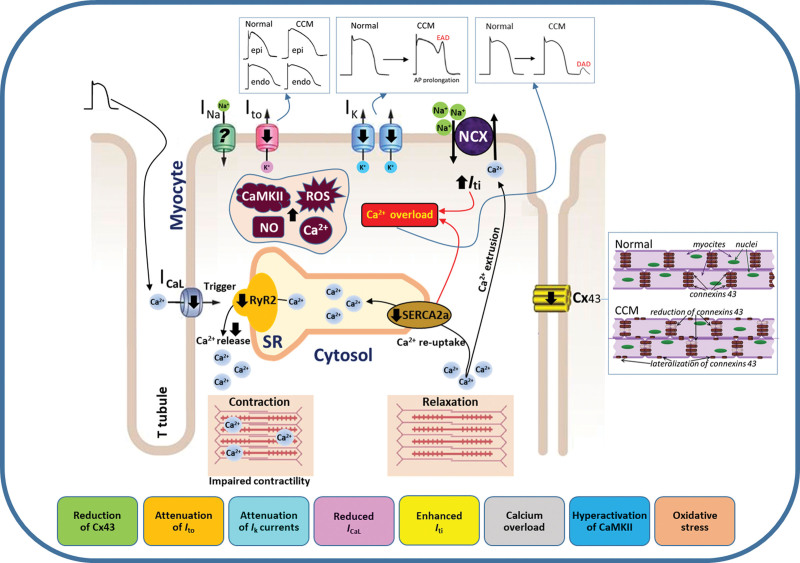
Electrical remodeling and arrhythmogenic mechanisms in Chagas cardiomyopathy (CCM). The infection with Trypanosoma cruzi (T. cruzi) initiates a complex sequence of alterations in cardiac myocytes that significantly disrupts the heart’s rhythm and contractile function. CCM is characterized by a cascade of events that include impaired calcium influx through attenuated L-type calcium channels (ICa-L), which is essential for triggering the release of calcium from the sarcoplasmic reticulum (SR) through the RyR2 (ryanodine receptor type 2). This disruption leads to insufficient release of calcium ions during systole, affecting myocardial contractility. Concurrently, the reuptake of calcium by the SR during diastole, mediated by a dysfunctional SERCA2a (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase) pump, causes a harmful cytosolic calcium overload. Additionally, disruptions in the function of potassium channels have also been observed. There is a marked attenuation of the transient outward potassium current (Ito) and the delayed rectifier potassium current (Ik) that are essential for the repolarization phase of the cardiac action potential (AP). This dysfunction results in a prolonged AP duration, creating a favorable environment for early afterdepolarizations (EADs). In parallel, the heart’s electrical connectivity is impaired due to a reduction in Cx43 (connexin-43) expression, disrupting the spread of electrical impulses and leading to disordered anisotropic conduction and desynchronized myocardial contractions. The compromised ionic balance triggers the activation of compensatory mechanisms, such as the sodium-calcium exchanger (NCX), which acts to expel excess calcium from the cell. This compensatory increase, depicted as enhanced transient inward current (Iti) in the diagram, may inadvertently become counterproductive, potentially precipitating additional electrical irregularities that can provoke delayed afterdepolarizations (DADs) during the heart diastolic phase. Oxidative stress further aggravates the situation, with increased levels of reactive oxygen species (ROS) and NO promoting the hyperactivation of CaMKII (Ca2+/calmodulin-dependent protein kinase II). This enzyme, when overstimulated, can modify the function of ion channels and calcium-handling proteins, further promoting the development of arrhythmias. In summary, arrhythmogenesis in CCM is characterized by calcium mishandling, ionic current imbalances, reduced intercellular communication, and oxidative stress. This comprehensive depiction of electrical remodeling may provide insights into the potential therapeutic targets for mitigating the progression of cardiac arrhythmias and myocardial dysfunction in CCM.
Connexin Remodeling
Gap junctions, particularly Cx43, are crucial for uniform anisotropic conduction in the heart, ensuring synchronized cardiac muscle contraction.22 Studies in both murine23 and human24 tissues have demonstrated that T. cruzi infection leads to reduction and lateralization of Cx43 expression in gap junctions, which are linked to a high proarrhythmic substrate and can also induce myocardial contractile dysfunction. Elevated levels of proinflammatory cytokines, such as TGF-β and IL-1β, contribute to this reduction. Moreover, genetic mutation with loss of function in Cx43 has been associated with sudden death in human infants25 and the development of bundle branch block,26 both of which are observed in patients with CCM.5,9 It is also important to note that acute gap junction uncoupling, in which direct cell-to-cell electrical communication is disrupted, causes slowing of the spread of electrical excitation, which facilitates the occurrence of malignant arrhythmias, electromechanical dysfunction, and heart failure.27
Potassium Current Remodeling
Alterations in the transient outward potassium current (Ito) and in the delayed K+ current (Ik) within cardiomyocytes can significantly impact cardiac electrophysiology and lead to arrhythmias.28,29 Ito, which is responsible for the initial phase of repolarization, is represented by a characteristic notch in the early phase of the human action potential (AP) repolarization. In the normal heart, there is an intrinsic Ito density gradient: it is more pronounced in the epicardium and less expressed in the endocardium. Of note, the loss of the notch gradient, particularly in the right ventricle, has been associated with the appearance of life-threatening arrhythmias in humans with diseases of distinct etiologies, such as the Brugada syndrome.30 The reduction of Ito and Ik in T. cruzi infection, which results in the prolongation of AP duration, involves a complex interplay of several key mechanisms and was observed in the setting of intense inflammatory infiltrates affecting the heart, in both dog and mice models of T. cruzi induced myocarditis.28,31–39
The neurohormonal activation, particularly alterations in the adrenergic pathway influenced by factors such as noradrenaline production, plays a significant role in modulating these potassium currents, especially Ito. Moreover, the infection-induced immune response leads to heightened oxidative stress and increased production of NO and ROS within the heart tissue.40 These biochemical changes can attenuate the potassium currents by impacting the channels’ structure and function. During the chronic phase of the infection, TNF-α38 can also downregulate Kv4.3 channels and function,28,34,35 which are pivotal for Ito, and potentially influence Ik channels, demonstrating the profound effect of cytokine-mediated inflammatory and immune responses on cardiac cellular functions and ion channel expression.
Additionally, specific signaling pathways, such as CaMKII (Ca2+/calmodulin-dependent protein kinase II), are also implicated in this process.41 CaMKII, which is hyperactivated in CCM (and will be discussed in detail later), can directly modulate potassium channel function and expression, further influencing the cardiac AP and the heart’s electrical stability.
Despite the lack of direct evidence linking potassium current disruptions to arrhythmic patients with CCM, it seems plausible to speculate that in those patients, a more severe pathophysiological scenario may indeed be present, similar to what has been described in end-stage heart failure of other etiologies associated with increased circulating TNF-α levels, and in whom the attenuation of Ito was already fully documented.42–44
L-Type Calcium Current Remodeling
L-type calcium current (ICa-L) is crucial for the plateau phase of the cardiac AP. This current is responsible for the calcium influx that triggers the release of calcium from the sarcoplasmic reticulum (SR), which, in turn, plays a pivotal role in the contractile function of the cardiomyocytes. In T. cruzi infection, there is a significant reduction in ICa-L density across the heart, affecting both the right and left ventricles during the acute and chronic phases of CD.31–34,36 This widespread attenuation of ICa-L also disrupts the heart’s electrical stability and contractile function. Studies in the mice model have demonstrated that NO and PI3Kinase (phosphoinositide 3-kinase) pathways are key contributors to ICa-L attenuation.35,36,45,46 From these studies, it is plausible to assume that infected cardiomyocytes, through activation of IFN-α receptors, have enhanced NO production through iNOS, which activates the PIK3kinase pathway, resulting in attenuation of ICa-L, a concept warranting further investigation.
Additional pathways may be involved in the attenuation of ICa-L during T. cruzi infection. NO, through activation of cyclic guanosine monophosphate production, has been reported to regulate the cardiac Cav1.2 channels by various mechanisms, which could lead to activation or inhibition of ICa-L.47,48 In one study, it was reported that cardiomyocytes isolated from mice infected with T. cruzi, but lacking the NOX2−/− (nicotinamide adenine dinucleotide phosphate oxidase 2 enzyme), have neither attenuation of ICa-L nor the Ito and Ik alterations, compared with normal infected mice.37 Importantly, NOX2 is fundamental to global ROS generation in cardiomyocytes during stress conditions,49 and this enzyme is a relevant source of ROS during T. cruzi infection.50
Interestingly, increased availability of NO was detected in isolated cardiomyocytes of infected NOX2−/− mice, compared with all other groups studied, an effect shown to be associated with an arrhythmogenic cellular profile.35 Thus, the interaction between NOX2-derived ROS and NO in the context of CCM is intricate and not fully understood. On the one hand, the absence of NOX2 (and, thus, lower ROS production) seems to protect against changes in heart ion currents that are typically seen with T. cruzi infection. In contrast, the resulting increase in NO levels associated with NOX2 deficiency could pose risks for the induction of heart rhythm disturbances.
CaMKII, Calcium Overload, Early Afterdepolarizations, and Delayed Afterdepolarizations
The role of CaMKII, a multifunctional enzyme that modulates various aspects of cardiomyocyte function, was studied in an experimental model of CCM.41 In this model, where hyperactivation of CAMKII is triggered by T. cruzi infection, most mice exhibited recurrent arrhythmic manifestations, such as triggered activity from ectopic foci, including atrial and ventricular extrasystoles, along with other ECG abnormalities commonly observed in patients with CCM. Furthermore, these cardiomyocytes demonstrated a highly arrhythmogenic profile, notably characterized by AP alternans, a beat-to-beat oscillation in AP duration. This phenomenon is linked to impaired coupling between ICa-L and RyR2 (ryanodine receptor type 2), potentially exacerbating the alternans phenotype, which was previously documented to occur in experimental CCM.35
AP alternan is believed to contribute significantly to the prolongation of AP duration and to the dispersion of the QT interval, thereby creating a favorable environment for arrhythmias.51 Notably, an increase in QT interval dispersion is an adverse prognostic indicator in patients with CCM, underscoring the proarrhythmic nature of AP alternans.52
Hyperactivation of CaMKII may result from increased oxidative stress and calcium overload, conditions also described in human CCM cases.53,54 Pharmacological inhibition of CaMKII has been shown to block arrhythmogenic mechanisms, such as AP alternans and Iti, a transient inward current characterized by a brief flow of ions into the cardiomyocyte during diastole, influencing the late phase of the cardiac AP in infected cardiomyocytes. Moreover, inhibiting the Ca2+/CaM (Ca2+ bound calmodulin)-CaMKII axis with the molecule KN-93 reversed the arrhythmogenic propensity in T. cruzi-infected ex vivo hearts, thus corroborating previously reported in vitro findings.41 The important role of CaMKII in CCM pathogenesis is further highlighted by its established hyperactivation in various human heart diseases through excessive ROS, contributing to diverse arrhythmias and SCD.55
Another proposed mechanism for malignant arrhythmias in CCM involves afterdepolarizations, specifically early afterdepolarizations (EADs) and delayed afterdepolarizations (DADs), arising from multiple foci and spreading through cardiac tissue. These phenomena, well-documented in experimental T. cruzi infection,37,41,56 are supported by a reduction in potassium currents, which extends AP repolarization and is commonly related to EADs. This reduction in potassium current is consistently observed in T. cruzi experimental infections. Additionally, other currents, such as the late sodium current, might also play a significant role, akin to their contribution to hereditary arrhythmogenic cardiomyopathies, such as the long-QT syndrome type-3,57 although their involvement in CCM needs further evaluation.
DADs are primarily associated with Iti, resulting from cytoplasmic calcium overload in cardiomyocytes, leading to a depolarizing influence adjacent to the sodium-calcium exchanger (NCX). Increased diastolic calcium concentration, a hallmark of T. cruzi infection in both experimental models37,56 and humans53 with CCM, clearly exacerbates this condition.
Isolated cardiomyocytes from T. cruzi-infected mice exhibited numerous EADs and DADs, attributed to enhanced NCX and Iti, both modulated by CaMKII.41 Notably, oxidative stress present in early CCM can activate CaMKII, contributing to the attenuation of the potassium current. Thus, the low-grade but persistent inflammatory response in early CCM likely amplifies cardiomyocyte oxidative stress, altering intracellular signaling pathways and leading to CaMKII hyperactivation. The hyperactivation of this enzyme, in turn, alters ion current functions in cardiomyocytes, modifying cellular excitability. The aforementioned impaired calcium dynamics coincide with reduced potassium currents in T. cruzi experimental infections,32,35,36 which is also associated with a depolarized membrane potential in isolated cardiomyocytes. A more depolarized resting membrane potential increases the likelihood of afterdepolarization-triggered activities by lowering the threshold amplitude for EADs/DADs, thus facilitating the spread of arrhythmogenic events to neighboring cells.
Finally, phase 2 reentry, a potential mechanism for life-threatening arrhythmias in CCM, involves the electrotonic spread of current from areas displaying spike-and-dome APs, reexciting regions where the AP dome is lost. This reentry is facilitated by the heterogeneous alteration of potassium currents, especially Ito, crucial for the spike-and-dome AP shape in humans.58 Experimental T. cruzi infection causes attenuation of ICa-L and provides a favorable environment for phase 2 reentry. This phenomenon, coupled with AP alternans, also observed in experimental CD, may exacerbate reexcitation.51 The similarity between the arrhythmogenic mechanisms of phase 2 reentry in CCM and Brugada syndrome, where structural cardiac remodeling is absent, underscores the need for focused research on phase 2 reentry’s role in CCM.
Iti and NCX
As previously mentioned, a prominent feature in CD is the occurrence of diastolic calcium overload within cardiomyocytes, a condition that is consistent across human patients53,54 and animal models,37,41,56 manifesting in both the acute and chronic phases of the disease. Central to these disturbances is the enhancement of Iti, which is intricately closely linked with the increased function of NCX, a critical membrane protein that regulates the exchange of sodium and calcium ions across the cardiomyocyte membrane.
In another investigation, a tachycardia protocol mimicking an AP from infected and noninfected mice was simulated,41 and enhanced Iti was observed in CD cardiomyocytes compared with control noninfected cardiomyocytes. This finding has significant implications for therapeutic strategies targeting electrical remodeling in CCM. It indicates that simply restoring the AP waveform is not sufficient to stabilize the diastolic membrane potential, which is often found depolarized in CCM. Addressing only the AP waveform falls short of correcting the arrhythmogenic propensity inherent to CCM, pointing to the need for a more comprehensive therapeutic approach.
Moreover, the combined effects of diastolic calcium overload, enhanced Iti, and increased NCX function lead to a prolongation of the AP duration during the repolarization phase. This slower repolarization process is crucial because it keeps the cardiomyocytes in a prolonged state of vulnerability.59 Specifically, such disturbances can lead to DADs, initiating reentrant circuits, a primary mechanism behind the arrhythmias observed in CCM.
In addition, using a well-known blocker of NCX current (INCX; SEA0400), an abnormal AP waveform was reversed and led to attenuation of Iti, suggesting an important involvement of NCX in the arrhythmogenesis seen in CCM.56 Finally, Iti also plays a significant role in other arrhythmogenic diseases associated with SCD such as congenital long-QT syndrome type-3.57
AUTONOMIC REMODELING IN CCM
The first suggestion that the cardiac autonomic system was involved in the arrhythmic manifestations of CCM appeared when Carlos Chagas and the cardiologist Eurico Villela described a blunted chronotropic response to atropine in patients with CCM along with severe ECG alterations, such as ventricular arrhythmias and atrioventricular block.60 At the time, they hypothesized that cardiac autonomic impairment was involved in the causation of sudden death that they observed in patients with CCM. The mechanistic underpinnings of that hypothesis remained elusive until recently when significant work was done to further understand the cardiac autonomic remodeling observed in CCM.
Pathological Evidence of Cardiac Autonomic Denervation
At autopsy, patients with CCM were found to have intense neuronal depopulation in the parasympathetic cardiac intramural ganglia.61,62 Investigations in animal models of experimental infection with T. cruzi also showed marked cardiac neuronal depopulation, with parasitism associated with intramural parasympathetic periganglionitis and degeneration of neural cells and fibers.63–66
Although parasympathetic neuronal depopulation is not specific to CD, several studies directly comparing autopsy materials from patients with inflammatory rheumatic and noninflammatory heart diseases (endomyocardial fibrosis, dilated cardiomyopathy, and hypertensive cardiomyopathy) showed that cardiac intramural denervation was much more prominent in autopsied patients with CCM.67–69 There is evidence both in humans and experimentally infected animals that at least 3 pathogenetic mechanisms are responsible for neuronal loss in Chagas heart disease that is deemed to occur predominantly during the acute phase of the infection: necrosis induced by direct parasitism of neurons, degeneration caused by periganglionic inflammation, and antineuronal autoimmune reaction.70,71
Evidence of Functional Abnormalities Caused by Cardiac Autonomic Denervation
Studies have demonstrated that in most cases, individuals with CD are deprived of the tonic inhibitory action normally exerted by the parasympathetic system on the sinus node.72 As a consequence, patients with CD constitute a natural model for studies of an autonomic remodeling of the heart functionality73,74 because they lack the parasympathetic mediated mechanism to respond with rapid bradycardia or tachycardia to transient changes in blood pressure or venous return.72
The dysautonomia in Chagas patients can be typically detected before the development of ventricular dysfunction and in all phases and forms of the disease, including the indeterminate and digestive forms.75–77 In addition, it is mostly irreversible and distinct from the nonspecific autonomic impairment that occurs in heart failure of whatever cause, which is due to neurohumoral activation and postsynaptic desensitization of neural pathways78,79 and has been shown to be at least partially reversible with clinical compensation of heart failure.80
Consequences of the Autonomic Remodeling in Chronic CCM
Given that neuronal depopulation and aperistalsis are considered to be the essential pathogenetic mechanism of digestive CD,81,82 it was postulated that CCM would be caused by lack of vagal influence on the heart for 2 reasons.83,84 First, it was thought that long-standing autonomic imbalance would eventually lead to catecholamine-induced cardiomyopathy.61,62 Second, it was hypothesized that structural changes would occur due to impairment of the heart to adapt to transient changes in venous return and blood pressure.72,85 However, tilt table tests performed under baseline conditions and after selective blockade with atropine and propranolol showed that although less conspicuous, there is also impairment of the adrenergic innervation of the sinus node in chronic CD.85 These findings showing attenuated sympathetically mediated sinus node responses were corroborated by studies employing power spectrum analyses focusing on the depressed vagal and adrenergic influences that are responsible for reduced heart rate variability in patients with CCM.86,87
Nevertheless, potentially even more important is the role of autonomic remodeling in the development of ventricular arrhythmias. There is now evidence of disturbances of sympathetic innervation at the ventricular level, which are clearly associated with serious ventricular arrhythmias. These investigations used single-photon emission computed tomography myocardial scintigraphy with meta-iodine-benzyl-guanidine labeled by 123Iodine to evaluate adrenergic myocardial innervation. The findings revealed diminished tracer uptake in areas that typically later exhibit perfusion defects and wall motion abnormalities. This reduced uptake was observed even in individuals presenting with the indeterminate form of CD.88,89 In fact, the presence and severity of ventricular arrhythmias in patients with CCM have been shown to correlate with regional sympathetic myocardial denervation even better than with fibrosis, another hallmark of this arrhythmogenic disease.90,91
These findings suggest that autonomic remodeling-related disturbances could serve as early arrhythmia triggers, even before the development of significant fibrosis, which is a more definitive mechanism for the onset of malignant ventricular arrhythmias but typically occurs in later stages of the disease. This hypothesis is supported by data from the Syrian hamster model of T. cruzi infection, showing that impaired ventricular wall motion is more closely dependent on histopathologic inflammatory changes, rather than on fibrotic replacement of the myocardial tissue.92 Furthermore, recent studies focusing on the prognostic meaning of dysautonomic remodeling as detected using spectral analysis of heart rate variability reported on the association of the autonomic derangement involving both the parasympathetic and the adrenergic limbs, with an elevated RASSI score, a major prognostic factor in CCM.93
MICROVASCULAR REMODELING IN CCM
The immune-mediated inflammatory reaction to parasitic persistence in the cardiac tissue triggers a remodeling of the coronary vessels at the microscopic and macroscopic levels.94 Histopathologic studies in autopsied humans with CCM demonstrated constriction of intramyocardial arterioles, intimal hyperproliferation, extensive capillary basement membrane thickening, focal myocytolysis—a form of cell death typically caused by repetitive ischemic injury—and reparative fibrosis.95–97 Additionally, in postmortem angiography of patients with CD, there is a striking reduction in the density of myocardial microvessels.98
A hallmark of CCM is the development of LV aneurysms, predominantly at the apex and the inferolateral regions, areas that represent watershed coronary territories.99,100 These regions may present marked anatomic and functional microvascular remodeling that is responsible for abnormal patterns of vasodilation and vasoconstriction, causing transient myocardial ischemia of low intensity and short duration that ultimately leads to aneurysm formation.101,102
Studies of Microvascular Derangements in Experimental Models of T. cruzi Infection
Studies focusing on the acute phase of in vitro animal models of T. cruzi infection have demonstrated the occurrence of myocardial ischemia attributed to multiple factors that include excessive local stimulation by catecholamines, occlusive platelet thrombosis and spasm causing focal vasoconstriction, neuraminidase induced platelet aggregation, endothelial microvascular hyperproliferation, direct damage caused by the parasite interaction with immune effector cells, increased production of endothelin and thromboxane-A2, and inhibition of cAMP endothelial protective role.103–108 In a hamster model of chronic T. cruzi infections, it was found that at 6 months, nearly 50% of the animals had extensive myocardial perfusion defects (MPDs) at rest on single-photon emission computed tomography imaging.92,109 In addition, histology showed that MPD areas had a clear topographical association with regions of inflammatory changes rather than regional transmural fibrosis. Dipyridamole compared with placebo showed a significant reduction of resting MPD in viable myocardium.110 However, this was neither associated with a reduction in myocardial inflammatory histological lesions nor with a reversal of LV systolic dysfunction. In contrast, experiments in mice and Syrian hamster models have shown improvement in perfusion derangements with the administration of dipyridamole and pentoxifylline, respectively.110,111
Evidence of Microvascular Remodeling Derangements from Clinical Studies
There is a paucity of evidence demonstrating abnormal endothelial and nonendothelial dysregulation of coronary flow at the epicardial level. However, significant evidence exists that establishes myocardial ischemia in patients with CCM even in the presence of angiographically normal coronary arteries.88,112–114 In a recent prospective study from Brazil, it was found that 15% of patients referred for diagnostic coronary angiograms had CD, with most of them not having evidence of obstructive epicardial coronary artery disease.115
Several studies have demonstrated both reversible and fixed MPD at various stages of CCM.116,117 Additionally, in patients with CCM and malignant ventricular arrhythmias, MPD regions have demonstrated local myocardial sympathetic denervation.118 It has also been found that in patients with the indeterminate form of CD, there is a reduction in coronary vasodilator flow reserve as demonstrated by stress echocardiography, as well as rest and stress-induced MPD and wall motion abnormalities.119,120 A longitudinal study of patients with CCM revealed that 68% of segments that showed reversibility on scintigraphy scans at baseline progressed to fixed perfusion abnormalities after a 5.6-year follow-up period.114 However, it is believed that the early phases of microvascular remodeling may be reversible as dipyridamole and isosorbide dinitrate, 2 coronary vasodilators, have been associated with short- and long-term improvements in the systolic LV function, even in the absence of improvement in symptoms.121,122 A preliminary study of aspirin plus verapamil in patients with CCM who had chest pain and evidence of MPD demonstrated a significant reduction of reversible perfusion defects and improvement of quality of life scores.123 These results have prompted further investigation into the role of vasodilation in a patient population, with an ongoing randomized controlled trial testing sildenafil on MPD in patients with CCM with normal epicardial coronary arteries.124
CONTRACTILE REMODELING IN CCM
Mechanical remodeling at the cellular and whole heart levels has been shown in both experimental models of T. cruzi infection and humans with CCM. It comprises abnormalities in both the relaxation and the contractile properties of the working myocardium.
Impairment in Relaxation
The ventricular diastolic dysfunction of CCM was first demonstrated in studies of patients with the indeterminate form of CD, which revealed no systolic abnormalities, but a striking elevation of LV end-diastolic pressure.113,125 The diastolic dysfunction identified by independent researchers through diverse echocardiographic techniques126,127 is likely the result of inflammatory and fibrotic alterations observed in endomyocardial biopsies, autopsies, or cardiac magnetic resonance imaging, even in patients with the indeterminate form of CD.128–130 The lusitropic dysfunction is also consistently described in models of CCM in infected mice, which is frequently associated with slower calcium transient decay, indicating a causal role for this phenomenon.35,37,41 Impaired actin-myosin interaction may also play a role.
Impairment in Systolic Function
Importantly, while systolic dysfunction in CD is typically classified as dilated cardiomyopathy, mild regional wall motion abnormalities often precede the deterioration of overall LV function. These abnormalities can be identified even in less common instances of asymptomatic individuals who have a normal ECG.131 The segmental wall motion systolic dysfunction leading to the contractile remodeling can progress from hypokinesis to akinesis, or dyskinesia, causing the characteristic aneurysms that predominate in the LV apical or inferolateral regions of watershed coronary supply.132
Another hallmark of CCM is that contractile systolic impairment involves both ventricles, and, consequently, both systemic and pulmonary congestion can occur concomitantly at later stages of the disease.127 Despite the fact that it is the severe global LV systolic impairment that connotes the worse prognosis in patients with CCM, it is relevant to emphasize that regional wall motion abnormalities are also an important predictor of death. A prospective study conducted in 1508 patients of the BENEFIT randomized trial (Benznidazole Evaluation for Interrupting Trypanosomiasis) showed that despite normal global LV systolic function, regional wall motion abnormalities identified patients at higher risk for a composite of hard adverse clinical outcomes, including death.133
Contractile Remodeling at the Cellular Level
Research using the Syrian hamster model to investigate chronic T. cruzi infections has shown that fibrosis is not necessarily the primary cause of LV wall motion abnormalities.92 Rather, it appears that segmental dysfunction could initially arise from inflammatory alterations, which then lead to disruptions in microvascular perfusion. Moreover, serial studies in humans showed that LV wall motion abnormalities are preceded by regional myocardial sympathetic denervation, another typical feature of CCM that is also involved in provoking malignant arrhythmias.89,92
Murine models of CCM have reported reduction in the amount of Ca2+ released from SR, and this was attributed to diminution of the expression of the calcium ion pump SERCA2a (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase), responsible for Ca2+ reuptake from the sarcoplasm back into the SR.35 Thus, reduced Ca2+ reuptake would lead to incomplete SR refilling, contributing to diminished cardiomyocyte contractile force, one of the hallmarks of CD. At the cellular level, appropriate organization of the excitation-contraction coupling is most likely to be altered in CCM, as already described in experimental models of the disease.35
Normal excitation-contraction coupling functionality relies on the anatomy of dyads, t-tubules, ryanodine receptors, and microtubules. Microtubule responses to stress (ie, excessive shear and long exposure to increased preload) result in increased ROS production in cardiomyocytes.134,135 Stretch-dependent ROS production through activation of NOX2 leads to enhanced and synchronous calcium release from the SR, such as that observed during varying mechanical loads.135 This mechanotransduction pathway contributes to contractile dysfunction, aberrant calcium signaling, and the generation of arrhythmias both when exacerbated or when impaired. There is also evidence that in CCM such structural remodeling occurs. In a murine model of CCM, an asynchronous calcium release from SR was already described.35
In another study using the NOX2−/− mice infected with T. cruzi, it was found that the absence of the enzyme, contrary to the initial hypothesis, led to impaired calcium dynamics and cardiomyocyte contraction in isolated cells, favoring the occurrence of arrhythmias in infected isolated cells compared with infected control mice.37
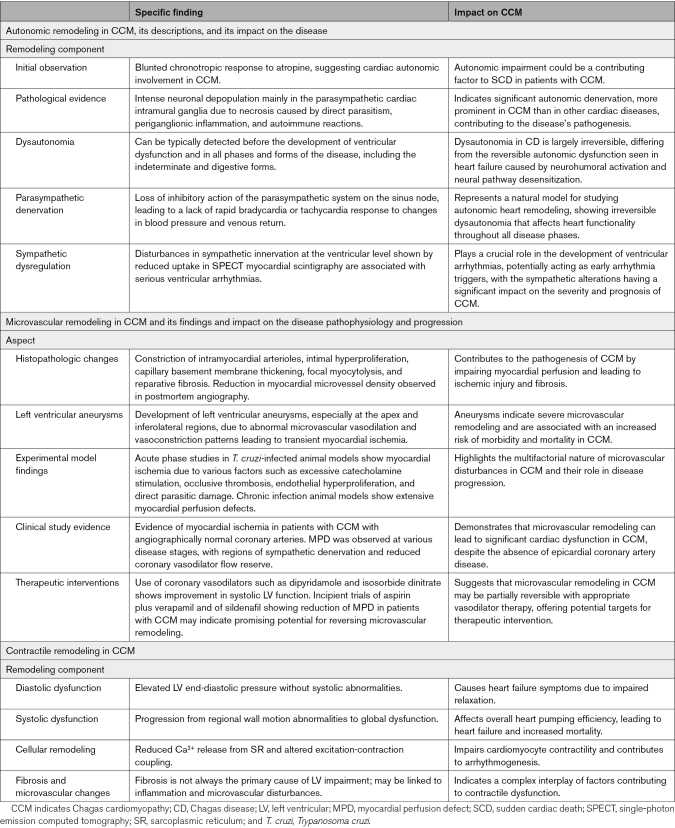
Table 3 summarizes the main insights related to autonomic, microvascular, and contractile remodeling processes in CCM.
Table 3.
Summary of Autonomic, Microvascular, and Contractile Remodelings in CCM
LINKING THE EXPERIMENTAL/CELLULAR DATA TO THE ARRHYTHMOGENIC MANIFESTATIONS OF CCM: FROM BENCH TO BEDSIDE
The full understanding of disease pathogenesis ideally requires a comprehensive knowledge of the molecular changes at the cellular scale, which are responsible for the observed organ-scale remodeling and, ultimately, lead to clinical manifestations. Unfortunately, at this point, we can only propose some general cellular mechanisms potentially related to cardiac arrhythmias in humans. To date, this knowledge is mostly based on experimental models of CD or extrapolation of data from other arrhythmogenic cardiomyopathies.
After T. cruzi infection, CCM takes several years to develop and to express its overt clinical manifestations. The range of arrhythmogenic clinical manifestations varies widely but can be classified as conduction system abnormalities (ie, sinus node dysfunction and atrioventricular conduction derangements) and ventricular arrhythmias (ie, isolated premature ventricular contractions, bigeminy, couplets, nonsustained VT, and, finally, VT and ventricular fibrillation).
Myocardial fibrosis, a most striking and peculiar hallmark of CCM, results from multiple diverse mechanisms, including inflammation directly linked to the tissue parasite persistence and to immune-mediated injury, myocytolytic necrosis induced by ischemia due to microvascular disturbances and, less importantly, apoptosis.136,137 Because it is multifactorial and diffuse, the essential triad of inflammation, cell death, and fibrosis described in experimental models of T. cruzi infection, as well as in human materials obtained from endomyocardial biopsy and autopsy studies, manifests initially as a focal microscopic process that ultimately is coalescent and assumes the form of an organ-scale macroanatomic disorder.138,139
Among others, 2 major remodeling consequences of myocardial fibrosis in CCM result from early contractile mechanical dysfunction that affects both the diastolic and the systolic ventricular performance126,140,141 and, no less conspicuously, the electrical properties of the whole heart, thereby causing various types of arrhythmia. Moreover, extensive ventricular areas exhibit myocardial fibrosis intermingled with abnormally functioning ischemic but still viable fibers. Of note, contractile disturbances have been reported using more sophisticated methods such as speckle-tracking echocardiography in humans with the indeterminate form of CD, even before a significant amount of coalescent fibrosis can be detected by the method of cardiac magnetic resonance.142 Such heterogeneous environments of dispersed electrical conduction properties are key phenomena, leading to focally abnormal patterns of refractoriness that cause unidirectional blocks and reentry circuits, which are the basic substrates for VT and ventricular fibrillation.
Before the expansion of fibrotic tissue in the heart, the most common arrhythmic findings in patients are triggered activities, probably due to the occurrence of focal excitation and spiral waves. It is plausible that the progression of the electrical remodeling at the clinical setting, thereby causing nonsustained or sustained VT, either monomorphic or polymorphic, happens together with the scar accumulation in the heart, thus providing a substrate for not only functional but also anatomic reentrant circuits.51,143 It is interesting to highlight that hyperactivation of CAMKII, together with increased diastolic calcium accumulation, may contribute to the activation of necrotic and apoptotic pathways in cardiomyocytes, leading to both reactive and reparative scar formation.144
In addition, with the progression of cardiomyocyte deaths, more inflammation occurs due to excessive production of pathogen-associated molecular patterns and damage-associated molecular patterns. This effect is particularly relevant because the intensity of inflammation is usually not proportional to the level of tissue parasitism, as found in experimental models of T. cruzi infection and in patients with CCM. From these studies, it is also apparent that additional mechanisms are involved in causing arrhythmias, such as microvascular derangements110,145 and sympathetic innervation abnormalities, which accompany the progression of CCM, as described above.89–91
In contrast to what is more often observed in ischemic cardiomyopathy (ie, malignant arrhythmia occurrence only at more advanced stages of the disease and with higher scar burden), in CCM, patients may experience SCD before any clinically apparent scar formation or contractile dysfunction as assessed by more conventional methods. It has been speculated that some of these catastrophic events may be related to higher susceptibility to oxidative stress and to an enhanced response of the immune system to T. cruzi infection. This speculation has been advanced based on recent experimental evidence supporting the concept that polymorphism of key genes involved in the immune response, following T. cruzi infection, favors the development of CCM and may be responsible for arrhythmogenesis. For example, it was reported that mutation in the RyR2 gene is associated with increased occurrence of polymorphic VT, particularly the catecholaminergic polymorphic VT.146
CONCLUSIONS
Despite some recent achievements in regard to the cellular and molecular basic mechanism of ventricular arrhythmias, the full understanding of the arrhythmogenic manifestations in patients with CCM is still incomplete. There is a relative paucity of literature findings to provide direct evidence of causality between the abnormal oxidative inflammatory profile found in patients with CCM and the remodeling of cardiomyocyte machinery responsible for the electrical behavior of the diseased heart. Moreover, most of the evidence is based on experimental models of T. cruzi infection; hence, a direct correlation of data from cellular and molecular investigations with research findings in human patients remains elusive and awaits further clarification.
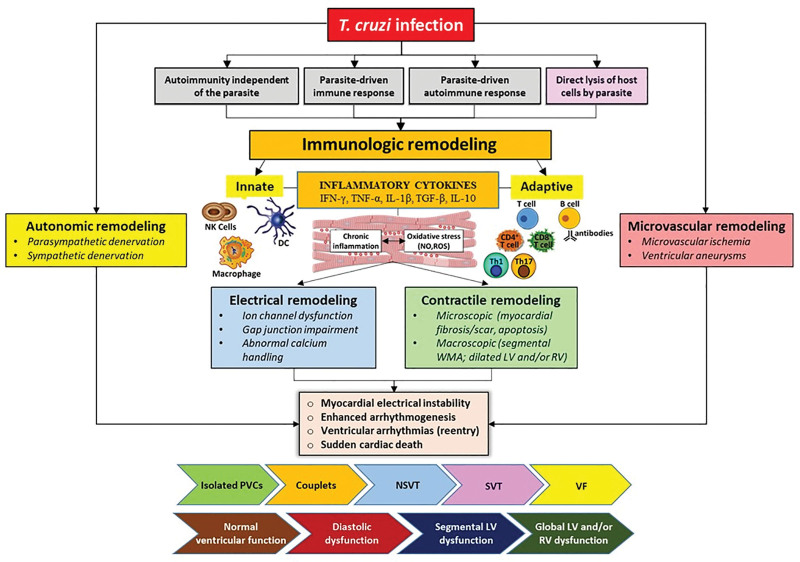
It should be acknowledged that the complex intrinsic pathophysiology of CCM entails remodeling effects acting simultaneously in the immune system, the cellular electrical conduction, the autonomic control, the coronary microvascular structure and function, and the contractile performance of the affected heart (Figure 5).
Figure 5.
Multifaceted cardiac remodeling in Chagas cardiomyopathy (CCM). The clinical course of CCM is characterized by a complex cascade of pathological remodeling processes that encompasses 5 distinct but interrelated phenomena: immunologic, electrical, structural, autonomic, and microvascular. The initial immune response to Trypanosoma cruzi (T. cruzi) infection is a combination of innate and adaptive mechanisms. Innate immunity involves dendritic cells (DCs), natural killer (NK) cells and macrophages, releasing a cascade of inflammatory cytokines such as IFN-γ (interferon gamma), TNF-α (tumor necrosis factor-alpha), IL (interleukin)-1β, TGF-β (transforming growth factor-beta), IL-12 and IL-10, while the adaptive immune response involves T cells and B cells. Although the immune response aims to control the infection, it also induces a state of persistent inflammation and oxidative stress, potentially leading to myocardial injury. Concurrently, the heart undergoes electrical remodeling. This is characterized by a disruption in ion channels’ function and gap junction connectivity, along with aberrant calcium handling, culminating in electrical instability. The consequence is an increased susceptibility to various forms of arrhythmias, ranging from benign premature ventricular contractions (PVCs) to serious ventricular arrhythmias, which can precipitate sudden cardiac death. As the heart faces persistent inflammation and imbalanced immune responses, structural changes ensue. On a microscopic level, there is an accumulation of myocardial fibrosis, scarring, and apoptosis of cardiac cells. Macroscopically, these changes manifest as segmental wall motion abnormalities (WMA) and dilatation of the left ventricle (LV) and right ventricle (RV). The autonomic nervous system is equally affected, undergoing early functional remodeling. The cardiac autonomic innervation, pivotal in modulating heart rate and rhythm, is disrupted through parasympathetic and sympathetic denervation, increasing the propensity for arrhythmogenesis and exacerbating the progression of cardiac dysfunction. Finally, the microcirculations are not spared in this pathogenic odyssey. Microvascular remodeling is characterized by ischemia in watershed zones between the main coronary arteries, leading to the formation of the characteristic aneurysmatic lesions at 2 principal sites of the left ventricle: the apex and the inferolateral wall. In summary, this figure succinctly captures the intricate and interconnected nature of cardiac remodeling induced by CCM, emphasizing the sequential development of ventricular arrhythmias as a hallmark of the disease. Yet, it is critical to acknowledge that ventricular arrhythmias do not always follow a predictable pattern in relation to myocardial changes, indicating a need for nuanced understanding in clinical assessment and management. This multidimensional model underscores the critical points for potential therapeutic intervention and highlights the necessity of a comprehensive approach to mitigate the adverse outcomes associated with CCM. NSVT indicates nonsustained ventricular tachycardia; ROS, reactive oxygen species; SVT, sustained ventricular tachycardia, and VF, ventricular fibrillation.
Treatment of patients with CCM remains challenging. Some of the new potential therapies for CCM lack information on its effects on the electrical function of cardiomyocytes, which makes it hard to optimize treatment guidelines, especially because CCM is a primarily arrhythmogenic pathological entity.2 To accomplish the goal of improving the therapeutic control of such a complex disease, a more comprehensive and integrated understanding of functional remodeling of the cardiovascular system, as proposed in this review, is necessary.
ARTICLE INFORMATION
Acknowledgments
This article was a solicited request by Circulation Research. D. Roman-Campos was invited to write a review article on electrical remodeling in Chagas cardiomyopathy.
Author Contributions
J.A. Marin-Neto and A. Rassi Jr proposed the innovative approach of broadening the scope to encompass 5 distinct remodeling processes. D. Roman-Campos, J.A. Marin-Neto, A. Santos-Miranda, and A. Rassi Jr equally contributed to the study’s conception, design, literature review, data analysis, and initial draft. N. Kong and A. D’Avila commented on previous versions of the article and revised it critically for important intellectual content. A Rassi Jr created all the figures and tables. All authors approved the final article.
Sources of Funding
This work was supported by the Government Official Non-Profitable Research Agency (FAPESP [Sao Paulo State Funding Agency]) grants 2021/05584-7 and 2020/14635-1 to D. Roman-Campos, FAPESP grant 2016/25403-9 to J.A. Marin-Neto, Fundação do Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG) grant APQ-01680-23 and Instituto Serrapilheira: n. 06/2022 grant APQ-05839-23 to Artur Santos-Miranda, and CNPq (National Council for Scientific and Technological Development) grants 304257/2020-6 and 310112/2023-0 to D. Roman-Campos.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- AP
- action potential
- BENEFIT
- Benznidazole Evaluation for Interrupting Trypanosomiasis
- Ca2+/CaM
- Ca2+ bound calmodulin
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- CCM
- Chagas cardiomyopathy
- CD
- Chagas disease
- Cx43
- connexin-43
- DAD
- delayed afterdepolarization
- EAD
- early afterdepolarization
- ICa-L
- L-type calcium current
- IFN-γ
- interferon gamma
- Ik
- delayed K+ current
- IL
- interleukin
- iNOS
- inducible nitric oxide synthase
- Iti
- transient inward current
- Ito
- transient outward potassium current
- LV
- left ventricular
- MPD
- myocardial perfusion defect
- NCX
- sodium-calcium exchanger
- NOX2−/−
- nicotinamide adenine dinucleotide phosphate oxidase 2 enzyme
- PI3Kinase
- phosphoinositide 3-kinase
- ROS
- reactive oxygen species
- RyR2
- ryanodine receptor type 2
- SCD
- sudden cardiac death
- SERCA2a
- sarcoplasmic/endoplasmic reticulum Ca2+-ATPase
- SR
- sarcoplasmic reticulum
- T. cruzi
- Trypanosoma cruzi
- TGF-β
- transforming growth factor-beta
- TNF-α
- tumor necrosis factor-alpha
- VT
- ventricular tachycardia
For Sources of Funding and Disclosures, see page 1393.
REFERENCES
- 1.Chagas C. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz. 1909;1:159–218. doi: 10.1590/s0074-02761909000200008 [Google Scholar]
- 2.Marin-Neto JA, Rassi A, Jr, Oliveira GMM, Correia LCL, Ramos Júnior AN, Luquetti AO, Hasslocher-Moreno AM, Sousa AS, Paola AAV, Sousa ACS, et al. SBC guideline on the diagnosis and treatment of patients with cardiomyopathy of Chagas disease - 2023. Arq Bras Cardiol. 2023;120:e20230269. doi: 10.36660/abc.20230269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irish A, Whitman JD, Clark EH, Marcus R, Bern C. Updated estimates and mapping for prevalence of Chagas disease among adults, United States. Emerg Infect Dis. 2022;28:1313–1320. doi: 10.3201/eid2807.212221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynn MK, Bossak BH, Sandifer PA, Watson A, Nolan MS. Contemporary autochthonous human Chagas disease in the USA. Acta Trop. 2020;205:105361. doi: 10.1016/j.actatropica.2020.105361 [DOI] [PubMed] [Google Scholar]
- 5.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X [DOI] [PubMed] [Google Scholar]
- 6.Rassi A, Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, Rassi GG, Hasslocher-Moreno A, Sousa AS, Scanavacca MI. Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med. 2006;355:799–808. doi: 10.1056/NEJMoa053241 [DOI] [PubMed] [Google Scholar]
- 7.Rassi Júnior A, Gabriel Rassi A, Gabriel Rassi S, Rassi Júnior L, Rassi A. Arritmias ventriculares na doença de Chagas. Particularidades diagnósticas, prognósticas e terapêuticas [Ventricular arrhythmia in Chagas disease. Diagnostic, prognostic, and therapeutic features]. Arq Bras Cardiol. 1995;65:377–387. [PubMed] [Google Scholar]
- 8.Sternick EB, Martinelli M, Sampaio R, Gerken LM, Teixeira RA, Scarpelli R, Scanavacca M, Nishioka SD, Sosa E. Sudden cardiac death in patients with Chagas heart disease and preserved left ventricular function. J Cardiovasc Electrophysiol. 2006;17:113–116. doi: 10.1111/j.1540-8167.2005.00315.x [DOI] [PubMed] [Google Scholar]
- 9.Rassi A, Jr, Rassi SG, Rassi A. Sudden death in Chagas’ disease. Arq Bras Cardiol. 2001;76:75–96. doi: 10.1590/s0066-782x2001000100008 [DOI] [PubMed] [Google Scholar]
- 10.Rassi A, de Rezende JM, Luquetti AO, Rassi A. Clinical phases and forms of Chagas disease. In: Telleria J, Tibayrenc M, eds. American Trypanosomiasis-Chagas Disease (Second Edition). Elsevier; 2017:653–686. [Google Scholar]
- 11.Higuchi Mde L, De Brito T, Martins Reis M, Barbosa A, Bellotti G, Pereira-Barreto AC, Pileggi F. Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic chagasic myocarditis: light microscopy and immunohistochemical findings. Cardiovasc Pathol. 1993;2:101–106. doi: 10.1016/1054-8807(93)90021-S [DOI] [PubMed] [Google Scholar]
- 12.Acevedo GR, Girard MC, Gómez KA. The unsolved jigsaw puzzle of the immune response in Chagas disease. Front Immunol. 2018;9:1929. doi: 10.3389/fimmu.2018.01929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhuri S, Rios L, Vázquez-Chagoyán JC, Garg NJ. Oxidative stress implications for therapeutic vaccine development against Chagas disease. Expert Rev Vaccines. 2021;20:1395–1406. doi: 10.1080/14760584.2021.1969230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magalhães LMD, Gollob KJ, Zingales B, Dutra WO. Pathogen diversity, immunity, and the fate of infections: lessons learned from Trypanosoma cruzi human-host interactions. Lancet Microbe. 2022;3:e711–e722. doi: 10.1016/S2666-5247(21)00265-2 [DOI] [PubMed] [Google Scholar]
- 15.Zingales B, Macedo AM. Fifteen years after the definition of Trypanosoma cruzi DTUs: What have we learned? Life (Basel). 2023;13:2339. doi: 10.3390/life13122339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pech-Canul AC, Monteón V, Solís-Oviedo RL. A brief view of the surface membrane proteins from Trypanosoma cruzi. J Parasitol Res. 2017;2017:3751403. doi: 10.1155/2017/3751403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-Mazliah D, Ward AI, Lewis MD. Host-parasite dynamics in Chagas disease from systemic to hyper-local scales. Parasite Immunol. 2021;43:e12786. doi: 10.1111/pim.12786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vespa GN, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machado FS, Martins GA, Aliberti JC, Mestriner FL, Cunha FQ, Silva JS. Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation. 2000;102:3003–3008. doi: 10.1161/01.cir.102.24.3003 [DOI] [PubMed] [Google Scholar]
- 20.Frade-Barros AF, Ianni BM, Cabantous S, Pissetti CW, Saba B, Lin-Wang HT, Buck P, Marin-Neto JA, Schmidt A, Dias F, et al. Polymorphisms in genes affecting interferon-γ production and Th1 T cell differentiation are associated with progression to Chagas disease cardiomyopathy. Front Immunol. 2020;11:1386. doi: 10.3389/fimmu.2020.01386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esper L, Utsch L, Soriani FM, Brant F, Esteves Arantes RM, Campos CF, Pinho V, Souza DG, Teixeira MM, Tanowitz HB, et al. Regulatory effects of IL-18 on cytokine profiles and development of myocarditis during Trypanosoma cruzi infection. Microbes Infect. 2014;16:481–490. doi: 10.1016/j.micinf.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 22.Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Carvalho AC, Tanowitz HB, Wittner M, Dermietzel R, Roy C, Hertzberg EL, Spray DC. Gap junction distribution is altered between cardiac myocytes infected with Trypanosoma cruzi. Circ Res. 1992;70:733–742. doi: 10.1161/01.res.70.4.733 [DOI] [PubMed] [Google Scholar]
- 24.Waghabi MC, Coutinho-Silva R, Feige JJ, Higuchi Mde L, Becker D, Burnstock G, Araújo-Jorge TC. Gap junction reduction in cardiomyocytes following transforming growth factor-beta treatment and Trypanosoma cruzi infection. Mem Inst Oswaldo Cruz. 2009;104:1083–1090. doi: 10.1590/s0074-02762009000800004 [DOI] [PubMed] [Google Scholar]
- 25.Van Norstrand DW, Asimaki A, Rubinos C, Dolmatova E, Srinivas M, Tester DJ, Saffitz JE, Duffy HS, Ackerman MJ. Connexin43 mutation causes heterogeneous gap junction loss and sudden infant death. Circulation. 2012;125:474–481. doi: 10.1161/CIRCULATIONAHA.111.057224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladenvall P, Andersson B, Dellborg M, Hansson PO, Eriksson H, Thelle D, Eriksson P. Genetic variation at the human connexin 43 locus but not at the connexin 40 locus is associated with left bundle branch block. Open Heart. 2015;2:e000187. doi: 10.1136/openhrt-2014-000187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leybaert L, De Smet MA, Lissoni A, Allewaert R, Roderick HL, Bultynck G, Delmar M, Sipido KR, Witschas K. Connexin hemichannels as candidate targets for cardioprotective and anti-arrhythmic treatments. J Clin Invest. 2023;133:e168117. doi: 10.1172/JCI168117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacioretty LM, Barr SC, Han WP, Gilmour RF, Jr. Reduction of the transient outward potassium current in a canine model of Chagas’ disease. Am J Physiol. 1995;268:H1258–H1264. doi: 10.1152/ajpheart.1995.268.3.H1258 [DOI] [PubMed] [Google Scholar]
- 29.Chiamvimonvat N, Chen-Izu Y, Clancy CE, Deschenes I, Dobrev D, Heijman J, Izu L, Qu Z, Ripplinger CM, Vandenberg JI, et al. Potassium currents in the heart: functional roles in repolarization, arrhythmia and therapeutics. J Physiol. 2017;595:2229–2252. doi: 10.1113/JP272883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660 [DOI] [PubMed] [Google Scholar]
- 31.Cruz JS, Machado FS, Ropert C, Roman-Campos D. Molecular mechanisms of cardiac electromechanical remodeling during Chagas disease: role of TNF and TGF-beta. Trends Cardiovasc Med. 2017;27:81–91. doi: 10.1016/j.tcm.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 32.Cruz JS, Santos-Miranda A, Sales-Junior PA, Monti-Rocha R, Campos PP, Machado FS, Roman-Campos D. Altered cardiomyocyte function and Trypanosoma cruzi persistence in Chagas disease. Am J Trop Med Hyg. 2016;94:1028–1033. doi: 10.4269/ajtmh.15-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esper L, Roman-Campos D, Lara A, Brant F, Castro LL, Barroso A, Araujo RR, Vieira LQ, Mukherjee S, Gomes ER, et al. Role of SOCS2 in modulating heart damage and function in a murine model of acute Chagas disease. Am J Pathol. 2012;181:130–140. doi: 10.1016/j.ajpath.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roman-Campos D, Sales-Junior P, Duarte HL, Gomes ER, Guatimosim S, Ropert C, Gazzinelli RT, Cruz JS. Cardiomyocyte dysfunction during the chronic phase of Chagas disease. Mem Inst Oswaldo Cruz. 2013;108:243–245. doi: 10.1590/0074-0276108022013019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roman-Campos D, Sales-Junior P, Duarte HL, Gomes ER, Lara A, Campos P, Rocha NN, Resende RR, Ferreira A, Guatimosim S, et al. Novel insights into the development of chagasic cardiomyopathy: role of PI3Kinase/NO axis. Int J Cardiol. 2013;167:3011–3020. doi: 10.1016/j.ijcard.2012.09.020 [DOI] [PubMed] [Google Scholar]
- 36.Roman-Campos D, Sales-Junior P, Santos-Miranda A, Joviano-Santos JV, Ropert C, Cruz JS. Deletion of inducible nitric oxide synthase delays the onset of cardiomyocyte electrical remodeling in experimental Chagas disease. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165949. doi: 10.1016/j.bbadis.2020.165949 [DOI] [PubMed] [Google Scholar]
- 37.Santos-Miranda A, Joviano-Santos JV, Ribeiro GA, Botelho AFM, Rocha P, Vieira LQ, Cruz JS, Roman-Campos D. Reactive oxygen species and nitric oxide imbalances lead to in vivo and in vitro arrhythmogenic phenotype in acute phase of experimental Chagas disease. PLoS Pathog. 2020;16:e1008379. doi: 10.1371/journal.ppat.1008379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roman-Campos D, Duarte HL, Sales PA, Jr, Natali AJ, Ropert C, Gazzinelli RT, Cruz JS. Changes in cellular contractility and cytokines profile during Trypanosoma cruzi infection in mice. Basic Res Cardiol. 2009;104:238–246. doi: 10.1007/s00395-009-0776-x [DOI] [PubMed] [Google Scholar]
- 39.Roman-Campos D, Sales-Junior P, Costa AD, Souza DS, Santos-Miranda A, Joviano-Santos JV, Ropert C, Cruz JS. Impact of IFN-γ deficiency on the cardiomyocyte function in the first stage of experimental Chagas disease. Microorganisms. 2022;10:271. doi: 10.3390/microorganisms10020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen JJ, Yachelini PC, Sembaj A, Manzur RE, Garg NJ. Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Radic Biol Med. 2006;41:270–276. doi: 10.1016/j.freeradbiomed.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 41.Santos-Miranda A, Costa AD, Joviano-Santos JV, Rhana P, Bruno AS, Rocha P, Cau SB, Vieira LQ, Cruz JS, Roman-Campos D. Inhibition of calcium/calmodulin (Ca2+/CaM)-calcium/calmodulin-dependent protein kinase II (CaMKII) axis reduces in vitro and ex vivo arrhythmias in experimental Chagas disease. FASEB J. 2021;35:e21901. doi: 10.1096/fj.202101060R [DOI] [PubMed] [Google Scholar]
- 42.Fernández-Velasco M, Ruiz-Hurtado G, Hurtado O, Moro MA, Delgado C. TNF-alpha downregulates transient outward potassium current in rat ventricular myocytes through iNOS overexpression and oxidant species generation. Am J Physiol Heart Circ Physiol. 2007;293:H238–H245. doi: 10.1152/ajpheart.01122.2006 [DOI] [PubMed] [Google Scholar]
- 43.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405 [DOI] [PubMed] [Google Scholar]
- 44.Näbauer M, Beuckelmann DJ, Erdmann E. Characteristics of transient outward current in human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:386–394. doi: 10.1161/01.res.73.2.386 [DOI] [PubMed] [Google Scholar]
- 45.Petroff MG, Kim SH, Pepe S, Dessy C, Marbán E, Balligand JL, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867 [DOI] [PubMed] [Google Scholar]
- 46.Kaur S, Sassano A, Joseph AM, Majchrzak-Kita B, Eklund EA, Verma A, Brachmann SM, Fish EN, Platanias LC. Dual regulatory roles of phosphatidylinositol 3-kinase in IFN signaling. J Immunol. 2008;181:7316–7323. doi: 10.4049/jimmunol.181.10.7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Wagner MB, Joyner RW, Kumar R. cGMP-dependent protein kinase mediates stimulation of L-type calcium current by cGMP in rabbit atrial cells. Cardiovasc Res. 2000;48:310–322. doi: 10.1016/s0008-6363(00)00178-4 [DOI] [PubMed] [Google Scholar]
- 48.Fischmeister R, Castro L, Abi-Gerges A, Rochais F, Vandecasteele G. Species- and tissue-dependent effects of NO and cyclic GMP on cardiac ion channels. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:136–143. doi: 10.1016/j.cbpb.2005.04.012 [DOI] [PubMed] [Google Scholar]
- 49.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202 [DOI] [PubMed] [Google Scholar]
- 50.Prolo C, Estrada D, Piacenza L, Benítez D, Comini MA, Radi R, Álvarez MN. Nox2-derived superoxide radical is crucial to control acute Trypanosoma cruzi infection. Redox Biol. 2021;46:102085. doi: 10.1016/j.redox.2021.102085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qu Z, Weiss JN. Cardiac alternans: from bedside to bench and back. Circ Res. 2023;132:127–149. doi: 10.1161/CIRCRESAHA.122.321668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salles G, Xavier S, Sousa A, Hasslocher-Moreno A, Cardoso C. Prognostic value of QT interval parameters for mortality risk stratification in Chagas’ disease: results of a long-term follow-up study. Circulation. 2003;108:305–312. doi: 10.1161/01.CIR.0000079174.13444.9C [DOI] [PubMed] [Google Scholar]
- 53.Mijares A, Espinosa R, Adams J, Lopez JR. Increases in [IP3]i aggravates diastolic [Ca2+] and contractile dysfunction in Chagas’ human cardiomyocytes. PLoS NeglTrop Dis. 2020;14:e0008162. doi: 10.1371/journal.pntd.0008162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.López JR, Espinosa R, Landazuru P, Linares N, Allen P, Mijares A. Disfunción de la [Ca(2+)] diastólica en cardiomiocitos aislados de pacientes chagásicos [Dysfunction of diastolic [Ca²+] in cardiomyocytes isolated from chagasic patients]. Rev Esp Cardiol. 2011;64:456–462. doi: 10.1016/j.recesp.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 55.Reyes Gaido OE, Nkashama LJ, Schole KL, Wang Q, Umapathi P, Mesubi OO, Konstantinidis K, Luczak ED, Anderson ME. CaMKII as a therapeutic target in cardiovascular disease. Annu Rev Pharmacol Toxicol. 2023;63:249–272. doi: 10.1146/annurev-pharmtox-051421-111814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santos-Miranda A, Joviano-Santos JV, Sarmento JO, Costa AD, Soares ATC, Machado FS, Cruz JS, Roman-Campos D. A novel substrate for arrhythmias in Chagas disease. PLoS NeglTrop Dis. 2021;15:e0009421. doi: 10.1371/journal.pntd.0009421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lindegger N, Hagen BM, Marks AR, Lederer WJ, Kass RS. Diastolic transient inward current in long QT syndrome type 3 is caused by Ca2+ overload and inhibited by ranolazine. J Mol Cell Cardiol. 2009;47:326–334. doi: 10.1016/j.yjmcc.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maoz A, Krogh-Madsen T, Christini DJ. Instability in action potential morphology underlies phase 2 reentry: a mathematical modeling study. Heart Rhythm. 2009;6:813–822. doi: 10.1016/j.hrthm.2009.02.043 [DOI] [PubMed] [Google Scholar]
- 59.Varshneya M, Mei X, Sobie EA. Prediction of arrhythmia susceptibility through mathematical modeling and machine learning. Proc Natl Acad Sci USA. 2021;118:e2104019118. doi: 10.1073/pnas.2104019118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chagas C, Villela E. Cardiac form of American trypanosomiasis. Mem Inst Oswaldo Cruz. 1922;14:5–61. doi: 10.1590/S0074-02761922000100001 [Google Scholar]
- 61.Koeberle F. Patogenia da moléstia de Chagas. Estudo dos órgãos musculares ocos. Rev Goiana Med. 1957;3:155–180. [Google Scholar]
- 62.Koeberle F. Cardiopathia parasympathicopriva. Munch Med Wochenschr. 1959;101:1308–1310. [PubMed] [Google Scholar]
- 63.Alcântara FG. Sistema neuro-vegetativo do coração na moléstia de Chagas experimental. Rev Goiana Med. 1961;7:111–126. [Google Scholar]
- 64.Tafuri WL, Brener Z. Lesões do sistema nervoso autônomo do camundongo albino na tripanosomíase cruzi experimental, na fase aguda [Lesions of the autonomic nervous system in albino mice in experimental trypanosomiasis cruzi in the acute phase]. Hospital (Rio J). 1966;69:371–383. [PubMed] [Google Scholar]
- 65.de Souza MM, Andrade SG, Barbosa AA, Jr, Macedo Santos RT, Alves VA, Andrade ZA. Trypanosoma cruzi strains and autonomic nervous system pathology in experimental Chagas disease. Mem Inst Oswaldo Cruz. 1996;91:217–224. doi: 10.1590/s0074-02761996000200018 [DOI] [PubMed] [Google Scholar]
- 66.Machado CR, Caliari MV, de Lana M, Tafuri WL. Heart autonomic innervation during the acute phase of experimental American trypanosomiasis in the dog. Am J Trop Med Hyg. 1998;59:492–496. doi: 10.4269/ajtmh.1998.59.492 [DOI] [PubMed] [Google Scholar]
- 67.Lopes ER. Estudo comparativo dos gânglios subepicárdicos nas cardiopatias chagásicas crônica, reumática e hipertensiva. Rev Inst Med Trop São Paulo. 1970;70:365–374. [PubMed] [Google Scholar]
- 68.Amorim DS, Olsen EG. Assessment of heart neurons in dilated (congestive) cardiomyopathy. Br Heart J. 1982;47:11–18. doi: 10.1136/hrt.47.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopes ER, Tafuri WL. Involvement of the autonomic nervous system in Chagas’ heart disease. Rev Soc Bras Med Trop. 1983;16:206–212. doi: 10.1590/S0037-86821983000400007 [Google Scholar]
- 70.Ribeiro dos Santos R, Marquez JO, Von Gal Furtado CC, Ramos de Oliveira JC, Martins AR, Köberle F. Antibodies against neurons in chronic Chagas’ disease. Tropenmed Parasitol. 1979;30:19–23. [PubMed] [Google Scholar]
- 71.Ribeiro dos Santos R, Hudson L. Denervation and the immune response in mice infected with Trypanosoma cruzi. Clin Exp Immunol. 1981;44:349–354. [PMC free article] [PubMed] [Google Scholar]
- 72.Amorim DD, Marin Neto JA. Functional alterations of the autonomic nervous system in Chagas’ heart disease. Sao Paulo Med J. 1995;113:772–784. doi: 10.1590/s1516-31801995000200007 [DOI] [PubMed] [Google Scholar]
- 73.Amorim DS, Manço JC, Gallo L, Jr, Marin Neto JA. Chagas’ heart disease as an experimental model for studies of cardiac autonomic function in man. Mayo Clin Proc. 1982;57:48–60. [PubMed] [Google Scholar]
- 74.Oliveira JS. A natural human model of intrinsic heart nervous system denervation: Chagas’ cardiopathy. Am Heart J. 1985;110:1092–1098. doi: 10.1016/0002-8703(85)90222-4 [DOI] [PubMed] [Google Scholar]
- 75.Marin-Neto JA, Bromberg-Marin G, Pazin-Filho A, Simões MV, Maciel BC. Cardiac autonomic impairment and early myocardial damage involving the right ventricle are independent phenomena in Chagas’ disease. Int J Cardiol. 1998;65:261–269. doi: 10.1016/s0167-5273(98)00132-6 [DOI] [PubMed] [Google Scholar]
- 76.Ribeiro AL, Moraes RS, Ribeiro JP, Ferlin EL, Torres RM, Oliveira E, Rocha MO. Parasympathetic dysautonomia precedes left ventricular systolic dysfunction in Chagas disease. Am Heart J. 2001;141:260–265. doi: 10.1067/mhj.2001.111406 [DOI] [PubMed] [Google Scholar]
- 77.Sousa AC, Marin-Neto JA, Maciel BC, Gallo L, Jr, Amorim DS. Cardiac parasympathetic impairment in gastrointestinal Chagas’ disease. Lancet. 1987;1:985. doi: 10.1016/s0140-6736(87)90340-0 [DOI] [PubMed] [Google Scholar]
- 78.Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med. 1971;285:877–883. doi: 10.1056/NEJM197110142851602 [DOI] [PubMed] [Google Scholar]
- 79.Colucci WS, Ribeiro JP, Rocco MB, Quigg RJ, Creager MA, Marsh JD, Gauthier DF, Hartley LH. Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta-adrenergic desensitization. Circulation. 1989;80:314–323. doi: 10.1161/01.cir.80.2.314 [DOI] [PubMed] [Google Scholar]
- 80.Marin-Neto JA, Pintya AO, Gallo Júnior L, Maciel BC. Abnormal baroreflex control of heart rate in decompensated congestive heart failure and reversal after compensation. Am J Cardiol. 1991;67:604–610. doi: 10.1016/0002-9149(91)90899-v [DOI] [PubMed] [Google Scholar]
- 81.Koeberle F. Enteromegaly and cardiomegaly in Chagas disease. Gut. 1963;4:399–405. doi: 10.1136/gut.4.4.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adad SJ, Cançado CG, Etchebehere RM, Teixeira VP, Gomes UA, Chapadeiro E, Lopes ER. Neuron count reevaluation in the myenteric plexus of chagasic megacolon after morphometric neuron analysis. Virchows Arch. 2001;438:254–258. doi: 10.1007/s004280000319 [DOI] [PubMed] [Google Scholar]
- 83.Meneghelli UG. Chagasic enteropathy. Rev Soc Bras Med Trop. 2004;37:252–260. doi: 10.1590/s0037-86822004000300012 [DOI] [PubMed] [Google Scholar]
- 84.Oliveira RB BC, Abràs A, Gállego M, Marin-Neto JA. An unknown and neglected disease. In: Pinazo-Delgado MJGJ, ed. Chagas Disease. a Neglected Tropical Disease. Springer Nature; 2020:1–26. [Google Scholar]
- 85.Marin Neto JA, Gallo L, Jr, Manco JC, Rassi A, Amorim DS. Mechanisms of tachycardia on standing: studies in normal individuals and in chronic Chagas’ heart patients. Cardiovasc Res. 1980;14:541–550. doi: 10.1093/cvr/14.9.541 [DOI] [PubMed] [Google Scholar]
- 86.Guzzetti S, Iosa D, Pecis M, Bonura L, Prosdocimi M, Malliani A. Impaired heart rate variability in patients with chronic Chagas’ disease. Am Heart J. 1991;121:1727–1734. doi: 10.1016/0002-8703(91)90019-e [DOI] [PubMed] [Google Scholar]
- 87.Emdin M, Marin-Neto JA, Carpeggiani C, Maciel BC, Macerata A, Pyntia A, Michelassi C, Gallo Jr L, Pola S, Marchesi C, et al. Heart rate variability and cardiac denervation in Chagas’ disease. J Amb Monit. 1992;5:251–257 [Google Scholar]
- 88.Simões MV, Pintya AO, Bromberg-Marin G, Sarabanda AV, Antloga CM, Pazin-Filho A, Maciel BC, Marin-Neto JA. Relation of regional sympathetic denervation and myocardial perfusion disturbance to wall motion impairment in Chagas’ cardiomyopathy. Am J Cardiol. 2000;86:975–981. doi: 10.1016/s0002-9149(00)01133-4 [DOI] [PubMed] [Google Scholar]
- 89.Gadioli LP, Miranda CH, Marin-Neto JA, Volpe GJ, Filho ACLB, Filho AP, Pintya AO, de Figueiredo AB, Simões MV. Regional myocardial sympathetic denervation precedes the development of left ventricular systolic dysfunction in chronic Chagas’ cardiomyopathy. J Nucl Cardiol. 2022;29:3166–3176. doi: 10.1007/s12350-021-02869-3 [DOI] [PubMed] [Google Scholar]
- 90.Miranda CH, Figueiredo AB, Maciel BC, Marin-Neto JA, Simões MV. Sustained ventricular tachycardia is associated with regional myocardial sympathetic denervation assessed with 123I-metaiodobenzylguanidine in chronic Chagas cardiomyopathy. J Nucl Med. 2011;52:504–510. doi: 10.2967/jnumed.110.082032 [DOI] [PubMed] [Google Scholar]
- 91.Gadioli LP, Miranda CH, Pintya AO, de Figueiredo AB, Schmidt A, Maciel BC, Marin-Neto JA, Simões MV. The severity of ventricular arrhythmia correlates with the extent of myocardial sympathetic denervation, but not with myocardial fibrosis extent in chronic Chagas cardiomyopathy: Chagas disease, denervation and arrhythmia. J Nucl Cardiol. 2018;25:75–83. doi: 10.1007/s12350-016-0556-6 [DOI] [PubMed] [Google Scholar]
- 92.de Oliveira LF, Romano MM, de Carvalho EE, Cabeza JM, Salgado HC, Fazan Júnior R, Costa RS, da Silva JS, Higuchi Mde L, Maciel BC, et al. Histopathological correlates of global and segmental left ventricular systolic dysfunction in experimental chronic Chagas cardiomyopathy. J Am Heart Assoc. 2016;5:e002786. doi: 10.1161/JAHA.115.002786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Silva LEV, Moreira HT, de Oliveira MM, Cintra LSS, Salgado HC, Fazan R, Jr, Tinós R, Rassi A, Jr, Schmidt A, Marin-Neto JA. Heart rate variability as a biomarker in patients with chronic Chagas cardiomyopathy with or without concomitant digestive involvement and its relationship with the Rassi score. Biomed Eng Online. 2022;21:44. doi: 10.1186/s12938-022-01014-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296 [DOI] [PubMed] [Google Scholar]
- 95.Vianna G. Contribuição para o estudo da anatomia patológica da “moléstia de Carlos Chagas” (esquisotripanose humana ou tireoidite parasitária). Mem Inst Oswaldo Cruz. 1911;3:276–294. doi: 10.1590/s0074-02761911000200004 [Google Scholar]
- 96.Torres CM. Myocytolysis and fibrosis of the myocardium in Chagas’ disease. Mem Inst Oswaldo Cruz. 1960;58:161–182. doi: 10.1590/s0074-02761960000200004 [DOI] [PubMed] [Google Scholar]
- 97.Ferrans VJ, Milei J, Tomita Y, Storino RA. Basement membrane thickening in cardiac myocytes and capillaries in chronic Chagas’ disease. Am J Cardiol. 1988;61:1137–1140. doi: 10.1016/0002-9149(88)90148-8 [DOI] [PubMed] [Google Scholar]
- 98.Ferreira CS, Lopes ER, Chapadeiro E, de Oliveira Almeida H, de Souza WF, da Silva Neto IJ. Coronariografia post mortem na cardite chagásica crônica. Correlação anátomo-radiológica [Post-mortem coronary angiography in chronic Chagas carditis, Anatomo-radiologic correlation]. Arq Bras Cardiol. 1980;34:81–86. [PubMed] [Google Scholar]
- 99.Oliveira JS, Mello De Oliveira JA, Frederigue U, Jr, Lima Filho EC. Apical aneurysm of Chagas’s heart disease. Br Heart J. 1981;46:432–437. doi: 10.1136/hrt.46.4.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Higuchi ML, Brito T, Parzianello LC, Fukasawa S, Ramires JA. Severe arteriolar dilatation and ischemic lesions in chronic Chagas’ cardiopathy: a 3D confocal laser microscopic study. J Am Coll Cardiol. 1998;31:383C.9462583 [Google Scholar]
- 101.Rossi MA. Microvascular changes as a cause of chronic cardiomyopathy in Chagas’ disease. Am Heart J. 1990;120:233–236. doi: 10.1016/0002-8703(90)90191-y [DOI] [PubMed] [Google Scholar]
- 102.Rossi MA, Tanowitz HB, Malvestio LM, Celes MR, Campos EC, Blefari V, Prado CM. Coronary microvascular disease in chronic Chagas cardiomyopathy including an overview on history, pathology, and other proposed pathogenic mechanisms. PLoS NeglTrop Dis. 2010;4:e674. doi: 10.1371/journal.pntd.0000674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rossi MA, Carobrez SG. Experimental Trypanosoma cruzi cardiomyopathy in BALB/c mice: histochemical evidence of hypoxic changes in the myocardium. Br J Exp Pathol. 1985;66:155–160. [PMC free article] [PubMed] [Google Scholar]
- 104.Factor SM, Cho S, Wittner M, Tanowitz H. Abnormalities of the coronary microcirculation in acute murine Chagas’ disease. Am J Trop Med Hyg. 1985;34:246–253. doi: 10.4269/ajtmh.1985.34.246 [DOI] [PubMed] [Google Scholar]
- 105.Andrade ZA, Andrade SG, Correa R, Sadigursky M, Ferrans VJ. Myocardial changes in acute Trypanosoma cruzi infection. Ultrastructural evidence of immune damage and the role of microangiopathy. Am J Pathol. 1994;144:1403–1411 [PMC free article] [PubMed] [Google Scholar]
- 106.Morris SA, Tanowitz H, Makman M, Hatcher VB, Bilezikian JP, Wittner M. Trypanosoma cruzi: alteration of cAMP metabolism following infection of human endothelial cells. Exp Parasitol. 1992;74:69–76. doi: 10.1016/0014-4894(92)90140-6 [DOI] [PubMed] [Google Scholar]
- 107.Libby P, Alroy J, Pereira ME. A neuraminidase from Trypanosoma cruzi removes sialic acid from the surface of mammalian myocardial and endothelial cells. J Clin Invest. 1986;77:127–135. doi: 10.1172/JCI112266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tanowitz HB, Burns ER, Sinha AK, Kahn NN, Morris SA, Factor SM, Hatcher VB, Bilezikian JP, Baum SG, Wittner M. Enhanced platelet adherence and aggregation in Chagas’ disease: a potential pathogenic mechanism for cardiomyopathy. Am J Trop Med Hyg. 1990;43:274–281. doi: 10.4269/ajtmh.1990.43.274 [DOI] [PubMed] [Google Scholar]
- 109.Lemos de Oliveira LF, Thackeray JT, Marin Neto JA, Dias Romano MM, Vieira de Carvalho EE, Mejia J, Tanaka DM, Kelly da Silva G, Abdalla DR, Malamut C, et al. Regional myocardial perfusion disturbance in experimental chronic Chagas cardiomyopathy. J Nucl Med. 2018;59:1430–1436. doi: 10.2967/jnumed.117.205450 [DOI] [PubMed] [Google Scholar]
- 110.Tanaka DM, de Oliveira LFL, Marin-Neto JA, Romano MMD, de Carvalho EEV, de Barros Filho ACL, Ribeiro FFF, Cabeza JM, Lopes CD, Fabricio CG, et al. Prolonged dipyridamole administration reduces myocardial perfusion defects in experimental chronic Chagas cardiomyopathy. J Nucl Cardiol. 2019;26:1569–1579. doi: 10.1007/s12350-018-1198-7 [DOI] [PubMed] [Google Scholar]
- 111.Pereira IR, Vilar-Pereira G, Moreira OC, Ramos IP, Gibaldi D, Britto C, Moraes MO, Lannes-Vieira J. Pentoxifylline reverses chronic experimental chagasic cardiomyopathy in association with repositioning of abnormal CD8+ T-cell response. PLoS NeglTrop Dis. 2015;9:e0003659. doi: 10.1371/journal.pntd.0003659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Torres FW, Acquatella H, Condado JA, Dinsmore R, Palacios IF. Coronary vascular reactivity is abnormal in patients with Chagas’ heart disease. Am Heart J. 1995;129:995–1001. doi: 10.1016/0002-8703(95)90122-1 [DOI] [PubMed] [Google Scholar]
- 113.Marin-Neto JA, Simões MV, Ayres-Neto EM, Attab-Santos JL, Gallo L, Jr, Amorim DS, Maciel BC. Studies of the coronary circulation in Chagas’ heart disease. Sao Paulo Med J. 1995;113:826–834. doi: 10.1590/s1516-31801995000200014 [DOI] [PubMed] [Google Scholar]
- 114.Hiss FC, Lascala TF, Maciel BC, Marin-Neto JA, Simões MV. Changes in myocardial perfusion correlate with deterioration of left ventricular systolic function in chronic Chagas’ cardiomyopathy. JACC Cardiovasc Imaging. 2009;2:164–172. doi: 10.1016/j.jcmg.2008.09.012 [DOI] [PubMed] [Google Scholar]
- 115.Campos FA, Magalhães ML, Moreira HT, Pavão RB, Lima Filho MO, Lago IM, Badran AV, Chierice JRA, Schmidt A, Marin Neto JA. Chagas cardiomyopathy as the etiology of suspected coronary microvascular disease. A comparison study with suspected coronary microvascular disease of other etiologies. Arq Bras Cardiol. 2020;115:1094–1101. doi: 10.36660/abc.20200381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hagar JM, Rahimtoola SH. Chagas’ heart disease in the United States. N Engl J Med. 1991;325:763–768. doi: 10.1056/NEJM199109123251103 [DOI] [PubMed] [Google Scholar]
- 117.Marin-Neto JA, Marzullo P, Marcassa C, Gallo Júnior L, Maciel BC, Bellina CR, L’Abbate A. Myocardial perfusion abnormalities in chronic Chagas’ disease as detected by thallium-201 scintigraphy. Am J Cardiol. 1992;69:780–784. doi: 10.1016/0002-9149(92)90505-s [DOI] [PubMed] [Google Scholar]
- 118.Sarabanda AV, Sosa E, Simões MV, Figueiredo GL, Pintya AO, Marin-Neto JA. Ventricular tachycardia in Chagas’ disease: a comparison of clinical, angiographic, electrophysiologic and myocardial perfusion disturbances between patients presenting with either sustained or nonsustained forms. Int J Cardiol. 2005;102:9–19. doi: 10.1016/j.ijcard.2004.03.087 [DOI] [PubMed] [Google Scholar]
- 119.Peix A, García R, Sánchez J, Cabrera LO, Padrón K, Vedia O, Choque HV, Fraga J, Bandera J, Hernández-Cañero A. Myocardial perfusion imaging and cardiac involvement in the indeterminate phase of Chagas disease. Arq Bras Cardiol. 2013;100:114–117. doi: 10.5935/abc.20130023 [DOI] [PubMed] [Google Scholar]
- 120.Rabelo DR, Rocha MO, de Barros MV, Silva JL, Tan TC, Nunes MC. Impaired coronary flow reserve in patients with indeterminate form of Chagas’ disease. Echocardiogr. 2014;31:67–73. doi: 10.1111/echo.12364 [DOI] [PubMed] [Google Scholar]
- 121.Kuschnir E, Sgammini H, Castro R, Evequoz C, Ledesma R. Miocardiopatía chagásica crónica: efectos del dipiridamol sobre la dinámica ventricular [Chronic Chagas’ cardiomyopathy: effects of dipyradamole on ventricular dynamics]. Arq Bras Cardiol. 1983;41:373–378 [PubMed] [Google Scholar]
- 122.Marin-Neto JA, Sousa AC, Maciel BC, Gallo Júnior L, Iazigi N. Avaliação angiocardiográfica nuclear do efeito do dinitrato de isosorbitol em pacientes chagásicos [Radionuclide angiocardiographic evaluation of the effect of isosorbide dinitrate in patients with Chagas’ disease]. Arq Bras Cardiol. 1988;51:367–371 [PubMed] [Google Scholar]
- 123.Pavão RB, Moreira HT, Pintya AO, Haddad JL, Badran AV, Lima-Filho MO, Lago IM, Chierice JRA, Schmidt A, Marin-Neto JA. Aspirin plus verapamil relieves angina and perfusion abnormalities in patients with coronary microvascular dysfunction and Chagas disease: a pilot non-randomized study. Rev Soc Bras Med Trop. 2021;54:e0181. doi: 10.1590/0037-8682-0181-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tanaka DM, Simões MV, Marin-Neto JA. Coronary microvascular dysfunction due to Chagas disease: where are we now? Expert Rev Cardiovasc Ther. 2023;21:379–387. doi: 10.1080/14779072.2023.2215983 [DOI] [PubMed] [Google Scholar]
- 125.Mady C, de Moraes AV, Galiano N, Décourt LV. Estudo hemodinâmico na forma indeterminada na doença de Chagas [Hemodynamic study of the indeterminate form of Chagas’ disease]. Arq Bras Cardiol. 1982;38:271–275. [PubMed] [Google Scholar]
- 126.Sousa AC, Marin-Neto JA, Maciel BC, Gallo Júnior L, Amorim Dde S, Barreto-Martins LE. Disfunção sistólica e diastólica nas formas indeterminada, digestiva e cardíaca crônica da moléstia de Chagas [Systolic and diastolic dysfunction in the indeterminate, digestive and chronic cardiac forms of Chagas’ disease]. Arq Bras Cardiol. 1988;50:293–299 [PubMed] [Google Scholar]
- 127.Nunes MCP, Badano LP, Marin-Neto JA, Edvardsen T, Fernández-Golfín C, Bucciarelli-Ducci C, Popescu BA, Underwood R, Habib G, Zamorano JL, et al. Multimodality imaging evaluation of Chagas disease: an expert consensus of Brazilian Cardiovascular Imaging Department (DIC) and the European Association of Cardiovascular Imaging (EACVI). Eur Heart J Cardiovasc Imaging. 2018;19:459–460n. doi: 10.1093/ehjci/jex154 [DOI] [PubMed] [Google Scholar]
- 128.Mady C, Barretto AC, Stolf N, Lopes EA, Dauar D, Wajngarten M, Martinelli Filho M, Macruz R, Pileggi F. Biópsia endomiocárdica na forma indeterminada da doenca de Chagas [Endomyocardial biopsy in the indeterminate form of Chagas’ disease]. Arq Bras Cardiol. 1981;36:387–390. [PubMed] [Google Scholar]
- 129.Lopes ER, Chapadeiro E, Andrade ZA, Almeida HO, Rocha A. Anatomia patolgica de coraçes de chagásicos assintomáticos falecidos de modo violento [Pathological anatomy of hearts from asymptomatic Chagas disease patients dying in a violent manner]. Mem Inst Oswaldo Cruz. 1981;76:189–197. doi: 10.1590/s0074-02761981000200010 [DOI] [PubMed] [Google Scholar]
- 130.Noya-Rabelo MM, Macedo CT, Larocca T, Machado A, Pacheco T, Torreão J, Souza BSF, Soares MBP, Ribeiro-Dos-Santos R, Correia LCL. The presence and extension of myocardial fibrosis in the undetermined form of Chagas’ disease: a study using magnetic resonance. Arq Bras Cardiol. 2018;110:124–131. doi: 10.5935/abc.20180016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverría LE, Dutra WO, Gascon J, Morillo CA, Oliveira-Filho J, et al. ; American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Stroke Council. Chagas cardiomyopathy: an update of current clinical knowledge and management: a scientific statement from the American Heart Association. Circulation. 2018;138:e169–e209. doi: 10.1161/CIR.0000000000000599 [DOI] [PubMed] [Google Scholar]
- 132.Sambiase NV, Higuchi ML, Benvenuti LA. Narrowed lumen of the right coronary artery in chronic Chagasic patients is associated with ischemic lesions of segmental thinnings of ventricles. Invest Clin. 2010;51:531–539. [PubMed] [Google Scholar]
- 133.Schmidt A, Dias Romano MM, Marin-Neto JA, Rao-Melacini P, Rassi A, Jr, Mattos A, Avezum A, Jr, Villena E, Sosa-Estani S, Bonilla R, et al. ; BENEFIT Investigators. Effects of trypanocidal treatment on echocardiographic parameters in Chagas cardiomyopathy and prognostic value of wall motion score index: a BENEFIT trial echocardiographic substudy. J Am Soc Echocardiogr. 2019;32:286–295.e3. doi: 10.1016/j.echo.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 134.Chen CY, Caporizzo MA, Bedi K, Vite A, Bogush AI, Robison P, Heffler JG, Salomon AK, Kelly NA, Babu A, et al. Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat Med. 2018;24:1225–1233. doi: 10.1038/s41591-018-0046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768 [DOI] [PubMed] [Google Scholar]
- 136.Rossi MA. Fibrosis and inflammatory cells in human chronic chagasic myocarditis: scanning electron microscopy and immunohistochemical observations. Int J Cardiol. 1998;66:183–194. doi: 10.1016/s0167-5273(98)00208-3 [DOI] [PubMed] [Google Scholar]
- 137.Figueiredo F, Marin-Neto JA, Rossi MA. The evolution of experimental Trypanosoma cruzi cardiomyopathy in rabbits: further parasitological, morphological and functional studies. Int J Cardiol. 1986;10:277–290. doi: 10.1016/0167-5273(86)90009-4 [DOI] [PubMed] [Google Scholar]
- 138.Rossi MA. Patterns of myocardial fibrosis in idiopathic cardiomyopathies and chronic Chagasic cardiopathy. Can J Cardiol. 1991;7:287–294. [PubMed] [Google Scholar]
- 139.Rossi MA. The pattern of myocardial fibrosis in chronic Chagas’ heart disease. Int J Cardiol. 1991;30:335–340. doi: 10.1016/0167-5273(91)90012-e [DOI] [PubMed] [Google Scholar]
- 140.Pazin-Filho A, Romano MM, Gomes Furtado R, de Almeida Filho OC, Schmidt A, Marin-Neto JA, Maciel BC. Left ventricular global performance and diastolic function in indeterminate and cardiac forms of Chagas’ disease. J Am Soc Echocardiogr. 2007;20:1338–1343. doi: 10.1016/j.echo.2007.04.029 [DOI] [PubMed] [Google Scholar]
- 141.Maciel BC, de Almeida Filho OC, Schmidt A, Marin-Neto JA. Ventricular function in Chagas’ heart disease. Sao Paulo Med J. 1995;113:814–820. doi: 10.1590/s1516-31801995000200012 [DOI] [PubMed] [Google Scholar]
- 142.Romano MMD, Moreira HT, Marin-Neto JA, Baccelli PE, Alenezi F, Klem I, Maciel BC, Kisslo J, Schmidt A, Velazquez EJ. Early impairment of myocardial deformation assessed by regional speckle-tracking echocardiography in the indeterminate form of Chagas disease without fibrosis detected by cardiac magnetic resonance. PLoS NeglTrop Dis. 2020;14:e0008795. doi: 10.1371/journal.pntd.0008795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qu Z, Weiss JN. Mechanisms of ventricular arrhythmias: from molecular fluctuations to electrical turbulence. Annu Rev Physiol. 2015;77:29–55. doi: 10.1146/annurev-physiol-021014-071622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang M, Zhang J, Zhang W, Hu Q, Jin L, Xie P, Zheng W, Shang H, Zhang Y. CaMKII-δ9 induces cardiomyocyte death to promote cardiomyopathy and heart failure. Front Cardiovasc Med. 2022;8:820416. doi: 10.3389/fcvm.2021.820416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tanaka DM, Fabricio CG, Marin-Neto JA, Barros-Filho ACL, Lopes CD, Oliveira LFL, Mejia J, Almeida RR, Batah SS, Nekolla SG, et al. Endothelial inflammatory activation is related to myocardial perfusion disturbance in experimental chronic Chagas disease. Eur Heart J. 2022;43:1. doi: 10.1093/eurheartj/ehac544.1692 [Google Scholar]
- 146.Shah M, Akar FG, Tomaselli GF. Molecular basis of arrhythmias. Circulation. 2005;112:2517–2529. doi: 10.1161/CIRCULATIONAHA.104.494476 [DOI] [PubMed] [Google Scholar]