Highlights
-
•
More than 70 % of patients with ovarian cancer experience a recurrence within 2 years from diagnosis.
-
•
Radiotherapy has proved to be an effective radical locoregional treatment in order to improve the “free-drug holiday” to the patients.
-
•
In this real-world study, carbon ion radiotherapy (CIRT) provided high objective response rate and durable local control with mild toxicity.
-
•
The achievement of a complete response and a small GTV resulted significantly associated with a better local control rate after CIRT.
Keywords: Carbon Ion Radiotherapy, Fallopian tube cancer, Ovarian Cancer, Oligometastases
Abstract
Introduction
In the multidisciplinary management of oligometastatic, persistent, or recurrent (MPR) ovarian cancer, radiotherapy (RT) is becoming a more and more worthwhile treatment to potentially improve the chronicity of the disease. Particle beam RT has proved to be effective in several gynecological malignancies, but so far no data are available for ovarian cancer.
Material and Methods
This is a real-world, retrospective, bi-institutional, single-arm study aimed to assess the effectiveness and the safety of carbon ion RT (CIRT) in this setting. The co-first endpoints are 1-year and 2-year actuarial local control (LC) rates and the objective response rate (ORR) defined on a “per lesion” basis. The secondary endpoint was toxicity. Actuarial outcomes were evaluated using the Kaplan-Meier method while potential predictors were explored using the Log-rank test. Bi-variable logistic regression was employed in the analysis of factors predicting the complete response on a per-lesion basis.
Results
26 patients accounting for a total of 36 lesions underwent CIRT with a total median dose of 52.8 Gy[RBE] (range: 39–64 Gy[RBE]). Five patients received CIRT for re-irradiation. No concomitant systemic therapies were administered during CIRT. Within 12 months after the treatment, 17 lesions (47 %) achieved complete response while 18 (50 %) obtained a partial response with an ORR of 97 %. The achievement of a complete response is related to the dose per fraction (>4.2 Gy[RBE], p = 0.04) and total dose (>52,8 Gy[RBE], p = 0.05). The 1-year LC was 92 % and the 2-year LC was 83 %, according to the achievement of a CR (p = 0.007) and GTV ≤ 14 cm3 (p = 0.024). No grade > 3 toxicities were recorded both in naïve and re-irradiated patients. PARP-i and anti-VEGF seemed not to exacerbate the risk of severe toxicities.
Conclusions
CIRT was effective and safe in MPR ovarian cancers, even in the case of re-irradiation. Largest cohort studies and longer follow-up are needed to confirm these data.
Introduction
Ovarian and fallopian tube cancers are the 7th most frequent cancer diagnosed worldwide and the 5th cause of death in women due to cancer after lung, breast, colorectal, and pancreas tumors. Currently, ovarian cancer ranks as the 1st cause of death due to gynecological cancer [1]. Up to 80 % of ovarian and fallopian tube cancers are diagnosed in the advanced stage and, despite all the improvements in cytoreductive surgery and the introduction of Bevacizumab in front-line chemotherapy, more than 70 % of women experience a recurrence within 2 years [2]. Traditionally, the management of recurrent and metastatic disease is systemic chemotherapy, selected according to patient performance status, previous toxicities, platinum sensitivity, histotype, mutational status (BRCA genes or homologous recombination deficiency), and type/site of recurrence [3]. However, the management of this setting would dramatically modify in the upcoming years due to the recent implementation of PARP- inhibitors (PARP-i) in the first and second line for BRCA mutated patients[3], [4], [5], [6], [7]. In this shifting context, radiotherapy (RT) is becoming a more and more intriguing radical locoregional treatment to be included in a multidisciplinary approach, in order to improve the free-drug interval to the patients[8]. The largest retrospective series focusing on the role of RT in oligometastatic, oligorecurrent and oligopersistent (MPR) disease showed an encouraging rate of complete response (CR) with a durable local control (LC)[9]. However, to the best of our knowledge, in this setting there are no data yet about the use of particle RT [10], [11], particularly carbon ion radiotherapy (CIRT) which has proved to be effective in several gynaecological malignancies [10], [11], [12]. This bi-institutional pilot study has the aim of defining the efficacy and safety of CIRT in the first real-world data set of MPR ovarian and fallopian tube cancers.
Material and methods
-
a.
Study design
This is a pilot, real-world, retrospective, bi-institutional, single-arm study including patients treated with CIRT at the National Center for Oncological Hadrontherapy (CNAO) in Pavia (Italy) and at the National Institutes for Quantum Science and Technology (QST) in Chiba (Japan) for an oligo-MPR disease. The two institutional review boards approved the study (CNAO OSS 48 2022 on the date 09/06/2022 and QST-N23-006 on the date 20/06/2023). According to recent literature on MPR we considered as: i)“ oligometastatic” a disease in which there are ≤ 5 synchronous lesions in any site; ii) “oligorecurrent” in case of less than 5 new or enlarging metastases in otherwise well-controlled disease status; iii) “oligopersistent” in case of less than 5 persistent lesions after a locoregional or systemic treatment [9], [13], [14]. The inclusion criteria were: i) age >/= 18 years; ii) histological diagnosis of primary ovarian/fallopian tube cancer (all histologies were allowed); iii) diagnosis of oligo-MPR disease; iv) patient candidates for a curative intent treatment in a multidisciplinary tumor board; iv) exclusion of eligibility for photon beam RT or other local approach and/or patient’s refusal; v) signed written informed consent for treatment and use of their clinical data for research purposes; vi) performance status according to Eastern Cooperative Oncology Group (ECOG) score = 0–1. The combination with other oncological systemic therapy, when indicated, was allowed.
The coprimary endpoints, defined on a “per lesion” basis, were the 1-year and 2-years actuarial local control (LC) rates and the objective response rate (ORR). The LC was the time interval between the end of CIRT and the evidence of a progression of disease (PD) in the field of treatment. The ORR was considered as the sum of complete response (CR) and partial response (PR) evaluated by dedicated imaging modalities: MRI (magnetic resonance imaging), computed tomography scan (CT), or positron emission tomography scan (PET). The acute (</= 6 months from the end of CIRT) and late (>6 months) toxicities were scored using RTOG/EORTC[15] and CTCAE version 5.0 scales [16] according to Center policy and represented the secondary endpoints.
-
b.
CIRT procedures and follow-up
CIRT in the treatment of oligo-MPR ovarian/fallopian tube cancers commenced in 2006 at QST and in 2019 at CNAO. Facility-specific protocols for this type of treatment followed the same principles in terms of plan preparation and optimization.
Tailor-made fixation cushions and thermoplastic shells were built for the immobilization of patients for acquiring a planning CT scan. For all lesions, the gross tumor volume (GTV) was defined on planning CT and rigidly fused with CT with contrast medium, and/or PET/CT and/or MRI. The clinical target volume (CTV) was obtained from the GTV with a margin set at 5 mm and, in the case of synchronous and really close GTV, a single CTV was obtained. According to the center policy: i) at QST the planning target volume (PTV) margins were considered based on the set-up margin, and personalized based on target and organ motion analysis (free breathing or four-dimensional respiratory-gated CT); ii) at CNAO positioning (±3 to 5 mm isocenter displacement) and range uncertainties (±3.5 %) were directly incorporated in a robust plan optimization to guarantee adequate CTV coverage; when respiratory motion was present, motion scenarios as depicted by four-dimensional respiratory-gated CTs were included in the optimization algorithm and optimized dose distributions on each respiratory phase were eventually inspected to verify the plan quality.
Concerning treatment plan preparation, CIRT beam treatments could be delivered with either a passive or an active irradiation technique. In the current study, passive irradiation used a broad radiation beam which was made uniform in 2-D by scatterers or wobbling magnets to cover the target volume, while the active method scans the tumor in 3-D using scanning magnets.
At QST beam delivery used both passive and active systems (the latter from 2012), while pencil-beam scanning beam delivery was implemented at CNAO from the beginning of its clinical activity. The delivered biological CIRT dose, which is defined as the physical dose multiplied by the Relative Biological Effect (RBE) and expressed in Gy[RBE], was calculated using different radiobiological models at the two facilities. In particular, the Microdosimetric Kinetic Model (MKM) was used at QST while the Local Effect Model I (LEM I) was used at CNAO. At the beginning of clinical activity at CNAO, LEM-based CIRT prescription doses for various body sites were translated from the NIRS-based system minimizing differences in physical dose distributions between treatment plans[17].
Both centres optimized CIRT plans with multiple beam ports and specific settings to take into account inter- and intra-fraction variations. In particular, for abdominal targets intra-fraction motion mitigation consisted of the acquisition of a 4DCT for patient simulation, 4D-robust optimization of treatment plans and gated dose delivery, combined with rescanning and abdominal compression. Weekly re-evaluative 4DCTs were used to assess the quality of plans throughout delivery. Based on these results, decisions on plan re-optimization and treatment implementation were made and, if necessary a Cone Beam CT was also scanned during treatment [18], [19], [20].
During treatment, clinical evaluation was performed at least once per week. After CIRT, patients were re-evaluated every 3–4 months either by a radiation oncologist or by a gynaecological oncologist with CT or/and MRI or/and PET/CT and tumor marker dosage; further radiological examinations were requested in case of suspicion progression.
Data management and Statistical analysis
Patients were pseudonymized and no personal identifiers were collected. Clinical data was transferred through a dedicated encrypted channel. A dedicated agreement was set up to manage data properly. All pseudonymized data were collected at CNAO into an electronic database. The data processing was performed by A.B., G.F. and K.M. Continuous variables were reported as median and interquartile ranges while counts and percentages were used to describe categorical variables. Actuarial outcomes were evaluated using the Kaplan-Meier method, while potential predictors were explored using the Log-rank test. Due to the expected low rate of local failure events, a multivariable analysis was not deemed reasonable. Bi-variable logistic regression was employed in the analysis of factors predicting the CR on a per-lesion basis. Quantitative variables were stratified based on their median value and adjusted in the logistic regression analysis for the binary variable identifying the radiobiological model (as well as the institution and ethnicity). 1y-LC, 2y-LC and ORR rates were provided with their binomial 95 % confidence intervals (95 % CI), and the two-sided type I error was set at 0.05. Statistical analyses were carried out using R version 4.0.1.
Results
Patient and tumor hallmarks
Twenty-six patients, accounting for a total of 36 lesions, underwent CIRT for oligo-MPR ovarian and fallopian tube cancers between 2006 and 2021. There were 58 % Asian and 42 % Caucasian patients. The median age at CIRT was 59.5 years (range: 44–81 years). Twenty-one patients were RT naïve, while 5 received CIRT for re-irradiation. As shown in Table 1, the primary histology included high-grade serous cells (N = 11; 42.3 %), mucinous (N = 3; 11.5 %), endometrioid (N = 2; 7.7 %), mixed mullerian (N = 5; 19.2 %), clear cells (N = 1; 3.8 %), undifferentiated (N = 1; 3.8 %) cancers. Lymph node lesions accounted for 42 % (N = 15), while parenchymal ones were 21 (abdominal = 14; pelvic = 5; brain = 2), for a total of 58 %. Data about genetic mutational status were available for 11 patients, 6 of them were wild type and the others carried the following mutation: BRCA2 (N = 1), BRCA1 (N = 2), FANCA (N = 1) and ERCC3 (N = 1). The volumes (GTVs) of lymph node lesions ranged between 1.2 and 157.69 cm3 while the parenchymal GTVs were between 1.02 and 256.83 cm3. The patient’s and tumour’s characteristics are shown in Table 1.
Table 1.
Tumor and treatment characteristics.
| Characteristics | N(%) |
|---|---|
| Histotype | |
|
11 (42.3) |
|
3 (11.5) |
|
2 (7.7) |
|
1 (3.8) |
|
5 (19.2) |
|
1 (3.8) |
|
3 (11.5) |
| Type of lesions | |
|
15(41.7) |
|
21(58.3) |
|
|
|
|
|
|
| Median (range) | |
| GTV volume | |
|
13.64 cm3 (1.2 -157.69 cm3) |
|
14.34 cm3 (1.02-256.83 cm3) |
| Doses | |
| Total dose | 52.8 Gy[RBE] (39-64 Gy[RBE]) |
| Number of fractions | 12 (10-16) |
Treatment characteristics
All patients underwent at least 1 cytoreductive surgery and at least 1 previous line of chemotherapy. No concomitant systemic therapies were administered during CIRT; PARP-I and anti-VEGF were stopped before CIRT and, overall, 4 patients received PARP-I (4 before and 2 also after) and 6 anti-VEGF before CIRT. Twenty-four women (92 %) received only one course of CIRT, whereas 2 (8 %) underwent a second course for an out-of-field recurrence. The median prescribed total dose was 52.8 Gy[RBE] (range:39–64 Gy[RBE]) in median 12 (range: 10–16) fractions. The median GTV was 14 cm3 (range:1.02–256.83), whereas the CTV was 72.92 cm3 (range: 7.9–256.83). In 5 cases, the synchronous MPR led to different and close GTVs that were treated in a single CIRT course and included in a single CTV.
Outcomes according to the endpoints
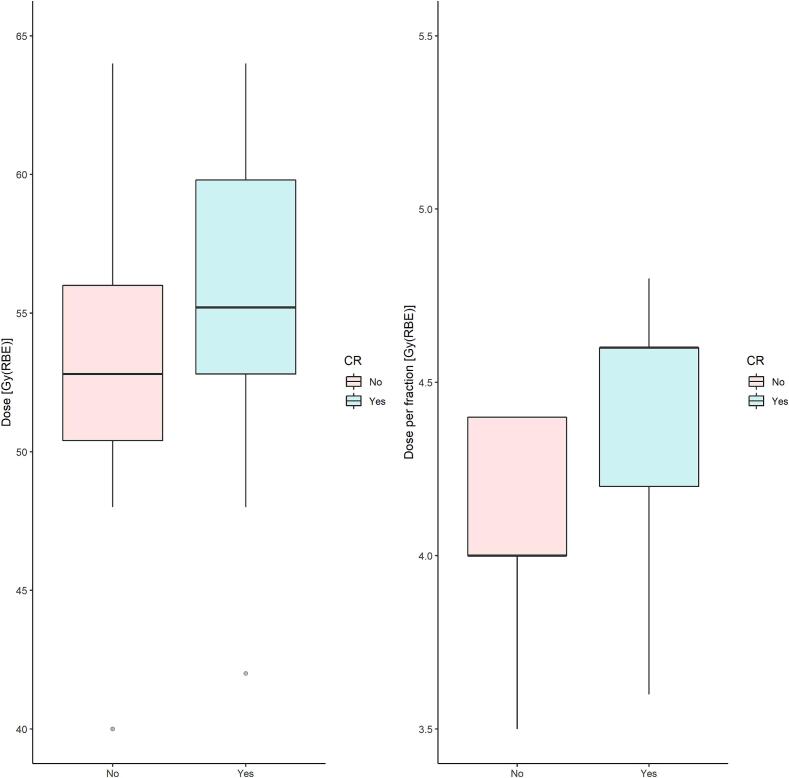
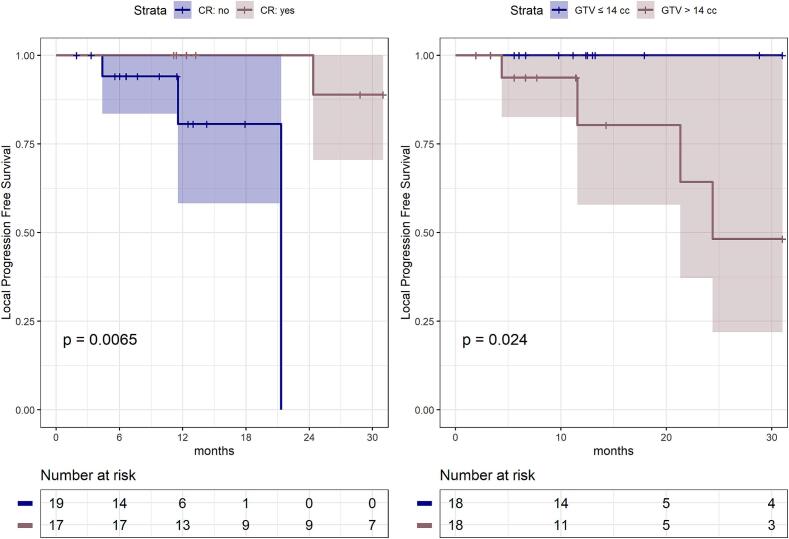
Within 12 months after CIRT, 17 lesions (47 %, 95 % CI: 31 %-64 %) achieved CR while 18 (50 %, 95 % CI: 34 %-66 %) a PR and 1 (3 %, 95 % CI: 0 %-8%) a PD. Overall, the ORR was 97 % (95 % CI: 92 %-100 %). We observed that the dose per fraction > 4.2 Gy[RBE] as well as the total dose > 52.8 Gy[RBE] were significantly related to the higher chance to achieve a CR (odds-ratio = 4.5, 95 %CI: 1.12–21.05, p = 0.04; odds-ratio: 4.05CI: 1.04–17.75, p = 0.05, respectively) as shown in Fig. 1. Age (>/≤ 60 years), GTV (>/≤ 14 cm 3), tumor site (lymph nodes vs parenchymal), different radiobiological models (LEM-1 vs MKM) applied for CIRT planning in the two centers along with differences in ethnicity (Asian vs Caucasian) appeared unrelated to the CR. After a median follow-up of 13 months (6–193 months), the 1-year LC was 92 % (95 % CI: 82 %- 100 %), and the 2-year LC was 83 % (95 % CI: 65 %-100 %). The high chance of achieving LC was unrelated to age (p = 0.92), dose per fraction (p = 0.14), total dose (p = 0.71), the radiobiological model used (p = 0.32), and tumor site (p = 0.62). However, the achievement of a CR (p = 0.007) as well as the GTV ≤ 14 cm3 (p = 0.024) resulted significantly associated with a better LC rate (Fig. 2).
Fig. 1.
Boxplot of the total dose and dose per fraction (Gy[RBE]) distributions, based on the achievement of complete response (CR).
Fig. 2.
Kaplan-Meier local progression-free survival curves, with respect to the complete response (CR) achievement and GTV ≤/> 14 cc.
With regards to toxicity, the treatment showed a high profile of safety, indeed CIRT was well tolerated and no interruption was needed. Only one case of grade 3 EORTC/RTOG enterocolitis in the late phase was observed and no grade ≥ 3 toxicities were recorded in re-irradiated patients (Table 2). PARP-i and anti-VEGF administered in 4 and 6 patients, respectively, seemed not to exacerbate the risk of severe toxicities. Patients with a BRCA mutation did not experience a grade ≥ 2 toxicity.
Table 2.
Toxicity.
| Toxiticy | Acute Toxicity | Late Toxicity |
|---|---|---|
| N (%) | N (%) | |
| All | 7 (26.9%) | 8 (30.8%) |
| Skin | ||
| G1 | 1 (3.8%) | 1 (3.8%) |
| Upper gastrointestinal disorders | ||
| G1 | 2 (7.69%) | - |
| Lower gastrointestinal disorder | ||
| G2 | - | 1 (3.8%) |
| G3 | - | 1 (3.8%) |
| Neuropathy | ||
| G1 | 1(3.8%) | 2(7.7%) |
| G2 | 3(11.53%) | 2(7.7%) |
| Lung | ||
| G1 | - | 1(3.8%) |
Discussion
In the last years, RT has taken on a pivotal role in the treatment of MPR ovarian and fallopian tube cancers showing a high safety profile, achieving a favourable LC and a promising rate of CR [9], [14], [21]. Even if several clinical trials investigated so far the role of CIRT in gynaecological tumors [12], there are no data concerning oligo-MPR ovarian/fallopian tube cancers. However, considering the radiobiological features of ovarian cancers[22] as well as the peculiar ballistic and radiobiological properties of carbon ions, resulting in an enhanced anti-tumor effect, especially in radioresistant histologies or tumors located in critical anatomical areas and surrounding high radiosensitive organs[23], CIRT might be theoretically advantageous in this setting.
To test this hypothesis, we evaluated for the first time the role of CIRT in MPR ovarian and fallopian tube cancers demonstrating the effectiveness and safety of this approach. Despite the small cohort and the short follow-up, CIRT provided in this real-world study a slightly better ORR compared to the largest photon beam data (97 % vs 89 % respectively)[9]. Within 12 months, 47 % of the lesions (95 % CI: 31 %-64 %) achieved CR that, among all clinical and RT parameters evaluated, seemed to be related only with the dose per fraction (>4.2 Gy[RBE]; odds-ratio = 4.5, 95 %CI: 1.12–21.05) and total dose (>52,8 Gy[RBE]; odds-ratio: 4.05 CI: 1.04–17.75). Moreover, the achievement of a CR emerged as a predictive factor for the LC (p = 0.007).
In oligometastatic settings, the achievement of high ORR has proved to be a pivotal goal of any locoregional treatment, including RT [24], allowing in the case of ovarian cancer to raise a “drug-free holiday”, crucial in the chronicization of the disease. In this “PARP-I era”, all efforts should be made to avoid a rechallenge with cytotoxic platinum-based chemotherapy, considering that platinum-sensitive MPR ovarian cancers are eligible for PARP-I [25]. This issue is crucial, knowing that, in the OReO/ENGOT Ov-38 trial the maintenance with olaparib rechallenge was effective in overall progression-free survival regardless of BRCA status [26]. Moreover, both research and real-life data suggested that response rates to chemotherapy for progression after PARP-I appear to be lower than those expected according to platinum-free interval, highlighting the need for new therapeutic options for this growing population[27], [28]. In this challenging scenario also entered our pilot study in which CIRT demonstrated a clinical benefit (defined in SBRT studies as the sum of ORR and SD after RT) of 97 %, as well as a high rate of LC (92 % and 83 % at 1 and 2 years, respectively) that well matched with the historical SBRT cohort [9], [14]. Differently from the SBRT series on gynaecological tumors [29], we experienced no acute nor late haematological toxicities and no grade > 3 adverse events.
Considering the availabilities of CIRT facilities worldwide and the encouraging SBRT data, the question is whether CIRT might play a role in MPR ovarian cancers and, if yes, how to accurately select patients to enrol for CIRT.
The selection of patients to treat with CIRT is the most important question considering its high cost compared to photons and the systemic characteristics of ovarian and fallopian tubes at the diagnosis. In our bi-institutional experience, indeed, only patients considered candidates for a locoregional approach by a multidisciplinary team, but for whom the other less expensive strategies (such as photon beam RT, and other regional approaches) were excluded or strongly refused by the patients, were evaluated for CIRT. For the natural story of the disease, it is not uncommon that a patient receives several courses of RT and, in these cases, the challenge is to deliver high doses to the GTVs but, at the same time, to spare the already radiotreated surrounding tissues. Moreover, BRCA mutation increases the risk of a high rate of toxicity after RT and, in this era of precision RT, all efforts should be made to reduce the morbidity of these treatments [30]. The physical hallmarks of particle radiotherapy, such as CIRT and protons, could guarantee an increased dose to the target minimizing the side effects on the surrounding organs, as well as in case of re-irradiation [31].
Moreover, MPR patients arrived from several systemic treatments and it appears crucial to reduce the risk of haematological toxicities to allow the maintenance treatment after CIRT. Besides, lymphopenia can be also a negative prognostic factor for cancer patients [32] and sparing the bone marrow and the circulating lymphocytes can be pivotal in the management of oligorecurrent and oligometastatic disease. Published data showed fewer chromosomal aberrations in peripheral blood lymphocytes in CIRT compared to photon RT patients, due to the volume of normal tissue exposed [33]; indeed CIRT reduces the risk of bone marrow morbidity and increases the possibility of triggering a significant immune response. The latter was essential in case of combination with immunotherapy, also for metastatic immune-cold tumors, such as ovarian cancers, in which RT seems to be able to reprogramme the tumor microenvironment inducing a systemic control of the disease when combined with immunotherapy[34].
In addition, the main rationale for using CIRT in ovarian cancers (and consequently for understanding how to better select patients to treat with heavy ions) could be found in the peculiar biology of this tumour and its response to different radiations [22]. In fact, after RT, the DNA double-strand breaks (DSBs) damage is mainly repaired by non-homologous end joining (NHEJ) and homologous recombination (HR) pathways. However, the cluster DNA DSBs produced by CIRT might activate also alternative and error-prone repair pathways [23], [35], one of which is based on microhomology-mediated recombination that follows the DNA damages [36]. It has been described that NHEJ is the choice pathway after photons, but HR significantly increased after CIRT [37] compared to photons in repair-deficient cell lines [38], [39]. In this context, Grosse et al [39] described a more radiosensitive effect of protons compared to photons in proliferating Chinese hamster ovary cells lacking HR proteins [39]. Moreover, Gerelchuluun et al [37] tested the response to gamma rays, proton, and carbon ions of hamster cell lines ovary AA8, lung fibroblast V79 and different sublines such as V3 and XR1 (lacking the NHEJ pathway), and irs1 and irs1SF cells (lacking the HR pathway). In this experiment, the authors demonstrated that wild-type cells were most radioresistant, followed by the HR-deficient irs1, NHEJ-deficient XR1, HR-deficient irs1SF, and NHEJ-deficient V3 cells to all radiation types examined. In particular, the response to gamma rays and protons was similar in all the cells, but the wild-type and HR-deficient cell lines were more strongly sensitized to CIRT than the NHEJ-deficient cell lines. Taken together, these data can be clinically significant for the stratification of MPR ovarian cancers carrying mutations in DNA, to treat with photons vs CIRT.
Another issue is whether particle therapy has additional benefit, and a better safety profile, compared to photons when combined with PARP-I. Bi et al. compared the radiosensitizing activity on BRCA1-proficient and BRCA1-deficient high-grade serous ovarian carcinoma cell lines, reporting a worse response to RT alone in BRCA1-proficient cells accompanied also by a lesser PARP-I radiosensitization effect, but a higher growth inhibition with Olaparib and RT in BRCA1-deficient tumors [40]. In the combined strategy with PARP-I and RT, the main concern is the risk of worsening RT-related toxicity [41] particularly in the case of rapidly proliferation tissues [42]. To note that currently there is no consensus about the PARP-I reduction or the optimal time to suspend PARP-I before and after RT in MPR ovarian cancers. In our clinical practice, PARP-I were stopped before the treatment and, similarly to the SBRT published data, [25] this approach seemed not to increase toxicity.
The retrospective nature, the small cohort, the absence of a comparison group and the short follow-up are undeniable limitations of the current study. Moreover, the small number of treated lesions and the missing data on the molecular status prevent an appropriate multivariable analysis in predictors evaluation. We have to acknowledge that the physician-reported toxicity score could have been biased and longer follow-up is needed to reduce the risk of underestimating the rate of late toxicities.
However, the homogeneity of the treatment, the methodology used in the analysis and the fact that this is, to the best of our knowledge, the first real-world assessment of the role of CIRT in this challenging setting, are the main strengths of the present bi-institutional analysis. The radiobiological data concerning the radioresistance/radiosensitivity of ovarian cancers according to their mutational status, the advantages of CIRT in radioresistant cells along with its efficacy and safety provided in the current study, might be the basis for future trials aimed to find a tailored RT strategy (SBRT vs CIRT) stratifying MPR ovarian cancers according to BRCA and HR status.
Conclusion
Herein it was described the efficacy of CIRT, accompanied by mild toxicity, in oligo-MPR ovarian and fallopian tube cancers. Further studies are needed to find the optimal selection criteria of patients to treat with CIRT and to guarantee the best-tumour-tailored personalized RT strategy. A strong collaboration between radiation oncologists, experts of SBRT and CIRT, medical oncologists and gynaecologists is warranted to guarantee the best RT option for ovarian cancer patients, according to the biology of the disease. Further collaborative studies might be worthwhile in the better comprehension of the underlying mechanisms of radioresistance and radiosensitivity in order to explore new treatment approaches.
Author contributions
Conceptualization and project development: A.B..; Methodology: A.B. and G.F.; Data collection: A.B. and M.K.; Statistical analysis: G.F; Data analysis interpretation: A.B, G.F, N.O and M.K.; Manuscript writing: A.B.; Review and Editing: All authors.; Supervision: N.O. and E.O. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that they have no funding and no financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to thank Dr Nadia Facchinetti, Dr Federica Serra and Dr Cristina Bono for their precious support.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.du Bois A., Pfisterer J. First-line therapy in ovarian cancer. Zentralbl Gynakol. 2004;126:312–314. doi: 10.1055/s-2004-820390. [DOI] [PubMed] [Google Scholar]
- 3.Pignata S, C Cecere S, Du Bois A, Harter P, Heitz F. Treatment of recurrent ovarian cancer. Ann Oncol Off J Eur Soc Med Oncol 2017;28:viii51–6. Doi: 10.1093/annonc/mdx441. [DOI] [PubMed]
- 4.Lin Q., Liu W., Xu S., Shang H., Li J., Guo Y., et al. PARP inhibitors as maintenance therapy in newly diagnosed advanced ovarian cancer: a meta-analysis. BJOG. 2021;128:485–493. doi: 10.1111/1471-0528.16411. [DOI] [PubMed] [Google Scholar]
- 5.Mittica G., Ghisoni E., Giannone G., Genta S., Aglietta M., Sapino A., et al. PARP Inhibitors in Ovarian Cancer. Recent Pat Anticancer Drug Discov. 2018;13:392–410. doi: 10.2174/1574892813666180305165256. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez Secord A., O’Malley D.M., Sood A.K., Westin S.N., Liu J.F. Rationale for combination PARP inhibitor and antiangiogenic treatment in advanced epithelial ovarian cancer: A review. Gynecol Oncol. 2021;162:482–495. doi: 10.1016/j.ygyno.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Bonadio R.C., Estevez-Diz M.D.P. Perspectives on PARP Inhibitor Combinations for Ovarian Cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.754524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadducci A., Aletti G.D., Landoni F., Lazzari R., Mangili G., Olivas P., et al. Management of ovarian cancer: guidelines of the Italian Medical Oncology Association (AIOM) Tumori. 2021;107:100–109. doi: 10.1177/0300891620966382. [DOI] [PubMed] [Google Scholar]
- 9.Macchia G., Lazzari R., Colombo N., Laliscia C., Capelli G., D’Agostino G.R., et al. A Large, Multicenter, Retrospective Study on Efficacy and Safety of Stereotactic Body Radiotherapy (SBRT) in Oligometastatic Ovarian Cancer (MITO RT1 Study): A Collaboration of MITO, AIRO GYN, and MaNGO Groups. Oncologist. 2020;25:e311–e320. doi: 10.1634/theoncologist.2019-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma V., Simone C.B., 2nd, Wahl A.O., Beriwal S., Mehta M.P. Proton radiotherapy for gynecologic neoplasms. Acta Oncol. 2016;55:1257–1265. doi: 10.1080/0284186X.2016.1205218. [DOI] [PubMed] [Google Scholar]
- 11.Taunk N. The role of proton therapy in gynecological radiation oncology. Int J Gynecol Cancer off J Int Gynecol Cancer Soc. 2022;32:414–420. doi: 10.1136/ijgc-2021-002459. [DOI] [PubMed] [Google Scholar]
- 12.Wang L., Wang X., Zhang Q., Ran J., Geng Y., Feng S., et al. Is there a role for carbon therapy in the treatment of gynecological carcinomas? A Systematic Review. Future Oncol. 2019;15:3081–3095. doi: 10.2217/fon-2019-0187. [DOI] [PubMed] [Google Scholar]
- 13.Macchia G., Jereczek-Fossa B.A., Lazzari R., Cerrotta A., Deodato F., Ippolito E., et al. Efficacy and safety of stereotactic body radiotherapy (SBRT) in oligometastatic/persistent/recurrent ovarian cancer: a prospective, multicenter phase II study (MITO-RT3/RAD) Int J Gynecol Cancer off J Int Gynecol Cancer Soc. 2022;32:939–943. doi: 10.1136/ijgc-2021-002709. [DOI] [PubMed] [Google Scholar]
- 14.Lazzari R., Ronchi S., Gandini S., Surgo A., Volpe S., Piperno G., et al. Stereotactic Body Radiation Therapy for Oligometastatic Ovarian Cancer: A Step Toward a Drug Holiday. Int J Radiat Oncol Biol Phys. 2018;101:650–660. doi: 10.1016/j.ijrobp.2018.03.058. [DOI] [PubMed] [Google Scholar]
- 15.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-c. [DOI] [PubMed] [Google Scholar]
- 16.US Department of Health and Human Services National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. n.d.
- 17.Fossati P., Molinelli S., Matsufuji N., Ciocca M., Mirandola A., Mairani A., et al. Dose prescription in carbon ion radiotherapy: A planning study to compare NIRS and LEM approaches with a clinically-oriented strategy. Phys Med Biol. 2012;57:7543–7554. doi: 10.1088/0031-9155/57/22/7543. [DOI] [PubMed] [Google Scholar]
- 18.Molinelli S., Vai A., Russo S., Loap P., Meschini G., Paganelli C., et al. The role of multiple anatomical scenarios in plan optimization for carbon ion radiotherapy of pancreatic cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2022;176:1–8. doi: 10.1016/j.radonc.2022.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Kubota Y., Okamoto M., Shiba S., Okazaki S., Matsui T., Li Y., et al. Robustness of daily dose for each beam angle and accumulated dose for inter-fractional anatomical changes in passive carbon-ion radiotherapy for pancreatic cancer: Bone matching versus tumor matching. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2021;157:85–92. doi: 10.1016/j.radonc.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Mori S., Zenklusen S., Inaniwa T., Furukawa T., Imada H., Shirai T., et al. Conformity and robustness of gated rescanned carbon ion pencil beam scanning of liver tumors at NIRS. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2014;111:431–436. doi: 10.1016/j.radonc.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Iftode C., DʼAgostino G.R., Tozzi A., Comito T., Franzese C., De Rose F., et al. Stereotactic Body Radiation Therapy in Oligometastatic Ovarian Cancer: A Promising Therapeutic Approach. Int J Gynecol Cancer off J Int Gynecol Cancer Soc. 2018;28:1507–1513. doi: 10.1097/IGC.0000000000001324. [DOI] [PubMed] [Google Scholar]
- 22.Barcellini A, Charalampopoulou A, De Cecco L, Fodor A, Rabaiotti E, Candotti G, et al. Ovarian Cancer Radiosensitivity: What Have We Understood So Far? Life (Basel, Switzerland) 2022;13. Doi: 10.3390/life13010006. [DOI] [PMC free article] [PubMed]
- 23.Tinganelli W., Durante M. Carbon Ion Radiobiology. Cancers (basel) 2020;12 doi: 10.3390/cancers12103022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willmann J., Vlaskou Badra E., Adilovic S., Christ S.M., Ahmadsei M., Mayinger M., et al. Stereotactic body radiotherapy to defer systemic therapy in patients with oligorecurrent disease. Clin Transl Radiat Oncol. 2022;37:12–18. doi: 10.1016/j.ctro.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palluzzi E., Marchetti C., Cappuccio S., Avesani G., Macchia G., Gambacorta M.A., et al. Management of oligometastatic ovarian cancer recurrence during PARP inhibitor maintenance. Int J Gynecol Cancer off J Int Gynecol Cancer Soc. 2022 doi: 10.1136/ijgc-2022-003543. [DOI] [PubMed] [Google Scholar]
- 26.Selle F., Asselain B., Montestruc F., Bazan F., Pardo B., Salutari V., et al. OReO/ENGOT Ov-38 trial: Impact of maintenance olaparib rechallenge according to ovarian cancer patient prognosis—An exploratory joint analysis of the BRCA and non-BRCA cohorts. J Clin Oncol. 2022;40:5558. doi: 10.1200/JCO.2022.40.16_suppl.5558. [DOI] [Google Scholar]
- 27.Frenel J.S., Kim J.W., Aryal N., Asher R., Berton D., Vidal L., et al. Efficacy of subsequent chemotherapy for patients with BRCA1/2-mutated recurrent epithelial ovarian cancer progressing on olaparib versus placebo maintenance: post-hoc analyses of the SOLO2/ENGOT Ov-21 trial. Ann Oncol off J Eur Soc Med Oncol. 2022;33:1021–1028. doi: 10.1016/j.annonc.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Gadducci A., Cosio S., Landoni F., Lissoni A.A., Zola P., Laudani M.E., et al. Response to Chemotherapy and Clinical Outcome of Patients With Recurrent Epithelial Ovarian Cancer After PARP Inhibitor Maintenance Treatment: A Multicenter Retrospective Italian Study. Anticancer Res. 2022;42:2017–2022. doi: 10.21873/anticanres.15681. [DOI] [PubMed] [Google Scholar]
- 29.Macchia G., Pezzulla D., Campitelli M., Laliscia C., Fodor A., Bonome P., et al. Efficacy and Safety of Stereotactic Body Radiation Therapy in Oligometastatic Uterine Cancer (MITO-RT2/RAD): A Large, Real-World Study in Collaboration With Italian Association of Radiation Oncology, Multicenter Italian Trials in Ovarian Cancer, and Mari. Int J Radiat Oncol Biol Phys. 2023;117:321–332. doi: 10.1016/j.ijrobp.2023.04.025. [DOI] [PubMed] [Google Scholar]
- 30.Pereira S, Orlandi E, Deneuve S, Barcellini A, Chalaszczyk A, Behm-Ansmant I, et al. The Normal, the Radiosensitive, and the Ataxic in the Era of Precision Radiotherapy: A Narrative Review. Cancers (Basel) 2022;14. https://doi.org/10.3390/cancers14246252. [DOI] [PMC free article] [PubMed]
- 31.Shiba S., Okonogi N., Kato S., Wakatsuki M., Kobayashi D., Kiyohara H., et al. Clinical Impact of Re-irradiation with Carbon-ion Radiotherapy for Lymph Node Recurrence of Gynecological Cancers. Anticancer Res. 2017;37:5577–5583. doi: 10.21873/anticanres.11991. [DOI] [PubMed] [Google Scholar]
- 32.Venkatesulu B.P., Mallick S., Lin S.H., Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51. doi: 10.1016/j.critrevonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Durante M., Yamada S., Ando K., Furusawa Y., Kawata T., Majima H., et al. X-rays vs. carbon-ion tumor therapy: cytogenetic damage in lymphocytes. Int J Radiat Oncol Biol Phys. 2000;47:793–798. doi: 10.1016/s0360-3016(00)00455-7. [DOI] [PubMed] [Google Scholar]
- 34.Herrera F.G., Ronet C., Ochoa de Olza M., Barras D., Crespo I., Andreatta M., et al. Low-Dose Radiotherapy Reverses Tumor Immune Desertification and Resistance to Immunotherapy. Cancer Discov. 2022;12:108–133. doi: 10.1158/2159-8290.CD-21-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yajima H., Fujisawa H., Nakajima N.I., Hirakawa H., Jeggo P.A., Okayasu R., et al. The complexity of DNA double strand breaks is a critical factor enhancing end-resection. DNA Repair (amst) 2013;12:936–946. doi: 10.1016/j.dnarep.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Sallmyr A., Tomkinson A.E. Repair of DNA double-strand breaks by mammalian alternative end-joining pathways. J Biol Chem. 2018;293:10536–10546. doi: 10.1074/jbc.TM117.000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerelchuluun A., Manabe E., Ishikawa T., Sun L., Itoh K., Sakae T., et al. The major DNA repair pathway after both proton and carbon-ion radiation is NHEJ, but the HR pathway is more relevant in carbon ions. Radiat Res. 2015;183:345–356. doi: 10.1667/RR13904.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fontana A.O., Augsburger M.A., Grosse N., Guckenberger M., Lomax A.J., Sartori A.A., et al. Differential DNA repair pathway choice in cancer cells after proton- and photon-irradiation. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2015;116:374–380. doi: 10.1016/j.radonc.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Grosse N., Fontana A.O., Hug E.B., Lomax A., Coray A., Augsburger M., et al. Deficiency in homologous recombination renders Mammalian cells more sensitive to proton versus photon irradiation. Int J Radiat Oncol Biol Phys. 2014;88:175–181. doi: 10.1016/j.ijrobp.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 40.Bi Y., Verginadis I.I., Dey S., Lin L., Guo L., Zheng Y., et al. Radiosensitization by the PARP inhibitor olaparib in BRCA1-proficient and deficient high-grade serous ovarian carcinomas. Gynecol Oncol. 2018;150:534–544. doi: 10.1016/j.ygyno.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Barcellini A, Loap P, Murata K, Villa R, Kirova Y, Okonogi N, et al. PARP Inhibitors in Combination with Radiotherapy: To Do or Not to Do? Cancers (Basel) 2021;13. Doi: 10.3390/cancers13215380. [DOI] [PMC free article] [PubMed]
- 42.de Haan R., van den Heuvel M.M., van Diessen J., Peulen H.M.U., van Werkhoven E., de Langen A.J., et al. Phase I and Pharmacologic Study of Olaparib in Combination with High-dose Radiotherapy with and without Concurrent Cisplatin for Non-Small Cell Lung Cancer. Clin Cancer Res an off J Am Assoc Cancer Res. 2021;27:1256–1266. doi: 10.1158/1078-0432.CCR-20-2551. [DOI] [PubMed] [Google Scholar]