Abstract
Background:
The prognostic significance of tumor-infiltrating immune cells in endometrial cancer is a subject of ongoing debate. Recent evidence increasingly suggests that these immune cells and cytokines, abundant in endometrial cancer tissues, play a pivotal role in stimulating the body inherent anti-tumor immune responses.
Methods:
Leveraging publicly accessible genetic data, we conducted an exhaustive 2-sample Mendelian randomization (MR) study. This study aimed to explore the causal links between 731 immunophenotypes and the risk of endometrial cancer. We thoroughly assessed the robustness, heterogeneity, and potential horizontal pleiotropy of our findings through extensive sensitivity analyses.
Results:
Our study identified 36 immunophenotypes associated with endometrial cancer risk. Specific immunophenotypes, such as the percentage of Naive-mature B-cells in lymphocytes (OR = 0.917, 95% CI = 0.863–0.974, P = .005), and HLA DR expression on CD14-CD16 + monocytes (OR = 0.952, 95% CI = 0.911–0.996, P = .032), exhibited a negative correlation with endometrial cancer. Conversely, CD127 expression on CD45RA + CD4 + in Treg cells (OR = 1.042, 95% CI = 1.000–1.085, P = .049), and CM CD4+%T in T cell maturation stages (OR = 1.074, 95% CI = 1.012–1.140, P = .018) showed a positive correlation. Reverse MR analysis linked endometrial cancer to 4 immunophenotypes, including a positive correlation with CD127-CD8br %T cell of Treg (OR = 1.172, 95% CI = 1.080–1.270, P = .0001), and negative correlations with 3 others, including CM CD4+%T cell (OR = 0.905, 95% CI = 0.832–0.984, P = .019).
Conclusion subsections:
Our findings underscore a significant causal relationship between immunophenotypes and endometrial cancer in bidirectional MR analyses. Notably, the CM CD4+%T immunophenotype emerged as potentially crucial in endometrial cancer development.
Keywords: endometrial cancer, immunology, Mendelian randomization analysis
1. Introduction
Endometrial cancer (EC), the most prevalent gynecological cancer in developed countries, is also known as uterine epithelial cell carcinoma. The 2020 Global Cancer Statistics report indicates that EC accounts for approximately 4.5% of all female cancers.[1] It is estimated to cause 97,370 deaths and 417,367 new cases worldwide each year.[2]The standard treatment for most EC patients involves surgical staging procedures, such as total hysterectomy and bilateral salpingo-oophorectomy, often supplemented with adjuvant chemotherapy or radiotherapy.[3]
The prognostic significance of tumor-infiltrating immune cells in EC remains a subject of debate, largely due to inconsistencies across studies, which often result from small sample sizes or varied prognostic indicators.[4] Some research suggests that EC patients with high densities of CD68 + tumor-associated macrophages (TAMs) exhibit poorer recurrence-free survival (RFS) and overall survival (OS) compared to those with lower TAM densities.[5] Conversely, other studies indicate that high densities of CD163 + TAMs in EC patients either bear no significant relation to survival outcomes or are only associated with RFS.[5–7] However, with the National Comprehensive Cancer Network (NCCN) endorsing the Cancer Genome Atlas Research Network (TCGA) molecular typing for EC in March 2020 and incorporating it into the guidelines for EC diagnosis and treatment, our understanding of EC pathogenesis continues to evolve. Furthermore, with over 50 clinical trials in various categories of EC immunotherapy listed on clinicaltrials.gov,[8] emerging evidence increasingly supports the role of immune cells and cytokines in EC tissues in stimulating endogenous anti-tumor immune responses. EC is predicted to be particularly responsive to immunotherapy compared to other gynecological malignancies, making it a focal point of current research.[9]
Mendelian randomization (MR) is an analytical method grounded in the principles of Mendelian independent distribution laws, predominantly used for epidemiological etiological inference. MR examines correlation results using genetic variants as proxies for an exposure,[10] and infers potential causality in order to assess the relationship between these proxies and the outcome.
In our study, a comprehensive 2-sample bidirectional MR analysis was employed to investigate the causal relationship between immunophenotypes and EC. This approach aims to elucidate the complex interactions between genetics, immune response, and the development of EC, offering potential pathways for future therapeutic interventions.
2. Materials and methods
2.1. Research design
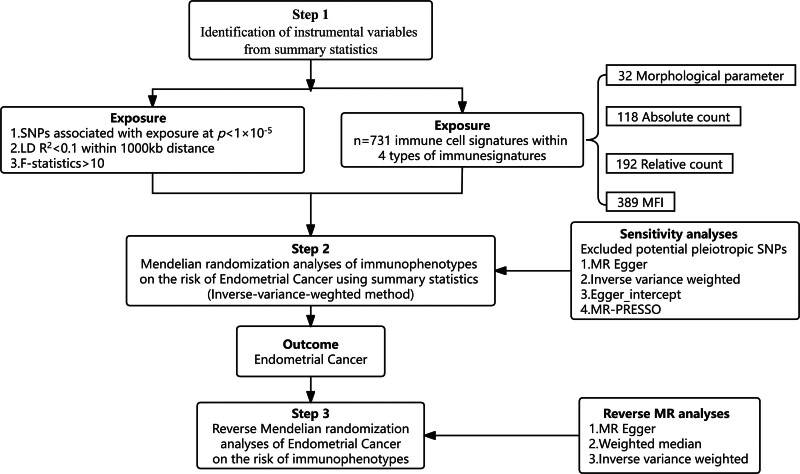
The comprehensive design of our study is illustrated in Figure 1. We conducted a 2-sample bidirectional MR analysis to explore the causal association between 731 immunophenotypes and EC. MR leverages genetic variations as proxies for risk factors, utilizing these variations as valid instrumental variables (IVs)[11] in establishing causal inferences. To be considered valid, these IVs must satisfy 3 critical criteria:
Figure 1.
The study design of the associations of immune cells and EC. EC = endometrial cancer, MR = Mendelian randomization, SNP = single nucleotide polymorphism.
Strong Association: There must be a robust association between the genetic variation and the exposure factors. This ensures that the genetic variation is a reliable proxy for the exposure of interest.
Independence from Confounding Factors: The genetic variation must be independent of any confounding factors that could affect both the exposure and the outcome. This criterion is essential to reduce the likelihood of bias in the causal inference.
Specific Pathway Influence: The genetic variation should influence the outcome exclusively through the exposure, and not via alternative pathways. This exclusivity is vital for attributing the observed effects on the outcome directly to the exposure.
Through these criteria, MR provides a powerful framework for inferring potential causal relationships, especially in complex scenarios where traditional observational studies may be susceptible to confounding factors or reverse causation.
2.2. Genome-wide association study (GWAS) data sources for EC
The GWAS data for EC was obtained from the Endometrial Cancer Association Consortium (ECAC),[12] encompassing 108,979 controls and 12,906 EC cases from European nations. Post quality control and imputation, approximately 9.47 million variants were analyzed. This analysis identified 9 new genome-wide significant loci, including a 12q24.12 locus previously recognized in meta-GWAS for endometrial and colorectal cancer. The specifics of the GWAS data sources for EC in this MR study are detailed in Table 1.
Table 1.
Details of the genome-wide association studies and datasets used in our analyses.
| Exposure or outcome | Sample size | Ancestry | Links for data download | PMID |
|---|---|---|---|---|
| Immunophenotypes | 731 immunological characteristics | European Ancestry | http://ftp.ebi.ac.uk/pub/databases/gwas/summary_statistics/(GCST90001391-GCST90002121) | 32929287 |
| Endometrial cancer | 108,979 controls and 12,906 EC cases | Endometrial Cancer Association Consortium | https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST006464/ | 30093612 |
2.3. Immunity-wide GWAS data sources
The GWAS data for 731 immunological characteristics were sourced from the GWAS catalog (serial numbers GCST90001391 to GCST90002121).[13] The immunophenotypes comprised 4 main feature types: absolute cell (AC) count (n = 118), morphological parameters (MP) (n = 32), relative cell (RC) count (n = 192), and median fluorescence intensity (MFI) indicating surface antigens amount (n = 389).[14]
The MFI, AC, and RC traits included B-cell, CDC, T cell maturation stage, monocyte, myeloid, TBNK (T cells, B-cells, natural killer cells), and Treg cell types, whereas MP trait covered CDC and TBNK types. The GWAS data, encompassing roughly 22 million variants, examined their effects on 731 immune cell traits in 3757 Sardinians,[15] identifying 122 significant independent associations across 70 loci (53 of which were new) and elucidating several molecules and mechanisms in cellular regulation. Details of these GWAS data sources for 731 immunological characteristics are presented in Table 1. This study was a reanalysis of previously collected and published publicly available data and therefore did not require additional ethical approval.
2.4. Selection of instrumental variables
Based on recent research,[13] we set the significance threshold for each immune trait IV and EC at 1 × 10−5. Using the “TwoSampleMR” software package, we defined linkage disequilibrium (LD) with an R2 < 0.001 threshold and set the aggregation distance at 10,000 kb within the EUR dataset of 1000 genomes. We extracted information related to the effect allele, effect size (β-value), standard error, and P value of each SNP. The proportion of variance explained (R2) and F-statistic were calculated to quantify instrumental strength using the formulae: R2 = 2 × MAF × (1 - MAF) × β,[2] F = R2 × (n - k - 1)/ [k × (1 - R2)], where “n” is the sample size, “k” the number of IVs, and “MAF” the minor allele frequency.[16,17]
2.5. Statistical analysis
We employed various methods including fixed/random effects inverse variance weighting (IVW), Weighted median, MR-Egger regression, Simple mode, and Weighted mode to estimate the potential causal relationship between immune cell phenotype and EC. The IVW method, known for providing the most accurate effect estimates, was our primary analysis tool.[18,19] The main causal estimate combined ratio estimates for each SNP, initially calculated using the Wald estimator and the Delta method.[20]
Cochran Q test and MR-Egger were utilized to test for heterogeneity among our selected SNPs. The random effects IVW method was applied in cases of heterogeneity (P < .05); otherwise, the fixed-effects IVW method was employed.[21] Sensitivity analyses were conducted to evaluate the robustness of associations, including the Weighted median method, which offers more reliable causal effect estimates when validated instruments are scarce.[22]
To detect horizontal pleiotropy, we employed MR-Egger regression, indicating its presence if the intercept term was significant (P < .05).[23] The MR-PRESSO test was also conducted to identify potential outliers, utilizing a global heterogeneity test. Additionally, scatterplots and funnel plots were used to ensure the results were unaffected by outliers and free from heterogeneity. All 731 immunophenotypes underwent MR analysis with EC. Those yielding positive results were further subjected to reverse MR analysis to determine which immunophenotypes are influenced by EC.
Bidirectional MR analysis was performed using R version 3.6.3, employing the “Mendelian Randomisation,” “TwoSampleMR,” “MR-PRESSO,” and “ggplot2” packages.
3. Results
3.1. Exploration of the causal effect of immunophenotypes on the onset of EC
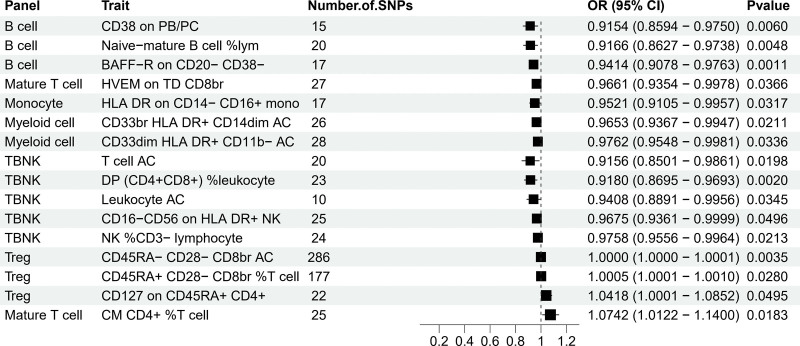
To analyze the causal relationship between 731 immunophenotypes and EC, 5 analytical methods were employed: MR-Egger, Weighted median, Inverse variance Weighted, Simple mode, and Weighted mode. This analysis identified 36 immunophenotypes correlated with EC, as detailed in Table S1, http://links.lww.com/MD/M489. We combined data from MR analyses after ensuring exposure and outcome concordance (Table S3, http://links.lww.com/MD/M491), and also provided outcome data for each SNP (Table S4, http://links.lww.com/MD/M492). To assess horizontal pleiotropy, heterogeneity, and sensitivity of each immunophenotype, we employed 3 methods: Inverse variance Weighted, MR-Egger, and MR-PRESSO, as shown in Table S1, http://links.lww.com/MD/M489. The selected results, based on the OR and P values from the MR analysis, are presented in Figure 2.
Figure 2.
Forest plots showed the exploration of the causal effect of immunophenotypes onset on EC by using IVW. CI = confidence interval, EC = endometrial cancer, IVW = inverse variance weighting.
Notably, several immunophenotypes demonstrated a negative correlation with EC, including CD38 on PB/PC, Naive-mature B-cell %lymphocyte, and BAFF-R on CD20- CD38- in B-cells; T cell AC, DP (CD4 + CD8+) %leukocyte, Leukocyte AC, CD16-CD56 on HLA DR+, and NK %CD3- lymphocyte in TBNK cells; HVEM on TD CD8br in Mature T cells; and CD33br HLA DR + CD14dim AC and CD33dim HLA DR + CD11b- AC in Myeloid; as well as HLA DR on CD14- CD16 + monocytes. Conversely, certain immunophenotypes, including CD45RA- CD28- CD8br AC in Treg, CD45RA + CD28- CD8br %T cell, CD127 on CD45RA + CD4+, and CM CD4+ %T cell in maturation stages of T cells, showed a positive correlation with the risk of EC. The robustness of these causal relationships was confirmed by the other 4 MR analysis methods and sensitivity analyses. The MR-Egger intercept and MR-PRESSO global test ruled out horizontal pleiotropy (Table S1, http://links.lww.com/MD/M489).
3.2. Exploration of the causal effect of EC on immunophenotypes
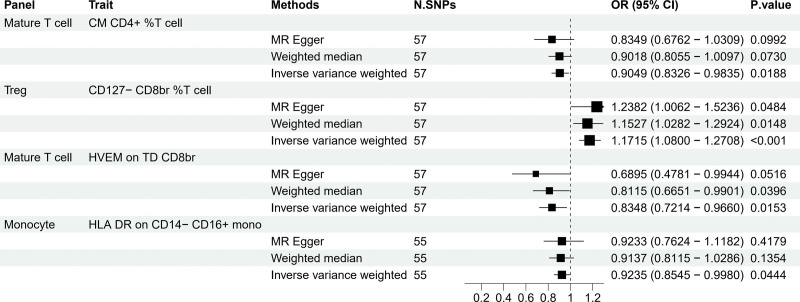
MR analysis indicated that EC impacted 4 immunophenotypes, as depicted in Figure 3. Methods like MR-Egger, Weighted median, and Inverse variance Weighted were used to analyze the causal relationship between EC and the 36 immunophenotypes identified earlier. EC positively correlated with CD127- CD8br %T cell of Treg cells, suggesting an increase in the expression of this immunophenotype with higher cancer risk (Fig. 4). In contrast, it showed negative correlations with HVEM on TD CD8br, CM CD4 + percent T cells, and HLA DR on CD14- CD16 + monocytes, indicating a decrease in the expression of these immunophenotypes with increased cancer risk (Fig. 4). The alignment of findings from MR-Egger and Weighted median with the IVW analysis, along with the intercept of MR-Egger and the global test of MR-PRESSO, confirmed the absence of horizontal pleiotropy (Table S2, http://links.lww.com/MD/M490).
Figure 3.
Forest plots showed the exploration of the causal effect of EC onset on immunophenotypes by using 3 methods. CI = confidence interval, EC = endometrial cancer.
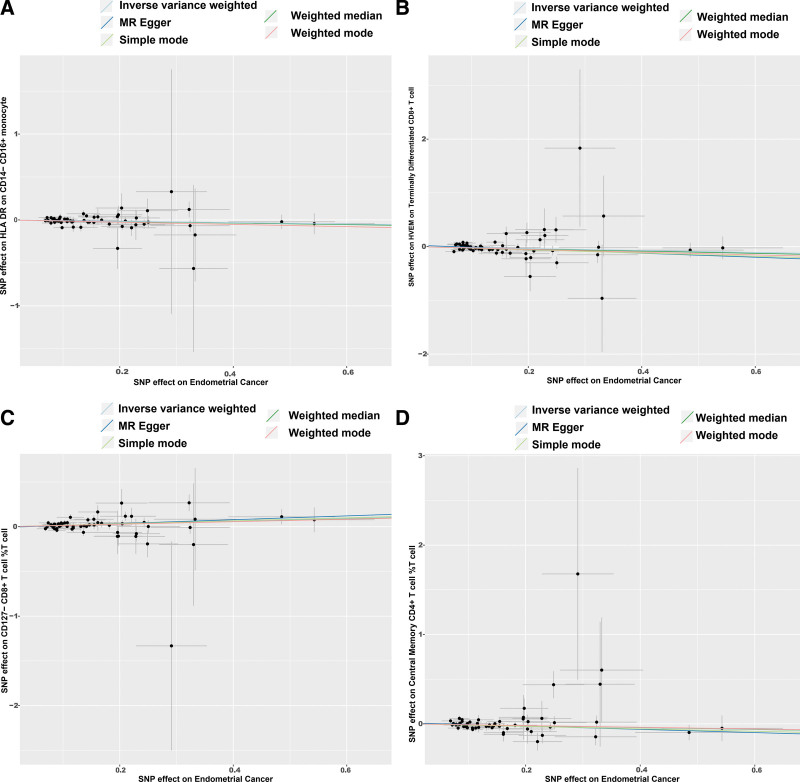
Figure 4.
Scatter plot of the associations of genetic variants with 4 immunophenotypes and the risk of EC. EC = endometrial cancer, MR = Mendelian randomization, SNP = single nucleotide polymorphism.
4. Discussion
Based on a large amount of publicly available genetic data, the causal relationship between 731 immunophenotypes and EC was examined, which is the first MR analysis to explore the causal relationship between multiple immunophenotypes and EC. In this study, among 4 types of immune traits (MFI, RC, AC, and MP), EC was found to have causal effects on 4 immunophenotypes (P < .05), and 36 immunophenotypes had significant causal effects on EC (P < .05).
When endometrial carcinogenesis occurs, endogenous or exogenous oncogenic factors can either directly modulate immune-related signaling pathways or host defensive inflammation, or indirectly alter the initial immune homeostasis through tumor-induced immune editing, leading to a dual role of immunity as both an anti-tumor and a tumor-promoting agent in the course of endometrial carcinogenesis.[8]
By generating T cell responses, killing cancer cells directly, and secreting immunoglobulins, B-cells impede the growth of tumors[24]; we found that Naive-mature B-cells exerted an inhibitory effect on EC, and Gunjan Mandal et al demonstrated that B-cell markers corresponded to prolonged survival in high-grade endometrioid tumors[25]; it was shown that the dimeric IgA of the B-cell binds to the polymerized immunoglobulin receptor, or pIgR, in the EC immune microenvironment. This interaction starts pro-apoptotic pathways, endoplasmic reticulum stress, and cell-intrinsic inflammation, all of which cause the tumor cells to die.[26]
Genetically altered to become effector cells that attack cancer cells, natural killer (NK) cells are highly effective at identifying and eliminating malignant cells,[27] We found that the vast majority of cellular phenotypes in the TBNK group of T cell AC, DP (CD4 + CD8+) %leukocyte, Leukocyte AC, CD16-CD56 on HLA DR + NK and NK %CD3- lymphocyte plays an inhibitory role in EC risk. In NK cells, CD4 + T cells identify MHC-class II molecularly restricted antigens, while CD8 + T cells identify MHC-class I molecules bound to antigens on the target cell surface and engage in immune function.[28] Empirical evidence suggests that cisplatin and rapamycin work in concert to enhance NK cell cytotoxicity and impede UEC growth in both IL-27-dependent and non-dependent ways,[29] suggesting that drugs that rely on the immune action of NK cells for the treatment of tumors have been used in EC.
The results showed that HLA DR on CD14- CD16 + Monocyte, CD33br HLA DR + CD14dim AC, CD33dim HLA DR + CD11b- AC of myeloid cells reduce the risk of EC; the human class II major histocompatibility complex (MHC) antigen HLA DR is constitutively expressed on the surface of B lymphocytes, monocytes, and macrophages. It plays a role in the presentation of antigen to CD4 + T cells, promoting T cell proliferation and stimulating B-cells to generate humoral immunity.[30] CD33 is a SIGLEC,-a transmembrane lectin that binds to sialic acid-and comprises of type 1 membrane proteins featuring 2 immunoglobulin structural domains. CD33 also possesses intracellular immune receptor tyrosine inhibitory motifs (ITIMs) crucial for immune processes.[31] Our opinion is supported by the study by Cristina Nieto-Jiménez et al which showed the effectiveness of CD33-containing tumor protest targets (TATs) in EC.[32]
Michel DuPage et al from the University of California, Berkeley, USA, discovered that in order for CXCR3-expressing Treg cells to interact with dendritic cells, co-stimulatory signaling was inhibited through the toxicity of T-lymphocyte antigen-4 (CTLA-4)-expressing CD 80 and CD 86, this failure to initiate CD8 + T cells limited the anti-tumor immune response.[33]
Studies have shown that regulatory T cells (Treg) can suppress the ongoing immune response in the endometrial microenvironment,[8] and our results show that the T cell phenotypes of CD127 on CD45RA + CD4 + CD45RA + CD28- CD8br, and CD45RA- CD28- CD8br AC and CM CD4+ %T cells play a significant role in EC. The affixation of Treg and DCs in an integrin-dependent manner has been demonstrated to specifically promote Treg proliferation, while Treg functions as an immune suppressor[34]; CD45RA is a heterozygous form of CD45 protein, and the CD45 molecule is encoded by a single gene in region 32 of the short arm of the first pair of chromosomes (lq32).
Lymphocyte growth and maturation, functional control, and signaling are all significantly influenced by CD45, and CD45RA + T cells are also known as “primitive” T cells, which have a suppressive immune effect.[35] Zhi wei Zhai et al found in a study of colorectal cancer that the proportion of CD4 + CD45RA + T cells increased significantly after surgery, suggesting that they play an important role in tumor immunity and have a close relationship to tumor staging and prognosis.[36]
Immune tolerance and immune response regulation are significantly aided by the cell surface receptor CD127; Hu Xiaoyu et al[37] Inflammation suppression of CD127 and its downstream STAT5 signaling pathway in monocytes, Thomas Kolben et al[38] found that CD4 + CD25 + CD127 Treg cells led to an increase in uterine enhanced invasion, migration and viability of EC cells, Using flow cytometry, Li Li et al examined the elevated proportion of peripheral blood bearing CD127 regulatory T cells in EC patients as a potential source of immune tolerance in EC.[39] We have shown that increased expression of CM CD4+ %T cells in mature-stage T cells promotes EC risk. It has been shown[40] that CD4 + T cells may be influenced by the tumor microenvironment TME, acting in association with bone marrow-derived suppressor cells MDSCs, tumor-associated macrophages TAMs, to produce cytokines for pro-tumor functions, and may also be transformed into cells such as Treg, Th1-Treg to produce immunosuppressive functions.
According to reverse magnetic resonance analysis, EC correlated negatively with CM CD4+%T cell and positively with CD127-CD8br%T HVEM on TD CD8br and HLA DR on CD14- CD16 + monocyte, TNF receptor family member HVEM (TNFRSF14) is expressed in immune cells by resting T and B-cells, NK cells, Treg, monocytes, and DC are strongly expressed; up-regulation of HVEM by tumor cells is involved in the suppression of anti-tumor immunity through the CD28 family BTLA belonging to, leading to disease progression and a poorer prognosis.[41]
The results have shown that increased expression of CM CD4+ %T cell in mature-stage T cells promotes EC risk, and Inverse MR analysis shows that increased EC risk suppresses its expression, which seems to be a paradoxical result, but in fact are 2 different response processes. The cellular phenotype of CM CD4+ %T cell, although pro-carcinogenic, leads to a decrease in its expression as tumor risk increases, suggesting that the body protects itself through some mechanism that inhibits the decline of this pro-carcinogenic factor? The stability of CM CD4+ %T cells was demonstrated by forest plot, scatterplot, and funnel plot (Fig. S1, http://links.lww.com/MD/M487 &S2, http://links.lww.com/MD/M488).
In summary, this study was based on published GWAS results for 2-sample MR analysis with a large sample size and high statistical efficiency. The conclusions of this study were based on genetic instrumental variables and causal inferences were made using multiple MR analyses. The results are robust and not confounded by horizontal pleiotropy and other factors.
This study has limitations, the analysis produced many positive results, and the significance of positive and negative correlations was not very clear, but most of the results analyzed are supported by the literature and data, indicating the reliability and accuracy of this bidirectional MR analysis, which is useful for clinical research. One of the other limitations is that this study is based on a European database, and whether it matches with the cellular immunity situation of EC in China is something to think about and further data support.
5. Conclusions
In conclusion, through comprehensive bidirectional MR analyses, the study shown that 36 immunophenotypes and EC are causally related, highlighting the complex pattern of interactions between the immune system and EC, which provides new avenues for studying the biological mechanisms and immunotherapy of EC. In particular, an immune cell phenotype CM CD4+ %T cell, which was expressed in both positive and negative MR, was found, suggesting that it may have a more important role in EC, but more research is required to determine its mechanism.
Acknowledgments
We thank the Endometrial Cancer Association Consortium (ECAC) for developing and curating their data resources of GWAS and we also thank Orrù V et al for providing Immunity-wide GWAS Data Sources for our analyses.
Author contributions
Conceptualization: Xinyun Zou.
Data curation: Xinyun Zou.
Formal analysis: Xinyun Zou, Hengdi Zhang.
Investigation: Xinyun Zou, Hengdi Zhang, Fangyuan Kong, Xuemei Jin.
Methodology: Xinyun Zou, Jinlan Shen.
Software: Jinlan Shen, Fangyuan Kong, Xuemei Jin, Ling Zhang.
Validation: Ling Zhang.
Writing – original draft: Xinyun Zou.
Writing – review & editing: Ling Zhang.
Supplementary Material
Abbreviations:
- AC
- absolute cell
- CI
- confidence interval
- EC
- endometrial cancer
- GWAS
- genome-wide association study
- IVW
- inverse variance weighting
- MFI
- median fluorescence intensities
- MP
- morphological parameters
- MR
- Mendelian randomization
- MR-PRESSO
- MR pleiotropy residual sum and outlier
- OR
- odds ratio
- RC
- relative cell
- SNPs
- single nucleotide polymorphisms
The study original contributions are contained in the article and Supplementary Material. Please contact the corresponding authors with any additional questions.
The studies included in our analysis were approved by the relevant institutional review boards, and participants provided informed consent.
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
How to cite this article: Zou X, Shen J, Zhang H, Kong F, Jin X, Zhang L. Association between immune cells and endometrial cancer: A bidirectional Mendelian randomization study. Medicine 2024;103:19(e38129).
Contributor Information
Xinyun Zou, Email: zouxy8700@163.com.
Jinlan Shen, Email: shenjl8800@163.com.
Hengdi Zhang, Email: zhangling1718@126.com.
Fangyuan Kong, Email: kfangyuan115@163.com.
Xuemei Jin, Email: youxiangjin1994@163.com.
References
- [1].Ministry of Health NZ. New cancer registrations 2018. https://www.health.govt.nz/publication/new-cancer-registrations-2018. [Google Scholar]
- [2].Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- [3].Crosbie EJ, Kitson SJ, McAlpine JN, et al. Endometrial cancer. Lancet (London, England). 2022;399:1412–28. [DOI] [PubMed] [Google Scholar]
- [4].Guo F, Dong Y, Tan Q, et al. Tissue infiltrating immune cells as prognostic biomarkers in endometrial cancer: a meta-analysis. Dis Markers. 2020;2020:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jiang X-f, Tang Q-l, Shen X-m, et al. Tumor-associated macrophages, epidermal growth factor receptor correlated with the triple negative phenotype in endometrial endometrioid adenocarcinoma. Pathol Res Pract. 2012;208:730–5. [DOI] [PubMed] [Google Scholar]
- [6].Soeda S, Nakamura N, Ozeki T, et al. Tumor-associated macrophages correlate with vascular space invasion and myometrial invasion in endometrial carcinoma. Gynecol Oncol. 2008;109:122–8. [DOI] [PubMed] [Google Scholar]
- [7].Salvesen HB, Akslen LA. Significance of tumour-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumour angiogenesis and prognosis in endometrial carcinomas. Int J Cancer. 1999;84:538–43. [DOI] [PubMed] [Google Scholar]
- [8].Cao W, Ma X, Fischer JV, et al. Immunotherapy in endometrial cancer: rationale, practice and perspectives. Biomarker Res. 2021;9. doi: 10.1186/s40364-021-00301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Di Tucci C, Schiavi MC, Faiano P, et al. Therapeutic vaccines and immune checkpoints inhibition options for gynecological cancers. Crit Rev Oncol Hematol. 2018;128:30–42. [DOI] [PubMed] [Google Scholar]
- [10].Sekula P, Del Greco MF, Pattaro C, et al. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016;27:3253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu Q, Ni J-J, Han B-X, et al. Causal relationship between gut microbiota and autoimmune diseases: a two-sample Mendelian randomization study. Front Immunol. 2022;12. doi: 10.3389/fimmu.2021.746998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].O’Mara TA, Glubb DM, Amant F, et al. Identification of nine new susceptibility loci for endometrial cancer. Nat Commun. 2018;9:3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Orrù V, Steri M, Sidore C, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52:1036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang C, Zhu D, Zhang D, et al. Causal role of immune cells in schizophrenia: Mendelian randomization (MR) study. BMC Psychiatry. 2023;23:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sidore C, Busonero F, Maschio A, et al. Genome sequencing elucidates Sardinian genetic architecture and augments association analyses for lipid and blood inflammatory markers. Nat Genet. 2015;47:1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu C, Zhu S, Zhang J, et al. Inflammatory bowel diseases, interleukin-6 and interleukin-6 receptor subunit alpha in causal association with cerebral cortical structure: a Mendelian randomization analysis. Front Immunol. 2023;14:1154746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kamat MA, Blackshaw JA, Young R, et al. PhenoScanner V2: an expanded tool for searching human genotype–phenotype associations. Bioinformatics. 2019;35:4851–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46:1734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Larsson SC, Traylor M, Malik R, et al. Modifiable pathways in Alzheimer’s disease: Mendelian randomisation analysis. Bmj. 2017:j5375. doi: 10.1136/bmj.j5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Del Greco M F, Minelli C, Sheehan NA, et al. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34:2926–40. [DOI] [PubMed] [Google Scholar]
- [22].Bowden J, Davey Smith G, Haycock PC, et al. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tokunaga R, Naseem M, Lo JH, et al. B cell and B cell-related pathways for novel cancer treatments. Cancer Treat Rev. 2019;73:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mandal G, Biswas S, Anadon CM, et al. IgA-dominated humoral immune responses govern patients’ outcome in endometrial cancer. Cancer Res. 2022;82:859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Qin M, Hamanishi J, Ukita M, et al. Tertiary lymphoid structures are associated with favorable survival outcomes in patients with endometrial cancer. Cancer Immunol Immunother. 2022;71:1431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gong Y, Klein Wolterink RGJ, Wang J, et al. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Waldmann TA, Dubois S, Miljkovic MD, et al. IL-15 in the combination immunotherapy of cancer. Front Immunol. 2020;11:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou W-J, Chang K-K, Wu K, et al. Rapamycin synergizes with cisplatin in antiendometrial cancer activation by improving IL-27–Stimulated Cytotoxicity of NK Cells. Neoplasia. 2018;20:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mispelbaum R, Hattenhauer ST, Held SAE, et al. Baseline immune signature score of Tregs × HLA-DR+CD4+ T cells × PD1+CD8+ T cells predicts outcome to immunotherapy in cancer patients. Front Immunol. 2022;13:1054161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hejazi M, Zhang C, Bennstein SB, et al. CD33 delineates two functionally distinct NK cell populations divergent in cytokine production and antibody-mediated cellular cytotoxicity. Front Immunol. 2022;12:798087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nieto‐Jiménez C, Sanvicente A, Díaz‐Tejeiro C, et al. Uncovering therapeutic opportunities in the clinical development of antibody‐drug conjugates. Clin Translat Med. 2023;13:e1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moreno Ayala MA, Campbell TF, Zhang C, et al. CXCR3 expression in regulatory T cells drives interactions with type I dendritic cells in tumors to restrict CD8 + T cell antitumor immunity. Immunity. 2023;56:1613–30.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Camirand G, Wang Y, Lu Y, et al. CD45 ligation expands Tregs by promoting interactions with DCs. J Clin Invest. 2014;124:4603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Weitering TJ, Melsen JE, van Ostaijen-ten Dam MM, et al. Normal numbers of stem cell memory T cells despite strongly reduced naive T cells support intact memory T cell compartment in ataxia telangiectasia. Front Immunol. 2021;12:686333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhai Z, Wang Z, Jin M, et al. Peripheral blood CD45RO+T cells is a predictor of the effectiveness of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Medicine (Baltimore). 2021;100:e26214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang B, Zhang Y, Xiong L, et al. CD127 imprints functional heterogeneity to diversify monocyte responses in inflammatory diseases. J Exp Med. 2022;219:e20211191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kolben T, Mannewitz M, Perleberg C, et al. Presence of regulatory T-cells in endometrial cancer predicts poorer overall survival and promotes progression of tumor cells. Cellular Oncol (Dordrecht). 2022;45:1171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li L, Li Y, Yin Z, et al. Increased frequency of regulatory T cells in the peripheral blood of patients with endometrioid adenocarcinoma. Oncol Lett. 2019;18:1424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chalmin F, Humblin E, Ghiringhelli F, et al. Transcriptional programs underlying Cd4 T cell differentiation and functions. Int Rev Cell Mol Biol. 2018;341:1–61. [DOI] [PubMed] [Google Scholar]
- [41].Demerlé C, Gorvel L, Olive D. BTLA-HVEM couple in health and diseases: insights for immunotherapy in lung cancer. Front Oncol. 2021;11:682007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.