Abstract
Background:
Augmentation and reshaping of body volume, particularly in the gluteal area, presents a significant challenge in aesthetic surgery. Hyaluronic acid (HA) fillers have emerged as an effective and safe tool for such indications, but literature examining nonsurgical gluteal reshaping with HA remains limited. This study aims to evaluate the long-term safety of using recommended volumes of HA body fillers for nonsurgical gluteal augmentation.
Methods:
A retrospective, observational study was carried out across multiple centers in Italy and the United Arab Emirates. The study involved participants between 22 and 53 years of age who underwent gluteal augmentation using HA body filler (HYAcorp MLF1/2) between 2017 and 2021, with up to 4 years and 7 months of follow-up. Participants and investigators independently evaluated the procedure’s effectiveness by comparing pre- and posttreatment photographs. The Global Aesthetic Improvement Scale was used to assess posttreatment satisfaction by both participants and investigators. All adverse effects (AEs) were recorded.
Results:
The study included a diverse group of 91 participants. No serious adverse events were reported, with the majority of AE occurring shortly after treatment and resolving in 1 week. AEs were more frequently observed in participants with previous treatments using different substances in the treatment area.
Conclusions:
The real-world application of HA body filler (HYAcorp MLF1/2) for gluteal augmentation in the participants of this study showed the treatment’s effectiveness, with no severe adverse events reported among the participants. High levels of satisfaction were reported among both participants and investigators.
Takeaways
Question: What is the long-term safety of hyaluronic acid (HA) body fillers for nonsurgical gluteal augmentation?
Findings: This multicenter study conducted in Italy and the United Arab Emirates analyzed the effectiveness and satisfaction of HA body filler (HYAcorp MLF1/2) used for gluteal augmentation in patients age 22–53 years of age, with a follow-up of up to 4 years and 7 months, showing high satisfaction and no serious adverse events.
Meaning: This study demonstrates that HA body fillers (HYAcorp MLF1/2) are safe and effective for nonsurgical gluteal augmentation, with high satisfaction rates and no serious adverse events observed among the participants.
INTRODUCTION
Over the past few decades, there has been an increasing demand for strategies that can enhance or modify body surfaces or shapes, driven by both medical and aesthetic considerations.1 There are three main reasons why individuals undergo gluteal augmentations: to enhance their aesthetics,2 to restore lost contours due to weight loss or aging,3 and to repair lipoatrophy due to the human immunodeficiency virus.4 Currently, there are five techniques used for gluteal augmentation: implant-based gluteal augmentation, autologous fat or dermal fat grafting, local tissue rearrangement or local flaps, hyaluronic acid (HA) gel injection or other nonpermanent filler injection, and silicone injection.5–7 Due to the high complication rate, invasiveness, extended downtime of other surgical procedures, and patients’ increasing request, nonsurgical gluteal volume correction has been investigated.7,8
Among all nonpermanent fillers currently available, HA stands out as a filler that integrates with the body and is widely used, offering numerous benefits.9 Naturally occurring HA in the body degrades quickly, so cross-linking modifications are needed to extend the lifespan of exogenous HA.10,11 Biphasic HA fillers are based on a heterogenous mixture of cross-linked highly elastic (high G′) gel particles obtained by the cross-linking process and dispersed in a noncross-linked HA vehicle. Biphasic HA fillers exhibit long-lasting effects, together with high elasticity, firmness, and lifting capacity, all accompanied by good safety, tolerability, easier injectability, and homogeneous distribution under the skin.12
The injection of these fillers for gluteal volume correction and reshaping is a minimally invasive technique that has been investigated.13,14 However, little has been published on nonsurgical gluteal volume correction and reshaping with HA body fillers.15
The main objective of the study was to assess long-term safety of injecting a recommended volume of two HA body fillers in nonsurgical gluteal volume correction and reshaping. Among secondary objectives, the aim of the study was to assess macromolecular HA efficacy, evaluate investigators’ and patients’ satisfaction, and provide recommendations on the administration technique.
MATERIAL AND METHODS
Patient Selection
This observational, multicentric, retrospective study in Italy (Milan and Rome) and the United Arab Emirates analyzed participants who received HA body fillers (HYAcorp MLF1/2; BioScience GmbH, Germany) for gluteal augmentation or contouring. The participants were seen as part of routine clinical practice in one of the three aesthetic clinics participating in the study. The data were part of the information already recorded in different databases of these clinics.
Each specialist decided to initiate HA body filler procedures based on the clinical need of each individual and according to the usual clinical practice. The exclusion criteria for injection were low-grade ptosis, body mass index less than 18 or more than 30, and those specified in the product’s instructions for use.16
Ninety-one participants older than 21 years underwent gluteal augmentation with HA body fillers (HYAcorp MLF1/2) from 2017 to 2022 with complete medical records with up to 4 years and 7 months follow-up (Table 1).
Table 1.
Follow-up Times
| Efficacy Outcomes | |
|---|---|
| Follow-up duration | |
| Mean follow-up (d ± SD) | 701.6 ± 490.9 |
| Follow-up > 2 (y), n | 44 |
| Follow-up ≥ 1 ≤ (y), n | 17 |
| Follow-up < 1 (y), n | 30 |
| Volume injected | |
| Mean volume, mL/session/side, (minimum–maximum) | 40.86 (20 - 70) |
| Mean total volume, cc/person/side ± SD | 119.89 ± 37.22 |
Filler Properties and Administration Technique
This study used HYAcorp MLF1/2 HA body fillers (CE 2409). These body fillers are absorbable, of middle-to-high viscosity, unequally sized, and of nonanimal origin, with a high purity level intended to restore lost volume and contour body surfaces.16
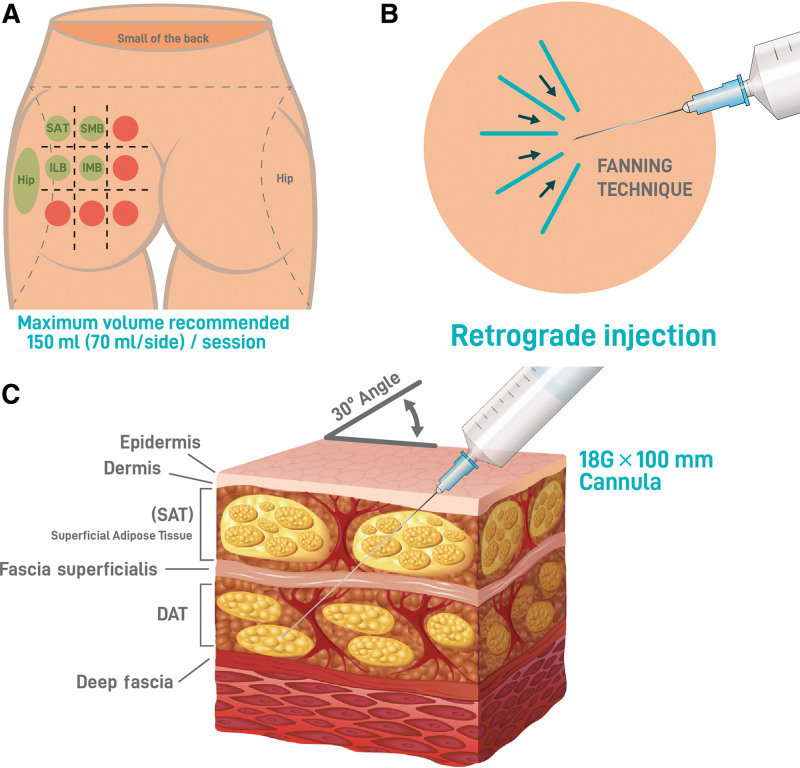
The approach to the patients’ needs and preferences was tailored, and with their input, the treatment area was determined. As for the application technique, HYAcorp MLF1/2 was implanted into the deep subcutaneous layer to provide volume by supporting with a vertical vectorial projection of the superior and inferior medial and lateral upper gluteus (Fig. 1A). For hip dip contouring, HYAcorp MLF1 is preferred for its lower particle size range, and it has been injected in a more superficial plane of the subcutaneous fat tissue. The technique used was retrograde injection with an 18G cannula in the deep fat layer of the gluteus (Fig. 1B and C).
Fig. 1.
Injection technique. A, Green circles represent the injection areas. The red circles indicate areas where injections are considered dangerous. B, Injection technique. The study used a fanning technique with retrograde injection as its method of injection. C, Infographic representation of the gluteal soft tissue layers. The injection layer DAT is indicated. DAT, deep adipose tissue; ILB, inferior lateral buttock; IMB, inferior medial buttock; SLB, superior lateral buttock; SMB, superior medial buttock.
Before the procedure, a prophylactic antibiotic oral treatment for 5 days and ibuprofen 600 mg twice daily for 7 days were prescribed to the patient. After taking the preprocedure pictures, the gluteus was marked according to the patient’s needs in an upright position. Subsequently, the patient lay prone, and the skin of the gluteal area was cleaned with an antiseptic solution (betadine or chlorhexidine). A sterile field was created with drapes, and 2 mL of lidocaine 2%, was injected at the cannula’s entry points, which were defined according to the target areas. A second entry point was made in each treated gluteal area to deposit cross-hatched product and achieve a three-dimensional augmentation. An 18G needle was used to create an opening in the skin that closes without stitches at the end of the procedure. An 18G 100-mm cannula was then inserted at a 30-degree angle, and 10 cc of lidocaine 2%, with or without epinephrine, was distributed superficially in the marked area to reduce patient pain.
After a few minutes, the procedure was started under sterility conditions to help prevent surgical site infections. The recommended injection volume per session did not exceed 70 mL of HA body filler per side. At the end of the treatment, a patch was used to close the entry points of the cannula. Taping around the treated area or gluteal enlargement garments was used for several days. Participants were advised to not shower or sleep face down for the first 3 days after treatment. Additionally, participants were advised to compulsory suspend physical activity for at least 2 weeks and to avoid saunas, steam rooms, tanning, and swimming for 3 weeks. Other prohibited treatments included skincare treatments such as retinoids, chemical peels, and laser treatments for at least a 2-week period to avoid inflammation of the injection site.
Study Endpoints
Patient demographic data such as gender and age were recorded. Information on the number and date of each session performed for each patient, along with the volume of HA injected per side, the region injected in each gluteus, the treatments performed in the area before the study, was collected.
Adverse effects (AEs) were analyzed as part of the safety analysis. The participants’ assessment of expected events was recorded. Late-onset AEs were defined as AEs that occur after 15 days postprocedure, whereas early AEs were those that occur within the first 15 days after injection. The safety analysis included all the participants treated with the study products.
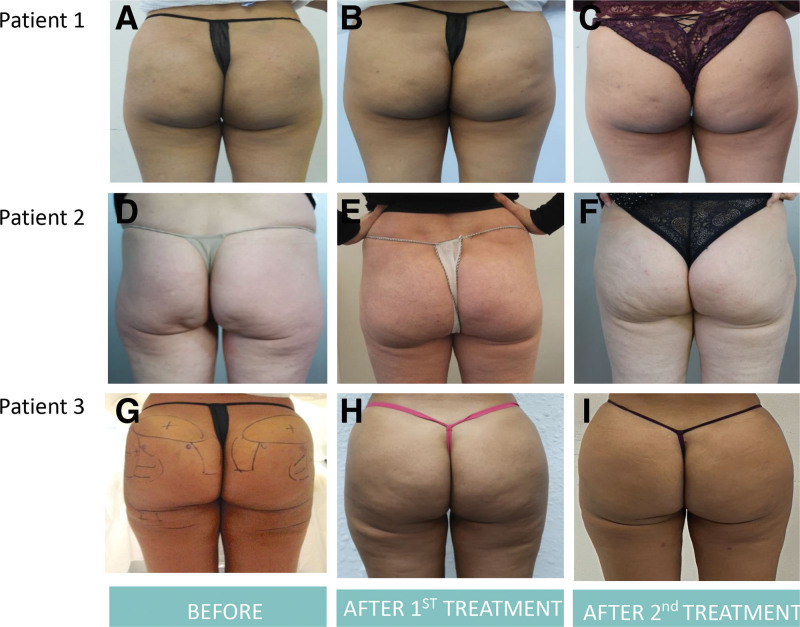
Effectiveness was assessed independently by the participants and the investigators at follow-up visits by comparing pre- and postprocedure photographs (Fig. 2). Posttreatment satisfaction was analyzed by the investigators and participants using the Global Aesthetic Improvement Scale17 at the last treatment. This five-point scale assesses overall satisfaction with the treatment, where 1 is “very much improved,” and 5 is “worsened.” All effectiveness analyses were performed on the intention-to-treat population, which included all the participants treated with the device.
Fig. 2.
Examples of photographs taken of the treated patients are provided. The figure displays three patients. A, D, and G, Patients before any treatments. B, E, and H, Patients after the first treatment. C, F, and I, Patients after the second treatment.
After identifying the participants in the database of the participating clinics and verifying that they met all inclusion criteria and no exclusion criteria, a dissociated code was assigned to each participant, and an electronic data collection form was completed. Finally, the investigators checked the validity of the data entered. Participants were not interviewed to collect additional data specific to the study.
Statistical Analysis
All data collected in the medical records were exported in an anonymized form. The data types of the different fields were heterogeneous, being numerical (age, volumes, and dates), Boolean (type of intervention, regions, and AE), or labels (places where the treatment was performed).
The Weka 3.8 Data Mining: Practical Machine Learning Tools and Techniques software18 was used for a preliminary visualization of the data and correlations between the descriptive data of the participants (gender, age, and previous treatments), the type of treatment performed (region, number of treatments, and the volume of HA body filler applied per treatment), and possible AEs. Cross and/or Pearson correlations were applied, depending on the data type used, using the NumPy Python module.19
First, a manual selection of the most significant differences and correlations was carried out. Subsequently, the significance of these results was corroborated by applying the t test with a confidence interval of 95%, confirming or rejecting the null hypothesis inferred from the observations. Both these calculations and the results’ representations were mAE with the modules SciPy 1.8.120 and Matplotlib 3.1.21
Ethical Aspects
The study was carried out under the principles of the Declaration of Helsinki and the legal regulations in force, respecting the ethical and legal standards applicable to this type of study. Due to the study’s retrospective design, only data already recorded in clinical history were collected. All the data were treated confidentially and dissociated, and it was impossible to associate, except by the site research team, the patient’s data with his or her person.
RESULTS
Patient Characteristics
A total of 91 participants were included, of which 81 (89%) were women, four (4.4%) were men, and six (6.6%) were transsexual. The mean age of the individuals analyzed was 33.5 (22–53) (minimum–maximum) years. Of the total participants recruited, 41 (45.5%) were treated at the center in Dubai (United Arab Emirates), 39 (42.86%) in Milan (Italy), and 11 (12.09%) in Rome (Italy).
Safety and Tolerability
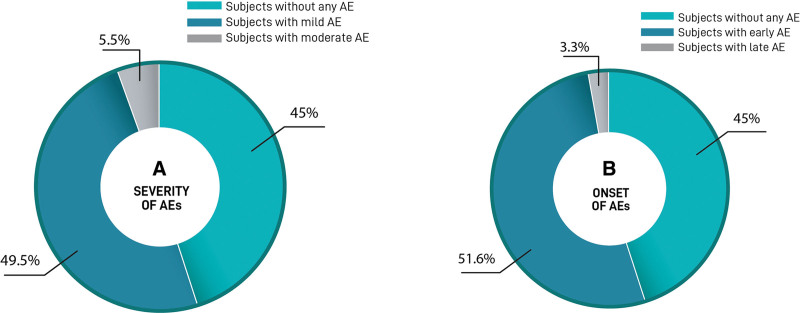
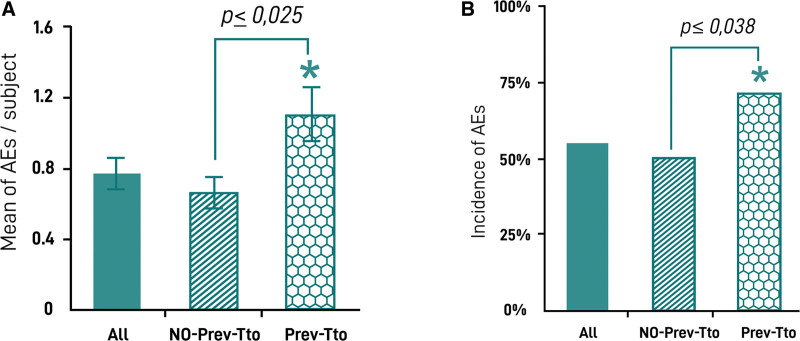
During the total follow-up period, 267 treatment sessions occurred in the 91 participants, and 69 AEs were reported in 50 participants. No serious AEs were reported during the follow-up period. Besides, 5.5% of the AEs were moderate according to the investigators’ criteria (nodules, infection, and allergy). Early AEs were the most common AEs, occurring in 47 (50.0%) participants versus only three (3.3%) late AEs (Fig. 3). The most common AEs was implant-site swelling which occurred in 25 (27.5%) participants, followed by bruising, itching pain, and mild inflammation. The incidence of AEs is shown in Table 2. It was observed that a significantly higher risk of AE in participants who had received a previous treatment in gluteal area with implants, fat, poly-L-lactic acid, or other HA (Fig. 4A) before the first HA injection with HYAcorp. Indeed, the probability of AE occurrence was 54.9% in all participants, 50% in naive, and 71.4% in those who had previously received greater than or equal to one surgical or nonsurgical treatment in the gluteus area. Moreover, participants who received a different previous treatment showed a significantly increased probability of developing more AEs compared with those who did not receive any different treatment in the gluteal area (Fig. 4B).
Fig. 3.
Incidence and type of adverse device effects. A, Severity of AE. The percentage of participants affected with mild to moderate AEs and participants not affected by any AE. B, Time of onset of AE. Percentage of participants affected with early or late-onset AEs after procedure. Color legend of each population is shown at the bottom.
Table 2.
HA Filler Safety Profile
| Subjects with AEs | |
|---|---|
| Total subjects with AEs, N (%) | 50 (54.9) |
| Early AEs | 47 (51.6) |
| Late AEs | 3 (3.3) |
| Incidence of AEs | |
| Total AEs, N (%) | 69 |
| Swelling | 25 (27.4) |
| Bruising | 14 (15.3) |
| Itching | 13 (14.2) |
| Pain | 8 (8.7) |
| Mild inflammation | 4 (4.4) |
| Nodules | 3 (3.3) |
| Infection | 1 (1.1) |
| Allergy | 1 (1.1) |
Fig. 4.
Incidence of AEs in participants with other previous treatments. A, Incidence of AEs in the whole cohort of participants (all), in a subcohort who did not receive a previous treatment (without previous treatment). B, Percentage of AEs per participant emerged in the study according to any previous treatment in the same area. *Statistically significant.
Treatment Recurrence
Of the 91 participants treated, five (5.5%) attended only one session; 25 (27.4%) had two sessions; 32 (35.2%) received three treatments; and, finally, 29 (31.9%) participants received four sessions. The enrolled participants were followed up for a mean of 701.6 days (SD 490.9). Of all treated participants, 44 were followed for more than 2 years, 17 between 1 and 2 years, and 30 during the first year (Table 1).
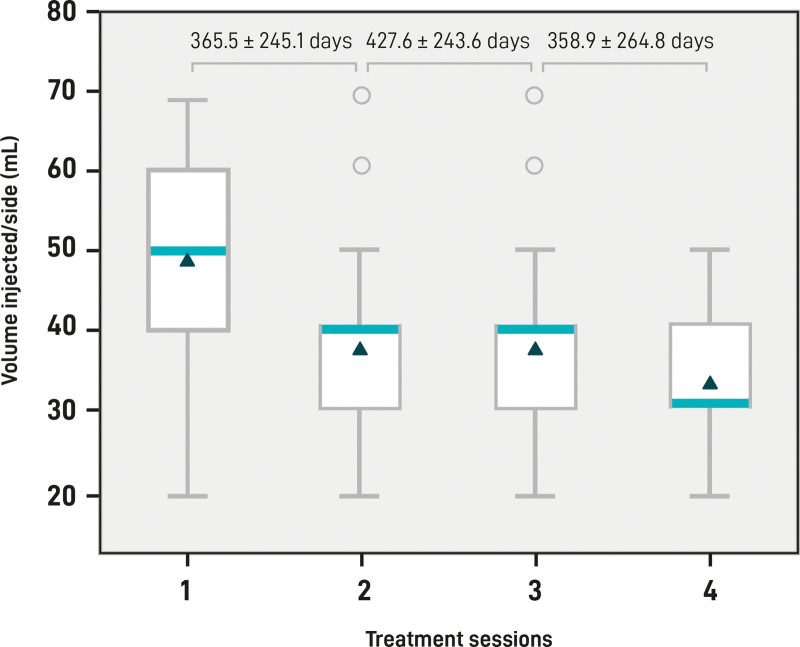
The mean volume of HA body filler injected per session per side was 40.86 mL (minimum–maximum, 20–70 mL), whereas the total mean volume injected per individual per side was 119.89 mL (SD 37.22) (Table 1). Throughout the follow-up, and to maintain product performance and patient satisfaction, it was observed that participants required less volume at subsequent visits to maintain the same result and/or, with the same injection volume, they obtained a satisfactory result for a longer period (Fig. 5).
Fig. 5.
HA filler volume injected. Box plots represent the lowest quartile, median, upper quartile, and highest observations of the mean volume (mL) injected in each session per side. Circles indicate the outliers. Mean and SD days of intervals between each session and the successive session are shown at the top of the plot.
Patient Satisfaction
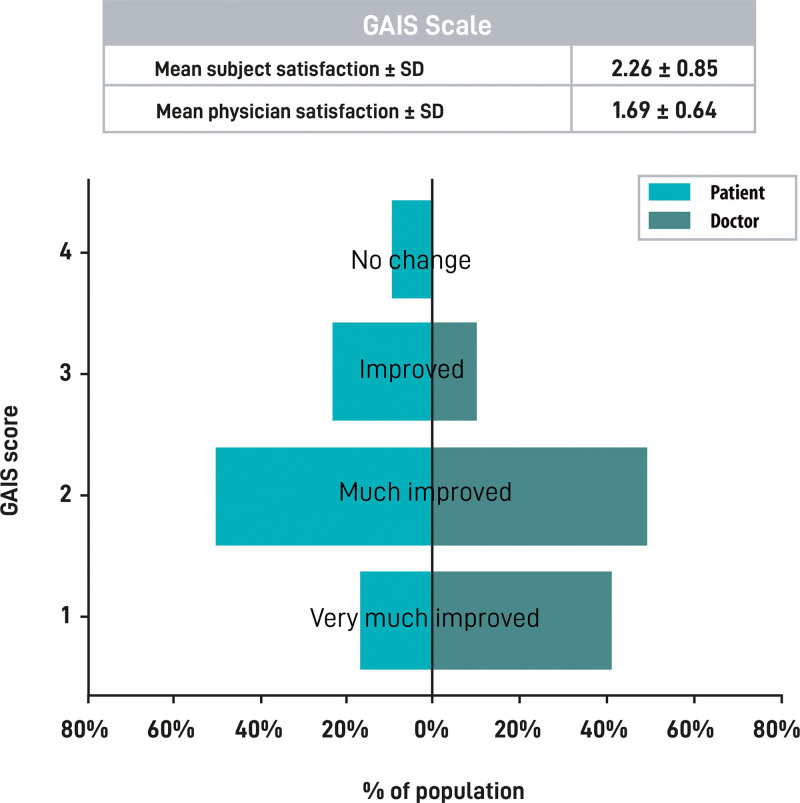
According to the patient-reported outcome tool, the Global Aesthetic Improvement Scale, the mean patient satisfaction score after the first treatment was 2.26 (SD 0.85) (much improved in 55% of the cases), whereas the investigators’ satisfaction was 1.69 (SD 0.64) (much improved in 51% of the cases and very much improved in 40% of the cases) (Fig. 6).
Fig. 6.
Treatment satisfaction. The table at the top of the figure represents the mean and SD of the participants’ and investigators’ GAIS scores. Score results were obtained from investigators and participants after completion of GAIS questionnaire after the procedure. Histogram represents the percentage of cases that assigned each GAIS score. Participants’ and investigators’ scores are shown in light green and dark green bars, respectively. GAIS, Global Aesthetic Improvement Scale.
DISCUSSION
The current retrospective observational study was conducted to extend the limit knowledge on the efficacy and safety of HA body filler treatment for nonsurgical gluteal volume correction and reshaping. For this purpose, we analyzed data from 91 participants in the database of three aesthetic clinics, two Italian and one in the United Arab Emirates.
A literature review (including gluteal augmentation techniques) analyzing data from 7834 participants revealed a high rate of complications after implant-based gluteal augmentation (30.5%) and the risk of serious complications such as fat embolism for fat grafting.15 Instances of fat embolism syndrome, though uncommon, have been reported in association with gluteal enhancement using HA, as noted in the medical literature. However, such complications are more frequently mentioned in the context of facial treatments.22–24
The use of HA gel injections for gluteal augmentation was investigated by two prospective studies,13,14 which showed that this is a safe and effective treatment for temporary aesthetic gluteal augmentation. Camenisch et al12 treated eight participants with a mean of 163 mL of HA per gluteus, reporting 56%, 36%, and 24% of gel remaining after 6, 12, and 24 months, respectively, located primarily in the subcutaneous fat. The treatment was well tolerated, with high participant and investigator perceptions.13 De Meyere et al13 administered HA injections to 61 patients, with each participant receiving up to a maximum volume of 400 mL, spread over one or two treatment visits. Notwithstanding the gradual degradation of the HA over time, a notable portion of the participants (40%) reported enhanced gluteal appearance and expressed satisfaction (33%) even after 2 years posttreatment.14
In a recent comprehensive analysis, Mortada et al reviewed the efficacy of HA injections in enhancing the gluteal area.25 According to these studies, the average volume of HA needed for satisfactory gluteal augmentation was 206.71 mL. This figure is quite close to the average volume used in the current study, which is 239.79 mL (approximately 119.89 ml per side). Similar to what is found in our study, the complication rate was very modest with only few complications observed in all the studies.25 In this study, it was observed that almost half of the participants (45.1%) did not experience AEs, and among those who did, the intensity was mainly mild. Moreover, almost all AEs were of early onset and rapid resolution. AEs postinjection observed in this study align with anticipated outcomes, as extensively outlined in the product’s instructions for use. Common reactions such as swelling, bruising, itching, and pain typically subside within 2-3 days. Additionally, other adverse events like mild inflammation, nodules, infection, and allergy (which represented 9.9% of the AEs in our study), usually resolve within a timeframe of 1-4 weeks, varying based on individual patient responses. AEs were more frequent in participants with a clinical history of autoimmune disease or previous treatments in the same area. Therefore, the key factors to ensure safe treatment revolve mainly around the quality of the product, the volume injected, the clinical history of the participant and the technique used. Indeed, when injecting HA filler in the buttock region, the preferred method involves targeting the deep subcutaneous tissue layer, due to the lack of large blood vessels or other high-risk structures in this area. This approach not only enhances safety but also ensures more efficient use of the product. This is particularly crucial for body regions, where the volume of product required is generally larger than that for facial treatments.15
In the Mortada et al25 review, the average effective duration of the HA filler treatment for gluteal augmentation was found to be about 16.16 months.25 In our study, notably, in cases where patient follow-up extended beyond 2 years, the treatment’s effectiveness appeared to last longer, around 24 months.
To the best of our knowledge, there is no published real-world data on HA body fillers in participants requiring gluteal augmentation with a larger sample size. Compared with a recent retrospective study,26 this study analyzes a larger sample size (43 versus 91, respectively) and includes a more diverse population (European and the United Arab Emirates patient cohorts). In addition, in this study, participants were followed up for a more extended period (6 versus 24 months on average).
Factors impacting HA filler’s persistence include product concentration, cross-linked technology, rheological parameters, water-binding capability, and injection technique,27,28 whereas other patient-dependent factors are the participant’s metabolism and daily activity. Additionally, a thorough patient evaluation and strategic procedure planning that accounts for cultural influences and the principles of aesthetic anatomy and morphometric considerations are crucial for fulfilling patient expectations.29
HA’s longevity in volume restoration depends on its resistance to breakdown by the body’s hyaluronidase and oxidative damage, with its effectiveness diminishing once below a critical level.30,31
Gel particle size, viscosity, and degree of cross-linking are key factors in determining the durability and effectiveness of HA fillers, particularly for large-volume applications like gluteal augmentation.32–34
On the other hand, Kim et al31 found that HYAcorp MLF2 has the best performance in terms of durability and maintaining localization without displacement, compared with a low-viscosity monophasic gel and a biphasic gel with smaller particle size such as Restylane.
So far, the cost-effectiveness of stabilized HA body filler for gluteal treatment has not been studied. However, the cost of this approach should be weighed against the participant’s wish not to undergo surgery and the fact that the HA injection procedure is generally more straightforward.14
We acknowledge that this study has several limitations. First, its design implies certain inherent limitations because it is a retrospective observational study performed in routine clinical practice. One of the limitations is that imaging evaluation, such as ultrasound, was not conducted before or after treatment. This is often the case in clinical practice where the use of imaging is not consistently applied. Another limitation, because the data were obtained from three centers in several countries, is that the different practices among them create uncontrolled variability. Finally, no data on comorbidities or the use of concomitant medication were collected in this study.
CONCLUSION
Our study indicates that using HA fillers for gluteal augmentation and reshaping is an effective method, yielding high patient satisfaction and a minimal rate of complications.
DISCLOSURES
Santacatterina, Fontenete, and Galera are employees at BioScience GmbH. BioScience supported the analysis of this study. The other authors have no financial interest to declare.
ACKNOWLEDGMENT
The authors thank Maria Romero, who provided medical writing assistance.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Footnotes
Published online 9 May 2024.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.The American Society for Aesthetic Plastic Surgery. Cosmetic surgery national data bank statistics. Aesthet Surg J. 2018;38:1–24. [DOI] [PubMed] [Google Scholar]
- 2.Lee EI, Thomas L, Roberts I, et al. Ethnic considerations in buttock aesthetics. Semin Plast Surg. 2009;23:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babuccu O, Gözil R, Özmen S, et al. Gluteal region morphology: the effect of the weight gain and aging. Aesthetic Plast Surg. 2002 M;26:130–133. [DOI] [PubMed] [Google Scholar]
- 4.Claude O, Bosc R, Pigneur F, et al. Treatment of HIV-infected subjects with buttock lipoatrophy using stabilized hyaluronic acid gel. Plast Reconstr Surg Glob Open. 2015;3:e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oranges CM, Haug M, Schaefer DJ. Body contouring. Plast Reconstr Surg. 2016;138:944e–945e. [DOI] [PubMed] [Google Scholar]
- 6.Hedén P, Sellman G, von Wachenfeldt M, et al. Body shaping and volume restoration: the role of hyaluronic acid. Aesthetic Plast Surg. 2009;33:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah B. Complications in gluteal augmentation. Clin Plast Surg. 2018;45:179–186. [DOI] [PubMed] [Google Scholar]
- 8.Necas J, Bartosikova L, Brauner P, et al. Hyaluronic acid (hyaluronan): a review. Vet Med. 2008;53:397–411. [Google Scholar]
- 9.Khunmanee S, Jeong Y, Park H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J Tissue Eng. 2017;8:2041731417726464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Micheels P, Sarazin D, Tran C, et al. Effect of different crosslinking technologies on hyaluronic acid behavior: a visual and microscopic study of seven hyaluronic acid gels. J Drugs Dermatol. 2016;15:600–606. [PubMed] [Google Scholar]
- 11.da Costa A, Biccigo DGZ, de Souza Weimann ET, et al. Durability of three different types of hyaluronic acid fillers in skin: Are there differences among biphasic, monophasic monodensified, and monophasic polydensified products? Aesthetic Surg J. 2017;37:573–581. [DOI] [PubMed] [Google Scholar]
- 12.Camenisch CC, Tengvar M, Hedén P. Macrolane for volume restoration and contouring of the buttocks: magnetic resonance imaging study on localization and degradation. Plast Reconstr Surg. 2013;132:522e–529e. [DOI] [PubMed] [Google Scholar]
- 13.De Meyere B, Mir-Mir S, Peñas J, et al. Stabilized hyaluronic acid gel for volume restoration and contouring of the buttocks: 24-month efficacy and safety. Aesthetic Plast Surg. 2014;38:404–412. [DOI] [PubMed] [Google Scholar]
- 14.Oranges CM, Tremp M, Di Summa PG, et al. Gluteal augmentation techniques: a comprehensive literature review. Aesthet Surg J. 2017;37:560–569. [DOI] [PubMed] [Google Scholar]
- 15.Lourenço LM, de Noronha MGO, Colla LA, et al. LL body contour technique—a new way of gluteal contouring and augmentation with hyaluronic acid filler. J Cosmet Dermatol. 2022;21:1967–1972. [DOI] [PubMed] [Google Scholar]
- 16.BioScience GmbH. HYacorp MLF1-2 instructions for use. 2022.
- 17.Narins RS, Brandt F, Leyden J, et al. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588–595. [DOI] [PubMed] [Google Scholar]
- 18.Frank E, Hall M, Trigg L, et al. Data mining in bioinformatics using Weka. Bioinformatics. 2004;20:79–81. [DOI] [PubMed] [Google Scholar]
- 19.Harris CR, Millman KJ, van der Walt SJ, et al. Array programming with NumPy. Nature. 2020;585:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virtanen P, Gommers R, Oliphant TE, et al. ; SciPy 1.0 Contributors. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. 2020;17:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter J. Matplotlib: a 2D graphics environment. Comput Sci Eng. 2007;9:90–95. [Google Scholar]
- 22.Uz I, Yalçinli S, Efe M. Fat embolism syndrome after gluteal augmentation with hyaluronic acid: a case report. Ulus Travma Acil Cerrahi Derg. 2020;26:960–962. [DOI] [PubMed] [Google Scholar]
- 23.Zhang YL, Chen Y, Sun ZS, et al. Retrospective study of vascular complications caused by hyaluronic acid injection. Aesthetic Plast Surg. 2023;47:2745–2753. [DOI] [PubMed] [Google Scholar]
- 24.Cho KH, Dalla Pozza E, Toth G, et al. Pathophysiology study of filler-induced blindness. Aesthet Surg J. 2019;39:96–106. [DOI] [PubMed] [Google Scholar]
- 25.Mortada H, Alkadi D, Saqr H, et al. Effectiveness and role of using hyaluronic acid injections for gluteal augmentation: a comprehensive systematic review of techniques and outcomes. Aesthetic Plast Surg. 2023;476:2719–2733. [DOI] [PubMed] [Google Scholar]
- 26.Santorelli A, Cerullo F, Salti G, et al. Gluteal augmentation with hyaluronic acid filler: a retrospective analysis using the BODY-Q scale. Aesthetic Plast Surg. 2022;47:1175–1181. [DOI] [PubMed] [Google Scholar]
- 27.Kablik J, Monheit GD, Yu LP, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35:302–312. [DOI] [PubMed] [Google Scholar]
- 28.De Boulle K, Glogau R, Kono T, et al. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol Surg. 2013;39:1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raposio E, Baldelli I, Vappiani M, et al. The ideal buttock: some aesthetic and morphometric considerations. Eur J Plast Surg. 2023;46:915–921. [Google Scholar]
- 30.Kobayashi T, Chanmee T, Itano N. Hyaluronan: metabolism and function. Biomolecules. 2020;10:1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YS, Choi JW, Park J-K, et al. Efficiency and durability of hyaluronic acid of different particle sizes as an injectable material for VF augmentation. Acta Otolaryngol. 2015;135:1311–1318. [DOI] [PubMed] [Google Scholar]
- 32.Heitmiller K, Ring C, Saedi N. Rheologic properties of soft tissue fillers and implications for clinical use. J Cosmet Dermatol. 202;20:28–34. [DOI] [PubMed] [Google Scholar]
- 33.Fagien S, Bertucci V, von Grote E, et al. Rheologic and physicochemical properties used to differentiate injectable hyaluronic acid filler products. Plast Reconstr Surg. 2019;143:707e–720e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six U.S. Food and Drug Administration-approved fillers. Plast Reconstr Surg. 2015;136:678–686. [DOI] [PubMed] [Google Scholar]