Abstract
Traditional practice favors total shoulder arthroplasty (TSA) for the treatment of primary glenohumeral osteoarthritis (PGHO) with an intact rotator cuff; however, the indications for reverse shoulder arthroplasty (RSA) have expanded to include PGHO. The purpose of this systematic review is to compare the mean differences in the range of motion and patient-reported outcomes between the TSA and RSA with an intact rotator cuff and to analyze the subgroup of the Walch type B2 glenoid. This IRB-exempt, PROSPERO-registered systematic review strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) guidelines. A literature search of five databases revealed 493 articles, of which 10 were included for quantitative synthesis. Level III evidence studies with the diagnosis of PGHO and ≥2 years of follow-up were included. Studies without preoperative and postoperative data were excluded. The Newcastle-Ottawa scale was used to evaluate the methodologic quality of the included studies. Preoperative and postoperative range of motion and patient-reported outcomes were collected. The random-effects model was employed, and p < 0.05 was considered statistically significant. There were a total of 544 and 329 studies in the TSA group and RSA group, respectively. The mean age in the TSA group and RSA groups were 65.36 ± 7.06 and 73.12 ± 2.40, respectively (p = 0.008). The percentages of males in the TSA and RSA groups were 73.2% and 51.1%, respectively (p = 0.02). The mean differences in forward elevation, external rotation in adduction, internal rotation scale, visual analog scale (VAS), American Shoulder and Elbow Surgeons (ASES) score, and Single Assessment Numeric Evaluation (SANE) scores were improved for both groups with no significant differences between the two. There were 9.6 times the revisions in the TSA group (8.8% vs. 0.91%; p = 0.014) and 1.5 times the complications in the TSA group (3.68% vs. 2.4%; p = 0.0096). Two hundred and forty-two glenoids were identified as Walch type B2 (126 in the TSA group and 116 in the RSA group). The mean ages in the B2 subgroup were 68.20 ± 3.25 and 73.03 ± 1.49 for the TSA and RSA, respectively (p = 0.25). The percentages of males in the B2 subgroup were 74.6% and 46.5% for the TSA and RSA groups, respectively (p = 0.0003). The ASES, SANE, forward elevation, and external rotation in the adduction results were descriptively summarized for this subgroup, with average mean differences of 49.0 and 51.2, 45.7 and 66.1, 77.6° and 58.6°, and 38.6° and 34.1° for the TSA and RSA groups, respectively. In the setting of primary glenohumeral osteoarthritis with an intact rotator cuff, the RSA has a similar range of motion and clinical outcomes but lower complication and revision rates as compared to the TSA. This may hold true in the setting of the B2 glenoid, although a high-powered study on this subgroup is required. Anatomic shoulder arthroplasty maintains an important role in select patients. Further studies are required to better elucidate the role of glenoid bone loss and posterior humeral head subluxation with regard to implant choice.
Keywords: evidence-based practice, surgical decision-making, glenohumeral arthritis, shoulder surgery, surgical outcomes, degenerative joint disease, shoulder replacement
Introduction and background
The indications for reverse shoulder arthroplasty (RSA) have grown substantially over the past few decades to include glenohumeral arthritis with significant glenoid bone deformity [1], irreparable rotator cuff tear without arthropathy [2], and complex proximal humerus fractures [3]. RSA is being performed at increasingly higher rates in the setting of primary glenohumeral osteoarthritis (PGHO) in elderly patients [2]. The number of RSAs performed yearly in the United States has surpassed the number of total shoulder arthroplasties (TSAs) both overall and for PGHO [4].
There is controversy in the setting of PGHO (intact rotator cuff) as to whether the TSA or RSA results in superior clinical outcomes. Proponents of the RSA cite the versatility and reproducibility of this procedure. A recent systematic review demonstrated that functional and patient-reported outcomes in RSA do not vary significantly based on the preoperative diagnosis [5]. However, historically, TSA has been the gold standard for patients with PGHO and an intact rotator cuff, given the excellent outcomes associated with restoring native biomechanics.
Glenoid wear is an important consideration in the evaluation of the patient. Walch et al. [6] described three glenoid wear patterns that have subsequently been expanded upon: A or concentric wear, B or posterior/eccentric wear, and C or dysplastic glenoids. B glenoids can be further described as B1 or monoconcave, B2 or biconcave glenoids, and B3 or monoconcave glenoids with posterior subluxation >70% or retroversion > 15% [7]. Glenoid deformity can influence treatment decisions with neoglenoid retroversion of greater than 27 degrees leading to an increased risk of glenoid component loosening [8]. However, the type B2 glenoid remains an indeterminate area where the decision between an RSA and TSA can be difficult [9]. The degree of bone deformities and surgeon comfort regarding handling glenoid eccentricity can influence this surgical decision [8,10,11]. However, the evidence to assist surgeons in making this clinical decision is limited. The purpose of this meta-analysis is to compare the mean differences between preoperative and postoperative range of motion and patient-reported outcomes between TSA and RSA with an intact rotator cuff and to analyze the subgroup of the Walch type B2 glenoid.
Review
Materials and methods
General
This IRB-exempt meta-analysis was registered with the International Prospective Register of Systematic 3.1.3 Patient-Reported Outcomes Reviews (PROSPERO No. CRD42022297549). The Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines were followed.
Search Strategy
A literature search was performed using the following keywords: Population: “primary glenohumeral osteoarthritis” OR “primary glenohumeral arthritis”; Intervention: “reverse shoulder arthroplasty” OR “RSA” OR “Grammont style replacement” AND "total shoulder arthroplasty" OR "Anatomic Shoulder Arthroplasty"; Outcomes: “patient-reported outcomes” OR “clinical outcomes” OR “functional outcomes” OR “radiographic outcomes”. PubMed, Cochrane, Web of Science Collection, Scopus, Google Scholar, MEDLINE, CINAHL, and Embase databases were searched. Search fields were varied until no new articles were collected at which point the search was considered exhaustive.
Study Screening and Selection
The search yielded 493 studies. Based on the title and abstract review, a total of 40 article abstracts were selected for review. Full-text screening of these articles was performed based on our inclusion/exclusion criteria (Table 1).
Table 1. Our inclusion and exclusion criteria as applied independently by three of the authors during the initial title/abstract review.
VAS: visual analog scale, ASES: American Shoulder and Elbow Surgeons, WOOS: Western Ontario Osteoarthritis of the Shoulder, SST: Simple Shoulder Test, SANE: Single Assessment Numeric Evaluation, DASH: Disabilities of the Arm, Shoulder, and Hand Questionnaire, SF-12 PCS: 12-item Short Form Health Survey Physical Composite Scale
| Inclusion criteria | Exclusion criteria |
| Level III evidence or higher | Systematic reviews, review papers, case reports, case series, and other level IV/level V studies |
| Ages 18-90 | Absence of preoperative and postoperative data |
| Diagnosis of primary glenohumeral osteoarthritis | Hemiarthroplasty, head replacement surgeries/stemless implants, recessed glenoid components |
| Follow-up of ≥2 years | Prior existing rotator cuff disease |
| Presence of any of the following outcome measures: VAS scores, satisfaction scores, ASES scores, WOOS scores, SST scores, constant-total scores, SANE scores, Quick DASH scores, SF-12 PCS scores, revision rate, complication rate, or postoperative range of motion | Prior shoulder arthroplasty |
The references in these articles were also hand-searched to identify any missing articles. This resulted in 10 articles included in the quantitative synthesis articles (Figure 1).
Figure 1. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram for our study.
Quality Assessment
To assess the included studies, guidelines from the Revised Assessment of Multiple SysTemAtic Reviews (R-AMSTAR) were followed [12]. The Newcastle-Ottawa scale was chosen considering that all included studies were cohort studies. Studies were evaluated based on the selection, comparability, and outcome criteria.
Data Collection
The data elements extracted from the articles fell into the categories of study basics, demographic data, glenoid type (when available), physical exam metrics, patient-reported outcomes, and complications. The variables under study basics included lead author, year published, journal of publication, study type, level of evidence, the risk of bias as determined above, and length of follow-up. The variables under demographic information were age, gender, date of publication, and country of origin of the publication. The physical examination metrics collected included forward elevation, abduction, external rotation in 90 of abduction (ER in Abd) external rotation in adduction (ER in Add), and internal rotation (IR). The variables under patient-reported outcomes included Visual Analog Scale (VAS) scores, Satisfaction Scores, American Shoulder and Elbow (ASES) Scores, Western Ontario Osteoarthritis of the Shoulder (WOOS) Scores, Simple Shoulder Test (SST) Scores, Constant-Murley (CM) Total Scores, Single Assessment Numeric Evaluation (SANE) scores, Quick DASH scores, and 12-item Short Form Health Survey Physical Composite Scale (SF-12 PCS) Scores. All documented complications and the duration of follow-up were also recorded.
Data Abstraction and Statistical Analysis
The data analysis was performed by a statistician at the University of Arizona Department of Biostatistics. Frequencies and proportions were calculated for categorical variables. Means and standard deviations were calculated for continuous variables. Data abstraction and figure creation were performed with Microsoft Excel V16.42. A two-sample T-test was used to compare the preoperative values for both range of motion and patient-reported outcomes. The random-effects model (REML) was used to compare the mean differences for the range of motion and patient-reported outcomes. The Mann-Whitney test was used to compare revision rates and complication rates. The I2 statistic, which explains the percentage of variation across studies that is due to heterogeneity rather than chance and should be included in all systematic reviews, was also calculated [13]. Forest plots were chosen to represent the data considering the homogeneity in reported outcomes between the ≥2 included studies for any given outcome measure. All two-tailed P values <0.05 were considered statistically significant. P-values were two-tailed with P < 0.05 considered statistically significant. Stata Statistical Software (release 17, StataCorp LLC, College Station, TX, 2021) was used for all statistical analyses.
Results
General
There were a total of 10 studies that met our inclusion and exclusion criteria (Table 2).
Table 2. A summary of the primary literature included in our study.
*This study contained 15 patients who were included in the total shoulder arthroplasty (TSA) group and 16 patients who were included in the reverse shoulder arthroplasty (RSA) group.
PGHO: primary glenohumeral osteoarthritis, TSA: total shoulder arthroplasty, RSA: reverse shoulder arthroplasty
| TSA and RSA, TSA, or RSA | Lead author last name | Year published | Journal | Study type | Level of evidence | PGHO shoulder number (intact cuff) | Walch type B2 shoulder number |
| TSA and RSA | Alentorn-Geli [9] | 2018 | Journal of Orthopaedic Surgery | Retrospective cohort study | III | 31* | 31 |
| TSA | Petri [14] | 2016 | Archives of Orthopedic Traumatology and Surgery | Retrospective cohort study | III | 78 | Could not determine |
| TSA | Sheth [15] | 2020 | Journal of Shoulder and Elbow Surgery | Prospective observational study | II | 111 | 111 |
| TSA | Neyton [16] | 2019 | Journal of Shoulder and Elbow Surgery | Retrospective cohort study | III | 155 | Could not determine |
| TSA | Hussey [17] | 2015 | Journal of Shoulder and Elbow Surgery | Retrospective cohort study | III | 148 | Could not determine |
| TSA | Nolte [18] | 2021 | The American Journal of Sports Medicine | Retrospective cohort study | III | 37 | Could not determine |
| RSA | Waterman [19] | 2020 | Journal of the American Academy of Orthopaedic Surgeons | Retrospective cohort study | III | 43 | 43 |
| RSA | Pettit [20] | 2021 | Journal of Shoulder and Elbow Surgery | Retrospective cohort study | III | 116 | 57 |
| RSA | Lindbloom [21] | 2019 | Journal of Shoulder and Elbow Surgery | Retrospective cohort study | III | 129 | Could not determine |
| RSA | Wall [22] | 2007 | The Journal of Bone and Joint Surgery | Prospective cohort observational study | II | 25 | Could not determine |
One study directly compared the outcomes of RSA and TSA [9], five studies provided outcomes after TSA for the treatment of PGHO [14-18], and four studies provided outcomes after RSA for the treatment of PGHO [19-22]. The results of our methodologic quality analysis with the Newcastle-Ottawa scale are provided in Table 3.
Table 3. Results of grading the clinical evidence using the Newcastle-Ottawa Scale from our included studies.
There were a total of 544 shoulders in our TSA group and 329 shoulders in our RSA group. Of these, 301 could be identified by Walch glenoid type, which included 10 B1 glenoids, 242 B2 glenoids, and 49 B3 glenoids. The mean ages in the TSA group and RSA groups were 65.36 ± 7.06 and 73.12 ± 2.40, respectively (P = 0.008). The percentages of males in the TSA and RSA groups were 73.2% and 51.1%, respectively (P = 0.02). There were no statistically significant differences between the TSA and RSA groups in terms of preoperative range of motion or patient-reported outcomes (Table 4).
Table 4. Comparison between our TSA and RSA groups preoperatively.
TSA: total shoulder arthroplasty, RSA: reverse shoulder arthroplasty, ER in Add: external rotation in adduction, VAS: visual analog scale, ASES: American Shoulder and Elbow Society, SANE: Single Assessment Numeric Evaluation
| Variable | TSA | RSA | P-Value |
| Forward elevation | 95.95° (8.37°) | 84.48° (6.96°) | 0.15 |
| ER in add | 13.93° (5.82°) | 21.94° (9.35°) | 0.21 |
| Internal rotation | 2.8 (0.85) | 2.74 (0.37) | 0.94 |
| VAS - pain | 5.00 (1.41) | 4.24 (1.73) | 0.64 |
| ASES | 44.58 (4.61) | 38.78 (8.03) | 0.38 |
| SANE | 44.4 (12.5) | 30.37 (0.467) | 0.19 |
Physical Examination
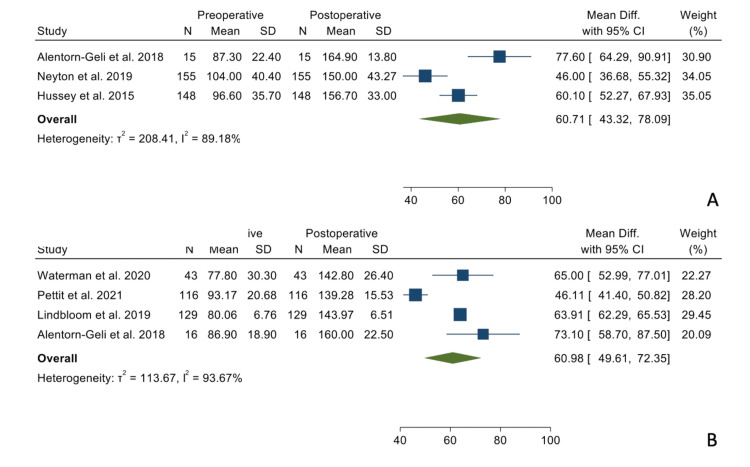
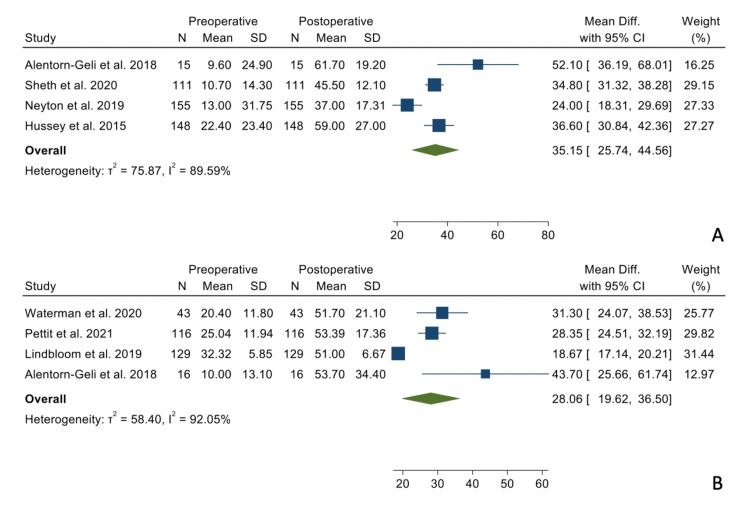
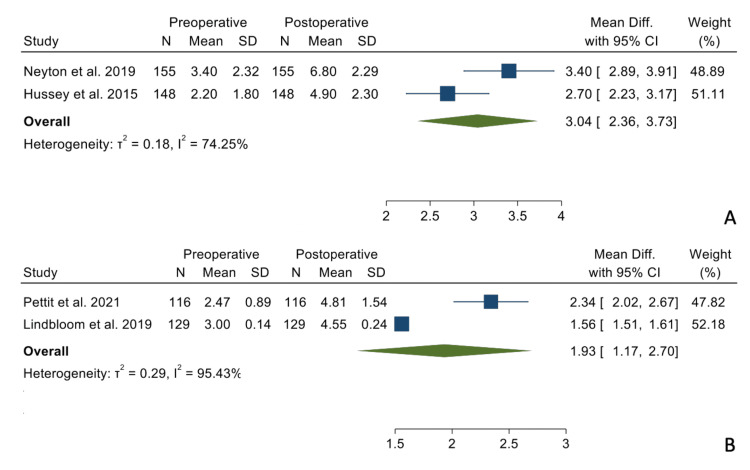
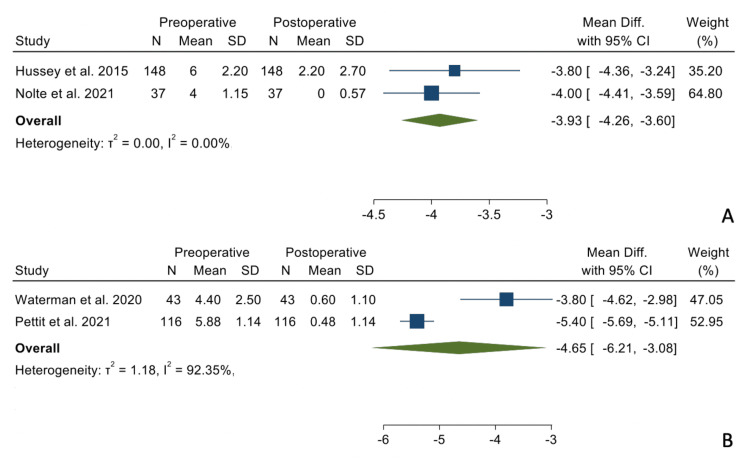
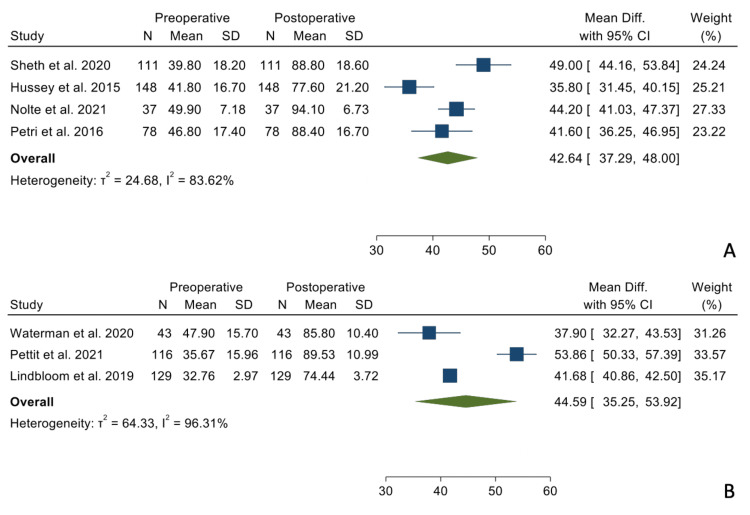
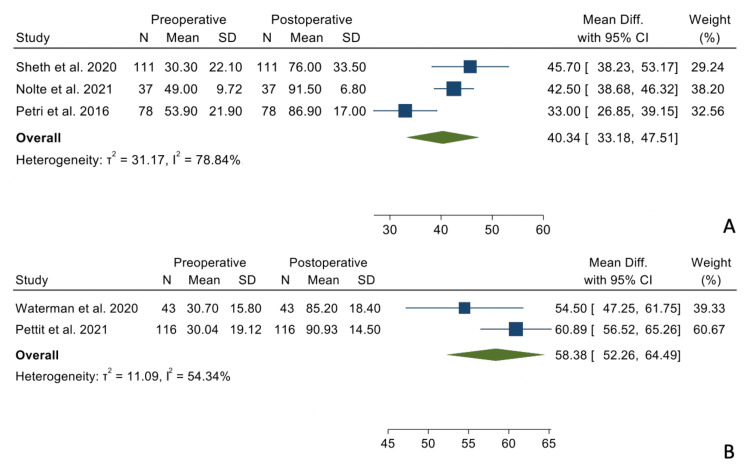
There were no statistically significant differences when comparing the mean differences between the preoperative and postoperative states between the two groups regarding forward elevation (Figure 2), external rotation in adduction (Figure 3), and internal rotation (Figure 4).
Figure 2. Mean differences in forward elevation between the TSA group (Panel A) and the RSA group (Panel B).
Figure 3. Mean differences in external rotation in adduction in the TSA group (Panel A) and the RSA group (Panel B).
Figure 4. Mean differences in internal rotation in the TSA (Panel A) and the RSA group (Panel B).
The summary data regarding mean differences between preoperative and postoperative data can be seen in Table 5.
Table 5. Summary of the mean differences between the preoperative and postoperative states for all outcomes of our study.
TSA: total shoulder arthroplasty, RSA: reverse shoulder arthroplasty, ER in Add: external rotation in adduction, VAS: visual analog scale, ASES: American Shoulder and Elbow Society, SANE: Single Assessment Numeric Evaluation
| TSA | RSA | ||||
| Variable | Mean difference | Minimum, maximum | Mean difference | Minimum, maximum | p-value |
| Forward elevation | 60.71° | 43.32°, 78.09° | 60.98° | 49.61°, 72.35° | 0.883 |
| ER in Add | 35.15° | 25.74°, 44.56° | 28.06° | 19.62°, 36.50° | 0.197 |
| Internal rotation | 3.04 | 2.36, 3.73 | 1.93 | 1.17, 2.70 | 0.308 |
| VAS - pain | -3.93 | -4.26, -3.60 | -4.65 | -6.21, -3.08 | 0.657 |
| ASES | 42.62 | 37.29, 48.00 | 44.59 | 35.25, 53.92 | 0.71 |
| SANE | 40.34 | 33.18, 47.51 | 58.38 | 52.26, 64.49 | 0.095 |
Patient-Reported Outcomes
There were no statistically significant differences when comparing the mean differences between the preoperative and postoperative states between the two groups regarding VAS-Pain scores (Figure 5), ASES scores (Figure 6), and SANE scores (Figure 7).
Figure 5. Mean differences in VAS-Pain scores in the TSA group (Panel A) and the RSA group (Panel B).
VAS: visual analog scale, TSA: total shoulder arthroplasty, RSA: reverse shoulder arthroplasty
Figure 6. Mean differences in ASES scores in the TSA group (Panel A) and the RSA group (Panel B).
ASES: American Shoulder and Elbow Surgeons, TSA: total shoulder arthroplasty, RSA: reverse shoulder arthroplasty
Figure 7. Mean differences in SANE scores in the TSA group (Panel A) and the RSA group (Panel B).
SANE: Single Assessment Numeric Evaluation, TSA: total shoulder arthroplasty, RSA: reverse shoulder arthroplasty
The summary data regarding mean differences between preoperative and postoperative data can be seen in Table 5.
Revisions
There were a total of 48 (8.8%) revisions in the TSA group and three revisions (0.91%) in the RSA group (P = 0.014).
Complications
There were a total of 20 (3.68%) complications in the TSA group and eight (2.4%) complications in the RSA group (P = 0.0096). The complications in the TSA group consisted of seven radiographic failures, six subscapularis failures, three posterior dislocations, two late rotator cuff insufficiencies, one arthrofibrosis, and one wound infection. The complications in the RSA group consisted of four acromial stress fractures, two base plate fractures, one periprosthetic infection, and one periprosthetic fracture.
Glenoid Type B2 Subgroup
Of our 873 glenoids, a total of 242 glenoids could be identified as Walch type B2 (126 in the TSA group and 116 in the RSA group; Table 2, column 8). The mean ages in the TSA and RSA groups were 68.20 ± 3.25 and 73.03 ± 1.49, respectively (P = 0.25). The percentage of males in the TSA and RSA groups was 74.6% and 46.5%, respectively (P = 0.0003). The results regarding ASES scores, SANE scores, forward elevation, and external rotation are summarized in Table 6.
Table 6. Descriptive summary of the available data from our included studies regarding the type B2 glenoid.
Asterisk (*) indicates that this data was not included in the weighted mean difference values given the lack of corresponding pre-operative data. Plus sign (+) indicates that this data was provided by the authors as mean differences as opposed to preoperative and postoperative data.
TSA: total shoulder arthroplasty, RSA: reverse shoulder arthroplasty
| TSA or RSA | Lead author last name | Walch type B2 shoulder number | Preoperative ASES scores | Postoperative ASES scores | Preoperative SANE scores | Postoperative SANE scores | Preoperative forward elevation | Postoperative forward elevation | Preoperative external rotation | Postoperative external rotation | |
| TSA | Alentorn-Geli [9] | 15 | Not available | 91.2 ± 6.7* | Not available | Not available | 87.3 ± 22.4 | 164.9 ± 13.8 | 9.6 ± 24.9 | 61.7 ± 19.2 | |
| TSA | Sheth [15] | 111 | 39.8 ± 18.2 | 88.8 ± 18.6 | 30.3 ± 22.1 | 76.0 ± 33.5 | Not available | Not available | 10.7 ± 14.3 | 45.5 ± 12.1 | |
| RSA | Alentorn-Geli [9] | 16 | Not available | 80.3 ±14.3* | Not available | Not available | 86.9 ± 18.9 | 160.0 ± 22.5 | 10.0 ± 13.1 | 53.7 ± 34.4 | |
| RSA | Waterman [19] | 43 | 49.2 ± 25.3+ | 71.8 ± 44.9+ | 60.0 ± 56.8+ | 30.8 ± 38.0+ | |||||
| RSA | Pettit [20] | 57 | 36.1 ± 17.0 | 88.8 ± 13.2 | 30.1 ± 19.0 | 91.9 ± 13.9 | 90.4 ± 21.0 | 140.1 ± 15.4 | 24.9 ± 10.7 | 56.2 ± 17.9 | |
| Average mean difference (TSA) | 49 | 45.7 | 77.6 | 38.6 | |||||||
| Average mean difference (RSA) | 51.2 | 66.1 | 58.6 | 34.1 | |||||||
Discussion
Our results demonstrated that TSA and RSA perform equally well in the setting of PGHO with an intact rotator cuff regarding forward flexion, external rotation in adduction, internal rotation, VAS-Pain scores, ASES scores, and SANE scores. There is a growing body of literature favoring the use of the RSA in many end-stage shoulder pathologies. Coscia et al. (2021) demonstrated no significant differences in the postoperative range of motion, ASES scores, and Constant-Murley scores after RSA in the treatment of massive cuff tears without osteoarthritis, cuff tear arthropathy, proximal humerus fractures, and PGHO [5]. As the RSA continues to evolve, indications have continued to expand with improved clinical results. Heifner et al. (2021) evaluated the use of RSA in PGHO [23]. These authors demonstrated an average postoperative ASES score of 77.8 and a revision rate of 3.1% after RSA in the setting of PGHO. This lends credibility to the use of the RSA in the treatment of PGHO but does not evaluate the optimal surgical solution in the patient with an intact rotator cuff or with a type B2 glenoid.
Kim et al. evaluated the TSA and the RSA in patients with PGHO and also found no difference concerning patient-reported outcomes [24]. However, these authors found a difference in external rotation and did not find a difference in terms of revision rate. There are, however, significant differences in methodology to consider when interpreting our differing results. In particular, Kim et al. included level IV evidence, only included articles with a direct comparison between RSA and TSA, and included articles without preoperative data [24]. In our study, only articles with preoperative and postoperative data were included in order to compare mean differences as the primary outcome measure as opposed to postoperative scores alone. It is important to consider these different methodologies when interpreting the difference in results from our study and the important work done by Kim et al. [24].
An interesting aspect of our clinical question is with regard to the management of glenoid bone deformity. In this setting, recent literature may favor the use of reverse shoulder arthroplasty. However, a variety of surgical options exist for the management of extensive glenoid bone deformity when using TSA including eccentric reaming [17], bone grafting [25], posteriorly augmented glenoid components [26,27], and the anterior-offset humeral head [28]. The current evidence does not seem to as strongly suggest the need for bone grafting severe glenoid bone deformity during an RSA, though the studies on this topic are limited [1].
Currently, it is difficult for shoulder surgeons to use the Walch glenoid type to the decision between TSA and RSA. In our study, there was a statistically significantly higher proportion of males in the TSA group (73.2%) as compared to the RSA group (51.1%). This may be multifactorial including gender variations in cuff atrophy and surgeon bias. However, preoperative range of motion and preoperative patient-reported outcomes did not differ significantly between the groups. Alentorn-Geli et al. quantified glenoid retroversion and posterior subluxation in the setting of the B2 glenoid between the TSA and RSA groups. They found no significant differences. In their study, they found significantly higher simple shoulder test scores and a significantly higher complication rate in the TSA group. In the present study, we also attempted to more closely look at the subgroup of patients who could be identified as Walch type B2. We demonstrated comparable changes in the ASES scores and external rotation, greater forward elevation changes in the TSA group, and greater SANE changes in the RSA group. Unfortunately, this was only possible for four of the included studies, for a total of 242 shoulders. Given the limited number of studies and heterogeneity of reported data, these results were performed solely with descriptive statistics only and thus should be interpreted with caution.
An important additional consideration in surgical decision-making is the longevity of the implants and revision options dictated by each surgical option. TSA lends the advantage of conversion to an RSA as an option in the future. However, the options in the setting of a primary RSA are revision RSA with further bone loss. This needs to be considered in the context of the current longevity data. A recent systematic review demonstrated l0-year survival rates of 94.6% and 94.4% for TSA and RSA in the context of PGHO, respectively [29]. In the context of equivalent survival rates, surgeons should give due consideration to the limitation of revision options for a primary RSA.
The results of the present study may suggest that both TSA and RSA may perform equally well in the setting of primary glenohumeral osteoarthritis with an intact cuff. However, when considering the ease of surgery and complexity of glenoid bone loss, the RSA may be the implant of choice in many scenarios. This may hold true in the setting of the type B2 glenoid as well; however, our limited subgroup in our study is insufficient to comment on this. Overall, when looking at PGHO with an intact rotator cuff, the RSA may be considered non-inferior to the TSA.
Limitations
Many of the studies included in this systematic review were retrospective in nature, which introduces a risk of confounding by surgical indication. There was an inhomogeneity of our groups with the group undergoing RSA being significantly older than those undergoing TSA. There was heterogeneity in terms of reported data with I2 statistics greater than 50% in most of our outcome measures, and thus the random effects model as opposed to a fixed effects model was chosen. Furthermore, ASES scores, forward flexion, and external rotation were reported in almost all studies, but the remaining outcome measures were not unanimously seen, and this should be considered when interpreting those results (IR, VAS, and SANE scores). Lastly, although we do descriptively summarize the performance of TSA and RSA in the included patients with a type B2 glenoid, ultimately, the patient number included in this subgroup was small, and we were not able to perform meaningful statistics with regard to the type B2 glenoid. Future research studies should aim to address this issue by separating the subjects into Walch B1 and Walch B2 groups to better understand the relationship between subluxation and retroversion on the performance of TSA and RSA.
Conclusions
In the setting of PGHO with an intact rotator cuff, the RSA has a similar range of motion and clinical outcomes, but lower complication and revision rates as compared to TSA. This may hold true in the setting of the B2 glenoid, although a high-powered study on this subgroup is required. Anatomic shoulder arthroplasty maintains an important role in select patients. Further studies are required to better elucidate the role of glenoid bone loss and posterior humeral head subluxation with regard to implant choice.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Neeraj Vij, Sailesh Tummala, Eahsan Shahriary, John Tokish, Shelden Martin
Acquisition, analysis, or interpretation of data: Neeraj Vij, Sailesh Tummala, Eahsan Shahriary
Drafting of the manuscript: Neeraj Vij, Sailesh Tummala, John Tokish, Shelden Martin
Critical review of the manuscript for important intellectual content: Neeraj Vij, Sailesh Tummala, Eahsan Shahriary, John Tokish, Shelden Martin
Supervision: John Tokish, Shelden Martin
References
- 1.Reverse total shoulder arthroplasty without bone-grafting for severe glenoid bone loss in patients with osteoarthritis and intact rotator cuff. McFarland EG, Huri G, Hyun YS, Petersen SA, Srikumaran U. J Bone Joint Surg Am. 2016;98:1801–1807. doi: 10.2106/JBJS.15.01181. [DOI] [PubMed] [Google Scholar]
- 2.A 10-year experience with reverse shoulder arthroplasty: are we operating earlier? Reams RC, Vigan M, Wright TW, King JJ, Werthel JD, Schoch BS. J Shoulder Elbow Surg. 2020;29:0–33. doi: 10.1016/j.jse.2020.04.040. [DOI] [PubMed] [Google Scholar]
- 3.Postoperative complication rates following total shoulder arthroplasty (TSA) vs. reverse shoulder arthroplasty (RSA): a nationwide analysis. Ross B, Wu V, McCluskey L, O’Brien M, Sherman W, Savoie F. Semin Arthroplasty JSES. 2020;30:83–88. [Google Scholar]
- 4.Utilization of reverse shoulder arthroplasty for the treatment of glenohumeral arthritis among American Board of Orthopaedic Surgery (ABOS) Part II candidates, 2008-2019. O’Reilly O, Day M, Skalitzky M, Gulbrandsen T, Patterson B. Semin Arthroplasty JSES. 2021:55–62. [Google Scholar]
- 5.Does preoperative diagnosis impact patient outcomes following reverse total shoulder arthroplasty? A systematic review. Coscia AC, Matar RN, Espinal EE, Shah NS, Grawe BM. J Shoulder Elbow Surg. 2021;30:1458–1470. doi: 10.1016/j.jse.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Morphologic study of the glenoid in primary glenohumeral osteoarthritis. Walch G, Badet R, Boulahia A, Khoury A. J Arthroplasty. 1999;14:756–760. doi: 10.1016/s0883-5403(99)90232-2. [DOI] [PubMed] [Google Scholar]
- 7.The evolution of the walch classification for primary glenohumeral arthritis. Jawa A, Shields MV. J Am Acad Orthop Surg. 2021;29:0–45. doi: 10.5435/JAAOS-D-20-00880. [DOI] [PubMed] [Google Scholar]
- 8.Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. Walch G, Moraga C, Young A, Castellanos-Rosas J. J Shoulder Elbow Surg. 2012;21:1526–1533. doi: 10.1016/j.jse.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Anatomic total shoulder arthroplasty with posterior capsular plication versus reverse shoulder arthroplasty in patients with biconcave glenoids: a matched cohort study. Alentorn-Geli E, Wanderman NR, Assenmacher AT, Sperling JW, Cofield RH, Sánchez-Sotelo J. J Orthop Surg (Hong Kong) 2018;26:1–8. doi: 10.1177/2309499018768570. [DOI] [PubMed] [Google Scholar]
- 10.Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. Mizuno N, Denard PJ, Raiss P, Walch G. https://pubmed.ncbi.nlm.nih.gov/23864178/ J Bone Joint Surg Am. 2013;95:1297–1304. doi: 10.2106/JBJS.L.00820. [DOI] [PubMed] [Google Scholar]
- 11.Eccentric reaming for B2 glenoids: history, preoperative planning, surgical technique, and outcome. Smith MJ, Loftis CM, Skelley NW. J Shoulder Elb Arthroplast. 2019;3 doi: 10.1177/2471549219870348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Research pearls: the significance of statistics and perils of pooling. Dhawan A, Brand JC, Provencher MT, Rossi MJ, Lubowitz JH. Arthroscopy. 2017;33:1099–1101. doi: 10.1016/j.arthro.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Quantifying heterogeneity in a meta-analysis. Higgins JP, Thompson SG. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Predictors for satisfaction after anatomic total shoulder arthroplasty for idiopathic glenohumeral osteoarthritis. Petri M, Euler SA, Dornan GJ, Greenspoon JA, Horan MP, Katthagen JC, Millett PJ. Arch Orthop Trauma Surg. 2016;136:755–762. doi: 10.1007/s00402-016-2452-6. [DOI] [PubMed] [Google Scholar]
- 15.Outcomes of anatomic shoulder arthroplasty performed on B2 vs. A1 type glenoids. Sheth MM, Morris BJ, Laughlin MS, Cox JL, Jones S, Elkousy HA, Edwards TB. J Shoulder Elbow Surg. 2020;29:2571–2577. doi: 10.1016/j.jse.2020.03.050. [DOI] [PubMed] [Google Scholar]
- 16.Mid- to long-term follow-up of shoulder arthroplasty for primary glenohumeral osteoarthritis in patients aged 60 or under. Neyton L, Kirsch JM, Collotte P, Collin P, Gossing L, Chelli M, Walch G. J Shoulder Elbow Surg. 2019;28:1666–1673. doi: 10.1016/j.jse.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 17.The effects of glenoid wear patterns on patients with osteoarthritis in total shoulder arthroplasty: an assessment of outcomes and value. Hussey MM, Steen BM, Cusick MC, et al. J Shoulder Elbow Surg. 2015;24:682–690. doi: 10.1016/j.jse.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 18.Total shoulder arthroplasty after previous arthroscopic surgery for glenohumeral osteoarthritis: a case-control matched cohort study. Nolte PC, Elrick BP, Arner JW, et al. Am J Sports Med. 2021;49:1839–1846. doi: 10.1177/03635465211006479. [DOI] [PubMed] [Google Scholar]
- 19.Comparative clinical outcomes of reverse total shoulder arthroplasty for primary cuff tear arthropathy versus severe glenohumeral osteoarthritis with intact rotator cuff: a matched-cohort analysis. Waterman BR, Dean RS, Naylor AJ, Otte RS, Sumner-Parilla SA, Romeo AA, Nicholson GP. J Am Acad Orthop Surg. 2020;28:0–8. doi: 10.5435/JAAOS-D-19-00493. [DOI] [PubMed] [Google Scholar]
- 20.Primary reverse total shoulder arthroplasty performed for glenohumeral arthritis: does glenoid morphology matter? Pettit RJ, Saini SB, Puzzitiello RN, Hart PJ, Ross G, Kirsch JM, Jawa A. J Shoulder Elbow Surg. 2022;31:923–931. doi: 10.1016/j.jse.2021.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Is there a relationship between preoperative diagnosis and clinical outcomes in reverse shoulder arthroplasty? An experience in 699 shoulders. Lindbloom BJ, Christmas KN, Downes K, et al. J Shoulder Elbow Surg. 2019;28:0–7. doi: 10.1016/j.jse.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Reverse total shoulder arthroplasty: a review of results according to etiology. Wall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. J Bone Joint Surg Am. 2007;89:1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 23.Glenohumeral osteoarthritis with intact rotator cuff treated with reverse shoulder arthroplasty: a systematic review. Heifner JJ, Kumar AD, Wagner ER. J Shoulder Elbow Surg. 2021;30:2895–2903. doi: 10.1016/j.jse.2021.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Is reverse total shoulder arthroplasty (rTSA) more advantageous than anatomic TSA (aTSA) for osteoarthritis with intact cuff tendon? A systematic review and meta-analysis. Kim H, Kim CH, Kim M, Lee W, Jeon IH, Lee KW, Koh KH. J Orthop Traumatol. 2022;23:3. doi: 10.1186/s10195-022-00625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and radiographic outcomes of total shoulder arthroplasty with bone graft for osteoarthritis with severe glenoid bone loss. Sabesan V, Callanan M, Ho J, Iannotti JP. J Bone Joint Surg Am. 2013;95:1290–1296. doi: 10.2106/JBJS.L.00097. [DOI] [PubMed] [Google Scholar]
- 26.Clinical and radiographic outcomes of a posteriorly augmented glenoid component in anatomic total shoulder arthroplasty for primary osteoarthritis with posterior glenoid bone loss. Ho JC, Amini MH, Entezari V, Jun BJ, Alolabi B, Ricchetti ET, Iannotti JP. J Bone Joint Surg Am. 2018;100:1934–1948. doi: 10.2106/JBJS.17.01282. [DOI] [PubMed] [Google Scholar]
- 27.Porous metal wedge augments to address glenoid retroversion in anatomic shoulder arthroplasty: midterm update. Sandow MJ, Tu CG. J Shoulder Elbow Surg. 2020;29:1821–1830. doi: 10.1016/j.jse.2020.01.101. [DOI] [PubMed] [Google Scholar]
- 28.Total shoulder arthroplasty with an anterior-offset humeral head in patients with a B2 glenoid. Chamberlain AM, Orvets N, Patterson B, Chalmers P, Gosselin M, Salazar D, Keener JD. JSES Int. 2020;4:638–643. doi: 10.1016/j.jseint.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.How long does a shoulder replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 10 years of follow-up. Evans J, Evans J, Craig R, et al. Lancet Rheumatol. 2020;2:539–548. doi: 10.1016/S2665-9913(20)30226-5. [DOI] [PubMed] [Google Scholar]